Preparation method of 3-(4-methoxyphenylthio)-1-methyl-1H-pyrrolyl-2,5-dione compound
A technology of methoxyphenylthio and methoxythiophenol, applied in the field of pharmaceutical intermediates and their preparation, can solve the problems of high price, excessively long reaction route, many by-products, etc., and achieves easy industrial production and reaction Simple operation and the effect of shortening the reaction time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
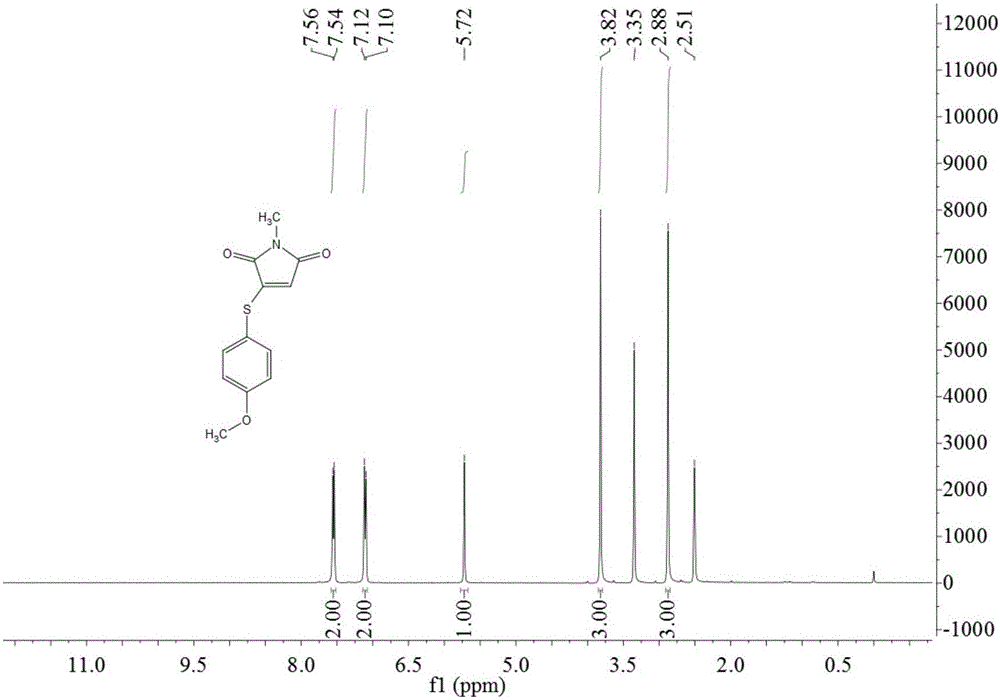
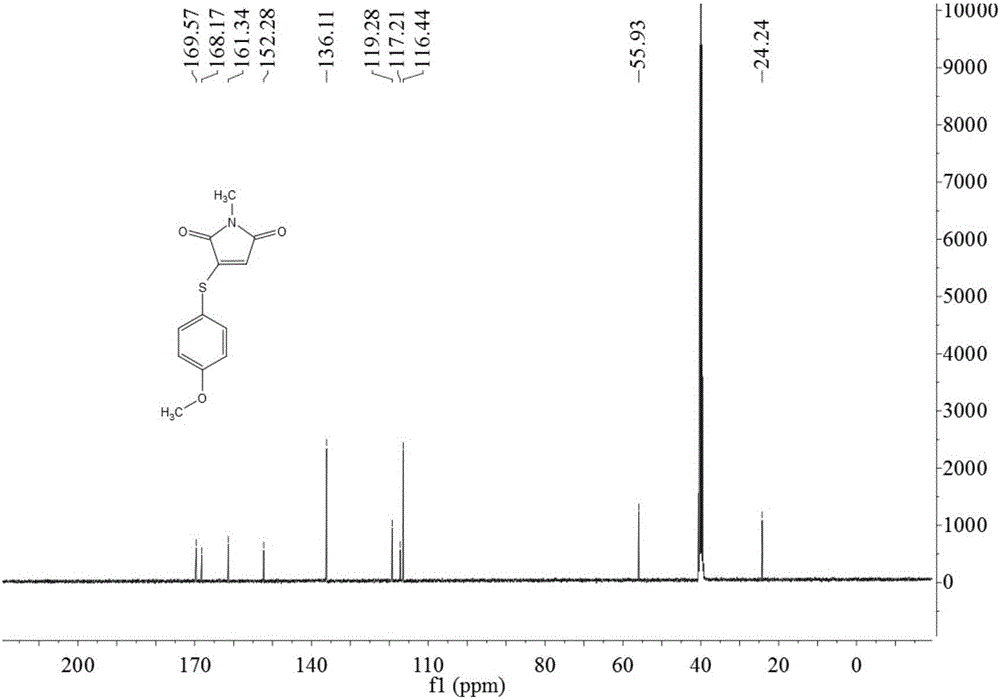
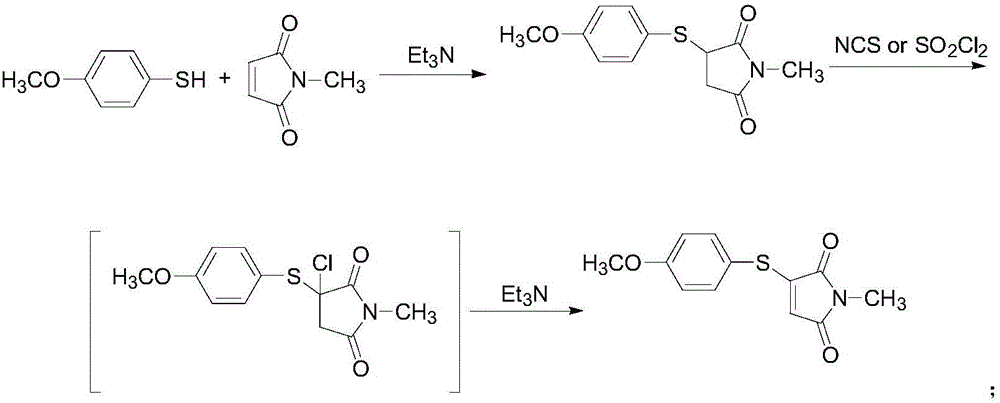
[0038] Take 14.0g (0.1mol) of 4-methoxythiophenol, 11.1g (0.1mol) of N-methylmaleimide, 1.90g (0.01mol) of cuprous iodide, and fluoboric acid (content 40%) Add 21.9g (0.1mol) into a 250mL round bottom flask, then add 100mL of DMSO, heat to 120°C and stir for 8h, after the reaction is complete, add 50mL of water, stir for 5 minutes, extract with ethyl acetate 300mL×3, and use anhydrous sodium sulfate for the organic phase After drying, the solvent was evaporated, and the obtained solid was recrystallized with 95% ethanol to obtain 16.0 g of yellow solid 3-(4-methoxyphenylthio)-1-methyl-1H-pyrrole-2,5-dione, yield 64.5%, mp: 96-98°C.
Embodiment 2
[0040] Take 35.0g (0.25mol) of 4-methoxythiophenol, 33.3g (0.30mol) of N-methylmaleimide, 13.3g (0.07mol) of cuprous iodide, and fluoboric acid (content 40%) Add 55.0g (0.25mol) to a 500mL round bottom flask, then add DMSO 200mL, heat to 150°C and stir for 8h, after the reaction is complete, add 200mL of water, stir for 5 minutes, extract with 500mL×3 ethyl acetate, and use anhydrous sulfuric acid for the organic phase The sodium was dried, the solvent was evaporated, and the obtained solid was recrystallized with 95% ethanol to obtain 53.8 g of a yellow solid 3-(4-methoxyphenylthio)-1-methyl-1H-pyrrole-2,5-dione. Rate 86.5%, mp: 95~97℃.
Embodiment 3
[0042]Take 10.5g (0.075mol) of 4-methoxythiophenol, 16.6g (0.15mol) of N-methylmaleimide, 14.2g (0.075mol) of cuprous iodide, and fluoboric acid (content 40%) Add 33.0g (0.15mol) into a 250mL round bottom flask, then add 120mL dimethylacetamide, heat to 120°C and stir for 8h, after the reaction is complete, add 100mL of water, stir for 5 minutes, extract with 150mL×3 ethyl acetate, organic phase Dry with anhydrous sodium sulfate, evaporate the solvent, and recrystallize the obtained solid with 95% ethanol to obtain a yellow solid 3-(4-methoxyphenylthio)-1-methyl-1H-pyrrole-2,5-dione 13.6g, yield 73.1%, mp: 96~99℃
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com