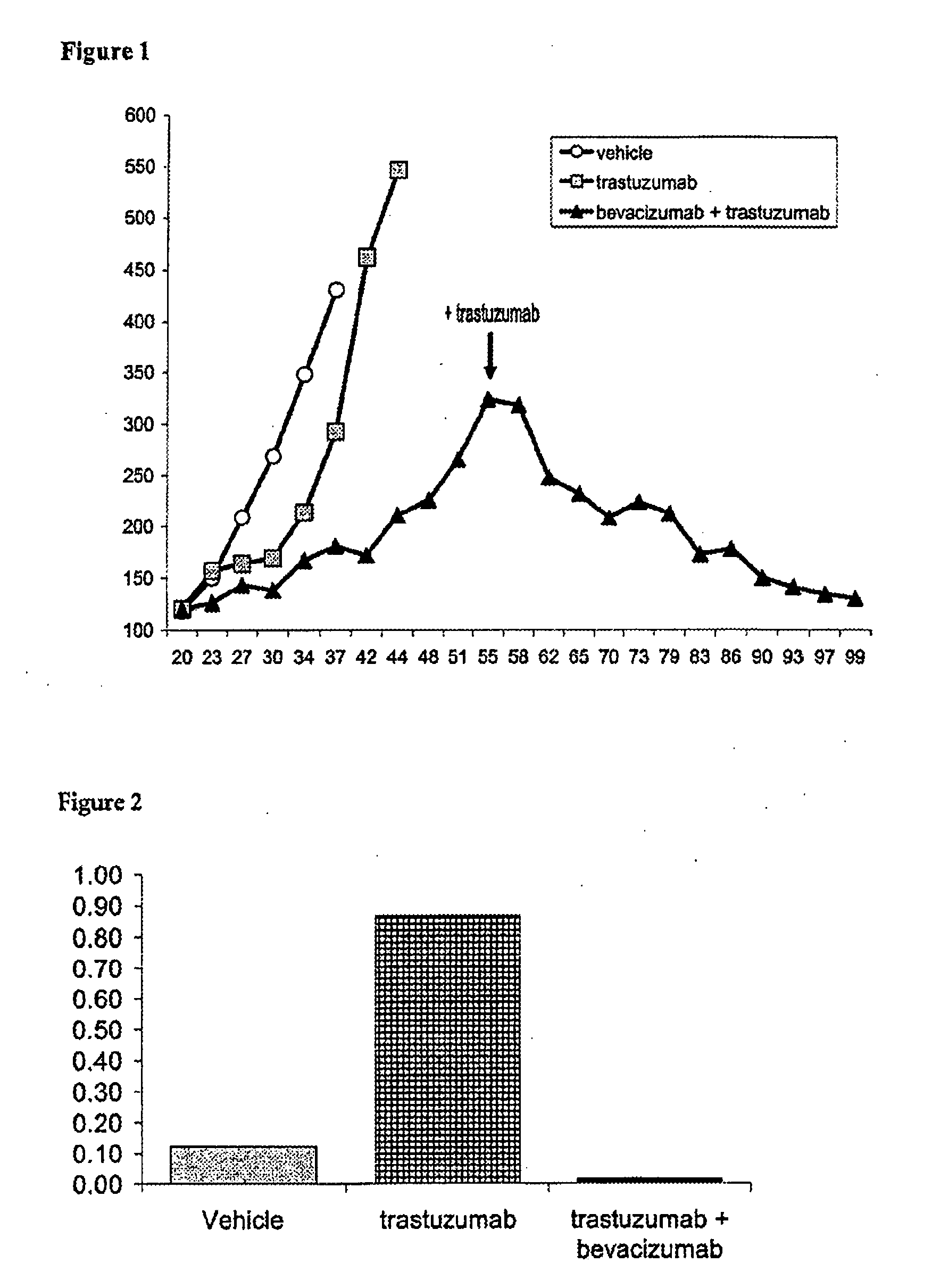
Tumor therapy with an antibody for vascular endothelial growth factor and an antibody for human epithelial growth factor receptor type 2
a technology of epithelial growth factor and antiher2 is applied in the field of combined therapy with antiher2 and antiher2 antibodies, which can solve the problems of poor prognosis and survival, her2 over-expression, etc., and achieve the effects of reducing metastasis, reducing metastasis, and reducing metastasis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0024]Al references cited herein are hereby incorporated by reference in their entirety.
Definitions
[0025]The term “VEGF” according to the invention refers to the vascular endothelial cell growth factor (Swiss-Prot No. P 15692), alternative splicing forms (see e.g. Leung, D. W., et al., Science, 246 (1989) 1306-1309; and Houck, K. A., et al., Mol. Endocrin. 5 (1991) 1806-1814) and active fragments, preferably N-terminal fragments thereof.
[0026]The term “anti-VEGF antibody” according to the invention is an antibody that binds specifically to VEGF. The preferred humanized anti-VEGF antibody or variant anti-VEGF antibody herein binds human VEGF with a Kd value of no more than about 1×10−8M and preferably no more than about 5×10−9M. Preferably the anti-VEGF antibody is a monoclonal antibody that binds to the same epitope as recombinant humanized anti-VEGF monoclonal antibody generated according to Presta, L. G., et al., Cancer Res. 57 (1997) 4593-4599. A preferred antibody is bevacizumab...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular mass | aaaaa | aaaaa |
| pH | aaaaa | aaaaa |
| weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com