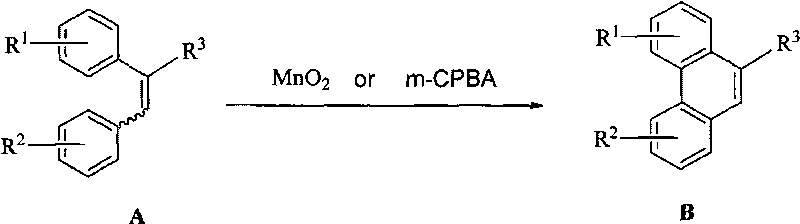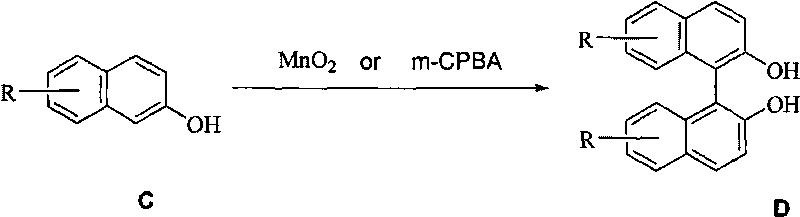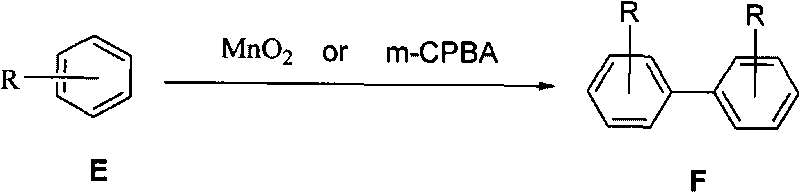Patents
Literature
67 results about "Phenanthrene derivative" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Effervescent granules and methods for their preparation
InactiveUS6649186B1Improve stabilityParticular regionPowder deliveryNervous disorderHYDROMORPHONE HYDROCHLORIDEAlpha adrenergic blockade
Disclosed here are effervescent granules having a controllable rate of effervescence. In some embodiments, the such granules comprise an acidic agent, an alkaline agent, a pharmacologically active agent, hot-melt extrudable binder capable of forming a eutectic mixture with the acidic agent and, optionally, a plasticizer. The effervescent granules are made by a hot-melt extrusion process. The present invention also provides a thermal heat process for preparing a pharmacologically active agent containing effervescent granule. In certain aspects, the granules contain pharmacologically active agents such as narcotics, antidiarrheal agents, antiviral agents, anxiolytic agents, a cholesterol lowering agent, an alpha adrenergic blocking agent, a phenanthrene derivative. By way of example, some of the narcotics that may be included in the granules and in the process of preparing the granules include, by way of example: phenanthrene derivatives (e.g., morphine sulfate), and morphine derivatives (e.g., hydromorphone hydrochloride).
Owner:ETHYPHARM SA
Effervescent granules and methods for their preparation
InactiveUS6488961B1Improve stabilityParticular regionPowder deliveryBiocideAnesthetic AgentHYDROMORPHONE HYDROCHLORIDE
Disclosed here are effervescent granules having a controllable rate of effervescence. In some embodiments, the such granules comprise an acidic agent, an alkaline agent, a pharmacologically active agent, hot-melt extrudable binder capable of forming a eutectic mixture with the acidic agent and, optionally, a plasticizer. The effervescent granules are made by a hot-melt extrusion process. The present invention also provides a thermal heat process for preparing a pharmacologically active agent containing effervescent granule. In certain aspects, the granules contain pharmacologically active agents such as narcotics, antidiarrheal agents, antiviral agents, anxiolytic agents, a cholesterol lowering agent, an alpha adrenergic blocking agent, a phenanthrene derivative. By way of example, some of the narcotics that may be included in the granules and in the process of preparing the granules include, by way of example: phenanthrene derivatives (e.g., morphine sulfate), and morphine derivatives (e.g., hydromorphone hydrochloride).
Owner:ETHYPHARM SA
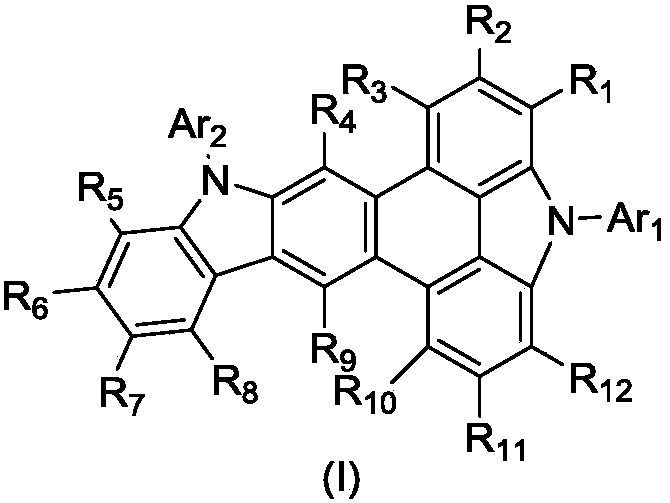
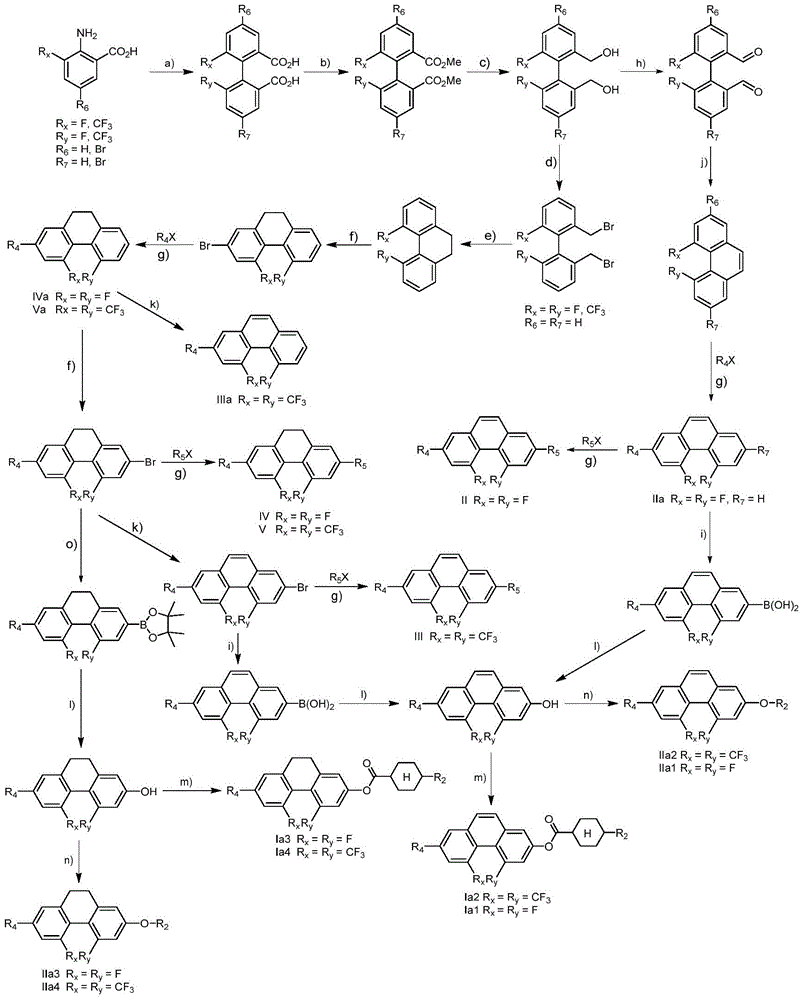
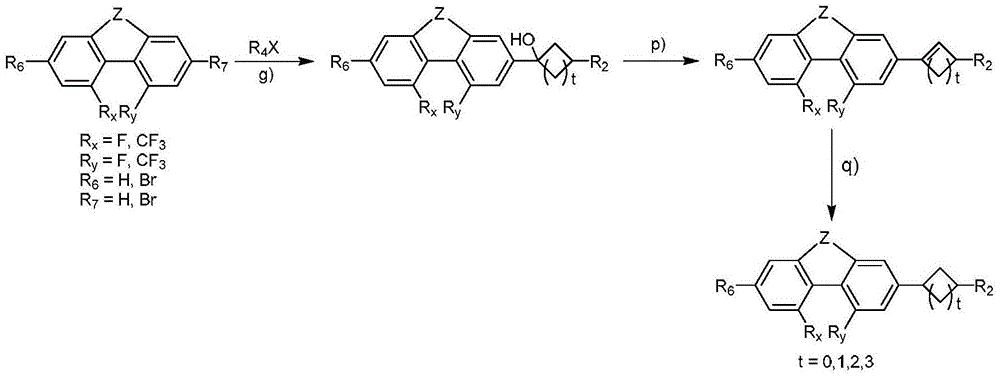
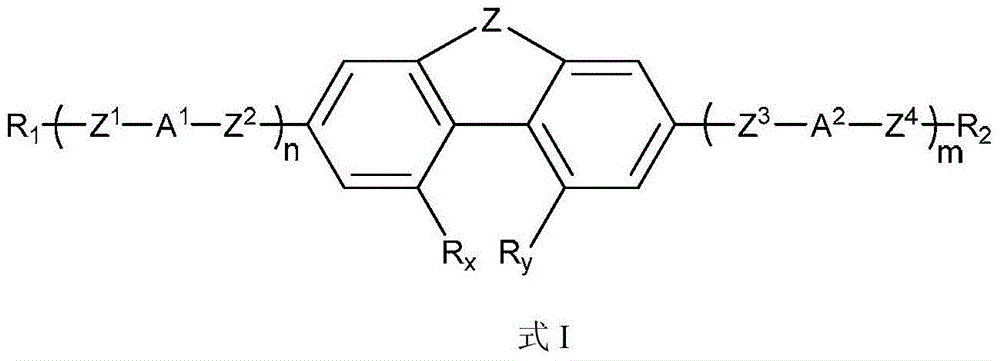
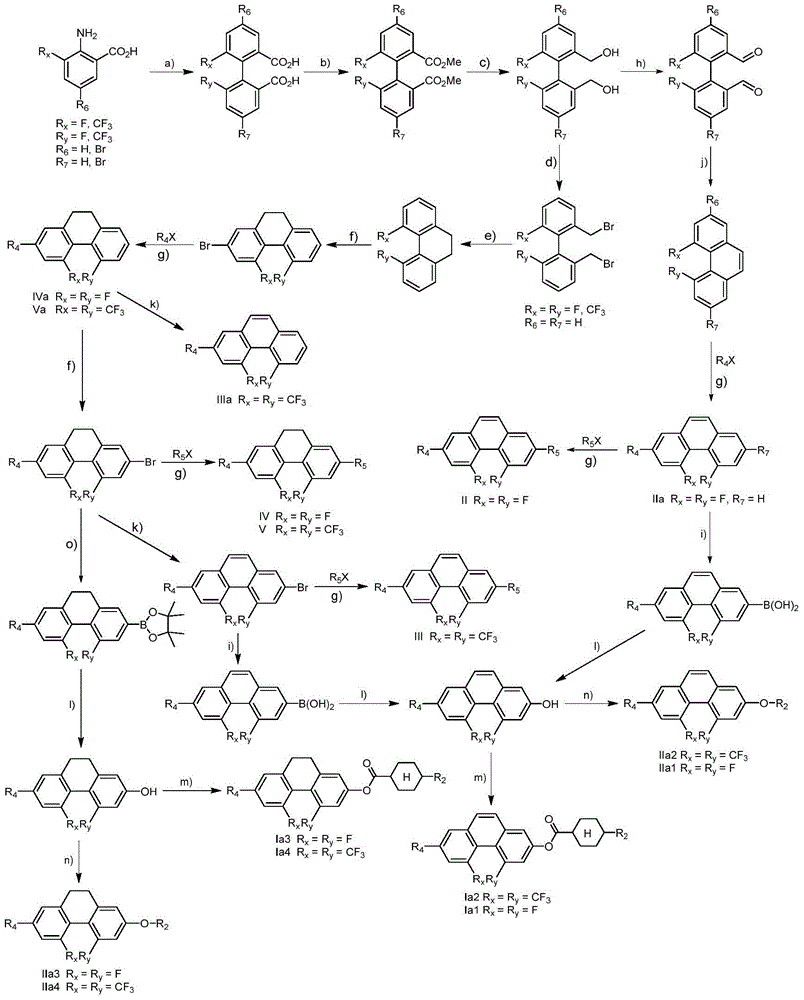
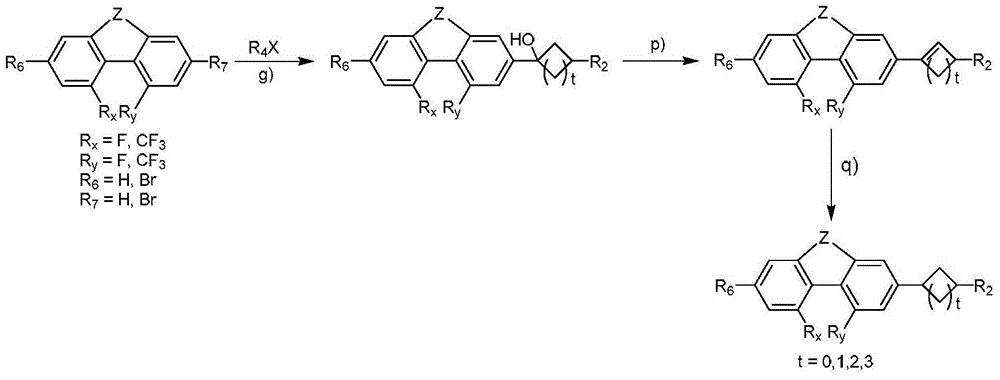
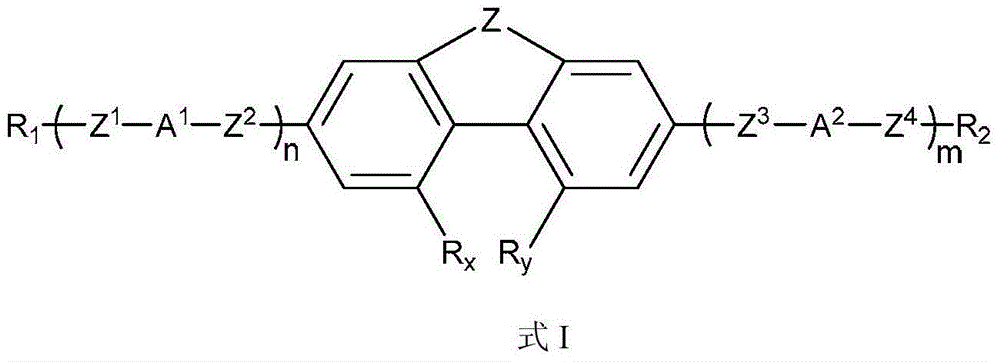
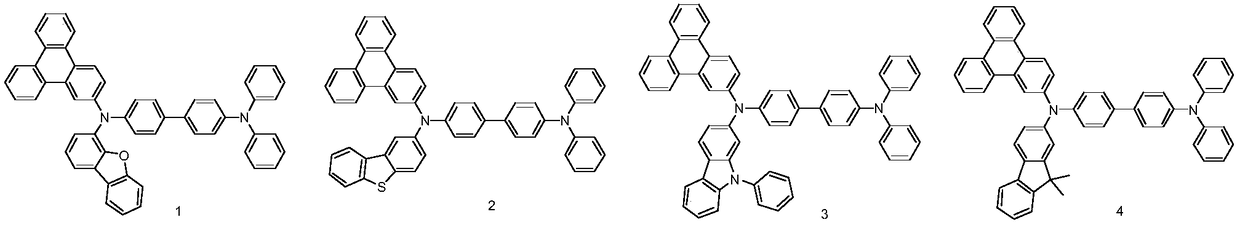
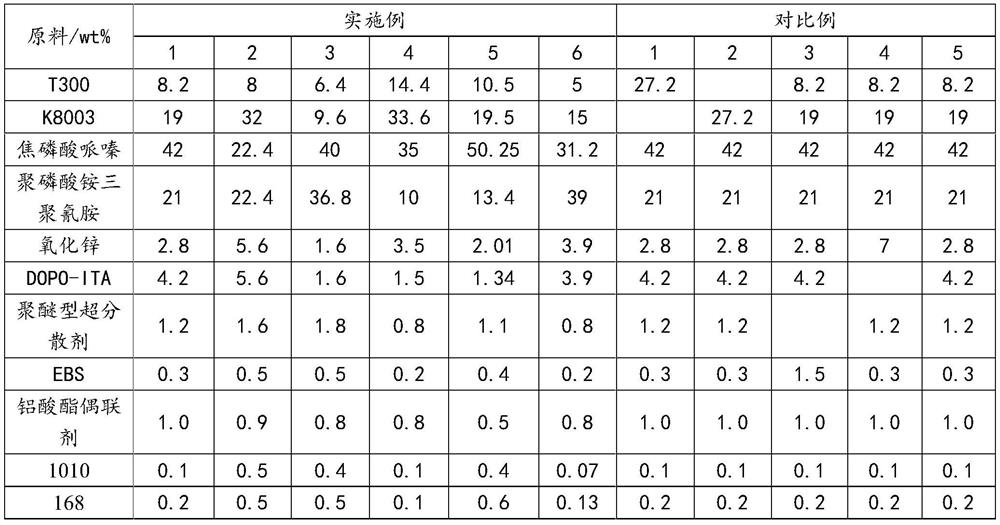
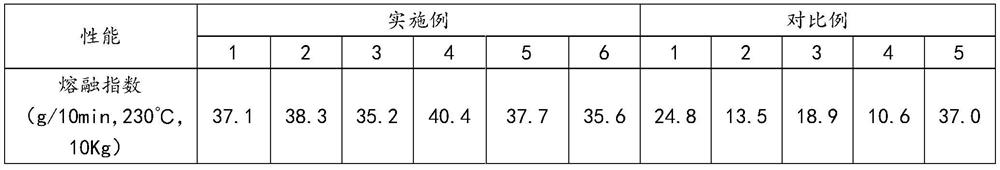
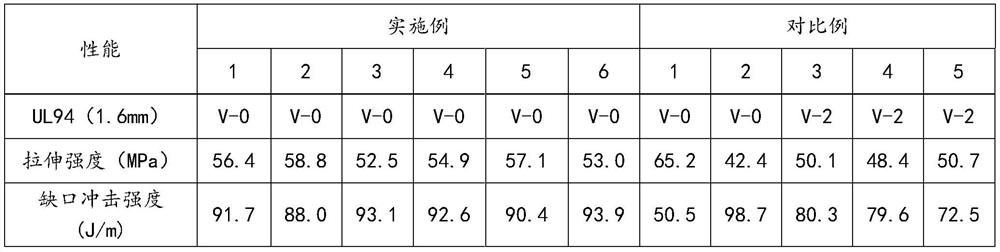
Phenanthrene derivatives and organic light-emitting diodes containing said phenanthrene derivative
ActiveUS20050176953A1Molecular structure is maintainedMaintain stabilityOrganic compound preparationSolid-state devicesPhotoluminescenceElectron transfer
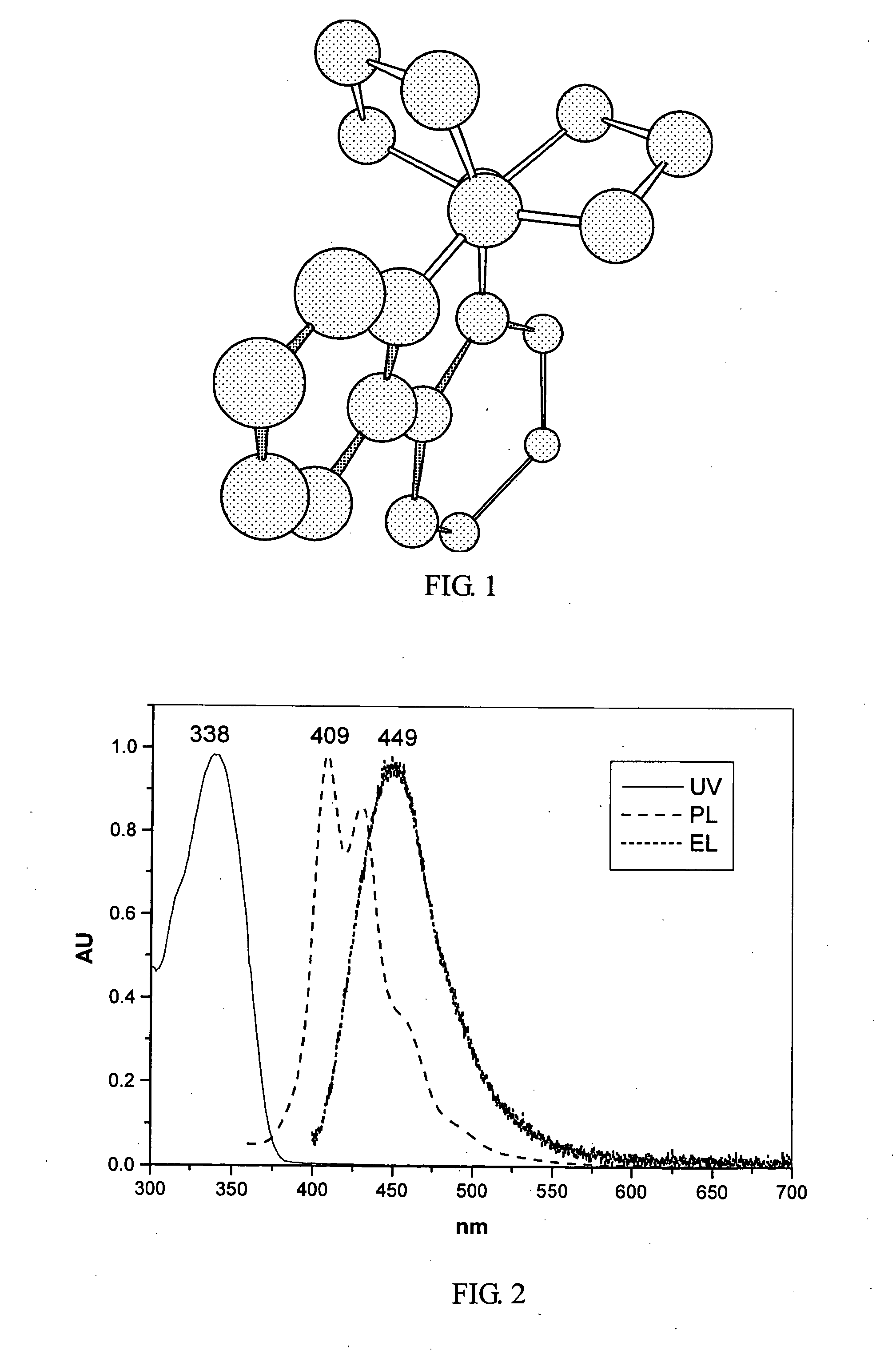
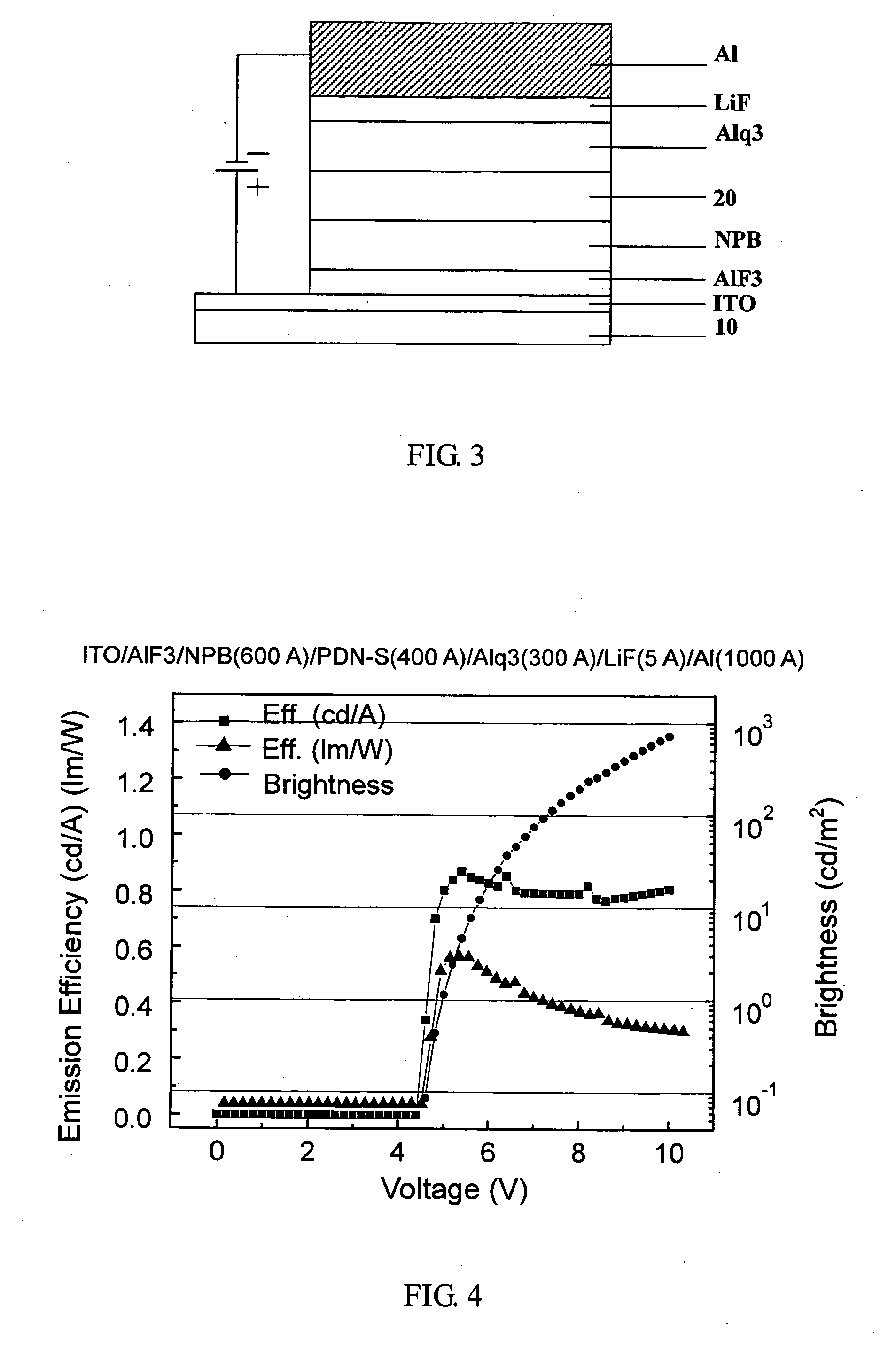
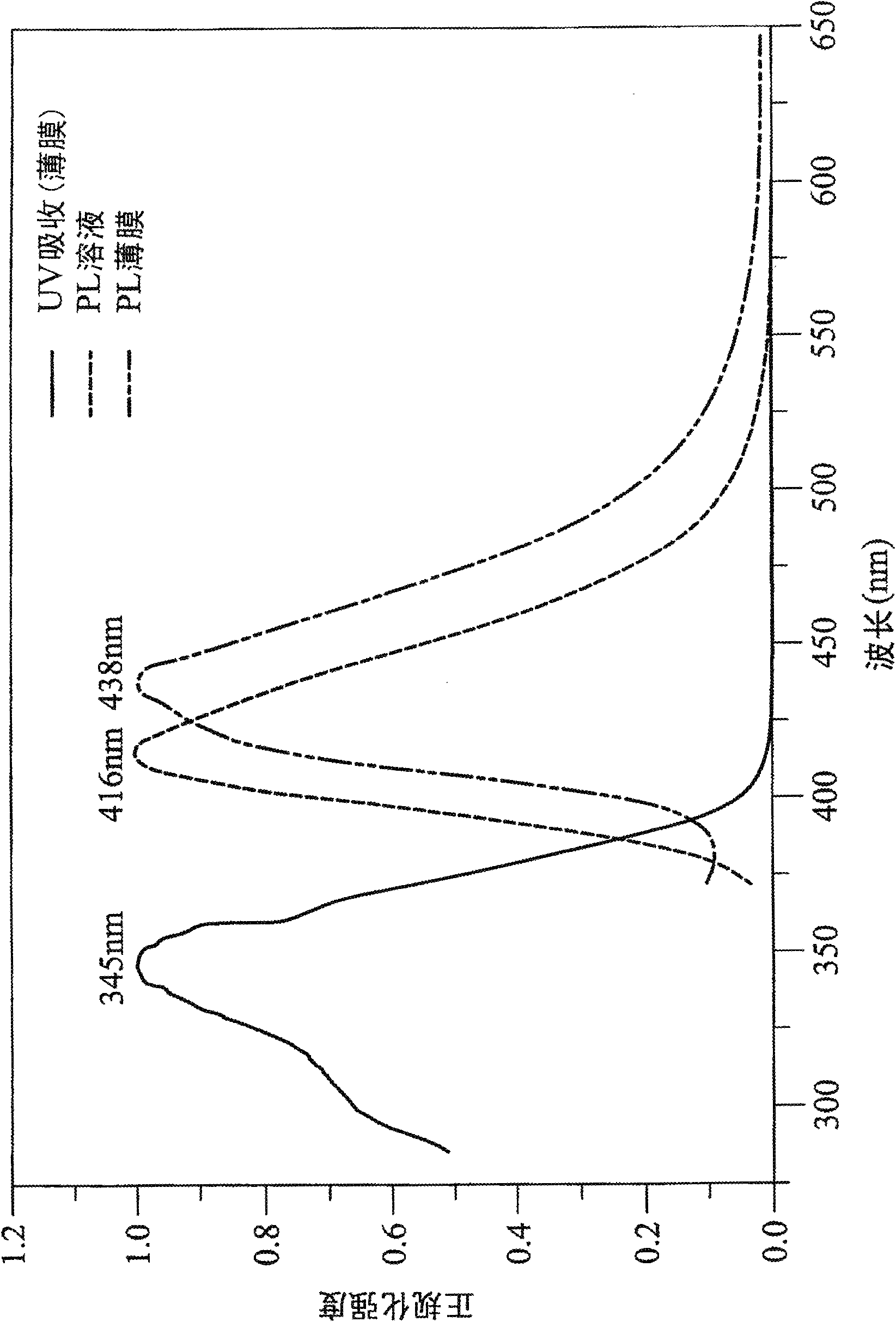
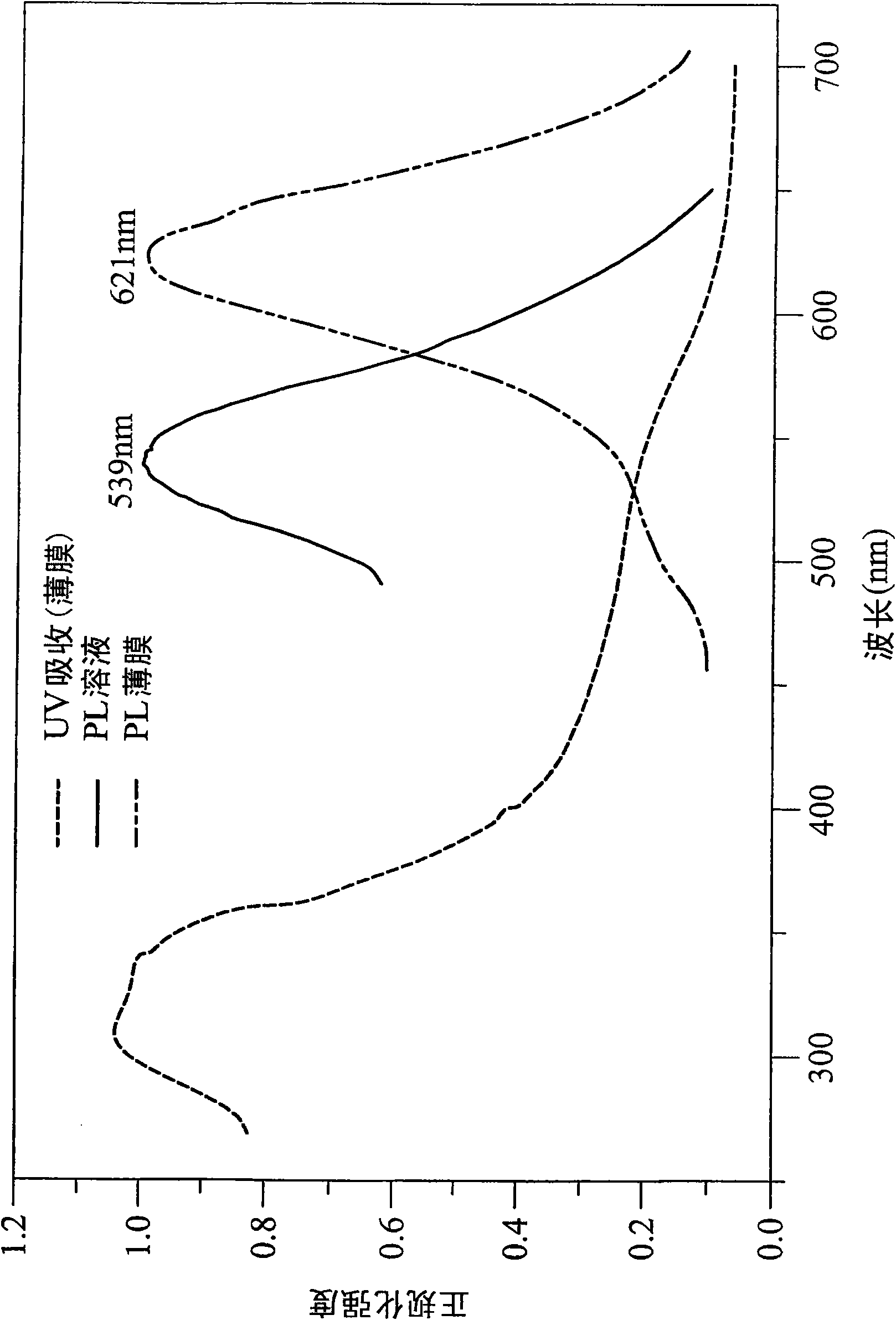
The present invention discloses a phenanthrene derivative having the following structure: wherein Ar1 and Ar2 independently are phenyl, nathphyl, heterocyclic group, polycyclic aromatic or polycyclic heterocyclic group with at least one conjugated substituent. The conjugated substituent can be an electron withdrawing group or electron donating group. The phenanthrene derivatives have semiconductor properties of electron transfer, electroluminescence (EL), and photoluminescence (PL). Intermolecular stacking can be avoided and electron-luminescent emission stability is enhanced when the derivatives are applied as a light-emitting material in organic EL devices due to the presence of the two stereo cyclopentane rings, such as a host compound or a dopant emitting blue light.
Owner:IND TECH RES INST
Organic phosphaphenanthrene derivatives, and preparation method and application thereof
ActiveCN105669760AImprove flame retardant performanceImprove thermal stabilityGroup 5/15 element organic compoundsPolyethylene terephthalate glycolPolyamide
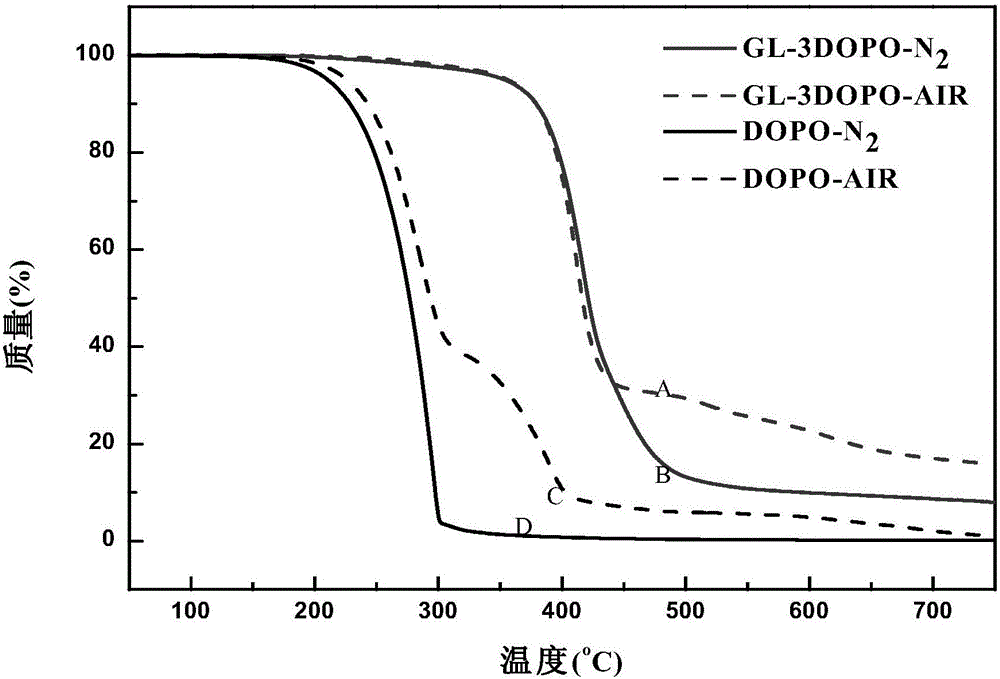
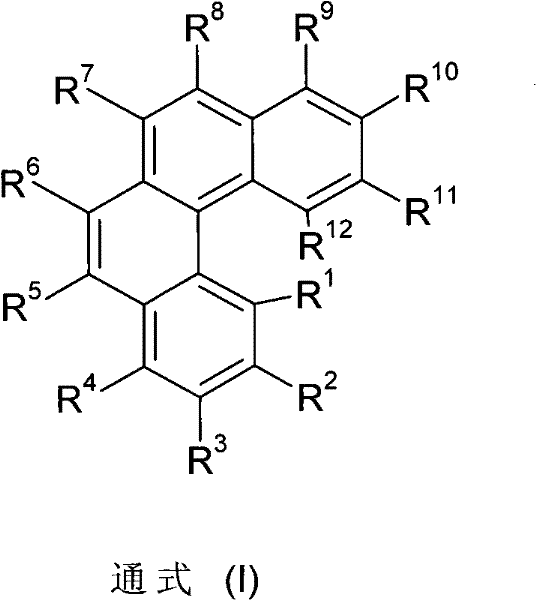
The invention discloses organic phosphaphenanthrene derivatives, and a preparation method and application thereof. The structural formula of the organic phosphaphenanthrene derivatives is disclosed as Formula I, wherein the group disclosed as Formula II is from carboxylic acid derivatives or acyl chloride derivatives, the group A is from organic phosphaphenanthrene compounds, and the group A is connected with the group disclosed as Formula II through a P-C bond. The preparation method of the organic phosphaphenanthrene derivatives comprises the following steps: carrying out esterification reaction on glycerol and carboxylic acid derivatives or acyl chloride derivatives to obtain glycerol ester derivatives; and under the action of a catalyst, carrying out reaction on the glycerol ester derivatives and organic phosphaphenanthrene compounds. The organic phosphaphenanthrene derivatives can enhance the flame retardancy of the polymer, and can enhance the flame retardancy of the prepared material when being added as a functional aid for preparing high-polarity engineering plastics of PET (polyethylene terephthalate), PBT (polybutylene terephthalate) and other polyesters and PA6 (polyamide 6), PA66 (polyamide 66) and other polyamides.
Owner:INST OF CHEM CHINESE ACAD OF SCI
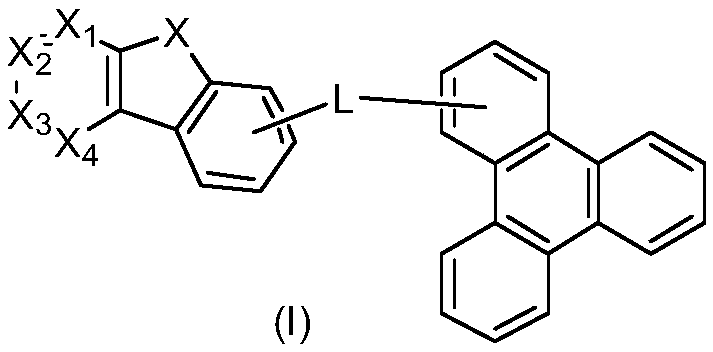
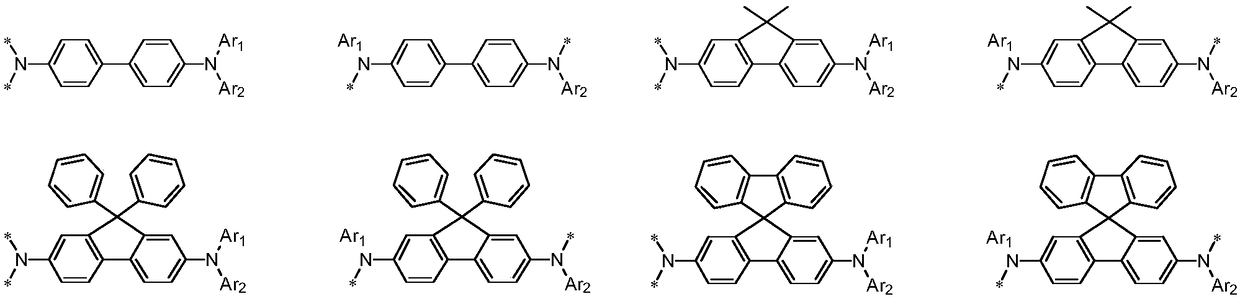
Benzo[c]phenanthrene derivative with electron donor-acceptor structure and application thereof and electroluminescent device
InactiveCN105503622AHigh electroluminescence efficiencyImprove thermal stabilitySilicon organic compoundsSolid-state devicesElectricityTriboluminescence
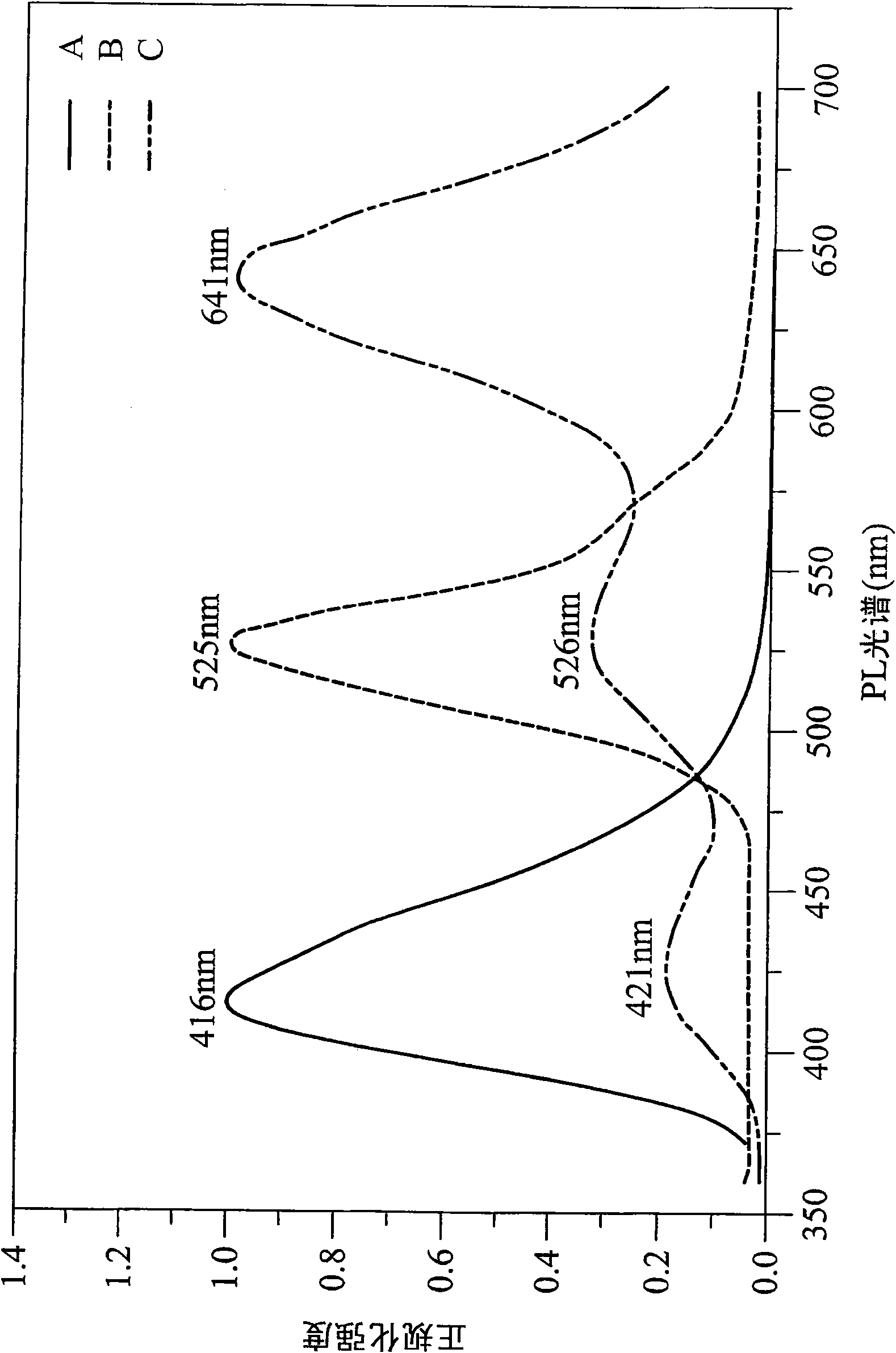
The invention belongs to the field of photoelectric material application science and technology, and particularly relates to a benzo[c]phenanthrene derivative with an electron donor-acceptor structure and application thereof and an electroluminescent device. The derivative takes benzo[c]phenanthrene as a core, and by linking different aromatic amine groups to different sites of the benzo[c]phenanthrene in a key mode or changing a bridged structure, an efficient fluorescence material with the double polarity is formed. The material has the advantages that the stability and conjugacy of large aromatic rings of the benzophenanthrene are utilized, distribution of electron clouds on the molecules is adjusted through the periphery groups, and therefore luminescent property can be effectively adjusted.
Owner:WUHAN SUNSHINE OPTOELECTRONICS TECH CO LTD
Materials for Organic Electroluminescent Devices
The present invention relates to substituted benzo[c]phenanthrene derivatives and to the production and to the use thereof in electronic devices, and to the electronic devices themselves. The present invention relates in particular to benzo[c]phenanthrene derivatives substituted with at least one aromatic unit or at least one diarylamino unit.
Owner:MERCK PATENT GMBH
Usage of oxidized aporphine derivative and composition thereof
ActiveCN101564391AEffective treatmentLow toxicityOrganic active ingredientsAntineoplastic agentsSide effectCurative effect
The invention belongs to the technical field of pharmacy and in particular relates to a usage of an oxidized aporphine derivative and a composition thereof. The invention discovers that the oxidized aporphine derivative can effectively inhibit the tumor proliferation and that when the oxidized aporphine derivative is used together with antitumor drugs to cure tumor, the synergy can be achieved, the cure effect can be enhanced, and the amount of the antitumor drugs in use can be reduced, thereby obviously reducing the toxic and side effects of the antitumor drugs.
Owner:XIANGBEI WELMAN PHARMA CO LTD
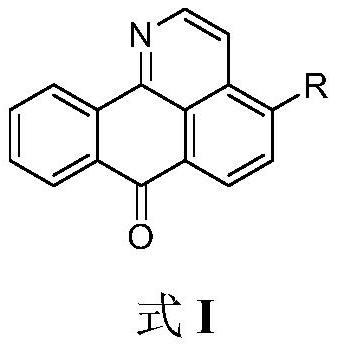
11-replaced oxoisoaporphine derivatives as well as synthetic method and application thereof
InactiveCN103923010AStrong inhibitory activityGood potential medicinal valueOrganic active ingredientsSenses disorderKetoneStructural formula
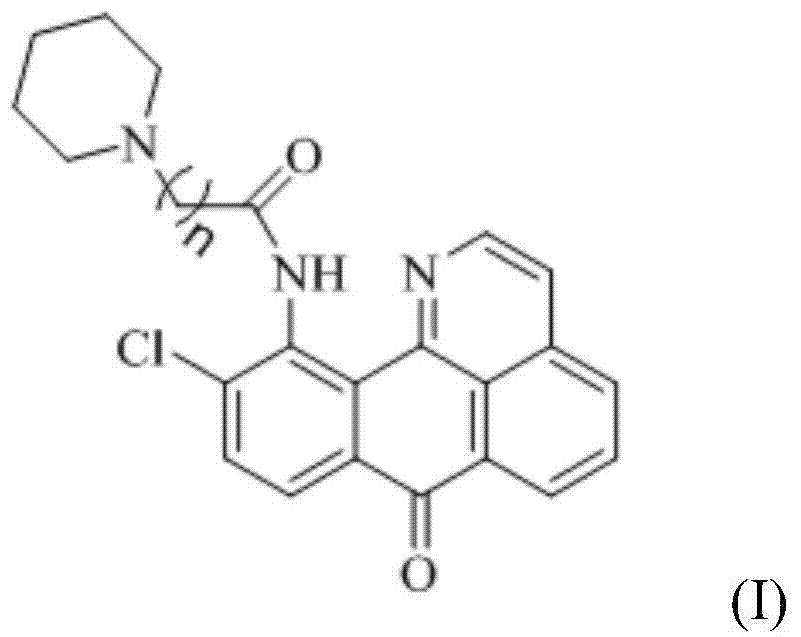
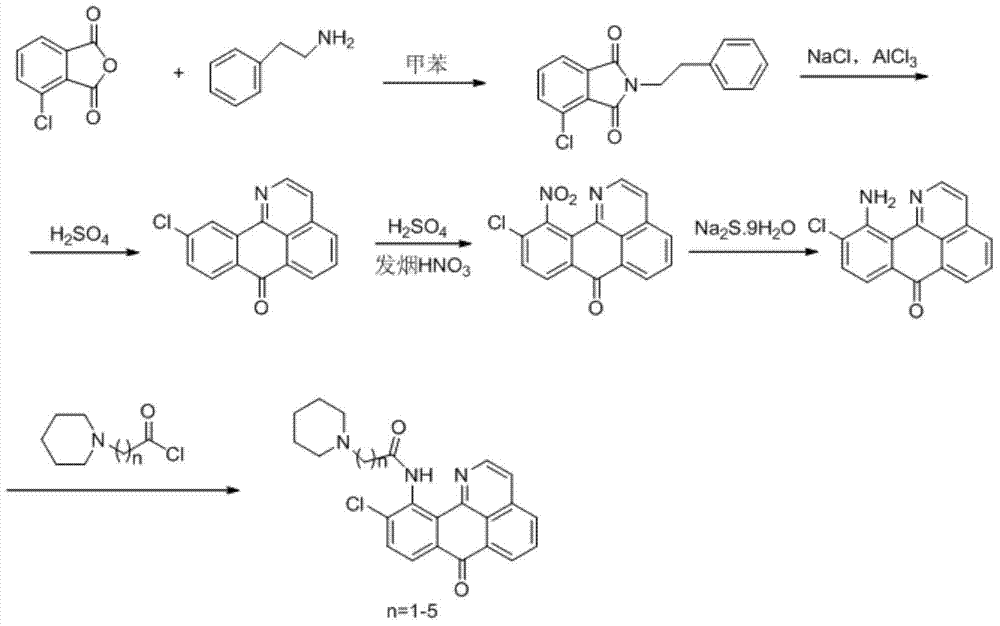
The invention discloses a series of 11-replaced oxoisoaporphine derivatives as well as a synthetic method and an application thereof. The synthetic method comprises the following steps: (1) carrying out ring closing reaction on 3-chlorophthalic anhydride and phenylethylamine as raw materials so as to construct a 10-Cl-1-azabenzanthrone parent body; (2) nitrating the parent body compound so as to obtain a 11-site nitrated product, and reducing the 11-site nitrated product so as to obtain 11-amino-10-chlorine-7H-dibenzoquinoline-7-ketone; and (3) reacting the 11-amino-10-chlorine-7H-dibenzoquinoline-7-ketone with an acyl chloride compound connected with piperidine so as to obtain a corresponding target product. Through study, the applicant finds that the series of derivatives have very strong inhibitory activity on acetylcholin esterase and are expected to be used for treating AD (Alzheimer Disease), cerebrovascular dementia and related diseases caused by cholinergic neurotransmitter reduction. The structural formula of the 11-replaced oxoisoaporphine derivatives is shown in descriptions.
Owner:GUANGXI NORMAL UNIV
Phenanthrene benzene derivative and polymer thereof, copolymer containing phenanthrene benzene derivative and luminous material composition
ActiveCN101654399AImprove solubilityInhibit aggregationGroup 3/13 element organic compoundsLuminescent compositionsSolubilityAlkoxy group
The invention relates to phenanthrene benzene derivatives with a structure of a formula (I), polymers thereof, copolymers containing the phenanthrene benzene derivatives and luminous material compositions, wherein R1, R2, R3 and R4 are independent substituted groups of alkyl or alkoxy with long chains. The phenanthrene benzene derivatives are co-synthesized by phenanthrene derivative molecules with stereo obstacles and benzene derivative molecules with high solubility, and can be copolymerized with other electrophilic or vugular conjugated molecules to form the copolymers of which energy gap is positioned between 1.8 and 3.0eV. The polymers of the phenanthrene benzene derivatives or the copolymers containing the phenanthrene benzene derivative can be doped with other luminous materials to form luminous materials emitting red, blue and green and full-color light.
Owner:IND TECH RES INST +1
Phenanthrene derivatives, applications thereof, and organic electroluminescent device
InactiveCN108299388AImprove thermal stabilityImprove luminous efficiencyOrganic chemistrySolid-state devicesOrganic solar cellOrganic layer
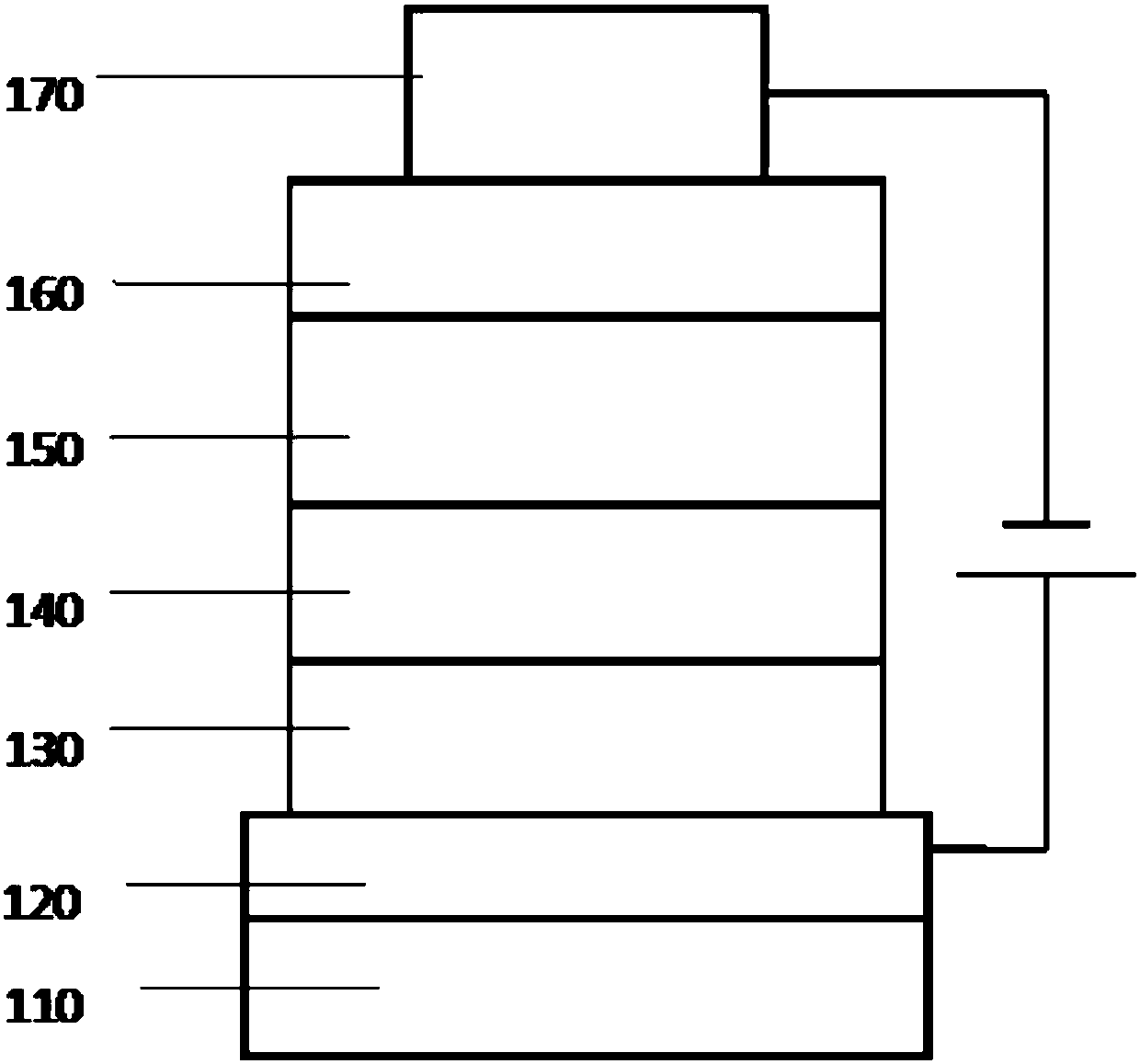
The invention provides phenanthrene derivatives represented by a formula shown in the description. The phenanthrene derivatives have the advantages of good thermal stability, high luminescent efficiency, and high luminescent purity, and can be applied to fields such as organic electroluminescent device, organic solar cell, organic thin film transistor, organic photoreceptor, or the like. The invention also provides an organic electroluminescent device, which comprises an anode, a cathode, and an organic layer. The organic layer comprises at least one of luminescent layer, hole injecting layer,hole transporting layer, hole blocking layer, electron injecting layer, and electron transporting layer and at least comprises a layer containing compounds represented by the structural formula I. The organic electroluminescent device prepared from the phenanthrene derivatives has the advantages of good electroluminescent efficiency, excellent color purity, and long service life.
Owner:SHANGHIA TAOE CHEM TECH CO LTD
2,7-dibromo-9-hydroxyl phenanthrene derivatives and preparation method thereof
InactiveCN102775279AReduce accumulationImprove solubilityOrganic chemistryOrganic compound preparationChemical synthesisPtru catalyst
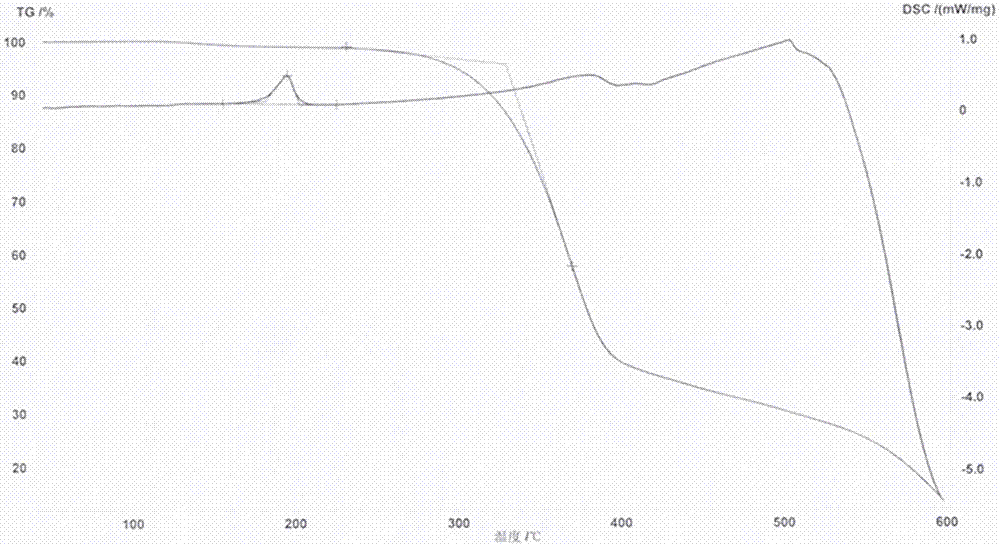
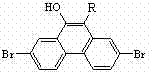
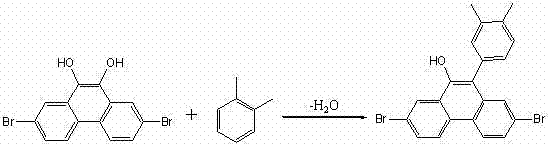
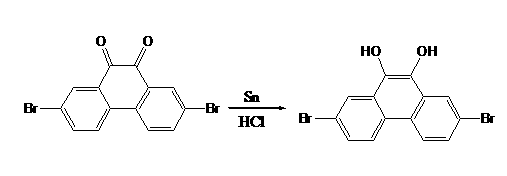
The invention discloses a series of 2,7-dibromo-9-hydroxyl phenanthrene derivatives with the following structural formula and a preparation method thereof, belonging to the field of organic chemical synthesis. The synthesis is realized by reacting 2,7-dibromo-9,10-dihydroxyl phenanthrene with an aromatic compound in the presence of a catalyst. R represents phenyl, C1-5 alkyl substituted phenyl or biphenyl. Since the sites 2, 7 and 9 of the phenanthrene derivatives are substituted and a large group is introduced to the site 10, the intermolecular accumulation can be effectively reduced, the derivatives obtain excellent electrochemical performance and high thermal stability, the blue luminescence property is improved, and the molecular dissolubility is also improved. The preparation technology is simple, the raw materials are easily available, the yield is high, and the derivatives are easy to purify and expected to be industrially applied to organic electroluminescence materials.
Owner:HENAN ACADEMY OF SCI CHEM RES INST CO LTD
2,7-dibromide-9,10 substituted-phenanthrene derivatives
InactiveCN103254044AReduce accumulationEliminate active hydrogenEther preparation by compound dehydrationChemical synthesisPtru catalyst
The invention discloses a series of 2,7-dibromide-9,10 substituted-phenanthrene derivatives having a structural formula as shown in the specification and a preparation method thereof, belonging to the field of organic chemical synthesis. Synthesis of the derivatives is realized by reacting 2,7-dibromide-9,10-dihydroxyl phenanthrene with alcohol compounds in the presence of a catalyst. According to the phenanthrene derivatives, substitution is carried out on sites 2,7,9 and 10, intermolecular accumulation can be effectively reduced, excellent electrochemical performance and high thermal stability are achieved, blue luminescence property of the phenanthrene derivatives is improved, and molecular dissolving property is also improved. A preparation process is simple, raw materials are available, yield is high, purification is easy, industrialization is hopeful, and the phenanthrene derivatives can be applied to organic electroluminescent materials. In a general formula, R1 and R2 are C1-C3 alkyls, and substituent groups are the same.
Owner:HENAN ACADEMY OF SCI CHEM RES INST CO LTD
Composition for forming liquid crystal layer, liquid crystal display device, and method for producing liquid crystal display device
InactiveUS20130135570A1Improve display qualityAvoid it happening againLiquid crystal compositionsNon-linear opticsOxygenComputational chemistry
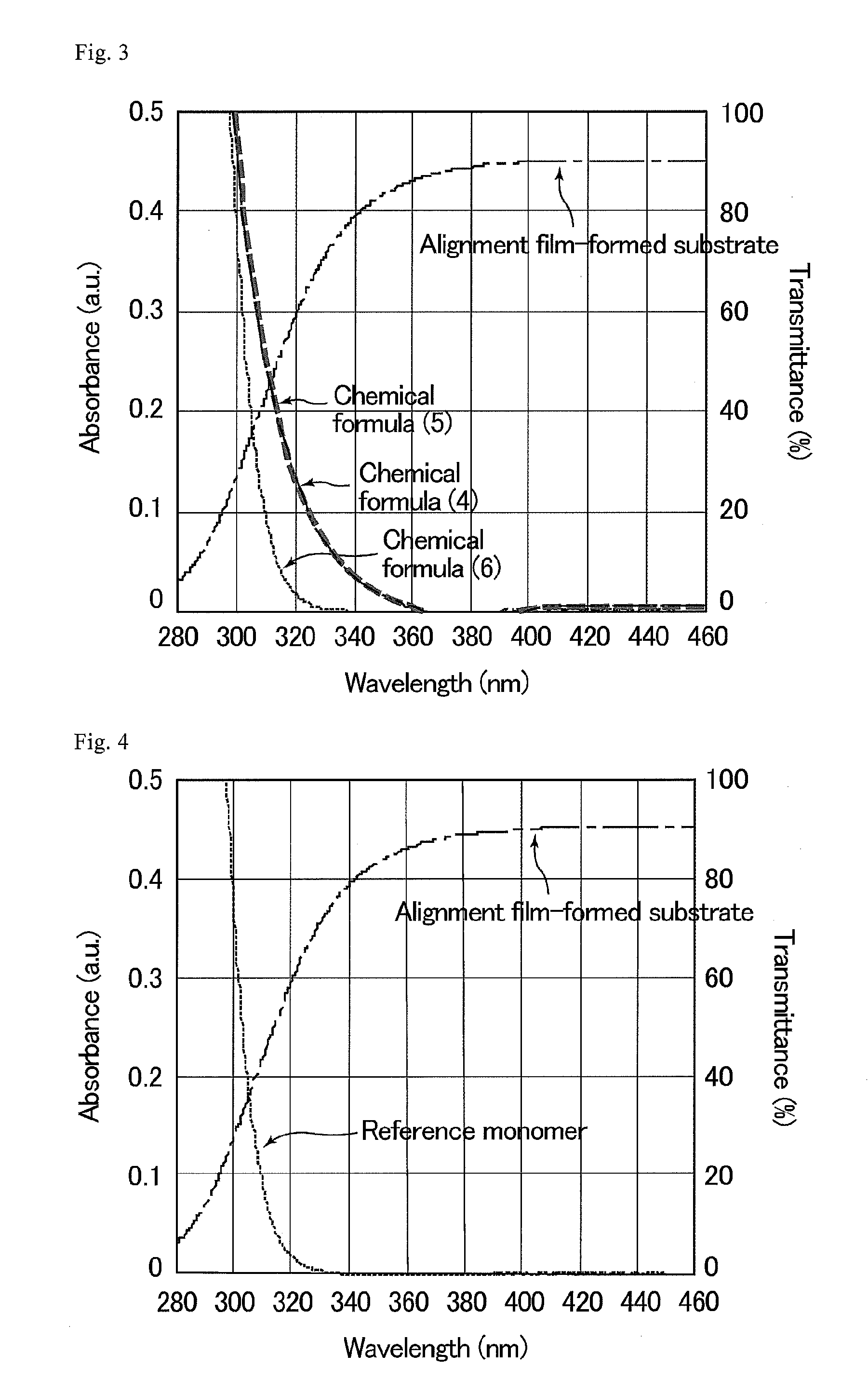
The present invention provides a composition for forming a liquid crystal layer from which a liquid crystal display device hardly generating image sticking can be obtained. The composition for forming a liquid crystal layer according to the present invention contains a liquid crystal material and one or two or more monomers, wherein at least one of the monomers is a phenanthrene derivative represented by the following chemical formula (1):the following chemical formula (2):or the following chemical formula (3):wherein R1 and R2 are identical or different, and each denote an -Sp-P group, a hydrogen atom, a halogen atom, a —CN group, a —NO2 group, a —NCO group, a —NCS group, an —OCN group, a —SCN group, a —SF5 group, or a straight-chain or branched-chain alkyl group having 1 to 12 carbon atoms; at least one of R1 and R2 denotes an -Sp-P group; P denotes a polymerizable group; Sp denotes a straight-chain, branched-chain or cyclic alkylene group or alkyleneoxy group having 1 to 6 carbon atoms, or a direct bond of both groups interposing Sp; a hydrogen atom which R1 and R2 have may be replaced by a fluorine atom or a chlorine atom; and a —CH2— group which R1 and R2 have, unless oxygen atoms, sulfur atoms and nitrogen atoms are mutually adjacent, may be substituted with an —O— group, a —S— group, a —NH— group, a —CO— group, a —COO— group, a —OCO— group, an —O—COO— group, a —OCH2— group, a —CH2O— group, a —SCH2— group, a —CH2S— group, a —N(CH3)— group, a —N(C2H5)— group, a —N(C3H7)— group, a —N(C4H5)— group, a —CF2O— group, a —OCF2— group, a —CF2S— group, a —SCF2— group, a —N(CF3)— group, a —CH2CH2— group, a —CF2CH2— group, a —CH2CF2— group, a —CF2CF2— group, a —CH═CH— group, a —CF═CF— group, a —C≡C— group, a —CH═CH—COO— group, or a —OCO—CH═CH— group.
Owner:SHARP KK +1
Phenanthrene derivative and organic light-emitting device thereof
InactiveCN108358919AGood electron transport propertiesHigh glass transition temperatureOrganic chemistrySolid-state devicesElectronic transmissionSynthesis methods
The invention provides a phenanthrene derivative and an organic light-emitting device thereof, and belongs to the technical field of organic photoelectric materials. The composition has the structureshown in the formula (I), the obtained phenanthrene derivative has the excellent electronic transmission capability, high glass-transition temperature and the effect of preventing crystallization by condensing phenanthrene and benzimidazole. The synthesis method is simple and easy to operate, the organic light-emitting device prepared by using the phenanthrene derivative has the advantages of being low in driving voltage and high in light-emitting efficiency, and is an organic light-emitting material with excellent performance.
Owner:CHANGCHUN HYPERIONS TECH CO LTD
4,5-di-substituted phenanthrene and hydrophenanthrene liquid crystal compound and preparation method thereof
ActiveCN104557481AQuick responseHD highlightsLiquid crystal compositionsOrganic compound preparationDielectric anisotropyViscosity
The invention discloses a 4,5-di-substituted phenanthrene and hydrophenanthrene liquid crystal compound and a preparation method thereof. A structural general formula of the compound is as shown in a formula I. Two benzene rings in 2,7-di-substituted and 4,5-di-substituted or 4,5-bi(trifluoromethyl) substituted phenanthrene derivatives cannot freely rotate; substituent groups are kept on the same side, can be spontaneously oriented, do not need to rotate to the same side under the action of an external electric field; the liquid crystal compound has the characteristics of being large negative dielectric anisotropy delta epsilon value, high response speed, high clearing point, low viscosity and the like, and is especially suitable for liquid crystal mixtures. The formula is as shown in the specification.
Owner:SHIJIAZHUANG CHENGZHI YONGHUA DISPLAY MATERIALS CO LTD
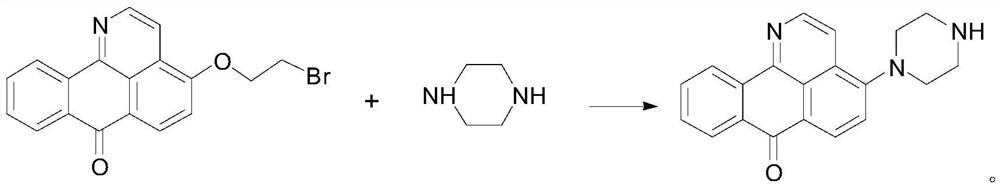
Isoaporphine oxide alkaloid derivative and application thereof
ActiveCN111825615AImprove biological activityStrong inhibitory activityOrganic chemistryAntineoplastic agentsPharmaceutical drugPerylene derivatives
The invention discloses an isoaporphine oxide derivative as shown in a formula I. R is as shown in the formula I. A is selected from-O-or a covalent bond, n is an integer from 0 to 4, and R1 is selected from-NR2R3; R2 is selected from H, and R3 is selected from C1-C3 linear chain or branched chain alkyl; A is selected from-O-, n is equal to 2 or 3, R1 is selected from the isoaporphine oxide alkaloid derivative, and compared with isoaporphine oxide, the inhibitory activity of the isoaporphine oxide alkaloid derivative on lung cancer, liver cancer and breast cancer is obviously improved, and thesolubility is increased. The invention also discloses an application of the isoaporphine oxide derivative in preparation of antitumor drugs.
Owner:CHINA PHARM UNIV
Benzophenanthrene derivative and organic light-emitting device thereof
InactiveCN109384706AImprove rigidityNot easy to age and deformOrganic chemistrySolid-state devicesOrganic light emitting deviceOrganic electroluminescence
The invention provides a benzophenanthrene derivative and an organic light-emitting device thereof, and relates to the technical field of organic photoelectric materials. The provided benzophenanthrene derivative has excellent hole transport performance and high heat resistance stability, needle holes cannot be formed in a formed film easily and the formed film cannot be aged or deform easily, thefilm is applied to the organic light-emitting device as a hole transport material, the light-emitting efficiency of the device can be improved, the service life of the device is prolonged, and furthermore, the driving voltage of the device can be reduced. The provided benzophenanthrene derivative can further serve as a covering layer, and thus, the light-emitting efficiency of the device is improved.
Owner:CHANGCHUN HYPERIONS TECH CO LTD
Halogen-free flame-retardant master batch for continuous long glass fiber reinforced polypropylene and preparation method of halogen-free flame-retardant master batch
The invention discloses a halogen-free flame-retardant master batch for continuous long glass fiber reinforced polypropylene and a preparation method of the halogen-free flame-retardant master batch. The halogen-free flame-retardant master batch consists of a polypropylene carrier, a halogen-free flame retardant, a hyperdispersant, a lubricant, a coupling agent and an antioxidant. The polypropylene carrier is a mixture of homo-polypropylene and co-polypropylene resin. The halogen-free flame retardant comprises a low-melting-point lubricating flame-retardant auxiliary agent, and the low-melting-point lubricating flame-retardant auxiliary agent is one or a mixture of several of a phosphaphenanthrene derivative or a phosphazene derivative with the melting point lower than 170 DEG C. The halogen-free flame-retardant master batch for the continuous long glass fiber added polypropylene is extruded at the extrusion temperature of 170 DEG C-210 DEG C, and is granulated by a special long strip granulator for LFTPP under the tension action of a traction machine to obtain the halogen-free flame-retardant master batch for the continuous long glass fiber added polypropylene. The halogen-free flame-retardant master batch can improve the processing fluidity of the continuous long glass fiber reinforced polypropylene material and the uniform dispersion of powder, so that a product prepared from the continuous long glass fiber reinforced polypropylene material has better uniformity. The invention also provides a preparation method of the halogen-free flame-retardant master batch.
Owner:ZHEJIANG XUSEN NON HALOGEN SMOKE FLAME RETARDANT CO LTD +1
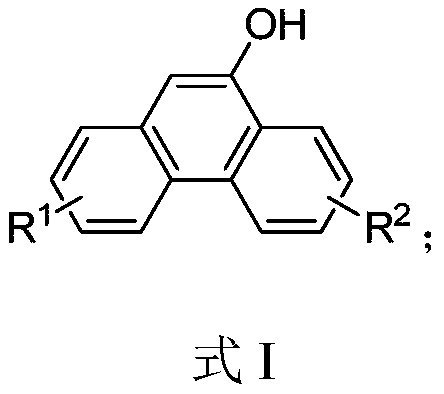
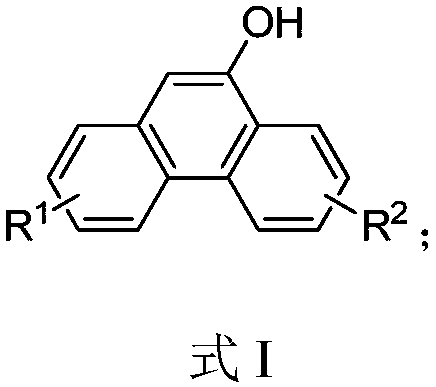
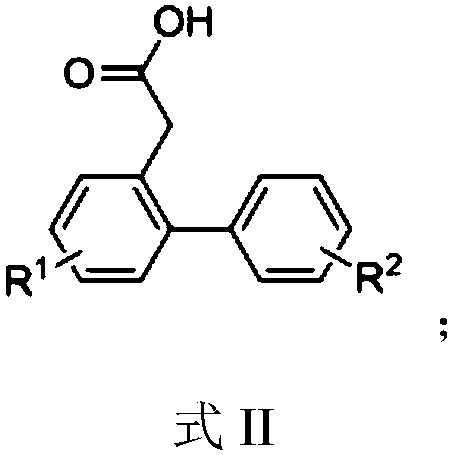
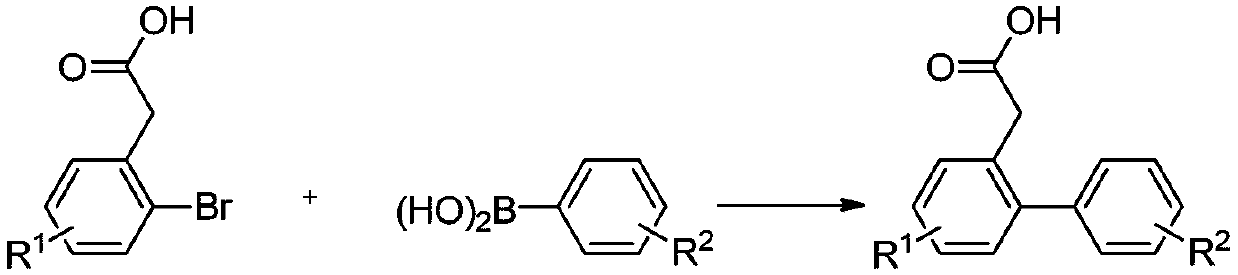
Hydroxyl-substituted phenanthrene derivative synthesis method
InactiveCN109574809AHigh yieldEasily hydrolyzedOrganic chemistryOrganic compound preparationSynthesis methodsTriflic acid
The invention relates to a hydroxyl-substituted phenanthrene derivative synthesis method, which includes taking a 2-carboxymethyl biphenyl compound as a raw material; and reacting under the action ofa catalyst to obtain a hydroxyl-substituted phenanthrene derivative, wherein the catalyst comprises one or two of trifluoromethanesulfonic acid or phosphorus pentoxide. Compared with the prior art, the synthesis method has the advantages of the cheap and easily-available catalyst, good universality of functional groups, simple and convenient operation, short reaction time, high reaction yield, mild reaction conditions and the like.
Owner:EAST CHINA UNIV OF SCI & TECH
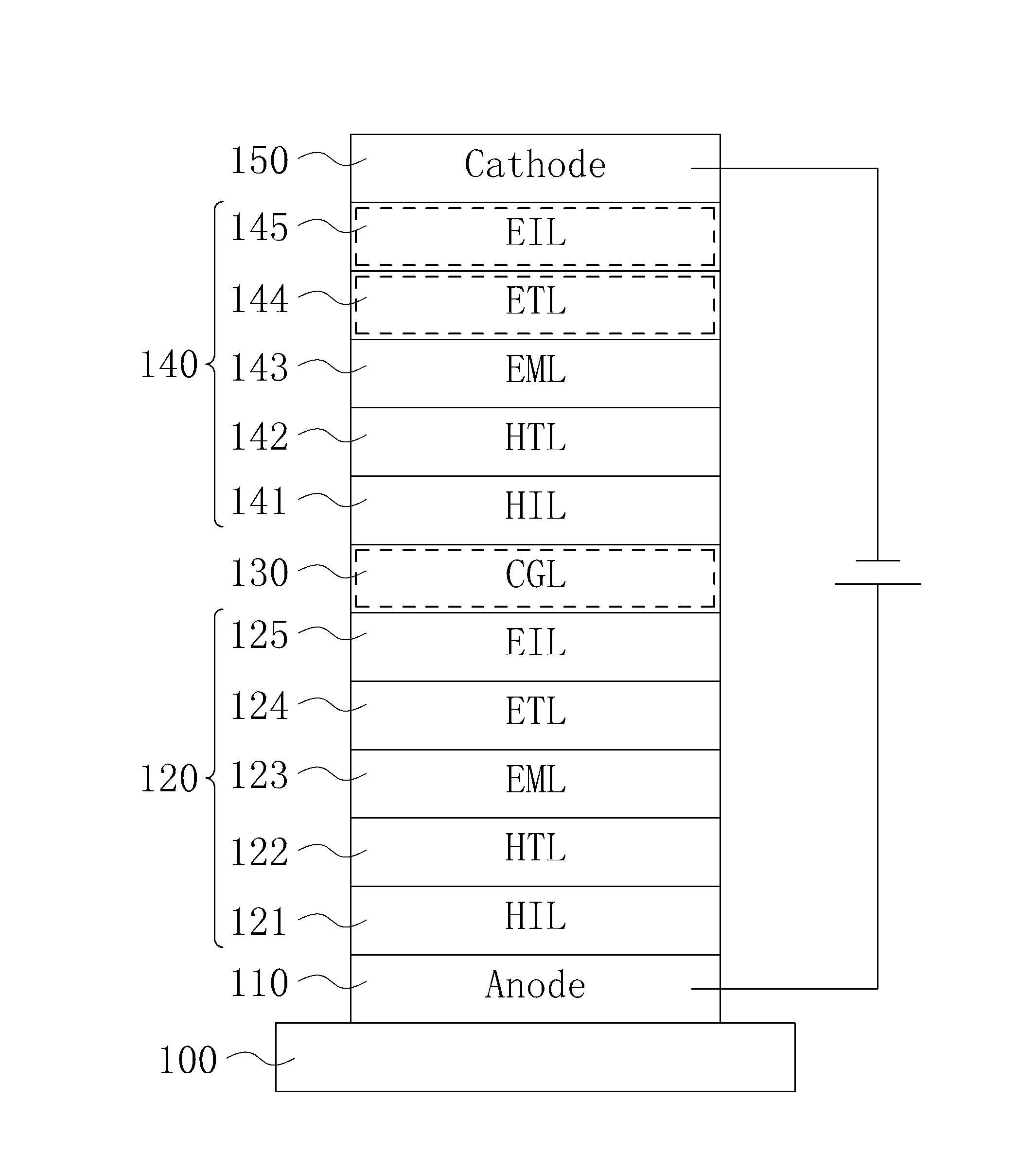
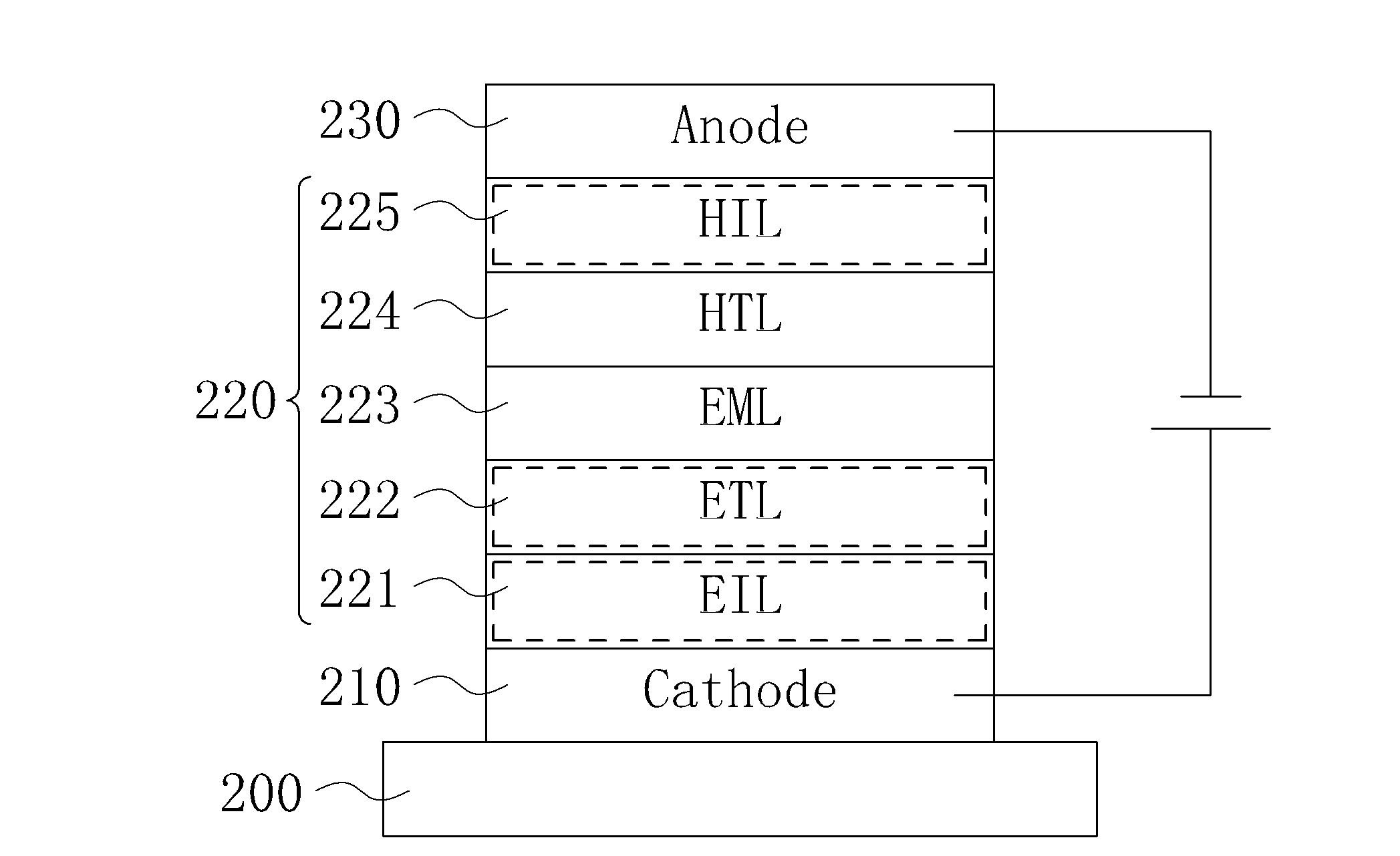
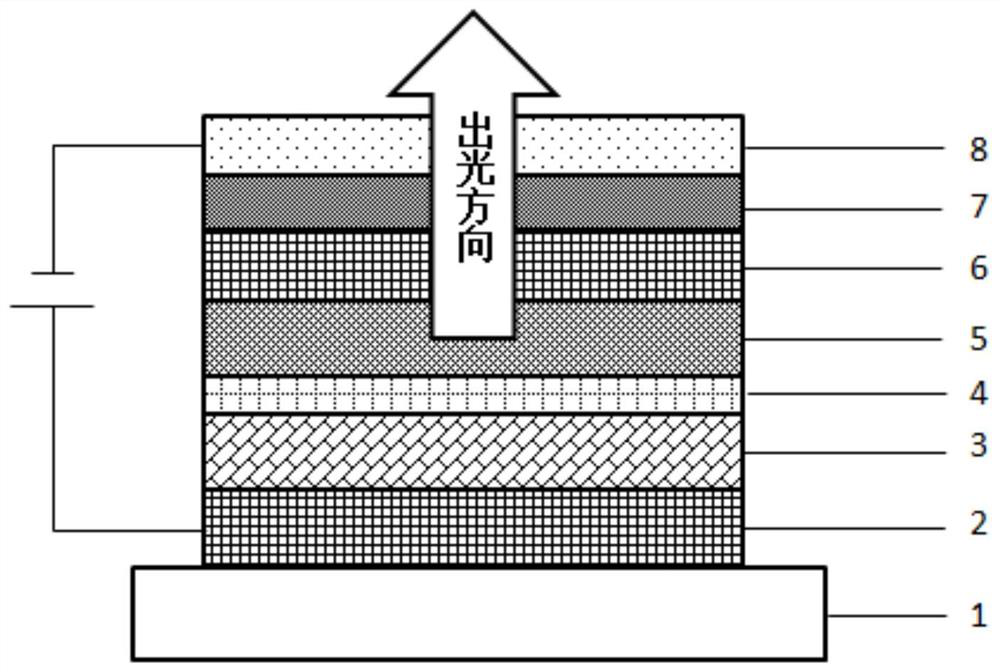
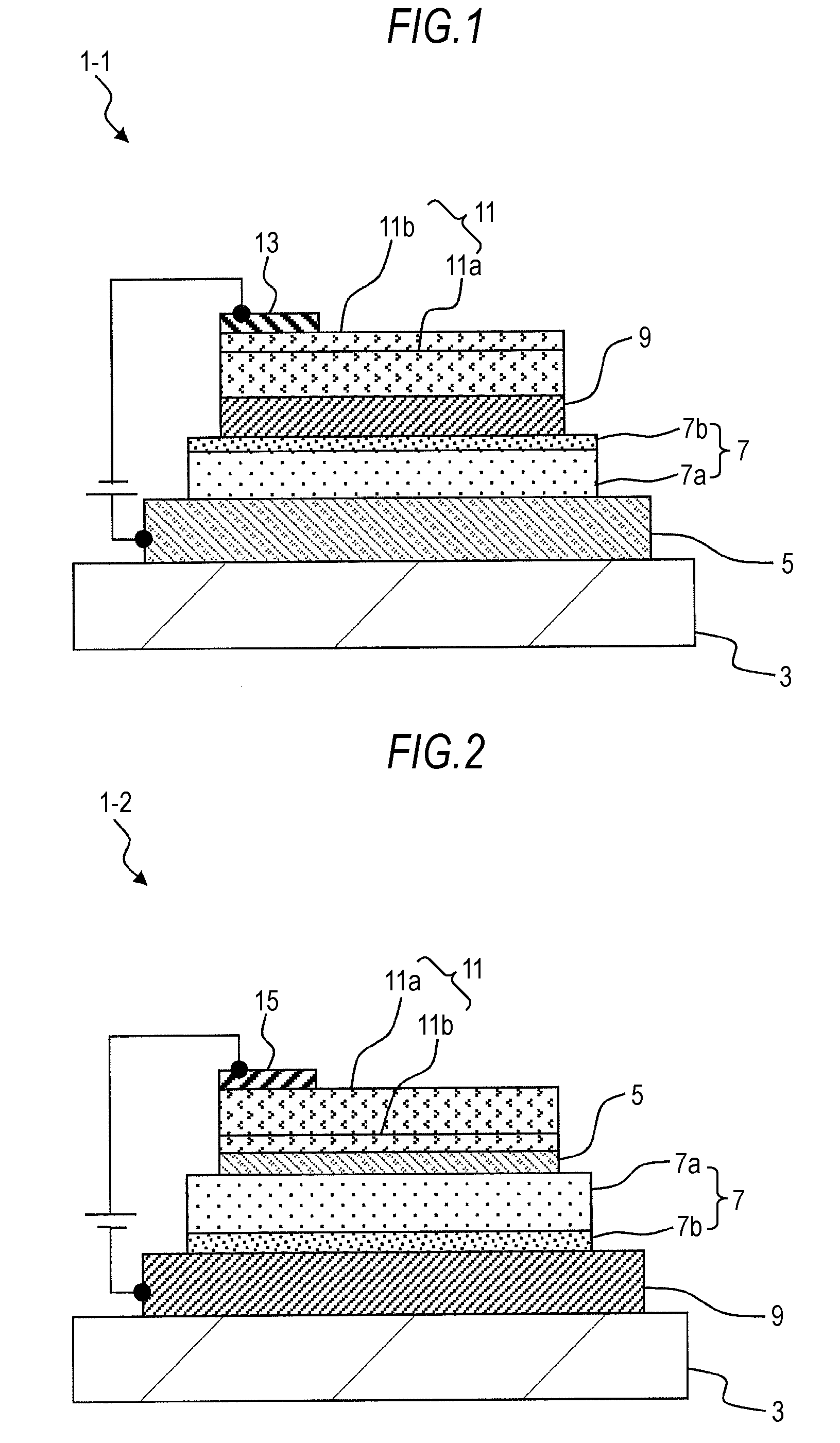
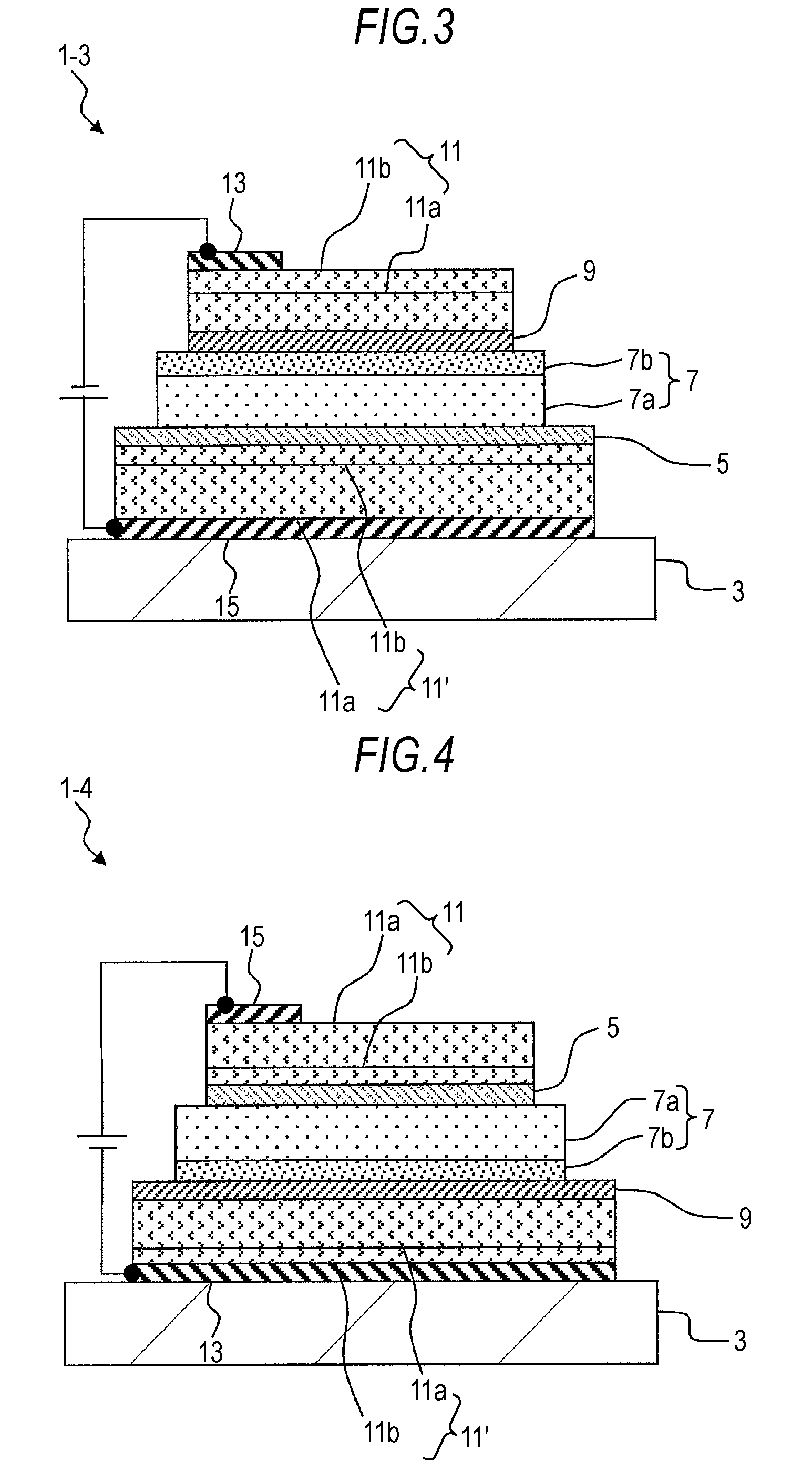
Organic electroluminescent device and display device
ActiveUS8586202B2Improve emission efficiencyImprove productivityDischarge tube luminescnet screensLamp detailsBenzimidazole derivativeBenzo(c)phenanthrene
Owner:JOLED INC
Method for the production of dibenz[c,e] [1,2]-oxaphosphorin derivatives, amino-dibenz[c,e] [1,2]-oxaphosphorin and also use thereof
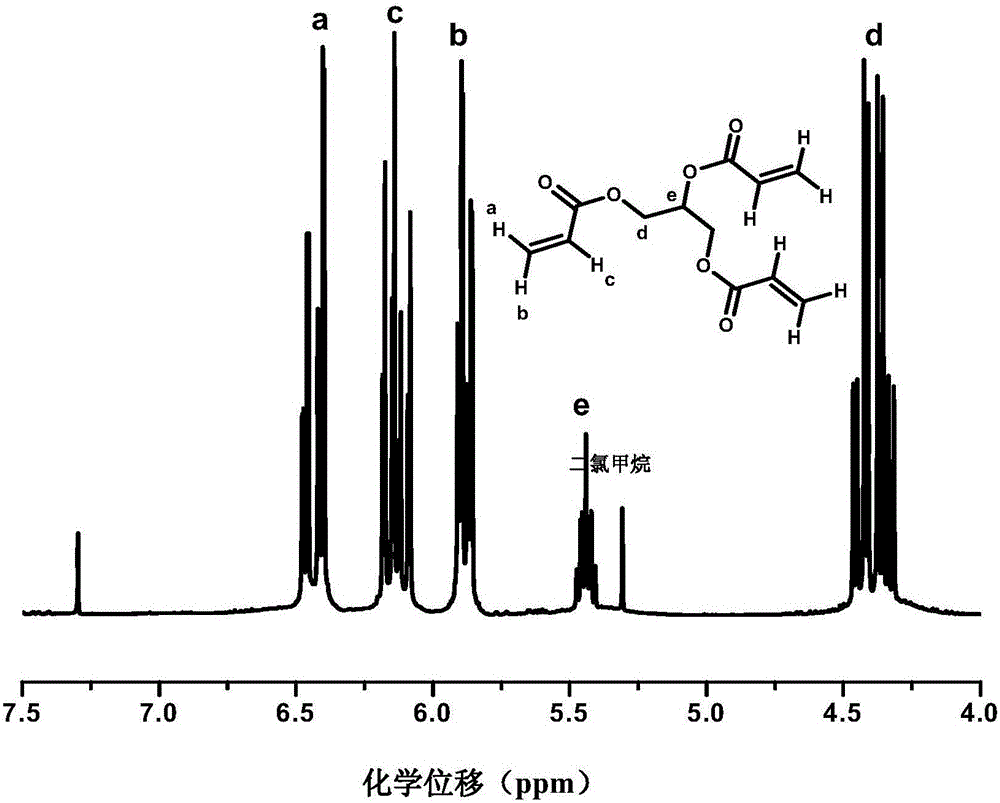
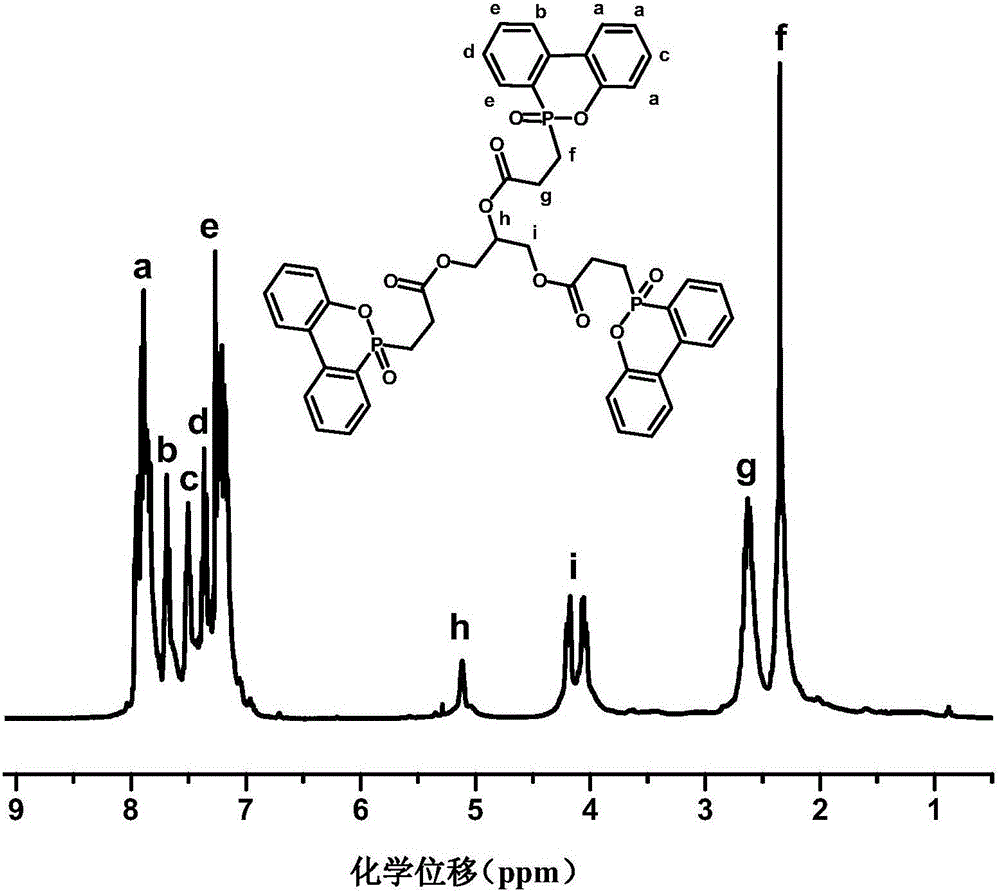
InactiveCN101648976AAvoid frequent occurrenceControls are responsiveGroup 5/15 element organic compoundsFire retardantPhotochemistry
Owner:EMS PATENT AG
Application of 6-aza-benzophenanthrene derivative to organic light-emitting element
InactiveCN103383990AReduce operating voltageImprove luminous efficiencyOrganic chemistrySolid-state devicesAza CompoundsElectric field
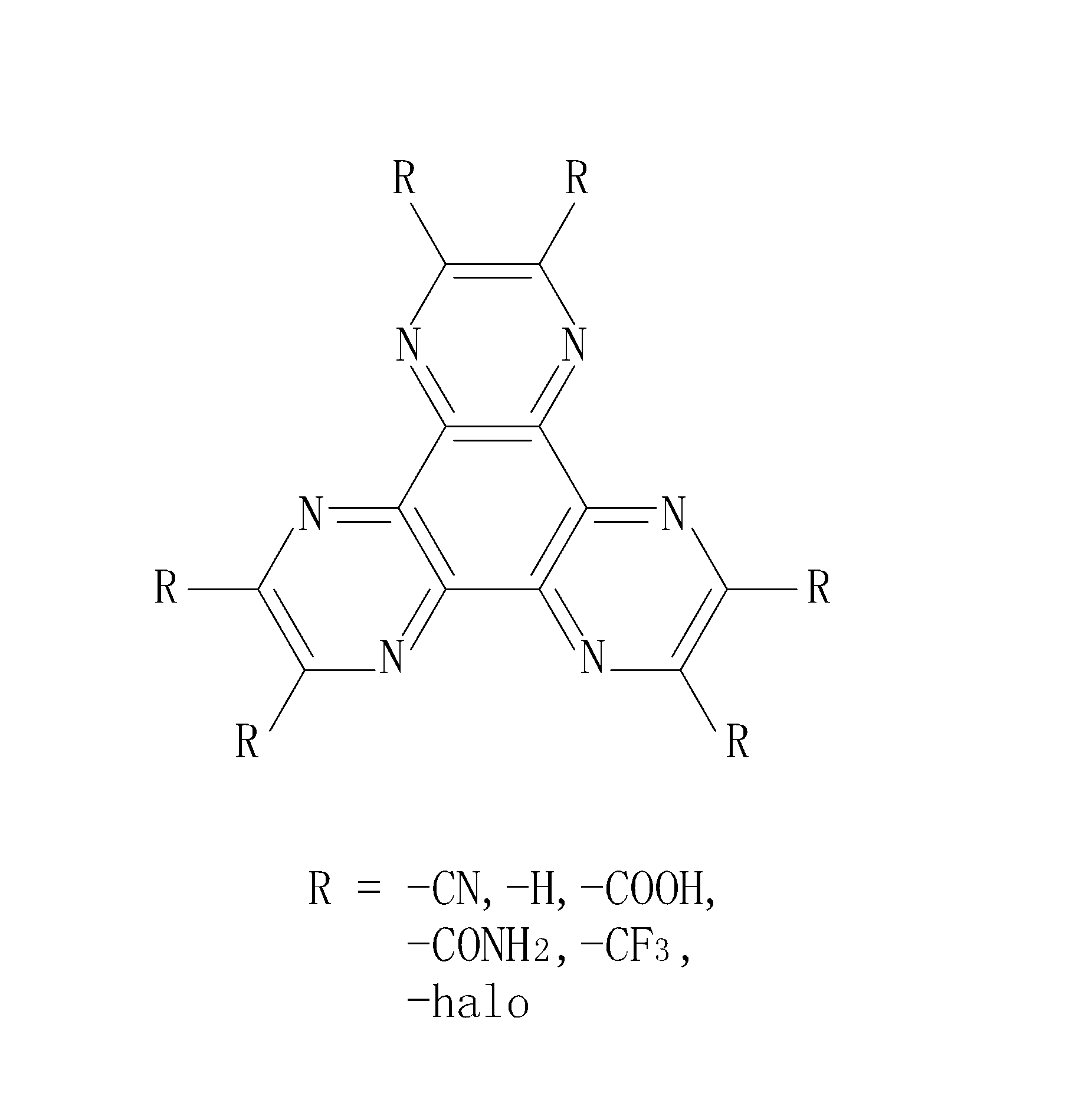
The invention provides an application of a 6-aza-benzophenanthrene derivative to an organic light-emitting element. The organic light-emitting element comprises a substrate, an anode, a cathode and at least one electroluminescence structure. The substrate is made of light-transmitting materials. The anode is electrically connected with a positive electrode of an external electric field and suitable for providing electron hole currents. The cathode is electrically connected with a negative electrode of the external electric field and suitable for providing electron currents. The electroluminescence structures are arranged between the anode and the cathode. At least one 6-aza-benzophenanthrene derivative layer is formed in each electroluminescence structure, wherein the material of the 6-aza-benzophenanthrene derivative layer comprises six functional groups R, and the functional groups are independently or simultaneously selected from a nitrile group (nitrile, -CN), a hydrogen group (hydrogen, -H), a carboxylic group (carboxylic, -COOH), a carboxamide group (carboxamide, -CONH2), a trifluoromethyl group (trifluoromethyl, -CF3) and a halogen group (halogen, -halo).
Owner:AU OPTRONICS CORP
Blue ray material with triaryl anthracene or triaryl phenanthrene structure and preparation method of blue ray material
InactiveCN103952148ADestruction of planarityNot easy to get close to each otherOrganic chemistryLuminescent compositionsBenzoxazoleFluorescence
The invention discloses a novel blue ray material with a 1,2,3-triaryl anthracene or 1,2,3-triaryl phenanthrene structure and a preparation method of the blue ray material. Derivatives of 1-(1-naphthyl)-2-(2-benzoxazolyl) acetylene and 1-(2-naphthyl)-2-(2-benzoxazolyl) acetylene are adopted as raw materials, a light ring addition reaction between hetero-aryl acetylenes is carried out in acetonitrile under irradiation of ultraviolet light. Maximum ultraviolet absorption wavelength of the novel triaryl anthracene or phenanthrene derivative is 280-300nm, shoulder peak of the novel triaryl anthracene or phenanthrene derivative extends to 380nm, and the novel triaryl anthracene or phenanthrene derivative can emit blue fluorescent lights with maximum emission wavelength of 380-400nm and shoulder peak extending to 500nm.
Owner:BEIJING UNIV OF CHEM TECH
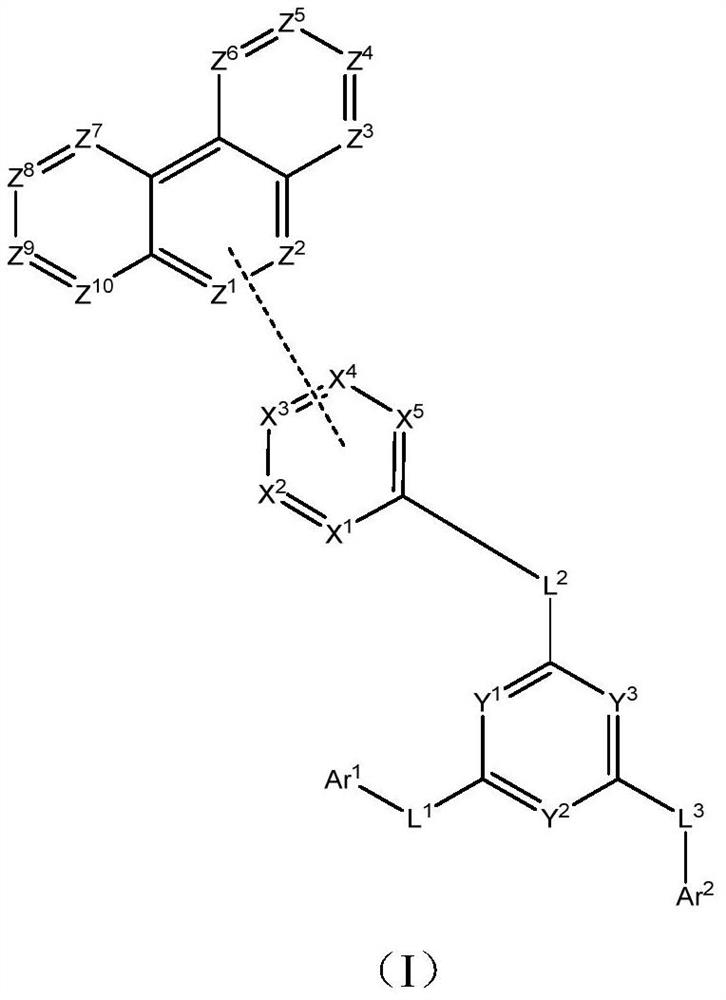
Compound, electron transport material, organic electroluminescent device and display device
PendingCN113816898AHigh bond energyConducive to solid accumulationOrganic chemistrySolid-state devicesDisplay deviceOrganic electroluminescence
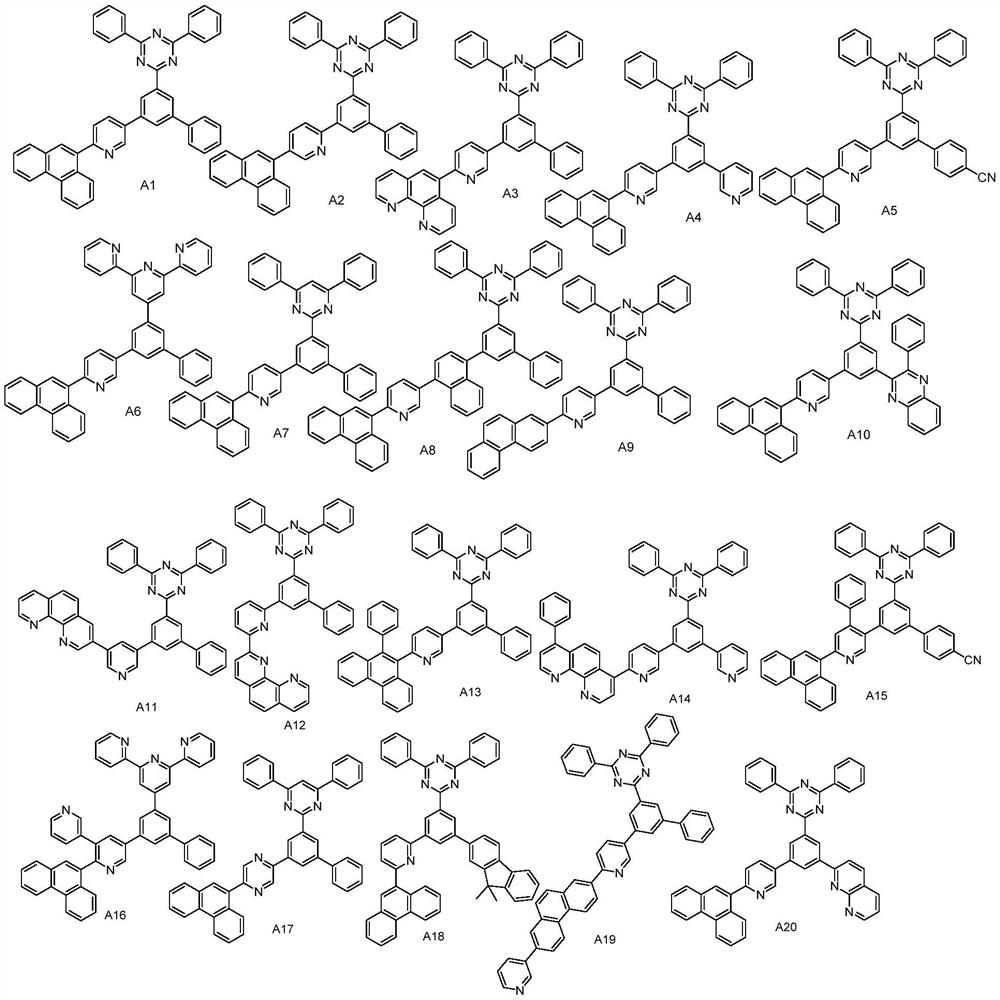
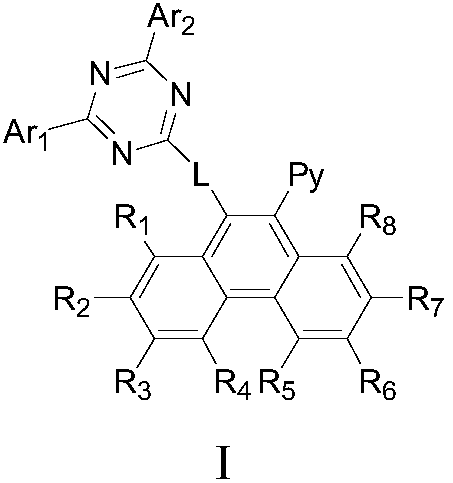
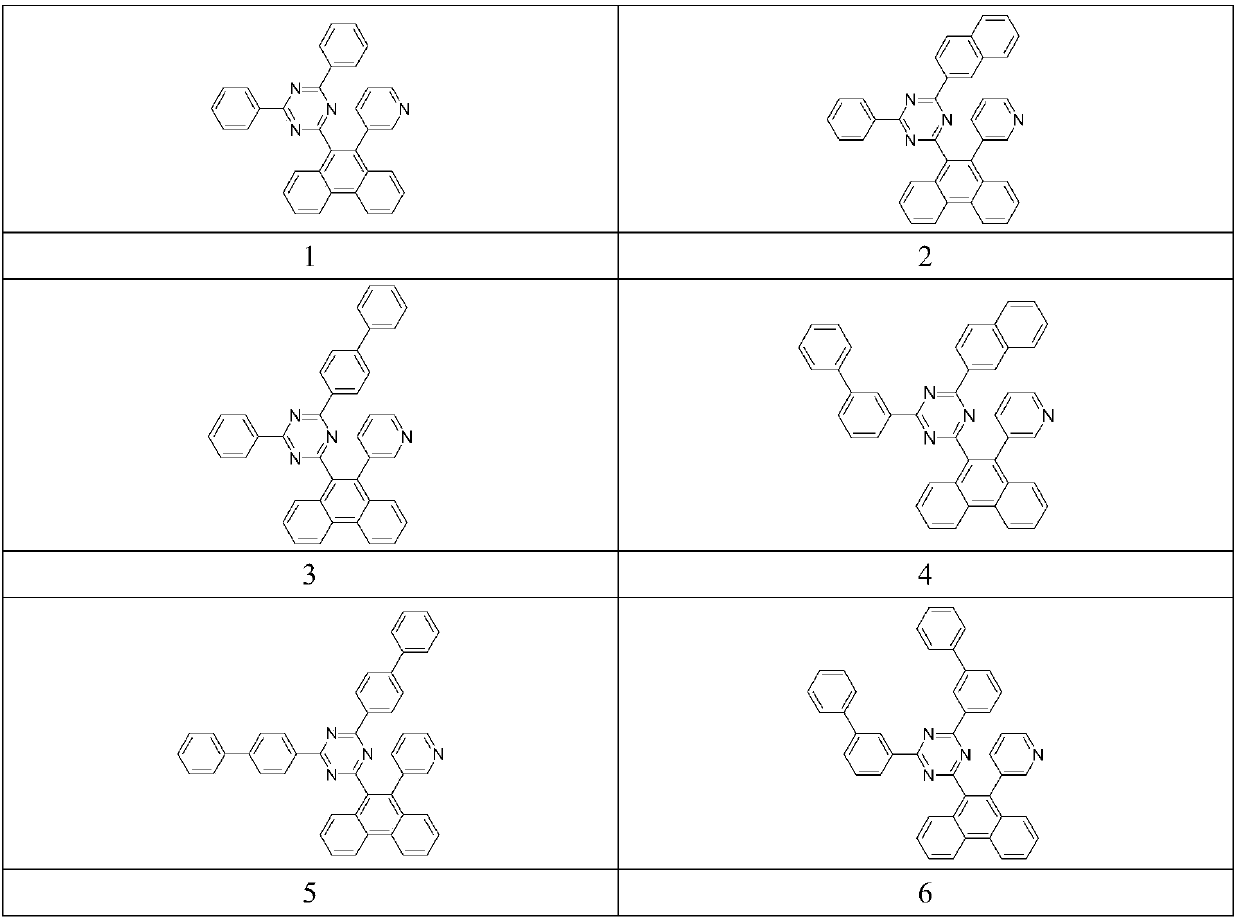
The invention provides a compound as shown in a general formula (I), wherein the compound can be used for an organic electroluminescent device as an electron transport material. The compound has a parent structure of a phenanthrene derivative linked electron-withdrawing fragment, has high bond energy between atoms, has good thermal stability, is beneficial to solid-state accumulation between molecules, and has strong electron transition ability. When the compound is used as an electron transport material, the driving voltage of an organic electroluminescent device is effectively reduced, the current efficiency of the organic electroluminescent device is improved, and the service life of the organic electroluminescent device is prolonged. The invention also provides an organic electroluminescent device containing the compound represented by the general formula (I) and a display device.
Owner:YANTAI XIANHUA CHEM TECH CO LTD +1
Manganese dioxide or m-chloroperoxybenzoic acid-participated oxidative coupling-prepared phenanthrene, dinaphthol and biphenyl derivative
The invention relates to manganese dioxide or m-chloroperoxybenzoic acid-participated oxidative coupling-prepared phenanthrene, dinaphthol and biphenyl derivative. The preparation method comprises the steps of: adding (E)-1, 2-di (phenyl-substituted) ethylene derivative or (z)-1, 2-di (phenyl-substituted) ethylene derivative or mixture of E / Z with any proportions into organic solvent to be dissolved; adding the manganese dioxide or the m-chloroperoxybenzoic acid (m-CPBA) again; stirring within the range from -30 DEG C to 80 DEG C, so that raw materials are completely reacted; adding water; separating liquid; drying an organic layer; evaporating the solvent to obtain a product phenanthrene derivative; and recrystallizing the product to obtain a pure product. The same method can be used forpreparing the dinaphthol derivative with 2-naphthol and preparing the biphenyl derivative with substituted benzene.
Owner:NANKAI UNIV
Triphenylene derivative and application thereof
InactiveCN107722021AImprove performanceImprove luminous efficiencyOrganic chemistrySolid-state devicesBenzeneAryl
The invention provides a triphenylene derivative and application thereof, and relates to the technical field of organic photoelectric materials. The compound takes triphenylene as a parent nucleus structure to modify different benzene rings with different benzene and nitrogen heterocyclic rings to further modify hydrogen of amino groups on the heterocyclic rings and hydrogen on the benzene rings with alkyl groups, substituted or non-substituted aryl groups and substituted or non-substituted condensed aryl groups, thereby obtaining a series of triphenylene derivatives. The preparation method issimple, and the raw materials are readily available; the triphenylene derivative is a matrix material with high performance, has hole transport capacity, and can be used as a hole transport materialapplied to an OLED device, so that the luminous efficiency of the device can be improved, and the service life of the device is prolonged.
Owner:CHANGCHUN HYPERIONS TECH CO LTD
A kind of synthetic method of phenanthrene derivatives substituted by hydroxy
InactiveCN109574809BImprove universalitySimple and fast operationOrganic chemistryOrganic compound preparationState of artSynthesis methods
The invention relates to a hydroxyl-substituted phenanthrene derivative synthesis method, which includes taking a 2-carboxymethyl biphenyl compound as a raw material; and reacting under the action ofa catalyst to obtain a hydroxyl-substituted phenanthrene derivative, wherein the catalyst comprises one or two of trifluoromethanesulfonic acid or phosphorus pentoxide. Compared with the prior art, the synthesis method has the advantages of the cheap and easily-available catalyst, good universality of functional groups, simple and convenient operation, short reaction time, high reaction yield, mild reaction conditions and the like.
Owner:EAST CHINA UNIV OF SCI & TECH
Polysubstitution phenanthrene derivative and preparation method thereof
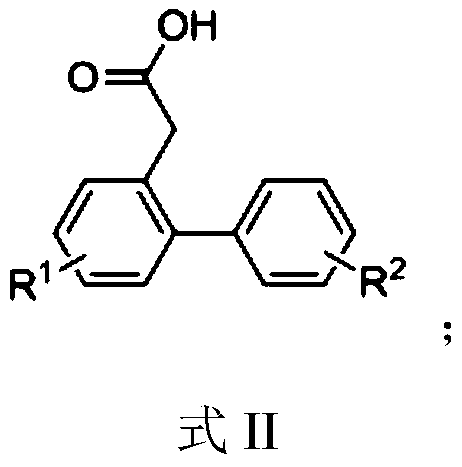
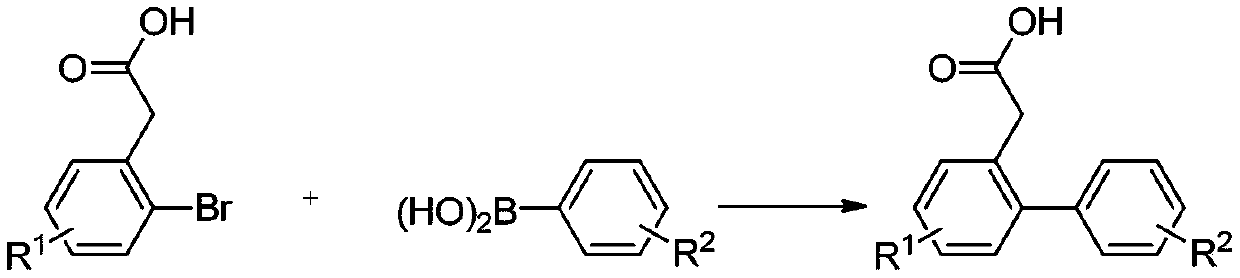
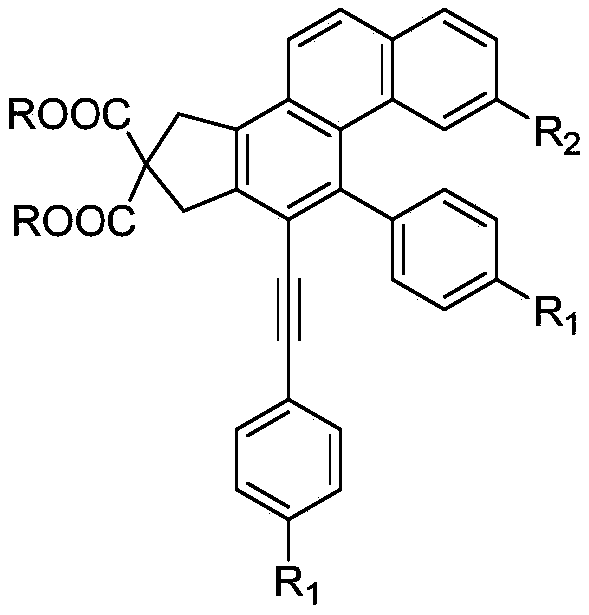
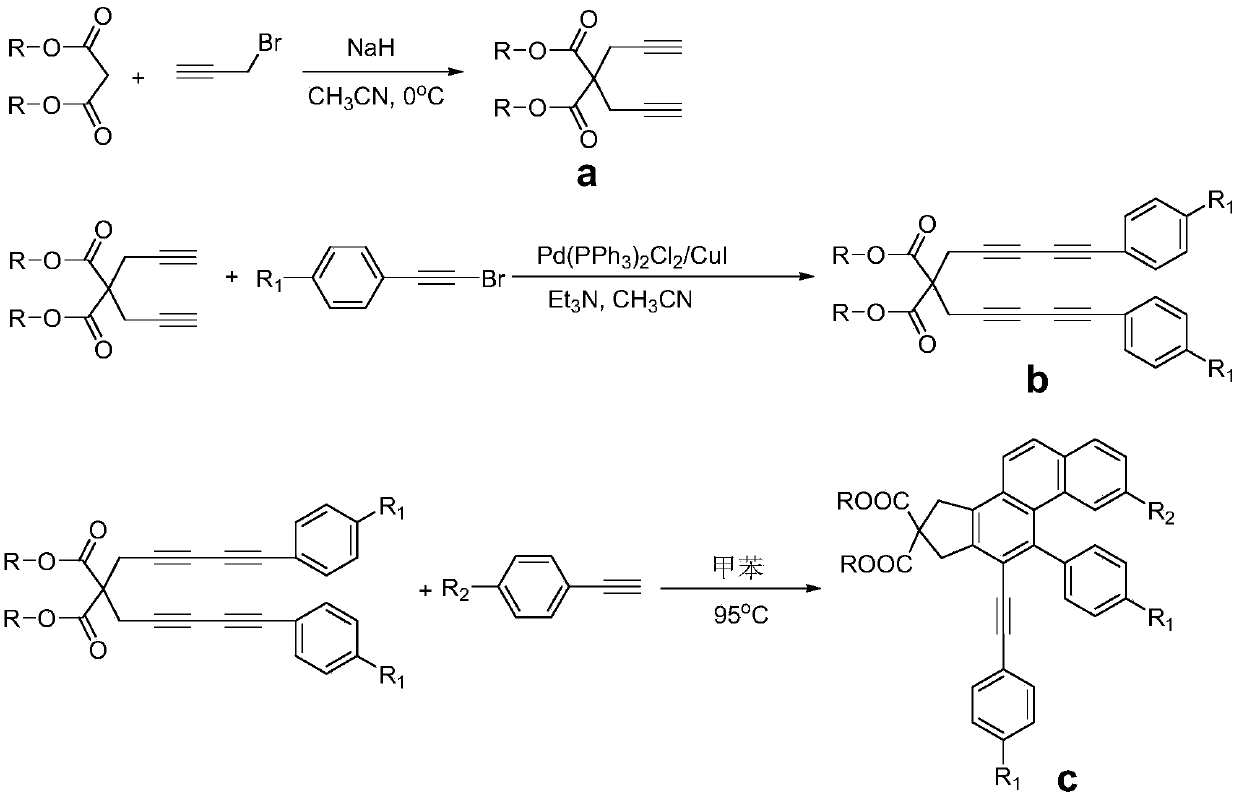
ActiveCN109651151AOvercome the shortcomings of limited expansionEasy to synthesizeOrganic compound preparationCarboxylic acid esters preparationBenzenePhenylacetylene
The invention discloses a polysubstitution phenanthrene derivative and a preparation method thereof. With different substitution phenylacetylene substrates, the polysubstitution phenanthrene derivative is constructed through a cascade reaction. The reaction overcomes the defects that in an existing reaction, a path is too long, the demands for substrates and reaction conditions are strict, and substitution functional groups are limited in expansion. During the reaction, substrate synthesis is simple, reagent is cheap, target molecules are obtained with high atom economy and environmental protection, and a quite valued approach is provided for industrialized production of the polysubstitution phenanthrene derivative.
Owner:ANHUI NORMAL UNIV
Synthetic method of phenanthrene, and phenanthrene derivative
ActiveCN109896920AEasy to prepareRaw materials are easy to obtainOrganic compound preparationHydrocarbonsOrganic acidAryl
The invention discloses a synthetic method of phenanthrene, and a phenanthrene derivative. The synthetic method comprises following steps: under nitrogen gas protection, in a toluene solution, an o-bromoiodobenzene compound, aryl boric acid, and a diaryl alkyne compound are taken as reaction raw materials, in the presence of palladium catalyst, organic phosphine ligand, an inorganic base, and an organic acid, heating backflow reaction is carried out, and separation is carried out so as to obtain phenanthrene and the phenanthrene derivative. The simple economical easily-available raw materialsare taken as substrates, palladium catalytic three-component cascade reaction is adopted, one-pot method is adopted to realize synthesis of phenanthrene and the phenanthrene derivative. The application prospect of phenanthrene and the phenanthrene derivate in the fields of biological industry, medical industry, and organic photoelectric material science is promising.
Owner:NANJING UNIV OF POSTS & TELECOMM
4,5-disubstituted phenanthrene and hydrophenanthrene liquid crystal compound and preparation method thereof
ActiveCN104557481BQuick responseHD highlightsLiquid crystal compositionsOrganic compound preparationDielectric anisotropyViscosity
The invention discloses a 4,5-di-substituted phenanthrene and hydrophenanthrene liquid crystal compound and a preparation method thereof. A structural general formula of the compound is as shown in a formula I. Two benzene rings in 2,7-di-substituted and 4,5-di-substituted or 4,5-bi(trifluoromethyl) substituted phenanthrene derivatives cannot freely rotate; substituent groups are kept on the same side, can be spontaneously oriented, do not need to rotate to the same side under the action of an external electric field; the liquid crystal compound has the characteristics of being large negative dielectric anisotropy delta epsilon value, high response speed, high clearing point, low viscosity and the like, and is especially suitable for liquid crystal mixtures. The formula is as shown in the specification.
Owner:SHIJIAZHUANG CHENGZHI YONGHUA DISPLAY MATERIALS CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com
![Benzo[c]phenanthrene derivative with electron donor-acceptor structure and application thereof and electroluminescent device Benzo[c]phenanthrene derivative with electron donor-acceptor structure and application thereof and electroluminescent device](https://images-eureka.patsnap.com/patent_img/3ec444d9-7b94-4312-ac09-a7e264cd9be3/HDA0000877054860000011.png)
![Benzo[c]phenanthrene derivative with electron donor-acceptor structure and application thereof and electroluminescent device Benzo[c]phenanthrene derivative with electron donor-acceptor structure and application thereof and electroluminescent device](https://images-eureka.patsnap.com/patent_img/3ec444d9-7b94-4312-ac09-a7e264cd9be3/HDA0000877054860000012.png)
![Benzo[c]phenanthrene derivative with electron donor-acceptor structure and application thereof and electroluminescent device Benzo[c]phenanthrene derivative with electron donor-acceptor structure and application thereof and electroluminescent device](https://images-eureka.patsnap.com/patent_img/3ec444d9-7b94-4312-ac09-a7e264cd9be3/HDA0000877054860000021.png)
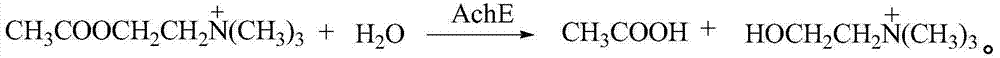
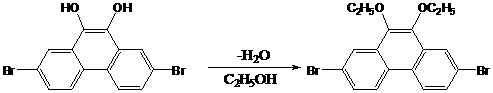
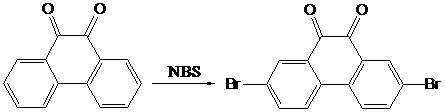
![Method for the production of dibenz[c,e] [1,2]-oxaphosphorin derivatives, amino-dibenz[c,e] [1,2]-oxaphosphorin and also use thereof Method for the production of dibenz[c,e] [1,2]-oxaphosphorin derivatives, amino-dibenz[c,e] [1,2]-oxaphosphorin and also use thereof](https://images-eureka.patsnap.com/patent_img/02f42171-e6e7-4034-9380-a047c9d31c86/A2009101610890002C1.PNG)
![Method for the production of dibenz[c,e] [1,2]-oxaphosphorin derivatives, amino-dibenz[c,e] [1,2]-oxaphosphorin and also use thereof Method for the production of dibenz[c,e] [1,2]-oxaphosphorin derivatives, amino-dibenz[c,e] [1,2]-oxaphosphorin and also use thereof](https://images-eureka.patsnap.com/patent_img/02f42171-e6e7-4034-9380-a047c9d31c86/A2009101610890002C2.PNG)
![Method for the production of dibenz[c,e] [1,2]-oxaphosphorin derivatives, amino-dibenz[c,e] [1,2]-oxaphosphorin and also use thereof Method for the production of dibenz[c,e] [1,2]-oxaphosphorin derivatives, amino-dibenz[c,e] [1,2]-oxaphosphorin and also use thereof](https://images-eureka.patsnap.com/patent_img/02f42171-e6e7-4034-9380-a047c9d31c86/A2009101610890003C1.PNG)