Novel antimicrobial compound and use thereof
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Isolation and Identification of Microorganisms
[0107]A Corallococcus coralloides M23 microorganism with Accession No. of KCTC18279P was isolated from a soil sample collected from the brook located at Imhakdong-ro, Incheon, Korea according to the method reported by Park et al. (S. Park, B. Lee, J. Kim, C. Lee, E. Jang and K. Cho, Kor. J. Microbiol. Biotechnol. 32: 218, 2004).
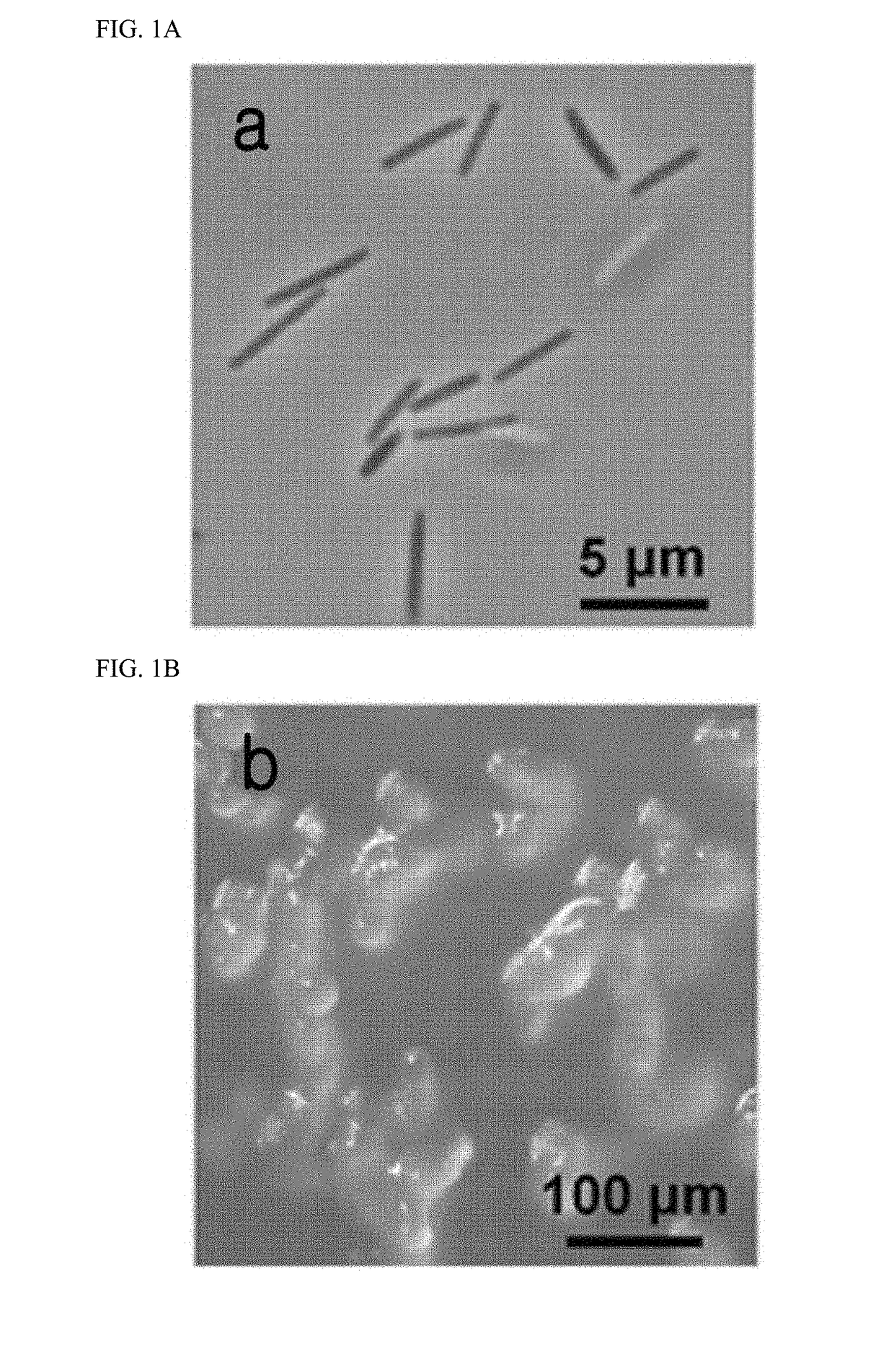
[0108]For the identification of the Corallococcus coralloides M23 microorganism, physiological and morphological tests were performed. As a result, it was confirmed that the M23 microorganism is a gram-negative Bacillus with a length of about 4 (FIG. 1a), which showed vegetative growth feeding on E. coli, and migrated with gliding motility. Additionally, the microorganism formed its unique fruiting bodies in WC medium (10 mM 3-[N-morpholino]propanesulfonic acid (pH 7.6), 0.1% CaCl2.2H2O, 1.5% agar) (Fig. 1b).
[0109]In order to perform the identification of microorganisms based on the nucleotide sequence of 16S rDNA...
example 2
Cultivation of Microorganisms
[0110]The surface of a piece of a filter paper, on which the spores of the Corallococcus coralloides M23 microorganism were present, was spread on the entire surface of VY / 3 medium (0.5% Baker's yeast, 0.1% CaCl2.2H2O, 10 mM MOPS (pH 7.6), 1.5% bacto-agar, 0.5 ppm cyanocobalamine). After wrapping, the medium was cultured for about 10 days until the formation of fruiting bodies was confirmed at 28° C.
[0111]CYS medium (0.5% casitone, 0.1% yeast extract, 0.3% soluble starch, 0.1% MgSO4.7H2O, 0.05% CaCl2, 50 mM HEPES (pH 7.6)) in an amount of 100 mL was added to a 500 mL Erlenmeyer flask, sterilized, and then an ST-trace element solution (0.01% MnCl2.4H2O, 0.004 CoCl2.6H2O, 0.0016% CuSO4.5H2O, 0.001% Na2MoO4.2H2O, 0.002% ZnCl2, 0.0005% LiCl, 0.0005% SnCl2.2H2O, 0.001% H3BO3, 0.002% KBr, 0.002% KI, 0.08% EDTA Na—Fe3+ salt (trihydrate)) and a vitamin B12 solution (0.05% cyanocobalamine) in an amount of 100 mL, respectively, were added to the sterilized CYS med...
example 3
Isolation and Purification of Coralmycins
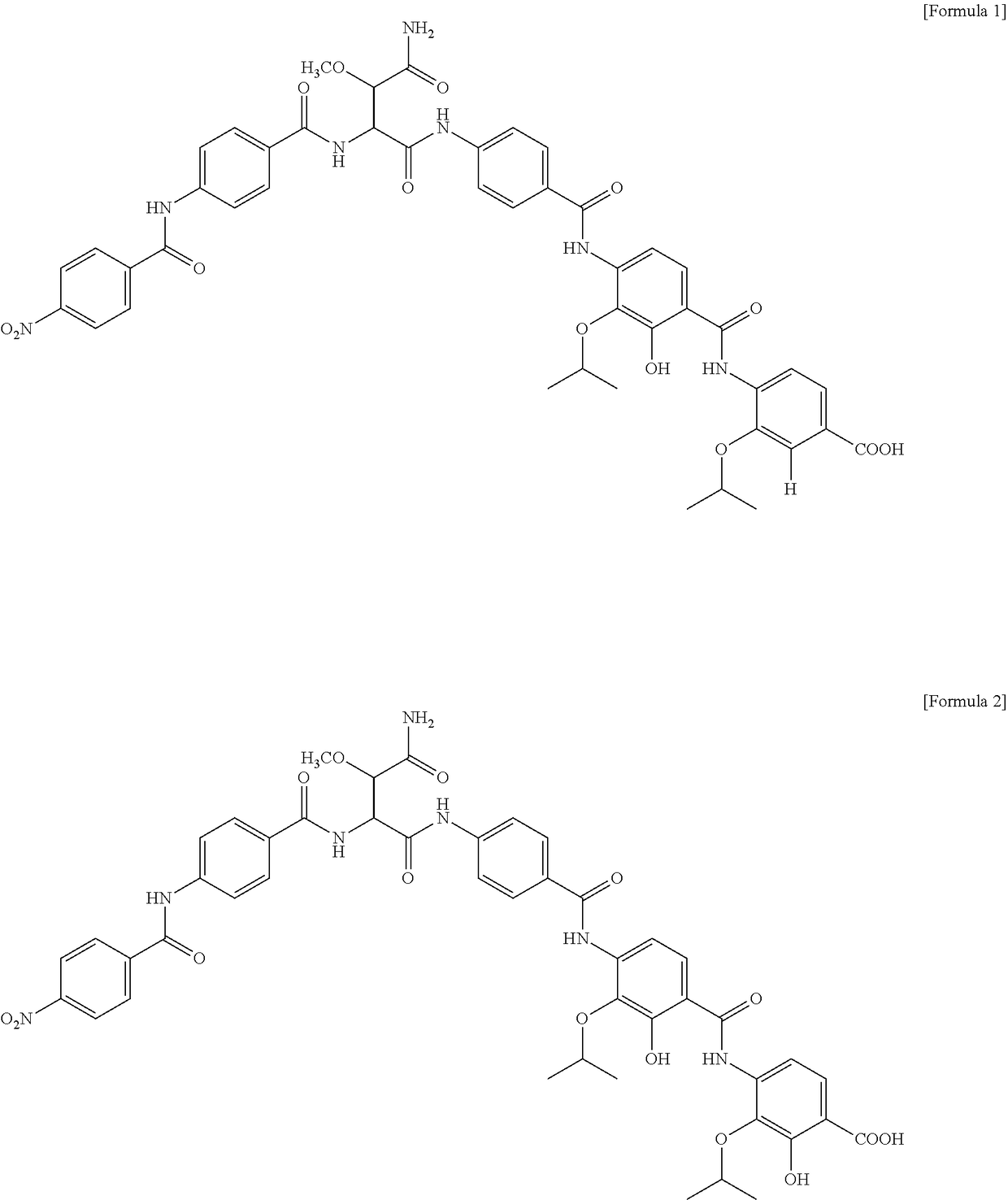
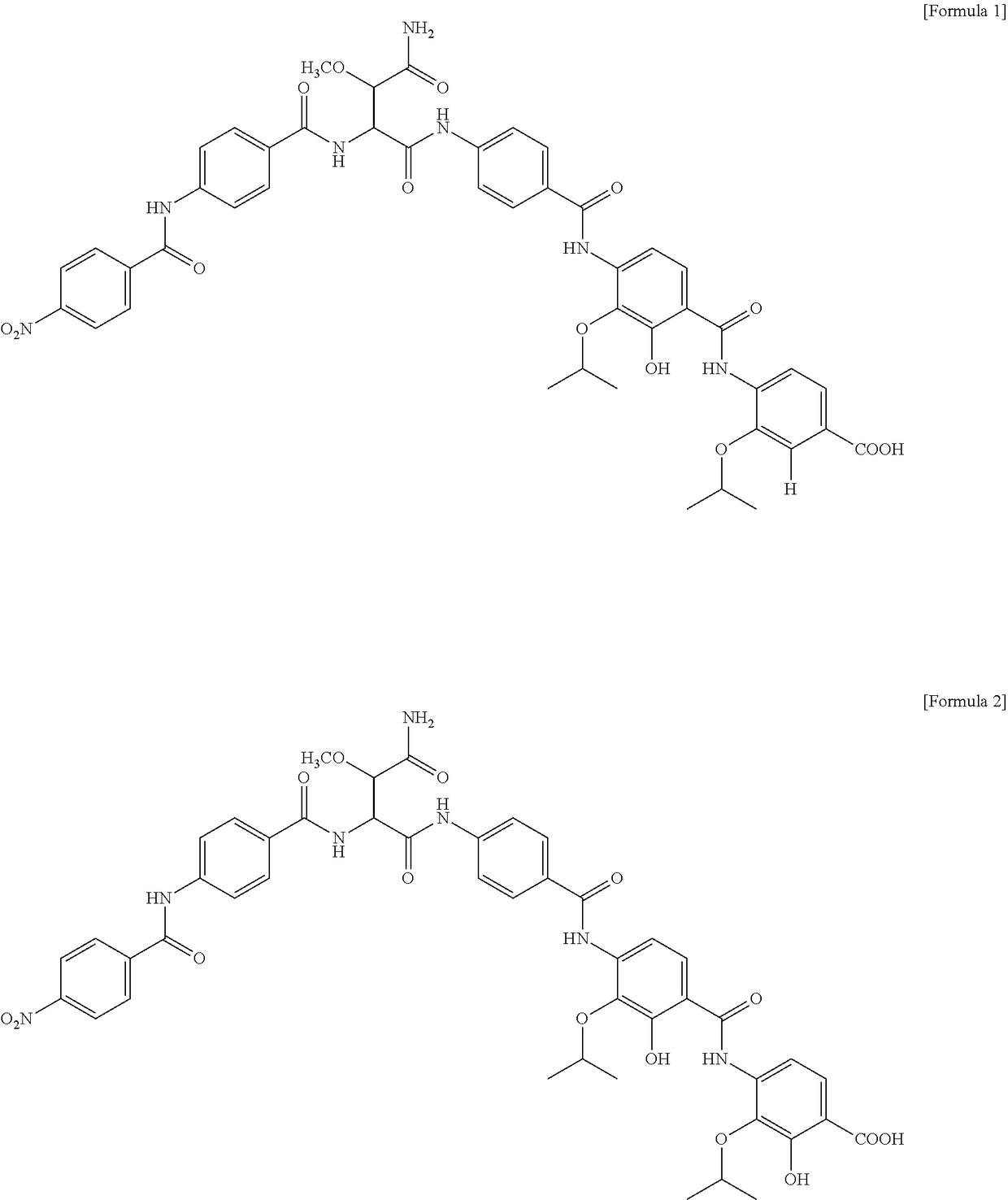
[0113]After the cultivation in Example 2, the amberlite XAD16 in the culture solution was recovered. Acetone was added to the amberlite XAD16 and the mixture was stirred to extract active ingredients from the amberlite XAD16. After evaporating acetone, the extract was extracted 3 times by solvent extraction using ethyl acetate. The thus-obtained ethyl acetate solvent layer containing active ingredients was concentrated under reduced pressure to remove ethyl acetate and the resulting residue was subjected to Sephadex LH-20 column chromatography using methanol as a solvent. The thus-obtained active fraction was concentrated under reduced pressure and then subjected to HPLC, in a condition where methanol:water is 50:50, to obtain compounds 1 and 2 of the active fraction.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Electrical resistance | aaaaa | aaaaa |
| Antimicrobial properties | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com