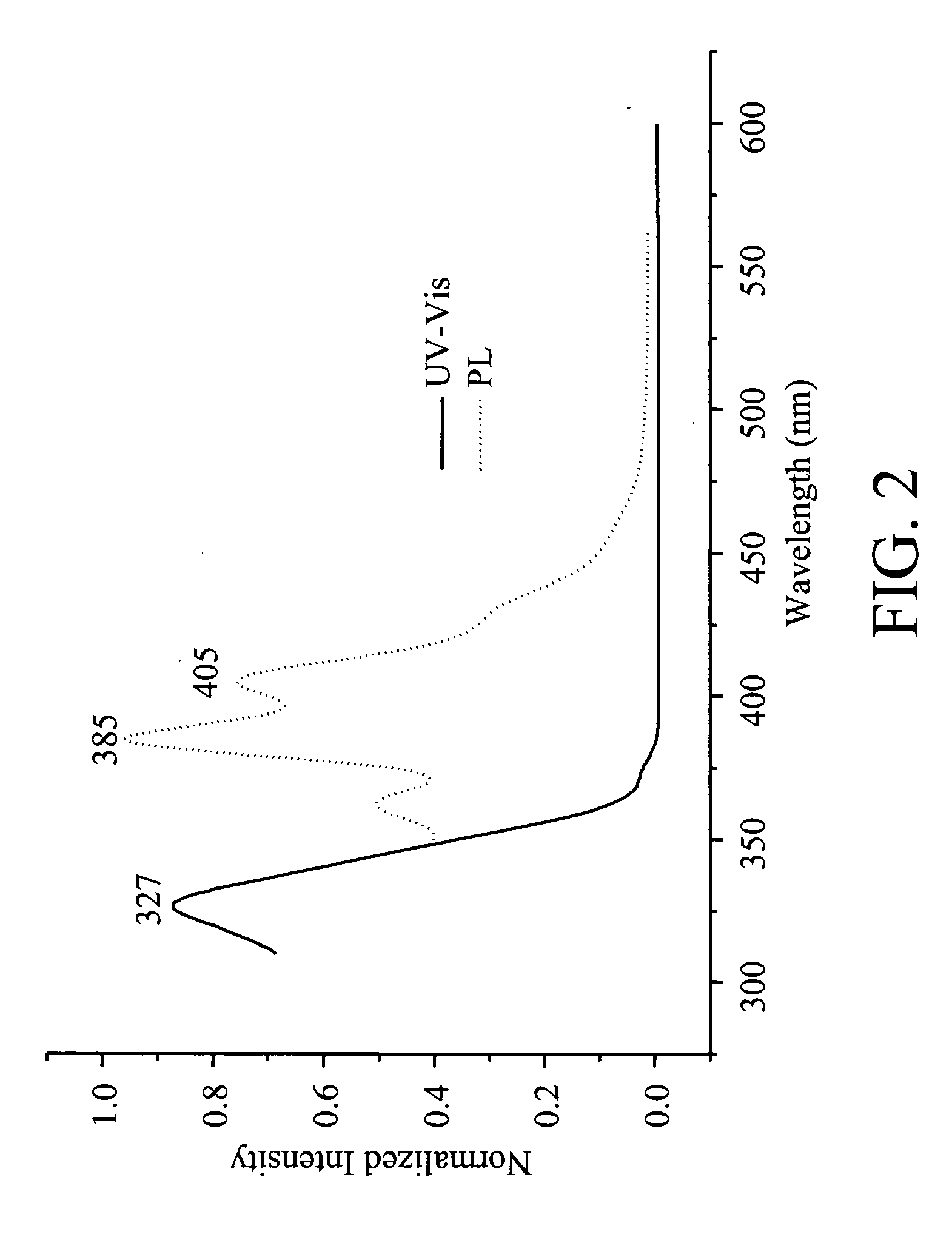
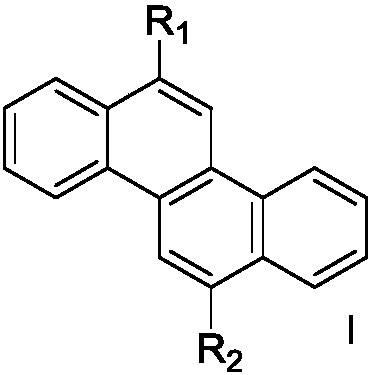
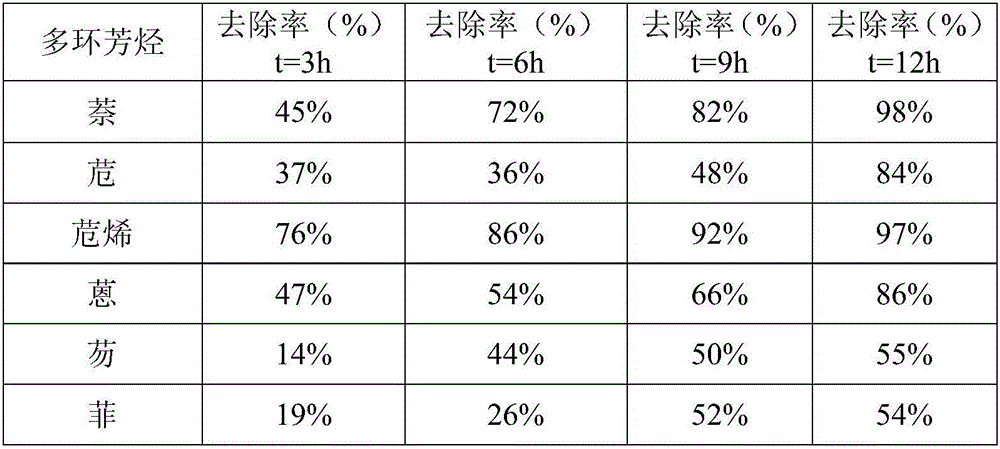
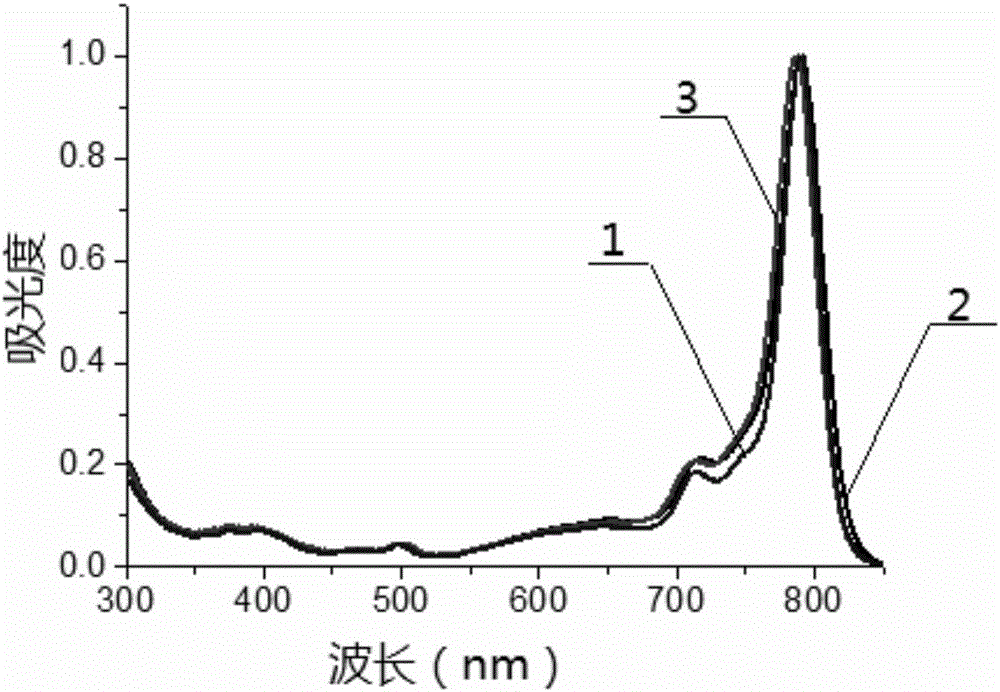
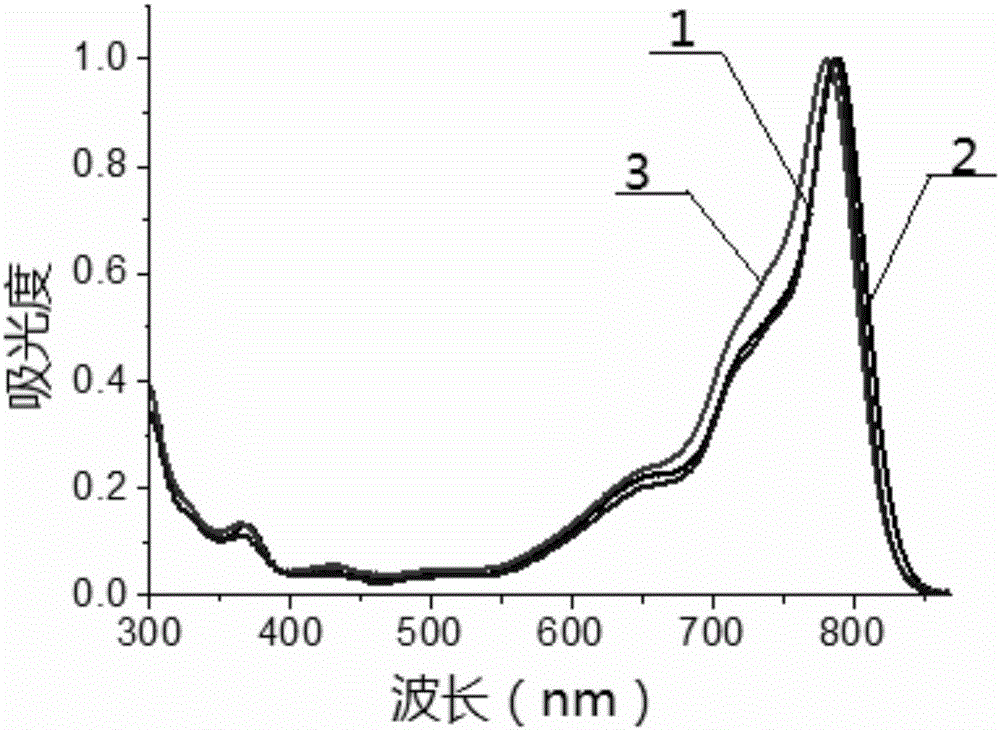
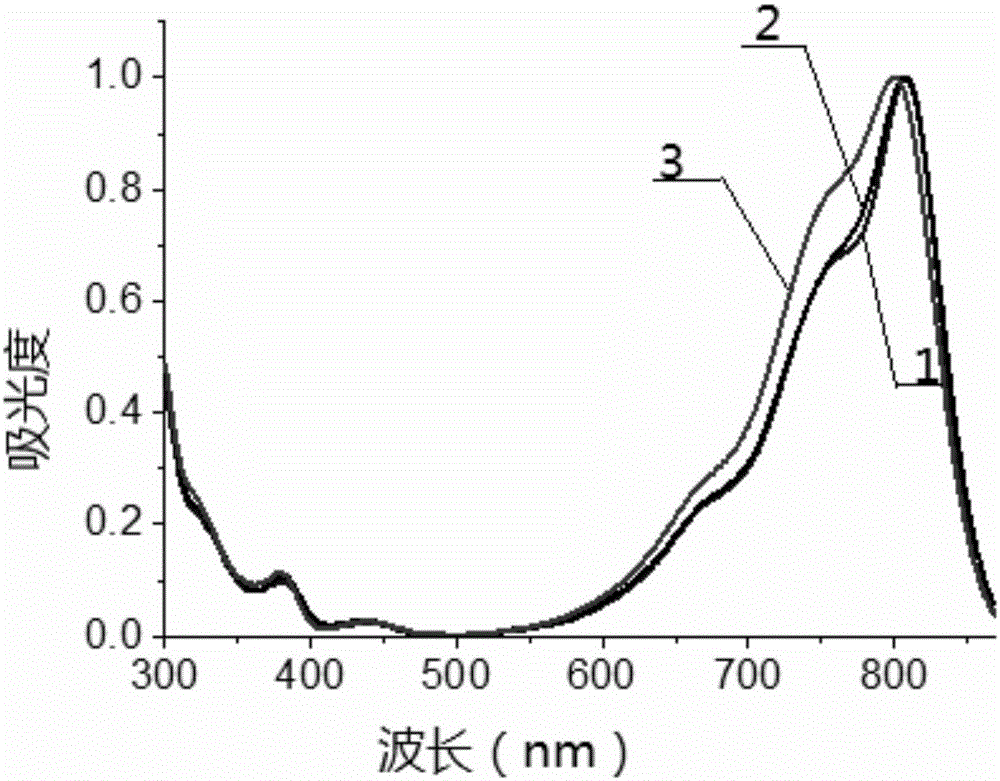
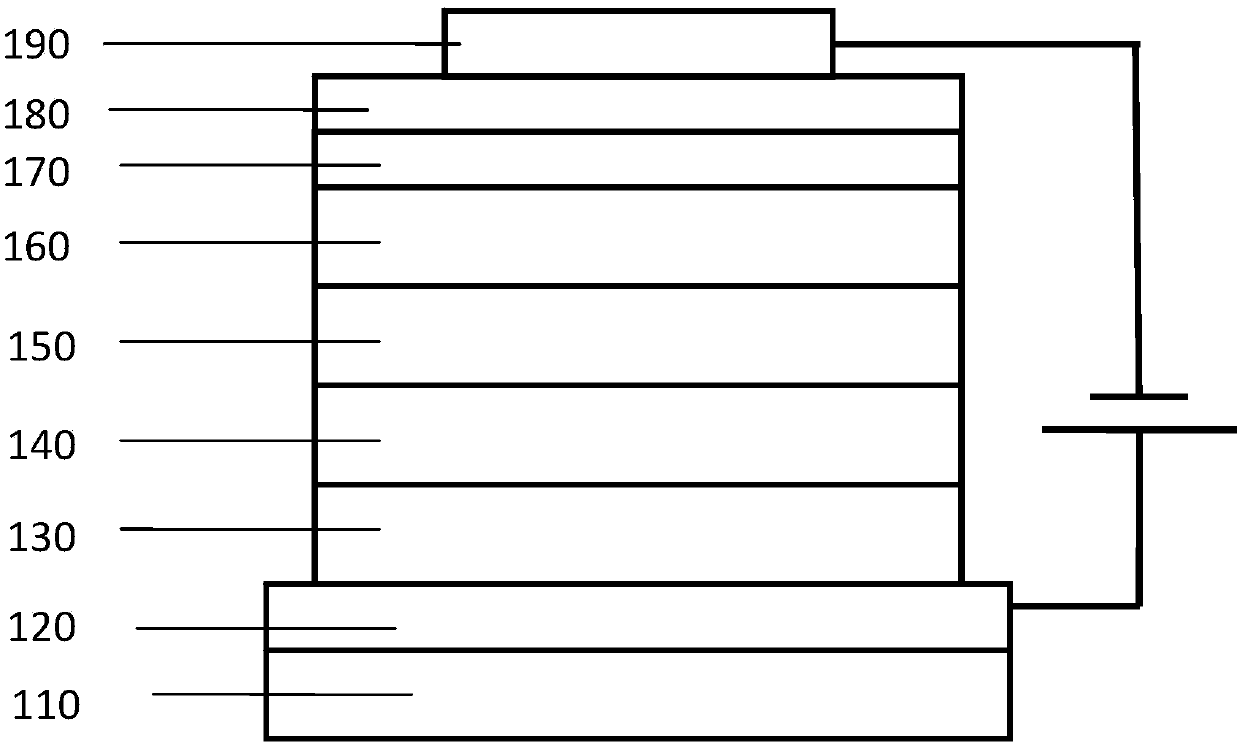
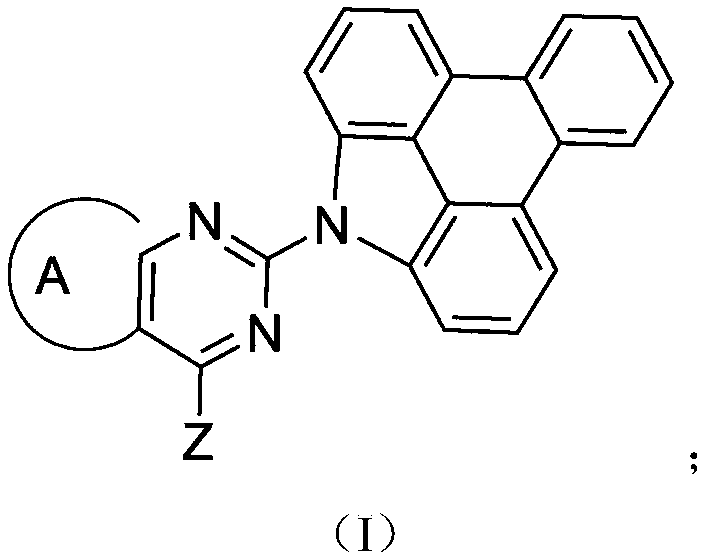
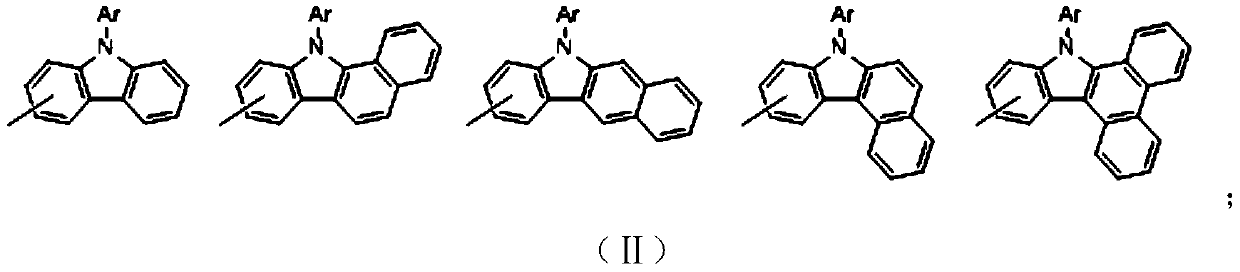
Patents
Literature
60 results about "Phenanthrenes" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
POLYCYCLIC AROMATIC HYDROCARBONS composed of three fused BENZENE rings.
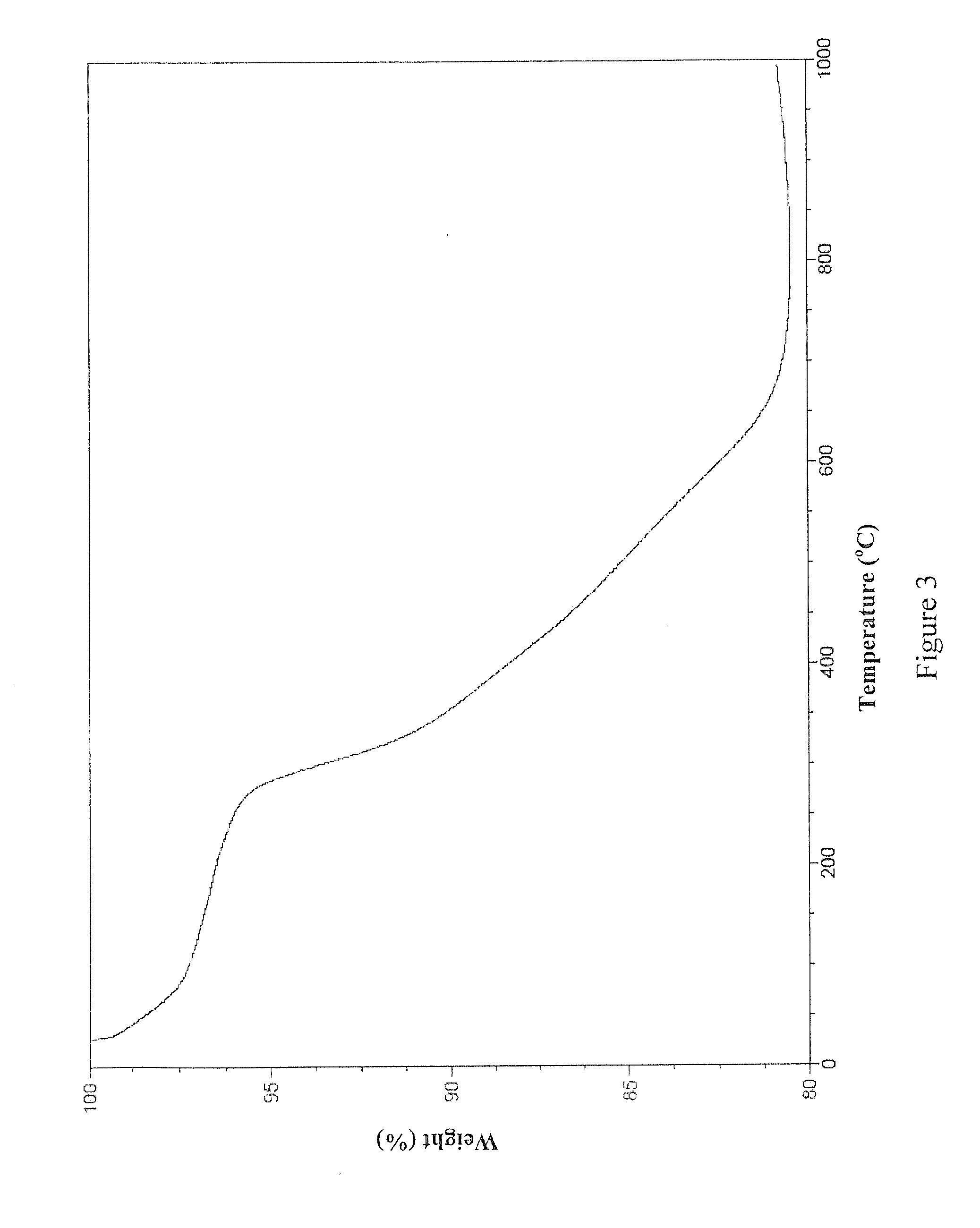
Mesoporous carbon and method of producing the same
InactiveUS20060263288A1Low plane resistanceImprove conductivityCarbon compoundsFinal product manufactureMesoporous silicaConductive materials
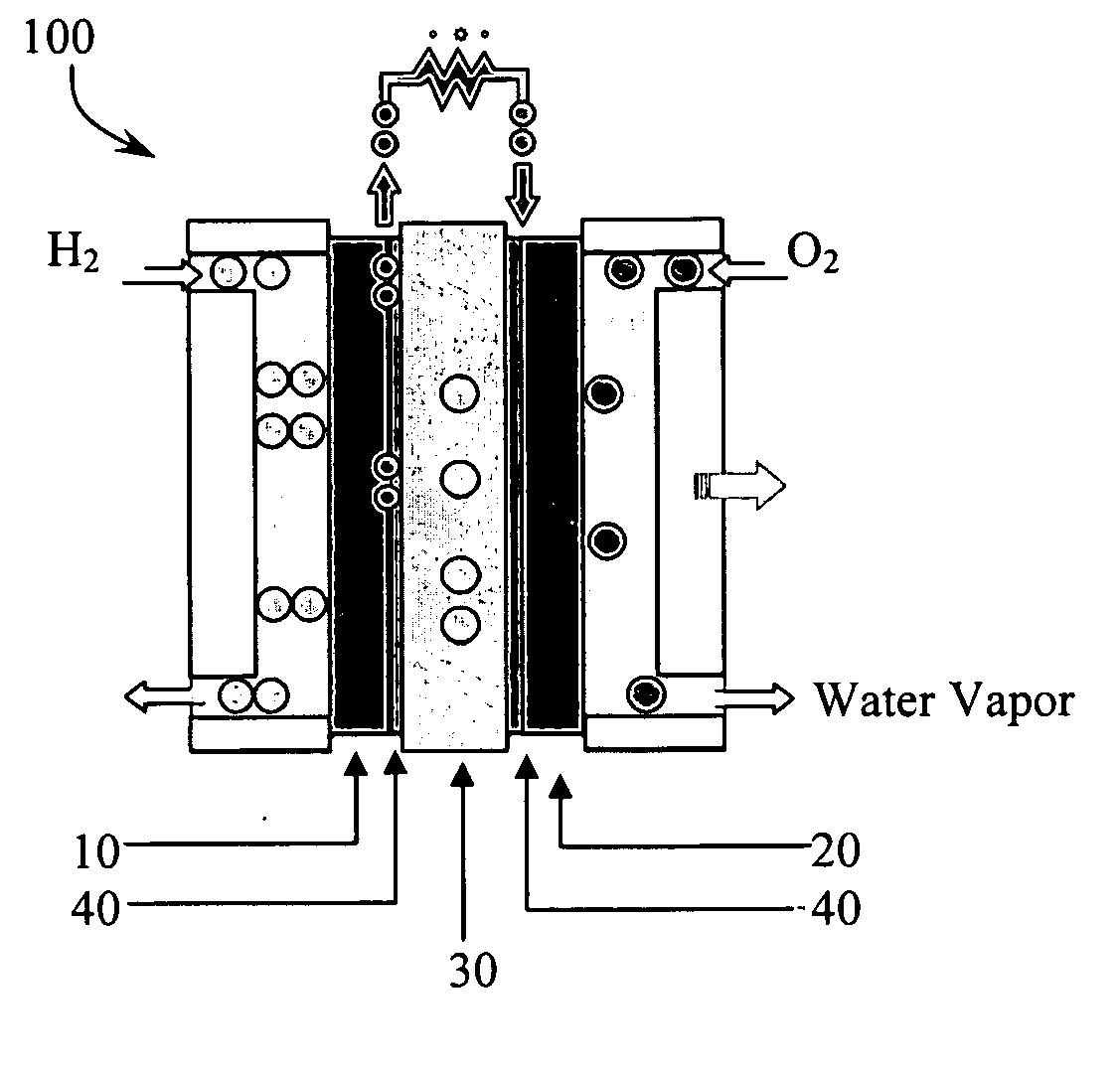
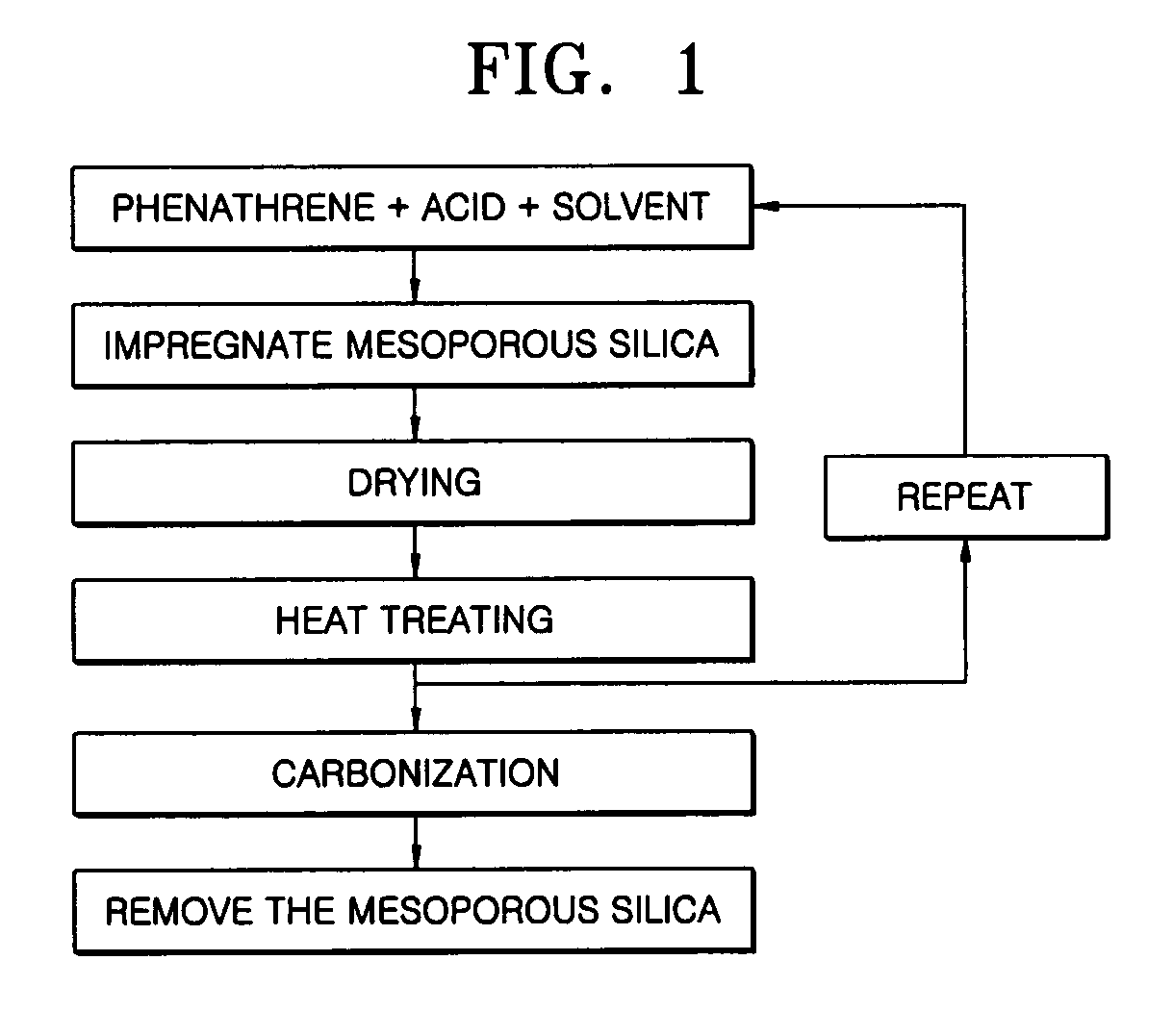
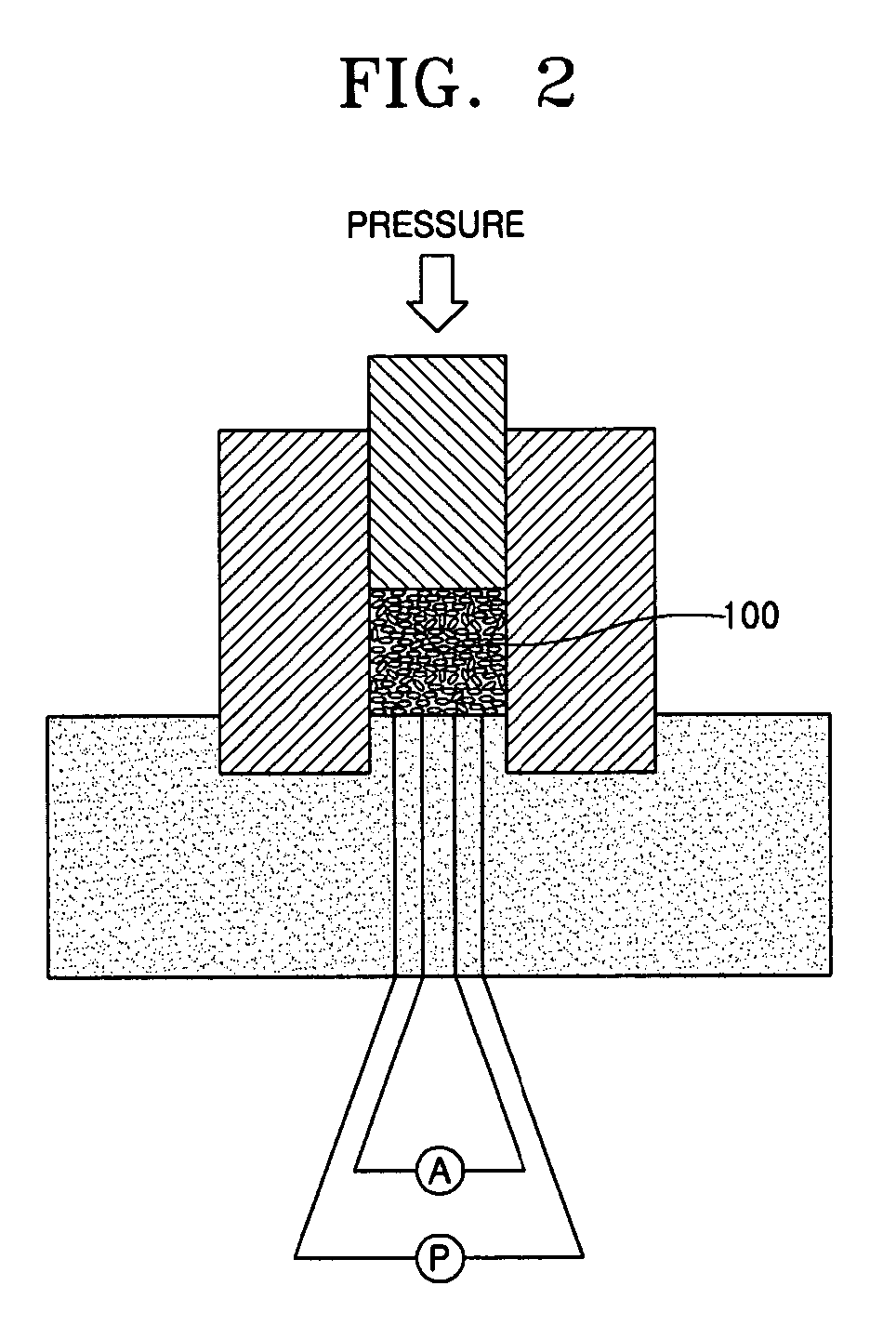
Provided are a mesoporous carbon and a method of preparing the same, where the mesoporous carbon is prepared using phenanthrene as a carbon source and a mesoporous silica as a template. The mesoporous carbon has a significantly low plane resistance, which can be obtained without sacrificing other physical properties, and thus obtains a high conductivity and effectively transfers electrical energy. Accordingly, a fuel cell electrode or a fuel cell which is produced using the mesoporous carbon as a conductive material has high efficiency. Furthermore, the mesoporous carbon may be used in various electrochemical devices as a conductive material.
Owner:SAMSUNG SDI CO LTD
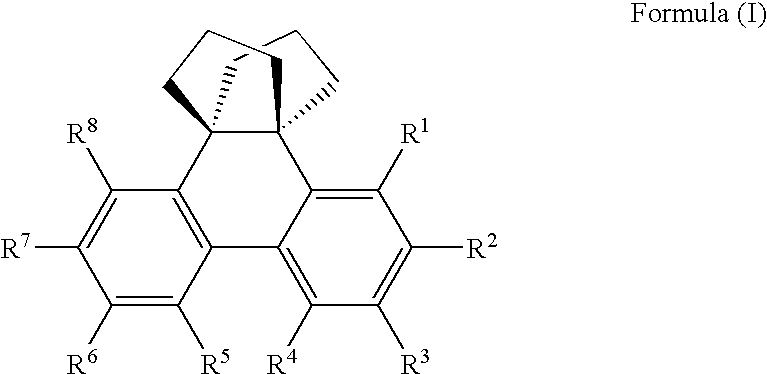
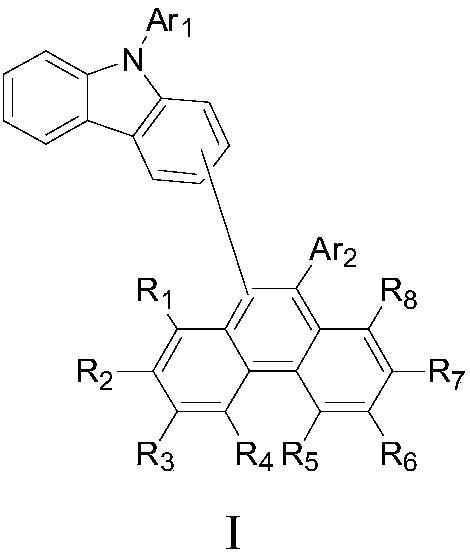
Phenanthrene compounds
ActiveUS20050176952A1Improve heat resistanceImprove stabilityOrganic compound preparationElectroluminescent light sourcesCompound aHalogen
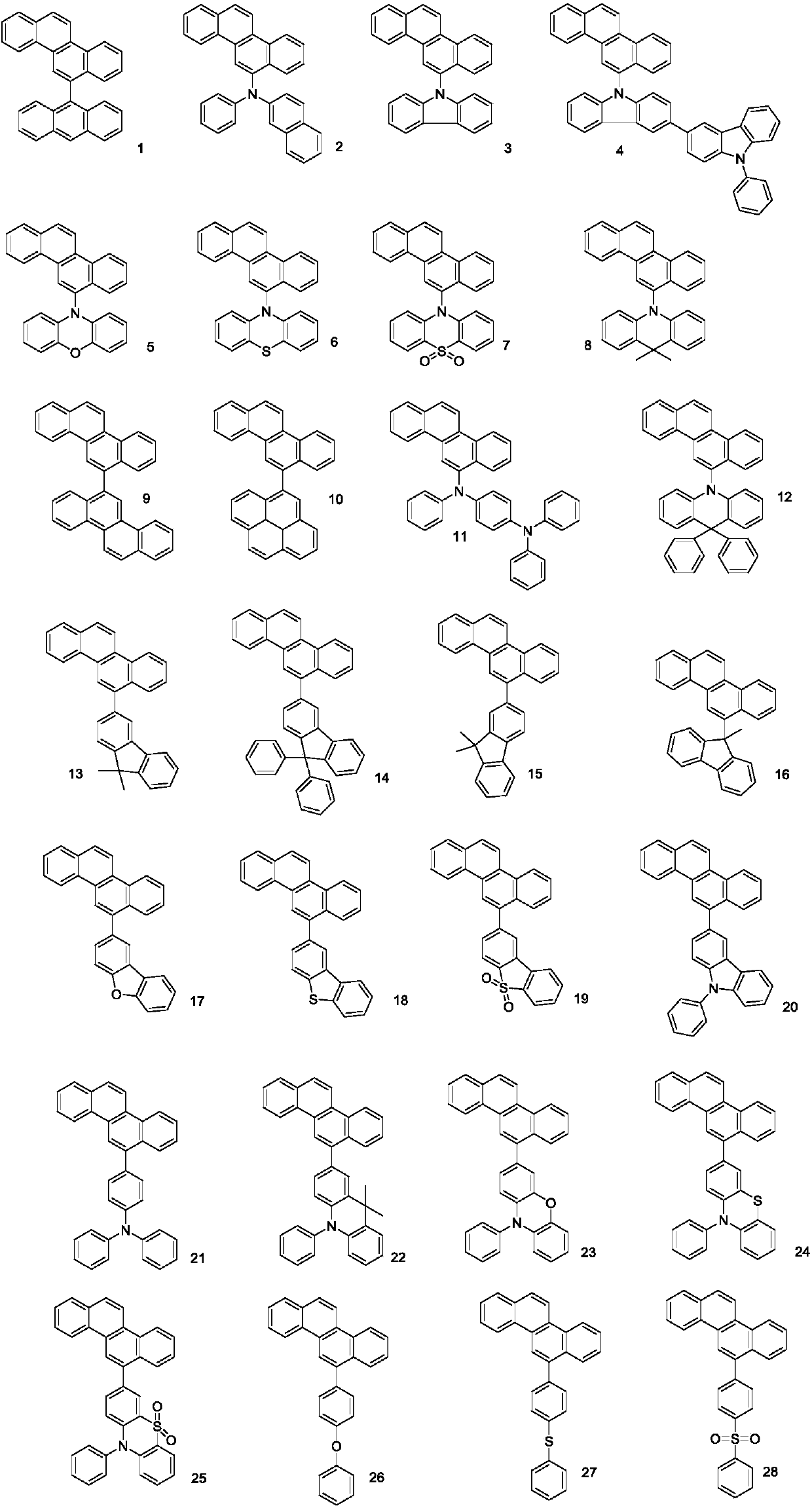
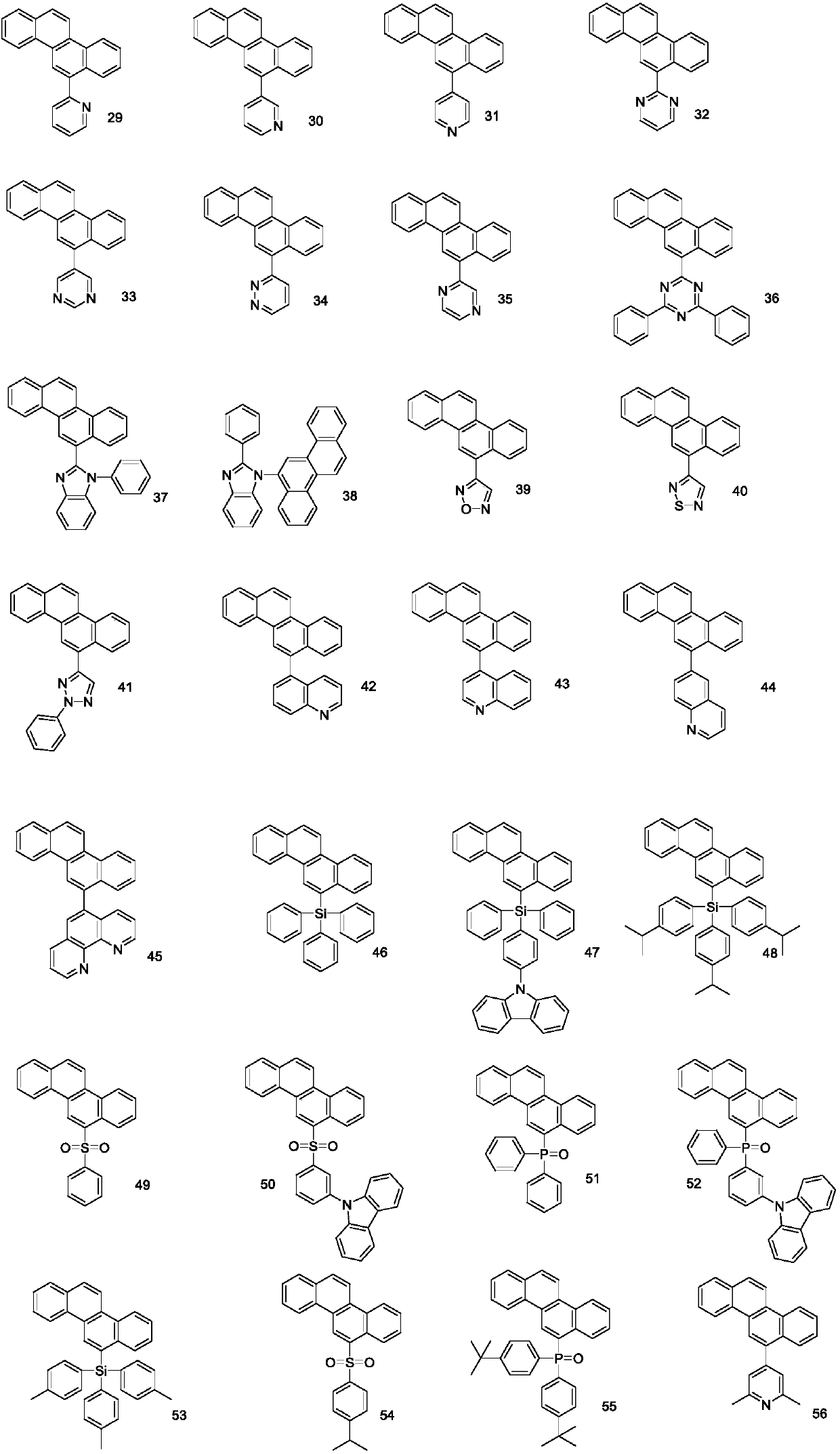
A phenanthrene compound represented by the formula (I): in which, R1, R2, R3, R4, R5, R6, R7, and R8 are identical or different and may each be hydrogen, halogen, C1-6 alkyl, C1-6 alkoxy, or a conjugated group. The phenanthrene compound has a polycyclic structure and semiconductor properties including electron transfer, electroluminescence, and photoluminescence.
Owner:IND TECH RES INST
Arthrobacter strain highly effectively degrading phenanthrene, and application thereof
ActiveCN103215204AReduce contentRealize harmless disposalBacteriaWater contaminantsPolycyclic aromatic hydrocarbonArthrobacter
The invention provides an arthrobacter strain highly effectively degrading phenanthrene, and an application thereof. The phenanthrene-degrading arthrobacter strain has a preservation number of CGMCC No. 6581. The strain provided by the invention can effectively degrade polycyclic aromatic hydrocarbon and heterocyclic substances in soil and oil sludge, and can be effectively applied in biodegradation treatments of petroleum-contaminated soil and PAHs pollutants in soil in loess highlands. Especially a phenanthrene degradation rate can be higher than 98%. The invention also provides a formula of a nutrient solution for the growth and degradation of the strain, and a method for applying the strain in petroleum-contaminated soil field reparation.
Owner:PETROCHINA CO LTD
Phenanthrene-containing heterocycle compound and preparation method and application in resisting plant virus
ActiveCN104412985AExcellent anti-plant virus activityEffectively prevent and treat viral diseasesBiocideOrganic chemistryTobacco mosaic virusChemical compound
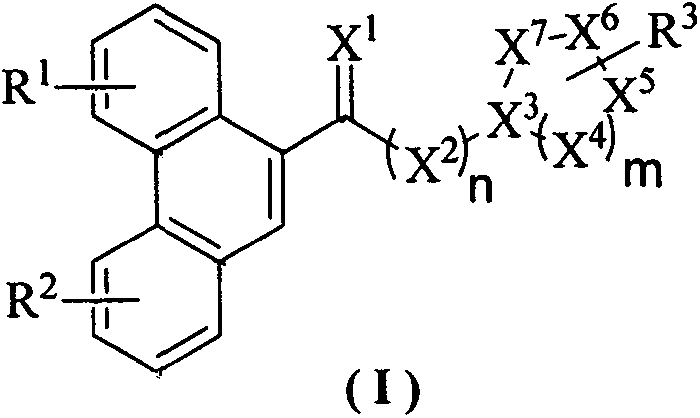
Disclosed are phenanthrene-containing heterocyclic compounds shown as general formula (I) and preparation method therefor and application thereof on pesticides. The synthetic method of the compounds is simple and easy to industrialize. The phenanthrene-containing heterocyclic compounds can be used as plant virucides to inhibit tobacco mosaic virus, pepper virus, rice virus, tomato virus, sweet potato virus, potato virus, melon virus, maize dwarf mosaic virus and the like, particularly to inhibit tobacco mosaic virus. R1, R2, R3, X1-X7, m and n in general formula (I) are as defined in description.
Owner:NANKAI UNIV
Efficient red light electroluminescence material processed by solution using naphthothiadiazole as luminous center
InactiveCN101591531AQuality improvementImprove efficiencyOrganic chemistrySolid-state devicesOxygenPyrrole
The invention belongs to the field of organic luminescence, and particularly relates to a red light electroluminescence material using naphthothiadiazole as a luminous center. The red light electroluminescence material can be prepared into a high electroluminescence film used for an organic electroluminescence device by using a solution processing method or an electrochemical polymerization method. The luminous center connected with an electroactive unit consists of an efficient naphthothiadiazole red light unit and efficient dark-blue light emitting units at two sides. The dark-blue light emitting units are fluorene, biphenyl, terphenyl, naphthalene, anthracene, phenanthrene and derivatives formed by mutually bonding the substances. The electroactive unit is carbazole, thiofuran, pyrrole, ethylene, ethyne, phenylamine, diphenylamine or triphenylamine. A connecting chain of the electroactive unit and the light emitting unit is an alkyl chain, an oxygen chain or an alkoxyl chain, and the electroactive unit and the light emitting unit also can be directly connected. The luminous efficiency of a device using the red light material reaches 5.8cd / A, and has high level in the red light devices prepared by electrochemical deposition.
Owner:JILIN UNIV
Novel organo-metal compounds
InactiveUS20060263621A1Drawback can be obviatedHigh refractive indexGroup 4/14 element organic compoundsPretreated surfacesCompound (substance)Film material
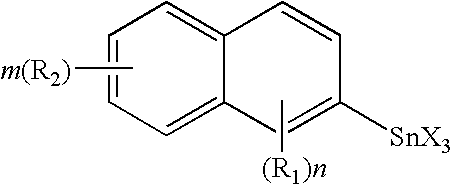
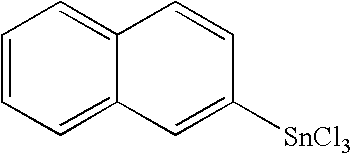
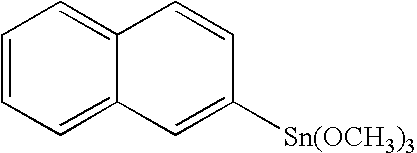
Organo-tin compound having the formula I: [R—(Y)a]b—SnX4-b wherein R stands for a polycyclic hydrocarbyl residue which optionally carries one or several substituents; Y stands for a bivalent linker group; X represents a hydrolysable group; a is an integer 0 or 1; and b is an integer 1 or 2. The preferably stands for an unsubstituted or substituted fused hydrocarbon ring system selected from naphthalene, antracene, phenanthrene, and pentacene. The novel compounds are useful for producing polymers which can be employed as thin film materials in optoelectronic devices.
Owner:BRAGGONE
Crosslinkable host materials
InactiveCN106715420AEasy to moveImprove efficiencyGroup 5/15 element organic compoundsFinal product manufacturePyridazinePhenanthroline
The invention relates to a crosslinkable organic molecule having a structure of the formula (1) and to the use thereof, wherein Ar is independently of one another, an unsaturated or aromatic carbo- or heterocyclic unit with 5 to 30 ring atoms, selected from the group consisting of naphthalene, anthracene, phenanthrene, pyrene, dihydropyrene, chrysene, perylene, fluoranthene, benzanthracene, tetracene, pentacene, benzpyrene, furan, benzofuran, isobenzofuran, thiophene, benzothiophene, isobenzothiophene, dibenzothiophene, pyrrole, indole, isoindole, carbazole, pyridine, quinoline, isoquinoline, acridine, phenanthridine, benzo-5,6-quinoline, benzo-6,7-quinoline, benzo-7,8-quinoline, phenothiazine, phenoxazine, pyrazole, indazole, imidazole, benzimidazol, naphthimidazole, phenanthrimidazole, pyridimidazole, pyrazine-imidazole, quinoxalinimidazole, oxazole, benzoxazole, naphthoxazole, anthroxazole, phenanthroxazole, isoxazole, isothiazole, 1,3-thiazole, benzothiazole, pyridazine, benzopyridazine, pyrimidine, benzpyrimidine, quinoxaline, pyrazine, phenazine, naphthyridine, azacarbazole, benzocarboline, phenanthroline, 1,2,3-triazole, 1,2,4-triazole, benzotriazole, 1,2,3-oxadiazole, 1,2,4-oxadiazole, 1,2,5-oxadiazole, 1,3,4-oxadiazole, 1,2,3-thiadiazole, 1,2,4-thiadiazole, 1,2,5-thiadiazole, 1,3,4-thiadiazole, 1,3,5-triazine, 1,2,4-triazine, 1,2,3-triazine, tetrazole, 1,2,3,4- oxatriazole, 1,2,3,4-oxatriazole, 1,2,4,5-tetrazine, 1,2,3,4-tetrazine, 1,2,3,5-tetrazin, purine, pteridine, indolizine, benzothiadiazole, indenocarbazole, indenofluorene, spirobifluorene, and indolocarbazole; D1 is a donor group having a structure of the formula (1a); and D2 is a donor group having a structure of the formula (1b).
Owner:SAMSUNG DISPLAY CO LTD
Phenanthrene compounds
ActiveUS6967255B2Increase resistanceImprove stabilityOrganic compound preparationElectroluminescent light sourcesCompound aHydrogen
A phenanthrene compound represented by the formula (I): in which, R1, R2, R3, R4, R5, R6, R7, and R8 are identical or different and may each be hydrogen, halogen, C1-6 alkyl, C1-6 alkoxy, or a conjugated group. The phenanthrene compound has a polycyclic structure and semiconductor properties including electron transfer, electroluminescence, and photoluminescence.
Owner:IND TECH RES INST
Thiol-containing compounds for the removal of elements from tissues and formulations therefor
ActiveUS20110160150A1Low toxicitySpeedGroup 4/14 element organic compoundsBiocidePolymerMercaptan compound
Methods and pharmaceutical formulations for ameliorating heavy metal toxicity and / or oxidative stress are disclosed, comprising administering pharmaceutically effective amounts of ligands according to the present disclosure. The ligands are of the general structure:where R1 comprises benzene, pyridine, pyridin-4-one, naphthalene, anthracene, phenanthrene or alkyl groups, R2 comprises hydrogen, alkyls, aryls, a carboxyl group, carboxylate esters, organic groups or biological groups, R3 comprises alkyls, aryls, a carboxyl group, carboxylate esters, organic groups or biological groups, X comprises hydrogen, lithium, sodium, potassium, rubidium, cesium, francium, alkyls, aryls, a carboxyl group, carboxylate esters, thiophosphate, N-acetyl cysteine, mercaptoacetic acid, mercaptopropionic acid, thiolsalicylate, organic groups or biological groups, n independently equals 1-10, m=1-6, Y comprises hydrogen, polymers, silicas or silica supported substrates, and Z comprises hydrogen, alkyls, aryls, a carboxyl group, carboxylate esters, a hydroxyl group, NH2, HSO3, halogens, a carbonyl group, organic groups, biological groups, polymers, silicas or silica supported substrates.
Owner:UNIV OF KENTUCKY RES FOUND
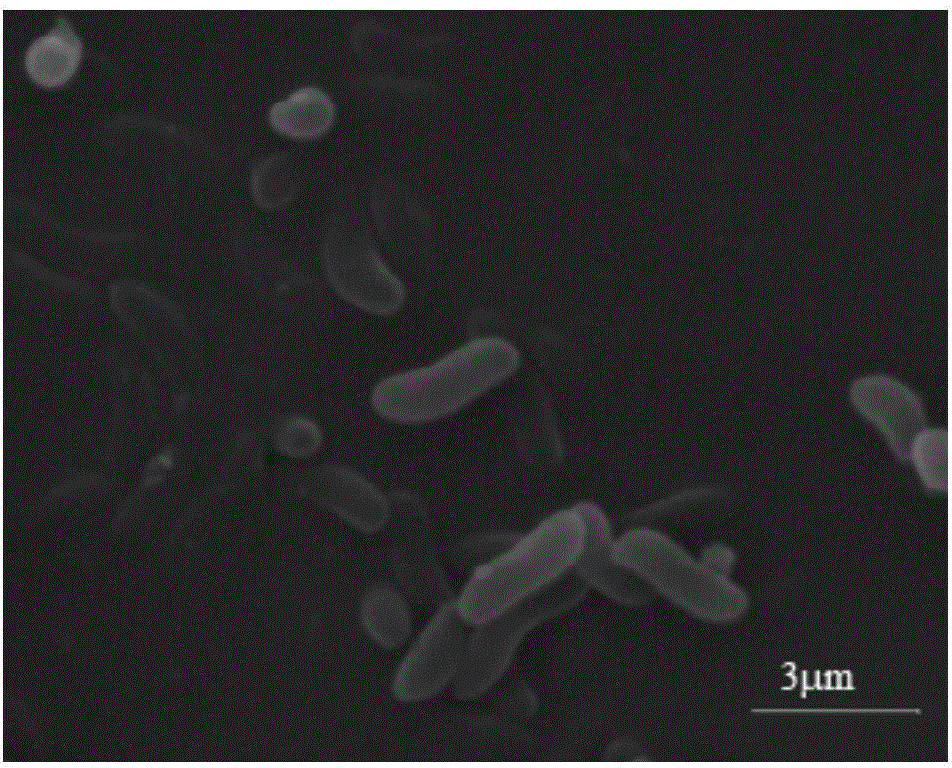
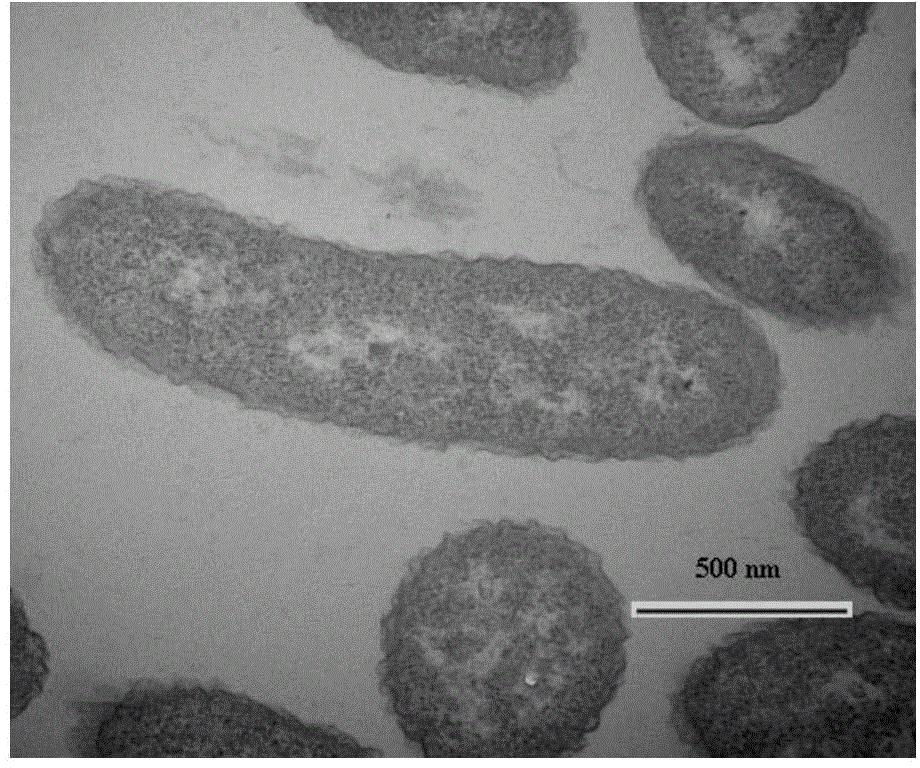
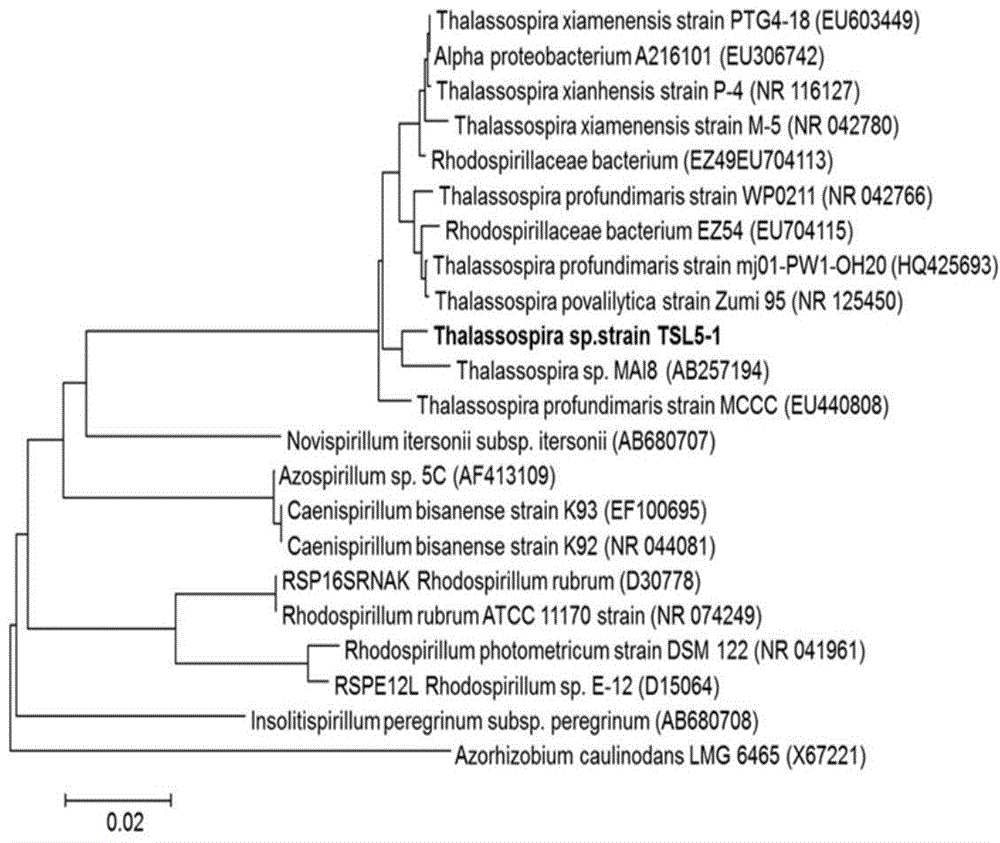
Thalassospira sp. capable of degrading polycyclic aromatic hydrocarbons under saline environment and application thereof
ActiveCN104789506AEfficient degradationBacteriaMicroorganism based processesPolycyclic aromatic hydrocarbonBenzanthracenes
The invention discloses thalassospira sp. capable of degrading polycyclic aromatic hydrocarbons under saline environment and application thereof. The preservation number of the provided thalassospira sp. TSL5-1 is CGMCC No. 10278. The invention further provides application of the thalassospira sp. or bacterial suspension thereof or culture product thereof or fermentation product thereof in polycyclic aromatic hydrocarbon degradation. Based on experiments, compared with the prior art, the thalassospira sp. TSL5-1 (CGMCC No. 10278) can grow and propagate under the saline environment by taking the polycyclic aromatic hydrocarbons, such as phenanthrene, pyrene, fluoranthene, benzopyrene and benzanthracene, as the unique carbon source and energy source.
Owner:TSINGHUA UNIV
Compound using anthracene and phenanthrene as core and application thereof to organic electroluminescence device
ActiveCN110294735ADeep singlet levelHigh singlet energy levelOrganic chemistrySolid-state devicesOrganic electroluminescencePhenanthrenes
The invention relates to a compound using anthracene and phenanthrene as a core and application thereof to an organic electroluminescence device. The compound uses the anthracene as the core; the structural general formula is shown as a formula in the description. The characteristics of difficult crystallization between molecules, difficult aggregation and good film forming performance are realized. When the compound is used as a material of the organic electroluminescence device, the current efficiency, the power efficiency and the external quantum efficiency of the device are greatly improved; meanwhile, the obvious effect is achieved on the service life prolonging of the device.
Owner:JIANGSU SUNERA TECH CO LTD
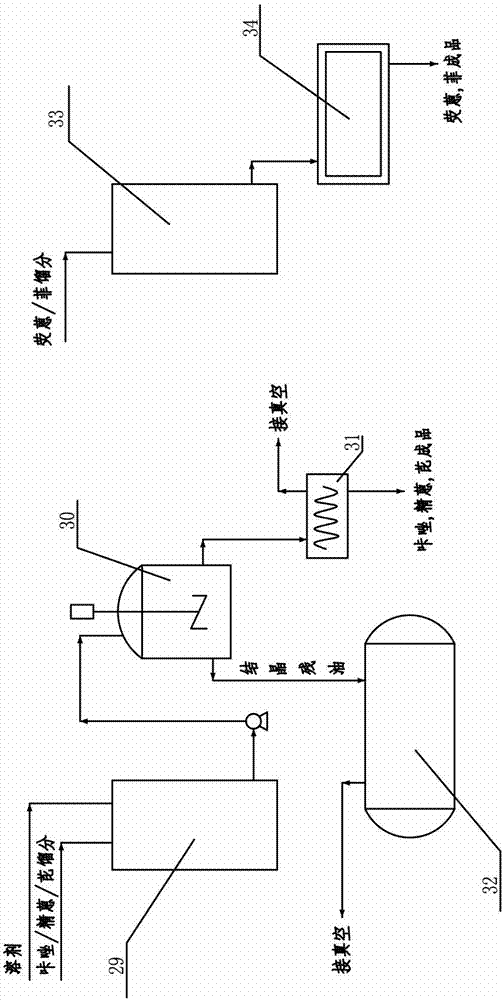
Device and process for extracting phenanthrene, fluoranthene and pyrene products in anthracene oil
ActiveCN104045506AEasy extractionEasy to operateChemical industryDistillation purification/separationCarbazoleDistillation
The invention belongs to the technical field of advanced treatment of anthracene oil and in particular relates to a device and process for extracting phenanthrene, fluoranthene and pyrene products in anthracene oil. The extracting device comprises an anthracene phenanthrene fraction extraction system and a carbazole fraction extraction system. The extracting device is characterized in that a fluoranthene / pyrene fraction extraction system, a solvent regeneration system, a phenanthrene fraction extraction system and a tail gas purification system are further arranged behind the carbazole fraction extraction system. The extraction device is added with a phenanthrene tower and a fluoranthene / pyrene tower on the previous distillation basis of refined anthracene and carbazole; the setting conditions of the two towers, and the material and filler of the towers are more convenient for extracting the phenanthrene, fluoranthene and pyrene products. The process flow is of four-furnace and four-tower continuous rectification, and capable of producing the anthracene phenanthrene fraction, the carbazole fraction, the fluoranthene fraction, the pyrene fraction and the phenanthrene fraction simultaneously; the process is convenient and flexible to operate, and capable of extracting the phenanthrene, fluoranthene and pyrene having the content of almost 40% in the anthracene oil without increasing more investment; the yield of the products can be more than 80%, and considerable economic benefits can be gained.
Owner:李信成
Method for simultaneously detecting content of 22 polycyclic aromatic hydrocarbon matters in cigarette smoke
ActiveCN106248835AGood effectLow requirements for matrix purityComponent separationAcenaphthyleneTrapping
The invention discloses a method for simultaneously detecting content of 22 polycyclic aromatic hydrocarbon matters in cigarette smoke. The method comprises the following steps: trapping total particular matters in cigarette main stream smoke by adopting a filter; extracting the trapping filter by utilizing an extraction agent; and detecting the filtered extract liquor by adopting gas chromatography-tandem mass spectrum (GC-MS / MS), and simultaneously detecting the contents of 22 polycyclic aromatic hydrocarbon matters, including indene, naphthalene, 1-methylnaphthalene, 2-methylnaphthalene, acenaphthylene, acenaphthene, fluorene, phenanthrene, anthracene, fluoranthene, pyrene, benzo [a] anthracene, chrysene, benzo [b] fluoranthene, benzo [k] fluoranthene, benzo [j] fluoranthene, benzo [e] pyrene, benzo [a] pyrene, perylene, indene [1,2,3-cd] perylene, dibenzo [a, h] anthracene, benzo [g, h, i] pyrene, and the like, in cigarette main stream smoke. The method fills the blank of the prior art, has the characteristics of simple, convenient and quick sample pretreatment, high detection accuracy and repeatability and large analysis test flux and is fit for quick analysis of mass samples.
Owner:YUNNAN TOBACCO QUALITY SUPERVISION MONITORING STATION
Organic electroluminescent material and organic light-emitting device thereof
InactiveCN108218664AIncrease in sizeEffectively interruptSilicon organic compoundsGroup 5/15 element organic compoundsPhosphine oxideElectron
The invention provides an organic electroluminescent material and an organic light-emitting device thereof, and belongs to the technical field of organic photoelectric materials. A structure of the organic electroluminescent material, provided by the invention, contains a chrysene structure, wherein chrysene, anthracene, phenanthrene and fluorene are common main structures. The organic electroluminescent material has a high triplet energy level, but the organic electroluminescent material is easy to crystallize. The organic electroluminescent material increases molecular volume by linking other aryl or heteroaryl groups, and effectively avoids crystallization. In addition, by linking the sulfone, phosphine oxide, silicon and other groups in a molecule, a conjugated structure is effectivelyinterrupted, the triplet energy level is higher, and an energy barrier between HOMO and LOMO is larger, so that the organic electroluminescent material is more favorable for being used as a blue light-emitting material; in addition, attachment of groups such as sulfone or phosphine oxide facilitates electron transport and balances carrier transport. Drive voltage is reduced. Compared with the prior art, the organic electroluminescent material is applied to the organic light-emitting device, particularly to be used as the blue light-emitting material in a light-emitting layer, has relatively high luminous efficiency and a low driving voltage.
Owner:CHANGCHUN HYPERIONS TECH CO LTD
Oxidation removing method for polycyclic aromatic hydrocarbon in soil through Fenton reagent bag
InactiveCN105855286AEfficient removalReduce processing timeContaminated soil reclamationAcenaphthylenePolycyclic aromatic hydrocarbon
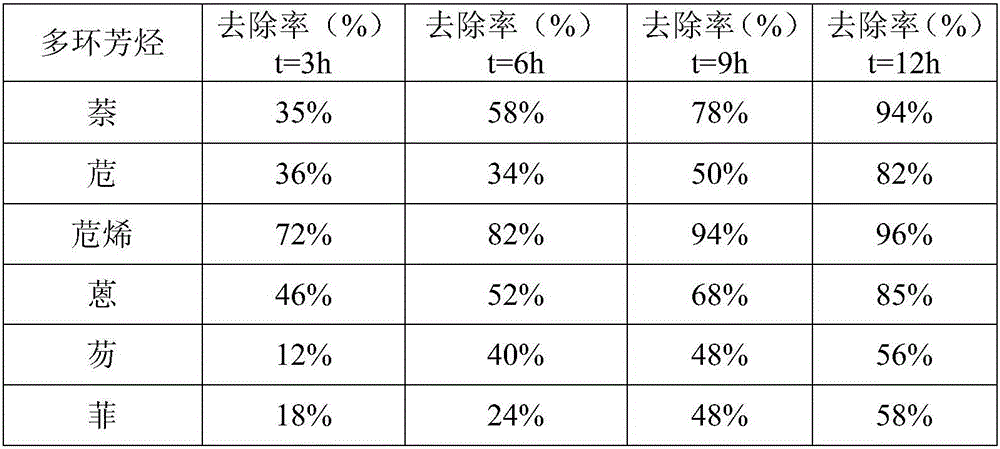
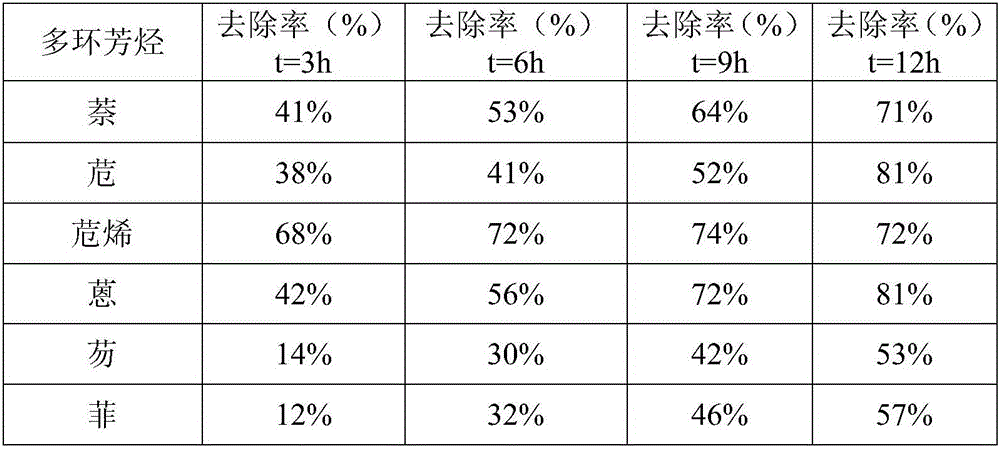
The invention discloses a method for oxidizing and removing polycyclic aromatic hydrocarbons in soil by using a Fenton reagent bag, and belongs to the technical field of soil restoration. The method is to mix the air-dried soil to be tested with distilled water to make mud, add acid reagents to the mud to adjust the pH value of the mud to 3-5 to obtain acid mud, add Fenton reagent packs to the acid mud and stir evenly to obtain Slurry reaction liquid, then the mud reaction liquid is left to react, and the polycyclic aromatic hydrocarbons in the soil can be removed after the reaction; wherein the Fenton reagent package is made of FeSO 4 、H 2 o 2 and K 2 C 2 o 4 composition. This method can effectively remove naphthalene, acenaphthene, acenaphthylene, anthracene, fluorene and phenanthrene in soil, and the treatment time is shortened and the removal rate is improved compared with the traditional Fenton reagent.
Owner:NANJING AGRICULTURAL UNIVERSITY
Beta-phenanthrene azaBODIPY dye and preparation method and application thereof
InactiveCN106117256ALong emission wavelengthNarrow emission peakEchographic/ultrasound-imaging preparationsAzo dyesHydrogenAlkoxy group
The invention discloses a beta-phenanthrene azaBODIPY dye and a preparation method and application thereof. The structural formula of the beta-phenanthrene azaBODIPY dye is as shown in the formula (I) (please see the formula in the description), wherein R1 is alkoxy groups of C1-C6, R2 and R4 are independently selected from alkoxy groups of C1-C6 or alkyl groups of C1-C6, and R3 and R5 are independently selected from hydrogen or alkoxy groups of C1-C6. By means of the design, the prepared beta-phenanthrene azaBODIPY dye of the structure shown in the formula (I) has large absorption wavelength in actual use, narrow absorption peaks and emission peaks and excellent molar absorption coefficient, and thereby having potential application in optical sensors and photoacoustic imaging.
Owner:ANHUI NORMAL UNIV
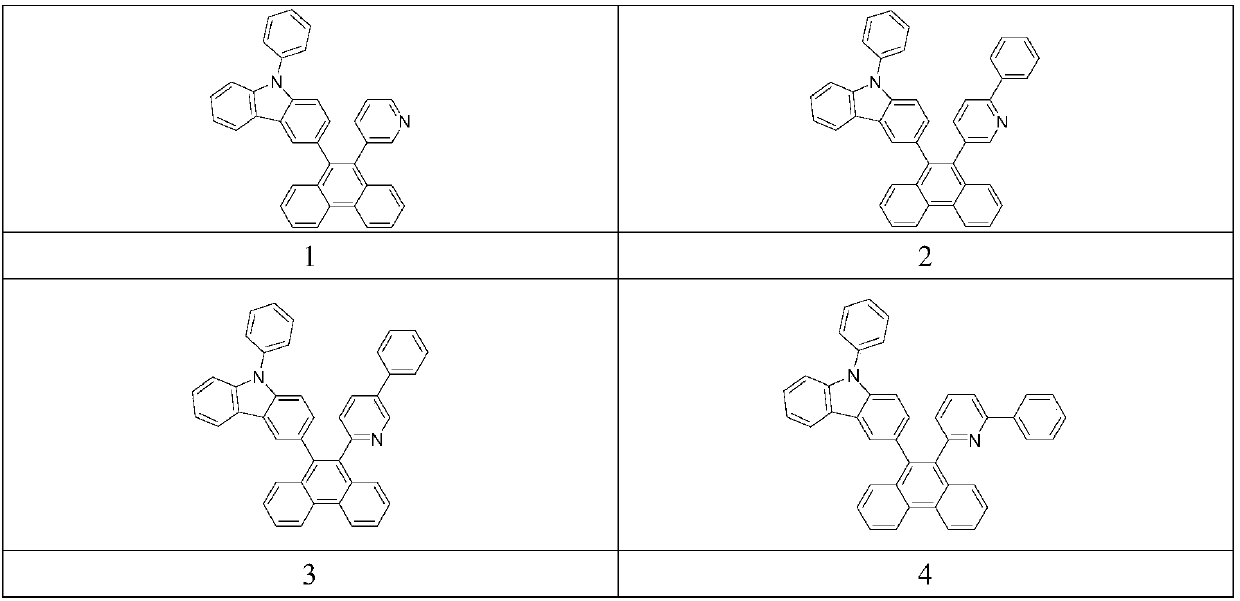
Compounds based on phenanthrene and carbazole, applications thereof, and organic electroluminescent device
InactiveCN108299389AGroup 5/15 element organic compoundsSolid-state devicesOrganic solar cellOrganic layer
The invention provides compounds based on phenanthrene and carbazole. The compounds are represented by a formula shown in the description. The compounds have the advantages of good thermal stability,high luminescent efficiency, and high luminescent purity, and can be applied to fields such as organic electroluminescent device, organic solar cell, organic thin film transistor, organic photoreceptor, or the like. The invention also provides an organic electroluminescent device, which comprises an anode, a cathode, and an organic layer. The organic layer comprises at least one of luminescent layer, hole injecting layer, hole transporting layer, hole blocking layer, electron injecting layer, and electron transporting layer and at least comprises a layer containing compounds represented by thestructural formula I. The organic electroluminescent device prepared from the compounds has the advantages of good electroluminescent efficiency, excellent color purity, and long service life.
Owner:SHANGHIA TAOE CHEM TECH CO LTD
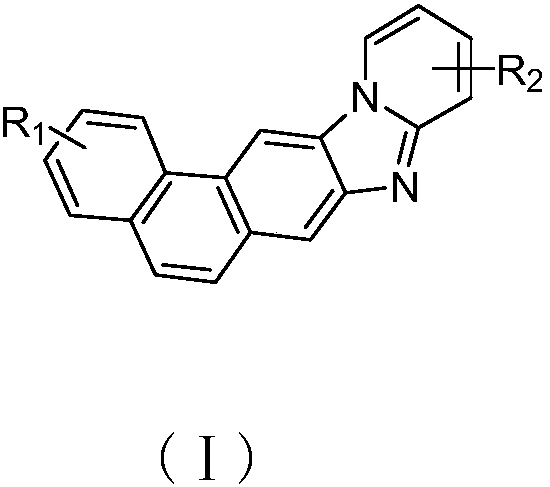
Phenanthrene derivative and organic light-emitting device thereof
InactiveCN108358919AGood electron transport propertiesHigh glass transition temperatureOrganic chemistrySolid-state devicesElectronic transmissionSynthesis methods
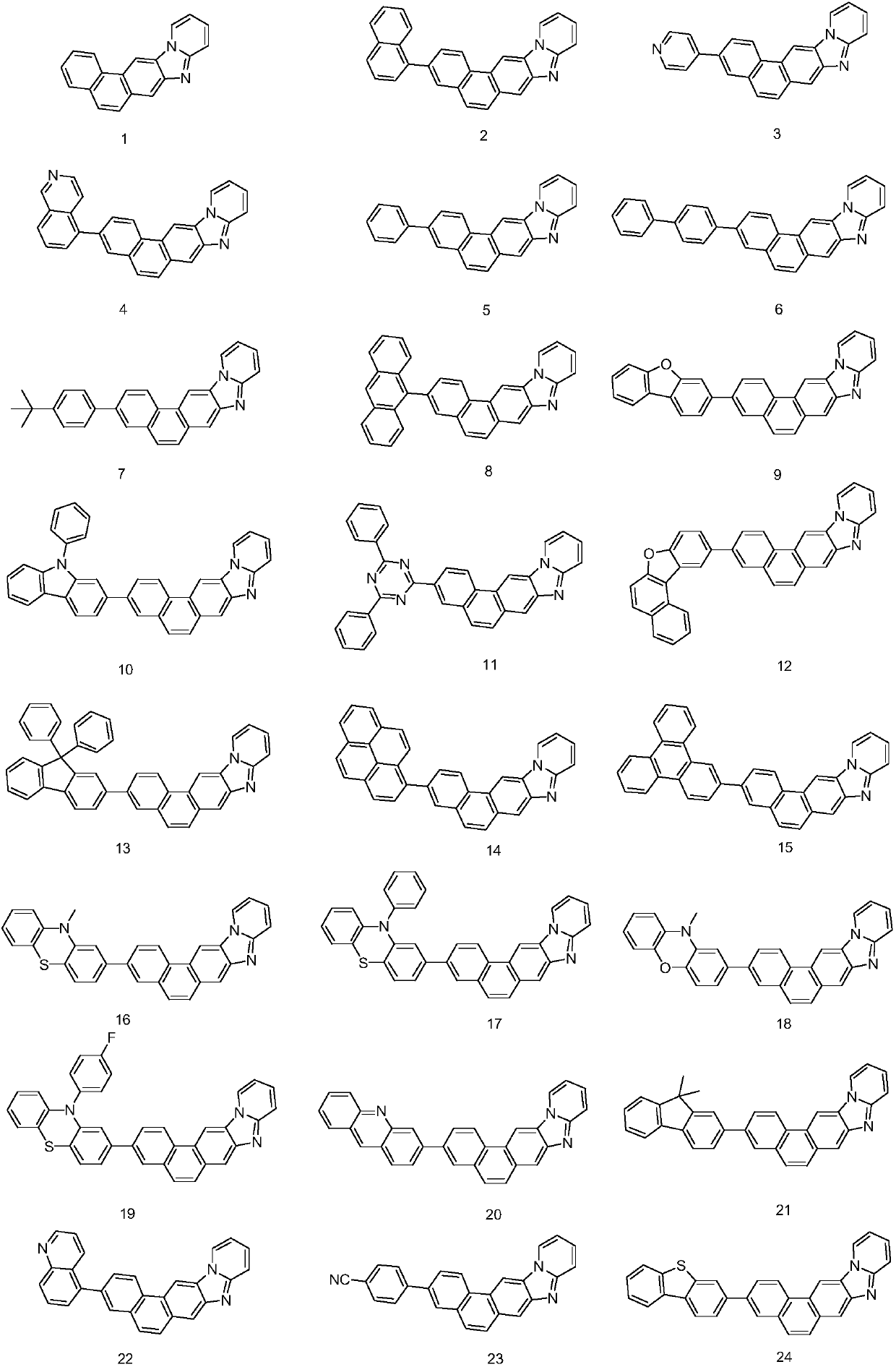
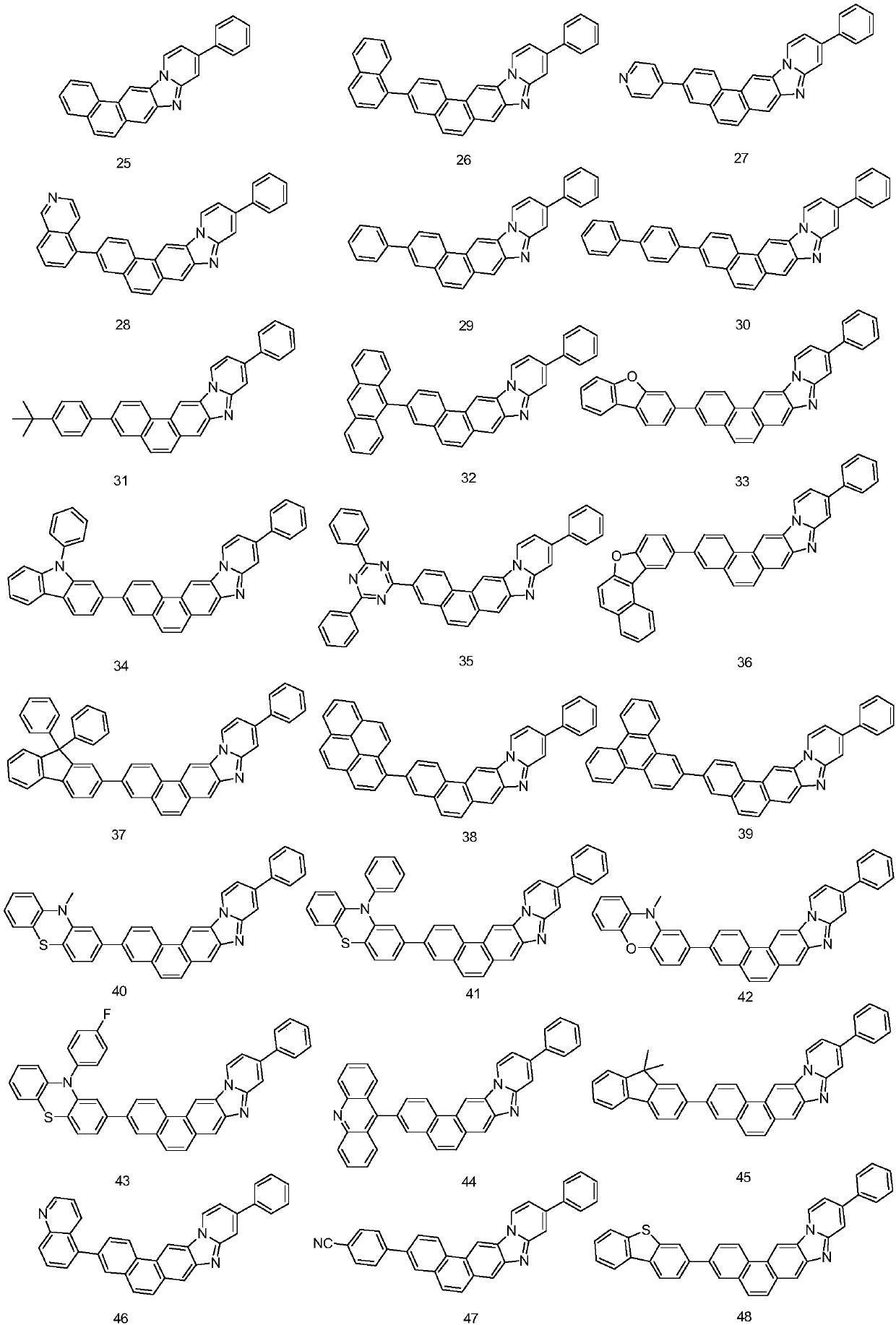
The invention provides a phenanthrene derivative and an organic light-emitting device thereof, and belongs to the technical field of organic photoelectric materials. The composition has the structureshown in the formula (I), the obtained phenanthrene derivative has the excellent electronic transmission capability, high glass-transition temperature and the effect of preventing crystallization by condensing phenanthrene and benzimidazole. The synthesis method is simple and easy to operate, the organic light-emitting device prepared by using the phenanthrene derivative has the advantages of being low in driving voltage and high in light-emitting efficiency, and is an organic light-emitting material with excellent performance.
Owner:CHANGCHUN HYPERIONS TECH CO LTD
Process for preparation of spirofluorenols
InactiveUS20060025636A1Efficient methodHigh selectivitySilicon organic compoundsOrganic compound preparationSilyleneFluorenone
A spirofluorenol such as 3′,9′-dimethoxy-5′-hydroxyspiro[(1H-cyclopent[d,e,f]phenanthrene)-1,7′-benzo [c] fluorene] is produced by protecting a hydroxyl group bonded to a particular fluorenone compound such as 3,9-dimethoxy-5-hydroxybenzo[c]fluorene-7-one with “a substituted silyl group in which the sum of carbon atoms of substituents bonded to a silicon atom is 5 to 12”, such as a t-butyldimethylsilyl group, then, reacting the fluorenone compound with a particular organometal compound such as 1-lithiophenanthrene so as to be transformed into a spiro form and, then, removing the protection therefrom. This method makes it possible to efficiently produce the spirofluorenol which is useful as a starting material for producing photochromic compounds.
Owner:TOKUYAMA CORP
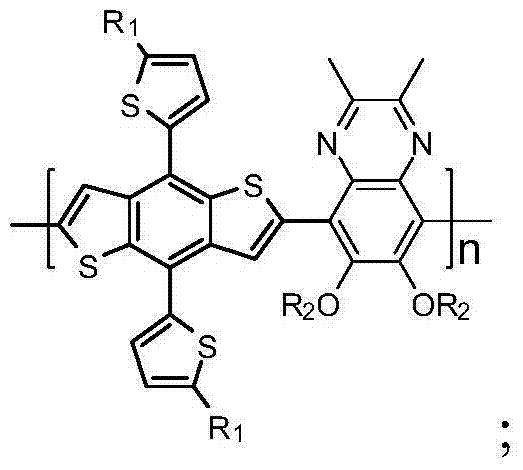
Quinoxaline based copolymer, preparation method and applications thereof
InactiveCN104513367AImprove solubilityImprove film formationSolid-state devicesSemiconductor/solid-state device manufacturingSolubilityPolymer dissolution
The invention belongs to the field of organic solar cell materials, and discloses a quinoxaline based copolymer, a preparation method and applications thereof. The structural formula of the copolymer is shown in the description, in the formula, the R represents a C1-C20 alkyl group and the n represents an integer in a range of 34 to 91. In the provided quinoxaline based copolymer, quinoxaline is an excellent electron acceptor unit and is advantageously for being applied to organic solar cells. The phenyl ring is modified by an alkyloxy chain so as to improve the solubility and film-forming ability of the copolymer. Phenanthrene is a compound with a large planar rigid structure, and thus has high thermal stability and a strong fluorescence performance. The light conversion rate of an organic solar cell device can be improved by applying the provided quinoxaline based copolymer to the solar cells of the organic solar cell device.
Owner:OCEANS KING LIGHTING SCI&TECH CO LTD +2
Thiol-containing compounds for the removal of elements from contaminated milieu and methods of use
Sulfur-containing ligands and methods of their utilization for binding metals and / or main group elements and removing them from fluids, solids, gases and / or tissues are disclosed. The ligands are of the general structure (A): or structure (B) where R1 comprises benzene, pyridine, pyridin-4-one, naphthalene, anthracene, phenanthrene or alkyl groups, R2 comprises hydrogen, alkyls, aiyls, a carboxyl group, carboxylate esters, organic groups or biological groups, R3 comprises alkyls, aryls, a carboxyl group, carboxylate esters, organic groups or biological groups, X comprises hydrogen, lithium, sodium, potassium, rubidium, cesium, francium, alkyls, aryls, a carboxyl group, carboxylate esters, thiophosphate, N-acetyl cysteine, mercaptoacetic acid, mercaptopropionic acid, thiolsalicylate, organic groups or biological groups, n independently equals 1-10, m = 1 -6, Y comprises hydrogen,; polymers, silicas or silica supported substrates, and Z comprises hydrogen, alkyls, aryls, a carboxyl group, carboxylate esters, a hydroxyl group, NH2, HSO3, halogens, a carbonyl group, organic groups, biological groups, polymers, silicas or silica supported substrates.
Owner:UNIV OF KENTUCKY RES FOUND
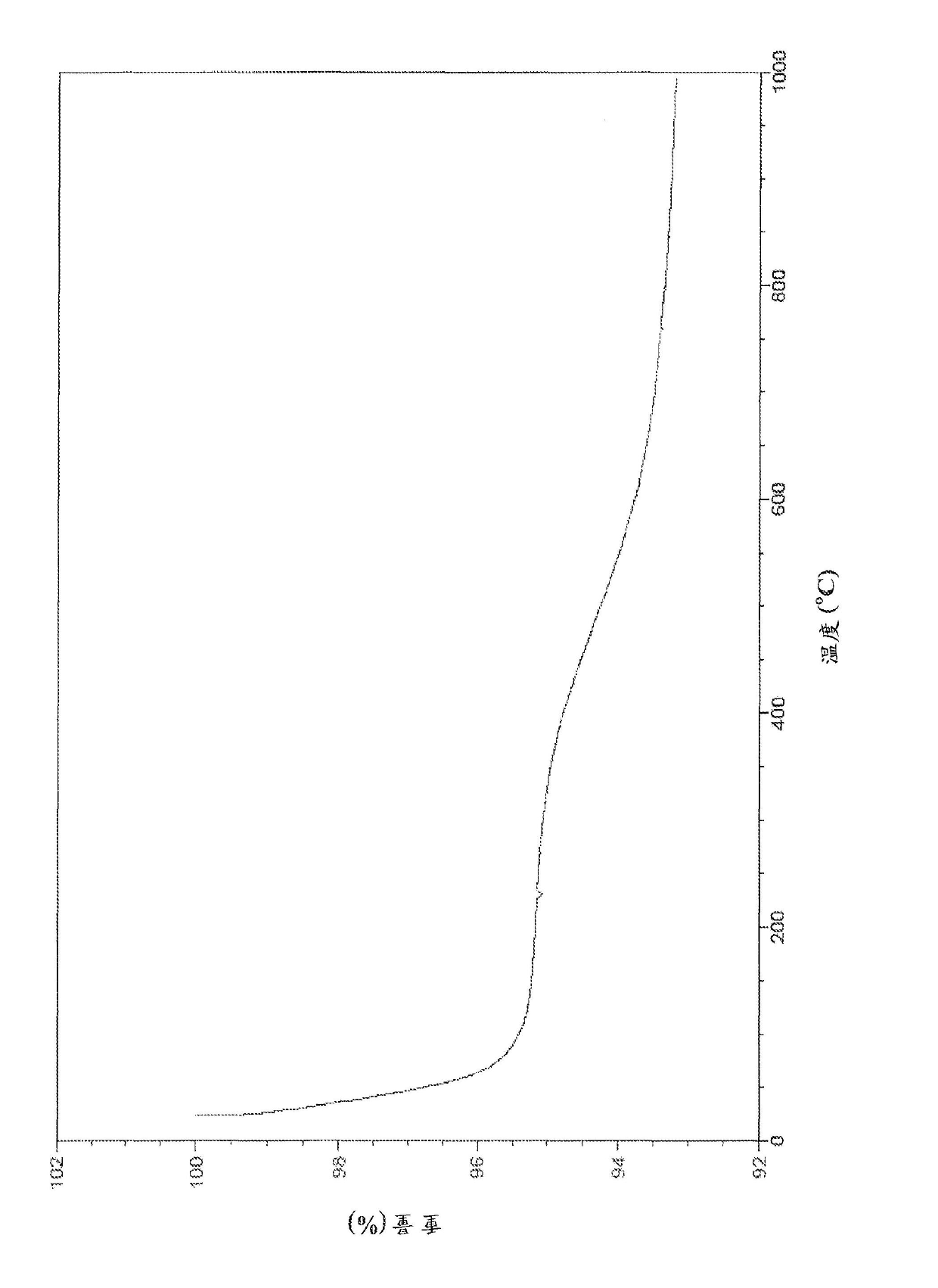
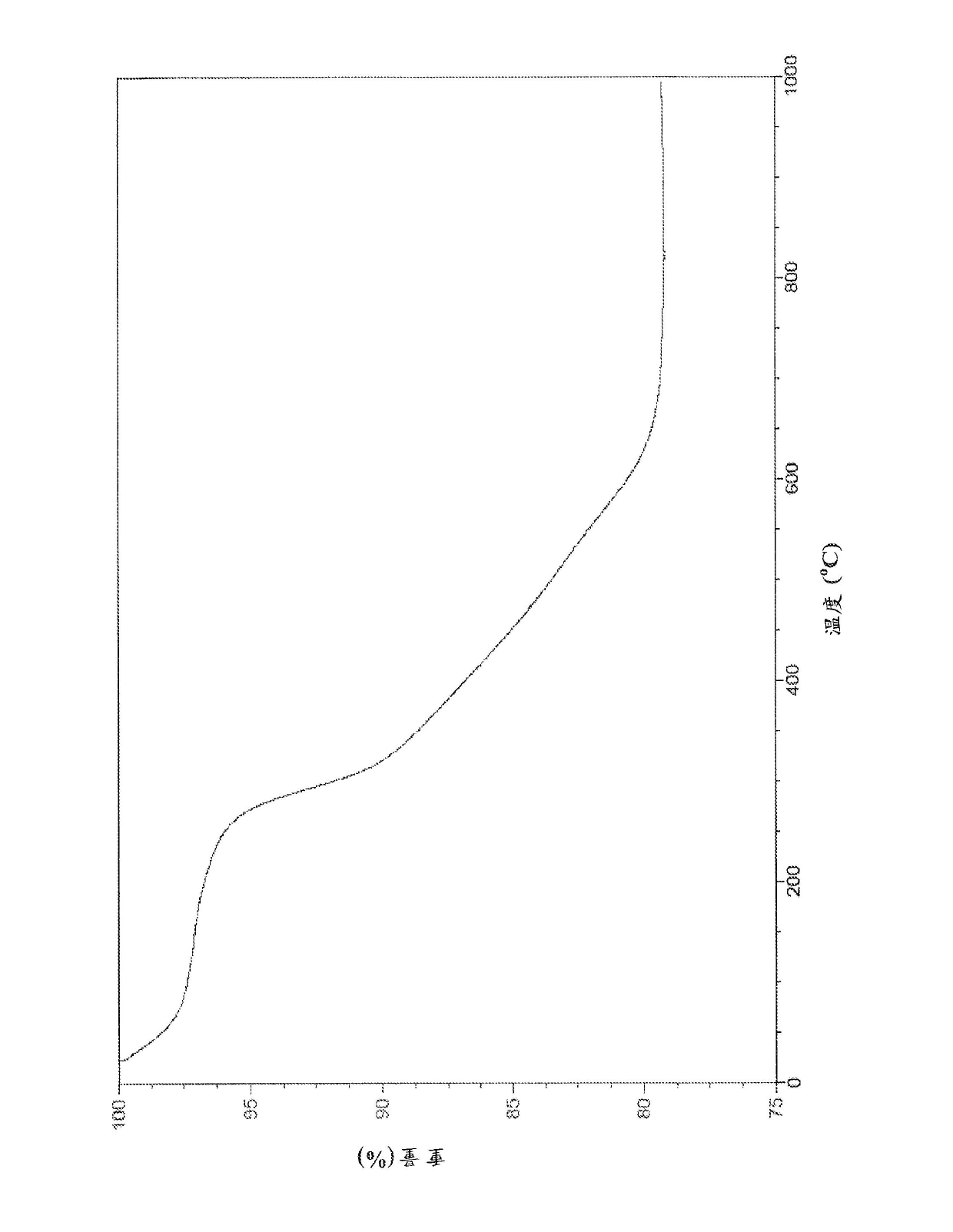
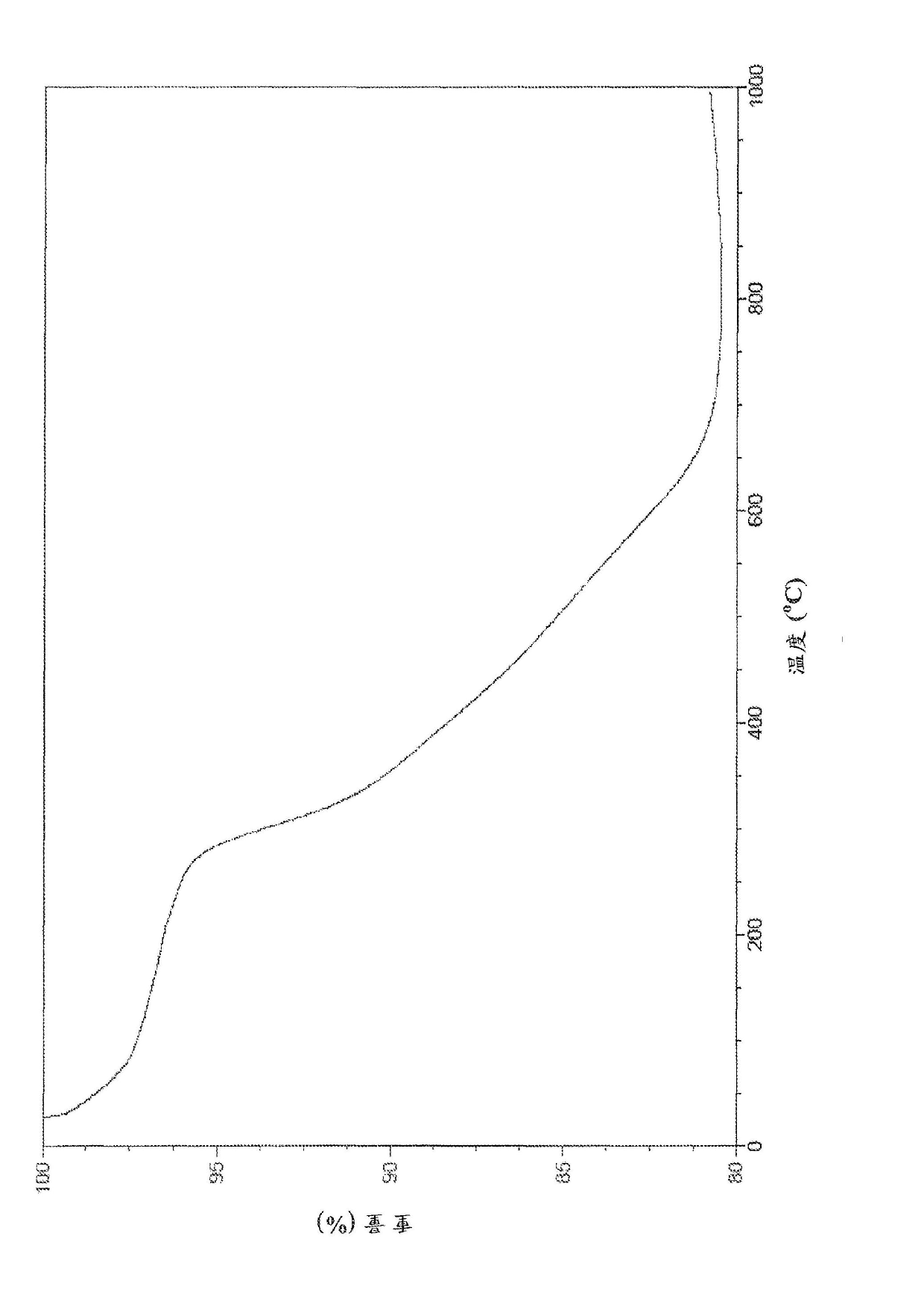
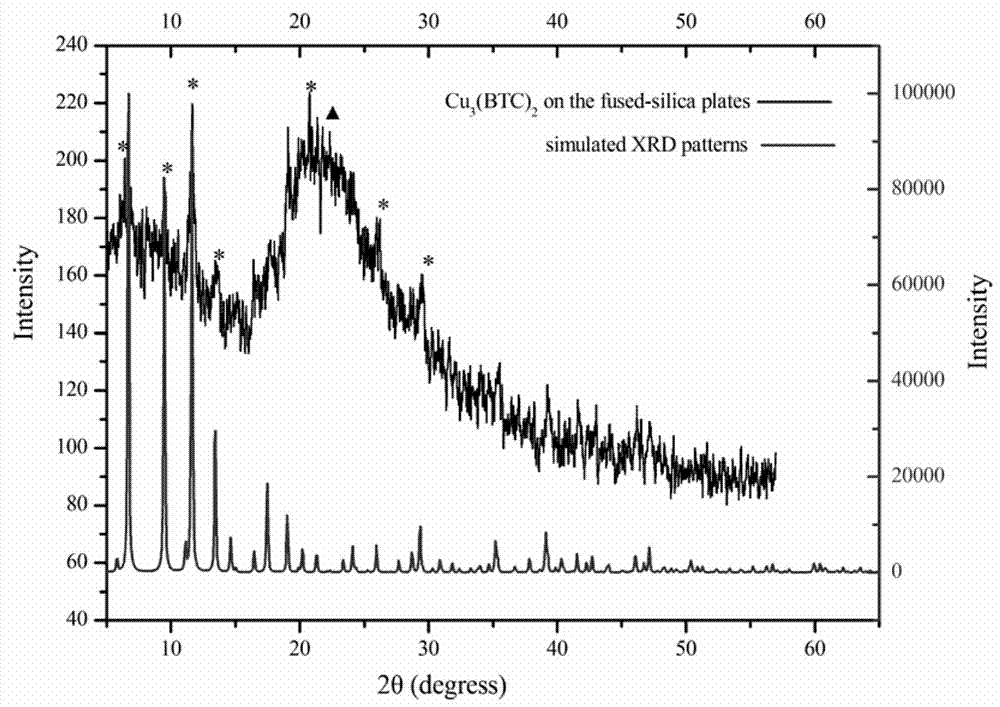
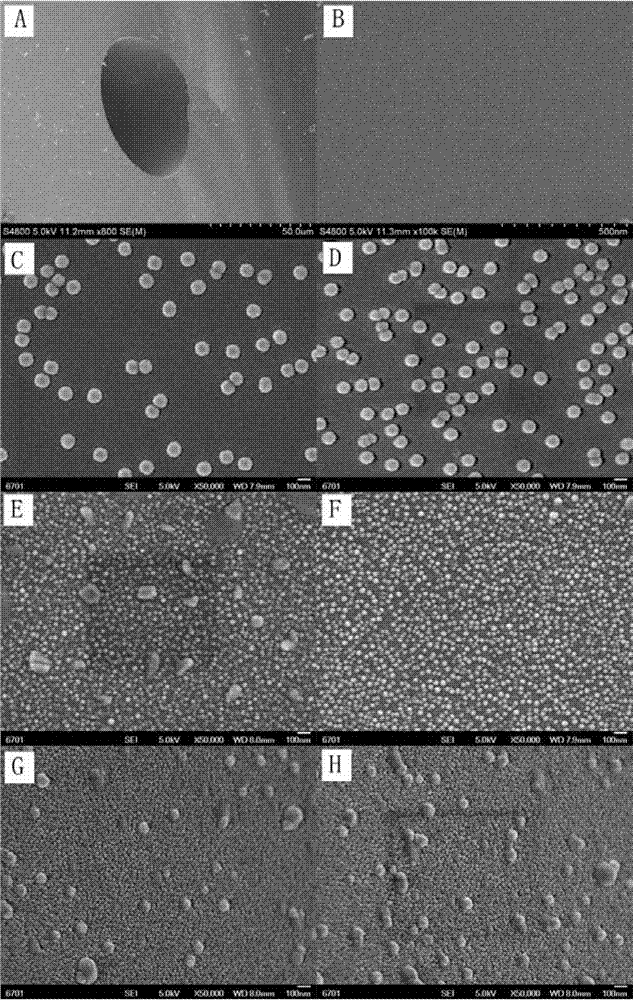
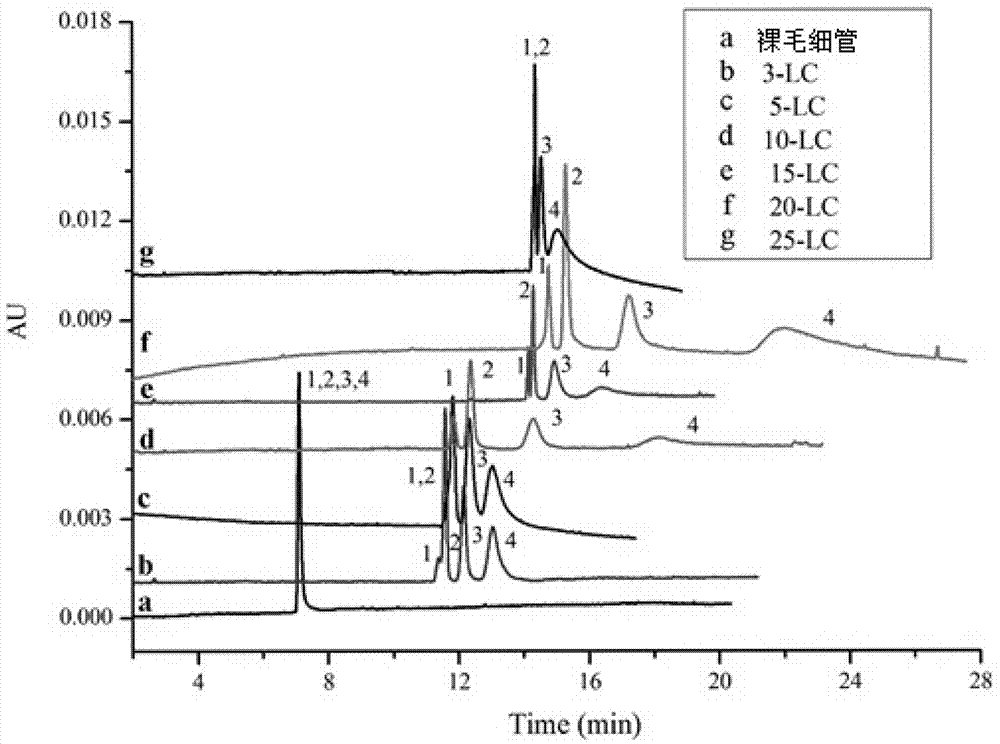
Cu3(BTC)2-modified capillary tube as well as preparation method and application thereof
ActiveCN104722099AEasy to separateIon-exchange process apparatusOther chemical processesBenzeneAlcohol
The invention discloses a Cu3(BTC)2-modified capillary tube. The inner wall of the capillary tube is modified with a metal organic framework material Cu3(BTC)2. A preparation method for the Cu3(BTC)2-modified capillary tube comprises the following process: filling the capillary tube with a gamma-glycidoxy propyl trimethoxy silane-imino sodium diacetate solution, preserving the temperature at 90-100 DEG C for 10-12 hours, and then cleaning the capillary tube; at 60-80 DEG C, filling the capillary tube processed in the step (2) with a copper acetate alcohol solution, contacting for 10-20 minutes, cleaning the capillary tube with alcohol, filling the capillary tube with a trimesic acid alcohol solution, cleaning the capillary tube with alcohol after contacting for 20-40 minutes, and repeating the operation for 3-40 minutes. The capillary tube disclosed by the invention can be used for improving separating effects of neutral micromolecules of benzene, naphthalene, acenaphthene, phenanthrene and the like.
Owner:LANZHOU UNIVERSITY
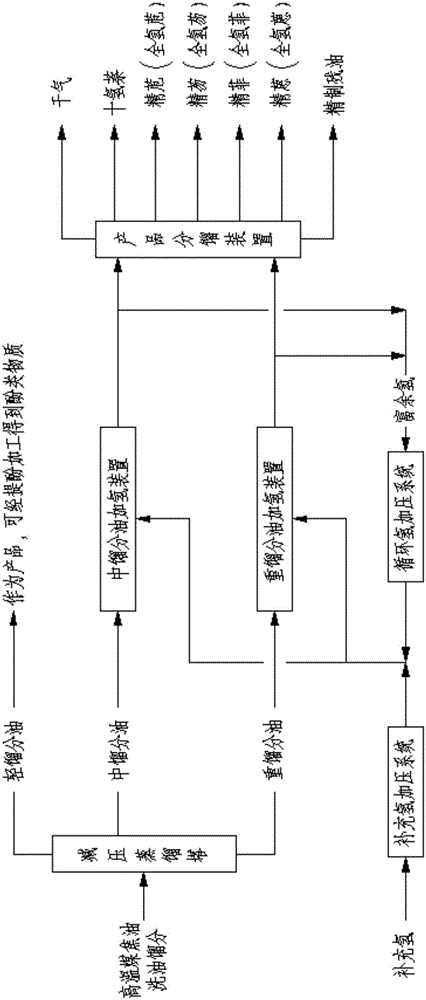
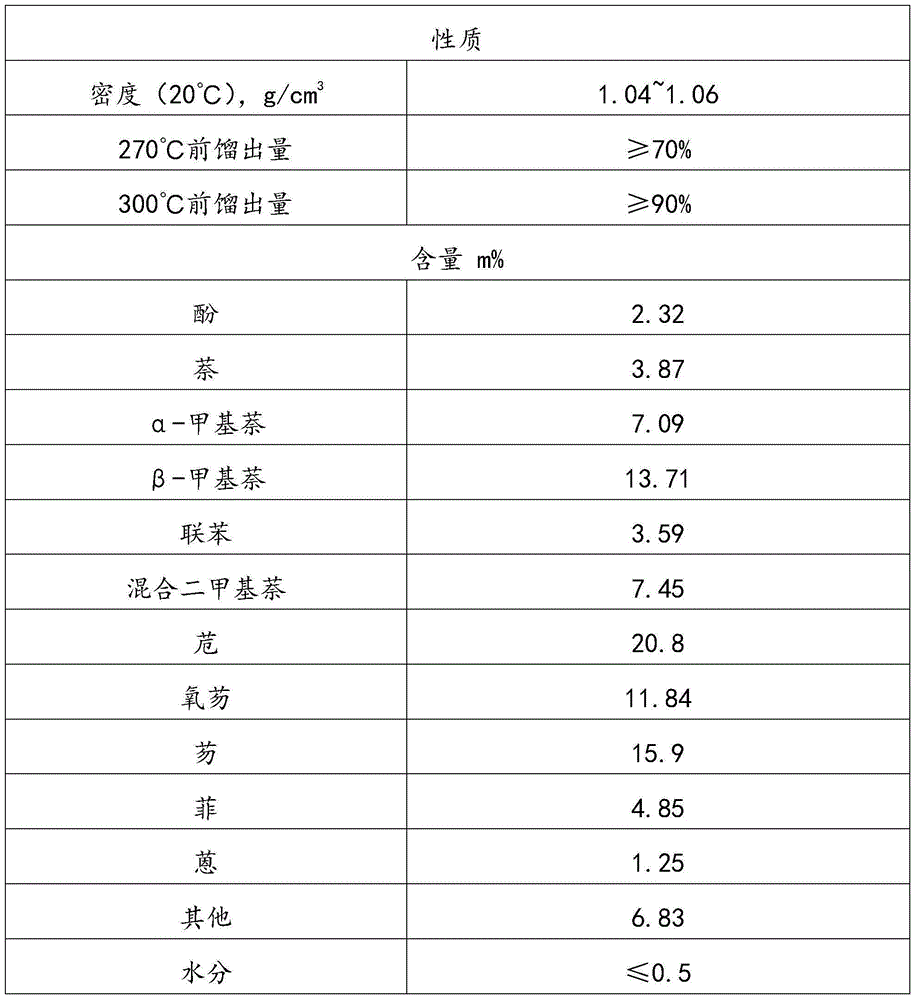
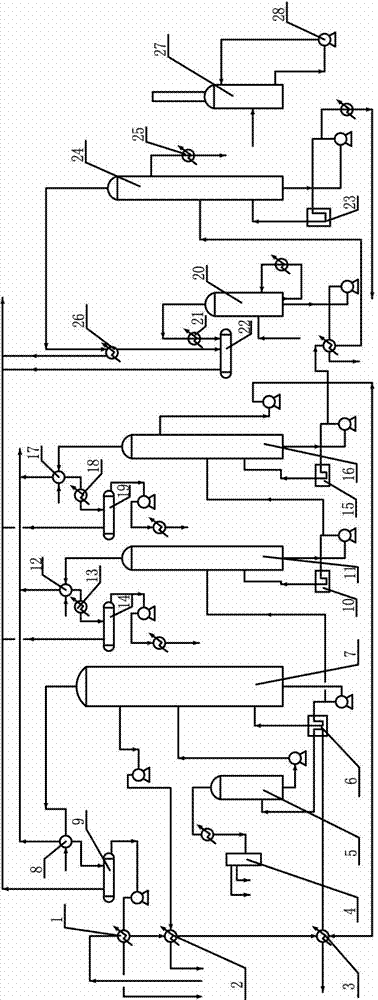
High-temperature coal tar washing oil fraction combination processing method
ActiveCN105018140AComponents are not highImprove completenessTreatment with hydrotreatment processesChemical industryFractionation
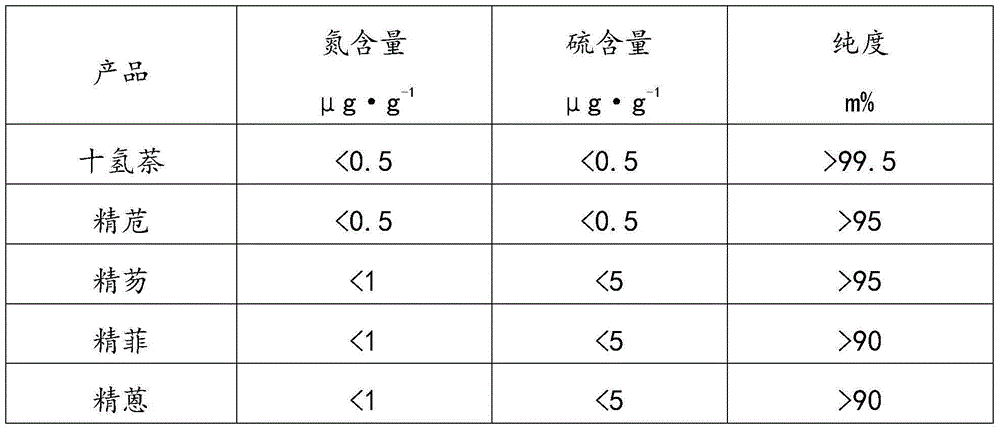
The invention relates to the field of petroleum and chemical industry, especially to a high-temperature coal tar washing oil fraction combination processing method. A high-temperature coal tar washing oil fraction undergoes pre-fractionation to obtain a light distillate, an intermediate distillate and a heavy distillate. The light distillate can undergo dephenolization treatment so as to obtain a phenolic substance. The intermediate distillate and the heavy distillate respectively undergo hydrotreatment, and hydrogenation products can undergo fractionation to obtain decahydronaphthalene, refined acenaphthene, refined fluorene, refined phenanthrene and refined anthracene or perhydroacenaphthene, perhydrofluorene, perhydrophenanthrene and perhydroan thracene. The invention provides a new clean and efficient processing method of a high-temperature coal tar washing oil fraction to increase new products with high added value.
Owner:程志宇
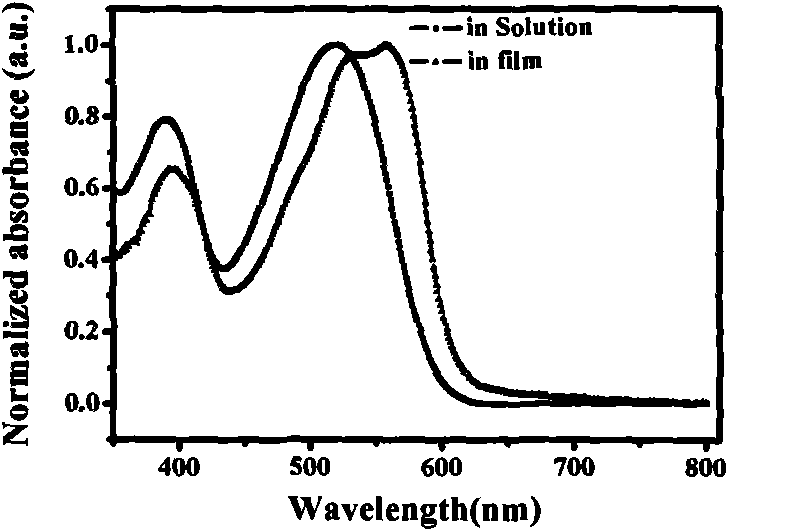
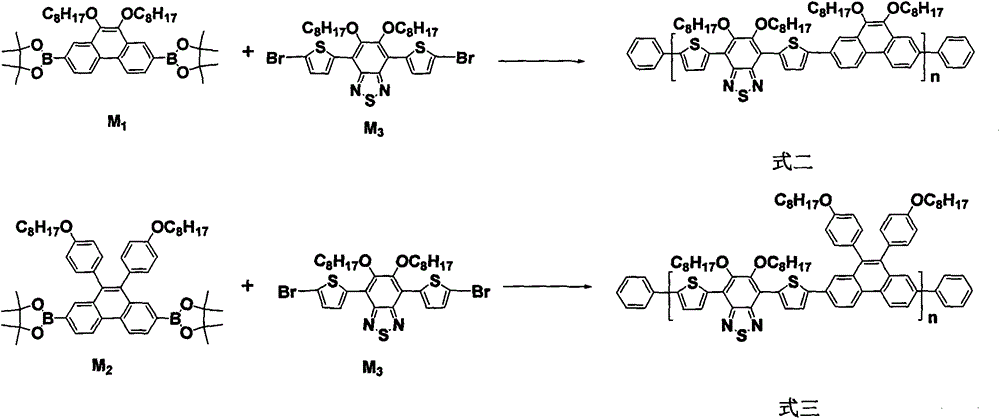
9, 10-disubstituted phenanthrene based conjugated polymer, preparation method and application thereof in organic optoelectronic devices
InactiveCN104629005ASolid-state devicesSemiconductor/solid-state device manufacturingOrganic field-effect transistorOrganic semiconductor
The invention discloses a 9, 10-disubstituted phenanthrene based conjugated polymer, a preparation method and application thereof in organic optoelectronic devices. The invention relates to a 9, 10-disubstituted phenanthrene based conjugated polymer, a preparation method and application thereof as an active layer material in organic optoelectronic devices like polymer solar cells and organic field effect transistors. The general formula is shown as formula I. The 9, 10-disubstituted phenanthrene based conjugated polymer provided by the invention has very good planarity and excellent sunlight capture capability and hole transport capability. With good processability, thermal stability, charge transport, and light absorption, the conjugated polymer solution is an ideal organic semiconductor material for organic electronic devices like solar cells, field-effect transistors and light-emitting diodes. The general formula is shown as the specification. (formula I).
Owner:BEIJING NORMAL UNIVERSITY
Device and process for extracting phenanthrene, fluoranthene and pyrene products in anthracene oil
ActiveCN104045506BEasy extractionEasy to operateChemical industryDistillation purification/separationCarbazoleDistillation
The invention belongs to the technical field of advanced treatment of anthracene oil and in particular relates to a device and process for extracting phenanthrene, fluoranthene and pyrene products in anthracene oil. The extracting device comprises an anthracene phenanthrene fraction extraction system and a carbazole fraction extraction system. The extracting device is characterized in that a fluoranthene / pyrene fraction extraction system, a solvent regeneration system, a phenanthrene fraction extraction system and a tail gas purification system are further arranged behind the carbazole fraction extraction system. The extraction device is added with a phenanthrene tower and a fluoranthene / pyrene tower on the previous distillation basis of refined anthracene and carbazole; the setting conditions of the two towers, and the material and filler of the towers are more convenient for extracting the phenanthrene, fluoranthene and pyrene products. The process flow is of four-furnace and four-tower continuous rectification, and capable of producing the anthracene phenanthrene fraction, the carbazole fraction, the fluoranthene fraction, the pyrene fraction and the phenanthrene fraction simultaneously; the process is convenient and flexible to operate, and capable of extracting the phenanthrene, fluoranthene and pyrene having the content of almost 40% in the anthracene oil without increasing more investment; the yield of the products can be more than 80%, and considerable economic benefits can be gained.
Owner:李信成
Photoelectric conversion element, and solar cell using the same
InactiveUS20180122585A1Low moisture resistanceImprove moisture resistanceLight-sensitive devicesOrganic chemistryHole transport layerSolar cell
Provided is a photoelectric conversion element including a first electrode that includes a photosensitive layer, which includes a light absorbing agent, and a second electrode. The light absorbing agent includes a compound having a perovskite-type crystal structure including a cationic organic group A, a cation of a metal atom M other than the element of Group 1, and an anion of an anionic atom X, a hole transport layer, which includes a hole transporting material, is provided between the first electrode and the second electrode, the hole transporting material includes a compound including a condensed polycyclic aromatic group having a number of rings of 4 or greater, at least two rings thereof are hetero rings, and the condensed polycyclic aromatic group includes at least one structure selected from the group consisting of benzene, naphthalene, and phenanthrene.
Owner:FUJIFILM CORP
Organic compound and photoelectric conversion element
ActiveUS20190119258A1Improve thermal stabilityNo absorption in a visible light regionTelevision system detailsOrganic chemistryOrganic compoundNaphthalene
An organic compound represented by the following general formula [1] is excellent in thermal stability.In the general formula [1], R1 to R12 are each independently selected from the group consisting of a hydrogen atom and a substituent. Symbol A is selected from the group consisting of a divalent residue of naphthalene, phenanthrene, fluorene, benzofluorene, dibenzofluorene or spirofluorene, and a heteroarylene group having 4 to 12 carbon atoms. Symbol B is selected from the group consisting of an arylene group having 6 to 25 carbon atoms and a heteroarylene group having 4 to 12 carbon atoms. Symbol n is an integer of 0 to 3.
Owner:CANON KK
Red phosphorescent compound, and organic luminescent devices prepared from red phosphorescent compound
ActiveCN110330481AHigh color purityIncrease brightnessOrganic chemistrySolid-state devicesLight-emitting diodeNaphthalene
The invention relates to a red phosphorescent compound, and organic luminescent devices prepared from the red phosphorescent compound, and more specially relates to a soluble phosphorescent compound high in color purity, brightness, and luminescent efficiency, and OLED devices using the soluble phosphorescent compound. The structure formula of the red phosphorescent compound is disclosed by formula I in the invention, wherein Z is used for representing a structure independently selected from structures disclosed in the invention, Ar is used for representing one structure independently selectedfrom C6-30 aryl, and C2-30 heteroaryl, A is used for representing one structure independently selected from benzene, naphthalene, phenanthrene, and triphenylene; the C6-30 aryl is one selected from phenyl, naphthyl, terphenyl, and phenanthryl; and C2-30 heteroaryl is one selected from pyridyl, dipyridyl, quinolyl, isoquinolyl, phenanthrolinyl, and triazinyl. The compound represented by formula (I) is adopted to prepare a luminescent layer of oganic light emitting diodes, and excellent color purity, brightness, and prolonged durability effect are achieved.
Owner:ZHEJIANG HUADISPLAY OPTOELECTRONICS CO LTD
Thiol-containing compounds for the removal of elements from tissues and formulations therefor
ActiveUS8575218B2Ability to treatLow toxicityBiocideGroup 4/14 element organic compoundsPolymerMercaptan compound
Methods and pharmaceutical formulations for ameliorating heavy metal toxicity and / or oxidative stress are disclosed, comprising administering pharmaceutically effective amounts of ligands according to the present disclosure. The ligands are of the general structure:where R1 comprises benzene, pyridine, pyridin-4-one, naphthalene, anthracene, phenanthrene or alkyl groups, R2 comprises hydrogen, alkyls, aryls, a carboxyl group, carboxylate esters, organic groups or biological groups, R3 comprises alkyls, aryls, a carboxyl group, carboxylate esters, organic groups or biological groups, X comprises hydrogen, lithium, sodium, potassium, rubidium, cesium, francium, alkyls, aryls, a carboxyl group, carboxylate esters, thiophosphate, N-acetyl cysteine, mercaptoacetic acid, mercaptopropionic acid, thiolsalicylate, organic groups or biological groups, n independently equals 1-10, m=1-6, Y comprises hydrogen, polymers, silicas or silica supported substrates, and Z comprises hydrogen, alkyls, aryls, a carboxyl group, carboxylate esters, a hydroxyl group, NH2, HSO3, halogens, a carbonyl group, organic groups, biological groups, polymers, silicas or silica supported substrates.
Owner:UNIV OF KENTUCKY RES FOUND
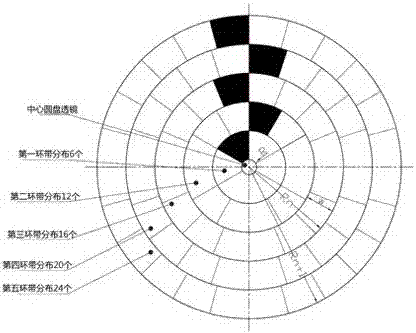
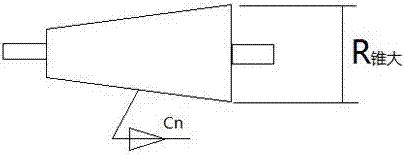
Method for segmented manufacturing of super-large aperture Fresnel lens
InactiveCN107015299AOvercome the disadvantage of limited sizeGuaranteed accuracyLensFresnel lensFine structure
The invention discloses a method for segmentally manufacturing an ultra-large-diameter Fresnel lens, and relates to the field of Fresnel lens manufacturing and processing. Specifically, it includes the following steps: Determine the diameter of the ultra-large-diameter Fresnel lens; Divide the ultra-large-diameter Fresnel lens to be produced into several annular zones; Determine the number of spliced bodies on each annular zone; According to the maximum cone that can be processed The size of the big end of the roller and the position of the annular belt; determine the taper and size of the tapered roller; use the tapered roller to make partial Fresnel lenses, that is, individual spliced bodies; splice the partial Fresnel lenses, that is, spliced bodies, into a complete Fresnel lens. The invention can manufacture large-sized and ultra-large-sized Fresnel lenses, and ensures the accuracy of microstructure molding.
Owner:CHENGDU FSCREEN SCI TECH
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com