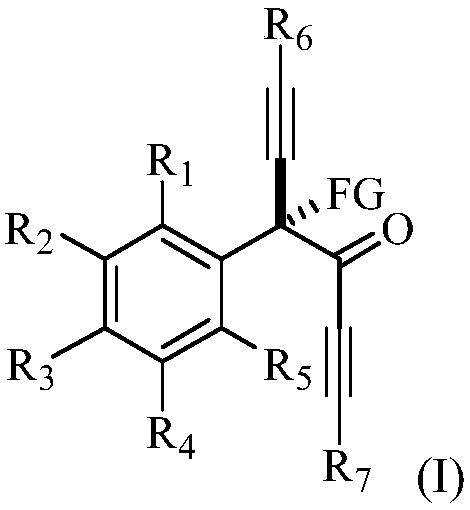
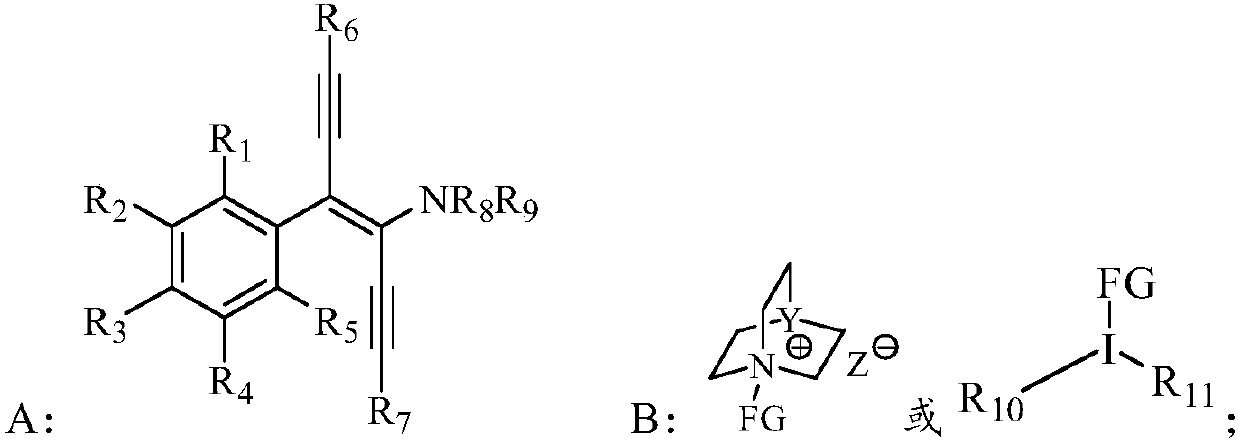
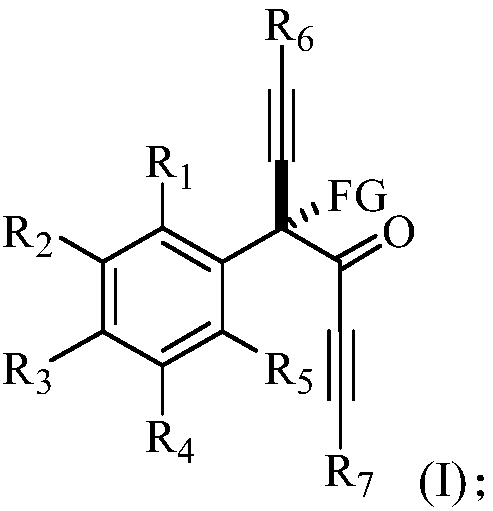
Alpha-tetra-substituted chiral acetyenic ketone compound, as well as preparation method and application thereof
A compound and four-substitution technology, applied in the field of chiral compound synthesis, can solve the problems of unfavorable subsequent conversion of products, limited product application range, harsh preparation conditions, etc., achieve safe and controllable reaction process, avoid heating or high pressure conditions, simplify The effect of the operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0116] This example provides a chiral R-4-fluoro-4-phenyl-1,6-bis(triisopropylsilyl)-n-hexane-1,5-diyn-3-one and its preparation method . The structural formula of the R-4-fluoro-4-phenyl-1,6-bis(triisopropylsilyl) n-hexane-1,5-diyn-3-one is shown in the following molecular structural formula I1:
[0117]
[0118] Its preparation steps are as follows:
[0119] Add homo-S-3,3'-bis(2,4,6-triisopropylphenyl)-6,6'-dinitro-binaphthyl phosphate (0.005mmol, 0.1 eq), sodium carbonate (0.10mmol, 2.0eq), 1-chloromethyl-4-fluoro-1,4-diazabicyclo[2.2.2]octane bis(tetrafluoroborate) salt (0.06mmol, 1.2 eq) and 0.5 mL of anhydrous m-xylene, replaced with argon three times, sealed the reaction test tube and stirred at room temperature for 10 min, then placed it in a -30°C cold trap. (R,trans)-3-tert-butyldiphenylsilyloxy-1-(4-phenyl-1,6-bis(triisopropylsilyl)n-hexyl-3-ene-1, A solution of 5-diyne-3-)pyrrolidine (0.05mmol, 1.00eq) in m-xylene was slowly added to the reaction system, an...
Embodiment 2
[0121] This example provides a chiral R-4-fluoro-4-(m-methylphenyl)-1,6-bis(triisopropylsilyl)-n-hexane-1,5-diyne-3- Ketones and methods for their preparation. The structural formula of the R-4-fluoro-4-(m-methylphenyl)-1,6-bis(triisopropylsilyl)-n-hexane-1,5-diyn-3-one is as follows molecular structure formula I2 Show:
[0122]
[0123] Its preparation steps are as follows:
[0124] Add homo-S-3,3'-bis(2,4,6-triisopropylphenyl)-6,6'-dinitro-binaphthyl phosphate (0.005mmol, 0.1 eq), sodium carbonate (0.10mmol, 2.0eq), 1-chloromethyl-4-fluoro-1,4-diazabicyclo[2.2.2]octane bis(tetrafluoroborate) salt (0.06mmol, 1.2 eq) and 0.5 mL of anhydrous m-xylene, replaced with argon three times, sealed the reaction test tube and stirred at room temperature for 10 min, then placed it in a -30°C cold trap. (R,trans)-3-tert-butyldiphenylsilyloxy-1-(4-m-methylphenyl-1,6-bis(triisopropylsilyl)n-hexyl-3-ene The m-xylene solution of -1,5-diyne-3-)pyrrolidine (0.05mmol, 1.00eq) was slowly ...
Embodiment 3
[0126] This example provides a chiral R-4-fluoro-4-(p-methylphenyl)-1,6-bis(triisopropylsilyl)-n-hexane-1,5-diyne-3- Ketones and methods for their preparation. The structural formula of the R-4-fluoro-4-(p-methylphenyl)-1,6-bis(triisopropylsilyl)-n-hexane-1,5-diyn-3-one is shown in molecular structural formula I3 Show:
[0127]
[0128] Its preparation steps are as follows:
[0129] Add homo-S-3,3'-bis(2,4,6-triisopropylphenyl)-6,6'-dinitro-binaphthyl phosphate (0.005mmol, 0.1 eq), sodium carbonate (0.10mmol, 2.0eq), 1-chloromethyl-4-fluoro-1,4-diazabicyclo[2.2.2]octane bis(tetrafluoroborate) salt (0.06mmol, 1.2 eq) and 0.5 mL of anhydrous m-xylene, replaced with argon three times, sealed the reaction test tube and stirred at room temperature for 10 min, then placed it in a -30°C cold trap. (R,trans)-3-tert-butyldiphenylsilyloxy-1-(4-p-methylphenyl-1,6-bis(triisopropylsilyl)n-hexyl-3-ene The m-xylene solution of -1,5-diyne-3-)pyrrolidine (0.05mmol, 1.00eq) was slowly a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com