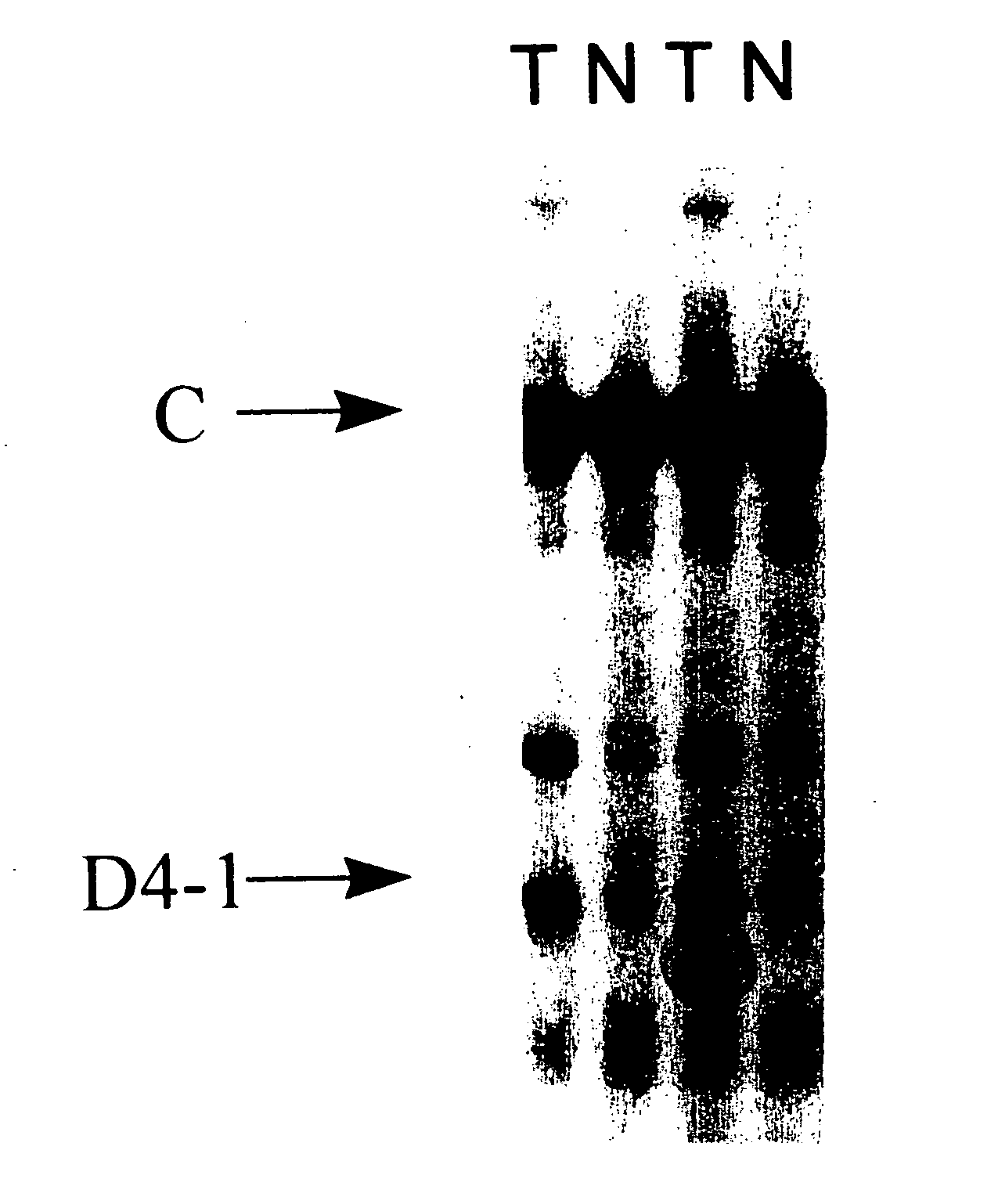
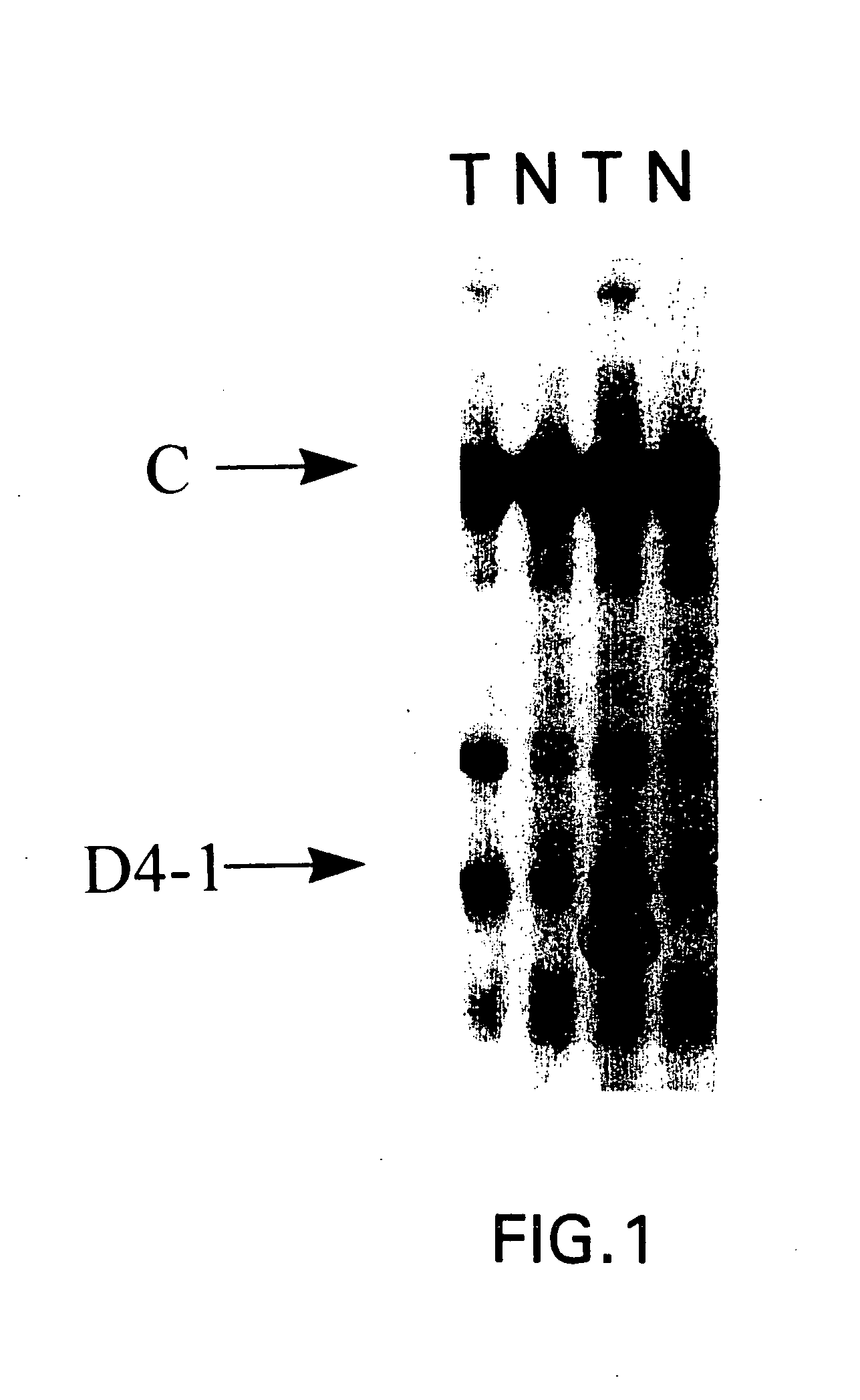
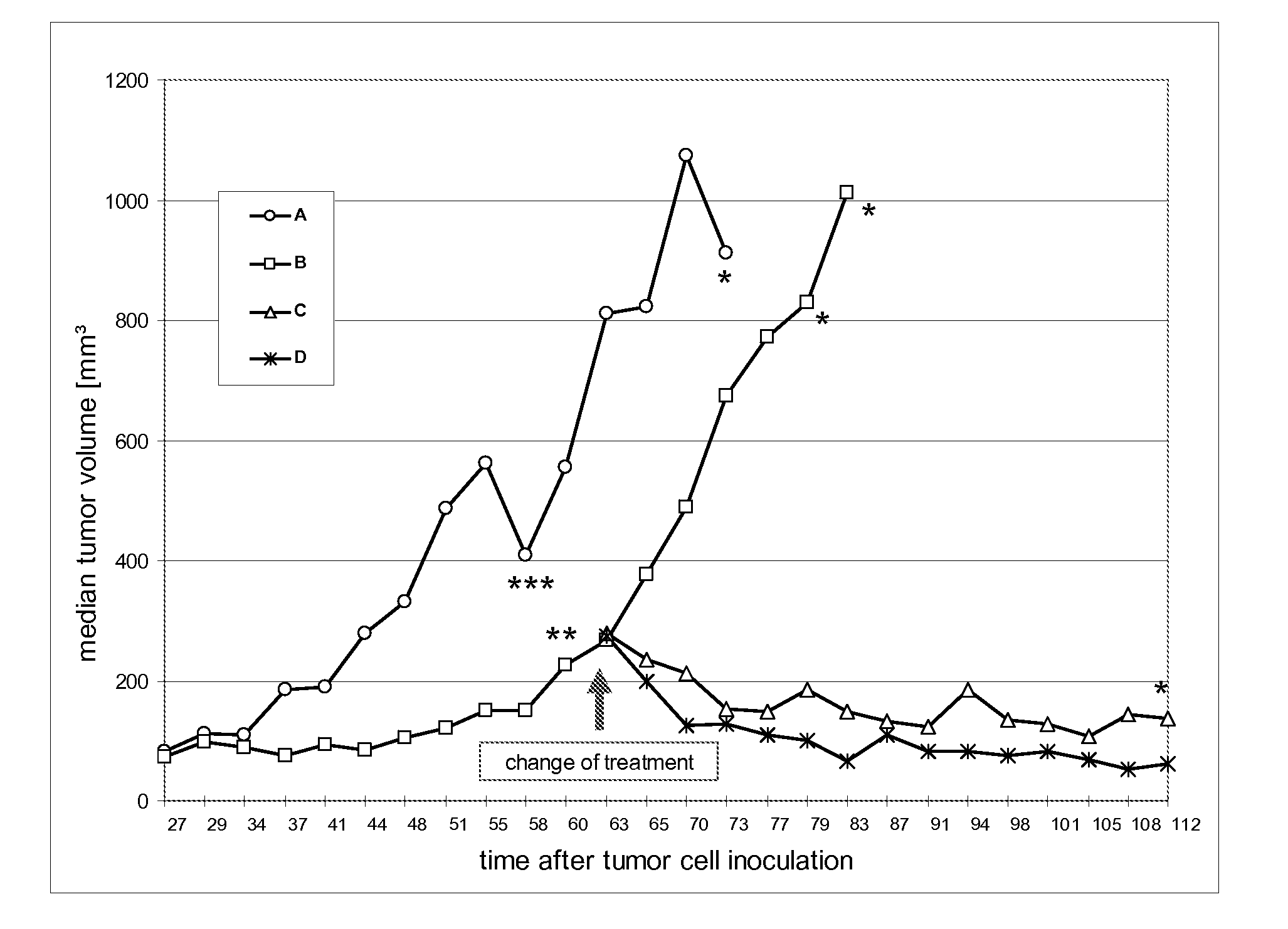
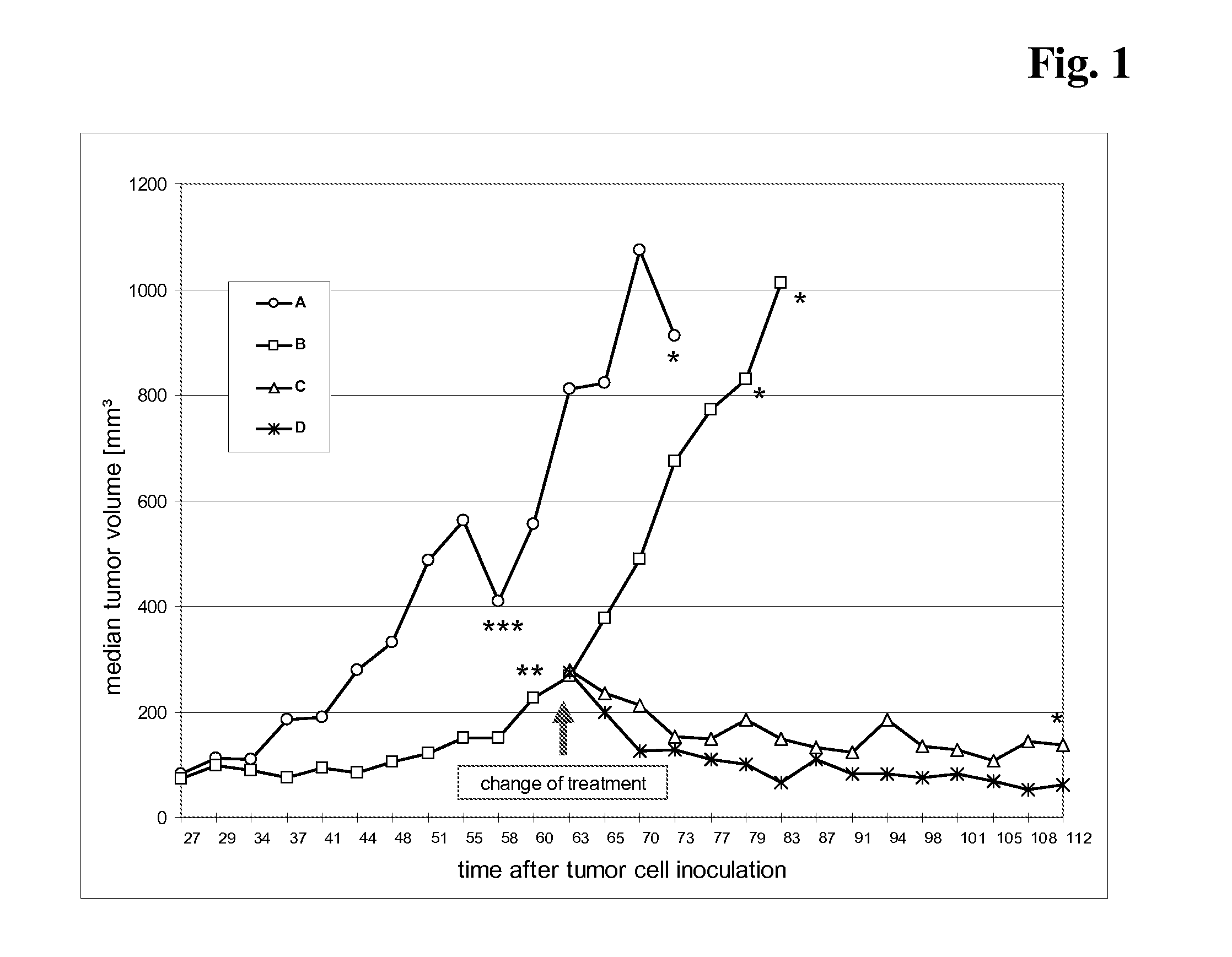
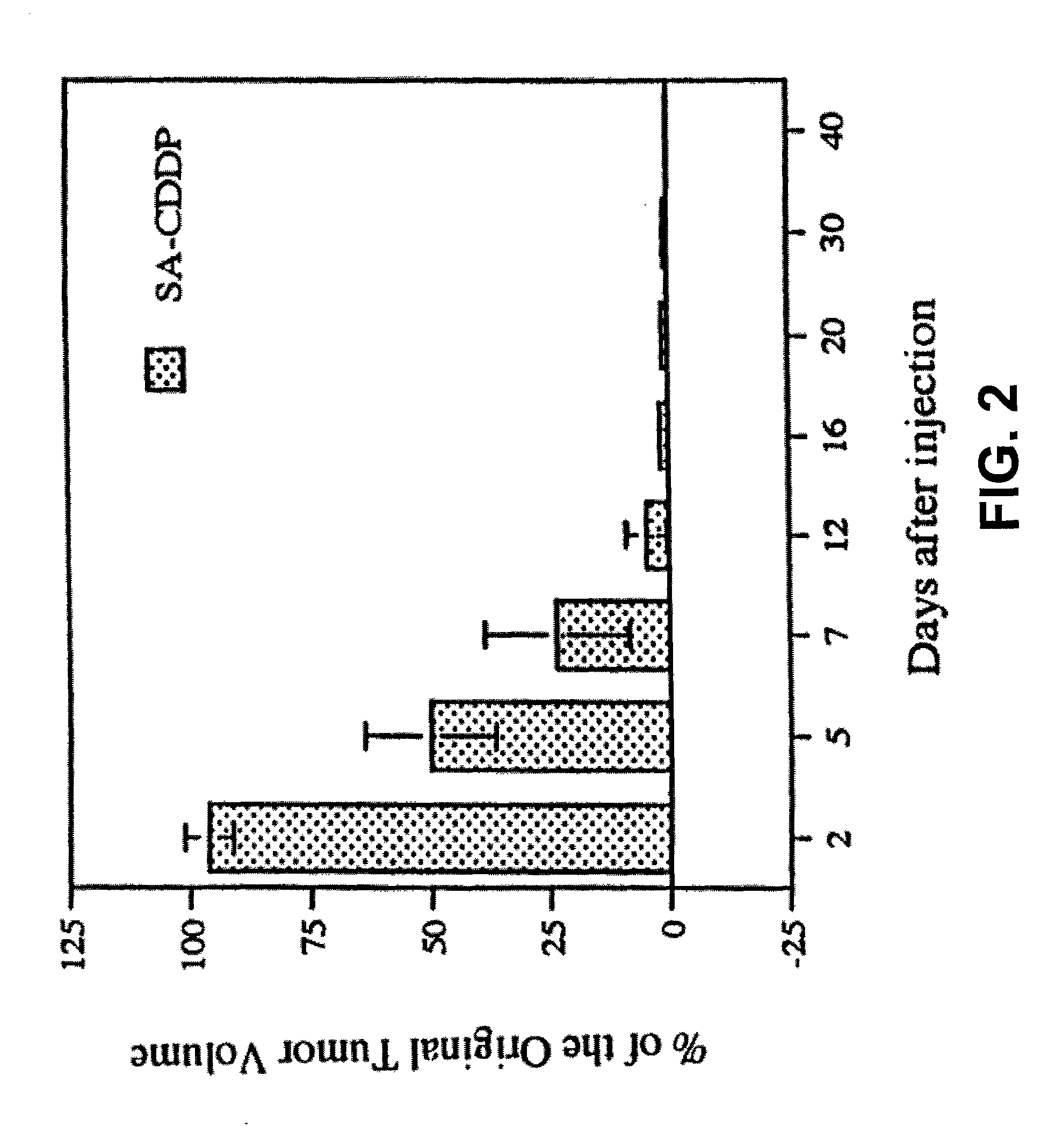
Patents
Literature
2216 results about "Tumor therapy" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Depending on the type and stage of cancer, treatments to eradicate the tumor or slow its growth may include some combination of surgery, radiation therapy, chemotherapy, hormone therapy or immunotherapy.
Methods and Antibody Compositions for Tumor Treatment
ActiveUS20150266966A1Decreased killing of T-cellsReduce the burden onImmunoglobulins against cell receptors/antigens/surface-determinantsAntibody ingredientsDiseaseCD20
The present invention provides bispecific antibodies that bind to CD3 and tumor antigens and methods of using the same. According to certain embodiments, the bispecific antibodies of the invention exhibit reduced effector functions and have a unique binding profile with regard to Fcγ receptors. The bispecific antibodies are engineered to efficiently induce T cell-mediated killing of tumor cells. According to certain embodiments, the present invention provides bispecific antigen-binding molecules comprising a first antigen-binding domain that specifically binds human CD3, a second antigen-binding molecule that specifically binds human CD20, and an Fc domain that binds Fcγ receptors with a specific binding pattern. In certain embodiments, the bispecific antigen-binding molecules of the present invention are capable of inhibiting the growth of B-cell or melanoma tumors expressing CD20. The bispecific antibodies of the invention are useful for the treatment of various cancers as well as other CD20-related diseases and disorders.
Owner:REGENERON PHARM INC
Method for diagnosis and treatment of prostate cancer
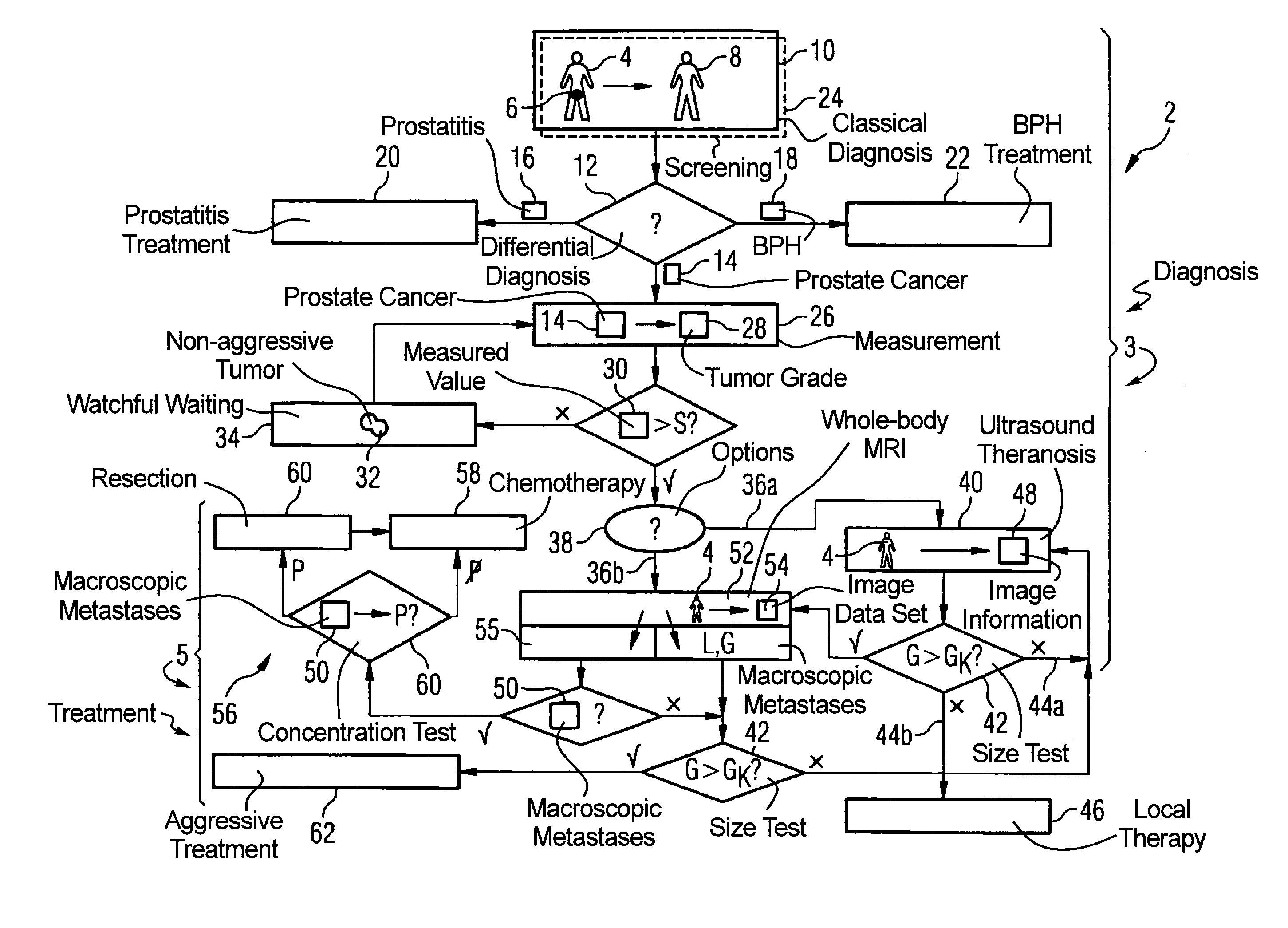
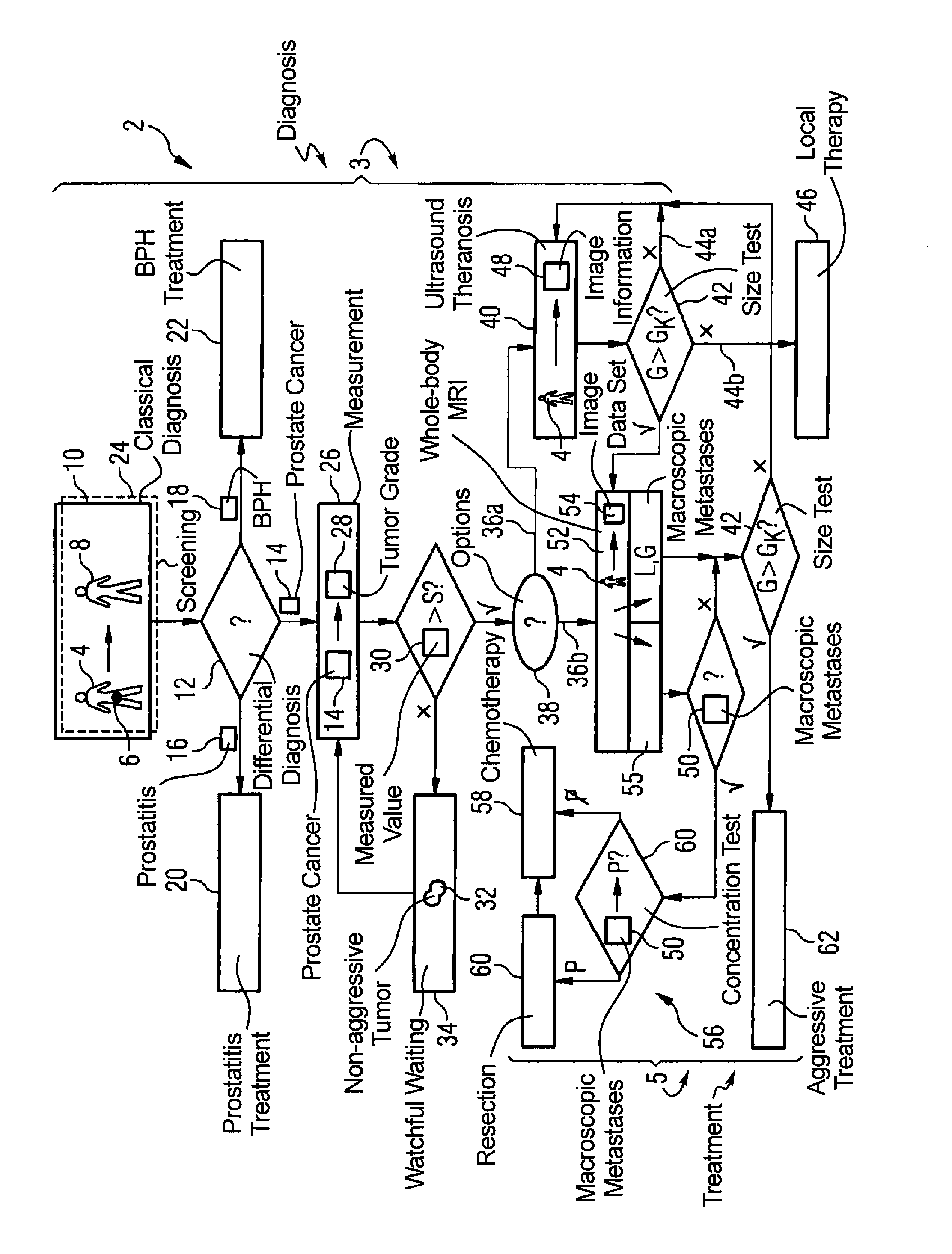
InactiveUS20090062645A1Easy to useUltrasound therapyOrgan movement/changes detectionLymphatic SpreadTumor therapy
In a method for diagnosis and treatment of a patient with a tumor relating to prostate cancer, the following steps are implemented. A differential diagnosis of prostate cancer versus prostatitis and / or BPH is conducted on a patient using a cost-effective diagnosis method. If prostate cancer is diagnosed in the patient using a cost-effective measurement method, a characteristic value for the tumor aggressiveness of the prostate cancer is determined. A watchful waiting treatment is implemented with the patient given a characteristic value below a predeterminable first limit value. The size and position of the tumor is determined using a cost-effective method given a characteristic value above the first limit value. A cost-effective ultrasonic theranosis or a conventional therapy is conducted for a size below a second predeterminable limit value. The presence of metastases in the patient is checked, with a cost-intensive method generating image information, for a size above the second limit value. A metastasis treatment is implemented in the event that metastases are present. In the event that no metastases are present, a tumor treatment based on the aforementioned image information generated is implemented.
Owner:SIEMENS AG
Tumor targeting drug-loaded particles
A composition for delivering a tumor therapeutic agent to a patient includes a fast-release formulation of a tumor apoptosis inducing agent, a slow-release formulation of a tumor therapeutic agent, and a pharmaceutically acceptable carrier. An apoptosis-inducing agent in a pharmaceutically acceptable carrier may be administered before or concomitantly therewith. Nanoparticles or microparticles (e.g., cross-linked gelatin) of the therapeutic agent (e.g., paclitaxel) also may be used. The nanoparticles or microparticles may be coated with a bioadhesive coating. Microspheres that agglomerate to block the entrance of the lymphatic ducts of the bladder to retard clearance of the microparticles through the lymphatic system also may be employed. This invention also uses drug-loaded gelatin and poly(lactide-co-glycolide) (PLGA) nanoparticles and microparticles to target drug delivery to tumors in the peritoneal cavity, bladder tissues, and kidneys.
Owner:AU JESSIE L S +1
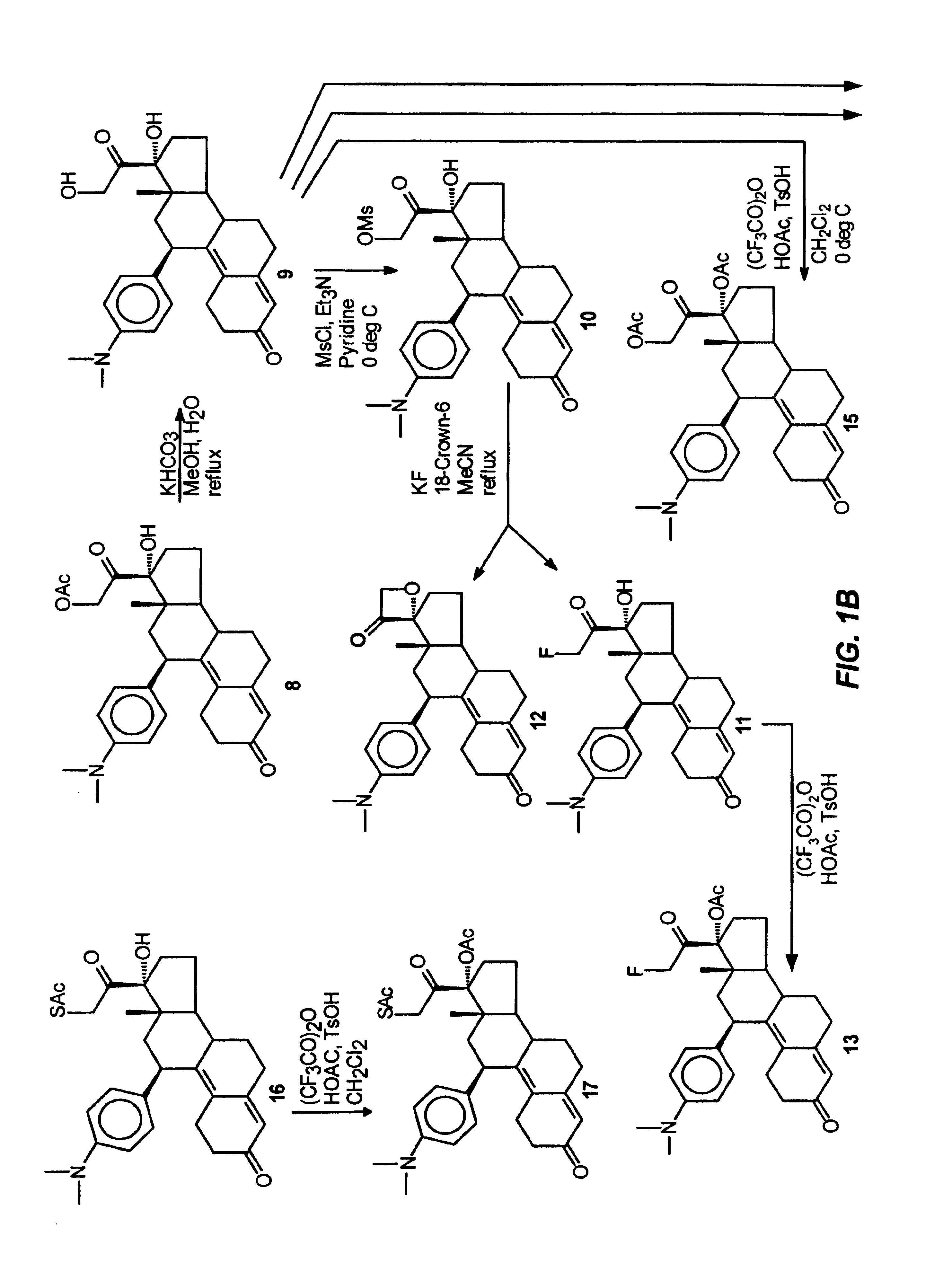
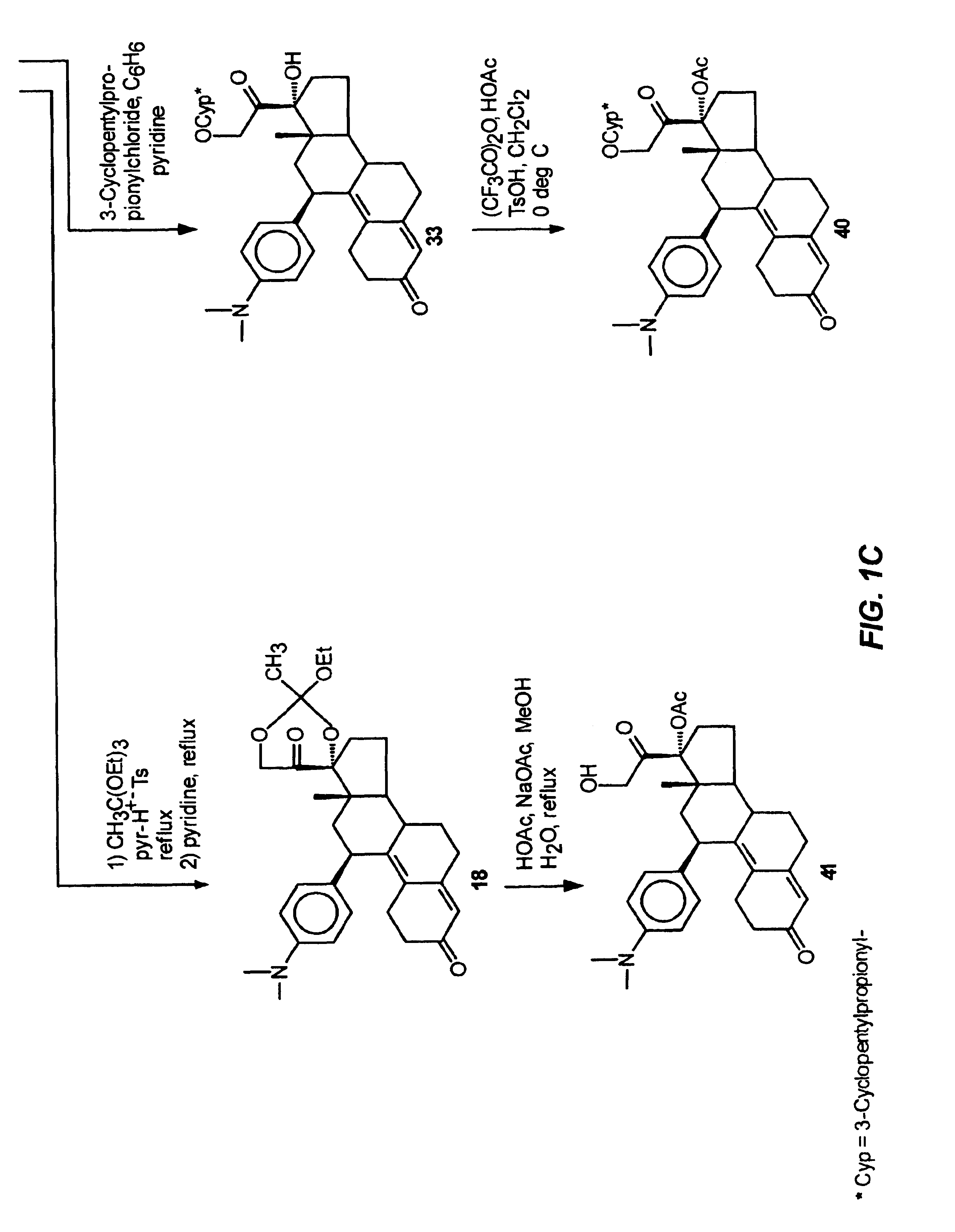
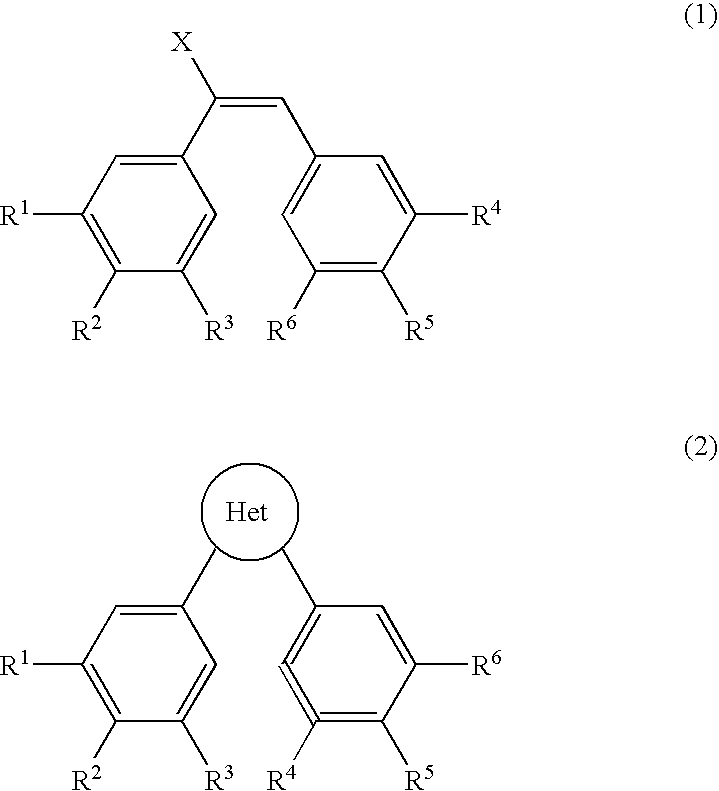
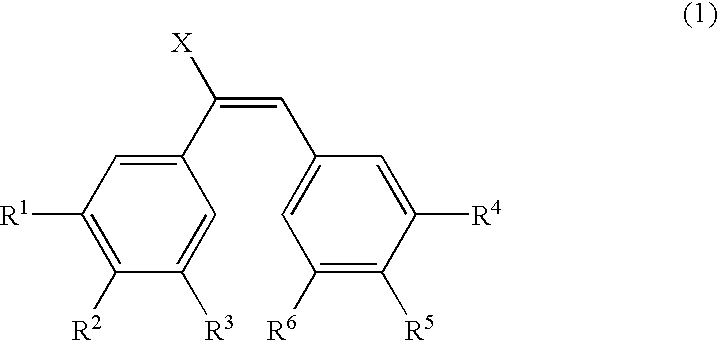
Structural modification of 19-norprogesterone I: 17-α-substituted-11-β-substituted-4-aryl and 21-substituted 19-norpregnadienedione as new antiprogestational agents
The present invention relates, inter alia, to compounds having the general formula: in which: R1 is a member selected from the group consisting of —OCH3, —SCH3, —N(CH3)2, —NHCH3, —NC4H8, —NC5H10, —NC4H8O, —CHO, —CH(OH)CH3, —C(O)CH3, —O(CH2)2N(CH3)2, and —O(CH2)2NC5H10; R2 is a member selected from the group consisting of hydrogen, halogen, alkyl, acyl, hydroxy, alkoxy (e.g., methoxy, ethoxy, vinyloxy, ethynyloxy, cyclopropyloxy, etc.), acyloxy (e.g., acetoxy, glycinate, etc.), alkylcarbonate, cypionyloxy, S-alkyl, —SCN, S-acyl and —OC(O)R6, wherein R6 is a functional group including, but not limited to, alkyl (e.g., methyl, ethyl, etc.), alkoxy ester (e.g., —CH2OCH3) and alkoxy (—OCH3); R3 is a member selected from the group consisting of alkyl, hydroxy, alkoxy and acyloxy; R4 is a member selected from the group consisting of hydrogen and alkyl; and X is a member selected from the group consisting of ═O and ═N—OR5, wherein R5 is a member selected from the group consisting of hydrogen and alkyl.In addition to providing the compounds of Formula I, the present invention provides methods wherein the compounds of Formula I are advantageously used, inter alia, to antagonize endogenous progesterone; to induce menses; to treat endometriosis; to treat dysmenorrhea; to treat endocrine hormone-dependent tumors; to treat meningiomas; to treat uterine leiomyomas; to treat uterine fibroids; to inhibit uterine endometrial proliferation; to induce cervical ripening; to induce labor; and for contraception.
Owner:HEALTH & HUMAN SERVICES THE GOVERNMENT OF THE US SEC THE DEPT OF
Treatment of B-cell associated diseases such as malignancies and autoimmune diseases using a cold anti-CD20 antibody/radiolabeled anti-CD22 antibody combination
InactiveUS20050112060A1Enhanced killing and depletionPrevent and inhibit relapseRadioactive preparation carriersAntibody ingredientsAutoimmune conditionRegimen
Owner:BIOGEN INC
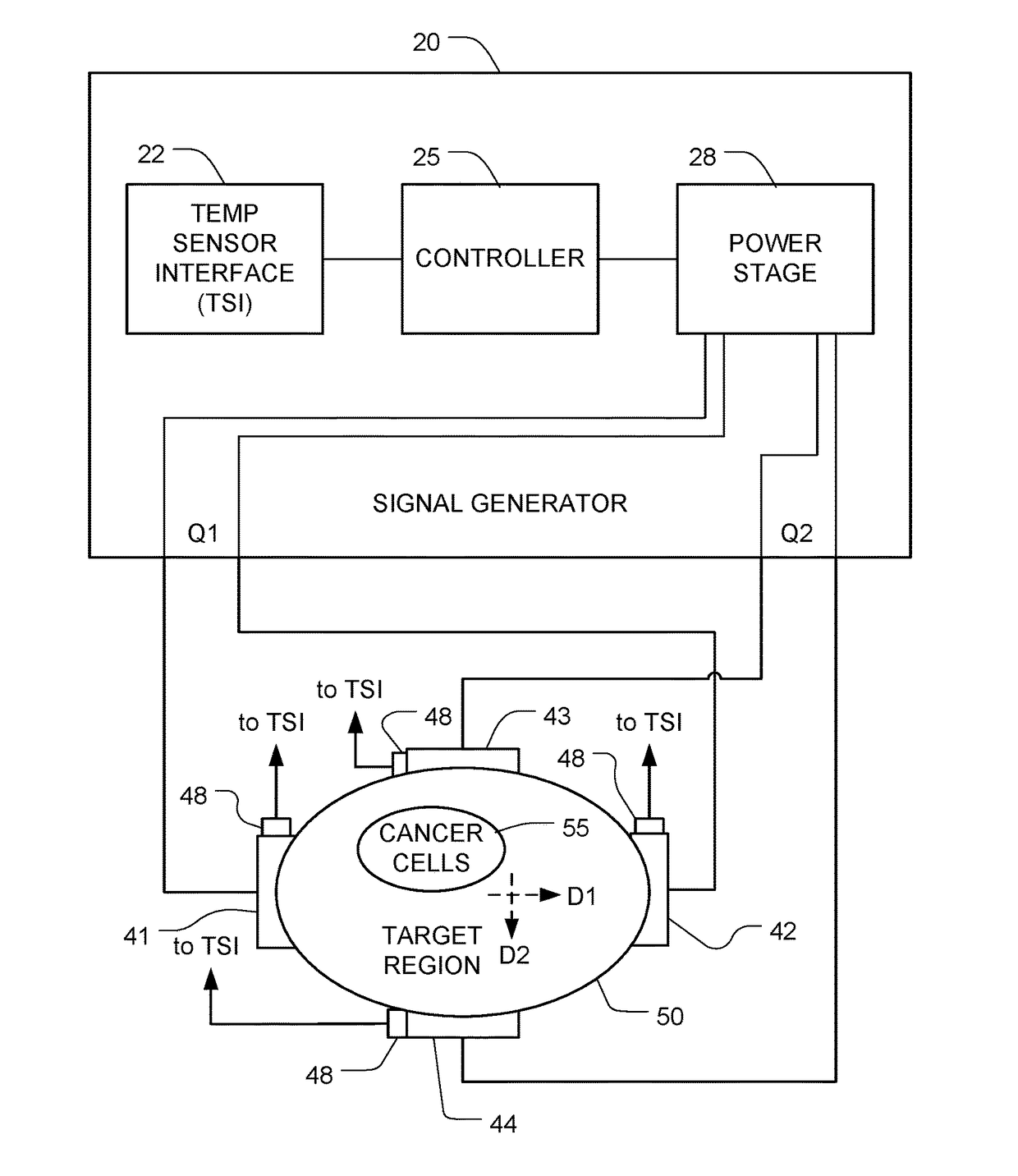
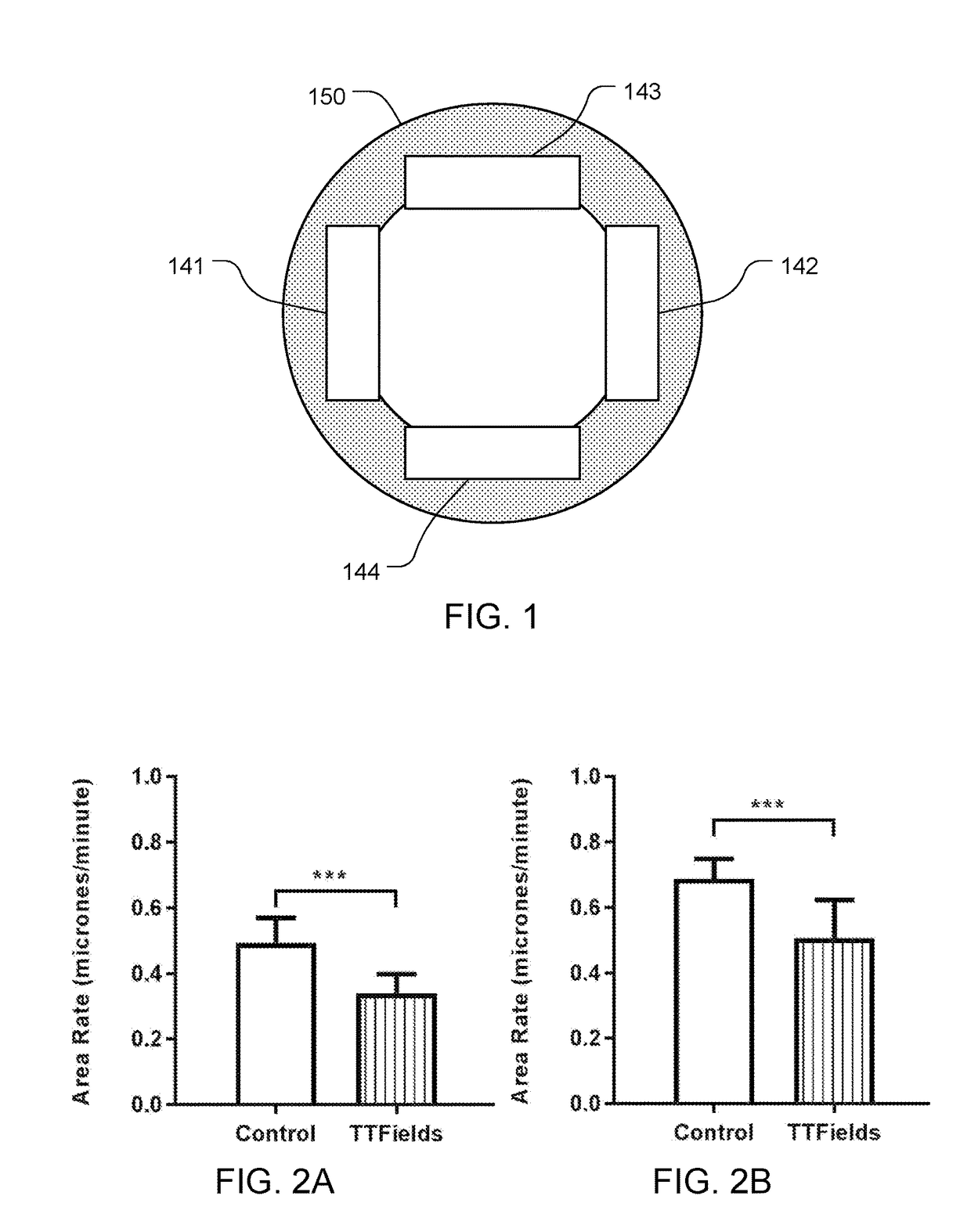
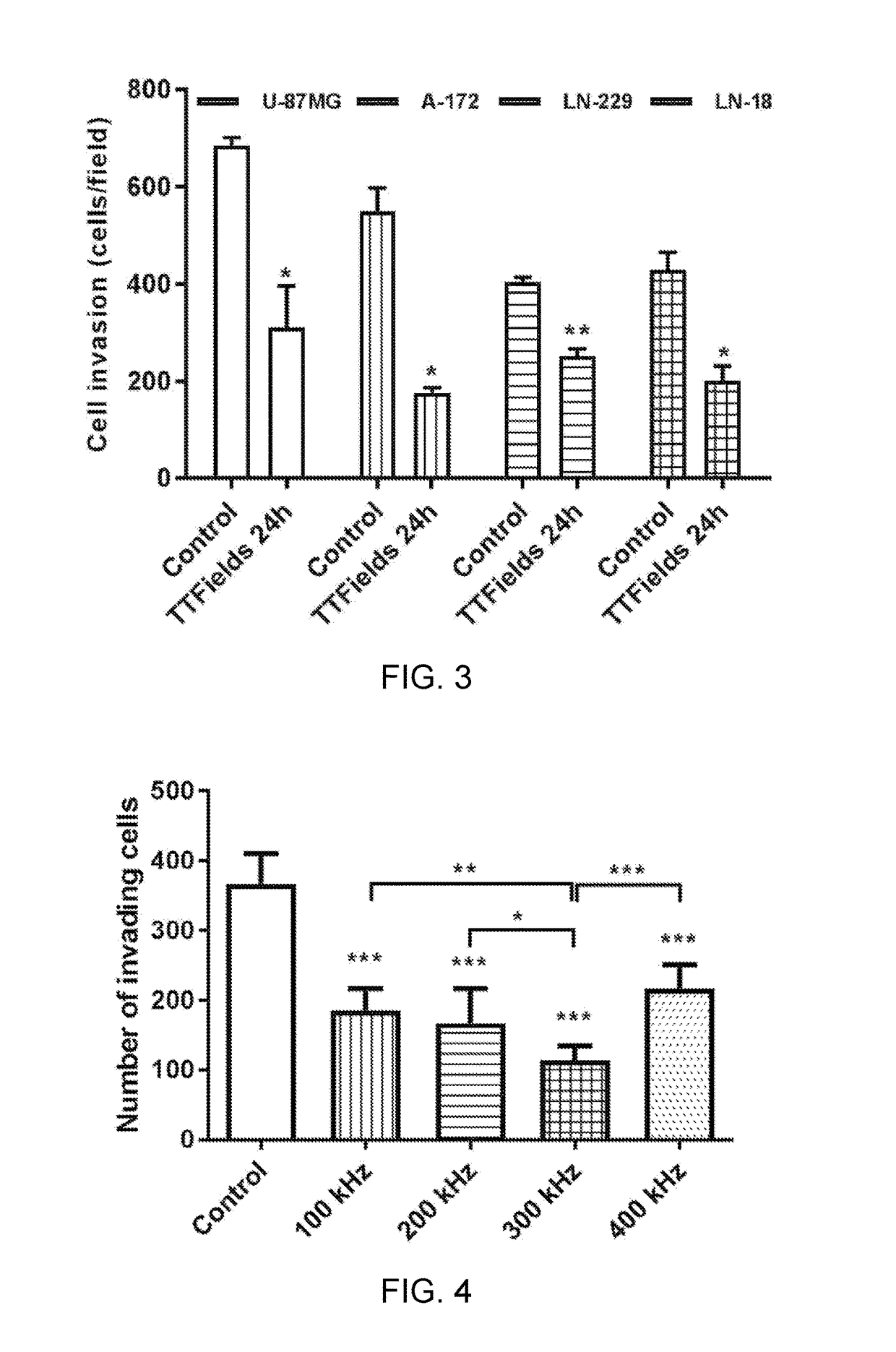
Reducing Motility of Cancer Cells Using Tumor Treating Fields (TTFields)
ActiveUS20170281934A1Reduce exerciseAvoid spreadingOrganic active ingredientsElectrotherapyCancer cellMotility
The spreading of cancer cells in a target region can be inhibited by imposing a first AC electric field in the target region for a first interval of time, with a frequency and amplitude selected to disrupt mitosis of the cancer cells; and imposing a second AC electric field in the target region for a second interval of time, with a frequency and the amplitude selected to reduce motility of the cancer cells. The amplitude of the second AC electric field is lower than the amplitude of the first AC electric field.
Owner:NOVOCURE GMBH
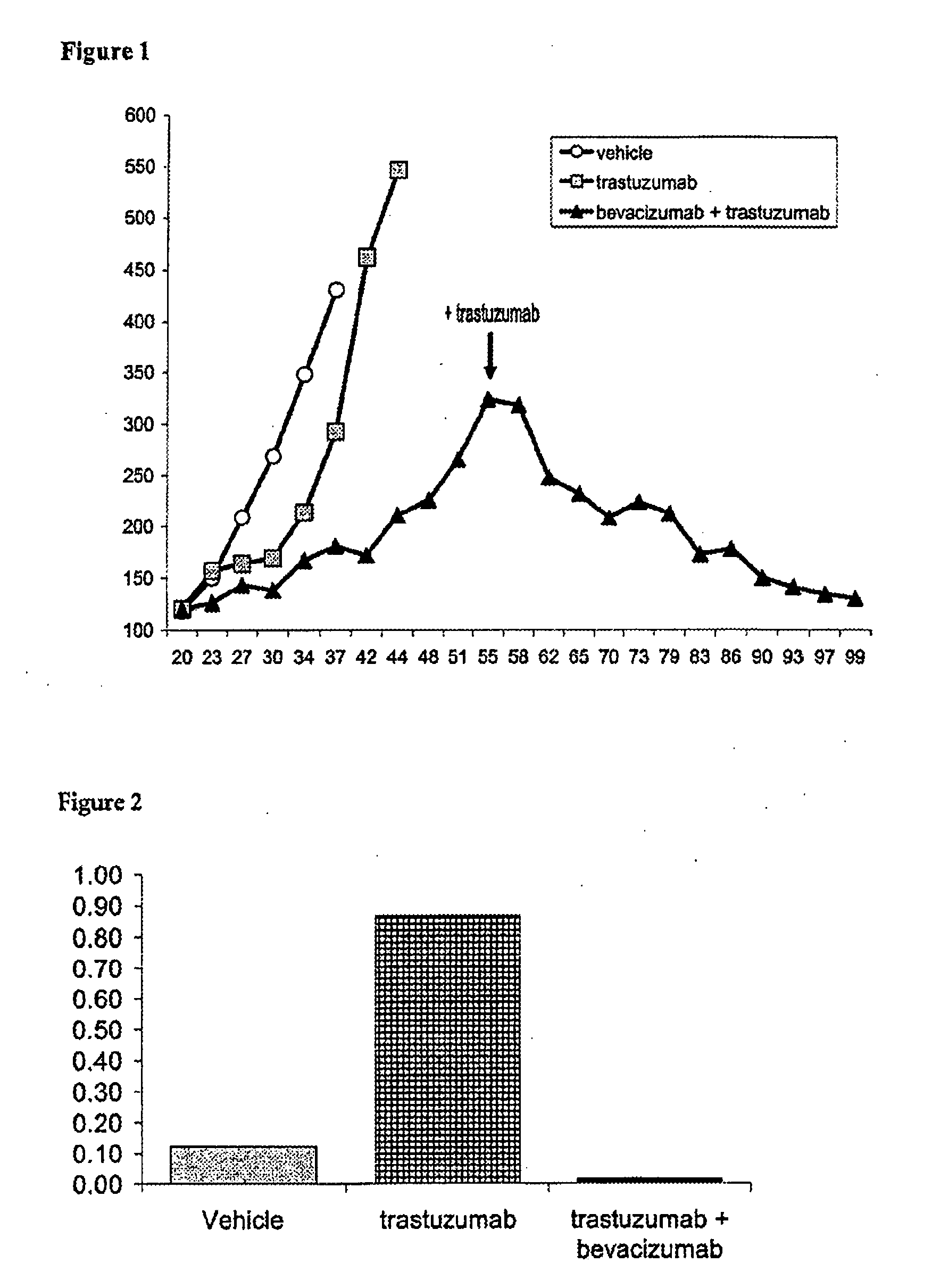
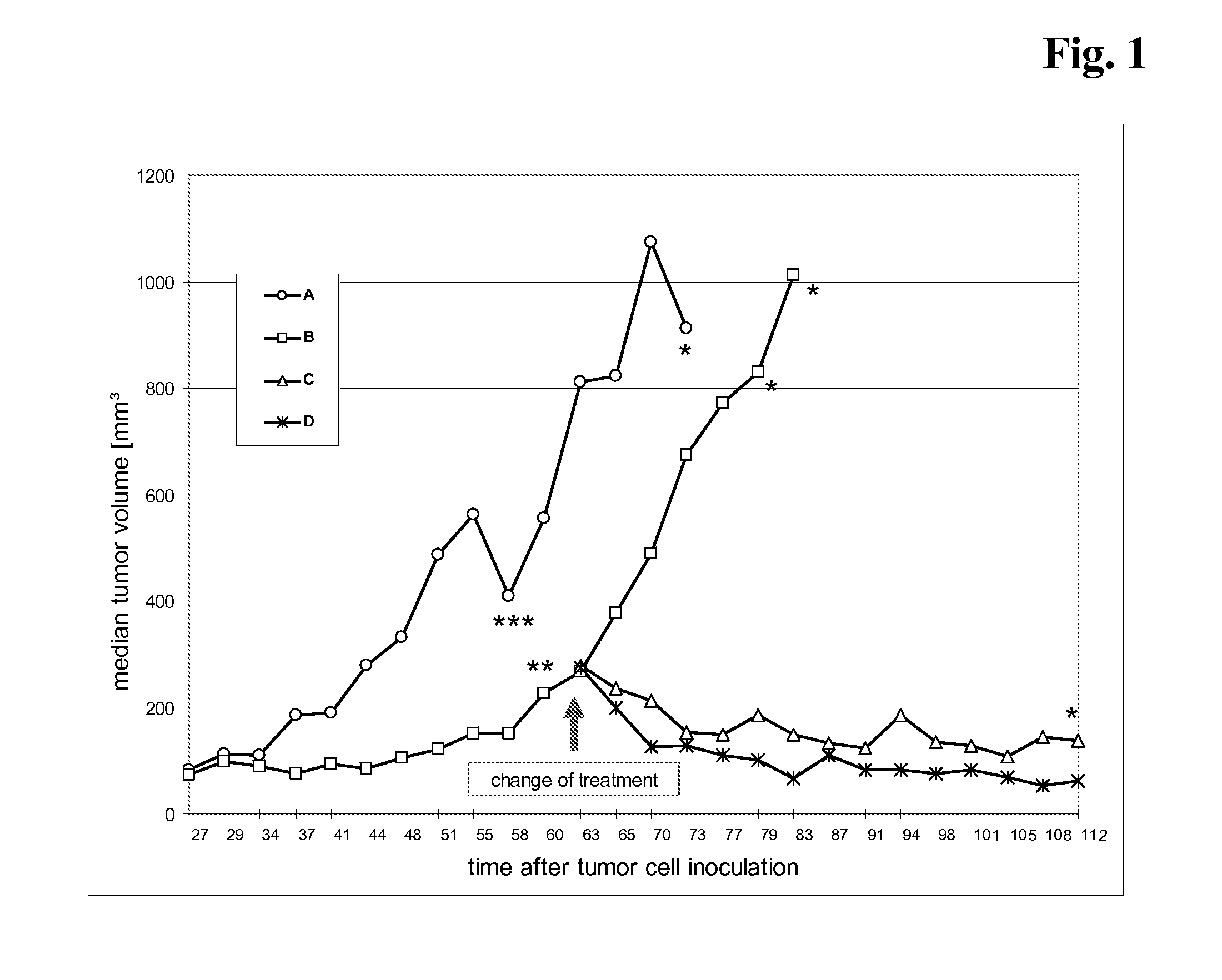
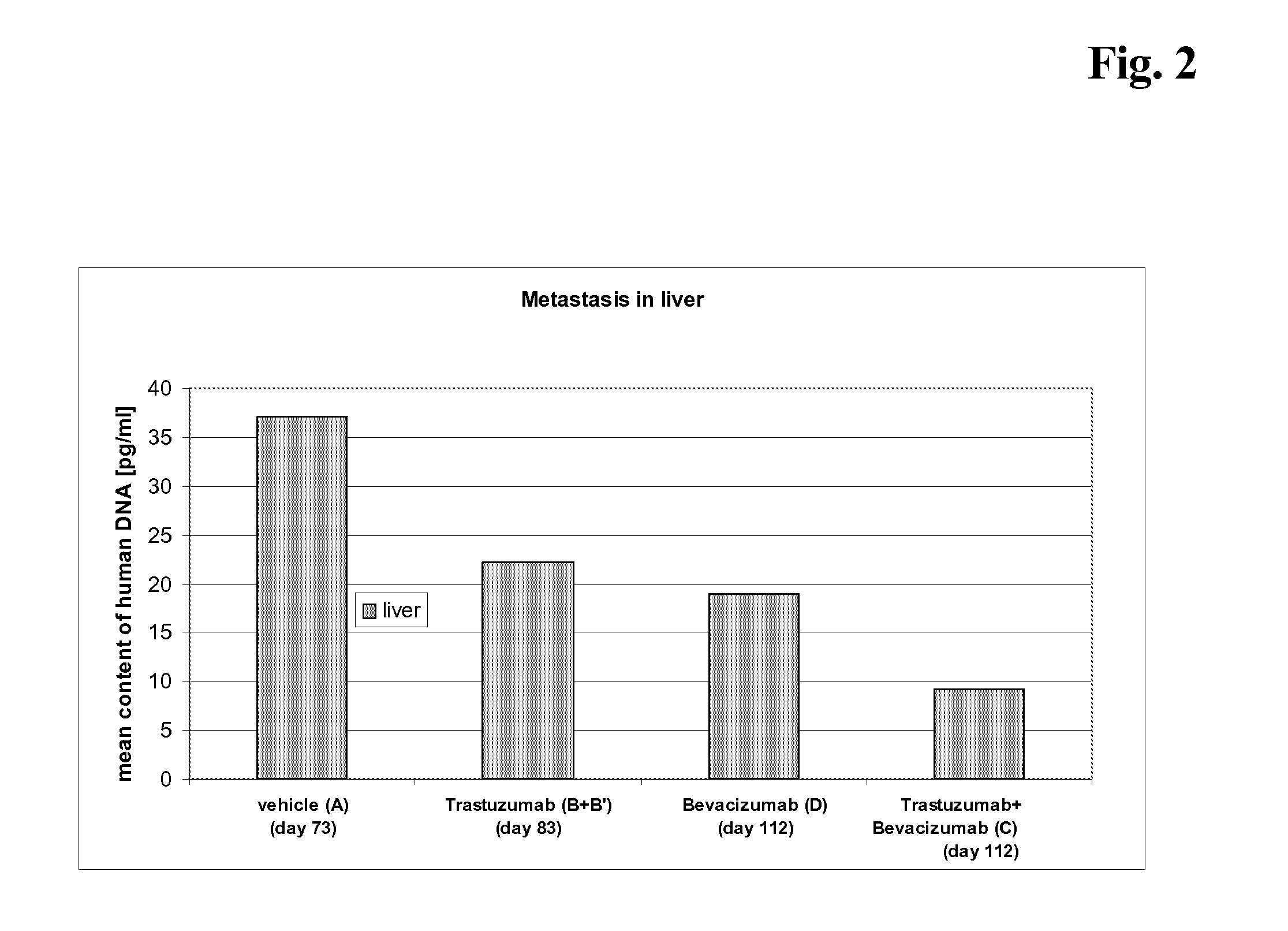
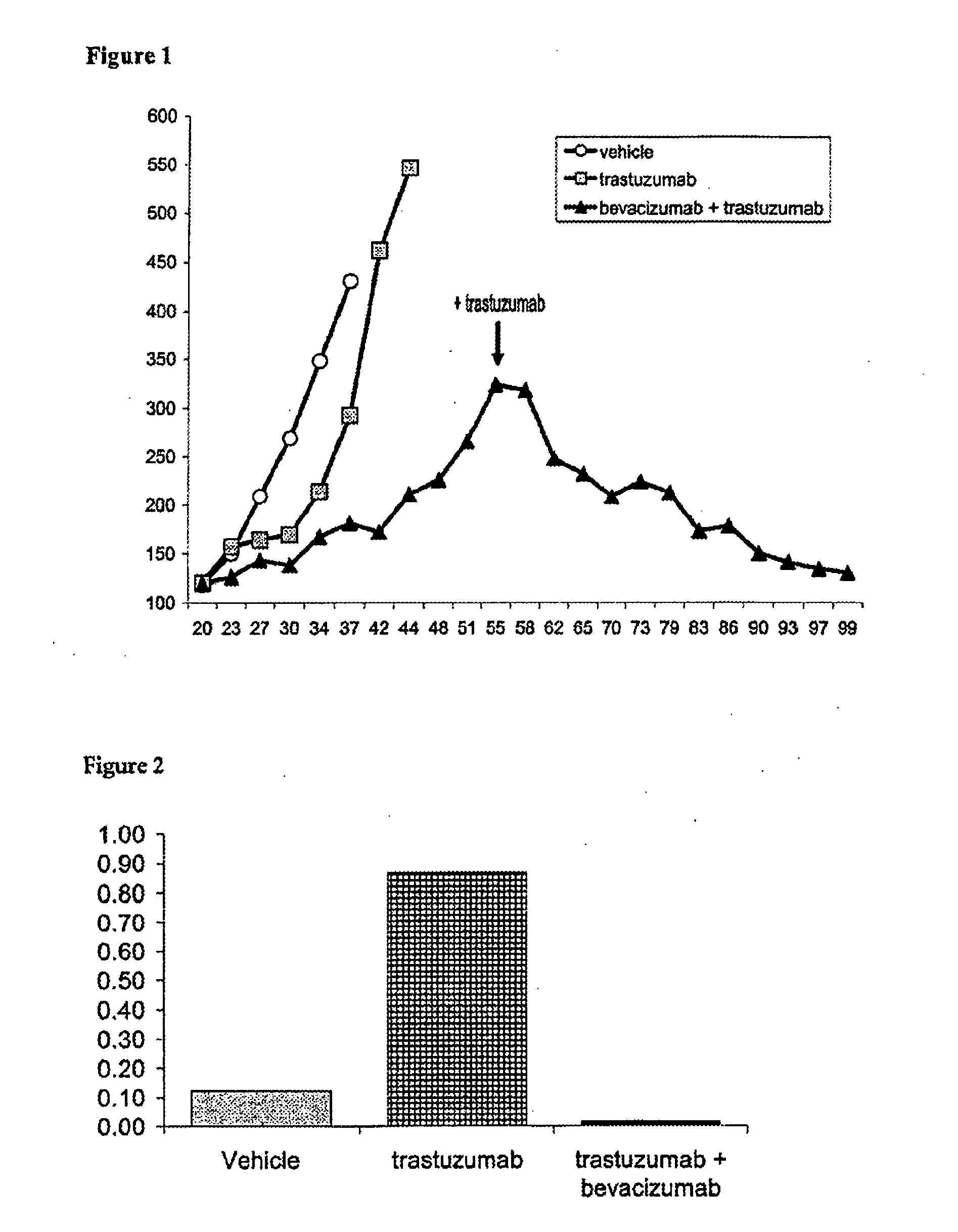
Tumor therapy with an antibody for vascular endothelial growth factor and an antibody for human epithelial growth factor receptor type 2
InactiveUS20070224203A1Prolong survival timeProlong progression-free survivalImmunoglobulins against growth factorsAntibody ingredientsDiseaseTumor therapy
The present invention provides a method of treating a breast cancer disease in a patient who has failed prior treatment with an anti-VEGF antibody, comprising administering to the patient a therapeutically effective amount of an anti-HER2 antibody while continuing said anti-VEGF antibody therapy. The invention also provides corresponding pharmaceutical kits and pharmaceutical compositions.
Owner:F HOFFMANN LA ROCHE & CO AG
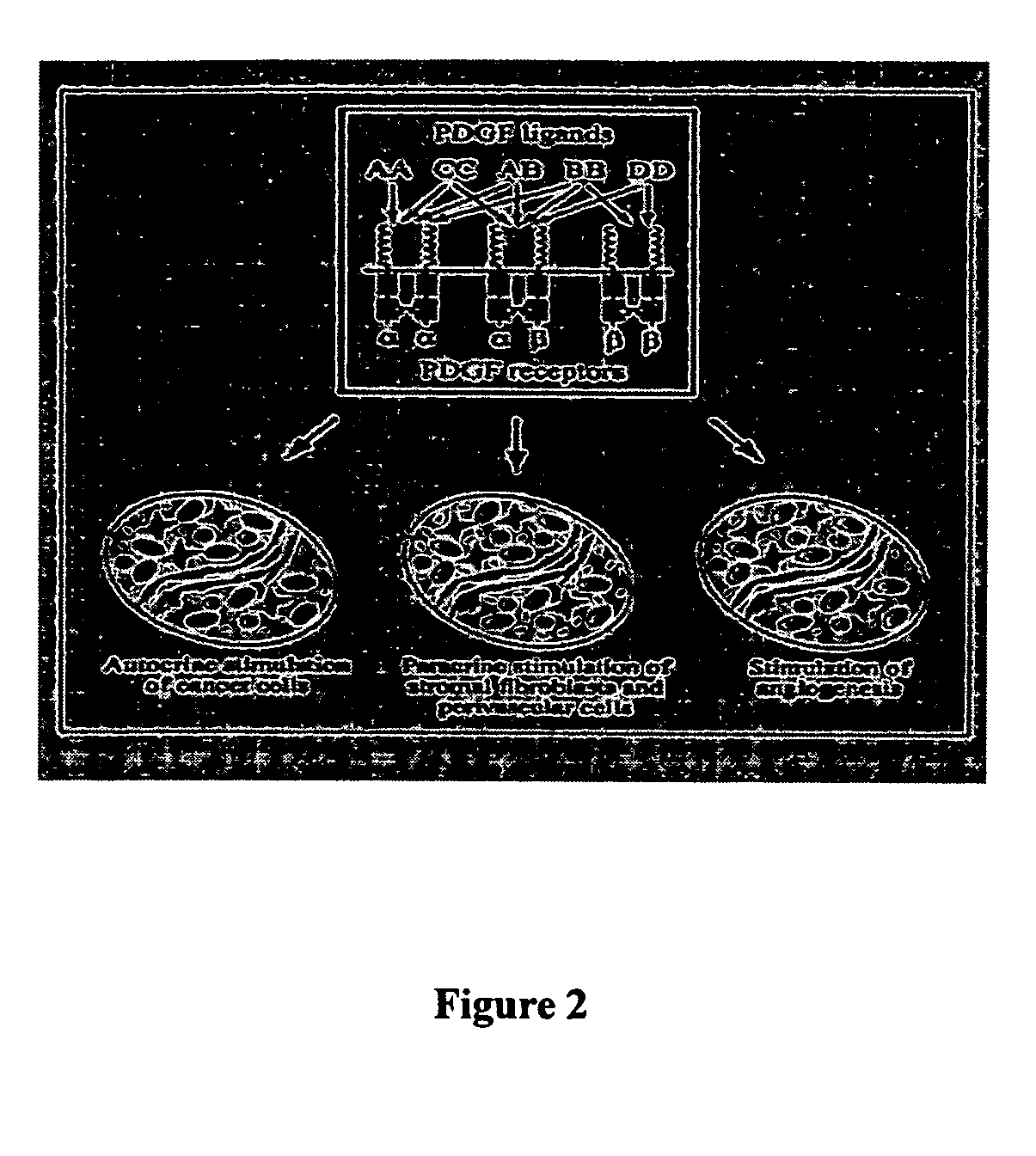
Stabilized aptamers to platelet derived growth factor and their use as oncology therapeutics
Materials and methods are provided for producing and using aptamers useful as oncology therapeutics capable of binding to PDGF, PDGF isoforms, PDGF receptor, VEGF, and VEGF receptor or any combination thereof with great affinity and specificity. The compositions of the present invention are particularly useful in solid tumor therapy and can be used alone or in combination with known cytotoxic agents for the treatment of solid tumors. Also disclosed are aptamers having one or more CpG motifs embedded therein or appended thereto.
Owner:ARCHEMIX CORP
Cytotoxic CD44 antibody immunoconjugates
The invention relates to novel conjugates of CD44 antibodies with cytotoxic compounds, pharmaceutical compositions comprising such compounds, and their use in tumor therapy.
Owner:BOEHRINGER INGELHEIM INT GMBH
Nr-CAM gene, nucleic acids and nucleic acid products for therapeutic and diagnostic uses for tumors
InactiveUS20060269558A1Microbiological testing/measurementAntibody ingredientsGene expressionTransformed cell
The present invention relates to the identification of a novel role of Nr-CAM in cell transformation and aberrant cellular proliferation. In particular, the present invention relates to the altered gene expression of Nr-CAM in a number of primary tumors and cell lines derived from tumors, in addition to, the altered gene expression of ligands for Nr-CAM. Further, the present invention relates, in part, to the Applicants' surprising discovery that the inhibition of Nr-CAM gene expression or the inhibition of Nr-CAM activity in transformed cells reverses the transformed phenotype.
Owner:MURPHY GERALD +3
Tumor therapy with an Anti-vegf antibody
InactiveUS20080050385A1Prevents and reduces metastasisImmunoglobulins against growth factorsAntibody ingredientsAntiendomysial antibodiesTumor therapy
The present invention provides a method of treating a patient suffering from relapsed HER2 positive cancer with an anti-VEGF antibody during or after treatment with an anti-HER2 antibody. The invention also provides a kit comprising an anti-VEGF antibody.
Owner:F HOFFMANN LA ROCHE INC
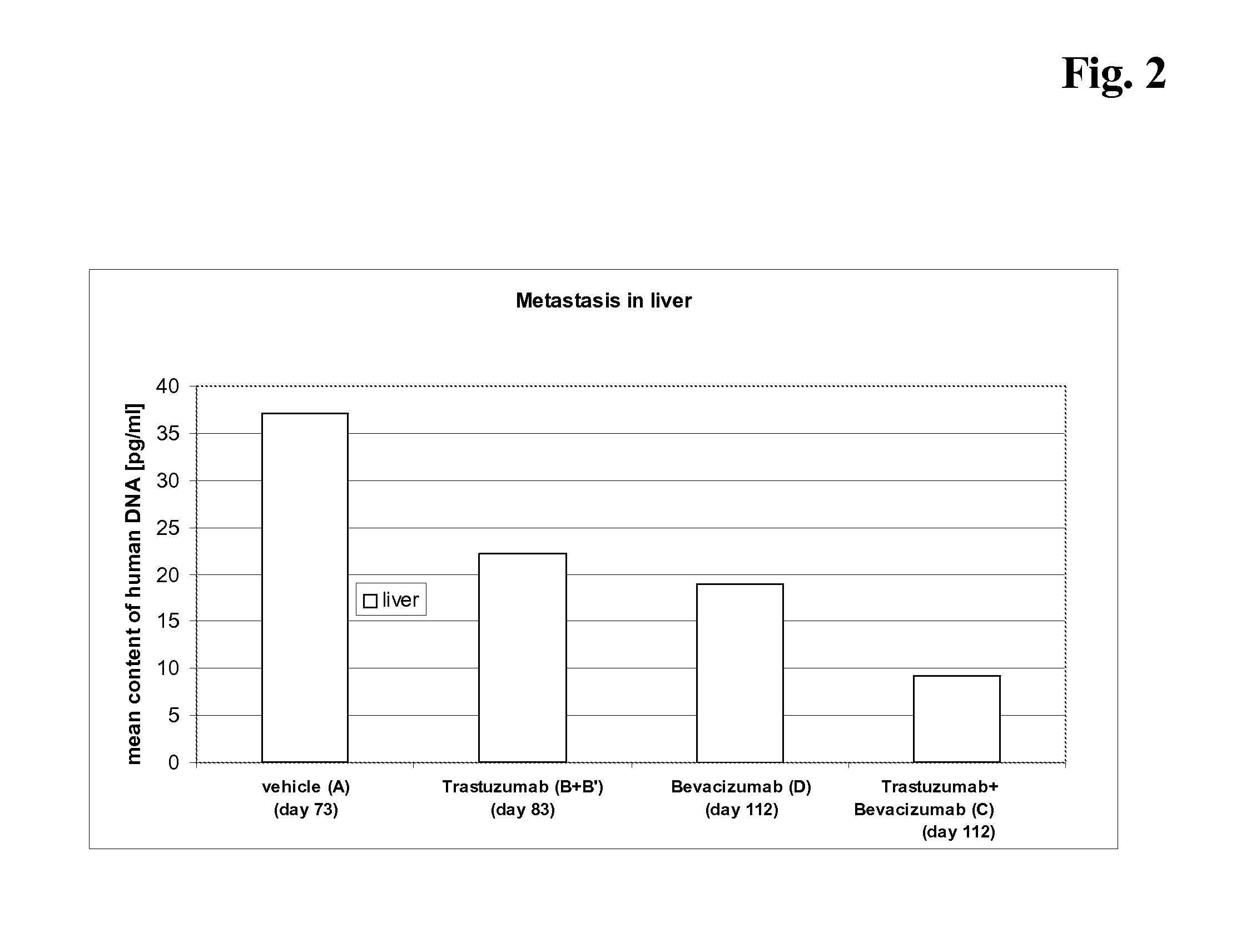
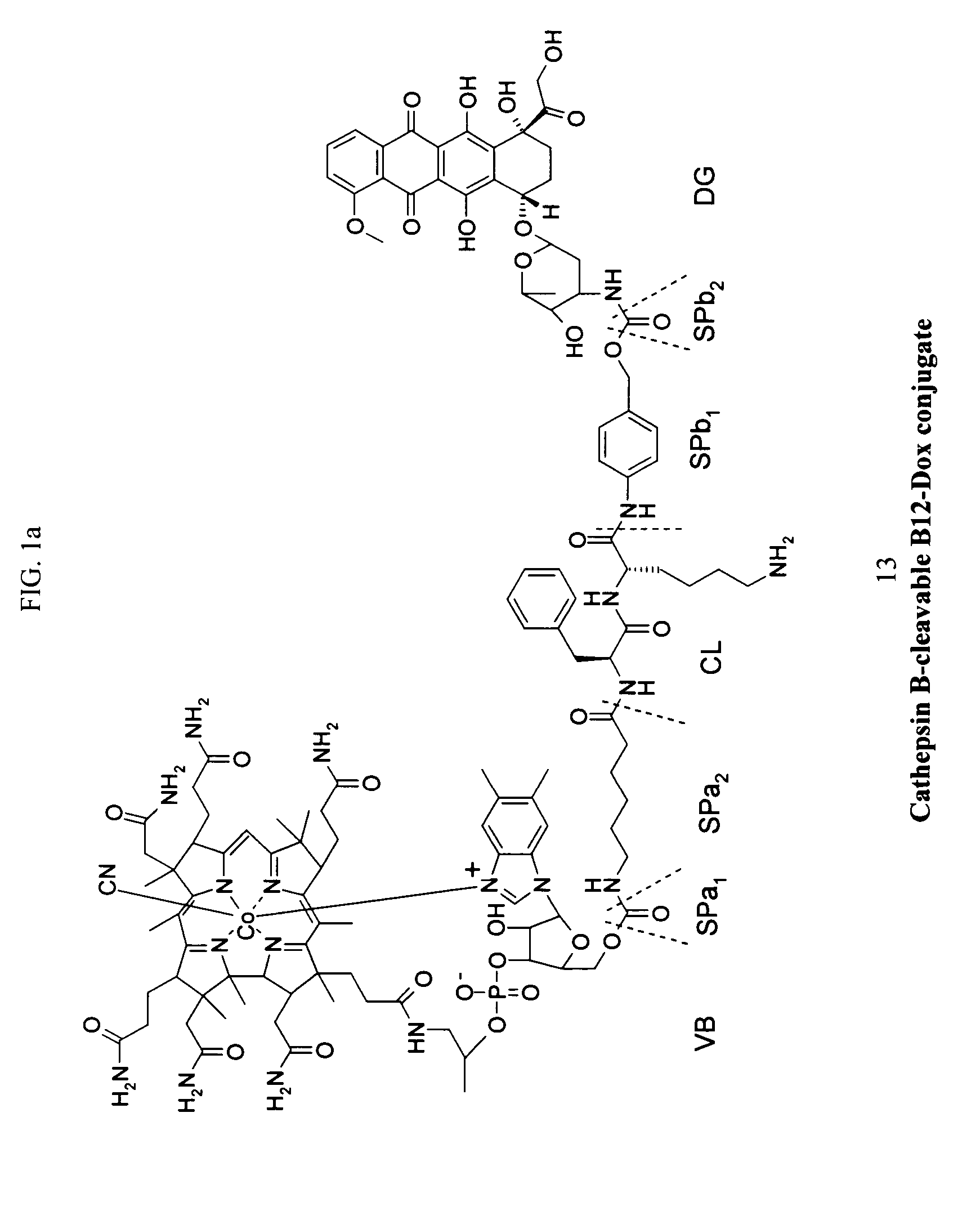
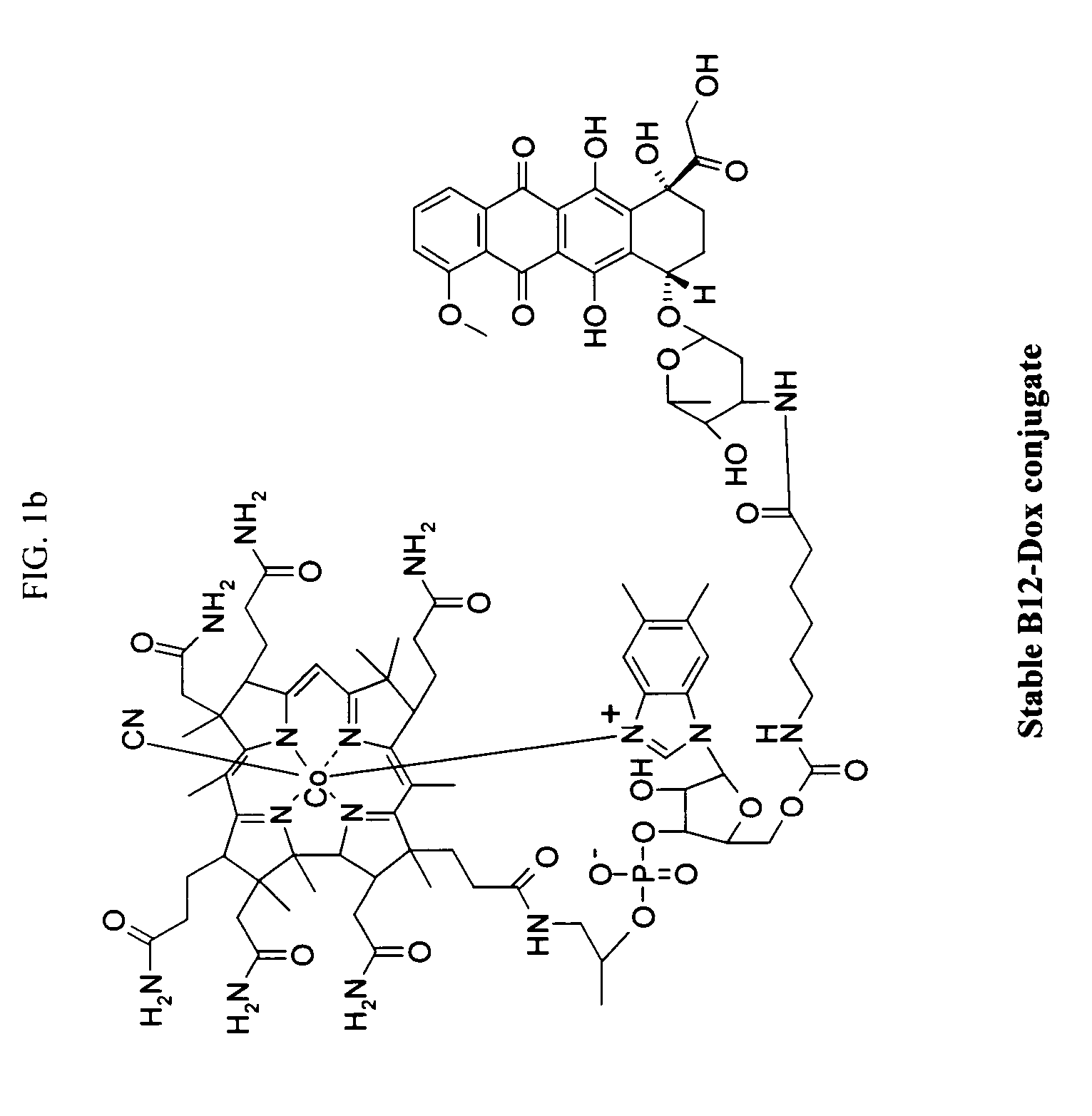
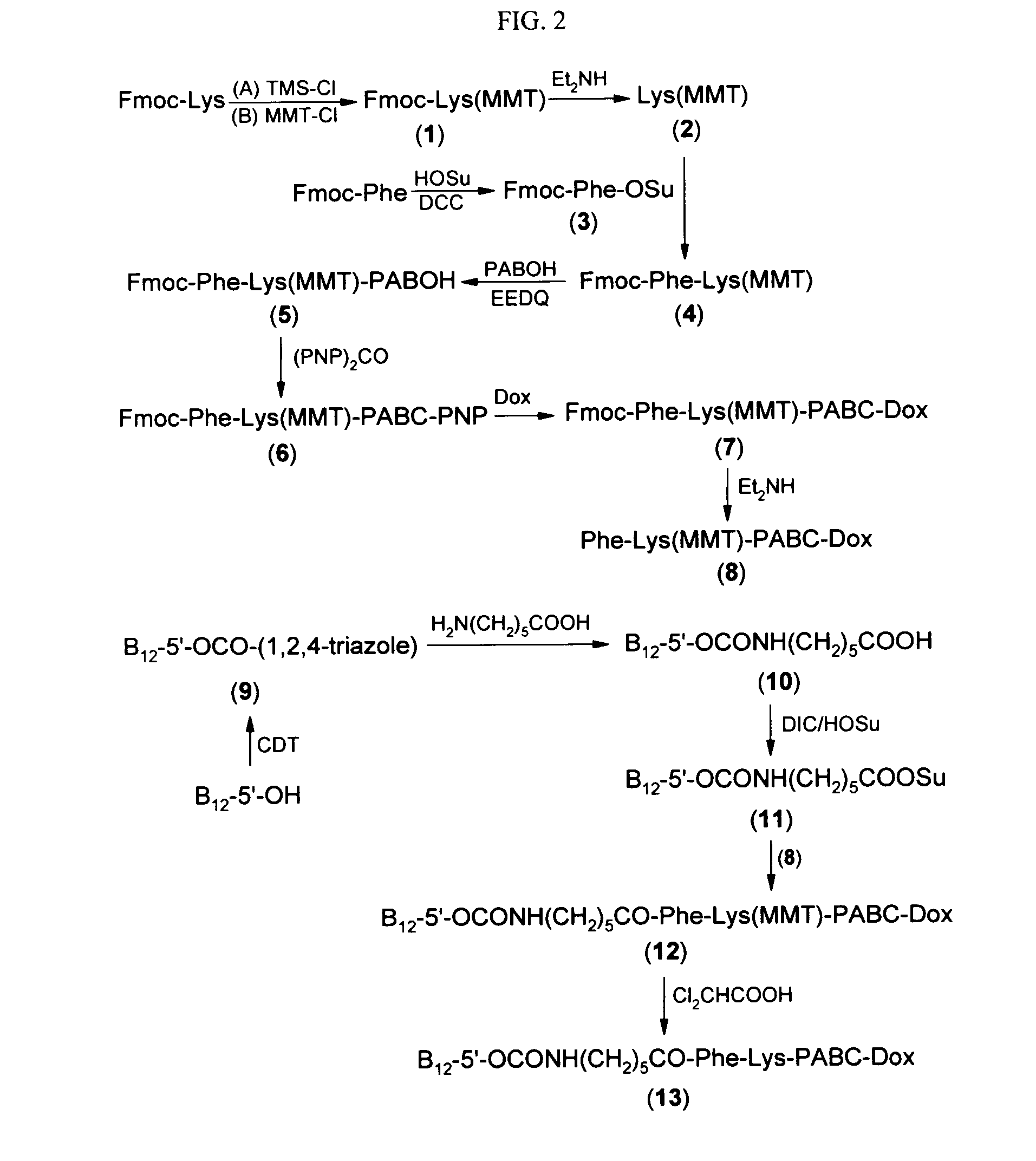
Cobalamin conjugates for anti-tumor therapy
The present invention provides a cobalamin-drug conjugate suitable for the treatment of tumor related diseases. Cobalamin is indirectly covalently bound to an anti-tumor drug via a cleavable linker and one or more optional spacers. Cobalamin is covalently bound to a first spacer or the cleavable linker via the 5′-OH of the cobalamin ribose ring. The drug is bound to a second spacer of the cleavable linker via an existing or added functional group on the drug. After administration, the conjugate forms a complex with transcobalamin (any of its isoforms). The complex then binds to a receptor on a cell membrane and is taken up into the cell. Once in the cell, an intracellular enzyme cleaves the conjugate thereby releasing the drug. Depending upon the structure of the conjugate, a particular class or type of intracellular enzyme affects the cleavage. Due to the high demand for cobalamin in growing cells, tumor cells typically take up a higher percentage of the conjugate than do normal non-growing cells. The conjugate of the invention advantageously provides a reduced systemic toxicity and enhanced efficacy as compared to a corresponding free drug.
Owner:INFLABLOC PHARMA
Tumor therapy with an Anti-vegf antibody
InactiveUS20100285010A1Prevents and reduces metastasisImmunoglobulins against growth factorsAntibody ingredientsAfter treatmentAnti vegf antibody
Owner:FRIESS THOMAS +2
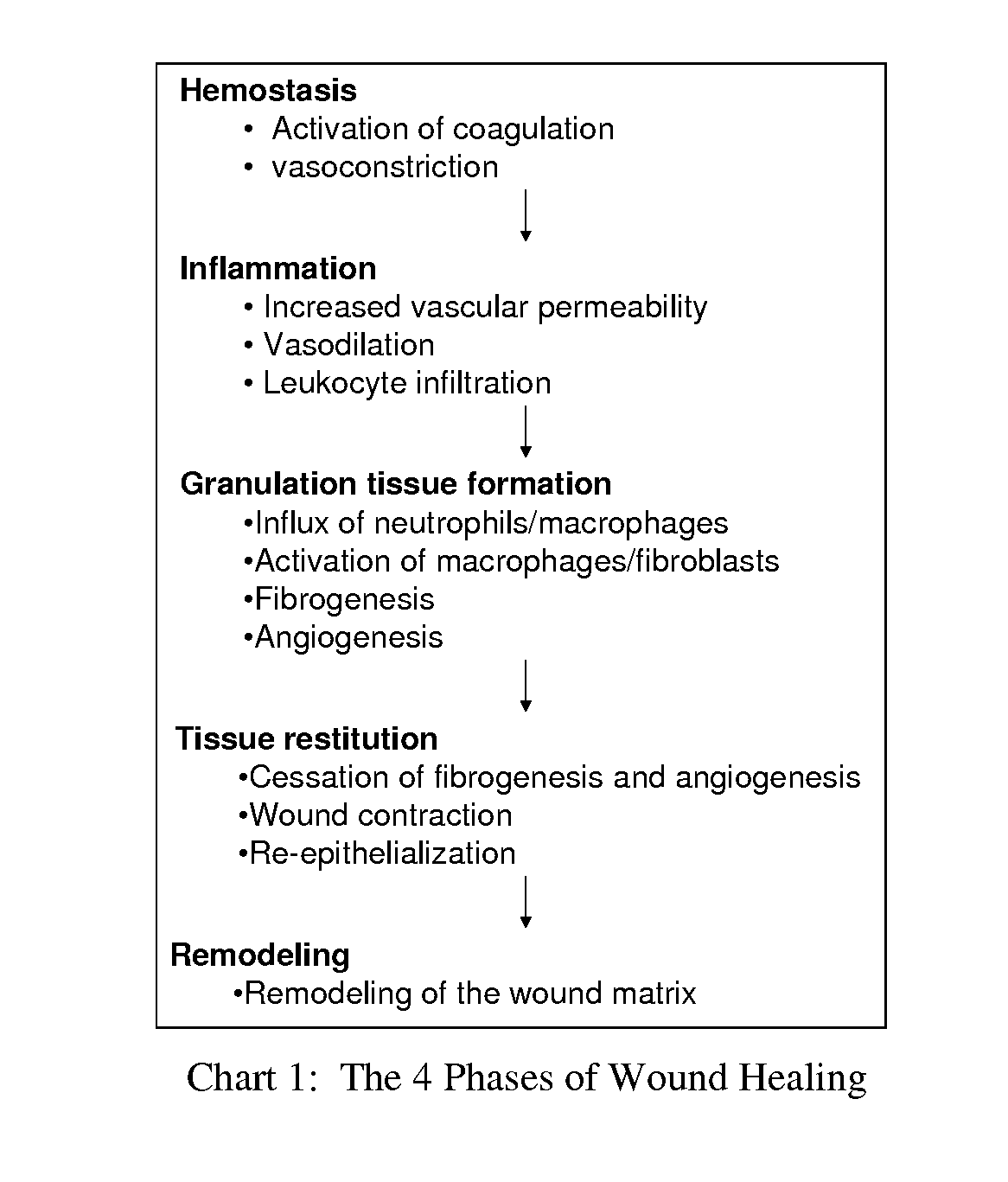
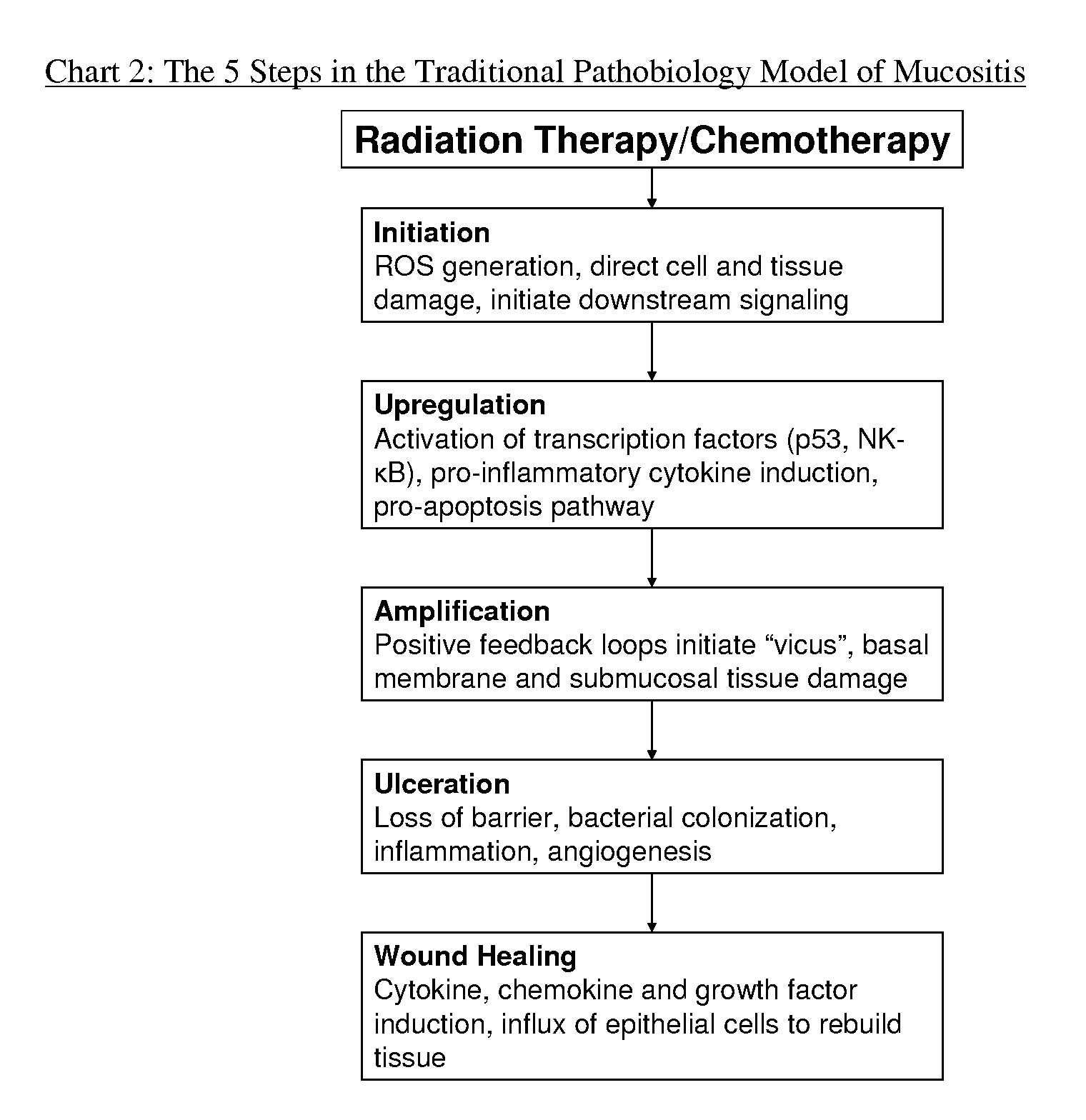
Compositions and methods for mucositis and oncology therapies
In alternative embodiments, this invention provides compositions and methods for treating cancer or any condition caused by dysfunctional cells, side effects from treatments for cancer or any condition caused by dysfunctional cells, e.g., mucositis therapies (e.g., for oral mucositis; digestive mucositis; esophageal mucositis; intestinal mucositis). In alternative embodiments, the invention provides cytoprotection products that may be used either alone or in combination with other medical therapies such as cancer chemotherapies and radiation therapies.
Owner:VICUS THERAPEUTICS
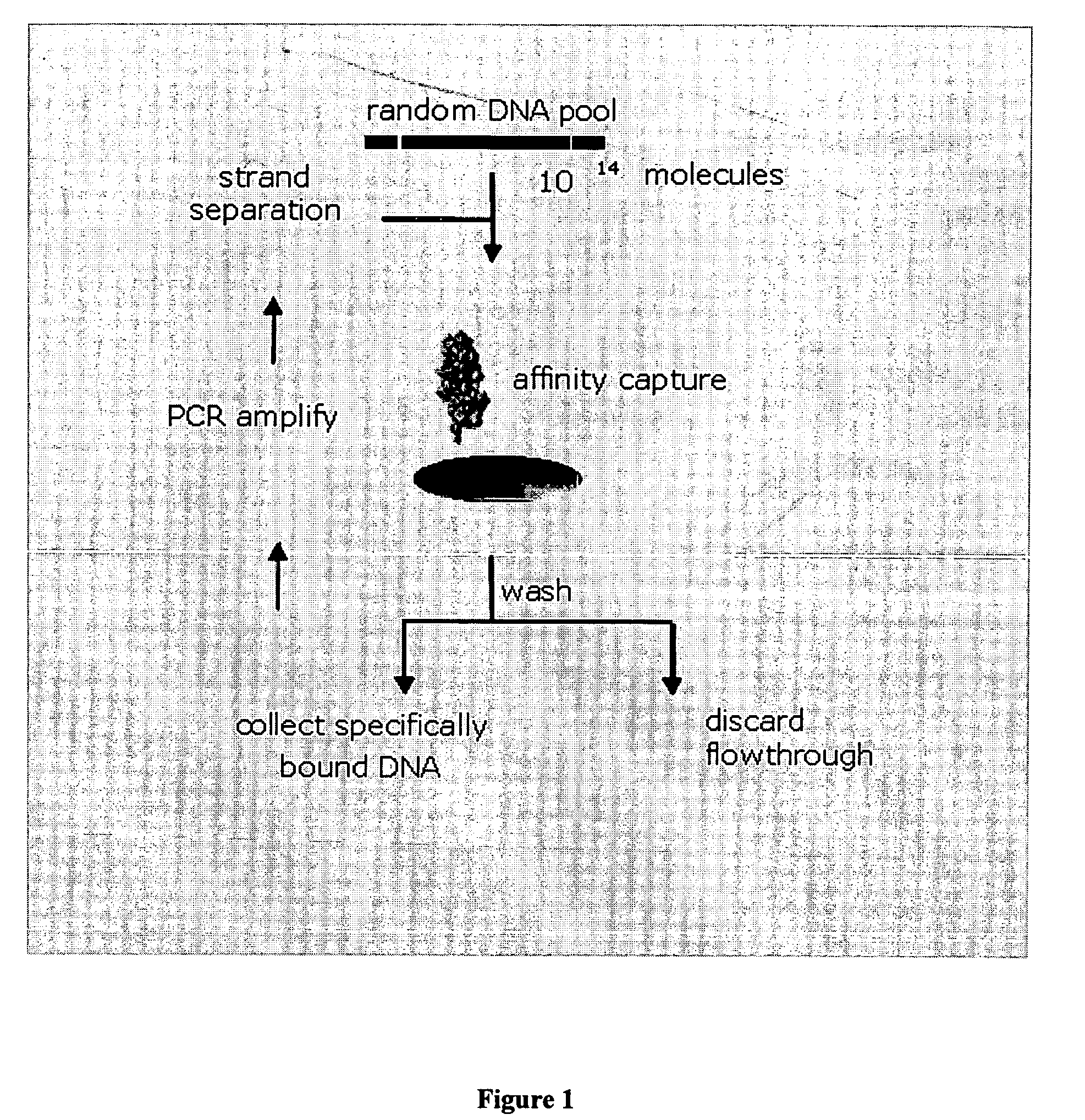
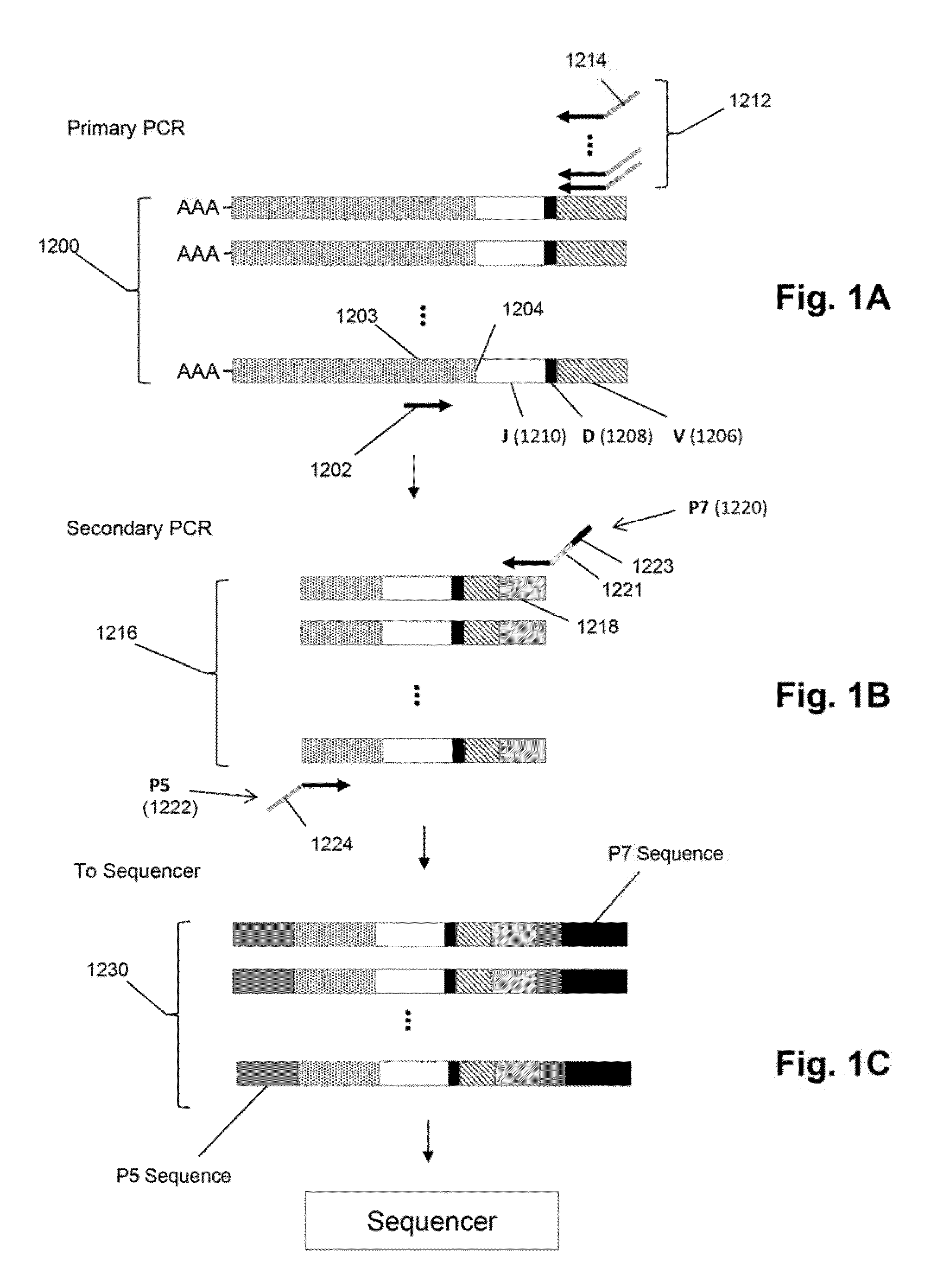
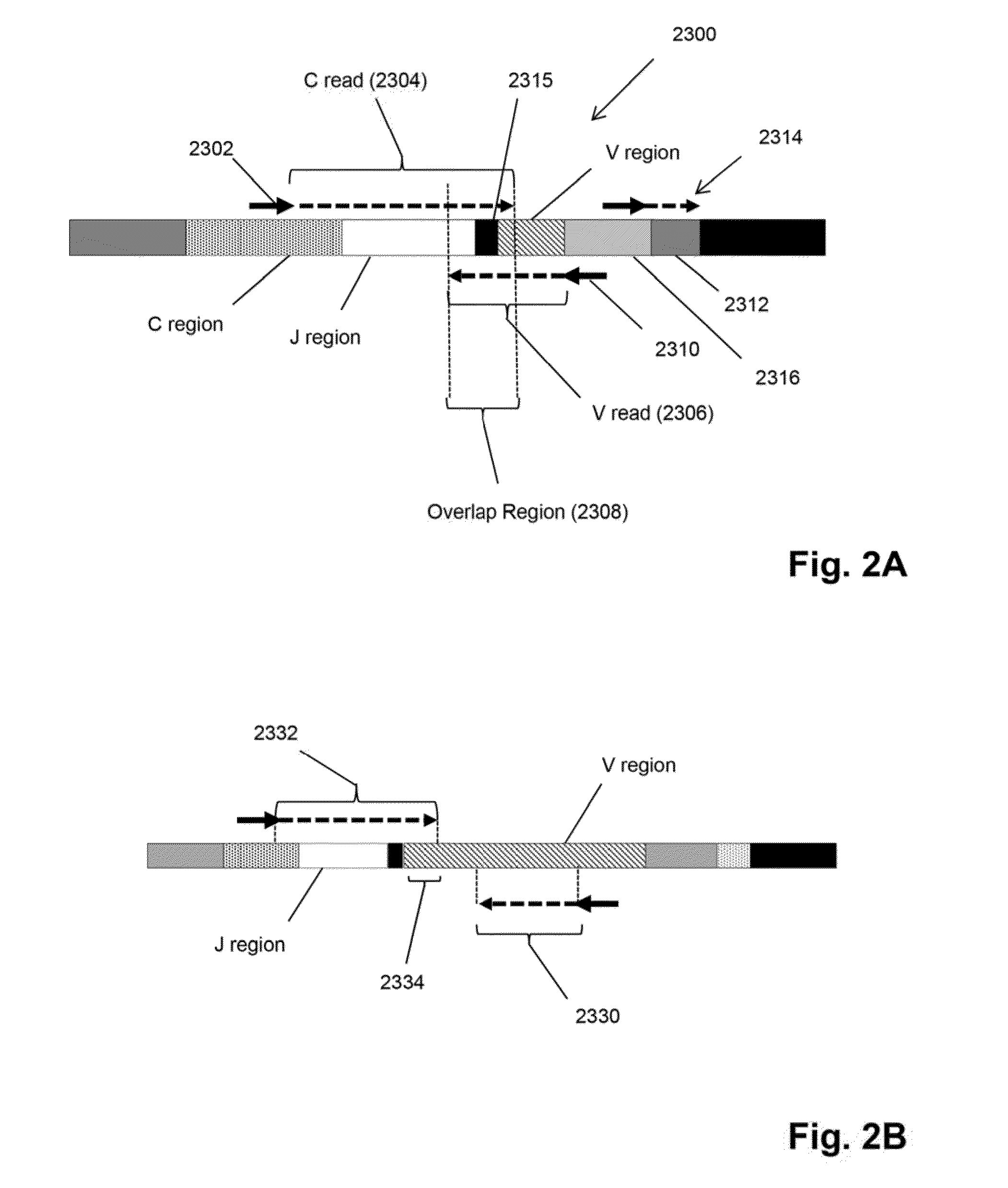
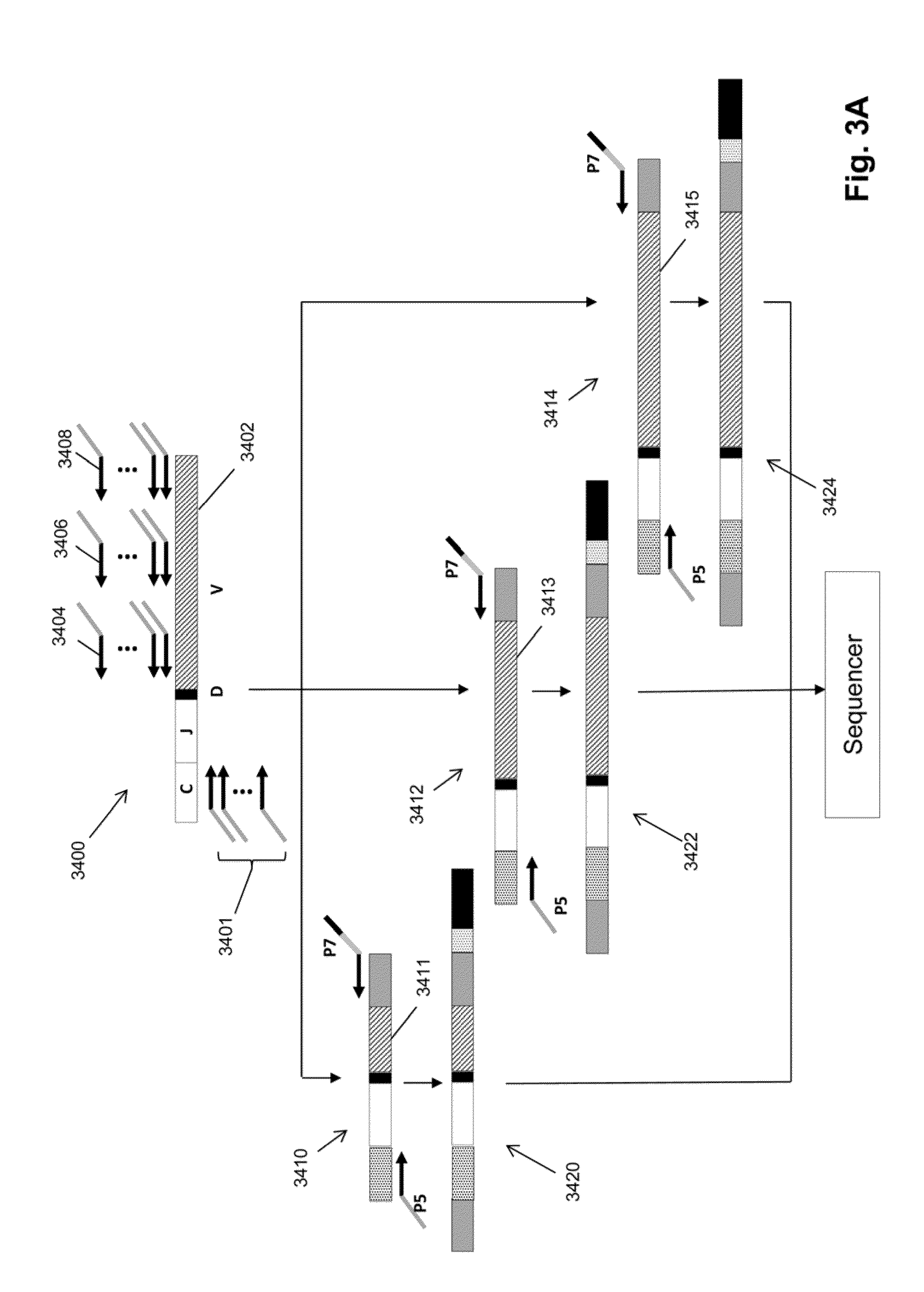
Monitoring treatment-resistant clones in lymphoid and myeloid neoplasms by relative levels of evolved clonotypes
The invention is directed to a method of monitoring or detecting treatment-resistant clones in a patient being treated for a lymphoid or myeloid neoplasm from which patient-specific correlating clonotypes have been identified. In some embodiments, such method includes the steps of obtaining a sample from the patient comprising T-cells and / or B-cells; amplifying molecules of nucleic acid from the T-cells and / or B-cells of the sample, the molecules of nucleic acid comprising recombined DNA sequences from T-cell receptor genes or immunoglobulin genes; sequencing the amplified molecules of nucleic acid to form a clonotype profile; determining from the clonotype profile a level of each correlating clonotype and clonotypes clonally evolved therefrom; and correlating a presence of a treatment-resistant clone of the neoplasm with a change in relative levels of the correlating clonotypes and clonotypes clonally evolved therefrom. In part, the invention permits one to distinguish between cases where treatment is effective but insufficiently intense and cases where a cancer clone arises that is resistant to a current treatment approach.
Owner:ADAPTIVE BIOTECH
Anti-tumor composition
InactiveUS6992106B2Good curative effectEasy to optimizeBiocideHalogenated hydrocarbon active ingredientsPlatinumMedicine
The present invention provides composition having as active ingredients a stilbene derivative and a platinum coordination compound which is highly efficacious and highly safe for treating tumors, particularly for the treatment of solid or malignant tumors and thus methods of cancer and tumor treatment using the composition are also provided.
Owner:AJINOMOTO CO INC
Local regional chemotherapy and radiotherapy using in situ hydrogel
Owner:BOARD OF RGT THE UNIV OF TEXAS SYST
Enhanced-Flexibility Transducer Arrays for Delivering TTFields (Tumor Treating Fields)
A transducer array for use in tumor-treating fields (TTFields) therapy is particularly suited for use in treating abdominal or thoracic cancers. The transducer array has features that increase its flexibility and adhesion to the patient's skin, including a branching configuration and a correspondingly branching top covering adhesive-backed layer. Additionally, a skin-level adhesive layer is provided beneath the flex circuit to which the electrode elements are attached, to help ensure thorough, lasting adhesion of the transducer array to the patient's skin over the course of treatment.
Owner:NOVOCURE GMBH
Methods of gene therapy using herpes viral vectors expressing GM-CSF
A genetically disabled mutant virus has a genome which is defective in respect of a selected gene that is essential for the production of infectious new virus particles, and which carries heterologous genetic material encoding an immunomodulatory protein such as GM-CSF, IL-2, or others, such that the mutant virus can infect normal host cells and cause expression of immunomodulatory protein, but the mutant virus cannot cause production of infectious new virus particles except when the virus infects recombinant complementing host cells expressing a gene that provides the function of the essential viral gene; the site of insertion of the heterologous genetic material encoding the immunomodulatory protein preferably being at the site of the defect in the selected essential viral gene. Uses include prophylactic and therapeutic use in generating an immune response in a subject treated therewith; use in the preparation of an immunogen such as a vaccine for use in tumor therapy; use in the in-vitro expansion of (e.g. virus-specific) cytotoxic T cells; and therapeutic or prophylactic use in corrective gene therapy.
Owner:CANTAB PHARMA RES
Stabilized aptamers to platelet derived growth factor and their use as oncology therapeutics
Materials and methods are provided for producing and using aptamers useful as oncology therapeutics capable of binding to PDGF, PDGF isoforms, PDGF receptor, VEGF, and VEGF receptor or any combination thereof with great affinity and specificity. The compositions of the present invention are particularly useful in solid tumor therapy and can be used alone or in combination with known cytotoxic agents for the treatment of solid tumors. Also disclosed are aptamers having one or more CpG motifs embedded therein or appended thereto.
Owner:ARCHEMIX CORP
Tumor-targeted drug delivery systems and uses thereof
InactiveUS20040258747A1Improved treatment of cancerImprove localizationOrganic active ingredientsBiocideTumor targetDelivery vehicle
The present invention relates to targeted delivery systems for delivering therapeutic agents to tumor. The invention further relates to methods of delivering a therapeutic agent to a tumor for the prevention and treatment of cancer by killing tumor cells and tumor-associated endothelial cells. In particular, the present invention provides a tumor-targeted drug delivery system comprising a NGR-containing molecule linked to a delivery vehicle encapsulating a therapeutic agent, preferably a drug, such as a cytotoxic agent or a chemotherapeutic agent. Specifically, the delivery systems of the present invention are capable of delivering an increased amount of therapeutic agent to a tumor as compared to other delivery systems. In particular, the delivery systems of the present invention are capable of accumulating a higher amount of therapeutic agent in a tumor, or in the vicinity of a tumor cell or tumor-supporting cell, resulting in exposure of the tumor cell and tumor-associated endothelial cell to therapeutic levels of the agent for a longer period of time as compared to other delivery systems. The present invention also describes pharmaceutical compositions comprising the delivery systems of the present invention. The present invention further relates to a tumor treatment comprising an increased amount of therapeutic agent delivered by the system of the present invention as compared to other delivery systems. The delivery systems and pharmaceutical compositions can be administered to a subject, preferably a human, alone or in combination, sequentially or simultaneously, with other prophylactic or therapeutic agents and / or anti-cancer treatments.
Owner:OSPEDALE SAN RAFFAELE SRL +2
Method for increasing therapeutic gain in radiotherapy and chemotherapy
InactiveUS20050272644A1Inhibit tumor growthReduced radiation-induced normal tissue fibrosisBiocideAntipyreticAbnormal tissue growthTumor therapy
The present invention provides compositions and methods for increasing therapeutic gain in radiotherapy and chemotherapy for proliferating malignant or nonmalignant disease to produce high probability of tumor control with low frequency of sequelae of therapy by administering a therapeutically effective amount of a histone deacetylase inhibitor. The compounds are capable of simultaneously stimulating the epithelium regrowth, inhibiting the fibroblast proliferation, decreasing the collagen deposit, suppressing the fibrogenic growth factor, subsiding the proinflammatory cytokine and modulating the expression of cell cycle genes, tumor suppressors and oncogenes, and are useful to increase the therapeutic gain in radiotherapy and chemotherapy, which results in decrease of skin swelling and inflammation, promotion of epithelium healing in mucosa and dermis, decrease of xerostomia, prevention / reduction of severity of plantar-palmar syndrome, prevention of tissue fibrosis, ulceration, necrosis and tumorigenesis, and increase of tumor growth inhibition and tumor therapy effectiveness.
Owner:SUNNY PHARMTECH
Anti-PD1 monoclonal antibody, pharmaceutical composition and uses thereof
ActiveCN106977602ABinding blockRelief of immunosuppressionImmunoglobulins against cell receptors/antigens/surface-determinantsAntibody ingredientsMolecular ImmunologyAntigen Binding Fragment
The present invention belongs to the field of tumor treatment and molecular immunology, and relates to an anti-PD1 monoclonal antibody, a pharmaceutical composition and uses thereof. In particularly, the present invention relates to a monoclonal antibody or an antigen-binding fragment thereof, wherein the heavy chain variable region of the monoclonal antibody comprises CDR having an amino acid sequence represented by SEQ ID NO:9-11, and / or the light chain variable region of the monoclonal antibody comprises CDR having an amino acid sequence represented by SEQ ID NO:12-14. According to the present invention, the monoclonal antibody can well and specifically bind to PD1, can specifically release the immunosuppression of PD1 on body, and can activate T lymphocytes.
Owner:CTTQ AKESO (SHANGHAI) BIOMED TECH CO LTD
Screening assays and methods of tumor treatment
InactiveUS20060015952A1Growth inhibitionBiological material analysisSkeletal disorderAbnormal tissue growthPrimary tumor
The invention relates generally to the screening of candidate molecules for the treatment of tumor metastasis, and treatment methods using such molecules. Thus, the invention includes a method of screening comprising the steps of: (1) administering a plurality of test substances to a non-human syngeneic immunocompetent animal model bearing at least one soft tissue or bone metastasis, in the presence or absence of a primary tumor; (2) determining the effects of the test substances on the soft tissue or bone metastasis and growth of the primary tumor, if present; and (3) identifying a test substance that inhibits the growth of a soft tissue or bone metastasis, without adverse effect on the status of the primary tumor, if present.
Owner:GENENTECH INC
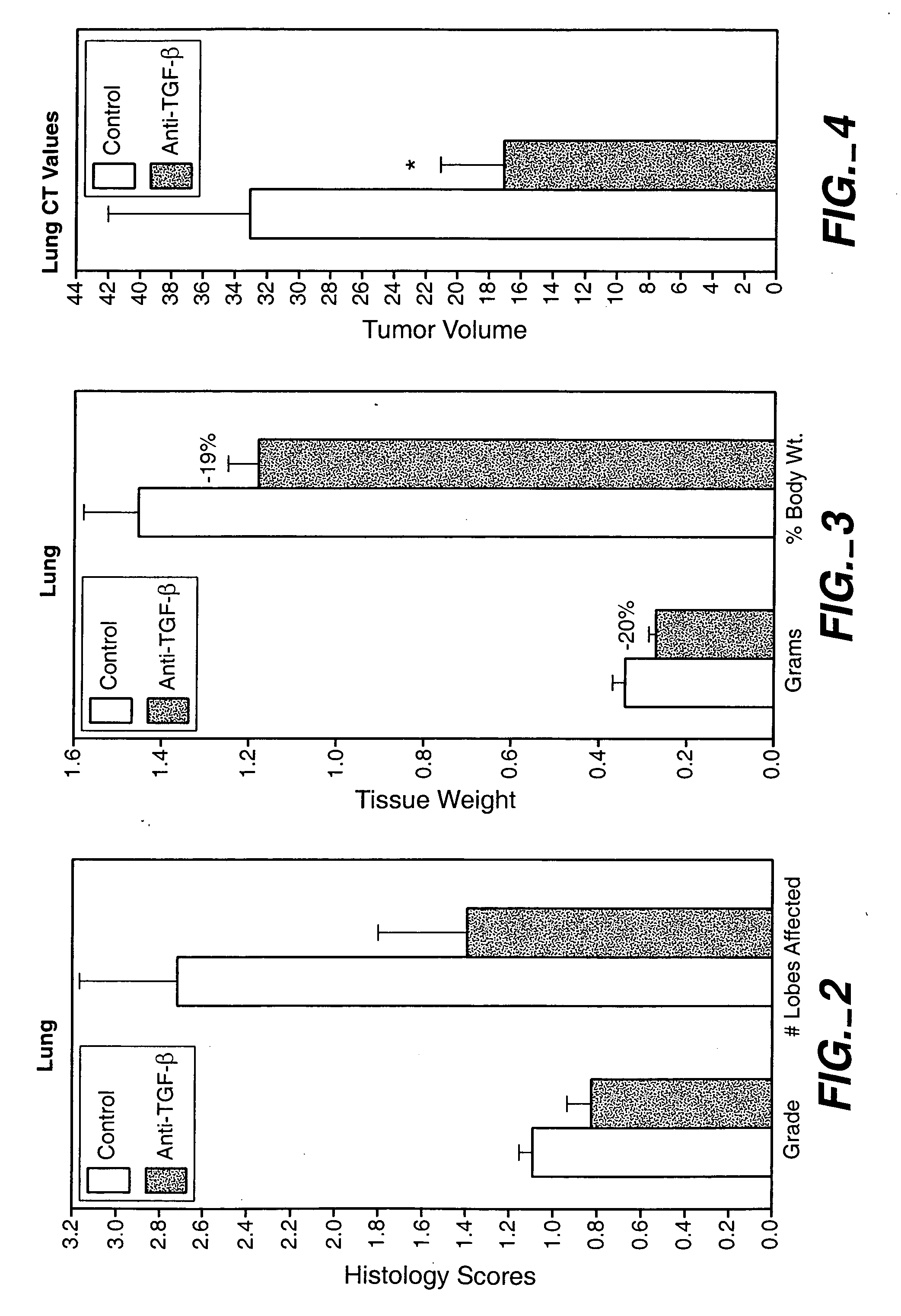
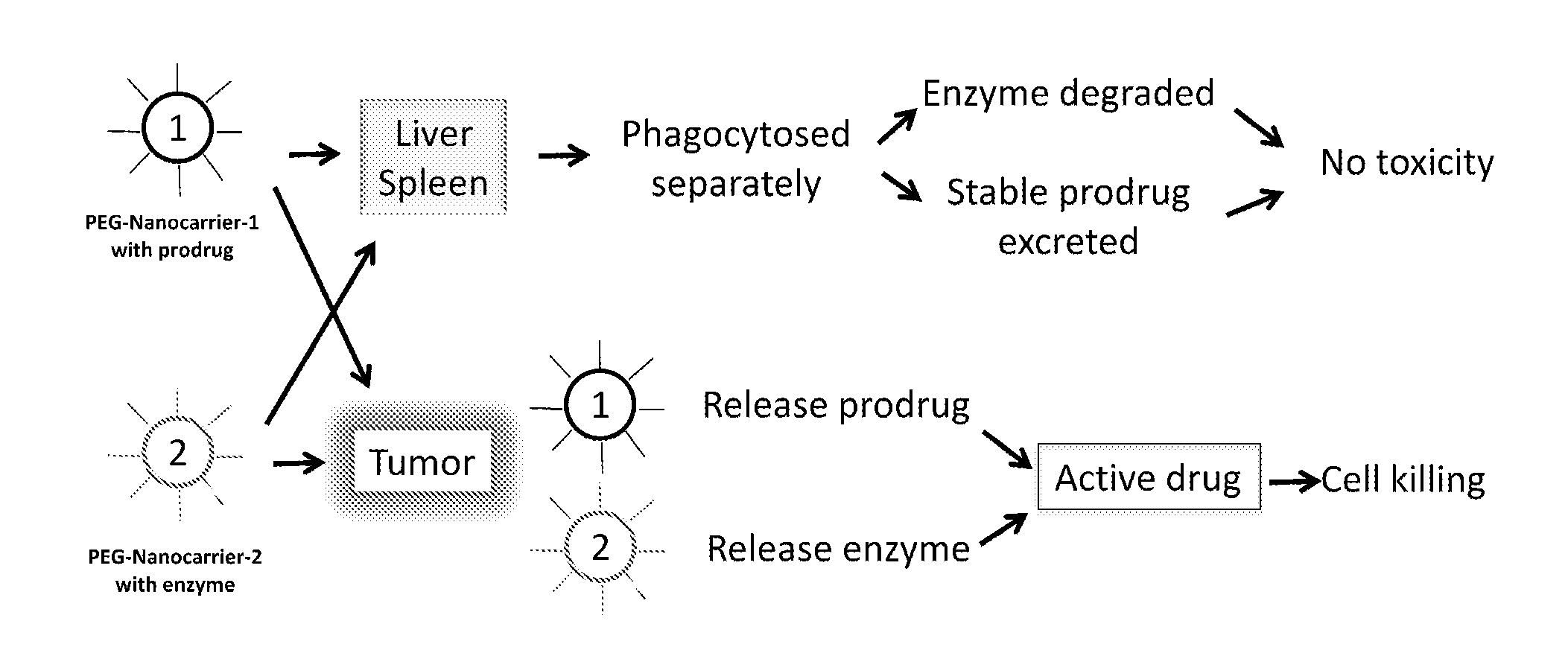
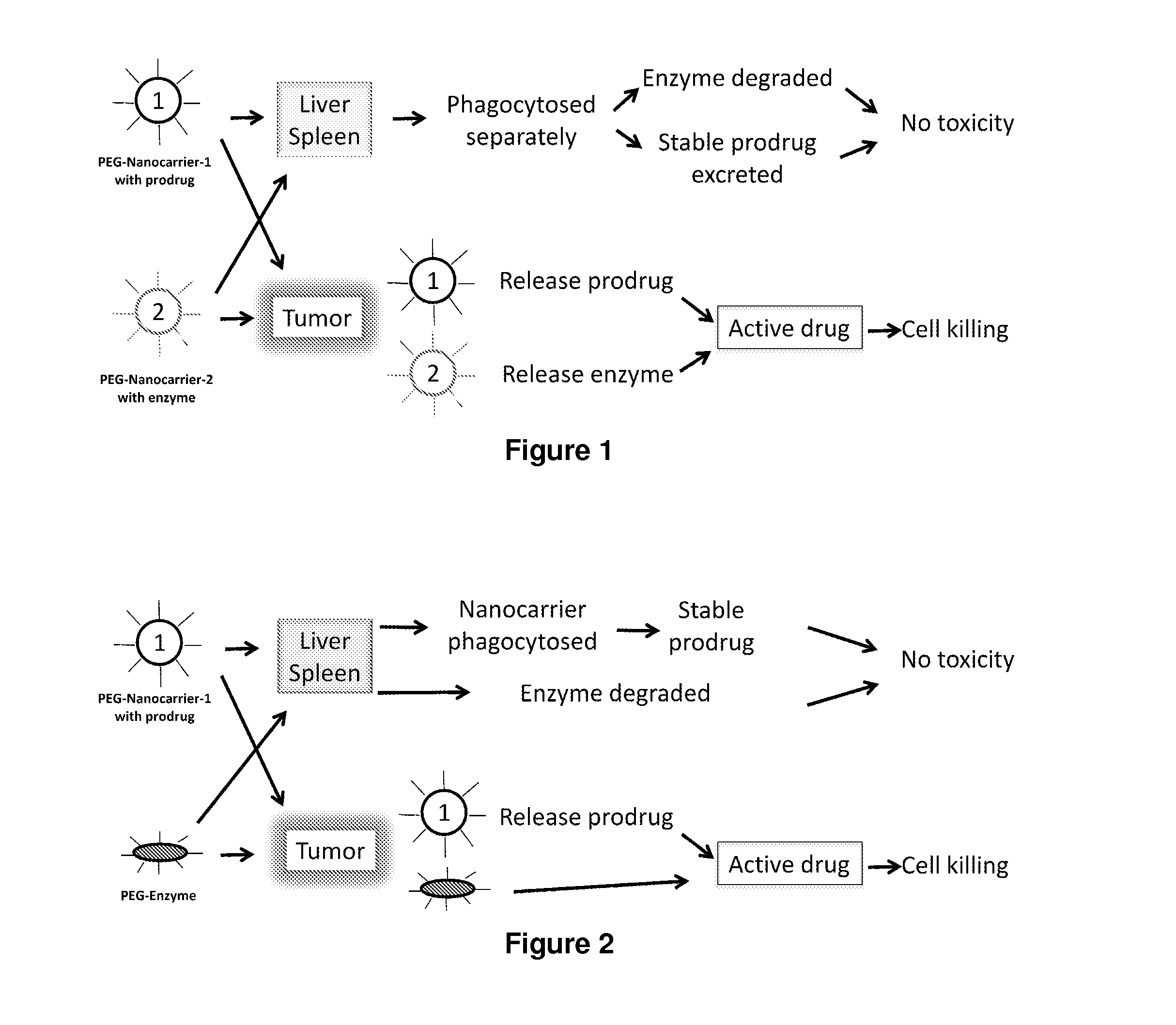
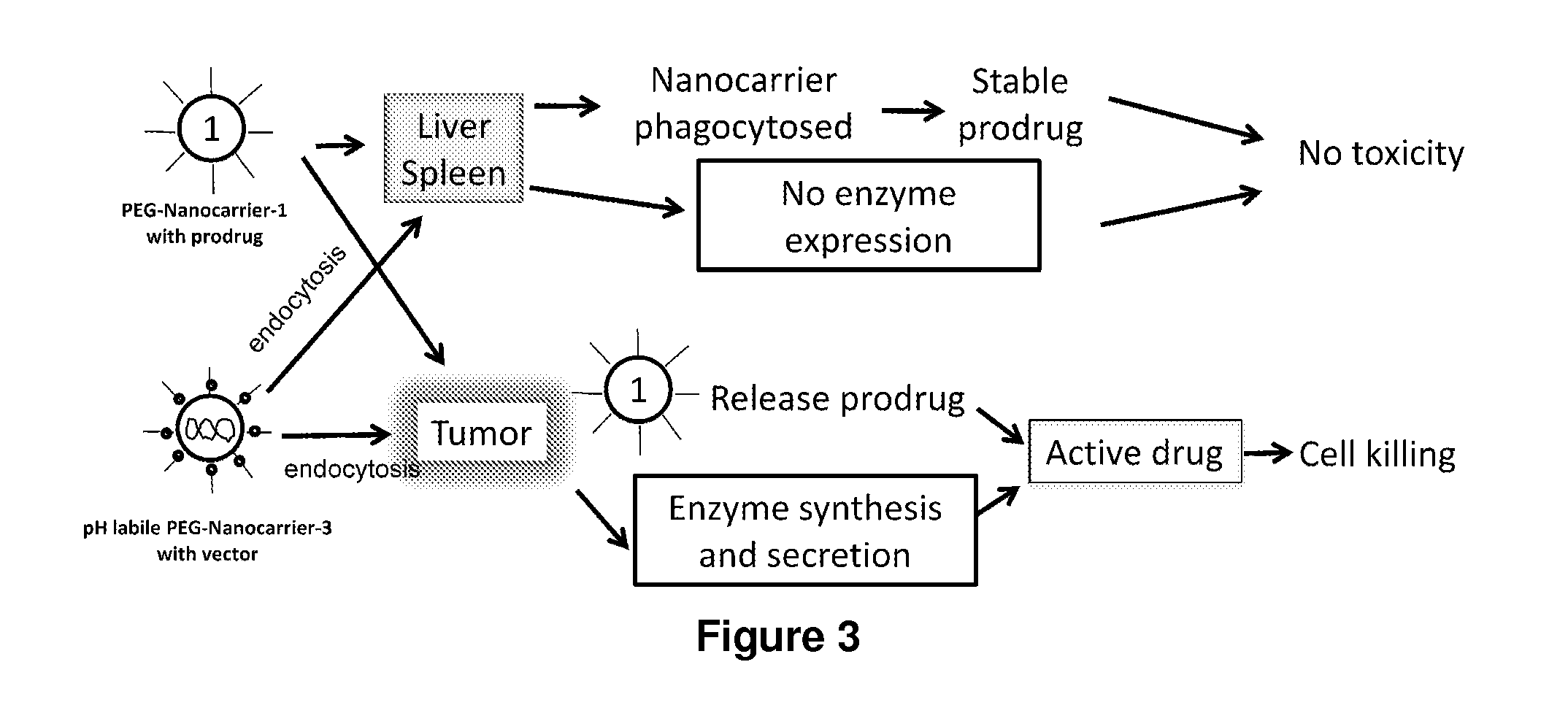
Two-step targeted tumor therapy with prodrug encapsulated in nanocarrier
InactiveUS20110217363A1Reduce activationIncrease targeted activationOrganic active ingredientsPeptide/protein ingredientsNanocarriersTumor therapy
A two-step targeted tumor therapeutic method is described comprising nanocarrier with prodrug encapsulated thereto and prodrug activating enzyme. The enzyme is either encapsulated in nanocarrier, or not encapsulated, or synthesized in the tumor. With the method, the prodrug released from the nanocarrier is activated by the enzyme mainly in the tumor.
Owner:BIONANOX
Monoclonal antibody for anti-human CEA, a composition containing same and application thereof
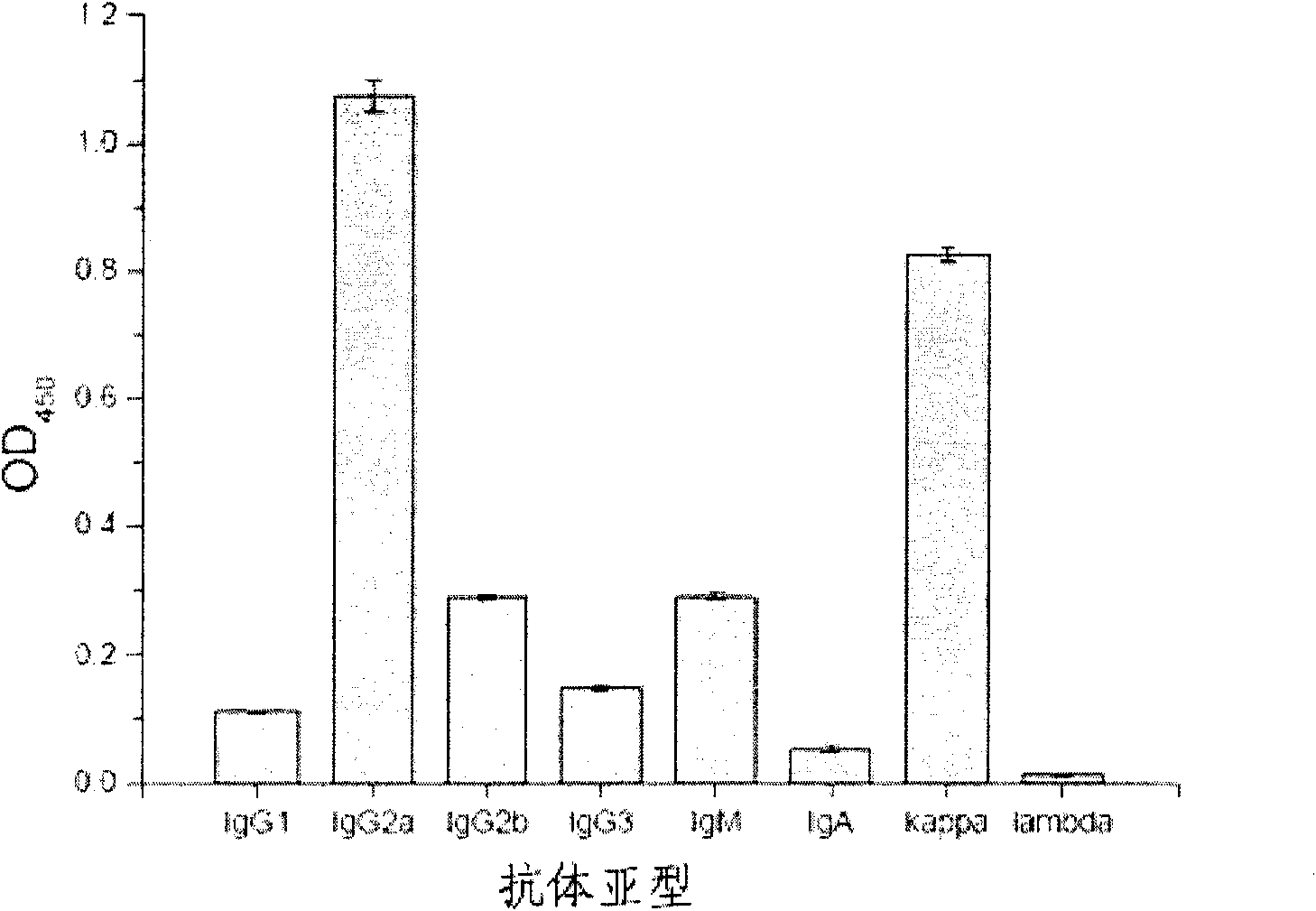
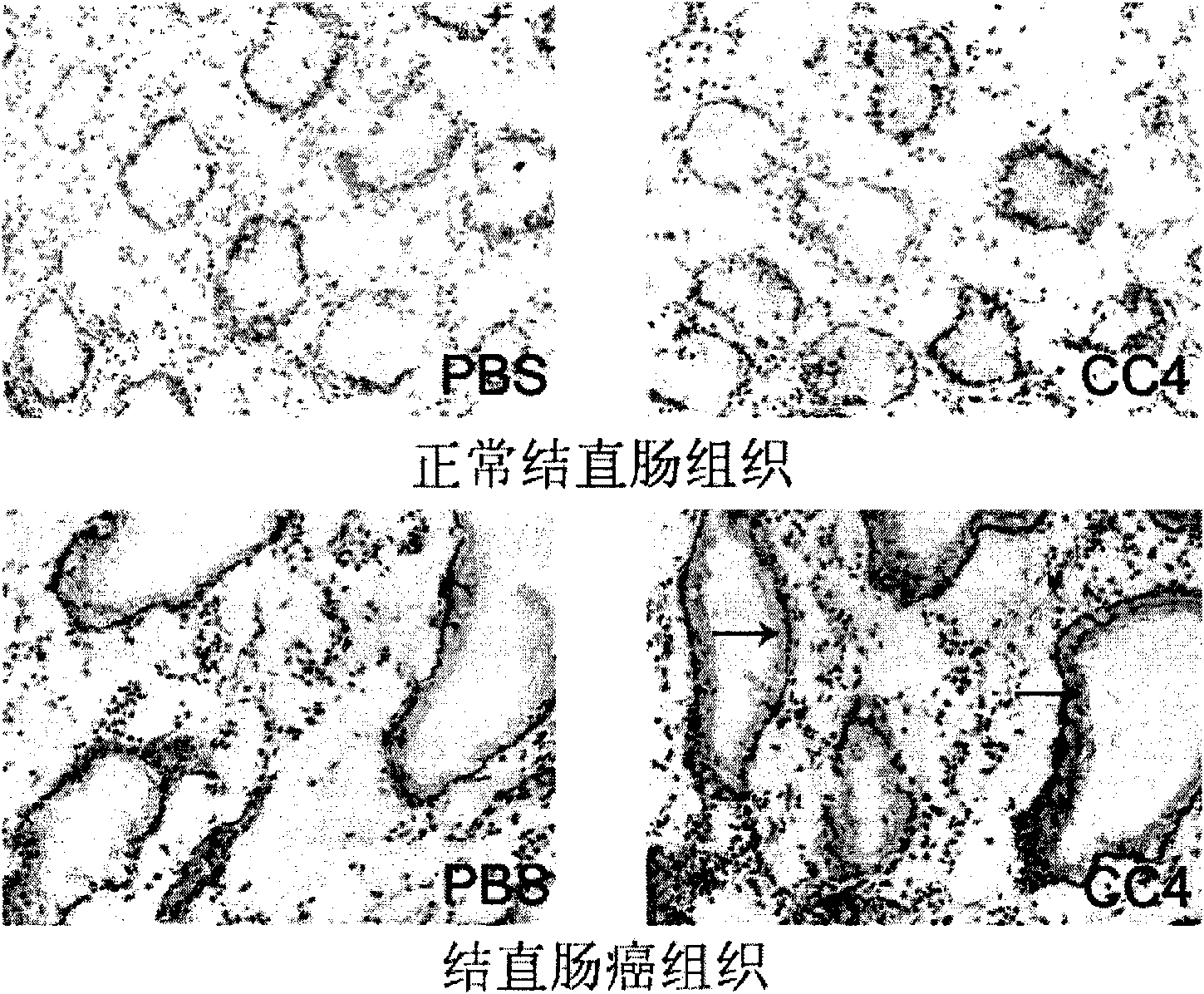
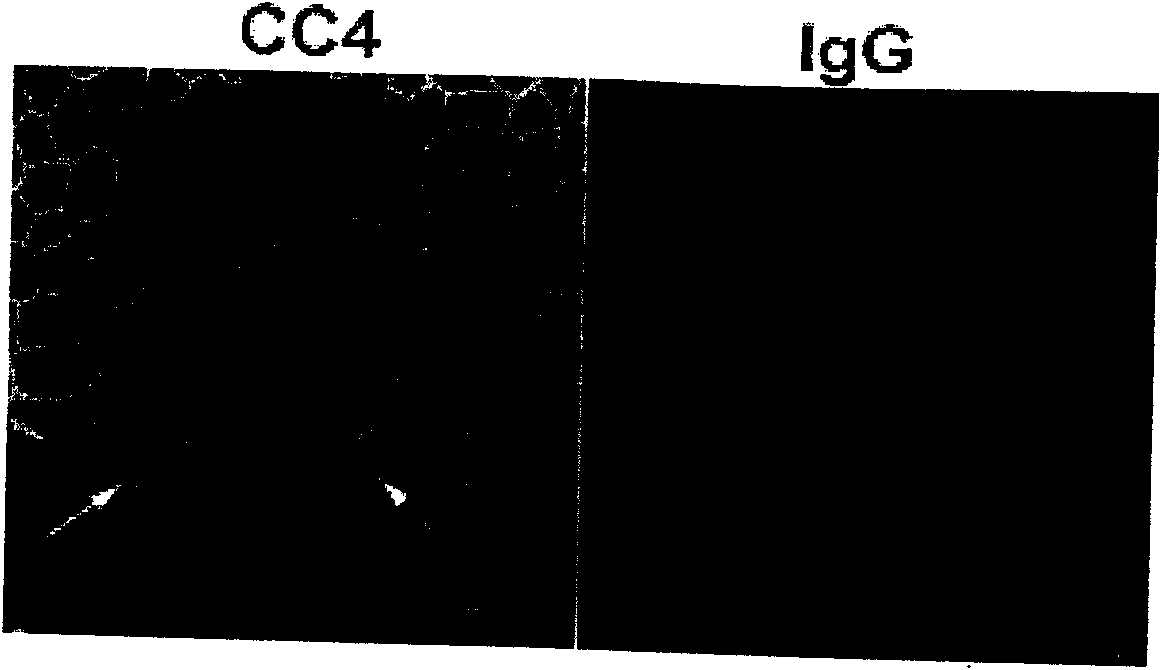
The invention relates to a mouse monoclonal antibody of anti-human carcino-embryonic antigen (CEA) resisting colorectal neoplasm activity, prepared by a biological technology. The method comprise a method for detecting CEA family protein by utilizing the antigen-combining capacity specificity of the mouse monoclonal antibody CC4 of anti-human CEA and a neoplasm treatment method by utilizing the colorectal neoplasm activity. The antibody can be used for specifically recognizing the human CEA at molecular, cellular and texture levels and recognizing a special antigen epiposition widely distributed in the CEA family protein. The CC4 is specifically combined with colorectal neoplasm texture at the texture level and presents stronger capabilities of restraining neoplasm growth, neoplasm metastasis, neoplasm invasion in an animal model and at the cellular level. The antibody and detection and treatment methods based on the antibody can become an effective neoplasm diagnosis and treatment tool in basal research or clinical application.
Owner:INSITUTE OF BIOPHYSICS CHINESE ACADEMY OF SCIENCES
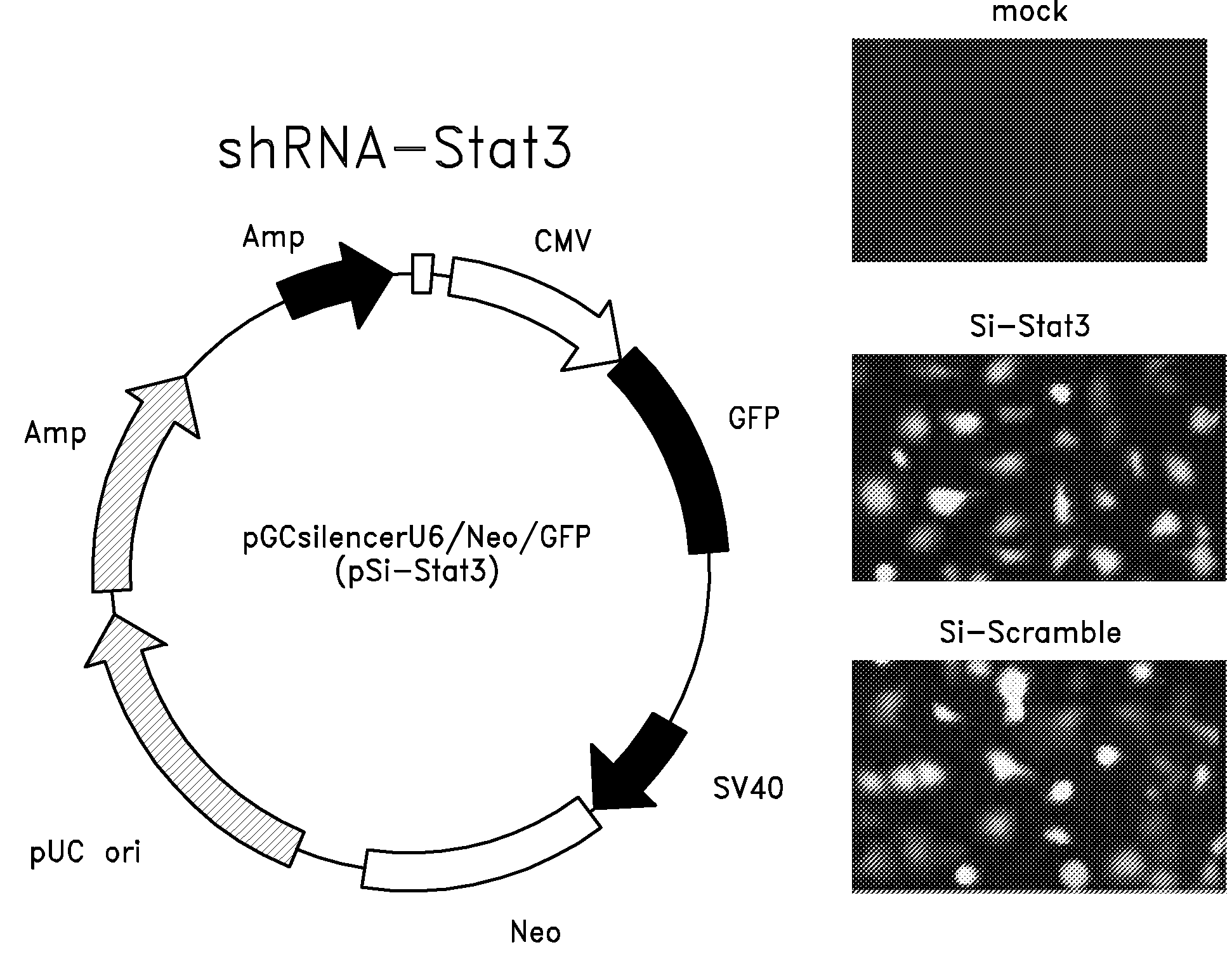
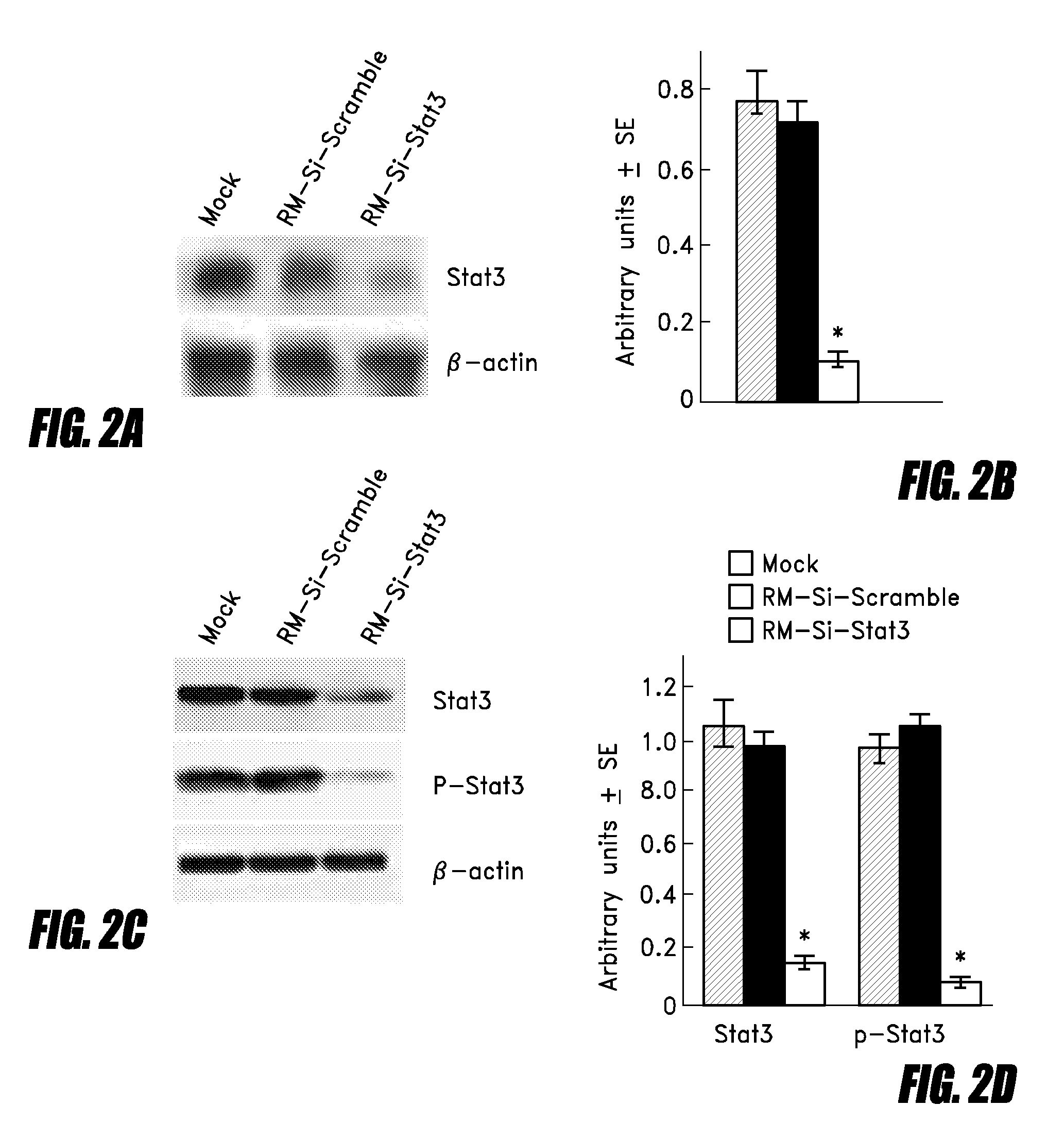
Attenuated salmonella as a delivery system for sirna-based tumor therapy
InactiveUS20090208534A1Promote growthConducive to survivalBacteriaSpecial deliveryTumor targetTumor targeting
The invention relates to an attenuated Salmonella sp. that is capable of targeting a solid tumor when administered in vivo comprising a short hairpin (sh) RNA construct, and methods of inhibiting the growth or reducing the volume of a solid tumor cancer comprising administering an effective amount of an attenuated Salmonella sp. to a patient having a solid tumor cancer, wherein said attenuated Salmonella sp. is a tumor targeting attenuated Salmonella sp. expressing a short hairpin (sh) RNA which attenuated Salmonella sp. is capable of inhibiting the growth or reducing the volume of the solid tumor cancer when administered in vivo.
Owner:JILIN UNIV +2
Tumor therapy with an antibody for vascular endothelial growth factor and an antibody for human epithelial growth factor receptor type 2
InactiveUS20110064736A1Prolong survival timeProlong progression-free survivalImmunoglobulins against growth factorsAntibody ingredientsDiseaseFactor ii
The present invention provides a method of treating a breast cancer disease in a patient who has failed prior treatment with an anti-VEGF antibody, comprising administering to the patient a therapeutically effective amount of an anti-HER2 antibody while continuing said anti-VEGF antibody therapy. The invention also provides corresponding pharmaceutical kits and pharmaceutical compositions.
Owner:F HOFFMANN LA ROCHE & CO AG
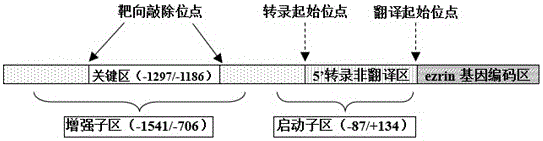
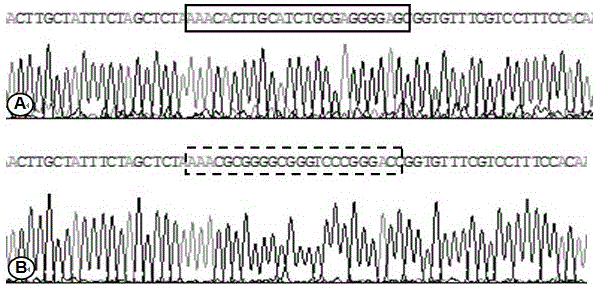
Method for CRISPR/Cas9 targeted knockout of human ezrin gene enhancer key region and specific gRNA thereof
InactiveCN106434663AAnimals/human peptidesVector-based foreign material introductionTumor therapyEsophagus Cancers
The invention belongs to the field of molecular biology, and particularly relates to a method for CRISPR / Cas9 targeted knockout of a human ezrin gene enhancer key region and specific gRNA thereof. In the method, according to the design principle of CRISPR / Cas9, two target sites are designed at the upstream and the downstream of the human ezrin gene enhancer key region, corresponding oligonucleotide sequences are synthesized and then connected to a carrier pX459 to construct recombinant plasmids, the recombinant plasmids are transfected with a human esophagus cancer cell line, and then specific knockout of the human ezrin gene enhancer key region can be achieved. The method and the specific gRNA have great significance for study on clinical tumor therapy with the human ezrin gene enhancer as the target.
Owner:ZUNYI MEDICAL UNIVERSITY
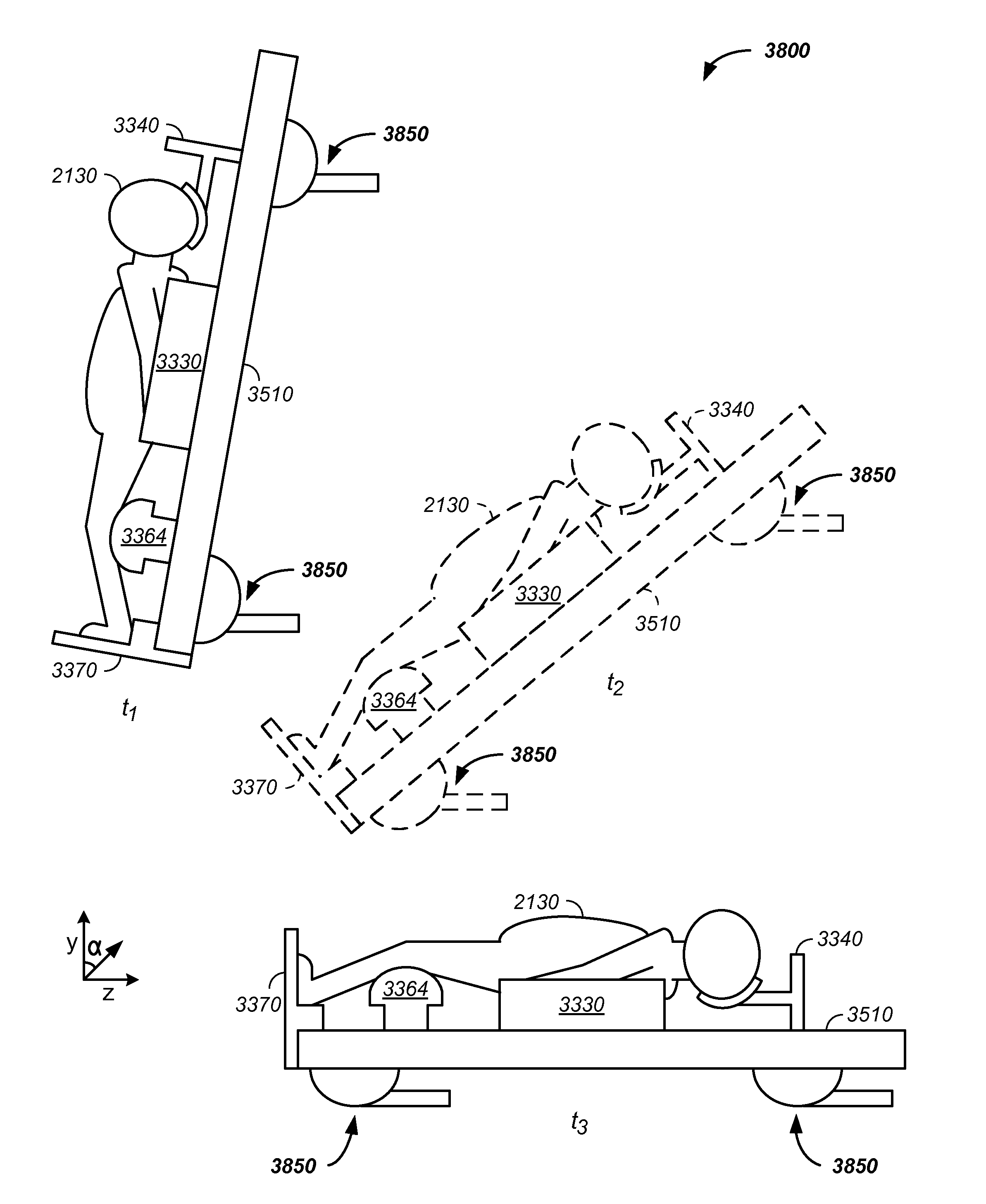
Charged particle treatment, rapid patient positioning apparatus and method of use thereof
ActiveUS20130217946A1Rapid positioningPatient positioning for diagnosticsMagnetic resonance acceleratorsTumor therapyEngineering
The invention comprises a charged particle beam, rapid patient positioning system including steps of: positioning a patient relative to a table in a substantially vertical orientation, optionally constraining motion of the patient with one or more constraints, transitioning the table through a semi-vertical orientation, such as with a robot arm, and orientating the patient and table in a substantially horizontal orientation, such as in a position for tumor therapy. Preferably, the robot arm is in common with an arm used to move the patient in traditional proton therapy. Optionally, the robot arm is used to re-orientate the patient into a substantially vertical orientation at the conclusion of a charged particle therapy session.
Owner:BALAKIN ANDREY VLADIMIROVICH +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com