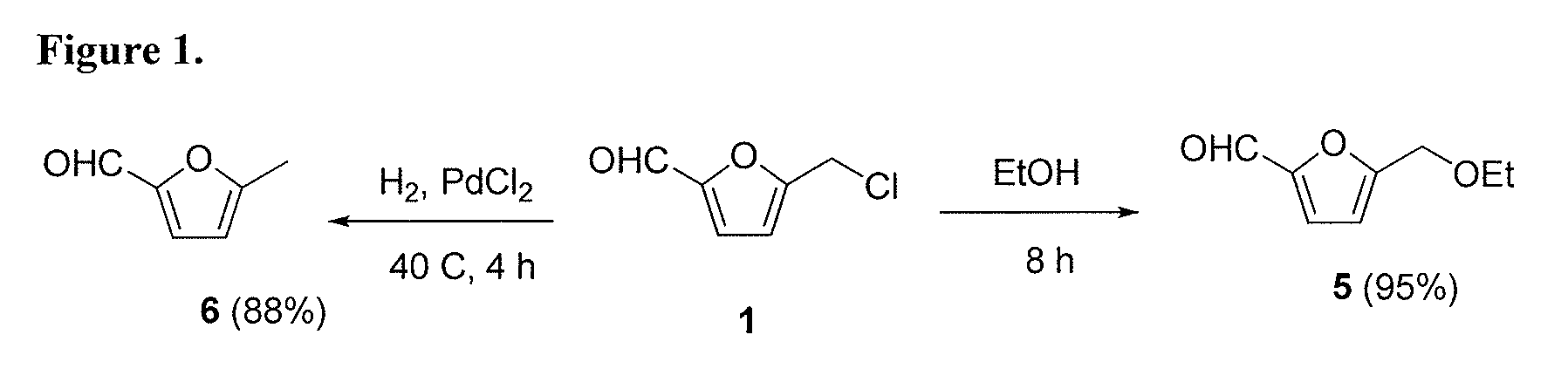
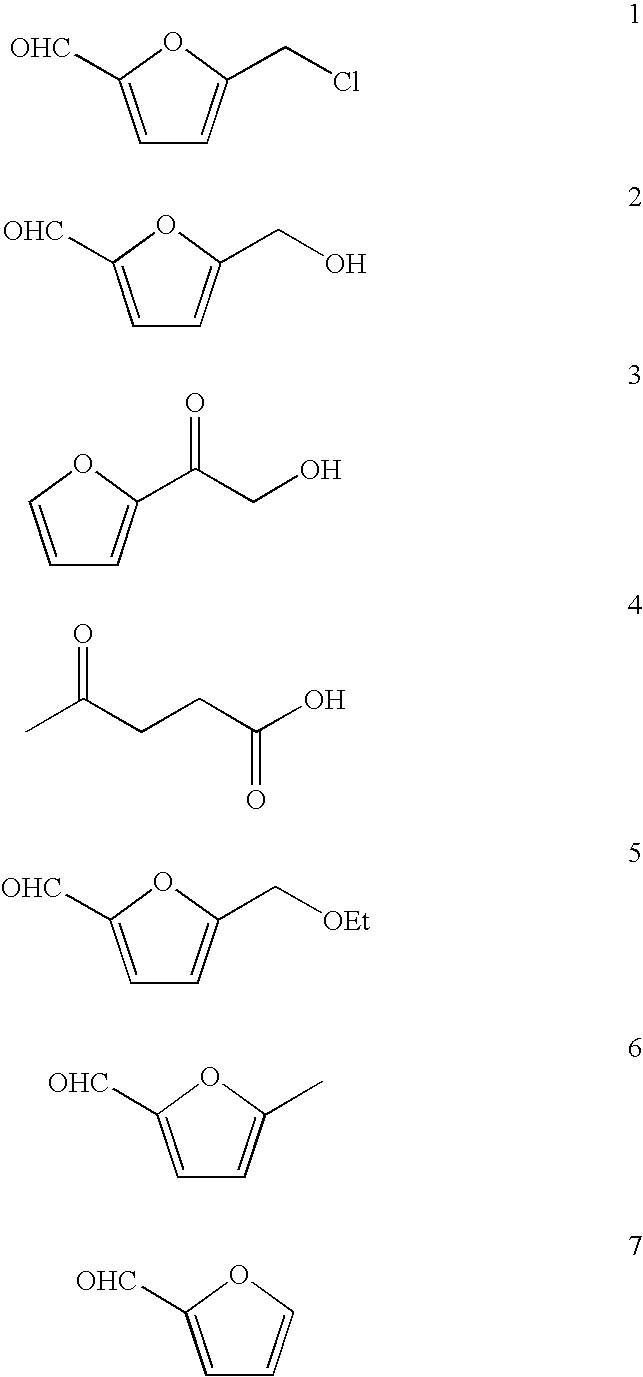
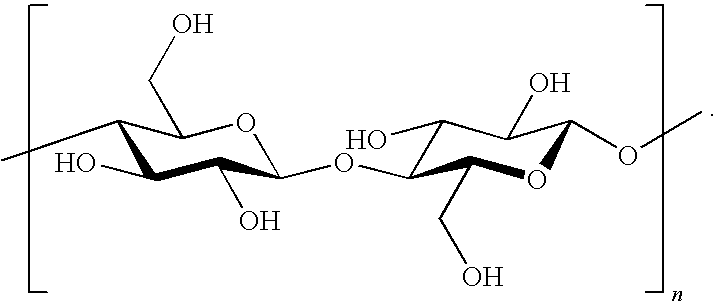
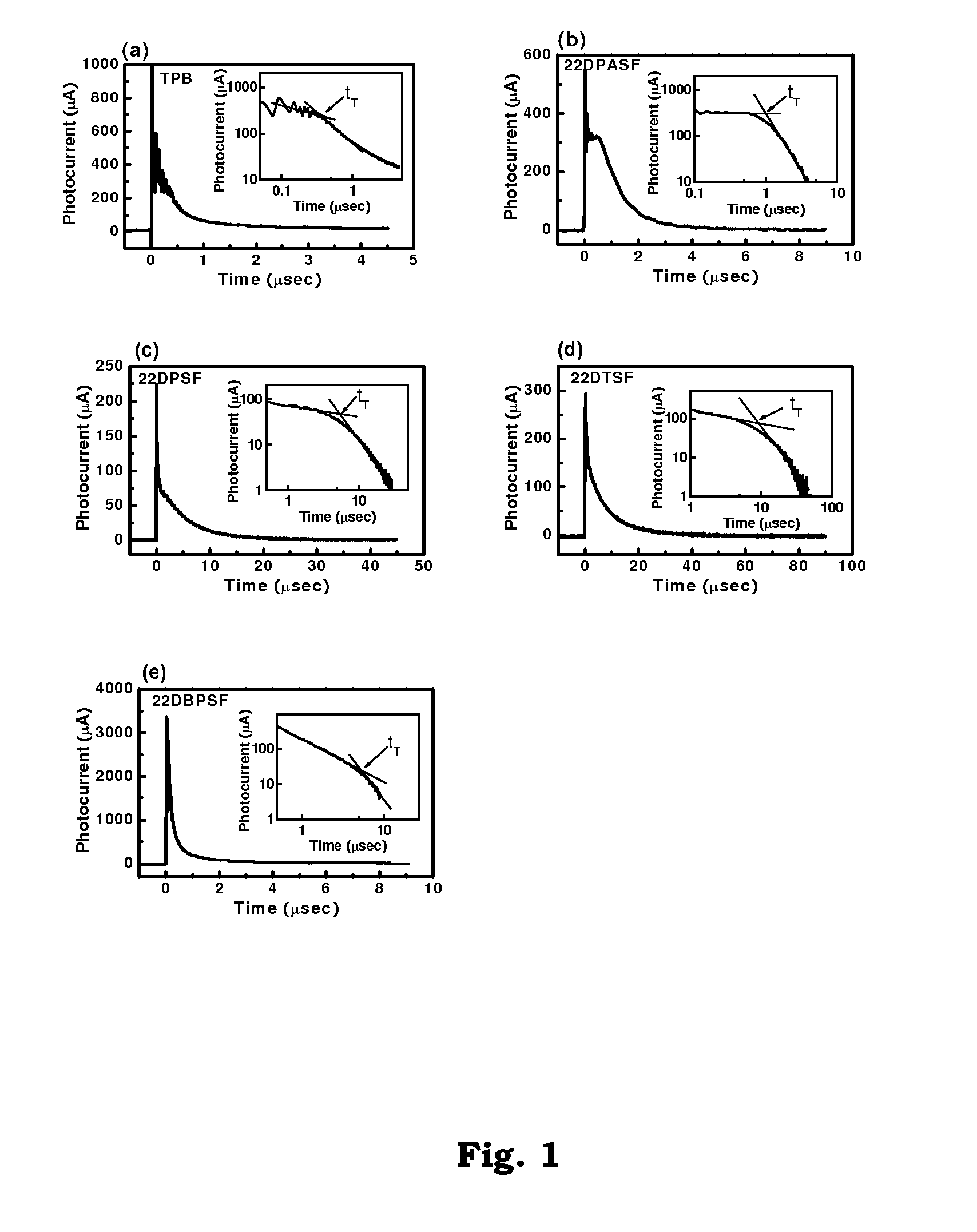
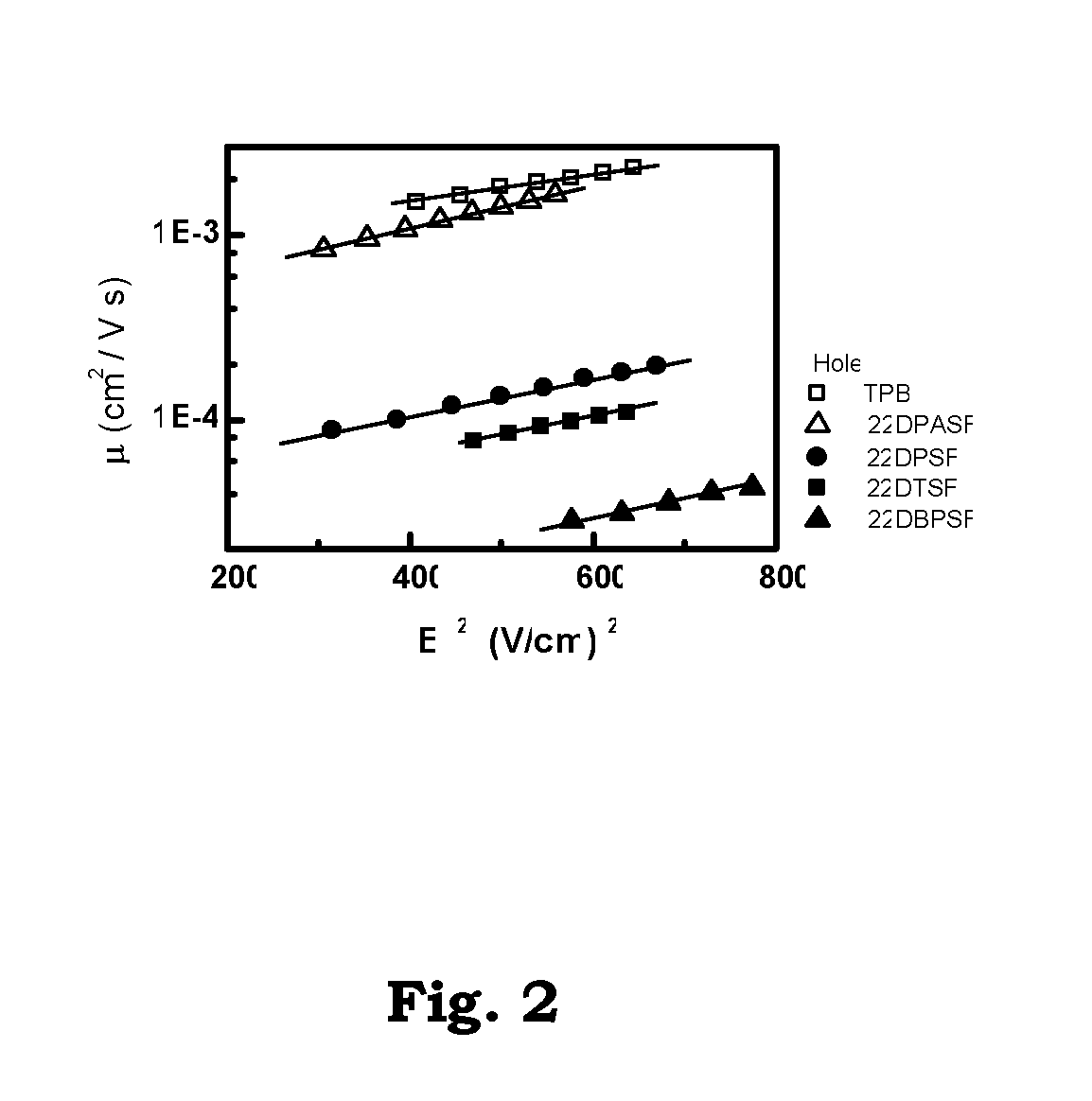
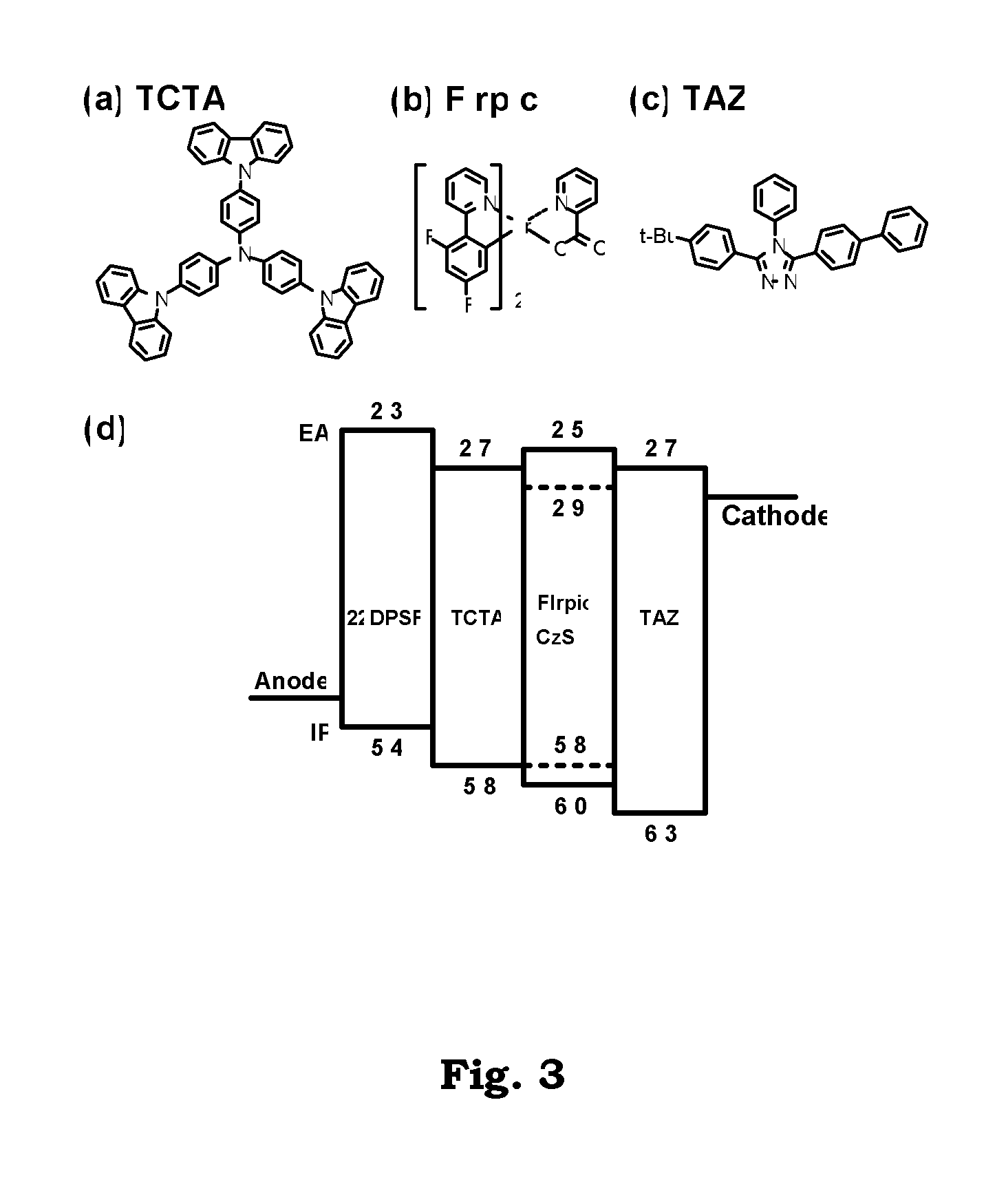
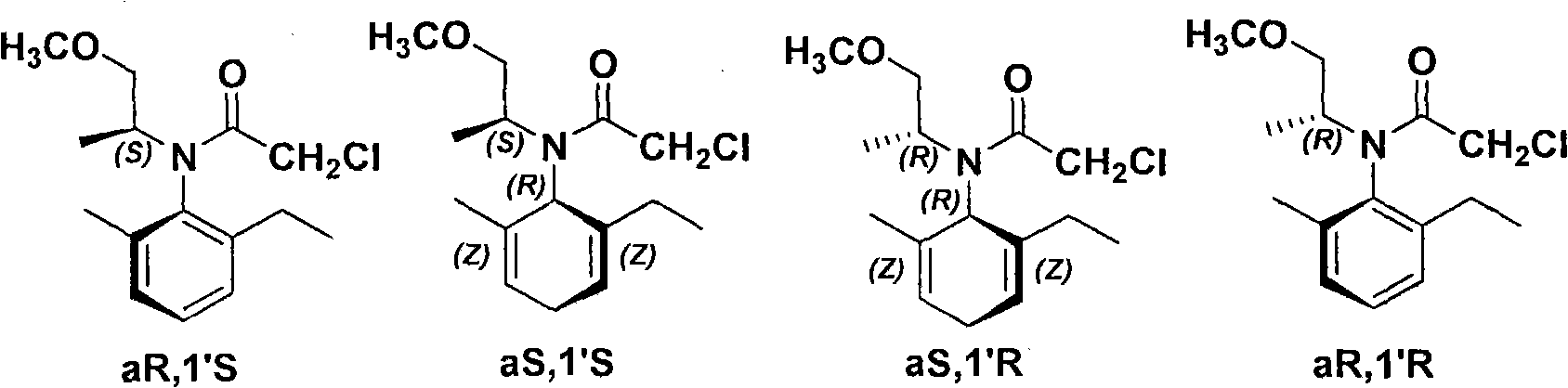
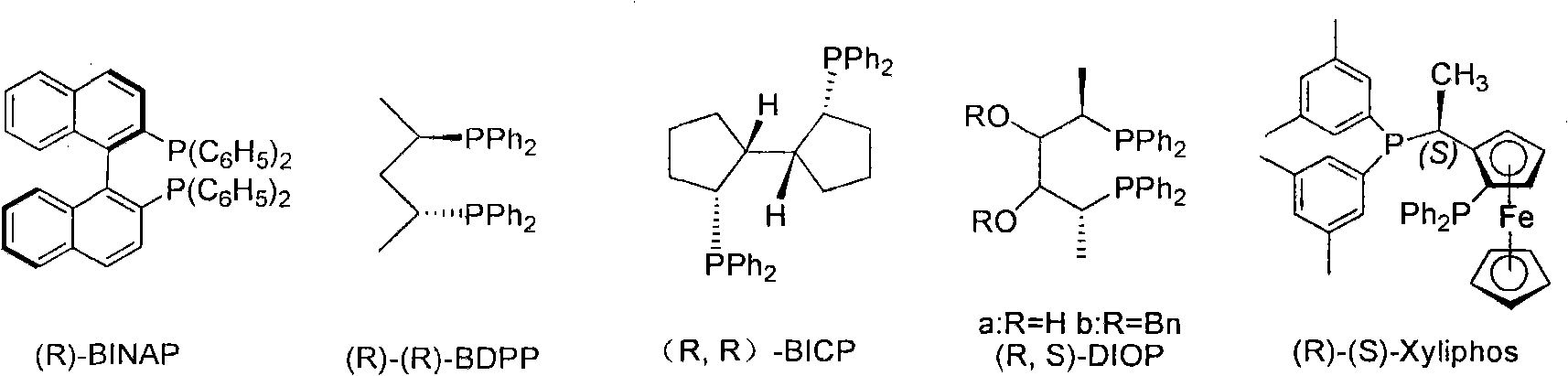
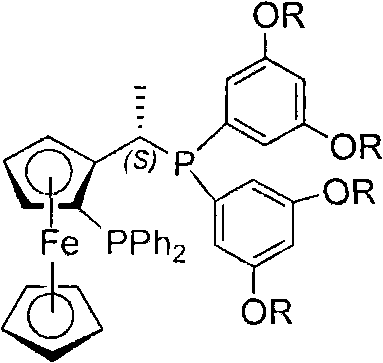
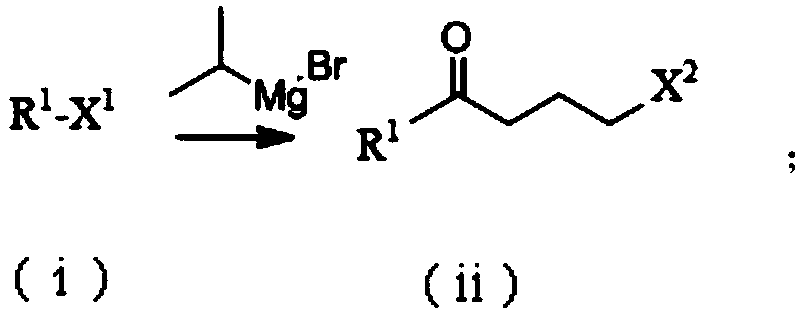
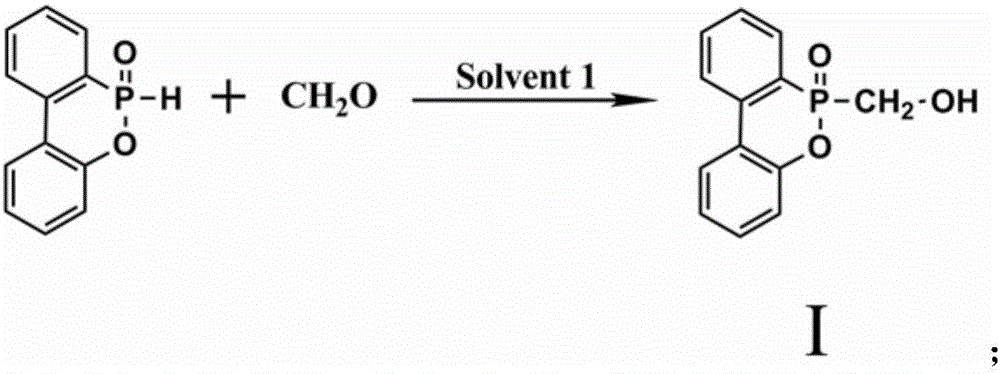
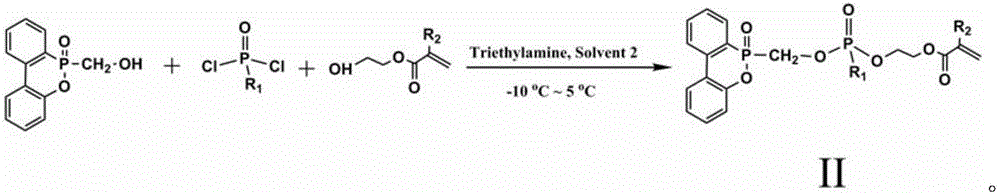
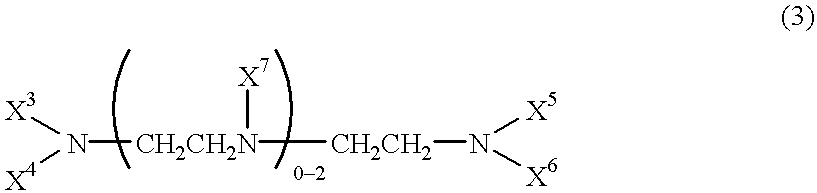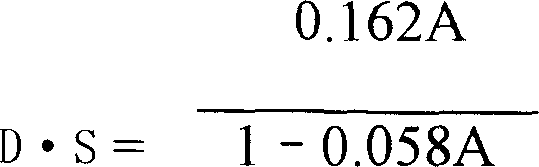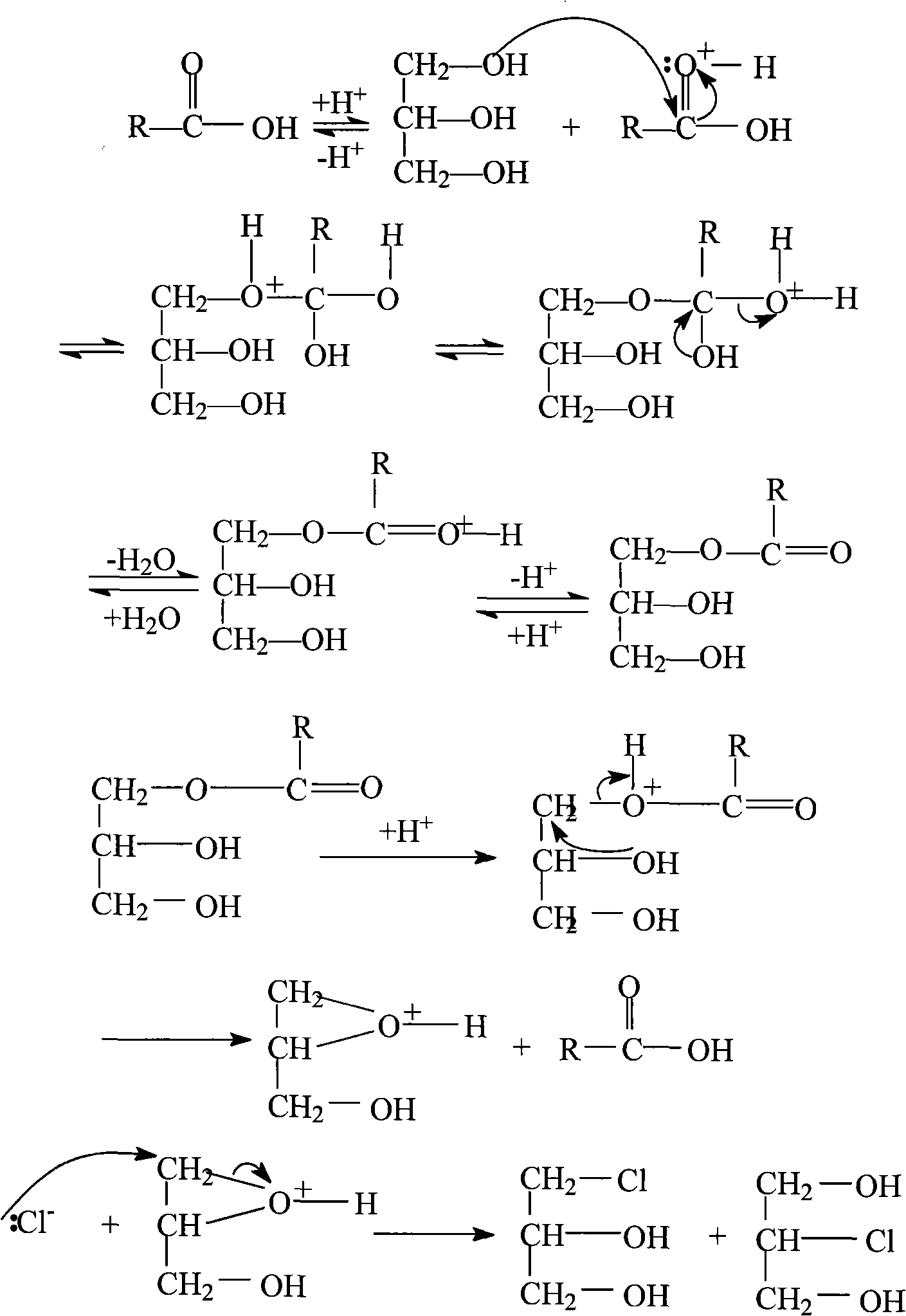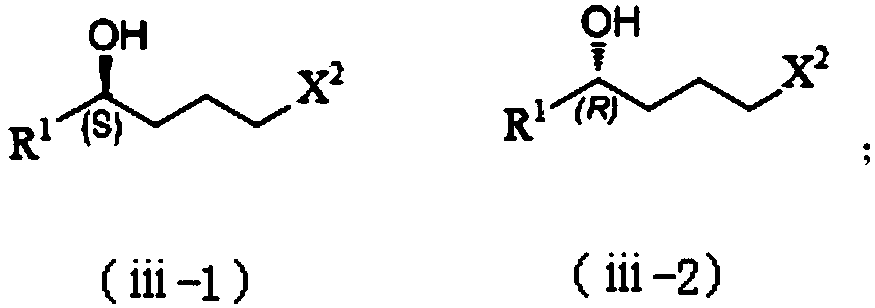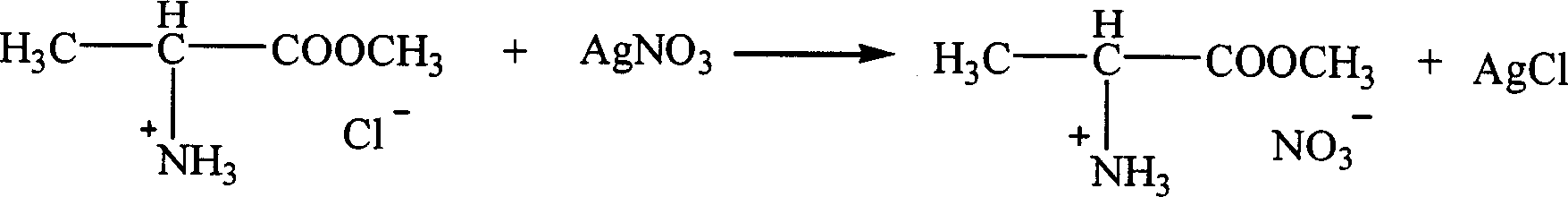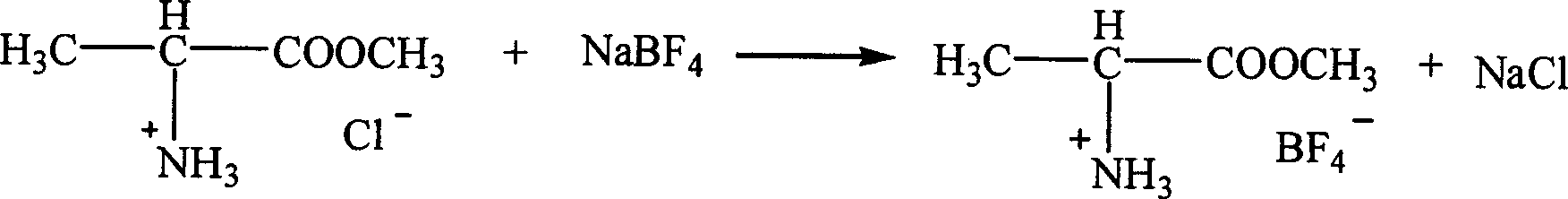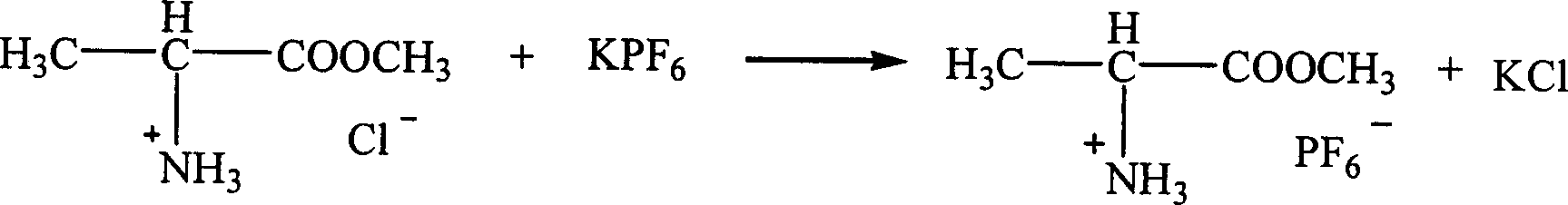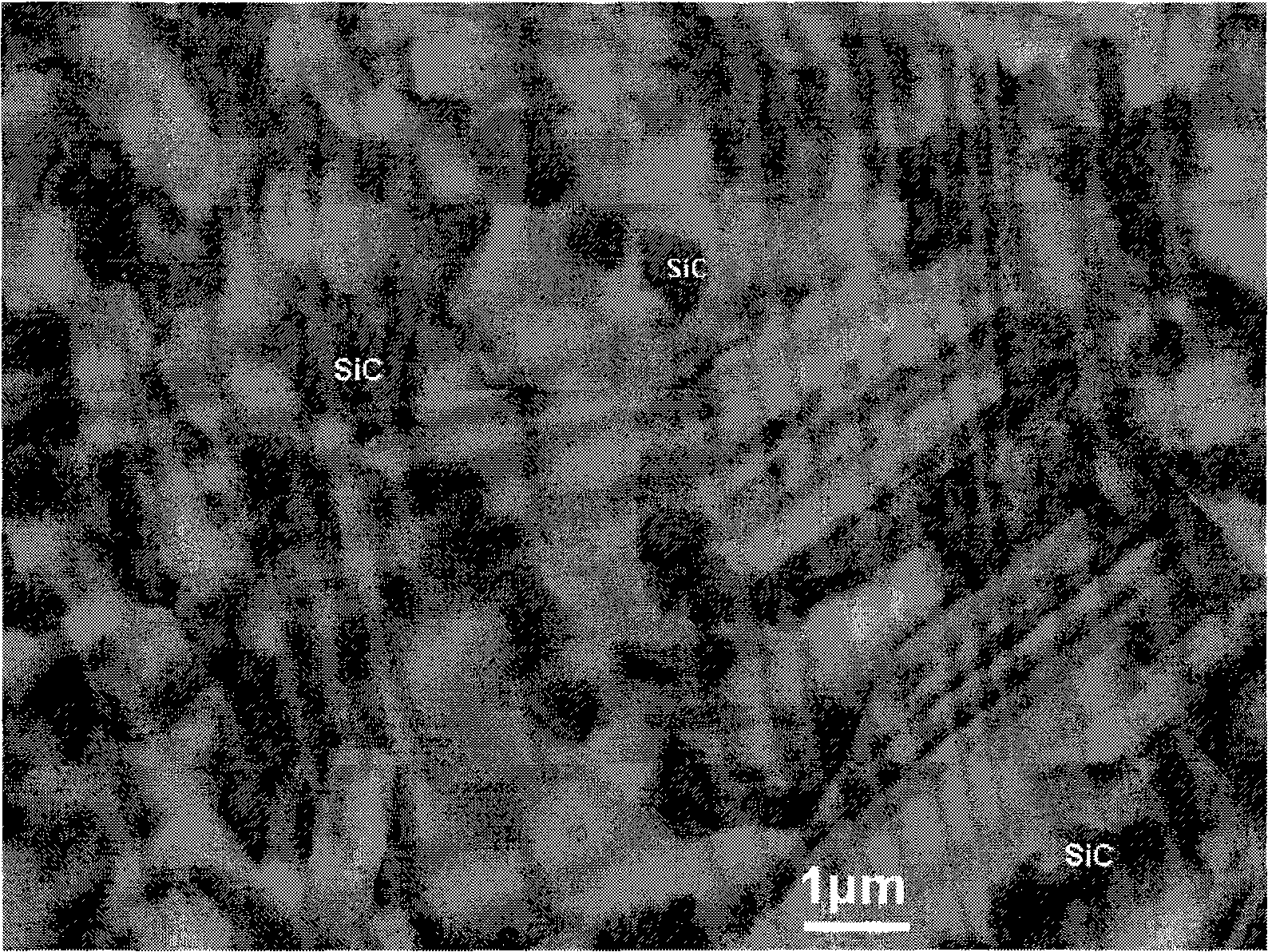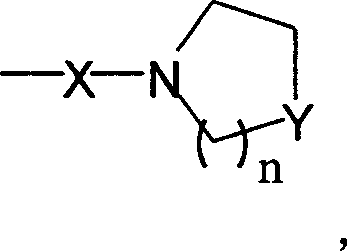Patents
Literature
2642 results about "Substitution reaction" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Substitution reaction (also known as single displacement reaction or single substitution reaction) is a chemical reaction during which one functional group in a chemical compound is replaced by another functional group. Substitution reactions are of prime importance in organic chemistry. Substitution reactions in organic chemistry are classified either as electrophilic or nucleophilic depending upon the reagent involved. There are other classifications as well that are mentioned below.
2-2′-disubstituted 9,9′-spirobifluorene-base triaryldiamines and their application
ActiveUS7714145B2Simple wayEasy to practiceSilicon organic compoundsDischarge tube luminescnet screensOrganic light emitting deviceSubstitution reaction
The present invention discloses synthesis of 2,2′-disubstituted 9,9′-spirobifluorene-based triaryldiamine. First, 2,2′-diamino-9,9′-spirobifluorene, a Pd-catalyst as auxiliary and aryl halide BX are provided, wherein X is selected from the group consisting of: Cl, Br and I, B comprises one of the following group: aryl moiety, hetero cycle, multiple fused ring, multiple fused ring with hetero atom(s). Next, a substitution reaction is performed to react the 2,2′-diamino-9,9′-spirobifluorene with the aryl halide BX to produce the 2,2′-disubstituted 9,9′-spirobifluorene-based triaryldiamines. In addition, the present invention discloses organic light emitting devices comprising hole transporting material comprising 2,2′-bis(N,N-disubstituted amino)-9,9′-spirobifluorenes.
Owner:E RAY OPTOELECTRONICS TECH
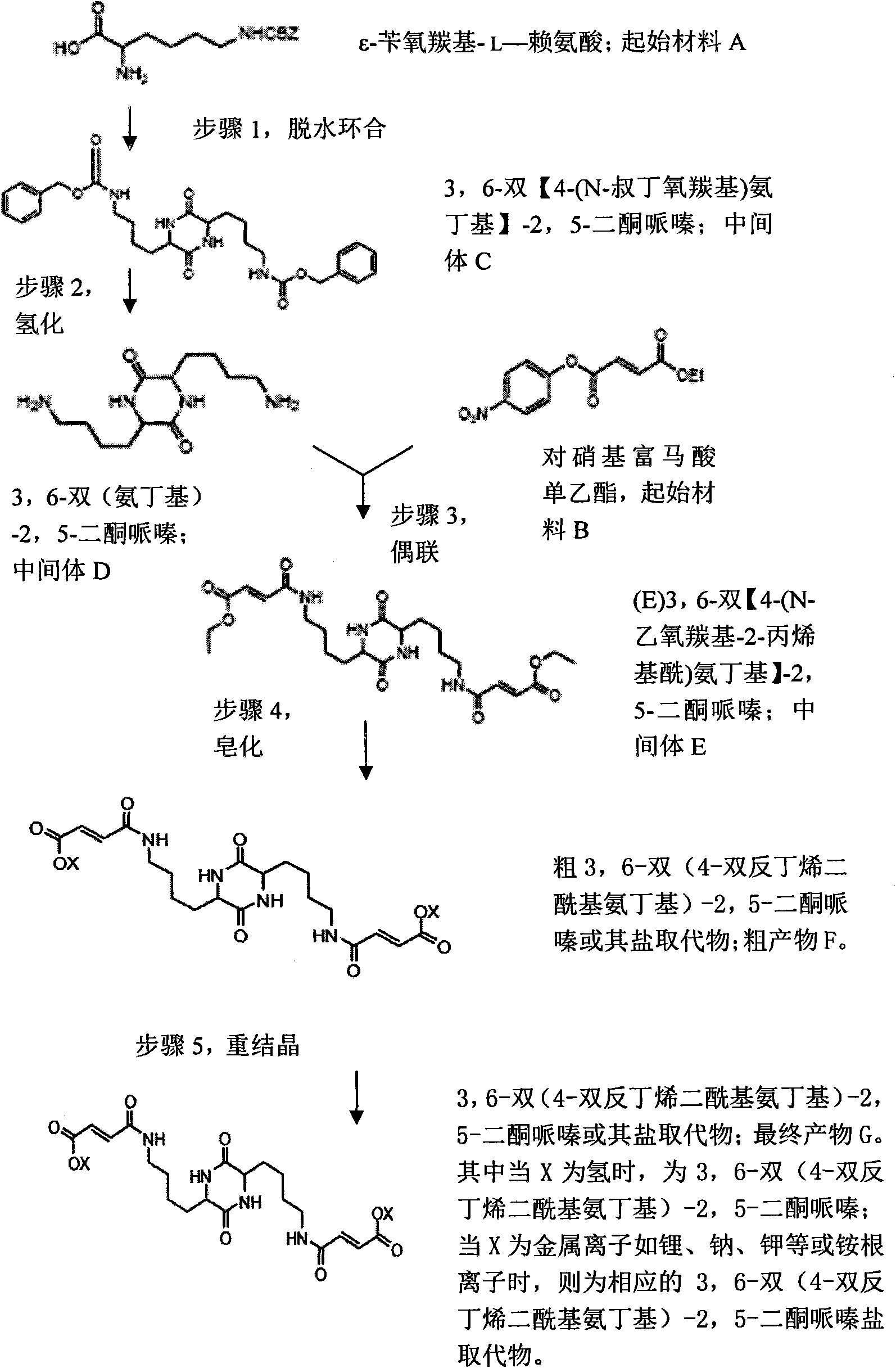
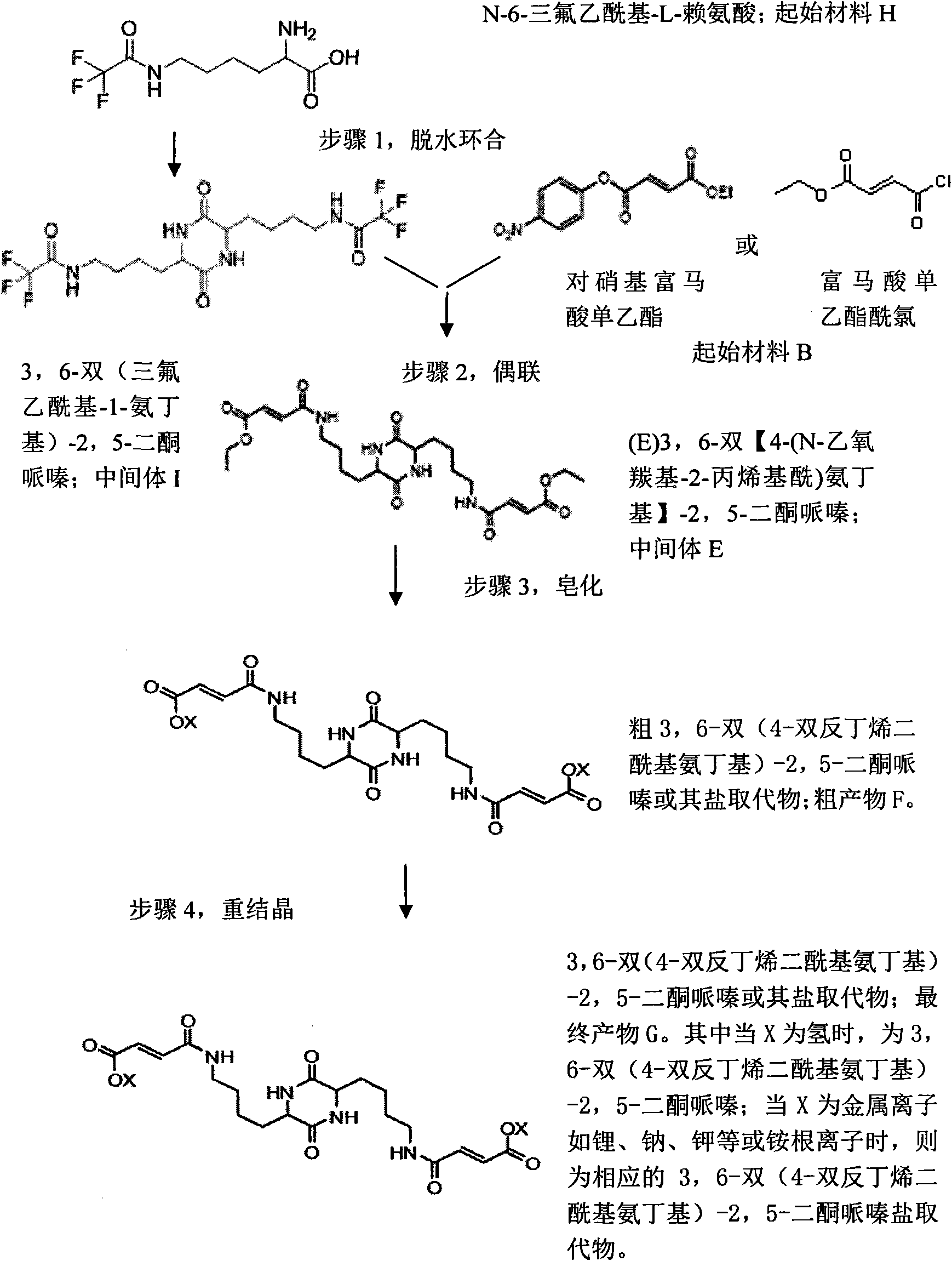
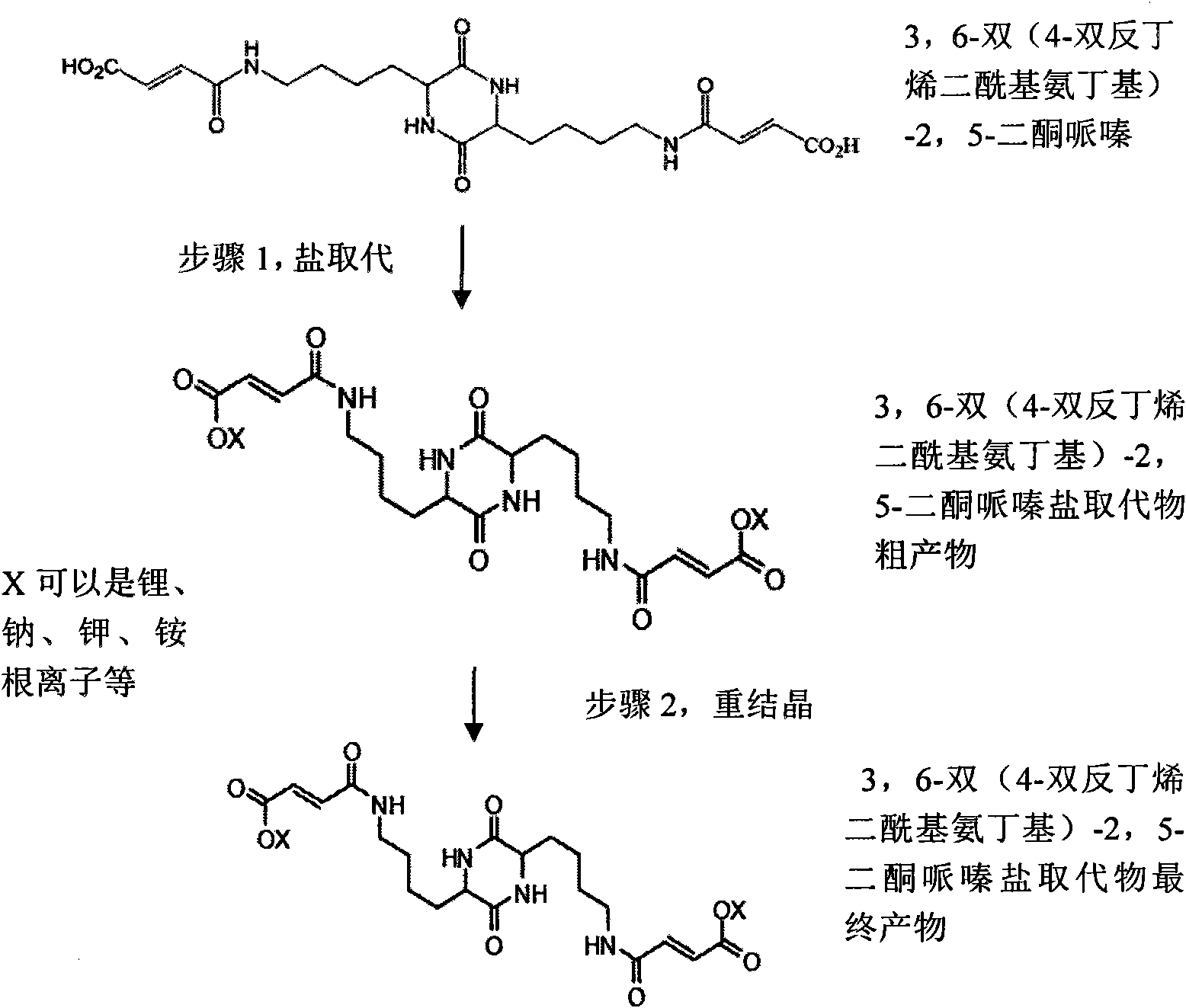
Synthetic methods of 3,6-bis(4-bisfumaroyl aminobutyl)-2,5-diketopiperazine and salt substitute thereof
The invention relates to two synthetic methods of 3,6-bis(4-bisfumaroyl aminobutyl)-2,5-diketopiperazine and a salt substitute thereof. The first synthetic method comprises the following steps of: obtaining a final product through the steps of cyclodehydration, hydrogenation, coupling, saponification, recrystallization and the like by using epsilon-benzoyloxycarbonyl-L-lysine and p-nitryl monoethyl fumarate as starting materials. The second synthetic method comprises the following steps of: obtaining the final product through the steps of cyclodehydration, coupling, saponification, recrystallization and the like by using N-6-trifluoroacetyl-L-lysine and the p-nitryl monoethyl fumarate (or p-nitryl monoethyl fumarate acyl chloride) as the starting materials. Meanwhile, the salt substitute of the 3,6-bis(4-bisfumaroyl aminobutyl)-2,5-diketopiperazine can also be generated by directly carrying out substitution reaction on the 3,6-bis(4-bisfumaroyl aminobutyl)-2,5-diketopiperazine as a reaction product and corresponding salt.
Owner:于清
High-yield conversion of cellulosic biomass into furanic biofuels and value-added products
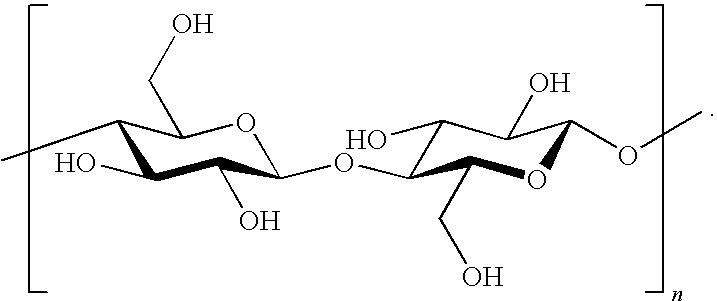
Paper, cotton, corn stover, straw, and wood are converted into furanic products in high yields (based on their cellulose content) using a simple, inexpensive process involving concurrent hydrolysis, dehydration, and substitution reactions coupled with continuous extraction into an organic phase. In a simultaneous process, the hemicellulose fraction of these substrates is converted into furfural, and together these constitute an efficient means for the total exploitation of the carbohydrate content of biomass.
Owner:RGT UNIV OF CALIFORNIA
Method for producing modified conjugated diene polymer/copolymer, modified conjugated diene polymer/copolymer, and rubber composition and tier using the same
ActiveUS20110146877A1Increased durabilityImprove rolling resistanceSpecial tyresPneumatic tyre reinforcementsSilane compoundsPolymer science
The invention provides a method for producing a modified conjugated diene (co)polymer, the method including comprising: a modification reaction step including causing an organic silane compound to react with a conjugated diene (co)polymer having an active site at the active site, the organic silane compound having a characteristic group for forming a silanol group through hydrolysis and, in the vicinity of the characteristic group, (i) a functional group which binds the organic silane compound to the conjugated diene (co)polymer via addition to or substitution at the active site and which promotes reaction between the silanol group and a reinforcing filler after the addition or substitution reaction, or (ii) a functional group which promotes reaction between the silanol group and a reinforcing filler, and a hydrolyzation step performed after the modification reaction step; a modified conjugated diene (co)polymer having, at a molecular end of the conjugated diene (co)polymer, a silanol group, and a functional group in the vicinity of the silanol group, the functional group accelerating reaction between the silanol group and the reinforcing filler; a rubber composition containing the (co)polymer and carbon black having specific characteristics; and a tire formed from the rubber composition.
Owner:BRIDGESTONE CORP
Non-electrolytic gold plating liquid and non-electrolytic gold plating method using same
InactiveUS6287371B1Improve adhesionInhibits excess local etching or corrosionAnti-corrosive paintsLiquid/solution decomposition chemical coatingEtchingCobalt
The present invention provides an excellent non-electrolytic gold plating liquid which produces a gold plating layer firmly adhered to a surface selected from the group consisting of nickel, cobalt, palladium or a metal alloy containing nickel, cobalt or palladium, as well as a method for performing a non-electrolytic gold plating method using the non-electrolytic gold plating liquid. The non-electrolytic gold plating liquid comprises:(1) a water-soluble gold compound;(2) a complexation agent which stabilizes a gold ion in the plating liquid, but does not substantially dissolve nickel, cobalt or palladium; and(3) an anti-gold deposit agent which inhibits excess local etching or corrosion by substitution reaction between the metal surface and gold during the gold plating.
Owner:LEARONAL JAPAN
High-yield conversion of cellulosic biomass into furanic biofuels and value-added products
Paper, cotton, corn stover, straw, and wood are converted into furanic products in high yields (based on their cellulose content) using a simple, inexpensive process involving concurrent hydrolysis, dehydration, and substitution reactions coupled with continuous extraction into an organic phase. In a simultaneous process, the hemicellulose fraction of these substrates is converted into furfural, and together these constitute an efficient means for the total exploitation of the carbohydrate content of biomass.
Owner:RGT UNIV OF CALIFORNIA
2,2'-Disubstituted 9,9'-Spirobifluorene-based Triaryldiamines and Their Application
ActiveUS20070262703A1Simple wayEasy to practiceSilicon organic compoundsDischarge tube luminescnet screensOrganic light emitting deviceSubstitution reaction
The present invention discloses synthesis of 2,2′-disubstituted 9,9′-spirobifluorene-based triaryldiamine. First, 2,2′-diamino-9,9′-spirobifluorene, a Pd-catalyst as auxiliary and aryl halide BX are provided, wherein X is selected from the group consisting of: Cl, Br and I, B comprises one of the following group: aryl moiety, hetero cycle, multiple fused ring, multiple fused ring with hetero atom(s). Next, a substitution reaction is performed to react the 2,2′-diamino-9,9′-spirobifluorene with the aryl halide BX to produce the 2,2′-disubstituted 9,9′-spirobifluorene-based triaryldiamines. In addition, the present invention discloses organic light emitting devices comprising hole transporting material comprising 2,2′-bis(N,N-disubstituted amino)-9,9′-spirobifluorenes.
Owner:E RAY OPTOELECTRONICS TECH
Method for preparing rosuvastatin calcium midbody
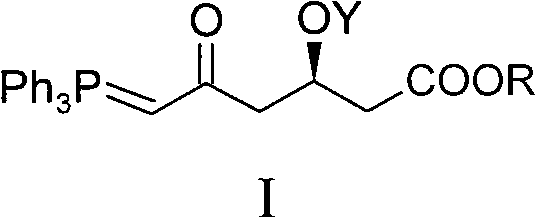
ActiveCN101735272AHigh yieldReduce pollutionGroup 5/15 element organic compoundsBulk chemical productionWittig reactionGrignard reaction
The invention discloses a method for preparing a rosuvastatin calcium midbody, namely a compound (R is C1-C10 alkyl, and Y is a hydroxyl protecting group) shown as the general formula I. Chloroethylene and R-epoxy chloropropane as initial raw materials are carried out seven steps of reaction, such as Grignard reaction, sodium cyanide nucleophilic substitution reaction, alcoholysis reaction, hydroxyl protection, oxidizing reaction, methylchloroformate acylation reaction and Wittig reaction to prepare the compound shown as the general formula I. The method has mild condition, simple and convenient operation, stable process, low cost and easy acquisition of raw materials, high product yield, easy disposal of the three wastes, less environmental pollution, low preparation cost and suitability for industrialized large-scale production.
Owner:JIANGXI DONGBANG PHARMA
High substitution degree carboxymethyl indianbread polysaccharide and its preparation method and uses
ActiveCN1970579AIncrease the reaction concentrationIncreased degree of carboxymethyl substitutionAntibacterial agentsOrganic active ingredientsSolubilityAlcohol
The invention discloses a carboxymethyl pachyman (CMP) with high-degree of substitution and making method and application, which is characterized by the following: adopting water or water alcohol solution as dielectric; proceeding substitution reaction for pachyman, chloroacetic acid and fitful excessive sodium hydroxide to obtain CMP without vibrating technique and equipment; improving CMP D / S and solubility to reach injection need.
Owner:HUNAN BUTIAN PHARMA
Preparation method of vinyl sulfate
The invention discloses a preparation method of vinyl sulfate. The preparation method comprises the following steps: (1) substitution reaction: carrying out substitution reaction on thionyl chloride and ethanediol used as raw materials, washing the reaction solution with deionized water to a neutral state, standing, stratifying, and separating to obtain vinyl sulfite; (2) oxidation reaction: adding dichloromethane and a ferric sulfate solution into the vinyl sulfite, cooling to 0-5 DEG C, dropwisely adding a sodium percarbonate solution to obtain a water-phase / organic-phase-coexistent reaction solution, standing the reaction solution, stratifying, separating out the water phase, and carrying out reduced pressure distillation on the organic phase, thereby obtaining the vinyl sulfate crude product; and refinement: recrystallizing the vinyl sulfate crude product by using dichloromethane to obtain the high-purity vinyl sulfate. The preparation method has the characteristics of high mole yield, high product purity, low moisture content, low acid value and low preparation cost. The mole yield can reach 85% or above, the purity can reach 99% or above, the moisture content is less than or equal to 60 ppm, and the acid value is less than or equal to 60 ppm.
Owner:江苏瀚康新材料有限公司
Method for producing modified conjugated diene polymer/copolymer, modified conjugated diene polymer/copolymer, and rubber composition and tier using the same
ActiveCN102026826AImprove rolling resistance performanceIncreased durabilitySpecial tyresWheelsPolymer sciencePolymer bonding
Provided are a method for producing a modified conjugated diene polymer / copolymer including a modified reaction step for causing an organosilane compound having a characteristic group for producing a silanol group by hydrolysis and, in the vicinity of the characteristic group, (i) a functional group for combining the organosilane compound with the conjugated dione polymer / copolymer by performing an addition or substitutive reaction on an active site and, after the reaction, prompting a reaction between the silanol group and a reinforcing filler or (ii) a functional group for prompting the reaction between the silanol group and the reinforcing filler to react with the active site of a conjugated dione polymer / copolymer having the active site, and a hydrosis step performed after completing the modified reaction step; a modified conjugated diene polymer / copolymer having, at an end of a molecular of a conjugated diene polymer / copolymer, a silanol group and, in the vicinity of the silanol group, a functional group for prompting a reaction between the silanolgroup and a reinforcing filler; and a rubber composition having the modified conjugated polymer / copolymer and carbon black of a specific property; and a tier using the rubber composition.
Owner:BRIDGESTONE CORP
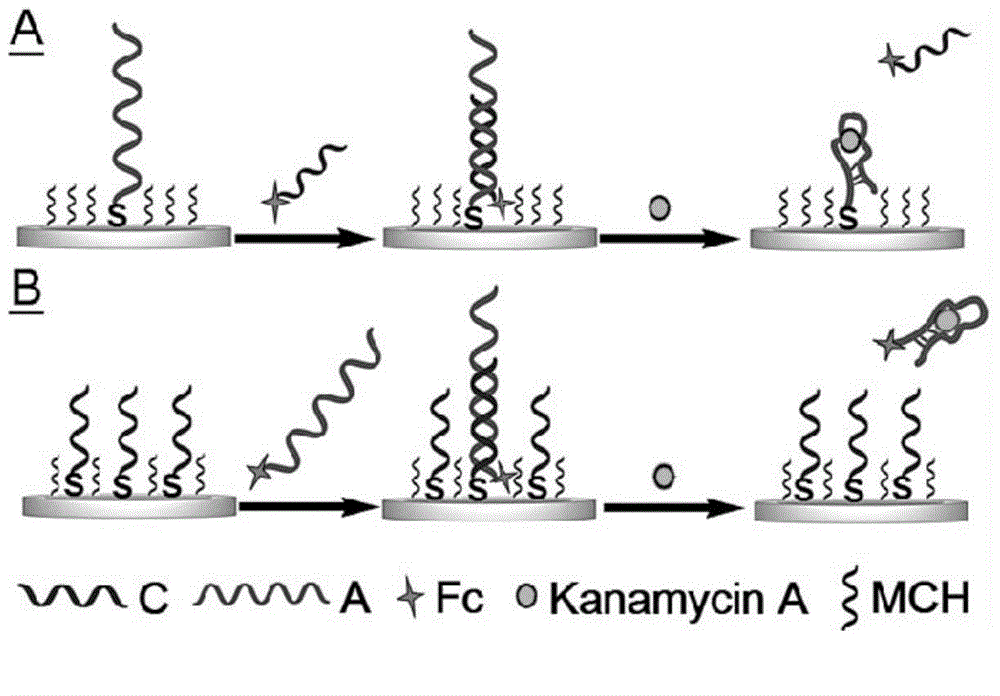
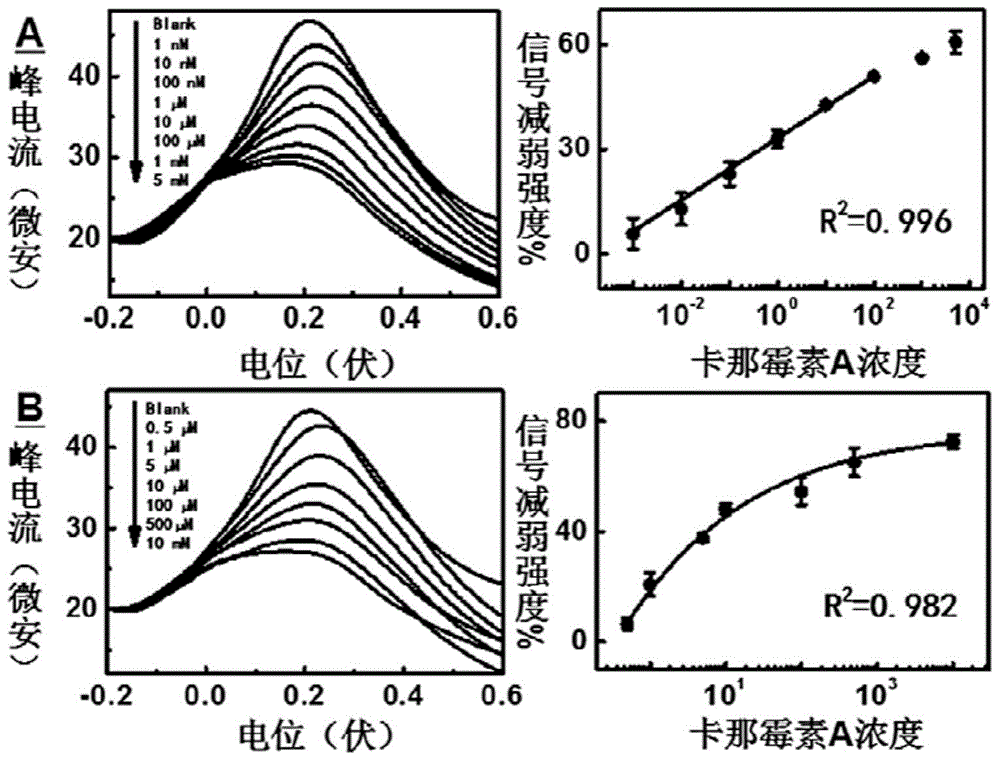
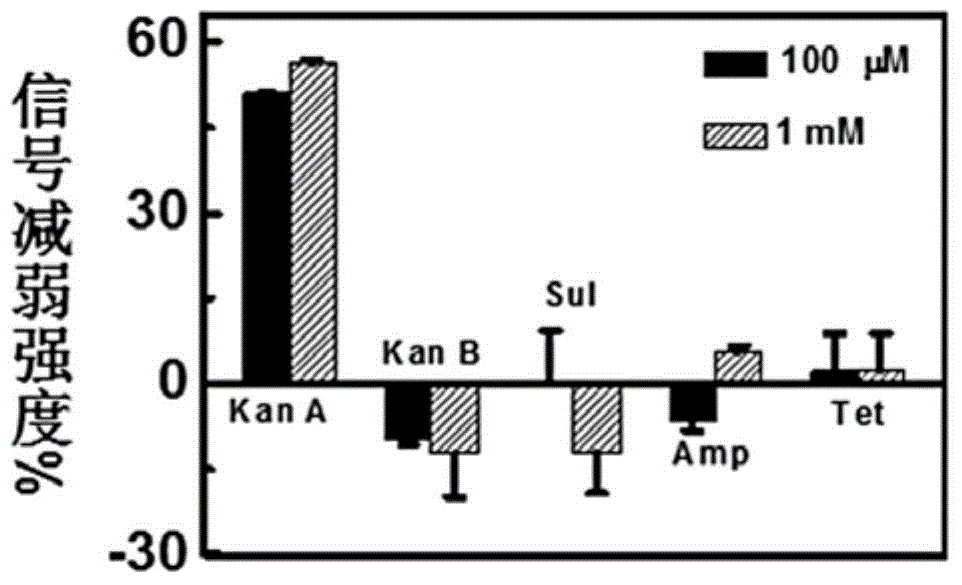
Aptamer electrochemical sensor used for kanamycin A detection and production and application methods of aptamer electrochemical sensor
ActiveCN104880498ASimple designImprove general performanceMaterial electrochemical variablesAnti jammingKanamycin
The invention relates to a kanamycin A aptamer electrochemical sensor based on signal probe chain substitution reaction and production and application methods of the kanamycin A aptamer electrochemical sensor. SD-EAB consists of a sulfhydryl-modified capture probe (an aptamer or a short complementary chain) and a signal probe (a short complementary chain or an aptamer) which is complementary to and is hybridized with the capture probe and has an oxidation-reduction mark. When kanamycin A exists, the signal probe is substituted and released from the surface of an electrode, so as to result in decreasing of current, and a decrease value of current is proportional to a logarithm of the concentration of the kanamycin A. The signal transduction of the kanamycin A aptamer electrochemical sensor provided by the invention is only caused by affine competition among target molecules, the short complementary chain and the aptamer, and is not associated with a conformation state of the aptamer, so that the generality of the aptamer is greatly improved. The SD-EAB has the advantages of high sensitivity, good specificity, wide dynamic interval and strong anti-jamming capability, without additional reagents.
Owner:CAPITAL NORMAL UNIVERSITY +1
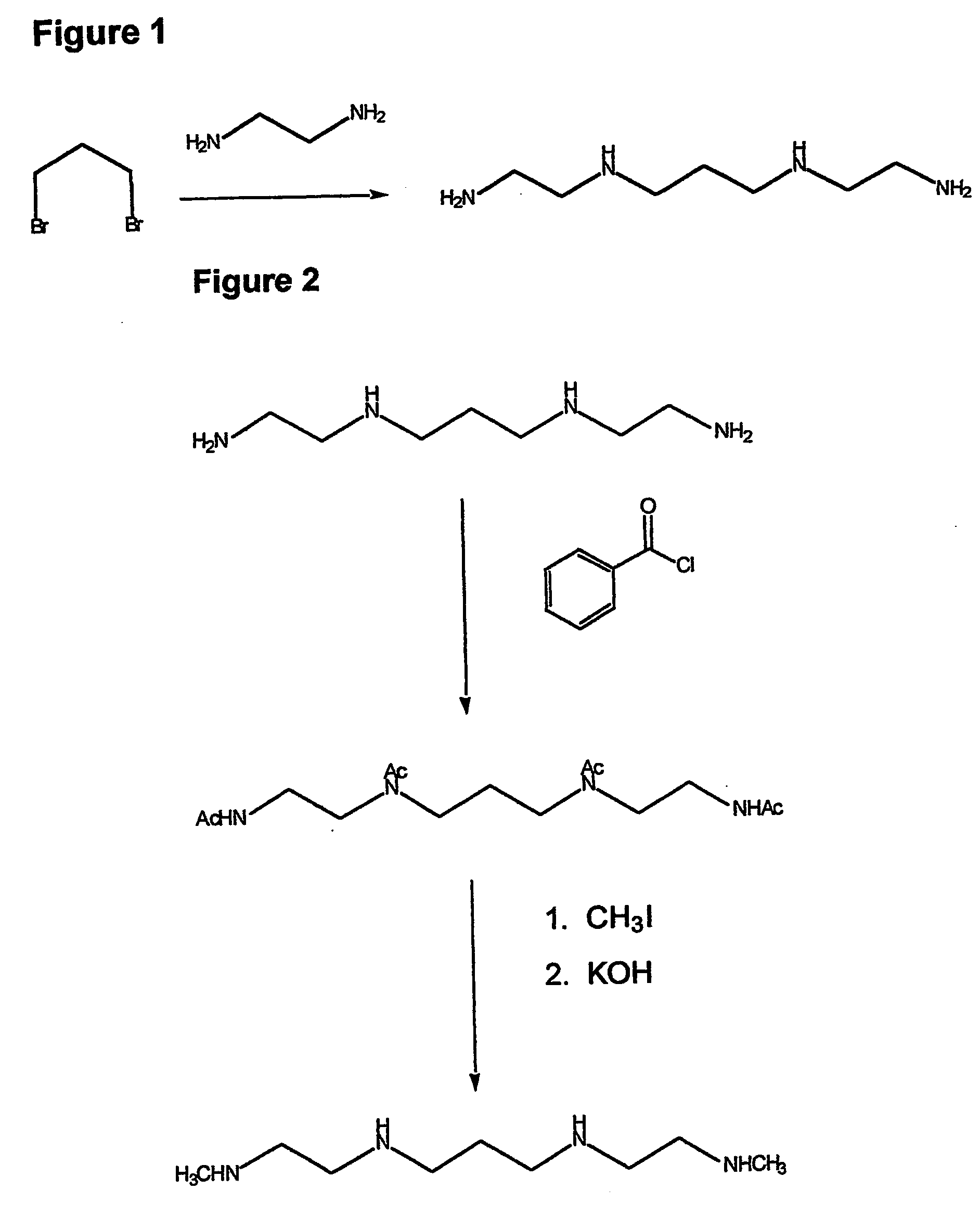
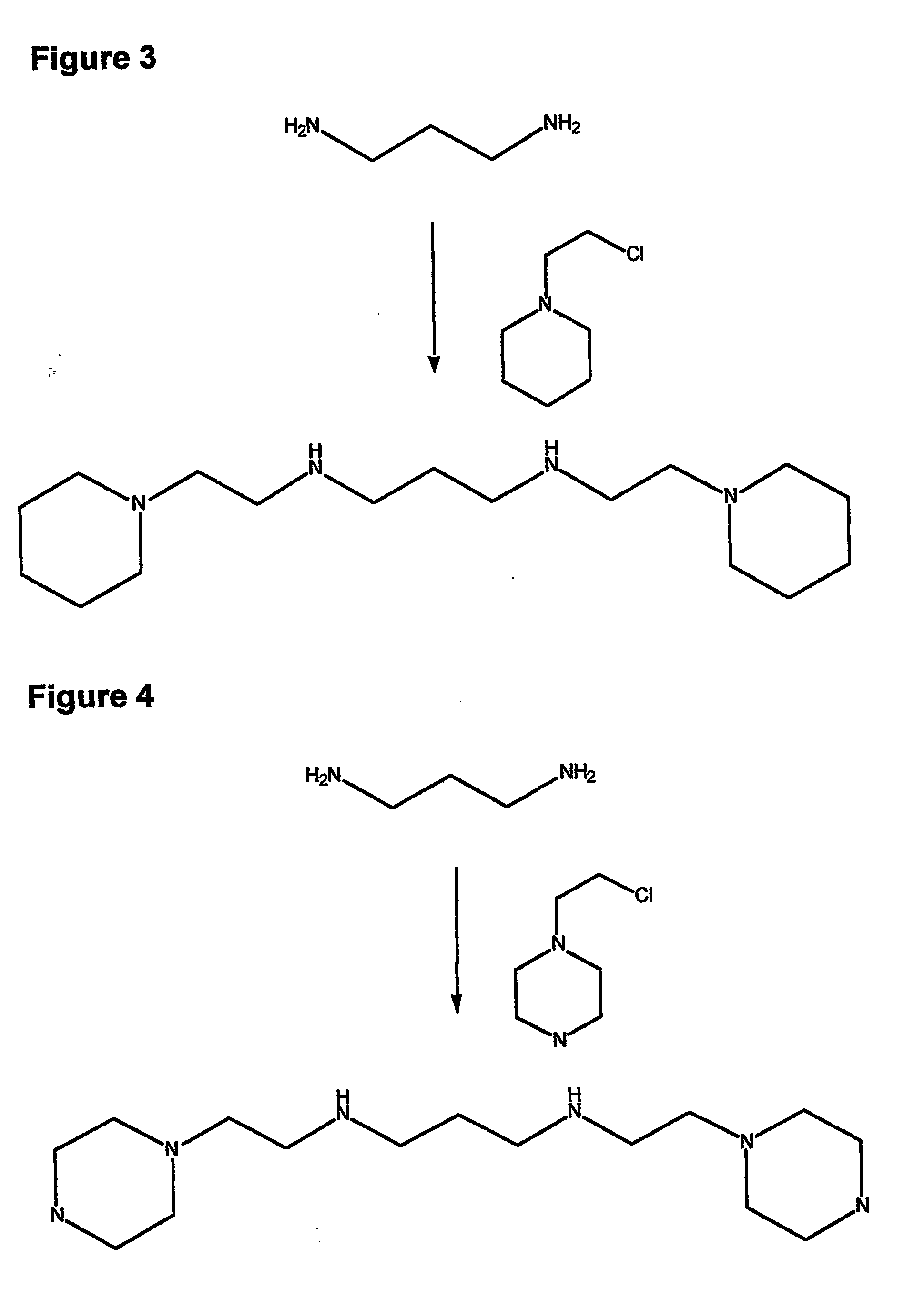
Composition, synthesis and therapeutic applications of polyamines
InactiveUS20050085555A1Chromium concentration were decreasedIncrease excretionBiocideGroup 5/15 element organic compoundsAntidoteRisk stroke
This invention relates to a process of synthesis and composition of open chain (ring), closed ring, linear branched and or substituted polyamines, polyamine derived tyrosine phosphatase inhibitors and PPAR partial agonists / partial antagonists via a series of substitution reactions and optimizing the bioavailability and biological activities of the compounds. Polyamines prevent the toxicty of neutoxins and diabetogenic toxins including paraquat, methyphenyl pyridine radical, rotenone, diazoxide, streptozotocin and alloxan. These polyamines can be to treat neurological, cardiovascular, endocrine acquired and inherited mitochondrial DNA damage diseases and other disorders in mammalian subjects, and more specifically to the therapy of Parkinson's disease, Alzheimer's disease, Lou Gehrig's disease, Binswanger's disease, Olivopontine Cerebellar Degeneration, Lewy Body disease, Diabetes, Stroke, Atherosclerosis, Myocardial Ischemia, Cardiomyopathy, Nephropathy, Ischemia, Glaucoma, Presbycussis, Cancer, Osteoporosis, Rheumatoid Arthritis, Inflammatory Bowel Disease, Multiple Sclerosis and as Antidotes to Toxin Exposure.
Owner:MURPHY MICHAEL A
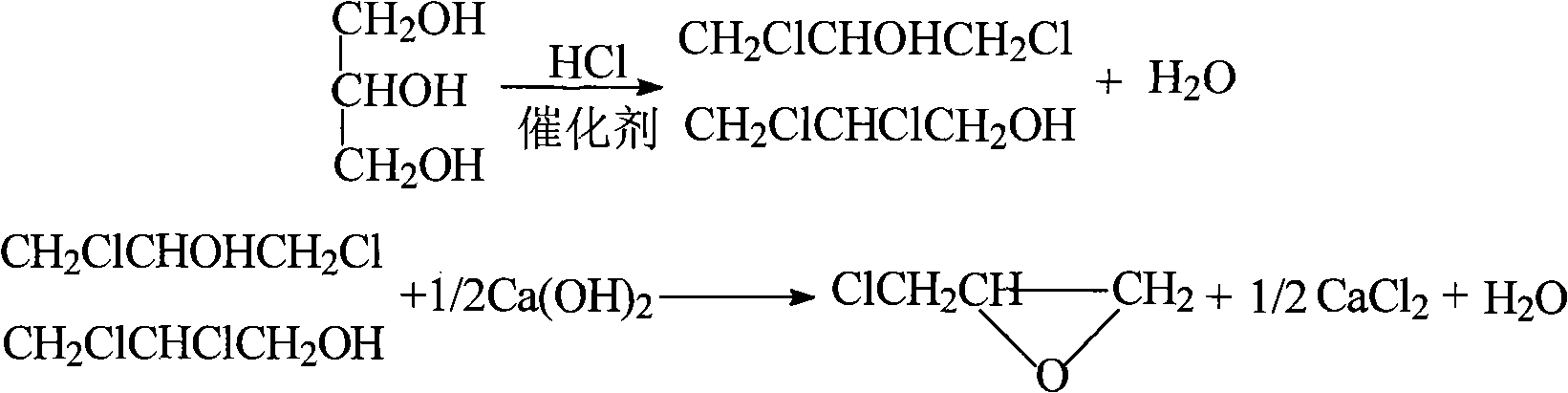
Method for continuously preparing epichlorohydrin by glycerine reaction fractional distillation
InactiveCN101337950AEfficient separationGuaranteed purityOrganic chemistryOrganic-compounds/hydrides/coordination-complexes catalystsBiodiesel1-Propanol
The invention relates to a method for preparing epoxy chloropropane which is obtained through successive reaction and distillation coupling technology after glycerine that is the by-product of biological diesel oil reacts with chlorine hydride through substitution reaction under homogeneous multielement catalysis and further performs saponification cyclization reaction. The method comprises the following steps: (1) chlorine hydride is pumped in glycerine that is the by-product of biological diesel oil for reaction under homogeneous multielement catalysis, and the mixture composed of 1, 3-dichloro-2-propanol and 2, 3-dichloro-1-propanol is prepared through the technologies of continuous feeding, continuous catalyzed chlorination and continuous rectification; (2) the isomeride mixture solution of 1, 3-dichloro-2-propanol and 2, 3-dichloro-1-propanol performs the saponification cyclization to generate a product of epoxy chloropropane in alkaline solution. The method has the advantages of mild reaction condition is mild, high catalyst activity, dedicated catalytic performance, high product selectivity, process 'cleaning', easy separation, continuous operation, environment-friendliness, etc.
Owner:JIANGSU POLYTECHNIC UNIVERSITY
Chiral diphosphite ligand and iridium composite catalyst and preparation thereof method and application to asymmetrical hydrogenization synthesis (S)-metolachlor
ActiveCN101857612AOrganic compound preparationOrganic-compounds/hydrides/coordination-complexes catalystsAlkaneDiphosphines
The invention relates to a kind of chiral diphosphite ligands, an iridium composite catalyst thereof, a preparation method and application thereof. The ligands are obtained through using chiral (R)-(S)-1-dimethylamino ethyiferroene as raw materials to react with diphenyl phosphonium chloride under the effect of butyl lithium and then to carry out displacement reaction with diaryl phosphine alkane. The chiral diphosphite ligands respectively act with homotropilidene compositions of iridous chloride, tetrabutyl ammonium iodide and glacial acetic acid, and imine asymmetrical hydrogenization catalysts can be obtained. When the iridium-diphosphine catalysts are used for catalyzing 2-methyl-6-ethyl-N-methylene aniline (EMA-imine) hydrogenization reaction, (S)-N-(1-anisyl-2-propyl)-2-methyl-6- ethylaniline ((S)-NNA) can be obtained, and the antimer excessive value (ee) can reach 86.5 percent. The (S)-NNA and chloracetyl chloride carry out acylation reaction to obtain (S)-metolachlor with the ee value of 86 percent. Thereby, the ligands provided by the invneiton can be used for synthesizing chiral herbicidal chemicals of (S)-metolachlor.
Owner:NANJING UNIV OF TECH +2
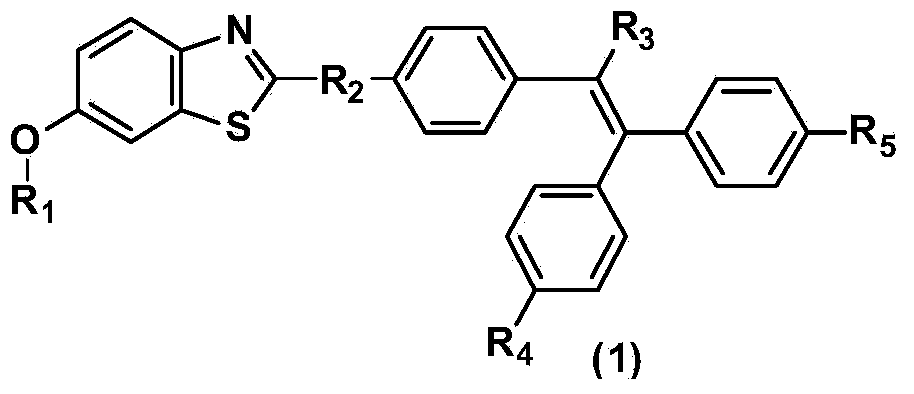
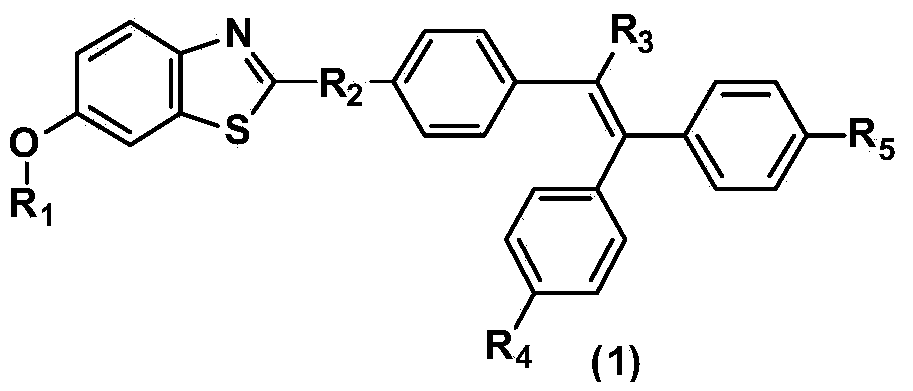
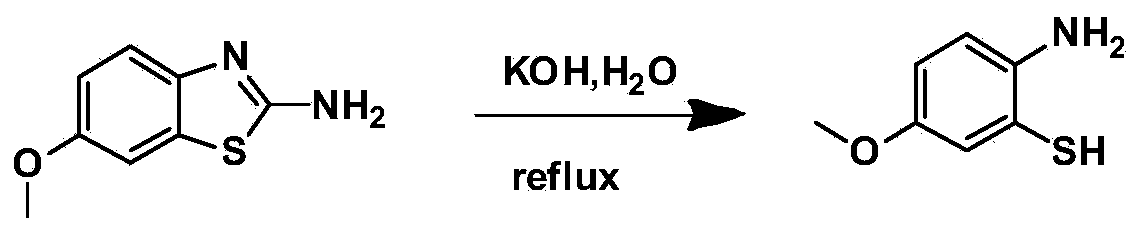
Benzothiazole derivative containing triphenylethylene or tetraphenylethylene structure and having aggregation-induced emission property and preparation method and application thereof
ActiveCN103804318AWeak luminous intensityLow costOrganic chemistrySolid-state devicesLuminous intensityPhotochemistry
The invention discloses a benzothiazole derivative containing a triphenylethylene or tetraphenylethylene structure and having aggregation-induced emission property and a preparation method and application thereof. The preparation method comprises the following steps: with 2-amino-6-methoxybenzothiazole as a start raw material, performing a ring-opening reaction under an alkaline condition to generate 2-amino-5-methoxythiophenol; performing a ring-closing reaction with an aromatic aldehyde compound containing a triphenylethylene or tetraphenylethylene structure to obtain a methoxy-containing benzothiazole derivative, or performing demethylation to obtain a hydroxyl-containing benzothiazole derivative, wherein the compound also can be modified through a group substitution reaction to generate a benzothiazole derivative containing other functional groups. The benzothiazole derivative disclosed by the invention has relatively low luminous intensity in a solution, emits strong fluorescence in an aggregation state or solid state, and belongs to good aggregation-induced emission materials; the synthesis is relatively simple, and the cost of the raw materials is low, so that large-scale commercial production is easy to realize; the benzothiazole derivative plays an important role in the fields of electroluminescent devices, fluorescent probes, fluorescent switches, organism imaging and the like.
Owner:SUN YAT SEN UNIV
Composite stopping agent preventing coal spontaneous combustion based on low-temperature oxidation mechanism of coal
InactiveCN102966369AStable in natureEasy to useOther chemical processesDust removalPolyethylene glycolSolvent
The invention relates to a composite stopping agent preventing coal spontaneous combustion based on a low-temperature oxidation mechanism of coal, which belongs to the stopping agent preventing coal spontaneous combustion. The composite stopping agent is characterized by comprising the components of catechinic acid, nontoxic phosphite ester, polyethylene glycol 200, lauryl sodium sulfate and solvent-water. The concentration of raw materials in the solvent-water by mass percent is 0.5-1% of catechinic acid, 0.5-1% of nontoxic phosphite ester, 4-7% of polyethylene glycol 200 and 0.5-1% of lauryl sodium sulfate. The stopping agent can not only be in substitution reaction with methyl and methylene in coal to generate stable ether matters, but also be interacted with free radicals of alkyl peroxides generated by oxidization of methyl and methylene in the coal. The free radicals of peroxides are destroyed, so that generation and activity of active groups such as methyl, methylene and the like in coal spontaneous combustion can be inhibited, therefore, the spontaneous combustion character of coal is fundamentally changed and spontaneous combustion of coal is inhibited. Meanwhile, the stopping agent has the physical moisturizing function and can lock water injected in the coal better, so as to prevent coal spontaneous combustion better in the physical inhibition respect.
Owner:CHINA UNIV OF MINING & TECH +1
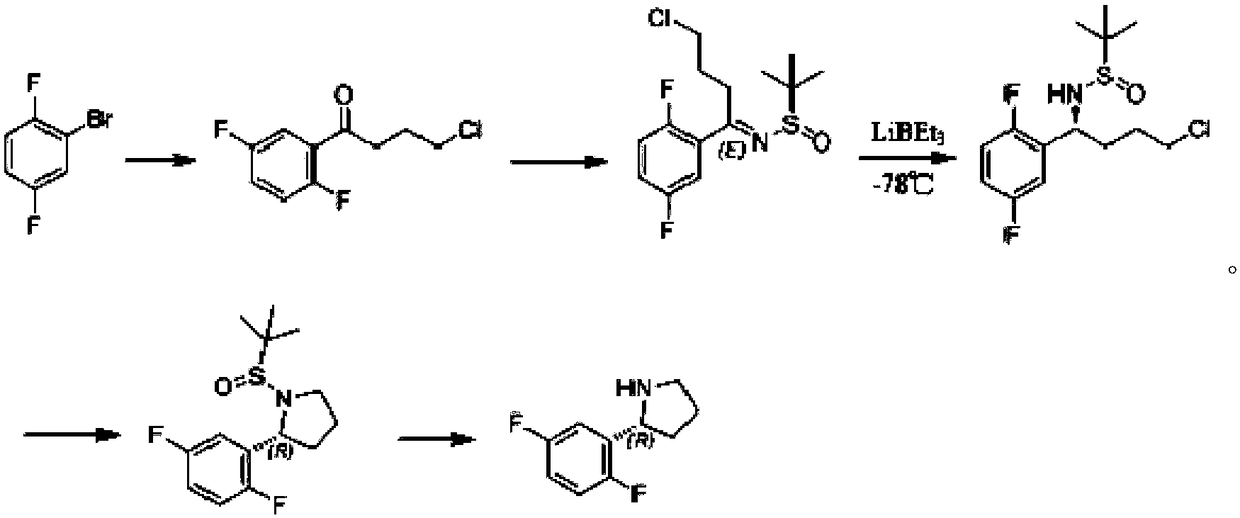
Synthesis process of chiral pyrrolidine and intermediates
The invention discloses a synthesis process of chiral pyrrolidine and intermediates. The synthesis process adopts 2, 5-difluorohalobenze or 5-fluoro-3 halogenated pyridine as the raw material substrate, and employs a chiral catalyst to induce chiral reaction so as to reduce a ketone compound into a corresponding chiral alcohol compound, and then carries out substitution reaction to introduce an easily leavable group to an alcoholic hydroxyl group so as to facilitate ring formation reaction (or carry out ring formation reaction directly). The process provided by the invention has the advantagesof significant increase of product yield and reduction of cost, also the reaction temperature is mild, and the process is easy to control and industrialize.
Owner:上海鑫凯化学科技有限公司
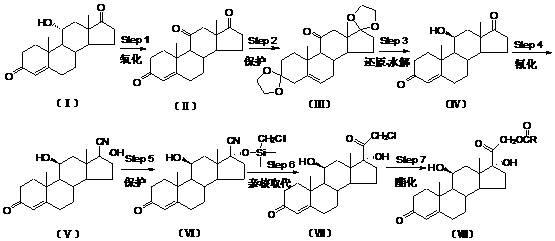
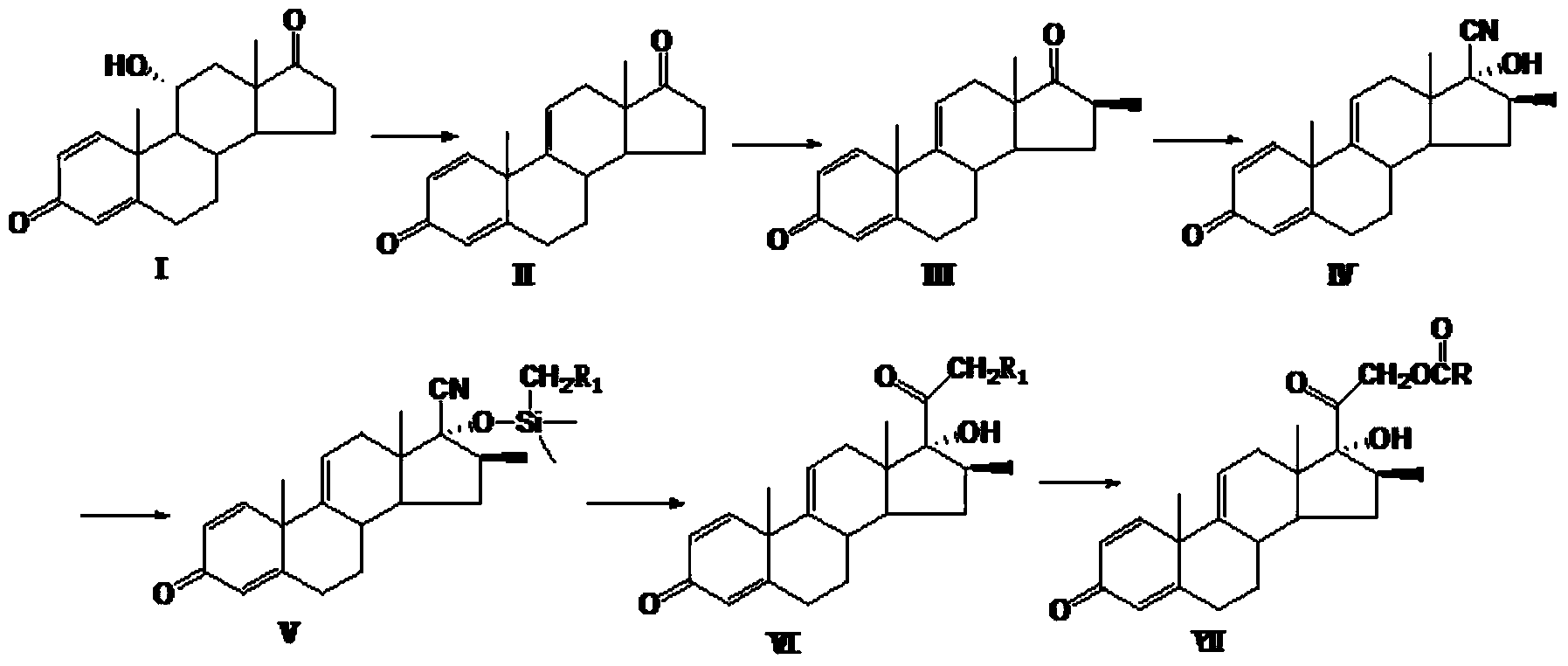
Preparation method of hydrocortisone acetate or analogue thereof
ActiveCN102603842AStarting materials are readily availableHigh and stable yieldSteroidsSNiStructural formula
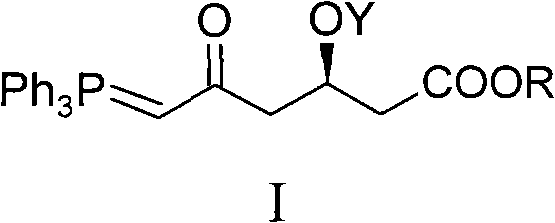
The invention relates to a preparation method of hydrocortisone acetate or an analogue thereof. Hydrocortisone acetate or the analogue thereof has a structural formula represented as a formula VIII. According to the invention, a compound I is subject to an oxidation reaction, a carbonyl protection reaction, a reduction and hydrolysis reaction, a cyano substitution reaction, a silicon alkyloxy protection reaction, an intramolecular nucleophilic substitution reaction, and a substitution reaction, such that hydrocortisone acetate or the analogue thereof is prepared. The initial raw materials areeasy to obtain, and the yield is high and stable. The compound I is represented by a formula I, wherein R is H or alkyl.
Owner:HUNAN NORCHEM PHARMACEUTICAL CO LTD
Ion liquid of amino acid ester cation and its preparation method
InactiveCN1621152AEasy to getLow priceOrganic compound preparationOrganic-compounds/hydrides/coordination-complexes catalystsTetrafluoroborateAmino acid
The present invention is ion liquid of amino acid ester cation and its preparation process, and belongs to the field of new chemical material and its preparation technology. The ion liquid of amino acid ester cation is prepared with amino acid ester hydrochloride and through the substitution reaction with nitrate, tetrafluoroborate, hexafluorophosphate, bistrifluoromesyl imine salt or thiocyanate or the direct addition reaction with aluminum trichloride, ferric trichloride or zinc chloride, and the separation. The ion liquid thus prepared has the characteristic of chiral matter except the ion liquid characteristics, has low cost, simple preparation process and no pollution. The present invention is suitable for industrial production and is expected to become important green chemical material.
Owner:PEKING UNIV
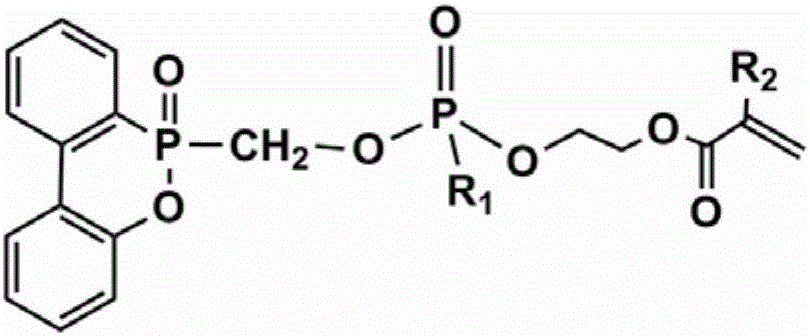
DOPO-based flame retardant, and preparation method thereof
ActiveCN106243385AHigh molecular weightImprove flame retardant performanceGroup 5/15 element organic compoundsGas phaseResin matrix
The invention discloses a DOPO-based flame retardant, and a preparation method thereof. According to the preparation method, 9,10-dihydro-9-oxa-10-phosphaphenanthrene 10-oxide and paraformaldehyde are taken as raw materials, and a precursor I is synthesized via substitution reaction; active hydroxide radicals of the precursor I, phenyl dichlorophosphate, and hydroxyethyl acrylate are subjected to substitution / esterification reaction so as to prepare a flame retardant which is high in phosphorus content and possesses active double bonds; and the flame retardant is added into an unsaturated polyester resin matrix so as to obtain a flame-retardant unsaturated polyester resin composite material. According to the DOPO-based flame retardant, flame resistance of the unsaturated polyester resin matrix is improved via a gas phase and a condensed phase, problems of conventional additive flame retardants that compatibility with polymer matrix is poor, migration is easily caused, and flame retardant efficiency is low are solved. When the DOPO-based flame retardant is applied to inflaming retarding of unsaturated polyester resin, material vertical flame rating is capable of reaching V-0 grade at a relatively low adding amount, and flame resistance is excellent. The DOPO-based flame retardant is high in purity and yield, and aftertreatment is simple.
Owner:SOUTH CHINA UNIV OF TECH
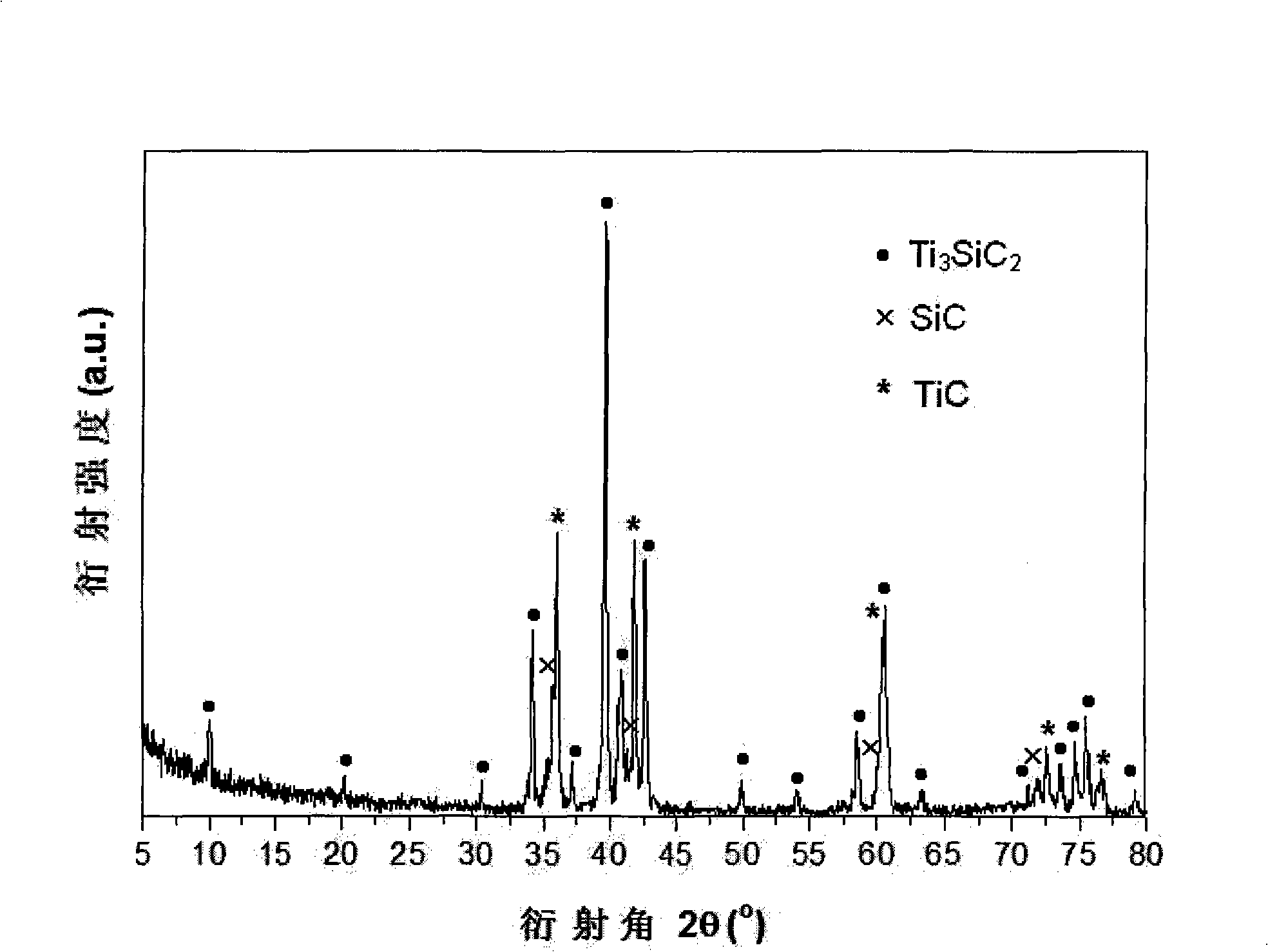
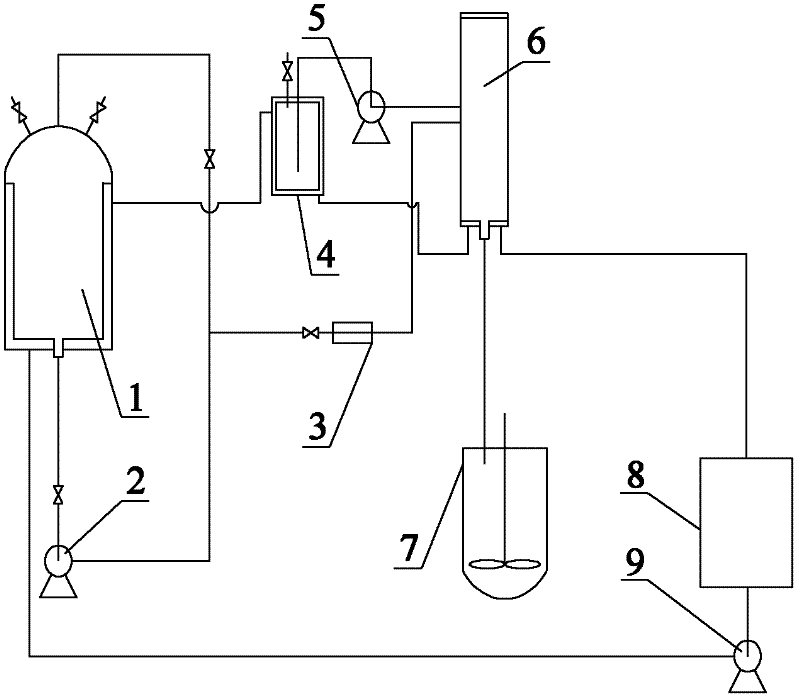
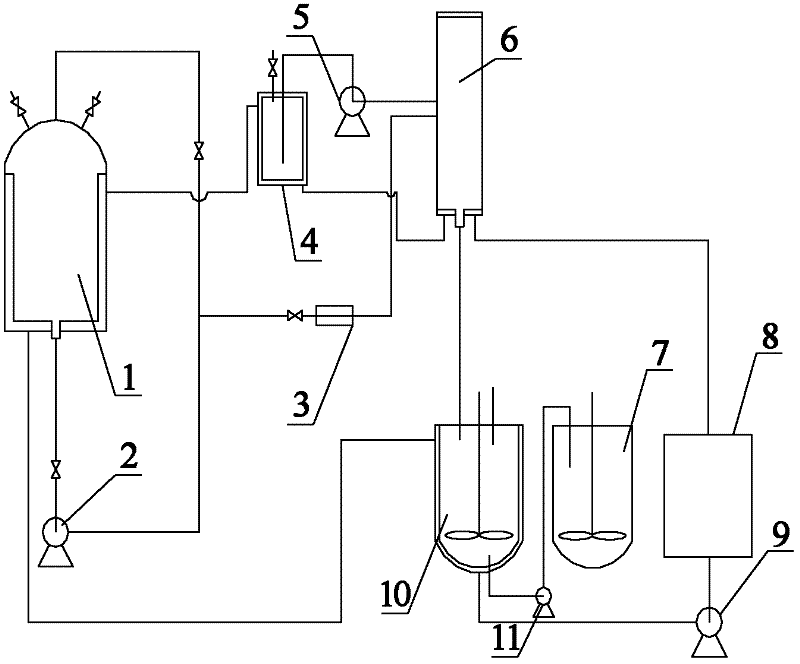
Method for preparing SiC/Ti3SiC2 with substitution reaction hot press in situ
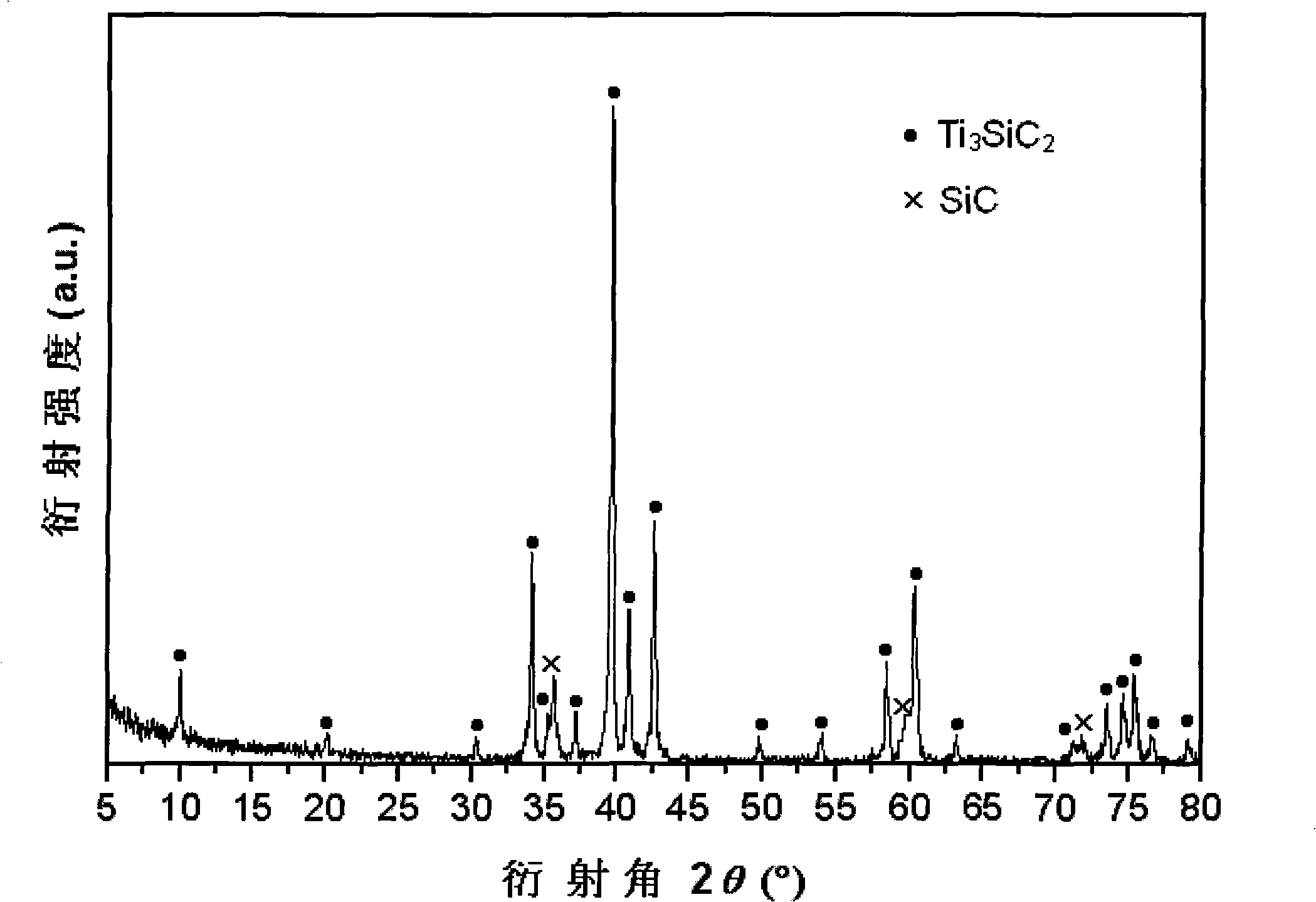
The present invention provides a composite material SiC / Ti3SiC2 that is synthesized by displacement reaction of original positions, and a hot-pressing preparation method thereof. Si powder and TiC powder are used as raw materials; Al is used as an additive for the reaction; the molar ratio between Si, TiC and Al is equal to 2 to 3 to from 0.2 to 1; after being mixed for 10 hours in a dry way, the mixed materials are arranged in a graphite die that is then arranged in a hot-pressing furnace; with the protection of argon gas, the temperature of the furnace is raised to be between 1350 and 1550 DEG C at the rate of 20 to 50 DEG C per minute; at the same time, the pressure of 20 to 40MPa is exerted in the die; the temperature is maintained for 1 to 4 hours; thus the composite material of SiC / Ti3SiC2 block can be prepared. The preparation method has the advantages of low costs of the composite material of synthesized SiC / Ti3SiC2, no phase of impurities of TiC, tiny particles of SiC that are evenly distributed in the matrix, stable technological parameters and suitability for large-scale production.
Owner:BEIJING JIAOTONG UNIV
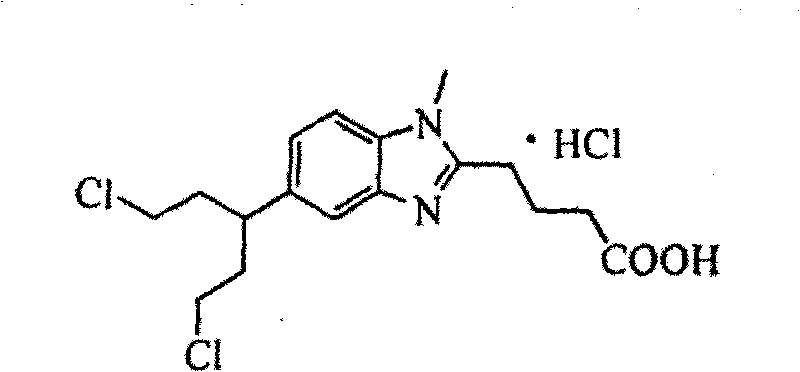
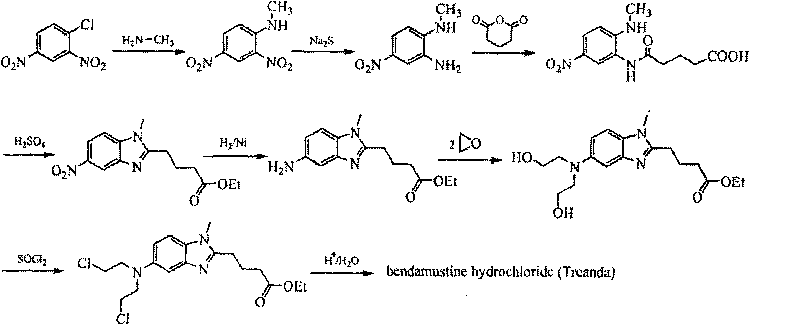
Method for synthesizing highly-pure bendamustine hydrochloride
ActiveCN101691359AComply with drug quality standardsShort synthesis cycleOrganic chemistryBendamustine hydrochlorideEthylene oxide
The invention discloses a method for synthesizing highly-pure bendamustine hydrochloride, which comprises the following steps: taking [1-methy-2(4'-ethyl butyrate)-5-amino]-1H-benzimidazole as a raw material; and orderly reacting the [1-methy-2(4'-ethyl butyrate)-5-amino]-1H-benzimidazole with ethylene oxide and phosphorus oxychloride through the four steps of substitution, hydrolysis, salification and refinement so as to produce the bendamustine hydrochloride. The method in the invention is mild in condition, simple in operation, short in synthesis period and suitable for scaled-up industrial production; and the purity of the synthesized bendamustine hydrochloride is over 99.5 percent, while monomeric impurity is below 0.1 percent, which meet the quality standards of raw material medicament.
Owner:深圳万乐药业有限公司
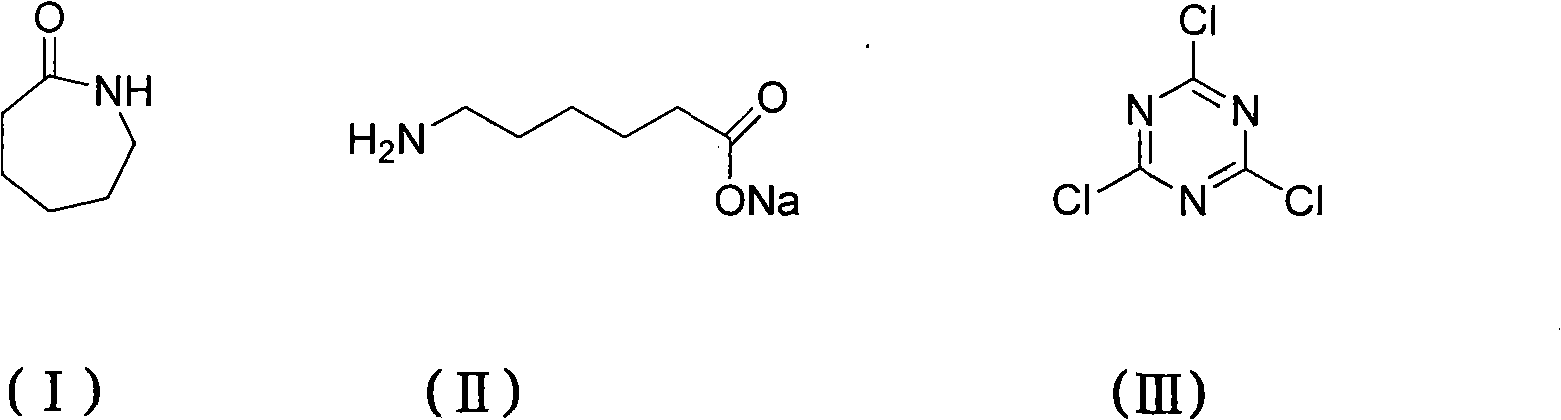
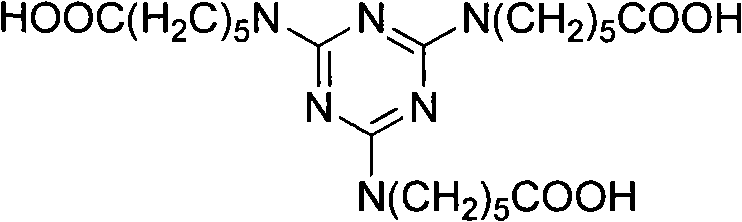
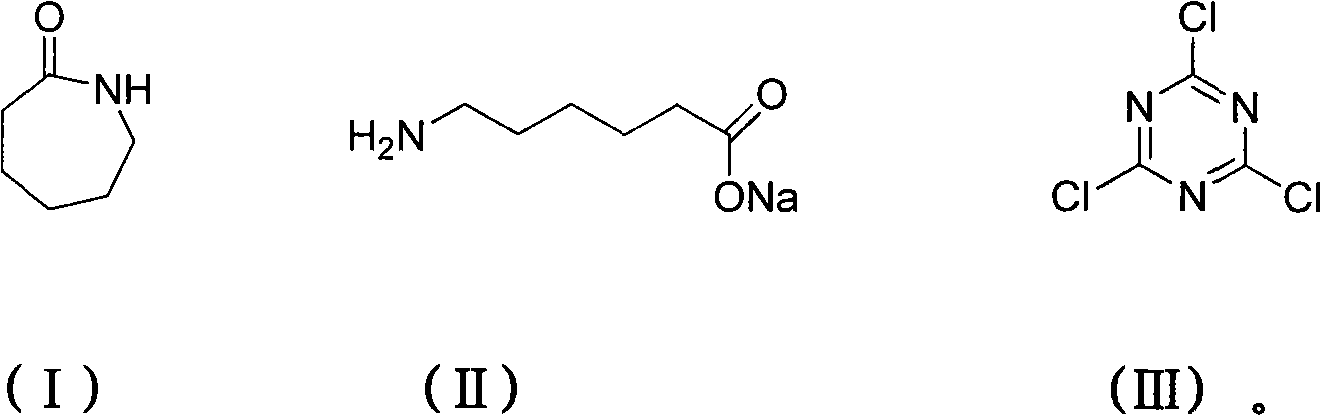
Method for preparing 2,4,6-tri(amino caproyl)-1,3,5-triazine
InactiveCN101973949AThe reaction is easy to operateMild reaction conditionsOrganic chemistryCarboxylic acidSolvent
The invention discloses a method for preparing an organic ternary polycarboxylic acid dustless antirust additive 2,4,6-tri(amino caproyl)-1,3,5-triazine by adopting a one-pot method, which comprises the following steps of: ring-opening caprolactam with alkali to generate carboxylate of aminocaproic acid first; then performing a substitution reaction by the carboxylate of aminocaproic acid and cyanuric chloride; and finally, acidizing with hydrochloric acid to prepare derivatives of organic ternary polycarboxylic acid. The preparation method is free from a toxic organic solvent and employs common water as a solvent for reaction. The preparation method ha the advantages of simple process operation, low production cost and low environmental pollution and is more suitable for large scale industrial production.
Owner:TIANJIN NORMAL UNIVERSITY
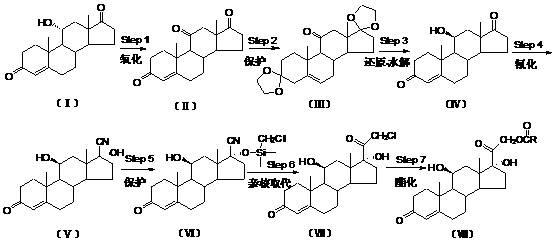
Preparation method for betamethasone intermediate or its analogue
ActiveCN103641878AStarting materials are readily availableHigh and stable yieldSteroidsSNiBetamethasone
The invention relates to a preparation method of a steroid hormone medicinal intermediate, in particular to a preparation method for a betamethasone intermediate or its analogue. The betamethasone intermediate or its analogue is prepared from a compound I by means of elimination reaction, methylation reaction, cyano-group substitution reaction, siloxy protective reaction, intramolecular nucleophilic substitution reaction and esterification reaction. The method provided by the invention has the characteristics of cheap raw materials, and high and stable yield. The reaction route is as the following, wherein R1 is Cl or Br, and R is H or hydrocarbonyl of C1-C10.
Owner:HUNAN NORCHEM PHARMACEUTICAL CO LTD
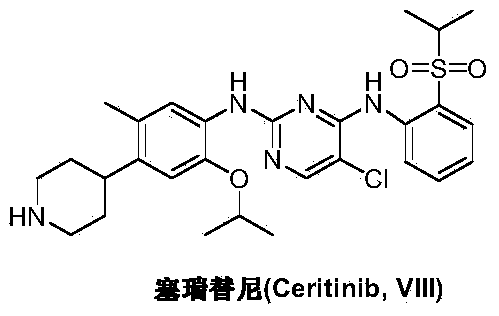
Preparation method of ceritinib and intermediate
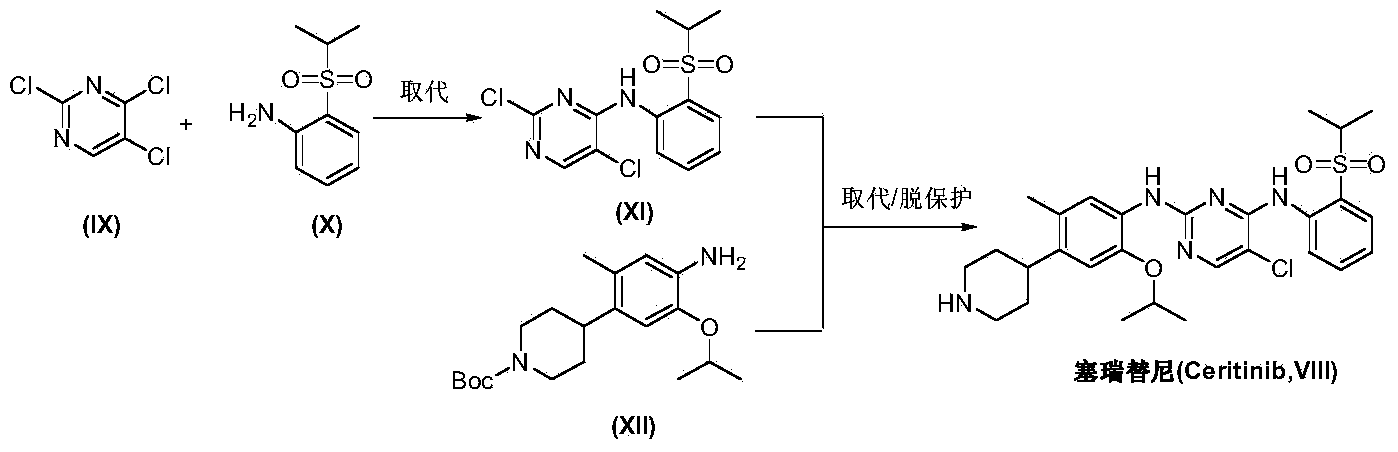
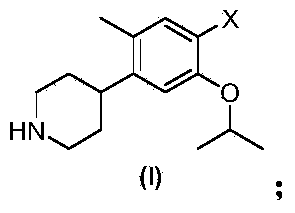
The invention discloses an intermediate 2-isopropoxy-5-methyl-4-(piperidyl-4-yl)halogeno-benzene (I) for preparing ceritinib and a preparation method thereof. The preparation method comprises the following steps: carrying out catalytic hydrogenation on the raw material 4-(5-isopropoxy-2-methyl-4-nitrophenyl)pyridine (II) to obtain 2-isopropoxy-5-methyl-4-(piperidyl-4-yl)aniline (III); and carrying out Sandmeyer reaction on the compound (III) to obtain the 2-isopropoxy-5-methyl-4-(piperidyl-4-yl)halogeno-benzene (I). The invention also discloses a preparation method of ceritinib. The 2-isopropoxy-5-methyl-4-(piperidyl-4-yl)halogeno-benzene (I) used as the raw material is sequentially subjected to substitution, reduction and substitution reaction to obtain the ceritinib (VIII). The preparation method has the advantages of simple technique, mild conditions and fewer side reactions, and is suitable for industrial amplification.
Owner:SCI GENERAL MATERIAL & CHEM
Fluoride alkoxycyclotriphosphazene derivative, preparation and use thereof
InactiveCN101407527AExcellent flame retardant finishing effectImprove flame retardant performanceGroup 5/15 element organic compoundsFibre treatmentAlcoholEmulsion
The invention discloses a fluorine-containing alkoxy ring triphosphonitrile derivative and a preparing method thereof, belonging to the technical field of macromolecule synthesis and textile finishing, and the derivative is used for a multifunctional finishing agent of textile. The method takes fluorine-containing alcohol as a material for reacting with hexachlor ring triphosphonitrile for substitution to synthesize the fluorine-containing alkoxy ring triphosphonitrile derivative; and the fluorine-containing alkoxy ring triphosphonitrile derivative is emulsified by choosing a suitable emulsifier and optimizing emulsion formula, thus obtaining the multifunctional finishing agent of textile with good stability. By adopting the finishing process of rolling-baking-toasting, the textile after finishing has not only good flame-retardant finishing effect but also certain water proof and oil proof effect owning to the introduction of fluoride in the phosphonitrile flame retardant.
Owner:SUZHOU UNIV
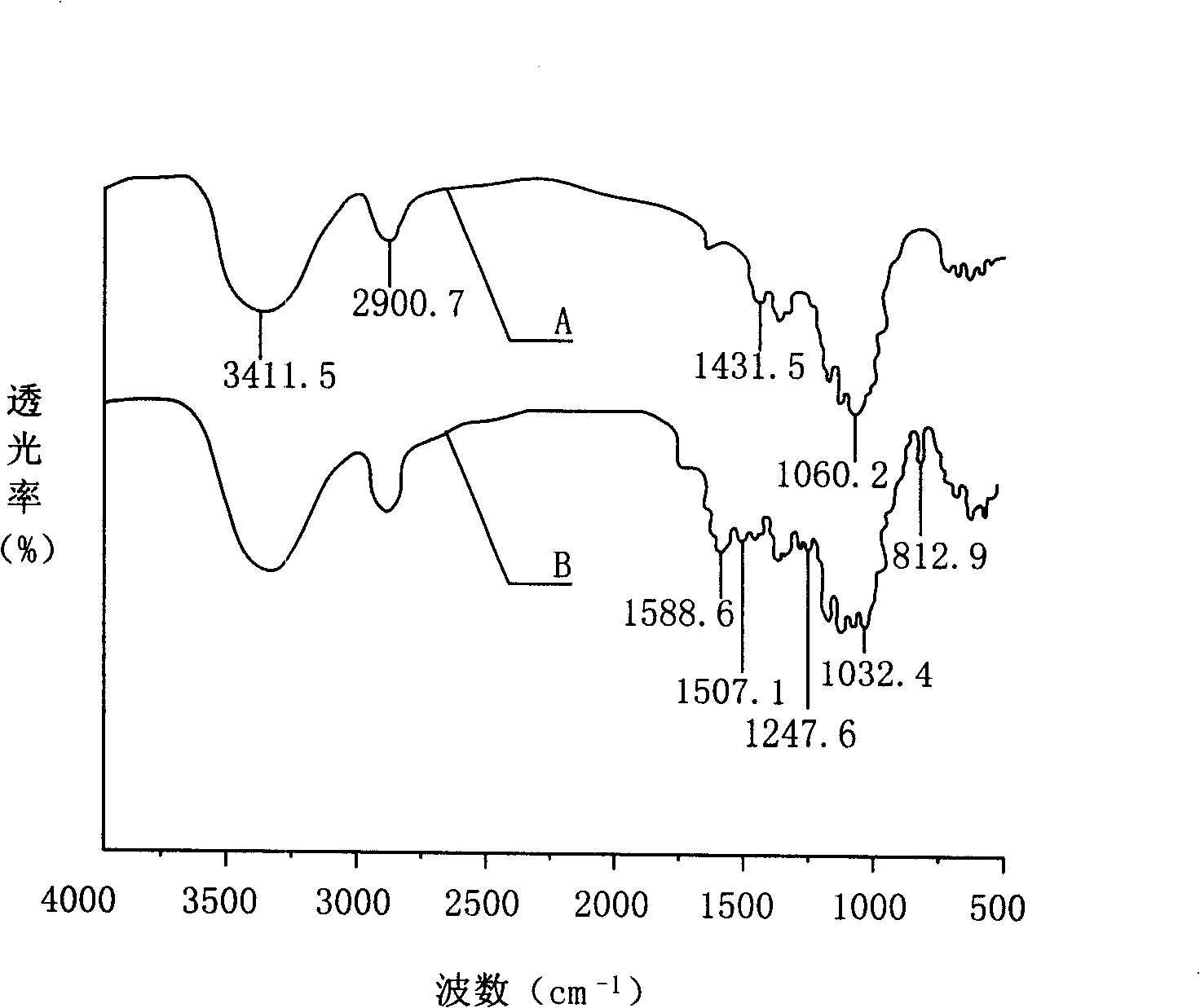
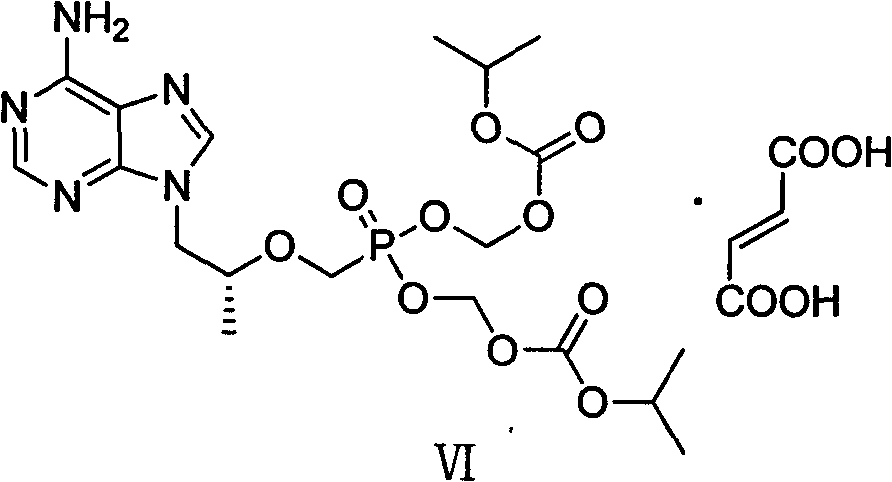
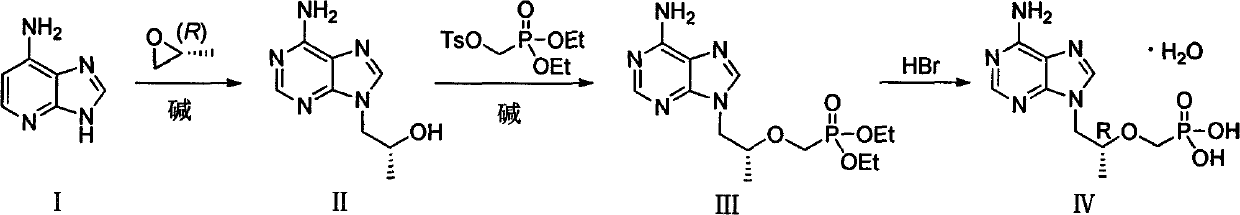
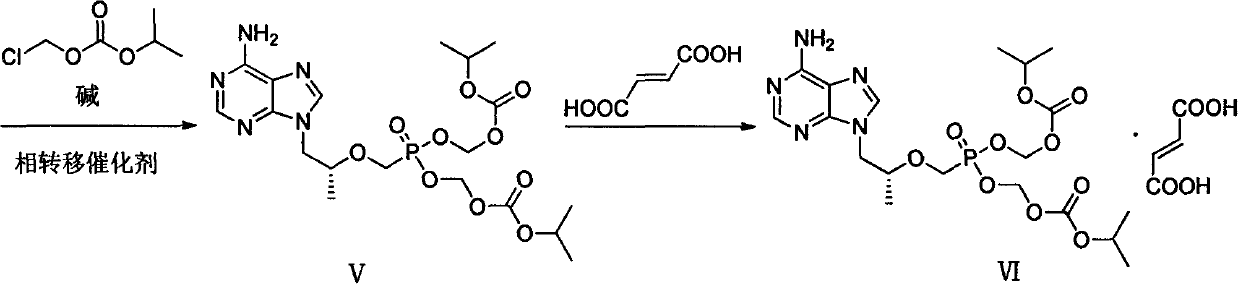
Preparation method of antiviral medicine
ActiveCN103374038ARaw materials are easy to getSimple process routeGroup 5/15 element organic compoundsCarboxylic acid salt preparationAntiviral drugBenzenesulfonates
The invention discloses a preparation method of anantiviral medicine tenofovirdisoproxil fumarate. The preparation method disclosed by the invention comprises the following steps of: performing addition reaction on adenine serving as raw material and (R)-epoxypropane in the presence of alkali; then, performing substitution reaction with (diethyoxyl phosphoracyl) methyl-4-methyl benzenesulfonate; then, hydrolyzing by using a hydrobromic acid solution; crystallizing to obtain tenofovir monohydrate; and reacting the product tenofovir monohydrate with chloromethyl isopropyl carbonate and fumaric acid to obtain tenofovirdisoproxil fumarate. The selected initial raw material is low in cost and easily available, and the synthetic line is simplified and the utilization ratio of the raw material and the total yield are improved. The intermediate obtained in the reaction is purified by the recrystallization method, so that the yield is high, less three-wastes are generated in the reaction process, and the cost is low; therefore, the preparation method is favorable for industrial production.
Owner:GUANGZHOU BAIYUNSHAN PHARMA HLDG CO LTD BAIYUNSHAN PHARMA GENERAL FACTORY
Method for preparing brominated butyl rubber
InactiveCN102382223ADwell time is easy to controlGood microscopic mixing effectPolymer scienceBromine
The invention discloses a method for preparing brominated butyl rubber, and belongs to the technical field of brominated butyl rubber. The method comprises the following steps that raw materials of a brominated butyl rubber solution and a bromine solution are added into a reinforced mixing reactor according to a ratio and undergo a replacement reaction of bromine on an allyl group, wherein stay time of the raw materials in the reinforced mixing reactor is controlled; and the products obtained by the previous step are fed into a stirring tank, are washed, are added with a stabilizing agent, are subjected to solvent flash evaporation, and are dried to form brominated butyl rubber products. The method realizes high-efficiency, large-scale, continuous and stable preparation of a brominated butyl rubber product having bromine content of 1.0 to 2.3 wt% and unsaturation of 0.6 to 1.6%, greatly reduces stay time, greatly reduces a reactor volume, and reduces a total operation cost.
Owner:BEIJING UNIV OF CHEM TECH +1
Dual functions ligand compound of chirality dioxazoline, preparation and application
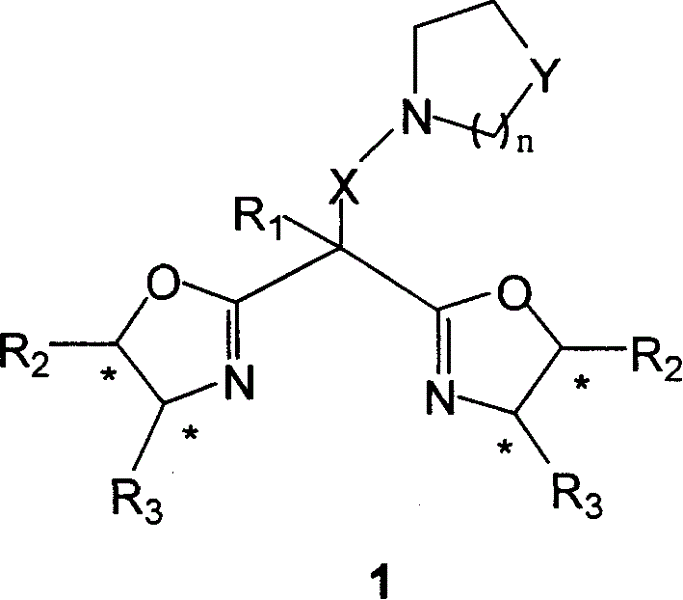
InactiveCN1626524AEasy to getEasy to manufactureOrganic chemistryOrganic-compounds/hydrides/coordination-complexes catalystsChemical compoundSubstitution reaction
A bifunctional chiral bioxazoline ligand used as the catalyst for asymmetrical reactions, such as cyanosiliconizing reaction, is prepared from propylbinitrile or its derivative through substitution reaction and cyclizing reaction on chiral aminoalcohol, or from the bioxazoline methane through substitution reaction.
Owner:PEKING UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com