Preparation process of lithium aluminium hydride reduction
A technology of aluminum lithium hydride and aluminum lithium, which is applied in the direction of various metal hydrides, can solve the problems of unsafety and many by-products, and achieve the effect of simple production method, complete utilization and reduced production cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0018] Weigh 241.43g of aluminum chloride hexahydrate and 42.41g of lithium chloride, add water to dissolve, add a certain amount of sodium carbonate solution, wait until the precipitation is complete, put it aside for suction filtration, dry the solid and then calcinate at a high temperature of 500-600°C After decomposing for 4 hours, the oxide was obtained, the oxide was in the autoclave, and CaH was added 2 As a catalyst, at 180°C and 20kg / cm 2 After hydrogenation of the solid under hydrogen pressure for 4-5 hours, a mixed element of aluminum and lithium is obtained. Adding catalyst naphthalene and TiCl in the organic solvent tetrahydrofuran 4 , hydrogenation at normal temperature and pressure by introducing hydrogen gas for 4-5 hours, and the lithium aluminum hydride product can be obtained.
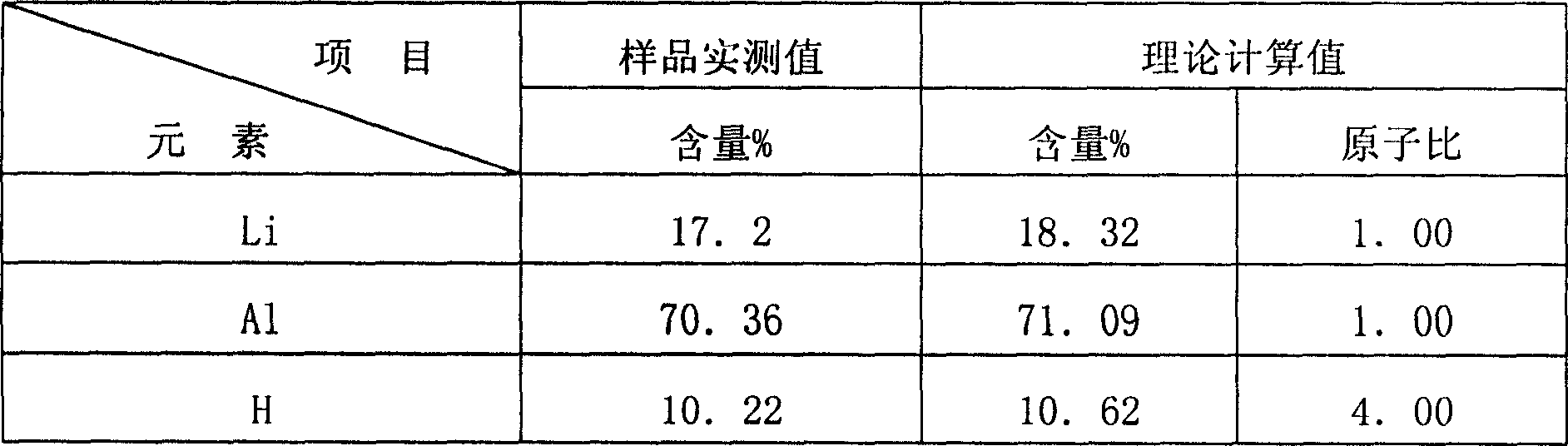
[0019] Product analysis: The content analysis of Li, Al and H was carried out on this product. The analysis of Li was carried out by atomic absorption method; the analysis of Al wa...
Embodiment 2
[0022] Weigh 241.43g of aluminum chloride hexahydrate and 42.41g of lithium chloride, add water to dissolve, add a certain amount of sodium oxalate solution, wait until the precipitation is complete, put it aside for suction filtration, dry the solid and then calcinate at a high temperature of 500-600°C After decomposing for 4 hours, the oxide was obtained, the oxide was in the autoclave, and CaH was added 2 As a catalyst, at 180°C and 20kg / cm 2 After hydrogenation of the solid under hydrogen pressure for 4-5 hours, a mixed element of aluminum and lithium is obtained. Adding catalyst naphthalene and TiCl in the organic solvent tetrahydrofuran 4 , hydrogenation at normal temperature and pressure by introducing hydrogen gas for 4-5 hours, and the lithium aluminum hydride product can be obtained.
Embodiment 3
[0024] Weigh 375g of aluminum nitrate and 69g of lithium nitrate, add water to dissolve, add a certain amount of sodium oxalate solution, wait until the precipitation is complete, put it aside for suction filtration, and after the solid is dried, it is calcined at 500-600°C for 4 hours and then oxidized. material, the oxide in an autoclave, and adding CaH 2 As a catalyst, at 180°C and 20kg / cm 2 After hydrogenation of the solid under hydrogen pressure for 4-5 hours, a mixed element of aluminum and lithium is obtained. Adding catalyst naphthalene and TiCl in the organic solvent tetrahydrofuran 4 , hydrogenation at normal temperature and pressure by introducing hydrogen gas for 4-5 hours to obtain the lithium aluminum hydride product.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com