Pharmaceutical composition containing sulbactam and avibactam and application of pharmaceutical composition
A composition and medicine technology, applied to a pharmaceutical composition comprising avibactam and sulbactam, the application field in the treatment of bacterial infections, can solve the problem of less clinical application experience, high probability of adverse reactions, and low bacterial drug resistance Clinical efficacy and other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
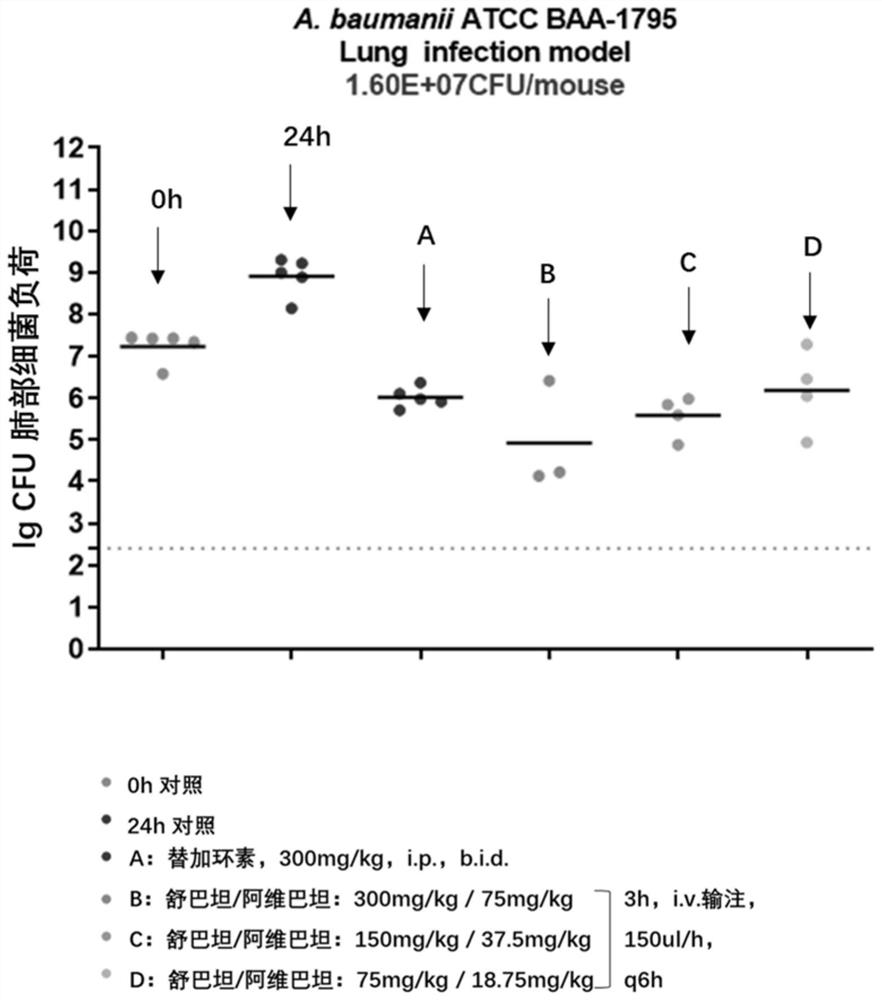
Image
Examples
Embodiment approach
[0217] 1. A pharmaceutical composition comprising sulbactam or a pharmaceutically acceptable salt thereof and avibactam or a pharmaceutically acceptable salt thereof, wherein the sulbactam or a pharmaceutically acceptable salt thereof The unit dose ratio of the avibactam or its pharmaceutically acceptable salt is about 8:1 to about 4:1, and the unit dose of the sulbactam or its pharmaceutically acceptable salt is about 1- 4g, the unit dose of the avibactam or its pharmaceutically acceptable salt is about 0.125-1g, and optionally a pharmaceutically acceptable carrier.
[0218] 2. The pharmaceutical composition according to embodiment 1, wherein the unit dosage ratio of sulbactam or a pharmaceutically acceptable salt thereof and avibactam or a pharmaceutically acceptable salt thereof is about 4:1.
[0219] 3. The pharmaceutical composition according to any one of embodiments 1-2, wherein the unit dose of sulbactam or a pharmaceutically acceptable salt thereof is about 1-3 g.
...
Embodiment 1
[0346] Example 1 Minimal inhibitory concentration test of sulbactam sodium and avibactam sodium to the first series of Acinetobacter baumannii clinical strains
[0347] The minimum inhibitory concentration (MIC) was determined based on the requirements of Clinical and Laboratory Standards Institute (CLSI) M07 (for aerobic bacteria).
[0348] 1. Test drug (combination):
[0349] Table 1 Test Drugs (Combinations)
[0350] compound (combination) Maximum concentration (μg / mL) Minimum Concentration (μg / mL) Sulbactam Sodium 64 0.06 Avibactam Sodium 32 0.5
[0351] 2. Test strains:
[0352] Table 2 Test strains (10 clinical strains in total)
[0353] Bacteria name Strain number culture medium Acinetobacter baumannii ATCC 19606 a Acinetobacter baumannii TNP041702 a Acinetobacter baumannii TNP041703 a Acinetobacter baumannii TNP041704 a Acinetobacter baumannii TNP041705 a Acinetobacter baumann...
Embodiment 2
[0369] Example 2 Sulbactam sodium and avibactam sodium are tested for the minimum inhibitory concentration of the second series of Acinetobacter baumannii
[0370] The minimum inhibitory concentration (MIC) was determined based on the requirements of Clinical and Laboratory Standards Institute (CLSI) M07 (for aerobic bacteria).
[0371] 1. Test drug (combination):
[0372] Table 5 Test Drugs (Combinations)
[0373] compound (combination) Maximum concentration (μg / mL) Minimum Concentration (μg / mL) Sulbactam Sodium 128 0.125 Avibactam Sodium 128 0.125 Sulbactam Sodium / Avibactam Sodium=1:1 64+64 0.063+0.063 Sulbactam Sodium / Avibactam Sodium=2:1 64+32 0.063+0.031 Sulbactam Sodium / Avibactam Sodium=4:1 64+16 0.063+0.016 Tigecycline 32 0.031 polymyxin 32 0.031
[0374] 2. Test strains:
[0375] Table 6 Test strains (20 strains in total)
[0376]
[0377]
[0378] Medium: a, CAMHB (cation adjusted Mueller-Hint...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com