Thermal stability improved xylanase XynAS9-m mutant V81P/G82E as well as gene and application thereof
A kind of xylanase mutation, xylanase technology, applied in the field of genetic engineering and enzyme engineering, can solve problems such as poor thermal stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
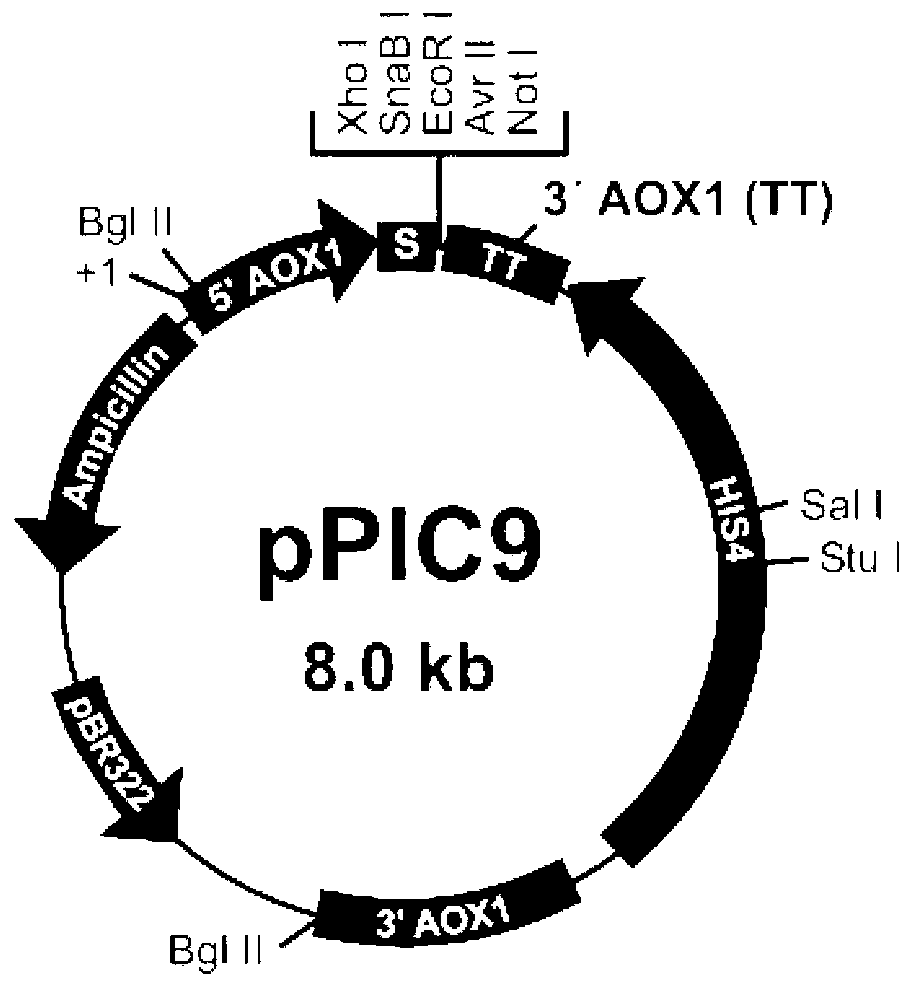
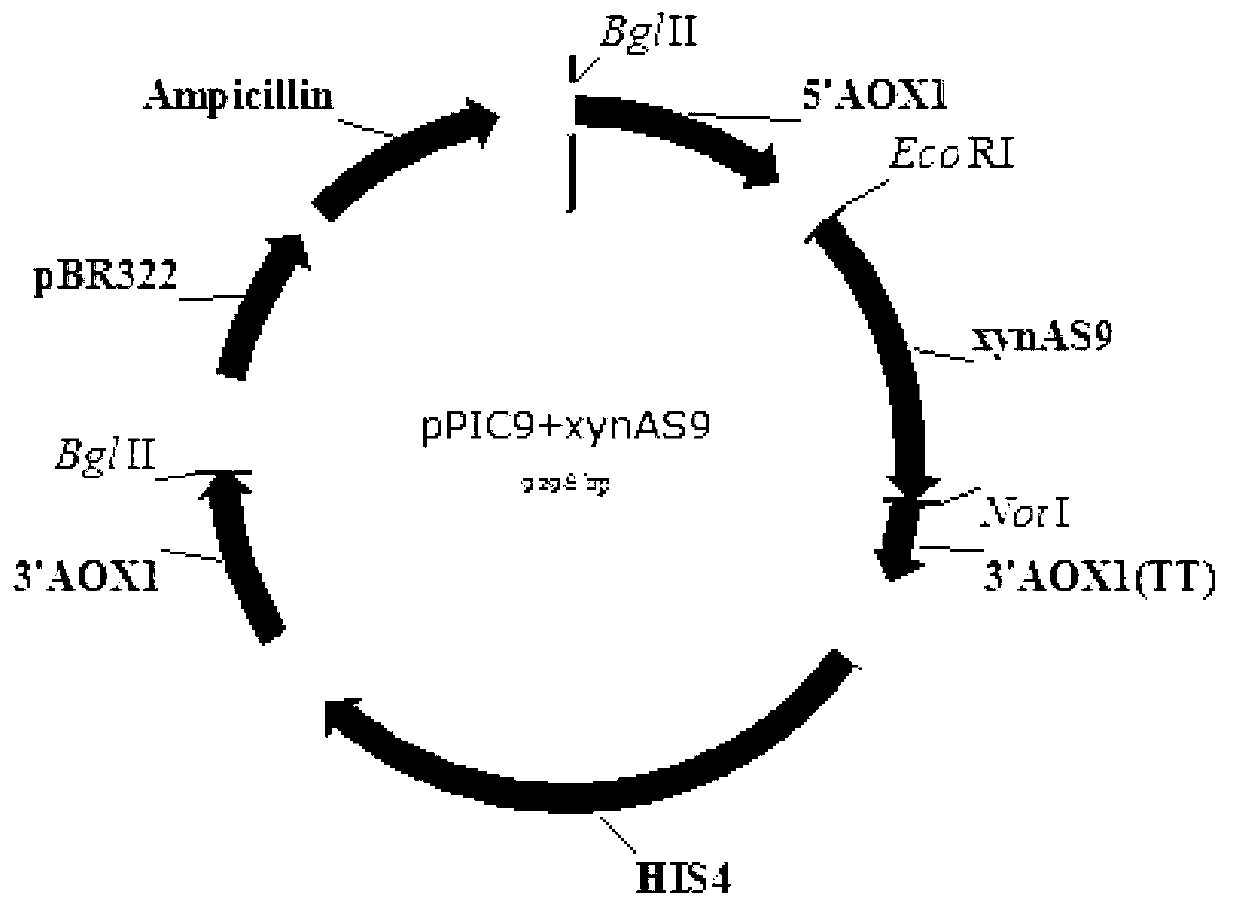
Image
Examples
Embodiment 1
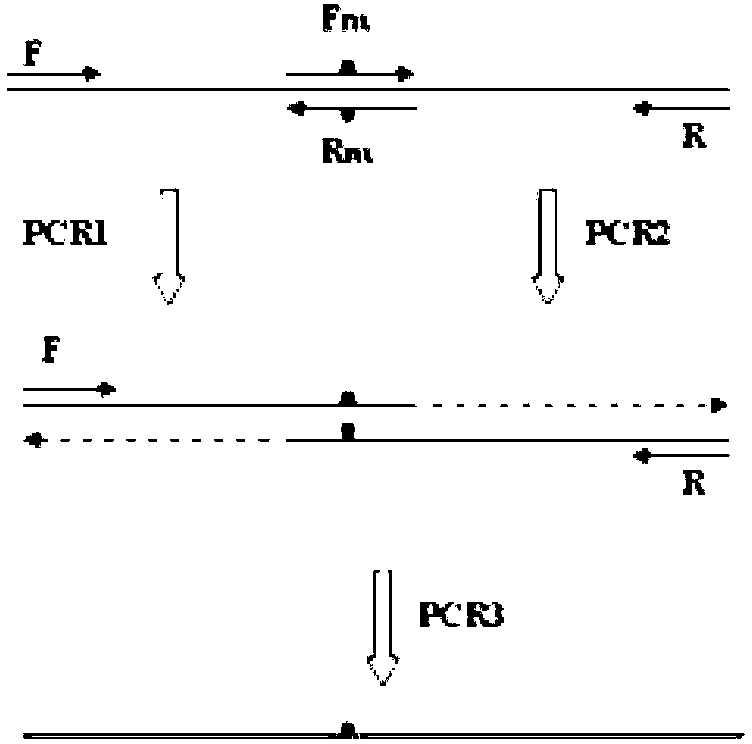
[0139] 1. Acquisition of mutant genes:
[0140] The gene sequence (SEQ ID NO.2) of xylanase XynAS9-m derived from Streptomyces sp.S9 was transformed, the mutation was introduced by means of Overlap PCR, and it was sequenced to obtain the mutant gene.
[0141] The mutation includes six PCR primers: S9BF, S9BR, V81P / G82E-F, V81P / G82E-R,
[0142] D185P / S186E-F, D185P / S186E-R.
[0143] The primer sequences are as follows:
[0144] S9BF:5'-TA GAATTC GACACCGCCACCCTGGGCGAACT-3'
[0145] S9BR: 5'-TAT GCGGCCGC CTACGCCGAAGTCCCGGACGGC-3'
[0146] V81P / G82E-F:5'-GCCAGATCACCcccgaaAACACCATGAAGT-3'
[0147] V81P / G82E-R:5-ACTTCATGGTGTTttcgggGGTGATCTGGC-3'
[0148] D185P / S186E-F:5'-AGAAGATCGGCcccgagTACATCG-3';
[0149] D185P / S186E-R:5'-CGATGTActcgggGCCGATCTTCT-3'
[0150] The underline represents the restriction enzyme cutting sites EcoRI and NotI, and the lowercase letters represent the mutant bases. The overlapping extension PCR method is completed through 3 PCR reactions. Take the...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com