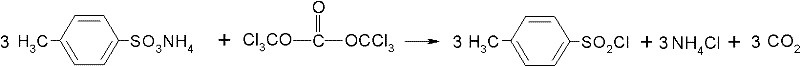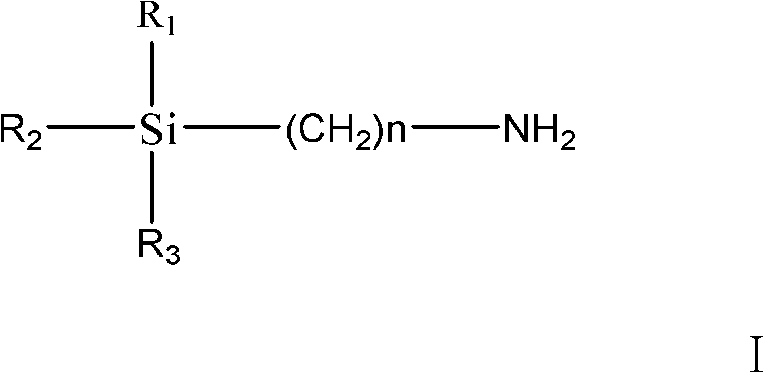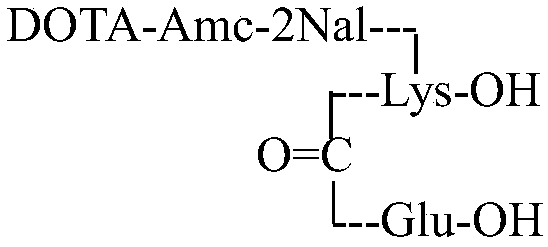Patents
Literature
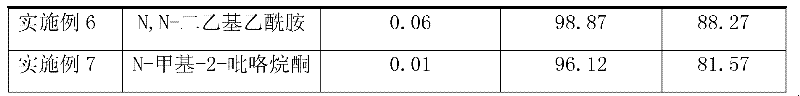
454 results about "Triphosgene" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Triphosgene (bis(trichloromethyl) carbonate (BTC), C₃Cl₆O₃) is a chemical compound that is used as a safer substitute for phosgene, because, at room temperature, it is a solid crystal, as opposed to phosgene, which is a gas. Triphosgene crystals decompose above 200 °C.
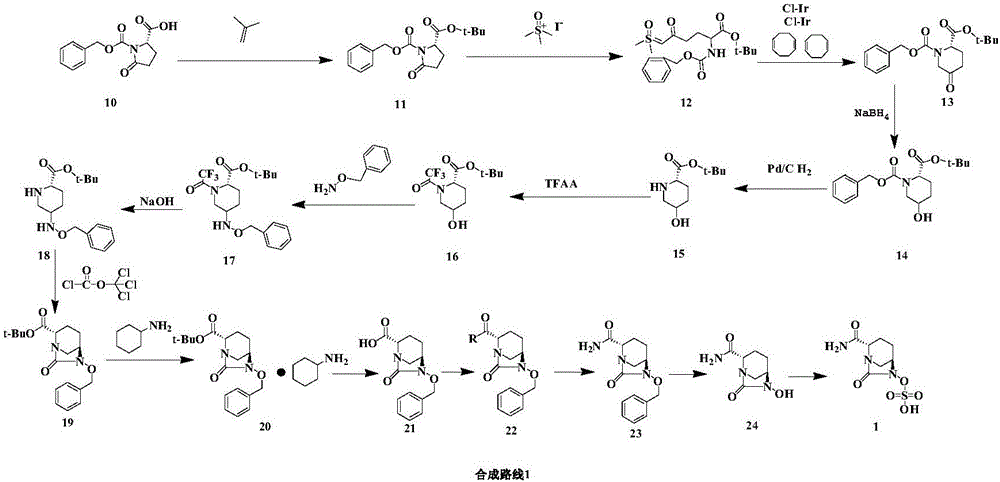
Method for preparing avibactam sodium through one-pot method
The invention discloses a method for preparing avibactam sodium through a one-pot method. The method comprises the following steps: by taking (2S,5R)-5-[(benzyloxy)amino]pyridine-2-ethyl carboxylate oxalate as a starting material, firstly generating a compound II by reacting with triphosgene, hydrolyzing, adding ammonia water to perform ammoniation, thereby obtaining a compound III, performing hydrogenation by taking formic acid, ammonium formate or hydrazine hydrate as a hydrogen donor in hydrogenation reaction, and then salifying to obtain the avibactam sodium. The avibactam sodium is prepared by use of the one-pot method, the raw material is cheap and easy to obtain, the reaction condition is mild, the operation is simple, the safety is higher, the yield is high, the purity is good, and the method is suitable for large scale industrial production. Formulae are shown in the description.
Owner:QILU TIANHE PHARMA
Synthetic method of malononitrile
InactiveCN103044286AHigh purityImprove stabilityPreparation by carboxylic acid amide dehydrationPtru catalystDistillation
The invention discloses a synthetic method of malononitrile. Cyanoacetamide reacts with triphosgene in the presence of a catalyst to synthesize malononitrile, wherein the catalyst is any one substance or a mixture of multiple substances selected from N,N-dimethyl formamide, sodium chloride, pyridine and triethylamine. The method provided by the invention employs the triphosgene as a dehydrating agent, and the triphosgene is low in cost, available and high in stability, and facilitates storage and transportation; the reaction products of the cyanoacetamide and the triphosgene only include carbon dioxide and hydrogen chloride gases except for the malononitrile, without solid waste, and therefore, after the reaction is completed, the steps of removing the solid waste by means of filtering, centrifuging and the like are not needed; the reaction mixture is directly subjected to reduced pressure distillation after the solvent is recovered so that the malononitrile having the purity of higher than 98% can be obtained; and the posttreatment is simple.
Owner:CHONGQING UNISPLENDOUR CHEM
Method for preparing sorafenib
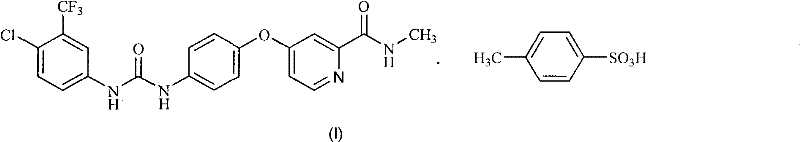
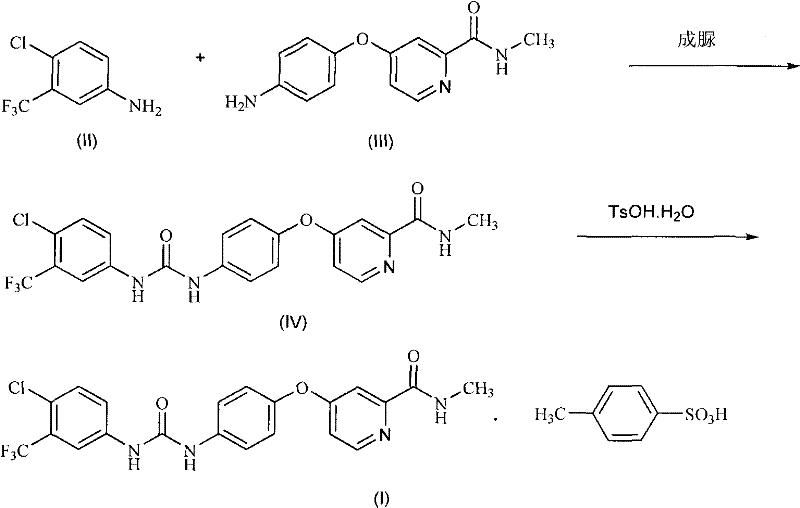
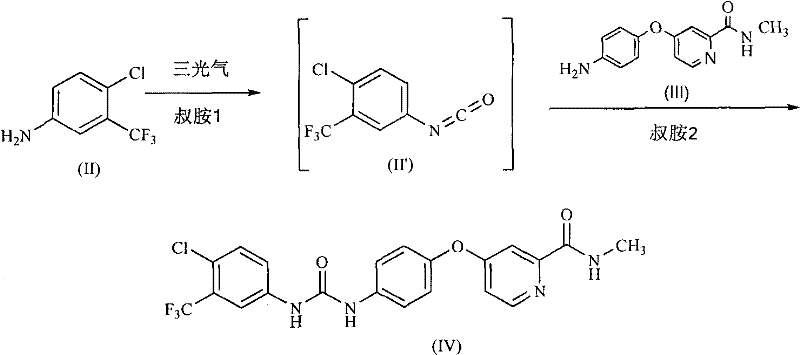
The invention discloses a method for preparing sorafenib, which comprises the steps of: 1) reacting triphosgene with 3-trimethyl fluoride-4-chloroaniline (II) in an inert solvent 1 under the existence of tertiary amine 1, thus obtaining a solution containing 3-trimethyl fluoride-4-chlorphenyl isocyanate (II'), wherein isolation and purification are not needed; and 2) reacting the 3-trimethyl fluoride-4-chlorphenyl isocyanate (II') with 4-(4-aminophenoxy)-N-methyl-2-pyridine carbonxamide (III) in an inert solvent 2 under the existence of tertiary amine 2, thus obtaining sorafenib (IV). The tertiary amine 1 and the tertiary amine 2 are the same or different, the inert solvent 1 and the inert solvent 2 are the same or different, and the feeding sequence of the 3-trimethyl fluoride-4-chloroaniline (II) and the 4-(4-aminophenoxy)-N-methyl-2-pyridine carbonxamide (III) can be interchangeable. The method is simple and convenient to operate, short for reaction time free from separation of intermediates with high reactivity, as well as is free from special equipment and conditions, and high in yield.
Owner:SHANGHAI INST OF PHARMA IND CO LTD
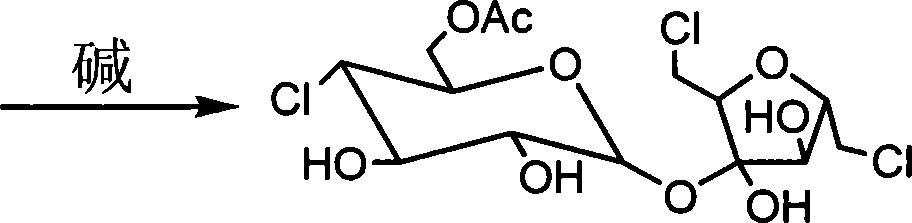
Method for synthesizing environment-friendly sucralose
InactiveCN101177437AOvercome the disadvantage of large pollutionOvercome the disadvantages of multiple and low chlorination yieldSugar derivativesSugar derivatives preparationSucroseAcetic anhydride
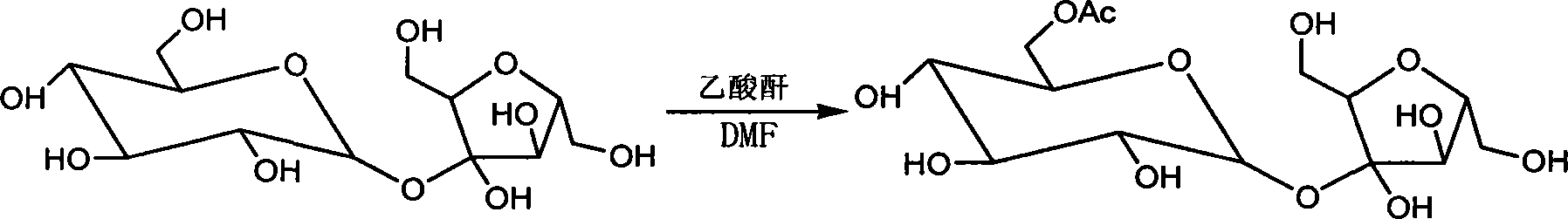
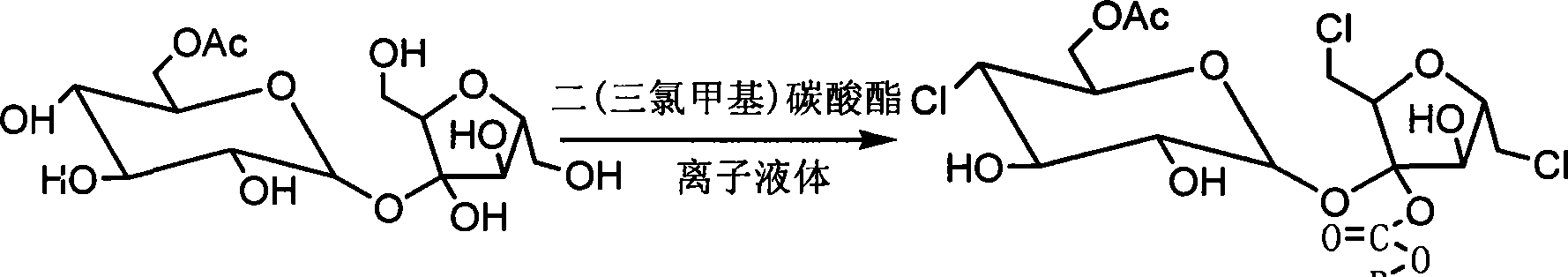
The invention discloses a sucralose green synthesizing method, which uses cane sugar as raw materials, adds ionic liquid for esterification reaction with acetic anhydride under the action of acid catalyst to generate cane sugar-6-acetic ester, which is chloro-substituted by solid triphosgene and degreased in ionic liquid to generate the sucralose. The invention has the advantages that the process is environment-friendly, the product quality is excellent, the operation is simple and the production cost is low, which is an ideal process for industrialized production.
Owner:ZHEJIANG APELOA JIAYUAN PHARMA
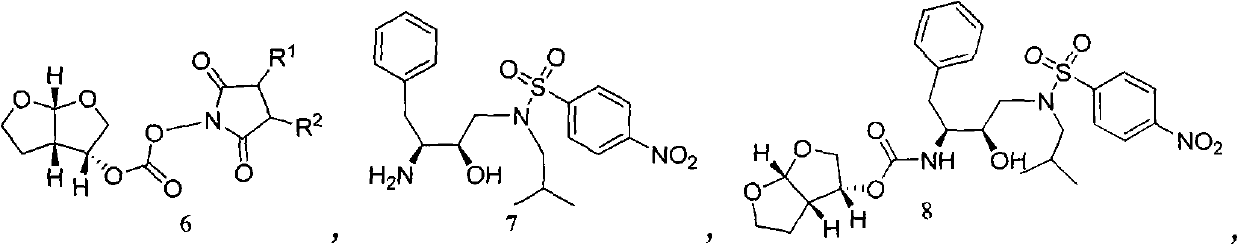
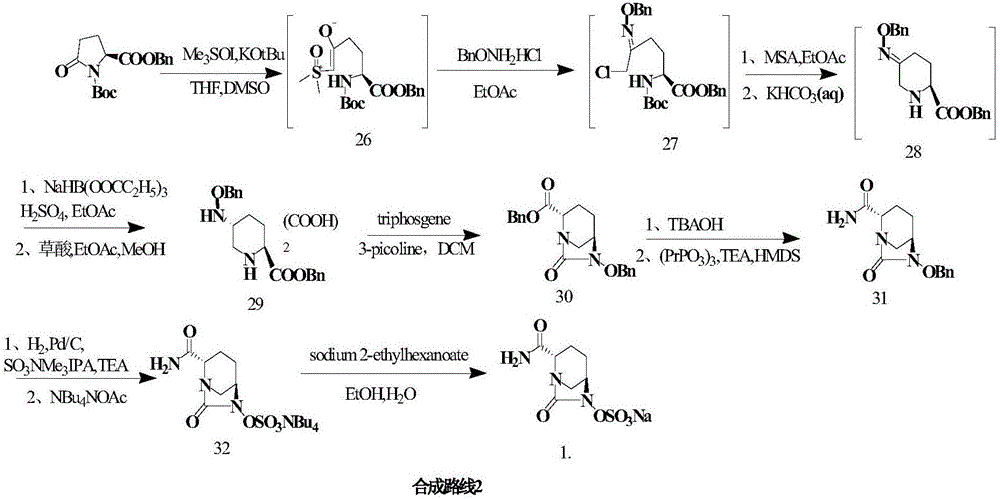
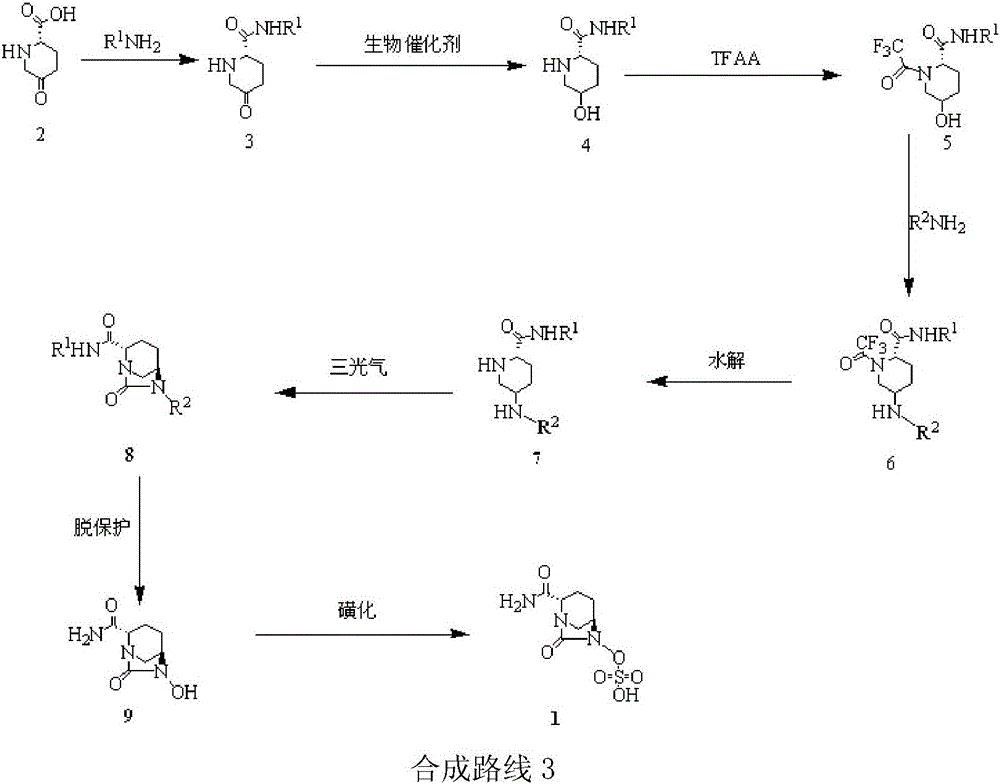
Method for synthesizing beta-lactamase inhibitor Avibactam
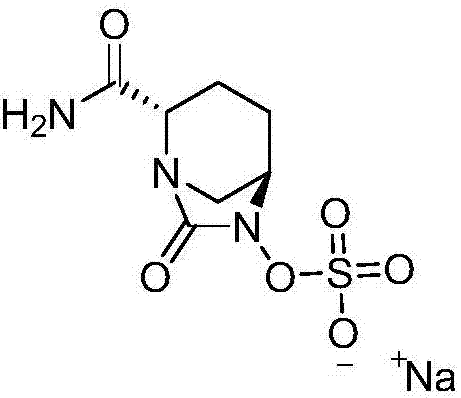
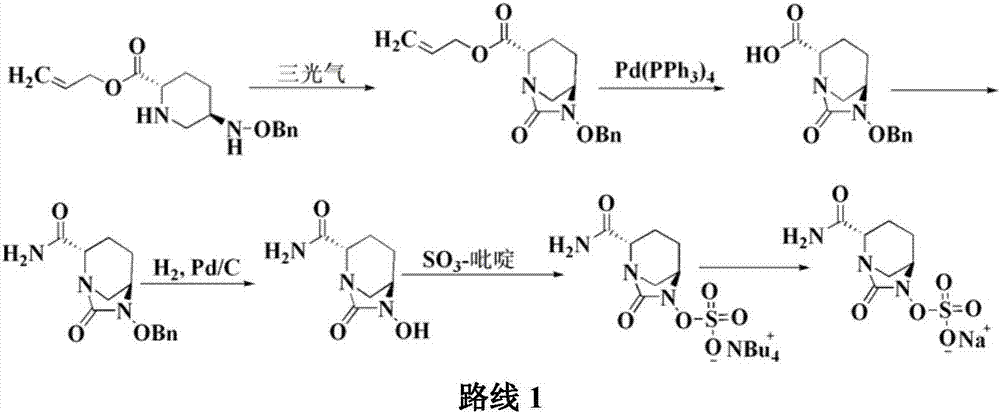
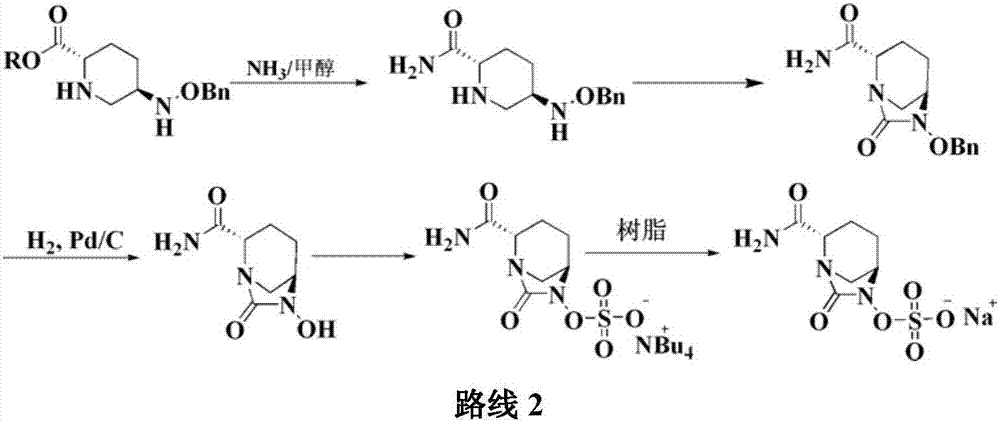
The invention relates to a method for synthesizing a beta-lactamase inhibitor Avibactam and belongs to the technical field of preparation of beta-lactamase inhibitors. The method disclosed by the invention comprises the following steps: (1) taking a compound 2 as a raw material, and reacting with R<1>NH2 so as to produce a compound 3; (2) enabling the compound 3 and a biocatalyst to produce a compound 4 in an organic solvent in the presence of glucose or sucrose; (3) enabling the compound 4 to react with trifluoroacetic anhydride so as to obtain a compound 5; (4) enabling the compound 5 to react with R<2>ONH2 so as to produce a compound 6; (5) hydrolyzing the compound 6 under alkaline conditions so as to produce a compound 7; (6) enabling the compound 7 to react with triphosgene so as to produce a compound 8; (7) enabling the compound 8 to react with ammonium formate in the presence of a catalyst so as to produce a compound 9 in the organic solvent, wherein the catalyst is a Pd / C system; and (8) enabling the compound 9 to react with a sulfonating agent, thereby obtaining the product 1. The method disclosed by the invention is stable in process, high in yield, simple and safe in operation and low in production cost.
Owner:YIYUAN XINQUAN CHEM
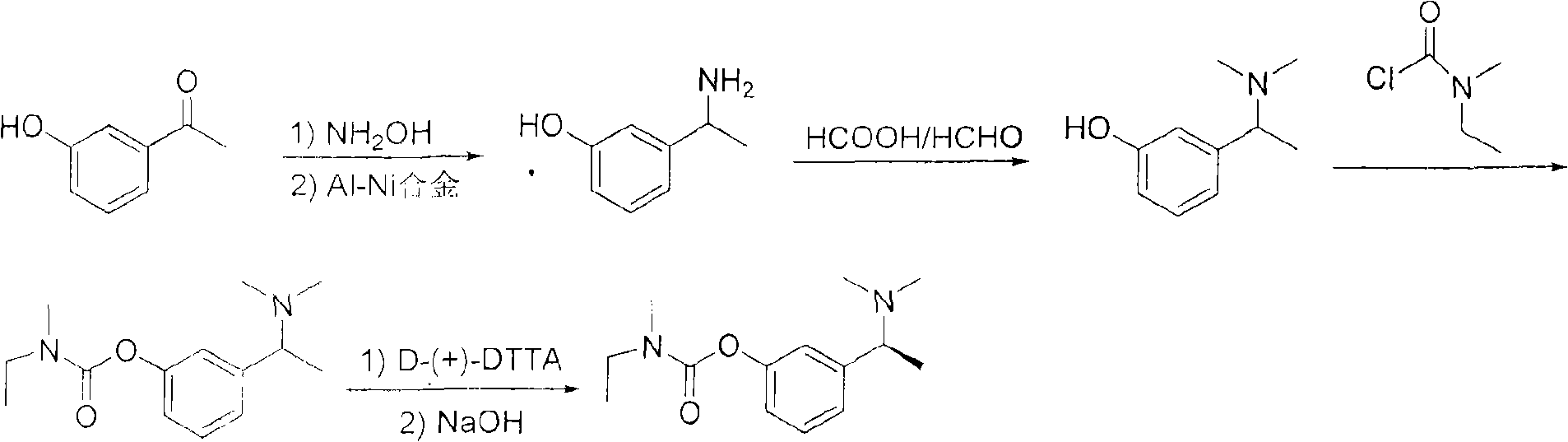
Method for preparing rivastigmine hydrogen tartrate and application thereof
InactiveCN101580482AEasy to operateLow costNervous disorderCarbamic acid derivatives preparationPhosgeneTriphosgene
The invention relates to a method for preparing rivastigmine hydrogen and tartrate thereof, which comprises the following steps: taking metamethoxyacetophenone as an initial raw material, and obtaining 1-(3-methoxyphenyl)ethanol by the reduction; then performing the chlorination to obtain 1-(chloroethyl)-3-methoxyphenyl; then reacting the1-(chloroethyl)-3-methoxyphenyl with dimethylamine hydrochloride to obtain 1-(3-methoxyphenyl)-N, N-dimethylethanamine; demethylating the reaction product to obtain 3-[1-(dimethylamino)ethyl]phenol; then performing salt formation resolution with (s)-(+)-camphor-10-sulfonic acid, recrystallizing, and dissociating to obtain (s)-3-[1-(dimethylamino)ethyl]phenol; then taking ethylamine as a raw material to react with ethyl formate to obtain formylethylamine; then reacting the formylethylamine with phosphorus oxychloride to obtain an imine intermediate; reducing the imine intermediate by sodium borohydride to obtain ethyl methyl amine; then reacting the ethyl methyl amine with triphosgene to obtain N-methyl-N-ethylformyl chloride; and finally using (s)-3-[1-dimethylamino)ethyl]phenol to condensate with the N-methyl-N-ethylformyl chloride, and then performing salt formation with levotartaric acid to obtain the rivastigmine hydrogen tartrate. The method has the advantages of easily-obtained raw materials, simple and convenient operation, low cost, high yield and small pollution, and is a brandnew synthesis route at present.
Owner:SHENYANG PHARMA UNIVERSITY
Synthetic method for N(2)-L-alanyl-L-glutamine dipeptide
InactiveCN1683391AEasy to separate and purifyThe synthesis process is simpleDipeptidesL-alanyl-l-glutamineDipeptide
The present invention relates to synthesis of dipeptide containing amino acid, and provides a kind of synthesis process of N(2)-L-alanyl-L-glutamine dipeptide with low material cost, simplicity, high yield, no need of separating and purifying intermediate product, easy product separation and purification and environment friendship. The synthesis process includes the reaction of amino acid with protected N-terminal with phosphorus triphenyl oxide and triphosgene in organic solvent to form active ester; the reaction of the active ester with glutamine in water solution of inorganic alkali; acidifying with inorganic acid and eliminating N-terminal protecting radical.
Owner:XIAMEN UNIV
Synthesis of amphipathic block antibacterial peptide as well as preparation method and application of assembly of amphipathic block antibacterial peptide
InactiveCN106750262AImprove antibacterial propertiesRaw materials are cheap and easy to getChemical/physical/physico-chemical microreactorsPharmaceutical non-active ingredientsOrganic solventBiological materials
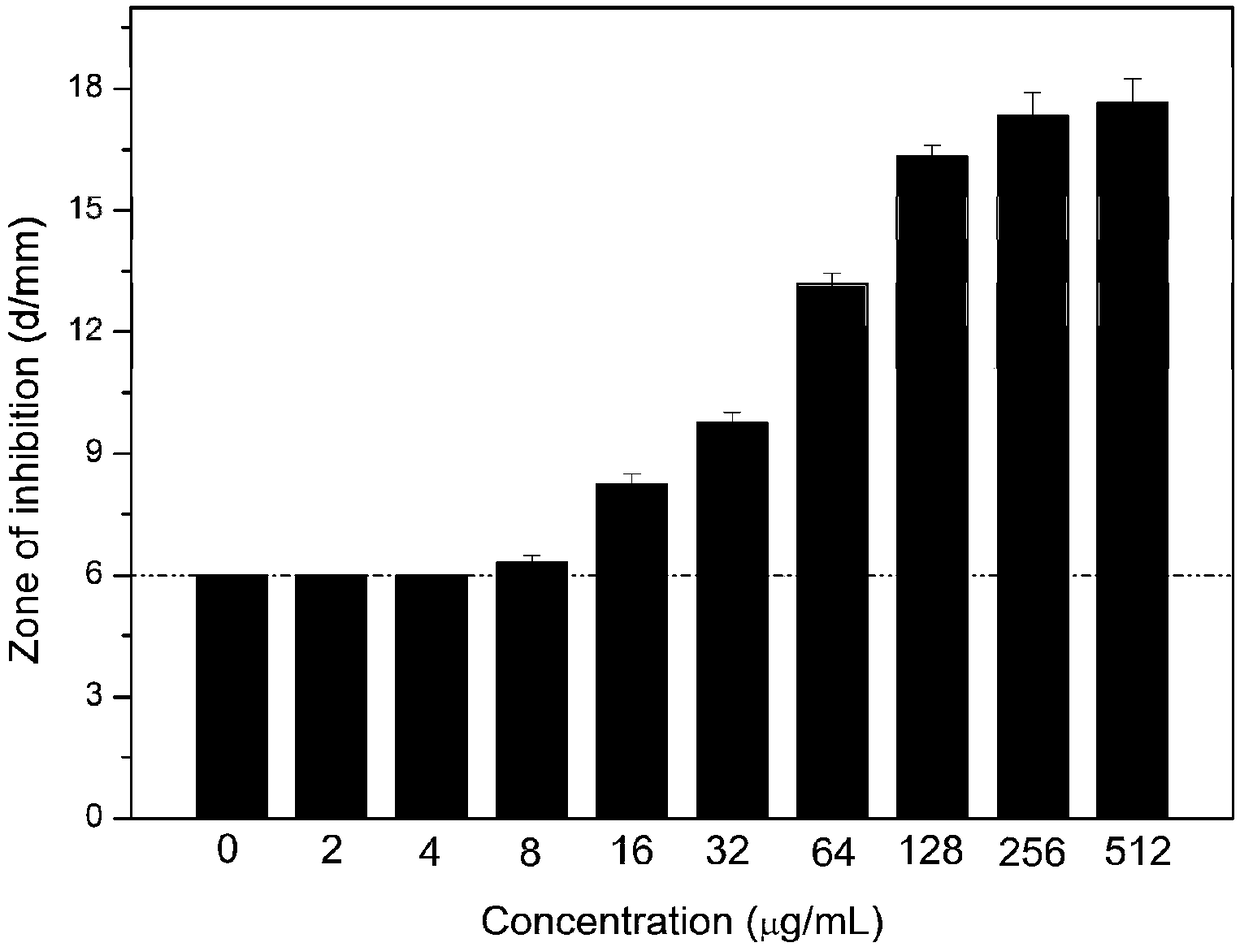
The invention provides synthesis of amphipathic block antibacterial peptide as well as a preparation method and application of an assembly of the amphipathic block antibacterial peptide. The amphipathic block antibacterial peptide is prepared from hydrophilic amino acid, hydrophobic amino acid, triphosgene, an organic solvent, an initiator, a de-protection agent and a precipitant. The amphipathic block antibacterial peptide provided by the invention has broad-spectrum and excellent antibacterial properties, is an economic, low-toxic and stable biological material, has wide application prospects and values, is low in raw material price, easy in raw material obtaining and low in cost, and in addition, the synthesis is simple in route and controllable in condition.
Owner:TONGJI UNIV
Preparation method of novel hyperbranched antibacterial peptide polymer
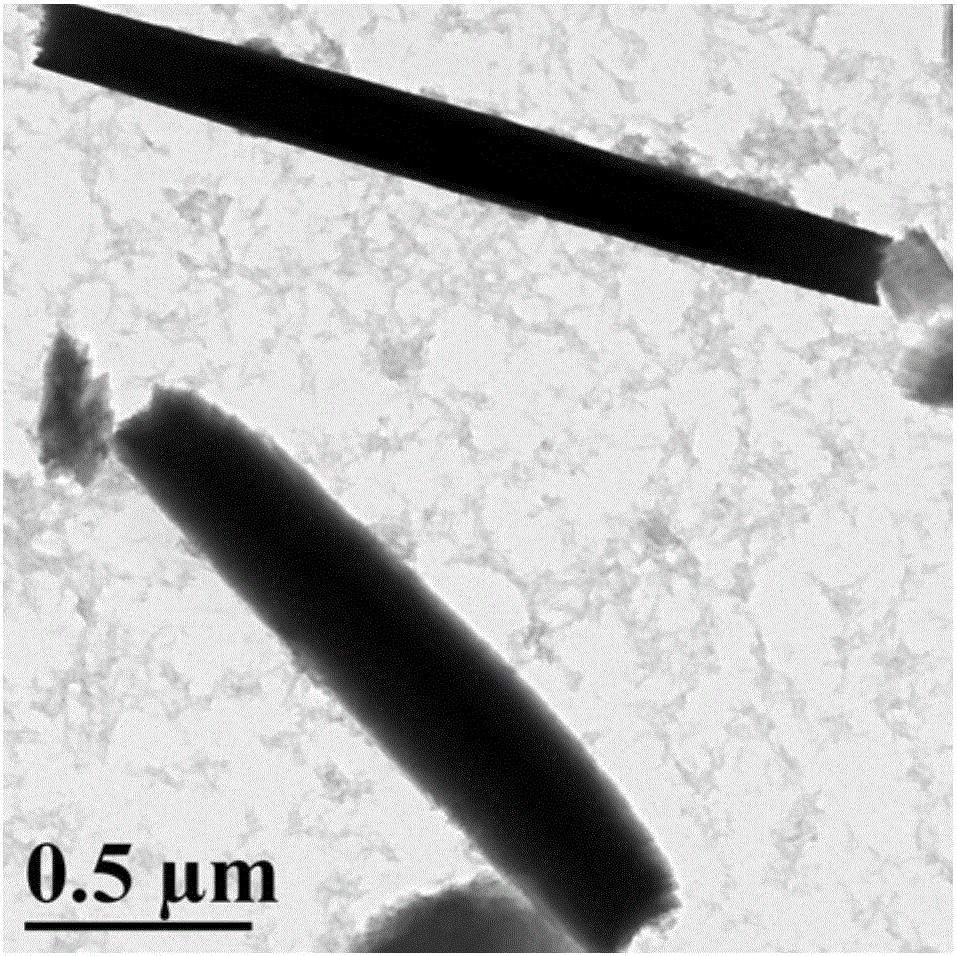
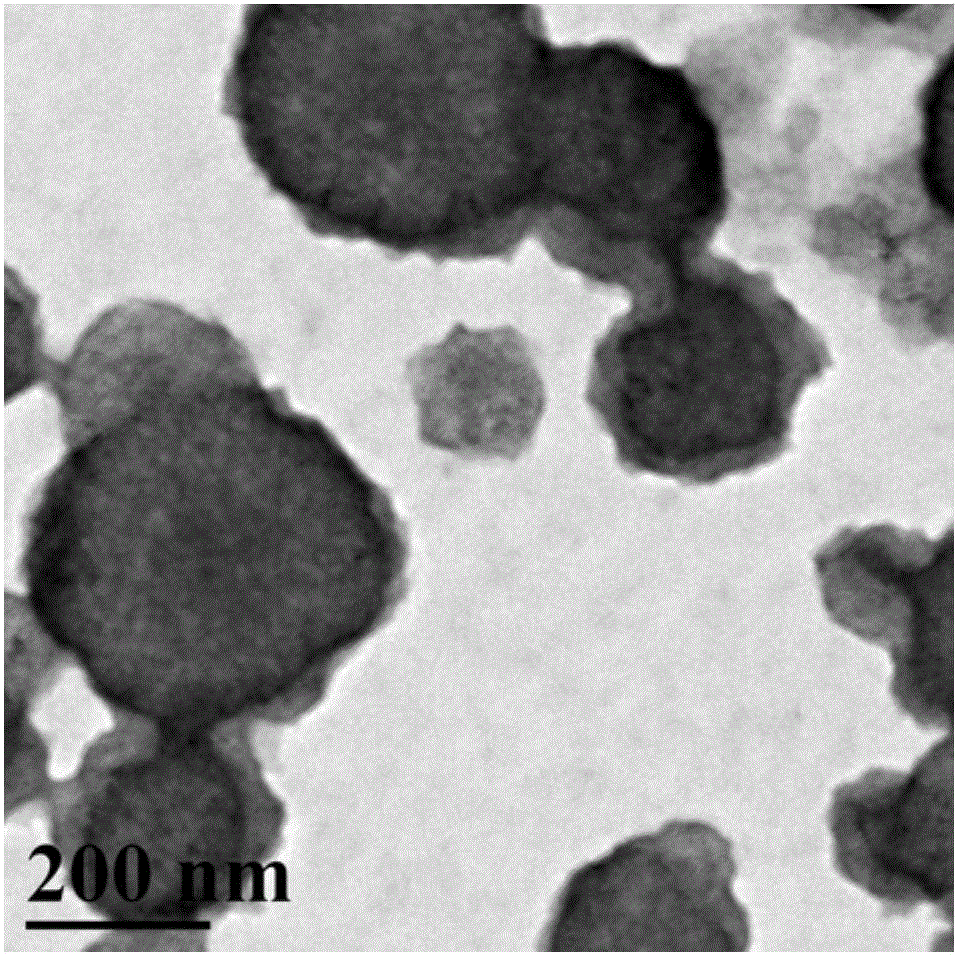
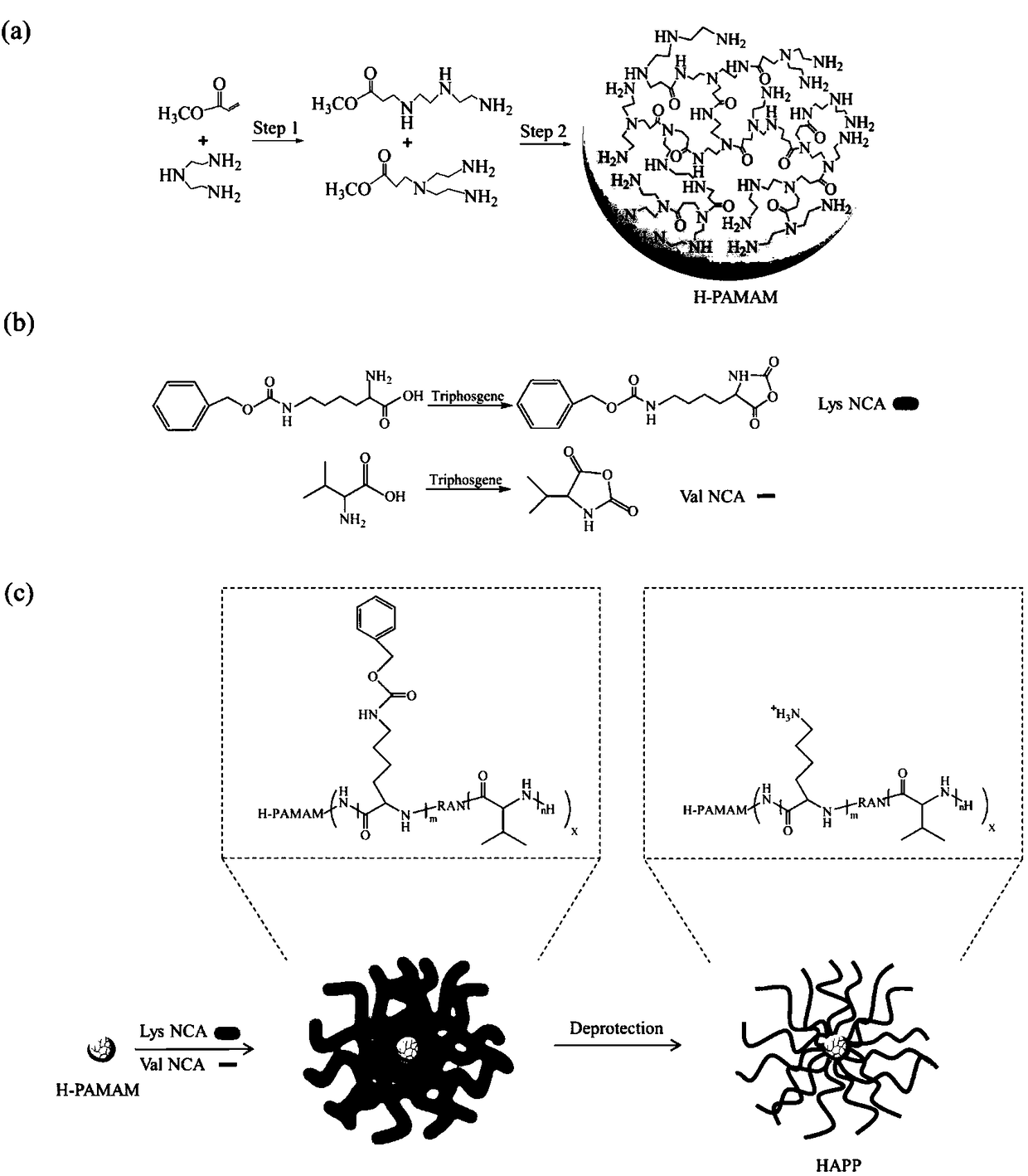
InactiveCN108329467AImprove antibacterial propertiesGood biocompatibilityAntibacterial agentsPeptide/protein ingredientsDiethylenetriamineCarboxylic acid
The invention discloses a preparation method of a novel hyperbranched antibacterial peptide polymer. The preparation method comprises the following steps: 1) synthesizing an amino-terminated hyperbranched polyamidoamine: preparing an amino-terminated low-molecular-weight hyperbranched polyamidoamine through a Michael addition reaction of methyl acrylate and diethylenetriamine; 2) synthesizing lysine-N-carboxylic acid anhydride and valine-N-carboxylic acid anhydride: taking the amino-terminated hyperbranched polyamidoamine as a core, and conducting a reaction of benzyloxycarbonyl lysine with triphosgene and a reaction of valine with triphosgene to obtain the lysine-N-carboxylic acid anhydride and the valine-N-carboxylic acid anhydride; and 3) synthesizing the hyperbranched antibacterial peptide polymer: grafting the lysine-N-carboxylic acid anhydride and the valine-N-carboxylic acid anhydride onto the terminated amino groups of the hyperbranched polyamidoamine through polypeptide chainsformed by ring opening polymerization, and carrying out amino group depretection and dialysis to prepare the antibacterial peptide polymer with a hyperbranched structure. The preparation method has the beneficial effects that the preparation method is simple and convenient, the raw materials are easy to obtain, the synthesis cost is low, and the production is convenient for large-scale production.
Owner:UNIVERSITY OF CHINESE ACADEMY OF SCIENCES
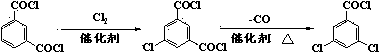
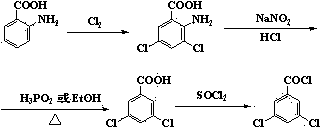
Method for synthesizing 3,5-dichlorobenzoyl chloride
ActiveCN103508880AGood effectAvoid recyclingOrganic compound preparationCarboxylic compound preparationSulfonyl chlorideWater chlorination
The invention discloses a method for synthesizing 3,5-dichlorobenzoyl chloride. The method comprises the following steps: carrying out sulfonation on benzoyl chloride by sulfur trioxide to obtain 5-chloroformyl metaphenylene disulfonic acid; carrying out chlorination by catalyzed triphosgene to obtain 5-chloroformyl metaphenylene disulfuryl chloride; finally, carrying out removal of sulfur dioxide chlorination to obtain 3,5-dichlorobenzoyl chloride. Compared with an existing preparation method, the method disclosed by the invention has the characteristics of stable chlorinating agent, high production safety, small amount of three wastes, no requirement for recovering and treating a great amount of dangerous byproducts, no requirement for refining a coarse product, low production cost and the like and is suitable for industrial production.
Owner:江西吉翔医药化工有限公司
Process for the preparation of sucralose by the chlorination of sugar with triphosgene (BTC)
In one embodiment of the invention a method to prepare sucralose-6-acylate through chlorinating sucrose-6-acylater by BTC in the process of sucralose preparation is disclosed. In this embodiment a Vilsmeier reagent is firstly prepared below 0° C. by dissolving BTC in DMF or in component solvent, containing DMF, toluene, dichloroethane, chloroform and carbon tetrachloride. Consequently, sucrose-6-ester was chlorinated by Vilsmeier reagent. BTC can also be dissolved in one or several organic solvent such as toluene, dichloroethane, chloroform and carbon tetrachloride, and added to a DMF solution of sucrose-6-acylate for chlorination. Sucralose was prepared through de-esterifying the obtained sucralosed 6-ester using sodium methoxide / methanol or sodium ethoxide / ethanol.
Owner:MAMTEK INT
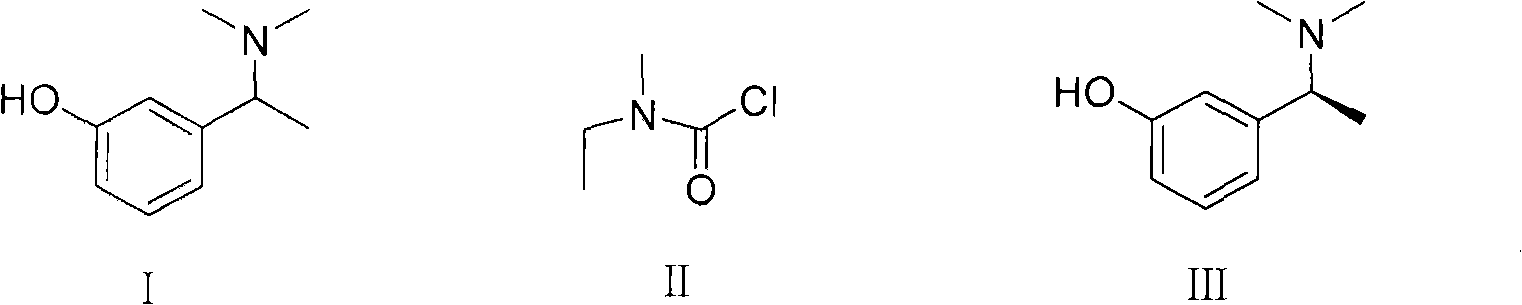
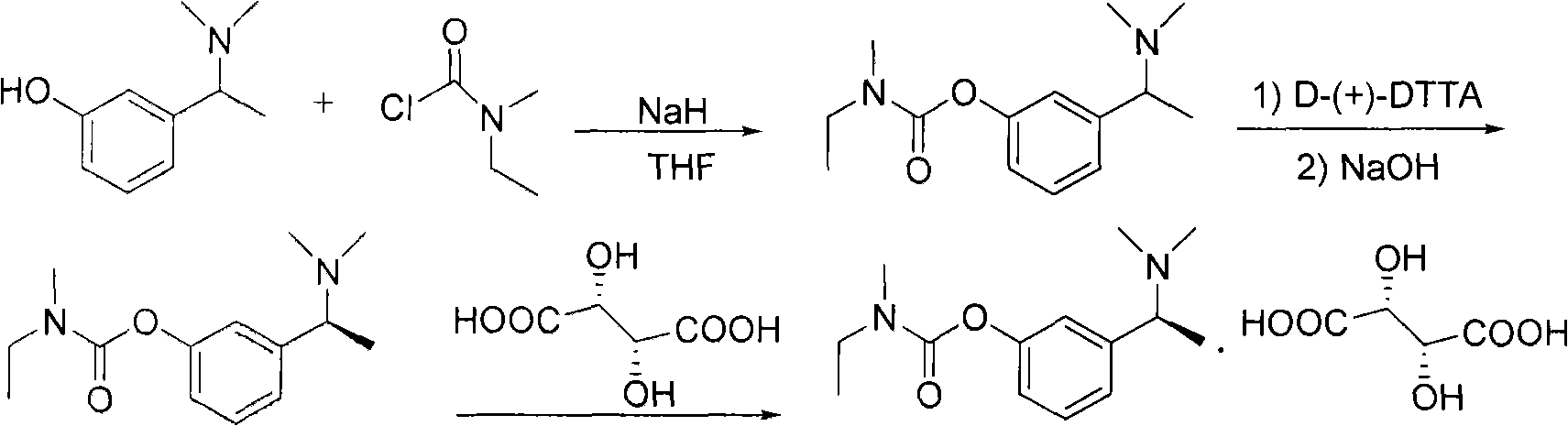
4-[4-(substituted phenyl) piperazine piperazinyl-1]-butylcarbamic acid substituted aromatic ester derivative and preparation method thereof
ActiveCN103073524AHigh affinityEffective treatmentNervous disorderOrganic chemistryReaction intermediateStructural formula
The invention discloses a phenyl piperazidine heterocyclic medicinal compound. The compound has high affinity to a dopamine D3 receptor, so that the compound can be used for treating addiction to and dependence on medicines such as cocaine, and a central nervous system disorder relevant to the addiction and the dependence. The compound is a 4-[4-(substituted phenyl) piperazine piperazinyl-1]-butylcarbamic acid substituted aromatic ester derivative with a structural formula as Formula (1) as shown in the specification. A synthetic method of the derivate comprises the steps that substituted aniline and 2-(beta-chloroethyl) amine hydrochloride reacts in a solvent by taking inorganic base as an acid-binding agent to form corresponding substituted phenyl piperazidine hydrochloride 1; substituted phenyl piperazidine hydrochloride 1 and N-(delta-bromobutyl) phthalimide react in acetonitrile by taking K2CO3 as an acid-binding agent and under catalysis of KI to form a reaction intermediate 2; the intermediate 2 is subjected to hydrazinolysis to form an intermediate 3; and the intermediate 3 and an intermediate 5 are condensed by taking triethylamine as an acid-binding agent and a catalyst to form a target product I. The intermediate 5 is obtained in a manner that triphosgene and substituted aromatic phenol conduct partial condensation reaction in methylene chloride.
Owner:宁波市微循环与莨菪类药研究所 +1
Environment friendly method for preparing DMF (Dimethyl Formamide) solution of 2-hydroxy-benzonitril, DMF solution of 2-hydroxy-benzonitril and application thereof
ActiveCN102516122AReduce manufacturing costOrganic compound preparationPreparation by carboxylic acid amide dehydrationInorganic saltsWastewater
The invention discloses an environment friendly method for preparing a DMF (Dimethyl Formamide) solution of 2-hydroxy-benzonitril, which is characterized in that triphosgene and salicylamide are respectively added into DMF to be fully reacted. With the preparation method disclosed by the invention, more than 98% of 2-hydroxy-benzonitril (excluding solvent) can be obtained, the chemical conversion and the selectivity are both greater than 98%, and no problems exist if the 2-hydroxy-benzonitril is directly applied for the next step of reaction. In the process of production, no polluting waste gas and waste water are discharged, scraps are inorganic salts which can directly used or organic amine hydrochlorides which can be recycled, the comprehensive production cost of the 2-hydroxy-benzonitril is greatly reduced, and the production process is environment friendly.
Owner:上海禾本药业股份有限公司
Method for synthesizing triphenyl phosphine dichloride
ActiveCN1660862AEasy to transportEasy to storeGroup 5/15 element organic compoundsOrganic solventTriphenylphosphine oxide
A process for synthesizing phosphine triphenyldichloride features the reaction between phosphine triphenyloxide and triphosgene or biphosgene in organic solvent under existance of the nucleophilic catalyst chosen from N,N-dimethyl formamide, triethylamine, pyridine, etc.
Owner:ZHEJIANG UNIV +3
9-fluorenylmethyl chloroformate preparation method
InactiveCN103408427ASimple processMild conditionsOrganic compound preparationCarbonic/haloformic acid esters preparationChloroformateSolvent
The present invention relates to a 9-fluorenylmethyl chloroformate preparation method, particularly to an improved method for preparing 9-fluorenylmethyl chloroformate from 9-fluorenylmethanol. The method comprises the following steps: 1) adding chloroform, 9-fluorenylmethanol and triphosgene to a reactor, and stirring for 30 min, wherein raw materials comprise 9-fluorenylmethanol, triphosgene, chloroform and 4-dimethylaminopyridine, and a ratio of the 9-fluorenylmethanol to the triphosgene to the chloroform to the 4-dimethylaminopyridine is 20 g:46 g:200 ml:18.5 g; 2) in an ice bath, adding a chloroform solution of 4-dimethylaminopyridine in a dropwise manner, and carrying out a reaction for 2-4 h; and 3) filtering the reactant in the reactor to obtain a white solid and the filtrate, and sequentially carrying out pressure reduction solvent removing, cryogenic crystallization, organic solvent washing and drying on the filtrate to obtain the white solid 9-fluorenylmethyl chloroformate. The preparation method has characteristics of mild reaction conditions, low cost, simple process, and easy industrialization.
Owner:ZHANG JIA GANG VINSCE BIO PHARM
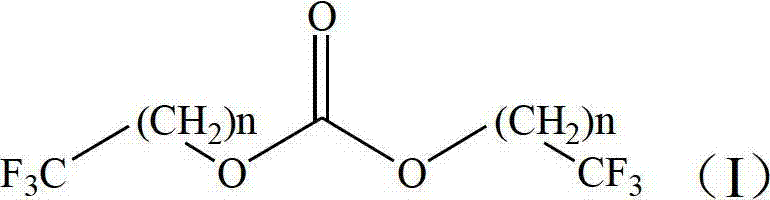
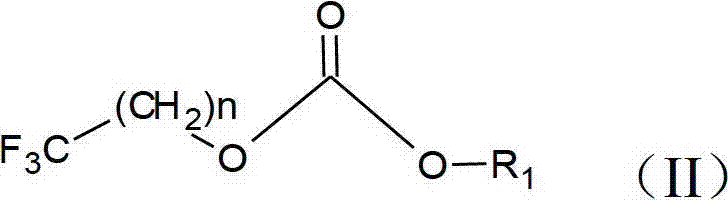
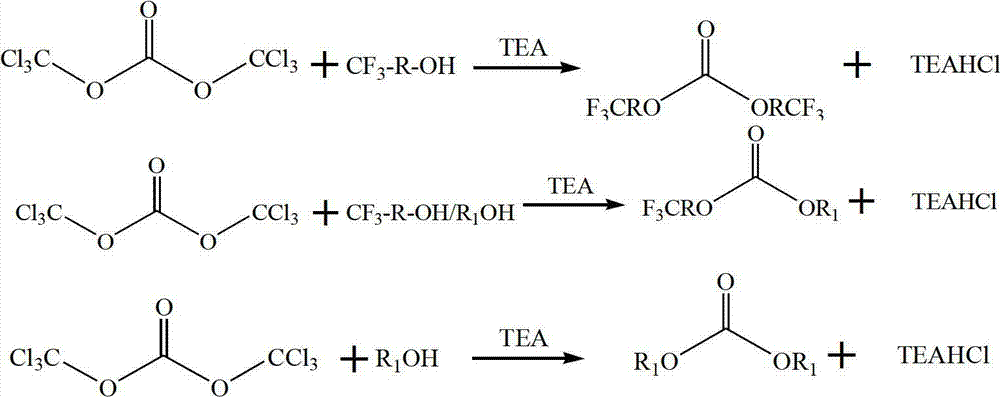
Preparation method of trifluoromethyl straight-chain carbonate
ActiveCN102775312AImprove stabilityImprove thermal stabilityCarbonic/haloformic acid esters purification/separationPreparation from phosgene or haloformatesAlcoholBoiling point
The invention discloses a preparation method of trifluoromethyl straight-chain carbonate, which comprises the following preparation steps: mixing trifluoromethyl saturated monohydric alcohol or a mixture of trifluoromethyl saturated monohydric alcohol and saturated straight-chain monohydric alcohol with triphosgene; regulating the temperature to 25-80 DEG C in the presence of an organic amine acid-binding agent, and reacting for 1-10 hours to obtain a trifluoromethyl straight-chain carbonate mixed solution; and filtering, separating, and performing distilling purification on the trifluoromethyl straight-chain carbonate mixed solution to obtain the trifluoromethyl straight-chain carbonate. The trifluoromethyl straight-chain carbonate prepared by the process is a high voltage type solvent for a novel power lithium battery, and can greatly enhance the heat stability, cycle performance and high voltage property of the power lithium battery. The preparation method has the advantages of simple process, high yield and low cost; and meanwhile, the triphosgene is high in stability and is decomposed a little at the boiling point of 200 DEG C. Thus, the preparation process has no serious problems on safety, environmental protection and the like.
Owner:ZHANGJIAGANG HUASHENG CHEM CO LTD
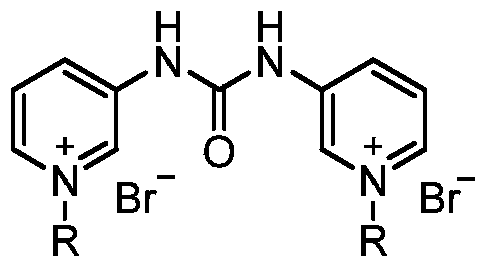
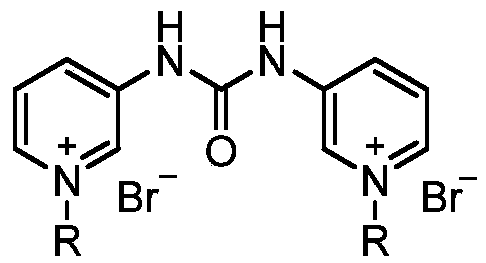
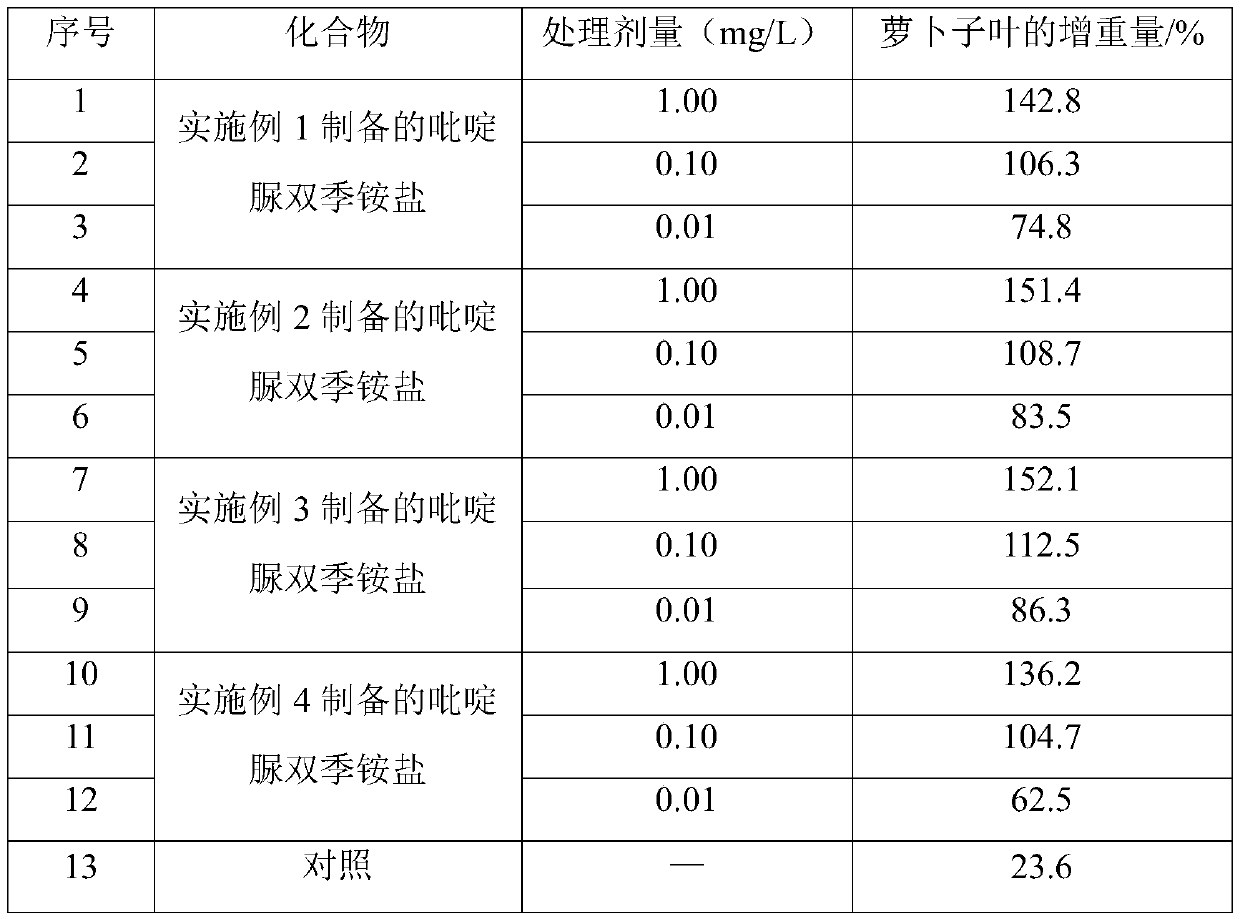
Pyridylurea biquaternary ammonium salt as well as preparation method and application thereof
ActiveCN105503711APromote divisionGood water solubilityPlant growth regulatorsBiocideSolubilitySolvent free
The invention relates to a pyridylurea biquaternary ammonium salt as well as a preparation method and application thereof. The preparation method of the pyridylurea biquaternary ammonium salt comprises the following steps: using 3-aminopyridine as a staring material, conducting reaction on 3-aminopyridine and triphosgene to obtain 1,3-bi(3-pyridyl)urea, and conducting reaction on 1,3-bi(3-pyridyl)urea and alkyl bromide under the solvent-free condition to prepare the pyridylurea biquaternary ammonium salt. According to the method, a solvent-free method is adopted to prepare the pyridylurea biquaternary ammonium salt, so that the environment can be protected, and the yield is relatively high. The provided pyridylurea biquaternary ammonium salt can be used as a plant growth regulator to promote plant cell division, and the problem that the traditional phenylurea plant growth regulator is poor in water solubility is solved.
Owner:SHANXI UNIV
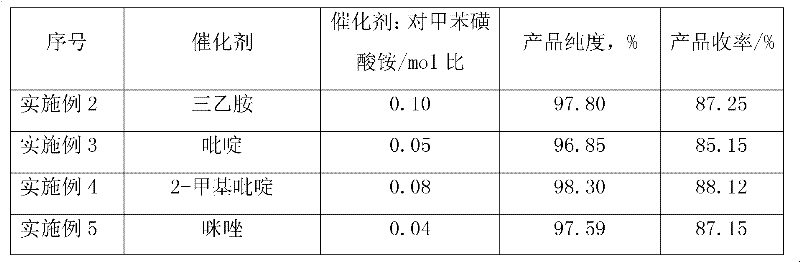
Method for preparing paratoluensulfonyl chloride
The invention discloses a method for preparing paratoluensulfonyl chloride, which comprises the following step of: reacting paratoluenesulfonic acid ammonium salt with bis(trichloromethyl) carbonate (commonly called triphosgene) in an inert organic solvent under the condition that organic alkali is used as a catalyst to synthesize the paratoluensulfonyl chloride. The preparation method has the advantages that: raw materials are conveniently and readily available, the process is simple and suitable for scale-up production, reaction conditions are mild, and a product is easy to purify. The prepared paratoluensulfonyl chloride is an important fine chemical product and can be used for preparing a dye intermediate, synthesizing intermediates of more than ten kinds of antibacterial medicines and anti-inflammatory medicines such as betamethasone, sulfamylon and the like and synthesizing plastic plasticizers, resin, coatings, pesticides and light-sensitive materials.
Owner:SINOCHEM LANTIAN +1
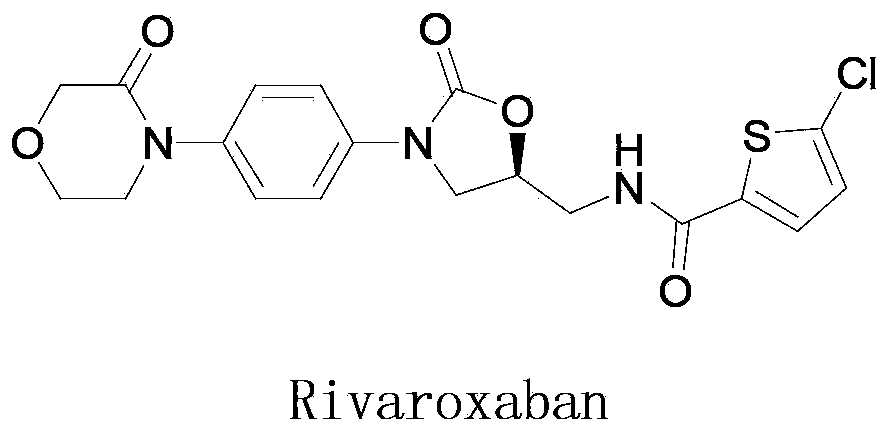
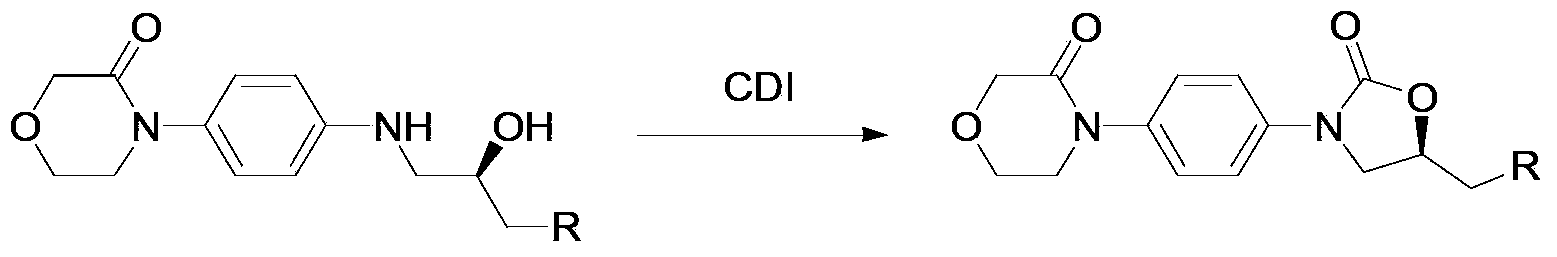
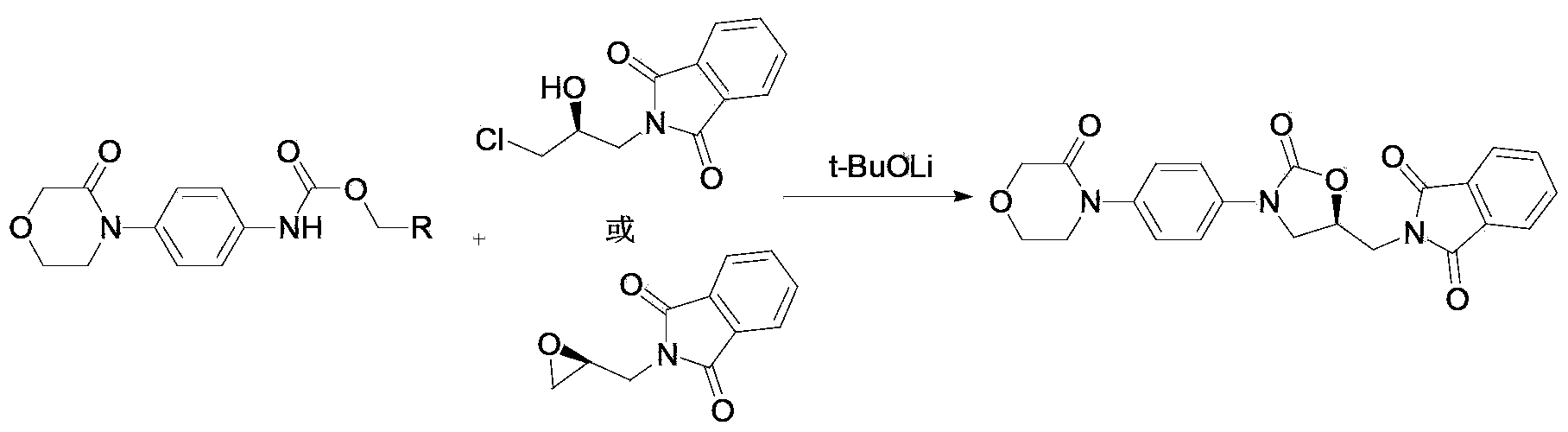
Preparation method for rivaroxaban intermediate
The invention provides a preparation method for a rivaroxaban intermediate. The preparation method is characterized in that a compound represented by formula 1 and triphosgene are used as raw materials and undergo a reaction in an organic solvent at a temperature of 40 to 100 DEG C for 1 to 10 h under the action of an organic amine catalyst, then pressure reduction is carried out to remove the solvent, and an obtained crude product is subjected to refining in the organic solvent so as to prepare a compound represented by formula 2, wherein the formula 1 and the formula 2 are described in the specification. According to the invention, the starting raw materials are easily available on the market and are cheap; triphosgene is used to replace expensive carbonyldiimidazole, CO2 and HCL gas are generated after the reaction, a product is easy to purify, and reaction yield and purity are effectively improved; triphosgene is used to replace expensive carbonyldiimidazole, HCL gas generated after the reaction can be utilized after recovery, so the purpose of green production is achieved; operation process is simple, and rigor conditions like low temperature, no water and no oxygen are not needed.
Owner:ZHEJIANG LIAOYUAN PHARM CO LTD
New method for preparing cefuroxime sodium compound
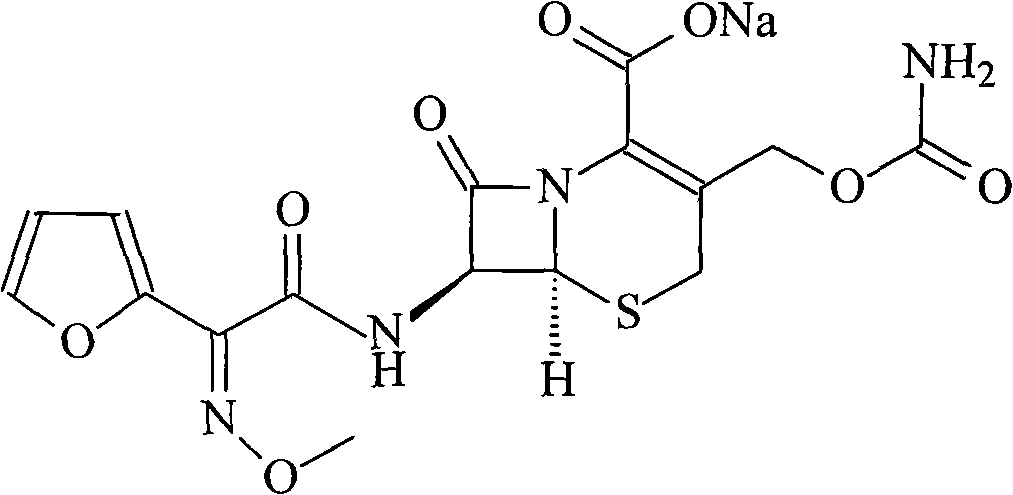
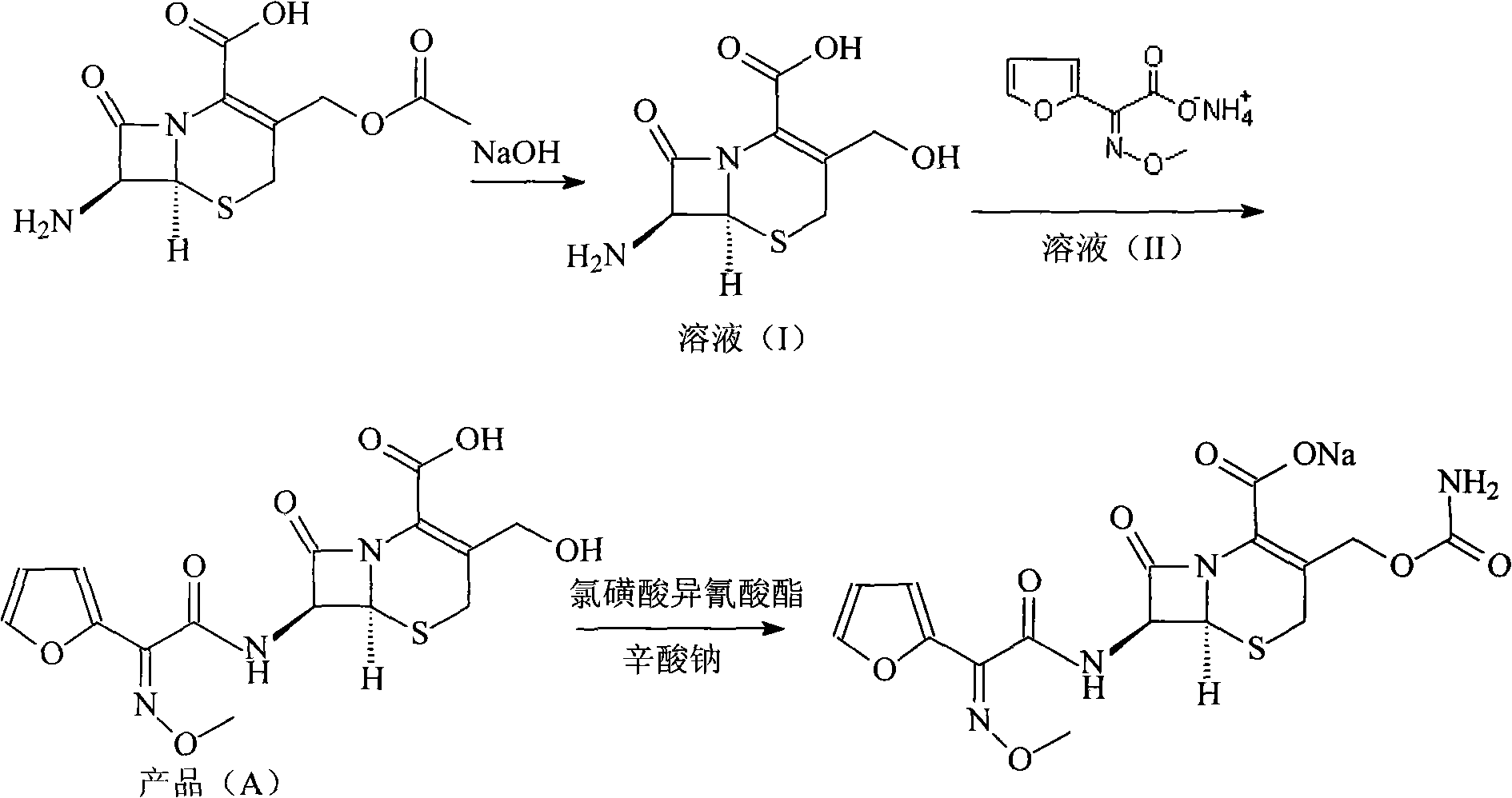
ActiveCN101671349AImprove responseSuitable for large-scale industrial productionOrganic chemistryOrganic-compounds/hydrides/coordination-complexes catalystsCephalosporanic AcidsTriphenylphosphine oxide
The invention relates to a new method for preparing a cefuroxime sodium compound. A target product is prepared by using triphosgene and triphenylphosphine oxide as catalysts, reacting 7-amino-cephalosporanic acid with (Z)-methoxyl imido furylacetic acid ammonium salt and sequentially adding chlorosulfonyl isocyanate and sodium iso-octoate for reaction. The cefuroxime sodium compound prepared by the method is greatly enhanced in purity and yield coefficient and has advantages of inexpensive using materials, simple synthesizing technology and equipment and easy production separation and purification.
Owner:灵康药业集团股份有限公司
Preparation method for phthalyl chloride, m-phthaloyl chloride and paraphthaloyl chloride
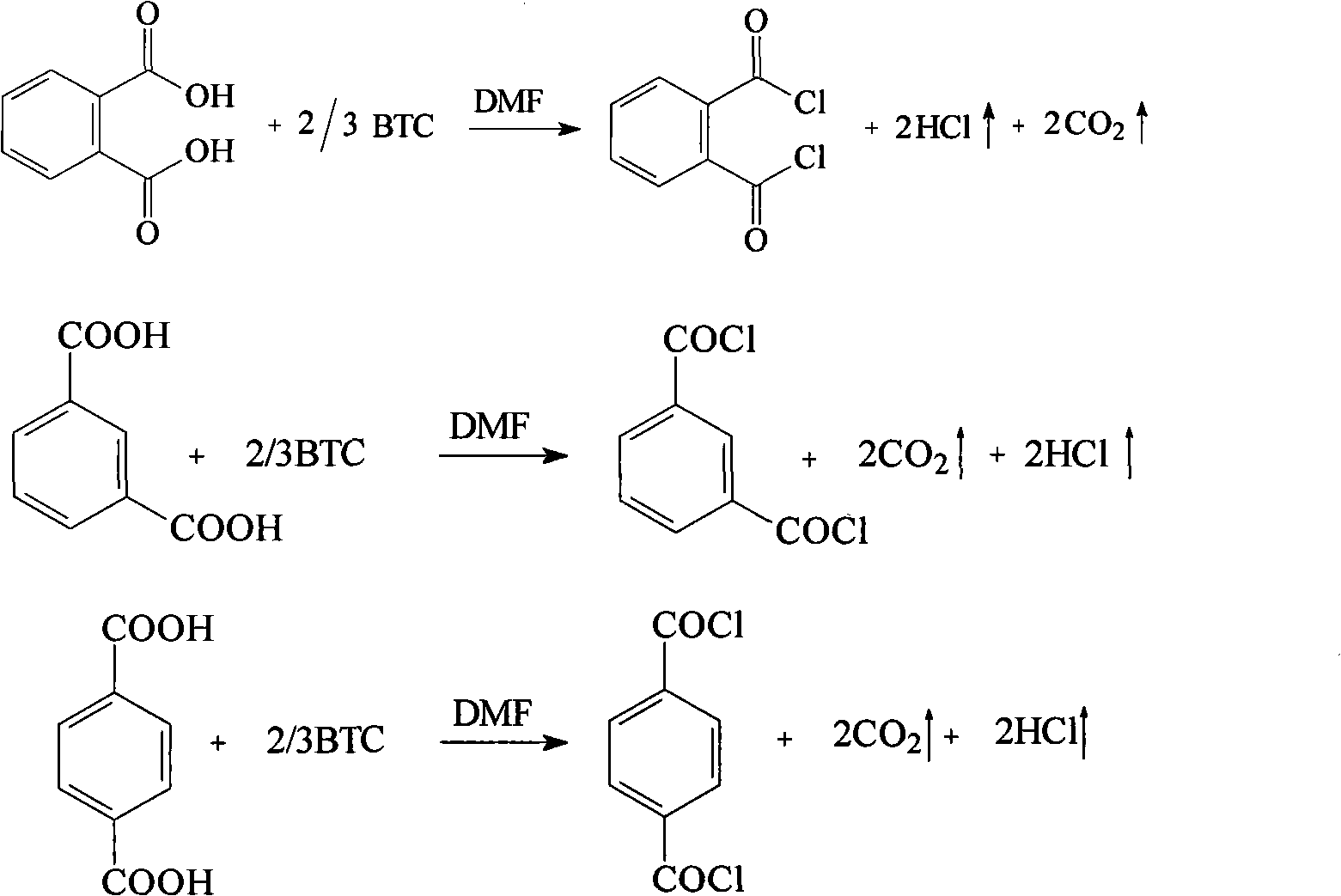
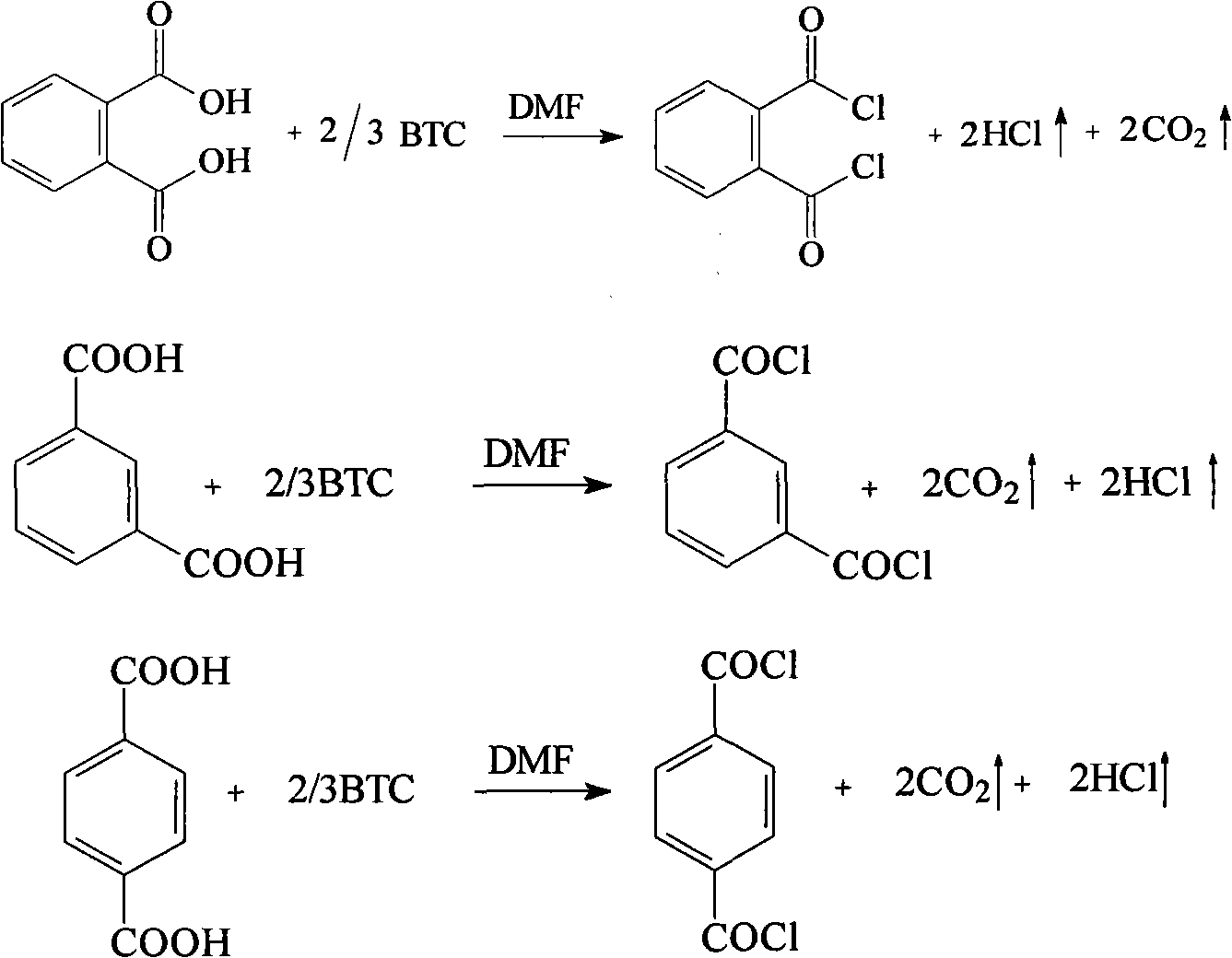
InactiveCN101805257AReduce usageReasonable process conditionsOrganic compound preparationCarboxylic compound preparationReaction temperatureEthane Dichloride
The invention relates to a preparation method for phthalyl chloride, m-phthaloyl chloride and paraphthaloyl chloride. In the method, benzene dicarboxylic acid and triphosgene are taken as raw materials and mixed for catalytic reaction in an organic solvent of 1, 2-dichloroethane, the mol ratio for the catalytic reaction is 0.5-0.9, the catalyst is N, the dosage of N-dimethyl formamide is 0.1-0.6 time of the weight of the raw material benzene dicarboxylic acid, the catalytic reaction lasts for 2-5 hours, and the reaction temperature is the reflux temperature of the solvent 1, 2-dichloroethane. Compared with the prior art, the invention avoids using toxic and hazardous thionyl chloride, phosphorus oxychloride and phosgene and the like. Therefore, the invention has the advantages of reasonable process conditions, simple and safe operation, high reaction yield, low manufacture cost and easy realization of industrial production, and has high implementation value, social benefit, economic benefit and environmental benefit.
Owner:TAIYUAN UNIV OF TECH
Process for synthesizing carbonochloridic acid 9-fluorene methyl ester
InactiveCN101245001ASimple processHigh yieldPreparation from phosgene or haloformatesN dimethylformamidePhosgene
The invention provides a method for synthesizing chlorinated formic acid 9-fluoren methyl, which comprises the following steps: (1) 9-fluoren methyl and triphosgene are reacted for 0.5 to 3 hours in ice bath, under the existence of catalyst and in organic solvent; the catalyst is chosen from N with nucleophilicity, N-dimethylformamide, acetone, diisopropyl ethyl amine, diethylamine, triethylamine, ethyleendiamine, putrescine, imidazole, and the solvent is chosen from benzene, toluene, cyclohexane, hexane, heptane, cyclopentane, dichloromethane, chloroform and the compound thereof; (2) the temperature raises to 20 to 50 DEG C, and the mixture reacts for 1 to 5 hours. The method for synthesizing chlorinated formic acid 9-fluoren methyl adopts safe solid phosgene as reactant, and is simple in technique, convenient in safety, mild in condition, high in yield and easy in realization of industrialization production; the solvent can be used repeatedly after being processed simply, and the products do not need to be refined, and purity is up to more than 98 percent.
Owner:BAOSHAN IRON & STEEL CO LTD
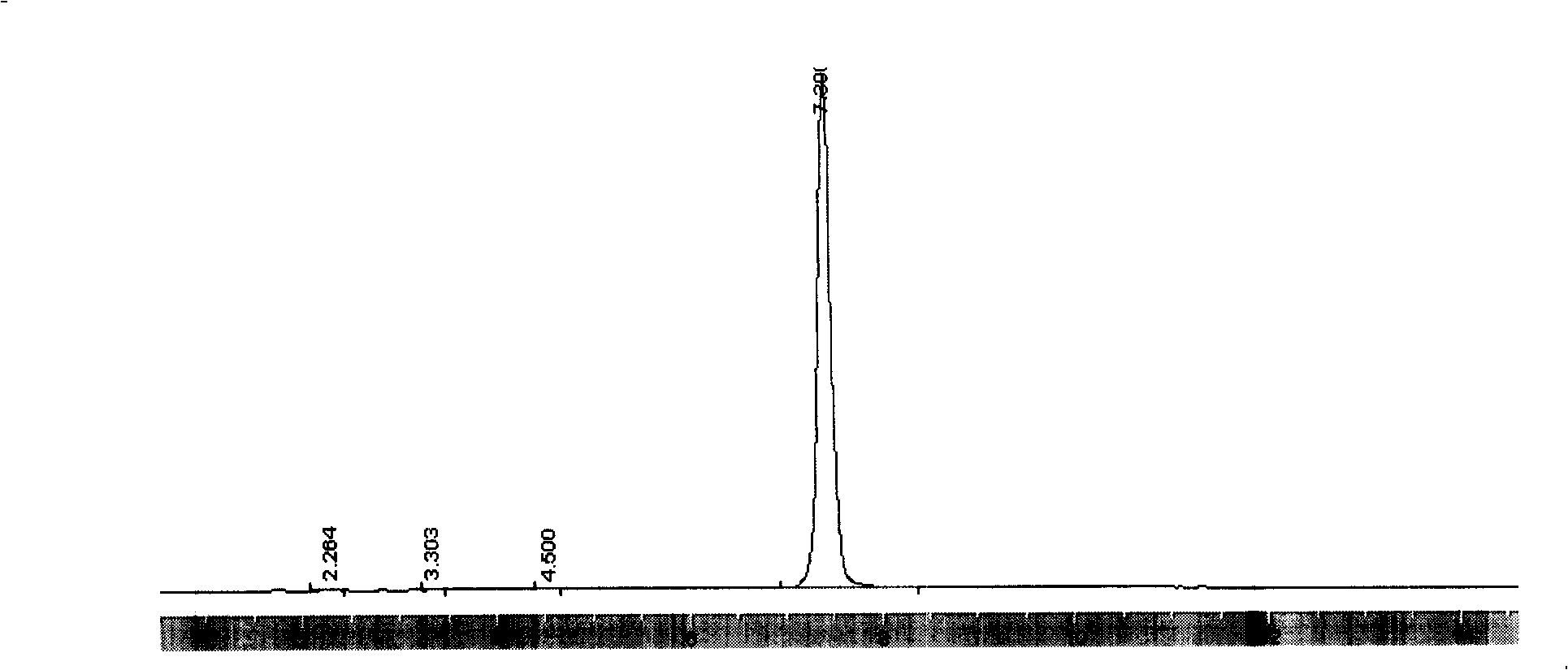
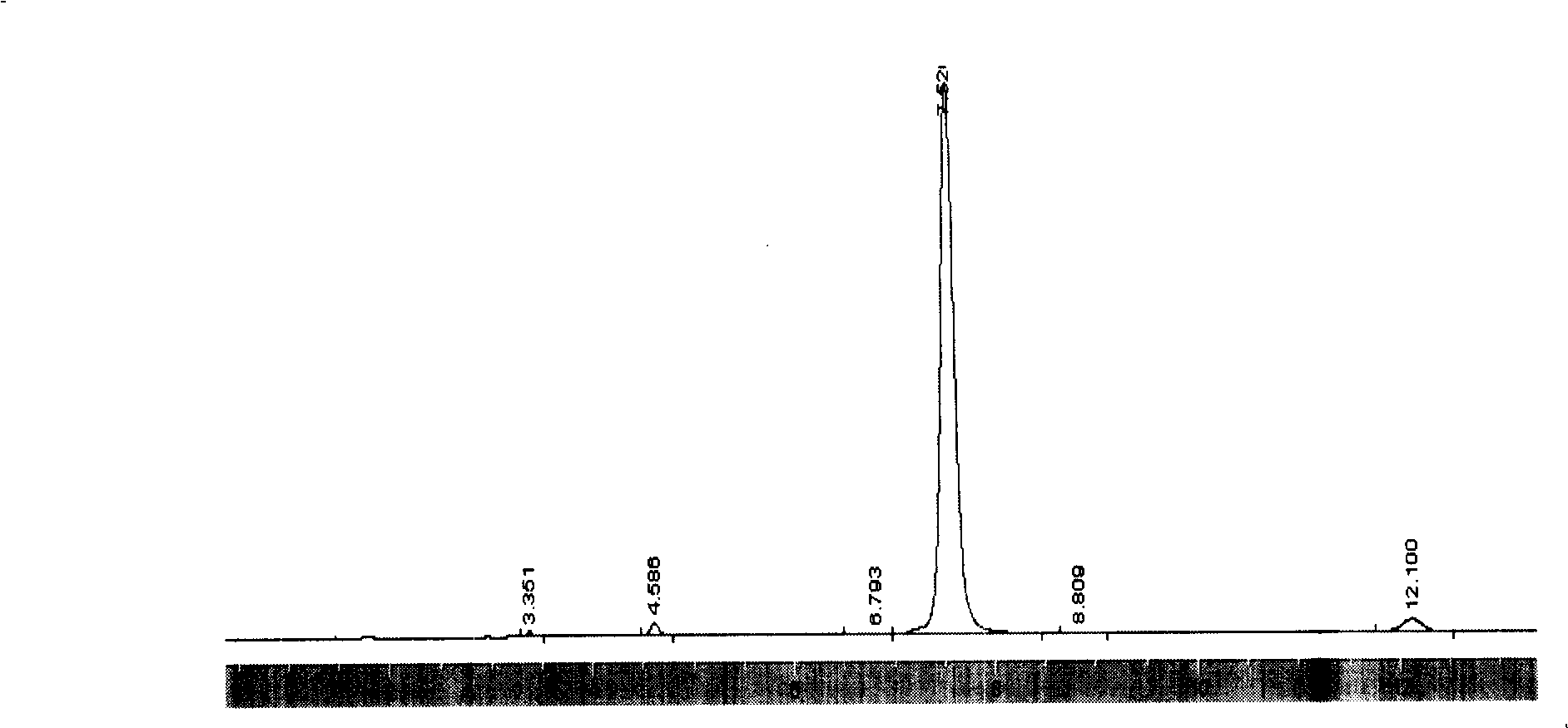
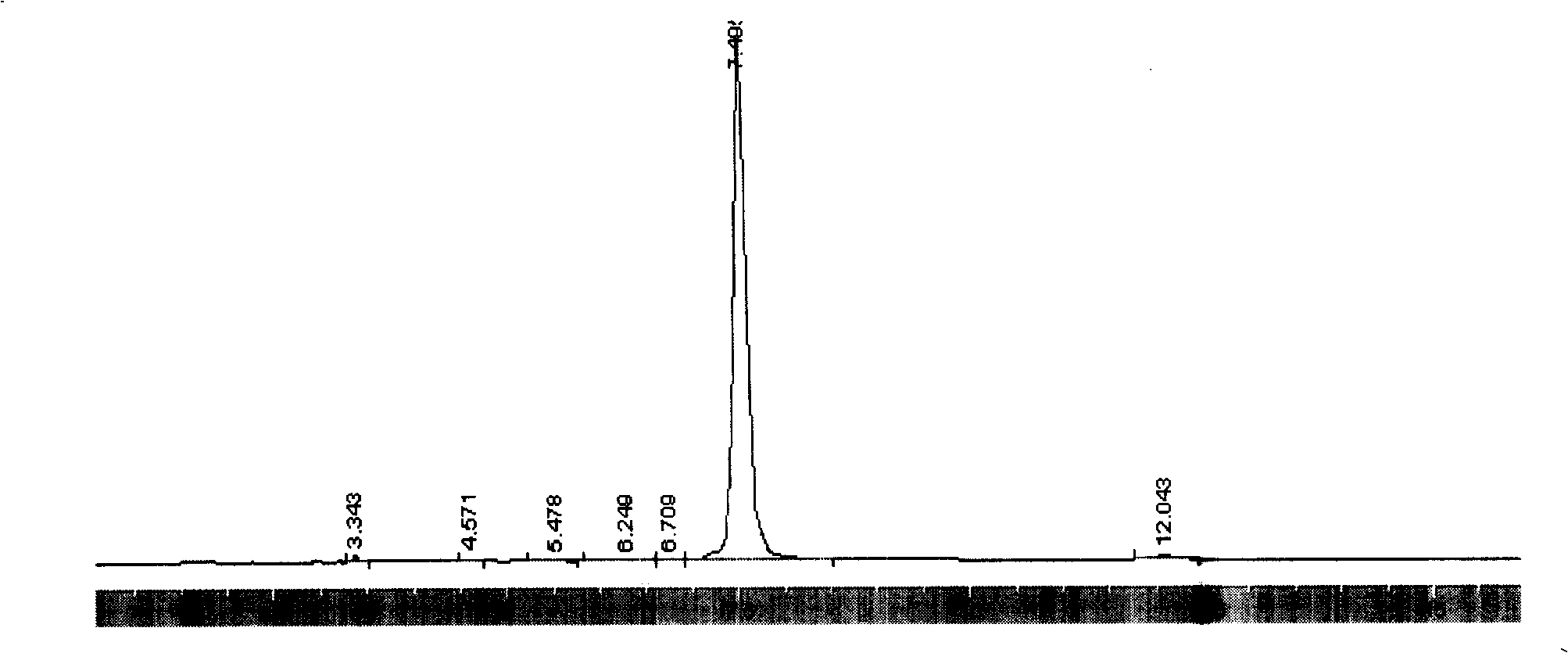
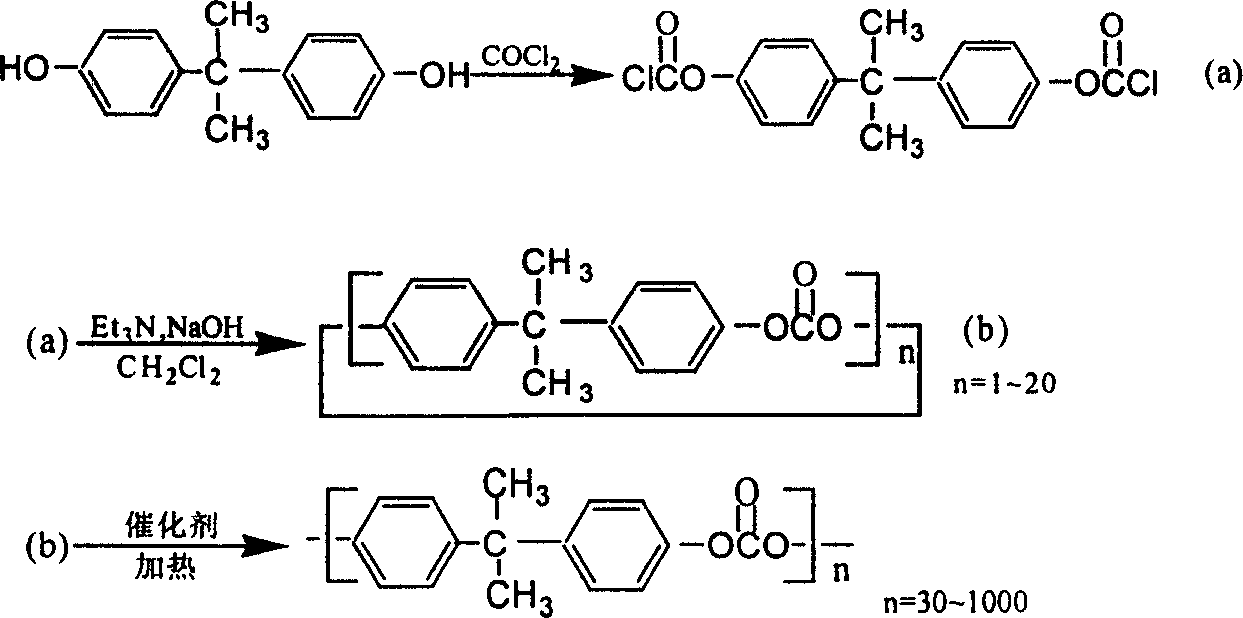
Synthesis process for polycarbonate with super high molecular weight
This invention discloses a synthetic method of extra high molecular weight polycarbonate. Materials are three phosgene and bisphenol A, it includes condensation polymerization, washing, sedimentation and separating each unit processes. The condensation polymerization is that soduim salt water solution of bisphenol an or its soduim salt solid is proceeded liquid-liquid two-phase polycondensation or solid-liquid two-phase suspension polycondensation 3-5 hours under temperature 20-450C with dichloromethane solution of three phosgene with phase transfer catalyst and tertiary amine and spreading agent. The mole ratio of bisphenol an and three phosgene is 1:0.3-0.5. Viscosity-average molecular weight of polycarbonate >70,000, yield that count by bisphenol A >= 98% and by three phosgene >= 85%. The reaction is milden, smooth and is reacted at normal temperature and normal pressure, and operation and control is simple, security is high, and it is appropriate to industrial production.
Owner:HEFEI UNIV OF TECH
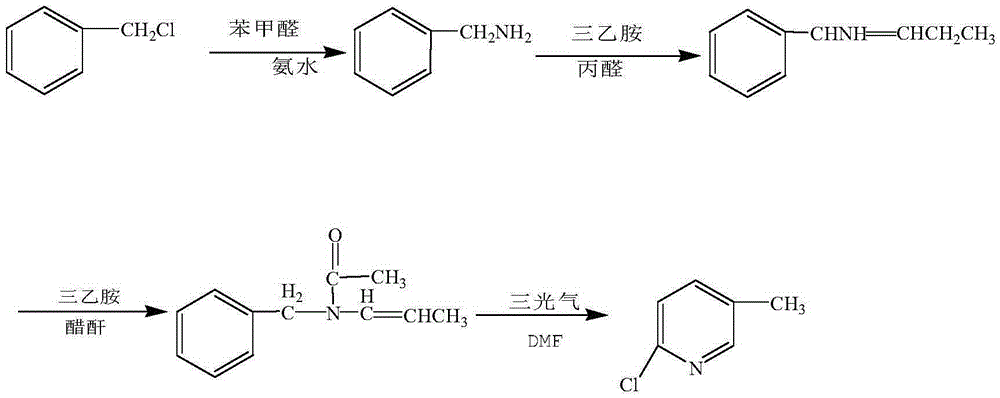
Preparation method of 2-chloro-5-picoline
The invention relates to a preparation method of 2-chloro-5-picoline. According to the method, benzyl chloride is taken as a raw material, benzaldehyde is taken as an inhibitor, ammonia is taken as an aminating agent, a product has a condensation reaction with propionaldehyde under the catalysis of an organic base, then a product has acetylation with an acetylation reagent, finally, a product performs cyclization under actions of N,N-dimethylformamide and triphosgene, and the target product 2-chloro-5-picoline is obtained through purification. The product purity is higher than or equal to 99.5%, and the total molar yield higher than 80% is realized. The preparation method of 2-chloro-5-picoline has good reaction selectivity, high yield and few three wastes, is simple to operate and facilitates industrialized production.
Owner:SHANGHAI JINJING CHEM CO LTD
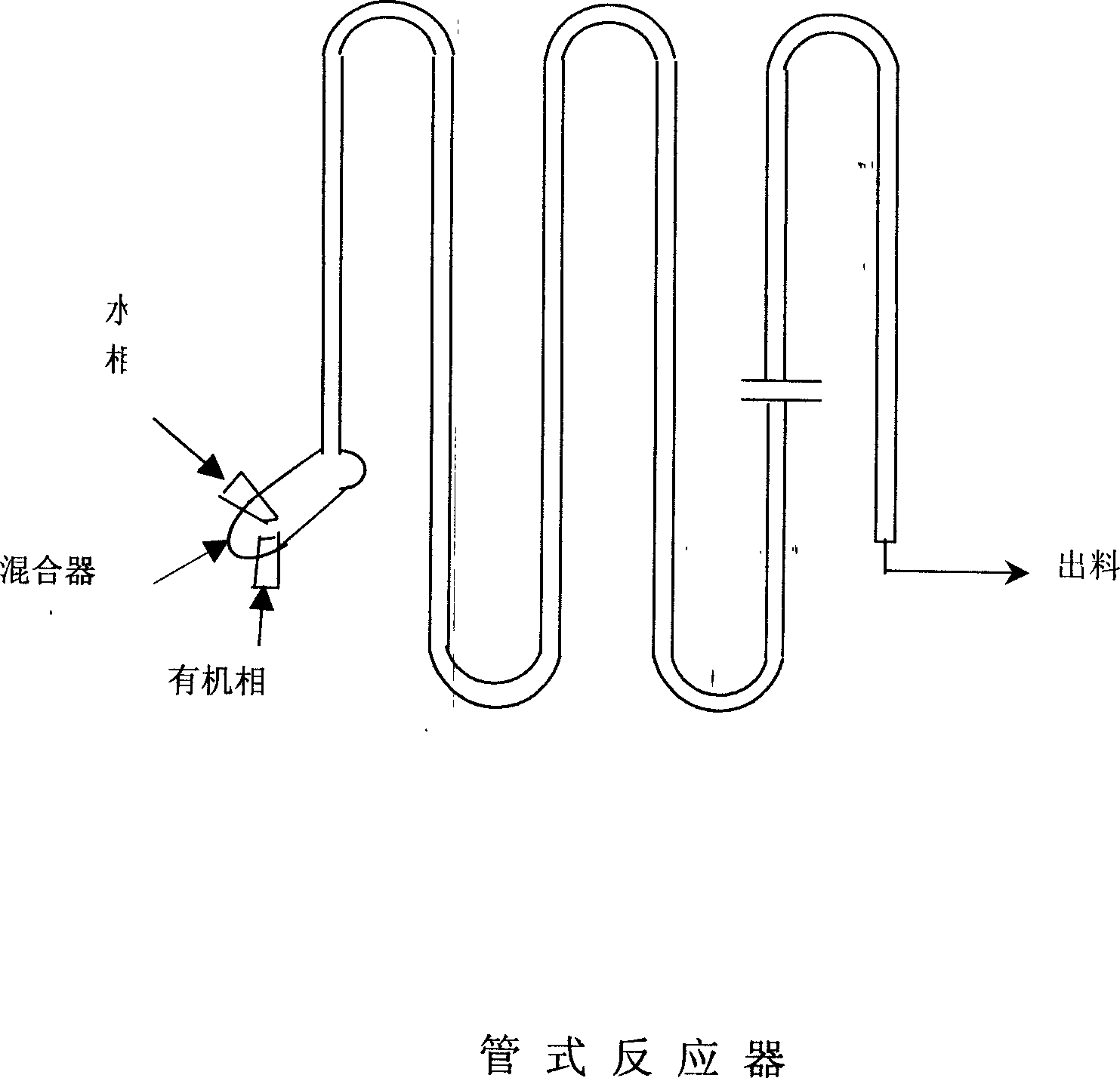
Production of diphenyl carbonate by triphosgene or solid phosgene
InactiveCN101074193AEliminate running, dripping and leakingHigh recovery ratePreparation from organic carbonatesCarbonate esterPolycarbonate
Production of diphenyl carbonate from trichloromethyl-carbonate ester adopts tubular reactor to increase contact effect and has more yield.
Owner:赵云
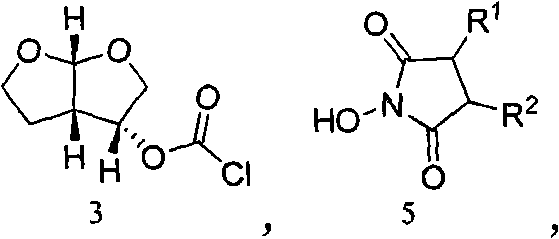
Preparation method of Darunavir intermediate
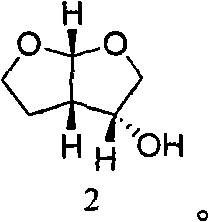
The invention relates to the technical field of heterocyclic chemistry, especially relates to a condensed ring system containing oxygen atoms as the only heterocycle atoms, concretely discloses a preparation method of a Darunavir intermediate. The method comprises the following steps: obtaining a compound of formula (3) by using (3R,3aS,6aR)-hexahydro-furo[2,3-b]furan-3-ol as a raw material to react with triphosgene under alkaline conditions, then directly reacting with a compound of formula (7) to obtain a compound of formula (8); or obtaining a compound of formula (3) to react with an N-hydroxyl compound to prepare active ester, and then reacting with the compound of formula (7) to obtain the compound of formula (8).
Owner:ZHEJIANG JIUZHOU PHARM CO LTD
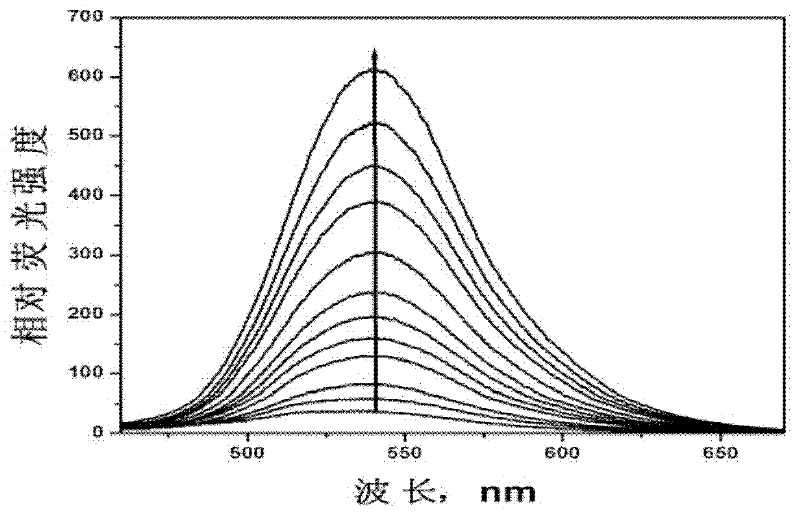
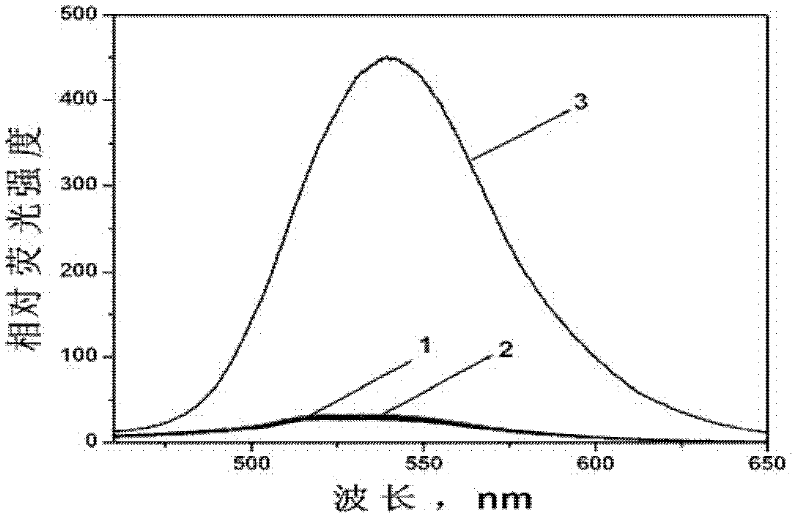
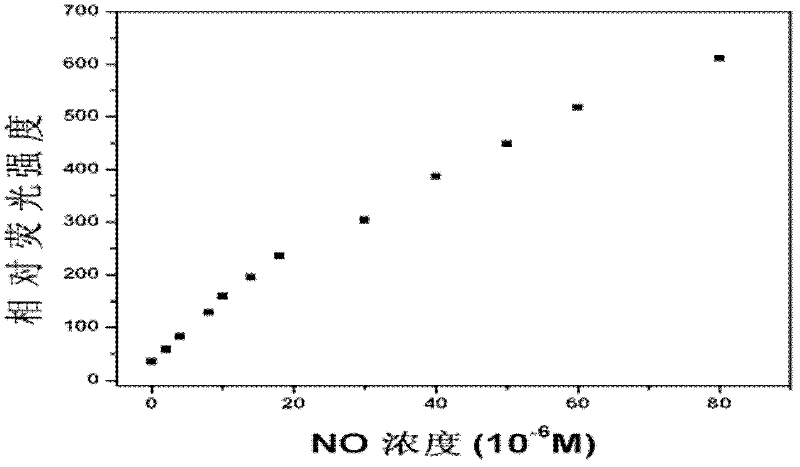
Fluorescent probe for detecting nitrogen monoxide and preparation method thereof
InactiveCN102516987AReduce distractionsFluorescence enhancementOrganic chemistryFluorescence/phosphorescenceAcetic anhydrideFluorescence
The invention relates to a fluorescent probe and in particular relates to a fluorescent probe for detecting nitrogen monoxide and a preparation method thereof. The probe uses 4-amino-1,8-naphthalimide as a basic molecular structure and carbamided o-phenylenediamine as a compound with nitrogen monoxide recognition groups. The preparation method comprises the following steps: adding 4-amino-1,8-naphthoic anhydride and an organic solvent into a container, then adding 2-(2-aminoethoxyl)ethanol, heating for reaction, and cooling; adding a saline solution, centrifuging to obtain an intermediate A, then dissolving the intermediate A in pyridine, and adding acetic anhydride to react; performing rotary evaporation, and adding water for extraction; performing rotary evaporation to separate out an intermediate B; adding the intermediate B, diisopropylethylamine and toluene into a container to obtain a mixed solution, then adding the mixed solution in a solution containing triphosgene and toluene, heating for refluxing, and cooling; adding dichloromethane, carrying out centrifugal separation to obtain a supernatant, and then adding the supernatant into a solution containing o-phenylenediamine and dichloromethane to react; performing rotary evaporation, and adding water for extraction; and drying the obtained ethyl acetate, and performing rotary evaporation to separate out the product.
Owner:XIAMEN UNIV
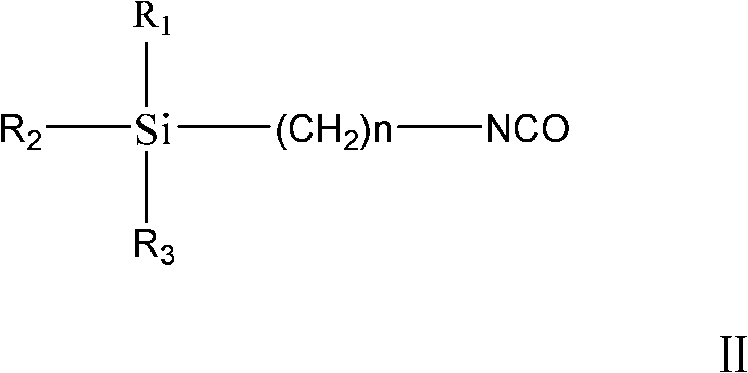
Method of preparing trialkoxysilanes isocyanic ester
InactiveCN101492468ALow priceReduce manufacturing costGroup 4/14 element organic compoundsOrganic solventPhosgene
The invention discloses a preparation method of trialkoxy siloyl isocyanate, comprising the following steps: (1) chlorine catching agent is dissolved in water and then is added into a reaction device to be stirred, wherein, the temperature is controlled at -5-0 DEG C; (2) triphosgene and trialkoxysilane amine are respectively dissolved in organic solvent and then respectively added into the reaction device and react by stirring for 10-20min, wherein, the temperature is controlled at -5-10 DEG C; (3) the phase separation is carried out on the reacted solution, the organic solvent is used for extracting water phase for 1-3 times, the drying is carried out after organic phases are annexed, and the organic solvent is extracted, filtered and removed; (4) after the vacuum distillation, the trialkoxy siloyl isocyanate is obtained. The chlorine catching agent used by the invention has the advantages of cheap price and low production cost. The used triphosgene has the advantages of no toxicity, safety and reliability.
Owner:常州胜杰生命科技股份有限公司
Preparation method of ponazuril
InactiveCN102936227AOperational securityRaw materials are easy to getOrganic chemistryCoccidiosisNitrobenzene
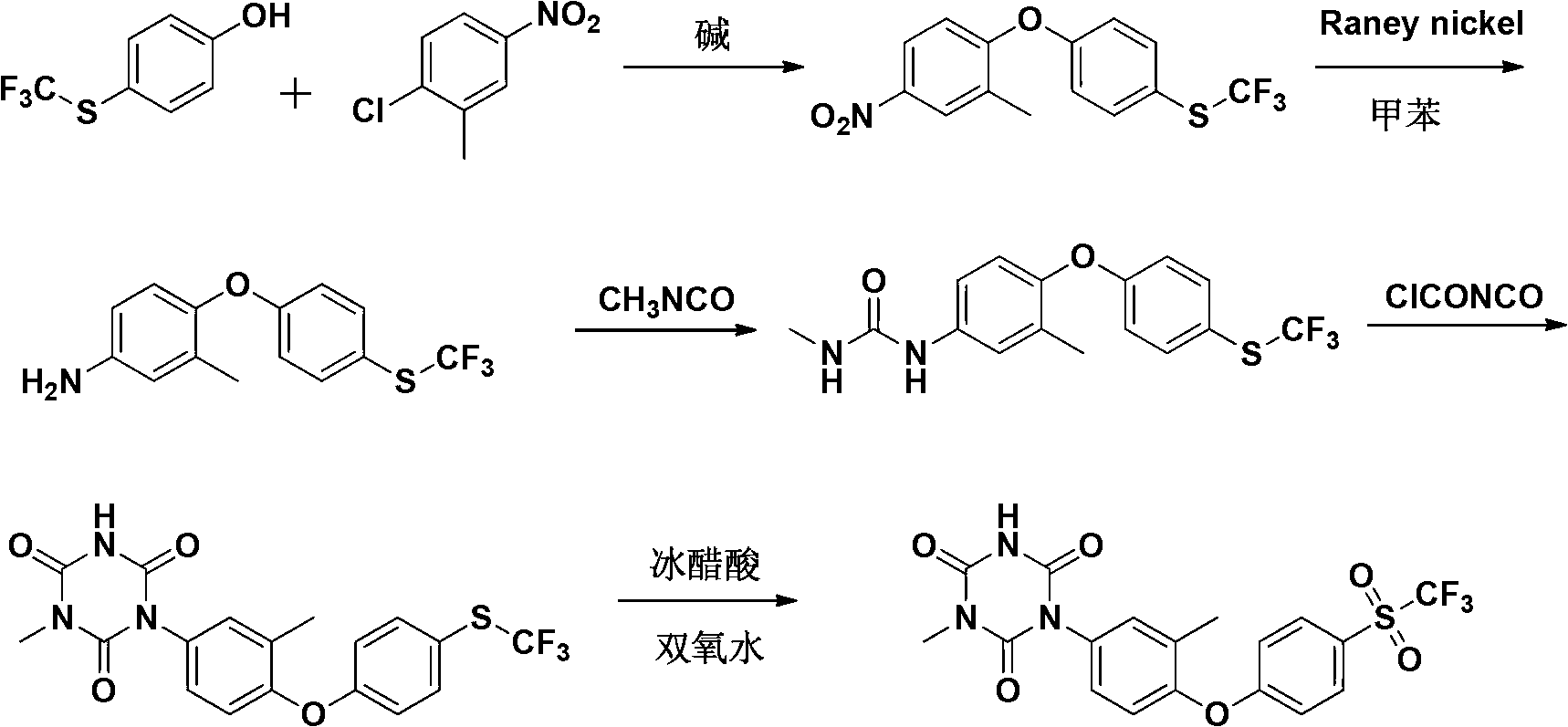
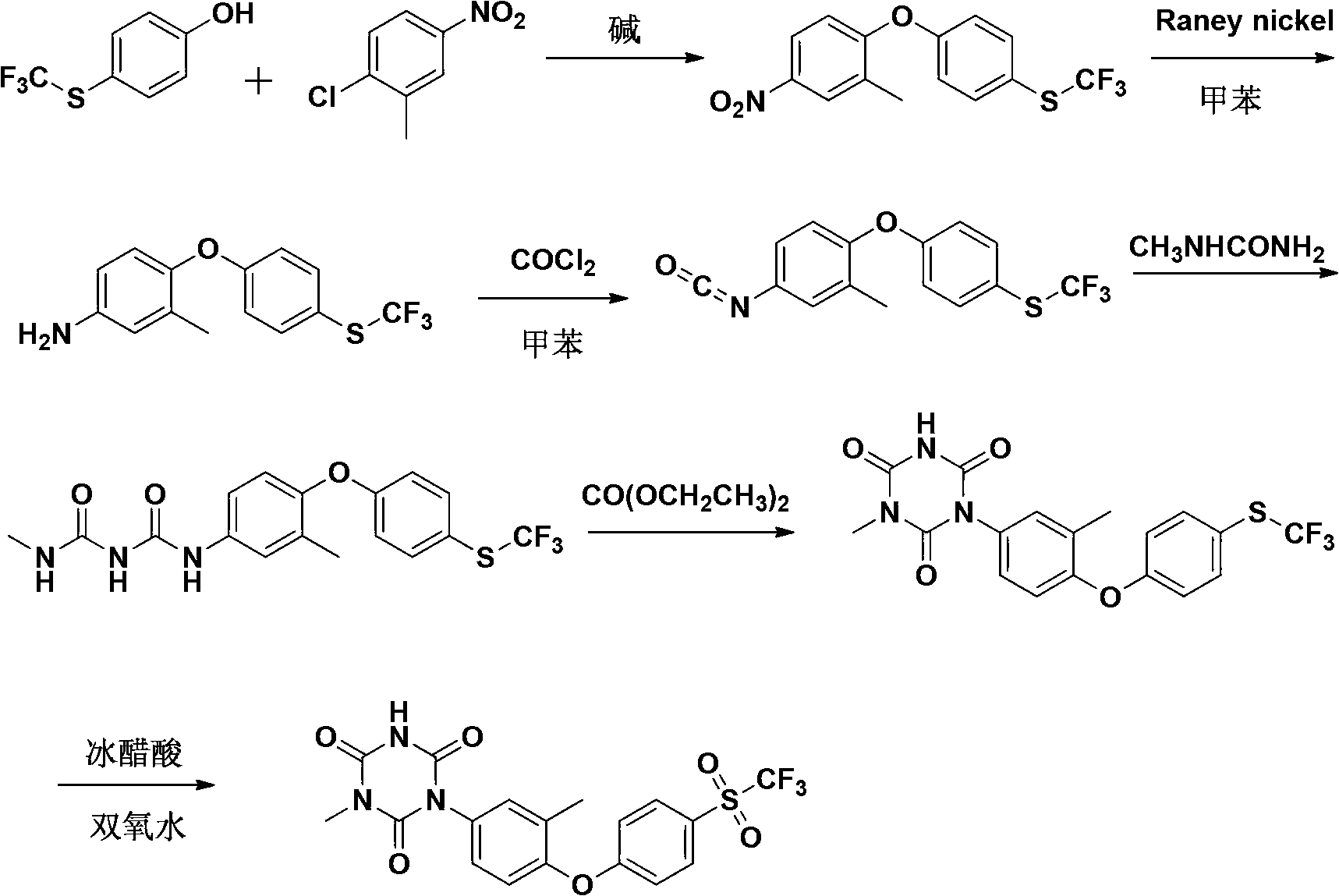
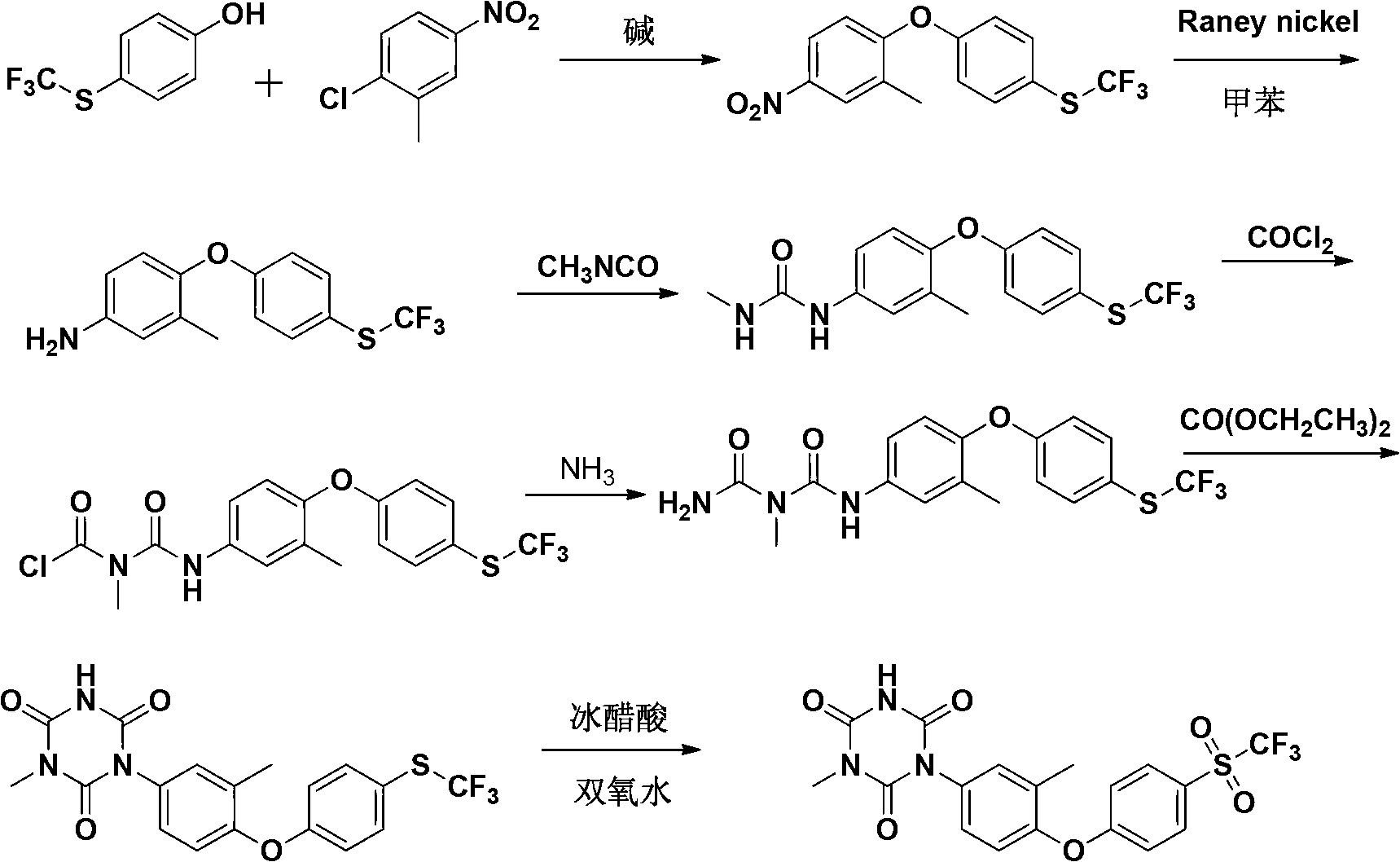
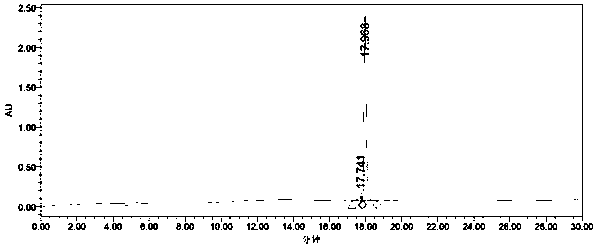
The invention relates to a preparation method of ponazuril which is an anti-coccidiosis drug. The method includes syntheses of 3-methyl-4-(4-phenoxy trifluoromethyl sulfide)-nitrobenzene, 3-methyl-4-(4-phenoxy trifluoromethyl sulfide)-aniline, 3-methyl-4-(4-phenoxy trifluoromethyl sulfide)-phenyl isocyanate, 1-[3-methyl-4-(4-phenoxy trifluoromethyl sulfide)-m-methylphenyl]-1,3,5-triazine-2,4,6-trione and ponazuril. Dangerous articles such as raney nickel, methyl isocyanate, chloroformylisocyanate, phosgene, triphosgene and hydrazine hydrate are not used during the reaction processes, and the operation is safe and reliable; raw materials are easy to obtain, the production cost and pollution are low, and the production process is environment-friendly; and the preparation method is simple, special equipment is not needed, a total yield of ponazuril product can reach above 60%, and large-scaleindustrial production is facilitated.
Owner:QILU ANIMAL HEALTH PROD +1
Solid-phase preparation method of efficient prostate-specific membrane antigen ligand PSMA-617
ActiveCN109134602AReduce the feed ratioReduce usagePeptide preparation methodsBulk chemical productionAntigenProstate specific membrane
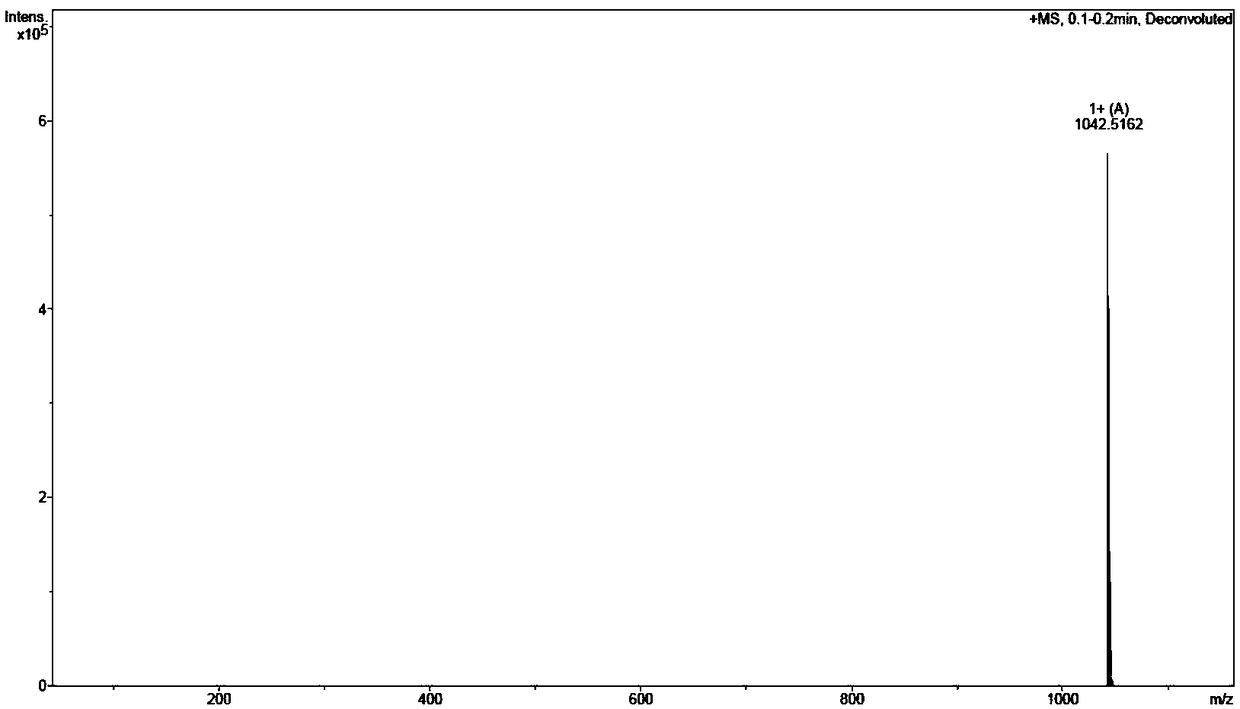
The invention discloses a solid-phase preparation method of an efficient prostate-specific membrane antigen ligand PSMA-617. By a solid-phase polypeptide synthesis method of amino acid protected by Fmoc, hydroxyl resin serves a starting point, and PSMA-617 sequences are sequentially synthesized. According to the method, high-risk reagent triphosgene used in the prior art is avoided, Pd(Ph3P)4 is omitted, synthesis safety is improved, the purity of an end-product is higher than 98.5%, the method has the advantages of low cost, less waste liquid, high efficiency and safety and easiness in purification, and large-scale and industrial production is facilitated.
Owner:LANZHOU UNIVERSITY
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com

![4-[4-(substituted phenyl) piperazine piperazinyl-1]-butylcarbamic acid substituted aromatic ester derivative and preparation method thereof 4-[4-(substituted phenyl) piperazine piperazinyl-1]-butylcarbamic acid substituted aromatic ester derivative and preparation method thereof](https://images-eureka.patsnap.com/patent_img/ccda6d25-26d1-4761-b15f-5f57af727bcc/BDA00002781366000011.PNG)
![4-[4-(substituted phenyl) piperazine piperazinyl-1]-butylcarbamic acid substituted aromatic ester derivative and preparation method thereof 4-[4-(substituted phenyl) piperazine piperazinyl-1]-butylcarbamic acid substituted aromatic ester derivative and preparation method thereof](https://images-eureka.patsnap.com/patent_img/ccda6d25-26d1-4761-b15f-5f57af727bcc/BDA00002781366000021.PNG)
![4-[4-(substituted phenyl) piperazine piperazinyl-1]-butylcarbamic acid substituted aromatic ester derivative and preparation method thereof 4-[4-(substituted phenyl) piperazine piperazinyl-1]-butylcarbamic acid substituted aromatic ester derivative and preparation method thereof](https://images-eureka.patsnap.com/patent_img/ccda6d25-26d1-4761-b15f-5f57af727bcc/BDA00002781366000041.PNG)