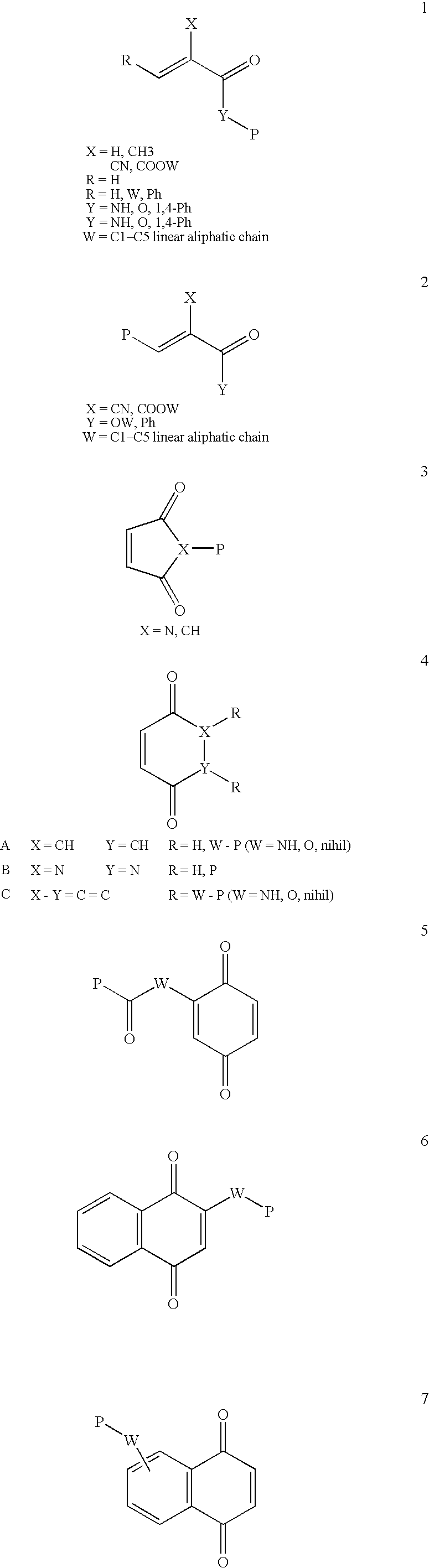
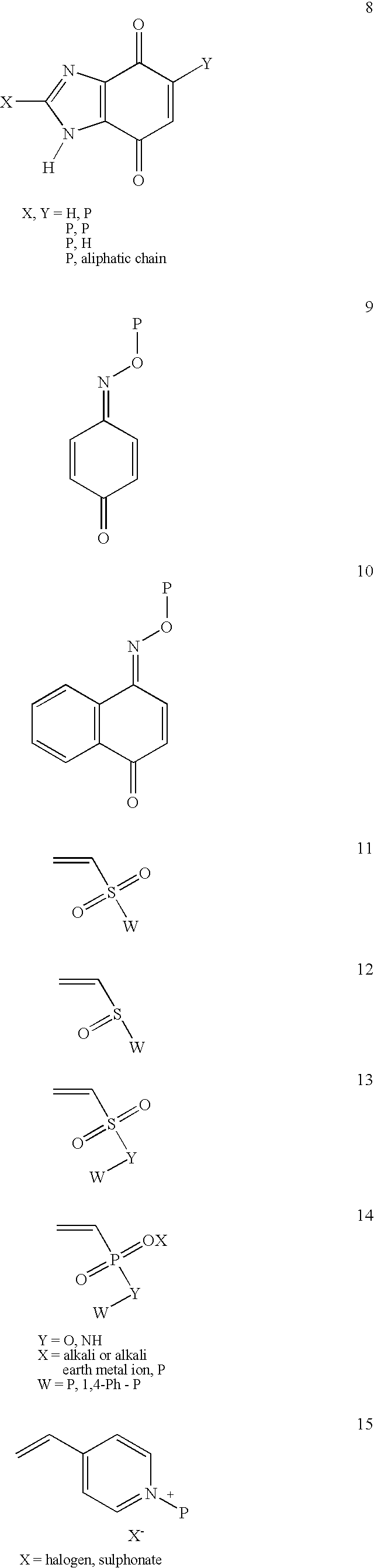
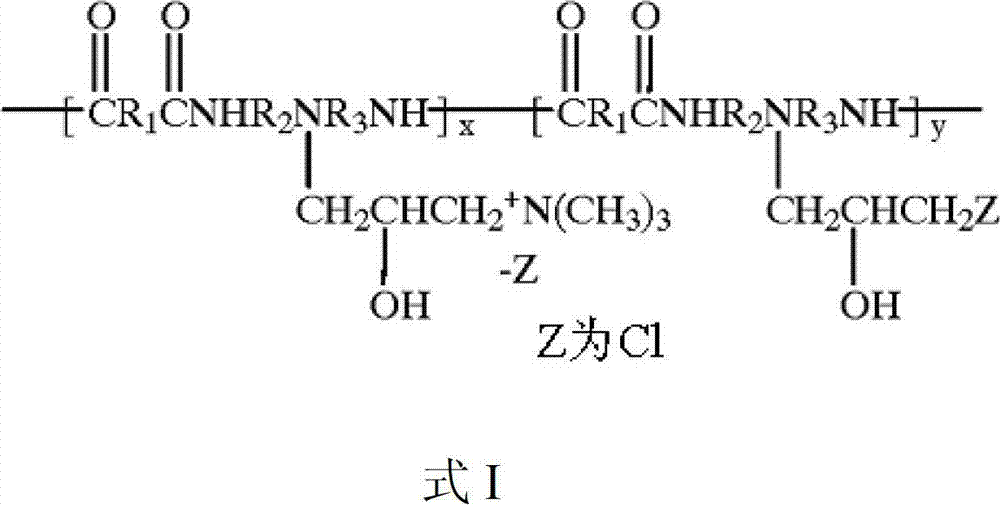
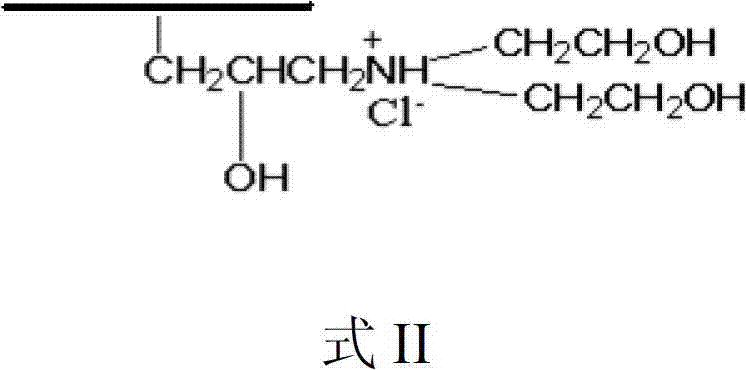
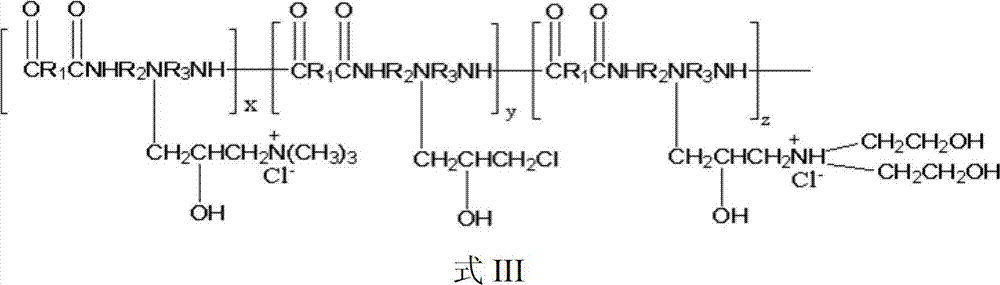
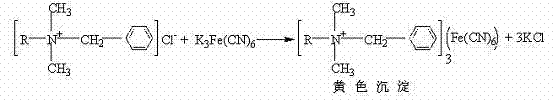
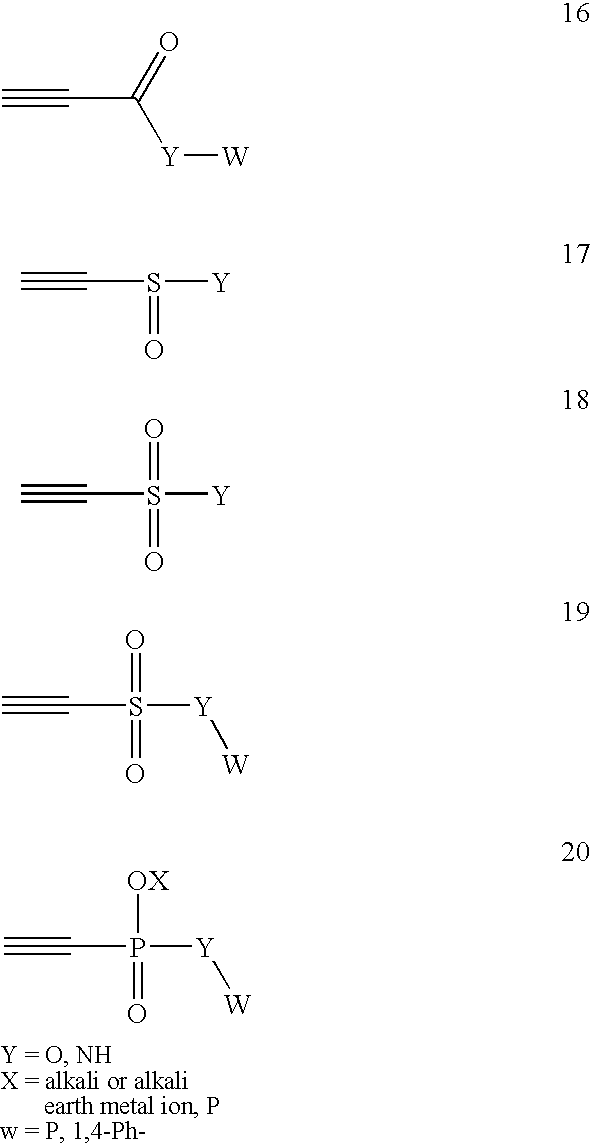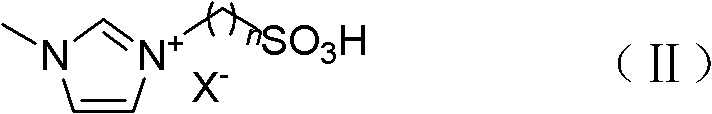Patents
Literature
535 results about "Nucleophilic addition" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
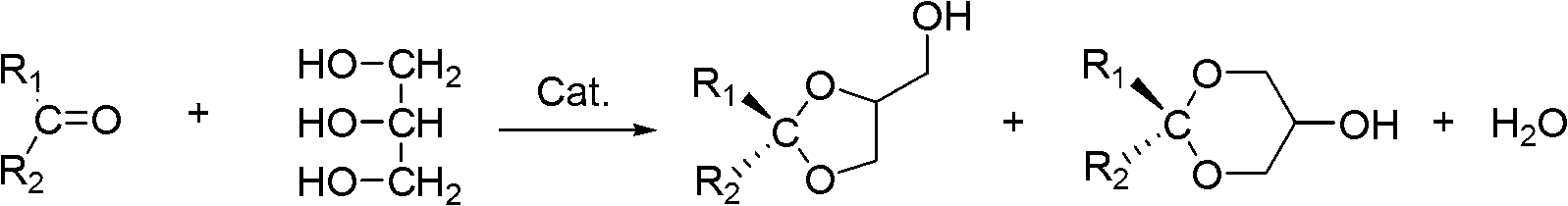
In organic chemistry, a nucleophilic addition reaction is an addition reaction where a chemical compound with an electron-deficient or electrophilic double or triple bond, a π bond, reacts with electron-rich reactant, termed a nucleophile, with disappearance of the double bond and creation of two new single, or σ, bonds. The reactions are involved in the biological synthesis of compounds in the metabolism of every living organism, and are used by chemists in academia and industries such as pharmaceuticals to prepare most new complex organic chemicals, and so are central to organic chemistry. Addition reactions require the presence of groups with multiple bonds in the electrophile(due to the fact that double bonds and even triple bonds can both lack electron rich sources): carbon–heteroatom multiple bonds as in carbonyls, imines, and nitriles, or carbon–carbon double or triple bonds. The lack of electron rich sources is due to the fact that these bonds are partially empty, even though they remain connected, since the region occupying the orbital is essentially dead. This electrophilic behavior is defined as empty space since everything inside is basically without any source of electricity except from outside the bond, since bonds tend to want to attract more to themselves(whether this be electric or non-electric can differ in most situations). The addition of the nucleophile means the continuous addition of a negative charge throughout the reaction, unless an electrophile also makes itself present to form a complete structure with no charge at all. The negative charge being continuous throughout the reaction until the formation of an intermediate, bearing the charge, thus is the addition reaction we have before us. Once this meets an electrophile, then the intermediate formed with the negative charge can thus be neutralized to form a complete structure via a type of bond.
Conjugate addition reactions for the controlled delivery of pharmaceutically active compounds
InactiveUS6958212B1Reducing and delaying onsetGood water solubilitySugar derivativesPeptide/protein ingredientsBiological materialsPolymer
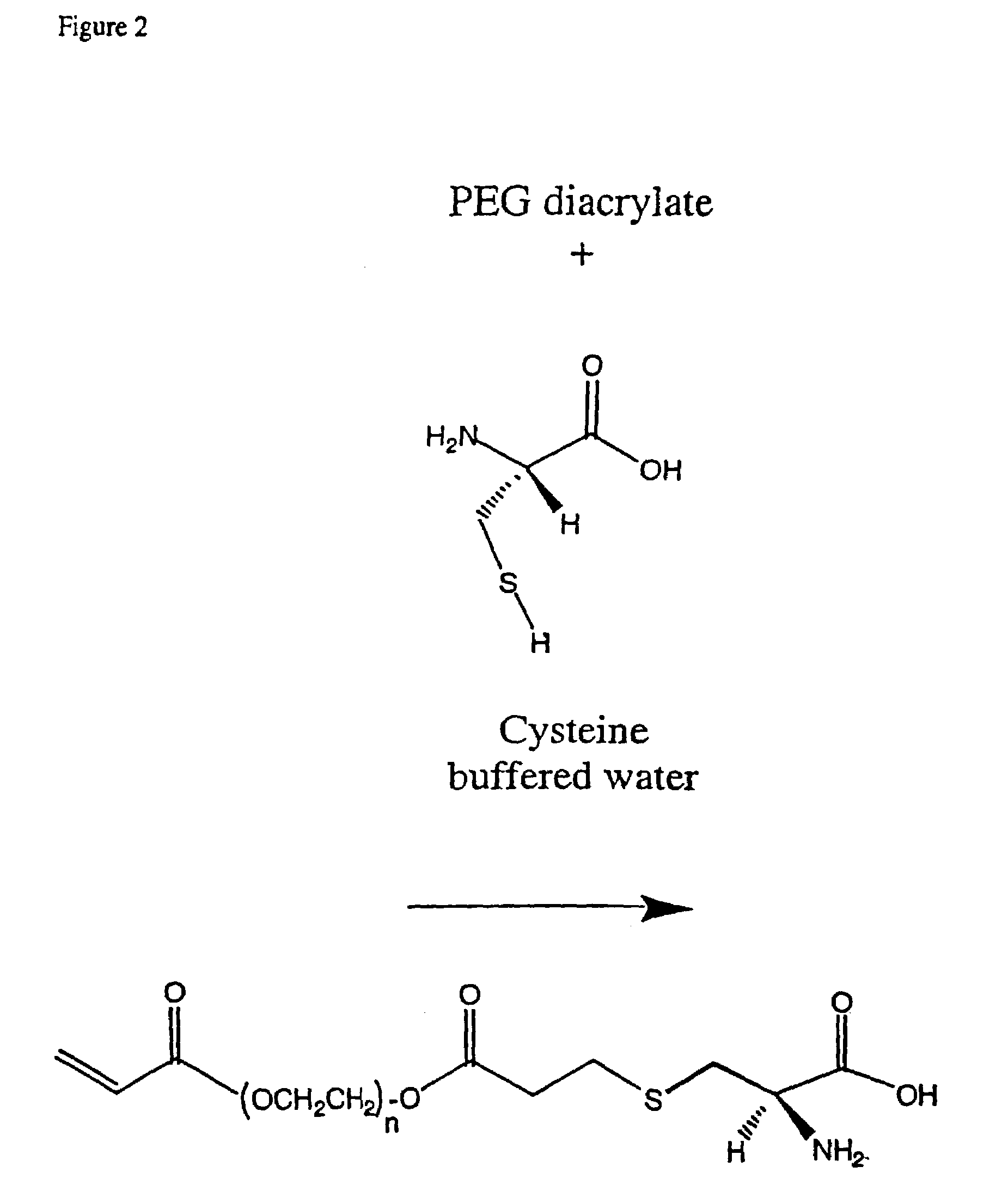
The invention features polymeric biomaterials formed by nucleophilic addition reactions to conjugated unsaturated groups. These biomaterials may be used for medical treatments.
Owner:ETH ZZURICH +1
Bioprosthetic tissue preparation with synthetic hydrogels
ActiveUS20050119736A1Reduce calcificationReduce stiffnessSuture equipmentsHeart valvesCross-linkIn situ polymerization
Methods for treating xenogenic tissue for implantation into a human body including in-situ polymerization of a hydrogel polymer in tissue, and tissue treated according to those methods, where the polymerization takes place in tissue that has not been fixed with glutaraldehyde. The polymerization may only fill the tissue, bind the polymer to the tissue, or cross-link the tissue through the polymer, depending on the embodiment. One method includes free radical polymerization of a first vinylic compound, and can include cross-linking through use of a second compound having at least two vinyl groups. Another method utilizes nucleophilic addition polymerization of two compounds, one of which can include PEG and can further include hydrolytically degradable regions. In one embodiment, applicants believe the in-situ polymerization inhibits calcification, and that the polymerization of tissue un-fixed by glutaraldehyde allows for improved penetration of the polymer. The methods find one use in the treatment of porcine heart valve tissue, intended to extend the useful life of the valves by inhibiting calcification. The incorporation of degradable hydrogel regions may initially fill the tissue and reduce any initial inflammatory response, but allow for later infiltration by cells to remodel the tissue.
Owner:MEDTRONIC INC
Conjugate addition reactions for the controlled delivery of pharmaceutically active compounds
InactiveUS7291673B2Reducing and delaying onsetGood water solubilityBiocideSurgical adhesivesPolymerControlled delivery
The invention features polymeric biomaterials formed by nucleophilic addition reactions to conjugated unsaturated groups. These biomaterials may be used for medical treatments.
Owner:UNIV ZURICH +1
Melt-blended protein composition
A melt-processed protein composition formed from a protein, plasticizer, and an electrophilic reagent is provided. The electrophilic reagent, for instance, may be selected to undergo a nucleophilic addition reaction with free sulfhydryl and / or thiyl radicals to help minimize the formation of disulfide crosslinking bonds that could otherwise lead to protein aggregation during melt processing. To enhance the degree to which the electrophilic reagent can limit crosslinking, a plasticizer is also employed that helps to mediate the adsorption of the electrophilic reagent into the internal structure of the protein, where it can be more stably retained. Furthermore, the temperature and shear rate employed during melt blending may also be selected to be relatively low to help limit polypeptide dissociation, thereby minimizing the impact of aggregation and embrittlement.
Owner:KIMBERLY-CLARK WORLDWIDE INC
Preparation method and application of magnetic silicon dioxide composite microsphere
ActiveCN103903827AEasy to separateSmall particle sizeMicrobiological testing/measurementInorganic material magnetismProtein targetMicrosphere
The invention relates to a preparation method and application of a magnetic silicon dioxide composite microsphere. Superparamagnetic ferroferric oxide nano particles of which the diameter ranges from 4 nm to 30 nm are prepared through a high-temperature pyrolysis method, a silicon dioxide shell of which the thickness ranges from 5 nm to 20nm covers the outer surfaces of the magnetic ferroferric oxide nano particles through a reverse microemulsion method, amination modification is conducted on the silicon dioxide surface, glutaraldehyde is used as a crosslinking arm, ligand protein is connected into, and protein separation is conducted through specific binding of ligand protein and target protein. The prepared magnetic microsphere is small in particle diameter and good in monodispersity, the composite microsphere with amine is large in specific surface area, nucleophilic addition is utilized, after the crosslinking arm glutaraldehyde is connected into, multiple kinds of ligand protein can be connected into, and then multiple kinds of target protein can be separated. The method is suitable for rapid separation and application of protein in biological samples, and has wide application prospect and great application value in the biomedical field and other fields.
Owner:HARBIN YICAI NEW MATERIAL
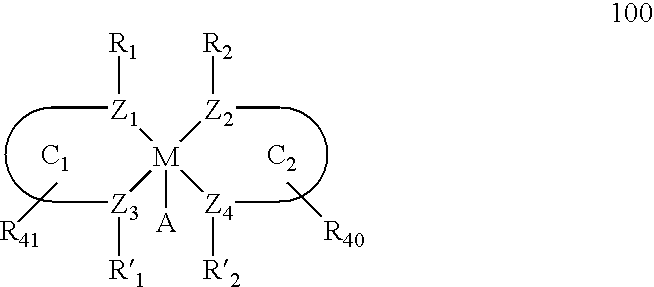
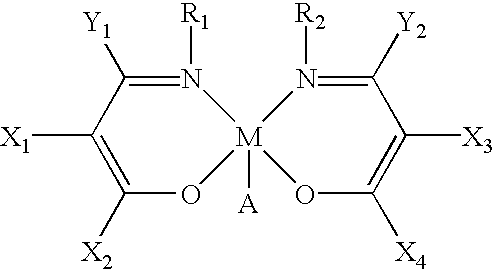
Main-group metal-based asymmetric catalysts and applications thereof
InactiveUS6844448B2Lactams preparationCarbamic acid derivatives preparationTetradentate ligandNucleophile
The present invention relates to a method and catalysts for the stereoselective addition of a nucleophile to a reactive π-bond of a substrate. The chiral, non-racemic catalysts of the present invention constitute the first examples of catalysts for nucleophilic additions that comprise a main-group metal and a tri- or tetra-dentate ligand.
Owner:PRESIDENT & FELLOWS OF HARVARD COLLEGE
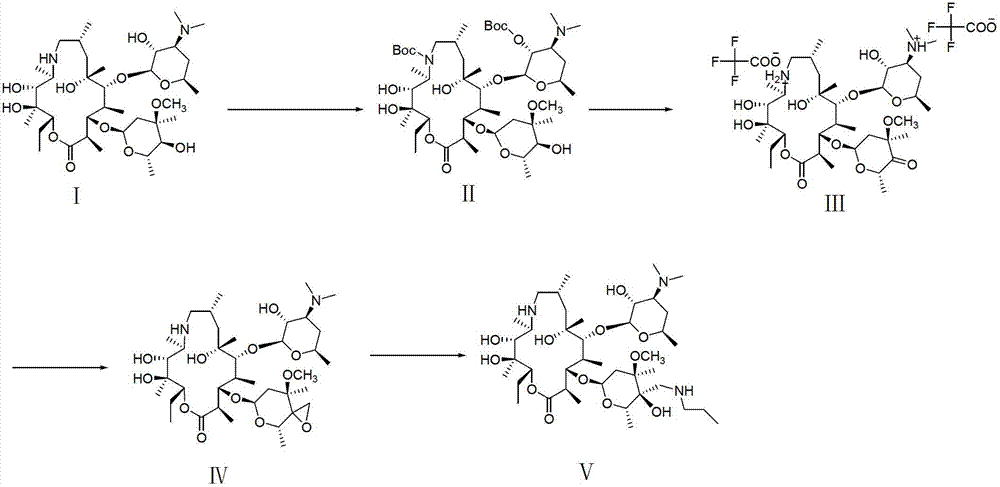
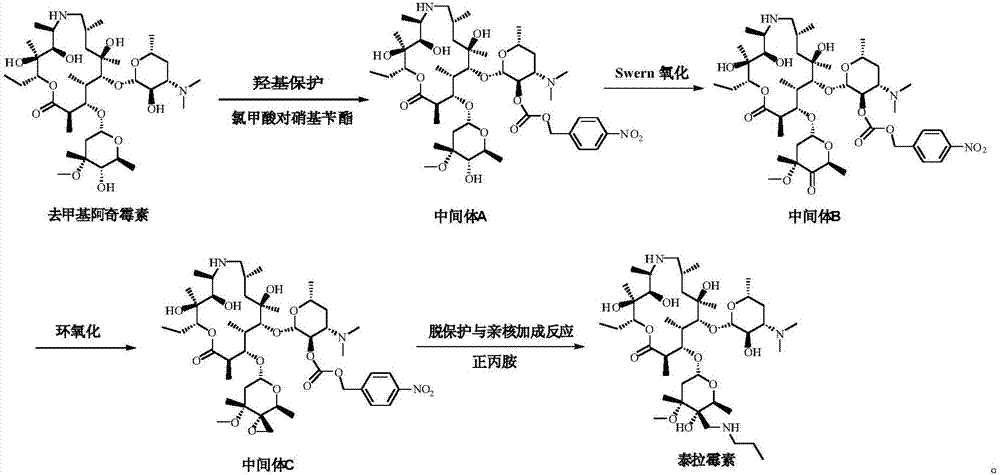
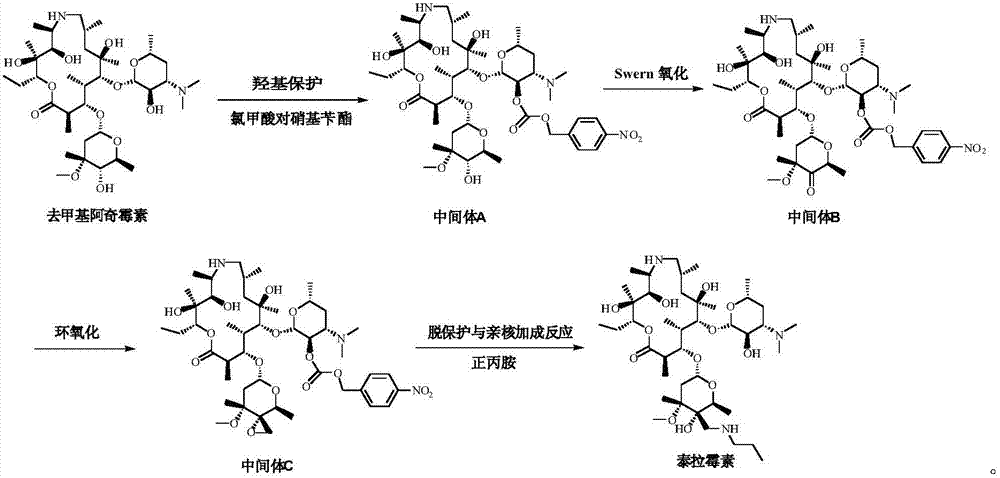
Tulathromycin intermediate and preparation method thereof, as well as preparation method of tulathromycin
ActiveCN102786569AReduce manufacturing costMild conditionsSugar derivativesSugar derivatives preparationEpoxyTert-Butyloxycarbonyl protecting group
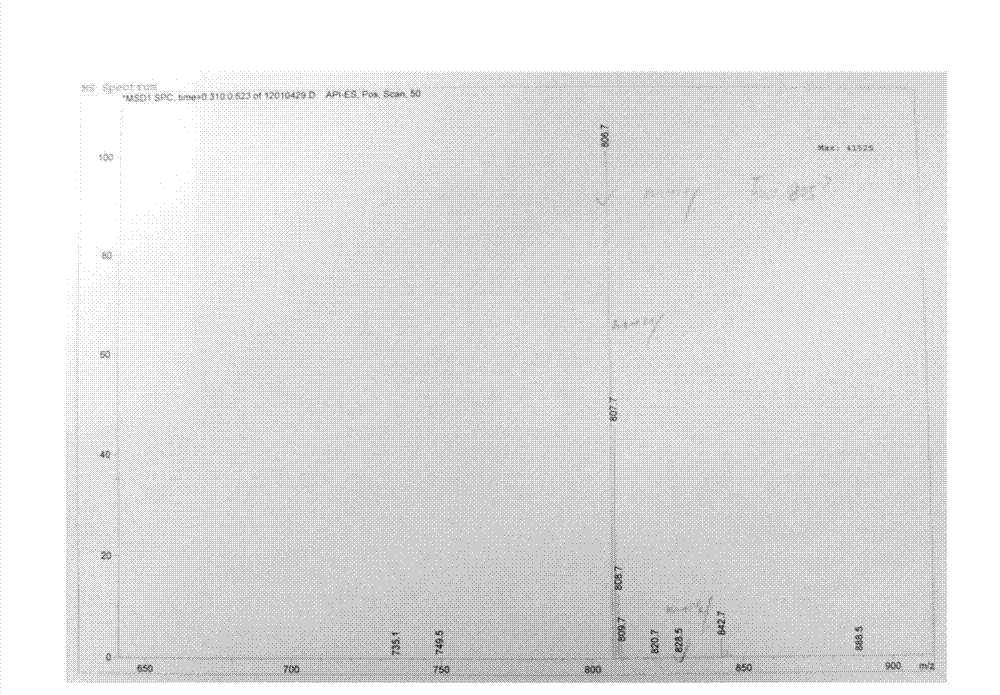
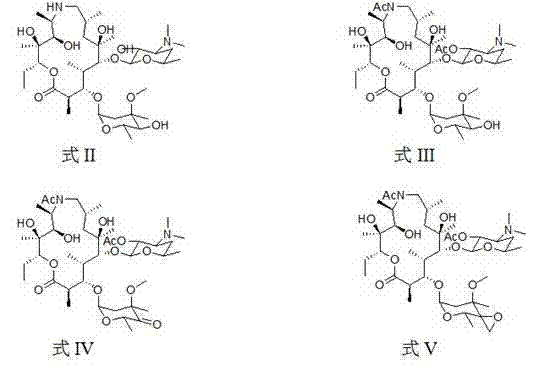
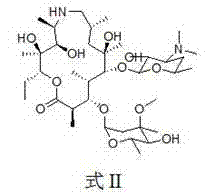
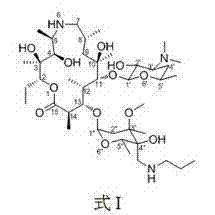
The invention provides a tulathromycin intermediate, a preparation method of the tulathromycin intermediate, and a preparation method of the tulathromycin. The preparation method of the tulathromycin has the advantages of mild condition, convenience for operation, and low cost. The preparation method of the tulathromycin comprises the following steps of: using azithromycin A as a raw material; protecting 2'-hydroxy and 6'-amino in the azithromycin A through di-tert-butyl dicarbonate so as to obtain double-protective azithromycin A; carrying out Swern oxidation to 4''-hydroxy to the double-protective azithromycin A; salifying along with trifluoroacetic acid; and synchronously removing boc t-butyloxycarbonyl to obtain the azithromycin A bitrifluoroacetic acid salt of 4''-carbonyl; and then reacting with trimethylsulfonium bromide to obtain 4''-epoxy compound; and finally carrying out nucleophilic addition on the 4''-epoxy compound by n-propylamine so as to obtain the phosphate of tulathromycin; and further neutralizing via alkaline to obtain the target compound tulathromycin; and synchronously obtaining the tulathromycin intermediate of azithromycin A bitrifluoroacetic acid salt of 4''-carbonyl.
Owner:TIANJIN ZHONGSHENG TIAOZHAN BIOTECH
A new method for preparing telamycin
ActiveCN102260306AAvoid catalytic hydrogenation methodsReduce manufacturing costSugar derivativesSugar derivatives preparationEpoxyAzithromycin
The invention discloses a novel method for preparing tulathromycin and relates to a semi-synthetic macrolide antibiotic. The method comprises the following steps: simultaneously protecting 2'-hydroxyl and 6a-amino of desmethyl azithromycin with acetyl, then carrying out oxidation and epoxidation on 4''-hydroxy, then removing the protecting groups under alkaline alcohol solution conditions, and carrying out nucleophilic addition on 4''-epoxy group with n-propylamine to obtain the target compound tulathromycin. Compared with the prior art, the method for preparing tulathromycin has the advantages of simple process, mild conditions, high yield and the like, and is beneficial to industrial production.
Owner:SHANDONG LUKANG SHELILE PHARMA
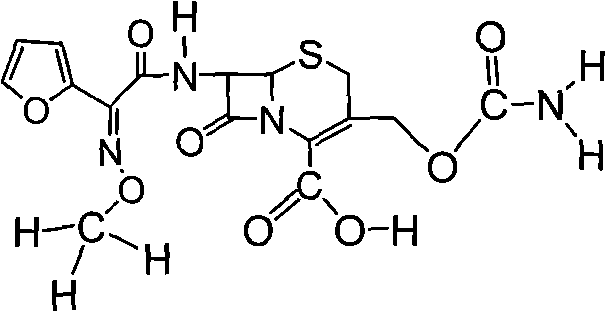
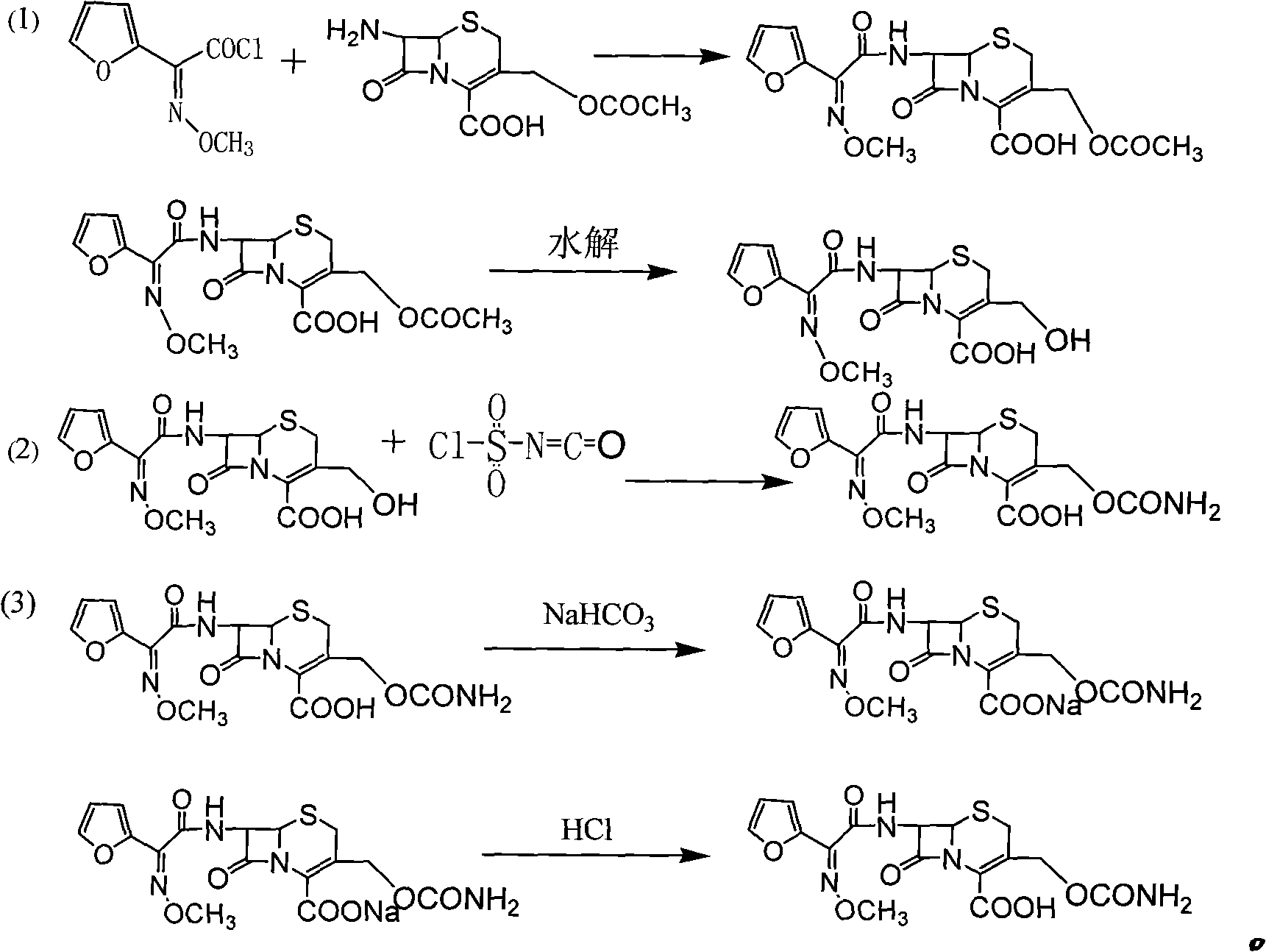
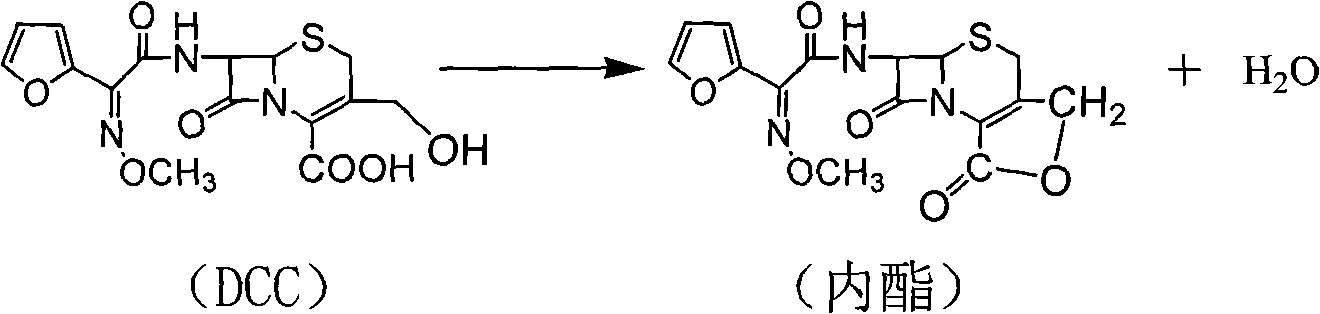
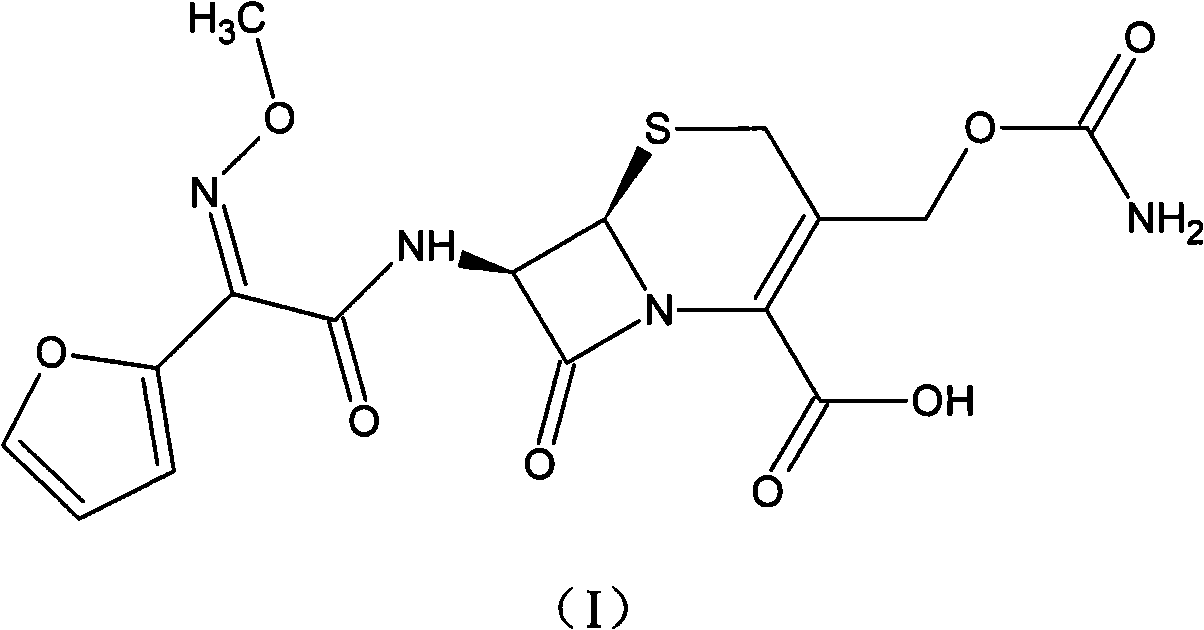
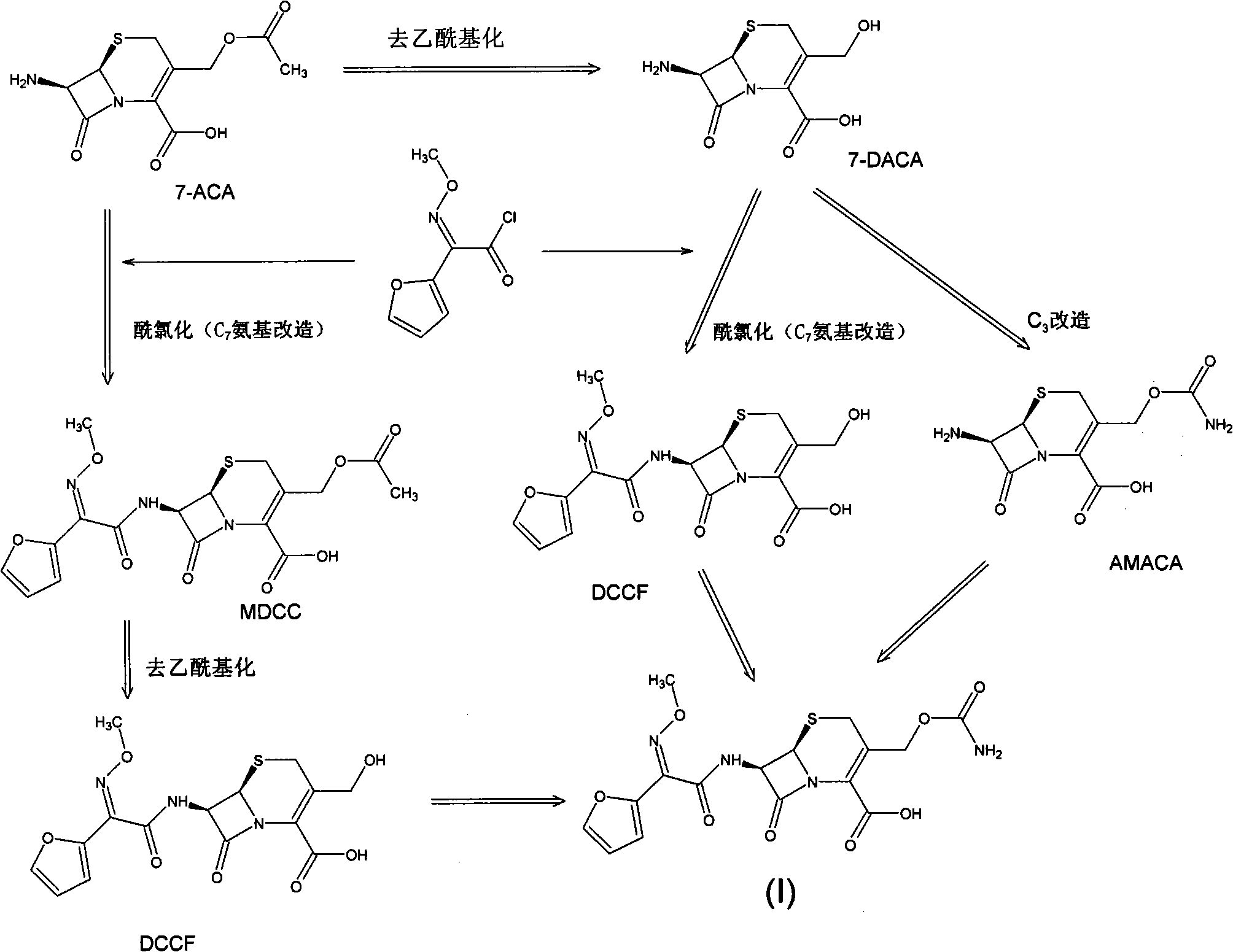
Preparation method of high-purity cefuroxime acid
The invention discloses a preparation method of high-purity cefuroxime acid which is an intermediate for synthesizing second-generation cephalosporins cefuroxime sodium and cefuroxime axetil. The preparation method comprises the following steps: based on 7-aminocephalosporanic acid (7-ACA) as a raw material, carrying out an N-acylation reaction on the 7-ACA and furoyl acetylcholine at the 7-position; at a low temperature, hydrolyzing 3-acetyl with a sodium hydroxide solution, crystallizing, filtering and drying so as to obtain the intermediate 3-deformamido cefuroxime acid (DCC); quantitatively adding the DCC in a tetrahydrofuran solvent, dropwise adding chlorosulfonyl isocyanate for a nucleophilic addition reaction so as to generate chlorosulfonyl cefuroxime acid, and adding purified water for hydrolysis so as to prepare a cefuroxime acid reaction liquid; adding sodium bicarbonate for salifying; removing by-reactant lactone and other unsaponifiable impurities in the reaction liquid with a ternary compound extracting agent of dichloromethane, ethyl acetate and tetrahydrofuran, layering, and adding hydrochloric acid in a water phase for acidification; adding the ternary compound extracting agent to extract and separate out the cefuroxime acid; and removing water-soluble impurities, crystallizing and filtering a distilled organic phase, and then drying so as to obtain the high-purity cefuroxime acid with the purity of more than or equal to 99%.
Owner:四平市精细化学品有限公司
Conjugate addition reactions for the controlled delivery of pharmaceutically active compounds
InactiveUS20060127352A1Good water solubilityReduce solubilitySugar derivativesPeptide/protein ingredientsCompound (substance)Biological materials
The invention features polymeric biomaterials formed by nucleophilic addition reactions to conjugated unsaturated groups. These biomaterials may be used for medical treatments.
Owner:ETH ZZURICH +1
Composition for forming reaction type polyurethane hot-melt adhesive and hot-melt adhesive
InactiveCN101423740AGood storage stabilityPolyureas/polyurethane adhesivesSimple Organic CompoundsHydrogen
The invention provides a composition for forming a reactive polyurethane hot melt adhesive, which contains polylol, isocyanate and a chain extendor, wherein the composition also contains a sealant, the sealant is an organic compound with at least one functional group containing active hydrogen, and the sealant can perform a nucleophilic addition reaction with an isocyanate group. The invention can greatly improve the storage stability of the polyurethane hot melt adhesive by adding the sealant into the composition for forming the polyurethane hot melt adhesive.
Owner:BYD CO LTD
Methyl pyridinium with organic two-photon absorption materia, and preparation method and application thereof
InactiveCN102702090APromote absorptionGood water solubilityOrganic chemistryMaterial analysis by observing effect on chemical indicatorSolubilitySolvatochromism
The invention relates to the field of organic nonlinear optical materials and relates to methyl pyridinium with an organic two-photon absorption material, and a preparation method and the application of the methyl pyridinium. The structural formula of the product is described in structural formula (I); the preparation method comprises the steps of taking 2,6-dimethyl pyridine as a raw material and finally obtaining a compound (I) by conducting oxidizing reaction, esterification reaction, methylolation reaction, halogenation reaction, nucleophilic addition reaction, Wittig reaction and the final methylation reaction. The raw materials are easy to access, and the synthesis steps are simple. The compound (I) has excellent two-photon absorption performance and good water solubility and is an ideal biological fluorescence probe; an obvious solvatochromism phenomenon easily occurs, the compound can be used for detecting water content in a common organic solvent, can also be used as a fluorescent probe for detecting the PH value in a solution system, has a series of advantages of high sensibility, quick response, identification by naked eyes and the like, and has an excellent application prospect.
Owner:SHANGHAI NORMAL UNIVERSITY
Method for preparing diphenolic acid in ionic liquid
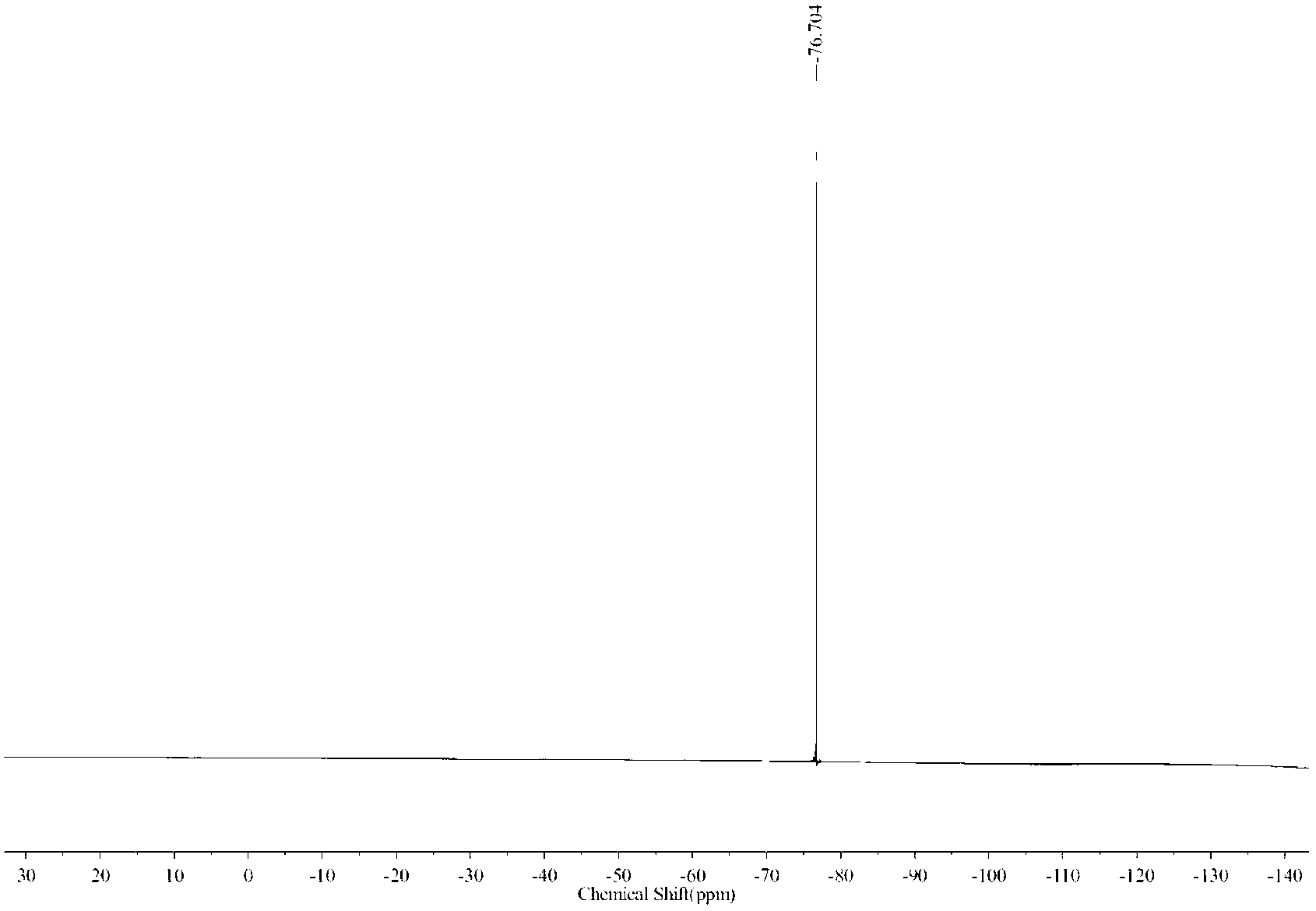
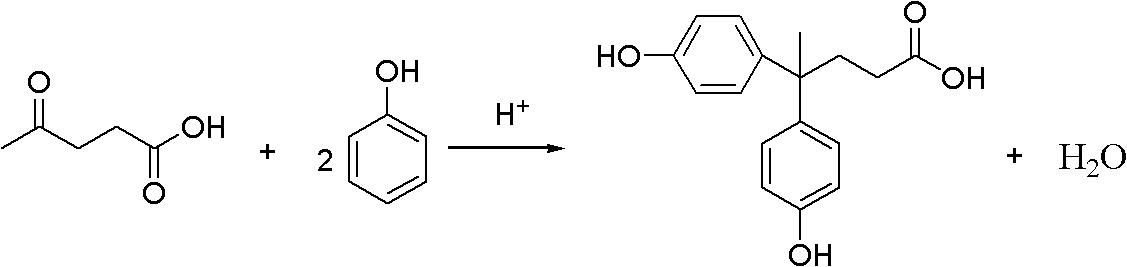
InactiveCN102584569AWell mixedMild reaction conditionsOrganic compound preparationCarboxylic compound preparationLevulinic acidPhysical chemistry
The invention discloses a method for preparing diphenolic acid in ionic liquid, which comprises the following steps in sequence: (1) uniformly mixing levulinic acid, carbolic acid and the ionic liquid to obtain mixture, wherein the structure of the ionic liquid at least contains one acidity group; and (2) conducting nucleophilic addition on the mixture to obtain diphenolic acid mixed liquor. Positive ions of the ionic liquid is selected from alkyl imidazole positive ions, sulfoacid substitution alkyl imidazole positive ions, allyl imidazole positive ions, alkyl pyridine positive ions and one of sulfoacid substitution alkyl pyridine positive ions, sulfoacid substitution alkyl urotropin positive ions and sulfoacid substitution alkyl triethylene diamine positive ions. The method adopts the ionic liquid to serve as a catalytic agent and reaction solvent and is not only simple and convenient in operation, soft in conduction, environment-friendly, low in equipment corrosivity, stable in performance and capable of repeatedly using, and the ionic liquid is easy to recover.
Owner:BEIJING FORESTRY UNIVERSITY
Preparation method of tulathromycin
ActiveCN106939029AImprove protectionAvoid catalytic hydrogenation methodsSugar derivativesSugar derivatives preparationAzithromycinChemical synthesis
The invention relates to the field of chemical synthesis and particularly relates to a preparation method of tulathromycin. The method comprises protecting a 2-hydroxyl group of demethyl azithromycin through 4-nitrobenzyl chloroformate, carrying out oxidation and epoxidation on a 4"-hydroxyl group, and carrying out deprotection and 4"-epoxy nucleophilic addition through n-propylamine to obtain tulathromycin. Comprised with the prior art, the preparation method has simple processes, mild conditions and a high yield, is free of palladium-carbon hydrodeprotection and is conducive to industrial production.
Owner:AMICOGEN CHINA BIOPHARM CO LTD
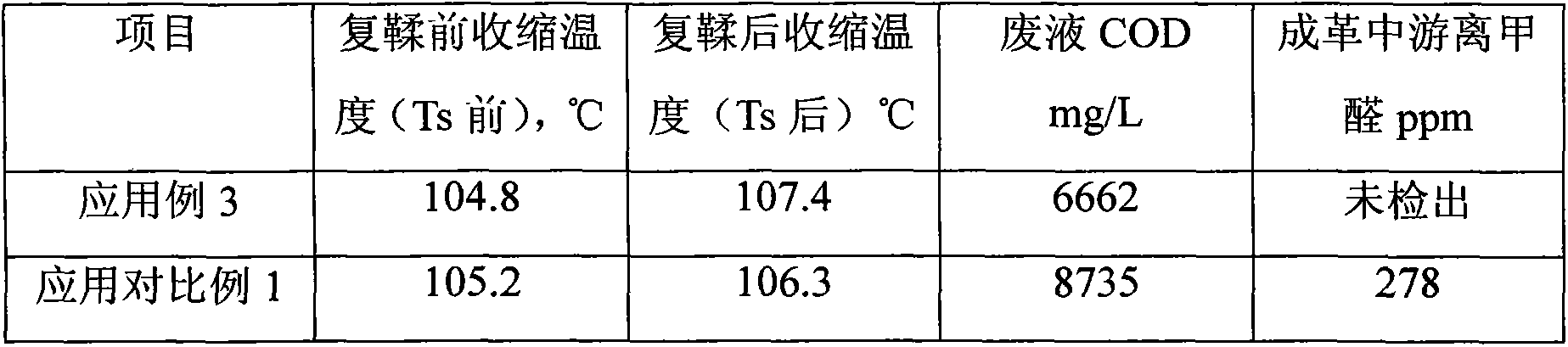
Formaldehyde-free amino resin retanning agent
The invention discloses a formaldehyde-free amino resin retanning agent. The formaldehyde-free amino resin retanning agent is characterized by being formed as follows: firstly, obtaining an intermediate containing hydroxyl and aldehyde group residue in molecule by using an amino monomer and glutaraldehyde through nucleophilic addition; then, obtaining the formaldehyde-free amino resin retanning agent by a series of reactions such as sulfonation, hydrolyzed collagen end-blocking, condensation and etherification. The formaldehyde-free amino resin retanning agent provided by the invention is an amino resin condensation product containing an aldehyde group, has retanning performance comparable with that of the conventional amino resin retanning agent, has an aldehyde group capable of performing covalent cross-linking reaction with the amino on collagenous fiber in molecule, and can be used for improving the humidity and heat-resistant stability of finished leather. Formaldehyde is not used in a preparation process of the formaldehyde-free amino resin retanning agent, so that harms of formaldehyde in the finished leather can be greatly lowered.
Owner:四川德赛尔新材料科技有限公司 +1
Bifunctional fluorescent probe adopting anthracene as matrix, as well as preparation method and application
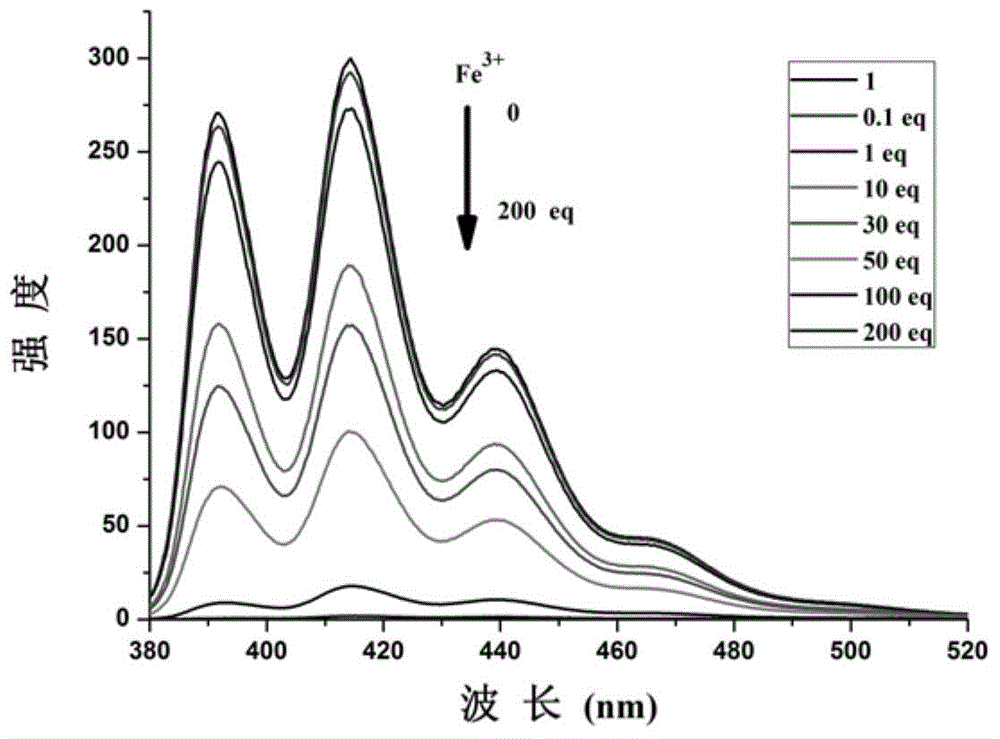
InactiveCN104788344AEnables fluorescence detectionEnables fluorescence-enhanced detectionUrea derivatives preparationOrganic compound preparationAnthraceneFluorescence
The invention provides a bifunctional fluorescent probe which is provided with the structure of the general formula I and adopts anthracene as the matrix. The preparation method of the bifunctional fluorescent probe comprises the following steps: conducting isocyanic acid esterification or isothiocyanic acid esterification on 9-anthracene methylamine; conducting the nucleophilic addition reaction to obtain the bifunctional fluorescent probe. The bifunctional fluorescent probe has the good fluorescence detection effect on iron ions; moreover, the system composed of the bifunctional fluorescent probe and the iron ions has the selective fluorescence enhanced detection effect on fluorine ions.
Owner:DALIAN UNIV OF TECH
High temperature yellowing resistant polyurethane and preparation method thereof
The invention provides a preparation method of high temperature yellowing resistant polyurethane. The method includes the steps of: (1) reacting diisocyanate with diol under the action of a catalyst to obtain a reaction product; (2) subjecting the reaction product to chain extension with a chain extender to obtain a chain extension product; and (3) reacting the chain extension product with epoxy resin to obtain high temperature yellowing resistant polyurethane. The method provided by the invention utilizes the reaction characteristics of isocyanato and hydroxyl to synthesize polyurethane that does not produce a quinoid structure, then utilizes the ring-opening reaction of epoxy group and carbamate in polyurethane to graft epoxy resin into a polyurethane chain, thus enhancing the thermal stability of carbamate bond, structural transformation and oxidation cannot occur to the carbamate bond under a high temperature condition, accordingly chromophoric group generation can be inhibited, thereby reaching the purpose of no yellowing of polyurethane under high temperature. The invention also provides the high temperature yellowing resistant polyurethane.
Owner:GUANGDONG UNIV OF TECH
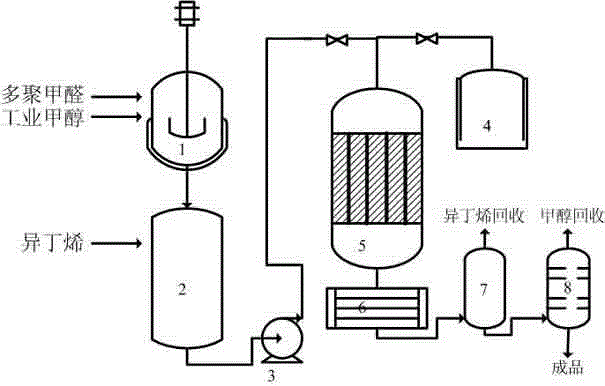
Method for continuously producing 3-methyl-3-butylene-1-alcohol
InactiveCN103333048ARealize continuous productionHigh catalytic efficiencyPreparation by oxygen reductionPhosphateFixed bed
The invention relates to a method for continuously producing 3-methyl-3-butylene-1-alcohol. The method comprises the steps of firstly dissolving polyformaldehyde in methanol; mixing the obtained solution with isobutylene to obtain a mixture; then pumping the mixture into a multitubular fixed bed reactor; contacting the mixture with gamma-Al2O3 dipped with an alkali metal phosphate catalyst dissolved by deionized water filled in the reactor; carrying out a nucleophilic addition reaction under the effect of the catalyst; then cooling reaction products; carrying out gas-liquid separation; and then rectifying and drying the separated solution. The method overcomes disadvantages of small contacting area of reaction mediums, low heat and mass transfer efficiency, high reaction pressure, many side reactions, low production efficiency, unstable quality and poor security and reliability and the like which are caused by adopting an intermittent tank reactor, so that continuous production of 3-methyl-3-butylene-1-alcohol by using isobutylene and polyformaldehyde as raw materials is realized.
Owner:JIANGSU SOBUTE NEW MATERIALS +1
Method for preparing cefuroxime acid
The invention discloses a method for preparing cefuroxime acid. The method comprises the following steps of: performing a chloride acylation reaction on 7-aminocephalosporanic acid (7-ACA) and methoxyiminofuran acetate serving as raw materials; performing deacetylation to synthesize DCCF; performing nucleophilic addition on the DCCF and chlorosulfonyl isocyanate (CSI) serving as a strong carbamoyl reagent to obtain chlorosulfonyl cefuroxime; and hydrolyzing the chlorosulfonyl cefuroxime to obtain cefuroxime acid. In the preparation method, the preparation process is simple, and the cefuroximeacid is crystallized by adopting aqueous solution, so that the loss of organic solvents is reduced; simultaneously, aids are added selectively in the reaction process to improve the quality of products, so that finished products with high purity and yield and good colors are obtained. The purity of the cefuroxime acid prepared by the method is more than 98.5 percent, and the weight yield is approximately 100 percent.
Owner:国药集团致君(苏州)制药有限公司
High-solid content polyamide polyamine epichlorohydrin wet strength agent, as well as preparation and application thereof
InactiveCN103030806AHigh solid contentHigh positive charge densityReinforcing agents additionPaper/cardboardOrganic chloride compoundPolyamide
The invention discloses a high-solid content polyamide polyamine epichlorohydrin wet strength agent, as well as a preparation method and an application thereof. The preparation method comprises the following steps: performing condensation on a dibasic acid and a diamine under the action of an acidic catalyst to get a polyamide polyamine intermediate; enabling the polyamide polyamine intermediate, an organic chloride and a cation active monomer or a mixture of the cation active monomer and sodium hydroxide to perform nucleophilic addition reaction to get a high-viscosity cation polymer; and further adding water-soluble hydroxy amine to react and regulating pH with an acid to get the high-solid content polyamide polyamine epichlorohydrin wet strength agent. The obtained wet strength agent has the advantages of high solid content above 40% (in percentage by weight), high charge density, stability in storage, great wet-strengthening performance and few chlorine-containing byproducts, and can be applied in papermaking wet-end chemistry process.
Owner:SOUTH CHINA UNIV OF TECH
Epoxy-resin-modified polyurethane waterproof paint and preparation method thereof
ActiveCN108707414AReduce polarityWeak polarityPolyurea/polyurethane coatingsEpoxyIsophorone diisocyanate
The invention discloses epoxy-resin-modified polyurethane waterproof paint and a preparation method thereof. The epoxy-resin-modified polyurethane waterproof paint comprises the following components according to the parts by weight: 80-100 parts of polyether glycol, 20-40 parts of isophorone diisocyanate, 30-50 parts of an epoxy resin, 2-6 parts of hydroxy-terminated poly-dimethylsiloxane, 0.04-0.2 parts of chloroplatinic acid, 0.6-1 part of a silane coupling agent, 2-6 parts of dimethylolpropionic acid, 15-25 parts of acetone, 4-10 parts of trimethylamine, 0.02-0.5 parts of dibutyltin dilaurate, 5-10 parts of water, and 30-60 parts of inorganic filler. The preparation method comprises the following steps: S1: modifying the epoxy resin; S2: under the protection of nitrogen in 65-75 DEG C,performing a nucleophilic addition reaction of the polyether glycol; S3: in 70 DEG C, copolymerizing reaction products in the steps S1 and S2 and the dimethylolpropionic acid; and S4: neutralizing andhomogenizing. The epoxy-resin-modified polyurethane waterproof paint is capable of using the mutual crosslinking modification of polydimethylsiloxane, the epoxy resin and polyurethane, synthesizing the advantages of three parties, and improving the structure stability and the mechanical property of a coating film. The produced polyurethane waterproof paint can be tightly adhered to a non-polar base plane, and excellent in waterproof anti-penetrability performance.
Owner:ANHUI LENCAQI BUILDING MATERIAL
DOPO (9,10-Dihydro-9-oxa-10-phosphaphenanthrene-10-oxide) derivative grafted siloxane flame retardant reaction type cable material and preparation method thereof
InactiveCN107022142AEvenly dispersedImprove hydrophobicityPlastic/resin/waxes insulatorsDouble bondFire retardant
The invention discloses a DOPO (9,10-Dihydro-9-oxa-10-phosphaphenanthrene-10-oxide) derivative grafted siloxane flame retardant reaction type cable material, which is prepared through the steps that firstly, KH-570-TiO2 particles are successfully synthesized by a sol-gel combined hydrothermal method; vinyl groups are introduced on the surfaces of the nanometer titania particles; ethenyltrimethoxysilan is used as a raw material to take a hydrolytic polycondensation reaction to prepare octavinyl polyhedral oligomeric silsesquioxane; the octavinyl polyhedral oligomeric silsesquioxane and DOPO take a nucleophilic addition reaction; an obtained DOPO derivative is used as a reaction type flame retardant; then, the flame retardant and nanometer TiO2 with double bonds are grafted on a polyethylene molecular chain by using peroxide as an initiator to obtain grafted aggregates; then, cross-linking reaction is performed in hot water to obtain the cable material. The reaction type flame retardant is connected with a polymer matrix through chemical bonds, so that the problem of compatibility is avoided; the influence on the mechanical property of the cable material is little; in addition, the flame retardant effect is durable.
Owner:ANHUI CHUNHUI INSTR CABLE GROUP
Formula and preparation method of cationic polymer
InactiveCN101817912ALow costLow market priceWaste water treatment from textile industryWater/sewage treatment by flocculation/precipitationIonic polymerizationPolymer
The invention provides a formula and a preparation method of a cationic polymer. The raw material formula of the cationic polymer comprises the following components in part by weight: water 5-50, acetone 3-25, amine 5-30, aldehyde 5-40, alkaline regulator 3-12 and acidic regulator 5-20. In the method, a water soluble cationic polymer is prepared from aldehyde, acetone and amine by a nucleophilic addition reaction, an aldol condensation reaction and a Michael addition reaction in the presence of acid and alkali catalysts in turn and by a two-step feeding process. The method has the advantages that: the raw material cost is low, the synthesis process is simple and easy to implement; the preparation conditions are mild and safe; and the obtained product has high cation content and has high decolorizing and flocculating effects on anionic dye waste water.
Owner:FUZHOU UNIV
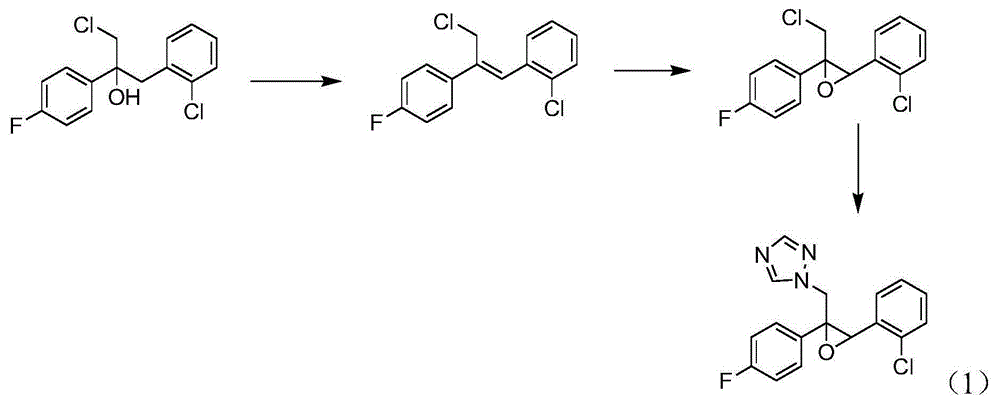
Epoxiconazole intermediate 1-chloro-3-(2-chlorophenyl)-2-(4-fluorophenyl)-2-propanol synthesis process
ActiveCN105130757AEasy to recycleProcess conditions are mild and controllableOrganic compound preparationHydroxy compound preparationPropanolChloride
The present invention discloses an epoxiconazole intermediate 1-chloro-3-(2-chlorophenyl)-2-(4-fluorophenyl)-2-propanol synthesis process, and belongs to the technical field of fine chemical industry. According to the process, 2-chlorobenzyl chloride and 2-chloro-4'-fluoroacetophenone are adopted as raw materials, diethoxymethane and toluene are adopted as solvents, and a two-step reaction comprising as a Grignard reaction and a nucleophilic addition reaction is performed to obtain the target product. According to the present invention, the high yield intermediate is obtained while the process operation is simplified, the raw material cost and the production cost are reduced, the environmental pollution is reduced, and the safety requirements of the industrial scale-up production are met.
Owner:内蒙古佳瑞米精细化工有限公司
Method for preparing propargyl alcohol by using micro-structural reactor
InactiveCN103896737AGood choiceMild reaction conditionsOrganic compound preparationHydroxy compound preparationGrignard reagentProduct selection
The invention provides a method for preparing propargyl alcohol by using a micro-structural reactor, which comprises the steps of by taking a simple Grignard reagent as a raw material, carrying out gas-liquid Grignard exchange reaction on the simple Grignard reagent and acetylene gas to generate a high-quality acetylene-based magnesium bromide Grignard reagent, carrying out nucleophilic addition reaction on the obtained acetylene-based magnesium bromide Grignard reagent and an electrophilic reagent in a continuous flow condition, and carrying out hydrolysis, separation and refining on the product to obtain a substitution propargyl alcohol product. The method provided by the invention has the advantages that the operation is simple, the raw material and the reagent are simple, cheap and easily available, the process is continuous, rapid and controllable, the condition is mild, the product has good selectivity and industrial production can be realized.
Owner:NANJING UNIV OF TECH
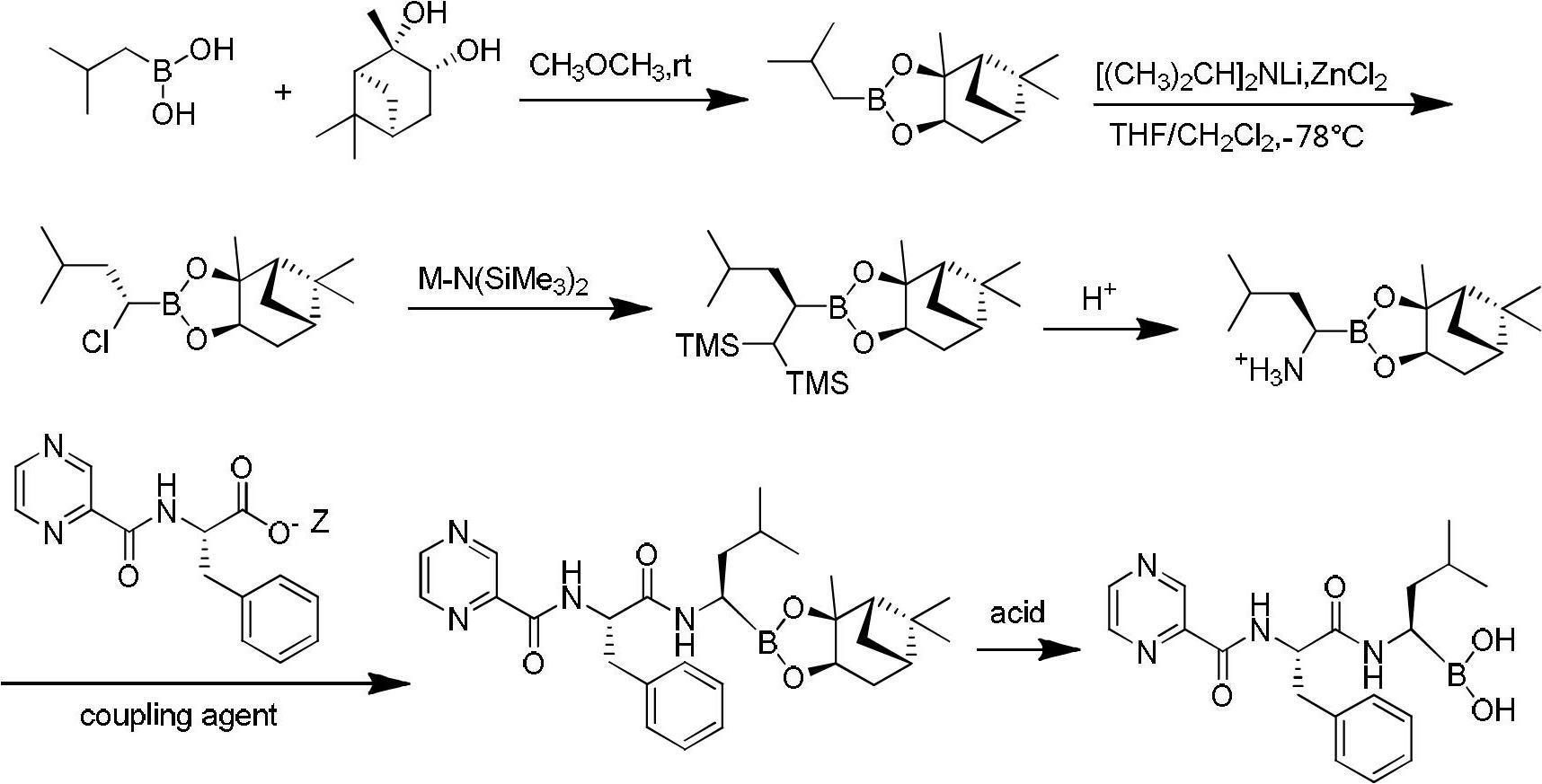
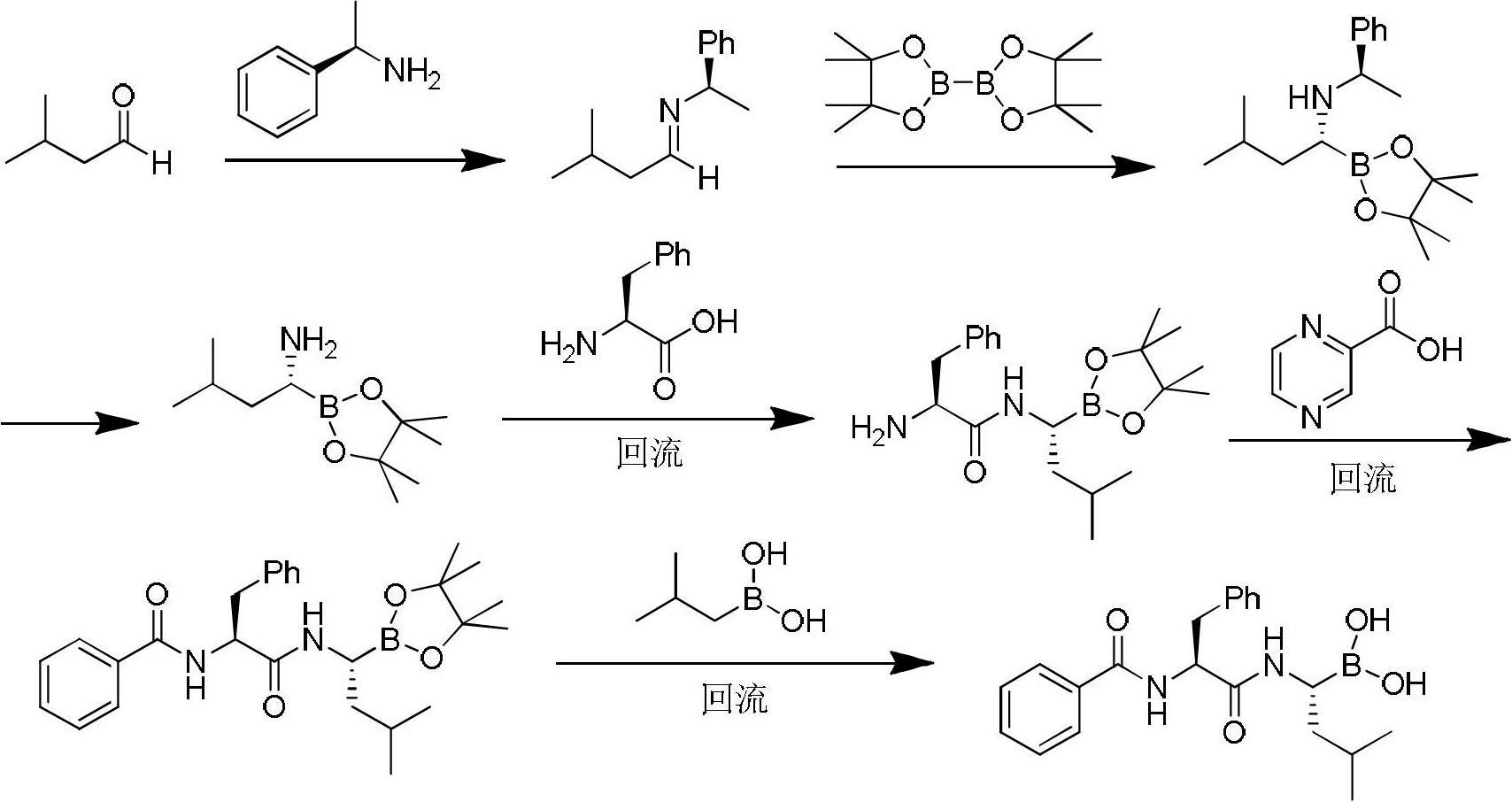
Synthetic method of bortezomib
The invention discloses a synthetic method of bortezomib, which comprises the following steps of: taking isovaleraldehyde as an initial raw material, taking (R)-methylpropane-2-sulfinamide as a chiral reagent, generating (R,E)-2-methyl-N-(3-methyl butylidene) propane-2-sulfinamide by a condensation and dehydration reaction, then carrying out a nucleophilic addition reaction with pinacol diboron so as to generate (R)-1-N-methylpropane sulfinyl-3-methyl butane-1-pinacol borate ester, afterwards hydrolyzing under an acidic condition so as to obtain pinacol-(R)-1-amino-3-methyl butane-1-borate ester hydrochloride, then reacting with (S)-3-phenyl-2-(pyrazine-2-formamido) propionic acid under the existence of a coupling agent and also hydrolyzing under the action of isobutyl borate so as to generate a final product of the bortezomib. According to the synthetic method of the bortezomib, the (R)-methylpropane-2-sulfinamide which is easy to obtain is used as the chiral induction reagent, so that an obtained intermediate enantiomorph has higher purity, and a bulk drug which is finally obtained has better quality.
Owner:HEFEI UNIV OF TECH
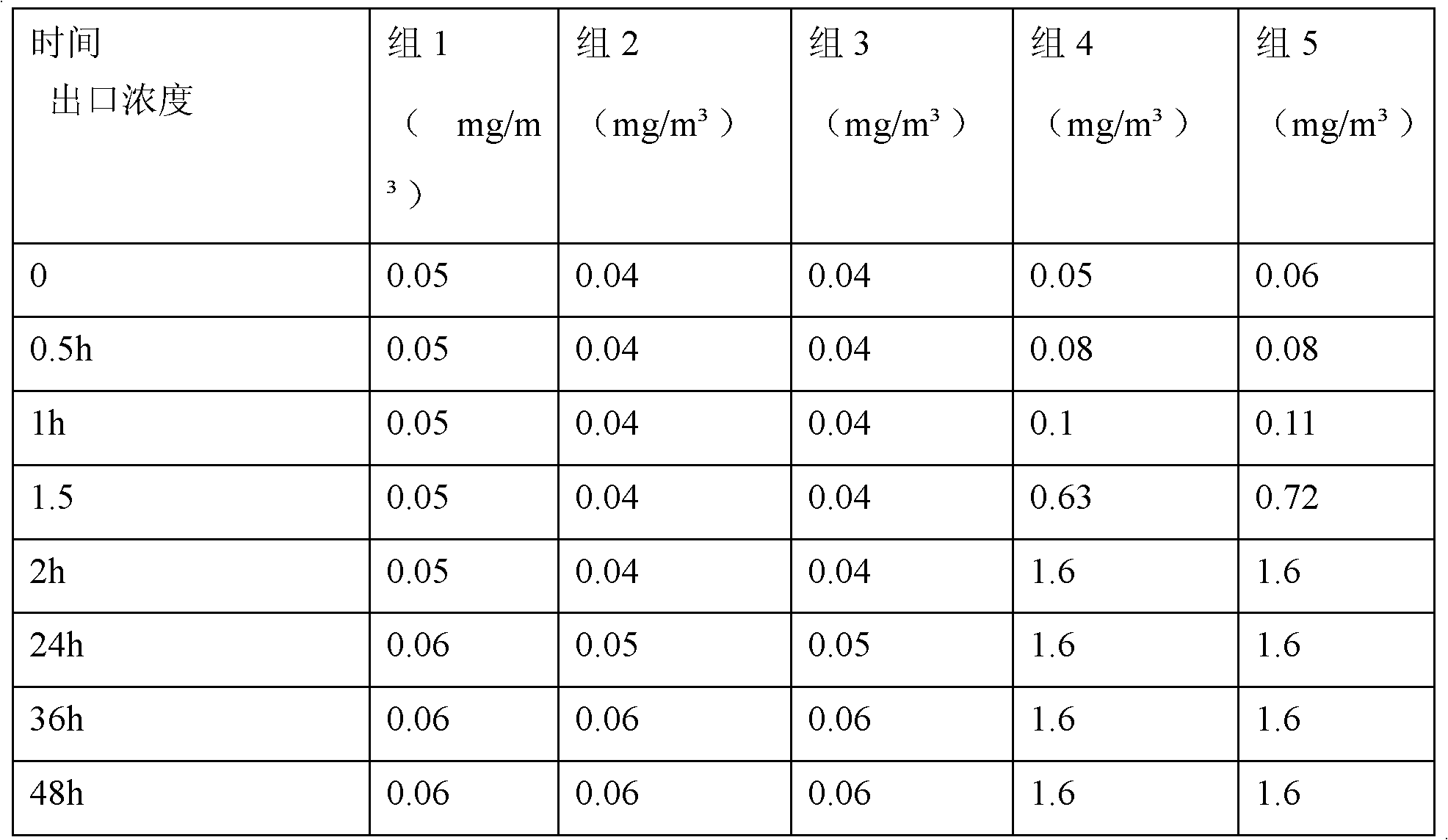
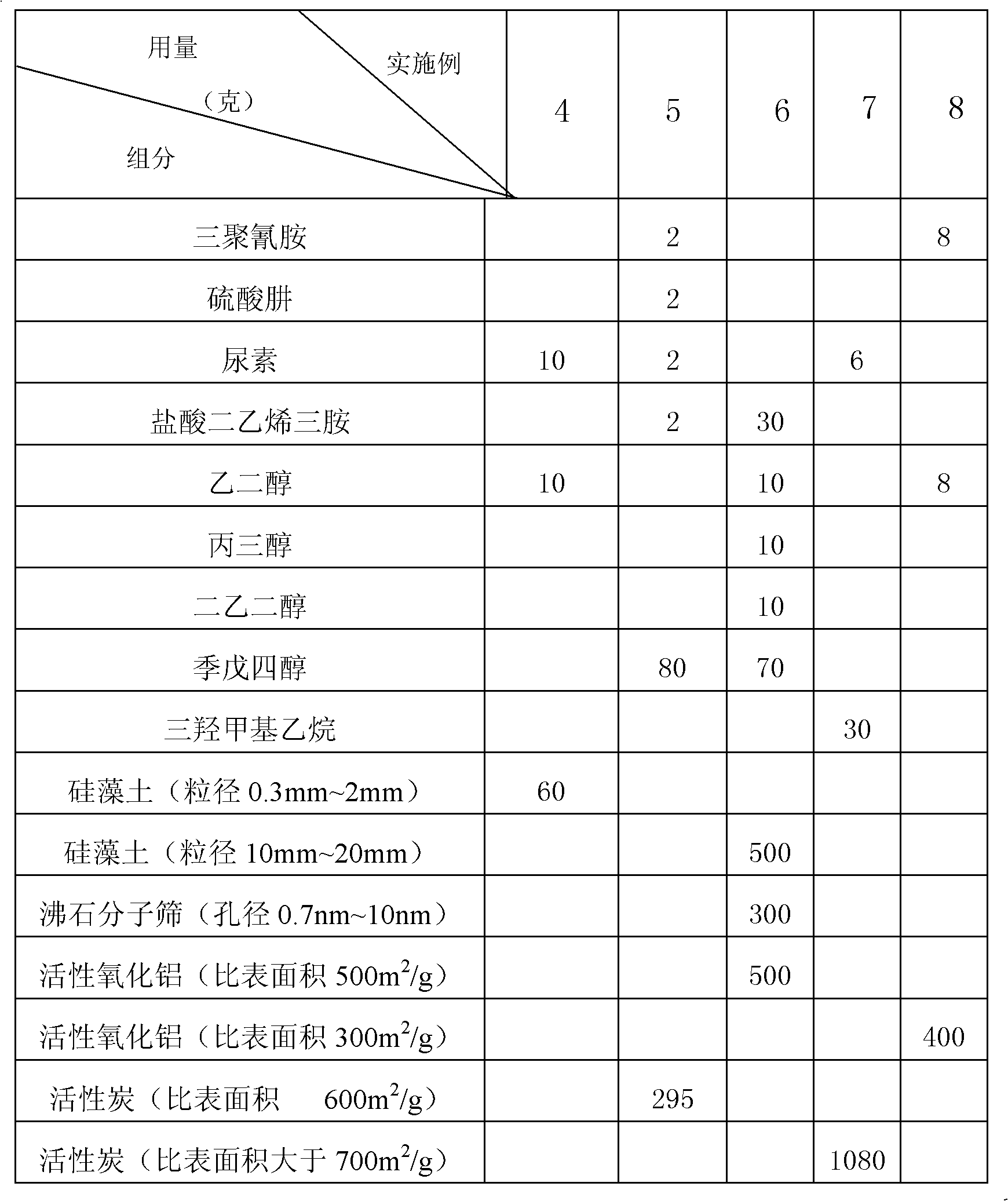
Polyfunctional formaldehyde adsorption material
InactiveCN102101040AImprove adsorption capacityImprove adsorption efficiencyOther chemical processesMass ratioNitrogen
The invention discloses a polyfunctional formaldehyde adsorption material. A polyfunctional nitrogen-containing compound with molecular weight of less than 200 is used as an active ingredient, and a small molecular alcohol substance is used as a synergist; and after the active ingredient and the synergist are mixed in a mass ratio of 1:1-10, the mixture is supported in a porous support. The small molecular polyfunctional nitrogen-containing compound and the small molecular polyalcohol synergist generate a stable three-dimensional netlike structural product by polyfunctional nucleophilic addition reaction, and the three-dimensional netlike structural product is supported on the porous support, so that the adsorption efficiency of the porous support is greatly enhanced, meanwhile, the formaldehyde molecules are locked in the three-dimensional netlike structure, and the defect of secondary release of the porous support is overcome.
Owner:河北亚太环境科技发展股份有限公司
Method for preparing glycerin shrinkage benzaldehyde
The invention discloses a method for preparing glycerin shrinkage benzaldehyde. The method comprises adding glycerin and benzaldehyde into ionic liquid serving as reaction media and catalysts to perform nucleophilic addition reaction, wherein anode ions and cathode ions of the ionic liquid at least contain one acid group. The method has the advantages of being environment-friendly, low in corrosion to devices, capable of effectively improving yield of the glycerin shrinkage benzaldehyde and the like. In addition, the catalysts can be repeatedly used.
Owner:BEIJING FORESTRY UNIVERSITY
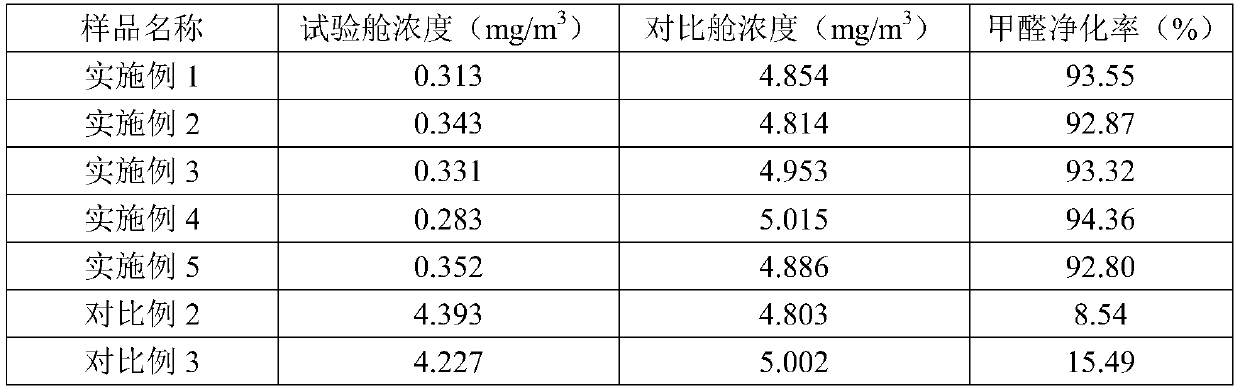
Modified activated carbon for purifying formaldehyde and preparation method thereof
PendingCN111013533AEfficient removal effectImprove long-term effectGas treatmentOther chemical processesActivated carbonPhysisorption
The invention relates to modified activated carbon for purifying formaldehyde and a preparation method thereof. A certain amount of an oxidizing agent and a nucleophilic addition agent are used for modifying the activated carbon according to a certain proportion through a specific process; the oxidation-reduction reaction of the oxidizing agent and formaldehyde, the addition reaction of the nucleophilic addition agent and formaldehyde and the physical adsorption of activated carbon are organically combined, so that formaldehyde in the air is quickly adsorbed on the activated carbon and is converted into substances harmless to a human body through chemical action, and the purpose of thoroughly purifying formaldehyde is achieved. The preparation method of the modified activated carbon purification material is simple and easy to operate, and experimental test results show that the prepared modified activated carbon purification material has adsorption and chemical conversion effects on formaldehyde gas and has the purification characteristics of rapidness, high efficiency, long effect and the like.
Owner:EAST CHINA UNIV OF SCI & TECH +1
Diaminobenzene-functionalized graphene nanomaterial and preparation method thereof
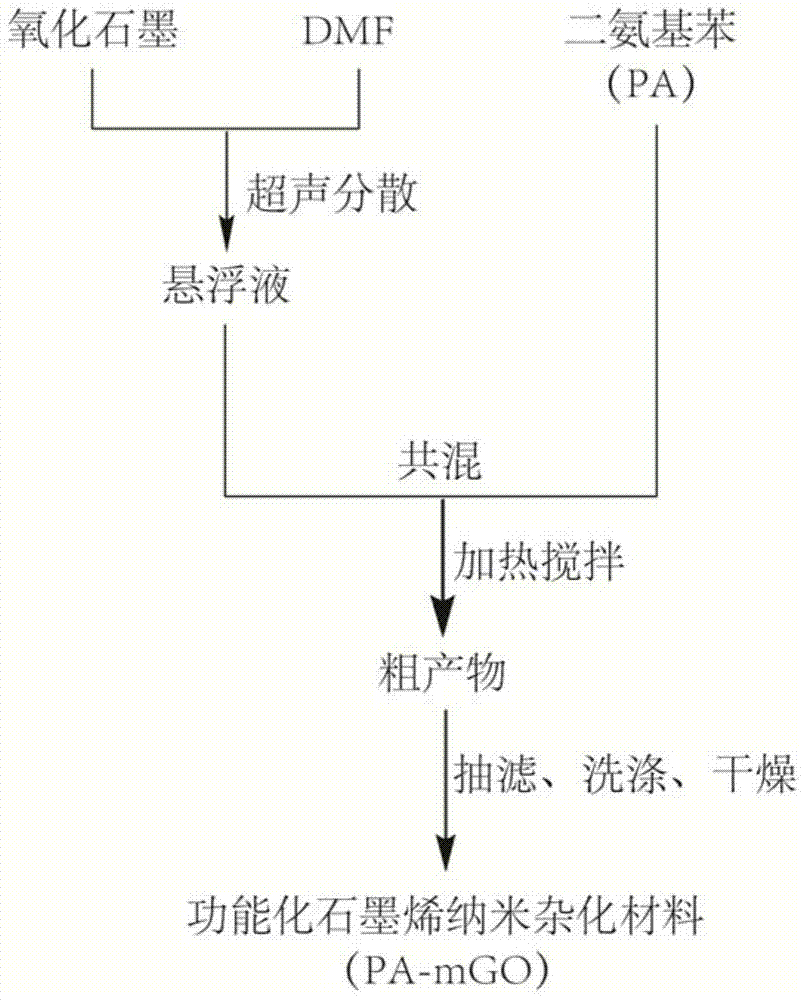
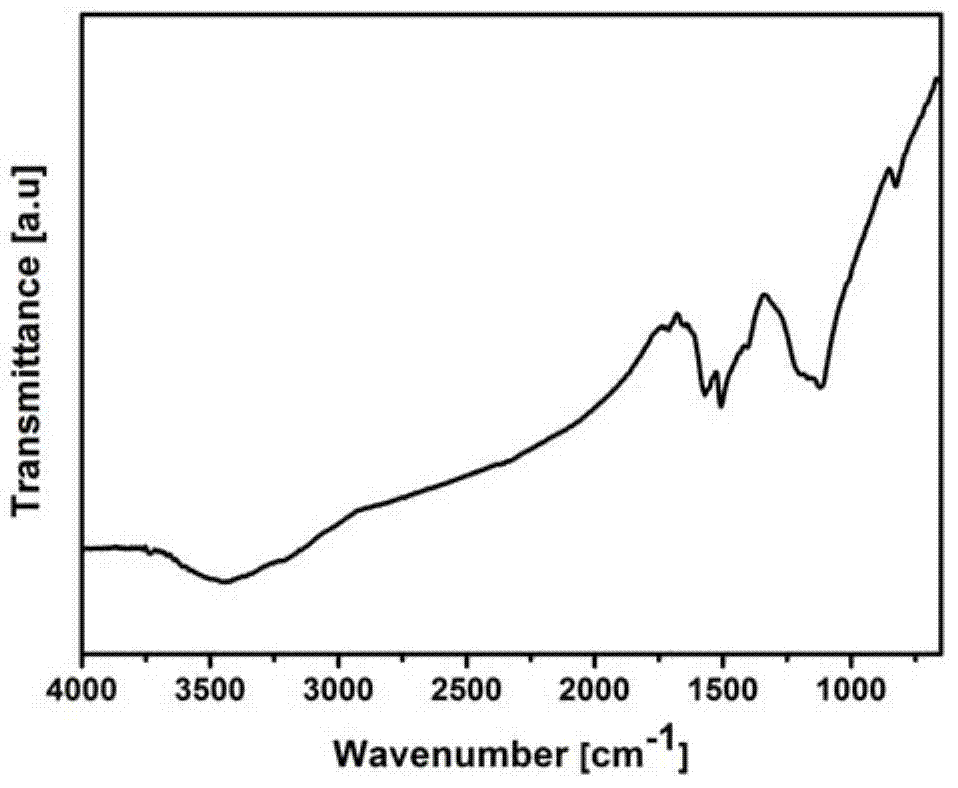
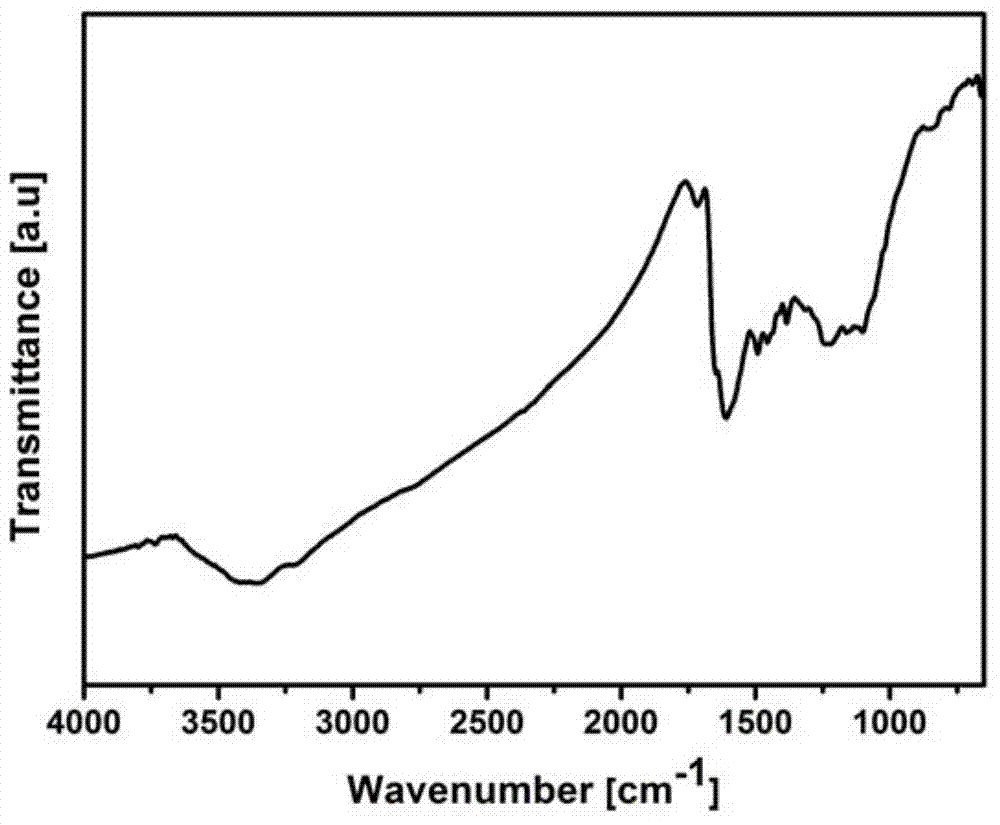
InactiveCN104761753AReduce usageAdjust thermal stabilityPigment treatment with non-polymer organic compoundsReaction temperatureNano hybrid
The invention discloses a diaminobenzene-functionalized graphene nanomaterial and a preparation method thereof. The functionalized graphene nanomaterial is of a nanometer hybrid structure established by modifying diaminobenzene on the surface and edge of graphene oxide by using a scientific comprehensive organic covalent nucleophilic addition and non-covalent electrostatic self-assembly synchronous functionalization technology. The thermal stability of the hybrid material synthesized by the invention is greatly improved as comparison with that of graphene oxide, and the covering amount of diaminobenzene on the surface of graphene oxide can be regulated through changing the reaction temperature; in addition, the synthesis step is simple and efficient; and the diaminobenzene-functionalized graphene nanomaterial is easily prepared on a large scale and is particularly suitable for application as a flame-retardant material and a polymer-based flame-retardant composite nanomaterial so as to have relatively good application prospects and economic benefits.
Owner:NANJING UNIV OF SCI & TECH
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com