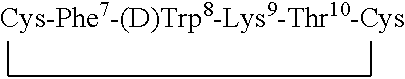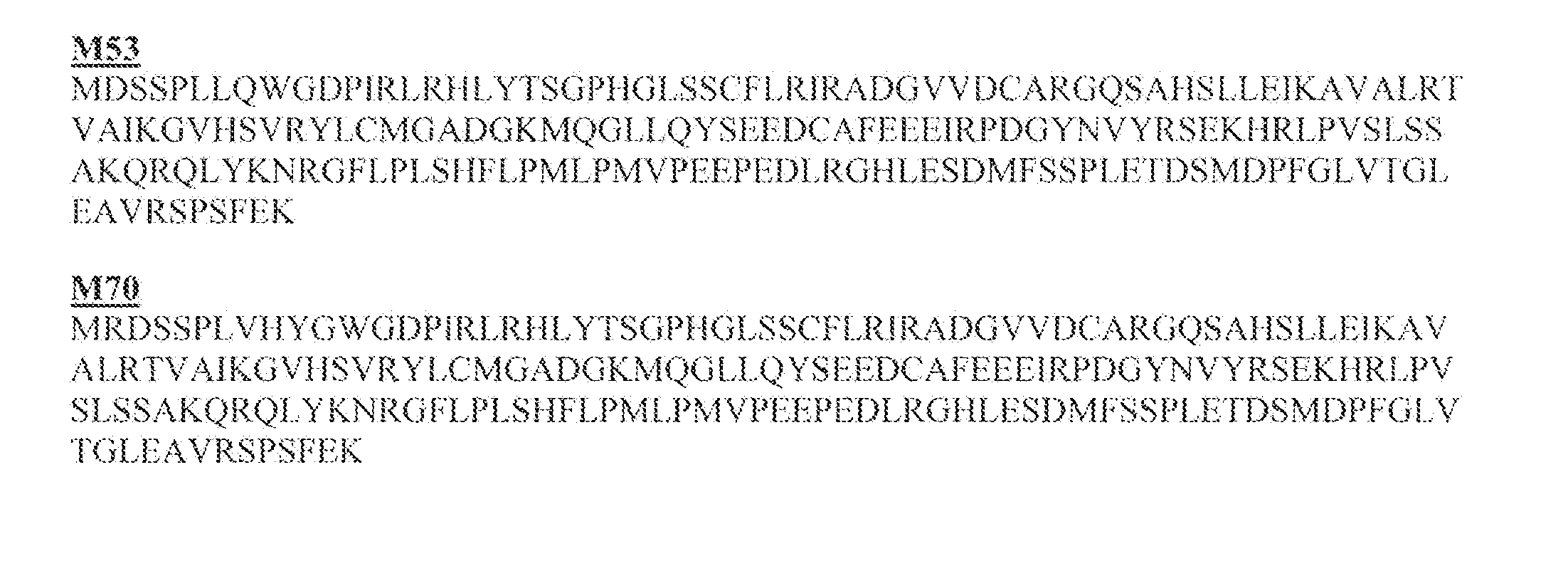Patents
Literature
1107 results about "Peptide sequence" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Peptide sequence, or amino acid sequence, is the order in which amino acid residues, connected by peptide bonds, lie in the chain in peptides and proteins. The sequence is generally reported from the N-terminal end containing free amino group to the C-terminal end containing free carboxyl group. Peptide sequence is often called protein sequence if it represents the primary structure of a protein.
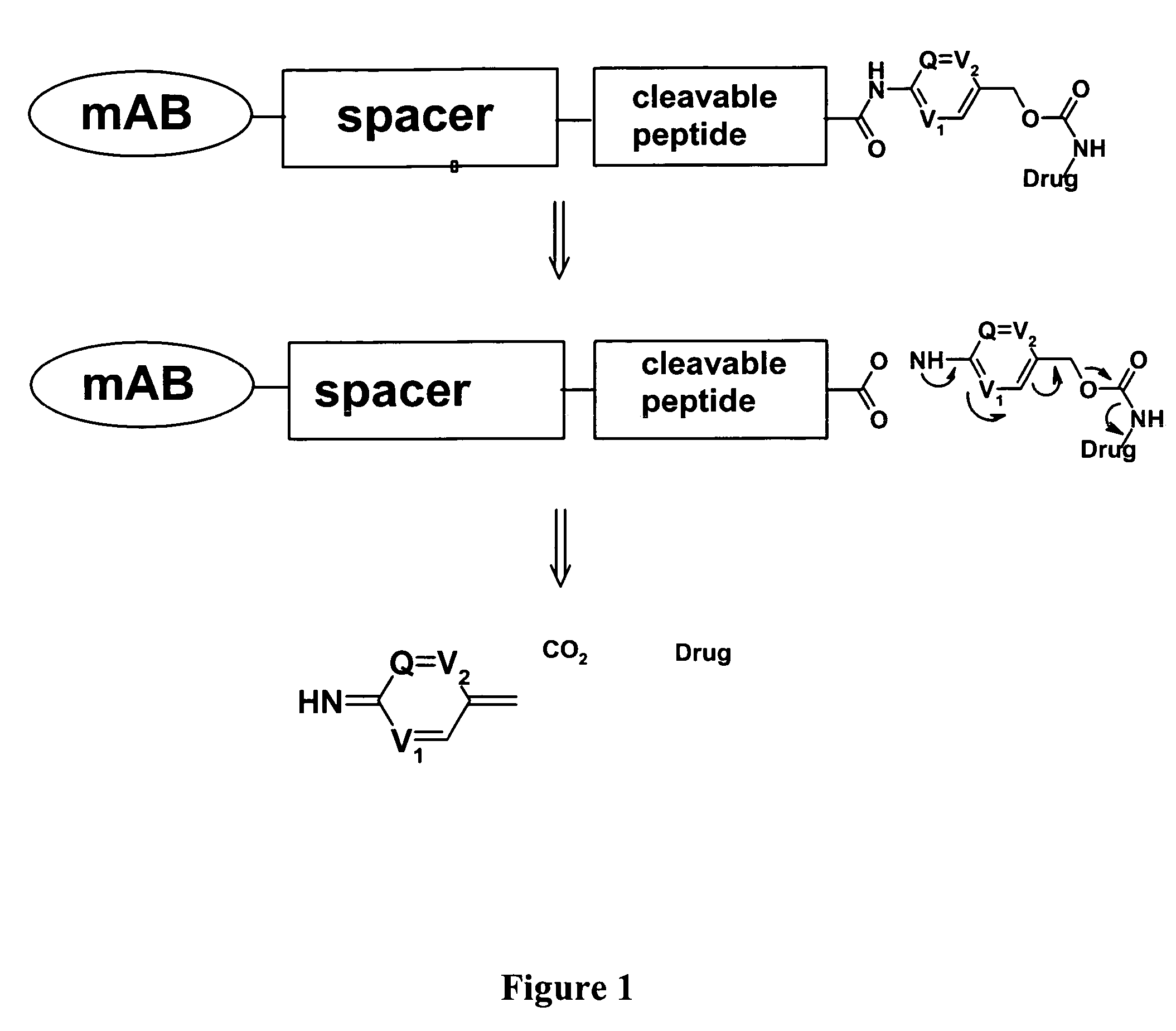
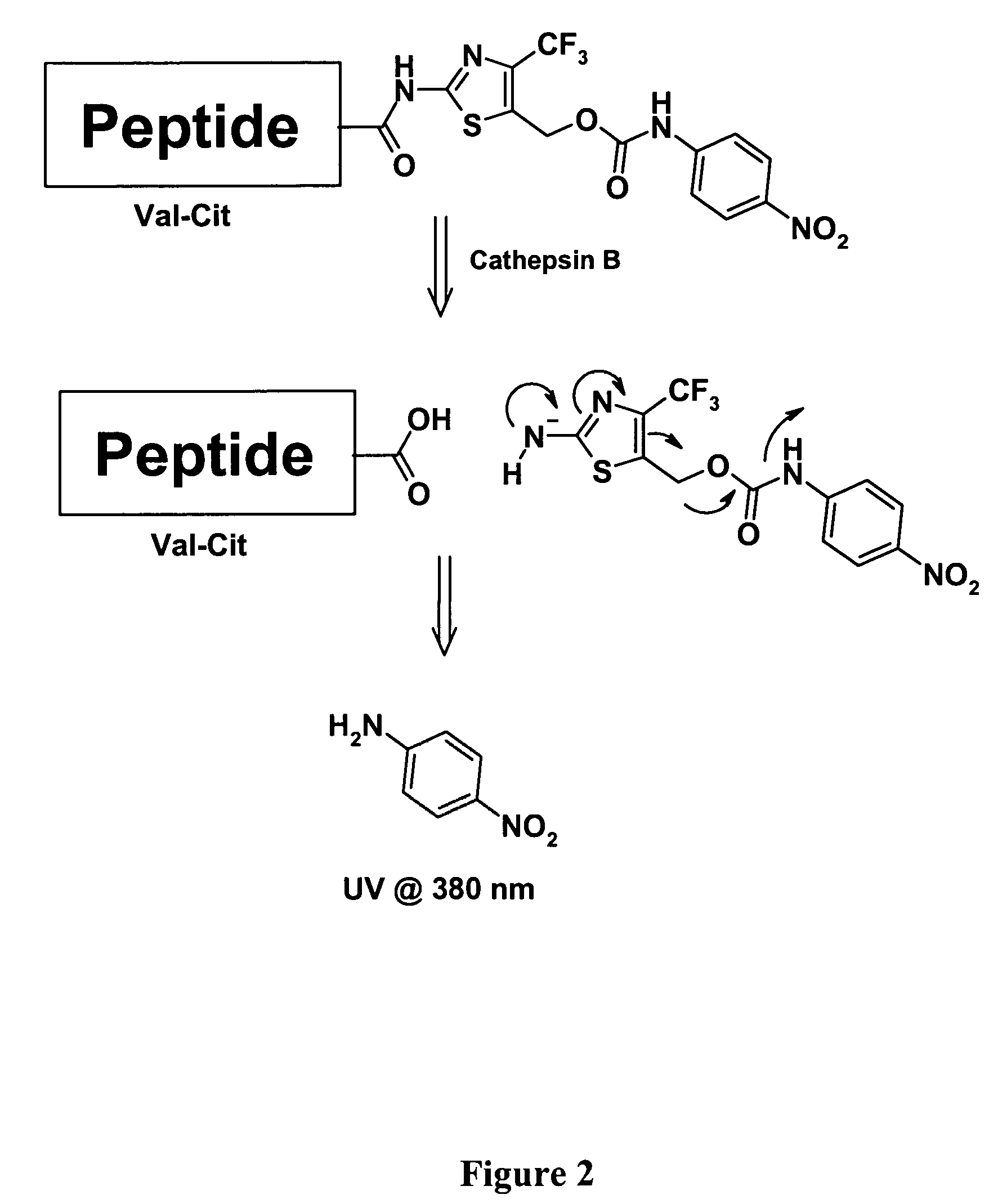
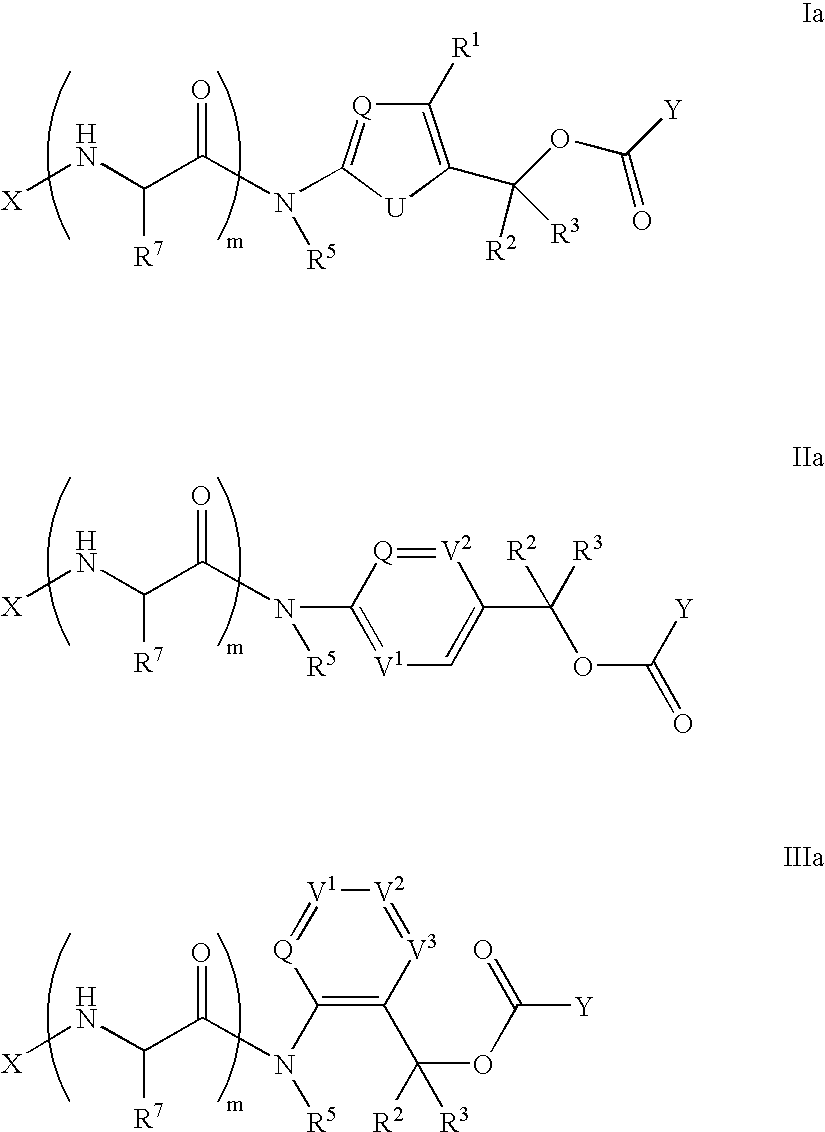
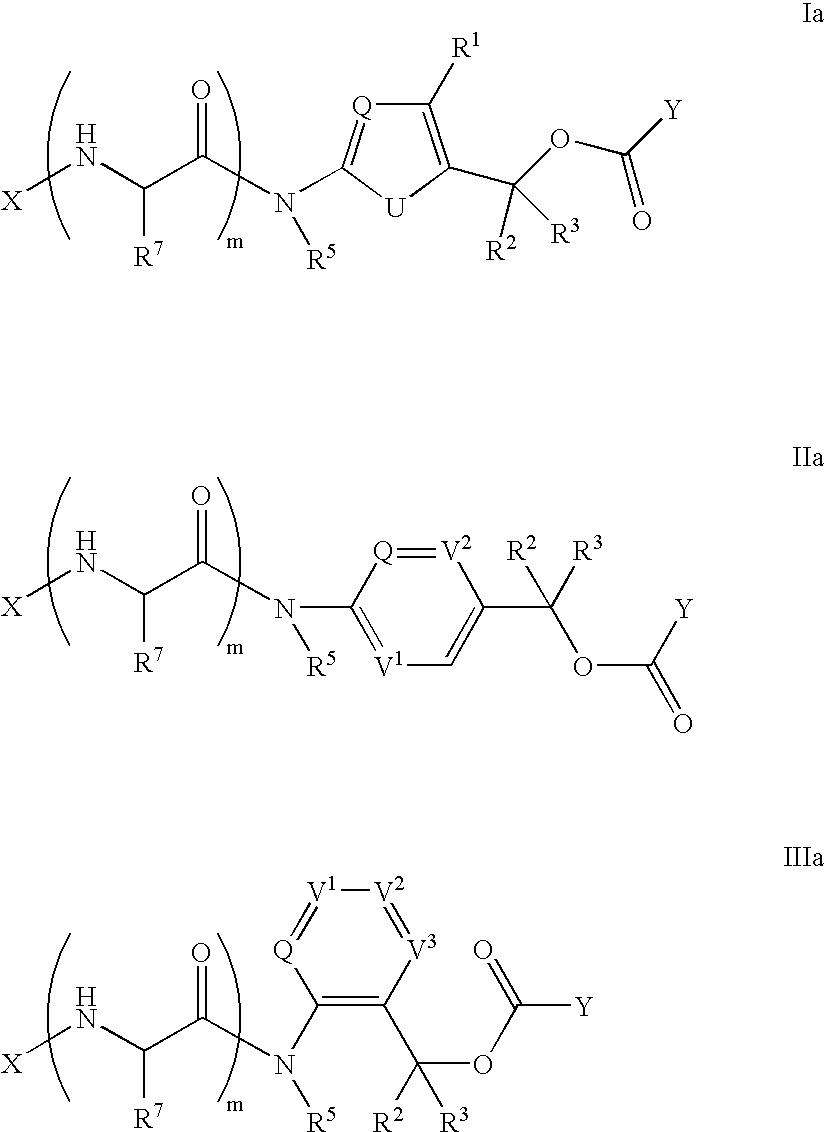
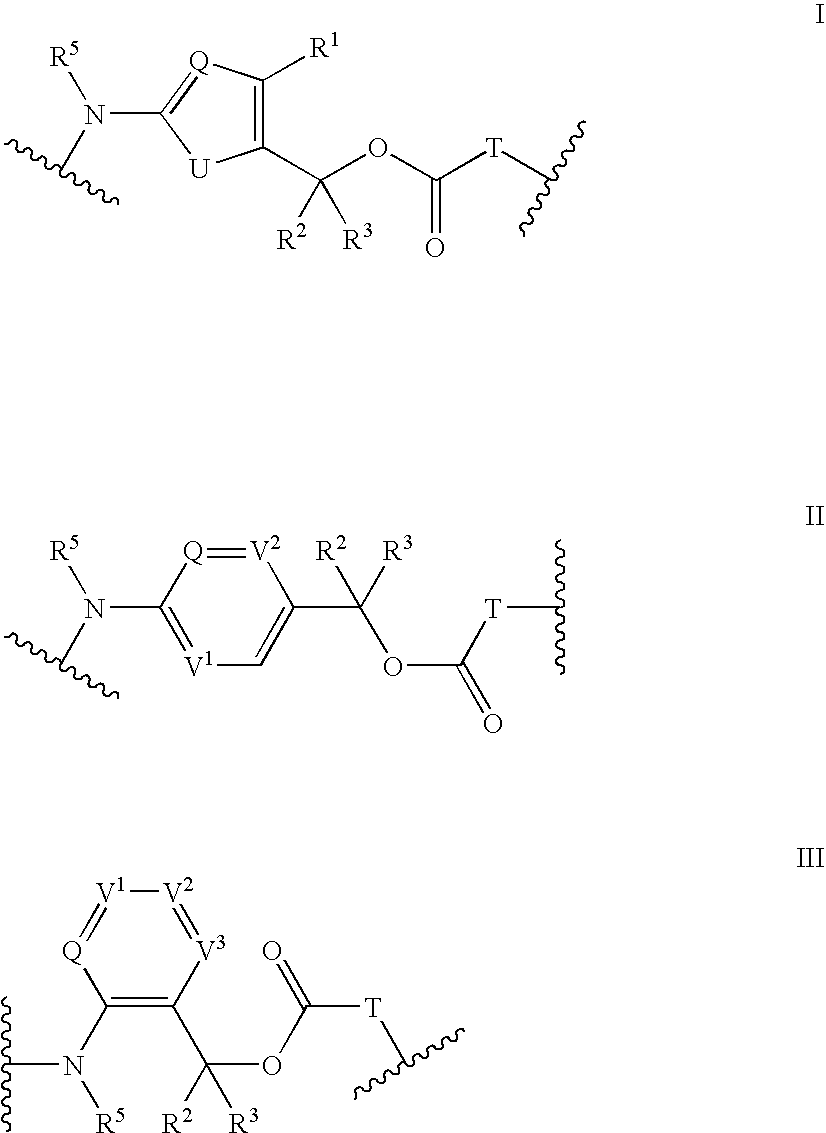
Heterocyclic self-immolative linkers and conjugates
The present invention provides heterocyclic linker compounds useful for linking drug moieties to ligands. The compounds also include drug-ligand conjugates comprising a ligand capable of targeting a selected cell population, and a drug connected to the ligand by a heterocyclic linker moiety. The linker moiety comprises a peptide sequence that is a substrate for an intracellular enzyme, for example a cathepsin, that cleaves the peptide at an amide bond. The peptide further contains a self-immolating moiety which connects the drug and the protein peptide sequence. Upon cleavage of the peptide sequence by an intracellular enzyme the self-immolating moiety cleaves itself from the drug moiety such that the drug moiety is in an underivatized and active form.
Owner:SEAGEN INC
Heterocyclic self-immolative linkers and conjugates
The present invention provides heterocyclic linker compounds useful for linking drug moieties to ligands. The compounds also include drug-ligand conjugates comprising a ligand capable of targeting a selected cell population, and a drug connected to the ligand by a heterocyclic linker moiety. The linker moiety comprises a peptide sequence that is a substrate for an intracellular enzyme, for example a cathepsin, that cleaves the peptide at an amide bond. The peptide further contains a self-immolating moiety which connects the drug and the protein peptide sequence. Upon cleavage of the peptide sequence by an intracellular enzyme the self-immolating moiety cleaves itself from the drug moiety such that the drug moiety is in an underivatized and active form.
Owner:SEAGEN INC
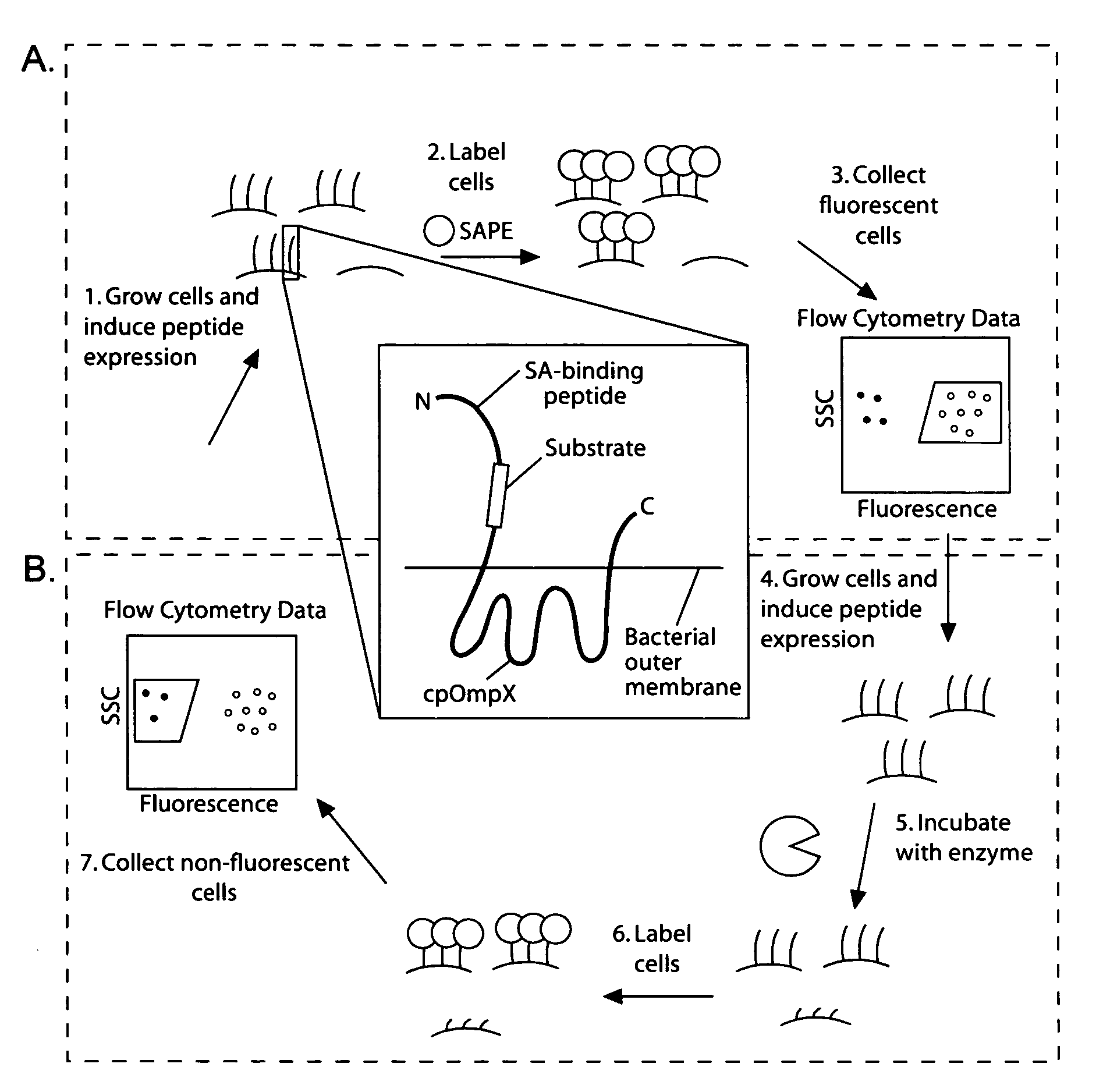
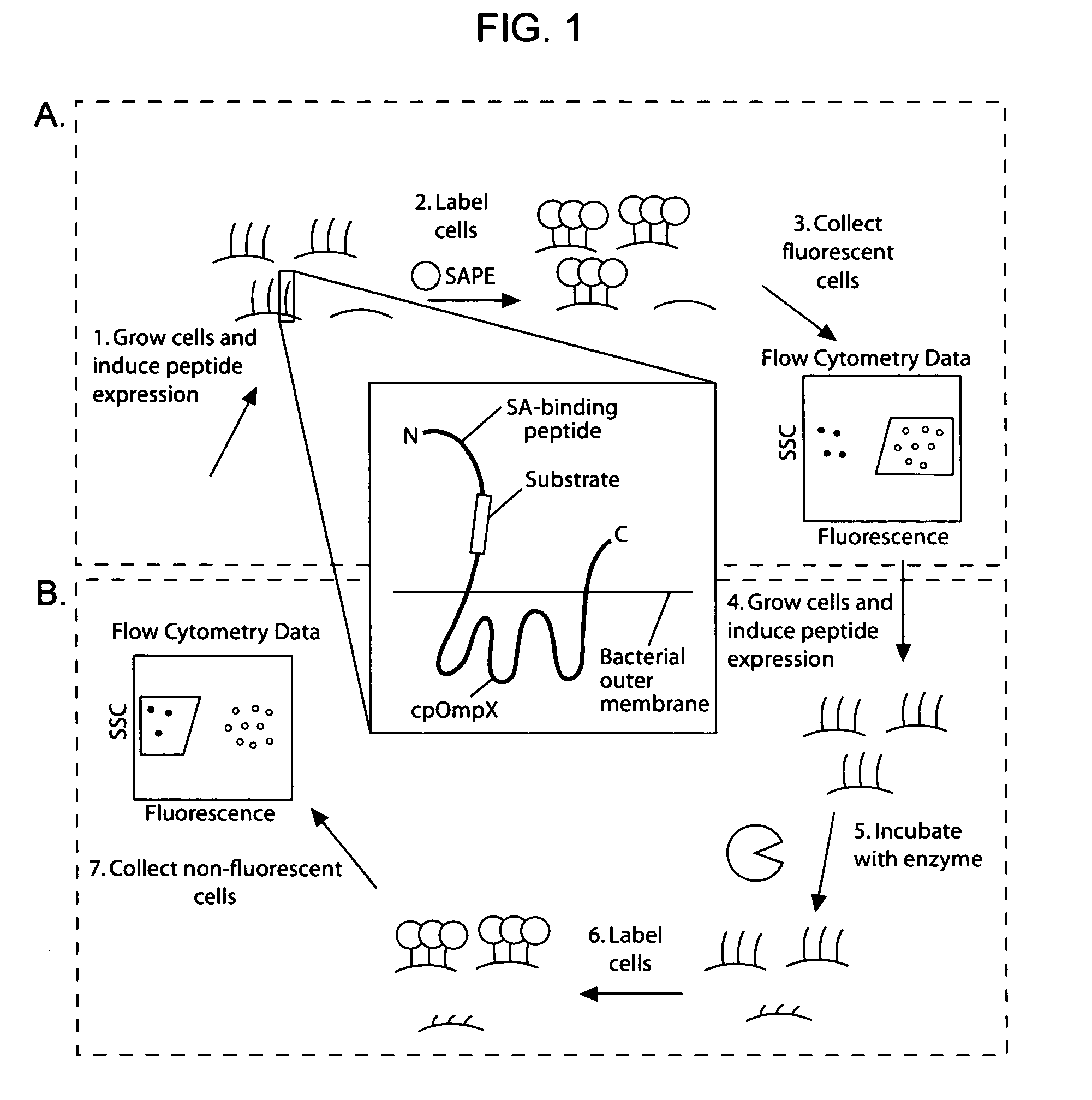
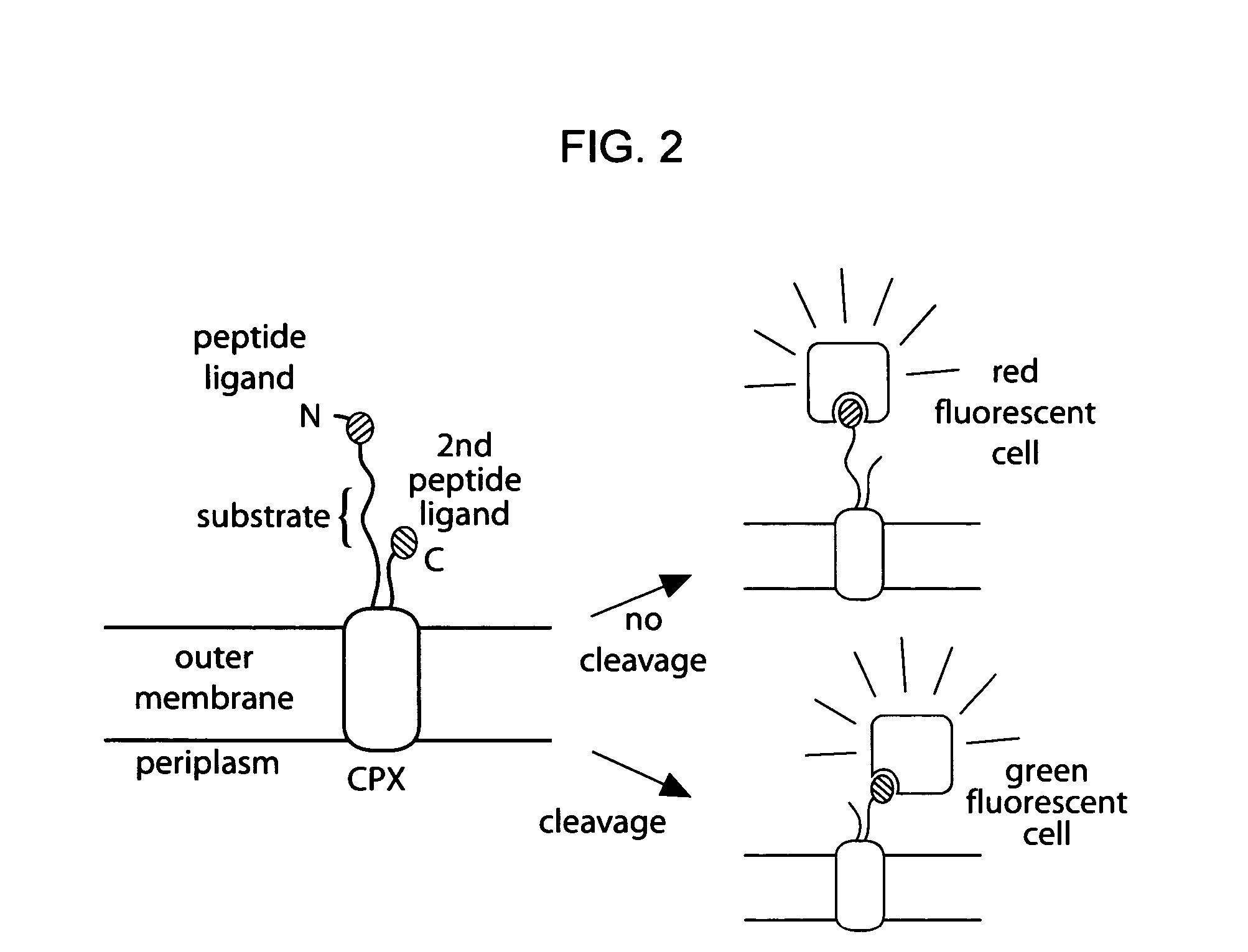
Cellular libraries of peptide sequences (CLiPS) and methods of using the same
The present invention provides compositions including peptide display scaffolds that present at least one candidate peptide and at least one detectable moiety in at least one of the N-terminal and C-terminal candidate peptide presenting domains that when expressed in a cell are accessible at a surface of the cell outermembrane. In addition, the present invention also provides kits and methods for screening a library of cells presenting the candidate peptides in peptide display scaffolds to identify a ligand for an enzyme.
Owner:RGT UNIV OF CALIFORNIA
Tumour-associated peptides binding to human leukocyte antigen (HLA) class i or ii molecules and related Anti-cancer vaccine
InactiveUS20090274714A1Organic active ingredientsPeptide/protein ingredientsHla class iiAdditive ingredient
The present invention relates to immunotherapeutic methods, and molecules and cells for use in immunotherapeutic methods. In particular, the present invention relates to the immunotherapy of cancer. The present invention furthermore relates to tumour-associated T-helper cell peptide epitopes, alone or in combination with other tumour-associated peptides, that serve as active pharmaceutical ingredients of vaccine compositions which stimulate anti-tumour immune responses. In particular, the present invention relates to two novel peptide sequences derived from HLA class II molecules of human tumour cell lines, which can be used in vaccine compositions for eliciting anti-tumour immune responses.
Owner:IMMATICS BIOTECHNOLOGIES GMBH
Metod of modulation of interaction between receptor and ligand
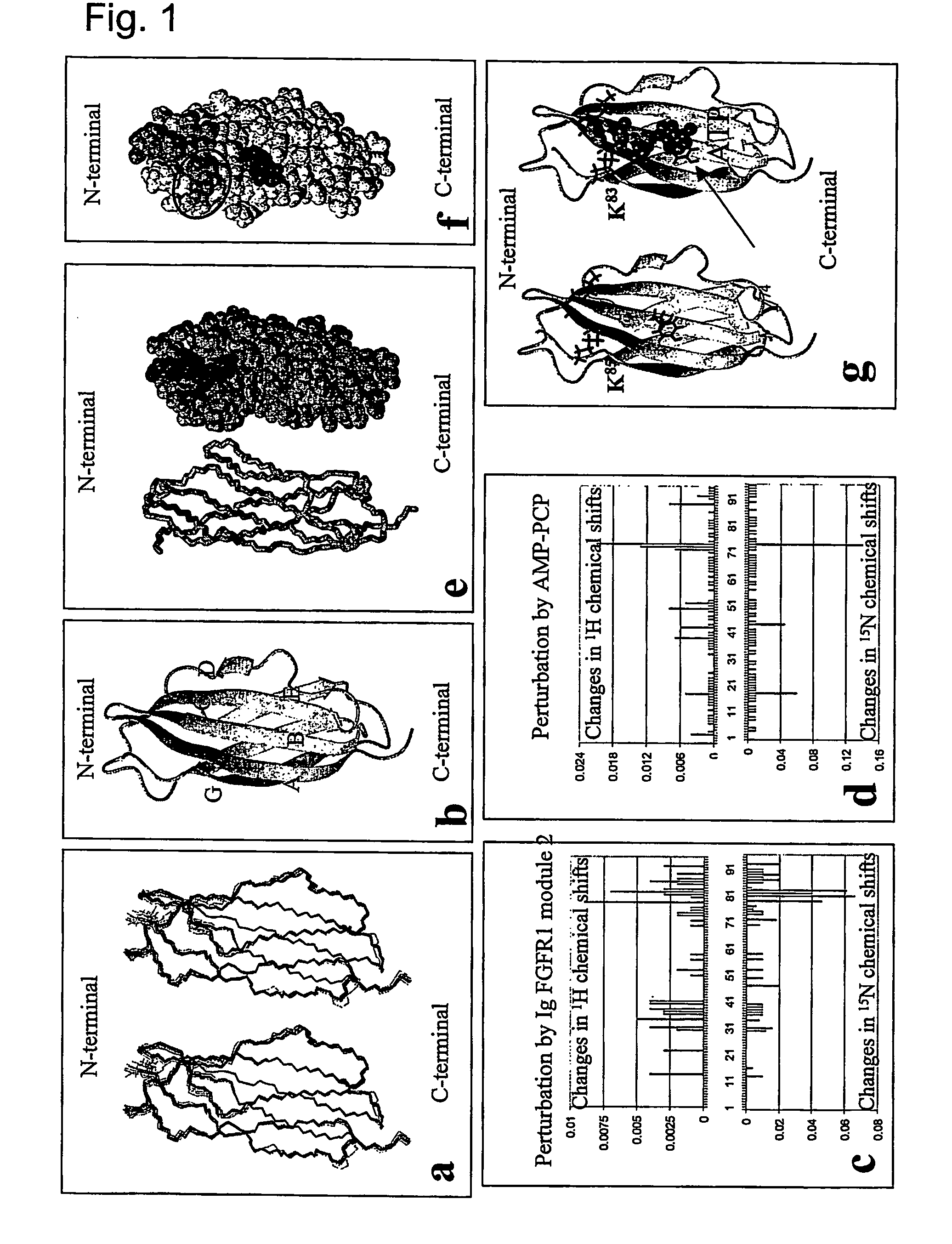
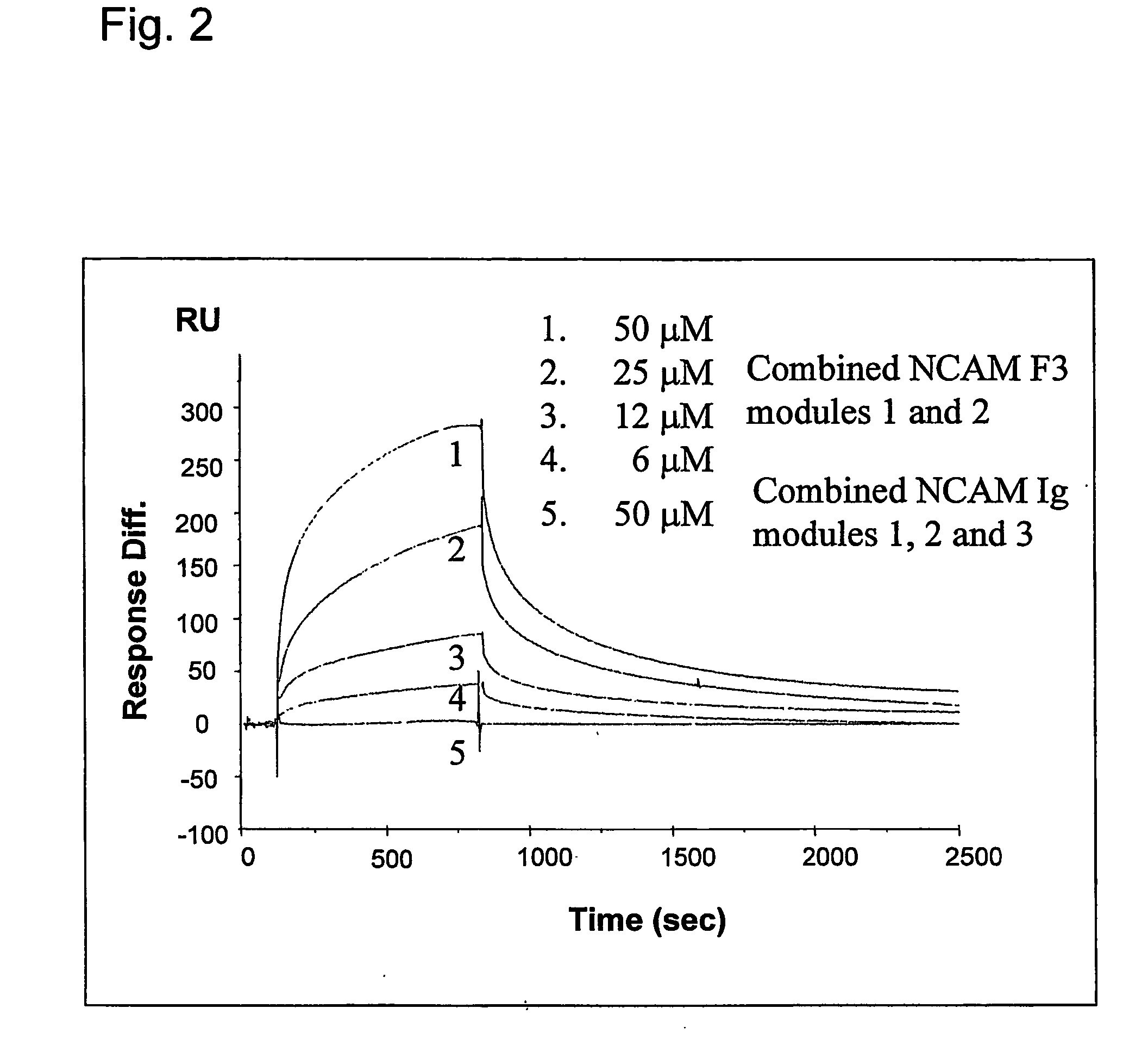
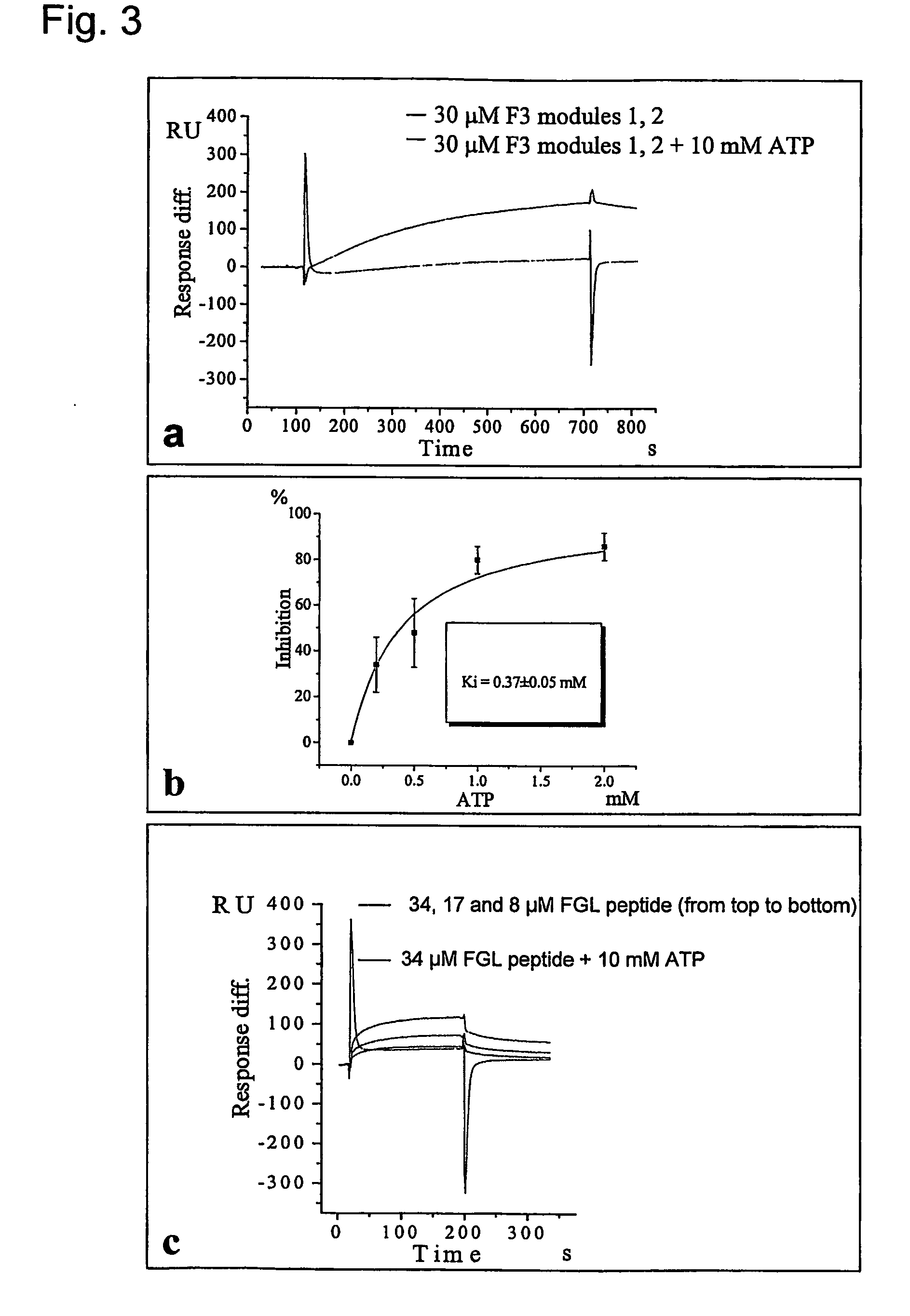
The present invention relates to a method for modulating the interaction between at least two proteins, wherein at least one of the two proteins is a functional cell-surface receptor and the other protein is the receptor ligand. The invention features a binding site of said functional cell-surface receptor on the receptor ligand and discloses a series of amino acid sequences, which are part of the structure of said binding site and / or involved in the interaction between the receptor and the ligand. Moreover, the present invention features methods for molecular design and screening of a candidate compound capable of modulating the interaction between the functional cell-surface receptor and receptor ligand through the described binding site, and provides a screening assay for identification of such a compound. The invention further describes an antibody capable of binding to the above binding site and / or to an epitope comprising an amino acid sequence essential for executing the receptor ligand interaction through said binding site. The invention also concerns a variety of uses of the disclosed methods, peptide sequences and antibodies. The invention in preferred embodiments concerns the binding site of the fibroblast growth factor receptor (FGFR) on FGFR ligands, compounds capable of modulating the receptor ligand interaction through said binding site, and antibody capable of recognition of said binding site.
Owner:ENKAM PHARMA
Pharmacologically active peptide conjugates having a reduced tendency towards enzymatic hydrolysis
InactiveUS20060063699A1Less susceptible to degradationLess susceptiblePeptide/protein ingredientsAntipyreticEnzymatic hydrolysisPeptide sequence
The invention is directed to a pharmacologically active peptide conjugate having a reduced tendency towards enzymatic cleavage comprising a pharmacologically active peptide sequence (X) and a stabilising petide sequence (Z) of 4-20 amino acid residues covalently bound to X.
Owner:ZEALAND PHARM AS
Conformationally constrained backbone cyclized peptide analogs
Novel backbone cyclized peptide analogs are formed by means of bridging groups attached via the alpha nitrogens of amino acid derivatives to provide novel non-peptidic linkages. Novel building units disclosed are Nα(ω-functionalized) amino acids constructed to include a spacer and a terminal functional group. One or more of these Nα(ω-functionalized) amino acids are incorporated into a peptide sequence, preferably during solid phase peptide synthesis. The reactive terminal functional groups are protected by specific protecting groups that can be selectively removed to effect either backbone-to-backbone or backbone-to-side chain cyclizations. The invention is specifically exemplified by backbone cyclized bradykinin antagonists having biological activity. Further embodiments of the invention are somatostatin analogs having one or two ring structures involving backbone cyclization.
Owner:DEVELOGEN ISRAEL
Enzymes and microorganisms having amidase activity for hydrolysing polyamides
The present invention relates to an enzyme with amidase activity, particularly towards substrates of the oligomer type derived from PA 6.6 (formula I) and / or PA 6 (formula II), said enzyme being characterized by a ratiowhich is greater than 2, preferably greater than or equal to 10 and particularly preferably greater than or equal to 50.More precisely, this enzyme consists of the peptide sequence corresponding to the attached sequence SEQ ID NO: 2.The invention further relates to the DNA which codes for this polypeptide SEQ ID NO: 2 and whose sequence corresponds to the attached sequence SEQ ID NO: 1.The invention further relates to the microorganisms capable of producing this enzyme and to the hydrolysis process in which this enzyme and / or these microorganisms are applied.
Owner:RHONE POULENC FIBERS & POLYMERES
Surgical adhesive compostion and process for enhanced tissue closure and healing
InactiveUS20070092483A1Promotes cellPromotes tissue in-growthPharmaceutical non-active ingredientsSynthetic polymeric active ingredientsPorositySide chain
A surgical tissue adhesive composition contains at least one 1,1-disubstituted electron-deficient olefin macromer. The adhesive composition of the invention has improved biocompatibility as well as controlled biodegradation characteristics and bioactivity. Adhesive co-monomer compositions contain at least one macromer with a pendant oligomer, polymer, or peptide chain as an acrylic ester of the reactive olefin. The polymers formed therefrom have a grafted brush-like nature. The composition is particularly useful for creating an adhesive bond at the junction of living tissue in surgical applications. The adhesive composition may further comprise co-monomer, co-macromer, cross-linker, or inter-penetrating polymer compounds containing peptide sequences that are bioactive or enzyme responsive. The peptide sequences are selected to promote tissue infiltration and healing in a particular biological tissue. The sequences may contain specific cell-adhesion, cell-signaling, and enzyme-cleavable domains. Furthermore, a degradable filler material may be included in the composition to create a reinforced composite. The filler preferably has a higher degradation rate than the polymer matrix, generating porosity upon degradation. The adhesive may further contain entrapped or incorporated drugs or biologics, including antibiotics or growth factors. The adhesive can be used to bind together the edges of living tissues during surgical procedures. The cured composition provides interfacial bonding and mechanical fixation while promoting tissue infiltration and replacement of the adhesive polymer.
Owner:POLLOCK POLYMER GROUP
Synthetic genes for plant gums and other hydroxyproline-rich glycoproteins
InactiveUS6639050B1Enhance molecular packingEasy to identifyBacteriaAntibody mimetics/scaffoldsBiotechnologyHydroxyproline
A new approach in the field of plant gums is described which presents a new solution to the production of hydroxyproline(Hyp)-rich glycoproteins (HRGPs), repetitive proline-rich proteins (RPRPs) and arabinogalactan-proteins (AGPs). The expression of synthetic genes designed from repetitive peptide sequences of such glycoproteins, including the peptide sequences of gum arabic glycoprotein (GAGP), is taught in host cells, including plant host cells.
Owner:OHIO UNIV TECH TRANSFER OFFICE TECH & ENTERPRISE BUILDING
Compositions, uses and methods for treatment of metabolic disorders and diseases
ActiveUS20130023474A1Lower Level RequirementsIncrease insulin sensitivityOrganic active ingredientsFungiDiseaseFGF19
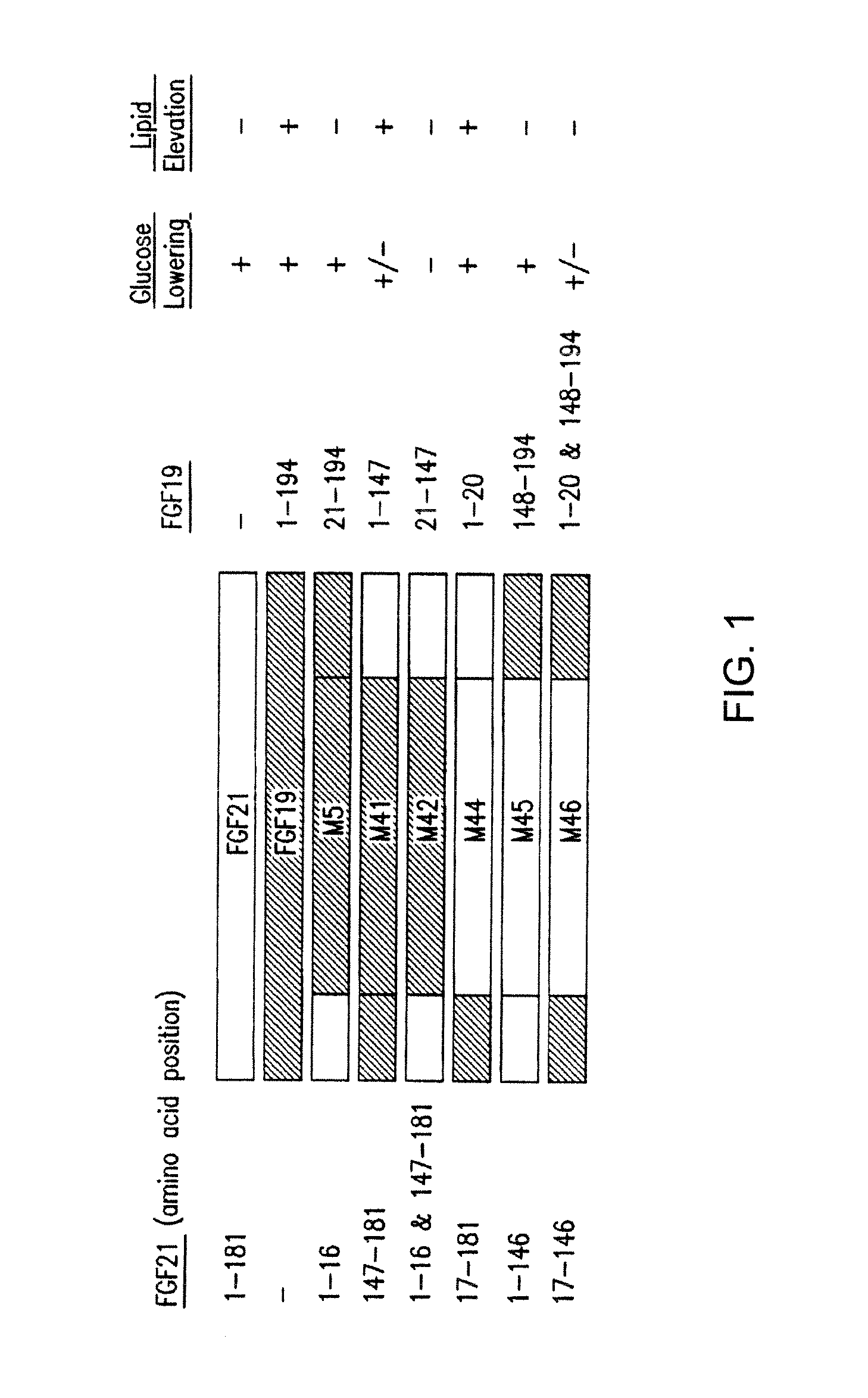
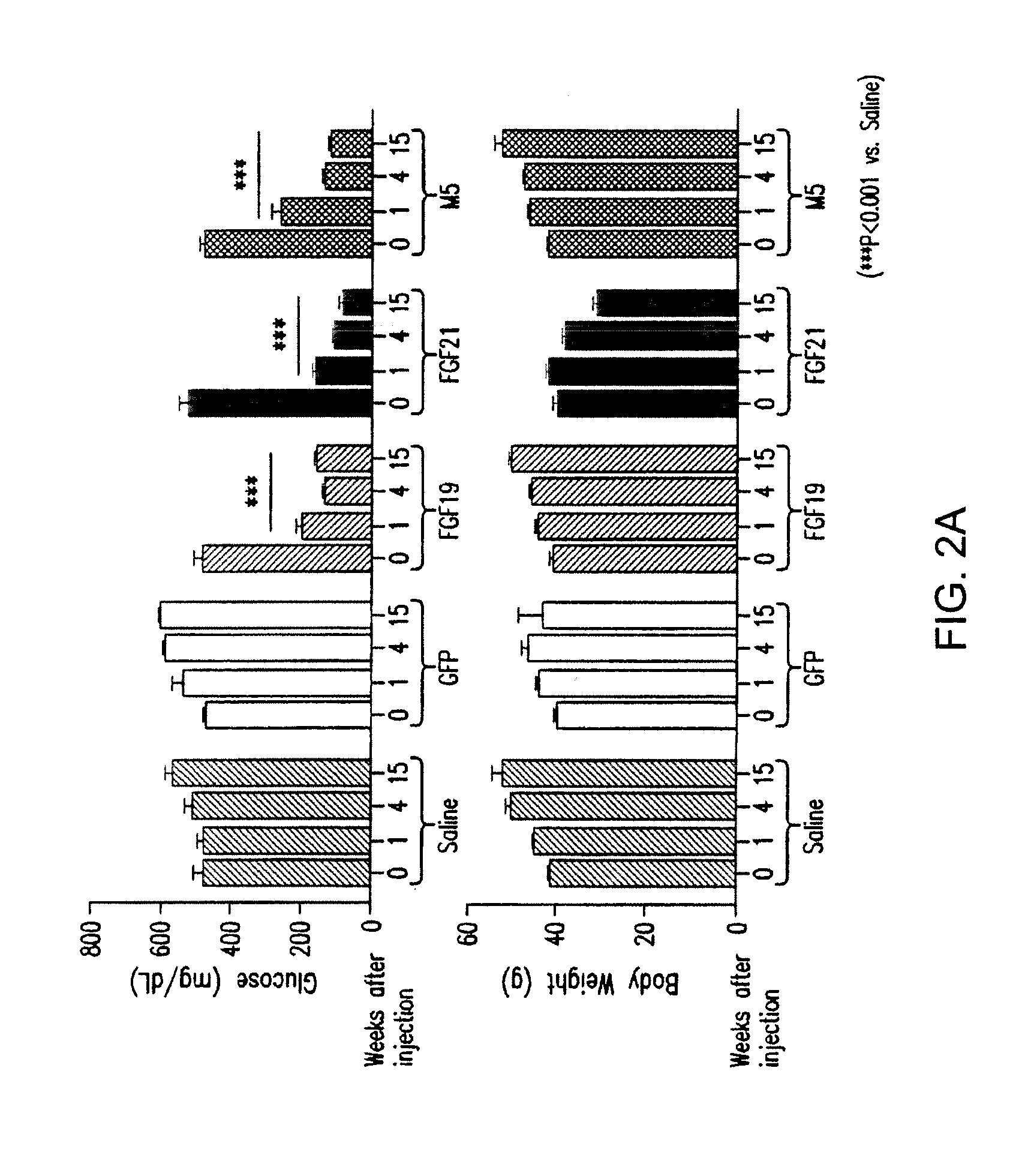
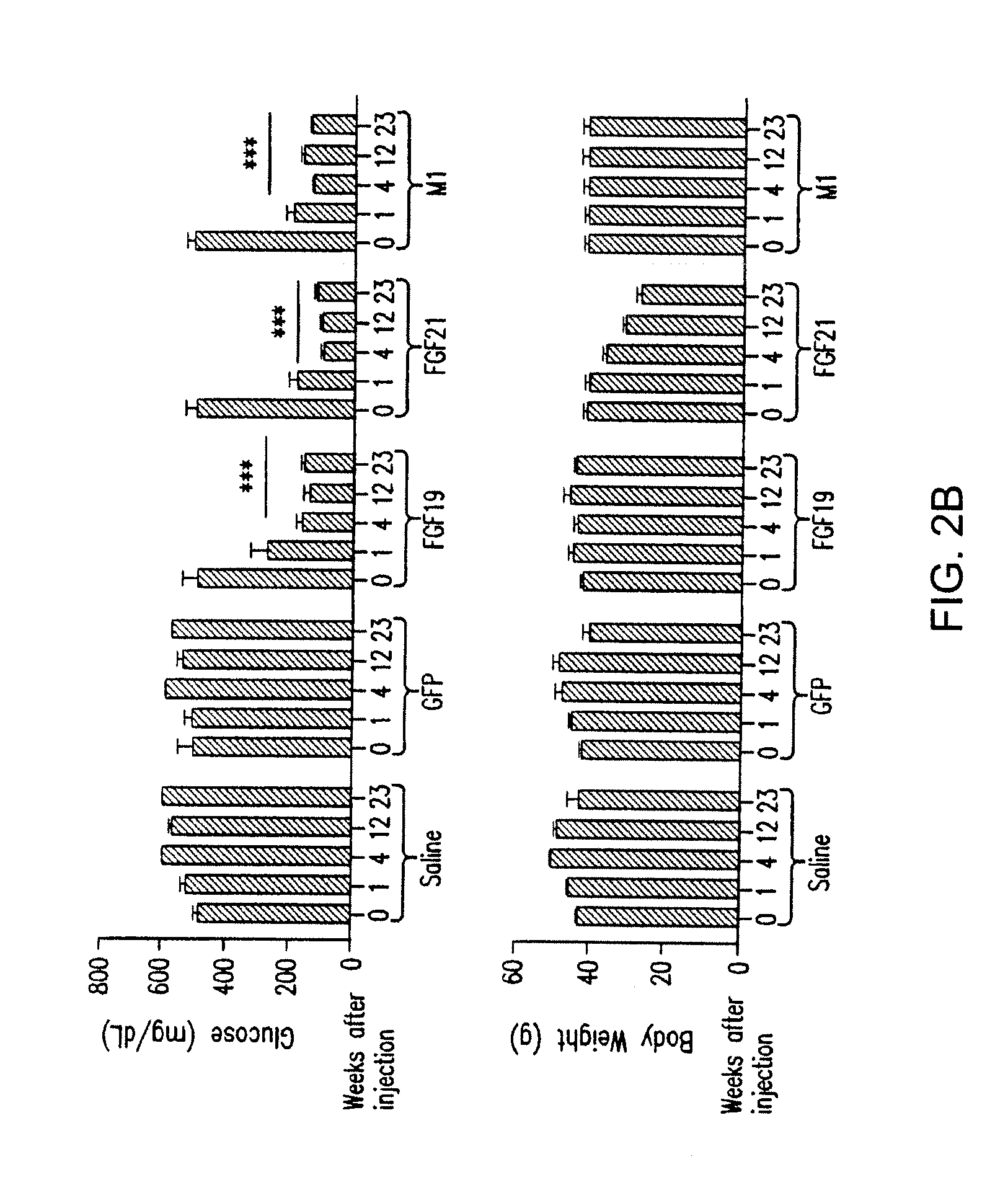
The invention relates to variants and fusions of fibroblast growth factor 19 (FGF19), variants and fusions of fibroblast growth factor 21 (FGF21), fusions of fibroblast growth factor 19 (FGF19) and / or fibroblast growth factor 21 (FGF21), and variants or fusions of fibroblast growth factor 19 (FGF19) and / or fibroblast growth factor 21 (FGF21) proteins and peptide sequences (and peptidomimetics), having one or more activities, such as glucose lowering activity, and methods for and uses in treatment of hyperglycemia and other disorders.
Owner:NGM BIOPHARMLS
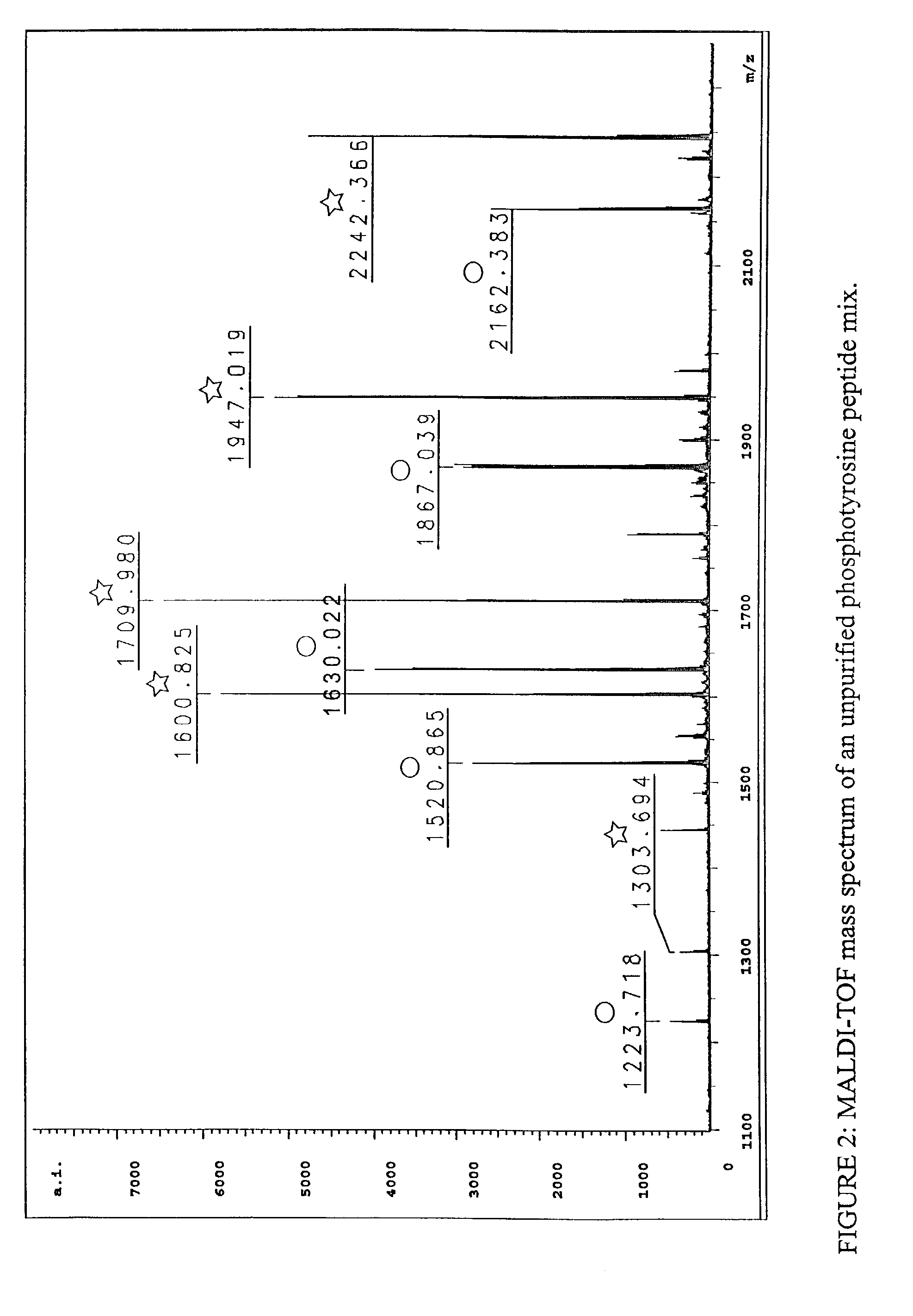
Automated identification of peptides
InactiveUS20020102610A1Quick identificationCharacter and pattern recognitionBiological testingPresent methodPost translational
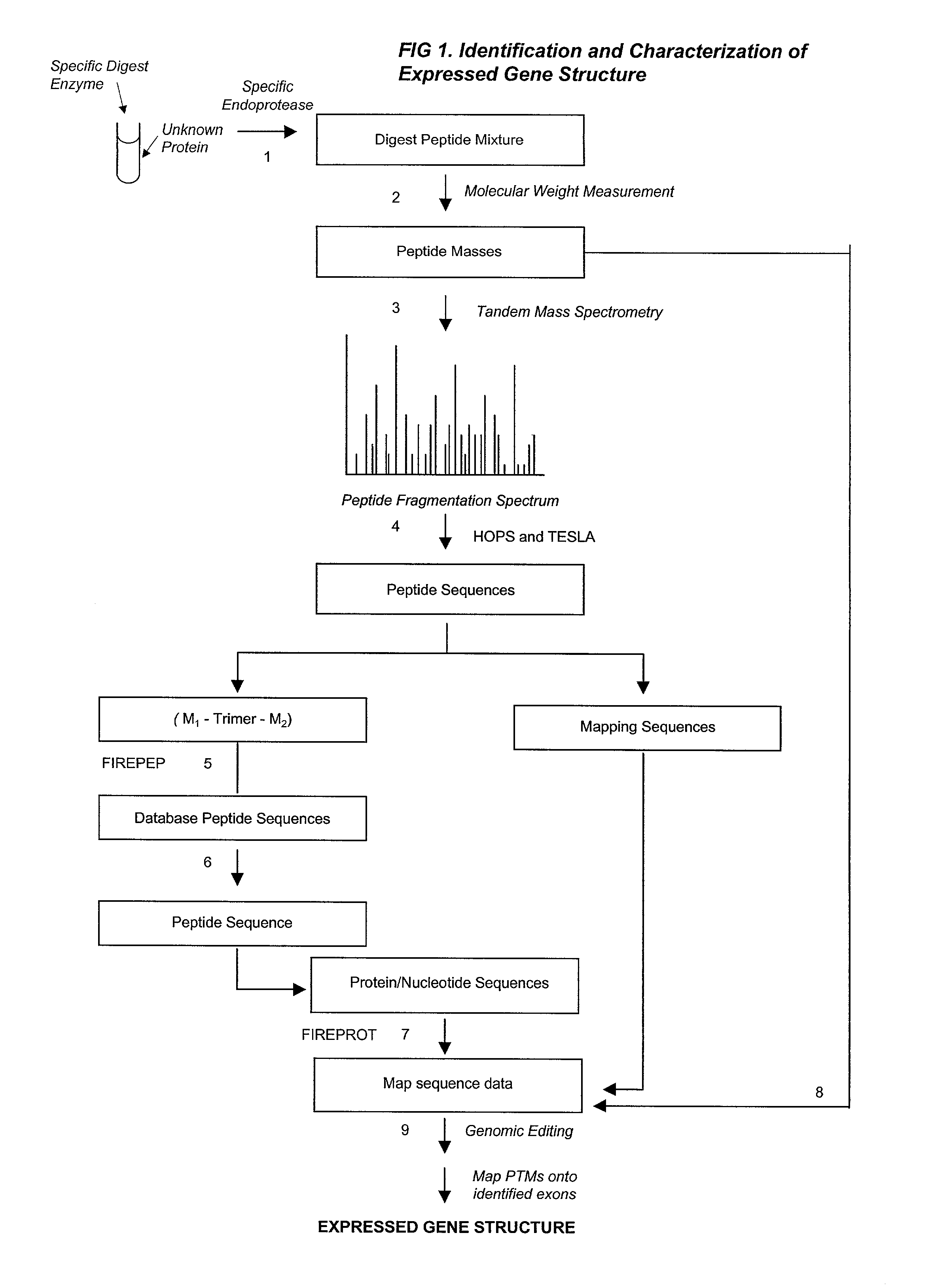
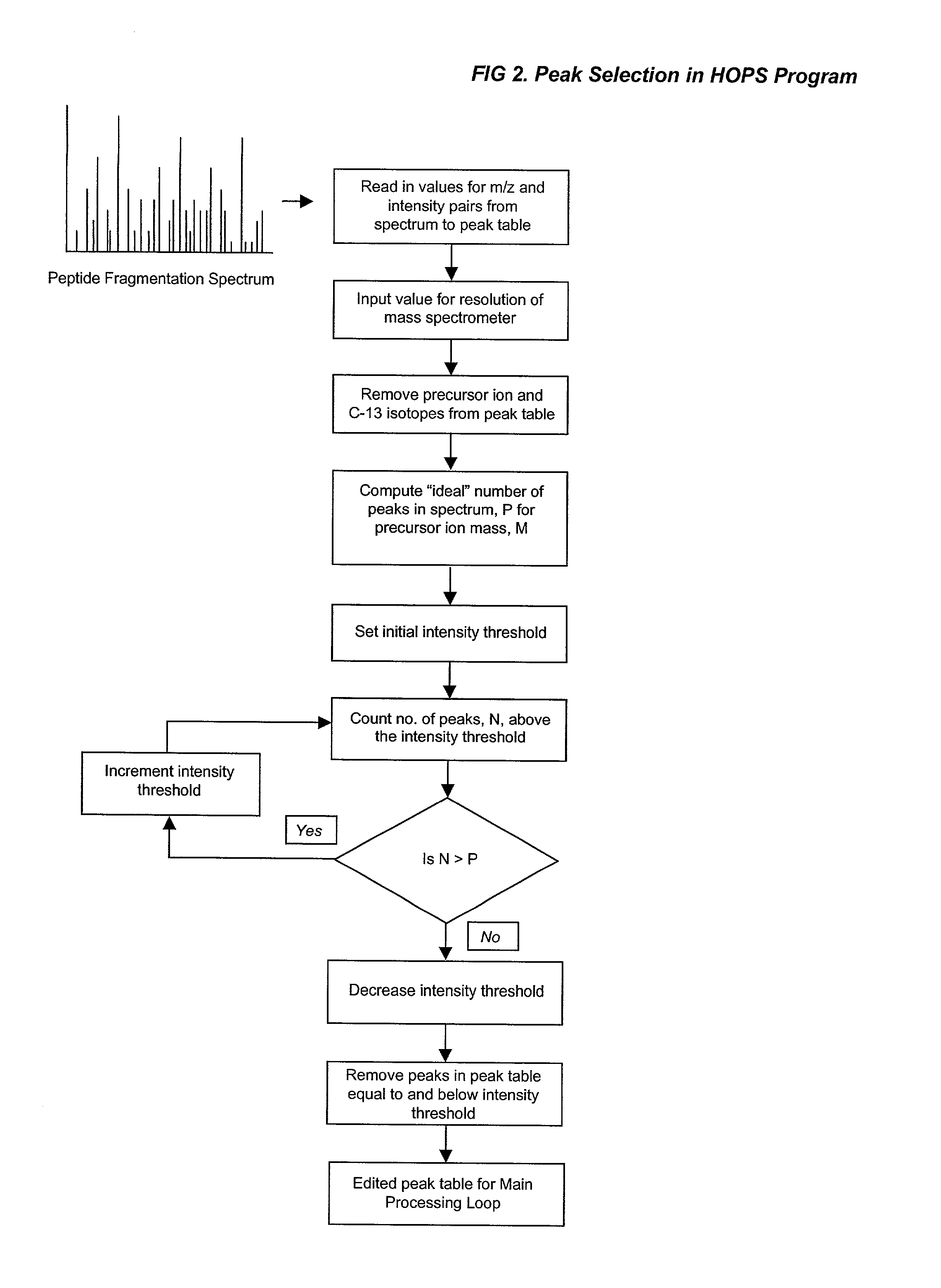
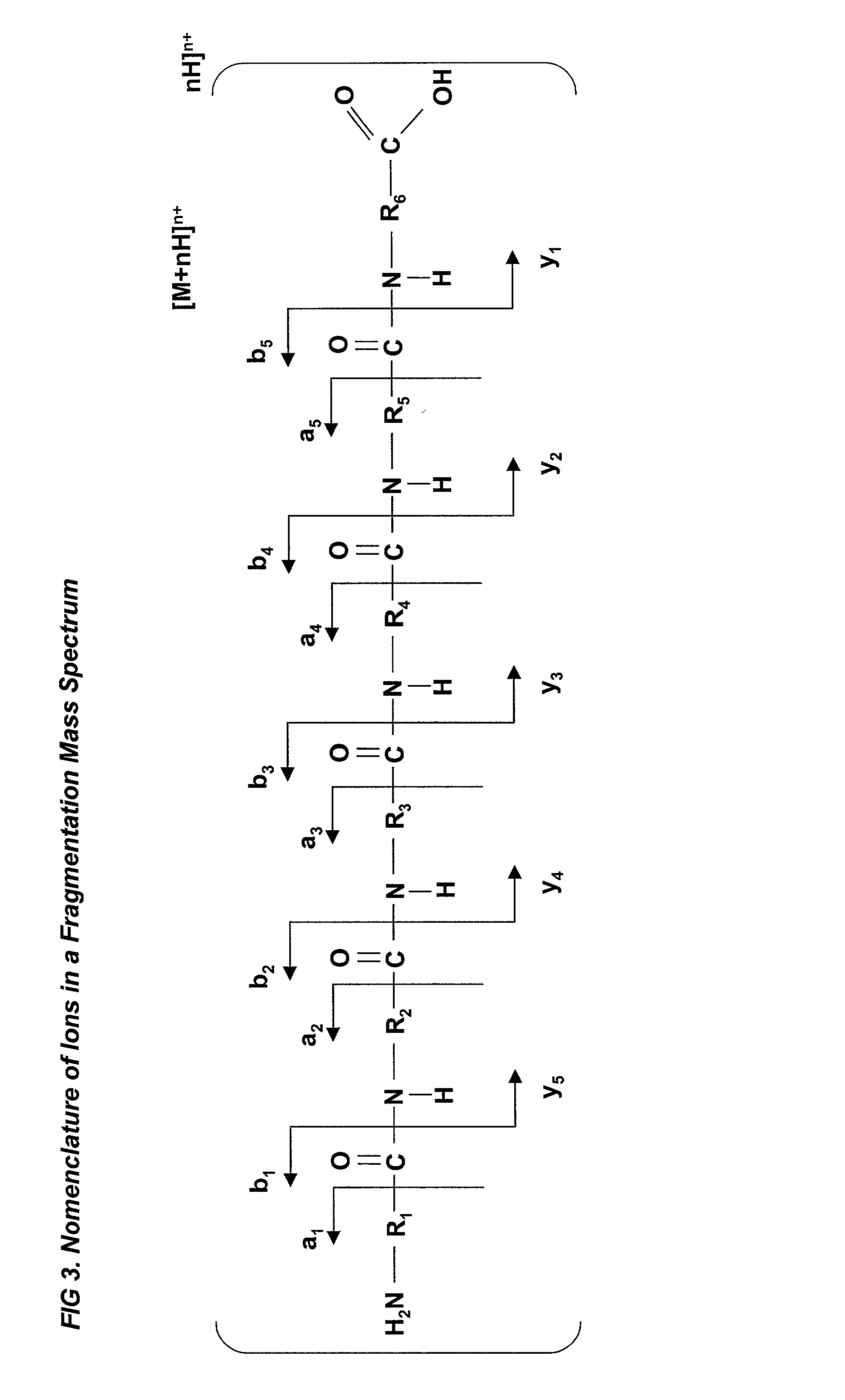
A fully automated, user-independent method is described for computer-mediated interpretation of data derived by mass spectrometry of an experimental peptide to identify and characterize a corresponding peptide sequence in a peptide database. The method identifies the corresponding sequence if it is present in the database, without the need for a skilled observer to choose from amongst a list of possible matches. By using an automated back-read process, the present method can uniquely identify a corresponding peptide sequence in a database based on a single matching peptide sequence. The method also permits mapping of mass spectral data to sequences in peptide or nucleotide databases for unambiguous identification of exons; determining a correct reading frame; identifying artefacts and errors in sequences; identifying mutations and polymorphisms; identifying post-translational modifications; and identifying exon-intron boundaries.
Owner:OXFORD GLYCOSCI UK
Peptide sequences specific for the hepatic stages of P. falciparum bearing epitopes capable of stimulating the T lymphocytes
The present invention relates to an in vitro diagnostic method for malaria in an individual comprising placing a tissue or a biological fluid taken from an individual in contact with a molecule or polypeptide composition, wherein said molecule or polypeptide composition comprises one or more peptide sequences bearing all or part of one or more T epitopes of the proteins resulting from the infectious activity of P. falciparum, under conditions allowing an in vitro immunological reaction to occur between said composition and the antibodies that may be present in the tissue or biological fluid, and in vitro detection of the antigen-antibody complexes formed. The invention further relates to a polypeptide comprising at least one T epitope from a liver-stage specific protein produced by P. falciparum and a vaccine composition directed against malaria comprising a molecule having one or more peptide sequences bearing all or part of one or more T epitopes resulting from the infectious activity of P. falciparum in the hepatic cells.
Owner:INST PASTEUR
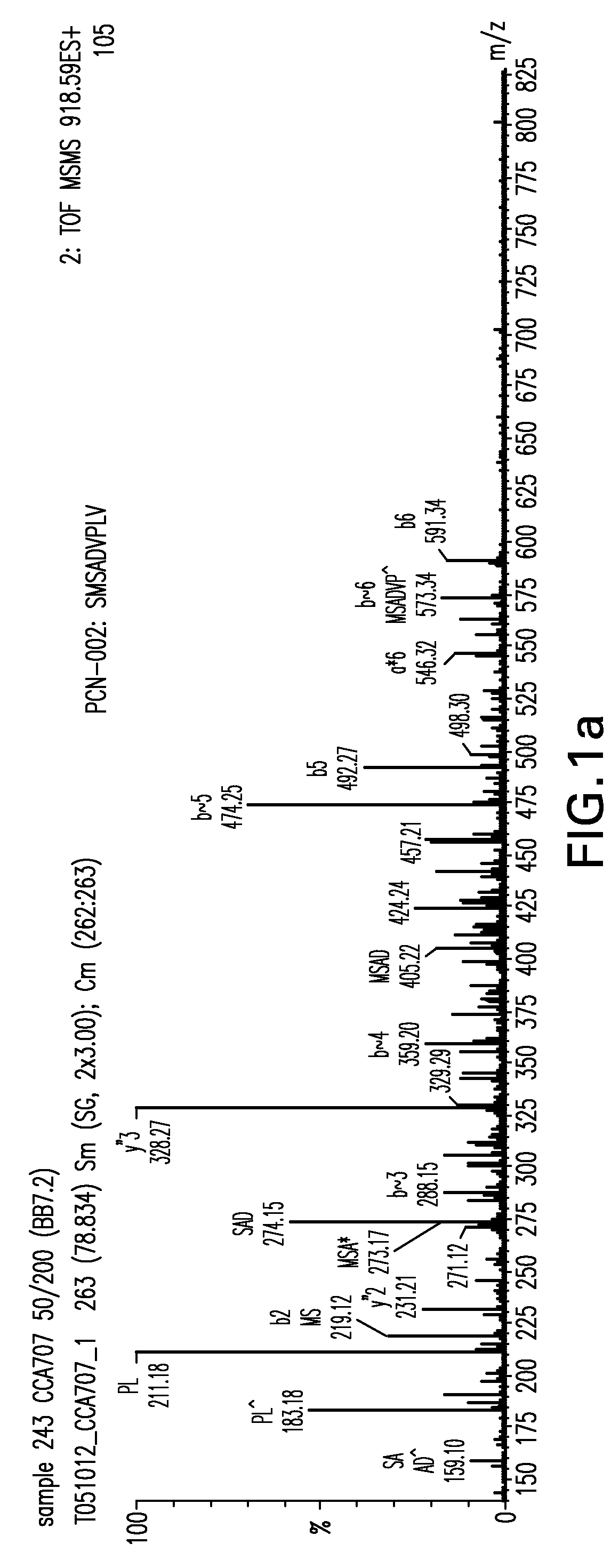
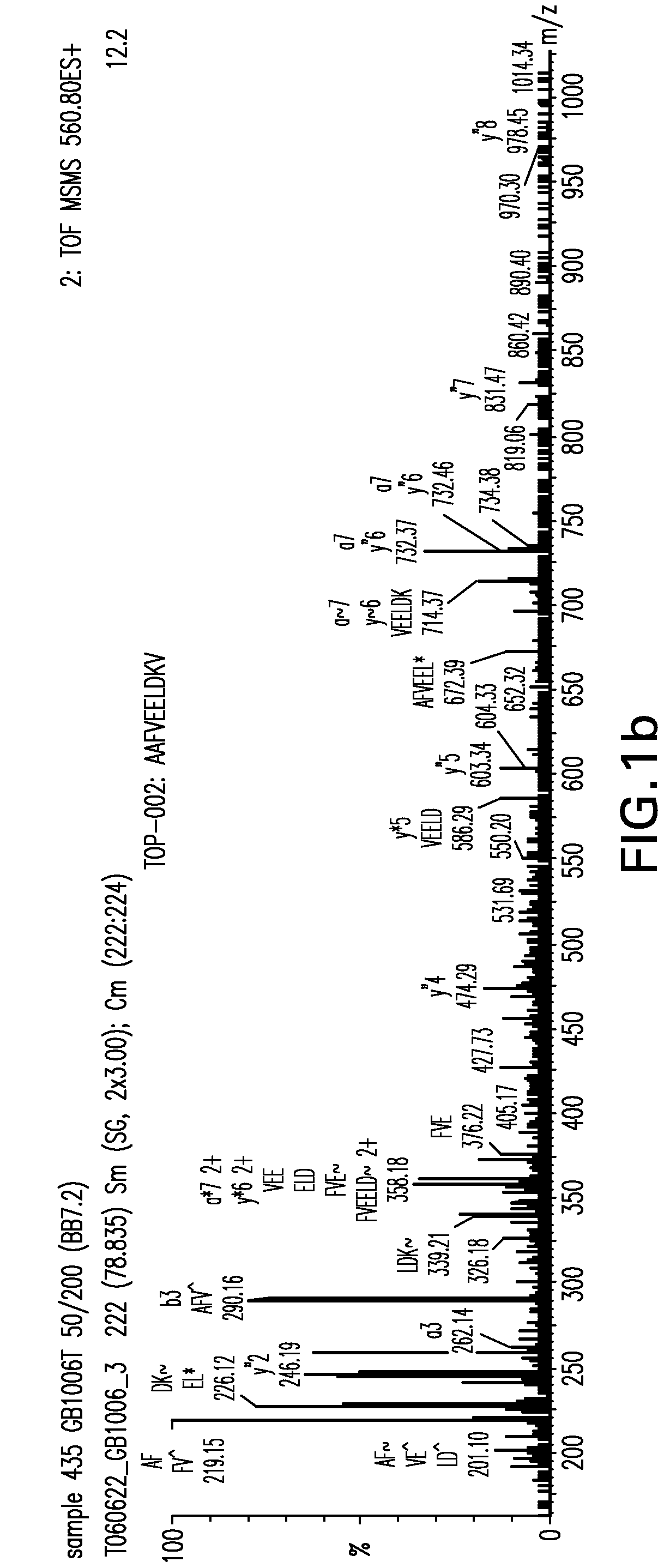
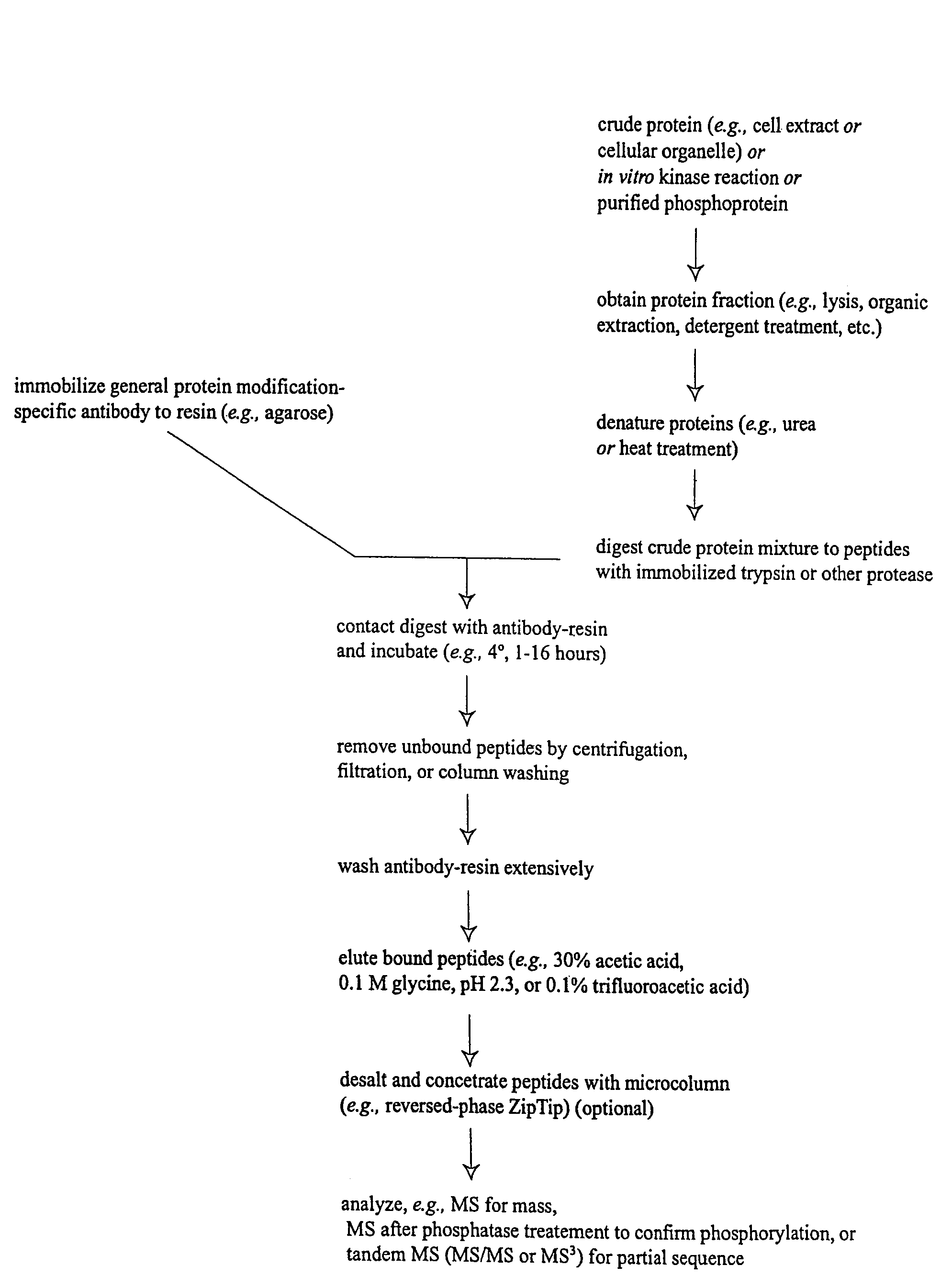
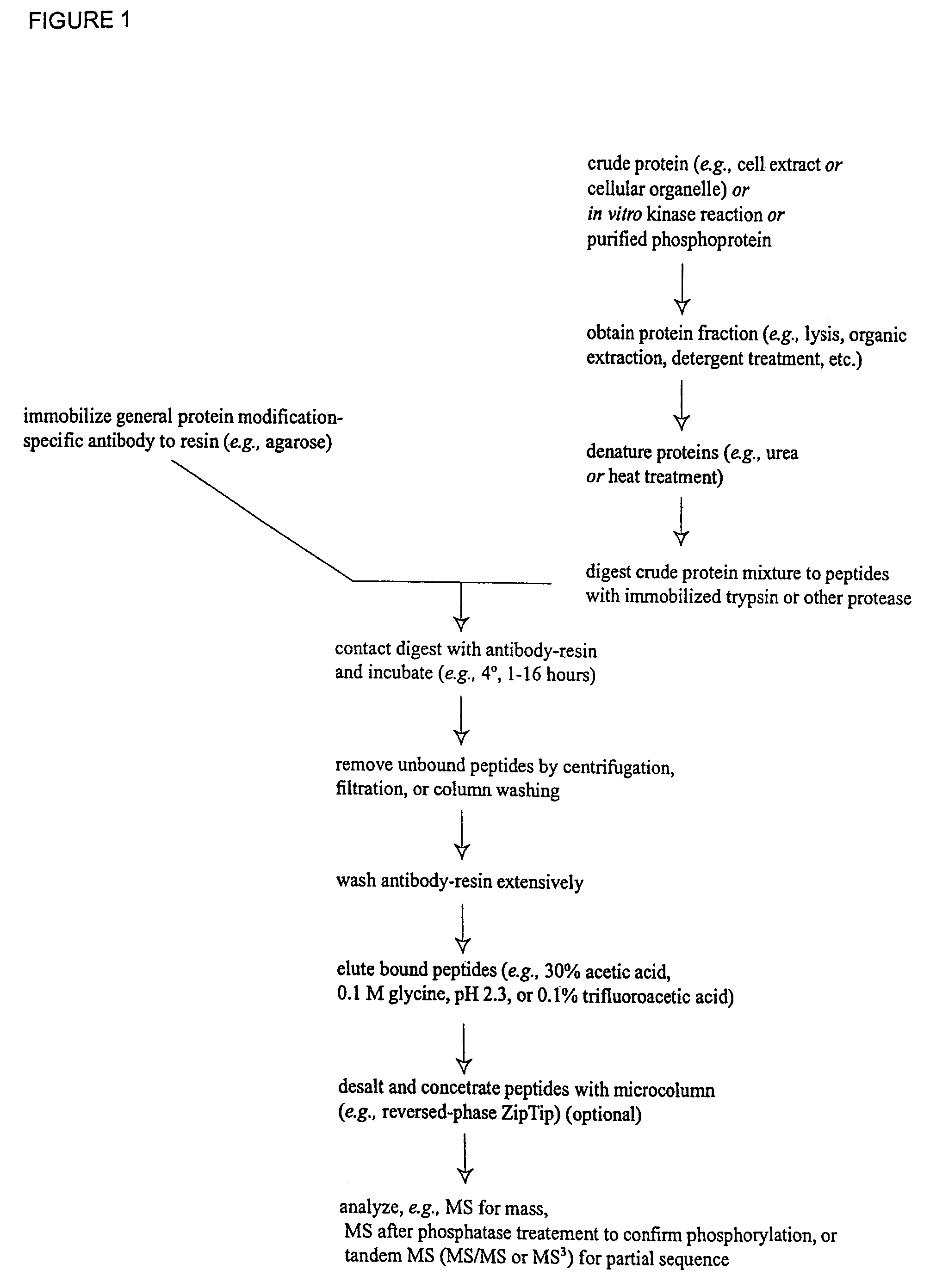
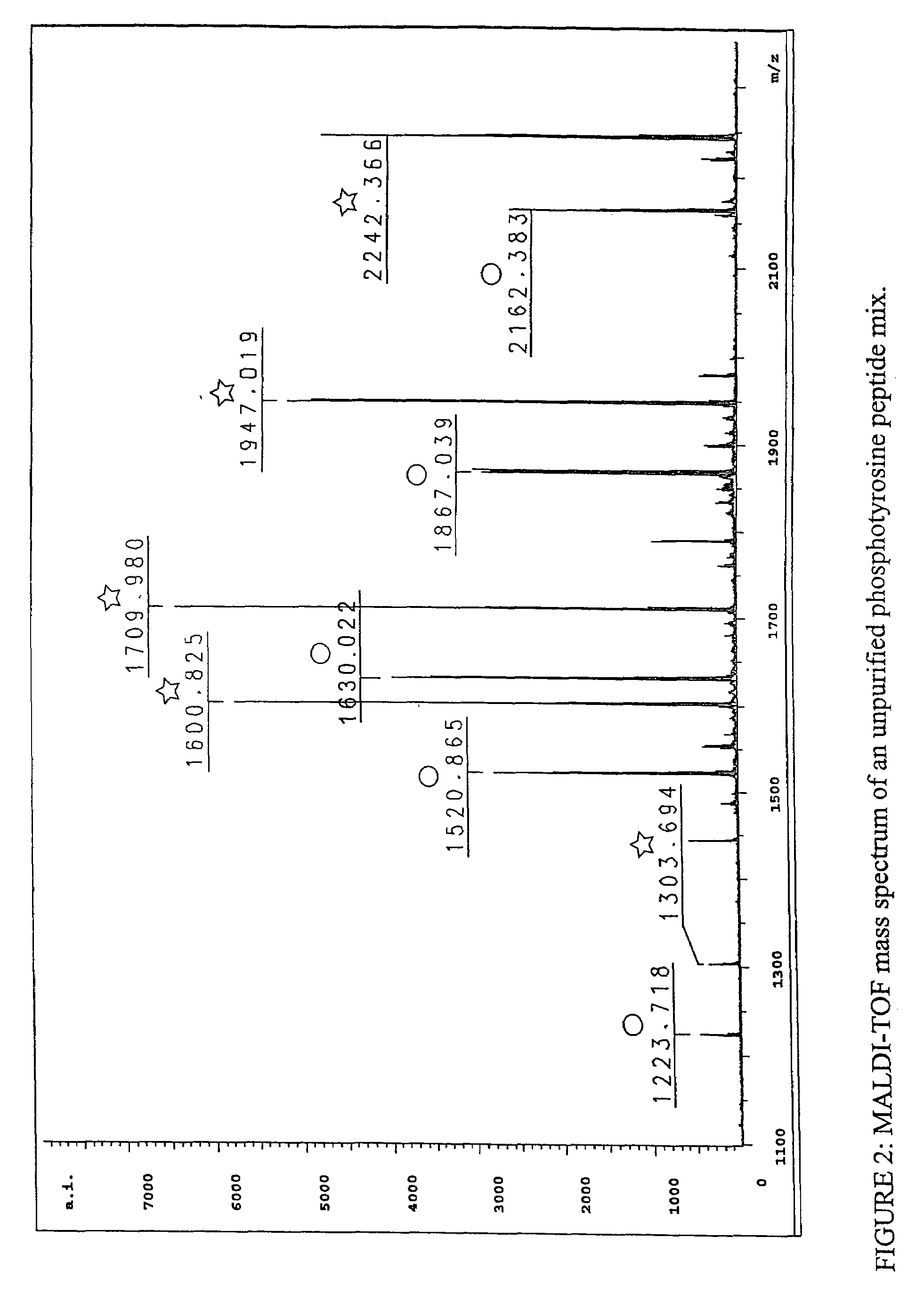
Immunoaffinity isolation of modified peptides from complex mixtures
InactiveUS7198896B2Rapid and efficient and direct isolationImprove automationIon-exchanger regenerationImmunoglobulinsBiological bodyPhosphorylation
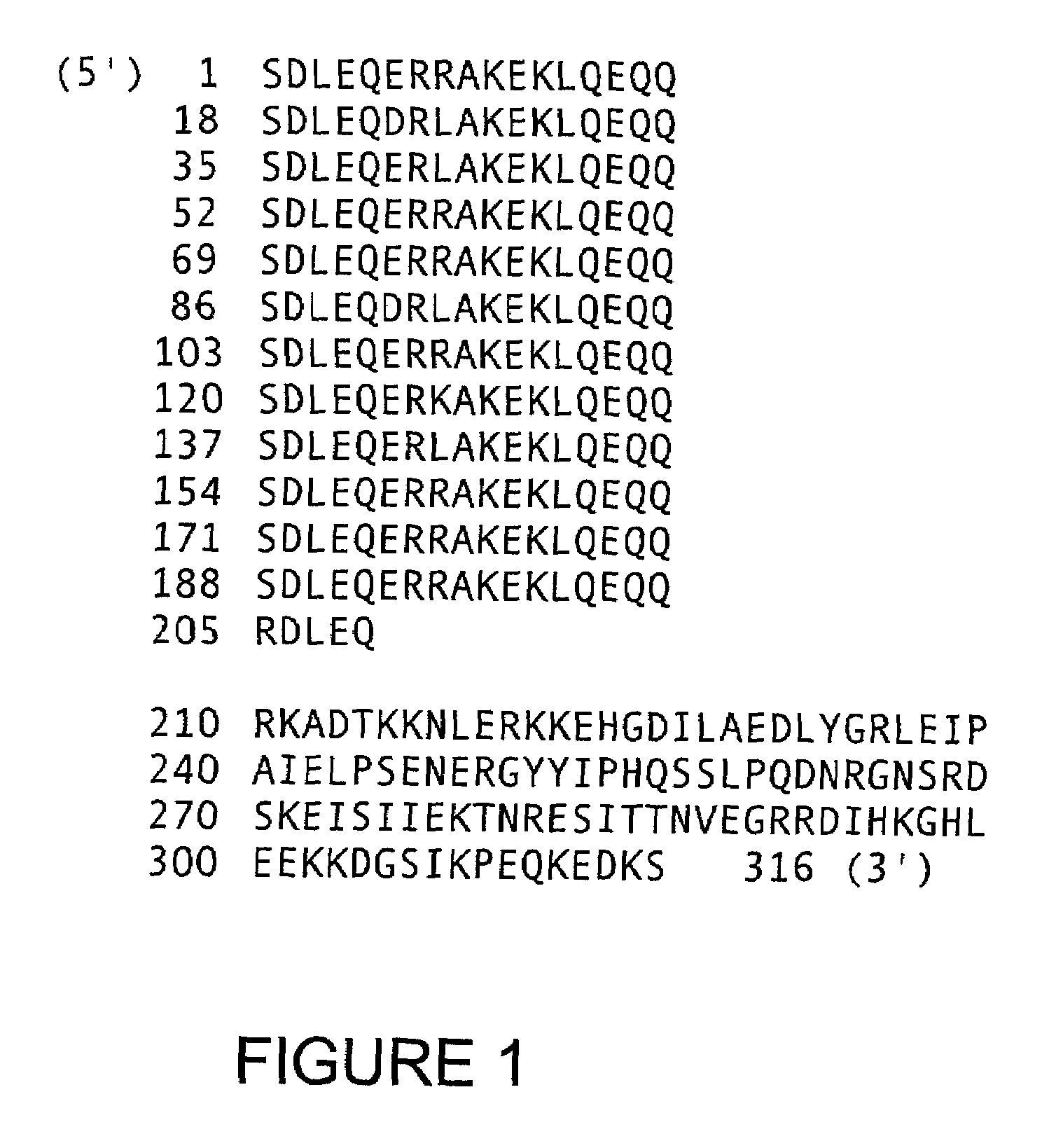
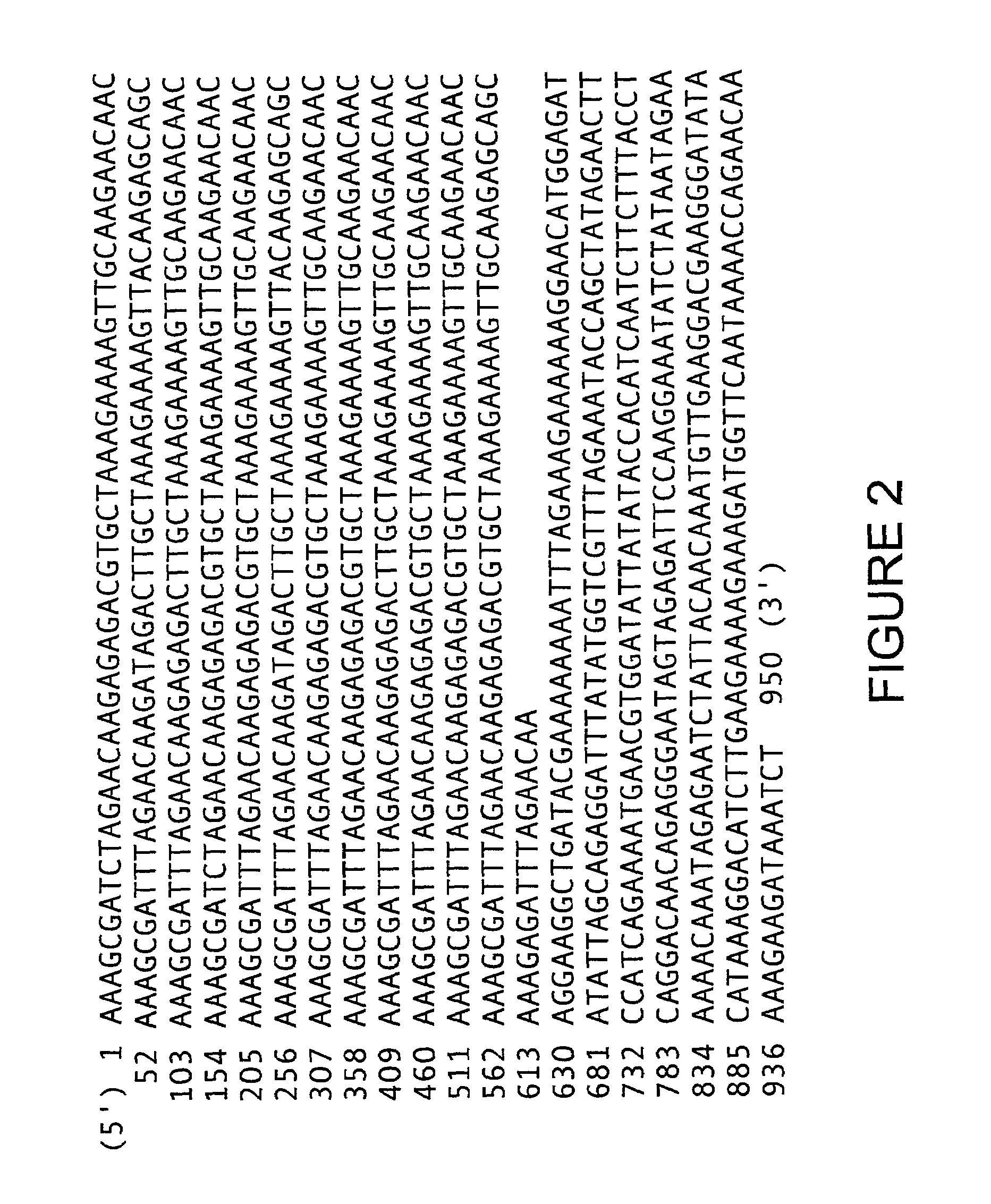
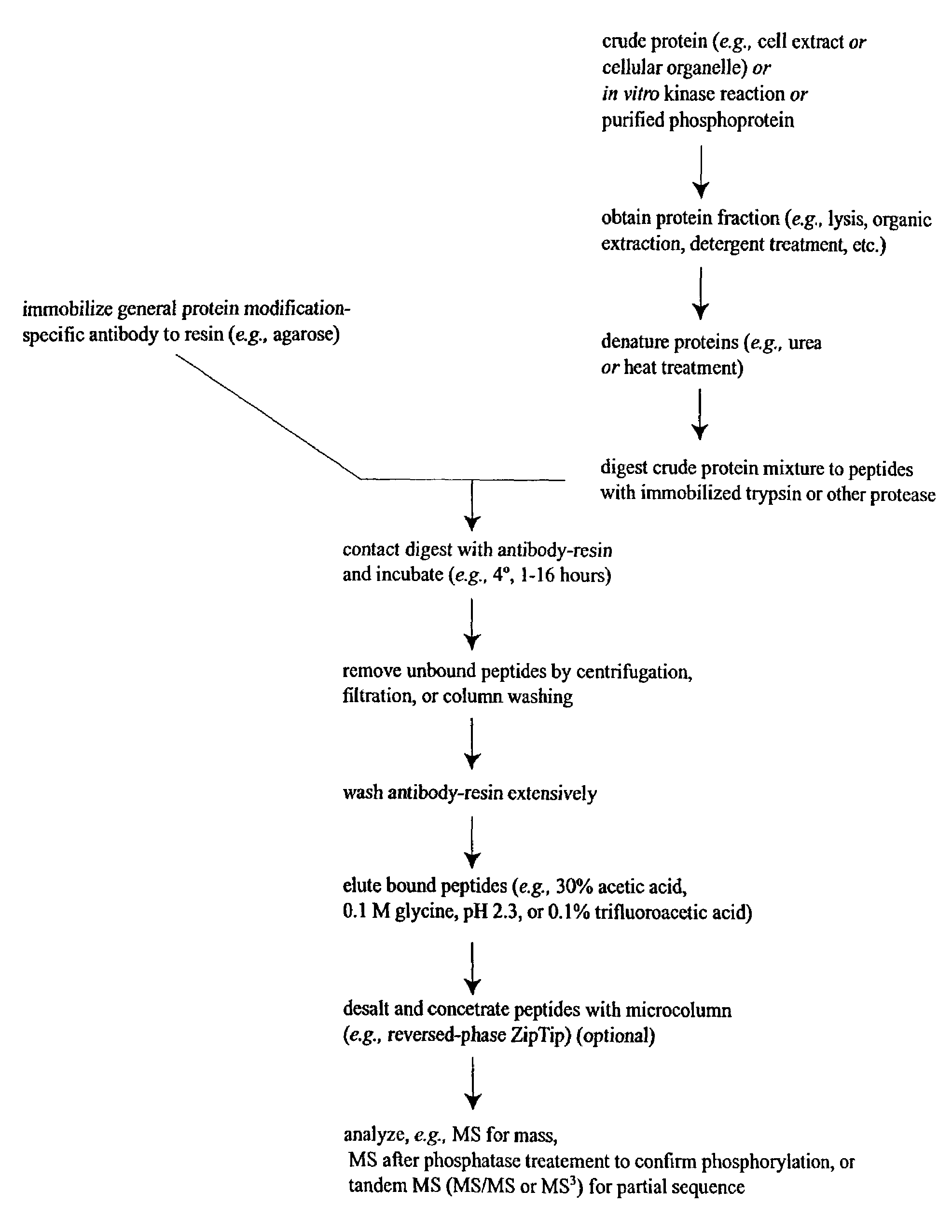
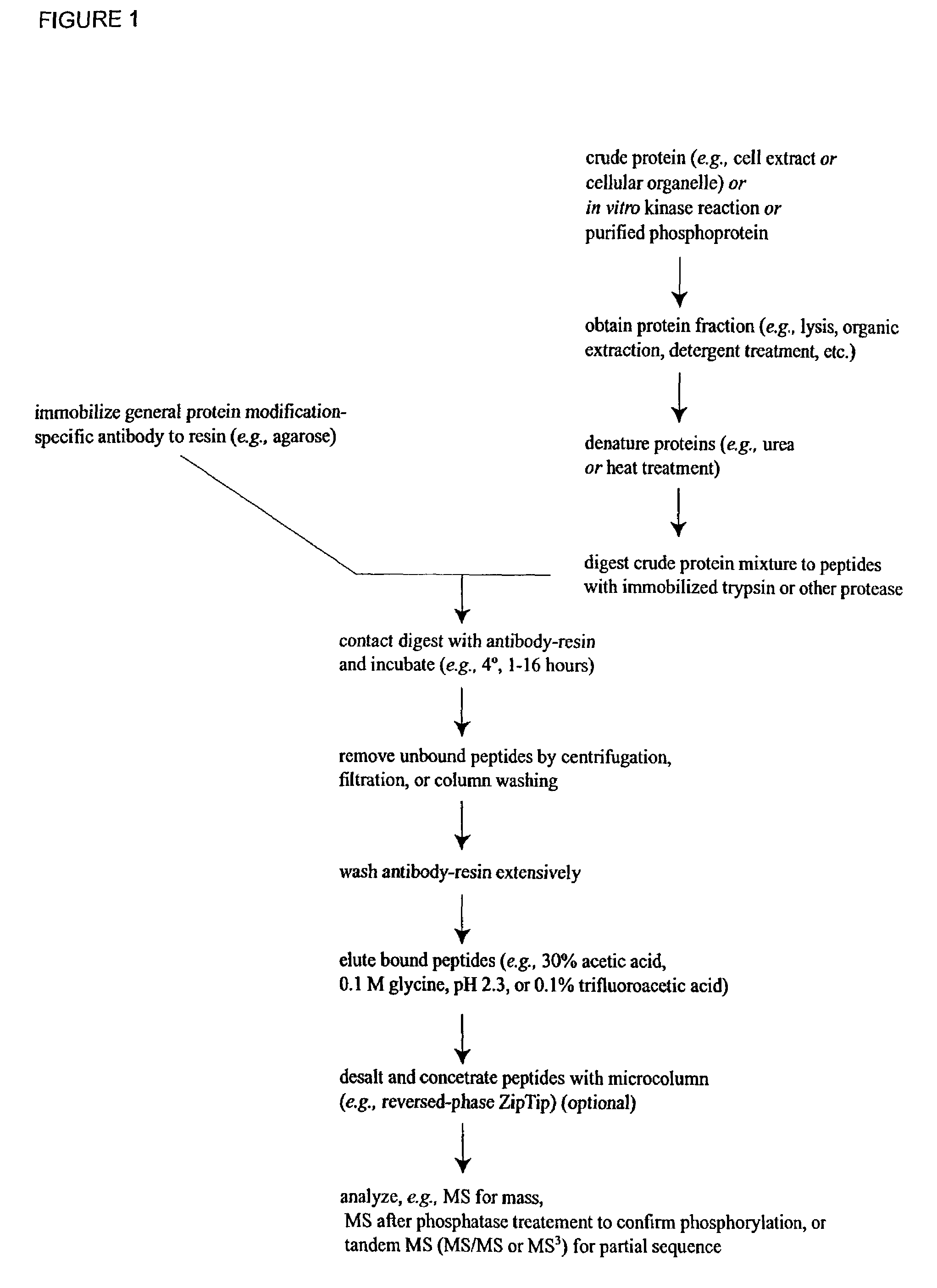
The invention provides methods for isolating a modified peptide from a complex mixture of peptides, the method comprising the steps of: (a) obtaining a proteinaceous preparation from an organism, wherein the preparation comprises modified peptides from two or more different proteins; (b) contacting the preparation with at least one immobilized modification-specific antibody; and (c) isolating at least one modified peptide specifically bound by the immobilized modification-specific antibody in step (b). The method may further comprise the step of (d) characterizing the modified peptide isolated in step (c) by mass spectrometry (MS), tandem mass spectrometry (MS—MS), and / or MS3 analysis, or the step of (e) utilizing a search program to substantially match the spectra obtained for the modified peptide during the characterization of step (d) with the spectra for a known peptide sequence, thereby identifying the parent protein(s) of the modified peptide. Also provided are an immunoaffinity isolation device comprising a modification-specific antibody, and antibodies against novel UFD1 and PTN6 phosphorylation sites.
Owner:CELL SIGNALING TECHNOLOGY
Compositions comprising variants and fusions of FGF19 polypeptides, and uses and methods thereof for treatment of metabolic disorders and diseases
ActiveUS8951966B2Lower Level RequirementsIncrease insulin sensitivityOrganic active ingredientsBacteriaDiseaseFGF19
The invention relates to variants and fusions of fibroblast growth factor 19 (FGF19), variants and fusions of fibroblast growth factor 21 (FGF21), fusions of fibroblast growth factor 19 (FGF19) and / or fibroblast growth factor 21 (FGF21), and variants or fusions of fibroblast growth factor 19 (FGF19) and / or fibroblast growth factor 21 (FGF21) proteins and peptide sequences (and peptidomimetics), having one or more activities, such as glucose lowering activity, and methods for and uses in treatment of hyperglycemia and other disorders.
Owner:NGM BIOPHARMLS
Novel immunogenic epitope for immunotherapy
ActiveUS20090136528A1Organic active ingredientsPeptide/protein ingredientsAdditive ingredientWilms' tumor
The present invention relates to peptides, nucleic acids and cells for use in immunotherapeutic methods. In particular, the present invention relates to the immunotherapy of cancer. The present invention furthermore relates to tumour-associated T-helper cell peptide epitopes, alone or in combination with other tumour-associated peptides that serve as active pharmaceutical ingredients of vaccine compositions which stimulate anti-tumour immune responses. The present invention relates to novel peptide sequences and their variants derived from HLA class I and class II molecules of human tumour cells which can be used in vaccine compositions for eliciting anti-tumour immune responses.
Owner:IMMATICS BIOTECHNOLOGIES GMBH
Immunoaffinity isolation of modified peptides from complex mixtures
InactiveUS7300753B2Rapid, efficient, and direct isolation (and subsequent characterization)Improve automationMicrobiological testing/measurementImmunoglobulins against animals/humansBiological bodyPhosphorylation
The invention provides methods for isolating a modified peptide from a complex mixture of peptides, the method comprising the steps of: (a) obtaining a proteinaceous preparation from an organism, wherein the preparation comprises modified peptides from two or more different proteins; (b) contacting the preparation with at least one immobilized modification-specific antibody; and (c) isolating at least one modified peptide specifically bound by the immobilized modification-specific antibody in step (b). The method may further comprise the step of (d) characterizing the modified peptide isolated in step (c) by mass spectrometry (MS), tandem mass spectrometry (MS—MS), and / or MS3 analysis, or the step of (e) utilizing a search program to substantially match the spectra obtained for the modified peptide during the characterization of step (d) with the spectra for a known peptide sequence, thereby identifying the parent protein(s) of the modified peptide. Also provided are an immunoaffinity isolation device comprising a modification-specific antibody, and antibodies against novel UFD1 and PTN6 phosphorylation sites.
Owner:CELL SIGNALING TECHNOLOGY
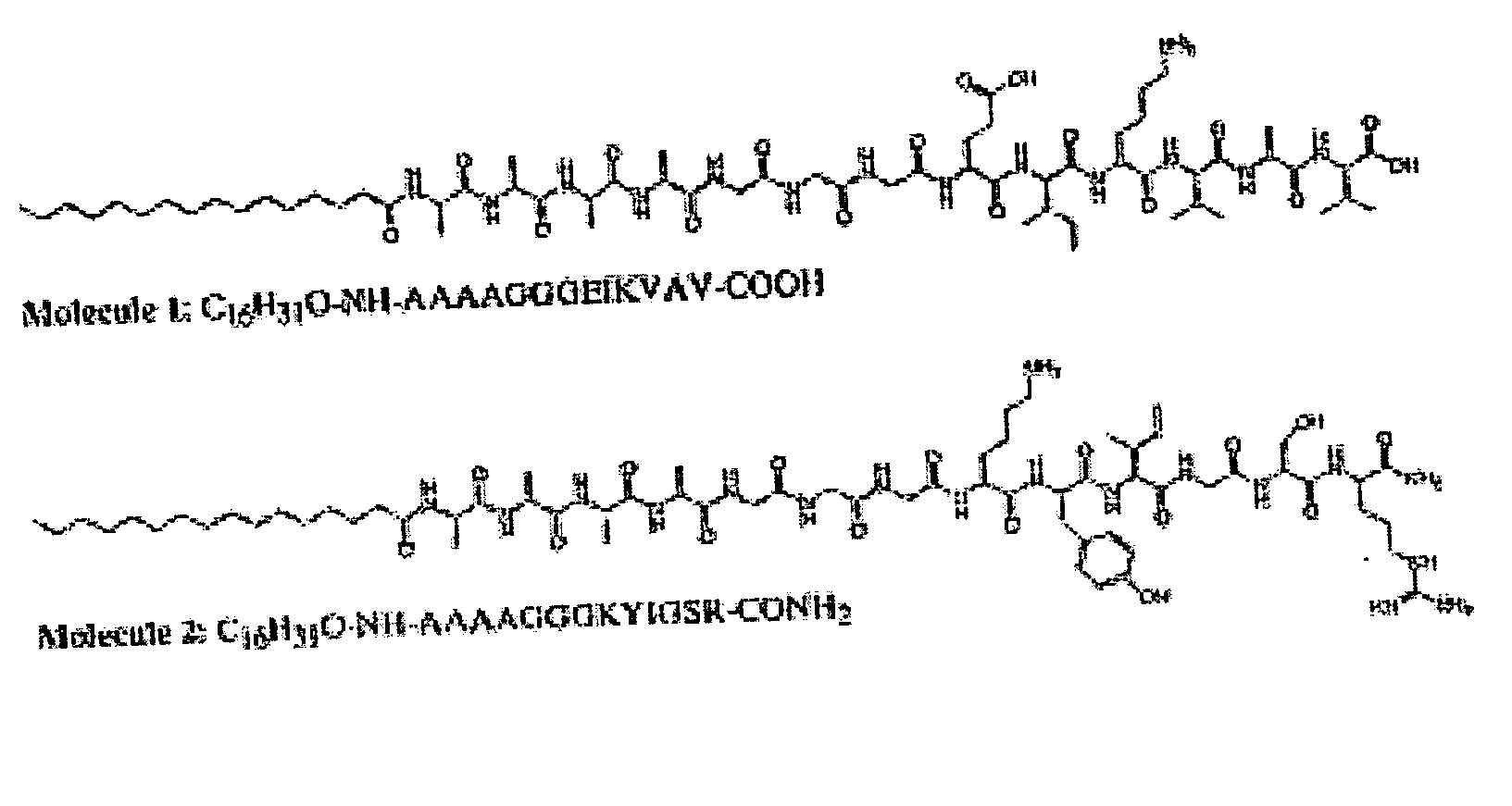
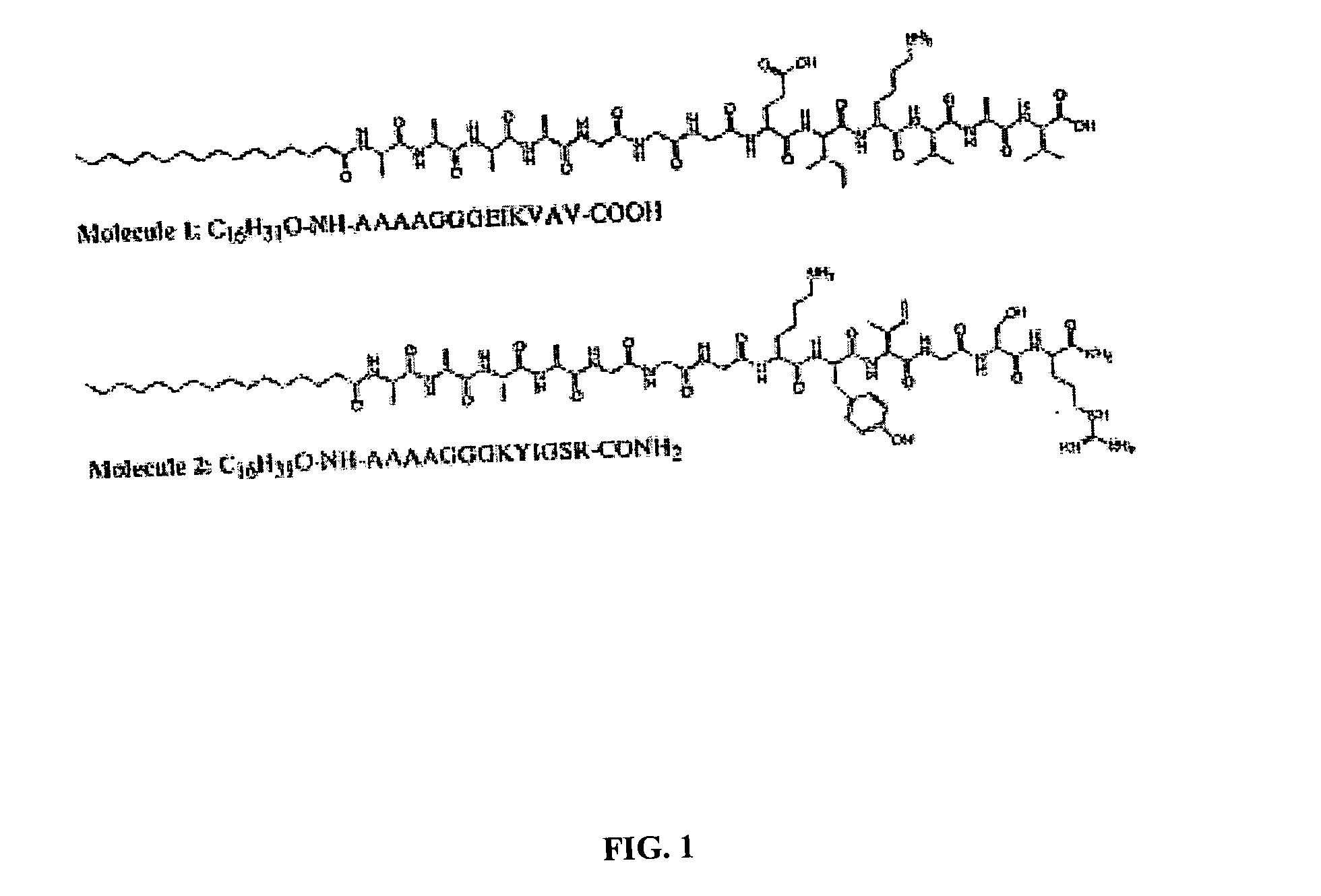
Self-assembled peptide-amphiphiles & self-assembled peptide nanofiber networks presenting multiple signals
InactiveUS20050272662A1Useful in reconstructionUseful in tissue repairMaterial nanotechnologyConnective tissue peptidesFiberPeptide sequence
The present invention provides a mixture of self-assembling peptide-amphiphiles with complementary charges whose design and function is patterned after proteins having biological functions. The oppositely charged peptide amphiphiles may be self-assembled by combining them in a charge equivalent ratio. Variations of structural peptide sequences in the oppositely charged peptide-amphiphiles enable the assembled nanofibers to exhibit two or more biologically relevant signals.
Owner:NORTHWESTERN UNIV
Modified alpha-MSH peptides and derivatives thereof
InactiveUS20050130901A1High activityReduce productionPeptide/protein ingredientsImmunoglobulinsMicroorganismMechanism of action
Novel peptides with antimicrobial activity are disclosed. The novel peptides are octomeric peptides modified from alpha-MSH. The modified alpha-MSH antimicrobial peptides disclosed herein may have enhanced activity against microbes over alpha-MSH due to modifications in peptide sequence and chirality of amino acids. Due an identified mechanism of action for antimicrobial activity in which cAMP accumulates in the microbial cell, it may be that microbes will not generate resistance to these modified alpha-MSH antimicrobial peptides.
Owner:ZENGEN
Tumor-associated Peptides Binding Promiscuously to Human Leukocyte Antigen (HLA) Class II Molecules
InactiveUS20080206216A1Organic active ingredientsPeptide/protein ingredientsHla class iiAdditive ingredient
The present invention relates to immunotherapeutic methods, and molecules and cells for use in immunotherapeutic methods. In particular, the present invention relates to the immunotherapy of cancer. The present invention furthermore relates to tumour-associated T-helper cell peptide epitopes, alone or in combination with other tumour-associated peptides, that serve as active pharmaceutical ingredients of vaccine compositions which stimulate anti-tumour immune responses. In particular, the present invention relates to 49 novel peptide sequences derived from HLA class II molecules of human tumour cell lines which can be used in vaccine compositions for eliciting anti-tumour immune responses.
Owner:IMMATICS BIOTECHNOLOGIES GMBH
Compositions and methods for treating hypophosphatasia
InactiveUS20070081984A1Extend your lifeWeight increaseHydrolasesPeptide/protein ingredientsBone tissueMembrane bound enzyme
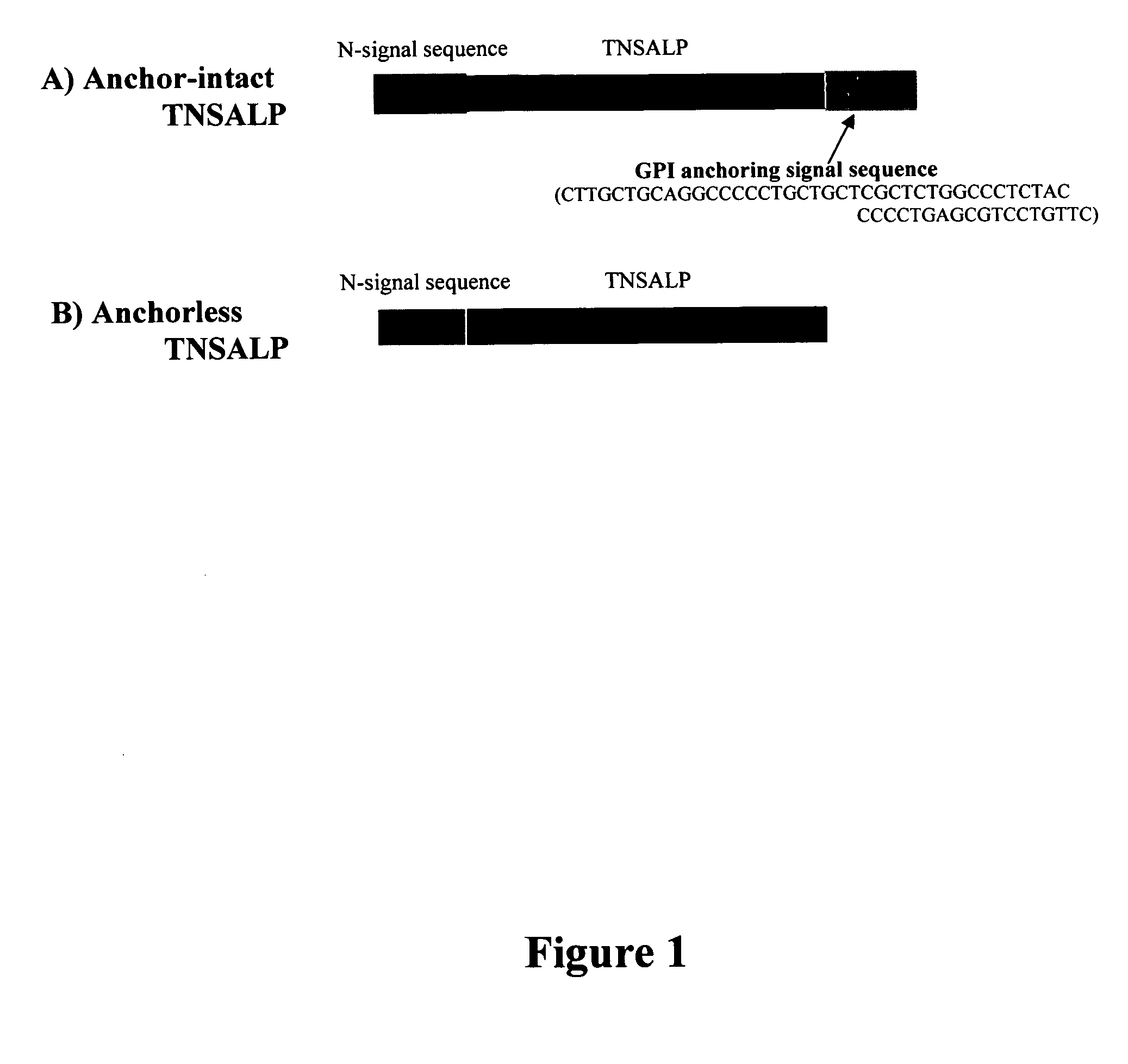
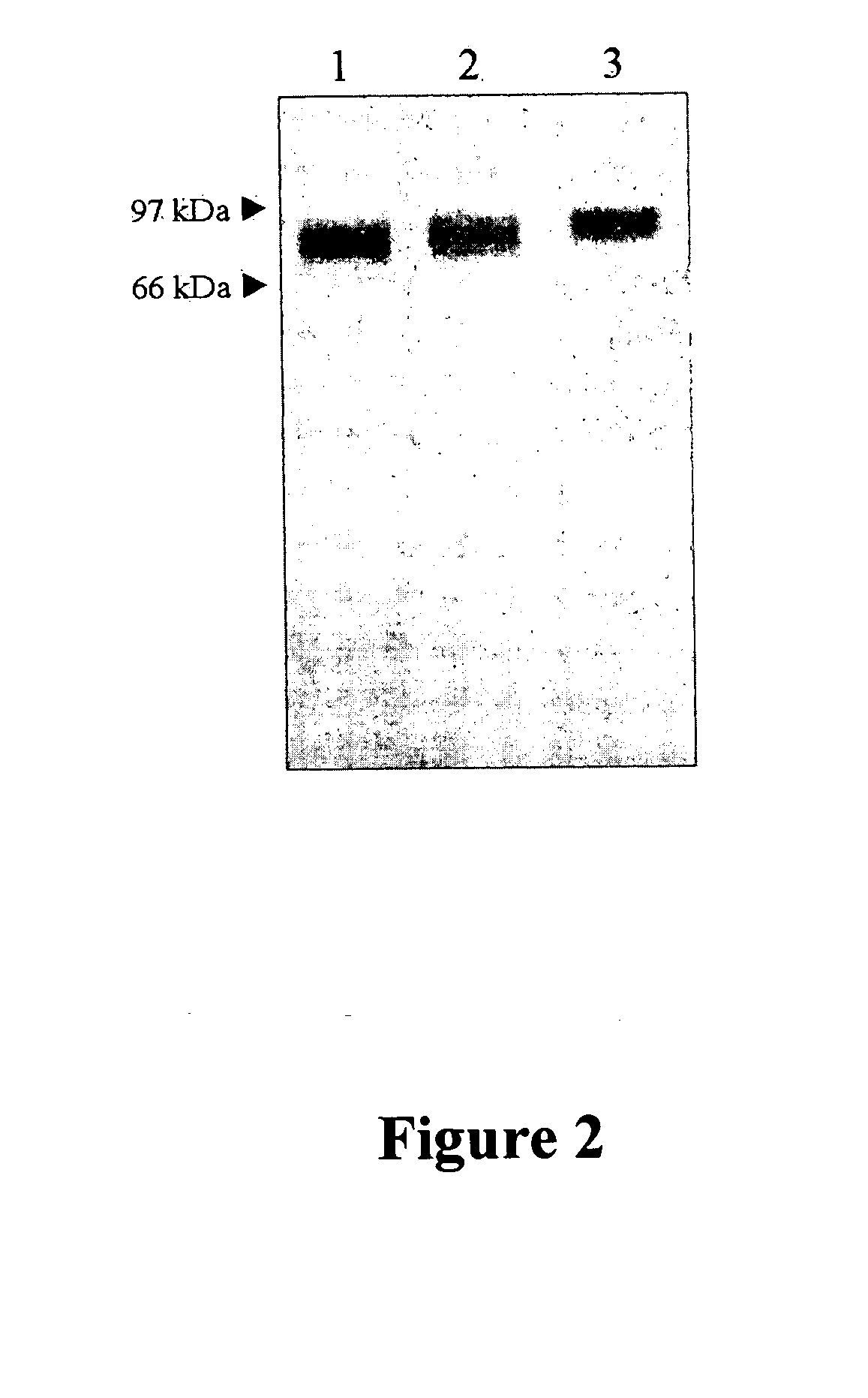
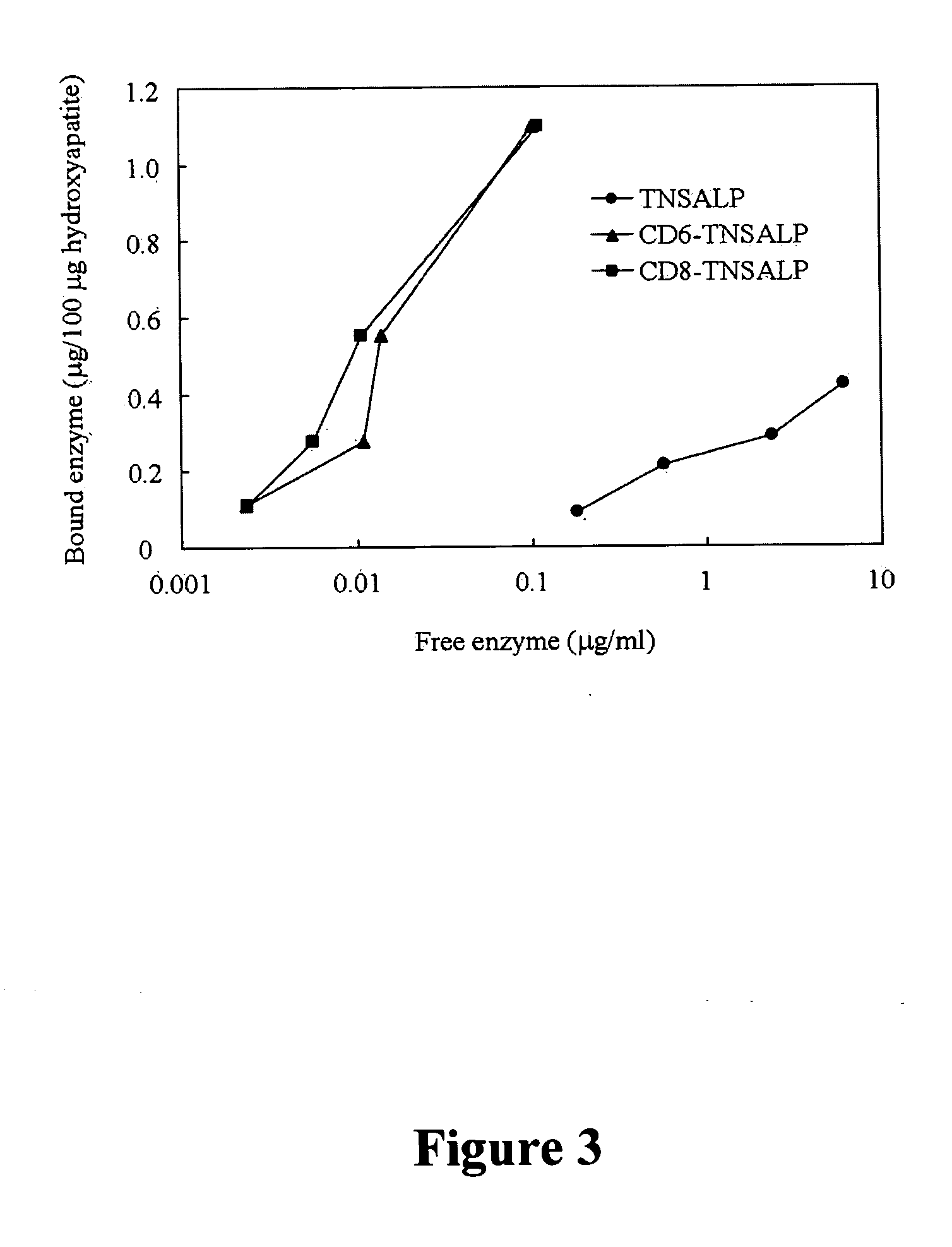
The present invention provides compositions and methods for use in enzyme replacement therapy. The inventors disclose a method of producing membrane bound enzymes in an active soluble form by eliminating the glycosylphosphatidylinositol (GPI) membrane anchor. In particular the inventors disclose a soluble active form of the membrane bound enzyme TNSALP which they produced by deleting the GPI anchor single peptide sequence. They have further shown that this composition is useful for treatment of hypophosphatasia. The inventors also disclose oligo acid amino acid variants thereof which specifically target bone tissue.
Owner:SAINT LOUIS UNIVERSITY +2
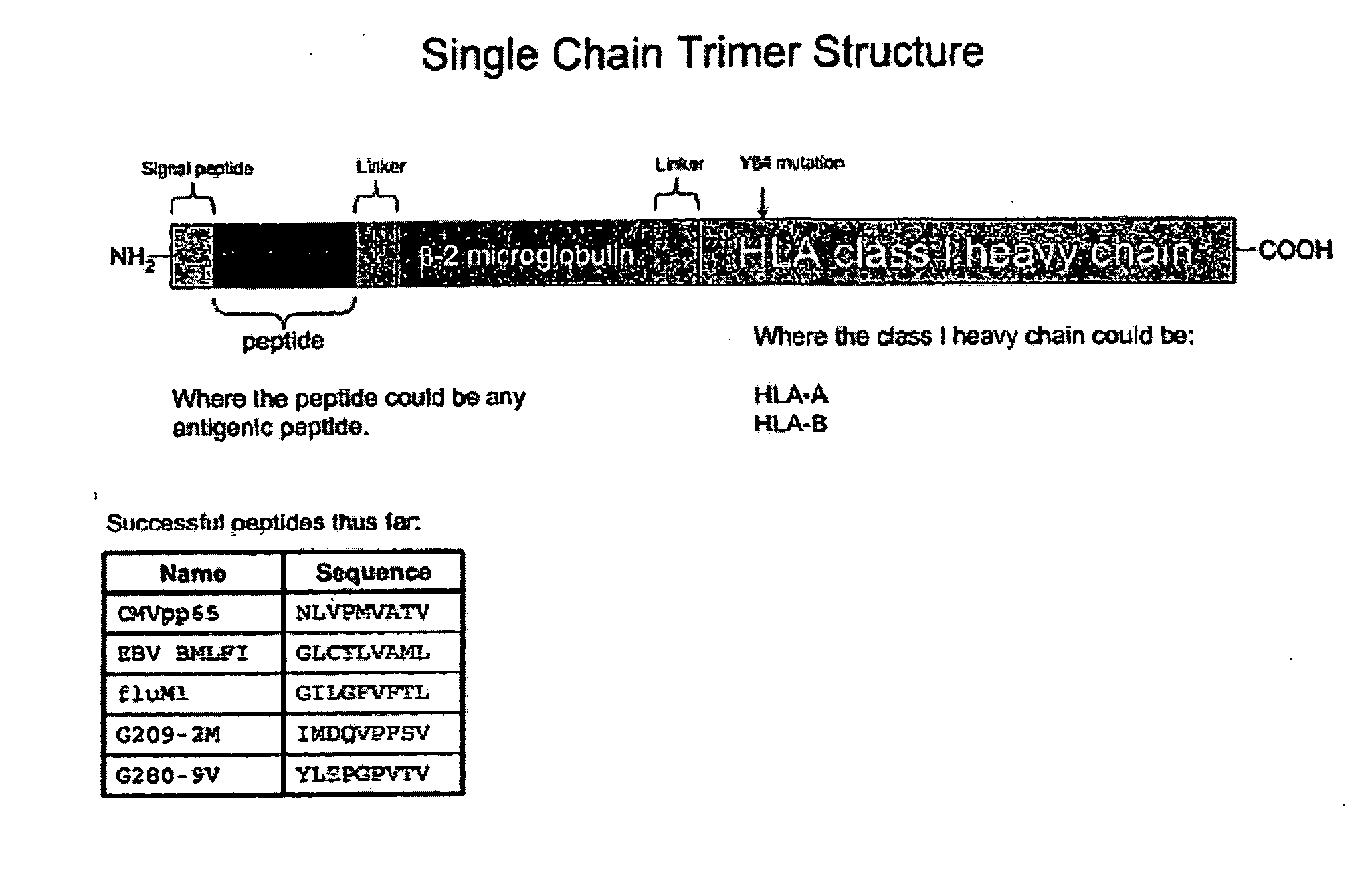
Single chain trimers and uses therefor
Owner:WASHINGTON UNIV IN SAINT LOUIS
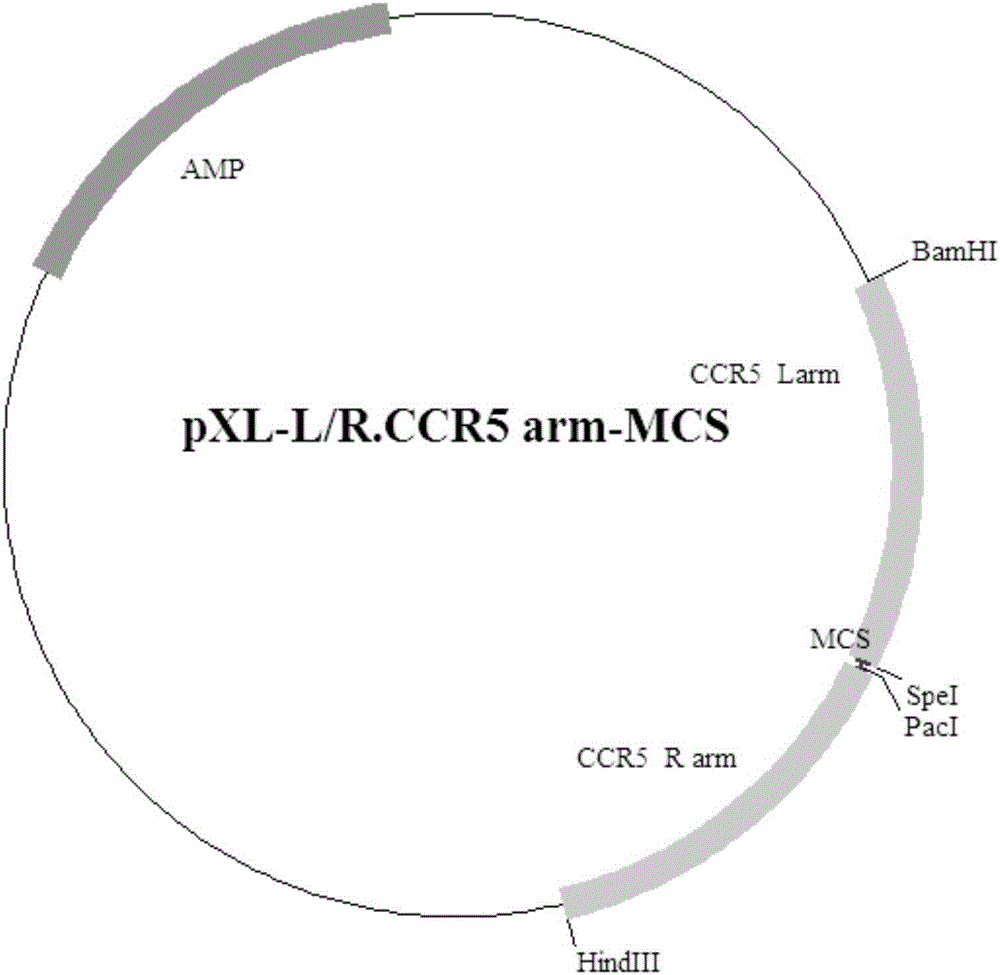
Exogenous gene knocking-in and integrating system on basis of CRISPR/Cas9, method for establishing exogenous gene knocking-in and integrating system and application thereof
ActiveCN106191116ANucleic acid vectorVector-based foreign material introductionTarget geneIntegrated systems
The invention provides an exogenous gene knocking-in and integrating system on the basis of CRISPR / Cas9, a method for establishing the exogenous gene knocking-in and integrating system and application thereof. The exogenous gene knocking-in and integrating system comprises vectors with report / donor functions and Cas9 expression vectors. Each report / donor vector comprises two target gent homologous arms and an exogenous sequence fragment positioned between the two target gene homologous arms; homologous sequences, which are positioned on a target gene, of the two target gene homologous arms of each report / donor vector are respectively positioned on two sides of a target sequence of the target gene and are connected with the target sequence of the target gene; the exogenous sequence fragments comprise promoters, resistant genes, shorn peptide sequences, report genes and polyA tails which are sequentially arrayed, two SSA repair homologous sequences of each resistant gene are inserted into the resistant gene, and the target sequence of each target gene is inserted in a space between the two corresponding SSA repair homologous sequences. The exogenous gene knocking-in and integrating system, the method and the application have the advantages that exogenous genes can be integrated with endogenous gene sequences in an efficient site-directed and accurately targeted manner, and double-chromosome allelic gene double-knocking-in can be efficiently carried out.
Owner:成都中科奥格生物科技有限公司
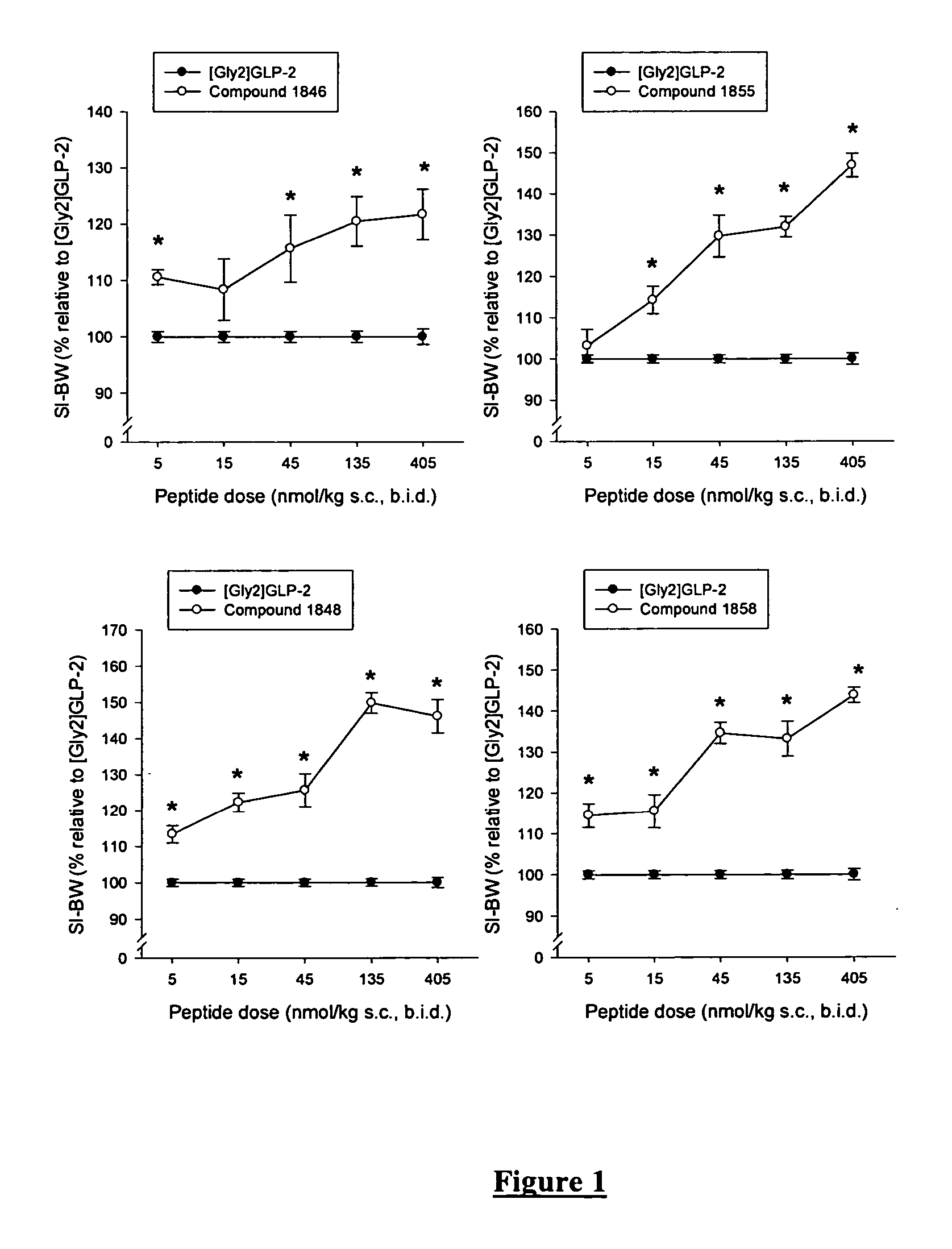
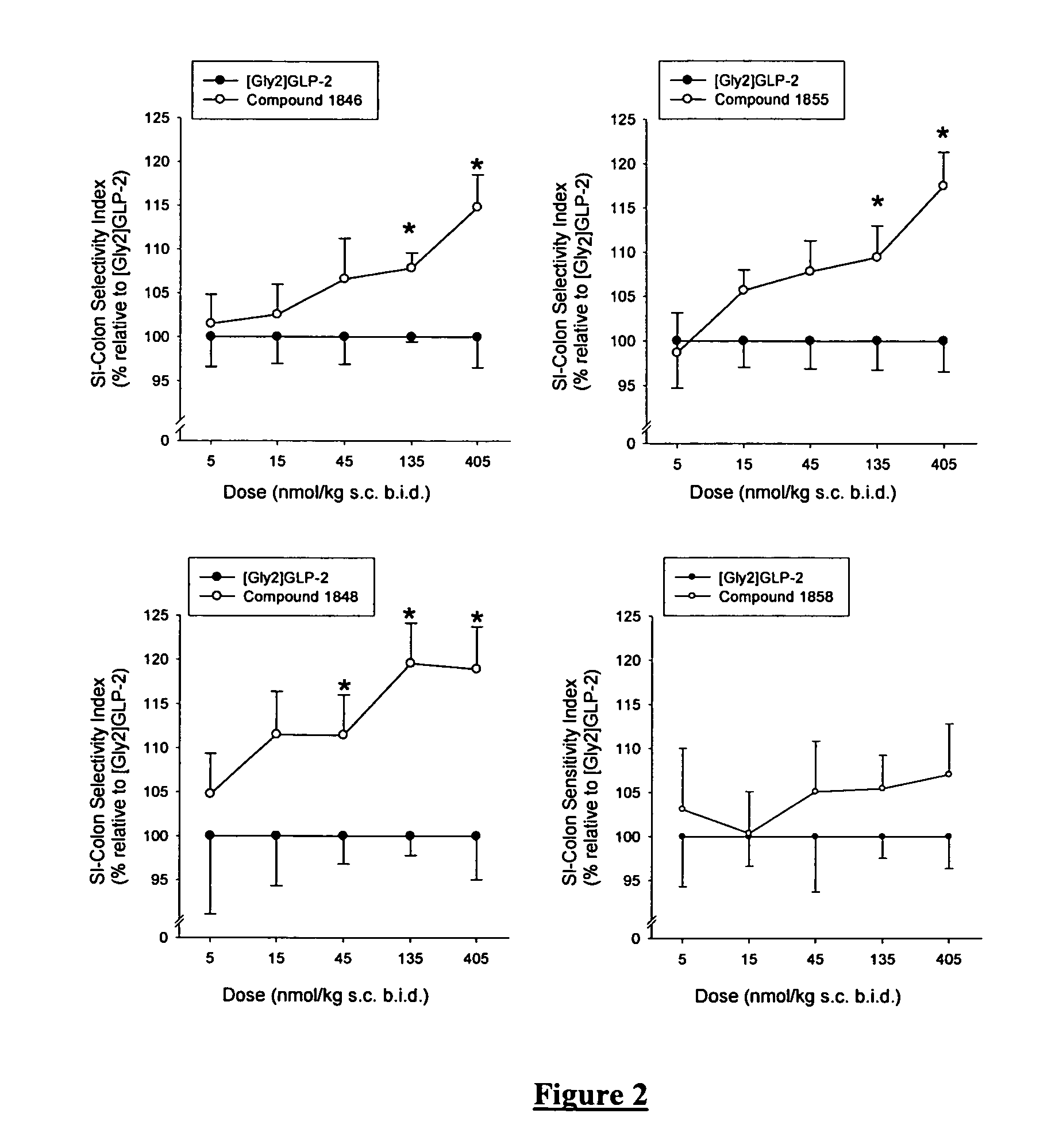
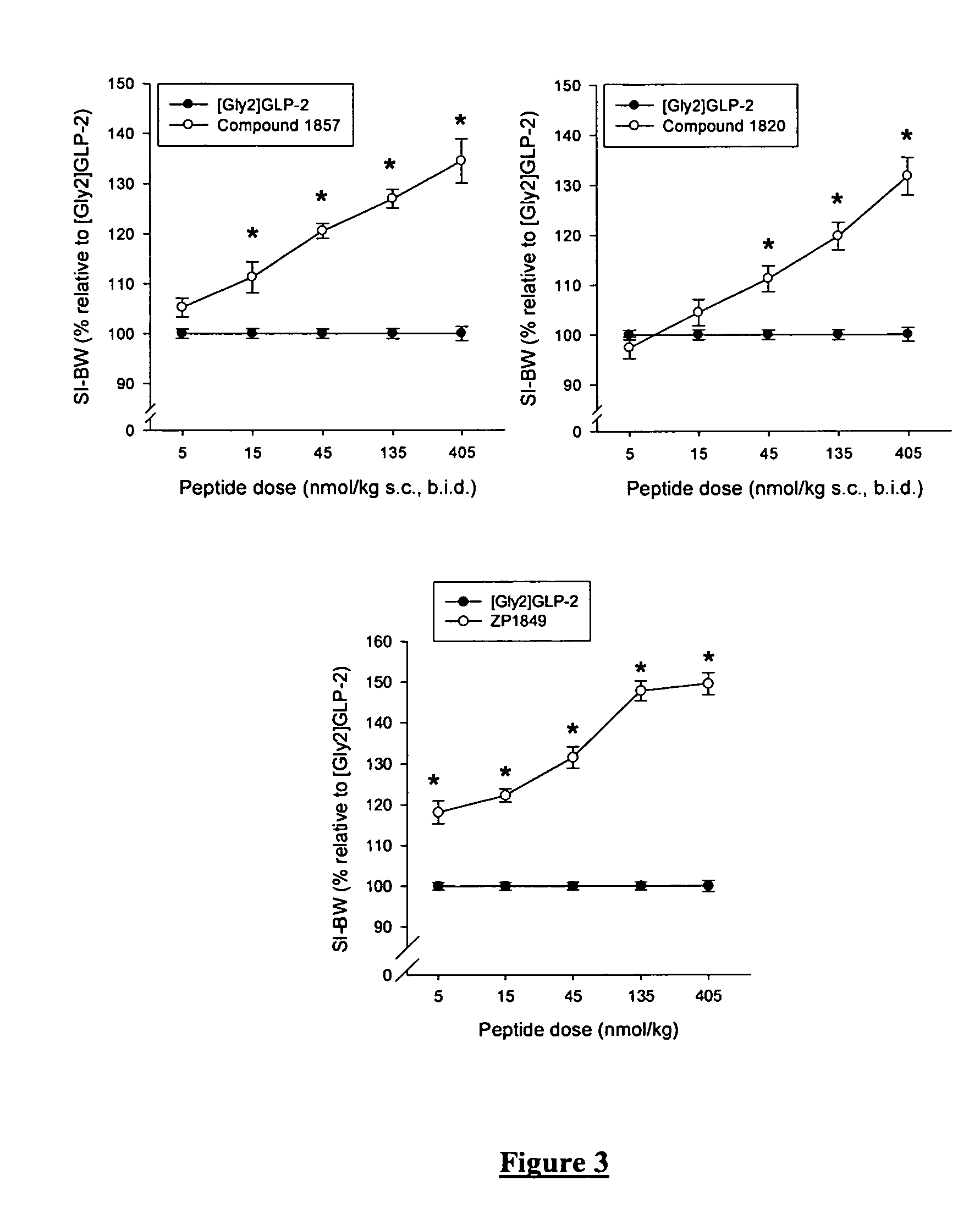
Glucagon-like-peptide-2 (GLP-2) analogues
ActiveUS20070117752A1Improve biological activityPreferential intestinal growth promoting activitySsRNA viruses negative-senseAntipyreticSide effectWild type
GLP-2 analogues are disclosed which comprise one of more substitutions as compared to [hGly2]GLP-2 and which improved biological activity in vivo and / or improved chemical stability, e.g., as assessed in in vitro stability assays. More particularly, preferred GLP-2 analogues disclosed herein comprise substitutions at one or more of positions 8, 16, 24 and / or 28 of the wild-type GLP-2 sequence, optionally in combination with further substitutions at position 2 (as mentioned in the introduction) and one or more of positions 3, 5, 7, 10 and 11, and / or a deletion of one or more of amino acids 31 to 33 and / or the addition of a N-terminal or C-terminal stabilizing peptide sequence. The analogues are particularly useful for the prophylaxis or treatment of stomach and bowel-related disorders and for ameliorating side effects of chemotherapy. Also disclosed are methods and kits for selecting a patient from populations suited for treatment with GLP-2 analogues.
Owner:ZEALAND PHARM AS
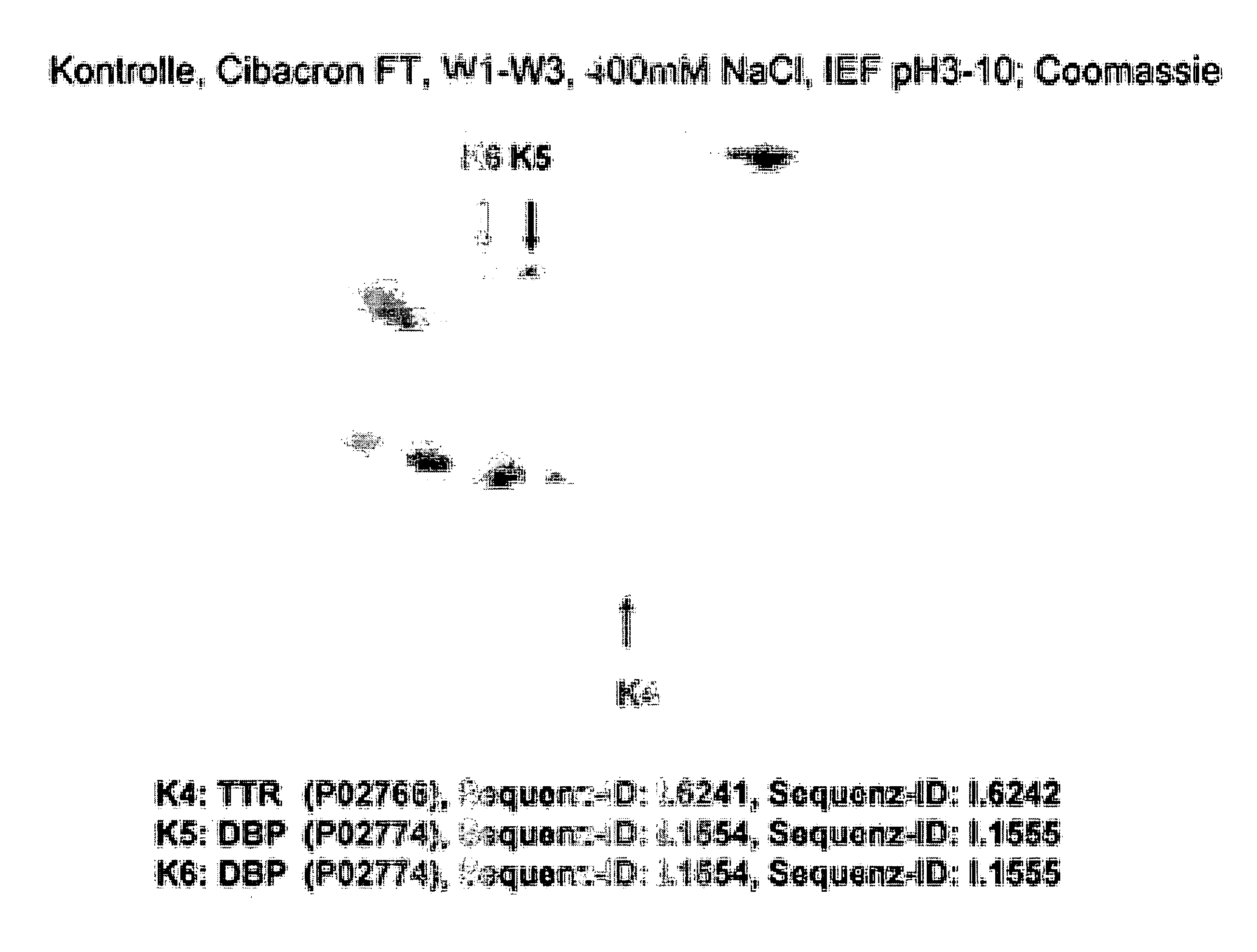
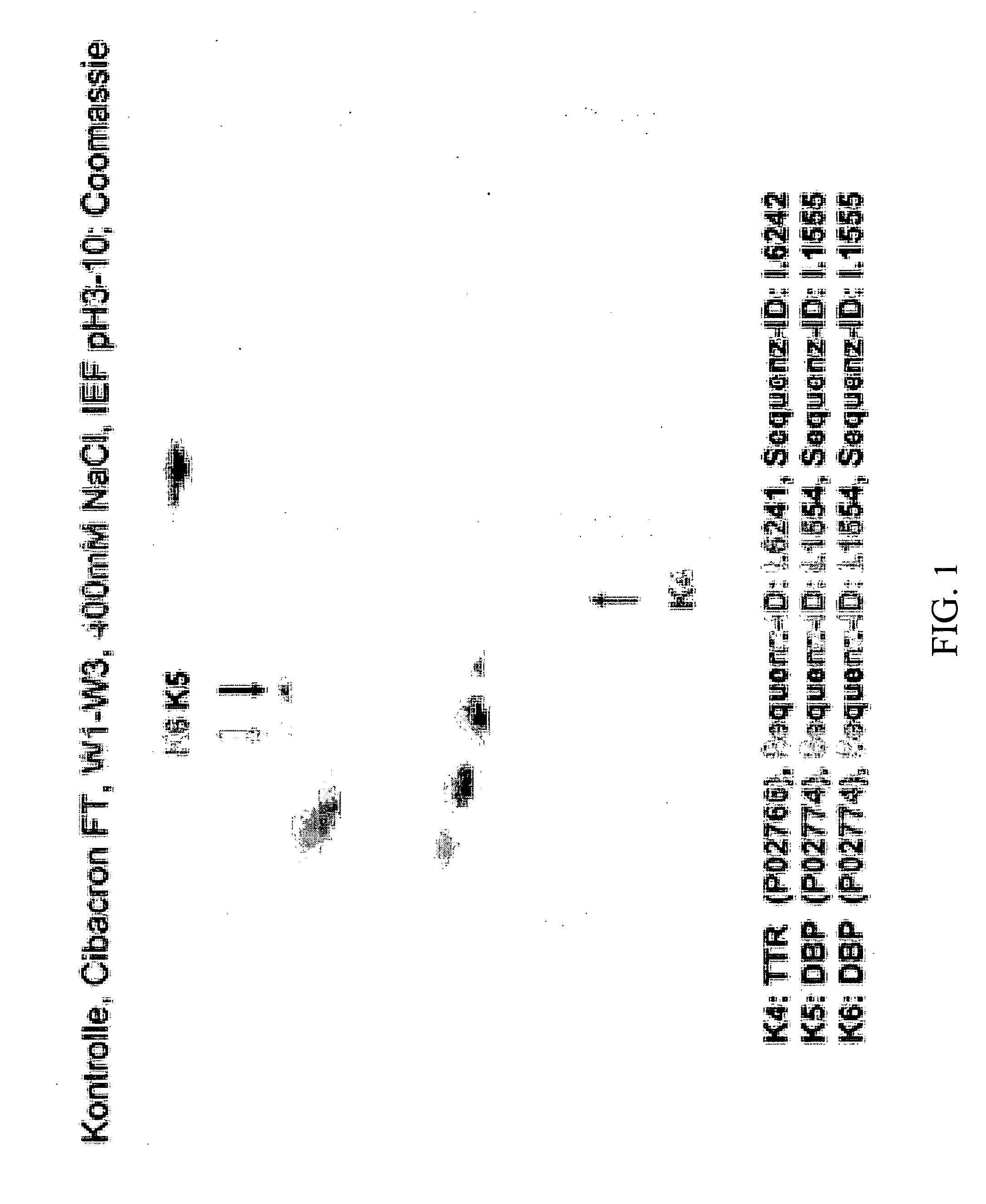
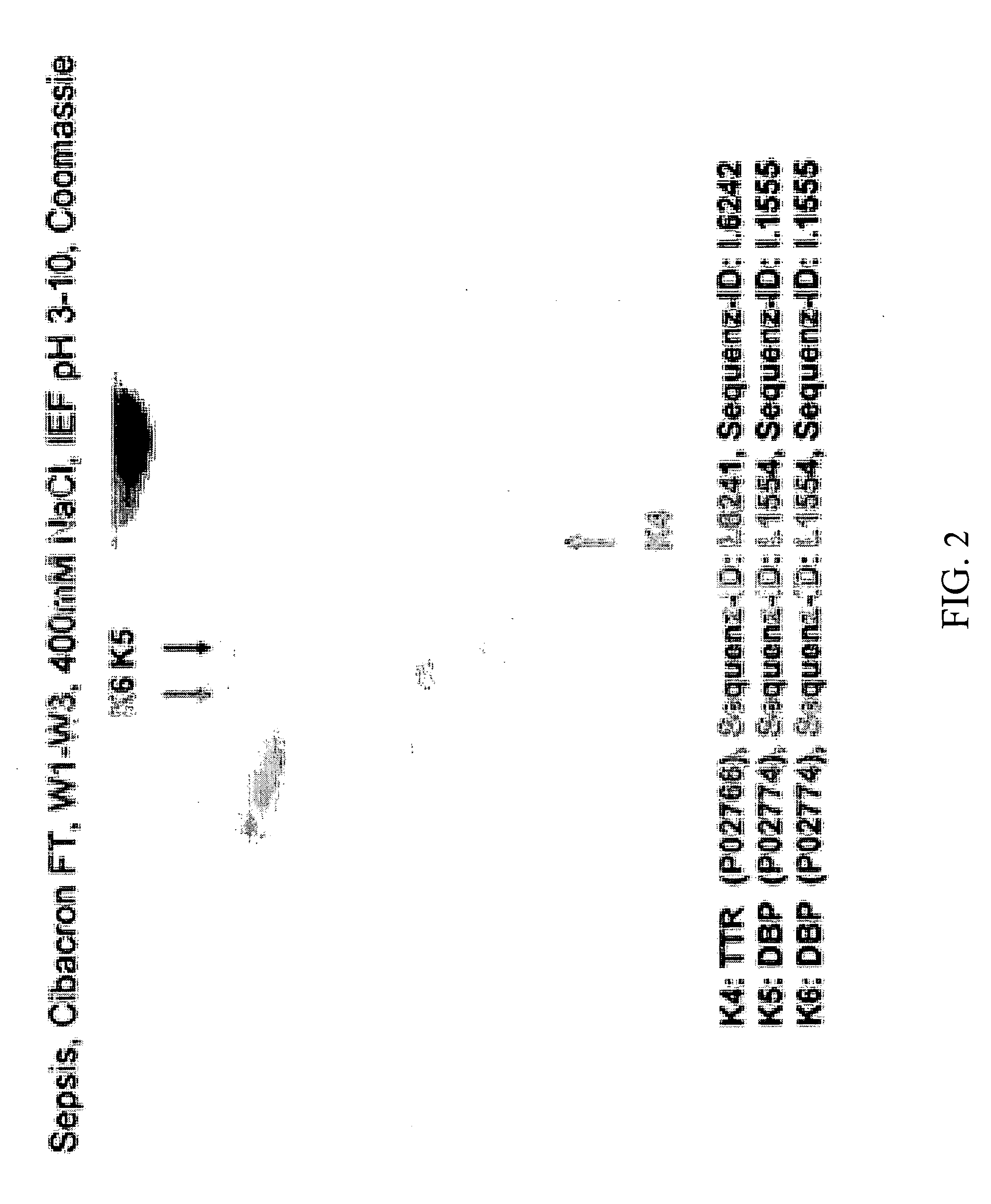
Method for Recognizing Acute Generalized Inflammatory Conditions (Sirs), Sepsis, Sepsis-Like Conditions and Systemic Infections
InactiveUS20080070235A1High riskMicrobiological testing/measurementNanoinformaticsActive agentWhole body
The present invention relates to a method for in vitro detection of SIRS, sepsis and / or sepsis-like conditions. This method renders the evaluation of the severity and / or the therapeutic progress of sepsis and severe infections, in particular sepsis-like systemic infections possible. Further, the present invention relates to the use of recombinantly or synthetically prepared nucleic acid sequences or peptide sequences derived therefrom as calibrator in sepsis assays and / or for the evaluation of the effect and the toxicity during screening of the active agents and / or the preparation of therapeutics for the prevention and treatment of SIRS, sepsis, sepsis-like systemic inflammatory conditions and sepsis-like systemic infections.
Owner:SIRS LAB
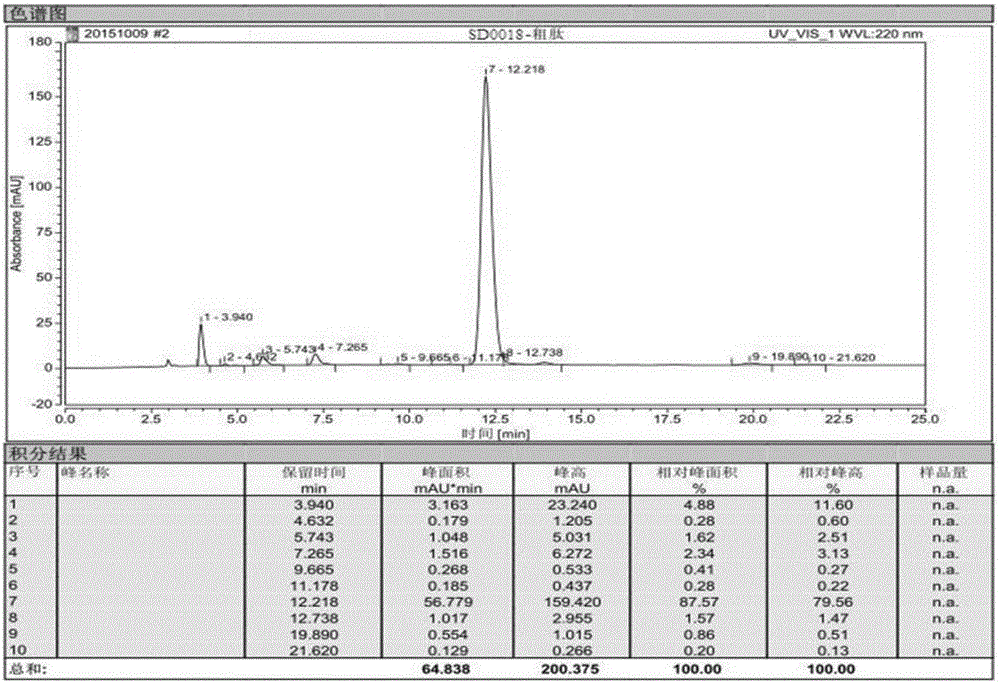
Synthesis method of semaglutide
ActiveCN106749613ALow costReduce the number of generatedPeptide preparation methodsBulk chemical productionSynthesis methodsPeptide sequence
The invention relates to a synthesis method of semaglutide. According to the method, a semaglutide product is synthesized by adopting a solid-liquid phase combination method, and three fragments are simultaneously synthesized in a synthesis manner of 16+6+9 fragments, and therefore, the synthesis time of the product is greatly shortened; moreover, by step-by-step analysis on synthesis factors of His-Ala-Glu-Gly-Thr-Phe-Thr-Ser-Asp-Val-Ser-Ser-Tyr-Leu-Glu-Gly, Gln-Ala-Ala-N6-[N-(17-carboxy-1-oxoheptadecyl-L-gama-glutamyl [2-(2-aminoethoxy) ethoxy] acetyl [2-(2-aminoethoxy) ethoxy] acetyl]-Lys-Glu-Phe, Ile-Ala-Trp-Leu-Val-Arg-Gly-Arg-Gly-OH and the like, the difficulty in synthesis of a peptide sequence in solid-phase synthesis is reduced, the problem of batch amplification in the solid-phase synthesis is solved, and the synthesis efficiency is improved; and as liquid-phase fragment synthesis is adopted, the purification difficulty is effectively reduced, and the production cost is greatly lowered. The synthesis method disclosed by the invention has the advantages that the synthesis time can be shortened by 40%, the cost of materials is lowered, the generation quantities of deletion peptide and hybrid peptide are decreased, and the synthesis method is suitable for industrial large-scale production.
Owner:SINOPEP ALLSINO BIOPHARMACEUTICAL CO LTD
Charged peptide-amphiphile solutions and self-assembled peptide nanofiber networks formed therefrom
InactiveUS7534761B1High total negative chargeLess structural stabilityBiocideMaterial nanotechnologyIonPeptide sequence
The present invention provides a system of self-assembling peptide amphiphiles with an absolute net charge of 3 or greater whose design and function may be patterned after proteins involved in vertebrate mineralization or other tissue forming processes. This molecular system preferably consists of a hydrophobic hydrocarbon tail attached to a relatively hydrophilic peptide sequence. Self-assembly of this peptide amphiphile may be induced through pH variation, divalent ion addition, or dehydration. Variations of structural peptide sequences in the peptide amphiphile may enable the assembled nanofibers to be reversibly cross-linked for more or less structural stability, or may allow for control of the rate of self-assembly.
Owner:NORTHWESTERN UNIV
Compositions and methods for treatment of metabolic disorders and diseases
InactiveUS20150291677A1Effective treatmentPromote sugar metabolismBacteriaPeptide/protein ingredientsDiseaseFGF19
The invention relates to variants and fusions of fibroblast growth factor 19 (FGF19), variants and fusions of fibroblast growth factor 21 (FGF21), fusions of FGF19 and / or FGF21, and variants or fusions of FGF19 and / or FGF21 proteins and peptide sequences (and peptidomimetics), having one or more activities, such as glucose lowering activity, and methods for and uses in treatment of hyperglycemia and other disorders.
Owner:NGM BIOPHARMLS
Method for enhancing targeting selectivity of administration system by modifying cell penetrating peptide
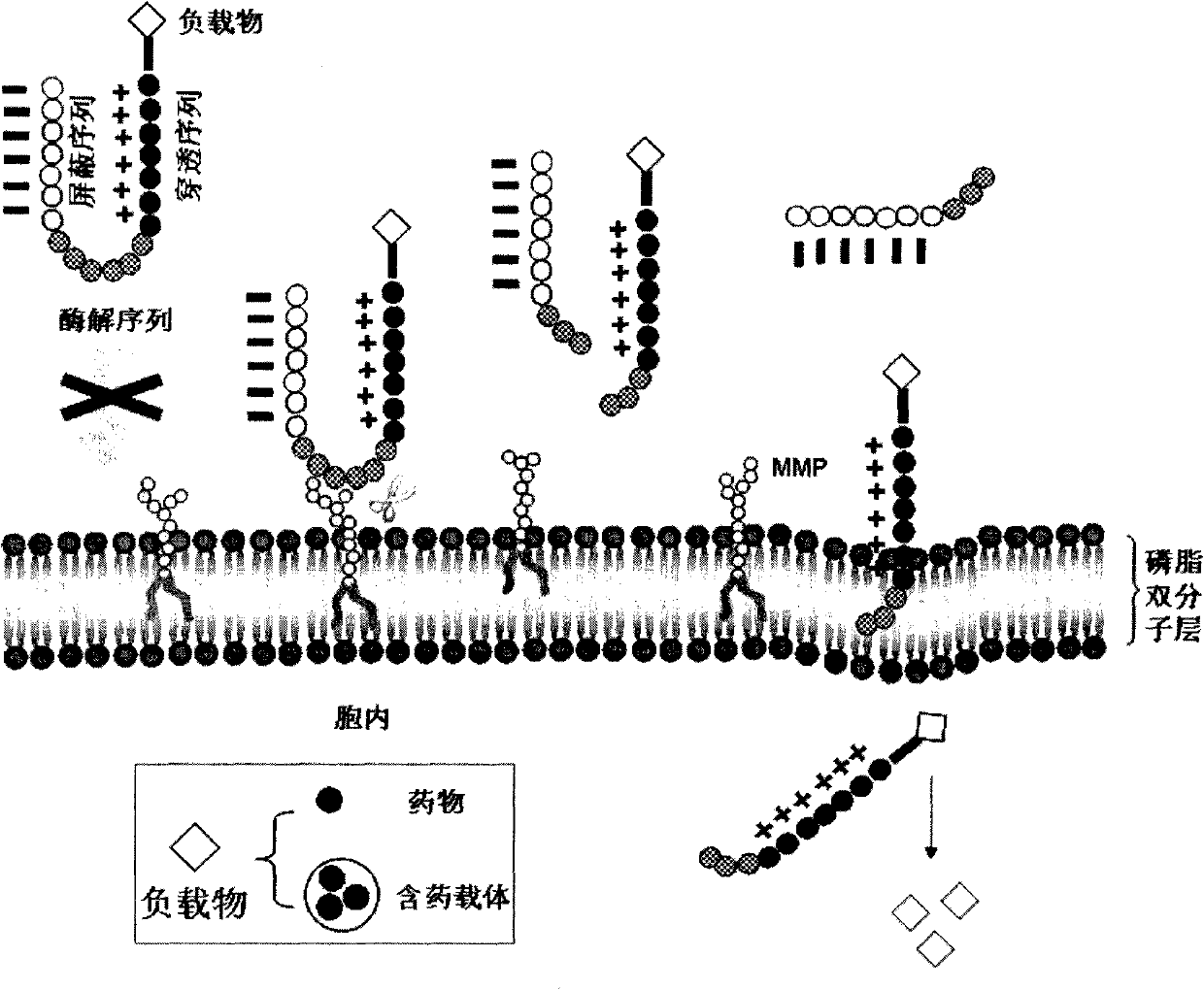
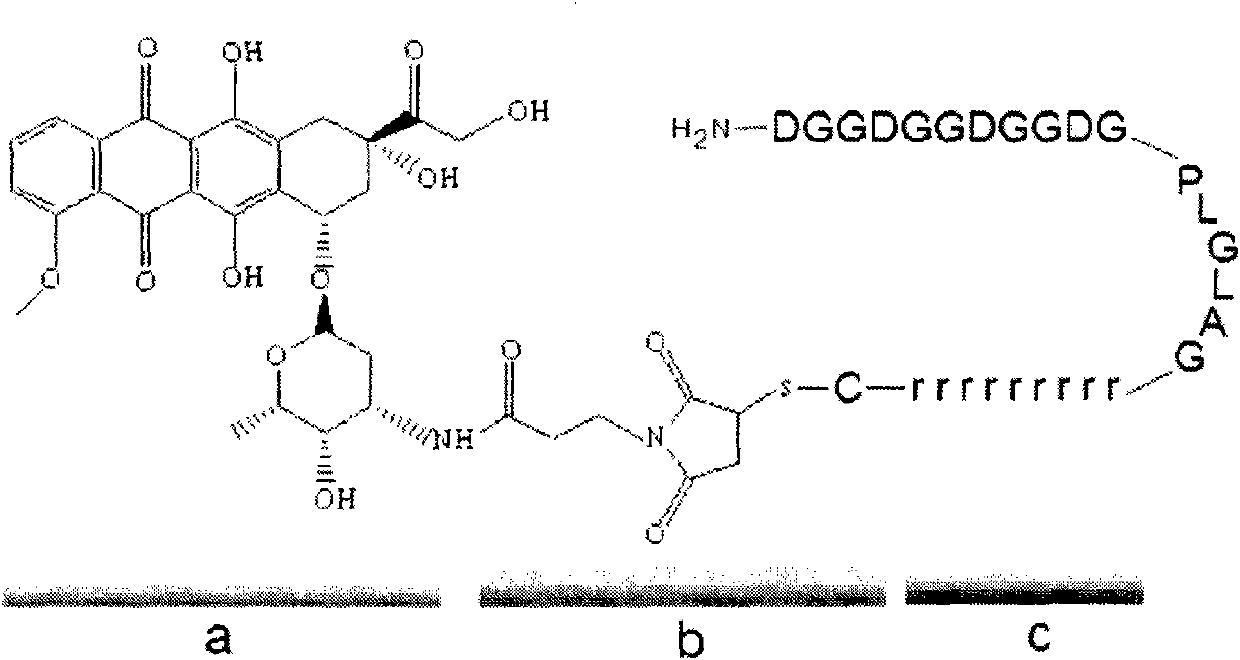
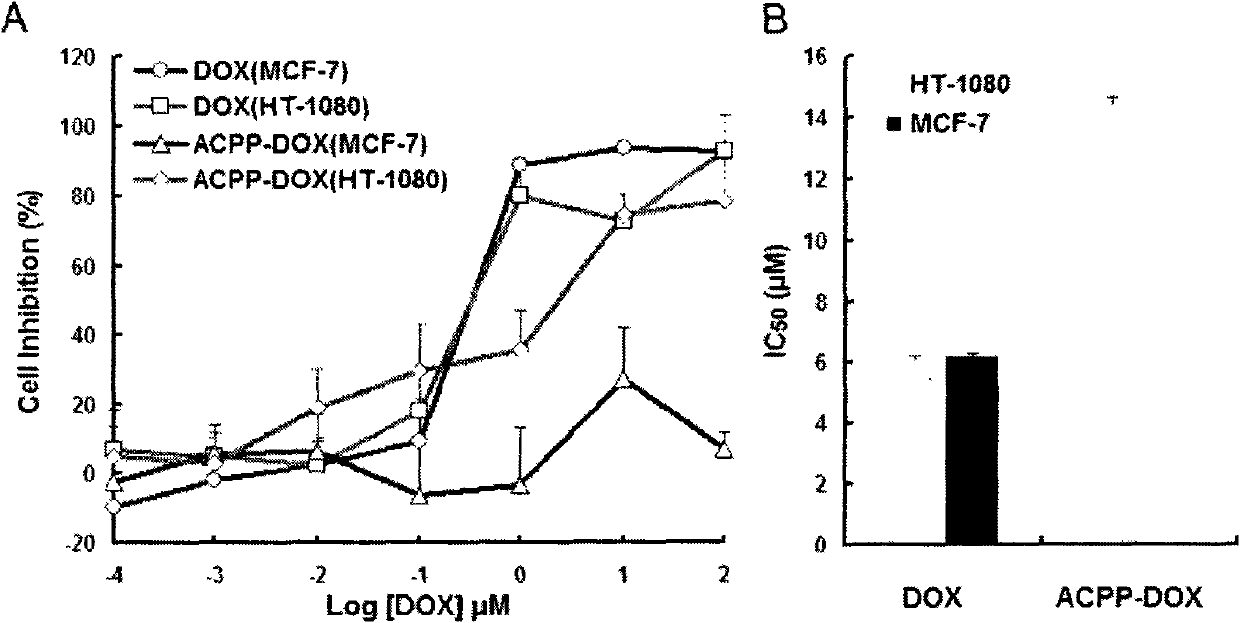
InactiveCN102552929AEliminate or reduce transmembrane effectAvoid damageIn-vivo radioactive preparationsPeptide/protein ingredientsCell membraneEnzyme system
The invention relates to modification of a cell penetrating peptide for realizing a low-toxicity administration system with a positive targeting selecting function. A shielding peptide, an enzymolysis substrate peptide and a cell-penetrating peptide are connected in sequence, so that an activatable cell penetrating peptide is formed; and a medicament and / or a tracer and / or a medicament carrier is connected or embedded or adsorbed to the cell penetrating peptide, so that an administration system is constructed. According to a shielding peptide sequence, positive charges carried on the surface of the administration system can be reduced or completely neutralized, the cell penetrating capability of the cell penetrating peptide is shielded, and the toxicity of the administration system on normal cells of an organism is lowered; and an enzymolysis substrate peptide sequence can be identified by enzyme systems secreted specifically by different pathological change tissue cells and fractured by enzyme hydrolysis, so that a cell penetrating peptide is released and is used for carrying a medicament and / or a medicament carrier through a cell membrane, and the medicament enters cells and is brought into play. The invention aims to actively convey an antitumor medicament to tumor tissues in a targeted way and make the antitumor medicament enter tumor cells to a larger extent by using the administration system which can be used for activating a cell penetrating function, so that the toxicity at a non-tumor position is lowered while the antitumor effect of the medicament is enhanced.
Owner:PEKING UNIV
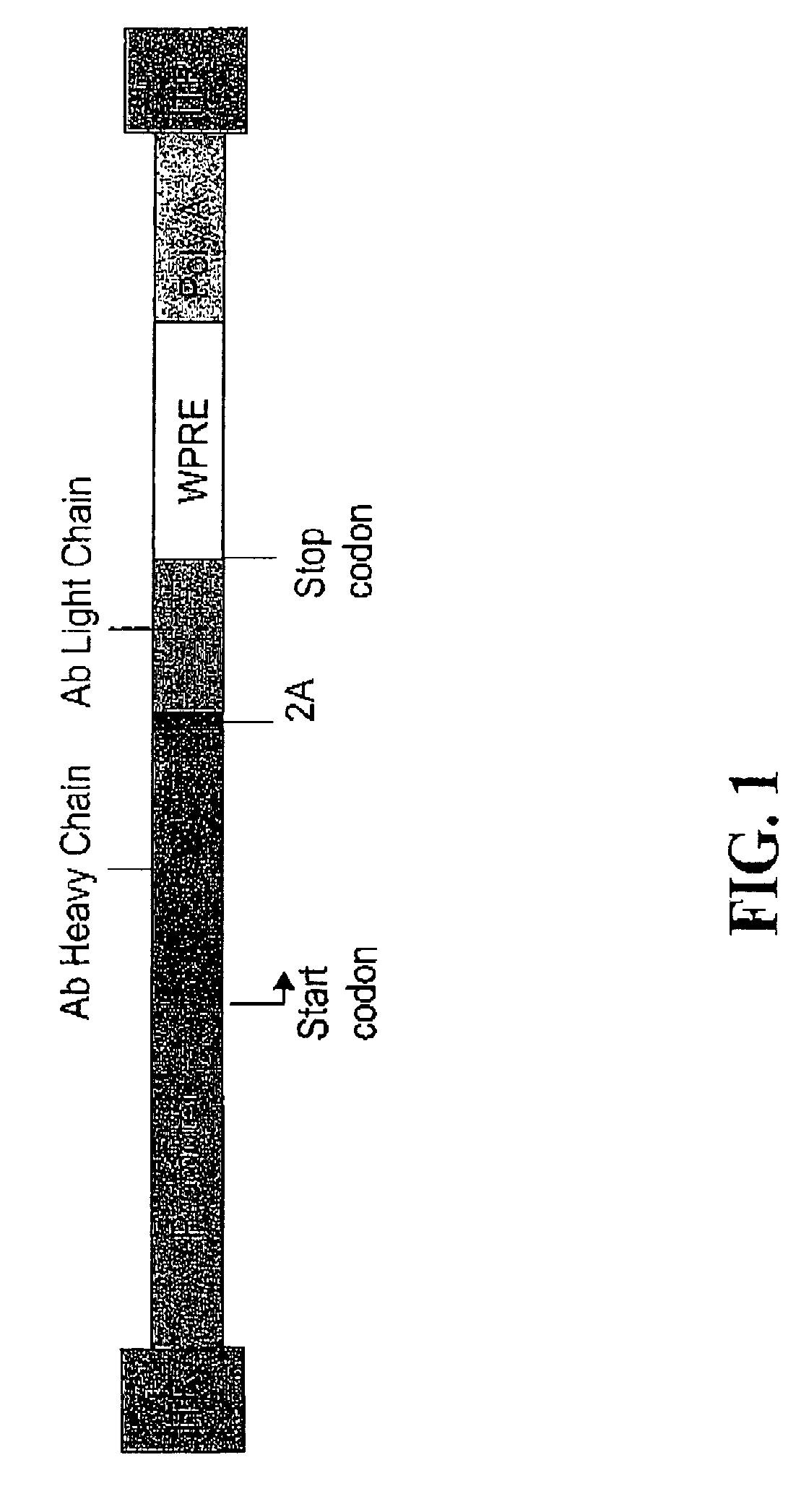
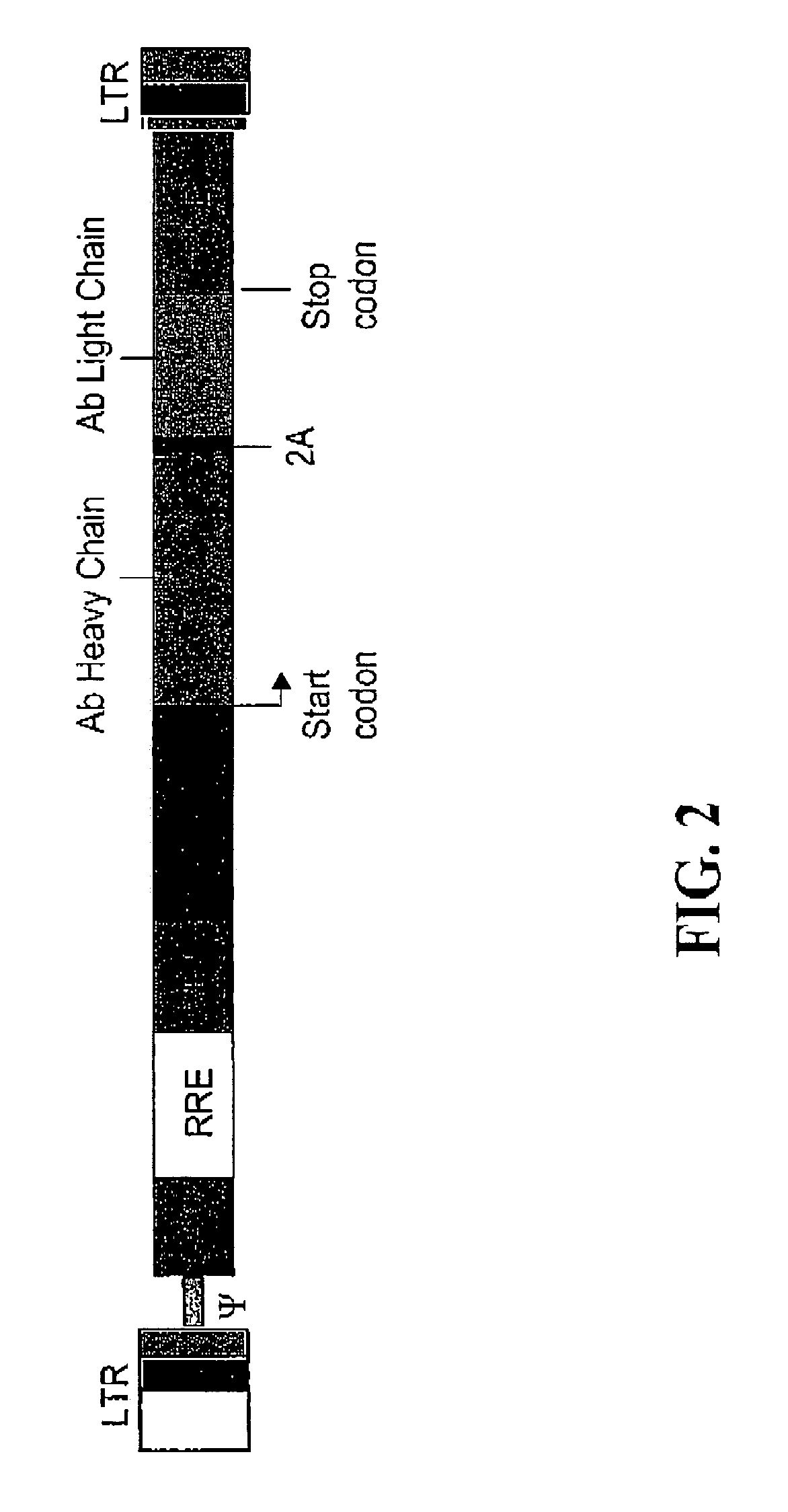
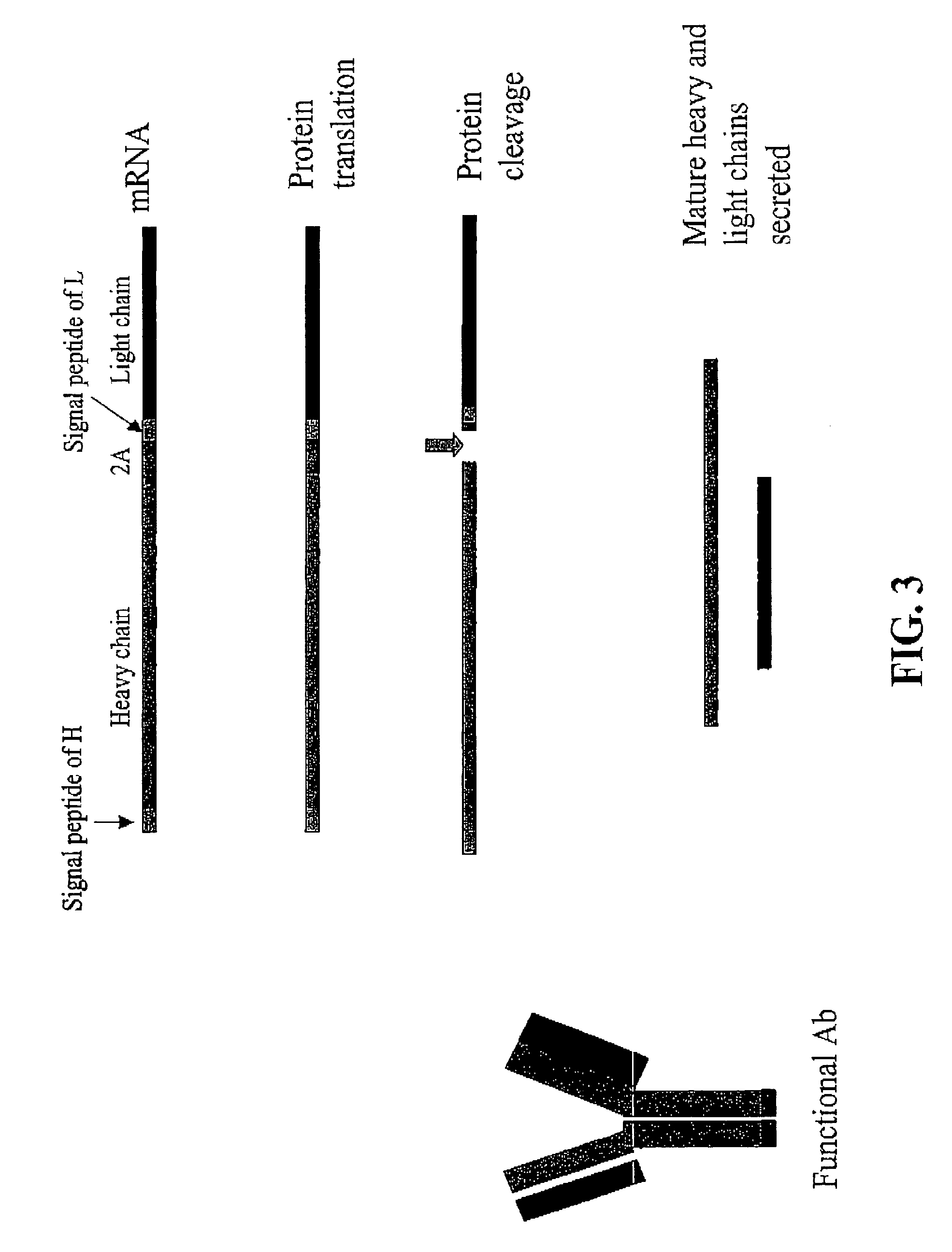
Compositions and methods for enhanced expression of immunoglobulins from a single vector using a peptide cleavage site
Single vector constructs for expression of an immunoglobulin molecule or fragment thereof and methods of making and using the same are described. The vectors comprise a self-processing cleavage sequence between a first and second immunoglobulin coding sequence allowing for expression of a functional antibody molecule using a single promoter. The vector constructs include the coding sequence for a self-processing cleavage site and may further include an additional proteolytic cleavage sequence which provides a means to remove the self processing peptide sequence from an expressed immunoglobulin molecule or fragment thereof. The vector constructs find utility in methods for enhanced production of biologically active immunoglobulins or fragments thereof in vitro or in vivo.
Owner:BIOSANTE PHARMA
Popular searches
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com