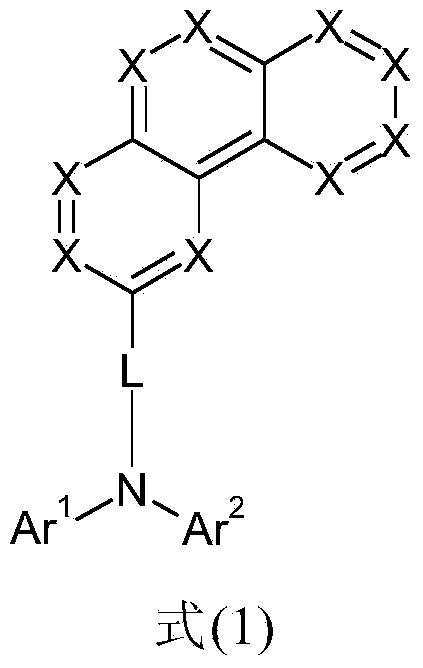
Phenanthrene compounds for organic electronic devices
A compound and atomic technology, applied in the field of organic compounds, can solve problems such as high working voltage and poor performance data
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0205] Synthesis of biphenyl-4-yl-(9,9-dimethyl-9H-fluoren-2-yl)phenanthrene-3-ylamine (1-1)
[0206]
[0207] Synthesis of raw material 3-bromophenanthrene
[0208]
[0209] Synthesis of 3-Aminophenanthrene
[0210] 50 g (227 mmol) of 3-acetylphenanthrene and 63.8 ml (790 mmol) of pyridine and 42 g (592 mmol) of hydroxyammonium chloride were dissolved in 300 mL of EtOH. The batch was heated to 75°C. After 1 hour of reaction the batch was cooled. The mixture was then partitioned between ethyl acetate and water, the organic phase was washed three times with water and washed with Na 2 SO 4 Dry and concentrate on a rotary evaporator. 300 ml of polyphosphoric acid was carefully added to the concentrated solution, and the mixture was heated at 75°C for 1 hour. The batch was then cooled to room temperature and poured carefully into ice water (300ml). The precipitated solid was filtered off with suction and rinsed with methanol. Finally, 800ml MeOH and 70ml concentrated...
Embodiment 13
[0226] Compound N*4'*-biphenyl-4-yl-N*4'*-dibenzofuran-4-yl-N*4*-phenanthrene-3-yl-N*4*-phenylbiphenyl- Synthesis of 4,4'-diamine (2-1)
[0227]
[0228] Dissolve 10g phenanthrene-3-ylphenylamine (37mmol), 21g biphenyl-4-yl-(4'-bromobiphenyl-4-yl)dibenzofuran-4-ylamine (37mol) in 500ml toluene Middle: degas the solution and wash with N 2 saturation. Then 1.5 ml (1.5 mmol) of tri-tert-butylphosphine solution and 0.17 g (0.74 mmol) of palladium(II) acetate were added, and thereafter 5.6 g of sodium tert-butoxide (56 mmol) were added. The reaction mixture was heated at boiling for 3 hours under a protective atmosphere. Thereafter the mixture was partitioned between toluene and water, the organic phase was washed three times with water and washed with Na 2 SO 4 Dry and evaporate on a rotary evaporator. After filtration of the crude product through silica gel with toluene, the remaining residue was recrystallized from heptane / toluene and finally sublimed under high vacuum,...
Embodiment 19
[0237] Synthesis of Compound Biphenyl-4-ylbiphenyl-2-yl-(9,9-Dimethyl-7-phenanthrene-3-yl-9H-fluoren-2-yl)amine (3-1)
[0238]
[0239] 3-(7-Bromo-9,9-dimethyl-9H-fluoren-2-yl)phenanthrene
[0240] Mix 52g (164mmol) of 7-bromo(9,9-dimethylfluoren-2-yl)boronic acid (CAS number: 1213768-48-9), 50g (164mmol) of 3-iodophenanthrene and 205ml of 2M NaHCO 3 The aqueous solution (327 mmol) was suspended in 800 ml of dimethoxyethane. 3.8 g (3.3 mmol) tetrakis(triphenyl)phosphine palladium(0) were added to the suspension, and the reaction mixture was heated under reflux for 16 hours. After cooling, the organic phase was separated, filtered through silica gel, washed 3 times with 300 mL of water and then evaporated to dryness. The crude product was filtered through silica gel with heptane / ethyl acetate (20:1) to afford 55 g (75%) of 3-(7-bromo-9,9-dimethyl-9H-fluoren-2-yl)phenanthrene .
[0241] Biphenyl-4-ylbiphenyl-2-yl-(9,9-dimethyl-7-phenanthrene-3-yl-9H-fluoren-2-yl)amine
...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com