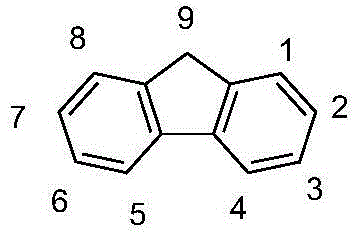
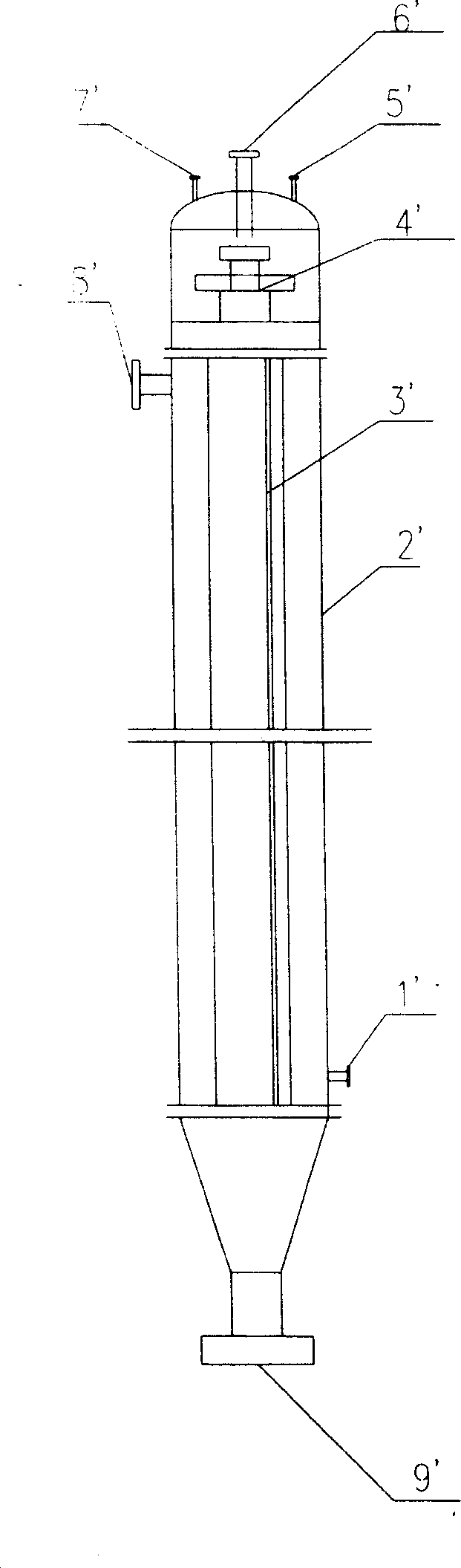
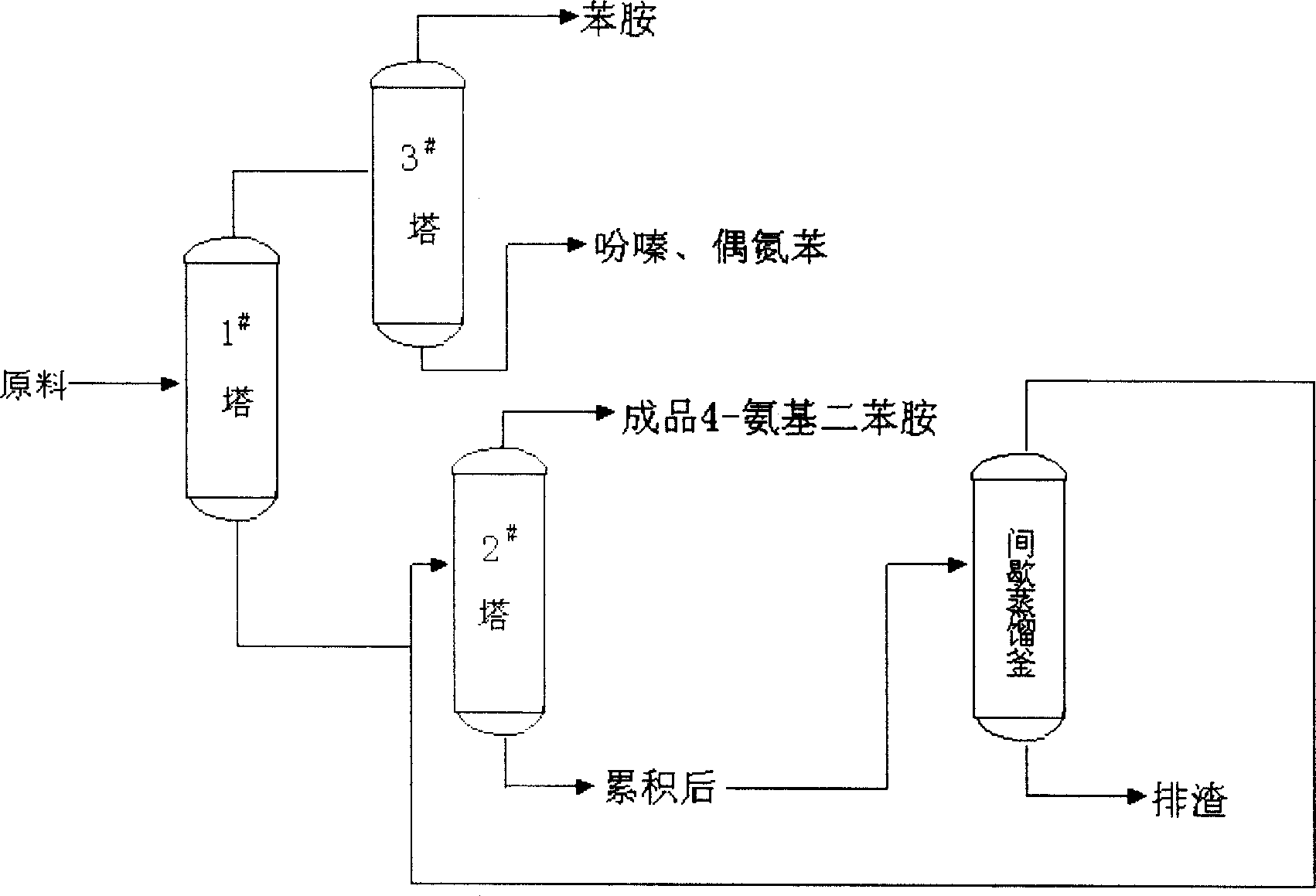
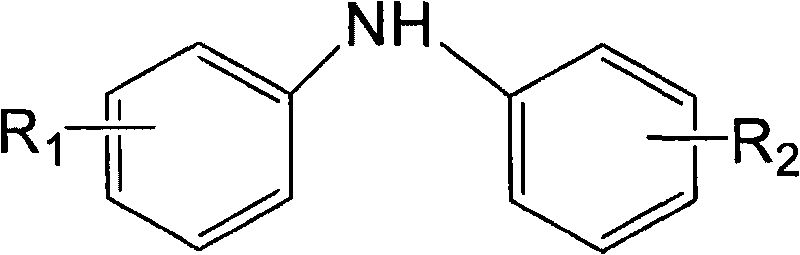
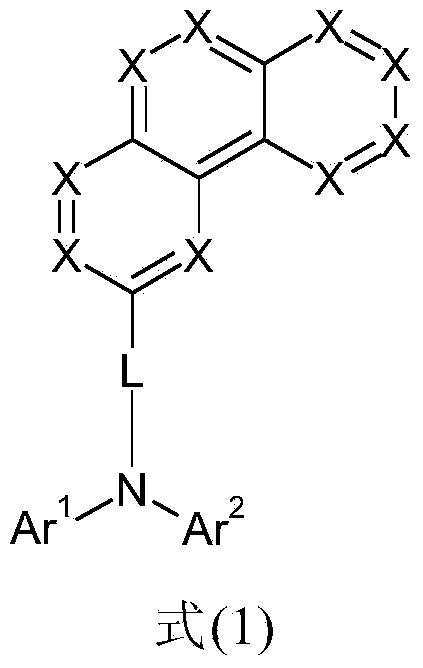
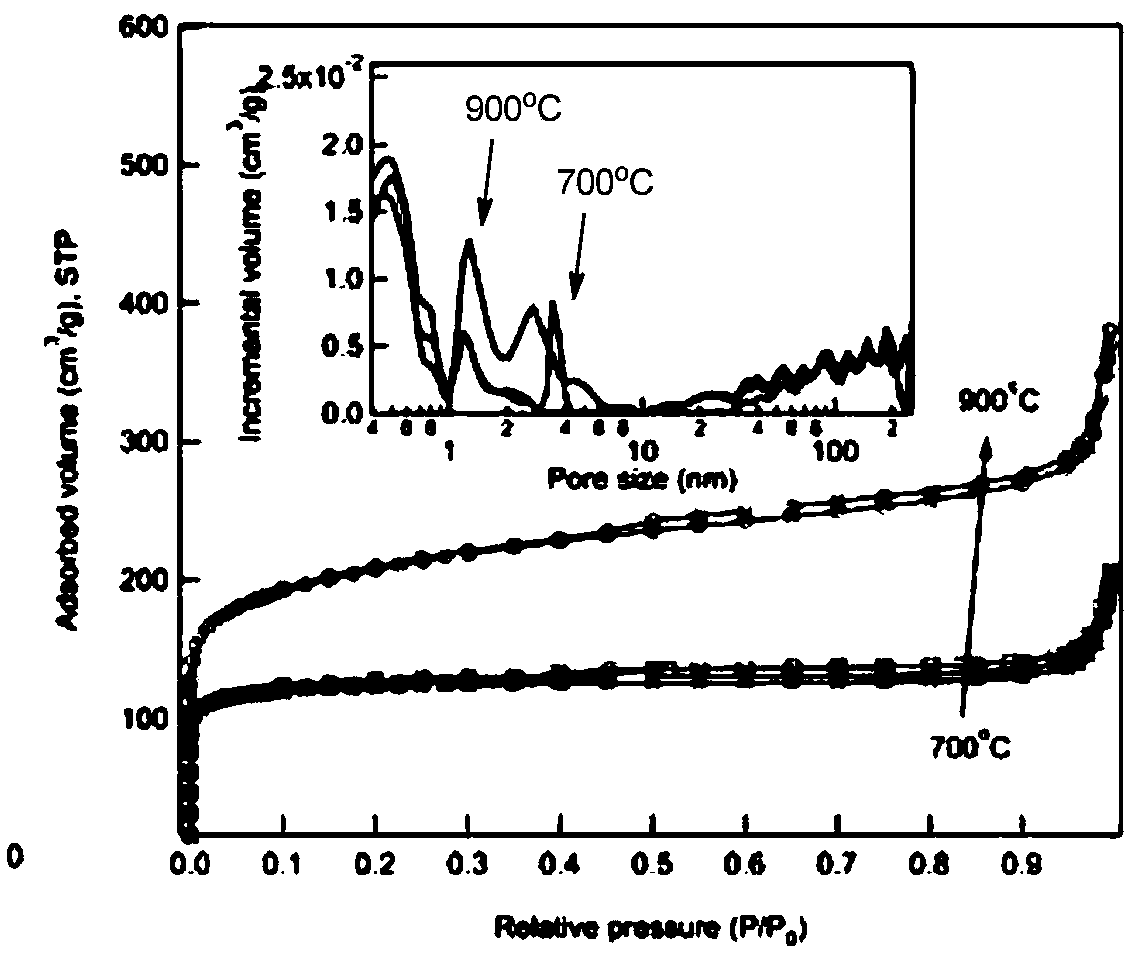
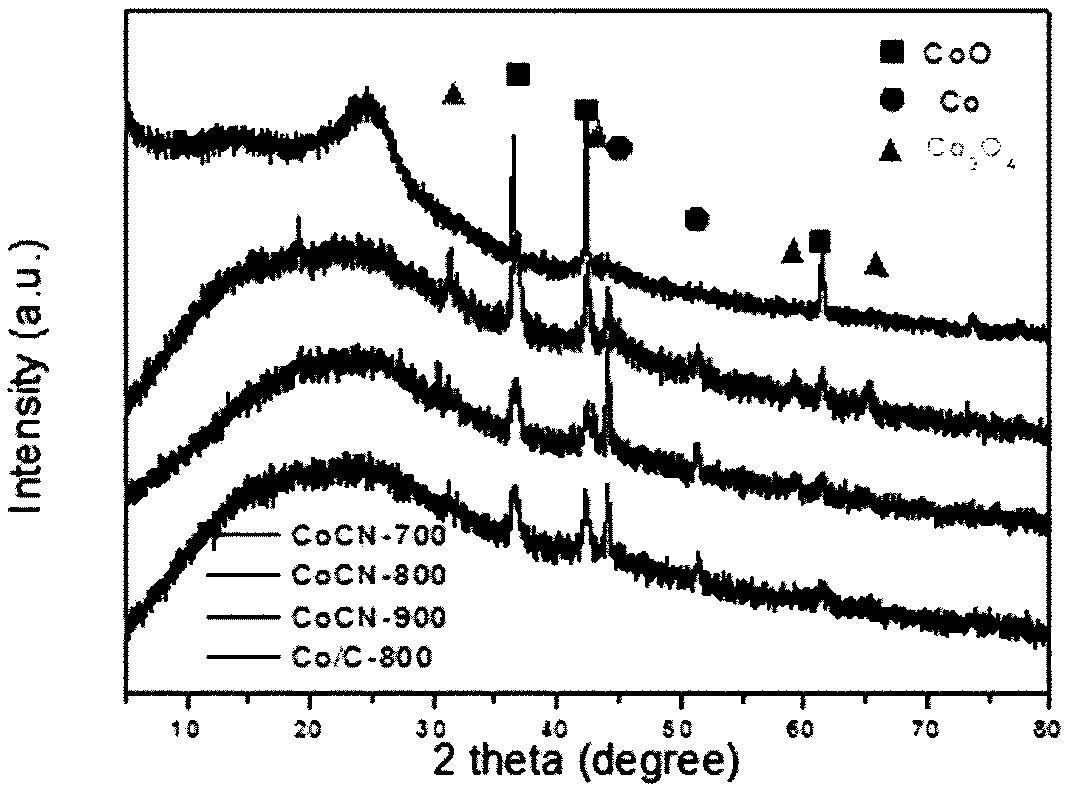
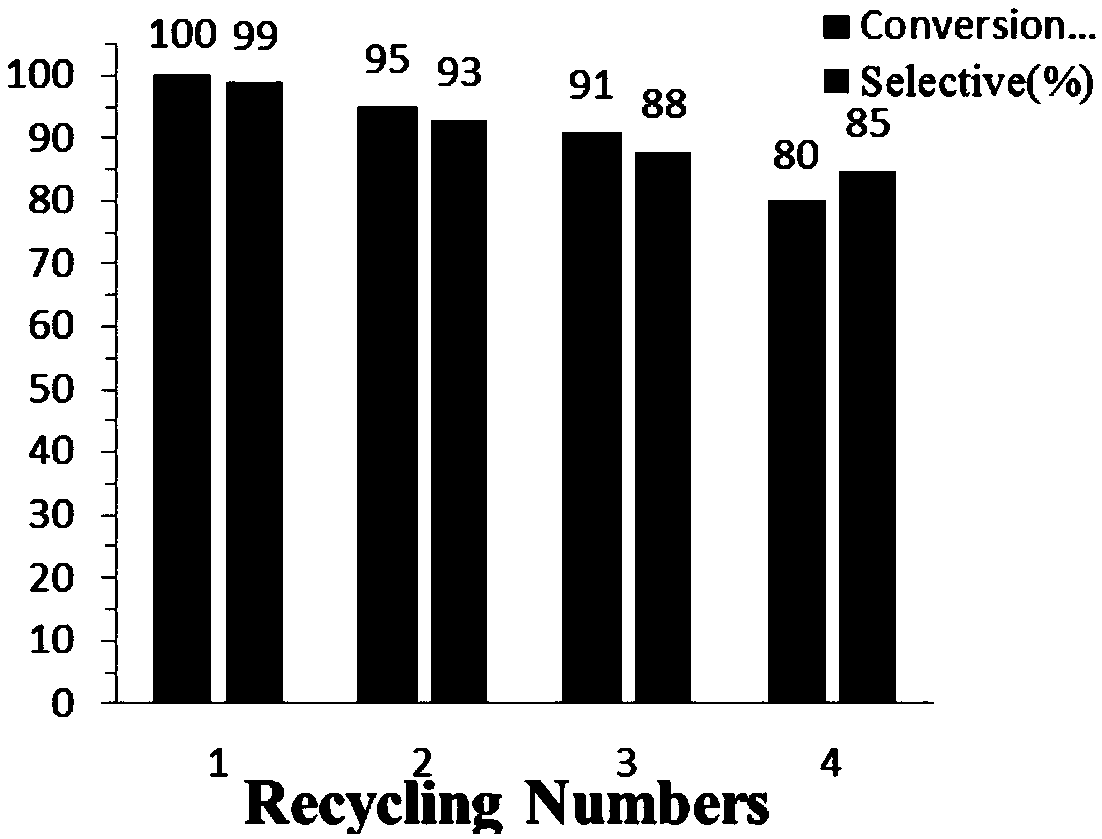
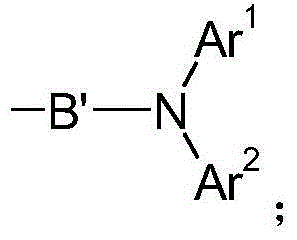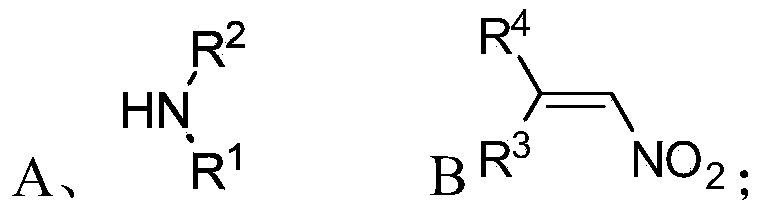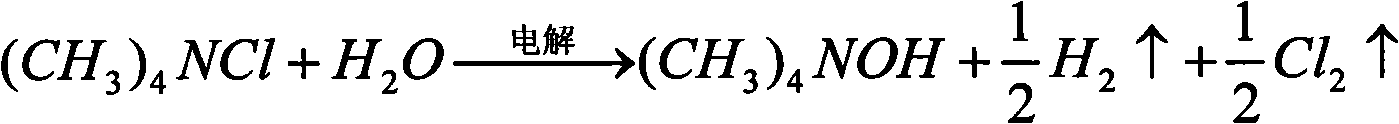Patents
Literature
441results about "Amino compound preparation by condensation/addition reactions" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Methods for making ethanolamine(s) and ethyleneamine(s) from ethylene oxide and ammonia, and related methods
ActiveUS20100087684A1Minimizes numberReduce equipment footprintAmino compound purification/separationOrganic compound preparationEthylenediamineEthylene oxide
The present invention relates to processes for the manufacture of one or more ethanolamines and one or more ethyleneamines starting from the reaction of ethylene oxide with ammonia to produce one or more ethanolamines and the conversion of the ethanolamine(s) to ethyleneamine(s). The present invention also relates to separating alkylethyleneamines from ethyleneamines.
Owner:DOW GLOBAL TECH LLC
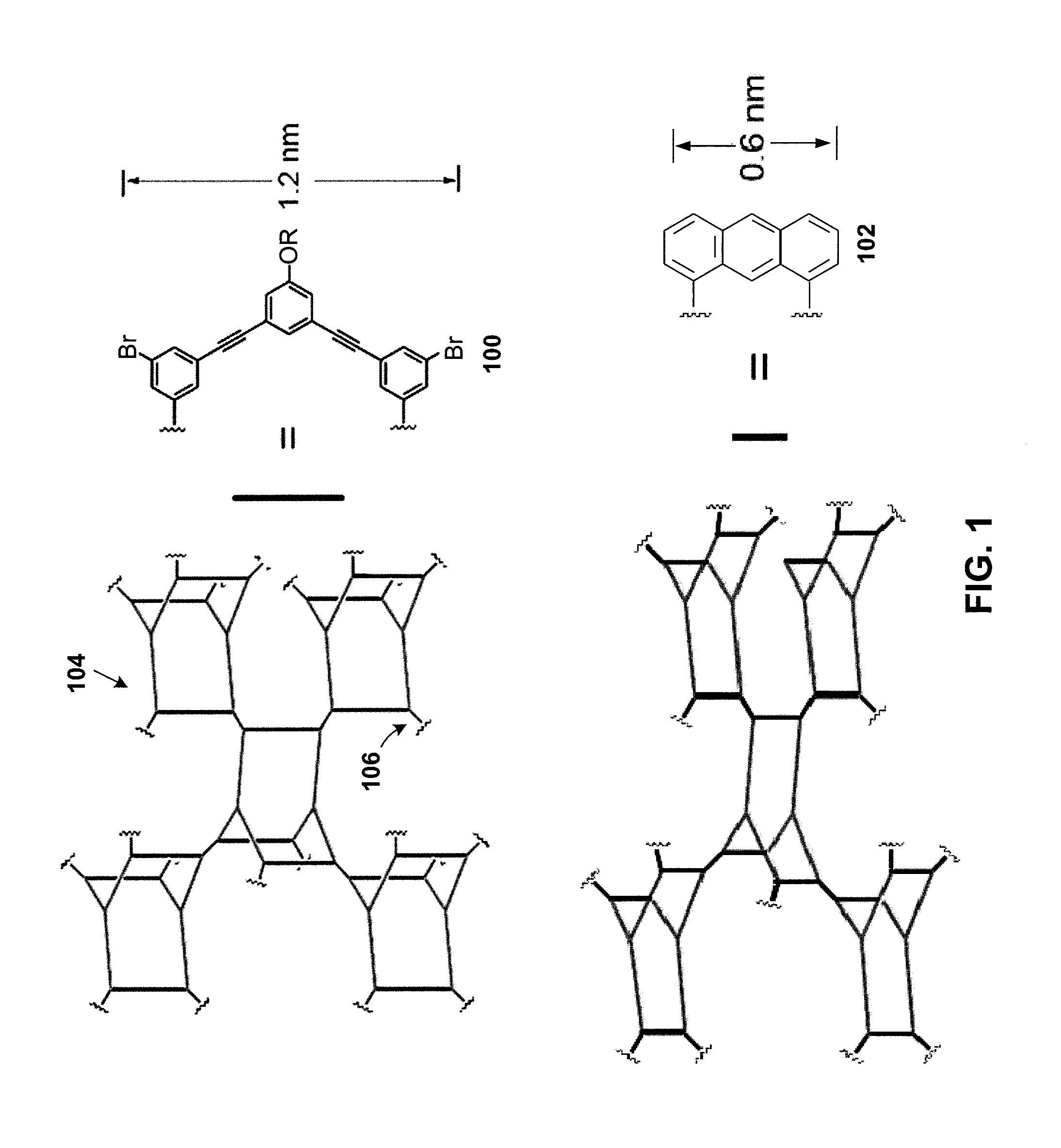
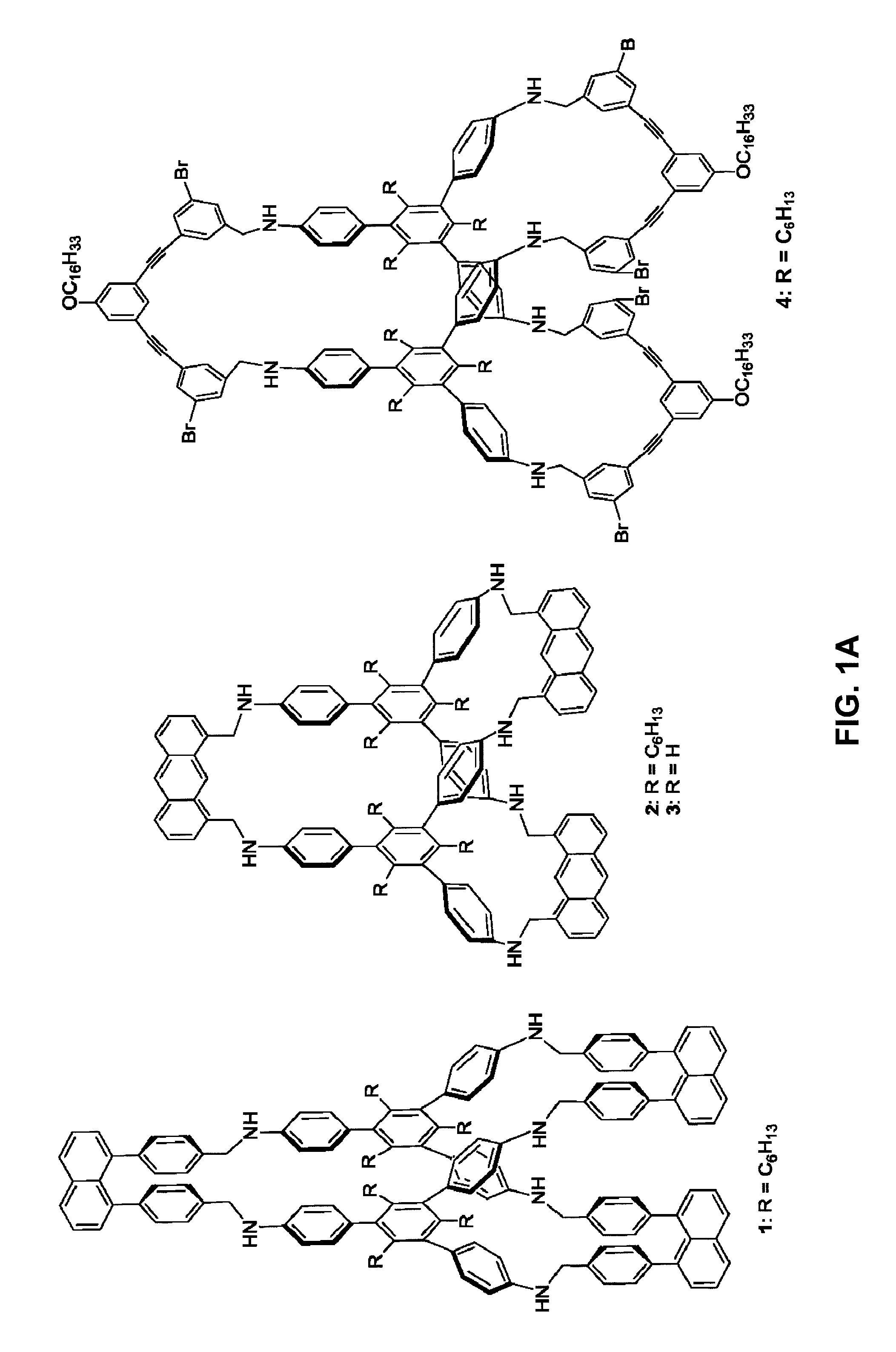
Organic Porous Materials Comprising Shape-Persistent Three-Dimensional Molecular Cage Building Blocks
ActiveUS20130047849A1Efficient separationEasy to synthesizeMaterial nanotechnologyGas treatmentMolecular physicsCatalysis
Porous bulk materials formed of shape-persistent, non-collapsible, three-dimensional molecular cage building blocks are presented that are useful for a variety of applications including gas separation / storage, sensing, and catalysis.
Owner:UNIV OF COLORADO THE REGENTS OF
Metal supported MOFs catalyst as well as preparation method and application thereof to PMDPTA synthesis
ActiveCN107774331AEasy to prepareStable structureCarboxylic acid nitrile preparationOrganic compound preparationMetal chlorideMetal nitrate
The invention provides a metal supported MOFs catalyst as well as a preparation method and application thereof to PMDPTA synthesis and specifically relates to the technical field of catalysts. The catalyst comprises a carrier and an active component; the carrier is an MOFs material; and the active component is single metal or a composite metal particle. The catalyst is prepared by using an excessimpregnation method comprising the steps: stirring at least one solution in metal chloride or metal nitrate and the MOFs material at room temperature for 24h, and carrying out suction filtration to obtain a catalyst precursor; and making the catalyst precursor and a reducing agent generate an effect to obtain the metal supported MOFs catalyst. The preparation method of the catalyst has the advantages such as simple preparation process and stable structure. The catalyst is used for synthesizing PMDPTA and has the advantages such as low cost, high product yield, greenness and environment friendliness.
Owner:ZHENXING FINE CHEM CO LTD
Aldehyde-amine formulations and method for making and using same
A novel method for producing amine-aldehyde sulfur scavenging compositions are disclosed, where the method comprises contacting an amine containing component and a aldehyde containing component in the presence of an alcohol at an amine to aldehyde ratio of between about 0.8 and 0.45 for a reaction time and at a reaction temperatures sufficient to produce an amine-aldehyde adduct product having a specific gravity between about 3% and 7% less than the specific gravity of a mixture of starting materials.
Owner:THE LUBRIZOL CORP
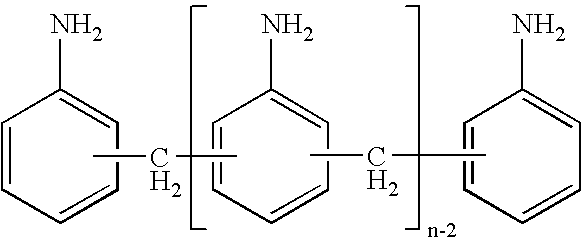
Process for the preparation of di- and polyamines of the diphenylmethane series
InactiveUS20060094897A1Organic compound preparationDiaryl/thriaryl methane dyesDiphenylmethaneAniline
The present invention relates to a process for the preparation of di- and polyamines of the diphenylmethane series, and to the preparation of di- and polyisocyanates of the diphenylmethane series from these di- and poly-amines. The di- and poly-amines of the diphenylmethane series are prepared by the reaction of aniline and formaldehyde in the presence of hydrochloric acid. In with the present invention, the hydrochloric acid employed contains less than 0.001 wt. % of metal ions which are divalent and / or more than divalent.
Owner:BAYER MATERIALSCIENCE AG
Fluorenes and electronic devices containing them
InactiveCN104603111ALow sublimation temperatureImprove temperature stabilitySilicon organic compoundsOrganic compound preparationCombinatorial chemistryFluorene
Owner:MERCK PATENT GMBH
Method of preparation of 4-aminodiphenylamine
InactiveUS6388136B1Easy accessHigh yieldAmino preparation from aminesOrganic compound preparationLiquid mediumOxygen
A method of preparing 4-aminodiphylamine through an intermediate preparation of 4-nitrodiphenylamine and / or 4-nitrosodiphenylamine and / or their salts by reaction of aniline with nitobenzene in a liquid medium at a temperature of 50 to 130° C., under normal or reduced pressure, in an inert atmosphere or in the presence of air oxygen, with subsequent hydrogenation of an intermediate of 4-nitrodiphenylamine and / or nitrosodiphenylamine and side products, and by isolation of 4-aminodiphenylamine and the side products of unconverted raw materials.
Owner:DUSLO A S
Aniline oligomer, its aliphatic polyester copolymer and their prepn
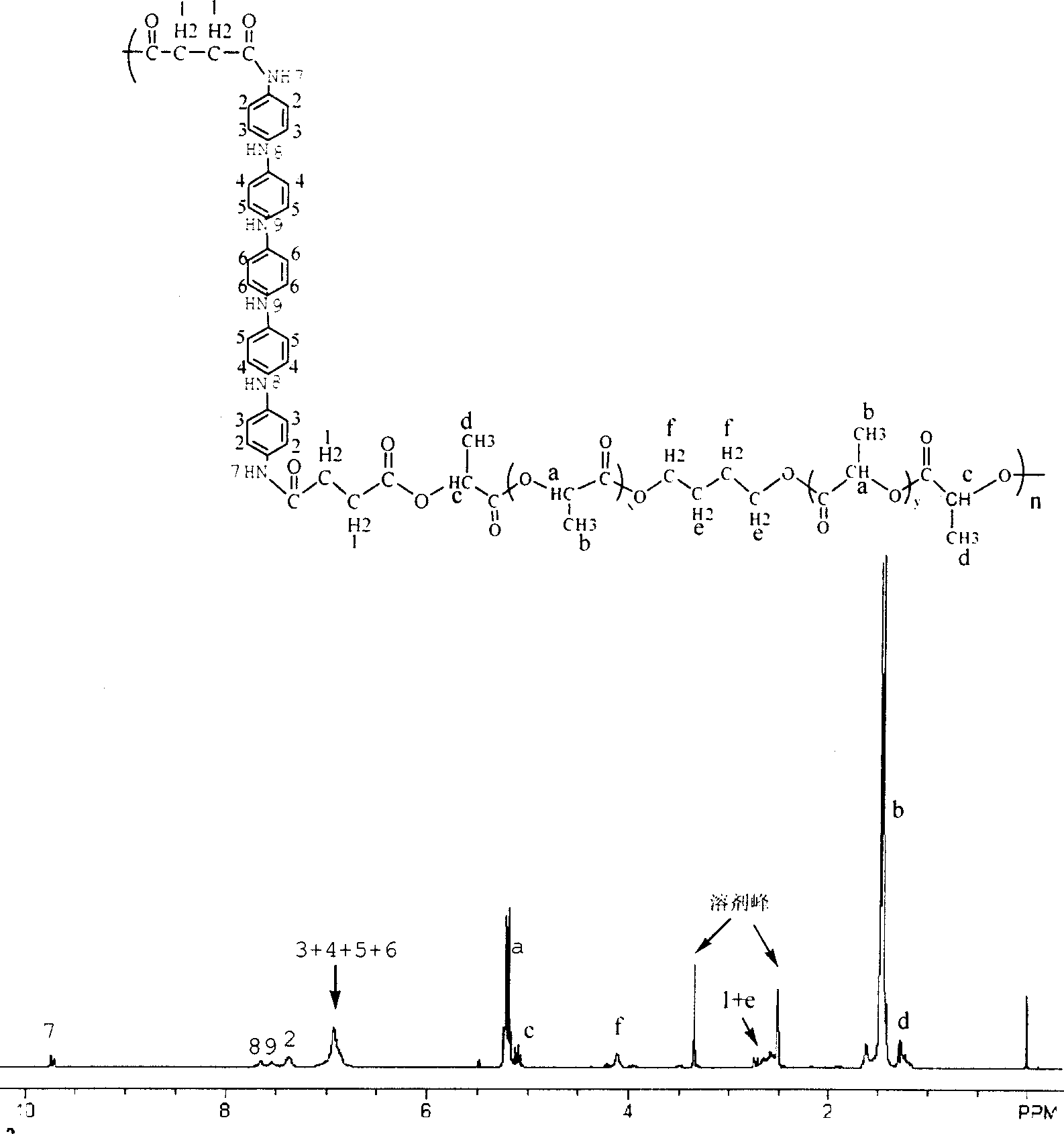
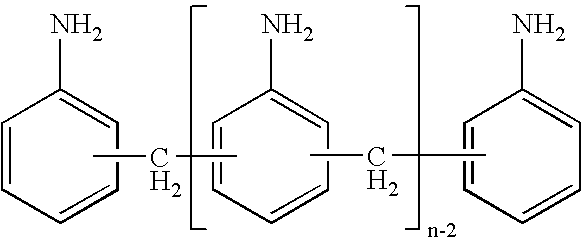
InactiveCN1887854AImprove electrical activityGood biocompatibilityAmino compound preparation by condensation/addition reactionsPolyesterPentamer
The present invention provides aniline oligomers, their aliphatic polyester copolymer and their preparation. Two kinds of aniline oligomers are first synthesized with N-phenyl-1, 4-p-phenylene diamine as material, and then copolymerized with aliphatic polyester to obtain electrically active biodegradable polymers. During the preparation, N-phenyl-1, 4-p-phenylene diamine has its end amido group protected with butanedioic anhydride and is then reacted with end amido aniline dimer and phenylene diamine to obtain aniline tetramer with one end carboxyl group and one end amino group and aniline pentamer with two end carboxyl groups; and the aniline oligomers are finally polycondensated with double hydroxyl group terminated aliphatic polyester to obtain the copolymers containing electrically active aniline oligomer block. The copolymers possess the advantages of both aniline oligomer and aliphatic polyester, and is used as biomedicine material mainly.
Owner:CHANGZHOU INST OF ENERGY STORAGE MATERIALS &DEVICES
Preparation method for aryl substituted p-phenylenediamine substance
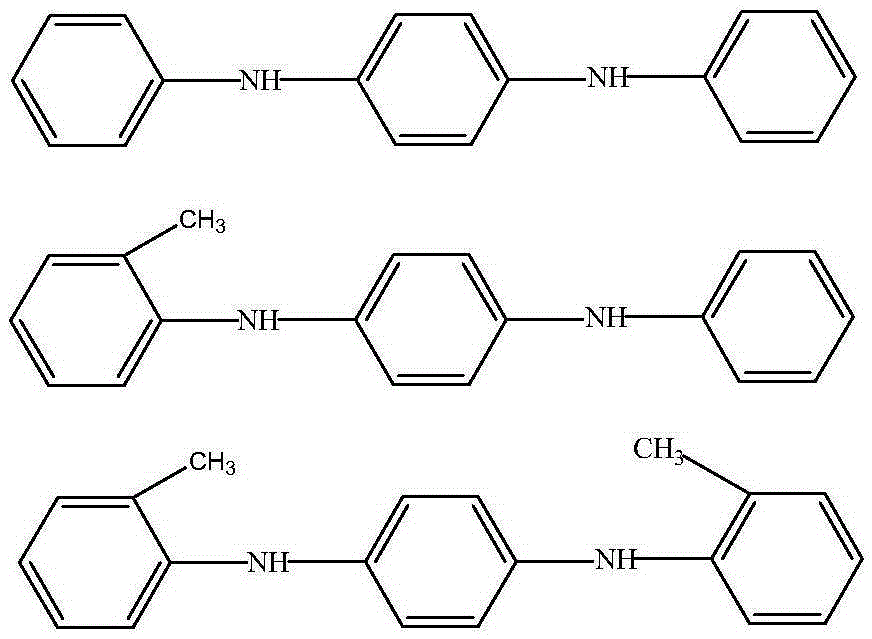
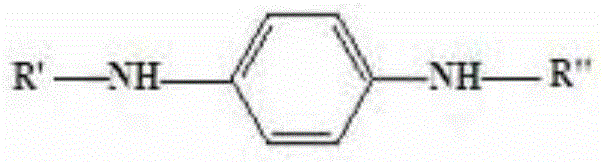
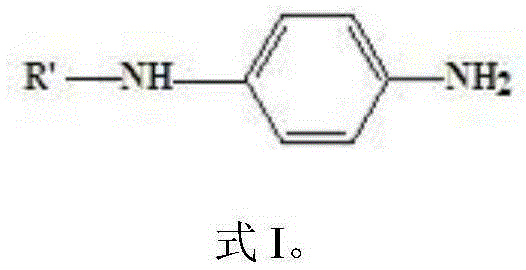
InactiveCN106608827AReduce the amount addedWill not corrodeAmino compound purification/separationAmino preparation from aminesCyclohexanoneCorrosion reaction
The invention provides a preparation method for an aryl substituted p-phenylenediamine substance. The aryl substituted p-phenylenediamine substance has the following structural formula defined in the specification, wherein R'' is phenyl or o-methyl phenyl, and R' and R'' are the same or different. The preparation method comprises the steps of carrying out a reaction of a raw material A and a raw materials B under the action of a hydrogen acceptor and a catalyst, to form the aryl substituted p-phenylenediamine substance, wherein the raw material A has the structural formula defined in the specification, the raw material B is cyclohexanone and / or o-methylcyclohexanone, and the hydrogen acceptor is a hydrogen acceptor which can accept hydrogen to be converted into the raw material B. The preparation method provided by invention has the advantages that the raw materials are cheap and easy to obtain, and reaction post-processing has no need of use of a lot of water. At the same time, the reaction conditions are mild, and equipment cannot be corroded. Therefore, the preparation method has the advantages of environmental protection and less pollution and can obtain better economic benefits.
Owner:JIANGSU SINORGCHEM TECH CO LTD
Process for preparing diamines and polyamines from the diphenylmethane series
ActiveUS7528283B2Organic compound preparationDiaryl/thriaryl methane dyesDiphenylmethanePolymer science
The invention provides an improved process for preparing diamines and polyamines from the diphenylmethane series by reacting aniline and formaldehyde in the presence of an acid catalyst, the improvement involving the aniline containing less than about 3 wt. % of diamines and polyamines from the diphenylmethane series, based on the weight of the aniline.
Owner:COVESTRO DEUTSCHLAND AG
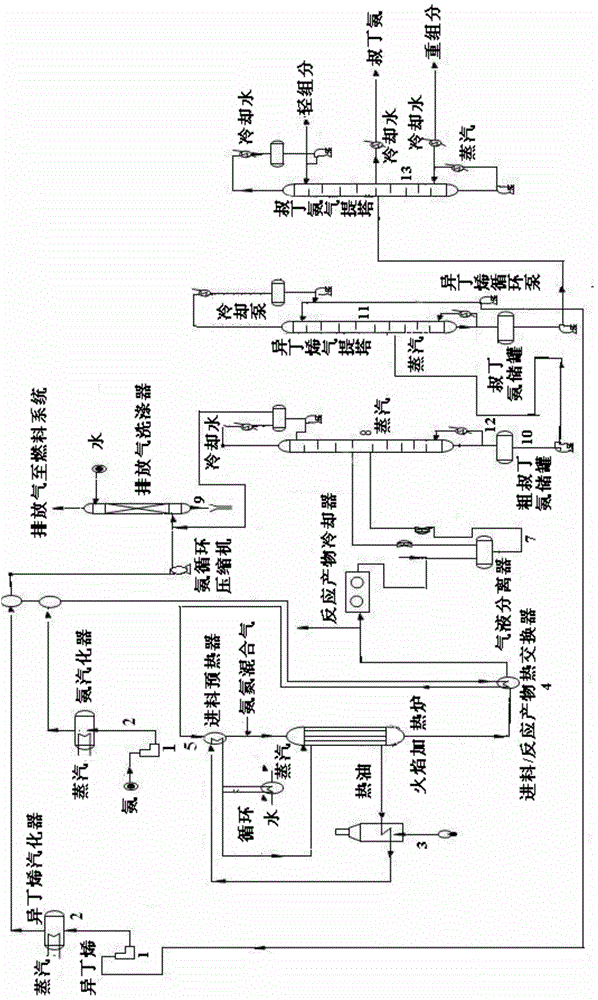
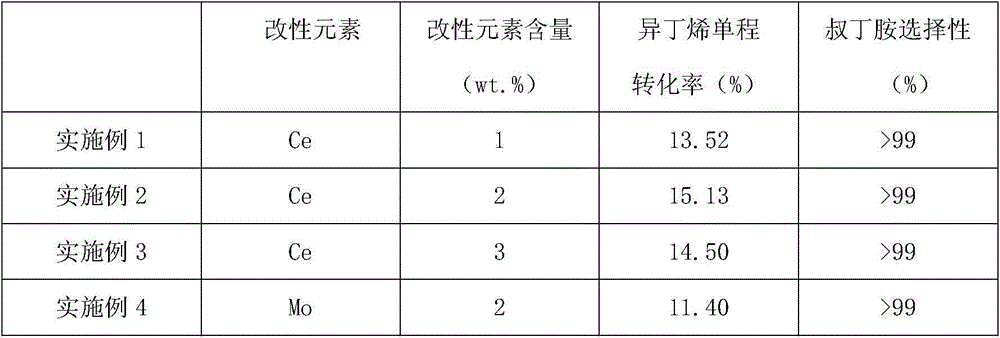
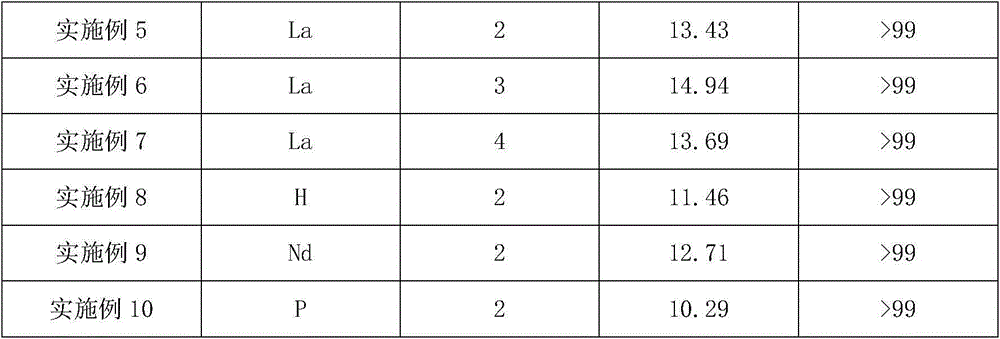
Method for producing tert-butylamine by direct catalytic amination of isobutene
ActiveCN104418754AAvoid pollutionInhibit aggregationOrganic-compounds/hydrides/coordination-complexes catalystsAmino compound preparation by condensation/addition reactionsRare-earth elementReaction temperature
The invention relates to a method for producing tert-butylamine by direct catalytic amination of isobutene, which is characterized by comprising the following steps: continuously injecting an olefin raw material and ammonia into a multitubular fixed bed reactor filled with a catalyst for direct amination reaction, wherein the feed mole ratio of the olefin raw material to ammonia is 1:0.5-1:4; and performing rectification separation of the reaction products to obtain tert-butylamine with purity of 99.9%. In the process of the invention, the molecular sieve catalyst is subjected to element modification so as to realize optimal adjustment of surface acidity, thus polymerization of the olefin is prevented, and the service life and the reaction selectivity of the catalyst are improved. The molecular sieve catalyst used in the invention is modified by rare earth elements or transition metal elements and organic halides and has improved catalytic performance, a special process operation method is adopted, thus the process of the invention is carried out at a low reaction temperature and reaction pressure, the olefin conversion rate and the amide selectivity are improved when compared with the prior art, and the service life of the catalyst is prolonged.
Owner:王荣发
Environment-friendly preparation method of tert-butylamine
InactiveCN102633647AExtended service lifeLow reaction temperatureMolecular sieve catalystsAmino compound preparation by condensation/addition reactionsPtru catalystArgon atmosphere
The invention discloses an environment-friendly preparation method of tert-butylamine. Isobutylene and liquid ammonia are directly subjected to amination under the action of a catalyst to prepare the tert-butylamine. The catalyst uses a Y-type zeolite molecular sieve as the matrix, and the active component elements and modifying elements account for 10-30%. The Y-type zeolite molecular sieve is modified by the following steps: exchanging the Y-type zeolite molecular sieves with an NH4Cl aqueous solution, washing, drying in a nitrogen or argon atmosphere, and carrying out heat treatment by roasting to obtain an H-type zeolite molecular sieve; and carrying out dipping treatment on the H-type molecular sieve with metal salt, drying in a nitrogen or argon atmosphere, roasting to obtain the modified Y-type zeolite molecular sieve. The modified Y-type zeolite molecular sieve catalyst must be activated before use. The invention has the advantages of mild reaction conditions, high selectivity, high yield, environment friendliness and low catalyst modifying cost, and is suitable for industrial production.
Owner:ZHEJIANG HUANGMA TECH
Method for preparing organic amine by directly aminating low-carbon olefin
InactiveCN101037389AAvoid pollutionInhibit aggregationMolecular sieve catalystsAmino compound preparation by condensation/addition reactionsRare-earth elementAlkaline earth metal
The invention discloses a method for directly amination to produce organic amine by the lower olefins including following steps: continuously feeding the lower olefins and ammonia into the reactor having the catalyzer for an amination reaction, collecting the reaction product, i.e. target organic amine. The catalyzer components and weight percentage comprises: molecular screen of 55-89%; binder of 10-44% which is selected from Al2O3; modified element of 1-10% which is selected from rare earth element, transition metal or alkaline earth metal element. The olefin percent conversion is more than 10%, and the amide selectivity is more than 99%. The inventive operation method benefits much to the catalytic properties. Compared to the current technology, the invention provides the catalyzer with a high activity, a reaction product with a good selectivity and an organic amine with a good quality.
Owner:EAST CHINA UNIV OF SCI & TECH
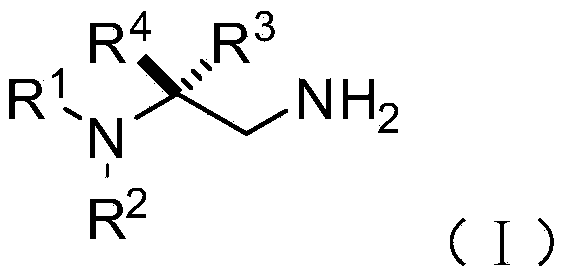
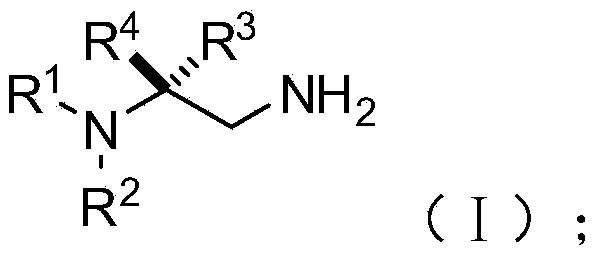
Chiral 1,2-diamine compound and preparation method and application thereof
ActiveCN105367427AAchieve strict metal-freeThe reaction process is safe and controllableOrganic compound preparationOrganic-compounds/hydrides/coordination-complexes catalystsCompound aNitroalkene
The invention discloses a chiral 1,2-diamine compound and a preparation method and application thereof. The molecular structure general formula of the chiral 1,2-diamine compound is shown as the general formula (I) in the specification. The preparation method of the chiral 1,2-diamine compound comprises the steps that an amine compound A and a nitroolefin compound B are added to a reaction system containing an n-heterocyclic carbine catalyst, an alkali reagent, a proton additive and a dewatering reagent for reacting and other steps. The chiral 1,2-diamine compound comprises chiral quaternary carbon atoms containing electrondrawing groups and amino groups, and can be widely used for synthesizing drug intermediates, especially heterocyclic ring structure compounds, and preparing functional materials. The preparation method is simple in process and low in requirement for reaction conditions, the reaction process is safe and controllable, the atom utilization rate and production efficiency are high, meanwhile, the enantioselectivity of products is efficiently guaranteed, and the environment pollution pressure of the methodology is low through the introduction of the small organic molecule asymmetric catalysis concept.
Owner:PEKING UNIV SHENZHEN GRADUATE SCHOOL
Process for preparing 4-amino diphenylamine
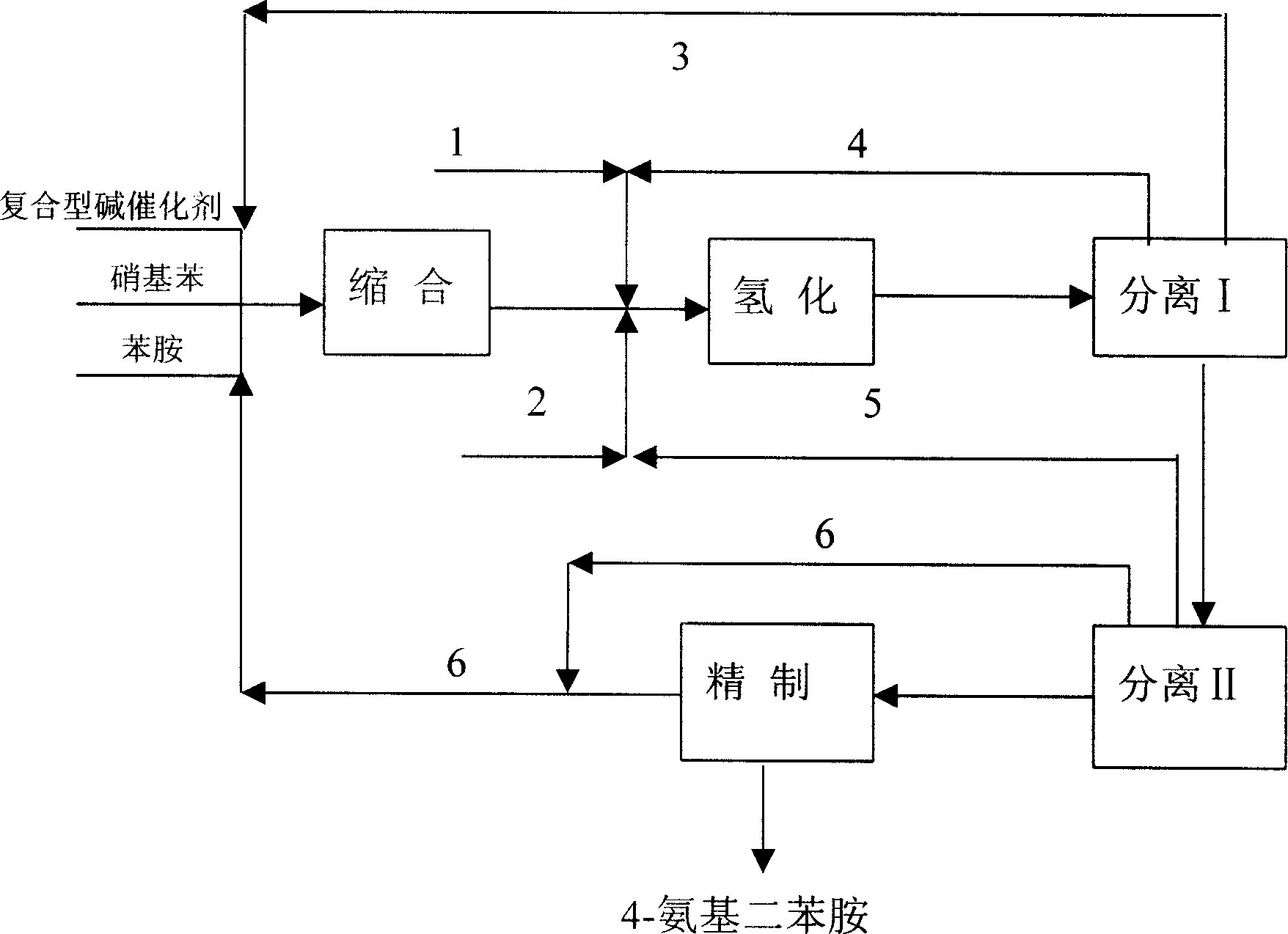
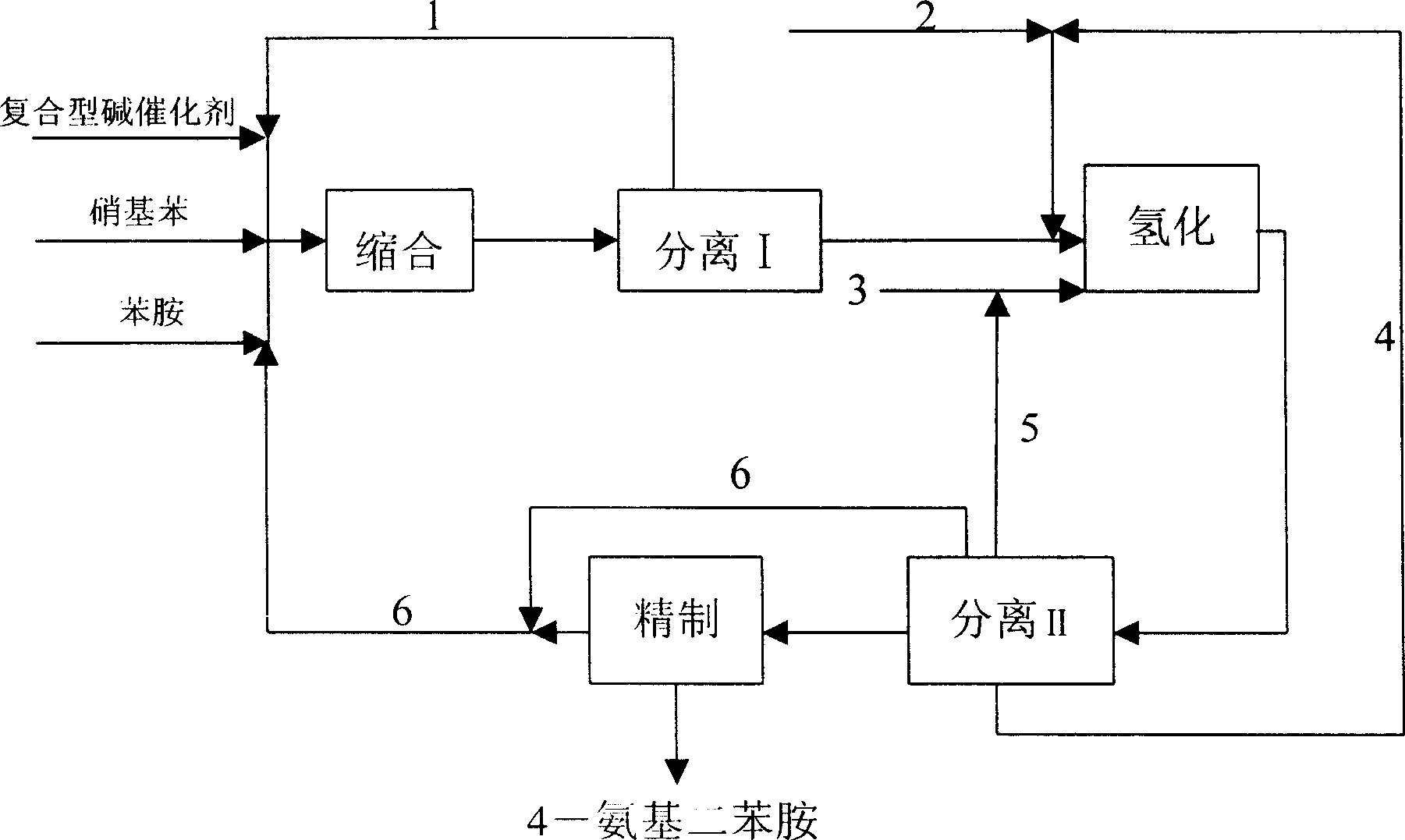
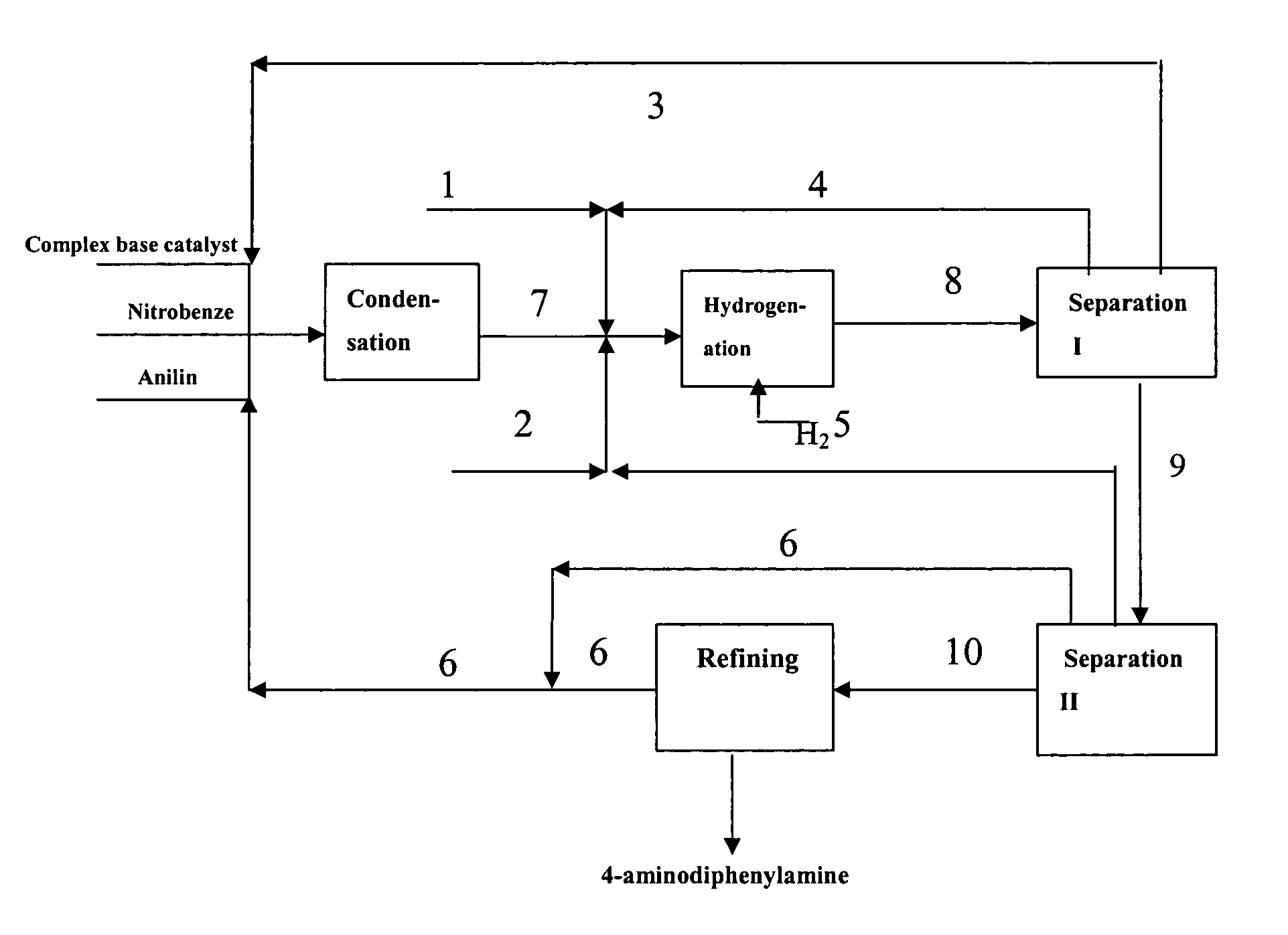
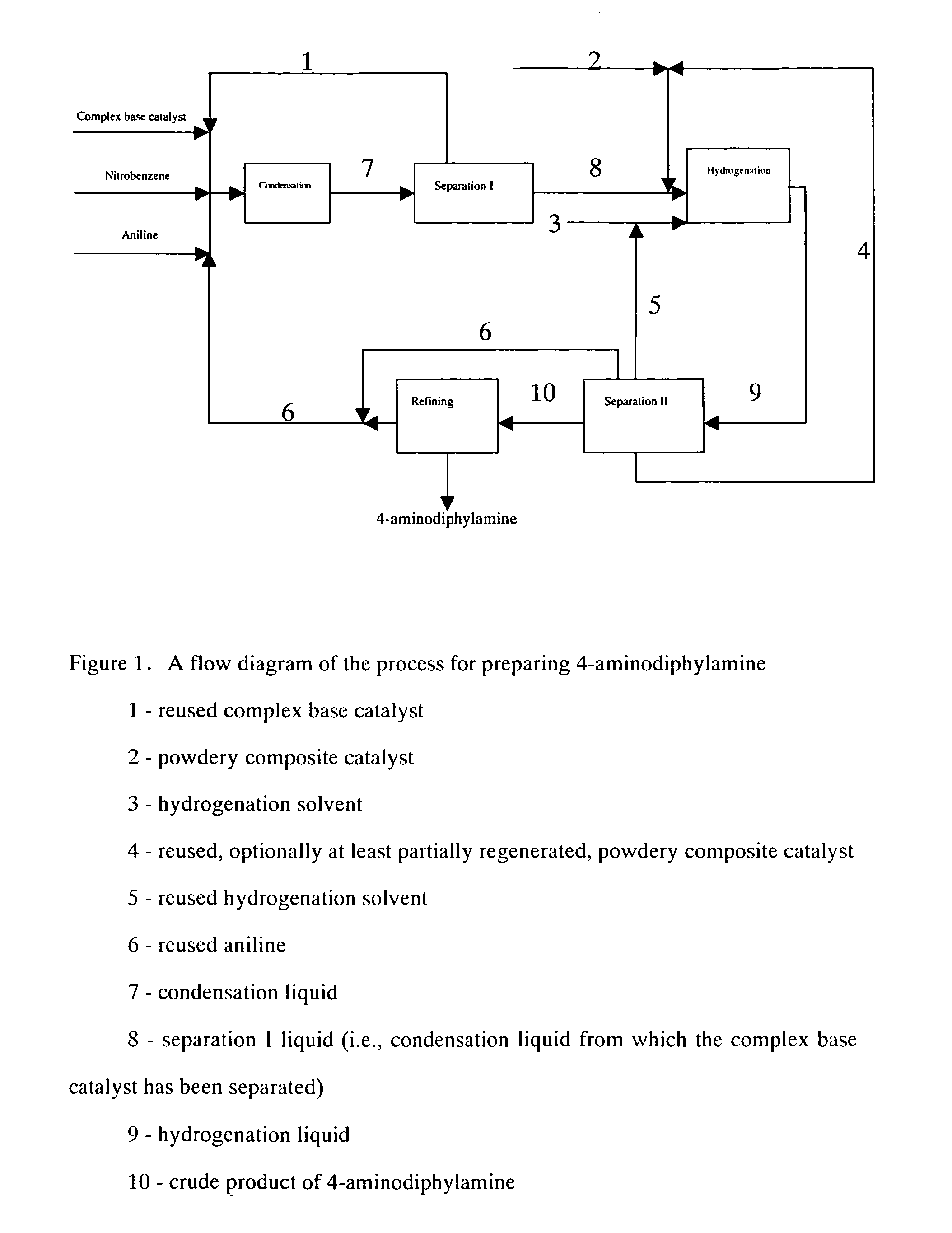
ActiveCN1721391AAvoid smallRaise the ratioAmino preparation from aminesChemical recyclingNitrobenzeneSolvent
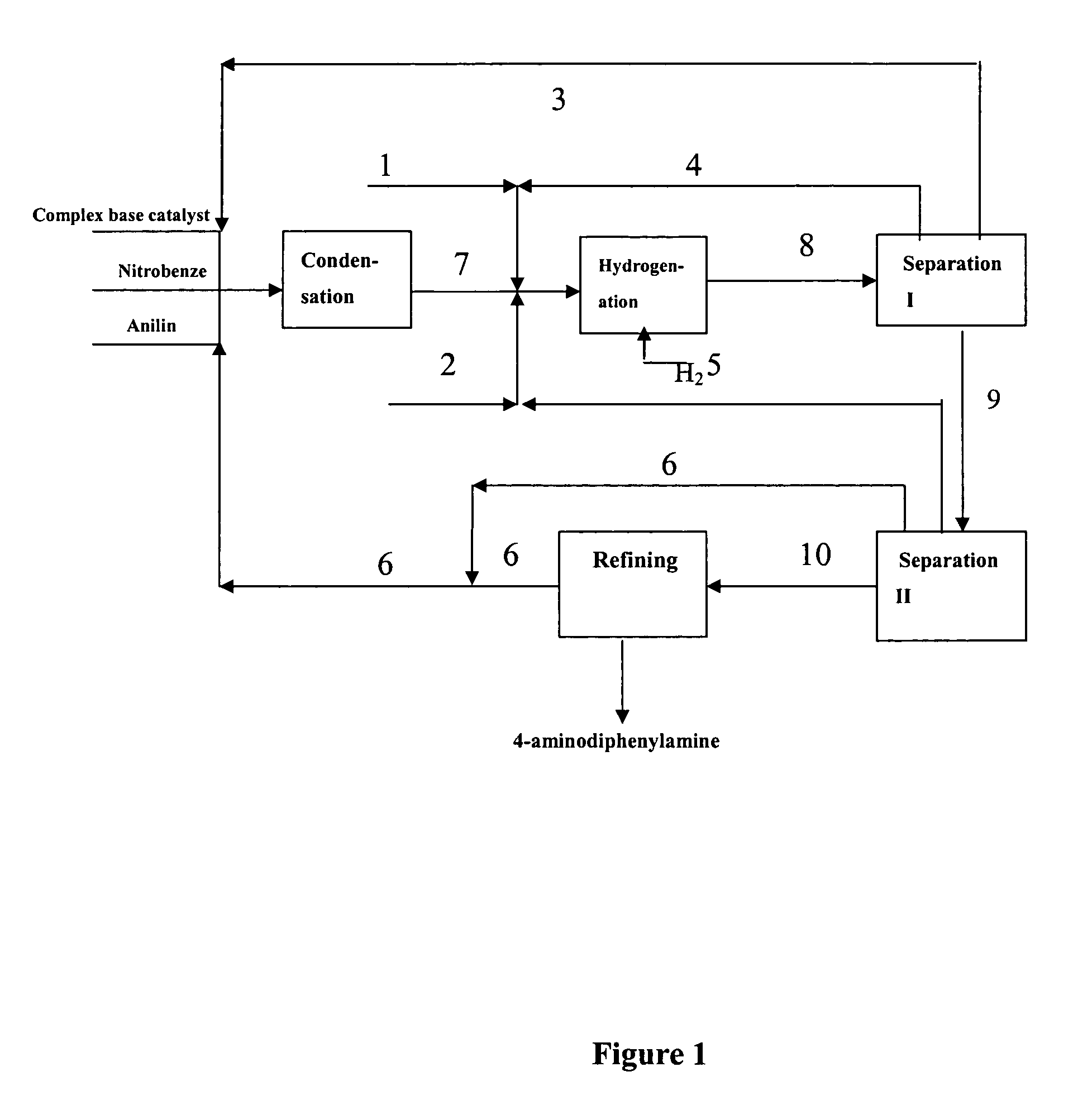
The present invention discloses production process of 4-amino diphenylamine with nitrobenzene and phenylamine as materials. The production process includes five steps of condensation; hydrogenation; separating, recovering and reusing composite alkali catalyst, and separating, recovering and reusing at least partial regenerated composite powdered catalyst; separating, recovering and reusing phenylamine and hydrogenation solvent; and refining. For the condensation and hydrogenation, composite alkali catalyst and composite powdered catalyst are adopted separately. The production process has mild reaction condition and less side products, may be completed continuously, and is suitable for industrial production. The production process has 4-amino diphenylamine yield industrial scale over 95 % and 4-amino diphenylamine purity over 99 wt%.
Owner:JIANGSU SINORGCHEM TECH CO LTD
Catalyst and diphenylamine alkylation method
ActiveCN101745423AReduce dosageIncrease dosageOrganic-compounds/hydrides/coordination-complexes catalystsAmino compound preparation by condensation/addition reactionsState of artAlkyl transfer
The invention provides a method for preparing a catalyst. The method comprises the steps of allowing aqueous solution of acid in a concentration of less than 20 percent by weight to be in contact with activated clay, dehydrating and drying the obtained product. The invention also provides a diphenylamine alkylation method, which comprises the steps of subjecting diphenylamine and diisobutylene to contact reaction under alkylation conditions in the presence of the catalyst in an inert gas atmosphere, wherein the catalyst is obtained by the method provided by the invention. According to the method provided by the invention, the catalyst is low in consumption, and increases the yield of final products compared with the prior art. In addition, the catalyst is good in reusability, can be directly used after the catalyst is removed from the final products, and can be repeatedly reused.
Owner:CHINA PETROLEUM & CHEM CORP +1
Phenanthrene compounds for organic electronic devices
ActiveCN104364245AHigh hole mobilityLow sublimation temperatureSilicon organic compoundsOrganic compound preparationPhenanthrene
The invention relates to specific phenanthrenes, the use of the compound in an electronic device, and an electronic device containing at least one of said compounds. The invention further relates to a method for producing the compound and a formulation and composition containing one or more of the compounds.
Owner:MERCK PATENT GMBH
Curing Agent For Low Temperature Cure Applications
ActiveUS20090259003A1Improved “ walk-on ” dry timeRapid hardness developmentIsocyanic acid derivatives preparationOther chemical processesEpoxyEndcapping
The present invention provides Mannich base derivatives of N,N′-dimethyl secondary diamine polymers including Mannich base derivatives of methylamine-terminated poly-(N-methylazetidine) and Mannich base derivatives of methylamine-terminated poly-(N-methylazacycloheptane). Amine curing agent compositions and amine-epoxy compositions containing Mannich base derivatives of N,N′-dimethyl secondary diamine polymers are also disclosed.
Owner:EVONIK OPERATIONS GMBH
Process for manufacturing diphenylamines
InactiveUS20070073086A1Organic compound preparationAmino preparation by hydrogen substitutionHeat sensitiveAniline
The invention teaches novel process steps for the rapid high yield manufacture of diphenylamines of the formula wherein R1 is selected from hydrogen, alkyl, and aryl; wherein each R2 and R3 is the same or different and each is independently selected from hydrogen, alkyl, alkoxy, aralkyl, dialkylamino, alkylarylamino and substituted or unsubstituted aryl, the substituents on aryl being each independently selected from alkyl (C1-C8), alkoxy (C1-C8), aroxy, aralkoxy and halogen; wherein n and m are each independently an integer from 1 to 5. Diphenylamines are key intermediates for the production of leuco dyes used in pressure-sensitive and heat-sensitive imaging systems. The process in at least one embodiment comprises reacting at elevated temperature an aryl halide with an aromatic amine in an organic solvent and aqueous alkaline solution and optionally in some embodiments, phase-transfer agent, followed by addition of catalytic amounts of bis[tri(t-butylphosphine)]palladium at a suitable temperature to rapidly form diphenylamine.
Owner:APPLETON PAPERS INC
Method for synthesizing amine and imine
InactiveCN103058805AHigh yieldOrganic compound preparationCarboxylic acid amides preparationPtru catalystNitrobenzene
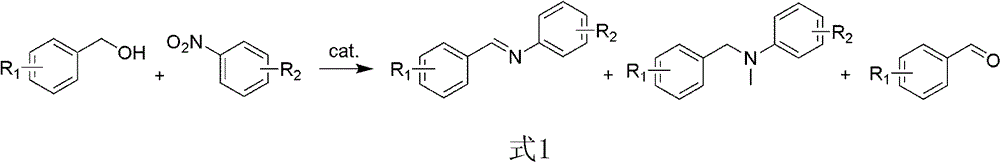
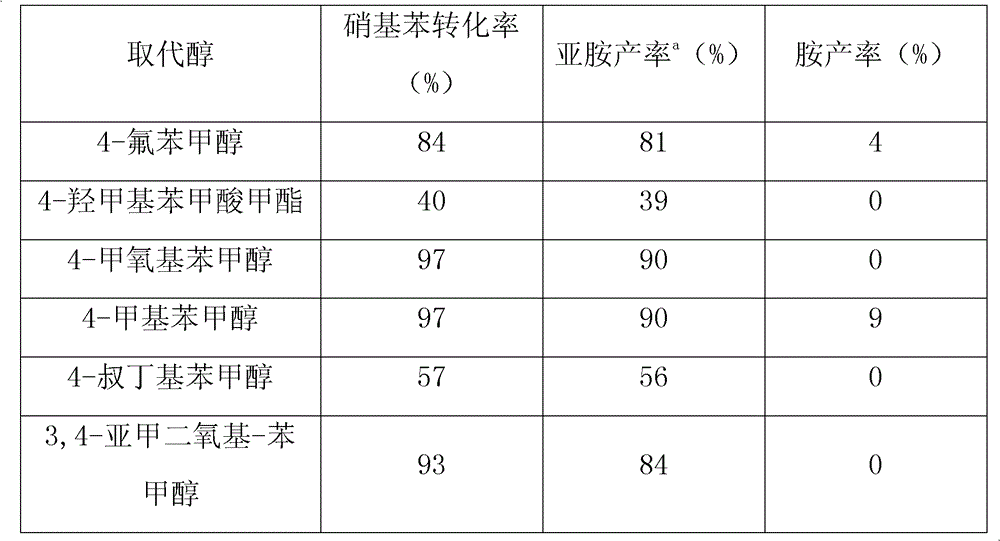
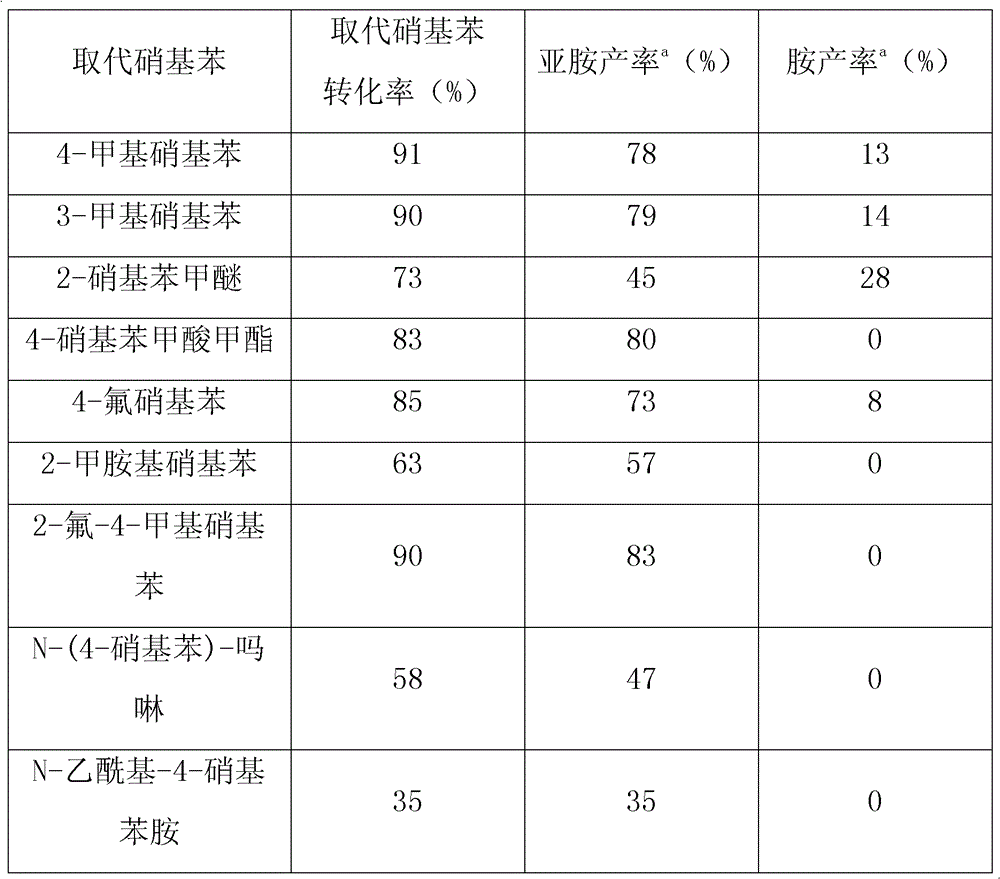
The invention provides a method for synthesizing N-benzalaniline and N-benzyl aniline, and relates to an efficient method for synthesizing N-benzalaniline and N-benzyl aniline through a continuous reaction on raw materials of aromatic alcohol and nitryl aromatic compound in the presence of a transition metal supported solid catalyst. The catalyst is loaded on hydrotalcite, hydroxyapatite and oxides by using palladium, gold or rhodium through an impregnation method or a precipitation method. The catalytic reaction is carried out in an inert atmosphere; conversion rate of nitrobenzene and total yield of N-benzalaniline and N-benzyl aniline can reach higher than 99% (imine yield of 93%, and amine yield of 7%). The catalyst can be used repeatedly. The method is applicable to a variety of substituted aromatic alcohols and substituted aromatic compounds, and can realize high conversion rate and high selectivity.
Owner:FUJIAN INST OF RES ON THE STRUCTURE OF MATTER CHINESE ACAD OF SCI
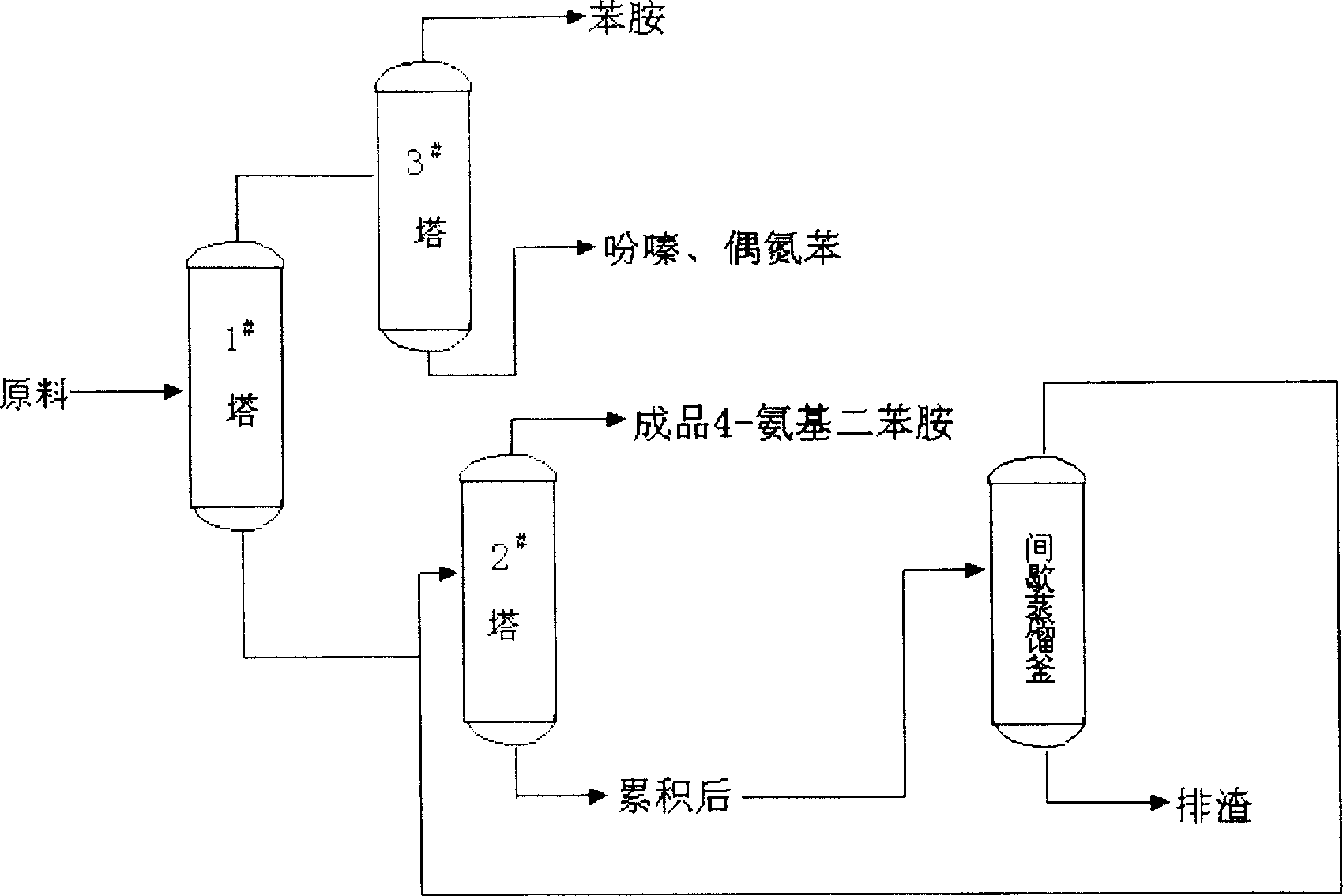
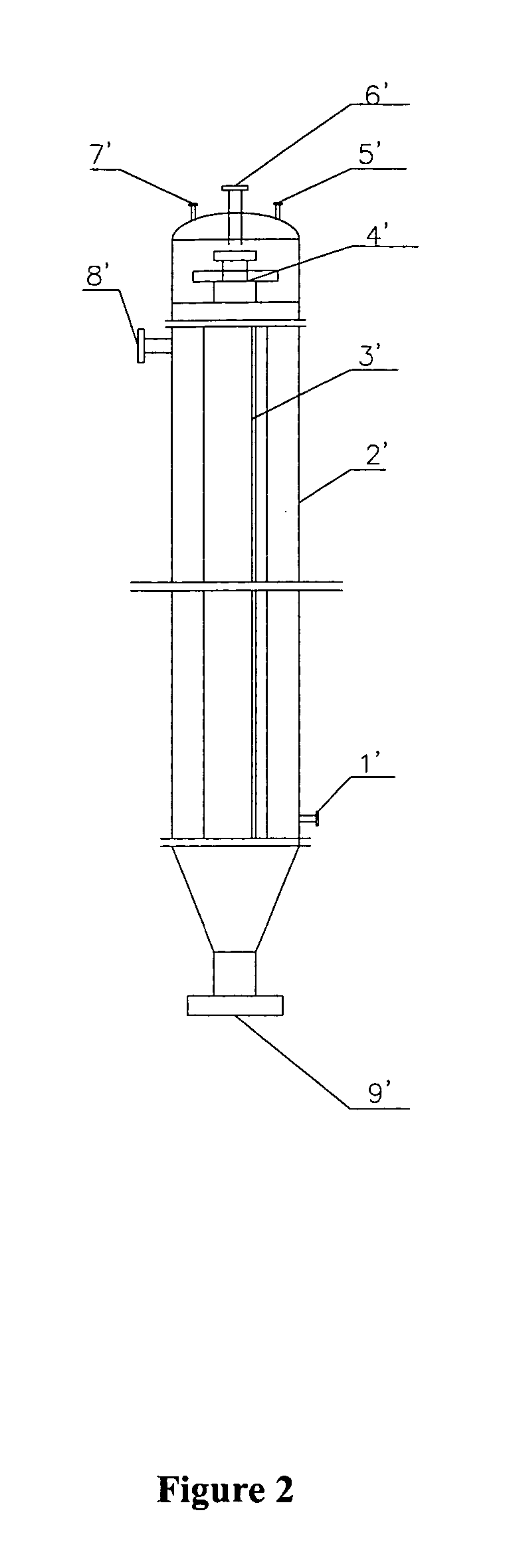
Method for continuous hydrogenation preparation of 4-amino diphenylamine
ActiveCN1470498ASolve the problem of single consumptionOrganic compound preparationMetal/metal-oxides/metal-hydroxide catalystsHigh concentrationHydrogenation reaction
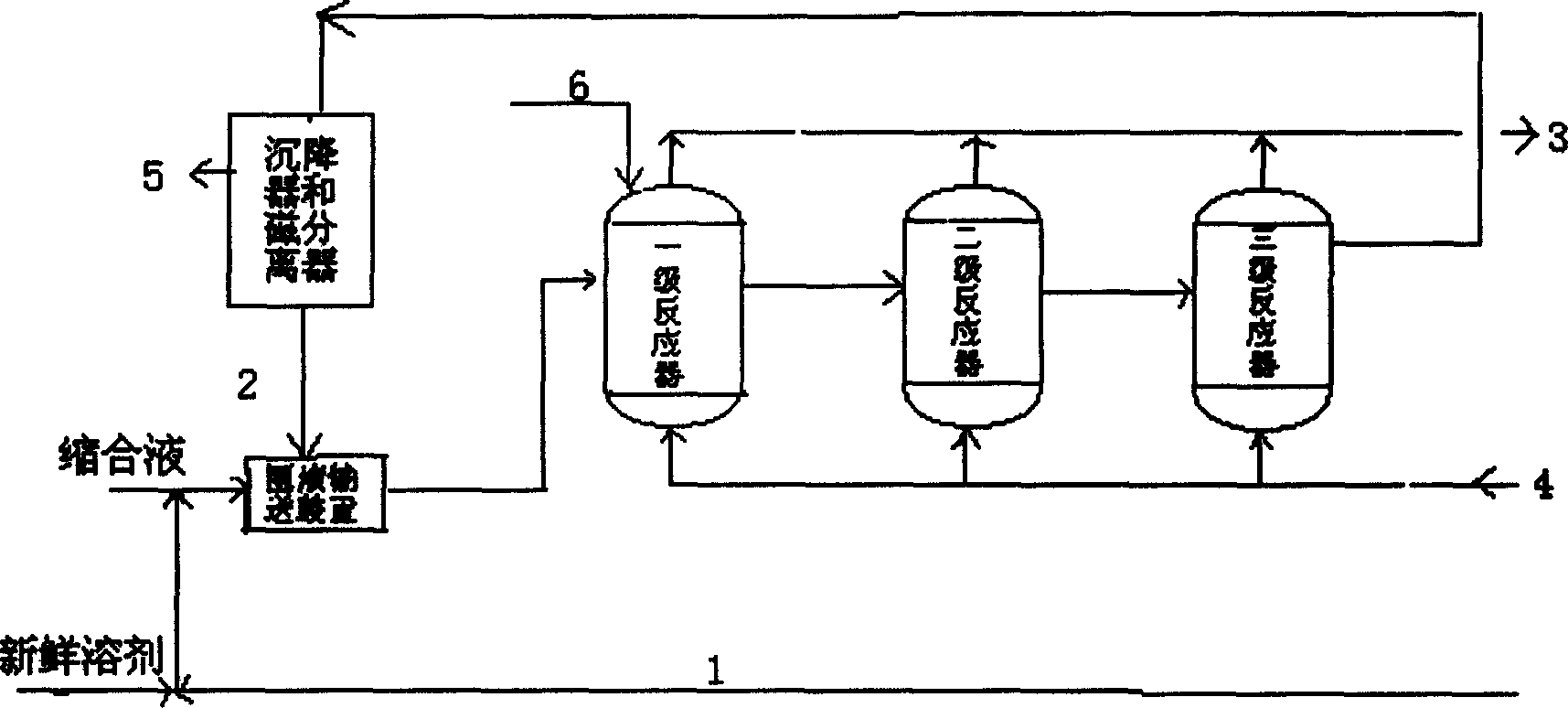
The present invention discloses a method for preparing 4-aminodiphenylamine by continuously hydronating condensate obtained from the reaction by using nitrobenzol and phenylamine as raw material and adopting compound powdered catalyst. i,e, adopts a catalyst and hydrogen gas circulation and solvent circulation combined new process to make the hydrogenation reaction be implemented under the condition of high-concentration, low-temp. and low pressure. The invented catalytic hydrogenation conversion rate can be up to 100%, and its selectivity is greater than 99%, and it is suitable for industrial production of 4-aminophenylamine.
Owner:JIANGSU SINORGCHEM TECH CO LTD
Process for preparing diamines and polyamines from the diphenylmethane series
ActiveUS20070179316A1Organic compound preparationDiaryl/thriaryl methane dyesDiphenylmethanePolymer science
The invention provides an improved process for preparing diamines and polyamines from the diphenylmethane series by reacting aniline and formaldehyde in the presence of an acid catalyst, the improvement involving the aniline containing less than about 3 wt. % of diamines and polyamines from the diphenylmethane series, based on the weight of the aniline.
Owner:COVESTRO DEUTSCHLAND AG
Process for preparing 4-amino diphenylamine
ActiveCN1721390AAvoid smallRaise the ratioAmino preparation from aminesAmino compound preparation by condensation/addition reactionsDecompositionNitrobenzene
The present invention discloses production process of 4-amino diphenylamine with nitrobenzene and phenylamine as materials. For the condensation and hydrogenation, corresponding composite alkali catalyst and composite powdered catalyst are adopted separately. The production process includes the successive steps of condensation, separation I, hydrogenation, separation II and refining, and may be completed continuously. The composite alkali catalyst is separated and reused before the hydrogenation step, and this avoids the heat decomposition of the composite alkali catalyst and increases the catalyst selecting range. The production process has 4-amino diphenylamine yield industrial scale over 95 % and 4-amino diphenylamine purity over 99 wt%.
Owner:JIANGSU SINORGCHEM TECH CO LTD
Process for preparing 4-aminodiphenylamine
ActiveUS20050240058A1Increase rangeHigh yieldAmino compound purification/separationOrganic compound preparationAniline4-aminodiphenylamine
A process for preparing 4-aminodiphenylamine using nitrobenzene and aniline as raw materials, a complex base catalyst as the condensation catalyst, and a powdery composite catalyst as the hydrogenation catalyst. The process has 5 process stages including condensation, hydrogenation, separation I, separation II, and refining.
Owner:SENNICS CO LTD
Mono-methylation method for amines compounds
InactiveCN101260045AHigh reaction conversion rateHigh selectivityAmino compound preparation by condensation/addition reactionsMethyl carbonateToxin
The invention relates to the monomethylation of amine-group compounds, in particular to a monomethylation method for amine-group compounds. The method is characterized in that primary amine or secondary amine is used as materials, and methyl carbonate (DMC) is used as a methylation reagent. The method has the advantages that: due to high conversion rate of material amine-group compounds and high selectivity of monomethylation products, target monomethylation products are high in yield. The reaction liquid is kept basic all the time, and compared with other methods, the method can hardly cause the problem of the corrosion of equipments; the toxin of the reagent is light, the reaction condition is mild and the risk is small; the reaction can be completed in one step, the operational path is simple and easy to practice; products are easy to separate, and reaction byproducts of the main reaction are easy to process.
Owner:抚顺市化工研究设计院
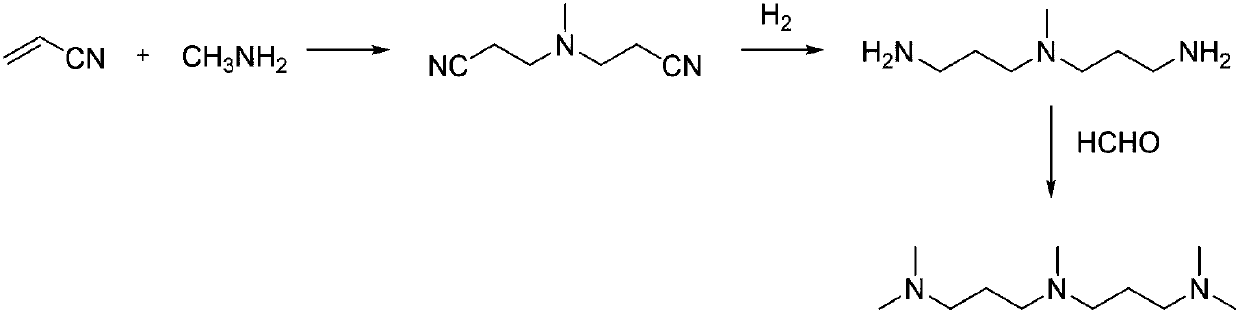
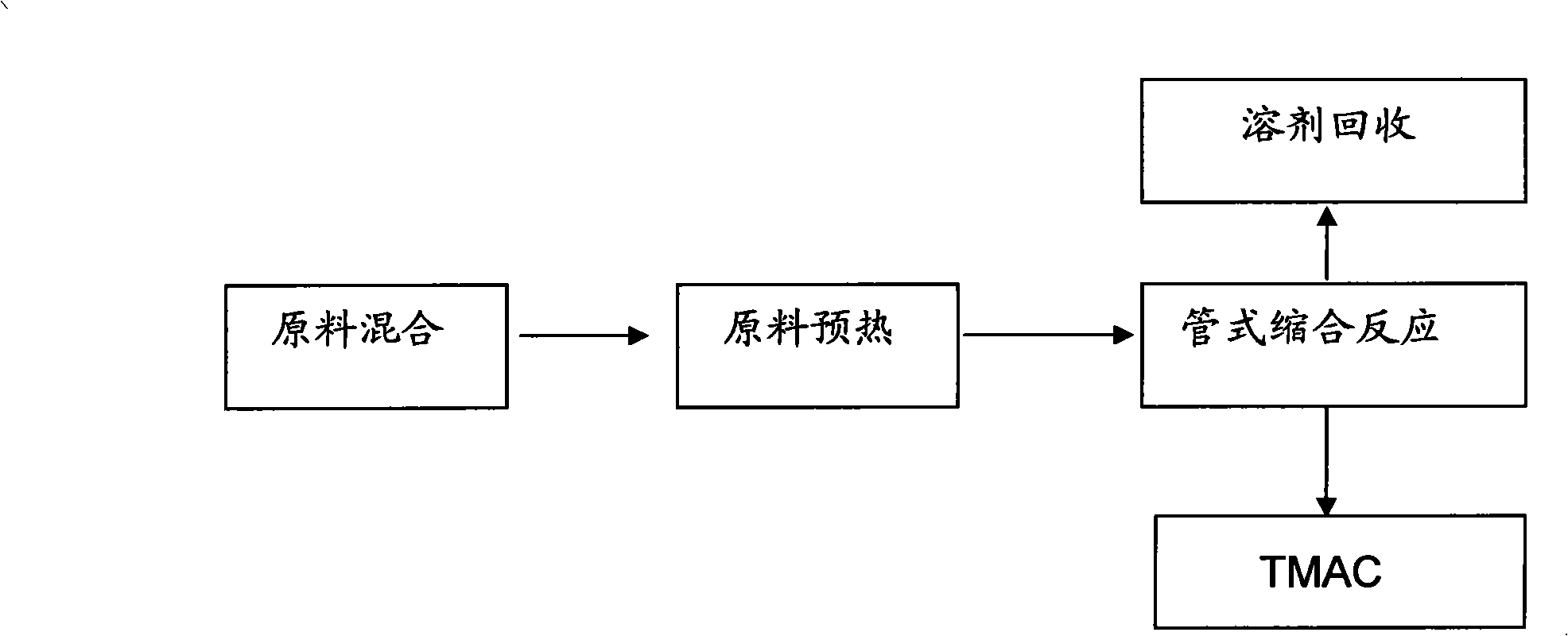
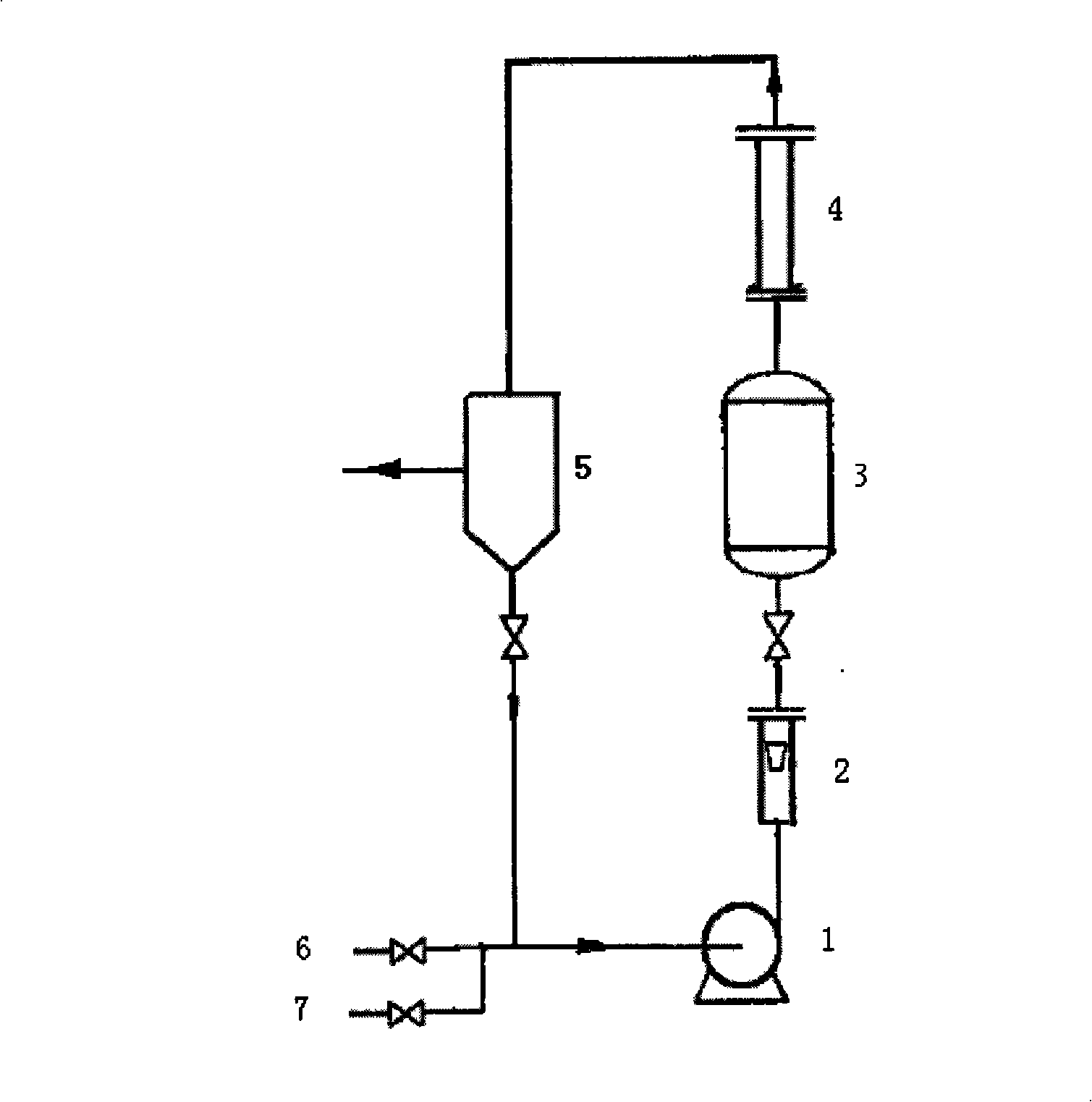
Method for preparing tetramethyl ammonium hydrogen carbonate with condensation reaction of pipe type reactor
InactiveCN101314572ASolve the disadvantages of batch reactionSolve production problemsOrganic compound preparationAmino compound preparation by condensation/addition reactionsSmall footprintSolvent
The invention discloses a method for preparing tetramethylammonium hydrogen carbonate through condensation reaction by a tubular reactor. The method comprises the following steps that: raw materials dimethyl carbonate (DMC) and trimethylamine (TMA) are mixed with a solvent for preheating with a mol ratio of 0.5-1.5 to 1, and then the mixture enters into the tubular reactor for condensation reaction so as to prepare the tetramethylammonium hydrogen carbonate; and the solvent after reaction is recovered and recycled. The method adopts continuous tubular reaction, and can effectively solve the problems of periodic kettle-type reaction; a device for implementing the method is simple and compact, has small floor space and high service efficiency, and saves the investment; the safety factor of the device is improved to a large extent; the product quality is stable; the production process is simple and concise; and the consumption and the cost are effectively reduced.
Owner:HANGZHOU GREENDA CHEM
Preparation method of cyclohexylamine
InactiveCN101161631ALow costOrganic compound preparationMetal/metal-oxides/metal-hydroxide catalystsAnilinePhenol
The present invention discloses a preparation method of cyclohexylamine, in which phenol, H2, and NH3 are adopted as the raw materials, the catalyst used by the present invention adopts gamma-Al2O3 as the carrier skeleton, and magnesia-alumina spinal carrier is made through impregnating the mixed solution of nitric magnesium and nitric aluminum, then the chloride palladium hydrochloric acid solution is impregnated to obtain the hydrogenated and aminated catalyst of Pd / Al2O3-MgO / Al2O3. Then the catalyst is added into an integral reactor, firstly activated under ordinary pressure through conducting the hydrogen gas, then phenyl, hydrogen gas and ammonia gas in proportion under about 180 DEG C are conducted to produce cyclohexylamine. Compared with the aniline hydrogenation reduction generally adopted both domestically and worldwide, the present invention with simple technics has the advantages of firstly low cost, with cheaper phenol to substitute for aniline as the raw material for the preparation of cyclohexylamine; secondly the catalyst with high activity and a long life ; thirdly high yield of product with high selectivity and 4, minor pollution, with capability to meet the principle of green chemical industrial technology.
Owner:HANERGY TECH
Process for preparing 4-aminodiphenylamine
ActiveUS7176333B2Amino compound purification/separationOrganic compound preparationHydrogenation reactionAniline
The present invention discloses a process for preparing 4-aminodiphenylamine, which process uses nitrobenzene and aniline as raw materials, a complex base catalyst as condensation catalyst and a powdery composite catalyst as hydrogenation catalyst, and comprises five process stages: condensation; separation I; hydrogenation; separation II; and refining. The process can be continuously carried out. By selecting a complex base catalyst to catalyze the condensation reaction and separating it prior to the hydrogenation, the problem that the complex base catalysts thermally decompose in the hydrogenation reaction is avoided, the selectable range of hydrogenation catalysts is largely enlarged so that it is possible to select cheaper hydrogenation catalyst, and the selection of production process and equipment is easier and further industrialization is easier. The complex base catalysts used in the present invention are inexpensive and have higher catalytic activity. The process can be carried out at mild conditions and can adapt to broad range of water content, by-product is less and conversion and selectivity are higher. The operational strength is low, no corrosive liquid is produced, and environment pollution is reduced. The purity of 4-aminodiphenylamine prepared can exceed 99 wt.-%, and the yield in the industrial production process can be over 95%.
Owner:NONGYUE WANG & GUANGQIANG SHI +1
Solid acid catalyst and preparation method and applications thereof
ActiveCN108654594AReduce manufacturing costLow costMetal/metal-oxides/metal-hydroxide catalystsAmino compound preparation by condensation/addition reactionsLanthanideSolid acid
The invention relates to a solid acid catalyst and a preparation method and applications thereof. The catalyst includes lanthanide rare earth metal ions, halogen and a carrier. The carrier includes aboron-silicon composite oxide and silicon oxide; and the boron-silicon composite oxide is an amorphous compound, and can be obtained by mixing boron source and silicon source compounds, adjusting pH to form gel, adding a surfactant as a pore forming agent during a gel synthesizing process, and performing hydrothermal reaction, hole expansion with ammonia, drying and roasting, etc. According to thecatalyst, the amorphous boron-silicon composite having a high specific surface area and specific pore size distribution is molded to form the carrier and a lanthanide rare earth metal oxide which canform a specific acid site and the halogen are added for modification. The catalyst can be used for direct amination of isobutene to prepare tert-butylamine; and high isobutene one-way conversion rateand tert-butylamine selectivity can be achieved, and the catalyst has good stability.
Owner:WANHUA CHEM GRP CO LTD
Method of synthesizing imine and amine compounds by means of borrowing-hydrogen reduction coupling
ActiveCN108689786ASimple and fast operationMild conditionsPhysical/chemical process catalystsCarboxylic acid nitrile preparationChemical industryPtru catalyst
The invention belongs to energy and chemical industry and particularly relates to a method of synthesizing imine and amine compounds by means of borrowing-hydrogen reduction coupling by using a nitrogen-doped hierarchical-porous biomass-based carbon material supported catalyst. The method includes the steps of: under a seal reaction condition, adding a nitro-aromatic hydrocarbon compound, benzyl alcohol compounds with different substituent groups, the supported catalyst, methylbenzene and potassium tert-butoxide; performing a reaction at 50-150 DEG C for 4-24 h, cooling the product to room temperature and filtering a reaction liquid to obtain the imine compound represented in the formula (1) or the amine compound represented in the formula (2). The raw materials of the catalyst are regenerable resources, are widely distributed, are green and environment-friendly, are easy to prepare and abundant in sources, and are low in cost; the catalyst can be recycled without deactivation and is stable to air, water and heat. By means of the supported metal catalyst, the conversion rate of the borrowing-hydrogen reduction coupling reaction on the nitro-compound and alcohol to prepare the iminecompounds is higher than 99%, and yield can reach 90-60%; the conversion rate of same to prepare the amine compounds is higher than 99%, and yield can reach 90-60%.
Owner:QINGDAO INST OF BIOENERGY & BIOPROCESS TECH CHINESE ACADEMY OF SCI
Popular searches
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com