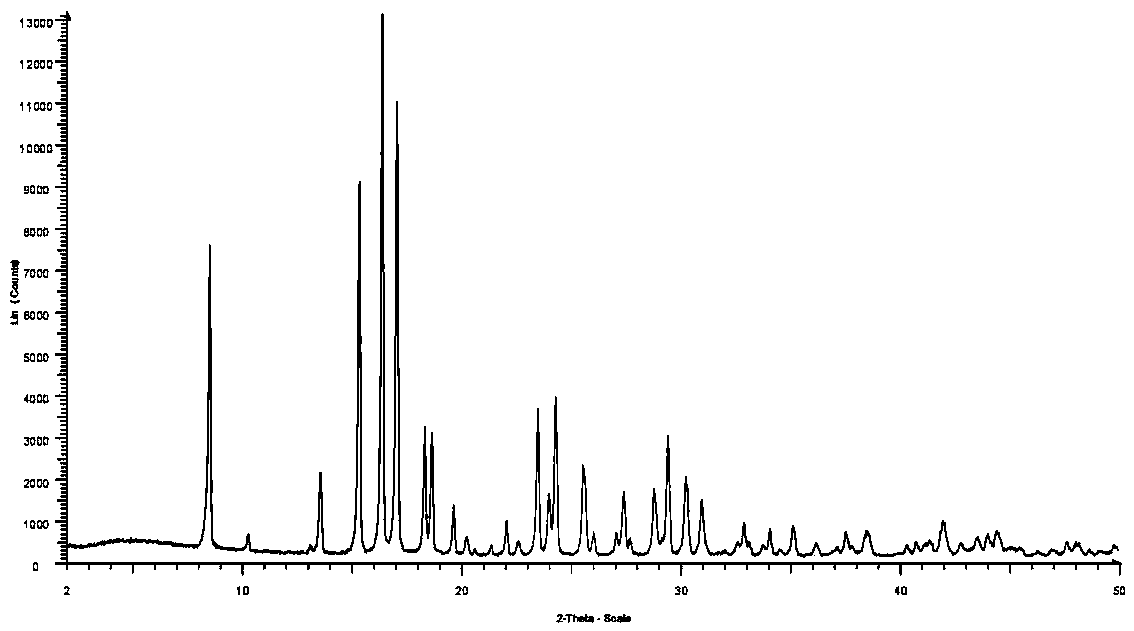
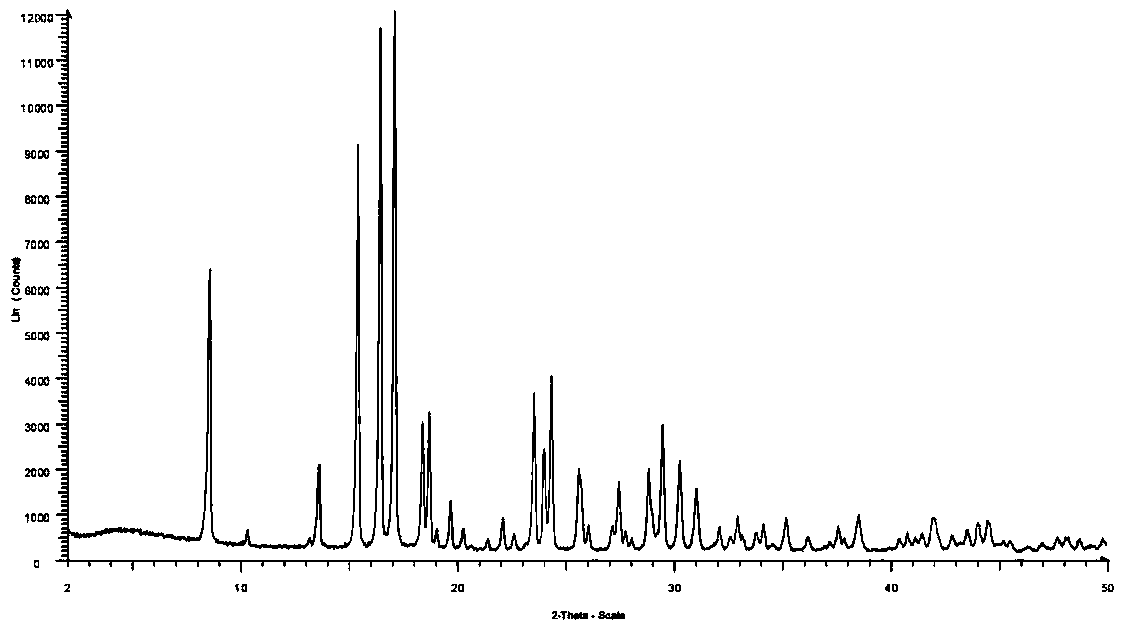
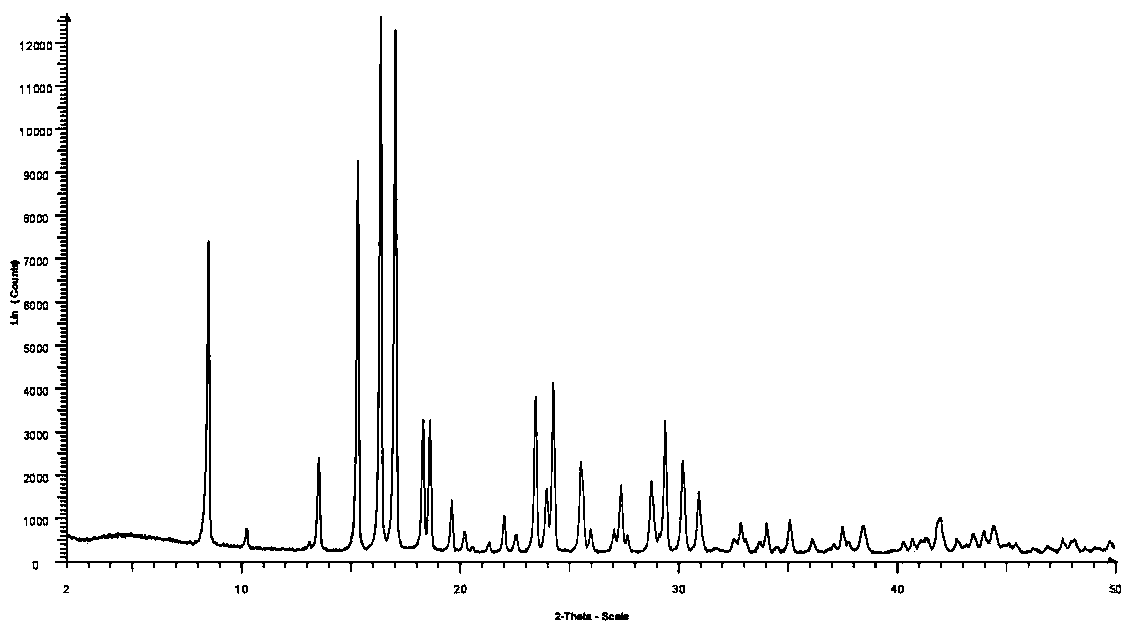
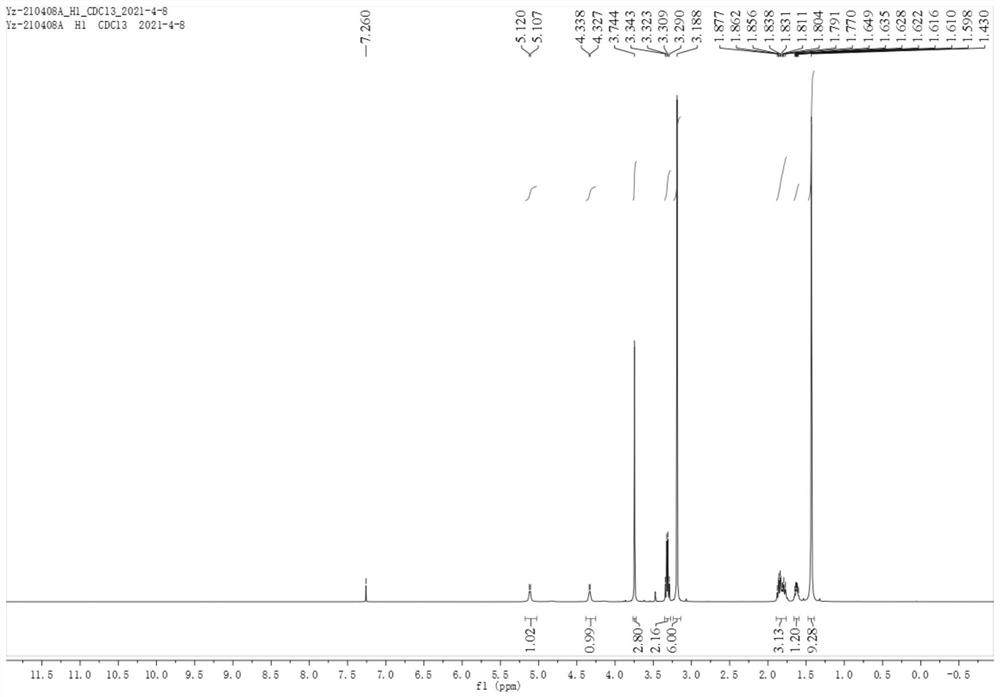
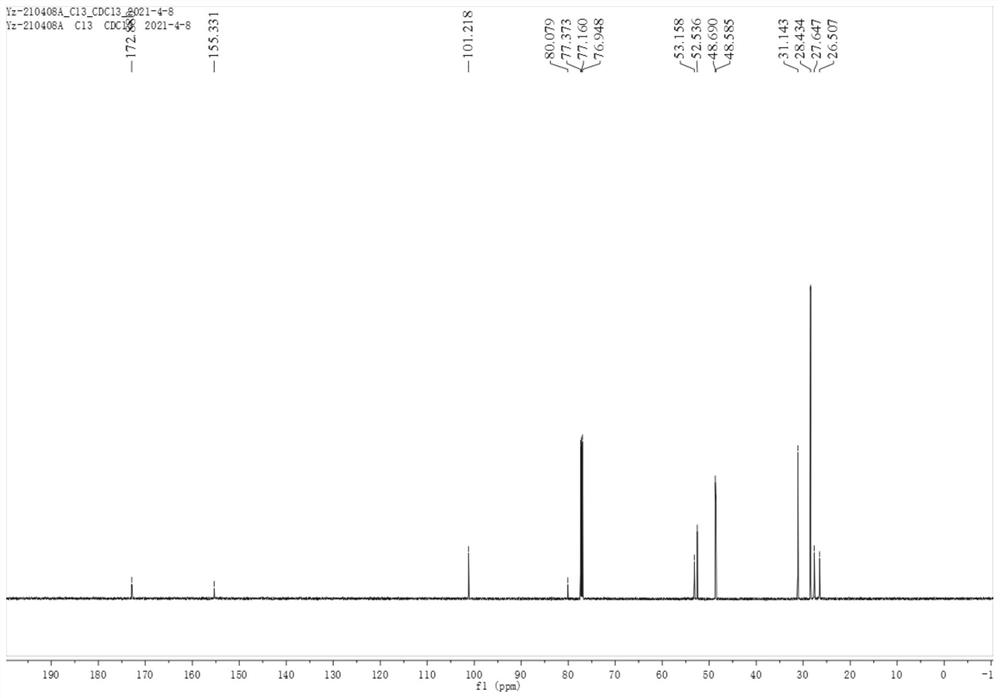
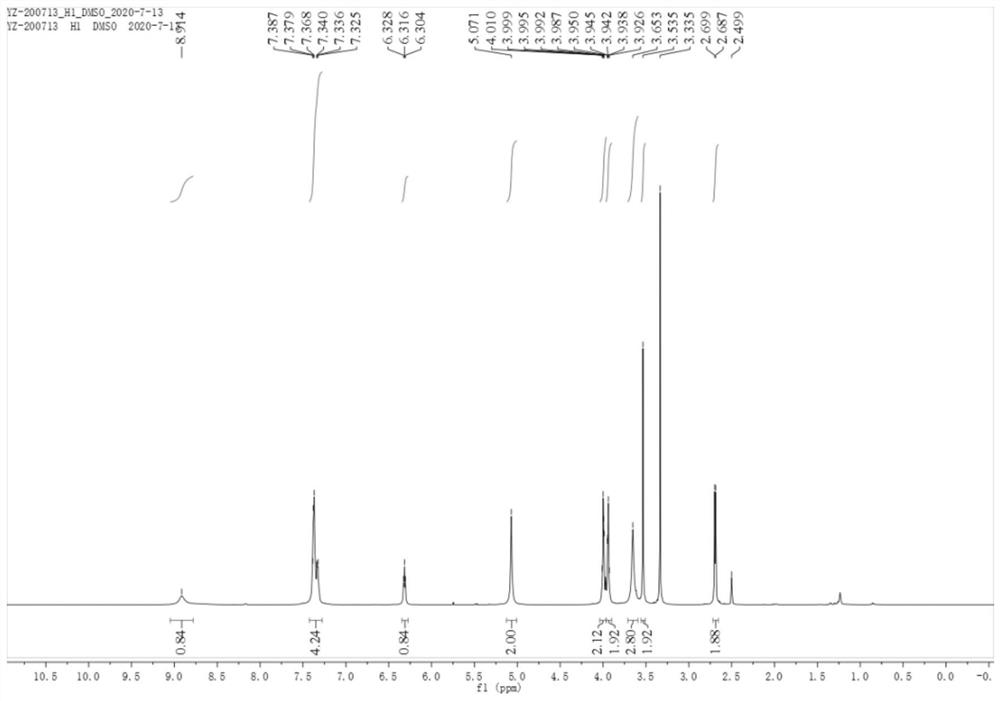
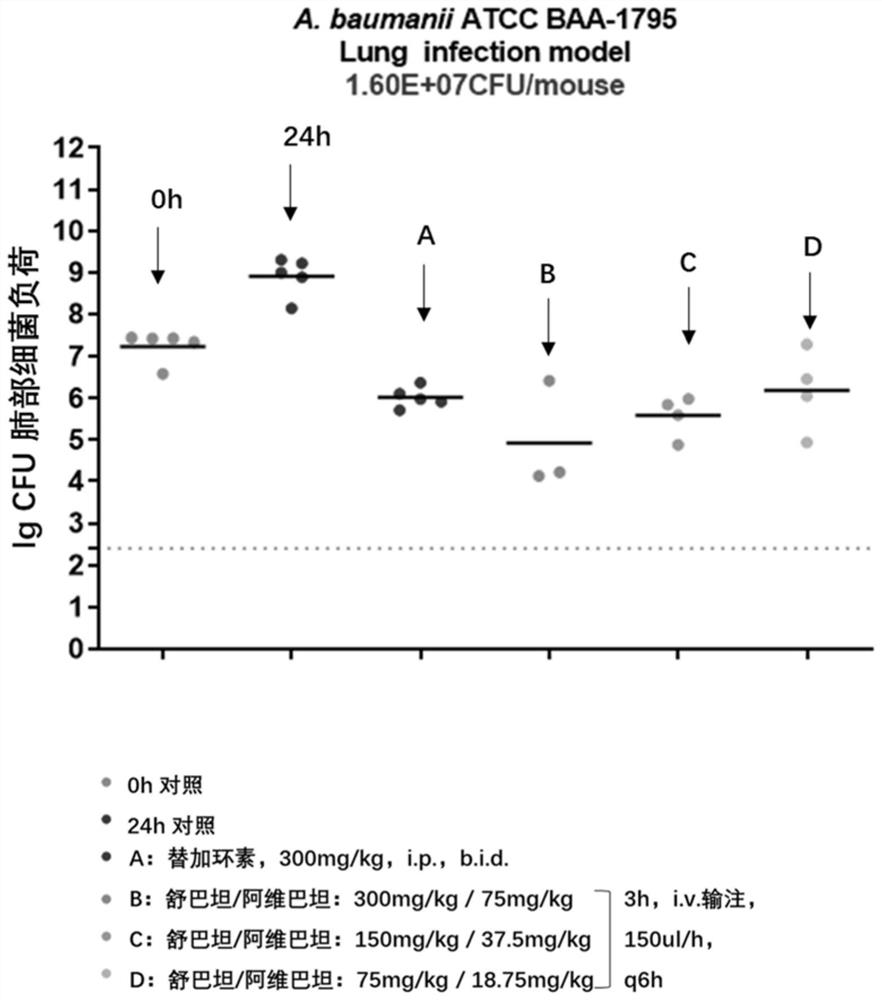
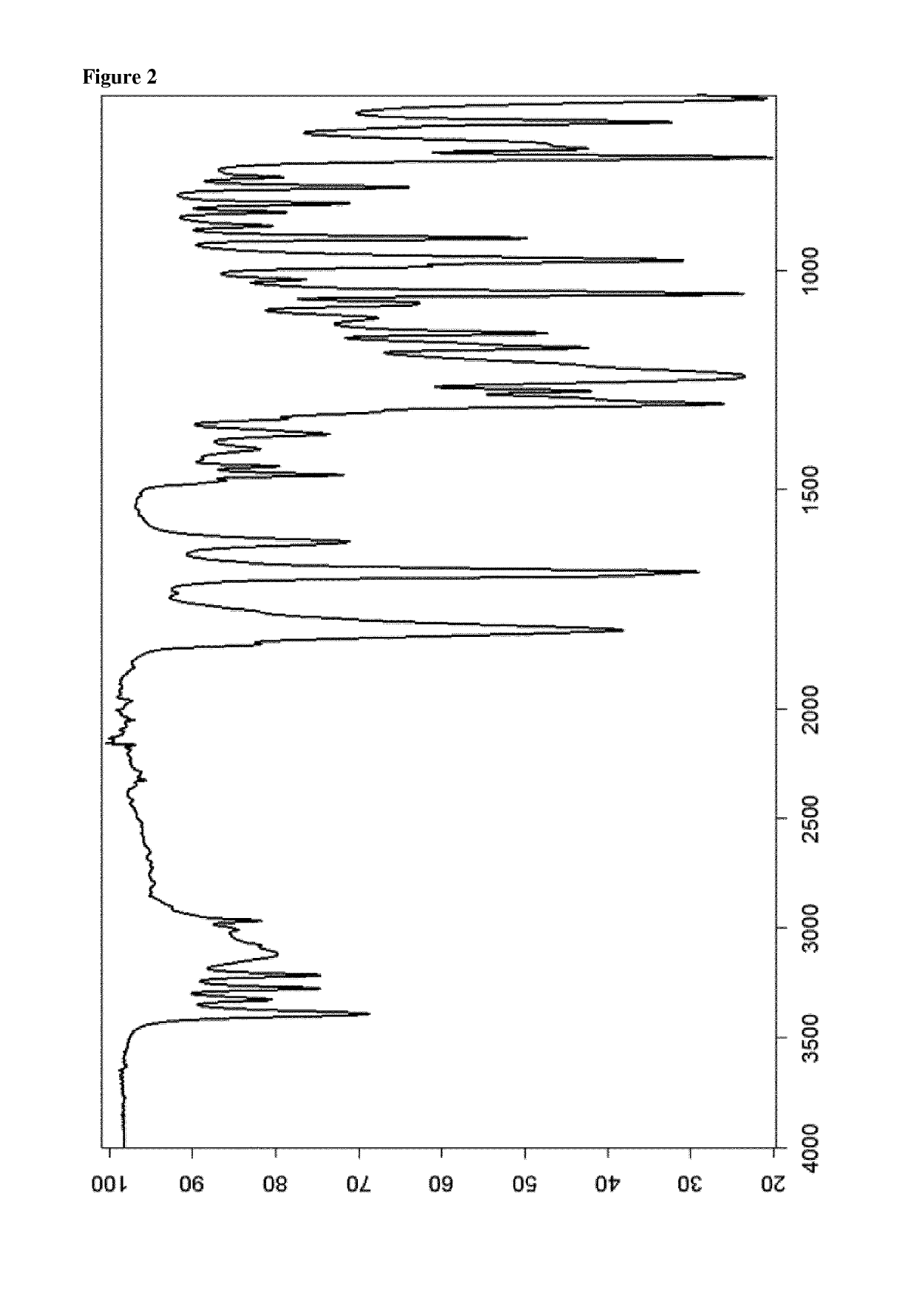
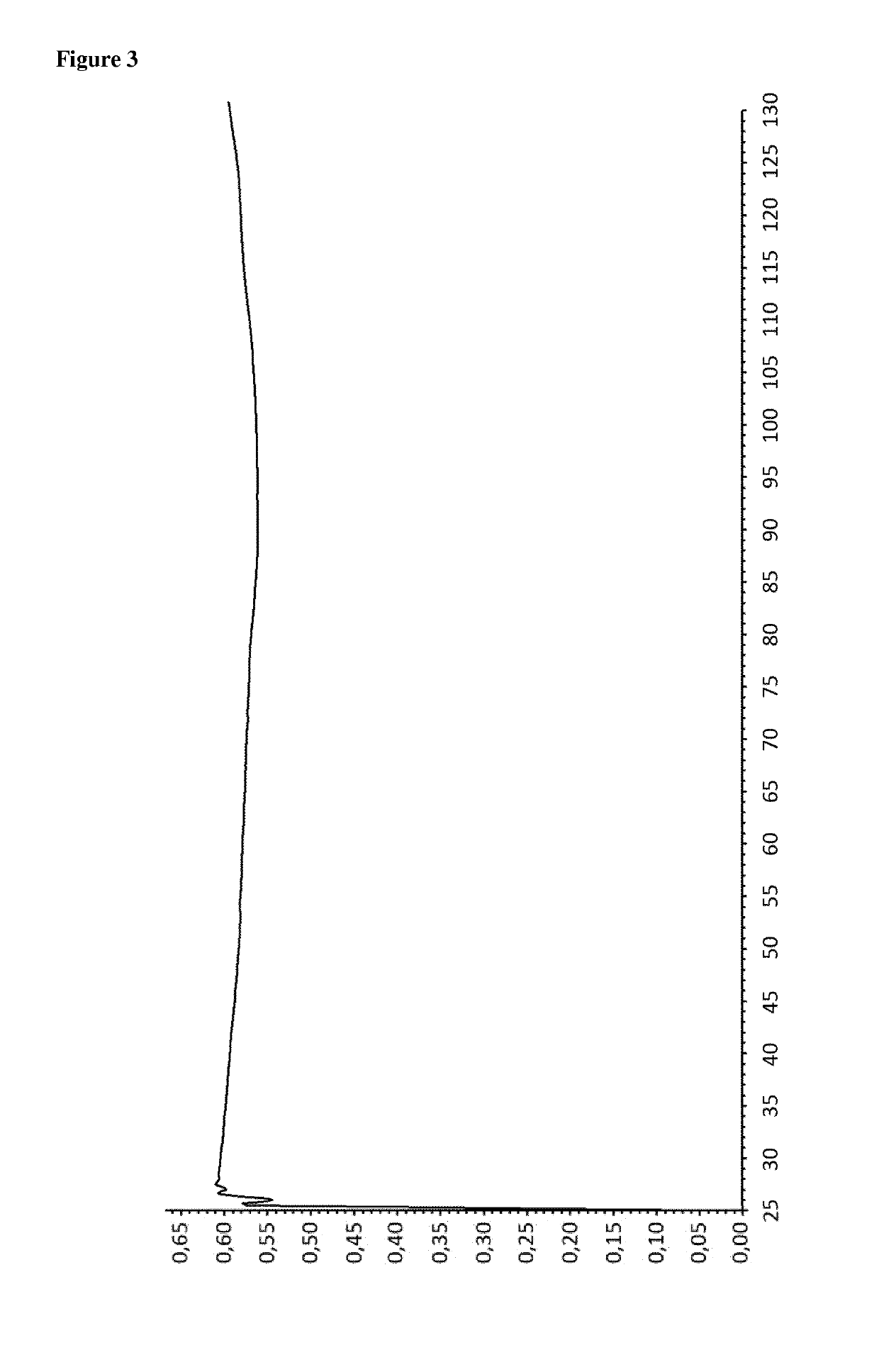
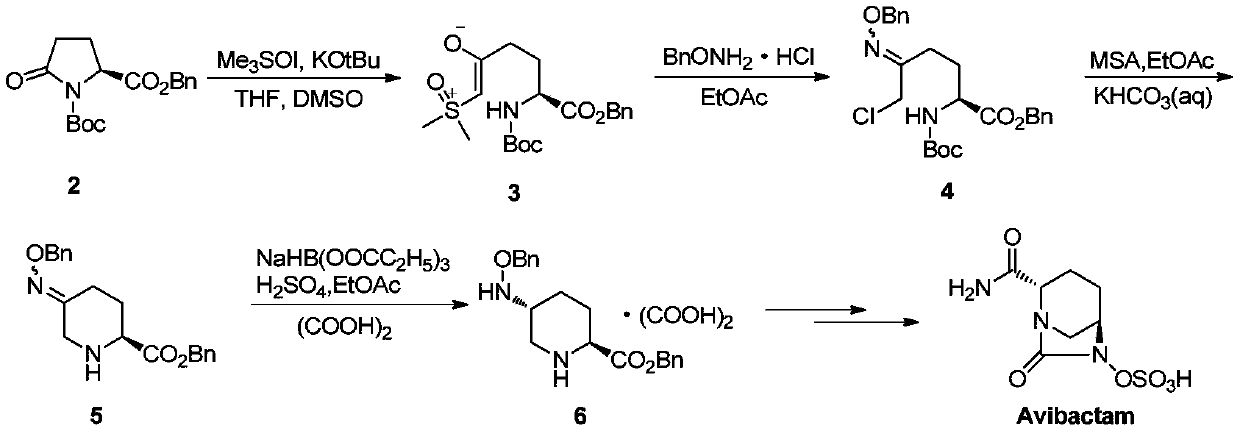
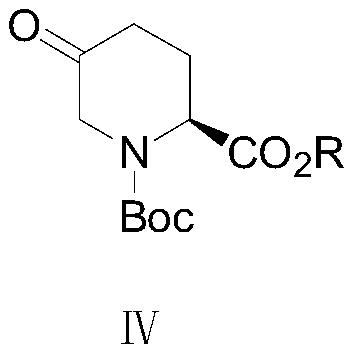
Patents
Literature
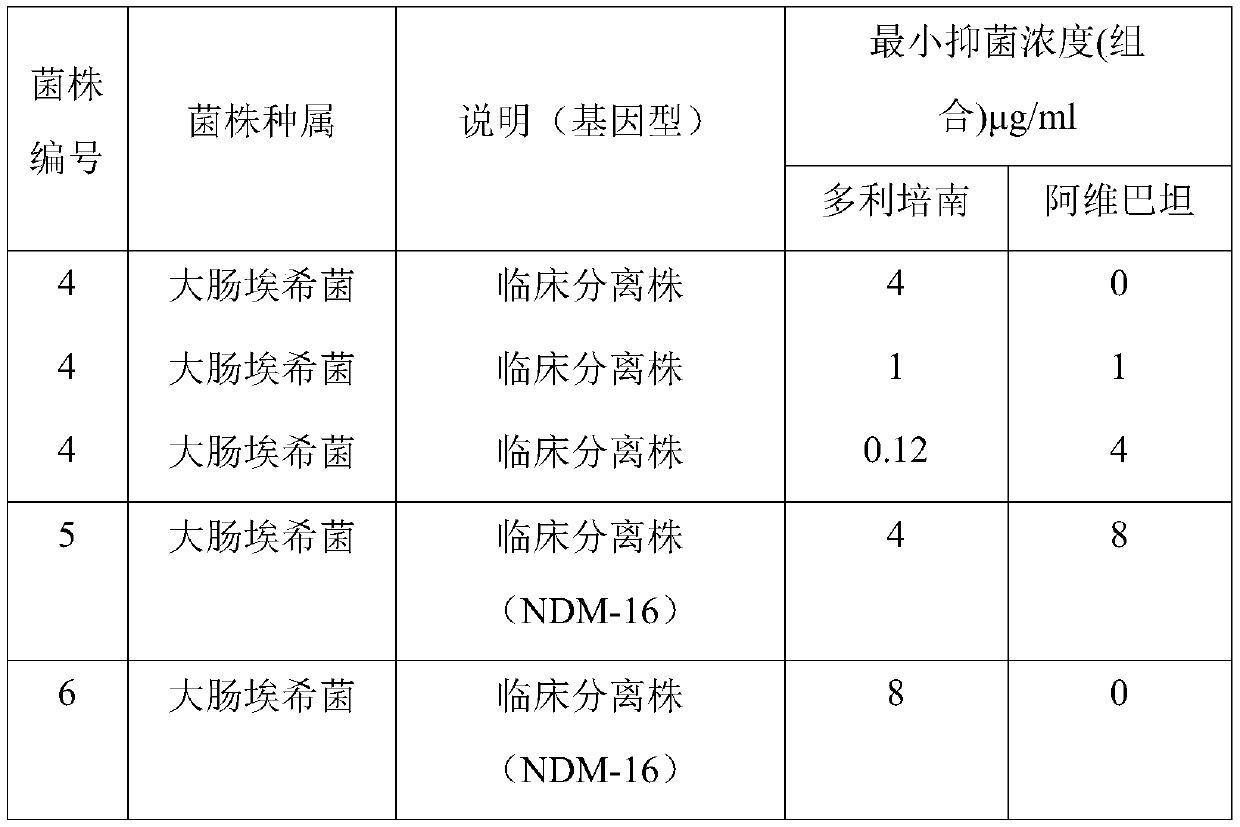
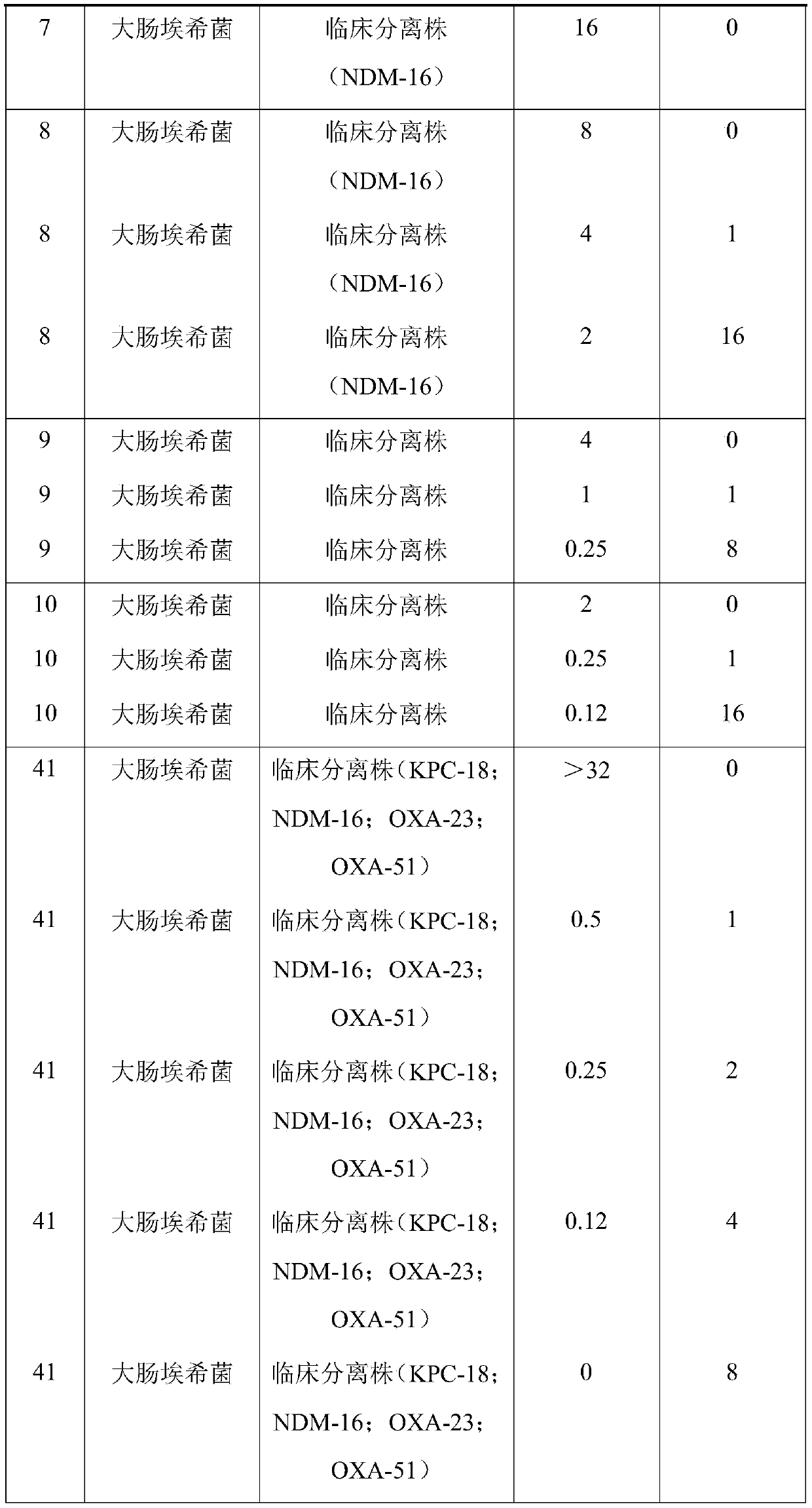
46 results about "Avibactam" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
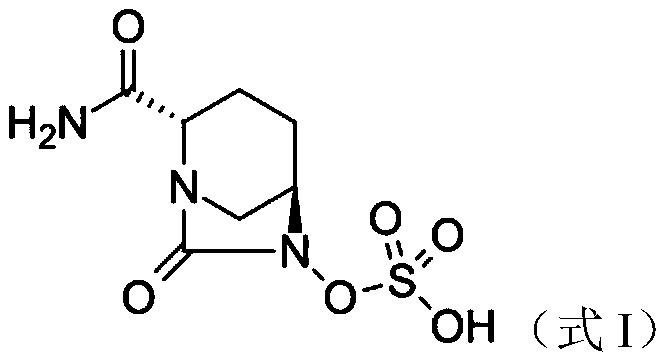
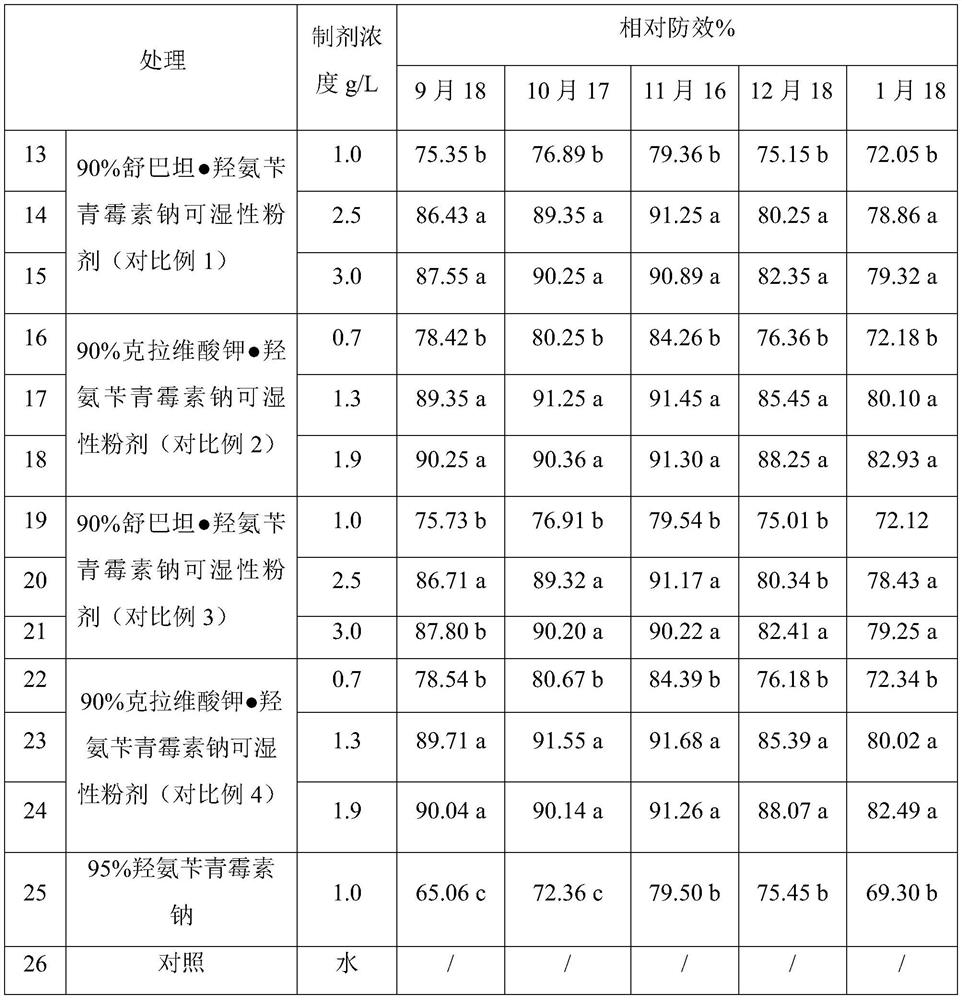
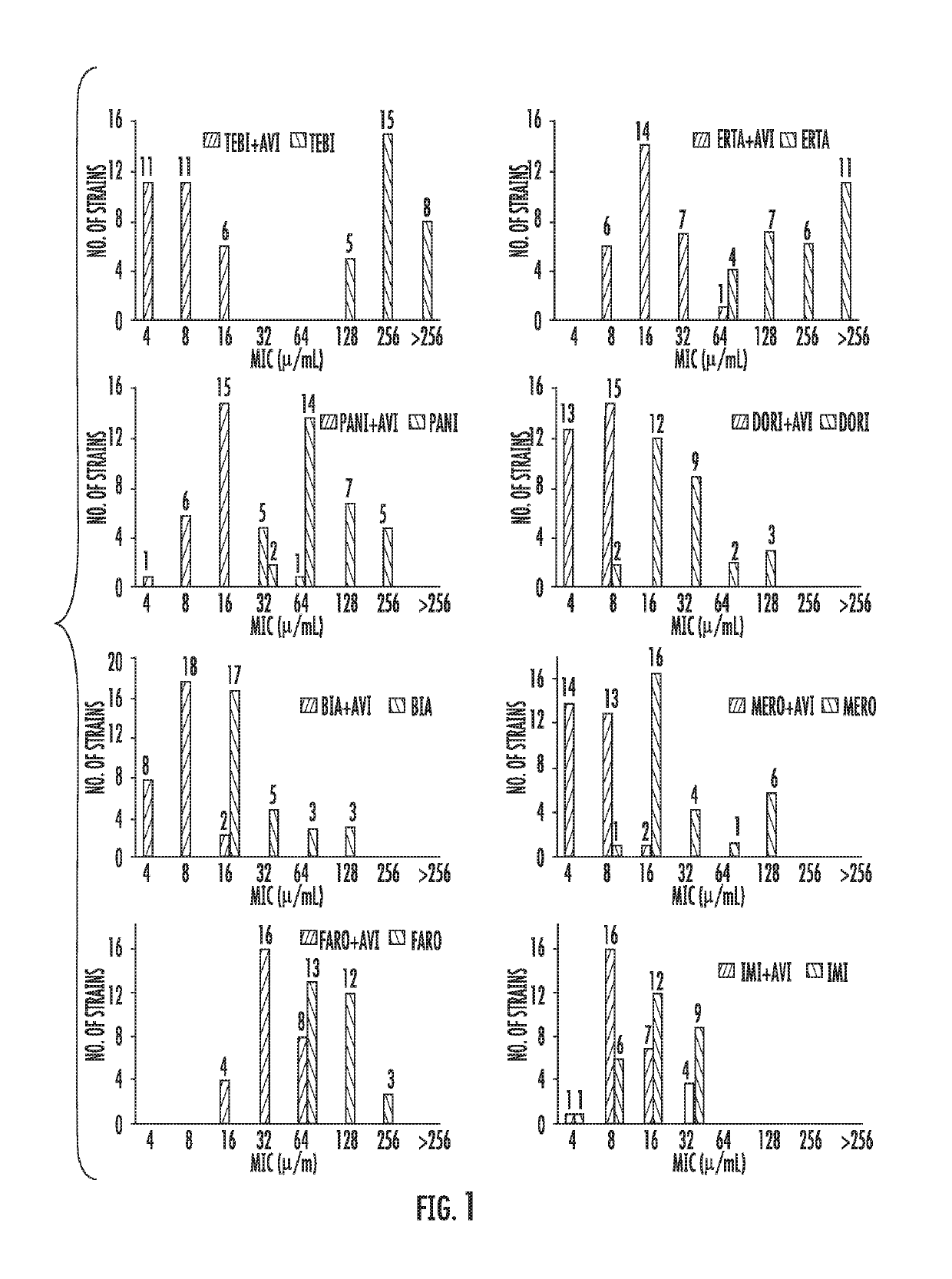
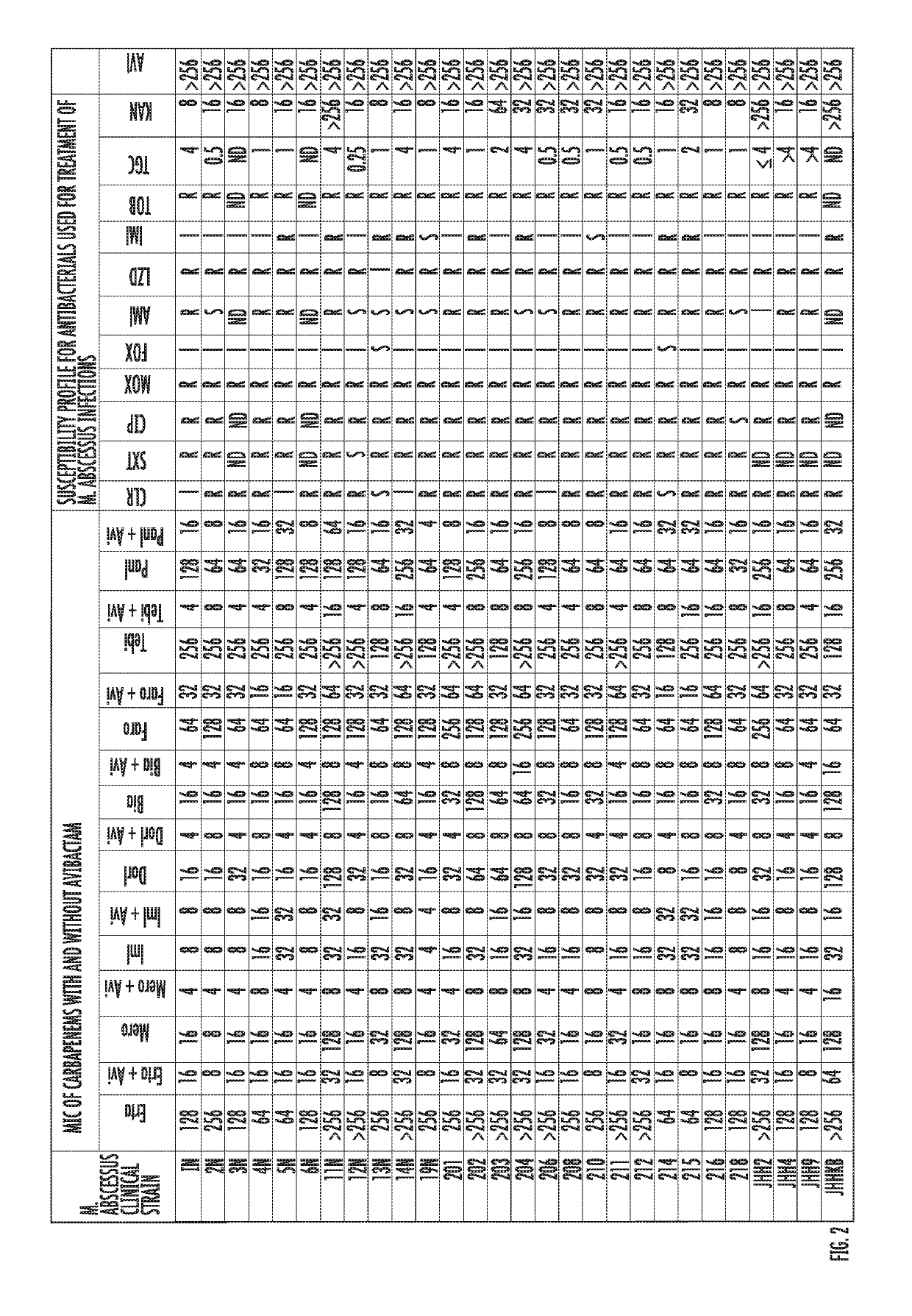
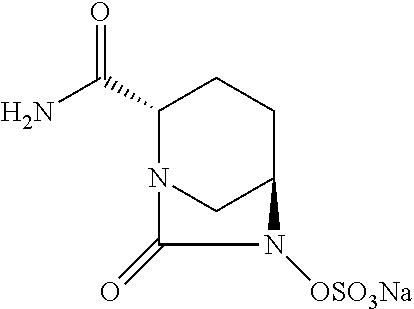
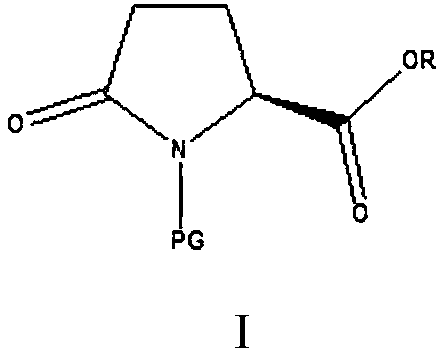
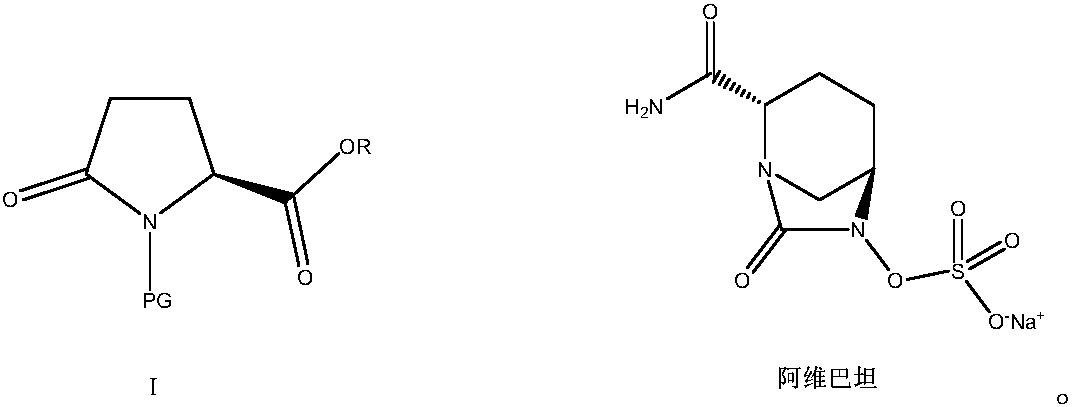
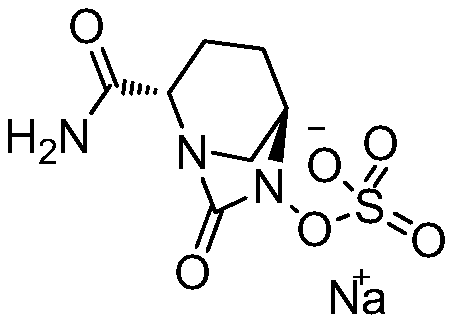
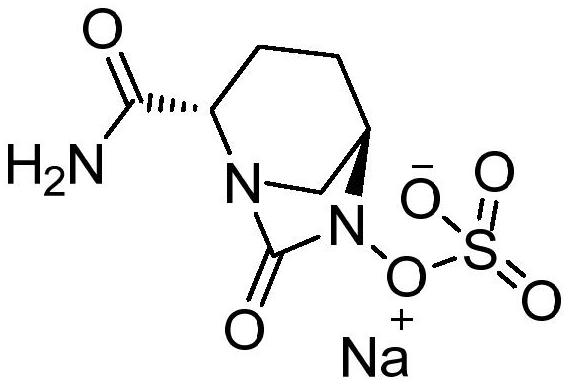
Avibactam is a non-β-lactam β-lactamase inhibitor developed by Actavis (now Teva) jointly with AstraZeneca. A new drug application for avibactam in combination with ceftazidime (branded as Avycaz) was approved by the FDA on February 25, 2015, for treating complicated urinary tract (cUTI) and complicated intra-abdominal infections (cIAI) caused by antibiotic resistant-pathogens, including those caused by multi-drug resistant Gram-negative bacterial pathogens.
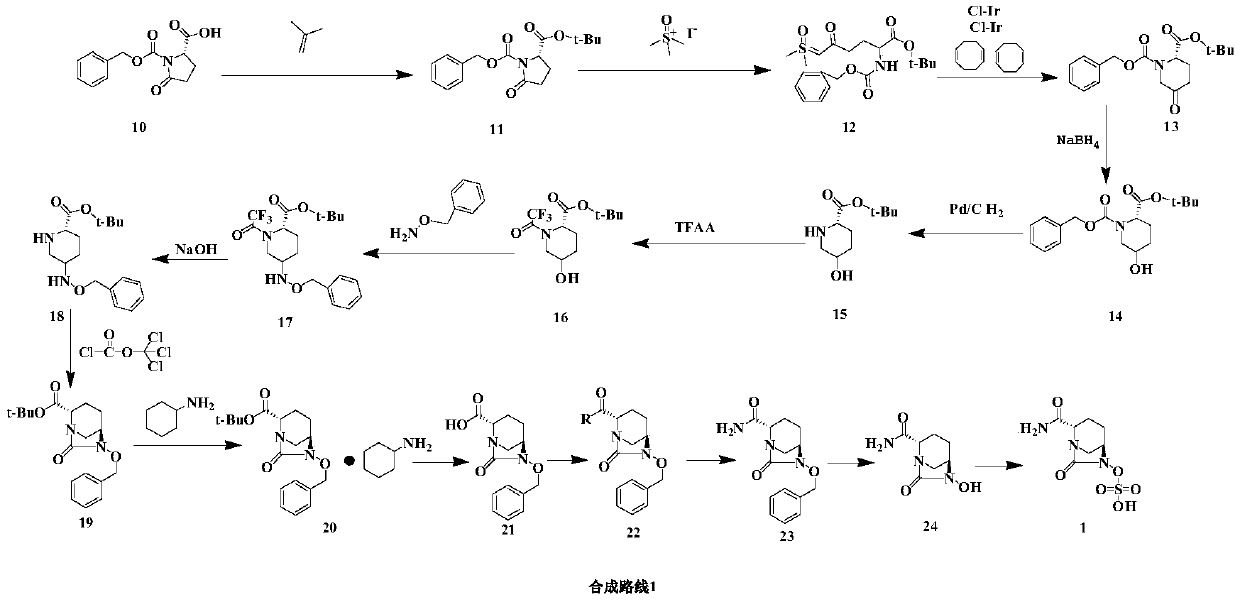
Method for synthesizing beta-lactamase inhibitor Avibactam
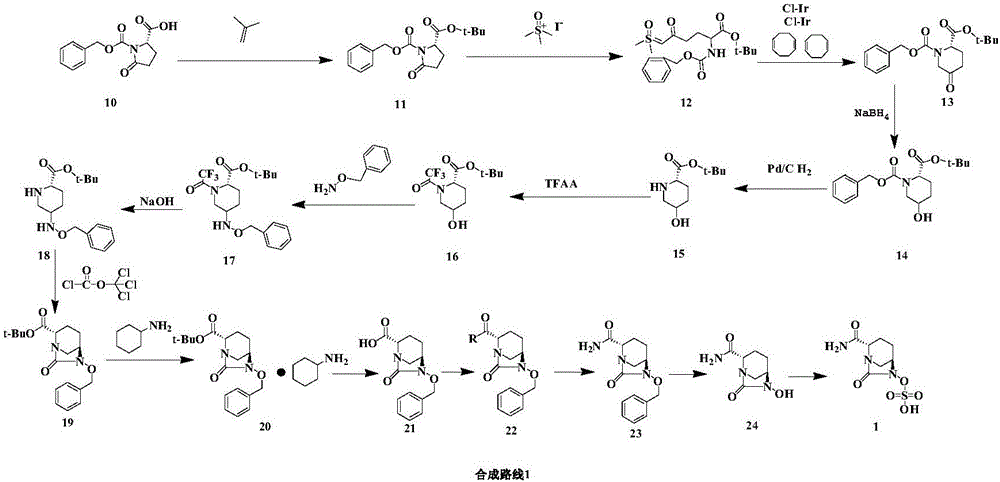
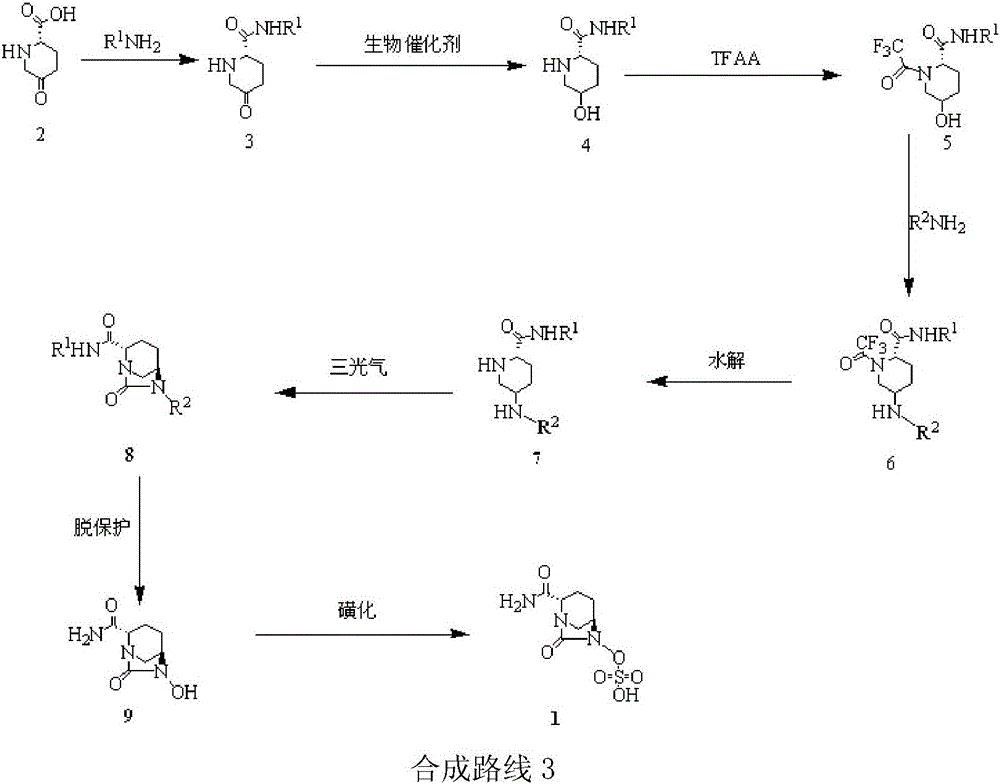
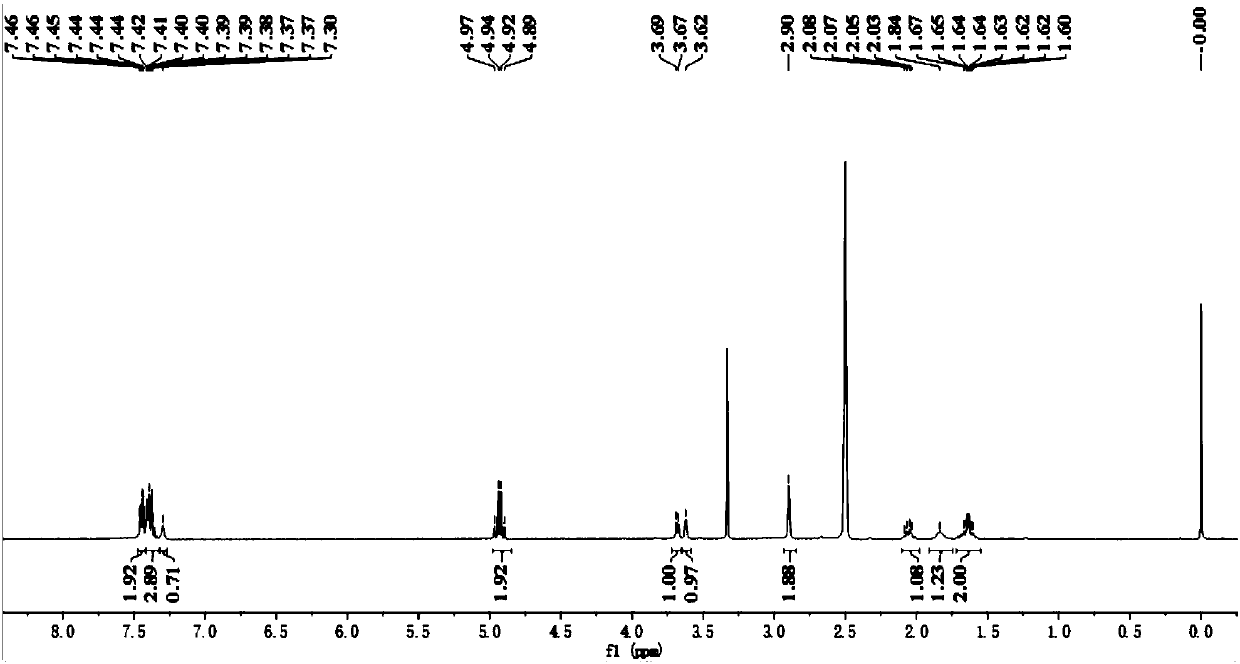
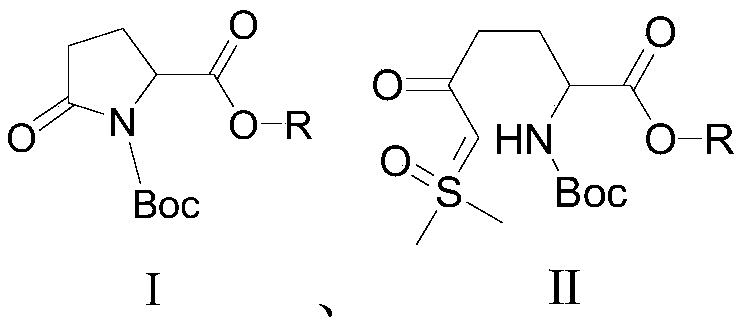
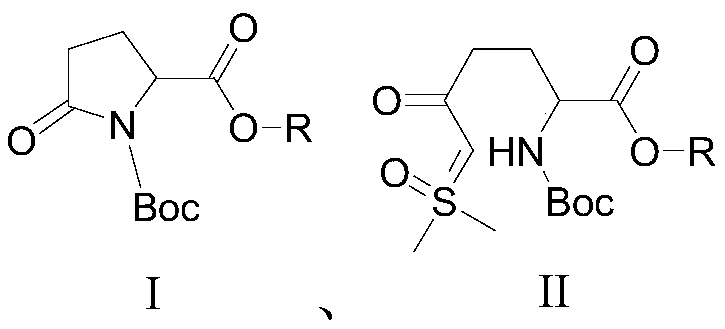
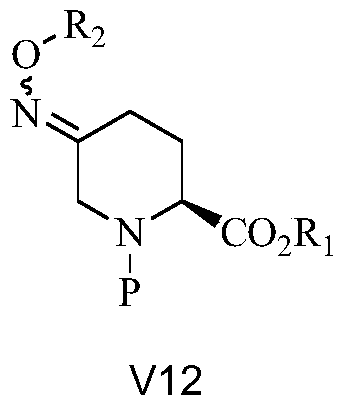
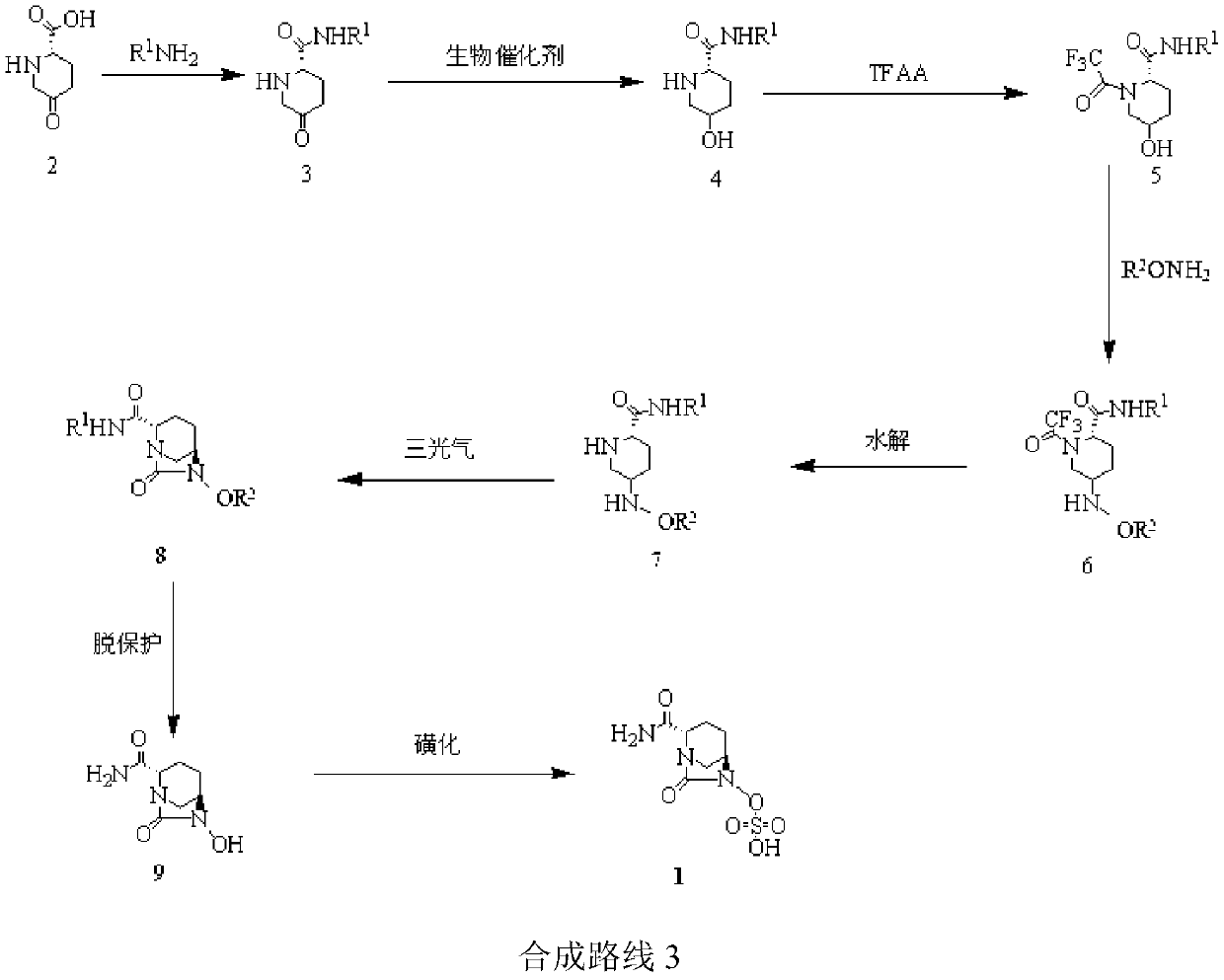
The invention relates to a method for synthesizing a beta-lactamase inhibitor Avibactam and belongs to the technical field of preparation of beta-lactamase inhibitors. The method disclosed by the invention comprises the following steps: (1) taking a compound 2 as a raw material, and reacting with R<1>NH2 so as to produce a compound 3; (2) enabling the compound 3 and a biocatalyst to produce a compound 4 in an organic solvent in the presence of glucose or sucrose; (3) enabling the compound 4 to react with trifluoroacetic anhydride so as to obtain a compound 5; (4) enabling the compound 5 to react with R<2>ONH2 so as to produce a compound 6; (5) hydrolyzing the compound 6 under alkaline conditions so as to produce a compound 7; (6) enabling the compound 7 to react with triphosgene so as to produce a compound 8; (7) enabling the compound 8 to react with ammonium formate in the presence of a catalyst so as to produce a compound 9 in the organic solvent, wherein the catalyst is a Pd / C system; and (8) enabling the compound 9 to react with a sulfonating agent, thereby obtaining the product 1. The method disclosed by the invention is stable in process, high in yield, simple and safe in operation and low in production cost.
Owner:YIYUAN XINQUAN CHEM
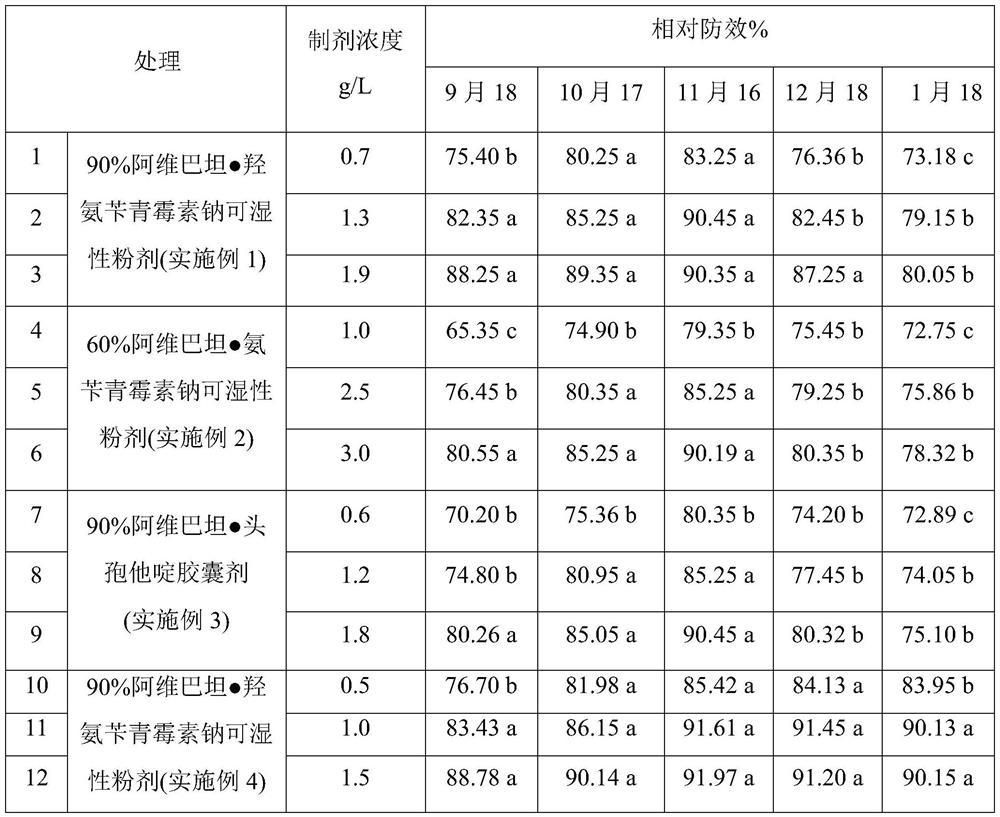
Antibiotic combination containing Avibactam and application of antibiotic combination
PendingCN110269857AImproved drug resistanceImprove survival rateAntibacterial agentsHeterocyclic compound active ingredientsMedicineDrug resistance
The invention belongs to the technical field of medicines, relates to an antibiotic combination containing Avibactam and an application of the antibiotic combination, provides an application of Avibactam or pharmaceutically acceptable salt and carbapenems antibiotics to preparation of medicines for treating or preventing bacterial infection, particularly an application of the Avibactam to preparation of medicines for treating infection caused by escherichia coli, pseudomonas aeruginosa, klebsiella pneumoniae, baumanii and enterobacter aerogenes so as to solve the problem of drug resistance of the carbapenems antibiotics.
Owner:CHIA TAI TIANQING PHARMA GRP CO LTD
Simple and convenient production method of avibactam
ActiveCN109956941AEase of industrial productionLow priceAntibacterial agentsOrganic active ingredientsFormateIon exchange
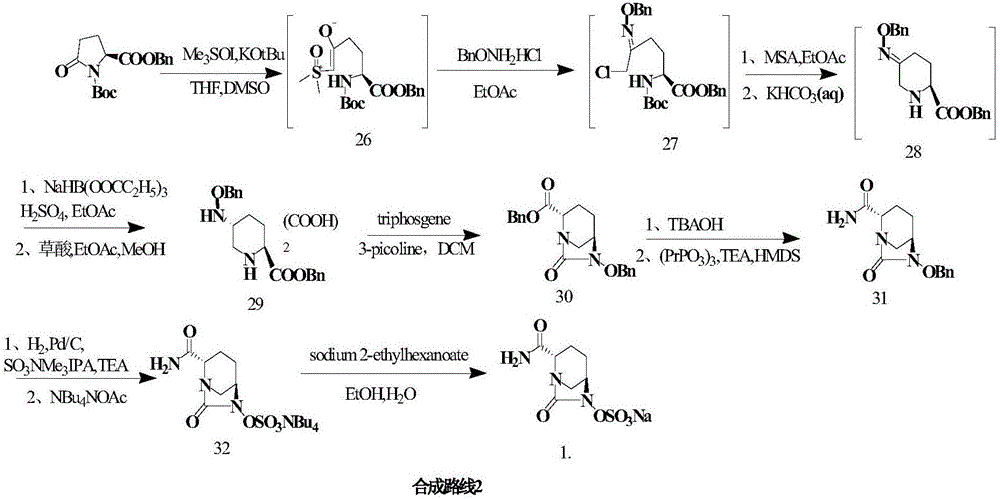
The invention provides a simple and convenient production method of avibactam. The simple and convenient production method of the avibactam comprises the steps of using piperidyl-5-keto-2S-formate IIas a raw material, making the piperidyl-5-keto-2S-formate II and O-protecting group hydroxylamine hydrochloride subjected to condensation reaction, and then obtaining 5R-substituted oxyaminopiperidyl-2S-formic acid V through reduction, chiral resolution and hydrolysis under an alkaline condition; and then making the 5R-substituted oxyamino piperidyl-2S-formic acid V and phosgene or triphosgene ordiphosgene subjected to cyclic urea reaction reaction, acylating chlorination reaction and amidation reaction through a one-pot method, obtaining {[(2S,5R)-2-formamyl-7-oxo-1,6-diazetidine[3.2.1]octane-6-yl]oxy}sulfonyl tetra-n-butylammonium salt VII through protecting group take-off, sulphating and tetrabutyl amination salification, and producing the avibactam I finally through ion exchange. Thesimple and convenient production method of the avibactam is simple and convenient in production route, easy to operate, low in raw material price, low in cost, small in three waste discharge, high inatom utilization rate, economical and environmentally friendly, and the yields of the various steps are high, so that the simple and convenient production method of the avibactam benefits industrial production of the avibactam.
Owner:XINFA PHARMA
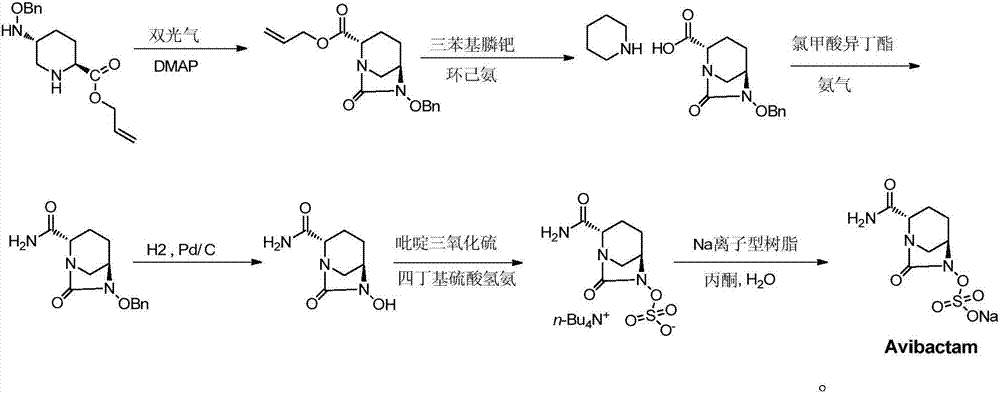
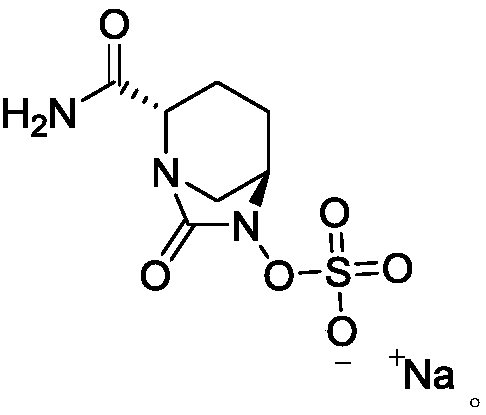
Preparation methods of sodium avibactam and intermediate compound thereof
InactiveCN107880042AEasy to operateAvoid high-difficulty and high-risk hydrogenation catalytic operationsOrganic chemistryCompound KSolvent
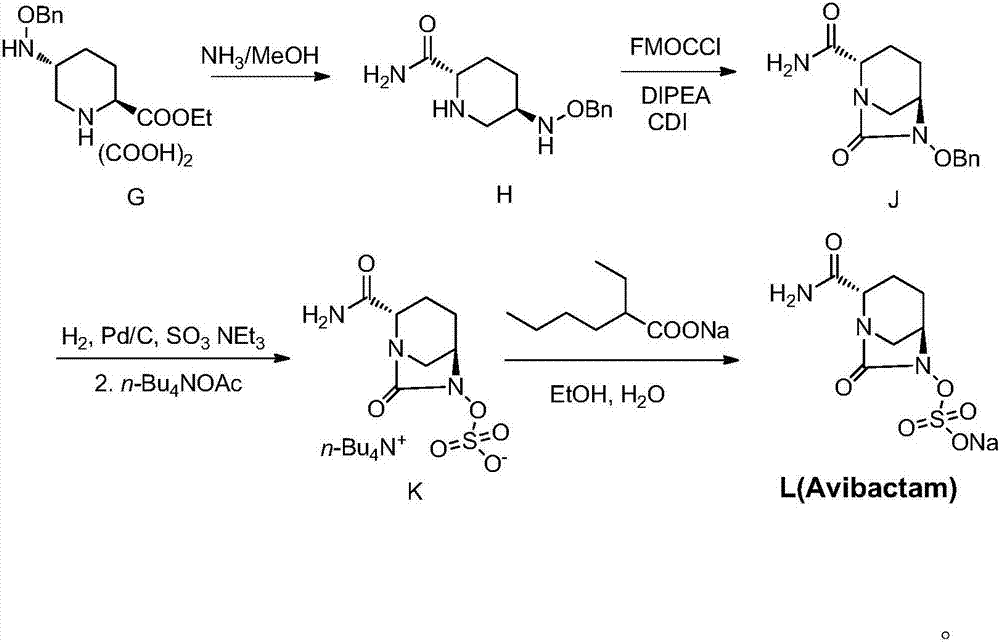
The invention provides a preparation method of sodium avibactam. The method comprises that sodium 2-ethylhexanoate reacts with an intermediate compound K, so that the sodium avibactam is obtained. Theinvention also provides a preparation method of the intermediate compound K. The preparation method comprises the following steps: (1) carrying out reaction on a compound J with ammonium formate, formic acid and triethylamine in presence of a catalyst, and removing benzyl, so that a compound K1 is obtained; (2) adding an acid binding agent, a sulfonation reagent and water into the K1 reaction liquid obtained in the step (1), and reacting; and (3) adding tetrabutylammonium acetate aqueous solution into the reaction liquid obtained in the step (2), stirring and reacting, then adding an extraction solvent, separating out an organic phase, drying, then distilling off the solvent, adding a crystallization solvent, stirring, and crystallizing, so that the compound K is obtained. The preparationmethods provided by the invention are low in cost, avoid a high-risk preparation method for debenzylation through hydrogenation, are high in safety performance, easy to operate and applicable to industrial production and have greater application value.
Owner:SHANGHAI SUNTECH PHARMA
Method for preparing amorphous avibactam sodium by spray-drying
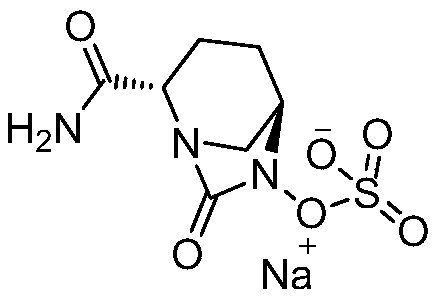
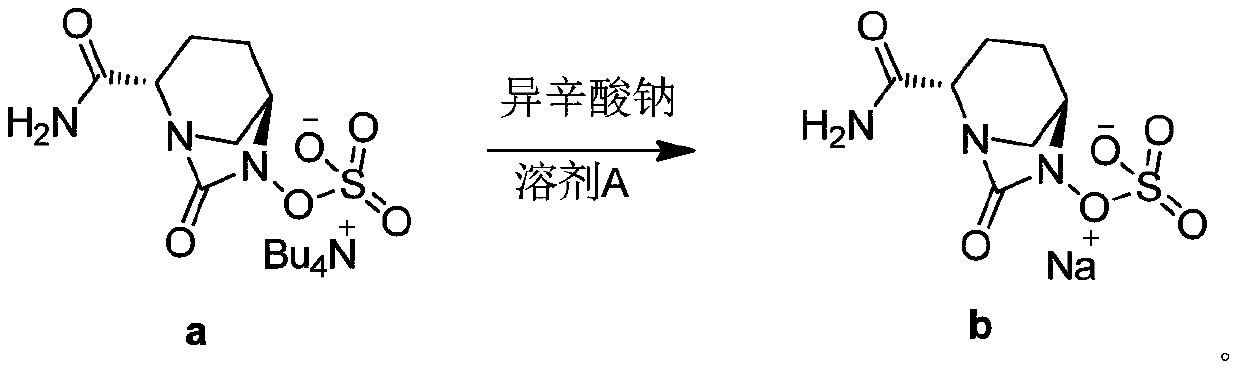
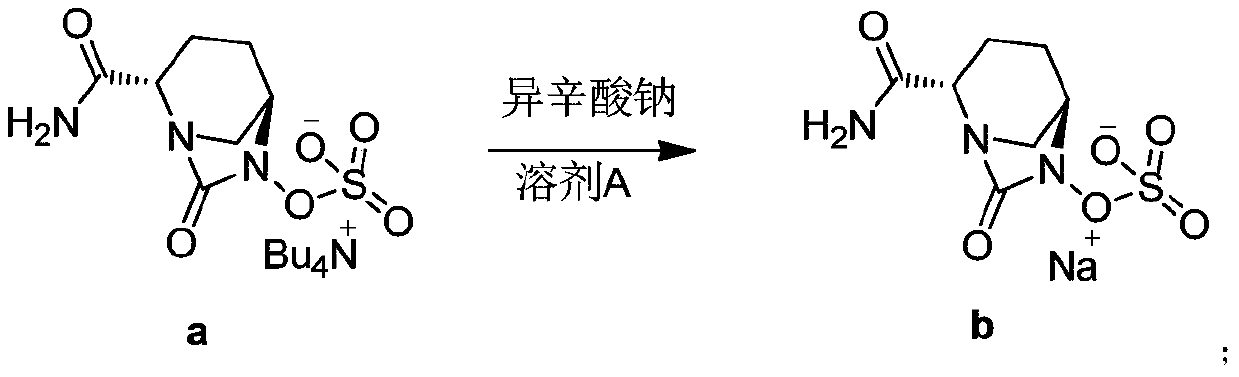
ActiveCN108409736AReduce generationThe method steps are simpleOrganic chemistryAvibactam sodiumPurified water
The invention discloses a method for preparing amorphous avibactam sodium by spray-drying. According to the invention, solution of sodium iso-octoate is dropwise added into solution of avibactam tetrabutylammonium salt, after dropwise adding is completed, the reaction is performed for 3 to 4h, and purified water is added for extraction; after a water phase is subjected to spray-drying, the amorphous avibactam sodium is obtained. The method disclosed by the invention is simple in step and easy for industrial production, the purity of a product is greater than or equal to 98.0%, and yield is greater than or equal to 90.0%.
Owner:山东安信制药有限公司
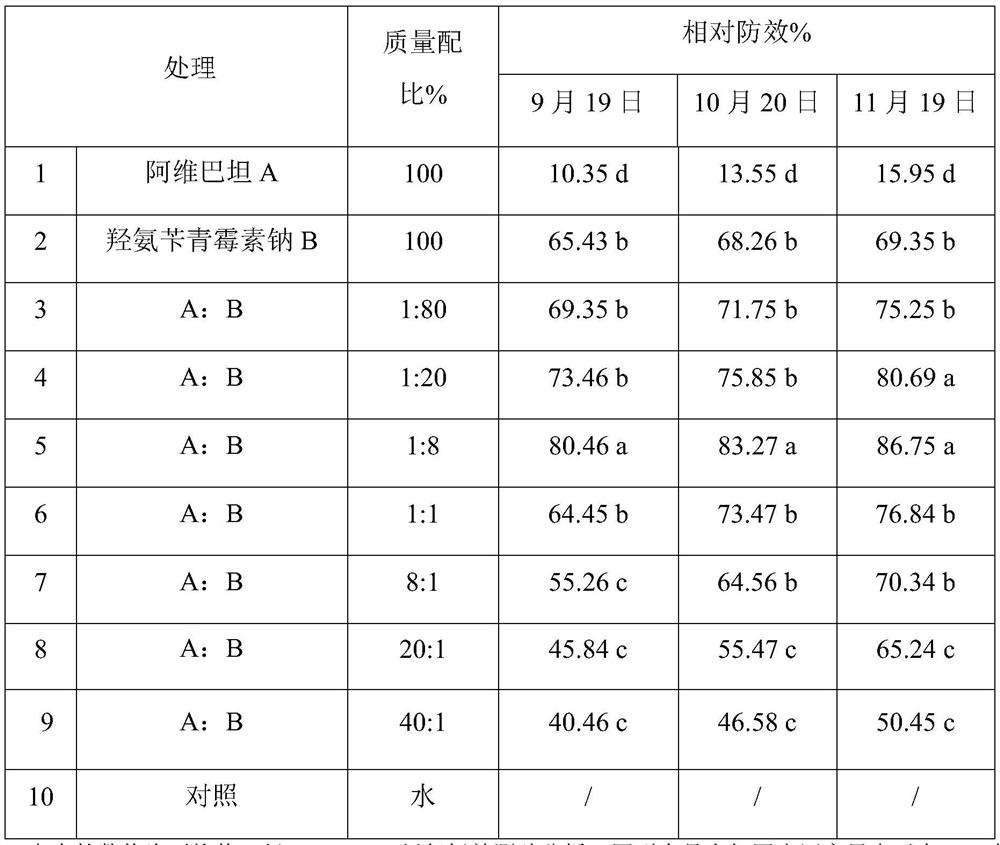
Avibactam and beta-lactam antibiotic compound synergistic composition
InactiveCN112450219AGood synergyReduce the risk of agricultural product quality and safetyBiocideDisinfectantsCombinatorial chemistryToxicology
The invention discloses an avibactam and beta-lactam antibiotic compound synergistic composition which comprises avibactam and beta-lactam antibiotics, and the mass ratio of the avibactam to the beta-lactam antibiotics is 1: 80-40: 1. The avibactam and beta-lactam antibiotic compound composition disclosed by the invention has an obvious synergistic effect, overcomes and delays rapid degradation ofbetalactam antibiotics in plants, reduces the application dosage and frequency, and obviously improves the prevention and treatment effect on citrus yellow shoot.
Owner:GUANGXI ZHUANG AUTONOMOUS REGION ACAD OF AGRI SCI
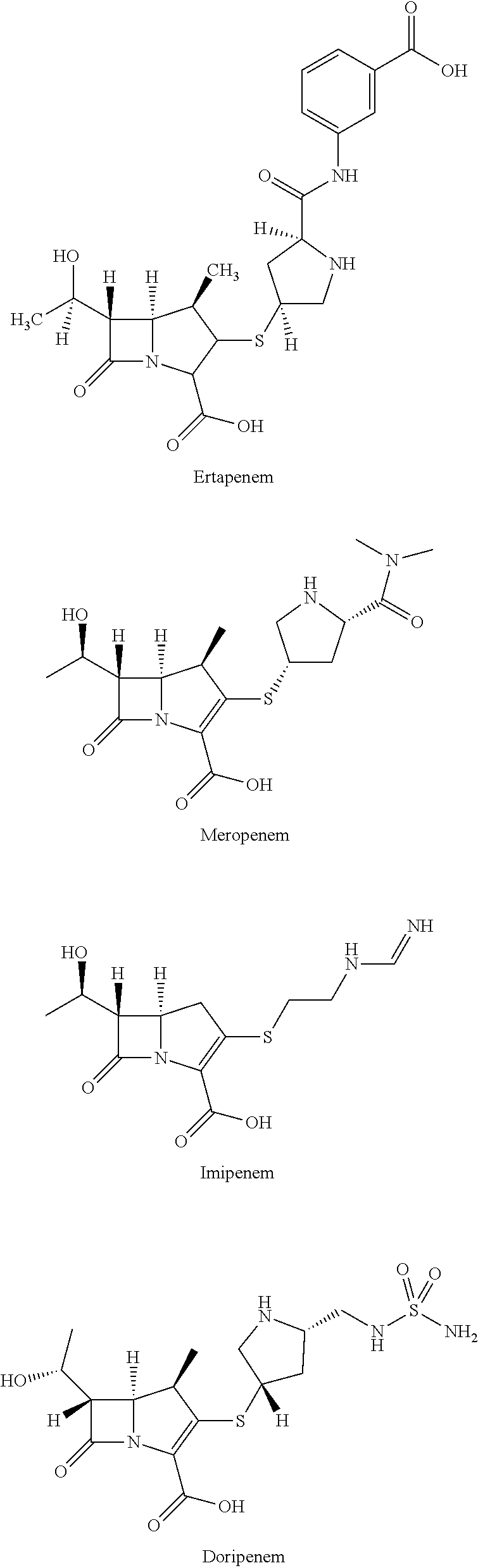
Avibactam and carbapenems antibacterial agents
ActiveUS10434089B2Reduce probabilityAntibacterial agentsOrganic active ingredientsMedicineAntibacterial agent
Described are methods of treating or preventing a bacterial infection by administering an antibacterial agent comprising a β-lactamase inhibitor and one or more carbapenem to a subject.
Owner:THE JOHN HOPKINS UNIV SCHOOL OF MEDICINE
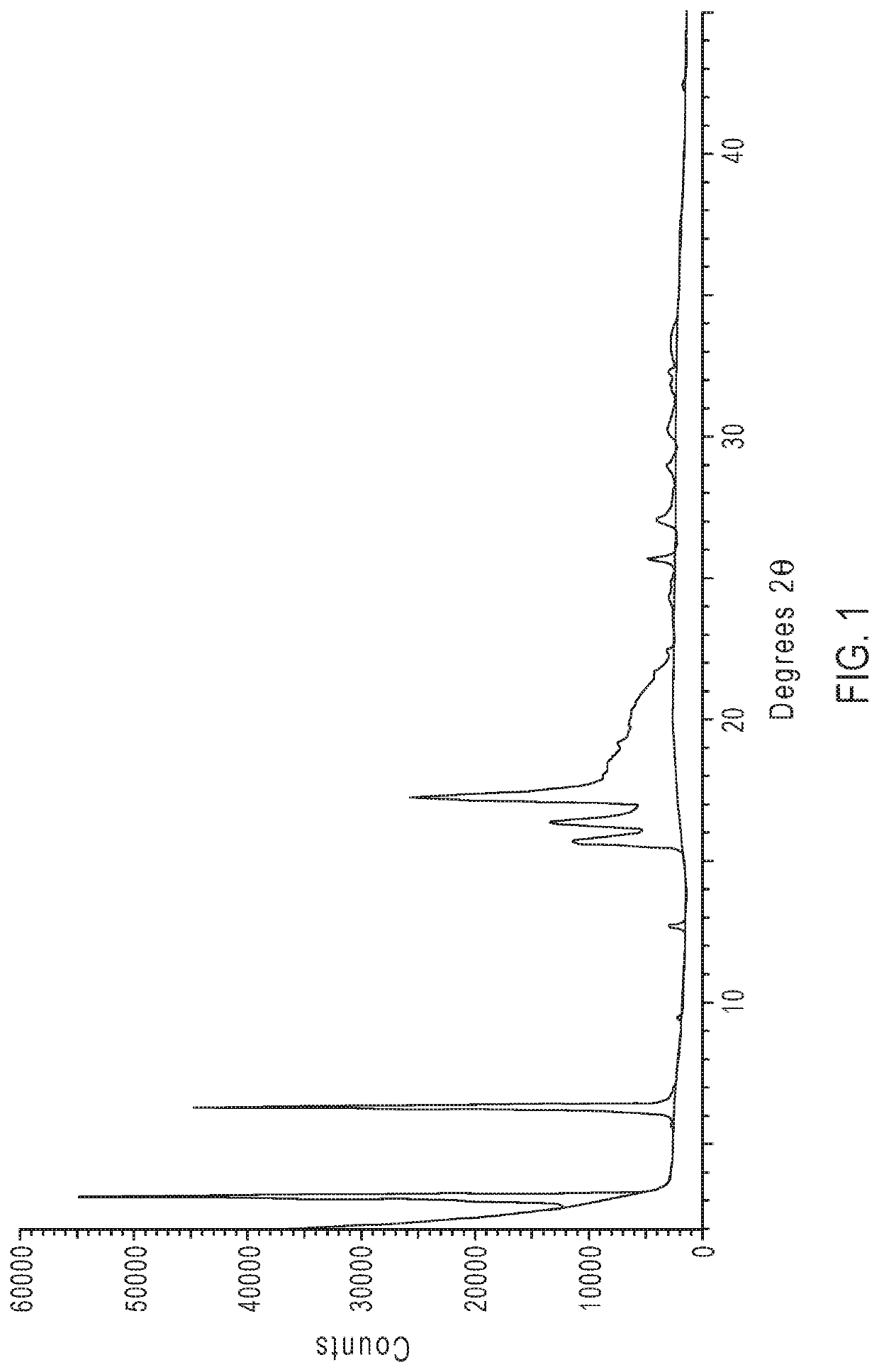
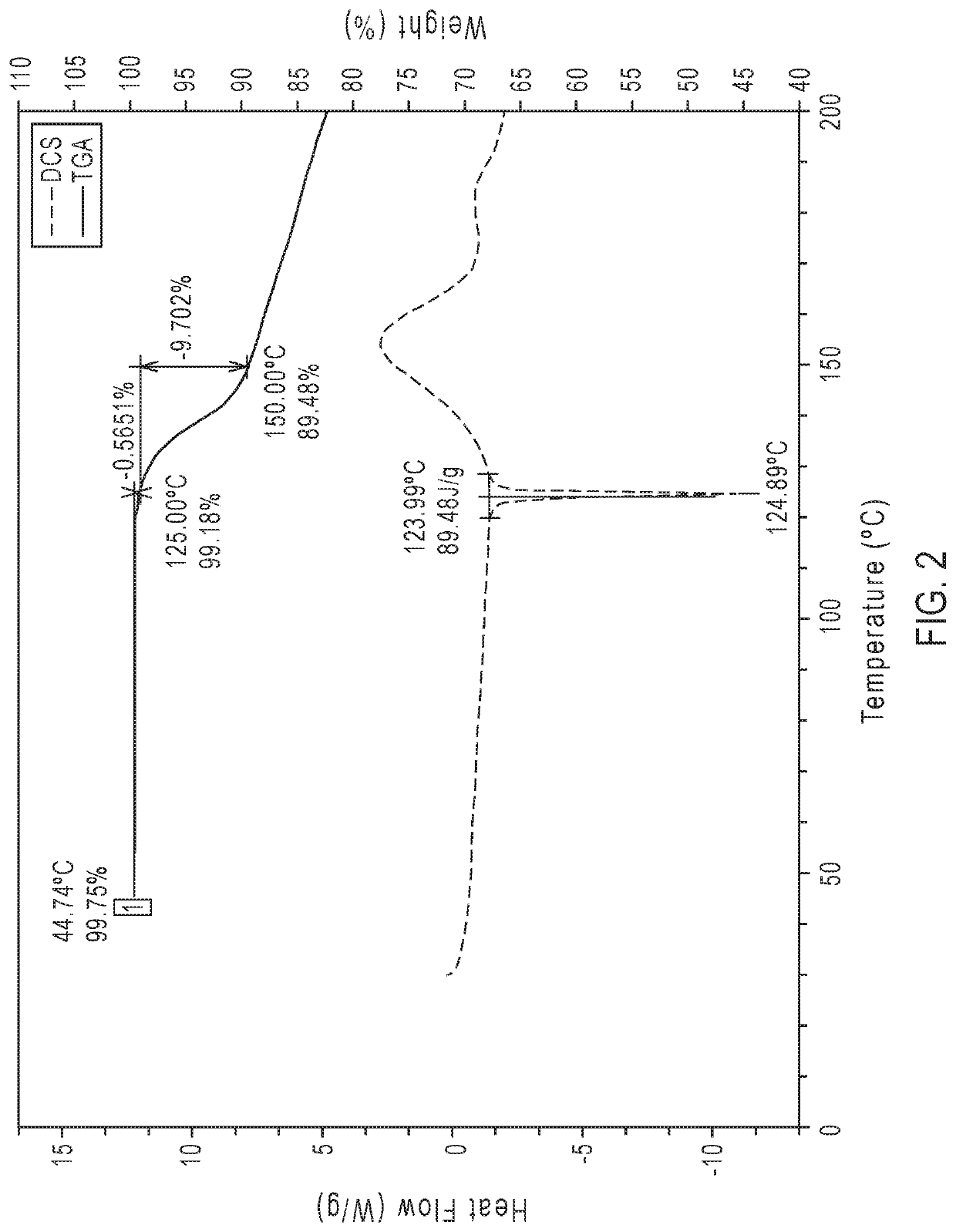
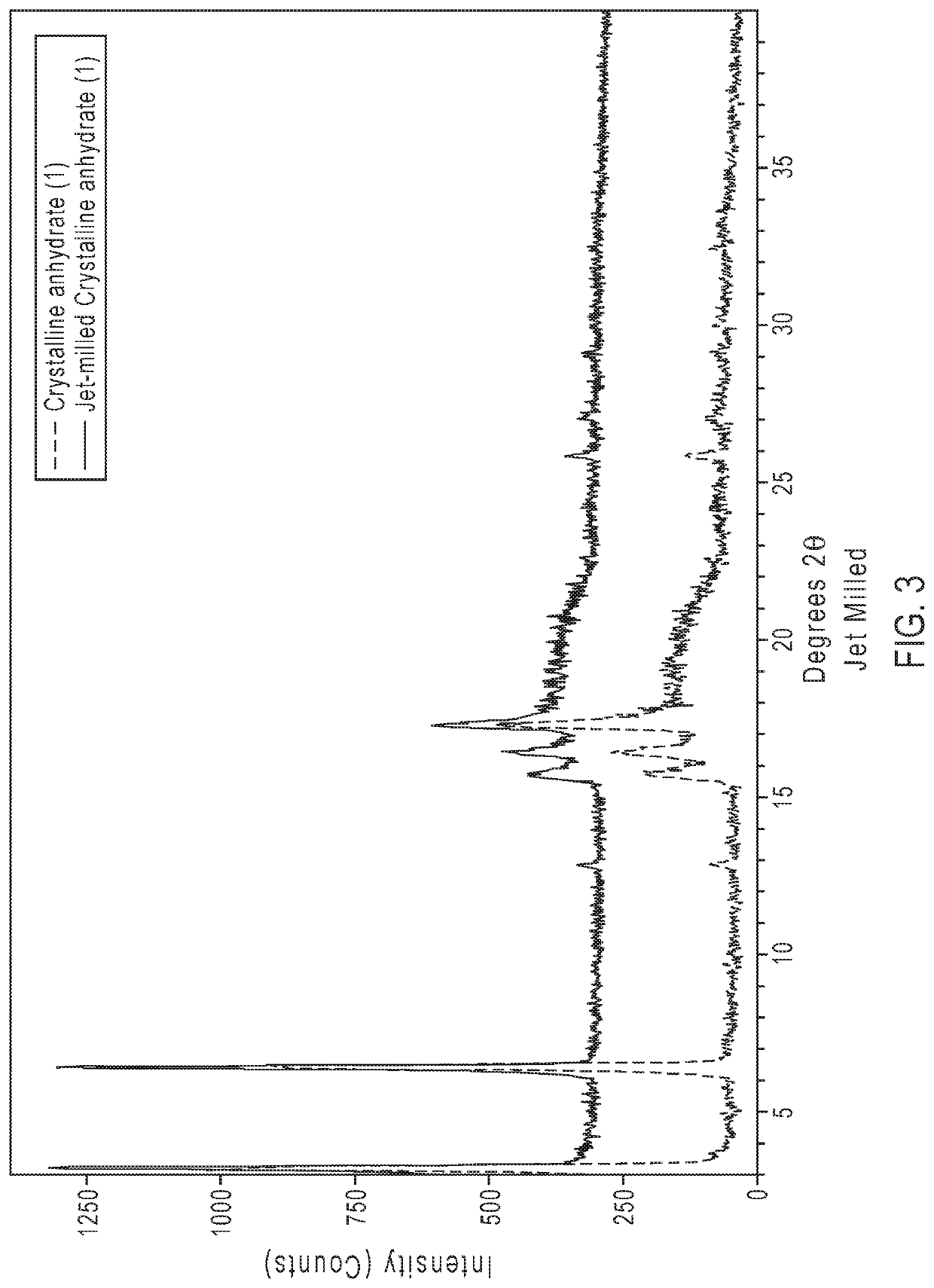
Crystalline form of an avibactam derivative
ActiveUS20200291022A1Antibacterial agentsOrganic active ingredientsPharmaceutical drugPerylene derivatives
Owner:ARIXA PHARMA INC
Combination Therapy for the Treatment of Nosocomial Pneumonia
The present invention relates to a method of treatment of nosocomial pneumonia using a combination of ceftazidime (a third generation cephalosporin) and avibactam (a novel β-lactamase inhibitor), optionally with one or more additional therapeutic agents.
Owner:ASTRAZENECA PHARMA LP +1
Compound pharmaceutical composition containing piperacillin and application thereof
ActiveCN107789355BMeet clinical needsAntibacterial agentsOrganic active ingredientsAntibacterial activityBULK ACTIVE INGREDIENT
The invention provides a compound pharmaceutical composition containing piperacillin and its application. In the compound pharmaceutical composition, piperacillin and avibactam are used as active ingredients, and both piperacillin and avibactam are in solid form exist. The pharmaceutical composition of the present invention has excellent antibacterial activity, especially has good effect on drug-resistant Streptococcus pneumoniae, and improves the efficacy by dozens of times compared with the existing piperacillin product.
Owner:XIANGBEI WELMAN PHARMA CO LTD
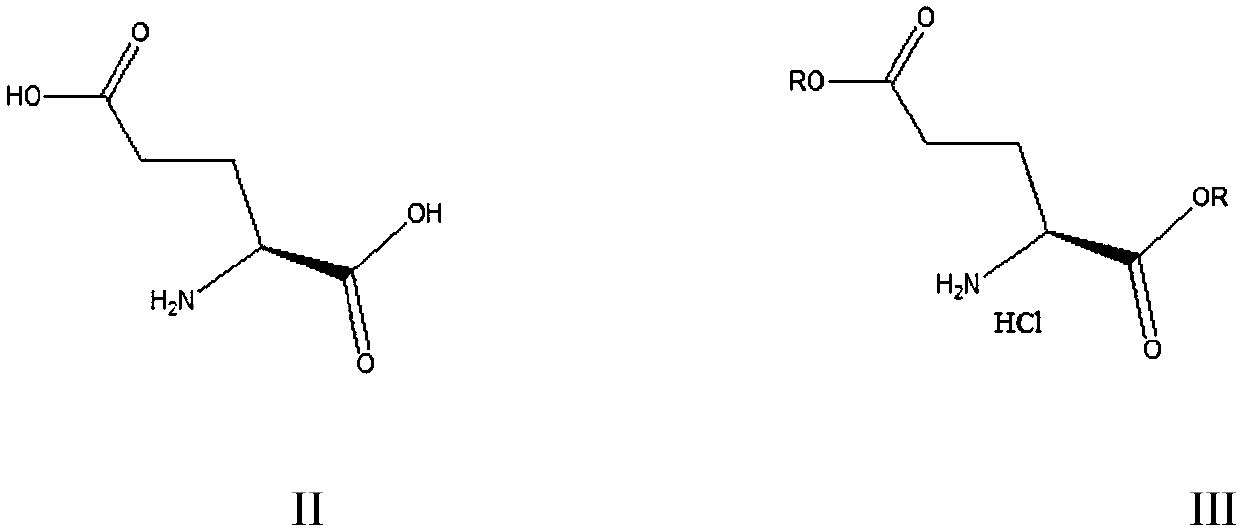
Green preparation method of N-substituted-L-pyroglutamate
InactiveCN107602436AReduce productionRaw materials are cheap and easy to getOrganic chemistryBulk chemical productionL-Pyroglutamic AcidSolvent
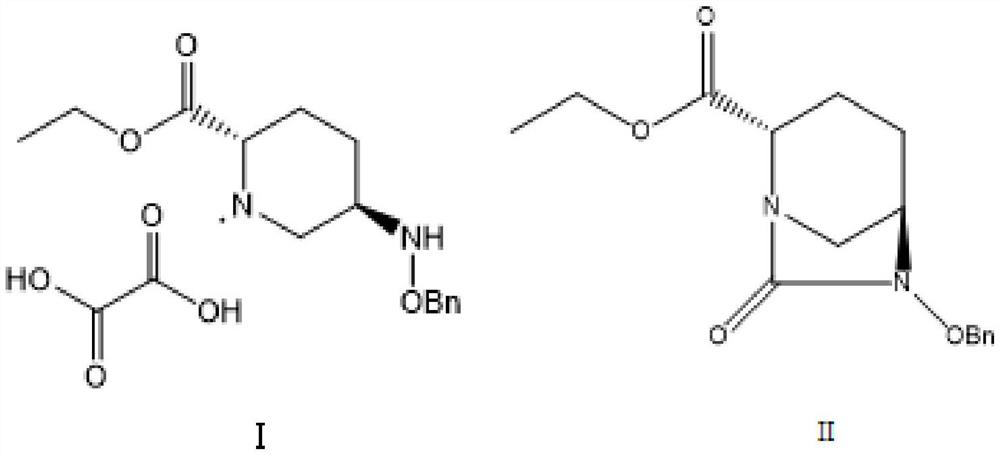
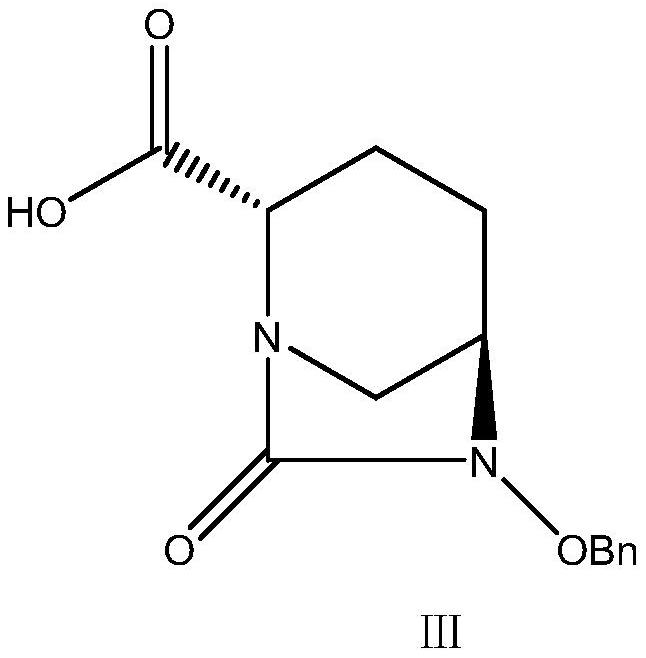
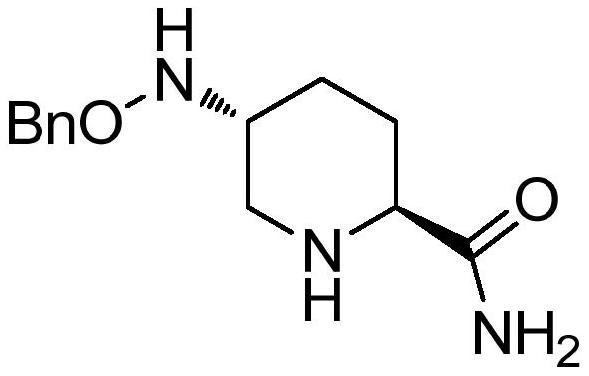
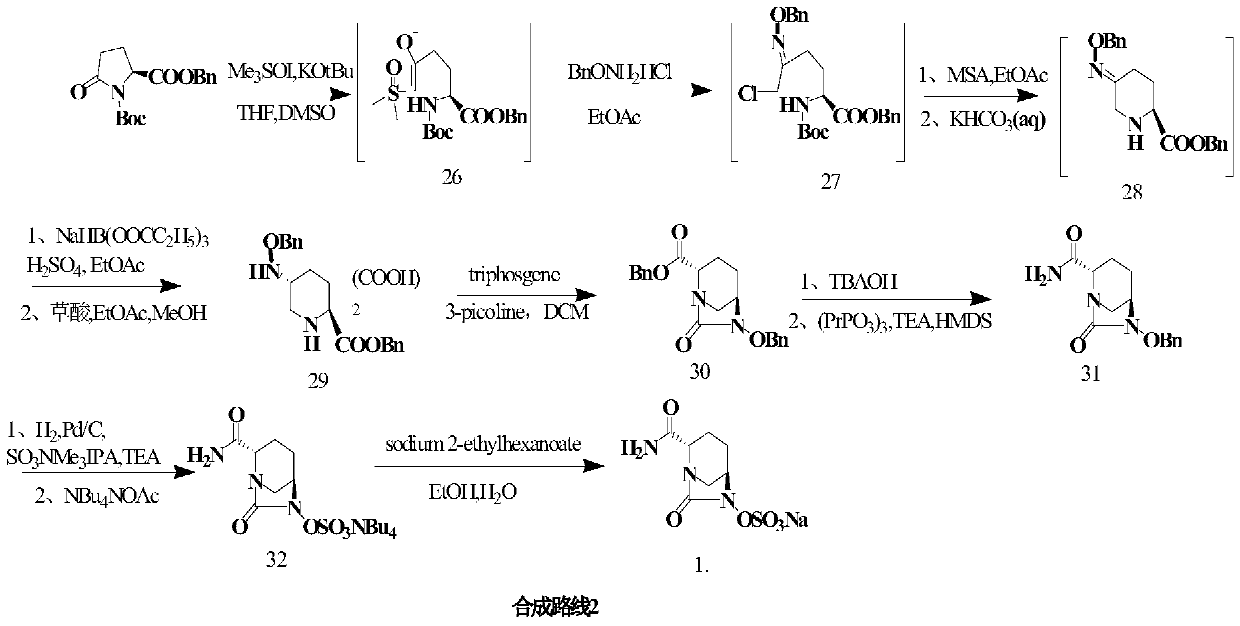
The invention provides a green preparation method of N-substituted-L-pyroglutamate. The method comprises the steps as follows: L-glutamic acid diester hydrochloride (III) is prepared from L-glutamic acid (II) as a starting material in the presence of an acidic reagent by an esterification reaction; then, L-glutamic acid diester hydrochloride (III) is subjected to N-substituted protective reactionwith an N-substituent protective reagent with a one-pot method in the presence of a base and a solvent, an N-substituted protective group is introduced, heating is performed for dealcoholization cyclization in molecules, and N-substituted-L-pyroglutamate as shown in the formula (I) is obtained. The method has the advantages of cheap and easily available raw materials, classic reaction types, shortprocess route, simple and convenient operation, small waste water amount, green and environment-friendly production process, high reaction yield and low product cost. 5R-benzyloxyaminopiperidine-2S-carboxylate, 5R-benzyloxyaminopiperidine-2S-formate ethanedioate and avibactam can be prepared from N-substituted-L-pyroglutamate as shown in the formula (I).
Owner:XINFA PHARMA
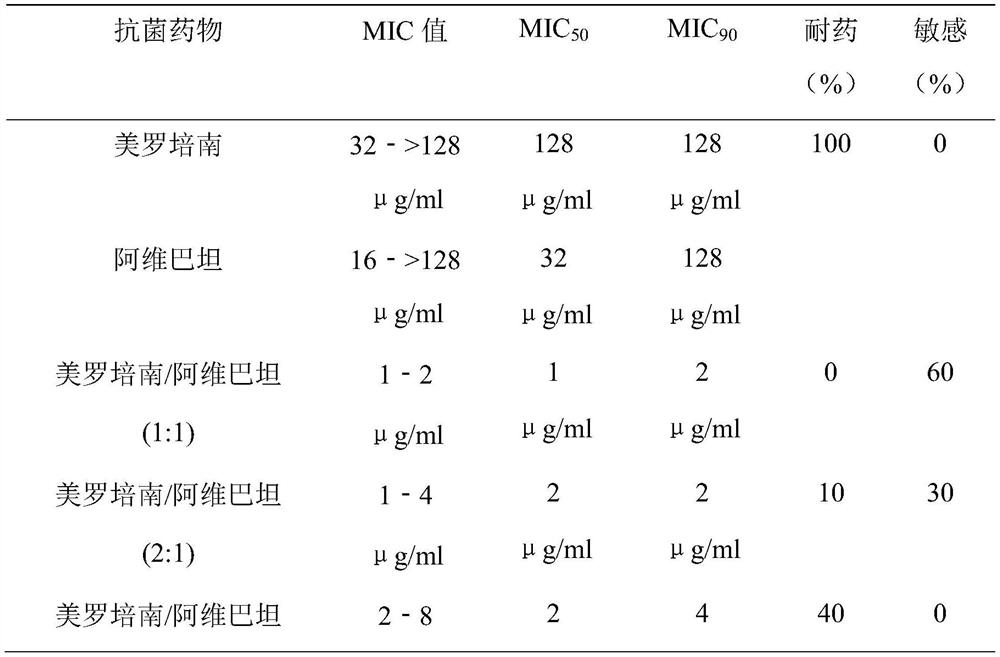
Antibacterial composition containing avibactam and meropenem and application thereof
The invention belongs to the technical field of medicines. The invention relates to an antibacterial composition containing avibactam and meropenem and application thereof. Particularly, the inventionprovides application of avibactam or a pharmaceutically acceptable salt thereof and meropenem in preparation of a medicine for treating or preventing bacterial infection, in particular to applicationin preparation of medicines for treating infection caused by escherichia coli, pseudomonas aeruginosa, klebsiella pneumoniae, acinetobacter baumannii and enterobacter aerogenes, and aims to solve theproblem of drug resistance of carbapenem antibiotics.
Owner:CHIA TAI TIANQING PHARMA GRP CO LTD
Anti-infective compound, preparations and application thereof
PendingCN108498519AIncreased sensitivityThe effect of CRKP is remarkableAntibacterial agentsOrganic active ingredientsResistant bacteriaMass ratio
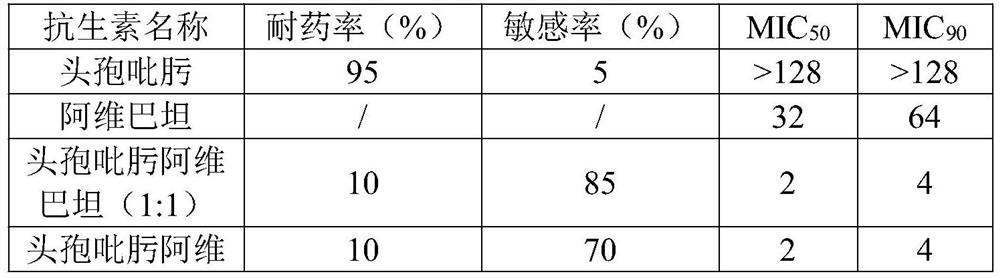
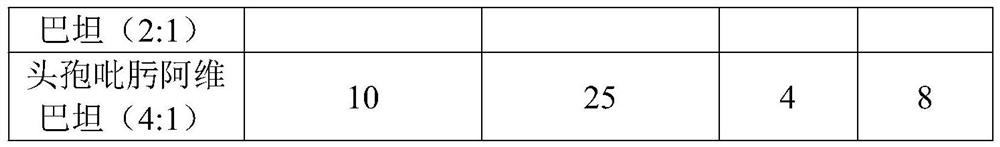
The invention belongs to the field of anti-infective medicines, and specifically discloses a cefepime and avibactam compound component for injection. The compound component is a mixture of cefepime and avibactam in a mass ratio of 8-1:1-8. The compound component has a good synergistic effect after mixing according to the above ratio, and the synergy rate of the cefepime and the avibactam is up to100%, which is better than other combinations; the sensitivity to drug-resistant bacteria can be significantly improved, MIC50 (>512 mcg / Ml) and MIC90 (>512 mcg / ml) of CRKP to the cefepime are reducedto MIC50 (4 mcg / ml) and MIC90 (32 mcg / ml), the activity is increased by 103 times and 16 times respectively, and the compound component greatly improves the effectiveness of drugs. Meanwhile, at least three molecules in the cefepime have tetravalent nitrogen atoms which can form zwitterions, the tissue distribution (especially brain tissues) is good, and the penetrating ability to an enterobacterium membrane is better than the same type of cephalosporin.
Owner:THE SECOND XIANGYA HOSPITAL OF CENT SOUTH UNIV
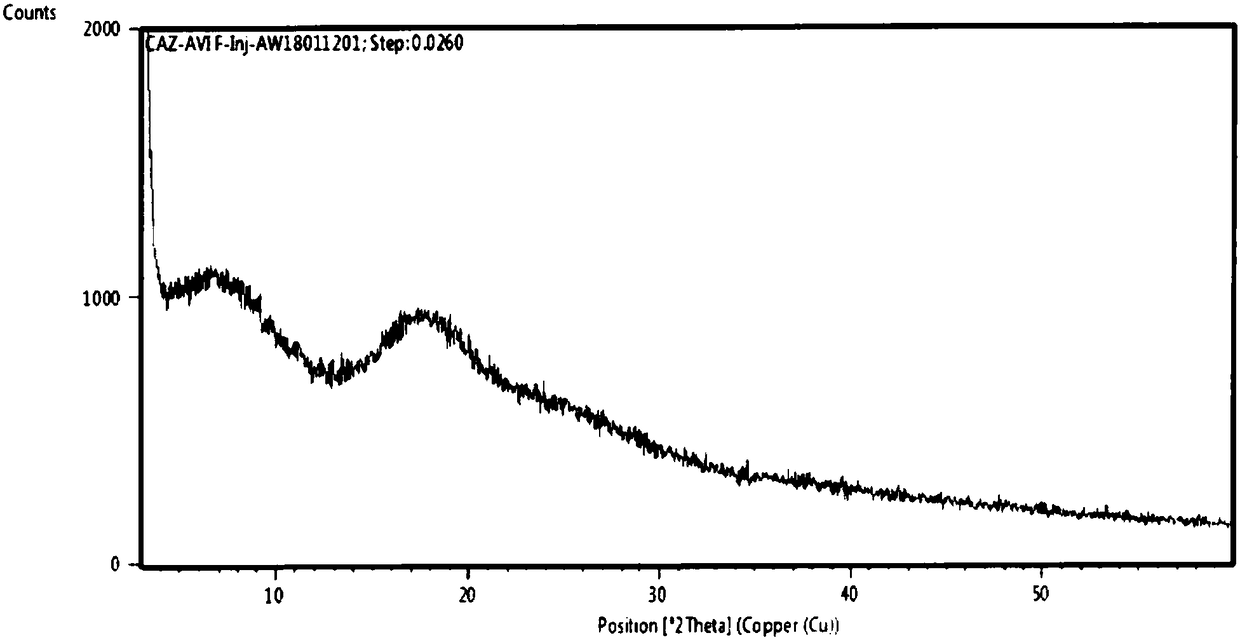
Method for preparing crystal form A or crystal form D type avibactam product through crystallization
The invention discloses a method for preparing a crystal form A or crystal form D avibactam product through crystallization. The method comprises the following steps: preparing an avibactam sodium salt solution by adopting a first solvent; weighing a second solvent; mixing the avibactam sodium salt solution and the second solvent by adopting a dropwise adding manner; after dropwise adding, stirring at room temperature; then cooling to 0 to 5 DEG C and continually stirring; filtering to obtain a filter cake; washing the filter cake by adopting the second solvent; after washing, drying in vacuumto obtain a white solid, namely the product. According to the method disclosed by the invention, the avibactam product is prepared through a crystallization manner; the yield is high, the operation method is simple and large-scale industrial production is easy to realize; the solvents used in a preparation process are adjusted, so that the crystal form A and crystal form D avibactam products canbe obtained respectively; when the large-scale industrial production is carried out, the avibactam products with different crystal forms can be obtained respectively only if simple solvent adjustmentis carried out; the method has a wide application range and a wide market prospect.
Owner:上海龙翔生物医药开发有限公司
Synthesis method of avibactam intermediate (I)
PendingCN113845434AMild conditionsSimple and fast operationOrganic compound preparationAmino-carboxyl compound preparationPtru catalystOrganic solvent
The invention provides a synthesis method of an avibactam intermediate (I). According to the method, an enamine substrate is subjected to asymmetric hydrogenation in an organic solvent in a hydrogen environment under the catalysis of a rhodium complex formed by a rhodium metal catalyst and a chiral diphosphine ligand to prepare the avibactam intermediate (I). The method has the advantages of mild reaction conditions, excellent enantioselectivity and diastereoselectivity, high chemical yield and potential industrial application value.
Owner:无棣融川医药化工科技有限公司 +1
Pharmaceutical composition containing sulbactam and avibactam and application of pharmaceutical composition
The application relates to a pharmaceutical composition comprising sulbactam or a pharmaceutically acceptable salt thereof and avibactam or a pharmaceutically acceptable salt thereof. The unit dose ratio of the sulbactam or the pharmaceutically acceptable salt thereof to the avibactam or the pharmaceutically acceptable salt thereof is from about 8: 1 to about 4: 1, the unit dose of the sulbactam or the pharmaceutically acceptable salt thereof is about 1-4 g, the unit dose of the avibactam or the pharmaceutically acceptable salt thereof is about 0.125-1 g, and a pharmaceutically acceptable carrier is included optionally. The present application also relates to methods of treating bacterial infections using the pharmaceutical composition.
Owner:TENNOR THERAPEUTICS (SUZHOU) LTD
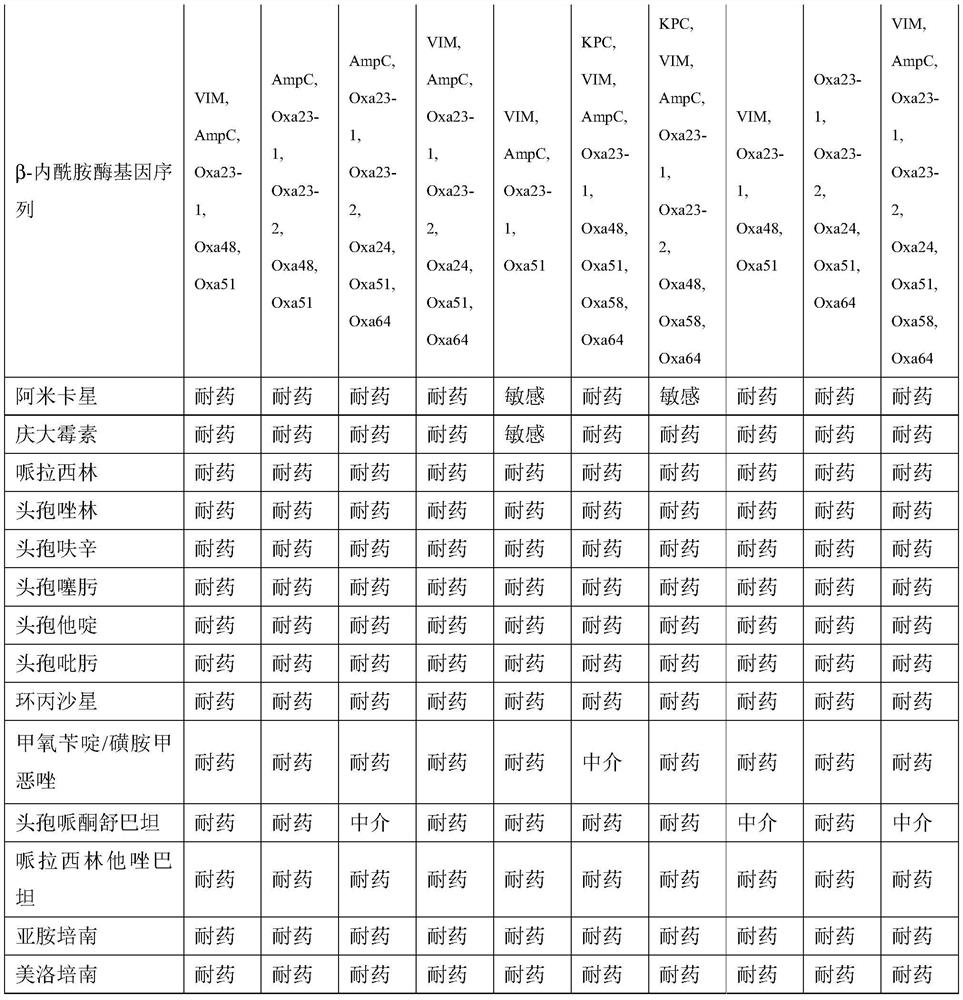
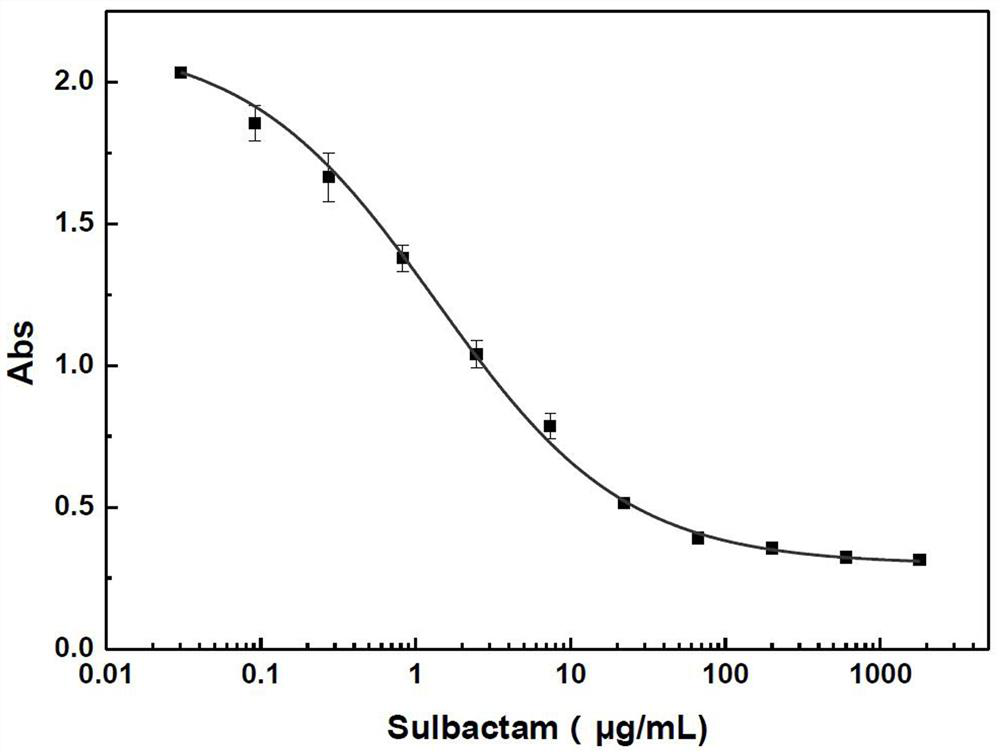
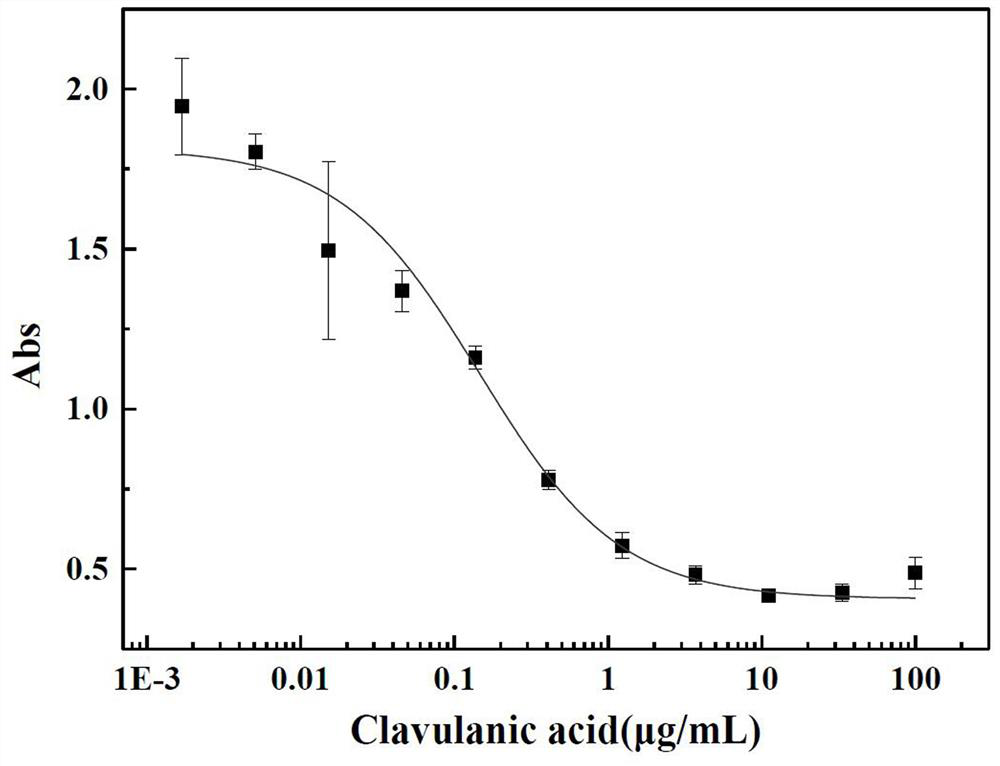
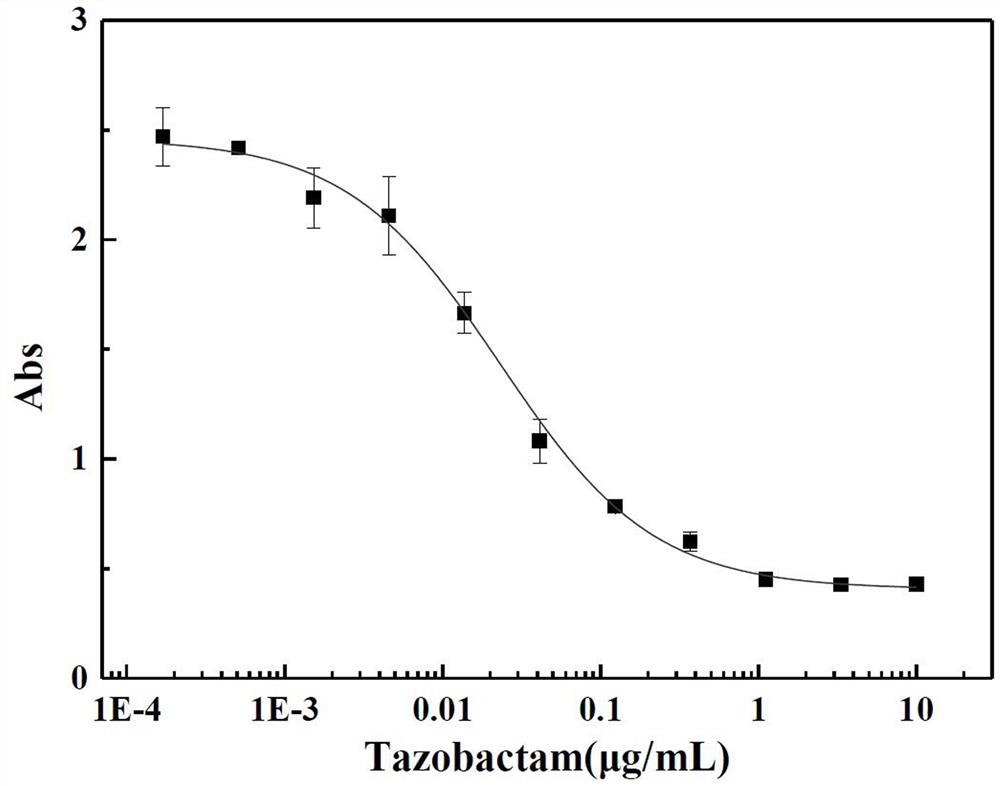
Kit for quantitatively detecting various beta-lactamase inhibitor residues and using method thereof
PendingCN112666155AEnables multi-residue detectionInhibition of color reactionMaterial analysis by observing effect on chemical indicatorBiotechnologyMilk sample
The invention discloses a kit for quantitatively detecting various beta-lactamase inhibitor residues and a using method thereof, which belong to the technical field of food safety rapid detection. The kit comprises a beta-lactamase solution, a beta-lactamase inhibitor standard substance solution, a developing stock solution, a buffer solution and a 96-hole black lightproof elisa plate, and can be used for quantitatively detecting multiple residues of sulbactam, clavulanic acid, tazobactam and avibactam in liquid milk at the same time. The detection limits of sulbactam, clavulanic acid, tazobactam and avibactam in the milk sample are respectively 4.7 mug / kg, 95.8 mug / kg, 2.0 mug / kg and 4.6 mug / kg; the linear ranges are respectively 34.2-622.8 mug / kg, 275.7-10233.3 mug / kg, 4.9-108.8 mug / kg and 9.6-118.7 mug / kg. The kit is wide in screening range, can effectively avoid the problem of missed detection caused by the fact that lawbreakers select and avoid sulbactam which is commonly detected and use beta-lactamase inhibitors of other varieties, and is particularly suitable for high-throughput screening.
Owner:GUANGDONG UNIV OF EDUCATION
Preparation method of avibactam intermediate
InactiveCN110590618AMild reaction conditionsEasy to operateOrganic chemistryOrganic compound preparationCalcium hydroxideLithium hydroxide
The invention discloses a preparation method of an avibactam intermediate. The method comprises the following step: performing a reaction on a compound I and trimethylsulfoxonium iodide in the presence of a base to prepare a compound II, wherein the structures of the compound I and the compound II are shown in the specification, R is one selected from the group consisting of methyl, ethyl, benzyl,tert-butyl and allyl, and the base is one or more selected from the group consisting of magnesium hydroxide, lithium hydroxide and calcium hydroxide. According to the preparation method of the avibactam intermediate, the method uses pyroglutamate with double protection as a raw material, the pyroglutamate reacts with the trimethylsulfoxonium iodide in the presence of the base to obtain the avibactam intermediate, and the method provided by the invention avoids the defects of potential safety hazard of a strong base, severe reaction condition requirements and strict process control requirements in the prior art, and has the advantages of mild reaction conditions, simple operation, high safety, and less reaction impurities.
Owner:JIANGXI FUSHINE PHARMA CO LTD
Preparation method of avibactam sodium
PendingCN111777607AHigh yieldSuitable for industrial productionOrganic chemistryBulk chemical productionSodium saltEster hydrolysis
The invention discloses a synthesis method of avibactam sodium. (2S,5R)-benzyloxyamino piperidine-2-ethyl formate oxalate (I) is used as an initial raw material; and the method comprises the followingsteps: reacting the raw material with a protecting group, carrying out carbonylation cyclization, carrying out hydrolysis of ester, ammoniating, sulfonating with a sulfur trioxide complex, salifyingwith an ammonium ion source, and salifying with a sodium salt to obtain avibactam sodium, and has the advantages of simple operation, easily controlled conditions, easy industrial production and wideapplication prospect.
Owner:HAINAN HAILING CHEMIPHARMA CORP
Method for synthesizing avibactam intermediate compound
PendingCN110437137ANo pollution in the processMild reaction conditionsOrganic chemistryBulk chemical productionOrganic chemistryReaction conditions
The invention relates to a method for synthesizing an avibactam intermediate compound. The specific synthetic route of the method is as described in the specification. According to a technical schemeof the invention, no precious metal catalyst is used, and no heavy metal pollution is produced; high yield and low cost are realized; and the method is mild in reaction condition and friendly to environment, and can realize industrial production.
Owner:CHENGDU CLIMB PHARMA TECH
A kind of avibactam intermediate, preparation method and application thereof
ActiveCN110078728BSimple post-processingSuitable for industrial mass productionOrganic chemistryCombinatorial chemistryAniline
The invention discloses an avibactam intermediate, and a preparation method of the avibactam intermediate. The preparation method comprises the steps of allowing a compound II to react with a sulfonation reagent in the presence of an alkaline agent and then react with the alkaline agent to form the avibactam intermediate, wherein the alkaline agent is trialkylamine, pyridine, alkylpyridine, N-alkylpiperidine, dialkyl phenylamine or dialkyl benzylamine; alkyl in trialkyl amine is C5-8 alkyl; alkyl in alkyl pyridine in C1-4 alkyl; alkyl in N-alkylpiperidine is C1-4 alkyl; alkyl in dialkyl phenylamine is C1-4 alkyl; and alkyl in dialkyl benzylamine is C1-4 alkyl. Compared with the traditional method, the method adopts the alkaline agent with appropriate alkalinity to substitute the traditional alkaline agent; the alkaline agent can react with the compound II in a step directly with the sulfonation reagent; the post-treatment is simple; and the method is more suitable for industrial mass production.
Owner:JIANGXI FUSHINE PHARMA CO LTD
Pharmaceutical composition and application thereof
PendingCN114177183AHigh synergy rateIncreased sensitivityAntibacterial agentsOrganic active ingredientsPharmaceutical medicineAvibactam
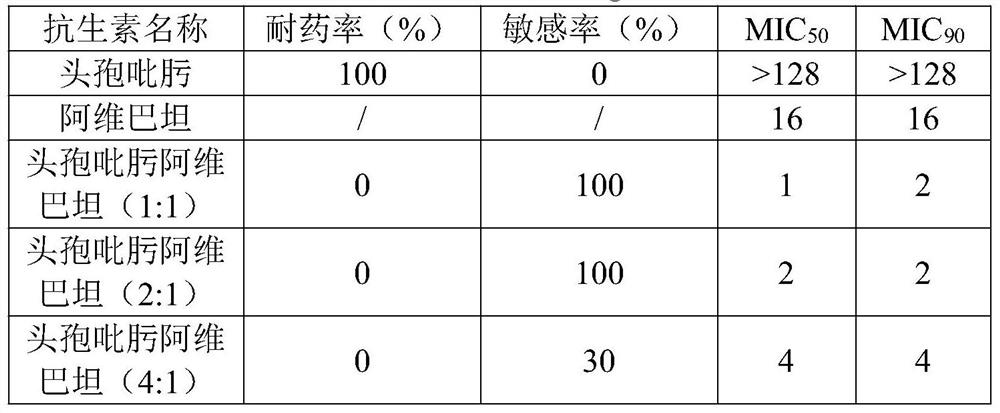
The invention belongs to the technical field of medicine application, and particularly discloses application of a medicine composition in preparation of a medicine for inhibiting A-class or D-class carbapenem drug-resistant klebsiella pneumoniae. The pharmaceutical composition comprises a component I and a component II, the component I is cefepime or pharmaceutically acceptable salt thereof, the component II is avibactam or pharmaceutically acceptable salt thereof, the component I and the component II are mixed according to the mass ratio of 1: 1-2: 1, and the synergistic rate of the component I and the component II is as high as 95%. According to the pharmaceutical composition, the sensitivity to the carbapenem-resistant klebsiella pneumoniae can be obviously improved, the sensitivity to the A type and D type carbapenem-resistant klebsiella pneumoniae is relatively high, and the pharmaceutical composition has a good application prospect in preparation of drugs for inhibiting the A type or D type carbapenem-resistant klebsiella pneumoniae.
Owner:南京力博维制药有限公司
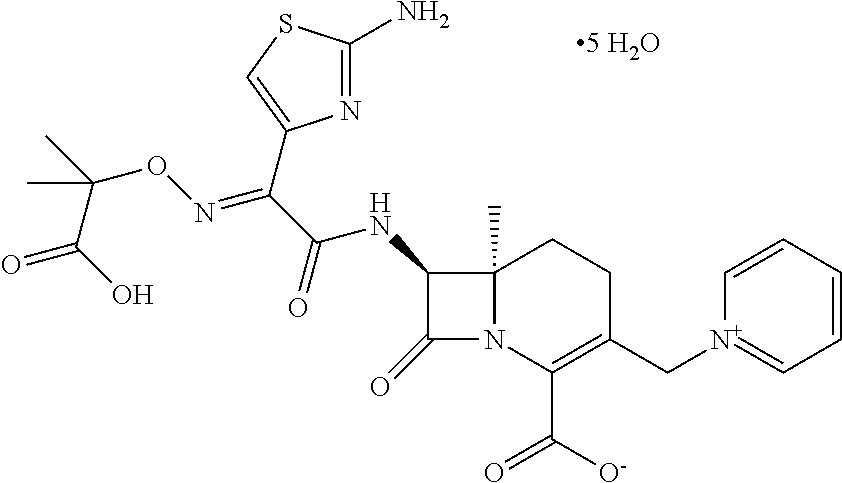
Avibactam Free Acid
The present invention relates to avibactam free acid, a method for preparing avibactam free acid and a method for preparing avibactam sodium by further reacting avibactam free acid. The invention further refers to a pharmaceutical composition comprising avibactam free acid, one or more alkaline sodium salt(s) and one or more beta-lactam antibiotic(s). The pharmaceutical composition of the present invention can be used as medicament, in particular for treatment and / or prevention of bacterial infections.
Owner:SANDOZ AG
A kind of recovery method of avibactam intermediate isomer
ActiveCN108373442BReduce pollutionEfficient recycling effectOrganic chemistryBulk chemical productionPtru catalystPalladium catalyst
Owner:TAIZHOU VOCATIONAL & TECHN COLLEGE
A kind of refining preparation process of high-purity avibactam
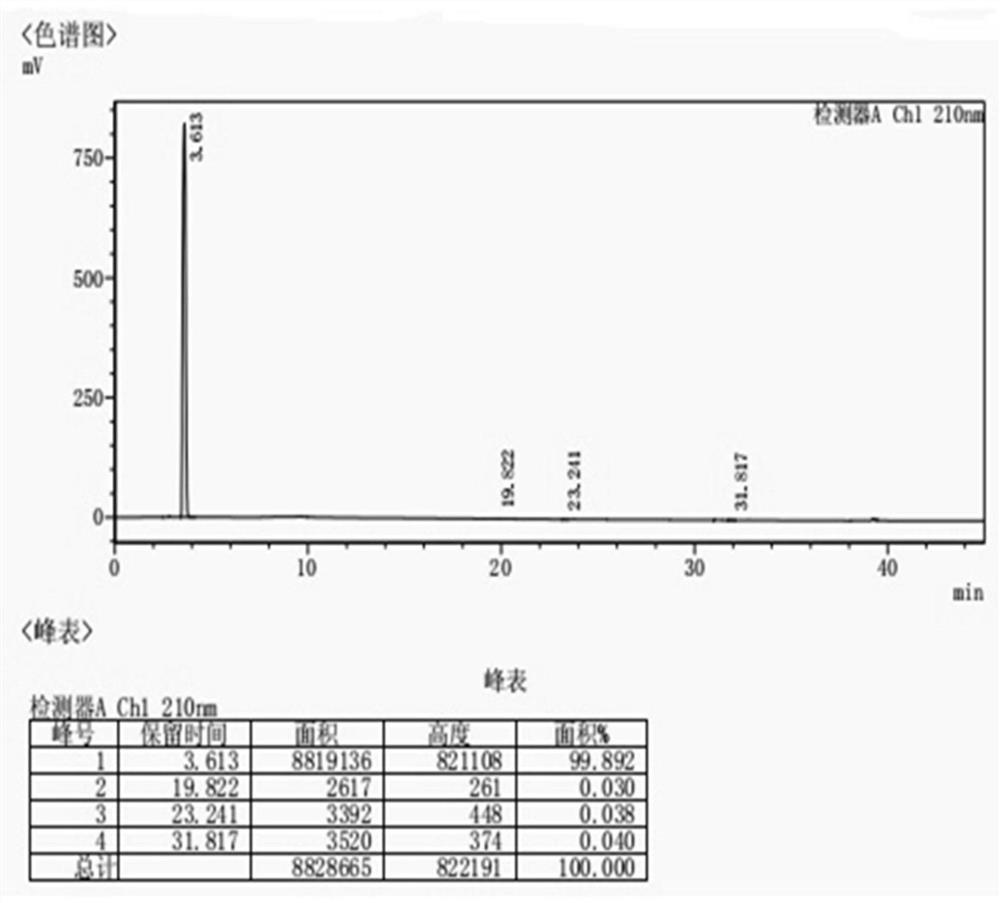
The invention discloses a refining preparation technology of high-purity avibactam. The refining preparation technology comprises the following steps: (1), adding an intermediate alpha and sodium isooctanoate into a solvent A, reacting for 10-15 hours, and treating to obtain crude avibactam; (2), adding the crude avibactam into a solvent B, heating for dissolving, and refining a mixture to obtainpurified avibactam, wherein the solvent B is at least one of anhydrous ethanol, acetone and 2-butanone. By the refining preparation technology, the 2-butanone is used as a purifying solvent for impurity removal, a relatively good technical effect is achieved, the contents of main impurity raw materials such as the intermediate alpha as well as technological impurities in the crude avibactam can besignificantly reduced, and HPLC detection shows that the purity of the avibactam reaches 99.7% or above and the impurity limits are lower than 0.1%.
Owner:GUANGXI ENANTIOTECH PHARM CO LTD
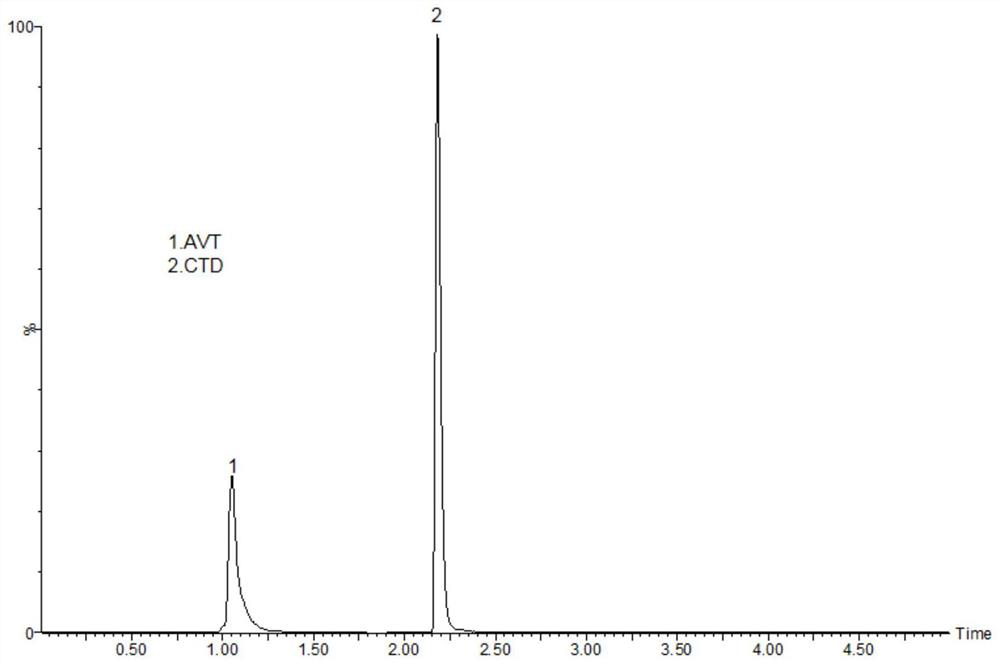
Method for detecting drug concentrations of avibactam and ceftazidime in blood plasma
Owner:南京品生医学检验实验室有限公司
The preparation method of avibactam intermediate
ActiveCN112125837BHigh yieldHigh purityOrganic chemistryOrganic-compounds/hydrides/coordination-complexes catalystsPtru catalystCombinatorial chemistry
The invention relates to a preparation method of an avibactam intermediate. The preparation method comprises the steps of: mixing a compound having a structure shown in formula 2, a chiral catalyst, an acid and a solvent, and stirring to prepare a compound having a structure shown in formula 1; the chiral catalyst having a structure shown in formula (I) Structure. The method has high yield, purity and chiral purity, and is environmentally friendly.
Owner:ENANTIOTECH CORP +1
A kind of convenient preparation method of avibactam
ActiveCN109956941BResidue reductionSimple stepsAntibacterial agentsOrganic active ingredientsFormateIon exchange
The invention provides a simple and convenient preparation method of avibactam, which uses piperidine-5-ketone-2S-formate II as a raw material, undergoes a condensation reaction with O-protecting group hydroxylamine hydrochloride, and then undergoes reduction, chiral Resolution and hydrolysis under alkaline conditions to obtain 5R-substituent oxyaminopiperidine-2S-carboxylic acid V; and then "one-pot" cyclic urealation, acyl chloride and amidation with phosgene, solid phosgene or diphosgene Reaction, through deprotection, sulfuric acid esterification, tetrabutylammonium into salt to obtain {[(2S,5R)-2-carbamoyl-7-oxo-1,6-diazacyclo[3.2.1] Octane-6-yl]oxyl}sulfonyl tetra-n-butylammonium salt VII, and finally avibactam I was obtained by ion exchange. The invention has simple preparation route, easy operation, low raw material price, low cost, low discharge of "three wastes", high atom utilization rate, economical and environmental protection, and high yield of each step, which is beneficial to the industrialized production of avibactam.
Owner:XINFA PHARMA
Synthetic method of β-lactamase inhibitor avibactam
Owner:YIYUAN XINQUAN CHEM
Avibactam and cefmenoxime compound powder injection for injection and preparation method thereof
PendingCN113413367AGood dispersionImprove solubilityAntibacterial agentsOrganic active ingredientsBiochemistryAvibactam sodium
The invention relates to an avibactam and cefmenoxime compound powder injection for injection and a preparation method thereof. The compound powder injection is prepared by well mixing cefmenoxime hydrochloride and avibactam sodium, tabletting and crushing. The powder injection disclosed by the invention is easy to disperse and dissolve when being prepared into an injection, is convenient to use, cannot easily generate beta-lactamase bacterial drug resistance while ensuring the content of cefmenoxime, and has a good market application prospect.
Owner:ZHEJIANG JIANFENG PHARM CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com