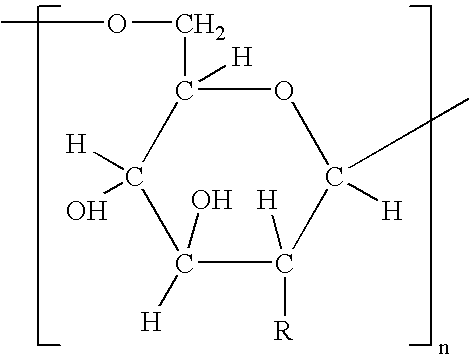
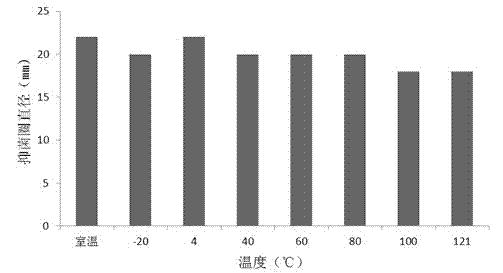
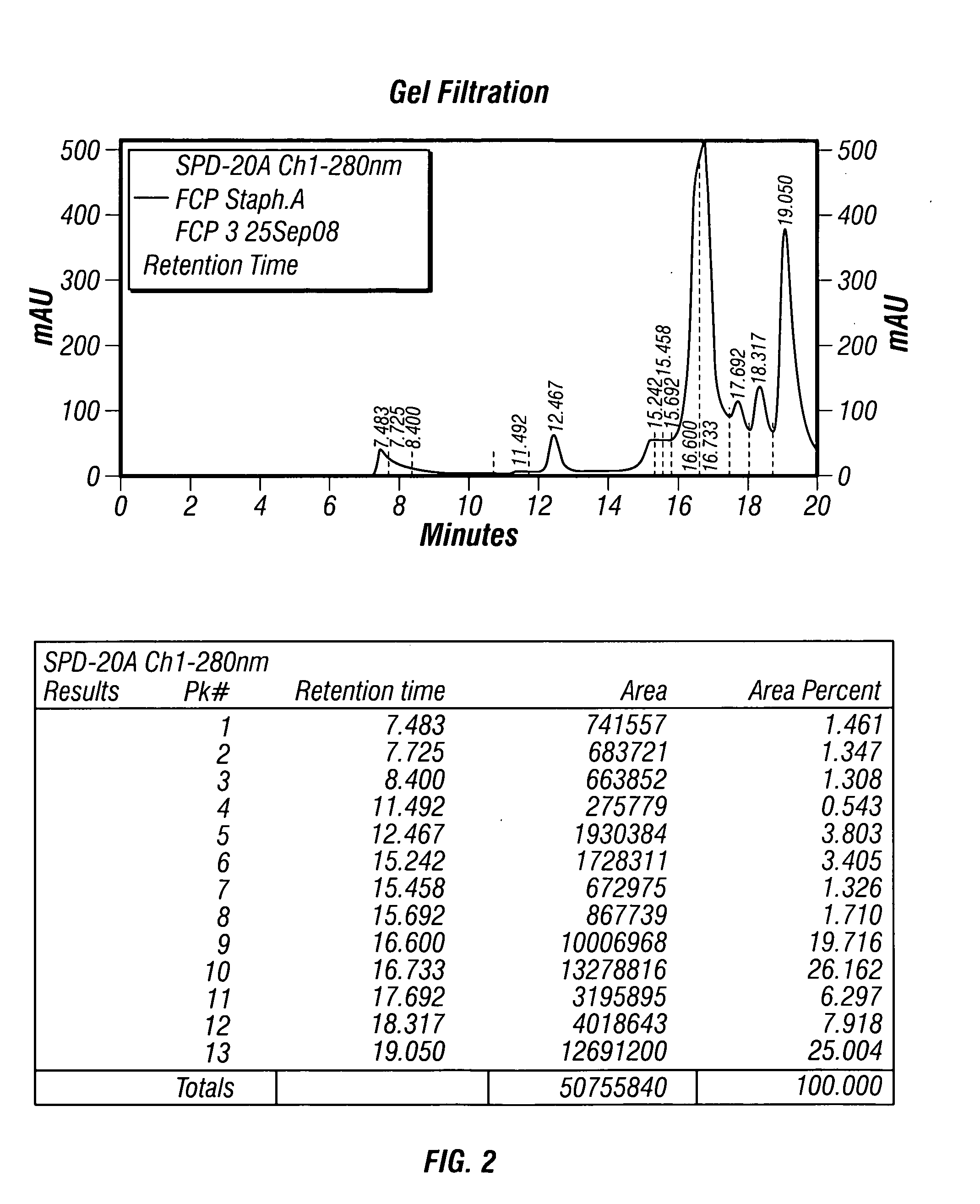
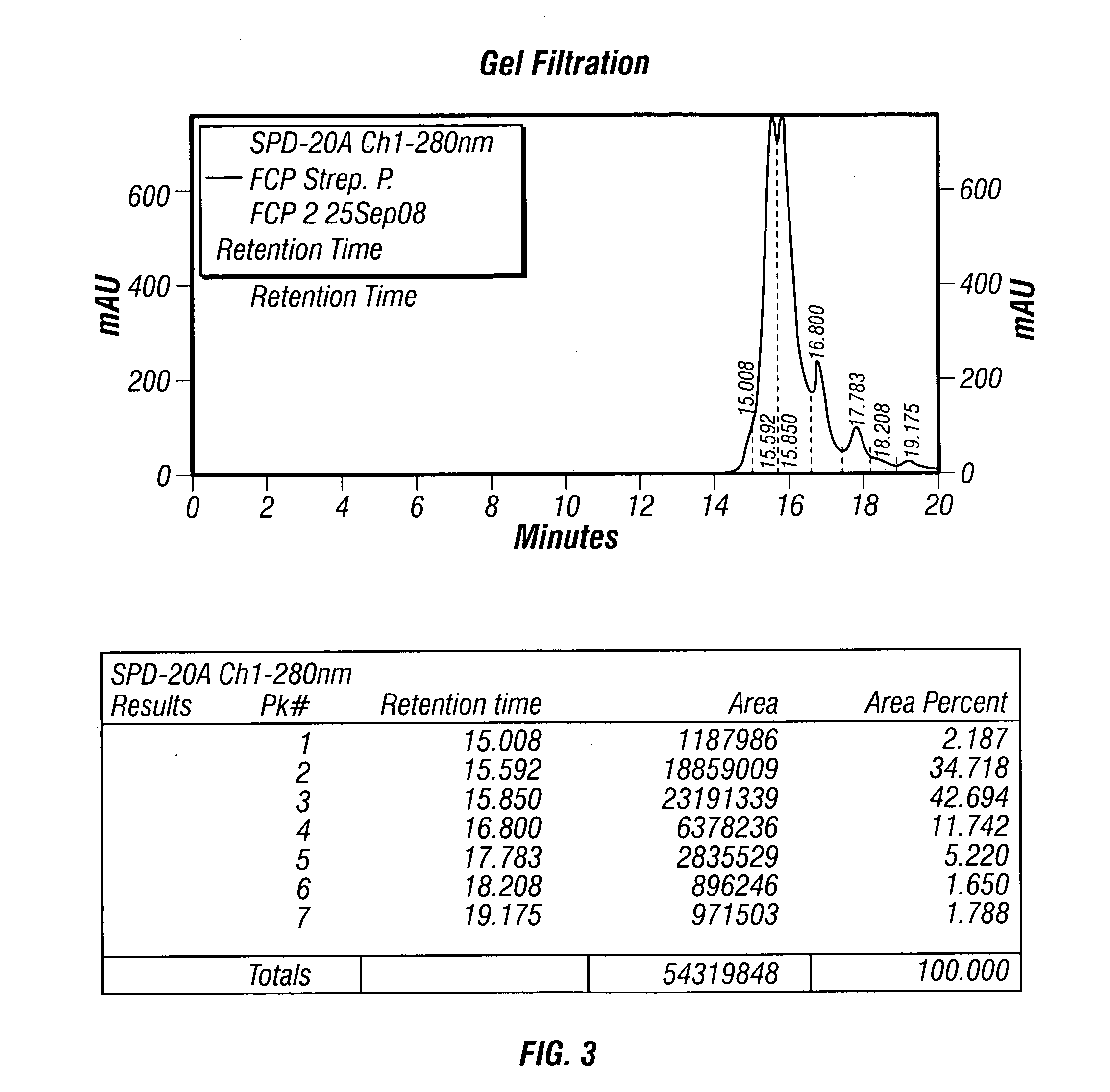
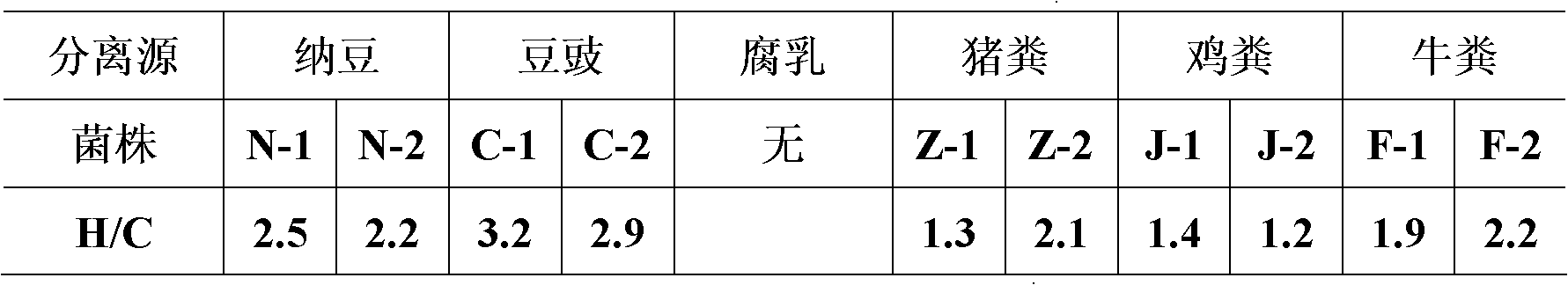
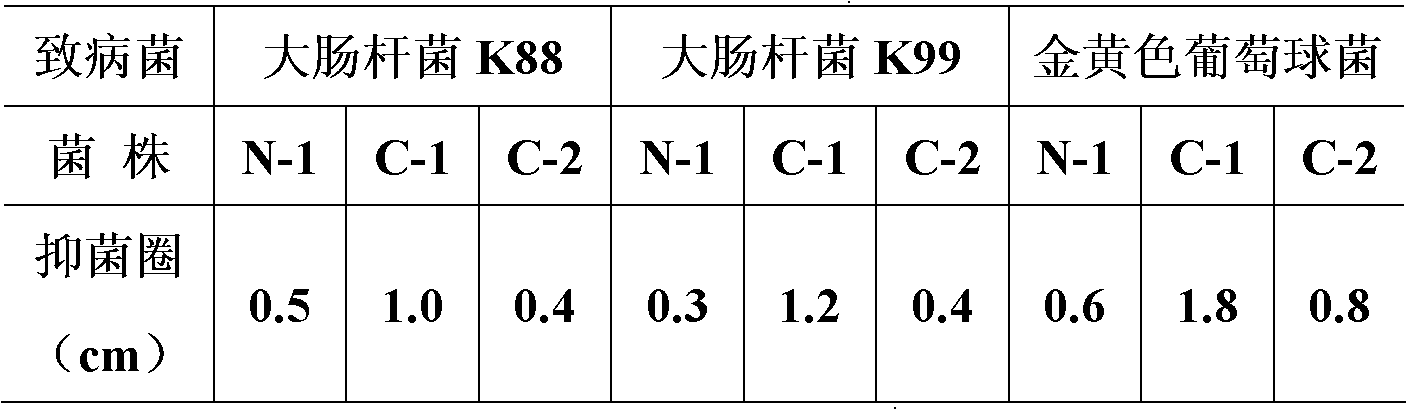
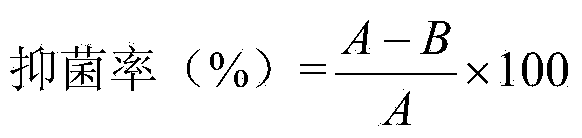Patents
Literature
6343 results about "Staphylococcus aureus bacteria" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
In medical literature, the bacterium is often referred to as S. aureus, Staph aureus or Staph a.. S. aureus appears as staphylococci (grape-like clusters) when viewed through a microscope, and has large, round, golden-yellow colonies, often with hemolysis, when grown on blood agar plates.
Collagen binding protein compositions and methods of use
InactiveUS6288214B1Prevent and lessen adhesionReduce adhesionAntibacterial agentsPeptide/protein ingredientsPassive ImmunizationsCarrier protein
Disclosed are the cna gene and cna-derived nucleic acid segments from Staphylococcus aureus, and DNA segments encoding cna from related bacteria. Also disclosed are Col binding protein (CBP) compositions and methods of use. The CBP protein and antigenic epitopes derived therefrom are contemplated for use in the treatment of pathological infections, and in particular, for use in the prevention of bacterial adhesion to Col. DNA segments encoding these proteins and anti-(Col binding protein) antibodies will also be of use in various screening, diagnostic and therapeutic applications including active and passive immunization and methods for the prevention of bacterial colonization in an animal such as a human. These DNA segments and the peptides derived therefrom are contemplated for use in the preparation of vaccines and, also, for use as carrier proteins in vaccine formulations, and in the formulation of compositions for use in the prevention of S. aureus infection.
Owner:TEXAS A&M UNIVERSITY
Nanosilver-containing antibacterial and antifungal granules and methods for preparing and using the same
InactiveUS6379712B1Improve solubilityPreventing mold build-upPowder deliveryOrganic active ingredientsEscherichia coliDisease
The present invention relates to nanosilver-containing antibacterial and antifungal granules ("NAGs"). The NAGs have longlasting inhibitory effect on a broad-spectrum of bacteria and fungi, which include, but are not limited to, Escherichia coli, Methicillin resistant Staphylococcus aureus, Chlamydia trachomatis, Providencia stuartii, Vibrio vulnificus, Pneumobacillus, Nitrate-negative bacillus, Staphylococcus aureus, Candida albicans, Bacillus cloacae, Bacillus allantoides, Morgan's bacillus (Salmonella morgani), Pseudomonas maltophila, Pseudomonas aeruginosa, Neisseria gonorrhoeae, Bacillus subtilis, Bacillus foecalis alkaligenes, Streptococcus hemolyticus B, Citrobacter, and Salmonella paratyphi C. The NAGs contain ground stalk marrow of the plant Juncus effusus L. which has been dispersed with nanosilver particles. The nanosilver particles are about 1-100 nm in diameter. Each of the nanosilver particles contain a metallic silver core which is surrounded by silver oxide. The present invention also provides a process for making the NAGs. The NAGs can be used in a variety of healthcare and industrial products. Examples of the healthcare products include, but are not limited to, ointments or lotions to treat skin trauma, soaking solutions or cleansing solutions for dental or women hygiene, medications for treating gastrointestinal bacteria infections, sexual related diseases, and eye diseases. Examples of industrial products include, but are not limited to, food preservatives, water disinfectants, paper disinfectants, construction filling materials (to prevent mold formation).
Owner:LEGEND WIN FINANCE
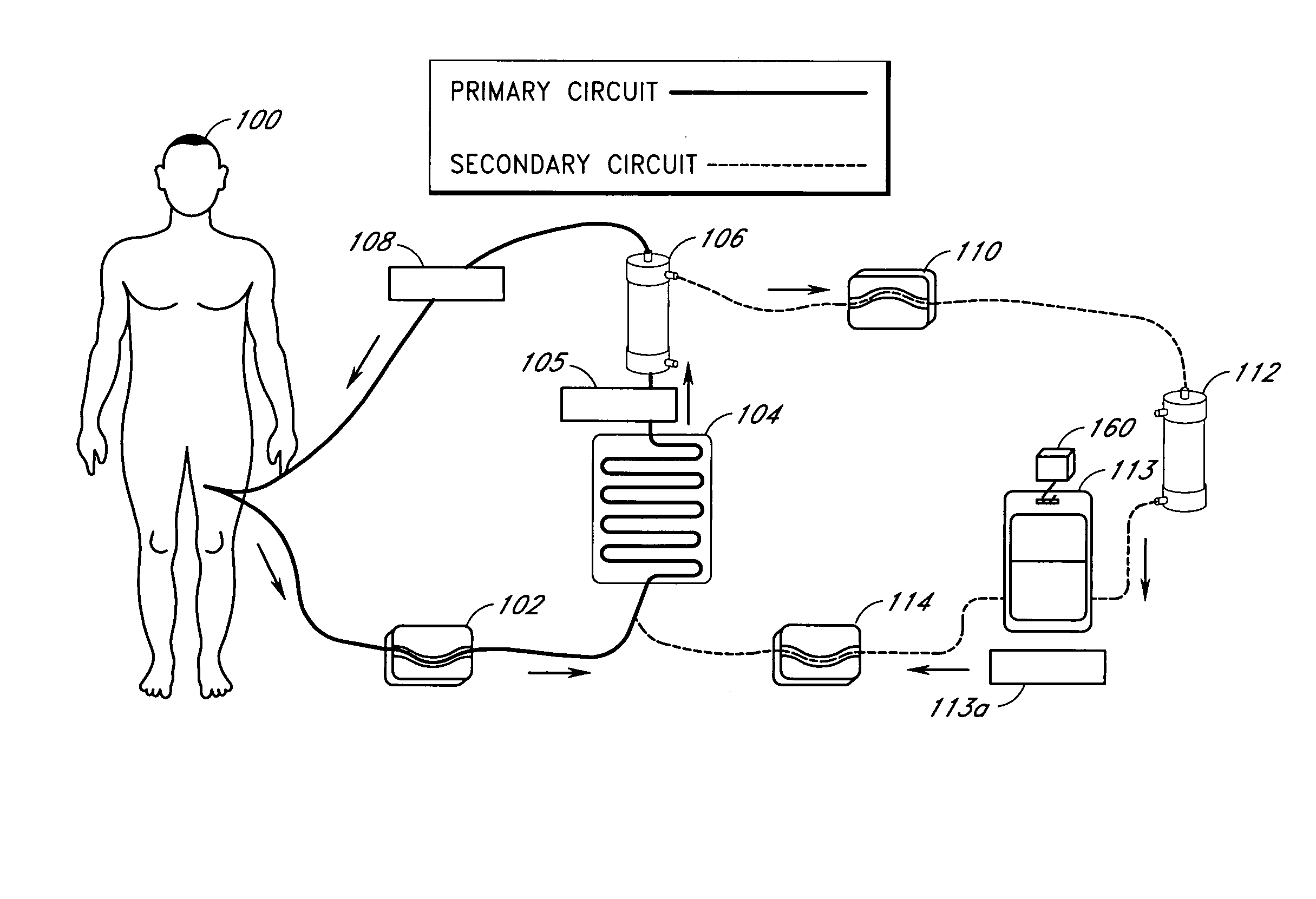
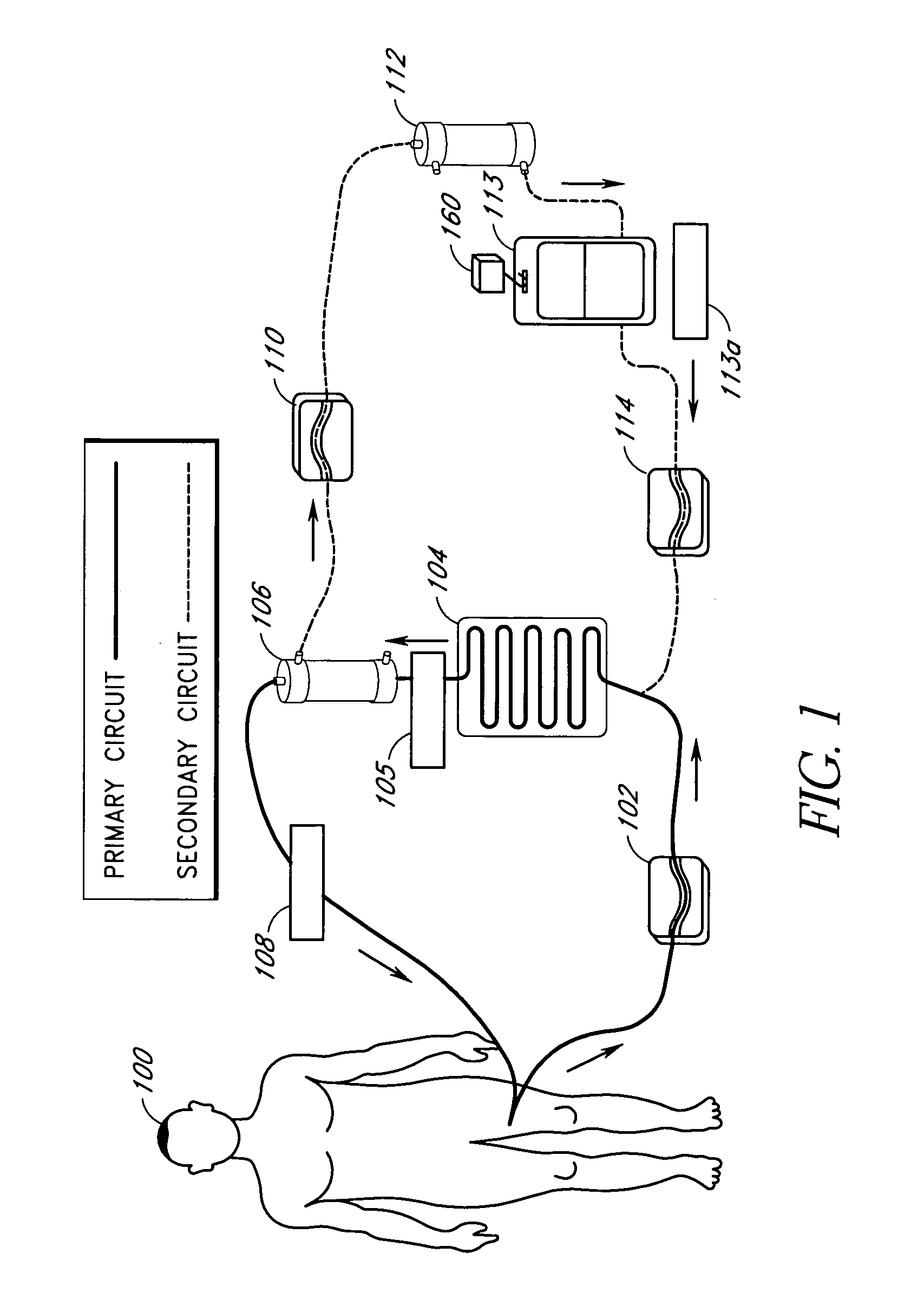
Septicemia prevention and treatment system
InactiveUS6193681B1Electrolysis componentsOther blood circulation devicesStaphylococcus cohniiFiltration
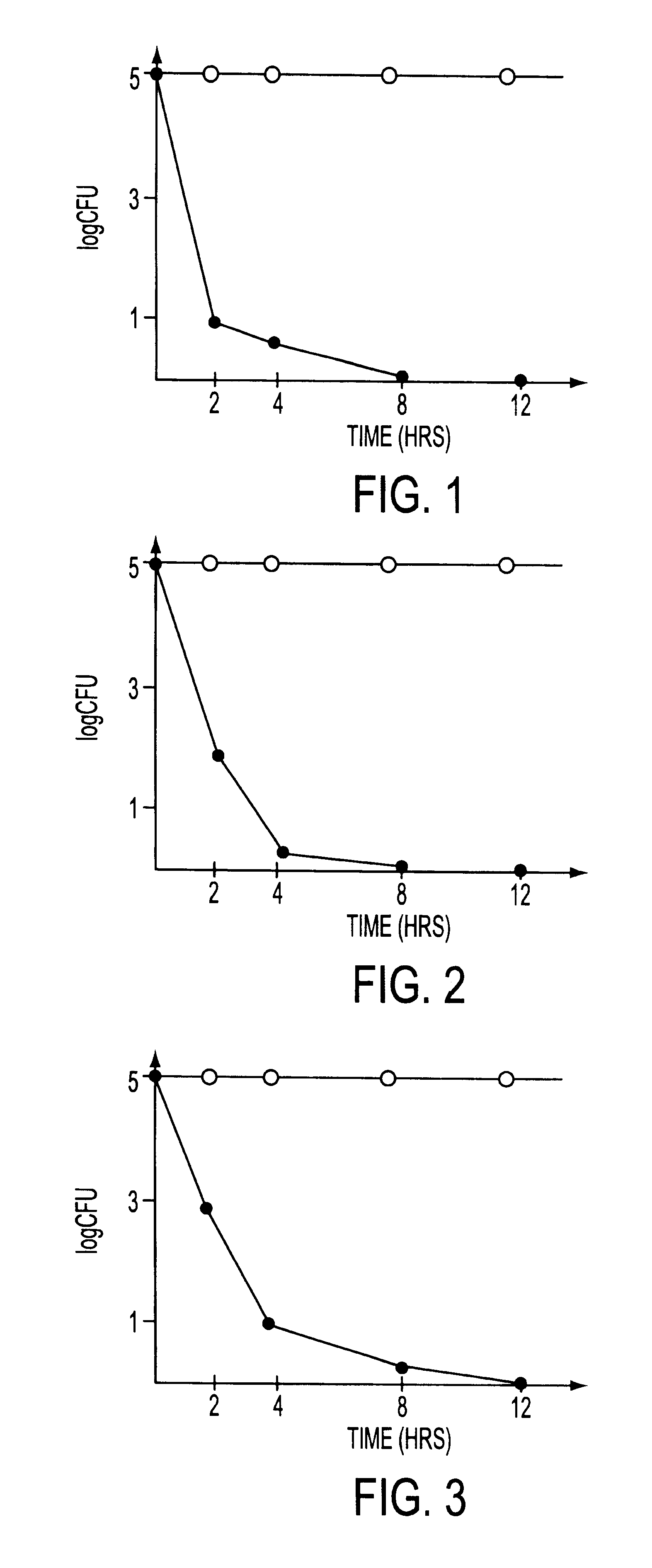
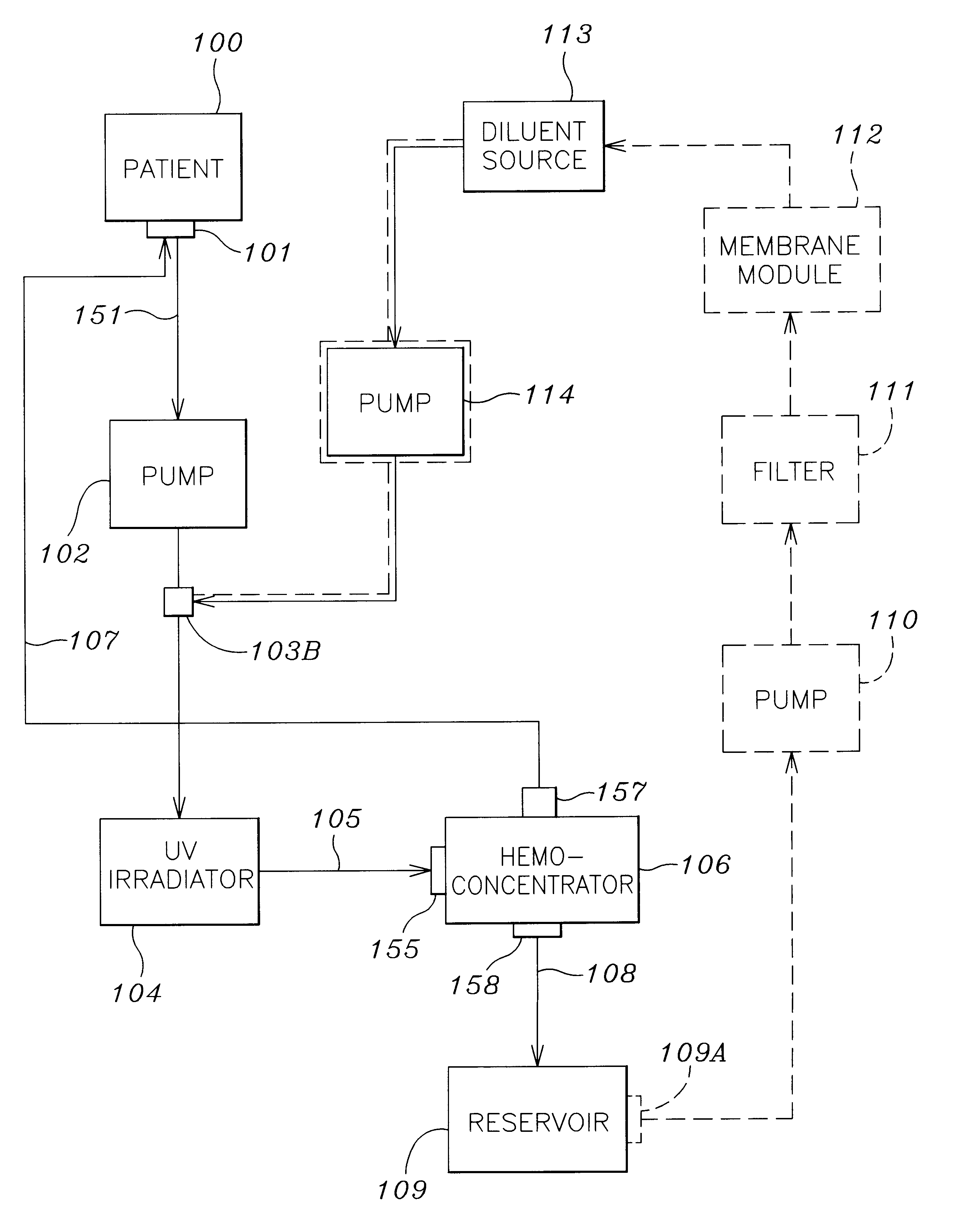
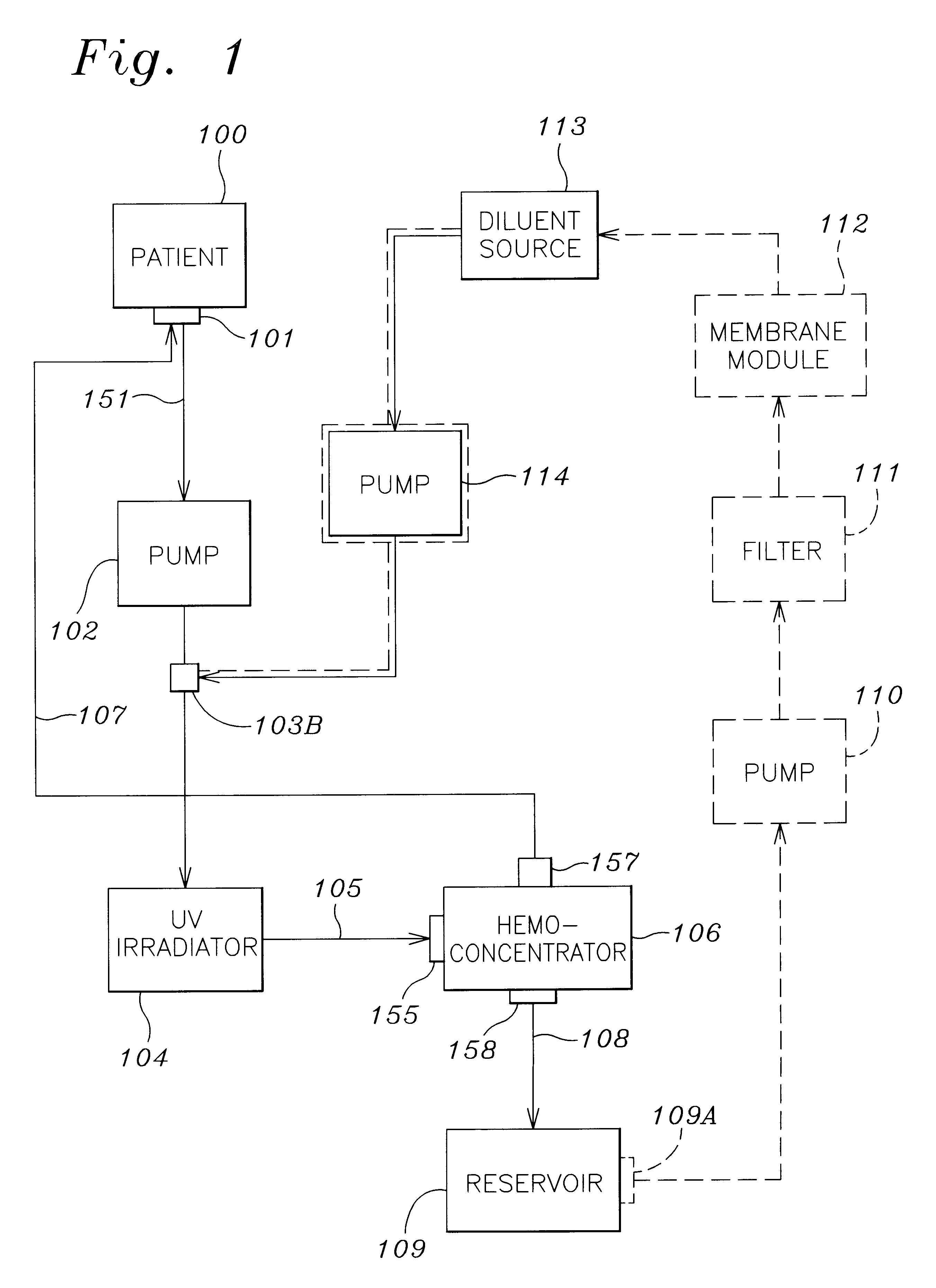
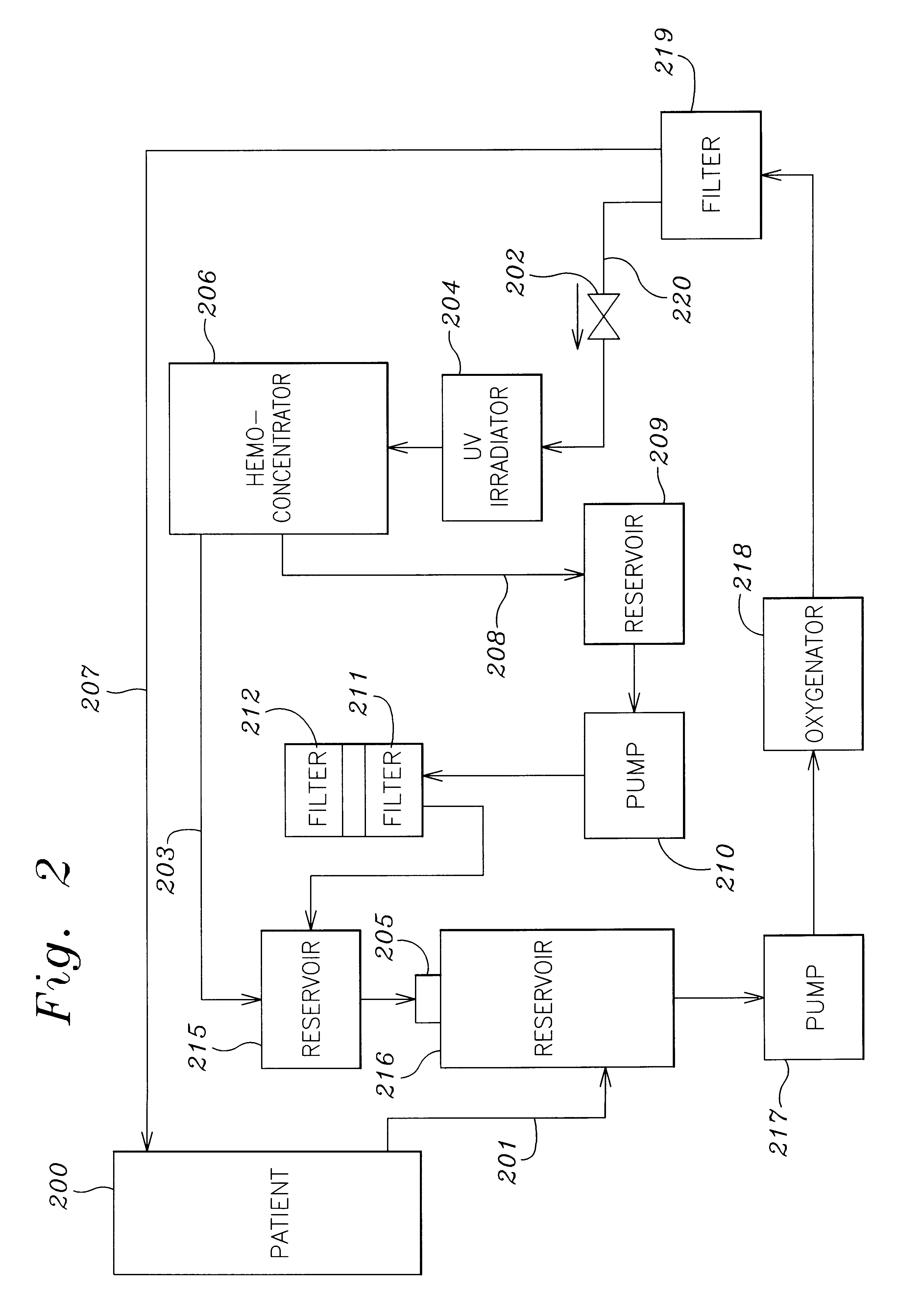
A method and apparatus for preventing and treating septicemia in patient blood. The extracorporeal system includes an anti-microbial device to kill at least 99% of bloodborne microorganisms, a hemoconcentrator / filtration unit to remove approximately 90% of target molecules from the patient blood and a filter unit to remove target molecules from patient blood from the sieved plasma filtrate. Target molecules are produced by microorganisms as well as the patient's cells and include endotoxins from gram negative bacteria, exotoxins from gram negative and gram positive bacteria, as well as RAP protein mediator from Staphylococcus aureus, and cell mediators such as tumor necrosis factor-alpha, and interleukin 1-beta, complement proteins C3a and C5a, and brandykinin.
Owner:HEMAVATION
Compositions and methods for characterizing and restoring gastrointestinal, skin, and nasal microbiota
ActiveUS20100074872A1Growth inhibitionFacilitate calorie uptakeBiocideMetabolism disorderBacteroidesDisease
The present invention relates to characterizing changes in mammalian bacterial gastrointestinal, cutaneous and nasal microbiota associated with antibiotic treatment and various disease conditions (such as asthma, allergy, obesity, metabolic syndrome, gastrointestinal reflux disease (GERD), eosinophilic esophagitis, gastro-esophageal junction adenocarcinomas (GEJAC), infections due to bacteria that are resistant to antibiotics, including Methicillin-resistant Staphylococcus aureus (MRSA), Clostridium difficile, vancomycin-resistant enterococci, etc.) and related diagnostic and therapeutic methods. Therapeutic methods of the invention involve the use of live bacterial inoculants that are capable of restoring healthy mammalian bacterial gastrointestinal, skin, and nasal microbiota.
Owner:NEW YORK UNIV
Composition for driving and killing mosquitoes and preparation method thereof
The invention relates to a composition for driving and killing mosquitoes. The composition is characterized by comprising the following substances by portions: 20 to 50 portions of natural essential oil, 10 to 35 portions of pyrethroid, 25 to 50 portions of solvent oil, 3 to 20 portions of surface active agent and 5 to 15 portions of antioxidant. The composition has the advantages of: (1) having no toxic side effect to body, having multiple action of driving mosquitoes, sterilizing and essence, being capable of reducing the cost of the product and bringing pleasant feeling to a user; (2) having different effective components with different action mechanism for target insects and also being capable of effectively retarding the drug resistance thereof; (3) having higher safety than chemosynthetic insecticide due to easy decomposition and low residue of the natural essential oil in natural environment; (4) having obvious control effect to mosquitoes, flies and cockroaches and being capable of effectively reducing the using amount of pyrethroid due to adding of the natural essential oil; and (5) having a certain action of restraining and killing various harmful bacterium such as golden staphylococcus aureus, shigella shigae and Pseudomonas aeruginosa due to adding of natural vegetable garlic oil.
Owner:江西山峰日化有限公司
Nanosilver-containing antibacterial and antifungal granules and methods for preparing and using the same
The present invention relates to nanosilver-containing antibacterial and antifungal granules ("NAGs"). The NAGs have longlasting inhibitory effect on a broad-spectrum of bacteria and fungi, which include, but are not limited to, Escherichia coli, Methicillin resistant Staphylococcus aureus, Chlamydia trachomatis, Providencia stuartii, Vibrio vulnificus, Pneumobacillus, Nitrate-negative bacillus, Staphylococcus aureus, Candida albicans, Bacillus cloacae, Bacillus allantoides, Morgan's bacillus (Salmonella morgani), Pseudomonas maltophila, Pseudomonas aeruginosa, Neisseria gonorrhoeae, Bacillus subtilis, Bacillus foecalis alkaligenes, Streptococcus hemolyticus B, Citrobacter, and Salmonella paratyphi C. The NAGs contain ground stalk marrow of the plant Juncus effuses L. which has been dispersed with nanosilver particles. The nanosilver particles are about 1-100 mn in diameter. Each of the nanosilver particles contain a metallic silver core which is surrounded by silver oxide. The present invention also provides a process for making the NAGs. The NAGs can be used in a variety of healthcare and industrial products. Examples of the healthcare products include, but are not limited to, ointments or lotions to treat skin trauma, soaking solutions or cleansing solutions for dental or women hygiene, medications for treating gastrointestinal bacteria infections, sexual related diseases, and eye diseases. Examples of industrial products include, but are not limited to, food preservatives, water disinfectants, paper disinfectants, construction filling materials (to prevent mold formation).
Owner:LEGEND WIN FINANCE
Enterococcus faecium ANSE228 and application thereof
ActiveCN102031235AIncrease production capacityReduce the death rateAntibacterial agentsBacteriaEscherichia coliStaphylococcus cohnii
The invention provides an Enterococcus faecium ANSE228 of which the collection number is CGMCC No.4082. The invention also provides application of the Enterococcus faecium ANSE228 to inhibition of salmonella pullorum and / or Escherichia coli and / or Staphylococcus aureus. The Enterococcus faecium ANSE228 is obtained by processes of repeated separation, purification, rejuvenation and the like, and has high biological activity, obvious probiotic property, high adversity resistance and the like. The invention also provides a microecological agent which contains the Enterococcus faecium ANSE228. When the microecological agent is added into drinking water and / or feeds for breeding animals, the Enterococcus faecium ANSE228 can be quickly activated and reproduced and a dominant beneficial flora can be formed after the Enterococcus faecium ANSE228 is fed into intestinal canals of the animals, and the Enterococcus faecium ANSE228 has the effects of reducing a harmful flora in the intestinal canals, adjusting microecological balance of the intestinal canals, substituting for medicaments such as antibiotic and the like, and improving weight increment of the animals and the utilization rate of the feeds.
Owner:科润生科技发展有限公司
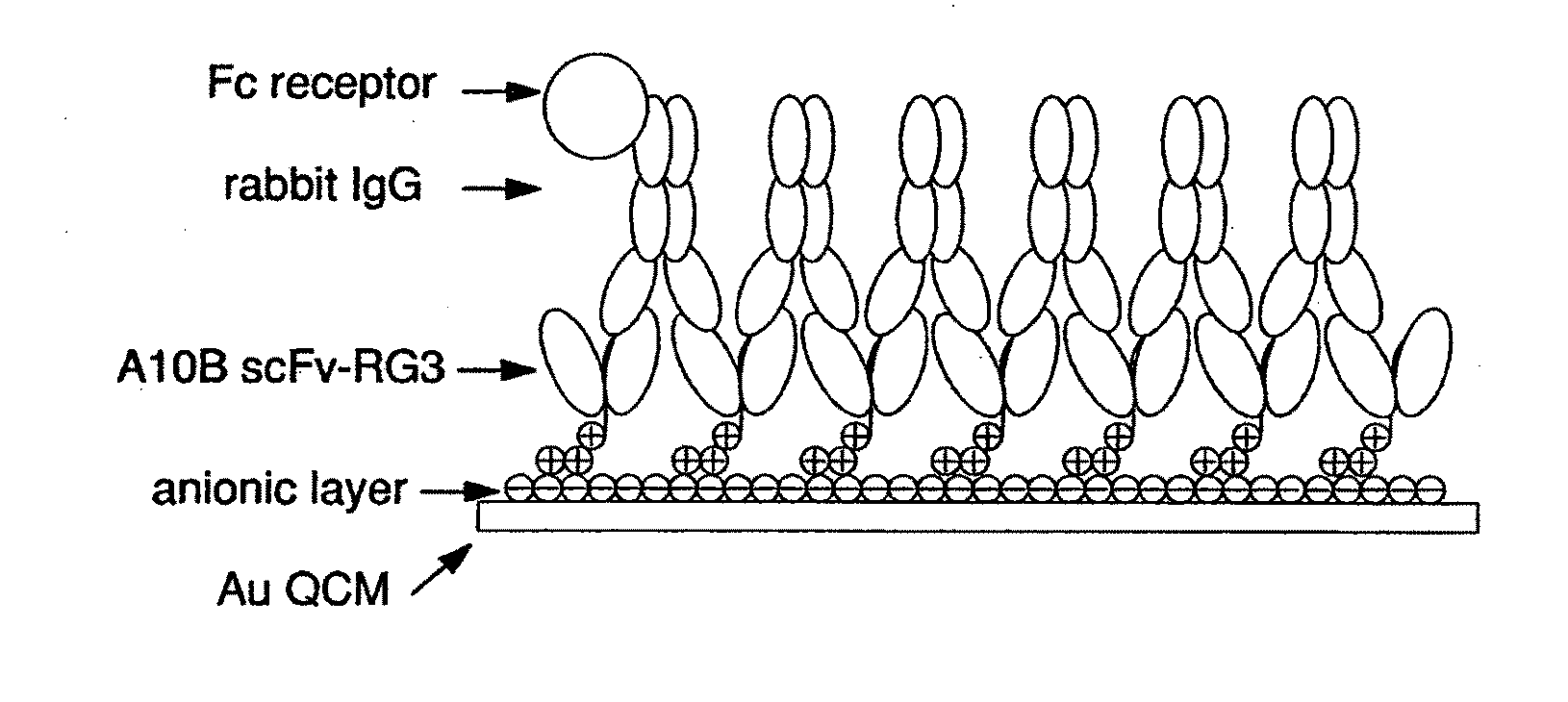
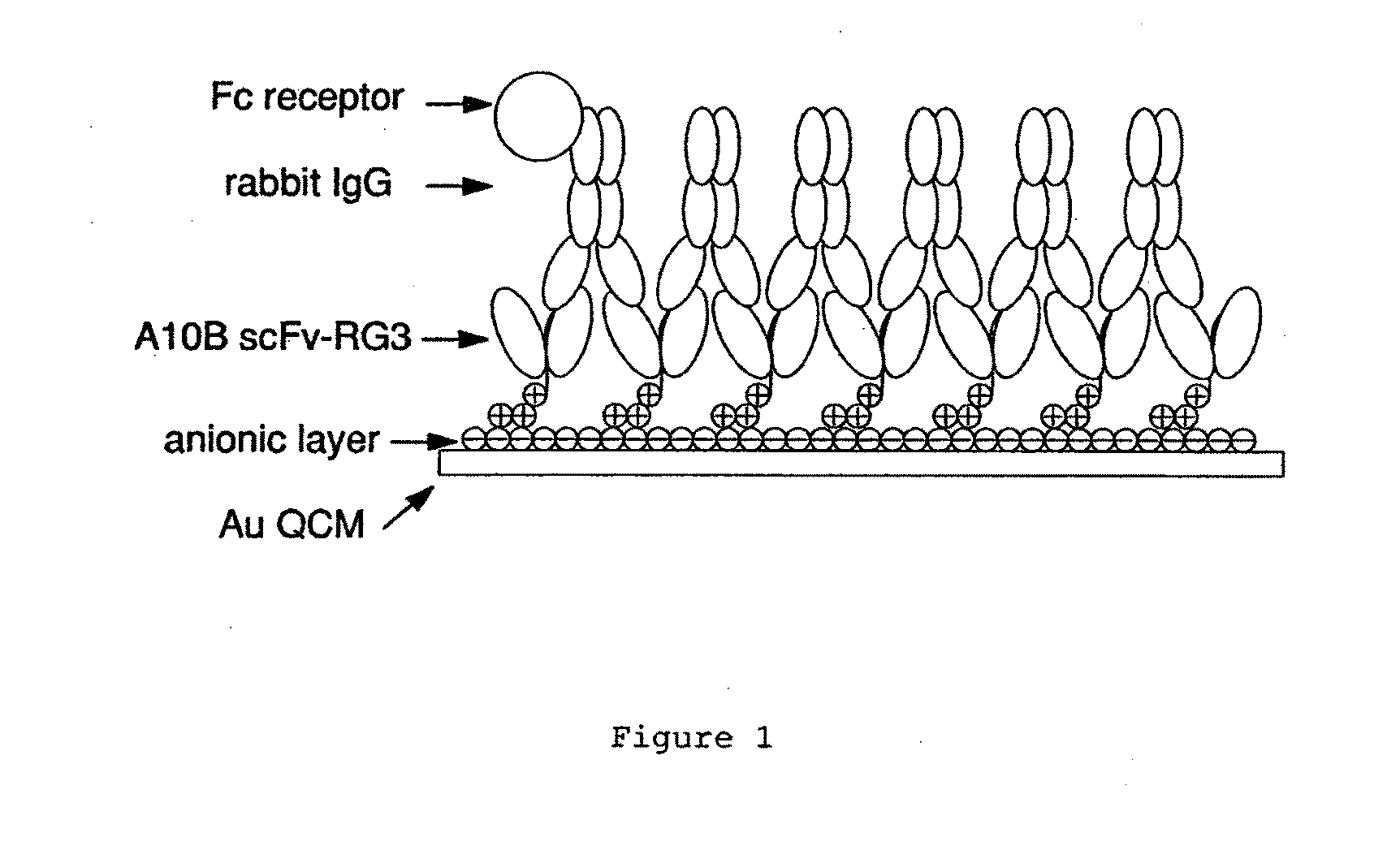
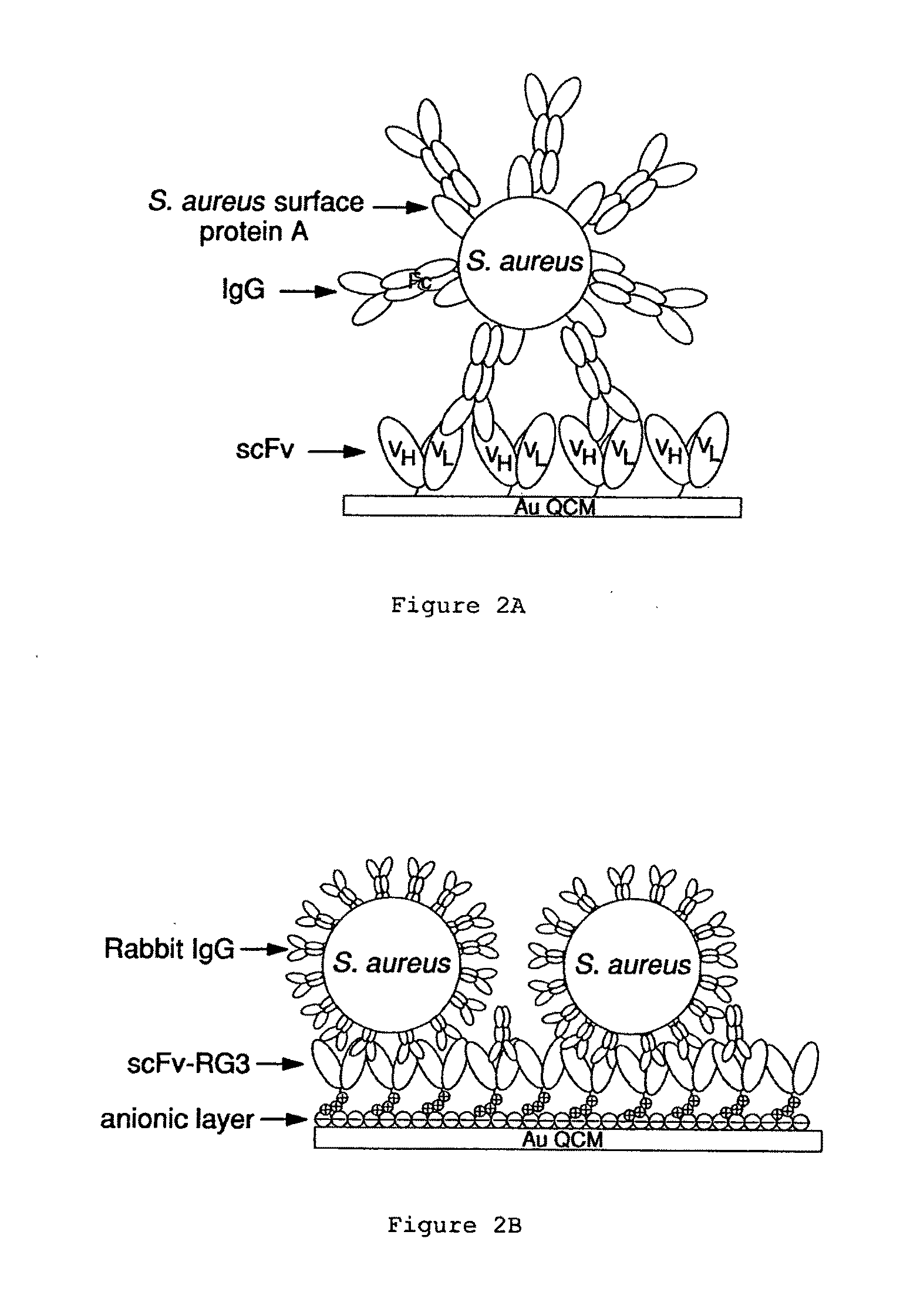
Immunosensors: scFv-linker design for surface immobilization
InactiveUS20110201032A1Bioreactor/fermenter combinationsMaterial nanotechnologySingle-Chain AntibodiesSide chain
An apparatus and methods for binding an analyte of interest in a sample are provided. The apparatus comprises a substrate with an exposed surface with an compound, that is electrostatically charged or capable of forming hydrogen bonds, provided bound to the solid substrate. A recombinant single chain antibody (scFv) molecule specific for the analyte of interest, having one or more amino acids with charged or hydrogen-bond forming sidechains in a linker polypeptide portion, is bound to the layer on the solid substrate. When the analyte of interest is present in the sample the scFv binds the analyte to the solid substrate. The apparatus can be used with an immunoglobulin layer to detect Fc receptors, so as to detect microorganisms such as Staphylococcus aureus having protein A or protein G.
Owner:OAKLAND UNIVESITY +1
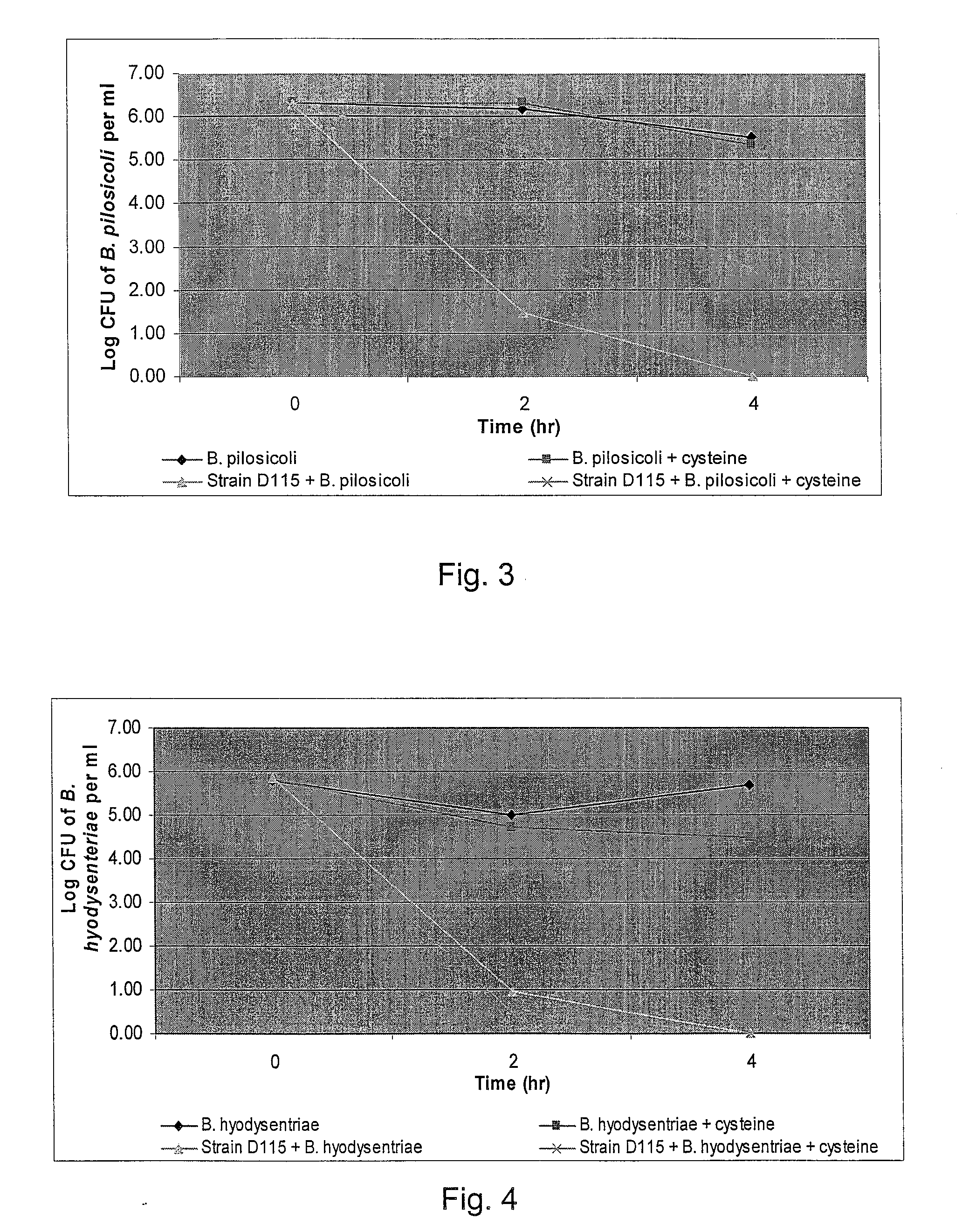
Broad-Spectrum Antibacterial and Antifungal Activity of Lactobacillus Johnsonii D115
The present invention demonstrated the potential use of Lactobacillus johnsonii D115 as a probiotic, as a prophylactic agent or as a surface treatment of materials against human and animal pathogens such as Brachyspira pilosicoli, Brachyspira hyodysenteriae, Shigella sonnei, Vibrio cholera, Vibrio parahaemolyticus, Campylobacter jejuni, Streptococcus pneumoniae, Enterococcus faecalis, Enterococcus faecium, Clostridium perfringens, Yersinia enterocolitica, Escherichia coli, Klebbsiella pneumoniae, Staphylococcus aureus, Salmonella spp., Bacillus cereus, Aspergillus niger and Fusarium chlamydosporum. The proteineous antimicrobial compound was partially characterized and found to be heat tolerant up to 121° C. for 15 min, and acid tolerant up to pH1 for 30 min at 40° C. The compound is also stable to enzymatic digestion, being able to retain more than 60% antimicrobial activity when treated with pepsin and trypsin.
Owner:KEMIN IND INC
Anti-bacterial water-based paint and preparation method thereof
InactiveCN102702889AImprove aging resistanceImprove the pulverization performanceBiocideAntifouling/underwater paintsWater basedEscherichia coli
The invention relates to anti-bacterial water-based paint and a preparation method thereof. The paint comprises the following components in parts by weight: 0.2-11 parts of anti-bacterial agent, 8-33 parts of nano material, 23-64 parts of water-based resin dispersoid and 0.75-18 parts of adhesive resin or plasticizer. The preparation method comprises the following steps: firstly preparing a nano silver anti-bacterial agent; mixing deionized water, the anti-bacterial agent, a wetting agent, a dispersing agent and a defoaming agent and uniformly mixing, adding the nano material, uniformly dispersing to obtain the water-based dispersoid; adding the obtained water-based dispersoid to the mixed emulsion or water-based resin dispersoid, then adding the adhesive resin or plasticizer and various conventional assistants, stirring and dispersing evenly; adding pigments or colorant; and supplementing water to obtain the anti-bacterial water-based paint. The long-acting broad-spectrum antibacterial water-based paint has high fungicidal efficiency (more than 99%) on escherichia coli, staphylococcus aureus, black varietas of bacillus subtilis and the like and can reduce the high concentrate of organic matters of formaldehyde to the range of specified concentration index.
Owner:ANHUI JINDUN PAINT
Polysaccharide vaccine for staphylococcal infections
ActiveUS20050118198A1Improving immunogenicityAntibacterial agentsOrganic active ingredientsNatural sourceIntracellular
The invention relates to compositions of a deacetylated poly N-acetylated glucosamine (dPNAG) of Staphylococci. The dPNAG may be isolated from natural sources or synthesized de novo. The invention also relates to the use of dPNAG as a vaccine for inducing active immunity to infections caused by Staphylococcus aureus, S. epidermidis, other related coagulase-negative or coagulase-positive Staphylococci, and other organisms carrying the ica (intracellular adhesion) locus. The invention further provides methods of use for antibodies directed to dPNAG, particularly for inducing passive immunity to the same class of infections.
Owner:THE BRIGHAM & WOMEN S HOSPITAL INC
Broad-spectrum antibacterial bacillus amyloliquefaciens strain and application thereof
ActiveCN103173397ASimple cultivation conditionsPromote growthBiocideBacteriaBiotechnologyBacillus amylolyticus
The invention relates to bacillus amyloliquefaciens NCPSJ7 and further relates to an application of the strain in treating plant diseases, diseases before and after harvesting fruits and vegetables as well as in preventing food-borne pathogenic bacteria and putrefying bacteria. The strain is preserved in China Center for Type Culture Collection (CCTCC) on March 22, 2013, wherein the preservation number is CCTCC NO: M2013098 and the strain is named as bacillus amyloliquefaciens NCPSJ7. The thallus and fermentation liquor of the strain disclosed by the invention has the effects of treating plant diseases caused by plant pathogenic fungus including antagonistic peach root rotten disease, fusarium wilt of cucumber, botrytis cinerea, jujube anthracnose, pear black spot, pear blue mould, apple brown rot, apple altermaria leaf spot, watermelon fusarium wilt and the like, as well as diseases after harvesting fruits and vegetables and food-borne pathogenic bacteria and putrefying bacteria including antagonistic staphylococcus aureus, salmonella paratyphi A, vibrio parahaemolyticus, yeast and the like; moreover, the bacillus amyloliquefaciens is broad in spectrum and antibacterial and great in potential in developing novel, efficient and natural biological control and biological preservative and fresh-keeping preparation.
Owner:INST OF AGRO FOOD SCI & TECH SHANDONG ACAD OF AGRI SCI
Sequences for detection and identification of methicillin-resistant Staphylococcus aureus (MRSA)
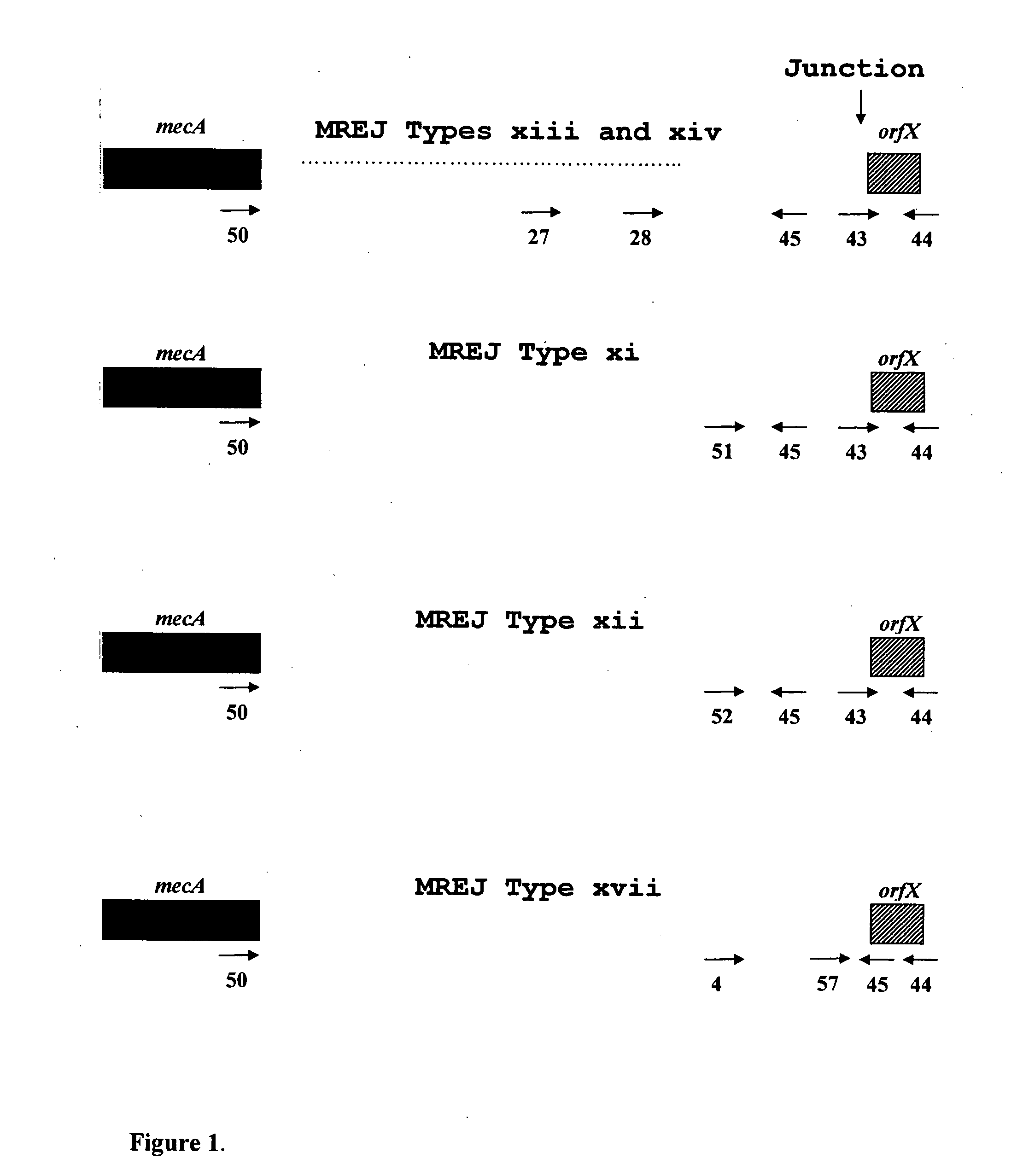
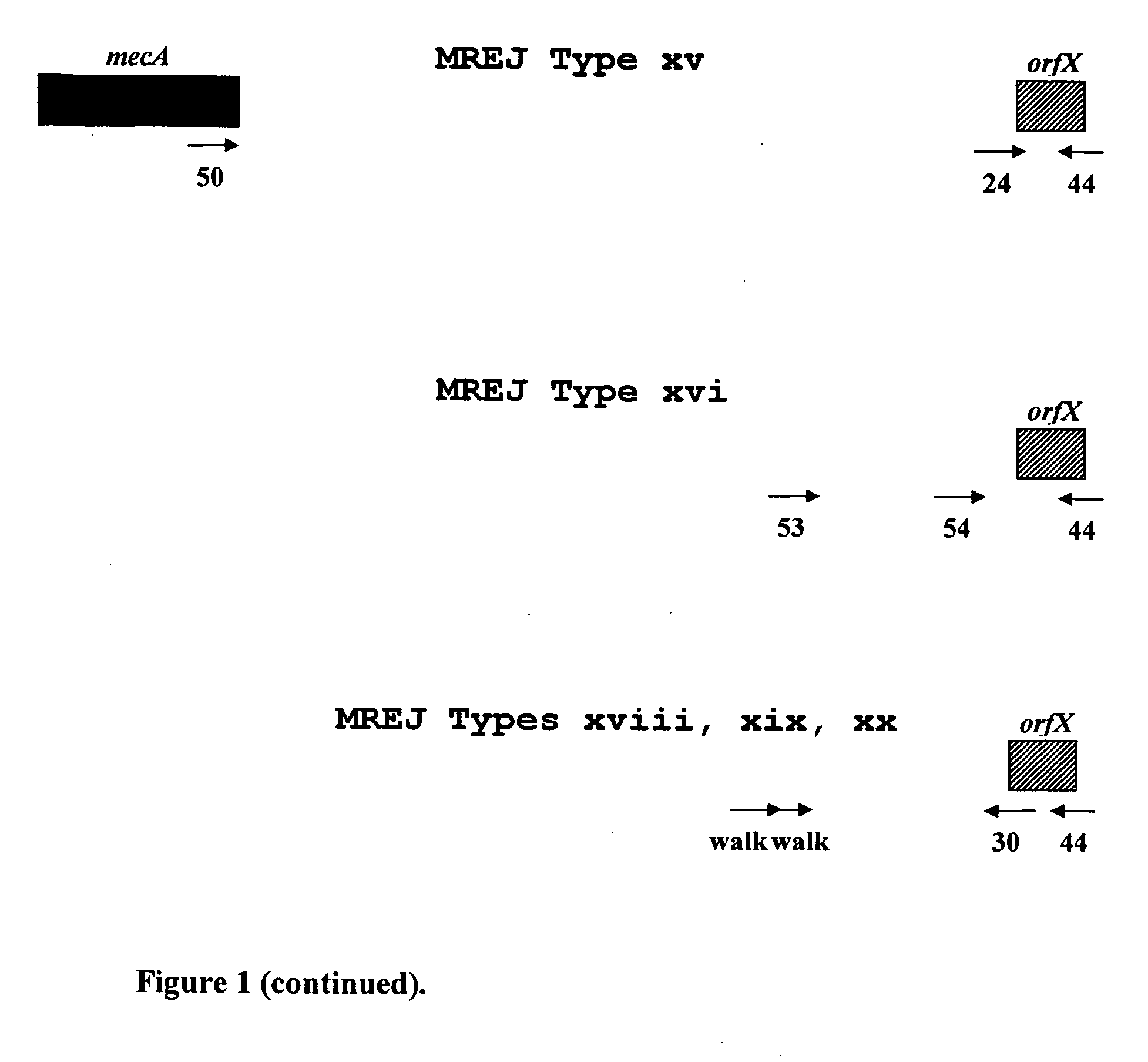
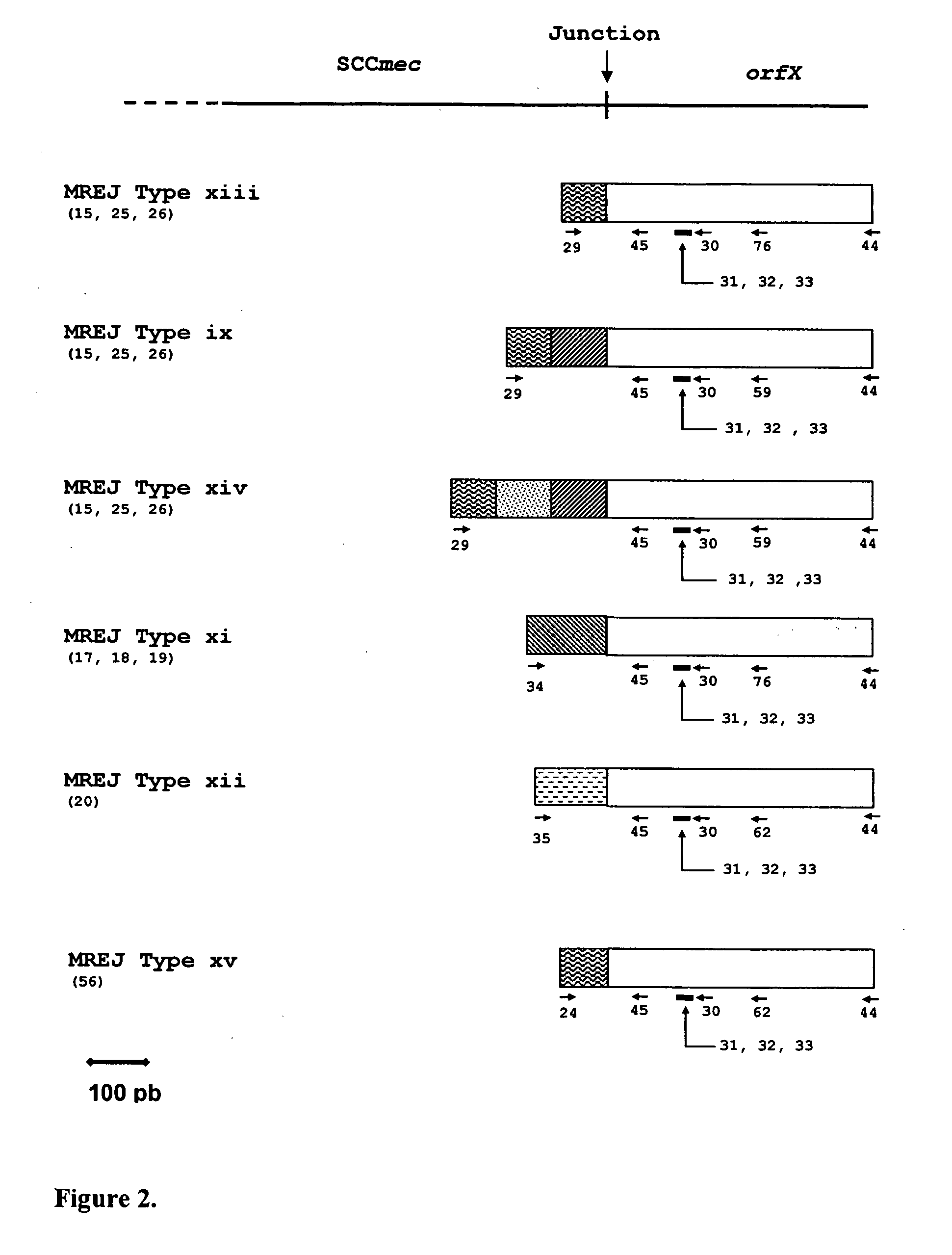
Described herein are novel SCCmec right extremity junction (MREJ) sequences for the detection and / or identification of methicillin-resistant Staphylococcus aureus (MRSA). Disclosed are methods and compositions based on DNA sequences for the specific detection of MREJ sequences designated types xi, xii, xiii, xiv, xv, xvi, xvii, xviii, xix, and xx for diagnostic purposes and / or epidemiological typing.
Owner:GENEOHM SCI INC
Chromatography matrices including novel staphylococcus aureus protein a based ligands
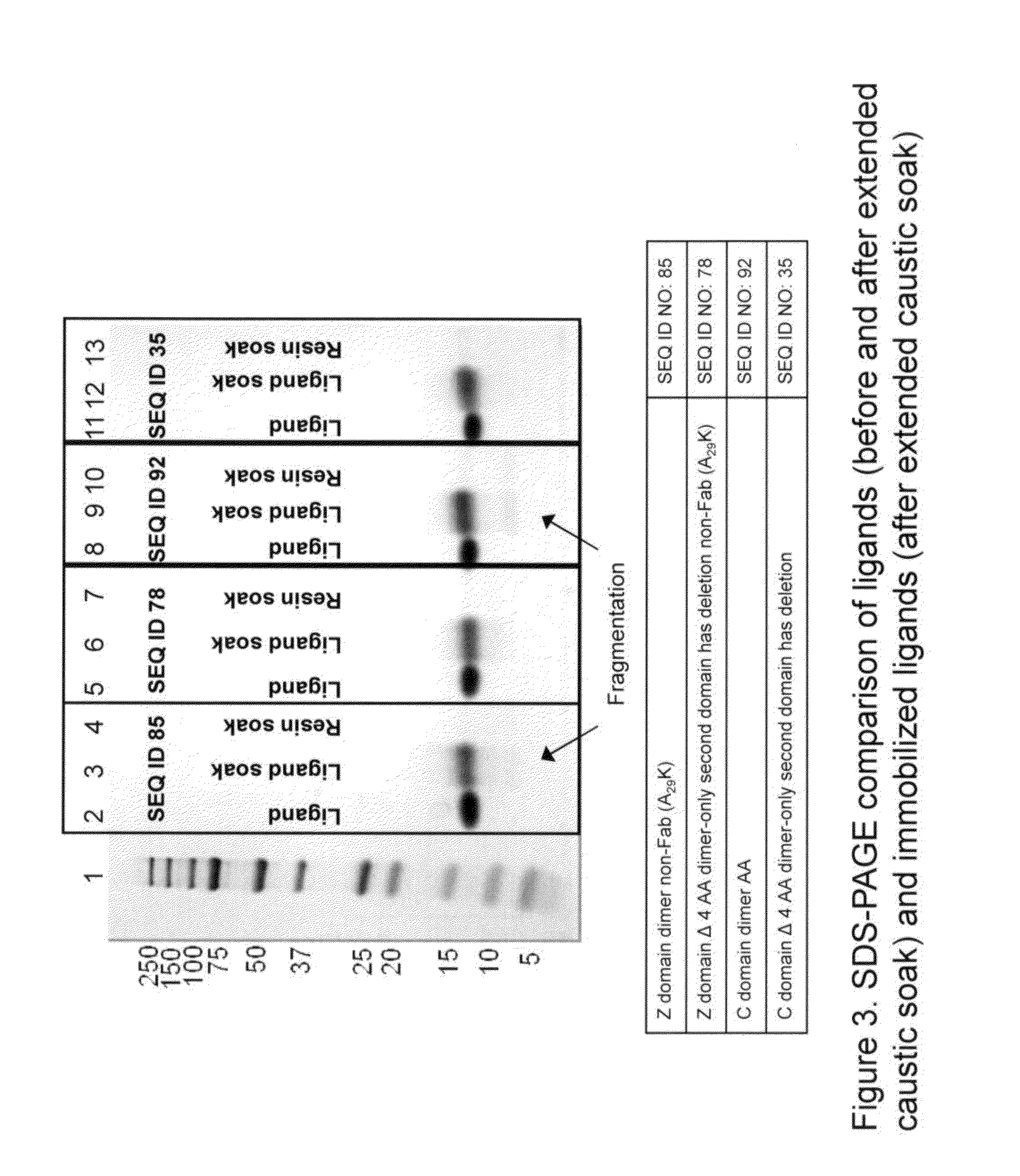
ActiveUS20130046056A1Reduce lossesLarge degree of fragmentationSolid sorbent liquid separationPeptide preparation methodsStaphylococcus aureusStaphylococcus aureus protein A
The present invention relates to chromatography matrices including ligands based on one or more domains of immunoglobulin-binding proteins such as, Staphylococcus aureus Protein A (SpA), as well as methods of using the same.
Owner:MILLIPORE CORP
Production process of high-quality ecological antibacterial health-care sock
ActiveCN103815555AGood health effectImprove antibacterial propertiesLiquid/gas/vapor article treatmentPanty-hoseYarnEscherichia coli
The invention discloses a production process of a high-quality ecological antibacterial health-care sock. The production process of the high-quality ecological antibacterial health-care sock comprises the following steps of yarn manufacturing, sock weaving, seam allowance processing, reinforcing, setting, pre-drying, water bathing, drying and package detection. The production process of the high-quality ecological antibacterial health-care sock adopts the method that natural cotton fibers, aloe fibers and modal fibers are interlaced and makes full use of the good antibacterial effect of the aloe fibers, and the bottom of the sock adopts a flat structure, so that the fabric can meet the requirements for high air permeability and comfort. The aloe fibers are used for replacing traditional common viscose, and aloe isocitric acid calcium and other matter have the functions of improving the constitution, strengthening the heart, promoting blood circulation, softening hardened arteries, lowering the cholesterol content and expanding the blood capillaries, and have a certain inhibition effect on escherichia coli and staphylococcus aureus. Compared with the prior art, the high-quality ecological antibacterial health-care sock has good health-care performance and antibacterial performance.
Owner:浙江丰悦针纺有限公司
Device and method for reducing inflammatory mediators in blood
InactiveUS7201730B2Reducing free radicals in a patient's bloodReduce concentrationSemi-permeable membranesSolvent extractionInterleukin 6Staphylococcus cohnii
A method and apparatus for preventing and treating septicemia in patient blood is provided. The extracorporeal system includes an antimicrobial device to inactivate at least 99% of bloodborne microorganisms, a hemoconcentrator / filtration unit to remove approximately 50–75% of target molecules from the patient blood and a filter unit to remove target molecules from patient blood from the sieved plasma filtrate. Target molecules are produced by microorganisms, as well as by the patient's cells. These molecules include endotoxins from Gram negative bacteria, exotoxins from Gram negative and Gram positive bacteria, as well as RAP protein mediator from Staphylococcus aureus, and cell mediators such as tumor necrosis factor-alpha, and interleukin 1-beta, interleukin 6, complement proteins C3a and C5a, and bradykinin.
Owner:HEMAVATION
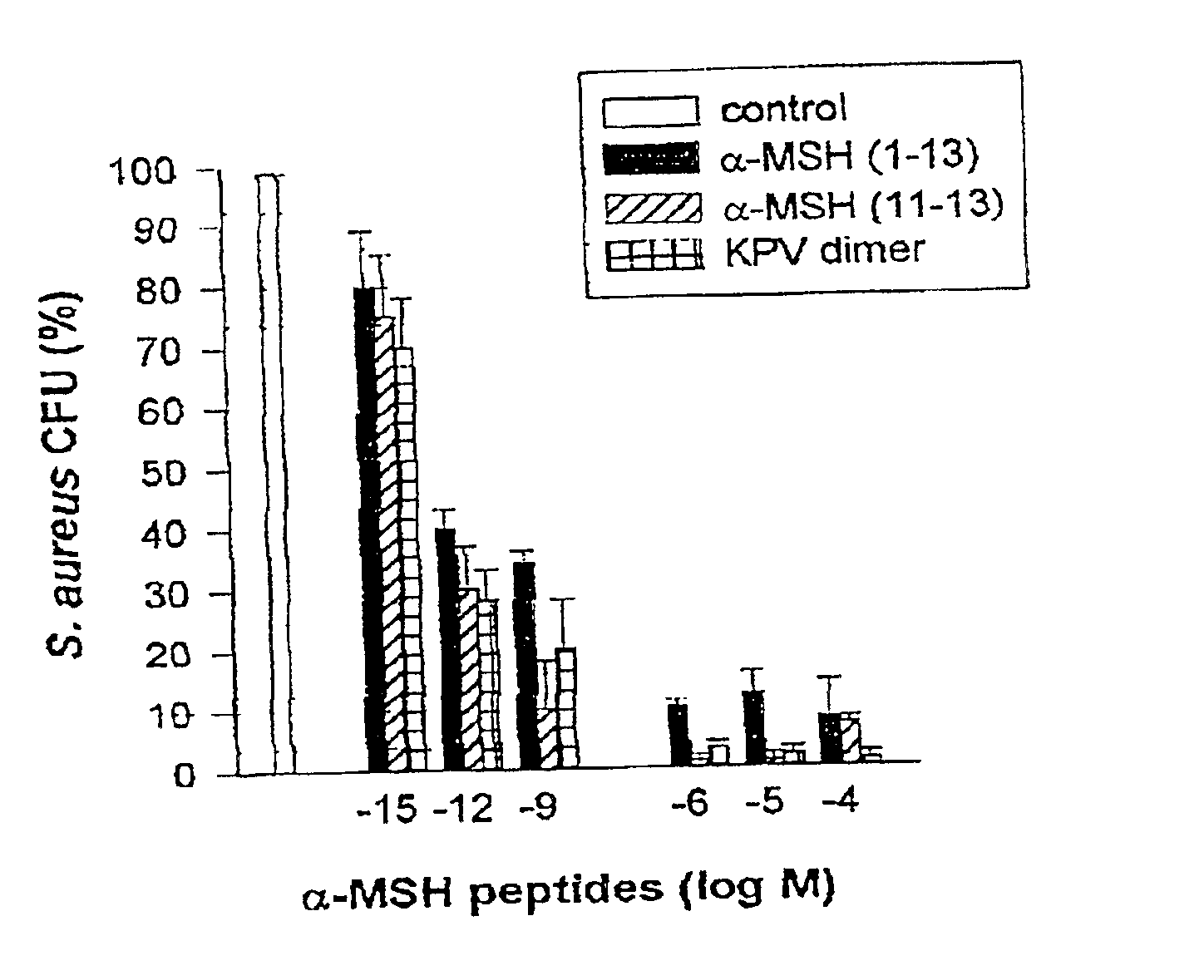
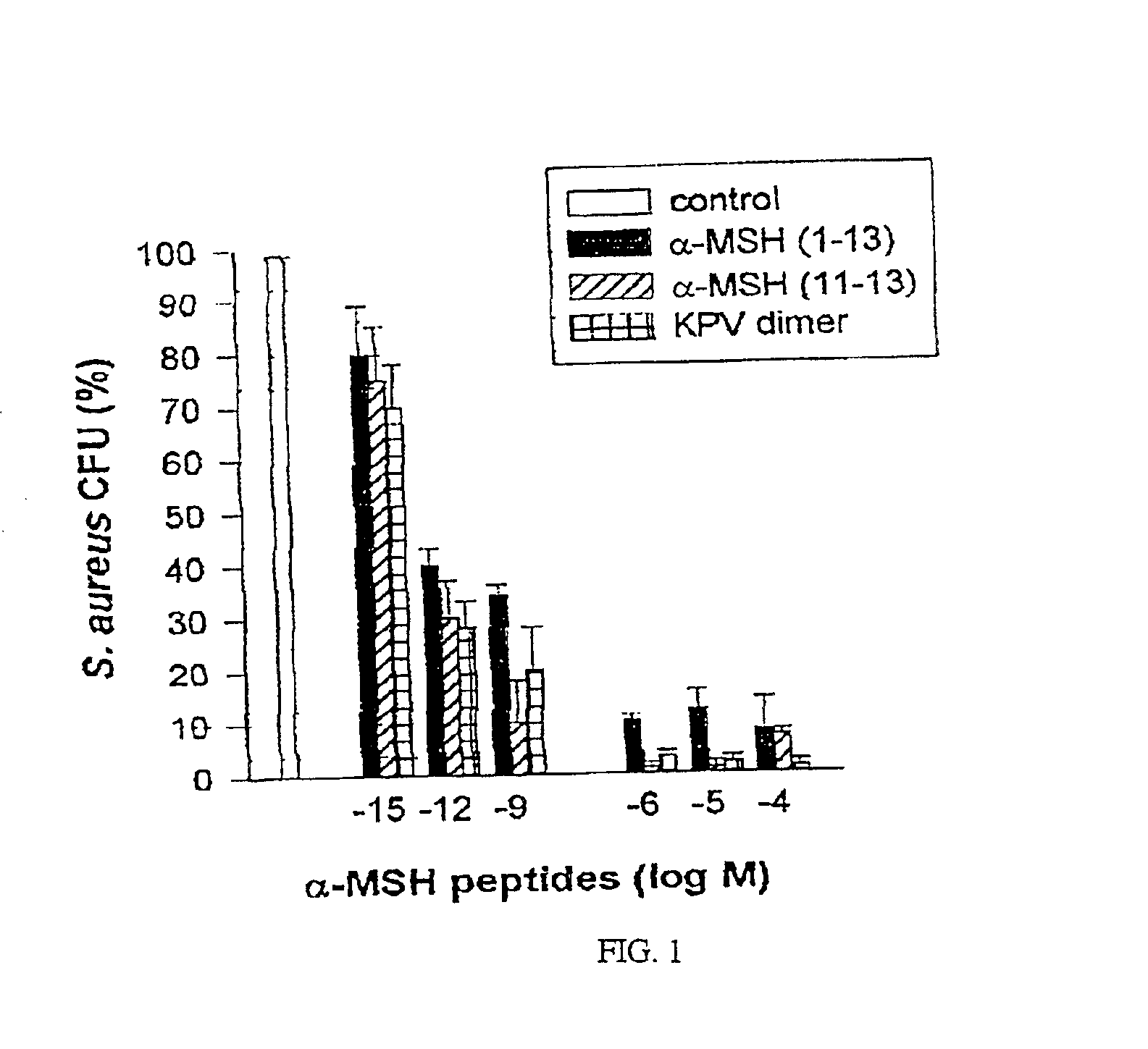
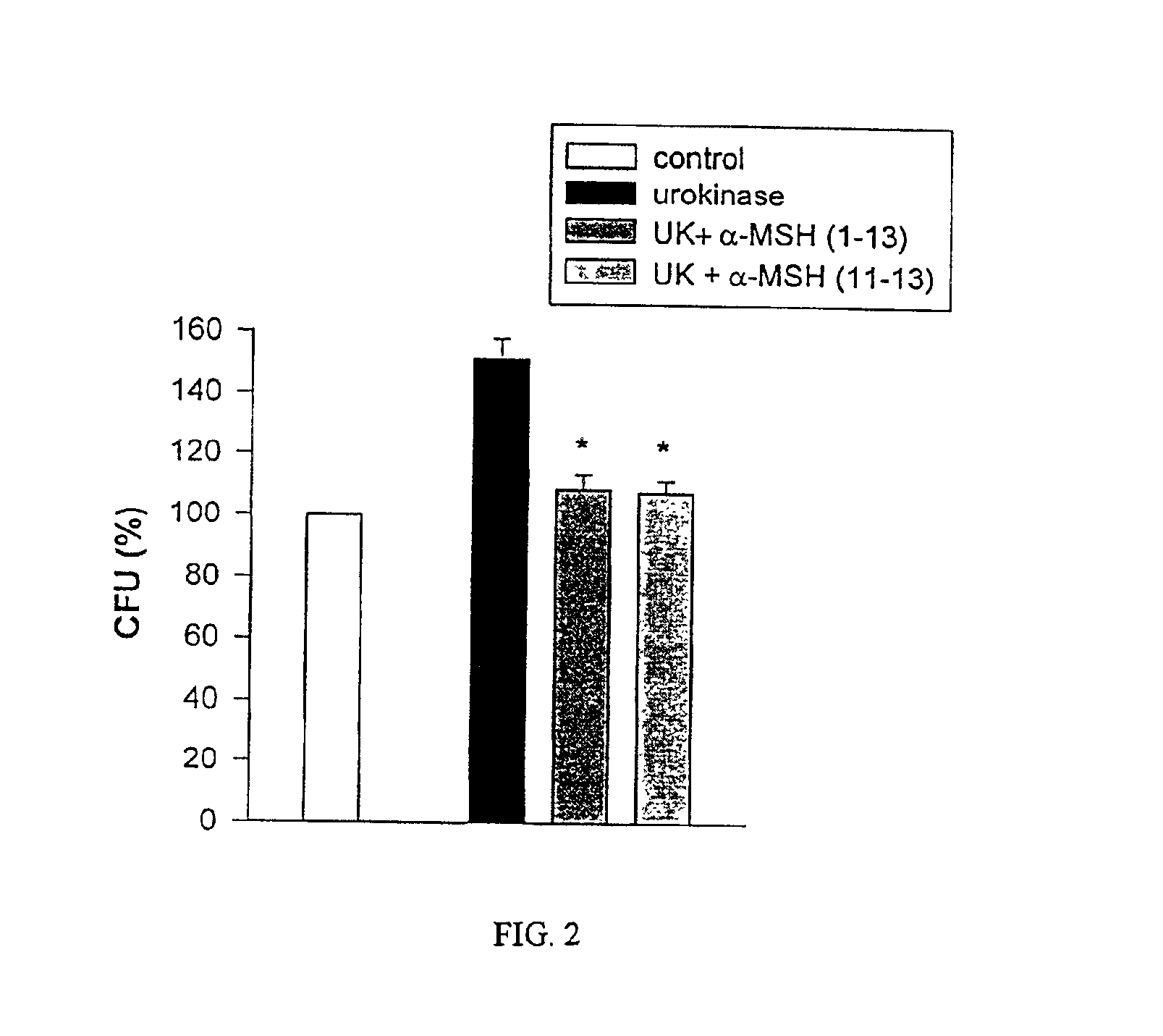
Antimicrobial amino acid sequences derived from alpha-melanocyte-stimulating hormone
Owner:MSH PHARMA INC
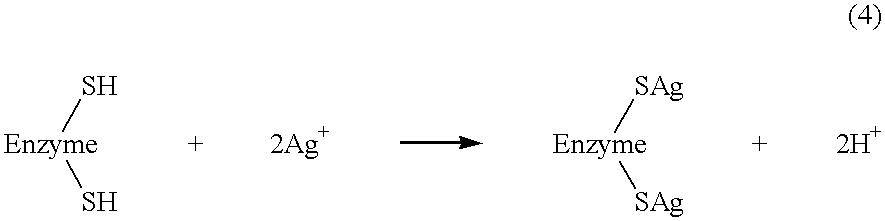
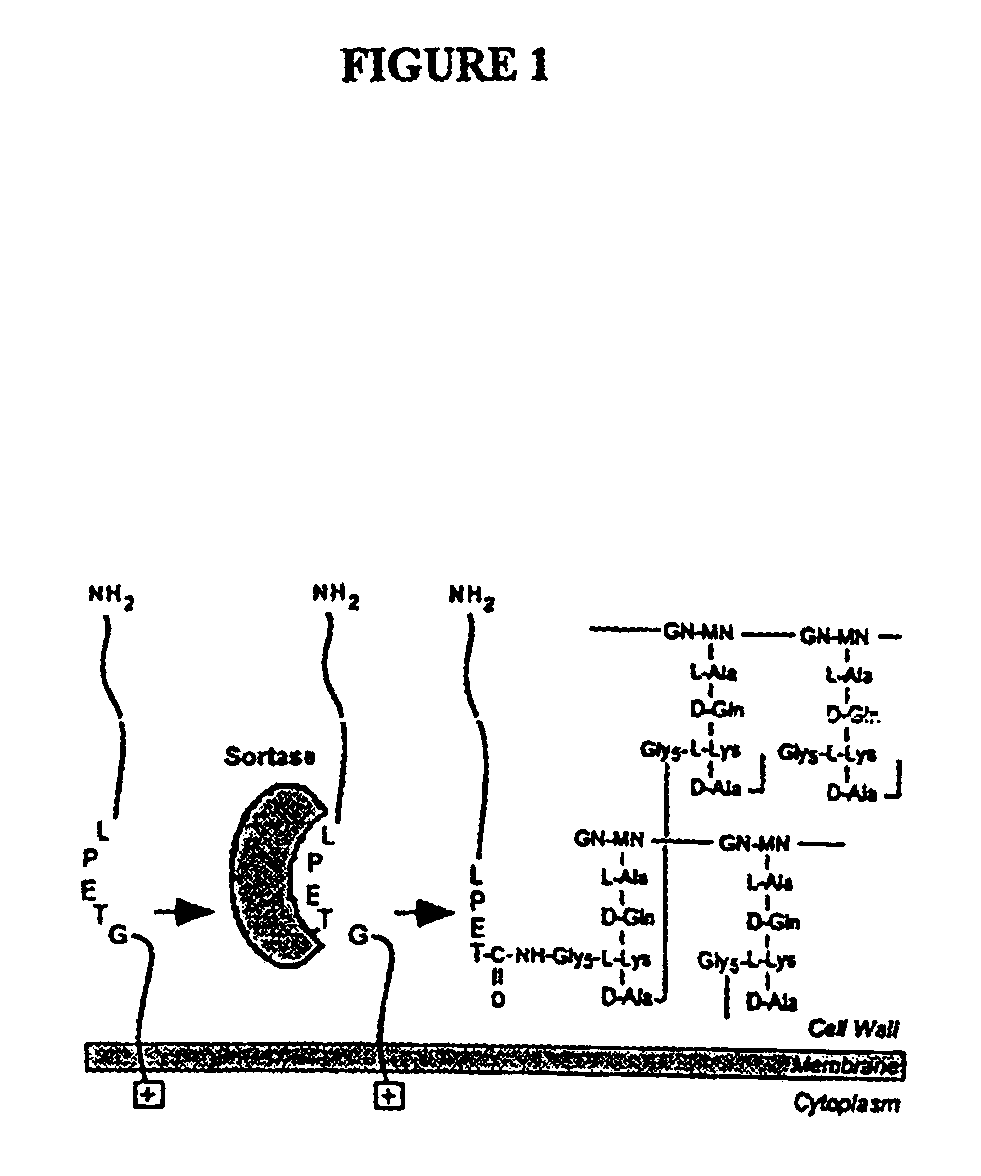
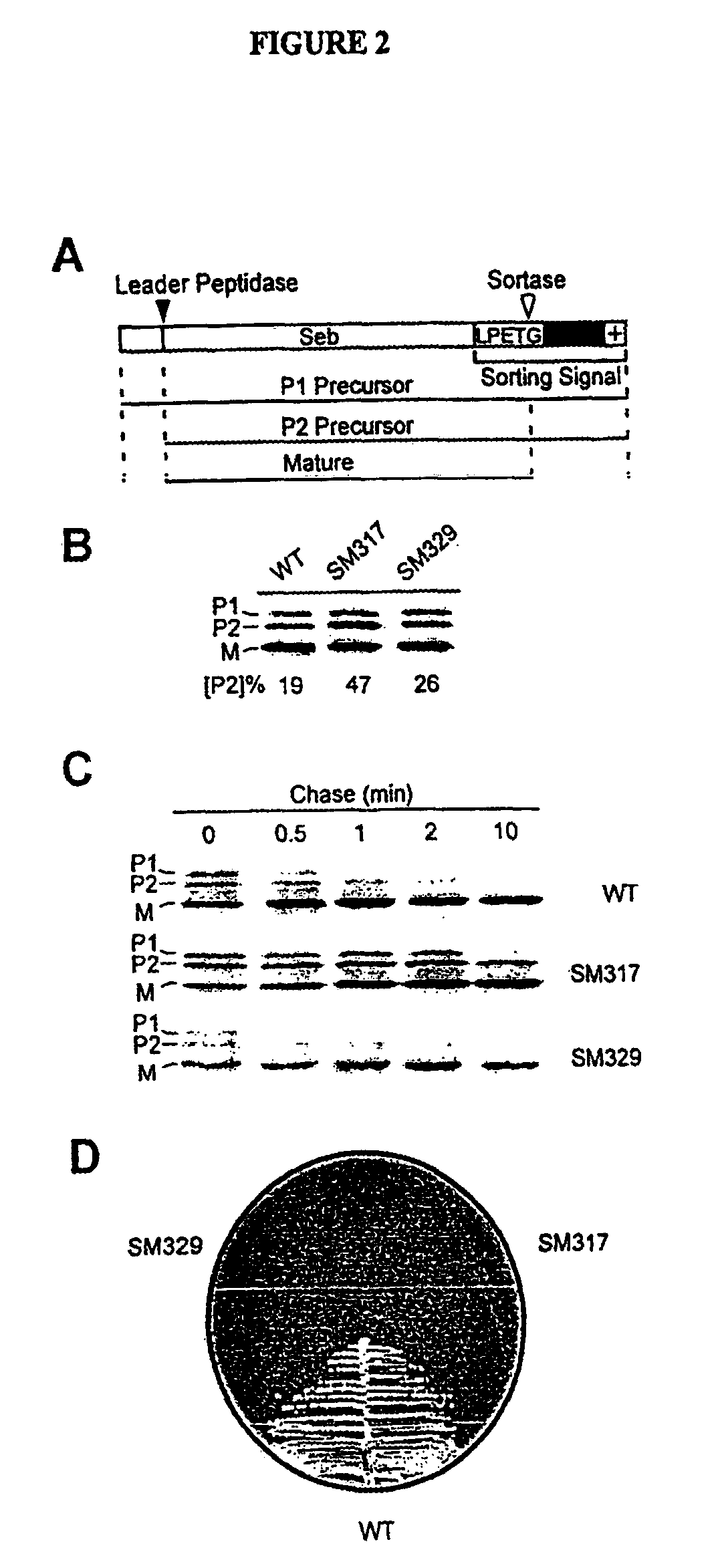
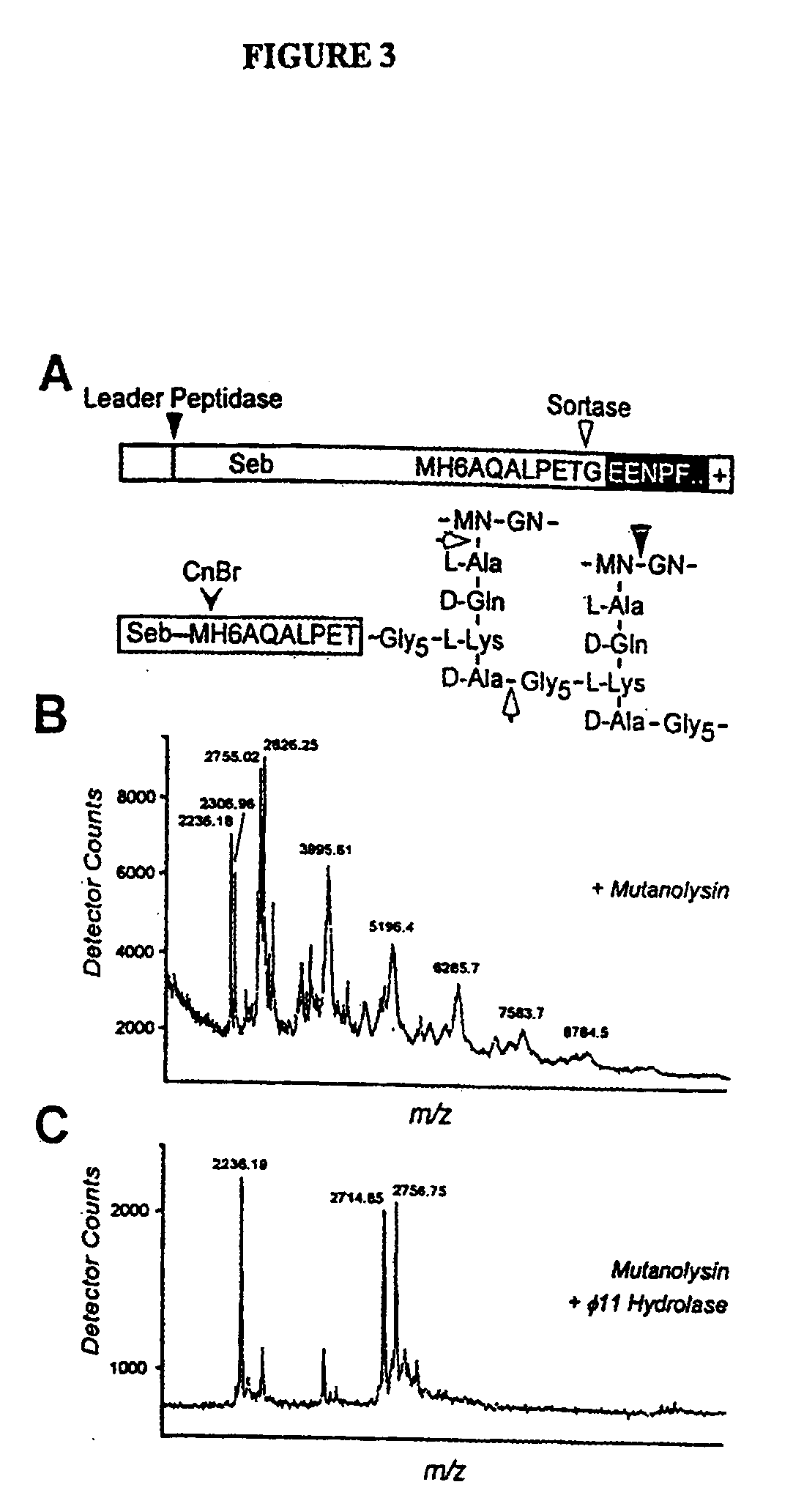
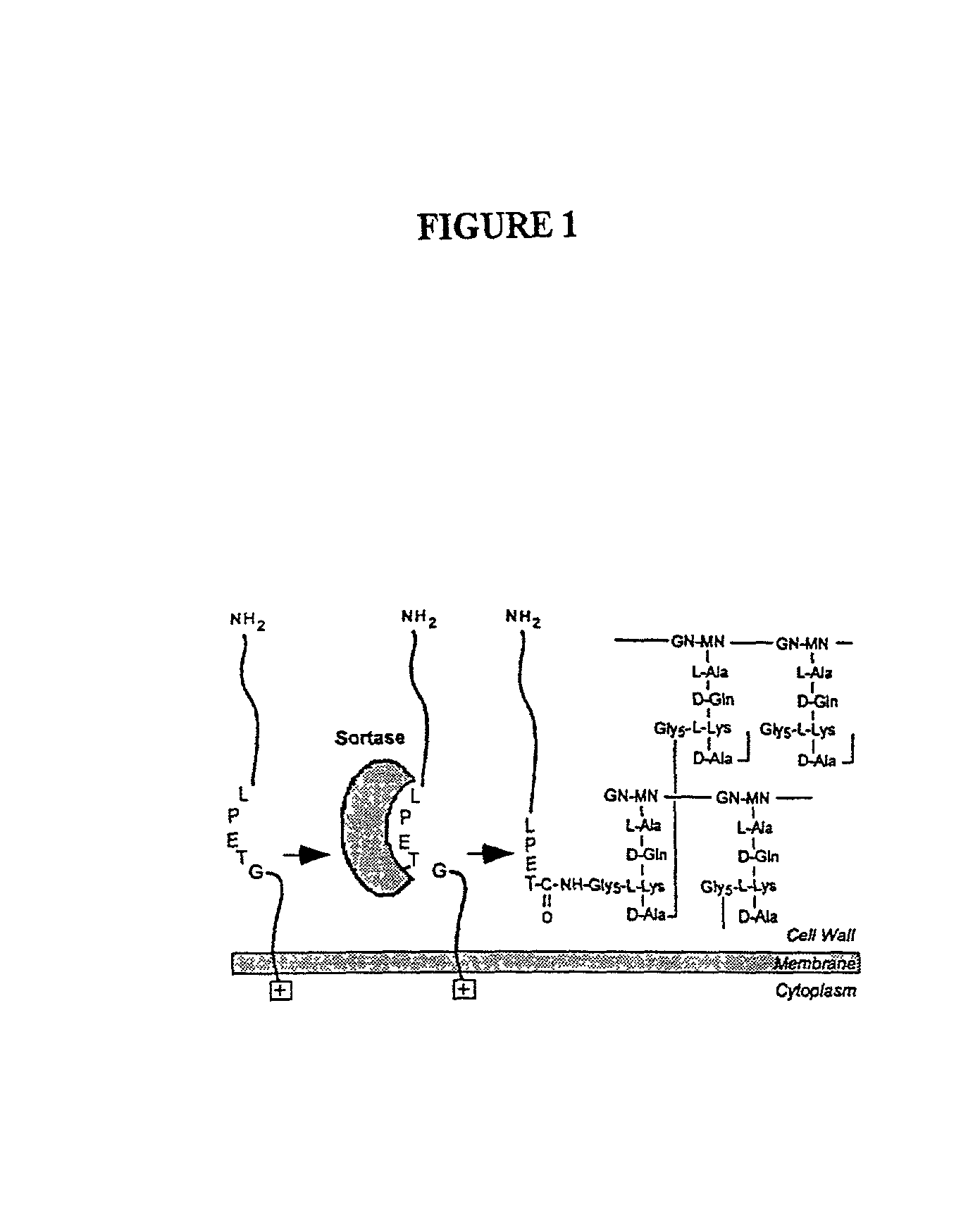
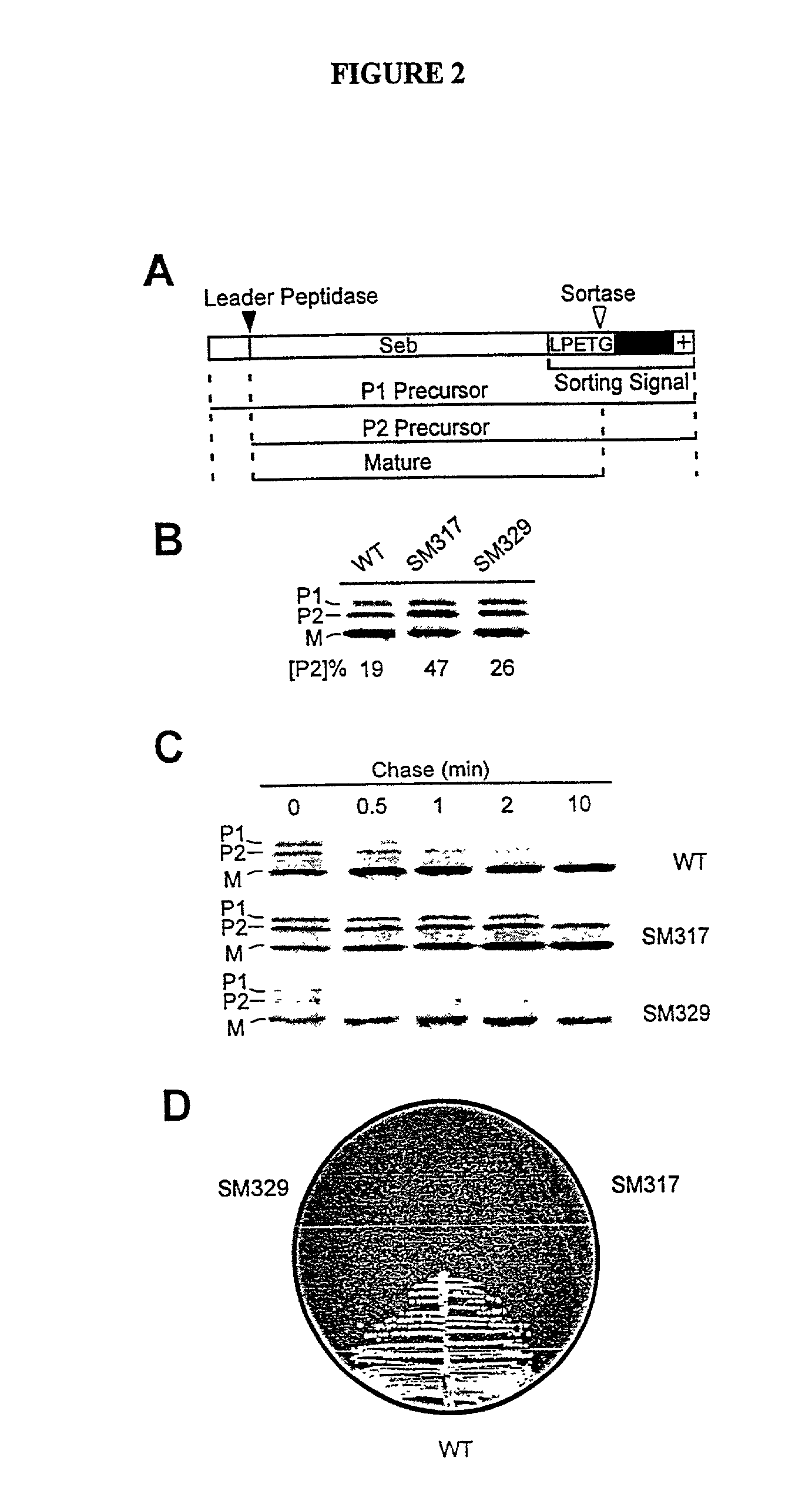
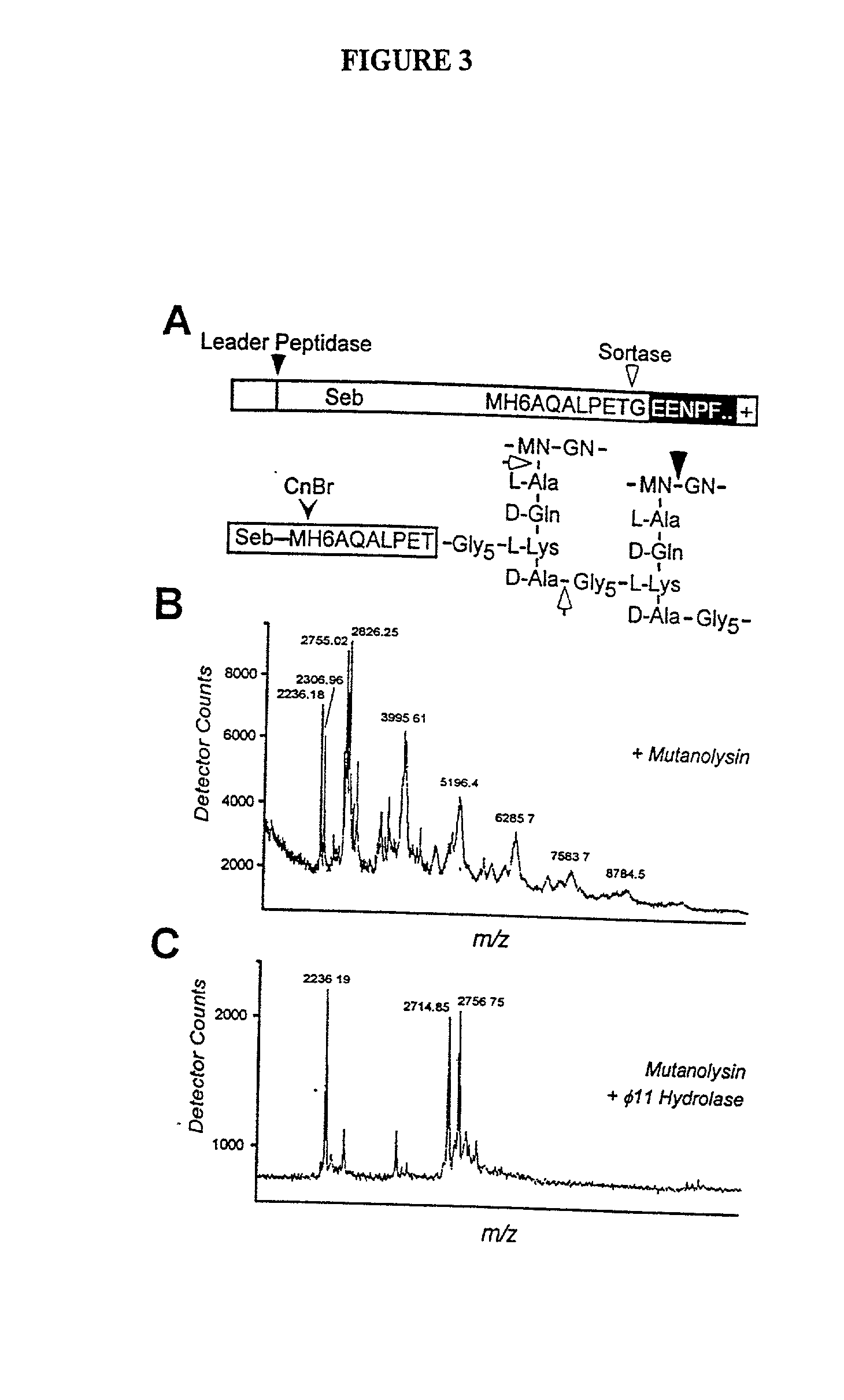
Identification of sortase gene
The present invention is a substantially purified sortase-transamidase enzyme from Gram-positive bacteria, such as Staphylococcus aureus. A specific sortase-transamidase enzyme disclosed has a molecular weight of about 29,076 daltons and catalyzes a reaction that covalently cross-links the carboxyl terminus of a protein having a sorting signal to the peptidoglycan of a Gram-positive bacterium, where the sorting signal has a a motif of NPQ / KTN / G therein. Variants of the enzyme, methods for cloning the gene encoding the enzyme and expressing the cloned gene, and methods of use of the enzyme, including for screening for antibiotics and for display of proteins or peptides on the surfaces of Gram-positive bacteria, are also disclosed.
Owner:RGT UNIV OF CALIFORNIA
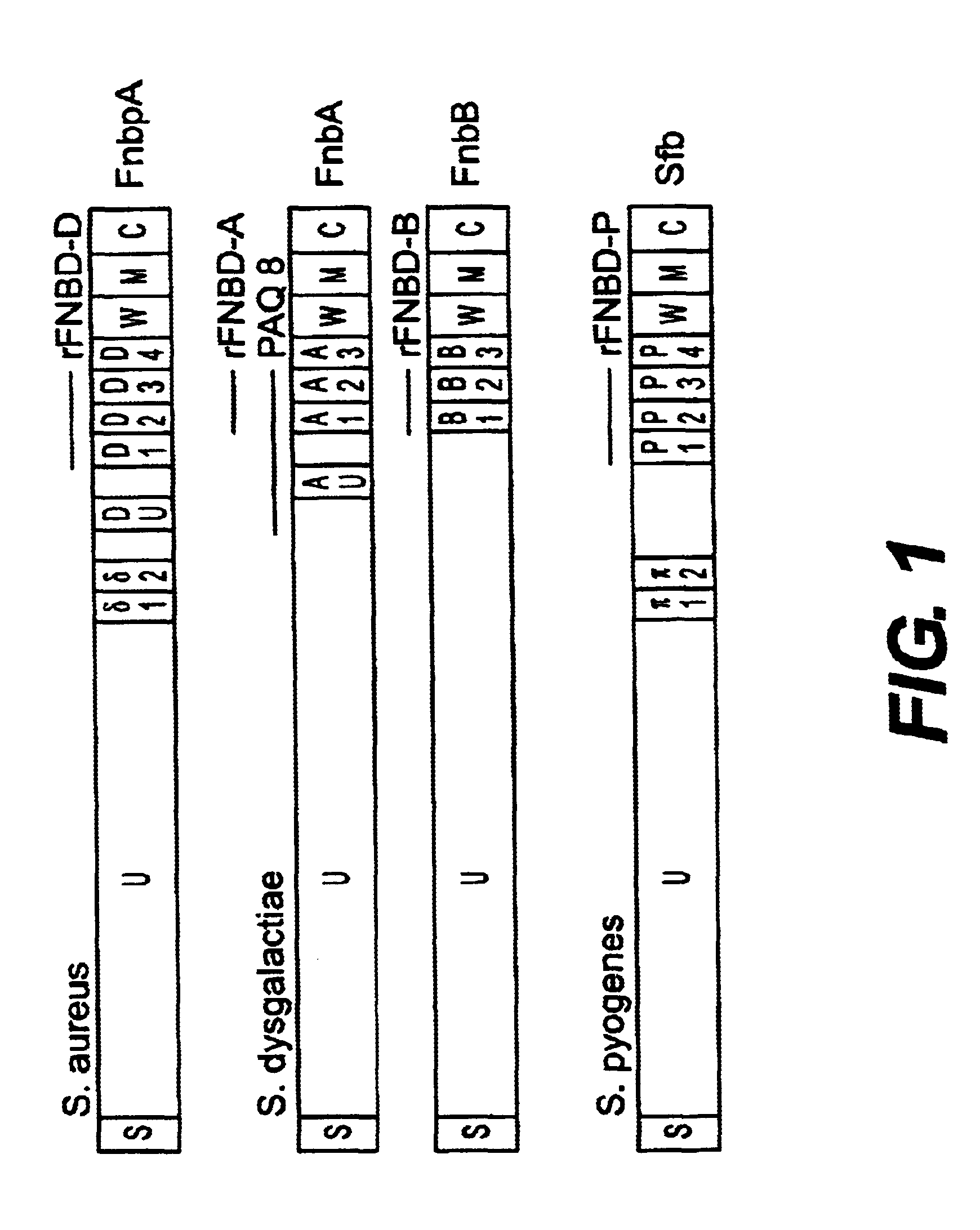
Fibronectin binding protein compositions and methods of use
InactiveUS6685943B1Avoid problemsOvercomes drawbackPeptide/protein ingredientsAntibody mimetics/scaffoldsPassive ImmunizationsStreptococcus pyogenes
Disclosed are antibodies that block the binding of fibronectin protein to fibronectin. Also disclosed are site specifically-mutated and truncated peptide epitopes derived from the fnbA and fnbB genes of Staphylococcus aureus, the fnba and fnbB genes of Streptococcus dysgalactiae, and the sfb gene of Streptococcus pyogenes, and nucleic acid segments encoding these peptides and epitopes. The anti-(fibronectin binding site) antibodies, peptides and epitopes that give rise to antibodies that block the binding of fibronectin binding proteins to fibronectin, and DNA segments encoding these proteins and are of use in various screening, diagnostic and therapeutic applications including active and passive immunization and methods for the prevention of streptococcal and staphylococcal colonization in animals or humans. These. DNA segments and the peptides derived therefrom are proposed to be of use directly in the preparation of vaccines and also for use as carrier proteins in vaccine formulations.
Owner:UNIVERSITY OF MANITOBA +2
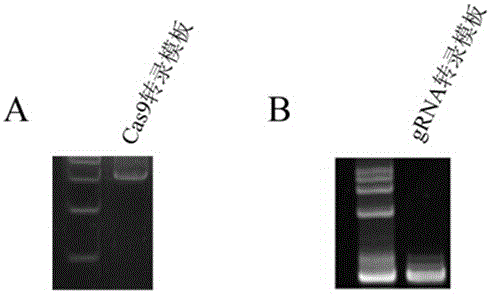
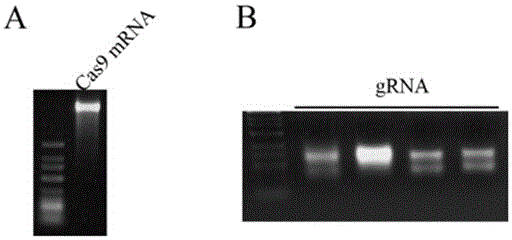
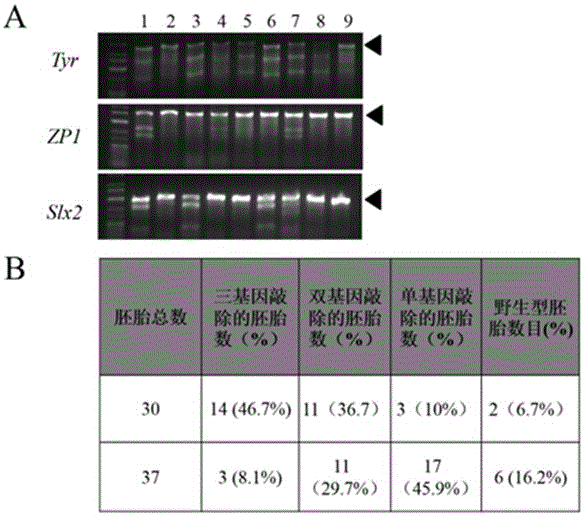
Preparation method of staphylococcus aureus CRISPR/Cas9 system and application of system in constructing mouse model
The invention discloses a preparation method of a staphylococcus aureus CRISPR / Cas9 system and an application of the system in constructing a genetically modified mouse model. The staphylococcus aureus CRISPR / Cas9 system is composed of two components, namely Cas9 mRNA and gRNA, wherein a preparation method of the Cas9 mRNA is achieved by adding a T7 promoter to the upstream region of original Cas9 coding DNA, and a preparation method of the gRNA is achieved by adding the T7 promoter to the upstream region of an original gRNA coding sequence. The staphylococcus aureus Cas9 mRNA and gRNA, which are injected to mouse fertilized embryos through micro-injection, can achieve gene editing and modification of various types, such as single-gene knockout, multi-gene knockout and / or gene knock-in and the like, on the mouse fertilized embryos; therefore, the CRISPR / Cas9 system has a good application prospect in the aspects of fertilized embryo gene editing and modification of such animals as mouse and the like as well as construction of animal models.
Owner:GUANGZHOU MAGIGEN BIOTECH
Identification of sortase gene
InactiveUS20030022178A1Fluorescence enhancementIncreased cleavageAntibacterial agentsFungiEnzyme GeneCarboxyl radical
The present invention is a substantially purified sortase-transamidase enzyme from Gram-positive bacteria, such as Staphylococcus aureus. The enzyme having a molecular weight of about 23,539 or about 29,076 daltons and catalyzing a reaction that covalently cross-links the carboxyl terminus of a protein having a sorting signal to the peptidoglycan of a Gram-positive bacterium, the sorting signal having: (1) a motif of LPX3X4G therein; (2) a substantially hydrophobic domain of at least 31 amino acids carboxyl to the motif; and (3) a charged tail region with at least two positively charged residues carboxyl to the substantially hydrophobic domain, at least one of the two positively charged residues being arginine, the two positively charged residues being located at residues 31-33 from the motif, wherein X3 is any of the twenty naturally-occurring L-amino acids and X4 is selected from the group consisting of alanine, serine, and threonine, and wherein sorting occurs by cleavage between the fourth and fifth residues of the LPX3X4G motif. Variants of the enzyme, methods for cloning the gene encoding the enzyme and expressing the cloned gene, and methods of use of the enzyme, including for screening for antibiotics and for display of proteins or peptides on the surfaces of Gram-positive bacteria, are also disclosed.
Owner:RGT UNIV OF CALIFORNIA
Affinity purified human polyclonal antibodies and methods of making and using them
The present invention describes a method for treating, removing or preventing a bacterial infection, which method comprises administering to a human suffering, suspected of suffering or at risk of suffering from Staphylococcus aureus (S. aureus) infection, a Streptococcus infection, Escherichia coli (E. coli) infection, Pseudomonas aeruginosa (P. aeruginosa) infection, Acinetobacter baumannii (A. baumannii) infection, Enterococcus faecium (E. faecium) infection and / or Clostridium difficile (C. difficile) infection, an effective amount of human polyclonal antibodies affinity purified from a human blood sample with an antigenic preparation comprising cellular and / or secreted antigen(s) from bacterial cells selected from S. aureus, a Streptococcus, E. coli, P. aeruginosa, A. baumannii, E. faecium, C. difficile or a combination thereof, and optionally, wherein said affinity purified human polyclonal antibodies are purified (e.g., as made more concentrated as compared to the starting or unpurified material) relative to the same human polyclonal antibodies in the unpurified or non-affinity-purified human blood sample, e.g., intravenous immunoglobulin (IVIG) sample, and / or also optionally, wherein said affinity purified human polyclonal antibodies are specific for the bacterial antigens used in the affinity purification, and / or further optionally wherein the affinity purified human polyclonal antibodies are substantially free of human antibodies that specifically bind to non-bacterial antigens in the human blood sample. Pharmaceutical compositions for treating bacterial infections, comprising an effective amount of human polyclonal antibodies affinity purified from a human blood sample with an antigenic preparation comprising cellular and / or secreted antigen(s) from S. aureus, Streptococcus, E. coli, P. aeruginosa, A. baumannii, E. faecium, C. difficile or a combination thereof, are also provided.
Owner:SCANTIBODIES LAB
Bacillus subtilis and feed additive and fermenting agent thereof
ActiveCN102178057AStrong stress resistanceImprove immunityAnimal feeding stuffEscherichia coliBiotechnology
The invention belongs to the field of biological techniques and relates to a bacillus subtilis strain and a feed additive and a fermenting agent thereof. The bacillus subtilis has high stress resistance and probiotic effect, so the bacillus subtilis can tolerate artificial gastric juice with a pH value of 2.0 and a concentration of 1 percent, artificial cholate at a concentration of 0.3 percent and a granulating temperature of 80 DEG C; and the bacillus subtilis has a strong inhibiting effect on Escherichia coli K88, Escherichia coli K99 and staphylococcus aureus, high cellulase producing capacity and ability of degrading cellulose. The biological feed additive prepared by using the bacillus subtilis provided by the invention can be used in place of part of antibiotics in livestock and aquatic product culture, improve immunity in animal, improve feed conversion rate and lower culture cost. The bacillus subtilis also can be used in fermentation of bean pulp, cotton meal, vegetable mealand the like, prevent the feed from mildewing, promote the digestion of cellulose in feed and improve the utilization rate of nutrients in the feed.
Owner:BEIJING DABEINONG TECH GRP CO LTD +1
Antibacterial finishing method for textile containing cellulose
ActiveCN103614927AImprove stabilityLess irritatingVegetal fibresMicroballoon preparationStaphylococcus aureusCellulose fiber
The invention relates to an antibacterial finishing method for textile containing cellulose. The method uses natural polymer chitosan and sodium alginate as wall materials; traditional Chinese medicine extracts are used as a core material and are subjected to encapsulation by a micro capsule technology; then an afterfinishing method is employed to treat the microcapsule to the partially carboxymethylated textile containing cellulose, so as to endow the textile with antibacterial performance and good wash fastness through electrostatic attraction between the carboxyl on cellulose fiber and chitosan, as well as the crosslinking effect of the cross-linking agent. The raw materials employed by the invention are green, environment-friendly, safe and high-efficiency, and the obtained textile has good antibacterial effect on common staphylococcus aureus, Eschierichia coli and bacillus subtilis.
Owner:成都艾蒂浮兰科技有限公司
Antibacterial mildewproof polylactic acid composition and preparation method thereof
ActiveCN102453315AImprove antibacterial propertiesGood anti-mildew effectEscherichia coliSodium Pyrithione
The invention relates to an antibacterial mildewproof polylactic acid composition and a preparation method thereof. The composition comprises the following mixed components: polylactic acid, an antibacterial mildewproof agent, and a dispersant; wherein on a basis of 100 parts by weight of polylactic acid, the composition comprises 0.3-2 parts by weight of antibacterial mildewproof agents, and 0-0.5 parts by weight of dispersants; the antibacterial mildewproof agent is a polyguanidine pyrithione antibacterial mildewproof agent which is obtained by mixing an aqueous solution of water-soluble polyguanidine inorganic acid salts or organic acid salts and an aqueous solution of sodium pyrithione; the molar ratio of the water-soluble polyguanidine inorganic acid salts or organic acid salts and sodium pyrithione is 1:(0.1-5). The polylactic acid composition of the invention has good antibacterial and mildewproof effect. The antibacterial effect on staphylococcus aureus and escherichia coli is up to 99.9%; the mildewproof grade for mold such as aspergillus niger, aspergillus terreus, paecilomyces varioti bainier, and the like reaches 0 grade; the composition has good water resistant stability, and has wide application prospects.
Owner:CHINA PETROLEUM & CHEM CORP +1
Air freshener and preparation method and application thereof
ActiveCN101884801AIrritatingIncrease relative volatilityGaseous substancesDeodrantsChemical synthesisSide effect
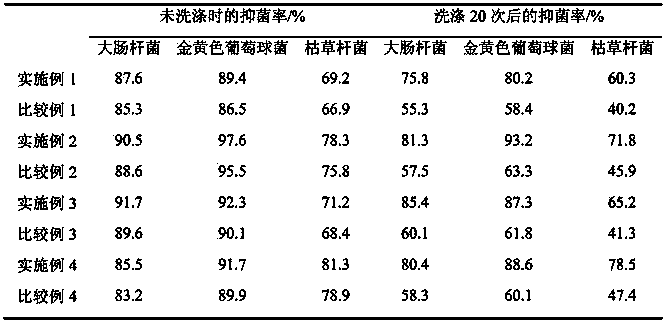
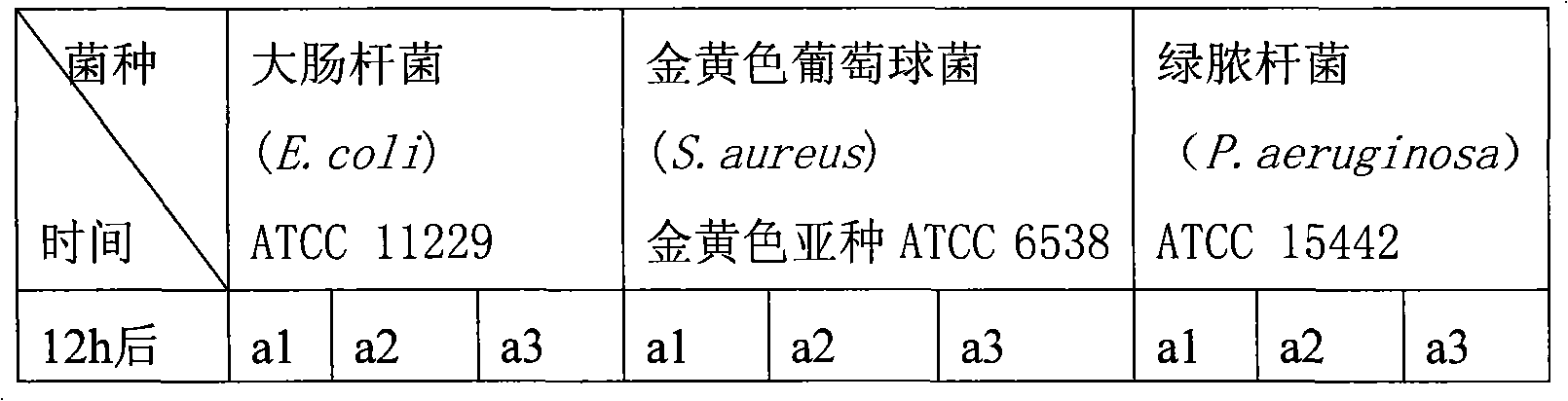
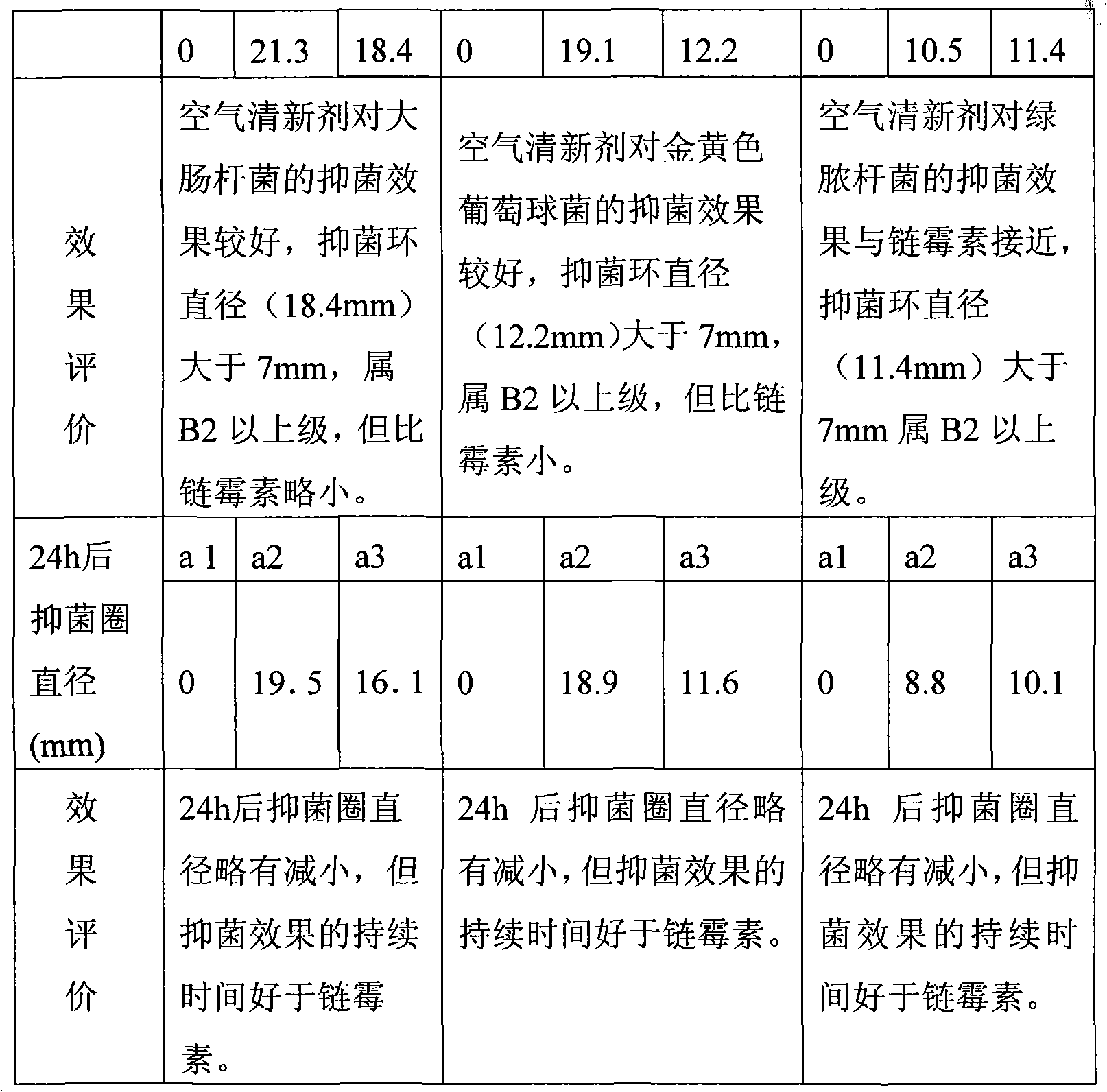
The invention discloses an air freshener and a preparation method and application thereof. The active ingredients of the air freshener comprise thyme essential oil, mint essential oil and tea tree oil. Bacteriostatic experiments prove the bacteriostatic effect of the air freshener, and the experimental results prove that the air freshener has the advantages of safety, high efficiency, no toxin, no irritation and environmental protection, has strong bacteriostatic and antibacterial effects, and can purify the air and improve the air quality. The air freshener can effectively suppress the activities of colibacillus and staphylococcus aureus in the air, has broad-spectrum antimicrobial activity, can play roles in completely improving the air quality and keeping indoor air fresh and clean, and has bacteriostatic activity similar to the streptomyces avermitilis. The air freshener is prepared from aromatic plant essential oil, such as lavender essential oil and rosemary essential oil instead of chemically synthesized essence, is non-toxic and harmless to human body, and has no irritating harm, sensitivity or other side effects.
Owner:INST OF BOTANY CHINESE ACAD OF SCI
Nano meso-porous titanium dioxide coating having high efficiency antibacterial and air purification function
InactiveCN101418151AEasy to preparePromote degradationAntifouling/underwater paintsPaints with biocidesUltraviolet lightsBULK ACTIVE INGREDIENT
The invention discloses a mesoporous nano titania coating with the functions of high-efficiency antibacterium and air purification. The compositions of the coating in percentage by weight are 1 to 10 percent of mesoporous nano titania, 20 to 60 percent of solvent, 20 to 55 percent of film-forming resin, 0.02 to 10 percent of dispersant and other auxiliary agent, and 10 to 30 percent of inorganic pigment filler. The coating takes the mesoporous nano titania or a modifier of the mesoporous nano titania as an active ingredient, has the functions of high-efficiency antibacterium and air purification after curing and film forming, can be widely applied to an internal wall and an external wall of a building. The mesoporous nano titania coating has simple preparation method and wide pollutant purification scope, has an eradicative rate of more than 85 percent on Escherichia coli, Staphylococcus aureus and Candida albicans within 1 hour, has a degradation rate of more than 90 percent on formaldehyde, benzene and toluene, can be excited to generate photocatalysis by faint ultraviolet light in sunlight or illumination light, and still has obvious effect on degradation of low-concentration pollutant.
Owner:NANJING UNIV OF TECH
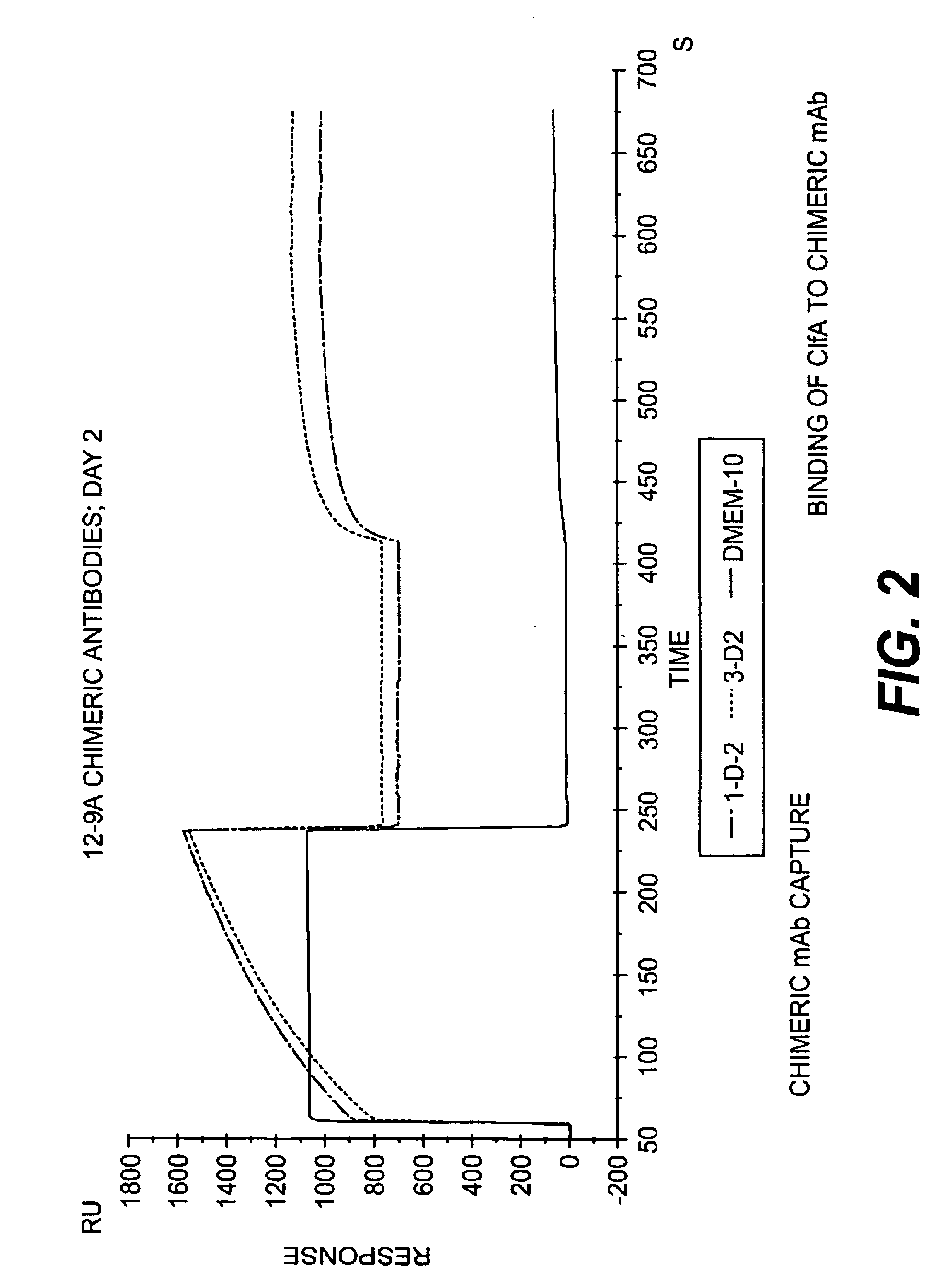
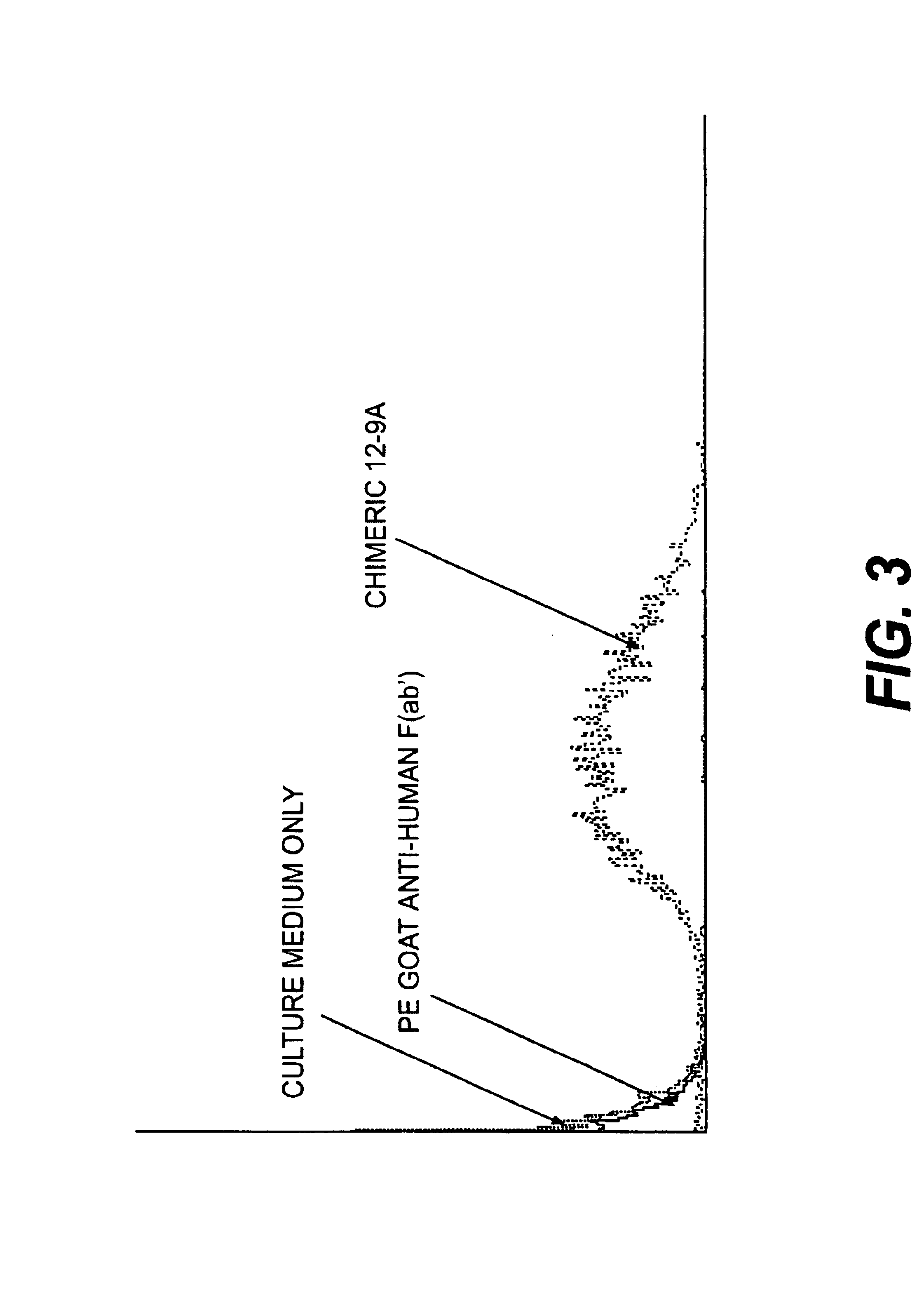
Monoclonal antibodies to the ClfA protein and method of use in treating or preventing infections
InactiveUS6979446B2Avoid stickingInhibiting or impairing the binding of the ClfA proteinAntibacterial agentsBacterial antigen ingredientsBacteroidesStaphylococcus cohnii
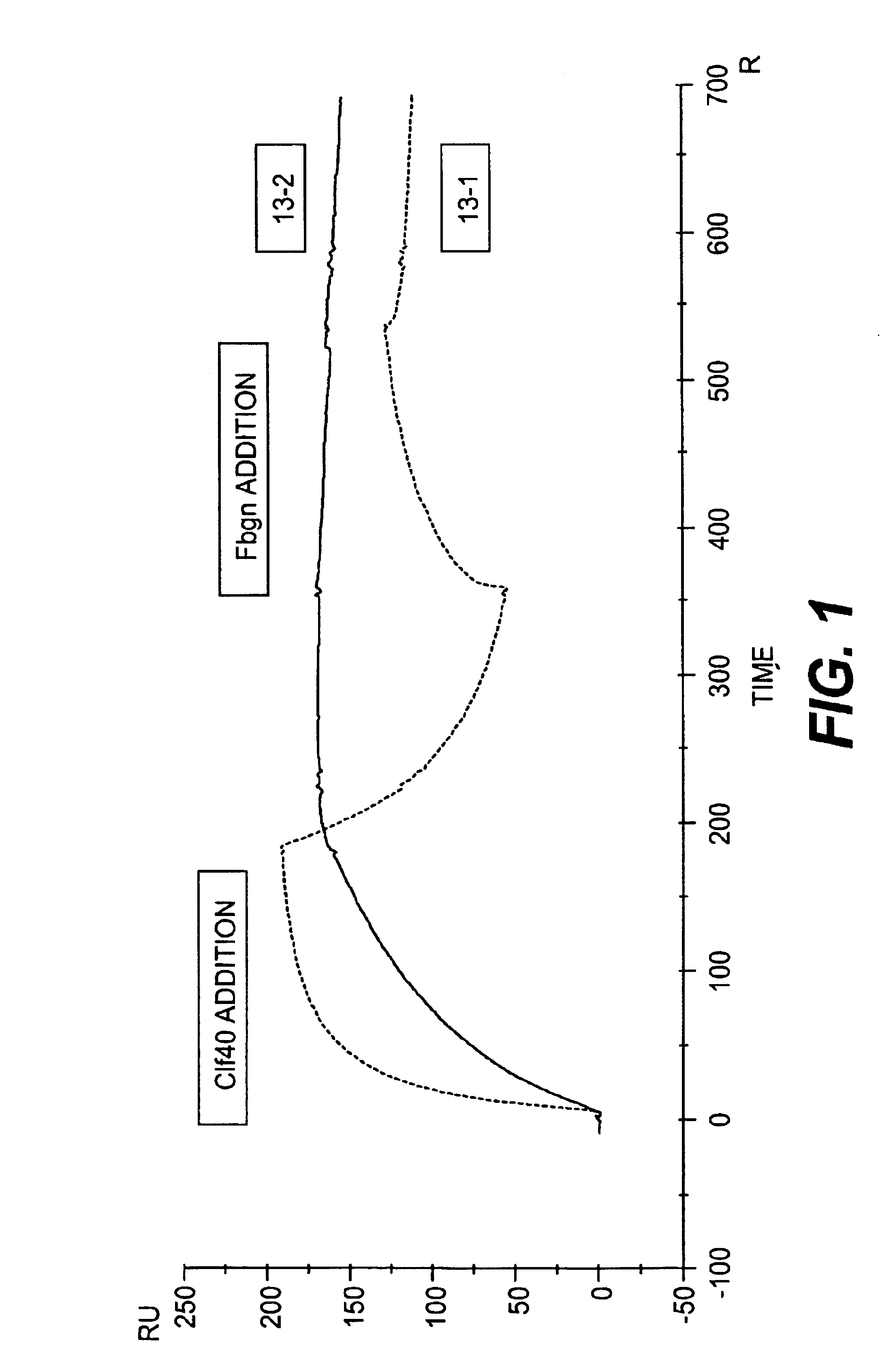
Monoclonal antibodies which can bind to the ClfA protein and which are generated from binding subdomains or active fragments of the ClfA protein from Staphylococcus aureus, including the active fragments proteins from its fibrinogen binding domain such as Clf40 protein, the Clf33 protein, or ClfA N3, are provided which can be useful in the treatment and protection against infection from staphylococcal bacteria such as Staphylococcus aureus. In addition, medical instruments can be treated using the monoclonal antibodies of the invention in order to reduce or eliminate the possibility of their becoming infected or further spreading the infection. In particular, the antibodies of the present invention are advantageous because they can prevent adherence of the bacteria to host cells by impairing or inhibiting the ability of S. aureus ClfA to bind to fibrinogen or fibrin, and thus can be utilized in methods or treating or preventing staphylococcal inventions.
Owner:INHIBITEX INC
Composite nanometer antibiotic material, preparation method and products thereof
PendingCN1568704AHas broad-spectrum antibacterial functionGood killing effectBiocideAnimal repellantsStaphylococcus aureusStaphylococcus aureus bacteria
A combined nano antiseptic material and its preparation method and products are disclosed. The advantages of the invention lie in 1.combining different antiseptic material and preparing combined nano antiseptic material, taking advantage of synergism and coupling effect of between nano particle, promoting the antiseptic ability 2.The combined antiseptic material has wide spectrum antiseptic function and killing effect to staphylococcus aureus etc. 3.The method can be used in producing sorts of bacteria and virus killing antiseptic material ,such as antiseptic fabric, latex etc.
Owner:TIANJIN UNIVERSITY OF TECHNOLOGY
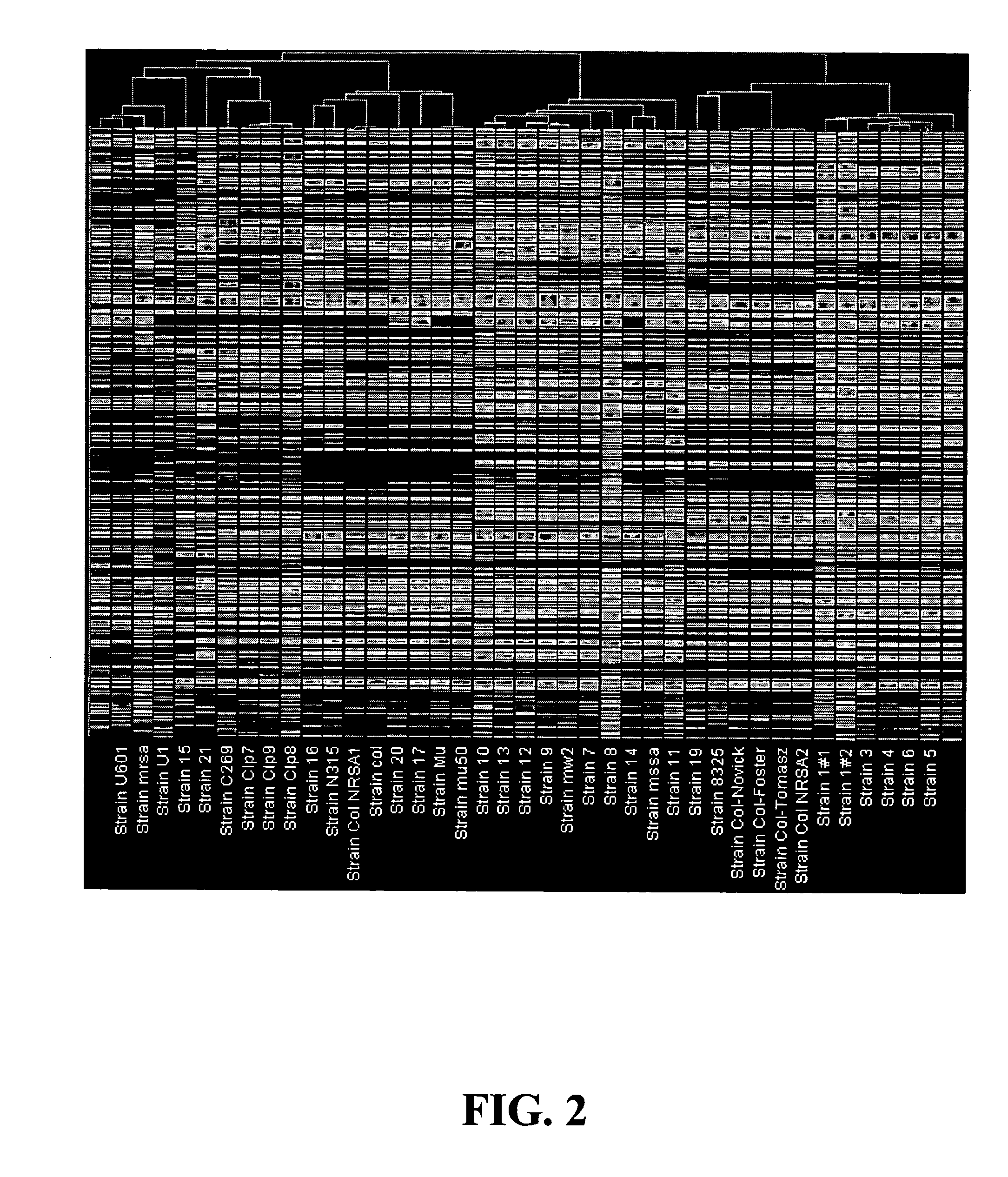
Nucleic acid arrays for detecting multiple strains of a non-viral species
InactiveUS20070031850A1Bioreactor/fermenter combinationsBiological substance pretreatmentsStaphylococcus aureusPolynucleotide
Nucleic acid arrays and methods of using the same for concurrent or discriminable detection of different strains of a non-viral species. In many embodiments, the nucleic acid arrays of the present invention include probes that are specific to different respective strains of a non-viral species. In many other embodiments, the nucleic acid arrays of the present invention include probes that are common to two or more different strains of the non-viral species. In one embodiment, the non-viral species is Staphylococcus aureus, and the different Staphylococcus aureus strains include COL, N315, Mu50, EMRSA-16, MSSA-476, and 8325 strains. In another embodiment, a nucleic acid array of the present invention includes polynucleotide probes capable of hybridizing under stringent or nucleic acid array hybridization conditions to respective sequences selected from SEQ ID NOs: 1 to 7,852, or the complements thereof.
Owner:WYETH
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com