Synthesis method of ketamine, derivative and intermediate thereof
A synthetic method and technology of ketamine, applied in the field of ketamine synthesis, can solve the problems of unfavorable industrial production, difficulty in mass production, low atom economy, etc., and achieve strong industrial prospects, mild reaction conditions, and high equipment requirements.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0084] Step 1: Dissolve 2-chlorophenylcyclopentyl ketone (4) (10.0g, 0.048mol) in 100mL absolute ethanol solution at room temperature, add potassium hydroxide (2.7g, 0.048mol) and triethyl phosphite The ester (12.0 g, 0.072 mol) was stirred and dissolved, and heated to 30° C. for 48 h under an oxygen atmosphere. After the reaction was completed, about 70 mL of absolute ethanol was concentrated under reduced pressure, 300 mL of water was added dropwise to precipitate a solid, stirred at room temperature for 1 h, filtered with suction, and dried at 50° C. for 10 h to obtain 2-(2-chlorophenyl)-2-hydroxycyclohexyl ketone ( Ⅲ) 8.9g, yield 83.0%, purity 97.15%.
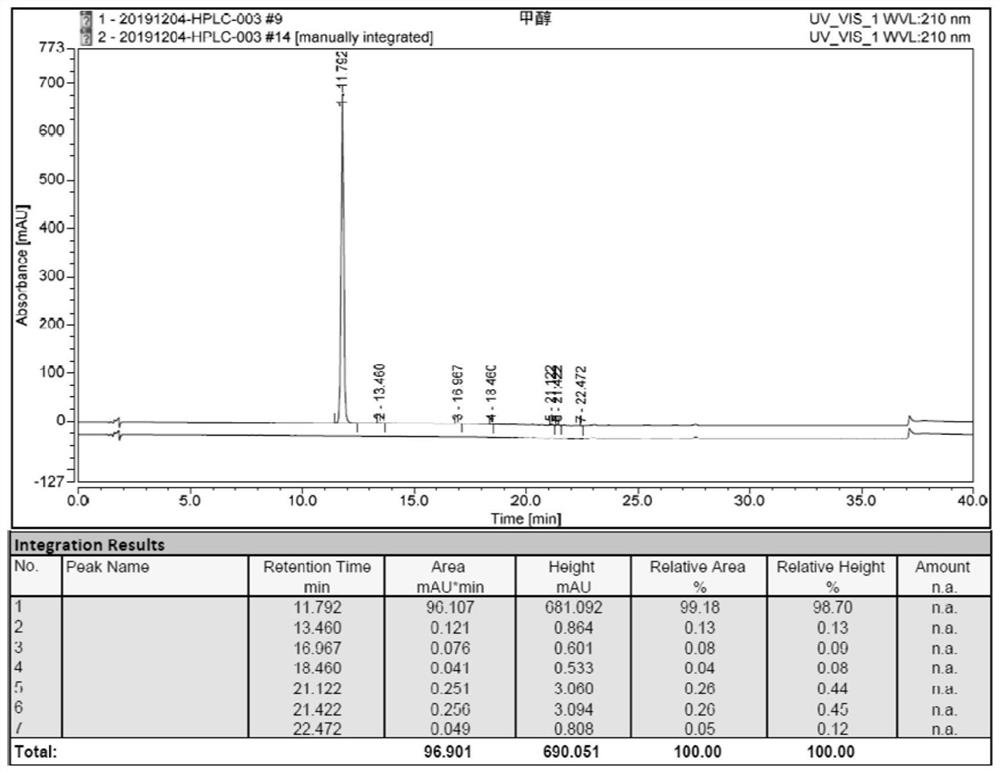
[0085] The HPLC chart of 2-(2-chlorophenyl)-2-hydroxycyclohexyl ketone (Ⅲ) figure 1 shown.
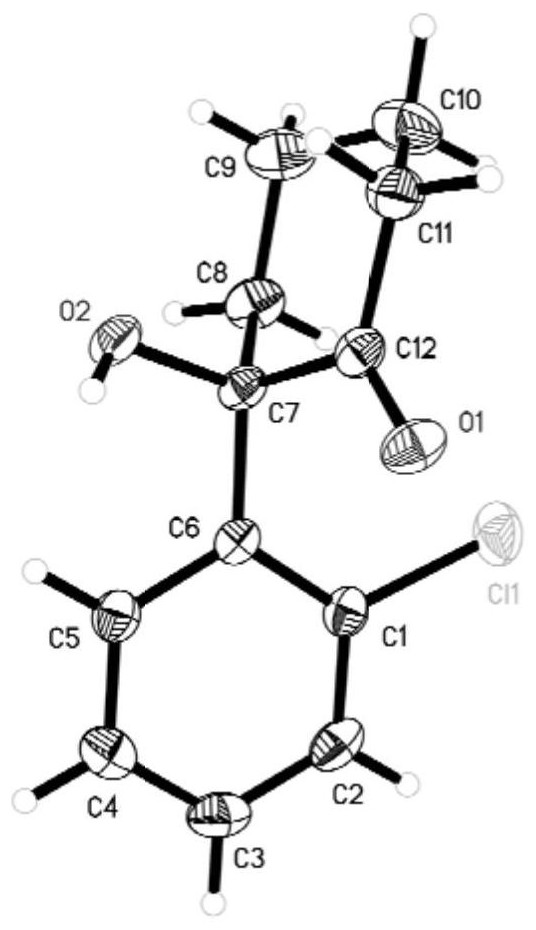
[0086] The H NMR spectrum of compound III is as follows: Figure 6 As shown, the specific data are as follows:
[0087] 1 H NMR (600MHz, CDCl3) δ7.68 (dd, J = 7.8, 1.0Hz, 1H), 7.42–7.33 (m, 2H), 7.28 (ddd, J = 21.5, 10.7, 4.3Hz...
Embodiment 2
[0099] Step 1: Dissolve 2-chlorophenylcyclopentyl ketone (10.0g, 0.048mol) in 100mL absolute ethanol solution at room temperature, add potassium hydroxide (2.7g, 0.048mol) and triethyl phosphite (12.0 g, 0.072mol) was stirred and dissolved, and the temperature was raised to 40° C. for 48 hours under an oxygen atmosphere. After the reaction, concentrate under reduced pressure to remove about 70mL of absolute ethanol, add dropwise 300mL of water to precipitate a solid, filter with suction, and dry at 50°C for 10h to obtain 8.8g of 2-(2-chlorophenyl)-2-hydroxycyclohexylketone (Ⅲ) , yield 82%, purity 98.35%.
[0100] Step 2: Dissolve 2-(2-chlorophenyl)-2-hydroxycyclohexyl ketone (6.0g, 0.027mol) in 50mL tetrahydrofuran solution at room temperature, add triethylamine (9.5g, 0.094mol), -10 Methanesulfonyl chloride (9.2 g, 0.08 mol) was added dropwise at °C for 1 h, then ammonia-methanol solution (40 mL, 0.27 mol) was added and the temperature raised to 15 °C for 3 h. After the rea...
Embodiment 3
[0108] Step 1: Dissolve 2-chlorophenylcyclopentyl ketone (10.0g, 0.048mol) in 100mL absolute ethanol solution at room temperature, add potassium carbonate (6.6g, 0.048mol) and sodium bisulfite (7.5g, 0.072mol) was stirred and dissolved, and the temperature was raised to 40° C. for 24 hours under an oxygen atmosphere. After the reaction, concentrate under reduced pressure to remove about 70mL of absolute ethanol, add dropwise 300mL of water to precipitate a solid, filter with suction, and dry at 50°C for 10h to obtain 6.0g of 2-(2-chlorophenyl)-2-hydroxycyclohexylketone (Ⅲ) , yield 55.7%, purity 98.1%.
[0109] Step 2: Dissolve 2-(2-chlorophenyl)-2-hydroxycyclohexyl ketone (3.0g, 0.013mol) in 25mL tetrahydrofuran solution at room temperature, add triethylamine (4.7g, 0.047mol), -10 Methanesulfonyl chloride (4.6 g, 0.04 mol) was added dropwise at °C for 1 h, then 25%-30% methylamine-ethanol solution (23 mL, 0.13 mol) was added and the temperature was raised to 30 °C for 1.5 h. ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com