Thermal stability improved mutant enzyme of D-psicose 3-epimerase and application thereof
A technology of epimerase and thermal stability, which is applied in the field of genetic engineering of enzymes and can solve problems such as limiting the scope of application
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] Example 1: DPE enzyme thermostability site-directed mutation analysis and mutant preparation method
[0023] Through the analysis of the 3D spatial structure of DPE enzyme, it was found that G109 is located in the β-turn region of the active site, and the stability of the enzyme molecule can be changed by changing the hydrophobicity of the 109-position amino acid. Therefore, the weaker hydrophobic glycine Gly was replaced by the stronger hydrophobic proline Pro to increase its thermal stability.
[0024] Construction of the pet22b(+)-G109P mutant plasmid by rapid PCR site-directed mutagenesis;
[0025] The construction of the pet22b(+)-G109P mutant plasmid: using the pet22b(+)-cb-dpe plasmid as a template, using the G109P mutant primer to obtain a product with a size of about 6.4kbp by PCR once, the PCR product was subjected to Dpn After I treatment, Escherichia coli DH5α competent cells were transformed, and transformants were selected for sequencing verification.
...
Embodiment 2
[0042] Example 2: Expression and purification method of mutant enzyme of Clostridium boltae (Clostridium boltae) DPE.
[0043] The mutant plasmid pet22b(+)-G109P verified by sequencing was transformed into Escherichia coli BL21(DE3) cells, and the positive transformants were picked and cultured overnight in LB medium at 37°C and 200rpm, and then cultured in LB medium at 37°C for 3 -4h until the OD value is 0.6-0.8, cool down to 25°C, add IPTG at a final concentration of 0.8mM to induce for 8h.
[0044] The fermentation broth was centrifuged at 4°C and 10000rpm for 20min to collect the bacterial cells. Add 15 mL Binding Buffer (50 mM Na 2 HPO 4 , 50 mM NaH 2 PO 4, 500 mM NaCl, adjust the pH to 7.4) to fully resuspend the bacteria, then place the centrifuge tube in an ice bath and put it into an ultrasonic cell disruptor. The conditions for ultrasonic disruption are: working time 1 s, stop time 2 s, total 20 min. Centrifuge the broken solution at low temperature and high s...
Embodiment 3
[0046] Example 3: Thermostability of DPE enzymes at 55°C
[0047] 1. DPE enzyme activity assay method: Add 700 μL of 100 g / L D-fructose to 1 mL of reaction system, 200 μL of diluted enzyme solution, and 100 μL of Co at a final concentration of 1 mM 2+ ion. Incubate at 55°C for 5 min, then boil for 10 min to terminate the enzyme reaction.
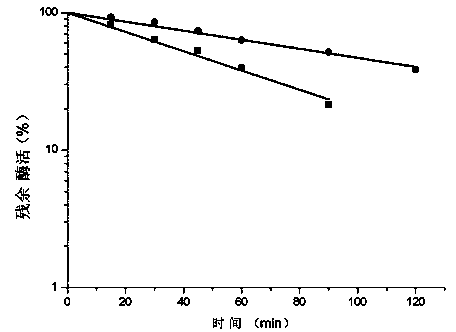
[0048] 2. Place the purified DPE enzyme solution in a 55°C water bath to keep it warm, and regularly measure the residual enzyme activity. Draw a curve of residual enzyme activity versus time (e.g. figure 1 ), according to the curve to get the half-life of DPE enzyme at this temperature.
[0049] Table 1. DPE enzyme thermostability at 55°C
[0050] t 1 / 2 (55℃) (min) t 1 / 2 (55℃) increase multiple wild-type DPE 42.9 1 G109P 91.5 2.13
[0051] Through measurement, it was found that the half-life of the mutant enzyme G109P at 55°C was greatly improved, from 42.9 min to 91.5 min, which was 2.13 times of th...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com