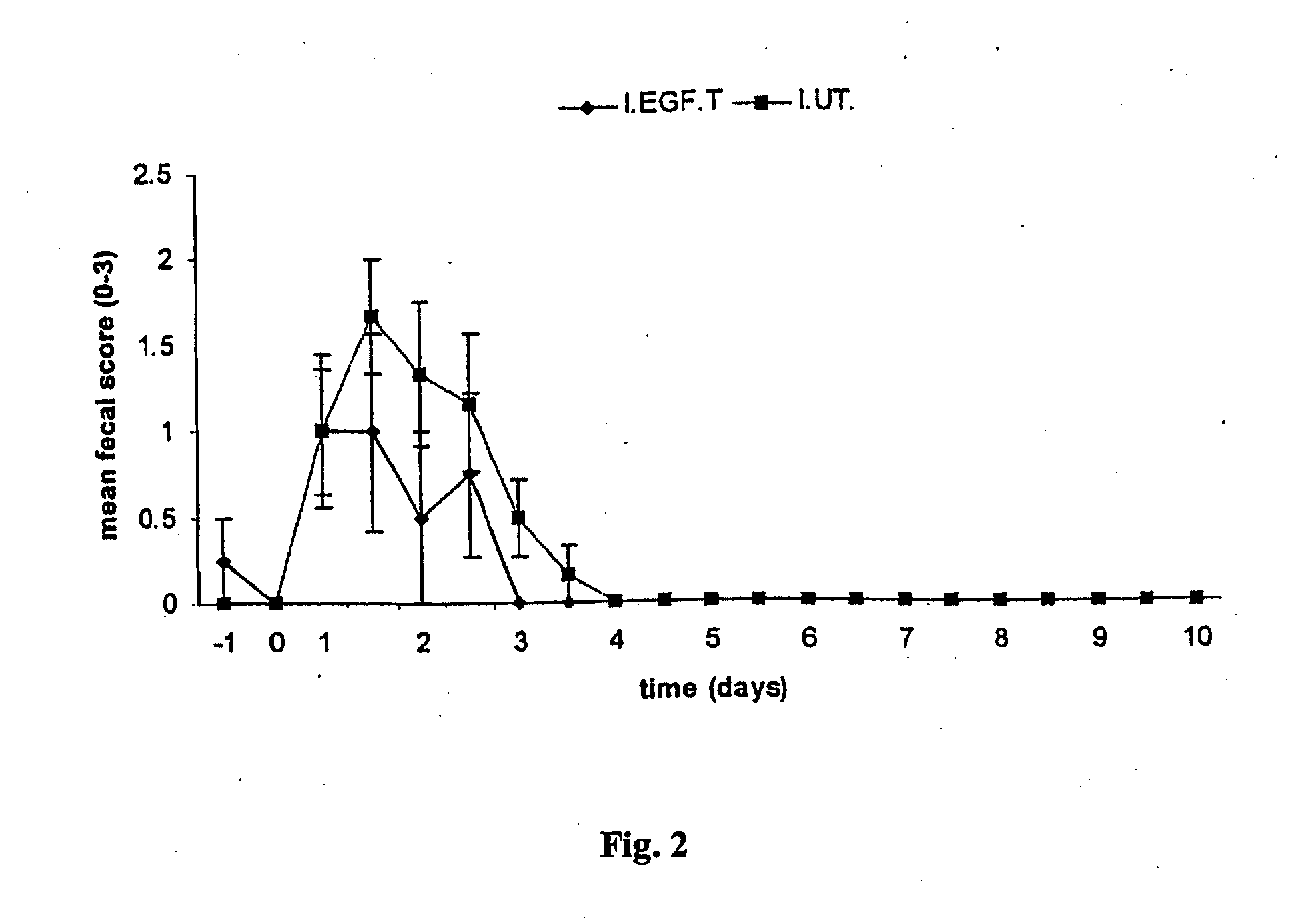
Patents
Literature
77 results about "Bacterial colonization" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Answer Wiki. Bacterial colonization is the presence of pathogenic bacteria on a body surface (like on the skin, mouth, intestines or airway) without causing clinical evidence of infection in the person. Colonization of bacteria develops when a pathogen grows on a host's tissues that would normally not allow such growth.
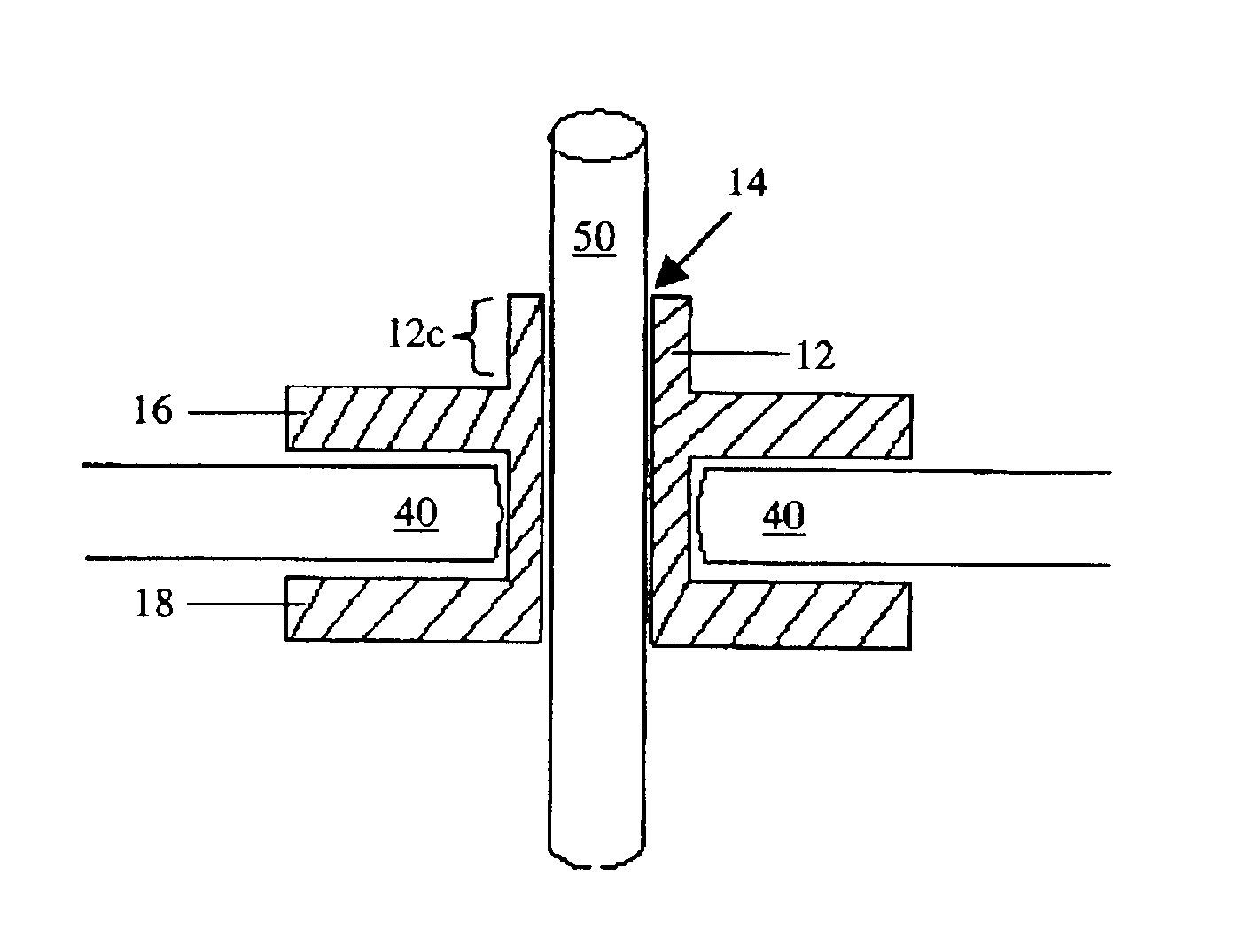
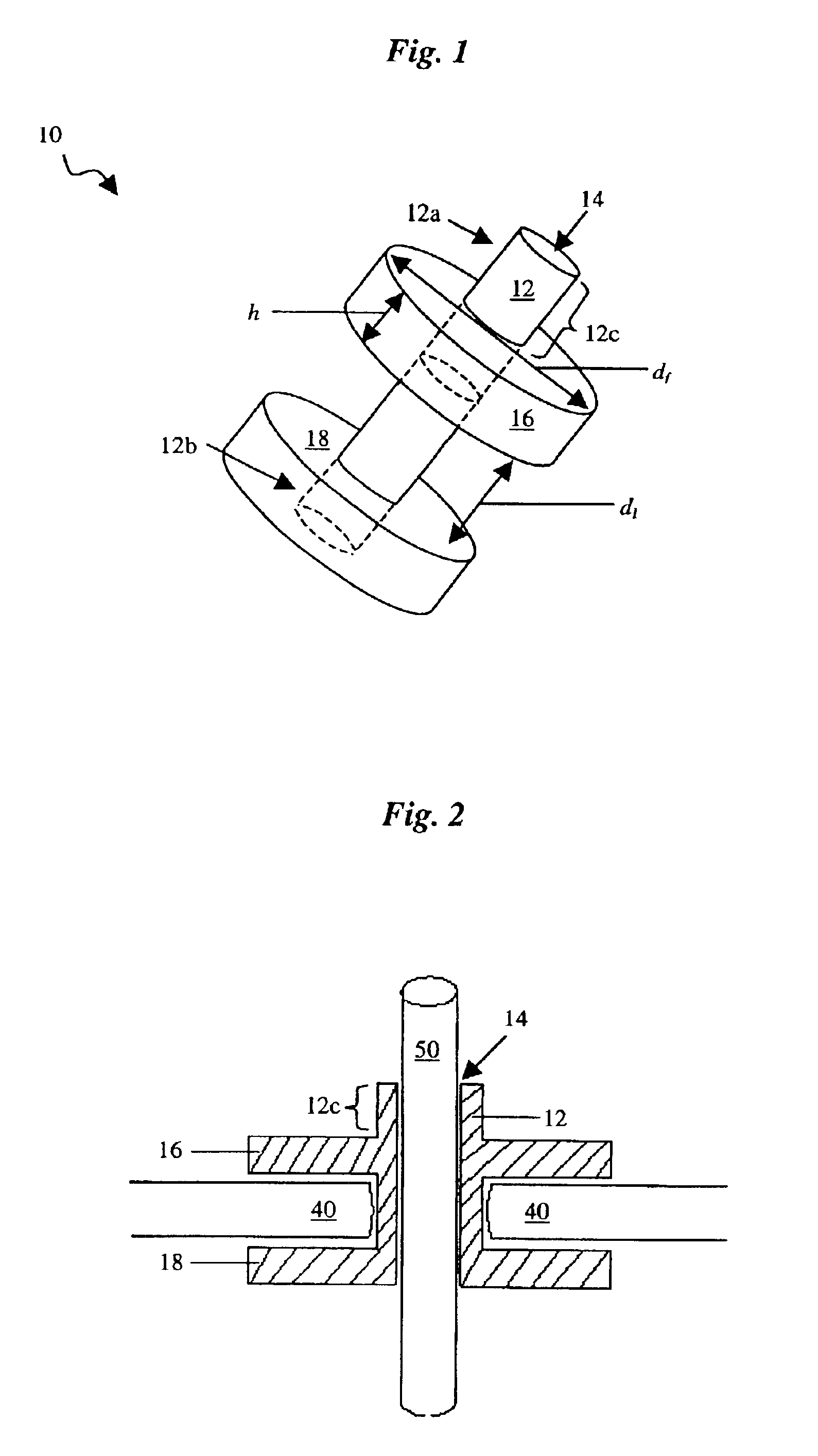
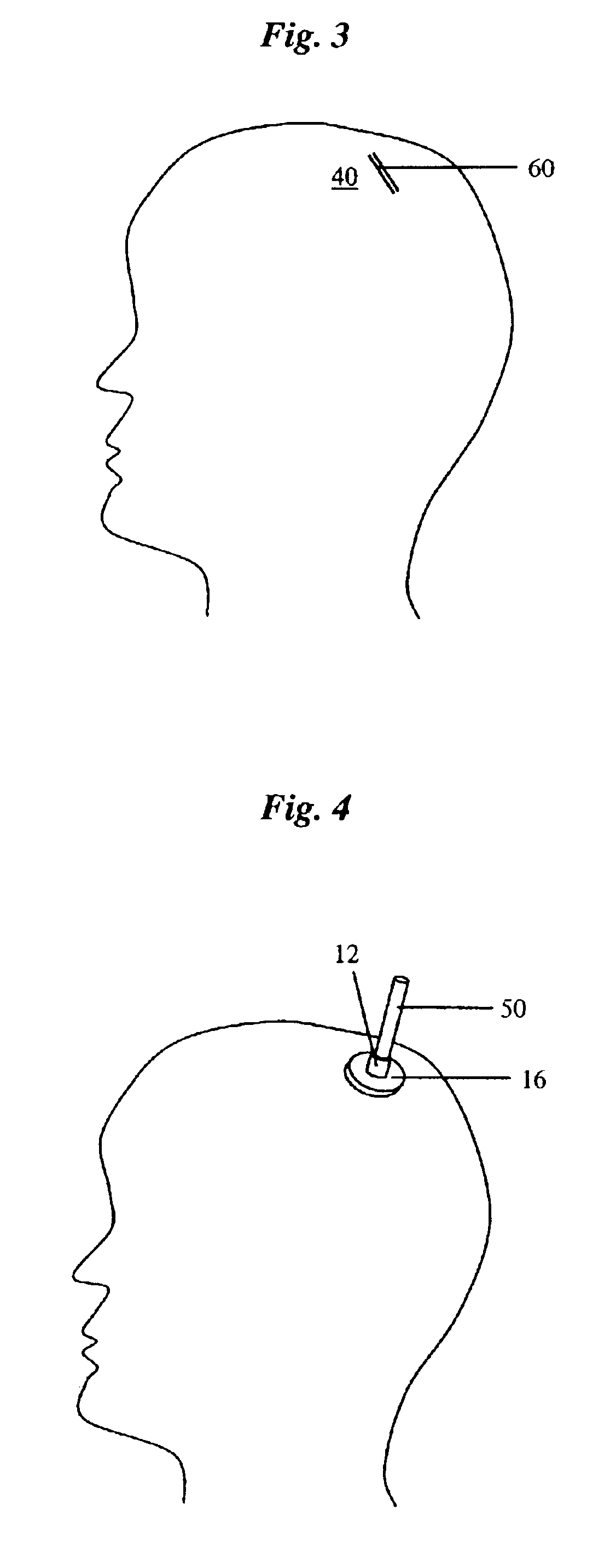
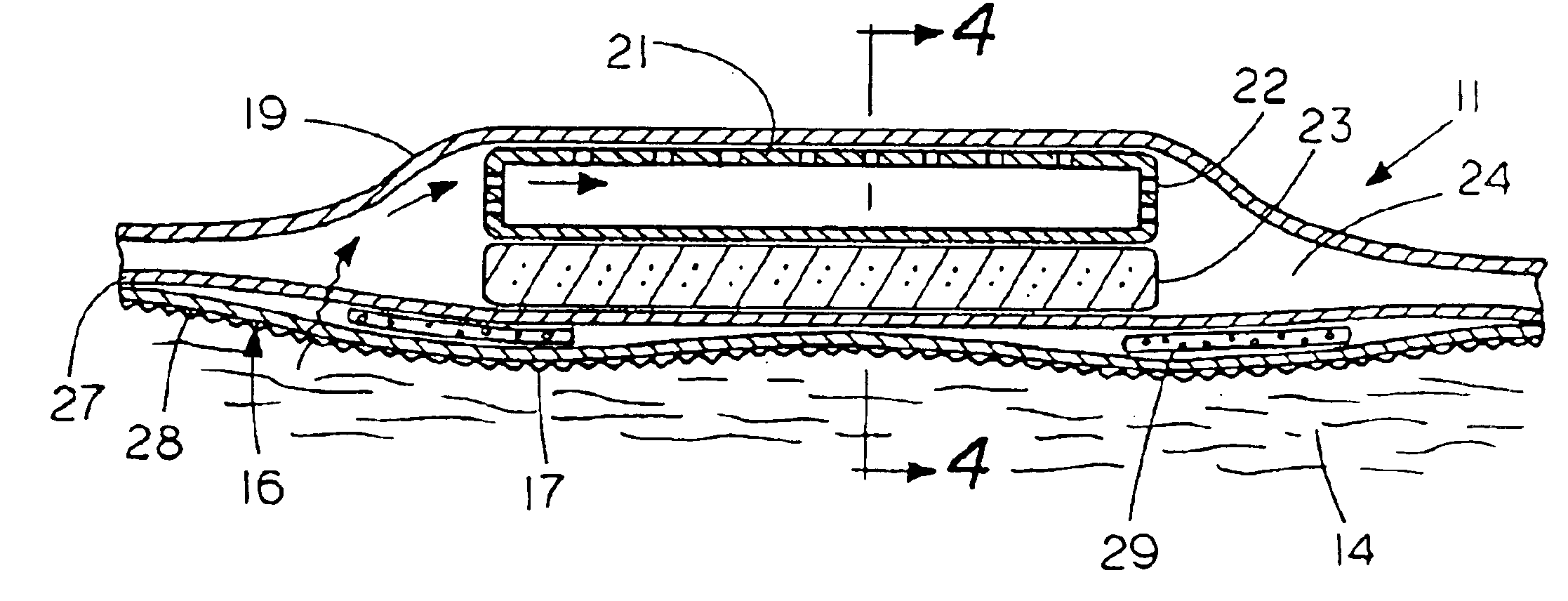
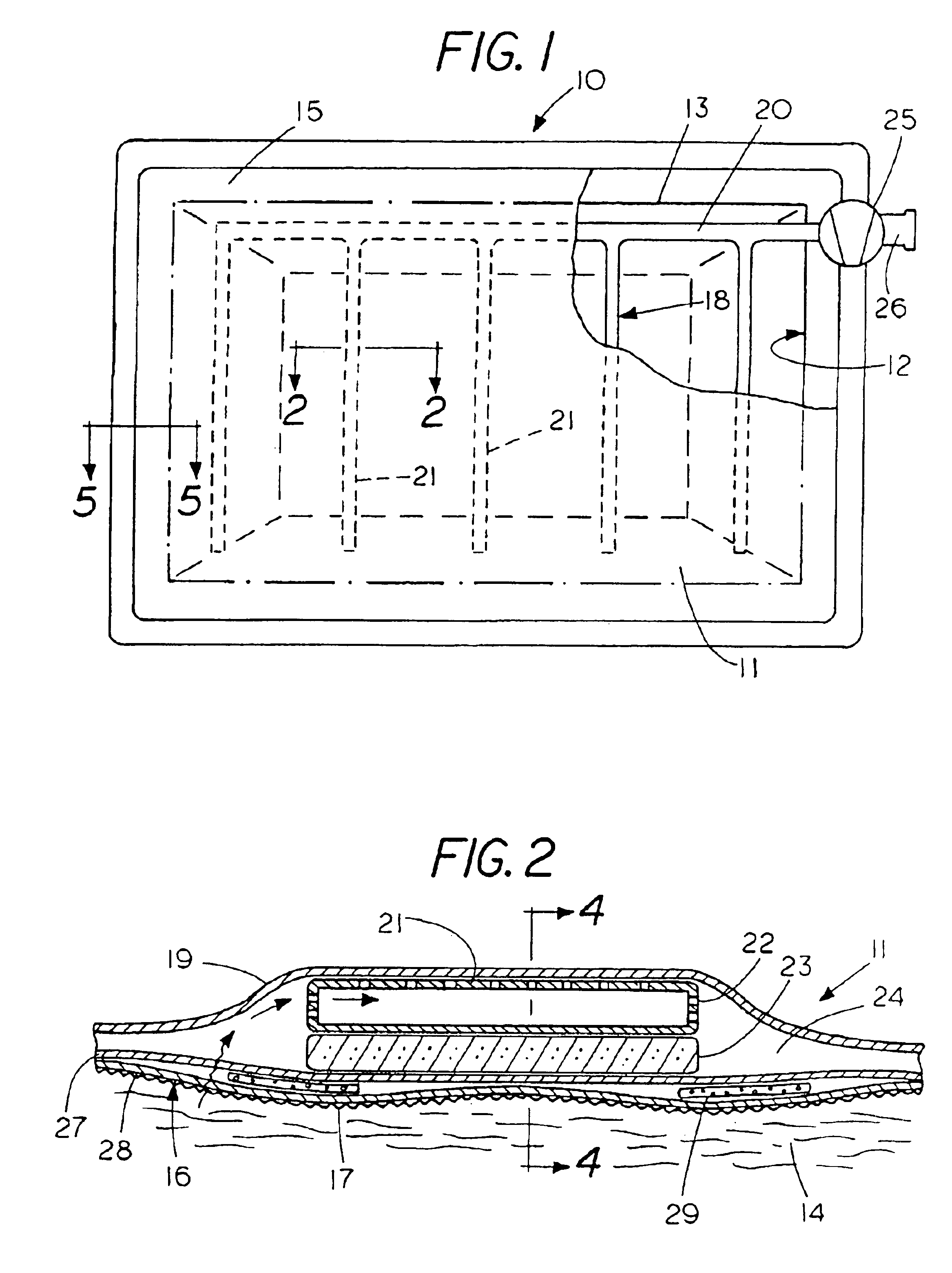
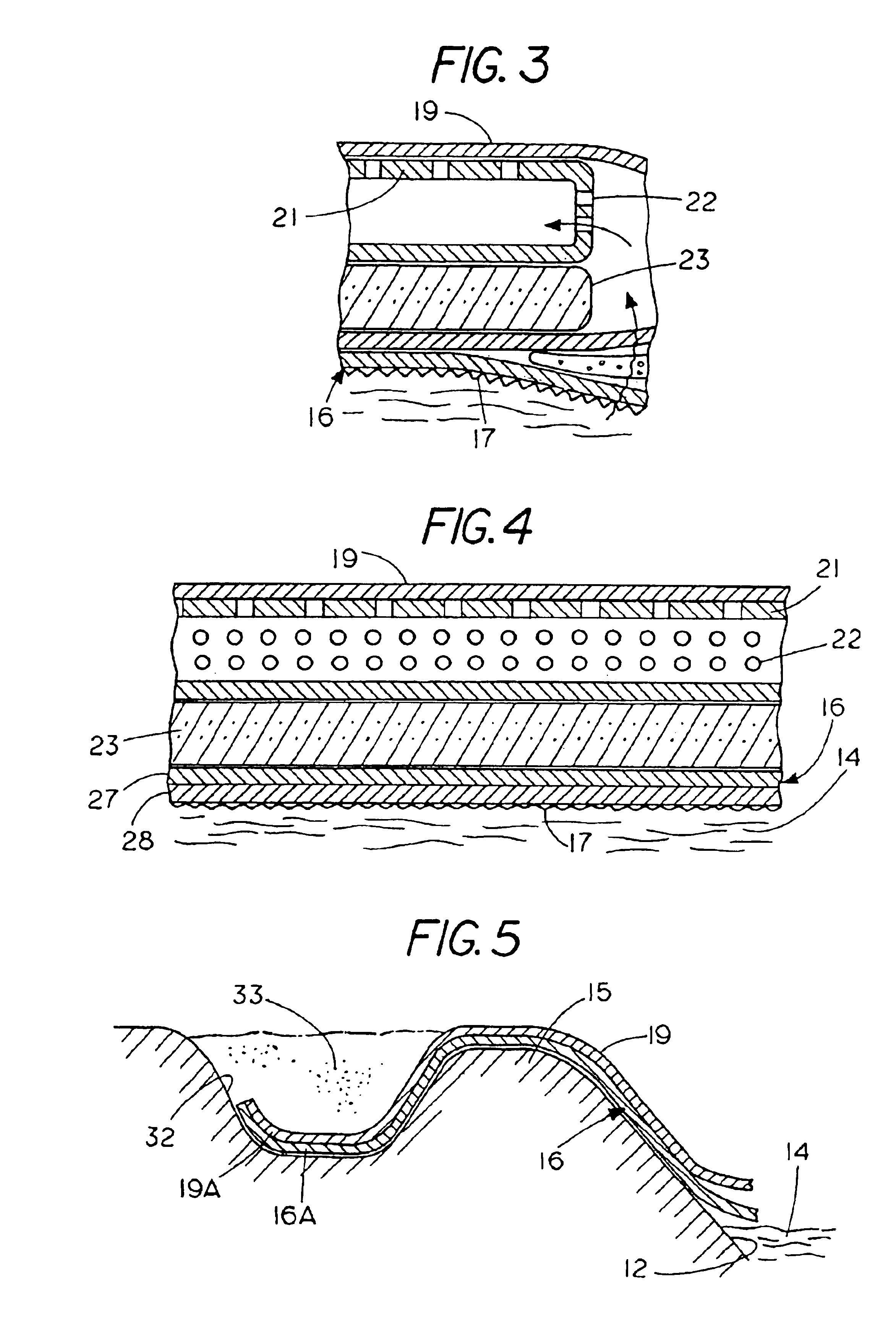
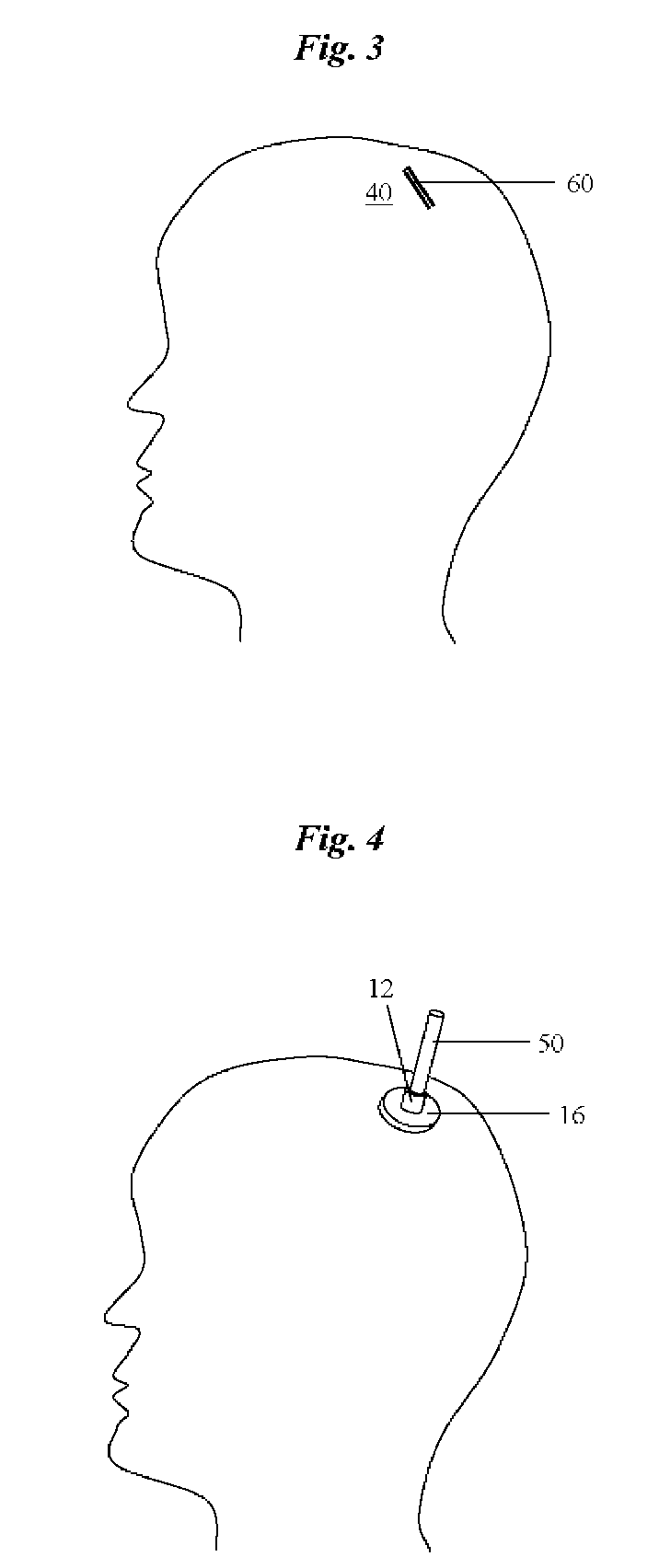
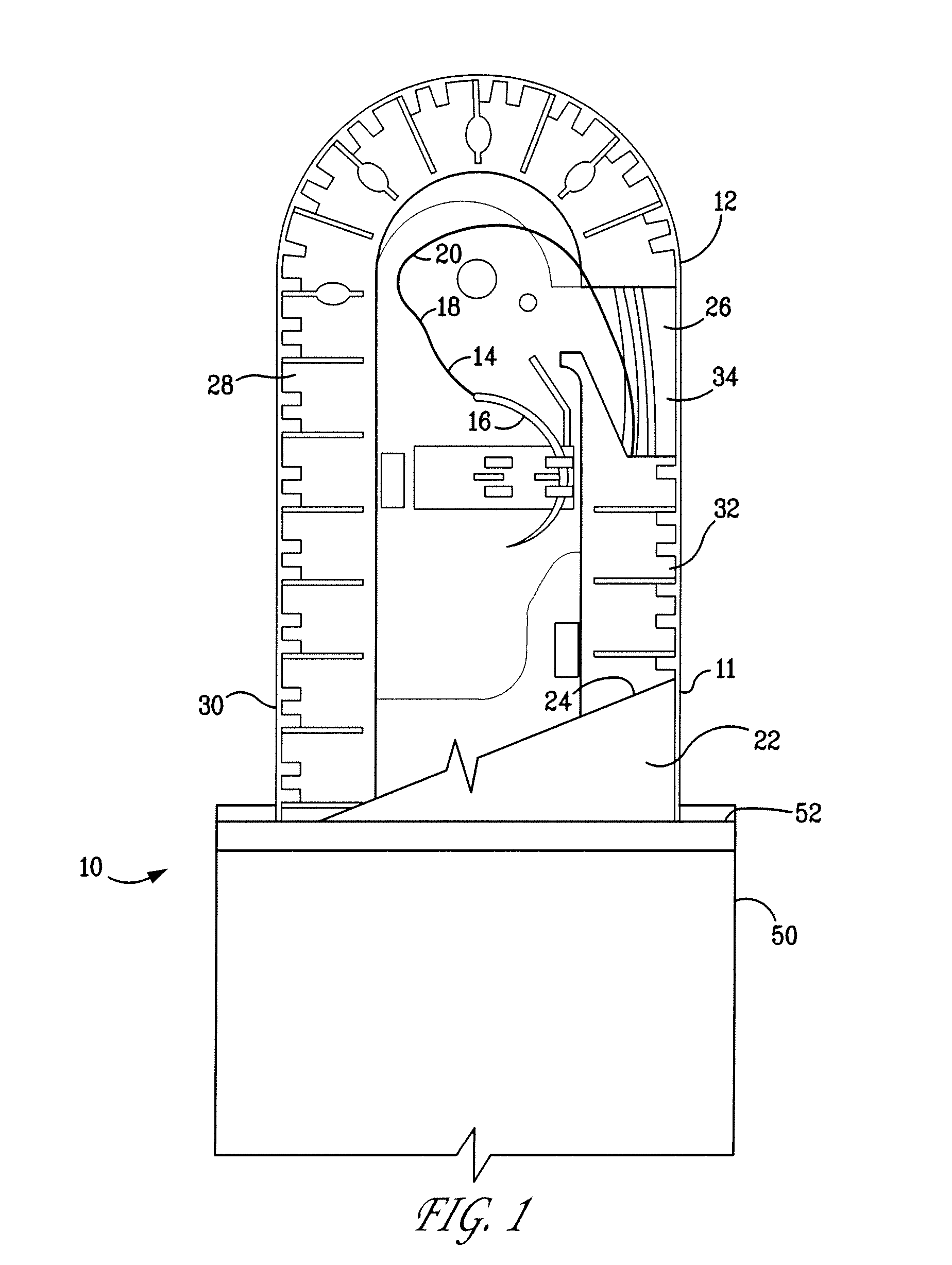
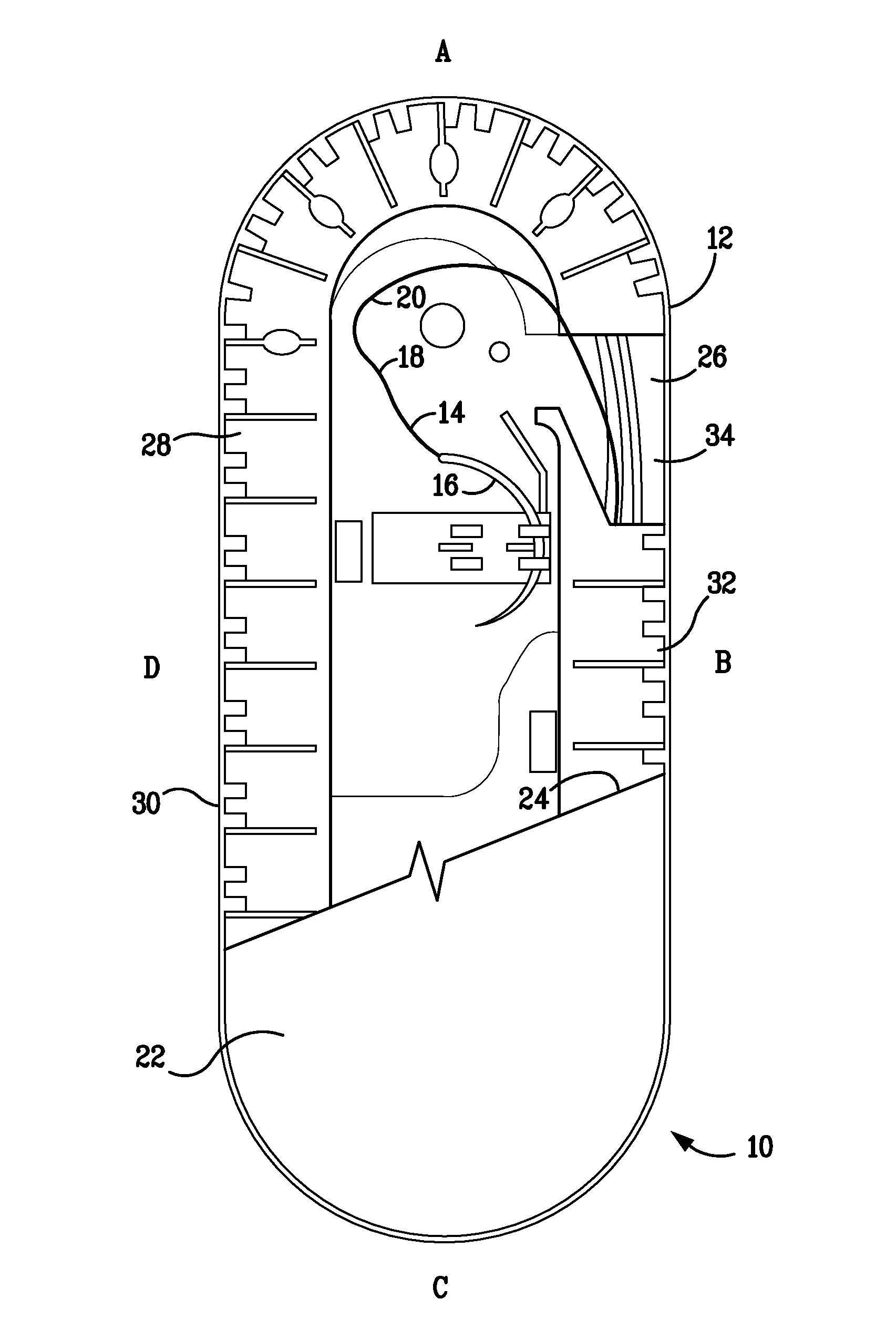
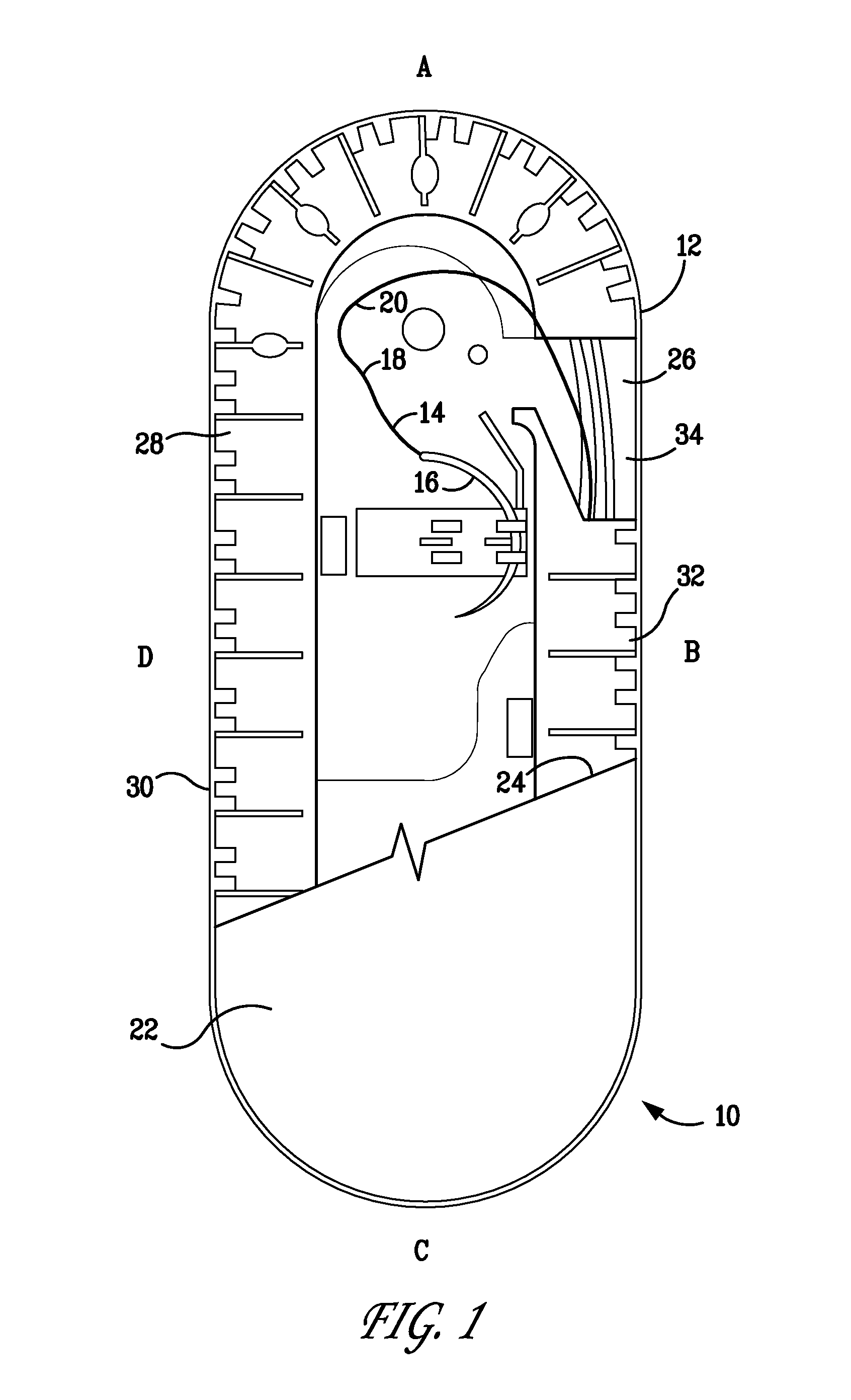
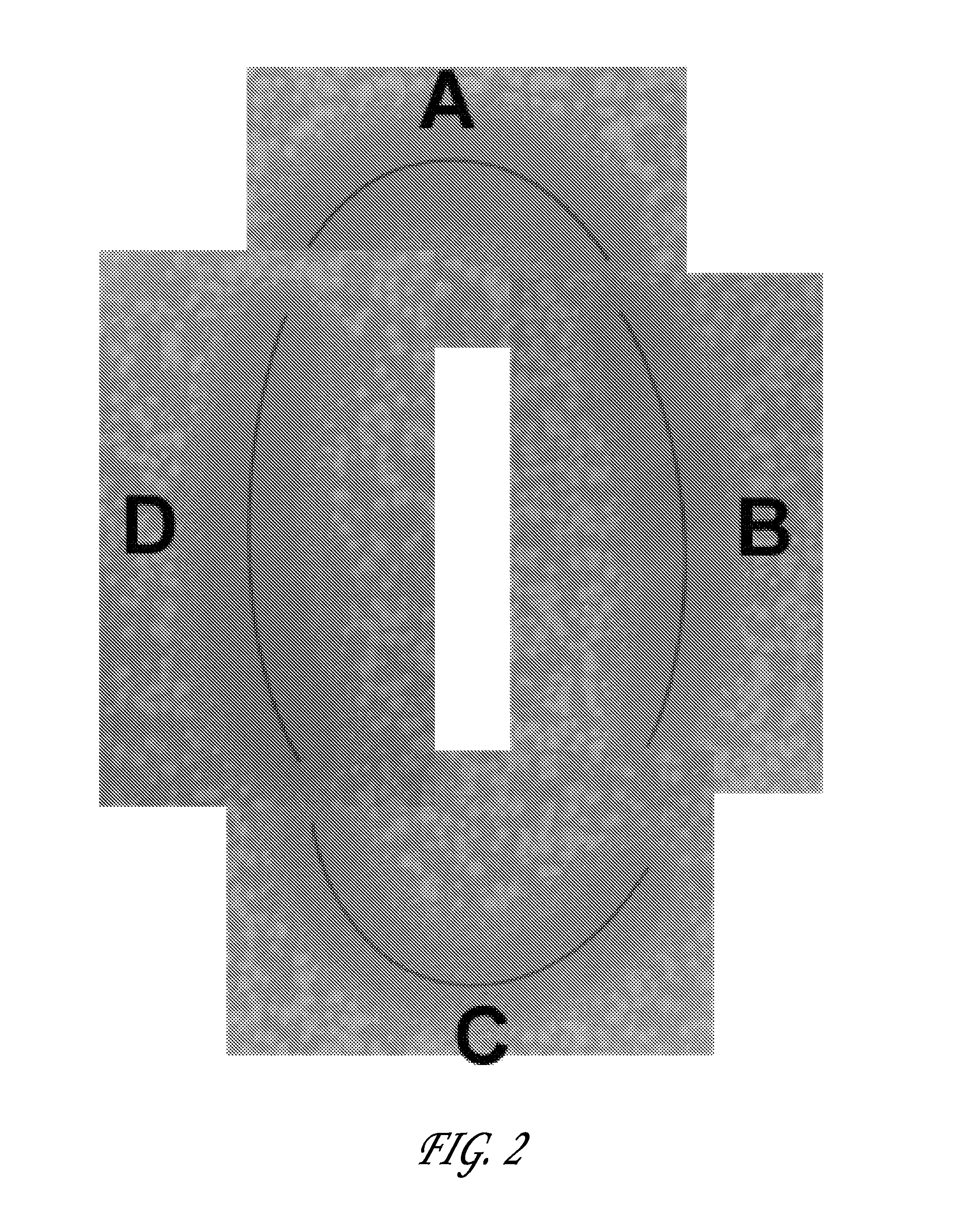
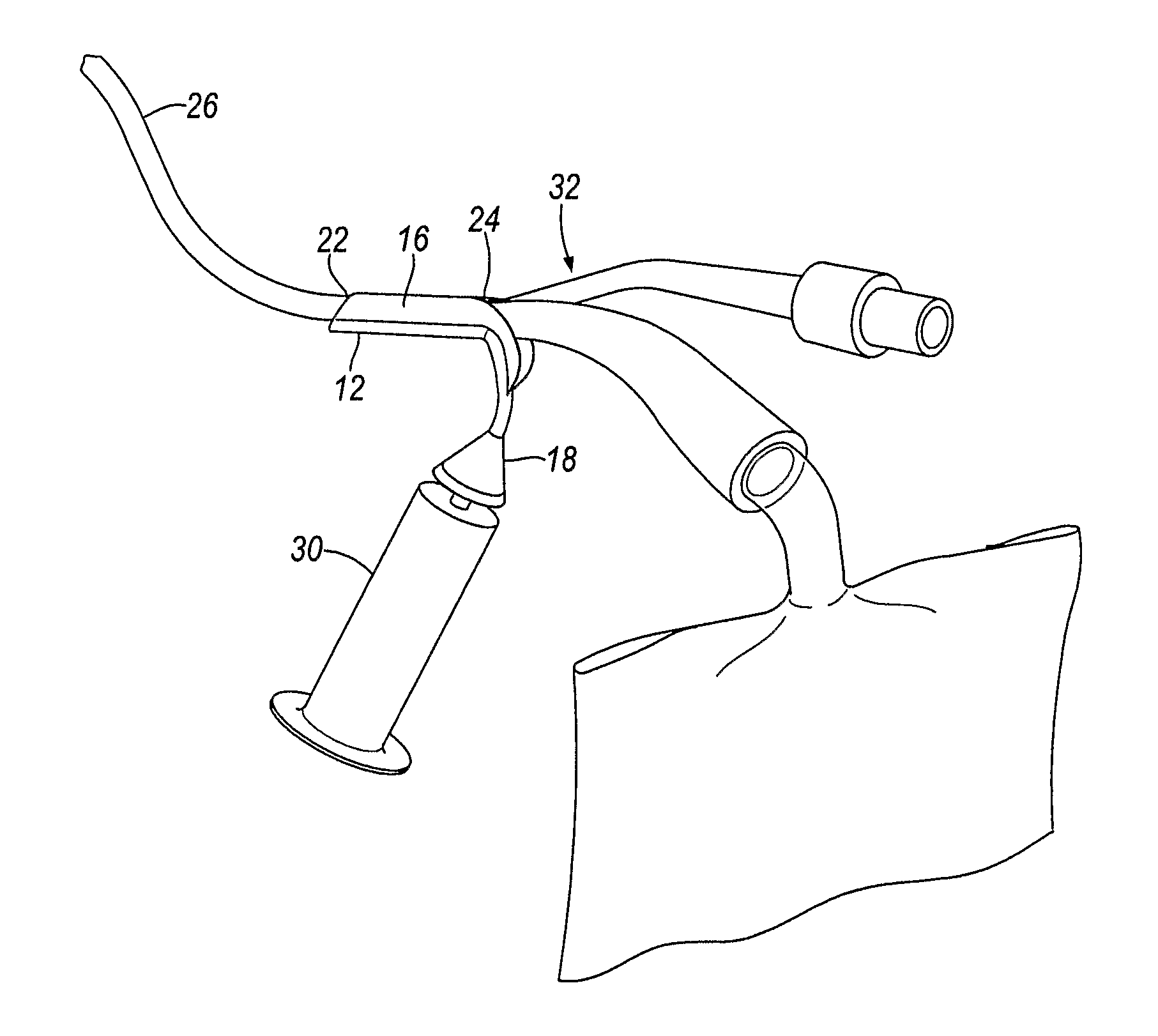
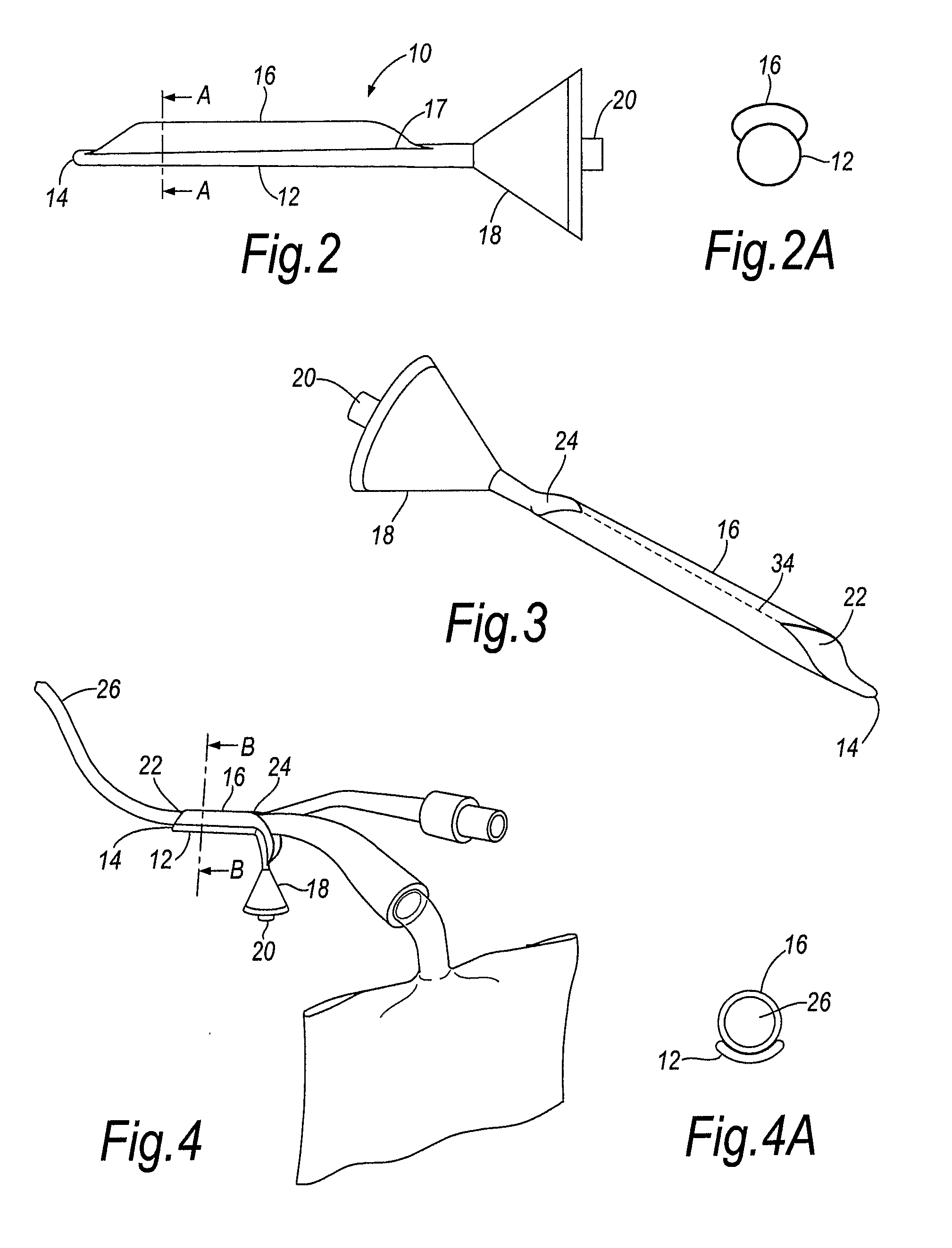
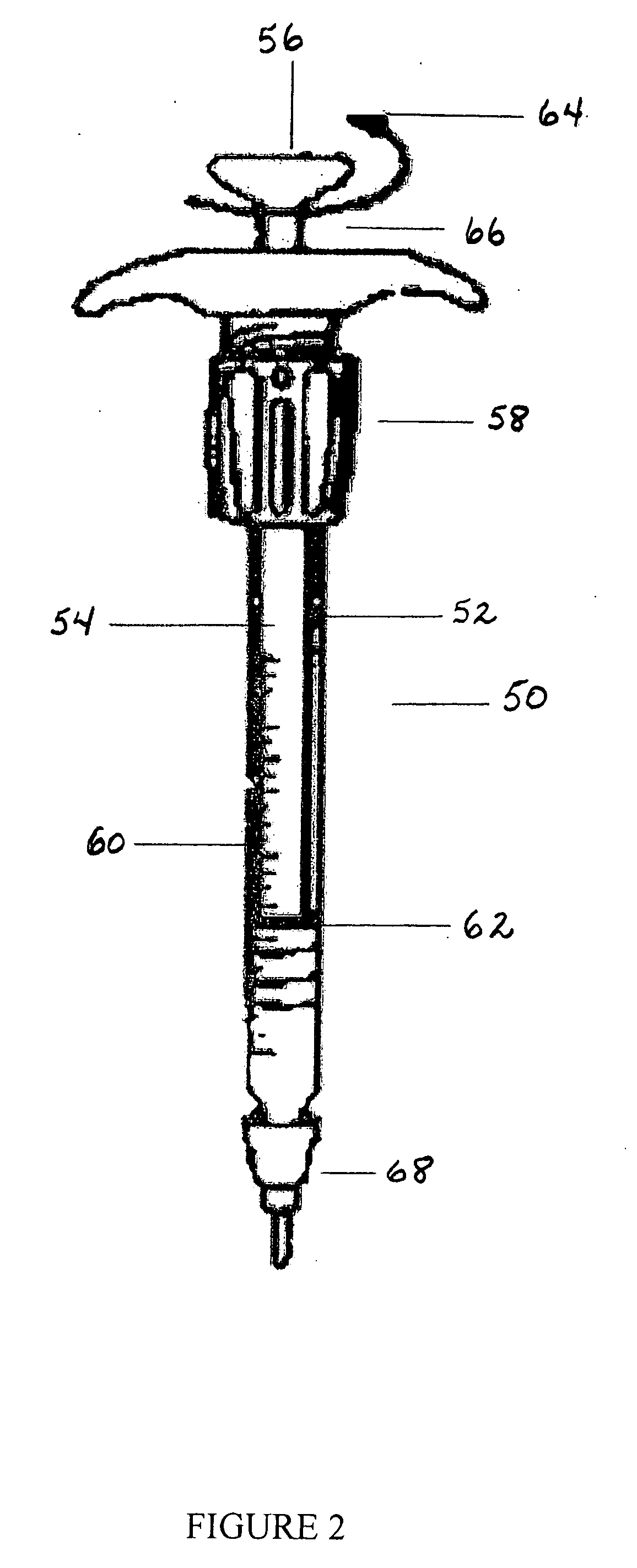
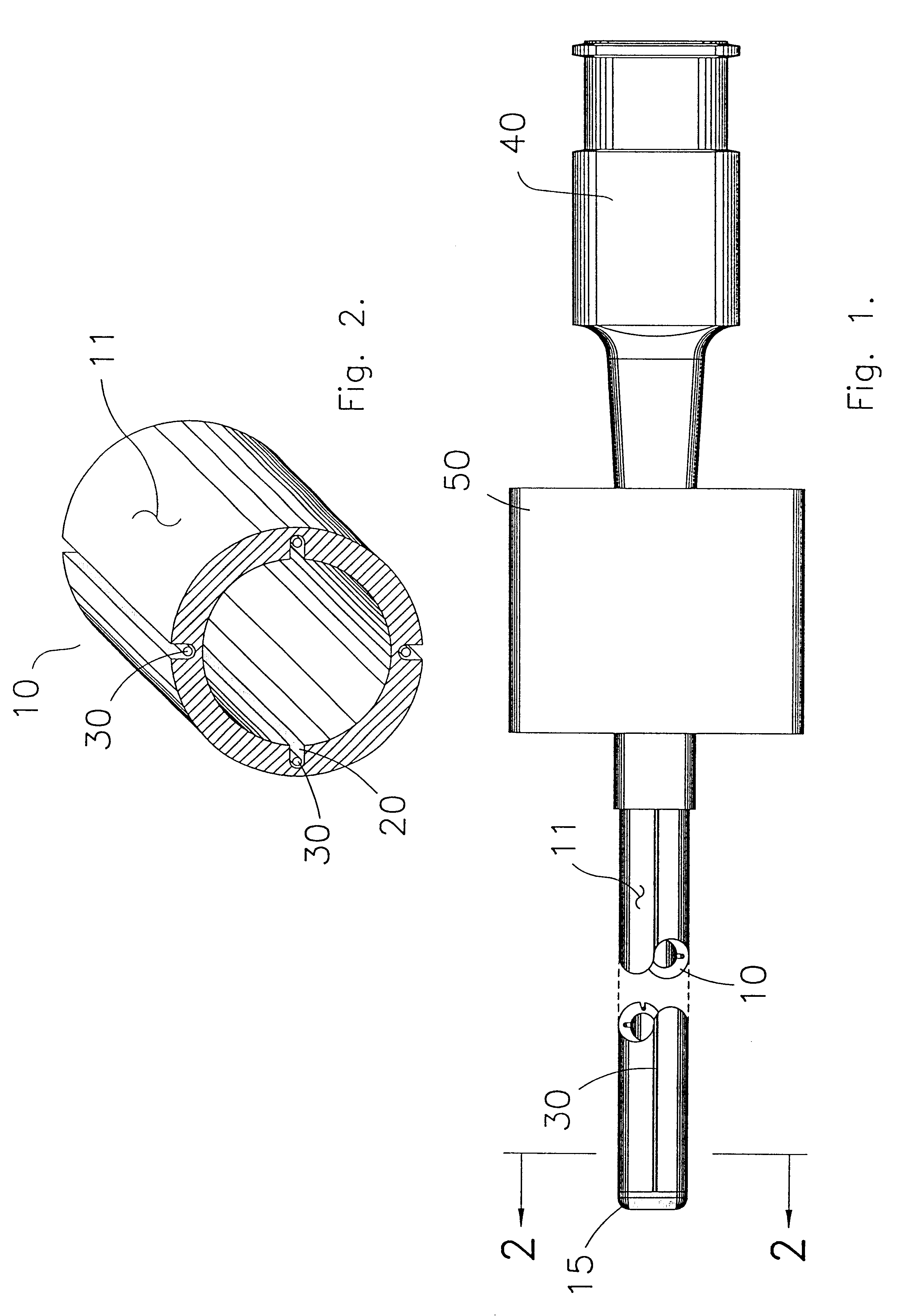
Percutaneous access device
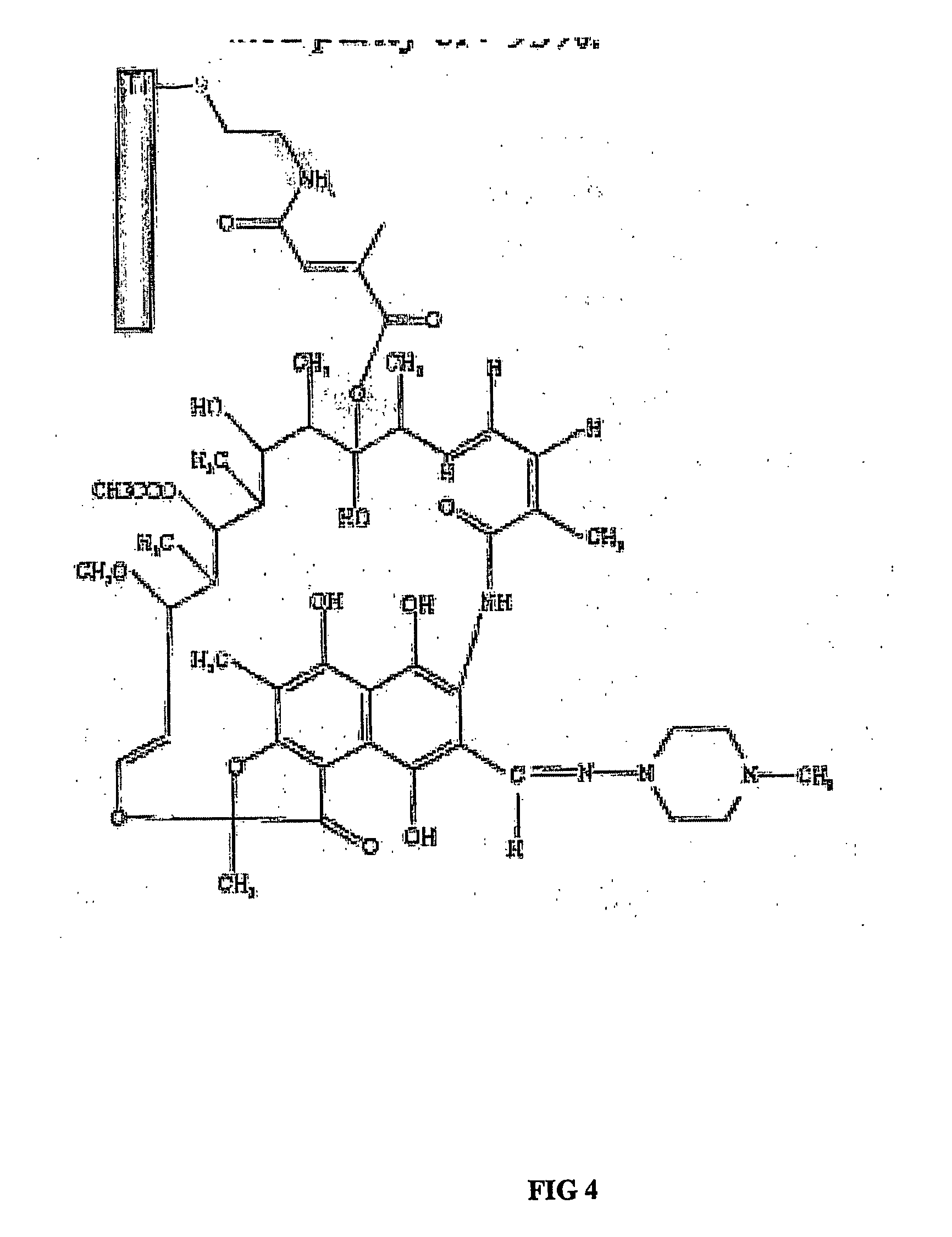
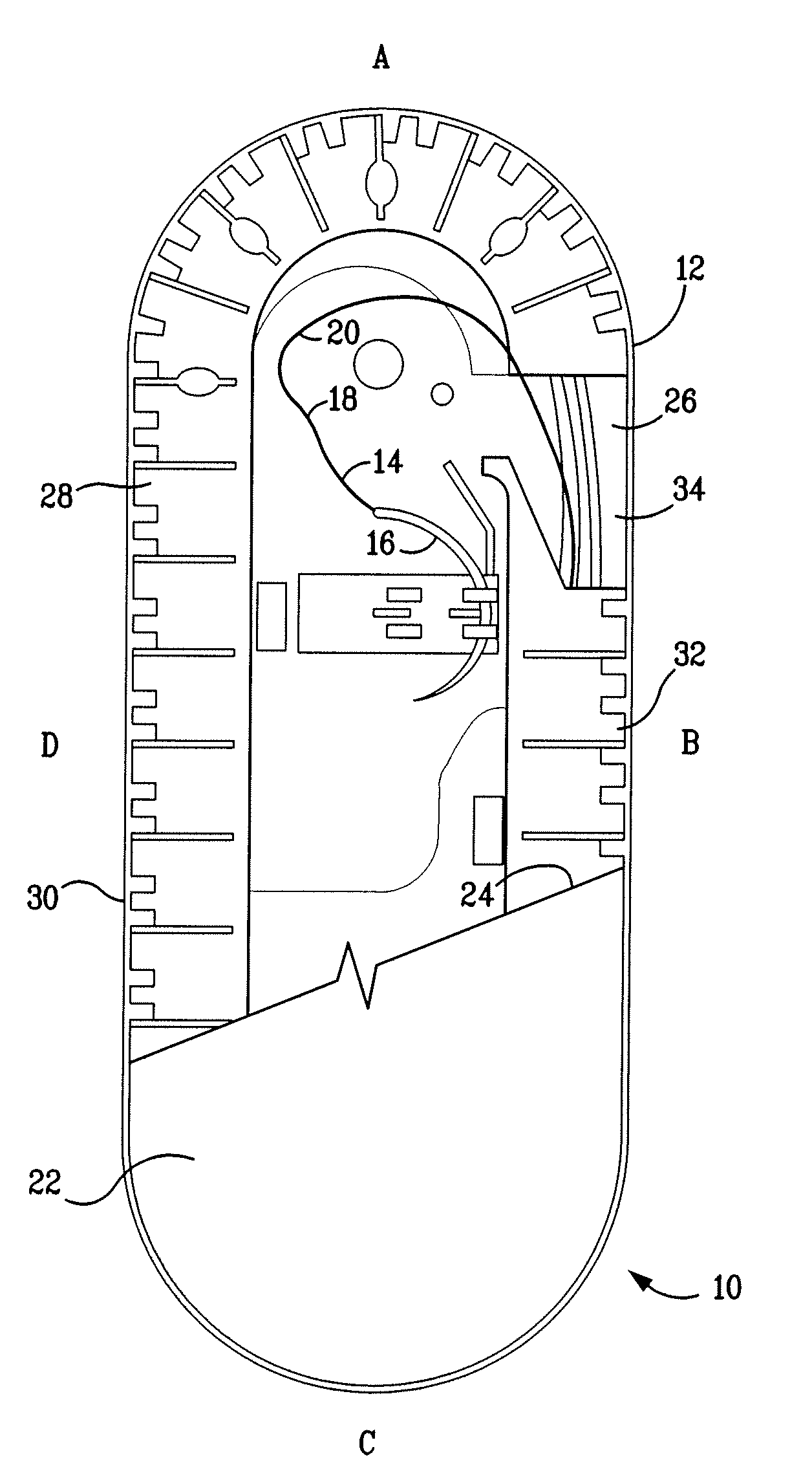
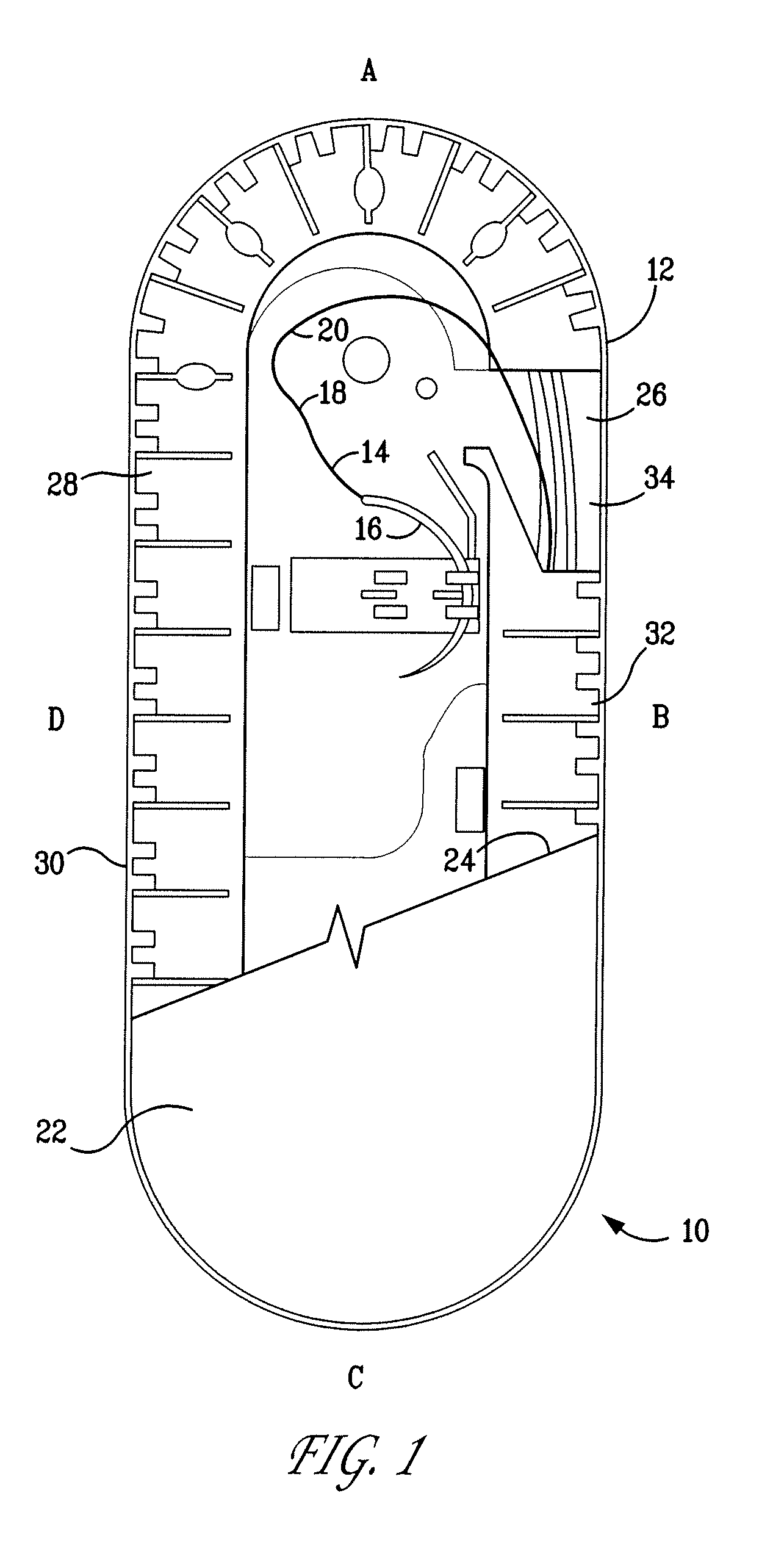
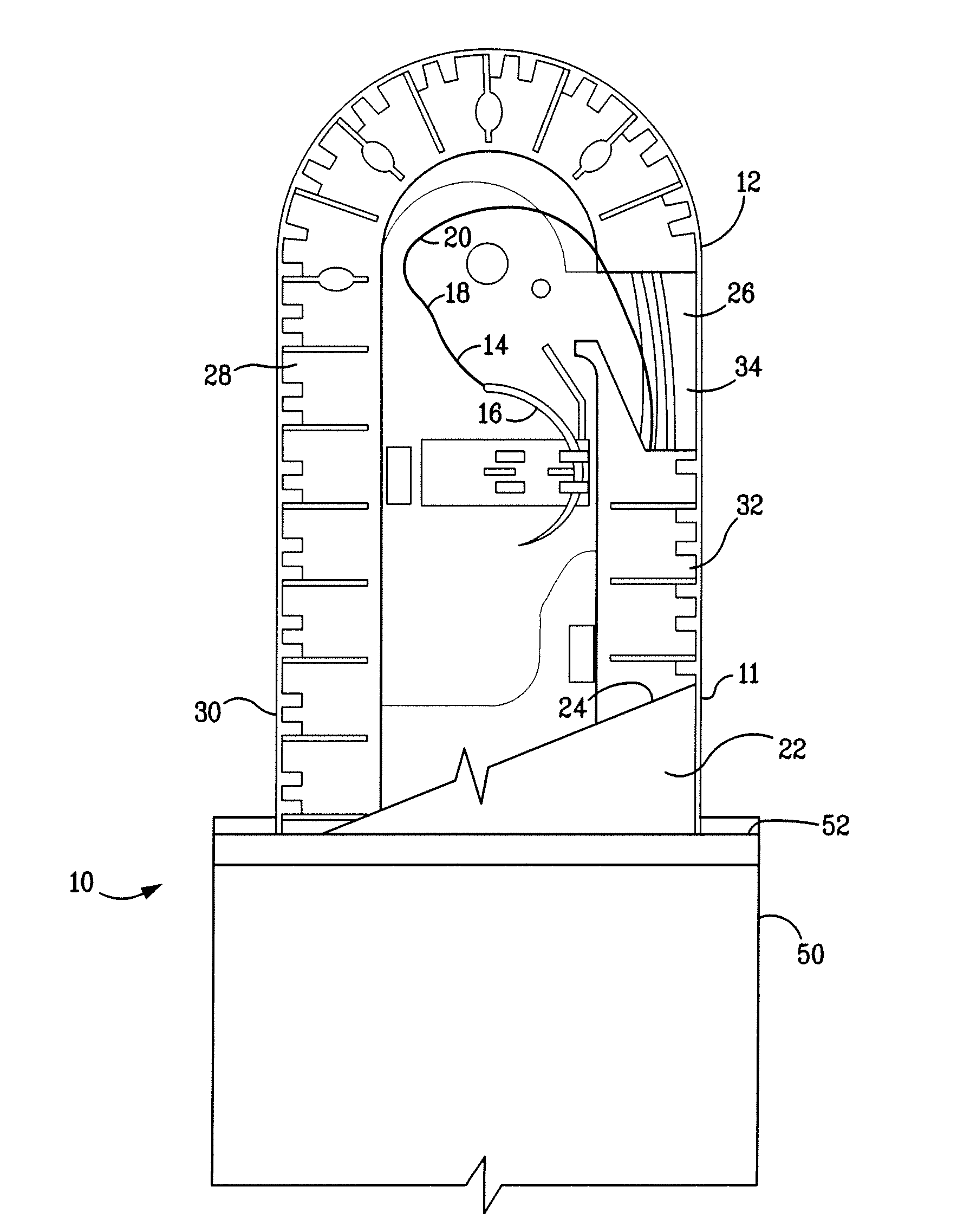
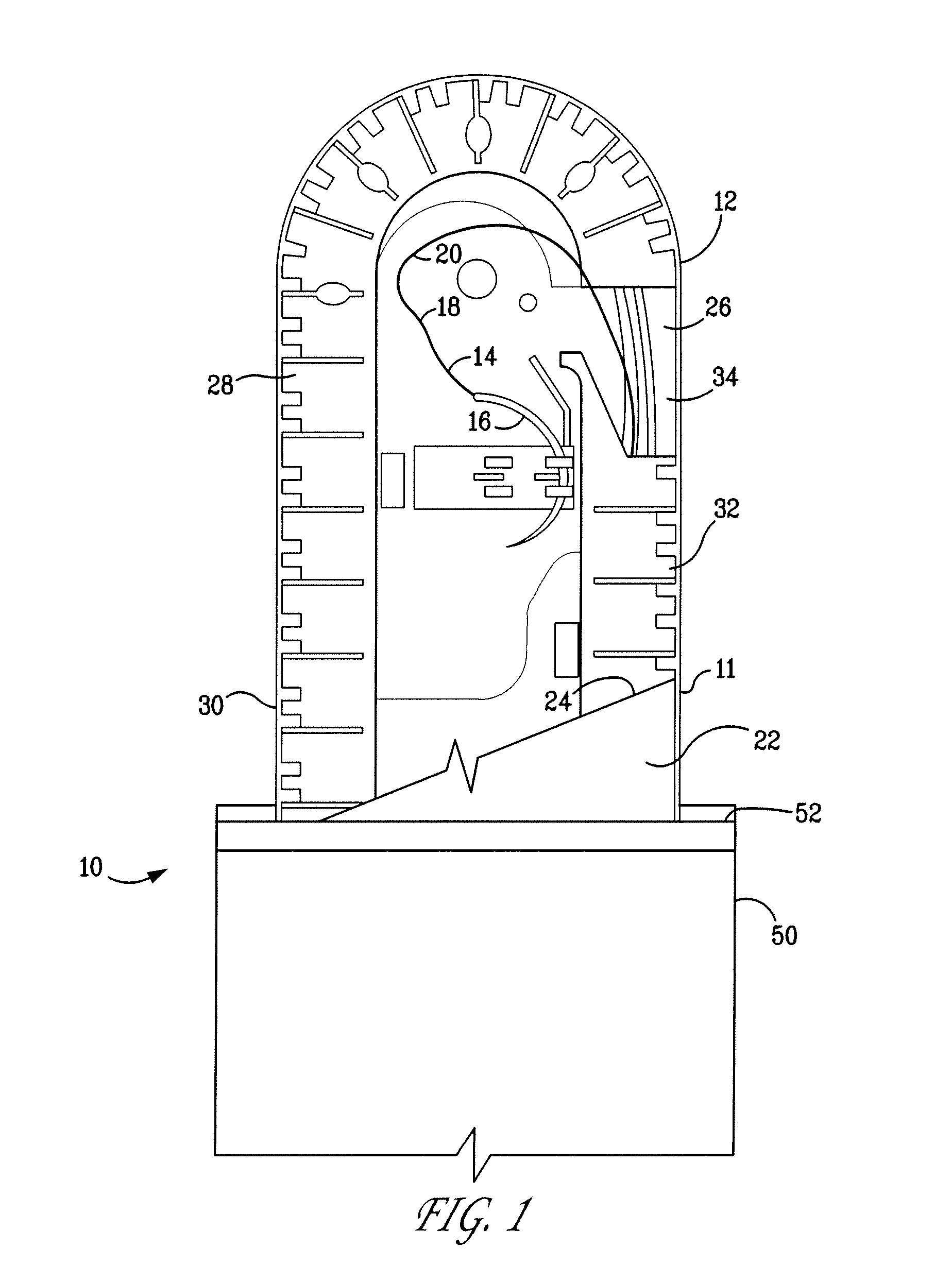
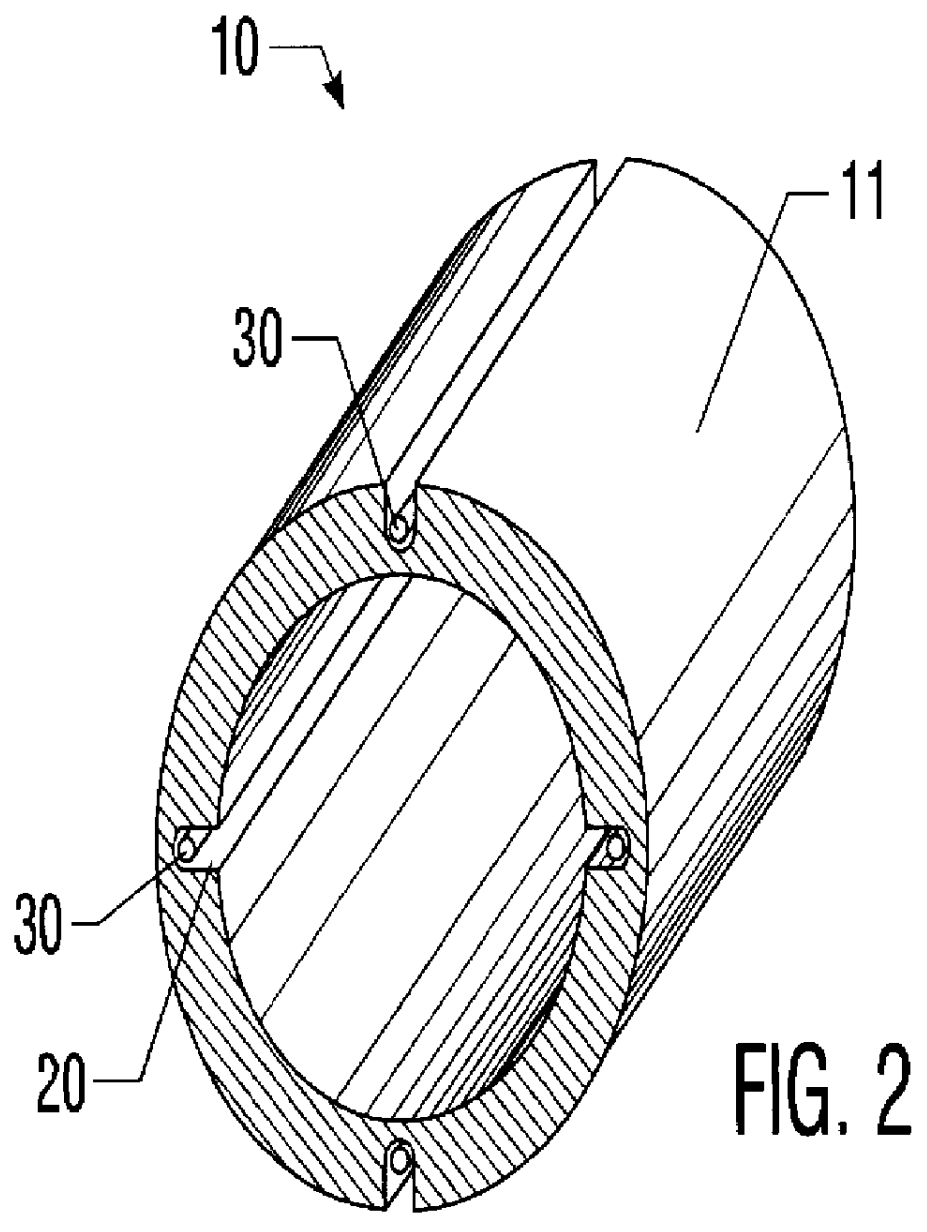
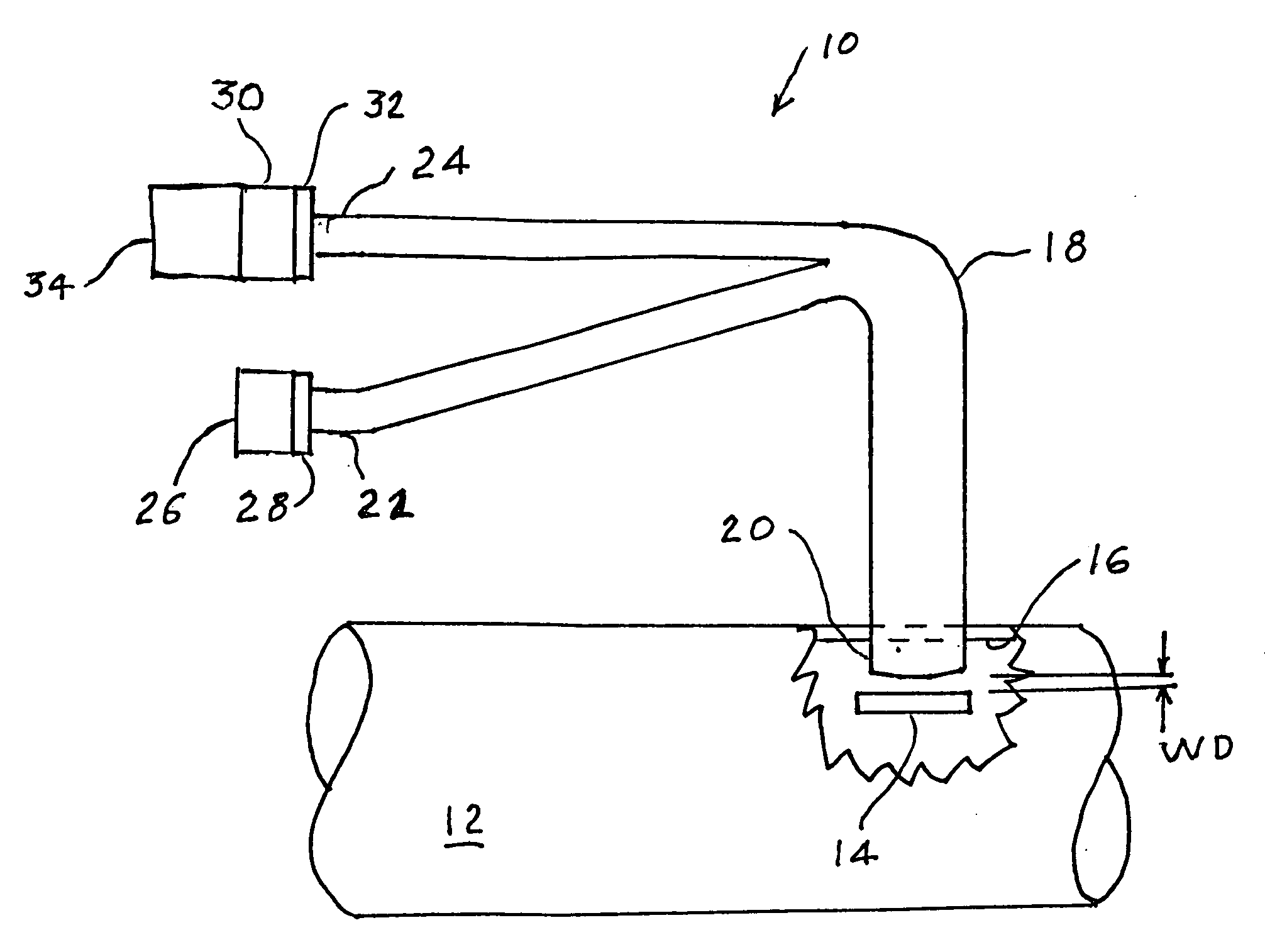
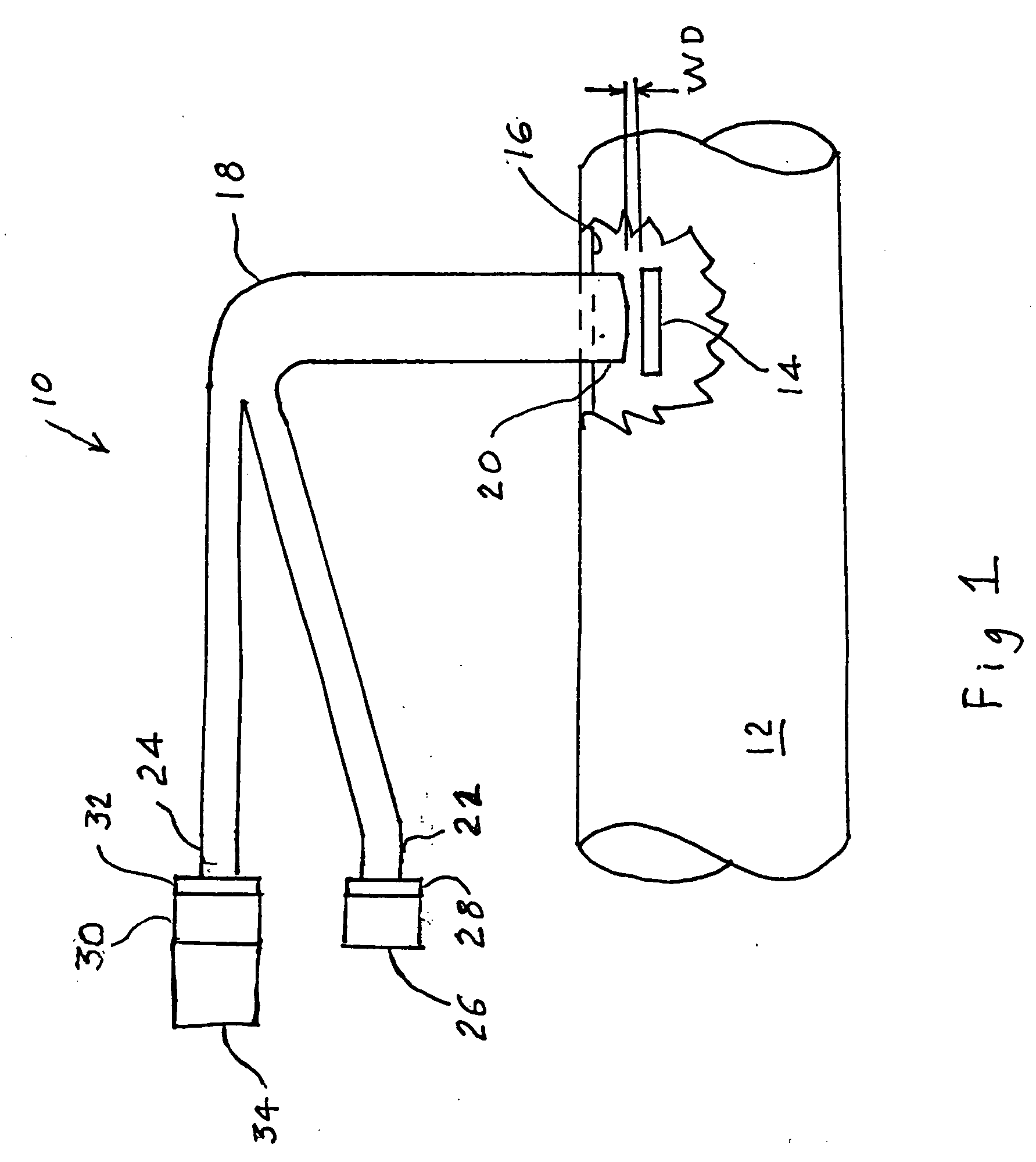
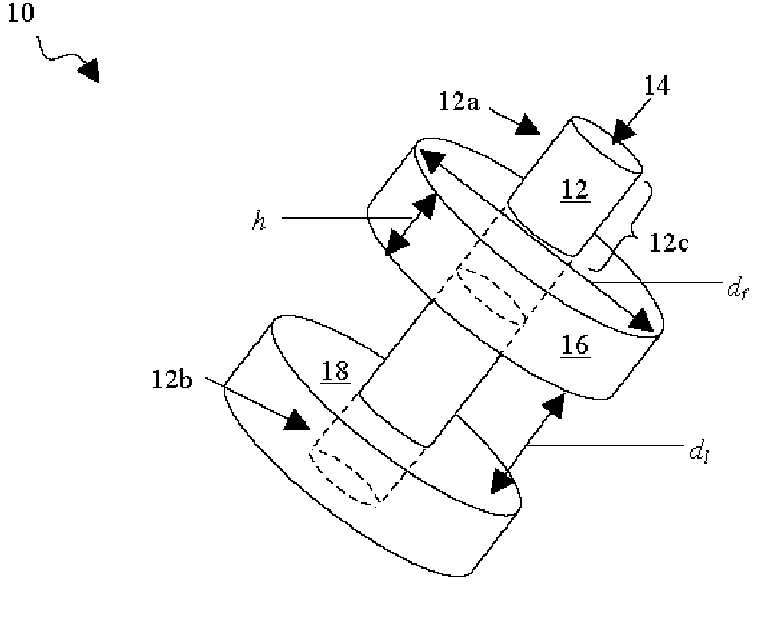
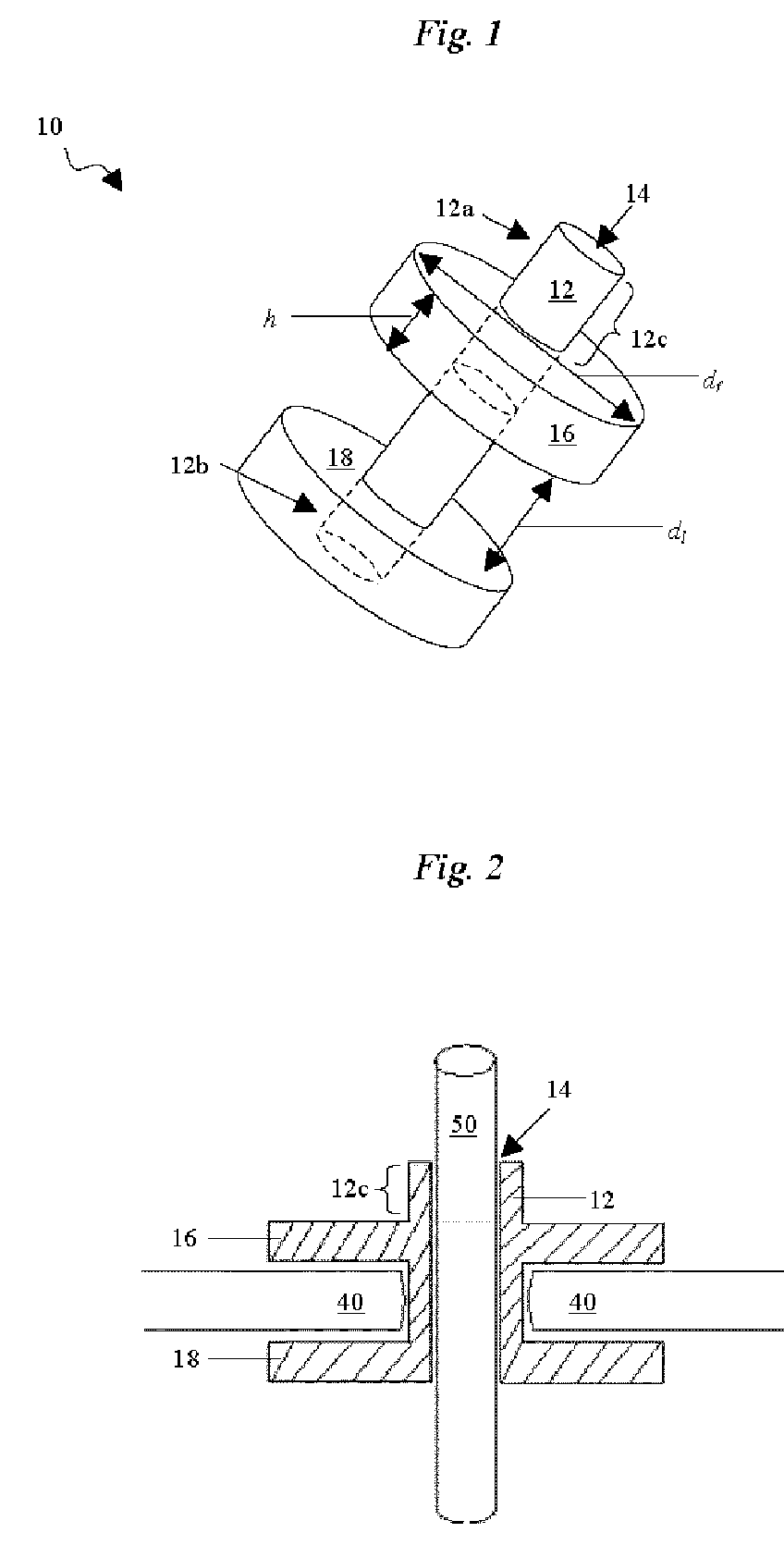
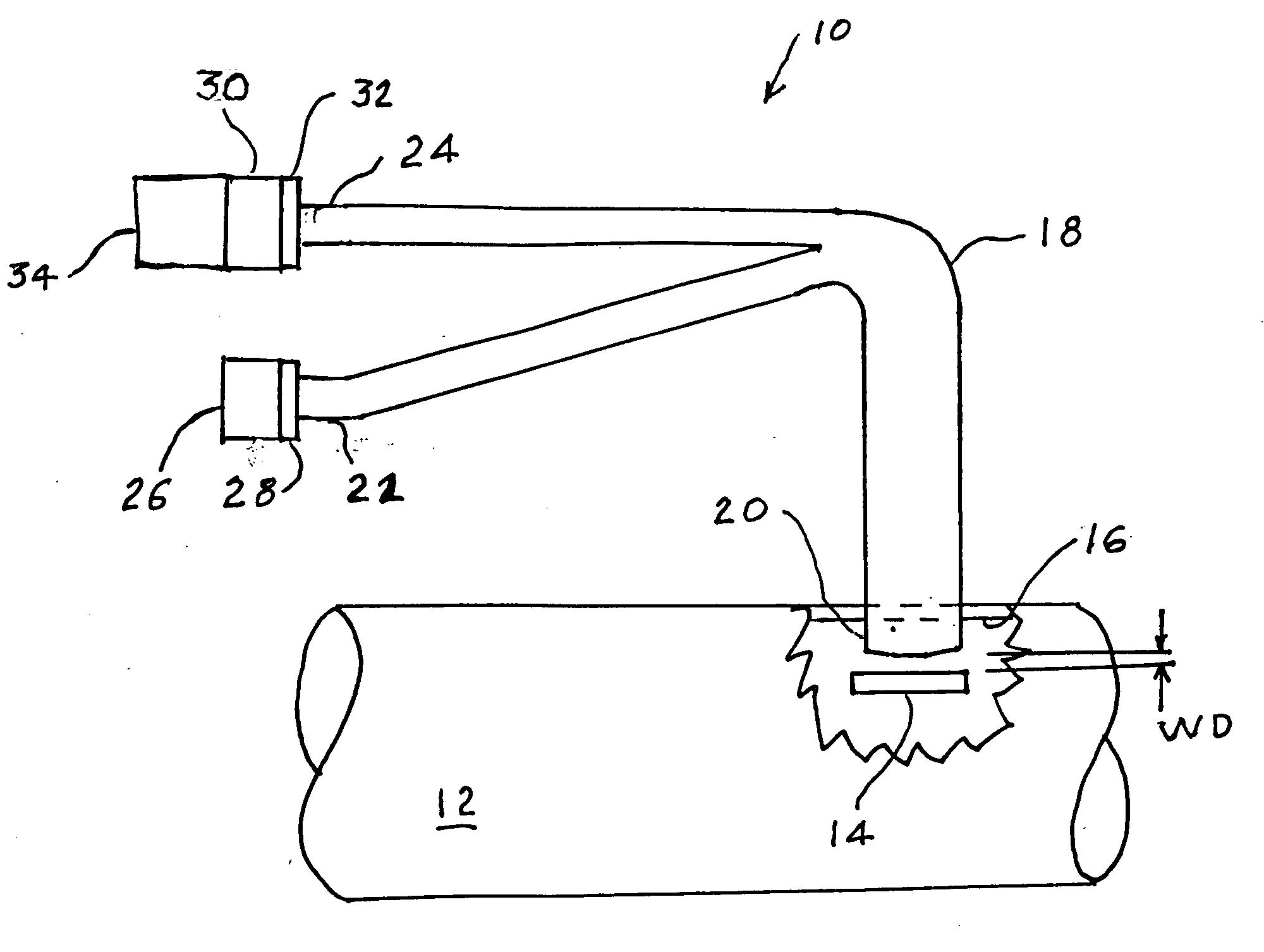
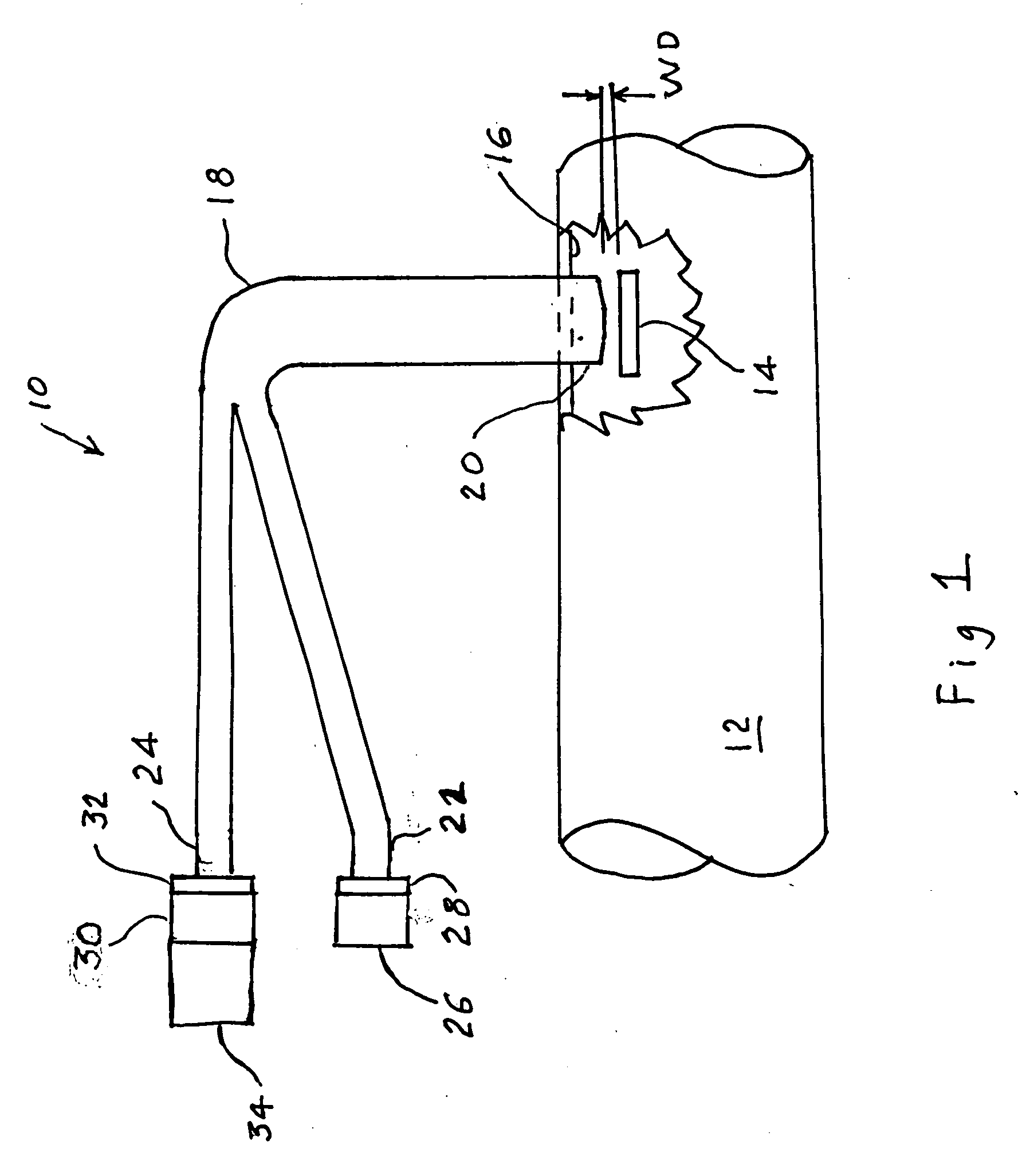
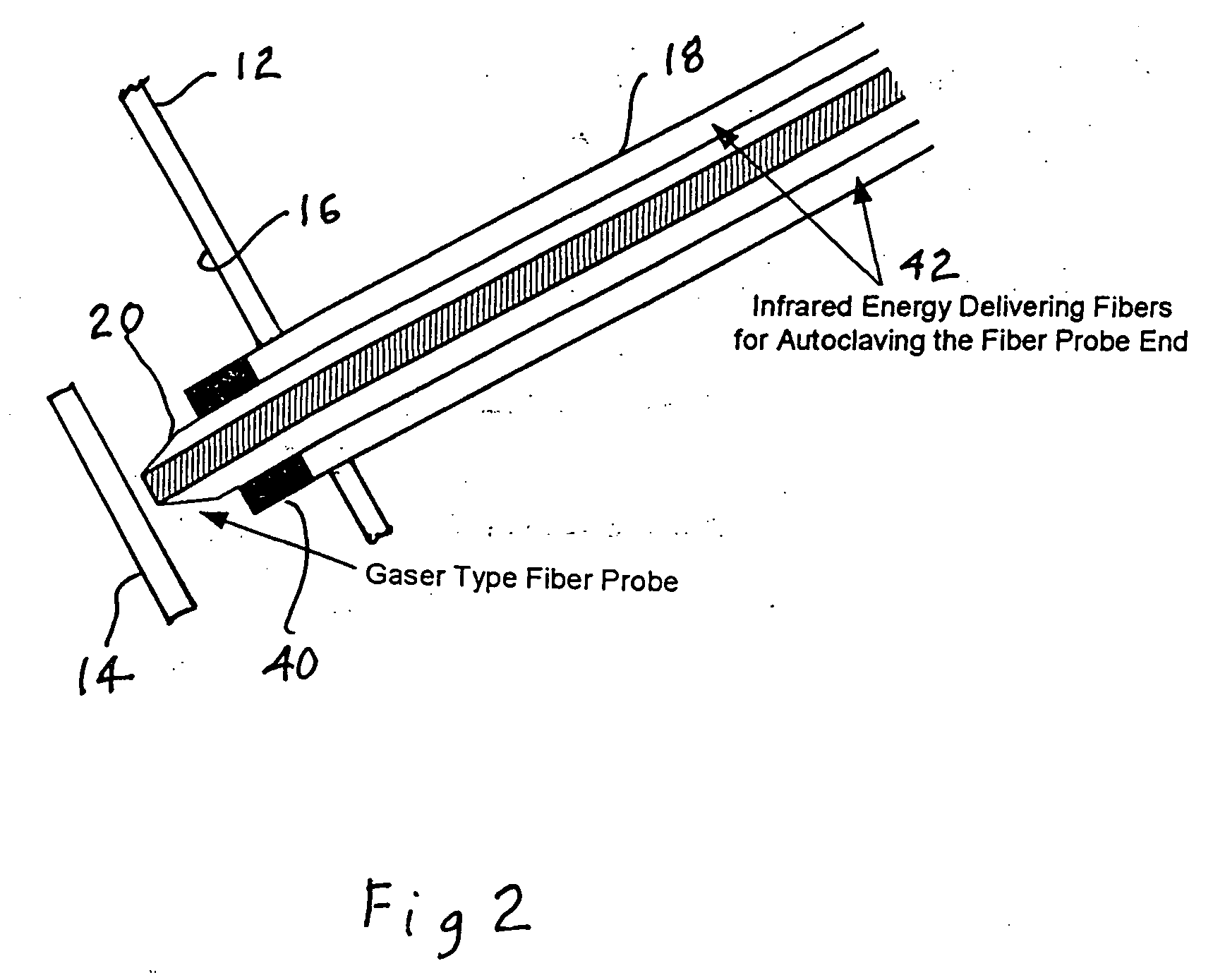
A percutaneous access device is provided including a sleeve having an inner lumen extending therethrough that is adapted to slidably receive a catheter, and at least one flange disposed around the sleeve and adapted to be positioned adjacent a tissue surface. In a preferred embodiment, the device includes a first flange disposed around the sleeve and adapted to be positioned adjacent a first tissue surface, and a second flange disposed around the sleeve and spaced apart from the first flange such that the second flange is adapted to be disposed adjacent a second tissue surface opposed to the first tissue surface. In use, the flanges 16, 18 are positioned on opposed sides of tissue so that the device 10 is effective to prevent tissue surrounding the percutaneous access device from coming into contact with the catheter as it is introduced through the sleeve 12. The device also includes an antimicrobial agent that is effective to protect against bacterial colonization on and around the access device, the catheter, and the tissue surface surrounding the access device.
Owner:INTEGRA LIFESCI SWITZERLAND SARL
Collagen binding protein compositions and methods of use
InactiveUS6288214B1Prevent and lessen adhesionReduce adhesionAntibacterial agentsPeptide/protein ingredientsPassive ImmunizationsCarrier protein
Disclosed are the cna gene and cna-derived nucleic acid segments from Staphylococcus aureus, and DNA segments encoding cna from related bacteria. Also disclosed are Col binding protein (CBP) compositions and methods of use. The CBP protein and antigenic epitopes derived therefrom are contemplated for use in the treatment of pathological infections, and in particular, for use in the prevention of bacterial adhesion to Col. DNA segments encoding these proteins and anti-(Col binding protein) antibodies will also be of use in various screening, diagnostic and therapeutic applications including active and passive immunization and methods for the prevention of bacterial colonization in an animal such as a human. These DNA segments and the peptides derived therefrom are contemplated for use in the preparation of vaccines and, also, for use as carrier proteins in vaccine formulations, and in the formulation of compositions for use in the prevention of S. aureus infection.
Owner:TEXAS A&M UNIVERSITY
Implants with attached silylated therapeutic agents
InactiveUS20060286140A1Promote osseointegrationPrevent bacterial growthAntibacterial agentsBiocideBiofilmAntibiotic Y
The present invention is directed to implants that include therapeutic molecules bonded to their surfaces. The therapeutic molecules interact with cells that are adjacent, near, or adhering to the implant. The covalently-bonded therapeutic molecules may be released from the implant surface by changes in pH or enzymes characteristic of cells adjacent to the implant. Preferably the covalently-bonded agents include an antibiotics that are released from the implant surface by bacteria and in this way ensures that the antibiotic is released at sites on the implant that would serve as centers for both bacterial colonization and biofilm formation.
Owner:SMART TECH INC (CA)
Packaged antimicrobial medical device and method of preparing same
ActiveUS20100078336A1Prevent bacterial growthSuture equipmentsSurgical furniturePolymer resinEngineering
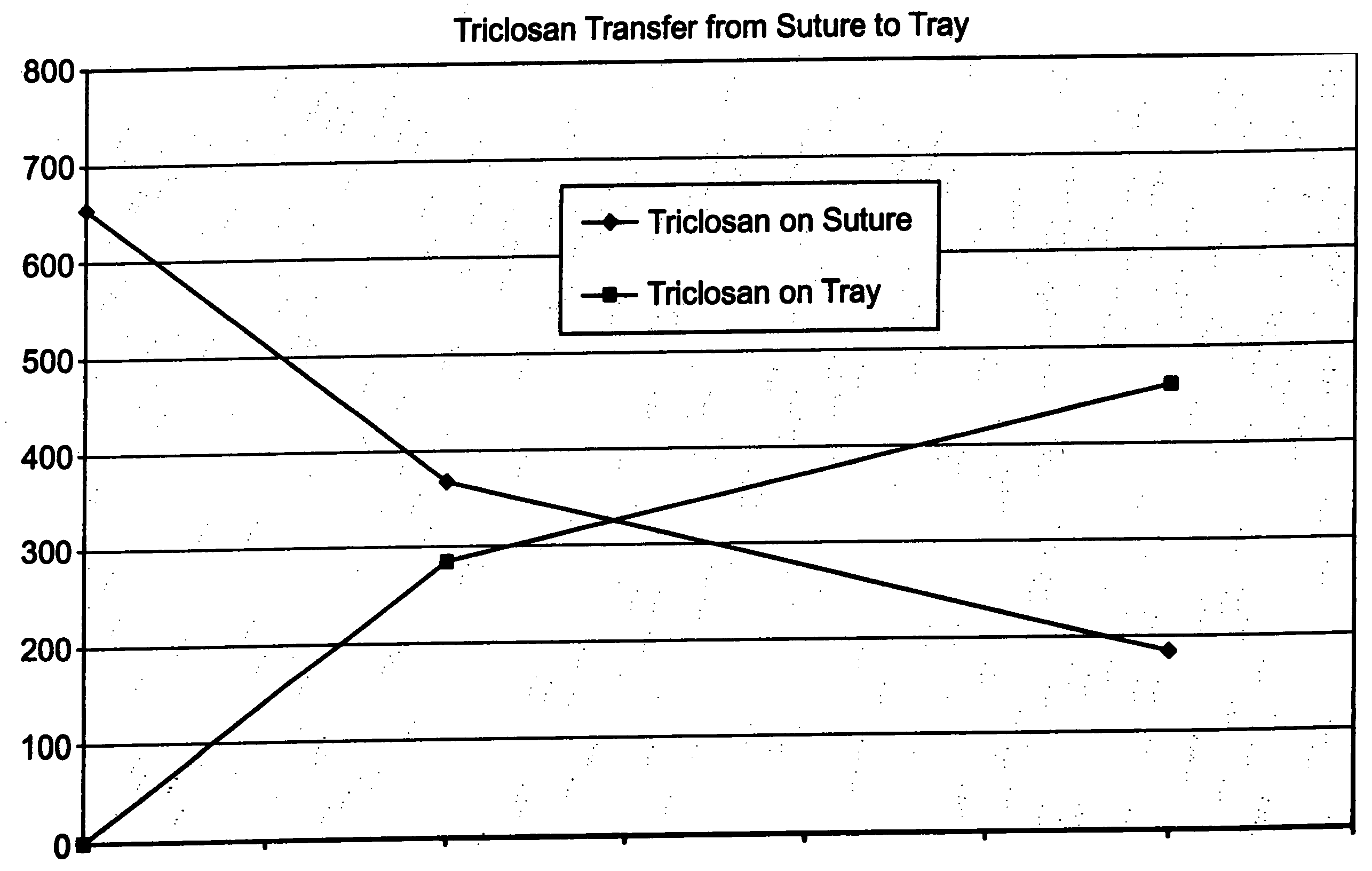
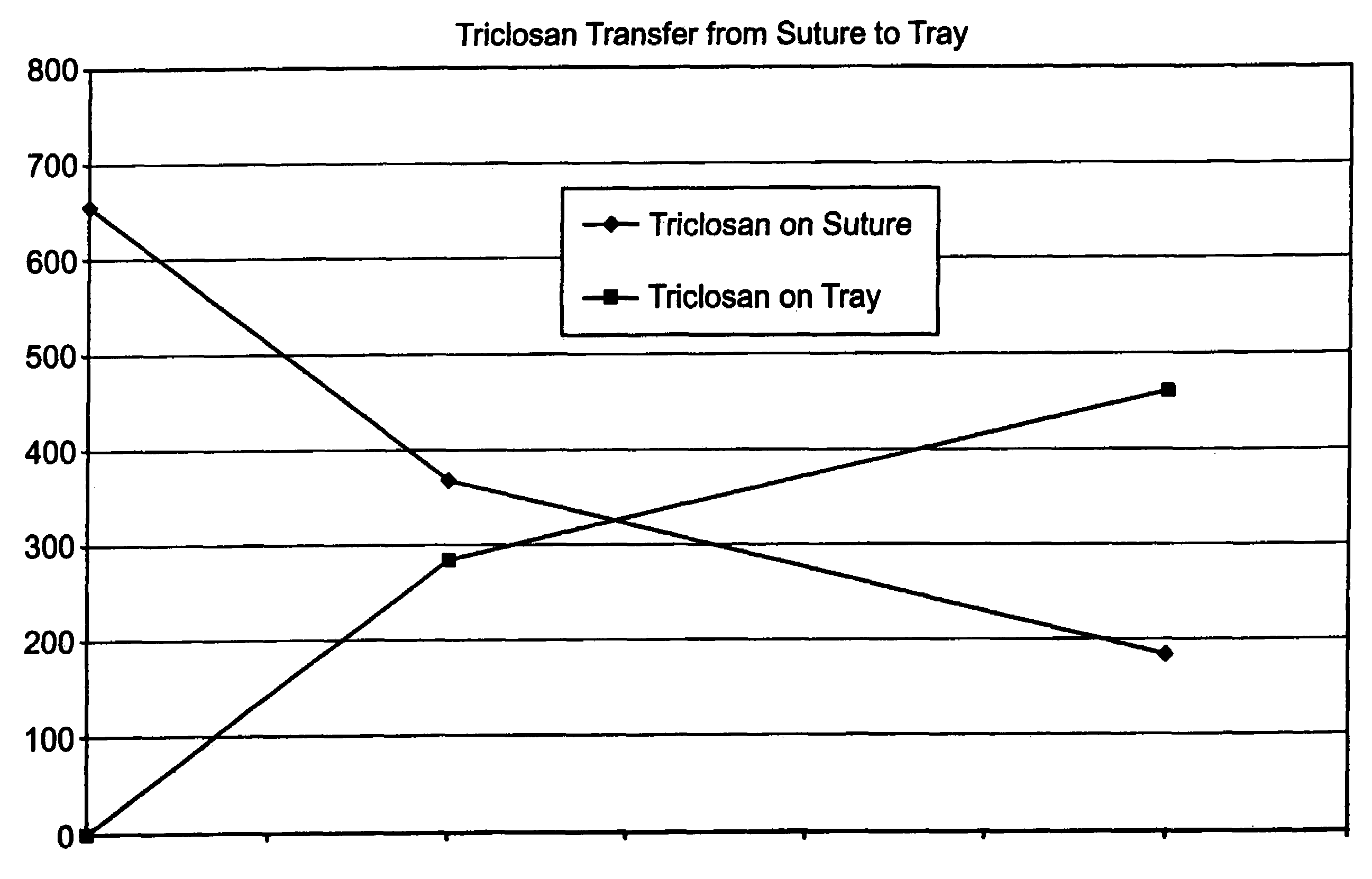
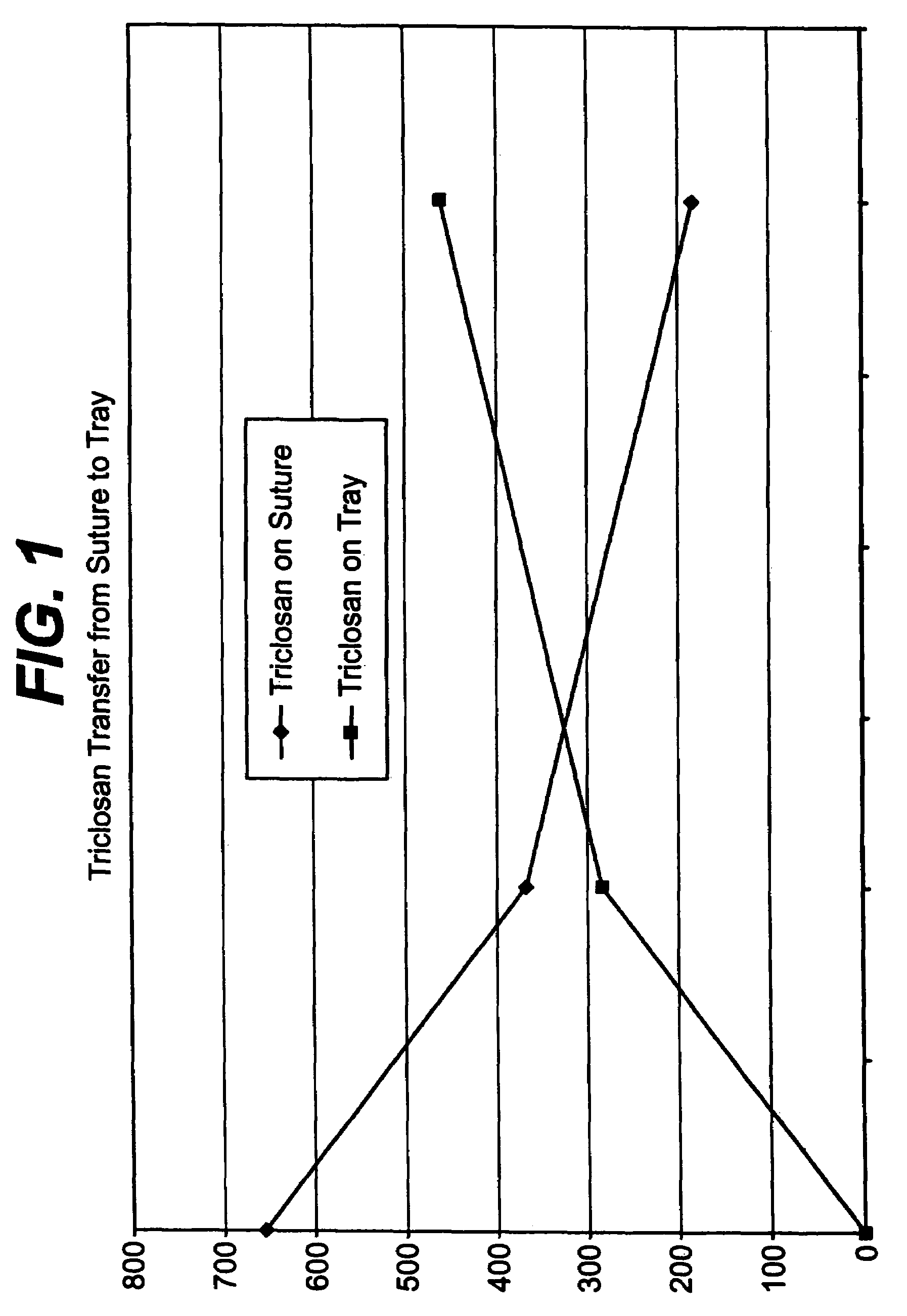
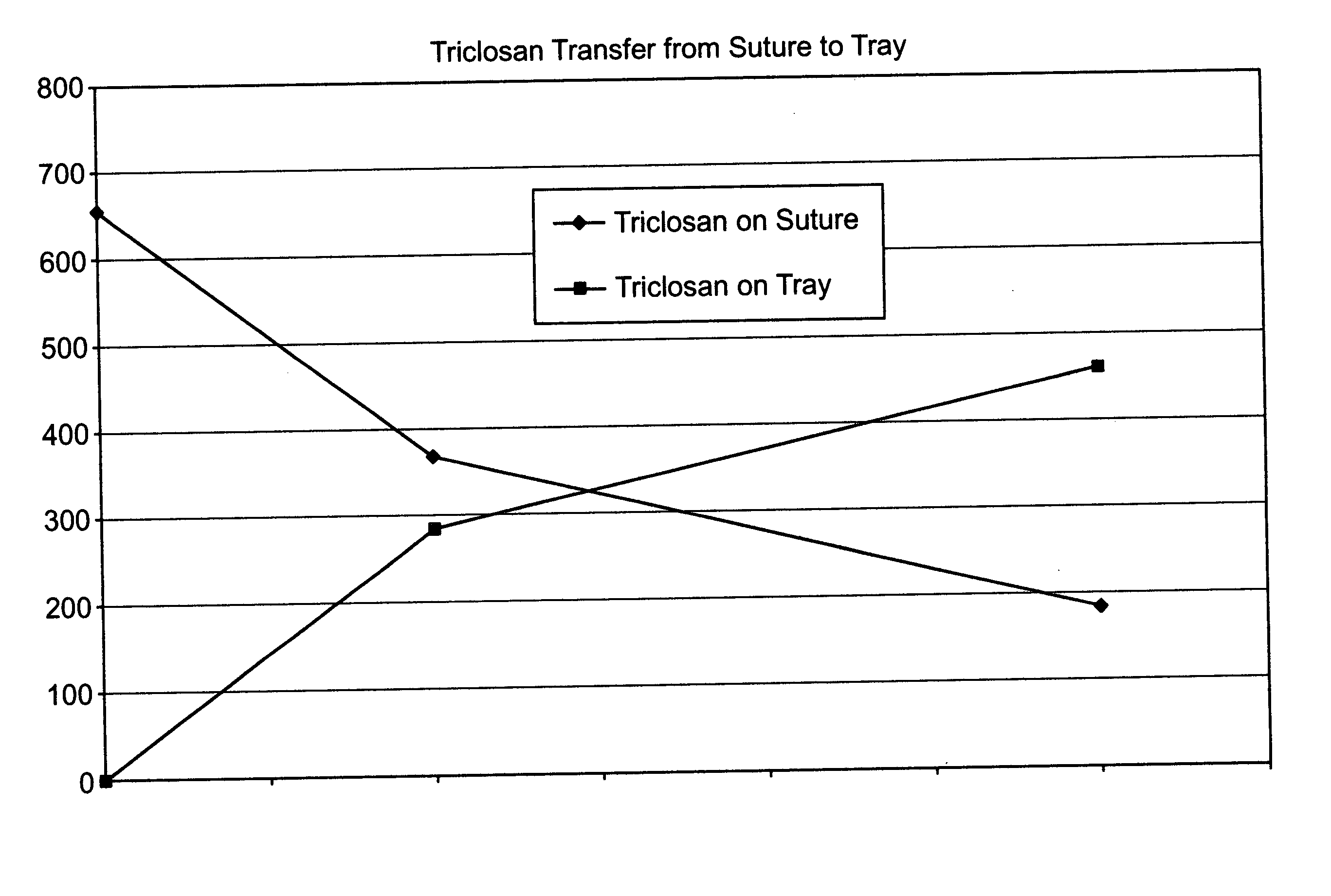
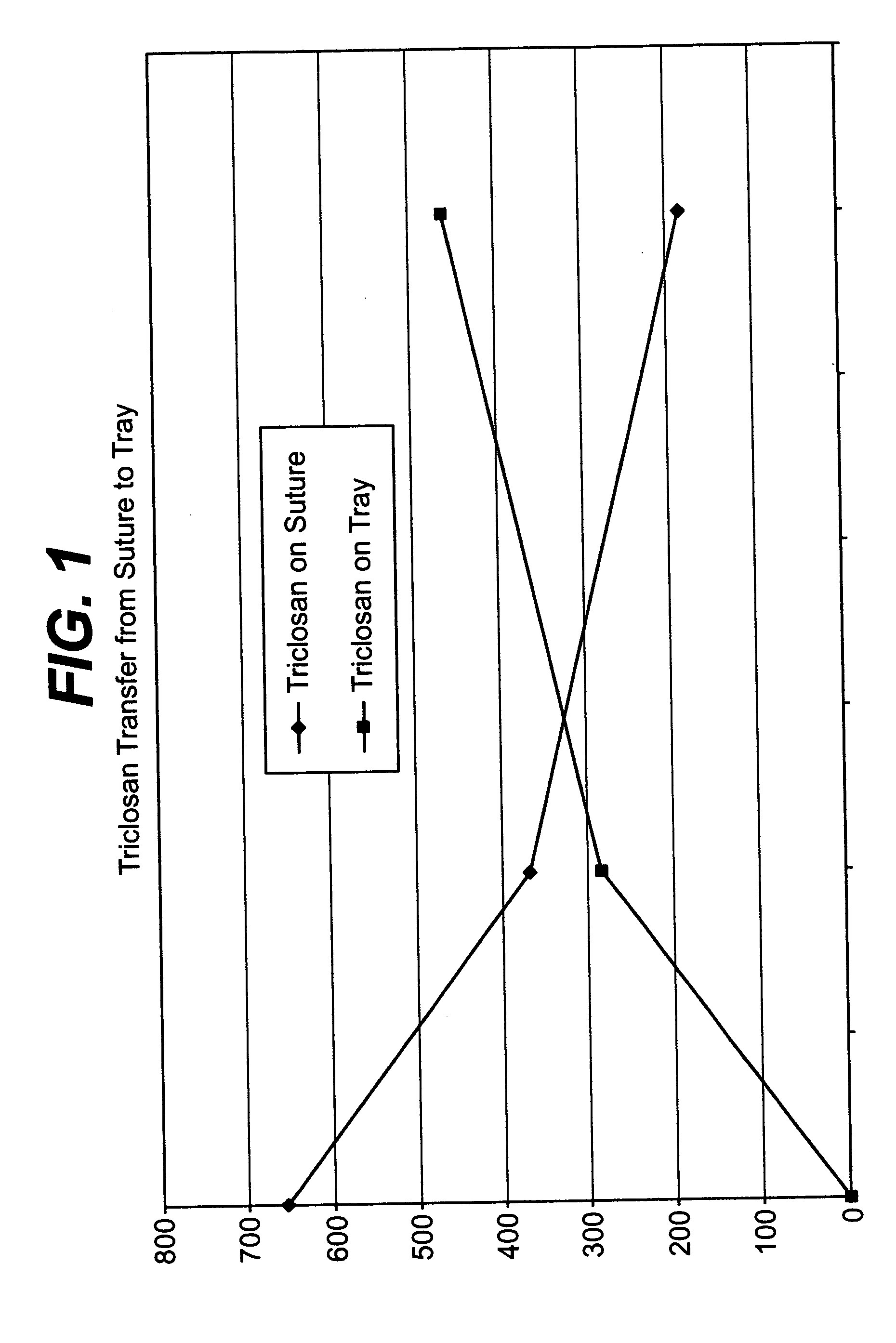
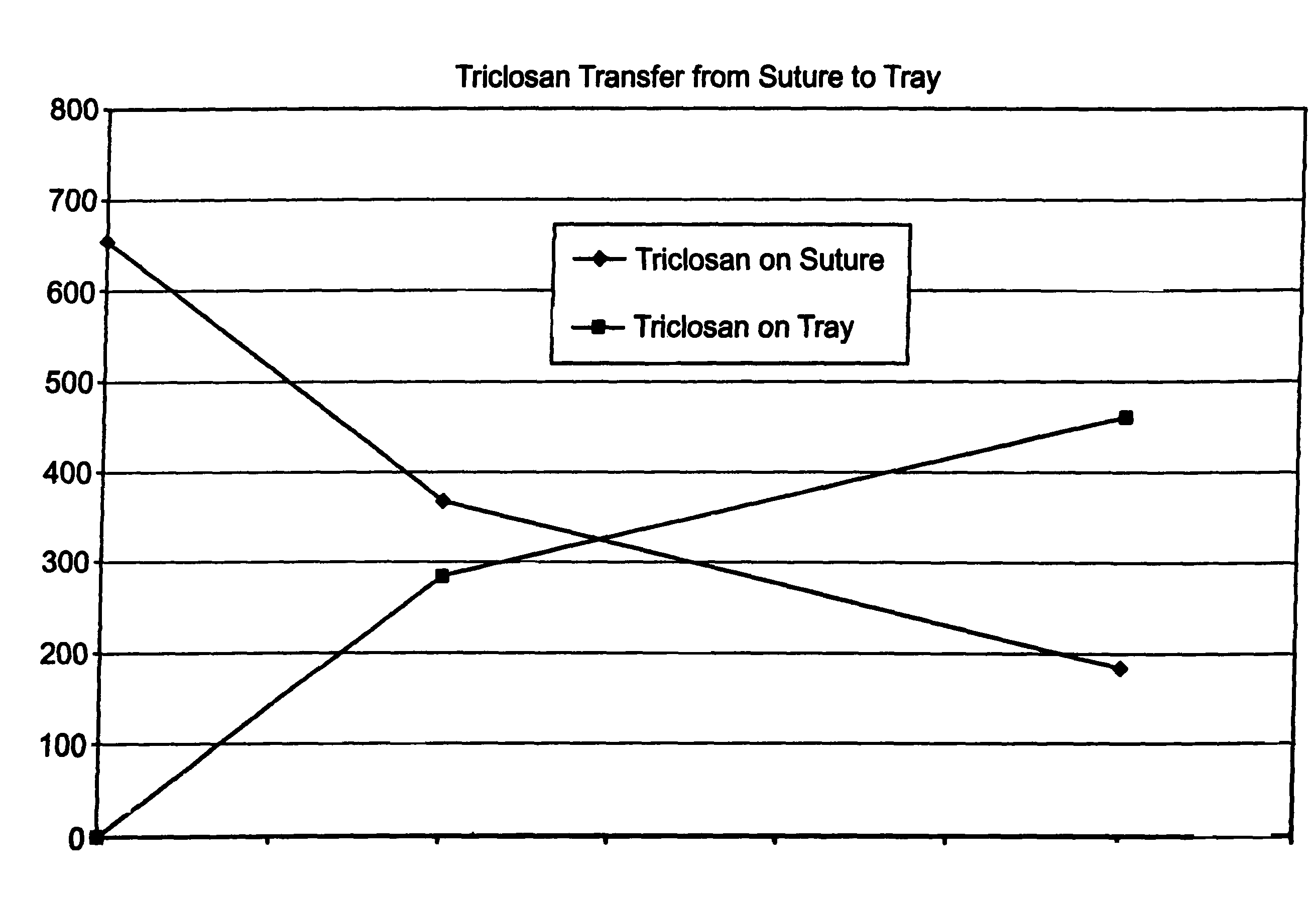
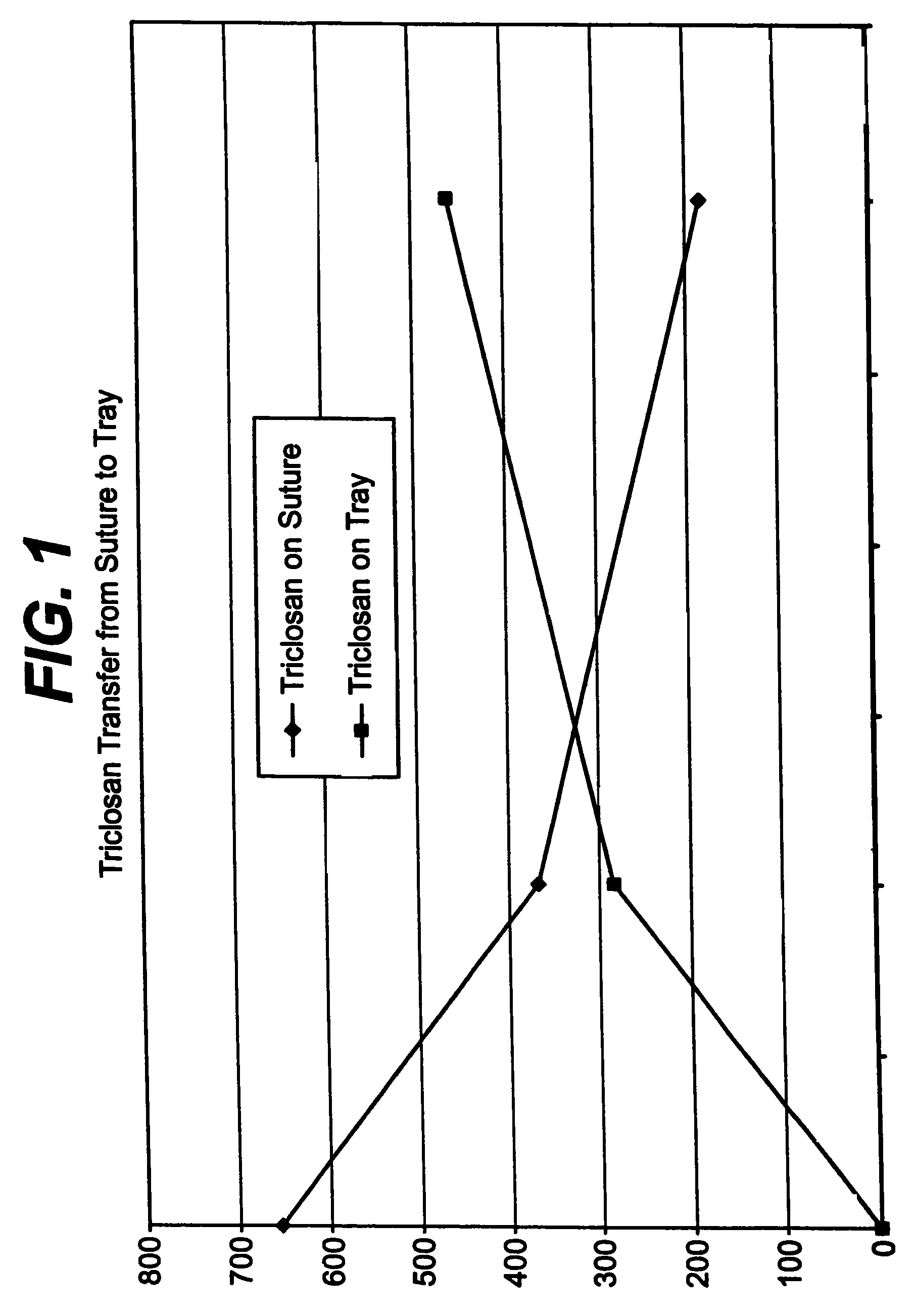
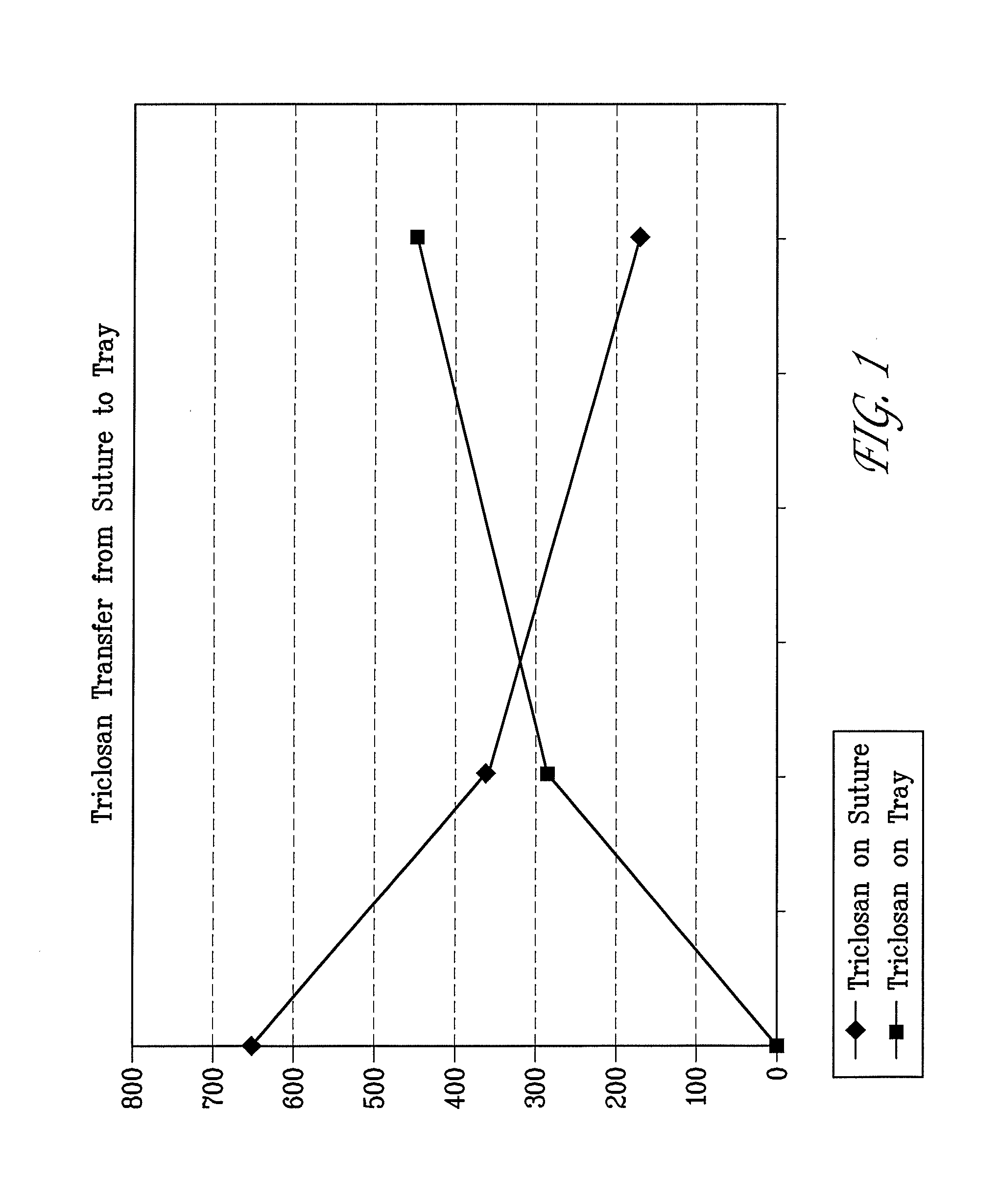
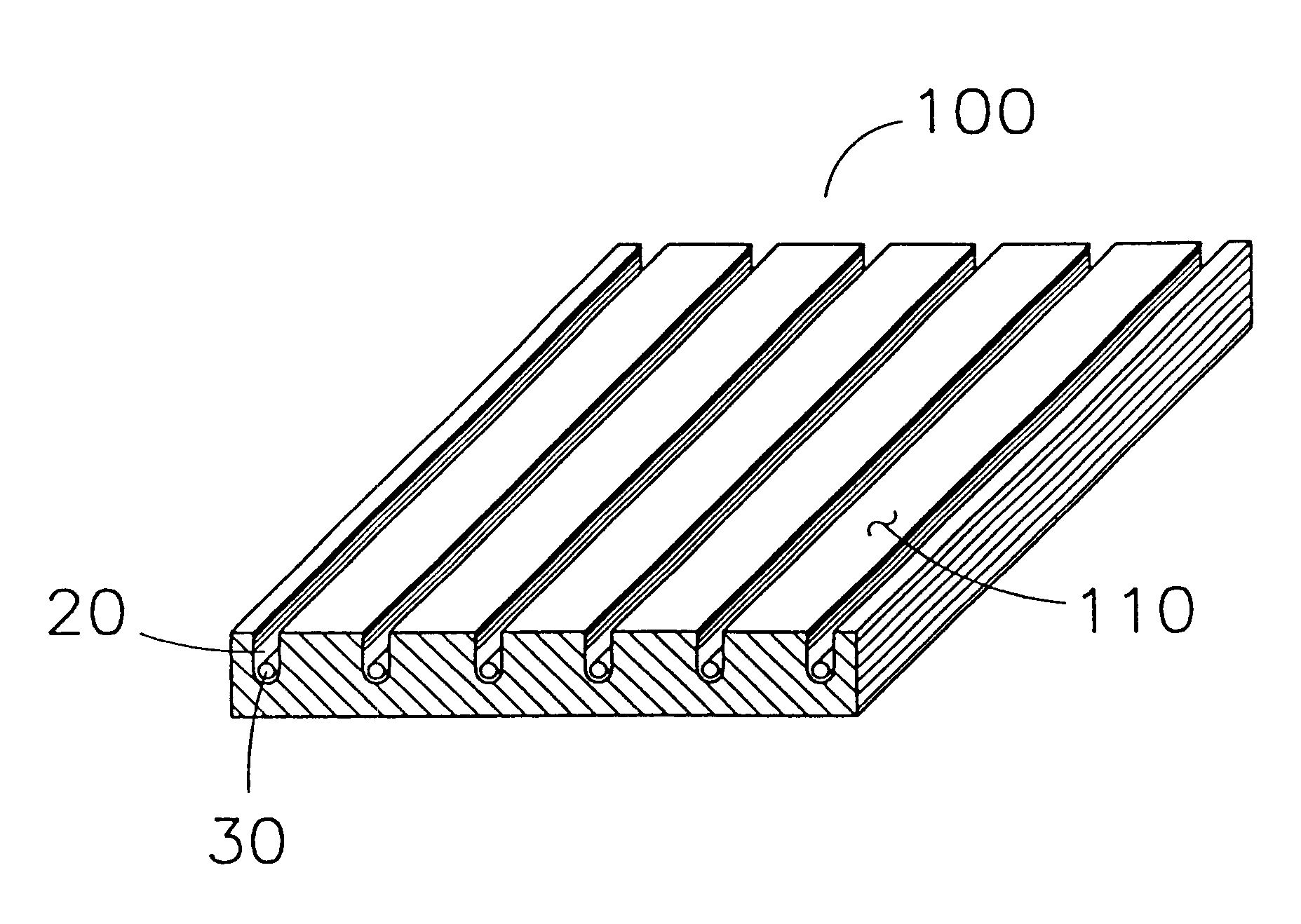
A method of making a packaged antimicrobial suture. The method includes the steps of providing a containment compartment molded from a polymeric resin comprising a polymeric material and an antimicrobial agent, positioning a suture within the containment compartment, the suture comprising one or more surfaces; covering the containment compartment having the suture in an outer package cover having an inner surface, and subjecting the outer package, the containment compartment and the suture to time, temperature and pressure conditions sufficient to vapor transfer an effective amount of the antimicrobial agent from the containment compartment to the suture, while retaining an effective amount of the antimicrobial agent on the containment compartment, thereby substantially inhibiting bacterial colonization on the suture and the containment compartment. A packaged antimicrobial suture is also provided.
Owner:ETHICON INC
Packaged antimicrobial medical device having improved shelf life and method of preparing same
ActiveUS20100163435A1Extended shelf lifeInhibition of colonizationSuture equipmentsSurgical furnitureMedicineBacterial colonization
A method of making a packaged antimicrobial suture having improved shelf life. The method comprising the steps of providing an inner package having a source of antimicrobial agent, providing an adsorbent material effective to adsorb a portion of the antimicrobial agent over time, positioning a suture within the inner package, the suture comprising one or more surfaces, covering the inner package with an outer package having an inner surface and subjecting the suture, the inner package and the inner surface of the outer package to time, temperature and pressure conditions sufficient to vapor transfer an effective amount of the antimicrobial agent from the antimicrobial agent source to the suture and the inner package, thereby substantially inhibiting bacterial colonization on the suture and the inner package, wherein the packaged antimicrobial suture exhibits improved shelf life. A packaged antimicrobial suture and a method of increasing the shelf life of a packaged antimicrobial medical device are also provided.
Owner:ETHICON INC
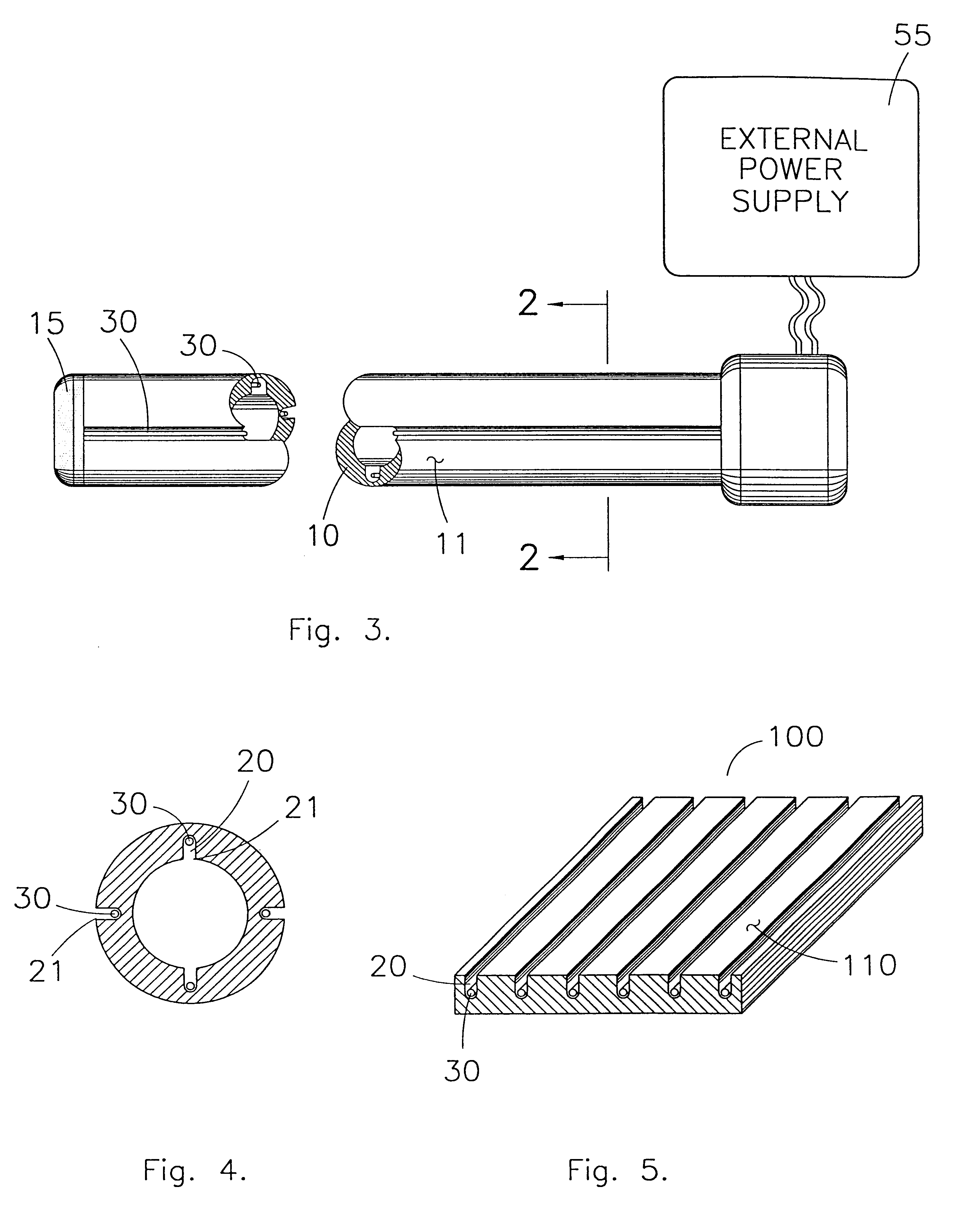
Electrode system in iontophoretic treatment devices
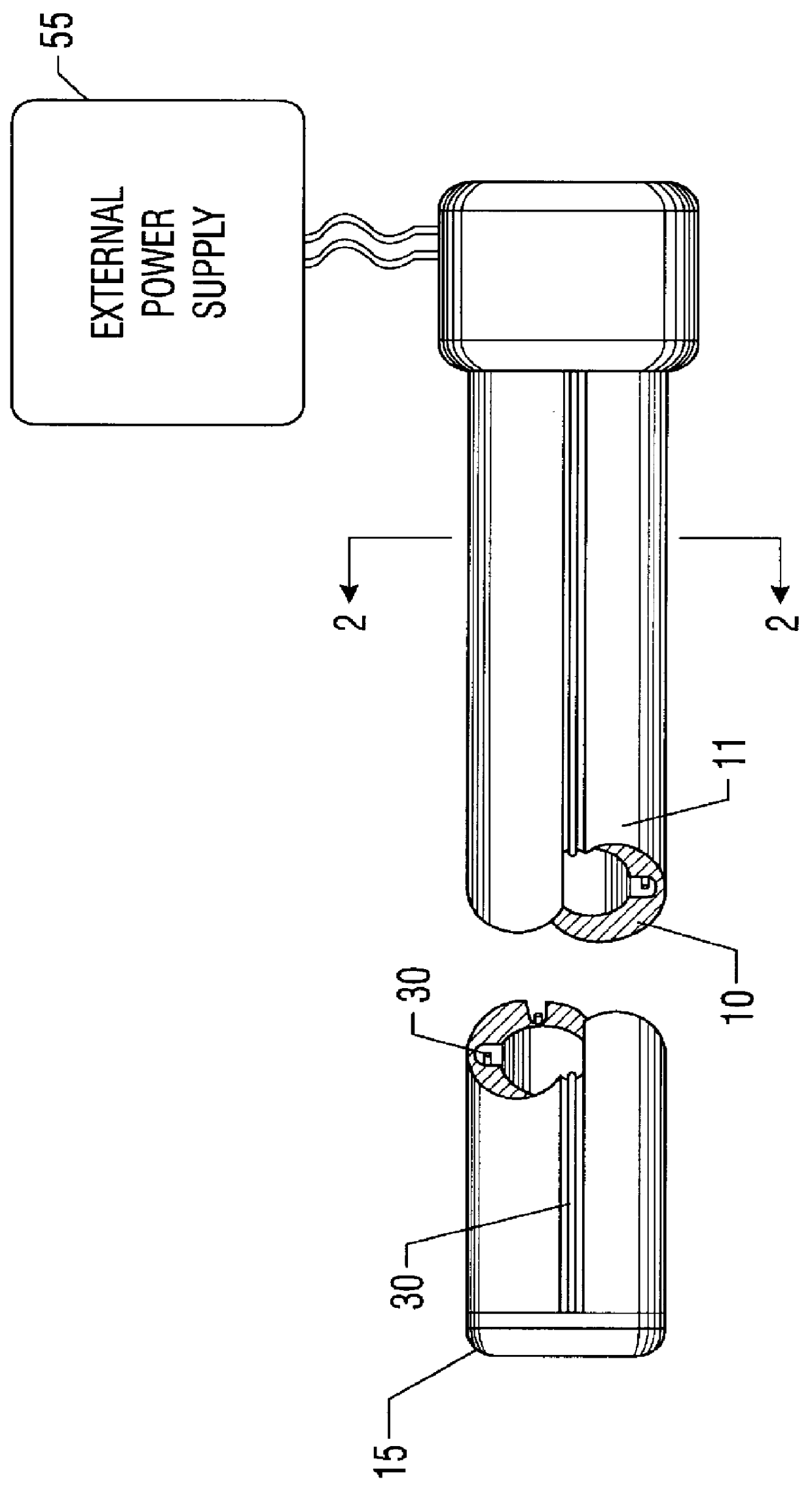
This invention relates to the field of medical devices employing electrophoresis to combat bacterial colonization. More particularly, it relates to an apparatus and method of providing an electrophoresis-type medical device that mitigates the burning of surrounding biological tissue. This invention uses grooves in the contact surface of the medical device for containing the electrodes which cause the electrophoresis to occur. The grooves allow the electrodes to contact biological fluid without contacting and hence burning the surrounding biological tissue. Through the use of this invention it is possible to provide an electrophoresis-type medical device while minimizing the burning of surrounding tissue.
Owner:BOARD OF RGT THE UNIV OF TEXAS SYST
Reduction in bacterial colonization by administering bacteriophage compositions
InactiveUS7459272B2Reduce and eliminate colonizationReduce riskAntibacterial agentsBiocideBacteroidesAcquired immunodeficiency
The present invention provides a method for reducing the risk of bacterial infection or sepsis in a susceptible patient by treating the susceptible patient with a pharmaceutical composition containing bacteriophage of one or more strains which produce lytic infections in pathogenic bacteria. Preferably, treatment of the patient reduces the level of colonization with pathogenic bacteria susceptible to the bacteriophage by at least one log. In a typical embodiment, the susceptible patient is an immunocompromised patient selected from the group consisting of leukemia patients, lymphoma patients, carcinoma patients, sarcoma patients, allogeneic transplant patients, congenital or acquired immunodeficiency patients, cystic fibrosis patients, and AIDS patients. In a preferred mode, the patients treated by this method are colonized with the pathogenic bacteria subject to infection by said bacteriophage.
Owner:INTRALYTIX
Method of preparing an antimicrobial packaged medical device
InactiveUS20060091034A1Prevent bacterial growthInhibit bacterial colonizationSuture equipmentsSurgical furnitureEtherBacterial colonization
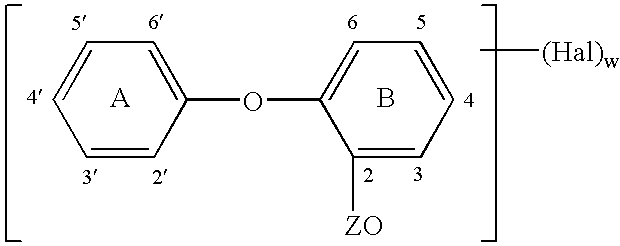
A method for making an antimicrobial suture comprising the steps of positioning an antimicrobial agent source within a package comprising an inner surface, said antimicrobial agent being selected from the group consisting of halogenated hydroxyl ethers, acyloxydiphenyl ethers, and combinations thereof; positioning a medical device within the package; and subjecting the package, the antimicrobial agent source and the medical device to time, temperature and pressure conditions sufficient to vapor transfer an effective amount of the antimicrobial agent from the antimicrobial agent source to the medical device, thereby substantially inhibiting bacterial colonization on the medical device. Alternatively, the packaged medical device is produced according to the steps of positioning a medical device within a package; exposing the package having the medical device to an antimicrobial agent source; and subjecting the package having the medical device and the antimicrobial agent source to time, temperature and pressure conditions sufficient to transfer an effective amount of the antimicrobial agent from the antimicrobial agent source to the medical device within the package, thereby substantially inhibiting bacterial colonization on the medical device.
Owner:ETHICON INC
Probiotic compounds from Lactobacillus GG and uses therefor
InactiveUS20070123460A1Bolster a cell's defenses against inflammationModulate signal transduction pathwaysPeptide/protein ingredientsAntipyreticBacteroidesLactobacillus GG
The invention provides methods and compositions for the treatment of inflammatory disorders, such as inflammatory bowel diseases (IBDs). The use of bacteria-free, probiotic-derived compounds instead of live bacteria provides a safety advantage over the use of live bacteria. In addition, the administration of isolated compounds will provide more reliable dosing, greater simplicity, and improved consistency than is found in administering probiotics, which is dependent on both establishing and maintaining bacterial colonization.
Owner:UNIVERSITY OF CHICAGO
Method of preparing a packaged antimicrobial medical device
A method of making a packaged antimicrobial suture comprising the steps of providing a containment compartment that is substantially free of an antimicrobial agent; positioning a suture within the containment compartment, said suture comprising one or more surfaces having an antimicrobial agent disposed thereon, said antimicrobial agent being selected from the group consisting of halogenated hydroxyl ethers, acyloxydiphenyl ethers, and combinations thereof; placing the containment compartment having the suture in an outer package; and subjecting the outer package, the containment compartment and the suture to time, temperature and pressure conditions sufficient to vapor transfer an effective amount of the antimicrobial agent from the suture to the containment compartment, while retaining an effective amount of said antimicrobial agent on the suture, thereby substantially inhibiting bacterial colonization on the suture and the containment compartment.
Owner:ETHICON INC
Bacteria sensor and method
InactiveUS20060254343A1Low costEasy to useBioreactor/fermenter combinationsBiological substance pretreatmentsBiofilm growthCatheter
Bacteria accumulations on the interior walls of a fluid conduit are detected by placing a bacterial target substrate in the conduit. The substrate is structured to allow bacteria to colonize it at at least the rate of accumulation expected on the conduit walls or at an accelerated rate in order to preempt normal bacteria accumulation on the walls. A bacteria getter may be used to accelerate bacterial colonization of the substrate. An excitation signal interrogating the substrate causes autofluorescence in the presence of bacteria, specifically from NADH and / or NADPH present. The autofluorescent emission is transmitted to a detector and processor. In one system when the presence of bacteria at a preset level is detected there is initiated a diversion of the fluid into an auxiliary subsystem during which the primary subsystem is remediated. In one configuration, a wall portion is transparent and the biofilm target substrate is integral with the transparent wall portion and the sensor head is attached to the outside of the transparent wall portion. It can be made as a removable and / or disposable cell in which the transparent wall portion is a glass plug that fits into a hole in the conduit. The biofilm target substrate can be a getter affinity surface formed on the inside surface of the glass plug. Various means are used for obtaining accelerated biofilm growth.
Owner:OPTECH VENTURES
Packaged antimicrobial medical device having improved shelf life and method of preparing same
ActiveUS20130264226A1Inhibit bacterial colonizationInhibition of colonizationSuture equipmentsSurgical needlesBiotechnologyMicrobial agent
A packaged antimicrobial suture. The packaged antimicrobial suture includes an inner package having a source of antimicrobial agent, the source of antimicrobial agent comprising a plurality of patches, each patch having a pair of antimicrobial material reservoirs; at least one suture positioned within the inner package, the at least one suture comprising one or more surfaces; and an outer package having an inner surface, the outer package having the inner package positioned within; wherein the at least one suture, the inner package and the inner surface of the outer package are subjected to time, temperature and pressure conditions sufficient to transfer an effective amount of the antimicrobial agent from the antimicrobial agent source to the at least one suture and the inner package, thereby substantially inhibiting bacterial colonization on the at least one suture and the inner package. A method of making a packaged antimicrobial suture having is also provided.
Owner:CILAG GMBH INT
Anaerobic digester
InactiveUS6855253B2Improve efficiencyQuality improvementGas production bioreactorsWaste based fuelSlurryEnvironmental engineering
An anaerobic digester and a method for treating organic waste and recovering a usable quality methane gas. A first cover of gas permeable material conducive to bacterial colonization covers the surface of a slurry of organic waste material in a containment vessel. The permeable cover acts as a media to support and encourage the growth of methanogenic bacteria. Gas collection apparatus is installed on the first cover. A second cover of gas impermeable material is installed over the gas collection apparatus. A gas collection space is formed between the two covers. Edges of the first and second covers are closed to inhibit the escape of gas from the gas collection space. Biogas produced as a result of anaerobic digestion activity permeates the gas permeable cover and enters the collection space. From the collection space the gas is drawn into the gas collection apparatus which can, for example, be comprised of a network of perforated ducts connected to a blower that draws gas out of the ducts for delivery to a storage or usage location.
Owner:BAUMGARTNER ENVIRONICS
Antimicrobial packaged medical device and method of preparing same
InactiveUS20050101993A1Prevent bacterial growthInhibit bacterial colonizationSuture equipmentsSurgical furnitureEtherBacterial colonization
A packaged medical device produced according to the steps of positioning an antimicrobial agent source within a package comprising an inner surface, said antimicrobial agent being selected from the group consisting of halogenated hydroxyl ethers, acyloxydiphenyl ethers, and combinations thereof; positioning a medical device within the package; and subjecting the package, the antimicrobial agent source and the medical device to time, temperature and pressure conditions sufficient to vapor transfer an effective amount of the antimicrobial agent from the antimicrobial agent source to the medical device, thereby substantially inhibiting bacterial colonization on the medical device. Alternatively, the packaged medical device is produced according to the steps of positioning a medical device within a package; exposing the package having the medical device to an antimicrobial agent source; and subjecting the package having the medical device and the antimicrobial agent source to time, temperature and pressure conditions sufficient to transfer an effective amount of the antimicrobial agent from the antimicrobial agent source to the medical device within the package, thereby substantially inhibiting bacterial colonization on the medical device.
Owner:ETHICON INC
Percutaneous access device
A percutaneous access device is provided including a sleeve having an inner lumen extending therethrough that is adapted to slidably receive a catheter, and at least one flange disposed around the sleeve and adapted to be positioned adjacent a tissue surface. In a preferred embodiment, the device includes a first flange disposed around the sleeve and adapted to be positioned adjacent a first tissue surface, and a second flange disposed around the sleeve and spaced apart from the first flange such that the second flange is adapted to be disposed adjacent a second tissue surface opposed to the first tissue surface. In use, the flanges 16, 18 are positioned on opposed sides of tissue so that the device 10 is effective to prevent tissue surrounding the percutaneous access device from coming into contact with the catheter as it is introduced through the sleeve 12. The device also includes an antimicrobial agent that is effective to protect against bacterial colonization on and around the access device, the catheter, and the tissue surface surrounding the access device.
Owner:INTEGRA LIFESCI SWITZERLAND SARL
Method of preparing an antimicrobial packaged medical device
A method for making an antimicrobial suture comprising the steps of positioning an antimicrobial agent source within a package comprising an inner surface, said antimicrobial agent being selected from the group consisting of halogenated hydroxyl ethers, acyloxydiphenyl ethers, and combinations thereof; positioning a medical device within the package; and subjecting the package, the antimicrobial agent source and the medical device to time, temperature and pressure conditions sufficient to vapor transfer an effective amount of the antimicrobial agent from the antimicrobial agent source to the medical device, thereby substantially inhibiting bacterial colonization on the medical device. Alternatively, the packaged medical device is produced according to the steps of positioning a medical device within a package; exposing the package having the medical device to an antimicrobial agent source; and subjecting the package having the medical device and the antimicrobial agent source to time, temperature and pressure conditions sufficient to transfer an effective amount of the antimicrobial agent from the antimicrobial agent source to the medical device within the package, thereby substantially inhibiting bacterial colonization on the medical device.
Owner:ETHICON INC
Collagen binding protein compositions and methods of use
InactiveUS20020102262A1Prevent diseaseSimple methodAntibacterial agentsPeptide/protein ingredientsPassive ImmunizationsCarrier protein
Disclosed are the cna gene and cna-derived nucleic acid segments from Staphylococcus aureus, and DNA segments encoding cna from related bacteria. Also disclosed are Col binding protein (CBP) compositions and methods of use. The CBP protein and antigenic epitopes derived therefrom are contemplated for use in the treatment of pathological infections, and in particular, for use in the prevention of bacterial adhesion to Col. DNA segments encoding these proteins and anti-(Col binding protein) antibodies will also be of use in various screening, diagnostic and therapeutic applications including active and passive immunization and methods for the prevention of bacterial colonization in an animal such as a human. These DNA segments and the peptides derived therefrom are contemplated for use in the preparation of vaccines and, also, for use as carrier proteins in vaccine formulations, and in the formulation of compositions for use in the prevention of S. aureus infection.
Owner:HOOK MAGNUS +2
Method of making a packaged antimicrobial suture
InactiveUS8112973B2Extended shelf lifeInhibition of colonizationSuture equipmentsSurgical furnitureMedicineBacterial colonization
Owner:ETHICON INC
Packaged antimicrobial medical device and method of preparing same
A method of making a packaged antimicrobial suture. The method includes the steps of providing a containment compartment molded from a polymeric resin comprising a polymeric material and an antimicrobial agent, positioning a suture within the containment compartment, the suture comprising one or more surfaces; covering the containment compartment having the suture in an outer package cover having an inner surface, and subjecting the outer package, the containment compartment and the suture to time, temperature and pressure conditions sufficient to vapor transfer an effective amount of the antimicrobial agent from the containment compartment to the suture, while retaining an effective amount of the antimicrobial agent on the containment compartment, thereby substantially inhibiting bacterial colonization on the suture and the containment compartment. A packaged antimicrobial suture is also provided.
Owner:ETHICON INC
Deploying linings in body cavities
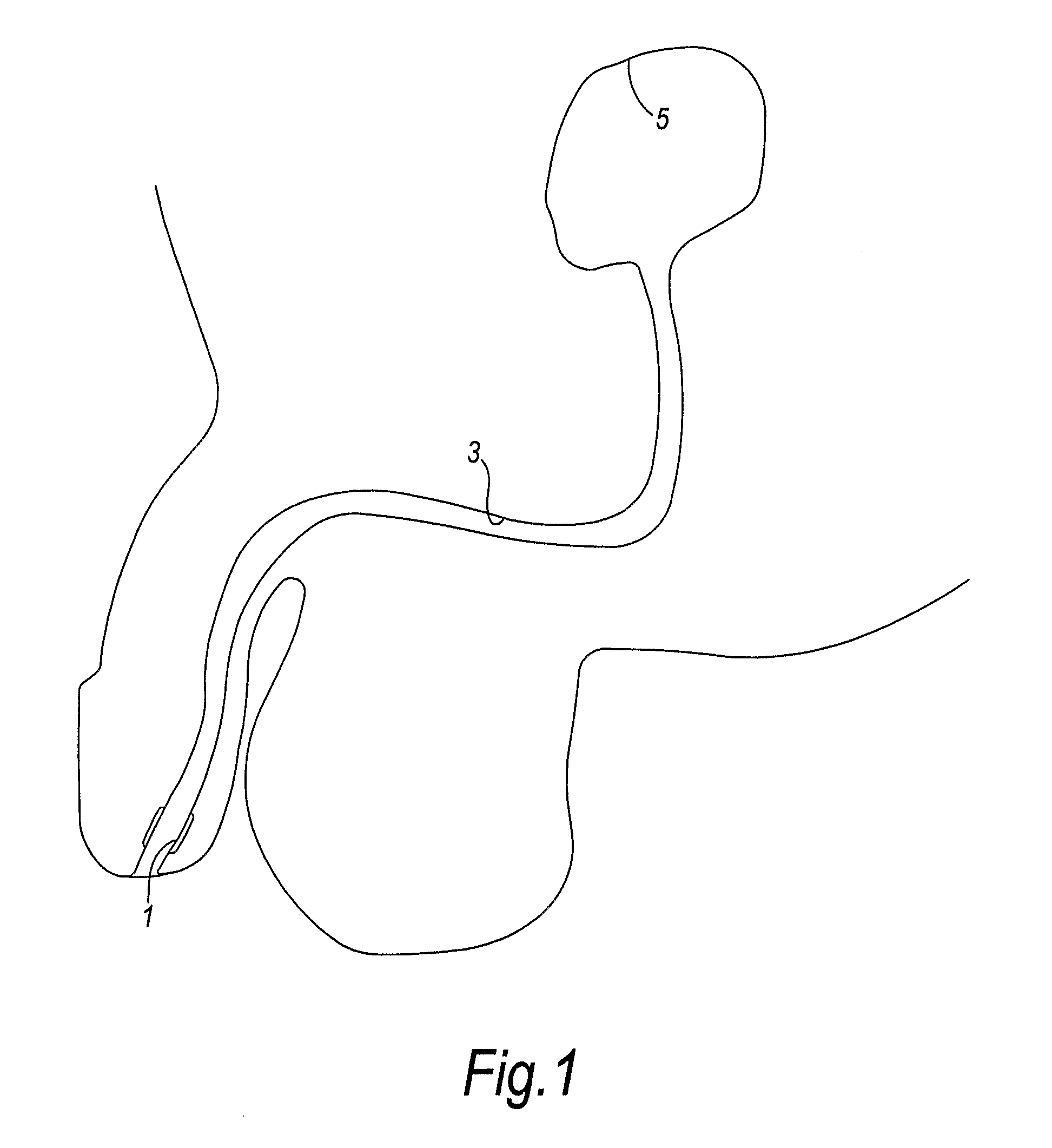
InactiveUS20110190736A1Convenient ArrangementEasy to insertWound drainsCatheterUpper urinary tract infectionUrethra
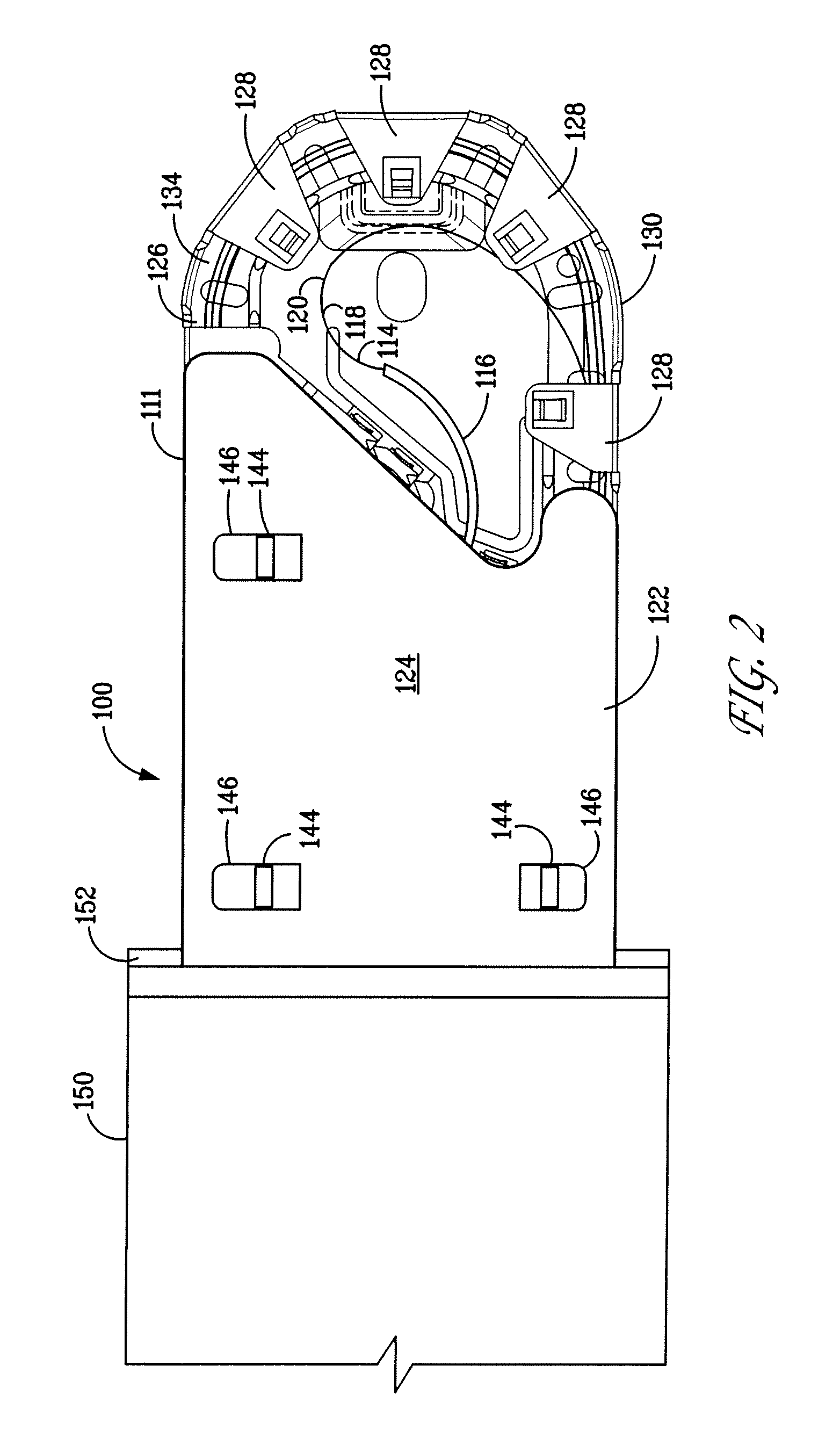
A cavity (3) such as the urethra of a patient is lined by providing a lining device that comprises an eversible chamber (12) and a liner (16) connected thereto. Fluid pressure is applied to the chamber (12) to cause the chamber (12) to evert and advance into the cavity (3) such that the chamber (12) carries the liner (16) into the cavity. A vacuum is then applied to the chamber (12) to cause it to deflate. A part, such as a catheter or cable, can then be inserted into the cavity through the liner (16). If the cavity is the urethra of a patient, the liner shields the catheter from the distal part (1) of the urethra which is colonised with bacteria, and therefore reduces the risk of a urinary tract infection.
Owner:SCOPEGUARD LTD
Bacteria sensor and method
InactiveUS20060159589A1Low costEasy to useAnalysis by electrical excitationOptically investigating flaws/contaminationBacterial colonizationExcitation signal
Bacteria accumulations on the interior walls of a fluid conduit are detected by placing a bacterial target substrate the conduit. The substrate structured to allow bacteria to colonize it at at least the rate of accumulation expected on the conduit walls or at an accelerated rate in order to preempt normal bacteria accumulation on the walls. A bacteria getter may be used to accelerate bacterial colonization of the substrate. An excitation signal interrogating the substrate causes autofluorescence in the presence of bacteria, specifically from NADH and / or NADPH present. The autofluorescent emission is transmitted to a detector and processor. In one system when the presence of bacteria at a preset level is detected there is initiated a diversion of the fluid into an auxiliary subsystem during which the primary subsystem is remediated.
Owner:OPTECH VENTURES
Method and apparatus for treating bacterial infections in devices
An apparatus and method for treating or preventing bacterial colonization of medical devices, or the host itself, comprise combining an RNAIII inhibiting peptide (RIP) with the medical device, optionally with an antibiotic. Further, RIP may be used in a lock technique, comprising adding sufficient solution comprising RIP to occupy a space within the device, which provides a high concentration of RIP at the actual or potential site of infection and prevents the space from filing with blood. The invention thus allows a clinician to maximize the amount of RIP and an antibiotic used to clean the medical device, while, at the same time, retaining the device within the host.
Owner:BALABAN NAOMI
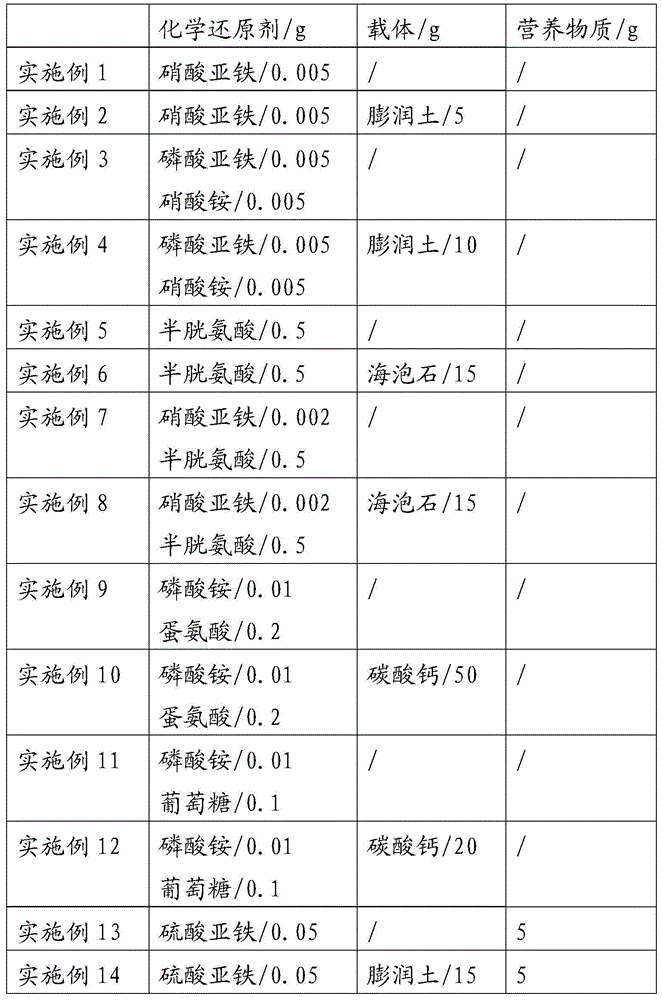
Chromium polluted soil composite microbial repairing agent, preparation method and application thereof
InactiveCN104611250APromote reductionReduce concentrationBacteriaContaminated soil reclamationCarbon oxideBacterial colonization
The invention provides a chromium polluted soil composite microbial repairing agent, which includes at least one chemical reductant and an active bacterium. The active bacterium at least contains one thermophilic-carbon oxide streptomycete. The invention also provides a preparation method of the chromium polluted soil composite microbial repairing agent. The method includes: mixing the chemical reductant, the active bacterium, an optional carrier and an optional nutrient evenly in proportion. The chromium polluted soil composite microbial repairing agent provided by the invention can quickly reduce the hexavalent chromium in soil and reduce the concentration of hexavalent chromium, is conducive to bacterial colonization growth, and the oxidation product of additives also provides rich nutrition for bacteria to promote the growth of bacteria, and the reduction effect and nutrition effect synergistically promote reduction of hexavalent chromium to nontoxic trivalent chromium so as to achieve the repairing goal. According to the technology, the repairing cost is low, and the project is convenient. The invention provides a new method for soil microbial repairing, and has very good popularization and application value.
Owner:SOOCHOW KH BIO SCI & TECH CO LTD KHB
Rehydration compositions comprising epidermal growth factor (EGF)
InactiveUS20110245171A1Promote wound repairEasy to transportPeptide/protein ingredientsDigestive systemHuman epidermal growth factor receptorAgonist
The invention comprises 1) an oral composition, including an oral rehydration composition or solution, comprising an effective amount of a compound selected from epidermal growth factor (EGF) and agonists to the epidermal growth factor receptor, 2) a kit comprising an oral rehydration composition containing an effective amount of a compound selected from epidermal growth factor and agonists to the epidermal growth factor receptor, and 3) methods for the treatment and prevention of dehydration and diarrhea, and enhancing intestinal healing, reducing bacterial colonization, reducing the incidence of weight loss, increasing food uptake, enhancing rehydration, and enhancing mucosal healing in an animal having diarrhea.
Owner:AB BIOPHARMA
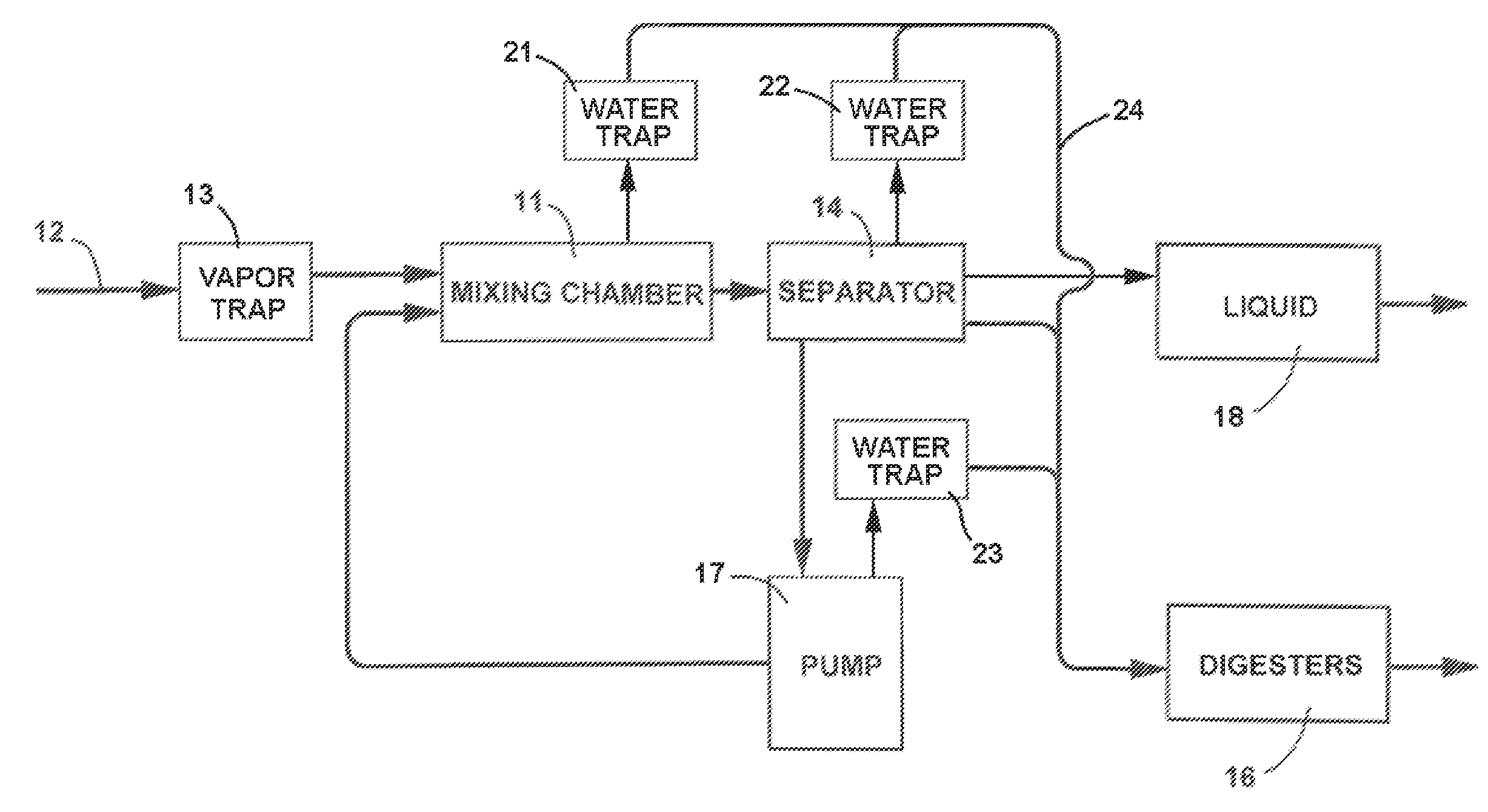
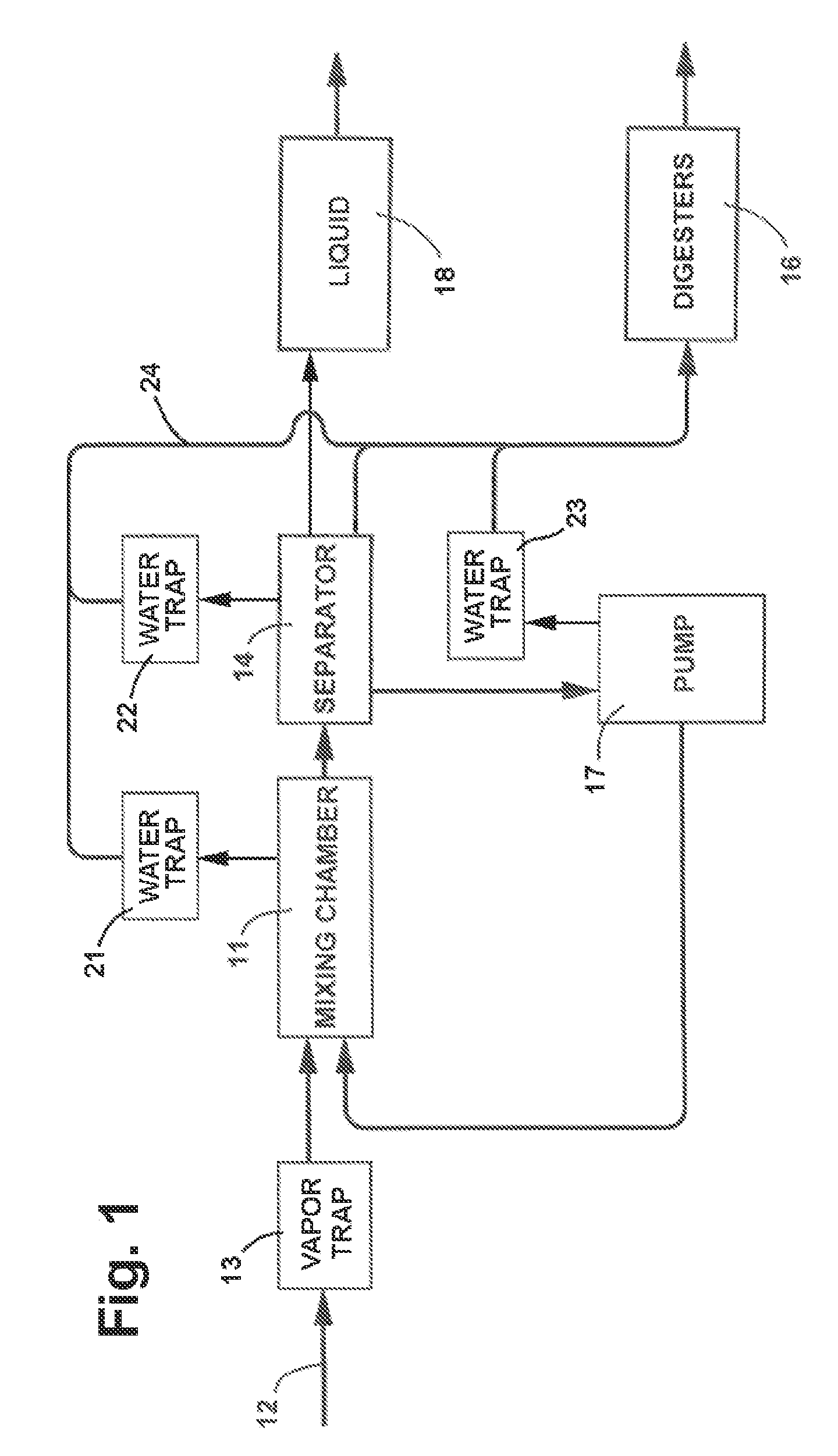
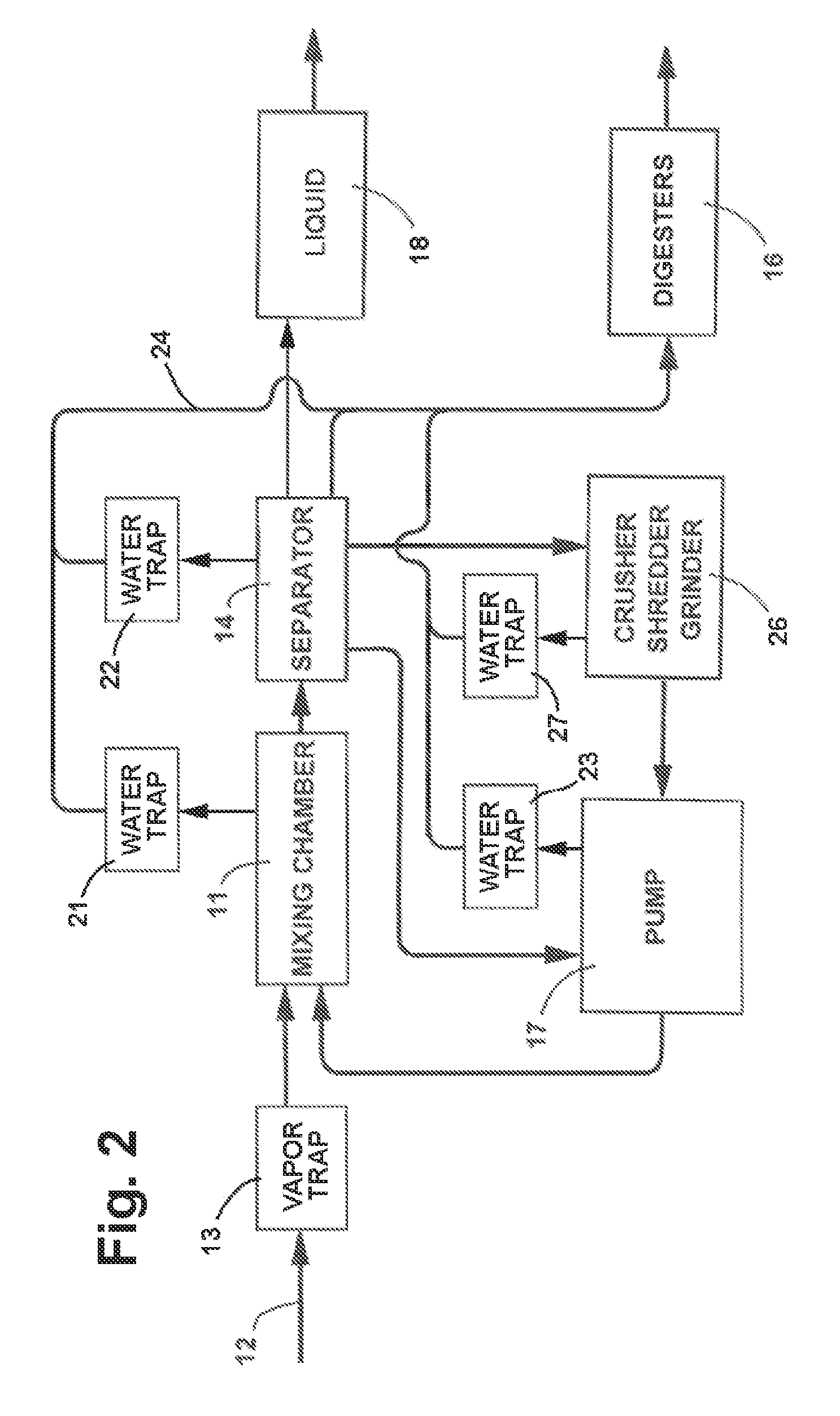
Anaerobic Wastewater Treatment System and Process Utilizing Solids Based Bacterial Colonization (SBBC)
InactiveUS20100213121A1Improve wastewater treatment efficiencyEnhancing growth and colonizing activitySpecific water treatment objectivesLavatory sanitorySolid baseBacterial colonization
Anaerobic Wastewater treatment system and process in which the influent is passed through a mixing chamber and then through a series of filters of progressively smaller size to separate the materials carrying the colonized bacteria from finer solids in the influent and separate the materials according to size in a completely enclosed and flooded environment. The materials from the filters are progressively reduced to a smaller size, the materials of smaller size are returned to the mixing chamber, and new influent is inoculated with the colonized bacteria carried by the materials returned to the mixing chamber.
Owner:MILLER III HERMAN P
Method of preparing an antimicrobial packaged medical device
A method for making an antimicrobial suture comprising the steps of positioning an antimicrobial agent source within a package comprising an inner surface, said antimicrobial agent being selected from the group consisting of halogenated hydroxyl ethers, acyloxydiphenyl ethers, and combinations thereof; positioning a medical device within the package; and subjecting the package, the antimicrobial agent source and the medical device to time, temperature and pressure conditions sufficient to vapor transfer an effective amount of the antimicrobial agent from the antimicrobial agent source to the medical device, thereby substantially inhibiting bacterial colonization on the medical device.
Owner:ETHICON INC
Electrode system in iontophoretic treatment devices
This invention relates to the field of medical devices employing electrophoresis to combat bacterial colonization. More particularly, it relates to an apparatus and method of providing an electrophoresis-type medical device that mitigates the burning of surrounding biological tissue. This invention uses grooves in the contact surface of the medical device for containing the electrodes which cause the electrophoresis to occur. The grooves allow the electrodes to contact biological fluid without contacting and hence burning the surrounding biological tissue. Through the use of this invention it is possible to provide an electrophoresis-type medical device while minimizing the burning of surrounding tissue.
Owner:BOARD OF RGT THE UNIV OF TEXAS SYST
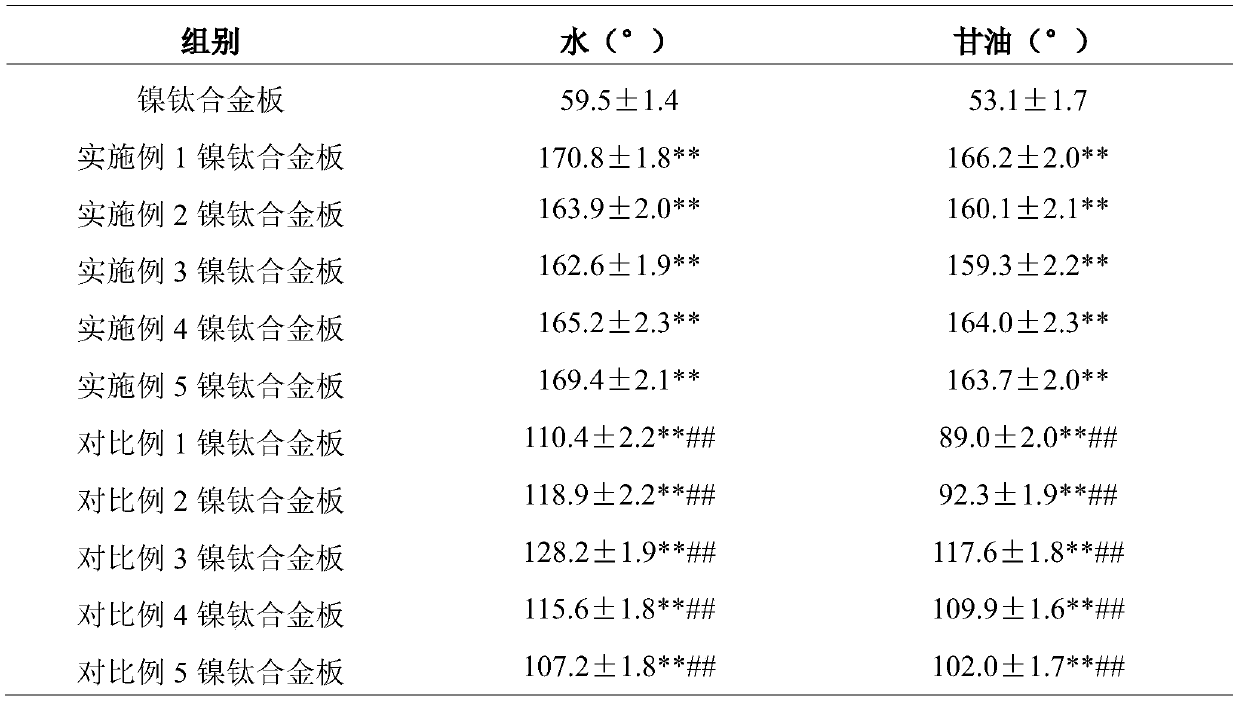
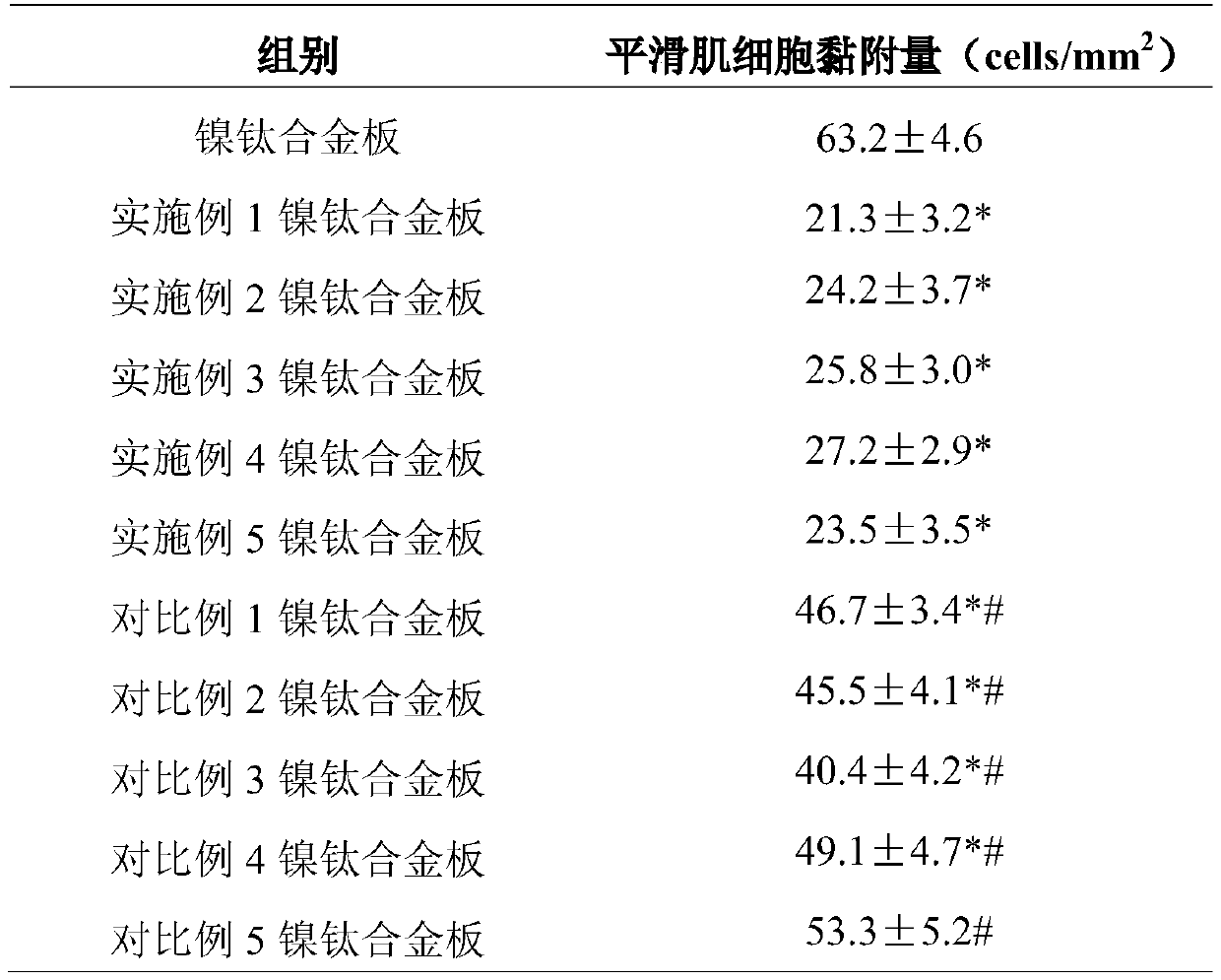
Fluorine-contained nanometer polymer modified nickel titanium alloy material with super-hydrophobic and oleophobic performances and preparation method thereof
ActiveCN110144593AExcellent superhydrophobicityExcellent super oleophobic propertiesMetallic material coating processesPolymer modifiedSilanes
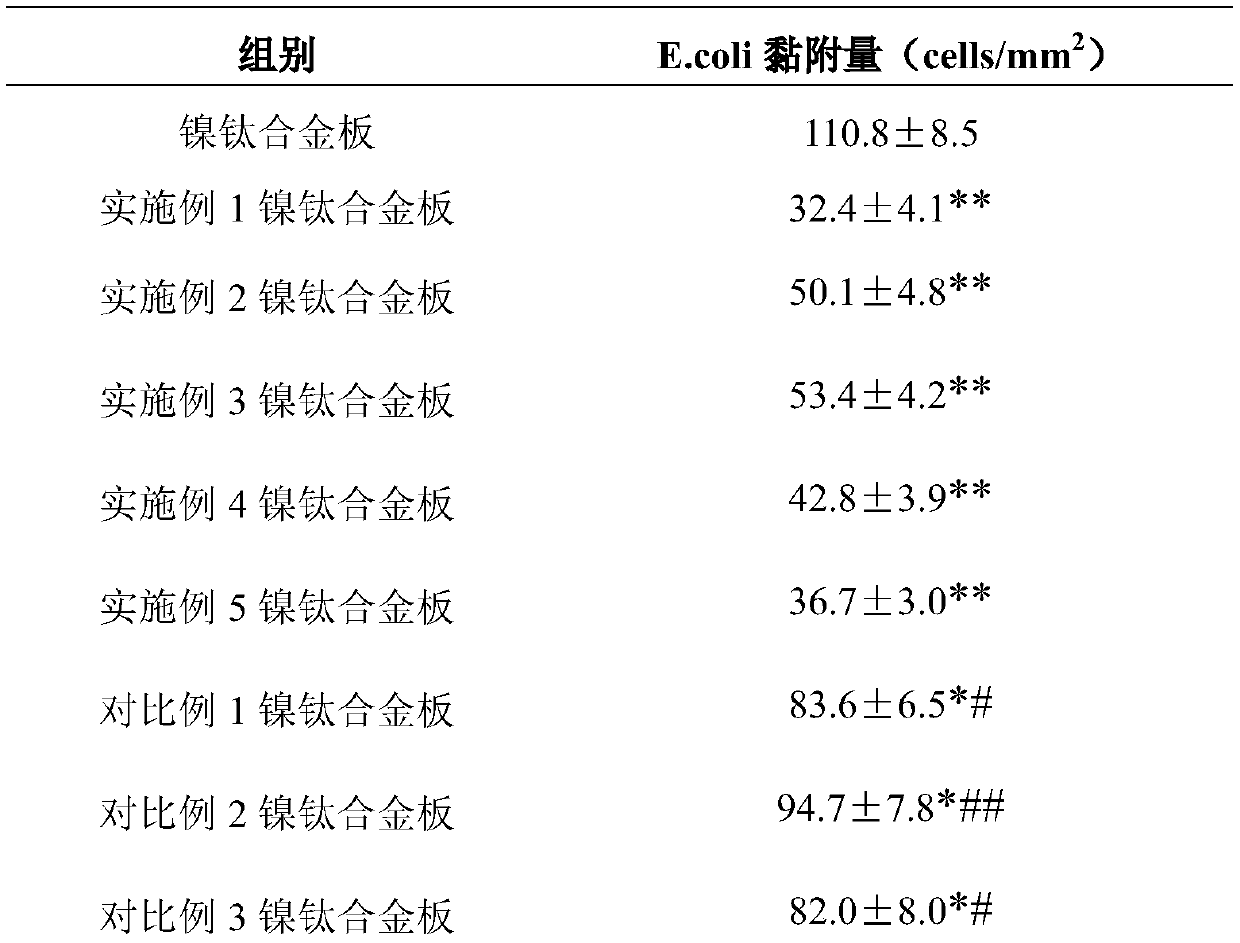
The invention relates to a fluorine-contained nanometer polymer modified nickel titanium alloy material with super-hydrophobic and oleophobic performances and a preparation method thereof. Mixed acidsolution and H2SO3 solution are used for twice etching in succession on the surface of a nickel titanium alloy material; and then, PFDAE, perfluorohexyl ethyl triethoxy silane, 3-triethylene oxide hexafluoropropanamide propylbetaine and ethanol solution of SiO2 nanoparticles are sprayed to obtain a surface-modified nickel titanium alloy material. Contact angle test results show that the surface ofthe modified nickel titanium alloy material is excellent in super-hydrophobic and oleophobic performances; and adhesion experiment results show that smooth muscle cells on the surface of the modifiednickel titanium alloy material are weak in adhering capacity and weaker in bacterial colonization capacity. The composite coating nickel titanium alloy material achieves unique advantages on the aspect of preparation of implantable medical devices, and can effectively inhibit the endangium hyperplasia to prevent the vascular restenosis and to inhibit the bacterial colonization and growth.
Owner:SHANGHAI FIRST PEOPLES HOSPITAL
Mildew proof antibacterial material with bionic micrometer structure surface and preparation method thereof
InactiveCN103043595AInhibition of colonizationPrevent adhesionSemi-permeable membranesFixed microstructural devicesEscherichia coliSpore
The invention discloses a mildew proof antibacterial material with a bionic micrometer structure surface. The material comprises the surface, a substrate and a topological feature printed on a surface material in press mode. The surface material of the material contains at least one polymer. Compared with the prior art, the material is capable of preventing bacteria from being planted and adhered to the surface according to the tests on multiple cells such as escherichia coli, staphylococcus aureus, vancomycin-proof enterococcus, pseudomonas aeruginosa and ulva spore and conducted in a laboratory. The material is capable of reducing 85%-99% of bacteria planting and adhesion relative to blank contrast samples with smooth surface and has strong mildew proof antibacterial functions.
Owner:于晓勇
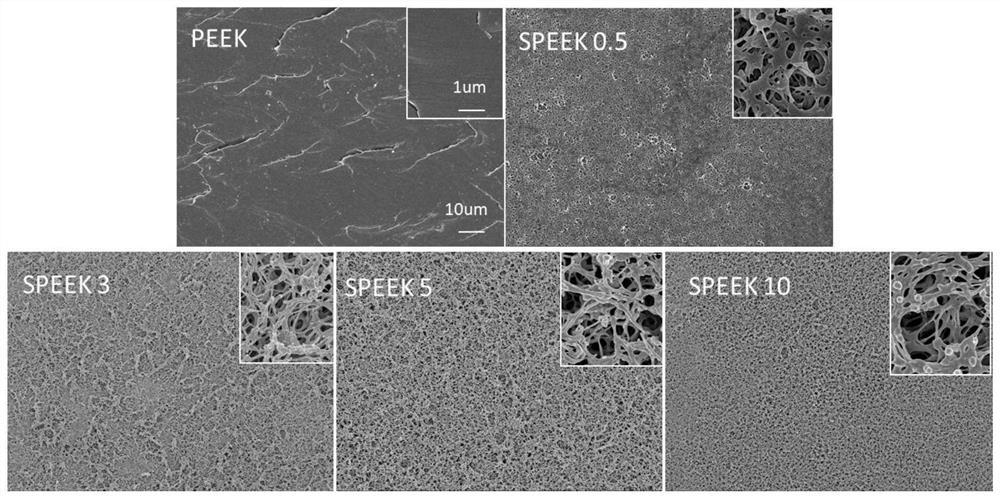
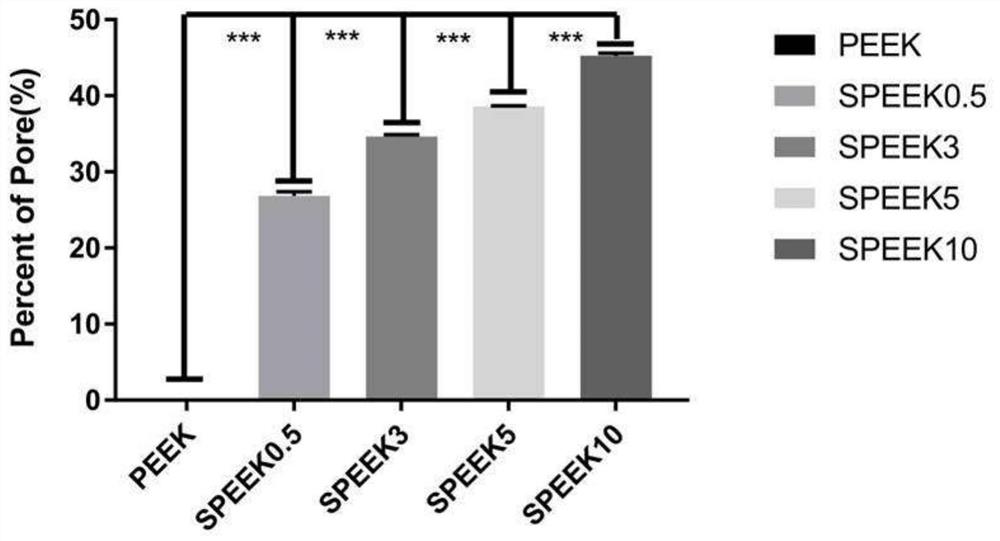
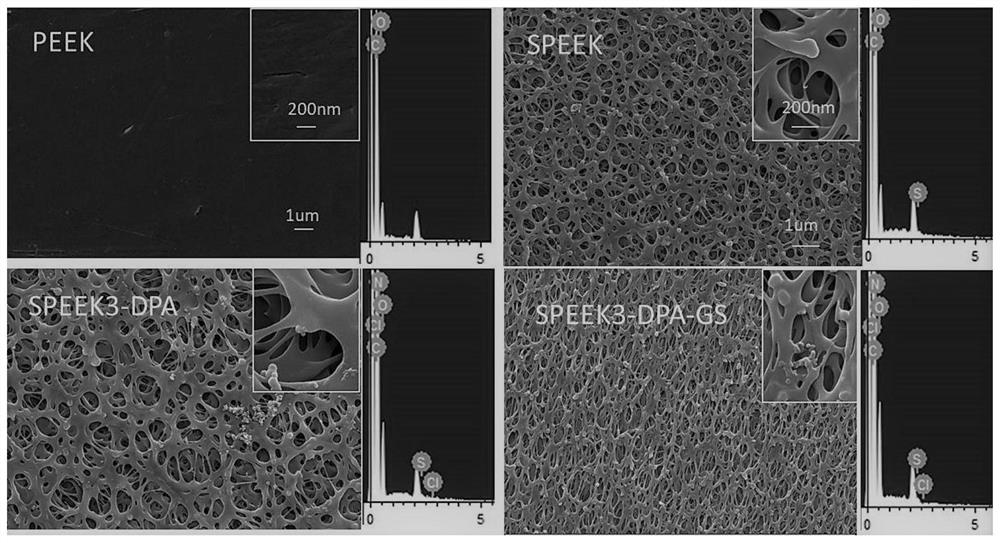
Polyether-ether-ketone three-dimensional porous and modified polydopamine/gentamicin for anti-bacteria and and anti-inflammation effects and promotion of osseointegration of implant
PendingCN112618791AAntibacterial detectionAntibacterialPharmaceutical delivery mechanismTissue regenerationOsseointegrationStaphyloccocus aureus
The invention discloses a simple porification and one-step deposition method of polyether-ether-ketone. The method uses a polydopamine and gentamicin coating to modify the surface of three-dimensional porous polyether-ether-ketone to realize the anti-bacteria effect and regulate macrophage polarization and inflammatory reaction, thereby preventing bacterial colonization and promoting osseointegration. In-vitro research results show that the polydopamine / gentamicin modified porous polyether-ether-ketone can more easily induce macrophages to differentiate into M2 types, has a relatively strong continuous killing effect on staphylococcus aureus and escherichia coli, has relatively high antibacterial and anti-inflammatory capabilities, and can be used as a novel implant material for clinical orthopedics. In-vivo experiment results show that the three-dimensional porous polyether-ether-ketone surface modified polydopamine / gentamicin can well control bacterial infection and promote osseointegration. The invention shows that the polyether-ether-ketone is porous, so that polydopamine and gentamicin can be immobilized at the same time, and the surface-activated polyether-ether-ketone has antibacterial, anti-inflammatory and osseointegration capabilities, and has relatively high application potential in regenerative medicine.
Owner:FUJIAN MEDICAL UNIV UNION HOSPITAL
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com