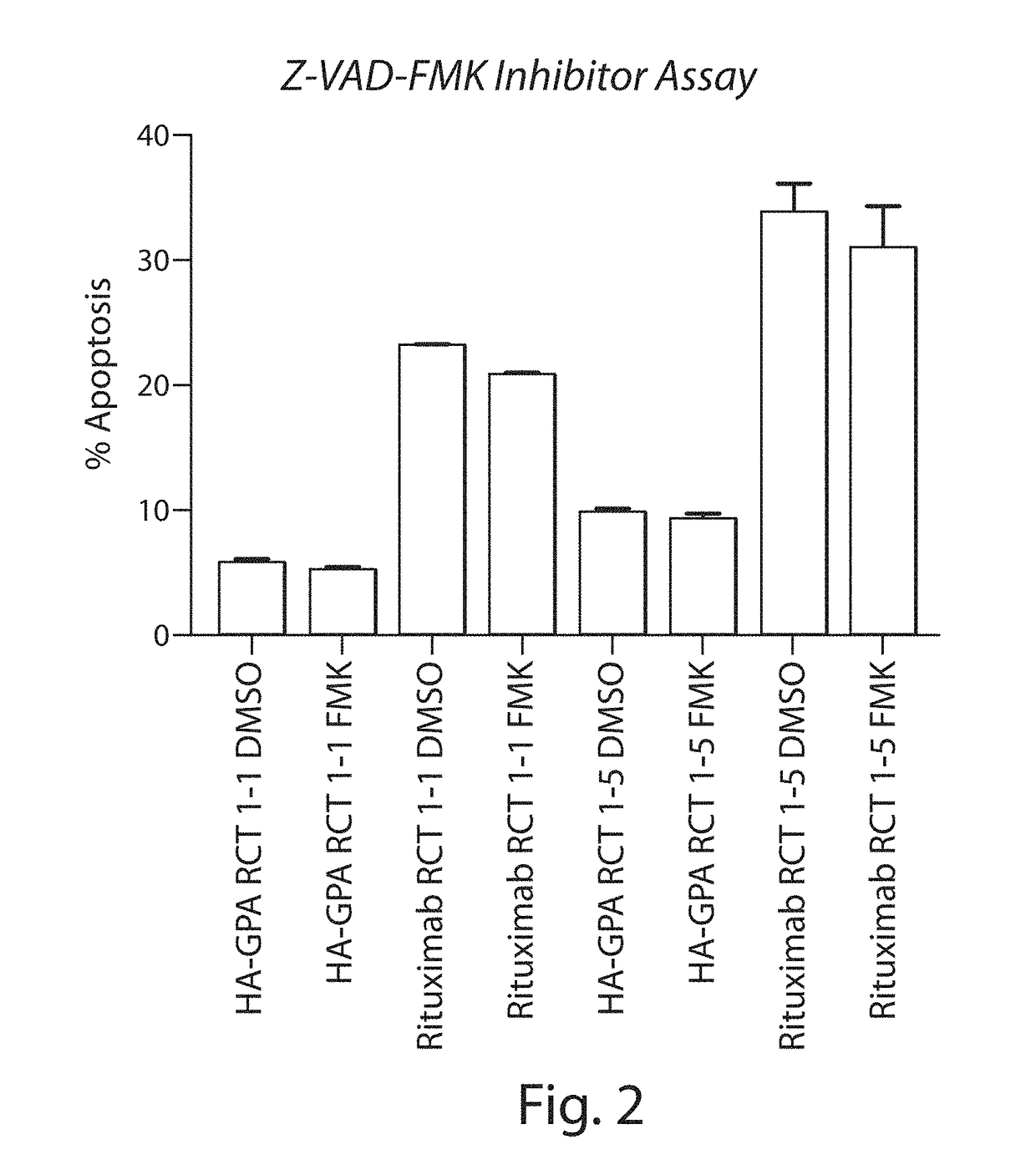
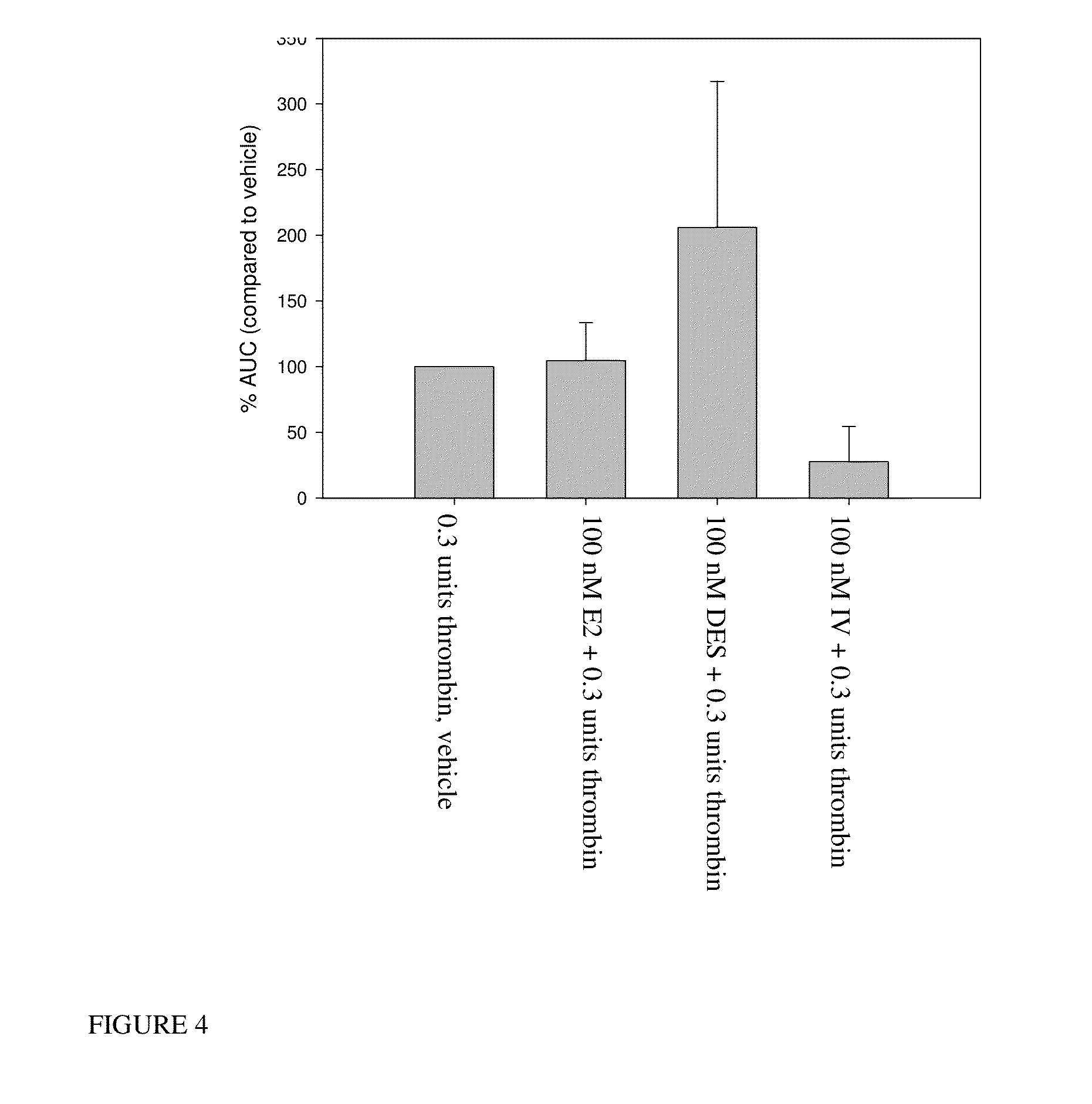
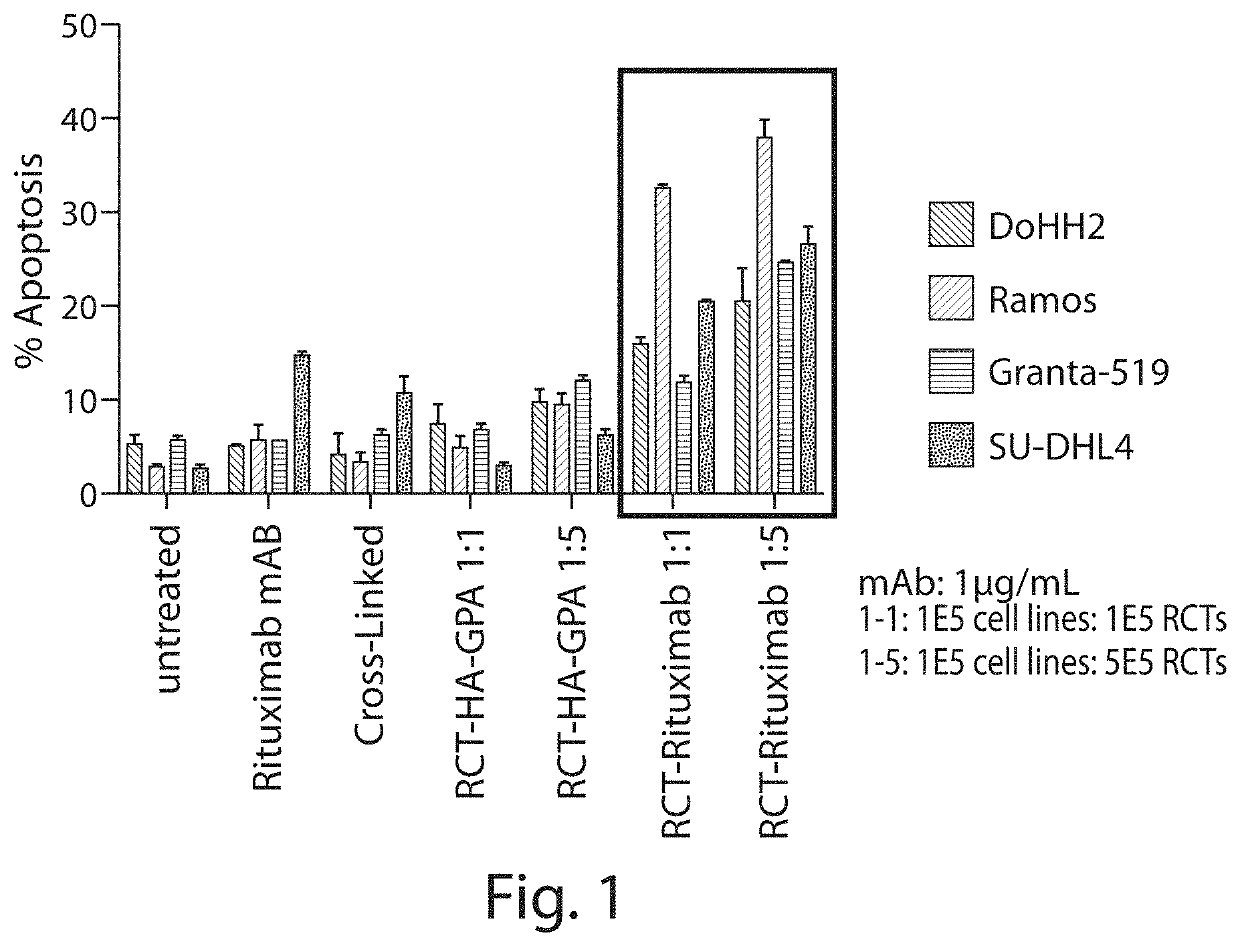
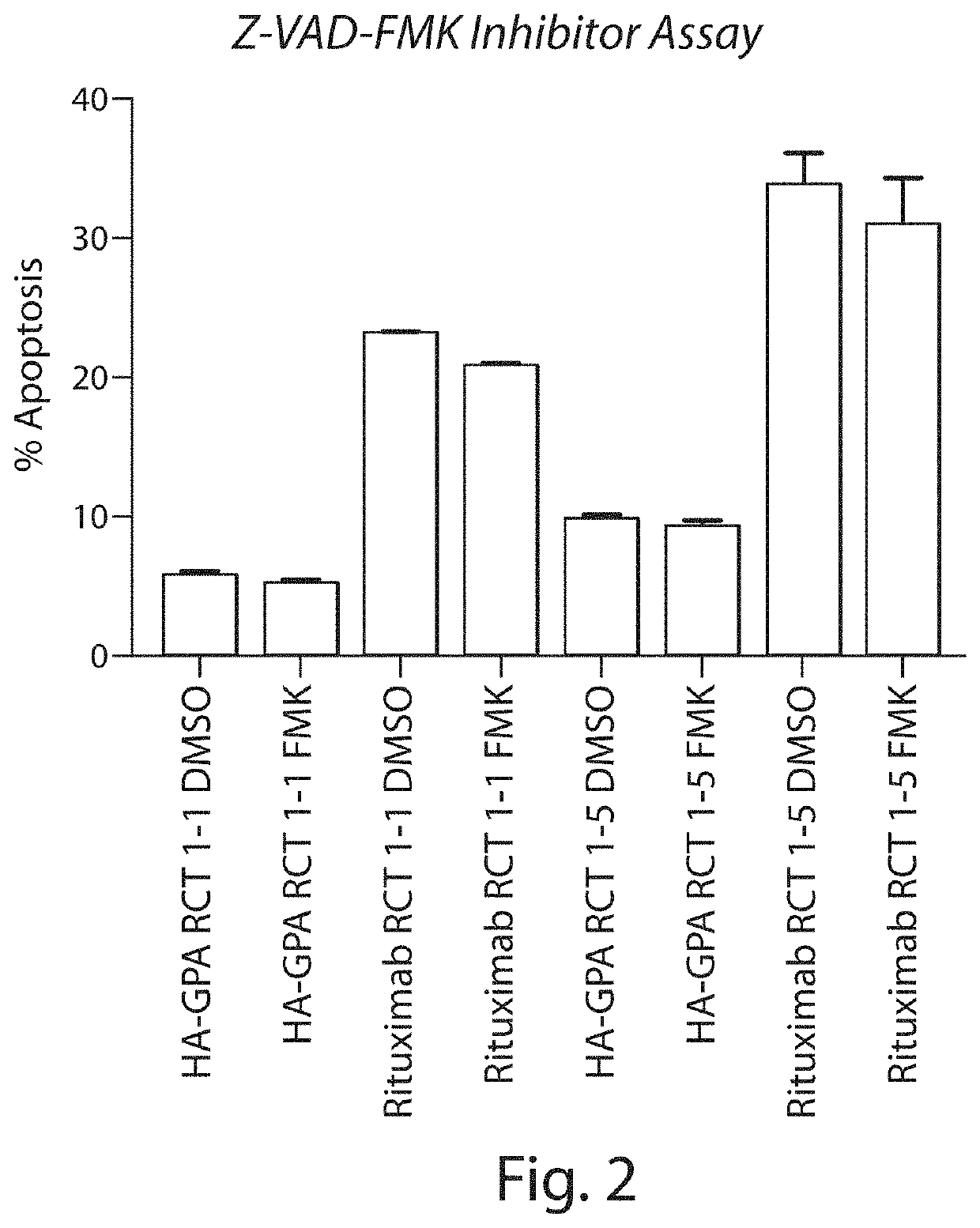
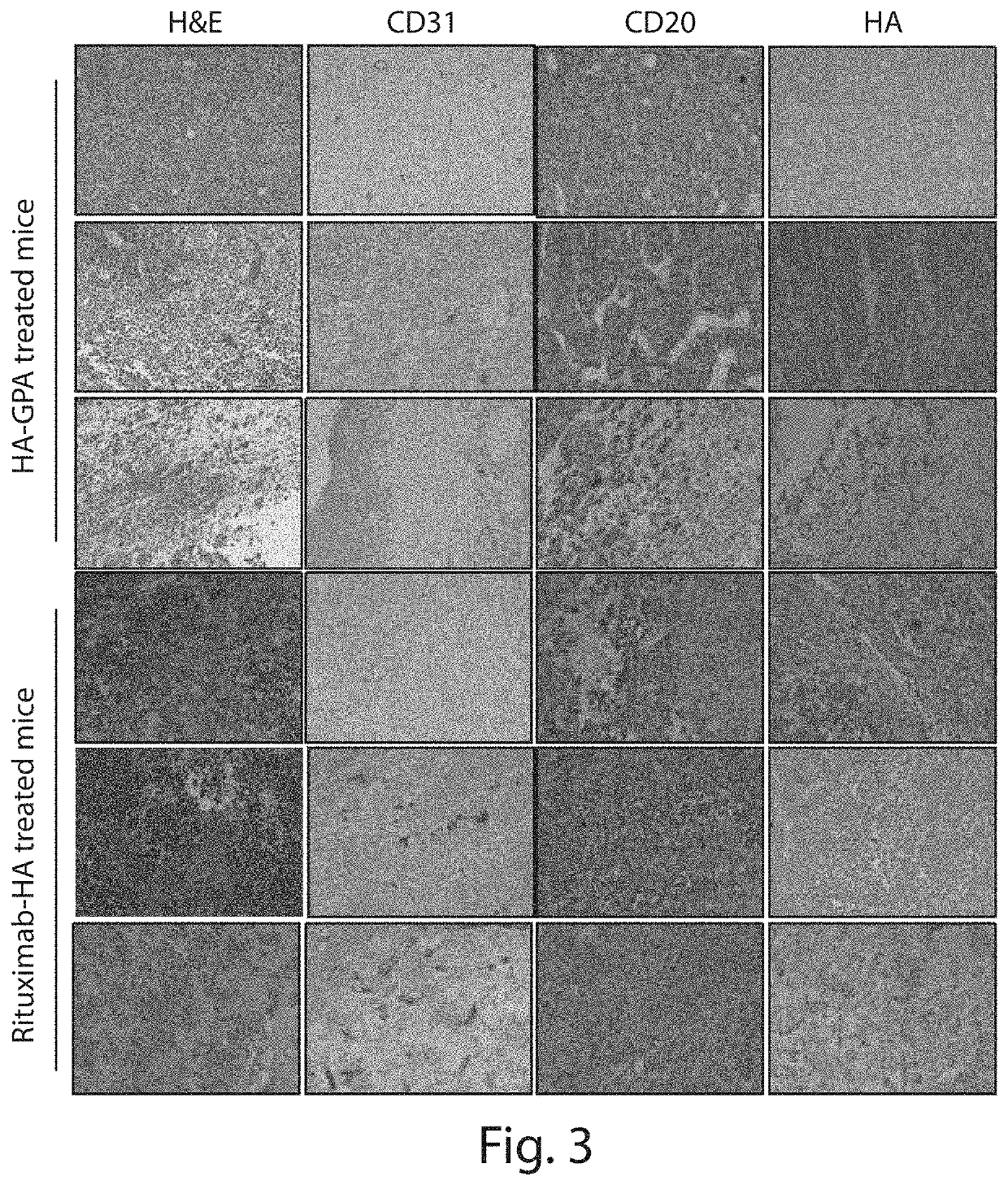
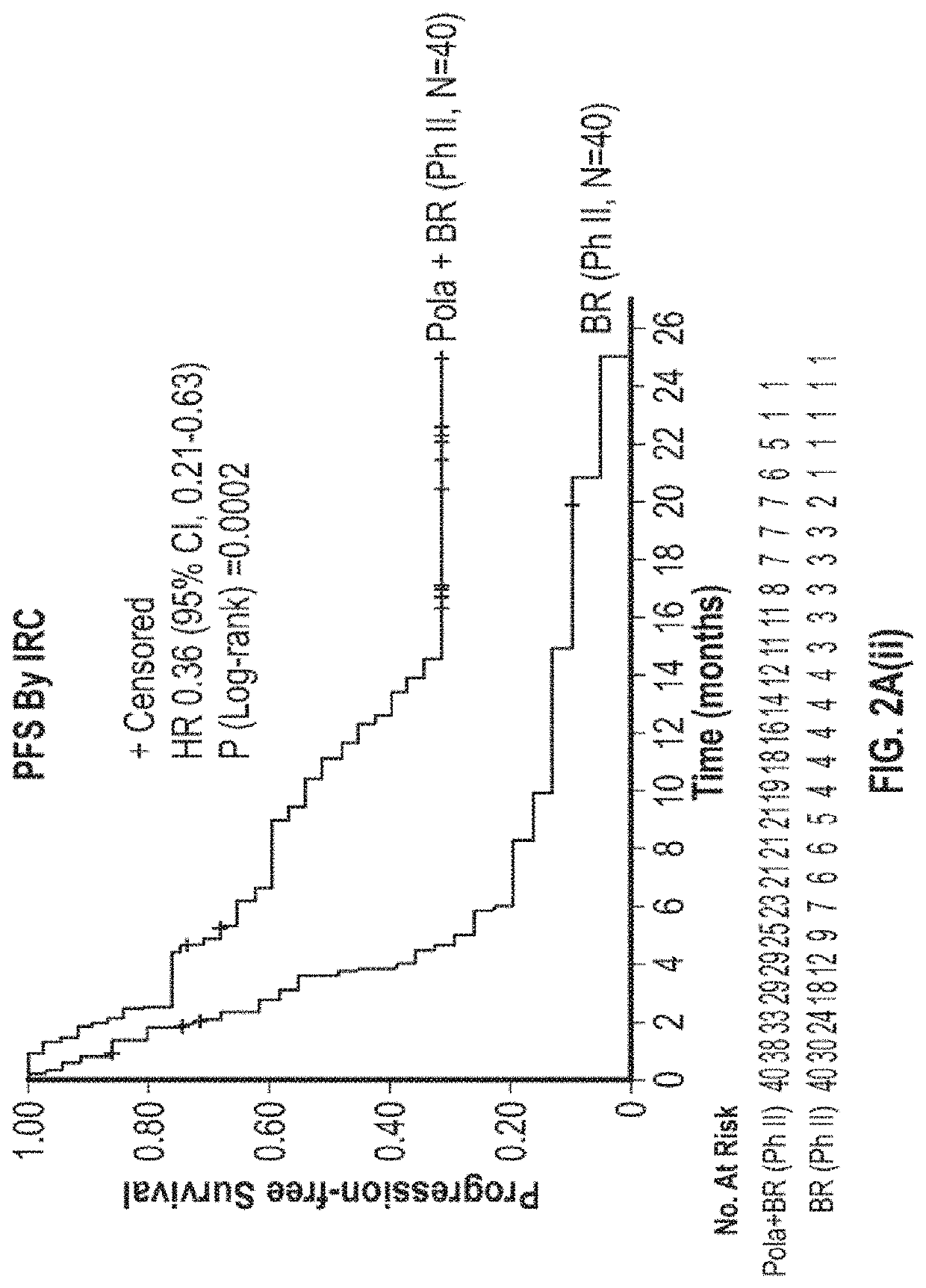
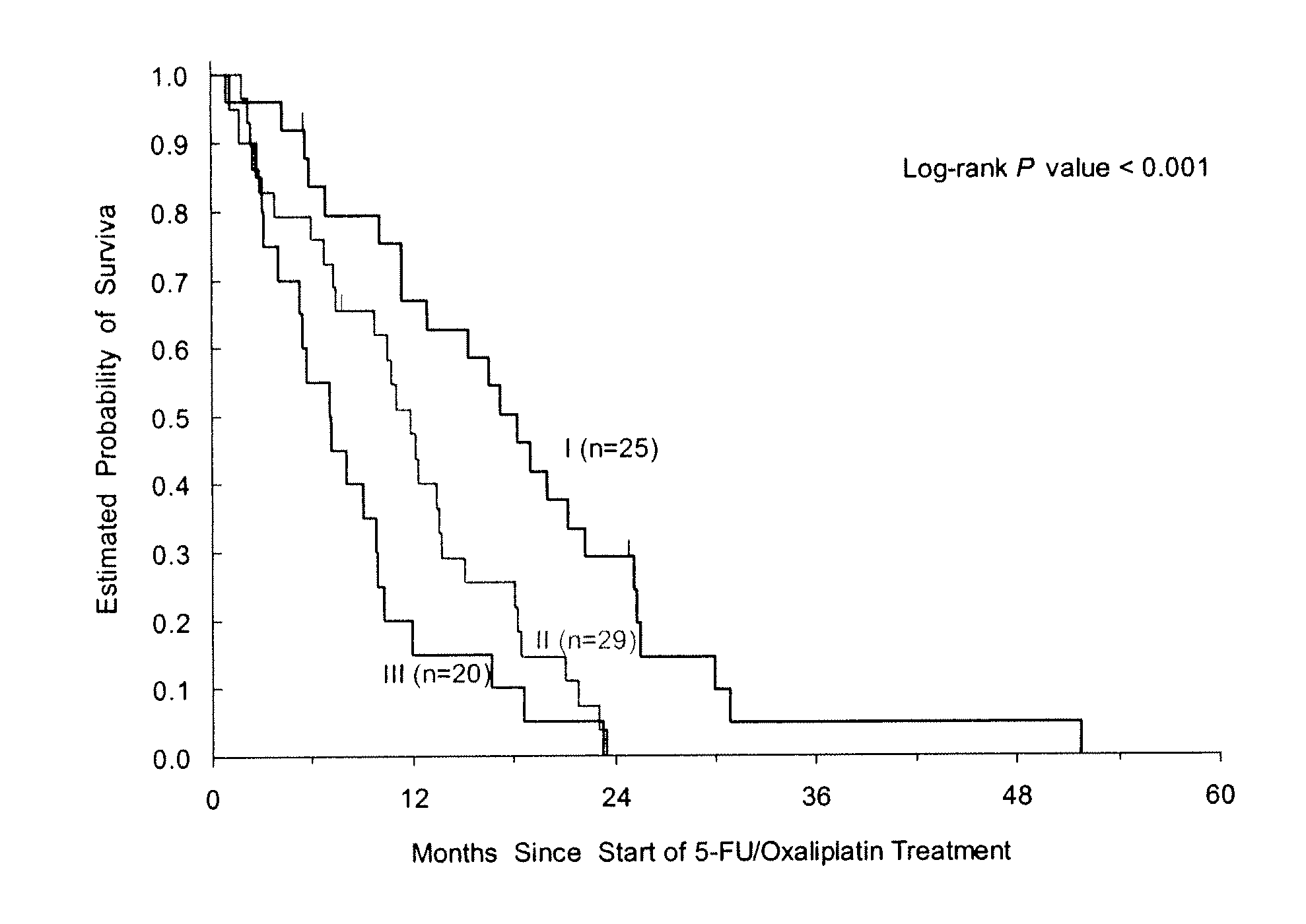
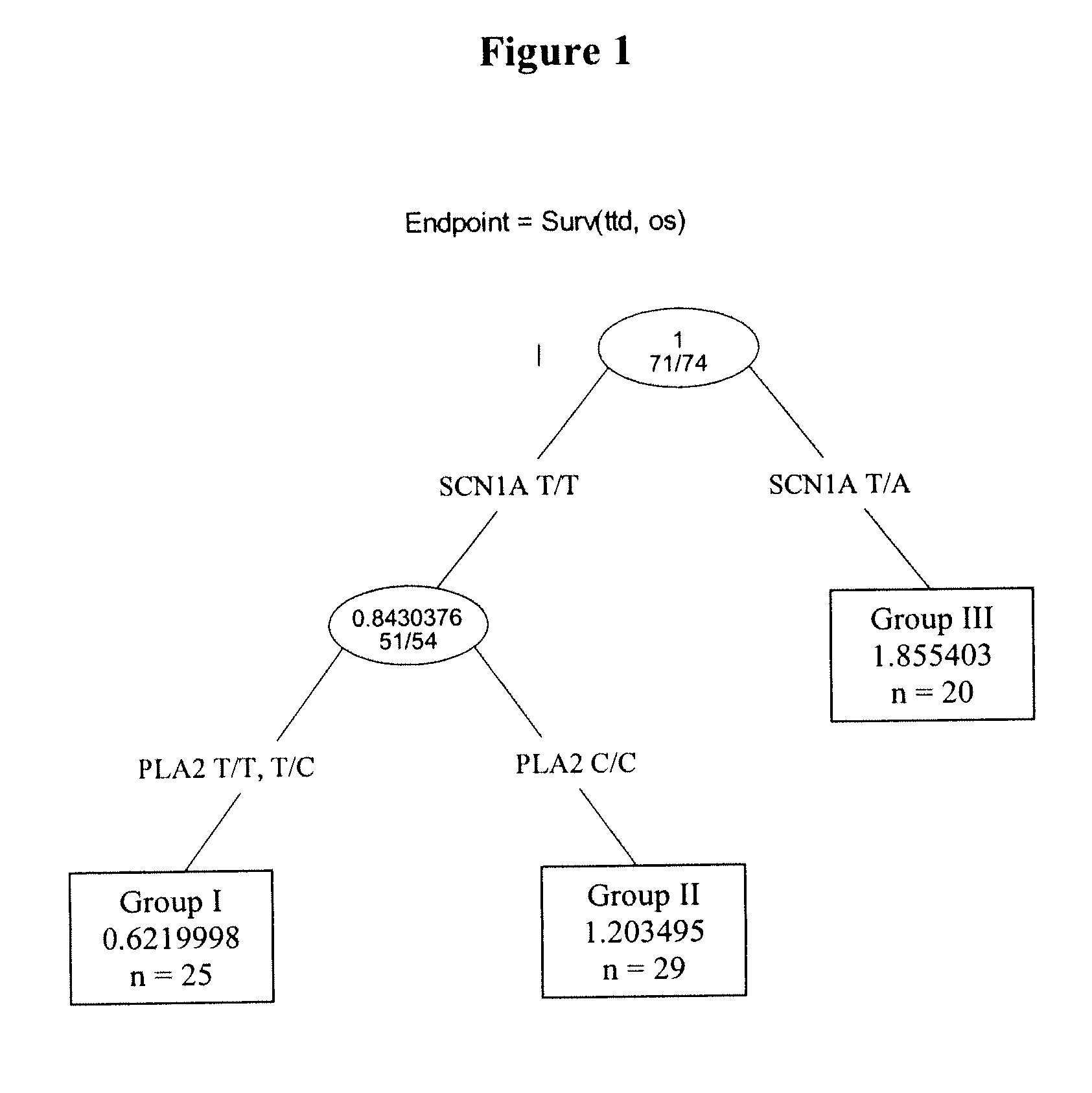
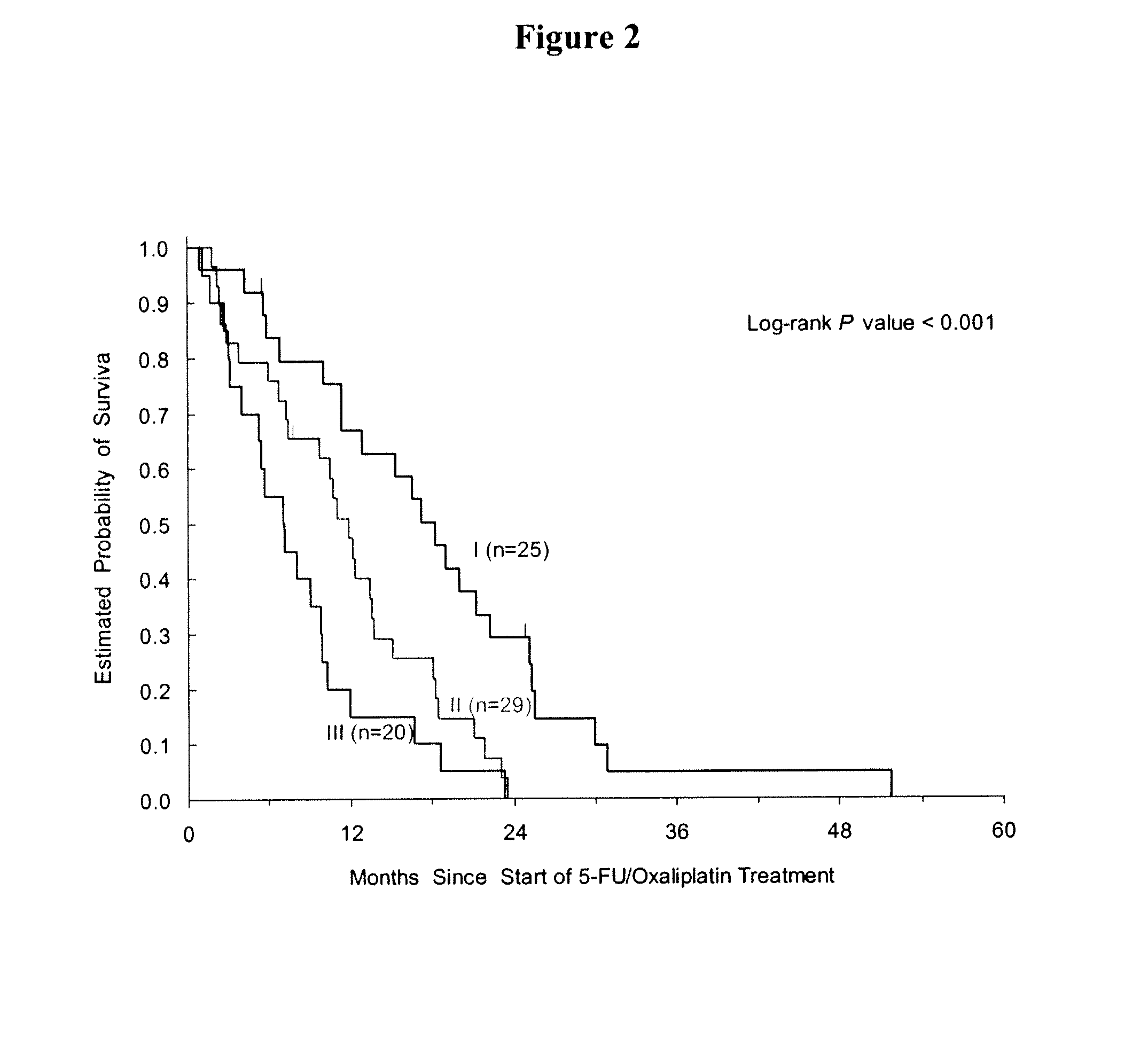
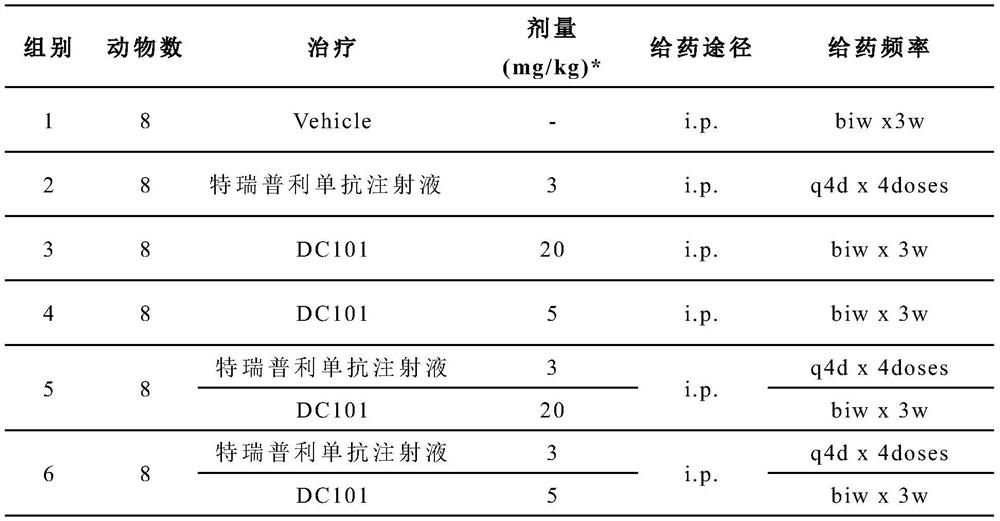
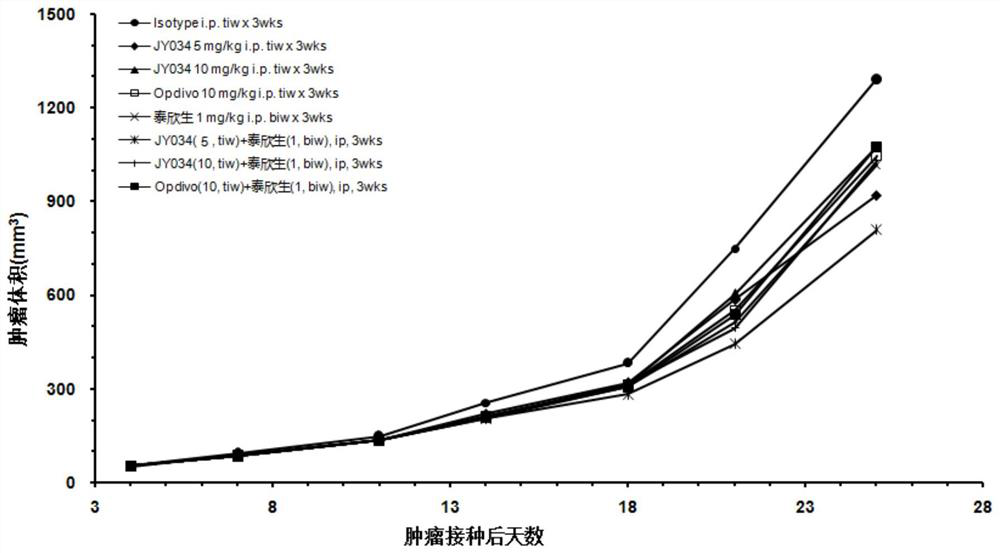
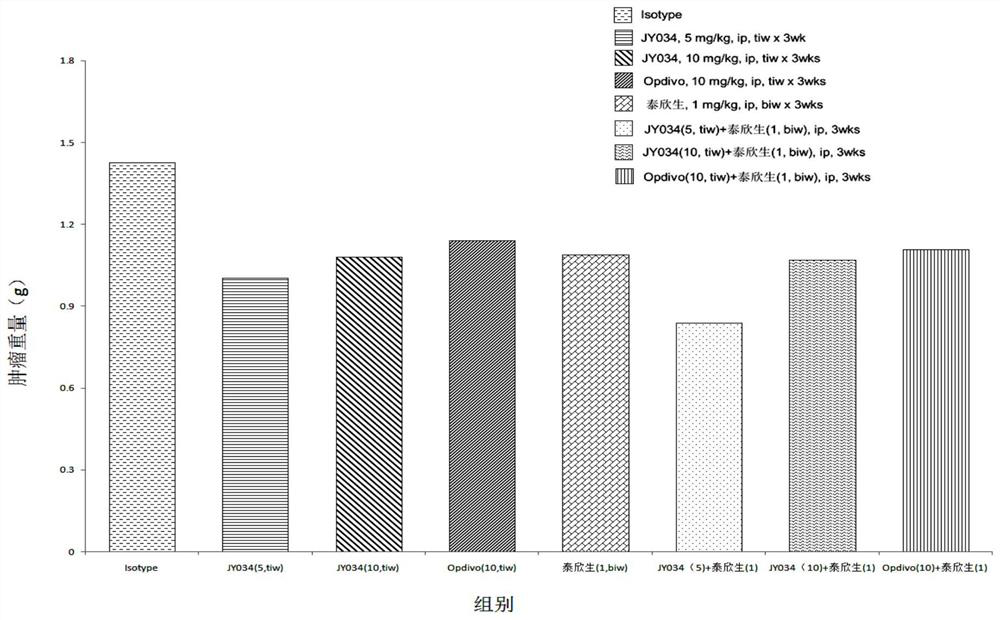
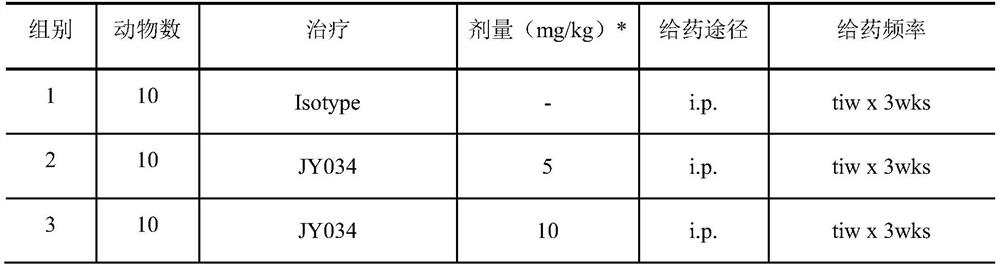
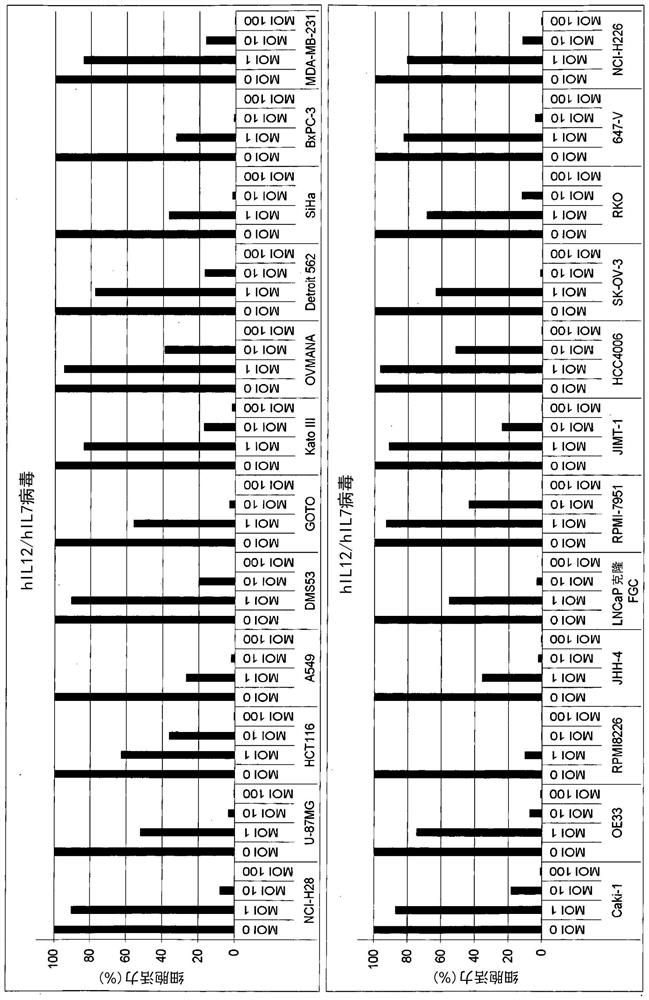
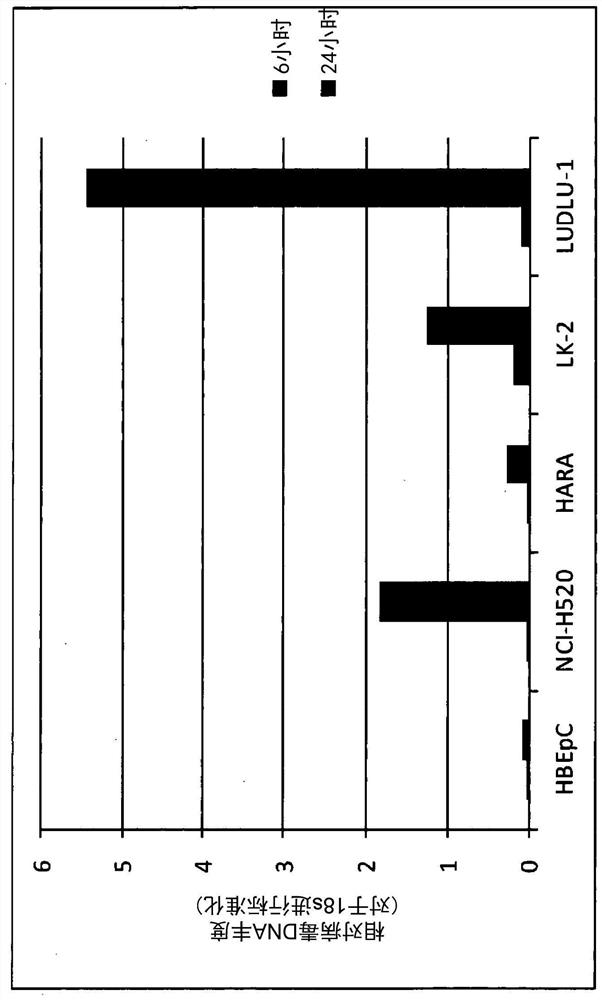
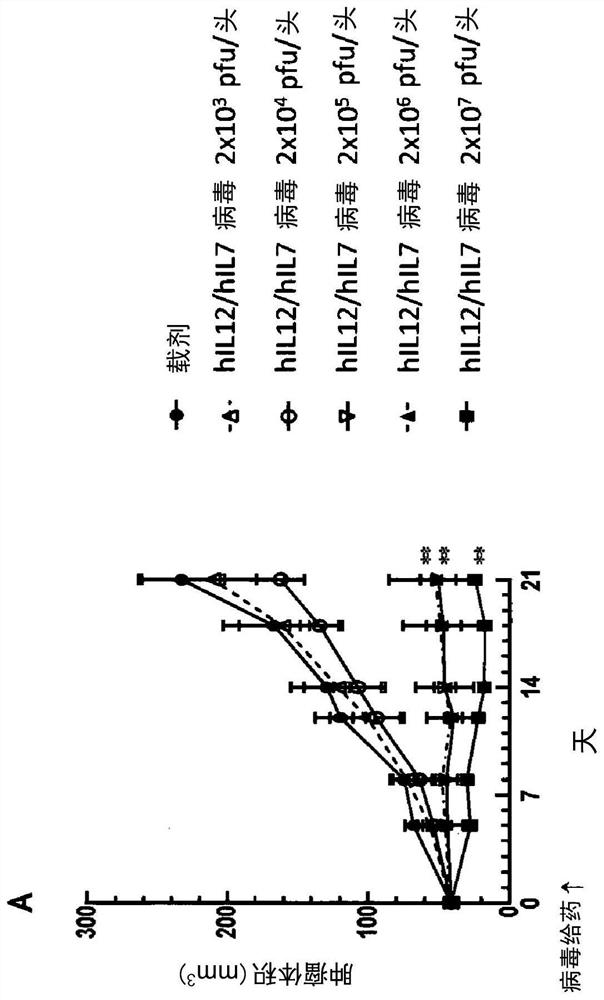
Patents
Literature
38results about How to "Prolong progression-free survival" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
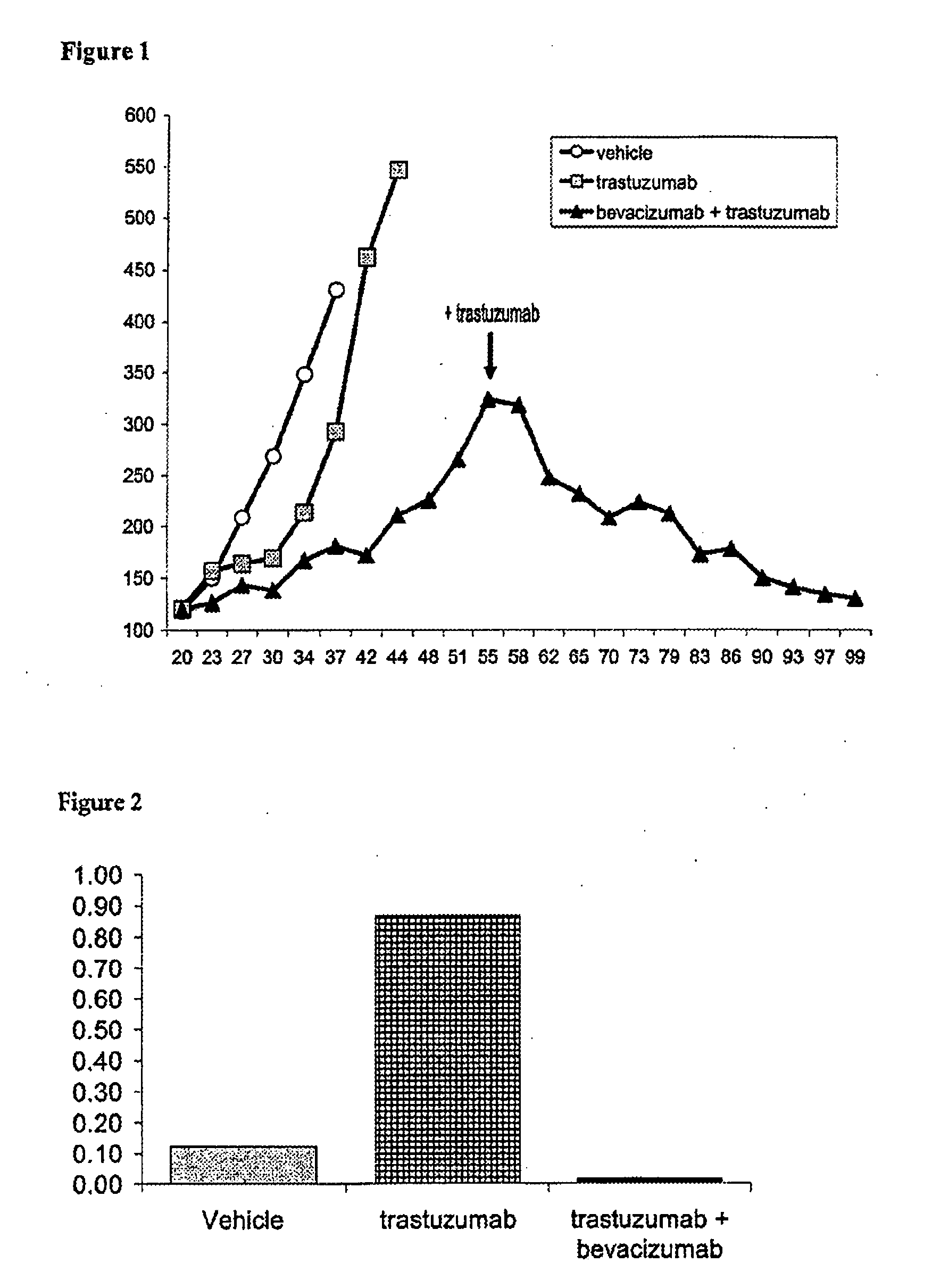
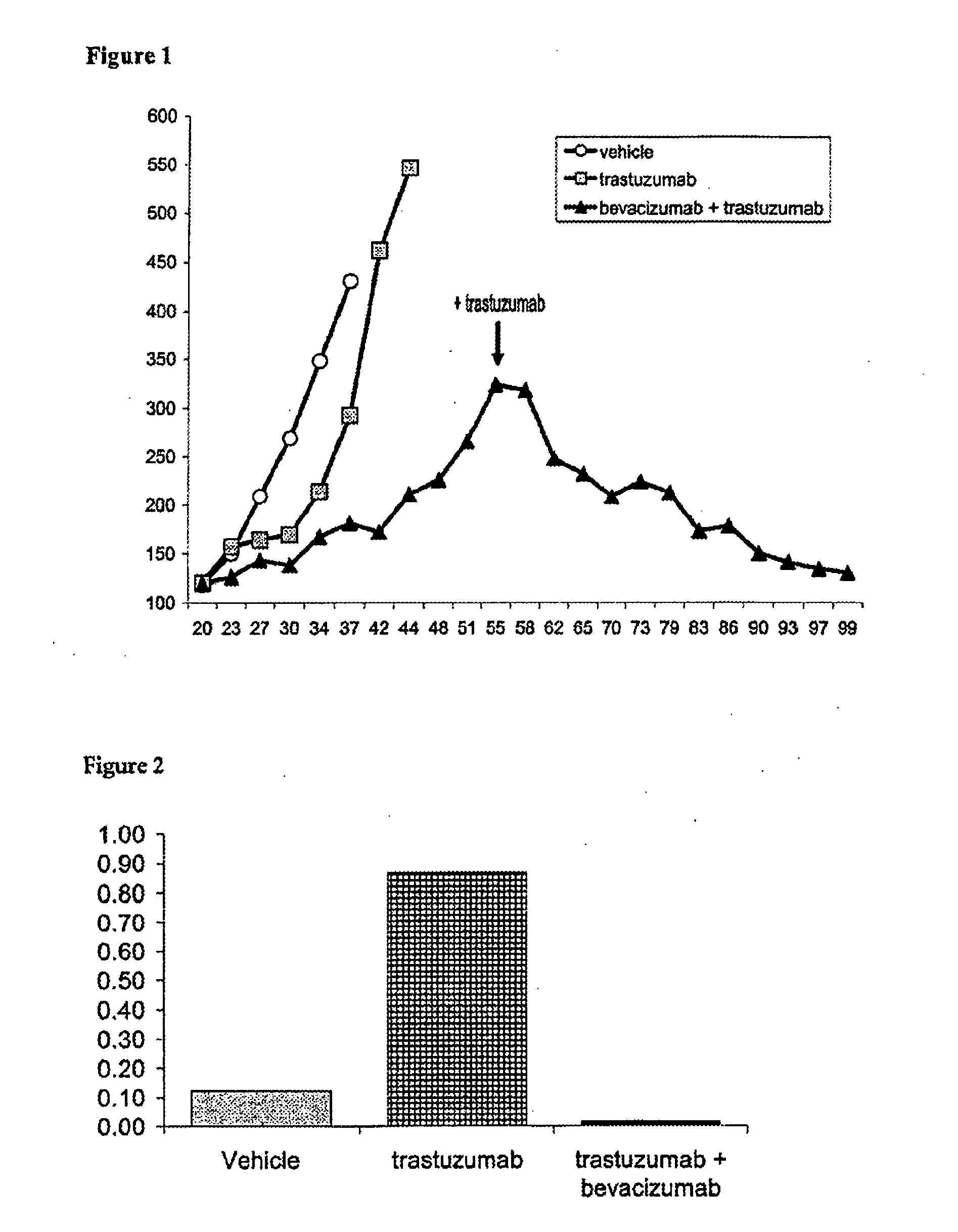
Tumor therapy with an antibody for vascular endothelial growth factor and an antibody for human epithelial growth factor receptor type 2
InactiveUS20070224203A1Prolong survival timeProlong progression-free survivalImmunoglobulins against growth factorsAntibody ingredientsDiseaseTumor therapy
The present invention provides a method of treating a breast cancer disease in a patient who has failed prior treatment with an anti-VEGF antibody, comprising administering to the patient a therapeutically effective amount of an anti-HER2 antibody while continuing said anti-VEGF antibody therapy. The invention also provides corresponding pharmaceutical kits and pharmaceutical compositions.
Owner:F HOFFMANN LA ROCHE & CO AG
Methods of treating cancer
ActiveUS20120070502A1High elongationLow toxicityPowder deliveryOrganic active ingredientsCarboplatinPlatinum
The present invention provides methods and compositions for treating non-small-cell lung cancer (NSCLC) by administering a) a composition comprising nanoparticles that comprise paclitaxel and an albumin and b) a platinum-based agent (e.g., carboplatin). The present application also provides methods of treating prostate cancer by administering to the individual a) an effective amount of a composition comprising nanoparticles comprising docetaxel and an albumin; and b) an effective amount of a steroid.
Owner:ABRAXIS BIOSCI LLC
Methods of treating cancer
ActiveUS20140072643A1Many symptomShorten the progressOrganic active ingredientsHeavy metal active ingredientsCarboplatinDocetaxel-PNP
The present invention provides methods and compositions for treating non-small-cell lung cancer (NSCLC) by administering a) a composition comprising nanoparticles that comprise paclitaxel and an albumin and b) a platinum-based agent (e.g., carboplatin). The present application also provides methods of treating prostate cancer by administering to the individual a) an effective amount of a composition comprising nanoparticles comprising docetaxel and an albumin; and b) an effective amount of a steroid.
Owner:ABRAXIS BIOSCI LLC
Tumor therapy with an antibody for vascular endothelial growth factor and an antibody for human epithelial growth factor receptor type 2
InactiveUS20110064736A1Prolong survival timeProlong progression-free survivalImmunoglobulins against growth factorsAntibody ingredientsDiseaseFactor ii
The present invention provides a method of treating a breast cancer disease in a patient who has failed prior treatment with an anti-VEGF antibody, comprising administering to the patient a therapeutically effective amount of an anti-HER2 antibody while continuing said anti-VEGF antibody therapy. The invention also provides corresponding pharmaceutical kits and pharmaceutical compositions.
Owner:F HOFFMANN LA ROCHE & CO AG
Uses for and article of manufacture including her2 dimerization inhibitor pertuzumab
InactiveUS20130095172A1Extend progression free survivalReduce riskOrganic active ingredientsImmunoglobulins against cell receptors/antigens/surface-determinantsVialVinorelbine
The present application describes uses for and articles of manufacture including Pertuzumab, a first-in-class HER2 dimerization inhibitor. In particular, the application describes methods for extending progression free survival in a HER2-positive breast cancer patient population; combining two HER2 antibodies to treat HER2-positive cancer without increasing cardiac toxicity; treating early-stage HER2-positive breast cancer; treating HER2-positive cancer by co-administering a mixture of Pertuzumab and Trastuzumab from the same intravenous bag; treating HER2-positive metastatic gastric cancer; treating HER2-positive breast cancer with Pertuzumab, Trastuzumab and Vinorelbine; treating HER2-positive breast cancer with Pertuzumab, Trastuzumab and aromatase inhibitor; and treating low HER3 ovarian, primary peritoneal, or fallopian tube cancer. It also describes an article of manufacture comprising a vial with Pertuzumab therein and a package insert providing safety and / or efficacy data thereon; a method of making the article of manufacture; and a method of ensuring safe and effective use of Pertuzumab related thereto. In addition the application describes an intravenous (IV) bag containing a stable mixture of Pertuzumab and Trastuzumab suitable for administration to a cancer patient.
Owner:GENENTECH INC
Methods of treating lung cancer
ActiveUS20160015681A1High elongationLow toxicityBiocideHeavy metal active ingredientsDiabetes mellitusCarboplatin
The present invention provides methods and compositions for treating non-small-cell lung cancer (NSCLC) by administering a) a composition comprising nanoparticles that comprise paclitaxel and an albumin and b) a platinum-based agent (e.g., carboplatin), wherein the individual has diabetes, has four or more metastatic sites, and / or is at least about 70 years old.
Owner:ABRAXIS BIOSCI LLC
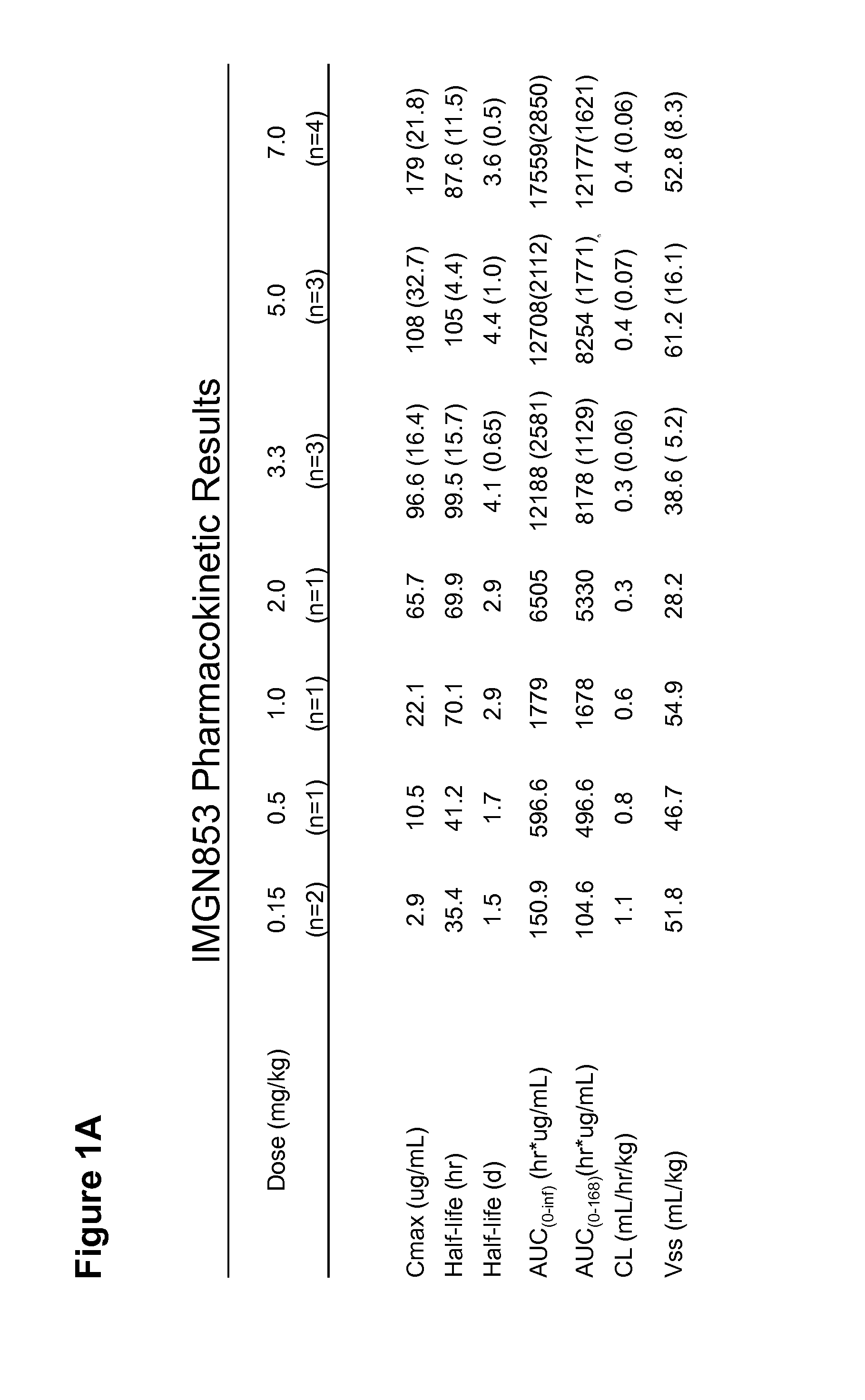
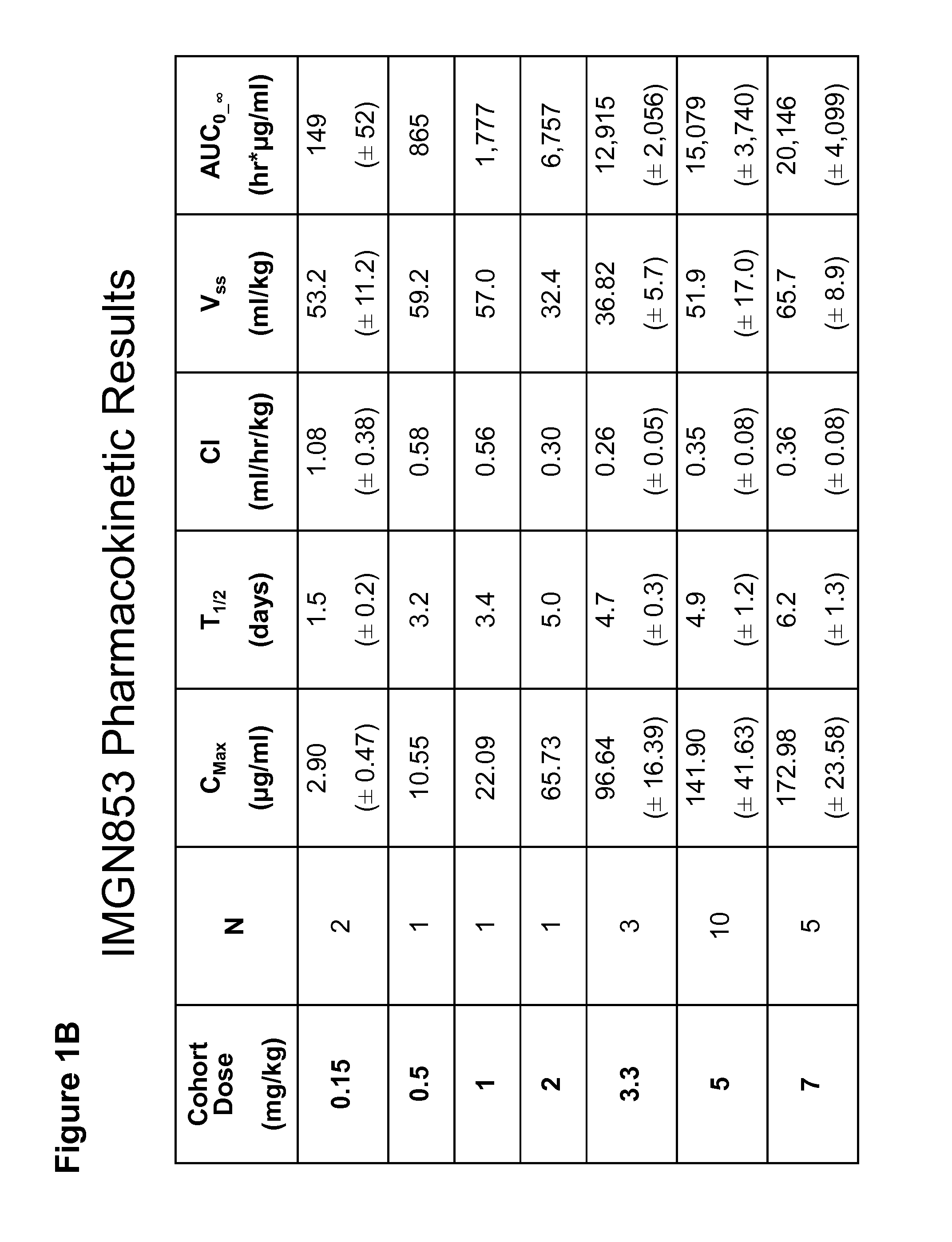
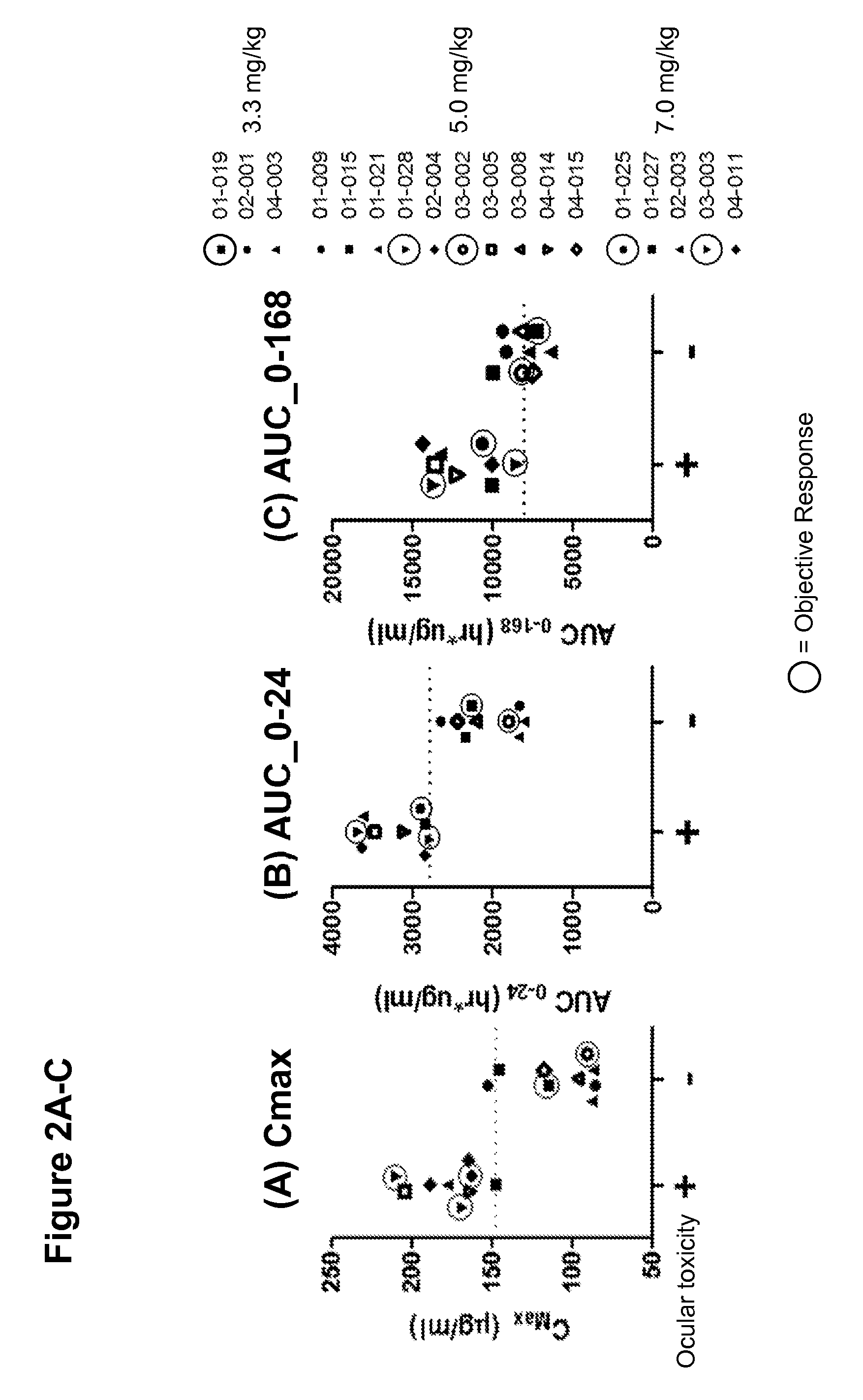
Anti-FOLR1 Immunoconjugate Dosing Regimens
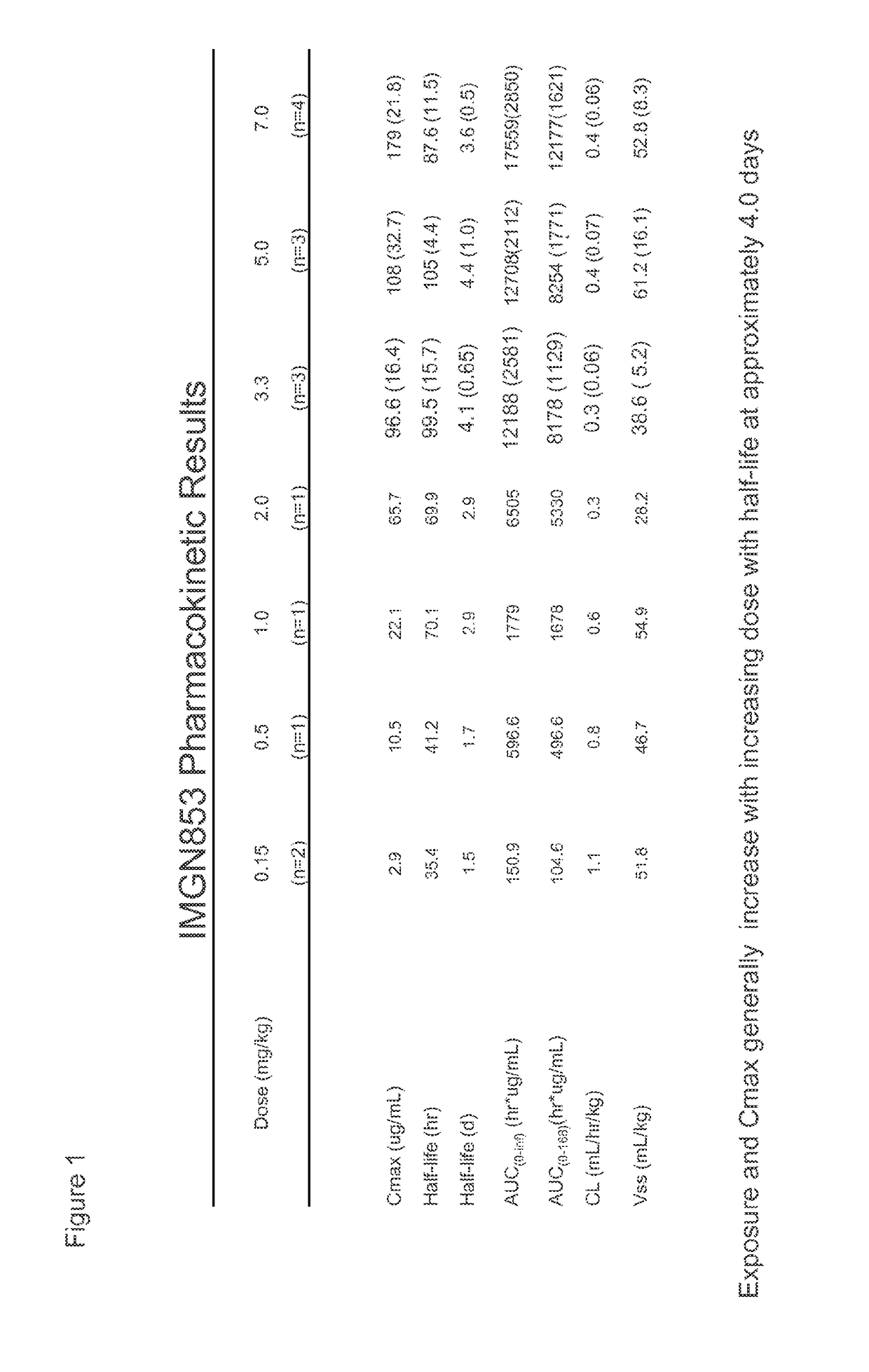
PendingUS20150132323A1High CmaxHigh initial auc valueImmunoglobulins against cell receptors/antigens/surface-determinantsAntibody ingredientsDosing regimenImmunoconjugate
Methods of administering immunoconjugates that bind to FOLR1 are provided. The methods comprise administering an anti-FOLR1 immunoconjugate to a person in need thereof, for example, a cancer patient, at a therapeutically effective dosing regimen that results in minimal adverse effects.
Owner:IMMUNOGEN INC
Compositions and methods related to cell systems for penetrating solid tumors
InactiveUS20180153989A1Improve securityImprovement of side effectsMammal material medical ingredientsImmunoglobulins against cell receptors/antigens/surface-determinantsErythroid cellCell system
The present disclosure provides, e.g., compositions and method for treating cancers, e.g., solid tumors. In embodiments, the compositions comprise an erythroid cell expressing an exogenous polypeptide, e.g., a polypeptide that promotes penetration of the erythroid cell into the solid tumor.
Owner:RUBIUS THERAPEUTICS
Peritumoral and intratumoral materials for cancer therapy
PendingUS20180021253A1Reduce tumor volumeProlong progression-free survivalOrganic active ingredientsPeptide/protein ingredientsSpecific immunityNeoplasm
Owner:PRESIDENT & FELLOWS OF HARVARD COLLEGE
Uses for and article of manufacture including her2 dimerization inhibitor pertuzumab
InactiveUS20160175438A1Prolong progression-free survivalReduce riskOrganic active ingredientsAntibody ingredientsHER2 Positive Breast CancerProgression-free survival
The present application describes uses for Pertuzumab, a first-in-class HER2 dimerization inhibitor. In particular, the application describes methods for extending progression free survival in a HER2-positive breast cancer patient population; combining two HER2 antibodies to treat HER2-positive cancer without increasing cardiac toxicity; and treating HER2-positive cancer by co-administering a mixture of Pertuzumab and Trastuzumab from the same intravenous bag.
Owner:GENENTECH INC
Immunogenic/therapeutic glycoconjugate compositions and uses thereof
PendingUS20160339089A1Improve the level ofLow antigenicityImmunoglobulins against animals/humansCancer antigen ingredientsDiseaseAdjuvant
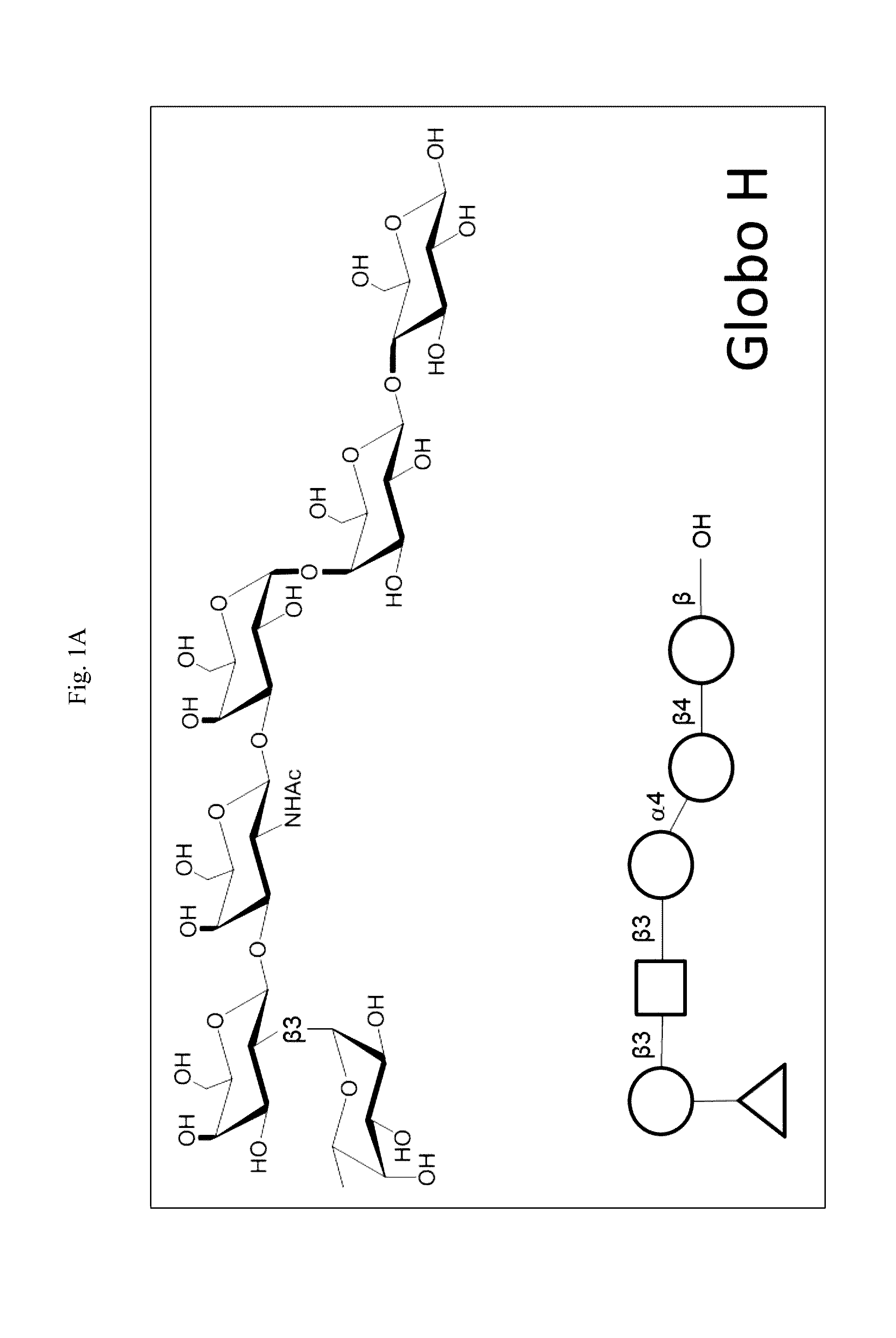
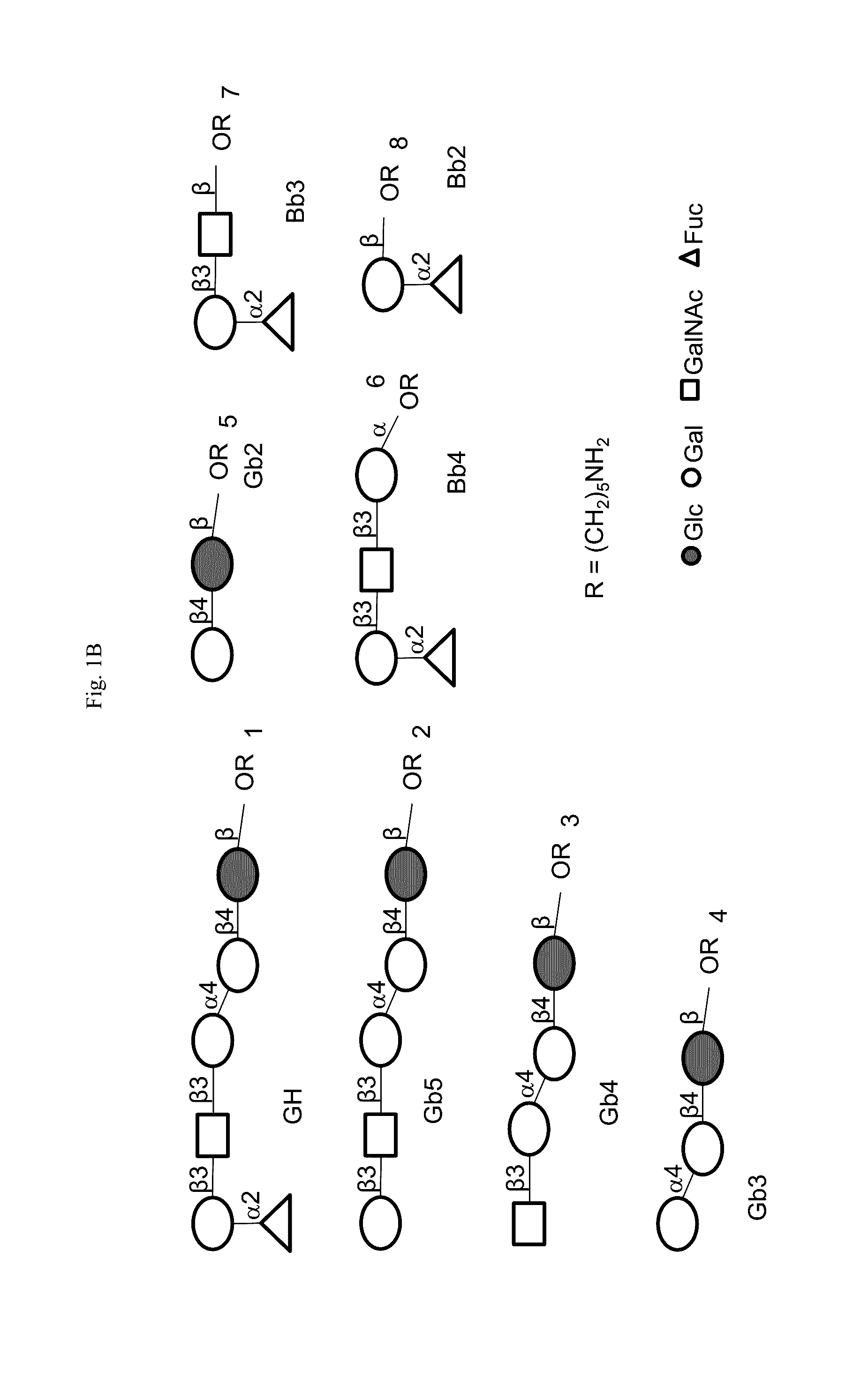
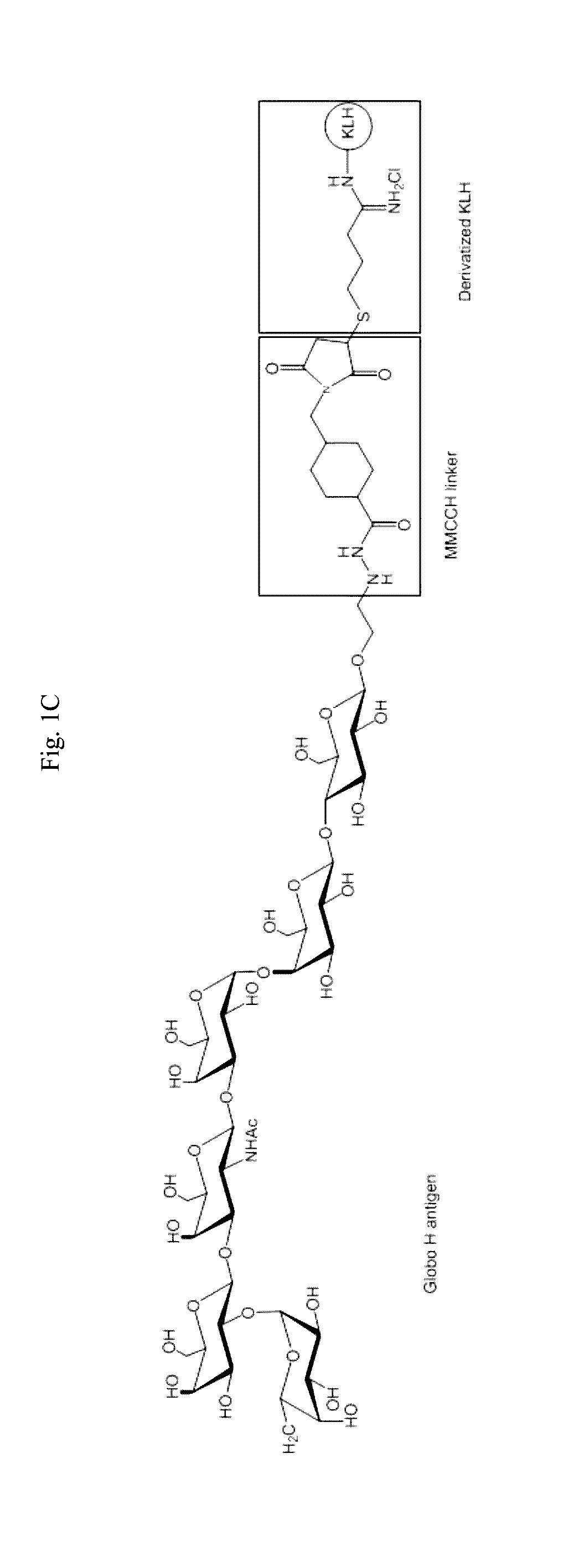
The present disclosure encompasses immunogenic / therapeutic compositions including Globo H-KLH glycoconjugates (OBI-822) and / or therapeutic adjuvants (OBI-821 / OBI-834) as well as methods of making and using the same to treat proliferative diseases such as cancer. The therapeutic conjugates include an antigen linked to a carrier. In particular the therapeutic conjugates include a Globo H moiety and a KLH moiety and / or a derivatized KLH moiety subunit optionally linked via a linker. The therapeutic compositions are in part envisaged to act as cancer vaccines for boosting the body's natural ability to protect itself, through the immune system from dangers posed by damaged or abnormal cells such as cancer cells. Exemplary immune response can be characterized by reduction of the severity of disease, including but not limited to, prevention of disease, delay in onset of disease, decreased severity of symptoms, decreased morbidity and delayed mortality.
Owner:OBI PHARMA
Anti-folr1 immunoconjugate dosing regimens
InactiveUS20170239367A1Minimizes unwanted side-effectsGain decreaseOrganic active ingredientsPharmaceutical delivery mechanismDosing regimenAdverse effect
Methods of administering immunoconjugates that bind to FOLR1 are provided. The methods comprise administering an anti-FOLR1 immunoconjugate to a person in need thereof, for example, a cancer patient, at a therapeutically effective dosing regimen that results in minimal adverse effects.
Owner:IMMUNOGEN INC
Estrogen receptor ligands and methods of use thereof
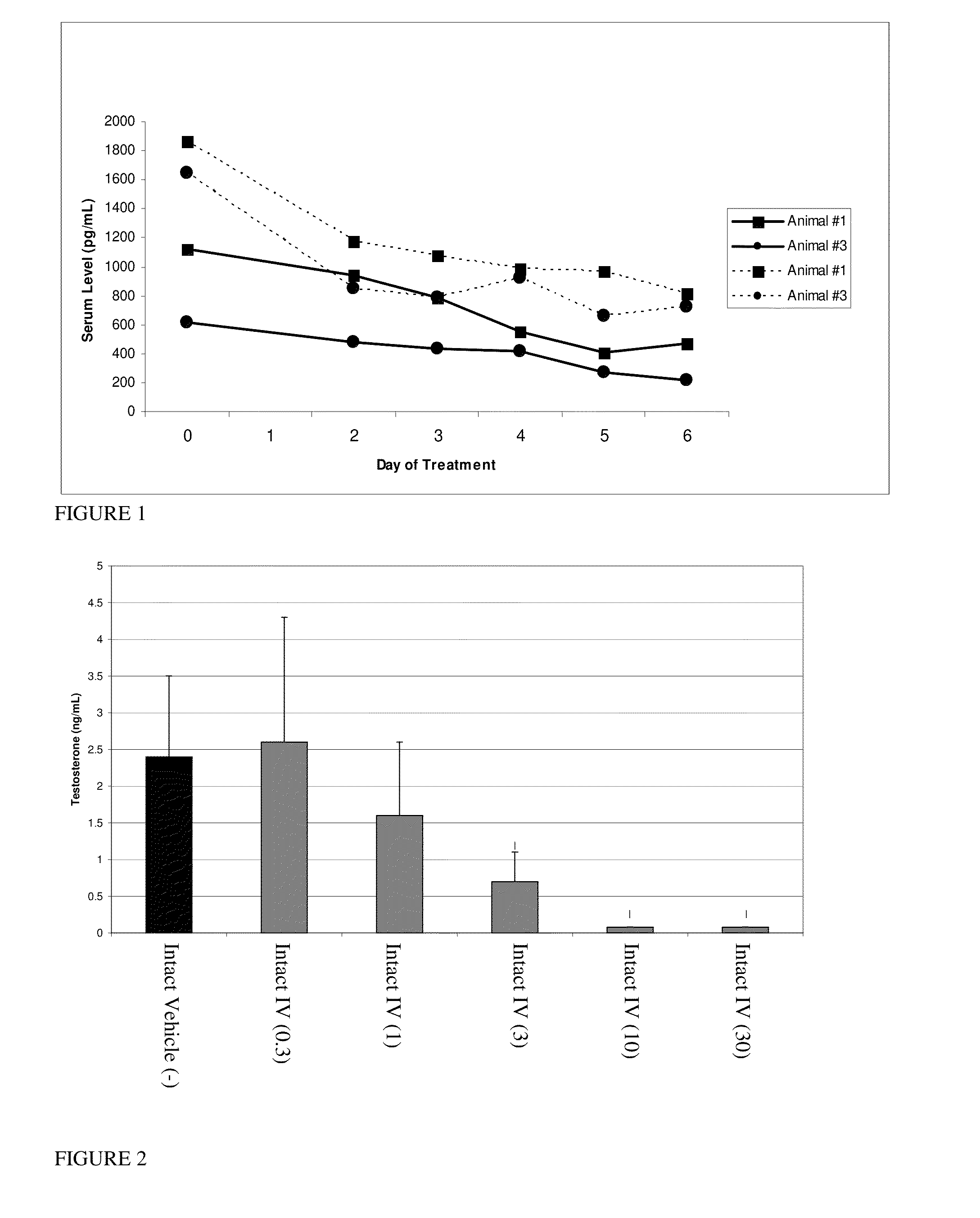
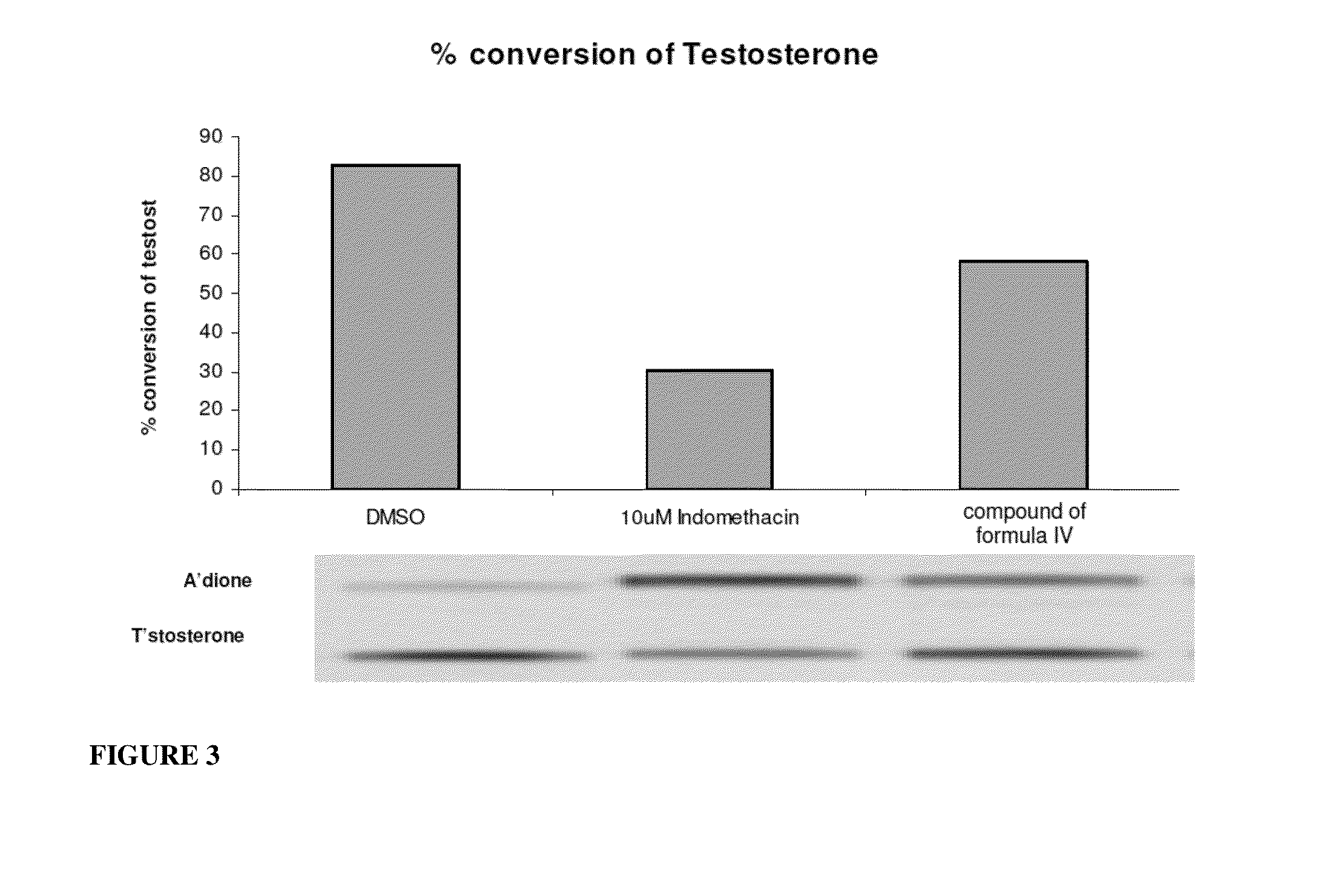
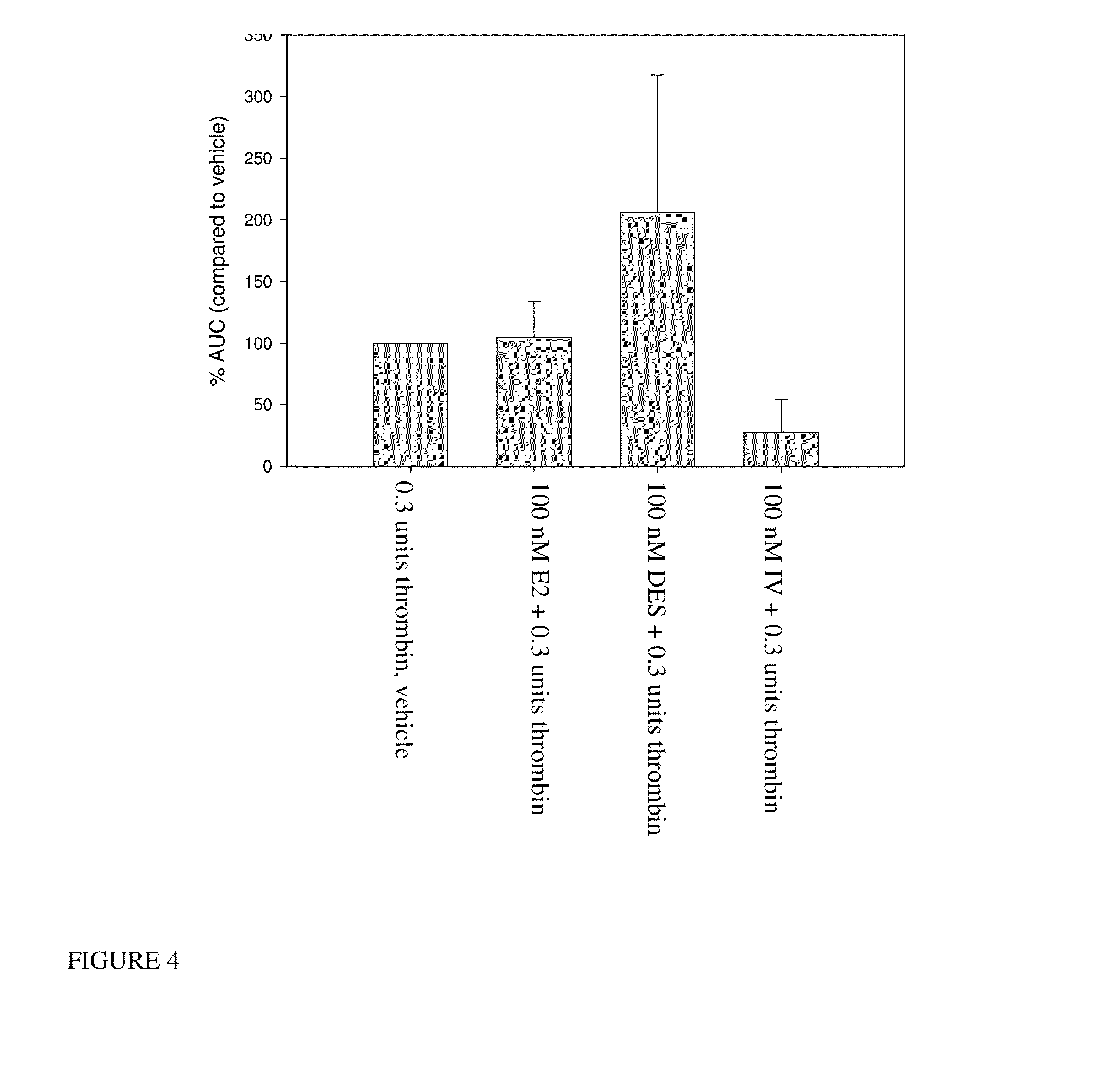
InactiveUS20140187641A1Prolong progression-free survivalImprove survivalBiocideOrganic chemistryGynecomastiaEstrogen receptor
The present invention relates to methods for reducing testosterone levels in a male subject and methods of treating, suppressing, reducing the incidence, reducing the severity, or inhibiting prostate cancer, advanced prostate cancer, castration resistant prostate cancer (CRPC), metastatic castration resistant prostate cancer (mCRPC), and methods of reducing high or increasing PSA levels and / or increasing SHBG levels in a subject suffering from prostate cancer, advanced prostate cancer, castration resistant prostate cancer (CRPC) and metastatic castration resistant prostate cancer (mCRPC). The compounds of this invention suppress free or total testosterone levels despite castrate levels secondary to ADT and reduce high or increasing PSA levels. This reduction in testosterone levels may be used to treat prostate cancer, advanced prostate cancer, CRPC and mCRPC without causing bone loss, decreased bone mineral density, increased risk of bone fractures, increased body fat, hot flashes and / or gynecomastia.
Owner:GTX INCORPORATED
Methods and compositions for immune dis-inhibition
InactiveUS20170038382A1Reduced activityReduce the amount requiredCompounds screening/testingCompound screeningAbnormal tissue growthCancer cell
The disclosure provides methods and compositions for immune dis-inhibition. In certain embodiments, the methods comprise administering an effective amount of an agent that decreases the amount of a soluble cytotoxic receptor or inhibits its activity. In certain embodiments, the agent inhibits the proliferation, growth, or survival of cancer cells, decreases the size or a tumor, or inhibits tumor growth.
Owner:NANOTICS LLC
Compositions and methods related to cell systems for penetrating solid tumors
InactiveUS20200345845A1Increase secretionProlong progression-free survivalImmunoglobulins against cell receptors/antigens/surface-determinantsMammal material medical ingredientsErythroid cellCell system
The present disclosure provides, e.g., compositions and method for treating cancers, e.g., solid tumors. In embodiments, the compositions comprise an erythroid cell expressing an exogenous polypeptide, e.g., a polypeptide that promotes penetration of the erythroid cell into the solid tumor.
Owner:RUBIUS THERAPEUTICS
Methods of treating lung cancer
ActiveUS10744110B2Many symptomShorten the progressPowder deliveryHeavy metal active ingredientsCarboplatinDiabetes mellitus
The present invention provides methods and compositions for treating non-small-cell lung cancer (NSCLC) by administering a) a composition comprising nanoparticles that comprise paclitaxel and an albumin and b) a platinum-based agent (e.g., carboplatin), wherein the individual has diabetes, has four or more metastatic sites, and / or is at least about 70 years old.
Owner:ABRAXIS BIOSCI LLC
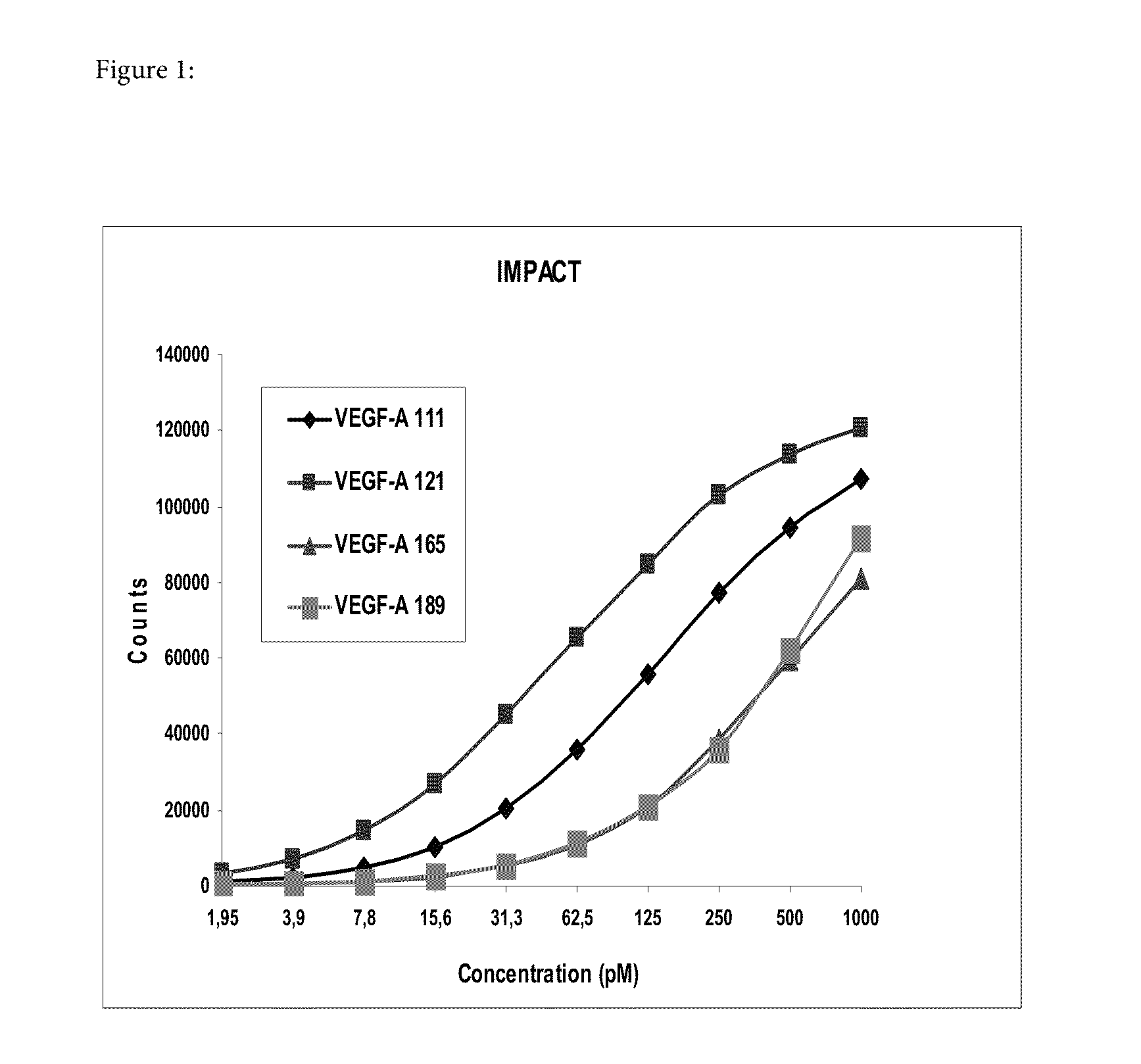
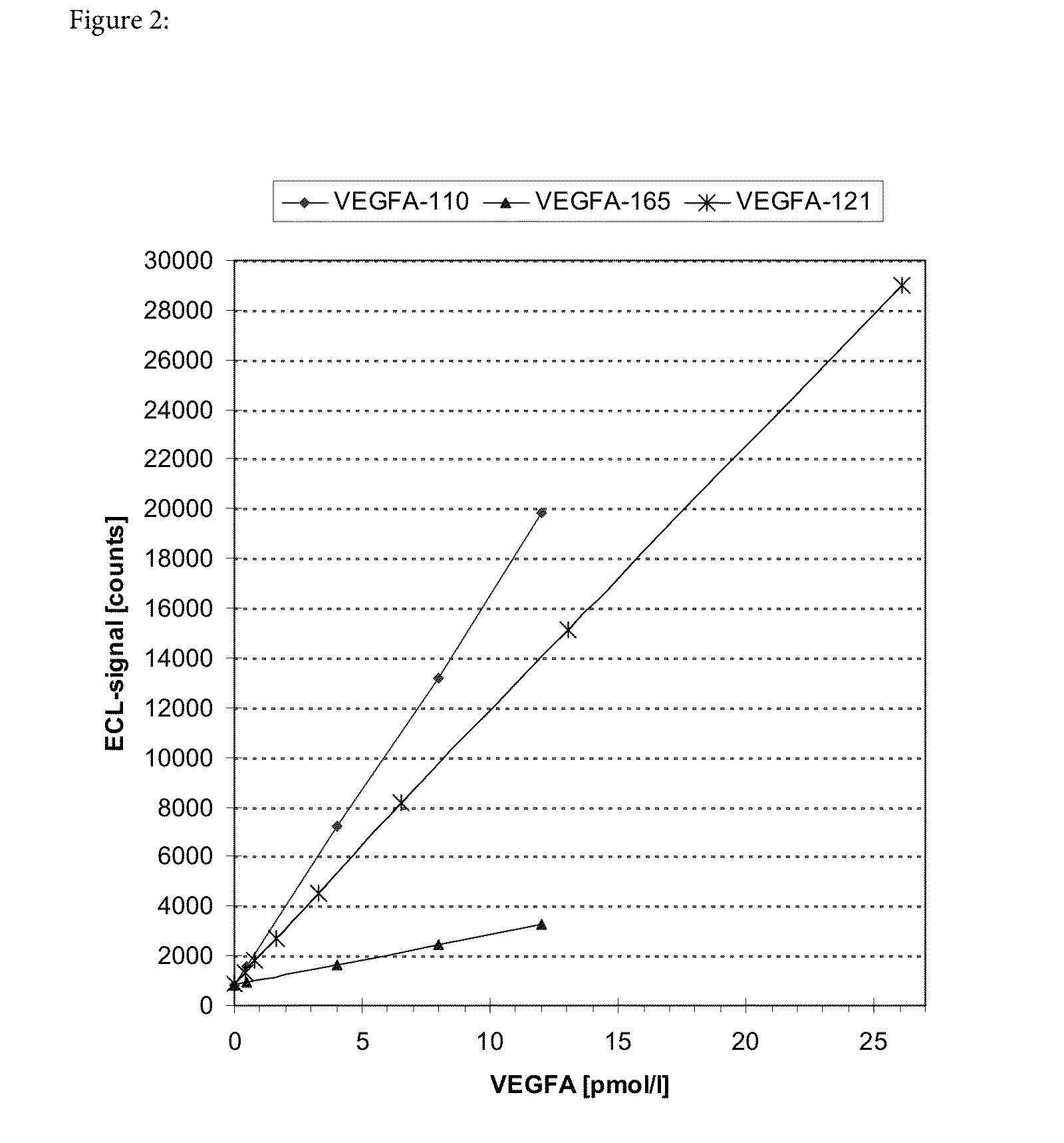
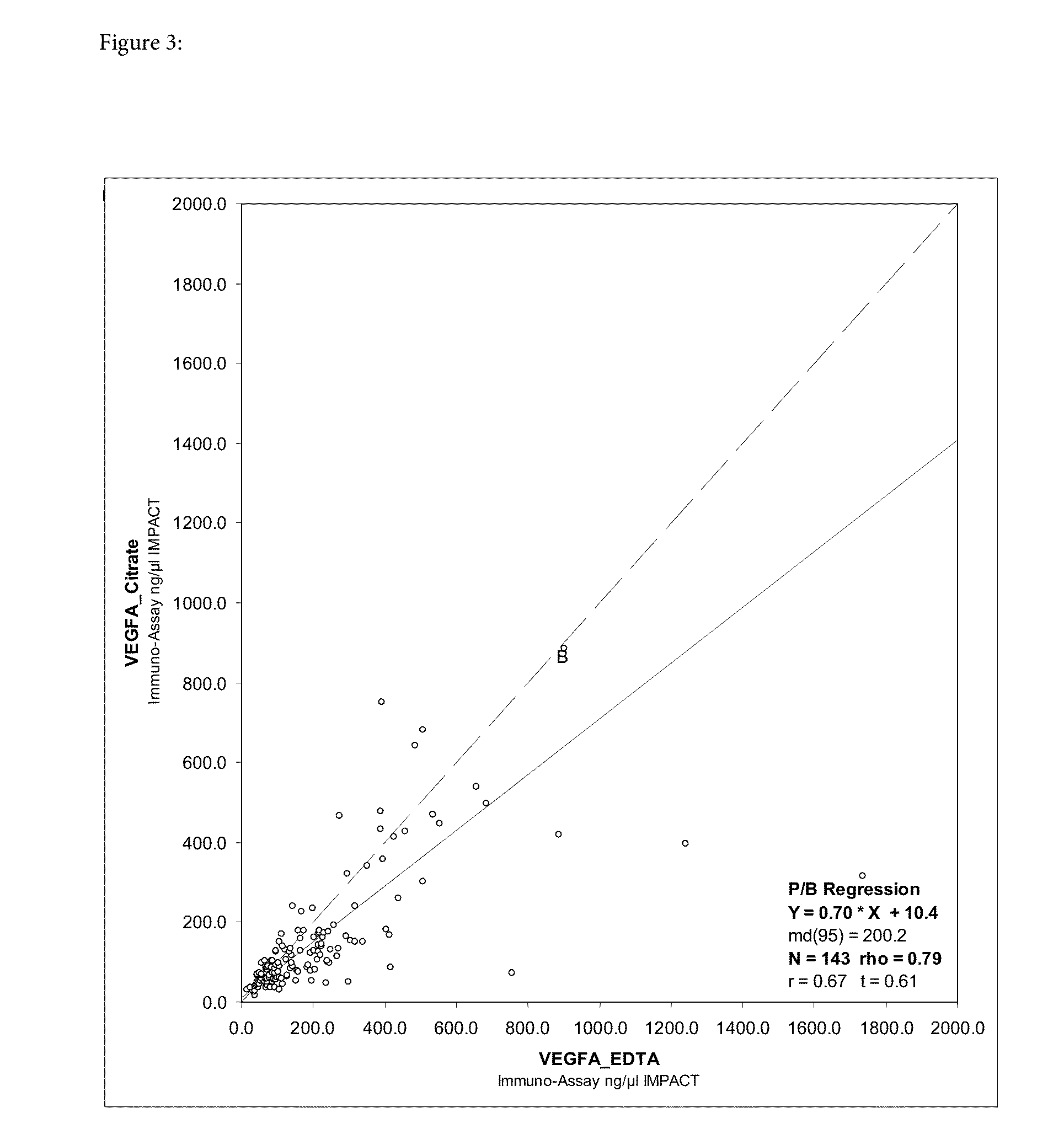
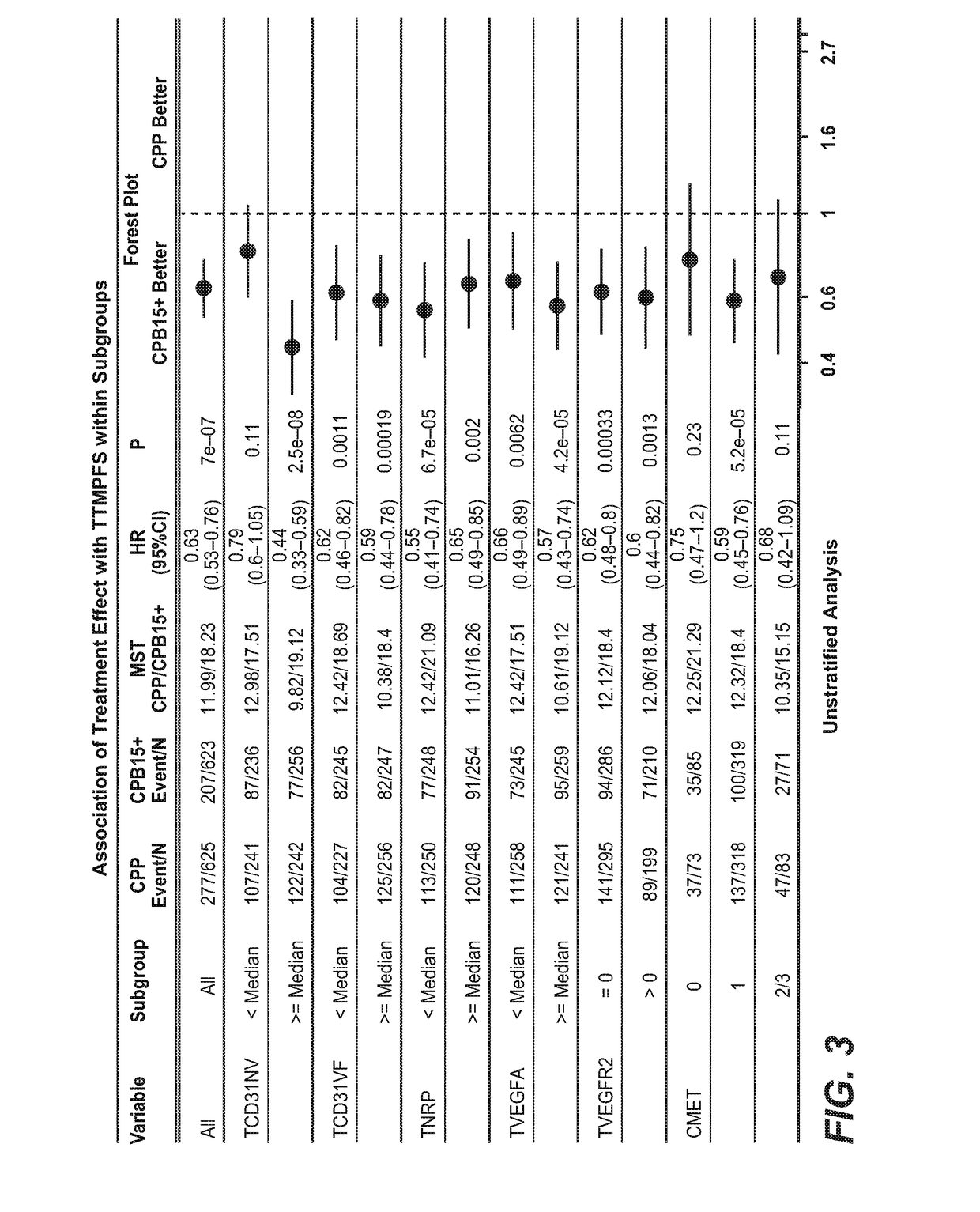
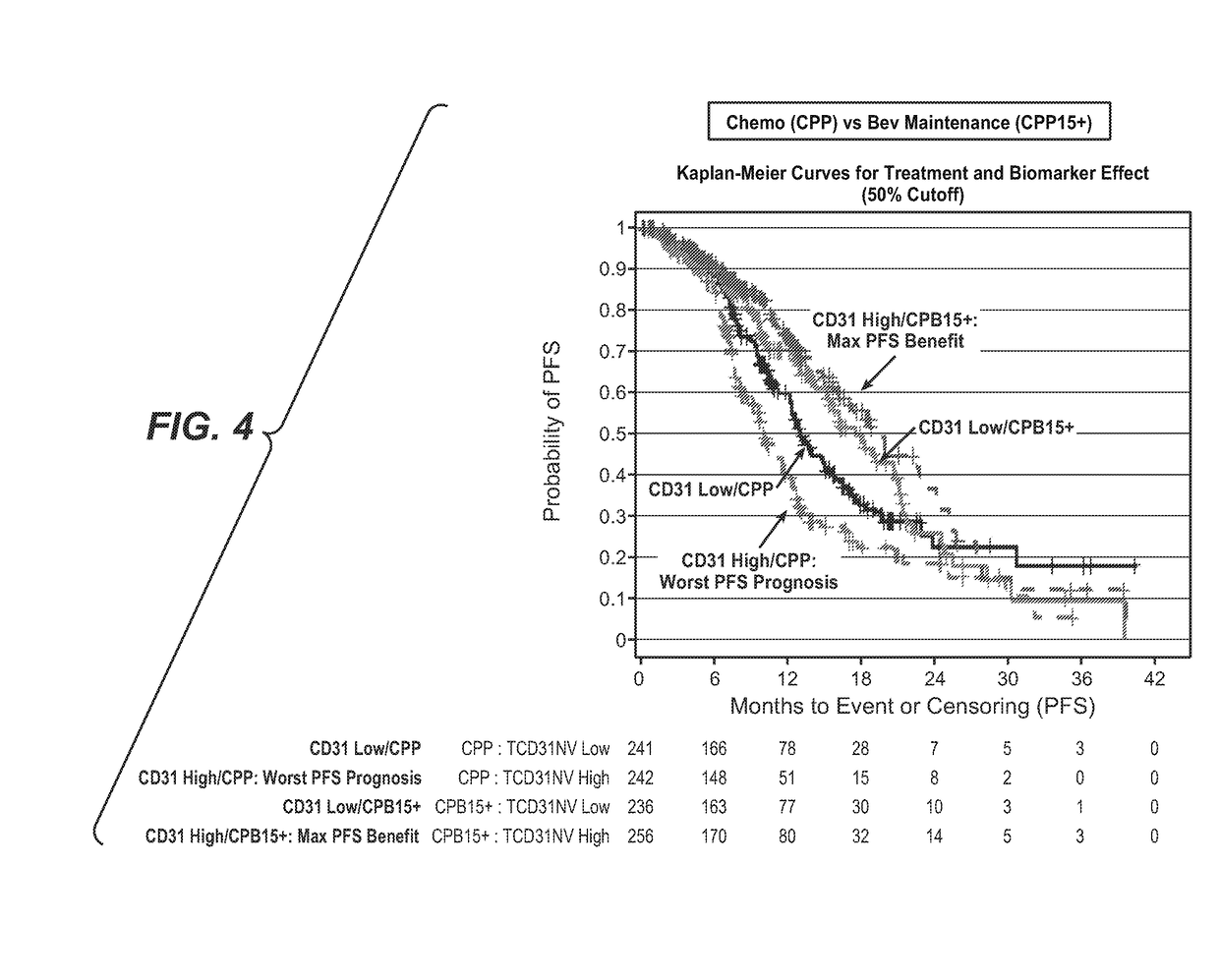
Blood plasma biomarkers for bevacizumab combination therapies for treatment of breast cancer
InactiveUS20140341893A1Improve treatment outcomesProlonged progression-free survivalOrganic active ingredientsBiological material analysisHER2 Positive Breast CancerChemotherapy regimen
The present invention provides methods for improving the treatment effect of a chemotherapy regimen of a patient suffering from HER2 positive breast cancer, in particular locally recurrent or metastatic HER2 positive breast cancer, by adding bevacizumab (Avastin®) to a chemotherapy regimen by determining the expression level, in particular the blood plasma expression level, of VEGFA and / or VEGFR2 relative to control levels of patients diagnosed with HER2 positive breast cancer, in particular locally recurrent or metastatic HER2 positive breast cancer. The present invention also provides for methods for assessing the sensitivity or responsiveness of a patient to bevacizumab (Avastin®) in combination with a chemotherapy regimen, by determining the expression level, in particular the blood plasma expression level, of VEGFA and / or VEGFR2 relative to control levels in patients diagnosed with HER2 positive breast cancer, in particular locally recurrent or metastatic HER2 positive breast cancer.
Owner:GENENTECH INC
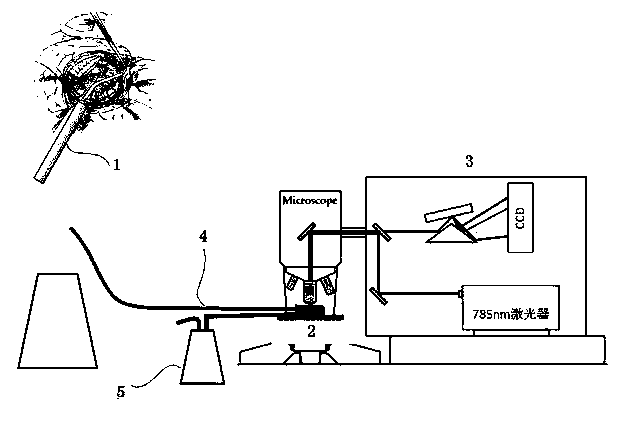
Integrated glioma surgery instrument
InactiveCN105496514AProlong median survival timeImproved prognosisIncision instrumentsRaman scatteringVacuum extractorReoperative surgery
The invention belongs to the technical field of medical instruments and relates to an integrated glioma surgery instrument CUSA-Raman Spectra, comprising a harmonic scalpel, a sample box, a Raman spectrometer, a connector, and a vacuum extractor; the harmonic scalpel is connected with the sample box through the connector, the sample box is fixed to a stage of the Raman spectrometer and also connected with the vacuum extractor. The harmonic scalpel in the surgery instrument can break tumor tissues that are sucked into the sample box through the connector, and finally analysis is carried out through the Raman spectrometer. Results of usage show that the surgery instrument can judge the property of the tumor tissues in real time during surgery and determine tumor peripheries for the expected surgical purposes, and the surgery instrument is simple in structure, low in cost, simple to manufacture and easy to popularize.
Owner:AFFILIATED HUSN HOSPITAL OF FUDAN UNIV
Anti-folr1 immunoconjugate dosing regimens
PendingUS20210155688A1Minimizes unwanted side-effectsHigh CmaxOrganic active ingredientsImmunoglobulins against cell receptors/antigens/surface-determinantsDosing regimenRegimen
Owner:IMMUNOGEN INC
PEPTIDE VACCINE THERAPY FOR TREATMENT OF FRa-EXPRESSING TUMORS
ActiveUS20170007687A1Reduce in quantityProlong progression-free survivalCell receptors/surface-antigens/surface-determinantsPeptide/protein ingredientsRegimenProtective immunity
Provided are methods for inducing and maintaining protective immunity against a tumor expressing FRα in a subject, comprising the administration of one or more peptide vaccines according to a particular dosages or particular dosage regimens.
Owner:THE HENRY M JACKSON FOUND FOR THE ADVANCEMENT OF MILITARY MEDICINE INC
Estrogen receptor ligands and methods of use thereof
InactiveUS20150087712A1Prolong progression-free survivalImprove survivalBiocideOrganic chemistryGynecomastiaEstrogen receptor
The present invention relates to methods for reducing testosterone levels in a male subject and methods of treating, suppressing, reducing the incidence, reducing the severity, or inhibiting prostate cancer, advanced prostate cancer, castration resistant prostate cancer (CRPC), metastatic castration resistant prostate cancer (mCRPC), and methods of reducing high or increasing PSA levels and / or increasing SHBG levels in a subject suffering from prostate cancer, advanced prostate cancer, castration resistant prostate cancer (CRPC) and metastatic castration resistant prostate cancer (mCRPC). The compounds of this invention suppress free or total testosterone levels despite castrate levels secondary to ADT and reduce high or increasing PSA levels. This reduction in testosterone levels may be used to treat prostate cancer, advanced prostate cancer, CRPC and mCRPC without causing bone loss, decreased bone mineral density, increased risk of bone fractures, increased body fat, hot flashes and / or gynecomastia.
Owner:GTX INCORPORATED
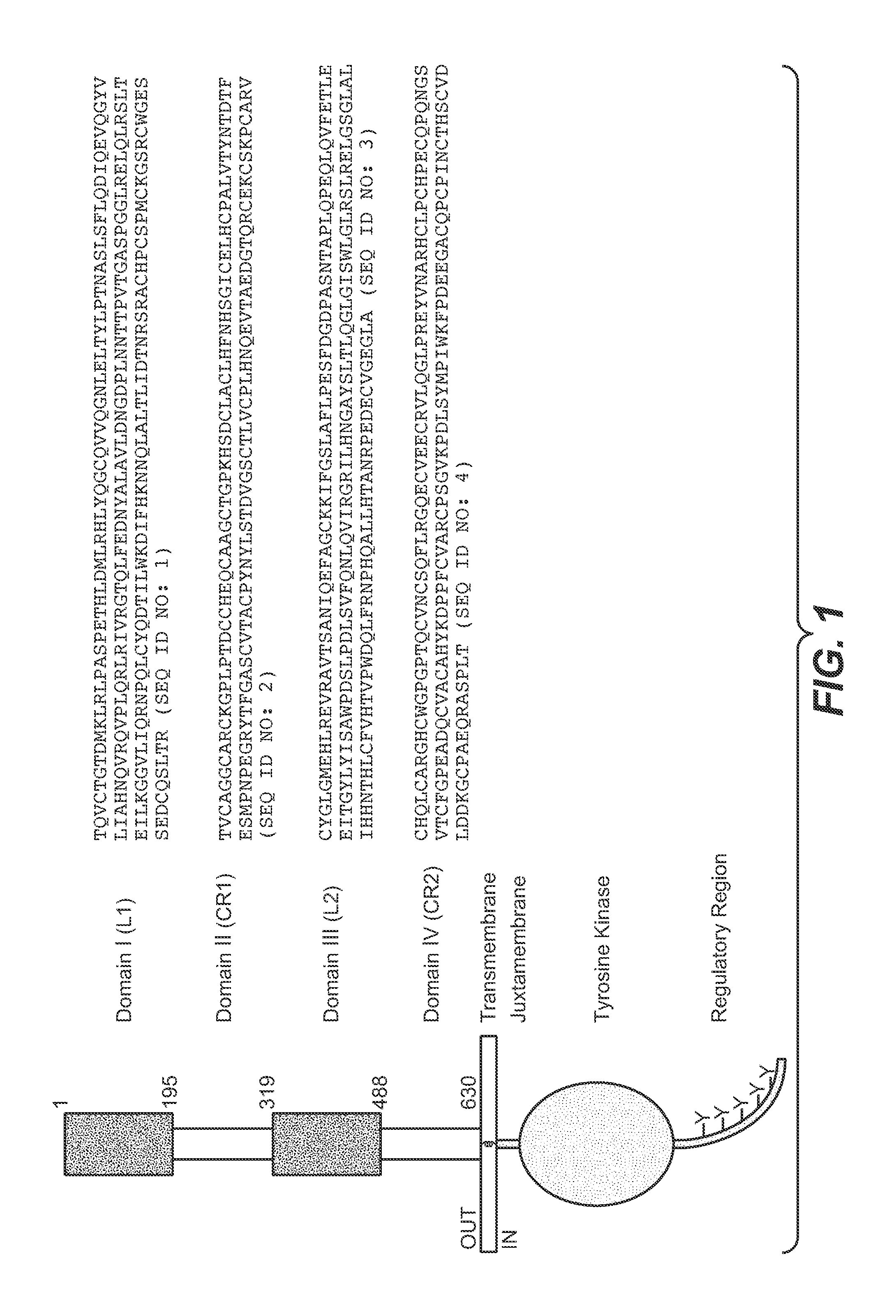
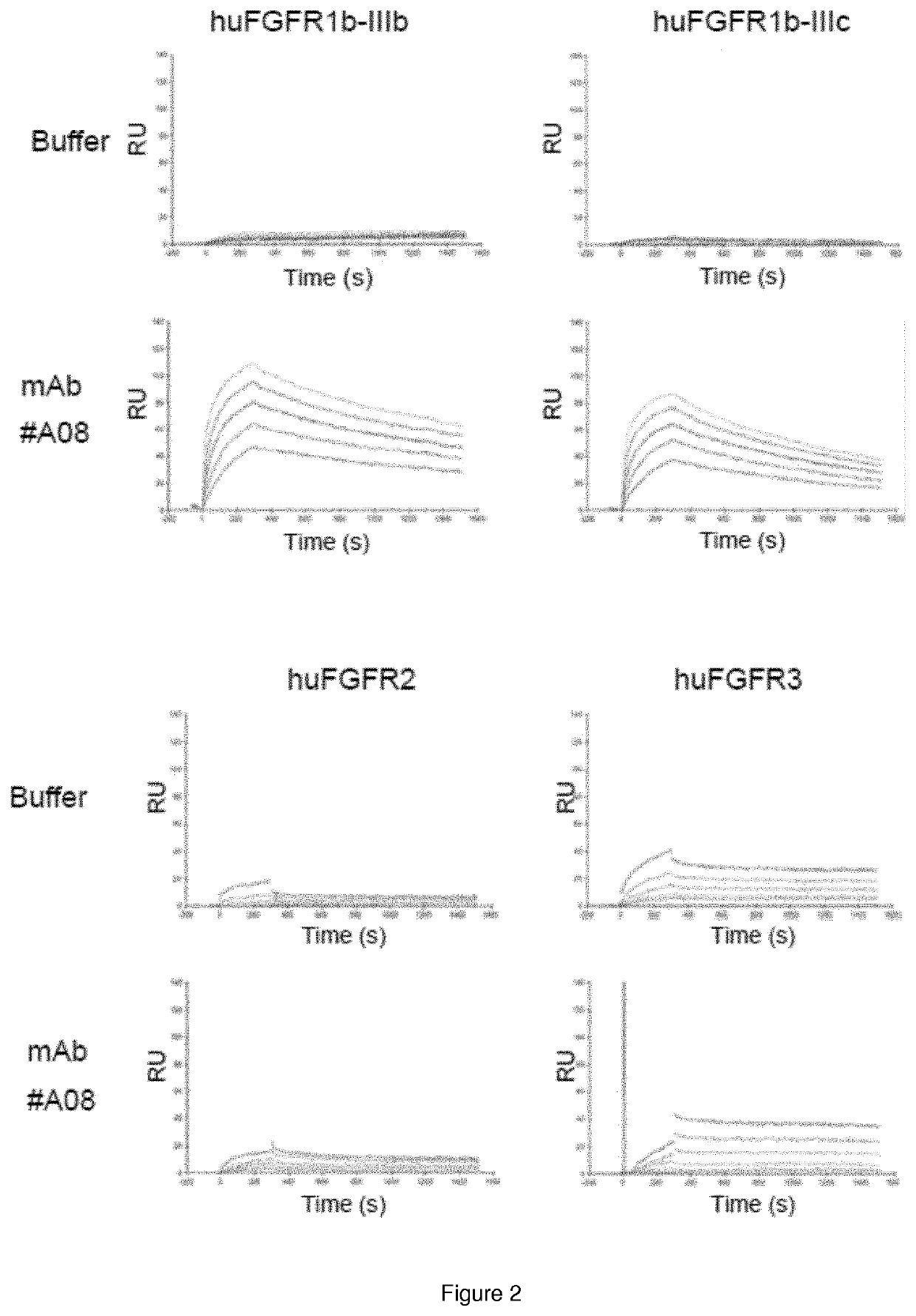
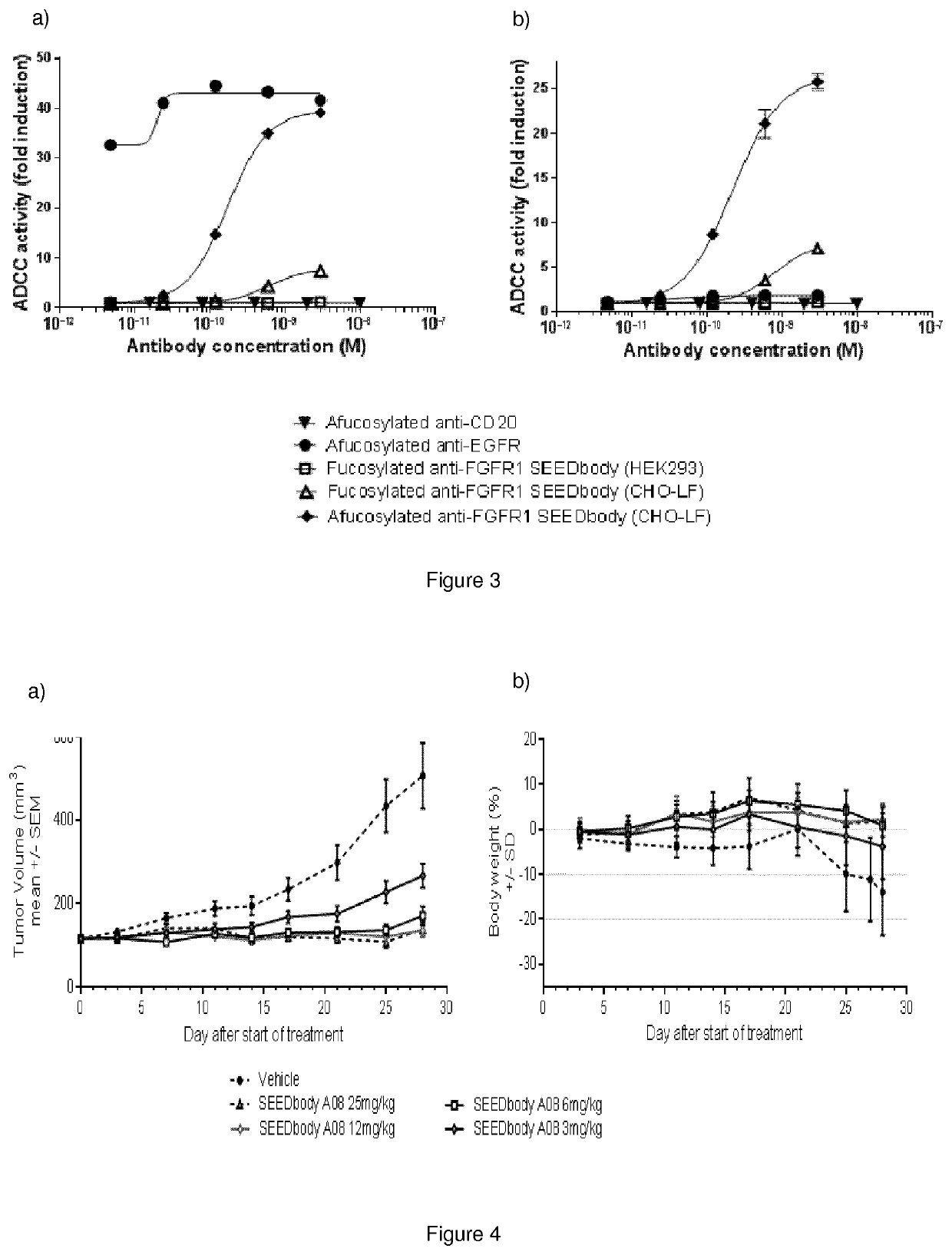
Monoclonal antibody directed to fgfr1
ActiveUS20190367620A1Increase flexibilityHigh affinityImmunoglobulins against cell receptors/antigens/surface-determinantsAntibody ingredientsMonoclonal antibodyMacaca cynomolgus
The present invention relates to antibodies with specificity for FGFR1. More particularly, the invention relates to monoclonal antibodies that bind specifically to and neutralize human, macaque and mouse forms of FGFR1 with high affinity. The invention also relates to nucleic acids encoding said antibodies, vectors for expression of these nucleic acids, and host cells for producing said antibodies. Further, the invention relates to the use of said antibodies in the diagnosis and / or treatment of cancers.
Owner:MERCK PATENT GMBH
Combination therapy of diffuse large b-cell lymphoma comprising an Anti-cd79b immunoconjugates, an alkylating agent and an Anti-cd20 antibody
PendingUS20220031861A1Speed upImprove survivalOrganic active ingredientsImmunoglobulins against cell receptors/antigens/surface-determinantsAlkylating antineoplastic agentAntiendomysial antibodies
Owner:GENENTECH INC +1
Gene Polymorphisms as Sex-Specific Predictors in Cancer Therapy
InactiveUS20100099720A1Extension of timeProlong progression-free survivalBiocideMicrobiological testing/measurementSex specificCancer therapy
The invention provides compositions and methods for determining the likelihood of gender-specific successful treatment with 5-FU / oxaliplatin or an equivalent of each thereof. The methods comprise determining the genomic polymorphism present in a predetermined region of a gene of interest and correlating the polymorphism to the predictive response. Patients identified as responsive are then treated with the appropriate therapy.
Owner:UNIV OF SOUTHERN CALIFORNIA
Methods for predicting and improving the survival of gastric cancer patients
InactiveUS20140099300A1Improved prognosisIncrease disease-free survivalOrganic active ingredientsBiocideCancer cellAnalyte
The present invention provides assays and methods for predicting the post-operative survival of a subject having an early stage gastric cancer after tumor surgery. The present invention also provides methods for treating a subject having an early stage gastric cancer by administering a combination therapy tailored to the signal transduction biomarkers that are activated in the cancer. In particular embodiments, the methods of the invention rely on the detection of the activation state or level of a specific combination of signal transducer analytes in a cancer cell obtained from the subject. Thus, the methods of the invention are particularly useful for predicting the survival or prognosis of a subject having an early stage gastric cancer and for guiding both pre- and post-operative treatment decisions by identifying subjects who would benefit from combination therapy as opposed to monotherapy.
Owner:NESTEC SA
Predicting response to a VEGF antagonist
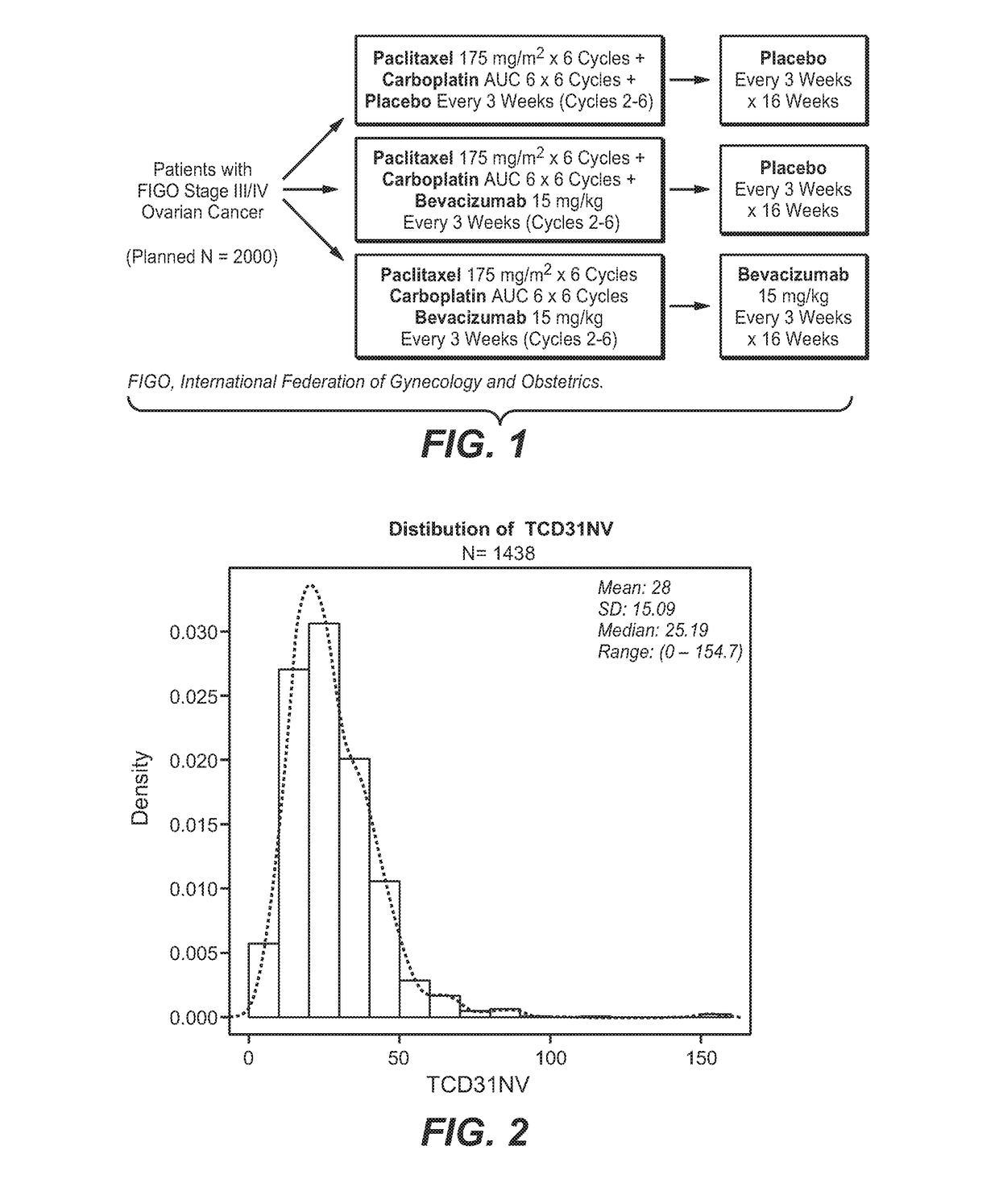
InactiveUS20170226198A1Prolong progression-free survivalImprove survivalHeavy metal active ingredientsOrganic active ingredientsRegimenBevacizumab Injection
The invention describes the use of high CD31 and / or tumor VEGFA as selection criteria for determining patient benefit or responsiveness to a VEGF antagonist, such as bevacizumab. The present invention also describes the use of high CD31 and / or tumor VEGFA as a selection criterion for treating cancer patients, such as ovarian cancer patients, who are undergoing a chemotherapy and / or anti-cancer therapy regimen, with a VEGF antagonist, such as bevacizumab.
Owner:GENENTECH INC
Engineered iga antibodies and methods of use
ActiveUS20210122834A1Strong cytotoxicityReducing glycosylationAntibody ingredientsImmunoglobulinsDiseaseAntiendomysial antibodies
Provided herein are engineered antibodies that comprise a modified IgA heavy chain constant region, pharmaceutical compositions, and methods of use. The engineered antibodies described herein comprise one or more amino acid substitution or deletion in a constant region of an IgA domain. Further provided herein are methods of treating disorders, including cancer, by administering an engineered IgA antibody described herein.
Owner:TIGA TX INC
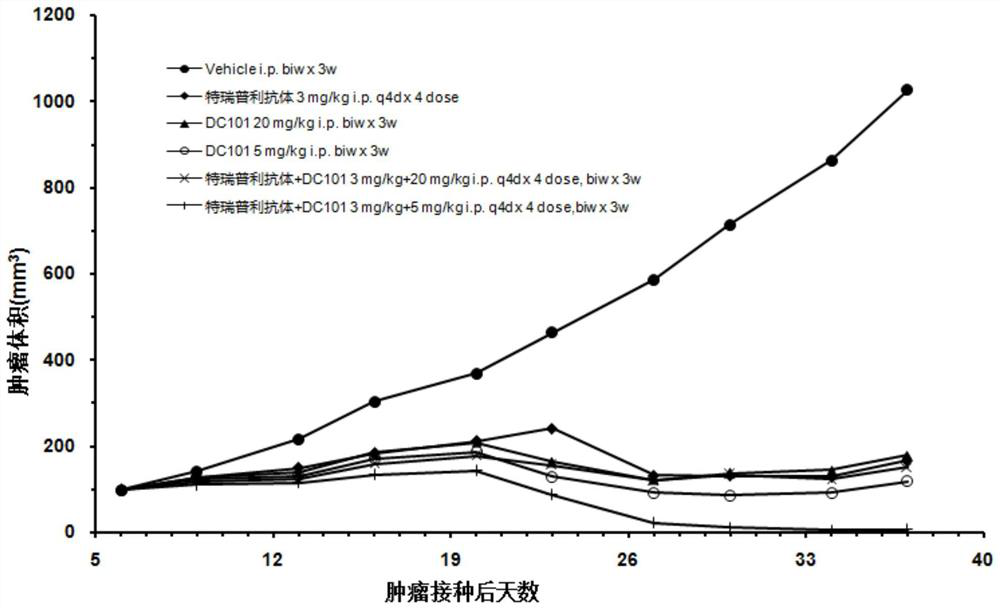
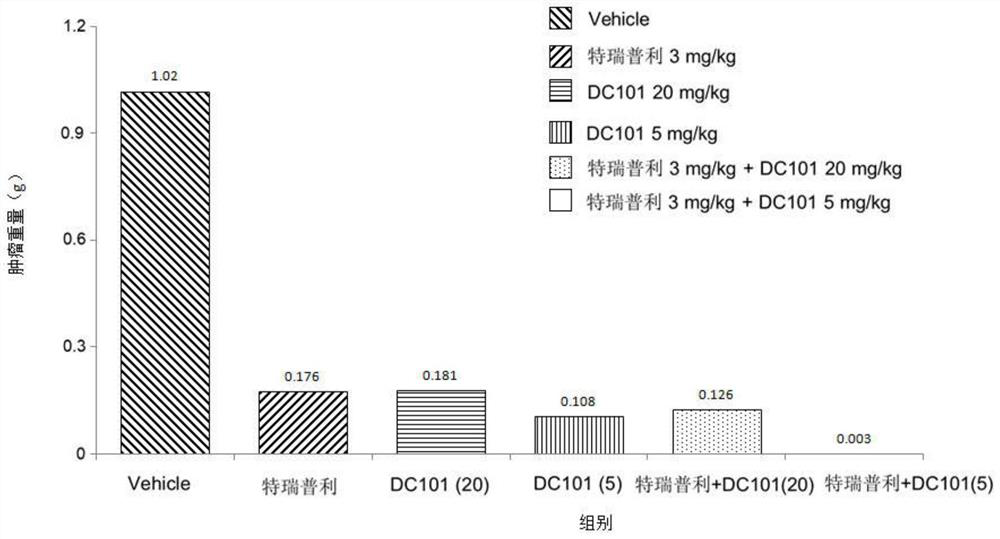
Drug combination composition for treating tumor diseases, and application thereof
PendingCN114081945AEffective treatmentProlong progression-free survivalAntibody ingredientsImmunoglobulinsDiseaseAntiendomysial antibodies
The invention relates to the field of biological medicines, and particularly provides a drug combination composition for treating tumor diseases. The composition comprises an anti-PD-1 monoclonal antibody taking PD-1 as a target spot and an anti-VEGFR-2 monoclonal antibody taking VEGFR-2 as a target spot. Through combined application of the anti-PD-1 monoclonal antibody and the anti-VEGFR-2 monoclonal antibody, not only is the medication safety higher, but also the treatment response duration is effectively prolonged, the progression-free lifetime and the total lifetime of cancer patients are prolonged, and the health condition and the life quality of the patients are improved. The drug combination composition can be used for treating non-small cell lung cancer, glioma, colorectal cancer, liver cancer, HER2 negative metastatic breast cancer, metastatic gastric adenocarcinoma, metastatic melanoma and metastatic renal cell carcinoma, and especially can be used for effectively treating non-small cell lung cancer and liver cancer.
Owner:BEIJING DONGFANG BIOTECH +1
Drug combination composition for treating tumor diseases and application
PendingCN114159557AProlong progression-free survivalImprove survivalImmunoglobulins against cell receptors/antigens/surface-determinantsAntibody ingredientsRectal carcinomaCARCINOMA BREAST
The invention relates to the field of biological medicines, and particularly provides a drug combination composition for treating tumor diseases, which comprises an anti-PD-1 monoclonal antibody taking PD-1 as a target spot and an anti-EGFR monoclonal antibody taking EGFR as a target spot. According to the invention, the anti-PD-1 monoclonal antibody and the anti-EGFR monoclonal antibody are combined for use, so that the medication safety is higher, the treatment response duration is effectively prolonged, the progression-free lifetime and the total lifetime of cancer patients are prolonged, the health condition and the life quality of the patients are improved, and the clinical application prospect is wide. The combined medicine composition can be used for treating non-small cell lung cancer, metastatic non-small cell lung cancer, glioma, colorectal cancer, liver cancer, hepatocellular carcinoma, metastatic hepatocellular carcinoma, HER2 negative metastatic breast cancer, metastatic gastric adenocarcinoma, metastatic colorectal cancer, metastatic melanoma, metastatic renal cell carcinoma and advanced esophageal squamous carcinoma. Advanced squamous non-small cell lung cancer or advanced head and neck squamous cell cancer.
Owner:BEIJING DONGFANG BIOTECH
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com