Mammalian-type glycosylation in plants by expression of non-mammalian glycosyltransferases
a technology of plant glycosylation and non-mammalian glycosylation, which is applied in the field of transgenic plant glycosylation, can solve the problems of lack of agents infectious to humans, lack of glycoprotein production in plants, and lack of plant glycoprotein characteristics
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
Identification of Putative Non-Mammalian β1,4 GalTs
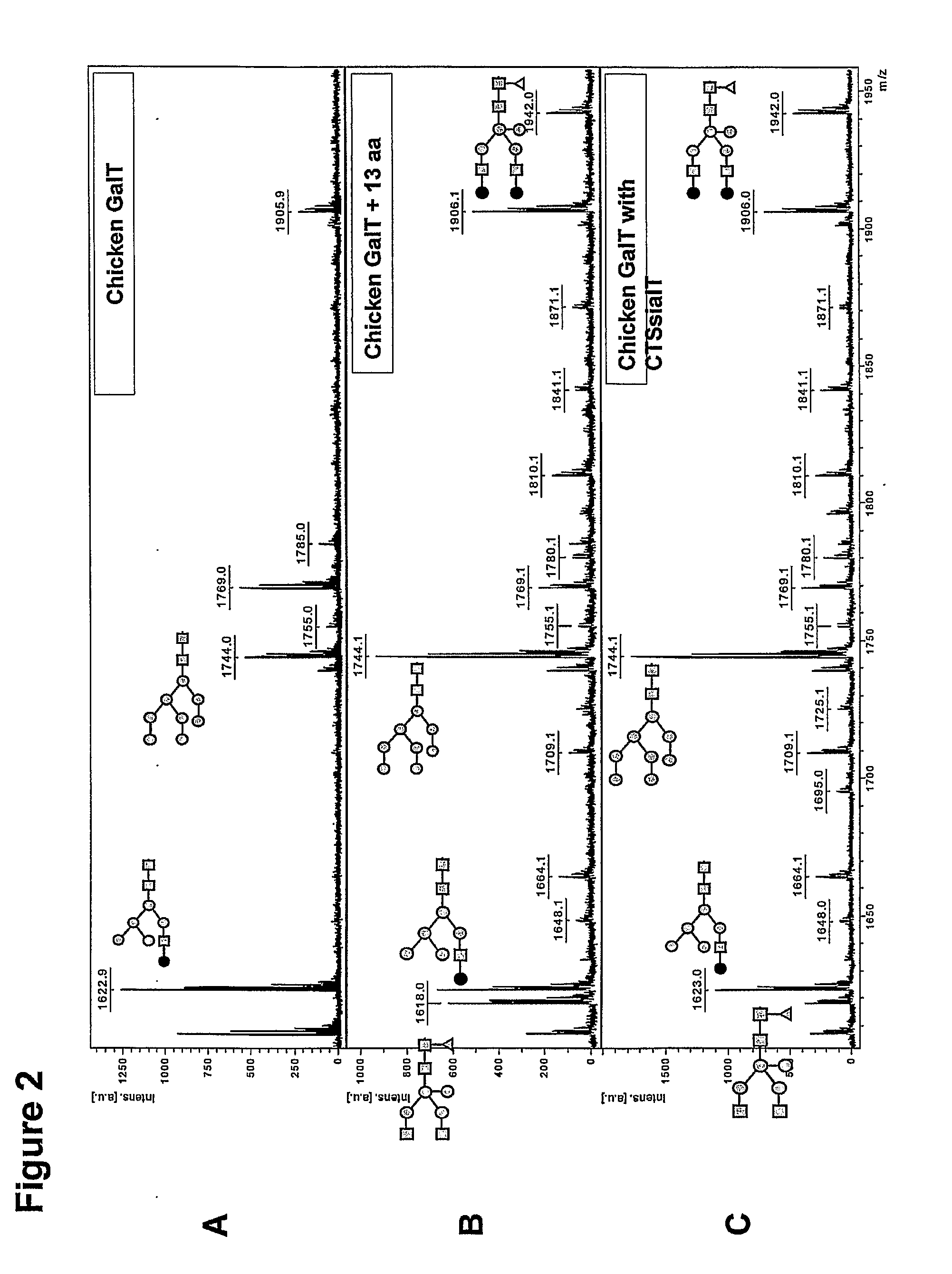
[0124]The family of β-1,4-galactosyltransferases (GalT) comprises at least seven members, of which several have been cloned but only very few have been characterized. The β-1,4-galactosyltransferases of human and bovine origin have been best characterized and were shown to be able to add a galactose residue in β-1,4-linkage to terminal GlcNAc residue on an N-glycan. Putative GalT genes involved in N-glycosylation were identified among non-mammalian species gene sequences based on homology. FIG. 1 shows a Clustal W alignment of mammalian GalT gene sequences (human, mouse and bovine) and non-mammalian putative GalT gene sequences (chicken, zebrafish and frog). Remarkably, the mammalian amino terminus (boxed, solid lines), which is cytosolic and adjacent to the transmembrane region (TM, boxed, dashed lines) and which putatively is involved in Golgi-localization is not conserved in GalT orthologs from non-mammalian origin, such as, for ...
example 2
Cloning and Expression of Genes Encoding the Full-Length Chicken β1,4-GalT1 Enzyme and Variants Thereof.
[0128]Putative chicken β1,4-GalT1 (GGal; GenBank accession U19890; SEQ Ggal, SEQ ID NO:2) has been cloned earlier, although it was not shown to be capable of galactosylating N-glycans (Shaper et al., J Biol Chem 272, 31389-31399, 1997). In our lab, the cDNA fragment comprising residues 114 to 362 (SEQ GgGal114-362, SEQ ID NO:3) has been amplified from chicken spleen total RNA using RT-PCR with primers GgalLEEVAST and GgGaldw (see Table 1). The resulting fragment containing the C-terminus was digested with Xho I and Bam HI and then cloned into plasmid pCASeco, the latter being a pUC19 derivative in which the Hin dIII and Eco RI sites flanking the multiple cloning site have been used to insert the sequence SEQ CASeco at the same time removing these two sites and the Eco 31I-site in the backbone. This plasmid pCASeco was digested with Xho I and Bam HI to accommodate the C-terminal GG...
example 3
Cloning and Expression of Genes Encoding the Full-Length Zebrafish β1,4-GalT1 Enzyme and Variants Thereof.
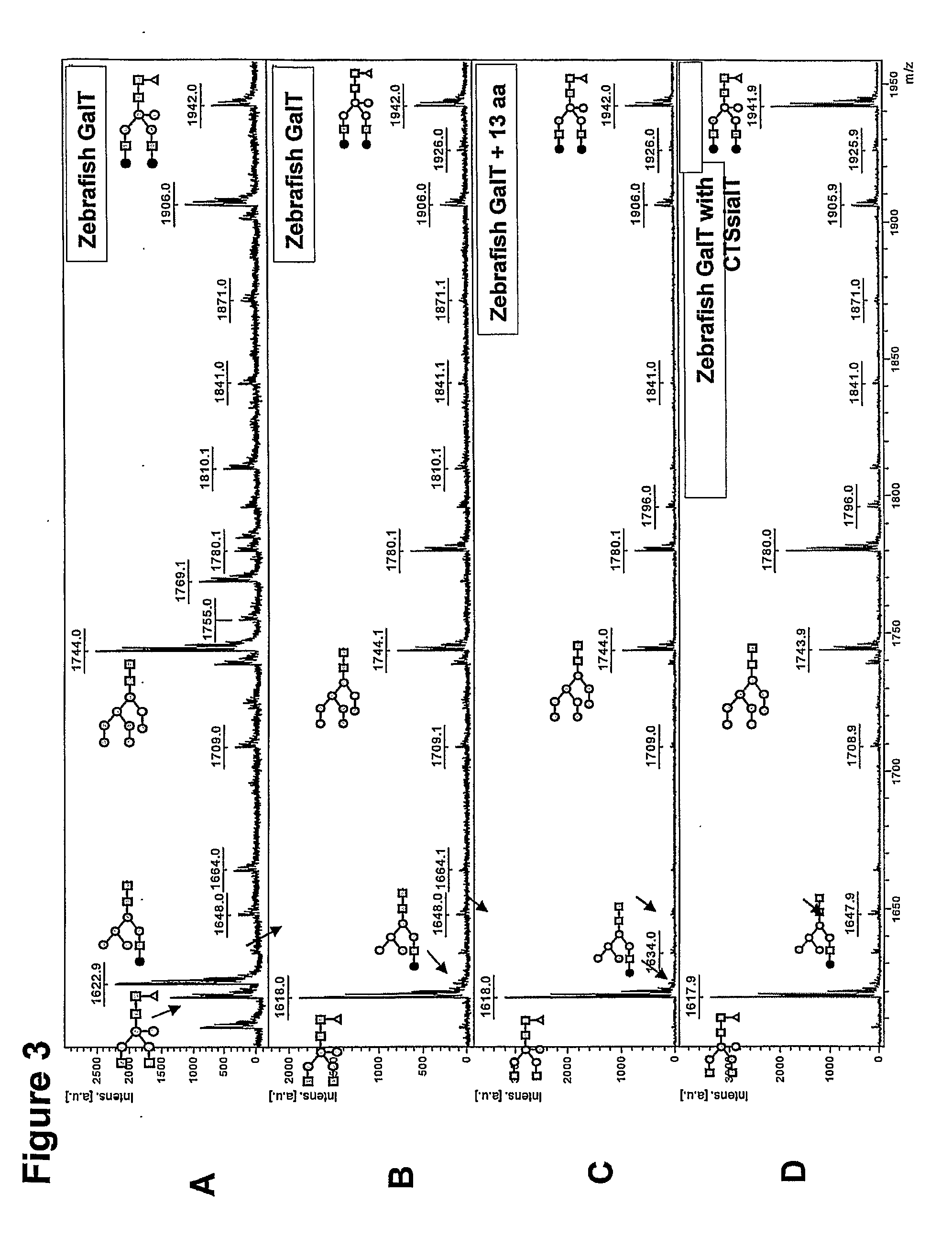
[0136]A putative zebrafish β1,4-GalT1 (Machingo et al., Dev Biol 297, 471-82, 2006; NM—001017730) has been identified, but its function has not been proven and the inventors believe the identification to be incorrect. Starting from whole zebrafish total RNA, a full-length DGal gene (SEQ Dgal, SEQ ID NO:13) has been amplified using RT-PCR with primers DrGalup and DrGaldw (see Table 2). The resulting fragment was digested with Xba I and Bam HI and then cloned into likewise digested plasmid pCASeco (see above). Sequencing showed that it was virtually identical to unidentified Genbank accession NM—001077259, albeit with three mutations as shown here (start at +1 and non-silent mutations underlined): T126C, T230G, G862C. Amino acid sequence comparison of Dgal (SEQ Dgal, SEQ ID NO:14) with putative zebrafish β1,4-GalT1 as published (Machingo et al., Dev Biol 297, 471-82, 2006; NM—0010...
PUM
| Property | Measurement | Unit |
|---|---|---|
| length | aaaaa | aaaaa |
| adhesion | aaaaa | aaaaa |
| nucleic acid | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com