Patents
Literature
261 results about "Methanesulfonyl chloride" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Methanesulfonyl chloride (mesyl chloride) is an organosulfur compound with the formula CH₃SO₂Cl. Using the organic pseudoelement symbol Ms for the methanesulfonyl (or mesyl) group CH₃SO₂, it is frequently abbreviated MsCl in reaction schemes or equations. It is a colourless liquid that dissolves in polar organic solvents but is reactive toward water, alcohols, and many amines. The simplest organic sulfonyl chloride, it is used to make methanesulfonates and to generate the elusive molecule sulfene (methylenedioxosulfur(VI)).
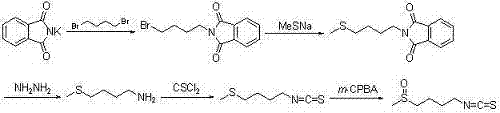
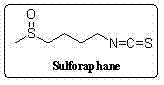
Synthetic method for sulforaphane
ActiveCN102249968AAvoid hydrazinolysisSimple and fast operationOrganic chemistryBulk chemical productionSulforaphaneSodium methanethiolate
The invention provides a synthetic method for sulforaphane and belongs to the field of drug synthesis. The method comprises the following steps that: after amino in 4-amino-1-butanol is protected by Boc groups, hydroxy in 4-amino-1-butanol is changed into methanesulfonyl ester by methanesulfonyl chloride, and then the resultant reacts with sodium methyl mercaptide to produce 4-methylthio butyl-1-tert-butoxycarbonylamide; Boc protective groups are removed under acidic condition to obtain 4-methylthio-1-butylamine; 4-methylthio-1-butylamine reacts with carbon disulfide for one hour in the presence of triethylamine and p-toluenesulfonyl chloride is added for treatment for half an hour to produce 4-methylthio butyl-1-isothiocyanate; and at last 4-methylthio butyl-1-isothiocyanate is oxidized by m-CPBA to produce sulforaphane. According to the invention, complex hydrazinolysis of phthalimide in aftertreatment is avoided and toxic thiophosgene is not needed in the preparation of isothiocyanate; overall yield of sulforaphane is 64%, substantially higher than the overall yield of 8% reported in literature; the whole preparation process is simple and time-saving and is suitable for large scale production.
Owner:江苏宁录科技股份有限公司
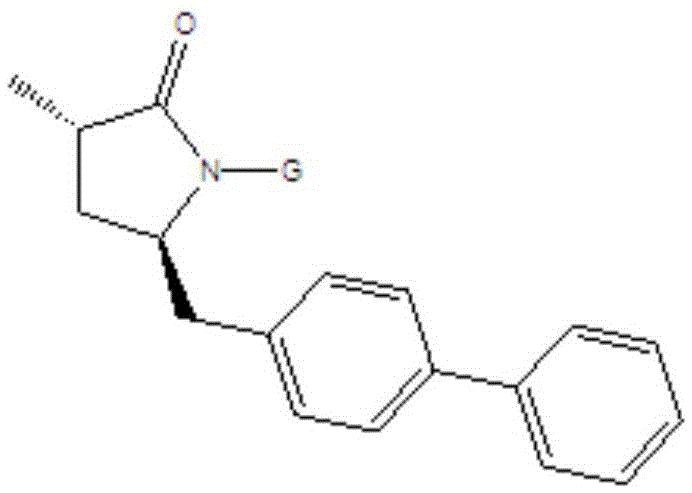
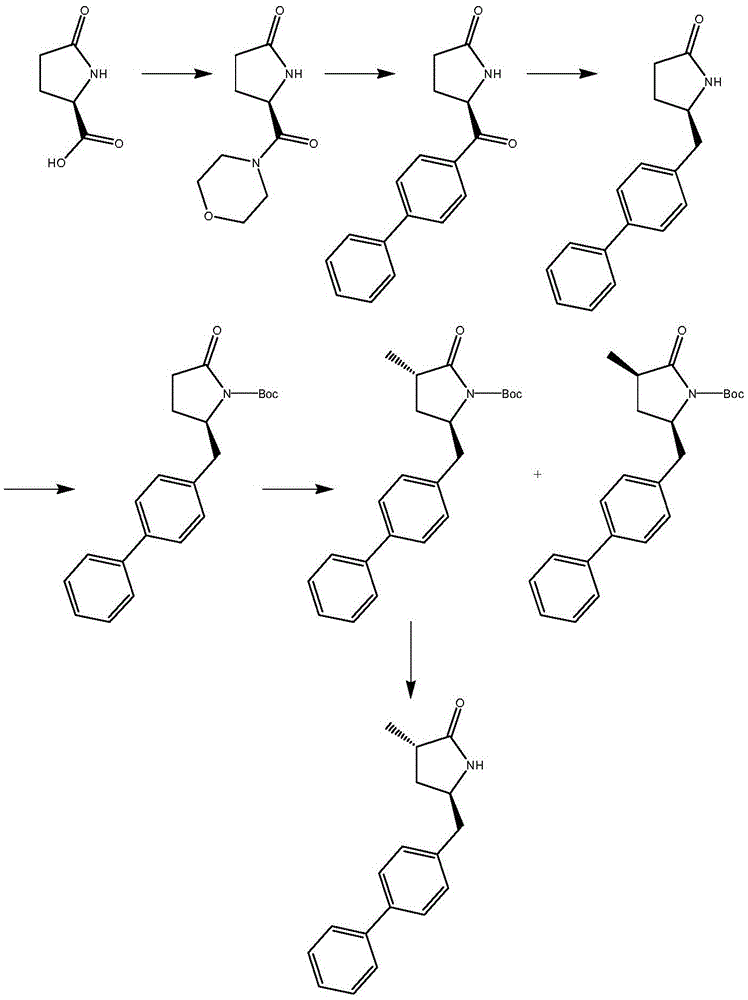
Preparation method for Sacubitril intermediate
InactiveCN105566194AHigh purityHigh yieldOrganic chemistry methodsPhenylmagnesium bromideMethyl methanesulfonate
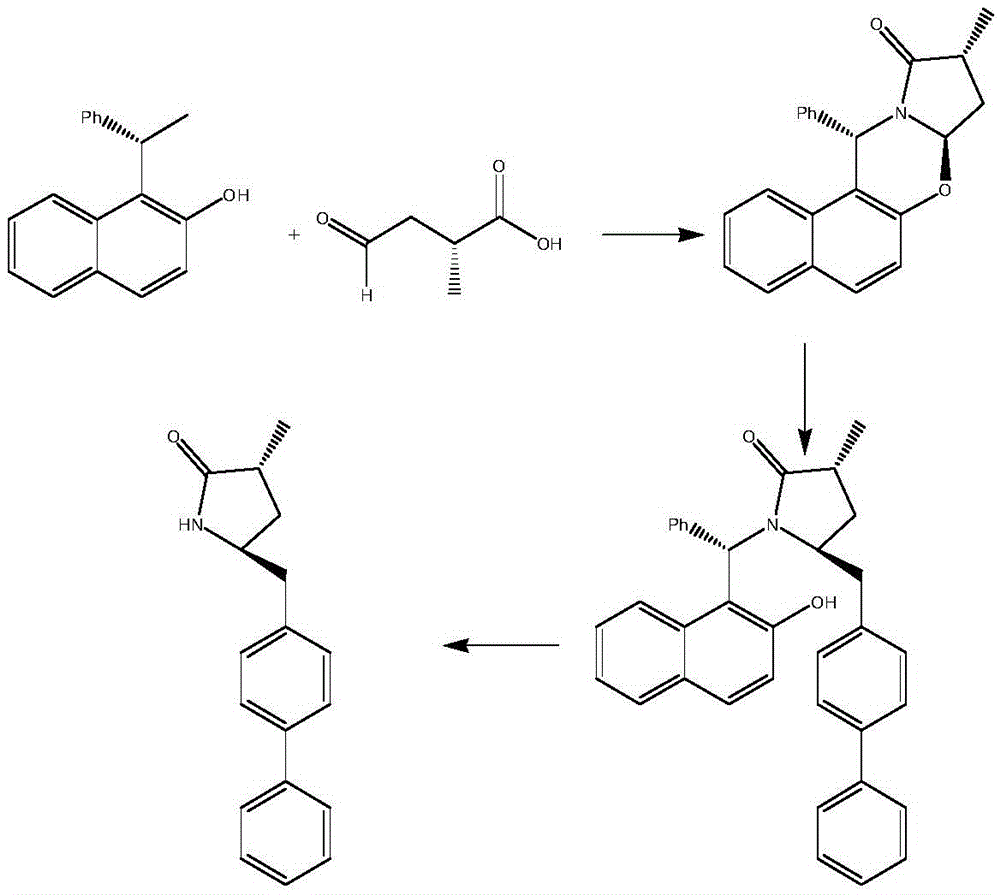
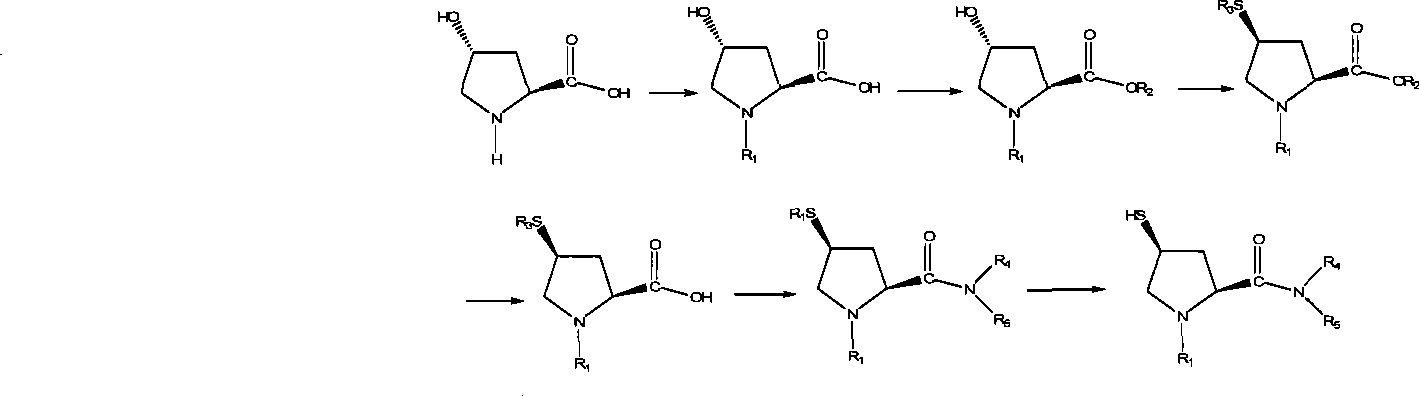
The invention relates to a preparation method for a Sacubitril intermediate. The preparation method comprises the following steps that (3R,5S)-5-(hydroxymethyl)-3-methyl-2-pyrrolidone is esterified with toluene sulfochloride or methanesulfonyl chloride to obtain (3R,5S)-5-(methyl p-toluenesulfonate)-3-methyl-2-pyrrolidinone or (3R,5S)-5-(methyl methanesulfonate)-3-methyl-2-pyrrolidinone; (3R,5S)-5-(methyl p-toluenesulfonate)-3-methyl-2-pyrrolidinone or (3R,5S)-5-(methyl methanesulfonate)-3-methyl-2-pyrrolidinone is coupled with 4-diphenylmagnesium bromide or 4-diphenylmagnesium chloride to obtain (3R,5S)-3-methyl-5-(1,1'-diphenyl-4-yl-methyl)-2-pyrrolidinone. According to the preparation method, the method is novel, the raw materials are easy to obtain, the technology is simple, and the purity and yield of the product are both very high.
Owner:张伯引
Preparation process of insulin sensitizer
InactiveCN104744282AHigh yieldAvoid conditions that are prone to hydrolysisOrganic compound preparationSulfonic acid esters preparationCyclohexanoneNitrogen gas
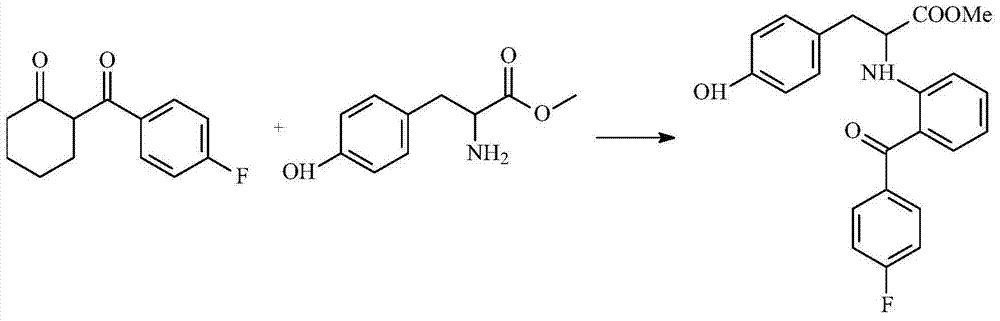
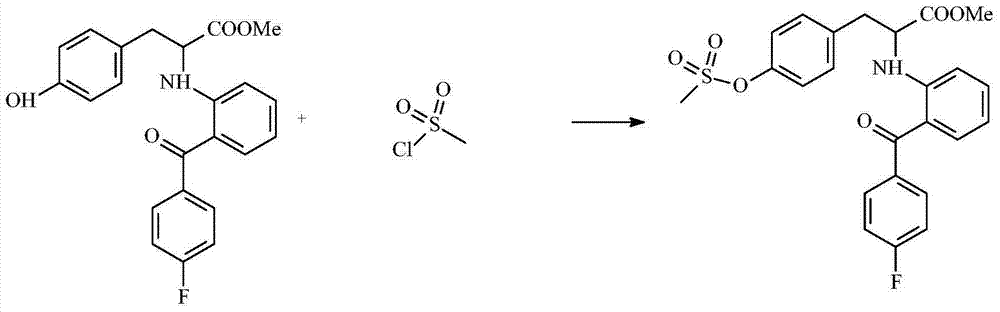
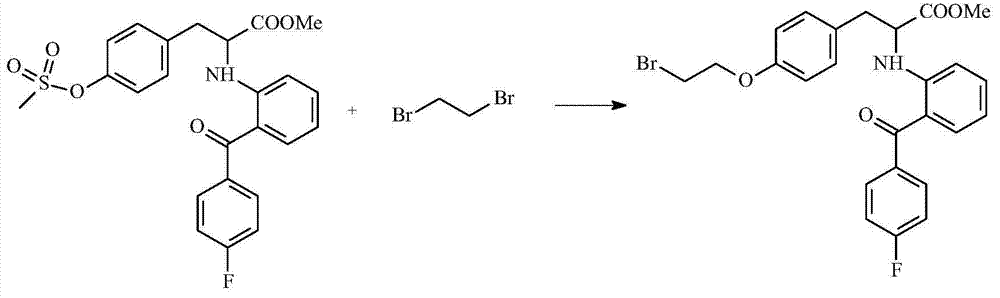
The invention discloses a preparation process of an insulin sensitizer. The preparation process comprises the following steps: mixing 2-(4-fluorobenzoyl) cyclohexanone, L-tyrosine methyl ester, dioxane and methylbenzene, filling nitrogen and reacting, carrying out a distilling procedure, and reacting with the addition of anisole and palladium / carbon so as to obtain a first product; mixing the first product with dichloromethane, adding methylsufonyl chloride and pyridine, reacting, distilling and removing dichloromethane so as to obtain a second product; mixing for reacting the second product with tetrahydrofuran and 1,2-dibromoethane, and thinning with water so as to obtain a third product; and mixing for reacting the third product with salt, cuprous chloride, carbazole, 8-hydroxyquinoline and dimethyl sulfoxide, thinning with water and extracting with ethyl acetate, distilling and removing ethyl acetate so as to obtain a final product, namely 2-[2-(4-fluorobenzoyl) aniline]-3-[4-(2-carbazolyl ethyoxyl) phenyl] methyl propionate. The yield of the final product is 47%; and in the process of joining the final product and carbazole, the circumstance of ester hydrolysis in a synthesis reaction of the novel insulin sensitizer, namely chiglitazar, is avoided.
Owner:NANTONG HENGSHENG FINE CHEM
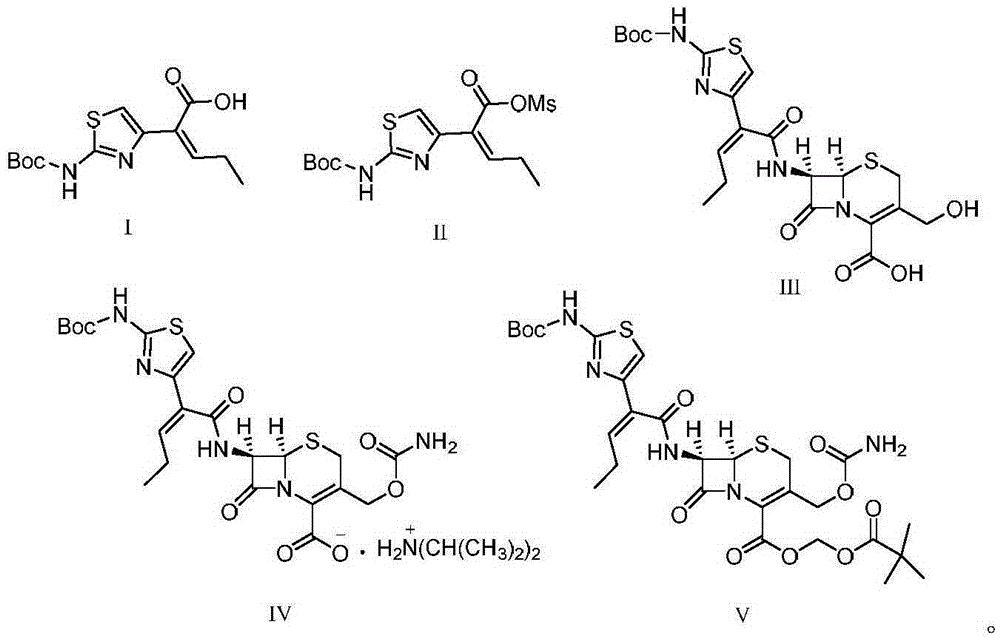
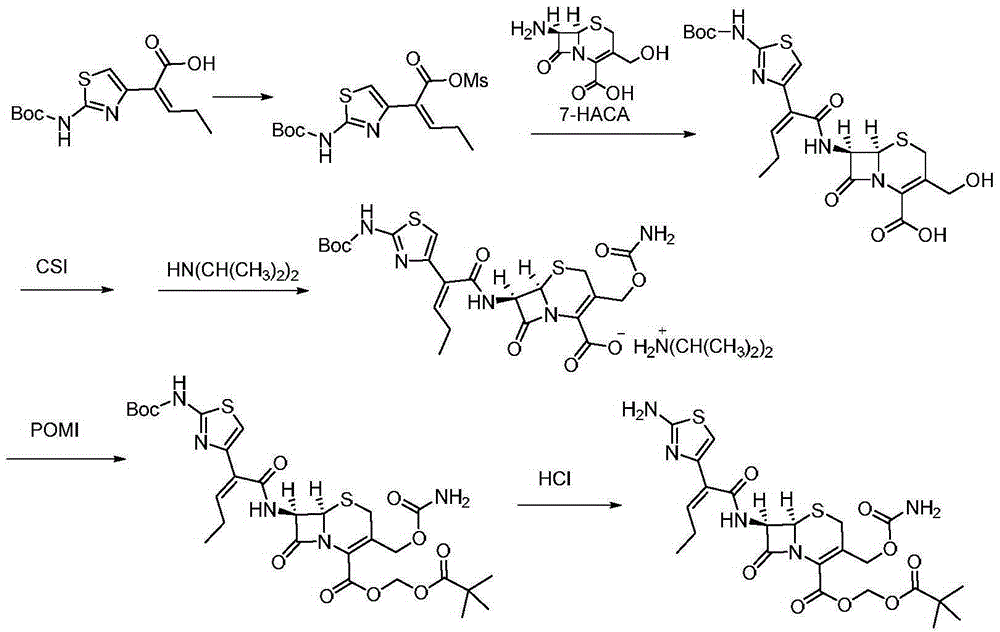
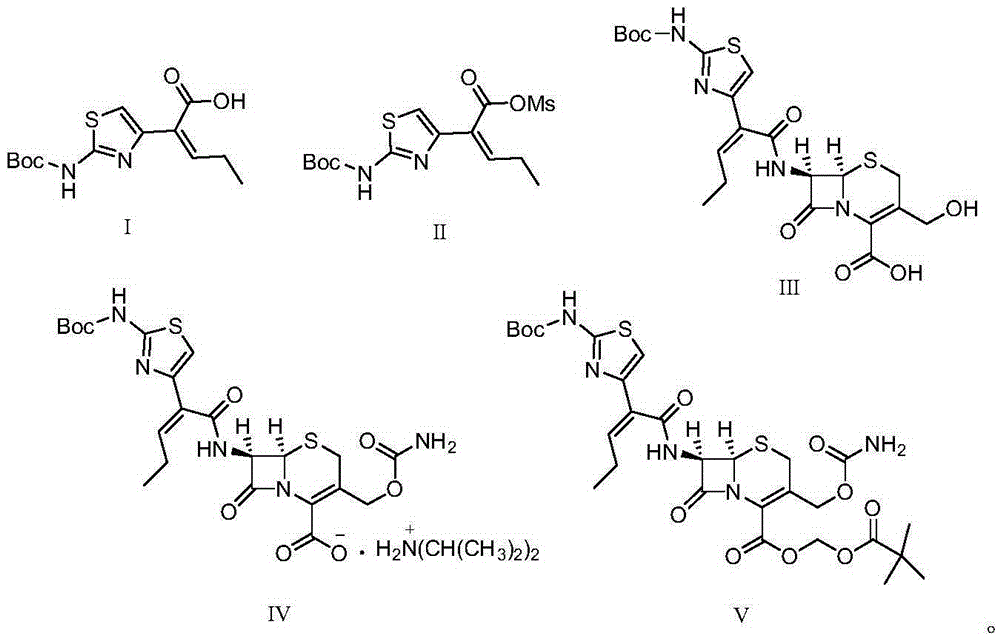
Preparation method for cefcapene pivoxil hydrochloride
ActiveCN105254649ASimple and fast operationConducive to follow-up reactionsOrganic chemistryBulk chemical productionMethanesulfonyl chlorideCefcapene pivoxil hydrochloride
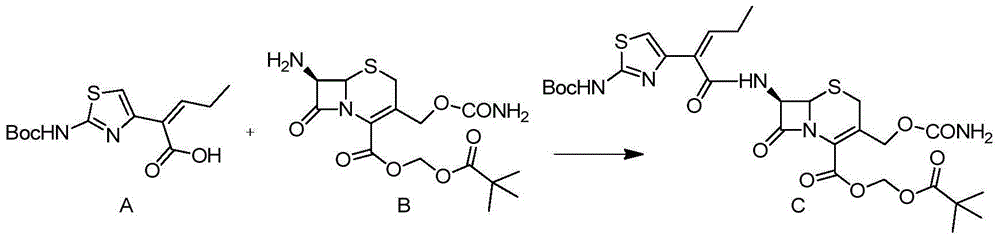
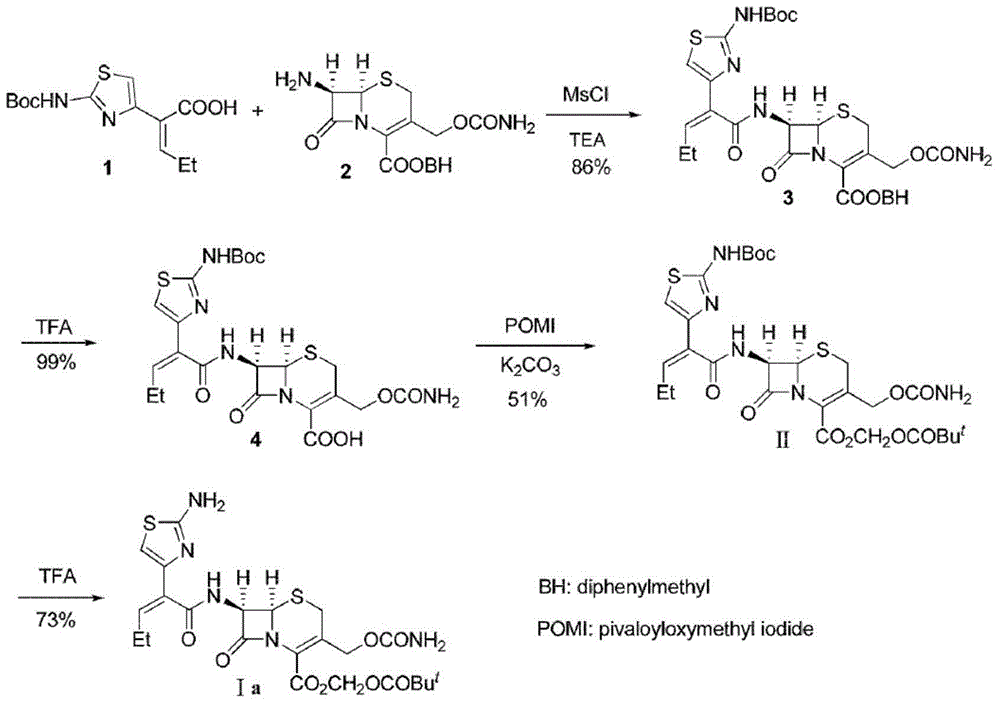
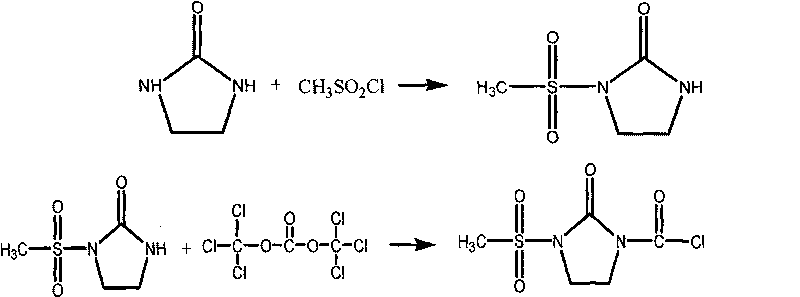
The invention discloses a preparation method for cefcapene pivoxil hydrochloride. The method comprises the following steps: (1) stirring and dissolving a compound which is shown as formula (I) in pyridine, adding methylsufonyl chloride to react to obtain a liquid which contains the compound shown as formula (II), placing the liquid at -15 DEG C to 0 DEG C for later use; (2) in the existence of proline and diisopropylamine, enabling 7-HACA and the liquid which contains the compound shown as the formula (II) to react in methyl alcohol to obtain the compound which is shown as formula (III); (3) adding diisopropylamine, enabling the compound which is shown as the formula (III) and chlorosulfonyl isocyanate to react, regulating the pH to 4 to 5, cooling the organic phase, and adding the diisopropylamine to obtain the compound which is shown as formula (IV); (4) in the existence of potassium phosphate and copper acetate, enabling the compound which is shown as the formula (IV) and iodomethyl pivalate to react in DMF (Dimethyl Formamide) to obtain the compound which is shown as formula (V); (5) removing protecting groups from the compound which is shown as the formula (V) in the methanol solution of hydrochloric acid to obtain the cefcapene pivoxil hydrochloride. According to the method, the product yield is greatly improved, and the method is suitable for industrial production.
Owner:湖北凌晟药业股份有限公司
Interrupter method for producing high-purity methyl sulfonyl chloride
InactiveCN1465564AEasy to operateHigh purityOrganic chemistryOrganic compound preparationSulfonyl chlorideMethanesulfonyl chloride
The present invention relates to a method for producing high-purity methyl sulfonyl chloride by using intermittent process. In the aqueous solution of hydrochloric acid it uses dimethyl disulfur and chlorine gas and makes them produce reaction to produce methyl sulfonyl chloride. Its reaction yield can be up to 97%, and its product purity is greater than or equal to 99.7%.
Owner:HEBEI YANUO CHEM IND
Synthesis method of Empagliflozin intermediate
InactiveCN108178751AHigh yieldImprove responseOrganic chemistry methodsIodine3-Hydroxytetrahydrofuran
The invention discloses a synthesis method of an Empagliflozin intermediate. The synthesis method uses 4-hydroxybenzyl chloride as a starting material to sequentially react with methanesulfonyl chloride and (S)-3-hydroxytetrahydrofuran to obtain compound III, then react with 4-iodoaniline to obtain compound IV, and finally react with cuprous chloride after diazotization to obtain (S)-3-(4-(5-iodine-2-chlorobenzyl)phenoxy) tetrahydrofuran. The raw materials used in the synthesis method are simple and easy to obtain, the operation steps are simple, the post-treatment is simple, the product yieldis high, and the method is suitable for industrial production.
Owner:YANGZHOU POLYTECHNIC INST
Preparation method for cefcapene pivoxil hydrochloride
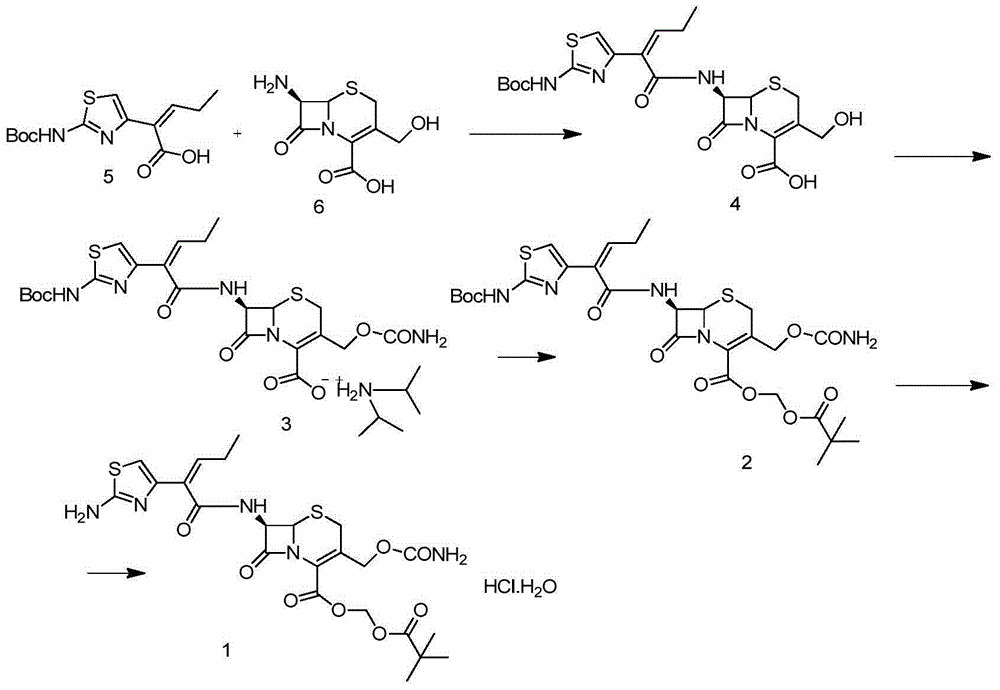
The invention belongs to the technical field of antibiotic synthesis, and relates to a preparation method for cefcapene pivoxil hydrochloride. The preparation method comprises the following steps: (1) reacting 7-ACA with sodium hydroxide in a solution with quaternary ammonium salt under the temperature of (-5 DEG C)-5 DEG C to generate 7-DACA; (2) adding (cefcapene pivoxil side chain acid, compound 5) into the solution containing 7-DACA, diisopropylamine and phenyltriethylammonium chloride under the temperature of 0-10 DEG C to react with a methylsufonyl chloride reaction solution under the temperature of (-15 DEG C)-0 DEG C to obtain a compound (4); reacting the compound 4 and chlorosulfonyl isocyanate to obtain a compound (3); further reacting the compound (3) with iodomethyl pivalate to obtain a compound (2); removing the protection base of the compound (2) in a hydrochloric acid methanol solution to obtain the cefcapene pivoxil hydrochloride (compound 1).
Owner:SHANDONG LUOXIN PARMACEUTICAL GROUP STOCK CO LTD
Method for synthesizing 1-chloroformyl-3-methyl sulfonyl-2-imidazo flavanone
InactiveCN101747278AHigh reaction yieldShort reaction timeOrganic chemistryN dimethylformamideReaction temperature
The invention provides a method for synthesizing 1-Chloro-formyl-3-methyl sulfonyl-2-imidazo flavanone, which comprises the steps of: (1) reacting methyl chloride and 2 - imidazo flavanone to produce methylsulfonyl-2-imidazo flavanone under the action of a catalyst; and (2) carrying out acylation reaction on the methylsulfonyl-2-imidazo flavanone and triphosgene in an organic solvent in the presence of a catalyst. The invention is technically characterized in that a catalyst is introduced in the step (1), thereby improving the reaction yield, wherein the catalyst can be triethylamine, pyridine, or N,N-dimethylformamide; and the reaction temperature and the dose of the solvent are adjusted in the step (2), thereby shortening the reaction time and reducing energy consumption, wherein the reaction temperature is between 15 DEG C and 25 DEG C, and the dose of the solvent is adjusted according to the ratio of the methylsulfonyl-2-imidazo flavanone to the organic solvent being 1g:5-10 ml.
Owner:YIYUAN XINQUAN CHEM
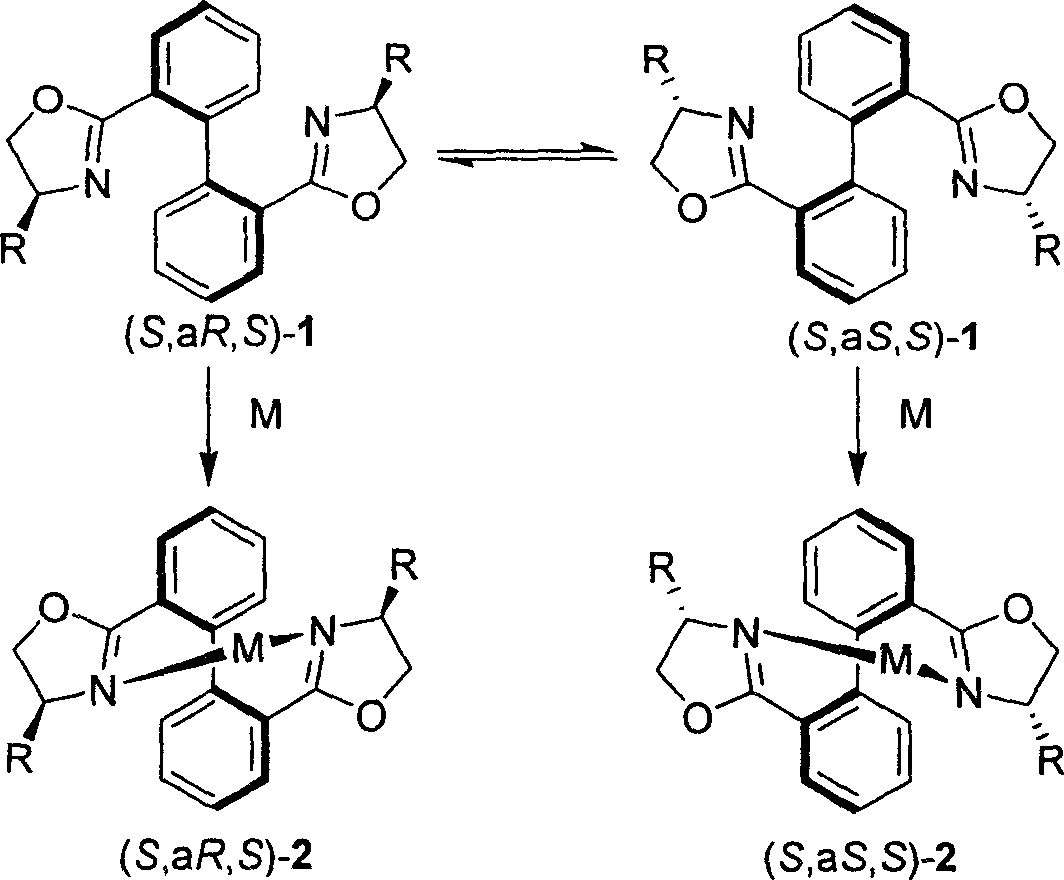
2,2', 6,6'-tetraoxazoline diphenyl ligancy and preparation process thereof
InactiveCN1793130AAvoid wasting resources and other issuesAvoid issues such as wasteOrganic chemistryCarboxylic acidMethanesulfonyl chloride
The invention relates to 2, 2í»,6, 6í»-4-oxazoline biphenyl ligand and the manufacture method that uses pyrene as raw material and oxidizing and opening loop in the oxidizing system of sodium periodate and ruthenium trichloride to gain 2, 2í», 6, 6í»-4-carboxylic acid biphenyl, reacting with thionyl chloride to gain acyl chloride that is dropped into the methylene chloride solution of spasmolytol and alkamine, reacting to gain product that takes reaction with methylsulfonyl chloride and spasmolytol to gain the target product. It has high reacting activity and solid selection and has great prospect. 2, 2í»,6, 6í»-4-oxazoline biphenyl ligand has the structure of R1=hydrogen, phenyl, benzyl group, or alkyl of 1-8 carbon; R2=hydrogen, phenyl, benzyl group, or alkyl of 1-8 carbon; R3=hydrogen, phenyl, benzyl group, or alkyl of 1-8 carbon; and R4=hydrogen, phenyl, benzyl group, or alkyl of 1-8 carbon.
Owner:SHANGHAI JIAOTONG UNIV
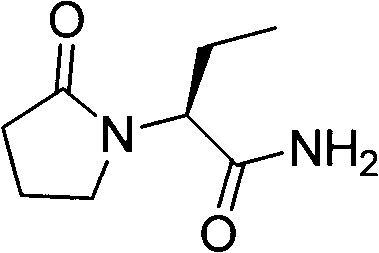
Novel synthetic method of antiepileptic drug levetiracetam
InactiveCN102863370ASuitable for industrial mass productionHigh yieldOrganic chemistryAcetic acidAntiepileptic drug
The invention belongs to the field of synthetic processes of chiral drugs and discloses a novel synthetic method of antiepileptic drug levetiracetam. (S)-alpha-ethyl-oxo-1-pyrrolidine acetic acid is used as an initial raw material and subjected to further reaction under the catalysis of methanesulfonyl chloride and the action of ammonia to prepare the levetiracetam. The novel synthetic method of the antiepileptic drug levetiracetam is simple in operating, few in by-products, high in yield and suitable for industrial large-scale production in factories.
Owner:SHANGHAI ECUST BIOMEDICINE CO LTD
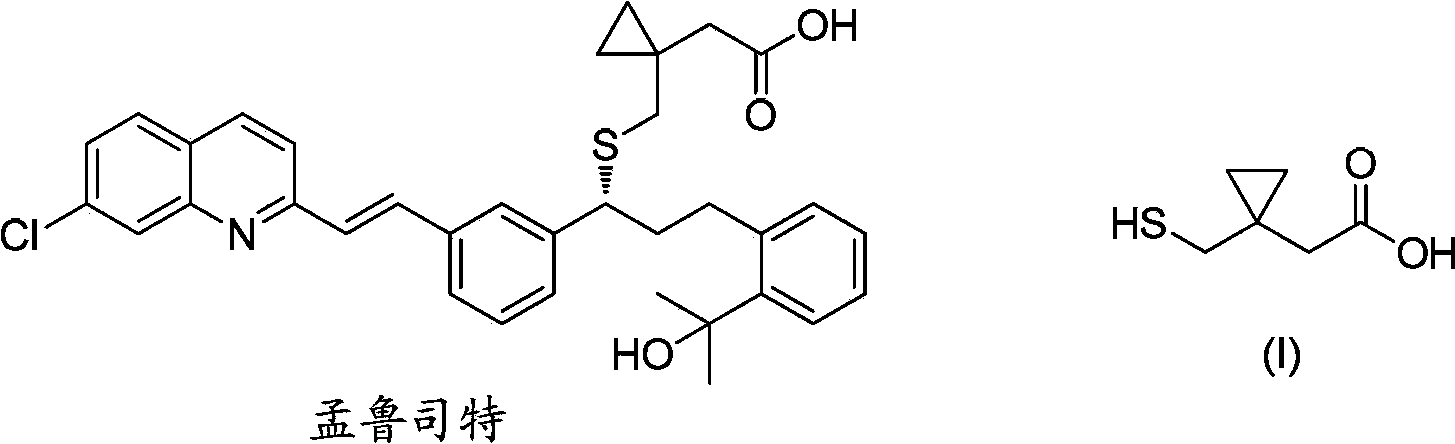
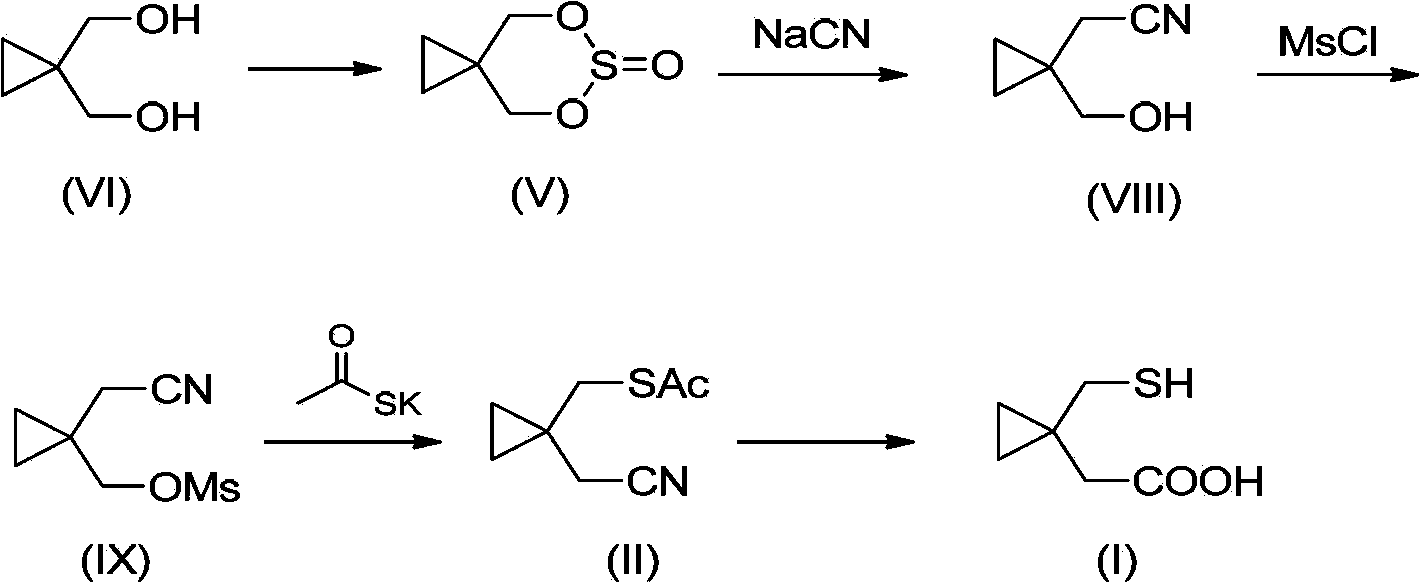
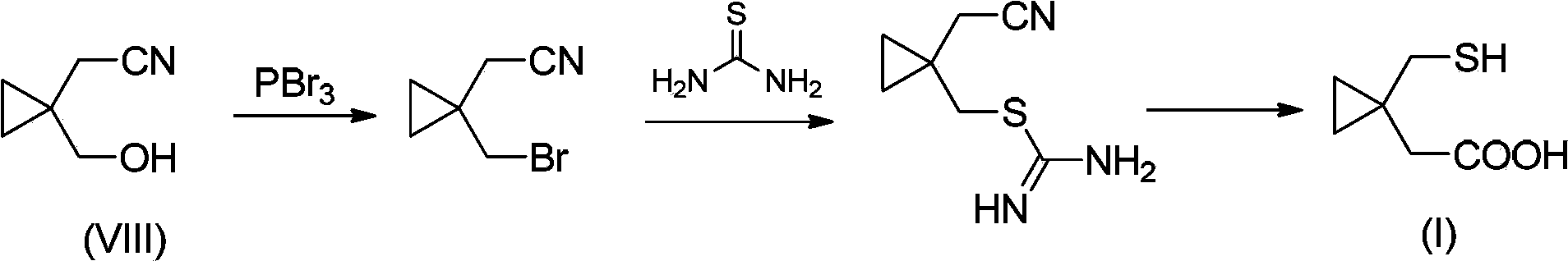
Preparation methods of 1-(mercaptomethyl)cyclopropyl acetic acid and intermediate thereof
InactiveCN103539714AIngenious designStarting materials are cheap and readily availableThiol preparationAcetic acidSulfite
The invention relates to a preparation method of 1-(mercaptomethyl)cyclopropyl acetic acid (I). The preparation method comprises the steps of carrying out ring opening on cyclopropanedimethanol cyclic sulfite (V) with potassium thioacetate, so that a compound (IV) is obtained; carrying out sulfonic acid esterification reaction on the compound (IV) and methanesulfonyl chloride or paratoluensulfonyl chloride to obtain a compound (III); carrying out cyano group substitution on the compound (III) to obtain a compound (II); and hydrolyzing the compound (II) under an alkaline condition so as to obtain the 1-(mercaptomethyl)cyclopropyl acetic acid (I), wherein R is a methyl or a p-methylphenyl. The invention further provides a preparation method of an 1-(mercaptomethyl)cyclopropyl acetic acid intermediate. The preparation methods of the 1-(mercaptomethyl)cyclopropyl acetic acid and the 1-(mercaptomethyl)cyclopropyl acetic acid intermediate are ingenious in design, initial raw materials are low in cost and easily available, and the technological process is simple and practicable, so that the production cost can be greatly reduced; the preparation methods are beneficial to industrial production and suitable for large-scale popularization and application.
Owner:SHANGHAI PUYI CHEM CO LTD
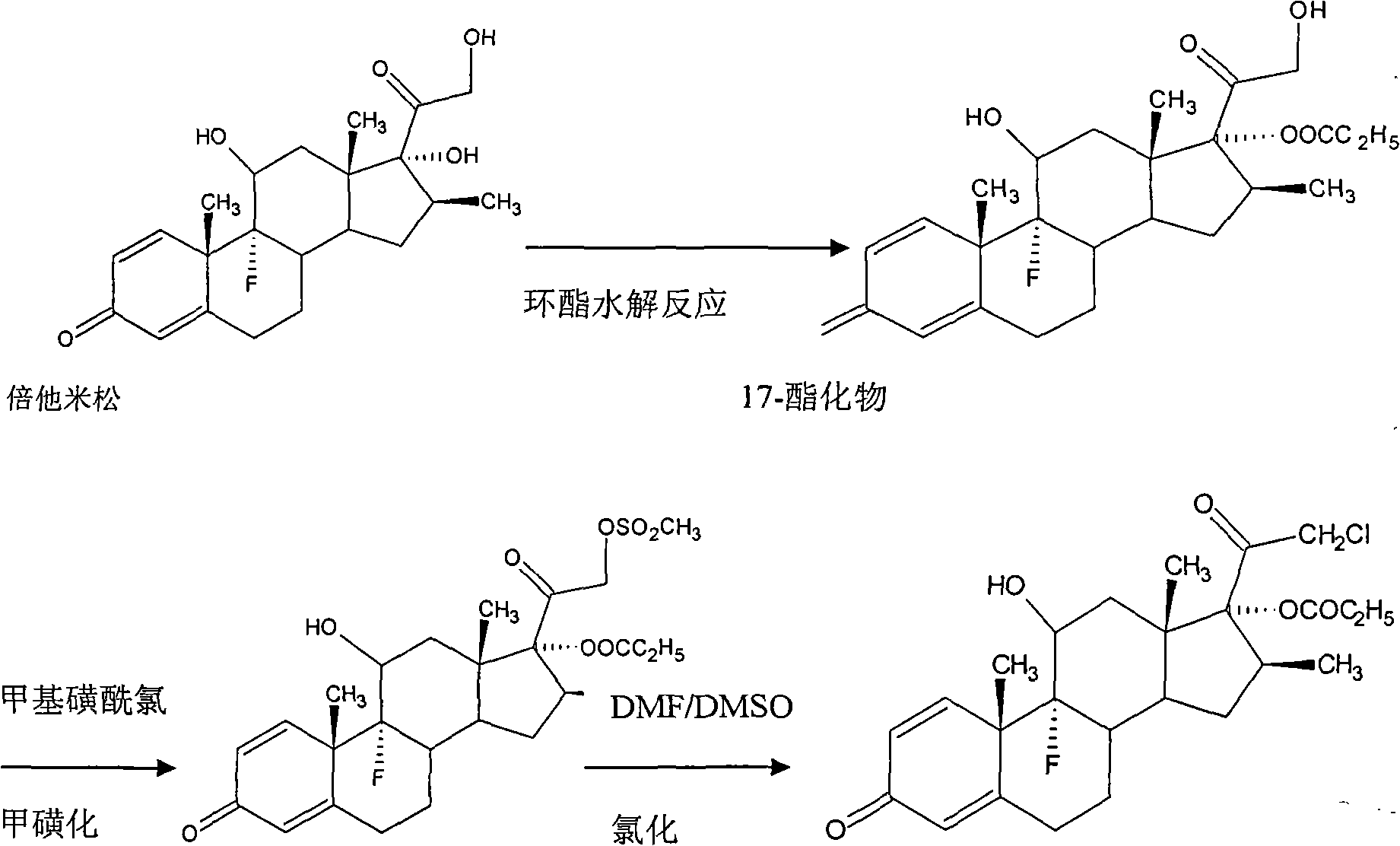
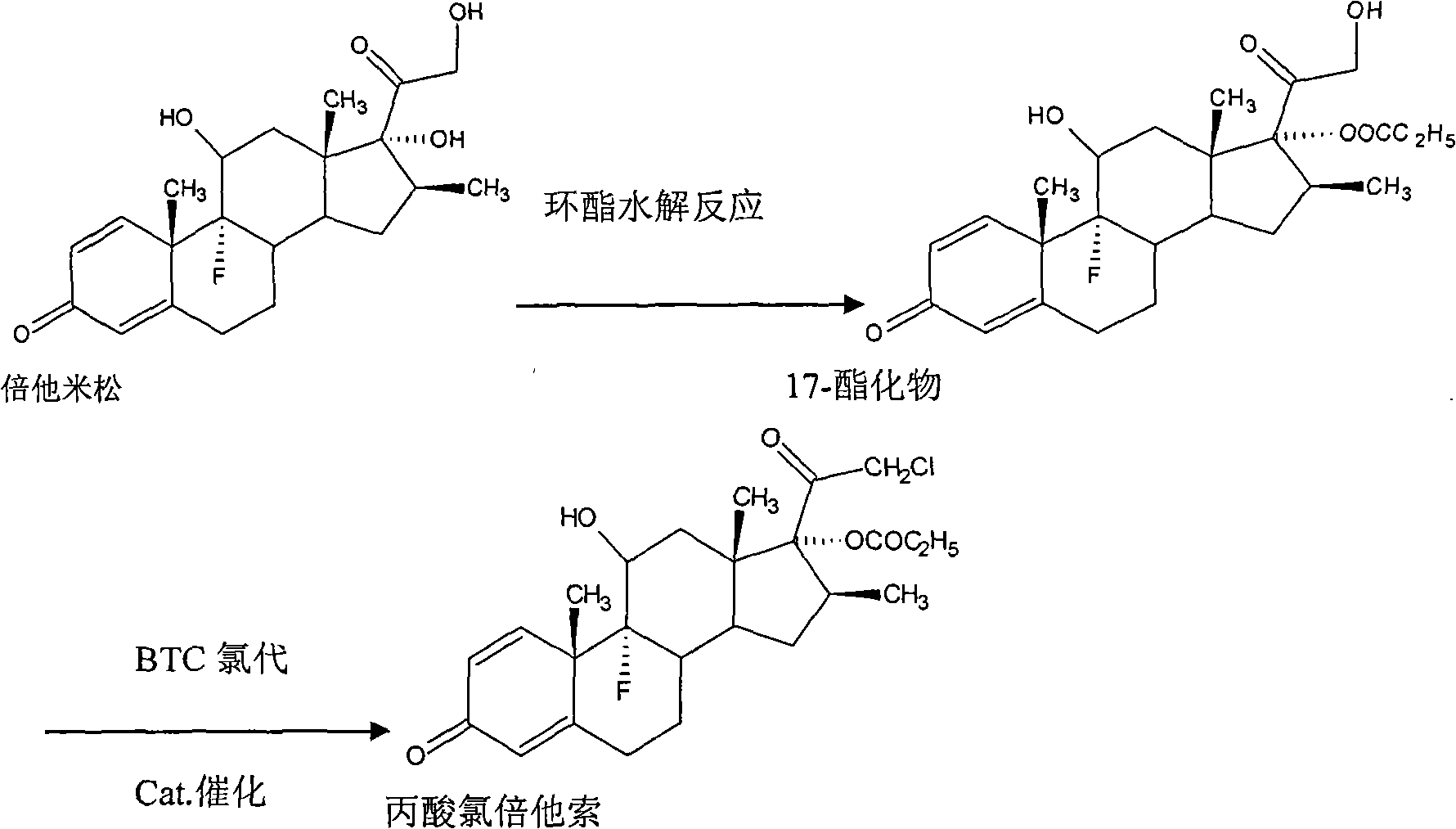
Method for synthesizing clobetasol propionate intermediate
ActiveCN101812107AReduce usageEmission reductionSteroidsReaction temperatureMethanesulfonyl chloride
The invention discloses a method for synthesizing clobetasol propionate, which belongs to the technical field of synthesis of steroid medicaments in the pharmaceutical chemistry and comprises the following steps: (1) dissolving betamethasone-17-ester into acetone solution; (2) adding a catalyst and BTC (bis(trichloromethyl) carbonate) for carrying out chloroacetic reaction at the reaction temperature of 30-50DEG C for 2-5 hours; and (3) after reaction, carrying out reduced pressure concentration, elutriation, filtering and drying. Compared with the traditional process, the yield can be improved by 6 percent, the cost is reduced by 20 percent and the quality is remarkably improved. The using amount of original auxiliary materials are reduced by 20 percent, the using amount of a toxic material of methanesulfonyl chloride can be reduced by 1 ton each year, the using amount of DMF (Dimethyl Formamide) can be reduced by 17 tons and the using amount of pyridine can be reduced by 6.3 tons, the economic cost can be totally saved by 366,000 yuan and waste water drain can be reduced by 310tons / year; and in addition, the invention greatly reduces the pressure for protecting the environment and can effectively reduce the hazard on human body and the pollution on the environment.
Owner:ZHEJIANG XIANJU XIANLE PHARMA
Phase-transfer catalysed formation of n-(substituted phenyl) sulfonamides in water
InactiveUS20160024028A1Promote rapid formationReduce hydrolysis rateOrganic chemistryChemical recyclingHydrogenOxygen
A new process for making agrochemically important N-[2,4-dichloro-5-[4-(difluoro methyl)-4,5-dihydro-3-methyl-5-oxo-1H-1,2,4-triazol-1-yl] phenyl]methanesulfonamide by reacting 1-(5-amino-2,4-dichlorophenyl)-4,5-dihydro-4-difluoromethyl-3-methyl-1,2,4-triazol-5(1H)-one and methanesulfonyl chloride in water using an inorganic base to dynamically control the reaction pH and in the presence of a phase transfer catalyst suspended in an 1:1 aromatic solution.
Owner:FRAMROZE BOMI P
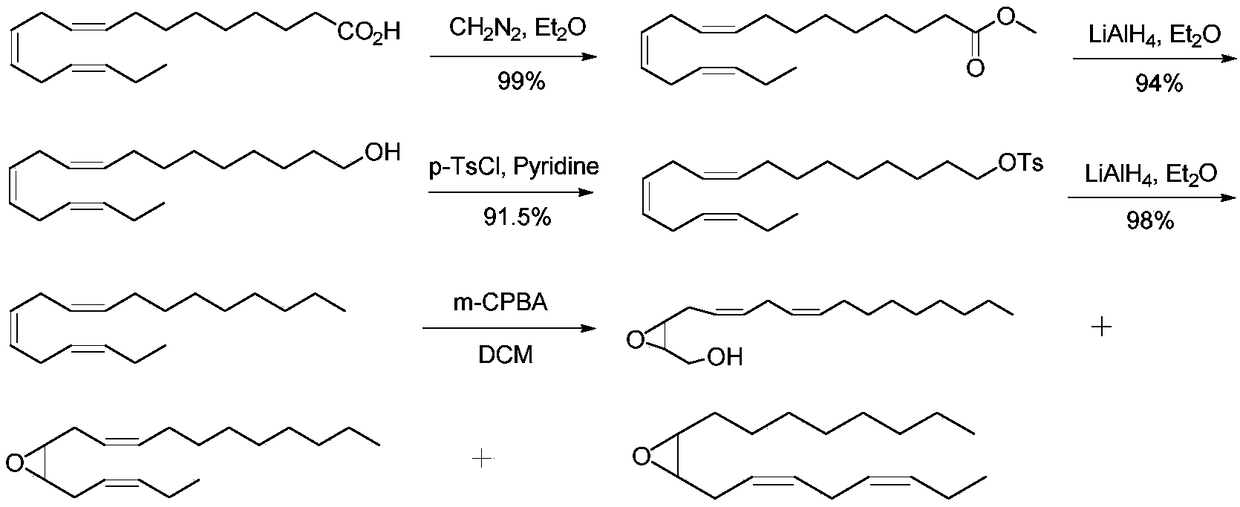
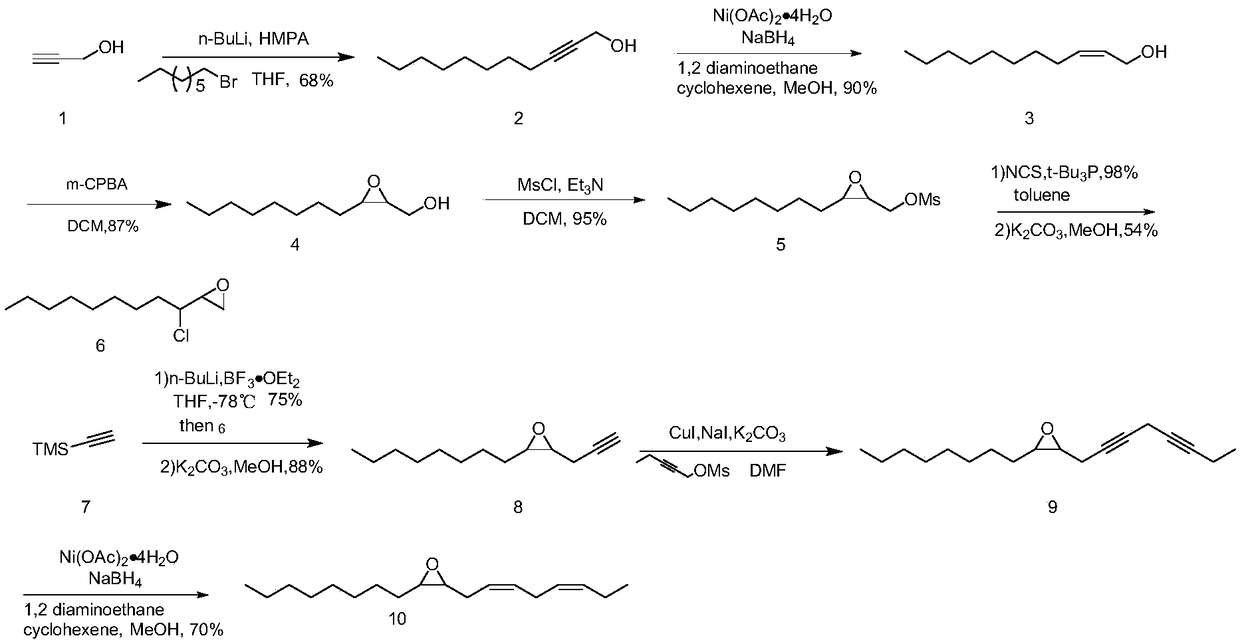
Synthesis method of (3Z,6Z)-9,10-epoxy-octadecadiene
InactiveCN109336846ARaw materials are cheap and easy to getSimple and fast operationOrganic chemistryEpoxySynthesis methods
The invention discloses a synthesis method of (3Z,6Z)-9,10-epoxy-octadecadiene which is one of tea geometrid sex pheromone components. The synthesis method comprises the following steps: coupling propynyl alcohol with bromooctane to produce undec-2-yn-1-ol, and catalytically hydrogenating the undec-2-yn-1-ol to obtain cis-undec-2-en-1-ol; performing a reaction on the cis-undec-2-en-1-ol and m-chloroperoxybenzoic acid to obtain undec-2,3-epoxy-1-ol; sulfonylating hydroxyl groups of the undec-2,3-epoxy-1-ol by p-methylsufonyl chloride to obtain p-methylsulfonyl ester; performing ring opening onan epoxidized compound under the conditions of tri-tert-butylphosphine and chlorosuccinimide to obtain 3-chloro-2-hydroxyundecyl methanesulfonate, and then performing ring closure under an alkali condition to obtain 3-chloro-1,2-epoxyundecane; then, performing ring opening under the conditions of n-butyl lithium and boron trifluorideetherate, and performing ring closure under the condition of potassium carbonate to obtain 2-octyl-3-(prop-2-yn-1-yl)oxirane; then, obtaining 2-(octane-2,5-diyn-1-yl)-3-octyloxirane under the condition of an iodo coupling reagent; and finally, performing hydrogenation catalysis to obtain a final product. The synthesis method is relatively low in cost, and is suitable for scale preparation.
Owner:CHANGZHOU UNIV +1
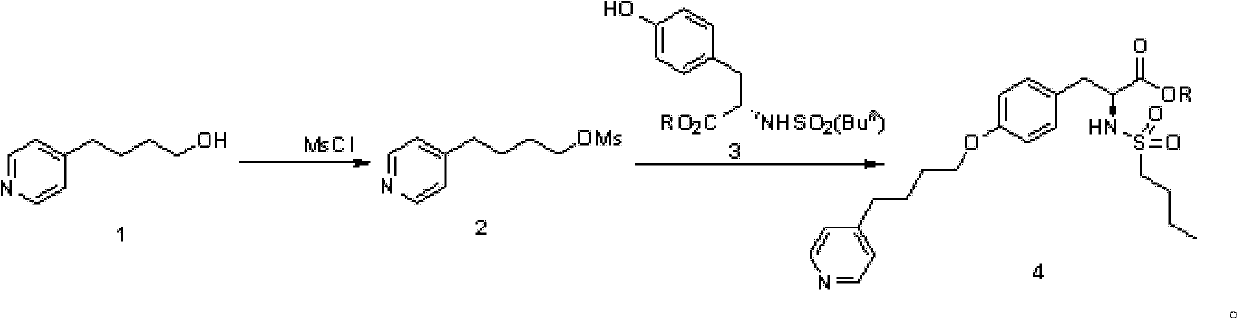
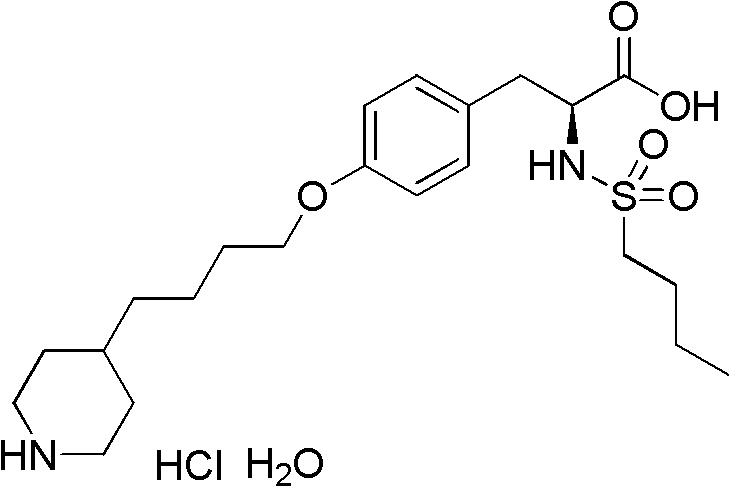
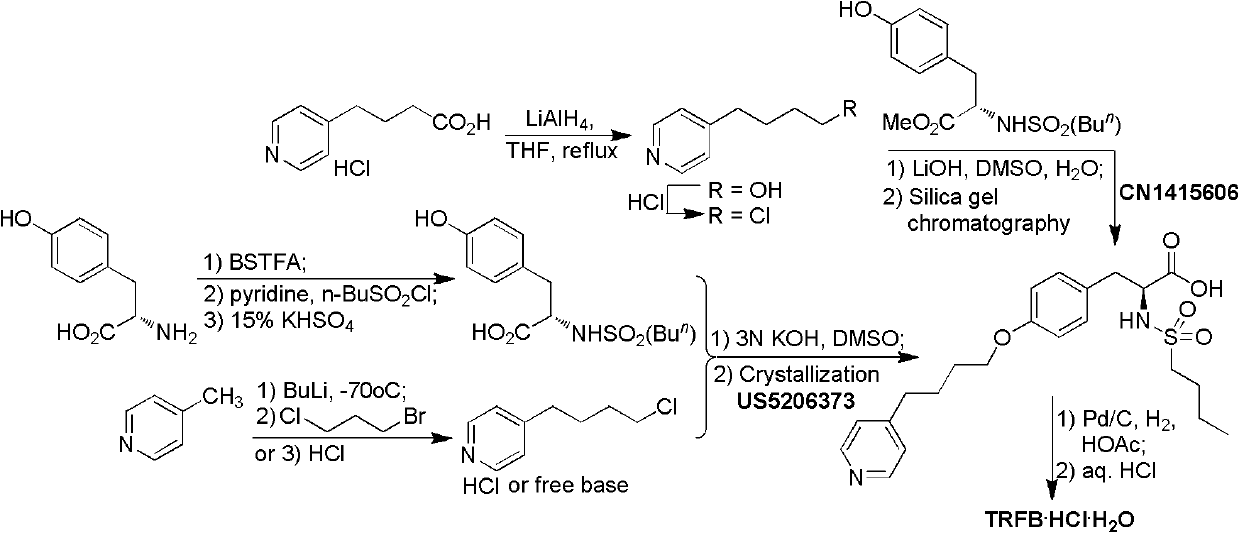
The preparation method of n-n-butylsulfonyl-o-(4-(4-pyridyl)-butyl)-l-tyrosinic acid alkyl ester
The preparation method of a kind of N-n-butylsulfonyl-O-(4-(4-pyridyl)-butyl)-L-tyrosinic acid alkyl ester that the present invention relates to comprises the following steps: the first step reaction : take pyridine butanol as the starting material, react with methanesulfonyl chloride under the action of alkali to obtain methanesulfonyl pyridine butanol; second step reaction: methanesulfonyl pyridine butanol under alkaline conditions, in the presence of phase transfer catalyst , and N-n-butylsulfonyl L-tyrosine alkyl ester condensation to N-n-butylsulfonyl-O-(4-(4-pyridyl)-butyl)-L-tyrosine alkyl ester. The reaction formula is as follows: the synthesis method of the present invention has few reaction steps, high total yield, mild reaction conditions, is suitable for industrial scale-up production, and avoids the use of dangerous and environmentally polluting reagents. Intermediate 2 was directly used in the next reaction without purification after simple treatment.
Owner:SHANGHAI JINGFENG PHARMA +1
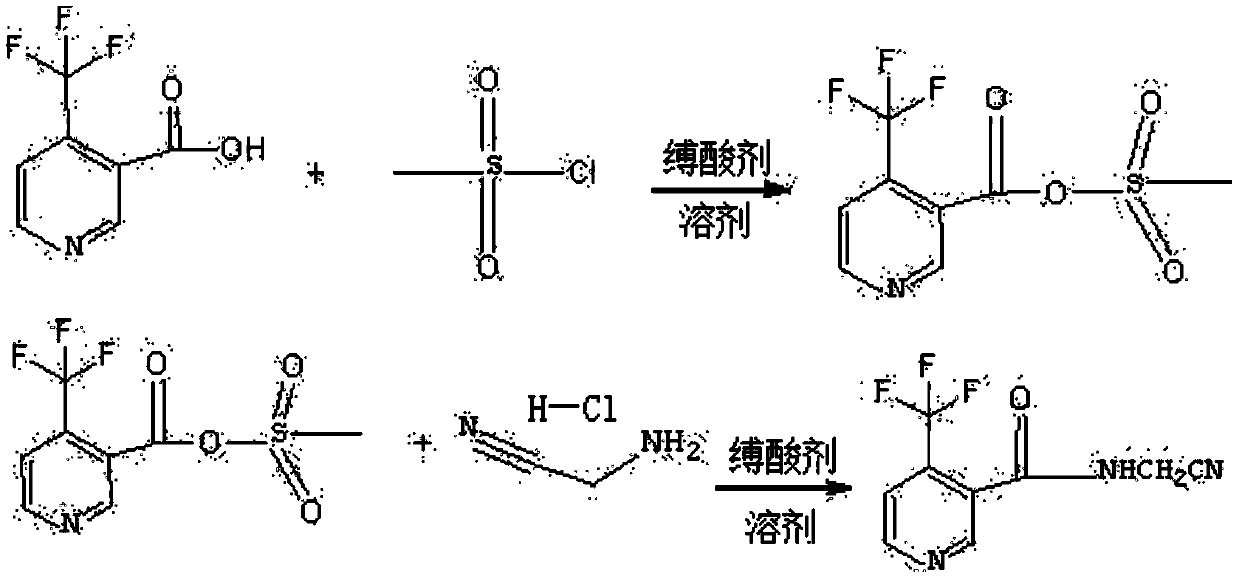
Synthesis method of N-cyanomethyl-4-(trifluoromethyl)-nicotinamide
PendingCN109851552AImprove conversion rateHigh purityOrganic chemistrySulfonyl chlorideSynthesis methods
The invention discloses a synthesis method of N-cyanomethyl-4-(trifluoromethyl)-nicotinamide, and belongs to the technical field of pesticides. According to the method, 4-trifluoromethylnicotinic acidand methane sulfonyl chloride are taken as the primary raw materials and carry out reactions in the presence of an acid binding agent and a solvent to prepare acid anhydrides; then in the presence ofan acid binding agent, acid anhydrides and amino acetonitrile hydrochloride carry out reactions to prepare N-cyanomethyl-4-(trifluoromethyl)-nicotinamide. 4-trifluoromethylnicotinic acid and methanesulfonyl chloride are taken as the primary raw materials to synthesize acid anhydrides at first, acid anhydrides are resistant to hydrolysis, then acid anhydrides react with amino acetonitrile hydrochloride to prepare N-cyanomethyl-4-(trifluoromethyl)-nicotinamide; the conversion rate is high (not less than 96%); the purity is high, and the yield is good (not less than 90%). Toxic phosgene is notused, the safety is greatly improved, moreover, the operation is simple, and the synthesis method is suitable for industrial production.
Owner:JINGBO AGROCHEM TECH CO LTD
Completely-biodegraded nanometer starch grafted poly glutamic acid benzyl ester
InactiveCN102219908AReduce hydrophilicityLow melting pointPharmaceutical non-active ingredientsEnd-groupClick chemistry
The invention provides a preparation method for completely-biodegraded nanometer starch grafted poly glutamic acid benzyl ester. The nanometer starch grafted poly glutamic acid benzyl ester is prepared by taking nanometer starch as raw materials and adopting a click chemistry method. The preparation method comprises the steps of: first, utilizing reaction activity of hydroxyl in the nanometer starch structure, reacting with methanesulfonyl chloride and sodium azide sequentially, and introducing azide group in molecules of nanometer starch; reacting N-carboxylic acid anhydride (BLG-NCA) of glutamic acid benzyl ester with propargylamine monomer, and preparing poly glutamic acid benzyl ester with tribond function as end group; and secondly, utilizing azide function group in the nanometer starch molecule and tribond function group in poly glutamic acid benzyl ester molecule to generate azide-alkynyl Husigen addition reaction to prepare nanometer starch poly glutamic acid benzyl ester grafted copolymer. The copolymer is prepared by the click chemistry method and is characterized in that the reaction has efficiency and controllability, the grafted rate of the poly glutamic acid benzyl ester is clear and controllable, the copolymer has a clear structure, the hydrophilcity of starch and hydrophobicity of poly glutamic acid benzyl ester can be improved, the melting point of the nanometer starch is lowered, and the copolymer materials can be used as fillers, bulking agents and drug carriers.
Owner:NORTHEAST NORMAL UNIVERSITY
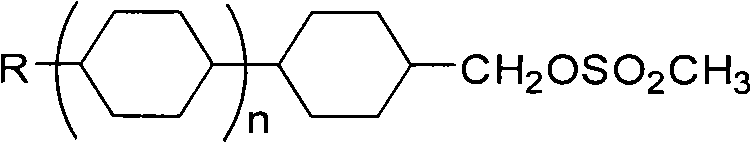
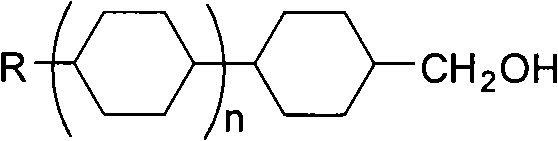
Intermediate for synthesizing butene liquid crystal and synthetic method thereof
ActiveCN101407482AThorough responseHigh puritySulfonic acid esters preparationMethanesulfonyl chlorideSolvent
The invention relates to an intermediate for synthesizing butene liquid crystal and a synthesizing method thereof. The intermediate for synthesizing butene liquid crystal has a general formula structure as the right, which is shown in a right formula (I) or alkyl of C1-C7 or alkoxy of C1-C7; when in a right formula (II), X is H or F, Z is H or F, Y=F or alkyl of C1-C7 or alkoxy of C1-C7 or -OCHF2 or -OCF3 or -CF3 or CHF2; and n=0 or 1. The synthesizing method lies in that corresponded raw material alcohol is reacted with methyl sulfonic acid chloride in solvent with the presence of catalyst. The synthesizing method has a fast reaction without other side products, the post treatment process is simple and handy and the obtained product has high yield coefficient and purity.
Owner:HEBEI MAIERSTON ELECTRONICS MATERIAL
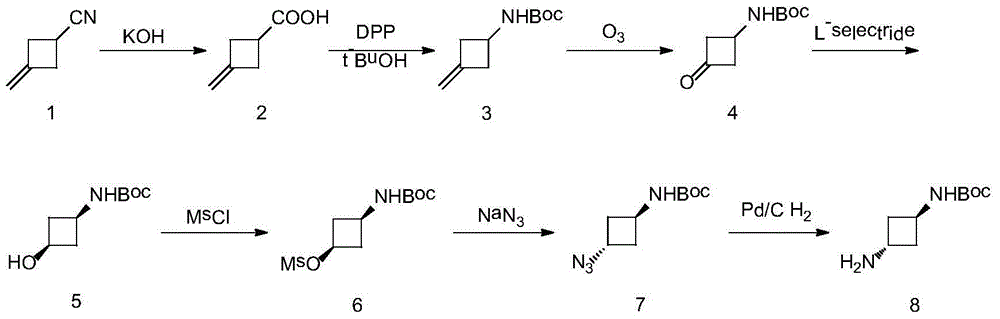
Preparation method of trans-N-Boc-1,3-cyclobutanediamine
InactiveCN104829492ASimple purification methodShort stepsCarbamic acid derivatives preparationOrganic compound preparationPurification methodsOxidative cleavage
The present invention is a preparation method of trans-N-Boc-1,3-cyclobutanediamine. The method uses easily available 3-methylene-cyclobutyl carbonitrile as a starting material, and conducts 7 steps of reaction including hydrolysis, Curtius rearrangement, oxicracking, reduction, methanesulfonyl chloride protection, substitution and hydrogenation. The invention has the technical characteristics of short reaction step, total yield of up to 49.8% and high purity of the final product (>99%) (the prior art comprises at least 8 steps, and the highest total yield is up to 19%); and N-Boc-1,3-cyclobutanediamine with transconfiguration is selectively synthesized. From the perspective of chemistry, the invention has the characteristics of few steps, simple reaction, high yield, feasible purification method of intermediate and simple operation, and has wide prospect in business application.
Owner:HEBEI UNIV OF TECH
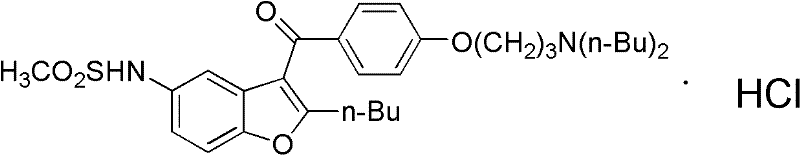
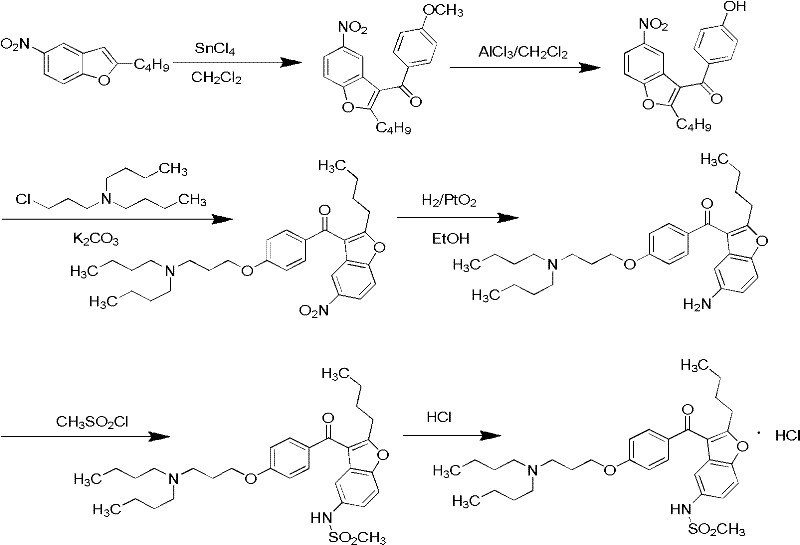
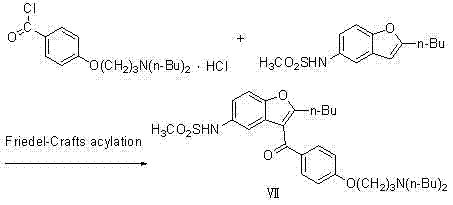
Method for preparing dronedarone hydrochloride
InactiveCN102382087ALess toxicLow process pollutionOrganic chemistrySulfonyl chlorideMethanesulfonyl chloride
The invention relates to a method for preparing dronedarone hydrochloride, comprising the following concrete steps of: (1) carrying out etherification reaction on 2-butyl-3-(4-hydroxy benzoyl)-5-nitro benzofuran and 1-bromine-3-chloropropane in an organic solvent so as to obtain 2-n-butyl-3-[4-(3-chlorine propoxy) benzoyl]-5-nitro benzofuran; (2) reducing to 2-n-butyl-3-[4-(3-chlorine propoxy) benzoyl]-5-amido benzofuran by utilizing a reductant; (3) carrying out sulfamide reaction with methyl sulfonyl chloride in the organic solvent so as to obtain 2-n-butyl-3-[4-(3-chlorine propoxy) benzoyl]-5-methyl sulfonamide benzofuran; (4) carrying out N-hydrocarbon reaction with di-n-butylamine so as to obtain the dronedarone; and (5) salifying the dronedarone hydrochloride and obtaining the dronedarone hydrochloride. The method provided by the invention has the advantages that the reaction route is simple, the raw material is cheap and easy to obtain and the reaction condition is mild and easy to control and is suitable for industrialized production.
Owner:NANJING UNIV OF TECH
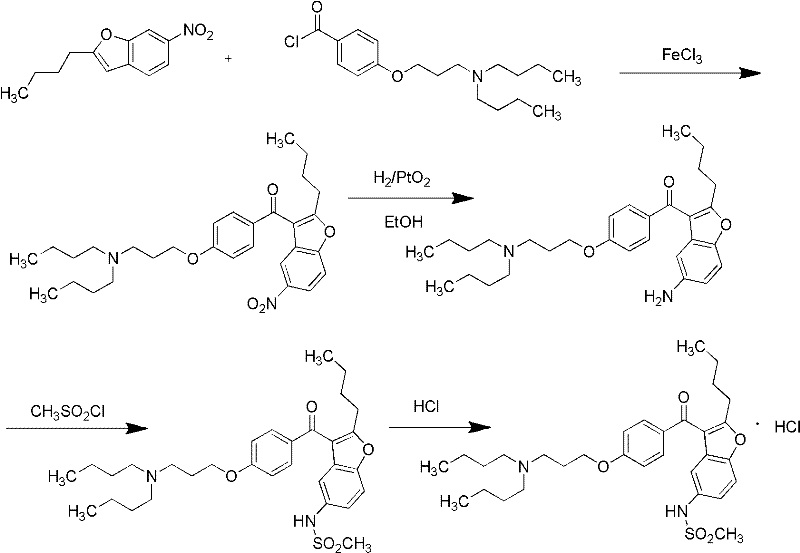
Preparation method for novel antiarrhythmic medicament of dronedarone
The invention discloses a preparation method for a novel antiarrhythmic medicament of dronedarone. The preparation method comprises the following steps of: (1) performing substitution reaction on a compound IV and dibutylamine in an organic solvent to obtain an intermediate compound V; (2) performing reduction reaction on the compound V in the organic solvent under the action of a catalyst to obtain an intermediate VI; and (3) performing sulfonylation reaction on the compound VI and methanesulfonyl chloride in the organic solvent to obtain the final product of dronedarone. The product prepared by the method has high purity, an operation method is simple, production cost is low, and the method has strong competitive power in markets, and is more suitable for industrialized production.
Owner:江苏万全特创医药生物技术有限公司
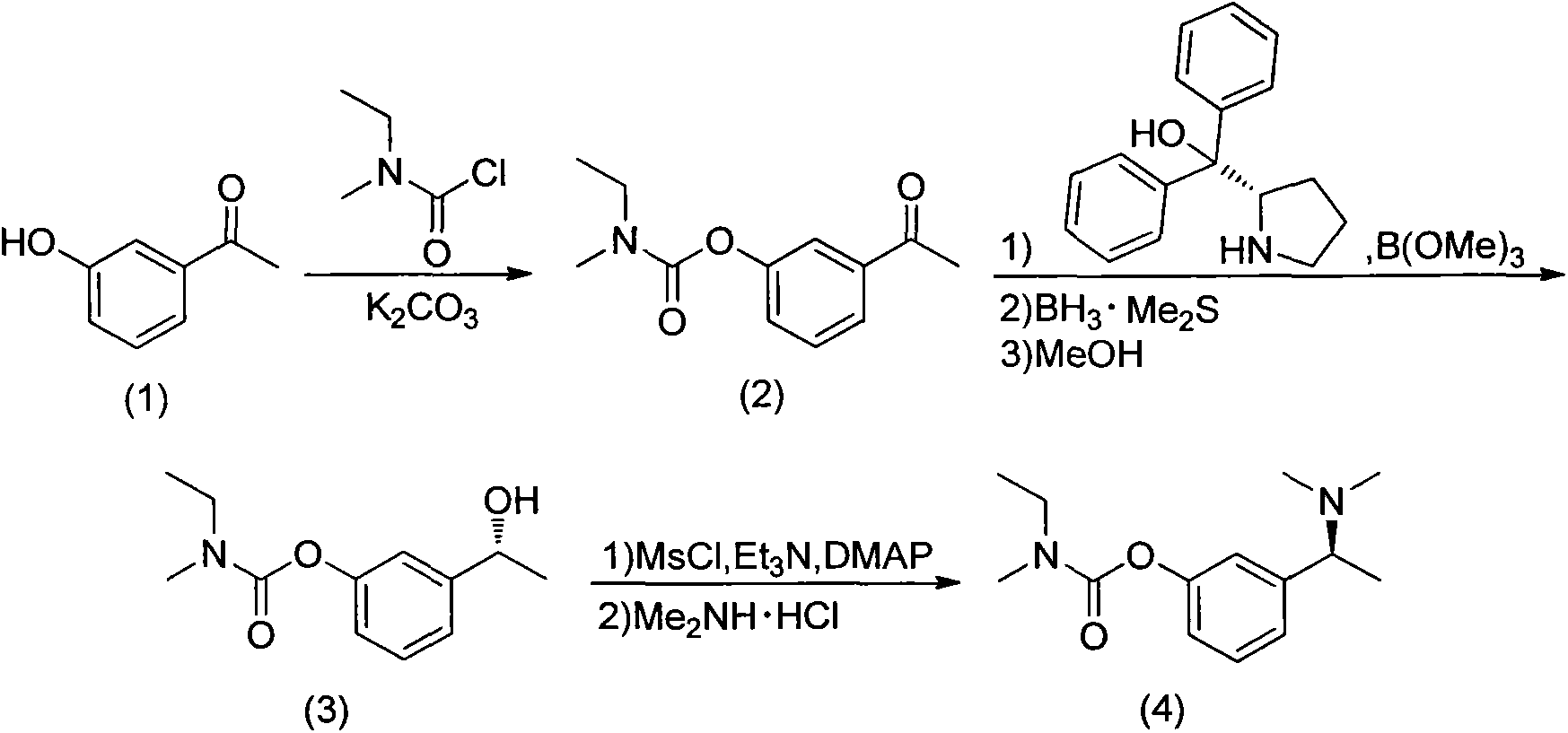
Synthesis process of (S)-rivastigmine
ActiveCN103304447AFew stepsHigh reaction yieldCarbamic acid derivatives preparationOrganic compound preparationMethyl carbamateFormate
The invention discloses an unsymmetrical synthesis method of (S)-rivastigmine. The method comprises the following steps of: reacting hydroxyacetophenone with methylethylcarbamoyl chloride to generate N-ethyl-N-methyl-carbamate 3-acetylphenyl formate, carrying out chiral reduction on the N-ethyl-N-methyl-carbamate 3-acetylphenyl formate under the catalysis effect of a complex of (S)-(-)-alpha, alpha-diphenyl prolinol and trimethyl borate, carrying out methanol dissociation to generate N-ethyl-N-methyl- carbamate 3-[(R)-1-hydroxyethyl]phenyl formate, and reacting the N-ethyl-N-methyl- carbamate 3-[(R)-1-hydroxyethyl]phenyl formate with methylsufonyl chloride and dimethylamine hydrochloride to obtain the (S)-rivastigmine. According to the method disclosed by the invention, a target product can be prepared from conventional reagents only through a three-step reaction, the reaction yield reaches 40% and is far higher than that by a racemic resolution method and that by other chiral synthesis methods, the application of virulent reagents and highly corrosive reagents are avoided in the process, and the unsymmetrical synthesis method is easy to operate, environment-friendly, low in production cost, and suitable for industrialized production.
Owner:YABAO PHARMA GRP CO LTD
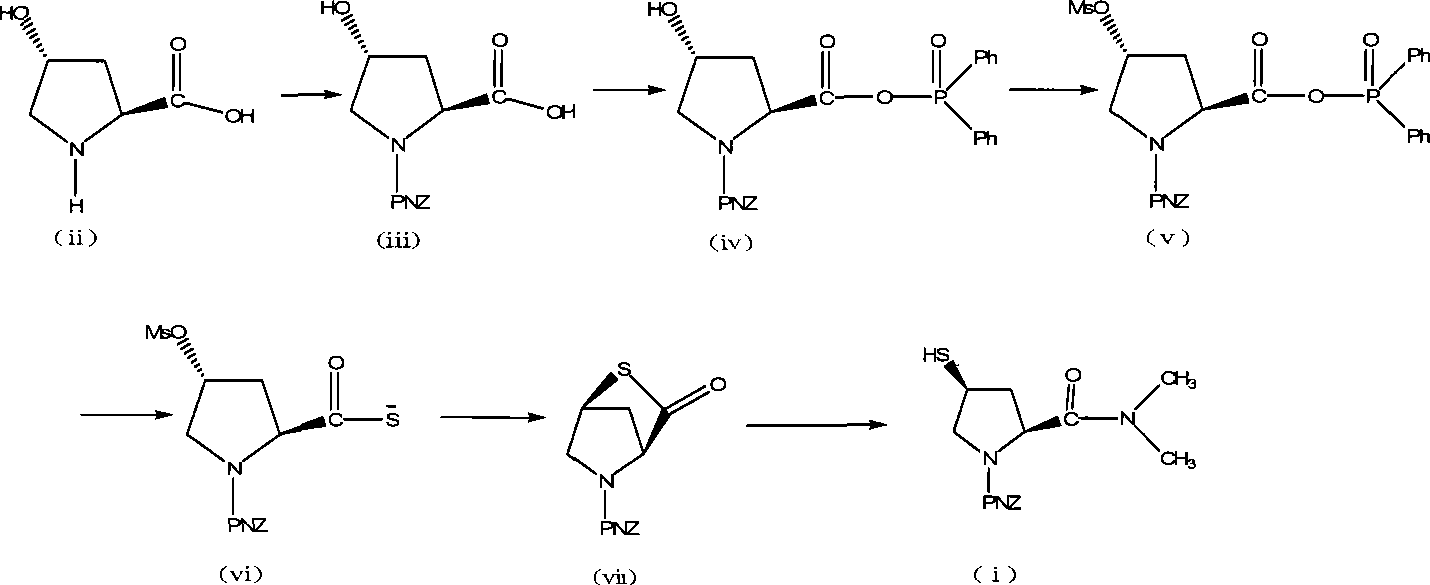
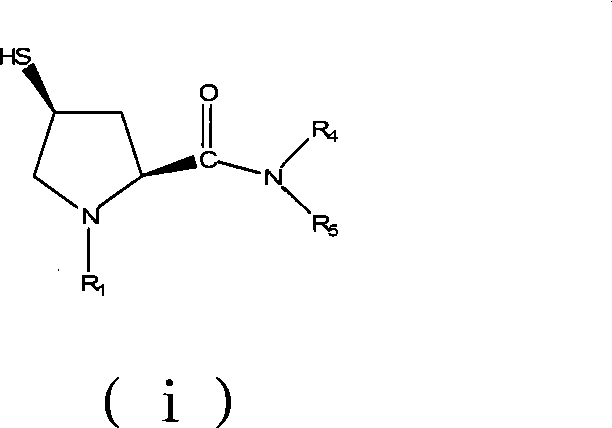
Preparation of carbapenem antibiotic side chain
ActiveCN101481346AEasy to purifyEasy to operateOrganic chemistrySide chainSODIUM SULFIDE NONAHYDRATE
The invention discloses a method for preparing side chains of carbapenems antibiotics, comprising the following steps: A) L-hydroxyproline (ii) reacts with 4-nitrobenzyl chloroformate and then is separated and crystallized to obtain a compound (iii); B) the compound (iii) reacts with diphenylphosphinyl chloride to obtain a compound (iv); the compound (iv) reacts with methanesulfonyl chloride to obtain a compound (v); the compound (v) reacts with sodium sulfide nonahydrate and is heated to 15-35 DEG C to react for 0.5-6h to obtain a compound (vii); C) the compound (vii) reacts with dimethyl amine hydrochloride and then is separated and crystallized to obtain a compound (i). The method simplifies the operation, ensures easy industrial production, reduces the production cost and leads the products to be easily purified; therefore, the obtained products have high purity and yield.
Owner:ZHEJIANG HISOAR CHUANNAN PHARMA +1
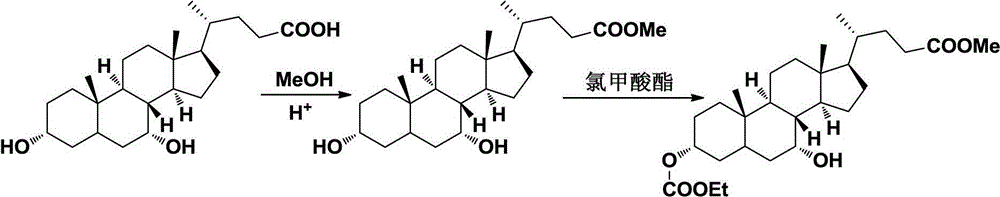
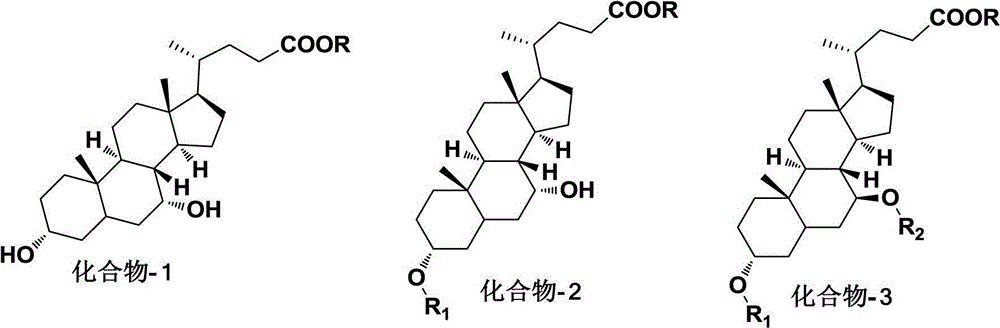
Novel synthesizing method for preparing ursodesoxycholic acid (UDCA) from chenodeoxycholic acid
ActiveCN102746359AShort production lineHigh yieldSteroidsBulk chemical productionChenodeoxycholic acidP-nitrobenzenesulfonyl chloride
The invention relates to a novel method for preparing UDCA from chenodeoxycholic acid. The method includes taking the chenodeoxycholic acid as a raw material, performing protection on carboxyl and selective protection on 3-carboxyl, then subjecting carboxyl at position 7 to a reaction through Mitsunobu or producing ester from paratoluensulfonyl chloride, methanesulfonyl chloride and paranitrobenzenesulfonyl chloride, and stripping off a protecting group to prepare the UDCA. Compared with other processes, the method has the advantages of being mild in reaction condition, simple in operation, high in efficiency, yield and optical purity and the like, and provided with a bright industrial production prospect.
Owner:HEFEI HUAFANG PHARMA SCI & TECH
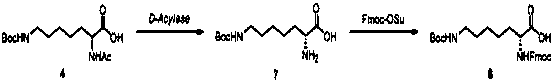
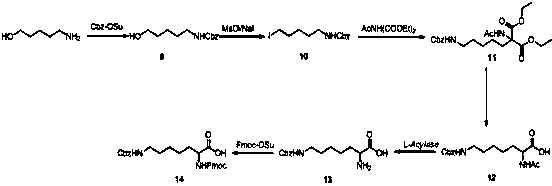
Synthesis method of double different protected amino acids
InactiveCN109824547AImprove general performanceLow priceCarbamic acid derivatives preparationOrganic compound preparationSynthesis methodsMethanesulfonyl chloride
The invention relates to a synthesis method of double different protected amino acids.The technical problems of harsh reaction conditions, inapplicability of production enlarging and the like in an existing synthesis method are mainly solved. According to the technical scheme, the synthesis method of double different protected amino acids comprises the following steps: one of Boc20, Alloc-Cl or Cbz-Osuis added to amino alcohol under the action of an alkaline reagent to obtain a compound 1; the compound 1 reacts with methanesulfonyl chloride or paratoluensulfonyl chloride to obtain an intermediate, then a halide is added into acetone, heating and refluxing are executed to obtain a compound 2; the compound 2 is condensed with diethyl acetamidomalonate under the action of an alkaline agent togenerate a compound 3; the compound 3 is dissolved in alcohol and water, an inorganic base is added, heating, hydrolyzing and decarboxylating are executed to obtain a compound 4; acetylase is added into deionized water to obtain a compound 5 through enzymolysis; amino acid protection is executed, wherein one of Fmoc-Osu, Cbz-OSu, Alloc-Cl or Boc20 is added into thecompound 5 under the action of an alkaline agent to generatea target compound A.
Owner:KANGHUA SHANGHAI DRUG RES DEV CO LTD
Method for synthesizing 1,2,6,7-tetrahydro-8H-indeno[5,4-b]furan-8-ketone
The invention provides a method for synthesizing 1,2,6,7-tetrahydro-8H-indeno[5,4-b]furan-8-ketone. The method comprises the following steps: a, performing the Claisen rearrangement reaction of a compound III in an appropriate solvent to generate a compound IV; b. supplying ozone to the compound IV at a low temperature to perform reaction, adding sodium borohydride or potassium borohydride to perform reduction reaction and obtaining a compound V; c, allowing the compound V to react with methanesulfonyl chloride or p-toluenesulfonyl chloride in pyridine so as to obtain a compound VI and a compound VII; and d, dissolving the compound VI and / or the compound VII in an appropriate reaction solvent, adding appropriate organic base, raising temperature to perform reaction, cooling the obtained product, adding diluted hydrochloric acid for washing, evaporating the solvent and obtaining a compound I, namely the 1,2,6,7-tetrahydro-8H-indeno[5,4-b]furan-8-ketone. The method has the advantages ofbrief synthesis route, simple operation, low cost, high yield and convenience for industrial production.
Owner:SHANGHAI PUYI CHEM CO LTD
Synthesis method of biochanin A
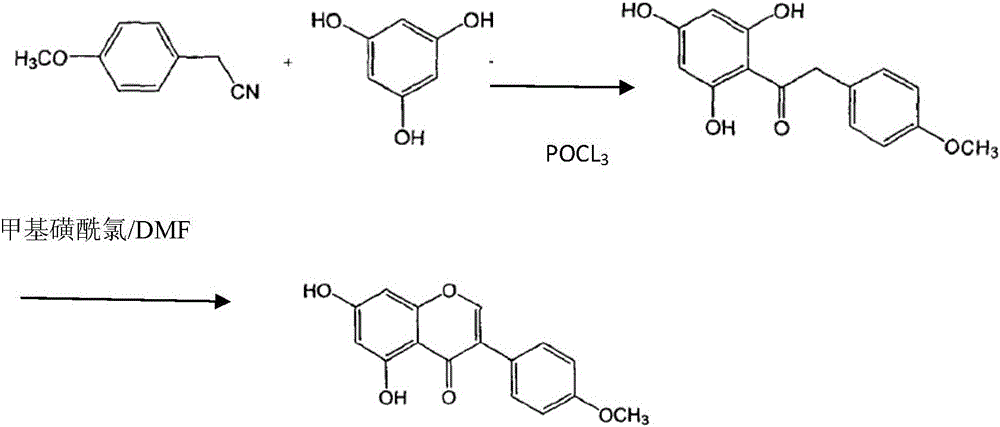
InactiveCN106220602ASimple processHigh yieldOrganic chemistrySynthesis methodsMethanesulfonyl chloride
The invention provides a synthesis method of biochanin A. The synthesis method includes: under room temperature, adding phosphorus oxychloride into phloroglucinol, 4-Methoxybenzyl cyanide and isopropyl ether, performing hydrolysis after reaction is completed, and decoloring with activated carbon to obtain an intermediate; allowing the obtained intermediate and methylsufonyl chloride to generate crude products of the biochanin A in DMF (dimethyl formamide) in a ring-closing manner; performing recrystallization on the obtained crude products of the biochanin A by adopting ethyl alcohol and decoloring with the activated carbon to obtain the biochanin A. The synthesis method is simple in process, low in cost, timesaving, high in yield, low in equipment corrosiveness and suitable for industrialized production.
Owner:SHAANXI JIAHE PHYTOCHEM
Method for preparing 2-azabicyclo[2.2.1]heptyl-5-ene-3-one
ActiveCN101417951ARich sourcesReduce manufacturing costOrganic compound preparationAmino compound preparationSodium bicarbonateSulfite salt
The invention discloses a preparation method of 2-azabicyclo(2.2.1)hept-5-alkene-3-ketone, which is an intermediate for the synthesis of antiviral medicine carbonyl ring nucleoside, which is characterized in that, an aqueous solution prepared by anhydrous sodium sulfite and sodium bicarbonate is added to a reactor; the temperature is controlled between 0 to 60 DEG C; a fixed amount of methanesulfonyl chloride is added by dropping, and the temperature is maintained to react for 2 to 4 hours to prepare methyl sulfonate solution; a fixed amount of ether solvent is added to the solution, and under a temperature ranging from 10 to 60 DEG C, cyanogens chloride is added; after 4 to 6 hours reaction, cyclopentadienyl is added by dropping; the pH value of the reaction solution is controlled between 1.5 to 3 to react for 3 to 5 hours; then sodium hydroxide is used for regulating the pH value to 7 to 9; after standing, the ether solvent is separated, and a fixed amount of methyl chloride is used for separated extraction 2-azabicyclo(2.2.1)hept-5-alkene-3-ketone; the methyl chloride is distilled and a crude product is obtained; the crude product is decolorized by activated carbon and recrystallized in ether solvent; 2-azabicyclo(2.2.1)hept-5-alkene-3-ketone with a purity of more than 99.6 percent is obtained after drying. The preparation method has the advantages of rich raw material sources, low cost, simple technological process, small emission of three wastes, benefits for industrial production and other advantages.
Owner:JILIN PURUITE BIOTECH CO LTD
Method for preparing antibacterial cefdinir
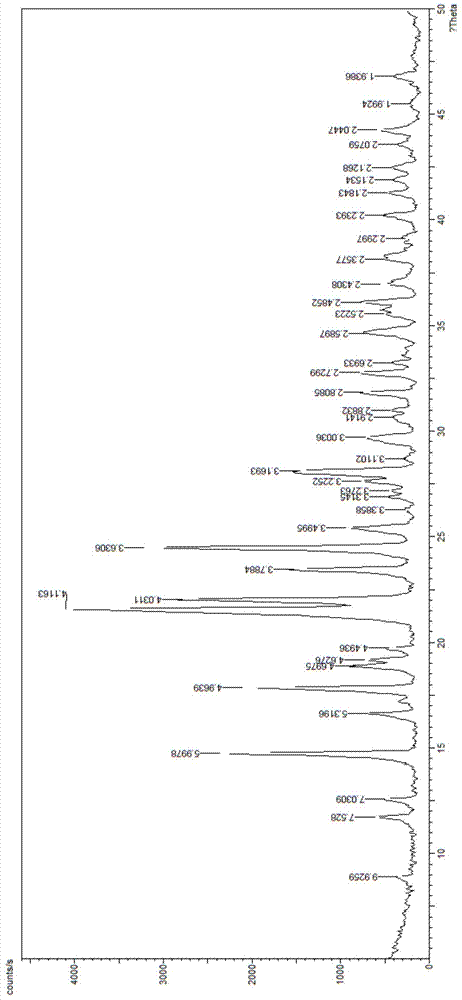
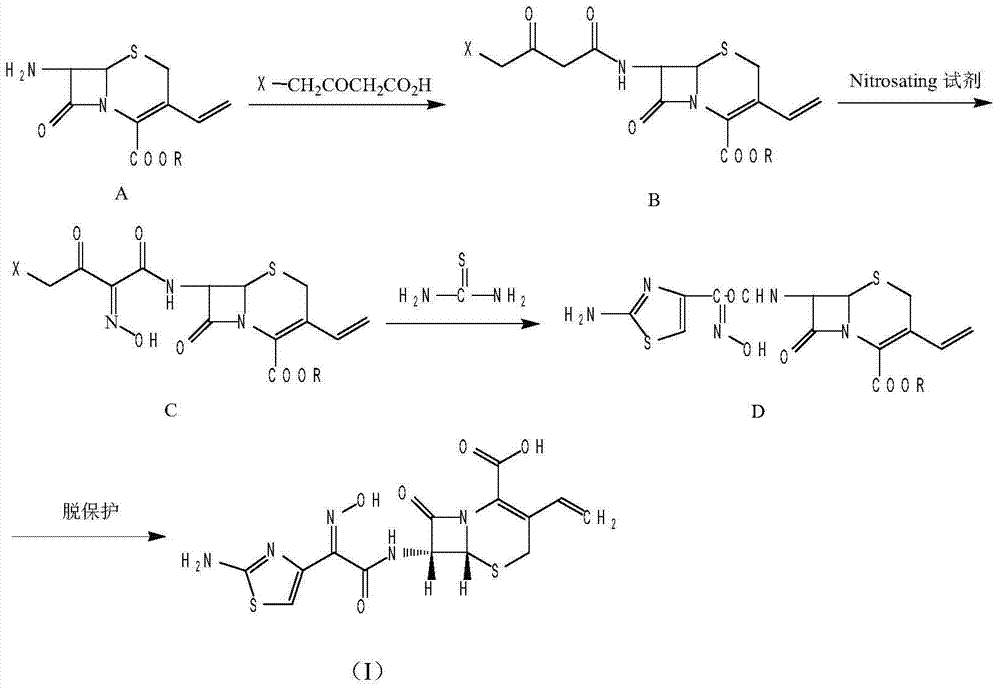
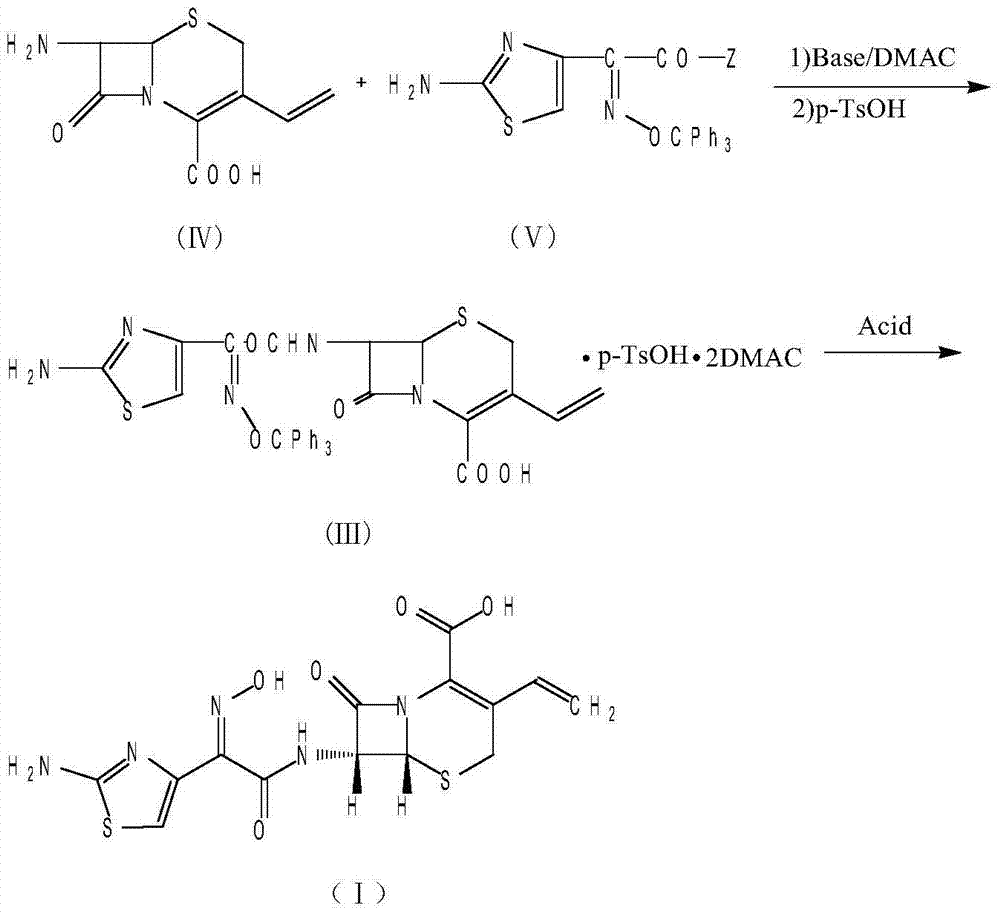
The invention provides a method for synthesizing antibacterial cefdinir. 2-(2-aminothiazole-4-yl)-2-hydroxyiMinoacetic acid and methylsufonyl chloride form anhydride; and anhydride reacts with 7-amino-3-vinyl-8-oxo-5-thia-azabicyclo [4.2.0]oct-2-ene-2-carboxylate, and cefdinir is obtained through hydrolysis. The method is simple in processing, high in yield and applicable to industrial production.
Owner:CHENGDU YILUKANG MEDICAL TECH & SERVICE
Synthesis method of S-metolachlor
InactiveCN102010346AOrganic compound preparationCarboxylic acid amides preparationSynthesis methodsMethyl lactate
The invention discloses a synthesis method of S-metolachlor, comprising the following steps: 1) reacting (R)-methyl lactate or (R)-ethyl lactate with methanesulfonyl chloride to generate (R)-2-(mesyloxy)propionate; 2) reacting the obtained (R)-2-(mesyloxy)propionate with 2-methyl-6-ethylphenylamine to obtain (S)-N-(2-methyl-6-ethyl phenyl) phenylalanine ester; 3) reducing an ester group into corresponding alcohol by a reducing agent; 4) obtaining acyamino alcohol by performing acylation on the alcohol and chloroacetic chloride; and 5) obtaining the S-metolachlor through methylation reaction. The S-metolachlor produced by the synthesis method is low in production cost and good in optical purity, the ee value reaches 90-93%, and the yield is up to 86-89%. The synthesis method is simple to operate and easy to realize industrialization.
Owner:江苏常隆化工有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com
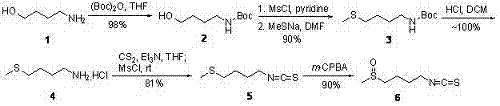
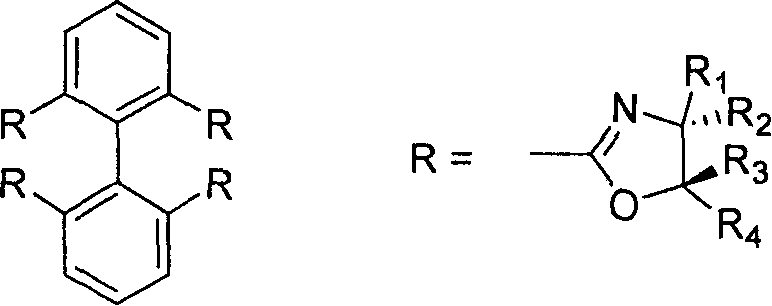
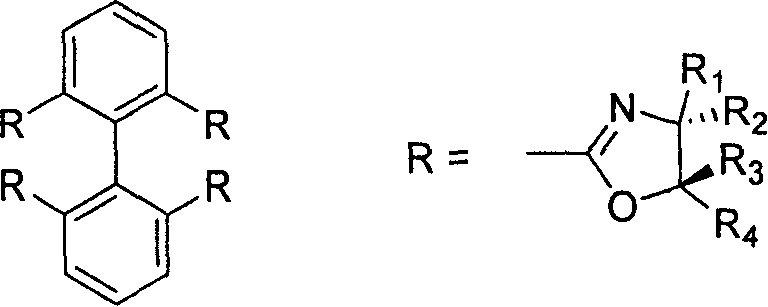

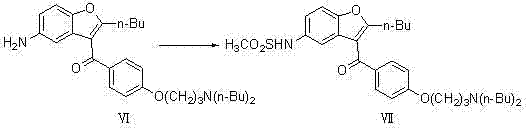
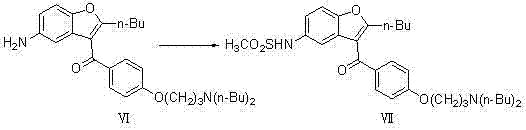
![Method for synthesizing 1,2,6,7-tetrahydro-8H-indeno[5,4-b]furan-8-ketone Method for synthesizing 1,2,6,7-tetrahydro-8H-indeno[5,4-b]furan-8-ketone](https://images-eureka.patsnap.com/patent_img/c0d08b2d-7c0b-48ef-8cfe-d6557b7ab8e7/A2008100427290002C1.PNG)
![Method for synthesizing 1,2,6,7-tetrahydro-8H-indeno[5,4-b]furan-8-ketone Method for synthesizing 1,2,6,7-tetrahydro-8H-indeno[5,4-b]furan-8-ketone](https://images-eureka.patsnap.com/patent_img/c0d08b2d-7c0b-48ef-8cfe-d6557b7ab8e7/A20081004272900041.PNG)
![Method for synthesizing 1,2,6,7-tetrahydro-8H-indeno[5,4-b]furan-8-ketone Method for synthesizing 1,2,6,7-tetrahydro-8H-indeno[5,4-b]furan-8-ketone](https://images-eureka.patsnap.com/patent_img/c0d08b2d-7c0b-48ef-8cfe-d6557b7ab8e7/A20081004272900051.PNG)
![Method for preparing 2-azabicyclo[2.2.1]heptyl-5-ene-3-one Method for preparing 2-azabicyclo[2.2.1]heptyl-5-ene-3-one](https://images-eureka.patsnap.com/patent_img/112b2583-6a73-484f-a31c-39a0c3939d47/a200710056212c00021.PNG)
![Method for preparing 2-azabicyclo[2.2.1]heptyl-5-ene-3-one Method for preparing 2-azabicyclo[2.2.1]heptyl-5-ene-3-one](https://images-eureka.patsnap.com/patent_img/112b2583-6a73-484f-a31c-39a0c3939d47/a200710056212c00031.PNG)
![Method for preparing 2-azabicyclo[2.2.1]heptyl-5-ene-3-one Method for preparing 2-azabicyclo[2.2.1]heptyl-5-ene-3-one](https://images-eureka.patsnap.com/patent_img/112b2583-6a73-484f-a31c-39a0c3939d47/a200710056212d00061.PNG)