Chitosan biguanide hydrochloride, preparation method and use thereof
The technology of chitosan hydrochloride and biguanide hydrochloride is applied in the field of chemical biology of renewable resources, and achieves the effects of strong antibacterial activity, rich source of raw materials and simple preparation method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0013] Embodiment 1: take by weighing 1.0g molecular weight between 1.0-300,000, deacetylation degree is 80-97% chitosan, add 20mL concentration and be the hydrochloric acid solution of 0.2-0.5mol / L, stir until completely dissolving, use Anhydrous ethanol precipitation, the precipitated solid was washed 2-3 times with ethanol, sucked dry, and vacuum-dried to obtain chitosan hydrochloride powder. Add water to the chitosan hydrochloride powder until it dissolves into a solution, add the guanidinating agent-dicyandiamide, which is 1 times the molar mass of the amino group, into the solution, and then stir at 100-120°C until the reaction is complete. After cooling, filter under reduced pressure, precipitate the filtrate with absolute ethanol, wash the filter cake 3 times with absolute ethanol after suction filtration, and dry in vacuum to obtain chitosan biguanide hydrochloride powder. Dissolve the obtained chitosan biguanide hydrochloride powder in water to prepare a solution wit...
Embodiment 2
[0014] Embodiment 2: Take 2.0g by weighing 2.0g degree of deacetylation is 80-97%, molecular weight is 0.5-15×10 4 Chitosan, add 50mL of hydrochloric acid solution with a concentration of 0.2-0.5mol / L, stir until completely dissolved, precipitate with absolute ethanol, wash the precipitated solid with ethanol for 3 times, drain, and dry in vacuum to obtain chitosan salt salt powder. Add water to the chitosan hydrochloride powder until it dissolves into a solution, add the guanidinating agent-dicyandiamide, which is 5 times the molar mass of the amino group, into the solution, and then stir at 100-120°C until the reaction is complete. After cooling, filter under reduced pressure, precipitate the filtrate with absolute ethanol, wash the filter cake twice with absolute ethanol after suction filtration, and dry in vacuum to obtain chitosan biguanide hydrochloride powder. The prepared chitosan biguanide The biguanide group forming the chitosan biguanide hydrochloride in the hydroc...
Embodiment 3
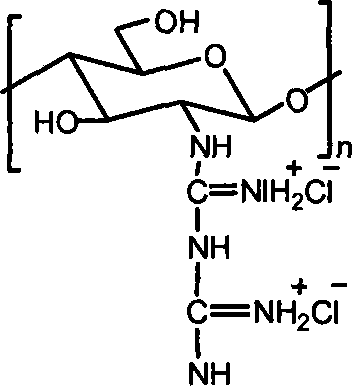
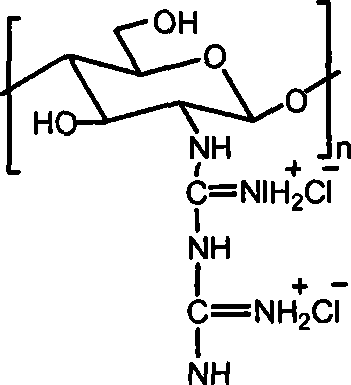
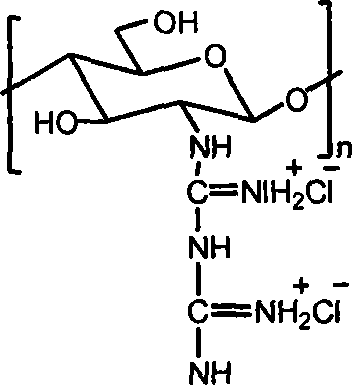
[0015] Embodiment 3: take by weighing 1.0g chitosan, add 30mL concentration and be the hydrochloric acid solution of 0.2-0.5mol / L, stir until dissolving, precipitate with absolute ethanol, the solid that separates out is washed with ethanol, drained, vacuum-dried, obtains Chitosan hydrochloride powder. Add water to the chitosan hydrochloride powder until it dissolves into a solution, add the guanidinating agent-dicyandiamide, which is 5 times the molar mass of the amino group, into the solution, and then stir at 100-120°C until the reaction is complete. After cooling, filter under reduced pressure, precipitate the filtrate with absolute ethanol, wash the filter cake with absolute ethanol after suction filtration, and dry in vacuum to obtain chitosan biguanide hydrochloride powder. The repeating unit of chitosan biguanide hydrochloride is 2-biguanide glucose hydrochloride, its structural formula is:
[0016]
[0017] In the prepared chitosan biguanide hydrochloride, the big...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com