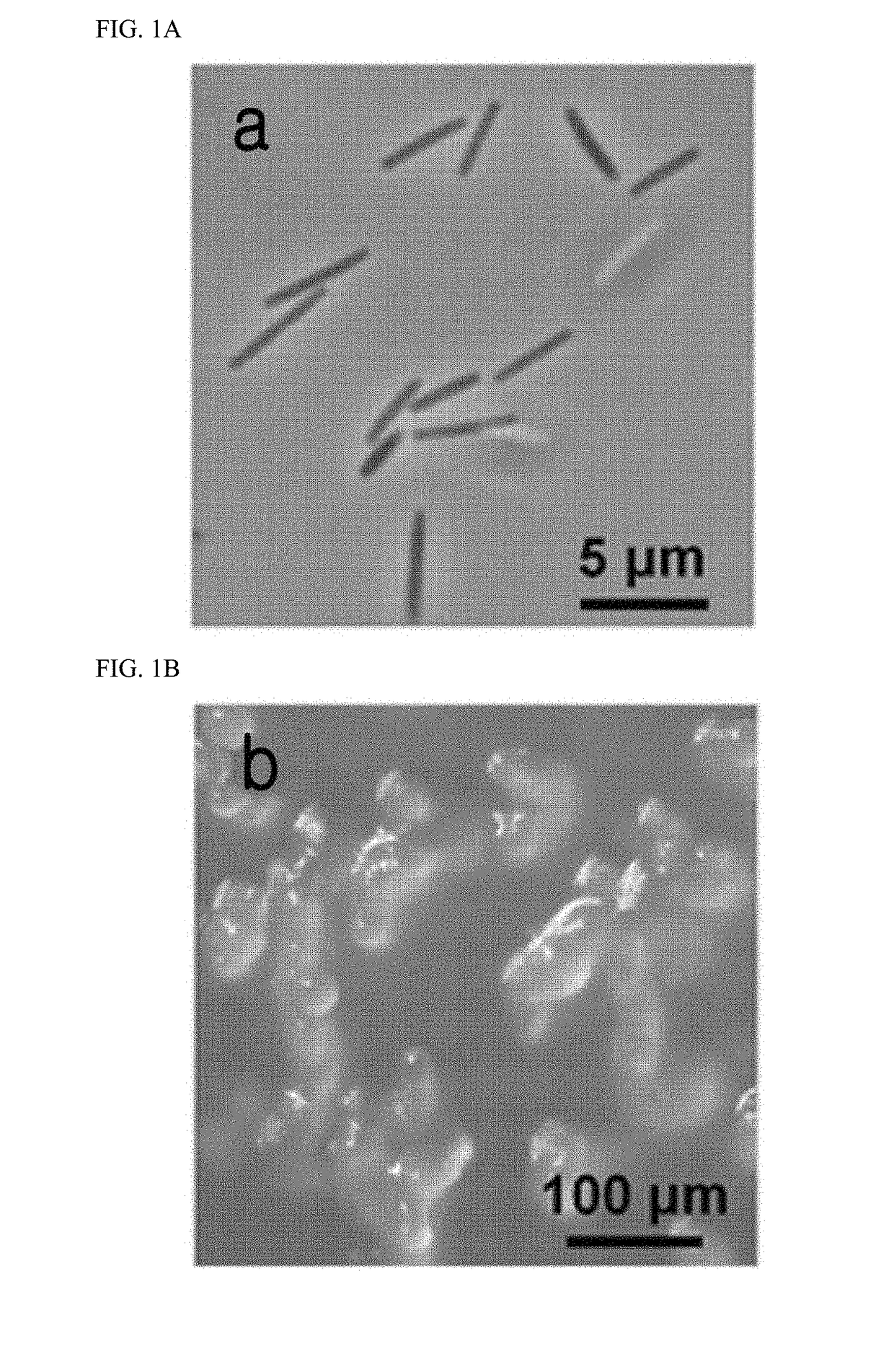
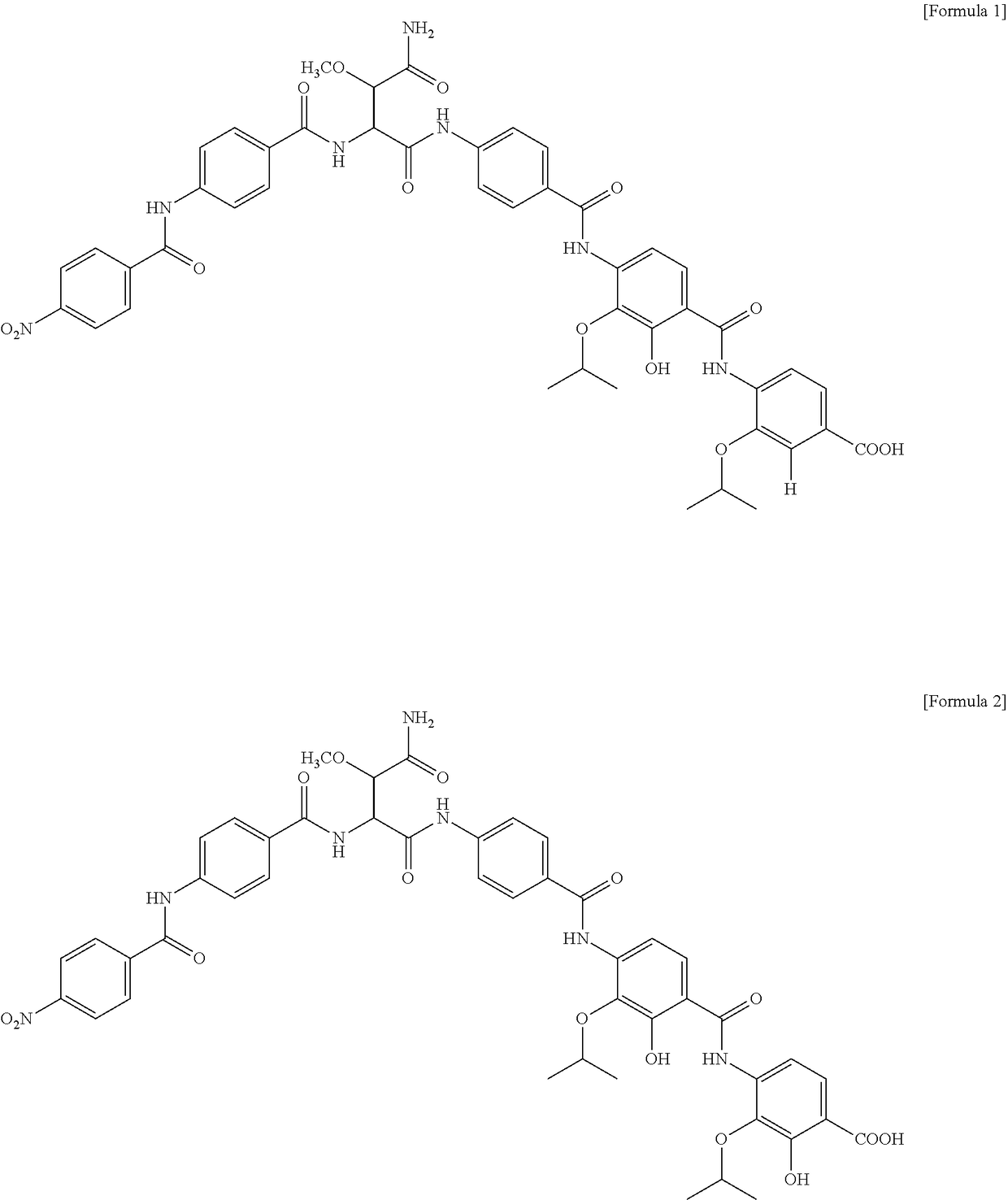
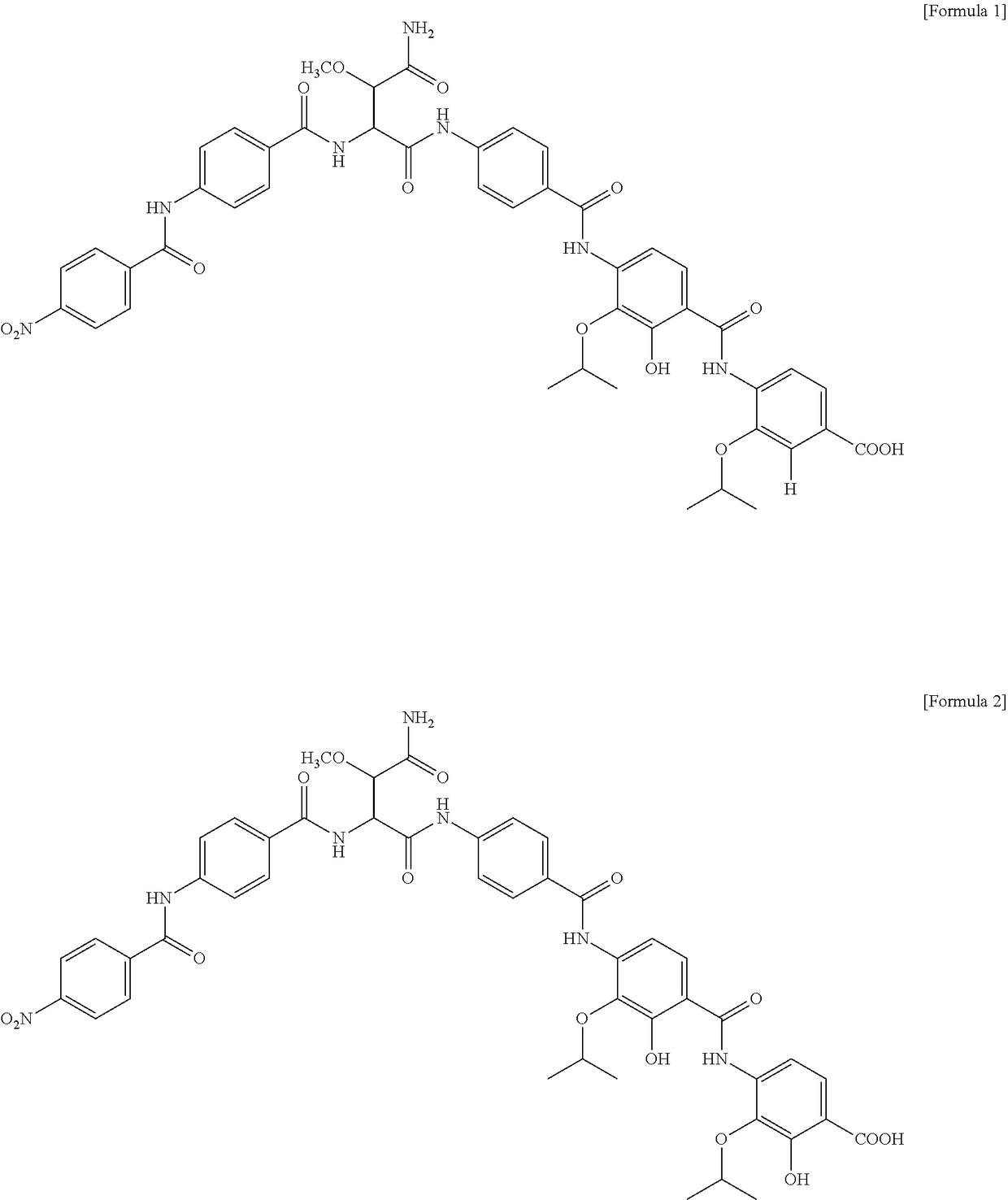
Patents
Literature
204 results about "Acinetobacter baumannii" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
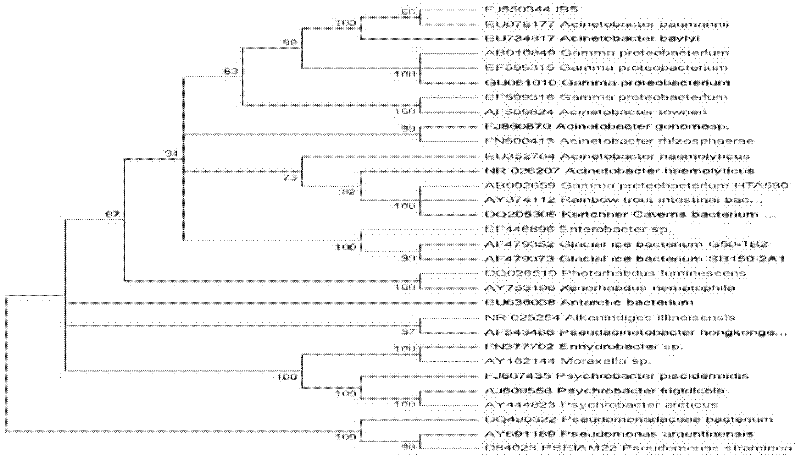
Acinetobacter baumannii is a typically short, almost round, rod-shaped (coccobacillus) Gram-negative bacterium. It is named after the bacteriologist Paul Baumann. It can be an opportunistic pathogen in humans, affecting people with compromised immune systems, and is becoming increasingly important as a hospital-derived (nosocomial) infection. While other species of the genus Acinetobacter are often found in soil samples (leading to the common misconception that A. baumannii is a soil organism, too), it is almost exclusively isolated from hospital environments. Although occasionally it has been found in environmental soil and water samples, its natural habitat is still not known.
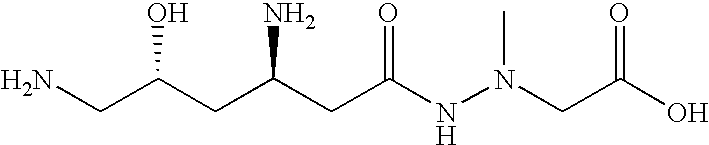
Affinity purified human polyclonal antibodies and methods of making and using them
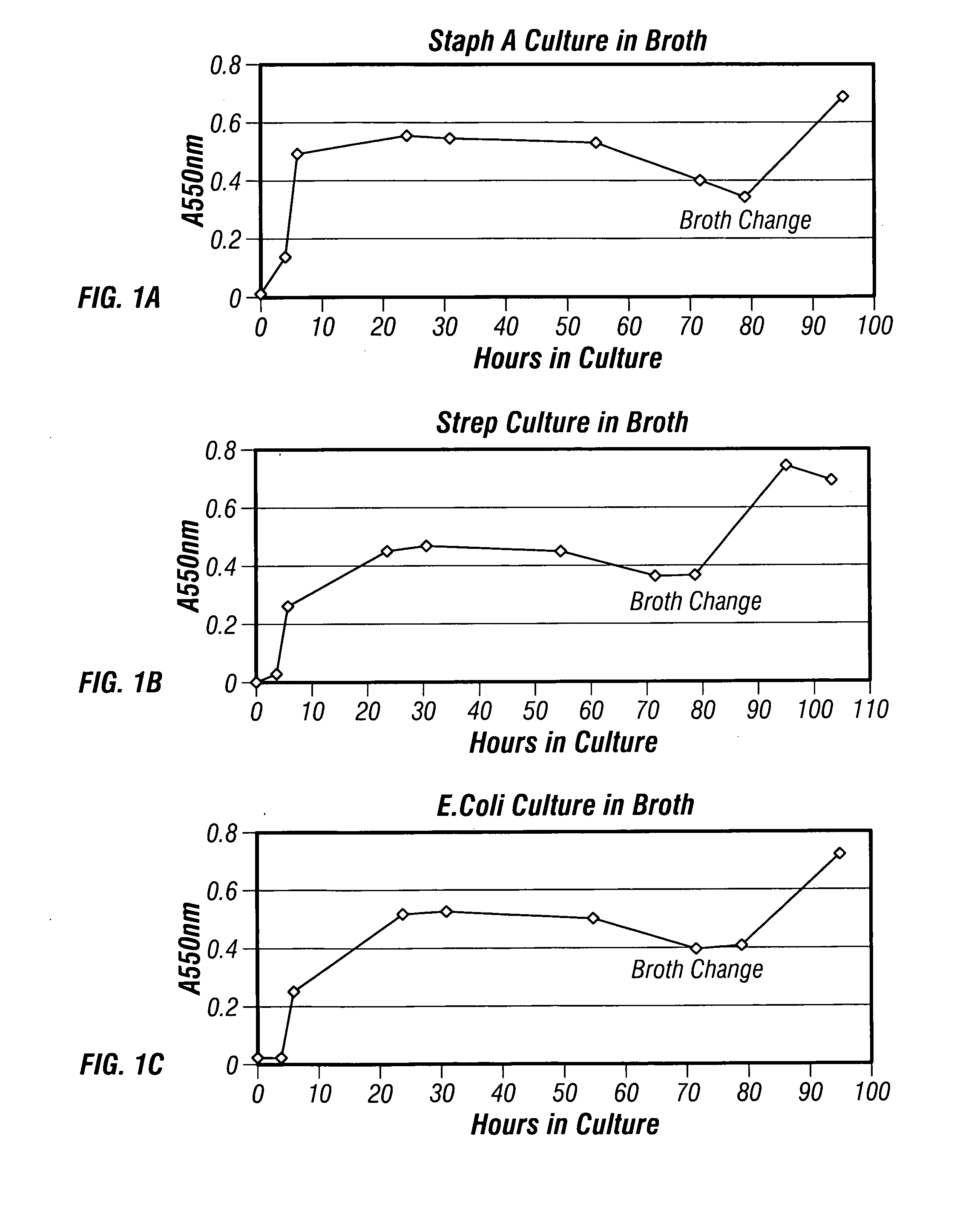
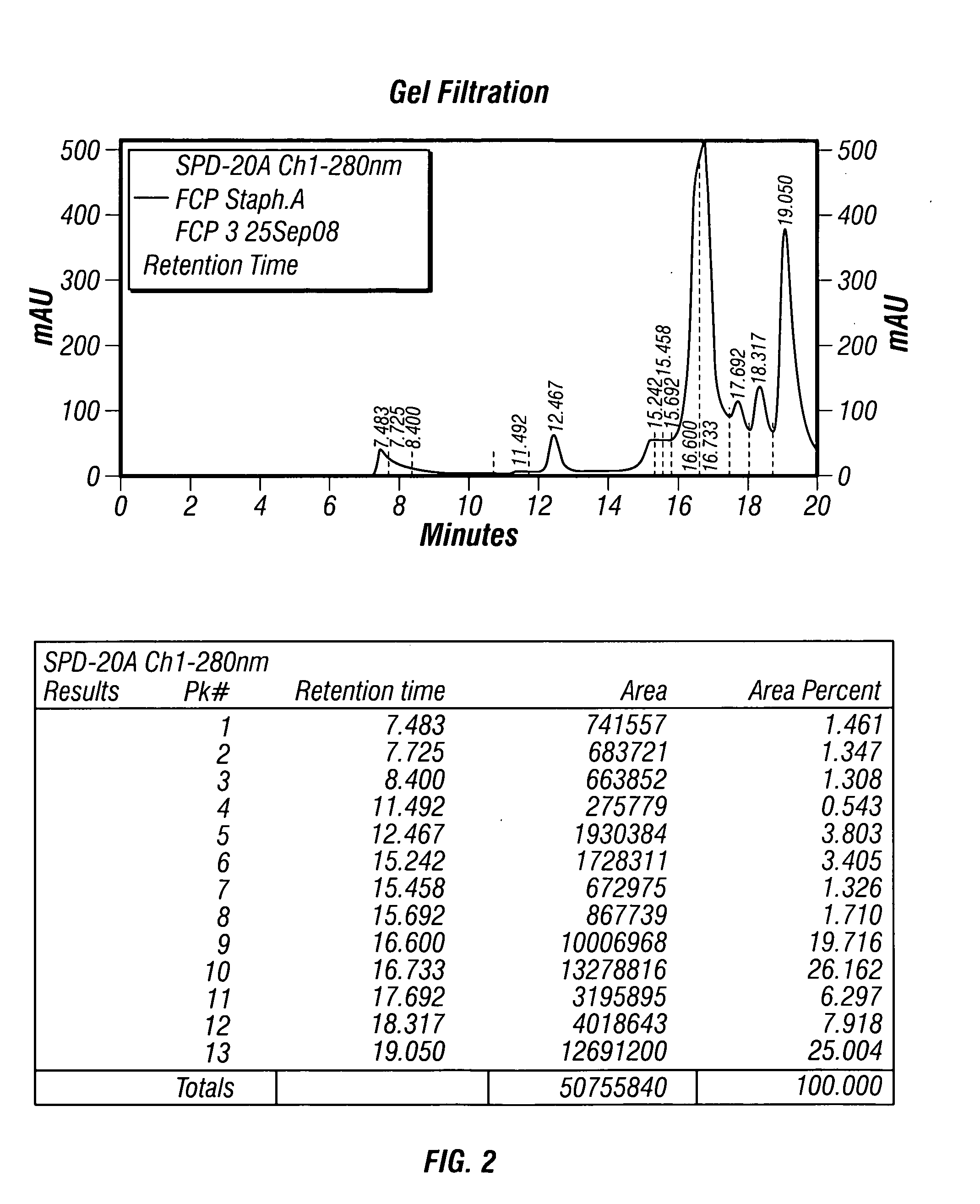
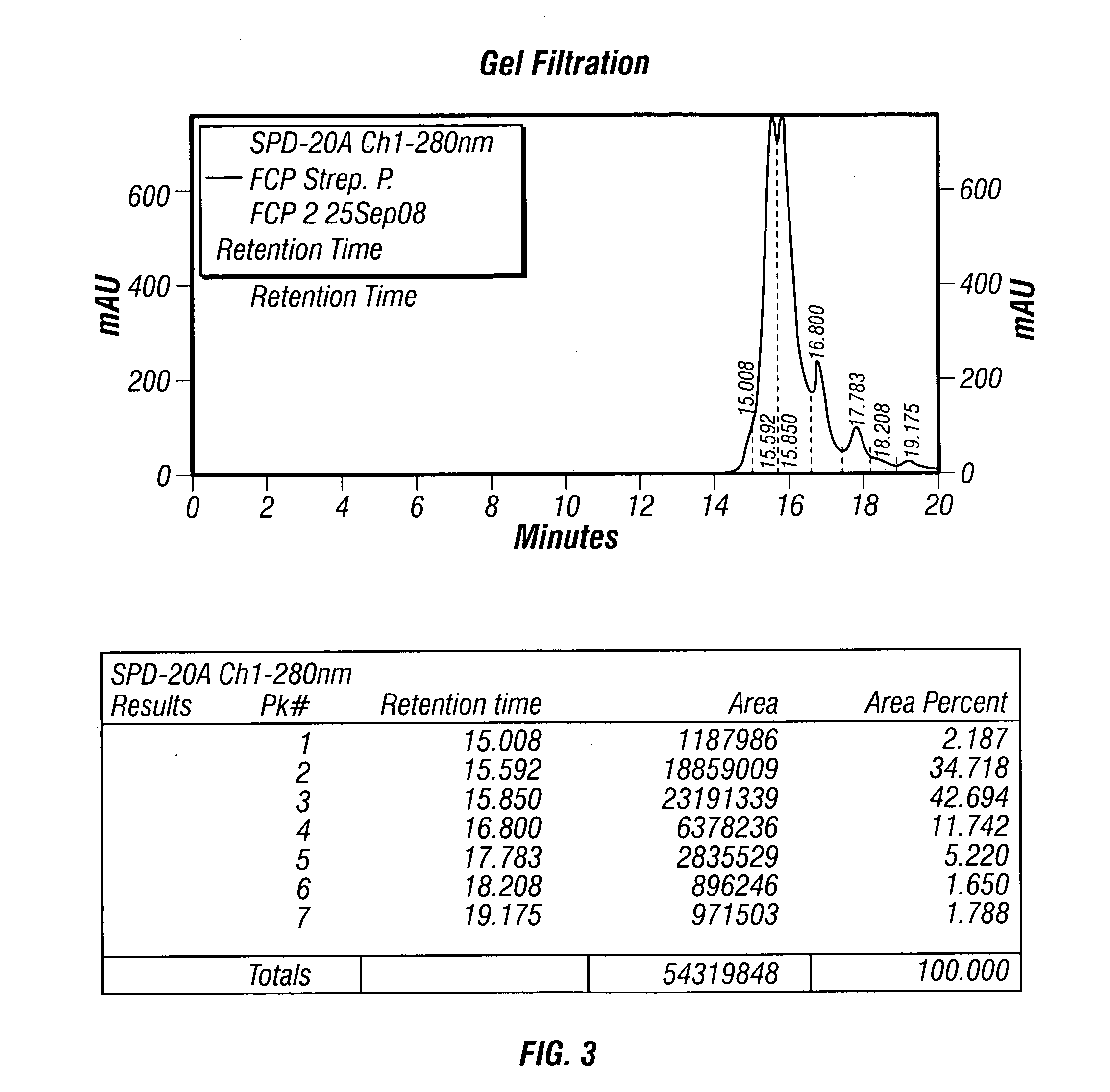
The present invention describes a method for treating, removing or preventing a bacterial infection, which method comprises administering to a human suffering, suspected of suffering or at risk of suffering from Staphylococcus aureus (S. aureus) infection, a Streptococcus infection, Escherichia coli (E. coli) infection, Pseudomonas aeruginosa (P. aeruginosa) infection, Acinetobacter baumannii (A. baumannii) infection, Enterococcus faecium (E. faecium) infection and / or Clostridium difficile (C. difficile) infection, an effective amount of human polyclonal antibodies affinity purified from a human blood sample with an antigenic preparation comprising cellular and / or secreted antigen(s) from bacterial cells selected from S. aureus, a Streptococcus, E. coli, P. aeruginosa, A. baumannii, E. faecium, C. difficile or a combination thereof, and optionally, wherein said affinity purified human polyclonal antibodies are purified (e.g., as made more concentrated as compared to the starting or unpurified material) relative to the same human polyclonal antibodies in the unpurified or non-affinity-purified human blood sample, e.g., intravenous immunoglobulin (IVIG) sample, and / or also optionally, wherein said affinity purified human polyclonal antibodies are specific for the bacterial antigens used in the affinity purification, and / or further optionally wherein the affinity purified human polyclonal antibodies are substantially free of human antibodies that specifically bind to non-bacterial antigens in the human blood sample. Pharmaceutical compositions for treating bacterial infections, comprising an effective amount of human polyclonal antibodies affinity purified from a human blood sample with an antigenic preparation comprising cellular and / or secreted antigen(s) from S. aureus, Streptococcus, E. coli, P. aeruginosa, A. baumannii, E. faecium, C. difficile or a combination thereof, are also provided.
Owner:SCANTIBODIES LAB
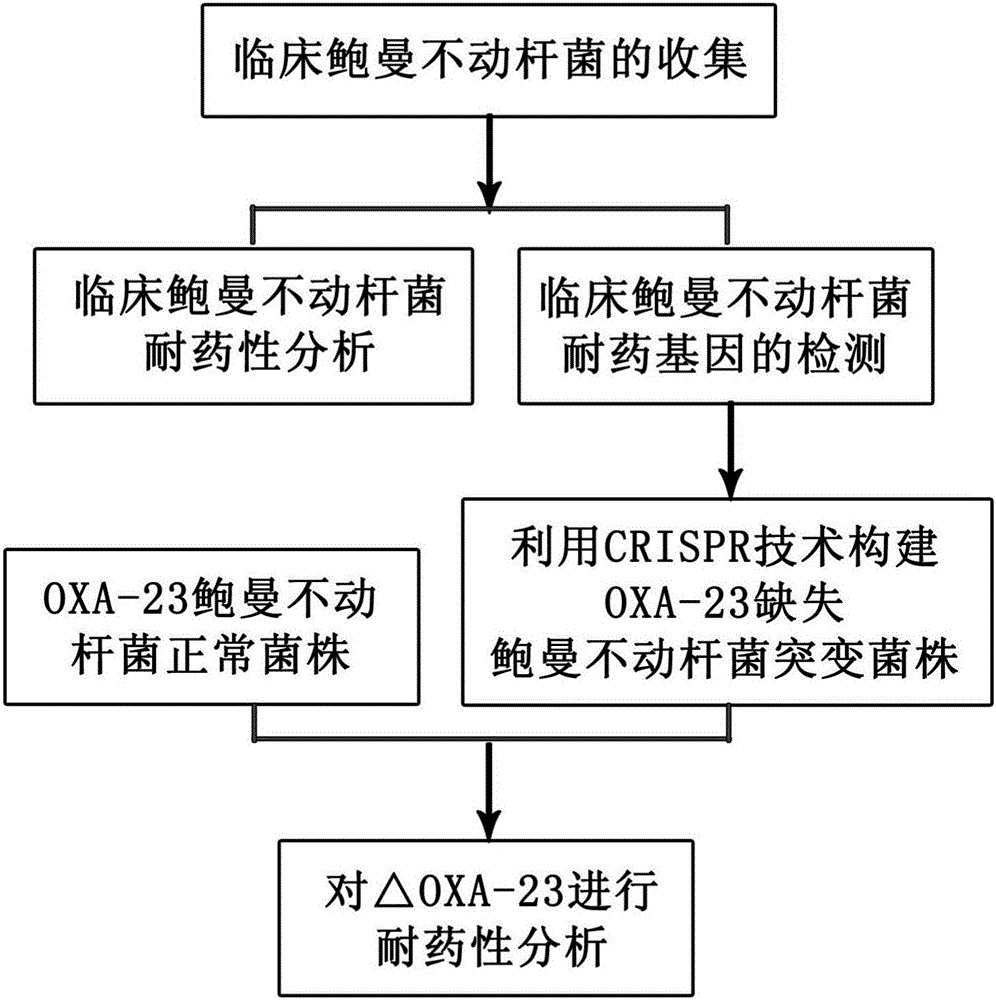
Method for deleting drug resistant genes of acinetobacter baumannii (AB) through CRISPR-Cas9
InactiveCN106544353ASensitivity reversalEasy to operateVectorsMicroorganism based processesBacteroidesMulti drug resistant
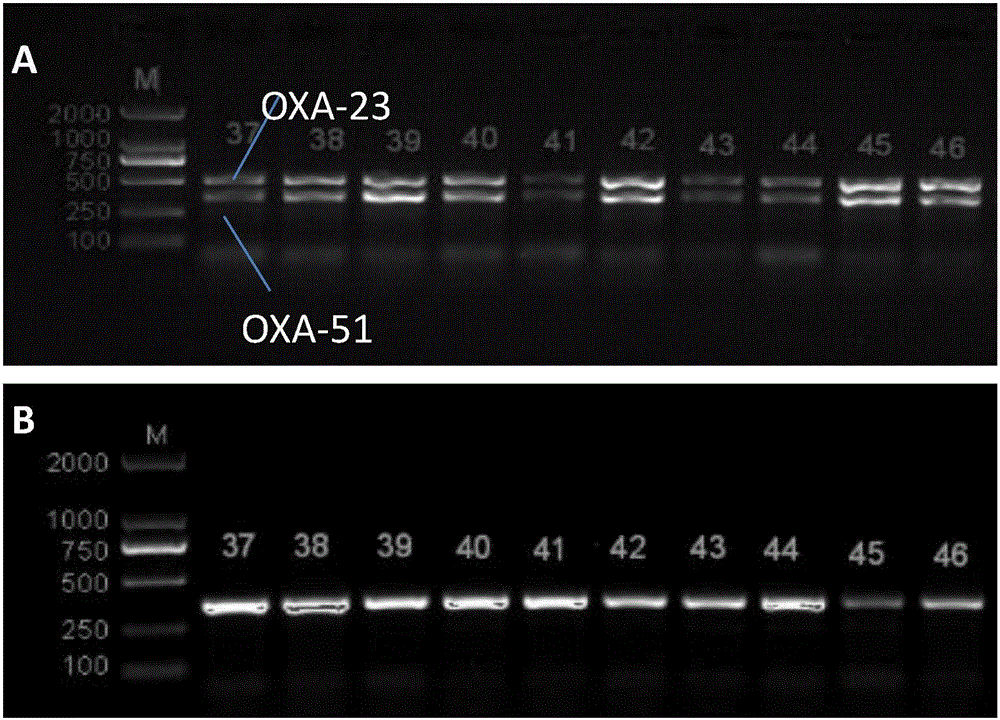
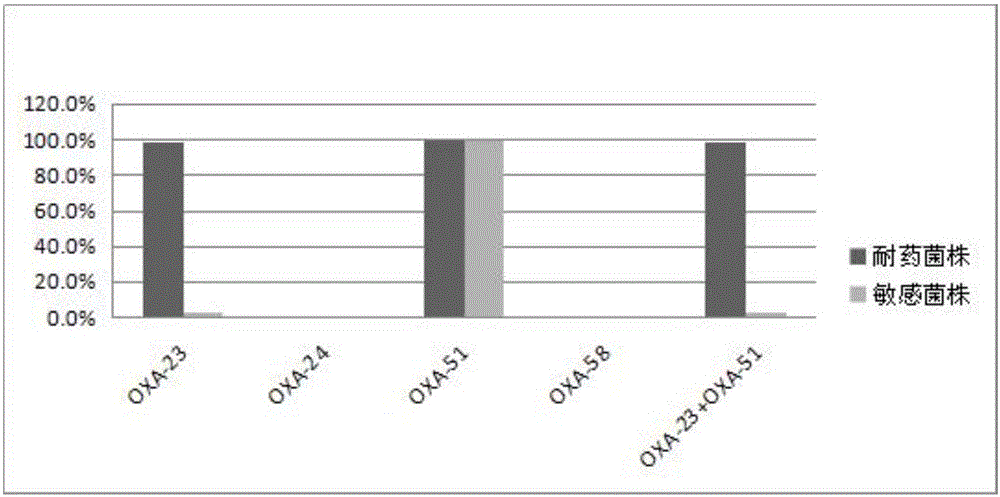
The invention discloses a method for deleting drug resistant genes of acinetobacter baumannii (AB) through CRISPR-Cas9. The method comprises the steps that firstly, AB is collected clinically, and drug resistance analysis and statistics are conducted after the AB is subjected to drug resistance measurement through a drug sensitive slip method; secondly, the multi-drug resistant AB is subjected to DNA extraction through a lysis-boiling method, and then amplification analysis is conducted by mean of well designed drug resistant gene primers of the AB; and thirdly, according to drug resistant gene detection in the former step, the AB containing an OXA-23 gene is selected, CRISPR / Cas9 and sgRNA plasmids are established and transferred into the AB containing the OXA-23 gene, an OXA-23 gene deletion AB mutant strain is established, and drug resistance analysis is conducted on the OXA-23 gene deletion AB mutant strain. The method is easy to operate and high in knocking-out efficiency, and a novel method and a novel concept are provided for preventing spreading of the drug resistant genes and treating drug resistant bacteria.
Owner:GENERAL HOSPITAL OF NINGXIA MEDICAL UNIV
Method for synergistically degrading oil producing wastewater by using petroleum degrading bacteria
InactiveCN103803714APlay a synergistic roleEfficient degradation rateWaste water treatment from quariesBiological water/sewage treatmentBacterial strainBeef extract
The invention provides a method for synergistically degrading oil producing wastewater by using petroleum degrading bacteria. The method comprises the following steps: (1) inoculating purified single pseudomonas aeruginosa, bacillus subtilis, pseudomonas mendocina and acinetobacter baumannii strains into a beef extract-peptone medium, and culturing for 18 hours so as to obtain seed bacterium liquid; (2) preparing an acclimation culture solution of the seed bacterium liquid; (3) adding the prepared acclimation culture solution to a fermentation medium according to a single strain bacterium liquid isopyknic ratio, and culturing; (4) putting fermented bacterium liquid in an aeration tank so as to treat the oil producing wastewater. The composite high-efficiency oil removal bacterial strain can be used for degrading more than 94% of hydrocarbon compounds in the oil producing wastewater; the selected strains are respectively high in crude oil degradation rate and have synergistic effect; the combined degradation efficiency of several bacteria for crude oil is considerably higher than the simple superimposed effect of the single effect of the bacteria.
Owner:CHANGZHOU UNIV
Method for restoring side slope ecology by adopting AB (Acinetobacter baumannii) bacterium plant growing bags
InactiveCN101904261APromote growthIncrease NPK levelsExcavationsCultivating equipmentsLandscape designMicroorganism
The invention relates to a method for restoring side slope ecology by adopting AB(Acinetobacter baumannii) bacterium plant growing bags, comprising the following steps of: placing substrates and seeds in plant growing bags sewn by adopting non-woven fabrics, obliquely palletizing the planting bags on a side slope by layers, and then curing for 30 days to accomplish side slope restoration. In the method, the AB bacterium plant growing bags are used as main materials for side slope restoration, thereby being beneficial to the activation and the long-term effectiveness of nutrients in the substrates; meanwhile, the quantity of microorganisms can be increased, and the microorganisms are used for decomposing organic matters and releasing mineral elements, thereby increasing the fertility of soil and being beneficial to the growth of greening plants on the side slope. The palletizing mode of the plant growing bags is beneficial to the utilization of moistures and simultaneously provides plastically space for vegetation regulation and control and landscape design at the later stage, thereby effectively promoting the progress of side slope restoration.
Owner:山合林(北京)水土保持技术有限公司
Biological agent for treating ammonia-nitrogen-containing wastewater and preparation method thereof
InactiveCN104630101APromote degradationIncrease contact areaBacteriaWater contaminantsPseudomonas fluorescensAniline
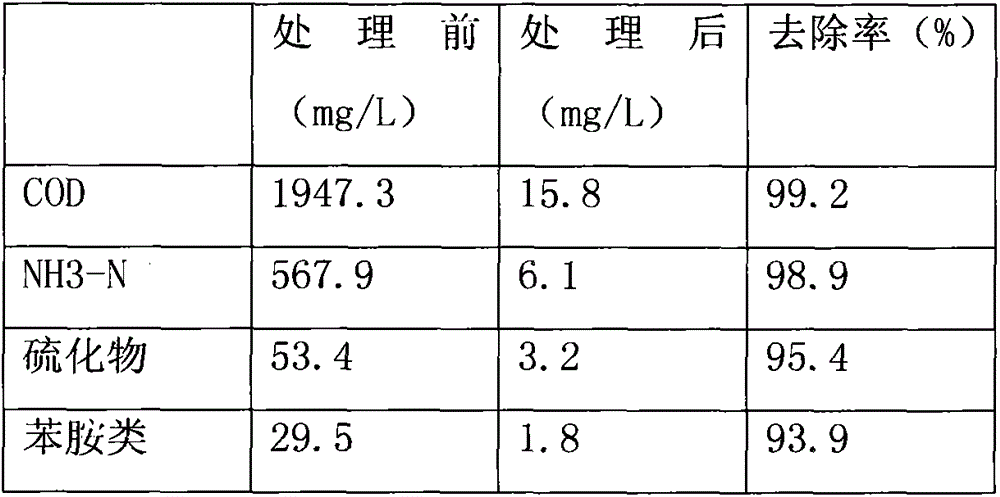
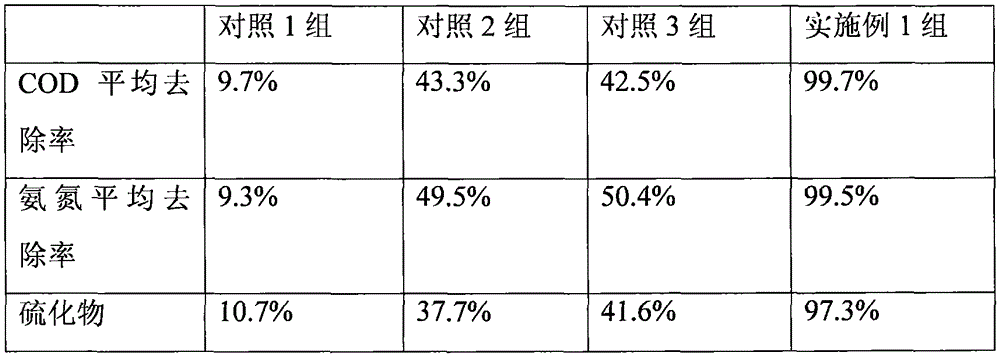
The invention belongs to the field of microorganisms and discloses a biological agent for treating ammonia-nitrogen-containing wastewater. The biological agent comprises rhodococcus ruber, micrococcus luteus, enterococcus faecalis, acinetobacter baumannii, arthrobacter crystallopoietes, thiobacillus denitrificans and pseudomonas fluorescens. The biological agent comprises multiple strains, has the advantages of reasonable compatibility and strong synergic effect and can be used for effectively removing ammonia nitrogen, sulfide, aniline substances and industrial COD in wastewater.
Owner:江苏睿智建筑工程有限公司
Bacteria capable of removing organic matter and ammonia nitrogen in micro-polluted water source water under low temperature and aerobic condition, and screening and taming method
InactiveCN101503665ANo accumulationBacteriaTreatment using aerobic processesWater sourceScreening method
The invention discloses bacteria capable of synchronously removing organic substances and ammonia nitrogen in water of a slightly polluted water source under low-temperature and aerobic conditions and a screening and domesticating method, which relate to bacteria and a screening and domesticating method and solve the problem that the prior bacteria are unsuitable for treating the slightly polluted water source as well as low temperature treatment. The bacteria SRA10 of the invention are acinetobacter lwoffi which belong to acinetobacter baumannii and are preserved in China General Microbiological Culture Collection Center on January 19th, 2009, with a preservation number of CGMCC No.2889. The screening method comprises the following steps: I, separating and purifying; II, preparing bacterial solution; III, screening and cultivating a bacterial solution; IV, screening and cultivating a bacterial solution with a live strain; V, repeating the step IV for 1 to 2 times, and stepwise domesticating the live strain; and VI, taking a bacterial solution of the domesticated strain, and rescreening the bacterial solution to obtain the bacteria. The bacteria obtained by screening and domesticating can process water of the slightly polluted water source under a condition of a low temperature of 2 to 10 DEG C.
Owner:HARBIN INST OF TECH
Protein of acinetobacter baumannii hypothetical protein A1S_1523 as well as preparation method and application of protein
ActiveCN104877019ADefend against deadly infectionElicit a protective immune responseAntibacterial agentsBacterial antigen ingredientsAdjuvantAmino acid
The invention relates to recombinant protein of A1S_1523 as well as a preparation method and application of the recombinant protein. The recombinant protein comprises A1S_1523 mature peptide, and an amino acid sequence of the recombinant protein is shown in SEQ ID NO. 3. The recombinant protein disclosed by the invention is high in expression quantity and convenient to separate and purify, is efficient and safe, can be directly matched with adjuvants for use, and can be used for preparing acinetobacter baumannii infection resistant subunit vaccines and related detection kits; proven by animal experiments, gene engineering recombinant monovalent subunit vaccines have good immune protective effects on acinetobacter baumannii infection resistance; and the recombinant protein can be used for laying a foundation for further researching combined vaccines and multi-subunit fusion vaccines, and can also play important roles in the development and application of prevention and treatment vaccines and diagnostic kits.
Owner:ARMY MEDICAL UNIV
Gene chip for detecting pathogens of lower respiratory tract and reagent kit
ActiveCN101748193AImprove accuracyGood repeatabilityMicrobiological testing/measurementAgainst vector-borne diseasesK pneumoniaeStaphylococcus aureus
The invention provides a gene chip for detecting pathogens of lower respiratory tract, which comprises a solid phase carrier and an oligonucleotide probe fixed on the solid phase carrier, wherein the oligonucleotide probe comprises a sequence selected from one or more DNA sequences of staphylococcus aureus, 16s-23s rDNA intergenic spacer region of Klebsiella pneumoniae or Acinetobacter baumannii, 16S gene of pseudomonas aeruginosa or haemophilus influenzae, gyrB gene of streptococcus pneumoniae or legionella pneumophila and copB gene of moraxella catarrhalis or the complementary DNA or RNA sequence. The invention further provides a reagent kit containing the gene chip. The utilization of the gene chip and the reagent kit can detect the pathogens of the lower respiratory tract; furthermore, the operation is simple, the accuracy is high and the repeatability is strong.
Owner:TIANJIN BIOCHIP TECH CO LTD
Disinfectant composition comprising phage
ActiveUS20110038840A1Reduce the amount requiredBioactivityBiocideAntisepticsAcinetobacter baumanniiBacteriophage
The present invention provides a disinfectant composition including a phage of Acinetobacter baumannii and a carrier. The present invention also provides a method for disinfecting a medical institute or a medical research institute, including the steps of applying an effective amount of a phage of Acinetobacter baumannii to the medical institute or the medical research institute for reducing amount of Acinetobacter baumannii in the medical institute or the medical research institute.
Owner:BUDDHIST TZU CHI GEN HOSPITAL
Gene chip for detecting several kinds of common pathogenic bacteria and its prepn process and kit
InactiveCN101045945AAccurate acquisitionNo pollution in the processMicrobiological testing/measurementAgainst vector-borne diseasesEscherichia coliBacillus pyocyaneus
The present invention relates to gene chip for detecting several kinds of common bacterial pathogens, and the gene chip includes solid carrier and oligonucleotide probe fixed on the carrier. The chip has up to 200 low density distributed sample points. The oligonucleotide detecting probe includes DNA' s selected from nucleotide sequences corresponding to the genomes of nine kinds of common bacterial pathogens, including Staphylococcus aureus, Staphylococcus epidermidis, bacillus pyocyaneus, colibacillus, etc. The said chip together with sample treating reagent, hybridizing reagent, color reagent and the specification constitutes the detection kit. The present invention has high detection efficiency.
Owner:IPE BIOTECHNOLOGY CO LTD
Antimicrobial Peptides
ActiveUS20110028386A1Inhibit microbial growthReduce the possibilityAntibacterial agentsBiocideStaphylococcus cohniiHemolysis
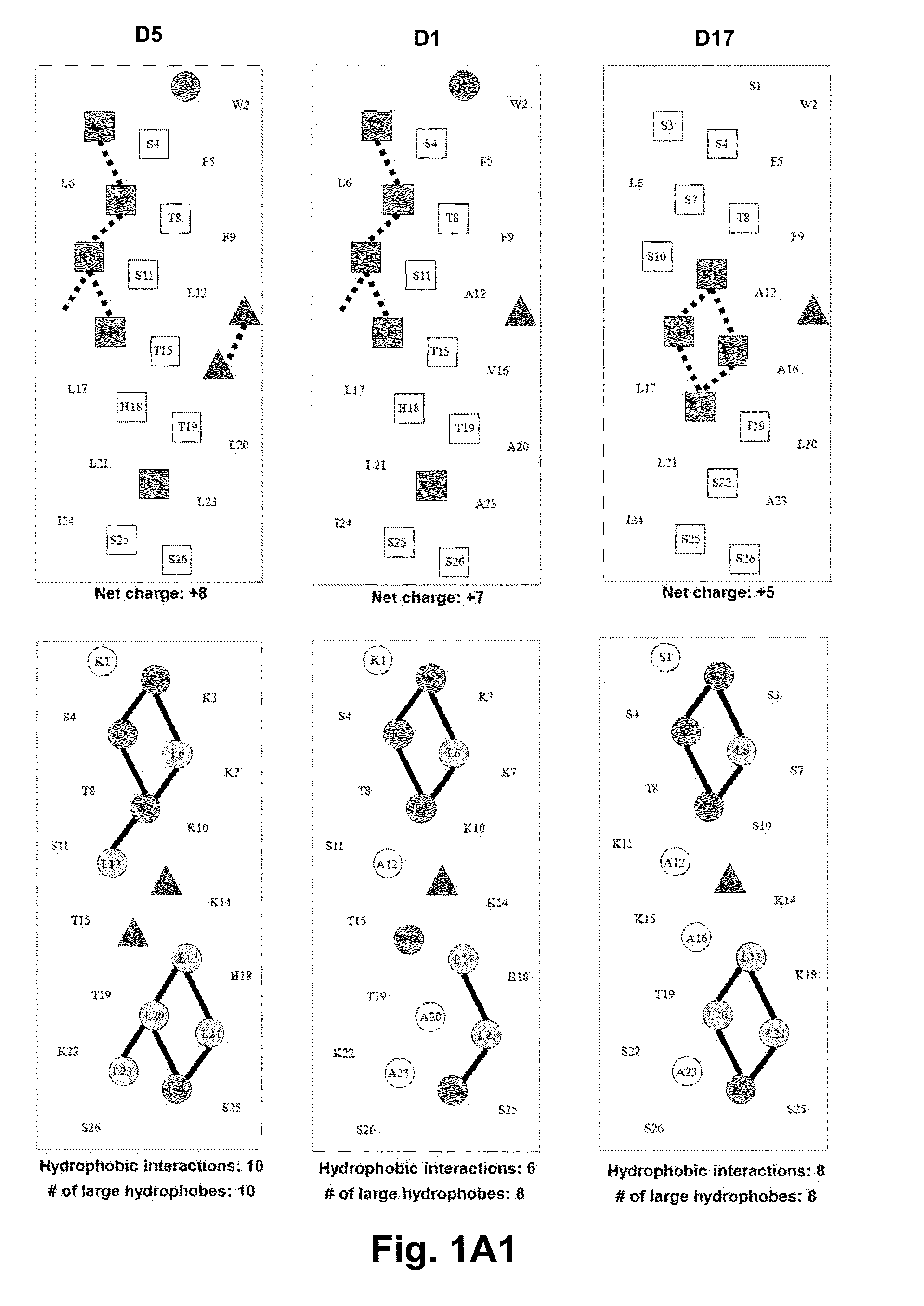
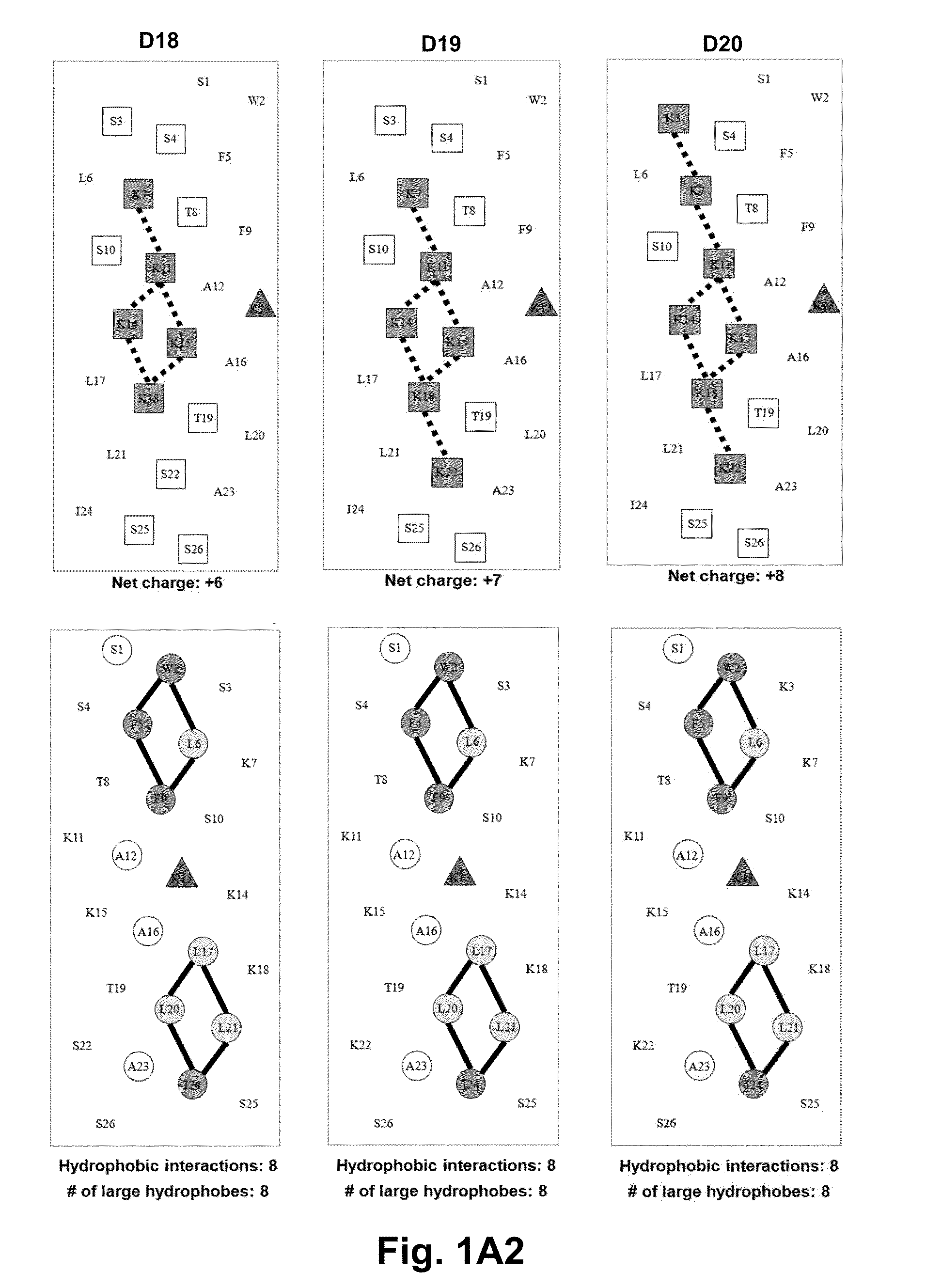
Disclosed are antimicrobial peptides with useful or superior properties such as antimicrobial activity, desirable levels of hemolysis, and advantageous therapeutic index against various microorganisms, especially Pseudomonas aeruginosa, Acinetobacter baumannii and Staphylococcus aureus. Also provided are methods of to control microbial growth and pharmaceutical compositions to treat or prevent microbial infections. Certain peptides are disclosed utilizing a structure-based rational modification of antimicrobial peptide D1, with single D- / L-amino acid substitutions or charged residue substitutions in or near the center of the peptide on the nonpolar or polar face, or peptides with one or more amino acids in the D configuration, and peptides with all amino acids in the D configuration. Modified peptide analogs herein can demonstrate one or more properties such as improved antimicrobial activity, specificity, and resistance to degradation. Compositions disclosed herein are useful as antibiotics, including as broad spectrum antibiotics.
Owner:UNIV OF COLORADO THE REGENTS OF
Acinetobacter baumannii capable of efficiently degrading imazamox
ActiveCN102250789AEfficient degradationHas a repairing effectBacteriaMicroorganism based processesMicrobiologyAcinetobacter baumannii
The invention dislcoses acinetobacter baumannii capable of efficiently degrading imazamox, which relates to acinetobacter baumannii. The invention provides an acinetobacter baumannii strain, which is capable of efficiently degrading imazamox and provides theoretical and microbial resource foundations for the environmental modification and degradation mechanism research of an imazamox weedicide. The acinetobacter baumannii capable of efficiently degrading imazamox, disclosed by the invention, is acinetobacter baumannii IB5 which is collected in the China General Microbiological Culture Collection Center (Address: No.3, Yard 1, Beichen Road West, Chaoyang District, Beijing) with the collection number CGMCC No.4728 on April 1, 2011. The acinetobacter baumannii IB5 is capable of efficiently degrading imazamox.
Owner:海林市中农国泰生物科技有限公司
Marine Bacillus amyloliquefaciens and uses thereof
ActiveCN104974950AThe effect of preventing and controlling aquatic pollution diseases is remarkableGood prospects for the development of biopesticidesAntibacterial agentsBacteriaBiotechnologyVibrio parahaemolyticus
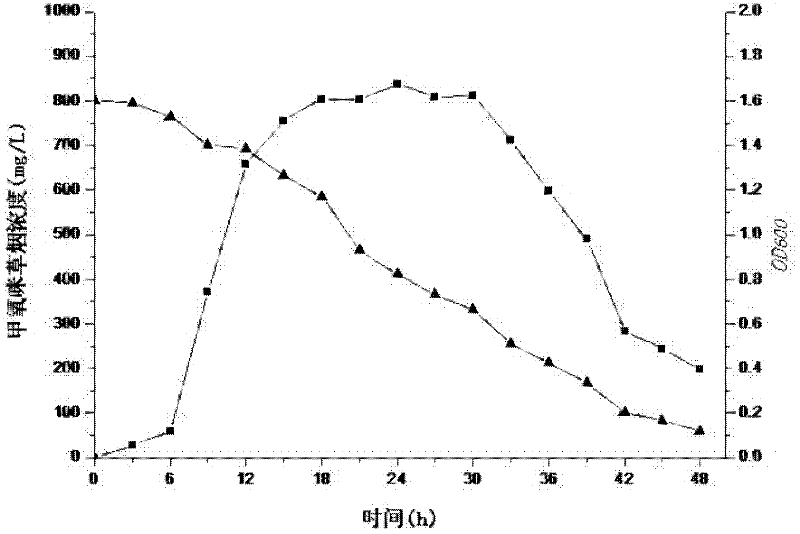
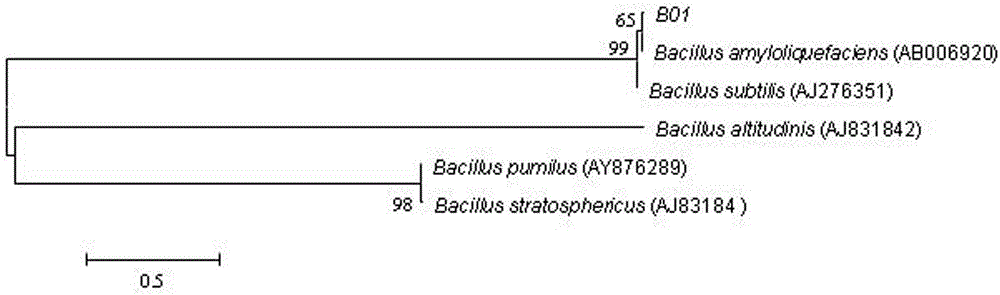
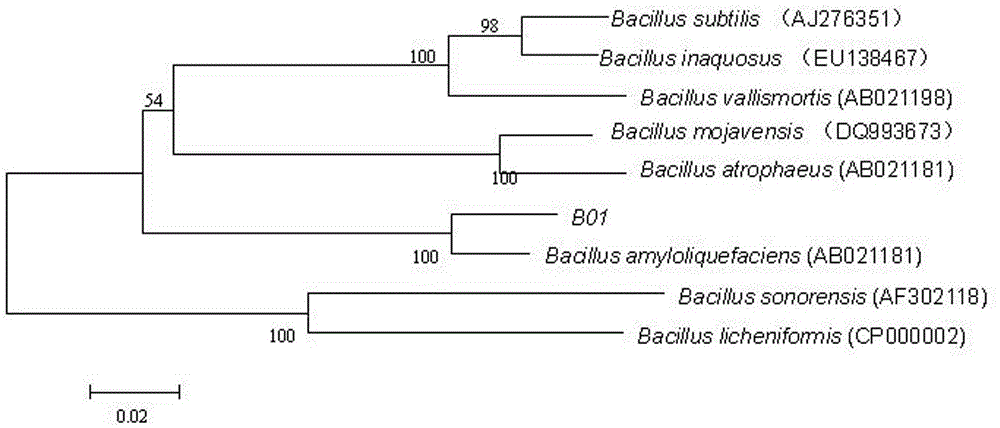
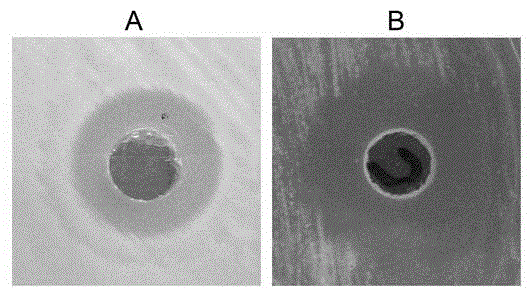
The present invention discloses a strain of marine Bacillus amyloliquefaciens B01, wherein the strain is preserved in the China general microbiological culture collection center on December 11, 2014, and has the preservation number of CGMCC No.10154. According to the present invention, the fermentation broth supernatant of the Bacillus amyloliquefaciens B01 can inhibit aquatic diseases caused by a variety of pathogenic bacteria such as Acinetobacter baumannii, Vibrio parahaemolyticus and the like, and the marine Bacillus amyloliquefaciens B01 has characteristics of no toxicity on human and animals, green environmental protection, aquatic pollution disease prevention and control effect, and good biological pesticide development prospects.
Owner:SHANDONG UNIV
Strain capable of degrading pyrethroid insecticide residues and application of strain
ActiveCN107058186AEfficient degradationPromote degradationBacteriaWater contaminantsMicrobiologyAcinetobacter baumannii
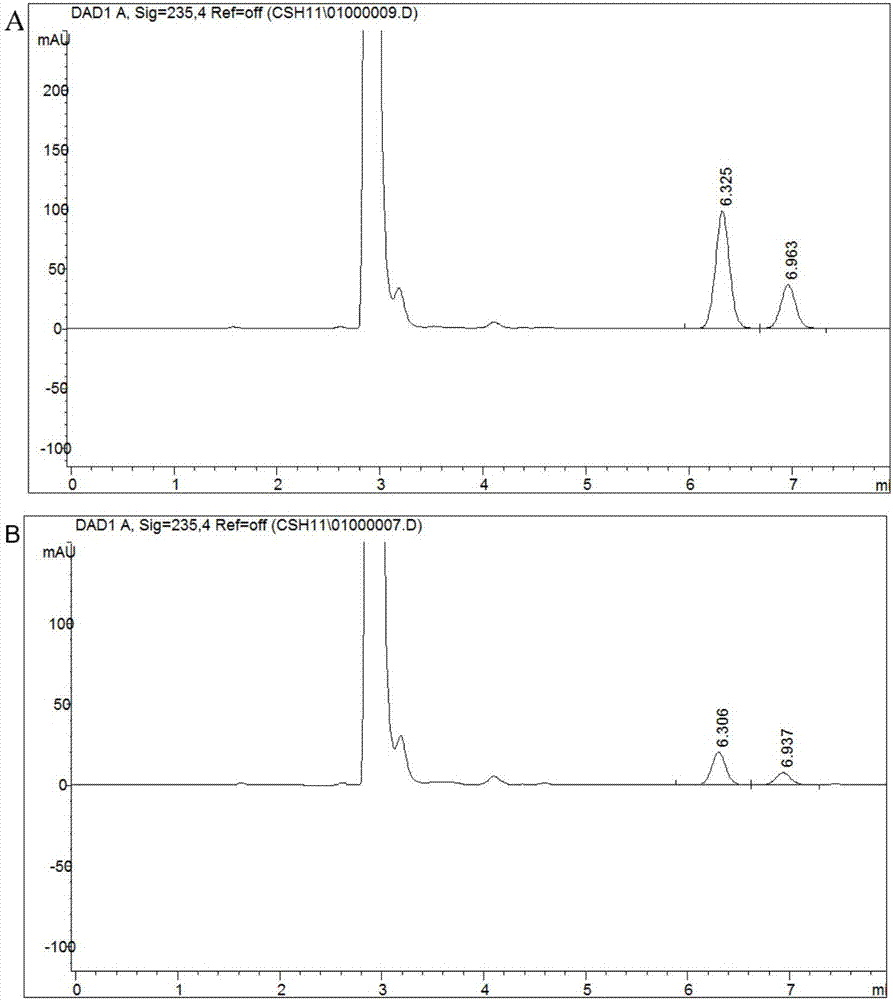
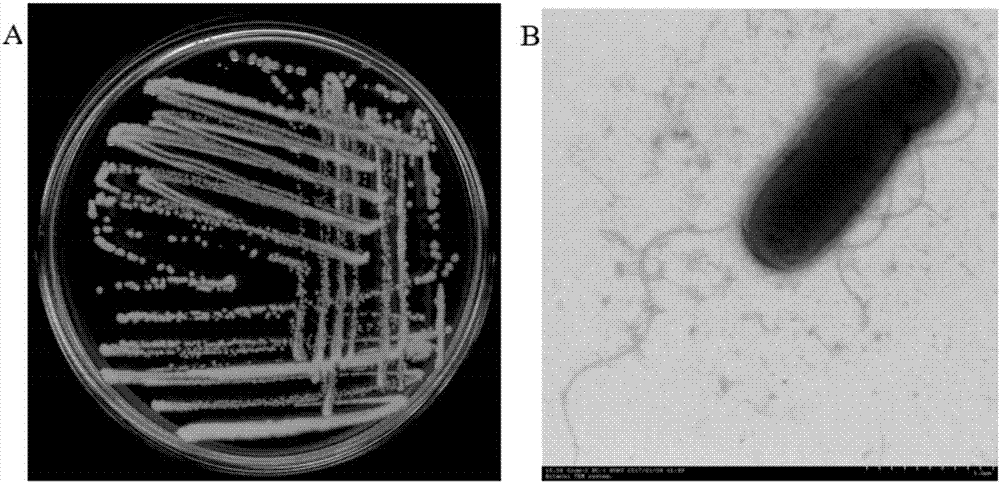
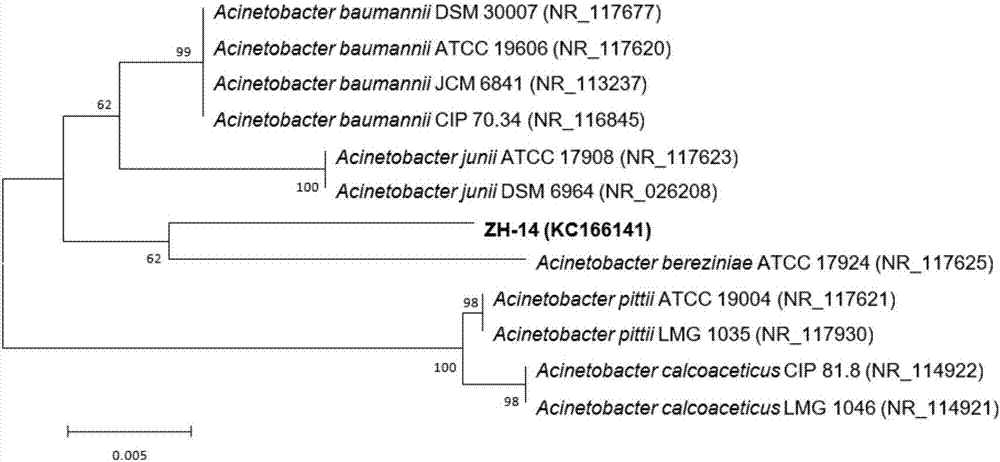
The invention discloses a strain capable of degrading pyrethroid insecticide residues and an application of the strain. The strain is an Acinetobacter baumannii strain ZH-14 and is preserved in CCTCC (China Center for Type Culture Collection) on November 28, 2016, and the preservation number is CCTCC NO: M 2016689. The strain has remarkable capacity of degrading the pyrethroid insecticide residues, and a liquid preparation prepared from the strain is low in production cost, convenient to use and suitable for treating residual pollution caused by a pyrethroid insecticide in natural environment such as water or soil and the like; the quantity of pyrethroid insecticide residues in water or soil can be reduced by 85% or more in a short time after direct application of the preparation, the problem of standard exceeding of the pyrethroid insecticide residues in agricultural production and the problem of environmental pollution can be solved, non-toxic and non-pollution green agricultural products are produced, and the strain has important theoretical guidance and practical application value.
Owner:SOUTH CHINA AGRI UNIV
Antimicrobial compositions, formulations and uses thereof
InactiveUS20110262508A1High activityLow toxicityAntibacterial agentsBiocideCytotoxicityAntimicrobial peptides
The present invention provides antimicrobial peptides and analogs thereof that are cytostatic or cytotoxic toward at least one gram-negative bacterium of the genus Acinetobacter including laboratory or clinical isolates of A. baumannii with low or non-detectable cytotoxicity against mammalian cells. Optionally, the antimicrobial peptides and analogs thereof are also cytotoxic or cytostatic against other unrelated bacteria such as Staphylococcus aureus.
Owner:DYNAMIC MICROBIALS
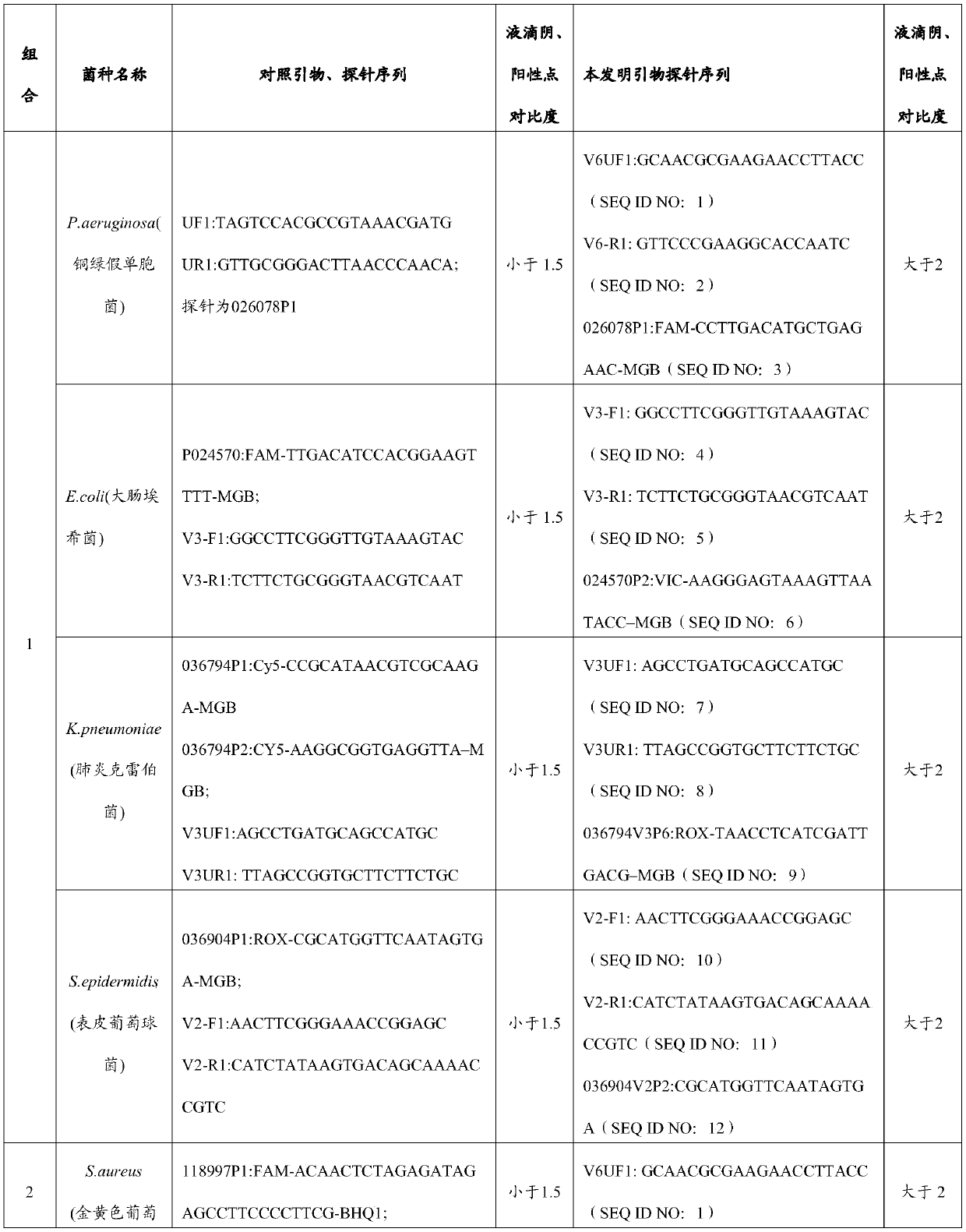
Primer and probe combination and kit for detecting human pathogenic bacteria
PendingCN110564824AShorten identification timeImprove positive detectionMicrobiological testing/measurementDNA/RNA fragmentationInfected patientTreatment effect
The invention relates to the technical field of medical detection, and in particular, relates to a primer and probe combination and a kit for detecting human pathogenic bacteria. The kit comprises theprimer and probe combination for detecting pseudomonas aeruginosa, escherichia coli, klebsiella pneumoniae, staphylococcus epidermidis, staphylococcus aureus, enterococcus faecium, human staphylococcus and acinetobacter baumannii. According to the kit, the primer and probe combination is adopted, multiple digital PCR is combined, the pathogenic bacteria types and the target nucleic acid copy number in the detection range can be rapidly and accurately identified, the detection method has high sensitivity, and positive detection of low-copy nucleic acid fragments of pathogenic bacteria is guaranteed; meanwhile, the variety of the pathogenic bacteria or the change of the copy number of the target nucleic acid fragment in the body of a pathogenic bacteria infected patient can be dynamically monitored, the treatment effect is assisted and evaluated in time, and reference is provided for optimization of a doctor clinical scheme.
Owner:PILOT GENE TECH HANGZHOU CO LTD
Method for quickly identifying bloodstream infection pathogenic bacteria
InactiveCN102453752ARapid determinationEfficient determinationMicrobiological testing/measurementAgainst vector-borne diseasesBacteroidesKlebsiella oxytoca
The invention belongs to the field of examination of microorganisms, and relates to a method for quickly identifying bloodstream infection bacteria by a high-resolution fusion curve method. The method comprises the following steps of: performing smear gram staining on a blood culture positive culture flask to distinguish negative bacteria and positive bacteria; selectively amplifying different 16S fragments for the negative bacteria and the positive bacteria; and analyzing by the high-resolution fusion curve method. Common bloodstream infection pathogenic bacteria comprise staphylococcus aureus, staphylococcus epidermidis, enterococcus faecalis, enterococcus faecium, Escherichia coli, Klebsiella pneumoniae, acinetobacter baumannii, pseudomonas aeruginosa, enterobacter cloacae, Serratia marcescens, Klebsiella oxytoca, acinetobacter lwoffii, enterobacter aerogenes, stenotrophomonas maltophilia and burkholderia cepacia. By the method, the common bloodstream infection pathogenic bacteria can be measured quickly, efficiently and accurately, so that strong evidences are provided and precious time is striven for subsequent treatment.
Owner:AFFILIATED HUSN HOSPITAL OF FUDAN UNIV +1
Microbial preparation for processing industrial fermentation wastewater
InactiveCN105274024APromote degradationGood removal effectFungiBacteriaStreptomycesIndustrial fermentation
The invention relates to a microbial preparation for processing industrial fermentation wastewater. The microbial preparation is prepared by mixing nitrosobacterium, Trichoderma longibrachiatum Rifai, aerobic denitrifying bacterium, acinetobacter baumannii, aspergillus niger, alcaligenes faecalis, Streptomyces lateritius, Candida albicans, Paenibacillus polymyxa and an adsorbent carrier. The microbial preparation contains multiple microbes having excellent ability of degrading non-biodegradable pollutants. Through reasonable compatibility between various strains, the microbial preparation has a good degradating effect and has a wide application prospect.
Owner:张胜平
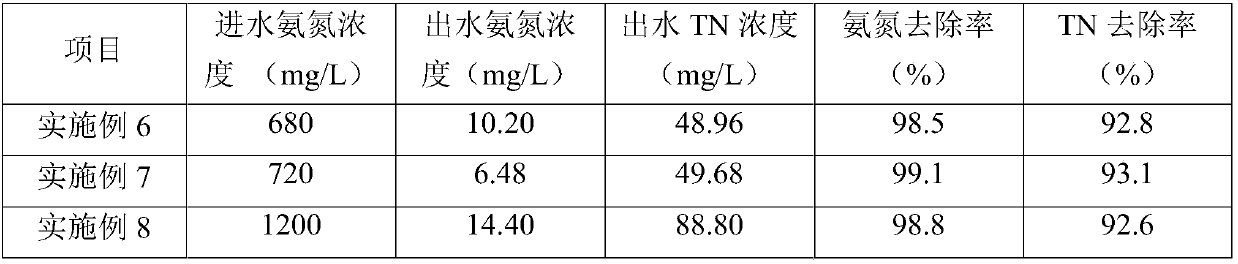
Biological preparation for treating high-concentration ammonia nitrogen wastewater and preparation method thereof
ActiveCN108034624AFast nitrification reactionHigh activityBacteriaUnicellular algaeHigh concentrationBacillus megaterium
The invention discloses a biological preparation for treating high-concentration ammonia nitrogen wastewater. Preparation raw materials of the biological preparation are prepared from the following components in parts by weight: 10 to 15 parts of chlorella DH2 algal liquid, 20 to 30 parts of pseudomonas hibiscus DT1 fermented bacterial liquid, 15 to 20 parts of bacillus megaterium NCT-2 fermentedbacterial liquid, 10 to 15 parts of acinetobacter baumannii AL-6 fermented bacterial liquid, 5 to 10 parts of photosynthetic bacteria fermented bacterial liquid, 1 to 3 parts of agar and 3 to 5 partsof polyvinyl alcohol. In addition, the invention also provides a preparation method of the biological preparation for treating high-concentration ammonia nitrogen wastewater. According to the biological preparation, ammonia nitrogen in the high-concentration ammonia nitrogen wastewater is efficiently removed by utilizing a synergistic effect of heterotrophic nitrifying and aerobic denitrifying bacteria and chlorella; further, a nitrification process and a denitrification process of the biological preparation are carried out in the same device; a procedure is simple; a different procedure combination does not need to be set, and not only is the treatment efficiency of the wastewater improved, but also the running cost is saved.
Owner:XIAMEN UNIV OF TECH
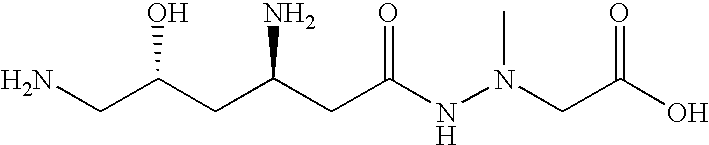
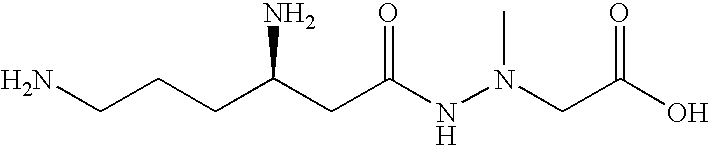
Administration of negamycin or deoxynegamycin for the treatment of bacterial infections
The invention provides a method for treating bacterial infections. In one aspect, the invention comprises orally administering a pharmaceutical composition to an animal, wherein the composition comprises a pharmaceutically acceptable excipient and an antibacterial effective amount of negamycin, or a pharmaceutically acceptable salt, prodrug or isomer thereof. An aspect of the invention also relates to a method of treating a bacterial infection, wherein the method comprises intravenously administering a pharmaceutical composition to an animal, and wherein the composition comprises a pharmaceutically acceptable excipient and an antibacterial effective amount of deoxynegamycin, or a pharmaceutically acceptable salt, prodrug or isomer thereof. An aspect of the invention also relates to a method of treating a bacterial infection, wherein the method comprises administering to an animal an antibacterial effective amount of negamycin or deoxynegamycin, or a pharmaceutically acceptable salt, prodrug or isomer thereof, and wherein the infecting bacteria are selected from a group of bacteria consisting of the following: Acinetobacter baumanii, Citrobacter freundii, Enterobacter aerogenes, haemophilus influenzae, Moraxella catarrhalis, Staphylococcus aureus MRSA, Staphylococcus aureus GISA, Staphylococcus epidermis, Streptococcus pneumoniae PenR, Streptococcus pneumoniae PenS and Streptococcus pyogenes.
Owner:VERSICOR
Microbial compound fungicide for treating oil base drilling cuttings as well as preparation method and application of microbial compound fungicide
ActiveCN104059867ALow costHarmless treatmentBacteriaMicroorganism based processesFungicideMicroorganism
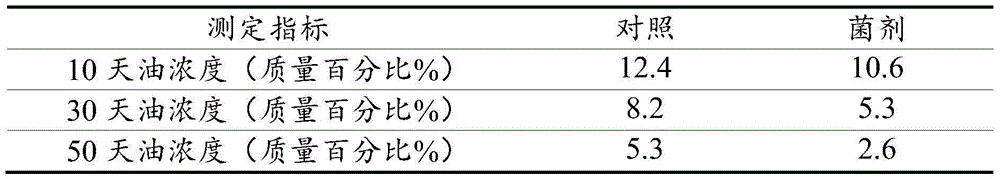
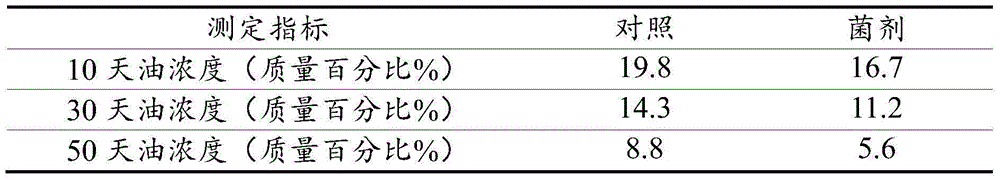
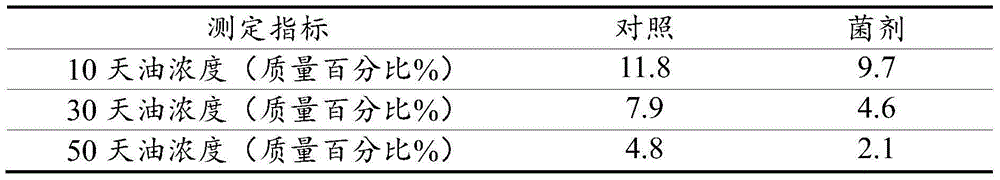
The invention relates to a microbial compound fungicide for treating oil base drilling cuttings as well as a preparation method and application of the microbial compound fungicide. The compound fungicide comprises pseudomonas aeruginosa with a collection number of CGMCC NO:8983 and acinetobacter baumannii with the collection number of CGMCCNO:8984. The invention further discloses the preparation method of the microbial compound fungicide and application of the compound fungicide in treatment of oil base drilling cuttings. The preparation method includes preparation methods of liquid and solid fungicides.
Owner:CHENGDU INST OF BIOLOGY CHINESE ACAD OF S +1
Plant pathogenic fungi antagonistic bacteria capable of generating siderophore and uses thereof
InactiveCN101230327AEffective controlGrowth inhibitionBiocideBacteriaAcinetobacter baumanniiMicrobiology
The invention discloses a plant pathogenic fungi antagonistic bacterium and the application thereof in the plant disease preventing and curing. The plant pathogenic fungi antagonistic bacterium is acinetobacter baumannii BEB3 CGMCC No.2244. The acinetobacter baumannii BEB3 CGMCC No.2244 of the plant pathogenic fungi antagonistic bacterium can prevent and cure plant diseases caused by banana blight germs, banana anthracnose germs, rice blast germs, rubber dactylarin wilt disease germs, mango anthracnose germs, and bamboo fusarium germs; in particular can symbiotic to banana, can exist stably in the banana body, and can prevent and cure banana anthracnose germs and banana blight germs.
Owner:ENVIRONMENT & PLANT PROTECTION INST CHINESE ACADEMY OF TROPICAL AGRI SCI
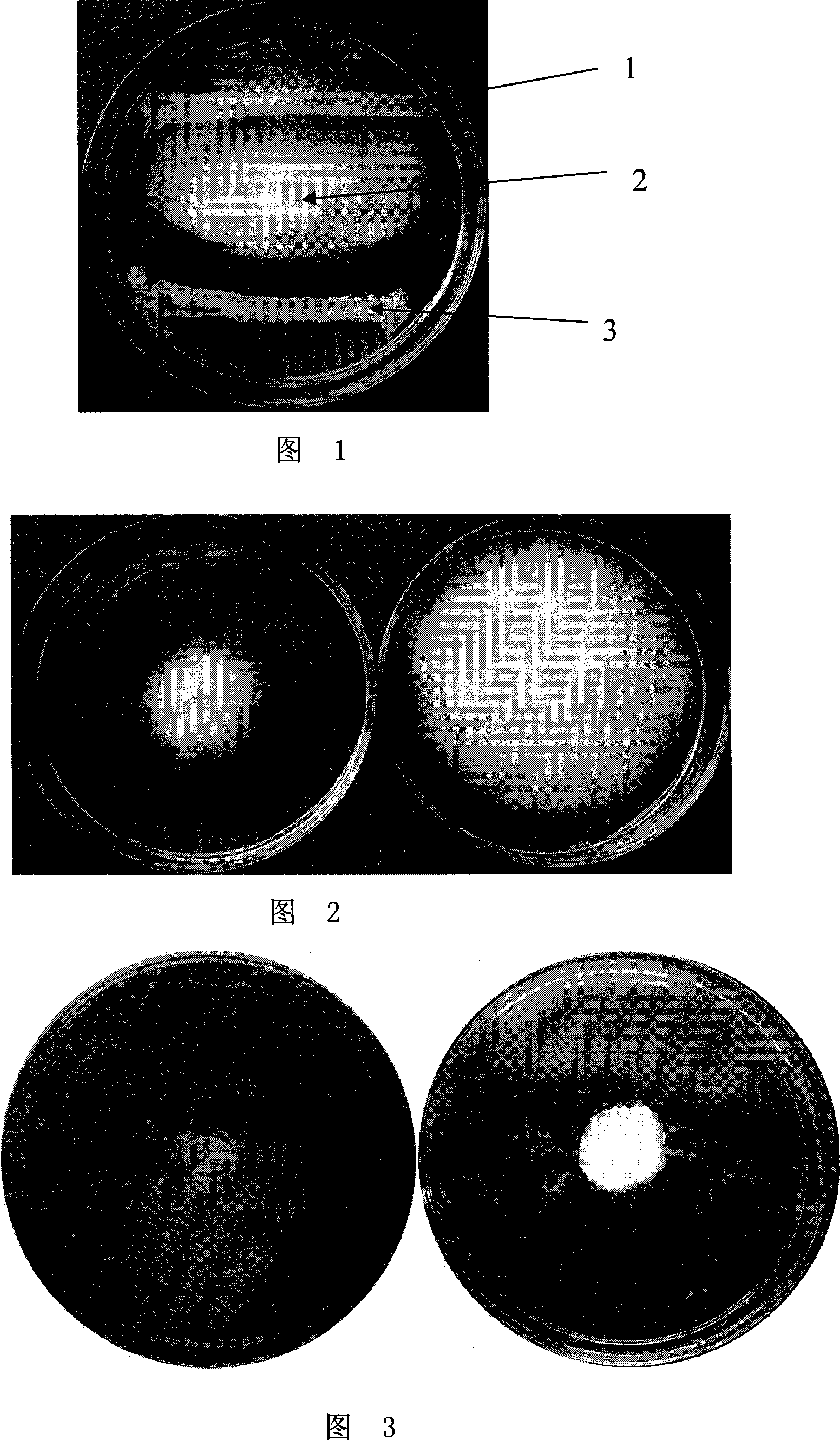
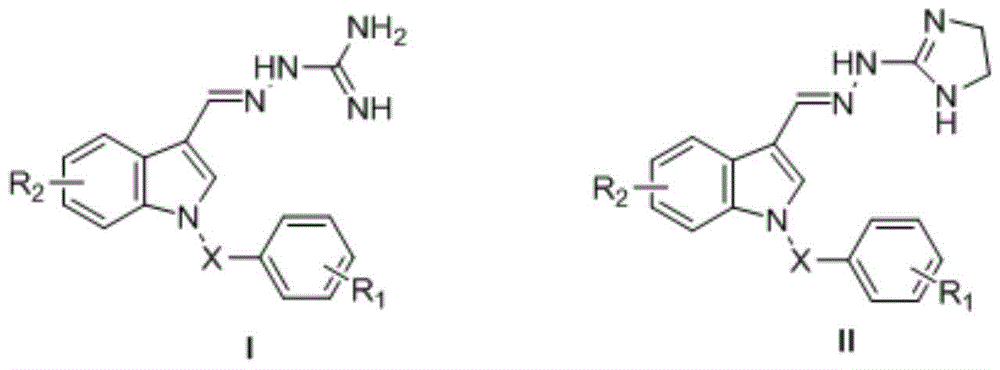
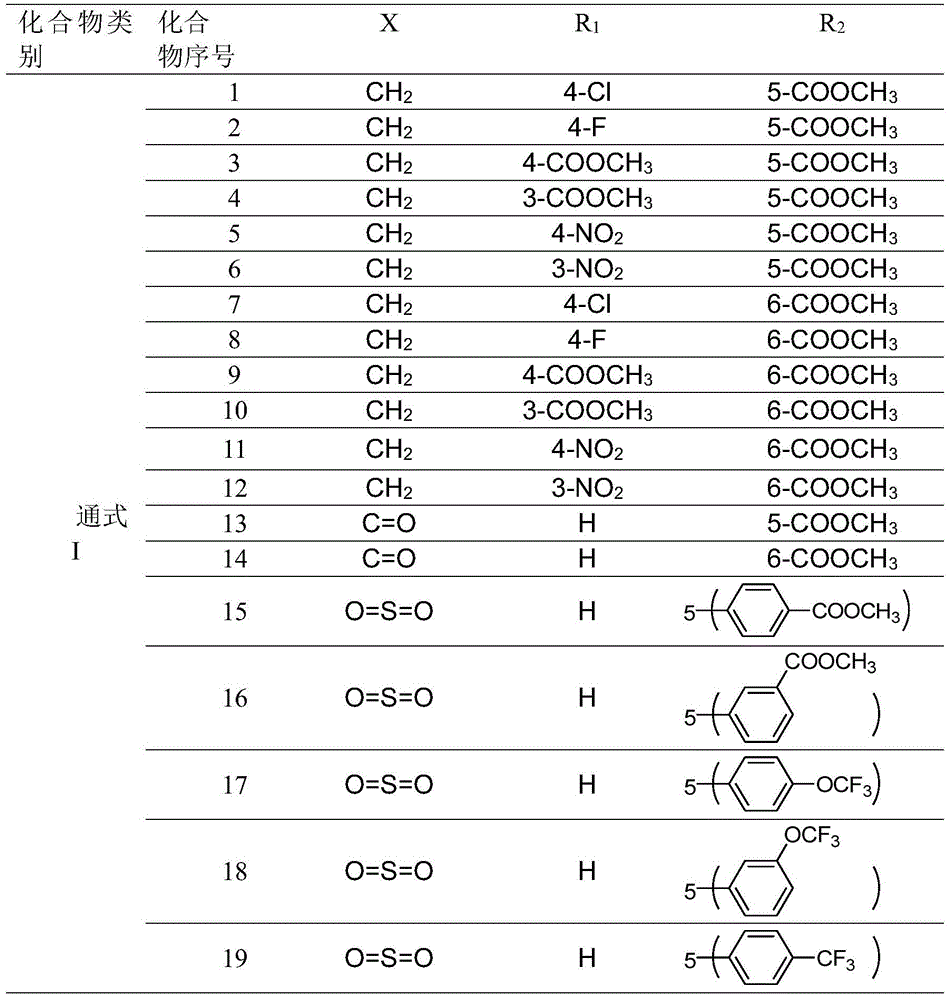
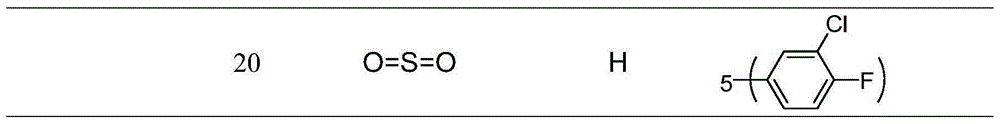
Indole compounds and preparation method and application thereof as drug-resistant bacteria resistant drugs
The invention discloses indole compounds represented by the general formula defined in the specification and salts and preparation method thereof, corresponding medical uses of the compounds and the salts thereof, and drugs and disinfectants containing the compounds and the salts thereof. A lot of experiments prove that the compounds and the salts thereof have good bacteriostatic and bactericidal activity on MRSA (drug-resistant staphylococcus aureus), MDR K.pneumonia (drug-resistant klebsiella pneumonia), and MDR A.baumanii (drug-resistant acinetobacter baumannii). In the formula, X is methylene, carbonyl or sulfonyl; R1 is fluorine, chlorine, bromine, methoxy acyl, nitro or hydrogen; R2 is methoxyl acyl, (p-methoxyl acyl)phenyl, (p-trifluoro oxy)phenyl, (p-trifluoro)phenyl, (m-methoxy acyl)phenyl, (m-trifluoro oxy)phenyl, (p-fluoro m-chloro)phenyl or hydrogen; and HA is inorganic acid or a part of organic acid with strong acidity, preferably hydrochloric acid or hydrobromic acid.
Owner:BEIFANG UNIV OF NATITIES
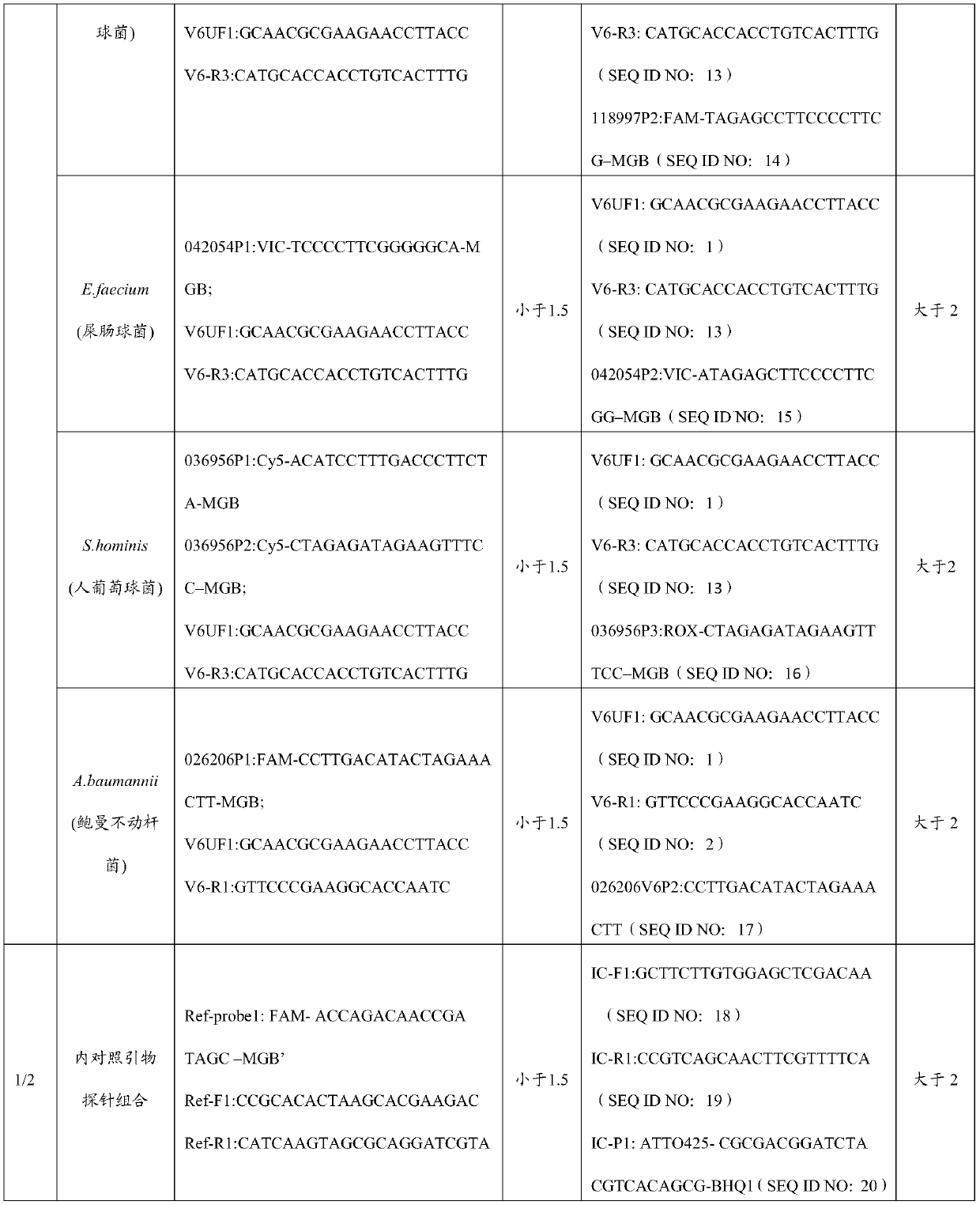
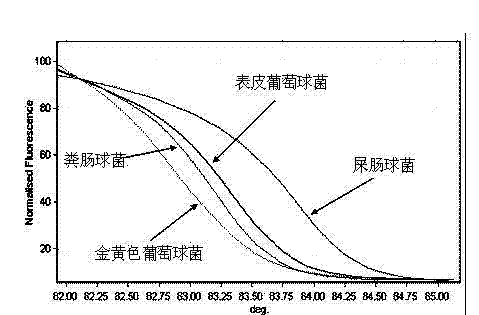
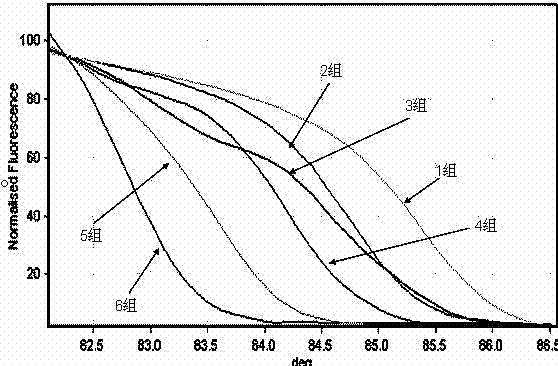
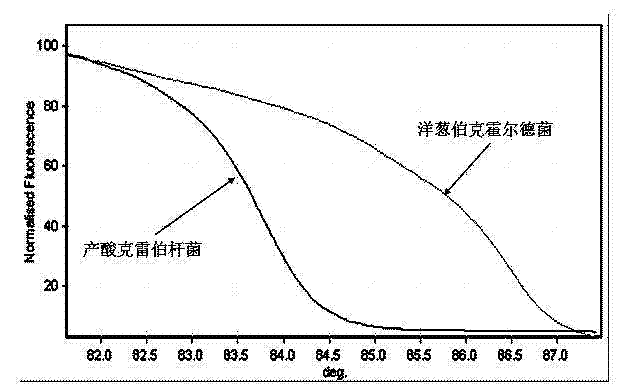
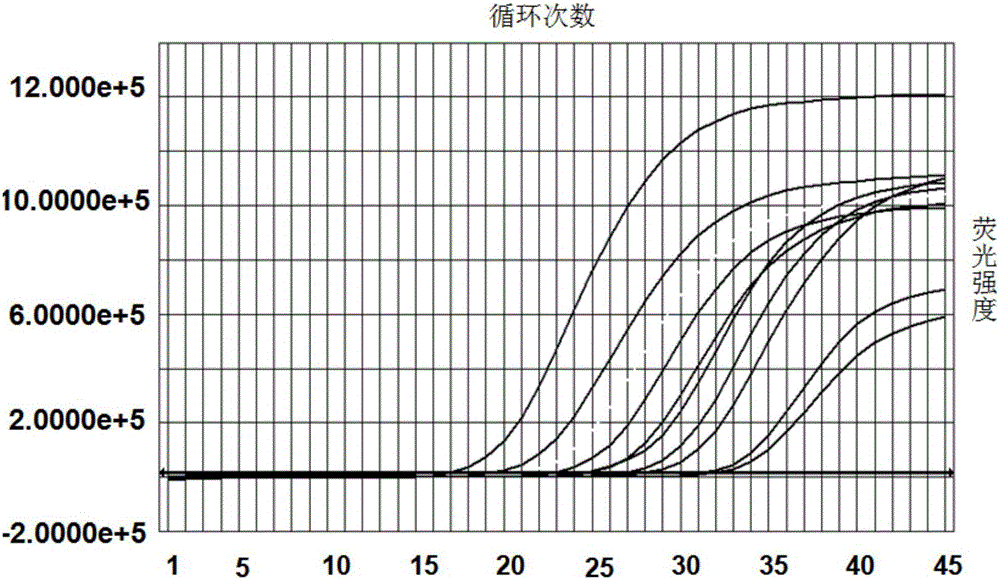
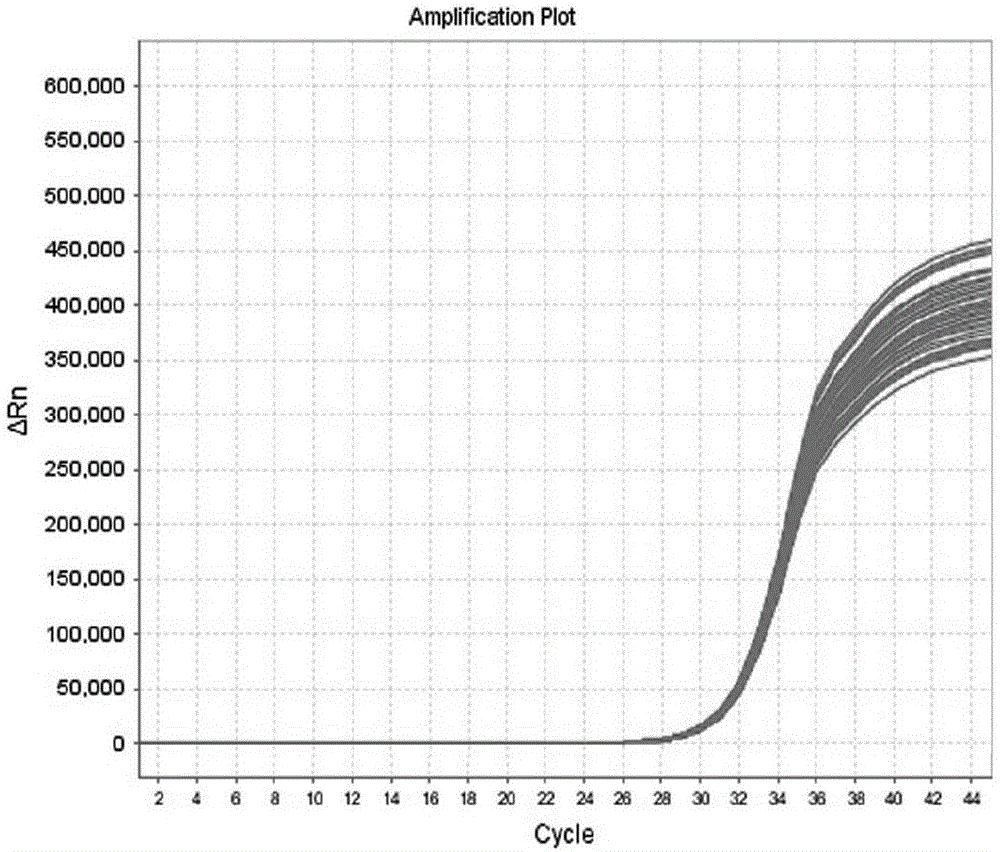
Kit and method for detecting Acinetobacter baumannii DNA
InactiveCN106434996AQuick destructionStrong specificityMicrobiological testing/measurementMicroorganism based processesPositive controlAcinetobacter baumannii DNA
The invention discloses a kit and method for detecting Acinetobacter baumannii DNA; the kit comprises: a nucleic acid release agent, a PCR (polymerase chain reaction) liquid, an enzyme mixed liquid, an Acinetobacter baumannii positive control, and an Acinetobacter baumannii negative control, wherein the PCR liquid is made from PCR buffer solution, deoxyribonucleoside, primers for amplifying targeted polynucleotide, a probe for amplifying targeted polynucleotide, primers for amplifying internally labeled fragments and a probe for detecting an internal label. By optimizing conditions in the embodiment of the invention, primers for amplifying targeted polynucleotide and probes for amplifying the targeted polynucleotide, both having optimal amplification, are screened out, the kit is capable of detecting not pathogens of Acinetobacter baumannii but various infections of Acinetobacter baumannii but, is highly specific, and accordingly has greatly improved detection sensitivity, accuracy and stability.
Owner:SANSURE BIOTECH INC
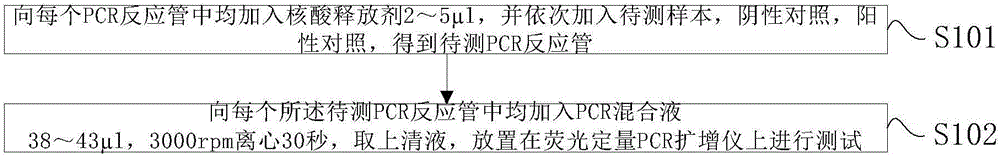
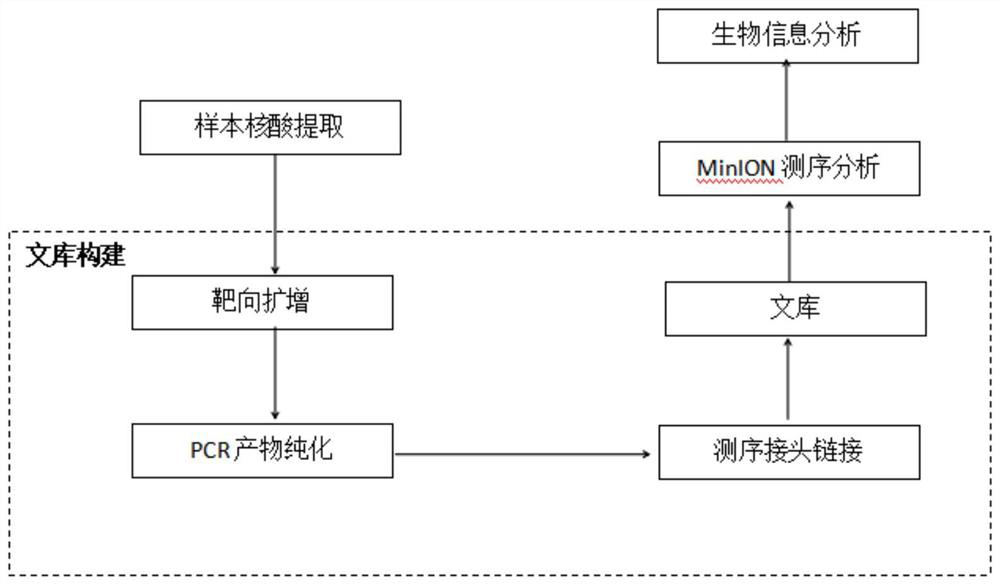
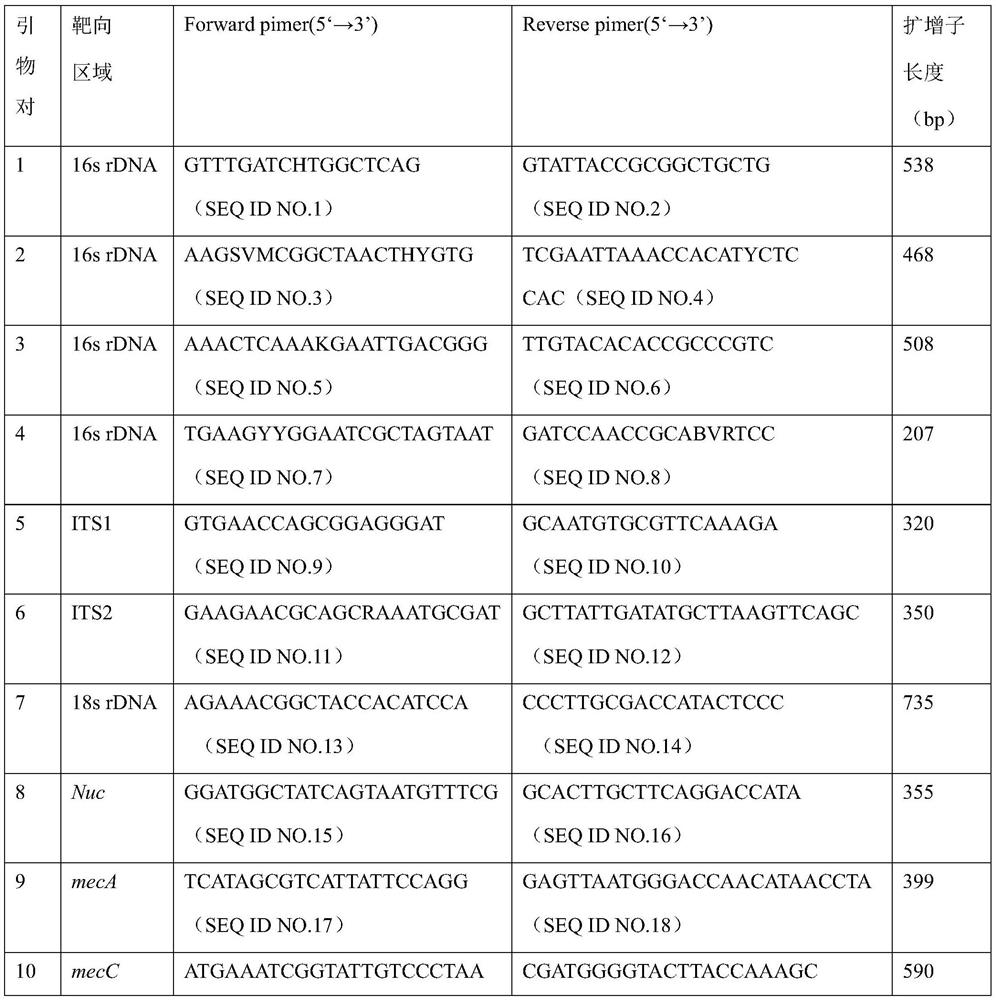
Primer group and kit for rapidly identifying respiratory tract microorganisms based on nanopore sequencing and application of primer group
ActiveCN112501268APrecise positioningImprove accuracyMicrobiological testing/measurementAgainst vector-borne diseasesPneumonitisEnterococcus
The invention discloses a primer group for detecting respiratory tract microorganisms based on a nanopore sequencing method. The nucleotide sequences of the primer group are as shown in SEQ ID NO:1-20. The microorganisms are streptococcus pneumoniae, staphylococcus aureus (resisting to methicillin), klebsiella pneumoniae, pseudomonas aeruginosa, acinetobacter baumannii, stenotrophomonas maltophilia, candida albicans, haemophilus influenzae, legionella pneumophila, enterococcus faecium, chlamydia psittaci, cryptococcus gattii, aspergillus fumigatus and pneumocystis jiroveci. According to the invention, sequencing optimization is carried out on different types of samples of the respiratory tract microorganisms, so that the detection method, the kit and the like are suitable for various typesof respiratory tract samples; and a targeted amplification method is adopted, so that the detection requirements of sputum, alveolar lavage and other sample types can be met. In addition, parallel sequencing can be performed, so that the flux of the detected samples is increased, the sequencing time is shortened, and the contradiction between the flux of detected pathogenic species and the cost and time is further relieved.
Owner:GUANGZHOU DARUI BIOTECH
Novel antimicrobial compound and use thereof
InactiveUS20170204052A1High antibacterial activityOrganic active ingredientsOrganic chemistryResistant bacteriaMicroorganism
The present invention relates to: coralmycin A and B, which are novel compounds exhibiting antimicrobial activity, an isomer thereof, a derivative thereof or a pharmaceutically acceptable salt thereof; a microorganism of the genus Corallococcus producing the same; and an antimicrobial use thereof.The coralmycin A and B have very strong antimicrobial activity against antibiotic-resistant bacteria such as MRSA, QRSA, VRE, VISA, etc.; Acinetobacter baumannii, which is a multidrug-resistant microorganism; and also against gram-positive microorganisms and gram-negative microorganisms. Therefore, the present invention can be very useful for prevention, treatment and alleviation of various microbial infections, and thus can be widely applied to the medical supply, quasi-drug, food, and feed industries.
Owner:KOREA RES INST OF BIOSCI & BIOTECH
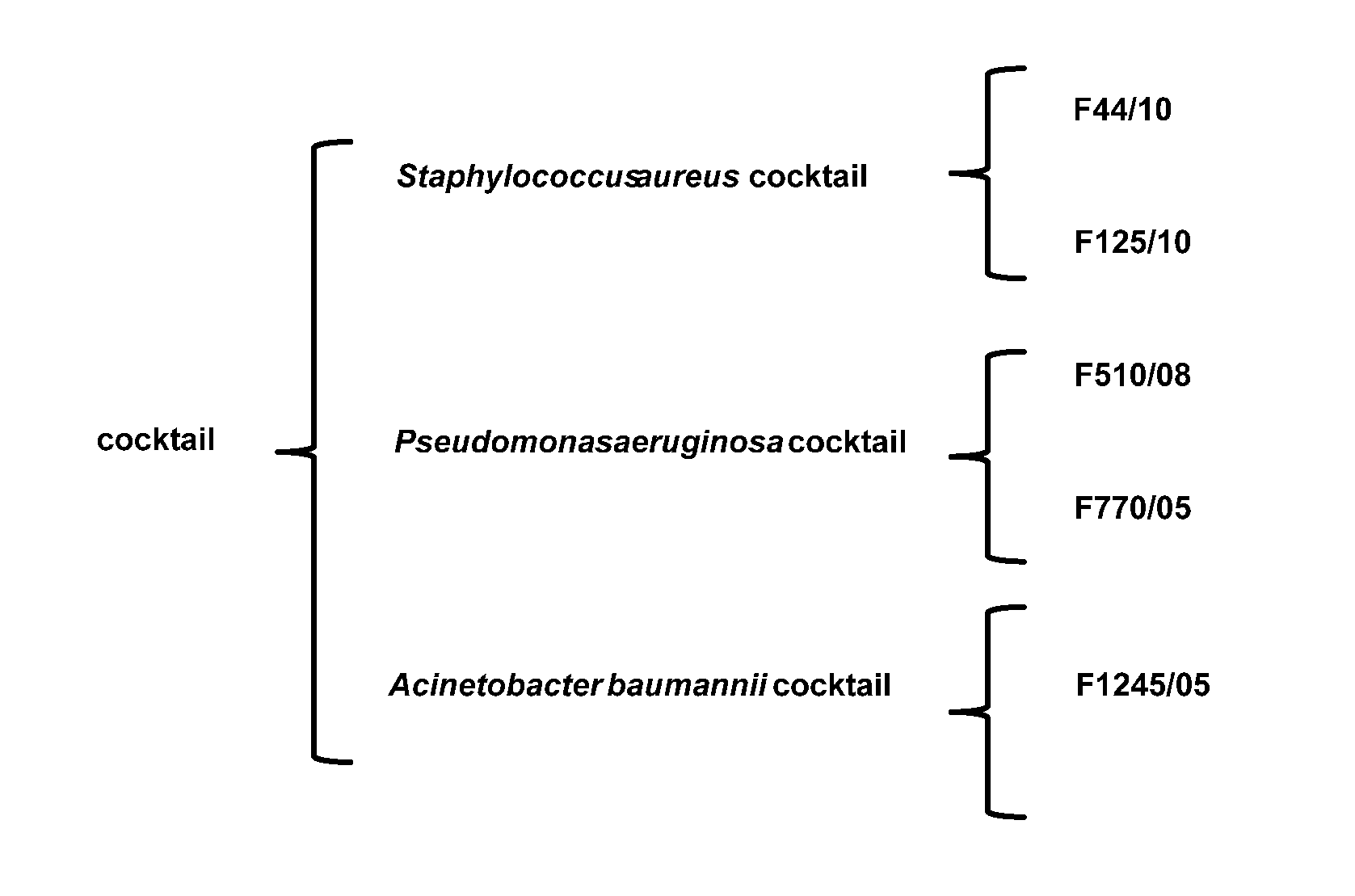
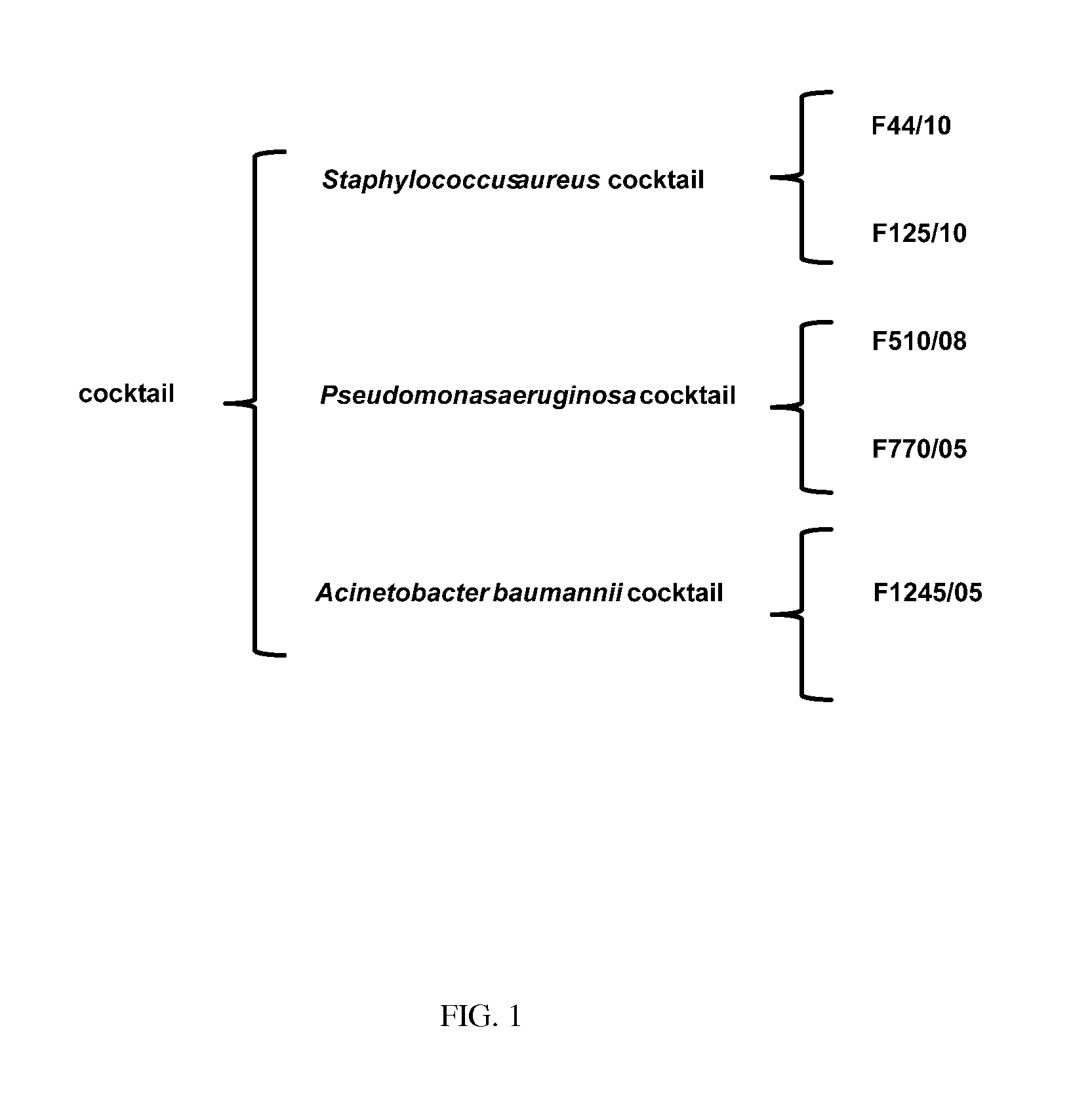
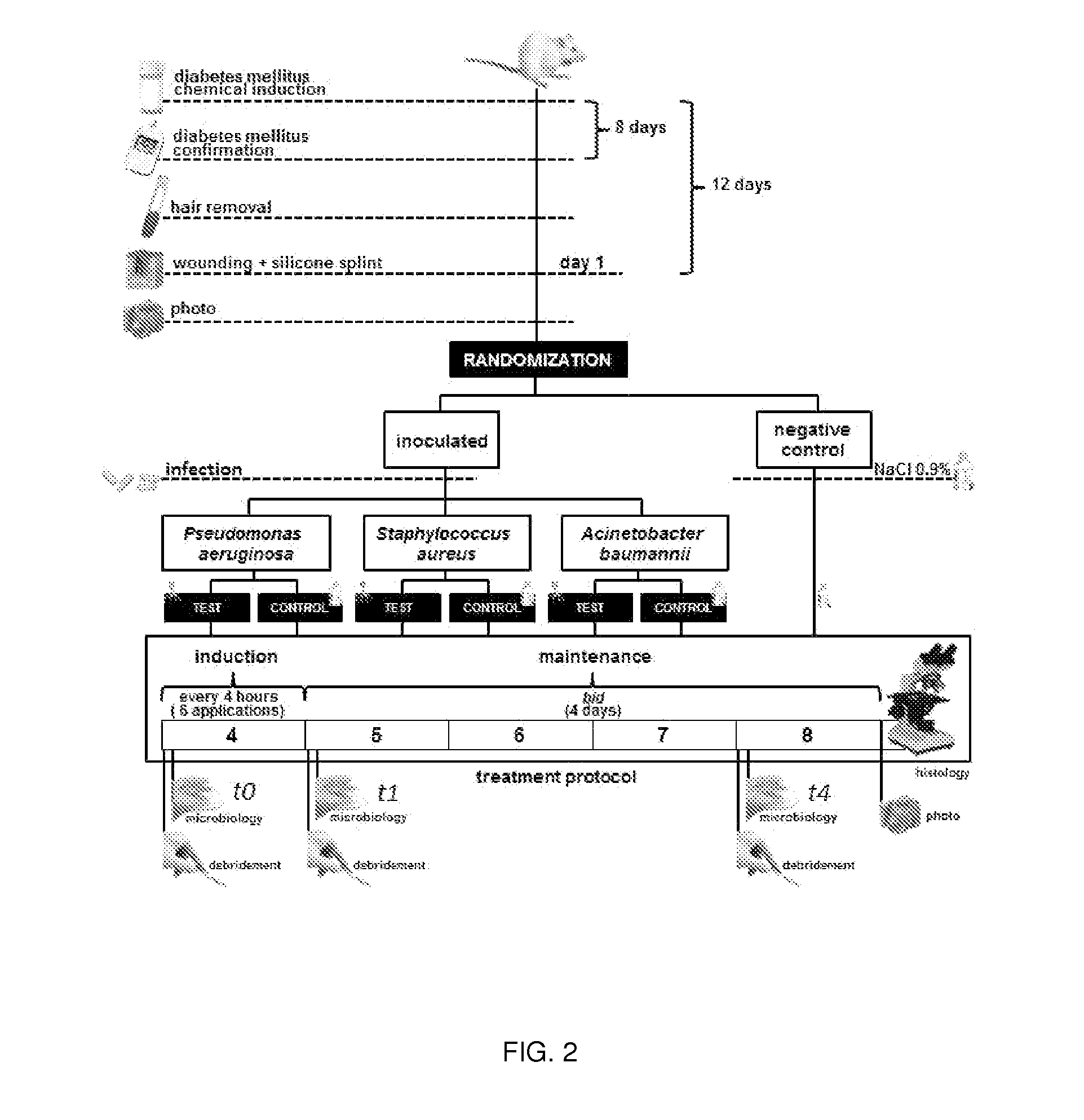
Compositions comprising cocktails of antibacterial phages and uses thereof for the treatment of bacterial infections
ActiveUS20150150919A1Shorten the progressEliminate and lessenAntibacterial agentsBiocideStaphylococcus aureusAntibiotic Y
The present invention is directed to the field of phage therapy for the treatment and control of bacterial infections, in particular diabetic foot infections. More specifically, the present invention is directed to novel cocktails of bacteriophage strains F44 / 10, F1 25 / 10, F770 / 05, F510 / 08, F1 245 / 05, and / or variants thereof; and methods of using same in the treatment and prevention of bacterial infections, including cutaneous ulcers associated with diabetic foot infections, caused by, e.g., Staphylococcus aureus, Pseudomonas aeruginosa, and / or Acinetobacter baumannii. The cocktails are used as pharmaceutical compositions either alone or in further combination with other therapies, e.g., antibiotics, growth factors, or other standard, as well as non-standard, therapies for diabetic foot infections.
Owner:TECHNOPHAGE INVESTIGACAO E DESENVOLVIMENTO EM BIOTECHA
Multiple PCR detection kit and method for identifying susceptible bacteria species for intracranial post-operation
InactiveCN106337087AEasy to operateImprove timelinessMicrobiological testing/measurementBacteroidesPositive control
The invention aims to provide a multiple PCR detection kit and a method for identifying susceptible bacteria species for intracranial post-operation to eliminate endogenous nucleic acid contamination. The detected bacteria species are the following common bacteria: staphylococcus aureus, enterococcus faecalis, streptococcus pneumoniae, escherichia coli, bacillus pyocyaneus, klebsiella pneumoniae and acinetobacter baumannii. Meanwhile, the invention provides the method to eliminate the endogenous nucleic acid contamination in a raw material of a PCR system and exclude a false positive experimental result of a PCR caused by the endogenous nucleic acid contamination. The detecting method using the kit has the advantages of that the operation is simple and fast, the detection cost is lowered, the detection result is accurate, and the false positive result is reduced. A negative control and a positive control are added in the amplification step, so that the judgment of false positive and false negative is effectively improved, and the detection accuracy is higher.
Owner:江苏睿玻生物科技有限公司
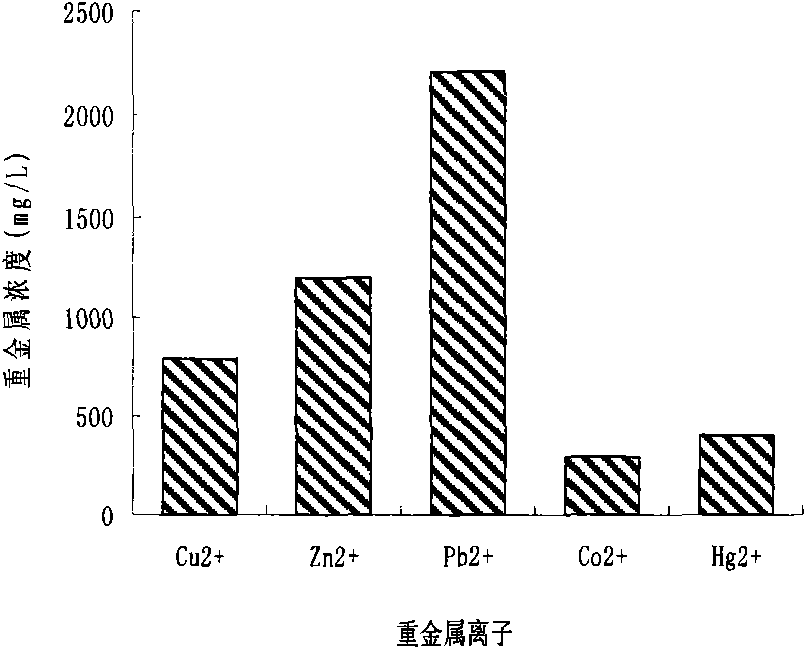
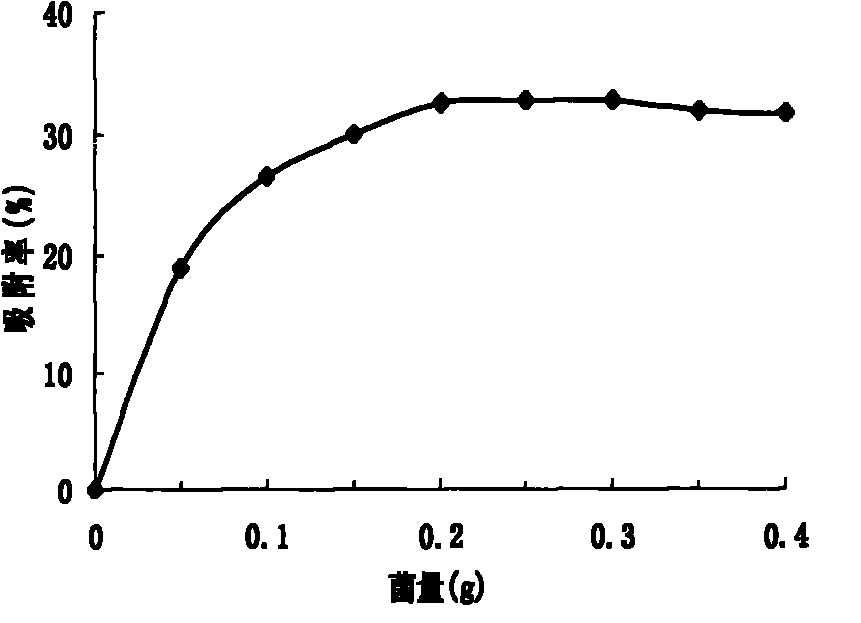
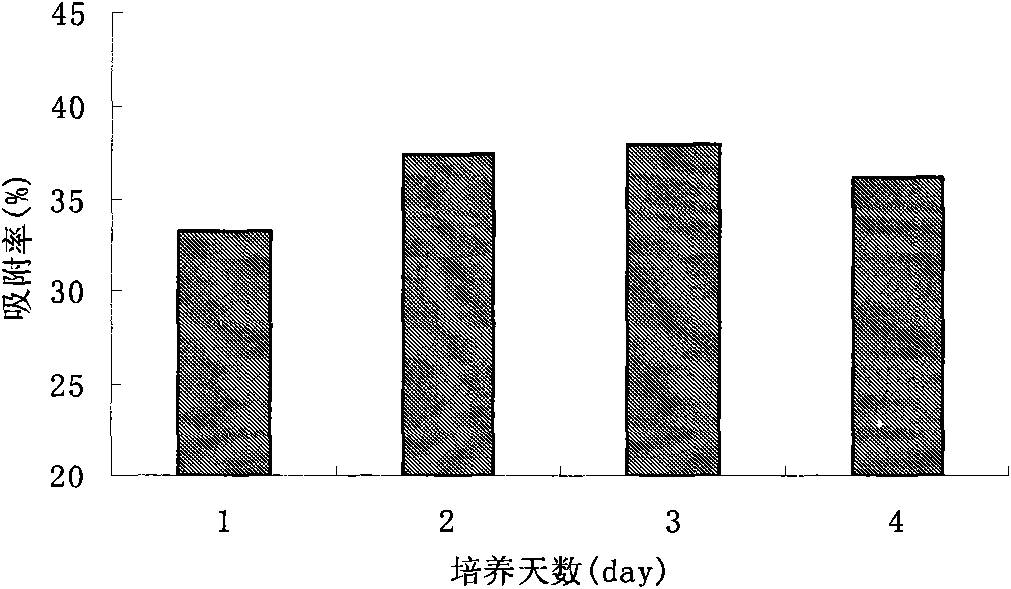
Acinetobacter and application thereof to biological treatment of heavy metal ions
InactiveCN101974448AHave tolerance propertiesHigh tolerance concentrationBacteriaWater contaminantsAcinetobacter baumanniiIon
The invention discloses acinetobacter baumannii R9 CGMCC No.3947 and application thereof to the adsorption of heavy metal ions. The acinetobacter baumannii is tolerant to five types of ions, namely, Hg<2+>, Cu<2+>, Co<2+>, Pb<2+> and Zn<2+>, wherein the tolerance concentrations to Pb<2+> and Zn<2+> are highest and are up to 2,200 mg / L and 1,500 mg / L respectively; the tolerances to Cu<2+> and Hg<2+> are up to 800 mg / L and 400 mg / L respectively; and the tolerance to Co<2+> is 300 mg / L. Through the method, lead ions are adsorbed by the growth of thalli and can be directly adsorbed by using wet thalli of which the maximum adsorption capacity is up to 39.56 mg / g. The acinetobacter has good adsorption effect in the application of the heavy metal ions and good application prospect.
Owner:THE INST OF MICROBIOLOGY XINJIANG ACADEMY OF AGRI SCI
LAMP (loop-mediated isothermal amplification) kit for detecting acinetobacter baumannii and special primers of LAMP kit
InactiveCN104450942AEasy to operateLow equipment requirementsMicrobiological testing/measurementDNA/RNA fragmentationDiseaseSocial benefits
The invention discloses an LAMP (Loop-mediated isothermal amplification) kit for detecting acinetobacter baumannii, special primers of the LAMP kit and an application of the LAMP kit to detection of the acinetobacter baumannii. The nucleotide sequence of the five special primers for performing LAMP detection for the acinetobacter baumannii in a sequence table is SEQ ID NO: 2(F3), SEQ ID NO: 3(B3), SEQ ID NO: 4(FIP), SEQ ID NO: 5(BIP) and SEQ ID NO: 6(LF). The acinetobacter baumannii can be rapidly, conveniently, efficiently, high-specifically and high-sensitively detected at the equal temperature, complicated instruments are omitted, and a novel technical platform for detecting the acinetobacter baumannii is provided. The LAMP kit can be used for screening and detecting the acinetobacter baumannii in livestock production units, basic medical and health units and disease prevention and control centers, has wide market prospects and high economic and social benefits and is suitable for wide-range popularization and application.
Owner:INST OF PLA FOR DISEASE CONTROL & PREVENTION +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com