Mutant of alpha-L-rhamnosidase from aspergillus terreus CCF 3059 and application thereof
A rhamnosidase and Aspergillus terreus technology, applied in the fields of molecular biology and enzyme engineering, can solve the problems of insufficient α-L-rhamnosidase activity, unfavorable substrate dissolution and product purification, poor thermal stability and the like , to achieve the effect of improving thermal stability, improving temperature stability, and improving thermal stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] 1. Obtaining the recombinant plasmid pPICZαA-MRha and determining the mutation site
[0024] Complete gene synthesis and optimization of Aspergillus terreus CCF 3059 α-L-rhamnosidase gene through yeast codon preference. Its nucleotide sequence is as SEQ ID NO: 8, which is ligated to pPICZαA plasmid, and the obtained recombinant plasmid is named To pPICZαA-MRha, transform Pichia pastoris KM71H.
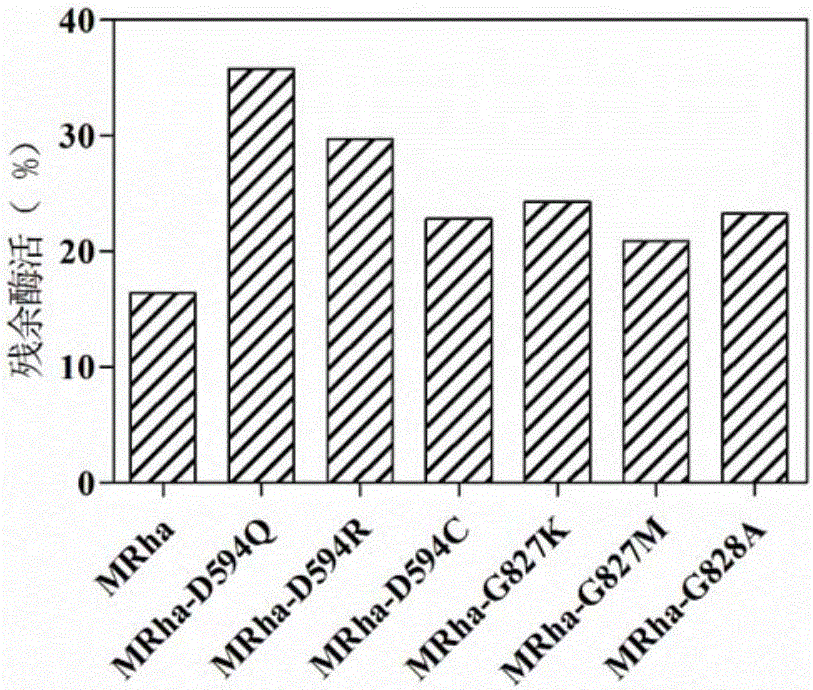
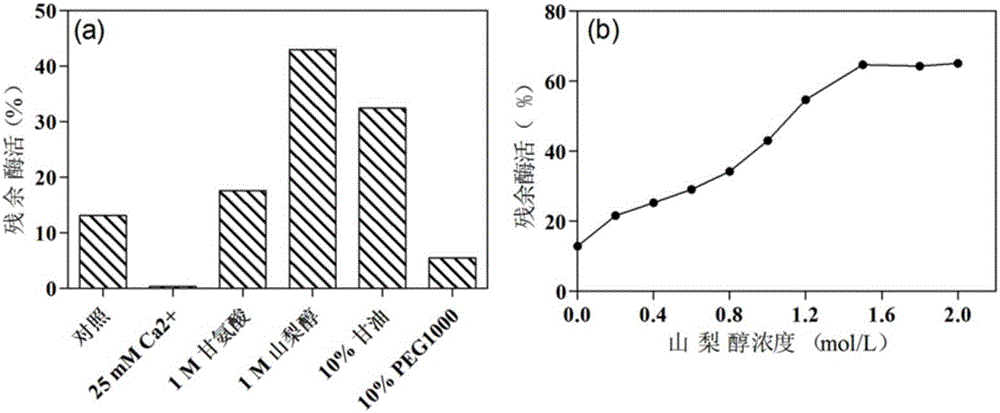
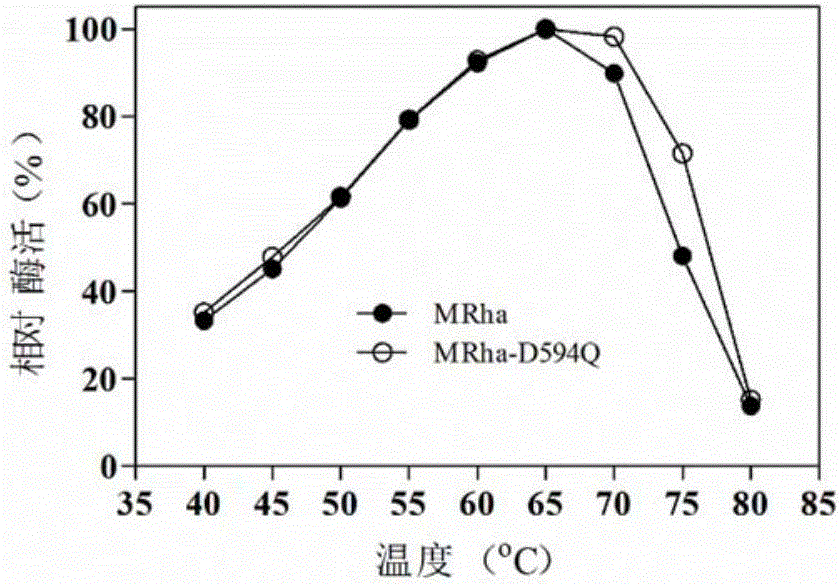
[0025] Studies have shown that the loop region of the enzyme protein is closely related to the thermostability of the enzyme. In addition, the larger the amino acid B-Factor value, the more unstable it is. Through homology modeling, the three-dimensional structure of Aspergillus terreus CCF 3059 α-L-rhamnosidase was obtained. Based on the above-mentioned theory, the mutation site was searched for, and the mutation of the mutation site was analyzed and screened using the bioinformatics software Discovery Studio. The mutants with lower potential energy were used as the research object...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com