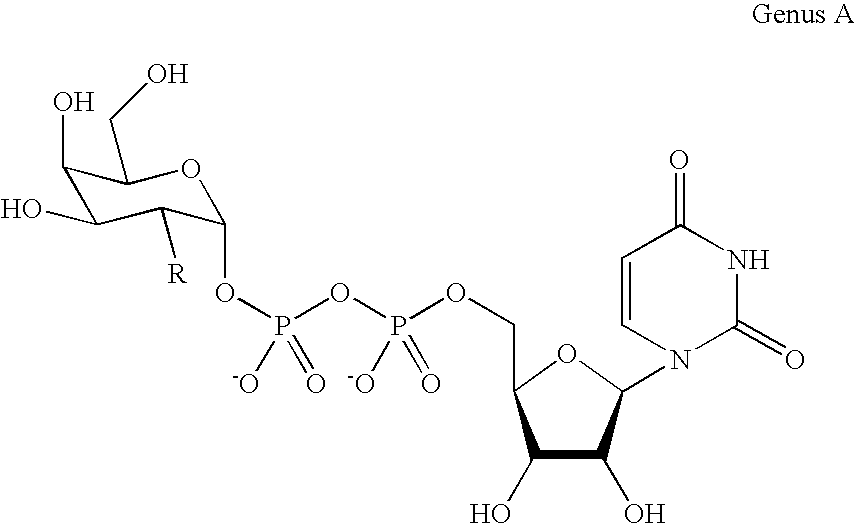
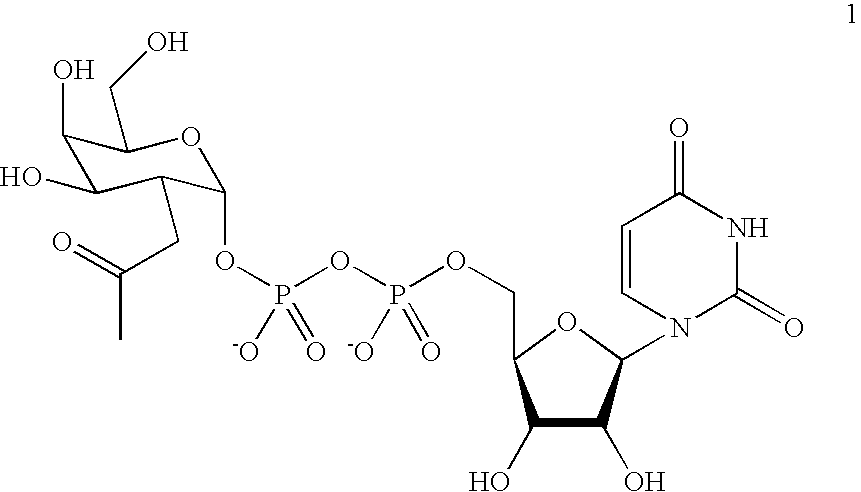
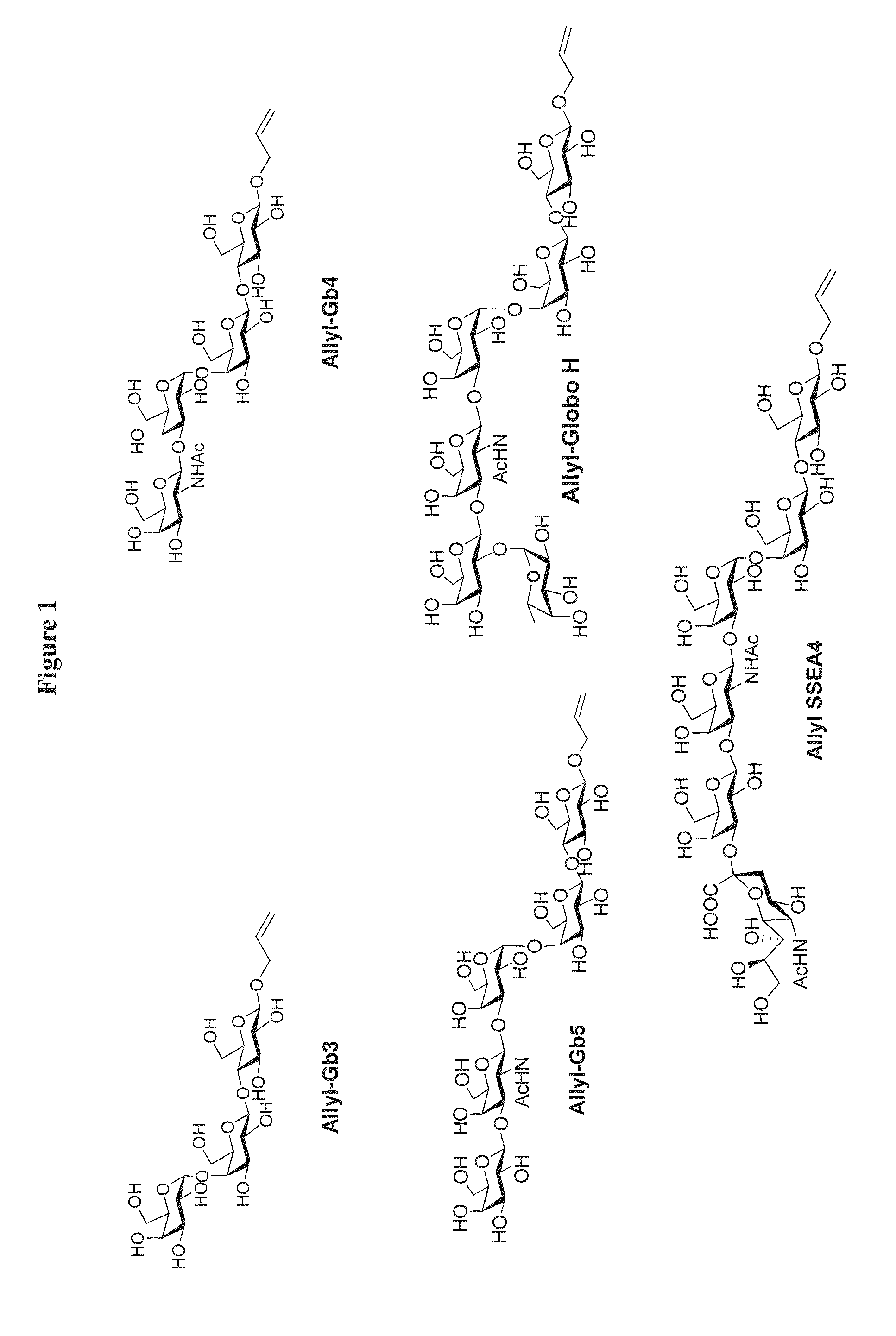
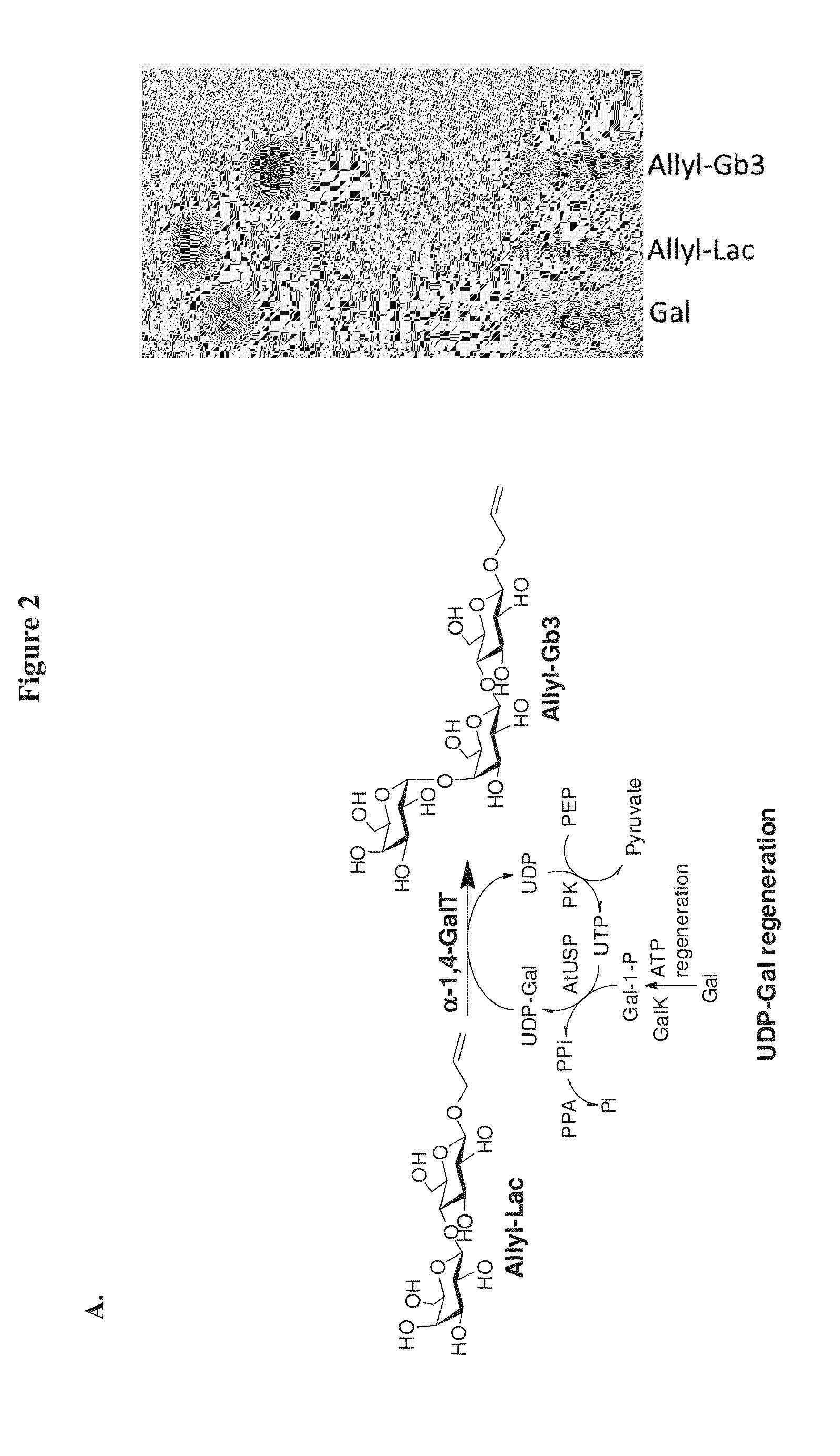
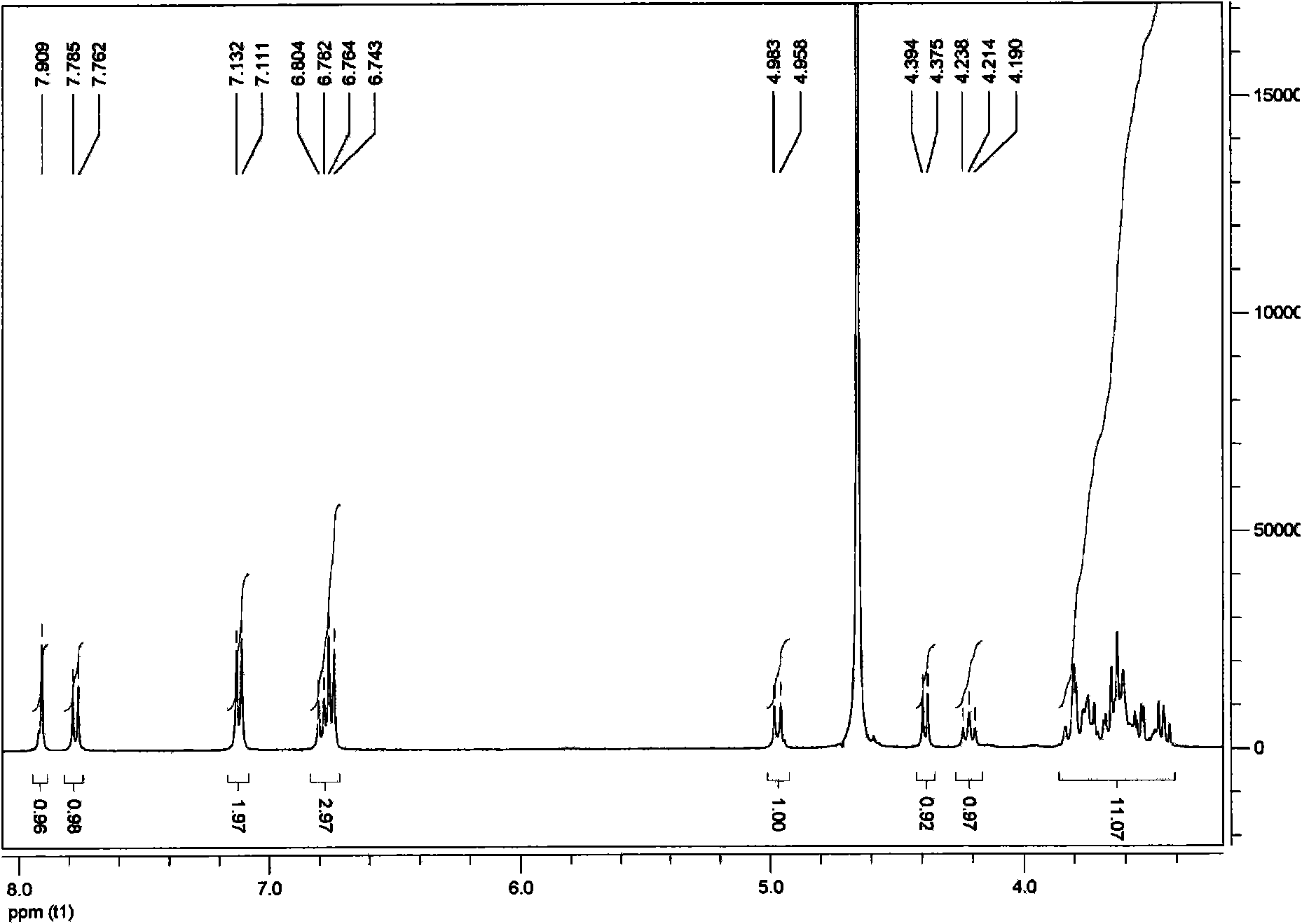
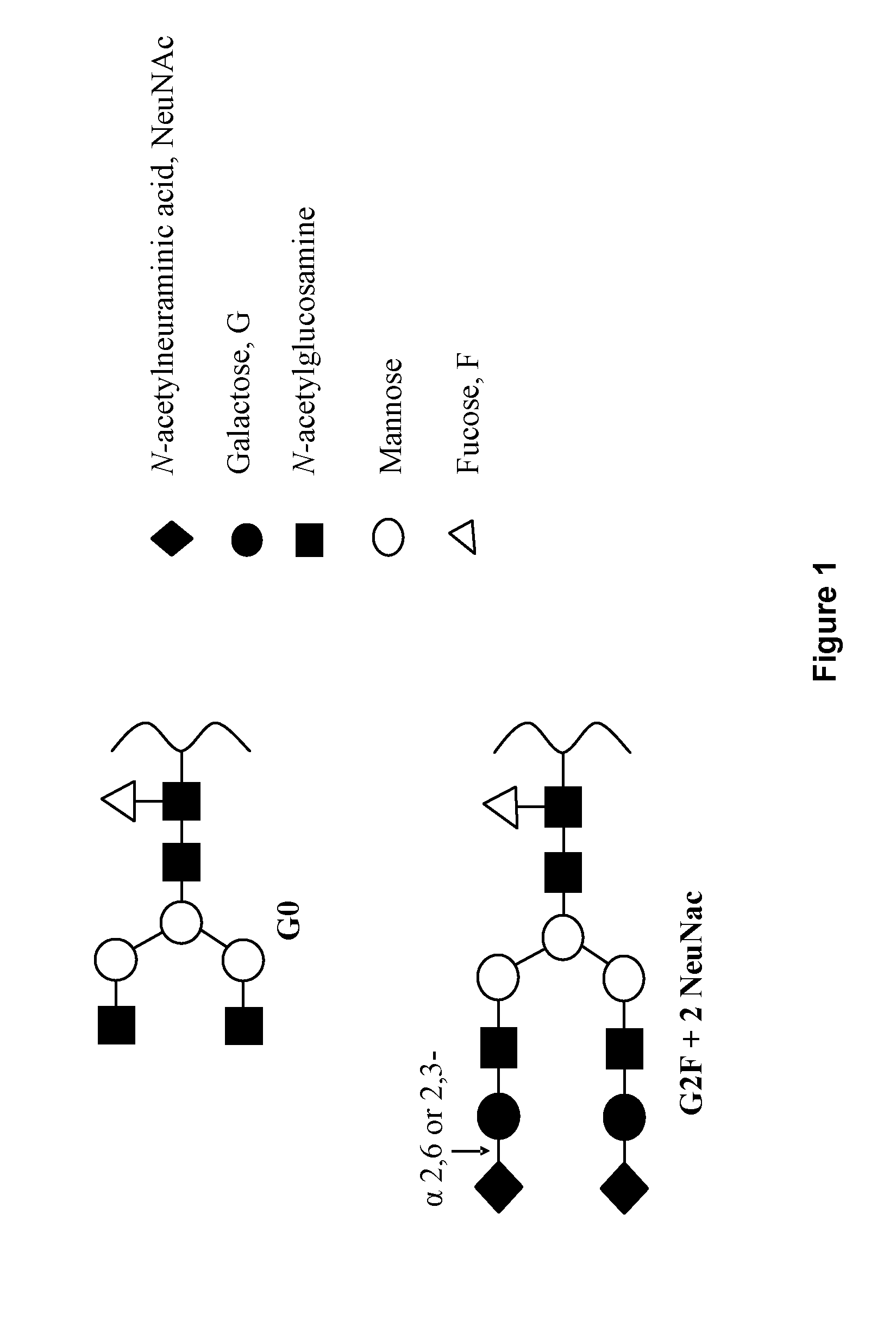
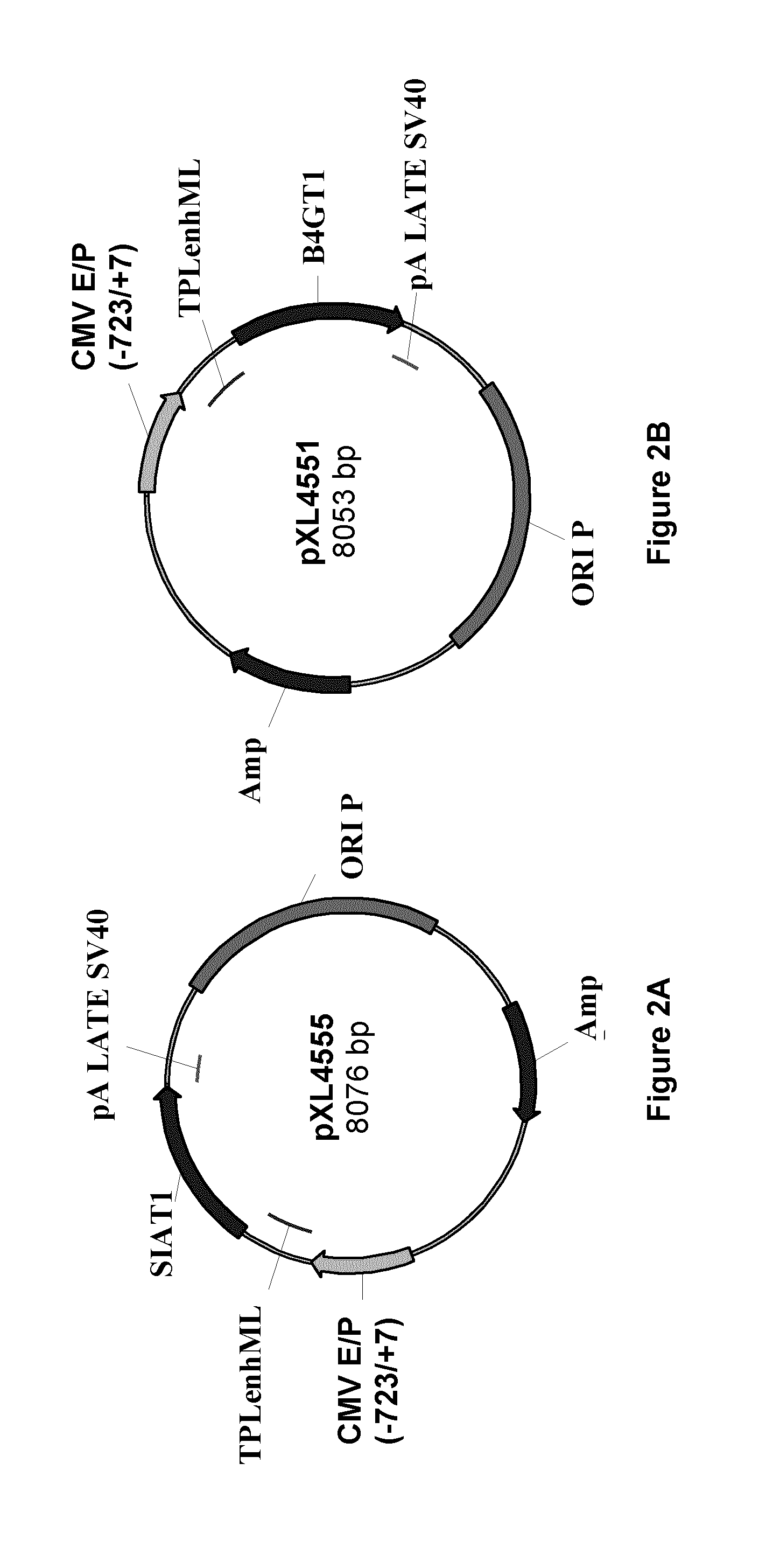
Patents
Literature
94 results about "Galactosyltransferase" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Galactosyltransferase is a type of glycosyltransferase which catalyzes the transfer of galactose. An example is B-N-acetylglucosaminyl-glycopeptide b-1,4-galactosyltransferase. The biosynthesis of disaccharides, oligosaccharides and polysaccharides involves the action of hundreds of different glycosyltransferases. These enzymes catalyse the transfer of sugar moieties from activated donor molecules to specific acceptor molecules, forming glycosidic bonds. A classification of glycosyltransferases using nucleotide diphospho-sugar, nucleotide monophospho-sugar and sugar phosphates (EC 2.4.1.-) and related proteins into distinct sequence based families has been described. This classification is available on the CAZy (Carbohydrate-Active EnZymes) web site. The same three-dimensional fold is expected to occur within each of the families. Because 3-D structures are better conserved than sequences, several of the families defined on the basis of sequence similarities may have similar 3-D structures and therefore form 'clans'.
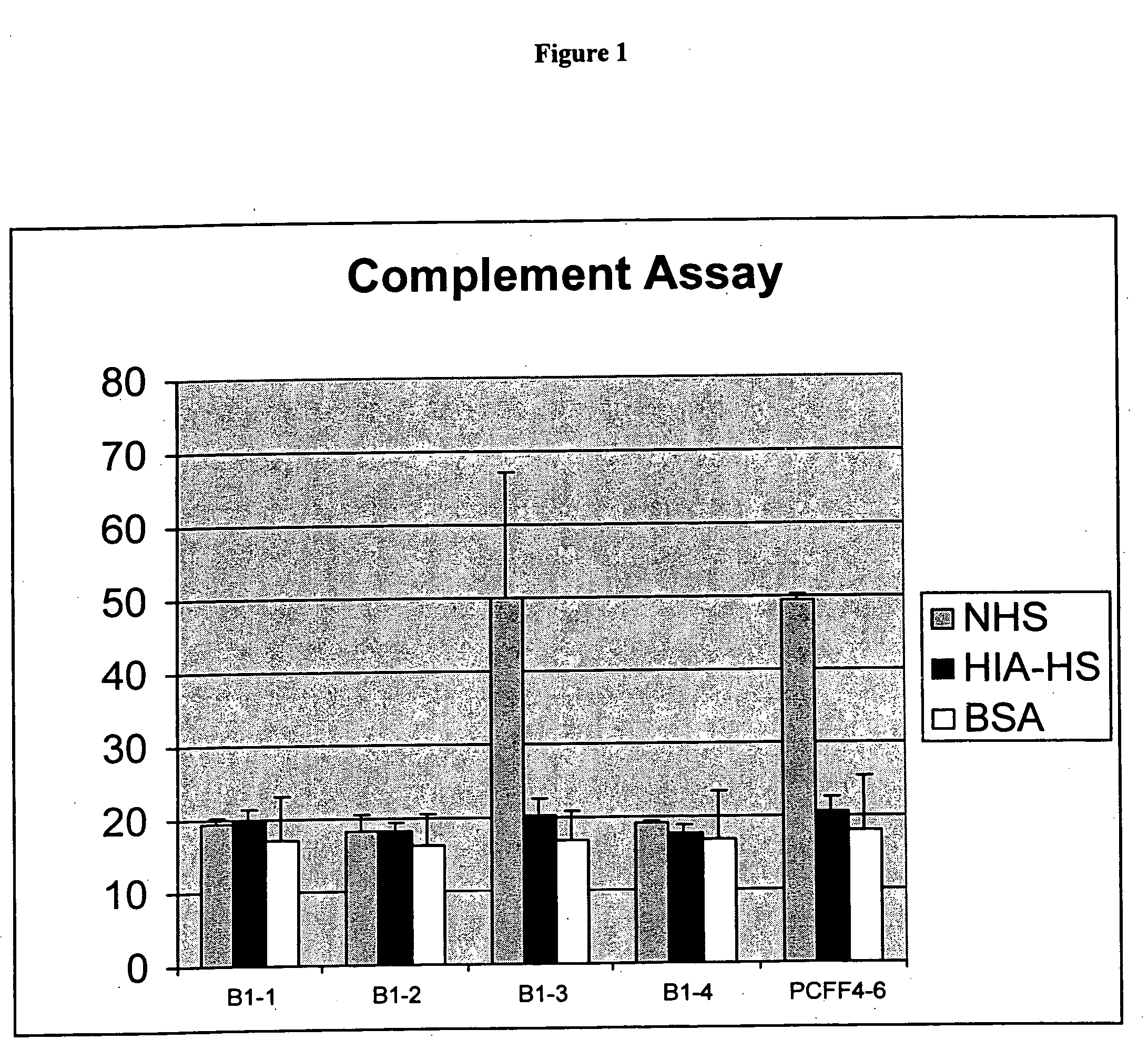
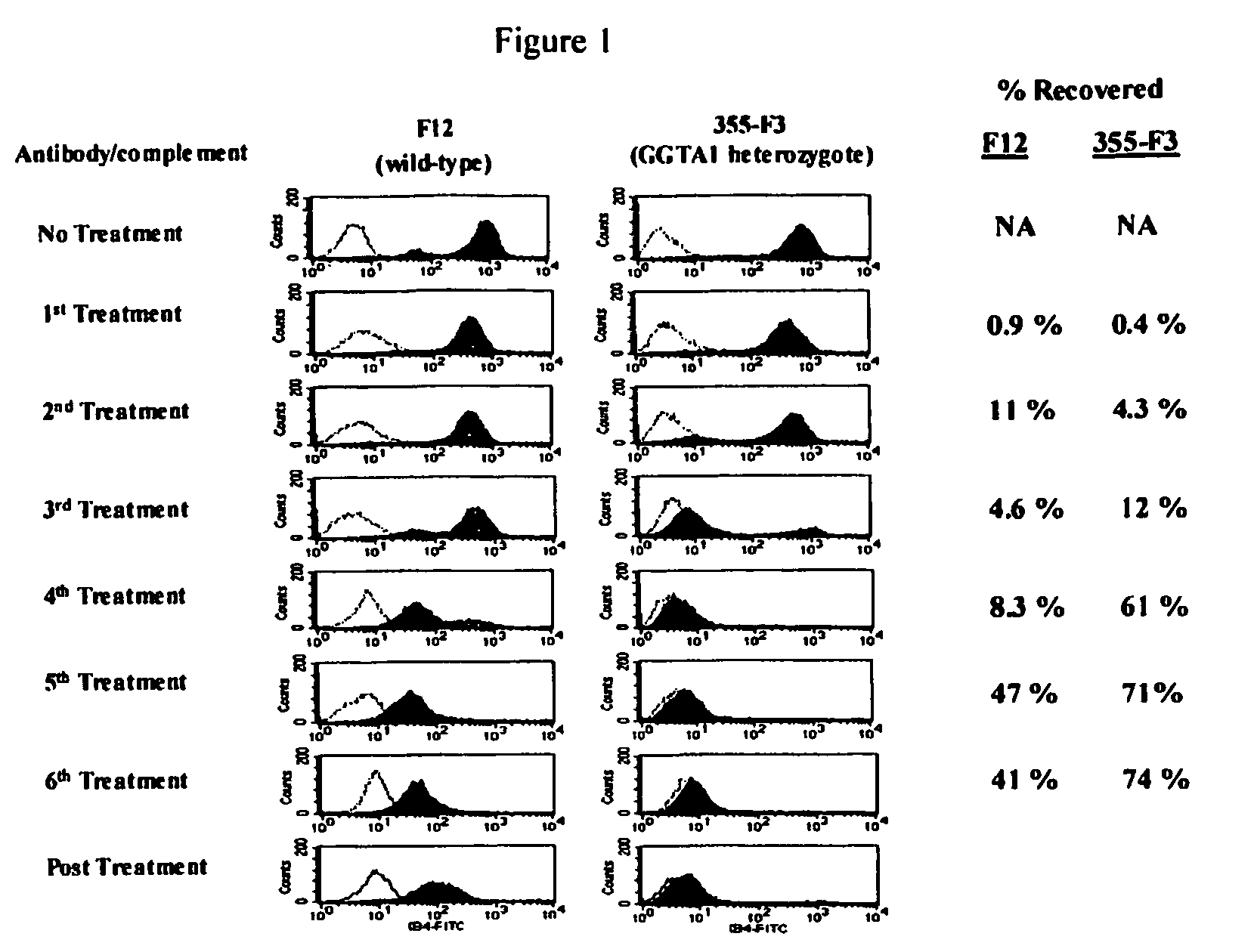
Tissue products derived from animals lacking any expression of functional alpha 1,3 galactosyltransferase
InactiveUS20050260176A1Prevent rejectionPromote wound healingBiocideGenetic material ingredientsTissue repairSkin repair
The present invention provides tissues derived from animals, which lack any expression of functional alpha 1,3 galactosyltransferase (alpha-1,3-GT). Such tissues can be used in the field of xenotransplantation, such as orthopedic reconstruction and repair, skin repair and internal tissue repair or as medical devices.
Owner:REVIVICOR INC
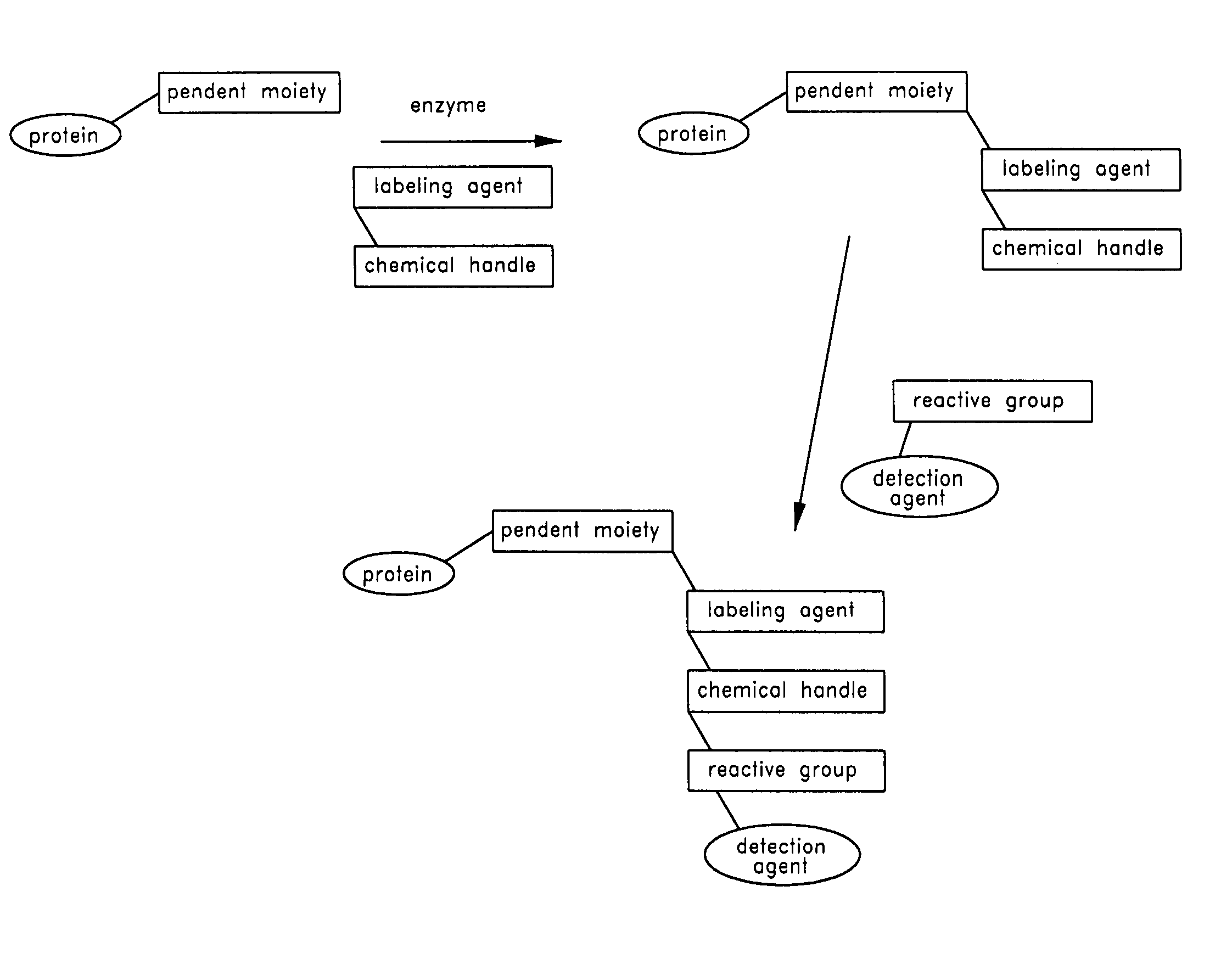
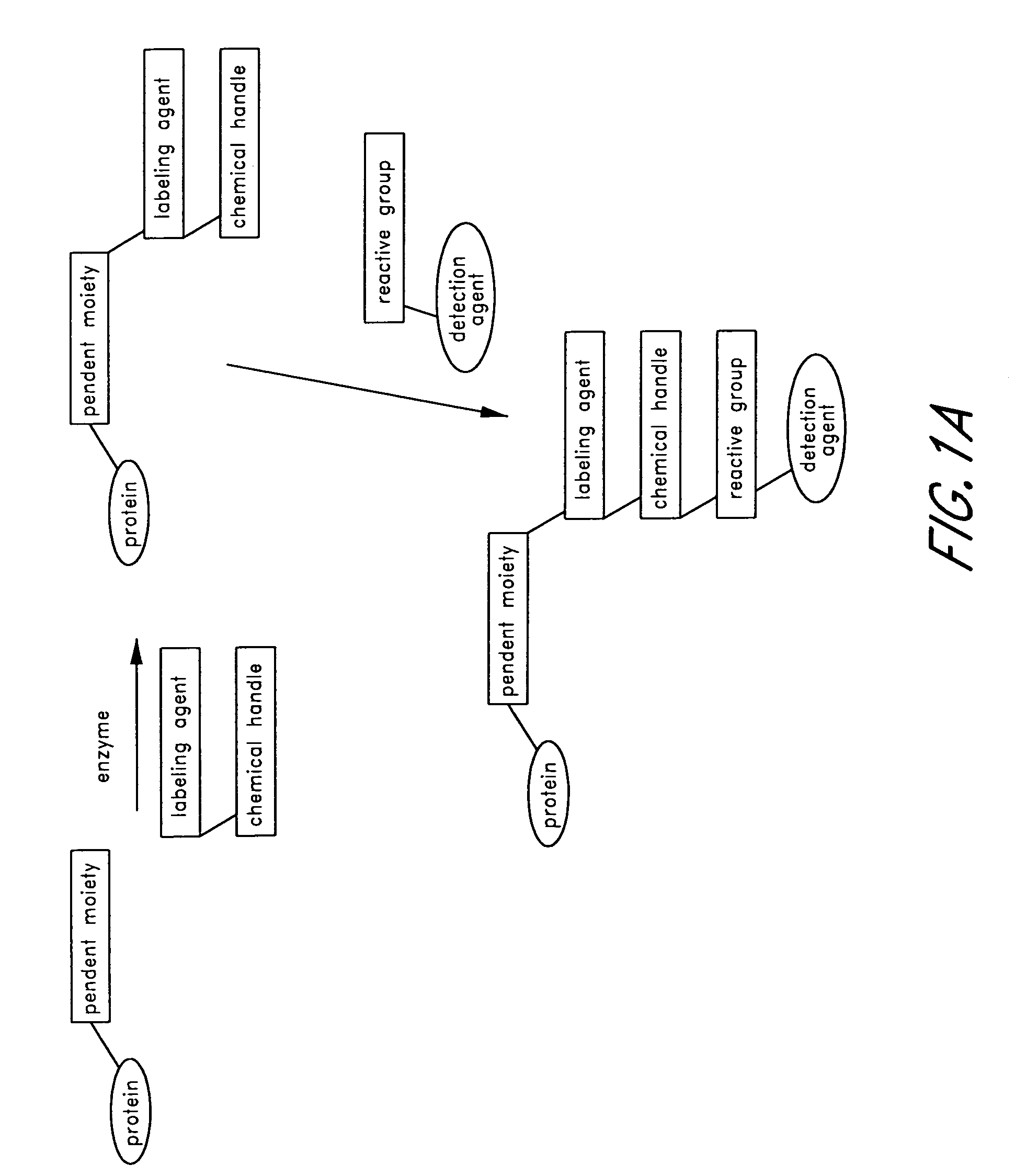
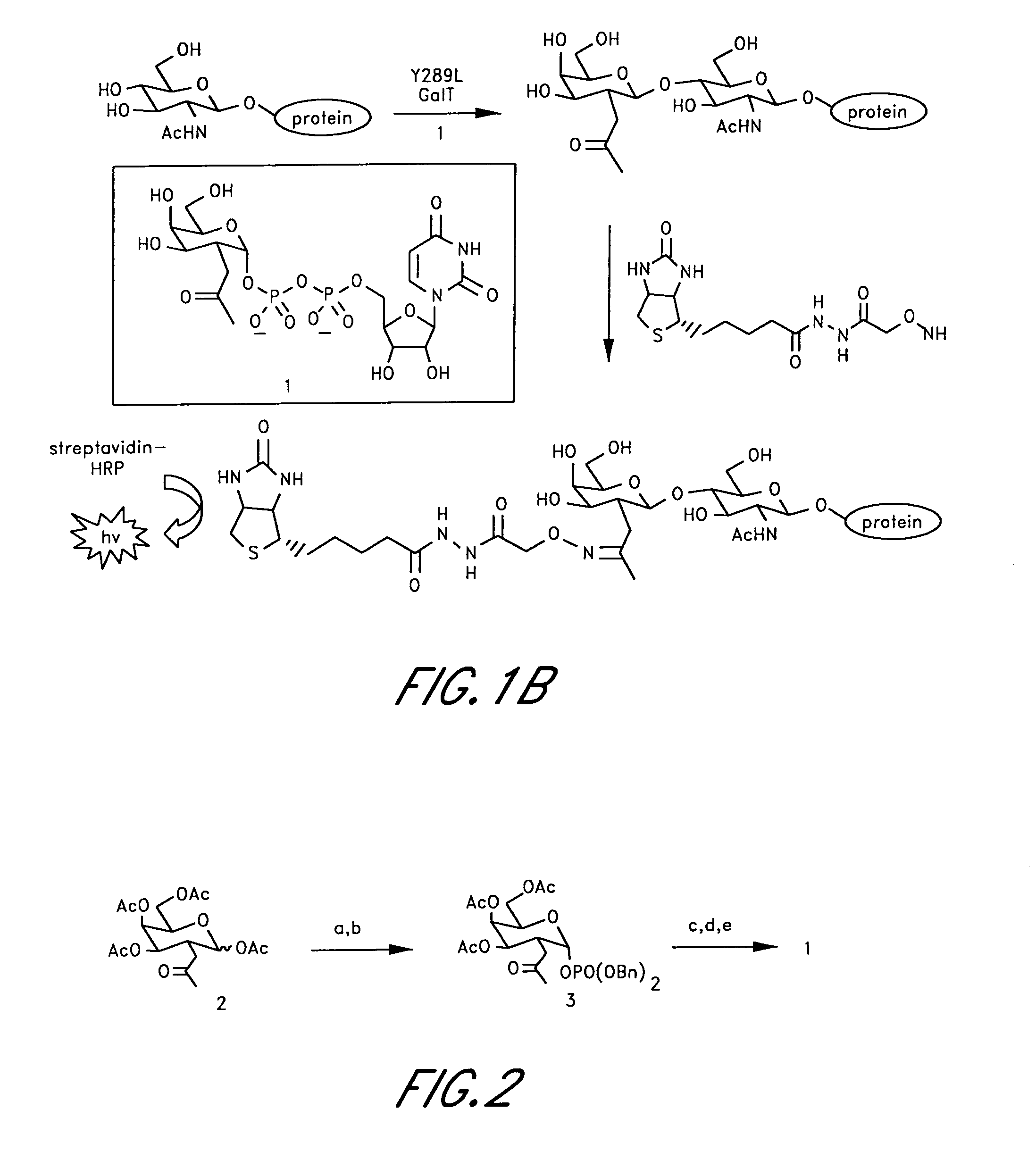
Method and compositions for the detection of protein glycosylation
ActiveUS20050130235A1Sugar derivativesMicrobiological testing/measurementPost translationalProtein insertion
The invention provides methods and compositions for the rapid and sensitive detection of post-translationally modified proteins, and particularly of those with post-translational glycosylations. The methods can be used to detect O-GlcNAc posttranslational modifications on proteins on which such modifications were undetectable using other techniques. In one embodiment, the method exploits the ability of an engineered mutant of β-1,4-galactosyltransferase to selectively transfer an unnatural ketone functionality onto O-GlcNAc glycosylated proteins. Once transferred, the ketone moiety serves as a versatile handle for the attachment of biotin, thereby enabling detection of the modified protein. The approach permits the rapid visualization of proteins that are at the limits of detection using traditional methods. Further, the preferred embodiments can be used for detection of certain disease states, such as cancer, Alzheimer's disease, neurodegeneration, cardiovascular disease, and diabetes.
Owner:CALIFORNIA INST OF TECH
Large scale enzymatic synthesis of oligosaccharides
ActiveUS20140051127A1Bioreactor/fermenter combinationsBiological substance pretreatmentsEnzymatic synthesisNucleotide
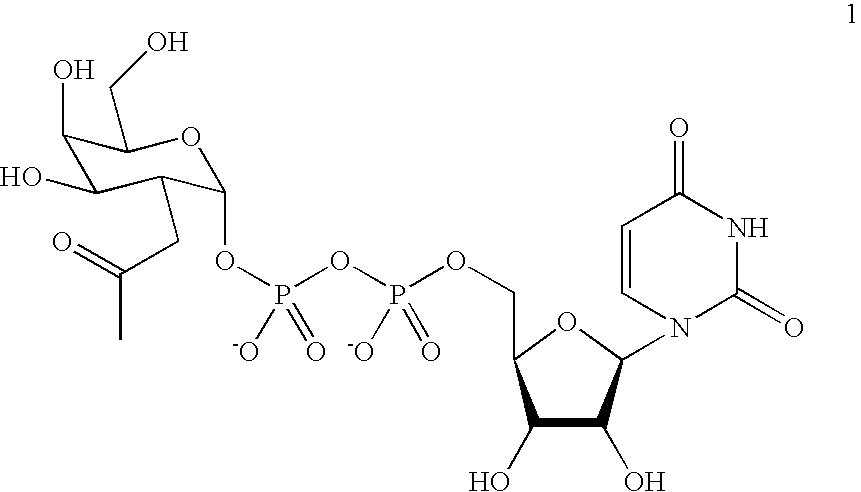
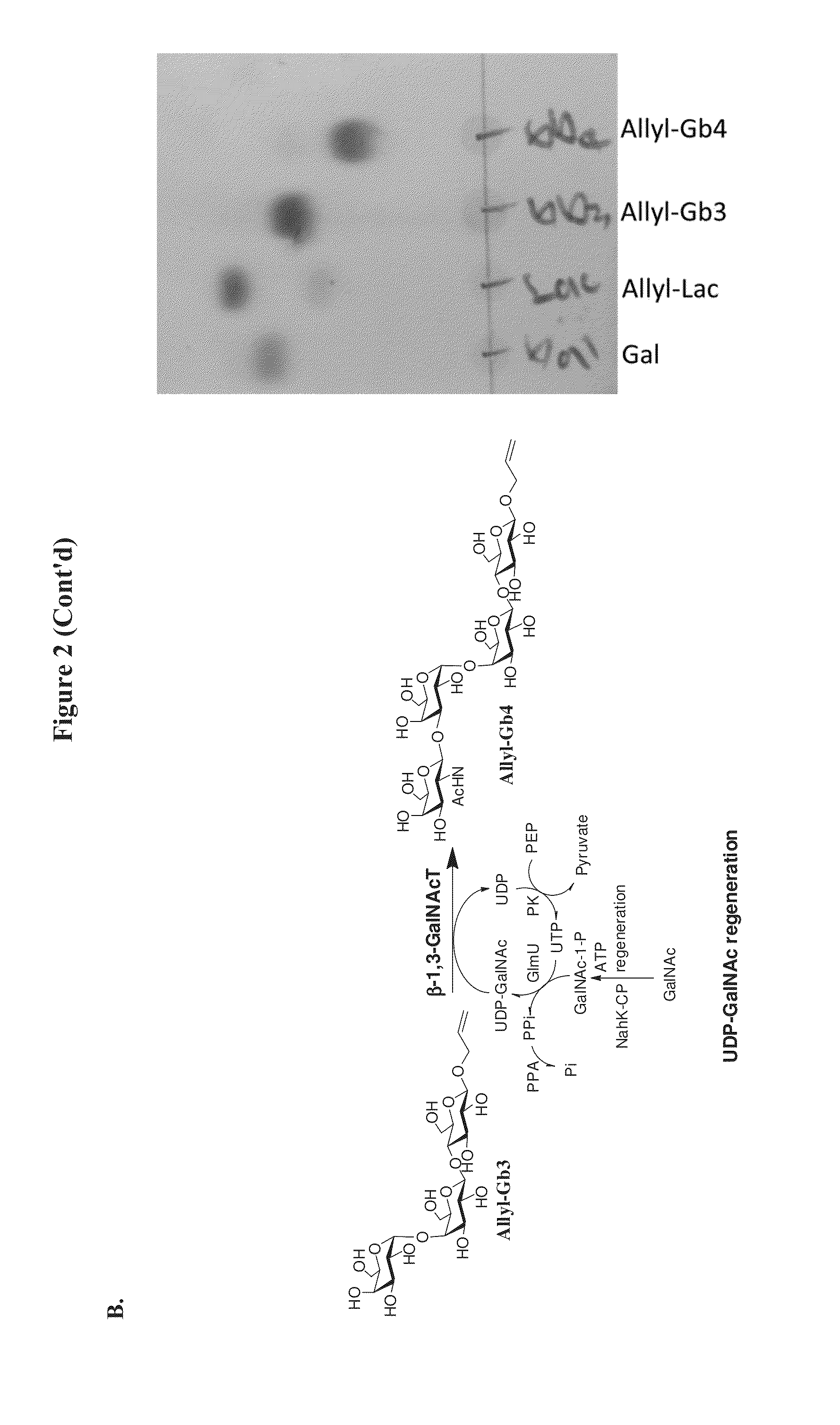
A novel UDP-Gal regeneration process and its combined use with a galactosyltransferase to add galactose to a suitable acceptor substrate. Also described herein are synthetic methods for generating Globo-series oligosaccharides in large scale, wherein the methods may involve the combination of a glycosyltransferase reaction and a nucleotide sugar regeneration process.
Owner:ACAD SINIC
Alpha(1,3)-galactosyltransferase knockout swine, tissues and organs
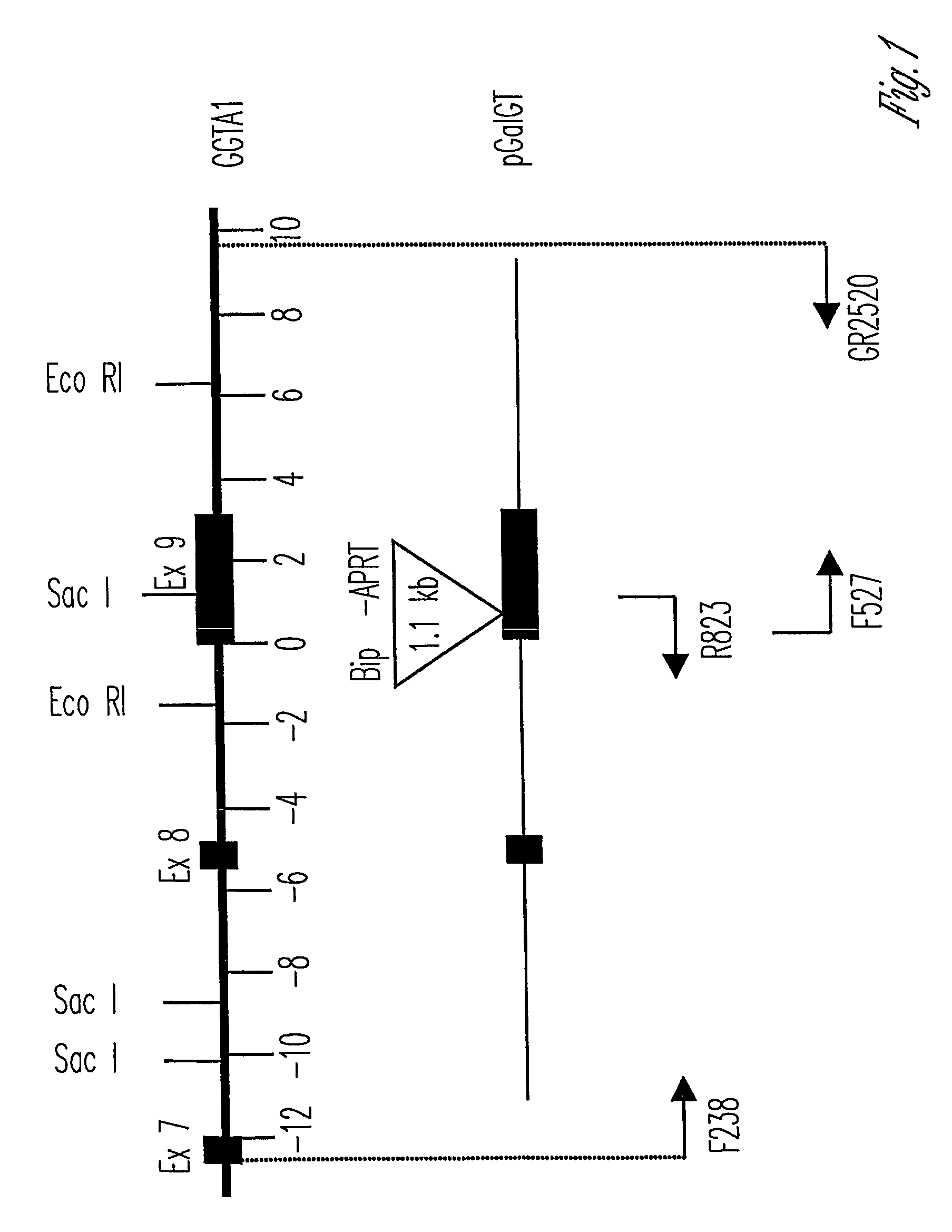
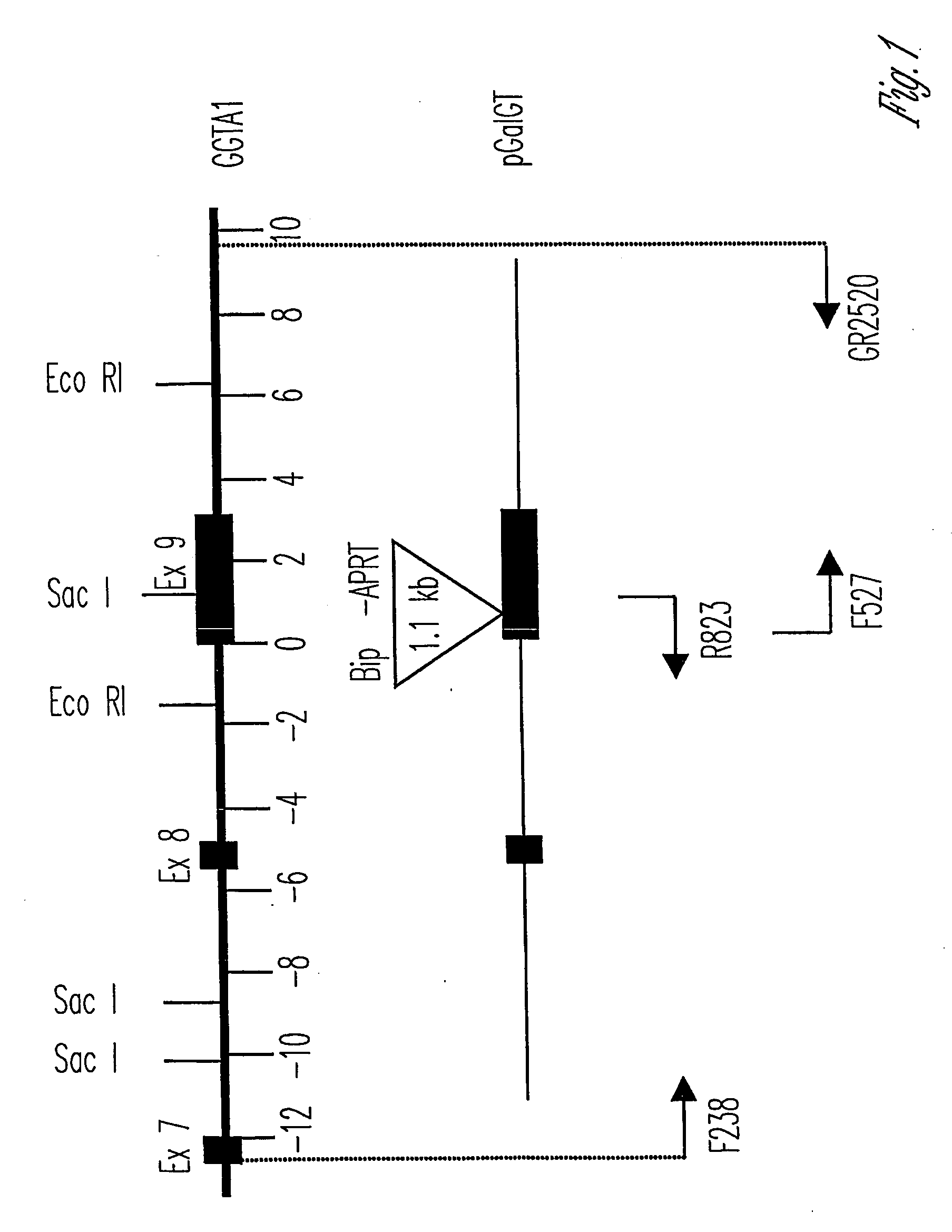
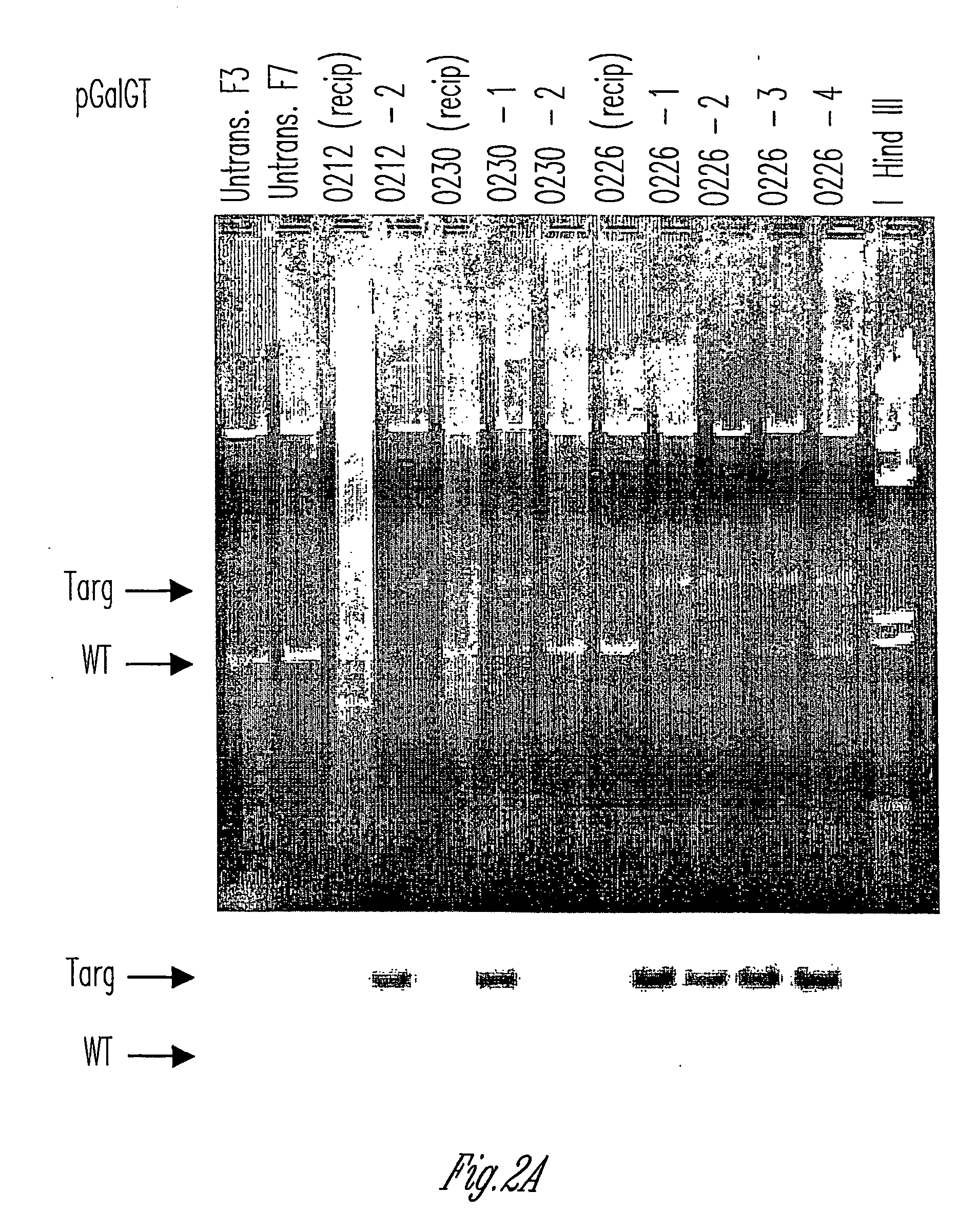
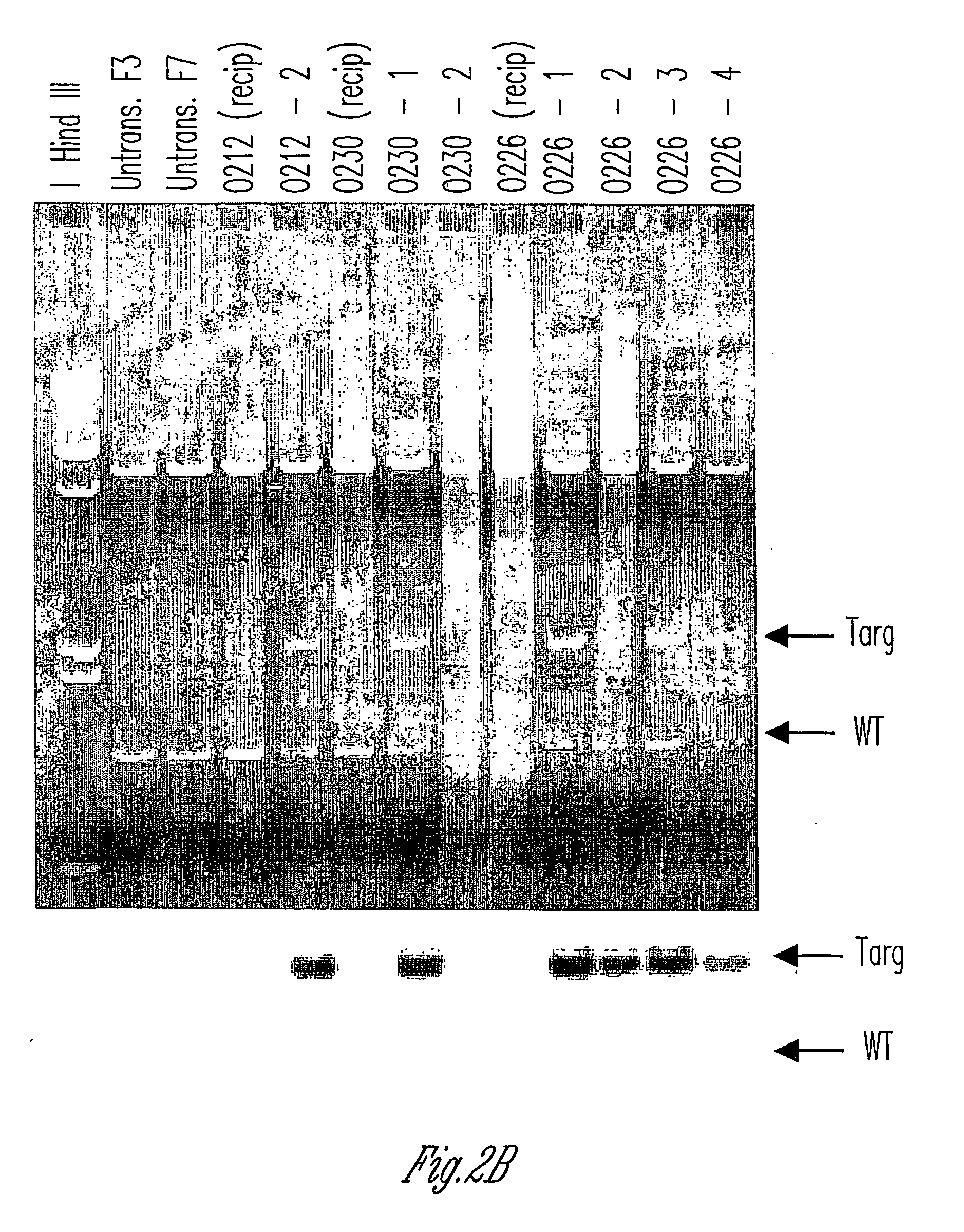
The invention relates to the genetic manipulation of non-human animals. More particularly, the invention relates to genetic manipulation of non-human animals to be used for xenotransplantation. The invention provides viable gene knockout swine including swine in which the α(1,3)-galactosyltransferase gene has been disrupted, methods for making such swine, and methods of using the tissues and organs of such swine for xenotransplantation.
Owner:IMMERGE BIOTHERAPEUTICS +1
Knockout swine and methods for making the same
The invention relates to the genetic manipulation of non-human animals. More particularly, the invention relates to genetic manipulation of non-human animals to be used for xenotransplantation. The invention provides viable gene knockout swine including swine in which the α(1,3)-galactosyltransferase gene has been disrupted, methods for making such swine, and methods of using the tissues and organs of such swine for xenotransplantation.
Owner:IMMERGE BIOTHERAPEUTICS +1
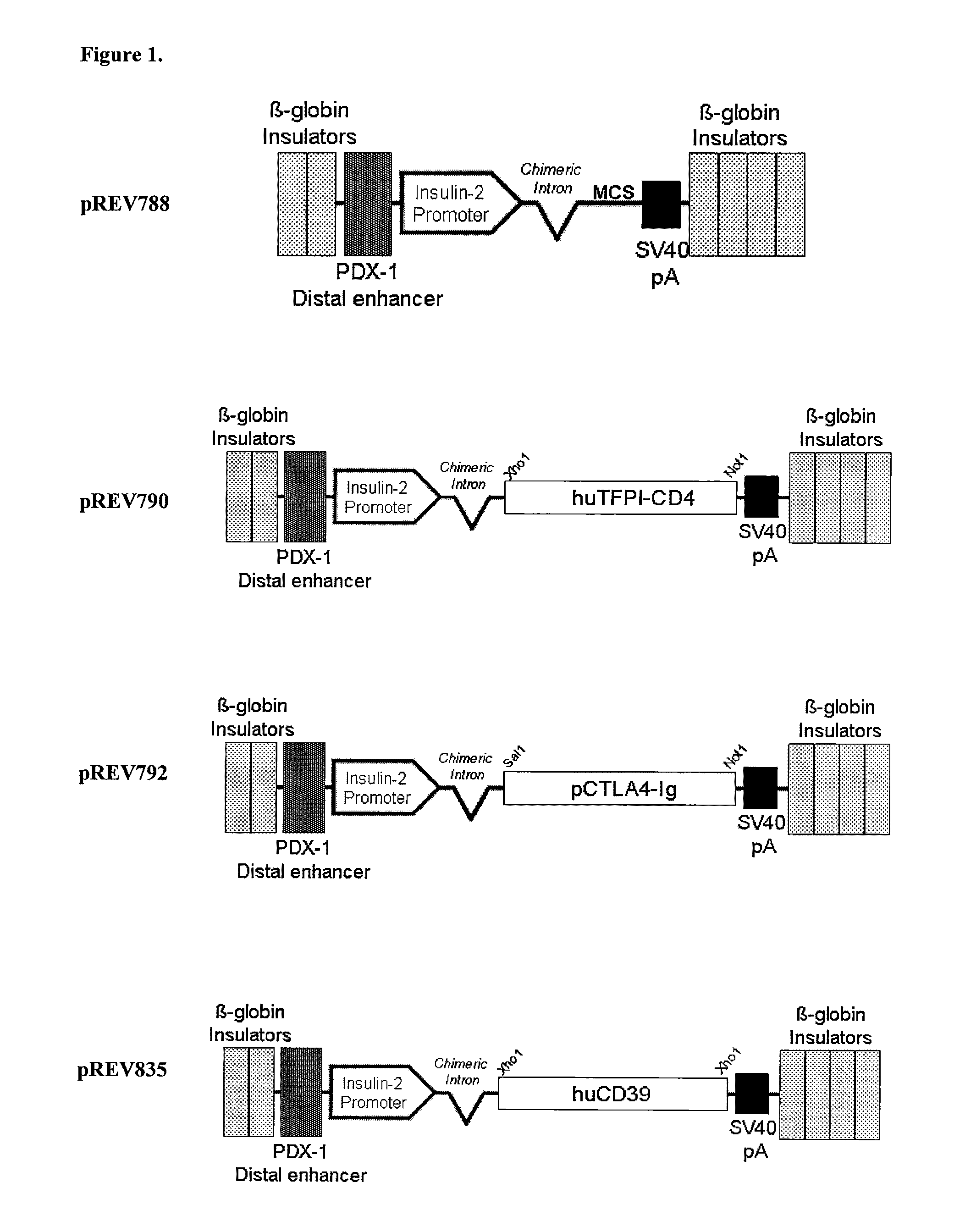
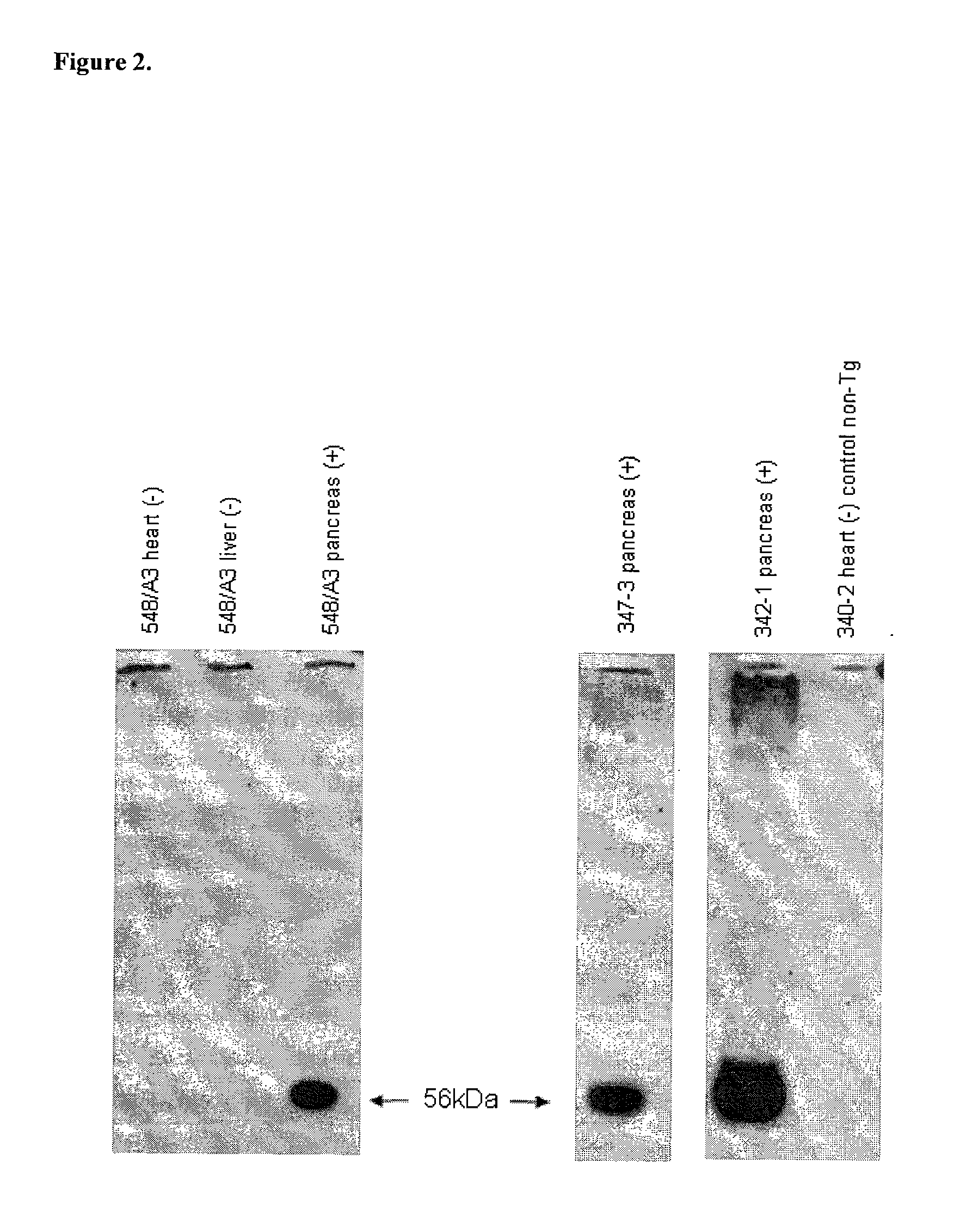
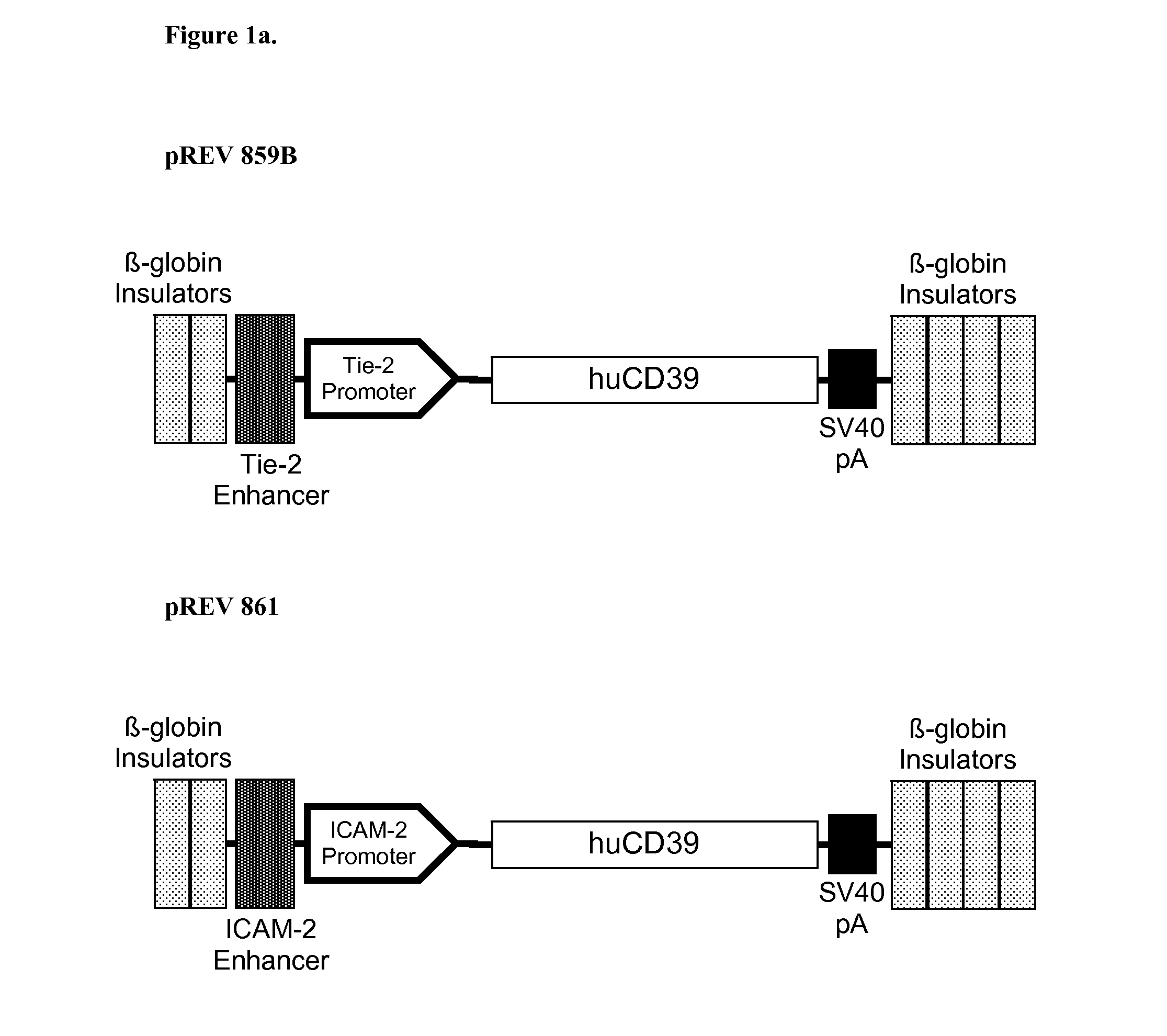
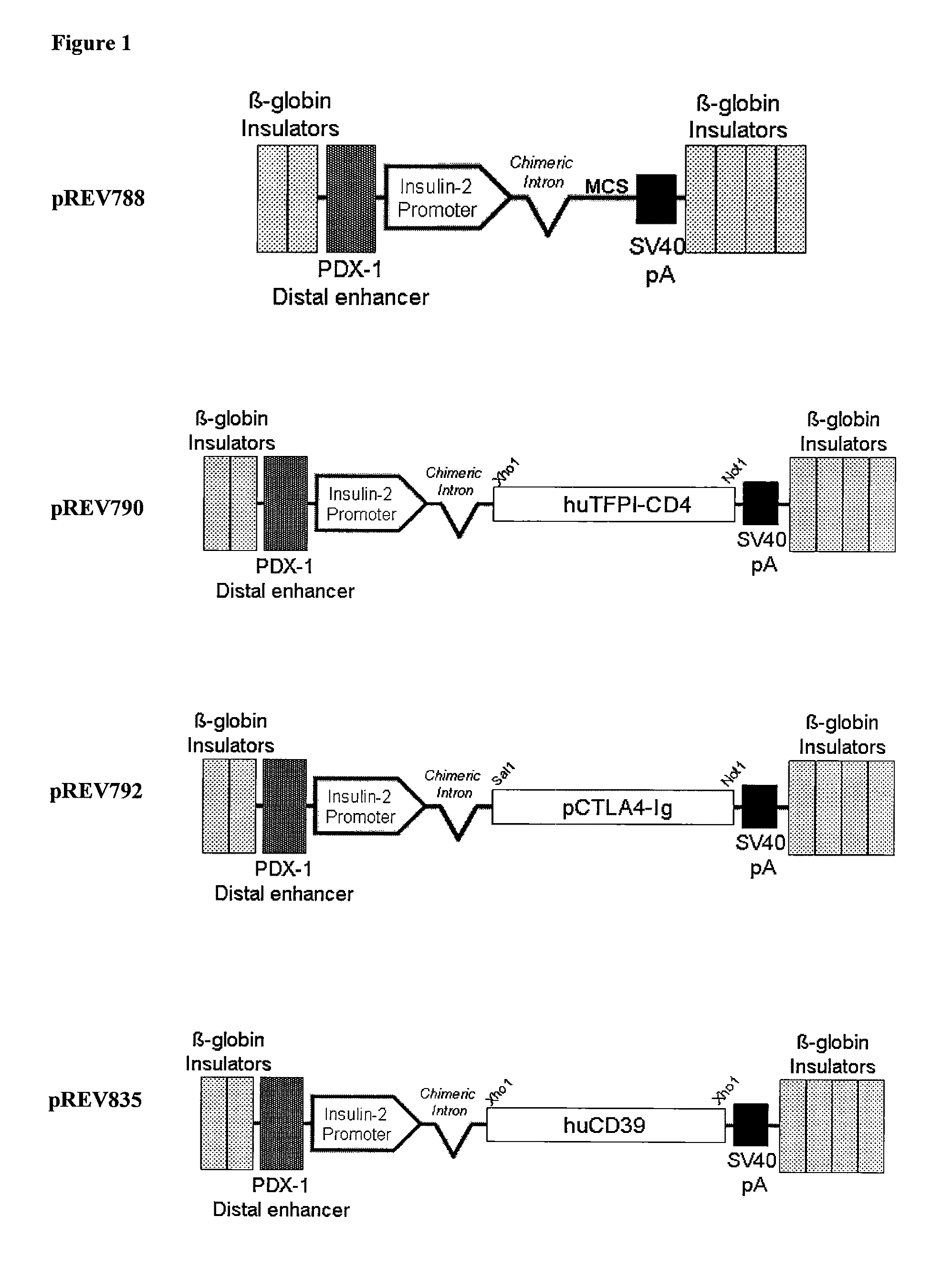
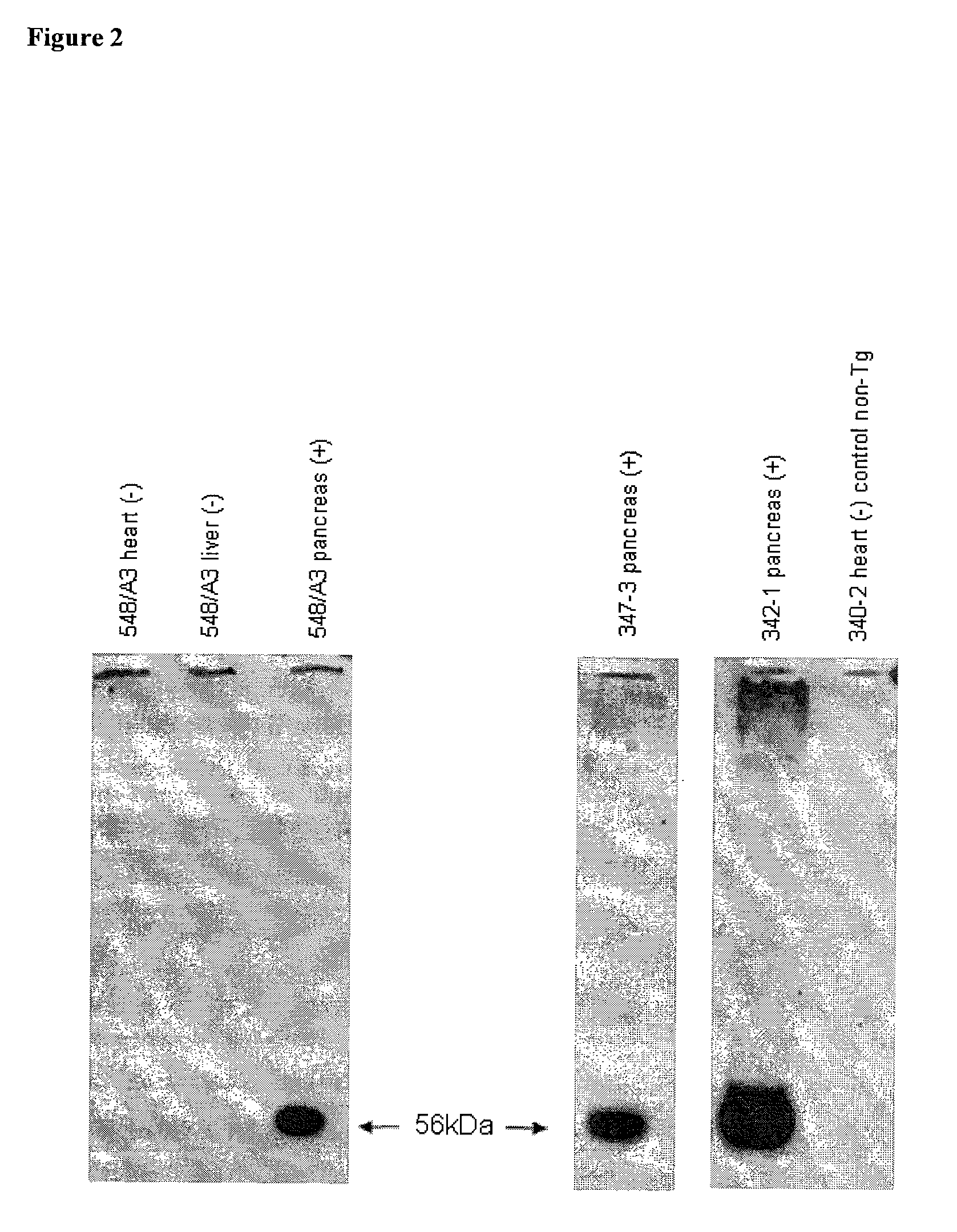
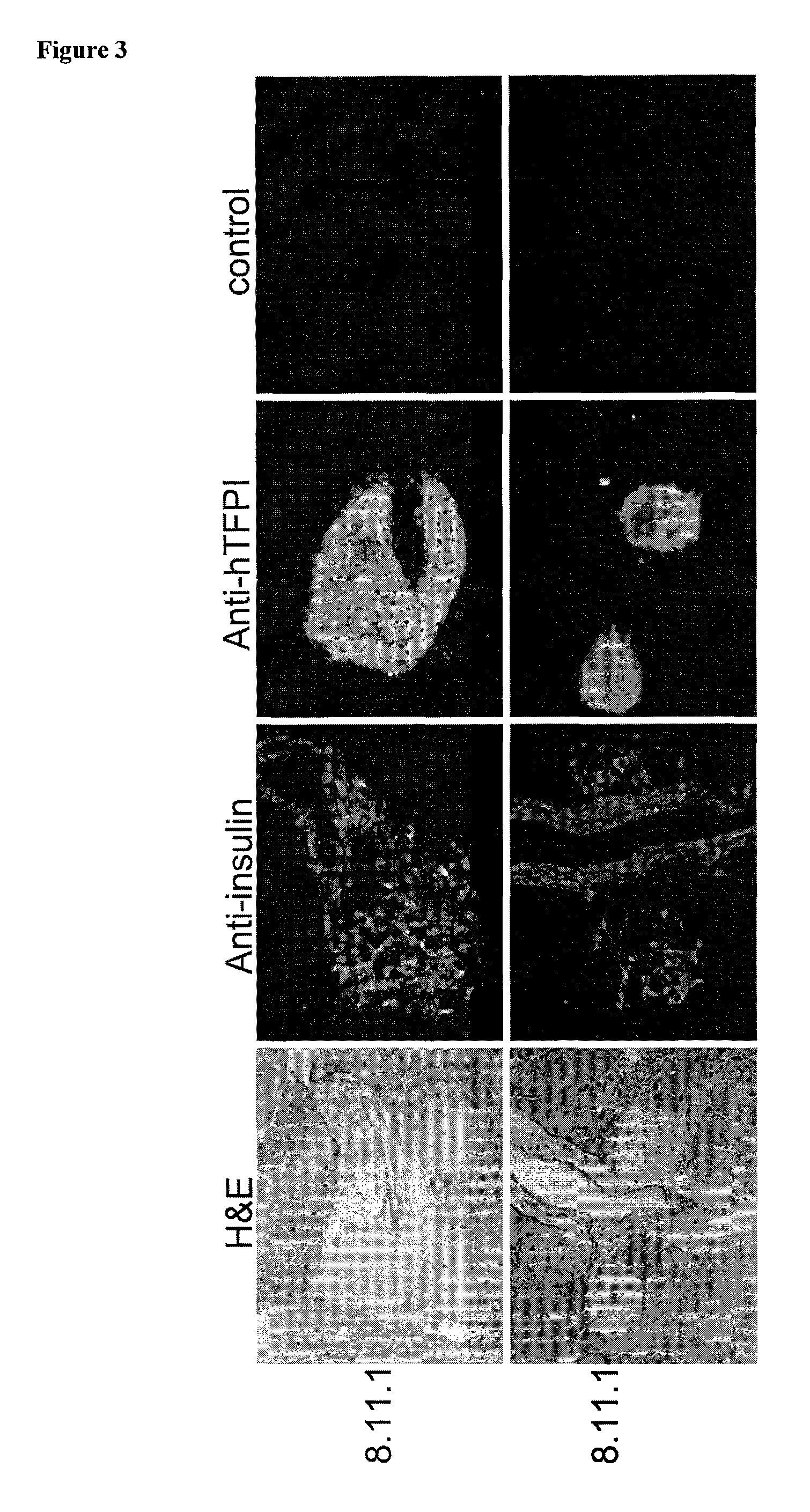
Multi-Transgenic Pigs for Diabetes Treatment
ActiveUS20110038841A1Reduce needInhibit the inflammatory responseBiocideVectorsTransgenePancreatic islets
The present invention provides certain animals, and in particular porcine animals, tissue and cells derived from these, which lack any expression of functional alpha 1,3 galactosyltransferase (αGT) and express one or more additional transgenes which make them suitable donors for pancreatic islet xenotransplantation. Methods of treatment and prevention of diabetes using cells derived from such animals are also provided.
Owner:REVIVICOR INC
Tissue products derived from porcine animals lacking any expression of functional alpha 1,3 galactosyltransferase
InactiveUS8106251B2Prevent rejectionPromote wound healingHeart valvesGenetic material ingredientsTissue repairSkin repair
The present invention provides tissues derived from animals, which lack any expression of functional alpha 1,3 galactosyltransferase (alpha-1,3-GT). Such tissues can be used in the field of xenotransplantation, such as orthopedic reconstruction and repair, skin repair and internal tissue repair or as medical devices.
Owner:REVIVICOR INC
Method and compositions for the detection of protein glycosylation
The invention provides methods and compositions for the rapid and sensitive detection of post-translationally modified proteins, and particularly of those with post-translational glycosylations. The methods can be used to detect O-GlcNAc post-translational modifications on proteins on which such modifications were undetectable using other techniques. In one embodiment, the method exploits the ability of an engineered mutant of β-1,4-galactosyltransferase to selectively transfer an unnatural ketone functionality onto O-GlcNAc glycosylated proteins. Once transferred, the ketone moiety serves as a versatile handle for the attachment of biotin, thereby enabling detection of the modified protein. The approach permits the rapid visualization of proteins that are at the limits of detection using traditional methods. Further, the preferred embodiments can be used for detection of certain disease states, such as cancer, Alzheimer's disease, neurodegeneration, cardiovascular disease, and diabetes.
Owner:CALIFORNIA INST OF TECH
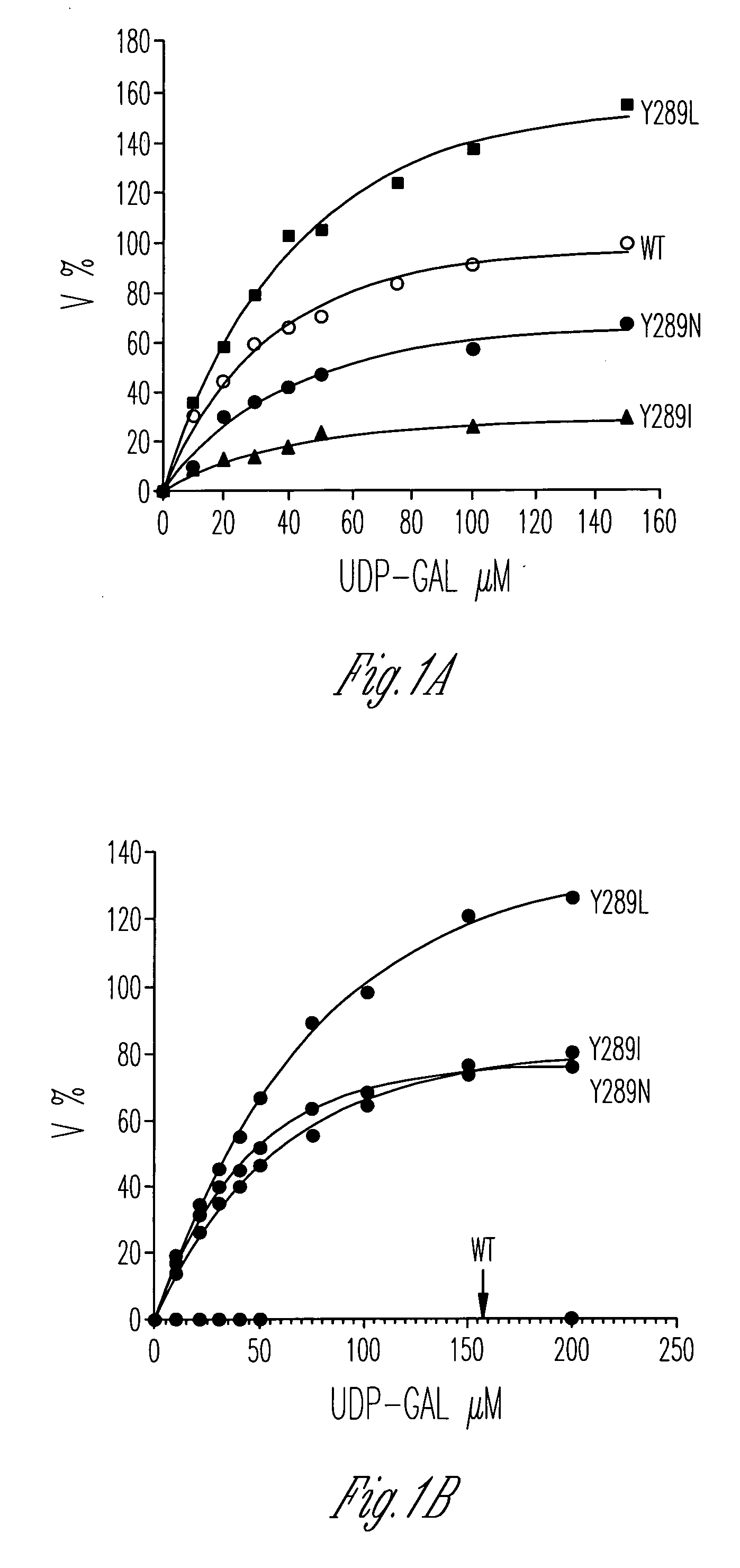
Catalytic domains of beta(1,4)-galactosyltransferase I having altered donor and acceptor specificities, domains that promote in vitro protein folding, and methods for their use
ActiveUS20060084162A1Improving immunogenicitySugar derivativesMicrobiological testing/measurementGreek letter betaMutant
Disclosed are methods and compositions that can be used to synthesize oligosaccharides; mutants of galactosyltransferases having altered donor and acceptor specificity; methods for increasing the immunogenicity of an antigen; and polypeptide stem regions that can be used to promote in vitro folding of polypeptides, such as the catalytic domain from a galactosyltransferase.
Owner:US DEPT OF HEALTH & HUMAN SERVICES
Plant Recombinant Human CTLA4IG and a Method for Producing the Same
InactiveUS20100189717A1Prolong half-life in vivoLow cost mass productionAntibody mimetics/scaffoldsHybrid cell preparationHalf-lifePlant cell
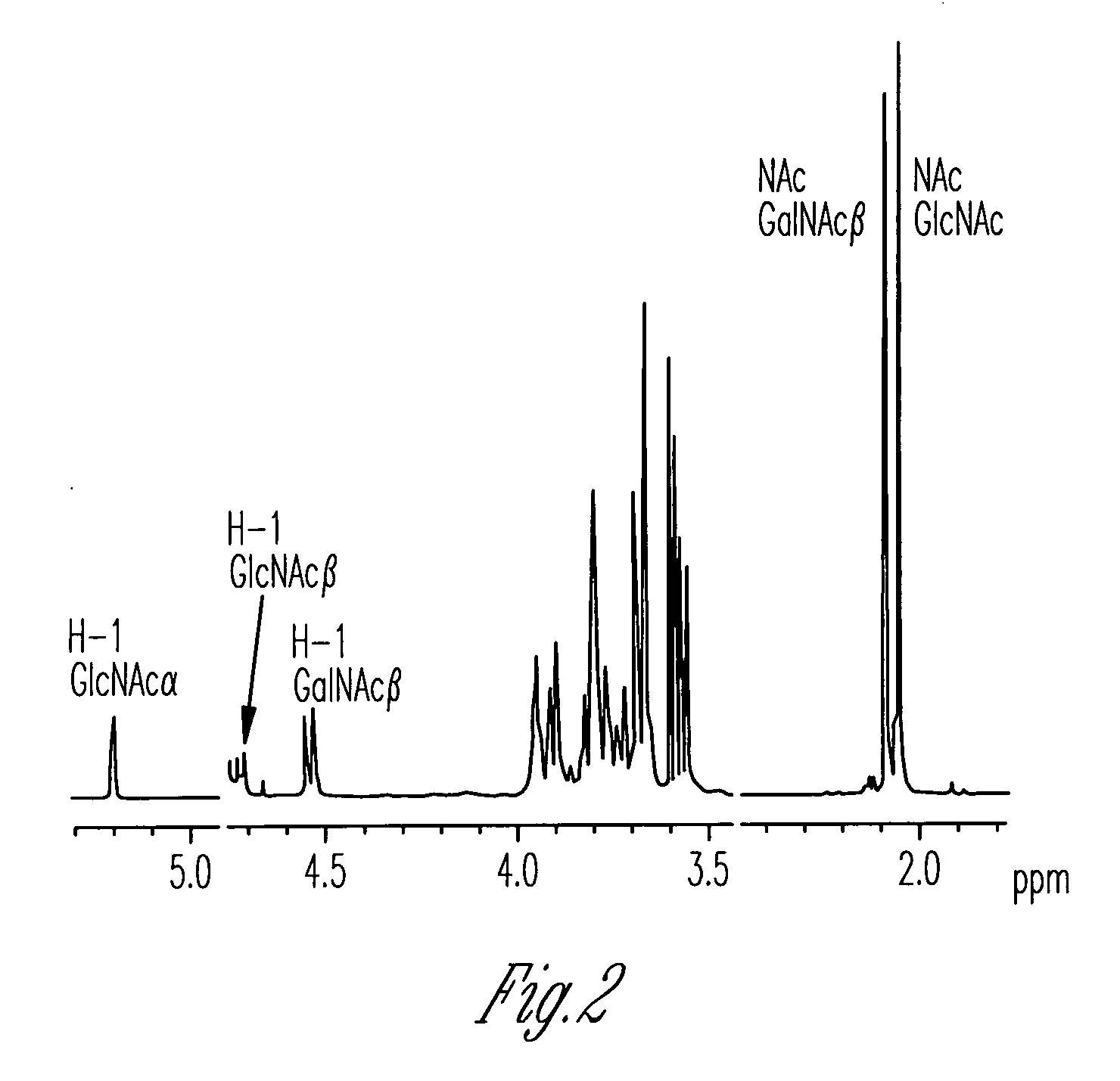
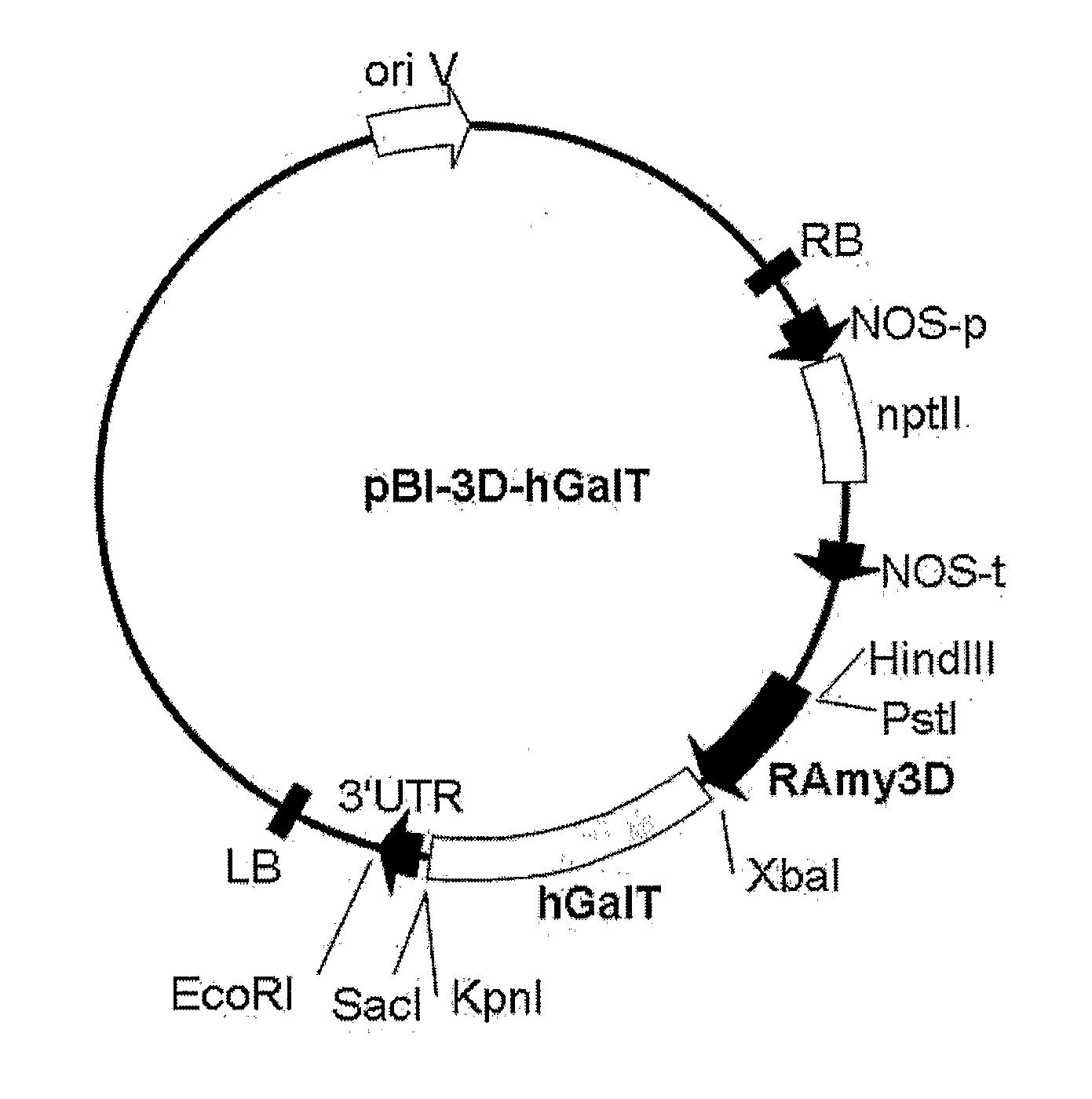
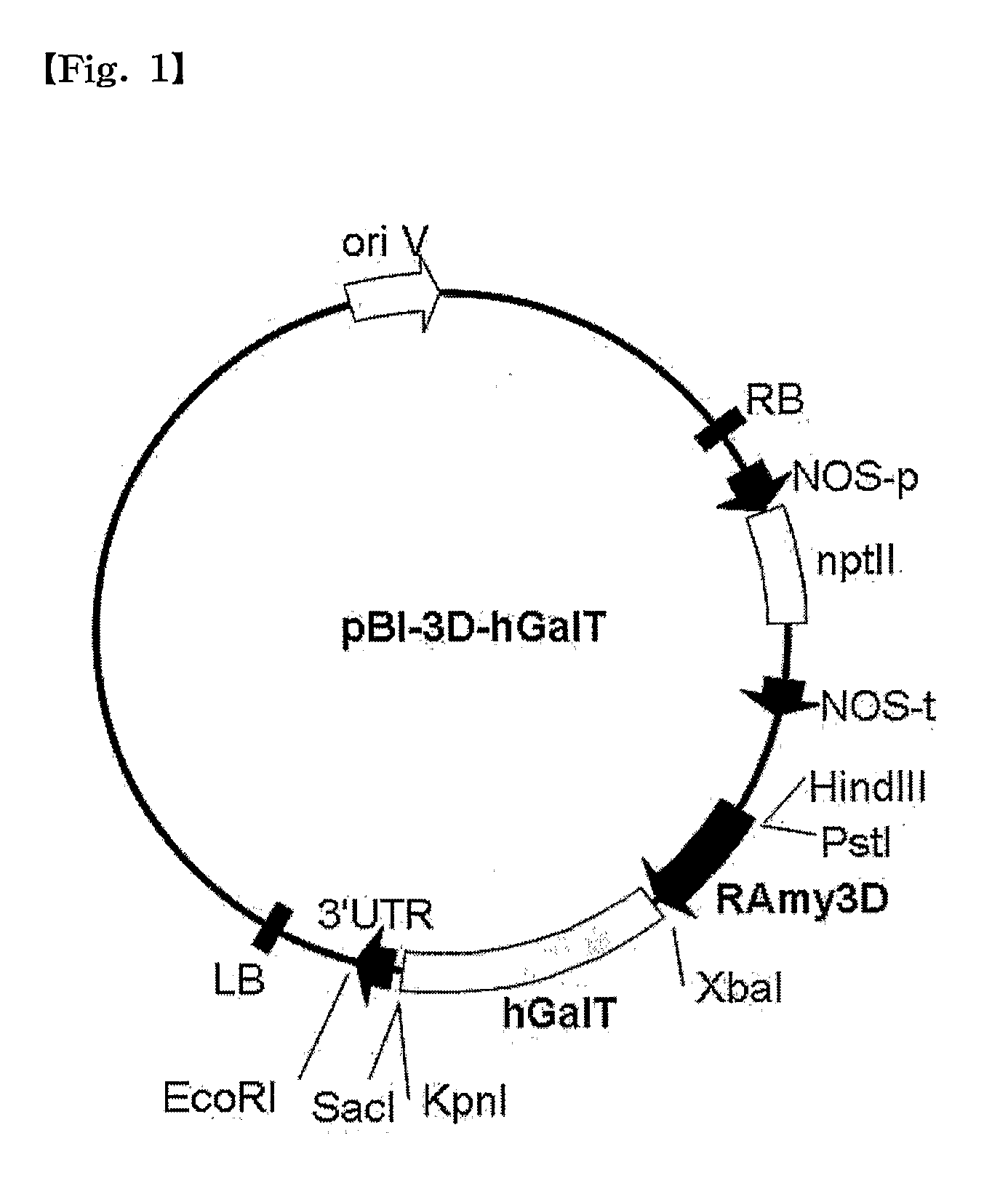
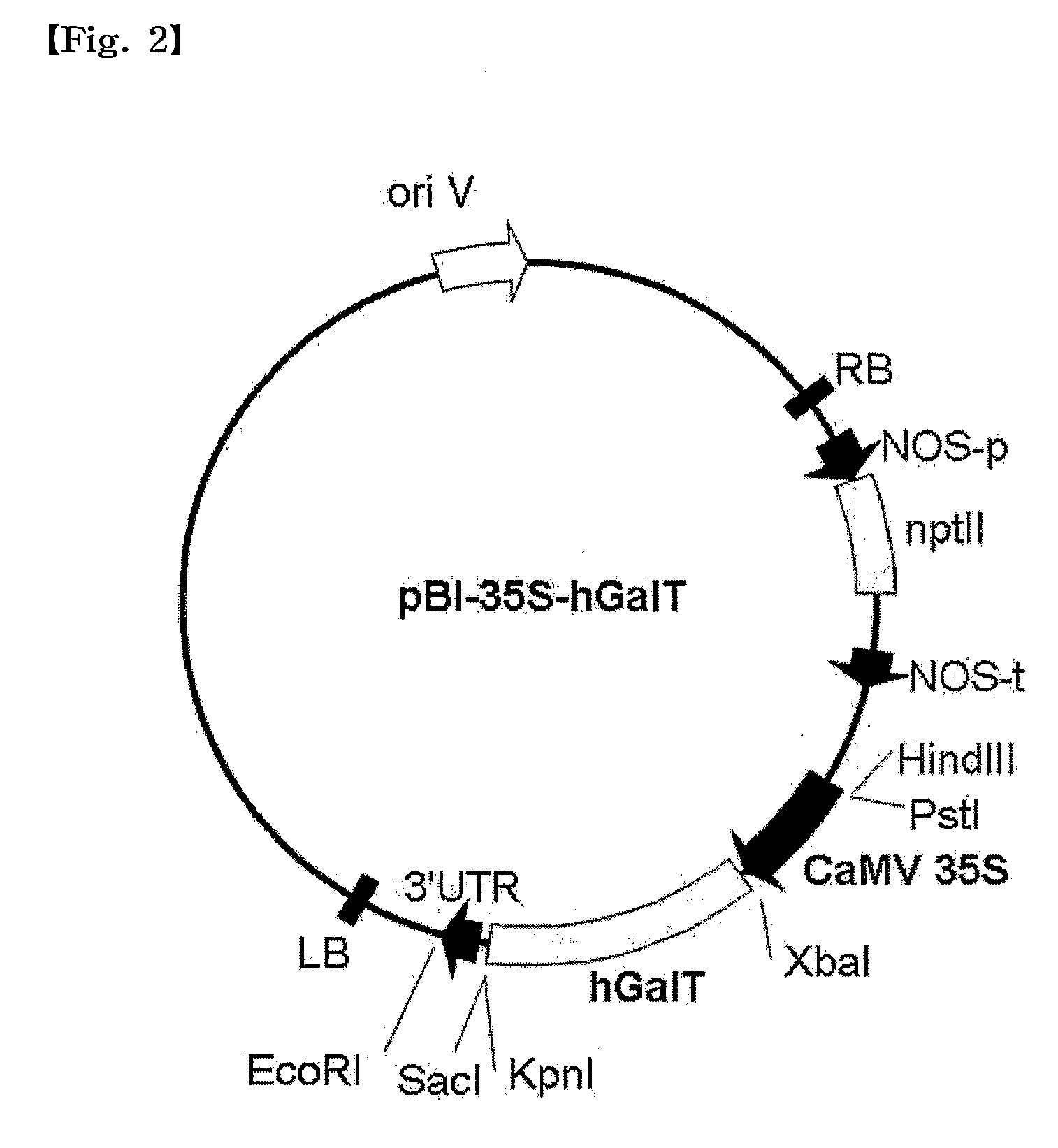
The present invention provides a recombinant vector pBI-3D-hGalT or pBI-35S-hGalT containing a human β1,4-galactosyltransferase gene; a cell line transformed with a recombinant vector pMYN414 containing a cytotoxic T-lymphocyte anti-gen A-immunoglobulin (CTLA4Ig) fusion protein gene and the recombinant vector pBI-3D-hGalT or pBI-355-hGalT; and a method for producing a plant-derived recombinant human CTLA4Ig (CTLA4Igp) fusion protein with a human glycan structure using the same. The plant cell-derived recombinant human CTLA4Ig fusion protein (CTLA4Igp), which has a human glycan structure and is produced according to the present invention, exhibits an improved in vivo half life as compared to conventional plant-derived proteins, due to the presence of a human-like glycan structure. Consequently, the present invention using the plant expression system enables low-cost mass production of a CTLA4Igp fusion protein having an immunosuppressive activity comparable to that of the CTLA4IgM fusion protein expressed in conventional animal cell expression systems.
Owner:BORYUNG PHARMA CO LTD
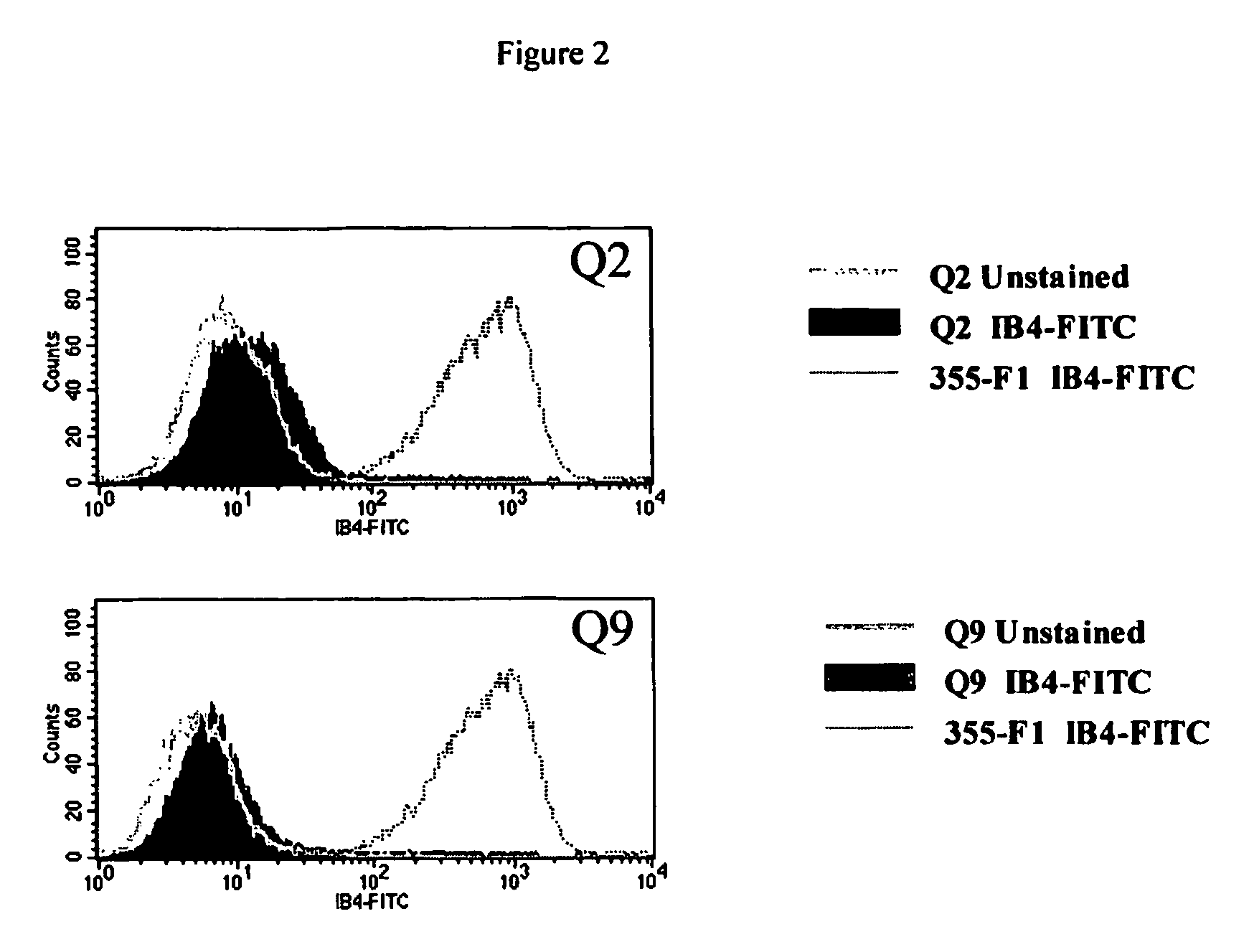
Method to enrich for alpha(1,3)-galactosyltransferase null pig cells
The invention relates to the genetic manipulation of non-human animals. More particularly, the invention relates to genetic manipulation of non-human animals to be used for xenotransplantation. The invention provides a method of selecting GGTA 1 null cells, a viable GGTA 1 null swine, methods for making such swine, and methods of using cells, tissues and organs of such swine for xenotransplantation.
Owner:IMMERGE BIOTHERAPEUTICS
Genetically Modified Pigs for Xenotransplantation of Vascularized Xenografts and Derivatives Thereof
ActiveUS20140017215A1Avoid damageReduce rejectionBiocideGenetic material ingredientsTransgeneBiology
The present invention provides certain donor animals, tissues and cells that are particularly useful for xenotransplantation therapies. In particular, the invention includes porcine animals, as well as tissue and cells derived from these, which lack any expression of functional alpha 1,3 galactosyltransferase (aGT) and express one or more additional transgenes which make these animals suitable donors for xenotransplantation of vascularized xenografts and derivatives thereof. Methods of treatment and using organs, tissues and cells derived from such animals are also provided.
Owner:REVIVICOR INC
Alpha(1,3)-galactosyltransferase null cells, methods of selecting and alpha(1,3)-galactosyl transferase null swine produced therefrom
ActiveUS20060242722A1New breed animal cellsMicrobiological testing/measurementHuman animalCell selection
The invention relates to the genetic manipulation of non-human animals. More particularly, the invention relates to genetic manipulation of non-human animals to be used for xenotransplantation. The invention provides a method of selecting GGTA 1 null cells, a viable GGTA 1 null swine, methods for making such swine, and methods of using cells, tissues and organs of such swine for xenotransplantation.
Owner:IMMERGE BIOTHERAPEUTICS
Porcine Animals Lacking Any Expression of Functional Alpha 1,3 Galactosyltransferase
InactiveUS20120255047A1Eliminating hyperacute rejectionTransferasesHybrid cell preparationCell biologyOrgan of Corti
The present invention is a porcine animal, tissue, organ, cells and cell lines, which lack any expression of functional alpha 1,3 galactosyltransferase (alpha1,3GT). These animals, tissues, organs and cells can be used in xenotransplantation and for other medical purposes.
Owner:PHELPS CAROL J +1
Tissue products derived from animals lacking any expression of functional alpha 1,3 galactosyltransferase
The present invention provides tissues derived from animals, which lack any expression of functional alpha 1,3 galactosyltransferase (alpha-1,3.GT). Such tissues can be used in the field of xenotransplantation, such as orthopedic reconstruction and repair, skin repair and internal tissue repair or as medical devices.
Owner:REVIVICOR INC
Method for modifying flavonoid glycoside compounds with galactosy transferase
The invention discloses a method for modifying flavonoid glycoside compounds with galactosy transferase. The flavonoid glycoside compounds are ubiquitous secondary metabolites in the nature and one of main active ingredients of medicinal plant, and have physiological activities of antibacterium, cancer resistance, antioxidation and the like. However, natural flavonoid glycoside compounds frequently have the defects of poor water solubility, poor drug effect and the like. The method solves the defects of relatively low solubility of the natural flavonoid glycoside compounds and takes the natural flavonoid glycoside compounds as a forerunner to improve the water solubility thereof by a glycosylation reaction, thereby improving the bioavailability thereof. The technical essential of the method is as follows: an enzymatic reaction is applied to the synthesis of polyglycoside of flavonoid compounds. Compared with chemical synthesis methods, the method has higher synthesis efficiency and lower cost. After glycosylation modification, part of drugs can improve drug effect and reduce toxic side effect; therefore, the method provides a new approach for the development of new drugs of the flavonoid compounds.
Owner:NANKAI UNIV
Method of production of sialylated antibodies
InactiveUS20140046032A1Dose be reduced and increasedHigh activityImmunoglobulins against animals/humansFermentationOligosaccharideAntibody
The present invention relates to a method for producing an IgG antibody, wherein at least 80% of the said antibody comprises a complex, bi-antennary oligosaccharide, which contains two sialic acid residues, attached to the Fc domain of the antibody. The said method comprises the steps of introducing a mutation in the Fc domain of the antibody, and expressing the mutant antibody in a cell which expresses a galactosyltransferase and a sialyltransferase activity.
Owner:SANOFI SA
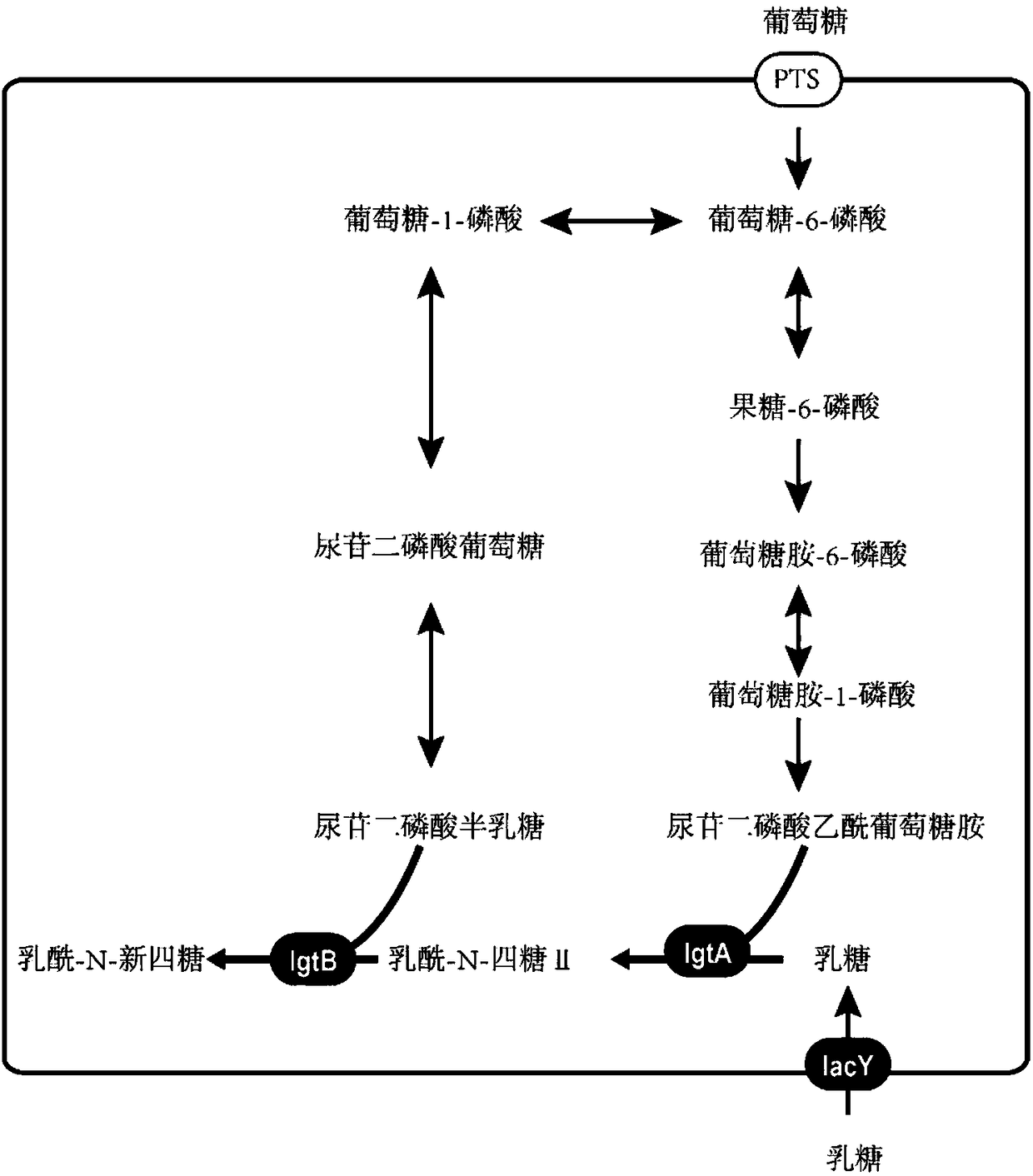
Recombinant bacillus subtilis for synthesizing lactyl-N-neotetraose and construction method and application of recombinant bacillus subtilis
The invention provides recombinant bacillus subtilis for synthesizing lactyl-N-neotetraose. The recombinant bacillus subtilis is integrated with lactose permease genes in a recombinant manner on bacillus subtilis 168 genome and converts plasmids which contain beta-1,3-N-glucoaminotransferase genes and beta-1,4-galactosyltransferase gene in bacillus subtilis 168. Uridine diphosphate galactose and acetylglucosamine uridine diphosphate in a metabolic pathway of the bacillus subtilis are used as precusor substances, lactose only needs to be added exogenously and is transferred into cells under theeffect of lactamase to synthesize the lactyl-N-neotetraose which is secreted to ectoenzyme. The accumulation amount of the lactyl-N-neotetraose synthesized by the recombinant bacillus subtilis reaches 1071 mg / L, and a foundation is laid for production of the lactyl-N-neotetraose through further metabolic engineering modified bacillus subtilis.
Owner:BRIGHT DAIRY & FOOD
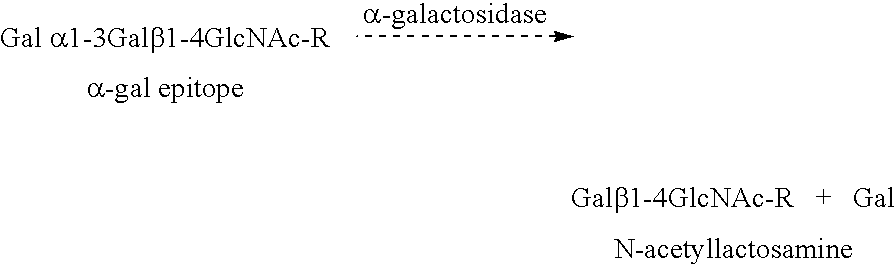
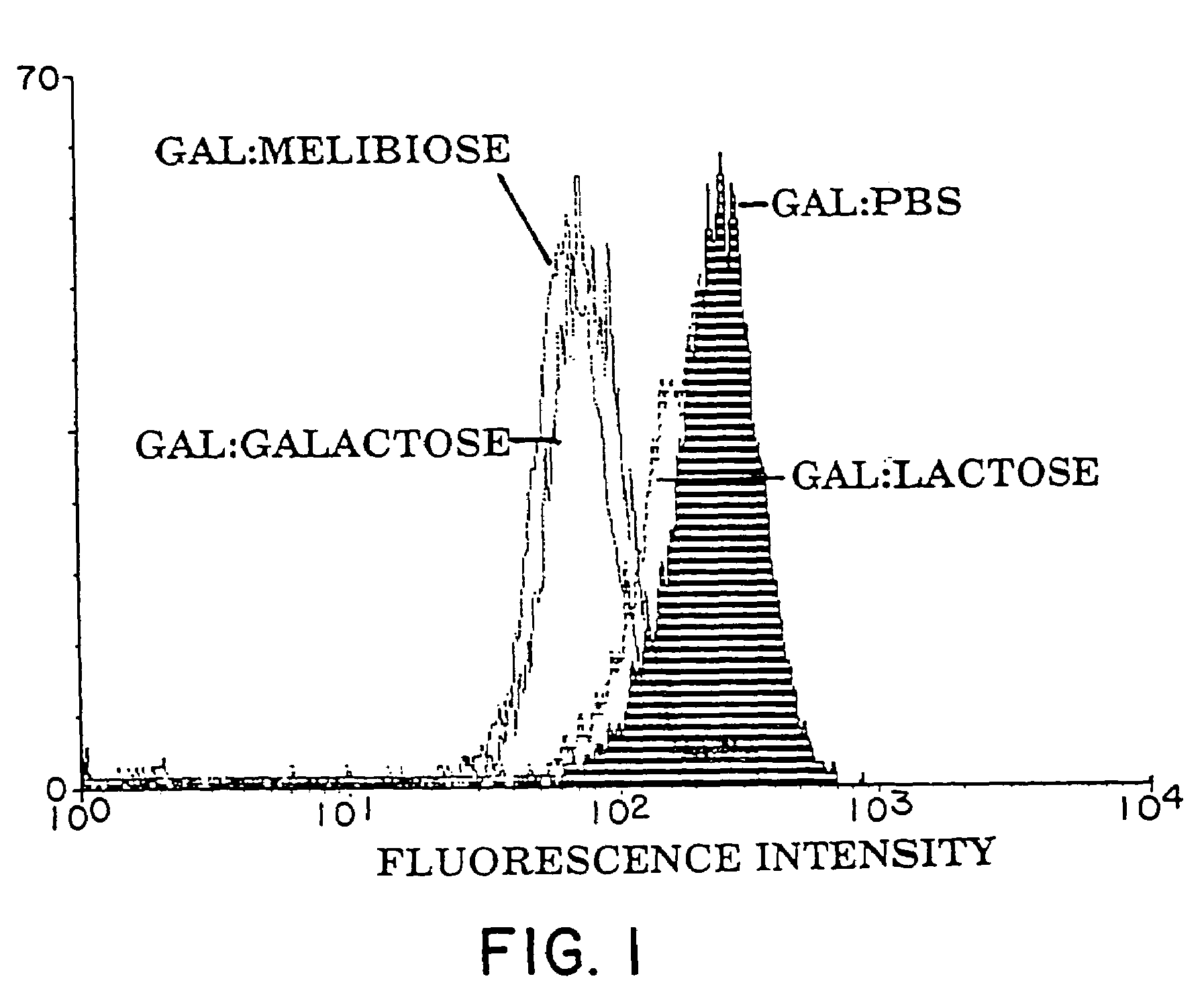
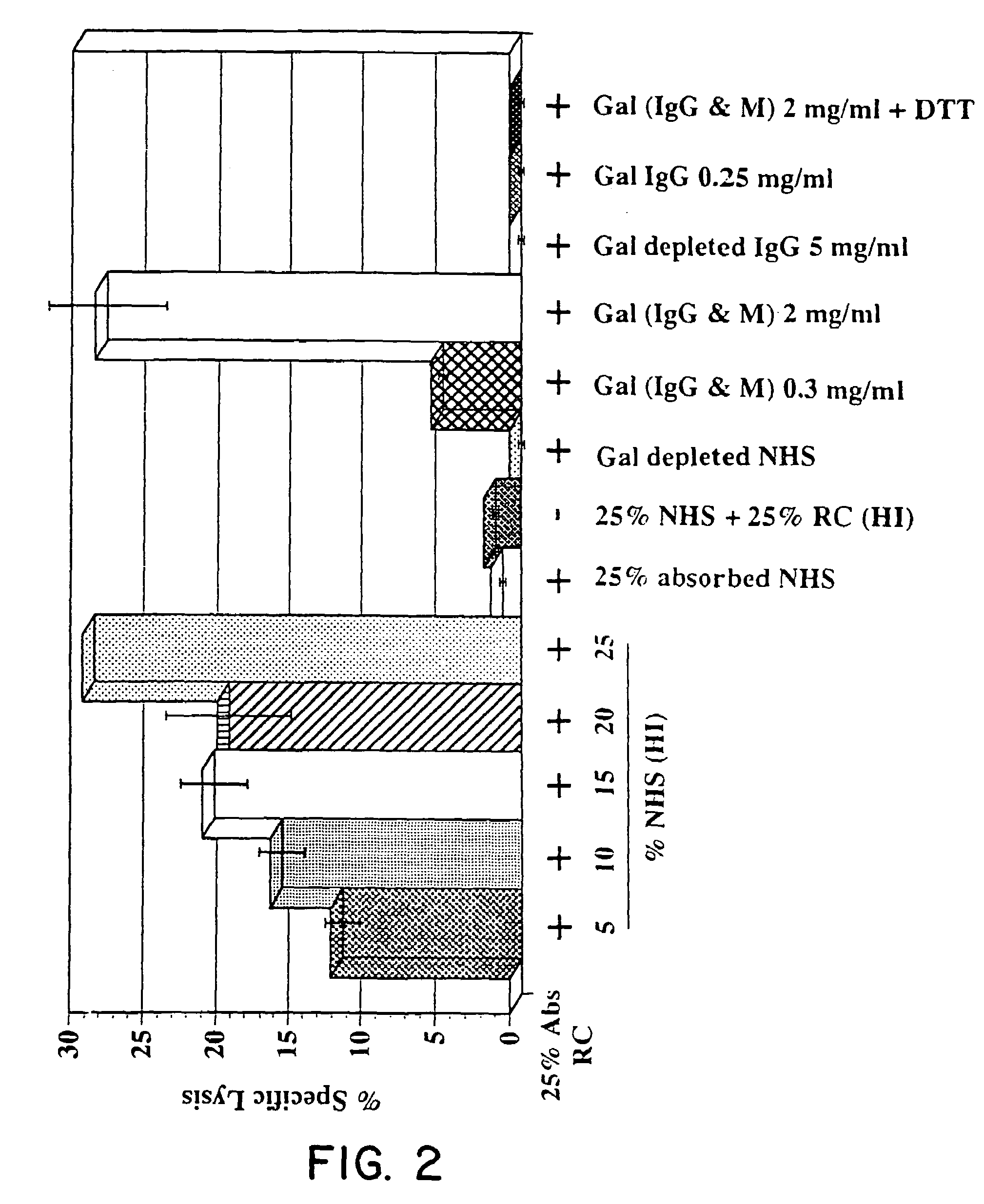
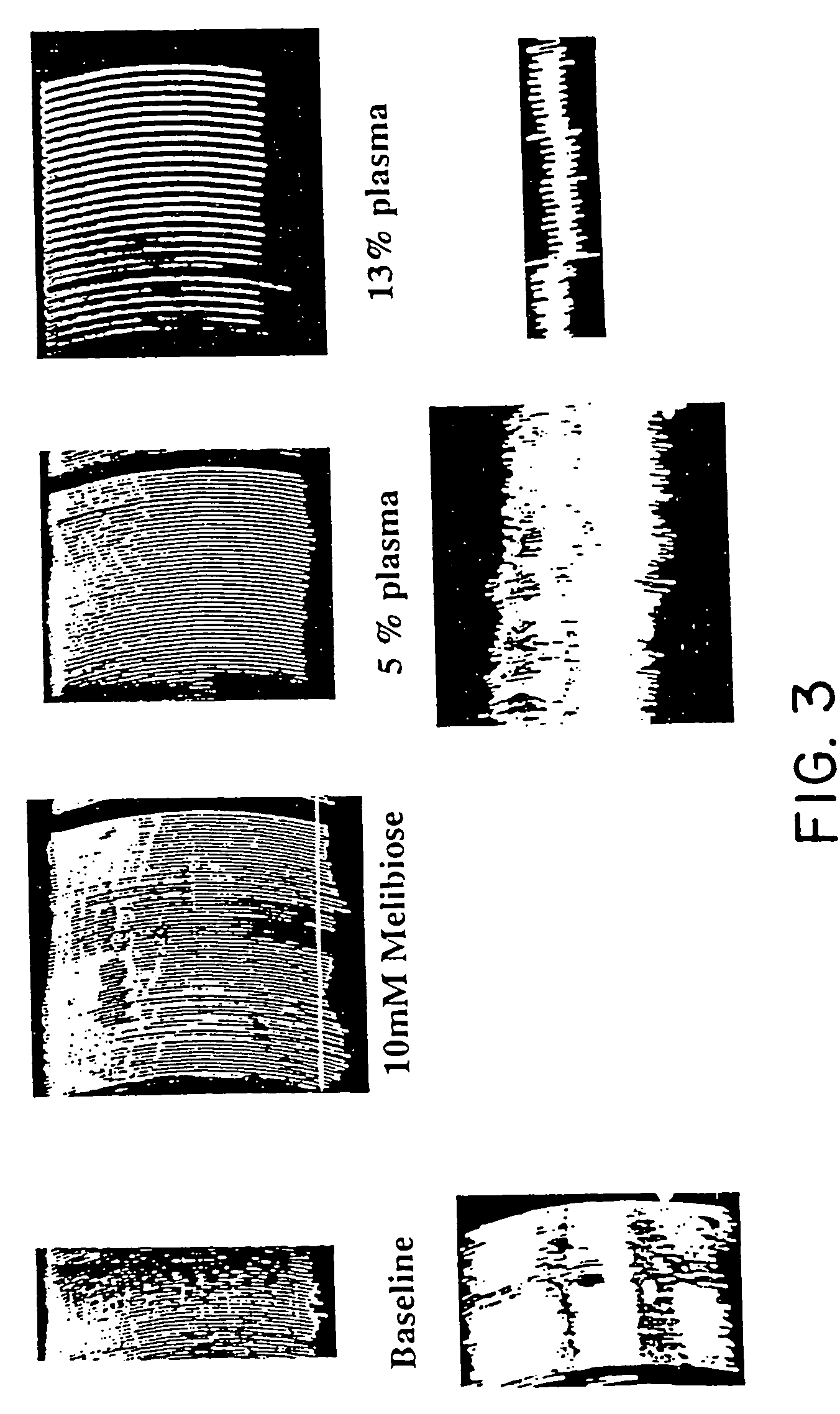
Tumor lesion regression and conversion in situ into autologous tumor vaccines by compositions that result in anti-Gal Antibody Binding
InactiveUS20060251661A1Reduce the overall heightBiocideSugar derivativesAntigenAbnormal tissue growth
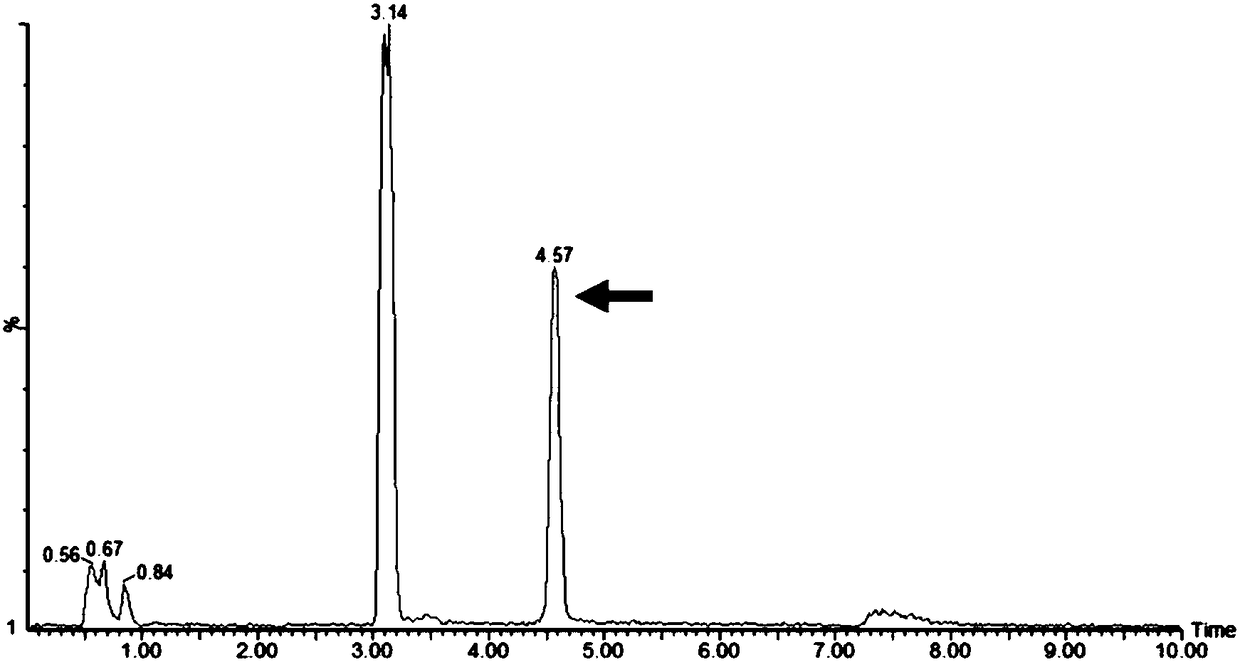
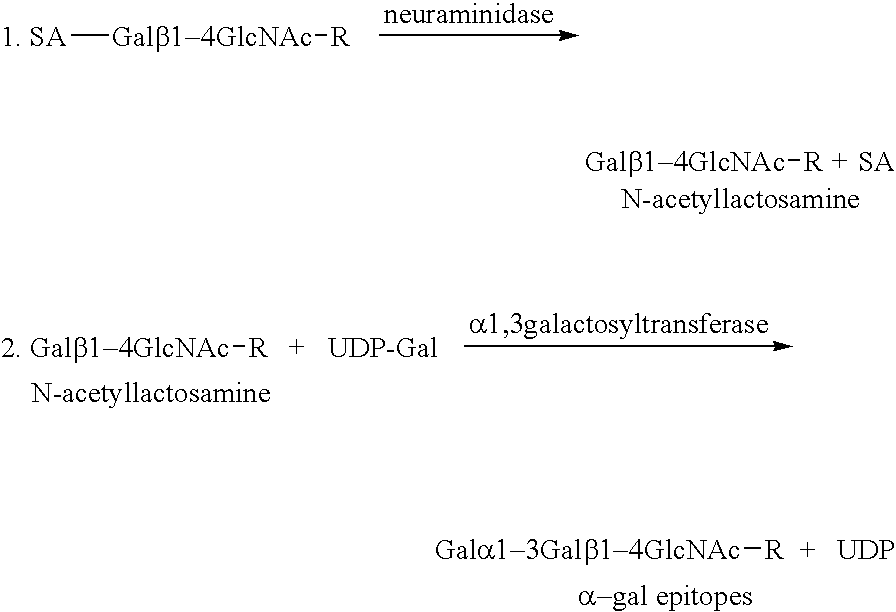
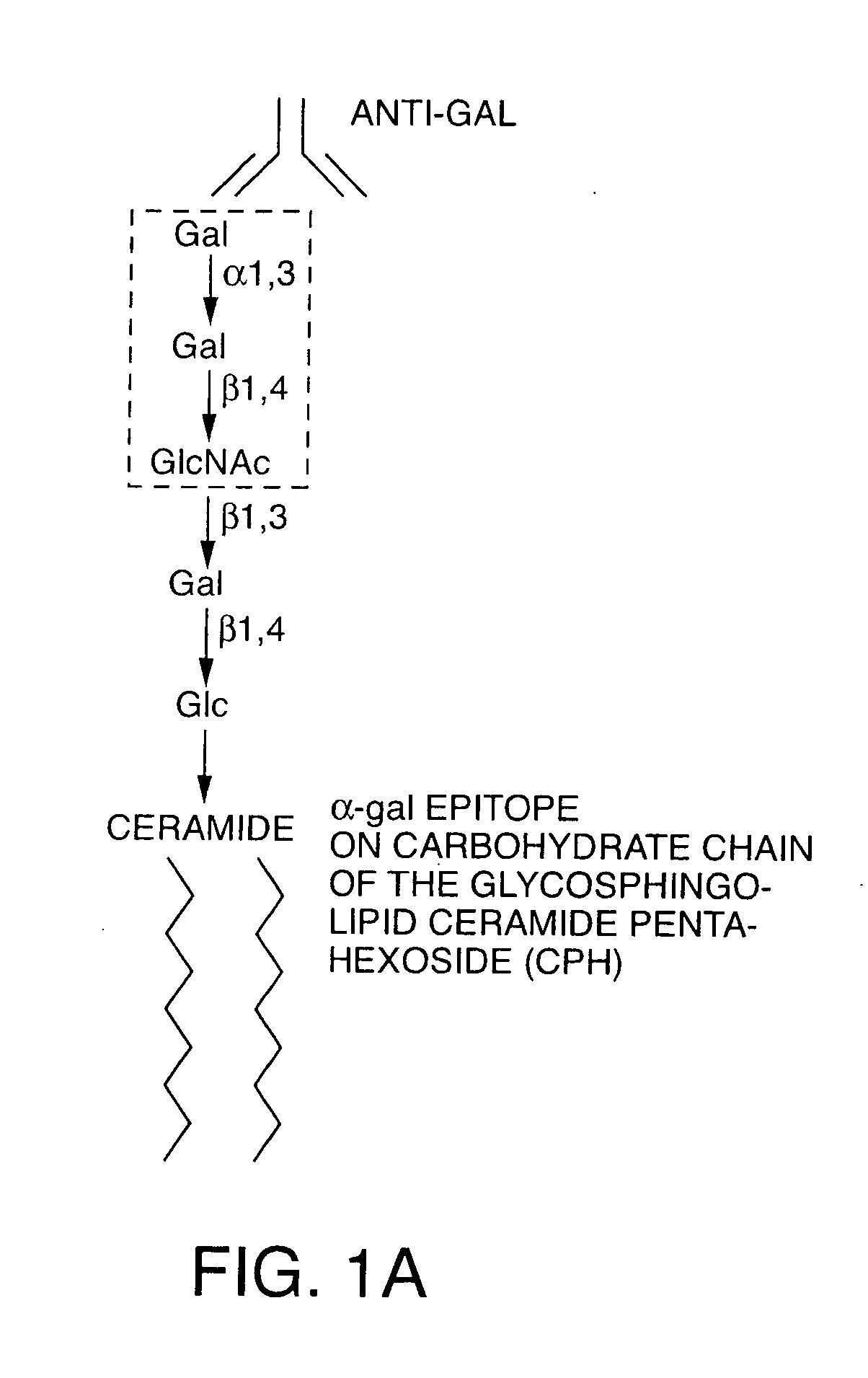
The present invention discloses that an intratumoral injection of: i) glycolipids with α-gal epitope; ii) gene vectors comprising an α1,3galactosyltransferase gene; or iii) a mixture of α1,3galactosyltransferase, neuraminidase, and uridine diphosphate galactose results in tumor regression and / or destruction. Binding of the natural anti-Gal antibody to de novo expressed tumoral α-gal epitopes induces inflammation resulting in an anti-Gal antibody mediated opsonization of tumor cells and their uptake by antigen presenting cells. These antigen presenting cells migrate to draining lymph nodes and activate tumor specific T cells thereby converting the treated tumor lesions into in situ autologous tumor vaccines. This therapy can be applied to patients with multiple lesions and in neo-adjuvant therapy to patients before tumor resection. In addition to the regression and / or destruction of the treated tumor, such a vaccine will help in the immune mediated destruction of micrometastases that are not detectable during the removal of the treated tumor.
Owner:UNIV OF MASSACHUSETTS MEDICAL SCHOOL
Cells deficient in cmp-n-acetylneuraminic acid hydroxylase and/or glycoprotein alpha-1,3-galactosyltransferase
ActiveUS20130004992A1Low immunogenicityImprove bioavailabilityAnimal cellsGenetically modified cellsCMP-N-acetylneuraminic acid hydroxylaseCMP-Neu5Ac
The present invention provides non-human mammalian cell lines that are deficient in CMP-Neu5Ac hydroxylase (Cmah) and / or glycoprotein alpha-1,3-galactosyltransferase (Ggta1). Also provided are methods for using the cells disclosed herein for producing recombinant proteins with human-like patterns of glycosylation.
Owner:SIGMA ALDRICH CO LLC
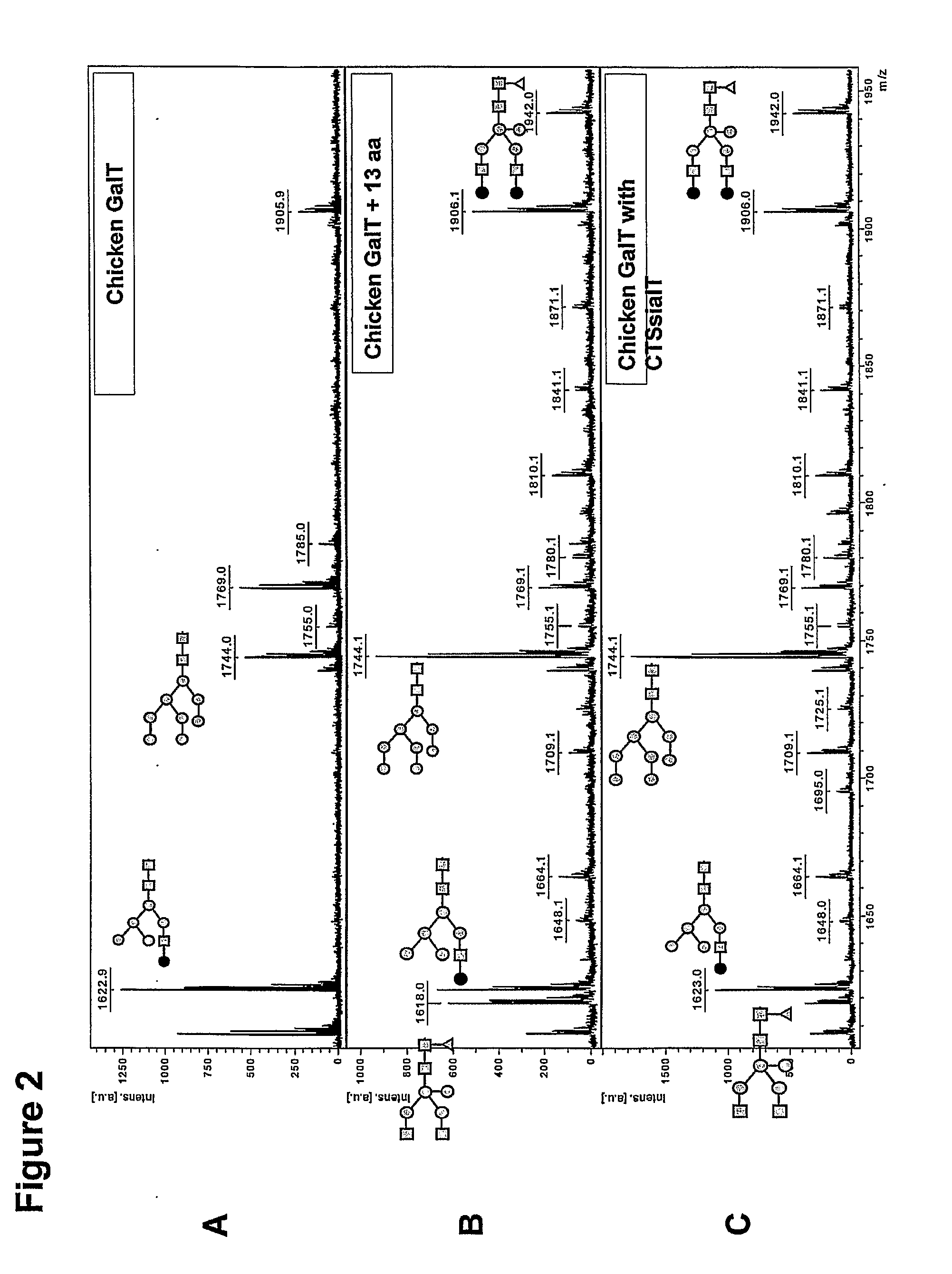
Mammalian-type glycosylation in plants by expression of non-mammalian glycosyltransferases
The present invention relates to non-mammalian β-1,4-galactosyltransferases that can be used in their wild-type or in modified forms. The invention further relates to transformed plants and plant cells expressing non-mammalian β-1,4-galacto-syltransferase and methods to produce glycoproteins with altered and preferably mammalian-type glycosylation. The invention additionally provides nucleic acid molecules and expression vectors of non-mammalian β-1,4-galactosyltransferases.
Owner:STICHTING DIENST LANBOUWKUNDIG ONDERZOEK
Multi-transgenic pigs for diabetes treatment
The present invention provides certain animals, and in particular porcine animals, tissue and cells derived from these, which lack any expression of functional alpha 1,3 galactosyltransferase (αGT) and express one or more additional transgenes which make them suitable donors for pancreatic islet xenotransplantation. Methods of treatment and prevention of diabetes using cells derived from such animals are also provided.
Owner:REVIVICOR INC
Immunochemically modified and sterilized xenografts and allografts
InactiveUS20070010897A1Improve integrityEasy to inactivateNew breed animal cellsTissue regenerationHeterograftsAlcohol
The invention provides an article of manufacture comprising a substantially non-immunogenic xenograft for implantation into humans. The xenograft is a body part dissected from a transgenic 1,3-α-galactosyltransferase gene-deficient animal, wherein the cells of the body part are dead. The invention also provides methods for preparing a xenograft by removing a body part from a transgenic animal, which is 1,3-α-galactosyltransferase gene-deficient, to provide a xenograft; optionally washing the xenograft in saline and alcohol; subjecting the xenograft to a cellular disruption treatment; optionally treating the xenograft with crosslinking agents, and optionally treating the xenograft with a proteoglycan-depleting factor. The invention further provides a method for sterilizing xenograft material, having the steps of obtaining substantially non-immunogenic xenograft material; treating the xenograft material with at least one crosslinking agent; and subjecting the crosslinked xenograft material to a radiation treatment.
Owner:APERION BIOLOGICS
Materials and methods for management of hyperacute rejection in human xenotransplantation
InactiveUS7201899B2Eliminating and reducing hyperacute rejectionReduce and eliminate hyperacute rejection responseOrganic active ingredientsPeptide/protein ingredientsPlant Germ CellsMammal
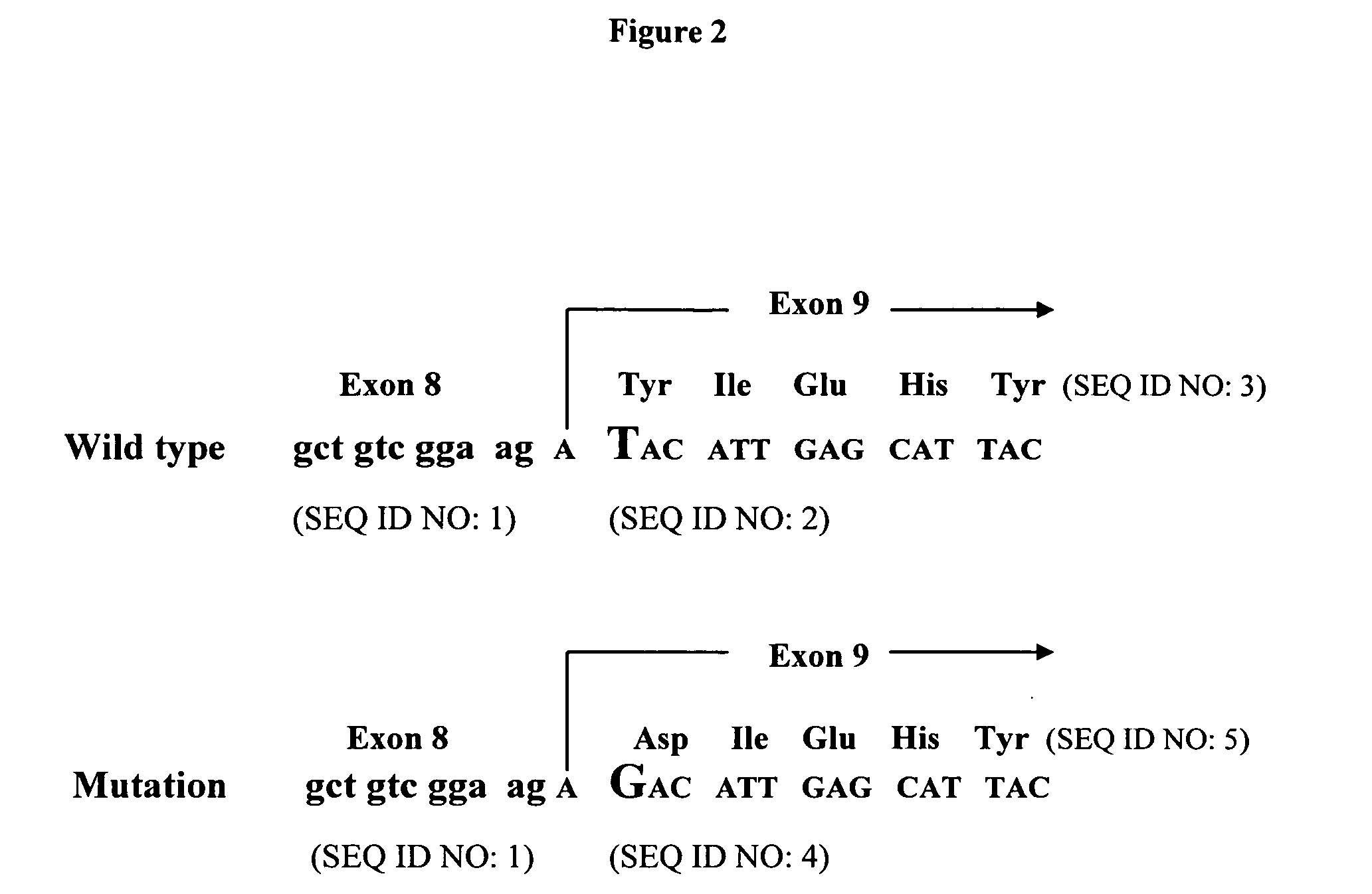
Human pre-formed xenoantibodies play an important role in the hyperacute rejection response in human xenotransplantation. Disclosed are materials and methods for removing or neutralizing such antibodies. Also disclosed are materials and methods for reducing or eliminating the epitopes in the donor organs that are recognized by such antibodies. Such epitopes are formed as the result of activity by the enzyme α-1,3 galactosyltransferase. The porcine gene encoding α-1,3 galactosyltransferase is disclosed, as are materials and methods for inactivating (“knocking out”) the α-1,3 galactosyltransferase gene in mammalian cells and embryos. Included are nucleic acid constructs useful for inactivating the α-1,3 galactosyltransferase gene in a target cell. Also disclosed is a novel leukemia inhibitory factor (T-LIF) that is useful for maintenance of embryonic stem cells and primordial germ cells in culture.
Owner:BRESAGEN +1
Mammalian-type glycosylation in plants by expression of non-mammalian glycosyltransferases
Owner:STICHTING DIENST LANBOUWKUNDIG ONDERZOEK
Porcine Animals Lacking Any Expression of Functional Alpha 1,3 Galactosyltransferase
InactiveUS20150106959A1Eliminating hyperacute rejectionTransferasesHybrid cell preparationCell biologyOrgan of Corti
The present invention is a porcine animal, tissue, organ, cells and cell lines, which lack any expression of functional alpha 1,3 galactosyltransferase (alpha1,3GT). These animals, tissues, organs and cells can be used in xenotransplantation and for other medical purposes.
Owner:REVIVICOR INC
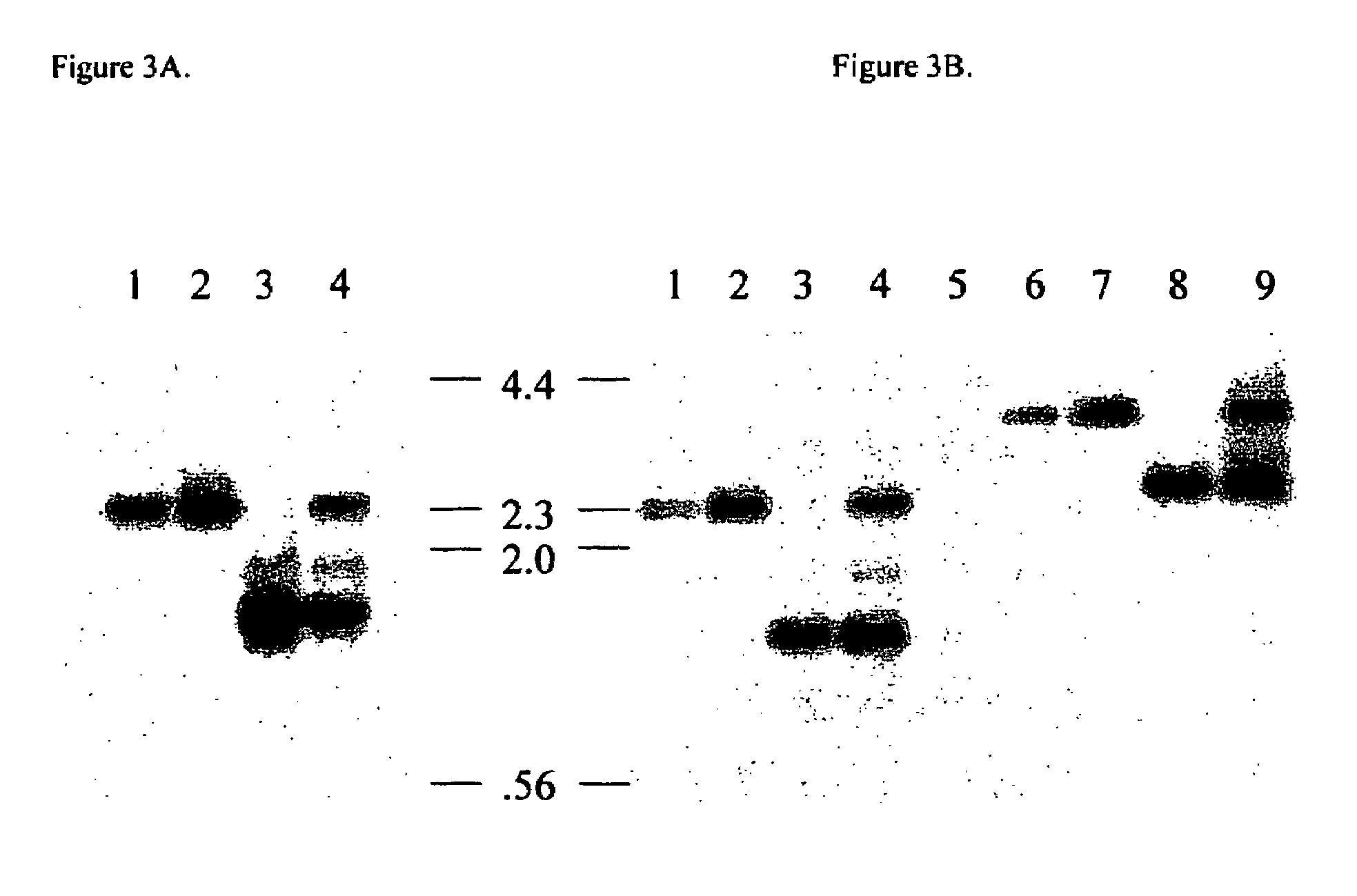
Alpha1-3 galactosyltransferase gene and promoter
The present invention provides a recombinant expression cassette comprising an α1-3 galactosyltransferase promoter operably linked to a polynucleotide for expression. The invention also provides a recombinant mutating cassette comprising a region of homology to an α1-3 galactosyltransferase genomic sequence. The cassettes can be employed to express foreign genes or to disrupt the native α1-3 galactosyltransferase genomic sequence, particularly within an animal. Thus, the invention also provides transgenic animals and methods for their production and use.
Owner:UNIVERSITY OF PITTSBURGH
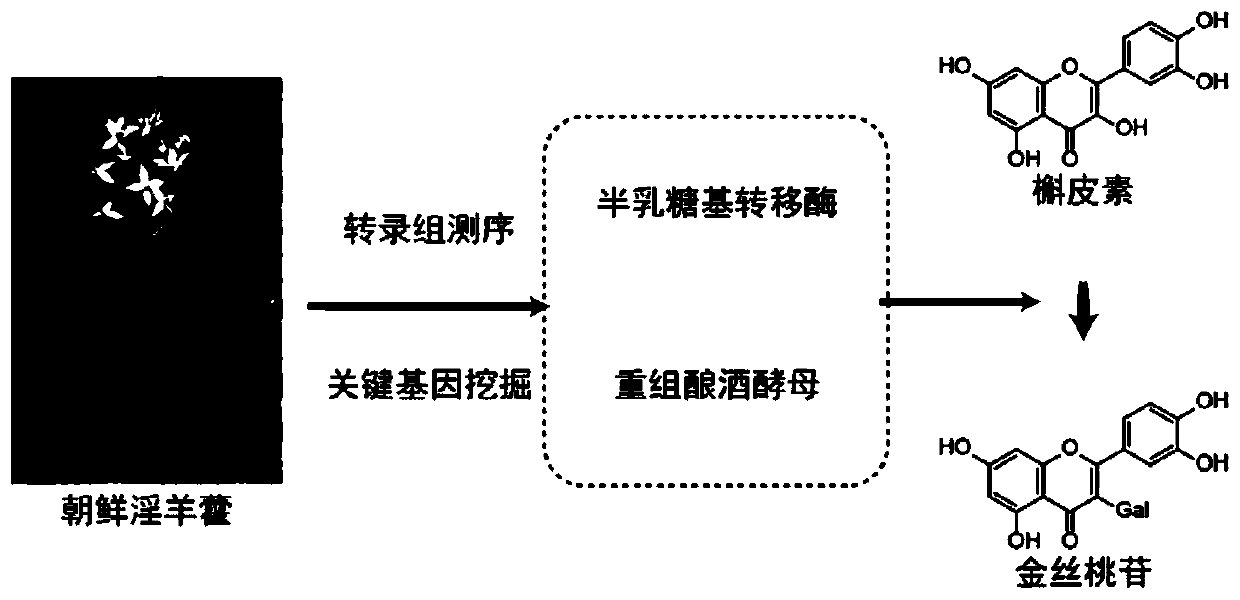
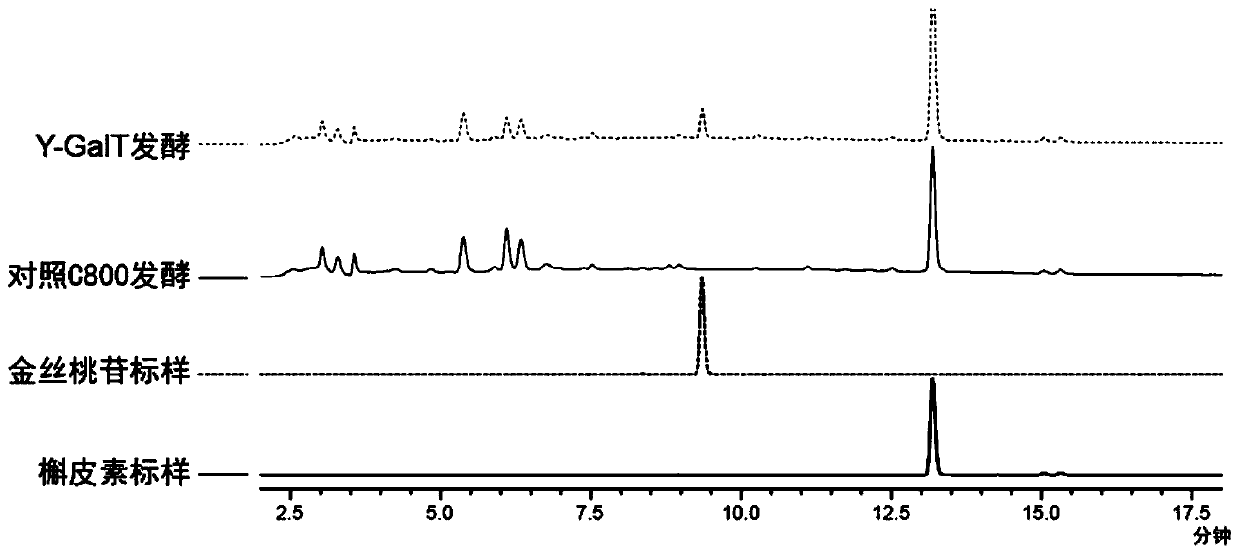
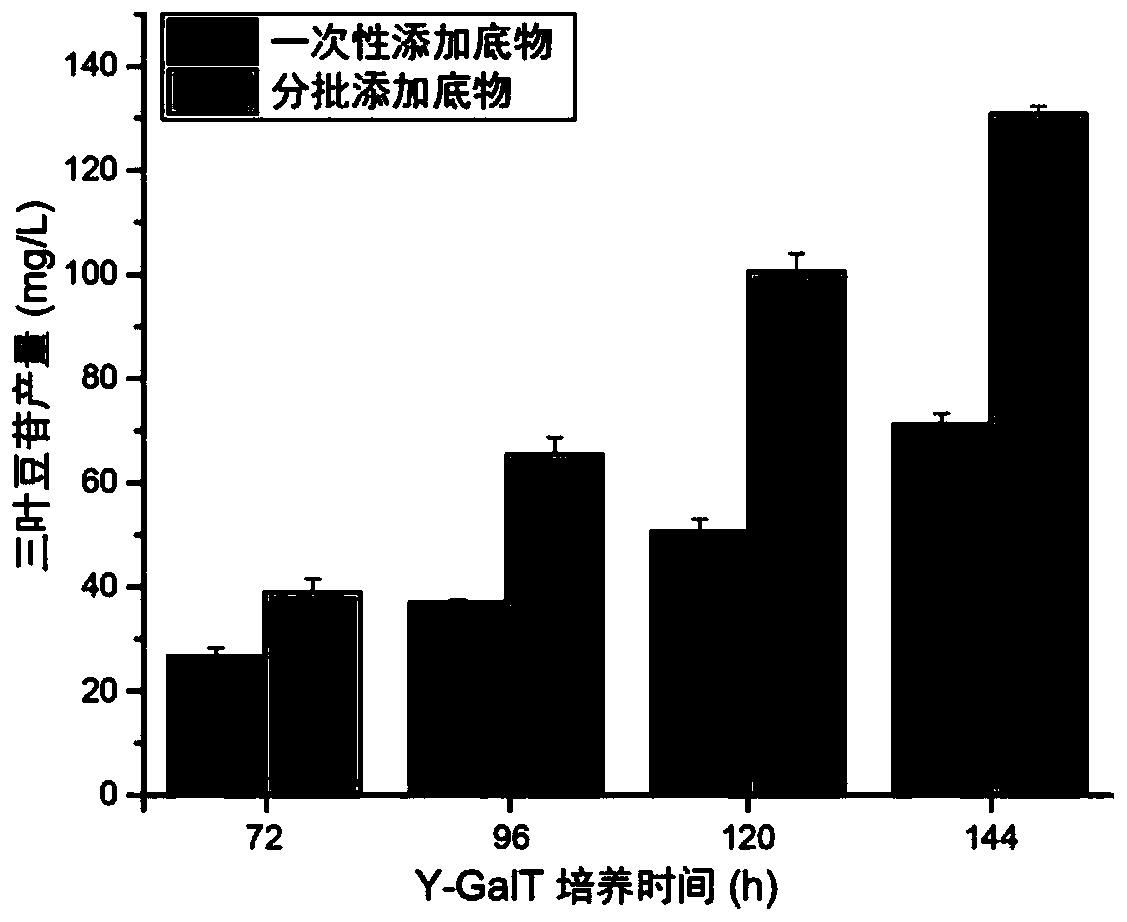
Epimedium source galactosyl transferase and application of epimedium source galactosyl transferase to preparation of hyperoside
The invention discloses epimedium source galactosyl transferase and an application of the epimedium source galactosyl transferase to the preparation of hyperoside, and belongs to the technical field of gene engineering and biological medicines. The invention provides galactosyl transferase and an application of recombinant saccharomyces cerevisiae expressing the galactosyl transferase to the catalytic synthesis of flavonoid compounds. The constructed recombinant saccharomyces cerevisiae can be enabled to catalyze the transfer of UDP-galactose to various flavone substrates such as quercetin, kaempferol, myricetin, dihydroquercetin, dihydrokaempferol, dihydromyricetin, fisetin, morin and icaritin.
Owner:JIANGNAN UNIV
Antitumor vaccination using allogeneic tumor cells expressing alpha (1,3)-galactosyltransferase
The invention relates to methods and compositions for causing the selective targeting and killing of tumor cells. Through ex vivo gene therapy protocols tumor cells are engineered to express an α (1,3) galactosyl epitope. The cells are then irradiated or otherwise killed and administered to a patient. The α galactosyl epitope causes opsonization of the tumor cell enhancing uptake of the opsonized tumor cell by antigen presenting cells which results in enhanced tumor specific antigen presentation. The animal's immune system thus is stimulated to produce tumor specific cytotoxic cells and antibodies which will attack and kill tumor cells present in the animal.
Owner:CENT IOWA HEALTH SYST
Catalytic domains of beta(1,4)-galactosyltransferase i having altered metal ion specificity
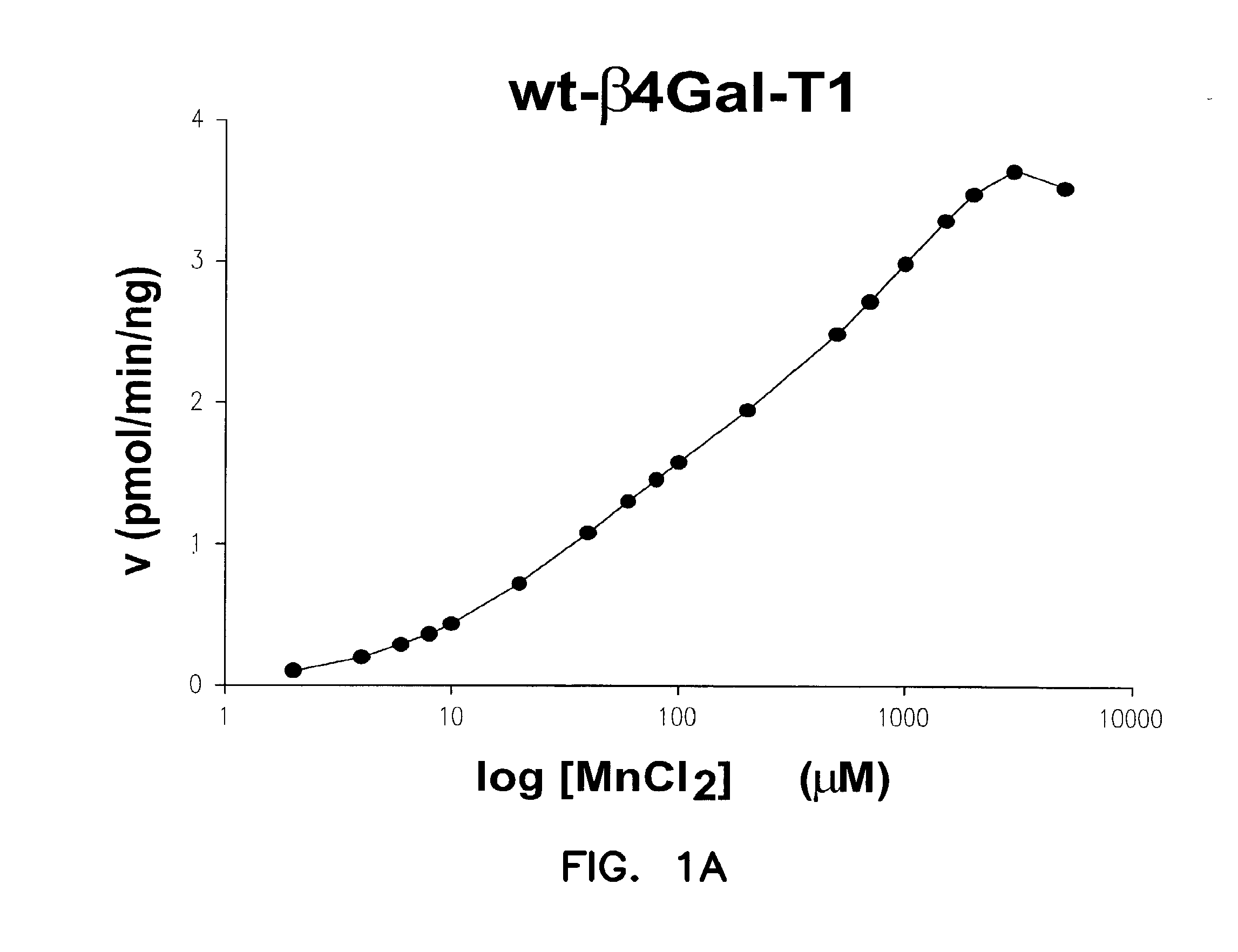
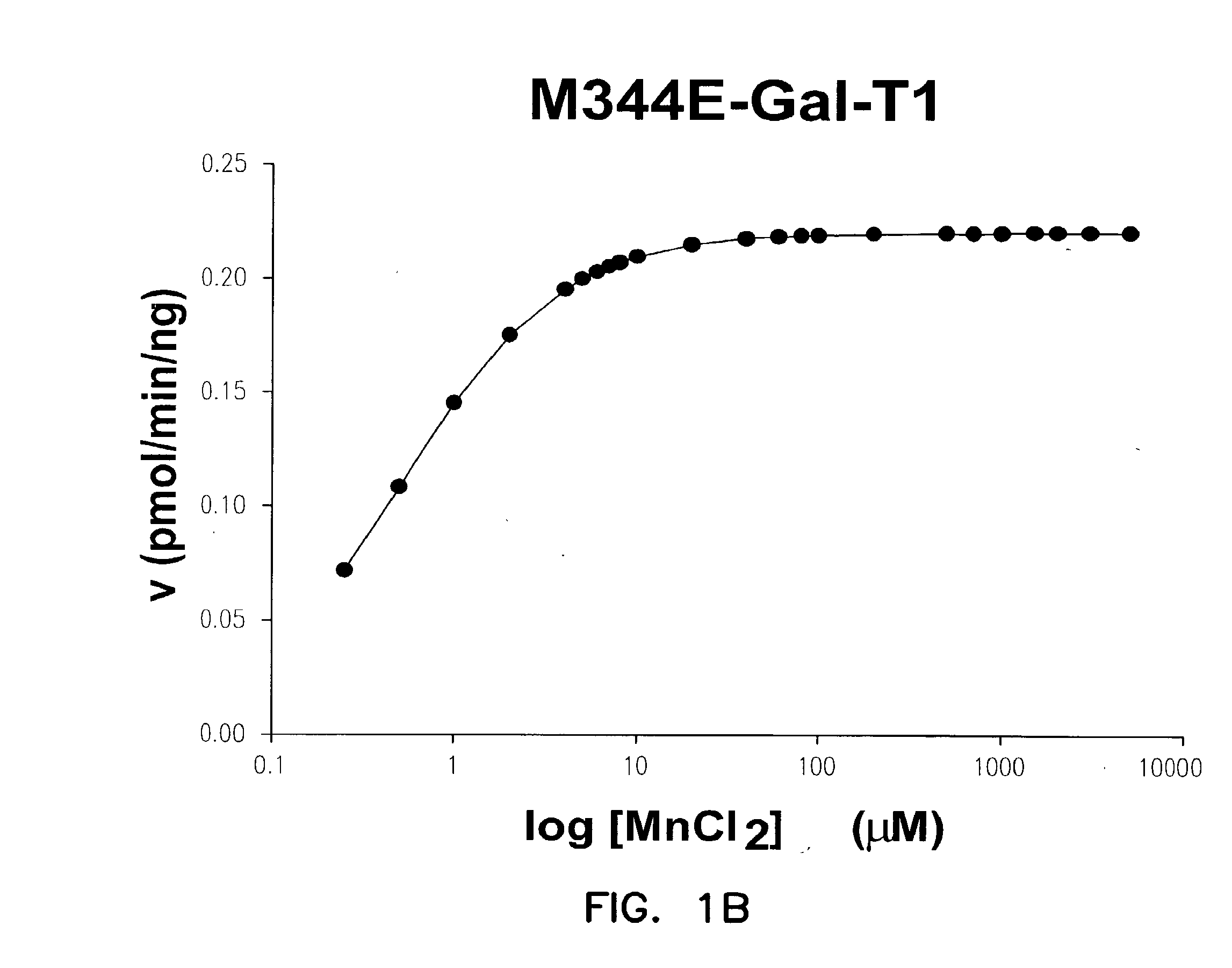
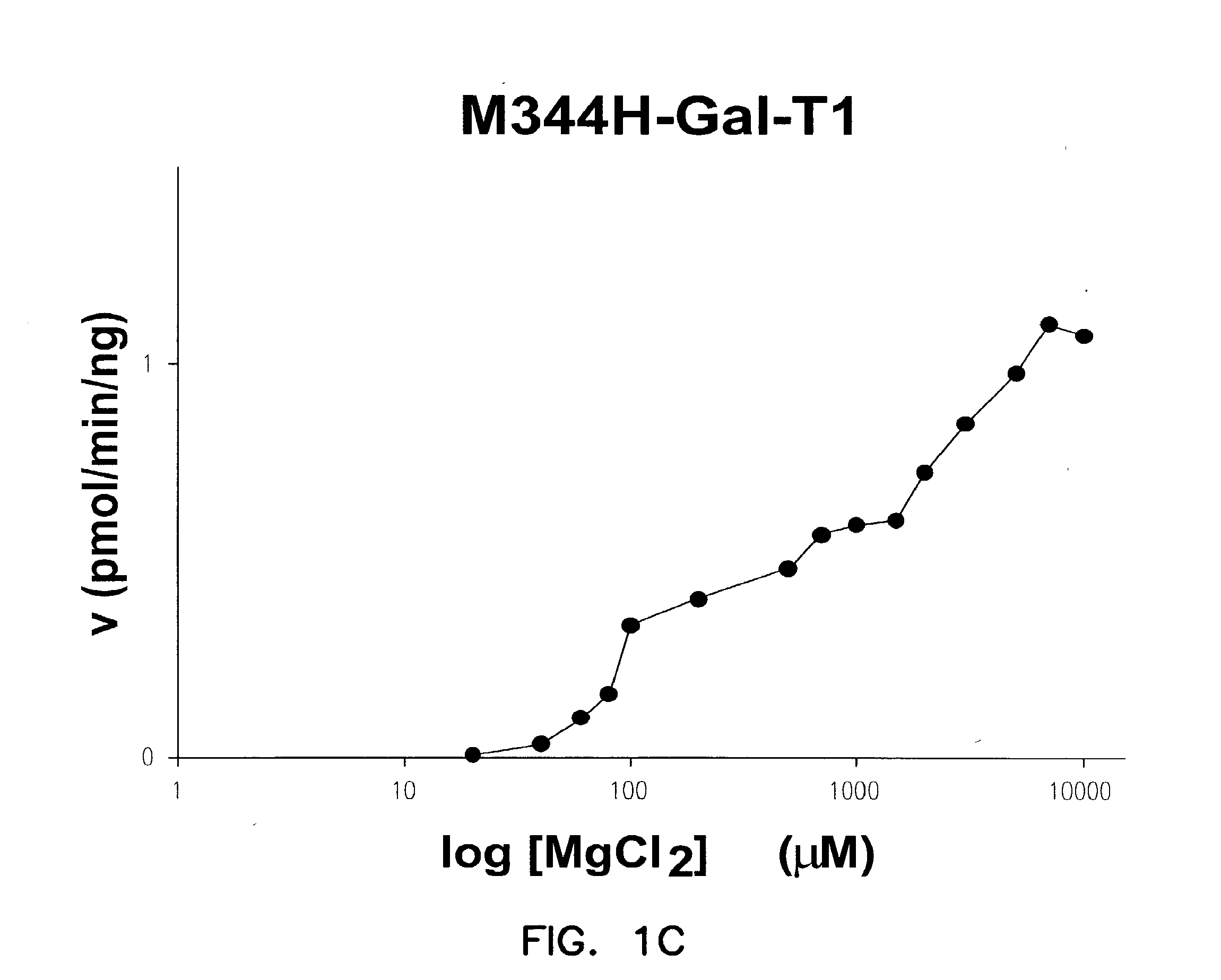
Disclosed are mutants of galactosyltransferases that can catalyze formation of oligosaccharides in the presence of magnesium; mutants of galactosyltransferases having altered donor and acceptor specificity which can catalyze formation of oligosaccharides in the presence of magnesium; methods and compositions that can be used to synthesize oligosaccharides; methods for increasing the immunogenicity of an antigen; and methods to stabilize platelets.
Owner:UNITED STATES OF AMERICA
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com