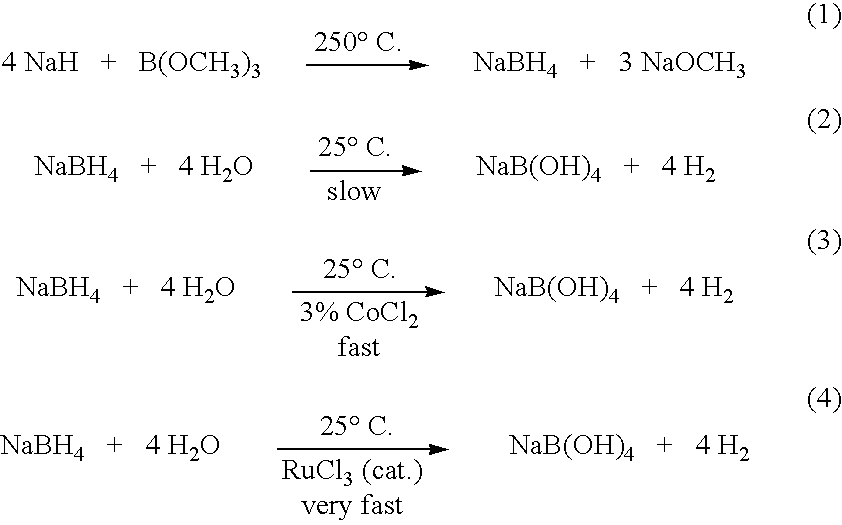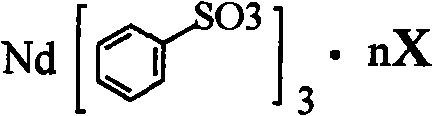Patents
Literature
510 results about "ALUMINUM HYDRIDE" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
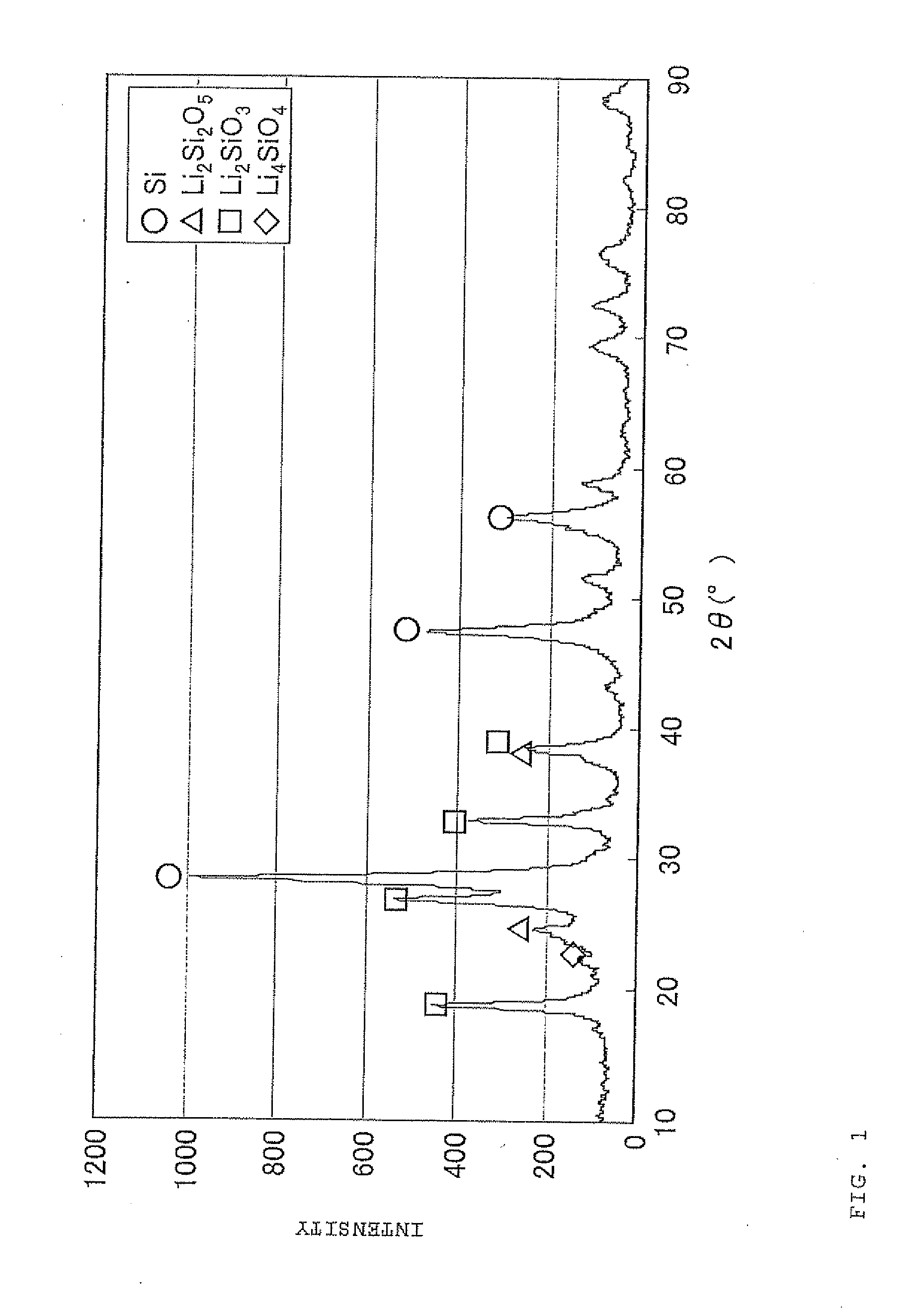
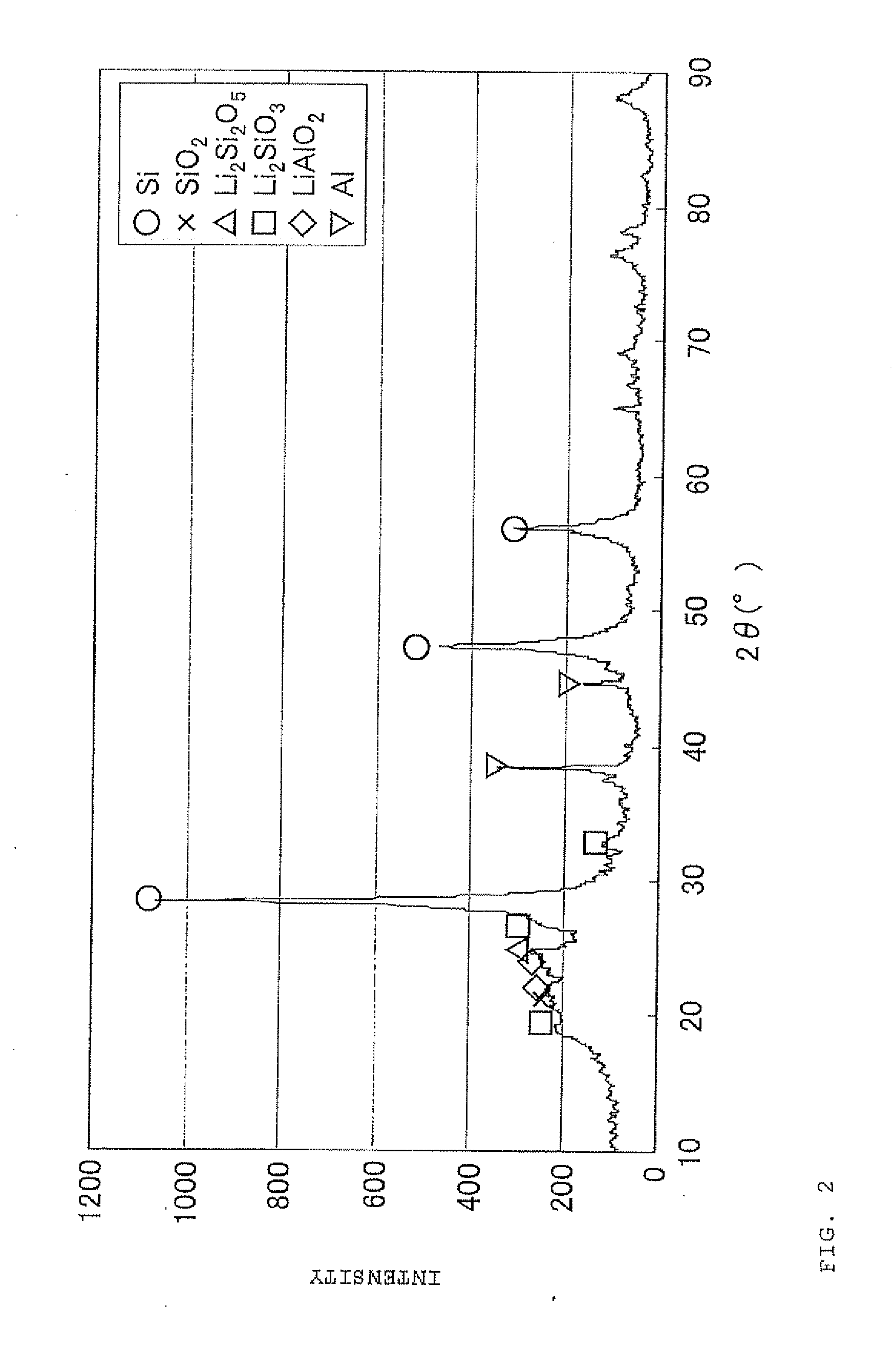
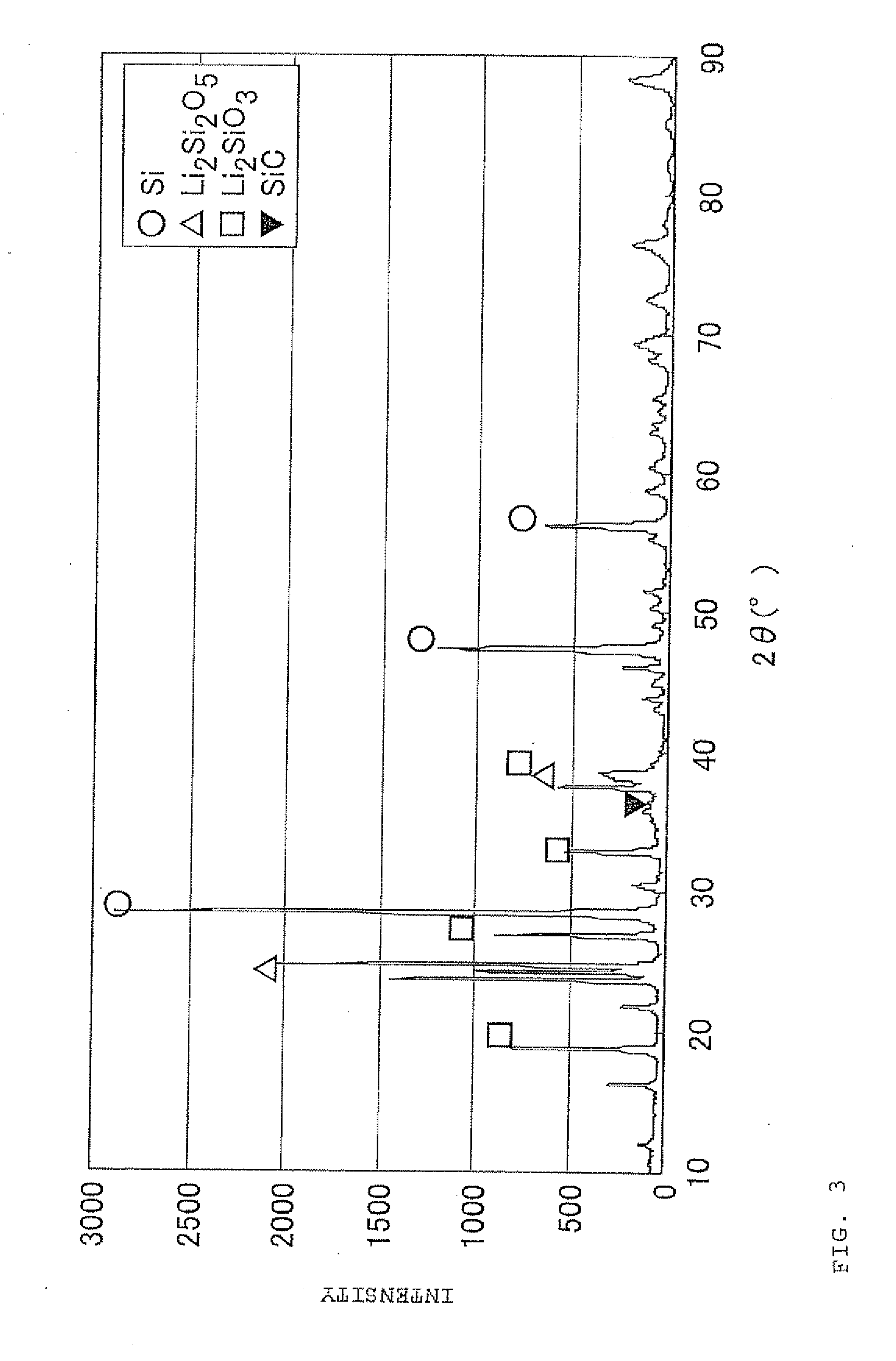
Negative electrode material for secondary battery with non-aqueous electrolyte, method for manufacturing negative electrode material for secondary battery with non-aqueous electrolyte, and lithium ion secondary battery
ActiveUS20110244333A1Cycle durability of negativeElectronic conductivity of negativeMaterial nanotechnologyElectrode thermal treatmentOxide compositeAtomic order
The present invention is a method for manufacturing a negative electrode material for a secondary battery with a non-aqueous electrolyte comprising at least: coating a surface of powder with carbon at a coating amount of 1 to 40 mass % with respect to an amount of the powder by heat CVD treatment under an organic gas and / or vapor atmosphere at a temperature between 800° C. and 1300° C., the powder being composed of at least one of silicon oxide represented by a general formula of SiOx (x=0.5 to 1.6) and a silicon-silicon oxide composite having a structure that silicon particles having a size of 50 nm or less are dispersed to silicon oxide in an atomic order and / or a crystallite state, the silicon-silicon oxide composite having a Si / O molar ratio of 1 / 0.5 to 1 / 1.6; blending lithium hydride and / or lithium aluminum hydride with the powder coated with carbon; and thereafter heating the powder coated with carbon at a temperature between 200° C. and 800° C. to be doped with lithium at a doping amount of 0.1 to 20 mass % with respect to an amount of the powder. As a result, there is provided a method for manufacturing a negative electrode material for a secondary battery with a non-aqueous electrolyte that enables a silicon oxide negative electrode material superior in first efficiency and cycle durability to conventional ones to be mass-produced (manufactured) readily and safely even in an industrial scale.
Owner:SHIN ETSU CHEM IND CO LTD
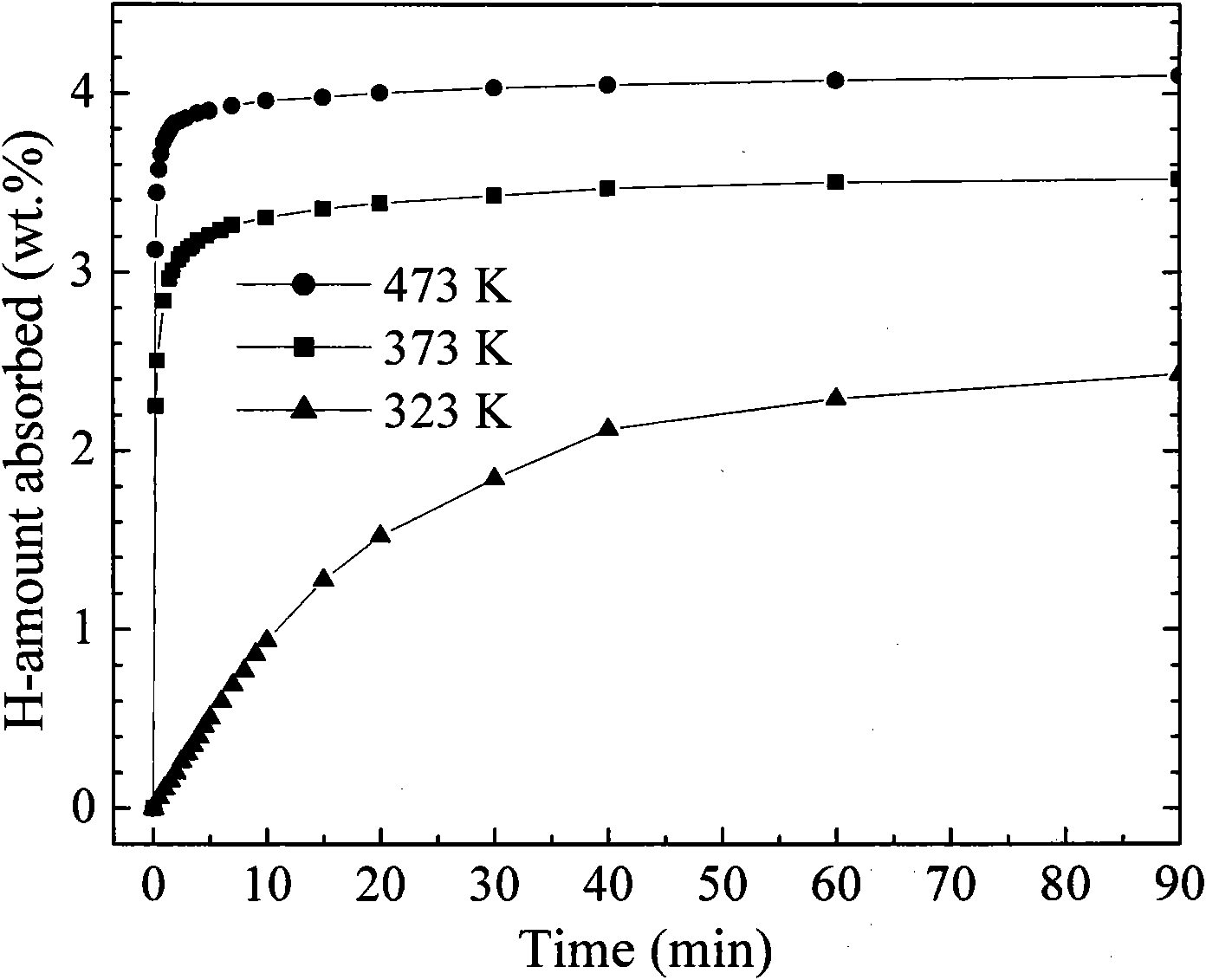
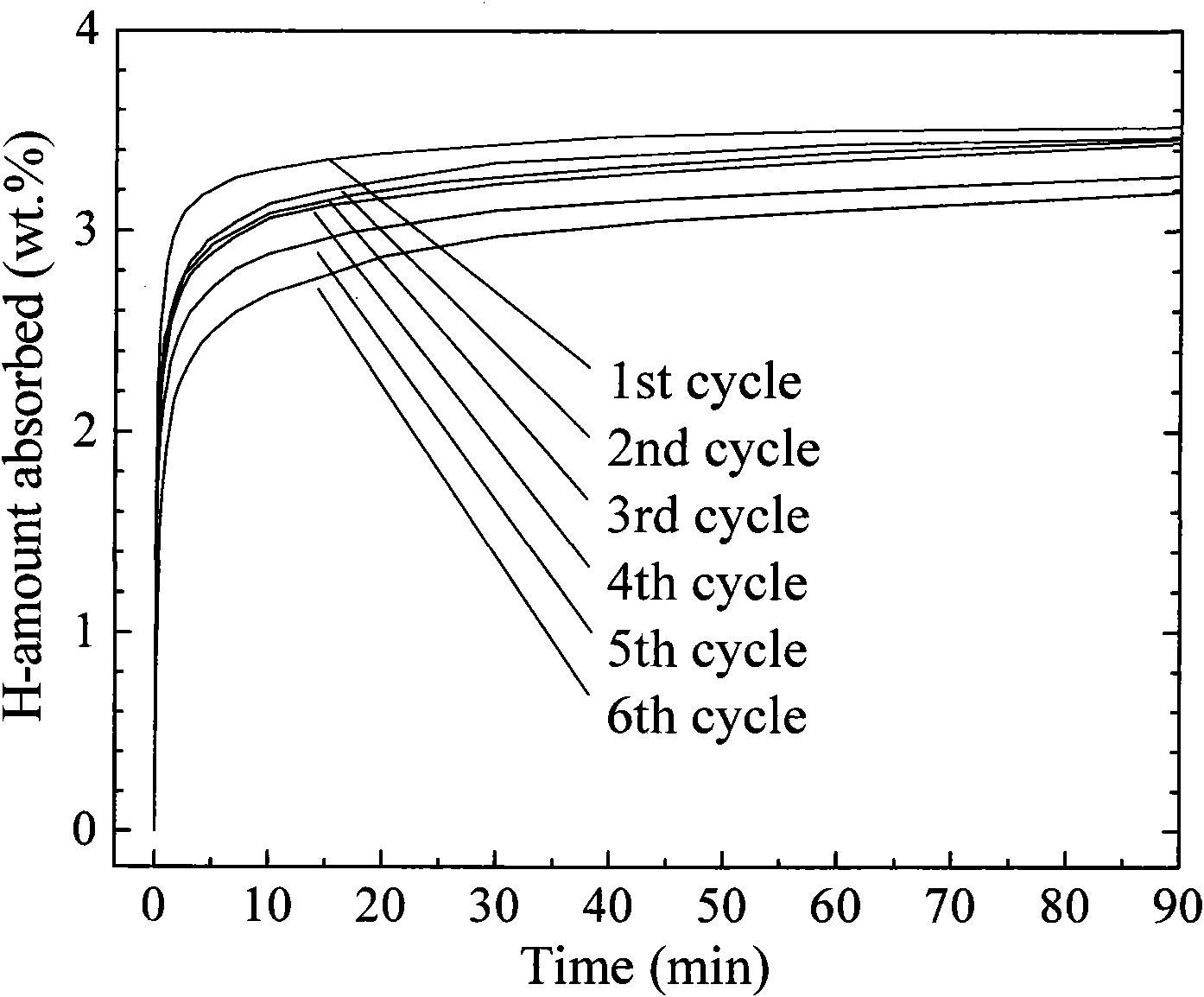
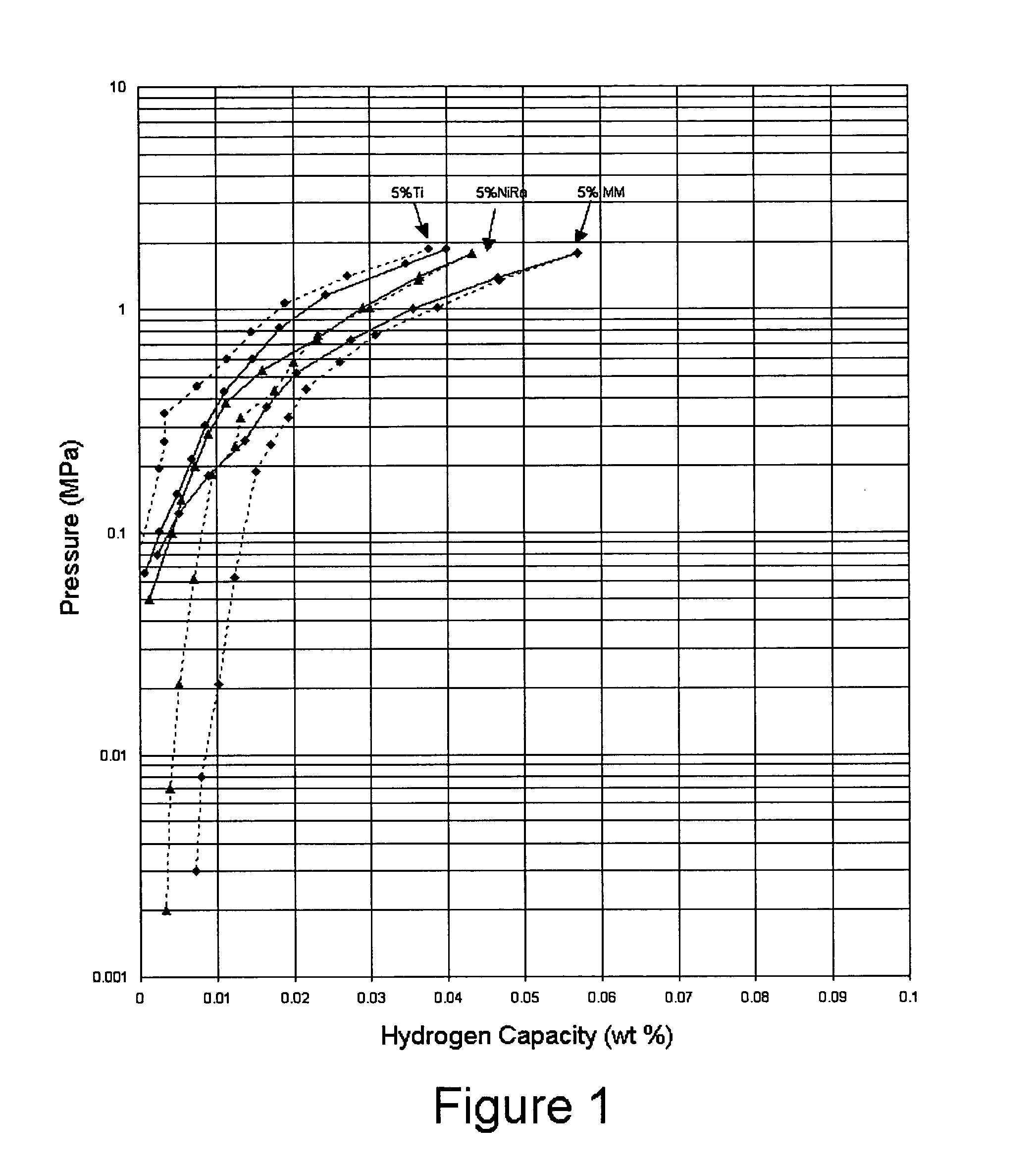
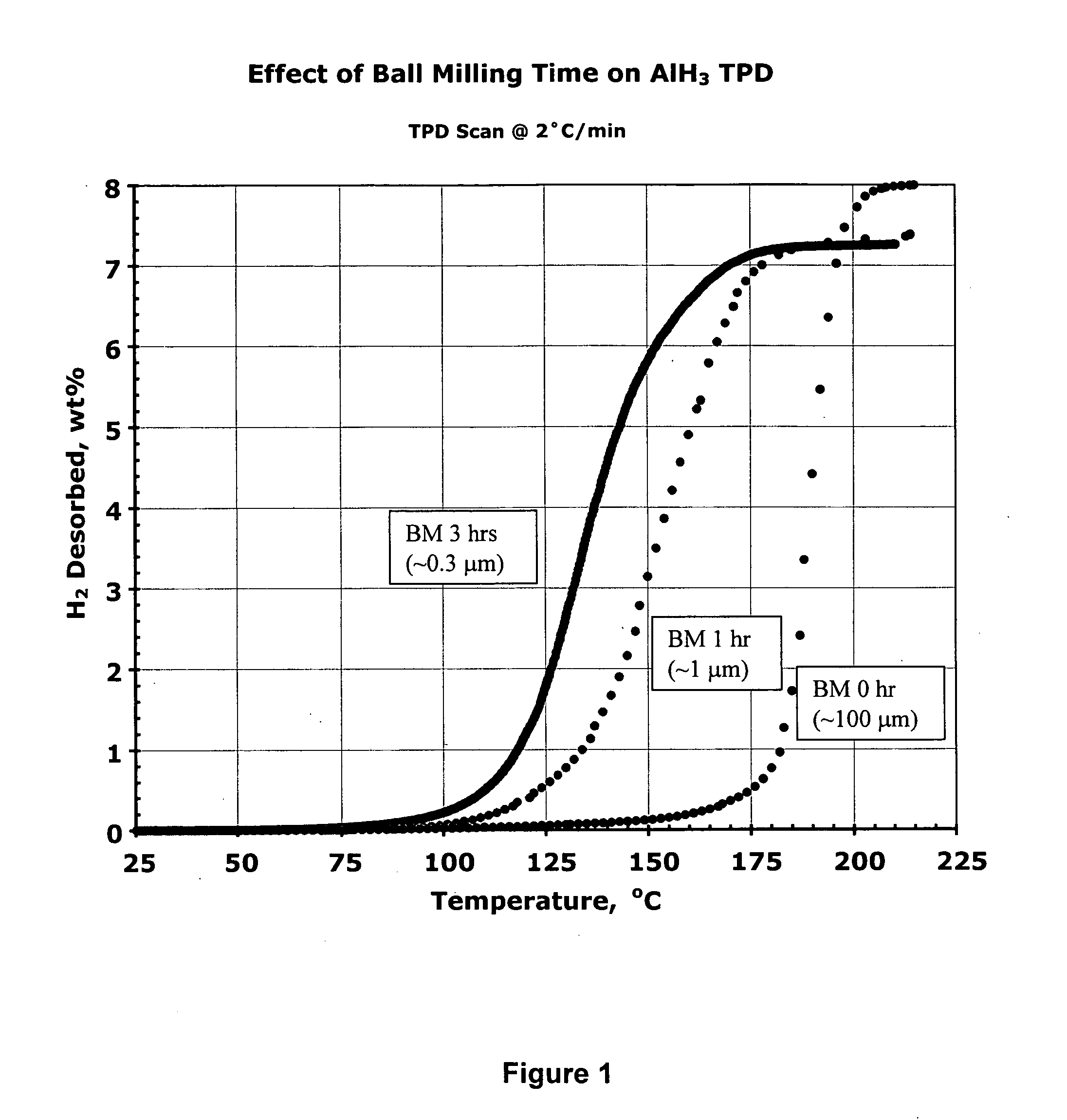
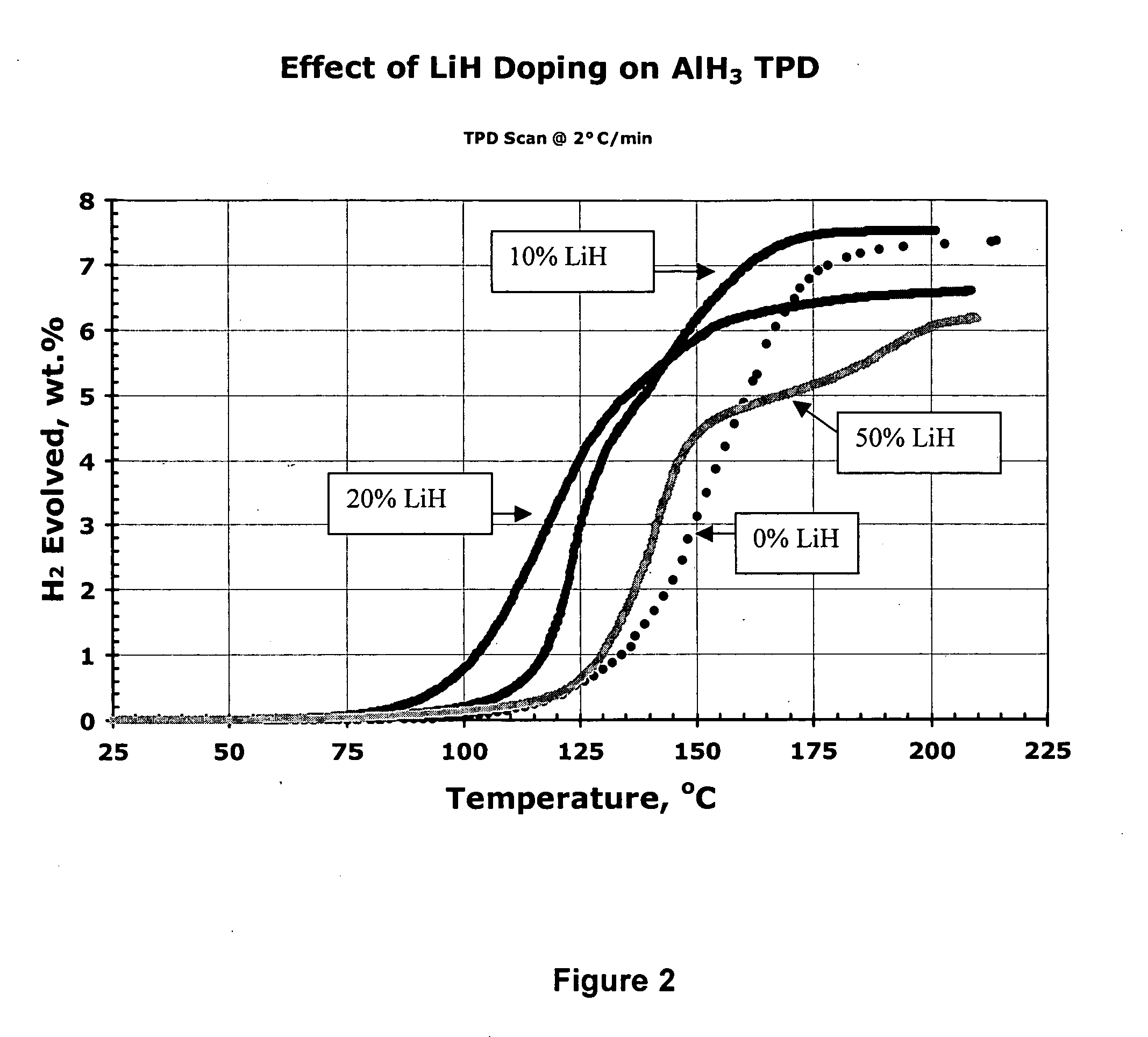
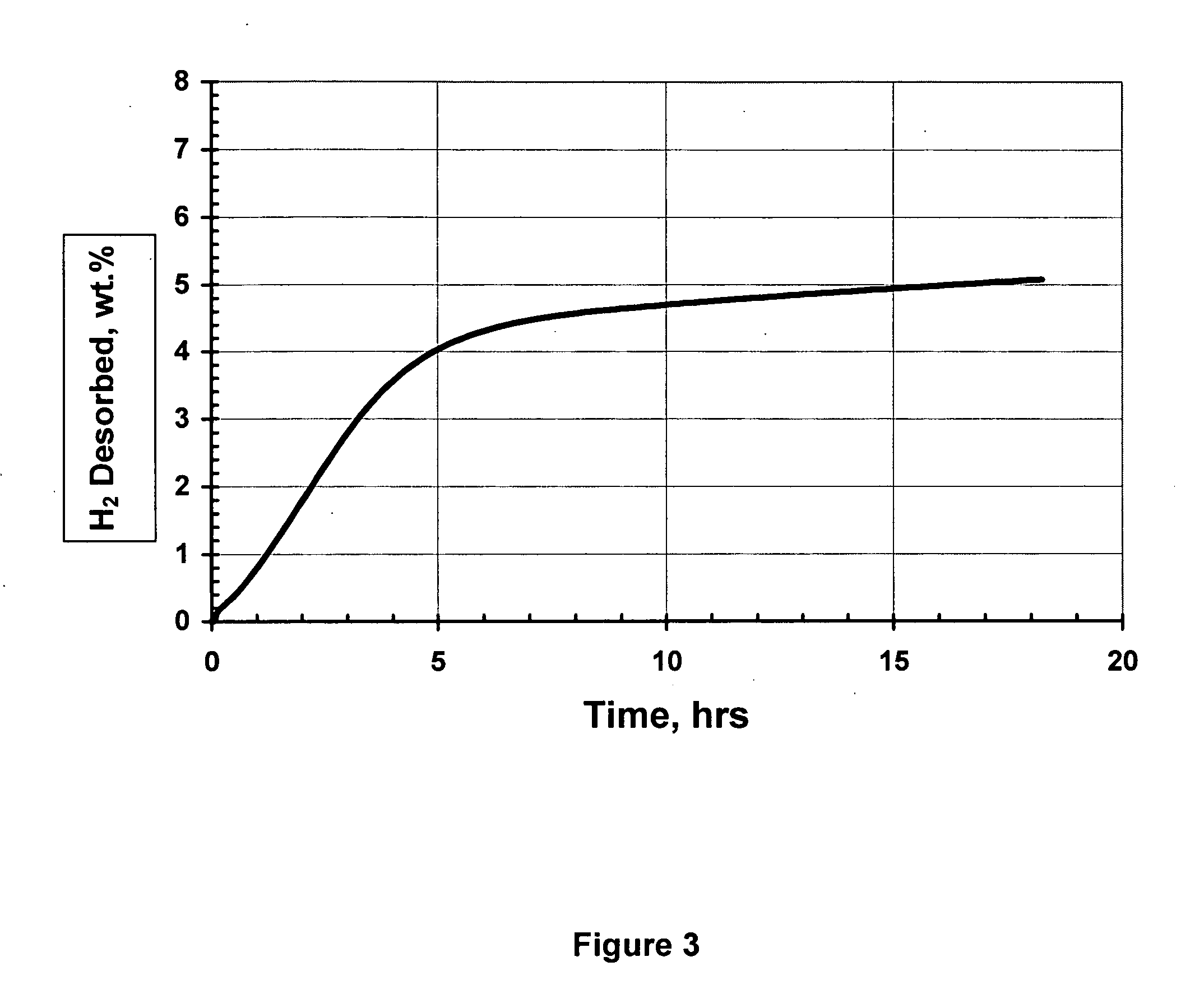
Activated aluminum hydride hydrogen storage compositions and uses thereof
InactiveUS20070025908A1Improve hydrogen storage performanceImproved desorption kineticsAlkali/alkaline-earth/beryllium/magnesium hydridesOther chemical processesHydrogen desorptionALUMINUM HYDRIDE
In one aspect, the invention relates to activated aluminum hydride hydrogen storage compositions containing aluminum hydride in the presence of, or absence of, hydrogen desorption stimulants. The invention particularly relates to such compositions having one or more hydrogen desorption stimulants selected from metal hydrides and metal aluminum hydrides. In another aspect, the invention relates to methods for generating hydrogen from such hydrogen storage compositions.
Owner:BROOKHAVEN SCI ASSOCS
Heavy metal free, environmentally green percussion primer and ordnance and systems incorporating same
A sensitized explosive that comprises an explosive precipitated onto a sensitizer. The explosive is CL-20, PETN, RDX, HMX, or mixtures thereof and the sensitizer is aluminum, titanium, zirconium, magnesium, melamine, styrene, lithium aluminum hydride, or mixtures thereof. The sensitized explosive is used in a percussion primer that includes a bismuth compound and a melt binder. The bismuth compound is bismuth oxide, bismuth subnitrate, bismuth tetroxide, bismuth sulfide, or mixtures thereof and the melt binder is a wax having a melting point above ambient temperature, trinitrotoluene, poly(3,3-bis(azidomethyl)oxetane), poly(3-azidomethyl-3-methyloxetane), ethyl-3,5-dinitrobenzoate, or mixtures thereof. A gun cartridge and other primer-containing ordnance assemblies employing the percussion primer are also disclosed. Methods of forming the sensitized explosive and the percussion primer are also disclosed.
Owner:ORBITAL ATK INC
Method for reducing 8-benzyl-2,8-diazabicyclo[4,3,0]nonane and chiral isomer thereof
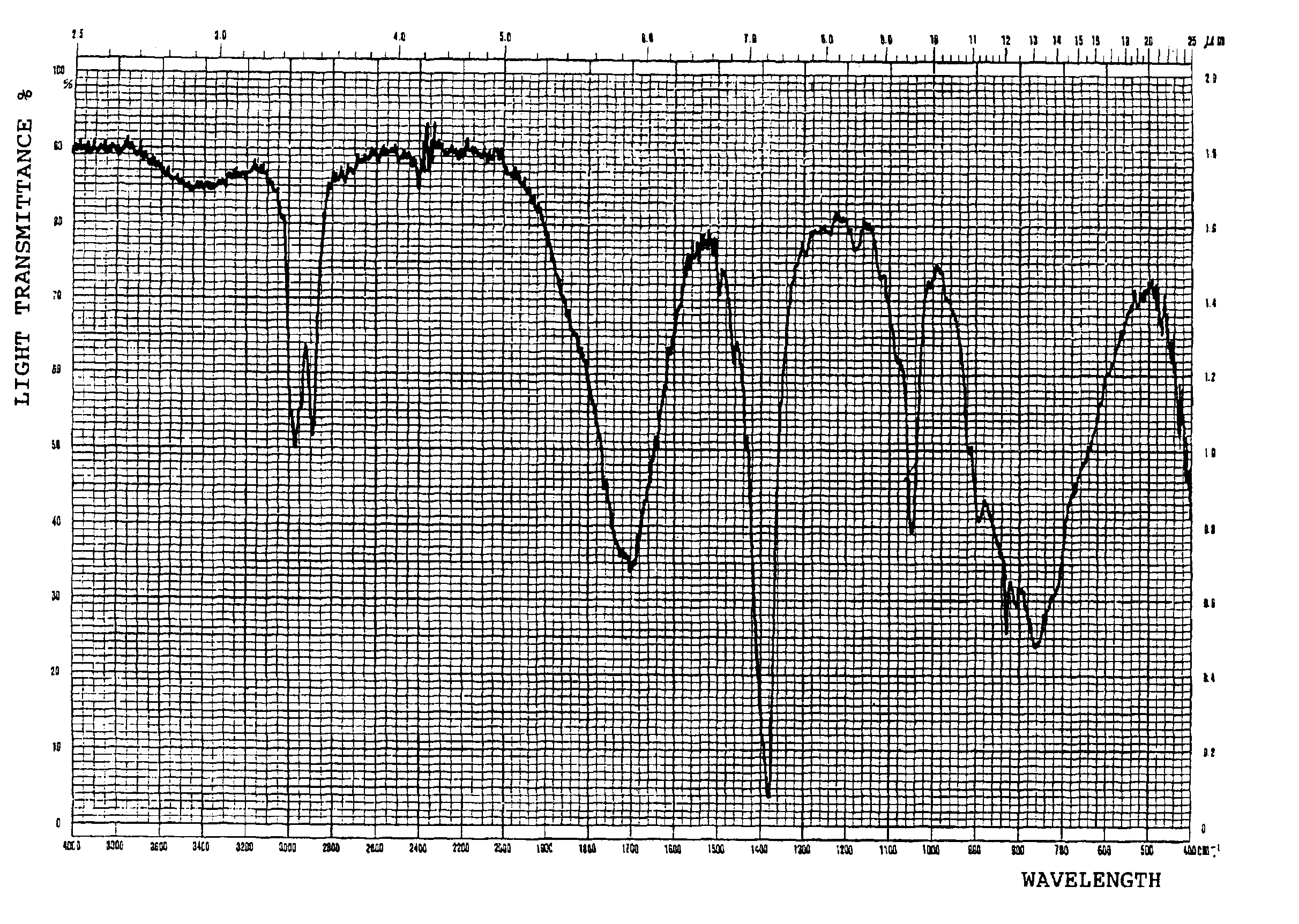
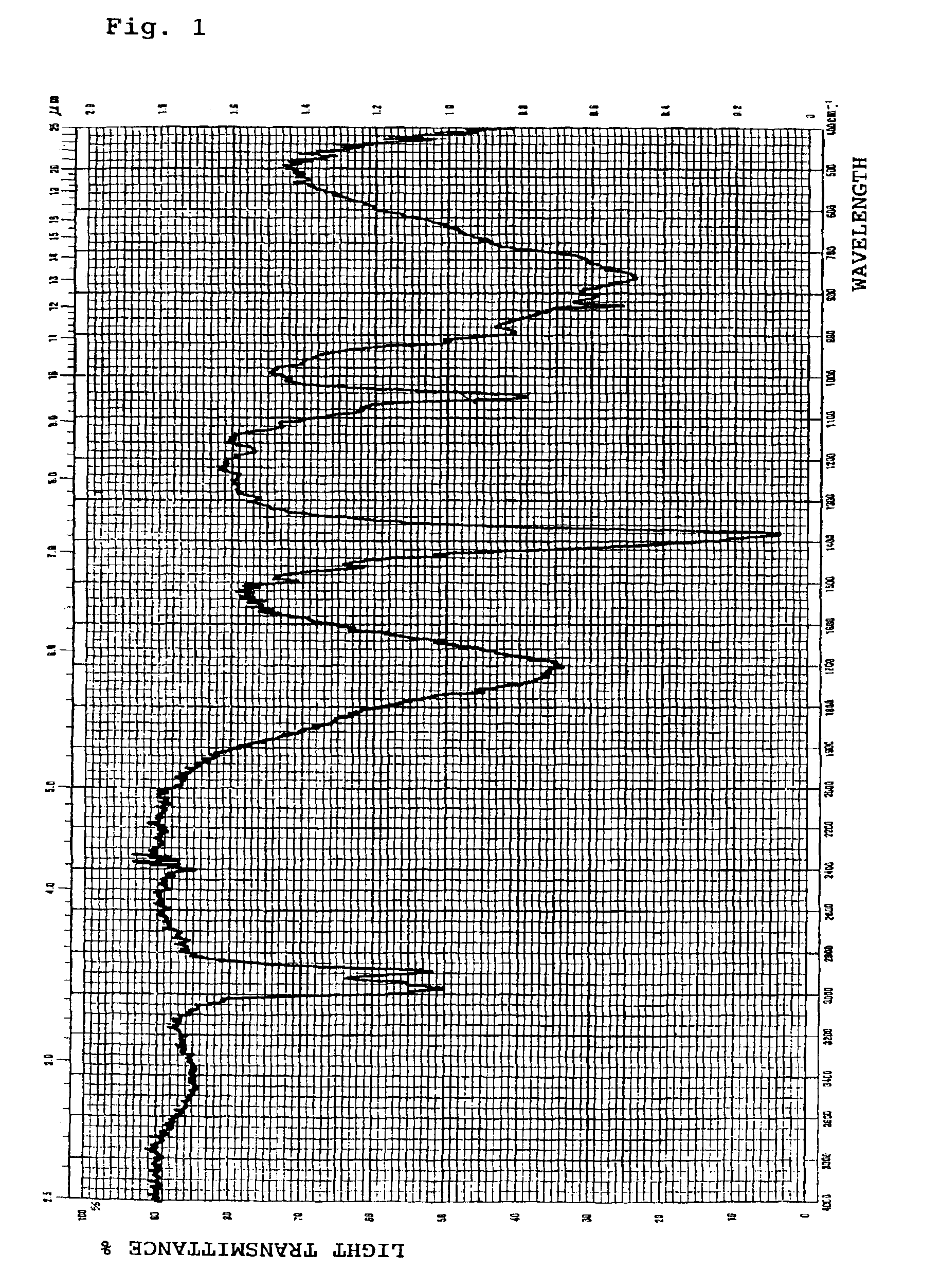
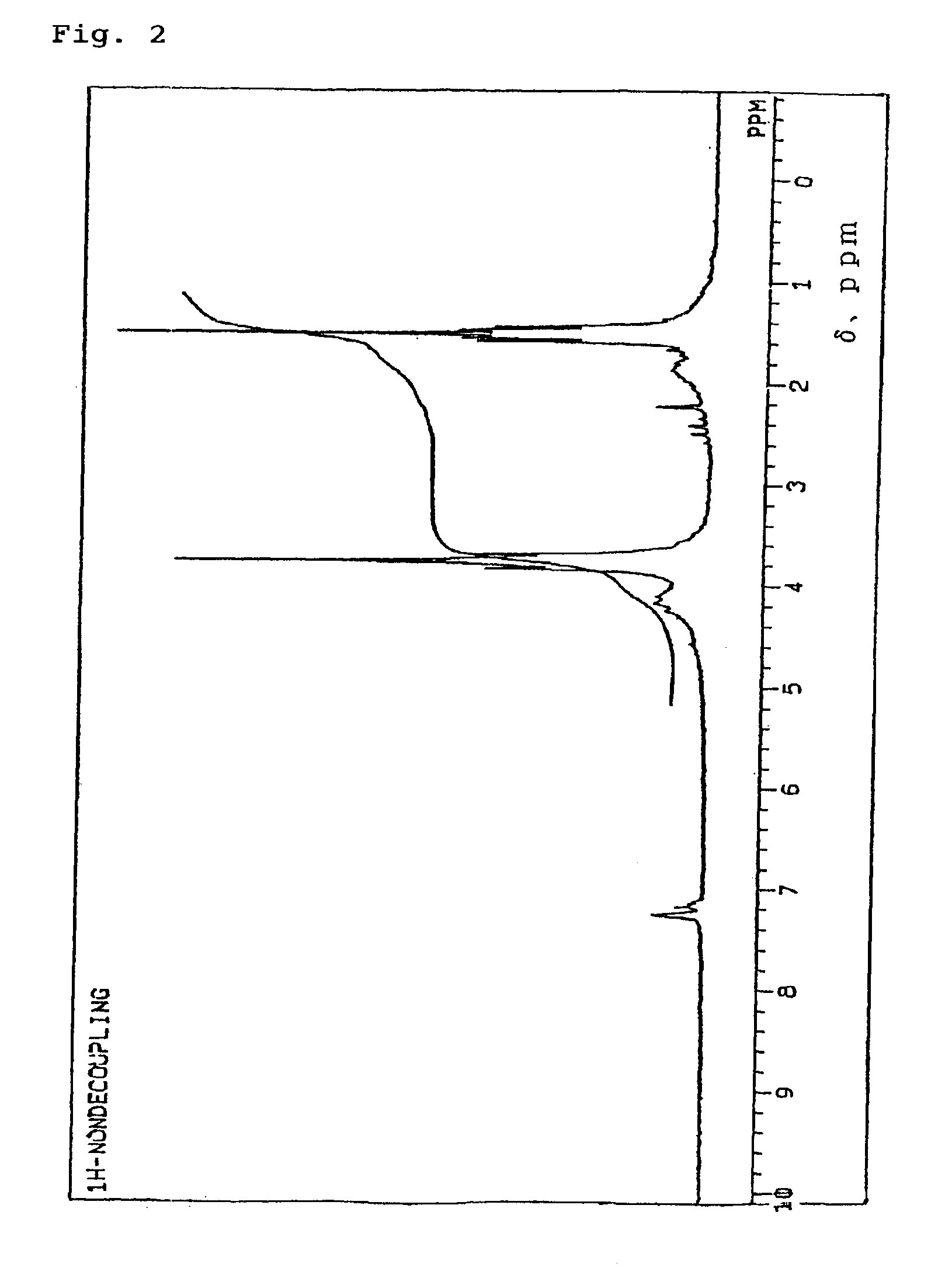
The invention discloses a method for reducing 8-benzyl-2,8-diazabicyclo[4,3,0]nonane and a chiral isomer thereof. The method comprises the steps of: taking 8-benzyl-7,9-dioxo2,8-diazabicyclo[4,3,0]nonane or (1S,6R)-8-benzyl-7,9-dioxo2,8-diazabicyclo[4,3,0]nonane as a raw material, and adopting a metal borohydride / BF3 reduction system for reduction to obtain a corresponding product, namely the 8-benzyl-2,8-diazabicyclo[4,3,0]nonane or (S,S)-8-benzyl-2,8-diazabicyclo[4,3,0]nonane. The method adopts the metal borohydride / BF3 reduction system for reduction, avoids the use of an expensive and dangerous reagent, namely lithium aluminum hydride, reduces production cost, improves the safety of the operation, and provides a safe and economical production method for industrial mass production of a moxifloxacin intermediate, namely the (S,S)-8-benzyl-2,8-diazabicyclo[4,3,0]nonane.
Owner:ZHEJIANG LIAOYUAN PHARM CO LTD
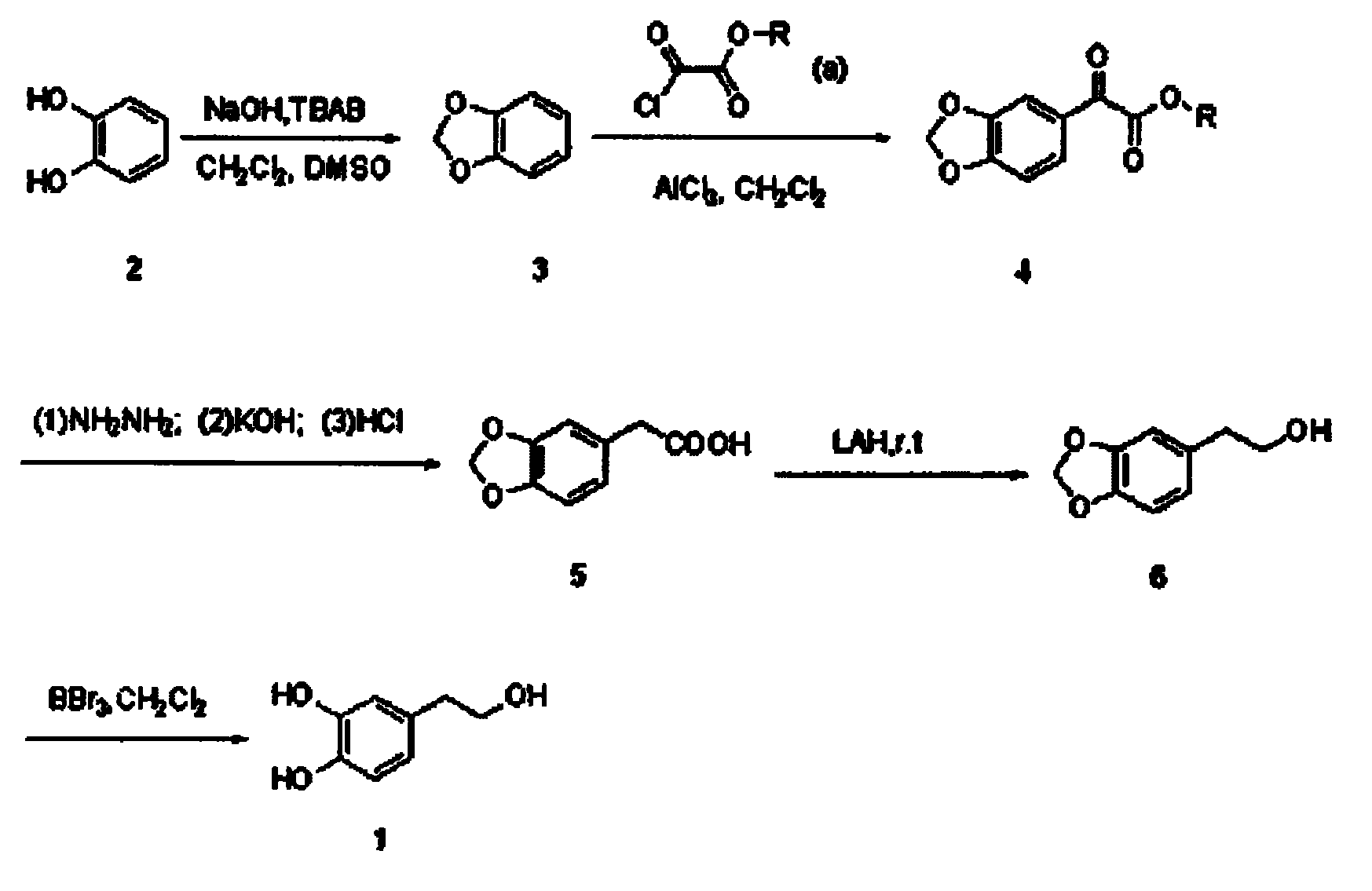
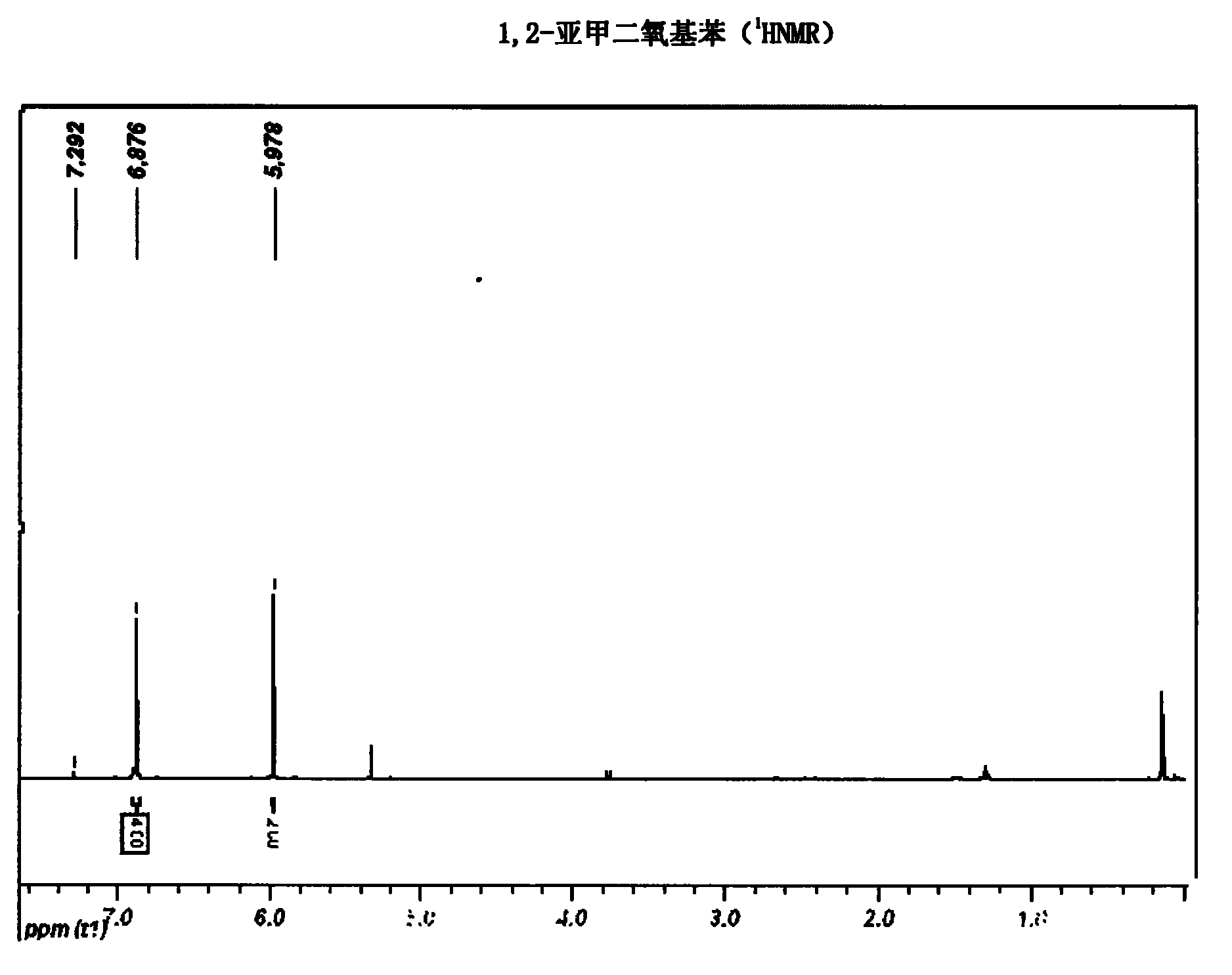
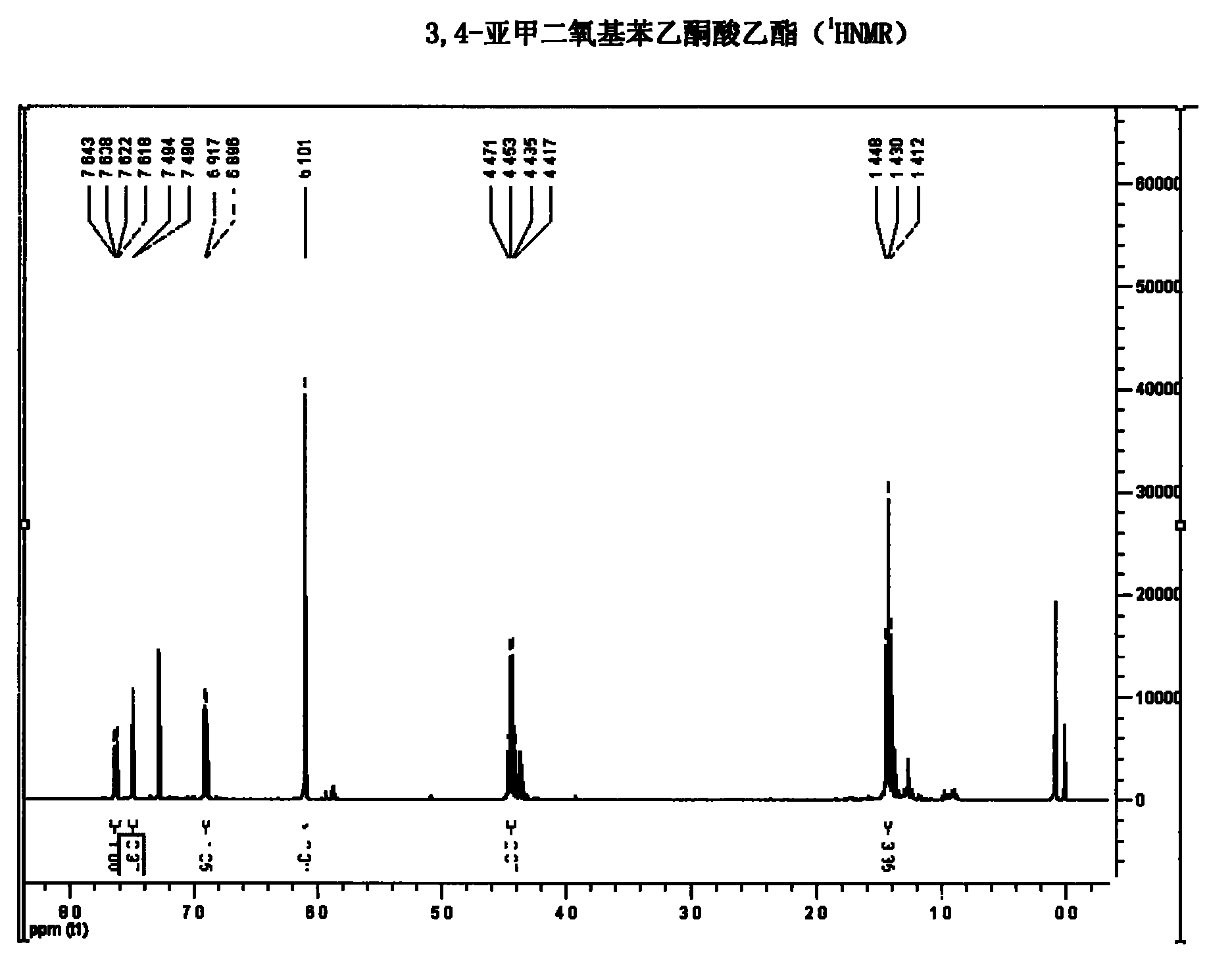
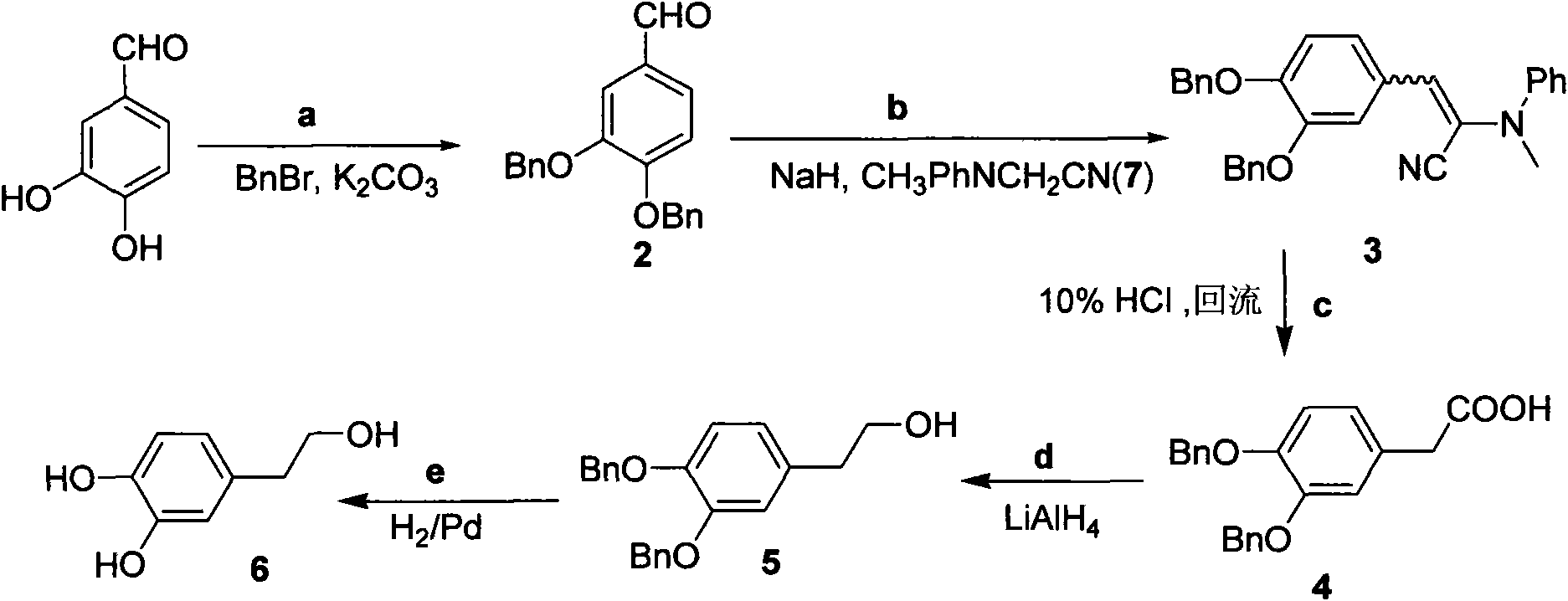
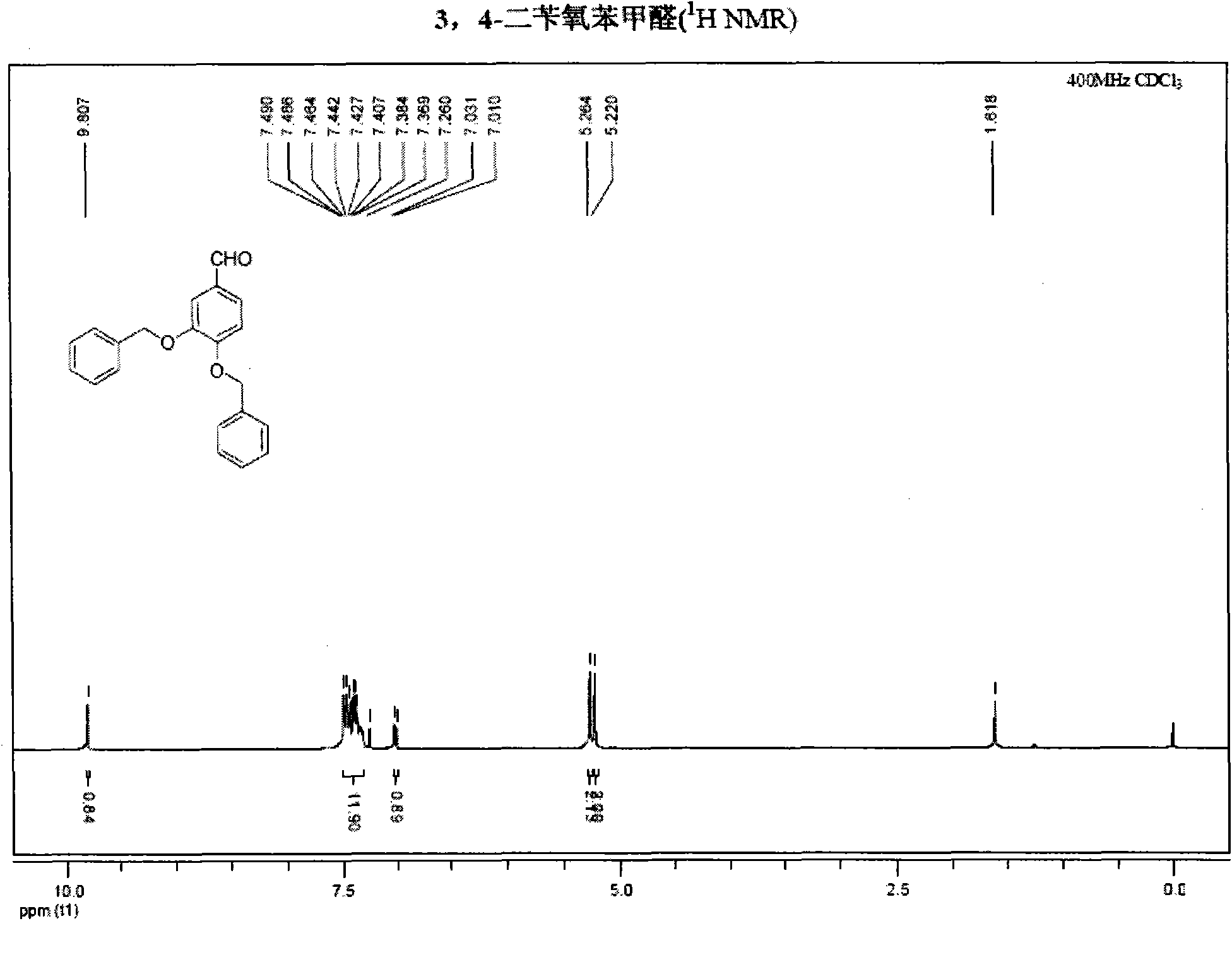
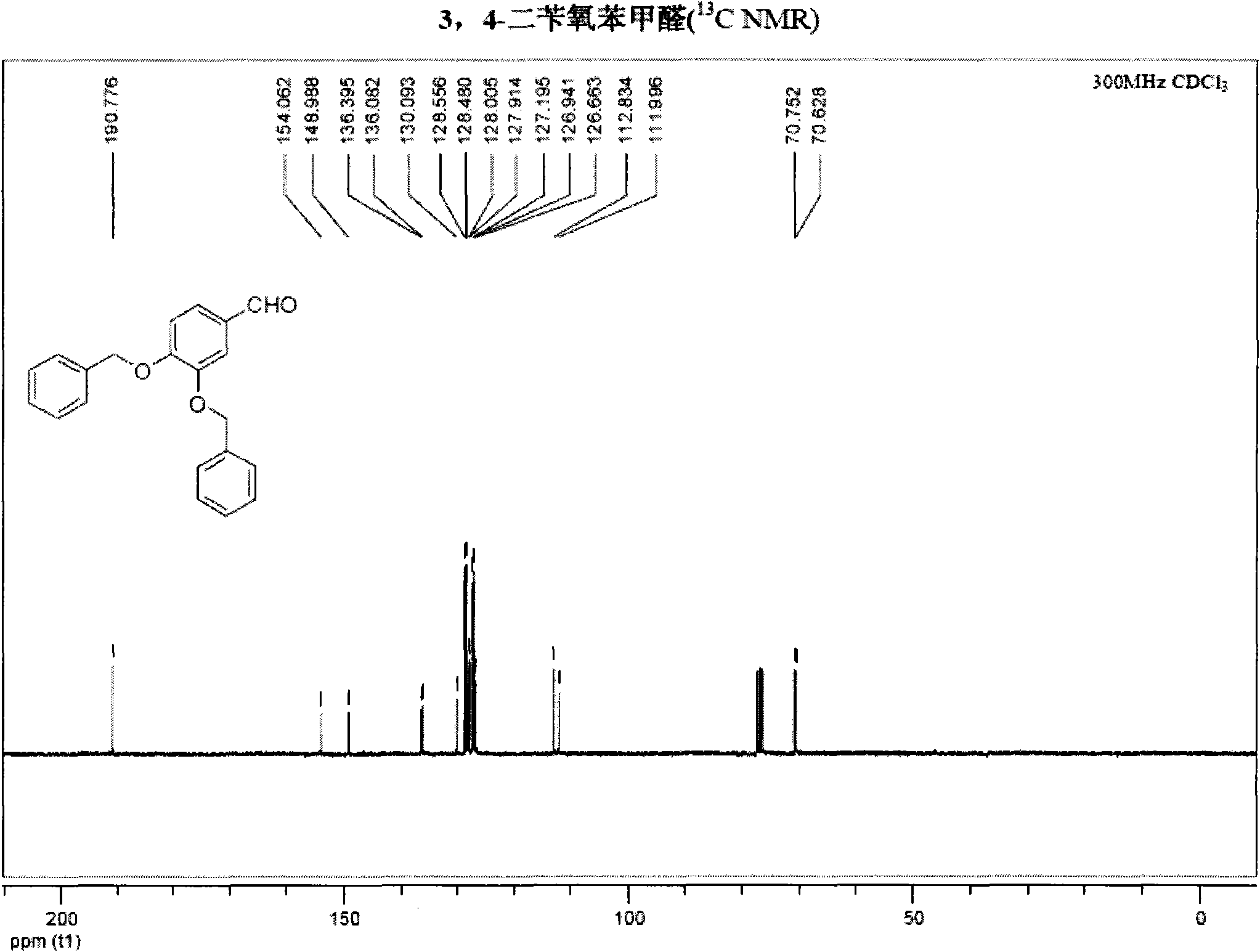
Synthetic method of hydroxytyrosol
InactiveCN103664536AMild reaction conditionsLower reaction costOrganic chemistryOrganic compound preparationChemical synthesisHydroxytyrosol
The invention belongs to the technical field of medicament synthesis, and in particular relates to a chemical synthetic method of hydroxytyrosol. The chemical synthetic method comprises the steps of (1) protecting two phenolic hydroxyl groups of catechol by using dichloromethane, and enabling catechol to react with dichloromethane to prepare 1,2-methylenedioxybenzene; (2) enabling 1,2-methylenedioxybenzene to react with various monoesters of oxalyl chloride to prepare 3,4-methylenedioxy phenylglyoxylic acid ester; (3) preparing 3,4-methylenedioxy phenylacetic acid by using 3,4-methylenedioxy phenylglyoxylic acid ester through a Wollff-kishner-Huang Minglong reduction reaction; and (4) reducing the 3,4-methylenedioxy phenylacetic acid by using lithium aluminum hydride, lithium borohydride or sodium borohydride to prepare 3,4-methylenedioxy phenethyl alcohol, and then removing methylene protection of the 3,4-methylenedioxy phenethyl alcohol by using boron tribromide or palladium on activated carbon to prepare hydroxytyrosol. A reactive reagent used in the synthetic method disclosed by the invention is easy to obtain and low in price, the reaction condition is mild, and the final yield of the whole reaction is 23%.
Owner:TIANJIN UNIVERSITY OF SCIENCE AND TECHNOLOGY
Method for synthesizing low-chloride polyphenylene sulfide resin
Owner:德阳科吉高新材料有限责任公司
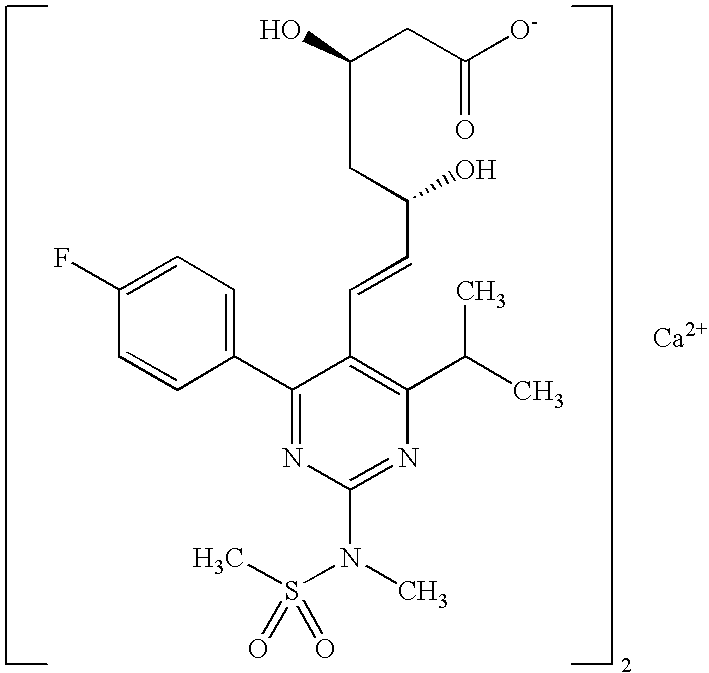
Synthetic Method and Intermediates of Rosuvastatin Calcium and Preparation Methods of Intermediates
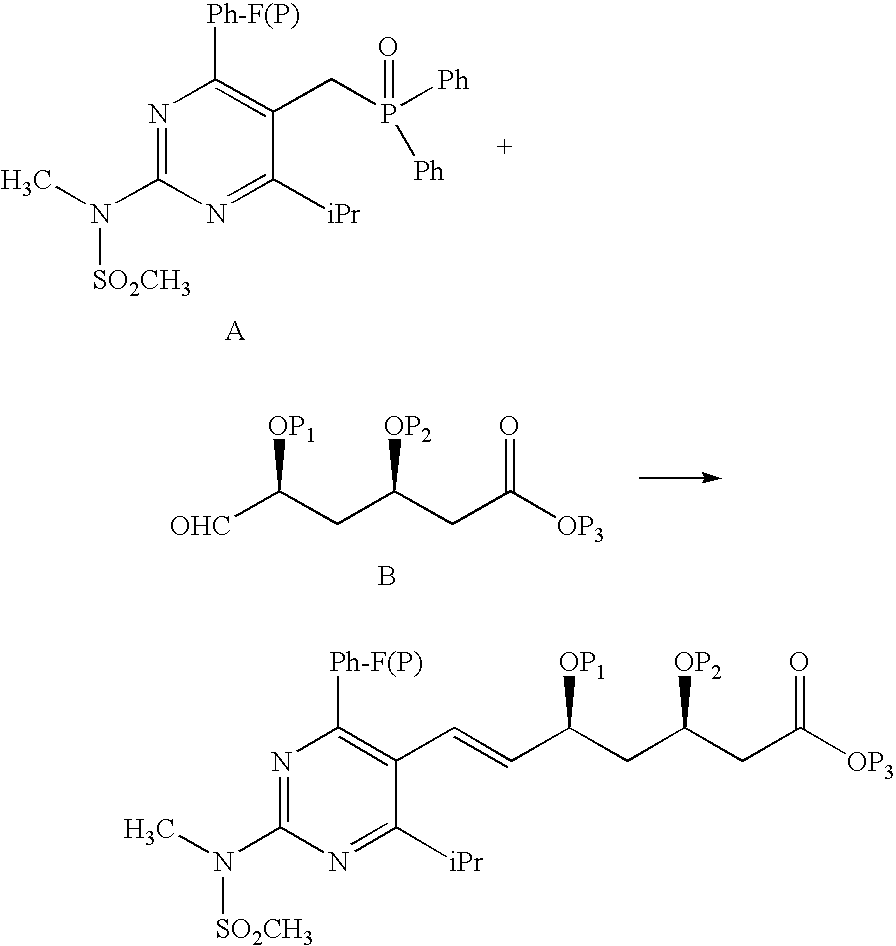
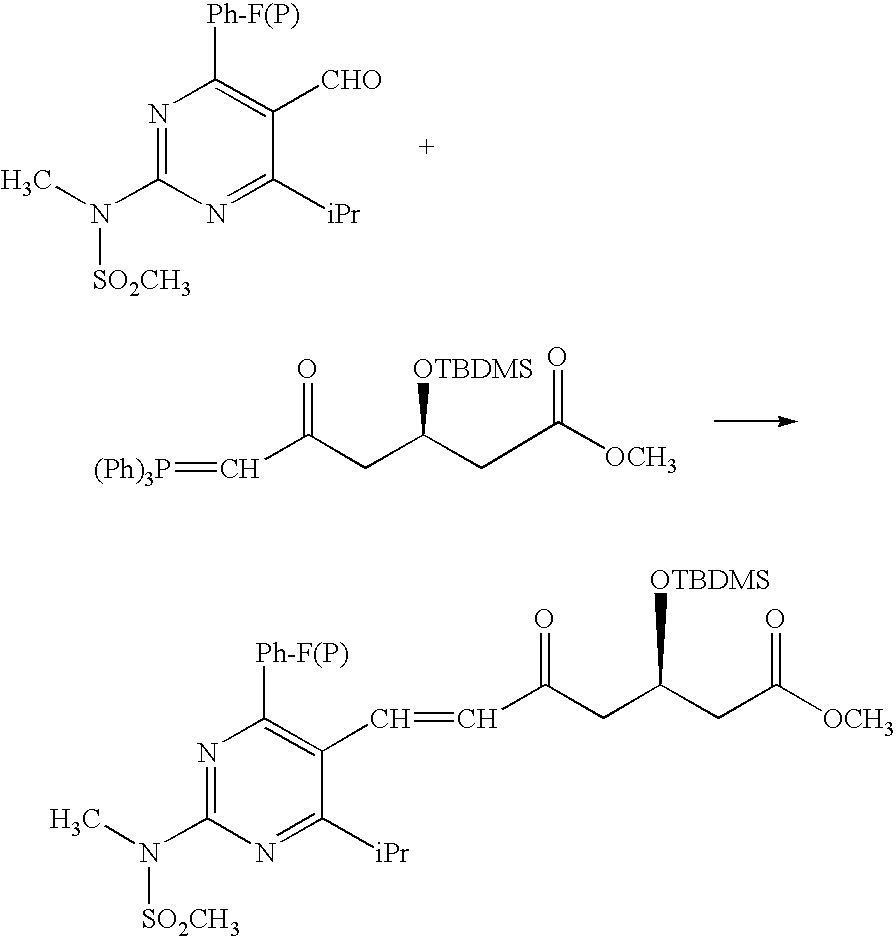
The present invention publicly discloses a synthetic method and intermediates of rosuvastatin calcium and synthetic methods of the intermediates. The synthetic method uses 4-4′-fluorophenyl-6-isopropyl-2-(N-methyl-N-methylsulfonylamino)pyridine-5-formaldehyde as the raw material, includes 4-4′-fluorophenyl-6-isopropyl-2-(N-methyl-N-methylsulfonylamino)pyridine-5-acrylonitrile (intermediate I) from a nitrilized reaction, and 4-4′-fluorophenyl-6-isopropyl-2-(N-methyl-N-methylsulfonylamino)pyridine-5-acraldehyde (intermediate II) from an aldehydized reaction of the intermediate I, and further goes through such unit processes as side-chain extension, ketone-group reduction, ethyl-group hydrolysis and neutralization reaction or decomposition reaction to obtain rosuvastatin calcium. The nitrilized reagent can be phosphate diethylacetonitrile, acetonitrile, etc.; the aldehyde reductant can be diisobutyl aluminum hydride, red aluminum, etc.; and the ketone-group reductant can be diethylmethoxyborane, NaBH4, KBH4, etc.
Owner:ANHUI QINGYUN PHARMA & CHEM
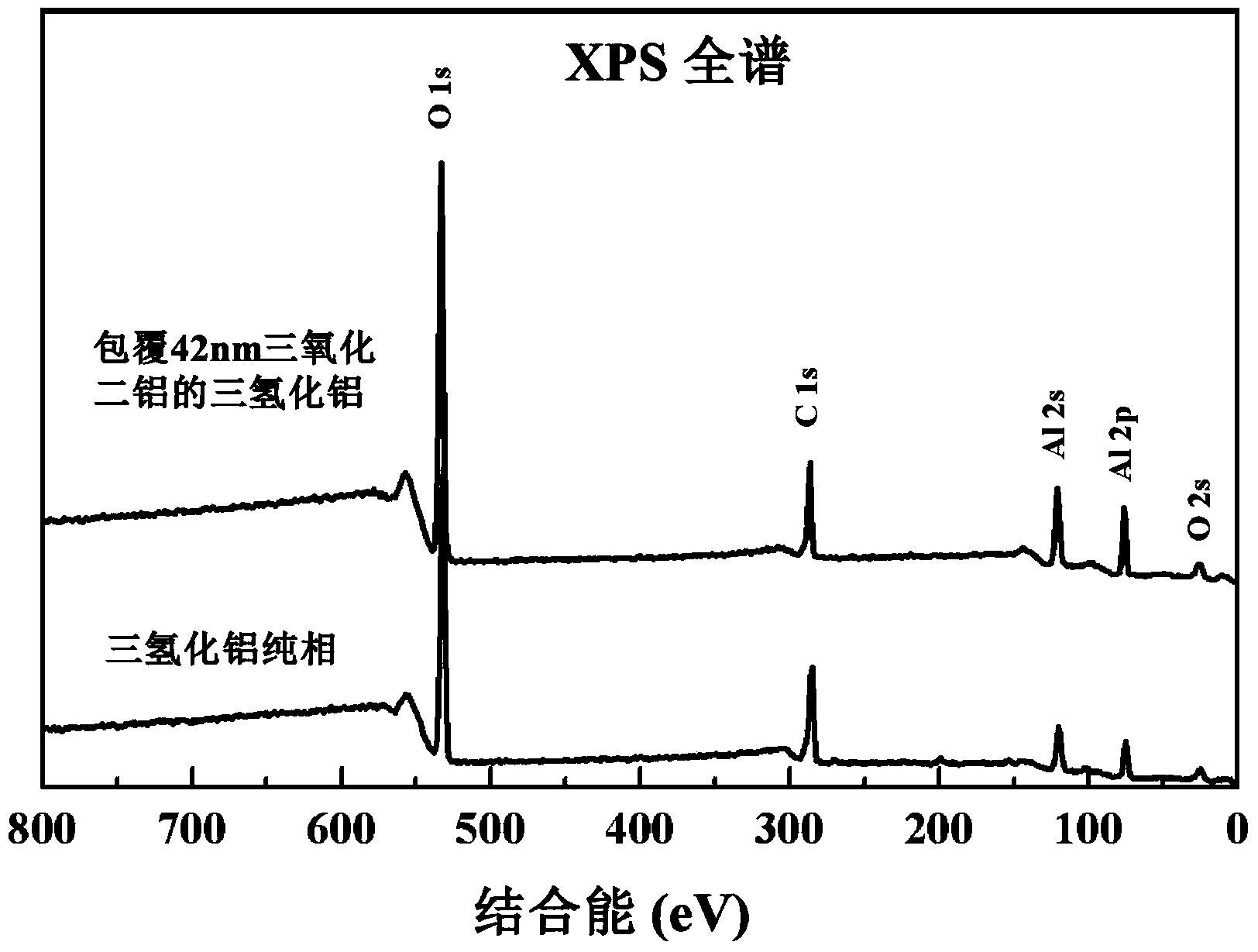
Aluminum hydride surface coating modification method
ActiveCN104046957AImprove stabilityReduce hydrogen contentChemical vapor deposition coatingHeat stabilityALUMINUM HYDRIDE
The invention discloses an aluminum hydride surface coating modification method. A metal oxide or a metal substance with a nanometer thickness is deposited on the aluminum hydride powder surface to clad the aluminum hydride powder surface by adopting an atomic layer deposition technology, so as to improve the heat stability of the aluminum hydride powder. The method comprises the following steps: S1, putting aluminum hydride powder into a cavity and vacuumizing; S2, introducing a fluidized gas after the cavity is heated to the set temperature and the temperature is even and stable, so that aluminum hydride is pre-dispersed; S3, atomic layer deposition reaction, carrying out the atomic layer deposition reaction when the temperature inside the cavity achieves 50-130 DEG C; S4, repeating the atomic layer deposition reaction for a plurality of times, so that the powder surface deposition thickness is continuously increased, and controlling the thickness of the metal oxide or the metal deposited on the surface of the aluminum hydride powder by controlling the circulating times of the deposition reaction. Thus, a cladding layer of which the cladding thickness is 1-1,000nm is cladded on the surface of the aluminum hydride powder, so as to achieve stabilization of powder.
Owner:HUAZHONG UNIV OF SCI & TECH
Method of controlled alcoholysis and regeneration of a borohydride
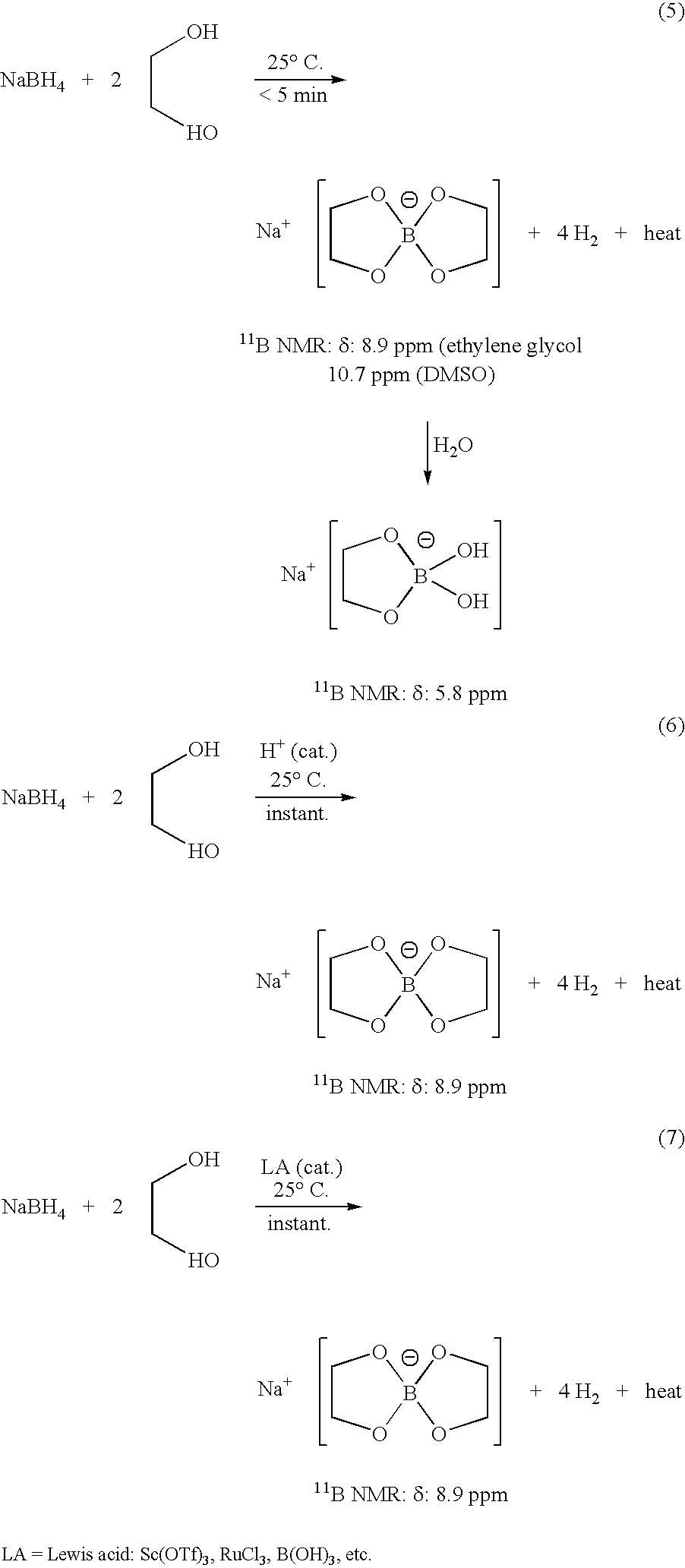
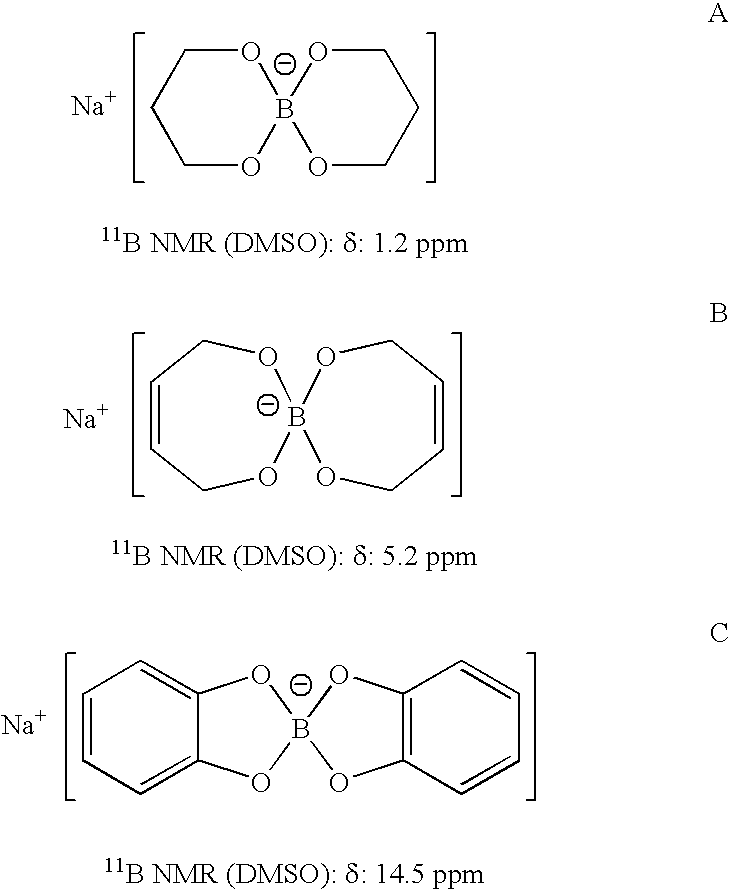
InactiveUS20050255024A1Efficient procedureEffective protocolMonoborane/diborane hydridesBoron/boridesSodium borohydrideBoric acid
Methods of controlled hydrolysis / alcoholysis and regeneration of a borohydride are disclosed. Examples of the present invention show that hydrolysis of sodium borohydride or lithium borohydride with dilute acid provides simultaneous generation of H2 and boric acid for recycling. Other examples of the present invention show methods for regenerating a borohydride by reacting an aluminum hydride to a borate compound to provide a regenerated borohydride.
Owner:PURDUE RES FOUND INC
Conductive film forming composition, conductive film, and method for forming the same
ActiveUS6953600B2Easy and inexpensiveSuitable for useSemiconductor/solid-state device detailsSolid-state devicesOrganic solventOptoelectronics
There are provided a conductive film forming composition capable of forming wiring or an electrode which can be suitably used in a variety of electronic devices, easily and inexpensively, a method for forming a film using the composition, a conductive film formed by the method, and wiring or an electrode which comprises the film.A conductive film forming composition comprising a complex of an amine compound and aluminum hydride and an organic solvent is applied on a substrate and then subjected to a heat treatment and / or irradiation with light, whereby a conductive film such as an electrode or wiring is produced.
Owner:JSR CORPORATIOON +1
Mg-based composite hydrogen storage material containing alkaline earth metals-aluminum hydride and preparation method thereof
InactiveCN101549854AImprove hydrogen storage performanceHigh hydrogen storage capacityHydrogen productionAlkaline earth metalHydrogen fuel cell
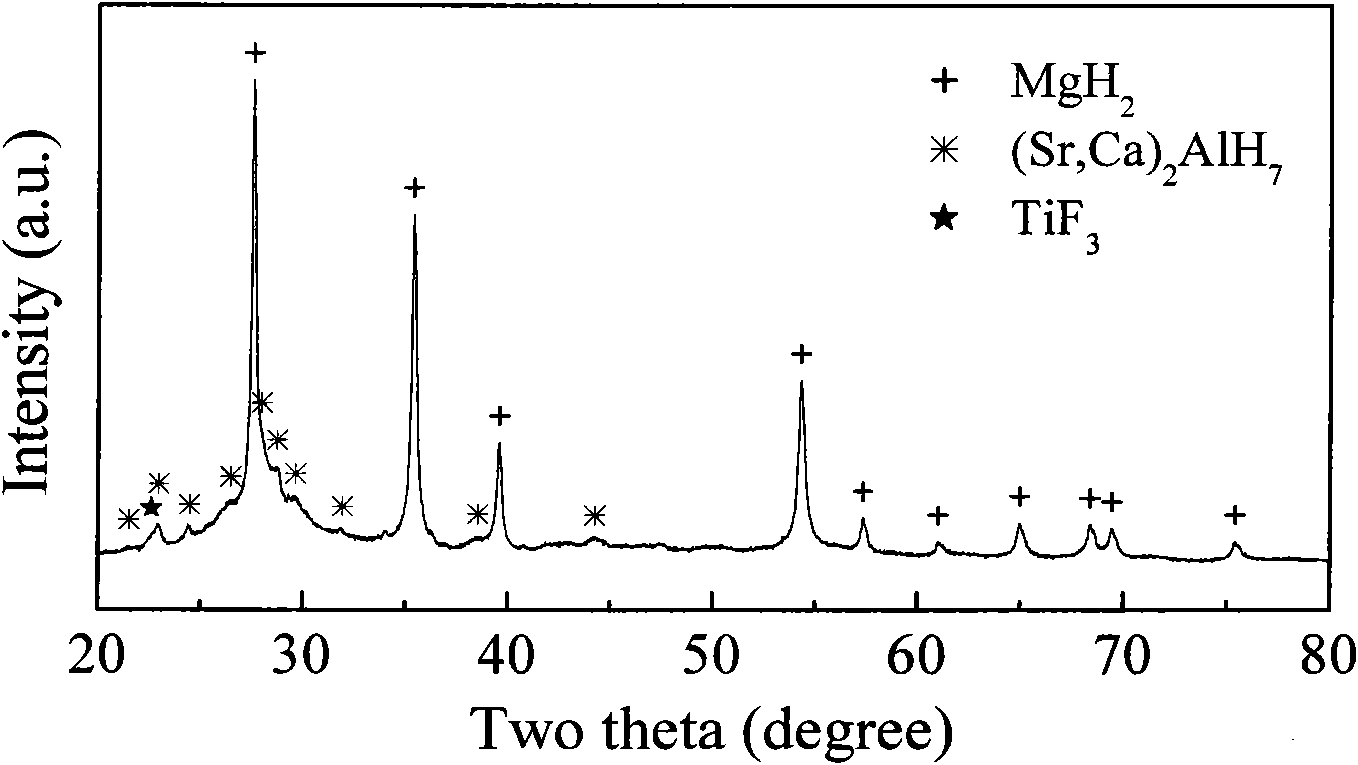
The invention provides an Mg-based composite hydrogen storage material containing alkaline earth metals-aluminum hydride and a preparation method thereof, which belongs to the technical field of hydrogen storage material. The chemical general formula of the hydrogen storage material is MgH2+x wt.%(Sr1-yCay)2AlH7+z wt.%TiF3, wherein x is more than or equal to 30 and less than or equal to 50, y is more than or equal to 0 and less than or equal to 0.5, and z is more than or equal to 2 and less than or equal to 10. The hydrogen storage material is gained by mechanically ball milling three materials powder of MgH2, (Sr, Ca)2AlH7 and TiF3, a planetary ball mill is adopted when ball milling, the ball material ratio is 15:1 to 20:1, the rotational speed is 350 to 400rpm, the ball milling time is 10 to 20h, the ball milling protecting atmosphere is argon gas or hydrogen gas, and the atmosphere pressure is 1 to 5atm. The invention has the advantages that: the preparation technique is simple, the provided composite hydrogen storage materials do not need to be activated, and have high hydrogen storage capacity, simultaneously have good low temperature hydrogen absorption dynamic performance and high hydrogen absorption and desorption cycling stability. The invention is applicable to the safe and efficient storage and transportation of the hydrogen, especially the hydrogen fuel cells and other fields.
Owner:ANHUI UNIVERSITY OF TECHNOLOGY
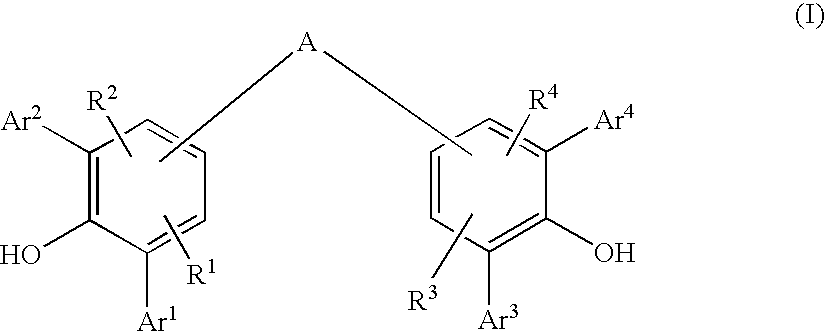
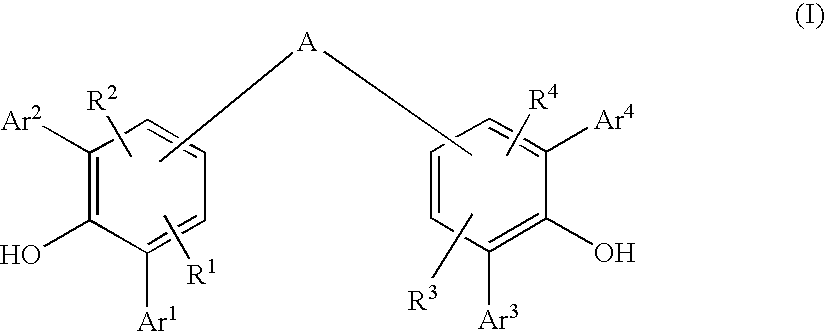
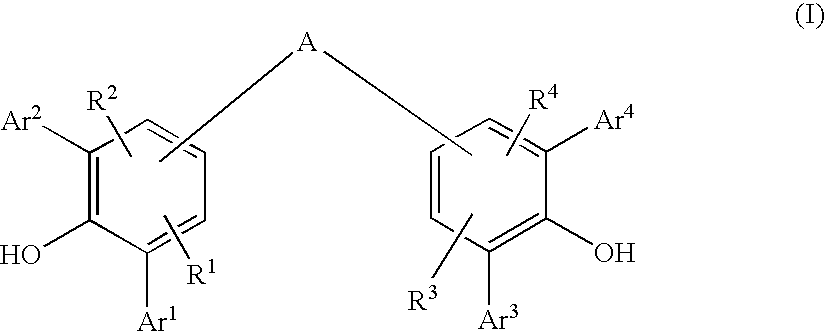
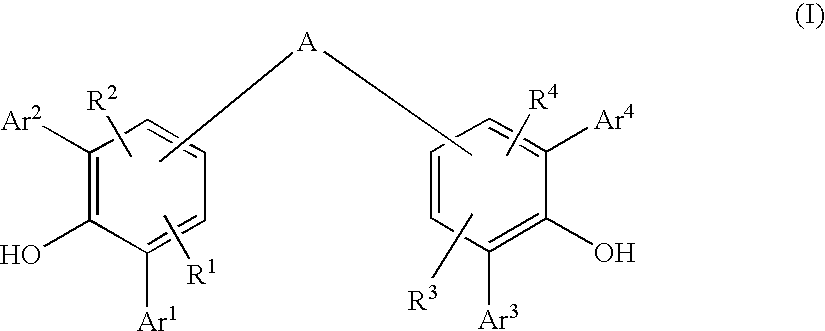
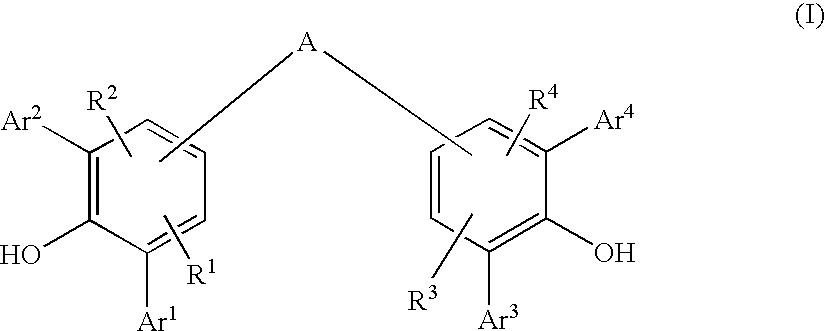
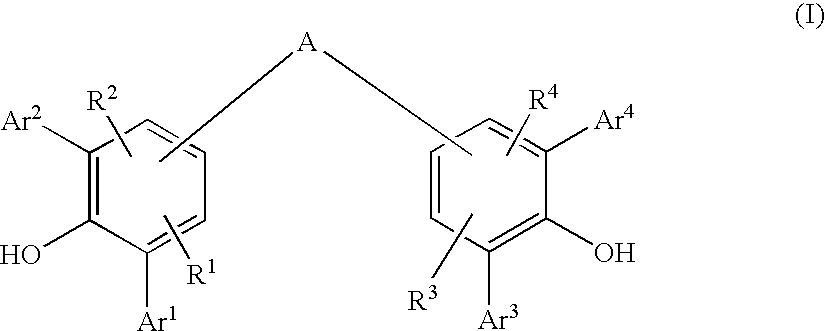
Diarylphenoxy Aluminum Compounds
ActiveUS20080167504A1Simple wayHigh purityGroup 3/13 element organic compoundsMentholALUMINUM HYDRIDE
The present invention relates to diarylphenoxyaluminum compounds which are obtainable by reacting a bis(diarylphenol) ligand of the formula (I)with an alkylaluminum compound and / or a complex aluminum hydride.The invention moreover relates to the use of such diarylphenoxyaluminum compounds as catalysts.Moreover, the invention relates to a method of producing isopulegol by cyclization of citronellal in the presence of diarylphenoxyaluminum compounds as catalysts.The invention also relates to a method of producing menthol by cyclization of citronellal in the presence of diarylphenoxyaluminum compounds as catalysts and subsequent hydrogenation.
Owner:BASF AG
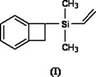
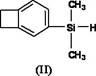
Silicon-containing benzocyclobutene monomers and preparation method thereof
InactiveCN102206229AImprove performanceLow toxicitySilicon organic compoundsLithium chlorideDistillation
The invention discloses silicon-containing benzocyclobutene monomers, such as dimethyl vinyl benzocyclobuten-1-ylsiloxane, and a preparation method thereof. The preparation method of the compound comprises: adding magnesium, anhydrous lithium chloride and lithium aluminum hydride into a reactor under water-free, anaerobic and nitrogen-protection conditions; dripping the tetrahydrofuran solution of 1-bromobenzocyclobutene with stirring, dripping the tetrahydrofuran solution of methylvinyldichlorosilane after the magnesium is reacted completely, and reacting at -20 to 40 DEG C for 3 to 24 hours; and adding water to terminate reaction, extracting with an organic solvent, drying an organic phase by using an organic salt drying agent, concentrating, and subjecting the concentrated material to reduced-pressure distillation or silica column chromatography to obtain a product. The compound contains reactive vinylsilyl and hydrosilyl reaction groups, and the high polymer material formed by reaction has high comprehensive performance and has great development potential and application prospect in fields of microelectronics, aerospace and national defense.
Owner:SOUTHWEAT UNIV OF SCI & TECH
High capacity hydrogen storage material based on catalyzed alanates
InactiveUS20050054525A1Improved kineticsReducing hydrogen storage capacityCatalyst protectionMultiple metal hydridesALUMINUM HYDRIDEHydrogen absorption
A complex aluminum hydride doped with a catalytic material adapted to increase the kinetics of hydrogen absorption / desorption of the aluminum hydride without reducing the hydrogen storage capacity of the aluminum hydride.
Owner:TEXACO OVONIC FUEL CELL
Reduction method of graphene oxide
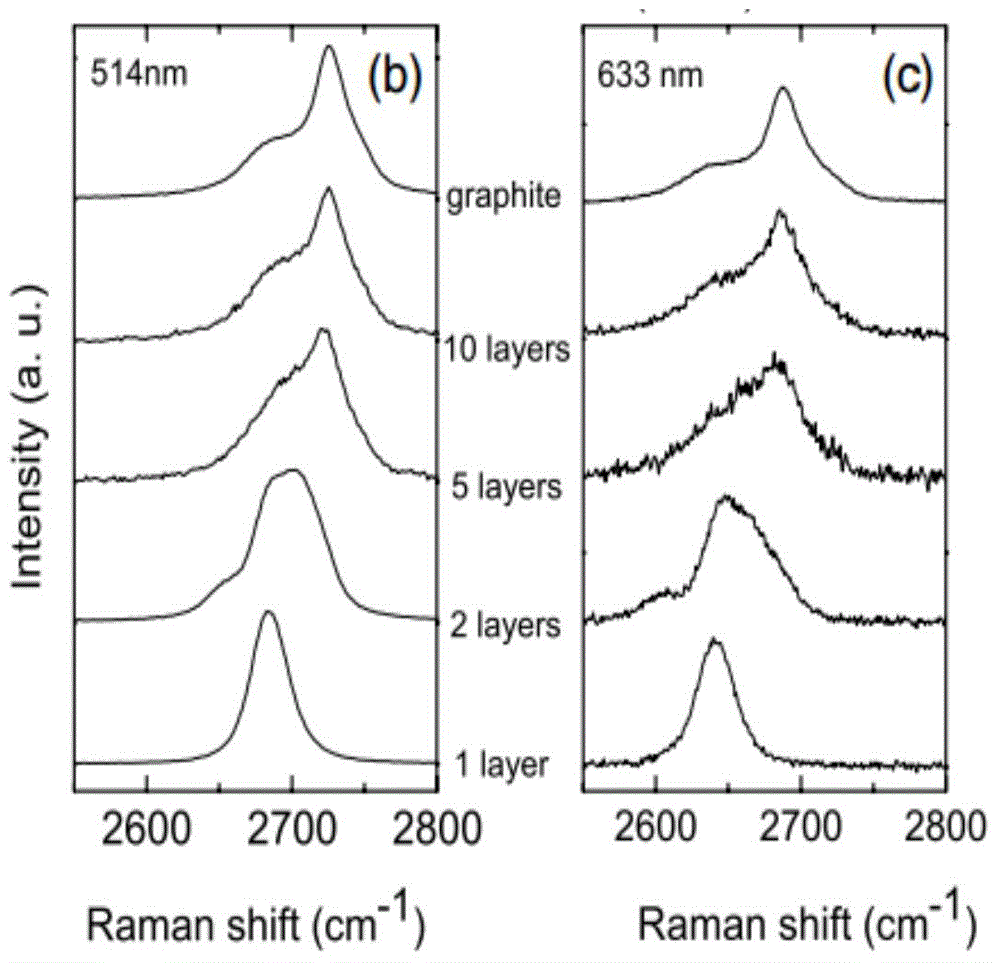
The invention discloses a reduction method of graphene oxide. According to the reduction method, with metal hydride as a reducing agent and lewis acid as a catalyst, graphene oxide is reduced at room temperature. The reduction method comprises the following steps: dispersing the graphene oxide in a solvent according to the dispersing concentration of 0.2mg / ml-4mg / ml by virtue of a magnetic stirring, high-shearing mixing or ultrasonic dispersing method; adding lewis acid, stirring and dispersing, wherein the adding concentration of the lewis acid is 1mM-30mM; adding metal hydride and stirring, wherein the dispersing concentration of the metal hydride is 10mM-300mM, and carrying out reduction reaction for 30 minutes to 12 hours at room temperature; filtering and washing by using a cleaning agent until the pH value is neutral, wherein the cleaning agent is pure water, and the metal hydride is one of lithium aluminum hydride, sodium borohydride and sodium hydride. The reduction method of the graphene oxide is efficient, cheap, non-toxic and pollution-free and capable of efficiently removing oxygen-containing functional groups out of graphene oxide, so that a hexatomic ring structure of the graphene oxide is recovered.
Owner:UNIV OF SHANGHAI FOR SCI & TECH
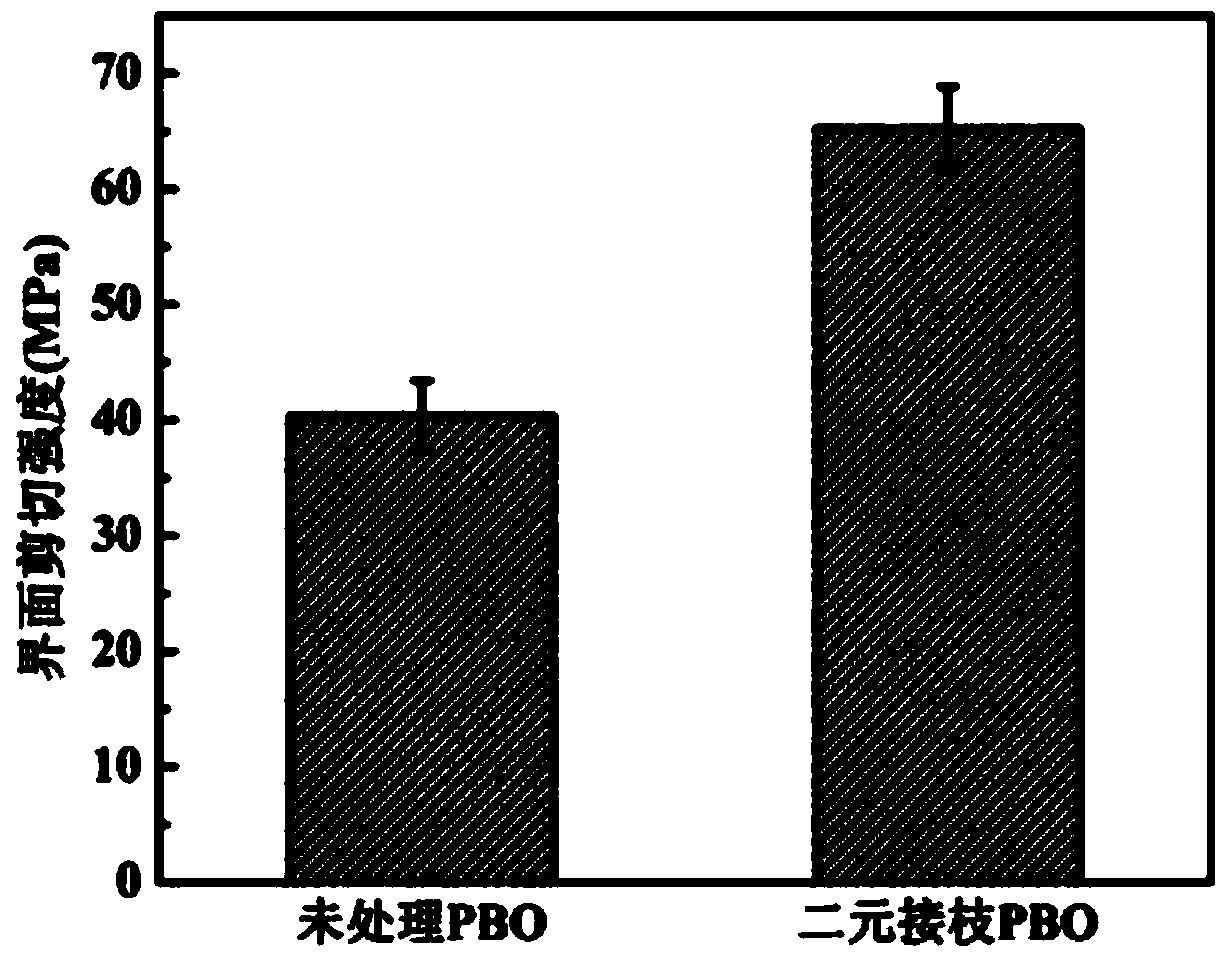
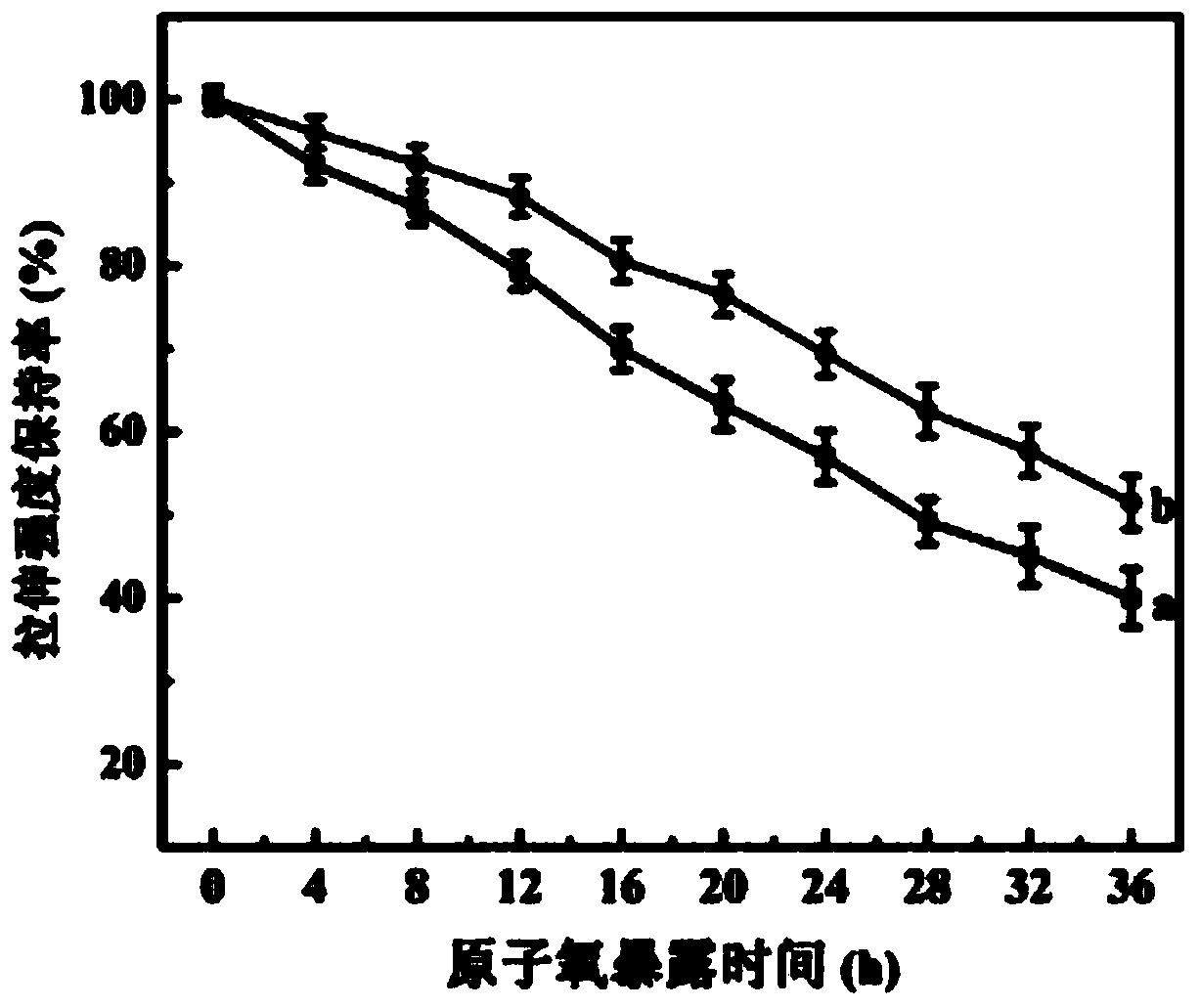
Preparation method for binary grafted modified PBO fiber
The invention relates to a preparation method for PBO fiber, in particular to a preparation method for binary grafted modified PBO fiber, and aims to solve the problem that the conventional PBO fiber has poor infiltration with matrix resin as the surface is inert and the fiber mechanical property is reduced as PBO fiber molecular chains break due to elemental oxygen. The preparation method comprises the following steps: 1, functionally processing graphene oxide; 2, performing activating treatment on PBO fiber; adding the PBO fiber into a lithium aluminum hydride-diethyl ether saturated solution to be subjected to hydroxyl functional processing; 4, grafting APTMS on the surface of the PBO fiber; 5, binarily grafting the graphene oxide on the surface of the PBO fiber. According to the invention, both the APTMS and the graphene oxide are grafted on the surface of the PBO fiber through a chemical grafting method, so that the infiltration of the PBO fiber is improved, and the binary grafted PBO fiber can keep higher tensile strength under the impact of elemental oxygen. The preparation method is mainly applied to the preparation of PBO fiber.
Owner:HARBIN INST OF TECH
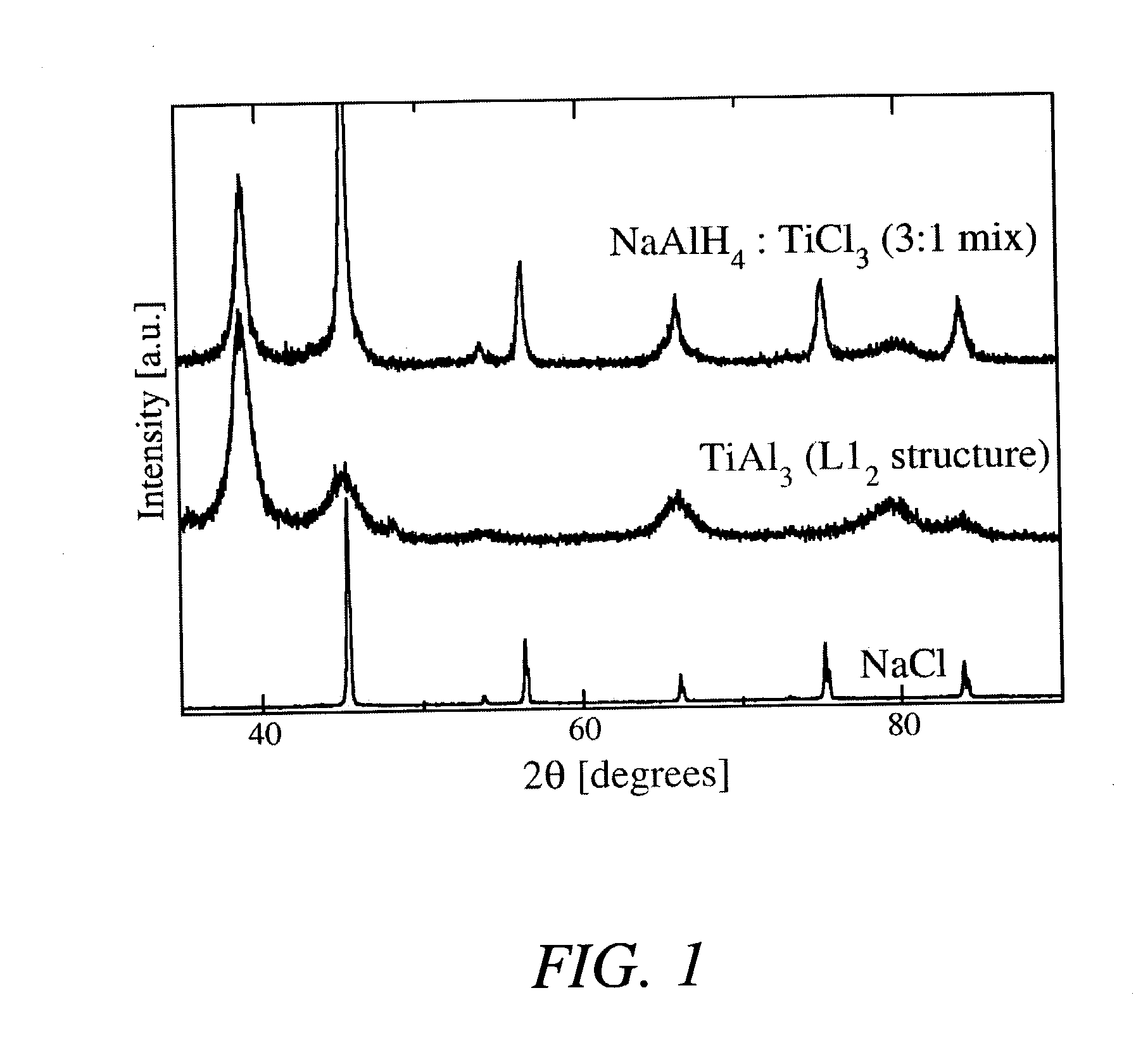
Direct synthesis of hydride compounds using a titanium aluminate dopant
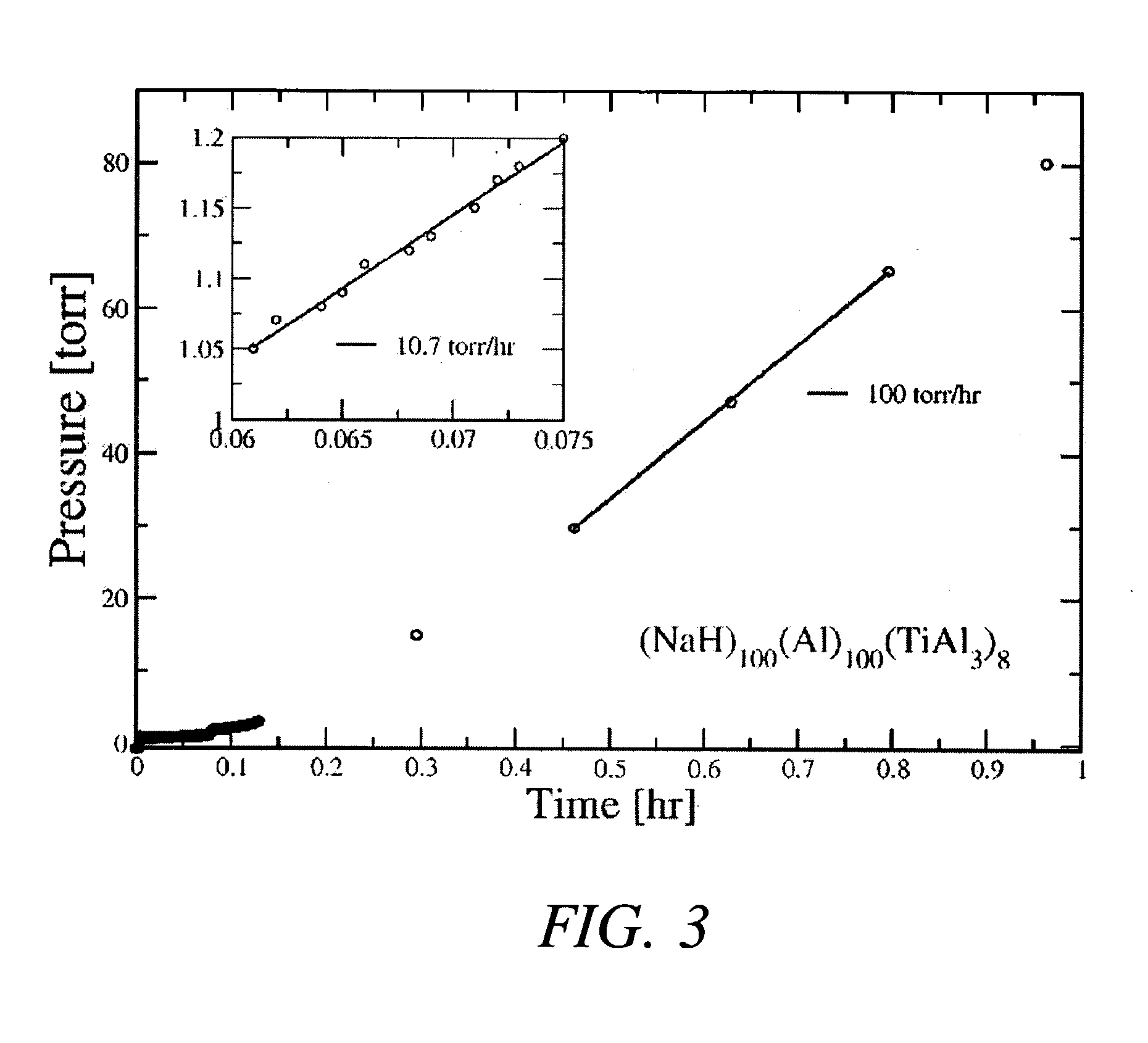
A method for directly preparing alkali metal aluminum hydrides such as NaAlH4 and Na3AlH6 from either the alkali metal or its hydride, and aluminum. The hydride thus prepared is doped with a small portion of TiAl3, in order to improve the reaction kinetics of the hydride compound thus formed. The process provides for mechanically mixing the dry reagents under an inert atmosphere followed by charging the mixed materials with high pressure hydrogen while heating the mixture to about 125° C.
Owner:SANDIA NAT LAB
Methods for forming wiring and electrode
ActiveUS7071084B2Suitable for useSemiconductor/solid-state device manufacturingNon-conductive material with dispersed conductive materialLight treatmentTitanium
There is provided a method for forming wiring or an electrode by coating a substrate with a composition comprising (A) a complex of an amine compound and a hydrogenated aluminum compound and (B) a titanium compound or a composition comprising the complex and (C) metal particles and subjecting the obtained coating film to heating and / or a light treatment. By the method, a film can be formed that uses a conductive film forming composition with which wiring and an electrode that can be suitably used for electronic devices can be formed easily and inexpensively.
Owner:JSR CORPORATIOON +1
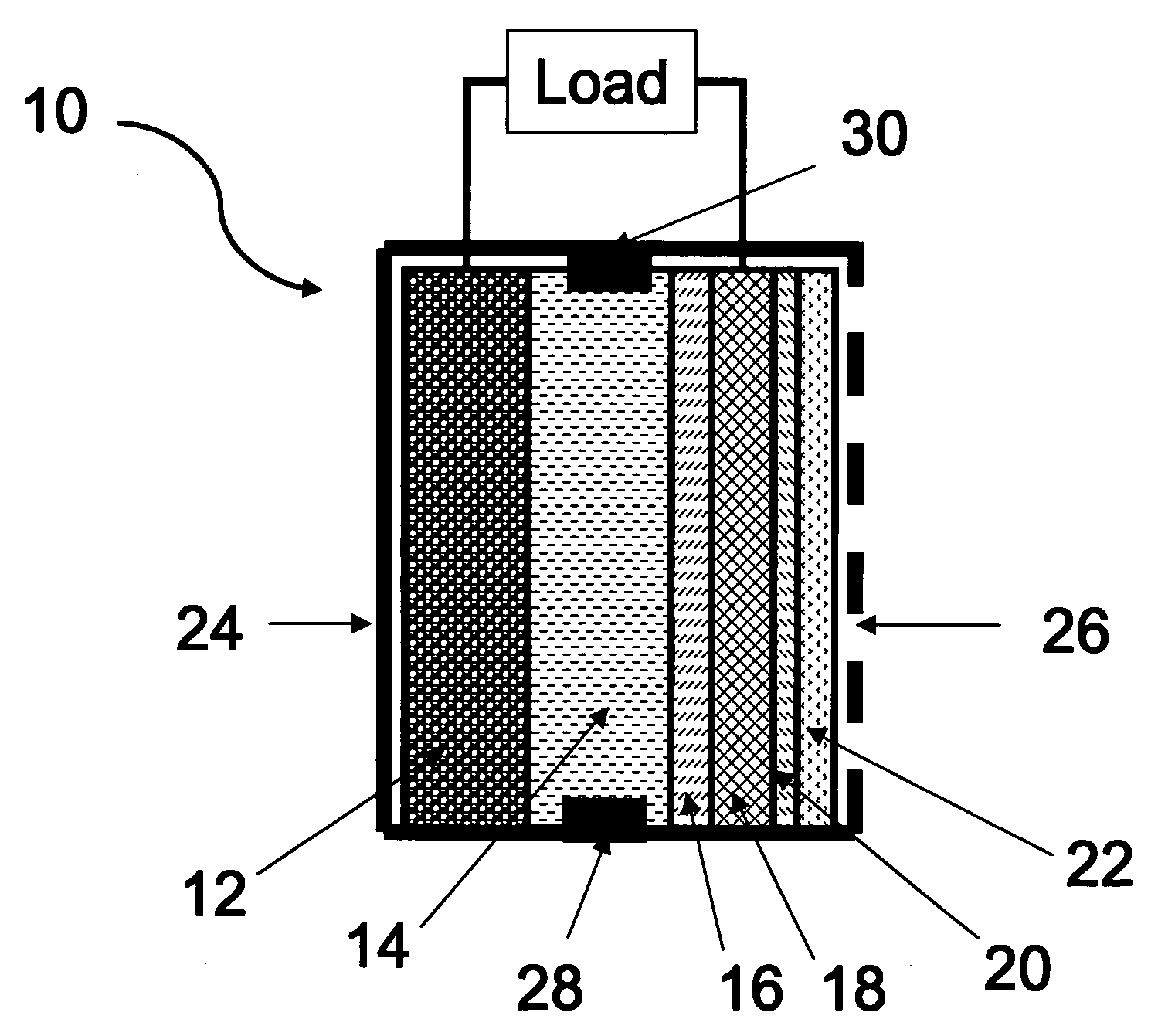
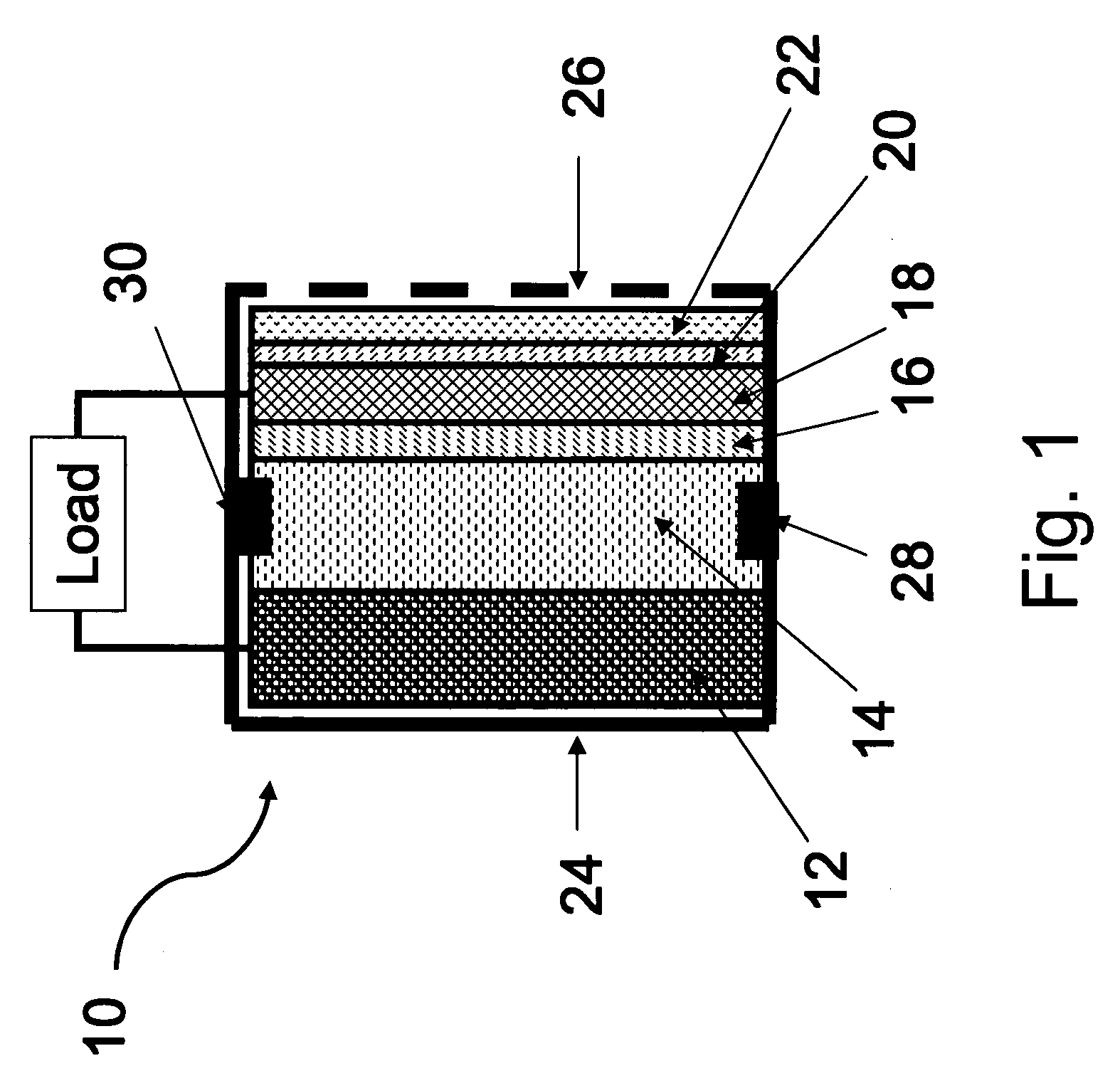
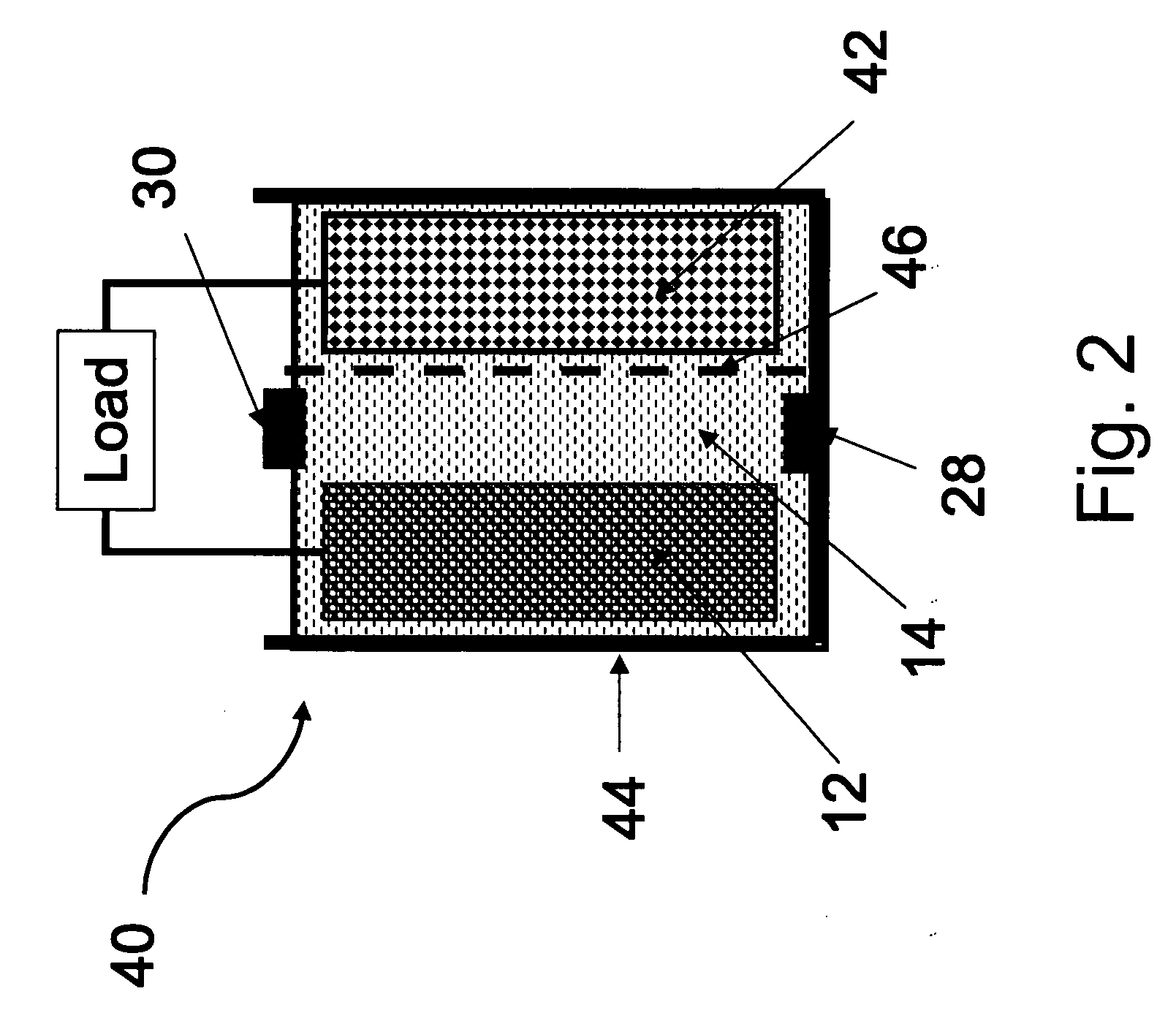
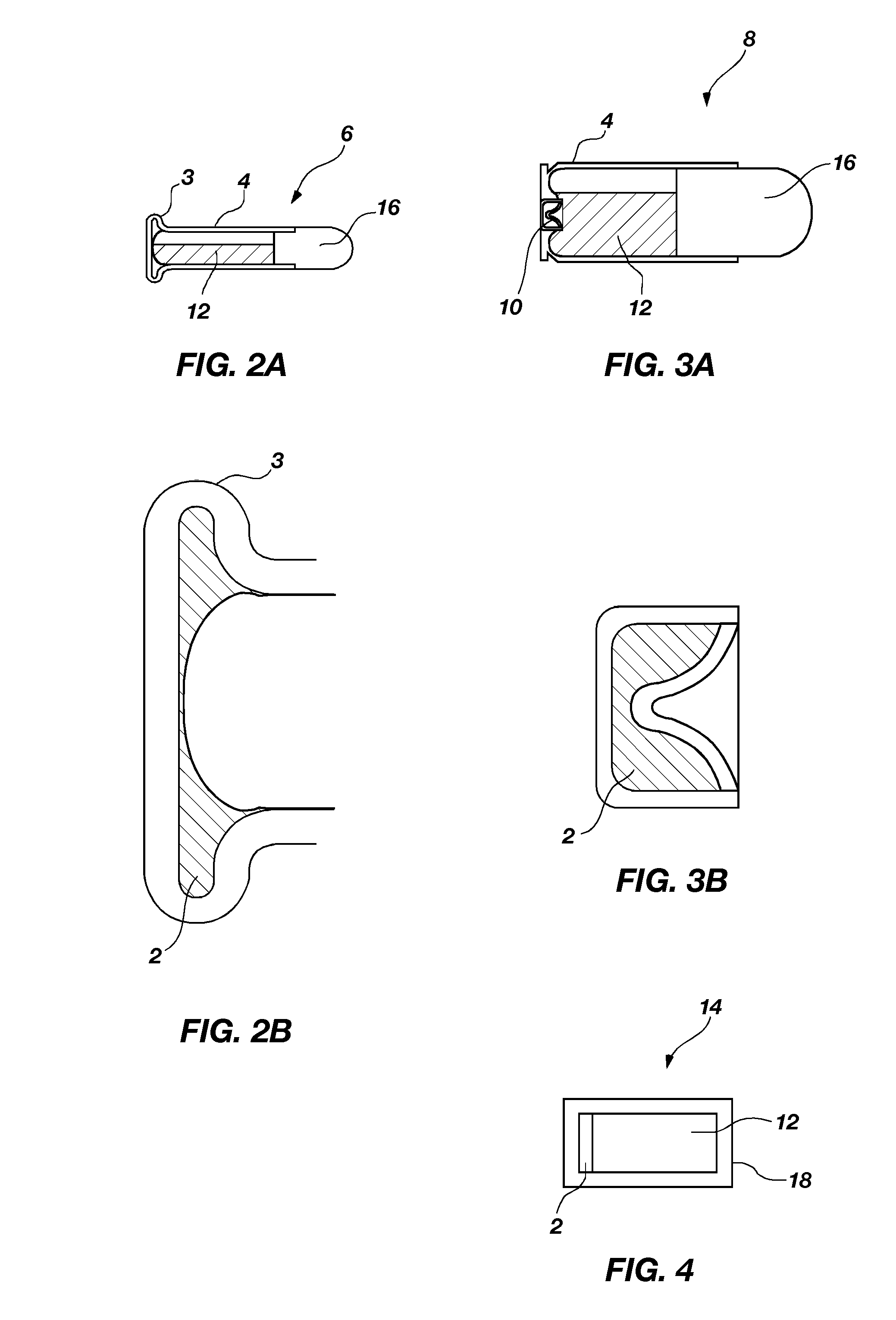
Primary aluminum hydride battery
InactiveUS20090325070A1Primary cell to battery groupingFuel and primary cellsConductive materialsAqueous electrolyte
A primary aluminum hydride cell and a battery formed with a plurality of the cells is described herein.. In some embodiments, the cells are constructed of:(a) an anode comprising aluminum hydride and a conductive material;(b) a cathode; and(c) an aqueous electrolyte.
Owner:GENERAL ELECTRIC CO
Method for preparing hydroxytyrosol
InactiveCN101891595AMild reaction conditionsFew reaction stepsOrganic chemistryOrganic compound preparationHydroxytyrosolBenzoyl bromide
The invention belongs to the field of medicinal synthesis, and particularly relates to a method for preparing hydroxytyrosol, which comprises the following steps of: (1) protecting free hydroxyl groups at 3 and 4 positions, on 3,4-dihydroxy benzaldehyde with benzyl, namely, reacting the 3,4-dihydroxy benzaldehyde with benzyl bromide to prepare 3,4-dibenzyloxybenzaldehyde; (2) reacting N-methylaniline acetonitrile with the 3,4-dibenzyloxybenzaldehyde to prepare 3-(3,4-dibenzyloxyphenyl)-2-(methylphenylamino) acrylonitrile, and hydrolyzing the 3-(3,4-dibenzyloxyphenyl)-2-(methylphenylamino) acrylonitrile under acidic condition to prepare a 3,4-dibenzylosyphenylacetic acid; (3) reducing a carboxyl group of the 3,4-dibenzylosyphenylacetic acid with lithium borohydride, lithium aluminum hydride or sodium borohydride to prepare 3,4-dibenzyloxyphenethyl alcohol; and (4) catalyzing the 3,4-dibenzyloxyphenethyl alcohol with a catalyst palladium / carbon to prepare the hydroxytyrosol. The reagents used in the method are easily obtainable and low in cost, reaction conditions are mild, and the final overall yield of the whole reaction reaches 50 to 60 percent.
Owner:SUZHOU UNIV
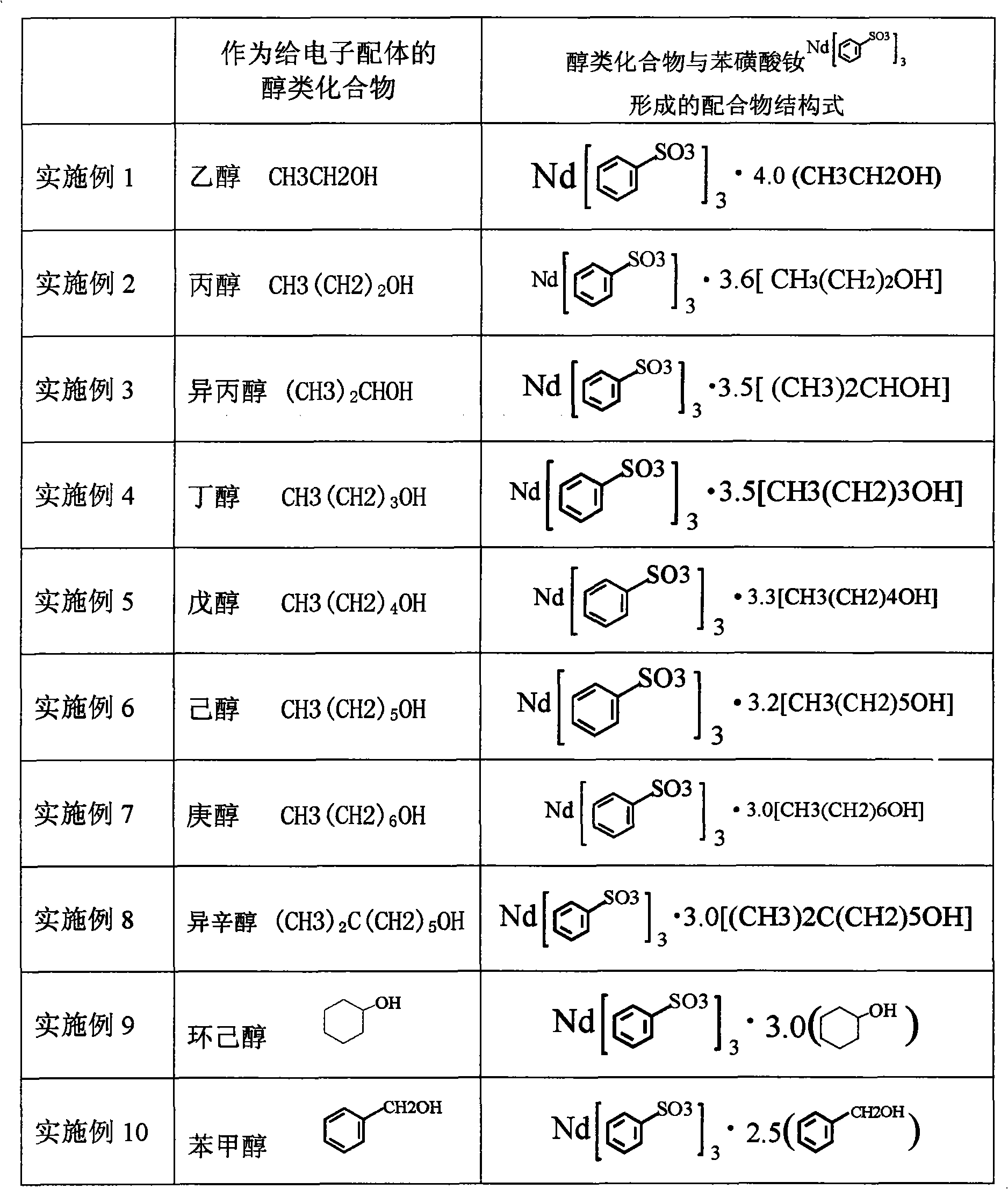
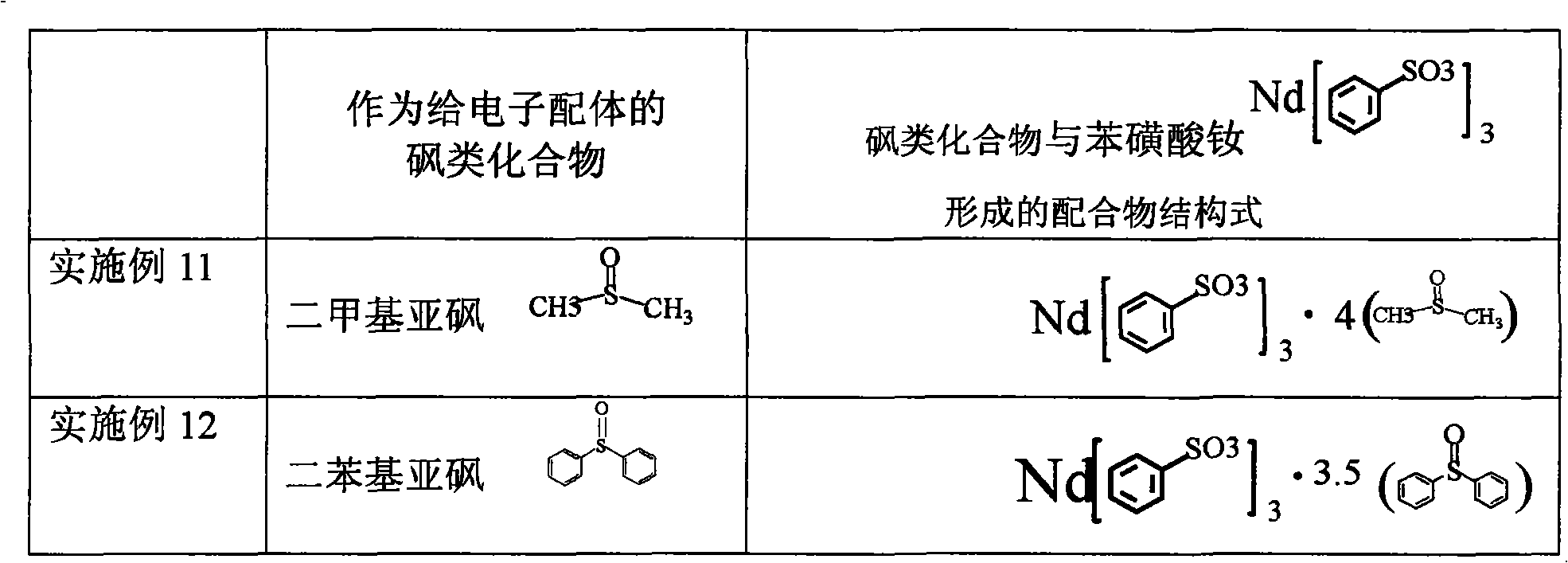
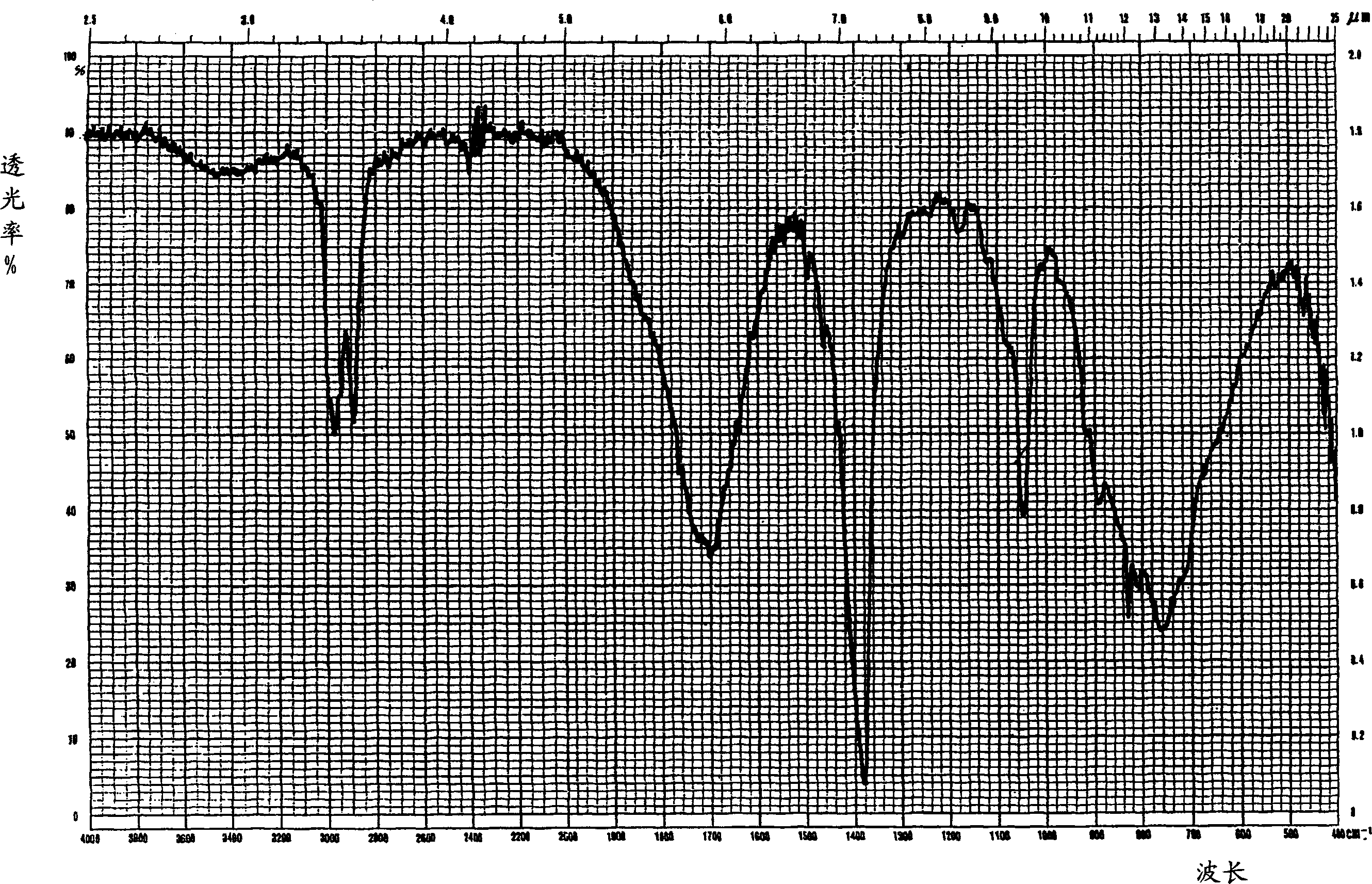
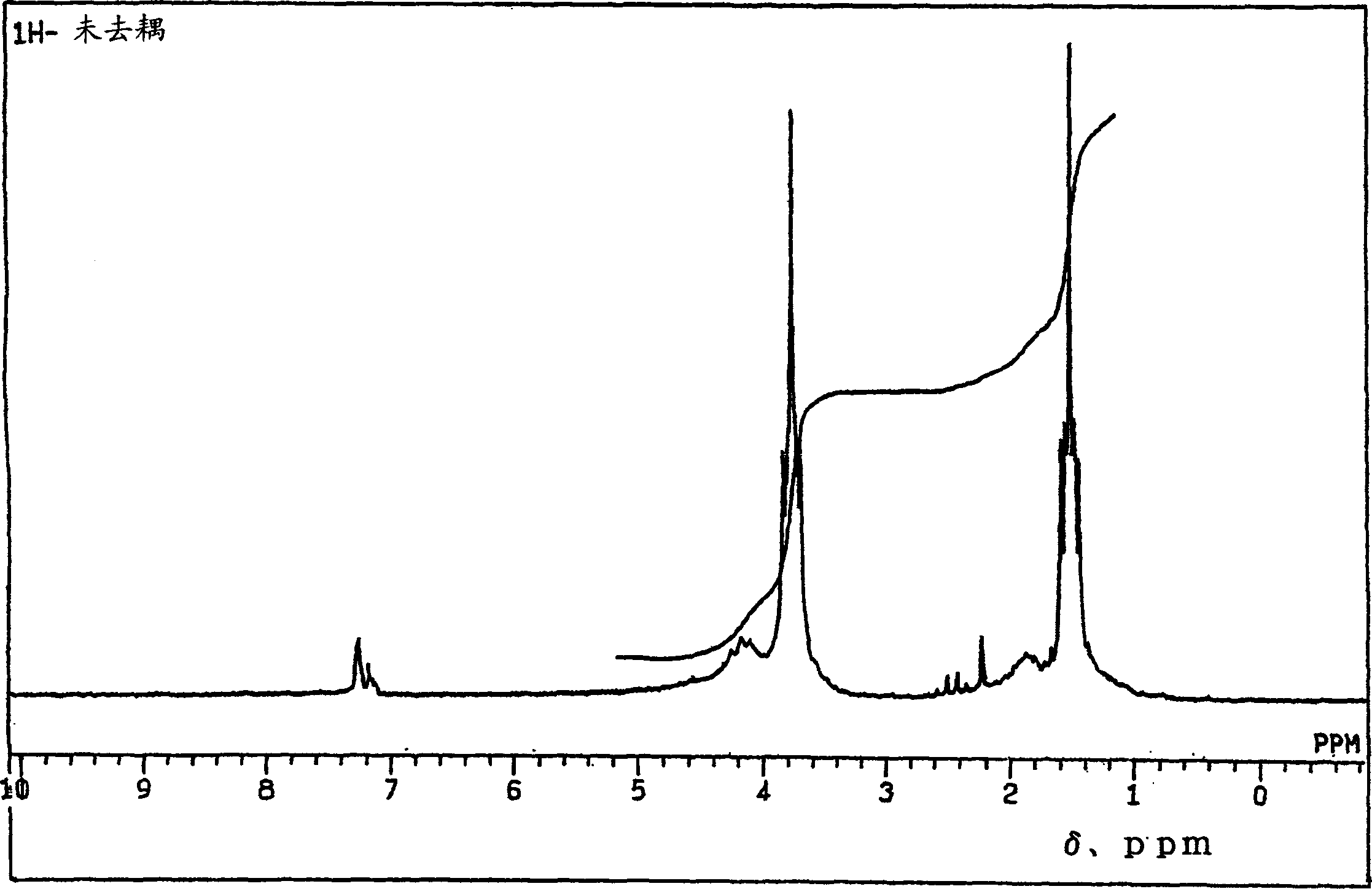
Sulfoacid rare earth catalyst for polymerizing high-cis-isoprene rubber and preparation method thereof
The invention relates to a sulfoacid rare earth catalyst for polymerizing high-cis-isoprene rubber and a preparation method thereof. The catalyst comprises benzenesulfonic acid neodymium rare earth complex and alkyl aluminum; the molar ratio of the alkyl aluminum to the rare earth neodymium in the benzenesulfonic acid neodymium rare earth complex is 15 to 40:1, wherein the benzenesulfonic acid neodymium rare earth complex comprises benzenesulfonic acid neodymium alcohol complex, benzenesulfonic acid neodymium sulphoxide complex, benzenesulfonic acid neodymium furan complex, benzenesulfonic acid neodymium amine complex, benzenesulfonic acid neodymium ether complex and benzenesulfonic acid neodymium ester complex; ligand comprises: alcohol compound, sulfoxide compound, furan compound, amine compound, ether compound and ester compound; and the alkyl aluminum comprises: 3-isobutyl aluminum, triethyl aluminum, diisobutyl aluminum hydride, or diethyl aluminum hydride. The sulfoacid rare earth complex catalyst is used for polymerizing isoprene, and can obtain the high-cis rare earth polyisoprene rubber with the cis 1,4 structure content more than 96.0 percent and the weight-average molecular weight more than 2.2 million.
Owner:CHANGZHOU INST OF ENERGY STORAGE MATERIALS &DEVICES
Composition used for rare earth catalyst, rare earth catalyst and its application
The invention provides a composition used for a rare earth catalyst. The composition contains alkyl aluminum and / or alkyl aluminum hydride, a rare earth compound and conjugated diene. The rare earth compound is a compound having a general formula as MR3.nL, wherein M is a rare earth metal, R is halogen, L is an electron-donating ligand, and the value of n is 1-3. Also, the alkyl aluminum and / or alkyl aluminum hydride, the rare earth compound and the conjugated diene are in a molar ratio of 0.05-1.5:0.01-0.05:1. The invention provides a rare earth catalyst, which is obtained by enabling the composition provided in the invention to contact an inert organic solvent mutually under the protection of an inert gas. Specifically, the contact condition enables the conjugated diene in the composition to polymerize so as to obtain a conjugated diene polymer with a polymerization degree of not less than 150. The invention also provides application of the catalyst in the polymerization of conjugated diene. The rare earth catalyst provided in the invention has good stability, and is in favor of synthesizing polymer products with stable quality and uniformity when it is used for conjugated diene polymerization.
Owner:CHINA PETROLEUM & CHEM CORP +1
Substance for forming electrically-conducting film and electrically-conducting film and its manufacturing method
InactiveCN1461779AConductive layers on insulating-supportsSemiconductor/solid-state device detailsOrganic solventOptoelectronics
There are provided a conductive film forming composition capable of forming wiring or an electrode which can be suitably used in a variety of electronic devices, easily and inexpensively, a method for forming a film using the composition, a conductive film formed by the method, and wiring or an electrode which comprises the film. A conductive film forming composition comprising a complex of an amine compound and aluminum hydride and an organic solvent is applied on a substrate and then subjected to a heat treatment and / or irradiation with light, whereby a conductive film such as an electrode or wiring is produced.
Owner:JSR CORPORATIOON
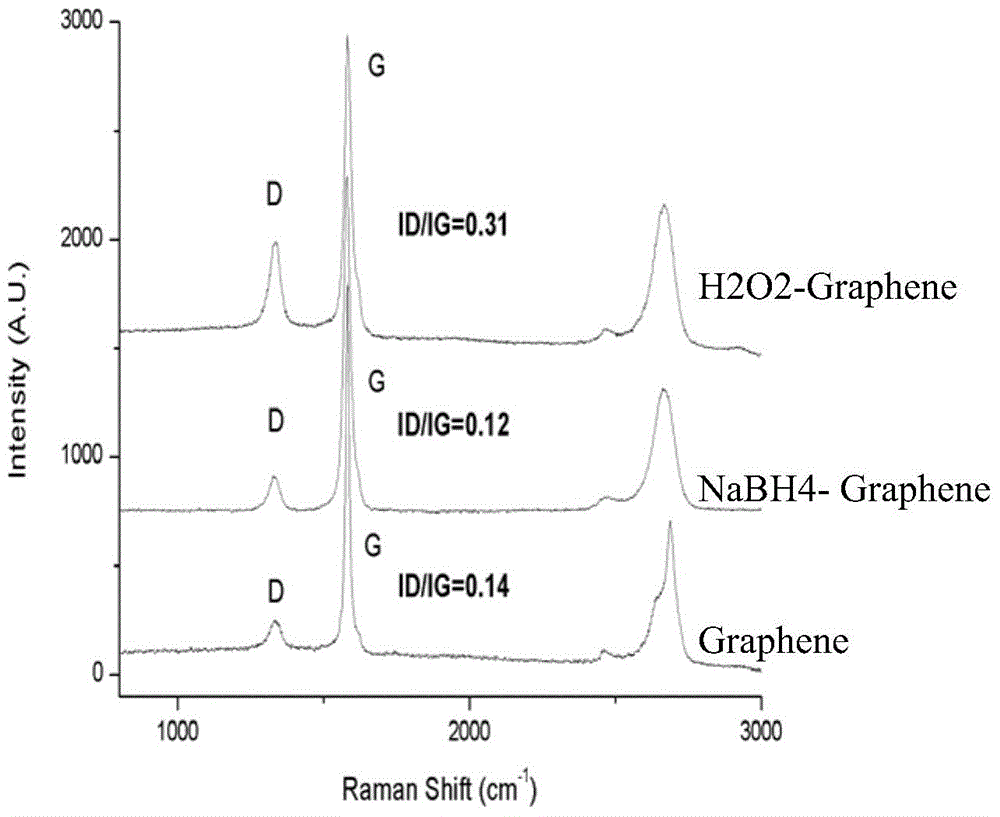
Graphene preparation method
ActiveCN103950919AInflation implementation time is shortImprove electrical performanceGrapheneFreeze-dryingALUMINUM HYDRIDE
The invention discloses a graphene preparation method. The method comprises the following steps: mixing a precursor first-order or second-order graphite intercalation compound with 1-10wt% of a reducing solution capable of generating a gas, and allowing the obtained mixture to stand for 1-5min to obtain highly expanded graphite, wherein the reducing solution is an aqueous solution of borohydride or a tetrahydrofuran solution of lithium aluminum hydride; pickling to remove impurities, filtering, carrying out ultrasonic dispersion of the obtained graphite in an aqueous solution or ethanol solution containing 0.5-5wt% of a dispersant for 0.5-3h to obtain a graphene dispersion; and freeze-drying the dispersion to obtain graphene powder, or adding a water-soluble high molecular polymer for co-precipitation to obtain a graphene-containing polymer master batch. The method has the advantages of realization of the repairing of the structural defect of graphene, mild reaction, fast reaction speed; and compared with an oxidative catalysis system, the method disclosed in the invention supplements the peeling of a reducing system in the graphene preparation process, so the graphene quality is further improved.
Owner:SOUTH CHINA UNIV OF TECH
Methods of forming a sensitized explosive and a percussion primer
A sensitized explosive that comprises an explosive precipitated onto a sensitizer. The explosive is CL-20, PETN, RDX, HMX, or mixtures thereof and the sensitizer is aluminum, titanium, zirconium, magnesium, melamine, styrene, lithium aluminum hydride, or mixtures thereof. The sensitized explosive is used in a percussion primer that includes a bismuth compound and a melt binder. The bismuth compound is bismuth oxide, bismuth subnitrate, bismuth tetroxide, bismuth sulfide, or mixtures thereof and the melt binder is a wax having a melting point above ambient temperature, trinitrotoluene, poly(3,3-bis(azidomethyl)oxetane), poly(3-azidomethyl-3-methyloxetane), ethyl-3,5-dinitrobenzoate, or mixtures thereof. A gun cartridge and other primer-containing ordnance assemblies employing the percussion primer are also disclosed. Methods of forming the sensitized explosive and the percussion primer are also disclosed.
Owner:NORTHROP GRUMMAN SYST CORP
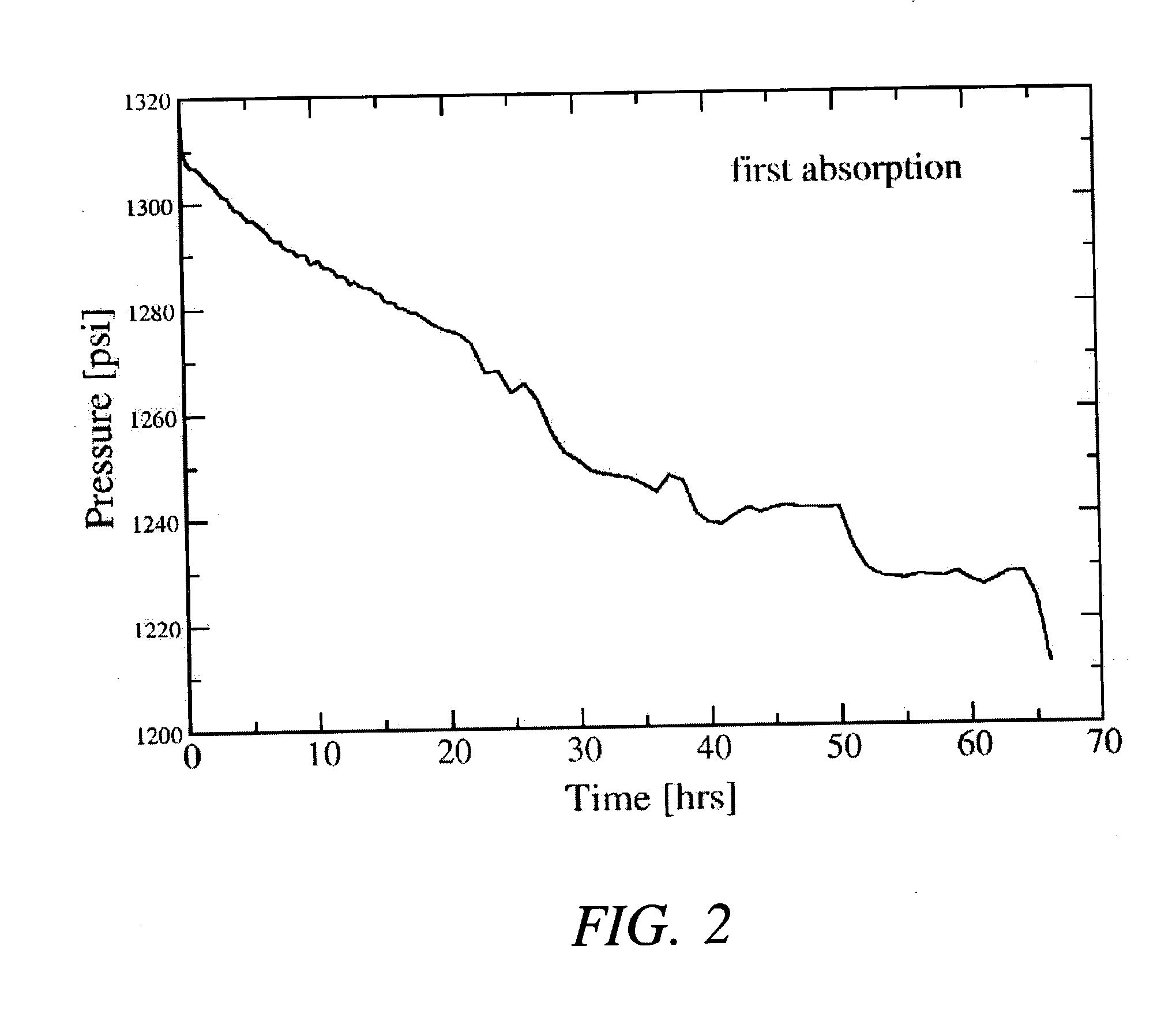
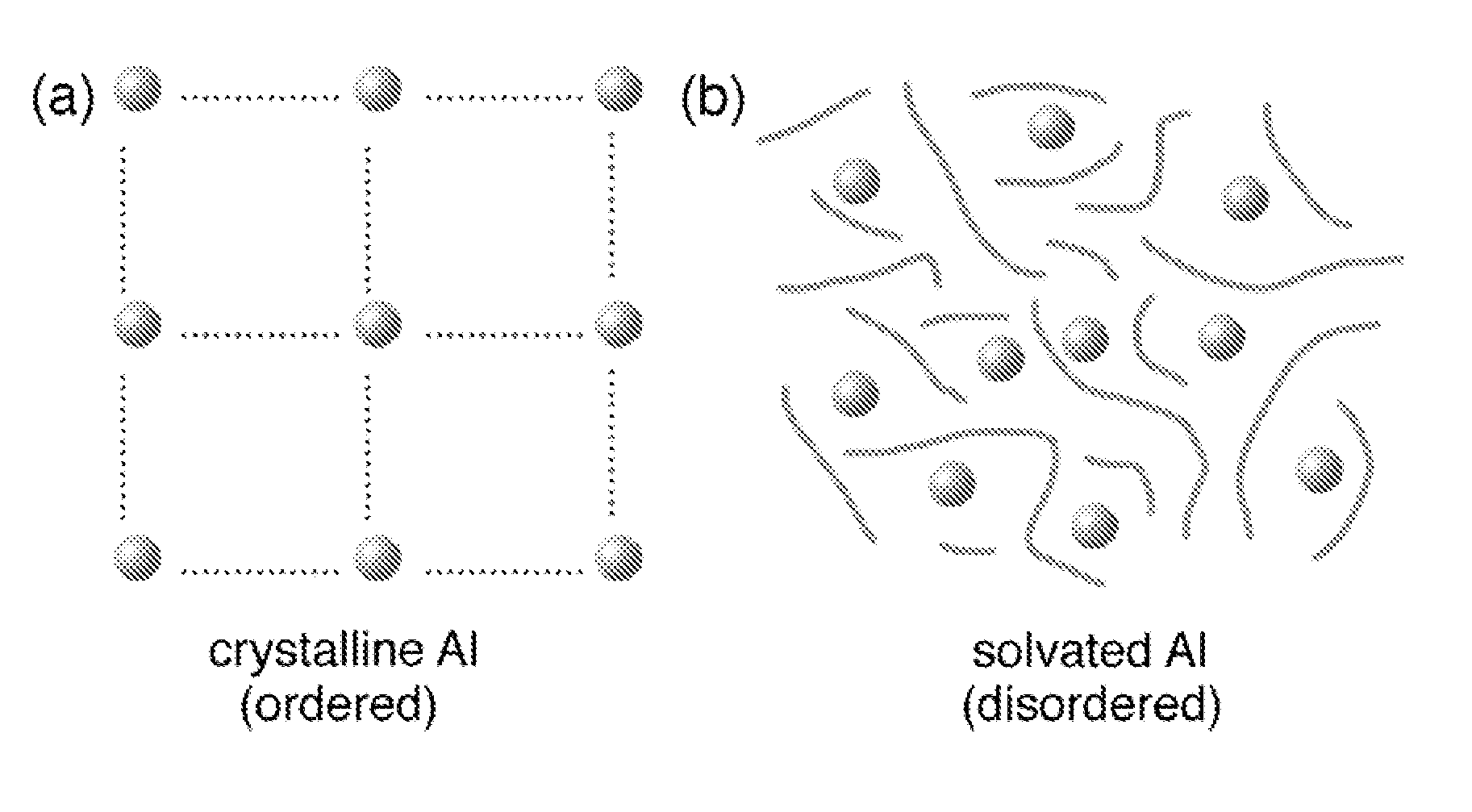
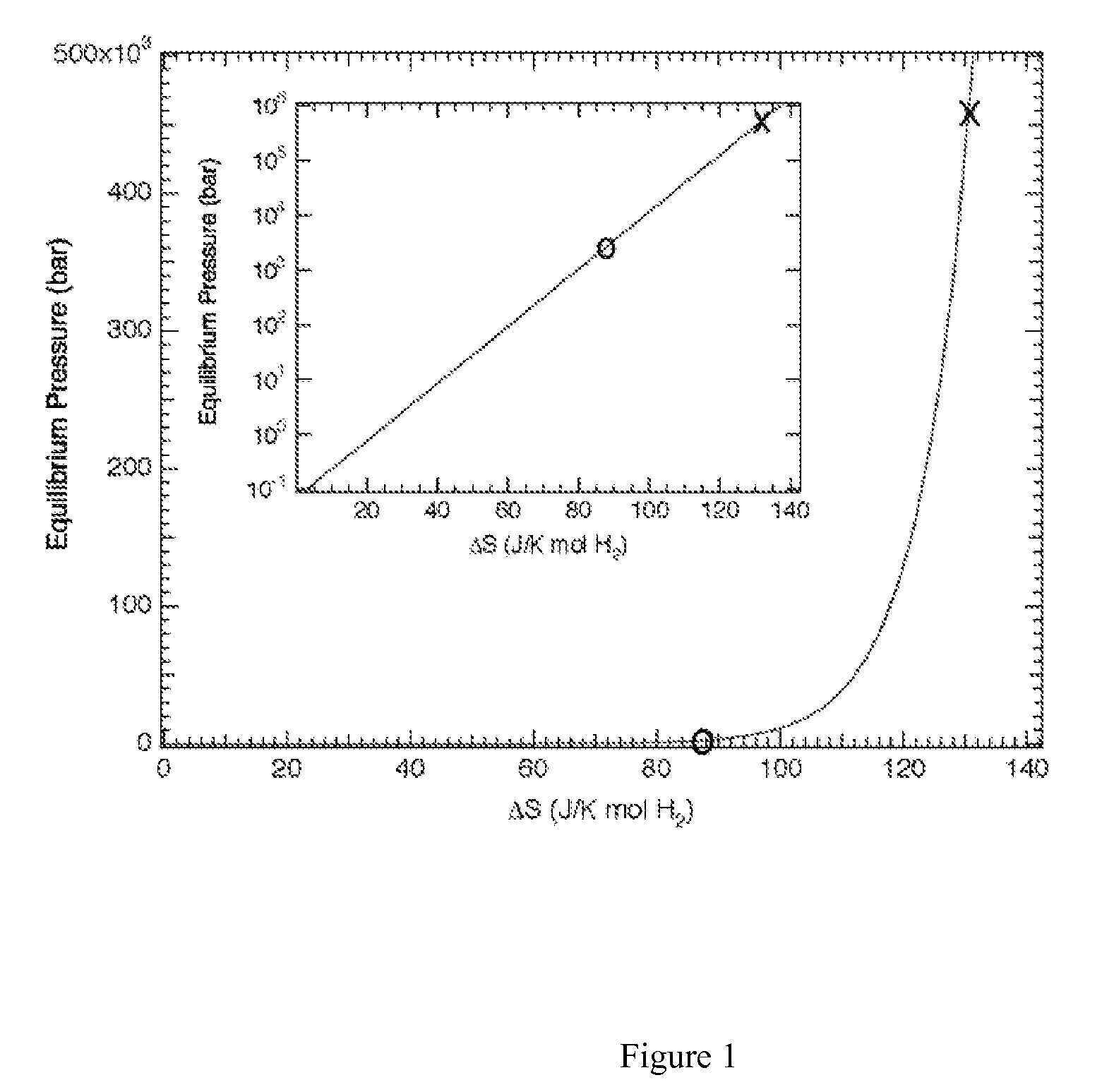
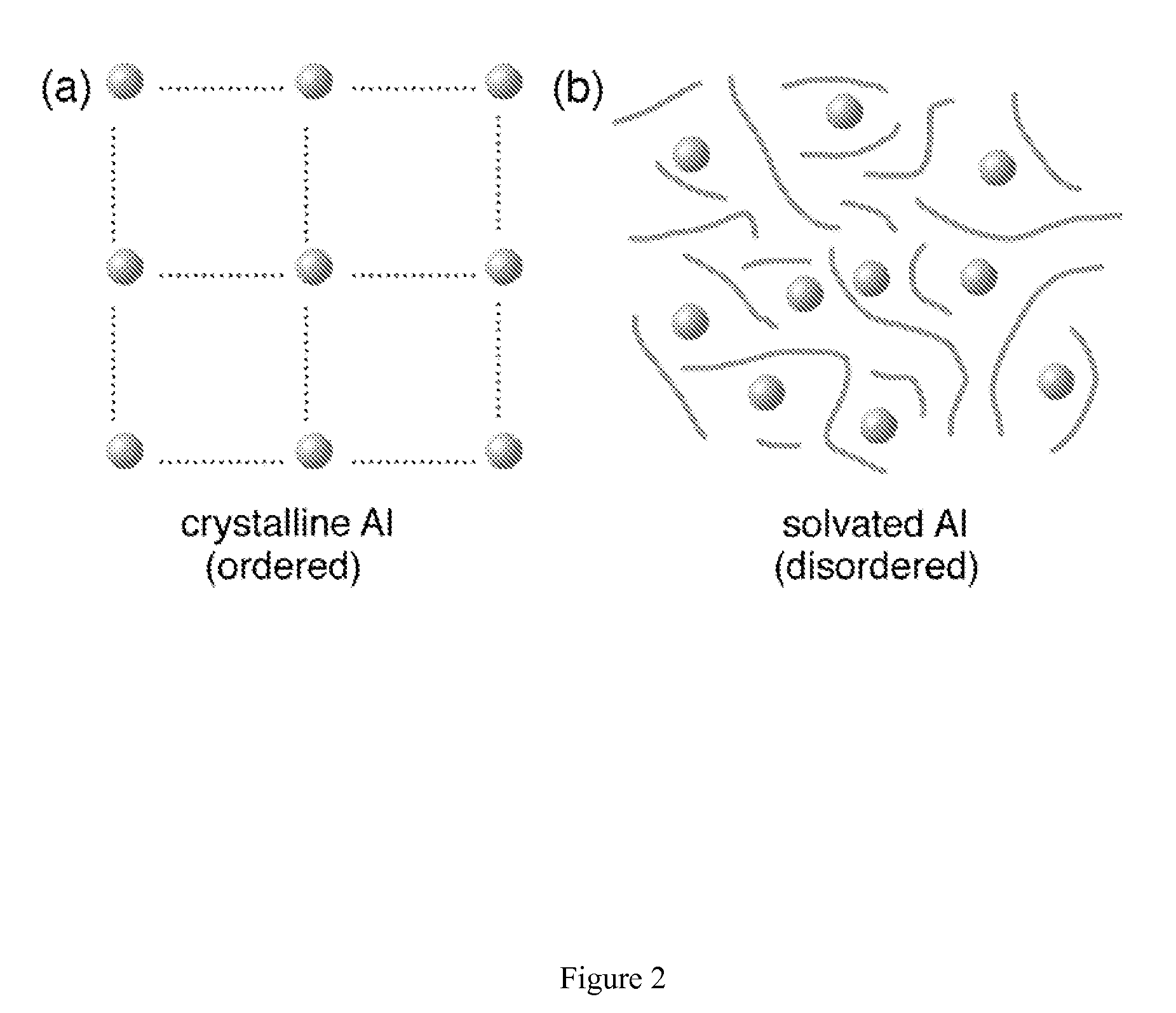
Regeneration of aluminum hydride
The present invention provides methods and materials for the formation of hydrogen storage alanes, AlHx, where x is greater than 0 and less than or equal to 6 at reduced H2 pressures and temperatures. The methods rely upon reduction of the change in free energy of the reaction between aluminum and molecular H2. The change in free energy is reduced by lowering the entropy change during the reaction by providing aluminum in a state of high entropy, by increasing the magnitude of the change in enthalpy of the reaction or combinations thereof.
Owner:BROOKHAVEN SCI ASSOCS
Method for synthesizing polyphenylene sulfide copolymer containing heteroaromatics
The present invention discloses a synthetic method of heteroaromatics polyphenyl sulfur ether. The present invention adopts sodium sulfide and paradichlorobenzene and 2-(4-chlorine phenyl group)-5-chlorine phenyl benzimidazole as raw materials and N-methyl-2 pyrrolidone as solvent for condensation and polymerization under the role of alkali metal lacquer solvent, so as to produce the polyphenyl sulfur ether resin. The mol ratio between sodium sulfide and paradichlorobenzene and 2-(4-chlorine phenyl group)-5-chlorine phenyl benzimidazole is 1:0.6-0.9:0.4-0.1. The sequential process mainly includes: in the process of sodium sulfide dehydration stage, assistant lithium aluminum hydride is added; in the condensation and polymerization stage, 2-(4-chlorine phenyl group)-5-chlorine phenyl benzimidazole is added. The product provided by the present invention with excellent indexes of relatively low dispersion coefficient and narrow molecular weight distribution and oxygen index can be made into paint, injection mold, fiber and membrane and be widely applied to the fields of machinery, chemicals, oil, electronic apparatus, war industry and airspace.
Owner:四川中科兴业高新材料有限公司
Aluminum catalyst for polyester synthesis, preparation method thereof and usage method thereof
The invention discloses an aluminum catalyst for polyester synthesis, a preparation method thereof and a usage method thereof. The catalyst which is aluminum glycol and is special for a polycondensation reaction in a polyester synthesis process of terephthalic acid and glycol is prepared through reacting aluminum / aluminum alcoholates / lithium aluminum hydride with glycol. The catalyst, which has less toxicity, allows the molecular weight of PET catalyzed by the catalyst to achieve more than 30,000, can coexist with polycondensation products, has no influence on the quality of the PET, allows requirements of environmental protection to be satisfied and requirements of activity to be guaranteed, has a high activity on the PET synthesizing reaction, and has low cost, is a novel catalyst for the condensation polymerization of PET synthesis.
Owner:CHANGZHOU INST OF CHEM +1
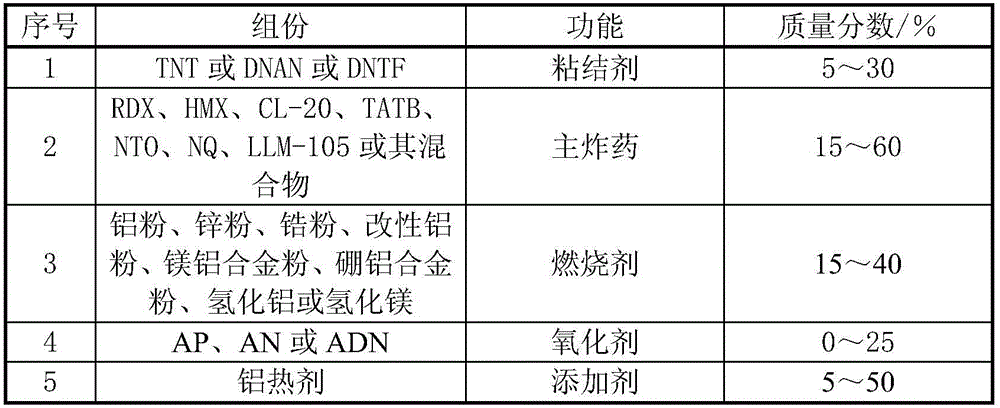
High-energy-density explosive mixture and preparation method thereof
The invention discloses a high-energy-density explosive mixture. A low-melting-point single-compound explosive is adopted and comprises, by mass, 5-30% of an adhesive, 15-60% of a main explosive, 15-40% of a combustion agent, 0-25% of an oxidizing agent and 5-50% of a high-density high-energy additive, wherein the adhesive is prepared from trinitrotoluene, 2,4-dimitroanisole and 3,4-dinitrofurazanofuroxan, the main explosive is prepared from RDX, HMX, CL-20, TATB, NTO, NQ, LLM-105 or the mixture thereof, the combustion agent is prepared from aluminum powder, zinc powder, zirconium powder, modified aluminum powder, magnalium powder, boron-aluminum alloy powder, aluminum hydride and magnesium hydride, the oxidizing agent is prepared from ammonium perchlorate, ammonium nitrate and ammonium dinitramide, and thermite is adopted as the high-density high-energy additive. The prepared explosive mixture has the high energy density, the density is larger than or equal to 2.0 g / cm<3>, and the heat of explosion in unit volume is larger than or equal to 16000 J / g.
Owner:INST OF CHEM MATERIAL CHINA ACADEMY OF ENG PHYSICS
Diarylphenoxy aluminum compounds
The present invention relates to diarylphenoxyaluminum compounds which are obtainable by reacting a bis(diarylphenol) ligand of the formula (I)with an alkylaluminum compound and / or a complex aluminum hydride.The invention moreover relates to the use of such diarylphenoxyaluminum compounds as catalysts.Moreover, the invention relates to a method of producing isopulegol by cyclization of citronellal in the presence of diarylphenoxyaluminum compounds as catalysts.The invention also relates to a method of producing menthol by cyclization of citronellal in the presence of diarylphenoxyaluminum compounds as catalysts and subsequent hydrogenation.
Owner:BASF AG
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com


![Method for reducing 8-benzyl-2,8-diazabicyclo[4,3,0]nonane and chiral isomer thereof Method for reducing 8-benzyl-2,8-diazabicyclo[4,3,0]nonane and chiral isomer thereof](https://images-eureka.patsnap.com/patent_img/d773f92e-9397-45a1-b63c-9d0abefeecf0/A2009101002270002C1.PNG)
![Method for reducing 8-benzyl-2,8-diazabicyclo[4,3,0]nonane and chiral isomer thereof Method for reducing 8-benzyl-2,8-diazabicyclo[4,3,0]nonane and chiral isomer thereof](https://images-eureka.patsnap.com/patent_img/d773f92e-9397-45a1-b63c-9d0abefeecf0/A20091010022700051.PNG)
![Method for reducing 8-benzyl-2,8-diazabicyclo[4,3,0]nonane and chiral isomer thereof Method for reducing 8-benzyl-2,8-diazabicyclo[4,3,0]nonane and chiral isomer thereof](https://images-eureka.patsnap.com/patent_img/d773f92e-9397-45a1-b63c-9d0abefeecf0/A20091010022700052.PNG)