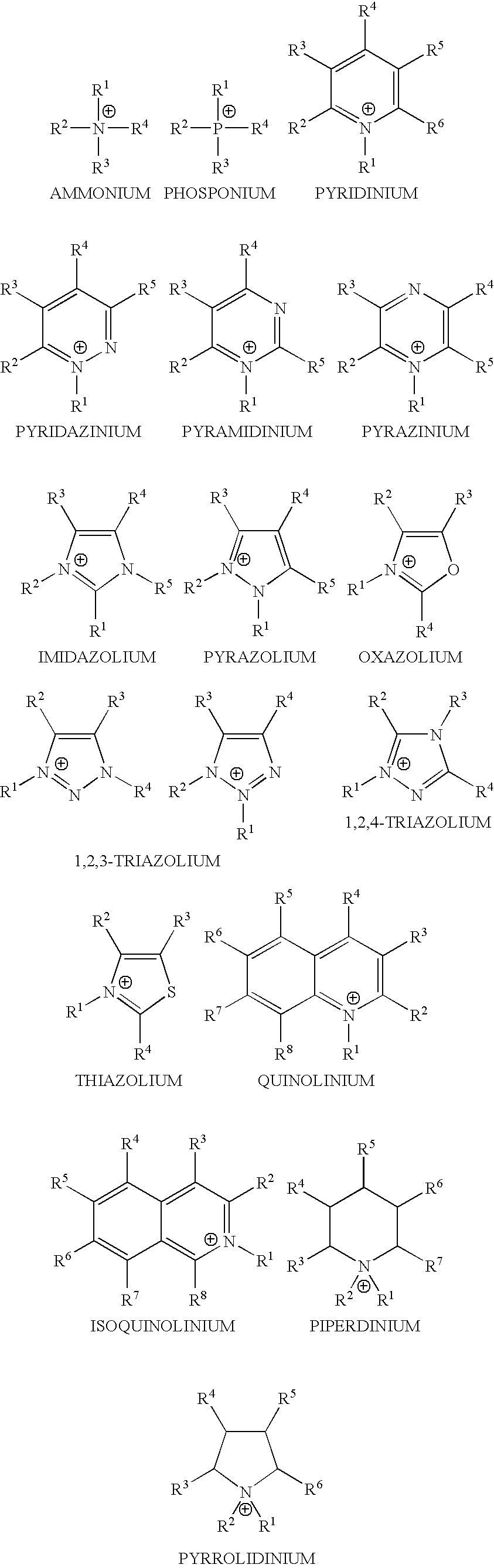
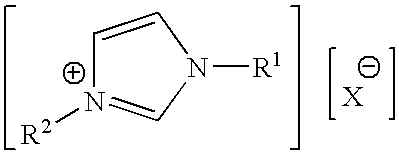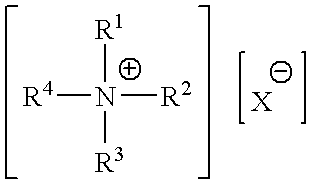Patents
Literature
720 results about "Collagen VI" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
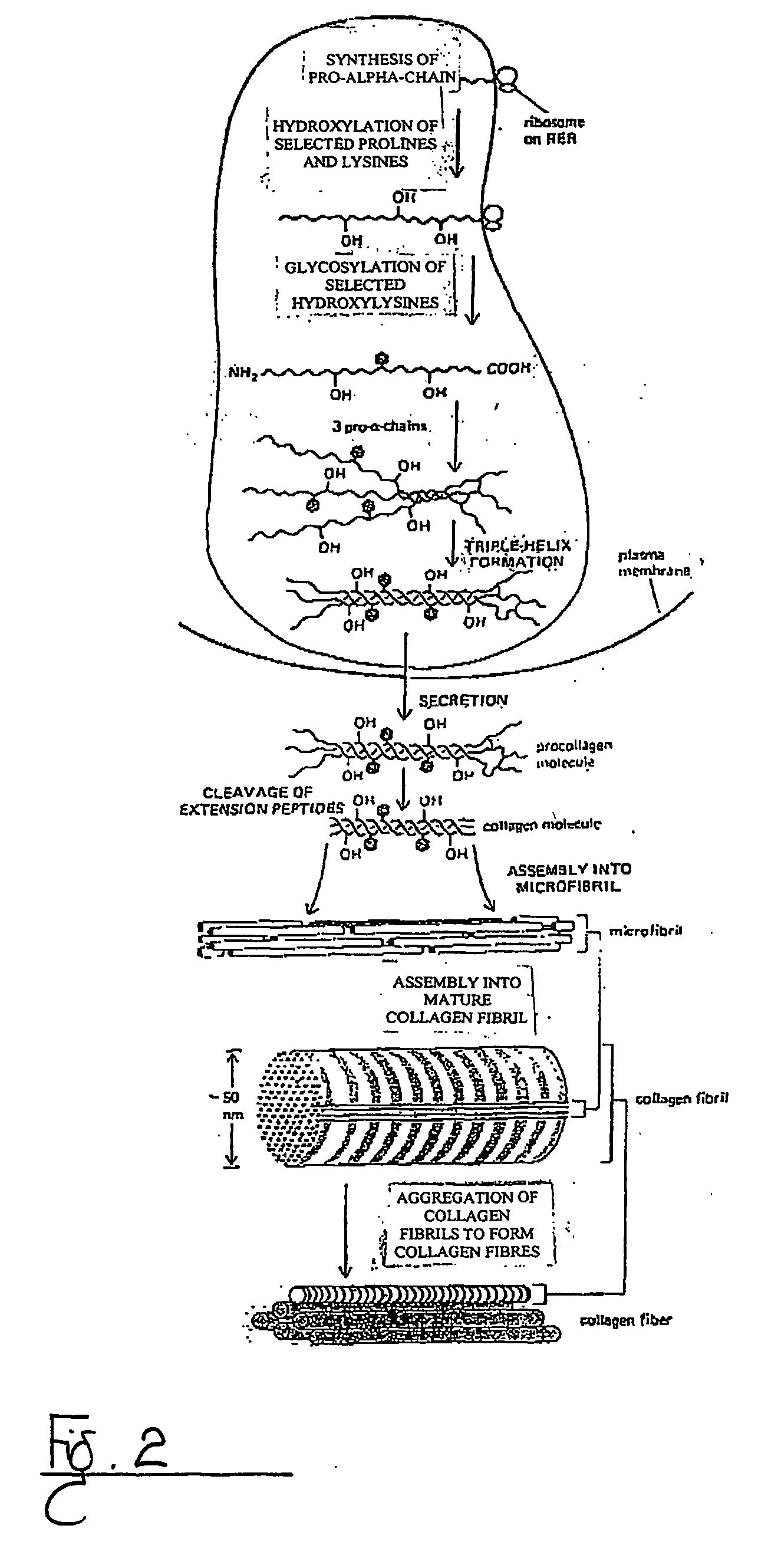
Collagen VI (ColVI) is a type of collagen primarily associated with the extracellular matrix of skeletal muscle. ColVI maintains regularity in muscle function and stabilizes the cell membrane. It is synthesized by a complex, multistep pathway that leads to the formation of a unique network of linked microfilaments located in the extracellular matrix (ECM). ColVI plays a vital role in numerous cell types, including chondrocytes, neurons, myocytes, fibroblasts, and cardiomyocytes. ColVI molecules are made up of three alpha chains: α1(VI), α2(VI), and α3(VI). It is encoded by 6 genes: COL6A1, COL6A2, COL6A3, COL6A4, COL6A5, and COL6A6. The chain lengths of α1(VI) and α2(VI) are about 1,000 amino acids. The chain length of α3(VI) is roughly a third larger than those of α1(VI) and α2(VI), and it consists of several spliced variants within the range of 2,500 to 3,100 amino acids.
Collagen binding protein compositions and methods of use
InactiveUS6288214B1Prevent and lessen adhesionReduce adhesionAntibacterial agentsPeptide/protein ingredientsPassive ImmunizationsCarrier protein
Disclosed are the cna gene and cna-derived nucleic acid segments from Staphylococcus aureus, and DNA segments encoding cna from related bacteria. Also disclosed are Col binding protein (CBP) compositions and methods of use. The CBP protein and antigenic epitopes derived therefrom are contemplated for use in the treatment of pathological infections, and in particular, for use in the prevention of bacterial adhesion to Col. DNA segments encoding these proteins and anti-(Col binding protein) antibodies will also be of use in various screening, diagnostic and therapeutic applications including active and passive immunization and methods for the prevention of bacterial colonization in an animal such as a human. These DNA segments and the peptides derived therefrom are contemplated for use in the preparation of vaccines and, also, for use as carrier proteins in vaccine formulations, and in the formulation of compositions for use in the prevention of S. aureus infection.
Owner:TEXAS A&M UNIVERSITY
Processes for the preparation of novel collagen-based supports for tissue engineering, and biomaterials obtained
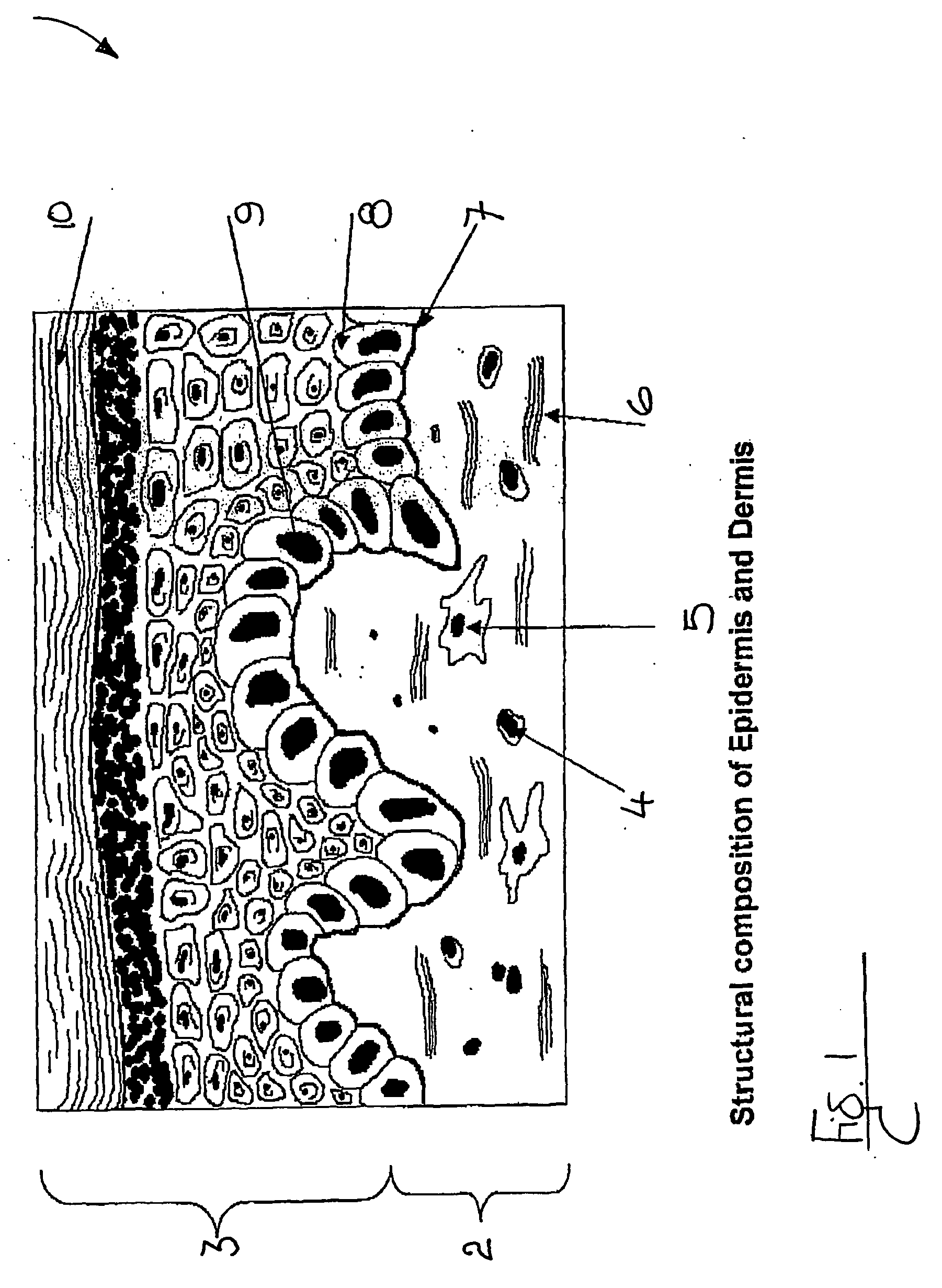
A composite product is disclosed as a collagen support comprising at least one porous collagen layer covered on at least one side with an essentially compact collagen membrane consisting either of a collagen film prepared by drying a collagen gel, preferably in air or a gaseous fluid, or of a very highly compressed collagen sponge. At least one of the two layers, i.e. the porous layer and the essentially compact membrane, may comprise normal, genetically modified or malignant living cells originating particularly from young or elderly subjects. This composite product is used as a collagen support for the manufacture of artificial skin intended especially for performing in vitro tests on the efficacy of potentially active substances or for reconstructing damaged areas of skin in vivo.
Owner:BASF BEAUTY CARE SOLUTIONS FRANCE SAS
Systems and methods for treating patients with collagen-rich material extracted from adipose tissue
InactiveUS20030162707A1Cosmetic preparationsConnective tissue peptidesBrown adipose tissueWhite adipose tissue
Compositions, methods, and systems are disclosed for using collagen-rich material, derived from adipose tissue, that is placed directly into a recipient along with such additives necessary to promote, engender, or support a therapeutic, structural, or cosmetic benefit. The compositions may be obtained during the course of a single surgical procedure, and may be administered to the patient immediately after adipose tissue is removed from a patient, such as within hours or days from being withdrawn from the patient.
Owner:LOREM VASCULAR PTE LTD
Recombinant humanized collagen and its preparation method
ActiveCN102443057ARealize series connectionEasy to produce by fermentationConnective tissue peptidesMicroorganism based processesPichia pastorisPichia stipitis
The invention discloses a recombinant humanized collagen. Its amino acid sequence is as shown in SEQNO.3 and has 599 amino acids. The molecular weight is 55.0kDa. A preparation method of the recombinant humanized collagen comprises the following steps of: constructing a gene engineering strain for the expression of the recombinant humanized collagen to obtain a Pichia pastoris engineering strain; carrying out fermentation culture on the engineering strain, and realizing inducible expression of the recombinant humanized collagen to obtain a recombinant humanized collagen fermentation broth; and purifying the fermentation broth to obtain the recombinant humanized collagen. The humanized collagen gene Ge1 designed in the invention is a brand-new sequence which is greatly shorter than a natural humanized collagen gene (Kbp) in length. By the preparation method, operation simplification at the molecular level is realized, and the gene engineering strain fermentation production of the collagen is more easily realized. The humanized collagen gene Ge1 contains characteristics of a natural humanized collagen gene. And simultaneously, its expressed protein has excellent characteristics of a natural humanized collagen.
Owner:JIANGSU JLAND BIOTECH CO LTD
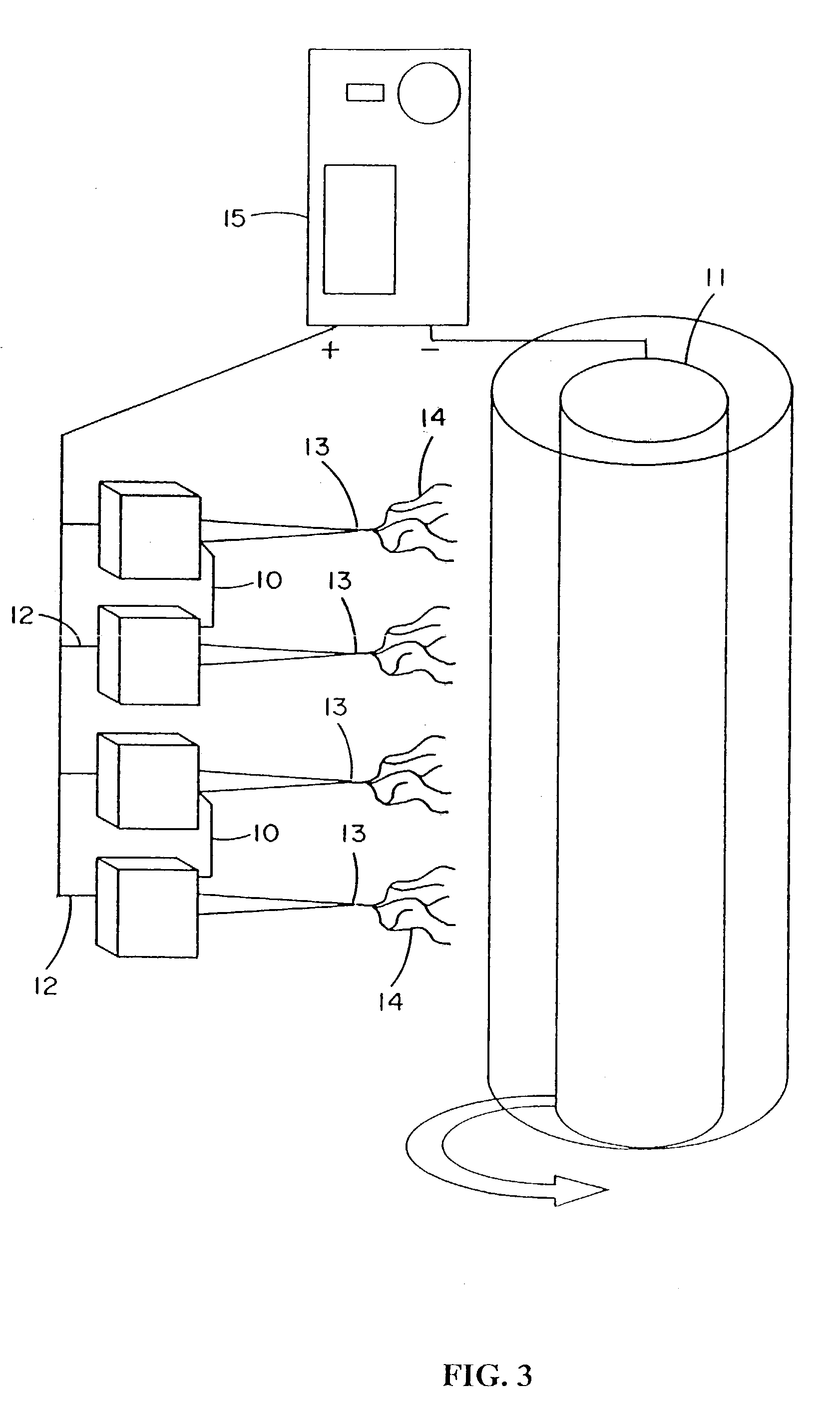
Electroprocessed collagen and tissue engineering
InactiveUS7615373B2Monocomponent protein artificial filamentPeptide/protein ingredientsCell-Extracellular MatrixECM Protein
The invention is directed to formation and use of electroprocessed collagen, including use as an extracellular matrix and, together with cells, its use in forming engineered tissue. The engineered tissue can include the synthetic manufacture of specific organs or tissues which may be implanted into a recipient. The electroprocessed collagen may also be combined with other molecules in order to deliver substances to the site of application or implantation of the electroprocessed collagen. The collagen or collagen / cell suspension is electrodeposited onto a substrate to form tissues and organs.
Owner:ORGANOGENESIS +1
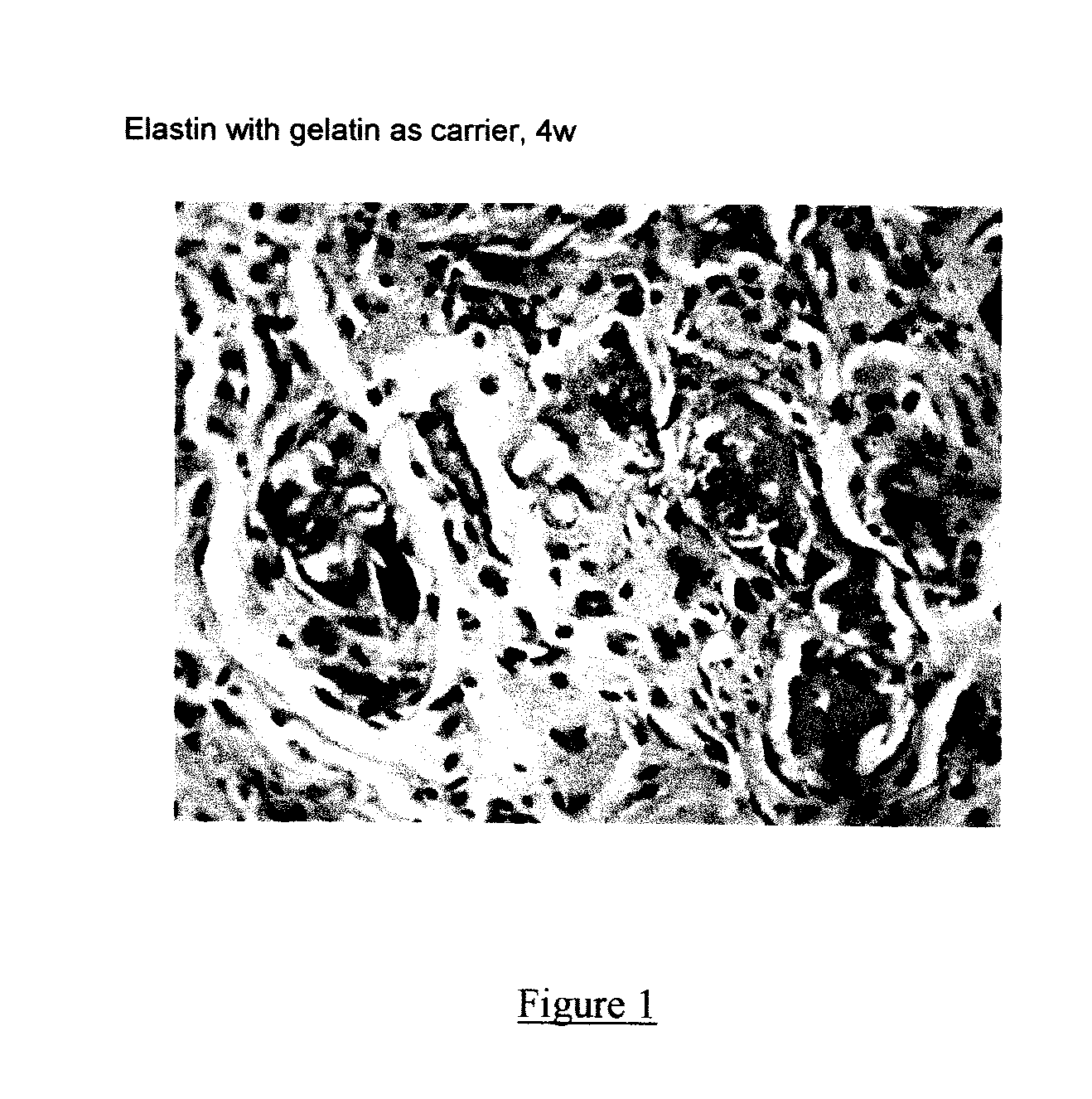
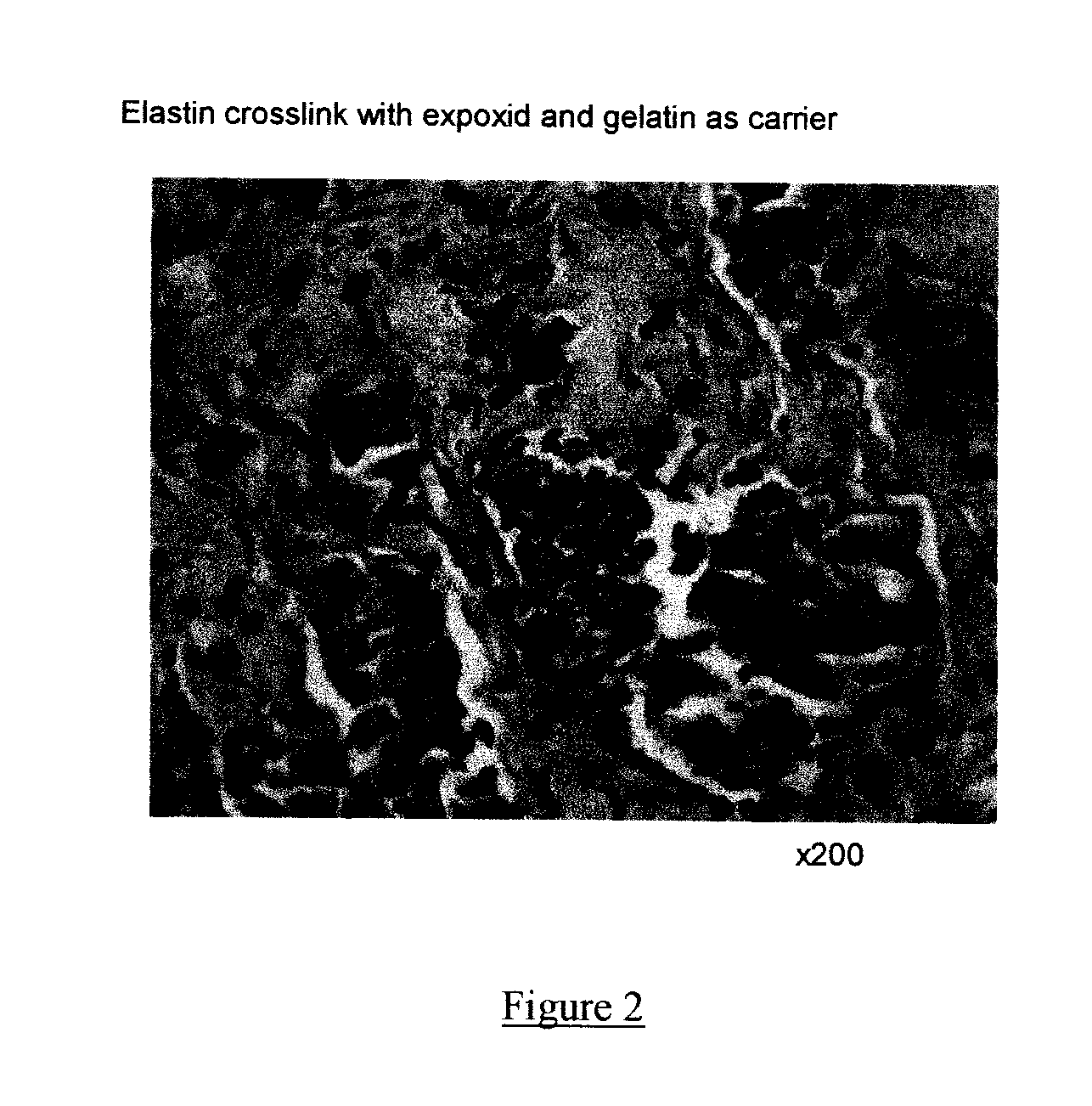
Composite containing collagen and elastin as a dermal expander and tissue filler
ActiveUS20120010146A1Easy injectionLesser tendency can be reactiveCosmetic preparationsPeptide/protein ingredientsTissue expansionCollagen VI
An injectable composition having dermal filling and tissue expanding activity comprises (1) a quantity of elastin sufficient to bring about dermal filling and tissue expansion when injected into a subject in need of dermal filling and tissue expansion, and (2) a pharmaceutically acceptable carrier. The composition can further comprise collagen, in other alternatives, the composition can further comprise hyaluronic acid, and one or more of the elastin, the collagen, and the hyaluronic acid, if present can be cross-linked, either intramolecularly or intermolecularly. The elastin, however, is the primary filler, even if collagen or hyaluronic acid are included in the composition
Owner:TRUELASTIN LAB INC
Adipose-derived stem cells and lattices
InactiveUS7470537B2Genetic material ingredientsMammal material medical ingredientsCell-Extracellular MatrixECM Protein
The present invention provides adipose-derived stem cells (ADSCs), adipose-derived stem cell-enriched fractions (ADSC-EF) and adipose-derived lattices, alone and combined with the ADSCs of the invention. In one aspect, the present invention provides an ADSC substantially free of adipocytes and red blood cells and clonal populations of connective tissue stem cells. The ADSCs can be employed, alone or within biologically-compatible compositions, to generate differentiated tissues and structures, both in vivo and in vitro. Additionally, the ADSCs can be expanded and cultured to produce molecules such as hormones, and to provide conditioned culture media for supporting the growth and expansion of other cell populations. In another aspect, the present invention provides an adipose-derived lattice substantially devoid of cells, which includes extracellular matrix material from adipose tissue. The lattice can be used as a substrate to facilitate the growth and differentiation of cells, whether in vivo or in vitro, into anlagen or even mature tissues or structures.
Owner:UNIVERSITY OF PITTSBURGH
Prion-free collagen and collagen-derived products and implants for multiple biomedical applications; methods of making thereof
InactiveUS6197935B1Preserve integrityPeptide/protein ingredientsImmunoglobulinsIn vivo biocompatibilityCollagen VI
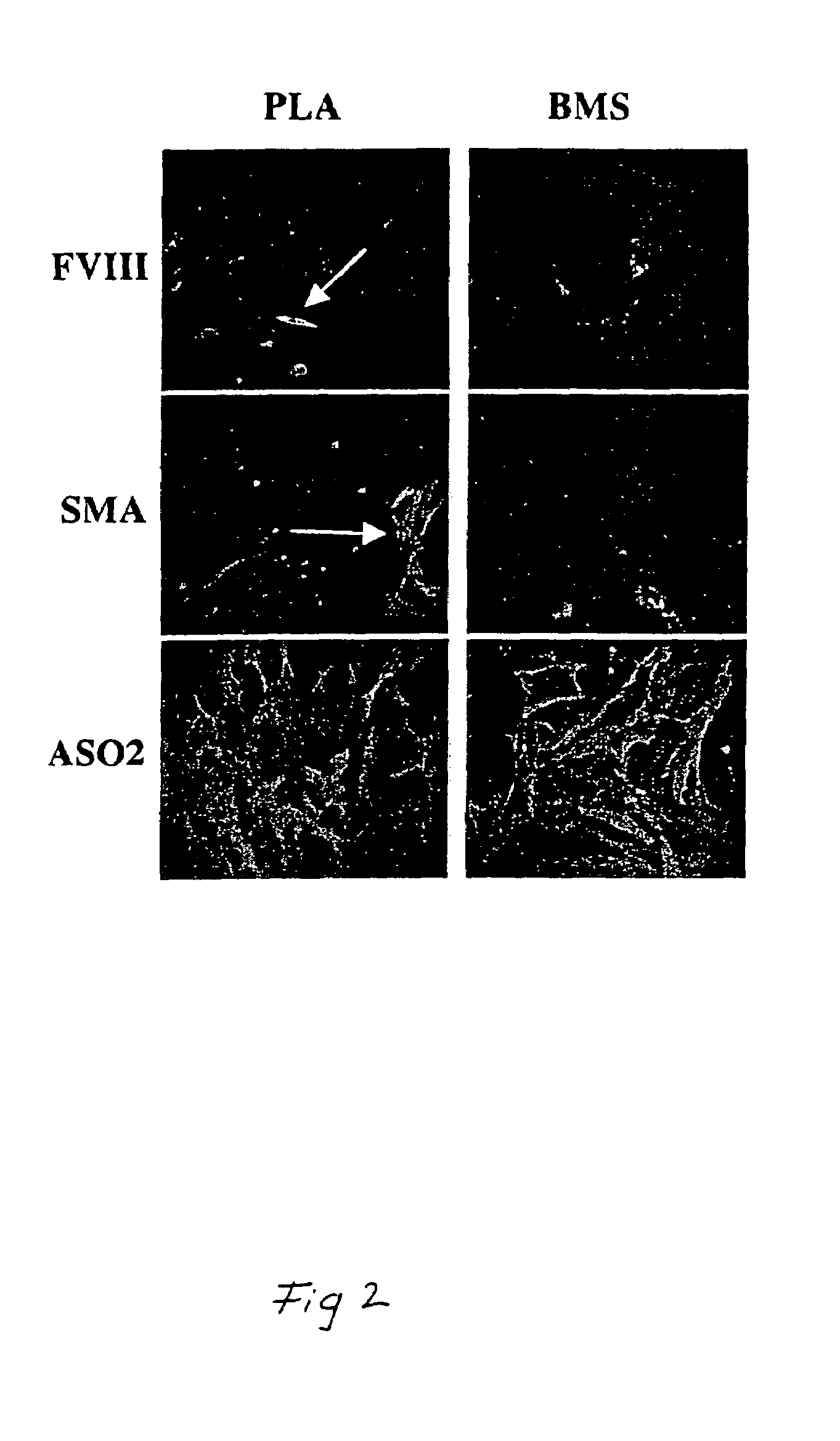
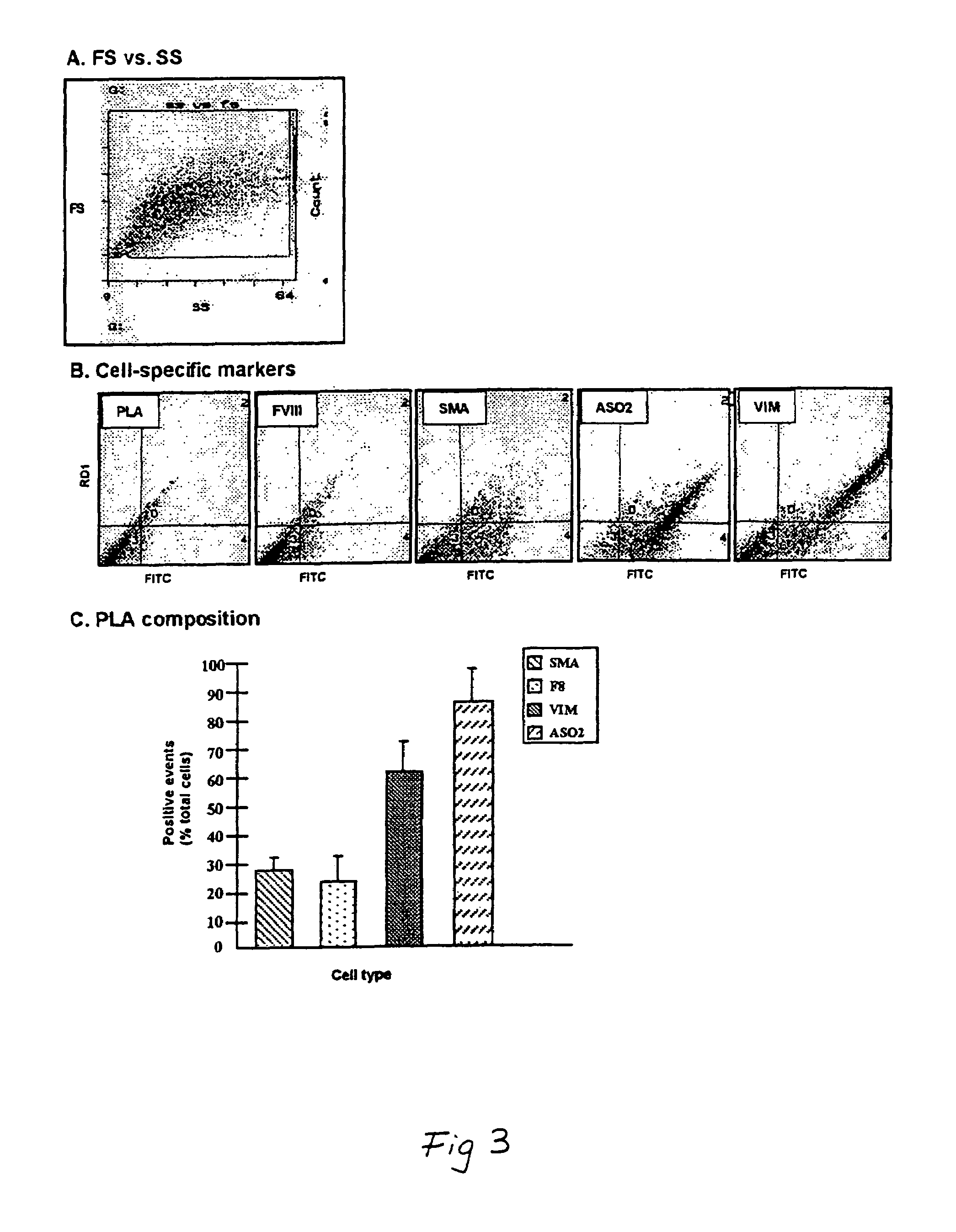
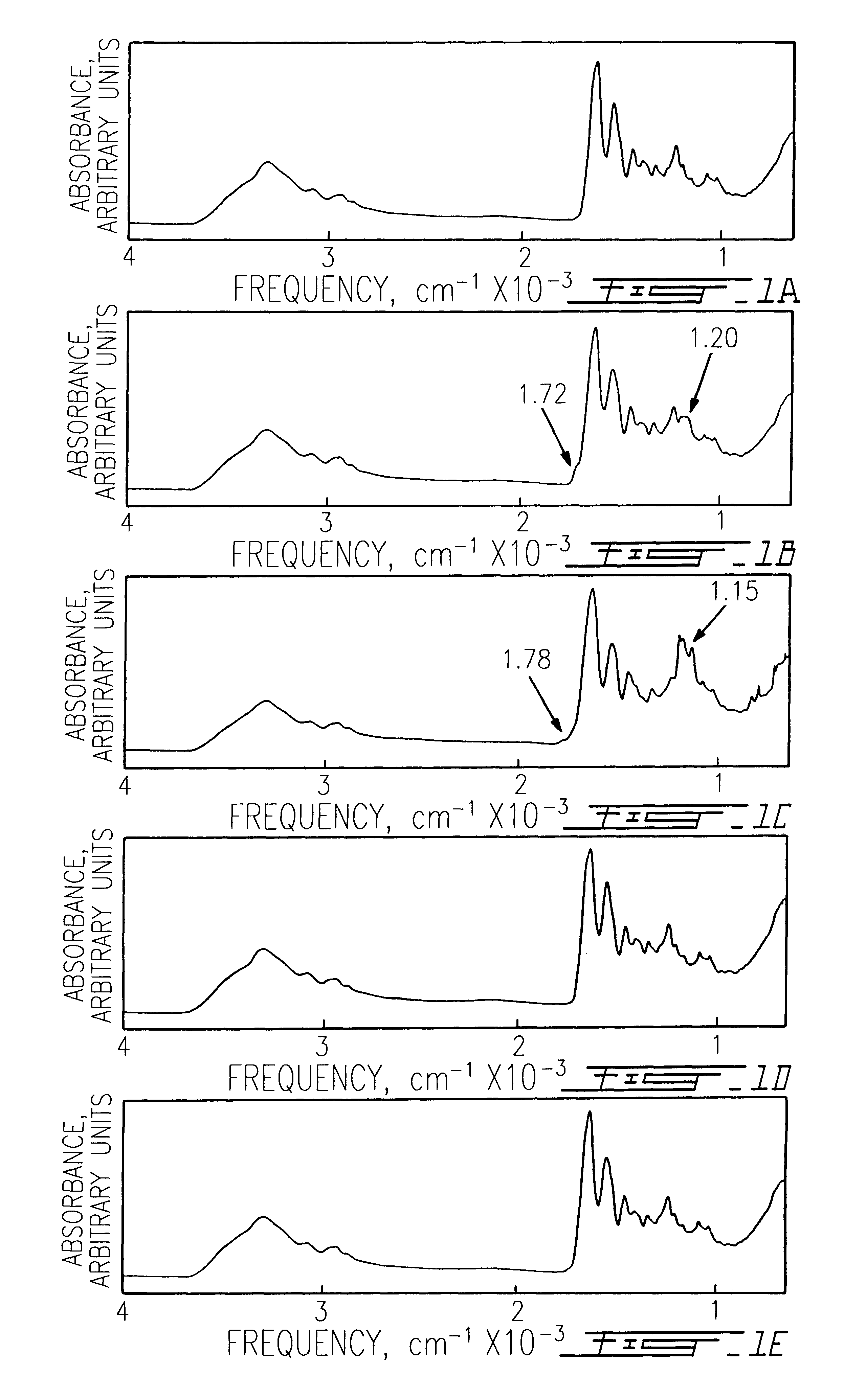
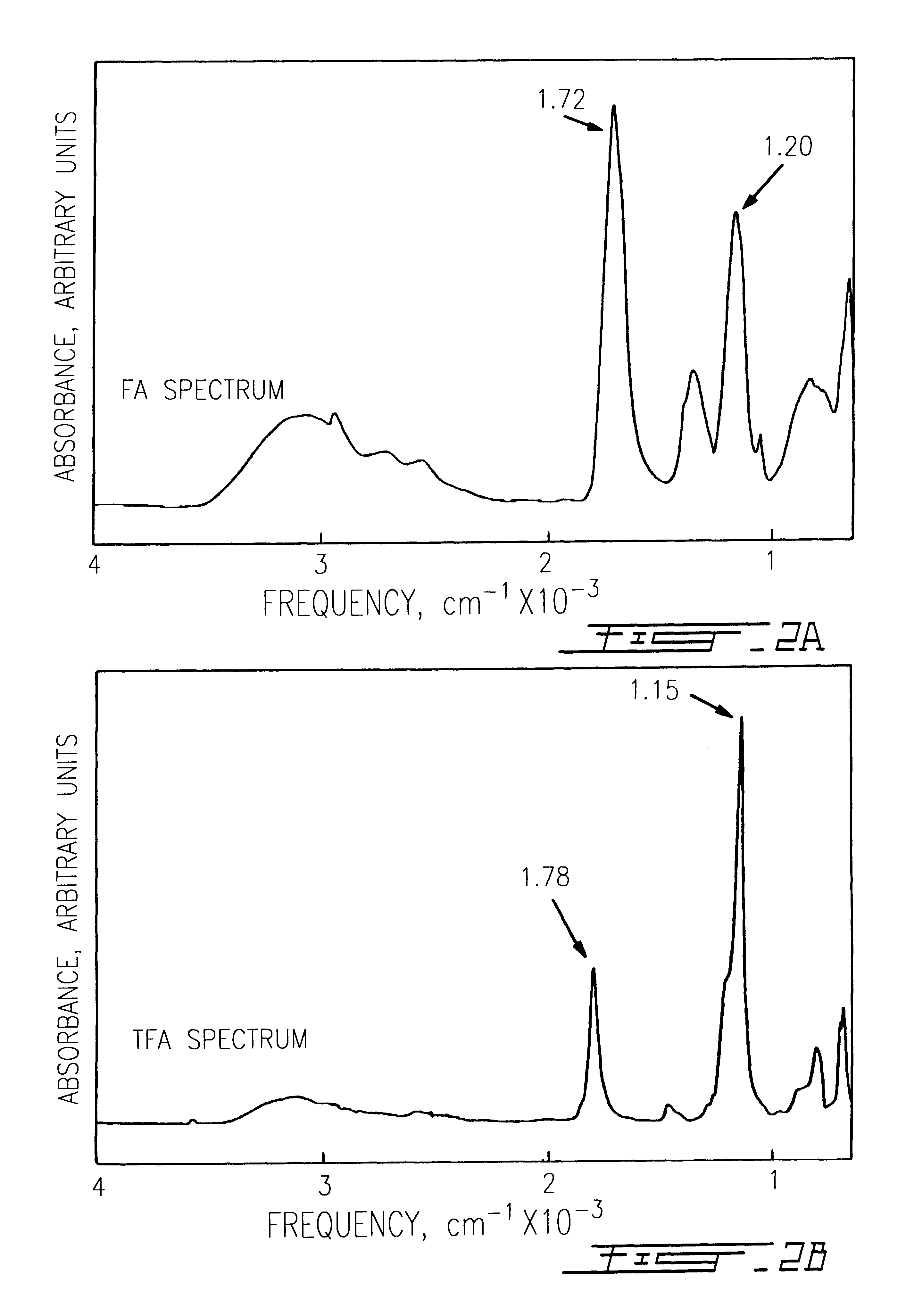
The use of collagen as a biomedical implant raises safety issues towards viruses and prions. The physicochemical changes and the in vitro and in vivo biocompatibility of collagen treated with heat, and by formic acid (FA), trifluoroacetic acid (TFA), tetrafluoroethanol (TFE) and hexafluoroiso-propanol (HFIP) were investigated. FA and TFA resulted in extensive depurination of nucleic acids while HFIP and TFE did so to a lesser degree. The molecules of FA, and most importantly of TFA, remained within collagen. Although these two acids induced modification in the secondary structure of collagen, resistance to collagenase was not affected and, in vitro, cell growth was not impaired. Severe dehydrothermal treatment, for example 110° C. for 1-3 days under high vacuum, also succeeded in removing completely nucleic acids. Since this treatment also leads to slight cross-linking, it could be advantageously used to eliminate prion and to stabilize gelatin products. Finally, prolonged treatment with TFA provides a transparent collagen, which transparency is further enhanced by adding glycosaminoglycans or proteoglycans, particularly hyaluronic acid. All the above treatments could offer a safe and biocompatible collagen-derived material for diverse biomedical uses, by providing a virus or prion-free product.
Owner:UNIV LAVAL
Composition for stabilizing corneal tissue during or after orthokeratology lens wear
Two types of compositions having an eye-drop delivery system are used during or after an orthokeratology procedure to prevent or retard relaxation of corneal tissue back to the original anterior curvature of the cornea. Each composition functions independently from the others and is a different approach of preparing a stabilizing agent. The first composition is directed to a biologically compatible composition comprising fibril associated collagens with interrupted triple helices (FACITs) and / or small leucine-rich repeat proteoglycans (SLRPs). The fibril associated collagen family includes various types of collagens, such as type VI, type XX, type XII, and type XIV. The small leucine-rich repeat proteoglycans family includes decorin, keratocan, biglycan, epiphycan, lumican, mimecan, and fibromodulin. The second composition includes the enzyme found as a normal component of tissues, plasma, or epidermis, such as transglutaminase.
Owner:EUCLID SYST CORP +2
Compositions and methods for stimulating synthesis of pro-collagen or collagen and hyaluronic acid
ActiveUS7722904B2Increase moistureImprove textureCosmetic preparationsBiocideSkin appearanceMedicine
A method for improving the appearance, texture and / or moisture of skin comprising administering a composition comprising Chia Seed oil, Opuntia ficus Indica extract, or both to stimulate collagen or hyaluronic acid synthesis, or decrease Interleukin-1b synthesis, in the skin.
Owner:ACCESS BUSINESS GRP INT LLC
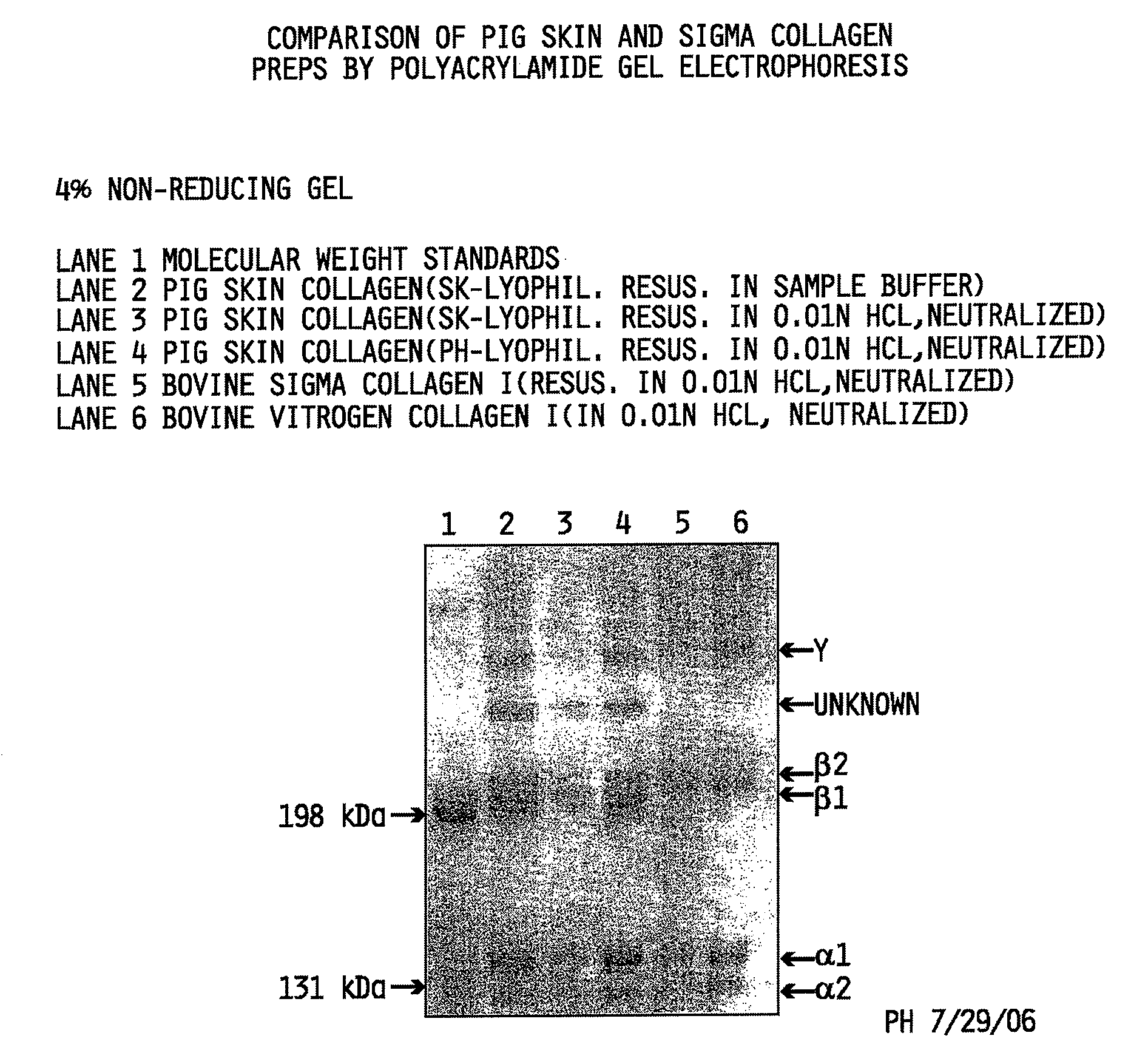
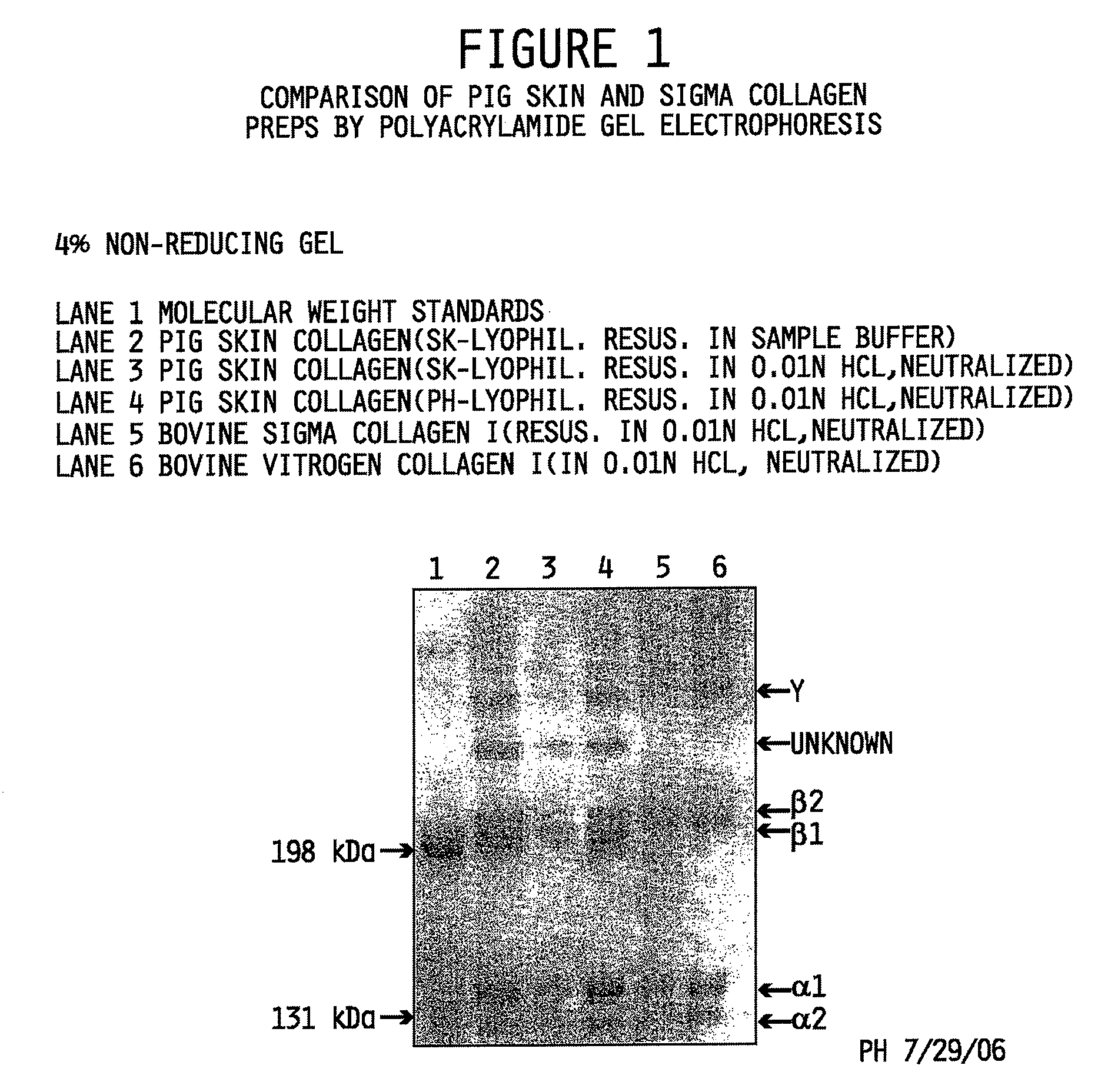
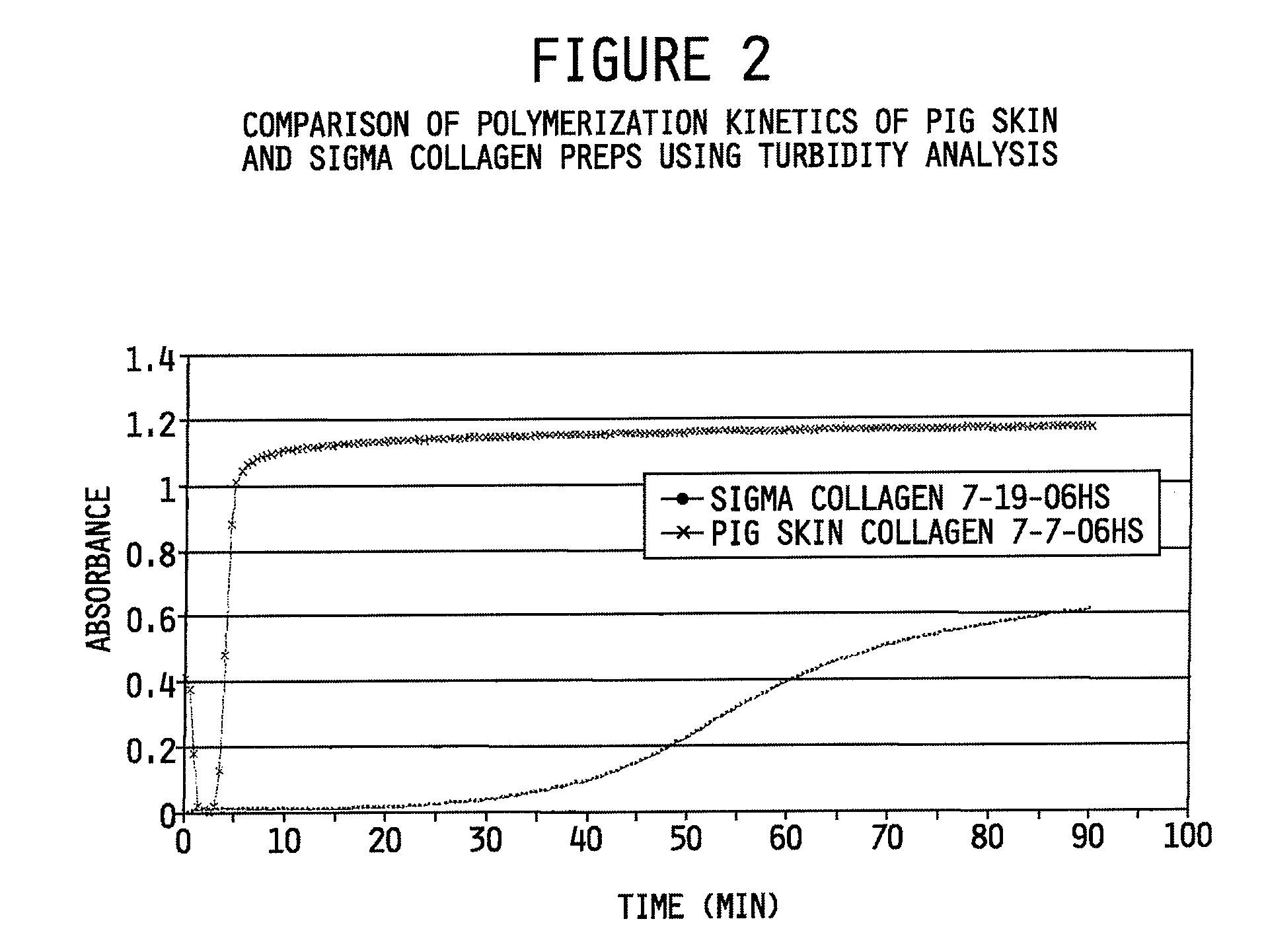
Collagen preparation and method of isolation
ActiveUS20080268052A1Facilitate hierarchical assemblyImprove mechanical propertiesPowder deliveryConnective tissue peptidesCollagen VIMechanical property
Collagen compositions, methods for preparing those collagen compositions, and graft compositions formed from those collagen compositions are provided. In particular, methods of isolating collagen that exhibits an enhanced rate of polymerization and enhanced microstructural and mechanical properties upon polymerization, such collagen compositions, and graft compositions formed from such collagen compositions are provided.
Owner:PURDUE RES FOUND INC
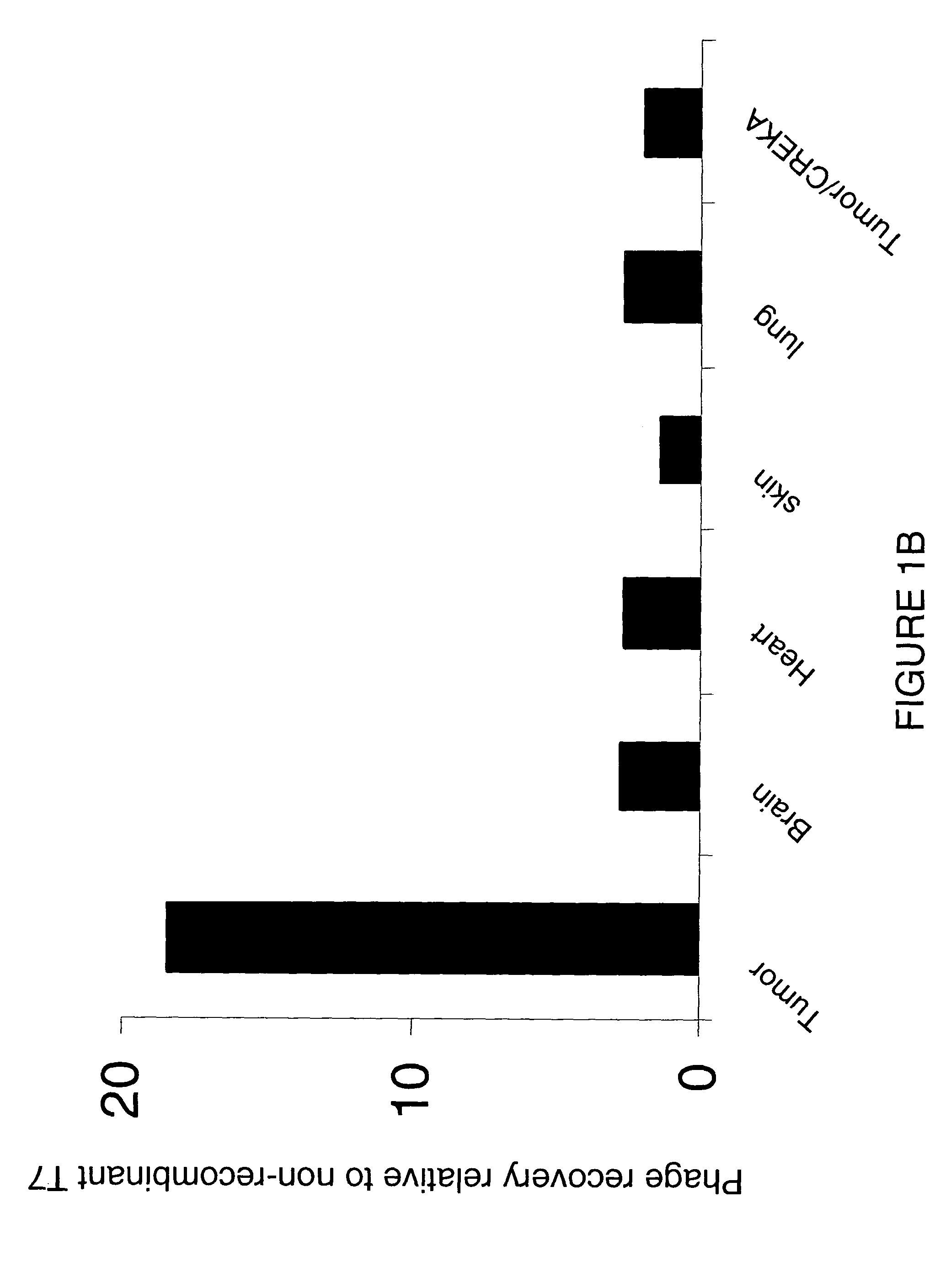
Collagen-binding molecules that selectively home to tumor vasculature and methods of using same
InactiveUS7488792B2Reduce in quantityReduce the numberHybrid immunoglobulinsPeptide/protein ingredientsTumor vasculatureWilms' tumor
Owner:BURNHAM INST FOR MEDICAL RES
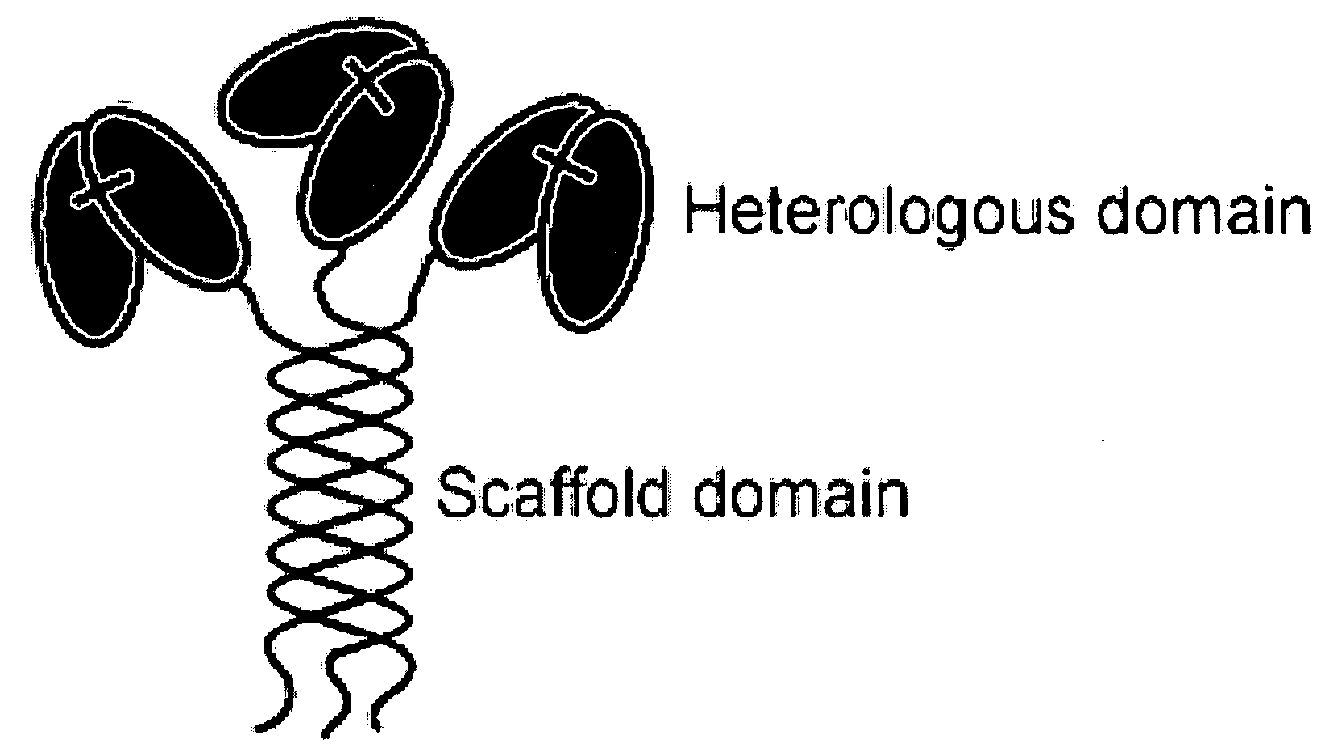
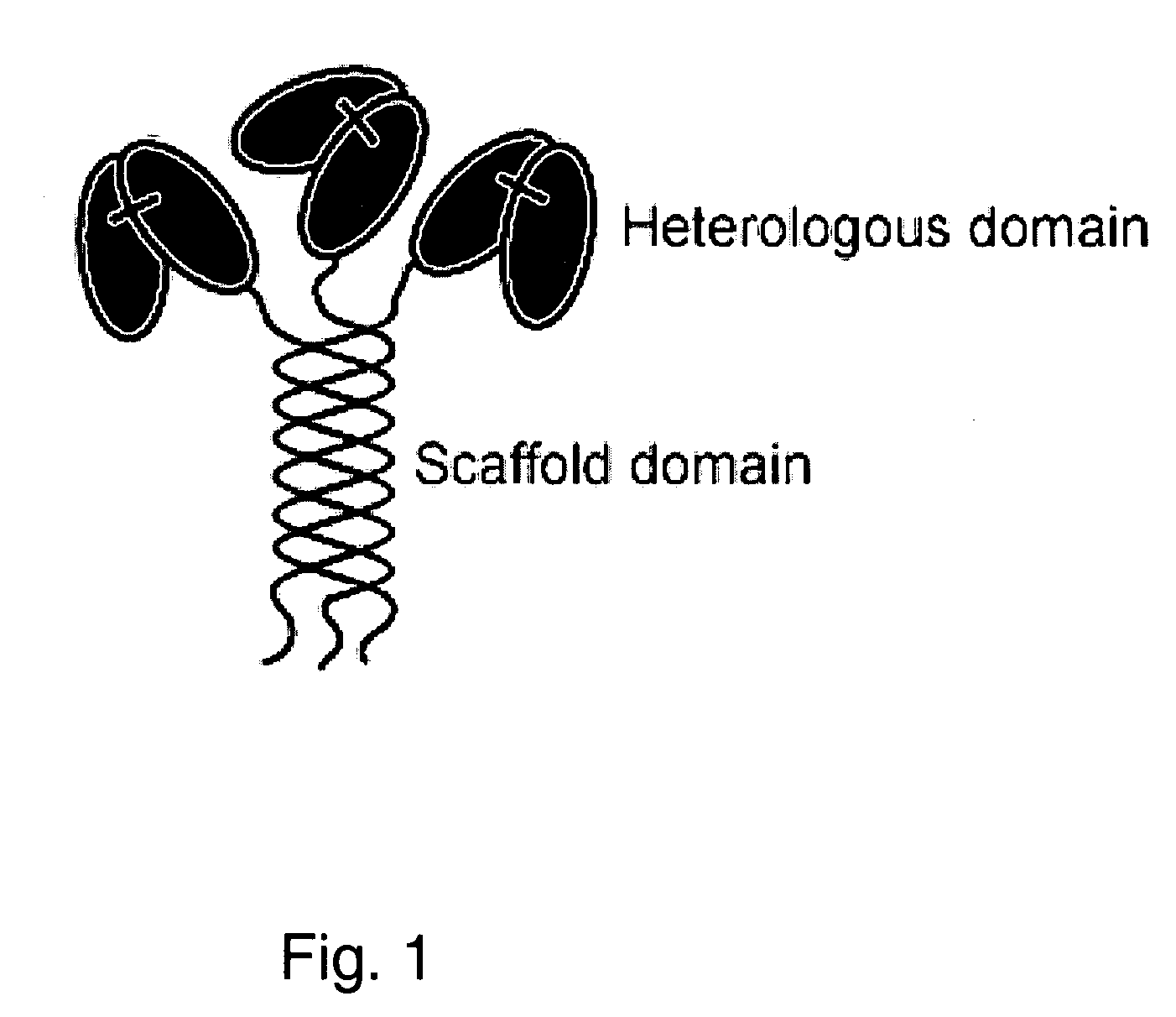
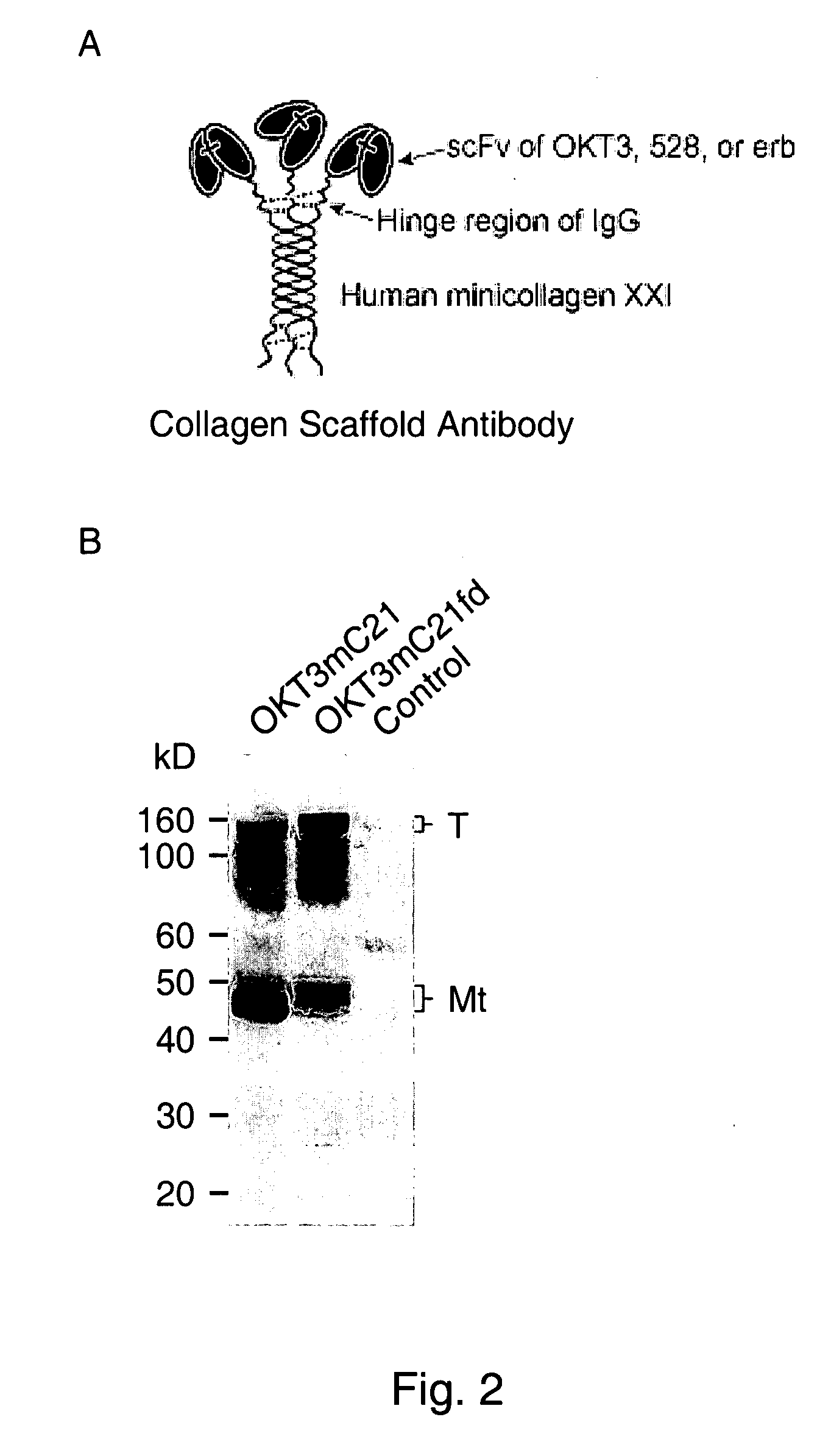
Trimeric collagen scaffold antibodies
ActiveUS20080176247A1High affinityHighly preventive effectAnimal cellsConnective tissue peptidesHeterologousEGF-like domain
A collagen scaffold domain, including a collagenous or collagen-like domain, which directs self-trimerization is provided. The collagen scaffold domain can be fused to one or more heterologous domains, such as an antibody domain. Methods for generating and using the scaffold domains and fusion proteins are also provided.
Owner:IND TECH RES INST
Collagen-binding proteins from Streptococcus pyogenes
InactiveUS7169902B2Avoid infectionInhibit bindingBacterial antigen ingredientsBacteriaStreptococcus pyogenesOrganism
Isolated proteins, designated Cpa1 and Cpa49, and their corresponding amino acid and nucleic acid sequences are provided which are useful in the prevention and treatment of infection caused by group A streptococcal bacteria such as Streptococcus pyogenes. These proteins have been observed to bind to collagen, and thus methods are provided, such as by administration of the proteins or antibodies generated thereto, whereby streptococcal binding of collagen can be inhibited, and streptococcal infection can be greatly reduced. In addition, medical instruments can be treated using the collagen-binding proteins of the invention in order to reduce or eliminate the possibility of their becoming infected or further spreading the infection. In particular, the proteins are advantageous because they may be used as vaccine components or antibodies thereof, and they may be administered to wounds or used to coat biomaterials in order to act as collagen blocking agents and reduce or prevent severe infection by group A streptococcal bacteria.
Owner:PODBIELSKI ANDREAS
Extracting biopolymers from a biomass using ionic liquids
Owner:PROCTER & GAMBLE C0MPANY THE +1
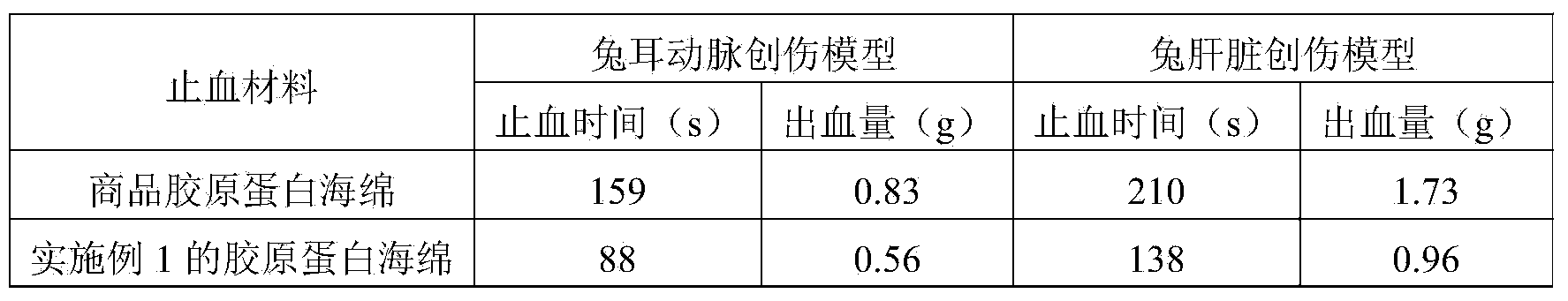
Preparation method of high-purity collagen protein sponge
InactiveCN103772734AGood removal effectHigh purityConnective tissue peptidesPeptide preparation methodsFreeze-dryingCollagen sponge
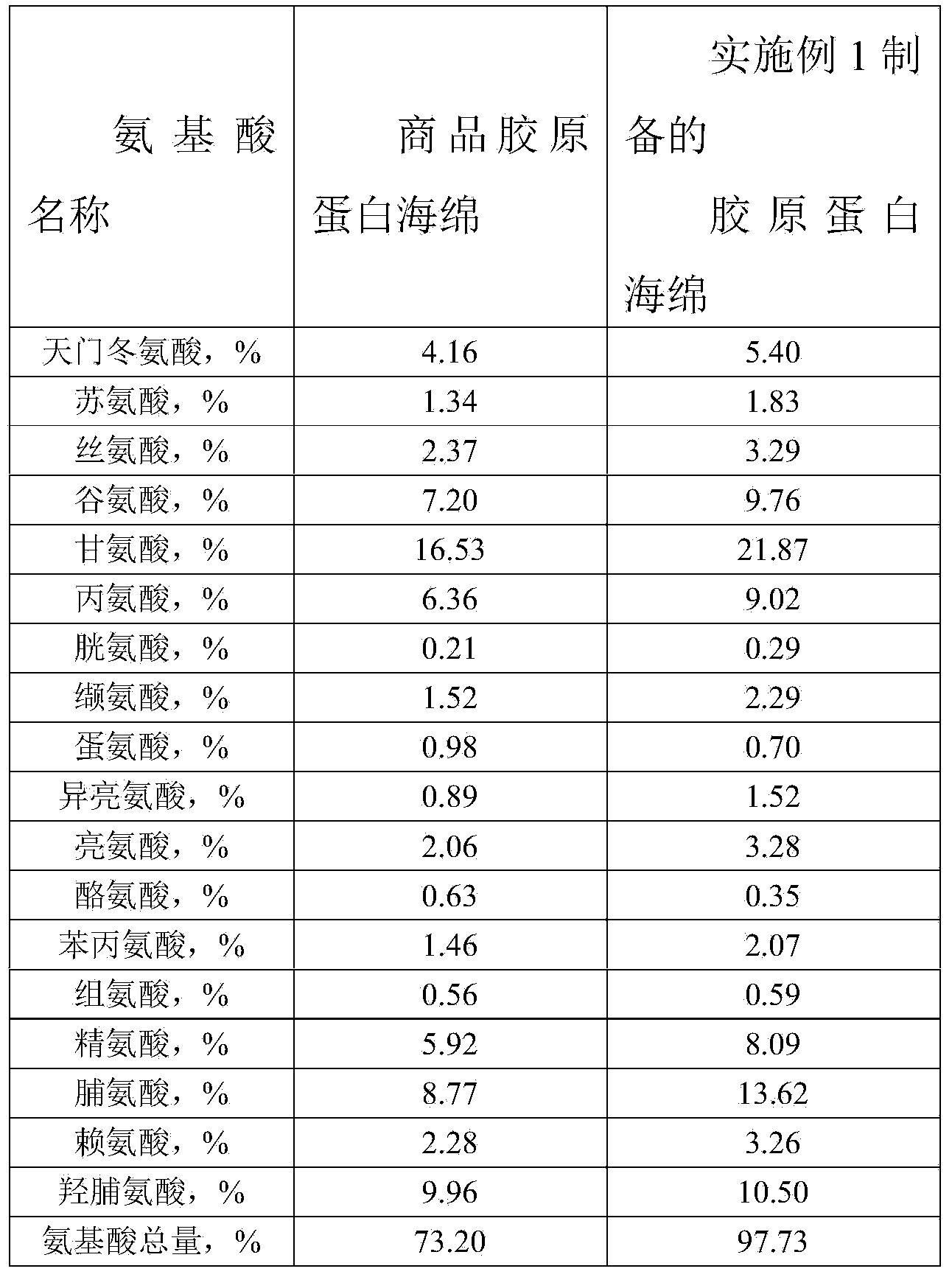
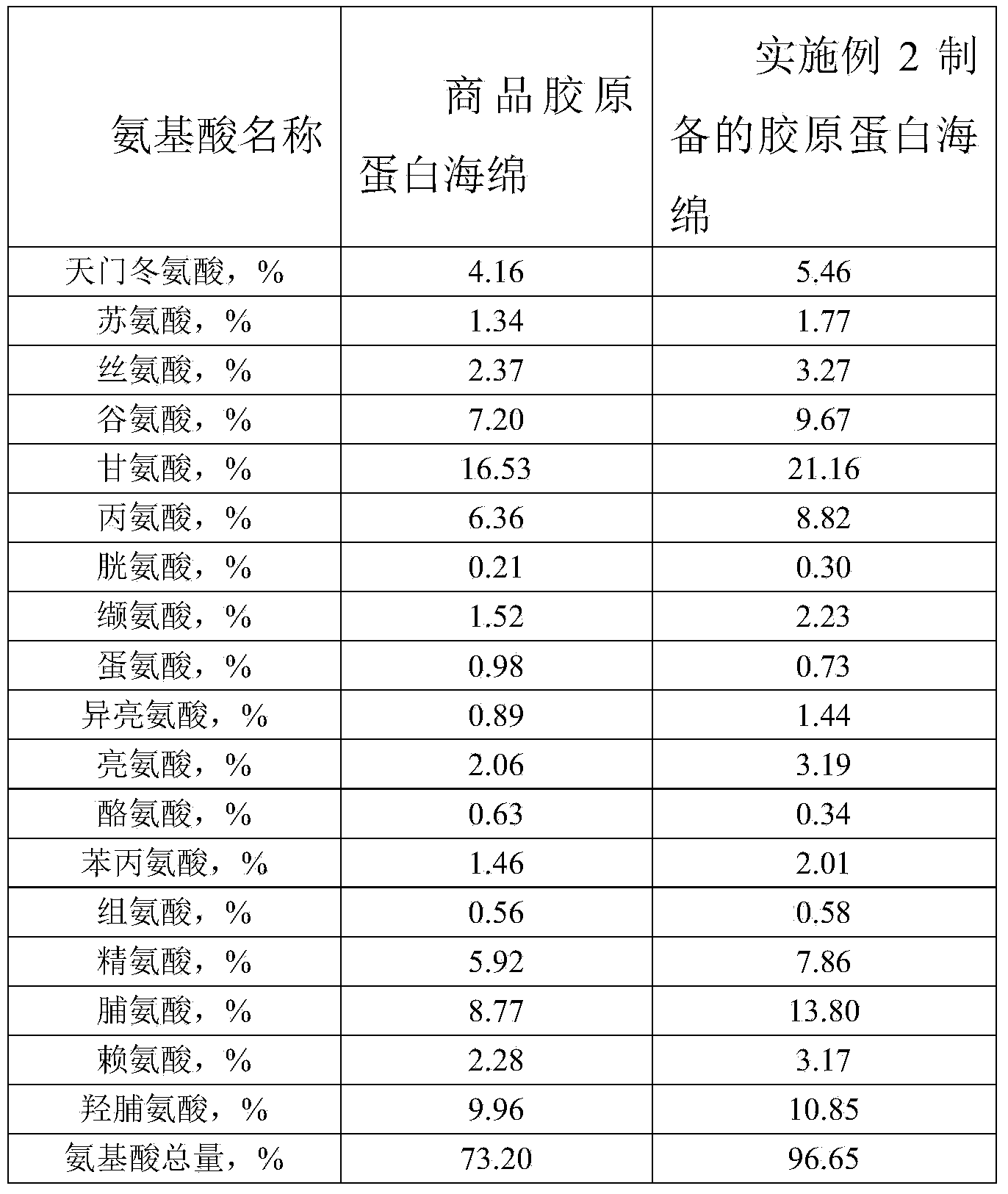
The invention provides a preparation method of high-purity collagen protein sponge, and relates to a preparation method of collagen protein sponge. The preparation method of the high-purity collagen protein sponge is used for solving the problems that the collagen protein sponge prepared by using a conventional method is long in production cycle and low in yield and purity and has poor hemostasis performance. The preparation method of the high-purity collagen protein sponge comprises the following steps: step one. pretreating fresh bovine heel tendons; step two. extracting collagen protein; step three. centrifuging; step four. salting out; step five. dissolving; step six. carrying out gradient dialysis; step seven. pre-freezing; step eight. carrying out freeze drying; and step nine. sterilizing. The final product prepared by using the method has a smooth and flat surface and relatively good hemostatic performance and is uniform in pore size distribution. The product has relatively high purity (the total amount of amino acids reaches 97.73%), an obvious hemostatic effect and no abnormal taste, is safe, non-toxic, high in yield and short in time; liquid is clear without impurities; the production cycle is shortened; the whole preparation process is carried out at a room temperature; the biological activity of the collagen protein is maintained; and the application of the high-purity collagen protein sponge in clinical is improved.
Owner:HARBIN INST OF TECH
Method for preparing collagen oligopeptide
InactiveCN102559826AWhite colorGreat tastePeptide preparation methodsFermentationFood industryProcollagen type i n-terminal peptide
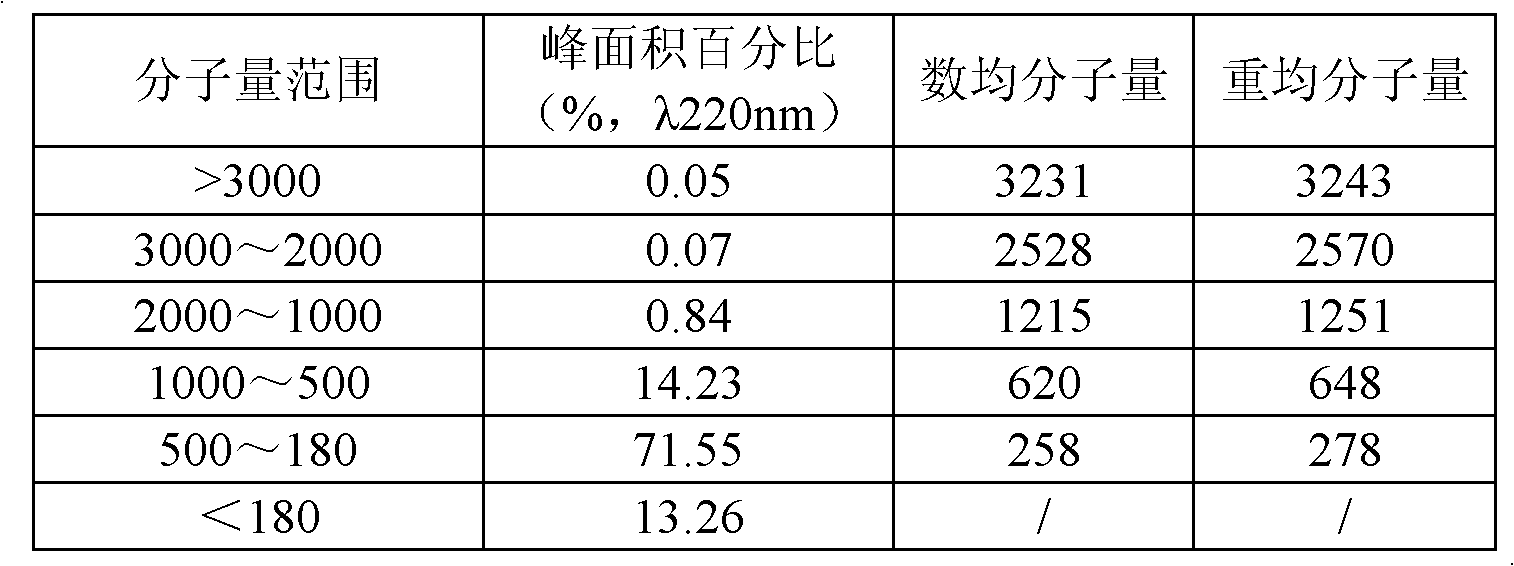
The invention discloses a method for preparing collagen oligopeptide. Animal skins are taken as raw materials, and are subjected to acid and surfactant pretreatment, enzymolysis, decoloration, membrane separation and drying to form the collagen oligopeptide. The relative molecular weight of the prepared collagen oligopeptide is mainly distributed below 1,000Dalton, and preferably, the Gaussian distribution peak area percentage of oligopeptide with the relative molecular weight of less than 1,000Dalton is over 85 percent and preferably over 90 percent. The method solves the general problems that the conventional collagen products have odor, are dark yellow and the like; and the obtained collagen oligopeptide powder is white, free of odor and good in mouthfeel, has high absorptivity and penetrability and can be widely applied to the cosmetics industry, the food industry and the like.
Owner:胡如桂
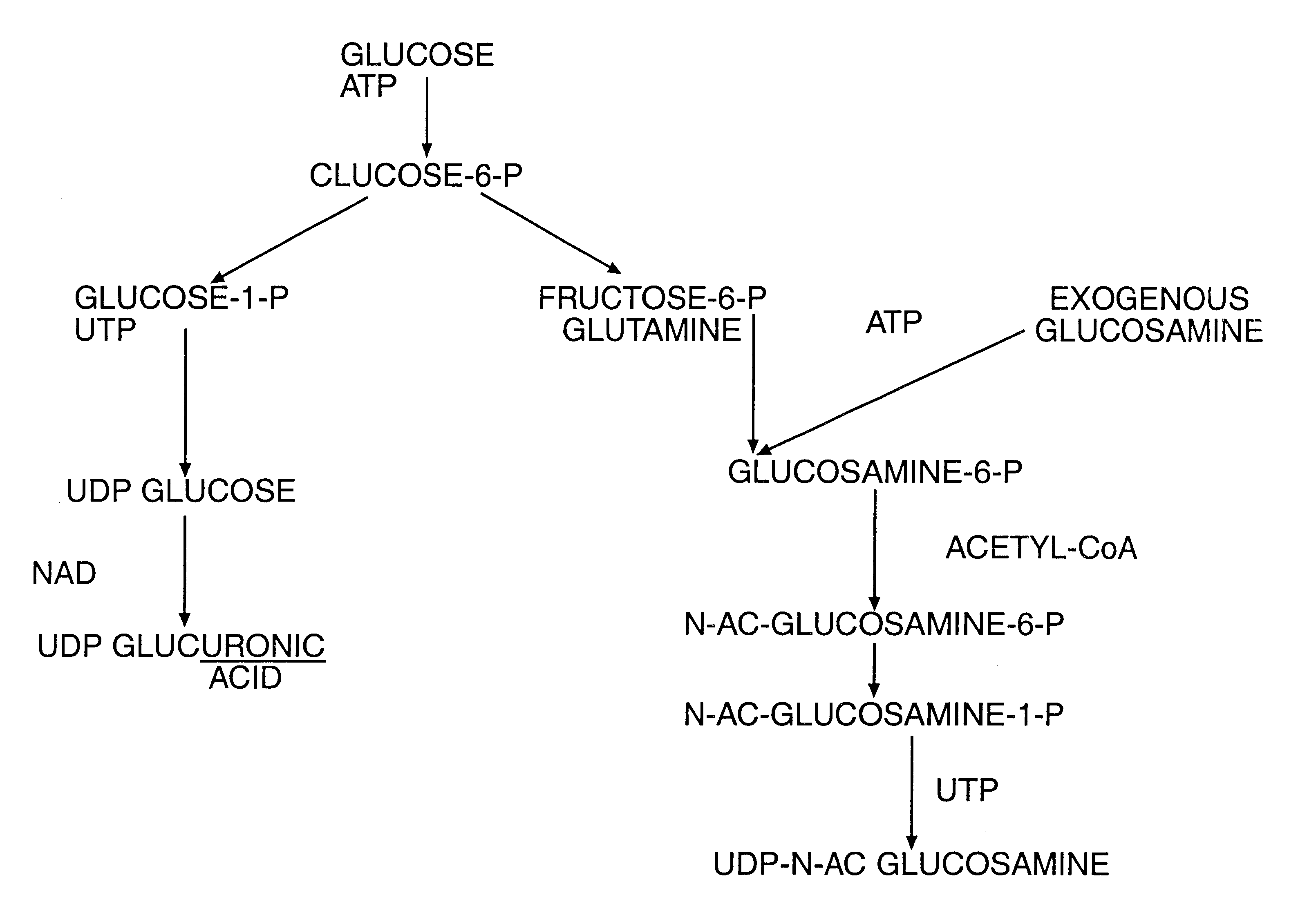
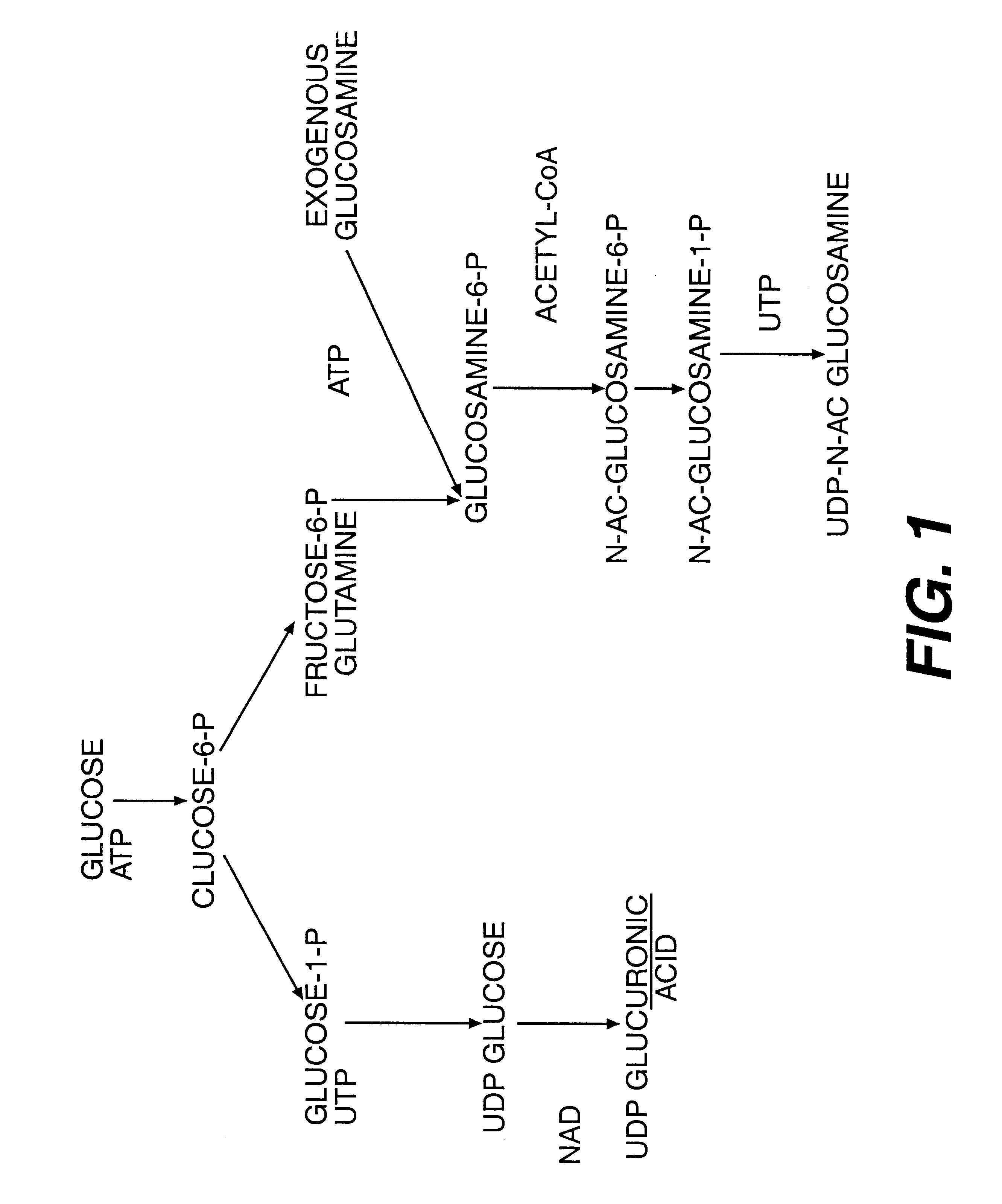
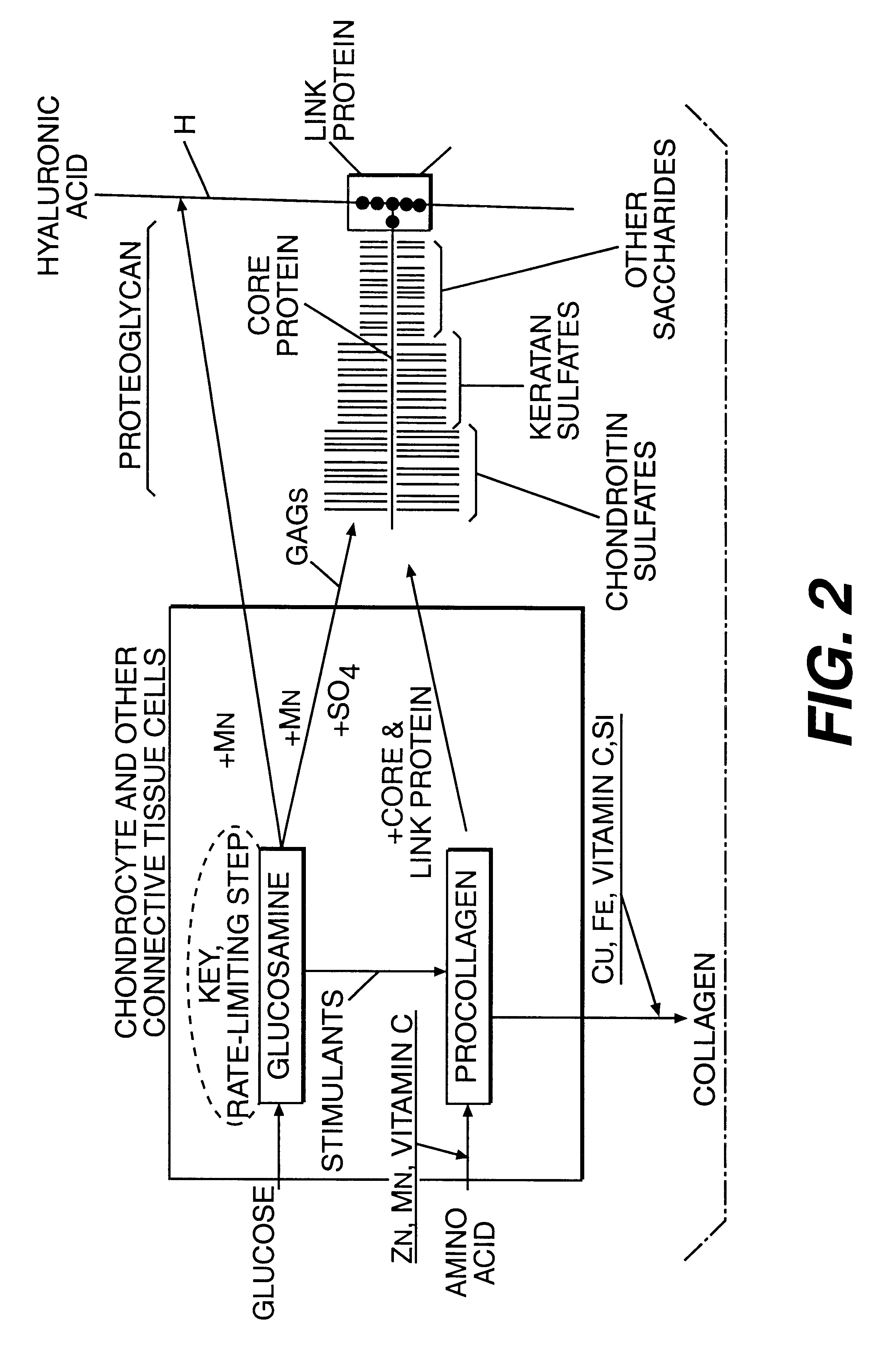
Aminosugar and glycosaminoglycan composition for the treatment and repair of connective tissue
Owner:NUTRAMAX LABORATORIES INC
Method for preparing collagen peptide by using cod skin
InactiveCN101407834AIncrease contentNo pollution in the processPeptide preparation methodsFermentationCollagen VIPollution
The invention relates to a method for preparing a collagen peptide by taking a cod skin as a material; in the method, the cod skin is selected as the material and is washed clearly by fresh water; 0.2 percent-0.5 percent mixed liquid of calcium hyduloxide and sodium hydroxide is added for dipping; then the cod skin is washed by fresh water until the pH value is neutral; after the water liquid of 0.3 percent to 0.6 percent diluted acid is added for dipping, the cod skin is washed by fresh water again until the pH value is neutral; the cod skin is cracked and is stirred and extracted in hot water for extracting a collagen; then undissolved substances are removed through filtering; the pH value of the extraction liquid of the collagen is adjusted to be neutral; under the stirring conditions, enzymes are added for carrying out zymohydrolysis and obtaining a zymohydrolysis liquid; and after the enzymes are eliminated and filtering is carried out, the powder of the collagen peptide is obtained through vacuum condensing and sponging drying. The method has the advantages of simple technique, less device investment, no environment pollution and being suitable for industrialization production. The collagen content of the powder of the prepared collagen peptide is higher; and the color, smell and biological properties are excellent. The powder of the collagen peptide can be broadly applied to the industries of health-care foods, medicines, and the like.
Owner:TAIXIANG GRP TECH DEV
Method of stimulating collagen formation
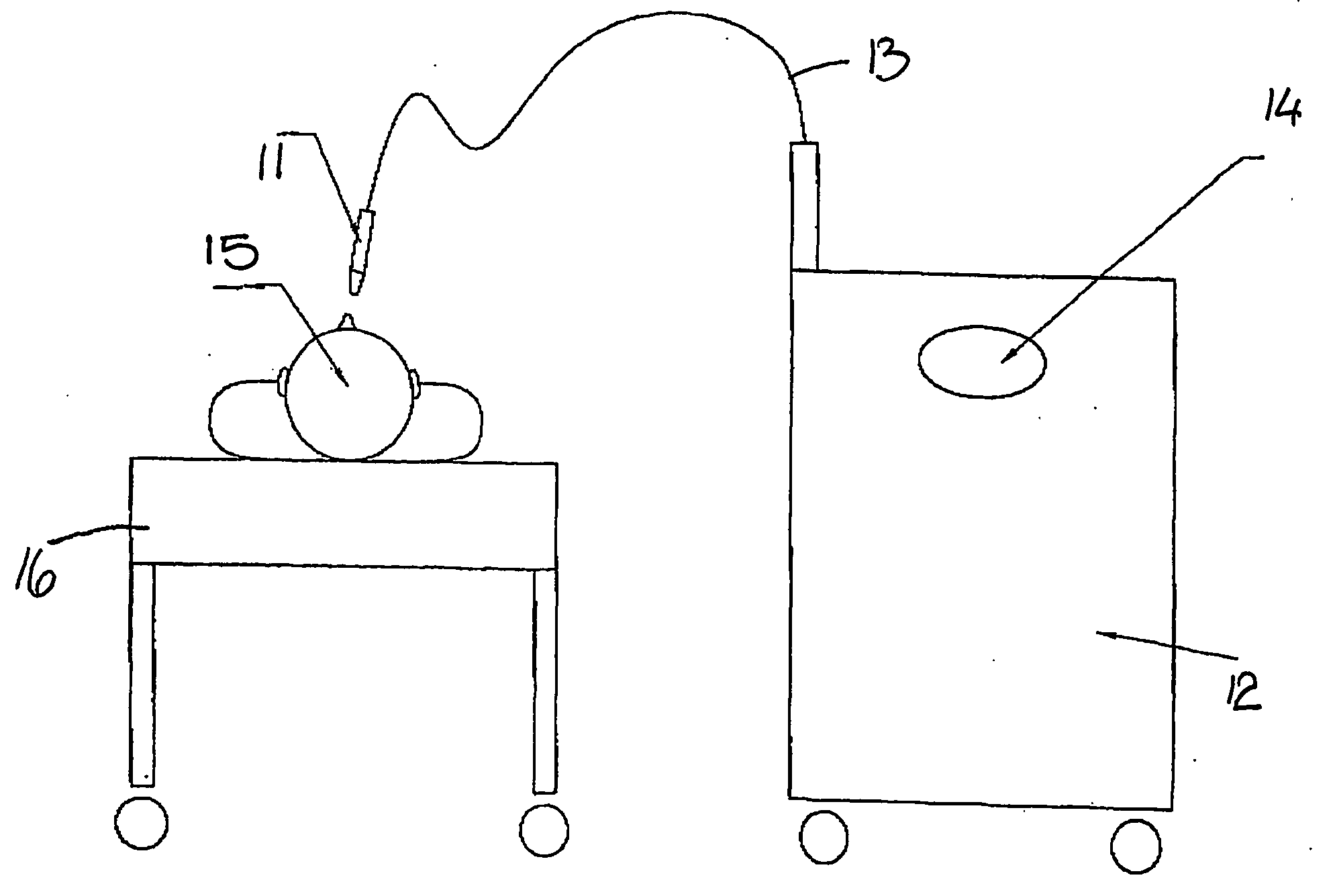
InactiveUS20040210275A1Improve cell activityIncreased collagen formationSurgical instrument detailsLight therapySkin treatmentsSurgery
A method of stimulating collagen formation in a selected area of mammalian skin in which the selected area is irradiated with a source of visible or near infra-red radiation. The radiation is absorbable by intracellular chromophores to enhance cellular activity and increase collagen formation. A skin treatment device for carrying out the method of the invention is also described.
Owner:CARL ZEISS MEDITEC AG
In vivo synthesis of connective tissues
InactiveUS7375077B2Overcome deficienciesBiocidePeptide/protein ingredientsLigament structureCollagen sponge
The in vivo synthesis of connective tissue by fibroblast or fibroblast precursor cells ensconced within a biocompatible scaffold is disclosed. The cells are preferably present in a biocompatible scaffold such as gelatin and placed between two other biocompatible scaffolds such as collagen sponges soaked with a collagenic amount of a member of the TGF-β family of proteins. This composition is then implanted in a host to produce cranial sutures, periodontal ligament or other fibrous tissue structures in vivo.
Owner:THE BOARD OF TRUSTEES OF THE UNIV OF ILLINOIS
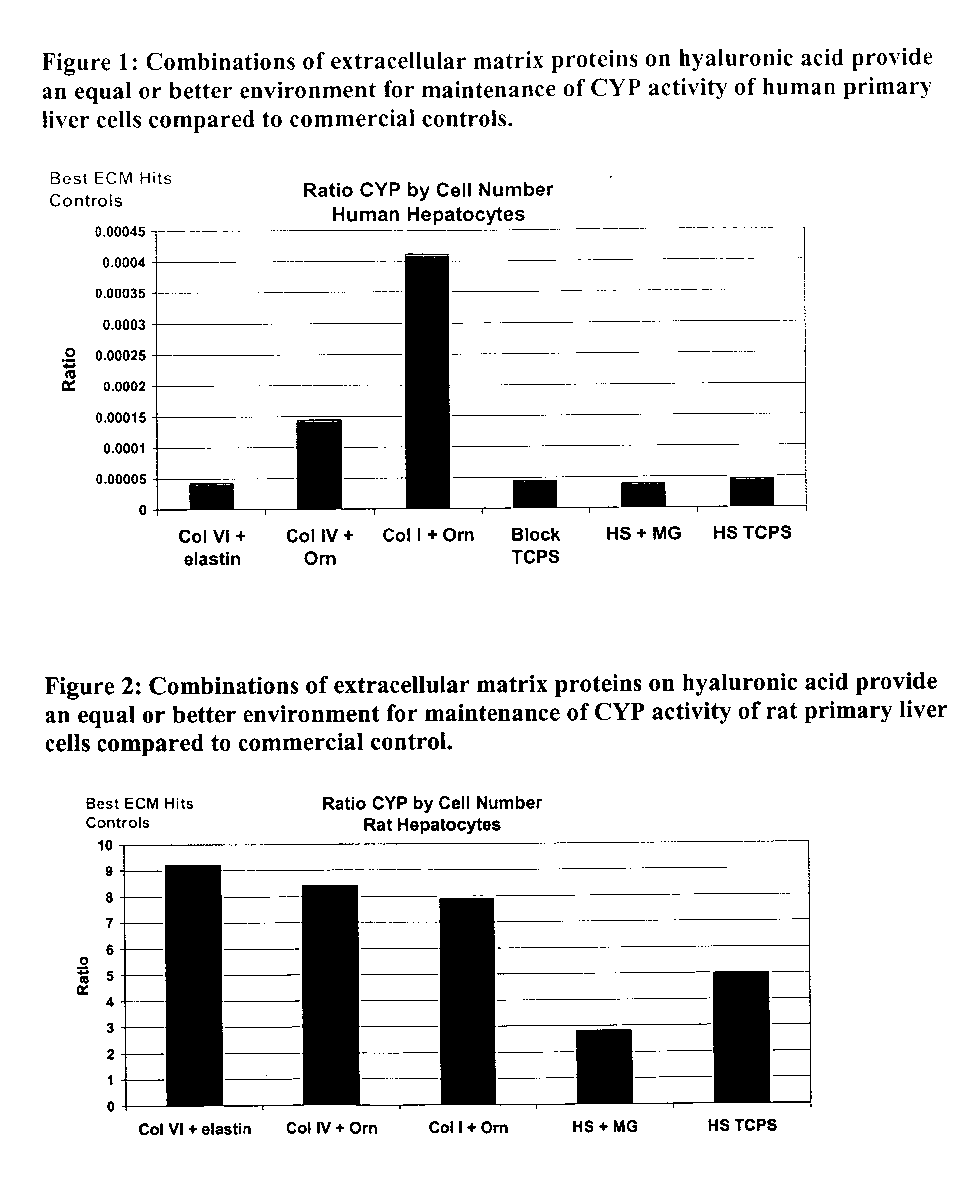
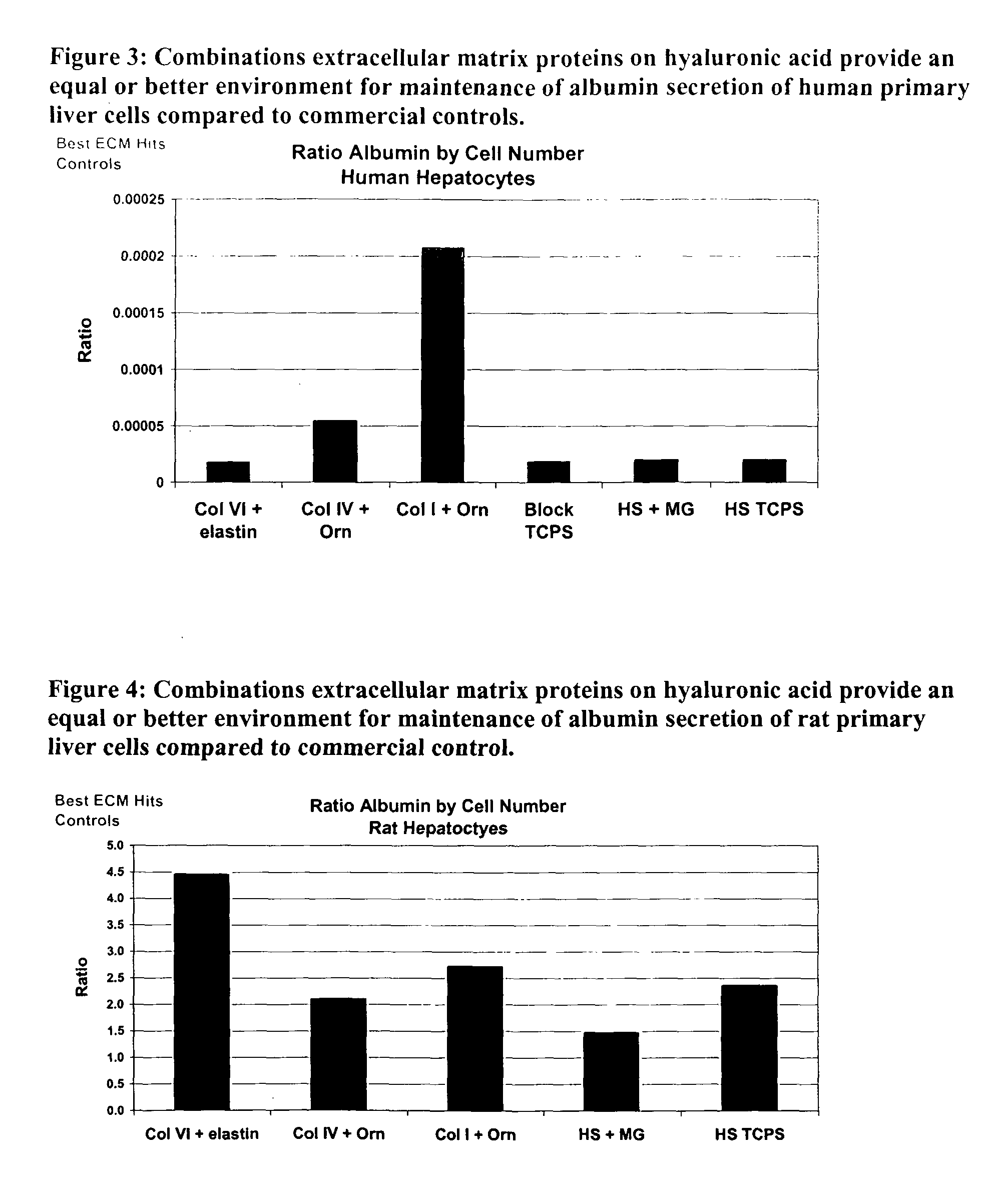
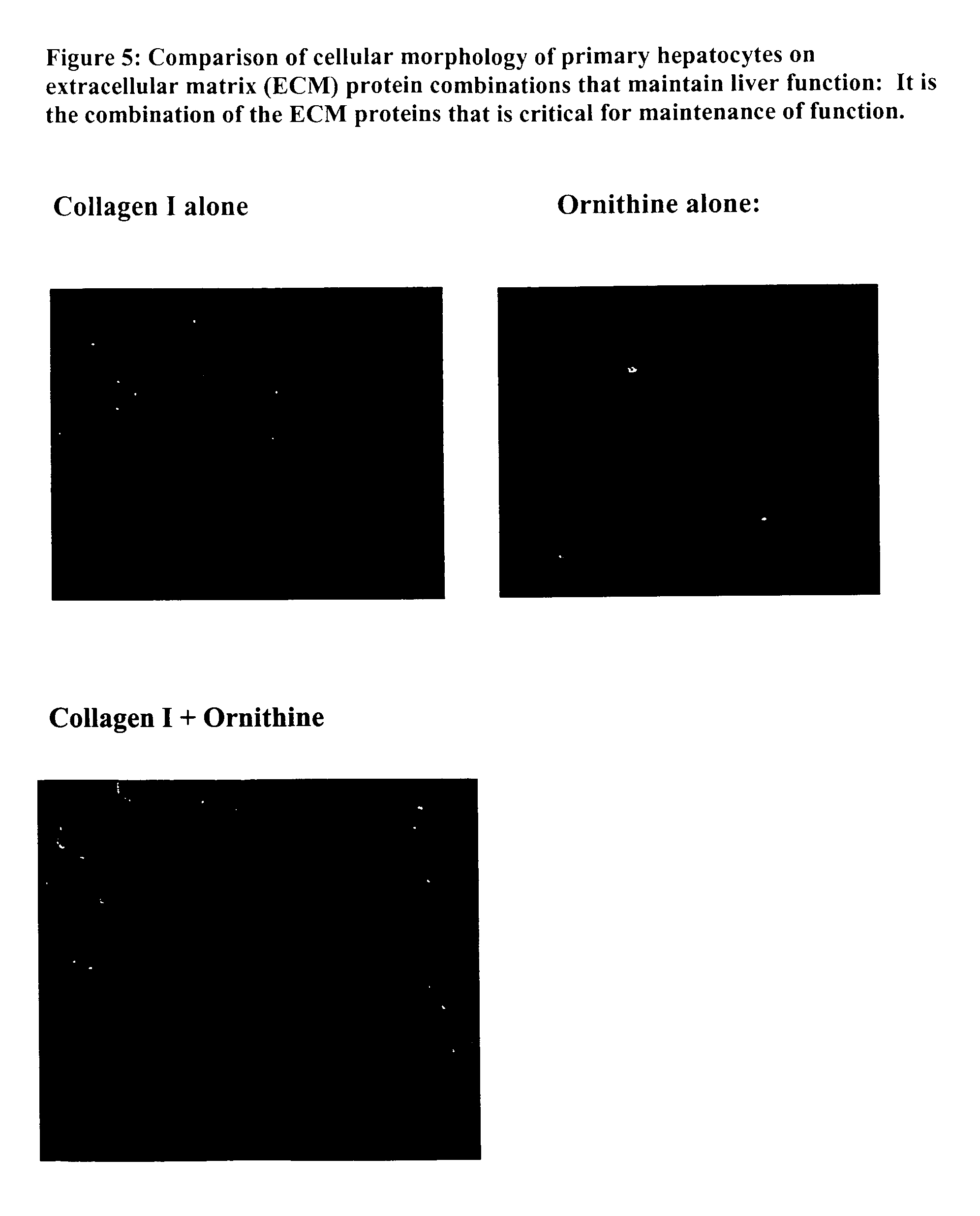
Environments that maintain function of primary liver cells
InactiveUS20050059150A1Good adhesionEasy maintenanceHepatocytesArtificial cell constructsECM ProteinL-Ornithine
Surfaces useful for cell culture comprise a support to which is bound a CAR material, and, bound to the CAR material, an ECM protein, or a biologically active fragment or variant thereof such as elastin, fibronectin, vitronectin, laminin, collagen I, collagen III, collagen IV, and collagen VI. Also, optionally present on the surface is an active factor, preferably a polycationic polymer or a biologically active fragment or variant thereof, such as polyethyleneimine (PEI), poly-D-lysine (PDL), poly-L-lysine (PLL), poly-D-ornithine (PDO) or poly-L-ornithine (PLO). This surface is used in cell culture to promote cell attachment, survival, and / or proliferation of primary liver cells. The invention also relates to methods utilizing this surface, such as methods for attachment, survival, and / or proliferation of cells. Further disclosed is the use of the surface in cell culture with serum-free medium. Methods of screening using the surface of the invention are also disclosed.
Owner:BECTON DICKINSON & CO
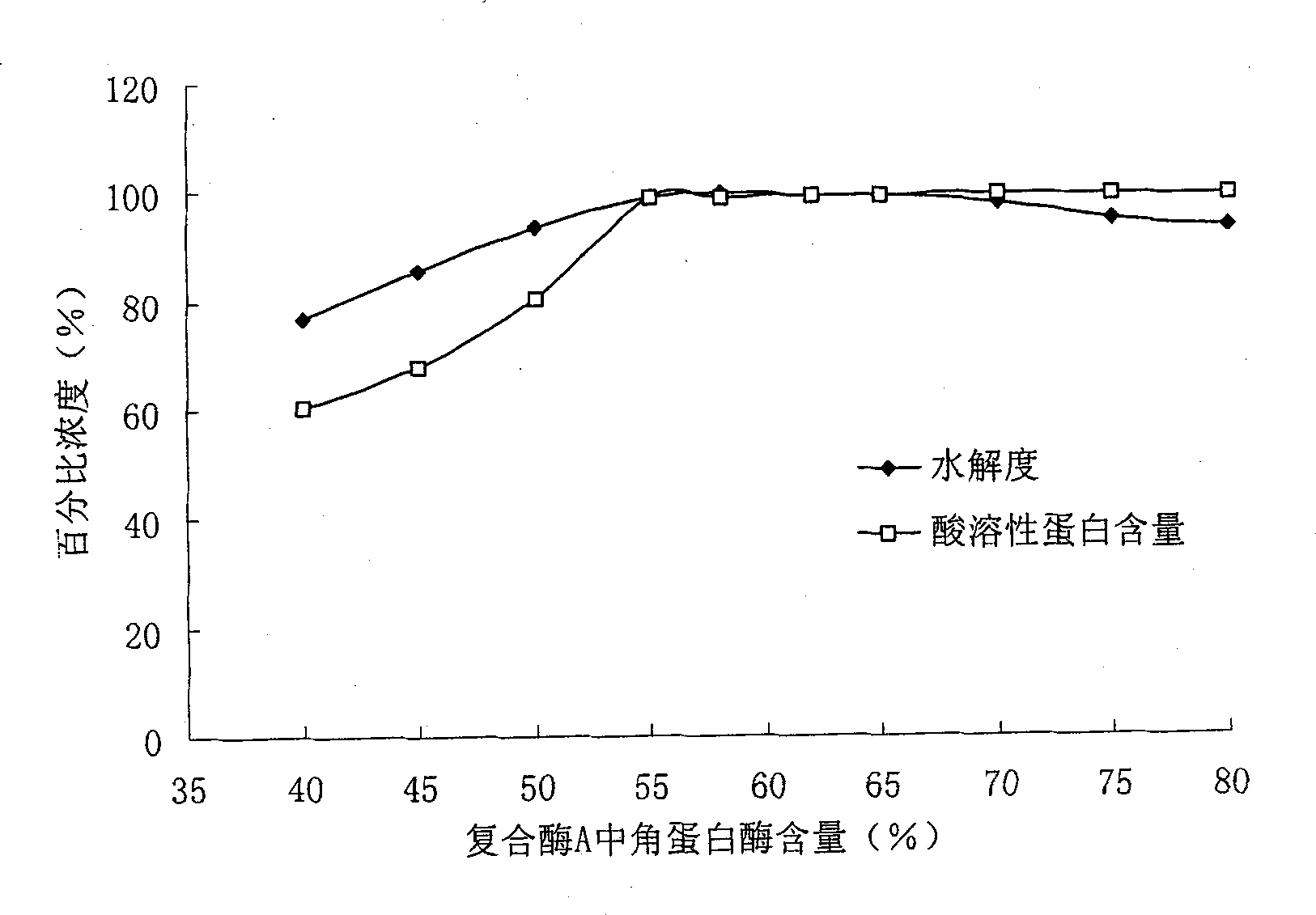
Collagen polypeptide, preparation method and application thereof
ActiveCN101787078AEliminate bitternessEliminate odorCosmetic preparationsConnective tissue peptidesSolubilityNitrogen
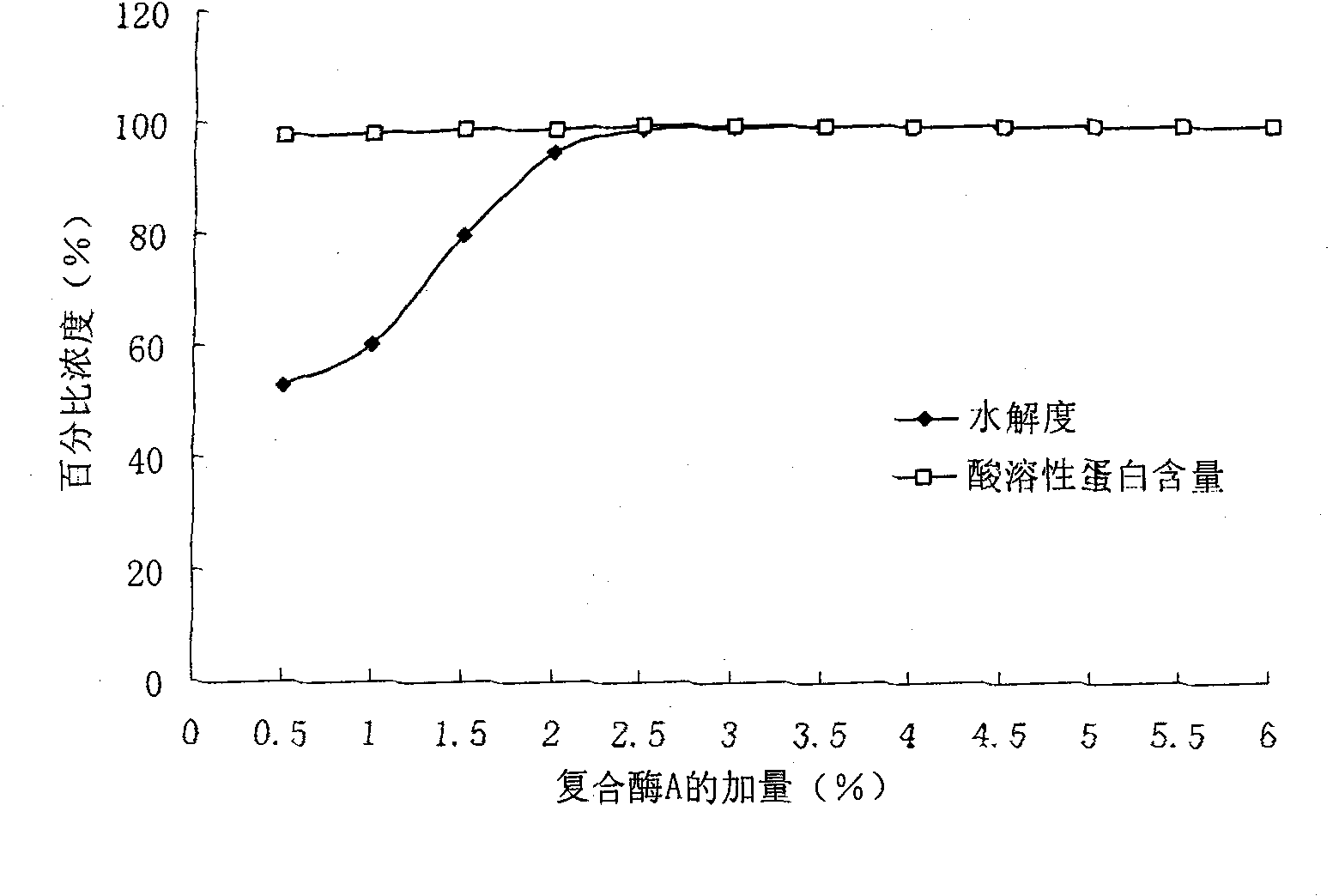
The invention discloses a collagen polypeptide, a preparation method and application thereof. The collagen polypeptide comprises the following components in percentage by weight: 45-55 w / w% of components with molecular weight of 6500+ / -100 Da, 20-25 w / w% of components with molecular weight of 1300+ / -100 Da, and 25-35 w / w% of components with molecular weight of below 1000 Da. The nitrogen solubility index of the collagen polypeptide is 98.5-99.9%. The preparation method disclosed by the invention comprises the following steps: mixing the raw materials with complex enzyme A, and hydrolyzing.
Owner:香港百特有限公司
Human umbilical cord mesenchymal stem cell exosome type external cream and preparation method thereof
InactiveCN107854418AEasy to get materialsA large amountCosmetic preparationsToilet preparationsFiberBase cream
The invention provides human umbilical cord mesenchymal stem cell exosome type external cream and a preparation method thereof, and aims at the solving the problem in effectively inducing human umbilical cord mesenchymal stem cells to massively synthesize and secrete mesenchymal stem cell exosome capable of specifically promoting epidermis cell and hypodermal cell to proliferate, divide and differentiate, promoting the formation of collagenous fibers, reticular fibers and elastic fibers, and adjusting the secreting functions of collagen and mucopolysaccharide. The cream comprises human umbilical cord mesenchymal stem cell exosome extracting liquid and a base cream formula based on the mass ratio ranging from (1:20) to (3:10), wherein the human umbilical cord mesenchymal stem cell exosome extracting liquid is prepared by performing hungry culture on P2-P5 generations of human umbilical cord mesenchymal stem cells and stimulating through epidermal growth factors. The prepared cream has the effects of improving the skin quality, resisting the aging of skin cells, recovering the normal structure and physiological function of the skin, reducing the skin atrophy exsiccosis, and moisturizing the skin tissue.
Owner:南京九圣生物医学科技股份有限公司
Preparation of agarose coated, solid agarose-collagen beads containing secretory cells
Biological agents such as secretory cells are encapsulated in a hydrophilic gel made of agarose or collagen-agarose and gelatin sponge-agarose combinations. In a preferred embodiment, semi-solid beads are formed from a suspension containing collagen, agarose and secretory cells such as pancreatic islets, the collagen is polymerized to form solid, agarose-collagen beads and the solid beads are coated with agarose. Coating is preferably by rolling the solid beads in about 5-10% agarose, contacting the rolled beads with mineral oil and washing oil from the beads. Beads containing secretory cells can be transplanted into a mammal to treat a condition caused by impaired secretory cell function.
Owner:THE ROGOSIN INST
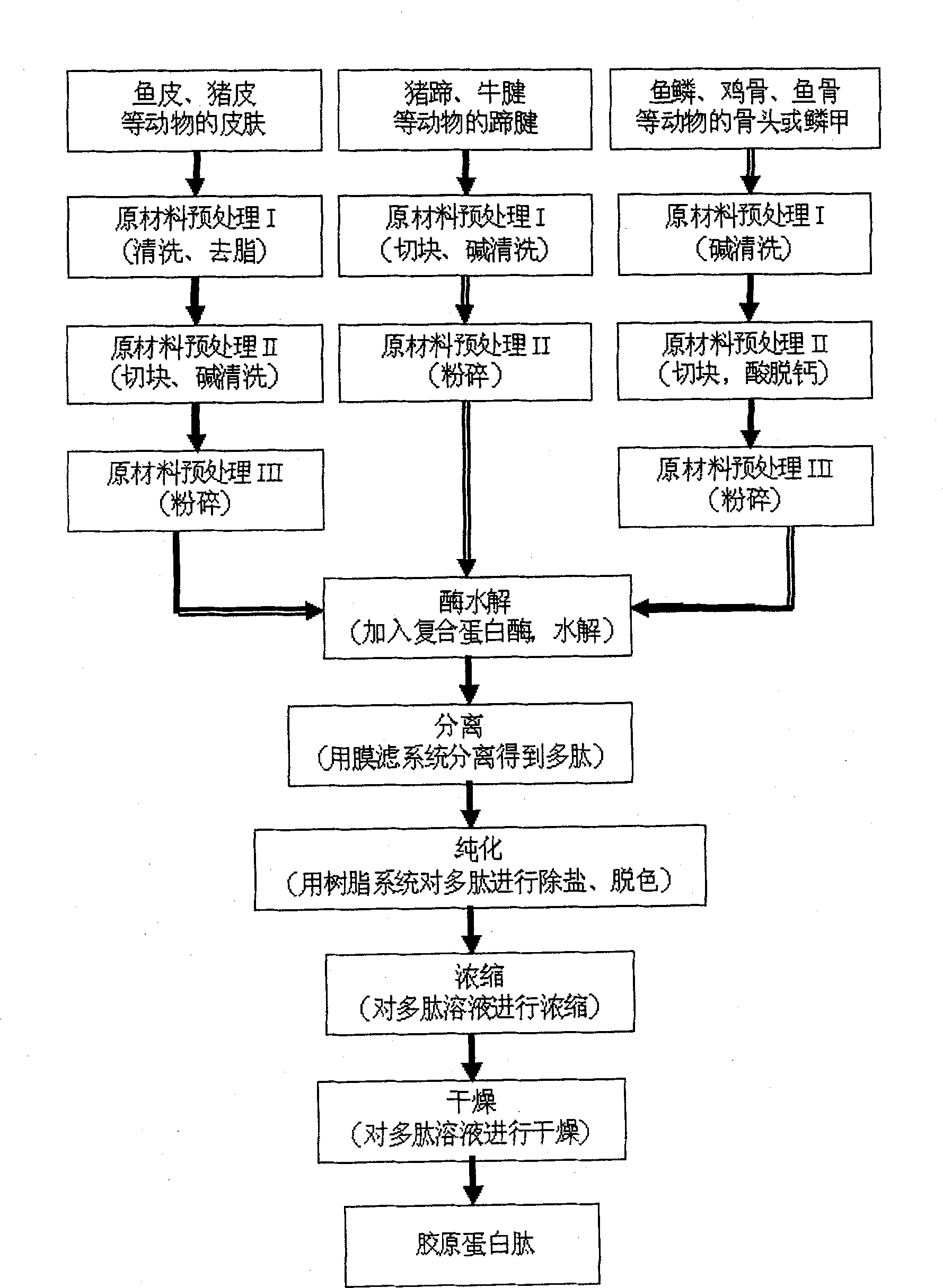
Technology of animal high-calcium powder, chondrine and collagen by composite enzyme method
InactiveCN1896262AReduce pollution indexReduce acid and alkali consumptionFermentationFiltrationCollagen VI
The present invention relates to a biological fermentative production technology of extracting high-calcium powder, chondroitin sulfate and collagen from animal cartilage which includes the following steps: the animal cartilage is simply broken into 5-20mm fragments, mixed with 1-4 times weight of water in the reaction kettle, heated to 100DEG C and kept for 1-2 hours to denature the proteins, then cold water is passed over into the kettle jacket to cool down the kettle to 40-50DEG C, compound enzyme in mass ratio of 1:0.001 which is mainly collagenase is then added, the mixture is stirred and hydrolyzed for 8-12 hours under the condition of 40-50DEG C, pH 7-8, bone residue that contains calcium phosphate is obtained after simple filtration through 100-meshed sieves; the filtrate is clarificated after fine filtration with filter press, then ethanol is added to the final concentration of 60-70%, chondroitin will be sedimentated, while remaining liquid is the mixture of collagen and ethanol. The sedimentated calcium phosphate is washed and dried and becomes calcium phosphate powder. The crystal sediment of chondroitin is purified by ethanol-washing. Collagen is separated after the mixture of collagen and ethanol goes through the ethanol regenerating column. This technology can produce different products with high purity from animal cartilage and solves the problems of pollution and single product of traditional technology.
Owner:郭秀明
Method for producing fish scale collagen protein
InactiveCN101418328AReduce churnHigh yieldConnective tissue peptidesPeptide preparation methodsProtein solutionFreeze-drying
The invention relates to a method for preparing fish scale collagen. The method comprises the following steps: fresh fish scales are taken as a material, undergo cleaning, drying and crushing, are soaked in NaOH solution for degreasing, and soaked in hydrochloric acid solution for deashing; the fresh fish scale is added with water of which the weight is 5 to 10 times of that of the fish scales, the pH value is adjusted to between 2 and 4 by acid solution, pepsin of which the weight accounting for the weight of the mixing solution is between 1 and 5 percent is added, the mixing solution undergoes enzymolysis at a low temperature for 4 to 10 hours, and enzyme is killed; enzymatic liquid is filtered twice to obtain extracting solutions, the extracting solutions are mixed, the mixing solution is decolored, sodium chloride of which the weight accounting for the weight of the mixing solution is between 5 and 15 percent is added into the mixing solution for overnight salting out, deposit is separated and collected, and acetic acid of which the weight accounting for the weight of the mixing solution is between 5 and 20 percent is added into the deposit for dissolution to obtain protein solution; the protein solution is dialyzed and desalted to obtain purified collagen liquid; and the collagen liquid is subjected to freeze-drying or spray-drying and micro-crushing to obtain solid particle. The method has the advantages of reasonable process, simple operation, safety, low cost, little loss of nutrient matters of the product, low ash content and high yield and purity of the collagen.
Owner:SHANDONG HOMEY AQUATIC DEV +1
Technique for preparing collagen of freshwater fish skin
InactiveCN101092449AEfficient removalHigh speedPeptide preparation methodsChemical recyclingAcetic acidFreeze-drying
This invention discloses a method for preparing freshwater fish skin collagen. The method comprises: (1) washing fish skin, draining, cutting, and soaking in 0.001-0.2 M alkali solution containing 0.005-2% (v / v) H2O2 for 3-48 h; (2) stirring at a high speed, washing and soaking with an organic reagent; (3) extracting with acid solution (pH = 1.0-5.0) containing 0.01-2% (m / v) pepsin for 12-64 h, and filtering with 30-100 mu.m membrane, and then with 1-30 mu.m membrane; (4) adding 5 M NaCl solution into the filtrate to 0.8-2.0 M, centrifuging, dissolving the precipitate in 0.001-0.1 M acetic acid, dialyzing with distilled water, and freeze-drying to obtain white and odorless collagen solid. The collagen can be widely used in food, cosmetic and biomedical fields.
Owner:NANJING AGRICULTURAL UNIVERSITY
Methods and compositions for enhancing collagen and proteoglycan synthesis in the skin
ActiveUS7598291B2Increase synthesisSustainable hydrationBiocideCosmetic preparationsAdditive ingredientIsoflavones
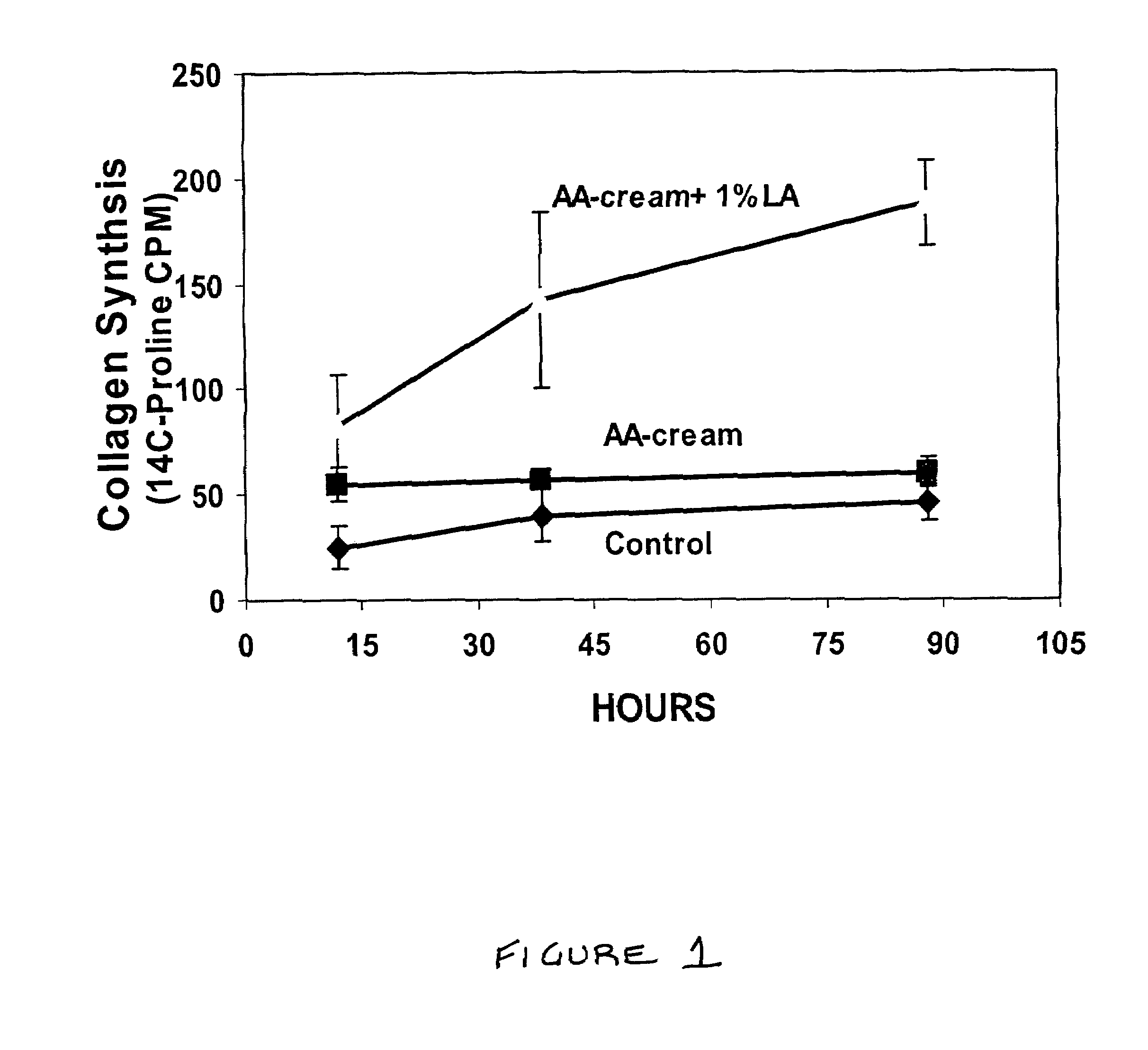
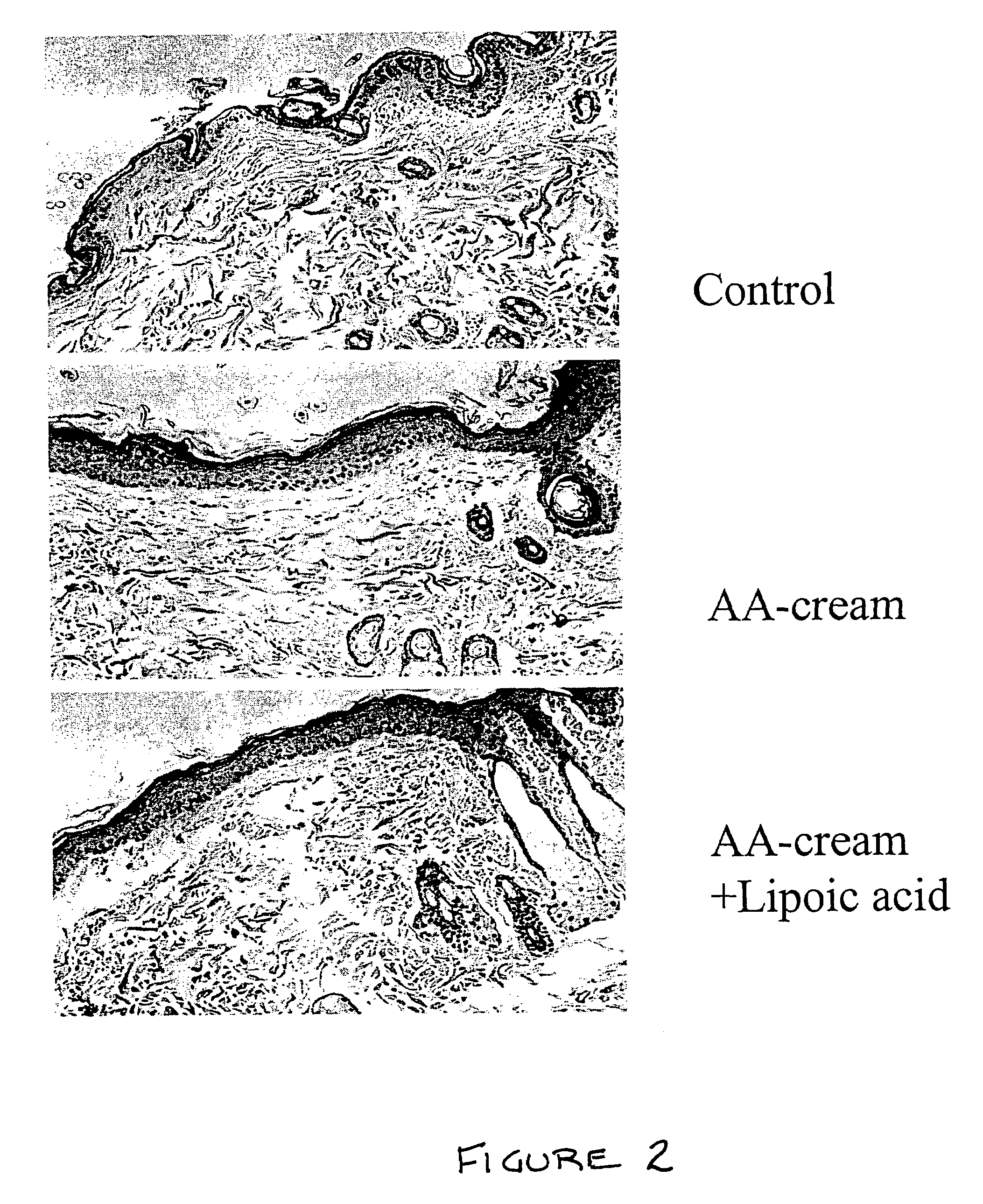
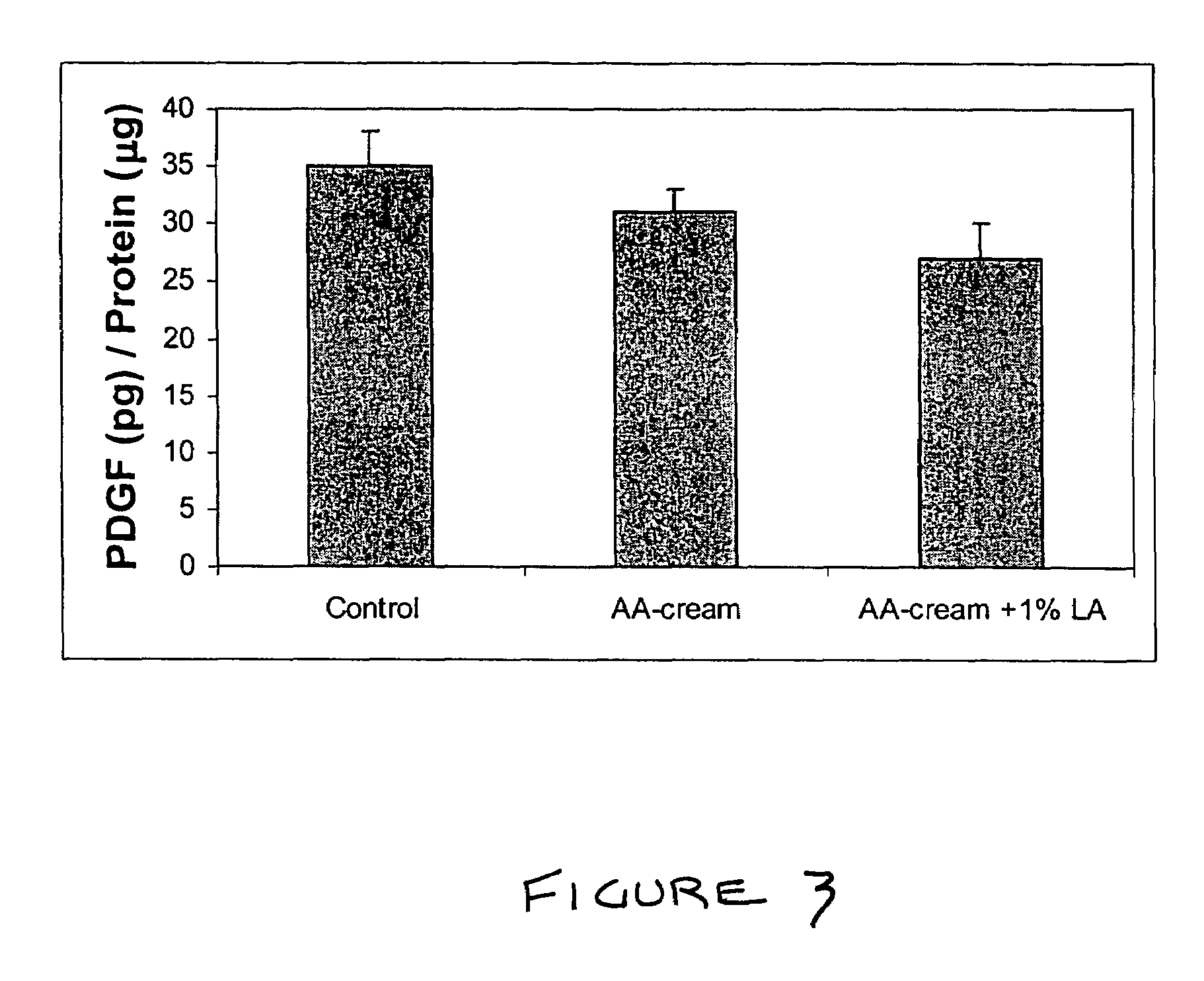
A composition for application to the skin can stimulate the in vivo synthesis of collagen and proteoglycans and improve the appearance of the skin, increasing its elasticity and fullness. In general, a composition according to the present invention comprises: (1) an antioxidant compound in a quantity sufficient to enhance collagen synthesis in the skin; (2) an organic penetrant in which the antioxidant compound is soluble in a sufficient quantity that a concentration of the antioxidant compound sufficient to enhance collagen synthesis can be applied topically and penetrate the skin; (3) a mixture of essential amino acids; (4) a supplemental source of sulfur; and (5) a topical pharmaceutically acceptable carrier. The antioxidant compound can be lipoic acid, a lipoic acid analogue or derivative, a bioflavonoid, a constituent of ginkgo, or an isoflavone. The organic penetrant is preferably benzyl alcohol. Other ingredients, such as esters of tocopherol and ascorbic acid, can be included.
Owner:NIMNI MARCEL +1
Fish collagen and method of producing same
InactiveUS6271350B1Easy to disassembleConnective tissue peptidesPeptide/protein ingredientsFiltrationSalt effect
A collagen is extracted and produced from a raw fish skin through the steps: a salt admixing step for admixing a salt (e.g. NaCl, KCl or the like) with a raw fish skin so as to allow non-collagen substances or portions (including fats and other tissue portions than a collagen portion) to be removed from the fish skin, while simultaneously degreasing and deodorizing the skin under the salt effect; a salt removal step for causing the admixed salt and non-collagen substances or portions to remove from the skin, achieving the fats removal and deodorization; a collagen extraction step for extracting a collagen (gelatinous) from the thus-treated skin; and a filtration step for filtering and refining the extracted collagen so as to obtain a refined collagen. The refined collagen may be dried and solidified. Thus, since the inexpensive salt is used for effective removal of non-collagen substances or portions to obtain a collagen portion, the method itself is simplified and economical. Further, the fish collagen obtained thereby, be it fluid or dried, is of a highly refined property, i.e. colorless, odorless and degreased, which is suited for food product elements and various industrial uses.
Owner:IHARA +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com