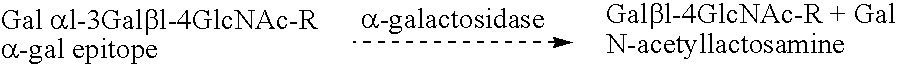Patents
Literature
57 results about "Heterografts" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
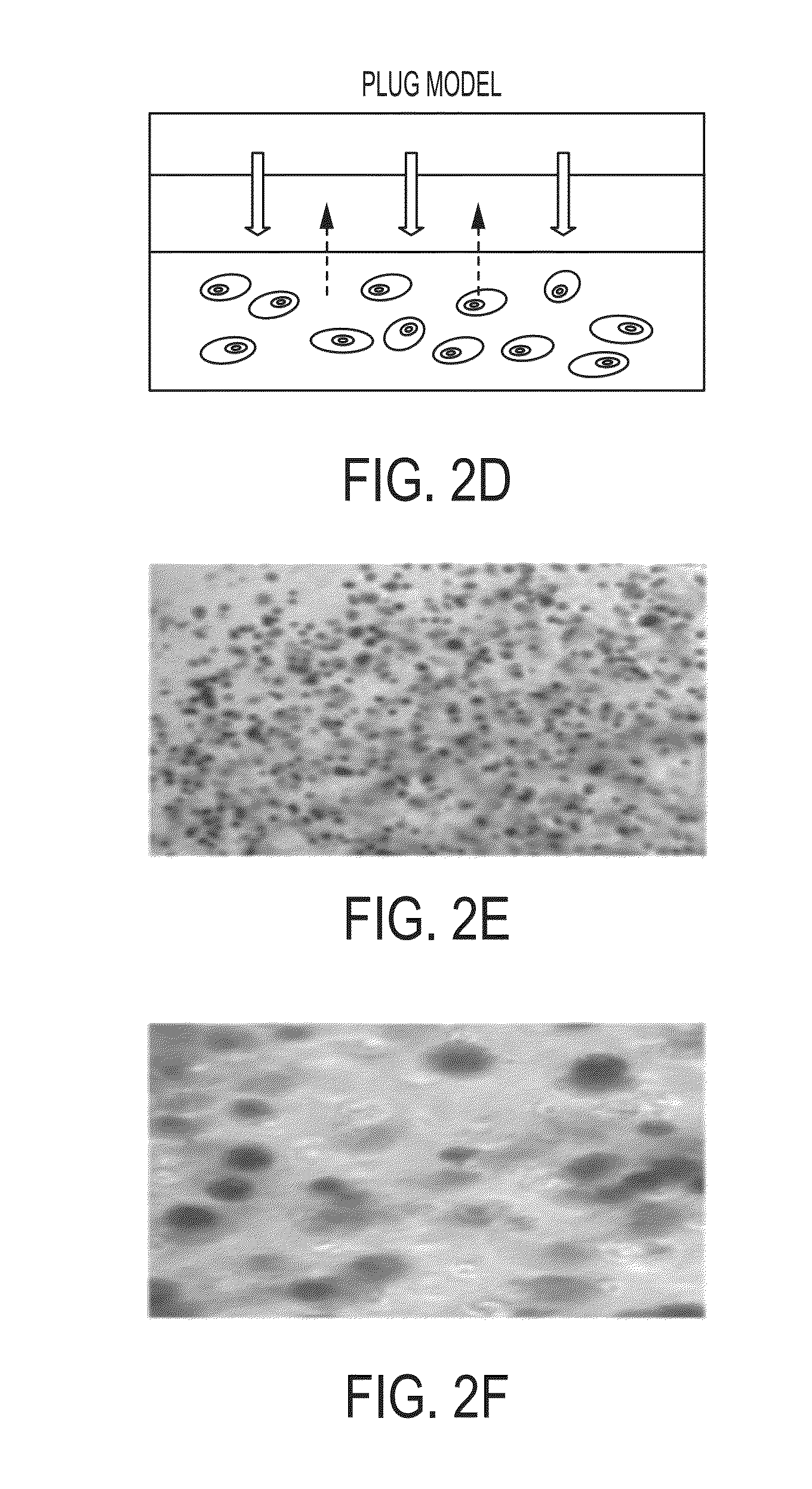
Tissues, cells or organs transplanted between animals of different species.
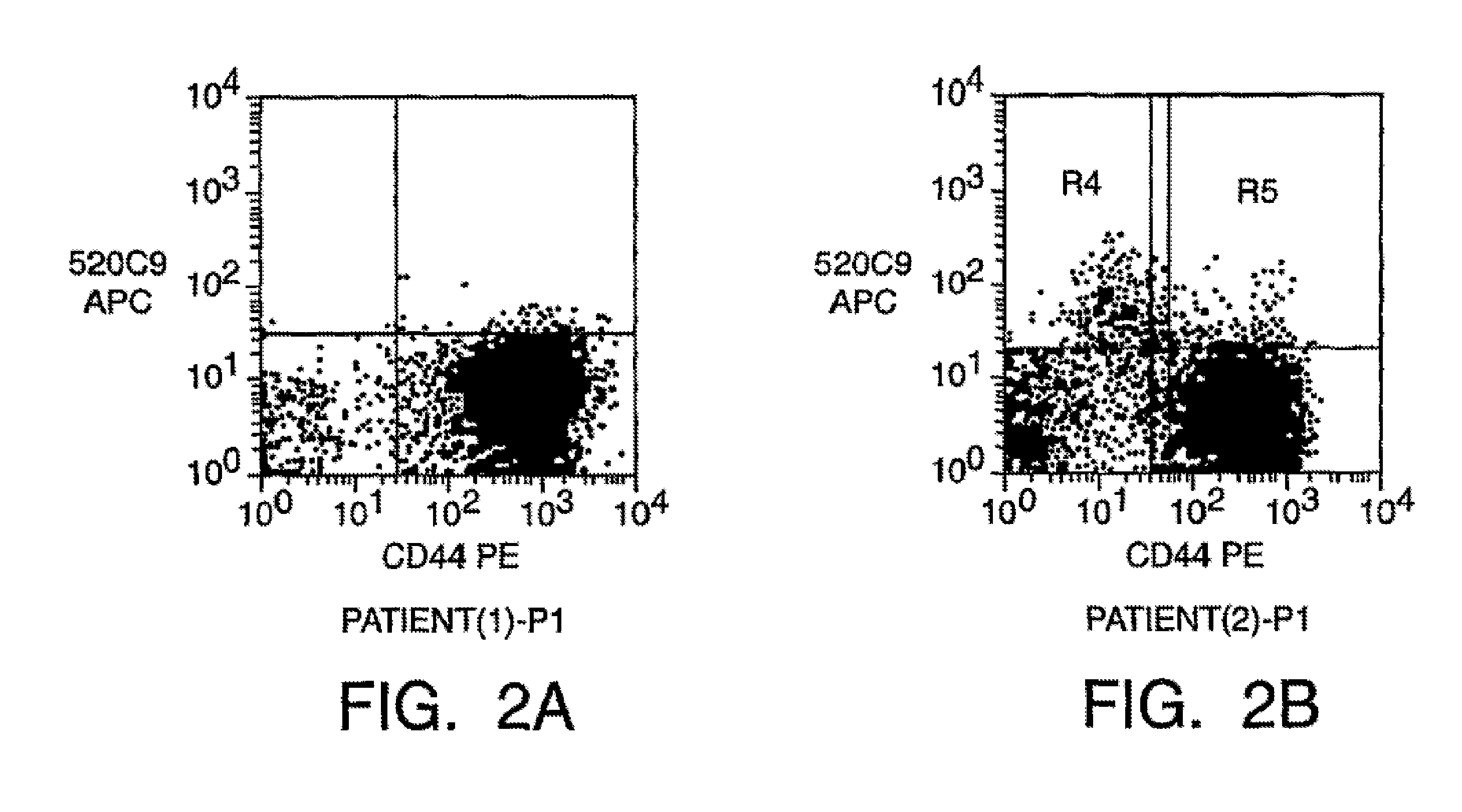
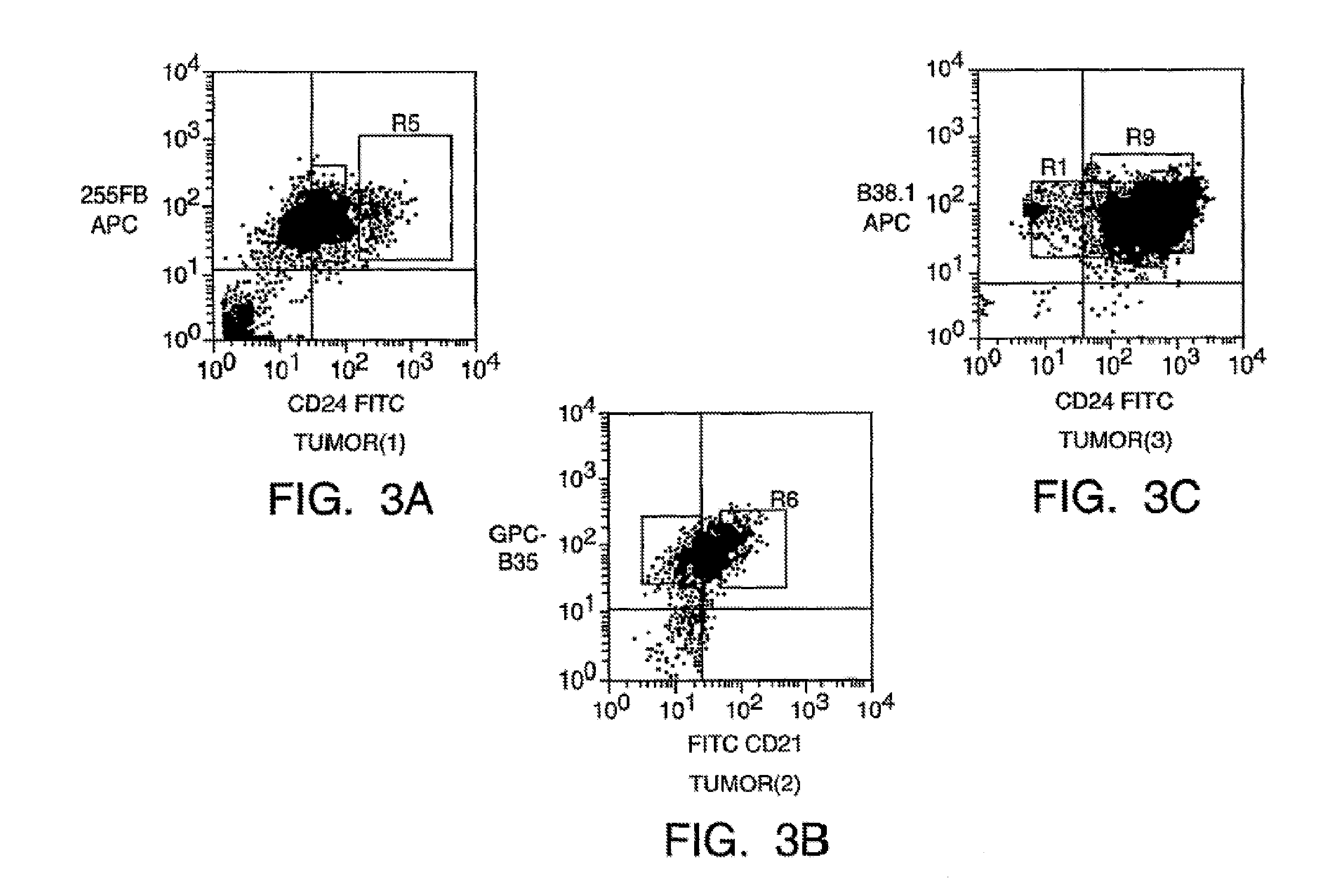
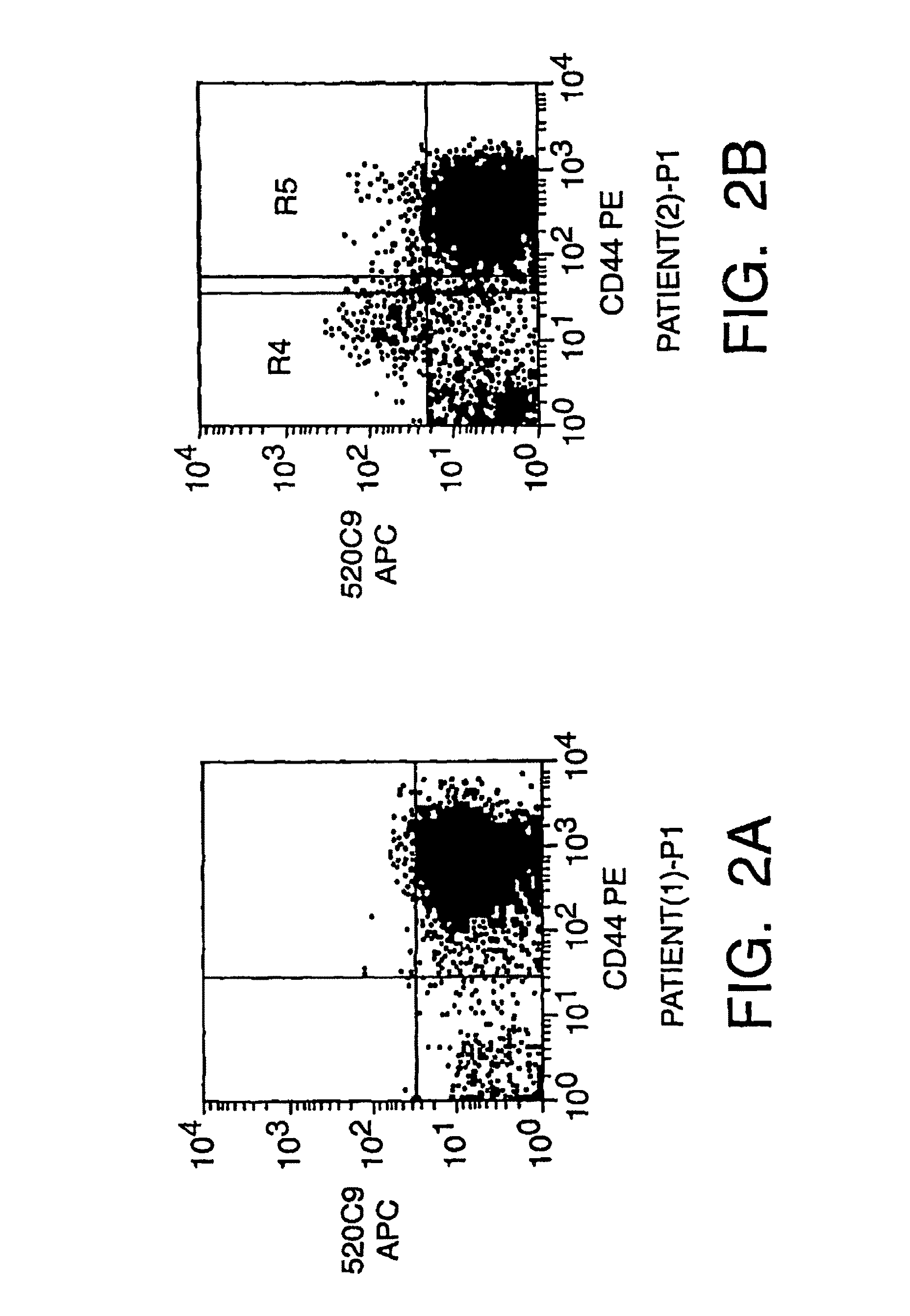
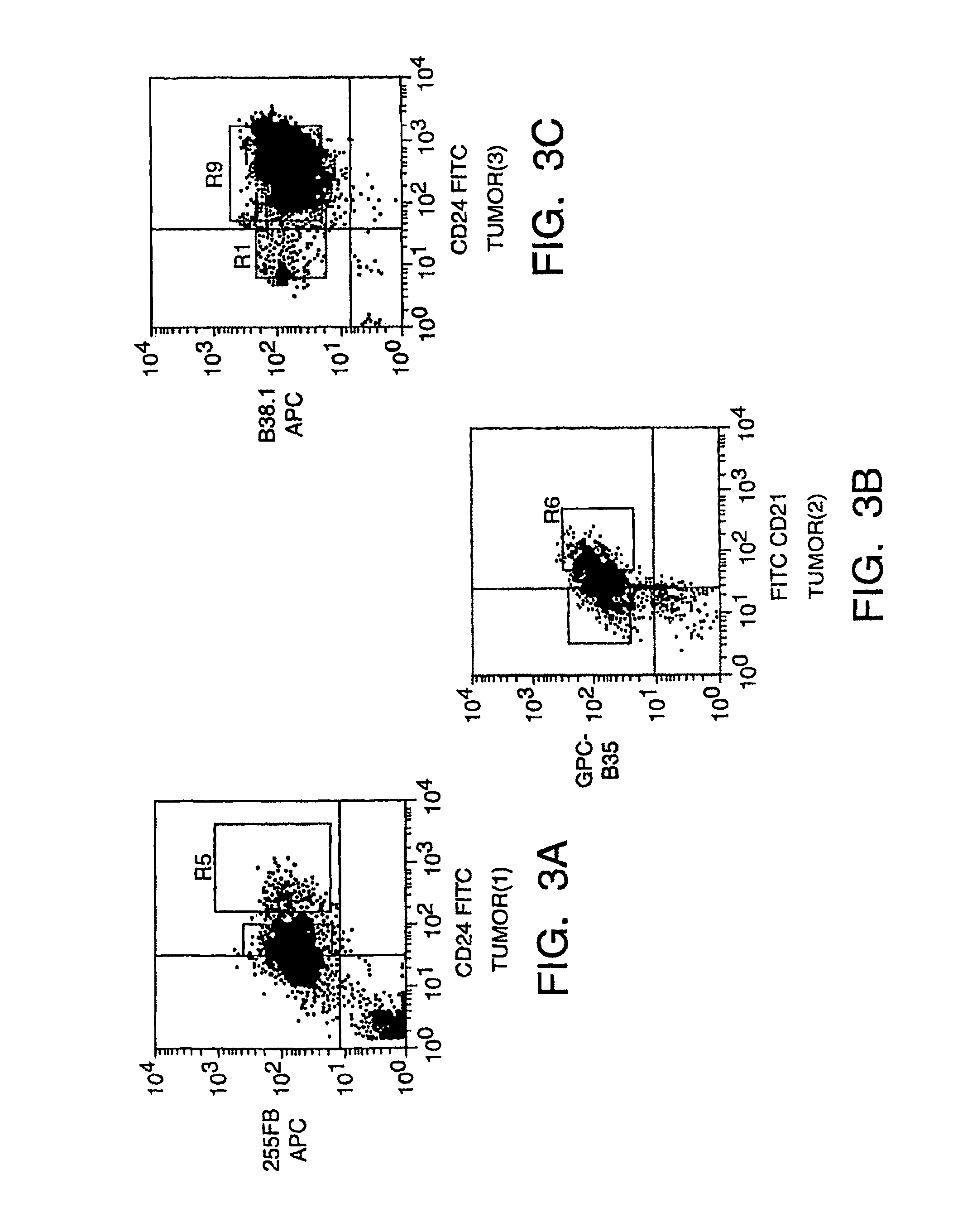
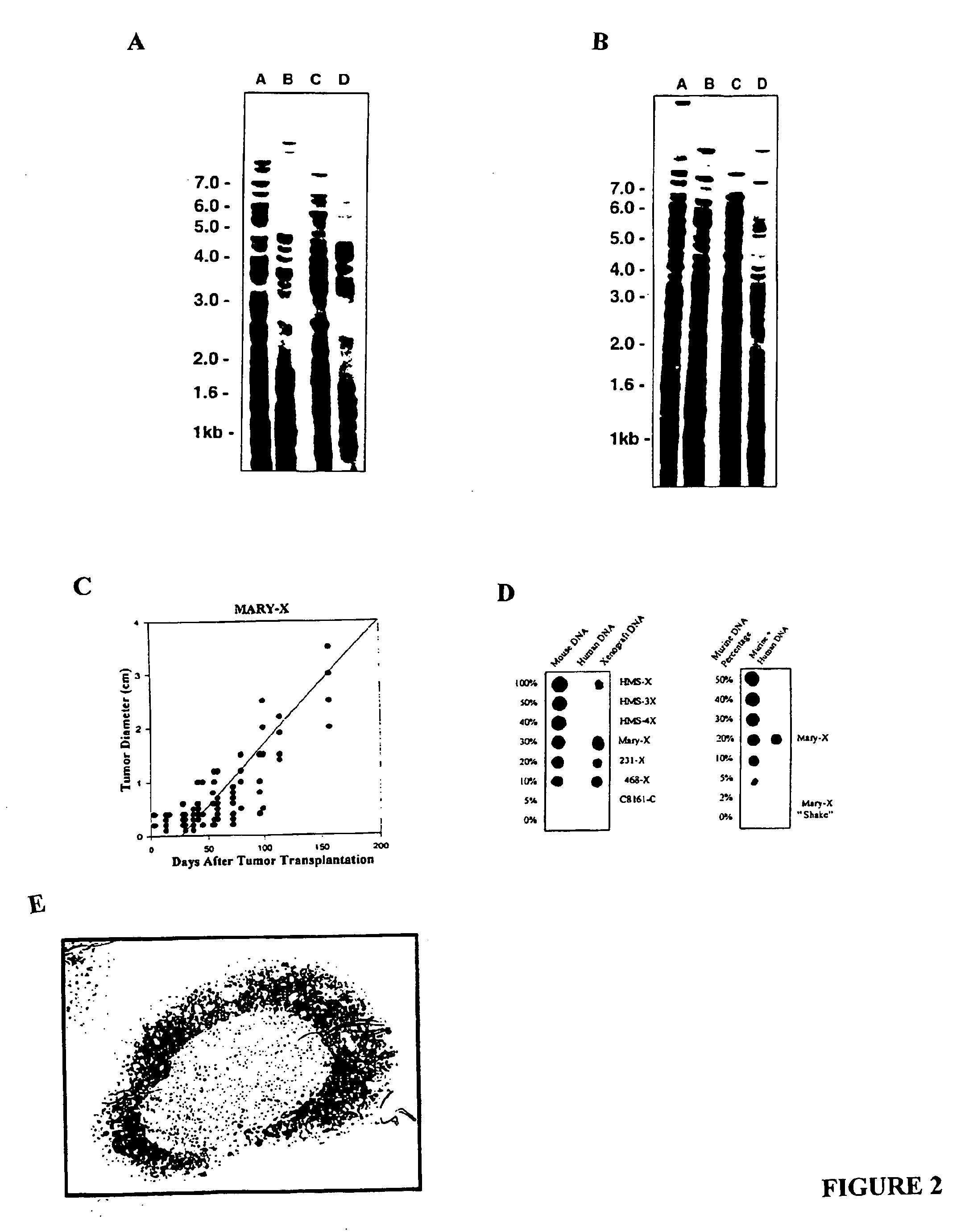
Isolation And Use Of Solid Tumor Stem Cells
InactiveUS20080178305A1Reduce spreadIncreased proliferationMaterial nanotechnologyMicrobiological testing/measurementAbnormal tissue growthMammary gland structure
A small percentage of cells within an established solid tumor have the properties of stem cells. These solid tumor stem cells give rise both to more tumor stem cells and to the majority of cells in the tumor that have lost the capacity for extensive proliferation and the ability to give rise to new tumors. Thus, solid tumor heterogeneity reflects the presence of tumor cell progeny arising from a solid tumor stem cell. We have developed a xenograft model in which we have been able to establish tumors from primary tumors via injection of tumor cells in the mammary gland of severely immunodeficient mice. These xenograft assay have allowed us to do biological and molecular assays to characterize clonogenic solid tumor stem cells. We have also developed evidence that strongly implicates the Notch pathway, especially Notch 4, as playing a central pathway in carcinogenesis.
Owner:ONCOMED PHARMA +1
Combining Radioimmunotherapy and Antibody-Drug Conjugates for Improved Cancer Therapy
InactiveUS20110070156A1Organic active ingredientsHybrid immunoglobulinsParanasal Sinus CarcinomaAntiendomysial antibodies
Described herein are compositions and methods of use of radionuclide-antibody conjugates (for RAIT) and drug-antibody conjugates (ADC). The combination of RAIT and ADC was more efficacious than either RAIT alone, ADC alone, or the sum of effects of RAIT and ADC. The unexpected synergy resulted in decreased tumor growth rate and increased survival, with a high incidence of tumor-free survival in Capan-1 human pancreatic cancer xenografts in nude mice.
Owner:IMMUNOMEDICS INC
Implantable graft to close a fistula
ActiveUS20070031508A1Facilitates formation of newAvoids surgical pain and attendant complicationSuture equipmentsSurgical furnitureHeterograftsFistula
An implantable graft, which may be inserted into a fistula tract to occlude the primary opening of the fistula, is provided. To prevent unintentional displacement of the graft or extrusion of the graft from the fistula of a patient, the graft may be provided with a cap that extends laterally from at least one end of the body of the graft, where the cap may be integral with the body of the graft, attachable to at least one end of the body of the graft, and / or moveable along the body of the graft. The graft may also have a tail that extends from one end of the body of the graft to assist in placement of the graft in a fistula tract. The graft may be an integral unit made of a single material, such as a heterograft material, or may include distinct components made of the same or different materials. Methods for closing a fistula tract are also provided.
Owner:COOK BIOTECH +1
Porcine animals lacking any expression of functional alpha 1, 3 galactosyltransferase
ActiveUS7795493B2Eliminating hyperacute rejectionTransferasesHybrid cell preparationHeterograftsXenotransplantation
The present invention is a porcine animal, tissue, organ, cells and cell lines, which lack any expression of functional alpha 1,3 galactosyltransferase (alpha1,3GT). These animals, tissues, organs and cells can be used in xenotransplantation and for other medical purposes.
Owner:REVIVICOR INC
Isolation and use of solid tumor stem cells
InactiveUS20110092378A1Promote resultsPromotes significant proliferationDiagnosticsMicrobiological testing/measurementPrimary tumorImmunodeficient Mouse
A small percentage of cells within an established solid tumor have the properties of stem cells. These solid tumor stem cells give rise to both more tumor stem cells and to the majority of cells in the tumor that have lost the capacity for extensive proliferation and the ability to give rise to new tumors. Thus, solid tumor heterogeneity reflects the presence of tumor cell progeny arising from a solid tumor stem cell.We have developed a xenograft model in which we have been able to establish tumors from primary tumors via injection of tumors in the mammary gland of severely immunodeficient mice. These xenograft assay have allowed us to do biological and molecular assays to characterize clonogenic solid tumor stem cells.We have also developed evidence that strongly implicates the Notch pathway, especially Notch 4, as playing a central pathway in carcinogenesis.
Owner:RGT UNIV OF MICHIGAN
Vascular graft sterilization and decellularization
ActiveUS20070260109A1Efficient removalOptimal biological functionalityTissue regenerationBlood vesselsBlood Vessel GraftingHeterografts
The present technology is related to the field of sterilization and decellularization of allografts or xenografts, specifically to processes that achieve effective removal of the cells contained within a vascular tissue matrix and an effective reduction in potential harmful organisms to create grafts suitable for human implantation. In some embodiments, vascular grafts, vascular tissue and / or blood vessels are contacted with cleaning solution under conditions suitable conditions to reduce immune reaction in patients. More specifically, the present technology is directed to sterilization and decellularization of vascular grafts.
Owner:RTI BIOLOGICS INC
Genetically modified heart valve xenografts
InactiveUS20090043383A1Increased durabilityLow immunogenicityBiocideHeart valvesHeterograftsNucleic acid sequencing
The invention relates to heart valve xenografts from transgenic pigs having a disruption of an α1-3 galactosyl transferase nucleic acid sequence and use of the xenografts for treating a patient.
Owner:MAYO FOUND FOR MEDICAL EDUCATION & RES
Modified organs and cells for xenotransplantation
InactiveUS7166278B2Maintain integrityMaintaining viabilityBiocidePeptide/protein ingredientsHeterograftsEngineered genetic
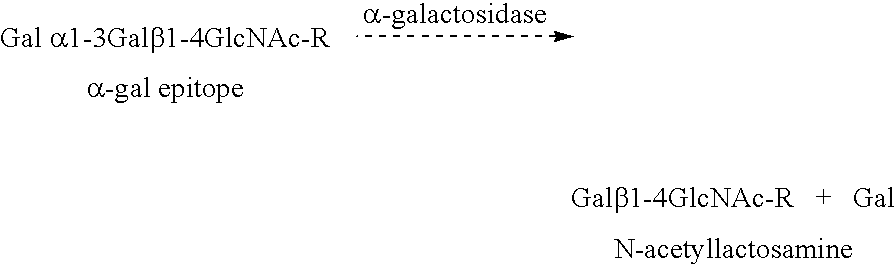
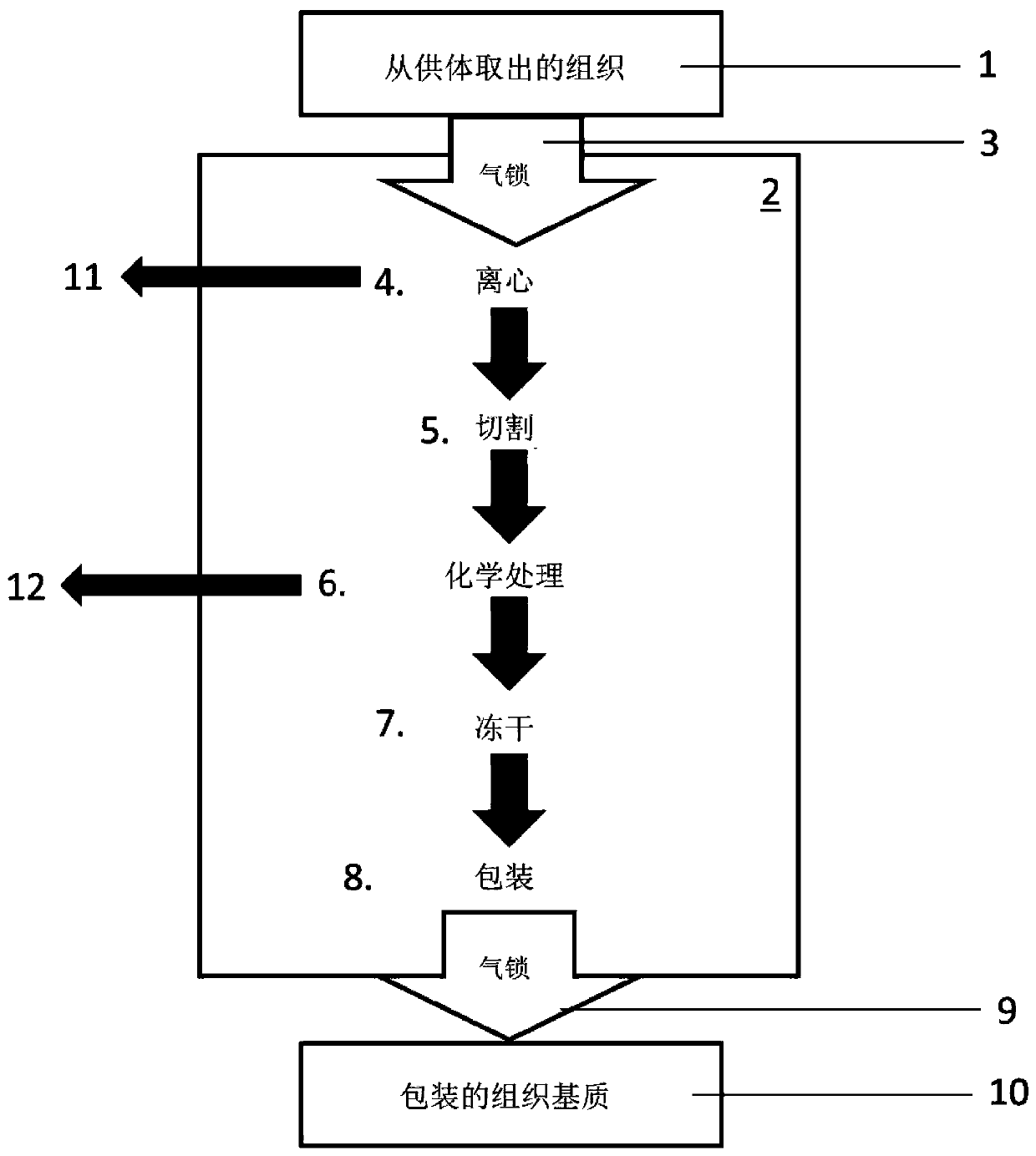
It has been discovered that there are at least two significant antigens present on the cells of animal species such as pigs that elicit an immune or inflammatory response immediately upon implantation into humans or contact with human serum. The first is an α-galactosyl (Gal) epitope, for example, Galα(1->3)Galβ(1->4)GlcNac (linear B type 2) or Galα(1->3) Galβ(1->4)Glc (linear B type 6). The second is an N-glycolylneuraminic acid (NeuGc) structure. By eliminating these epitopes, preferably by genetically engineering the animal so that the epitope is either not produced or is greatly reduced, or by chemical or enzymatic treatment of the animal's cells to remove the epitopes, it is possible to produce organs, tissues and cells suitable for xenotransplantation into humans. Cells can be rendered even more compatible by genetically engineering the animal to express a human complement regulatory protein (inhibitor), such as CD59, on its cells, or to express an excess of a pig complement regulatory protein.
Owner:RBC BIOTECH
Three-dimensional transglutaminase-crosslinked hydrogel for tumor engineering
InactiveUS20160178611A1Microbiological testing/measurementBiological testingHeterograftsDelivery vehicle
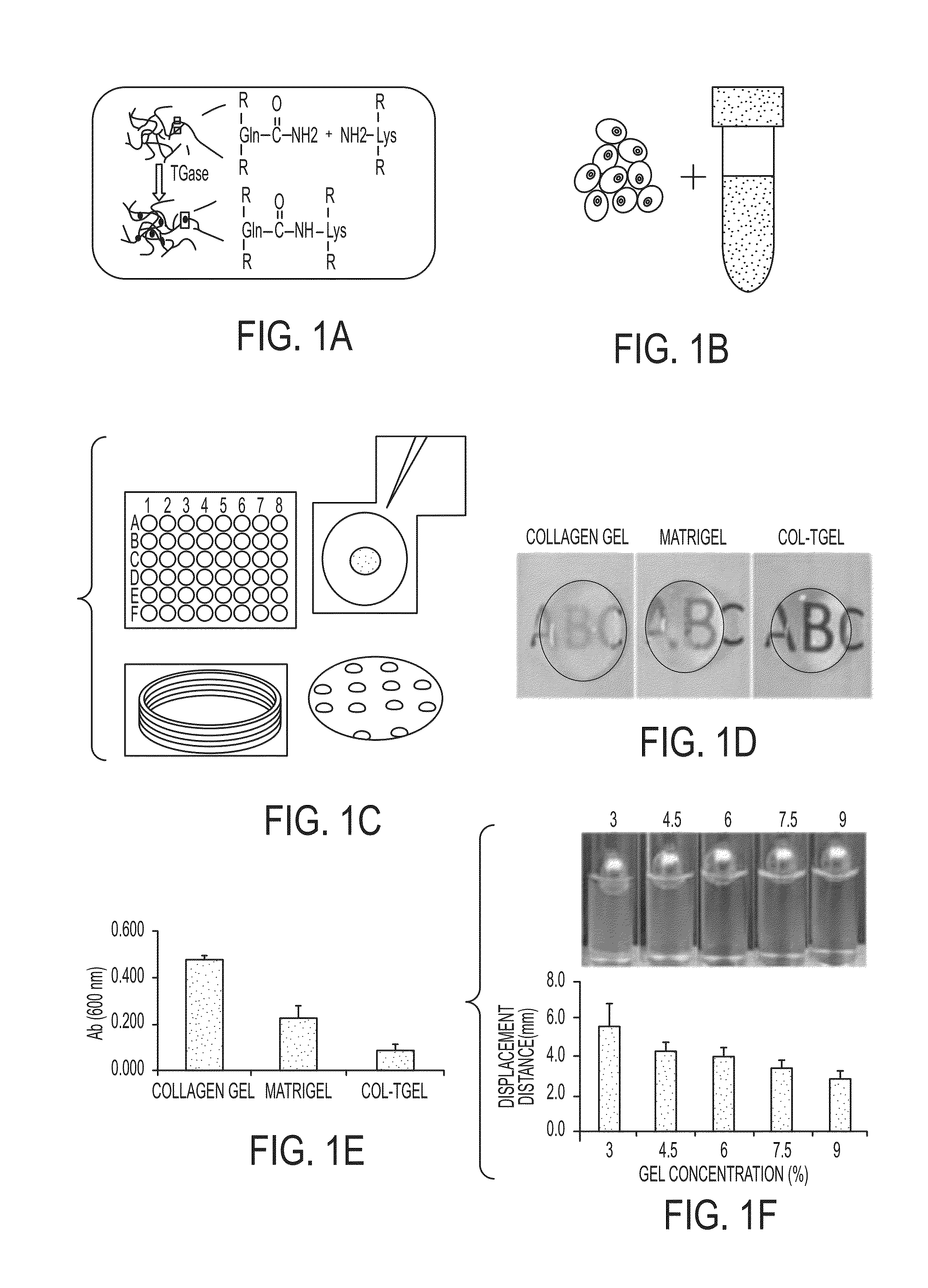
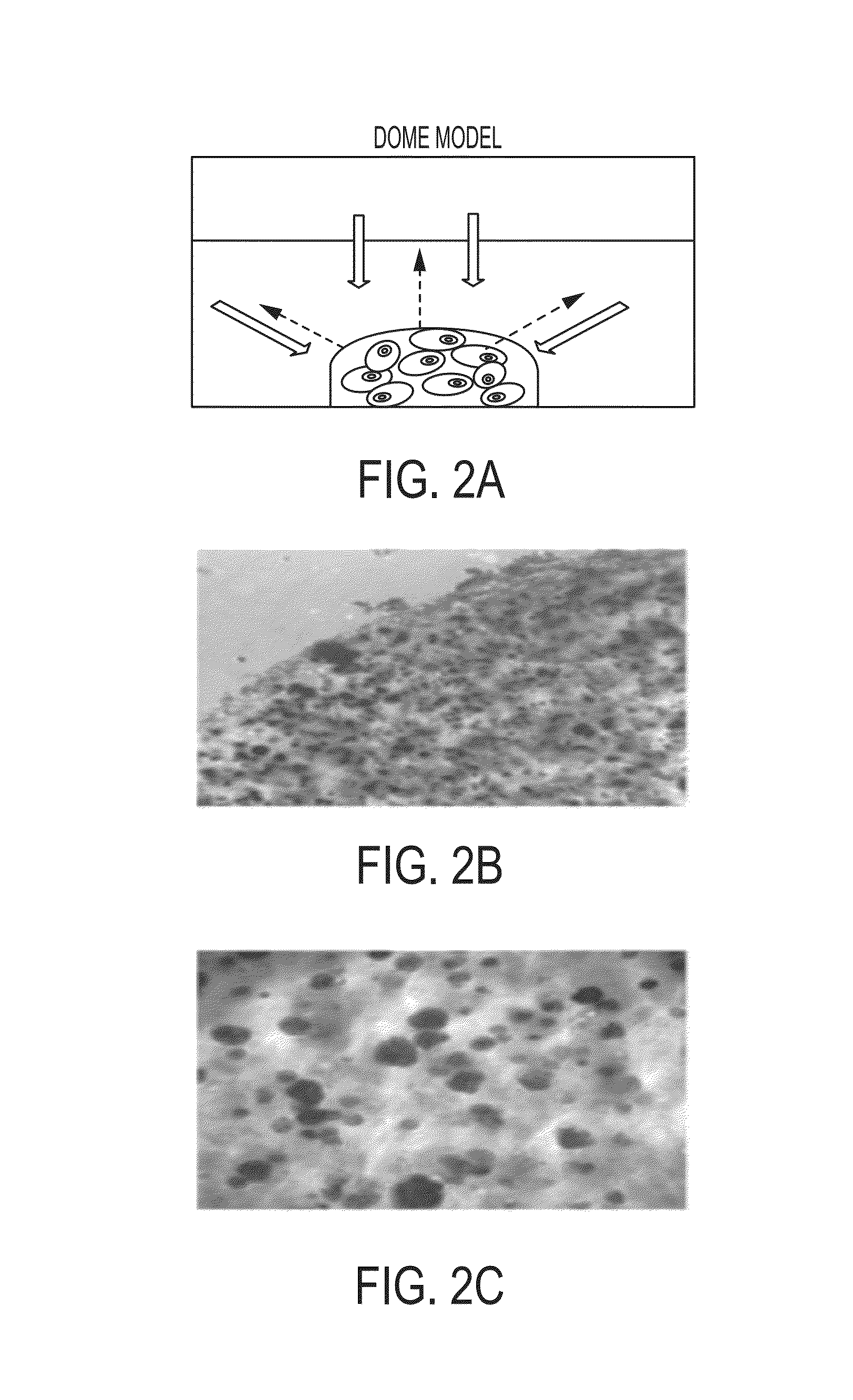
Development of a physiologically relevant 3D model system for cancer research and drug development represents quite a challenge. We have adopted a 3D culture system based on a transglutaminase-crosslinked gelatin gel (Col-Tgel) to mimic tumor 3D microenvironment. The system has several unique advantages over other alternatives which include cell-matrix interaction sites provided by collagen derived peptides, a 3D construct suitable for reproducing the solid tumor microenvironment including multicellular tumor spheroids and metabolic gradients. In addition the controllable gel stiffness provides a wide range of mechanical restrictions; and compatibility with imaging based screening due to its transparent properties. In addition the Col-Tgel provides a cure-in-situ delivery vehicle for tumor xenograft formation in animals with a high take rate. Overall, this unique 3D system could provide a platform to accurately mimic in vivo situations to study tumor formation and progression both in vitro and in vivo, as well as for screening antineoplastic drugs and assessing the occurrence of drug resistance related to cancer cell stress.
Owner:HAN BO +1
Immunochemically modified and sterilized xenografts and allografts
InactiveUS20070010897A1Improve integrityEasy to inactivateNew breed animal cellsTissue regenerationHeterograftsAlcohol
The invention provides an article of manufacture comprising a substantially non-immunogenic xenograft for implantation into humans. The xenograft is a body part dissected from a transgenic 1,3-α-galactosyltransferase gene-deficient animal, wherein the cells of the body part are dead. The invention also provides methods for preparing a xenograft by removing a body part from a transgenic animal, which is 1,3-α-galactosyltransferase gene-deficient, to provide a xenograft; optionally washing the xenograft in saline and alcohol; subjecting the xenograft to a cellular disruption treatment; optionally treating the xenograft with crosslinking agents, and optionally treating the xenograft with a proteoglycan-depleting factor. The invention further provides a method for sterilizing xenograft material, having the steps of obtaining substantially non-immunogenic xenograft material; treating the xenograft material with at least one crosslinking agent; and subjecting the crosslinked xenograft material to a radiation treatment.
Owner:APERION BIOLOGICS
Vascular graft sterilization and decellularization
ActiveUS7658706B2Effective sterilization and decellularizationEffective reduction in potential harmful organismsTubular organ implantsTissue regenerationVascular tissueBlood Vessel Tissue
The present technology is related to the field of sterilization and decellularization of allografts or xenografts, specifically to processes that achieve effective removal of the cells contained within a vascular tissue matrix and an effective reduction in potential harmful organisms to create grafts suitable for human implantation. In some embodiments, vascular grafts, vascular tissue and / or blood vessels are contacted with cleaning solution under conditions suitable conditions to reduce immune reaction in patients. More specifically, the present technology is directed to sterilization and decellularization of vascular grafts.
Owner:RTI BIOLOGICS INC
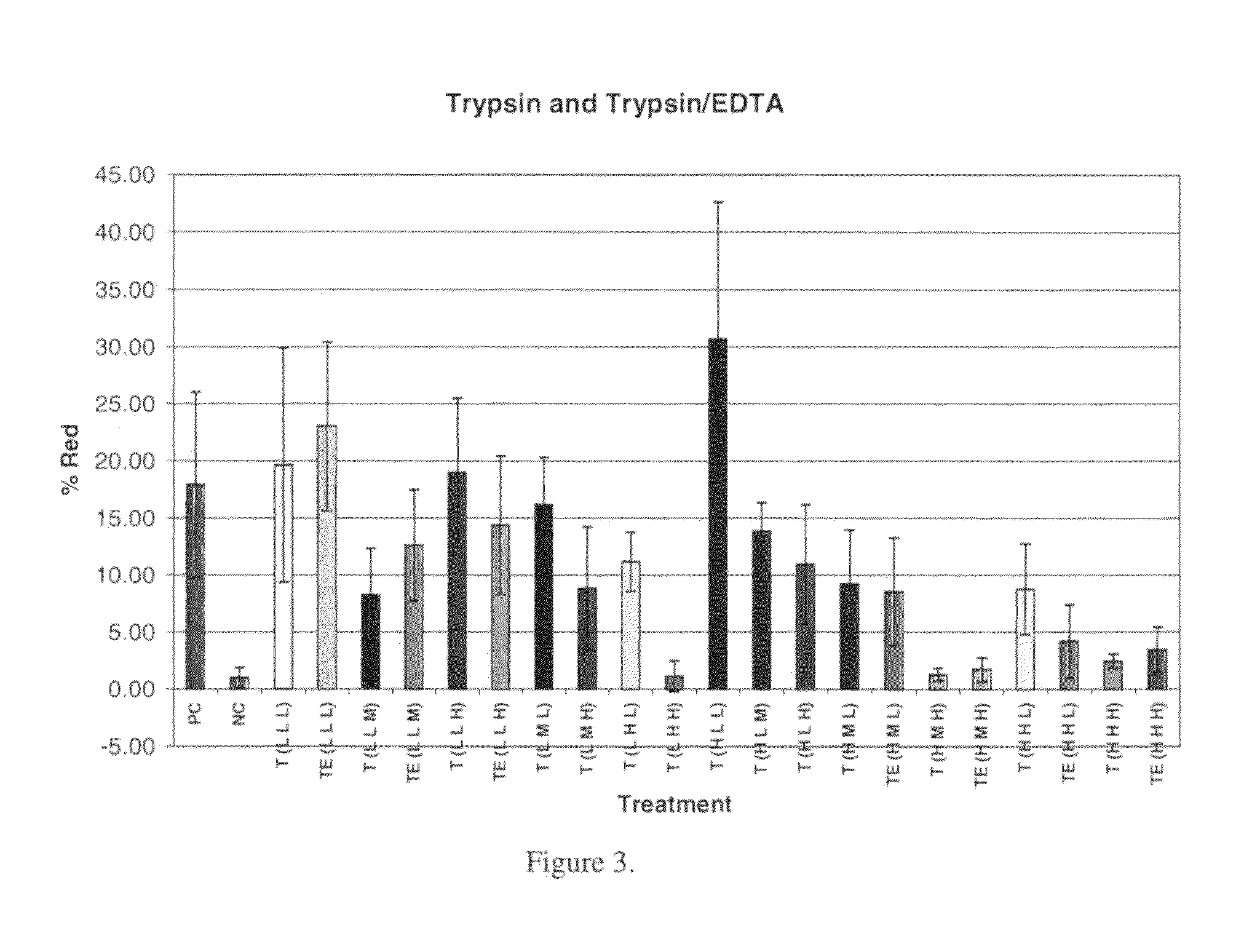
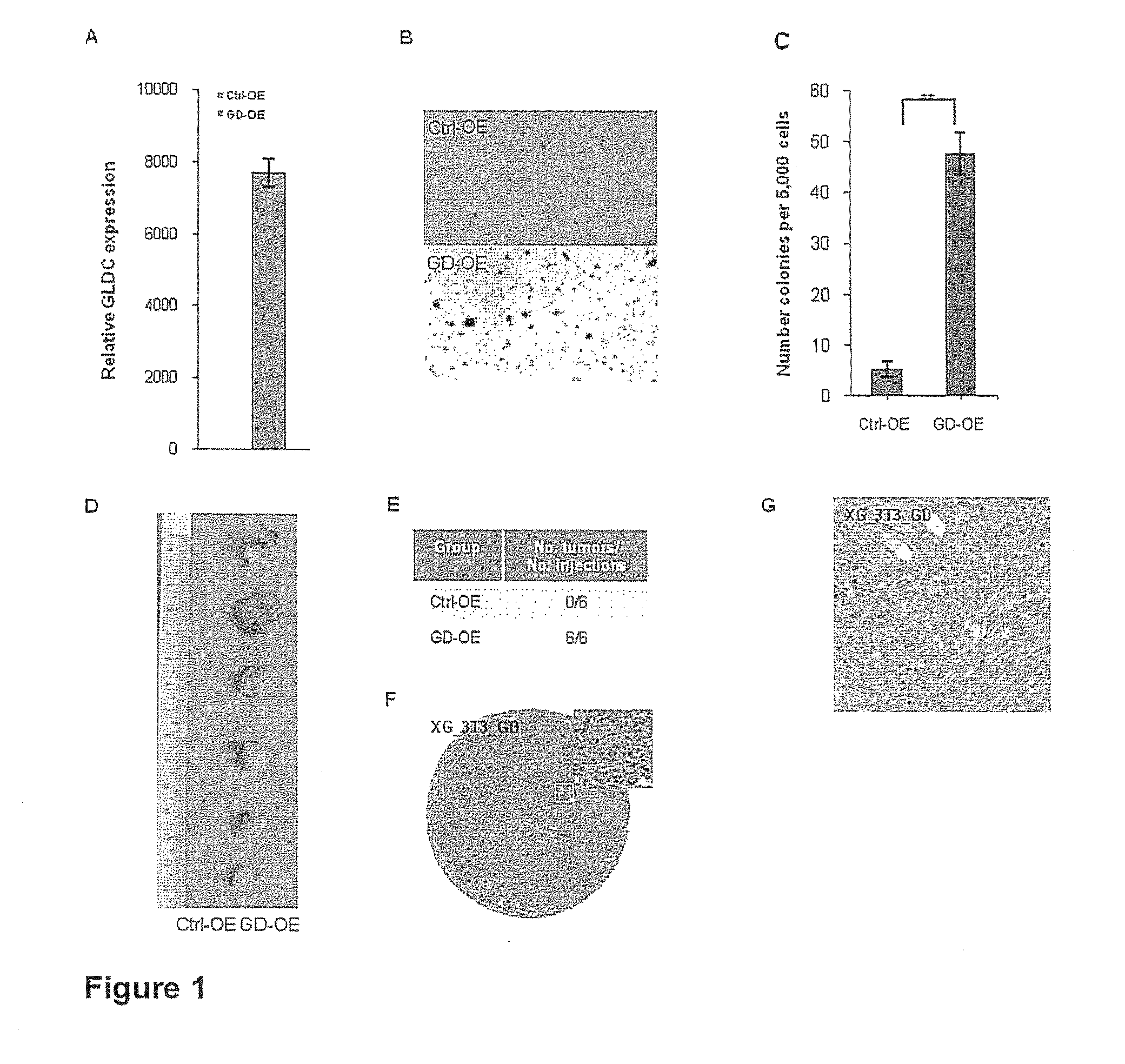
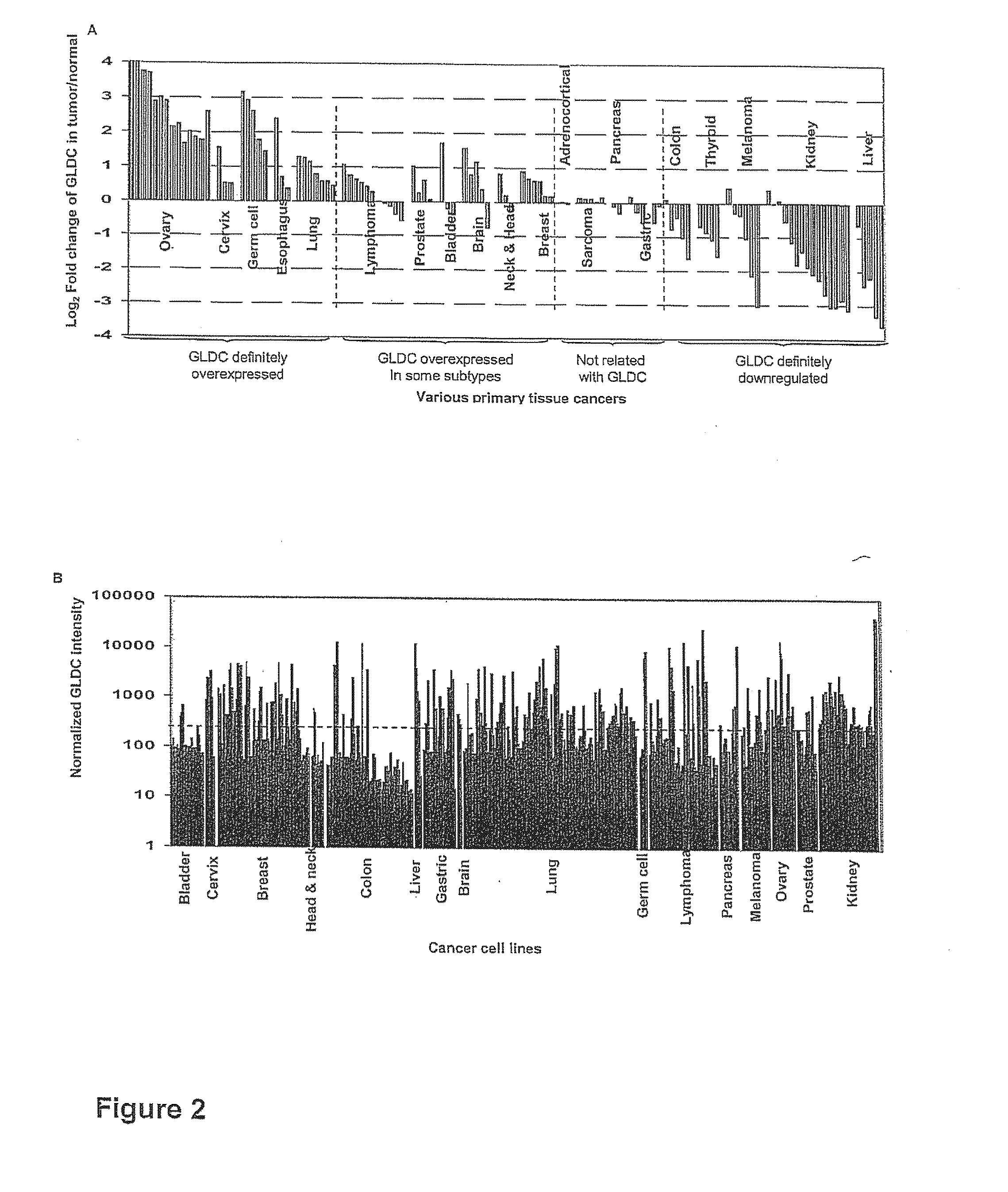
Targeting metabolic enzymes in human cancer
Targeting metabolic enzymes in human cancer Abstract Lung cancer is a devastating disease and a major therapeutic burden with poor prognosis. The functional heterogeneity of lung cancer (different tumor formation ability in bulk of tumor) is highly related with clinical chemoresistance and relapse. Here we find that, glycine dehydrogenase (GLDC), one of the metabolic enzyme involved in glycine metabolism, is overexpressed in various subtypes of human lung cancer and possibly several other types of cancers. GLDC was found to be highly expressed in tumor-initiating subpopulation of human lung cancer cells compared with non-tumorigenic subpopulation. By array studies we showed that normal lung cells express low levels of GLDC compared to xenograft and primary tumor. Functional studies showed that RNAi inhibition of GLDC inhibits significantly the clonal growth of tumor-initiating cells in vitro and tumor formation in immunodeficient mice. Overexpression of GLDC in non-tumorigenic subpopulation convert the cells to become tumorigenic. Furthermore, over-expression of GLDC in NIH / 3T3 cells and human primary lung fibroblasts can transform these cells, displaying anchorage-independent growth in soft agar and tumor-forming in mice. Not only is GLDC is expressed human lung cancer, it is also up-regulated in other types of cancer, such as colon cancer. RNAi knockdown of GLDC in colon cancer cell line, CACO-2 cells, can also inhibit the tumor formation in mice. Thus GLDC maybe a new metabolic target for treatment of lung cancer, and other cancers.
Owner:AGENCY FOR SCI TECH & RES
Viral vector driven mutant bacterial cytosine deaminase gene and uses thereof
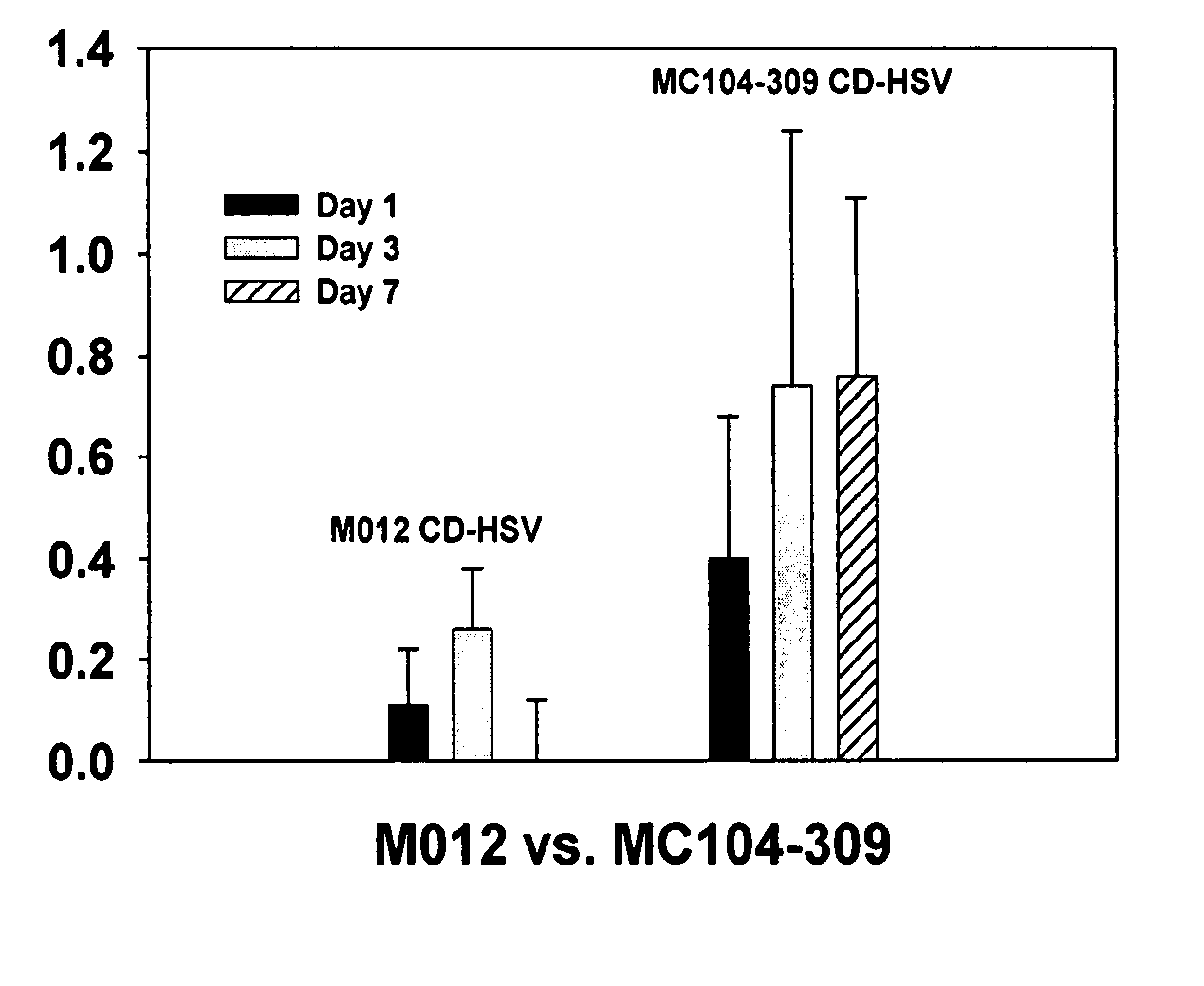
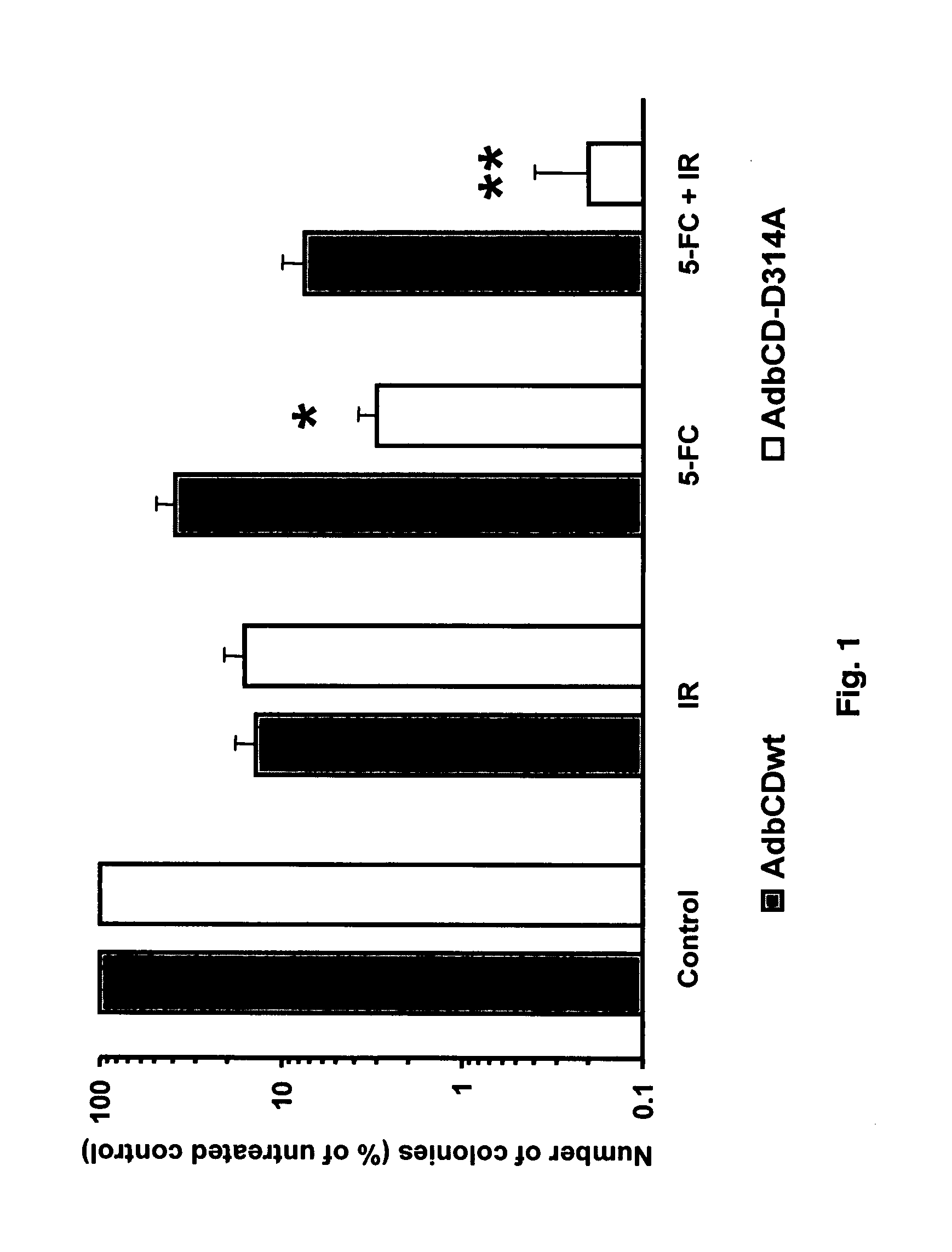
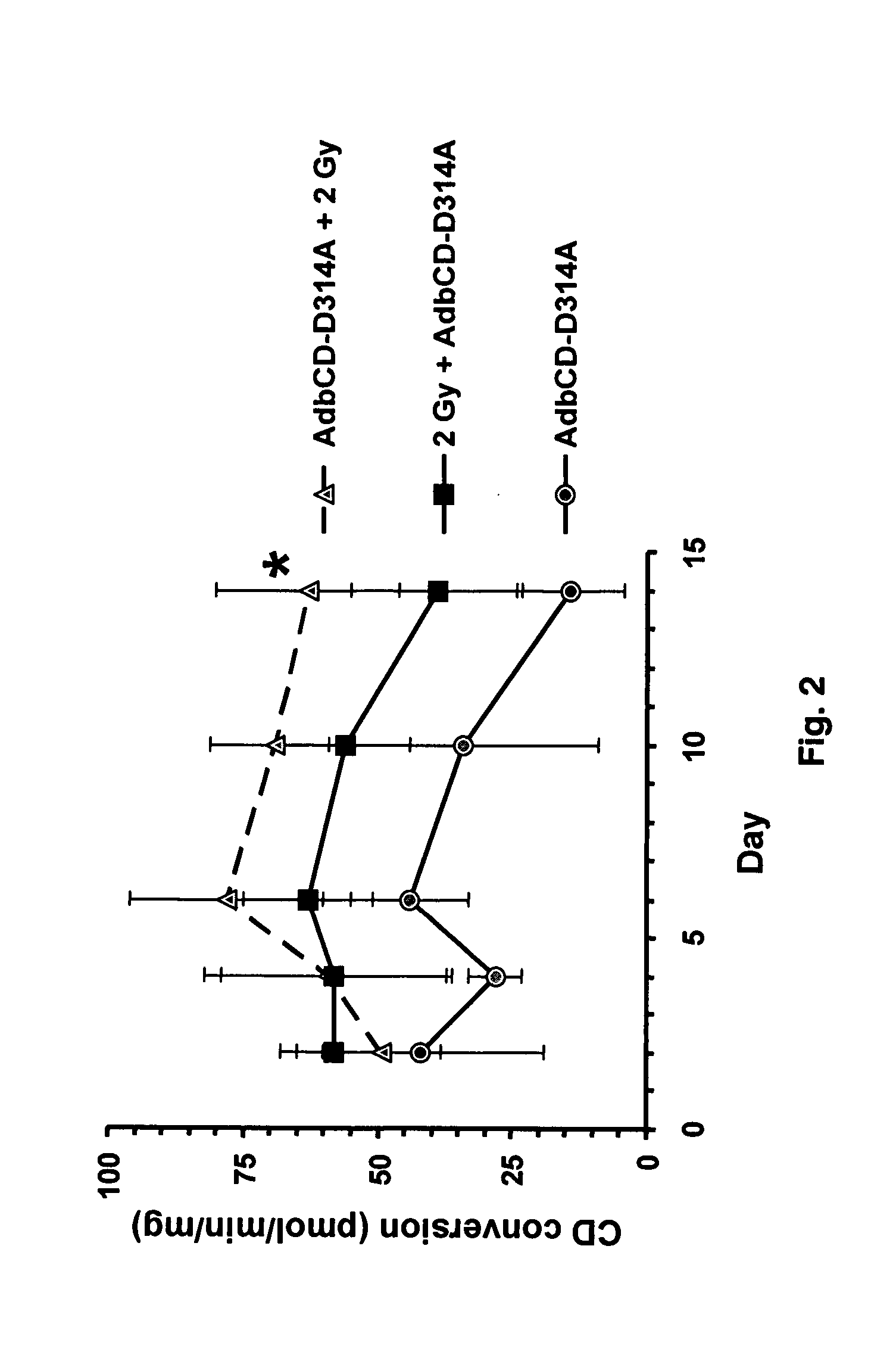
InactiveUS20070225245A1Low efficiencyGreat fold substrate preferenceVectorsHydrolasesCytosine deaminaseHuman glioma
The instant invention has developed viral vectors encoding a mutant bacterial cytosine deaminase (bCD) gene, which have a higher affinity for cytosine than wild type bCD (bCDwt). The purpose of the present invention was to evaluate cytotoxicity in vitro and therapeutic efficacy in vivo of these vectors in combination with the prodrug 5-FC and ionizing radiation against human glioma. The present study demonstrates that infection with the viral vector expressing the mutant cytosine deaminase gene resulted in increased 5-FC-mediated cell killing, compared with vectors expressing the wild-type gene. Furthermore, a significant increase in cytotoxicity following infection with viral vector expressing the mutant cytosine deaminase gene and radiation treatment of glioma cells in vitro was demonstrated as compared to infection with viral vector expressing the wild-type gene. Animal studies showed significant inhibition of subcutaneous or intracranial tumor growth of D54MG glioma xenografts by the combination of AdbCD-D314A / 5-FC with ionizing radiation as compared with either agent alone, and with AdbCDwt / 5-FC plus radiation. These data indicate that combined treatment with this mutant enzyme / prodrug therapy and radiotherapy provides a promising approach for cancer therapy.
Owner:BUCHSBAUM DONALD J +3
Xenograft heart valves
The invention provides an article of manufacture comprising a substantially non-immunogenic heart valve xenograft for implantation into humans. The invention further provides methods for preparing a heart valve xenograft by removing at least a portion of a soft tissue from a non-human animal to provide a xenograft; washing the xenograft in saline and alcohol; subjecting the xenograft to cellular disruption treatment; treating the xenograft with crosslinking agents, and digesting the xenograft with a proteoglycan-depleting factor and / or glycosidase. The invention also provides an article of manufacture produced by the above-identified method of the invention. The invention further provides a heart valve xenograft for implantation into a human including a portion of a heart valve from a non-human animal, wherein the portion has extracellular components and substantially only dead cells. The extracellular components have reduced proteoglycan molecules. Each of the xenografts of the invention are substantially non-immunogenic and have substantially the same mechanical properties as a corresponding native heart valve.
Owner:APERION BIOLOGICS
Sterile Autologous, Allogenic or Xenogenic Implant and the Method of its Production
ActiveUS20100291172A1Reduction of wound bleedingReduction of seepingAntibacterial agentsBiocideChemistryAliphatic alcohol
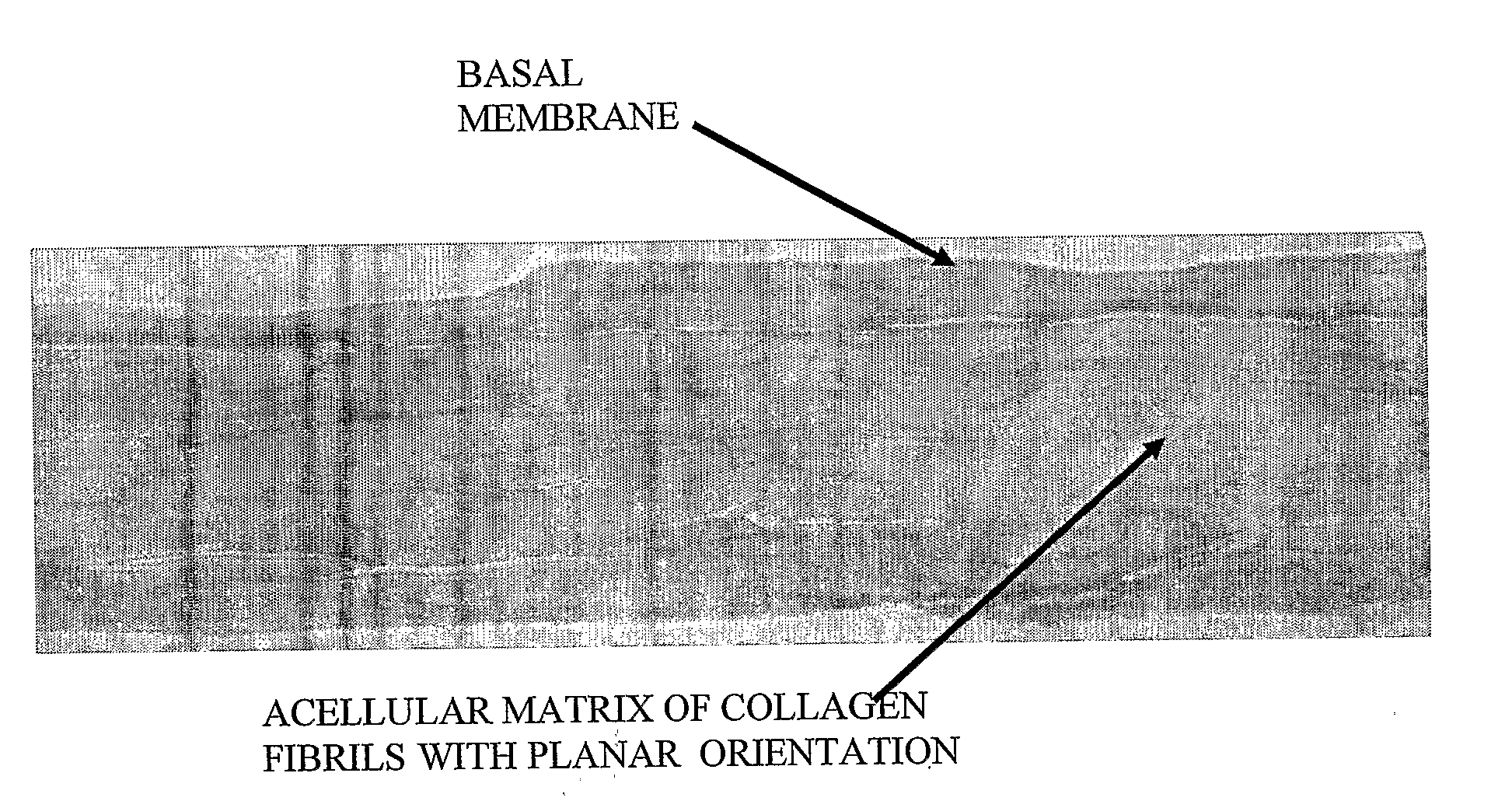
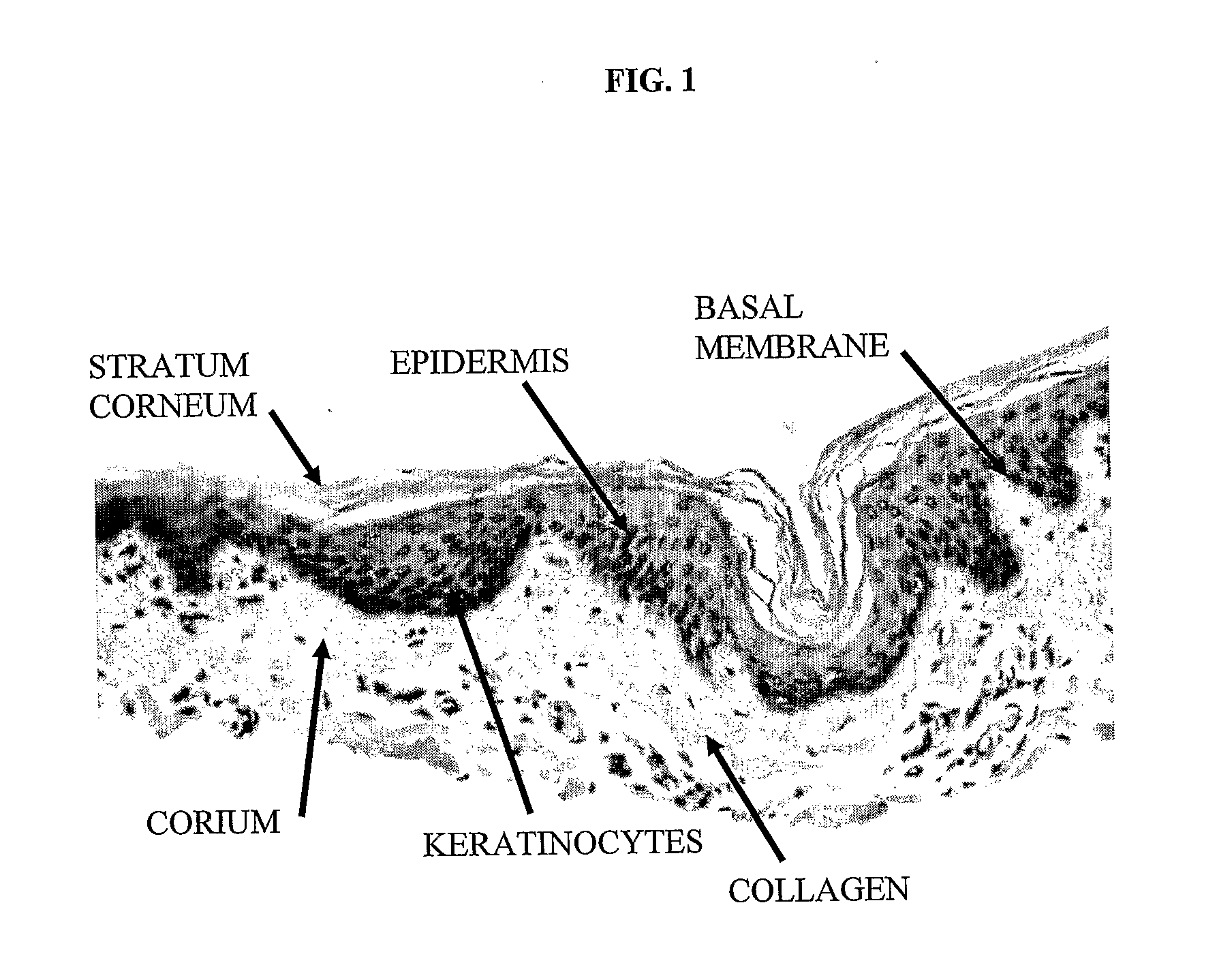
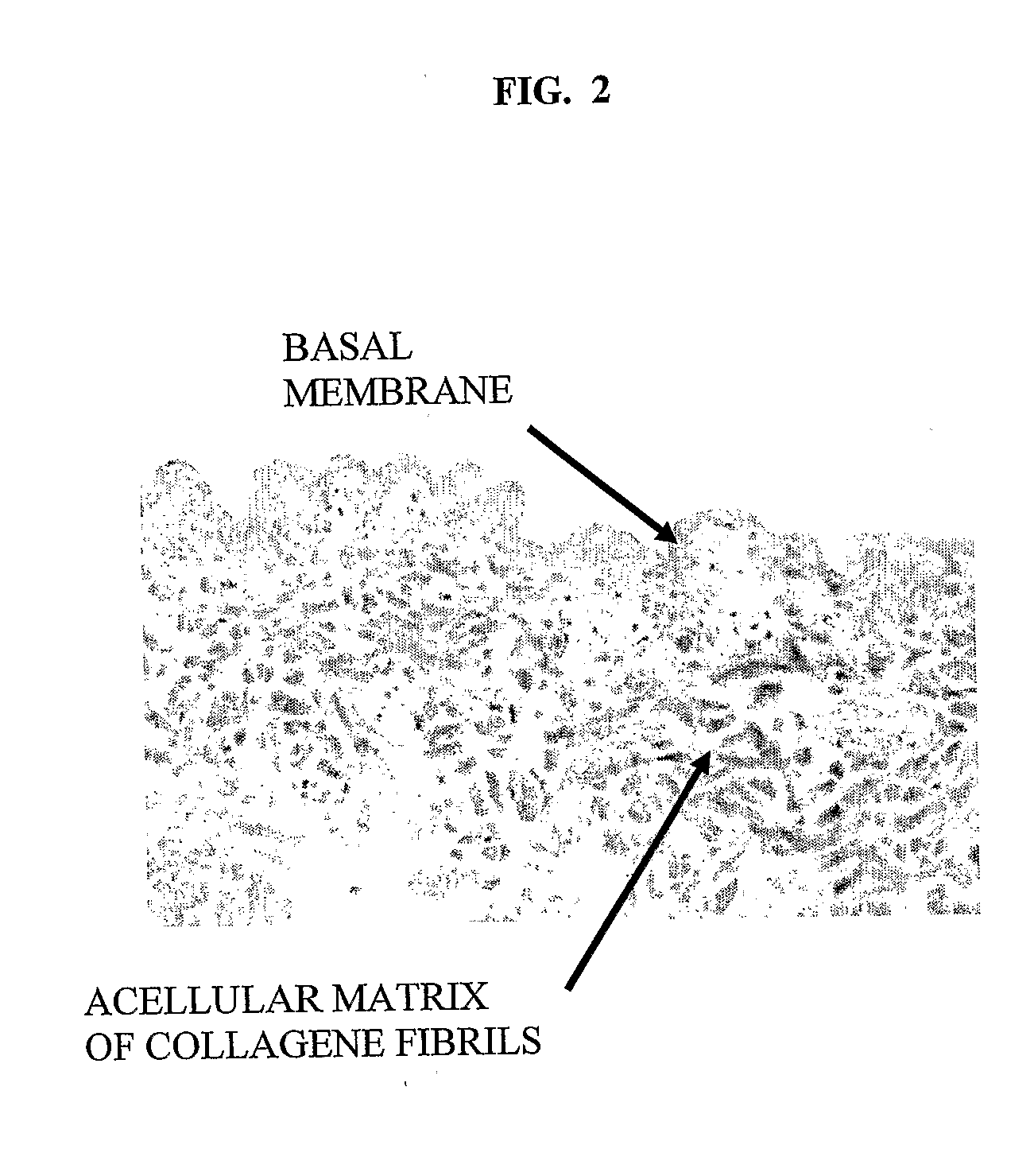
The subject of the invention is a sterile, dehydrated acellular implant, which during its rehydration by water or bodily fluids displays anisotropic expansion and can act as a substrate for adhesion, migration and growth of live cells. Collagen structures of the transplant are at least partially denatured through the action of heat or organic solvents, such as lower aliphatic alcohols and ketones, which simultaneously act as preservative and sterilization agents, especially for certain types of viruses. The implant is sterilized by radiation while in an substantially dehydrated state, preferably by accelerated electrons. The transplant can be derived from various animal tissues, especially mammalian tissues, such as human or porcine tissues. The tissues suitable for the invention can be, for example, skin, placenta, pericardium, peritoneum, intestinal wall, tendon, blood vessel, etc. The implant is suitable for use in human and veterinary medicine, for instance as a temporary wound and burn cover, for the repair, substitution and regeneration of tissues, and also as a substrate for laboratory cell cultivation.
Owner:MEDICEM TECH SRO
Lung cancer-targeted peptides and applications thereof
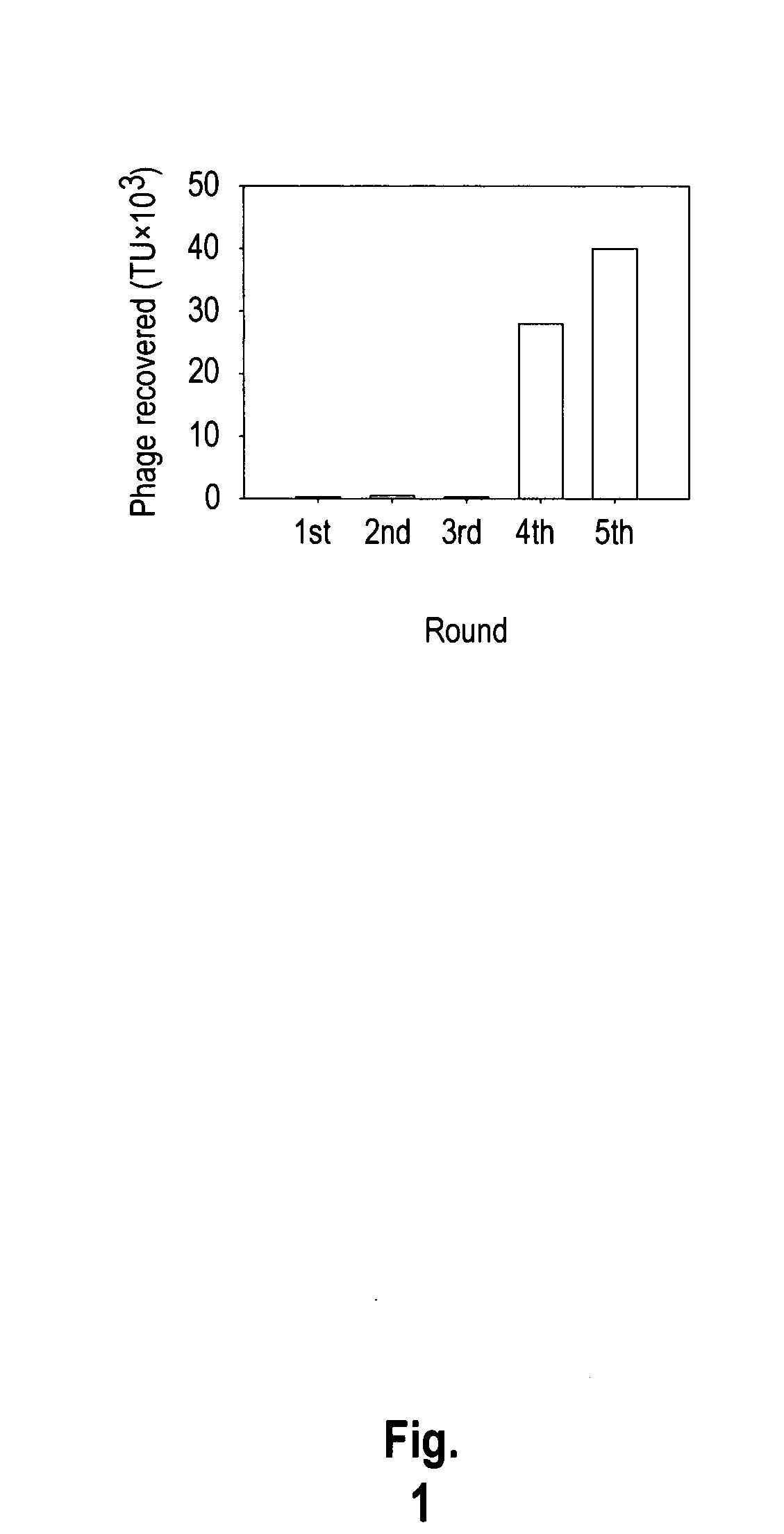
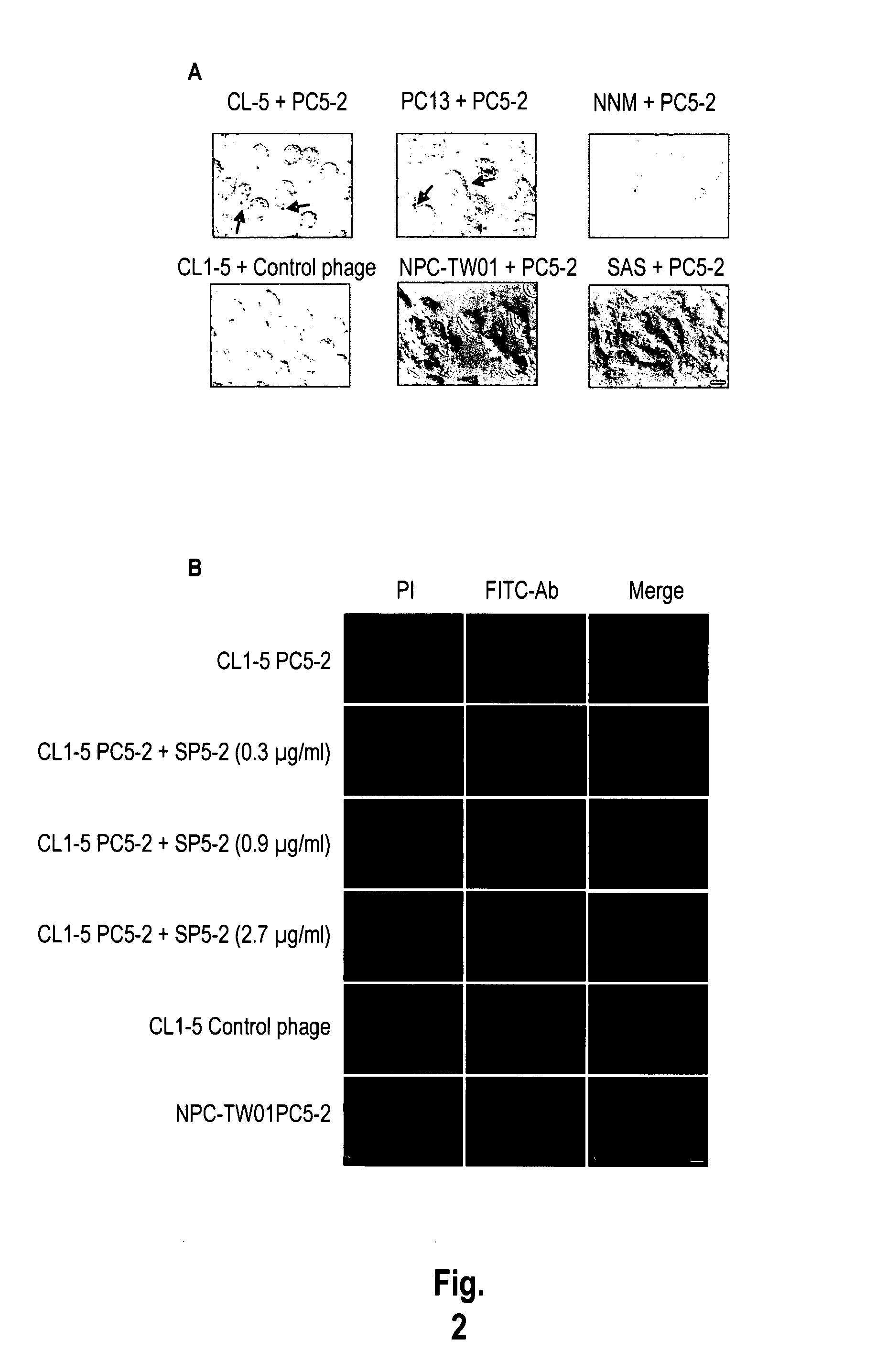
The invention provides nucleic acids, peptides, and antibodies for use in applications including diagnosis and therapy. The peptides target lung cancer and were identified by phage display. Targeting phage PC5-2 and synthetic peptide SP5-2 were both able to recognize human pulmonary tumor specimens from lung cancer patients. In SCID mice bearing NSCLC xenografts, the targeting phage was able to target tumor masses specifically. When the peptide was coupled to liposomes containing the anti-cancer drugs vinorelbine or doxorubicin, the efficacy of these drugs against human lung cancer xenografts was improved, the survival rate increased, and the drug toxicity was reduced.
Owner:ACAD SINIC +1
Immunosuppression
InactiveUS20060134124A1Improve survivalImprove toleranceAntibody mimetics/scaffoldsSnake antigen ingredientsTolerabilityHeterografts
The invention herein described relates to a method to improve the tolerance of a mammal, preferably a human, to a xenograft through immunisation of the recipient mammal with an immunogen comprising both a B cell epitope derived from porcine polypetides and T cell epitope. The invention also encompasses immunogenic compositions comprising said immunogens and methods to monitor the status of the xenograft.
Owner:ML LAB PLC
Generation of antigen specific T suppressor cells for treatment of rejection
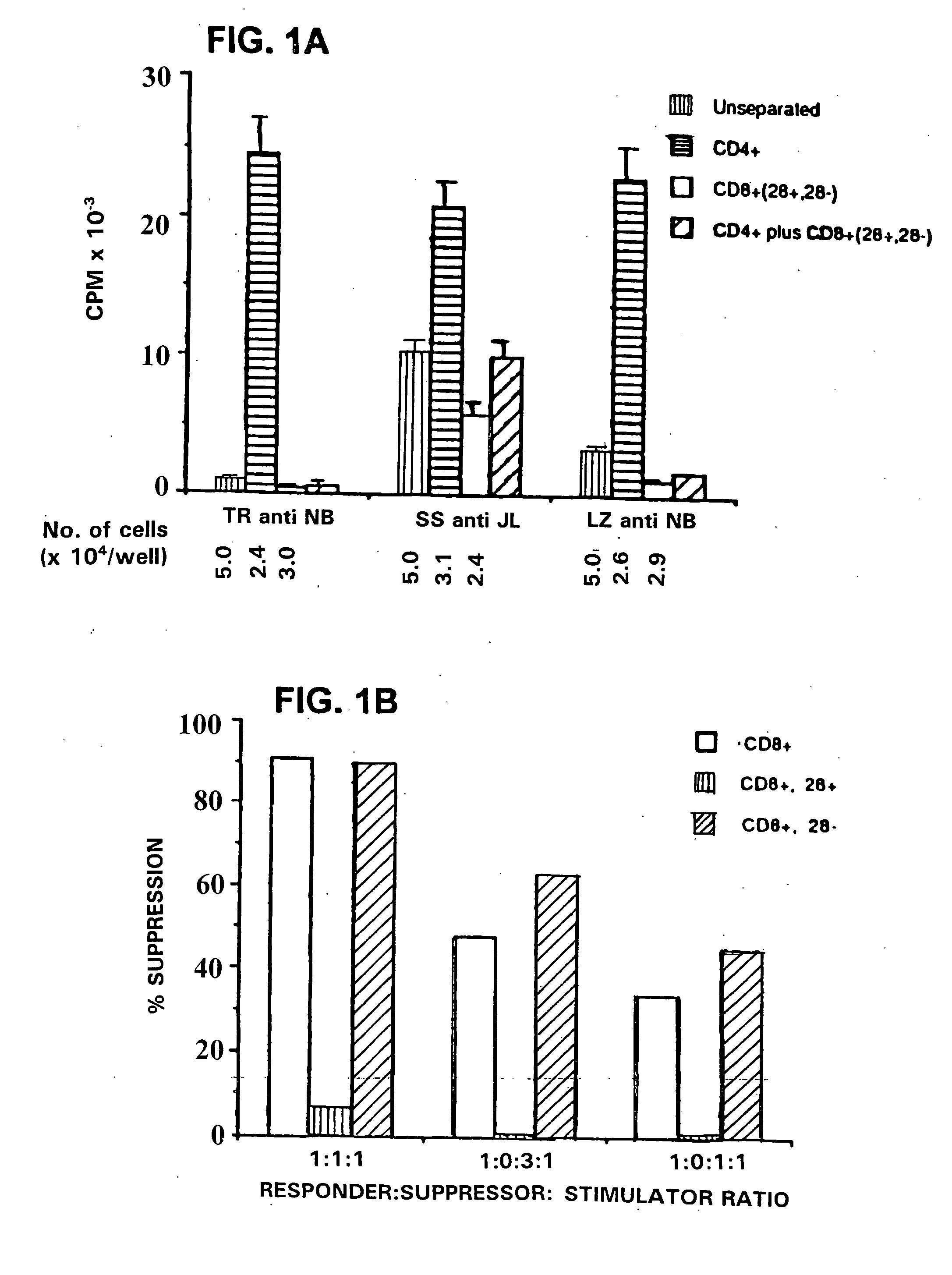
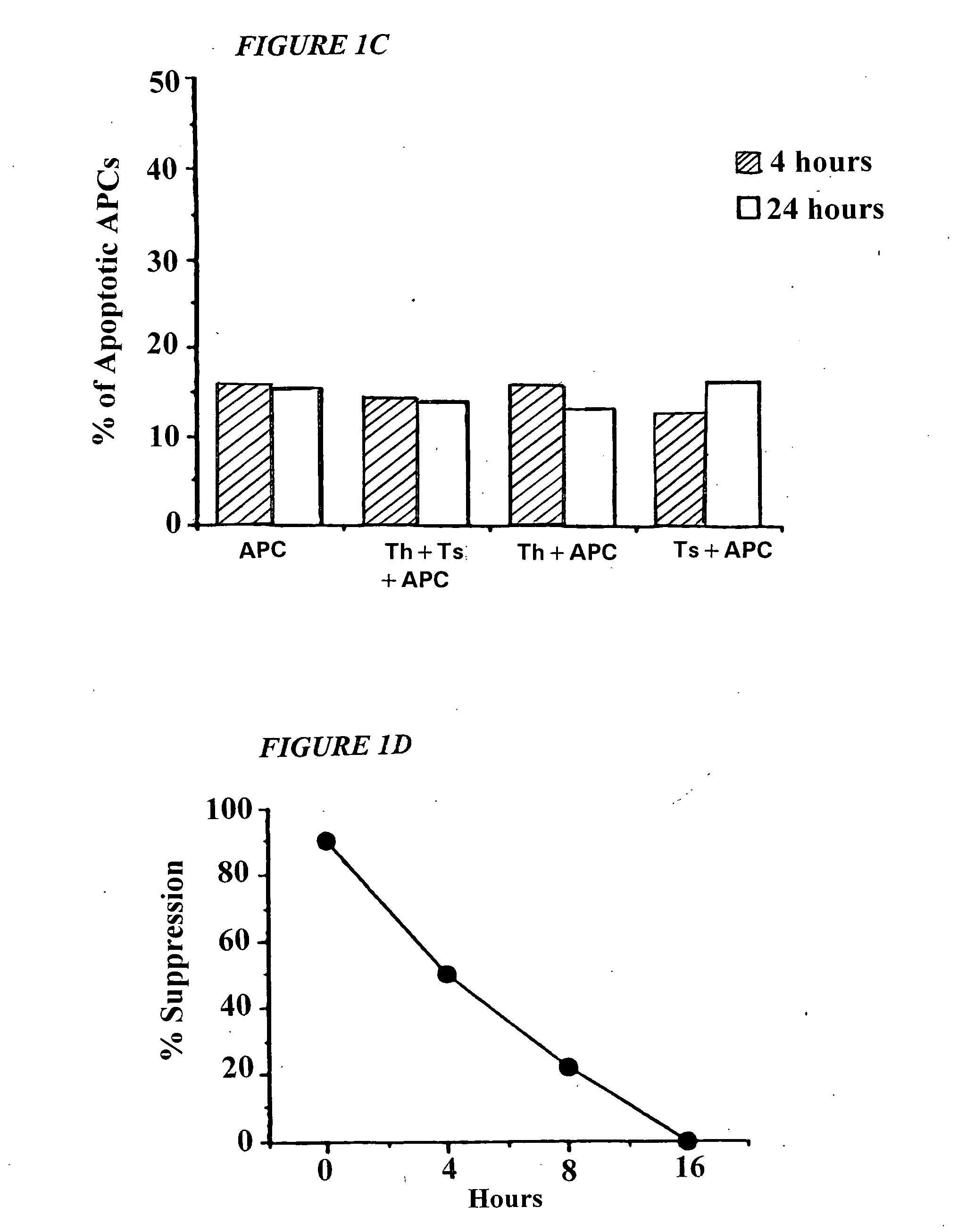
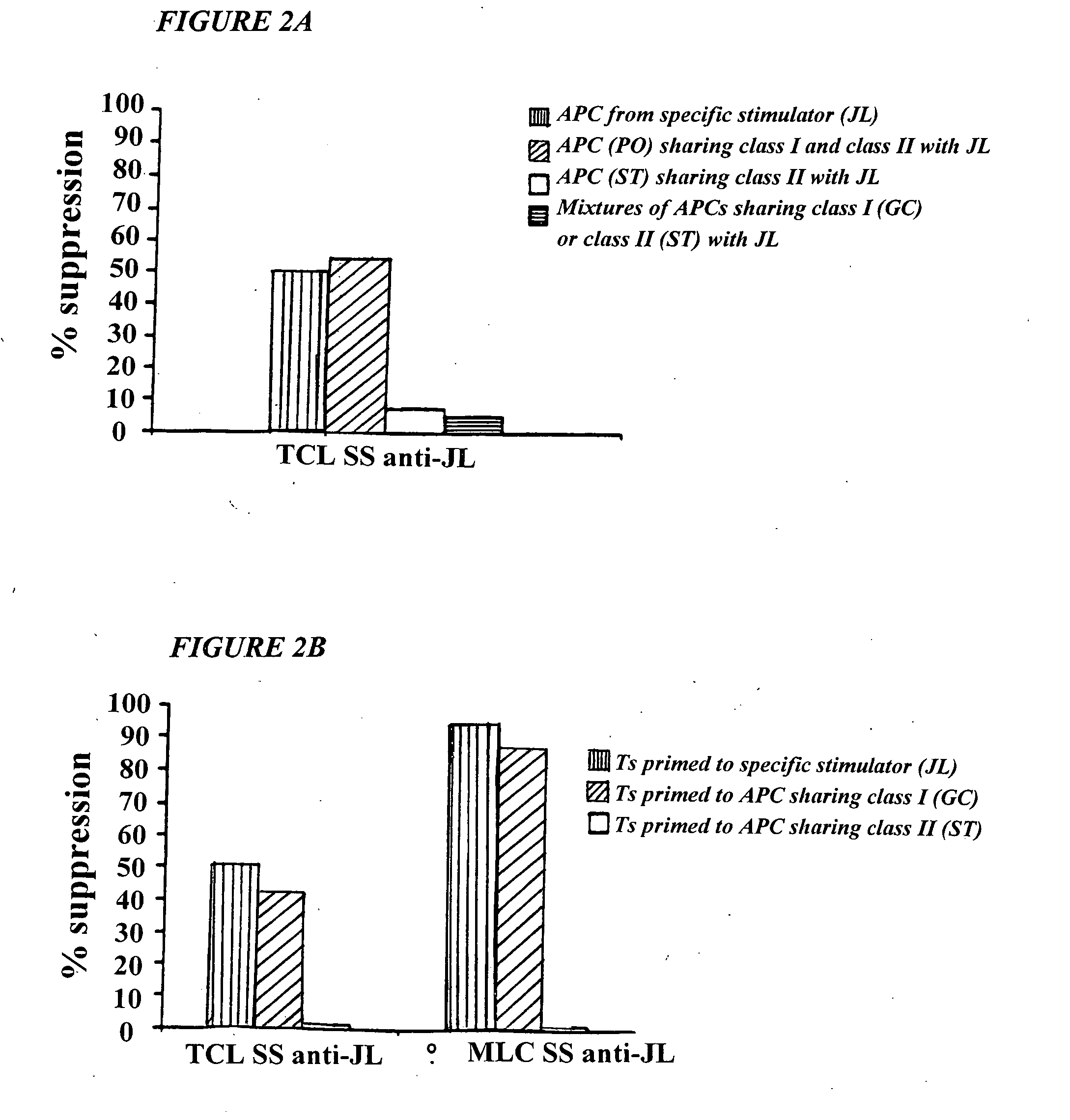
InactiveUS20050250161A1Preventing autoimmune diseaseReduce riskGenetically modified cellsMicrobiological testing/measurementDiseaseHeterografts
This invention provides a method of generating antigen specific allospecific human-suppressor CD8+CD28− T cells. This invention also provides a method of generating xenospecific human suppressor CD8+CD28− T cells. This invention further provides a method of generating allopeptide antigen specific human suppressor CD8+CD28− T cells. Methods of treatment for, reduction of risk of rejection of allografts and xenografts and autoimmune diseases using the human suppressor CD8+CD28− T cells so produced are also provides, as are methods of preventing rejection and autoimmune diseases, and vaccines comprising the produced suppressor T cells. Methods of diagnosis to determine whether a level of immuno-suppressant therapy requires a reduction are provided.
Owner:THE TRUSTEES OF COLUMBIA UNIV IN THE CITY OF NEW YORK
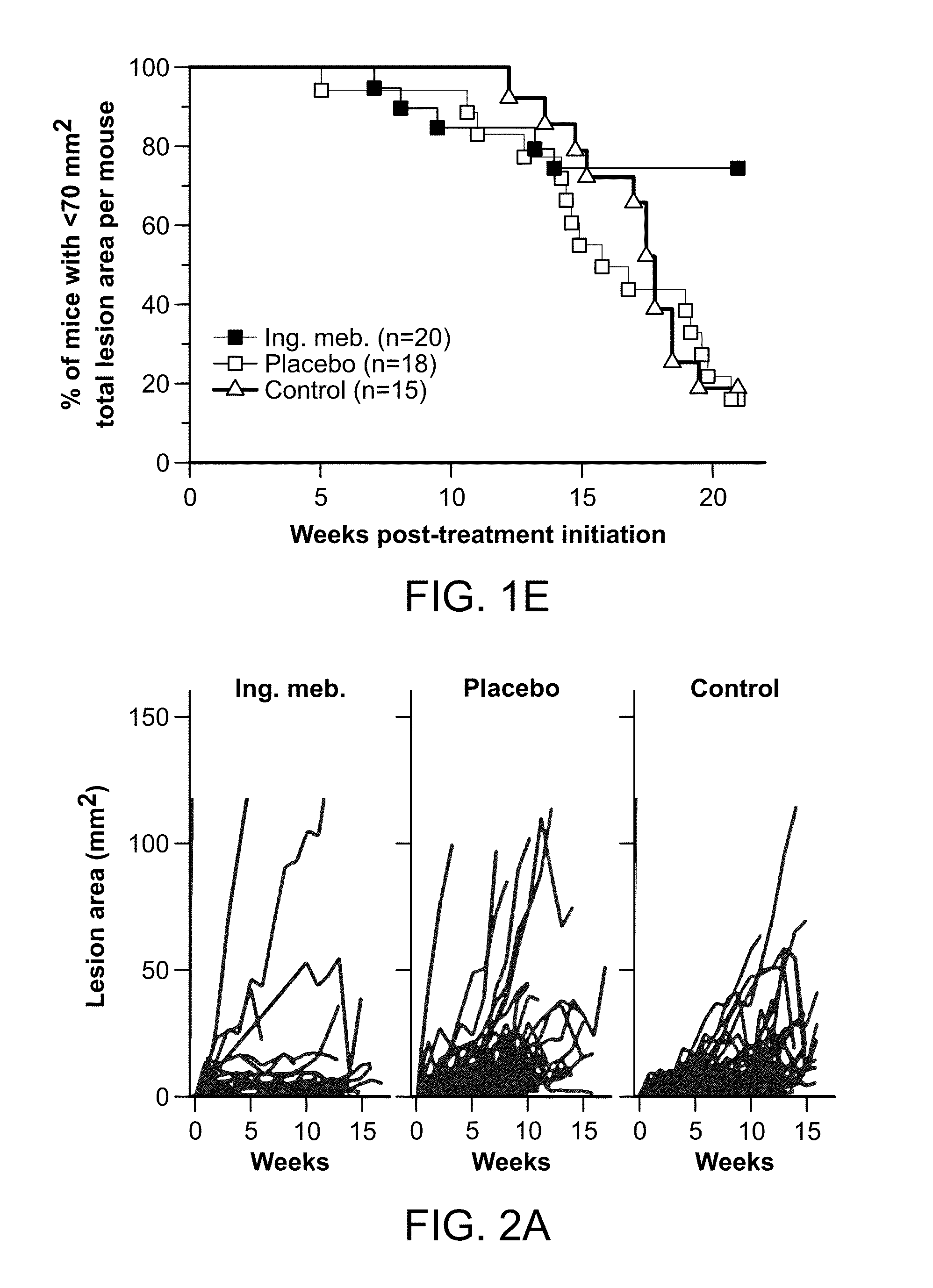
Methods for treating uv-damaged skin and scc tumors and for removing tattoos with topical ingenol mebutate
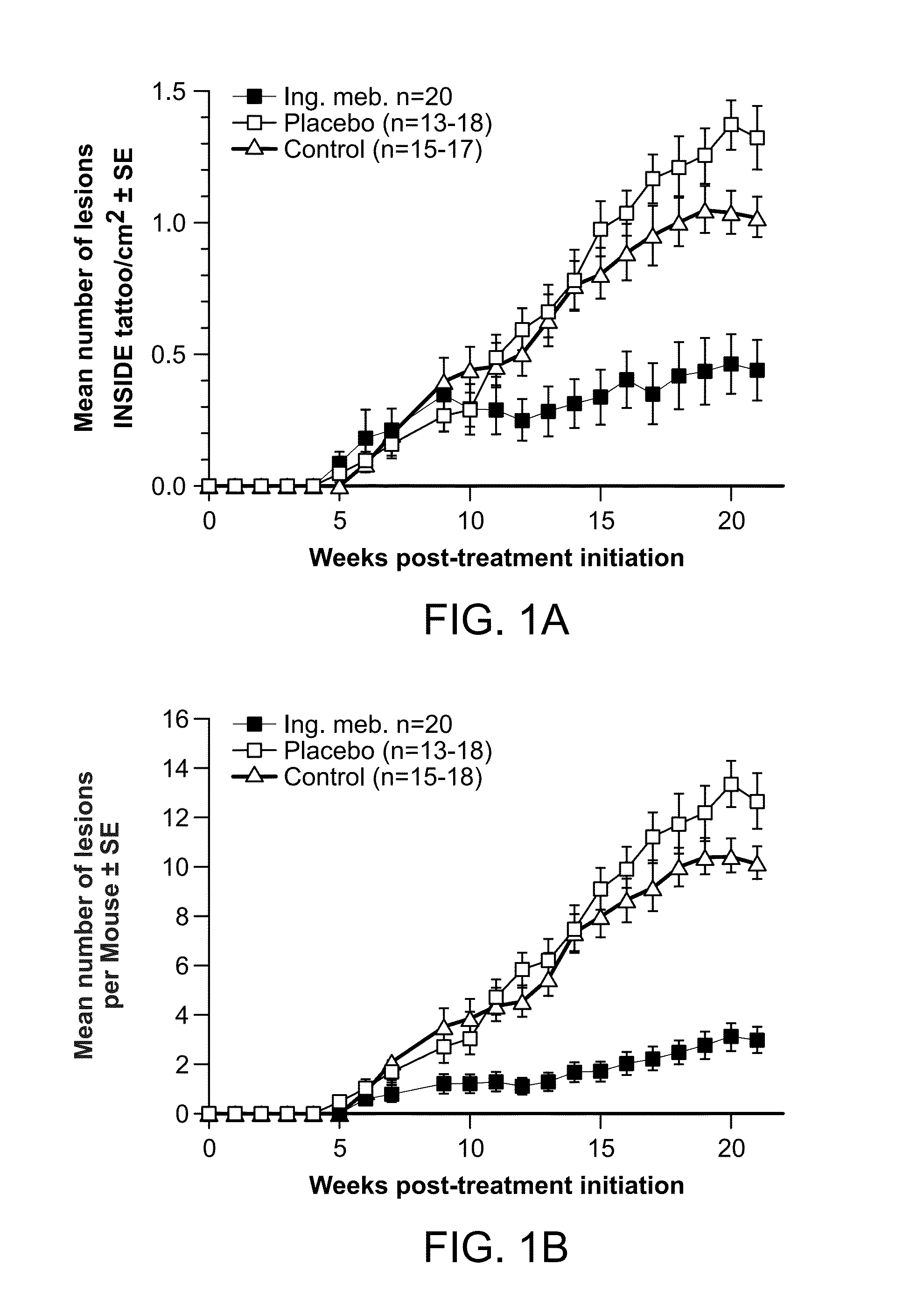
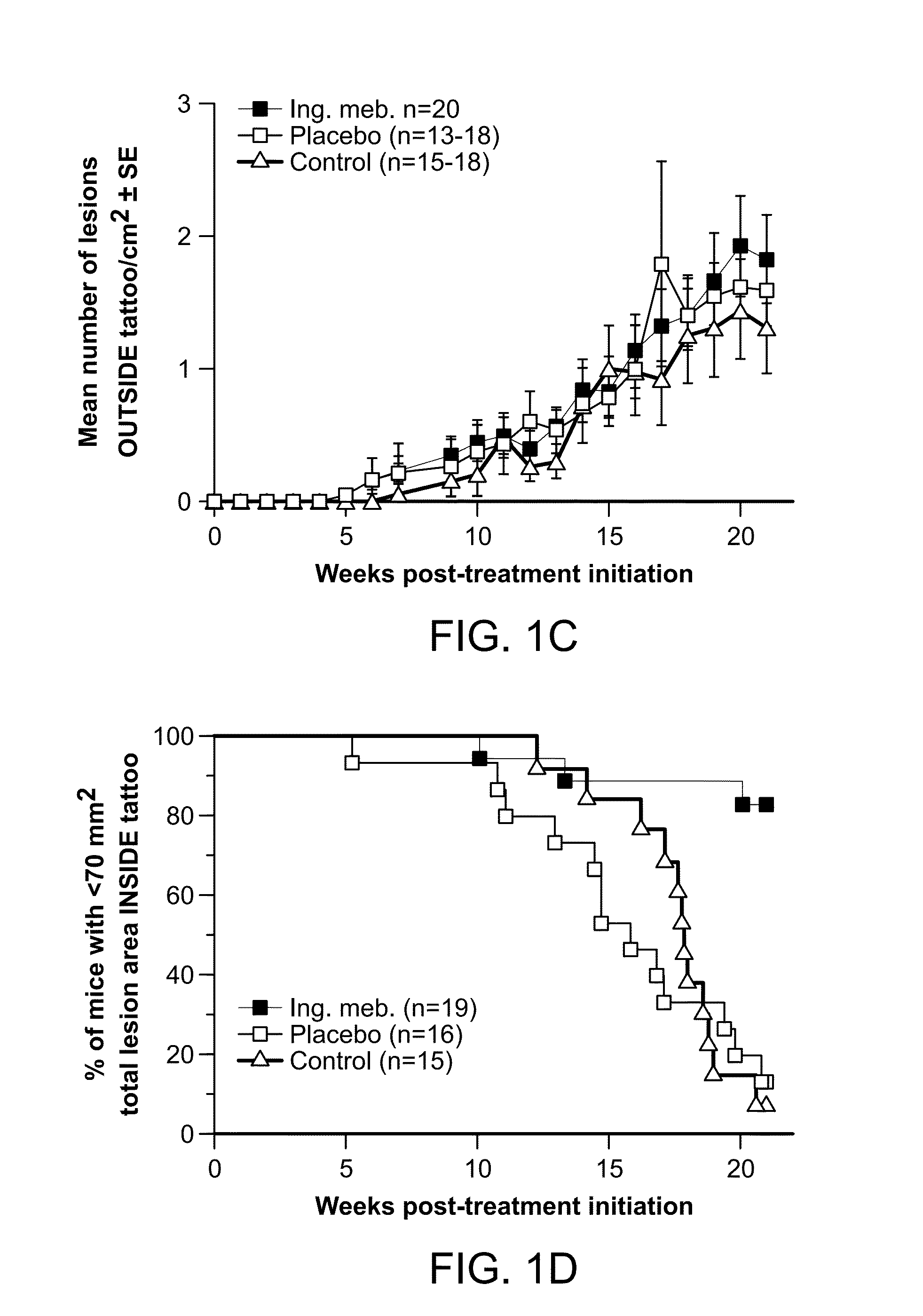
InactiveUS20140242012A1Reduce and cure nymberReduce in quantityCosmetic preparationsBiocideAbnormal tissue growthHeterografts
The present invention is directed to the prophylatic field treatment of photodamaged skin with topical ingenol mebutate. More specifically, the present invention concerns field-directed treatment of UV-damaged skin with topical ingenol mebutate for reducing the number of skin lesions that emerge from the UV-damaged skin over time. In addition, the present invention concerns field-directed treatment for removing photodamaged skin, mutated keratinocytes, cutaneous immunosuppressive environments and / or p53+ patches caused by UV with topical ingenol mebutate. By way of example, the present invention is directed to treating photodamaged skin with topical ingenol mebutate at about 0.05% concentration.The present invention is also concerned with the treatment of SCC tumors with topical ingenol mebutate for reducing the number of SCC tumors. By example, the present invention is directed to treating and curing SCC xenografts with topical ingenol mebutate at about 0.25% concentration.The present invention is further directed to a topical field-directed treatment for the removal of tattoos from skin with ingenol mebutate. By way of example, the present invention is directed to removing tattoos with topical ingenol mebutate at concentrations of up to about 0.25%.
Owner:LEO LAB
Xenograft soft tissue implants and methods of making
The present application is directed to the field of implants comprising soft tissue for use in implantation in humans. The soft tissue implants of the present application are preferably obtained from xenograft sources. The present application provides a chemical process that sterilizes, removes antigens from and / or strengthens xenograft implants. The present techniques yield soft tissue implants having superior structural, mechanical, and / or biochemical integrity. The present application is also directed to processes for treating xenograft implants comprising soft tissues such as dermis, and to implants produced by such processes.
Owner:TUTOGEN MEDICAL INC
Determining the capability of a test compound to affect solid tumor stem cells
InactiveUS8044259B2Reduce spreadIncreased proliferationMaterial nanotechnologyDrug screeningPrimary tumorImmunodeficient Mouse
A small percentage of cells within an established solid tumor have the properties of stem cells. These solid tumor stem cells give rise both to more tumor stem cells and to the majority of cells in the tumor that have lost the capacity for extensive proliferation and the ability to give rise to new tumors. Thus, solid tumor heterogeneity reflects the presence of tumor cell progeny arising from a solid tumor stem cell. We have developed a xenograft model in which we have been able to establish tumors from primary tumors via injection of tumor cells in the mammary gland of severely immunodeficient mice. These xenograft assay have allowed us to do biological and molecular assays to characterize clonogenic solid tumor stem cells. We have also developed evidence that strongly implicates the Notch pathway, especially Notch 4, as playing a central pathway in carcinogenesis.
Owner:ONCOMED PHARMA INC +1
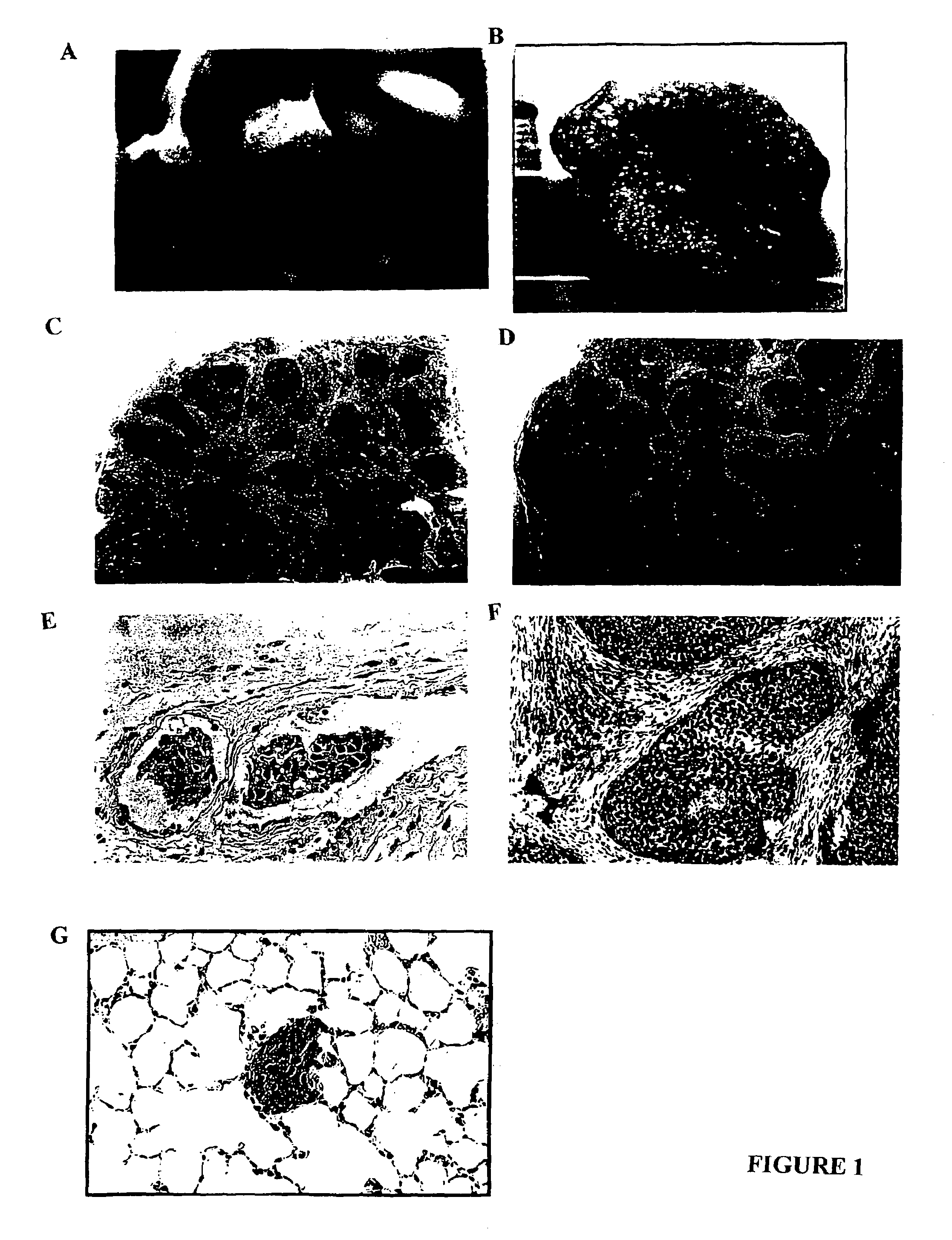
Human inflammatory breast carcinoma xenograft capable of lymphovascular invasion and methods for its use
InactiveUS6998513B1Prevention of lymphovascular invasionGrowth inhibitionCompounds screening/testingDiagnosticsHeterograftsLymphatic Spread
The present invention provides human transplantable inflammatory breast carcinoma xenografts. Such xenografts exhibit a number of unique characteristics which allows their use in experimental models of inflammatory carcinoma in order to dissect out the molecular basis of this phenotype. This experimental model of inflammatory carcinoma can be used to identify molecular targets for therapeutic intervention and to assess the efficacy of a broad spectrum of diagnostic and therapeutic agents. Specific animal models of inflammatory breast cancer are described as well as methods for evaluating diagnostic and therapeutic agents for treating inflammatory breast cancer. Methods for identifying molecules whose expression is modulated in inflammatory breast cancer are provided. In addition, methods for diagnosing and inhibiting the growth of inflammatory breast cancer metastases in vivo are provided.
Owner:RGT UNIV OF CALIFORNIA
Antigenic fusion protein carrying Galalpha 1,3Gal epitopes
InactiveUS20050255099A1Easy and cheap to produceSimple designOrganic active ingredientsPeptide/protein ingredientsEpitopeHeterografts
Owner:RECOPHARMA AB
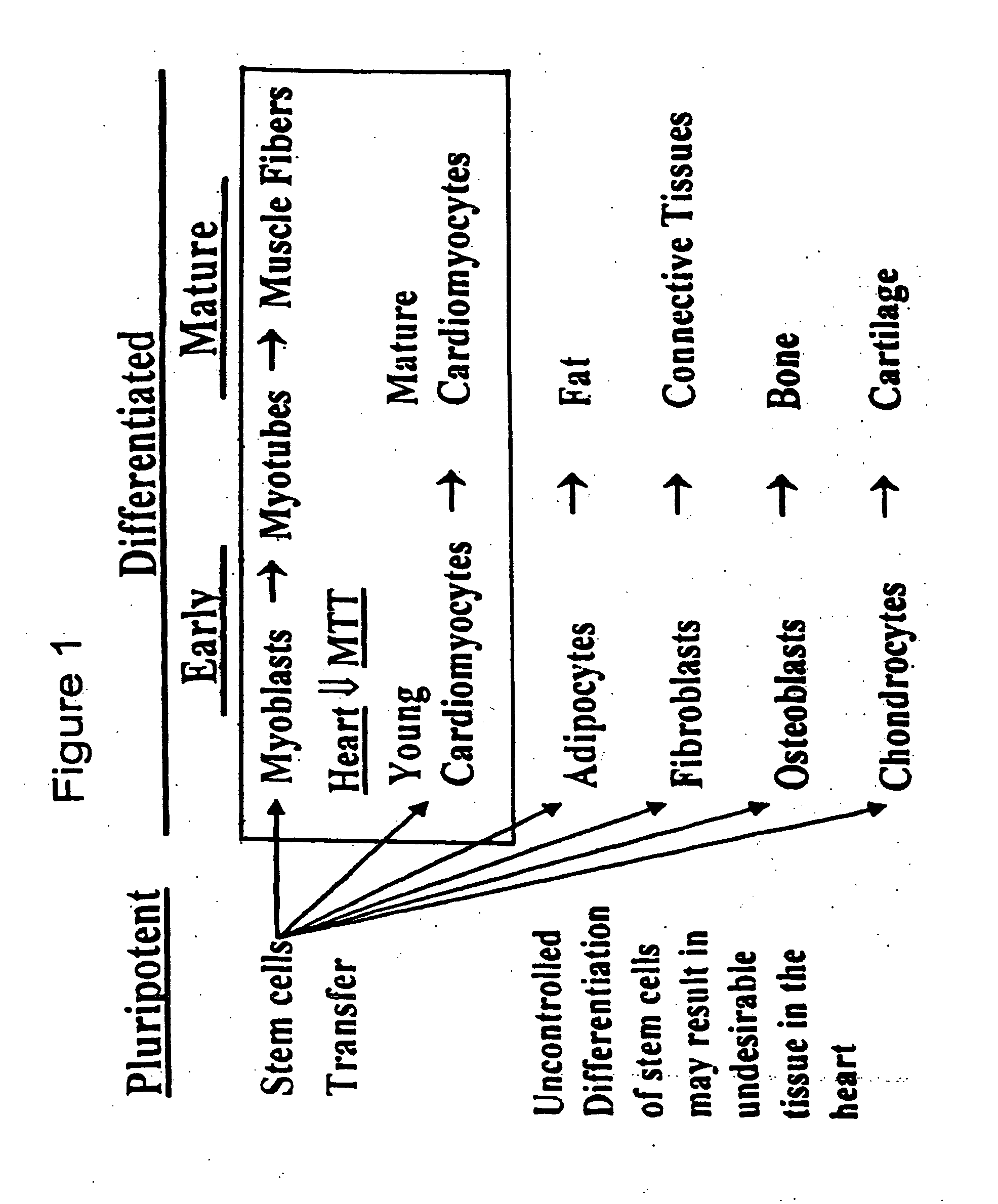
Myoblast treatment of diseased or weakened organs
InactiveUS20070009499A1Increase blood flowImprove bindingBiocidePeptide/protein ingredientsHeterograftsWeakness
Bioengineering the regenerative heart or other body organ in need of greater muscle mass or improved blood perfusion provides a novel treatment for organ weakness or failure. In the case of cardiac failure treatment, on May 14, 2002, a 55-year-old man suffering ischemic myocardial infarction received 25 injections carrying 465 million cGMP-produced pure myoblasts into his myocardium after coronary artery bypass grafting. Three myogenesis mechanisms were elucidated with 17 human / porcine xenografts using cyclosporine as immunosuppressant. Some myoblasts developed to become cardiomyocytes. Others transferred their nuclei into host cardiomyocytes through natural cell fusion. As yet others formed skeletal myofibers with satellite cells. De novo production of contractile filaments augmented heart contractility. Human myoblasts transduced with VEGF165 gene produced six times more capillaries in porcine myocardium than placebo. Xenograft rejection was not observed for up to 20 weeks despite cyclosporine discontinuation at 6 weeks.
Owner:LAW PETER
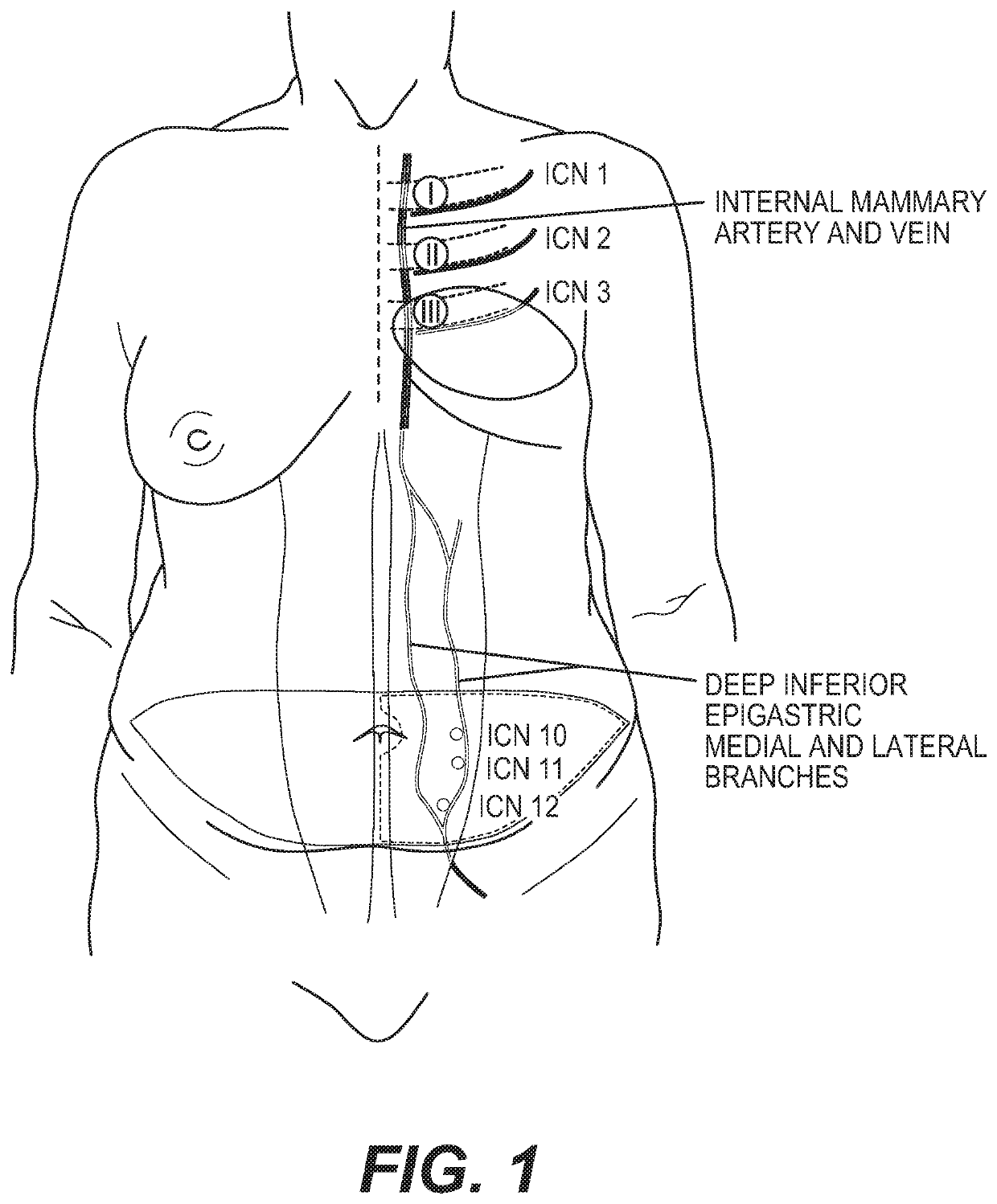
Materials and methods for nerve repair with animal-sourced grafts
ActiveUS20200155156A1Overcomes shortcomingPromote repairSurgeryTissue regenerationHeterograftsIntercostal nerves
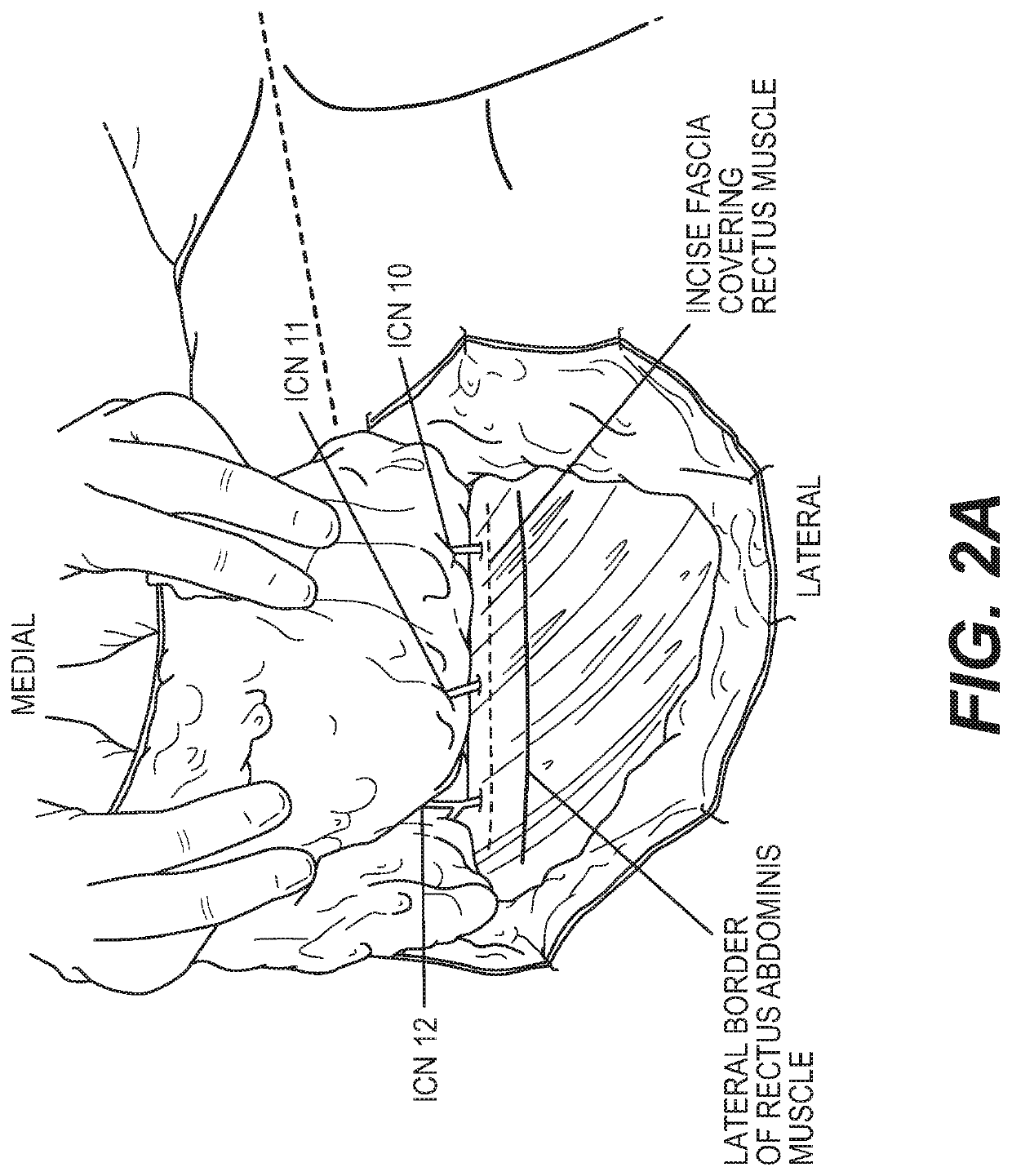
The subject invention pertains to materials, including sets of nerve grafts, for performing breast neurotization with xenograft nerves in breast surgeries, such as reconstructive breast surgery. Certain embodiments of the set of nerve grafts comprise at least two nerve grafts prepared from one or more nerves, such as one or more intercostal nerves (ICNs), obtained from one or more animal sources. Such animal-sourced nerve grafts may be used as xenografts in the reconstruction of nerve defects in humans, and in particular, animal-sourced ICN grafts may be used as xenografts in the reconstruction of ICN nerve defects in humans, including through use of the breast neurotization technique described herein. These animal-sourced nerve grafts may also be used in the reconstruction of nerve defects in animal recipients, including as xenografts, allografts and autografts.
Owner:AXOGEN CORP
Combined therapy of alpha-1-antitrypsin and temporal t-cell depletion for preventing graft rejection
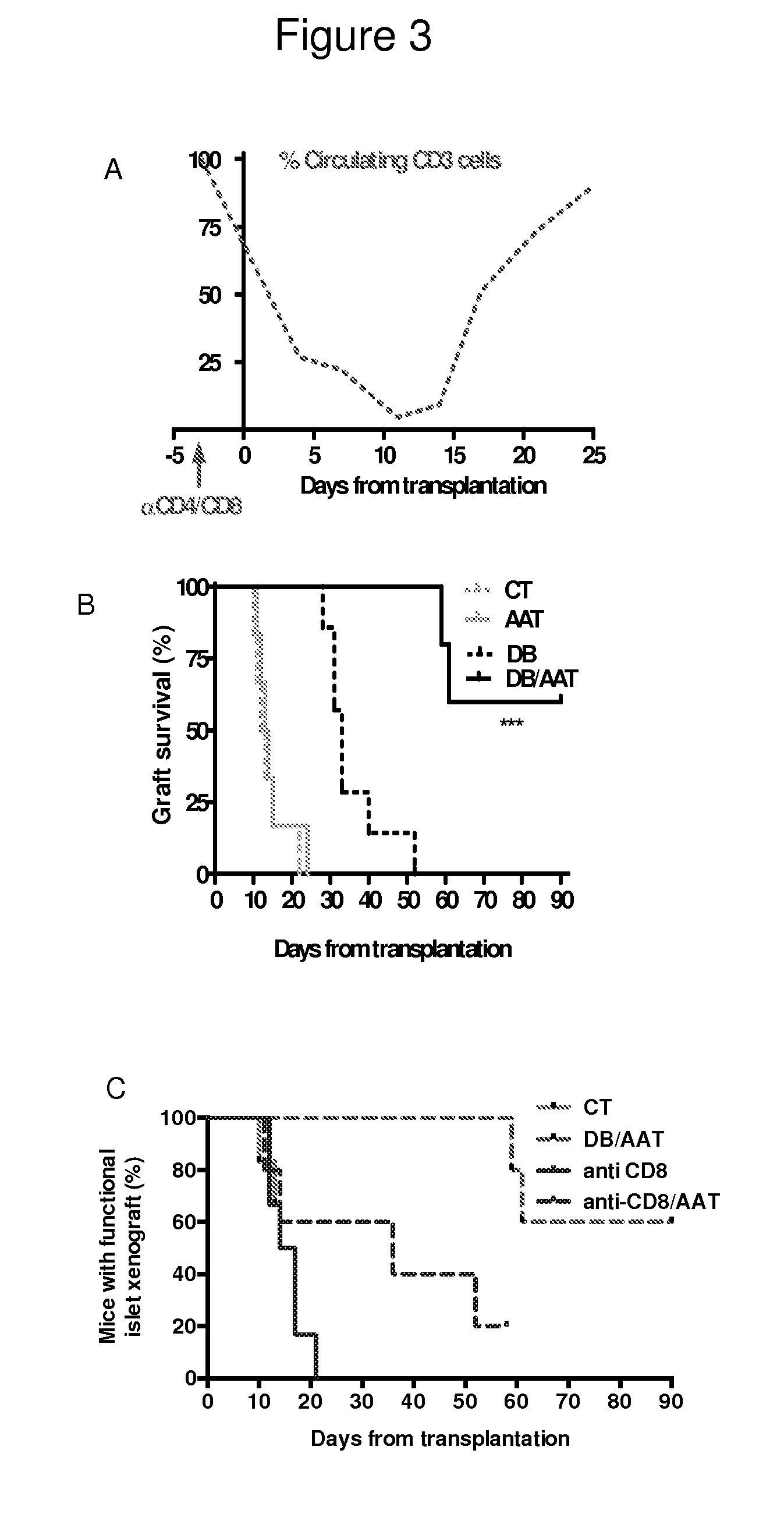
InactiveUS20150010581A1Prolonging xenograft survivalImprove survivalSaccharide peptide ingredientsAntibody ingredientsAntiendomysial antibodiesHeterografts
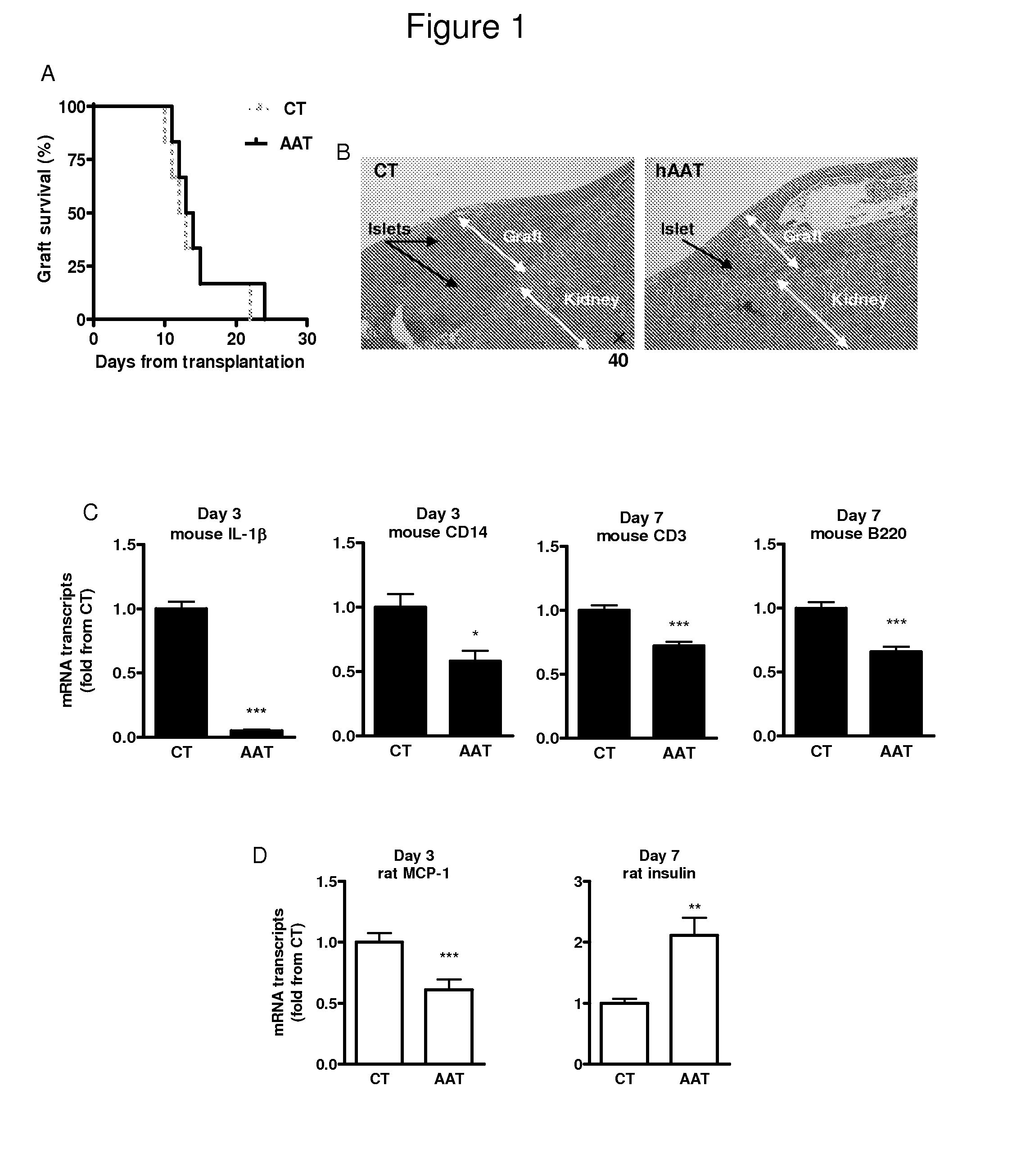
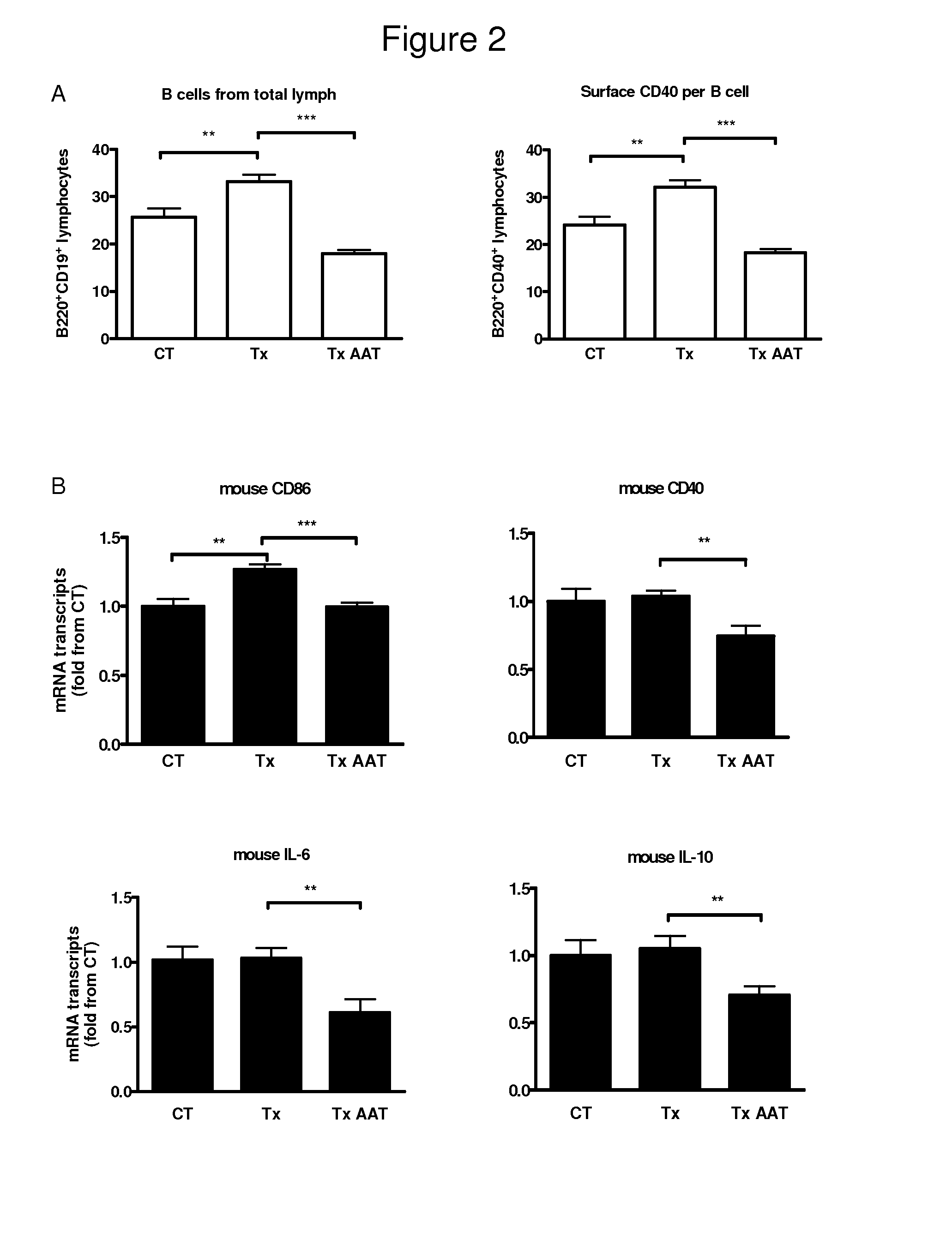
The present invention provides compositions and methods for the prevention and treatment of aggressive immune processes to life-saving grafts, such as xenograft rejection, and for attenuating host responses in transplantation of tissues, cells and organs. More specifically, the compositions and methods of the present invention relate to combined therapies comprising treatment of alpha-1-antitrypsin (AAT) and temporary T-cell depletion including but not limited to anti-CD4 and anti-CD8 antibodies.
Owner:BEN GURION UNIVERSITY OF THE NEGEV
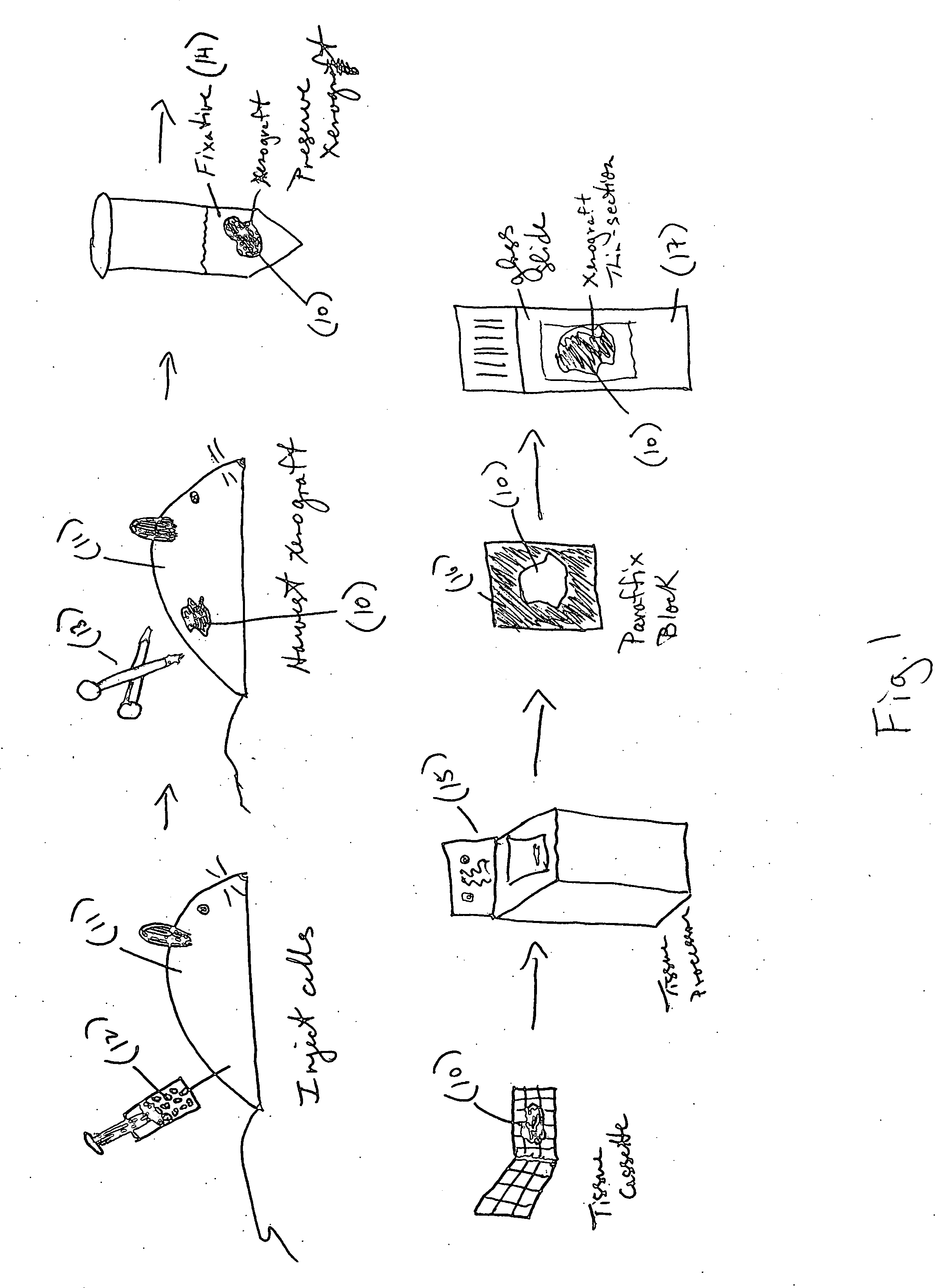
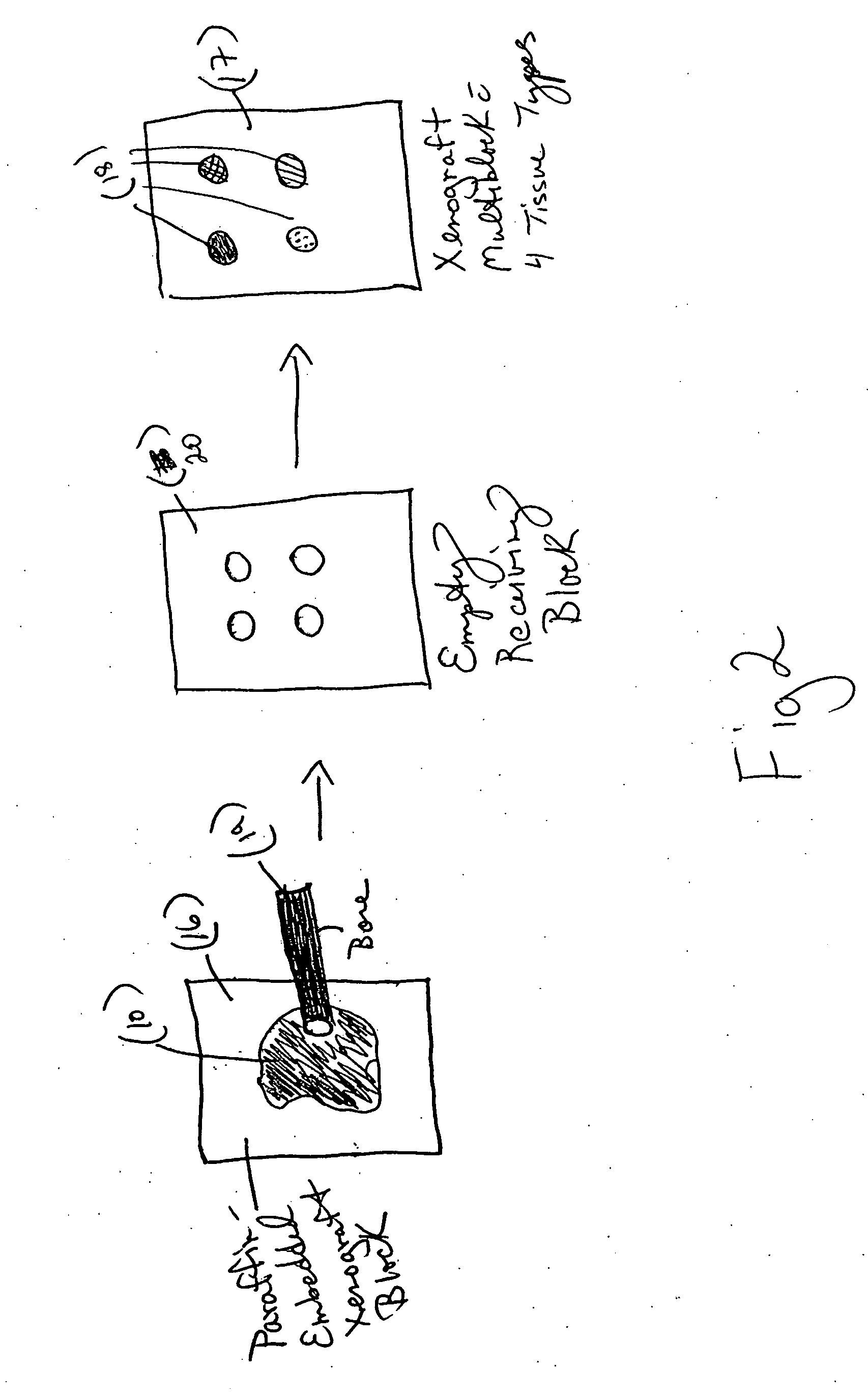
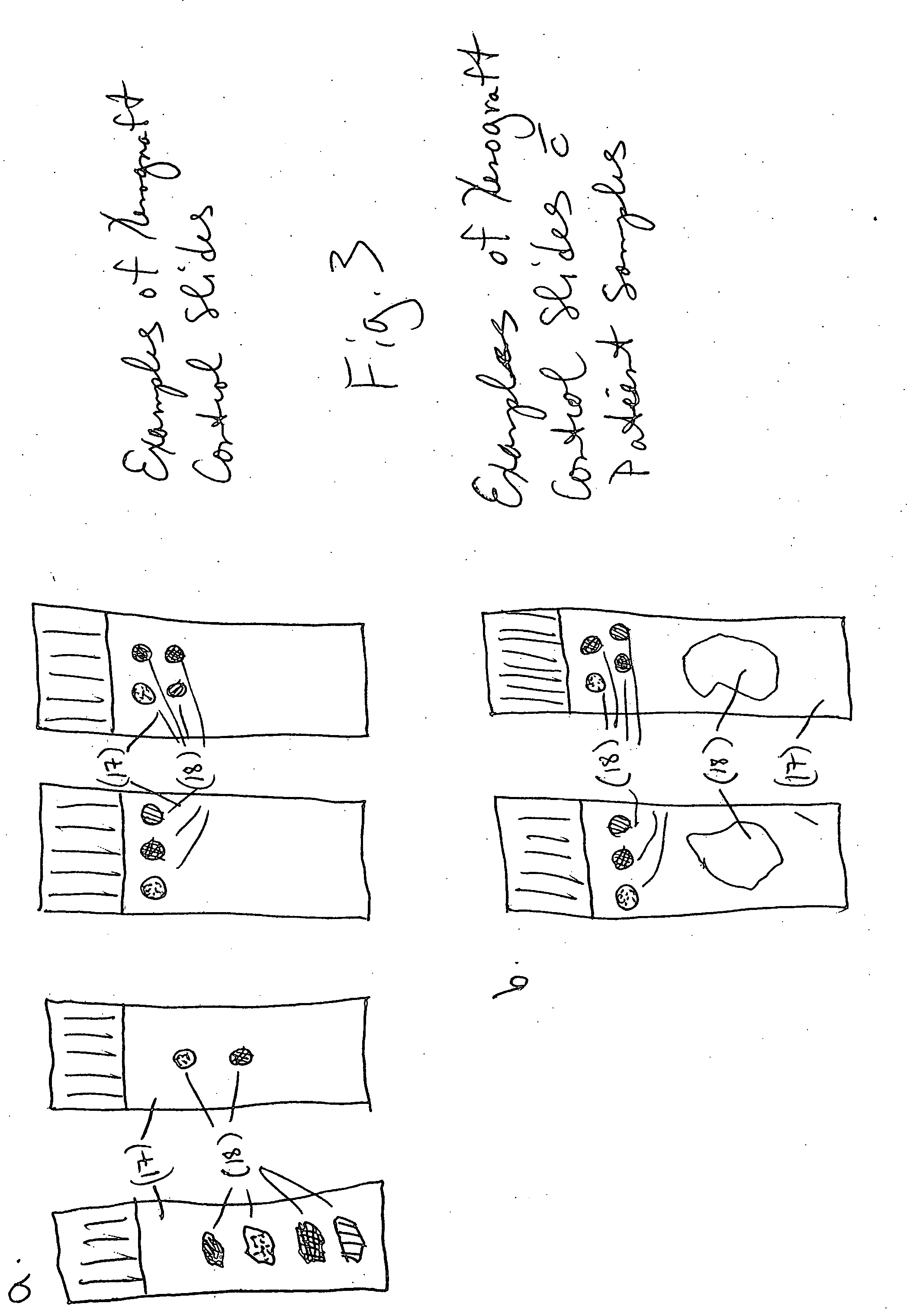
Xenograft tissue control for histology
InactiveUS20060246536A1Highly manufacturablePromote resultsPreparing sample for investigationVertebrate cellsAntigenHeterografts
An embodiment of the invention is a method of using a xenograft as a control tissue for histology, comprising staining both a patient and a xenograft-derived control sample under substantially similar staining conditions, and assessing the staining outcomes of the two to determine whether the stain was effective for the patient sample. A xenograft has never been used before in histology as a control, as far as the inventors know. The result of using a xenograft as a control is surprisingly advantageous. First, the cell lines grow and differentiate similarly to a human, taking on the general morphology of a real tissue sample. Second, because the same transformed cell line can be grown limitless times in SCID mice, the xenograft control is highly reproducible, leading to a consistent artificial control that is highly manufacturable and subject to genetic manipulation so that antigens or genetic elements may be embedded in the tissue. Another embodiment of the invention is directed generally to a method of making a tissue control substrate, comprising growing a xenograft from a mammalian transformed cell line in a host animal, removing the xenograft from the host animal, processing the xenograft thereby embedding the xenograft tissue in an embedding medium, and finally affixing the embedded xenograft sample onto a substrate. The substrate is generally a microscope slide. The xenograft control slide can then be stained side-by-side with a specimen sample in an automated slide stainer, and act as a control against which the staining quality can be compared. The xenograft control can also be used as a manual staining control. Determining whether the staining was effective for the patient specimen comprises judging the staining intensity of the xenograft control sample to determine if the expected degree and type of staining were realized in the control. If the expected type (nuclear, membranous, or cytoplasmic) and degree (0-4 scale) of staining are realized during the run, then the xenograft control indicates the staining process and reagents are working properly, and so the result in the patient specimen can be trusted. A further embodiment of the invention is a xenograft-derived control slide for histochemical use, comprising at least one xenograft control sample prepared for histological use, and a sample slide upon which the at least one xenograft control sample is affixed.
Owner:VENTANA MEDICAL SYST INC
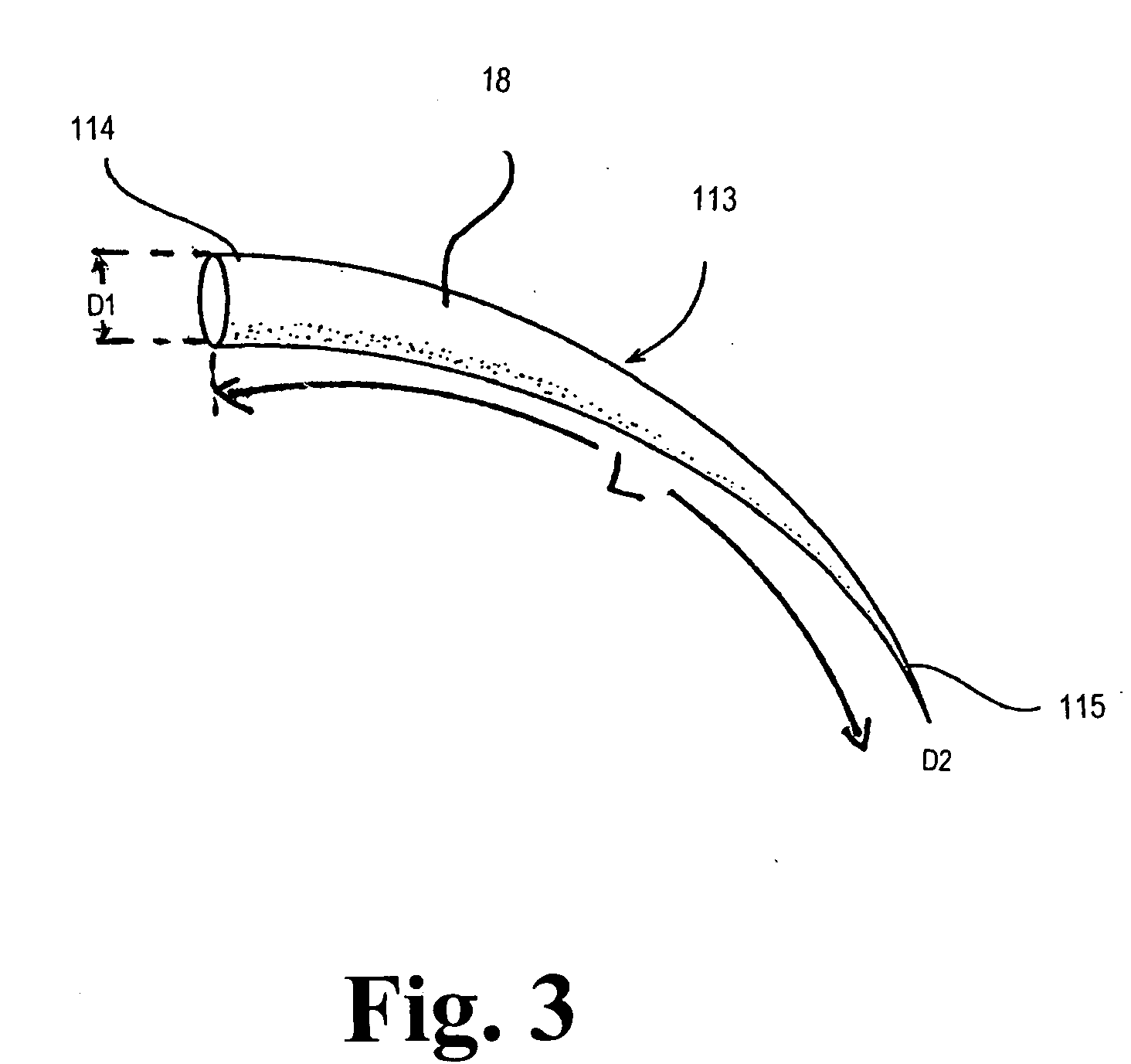
Bone Xenograft
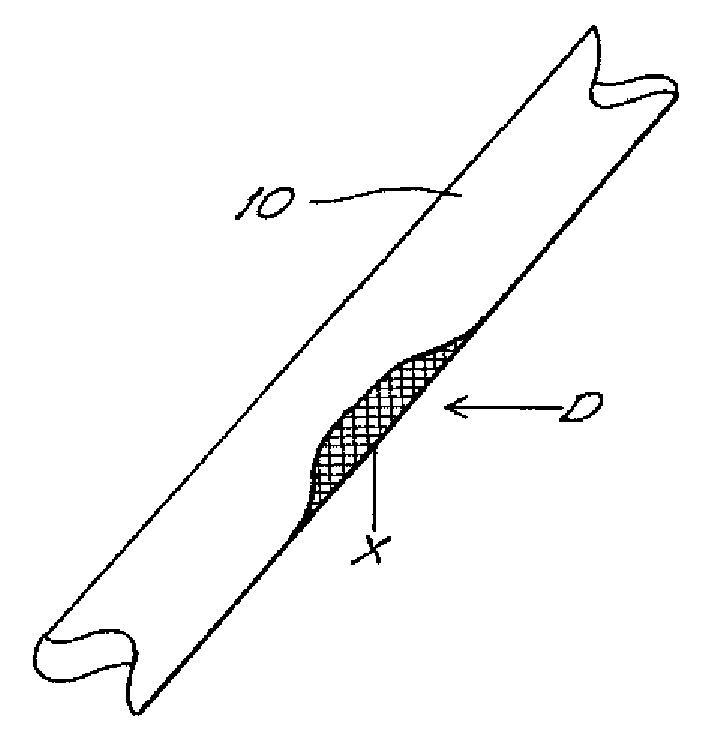
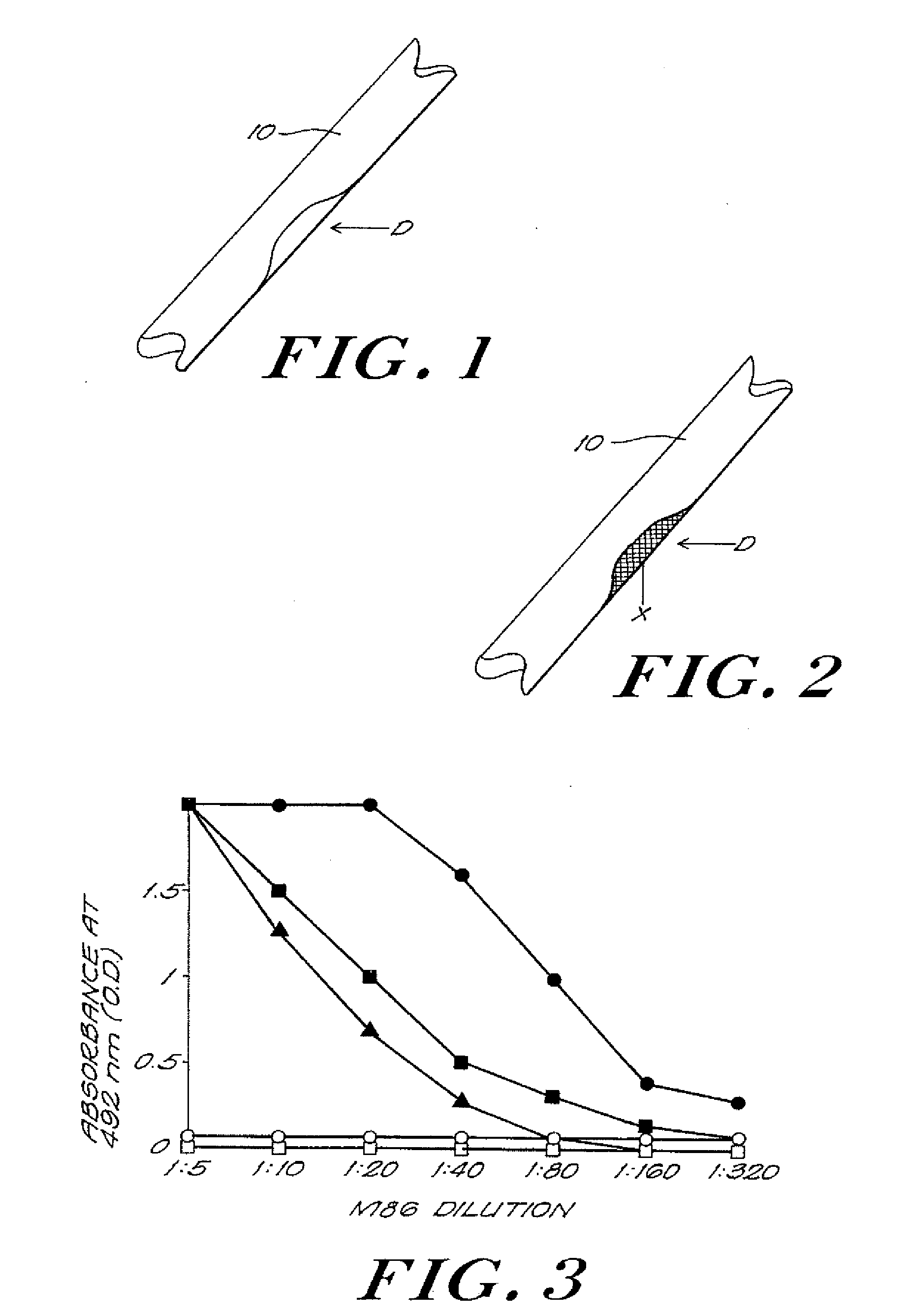
The invention provides an article of manufacture comprising a substantially non-immunogenic bone xenograft (X) for the implantation into a defect (D) located in a bone portion (10) of a human. The invention further provides methods for preparing a bone xenograft (X) by removing at least a portion of bone from a non-human animal to provide a xenograft (X); washing the xenograft (X) in saline and alcohol; and subjecting the xenograft (X) to at least one of the treatments including exposure to ultraviolet radiation, immersion in alcohol, ozonic, and freeze / thaw cycling. In addition to or in lieu of the above treatments, the methods include a cellular disruption treatment, and digestion of the carbohydrate moieties of the xenograft (X) with a glycosidase followed by treatment for sialylation.
Owner:APERION BIOLOGICS
Automated method for producing human or animal tissue for transplants
PendingCN110325225AEnsure Grading ContinuityGuaranteed traceabilityBioreactor/fermenter combinationsBiological substance pretreatmentsBiotechnologyHeterografts
The invention relates to a method for producing a tissue matrix for an allotransplantation or a xenotransplantation, according to which, once a biological tissue has been obtained, said tissue is classified, said tissue is treated and the tissue matrix produced is conditioned, all of said steps being implemented in an automated manner inside the same 'classified' reactor. The invention also relates to the reactor allowing the implementation of the method of the invention.
Owner:THERACELL CONSULTING SPRL
Method for Using Lowstrength Electric Field Network (LSEN) and Immunosuppressive Strategies to Mediate Immune Responses
InactiveUS20100111983A1Reduce intensityAccurate toleranceBioreactor/fermenter combinationsElectrotherapyHeterograftsTolerability
Application to an allograft or xenograft of a low strength electric field network (LSEN) together with an immuno-suppressive drug, gene and siRNA or other gene-based therapy is used to mediate the immune responses within an donor organ, tissue or cells, to prevent the acute and chronic rejection and to induce true tolerance, The gene(s) is locally transferred ex vivo in the time interval between harvest and implantation of allografts or xenografts before the implantation to introduce the long-term over expression of immunosuppressive and / or modulative molecules, or for down regulating alloreactive molecules in the donor organ, tissue or cells only and not in the recipient's whole body system.
Owner:RGT UNIV OF CALIFORNIA
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com