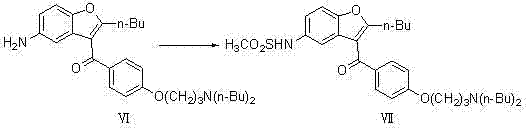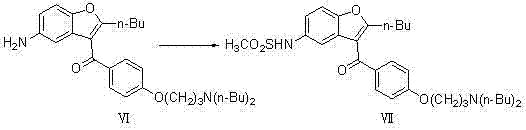Preparation method for novel antiarrhythmic medicament of dronedarone
An anti-arrhythmic and dronedarone technology, applied in the direction of organic chemistry and the like, can solve the problems of high preparation cost and no effective solution, and achieve the effects of low cost, good therapeutic effect and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] (1) Preparation of 2-butyl-3-{4-[3-(dibutylamino)propoxy]benzoyl-5-nitro}benzofuran (compound Ⅴ)
[0032]
[0033] In a 3000ml three-neck flask, add 1500ml toluene, 475g (1.0mol) 2-butyl-3-{4-[3-(methylsulfonyl)propoxy]benzoyl-5-nitro}benzofuran (Compound IV) was added to the reaction system, 5g (6.0mol) of di-n-butylamine was added, and reacted at 72°C for 30h; the solvent was evaporated to dryness, and the theoretical yield was 508g, which was directly used in the next reaction;
[0034] (2) Preparation of 2-butyl-3-{4-[3-(dibutylamino)propoxy]benzoyl-5-nitro}benzofuran (compound VI)
[0035]
[0036] In a 20 L autoclave, add 4800 ml of ethanol, 2.5 g (2.3 mmol) of 10% Pd / C, and react at 1.0 Mpa, and the concentrated solution of the previous step 2-butyl-3-{4-[3-( Dibutylamino)propoxy]benzoyl-5-nitro}benzofuran (compound V) was added to the reaction system, and reacted at 78°C for 8h; filtered with suction, evaporated part of the solvent under reduced pressure,...
Embodiment 2
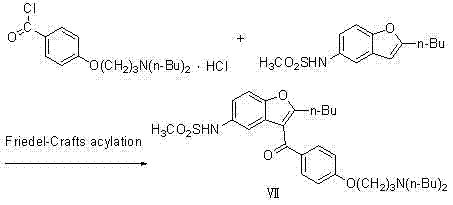
[0040] (1) Preparation of 2-butyl-3-{4-[3-(dibutylamino)propoxy]benzoyl-5-nitro}benzofuran (compound V)
[0041]
[0042] In a 3000ml three-neck flask, add 1500ml tetrahydrofuran, 475 g (1.0mol) 2-butyl-3-{4-[3-(methylsulfonyl)propoxy]benzoyl-5-nitro}benzo Add furan (compound IV) to the reaction system, add 4g (1.5mol) of di-n-butylamine, and react under reflux at 65°C for 45 hours; evaporate the solvent to dryness, and the theoretical yield is 508g, which is directly used in the next reaction;
[0043] (2) Preparation of 2-butyl-3-{4-[3-(dibutylamino)propoxy]benzoyl-5-nitro}benzofuran (compound VI)
[0044]
[0045] In a 3000ml three-necked flask, add 2400ml of methanol, 5.0g (4.6mmol) of 10% Pd / C, and react at 0.1Mpa, and the concentrated solution of the previous step 2-butyl-3-{4-[3-(di Butylamino)propoxy]benzoyl-5-nitro}benzofuran (Compound Ⅴ) was added to the reaction system, and reacted at 65°C for 20h; Gas, after obtaining solid filtration, desalination, toluene...
Embodiment 3
[0050] (1) Preparation of 2-butyl-3-{4-[3-(dibutylamino)propoxy]benzoyl-5-nitro}benzofuran (compound V)
[0051]
[0052] In a 10000ml three-necked flask, add 4750ml of dichloromethane, 475 g (1.0mol) of 2-butyl-3-{4-[3-(methylsulfonyl)propoxy]benzoyl-5-nitro} Benzofuran (compound IV) was added to the reaction system, 1030g (8.0mol) of di-n-butylamine was reacted at 39°C for 80h; the solvent was evaporated to dryness, and the theoretical yield was 508g, which was directly used in the next reaction;
[0053] (2) Preparation of 2-butyl-3-{4-[3-(dibutylamino)propoxy]benzoyl-5-nitro}benzofuran (compound VI)
[0054]
[0055] In a 20L autoclave, add 3500 ml of dichloroethane, 1 g (0.92 mmol) of 10% Pd / C, and react at 2.0 Mpa, and the concentrated solution of the previous step 2-butyl-3-{4-[3 -(dibutylamino)propoxy]benzoyl-5-nitro}benzofuran (compound V) was added to the reaction system, and reacted at 85°C for 8h; suction filtered, and part of the solvent was evaporated unde...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com