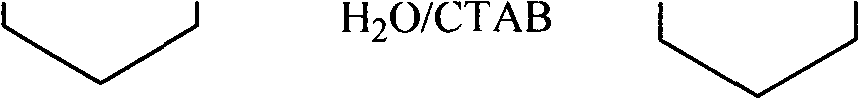Patents
Literature
232 results about "Lithium aluminium hydride" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Lithium aluminium hydride, commonly abbreviated to LAH, is an inorganic compound with the chemical formula LiAlH₄. It was discovered by Finholt, Bond and Schlesinger in 1947. This compound is used as a reducing agent in organic synthesis, especially for the reduction of esters, carboxylic acids, and amides. The solid is dangerously reactive toward water, releasing gaseous hydrogen (H₂). Some related derivatives have been discussed for hydrogen storage.
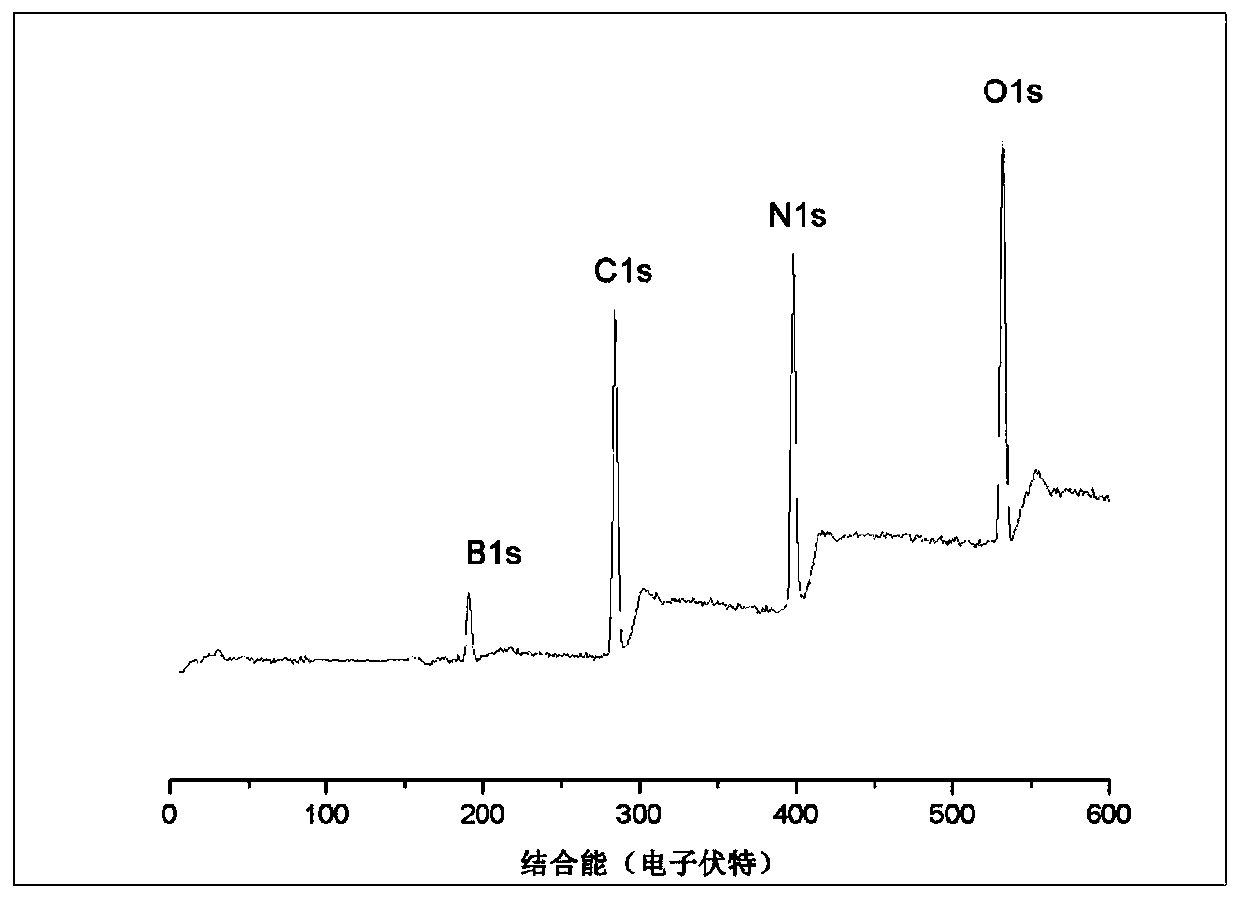
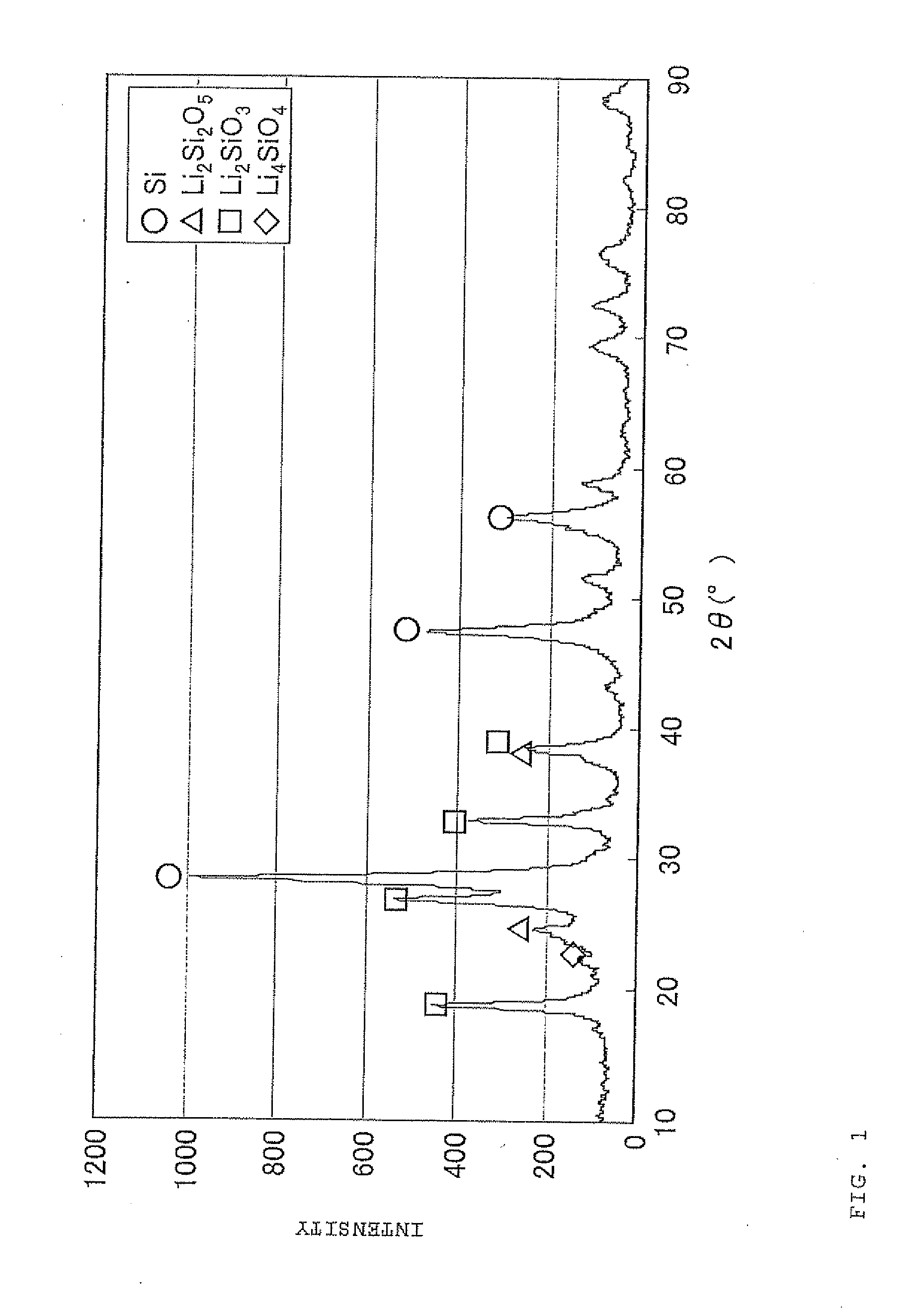
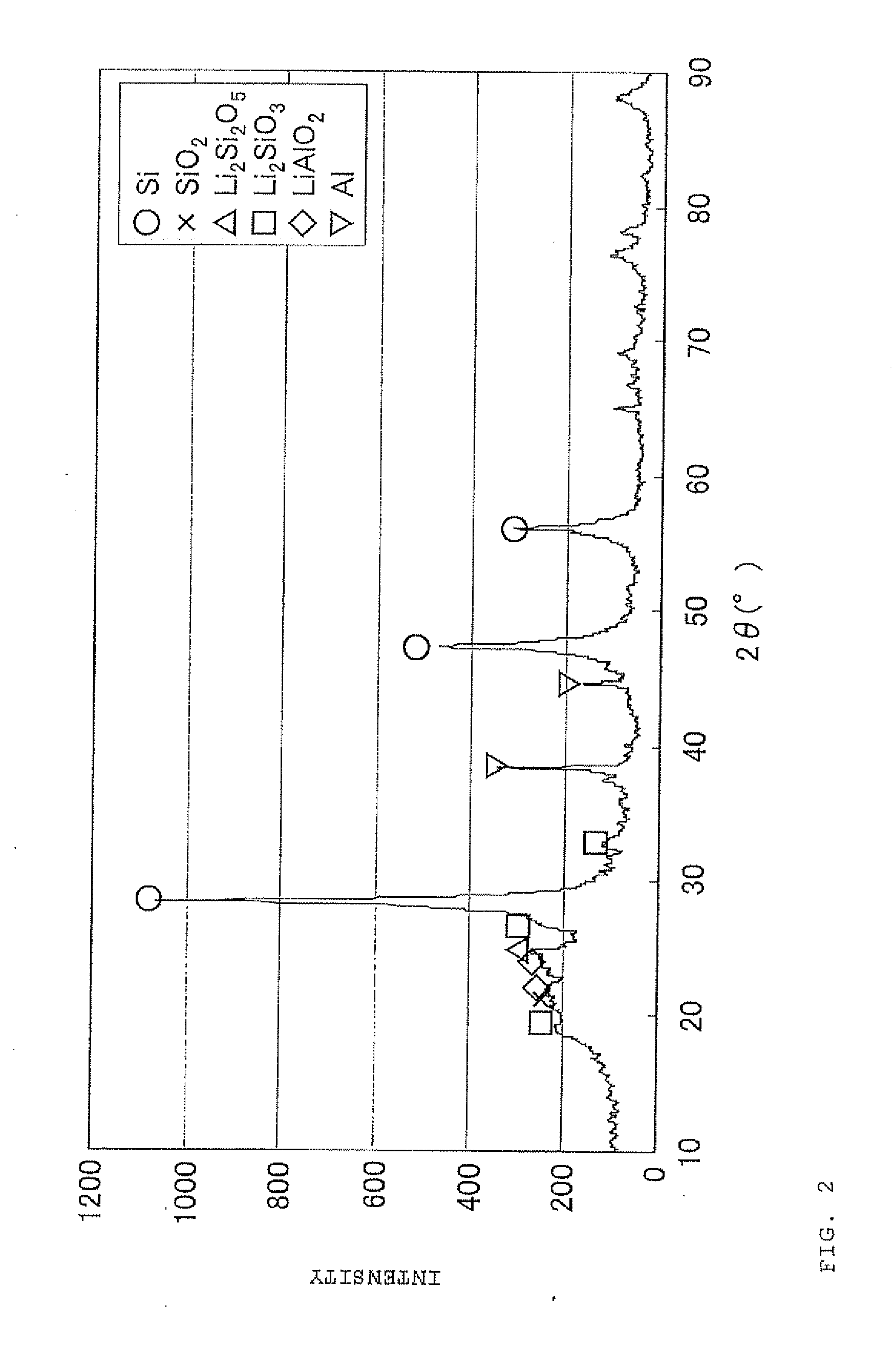
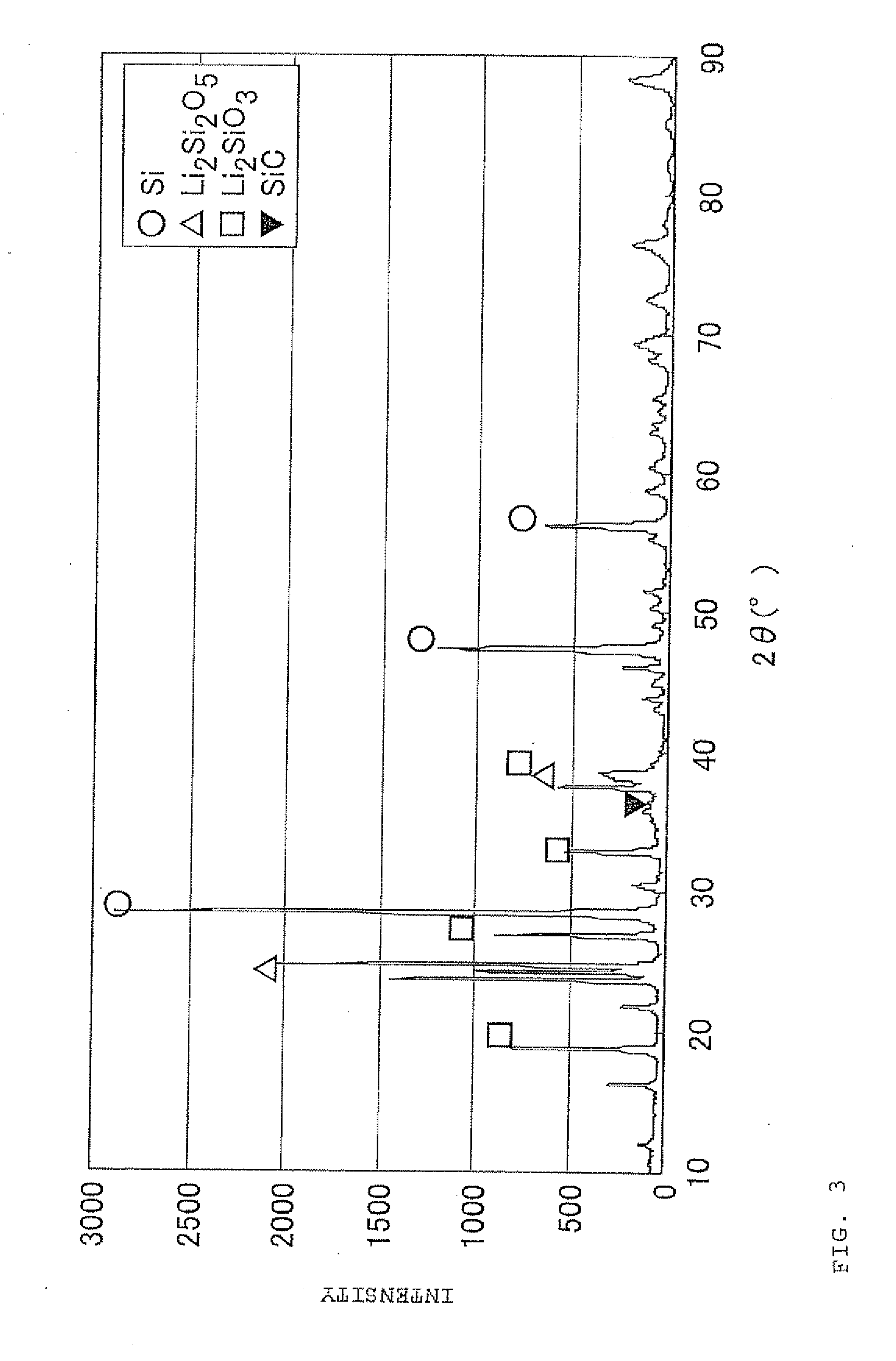
Negative electrode material for secondary battery with non-aqueous electrolyte, method for manufacturing negative electrode material for secondary battery with non-aqueous electrolyte, and lithium ion secondary battery
ActiveUS20110244333A1Cycle durability of negativeElectronic conductivity of negativeMaterial nanotechnologyElectrode thermal treatmentOxide compositeAtomic order
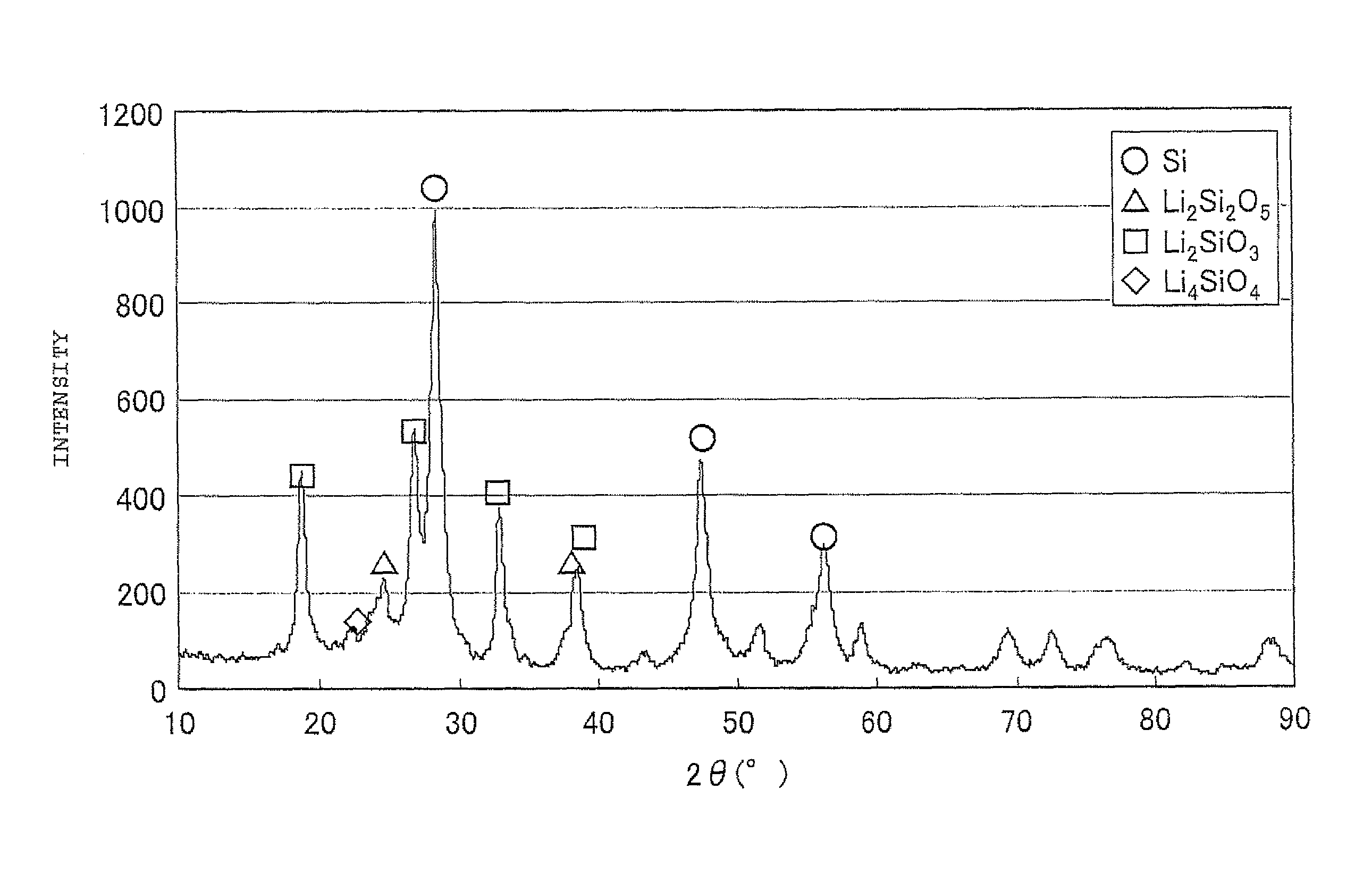
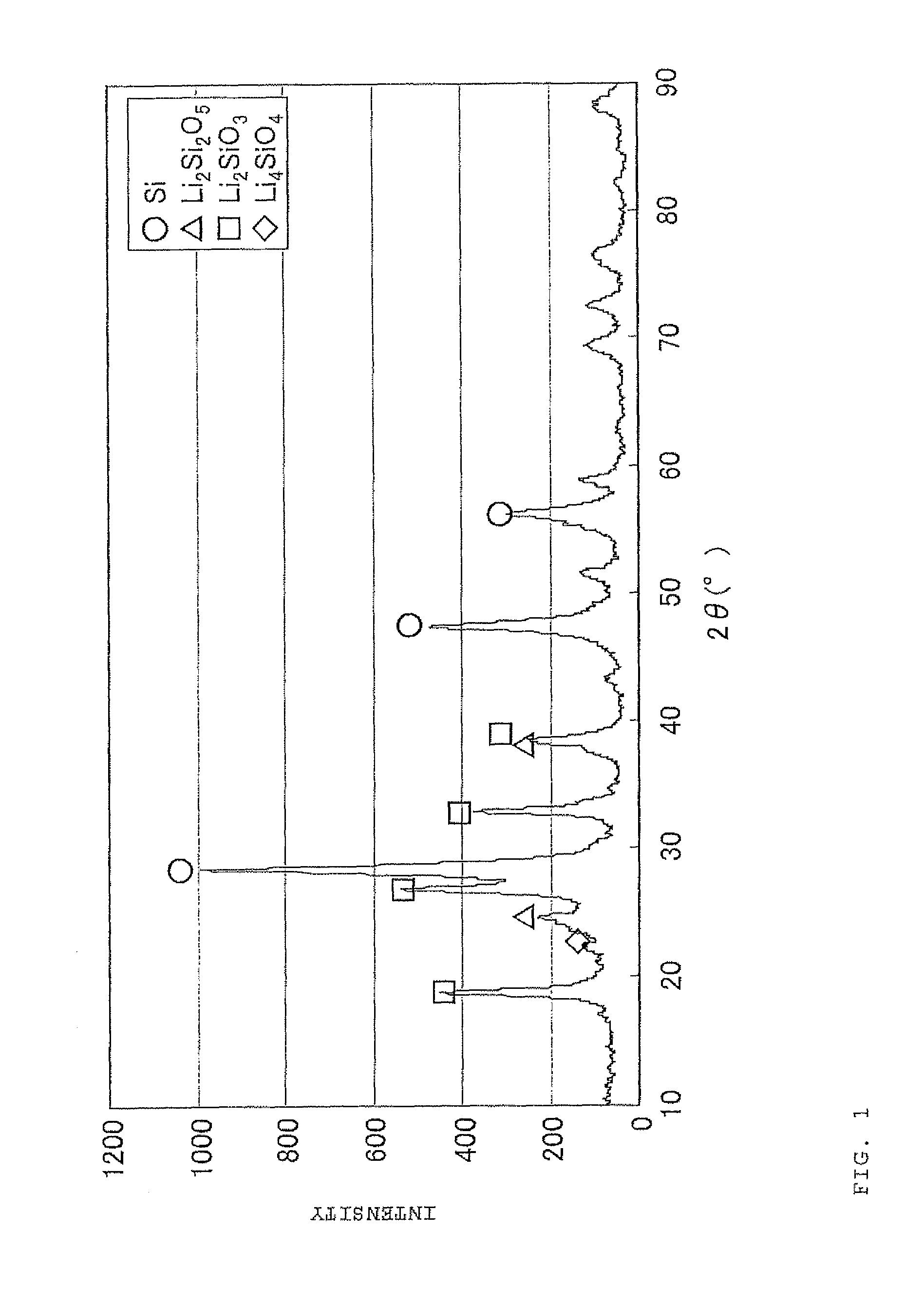
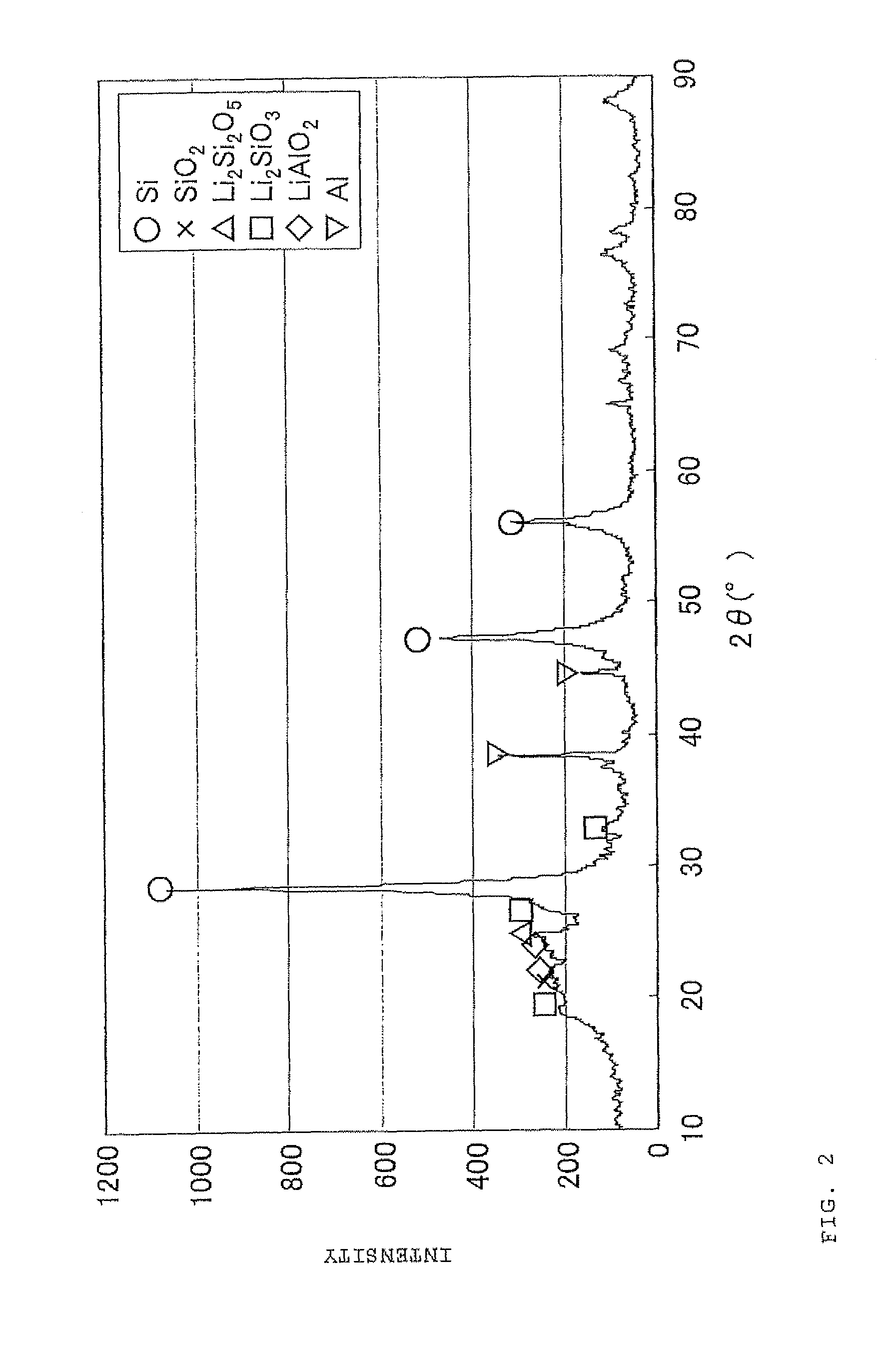
The present invention is a method for manufacturing a negative electrode material for a secondary battery with a non-aqueous electrolyte comprising at least: coating a surface of powder with carbon at a coating amount of 1 to 40 mass % with respect to an amount of the powder by heat CVD treatment under an organic gas and / or vapor atmosphere at a temperature between 800° C. and 1300° C., the powder being composed of at least one of silicon oxide represented by a general formula of SiOx (x=0.5 to 1.6) and a silicon-silicon oxide composite having a structure that silicon particles having a size of 50 nm or less are dispersed to silicon oxide in an atomic order and / or a crystallite state, the silicon-silicon oxide composite having a Si / O molar ratio of 1 / 0.5 to 1 / 1.6; blending lithium hydride and / or lithium aluminum hydride with the powder coated with carbon; and thereafter heating the powder coated with carbon at a temperature between 200° C. and 800° C. to be doped with lithium at a doping amount of 0.1 to 20 mass % with respect to an amount of the powder. As a result, there is provided a method for manufacturing a negative electrode material for a secondary battery with a non-aqueous electrolyte that enables a silicon oxide negative electrode material superior in first efficiency and cycle durability to conventional ones to be mass-produced (manufactured) readily and safely even in an industrial scale.
Owner:SHIN ETSU CHEM IND CO LTD
Method for reducing 8-benzyl-2,8-diazabicyclo[4,3,0]nonane and chiral isomer thereof
The invention discloses a method for reducing 8-benzyl-2,8-diazabicyclo[4,3,0]nonane and a chiral isomer thereof. The method comprises the steps of: taking 8-benzyl-7,9-dioxo2,8-diazabicyclo[4,3,0]nonane or (1S,6R)-8-benzyl-7,9-dioxo2,8-diazabicyclo[4,3,0]nonane as a raw material, and adopting a metal borohydride / BF3 reduction system for reduction to obtain a corresponding product, namely the 8-benzyl-2,8-diazabicyclo[4,3,0]nonane or (S,S)-8-benzyl-2,8-diazabicyclo[4,3,0]nonane. The method adopts the metal borohydride / BF3 reduction system for reduction, avoids the use of an expensive and dangerous reagent, namely lithium aluminum hydride, reduces production cost, improves the safety of the operation, and provides a safe and economical production method for industrial mass production of a moxifloxacin intermediate, namely the (S,S)-8-benzyl-2,8-diazabicyclo[4,3,0]nonane.
Owner:ZHEJIANG LIAOYUAN PHARM CO LTD
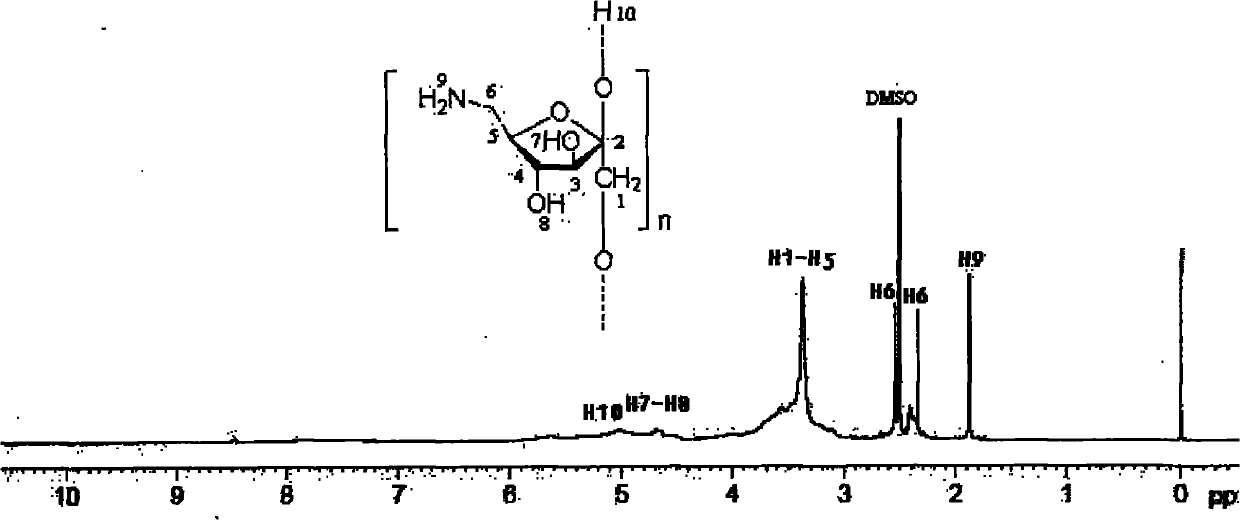
6-amino-6-deoxyinulin as well as preparation and application thereof
ActiveCN102060942AImprove high-value utilizationHigh reactivityOrganic active ingredientsAntinoxious agentsSulfonyl chlorideLeaving group
The invention relates to fields of daily chemicals and pharmaceuticals industry, in particular to 6-amino-6-deoxyinulin as well as preparation and application thereof. In the 6-amino-6-deoxyinulin shown as a formula (I), the average value range of n is 10-35. The preparation comprises the steps of undergoing a helogenation reaction of inulin or undergoing esterification of inulin and sulfonyl chloride, respectively undergoing a reaction of the product obtained through the helogenation reaction of the inulin or the esterification and sodium azide or azido lithium at a temperature of 40-70 DEG C for 8-24 hours to obtain 6-azido-6-deoxyinulin, and reducing the 6-azido-6-deoxyinulin by utilizing triphenylphosphine or lithium aluminium hydride to obtain the 6-amino-6-deoxyinulin. In the invention, the 6-amino-6-deoxyinulin is obtained through an effective synthetic means; an inulin six-position easily-leaving group is obtained through utilizing halogen to substitute the primary hydroxyl ofthe inulin or through the esterification of inulin and sulfonyl chloride; and the 6-amino-6-deoxyinulin with high substitution is obtained through azido nucleophilic substitution and reduction. The 6-amino-6-deoxyinulin synthesized by the preparation method provided by the invention is simple in the synthetic step and easy to popularize; and the required equipment and raw materials are easy to get. The formula (I) is shown in the specification.
Owner:YANTAI INST OF COASTAL ZONE RES CHINESE ACAD OF SCI
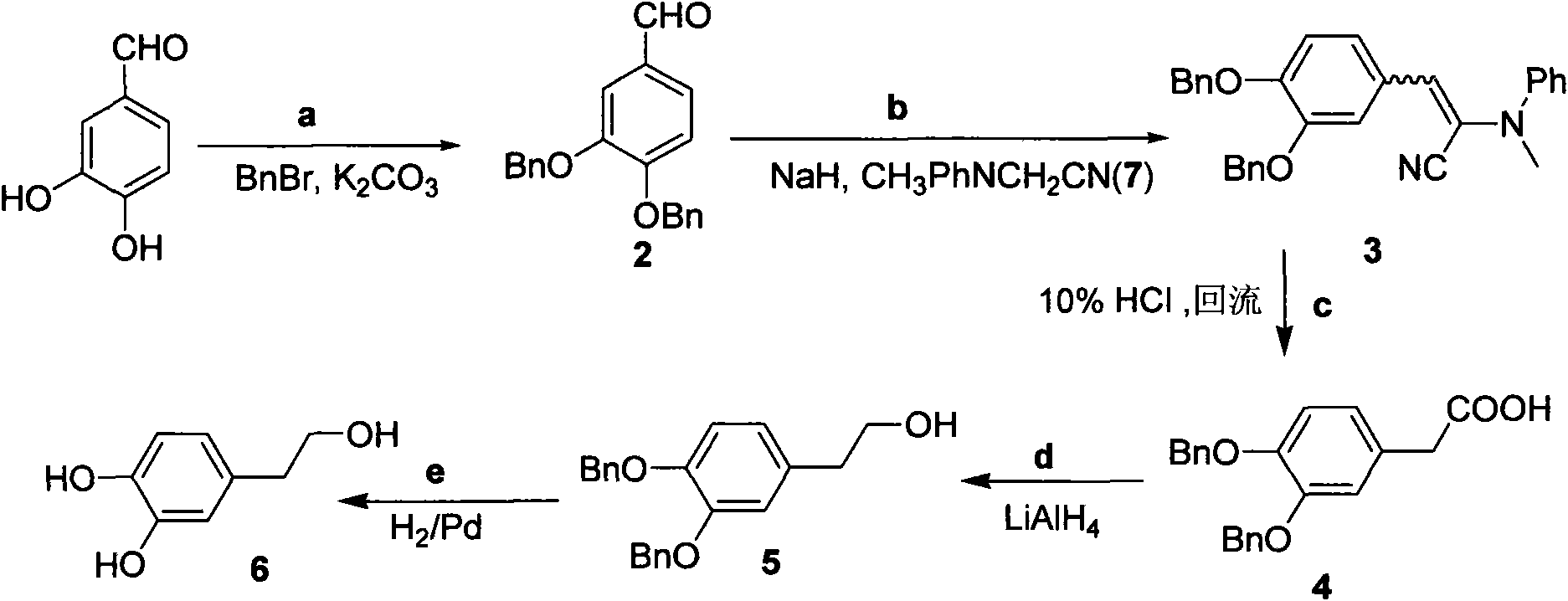
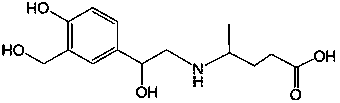
Method for preparing hydroxytyrosol
InactiveCN101891595AMild reaction conditionsFew reaction stepsOrganic chemistryOrganic compound preparationHydroxytyrosolBenzoyl bromide
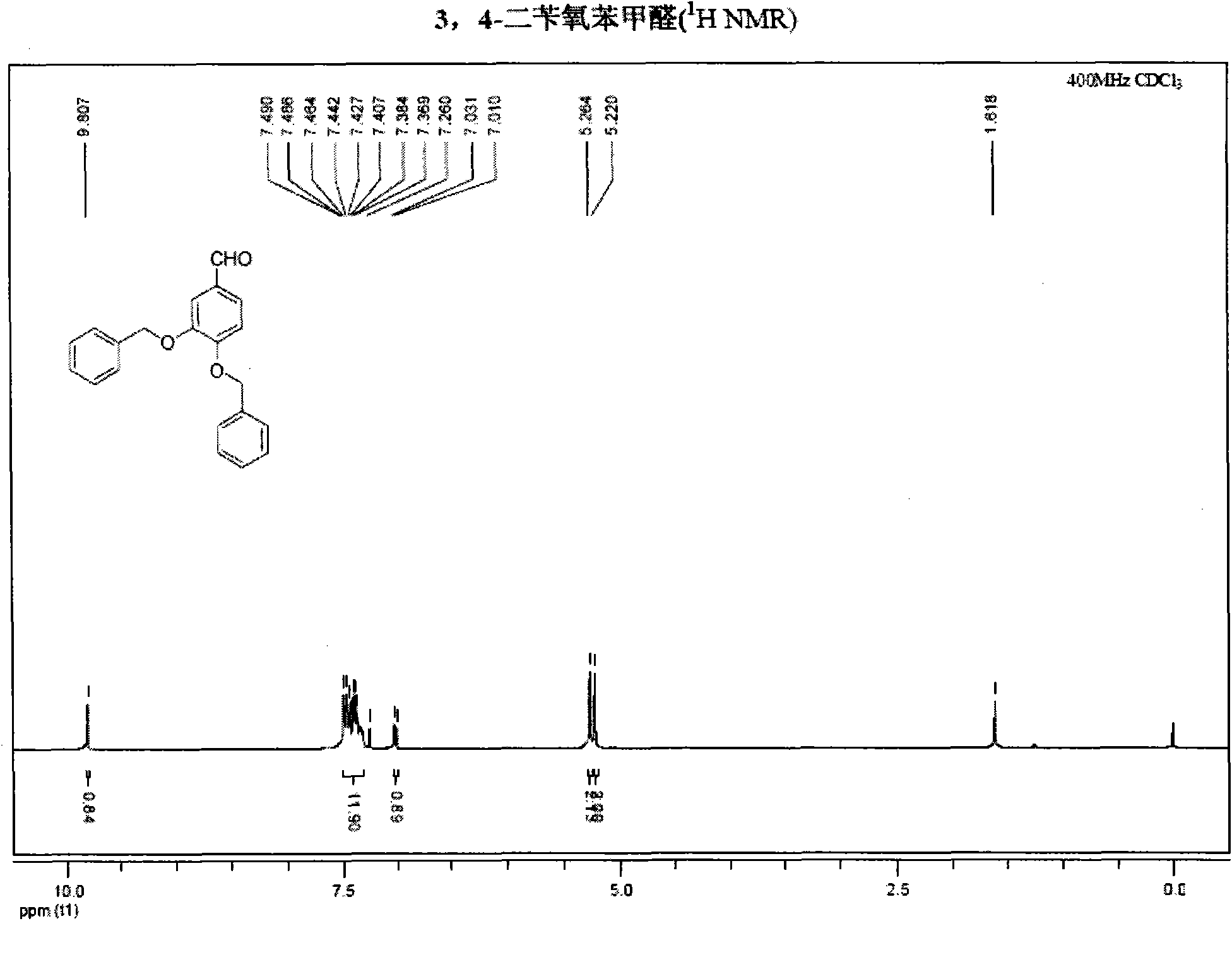
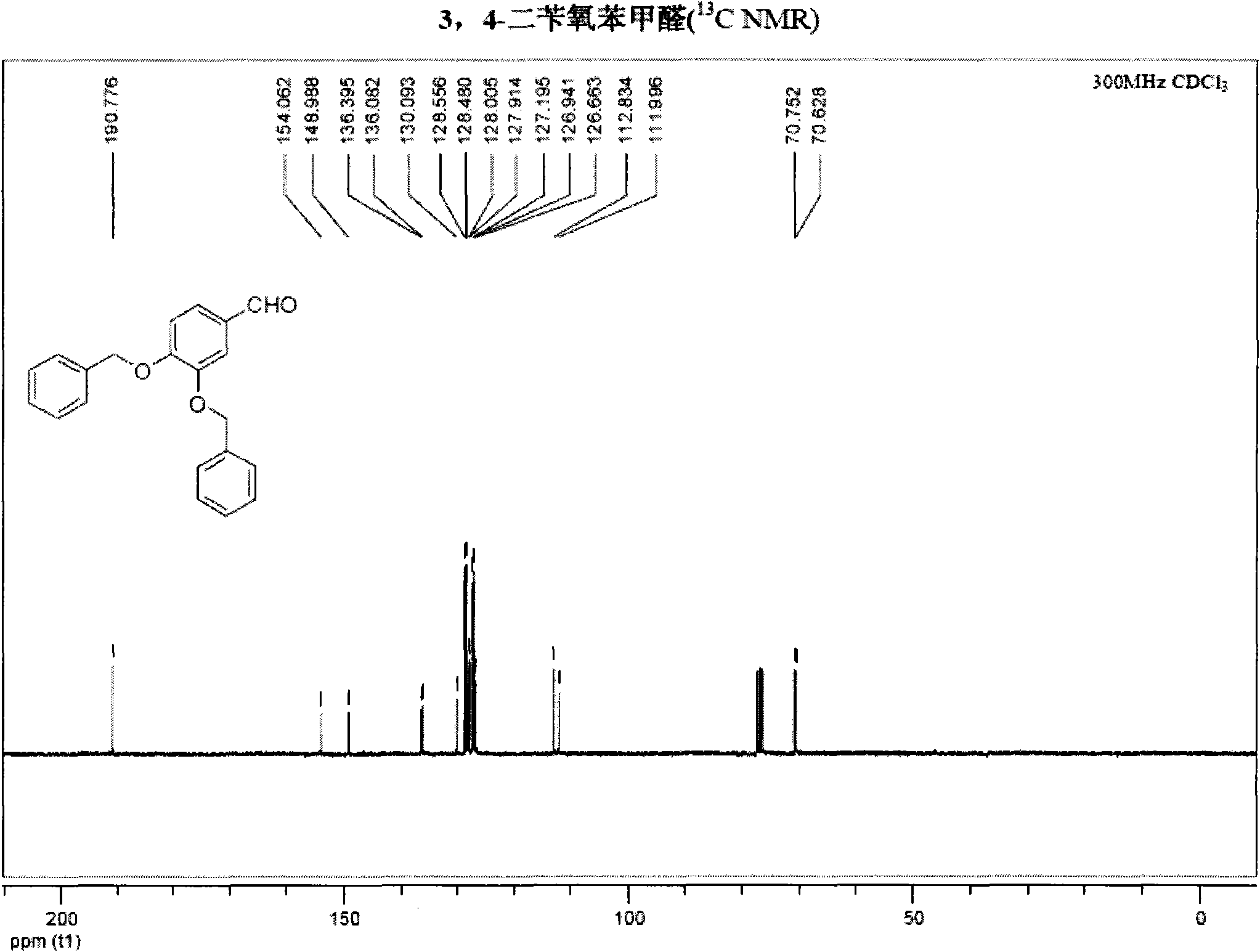
The invention belongs to the field of medicinal synthesis, and particularly relates to a method for preparing hydroxytyrosol, which comprises the following steps of: (1) protecting free hydroxyl groups at 3 and 4 positions, on 3,4-dihydroxy benzaldehyde with benzyl, namely, reacting the 3,4-dihydroxy benzaldehyde with benzyl bromide to prepare 3,4-dibenzyloxybenzaldehyde; (2) reacting N-methylaniline acetonitrile with the 3,4-dibenzyloxybenzaldehyde to prepare 3-(3,4-dibenzyloxyphenyl)-2-(methylphenylamino) acrylonitrile, and hydrolyzing the 3-(3,4-dibenzyloxyphenyl)-2-(methylphenylamino) acrylonitrile under acidic condition to prepare a 3,4-dibenzylosyphenylacetic acid; (3) reducing a carboxyl group of the 3,4-dibenzylosyphenylacetic acid with lithium borohydride, lithium aluminum hydride or sodium borohydride to prepare 3,4-dibenzyloxyphenethyl alcohol; and (4) catalyzing the 3,4-dibenzyloxyphenethyl alcohol with a catalyst palladium / carbon to prepare the hydroxytyrosol. The reagents used in the method are easily obtainable and low in cost, reaction conditions are mild, and the final overall yield of the whole reaction reaches 50 to 60 percent.
Owner:SUZHOU UNIV
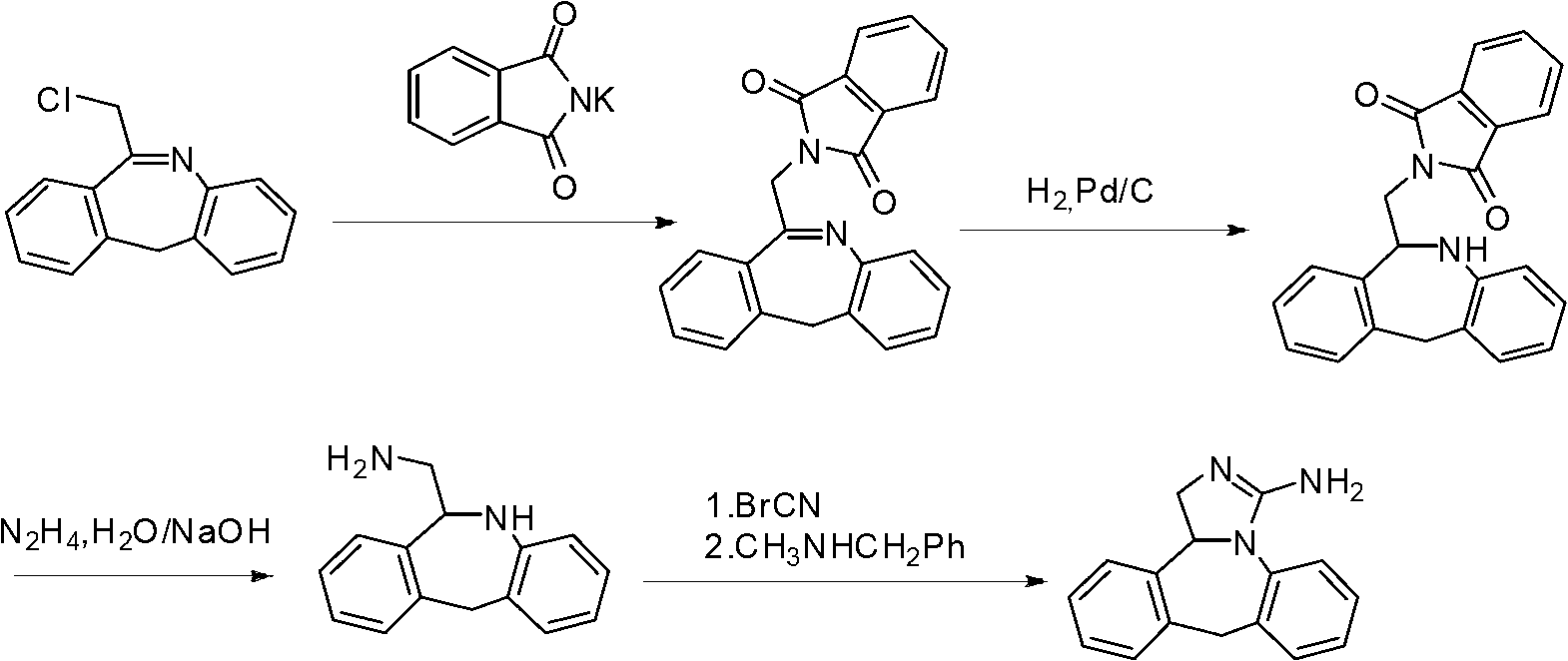
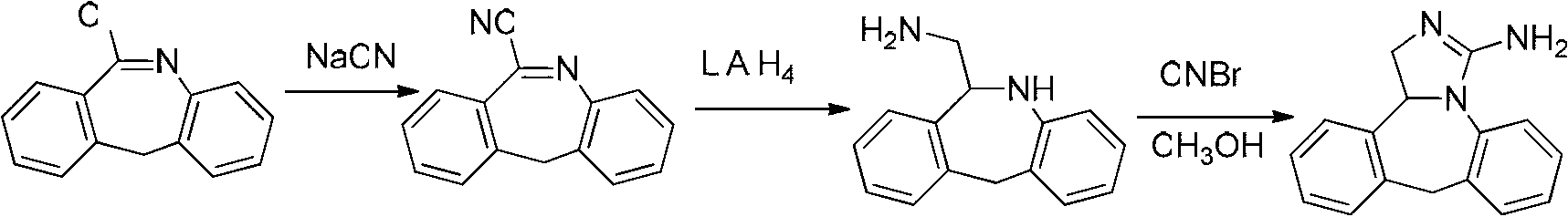
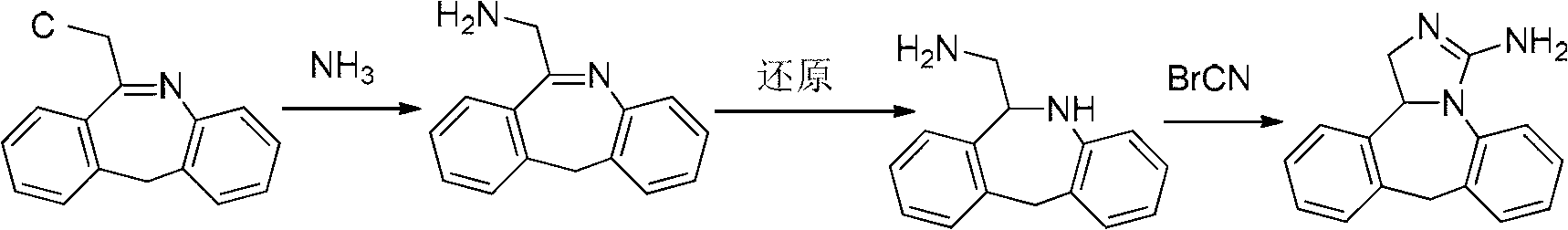
Synthesis method of epinastine
The invention discloses a synthesis method of epinastine. The synthesis method is implemented by taking 2-aminobenzophenone as a raw material and comprises the following steps of: reacting the 2-aminobenzophenone with a silane agent to obtain 2-benzylaniline; then, carrying out acylation reaction on the 2-benzylaniline and 2-chloroacetyl chloride to obtain N-(2-benzyl phenyl)-2-chloroacetamide; carrying out acidamide dehydration and cyclization on the N-(2-benzyl phenyl)-2-chloroacetamide under the action of a dehydrating agent to obtain 6-(chloromethyl)-11H-dibenzo[b,e] azepine; carrying out azidation reaction on the 6-(chloromethyl)-11H-dibenzo[b,e] azepine to obtain 6-(azido-methytbiphenyl)-11H-dibenzo[b,e] azepine; carrying out reduction on the 6-(azido-methytbiphenyl)-11H-dibenzo[b,e] azepine to obtain 6-(aminomethyl)-6,11-dihydro-1H-dibenzo[b,e] azepine; and finally, carrying out cyclization on the 6-(aminomethyl)-6,11-dihydro-1H-dibenzo[b,e] azepine and cyanogen bromide to obtain the epinastine. The synthesis method disclosed by the invention avoids the application of expensive and flammable lithium aluminium hydride and aluminium hydride as well as hypertoxic sodium cyanide, so that the operation is safer in industrial production, and the cost is reduced. The method is simple in process and high in yield, requires mild conditions, and is suitable for industrialized production.
Owner:GUANGZHOU INST OF BIOMEDICINE & HEALTH CHINESE ACAD OF SCI
Graphene preparation method
ActiveCN103950919AInflation implementation time is shortImprove electrical performanceGrapheneFreeze-dryingALUMINUM HYDRIDE
The invention discloses a graphene preparation method. The method comprises the following steps: mixing a precursor first-order or second-order graphite intercalation compound with 1-10wt% of a reducing solution capable of generating a gas, and allowing the obtained mixture to stand for 1-5min to obtain highly expanded graphite, wherein the reducing solution is an aqueous solution of borohydride or a tetrahydrofuran solution of lithium aluminum hydride; pickling to remove impurities, filtering, carrying out ultrasonic dispersion of the obtained graphite in an aqueous solution or ethanol solution containing 0.5-5wt% of a dispersant for 0.5-3h to obtain a graphene dispersion; and freeze-drying the dispersion to obtain graphene powder, or adding a water-soluble high molecular polymer for co-precipitation to obtain a graphene-containing polymer master batch. The method has the advantages of realization of the repairing of the structural defect of graphene, mild reaction, fast reaction speed; and compared with an oxidative catalysis system, the method disclosed in the invention supplements the peeling of a reducing system in the graphene preparation process, so the graphene quality is further improved.
Owner:SOUTH CHINA UNIV OF TECH
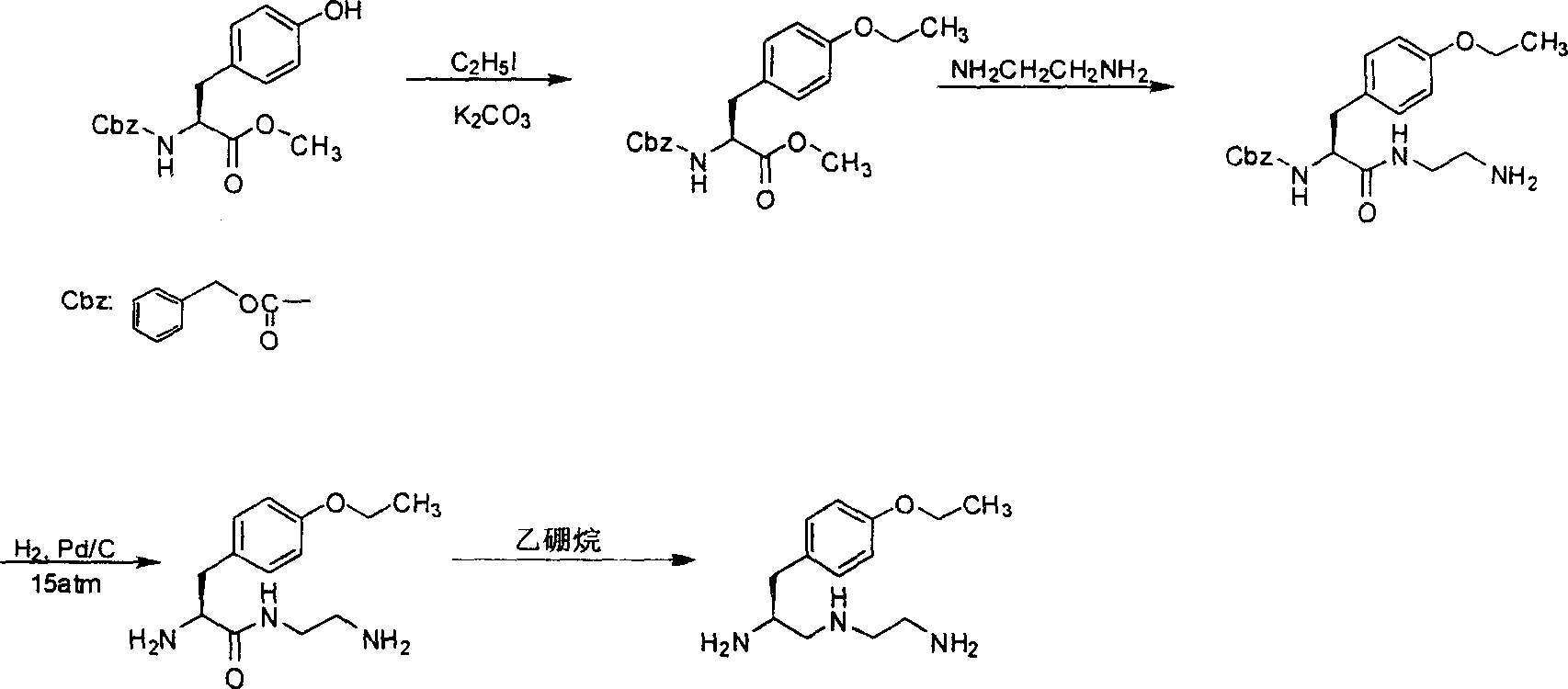
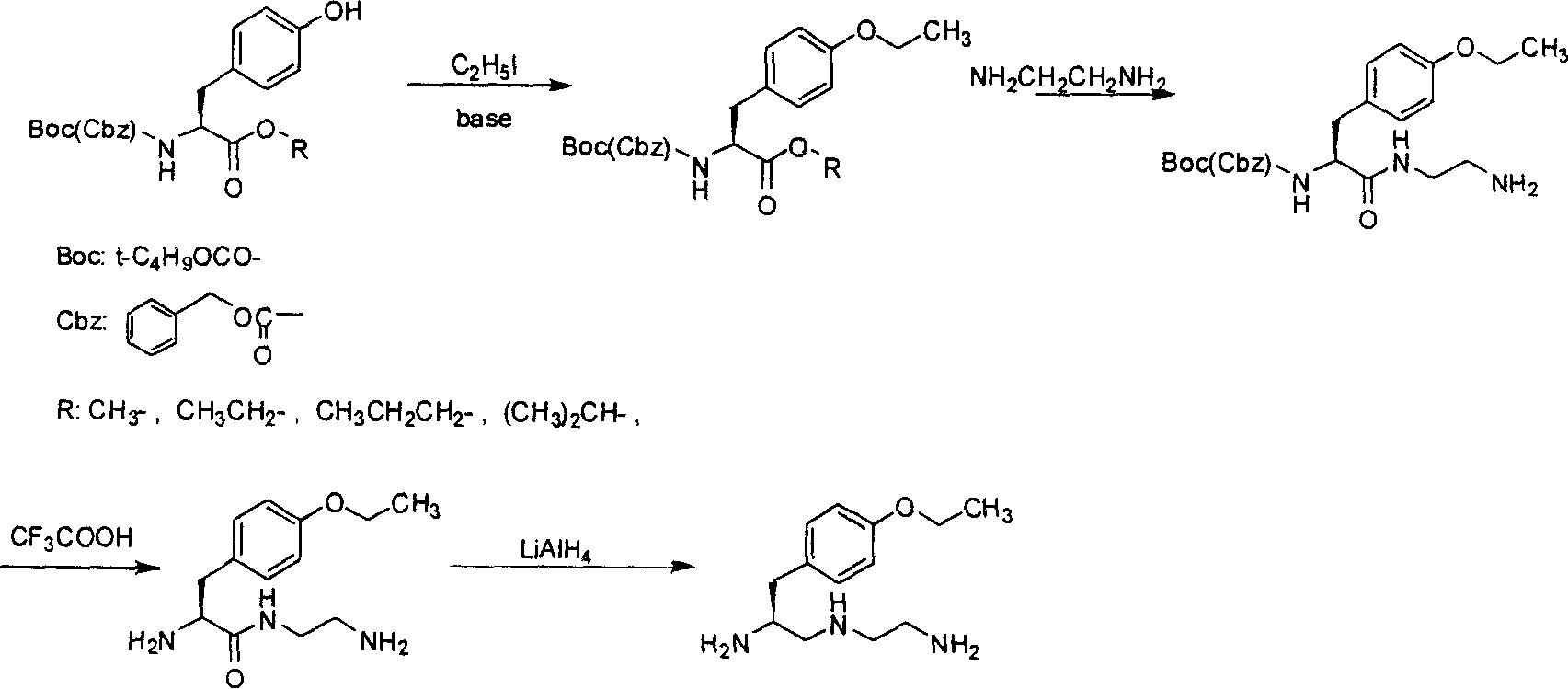
S-1-(4-ethoxybenzyl)-3-azapentane-1,5 diamine preparation method
ActiveCN101007775AImprove securityHigh yieldOrganic compound preparationCarboxylic acid amides preparationLithium hydroxideOrganic synthesis
The invention discloses a making method of S-1-(4-ethoxy benzyl)-3-aza pantane-1, 5 diamine in the organic synthetic technical domain, which comprises the following steps: 1reacting L-tyrosine alkyl ester protected by amino and ethyl iodide catalyzed by anhydrous carbonate alkali to produce L-tyrosine alkyl ester protected by O-ethyl-amino; 2. reacting L-tyrosine alkyl ester protected by O-ethyl-amino and anhydrous ethanediamine to do ester exchange to produce O-ethyl-L-tyrosinamide protected by N-(2-amino ethyl)-amino; 3. stripping protecting group of amino through trifluoroacetic acid; 4. using aluminium lithium hydroxide to reduce; obtaining the product. The invention reduces technical difficulty with mild reacting condition, which can prepare key intermediate of Gadoxetic acid disodium (Gd-EOB-DTPA).
Owner:CHIA TAI TIANQING PHARMA GRP CO LTD
Aluminum catalyst for polyester synthesis, preparation method thereof and usage method thereof
The invention discloses an aluminum catalyst for polyester synthesis, a preparation method thereof and a usage method thereof. The catalyst which is aluminum glycol and is special for a polycondensation reaction in a polyester synthesis process of terephthalic acid and glycol is prepared through reacting aluminum / aluminum alcoholates / lithium aluminum hydride with glycol. The catalyst, which has less toxicity, allows the molecular weight of PET catalyzed by the catalyst to achieve more than 30,000, can coexist with polycondensation products, has no influence on the quality of the PET, allows requirements of environmental protection to be satisfied and requirements of activity to be guaranteed, has a high activity on the PET synthesizing reaction, and has low cost, is a novel catalyst for the condensation polymerization of PET synthesis.
Owner:CHANGZHOU INST OF CHEM +1
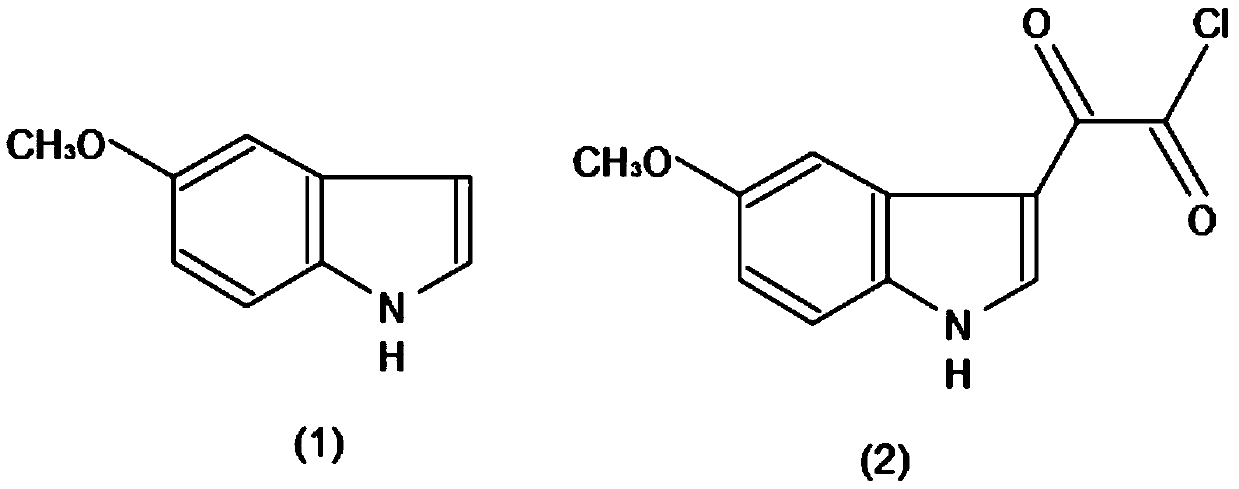
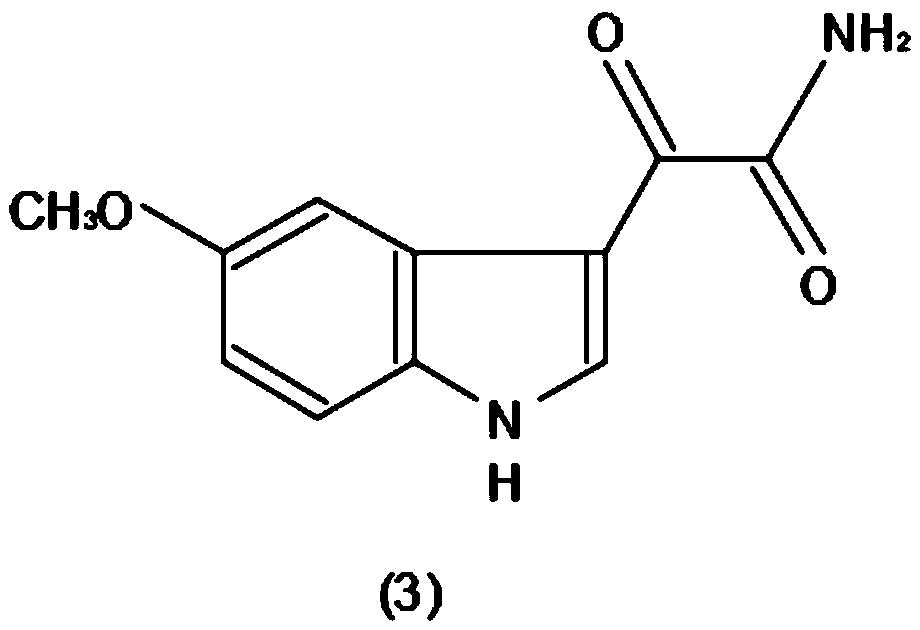
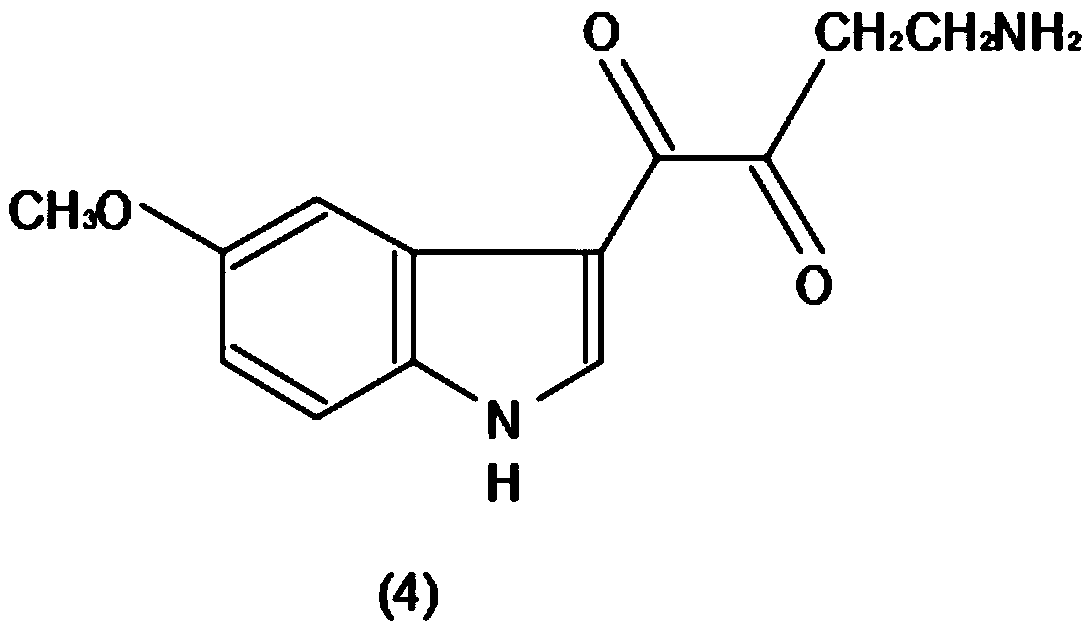
Method for efficiently producing melatonin
InactiveCN110229092AHigh total extraction rateShort operating cycleOrganic chemistryMelatonin synthesisState of art
The invention discloses a method for efficiently producing melatonin, relates to the technical field of melatonin synthesis, and aims to solve the problems that purity and an extraction rate of melatonin are relatively low and preparation cost is relatively high in the prior art. According to the method, 5-methoxy indole is taken as 100% of a raw material, carbonyl chloride accounts for 98% of theraw material, ammonium hydroxide accounts for 57% of a generation compound, and lithium aluminum hydride accounts for 80% of the generated compound. The 5-methoxy indole (1) is taken as a raw material, and a carbonyl chloride reaction solvent is added to acylate the 5-methoxy indole, so that a reaction is carried out to generate a compound (2); the generated compound (2) is stranded, and then anammonium hydroxide solvent is mixed with the generated compound (2) according to a certain usage amount to generate a compound (3); and after the compound (3) is generated, a lithium aluminum hydridereagent is added to carry out a reduction reaction on the compound (3) to generate a compound (4).
Owner:罗田县新普生药业有限公司
Method for preparing aluminum nanoparticles coated with dispersion stabilizers by liquid-phase chemical reduction method
InactiveCN103056388AAvoid reunionProvides antioxidant protectionNanotechnologyDispersityAluminium chloride
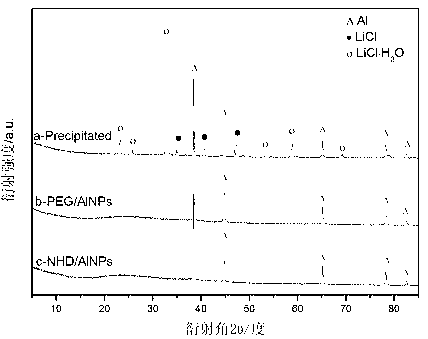
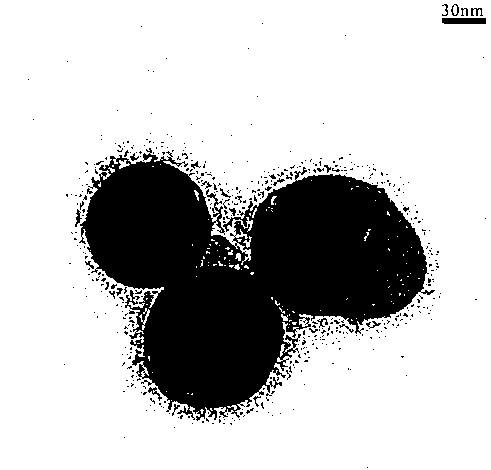
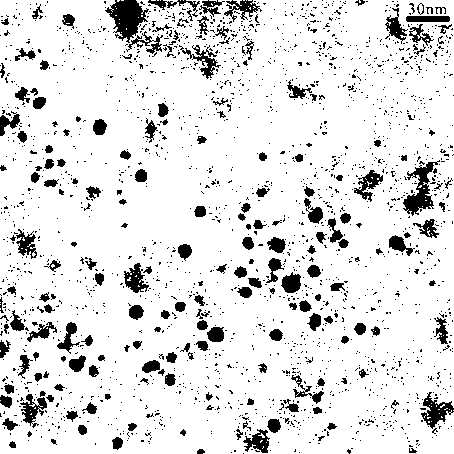
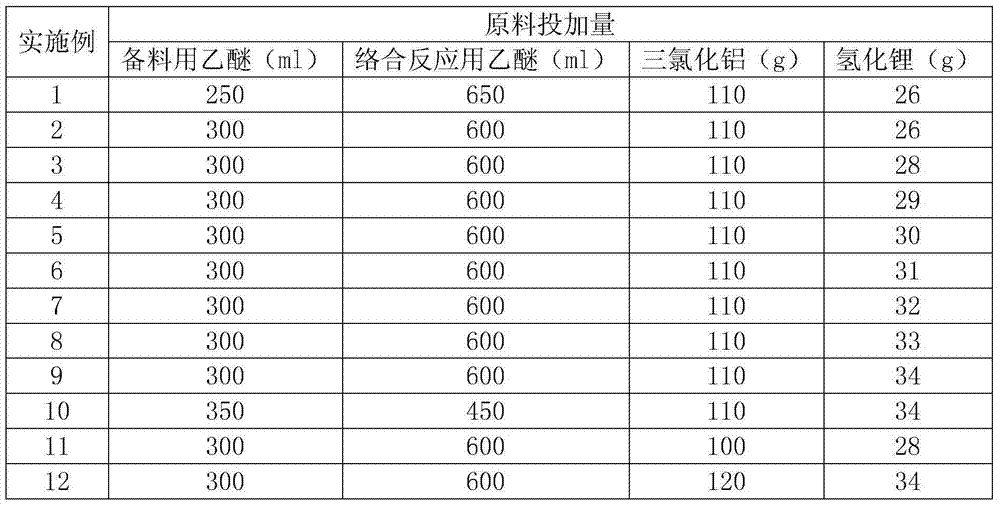
The invention discloses a method for preparing aluminum nanoparticles coated with dispersion stabilizers by a liquid-phase chemical reduction method. The method is characterized by including: purifying commercially available mesitylene; dispersing aluminum chloride in the mesitylene; and in the presence of nitrogen, adding polyethylene glycol or polyethylene glycol dimethyl ether serving as the dispersion stabilizer and lithium aluminum hydride in the mesitylene dispersed with the aluminum chloride according to the mass ratio of 1-2.5:0.5-1:0.5-1 among the aluminum chloride, the lithium aluminum hydride and the dispersion stabilizer, stirring for reacting 12-24 hours at the temperature of 164-166 DEG C, cooling, performing centrifugal separation, abandoning supernatant liquid, removing residual mesitylene solvents, washing by low-temperature methyl alcohol, performing ultrasonic washing and centrifugal separation, abandoning supernatant liquid and performing vacuum drying for lower materials so that the aluminum nanoparticles coated with the dispersion stabilizers are obtained. The prepared aluminum nanoparticles coated with the dispersion stabilizers are uniform in size and good in dispersity, have certain activity and are applicable to the fields of rocket propellants, explosives and powders, solar back plates and the like.
Owner:SOUTHWEAT UNIV OF SCI & TECH
Method for preparing electrolyte for lithium-ferrous disulfide battery by one-step method
The invention belongs to the technical field of primary lithium batteries, and discloses a method for preparing electrolyte for a lithium-ferrous disulfide battery by a one-step method. The method comprises the following steps: in inert atmosphere, adding elemental iodine into an organic solvent and stirring the organic solvent evenly at 0-5 DEG C; adding lithium aluminum hydride or lithium hydride and carrying out stirring reaction for 1-2 hours; heating the mixture to 40-60 DEG C and carrying out stirring reaction for 1-2 hours; and centrifuging and filtering the mixture to obtain the electrolyte for the lithium-ferrous disulfide battery. According to the preparation method, the electrolyte for the lithium-ferrous disulfide battery is synthesized by the one-step method; and no water is introduced in the entire preparation process, so that the cost is relatively low; and the lithium-ferrous disulfide battery prepared from the electrolyte prepared by the method is good in property.
Owner:EVE ENERGY CO LTD
Preparation process for lithium aluminum hydride-tetrahydrofuran solution
InactiveCN104759215AStrong reductionEasy to useMultiple metal hydridesMixing methodsMaterials preparationDistillation
The invention especially relates to a preparation process for a lithium aluminum hydride-tetrahydrofuran solution, which belongs to the field of preparation technology for chemical reagents. The preparation process is characterized by comprising the following steps: material preparation; complexation reaction; drying by distillation; and preparation of the lithium aluminum hydride-tetrahydrofuran solution. The preparation process has the following advantages: a reducing agent with extremely strong reduction capability is synthesized, and rapid development of organic synthesis technology, especially development of the pharmaceutical industry, can be effectively promoted; the lithium aluminum hydride-tetrahydrofuran solution is safe, simple and convenient to use; and finished lithium aluminum hydride-tetrahydrofuran solution products with different concentrations can be blended according to market demands so as to meet demands of different process conditions.
Owner:车荣睿
Novel method for preparing lithium aluminum hydride
InactiveCN102659080ASimple production methodReduce manufacturing costMultiple metal hydridesLithium chlorideDistillation
The invention relates to a novel method for preparing lithium aluminum hydride. The method comprises the following steps of: 1-, fully dissolving aluminum trichloride in anhydrous ether to prepare a diethyl ether solution of the aluminum trichloride; 2-, crushing lithium hydride particles into powder by adopting a screen mesh under the protection of nitrogen; putting the crushed lithium hydride powder into diethyl ether under the protection of the nitrogen; putting into a reaction kettle, and then evenly mixing to form a uniform lithium hydride and diethyl ether suspension for later use; 3-, mixing the diethyl ether solution of the aluminum trichloride prepared in the step (1) with the lithium hydride and diethyl ether suspension prepared in the step (2) for full reaction; and 4-, collecting clear liquid into a container after byproduct lithium chloride is deposited; performing static distillation to evaporate the diethyl ether in the clear liquid to dryness to obtain a required lithium aluminum hydride solid product. The novel method for preparing the lithium aluminum hydride is simple, safe and reliable, is low in production cost and good in product quality.
Owner:TIANJIN BEIDOUXING FINE CHEM
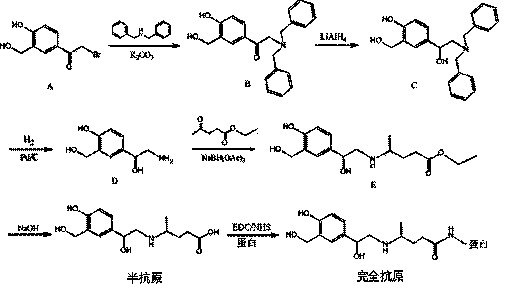
Synthesis method of structurally specific salbutamol complete antigen
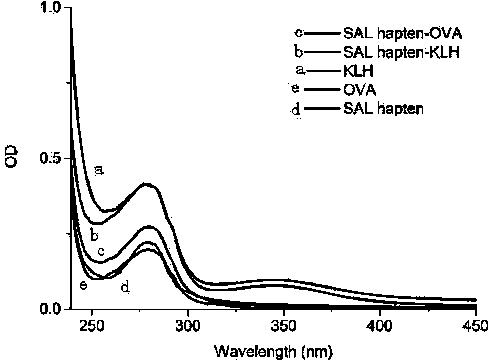
ActiveCN103755802AStrong specificityHigh sensitivityOvalbuminSerum albuminAntiendomysial antibodiesCarboxyl radical
The invention discloses a synthesis method of a structurally specific salbutamol complete antigen and belongs to the technical field of biochemical engineering. The synthesis method comprises the following steps: dissolving 2-bromo-1-[4-hydroxy-3-(hydroxymethyl)phenyl]aceto-1-ketone in acetone; after adding potassium carbonate, reacting with dibenzylamine to generate a compound B; generating a compound C under catalysis of lithium aluminium hydride; reacting with hydrogen under catalysis of Pd / C to generate a compound D; performing a reductive amination reaction with ethyl levulinate to generate a compound E; and hydrolyzing under alkaline condition, and recrystallizing to generate salbutamol hapten named as 4-((2-hydroxy-2-(4-hydroxy-3-(hydroxymethyl)phenyl)ethyl) amino) valeric acid. A complete antigen is obtained by coupling the carboxyl on the hapten and amidogen on carrier proteins. Experimental results show that the antiserum titer of animals immune to the complete antigen achieves 81,000, the limit of detection is 0.3ng / mL, and half inhibiting concentration is 3.2ng / mL; a generated antibody is high in specificity and sensitivity; the antigen or the antibody has a broad application prospect.
Owner:JIANGNAN UNIV
Preparation method of 2-methyl-3-biphenylmethanol
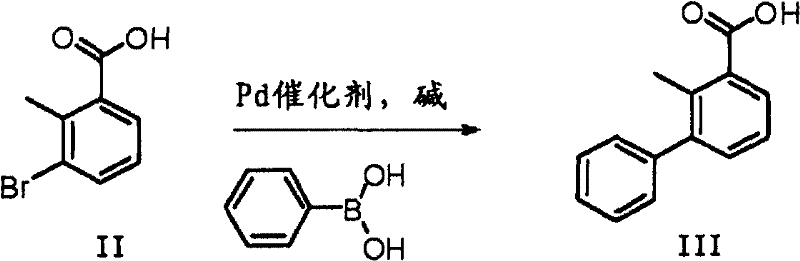
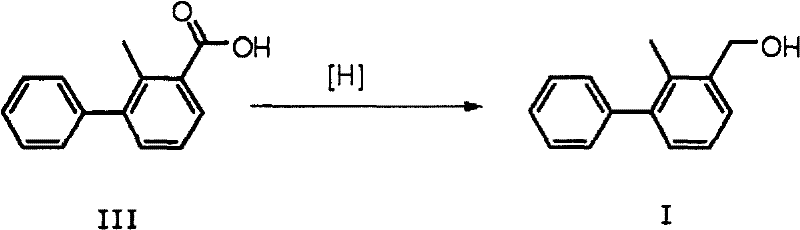
InactiveCN102603485ASimple processRelaxed reaction conditionsOrganic compound preparationHydroxy compound preparationBromineBoric acid
The invention discloses a preparation method of 2-methyl-3-biphenylmethanol, which comprises the following steps: carrying out a Suzuki coupling reaction between 3-bromo-2-methylbenzoic acid and phenyl substituted boric acid or phenyl substituted borate to obtain 3-phenyl-2-methylbenzoic acid, and then carrying out a reduction reaction to obtain 2-methyl-3-biphenylmethanol, wherein the Suzuki coupling reaction is carried out at 10-150 DEG C for 1-12 hours under the action of an alkali. 3-phenyl-2-methylbenzoic acid can be directly reduced with a reducing agent such as borane and lithium aluminium hydride to obtain 2-methyl-3-biphenylmethanol.
Owner:NUTRICHEM LAB CO LTD
Method synthesizing 4, 4, 4-trifluoro butanol
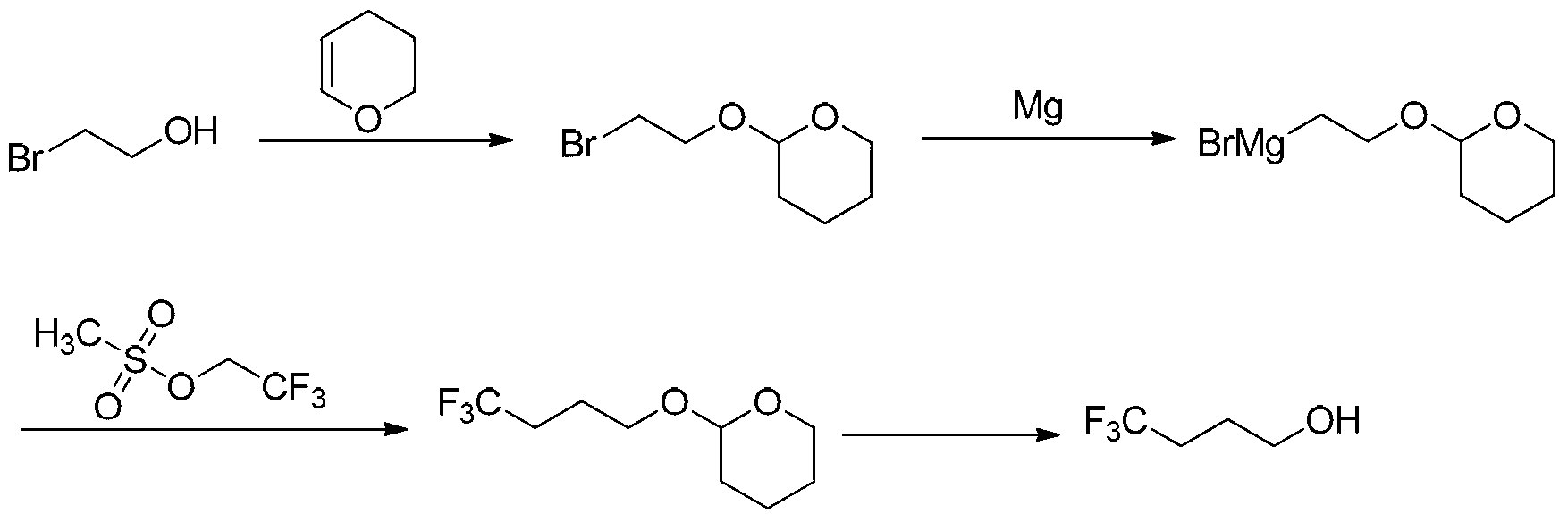
ActiveCN103265403AShort process routeMild reaction conditionsPreparation by hydrolysisAlcoholGrignard reagent
The invention discloses a method for synthesizing 4, 4, 4-trifluoro butanol, wherein the method comprises: using 2-bromoethanol as a raw material, protecting a alcoholic hydroxyl group with 3,4-dihydro pyrans, preparing a Grignard reagent, carrying out coupling reaction of the Grignard reagent with 2,2,2-trifluoro ethyl methanesulfonates, and finally obtaining 4,4,4-trifluoro butanol by deprotection reaction. The method has advantages of short technology route, mild reaction condition, cheap and easily available raw materials, high reaction overall yield and low production cost, is in favor of industrialization production, and avoids usage of expensive raw materials like trifluoro butyric acid and lithium aluminium hydride.
Owner:XIAN CAIJING OPTO ELECTRICAL SCI & TECH
Method for deacidizing and desulfurizing diesel fuel oil
ActiveCN101139530AReduce sulfur contentHigh recovery rateHydrocarbon oils refiningPotassiumSodium hydride
The invention relates to a method for reducing and desulfurizing diesel oil, which comprises such procedures as mixing diesel oil with proton solvent containing divalent transitional metal salt, then adding reducing agent, keeping under 10-40 DEG C to complete reaction; after reaction, keeping still for layering, collecting the oil layer and getting the desulfurized oil. The divalent transitional metal salt is a hydrochloride and sulfate of d-zone metals in the periodic table of elements; the proton solvent is water, methanol, alcohol or oxolane; the reducing agent is tetrahydro boron potassium, aluminum lithium hydride, tetrahydro boron sodium, aluminum sodium hydride or tetrahydro boron lithium. The mole proportion of the divalent transitional metal salt to the proton solvent is 0.1-10:100; the mole proportion of the reducing agent to the divalent transitional metal salt is 100:10-80; the proportion by weight of the reducing agent to the diesel oil is 1-30:100. The invention can be carried out under normal temperature and normal pressure, the operation condition is moderate, the reaction time is short, and the invention can effectively reduce the sulphur content in the diesel oil, and has no special requirement on the reaction devices; the invention is applicable for catalyzed and cracked diesel oil, hydrogenated and cracked diesel oil, straight-run diesel oil or their combinations.
Owner:BC P INC CHINA NAT PETROLEUM CORP +1
Negative electrode material for secondary battery having lithium-doped silicon-silicon oxide composite
ActiveUS8753774B2Superior in first efficiency and cycle durabilityMass-produced (manufactured) readily and safelyElectrode thermal treatmentLi-accumulatorsAtomic orderOxide composite
A method for manufacturing a negative electrode material for a secondary battery with a non-aqueous electrolyte, including coating a surface of a powder with 1 to 40 mass % carbon by heat CVD treatment under an organic gas and / or vapor atmosphere at a temperature between 800° C. and 1300° C., blending lithium hydride and / or lithium aluminum hydride with the carbon-coated powder; and heating the carbon-coated powder at a temperature between 200° C. and 800° C. to be doped with lithium at a doping amount of 0.1 to 20 mass %. The powder is composed of at least one of silicon oxide represented by general formula of SiOx (x=0.5 to 1.6) and a silicon-silicon oxide composite having a structure so that silicon particles having a size of 50 nm or less are dispersed to silicon oxide in an atomic order and / or a crystallite state, and having a Si / O molar ratio of 1 / 0.5 to 1 / 1.6.
Owner:SHIN ETSU CHEM CO LTD
Improved process for preparing alpha-aluminum hydride
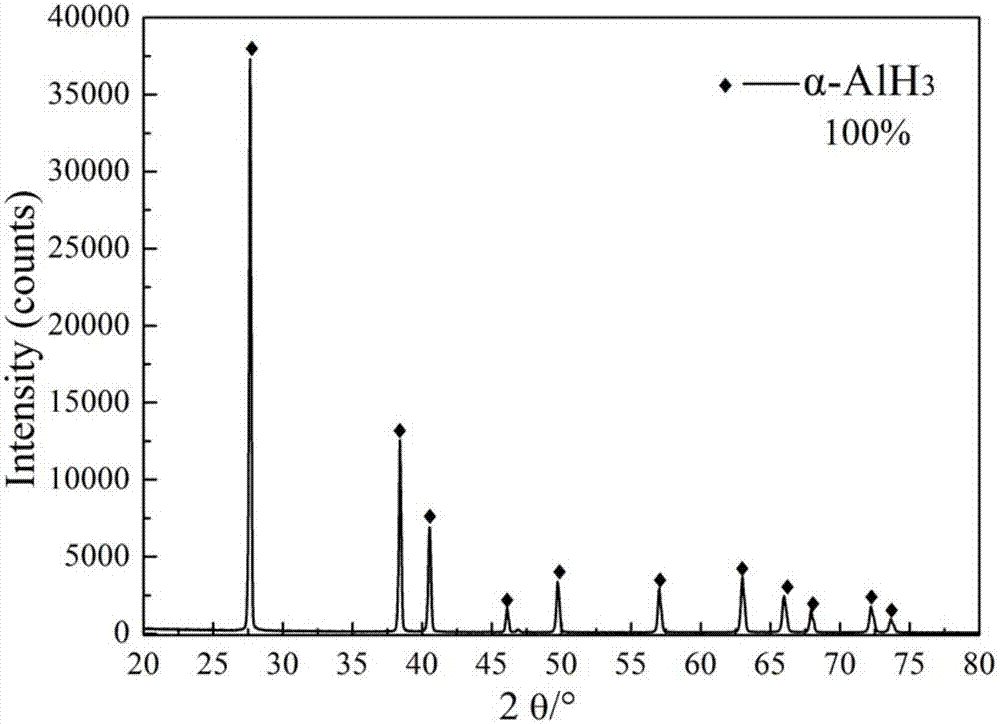
The invention discloses an improved process for preparing alpha-aluminum hydride. The improved process comprises the following steps: (1) refining absolute ether; (2) refining methylbenzene; (3) refining lithium aluminum hydride; (4) preparing a mixed solution of anhydrous AlCl3 and diethyl ether; (5) preparing a mixed solution of LiAlH4 and anhydrous AlCl3; and (6) preparing the alpha-aluminum hydride. The invention provides the improved process for preparing the alpha-aluminum hydride, graphene is used to activate a reaction solution in the process, the production is safe and reliable, the yield is high, the purity of the obtained alpha-aluminum hydride is high, and the distribution of the particle size of a product is even. A preparation method is low in reaction temperature, shorter in reaction time, low in energy consumption, low in equipment requirement, simple in operations and easy in control of the reaction conditions, and is favorable for industrial production. According to the alpha-aluminum hydride prepared with the method, the product purity is more than or equal to 99%, the particle size range is 5-20 microns, the direct cost is less than or equal to 4000 yuan per kilogram.
Owner:河南纳宇新材料有限公司
Preparation method of chloride alkyl hydrogen silane
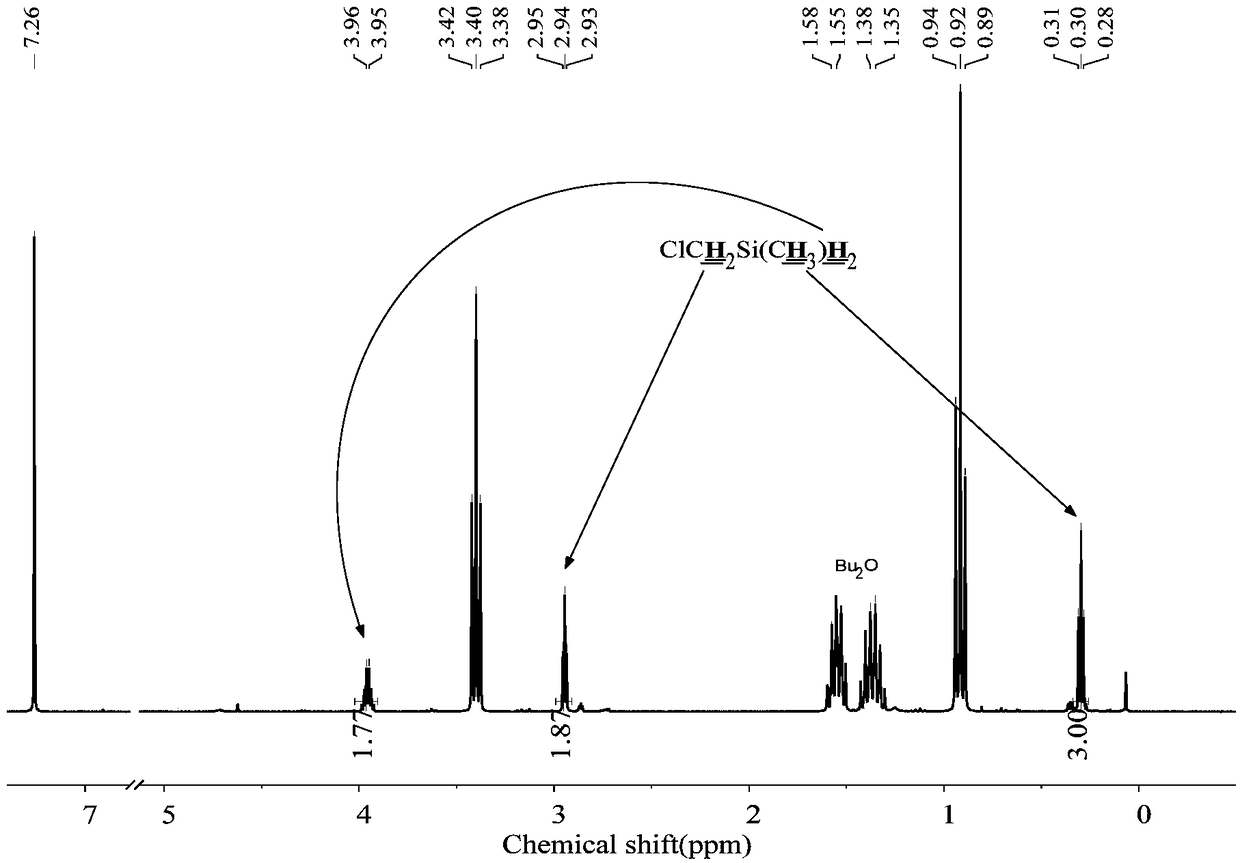
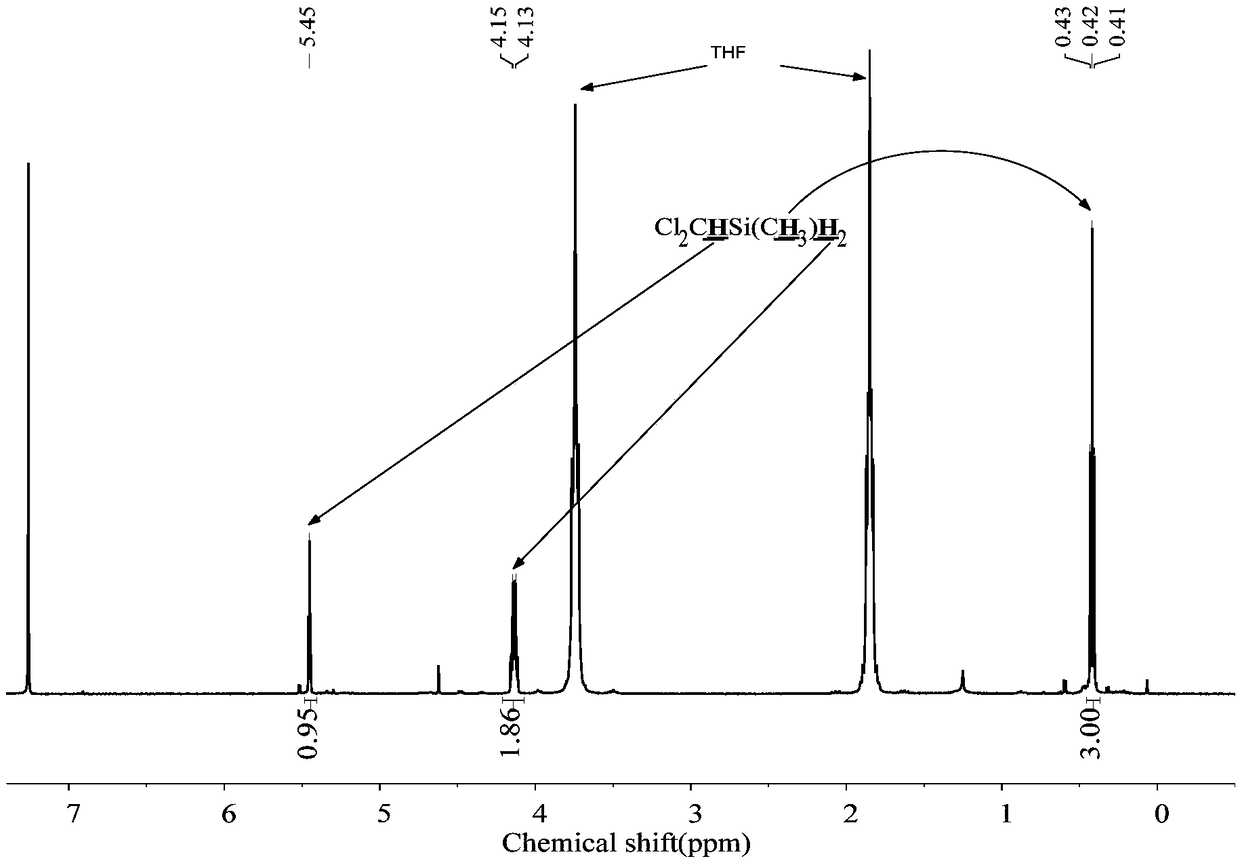
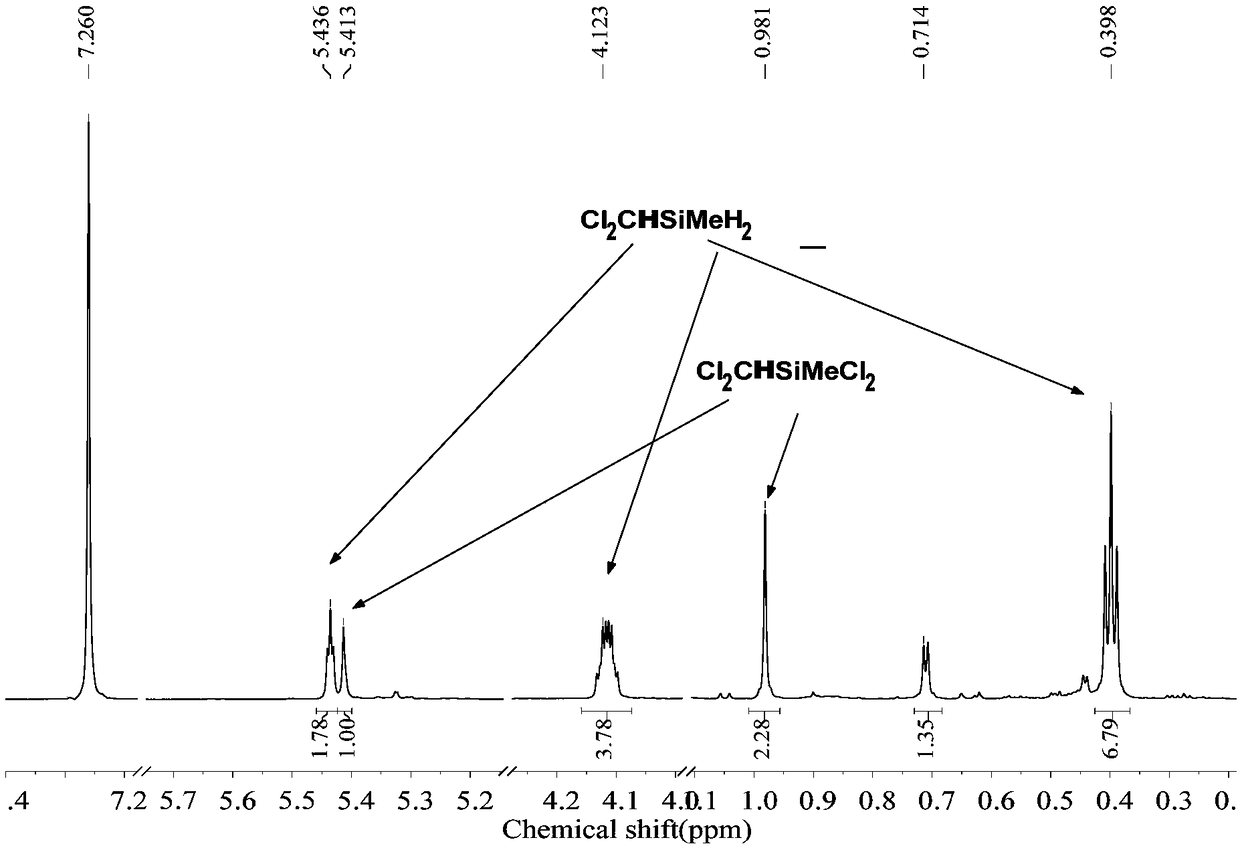
The invention discloses a preparation method of chloride alkyl hydrogen silane, belongs to the technical field of reduction of the chloride alkylchlorosilane, and solves the problem that in the priorart, the chloride alkyl hydrogen silane cannot be prepared through selective reduction of the chloride alkyl chlorosilane. In an ether solvent, under the catalysis of a catalyst, LiH is adopted as a reducing agent, the chloride alkyl chlorosilane is reduced to the chloride alkyl hydrogen silane; the catalyst is boron hydride, borohydride or lithium aluminum hydride. The preparation method can be used for preparing the chloride alkyl hydrogen silane.
Owner:INST OF CHEM CHINESE ACAD OF SCI
Purification method of chromatographic pure 1,4-dioxane
InactiveCN109438413AReach the index requirement of chromatographically pure 1,4-dioxaneReach the index requirement of pure 1,4-dioxaneOrganic chemistryPurification methodsALUMINUM HYDRIDE
The invention provides a purification method of chromatographic pure 1,4-dioxane. The method comprises the following steps of (1) eliminating 2-methyl-1,3-dioxolame impurities through hydrolysis; (2)performing adsorption: injecting the hydrolyzed 1,4-dioxane raw materials into a serially connected cation exchange resin adsorption column and active carbon adsorption column; adsorbing some aldehydes impurities in the 1,4-dioxane raw materials; (3) removing peroxides through the adsorption by a lithium aluminum hydride adsorption column; (4) performing drying and dewatering by a 4A molecular sieve; (5) performing rectification. According to the method, 1,4-dioxane industrial products with the content being 98.0 percent are used as raw materials; some adsorbable impurities can be eliminated through hydrolysis, the cation exchange resin adsorption column and the active carbon adsorption column; then, through heating treatment, the peroxides in the products are eliminated through the entering into a lithium aluminum hydride filling column. The method has the advantages that the reaction is complete; the safety is high. After the rectification, the final product content reaches 99.9 percent or higher; the moisture is less than or equal to 0.02 percent.
Owner:天津康科德医药化工有限公司
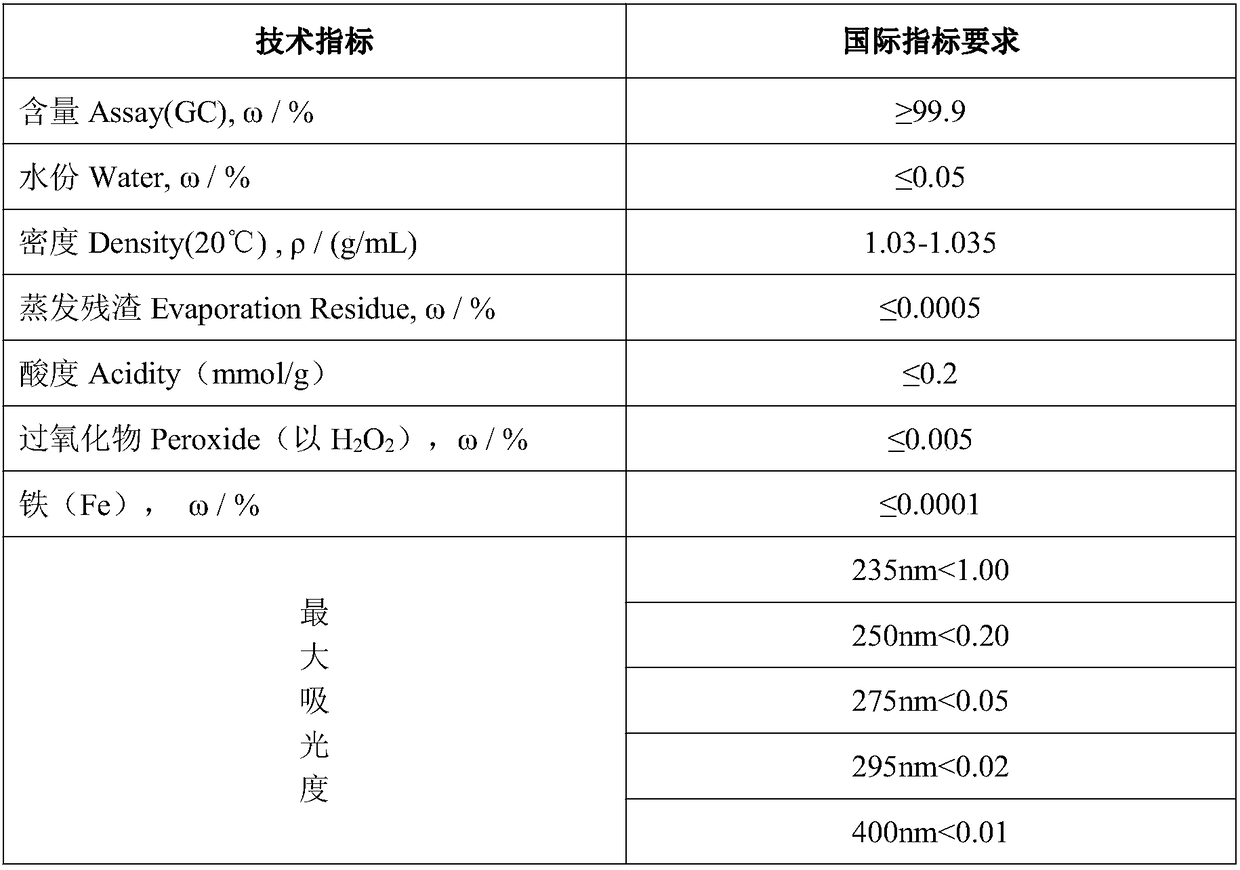
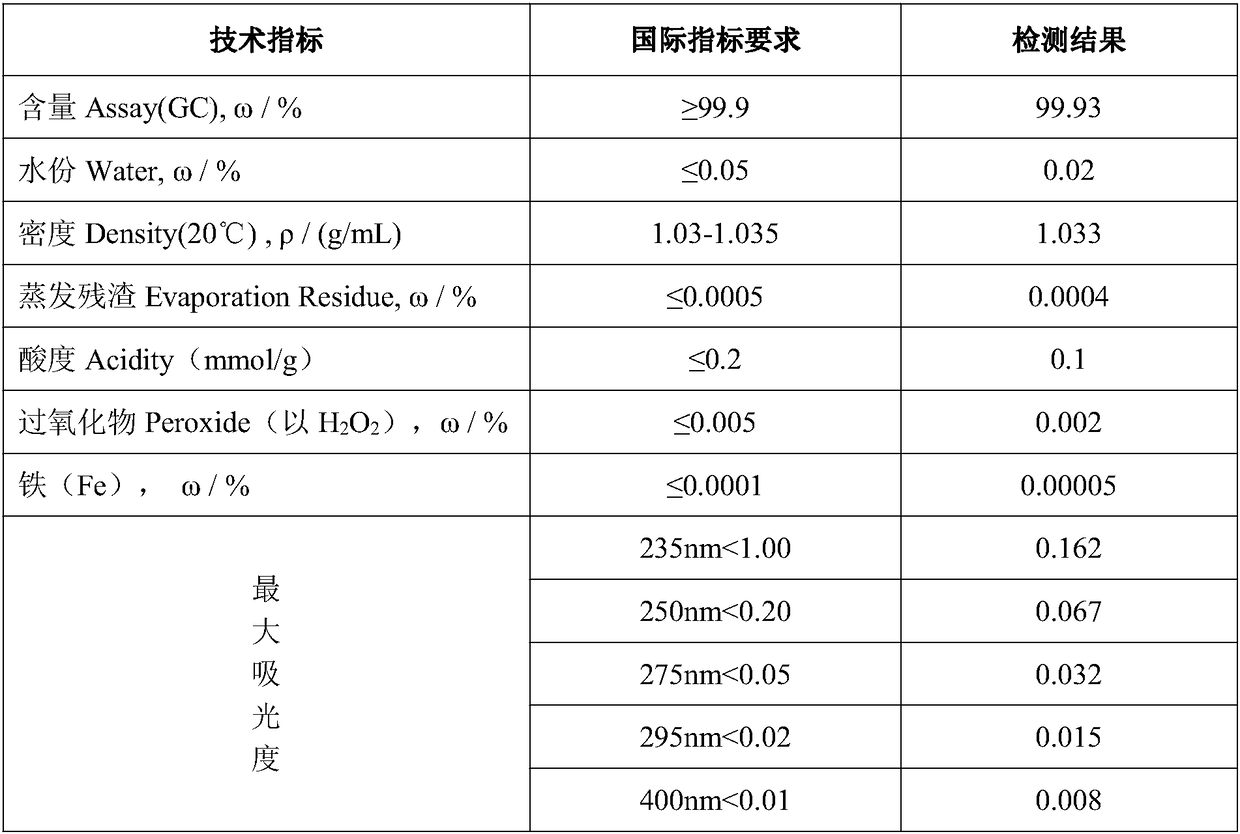
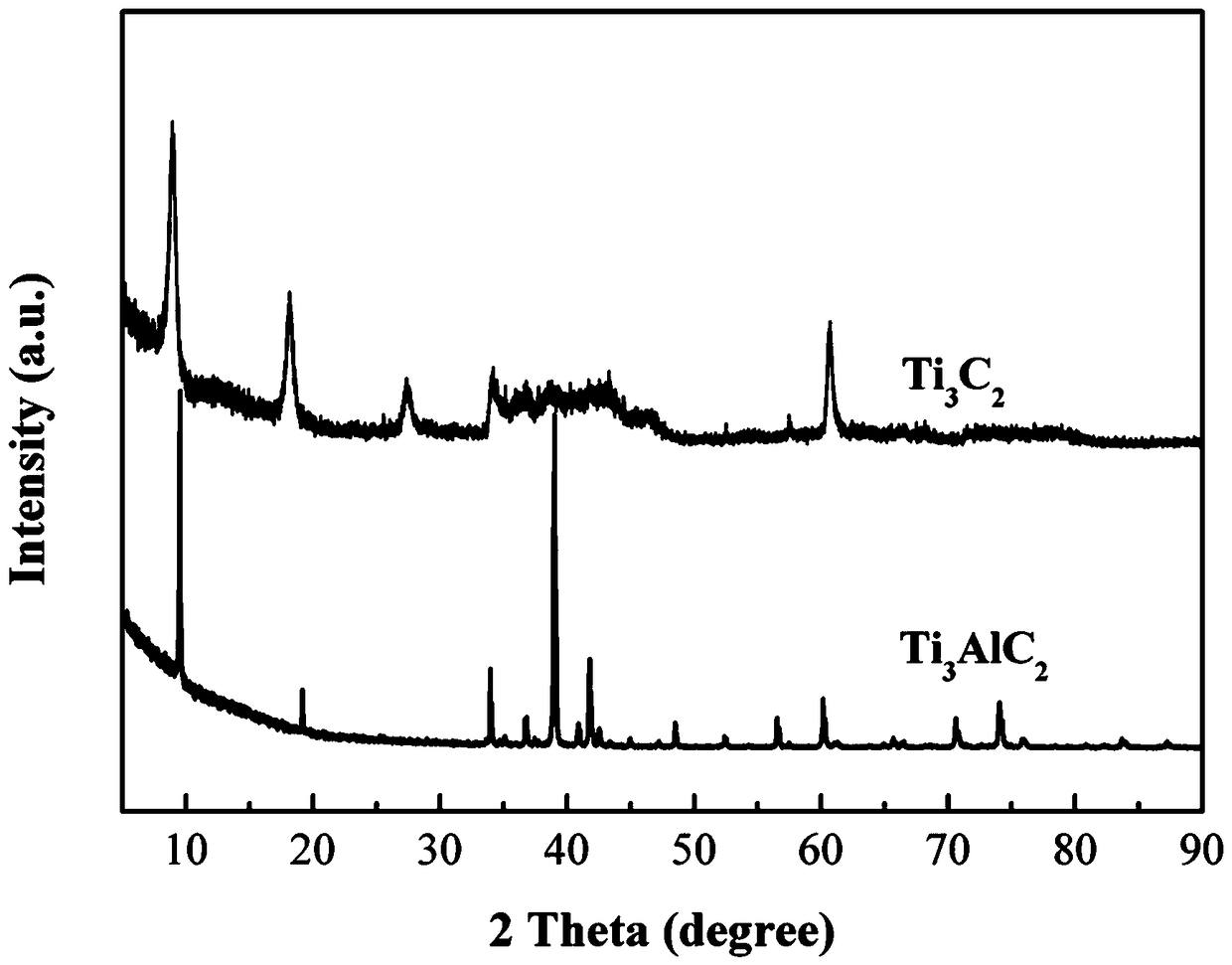
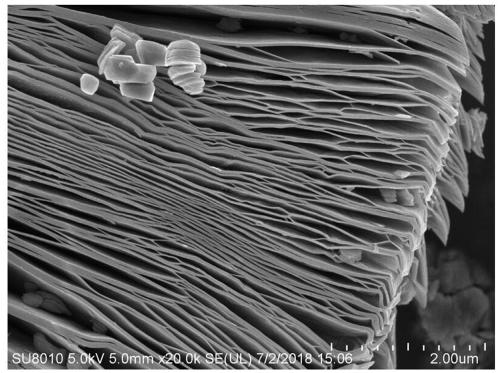
Two-dimensional titanium carbide-doped lithium aluminum hydride hydrogen storage material and preparation method thereof
ActiveCN109052403AImprove hydrogen storage performanceGood dehydrogenation kineticsReversible hydrogen uptakeMultiple metal hydridesHydrofluoric acidDehydrogenation
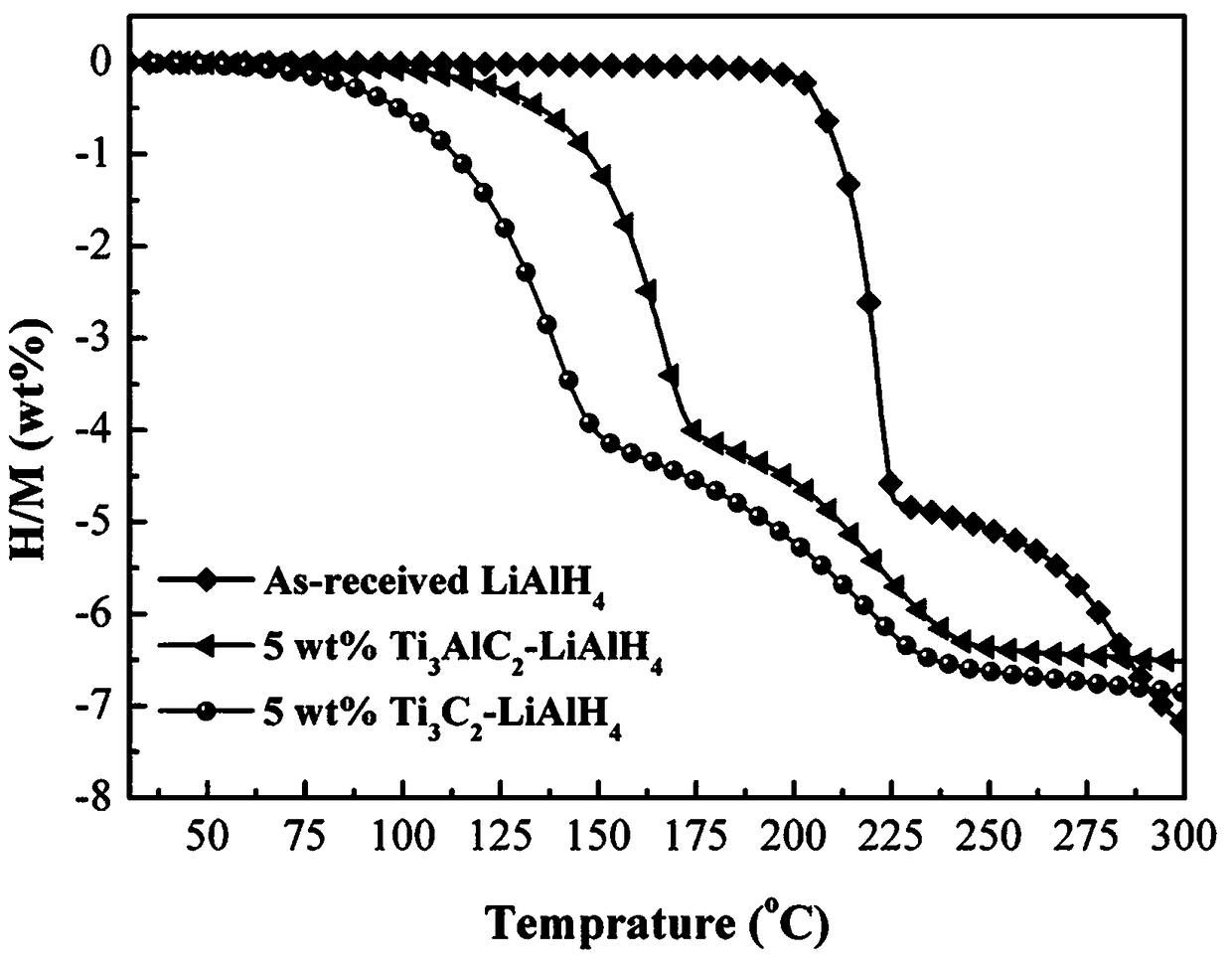
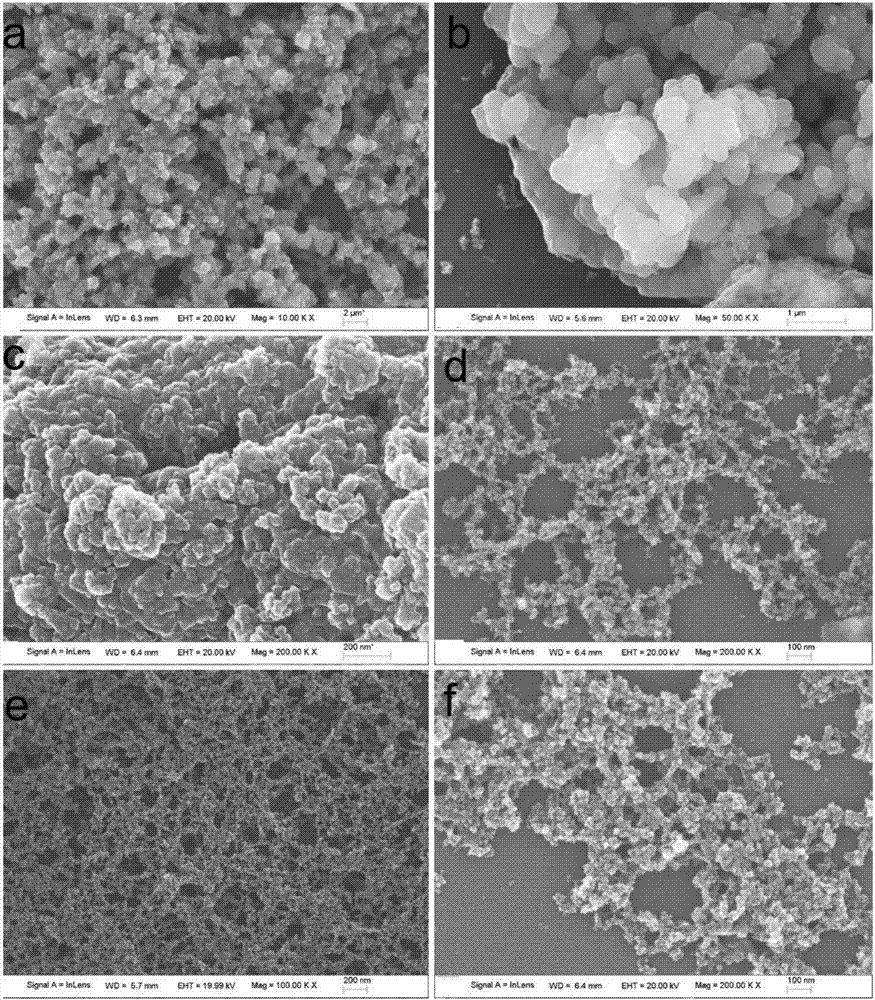
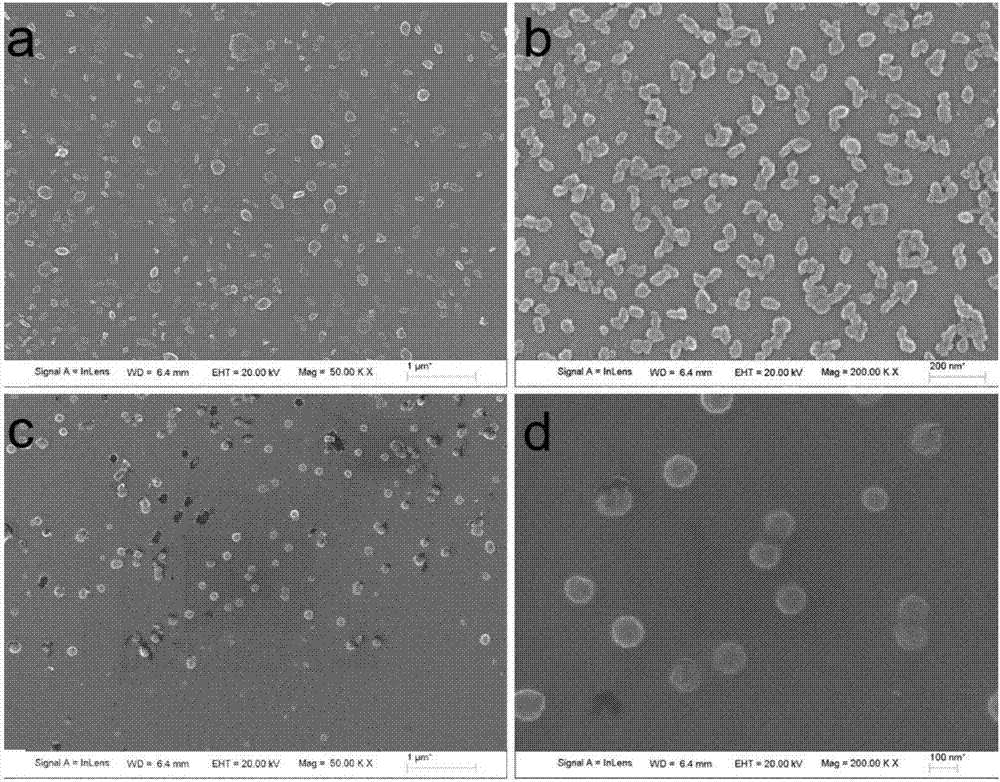
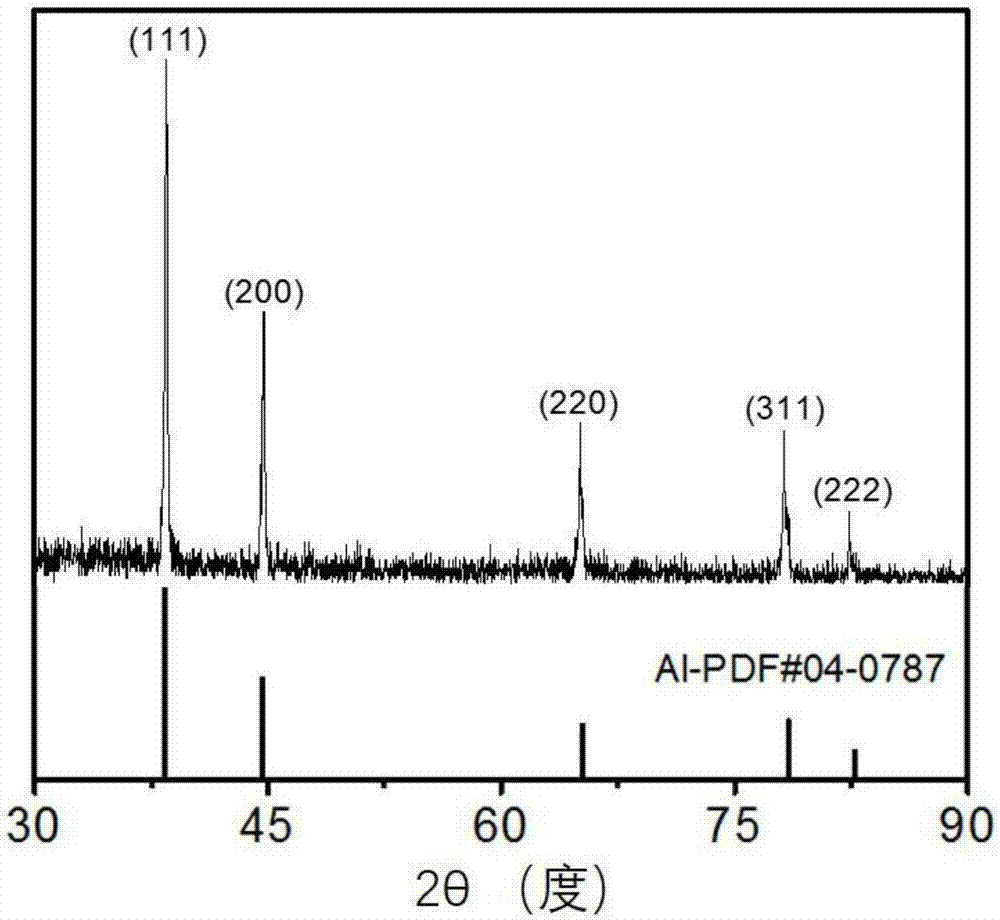
The invention discloses a two-dimensional titanium carbide-doped lithium aluminum hydride hydrogen storage material. The two-dimensional titanium carbide-doped lithium aluminum hydride hydrogen storage material is prepared by mixing lithium aluminum hydride and two-dimensional titanium carbide Ti3C2 and performing mechanical ball milling, wherein the two-dimensional titanium carbide Ti3C2 is prepared by carrying out a reaction between Ti3AlC2 and hydrofluoric acid. A preparation method of the material comprises the following steps: 1, preparing the two-dimensional Ti3C2; 2, preparing the two-dimensional titanium carbide-doped lithium aluminum hydride hydrogen storage material. Under the catalytic action of the two-dimensional Ti3C2, the hydrogen storage material has the initial dehydrogenation temperature of 43-68 DEG C, which is 129-154 DEG C lower than that of the pure lithium aluminum hydride, the total hydrogen release amount thereof reaches 4.6-7.2wt%, and the initial dehydrogenation temperature thereof is 148.2 DEG C lower than that of the original lithium aluminum hydride; at 150 DEG C, 3.7 wt% of hydrogen can be released within 15 minutes; at 200 DEG C, 5.3 wt% of hydrogencan be released within 15 minutes. Therefore, the hydrogen storage material has excellent hydrogen storage and release performance, and the prepared two-dimensional Ti3C2 can significantly improve thehydrogen release performance of the lithium aluminum hydride, so that the hydrogen storage material shows excellent hydrogen release performance at relatively low temperature.
Owner:GUILIN UNIV OF ELECTRONIC TECH
Aluminum nanometer particles and preparation method thereof
InactiveCN107225254AParticle size independently adjustableHigh particle contentMaterial nanotechnologyTransportation and packagingDissolutionOrganic reaction
The invention discloses aluminum nanometer particles and a preparation method thereof and belongs to the technical field of inorganic advanced nanometer materials. The diameter of each aluminum nanometer particle ranges from 15 nanometers to 1000 nanometers. The preparation method comprises the steps of adding an aluminum source to an organic reaction solution of ammonium salt, and raising the temperature to achieve dissolution; adding lithium aluminum hydride to the solution, and then making an obtained mixture react for 1-72 hours at the temperature of (100-165) DEG C, so that aluminum nanometer particle suspension liquid is obtained; and conducting solid-liquid separation, so that solids, namely the aluminum nanometer particles, are obtained. According to the preparation method of the aluminum nanometer particles, a simple and effective solvothermal method is adopted, so that the obtained aluminum nanometer particles are high in content and small in number of impurities.
Owner:BEIJING UNIV OF CHEM TECH
Synthetic method of chiral 2-phenylpyrrolidine
ActiveCN104592163ALow costEasy to operateOrganic chemistryChemical recyclingCarbamateTert-Butyloxycarbonyl protecting group
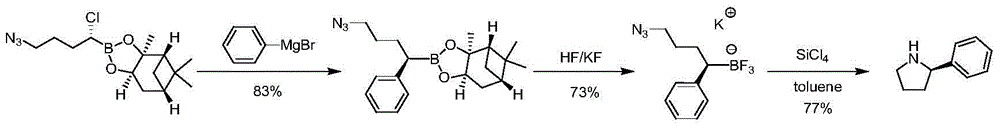
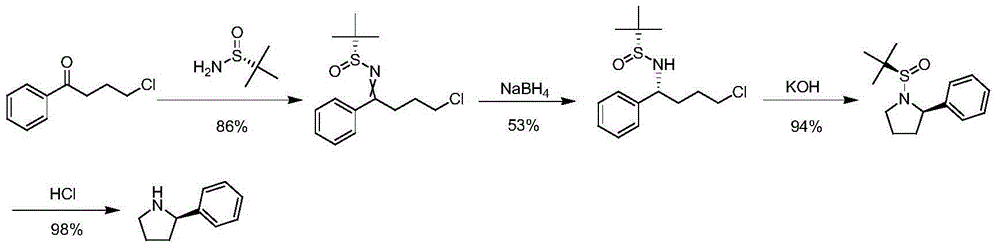
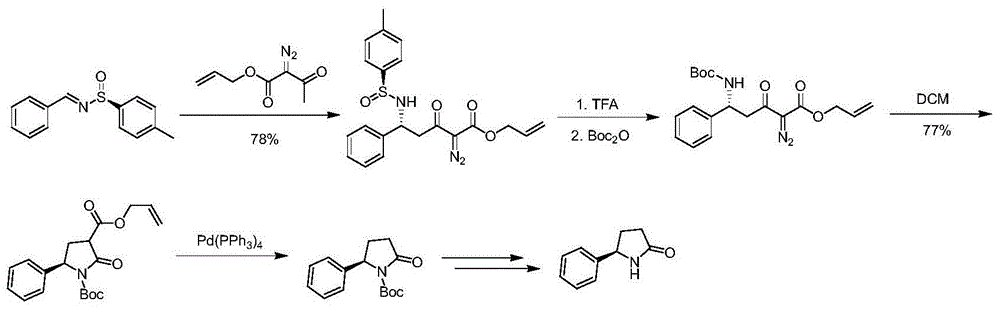
The invention discloses a synthetic method of chiral 2-phenylpyrrolidine. The synthetic method comprises the following steps: (1) butoxycarbonyl protective reaction: carrying out reaction on chiral 2-amino-phenylacetic acid and di-tert-butyl dicarbonate to obtain chiral 2-( butoxycarbonyl amino)-2-phenylacetic acid; (2) condensation and reduction reaction: carrying out condensation on the chiral 2-(butoxycarbonyl amino)-2-phenylacetic acid and 2, 2-dimethyl-1, 3-dioxane-4, 6-dione, and carrying out reduction by virtue of sodium borohydride to obtain chiral 2-(2, 2-dimethyl-4, 6-dicarbonyl-1, 3-dioxane-5-yl)-1-phenyl ethyl tert-butyl carbamate; (3) de-protection and heating ring closure reaction: carrying out de-protection and heating ring closure reaction on the chiral 2-(2, 2-dimethyl-4, 6-dicarbonyl-1, 3-dioxane-5-yl)-1-phenyl ethyl tert-butyl carbamate to obtain chiral 5-phenylpyrrolidine-2-ketone; and (4) reduction reaction: reducing the chiral 5-phenylpyrrolidine-2-ketone by virtue of lithium aluminium hydride to obtain the chiral 2-phenylpyrrolidine. The synthetic method is simple in operation, high in yield and cheap and easily acquired in raw materials and is suitable for industrial production.
Owner:ASTATECH CHENGDU BIOPHARM CORP
Graphene composite catalyst with good catalytic performance and preparation method thereof
InactiveCN109772284AGood dispersionHigh bonding strengthCatalyst activation/preparationSodium azideSodium hydroxide
The invention discloses a graphene composite catalyst with good catalytic performance and a preparation method thereof, and belongs to the technical field of catalyst preparation. The method includesthe steps that graphene oxide and deionized water are mixed and subjected to ultrasonic treatment, ammonia water is dropwise added to regulate the pH, sodium azide is added, a heating and stirring reaction is carried out, lithium aluminum tetrahydride is added, a heating and stirring reaction is carried out, and filtering and drying are carried out to obtain pretreated graphene oxide; alcohol, acid, petroleum ether, animal oil, tetrabutyl titanate and the pretreated graphene oxide are subjected to a high-temperature stirring reaction to obtain mixed liquor; a sodium hydroxide solution is addedinto the mixed liquor to regulate the pH, an emulgator and isocyanate are added, and stirring and mixing are carried out to obtain mixed homogenate; the mixed homogenate is put into an autoclave, pressurization, pressure maintaining and instantaneous depressurization are carried out, filtering and drying are carried out, and the graphene composite catalyst is obtained. The graphene composite catalyst has an excellent photocatalytic effect and a long service life, and can be widely applied to the field of catalysts.
Owner:周玉芳
Method for reducing acid into alcohol by sodium borohydride
ActiveCN102951978AReduce riskImprove reducibilityOrganic compound preparationHydroxy group formation/introductionPotassium borohydrideOrganic solvent
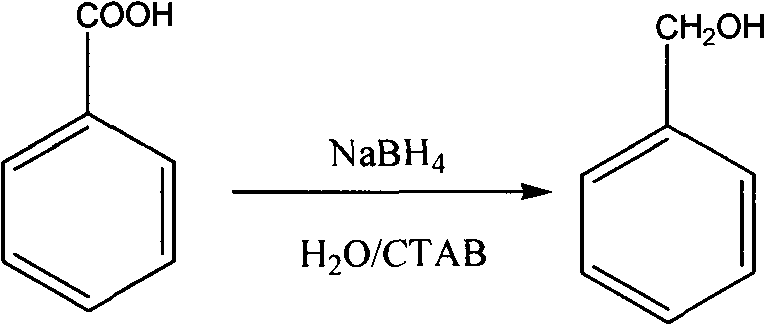
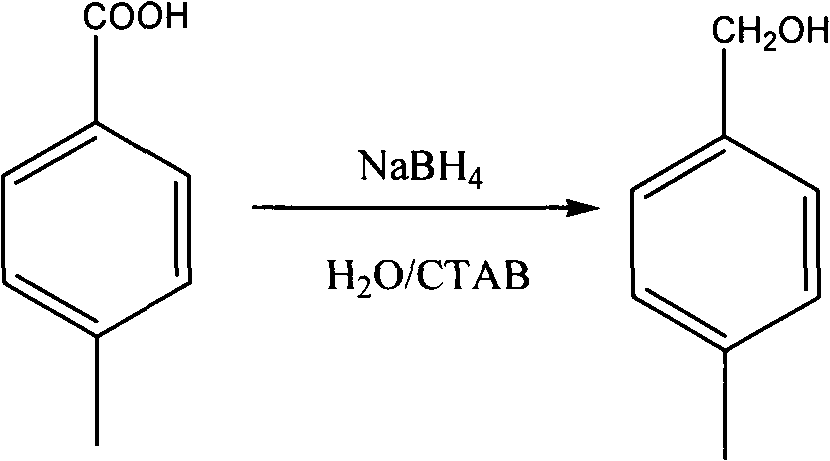
The invention discloses a method for reducing acid into alcohol by sodium borohydride. The reaction is carried out as follows: changing a reaction solvent and adding a phase transfer catalyst to reduce the acid into the alcohol by the sodium borohydride under the constant temperature by using sodium borohydride as a reducing agent, water as a solvent and cetyl trimethyl ammonium bromide as the phase transfer catalyst. By using the water as the solvent, the using of organic solvent is avoided, the pollution is reduced, and the environment protection is realized; and meanwhile, lithium aluminium hydride is replaced by the sodium borohydride, the process is simplified, and the large-scale industrial production is easily performed. The system has a good effect on fatty acid, and also has a good reduction effect on some aromatic and heterocyclic acids.
Owner:TIANJIN SCIPHARMACN
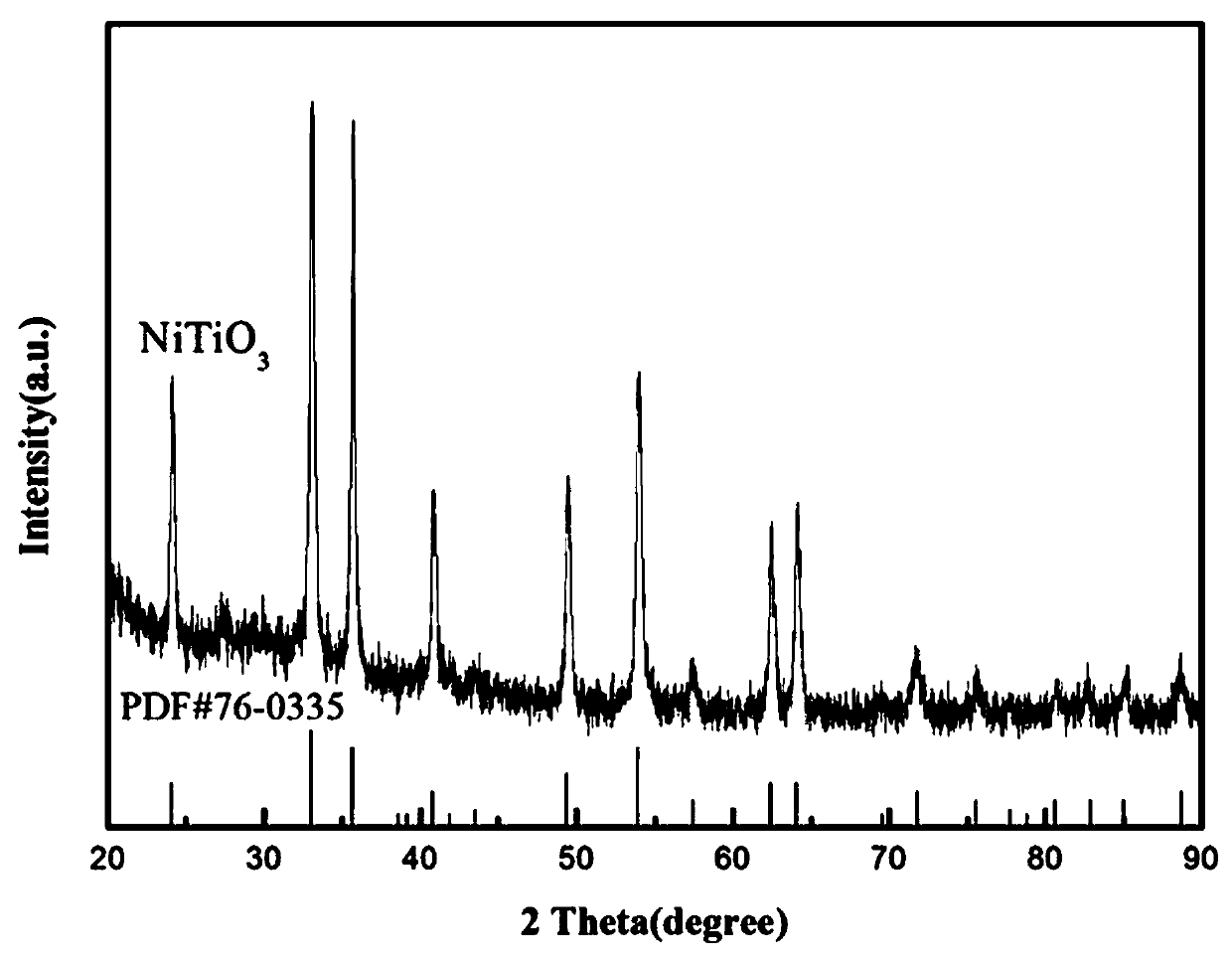
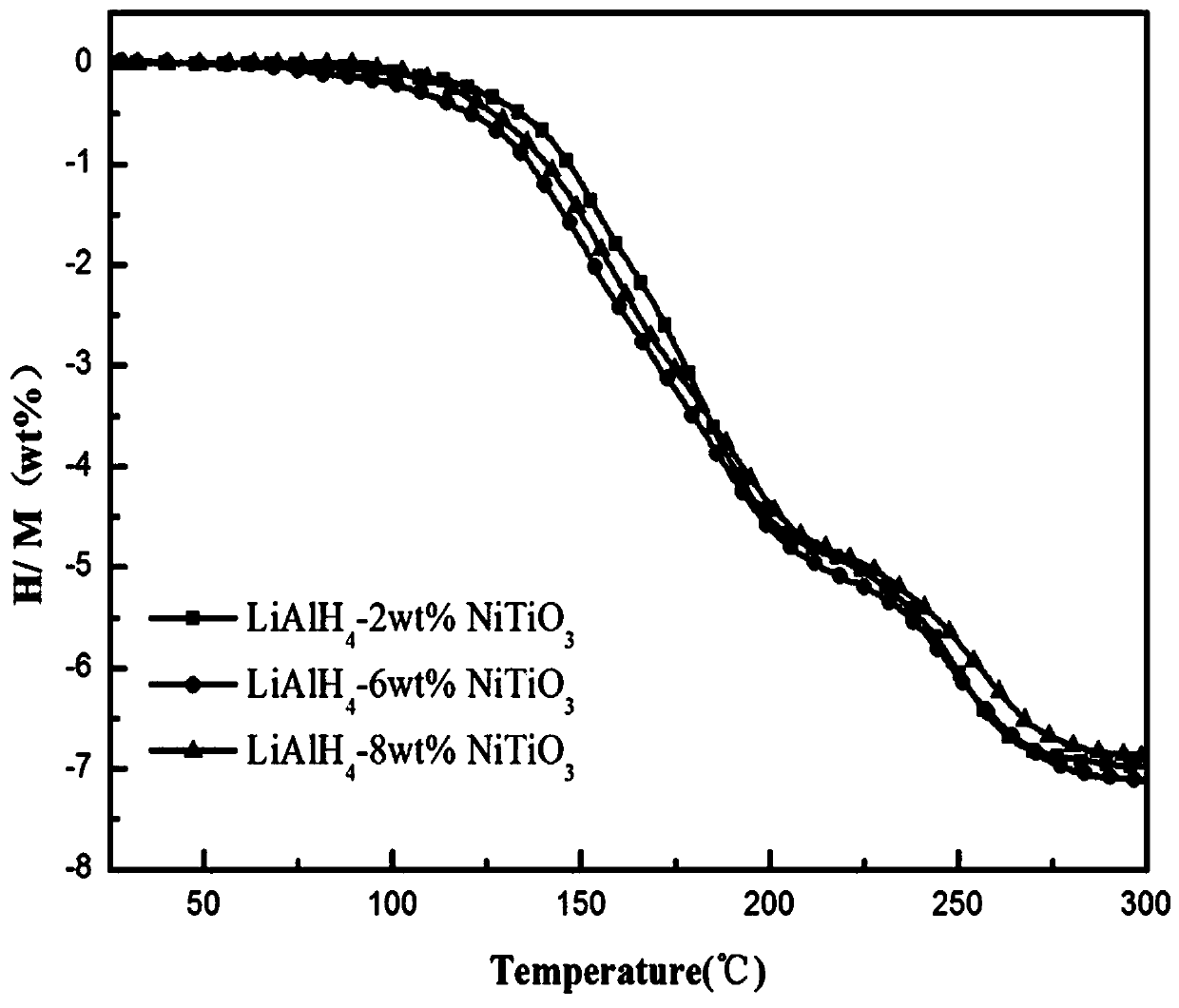
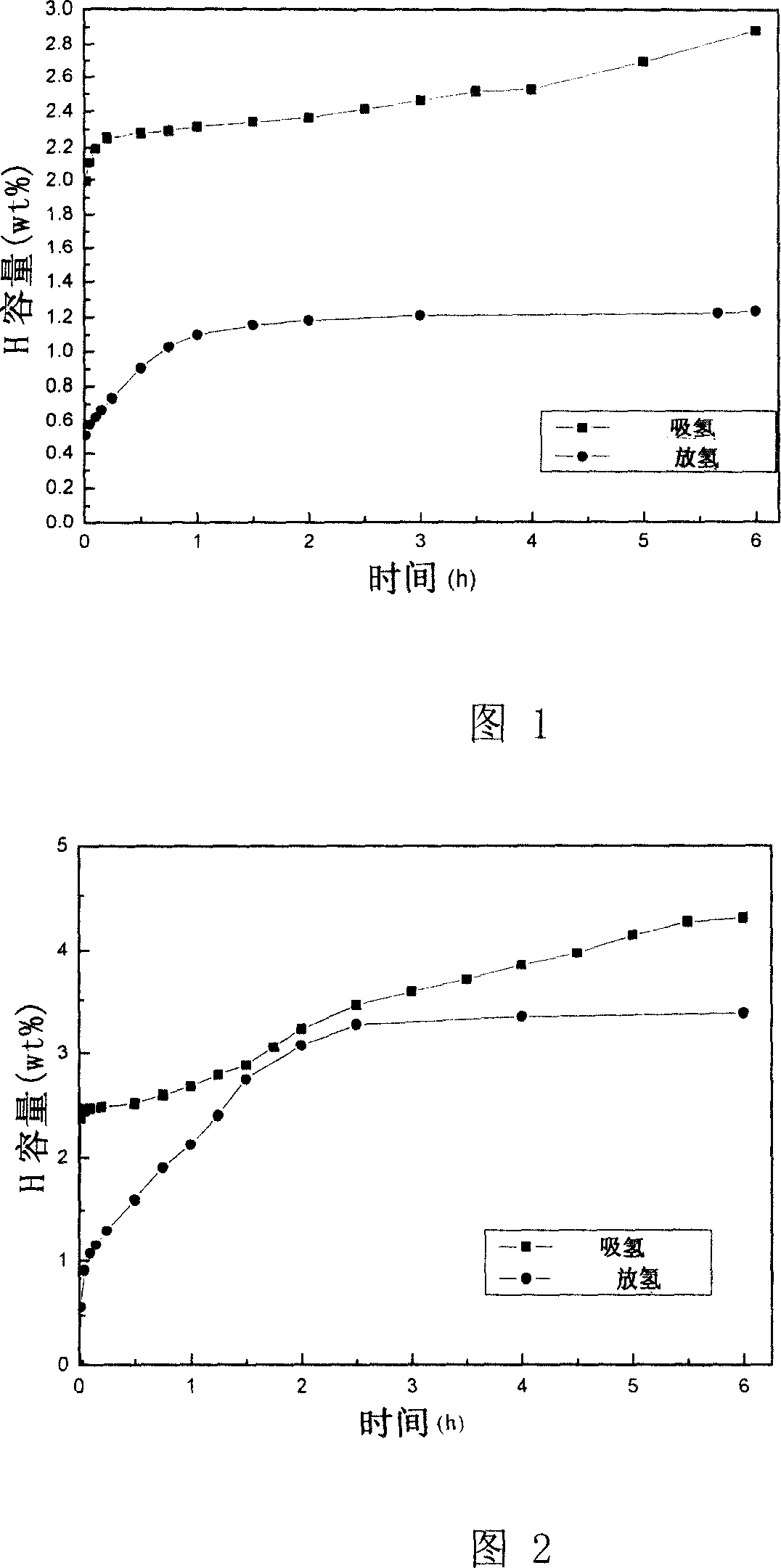
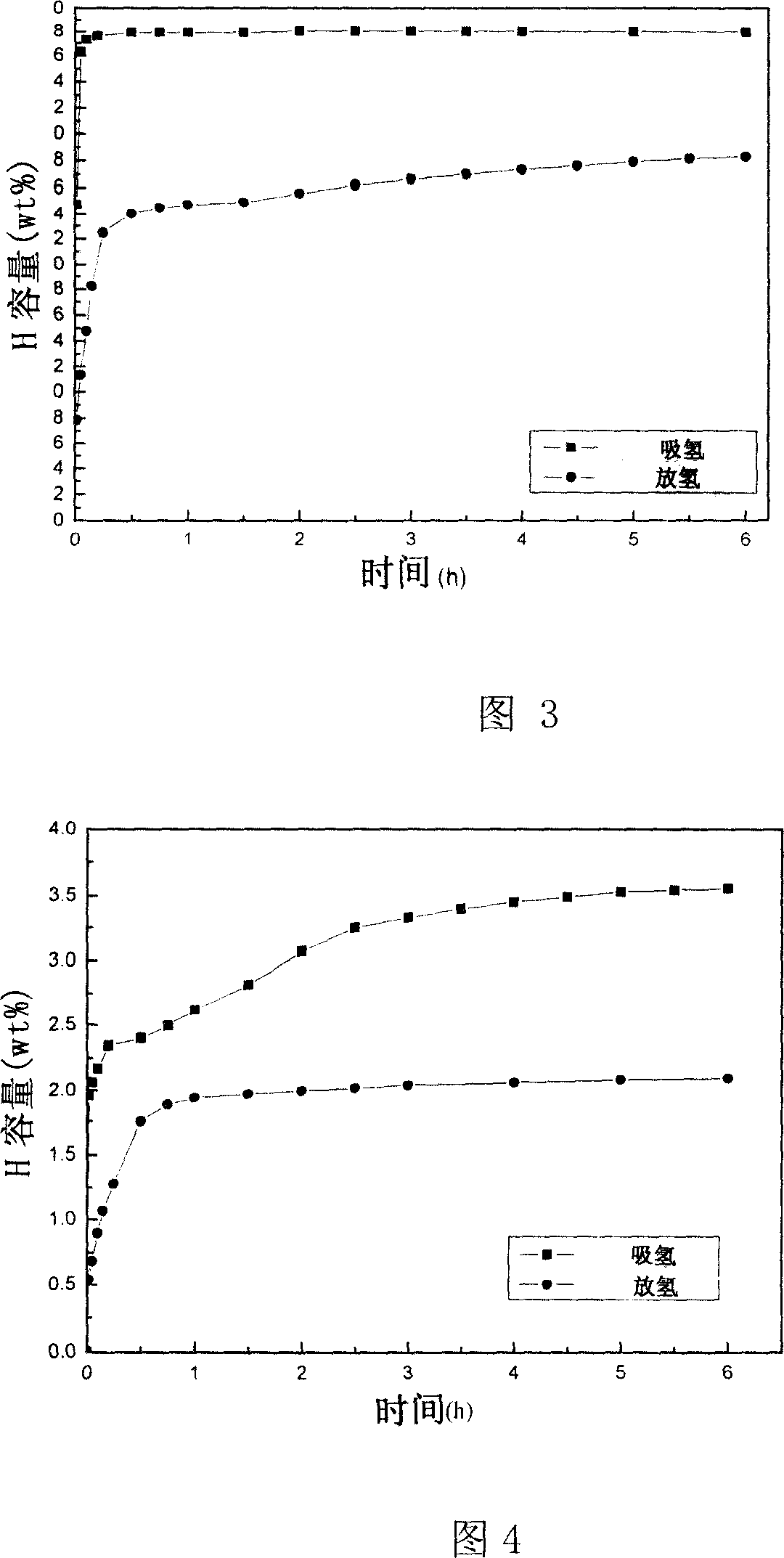
Nickel titanate doped lithium aluminum hydride hydrogen-storage material and preparation method thereof
ActiveCN110817791AControl the dehydrogenation processImproved hydrogen release performanceHydrogenPtru catalystPhysical chemistry
The invention discloses a nickel titanate doped lithium aluminum hydride hydrogen-storage material. The material is prepared by mixing and mechanically ball-milling lithium aluminum hydride and nickeltitanate NiTiO3, wherein the nickel titanate NiTiO3 is prepared by calcining precipitates generated by reaction of nickel chloride and butyl titanate in ethylene glycol, the nickel titanate NiTiO3 isin a rod shape with the length of 1-4 [mu]m and the width of 0.5-2 [mu]m, and the addition amount of the nickel titanate NiTiO3 accounts for 2-8 wt% of the total mass. The preparation method comprises the steps: 1) preparing rod-like nickel titanate; and 2) preparing the nickel titanate doped lithium aluminum hydride hydrogen-storage material. As application in the field of hydrogen storage, whenthe doping amount of the catalyst is 2 wt%, the hydrogen desorption temperature of the system is reduced to 95 DEG C, and the hydrogen desorption amount reaches 7.0 wt%; when the doping amount of thecatalyst is 6 wt%, the hydrogen desorption temperature of the system is reduced to 73 DEG C, and the hydrogen desorption amount reaches 7.2 wt%. The hydrogen storage material has the following advantages: 1, the hydrogen desorption performance of lithium aluminum hydride is effectively improved, and the hydrogen storage material has high hydrogen desorption capacity after a small amount of catalyst is added; and 2, the method has the advantages of low cost, simple preparation process, controllable reaction and the like.
Owner:GUILIN UNIV OF ELECTRONIC TECH
Coordination hydride catalyzed reversible hydrogen storage materials and method of preparing the same
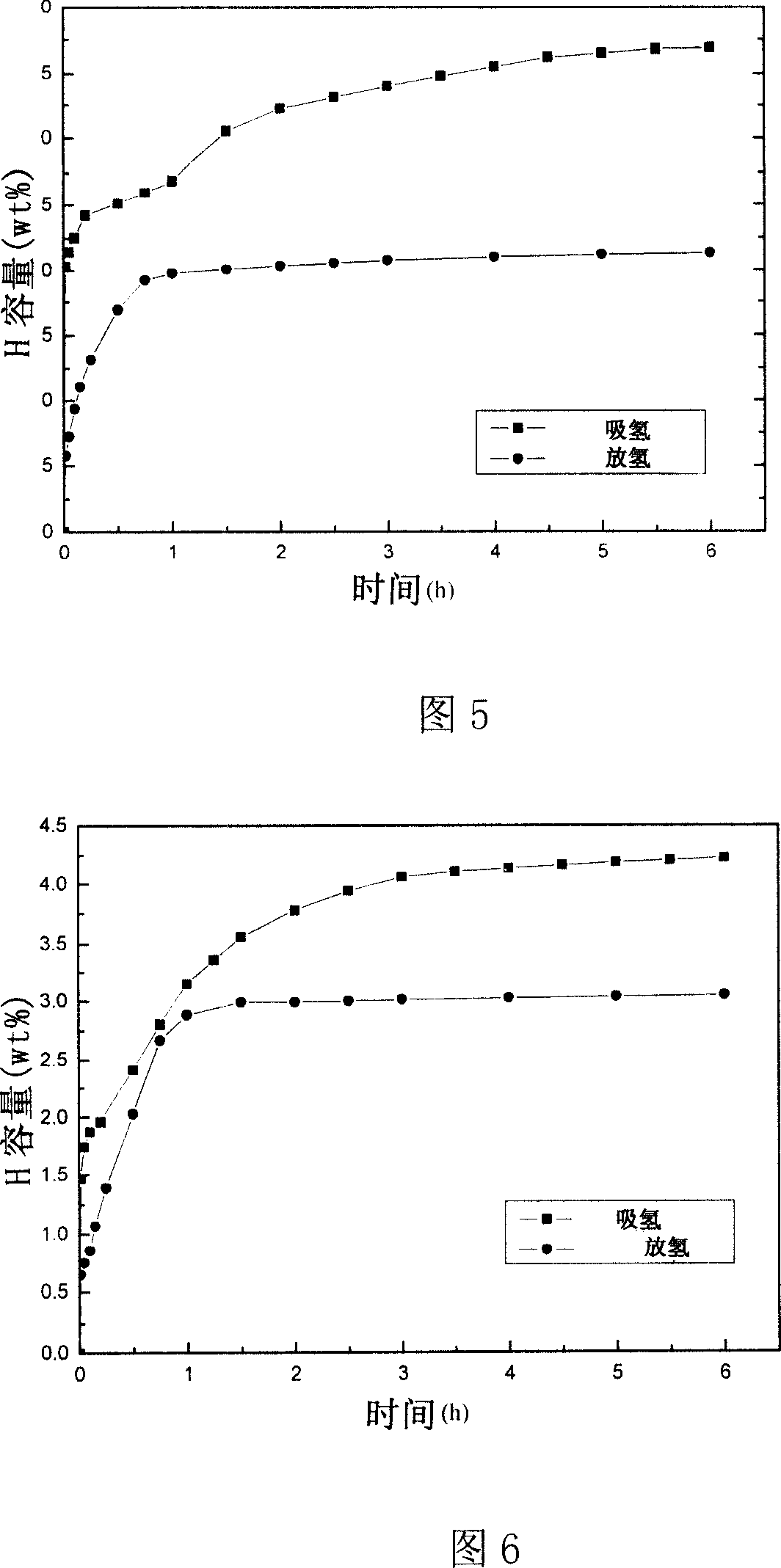
The invention relates to a novel high capacity hydrogen storage material, namely coordination hydride, the chemical formula is A(MH4)n. The invention adds titanium catalyst Ti(OC4H9)4 or TiC13 .1 / 3 AlCl3 in the classic coordination hydride lithium aluminium hydride and sodium aluminum hydride by the high energy ball mill method under the protection of hydrogen pressure, therefore reaching reversible storage hydrogen. The preparation method of the coordination hydride catalyzing reversible storage hydrogen seals the coordination hydride (LiA1H4 or NaA1H4), titanium catalyst Ti(OC4H9)4 or TiC13 .1 / 3 AlCl3 and steel ball in a stainless ball mill Pot, and mill ball with high energy under the protection of hydrogen pressure. The material can be used as a high capacity hydrogen storage material, the reversible hydrogen adsorption volume can reach above 4.0wt per cent at 150 DEG C., the reversible hydrogen adsorption volume to 0.1MPa hydrogen pressure also can reach above 3.0 wt per cent.
Owner:GENERAL RESEARCH INSTITUTE FOR NONFERROUS METALS BEIJNG
Preparation process of lithium aluminium hydride reduction
InactiveCN1559883AAvoid high temperature reactionAvoid hyperbaric reactionsMultiple metal hydridesDecompositionAqueous solution
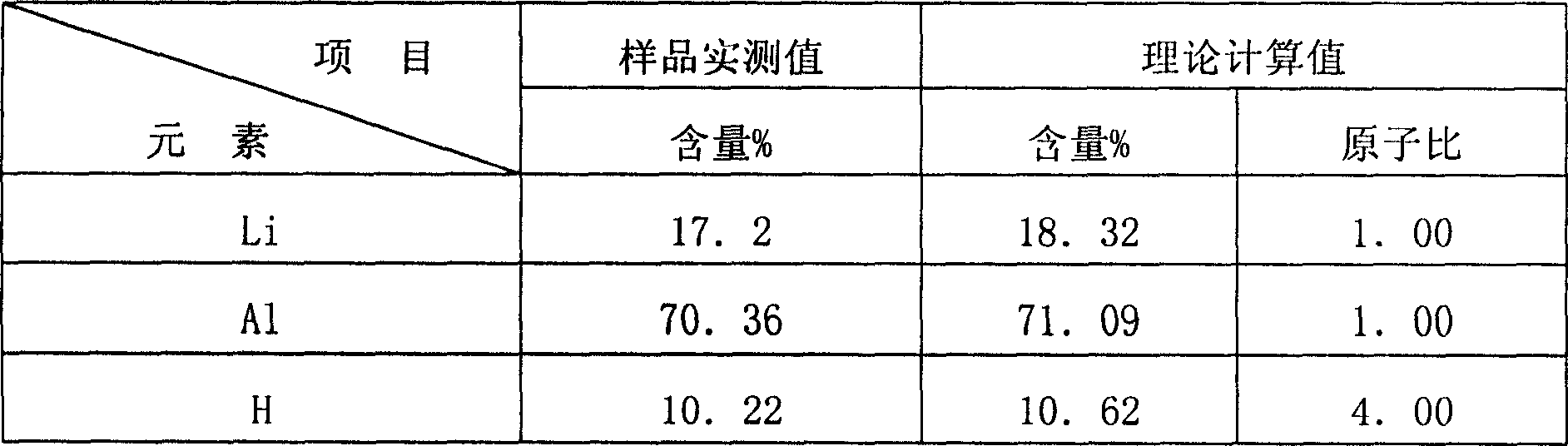
The invention relates to a metal hydride, concretely belonging to an aluminum lithium hydride making method, and its character: it adopts soluble aluminum salt and lithium salt as raw materials, and adds precipitator in water solution to coprecipitate Al3+ and Li+ ions; makes drying and high-temperature decomposition processing on the deposit to obtain an oxide; makes hydrogenation reduction on the oxide so as to obtain an aluminum-lithium mixed simple substance, and hydrogenates the mixed simple substance at normal temperature and normal pressure to obtain aluminum lithium hydride. It has low temperature in preparing course, operated at normal pressure, not only reduces energy consumption and eliminates potential danger but also the raw materials are easy to obtain and the technique is simple, and provides a new clean producing technique on friendly terms with environment for preparing aluminum lithium hydride.
Owner:SHANXI UNIV
Preparing method of BCN ceramic fiber
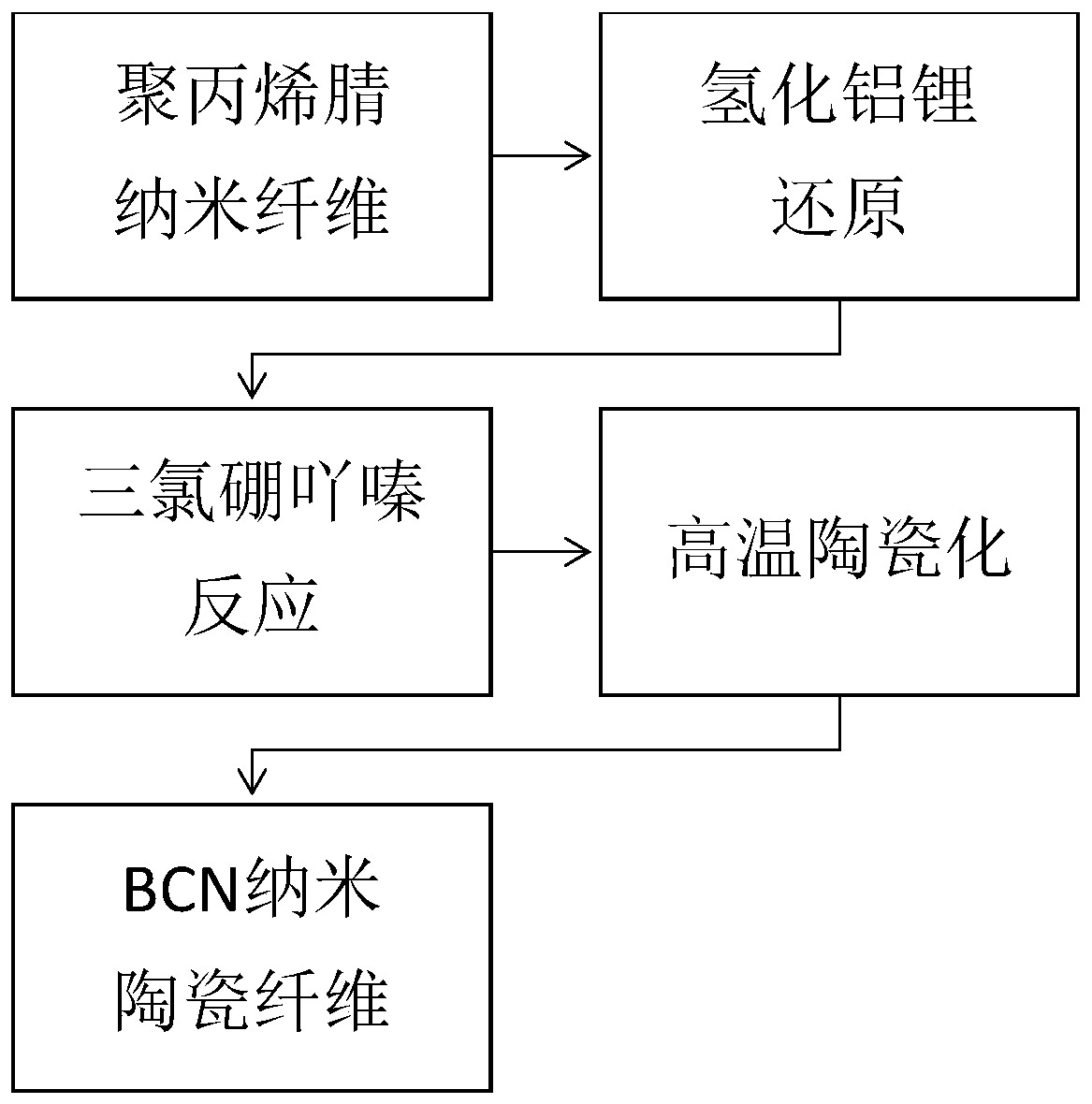
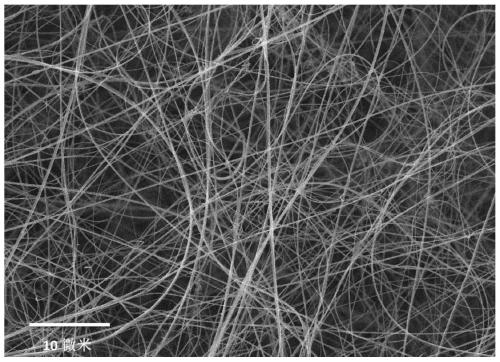
The invention discloses a preparing method of a BCN ceramic fiber. The method includes the steps of firstly, preparing a nanofiber template from polyacrylonitrile fiber; secondly, reducing a nitrile group into an amino group through lithium aluminium hydride; thirdly, making trichloroborazine react with the amino group to produce boron nitride; fourthly, obtaining the final BCN nanometer ceramic fiber through high-temperature processing. Compared with an existing nanofiber ceramic fiber, the preparing method is simple in technological process, no spinning and forming device is needed, and thestrict requirements of synthesizing and spinning of sensitive BCN ceramic precursors for the environment are avoided; meanwhile, the BCN nanometer ceramic fiber has the monofilament diameter of 50-500nm, the density of 0.1-0.2 mg / cm<3>, the heat conductivity of 20-30 W / m K and the dielectric constant of 8-12 and can be used as an excellent heat insulation material and wave absorption material.
Owner:NAT UNIV OF DEFENSE TECH
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com
![Method for reducing 8-benzyl-2,8-diazabicyclo[4,3,0]nonane and chiral isomer thereof Method for reducing 8-benzyl-2,8-diazabicyclo[4,3,0]nonane and chiral isomer thereof](https://images-eureka.patsnap.com/patent_img/d773f92e-9397-45a1-b63c-9d0abefeecf0/A2009101002270002C1.PNG)
![Method for reducing 8-benzyl-2,8-diazabicyclo[4,3,0]nonane and chiral isomer thereof Method for reducing 8-benzyl-2,8-diazabicyclo[4,3,0]nonane and chiral isomer thereof](https://images-eureka.patsnap.com/patent_img/d773f92e-9397-45a1-b63c-9d0abefeecf0/A20091010022700051.PNG)
![Method for reducing 8-benzyl-2,8-diazabicyclo[4,3,0]nonane and chiral isomer thereof Method for reducing 8-benzyl-2,8-diazabicyclo[4,3,0]nonane and chiral isomer thereof](https://images-eureka.patsnap.com/patent_img/d773f92e-9397-45a1-b63c-9d0abefeecf0/A20091010022700052.PNG)