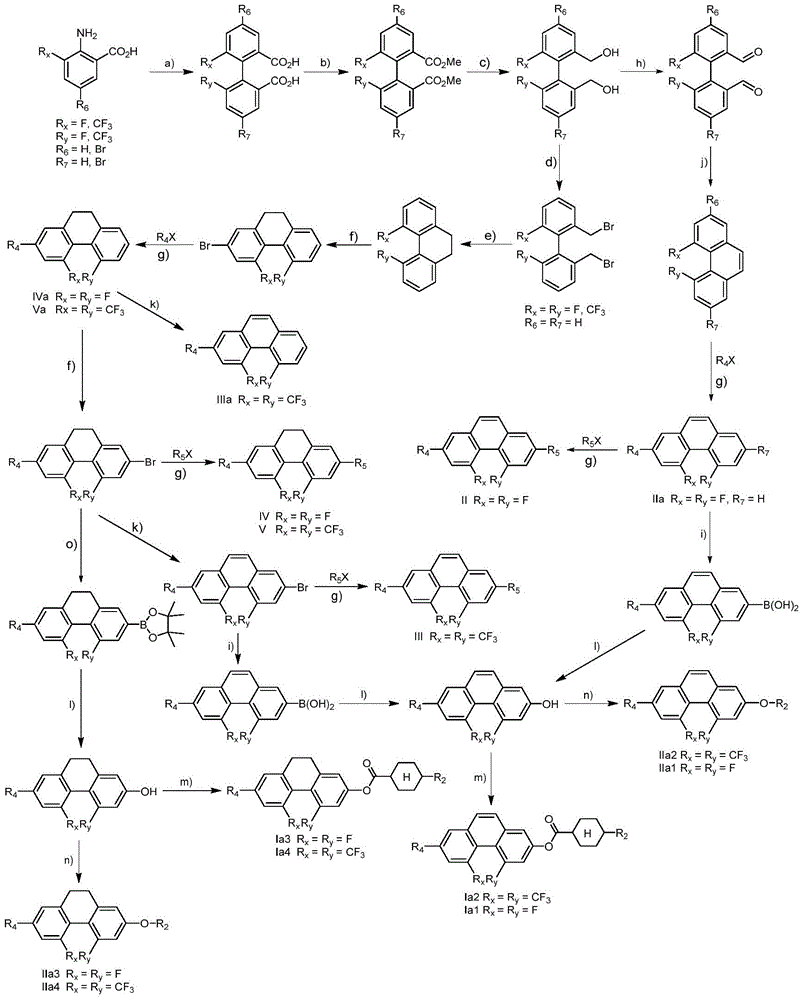
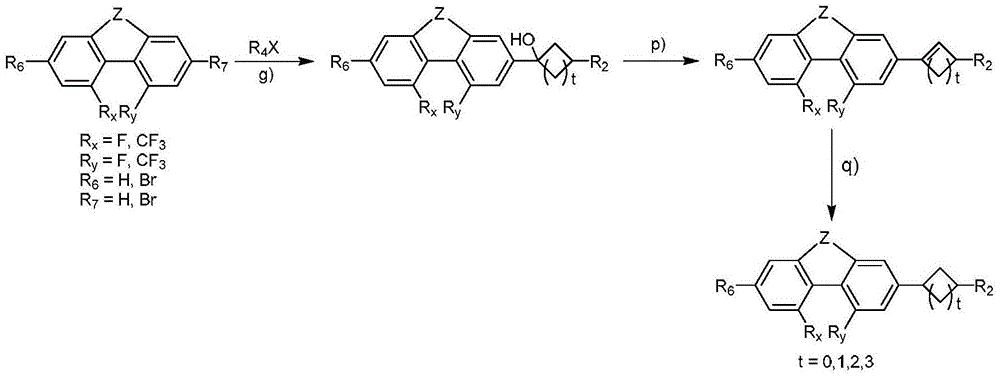
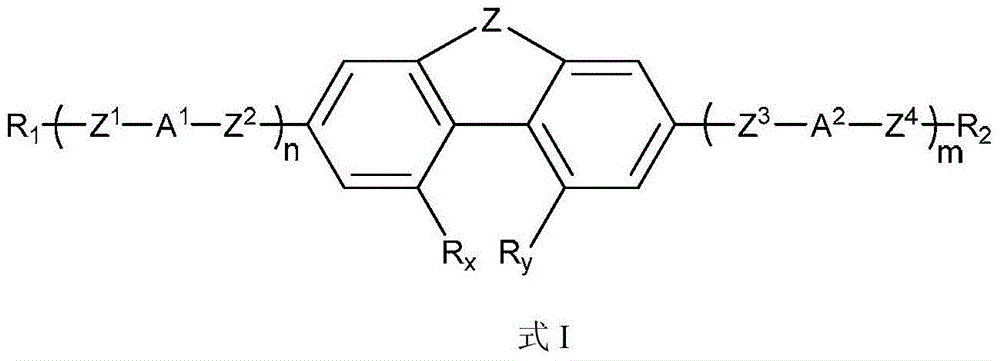
4,5-disubstituted phenanthrene and hydrophenanthrene liquid crystal compound and preparation method thereof
A technology of liquid crystal composition and compound, applied in the field of liquid crystal
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0080] Example 1 Compound 2-(2,3-difluoro-4-n-propylphenyl)-4,5-difluoro-7-(3,5-difluoro-4-trifluoromethoxyphenyl)phenanthrene preparation of
[0081]
[0082] The first step: the preparation of 2-amino-5-bromo-3-fluorobenzoic acid hydrobromide
[0083]
[0084] 153g of 2-amino-3-fluorobenzoic acid dispersed in 1500ml of chloroform, 175g of bromine dissolved in 150ml of chloroform was slowly added dropwise to the above chloroform solution, and stirred at room temperature overnight, filtered the next day, and the filter cake After washing with DCM and vacuum drying, 276 g of 2-amino-5-bromo-3-fluorobenzoic acid hydrobromide was obtained as a yellow powder.
[0085] The second step: Preparation of 4,4'-dibromo-6,6'-difluorobiphenyl-2,2'-dicarboxylic acid
[0086]
[0087] Dissolve 75g of copper sulfate pentahydrate in 300ml of water, add 230ml of 25% ammonia water under stirring, and cool to 0°C with an ice-salt bath. An aqueous solution prepared by 20.8 g of hydroxy...
Embodiment 2
[0115] Example 2 Preparation of compound 2-(3,5-difluoro-4-(trifluoromethoxy)phenyl)-4,5-difluorophenanthrene
[0116]
[0117] Step 1: Preparation of (4-bromo-6,6'-difluorobiphenyl-2,2'-yl)dimethanol
[0118]
[0119] 35g of 4,4'-dibromo-6,6'-difluorobiphenyl-2,2'-dicarboxylate was dissolved in 300ml of dry THF, and the clear solution was cooled to -20°C with a liquid nitrogen bath , slowly add 7g of lithium aluminum hydride in batches, keep stirring for 1 hour, slowly rise to room temperature, stir and react for 5 hours, add water and 20ml of 15% sodium hydroxide aqueous solution dropwise to the reaction solution to quench the reaction, suction filter , the filter cake was washed with THF, the filtrate was concentrated to dryness under reduced pressure, and the residue was separated and purified with a silica gel column to obtain 23.5 g of (4-bromo-6,6'-difluorobiphenyl-2,2'-yl)dimethanol, Yellow oil.
[0120] The second step: the preparation of 4-bromo-6,6'-difluoro...
Embodiment 3
[0134] Preparation of Example 3 Compound 4,5-bis(trifluoromethyl)-2-(3,4,5-trifluorophenyl)-9,10-dihydrophenanthrene
[0135]
[0136] Step 1: Preparation of 6,6'-bis(trifluoromethyl)biphenyl-2,2'-dicarboxylic acid
[0137]
[0138] According to the same operation method as the second step of Example 1, 6,6' can be prepared by replacing 2-amino-5-bromo-3-fluorobenzoic acid hydrobromide with 2-amino-3-trifluoromethylbenzoic acid -Bis(trifluoromethyl)biphenyl-2,2'-dicarboxylic acid, a yellow solid was obtained.
[0139] The second step: Preparation of methyl 6,6'-bis(trifluoromethyl)biphenyl-2,2'-dicarboxylate
[0140]
[0141] According to the same operation method as the third step of Example 1, 6,6'-bis(trifluoromethyl)biphenyl-2,2'-dicarboxylic acid was used instead of 4,4'-dibromo-6,6'- Difluorobiphenyl-2,2'-dicarboxylic acid can be used to prepare methyl 6,6'-bis(trifluoromethyl)biphenyl-2,2'-dicarboxylate as white crystals.
[0142] The third step: Preparation...
PUM
| Property | Measurement | Unit |
|---|---|---|
| isotropization temperature | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| isotropization temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com