Primer and probe combination and kit for detecting human pathogenic bacteria
A technology of pathogenic bacteria and kits, applied in the field of medical detection, can solve the problem that the detection sensitivity cannot reach the clinical level
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0058] Embodiment 1 Primer and probe combination of the present invention, reference primer and probe combination
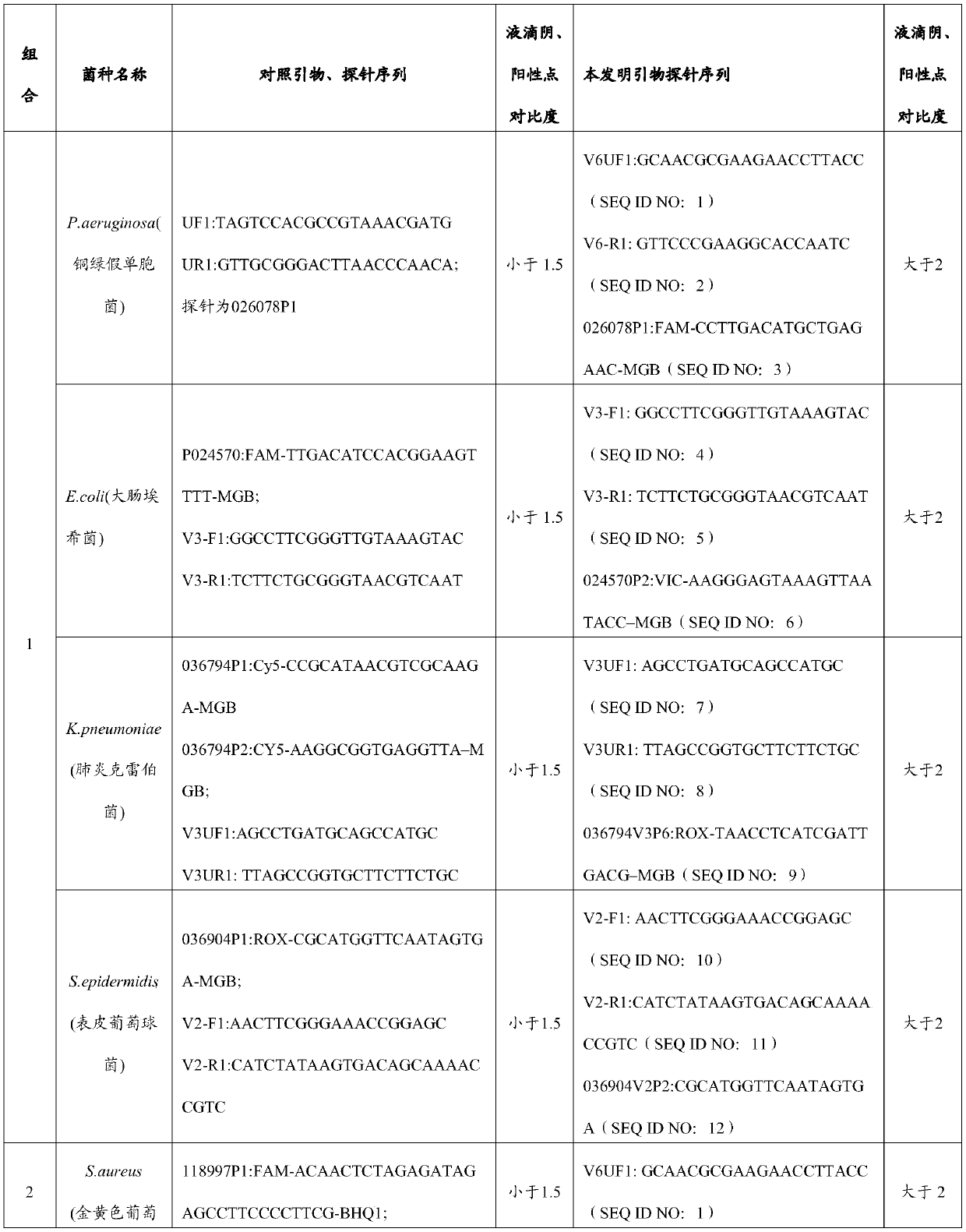
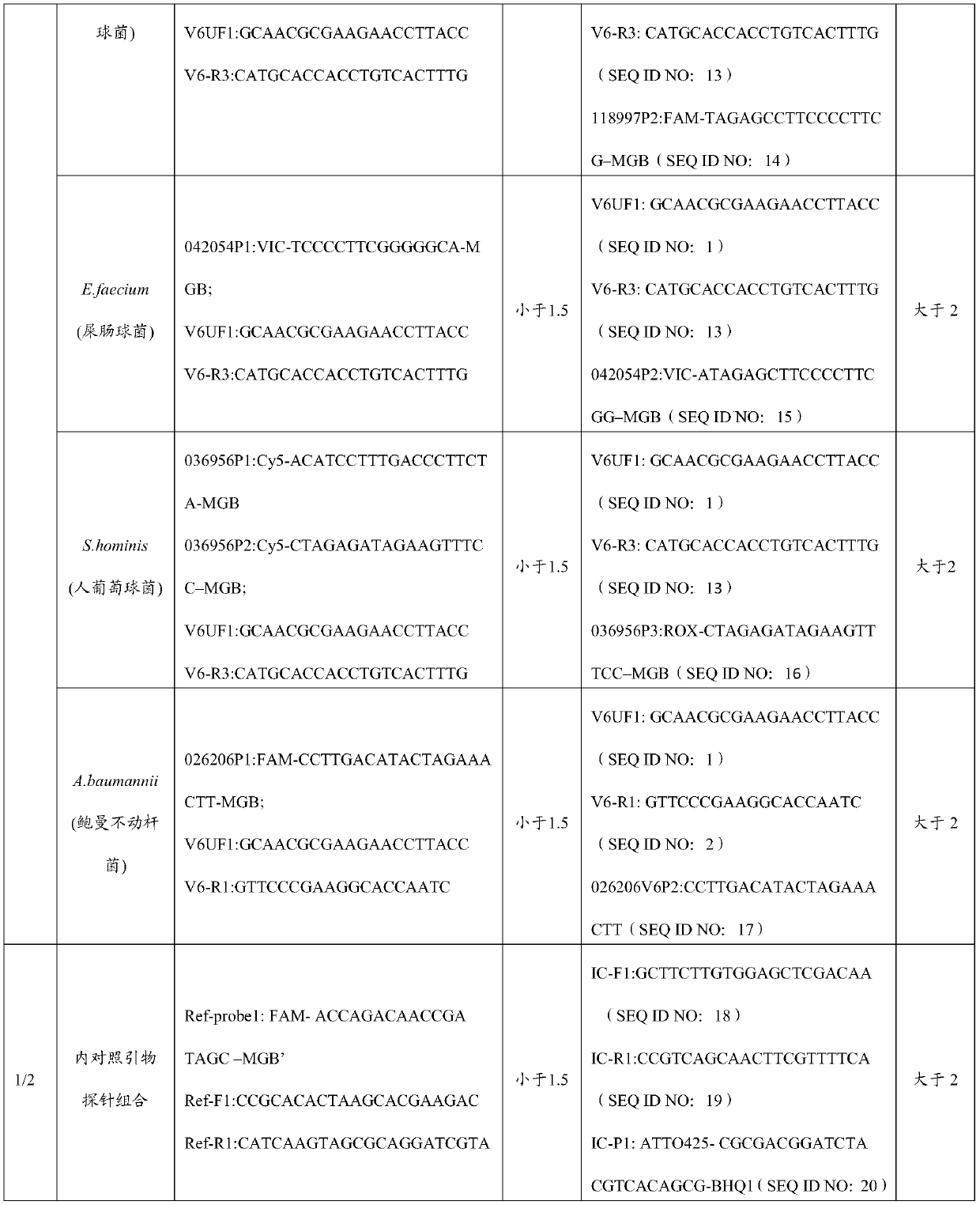
[0059] The sequences of primers and probes are listed in Table 1.
[0060] Table 1 Primer and Probe Combination Sequences
[0061]
[0062]
Embodiment 2
[0063] Embodiment 2 Kit of the present invention
[0064] The kit includes primers and probe combinations of the present invention in Table 1, sample extraction reagents, multiple digital PCR detection reagents, positive control substances, negative control substances, PCR chips, special oil for chips, ddH 2 O.
[0065] Among them, the negative control substance is ultrapure water, and the positive control substance is the standard substance corresponding to the target strain detected by the kit.
Embodiment 3
[0067] Utilize the kit of embodiment 2 to Pseudomonas aeruginosa, Escherichia coli, Klebsiella pneumoniae, Staphylococcus epidermidis, Staphylococcus aureus, Enterococcus faecium, Staphylococcus hominis and Baumannii in 84 cases of blood samples Acinetobacter was tested. Among them, 14 were positive samples and 70 were negative samples. At the same time, the blood culture method is used to determine the infection of the sample, which is the current industry gold standard.
[0068] The detection steps are:
[0069] 1. Nucleic acid extraction:
[0070] The instruments and equipment used in the nucleic acid extraction process are shown in Table 2, and the reagents are shown in Table 3.
[0071] Table 2 Instrument and Equipment List
[0072]
[0073] Table 3 Reagent name and storage
[0074] Reagent name storage Lysate A Store at 2-8°C Proteinase K Store at 2-8°C magnetic bead suspension Store at 2-8°C Washing solution A Store at 2-8...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com