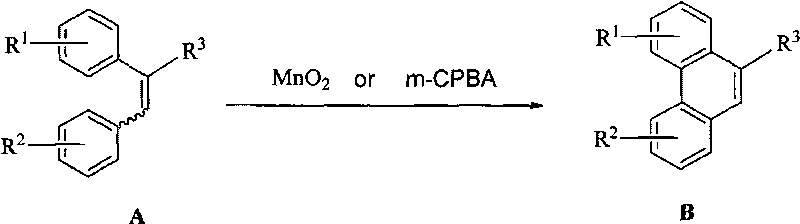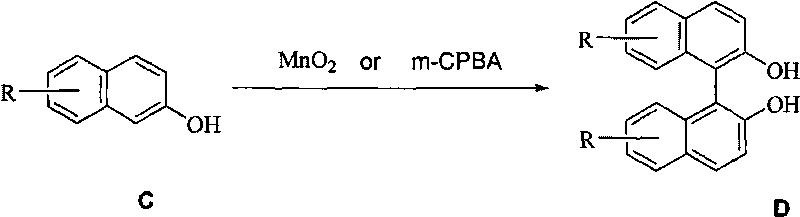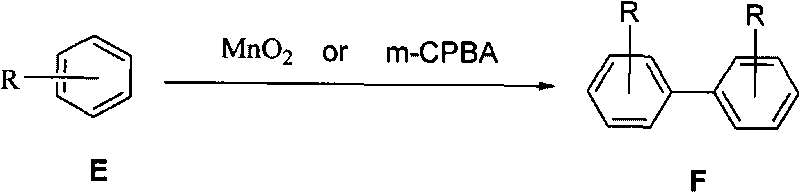Manganese dioxide or m-chloroperoxybenzoic acid-participated oxidative coupling-prepared phenanthrene, dinaphthol and biphenyl derivative
A technology of m-chloroperoxybenzoic acid and manganese dioxide, applied in chemical instruments and methods, organic cyclization, organic chemistry and other directions, can solve the problems of low reaction yield, harsh reaction conditions, high toxicity and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Embodiment 1: 2,3,6, the synthesis of 7-tetramethoxy-9-phenanthrene acid methyl ester:
[0030] Add 0.179g (0.5mmol) (E)-2,3-bis(3',4'-dimethoxyphenyl)methyl acrylate and 5mL trifluoroacetic acid into a 25mL four-neck flask, stir at room temperature to dissolve the raw materials, Add 0.044g (0.5mmol) MnO 2 , the mixture was stirred at room temperature for 2 hours, 50 mL of dichloromethane was added, washed with water, dried, filtered, and precipitated to obtain 2,3,6,7-tetramethoxy-9-phenanthrene carboxylate methyl ester. Conversion (100%) and yield (100%) were determined by HPLC. m.p.202-203℃ 1 H NMR (CDCl 3 , 400MHz) δ: 8.65(s, 1H), 8.43(s, 1H), 7.81(s, 1H), 7.77(s, 1H), 7.27(s, 1H), 4.14(s, 3H), 4.13(s , 3H), 4.08(s, 3H), 4.04(s, 3H), 4.02(s, 3H).
[0031] The following phenanthrene derivatives can be synthesized by the same method, but this does not limit the present invention.
[0032] 2,3,6,7-Tetramethoxy-9-phenanthrenemethylnitrile: Yield 99%, mp 266-268°C;...
Embodiment 2
[0044] Embodiment 2: 2,3,6, the synthesis of 7-tetramethoxy-9-phenanthrene acid:
[0045] Add 0.5mmol (E)-2,3-bis(3′,4′-dimethoxyphenyl)acrylic acid and 5mL trifluoroacetic acid to a 25mL four-neck flask, stir at room temperature to dissolve the raw materials, add 0.5mmol MnO 2 , the mixture was stirred at room temperature for 2 hours, 50 mL of dichloromethane was added, washed with water, dried, filtered, and precipitated to obtain 2,3,6,7-tetramethoxy-9-phenanthrene carboxylate methyl ester. Conversion (100%) and yield (100%) were determined by HPLC. m.p.285-287℃; 1 H NMR (DMSO, 300MHz) δ: 8.58(s, 1H), 8.43(s, 1H), 8.03(s, 1H), 7.99(s, 1H), 7.54(s, 1H), 4.08(s, 3H) , 4.07(s, 3H), 3.94(s, 3H), 3.93(s, 3H).
Embodiment 3
[0046] Example 3: Synthesis of 2,3,6,7-tetramethoxy-9-phenanthrene acid methyl ester:
[0047] Weigh 0.5 mmol of E, Z formula mixed 2,3-bis-(3',4'-dimethoxyphenyl)methyl acrylate with a molar ratio of 1:1 in a 25 mL four-necked flask, 5 mL of trifluoroacetic acid , stir at room temperature to dissolve the raw material, add 0.044g (0.5mmol) MnO 2 , the mixture was stirred at room temperature for 2 hours, 50 mL of dichloromethane was added, washed with water, dried, filtered, and precipitated to obtain 2,3,6,7-tetramethoxy-9-phenanthrene carboxylate methyl ester. Conversion (100%) and yield (100%) were determined by HPLC. m.p.202-203℃
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com