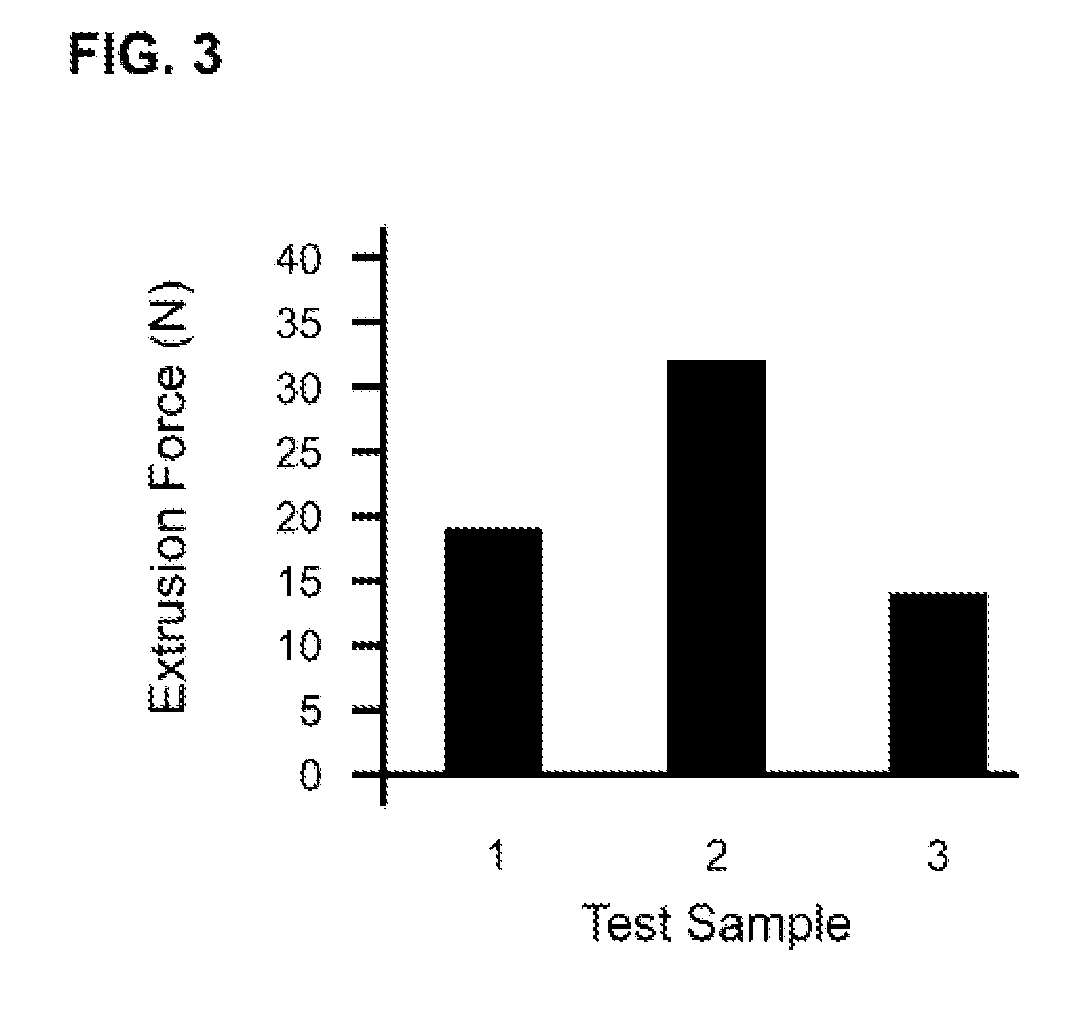
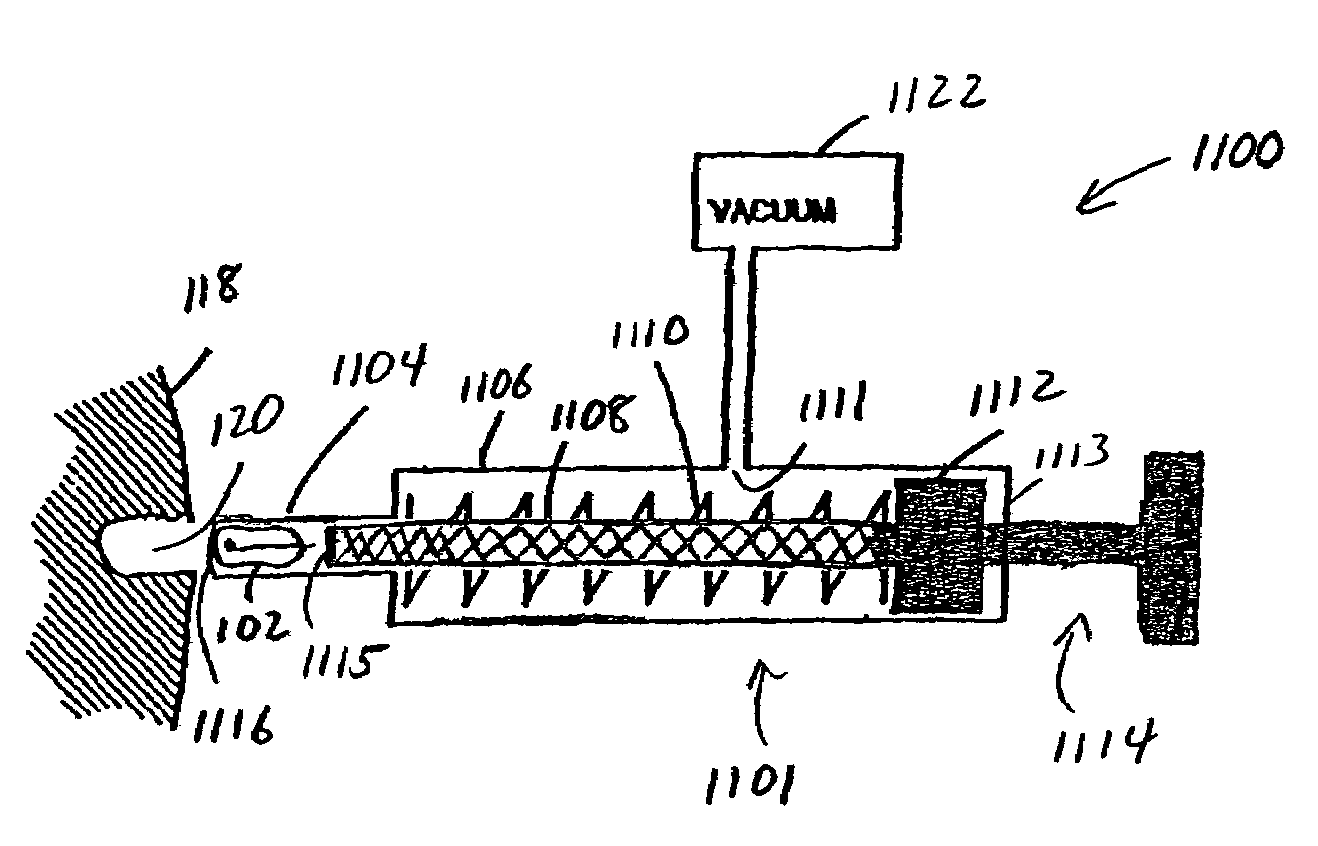
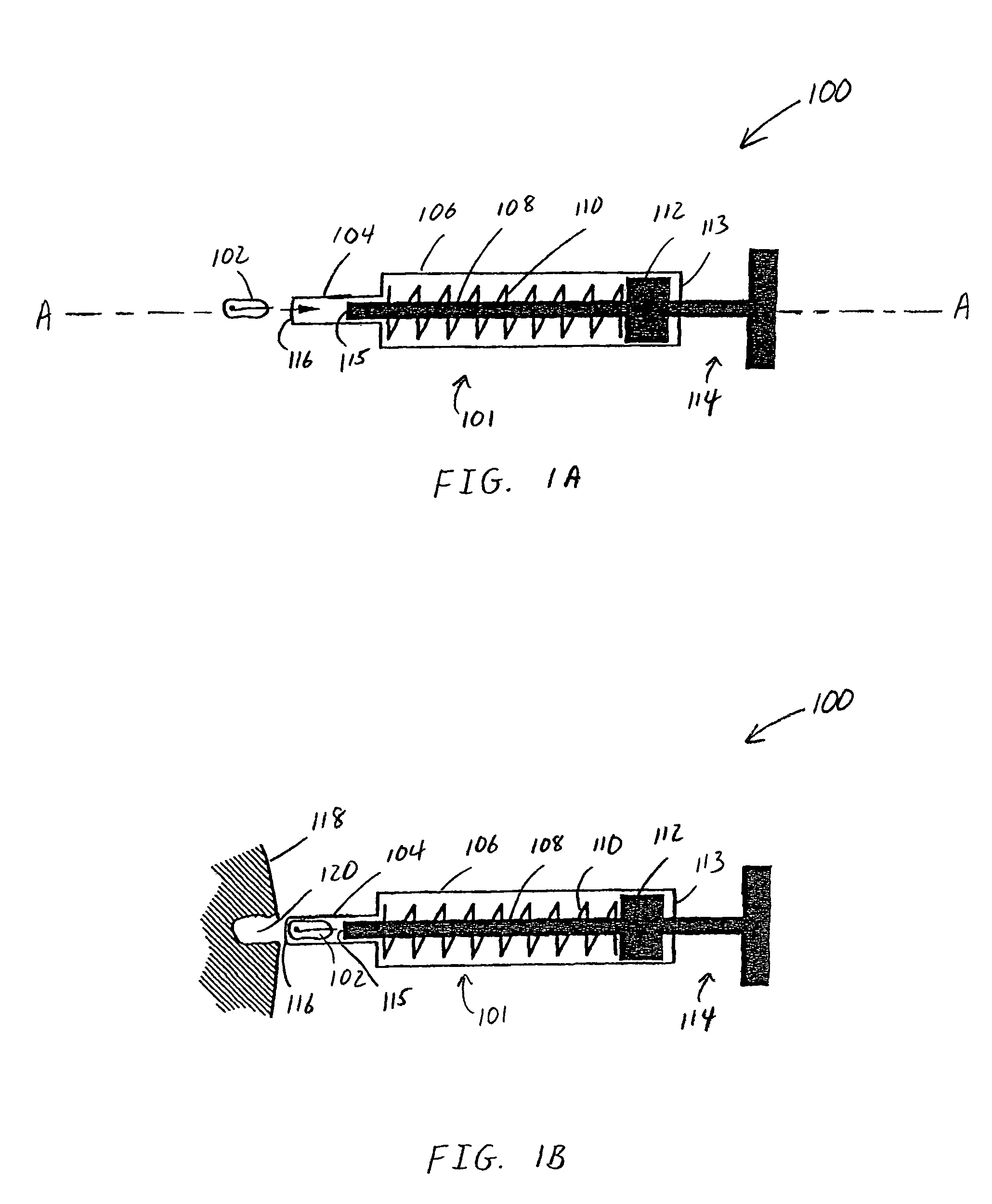
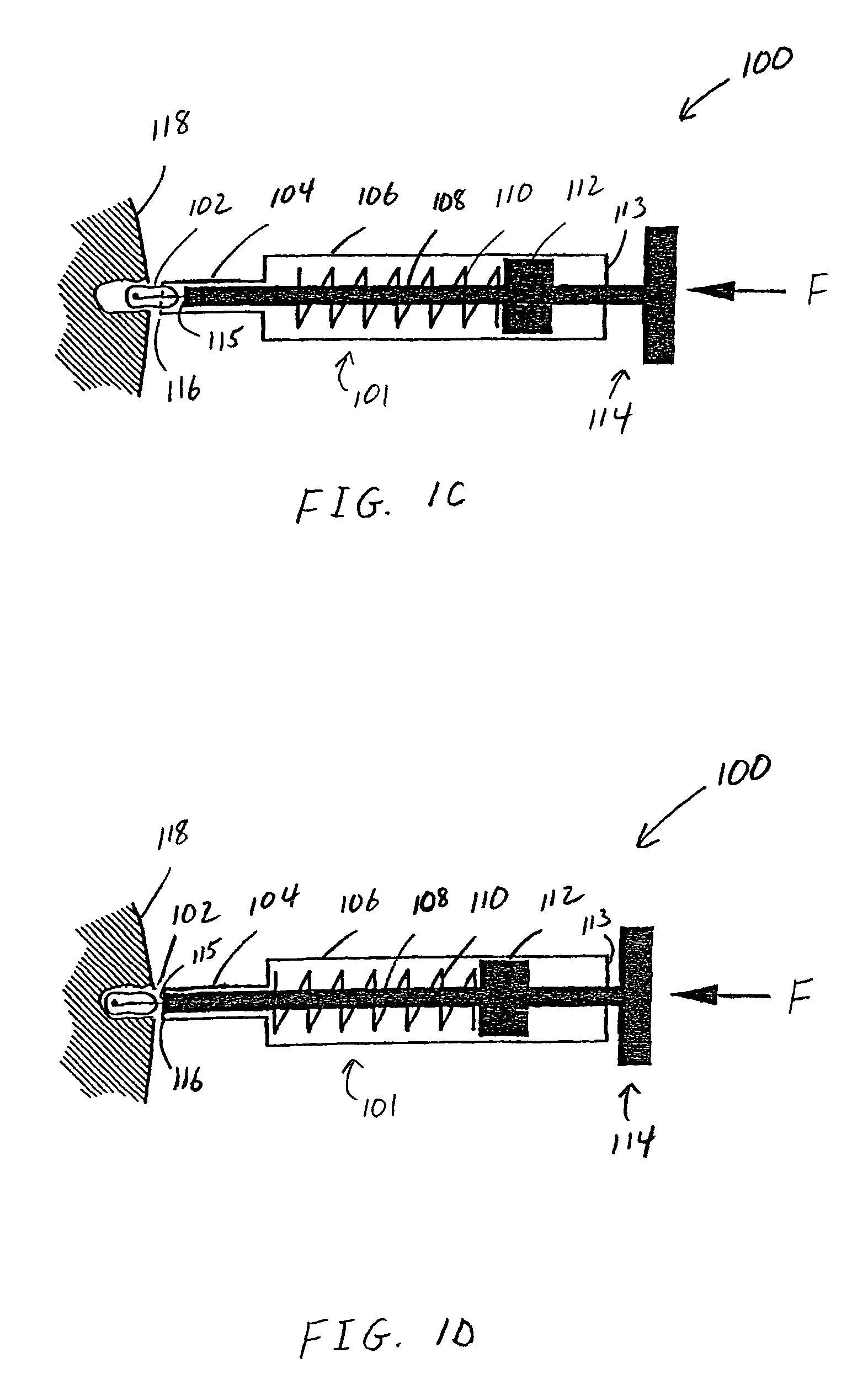
Patents
Literature
579results about "Skin implants" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
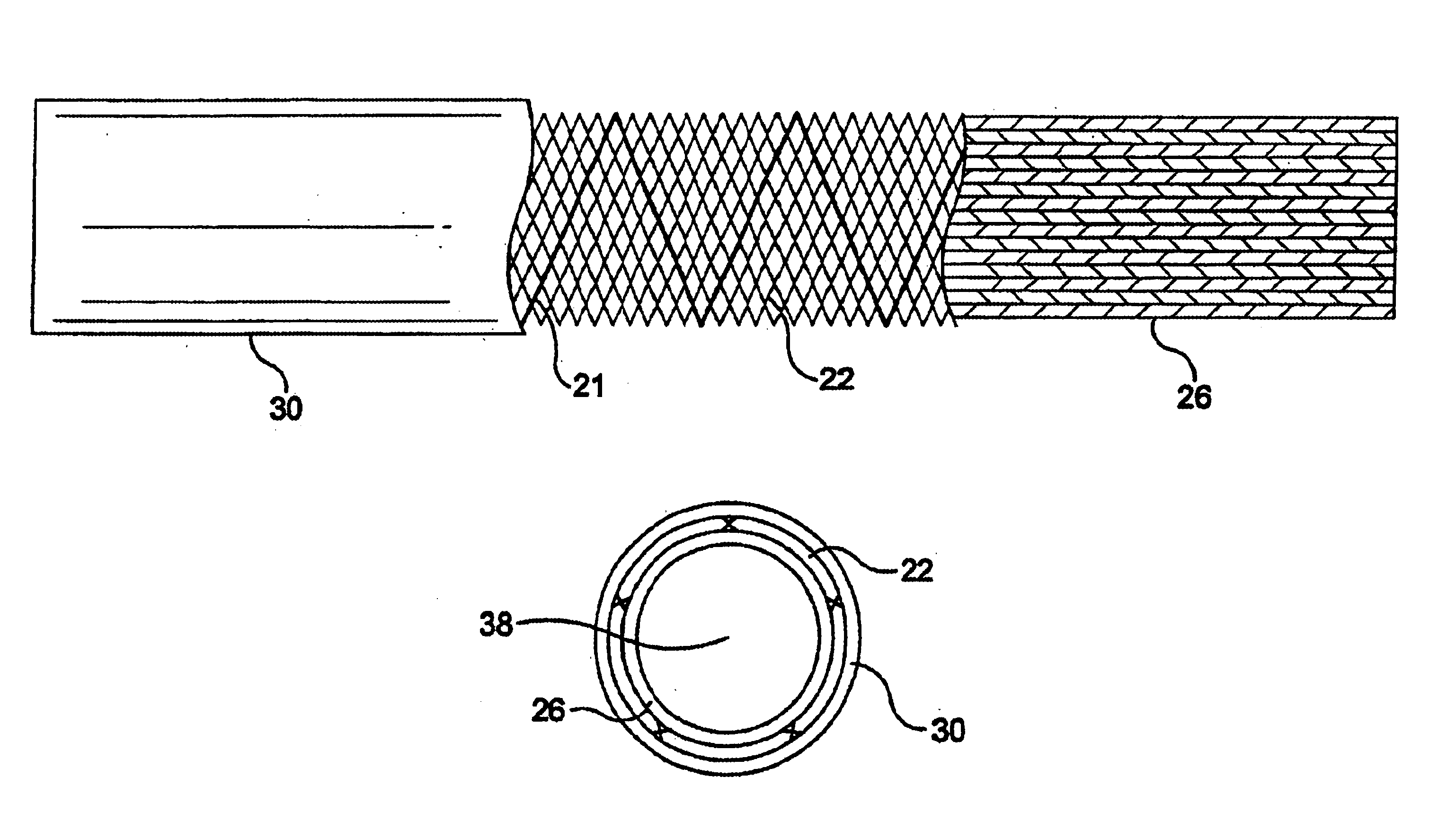
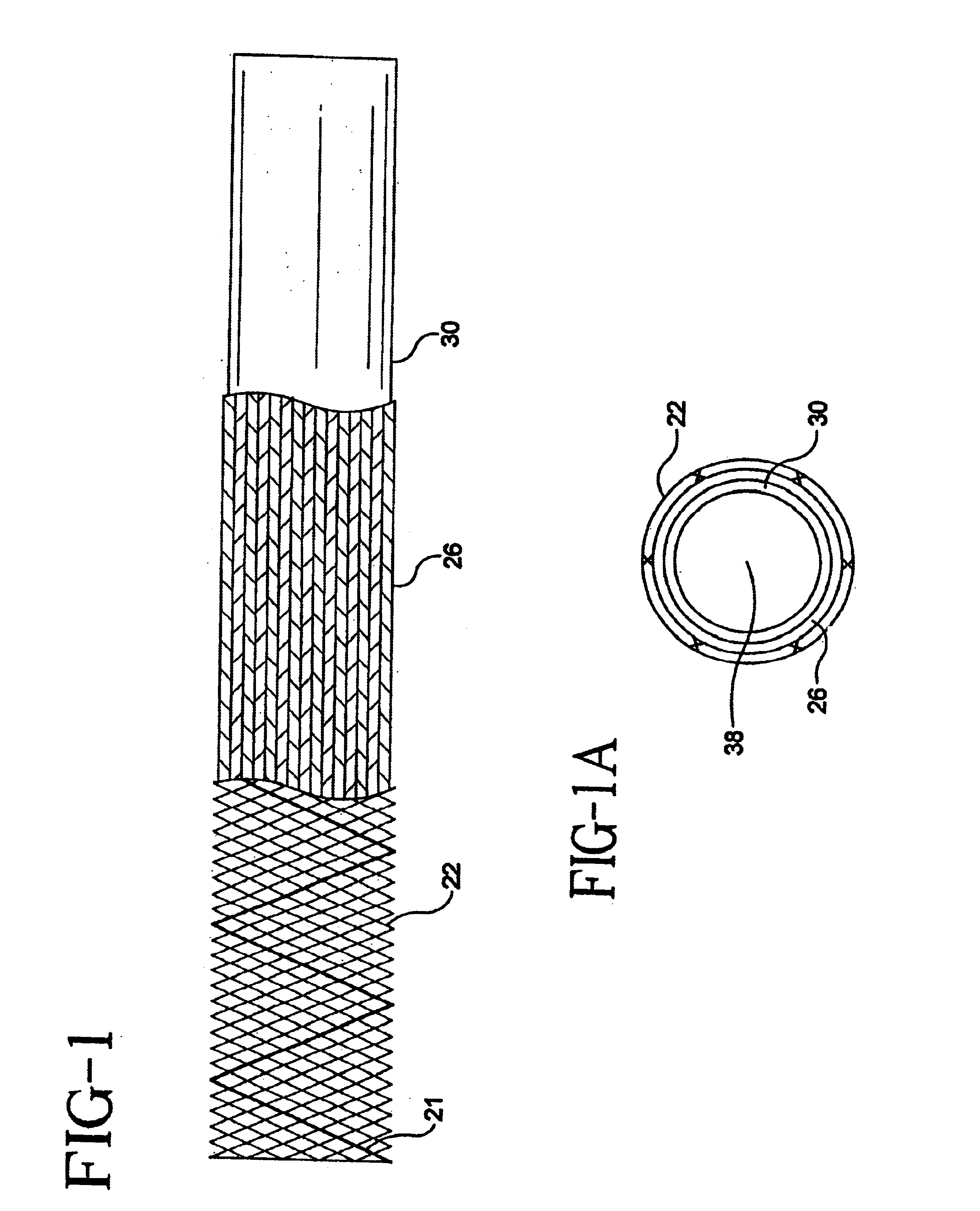
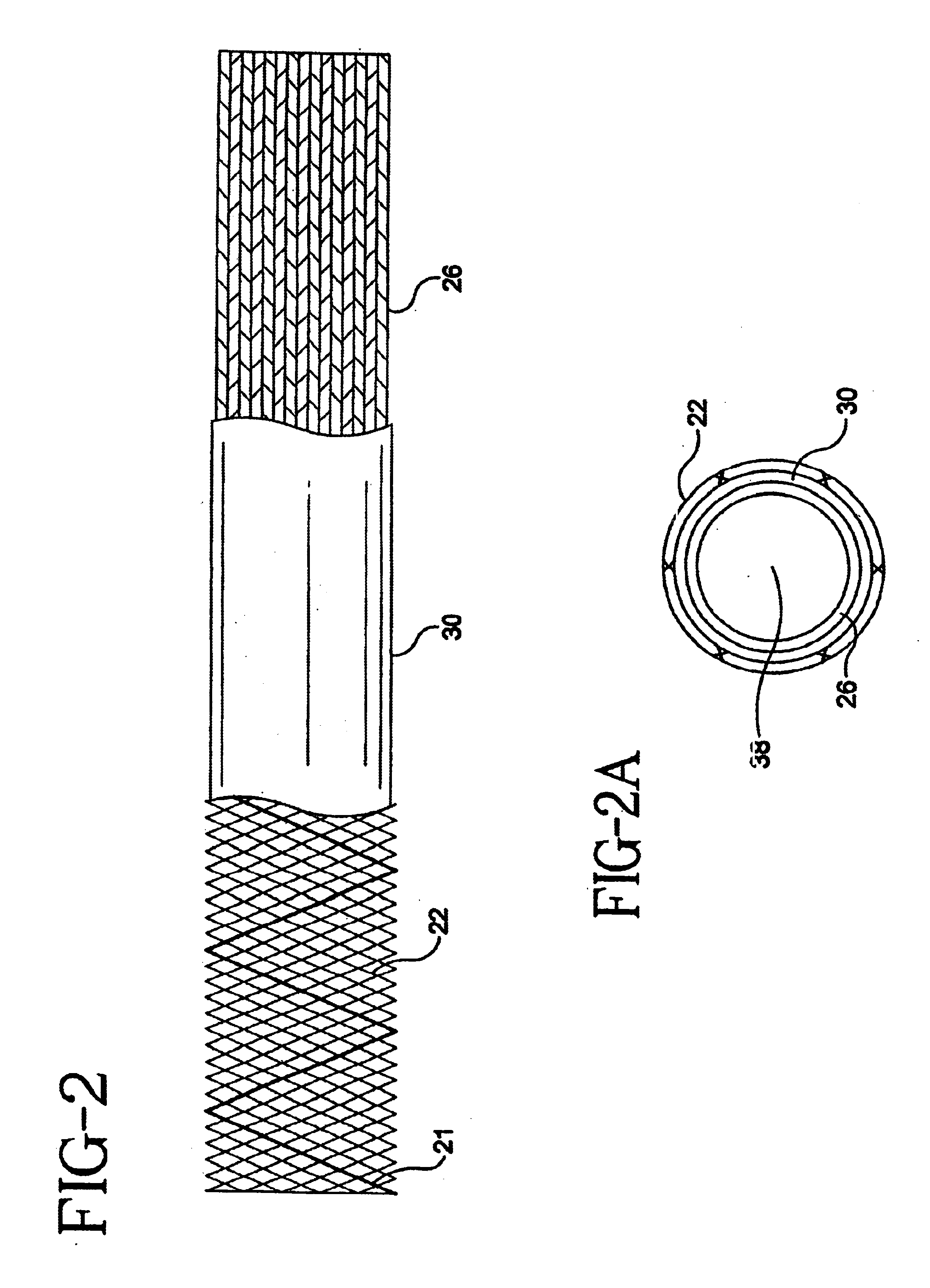
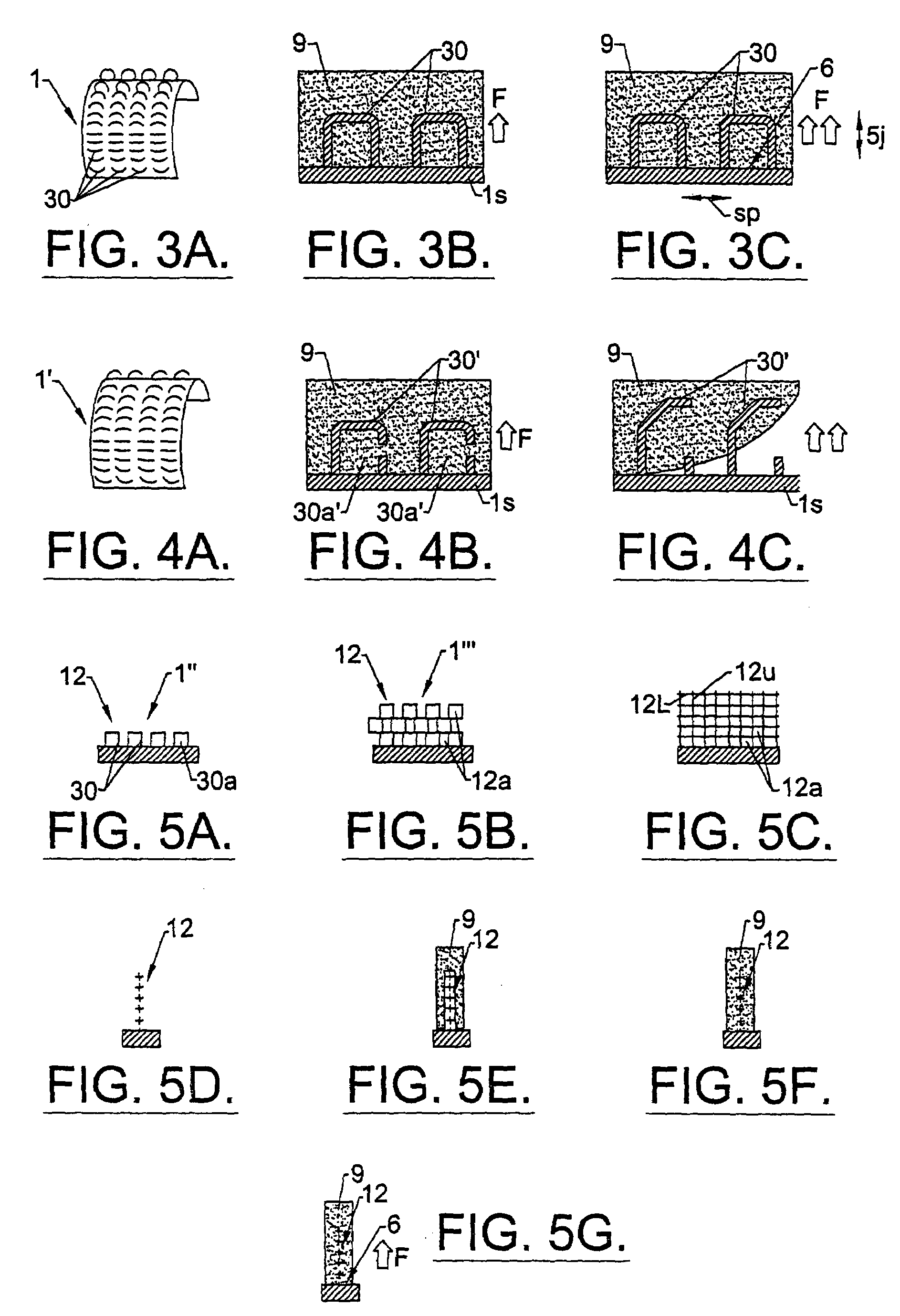
Method of preparation of bioabsorbable porous reinforced tissue implants and implants thereof
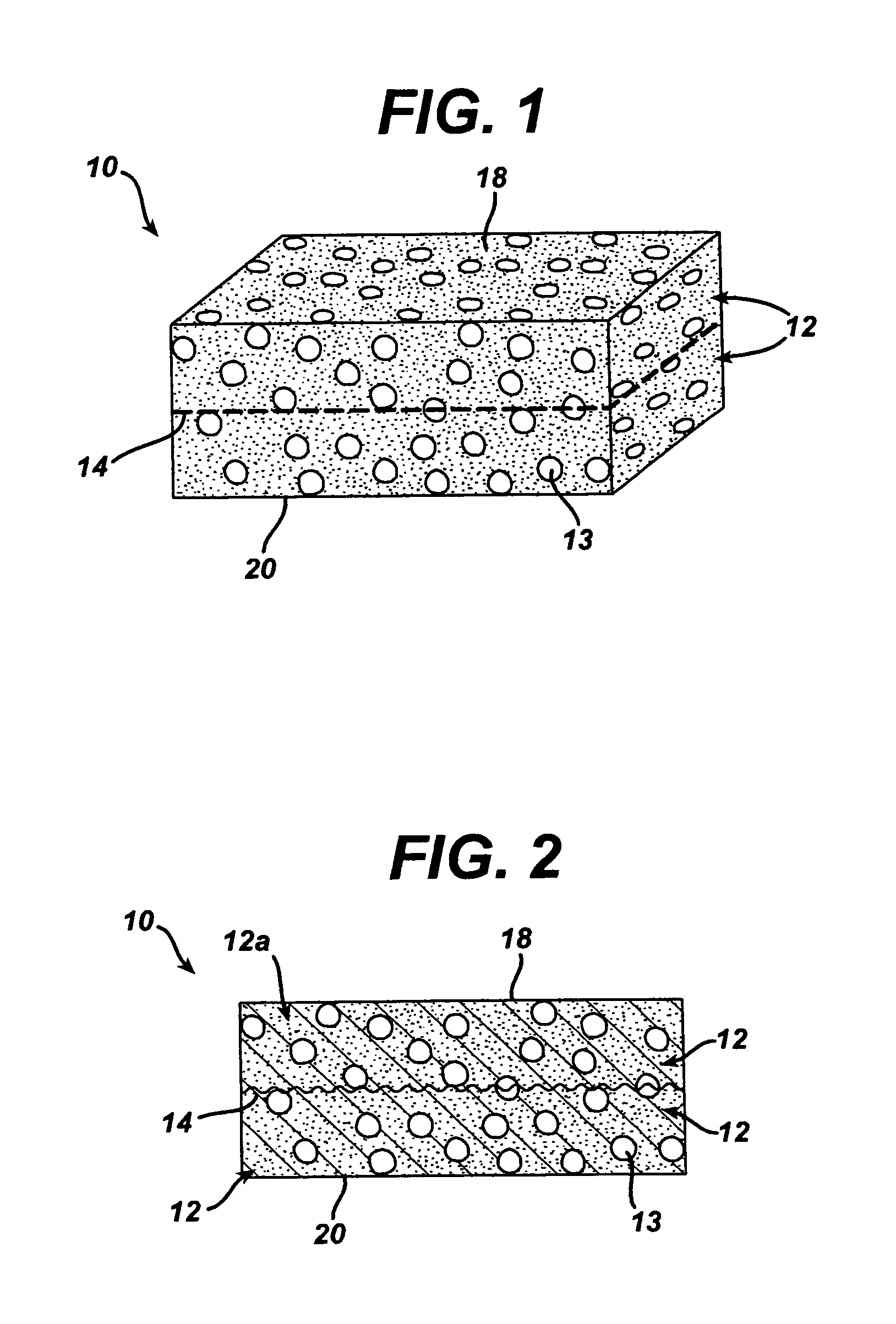
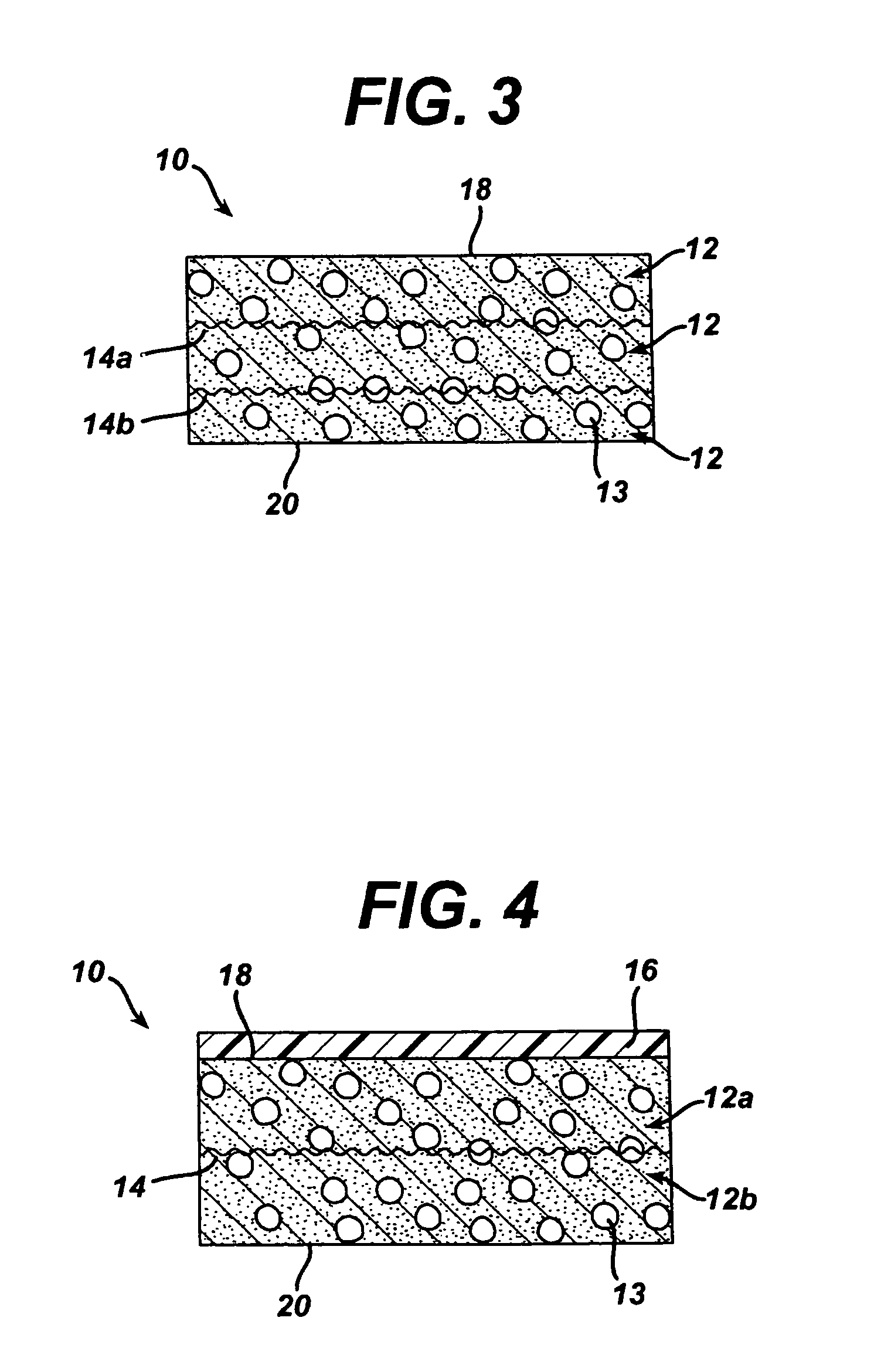
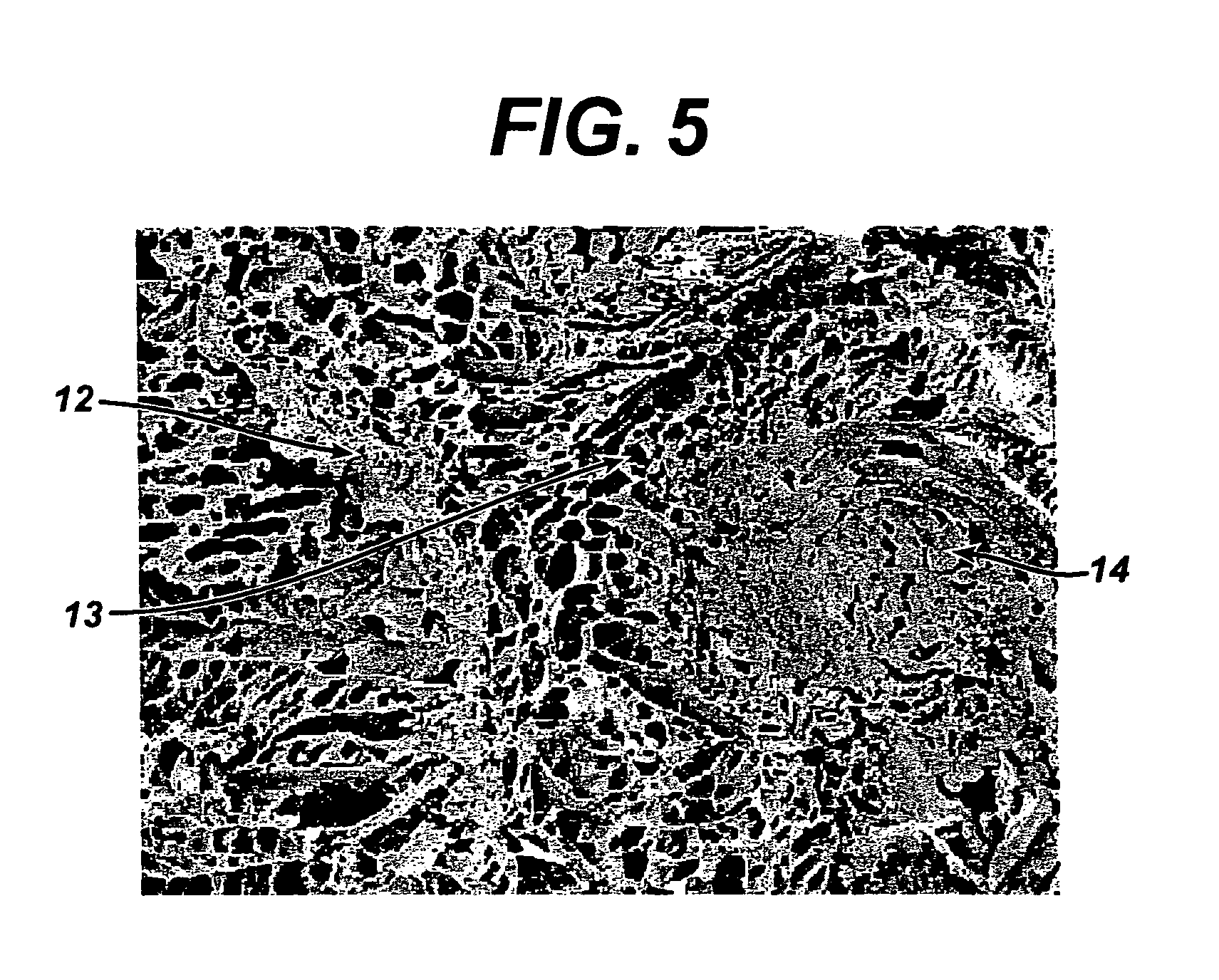
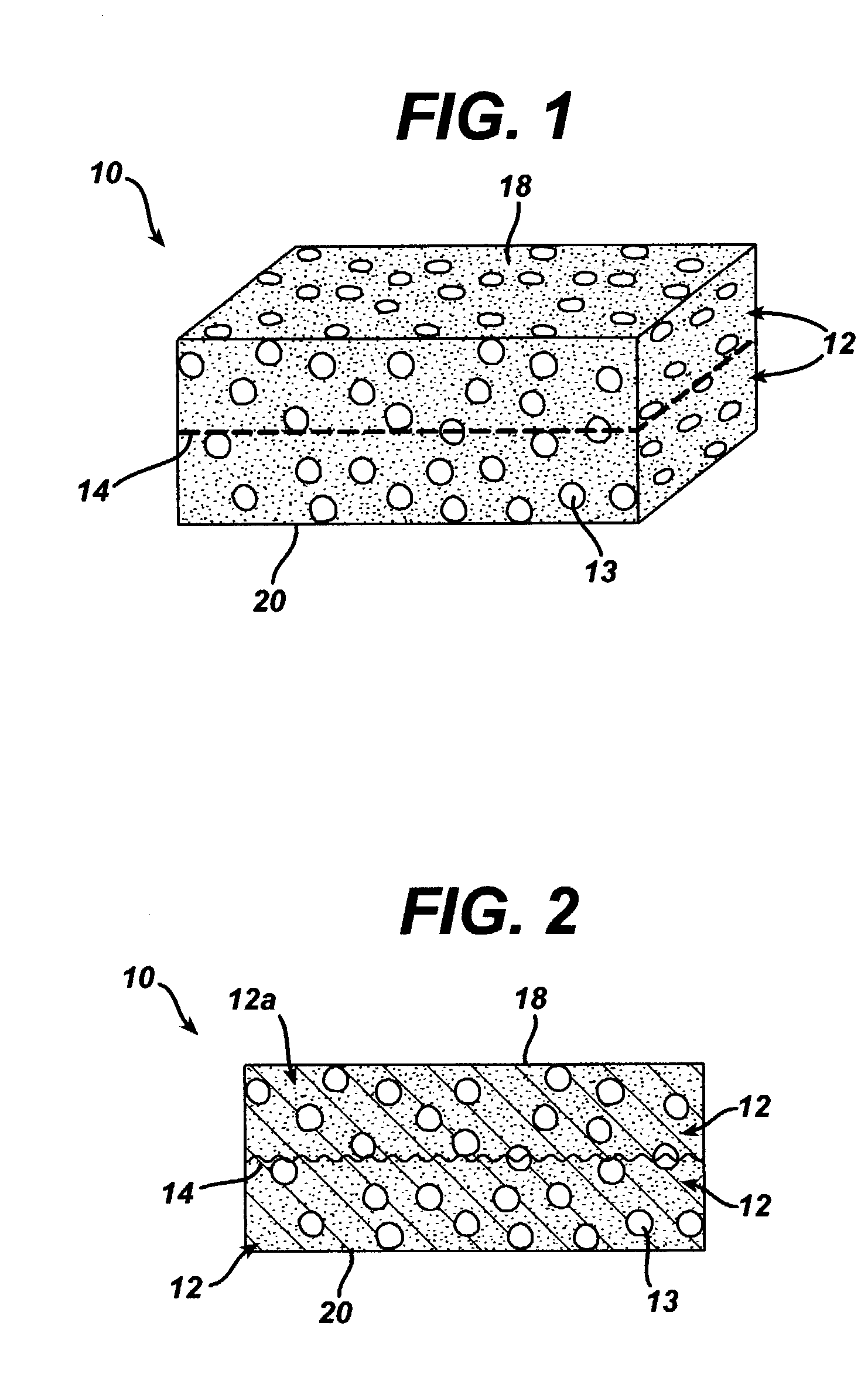
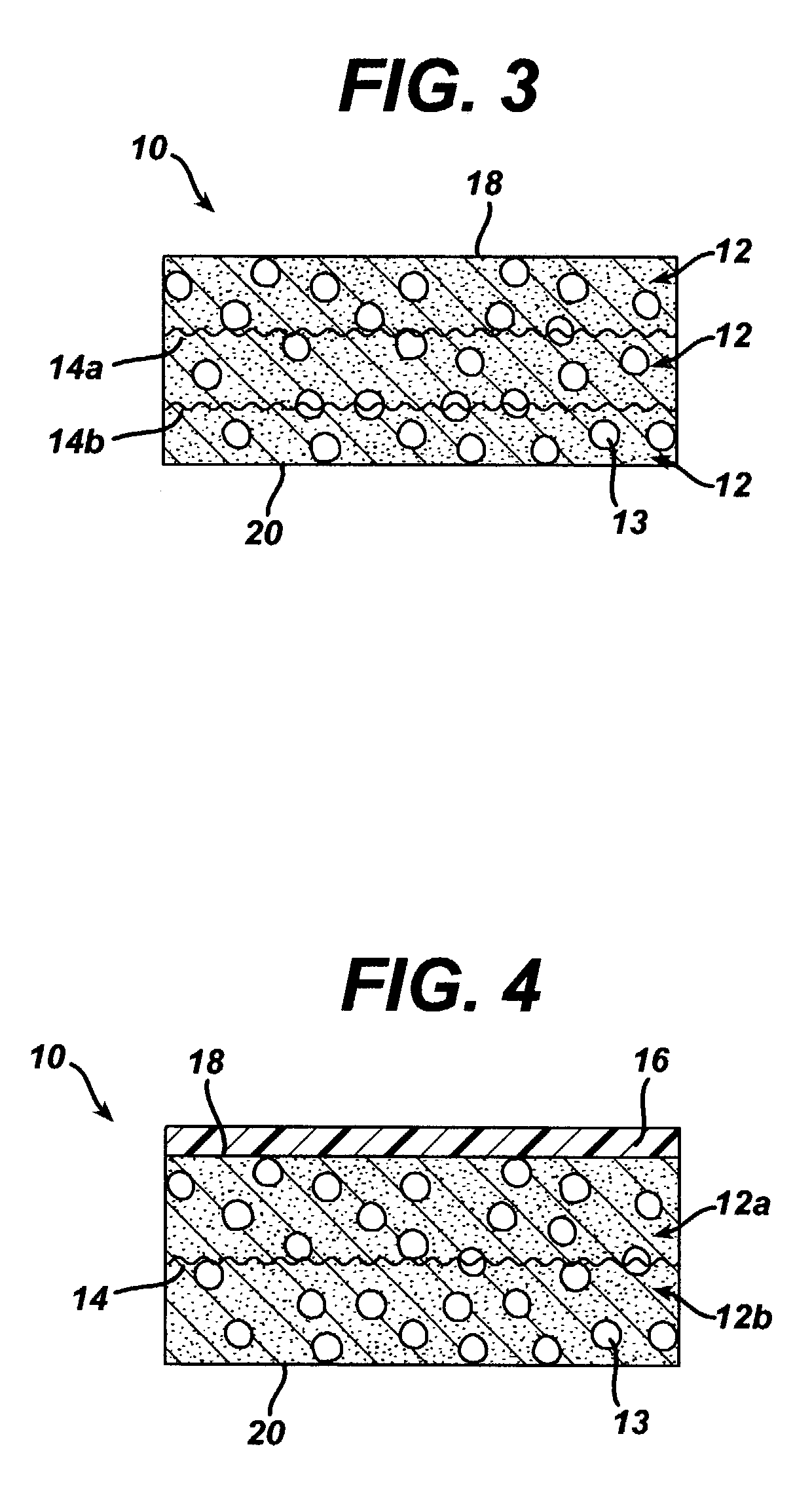
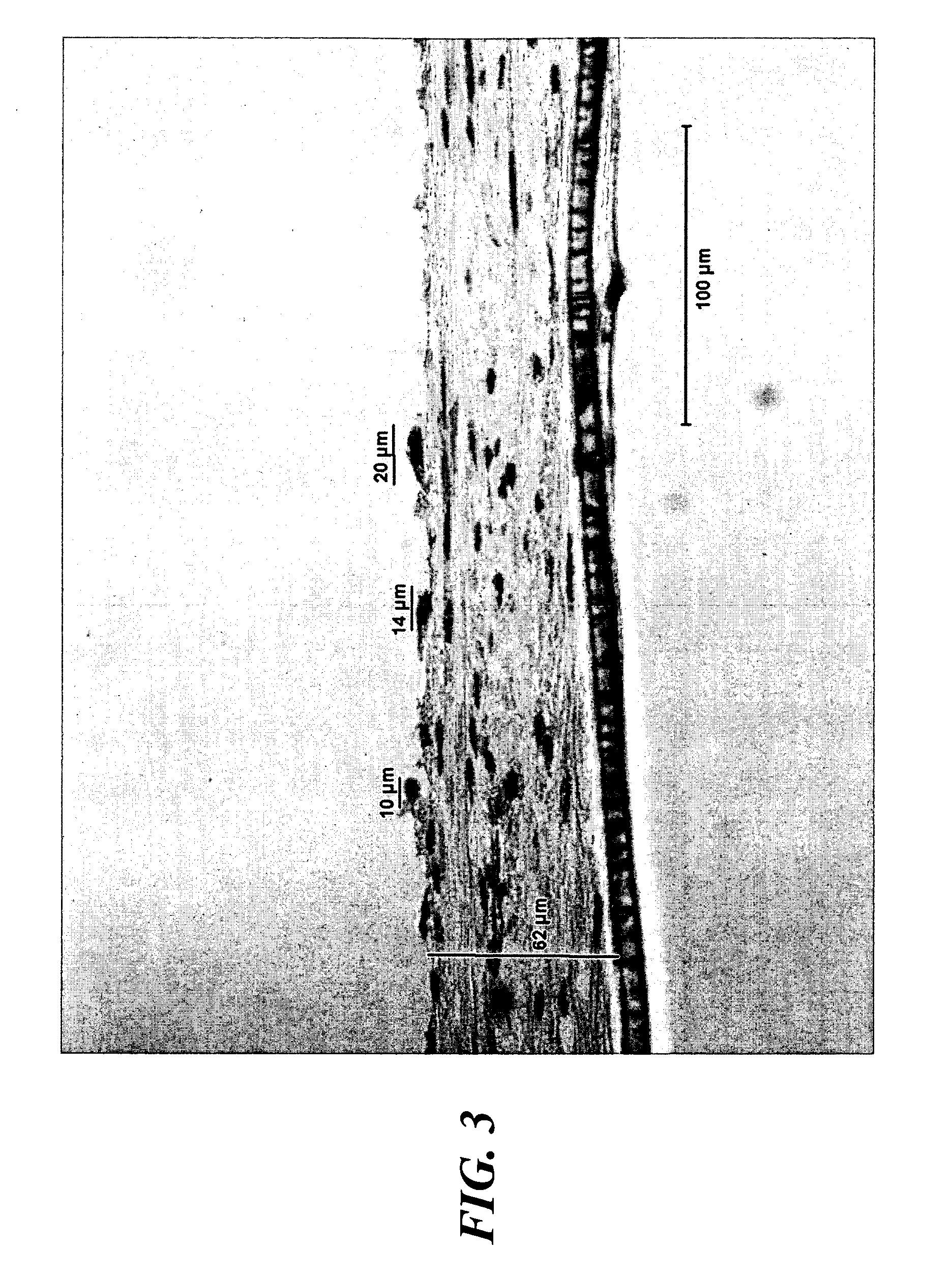
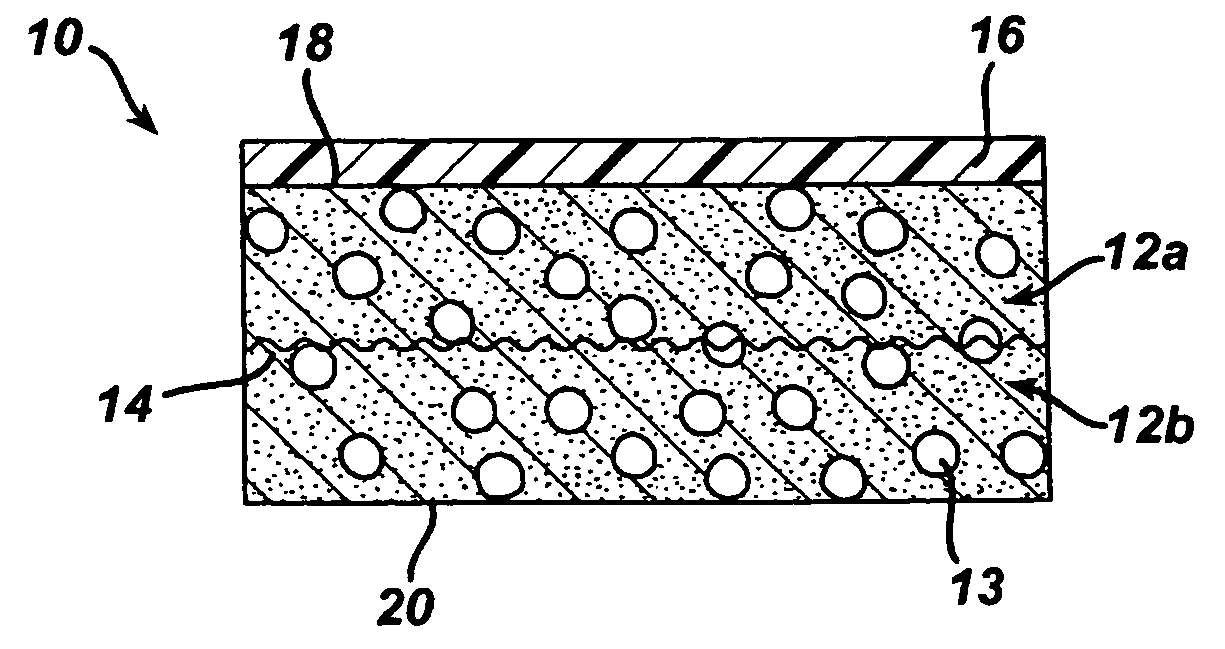
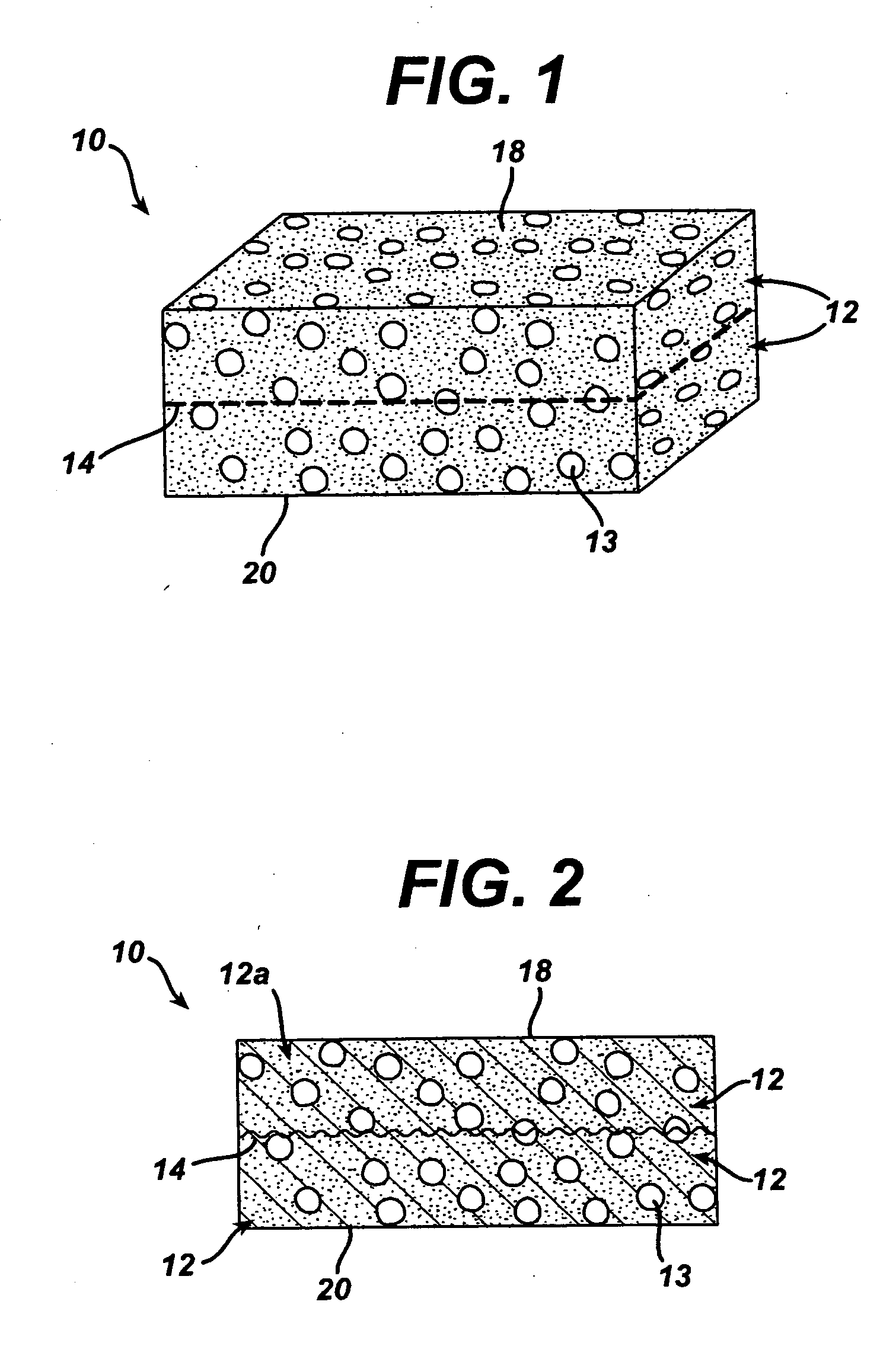
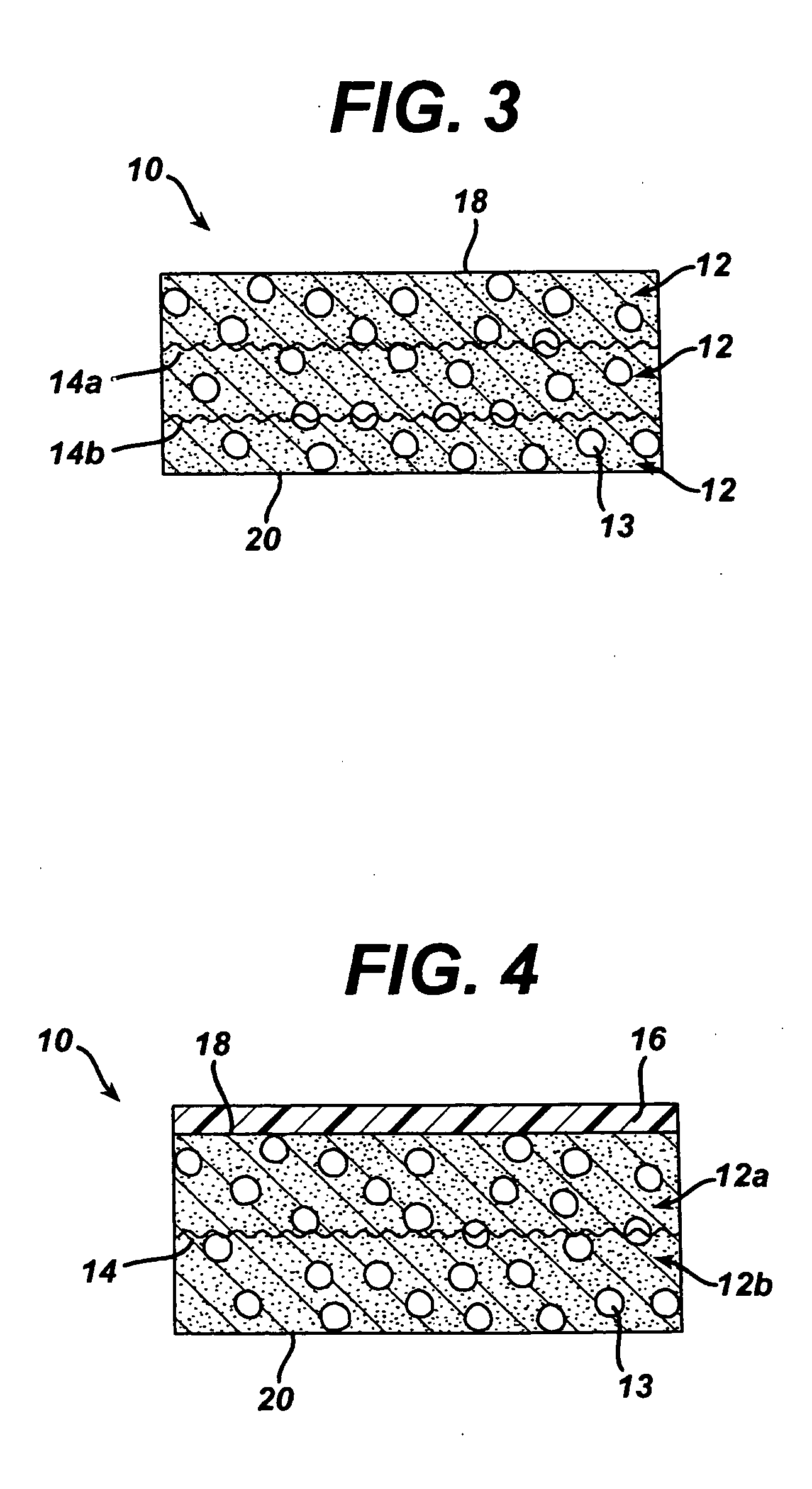
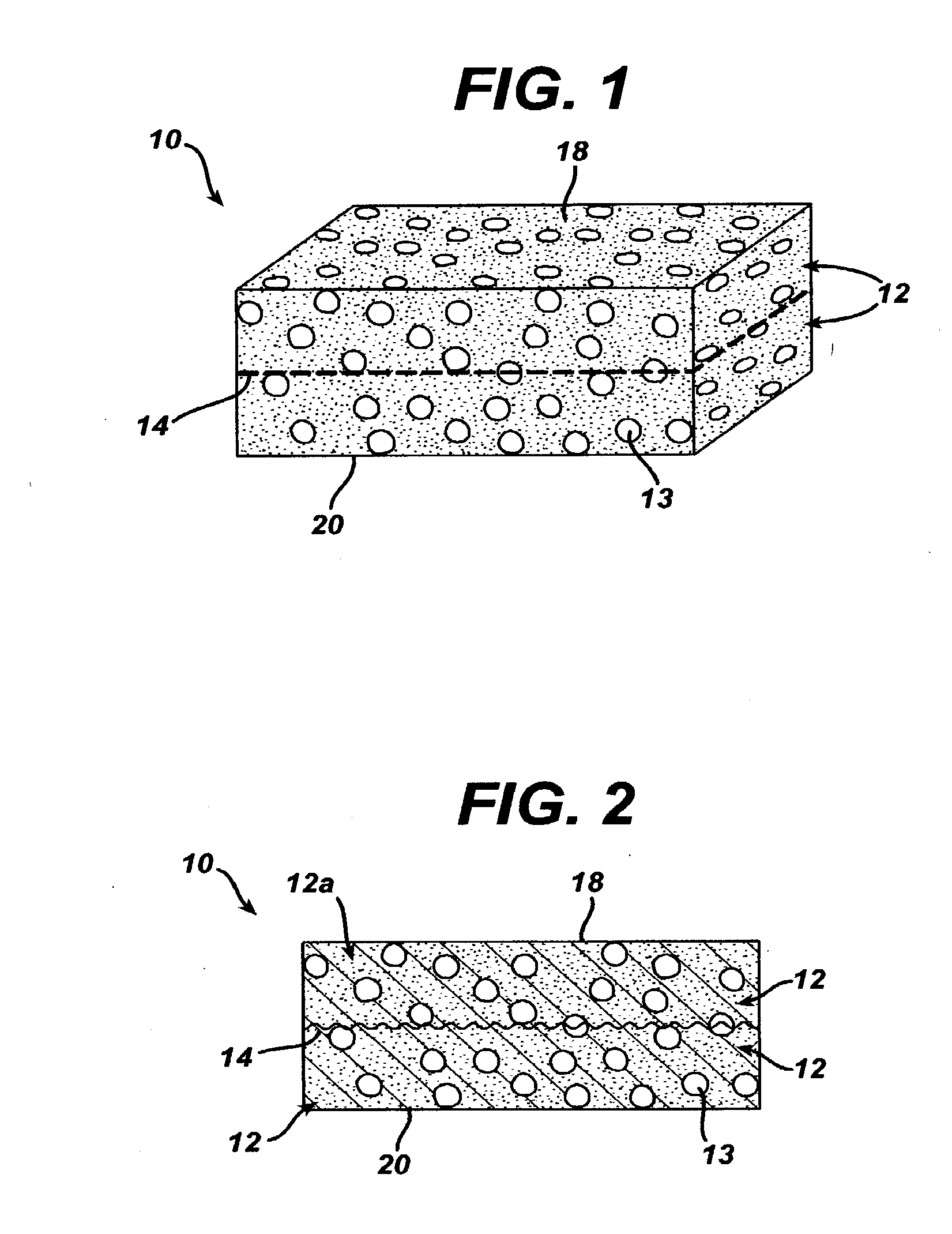
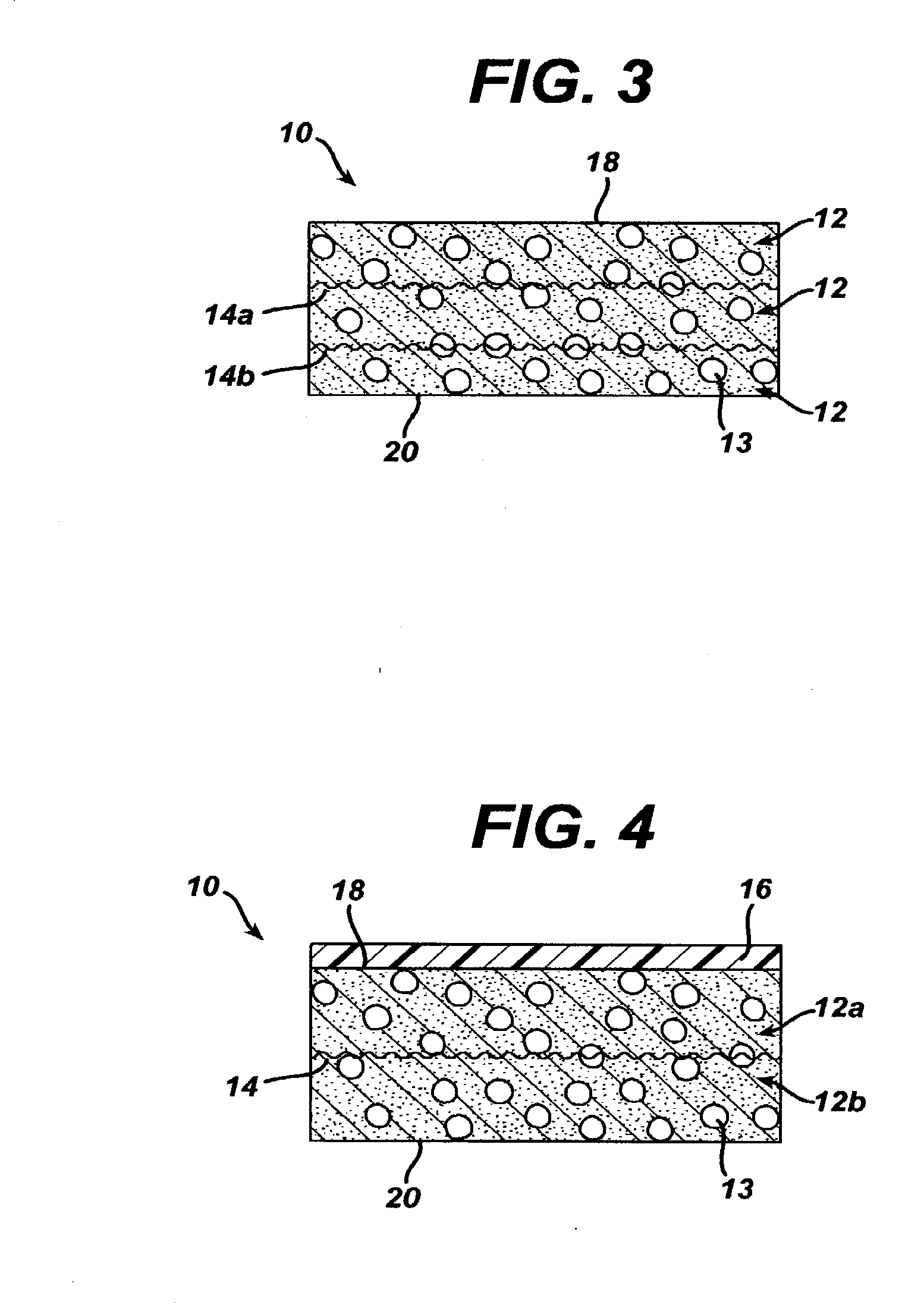
A biocompatible tissue implant. The tissue implant may be bioabsorbable, consists of a biocompatible polymeric foam. The tissue implant also includes a biocompatible reinforcement member. The polymeric foam and the reinforcement member are soluble in a lyophilizing solvent. The reinforcement may be annealed and / or coated.
Owner:DEPUY MITEK INC
Method of preparation of bioabsorbable porous reinforced tissue implants and implants thereof
Owner:DEPUY SYNTHES PROD INC
Multilayer scaffold
InactiveUS20170182211A1Avoid disease riskAvoids the potential ethical and religious barriers to the useSkin implantsTissue regenerationMedicinePlla scaffold
Owner:SMITH & NEPHEW PLC
Soft and calcified tissue implants
InactiveUS20050101957A1Minimize movementReduce degradationInternal osteosythesisSurgical adhesivesLigament structurePlastic surgery
Disclosed herein is processed dermis graft for use in orthopedic surgical procedures. Specifically exemplified herein is a processed dermis graft comprising one or more bone blocks having a groove cut into the surface thereof, wherein said groove is sufficient to accommodate a fixation screw. Also disclosed is a method of processing dermis that results in a dermis derived implant suitable to replace a tendon or ligament in a recipient in need thereof. Other compositions and applications of a dermis derived implant, and methods of manufacture and use, are disclosed.
Owner:RTI BIOLOGICS INC
Composition and method for the repair and regeneration of cartilage and other tissues
InactiveUS7148209B2Add supportImprove coagulation/solidificationBiocidePeptide/protein ingredientsAbnormal tissue growthRepair tissue
Owner:SMITH & NEPHEW ORTHOPAEDICS
Conformable tissue repair implant capable of injection delivery
A conformable tissue implant is provided for use in repairing or augmenting a tissue defect or injury site. The tissue implant contains a tissue carrier matrix comprising a plurality of biocompatible, bioresorbable granules and at least one tissue fragment in association with the granules. The tissue fragment contains one or more viable cells that can migrate from the tissue and populate the tissue carrier matrix. Also provided is a method for injectably delivering the tissue implant.
Owner:DEPUY SYNTHES PROD INC
Methods and compositions for organ and tissue functionality
Materials and methods for treating tissue defects in human or animal tissues using implantable cells are described. Further, culture techniques and factors for enhancing these procedures, and cell survival and adaptation are described. Many of the tissue defects may be treated with autologous cells, while applications involving non-autologous cells or stem cells are also described.
Owner:DASK TECH
Implant with high vapor pressure medium
InactiveUS20100222802A1Promote recoveryImprove the immunitySuture equipmentsUrinary bladderHydrostatic pressureProduct gas
An implant for use in a human or animal body can include a flexible housing with an outer wall and having a chamber therein. The implant can have at least one high vapor pressure medium within the chamber. The at one high vapor pressure medium can have a combined vapor pressure equal to or greater than about the average value of the hydrostatic pressure of the implantation site plus the skin tension of the housing minus the gas tension of the dissolved gasses present at the implantation site.
Owner:SOLACE THERAPEUTICS
Method of forming polysaccharide sponges for cell culture and transplantation
A polysaccharide sponge characterized by having: (i) an average pore size in the range between about 10 mum to about 300 mum; (ii) an average distance between the pores being the wall thickness of the pores in the range between about 5 mum to about 270 mum; and (iii) an E-modulus of elasticity being a measure of the rigidity of the sponge in the range of about 50 kPa to about 500 kPa.
Owner:BEN GURION UNIVERSITY OF THE NEGEV
Autogenic living scaffolds and living tissue matrices: methods and uses thereof
ActiveUS20050226856A1Preventing host rejectionThicker and strongBiocideSkin implantsTransdifferentiationOrganism
A 3-dimensional structure comprising suitable cells (or entities) and the ECM (or matrix) that has been completely produced and arranged by these cells (or entities) that promotes the differentiation, dedifferentiation and / or transdifferentiation of cells and / or formation of tissue in vitro and in vivo, while at the same time promoting cell growth, proliferation, migration, acquisition of in vivo-like morphology, or combinations thereof, and that 1. provides structural and / or nutritional support to cells, tissue, organs, or combinations thereof, termed an “Autogenic Living Scaffold” (ALS); or 2. is capable of being transformed into a more complex tissue (or matrix) or a completely different type of tissue (or matrix), termed a “Living Tissue Matrix” (LTM). Autogenic means it is self-produced. The living cells that produce the LTM or ALS, or are added to Autogenic Living Scaffolds, may be genetically engineered or otherwise modified. The matrix component of the ALS or LTM provides a structural framework for cells that guide their direction of growth, enables them to be correctly spaced, prevents overcrowding, enables cells to communicate between each other, transmit subtle biological signals, receive signals from their environment, form bonds and contacts that are required for proper functioning of all cells within a unit such as a tissue, or combinations thereof. The ALS or LTM may thus provide proper or supporting mechanical and chemical environments, signals, or stimuli to other cells, to the cells that produce the ALS, to surrounding tissue at an implantation site, to a wound, for in vitro and ex vivo generation and regeneration of cells, tissue and organs, or combinations thereof. They may also provide other cells with nutrients, growth factors, and / or other necessary or useful components. They may also take in or serve as buffers for certain substances in the environment, and have also some potential at adapting to new environments.
Owner:GENESIS TECH LTD
Method of preparation of bioabsorbable porous reinforced tissue implants and implants thereof
A biocompatible tissue implant. The tissue implant may be bioabsorbable, consists of a biocompatible polymeric foam. The tissue implant also includes a biocompatible reinforcement member. The polymeric foam and the reinforcement member are soluble in a lyophilizing solvent. The reinforcement may be annealed and / or coated.
Owner:DEPUY MITEK INC
Stent-graft-membrane and method of making the same
A braided self-expandable stent-graft-membrane made of elongated members forming a generally tubular body. A membrane layer and graft layer are disposed on a endoprosthesis such as a stent. The membrane layer is substantially impermeable to fluids. The outermost layer is biocompatible with the body tissue. The innermost layer is biocompatible with the fluid in the passage. An embodiment includes a graft layer disposed on the inside of a stent and a membrane layer disposed on the outside of the stent. The innermost layer is biocompatible with the fluid in the passage. The stent-graft-membrane is used at a treatment site in a body vessel or organ where it is desirous to exclude a first fluid located outside the endoprosthesis from reaching a second fluid located in the lumen. The membrane may be made of silicone or polycarbonate urethane. The graft may be braided, woven, spun or spray-cast PET, PCU, or PU fibers. The layers may include ePTFE or PTFE.
Owner:LIFEPORT SCI
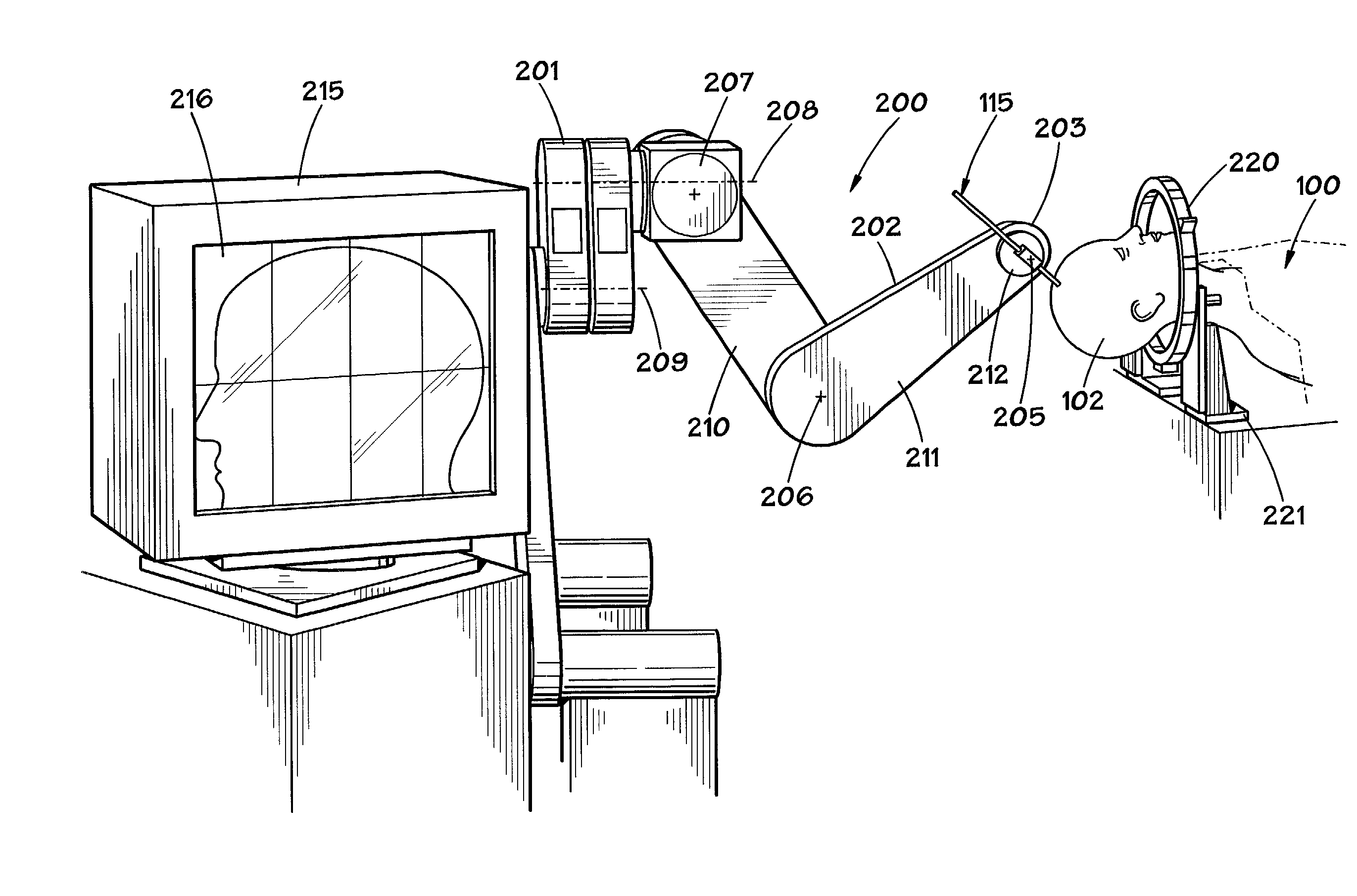
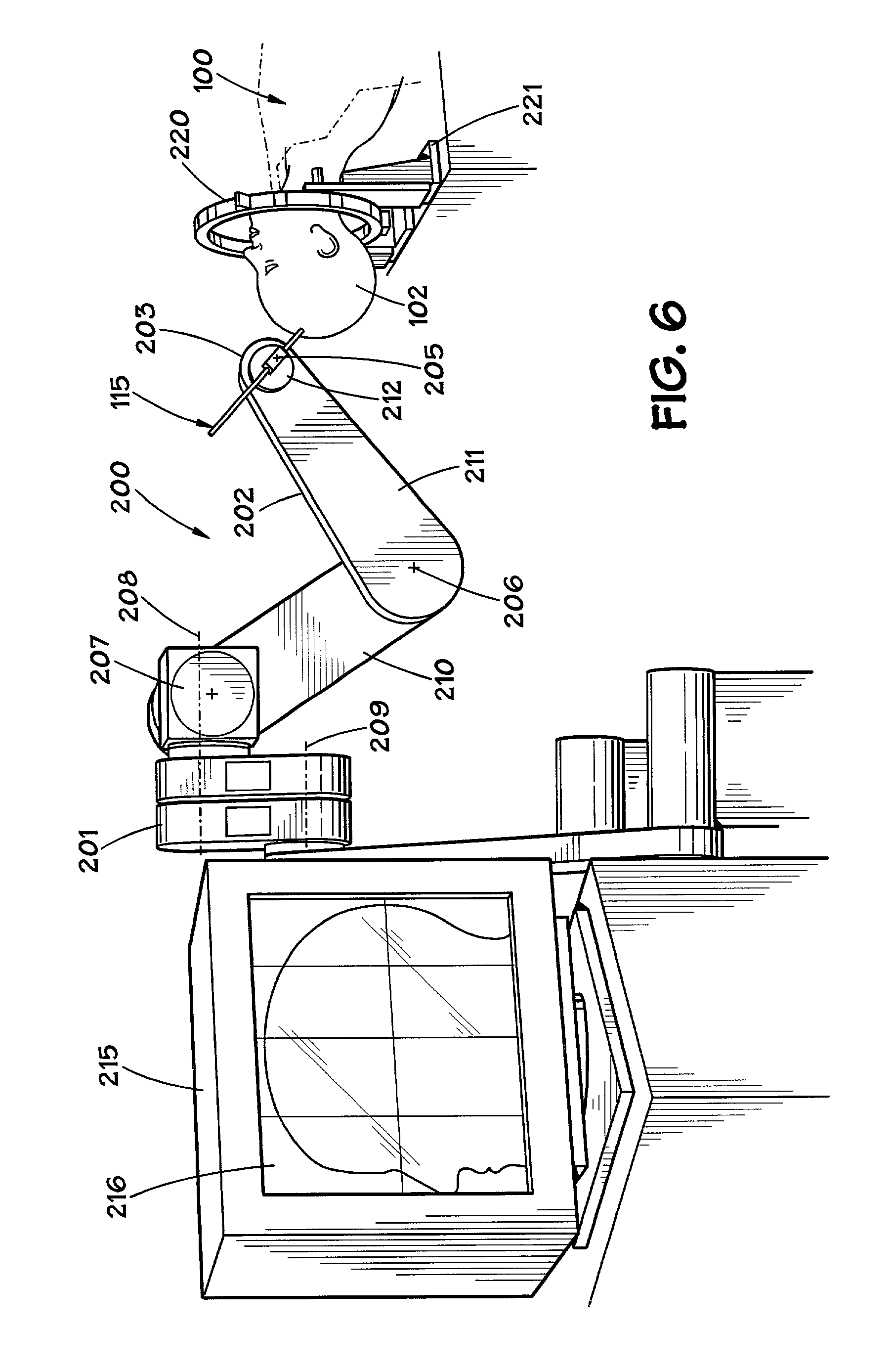
Hair transplantation method and apparatus
A hair transplantation method and apparatus utilizes a stereotactic robot, which includes a robotic arm, having a hair follicle introducer associated with the robotic arm.
Owner:RESTORATION ROBITICS INC
Tissue lockable connecting structures
InactiveUS7083648B2Strengthen the mechanical connectionInhibits tissueDental implantsSkin implantsSoft tissue infectionSubcutaneous tissue
Percutaneous skin access devices include a plurality of locked connecting units mounted to the exterior surface of an implantable medical object which, in position, is configured to penetrate the skin of a subject. The locked connecting units may be mounted directly onto the desired surface of the exterior of the device or may be held on a substrate sheet, which is mounted to the exterior surface of the device. In position, the locked connecting units engage with soft tissue which can include the skin to form a bio-junction layer which includes mechanical and bio-sealing connection between the device body and the soft tissue. The configuration at the bio-junction layer secures the medical object in location in the subject even for long-term indwelling applications in a manner, which inhibits soft tissue infection.The locked connecting units may be rigid or semi-rigid for longer-term indwelling applications, and semi-rigid and / or resilient for shorter term indwelling applications. The locked connecting units may take on the form of rings, hooks, or loops having aperture or gap width / length sizes of from about 0.2–4 mm. The rings, loops, or hooks may connect with any soft tissue including skin as well subcutaneous tissue. The rings, hooks, or loops may be released from the skin / tissue without requiring surgical cutting procedures.The locked connecting units may be configured as a semi-rigid mesh collar arranged about the primary body providing access to the subject such that it resides in the subject and engages with the skin (epidermal / dermal layer). The mesh collar can be described as a particular type of ring or loop structure as the mesh defines the gap provided in individual loop configurations. The mesh collar may be used alone, or in combination with the loops, rings, or hooks. A skin stop collar having increased rigidity may be disposed under the mesh collar.
Owner:EAST CAROLINA UNIVERISTY
Composition and method for the repair and regeneration of cartilage and other tissues
InactiveUS20060029578A1Add supportImprove coagulation/solidificationBiocideOrganic active ingredientsAbnormal tissue growthRepair tissue
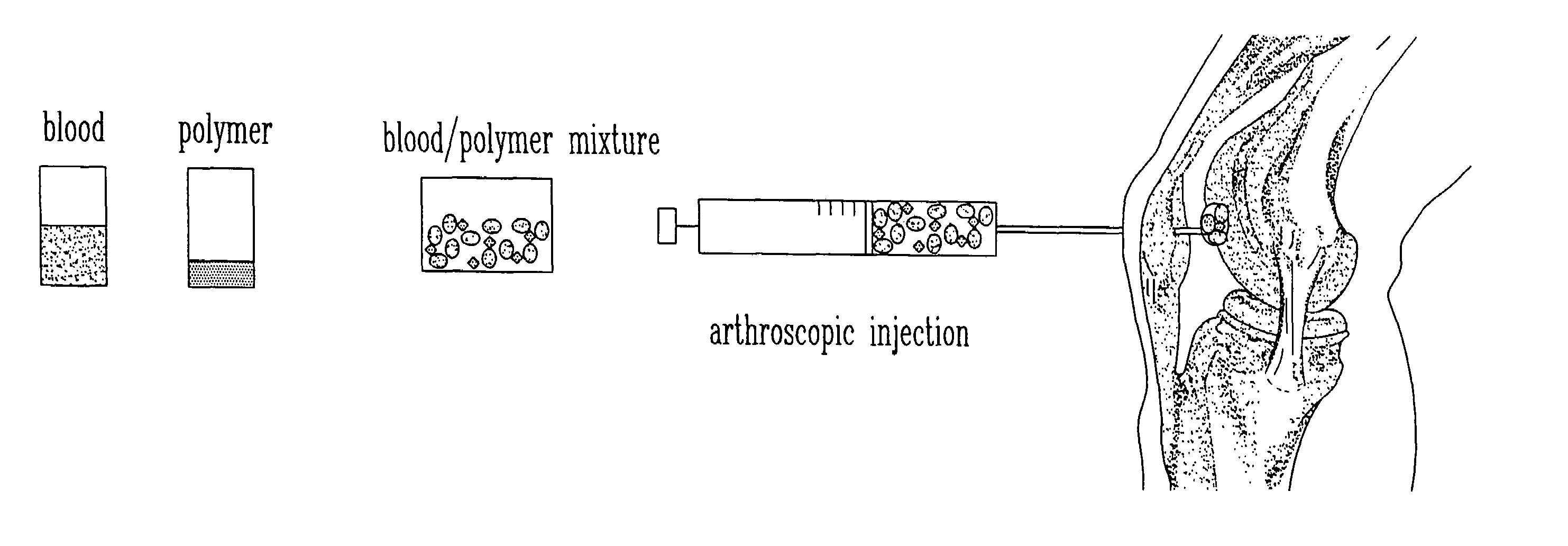
The present invention relates to a new method for repairing human or animal tissues such as cartilage, meniscus, ligament, tendon, bone, skin, cornea, periodontal tissues, abscesses, resected tumors, and ulcers. The method comprises the step of introducing into the tissue a temperature-dependent polymer gel composition such that the composition adhere to the tissue and promote support for cell proliferation for repairing the tissue. Other than a polymer, the composition preferably comprises a blood component such as whole blood, processed blood, venous blood, arterial blood, blood from bone, blood from bone-marrow, bone marrow, umbilical cord blood, placenta blood, erythrocytes, leukocytes, monocytes, platelets, fibrinogen, thrombin and platelet rich plasma. The present invention also relates to a new composition to be used with the method of the present invention.
Owner:SMITH & NEPHEW ORTHOPAEDICS
Follicular extraction method and device
ActiveUS20050267506A1Diminish and even eliminate shortcomingUnable to easily cut and inciseSkin implantsSurgical needlesTissue skinOvarian follicle
A method and device for the extraction of follicular units from a donor area on a patient. The method includes scoring the outer skin layers with a sharp punch, and then inserting a blunt punch into the incision to separate the hair follicle from the surrounding tissue and fatty layer. The method and device will significantly decrease the amount of follicular transection and increase the rate at which follicular units can be extracted.
Owner:HSC DEV
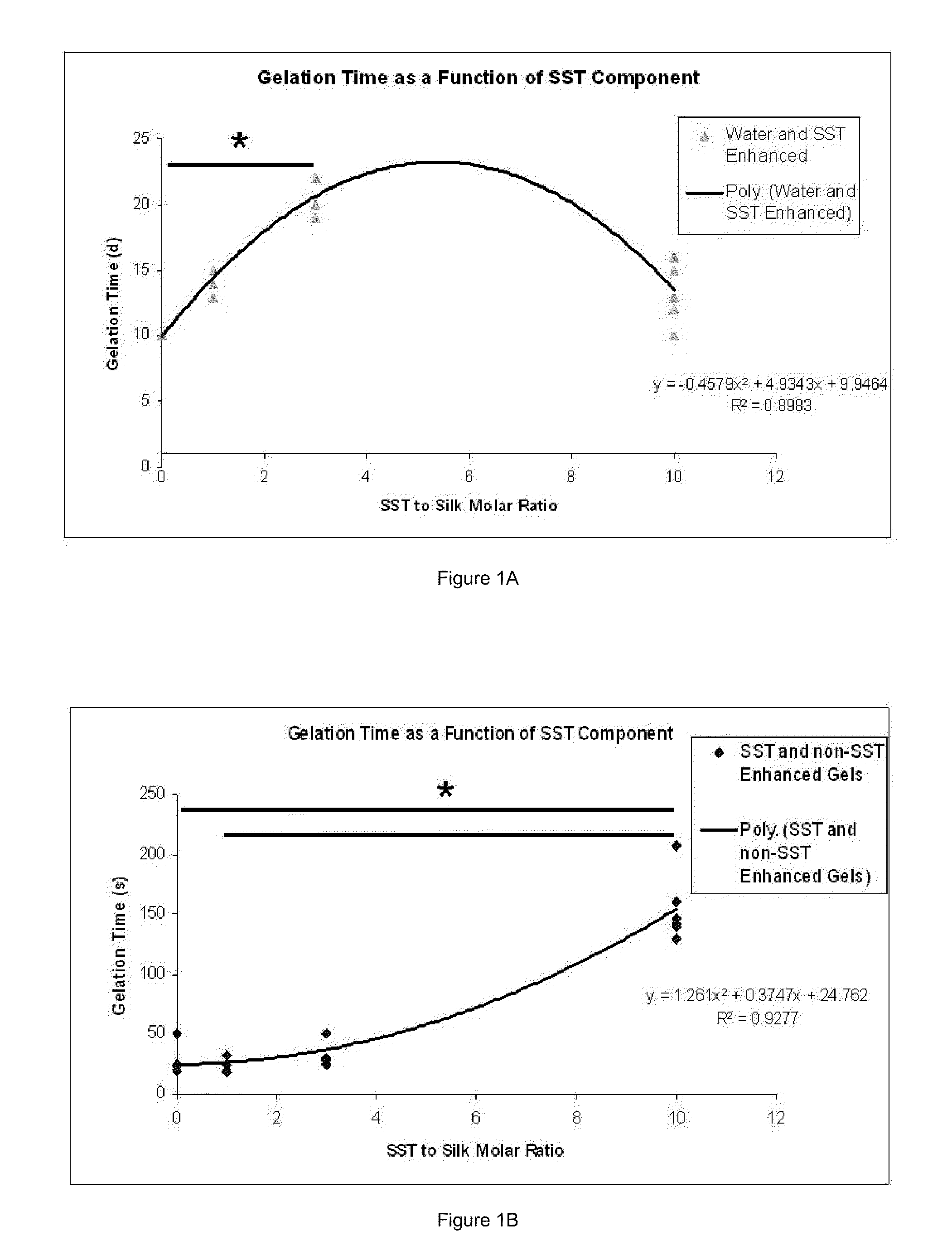
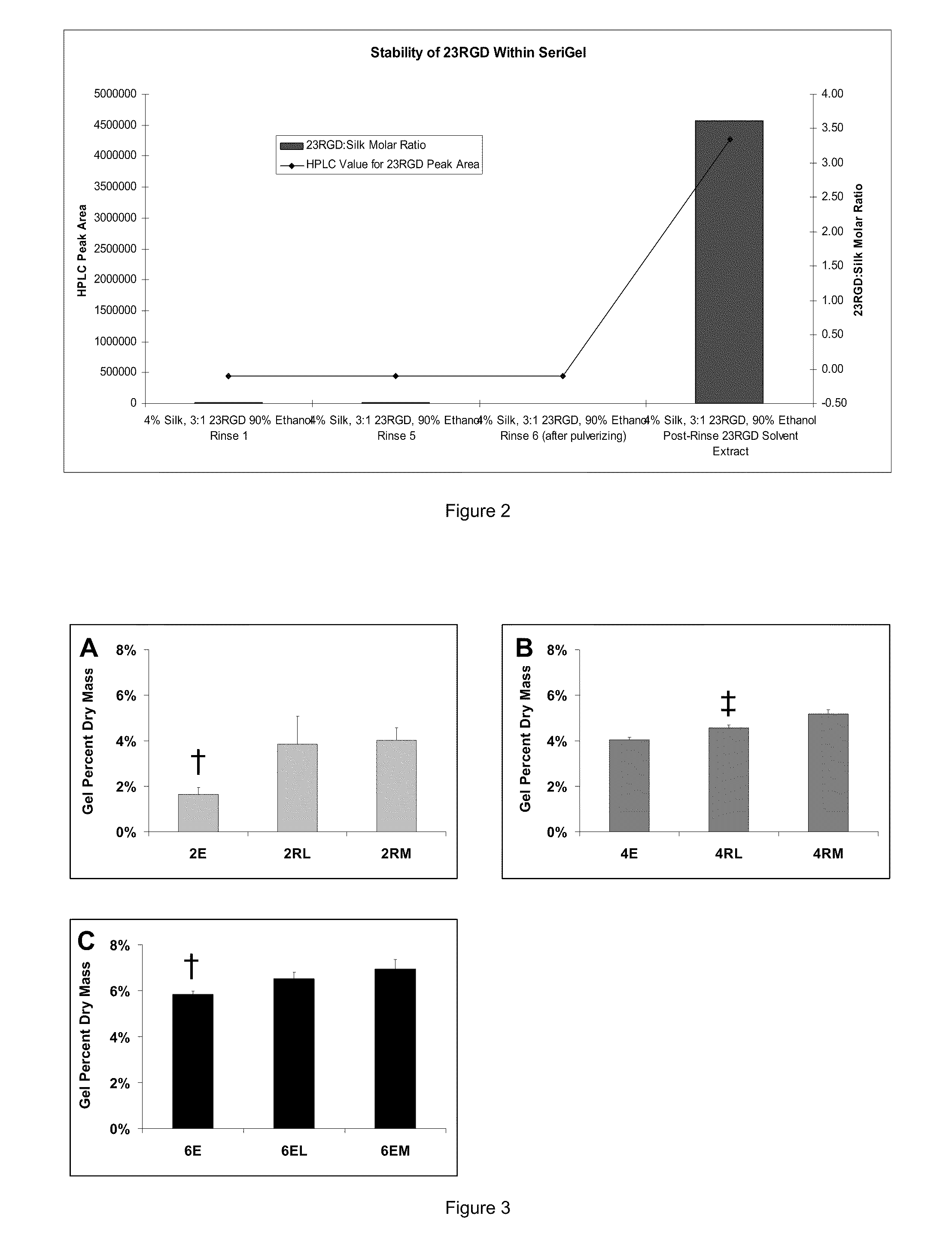
Silk Fibroin Hydrogels and Uses Thereof
InactiveUS20110008406A1Increase profitGood biocompatibilityBiocideCosmetic preparationsDiseaseFibronectin binding
The present specification provides for methods for purifying fibroins, purified fibroins, methods of conjugating biological and synthetic molecules to fibroins, fibroins conjugated to such molecules, methods of making fibroin hydrogels, fibroin hydrogels and fibroin hydrogel formulations useful for a variety of medical uses, including, without limitation uses as bulking agents, tissue space fillers, templates for tissue reconstruction or regeneration, cell culture scaffolds for tissue engineering and for disease models, surface coating to improve medical device function, or drug delivery devices.
Owner:ALLERGAN INC
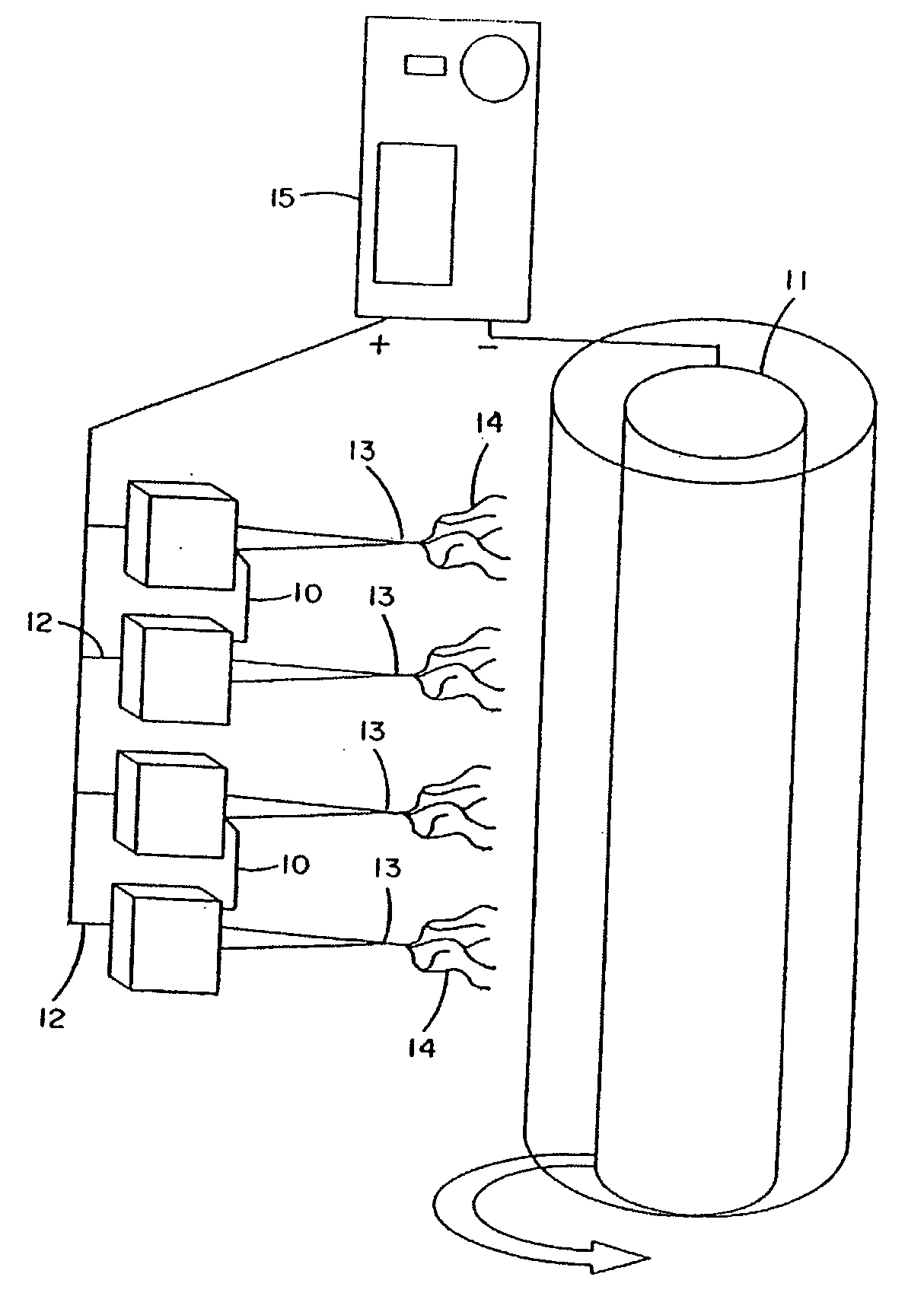
Sealants for Skin and Other Tissues
InactiveUS20070225631A1Prevent and reduce and eliminate flow of fluidSurgical adhesivesPeptide/protein ingredientsSealantTissue skin
The present invention relates to sealants for skin and other tissues. The sealants include an electroprocessed material. The sealants may contain more than one electroprocessed materials and may contain additional substances. The invention further relates to methods of making and using such sealants.
Owner:VIRGINIA COMMONWEALTH UNIV INTPROP FOUND INC +1
Processes for the preparation of novel collagen-based supports for tissue engineering, and biomaterials obtained
A composite product is disclosed as a collagen support comprising at least one porous collagen layer covered on at least one side with an essentially compact collagen membrane consisting either of a collagen film prepared by drying a collagen gel, preferably in air or a gaseous fluid, or of a very highly compressed collagen sponge. At least one of the two layers, i.e. the porous layer and the essentially compact membrane, may comprise normal, genetically modified or malignant living cells originating particularly from young or elderly subjects. This composite product is used as a collagen support for the manufacture of artificial skin intended especially for performing in vitro tests on the efficacy of potentially active substances or for reconstructing damaged areas of skin in vivo.
Owner:BASF BEAUTY CARE SOLUTIONS FRANCE SAS
Tissue products derived from animals lacking any expression of functional alpha 1,3 galactosyltransferase
InactiveUS20050260176A1Prevent rejectionPromote wound healingBiocideGenetic material ingredientsTissue repairSkin repair
The present invention provides tissues derived from animals, which lack any expression of functional alpha 1,3 galactosyltransferase (alpha-1,3-GT). Such tissues can be used in the field of xenotransplantation, such as orthopedic reconstruction and repair, skin repair and internal tissue repair or as medical devices.
Owner:REVIVICOR INC
Conformable tissue repair implant capable of injection delivery
ActiveUS20050113937A1Promote healingPromote new cell growthAntipyreticAnalgesicsTissue repairTissue defect
A conformable tissue implant is provided for use in repairing or augmenting a tissue defect or injury site. The tissue implant contains a tissue carrier matrix comprising a plurality of biocompatible, bioresorbable granules and at least one tissue fragment in association with the granules. The tissue fragment contains one or more viable cells that can migrate from the tissue and populate the tissue carrier matrix. Also provided is a method for injectably delivering the tissue implant.
Owner:DEPUY SYNTHES PROD INC
Elastin and elastin-based materials
It is a general object of the invention to provide a method of effecting repair or replacement or supporting a section of a body tissue. Specifically to provide an elastin or elastin-based biomaterial suitable for use as a stent, for example, a vascular stent, or as conduit replacement, as an artery, vein or a ureter replacement. The biomaterial can also be used as a stent or conduit covering or coating or lining. It is also an object of the invention to provide a method of securing an elastin or elastin-based biomaterial to an existing tissue without the use of sutures or staples.
Owner:PROVIDENCE HEALTH SYST OREGON AN OREGON NONPROFIT CORP +2
Artificial skin and elastic strain sensor
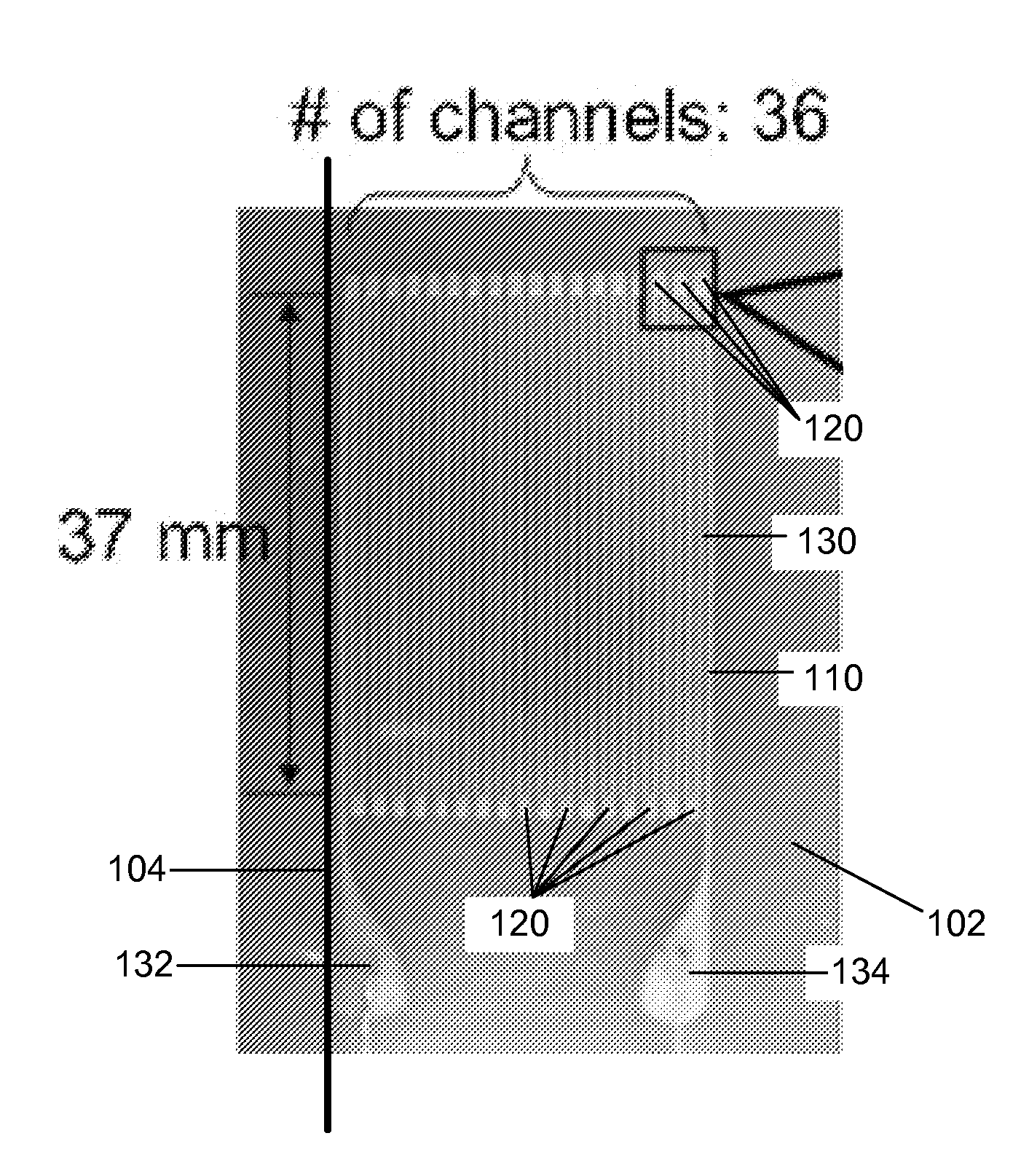
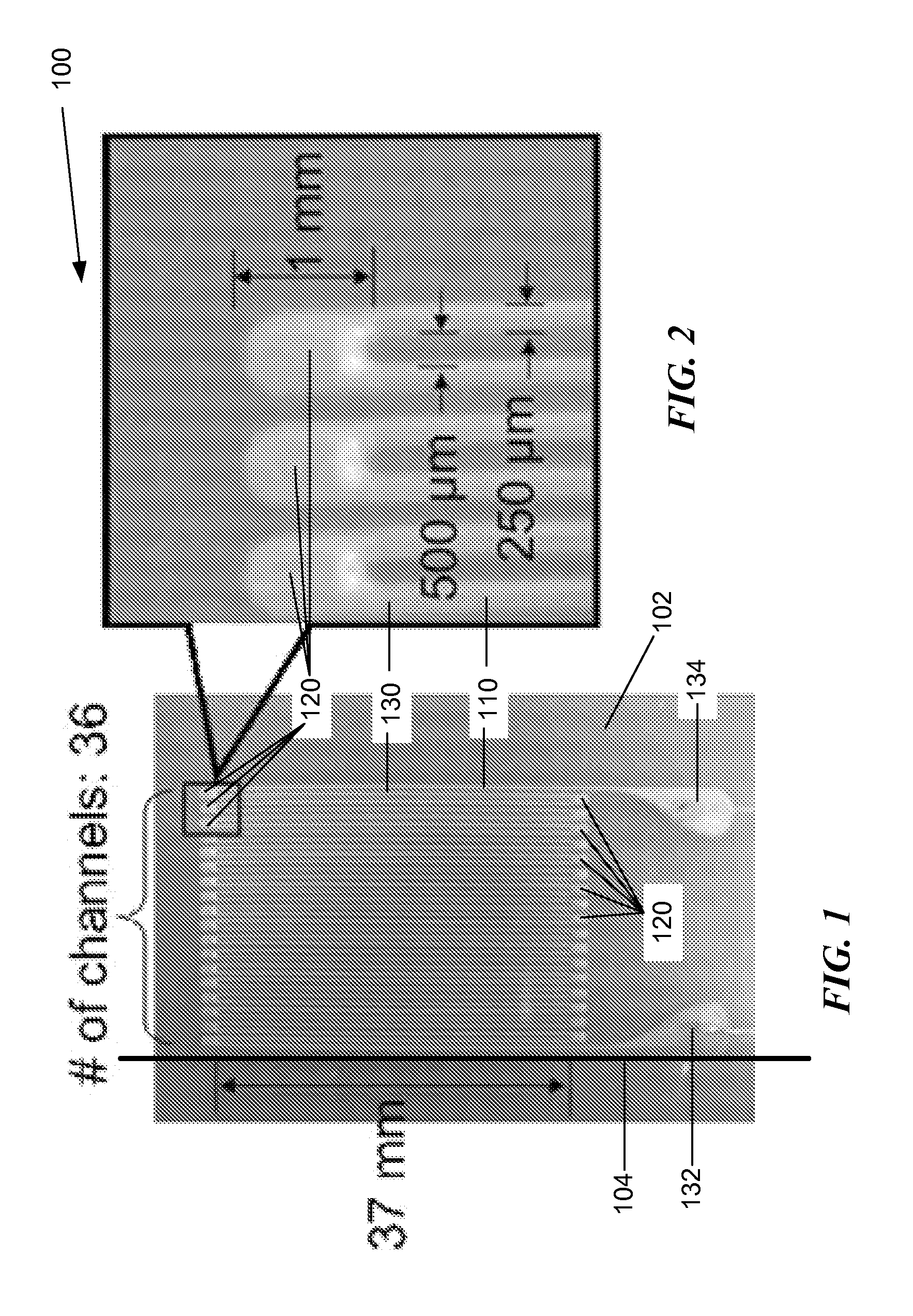
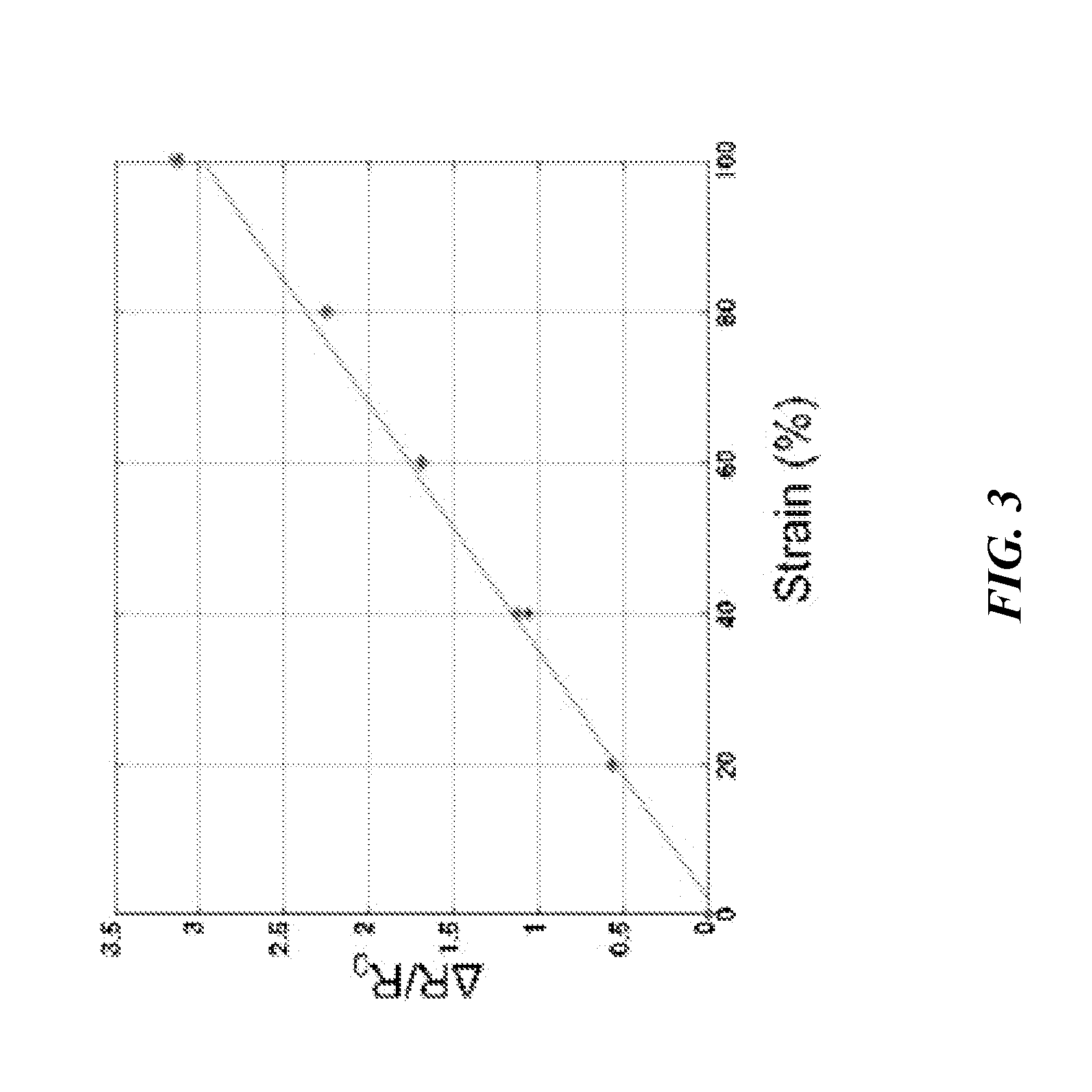
ActiveUS20140238153A1Sufficient cross-sectional areaHigh sensitivitySkin implantsSolesElectrical resistance and conductanceElastic substrate
An elastic strain sensor can be incorporated into an artificial skin that can sense flexing by the underlying support structure of the skin to detect and track motion of the support structure. The unidirectional elastic strain sensor can be formed by filling two or more channels in an elastic substrate material with a conductive liquid. At the ends of the channels, a loop port connects the channels to form a serpentine channel. The channels extend along the direction of strain and the loop portions have sufficiently large cross-sectional area in the direction transverse to the direction of strain that the sensor is unidirectional. The resistance is measured at the ends of the serpentine channel and can be used to determine the strain on the sensor. Additional channels can be added to increase the sensitivity of the sensor. The sensors can be stacked on top of each other to increase the sensitivity of the sensor. In other embodiments, two sensors oriented in different directions can be stacked on top of each other and bonded together to form a bidirectional sensor. A third sensor formed by in the shape of a spiral or concentric rings can be stacked on top and used to sense contact or pressure, forming a three dimensional sensor. The three dimensional sensor can be incorporated into an artificial skin to provide advanced sensing.
Owner:PRESIDENT & FELLOWS OF HARVARD COLLEGE
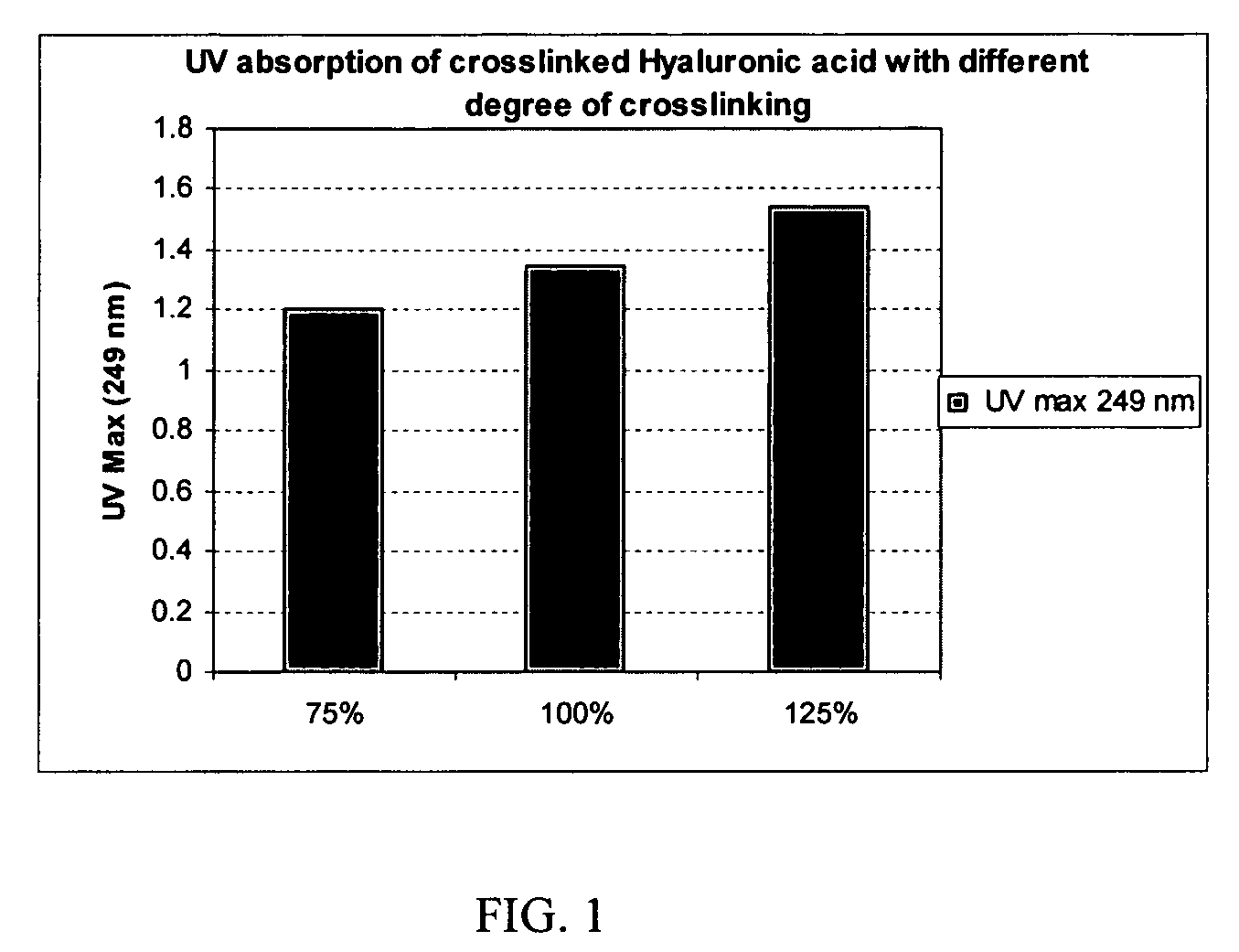
Crosslinked hyaluronic acid compositions for tissue augmentation
ActiveUS8124120B2Improve drug deliveryReduce frequencyAntibacterial agentsOrganic active ingredientsMedicineWater insoluble
Disclosed are hyaluronic acid (HA) compositions including crosslinked, water-insoluble, hydrated HA gel particles. Also disclosed are methods of making the HA compositions, and methods of using the HA composition to augment tissue in a subject.
Owner:ANIKA THERAPEUTICS INC
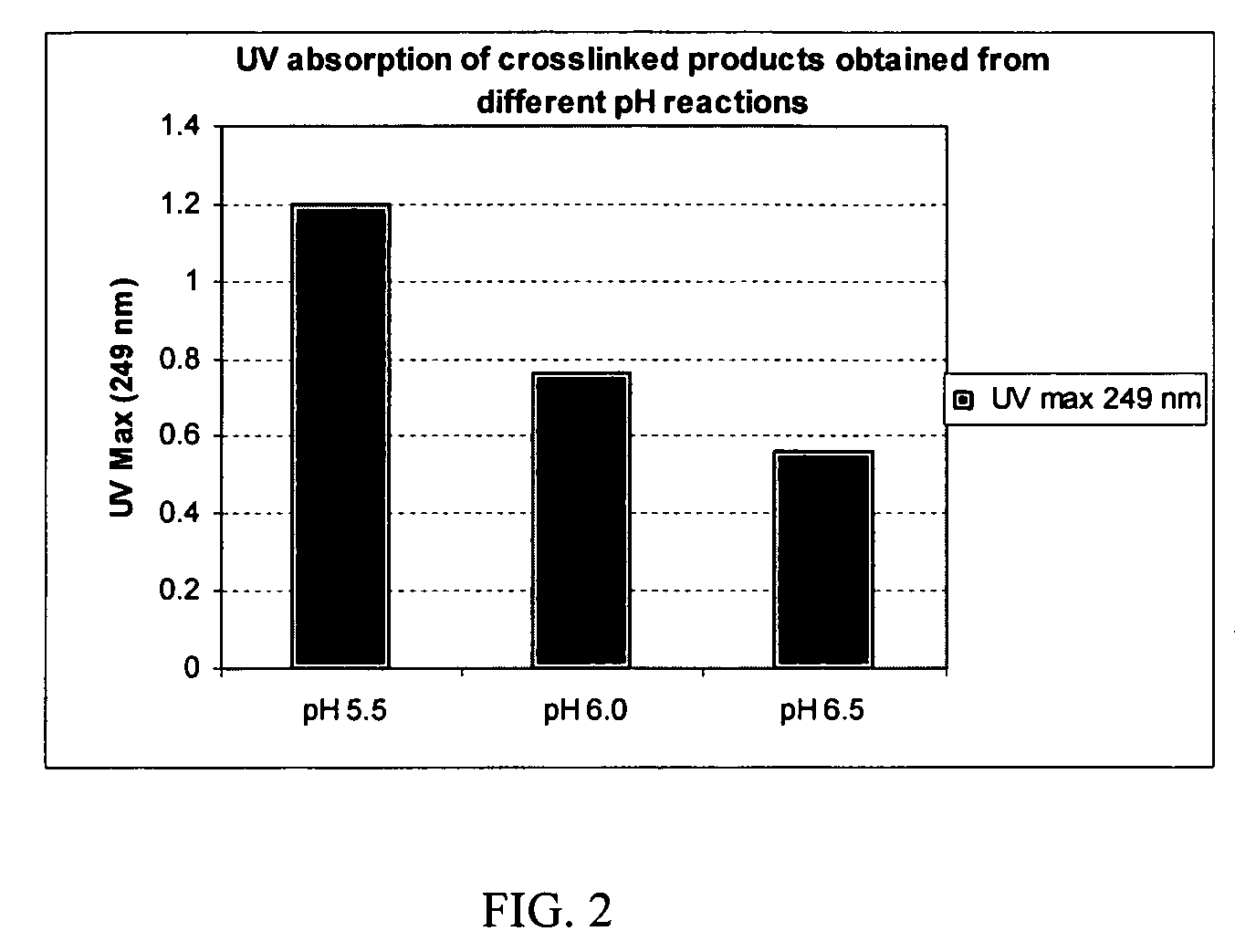
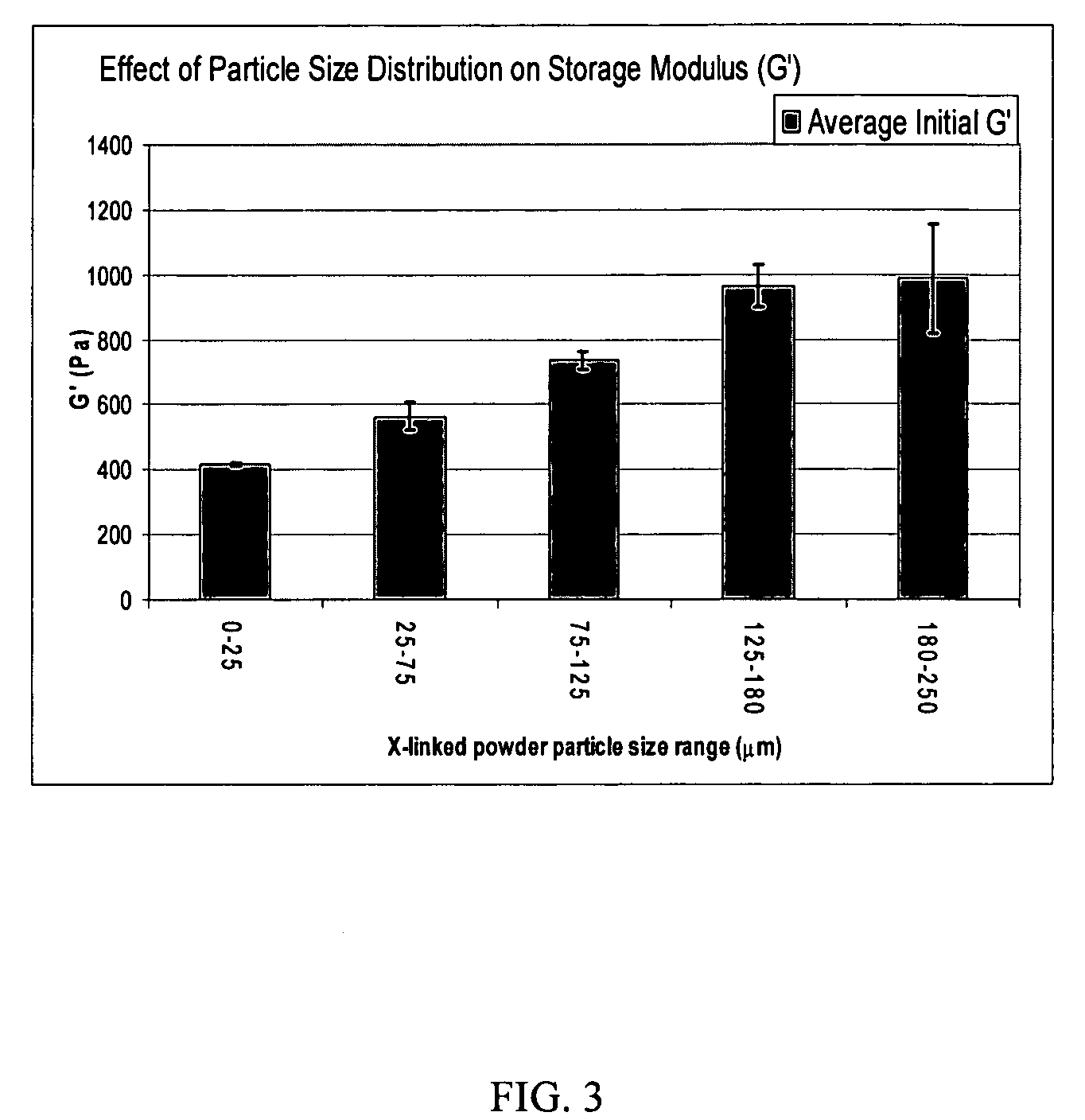
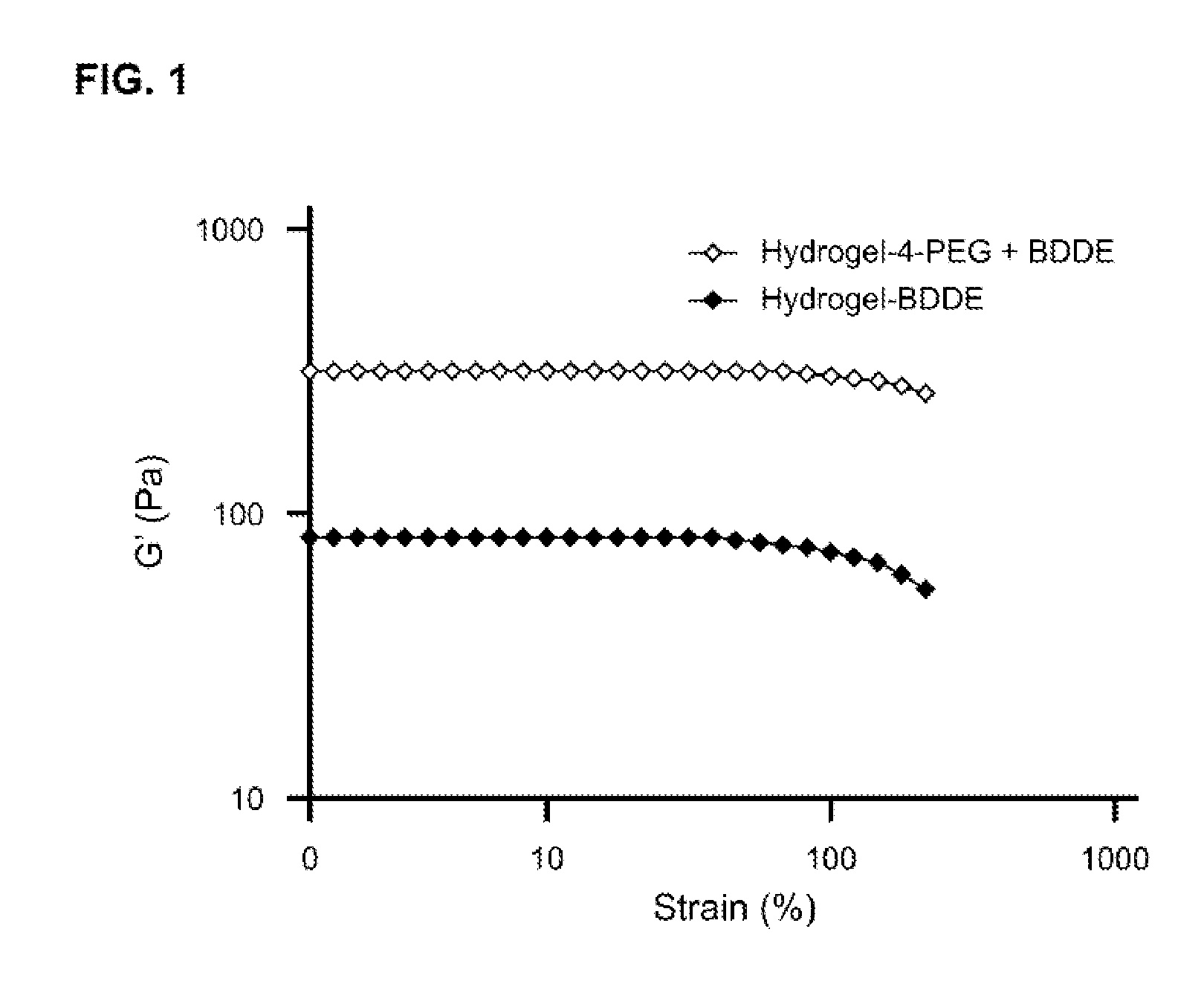
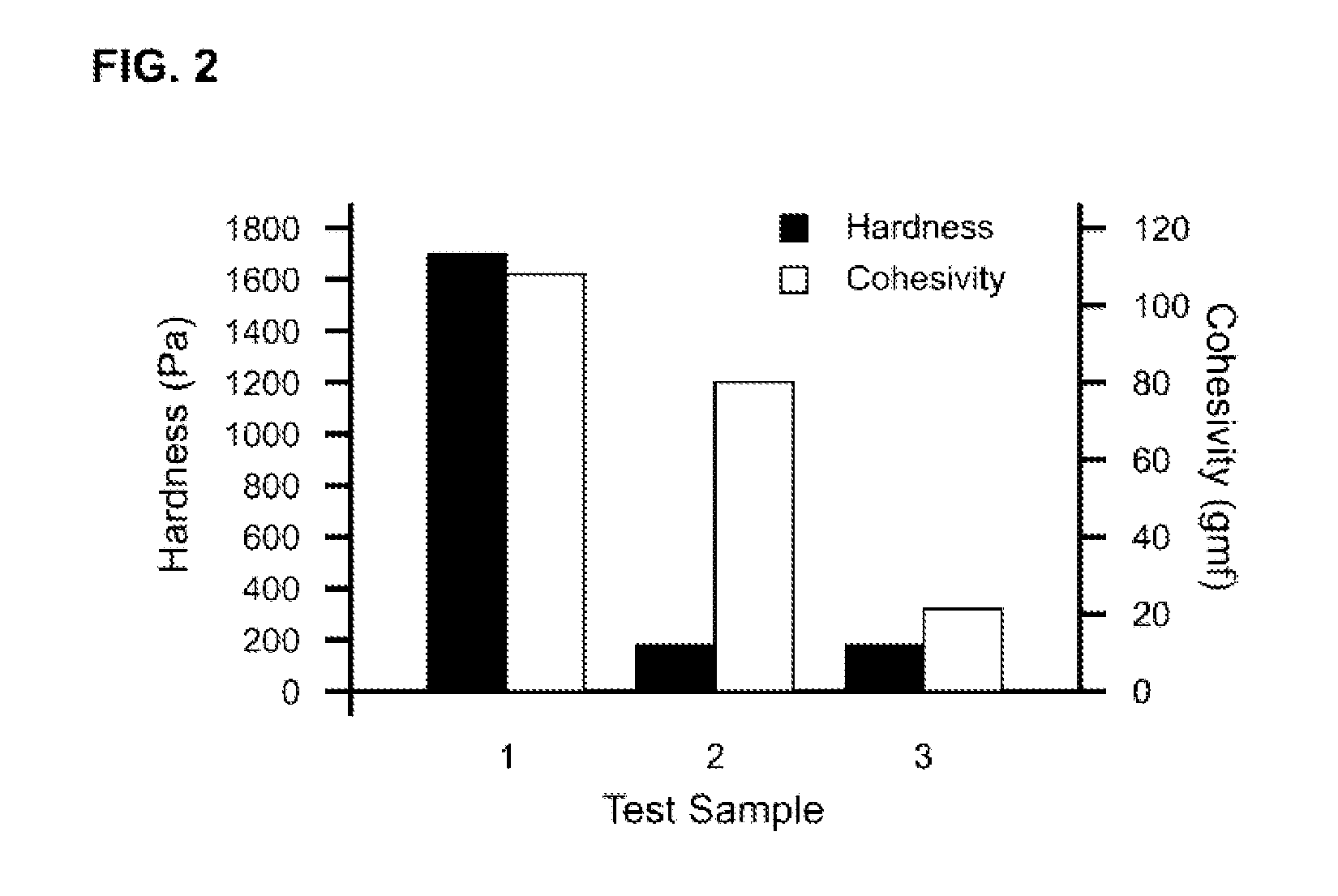
Tunably Crosslinked Polysaccharide Compositions
The present specification generally relates to multifunctional polyethylene glycol-based crosslinking agents, hydrogel compositions comprising a matrix polymer crosslinked with such crosslinking agents, and methods of treating a soft tissue condition using such hydrogel compositions.
Owner:ALLERGAN INC
Method and apparatus for transplanting a hair graft
Owner:RASSMAN WILLIAM R
Injectable hollow tissue filler
The present invention comprises a plurality of injectable hollow particulate fillers suspended in a biocompatible fluid carrier to significantly improve the clumping resistance and injectability of the composition. The hollow particulate fillers have a lower effective density and are able to suspend in the carrier without precipitation. The loss of skin volume as a result of aging, diseases, weight loss, and injury can lead to uneven skin surface (e.g. wrinkle, etc.). The uneven skin can be repaired by injecting appropriate amount of hollow fillers underneath the skin. Some cases of urinary incontinence occur when the resistance to urine flow has decreased excessively. Continence is restored by injecting the present invention to the urethra tissue to increase resistance to urine outflow. Similarly, the present invention allows for the control of gastric fluid reflux by submucosal injections of the fillers to the esophageal-gastric and gastric-pyloric junction. For patients with vesicoureteral reflux, it can be treated by injection of the present invention into patients' ureteral tissue. This invention can also be used to repair defective or inadequately functioning muscles of the anal sphincter by administering an effective amount of injectable hollow fillers into the defect or anal sinuses.
Owner:CHU JACK FA DE
Method of preparation of bioabsorbable porous reinforced tissue implants and implants thereof
Owner:CHUN IKSOO +5
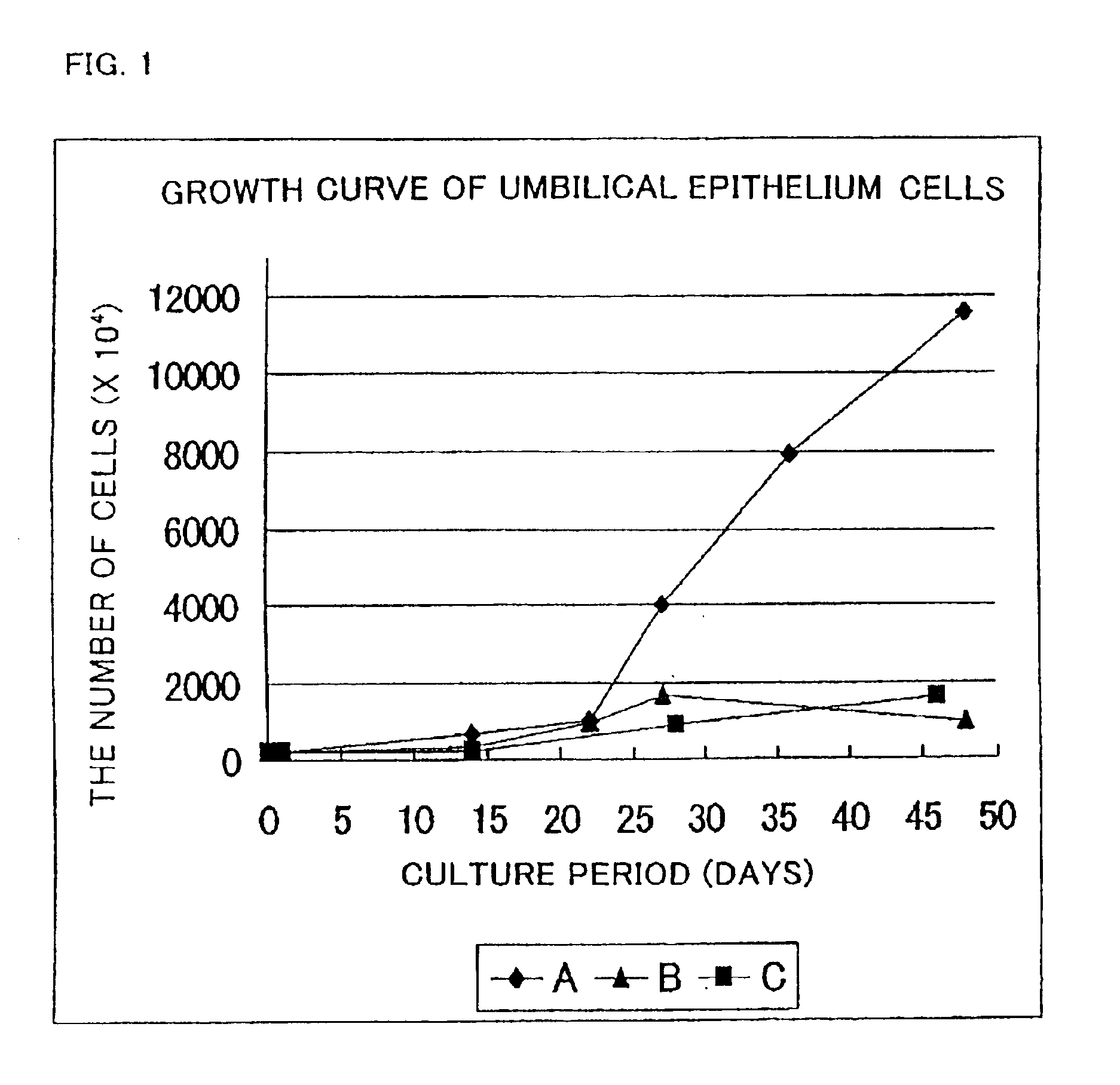
Cultured skin and method of manufacturing the same
InactiveUS6916655B2High successful grafting rateSkin implantsEpidermal cells/skin cellsEpitheliumFibroblast
A cultured skin and a grafting cultured skin sheet are provided, each of which is a cultured reconstructive skin with a high take rate using cells collectable from cells originated from tissue included in an umbilical cord such as tissue included in an umbilical cord originated from a human fetus. The grafting cultured skin stratified sheet is prepared by placing an epithelium sheet on the top surface of a cultured dermis. The cultured dermis includes as components a cultured skin containing cells originated from a tissue included in an umbilical cord, such as umbilical cells, more concretely, umbilical fibroblast cells, being separated and cultured, preferably in a collagen nonwoven fabric. On the other hand, the epithelium sheet is prepared by culturing and stratifying the umbilical cord epithelium cells.
Owner:NIPRO CORP
Method and apparatus for tissue expansion
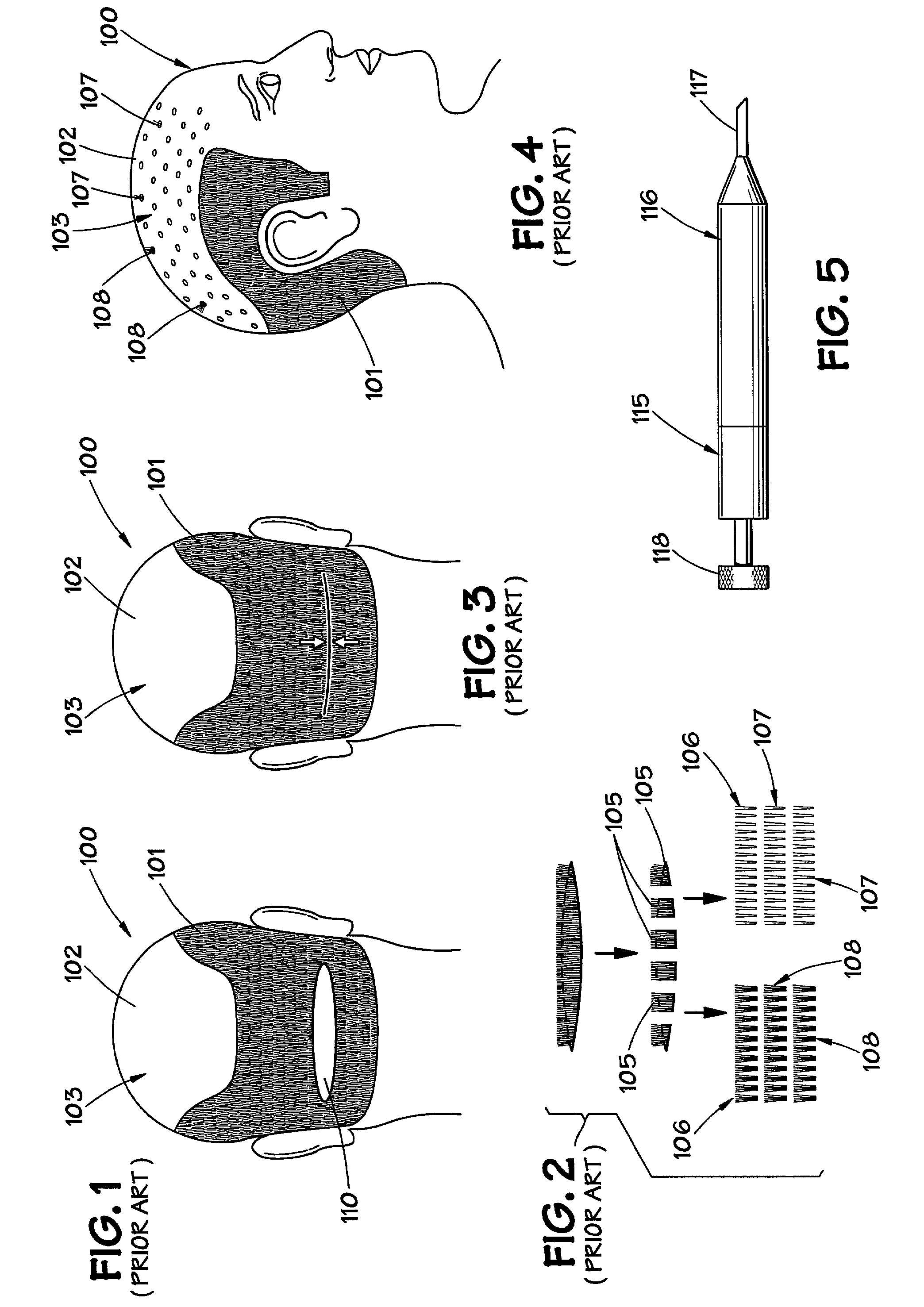
InactiveUS20110251602A1Reduce presenceReduced likelihoodSkin implantsIncision instrumentsMedicineTissue expansion
Exemplary embodiments of the present disclosure provide method and apparatus for facilitating stretching of a bio-logical tissue by forming a plurality of micro-slits in the tissue. Each micro-slit can be less than about 2 mm or less than about 1.5 mm long, or even less than about 1 mm, such that small gaps that can heal quickly can be formed when the tissue is stretched. The micro-slits can be formed using a plurality of cutting arrangements or an ablative laser. The micro-slits can be formed in various patterns, including staggered rows, circular or spiral patterns, or random patterns.
Owner:THE GENERAL HOSPITAL CORP
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com