Bifunctional fluorescent probe adopting anthracene as matrix, as well as preparation method and application
A fluorescent probe, dual-function technology, applied in the field of fine chemicals, to achieve the effects of cheap raw materials, good selectivity, and obvious color change
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Embodiment 1 prepares fluorescent probe 1
[0033] (1) Synthesis of 9-anthracene methyl isocyanate
[0034] Dissolve 828mg of 9-anthracenemethylamine in 80mL of dichloromethane at room temperature, then add dropwise 8mL of dichloromethane solution containing 1.2g of triphosgene, and stir at room temperature for 1 hour; then add dropwise dichloromethane solution containing 808mg of triethylamine Methane solution 8mL, react for 24 hours; add saturated sodium bicarbonate to it to adjust the pH to neutral, separate the liquid, extract the water layer with dichloromethane, separate the liquid, wash the organic layer with water, dry the organic layer with magnesium sulfate, and obtain the intermediate 9-anthracene methyl isocyanate 800mg, yield 85%.
[0035] 1 H-NMR (400MHz, CDCl 3 ),8.51(s,1H),8.27(d,2H,J=8.0Hz),8.06(d,2H,J=8.0Hz),7.62(t,2H,J=8.0Hz),7.52(t,2H ,J=8.0Hz),5.36(s,2H,CH 2 );
[0036] HRMS: Calcd for [M-NCO] + ,191.0861; Found, 191.0852.
[0037] (2) Synth...
Embodiment 2
[0041] Embodiment 2 prepares fluorescent probe 2
[0042] (1) Synthesis of 9-anthracene methyl isothiocyanate
[0043] Dissolve 414mg of 9-anthracenemethylamine in 20mL of dichloromethane, then add 520mg of calcium carbonate and 20mL of water, and finally add 322mg of thiophosgene, and react at room temperature for 12 hours. Add saturated sodium bicarbonate solution to the reaction solution to adjust the pH to neutral, extract with dichloromethane, separate the layers, wash the organic layer with water, dry the organic layer with magnesium sulfate, and obtain the intermediate 9-anthracenemethyl isothiocyanate after rotary evaporation 380 mg, yield 76%.
[0044] 1 H-NMR (400MHz, CDCl 3 ),8.54(s,1H),8.24(d,2H,J=8.0Hz),8.07(d,2H,J=8.0Hz),7.64(t,2H,J=8.0Hz),7.54(t,2H ,J=8.0Hz),5.60(s,2H).
[0045] (2) Synthesis of fluorescent probe 2
[0046] Dissolve 260 mg of 9-anthracenemethyl isothiocyanate obtained in the previous step in 30 mL of ammonia (0.7 mol / L) tetrahydrofuran solut...
Embodiment 3
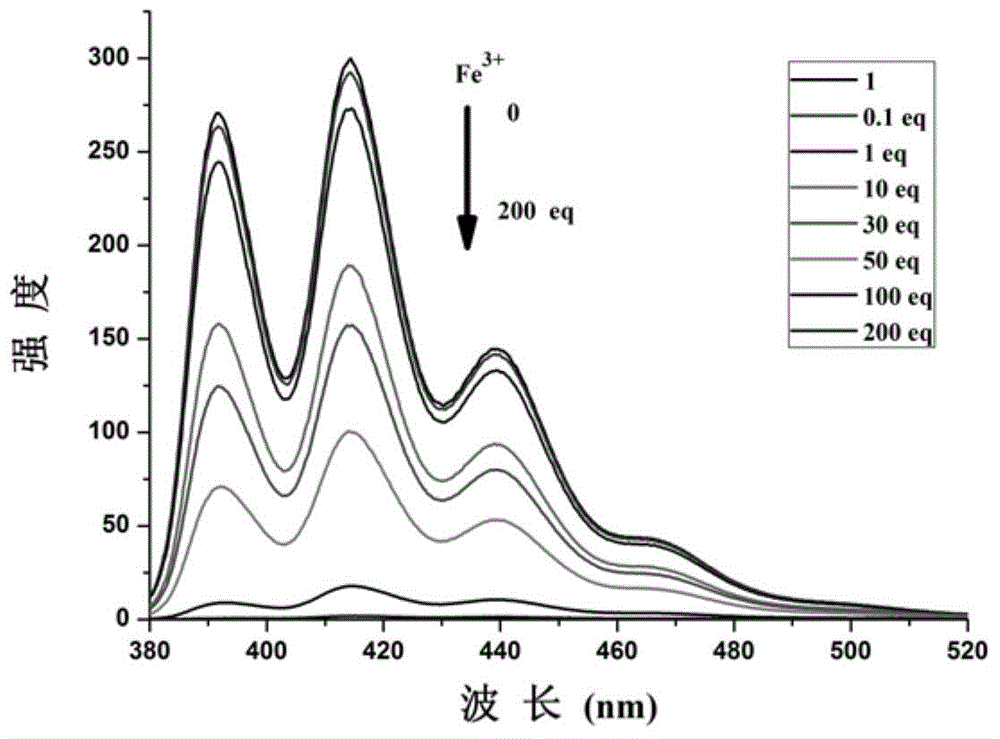
[0049] Embodiment 3. Fluorescent probe 1 and Fe under visible light and 365nm 3+ and F - effect
[0050] Accurately weigh 25 mg of fluorescent probe 1 and dissolve it in 10 mL of dimethyl sulfoxide, and take 1 mL of it and dissolve it in 99 mL of a mixed solution of dimethyl sulfoxide and water with a volume ratio of 1:1 to make a concentration of 10 -4 mol / L of test solution 1. Accurately measure 3mL of test solution 1 each time in three sample bottles of No. 1, No. 2 and No. 3, and then add 30 μL of ferric chloride with a concentration of 0.01mol / L to No. 2 and No. 3 sample bottles respectively Finally, add 30 μL of tetrabutylammonium fluoride dimethyl sulfoxide solution with a concentration of 0.01mol / L to sample bottle No. 3, and three samples of No. 1, No. 2 and No. 3 Shake the bottle evenly, and it can be obtained under visible light Figure 1a The results shown; can be obtained under UV 365nm Figure 1b The results shown.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com