Preparation method for betamethasone intermediate or its analogue
A technology of betamethasone and its analogs, which is applied in the field of preparation of steroid hormone drug intermediates, can solve the problems of high cost and long reaction route, and achieve the effects of stable yield, high yield and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
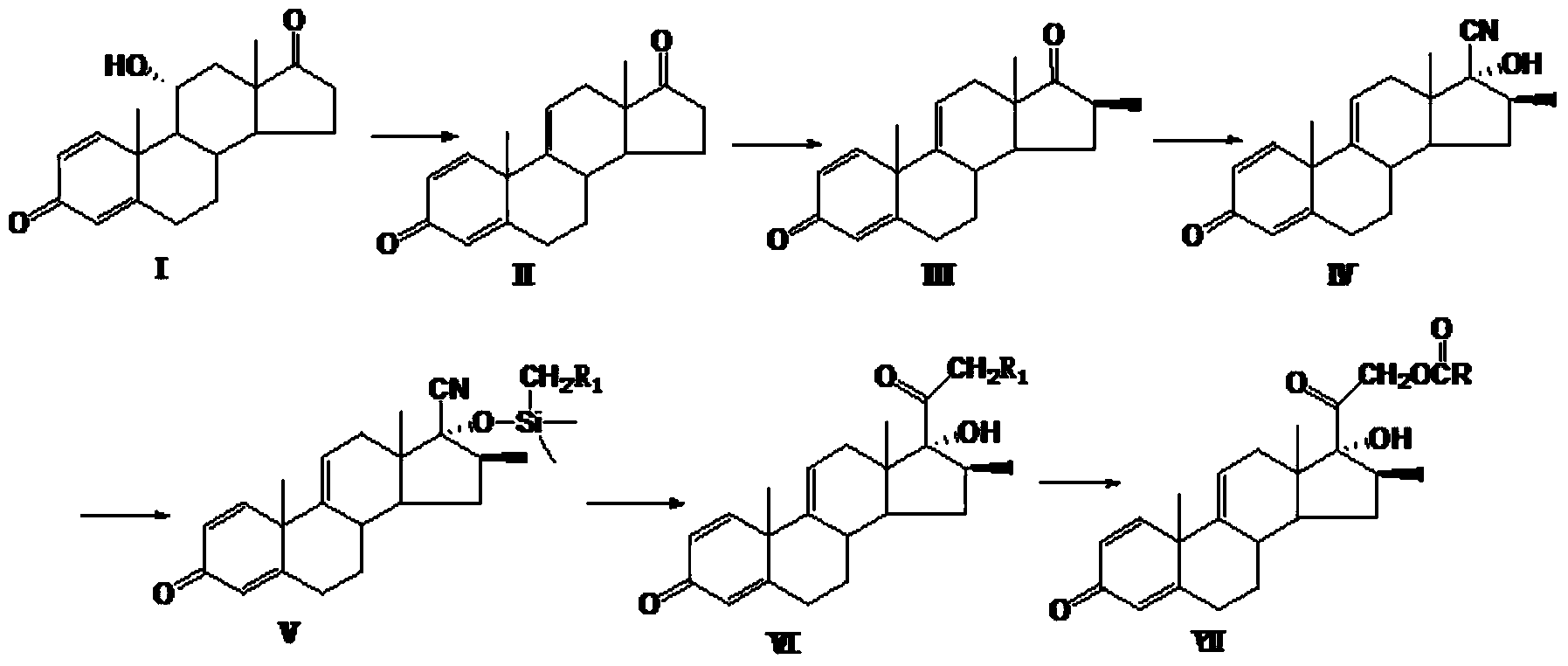
[0018] Elimination reaction
[0019] At room temperature, under the protection of nitrogen, add 400ml of anhydrous tetrahydrofuran to a clean and dry 500ml four-neck round-bottomed flask equipped with a thermometer, reflux condenser, and mechanical stirring, and add 80.0g of compound Ⅰ under stirring, and cool the system to 0 to 10°C , add 80.0 g of phosphorus pentachloride in batches at 0 to 10°C under temperature control, and keep warm at 0 to 10°C after the addition is complete. TLC followed the reaction until no raw material remained. The reaction system was added dropwise to 1 L of ice water, a large amount of solids were precipitated, and the stirring was continued for 2 hours. The system was suction-filtered, the filter cake was washed with water until it was neutral, and dried at 50°C to obtain 70.8 g of compound II with a yield of 88.5%. 96.8%.
[0020] methylation reaction
[0021] At room temperature, under the protection of nitrogen, weigh 50.0g of compound II ...
Embodiment 2
[0032] Elimination reaction
[0033] At room temperature, under the protection of nitrogen, add 450ml of anhydrous tetrahydrofuran to a clean and dry 500ml four-necked round-bottomed flask equipped with a thermometer, reflux condenser, and mechanical stirring, and add 80.0g of compound Ⅰ under stirring, and cool the system to 0 to 10°C , add 80.0 g of p-toluenesulfonyl chloride in batches under temperature control at 0 to 10°C, and keep warm at 0 to 10°C after the addition is complete. TLC followed the reaction until no raw material remained. The reaction system was added dropwise to 1 L of ice water, a large amount of solids were precipitated, and the stirring was continued for 2 hours. The system was suction-filtered, the filter cake was washed with water until it was neutral, and dried at 50°C to obtain 69.2 g of compound II with a yield of 86.5%. 96.2%.
[0034] methylation reaction
[0035] At room temperature, under the protection of nitrogen, weigh 50.0g of compound...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com