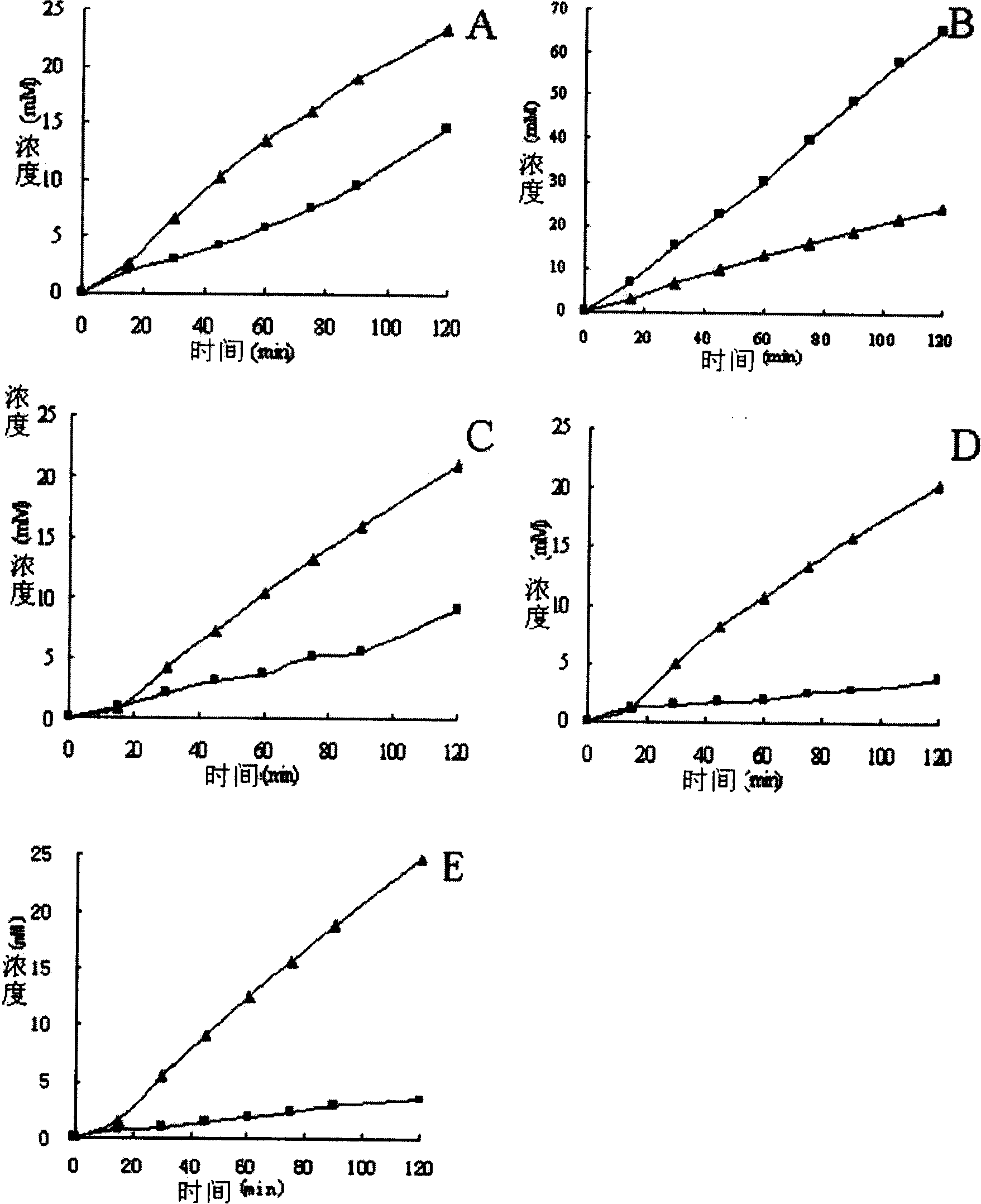
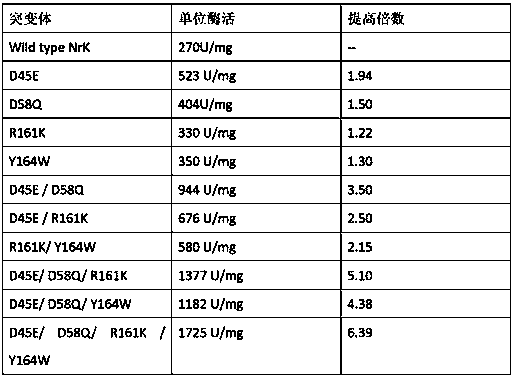
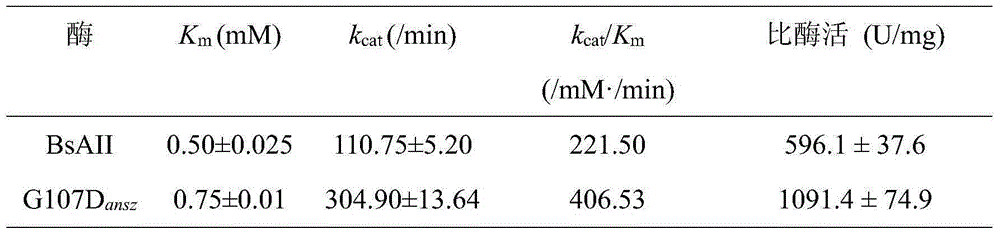
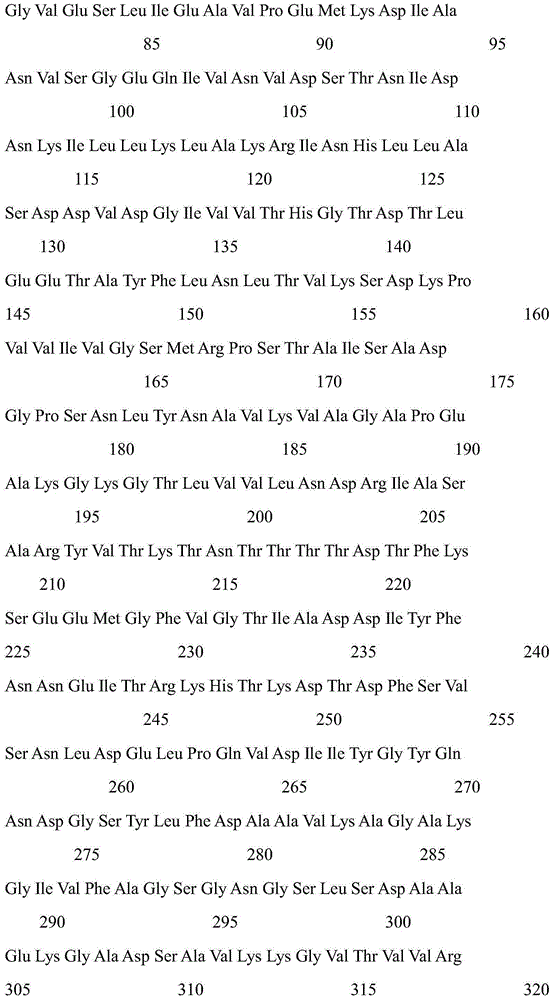
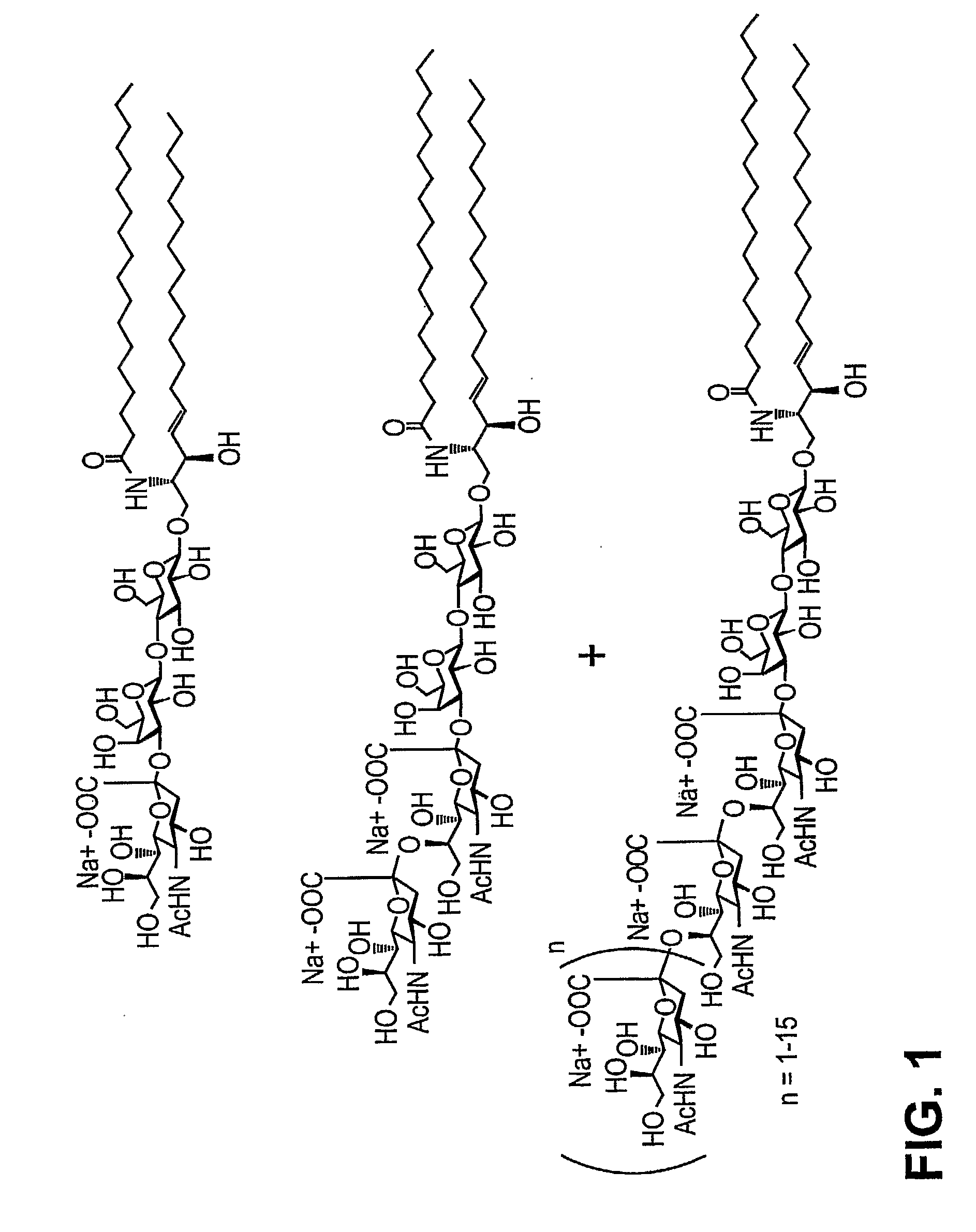
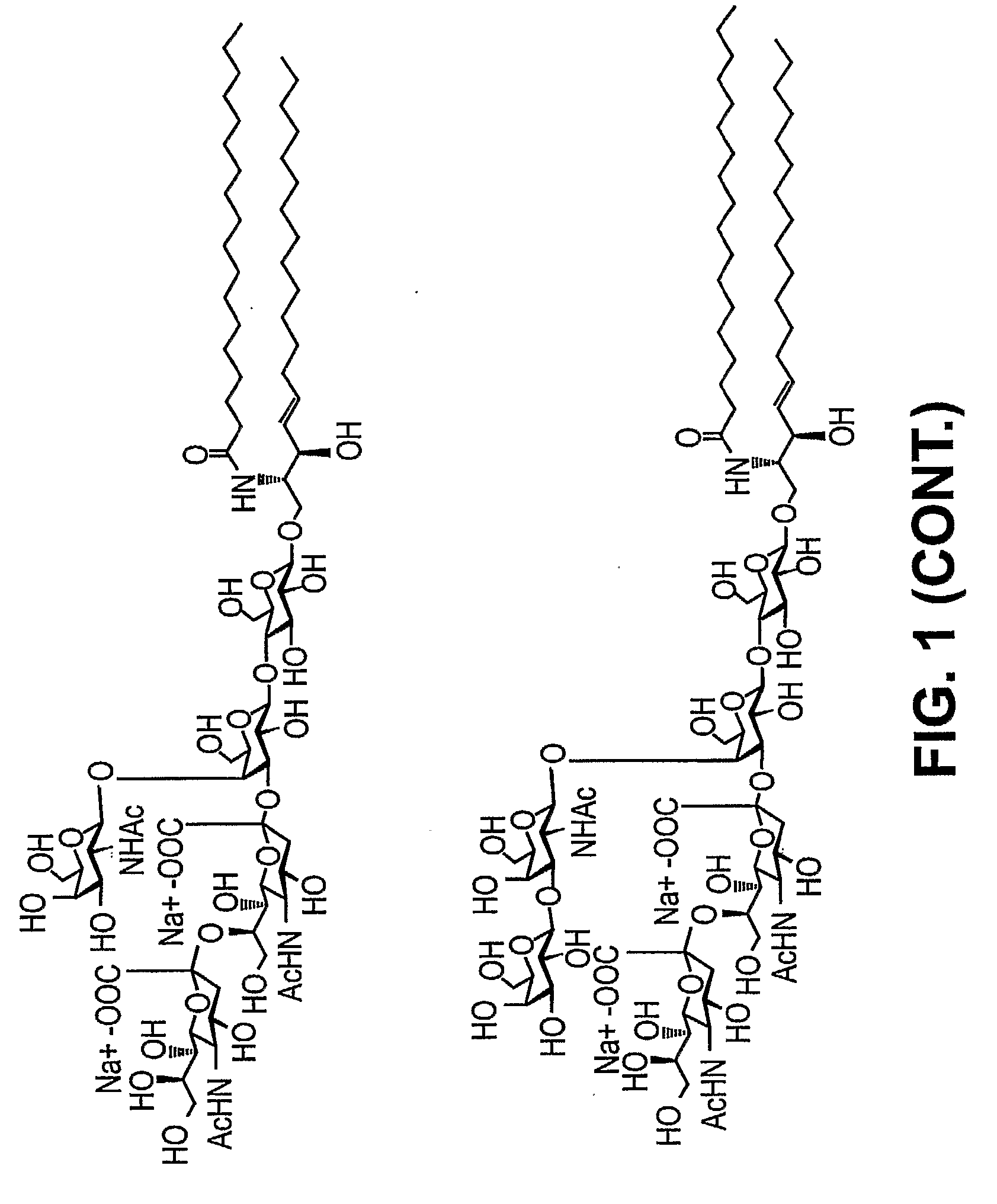
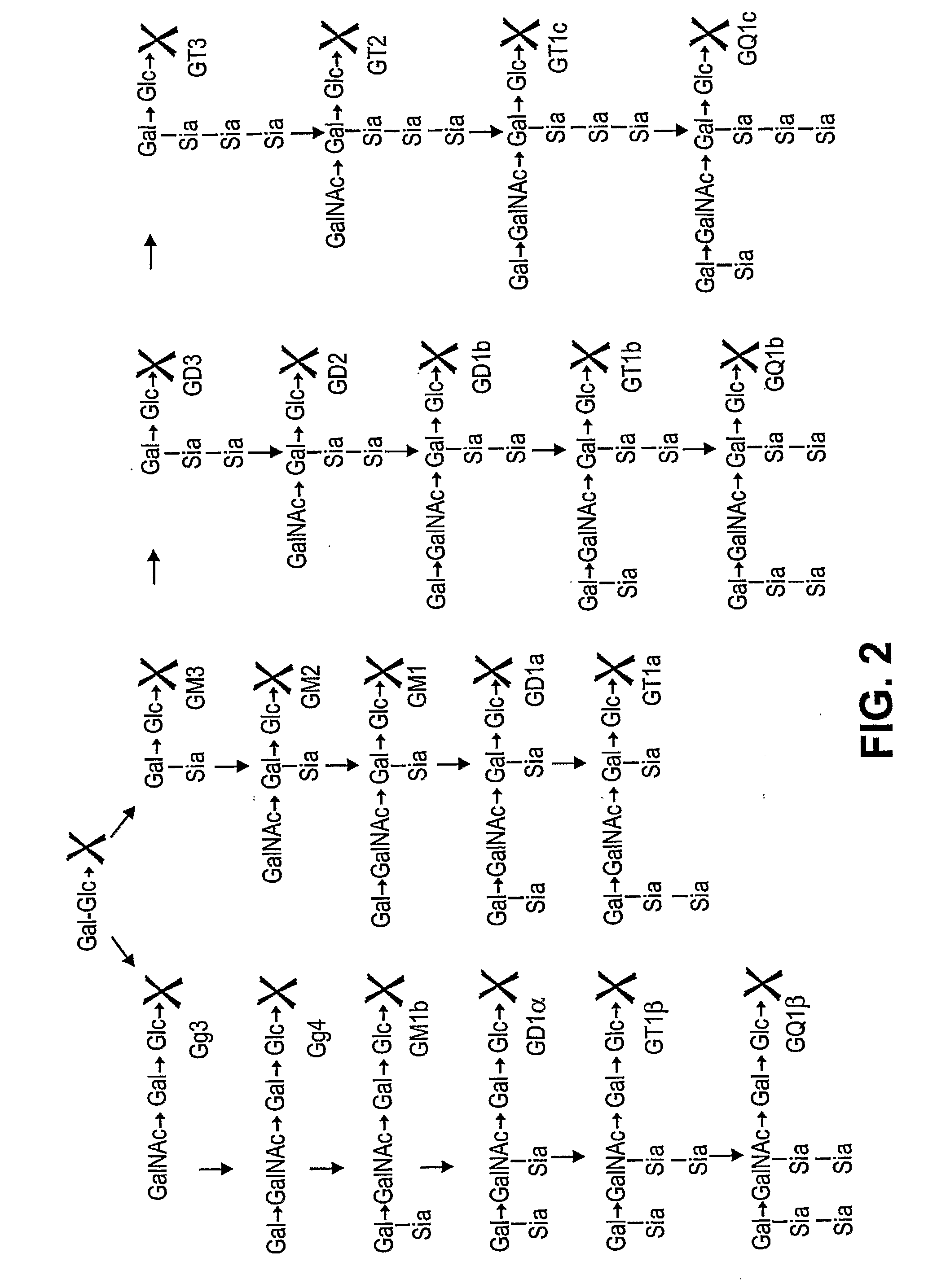
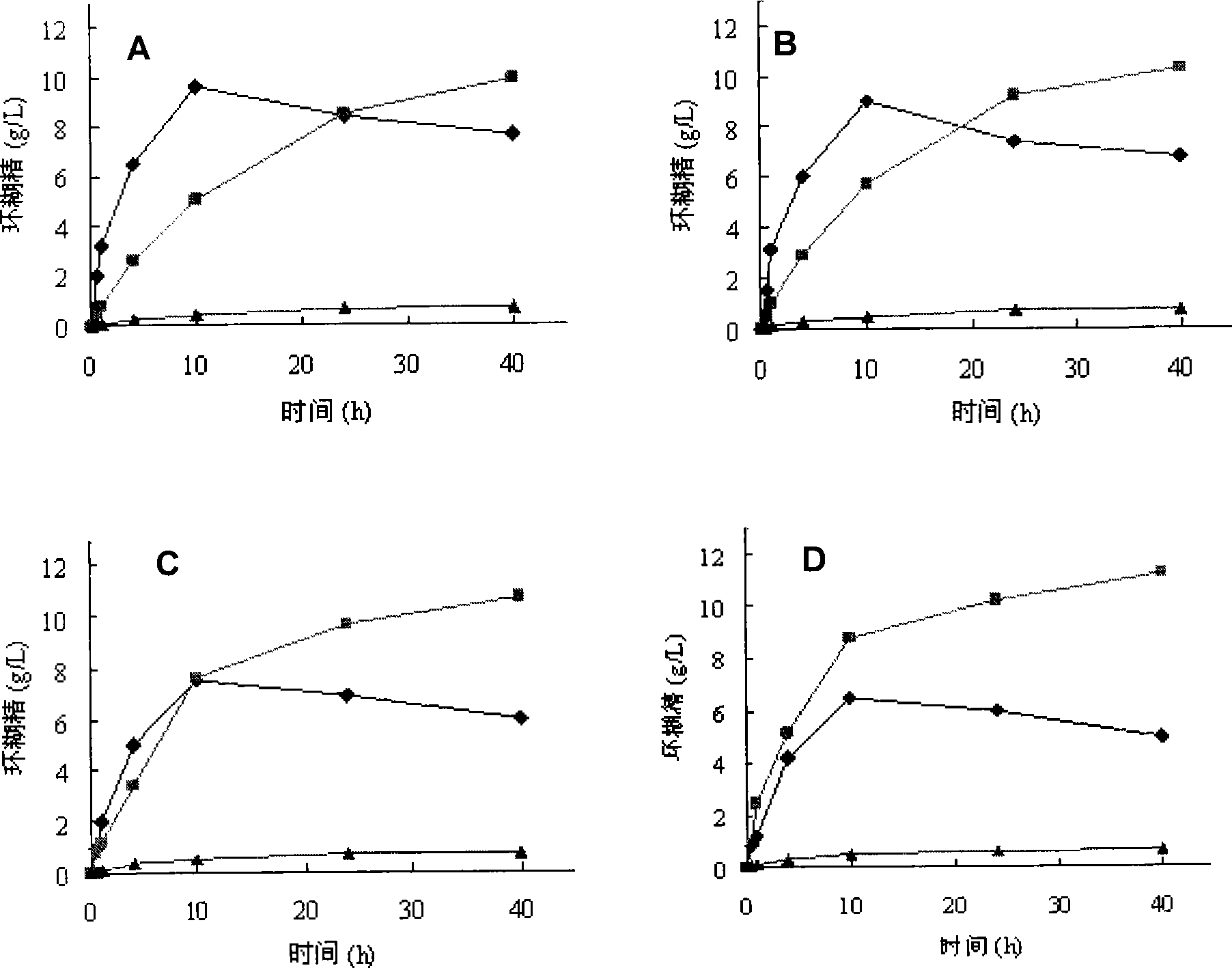
Patents
Literature
388 results about "Mutant enzyme" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Mouse Study Finds That Mutant Enzyme is Able to Help Protect DNA From Damage. Research has shown that when DNA damage occurs, a key enzyme — called ataxia telangiectasia mutated protein, or ATM — becomes activated.
Gene encoding resistance to acetolactate synthase-inhibiting herbicides
InactiveUS20060130172A1Inhibit growthInhibition of reproductionTransferasesOther foreign material introduction processesAcetolactate synthaseSulfonylurea
A mutant acetolactate synthase (ALS) enzyme that confers cross-resistance to all sulfonylurea, imidazolinone, pyrimidinyloxybenzoate, triazolopyrimidine and sulfonylamino-carbonyl-triazolinone herbicides is provided. The mutant enzyme contains an aspartic acid to glutamic acid substitution mutation at a newly identified conserved region of the ALS enzyme. A gene encoding the enzyme is also provided, as are transgenic plants that have been genetically engineered to contain and express the gene. The transgenic plants are cross-resistant to sulfonylurea, imidazolinone, pyrimidinyloxybenzoate, triazolopyrimidine and sulfonylamino-carbonyl-triazolinone herbicides.
Owner:VIRGINIA TECH INTPROP INC
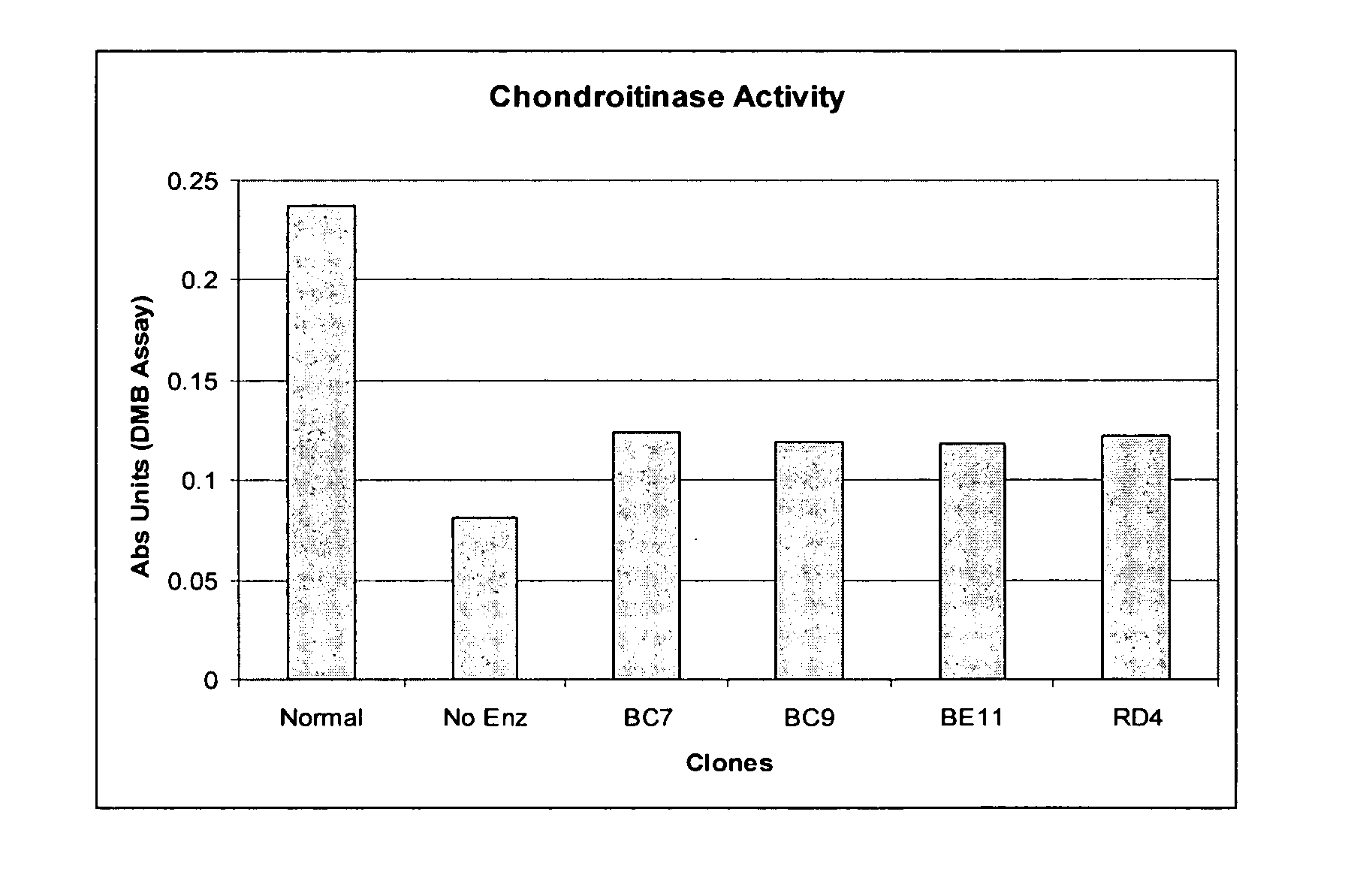
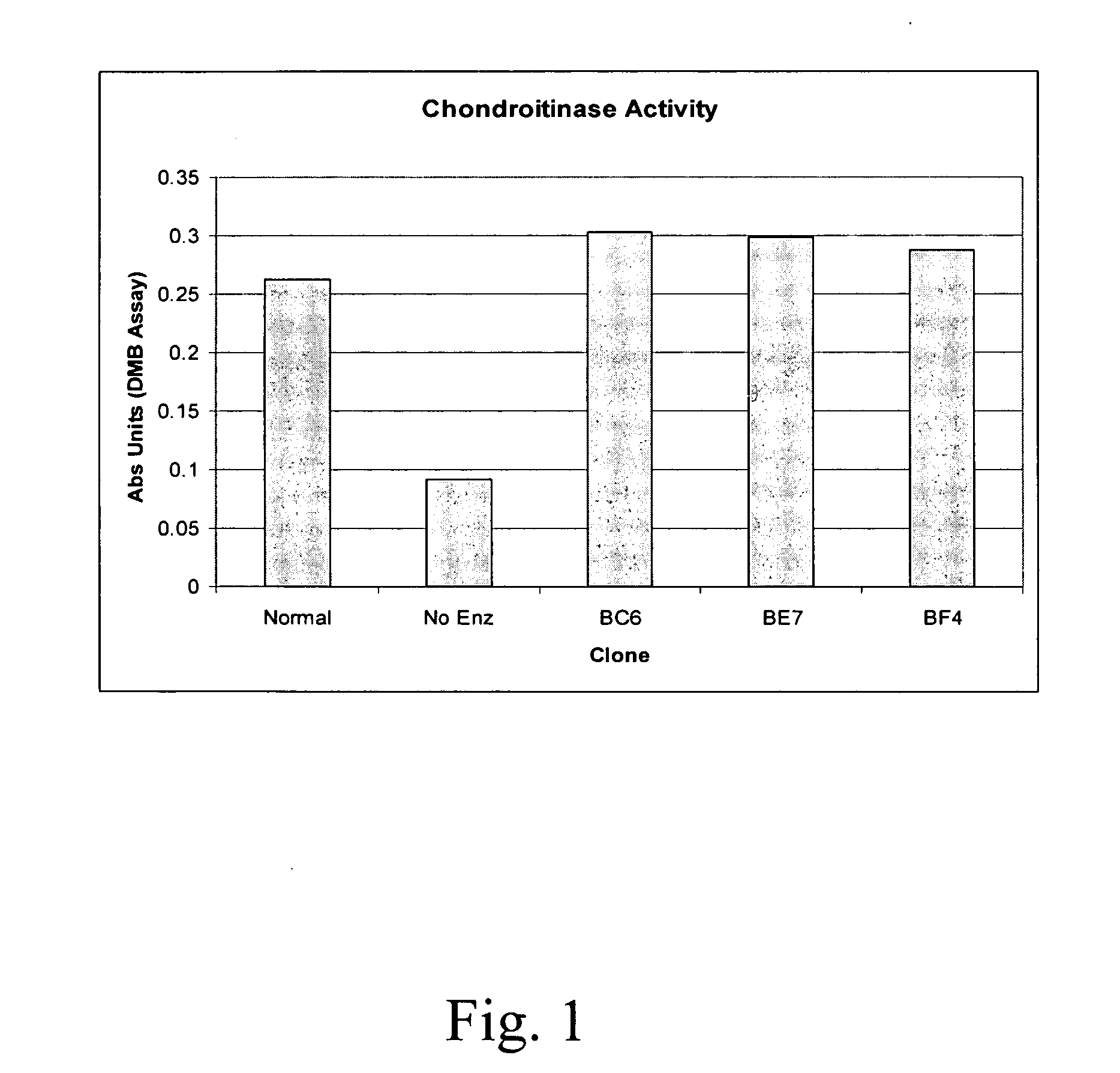
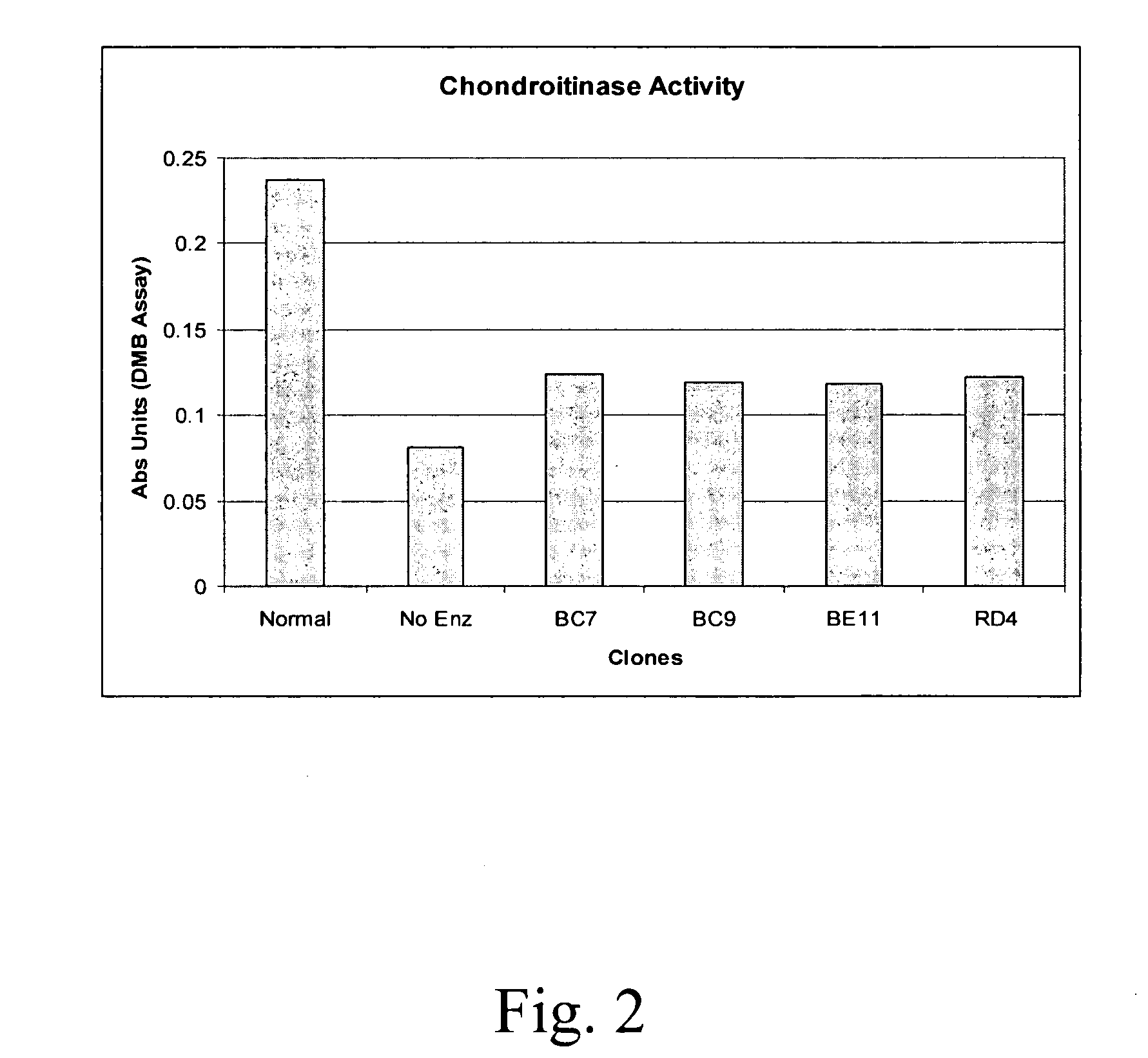
Compositions and methods of using chondroitinase ABCI mutants
One aspect of the present invention relates to mutants of chondroitinase ABCI. Such chondroitinase ABCI mutants exhibit altered chondroitin lyase activity or increased resistance to inactivation from stressors including exposure to UV light or heat. Methods of using chondroitinase ABCI mutant enzymes are also provided.
Owner:ACORDA THERAPEUTICS INC
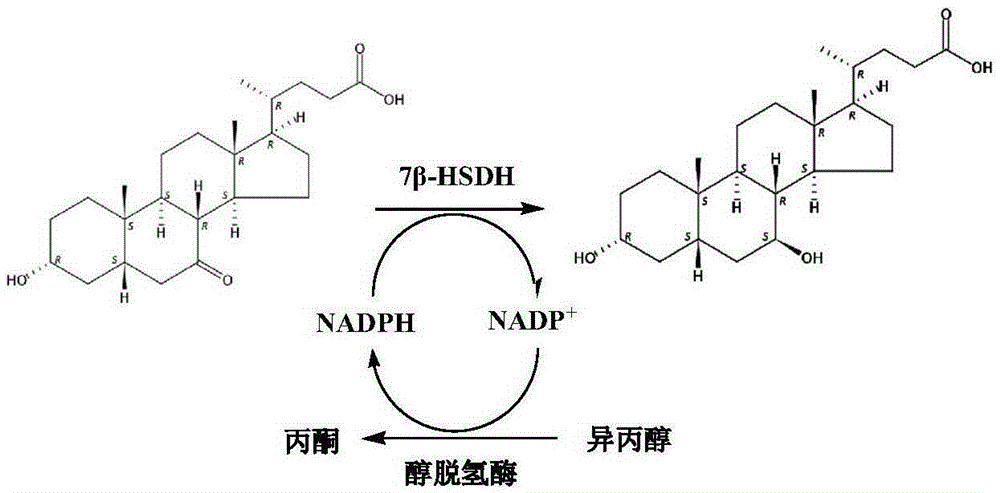
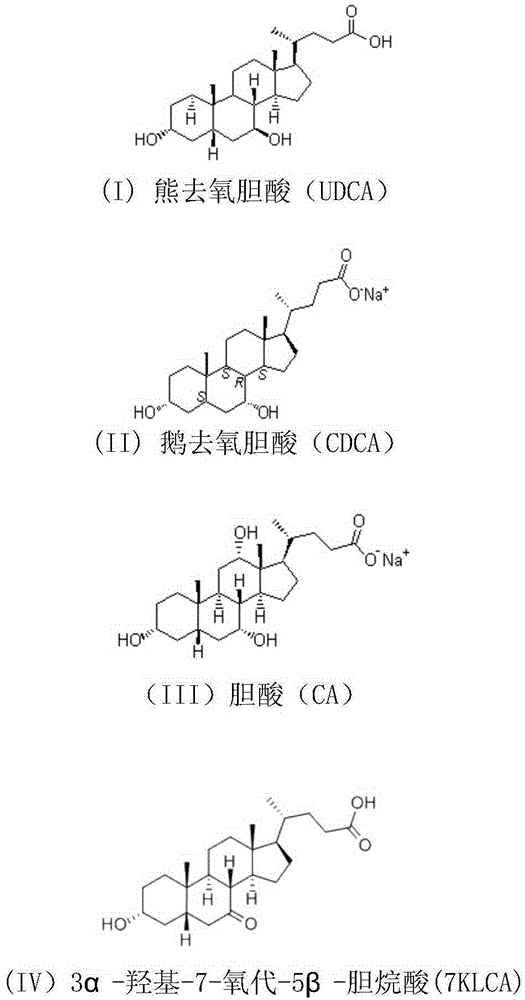
7beta-hydroxyl sterol dehydrogenase mutant and application thereof to preparation of ursodeoxycholic acid
ActiveCN108546691AEasy extractionEasy to manufactureOxidoreductasesGenetic engineeringSterolProtein engineering
The invention discloses a 7beta-hydroxyl sterol dehydrogenase mutant which is obtained by protein engineering and of which coenzyme preference is changed, a coding gene of the 7beta-hydroxyl sterol dehydrogenase mutant, a recombinant expression vector and a recombinant expression transformant which contain a sequence of the gene, a preparation method of a recombinant mutant enzyme preparation, andapplication of the recombinant mutant enzyme preparation to preparation of ursodeoxycholic acid. By co-enzyme regeneration of enzymic coupling, the recombinant mutant enzyme preparation disclosed bythe invention can efficiently utilize relatively cheap oxidized coenzyme I (NAD+) instead of very expensive oxidized coenzyme II (NADP+); asymmetric reduction of catalytic 7-hydroxyl lithocholic acideffectively reduces production cost; moreover, the recombinant mutant enzyme preparation has the advantages of simplicity for operation, mild reaction condition, environmental-friendliness, high yieldand the like, and has a good application prospect in preparation of ursodeoxycholic acid by epimerization of chenodesoxycholic acid.
Owner:EAST CHINA UNIV OF SCI & TECH +1
Mutant of 7 beta-hydroxyl steroid dehydrogenase, application of mutant and synthesis method
ActiveCN105274070ASuitable for industrial productionEasy to controlOxidoreductasesFermentationChemical synthesisCholic acid
The invention provides a mutant of 7 beta-hydroxyl steroid dehydrogenase, application of the mutant and a synthesis method. The mutant of the 7 beta-hydroxyl steroid dehydrogenase is characterized in that amino acid sequences of the mutant are Seq ID NO:4, and coded nucleotide sequences are Seq ID NO:3; or amino acid sequences of the mutant are Seq ID NO:6, and coded nucleotide sequences are Seq ID NO:5. The mutant, the application and the synthesis method have the advantages that cholic acid compounds, particularly ursodeoxycholic acid, can be catalytically synthesized by the efficient 7 beta-hydroxyl steroid dehydrogenase, mutant enzymes of the 7 beta-hydroxyl steroid dehydrogenase and coenzyme regeneration systems, accordingly, the substrate concentration can reach 100 g / L, the conversion rate is 99.2-99.5%, and the weight yield can reach 94-96%; and the enzymes can be inexpensively and easily obtained by the aid of a fermentation process, accordingly, the production cost and the product quality are superior to the production cost and the product quality of chemical synthesis methods, and the mutant and the synthesis method are applicable to industrial production.
Owner:苏州天绿生物制药有限公司
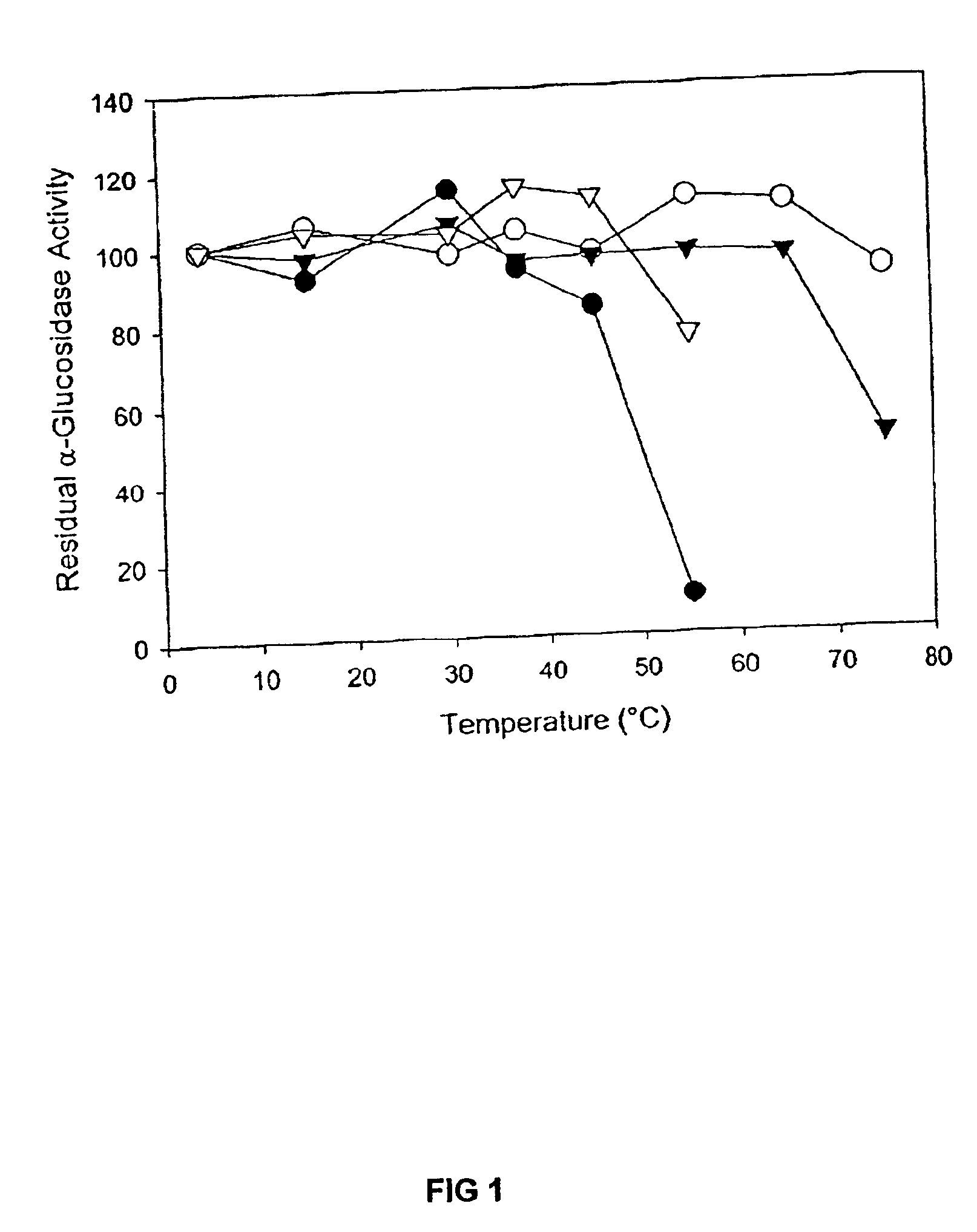
Modified barley α-glucosidase
Barley α-glucosidase is an important enzyme in the conversion of barley starch to fermentable sugars during the industrial production of ethanol, as in brewing and fuel ethanol production. The enzyme is, however, relatively thermolabile, a disadvantage for an enzyme useful in industrial processes which are preferably conducted at elevated temperatures. Site directed mutagenesis has been conducted to make mutant forms of barley α-glucosidase which have improved thermostability. The sites for this site-directed mutagenesis were selected by sequence comparisons with the sequences of other α-glucosidase proteins which are more thermostable. The recombinant mutant enzymes thus produced have been demonstrated to improve the thermostability of the enzyme.
Owner:WISCONSIN ALUMNI RES FOUND +1
Mutation penicillin G acylase, recombinant expression plasmid and transformation engineering strains thereof
ActiveCN101177688AImprove synthesis abilityMaximum conversion rate increaseBacteriaHydrolasesHydrolysatePolymerase L
The invention relates to a gene, mutant plasmid and engineering bacteria which have improved synthesis performance to penicillin G acylase and are obtained by a gene site-directed mutagenesis method, and mutant enzyme can also be obtained with improved synthesis performance to penicillin G acylase by fermenting and purifying the engineering bacteria. Two enzymes Kpn I and Pst I are firstly used for cutting pUC18 by the invention, then T4 polymerase is adopted to make the ends blunt, and pZ01 is obtained through self-linkage; the enzyme of EcoR I is used for cutting pZ01, and then connected with pEES102 that is also cut by the enzyme of EcoR I, thereby obtaining the recombinant plasmid pY020; the pY020 is adopted as a template plasmid, and TaKaRa MuTanBEST Kit is utilized for conducting the site-directed mutagenesis to B.megaterium PGA, thereby obtaining the mutant plasmid with improved synthesis performance to the penicillin G acylase. The mutant plasmid is transformed to bacillus subtilis to obtain the required engineering bacteria. The engineering bacteria are amplified and fermented, and the mutant enzyme with improved maximum conversion rate of 7-ADCA and the ratio of synthetic product / hydrolysate can be obtained after the engineering bacteria are purified.
Owner:SHANXI WEIQIDA PHARMA IND
Nicotinamide ribokinase mutant and application thereof
ActiveCN110373398AMild reaction conditionsStable energy cycle systemTransferasesFermentationSingle mutationNicotinamide mononucleotide
Owner:JIANGSU CHENGXIN PHARMA
L-asparaginase mutant with improved enzyme activity and construction method thereof
InactiveCN105062997AIncreased potential for industrial applicationsReduce generationBacteriaHydrolasesGlycineSpecific enzyme
The invention discloses an L-asparaginase mutant with the improved enzyme activity and a construction method thereof, and belongs to the field of gene engineering. According to the L-asparaginase mutant, on the basis of amino acid shown in the SEQ ID NO.2, the 107th glycine is mutated into aspartic acid. The obtained mutant is expressed in bacillus subtilis, fermentation is performed in a shake flask for 24 h and then the enzyme activity is 961 U / mL; the enzyme activity of the mutant is improved by 80%, the appetency of a substrate is decreased by 50% compared with protoenzyme, the catalytic efficiency is improved by 84%, and meanwhile the specific enzyme activity is improved by 83%. According to the L-asparaginase mutant, it is shown that the 107th amino acid residue has a great influence on the enzyme catalytic action, a certain foundation is provided for research on the enzyme catalytic mechanism, and the enzyme industrial application potential is improved.
Owner:JIANGNAN UNIV
Mutant Endoglycoceramidases With Enhanced Synthetic Activity
InactiveUS20090170155A1Simple synthesisExquisite selectivity of enzymatic reactionsBacteriaSugar derivativesReactive siteEndoglycoceramidase
The present invention relates to a novel endoglycoceramidase whose hydrolytic activity has been substantially reduced or eliminated, such that the enzyme is useful for synthesis of glycolipids from a monosaccharide or oligosaccharide and a ceramide. More specifically, the endoglycoceramidase is a mutant version of a naturally occurring endoglycoceramidase, preferably comprising a mutation within the active site or the nucleophilic site of the enzyme and more preferably comprising a substitution mutation of the Glu residue within the active site or the nucleophilic site. Also disclosed are a method for generating the mutant endoglycoceramidase and a method for enzymatically synthesizing glycolipids using this mutant enzyme.
Owner:SENEB BIOSCI +1
Mutant of cyclodextrin glucosyl transferase having highly beta-cyclodextrin yielding property and mutation method
InactiveCN101503680AEase of industrial productionStrong specificityTransferasesMicroorganism based processesWild typeSite-directed mutagenesis
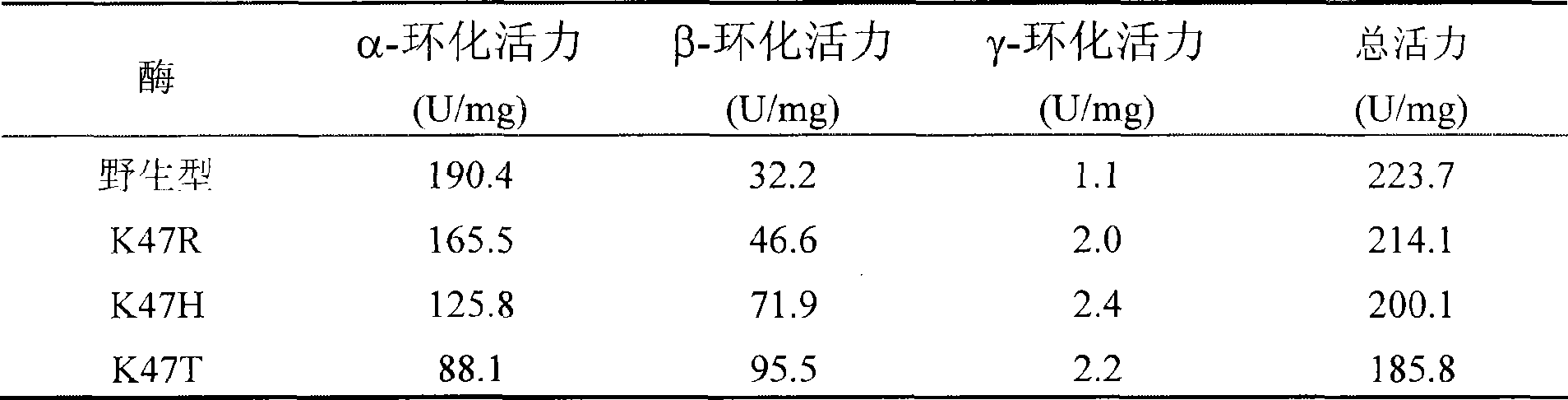
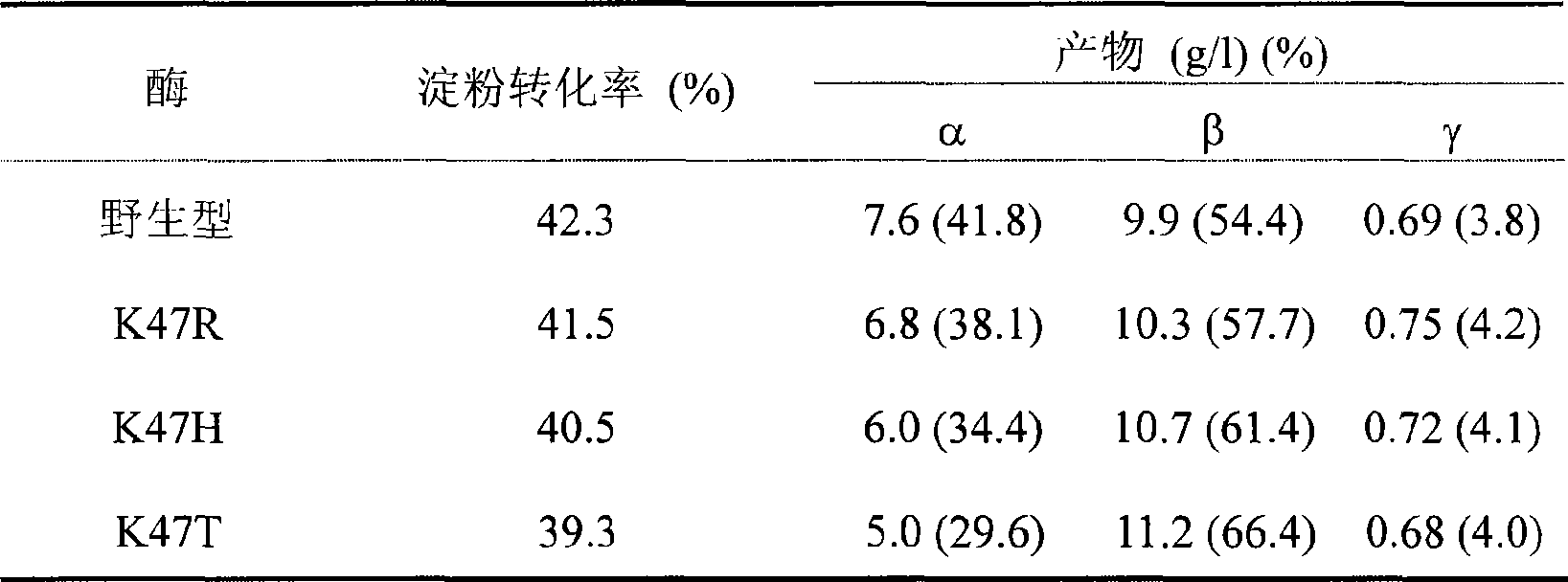
The invention relates to a mutant of a cyclodextrin glucosyltransferase with the capability of highly yielding beta-cyclodextrin and a mutation method, which belong to the fields of gene engineering and enzyme engineering. The invention improves the capability of the cyclodextrin glucosyltransferase (CGT enzyme for short) for producing the beta-cyclodextrin by a rite-directed mutagenesis method, provides a mutant proposal for improving the capability of CGT enzyme from Peanibacillus macerans JFB05-01 (CCTCC NO: M 208063) for producing the beta-cyclodextrin, and substitutes Lys on the 47 position of the CGT enzyme for Arg, His and Thr respectively; the beta-cyclodextrin production capacity of the obtained three mutant enzyme of K47R, K47H and K47T is improved compared with wild type CGT enzymes, wherein the mutant enzyme K47T is particularly obvious. The mutant enzymes are more favorable for industrial production of the beta-cyclodextrin than the wild type CGT enzymes.
Owner:JIANGNAN UNIV
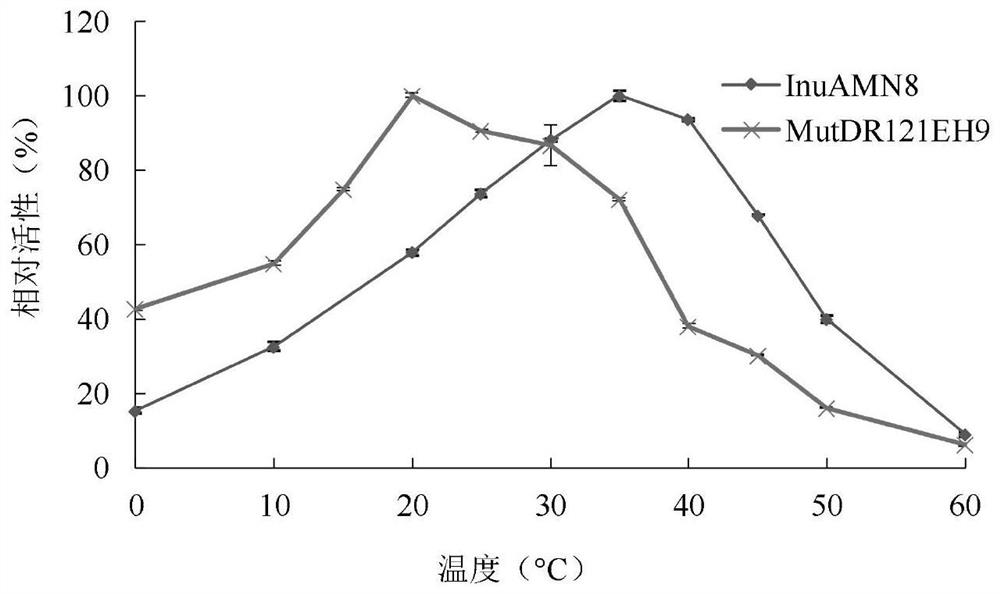
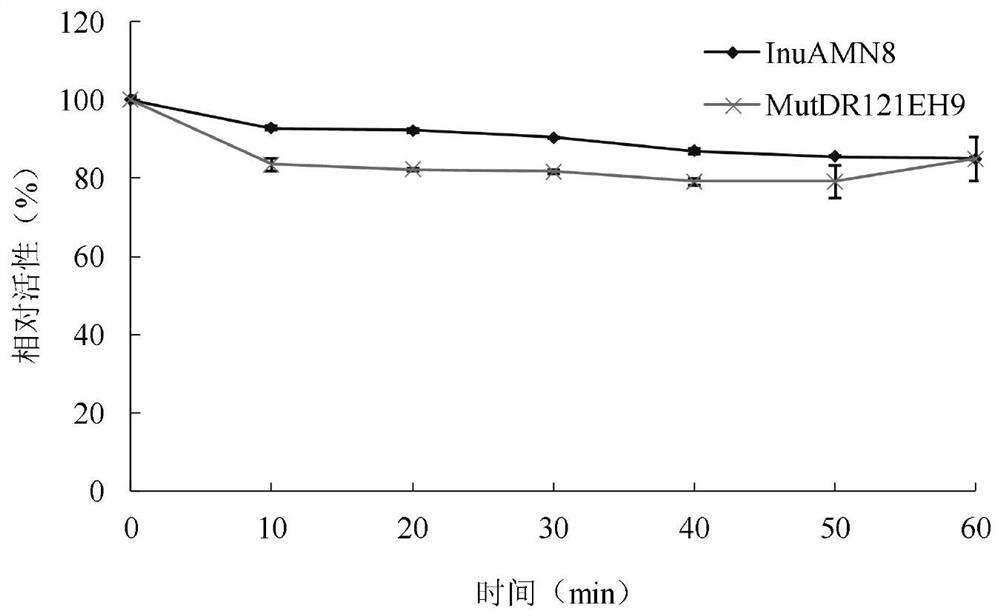
Exo-inulinase mutant MutDR121EH9 with improved low-temperature activity
The invention discloses an exo-inulinase mutant MutDR121EH9 with improved low-temperature activity, which has an amino acid sequence as shown in SEQ ID NO.1; the thermal activity and the thermal stability of the mutant MutDR121EH9 are changed, so that the mutant MutDR121EH9 has higher activity at low temperature, the thermal stability is reduced and the low-temperature activity is increased, and the use amount of enzyme is reduced or the reaction time is shortened during a low-temperature reaction; meanwhile, the degraded thermal stability makes the enzyme reaction process to be controlled through thermal treatment, wherein optimal temperature of the wild enzyme InuAMN8 is 35 DEG C, and the optimal temperature of the mutant enzyme MutDR121EH9 is 20 DEG C; after treatment at 50 DEG C, the enzyme activity of the wild enzyme InuAMN8 keeps 81% or above, and the enzyme activity of the mutant enzyme MutDR121EH9 is reduced from 32% to 14%. The mutant MutDR121EH9 disclosed by the invention can be applied to the industries of food, wine brewing, washing and the like.
Owner:YUNNAN NORMAL UNIV
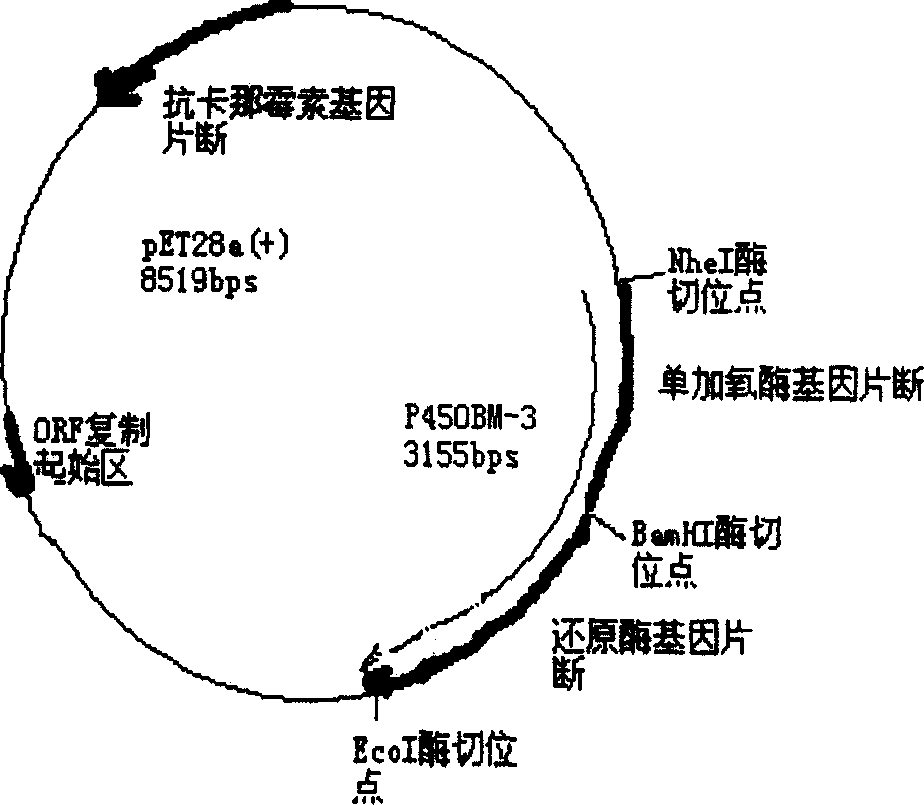
Cytochrome P450BM-3 monooxygehase varient gene and its use
Owner:ZHEJIANG UNIV
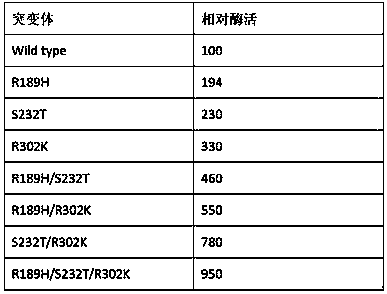
Nicotinamide phosphoribosyltransferase mutant and application thereof
ActiveCN110373397AMild reaction conditionsStable energy cycle systemMicroorganism based processesFermentationNicotinamide phosphoribosyltransferaseSingle mutation
The invention provides a nicotinamide ribosyltransferase mutant and application thereof. Compared with an amino acid sequence SEQID NO. 2, the difference of the amino acid sequence of the mutant is that the R189th, S232th and R302th sites in the amino acid sequence SEQID NO. 2 are subjected to single mutation or in-pair combined mutation or three combined mutation. Novel nicotinamide ribosyltransferase mutant enzyme is used for synthesis and preparation of beta-nicotinamide mononucleotide. The constructed nicotinamide ribosyltransferase mutant enzyme has the advantages that the enzyme cost islow, the transformation time is short, and the process operation is simple, and has a broad large-scale industrial application prospect.
Owner:KINGDOMWAY BIOTECH (JIANGSU) CO LTD +1
68th and 109th double mutant enzyme of D-psicose 3-epimerase and application thereof
InactiveCN103849612AHigh catalytic activityFunction increaseMicroorganism based processesOxidoreductasesGenes mutationGenetics
The invention provides a 68th and 109th double mutant enzyme of D-psicose 3-epimerase and an application thereof, and belongs to the technical field of enzyme genetic engineering. The invention discloses the mutant enzyme Y68I / G109P which is obtained by using the D-psicose 3-epimerase (called DPE enzyme for short) derived from clostridium bolteae ATCCBAA-613 as a parent and utilizing a gene mutation technology for respectively replacing 68th tyrosine Tyr and 109th glycine Gly with isoleucine Ile and praline Pro. The heat stability and catalytic activity of the mutant enzyme Y68I / G109P are improved, therefore, the mutant enzyme Y68I / G109P has important industrial application value.
Owner:JIANGNAN UNIV
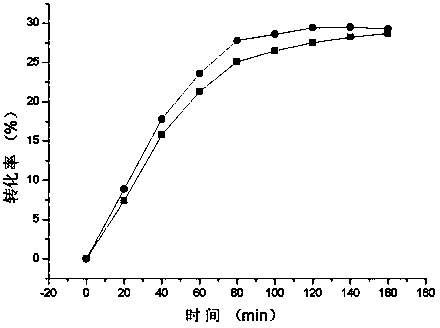
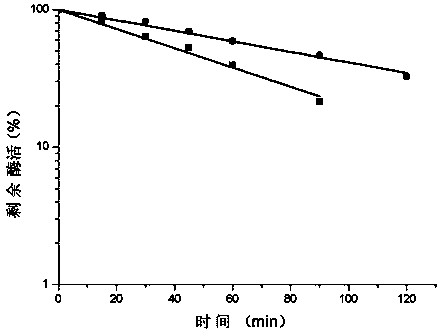
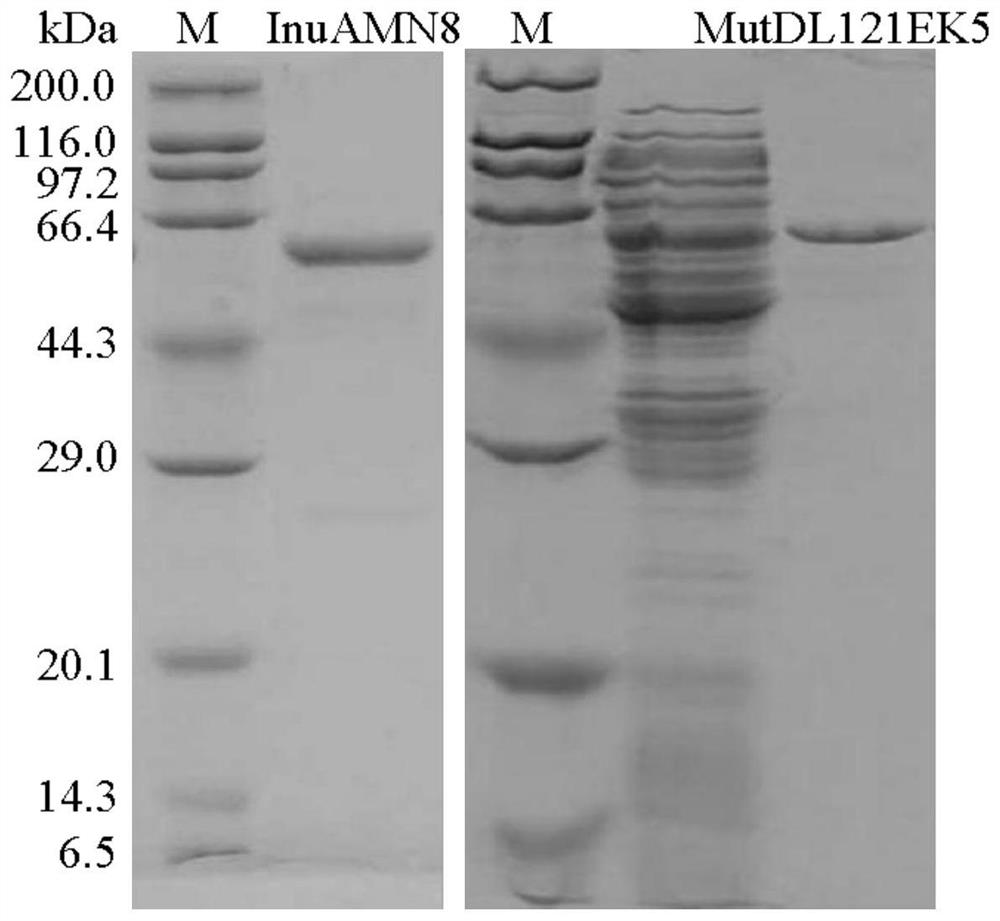
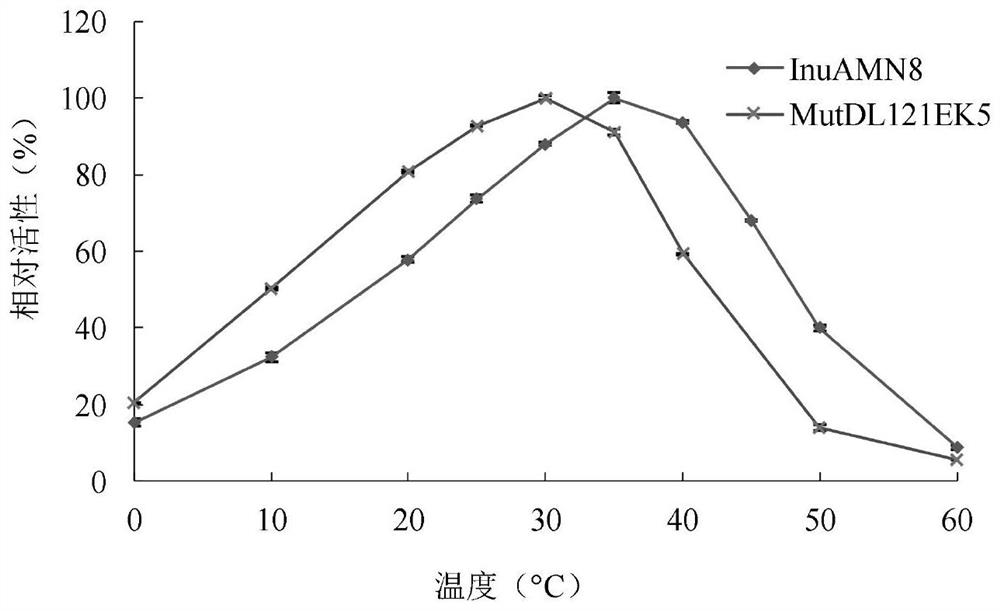
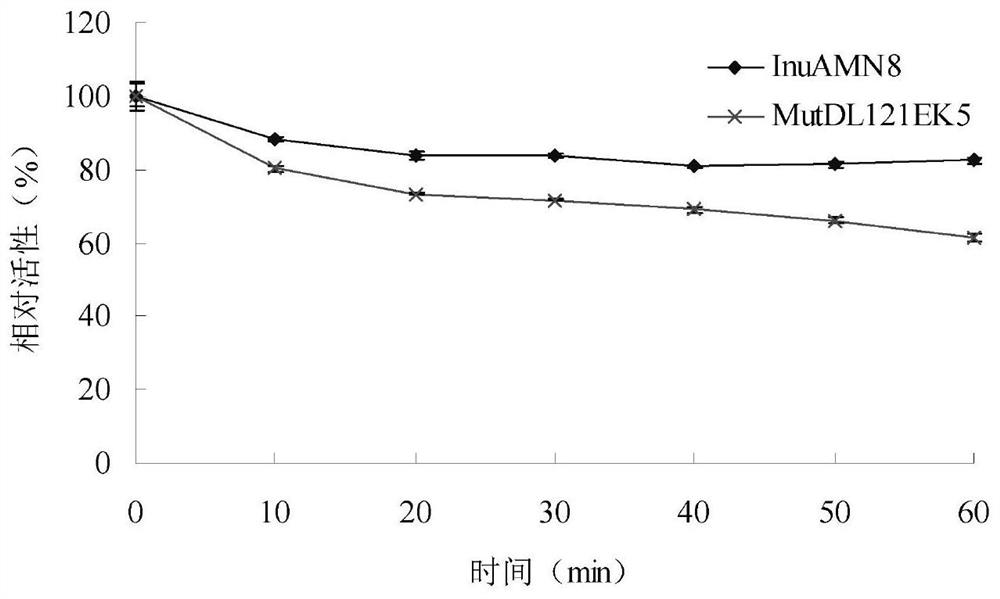
Low-temperature exoinulinase mutant MutDL121EK5 with improved low-temperature adaptability and application thereof
ActiveCN112852782AChange thermal stabilityHigh activityBacteriaMicroorganism based processesBiotechnologyThermal denaturation
The invention relates to the technical field of gene engineering and protein modification, and discloses a low-temperature exoinulinase mutant MutDL121EK5 with improved low-temperature adaptability and application thereof, the amino acid sequence of the mutant MutDL121EK5 is obtained by replacing DAAPL from the 121st site to the 125th site of wild exoinulinase InuAMN8 with five amino acids EEDRK, and the sequence of the MutDL121EK5 is shown as SEQ ID NO.1. Compared with a wild enzyme InuAMN8, the mutant enzyme MutDL121EK5 has the advantages that the low-temperature activity is improved, the mutant enzyme MutDL121EK5 is more easily subjected to thermal denaturation, the improvement of the low-temperature activity is beneficial to reducing the dosage of the enzyme or shortening the reaction time during low-temperature reaction, and the easy thermal denaturation is beneficial to controlling the reaction process of the enzyme through thermal treatment. The low-temperature exoinulinase mutant MutDL121EK5 disclosed by the invention can be applied to the industries of food, wine brewing, washing and the like.
Owner:YUNNAN NORMAL UNIV
D-psicose 3-epimerase mutant with improved catalytic efficiency
ActiveCN108018278AHigh catalytic efficiencyIncreased relative enzyme activityBacteriaMicroorganism based processesPsicoseMutant enzyme
The invention discloses a D-psicose 3-epimerase mutant with improved catalytic efficiency and belongs to the technical field of enzyme engineering. The Dorea sp. DPEase mutant enzyme A38E / G105A keepsthe optimal catalytic conditions. Under the optimal catalytic conditions, the relative enzyme activity of the enzyme for catalytic conversion of D-fructose as a substrate into D-psicose is improved by38.6%. The discovery has an important research value for the industrial production of D-psicose.
Owner:JIANGNAN UNIV
7beta-hydroxysterol dehydrogenase mutant and application of 7beta-hydroxysterol dehydrogenase mutant in ursodeoxycholic acid synthesis
ActiveCN107099516AEasy to separate and extractOvercoming the problem of inactivation processingOxidoreductasesGenetic engineeringChenodeoxycholic acidSubstrate concentration
The invention discloses a 7beta-hydroxysterol dehydrogenase mutant with increased activity and stability which is obtained through molecular evolution, recombinant expression plasmid containing the 7beta-hydroxysterol dehydrogenase mutant gene and a recombinant expression transformant and a preparation method of a recombinant mutant enzyme preparation, and the invention also provides an application of the recombinant mutant enzyme preparation in ursodeoxycholic acid synthesis. The 7beta-hydroxysterol dehydrogenase has excellent activity and heat stability, can efficiently catalyze asymmetric reduction of 7-carbonyl lithocholic acid to prepare the ursodeoxycholic acid; the 7beta-hydroxysterol dehydrogenase is subjected to immobilization and then is subjected to couple by an enzyme method with the immobilized 7beta-hydroxysterol dehydrogenase, epimerization of a substrate chenodeoxycholic acid with low cost can be directly catalyzed, ursodeoxycholic acid can be prepared through continuous conversion, and the operation is simple. Compared with the prior art reported currently, ursodeoxycholic acid prepared by hydroxysterol dehydrogenase through catalysis has the advantages of high substrate concentration, short reaction time, complete reaction, and high product purity, and has strong industrial application prospect.
Owner:EAST CHINA UNIV OF SCI & TECH +1
Thermal stability improved mutant enzyme of D-psicose 3-epimerase and application thereof
InactiveCN103849613AImprove thermal stabilityMicroorganism based processesIsomerasesIsomerasePsicose
The invention provides thermal stability improved mutant enzyme of D-psicose 3-epimerase and application thereof, and belongs to the technical field of enzyme genetic engineering. The mutant enzyme is characterized in that D-psicose 3-epimerase (DPE enzyme for short) sourced from clostridium ((i) Clostridium bolteae ( / i)) ATCC BAA-613 is used as a parent, and glycine Gly at the position 109 is replaced by proline Pro through the gene mutagenesis technique so as to obtain single mutant enzyme G109P; the stability of the mutant enzyme is improved; high industrial application value is brought.
Owner:JIANGNAN UNIV
Glutamate decarboxylase mutant establishment improving enzyme activity and application thereof
ActiveCN105255849AIncreased potential for industrial applicationsBacteriaFermentationGlutamate decarboxylaseTyrosine
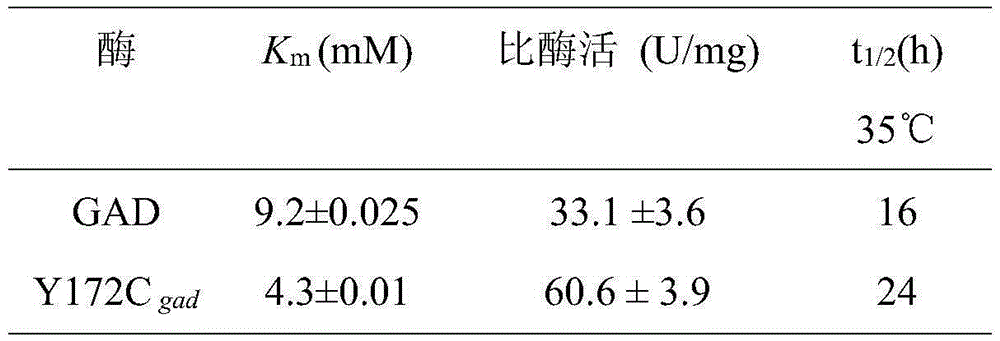
The invention discloses a glutamate decarboxylase mutant improving enzyme activity and an establishment method thereof, and belongs to the field of gene engineering. On the basis of an amino acid shown as SEQ ID NO.1, a 172 tyrosine is mutated to form cysteine. The obtained mutant is expressed in colibacillus, after being fermented for 24h in a shake flask, the enzyme activity is 28.6U / mL, the mutant enzyme activity is improved by 81 percent, compared with the original enzyme, the substrate affinity is reduced by 53 percent, the enzyme activity is improved by 83 percent, and the half-time period of the enzyme at 35 DEG C is increased from 16h to 24h. The recombinase is expressed in the colibacillus, and the glutamic acid is converted in a total cell manner for 18h to obtain 283.8g / L gamma-aminobutyric acid; the recombinase is expressed in glutamic acid coryneform bacteria, the glutamic acid is converted for 18h in a total cell manner to obtain 126.7g / L gamma-aminobutyric acid. The result shows that the 172 amino acid residue can severely influence the catalytic effect and stability of the enzyme, a foundation is set for researching the catalytic mechanism of the enzyme, and the industrial application potential of the enzyme is improved.
Owner:JIANGNAN UNIV
Novel endos mutant enzyme
ActiveUS20180208915A1Reduced hydrolysis activityLow production costHybrid cell preparationImmunoglobulinsHydrolysisMutant enzyme
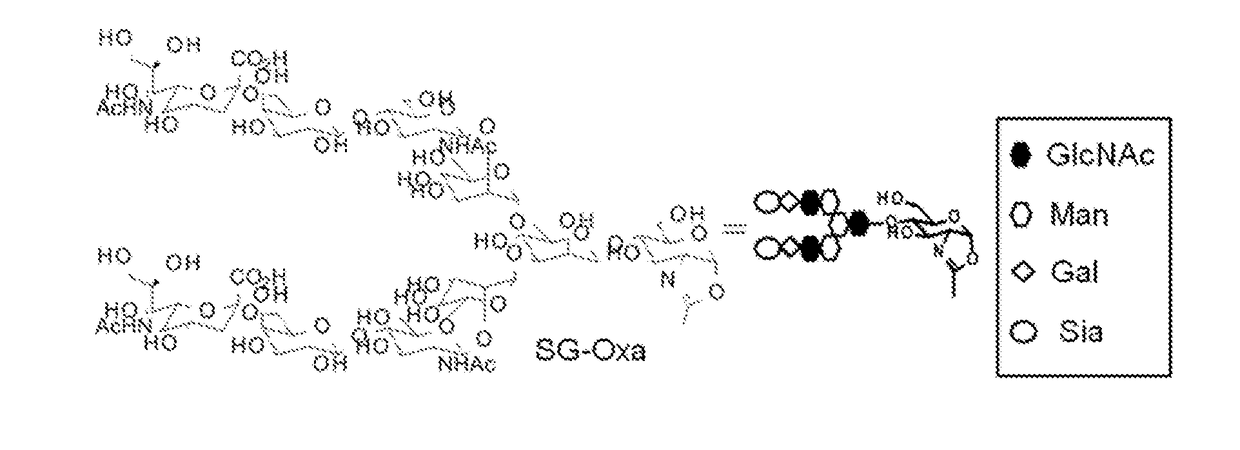
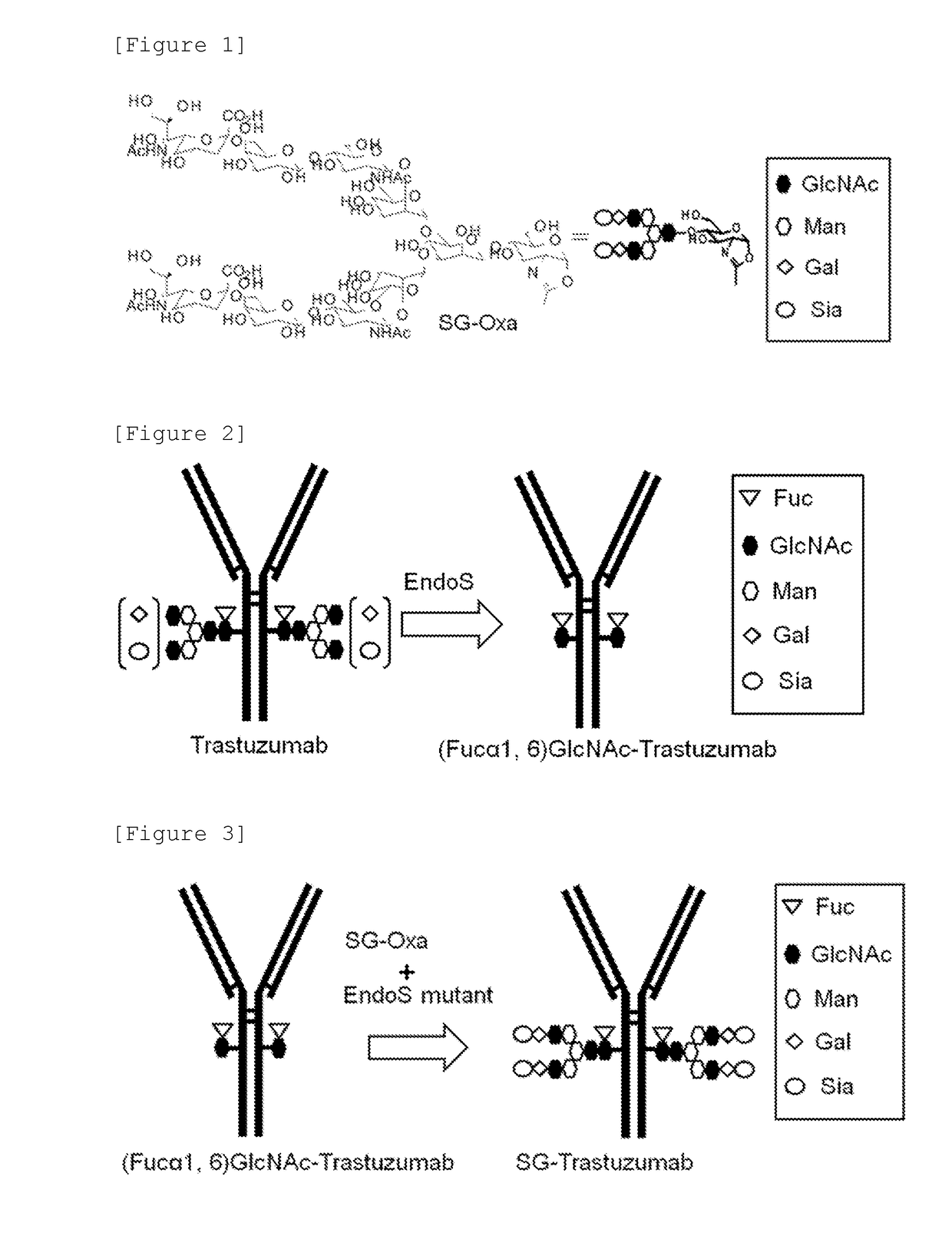
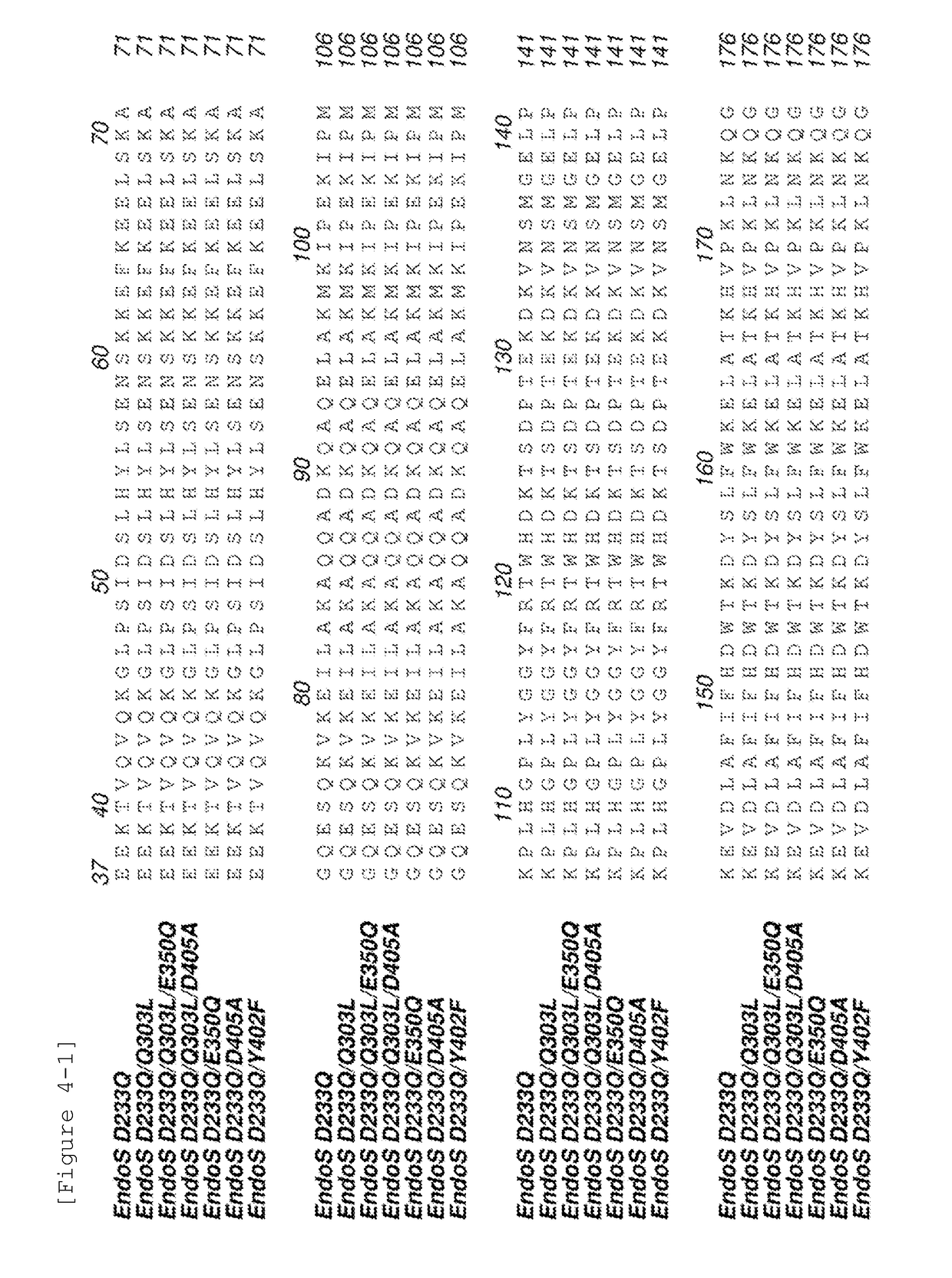
The present invention provides an EndoS mutant enzyme having an amino acid sequence of EndoS D233Q and further having a particular additional mutation and exhibiting a reduced hydrolysis activity, in comparison with the activity of EndoS D233Q, to an N-linked sugar chain (N297-linked sugar chain) linked to Asn at position 297 in IgG and a gene encoding the same.
Owner:DAIICHI SANKYO CO LTD
Low-temperature excision inulase mutant MutP126R stable at medium temperature
ActiveCN112725309AEasy to produceEasy to storeBacteriaMicroorganism based processesMutant enzymeAmino acid
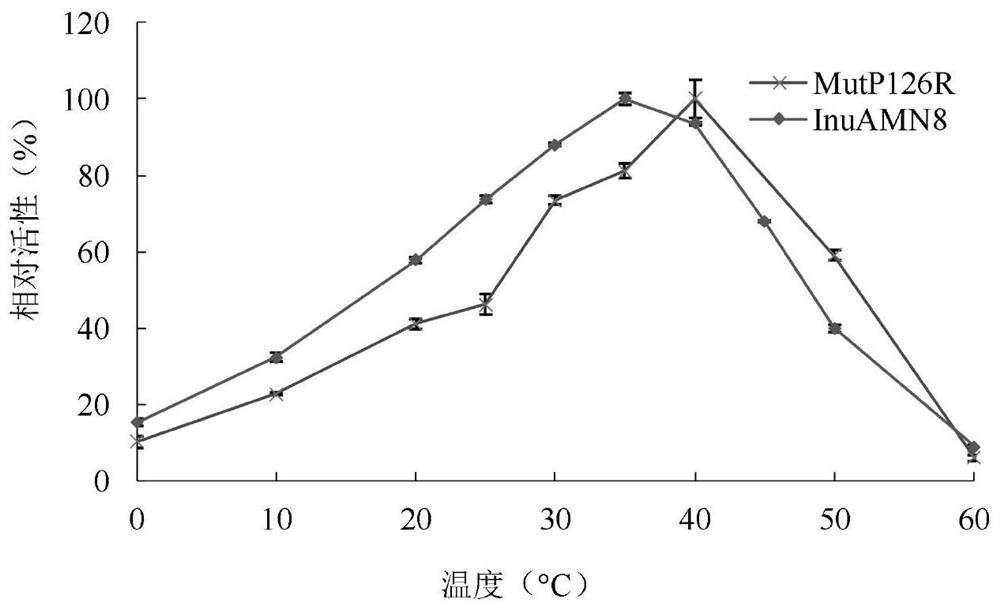
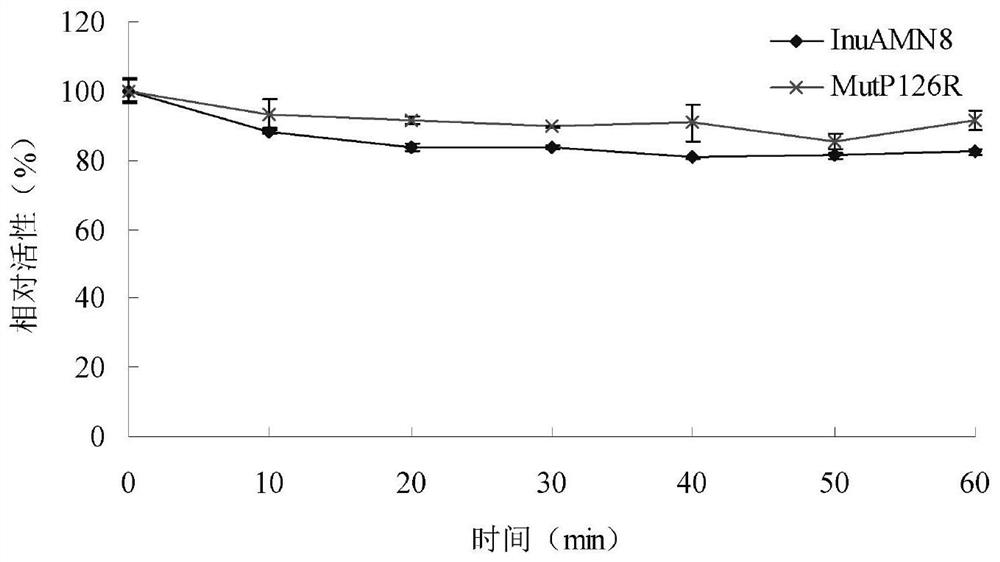
The invention discloses a low-temperature exoinulinase mutant MutP126R stable at medium temperature, the mutant MutP126R has an amino acid sequence as shown in SEQ ID NO.1, the thermal activity and thermal stability of the mutant MutP126R are changed, the optimal temperature is increased, the thermal stability is better, and the mutant MutP126R is beneficial to production, storage, transportation and the like of enzymes. The optimum temperature of the purified wild enzyme InuAMN8 is 35 DEG C, and the optimum temperature of the mutant enzyme MutP126R is 40 DEG C; after the wild enzyme InuAMN8 is treated at 55 DEG C for 10-60 minutes, the enzyme activity of the wild enzyme InuAMN8 is reduced from 70% to 17%, and the enzyme activity of the mutant enzyme MutP126R is reduced from 70% to 26%. The low-temperature excision inulase mutant MutP126R disclosed by the invention can be applied to the industries of food, wine brewing, biological energy and the like.
Owner:YUNNAN NORMAL UNIV
L-alanine dehydrogenase mutant zymoprotein and preparation method thereof
InactiveCN103074309AIncrease enzyme activityMicroorganism based processesEnzymesTurnover numberWild type
The present invention discloses L-alanine dehydrogenase mutant zymoprotein and a preparation method thereof. According to a homologous sequence secondary structure comparison result, site-specific mutagenesis of 73th lysine near an L-alanine dehydrogenase activity center of Bacilluspseudofirmus into alanine through PCR is performed to construct a mutant expression vector, and prokaryotic expression and Ni-NTA affinity chromatography purification are performed to obtain a mutant enzyme K73A, wherein specific activity of the obtained mutant enzyme K73A is 202% of specific activity the wild type, and other enzymatic properties are not changed. In addition, a turnover number Kcat on a substrate L-alanine by the mutant zymoprotein is 376.7 min<-1> and is 2.0 times the turnover number Kcat of the wild type, a turnover number Kcat on beta-NAD<+> by the mutant zymoprotein is 290.1 min<-1> and is 2.1 times the turnover number Kcat of the wild type, and the mutant zymoprotein can be applicable for production processes of production of pyruvic acid, L / D-alanine, and the like through improvement biology methods.
Owner:HEBEI NORMAL UNIV
Mutant of alpha-L-rhamnosidase from aspergillus terreus CCF 3059 and application thereof
ActiveCN106318957AReduce inactivation rateFavorable for biocatalytic conversionGenetic engineeringFermentationAgricultural scienceAlpha-L-rhamnosidase
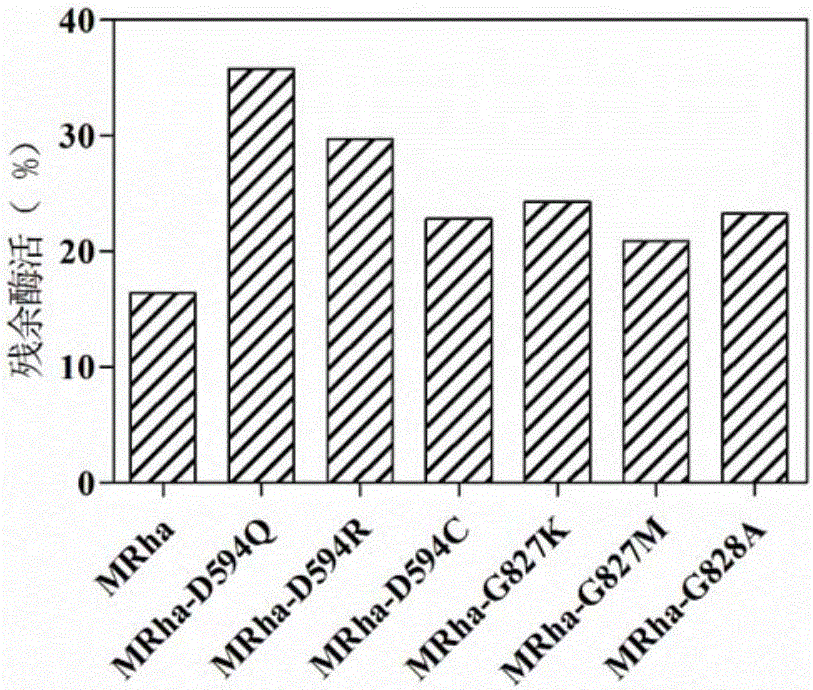
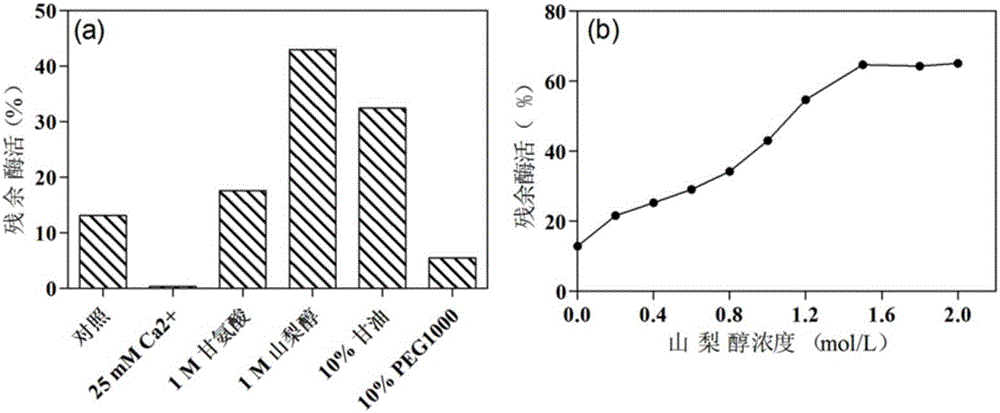
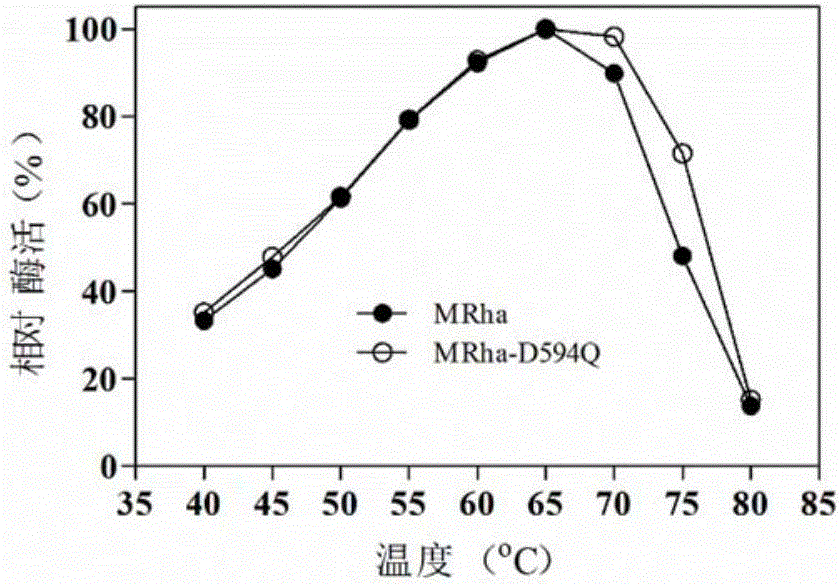
The invention provides a mutant of alpha-L-rhamnosidase from aspergillus terreus CCF 3059 and application thereof. The mutant comprises a gene D594Q shown in SEQ ID NO:2, a gene D594R shown in SEQ ID NO:3, a gene D594C shown in SEQ ID NO:4, a gene G827K shown in SEQ ID NO:5, a gene G827M shown in SEQ ID NO:6 and a gene G828A shown in SEQ ID NO:7. The mutant has the beneficial effects that the optimum temperatures of a mutant enzyme MRha-D594Q and a proenzyme MRha are 65 DEG C, but compared with the proenzyme MRha, the mutant enzyme MRha-D594Q still maintains higher enzymatic activity at 70 DEG C and 75 DEG C; the heat stability of the mutant enzyme MRha-D594Q can be further improved by adding sorbitol and is improved by 7.8 times in the half-life period at 70 DEG C.
Owner:NANJING FORESTRY UNIV
Method for increasing lipase expression through glycosylation modification as well as mutant enzyme and application thereof
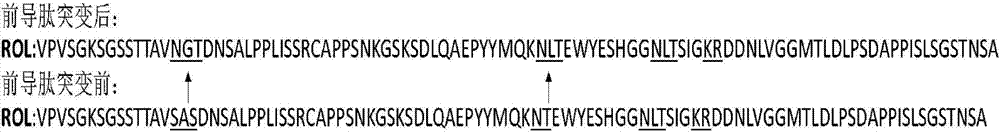
ActiveCN104762277ADoes not affect growthIncrease enzyme activityFungiHydrolasesExtracellular proteinsPeptide sequence
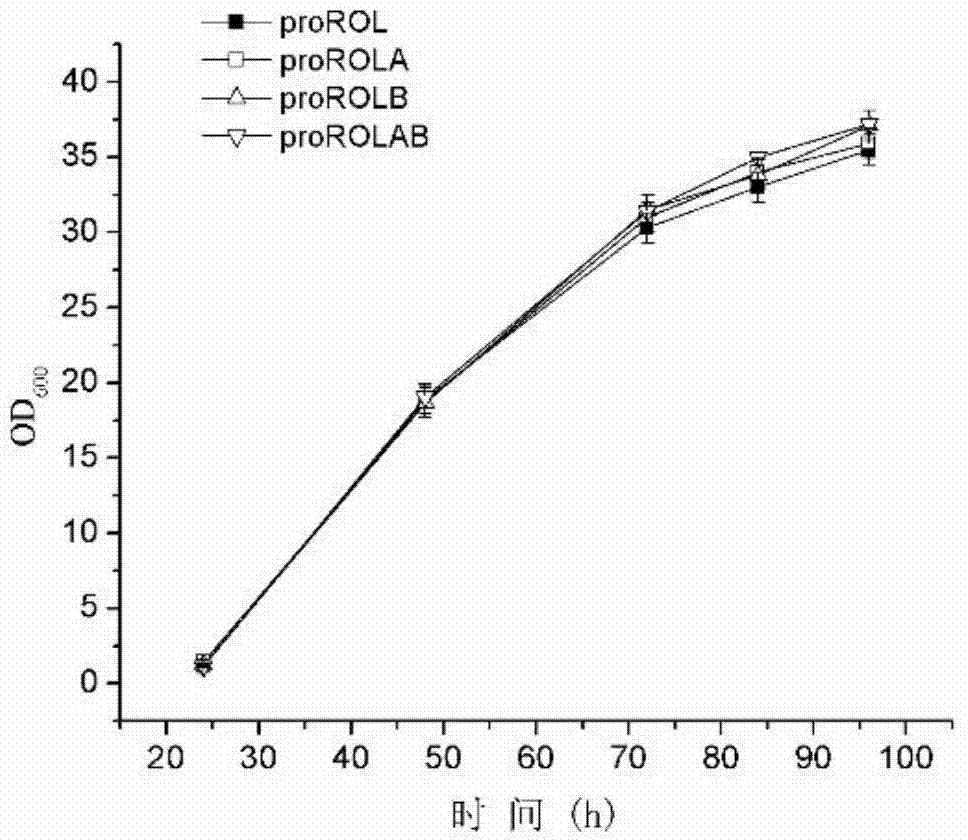
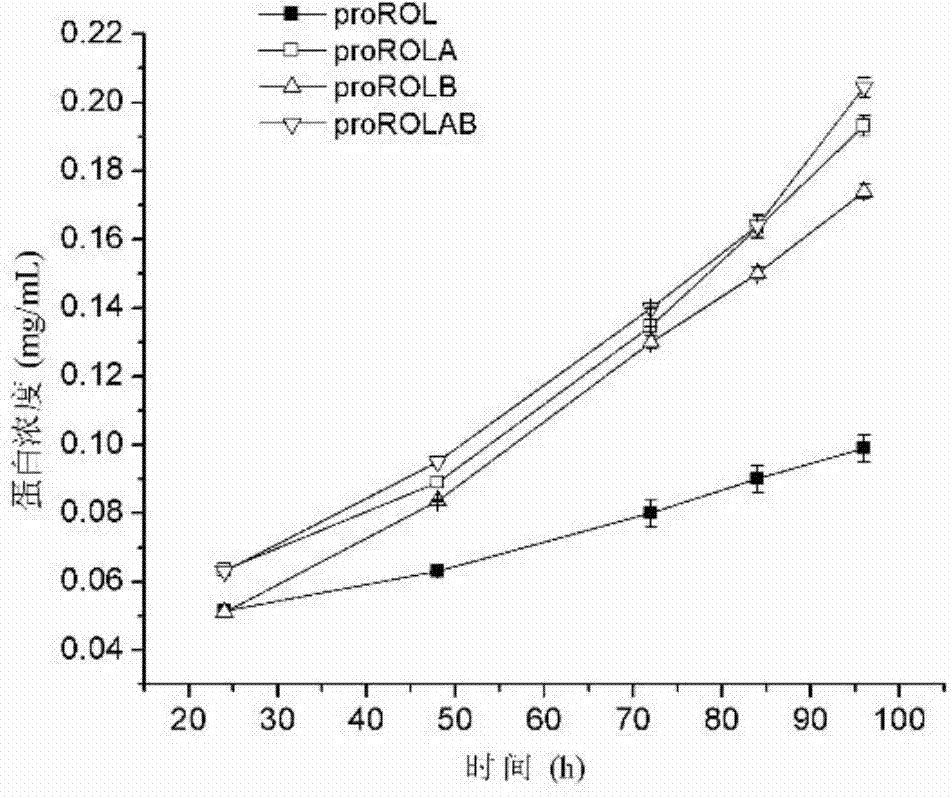
The invention discloses a method for increasing lipase expression through glycosylation modification as well as a mutant enzyme and an application thereof and belongs to the field of enzyme engineering. The N-glycosylation mutation is performed on a leading peptide sequence of rhizopus oryzae lipase, the SAS and / or NT amino acid are / is respectively modified to an N-glycosylation site NGT and / or NLT, the extracellular protein concentrations of the obtained mutant enzyme proROLA, proROLB and proROLAB are increased by 211%, 188% and 233% compared with those of the un-glycosylated proROL, the enzyme activities when culturing in a fermentation tank are respectively 8210 U.mL<-1>, 8457 U.ml<-1> and 9366 U.mL<-1>, and the un-mutated proROL extracellular enzyme activity is almost zero. The rhizopus oryzae lipase provided by the invention has obviously increased lipase enzyme activity and can be used in the fields such as food, chemical engineering and biological energy source.
Owner:TAIXING YIMING BIOLOGICAL PRODS
Glycosyl transferase mutant and method for catalytically synthesizing rebaudioside M by using glycosyl transferase mutant
ActiveCN113462670AIncrease productionHigh affinityBacteriaMicroorganism based processesSucrose synthetasePtru catalyst
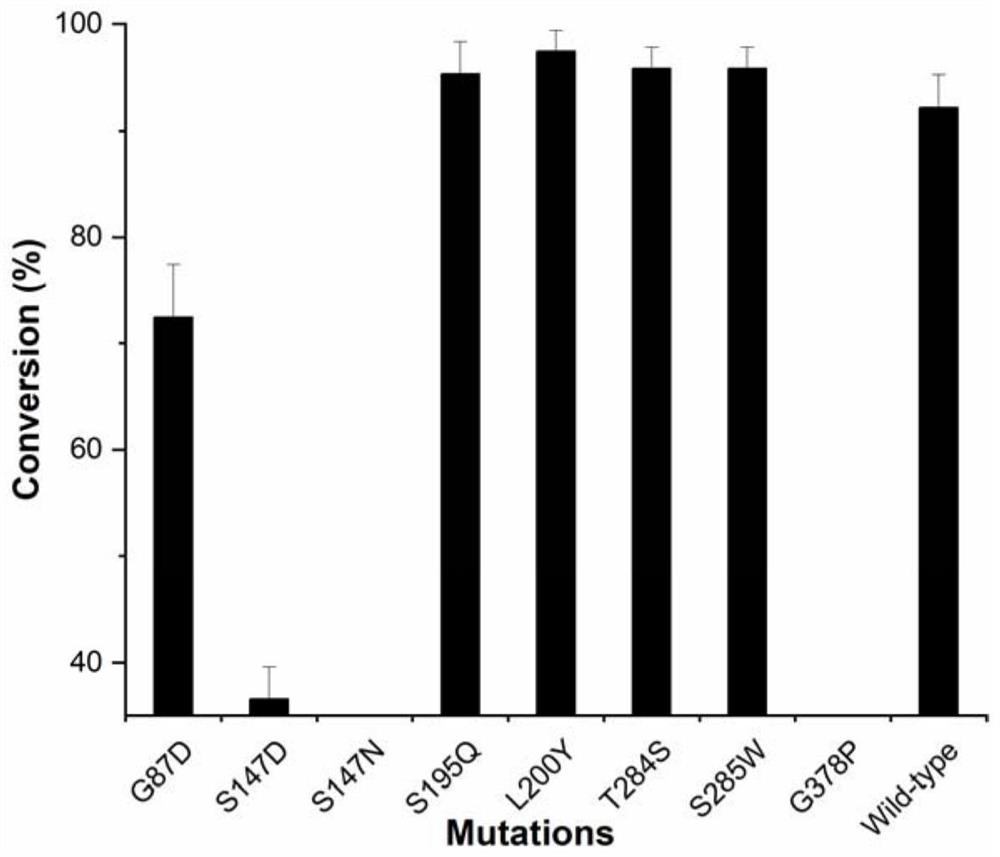
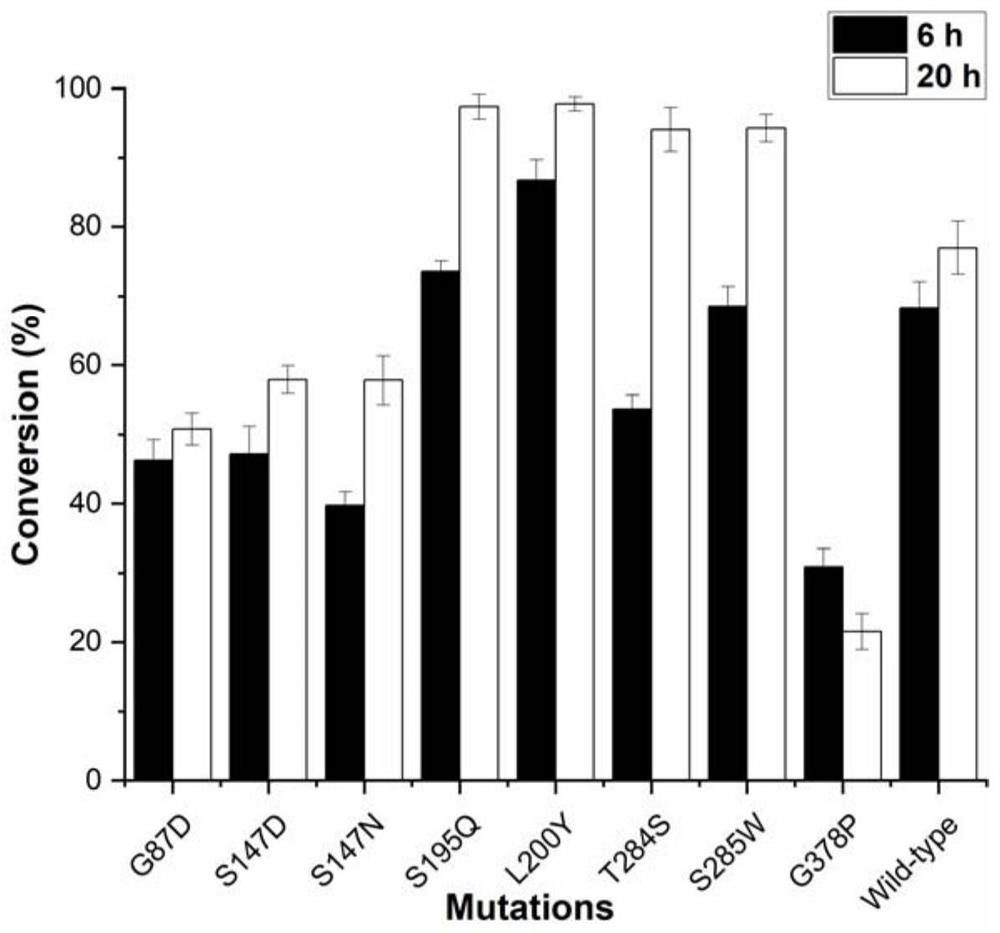
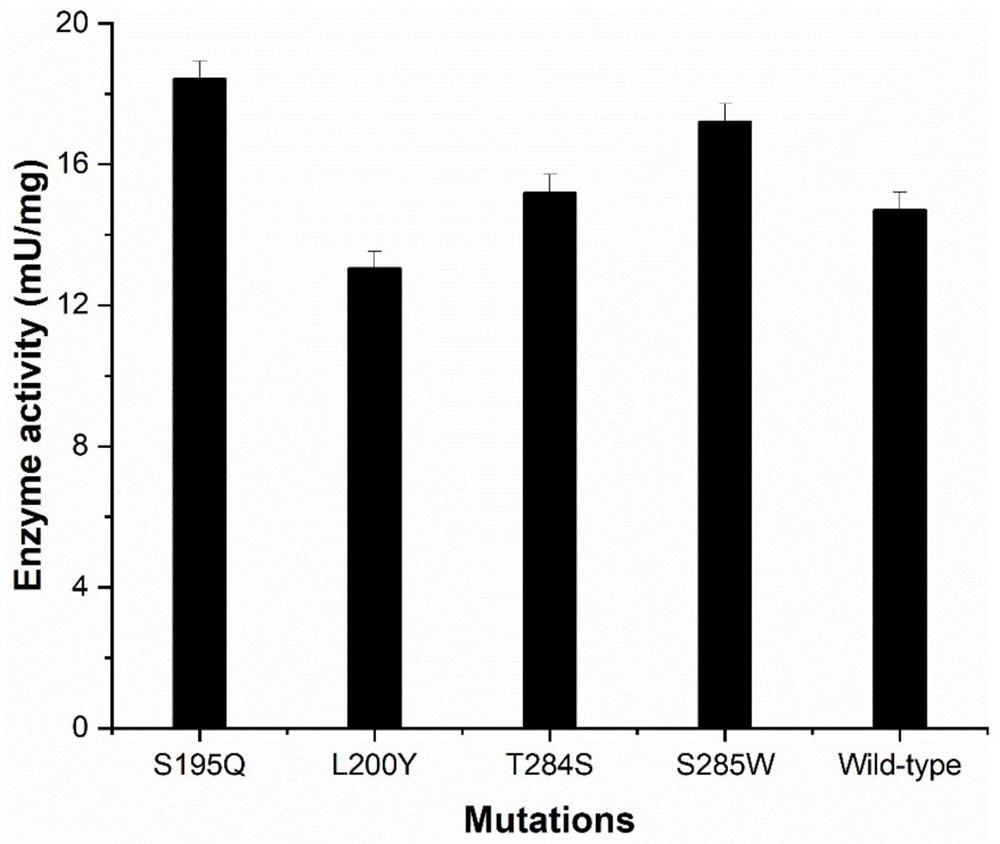
The invention discloses a glycosyl transferase mutant and a method for catalytically synthesizing rebaudioside M by using the glycosyl transferase mutant. The mutant is obtained by performing mutation on the basis of a glycosyl transferase amino acid sequence shown as SEQ ID NO: 1, performing induced expression on a mutant strain to obtain a mutant enzyme, and catalyzing 20g / L RebE to synthesize 12.8 g / L RebM by using the mutant enzyme as a catalyst and the enzymic method. The kinetic parameters of the mutant S195Q on rebaudioside E and rebaudioside D and the Michaelis constant of the mutant are 56.34 + / -2.02 mu M and 214.48 + / -14.54 mu M respectively, and are 1 / 3 and 2 / 5 of those of a wild type. The glycosyl transferase mutant is coupled with sucrose synthase to realize efficient catalytic synthesis of rebaudioside M. According to the present invention, the recombinant strain of the glycosyl transferase UGT76G1 or the mutant thereof and the sucrose synthase is constructed so as to achieve the efficient catalytic synthesis of the rebaudioside M; the method has the optimal yield in the current enzymatic catalytic synthesis experiment of rebaudioside M, and is green, environment-friendly and pollution-free.
Owner:XINGHUA GL STEVIA CO LTD
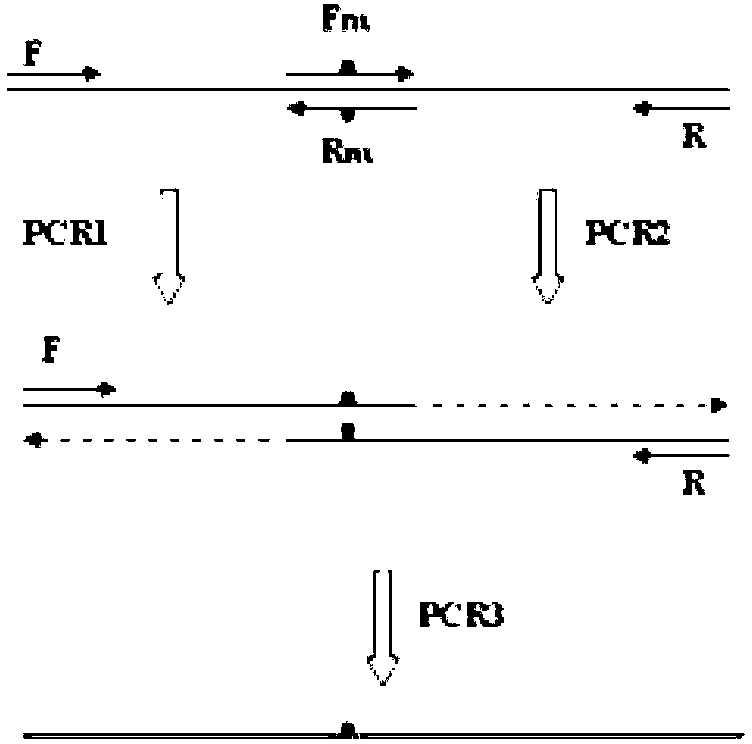
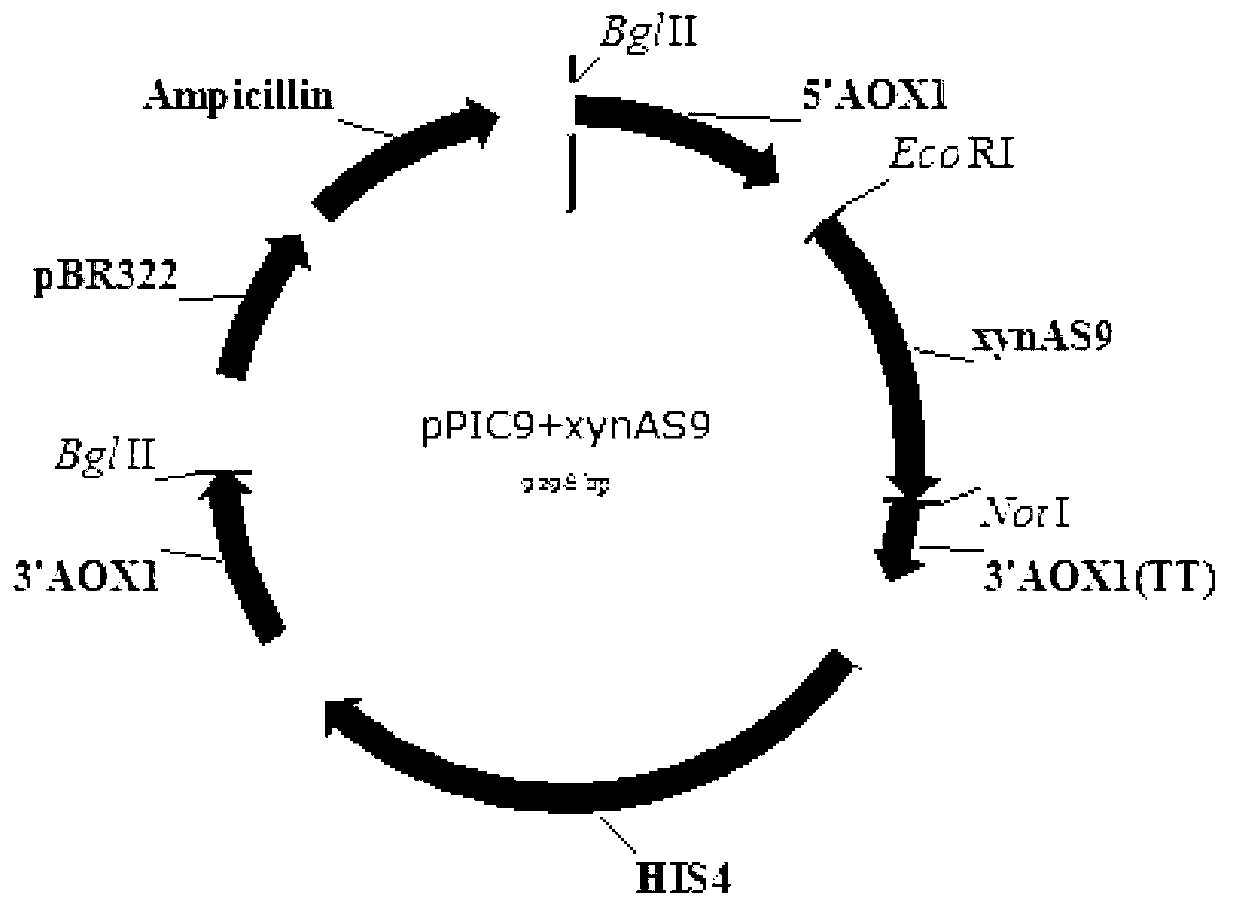
Thermal stability improved xylanase XynAS9-m mutant V81P/G82E as well as gene and application thereof
The invention belongs to the technical field of gene engineering and enzyme engineering and particularly relates to thermal stability improved xylanase XynAS9-m mutants V81P / G82E and V81P / G82E / D185P / S186E as well as a gene and application thereof. The xylanase XynAS9-m mutant V81P / G82E is a xylanase with an amino acid sequence shown as SEQ ID No.1, wherein the valine on the 81st position of the xylanase is mutated into proline, and the glycine on the 82nd position of the xylanase is mutated into glutamic acid. Furthermore, the valine on the 81st position of the xylanase is mutated into the proline, and the glycine on the 82nd position of the xylanase is mutated into the glutamic acid; and the aspartic acid on the 185th position of the xylanase is mutated into the proline, and the serine on the 186th position of the xylanase is mutated into the glutamic acid, so that the xylanase XynAS9-m mutant V81P / G82E / D185P / S186E is obtained. The thermal stability of the mutated enzyme obtained by the invention is remarkably improved, so that the mutant has a potential application value in industries such as paper pulp making, biological energy source and the like.
Owner:INST OF ANIMAL SCI OF CHINESE ACAD OF AGRI SCI
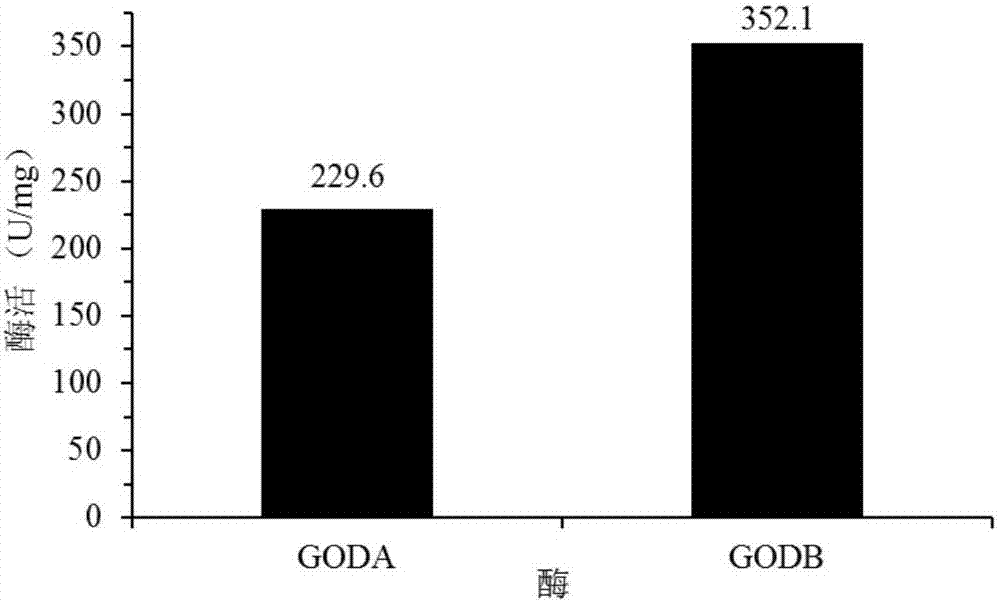
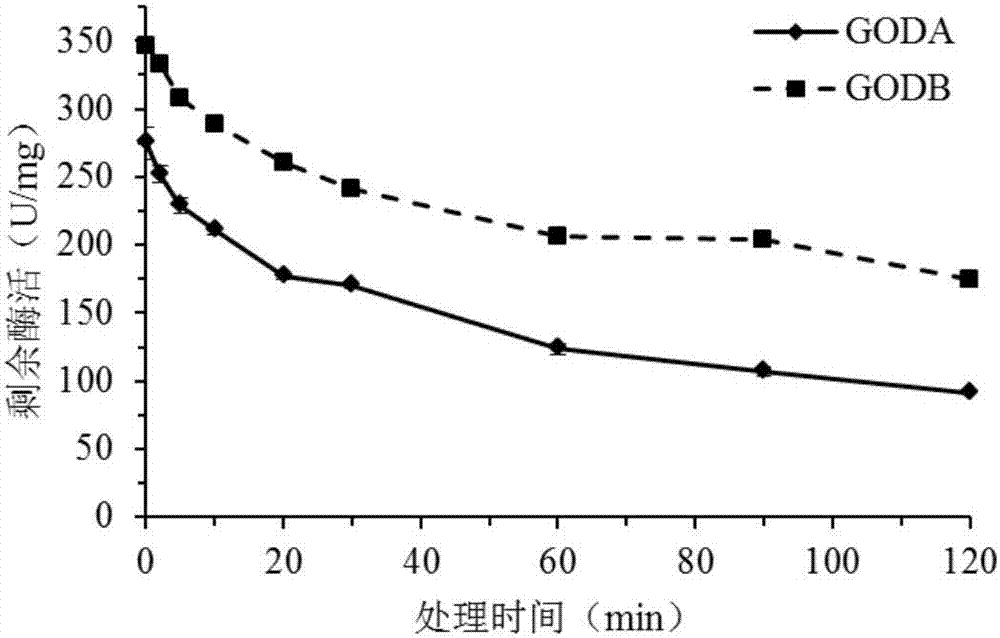
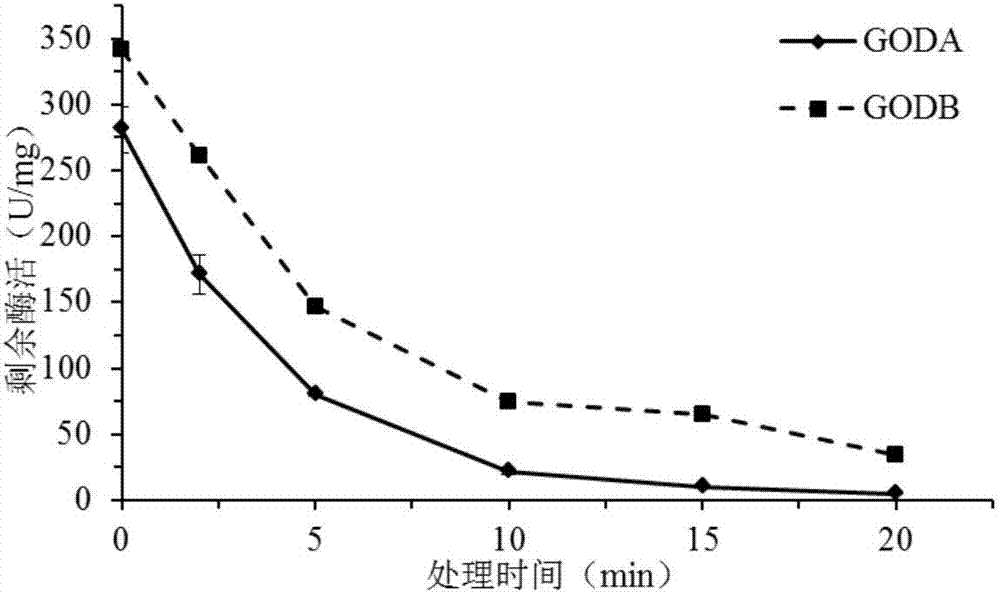
Glucose oxidase mutant and encoding gene and application thereof
ActiveCN107189991AMeet application needsFungiMicroorganism based processesAgricultural scienceHalf-life
The invention discloses a GODB mutant and an encoding gene and application thereof. The GODB mutant derives from GODA of Aspergillus niger, a female parent, and is obtained through point mutation Glu82Cys. The GODB mutant has the advantages that mutant enzyme activity is increased from 229.6 U / mg of a wild type to 352.1 U / mg, an increase of 53.3%, a half-life period at 60 DEG C is increased to 119 minutes from 51 minutes of the wild type, an increase of 133%, and accordingly the GODB mutant can meet the requirements of the fields such as food, medicine, feed and the textile industry and is promising in application prospect.
Owner:INST OF ANIMAL SCI OF CHINESE ACAD OF AGRI SCI
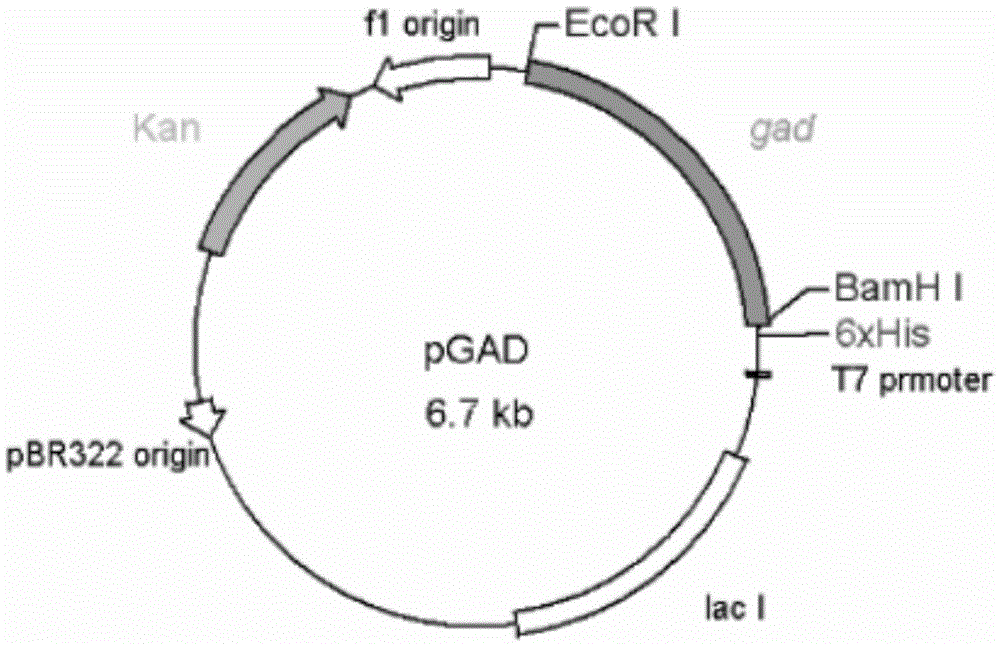
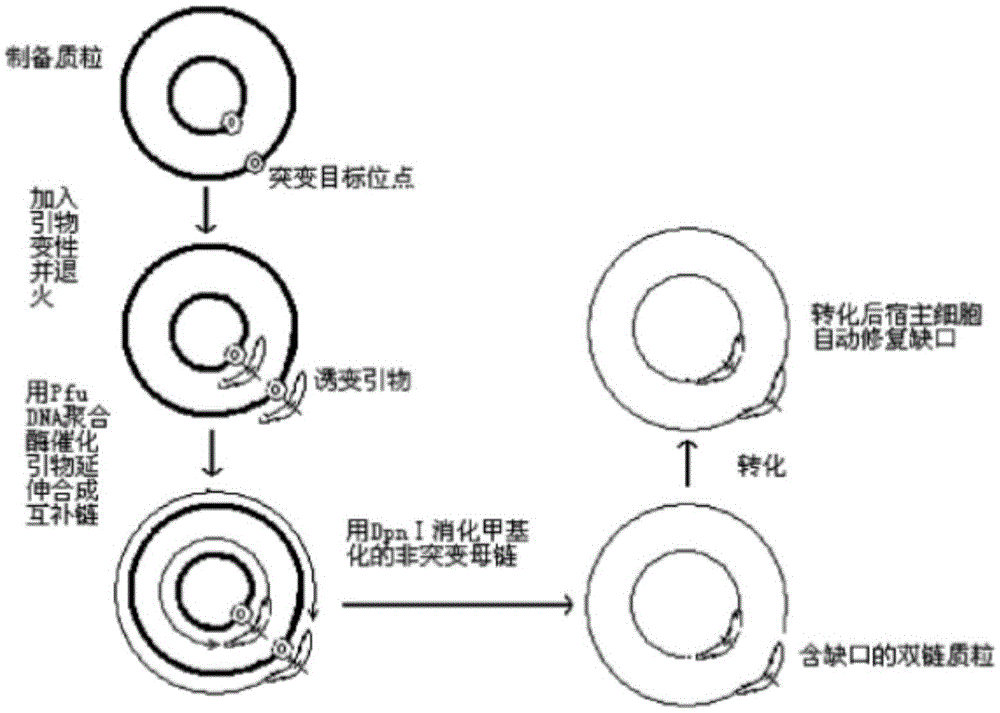
Glutamic acid decarboxylase mutant and preparation method thereof and application
InactiveCN105462949AGood thermal stabilityHigh application valueFermentationGenetic engineeringGlutamate decarboxylaseMutant
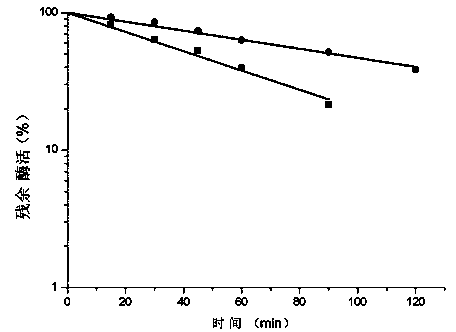
The invention discloses a glutamic acid decarboxylase mutant and a preparation method thereof and application. An amino acid sequence of the mutant is as shown in SEQ ID No. 2, and a nucleotide sequence is as shown in SEQ ID No. 1. The invention further discloses an expression unit containing genes for encoding the glutamic acid decarboxylase mutant, a recombinant plasmid and a transformant. According to information of comparison with a thermococcus kodakarensis glutamic acid decarboxylase (GAD) amino acid sequence, a method of site-directed mutagenesis is adopted to introduce proline residue at an amino acid locus corresponding to lactobacillus brevis GAD, and the thermal stability of the GAD is improved through rational design. The mutant has better thermal stability in the process of catalyzing L-glutamic acid or sodium salts thereof to generate gamma-aminobutyric acid and is favorable for industrial production of gamma amino acid butyric acid (GABA).
Owner:NINGBO INST OF TECH ZHEJIANG UNIV ZHEJIANG
Nitrile hydratase mutant, genetically engineered bacteria containing mutant and application of mutant
ActiveCN109593750AImprove thermal stabilityImprove toleranceBacteriaMicroorganism based processesGlycineHigh density
The invention discloses a nitrile hydratase mutant, genetically engineered bacteria containing the mutant and application of the mutant, and belongs to the technical field of enzyme engineering. According to the invention, a 47th glycine of the nitrile hydratase mutant alphaL6T / A19V / F126Y-betaM46K / E108R / S212Y (disclosed in the invention patent CN102216455A) mutates into asparagine; the obtained new mutant enzyme has better temperature tolerance and product tolerance, which is conducive to industrial production in the future; a recombinant strain containing the nitrile hydratase mutant is fermented at a high density, and nicotinonitrile is used as a substrate to perform whole-cell catalytic reaction to prepare nicotinamide. Compared with a chemical production method, the method is safe andclean in production process and free of environmental pollution; and compared with an enzymatic method, the method has low price of the substrate and high catalysis efficiency, obtains a final productnicotinamide at a yield of over 95% and a concentration up to 680 g / L, and simplifies separation and purification steps of the product.
Owner:JIANGNAN UNIV
Preparation method and application of pullulanase mutant PulB-d99-D436H
ActiveCN105441415AImprove featuresImproved fermentation productionBacteriaMicroorganism based processesBacillus naganoensisPullulan
The invention discloses a preparation method and application of a pullulanase mutant PulB-d99-D436H. The preparation method comprises the steps that 100-926 amino acid peptide fragments of bacillus naganoensis pullulanase are cut, aspartic acid (Asp) at the 436 locus of the amino acid peptide fragments is mutated into histidine (His), and then the novel pullulanase mutant is obtained through transformation. The preparation method and application of the pullulanase mutant PulB-d99-D436H have the advantages that the mutant enzyme has the excellent characteristics compared with an enzyme before mutation, wherein the enzyme expression quantity is 3.8 times of that of a parent enzyme, the optimum temperature is increased by 2.5 DEG C, and the catalytic activity is 1.36 times of that of the parent enzyme. The mutant gene is integrated into chromosomes of bacillus subtilis to construct and recombine the bacillus subtilis, and therefore pullulanase can be stably fermented and produced. The hydrolysis efficiency of starch and pullulan can be improved through the mutant enzyme.
Owner:南宁邦尔克生物技术有限责任公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com