Diparacyclic acetal compounds and their preparation methods and applications
A technology of compounds and cyclic acetals, which is applied in the direction of silicon organic compounds, chemical instruments and methods, compounds of group 4/14 elements of the periodic table, etc., can solve the problem of di-paracyclic acetal compounds that cannot undergo multiple substitution reactions Due to problems such as structural limitations, the reaction process is safe and controllable, the possibility of becoming a drug is improved, and the forward reaction rate is high.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0062] Correspondingly, the embodiment of the present invention also provides a preparation method of bis-cyclic acetal compounds. The method comprises the steps of:
[0063] S01. provide nucleophile compound, the general structural formula of the nucleophile compound is
[0064] S02. Provide allyl alcohol compounds, the general structural formula of the allyl alcohol compounds is
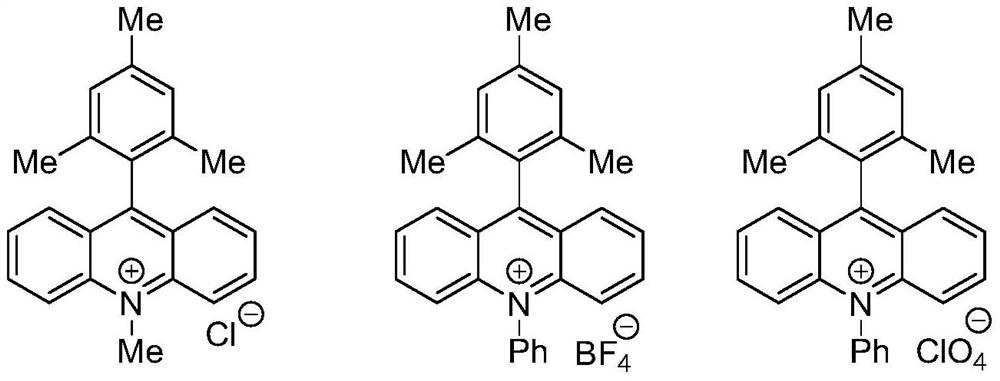
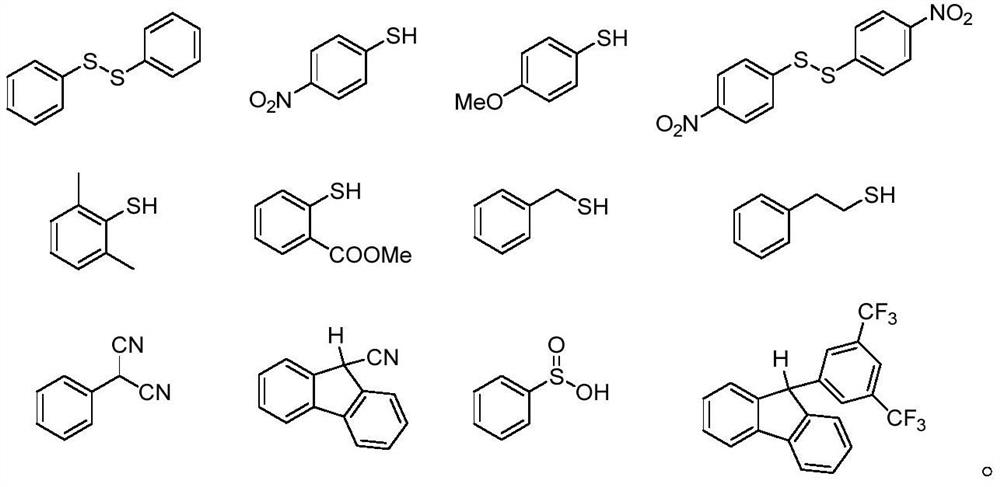
[0065] S03. Mixing the nucleophile compound, the allyl alcohol compound, the acridinium photocatalyst and the hydrogen transfer reagent, adding a solvent to dissolve to obtain a reaction solution;
[0066] S04. Carrying out the cyclization reaction of the reaction solution under the irradiation of visible light to obtain the bis-cycloacetal compound;
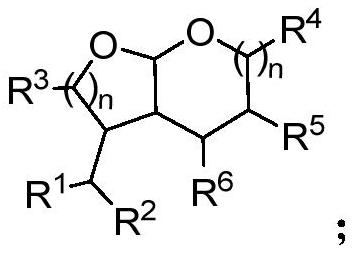
[0067] The general structural formula of the bis-cyclic acetal compound is where the R 1 , R 2 , R 3 each independently selected from C 1 -C 20 Alkyl, C 1 -C 20 Alkoxy, aryl, substituted aryl, heteroaryl, substituted heteroaryl, arylox...
Embodiment 1
[0090] This example provides 3-(4-methoxybenzyl)2,3,4,5-tetrahydrofuro[2,3-b]furan and its preparation method. The structural formula of the 3-(4-methoxybenzyl) 2,3,4,5-tetrahydrofuro[2,3-b]furan is shown in the following molecular structural formula I1:
[0091]
[0092] Its preparation steps are as follows: In a dry 8mL test tube, add a photocatalyst (0.01mmol, 0.05eq), diphenyl disulfide (0.04mmol, 0.2eq), 4-methyl Oxycinnamyl alcohol (0.2mmol, 1eq) and 3.0mL of anhydrous 1,2-dichloroethane, replaced by argon three times, after the reaction test tube was sealed, 2,3-dihydrofuran (0.8mmol, 4eq) was slowly added to the reaction The system was stirred for 18 hours at room temperature under blue light irradiation. After the reaction was completed, the reaction solution was spin-dried and separated by column chromatography to obtain the target product, a colorless oily liquid, with a yield of 60% and a dr value of 1.9:1. Correlation characterization analysis, the result is:...
Embodiment 2
[0094] This example provides a kind of 3-benzyl-2,3,4,5-tetrahydrofuro[2,3-b]pyran and its preparation method. The structural formula of the 3-benzyl-2,3,4,5-tetrahydrofuro[2,3-b]pyran is shown in the following molecular structural formula I2:
[0095]
[0096] Its preparation steps are as follows: in a dry 8mL test tube, add a photocatalyst (0.01mmol, 0.05eq), diphenyl disulfide (0.04mmol, 0.2eq), trans-cinnamon Alcohol (0.2mmol, 1eq) and 3.0mL of anhydrous 1,2-dichloroethane, replaced by argon three times, after the reaction test tube was sealed, 3,4-dihydro-2H-pyran (0.8mmol, 4eq) was added slowly The reaction system was stirred for 18 hours at room temperature under blue light irradiation. After the reaction was completed, the reaction solution was spin-dried and separated by column chromatography to obtain the target product, a colorless oily liquid, with a yield of 81% and a dr value of 1.8:1. Correlation characterization analysis, the result is: Cis-syn: 1 H NMR (...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com