Chiral 1,2-diamine compound and its preparation method and application
A technology for diamines and compounds, applied in the field of organic compound synthesis, can solve the problems of limited application range of 1,2-diamine compounds, harsh preparation conditions, complicated processes, etc., and meet the requirements of safe and controllable reaction process and reaction conditions. Low, simple process effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
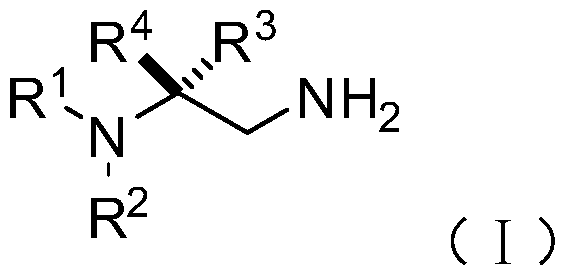
[0074] On the other hand, on the basis of the above-mentioned chiral 1,2-diamine compound of the present invention, the embodiment of the present invention also provides the chiral 1,2-diamine compound of the above molecular structure general formula (I). A preparation method of amine compound. The method comprises the steps of:
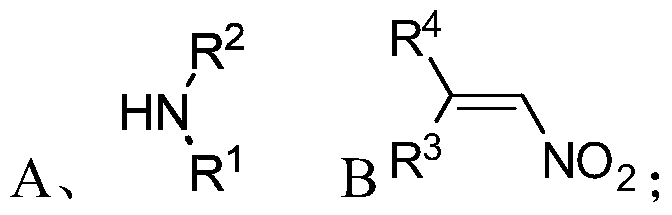
[0075] S01: respectively provide the fatty chain amine compound A and the nitroalkene compound B represented by the following structural formula:
[0076]
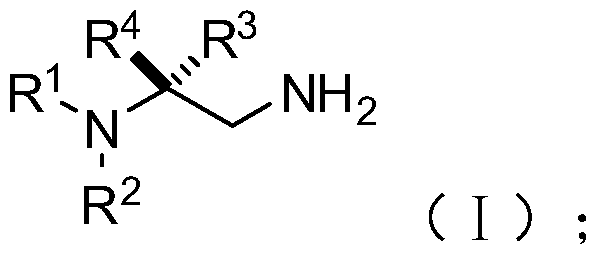
[0077] S02: adding the aliphatic chain amine compound A and the nitroalkene compound B into a reaction system containing an azacarbene catalyst, a proton additive, an alkali reagent and a water-absorbing additive to react at a temperature of -80-25°C to obtain The following structural general formula is a chiral 1,2-diamine compound shown in (I),
[0078]
[0079] Specifically, in the above step S01, R in the molecular structural formula of fatty chain amine compound A 1 , R 2 The repre...
Embodiment 1
[0100] This example provides (R)-N-benzyl-3,3,3-trifluoro-2-phenylpropyl-1,2-diamine and its preparation method. The structural formula of the (R)-N-benzyl-3,3,3-trifluoro-2-phenylpropyl-1,2-diamine is shown in the following molecular structural formula I1:
[0101]
[0102] Its preparation steps are as follows:
[0103] In a dry 10mL test tube, add mesitylene-substituted indenol-derived triazole carbene catalyst (0.01mmol, 0.2eq), 100mg pre-activated powdered molecular sieves and 0.4mL anhydrous toluene, argon replacement three times, add 0.01mmol LiHMDS was replaced with argon three times again, 2.2uL of hexafluoroisopropanol (0.02mmol, 0.4eq) was added, and the reaction tube was sealed and stirred at room temperature for 0.5h. β-trifluoromethyl substituted nitrostyrene (0.05mmol, 1.0eq) was dissolved in 0.2mL toluene and slowly added to the reaction system, and stirred at -78°C for 0.5 hours. Aliphatic chain amine reagent (0.1mmol, 2.0eq) was dissolved in 0.6mL and slo...
Embodiment 2
[0105] Embodiment 2 (chiral diamine precursor-nitro compound)
[0106] This example provides a kind of (R)-1,1,1-trifluoro-N-(2-methylphenyl)-3-nitro-2-phenylpropyl-2-amine and its preparation method . The structural formula of the (R)-1,1,1-trifluoro-N-(2-methylbenzyl)-3-nitro-2-phenylpropyl-2-amine is shown in the following molecular structural formula I2:
[0107]
[0108] Its preparation method refers to the preparation method of (R)-N-benzyl-1,1,1-trifluoro-3-nitro-2-phenylpropyl-2-amine in Example 1, the difference is that 2 - Methylbenzylamine (0.1 mmol) instead of benzylamine. The reaction solution was filtered through a glass dropper containing silica gel, rinsed with ether, the filtrate was spin-dried, and separated by column chromatography to obtain the target product precursor, a colorless oily liquid, with a yield of 67% and an ee value of 91%.
[0109] The product I2 prepared is subjected to characterization data analysis, and the result is: 1 HNMR (400MHz...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com