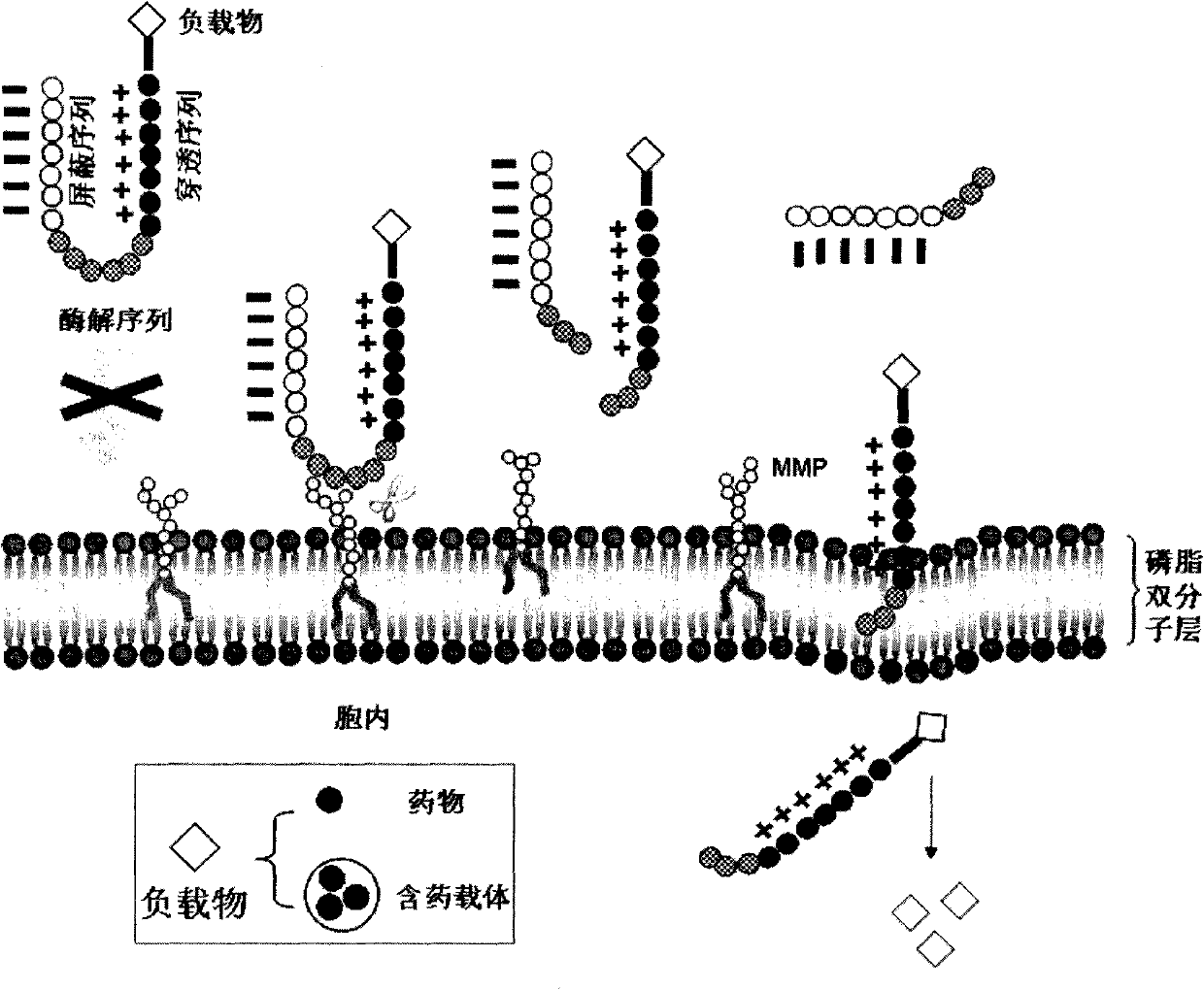
Method for enhancing targeting selectivity of administration system by modifying cell penetrating peptide
A drug delivery system, a technology for penetrating peptides, applied in pharmaceutical formulations, chemical instruments and methods, peptides, etc., can solve the problems of cytotoxicity, limited application, lack of cell selectivity, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Example 1: Enzymatic hydrolysis of cell-penetrating peptides that can activate
[0026] Design and synthesis of activating cell penetrating peptide, the sequence of activating cell penetrating peptide is EEEEEXPLGLAGRRRRRRXKC. Enzymatic hydrolysis of synthetic activatable cell penetrating peptides. Reversed-phase high performance liquid chromatography was used to monitor the enzymatic hydrolysis of type IV collagenase (containing MMP-2 and 9) on activating cell penetrating peptides at 37°C. Collect the enzymolysis components for matrix-assisted laser analysis and ionization time-of-flight mass spectrometry detection, and compare the sequence of the speculated enzymatic fragments. HPLC detects the peak position of the activatable cell-penetrating peptide and the changes in the spectrum after enzymatic hydrolysis. The activatable cell-penetrating peptide solutions of different concentrations all have an absorption peak at about 14.8 min (MALDI-TOF-MS detects the molecular w...
Embodiment 2
[0027] Example 2: Investigation on the anti-proliferation effect of activating cell penetrating peptide-doxorubicin copolymer on tumor cells
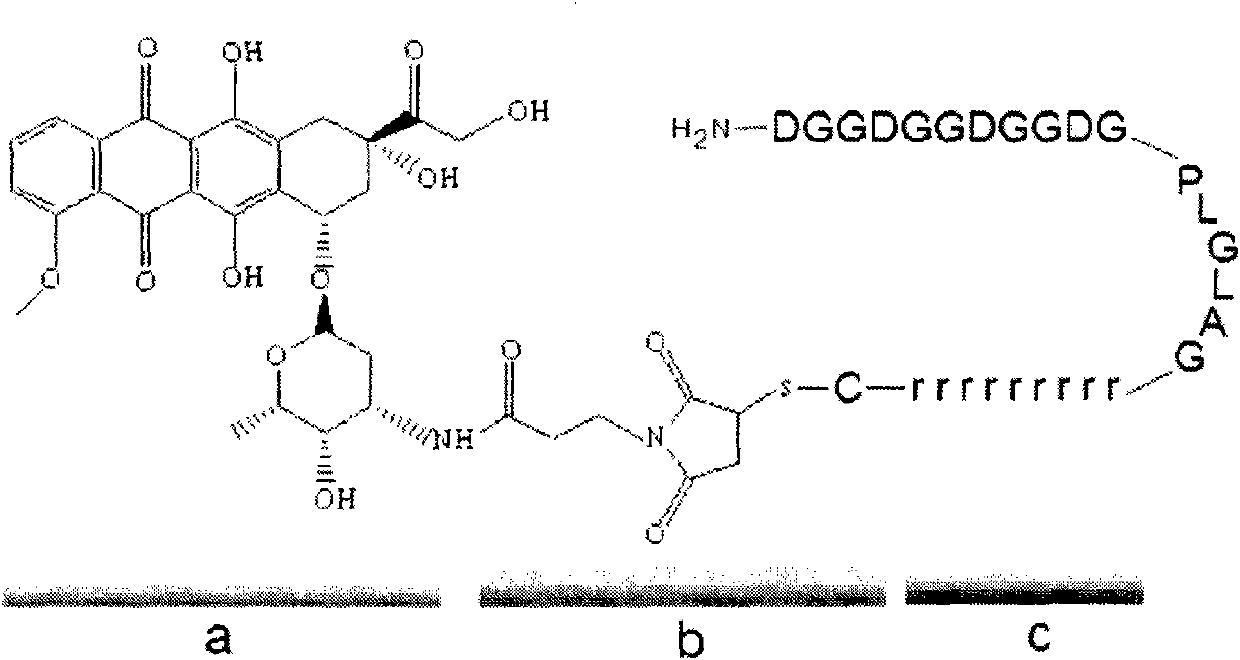
[0028] Can activate cell penetrating peptide, the sequence is DGDGDGDGGDGPLGLAGrrrrrrrrrC, and adriamycin through the intermediate linker 3-maleimidopropionic acid N-hydroxysuccinimide ester (CAS: 55750-62-4) chemical bond and form a copolymer (As attached figure 2 Shown). The specific process is as follows: dimethylformamide dissolves adriamycin and 3-maleimidopropionate N-hydroxysuccinimide ester respectively, adds triethylamine to adjust the pH value, stirs at room temperature and reacts for 2 hours and then pour In 50ml ether, wash the precipitated precipitate with anhydrous ether twice, centrifuge the precipitate and vacuum dry; take the precipitate and activating cell penetrating peptide to dissolve in dimethylformamide, add triethylamine, stir at room temperature for 2 hours Then it was poured into 10 ml of ether, the precipitated...
Embodiment 3
[0030] Example 3: Inhibitory effect of activating cell penetrating peptide-methotrexate copolymer on tumor cells
[0031] It can activate the cell penetrating peptide, the sequence is DGDDGGDGGDGPLGLAGrrrrrrrrrC, which forms a copolymer with methotrexate (as attached Figure 4 Shown).
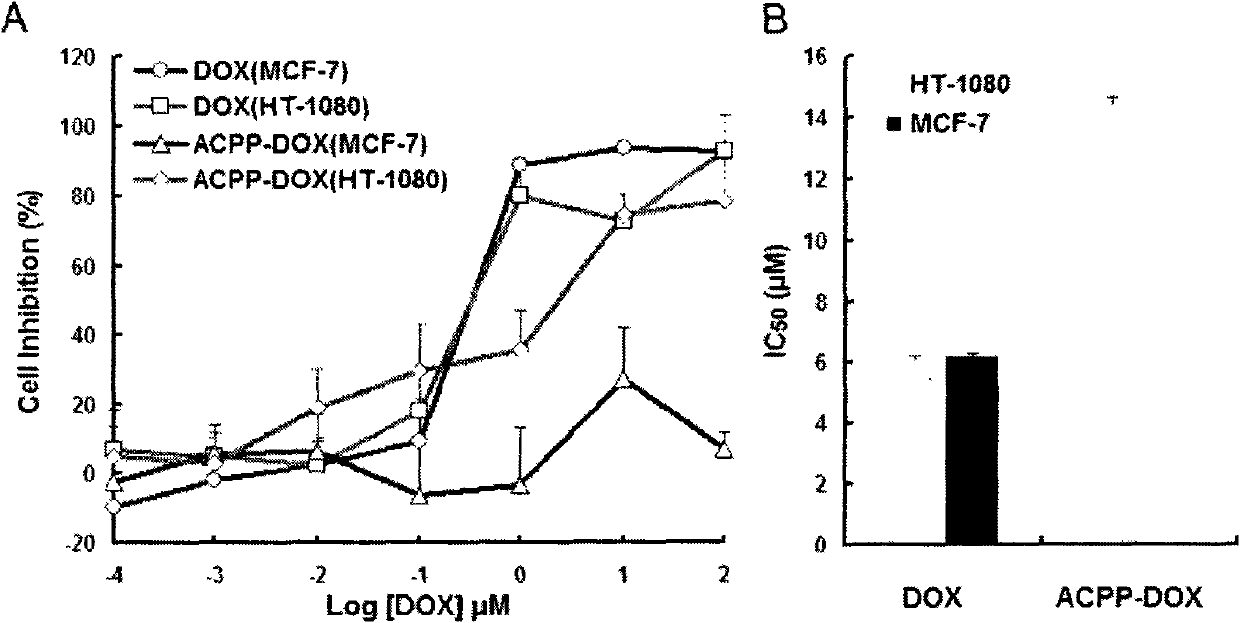
[0032] Human fibrosarcoma cell HT-1080, MMP enzyme is highly expressed, and has a specific degradation effect on the peptide PLGLAG; human breast cancer cell MCF-7, MMP enzyme expression is less. The two kinds of cells were cultured in appropriate culture medium to investigate the cytotoxicity of activating cell-penetrating peptide-methotrexate copolymer in HT-1080 and MCF-7 cells. The results showed that the free drug methotrexate has a strong cytotoxic effect in both types of cells, inhibiting cell growth; it can activate the cell penetrating peptide-methotrexate copolymer in HT-1080 cells and incubate The cell inhibition rate of 2h reached 20%, and the cell inhibition rate of 24h incubation was ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com