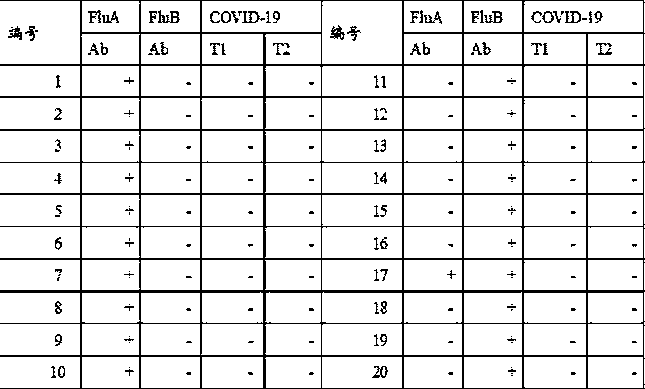
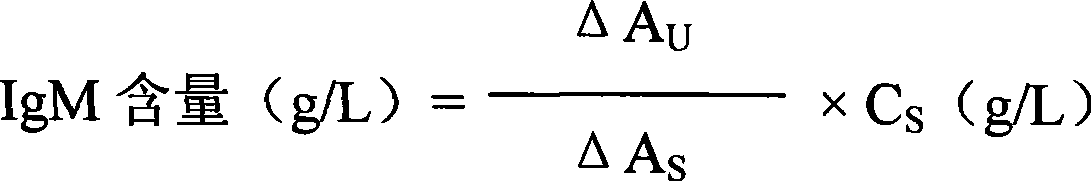Patents
Literature
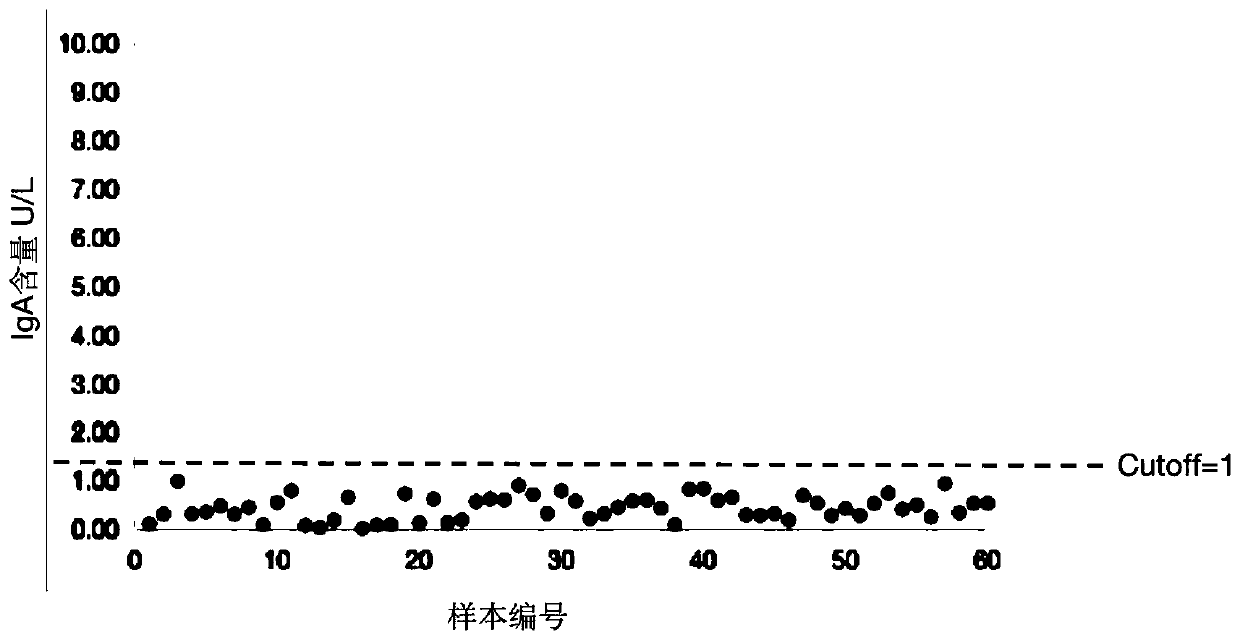
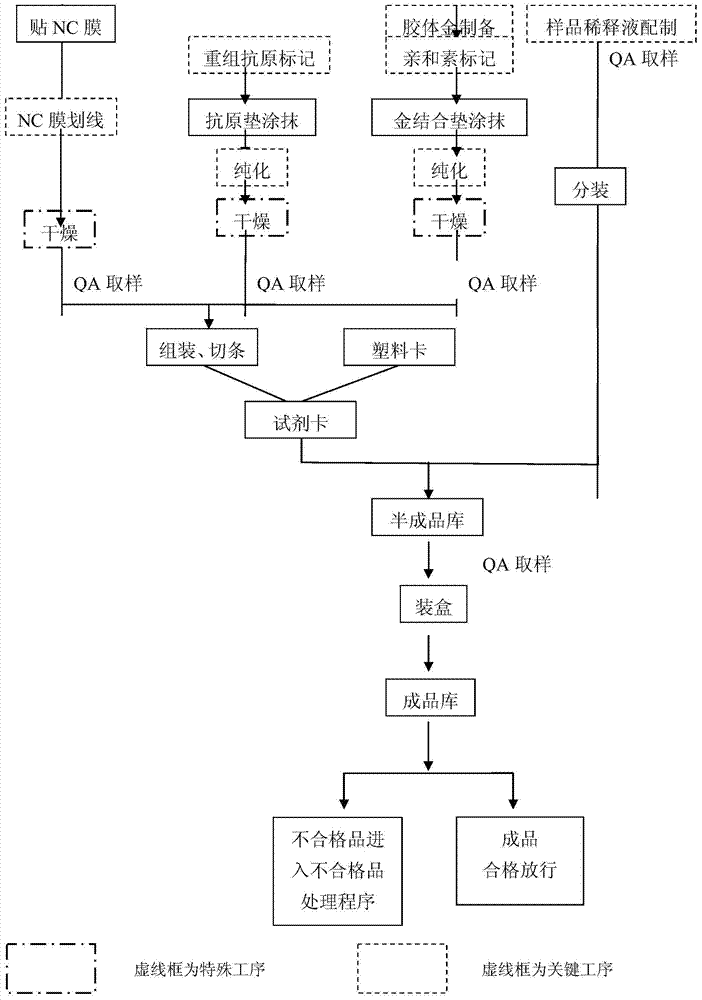
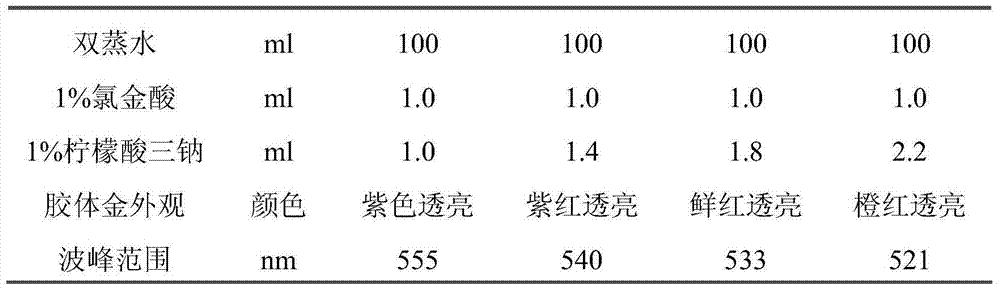
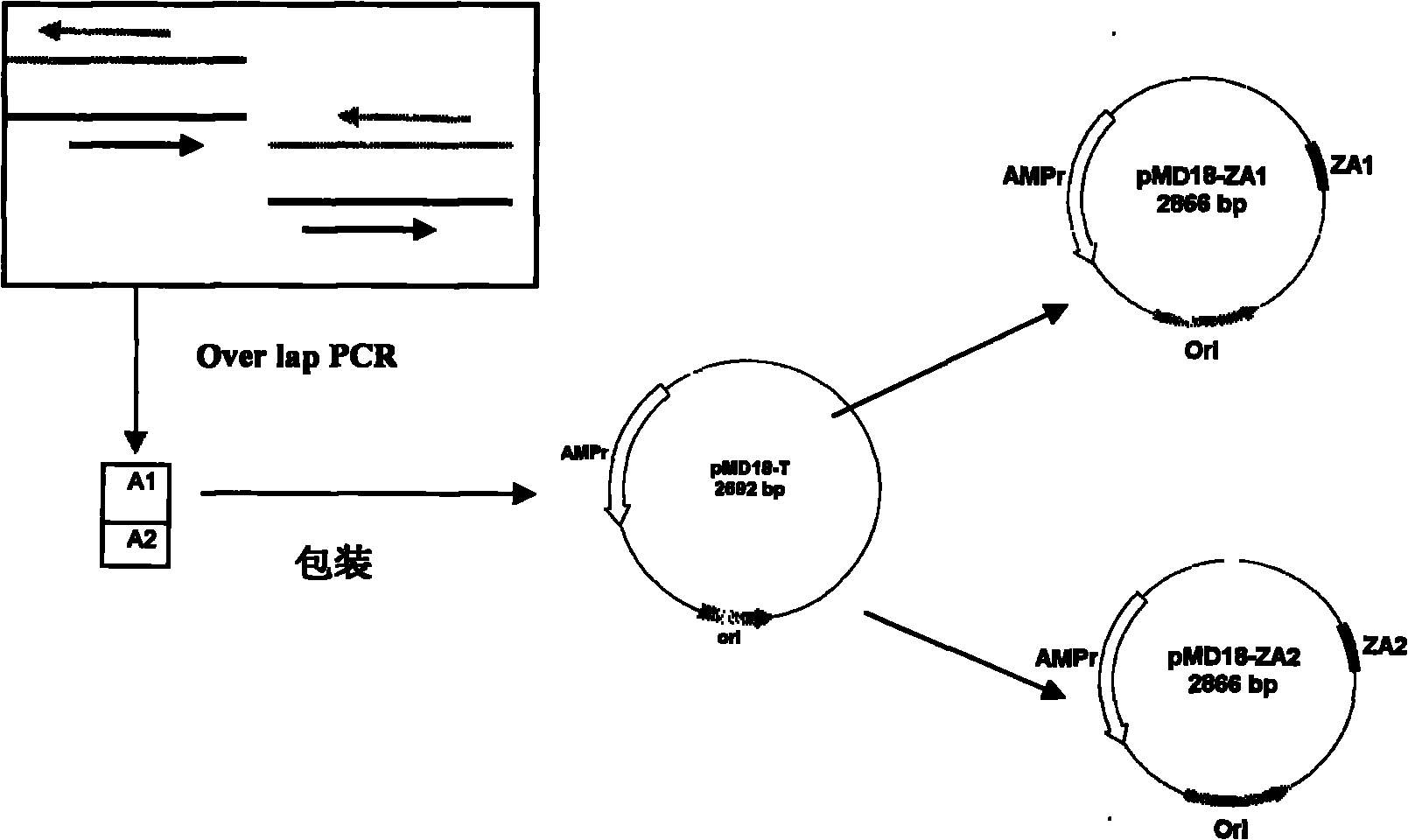
143 results about "IgA antibody" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
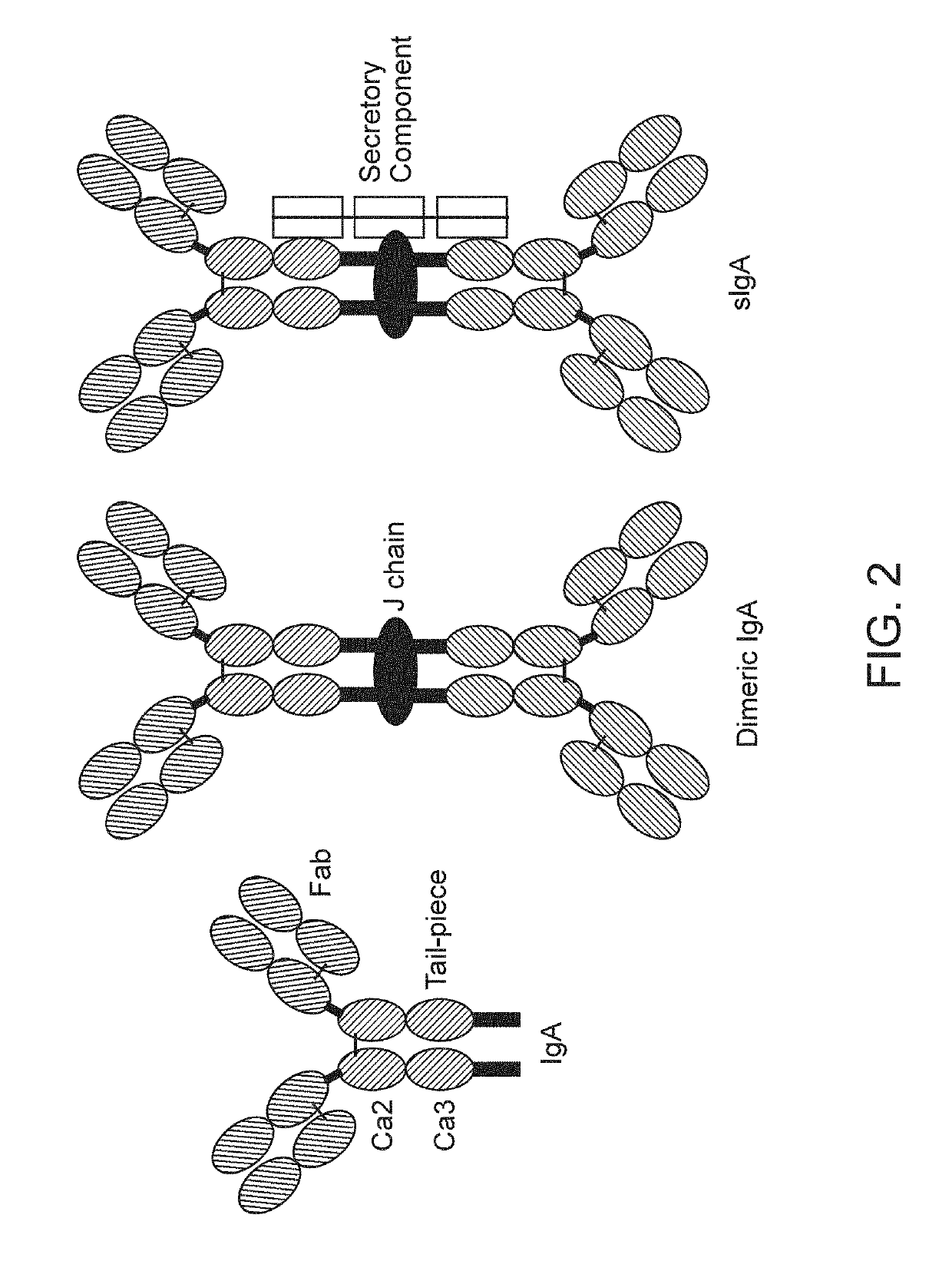
IgA is a polyvalent antibody that is translocated to mucosal surfaces as the first line of defense against infections. Most of the secreted IgA lines the mucosal surfaces including respiratory, digestive and genitourinary tracts to protect against pathogens while maintaining gut homeostasis.
Fusion protein construct and method for inducing HIV-specific serum IgG and secretory IgA antibodies in-vivo
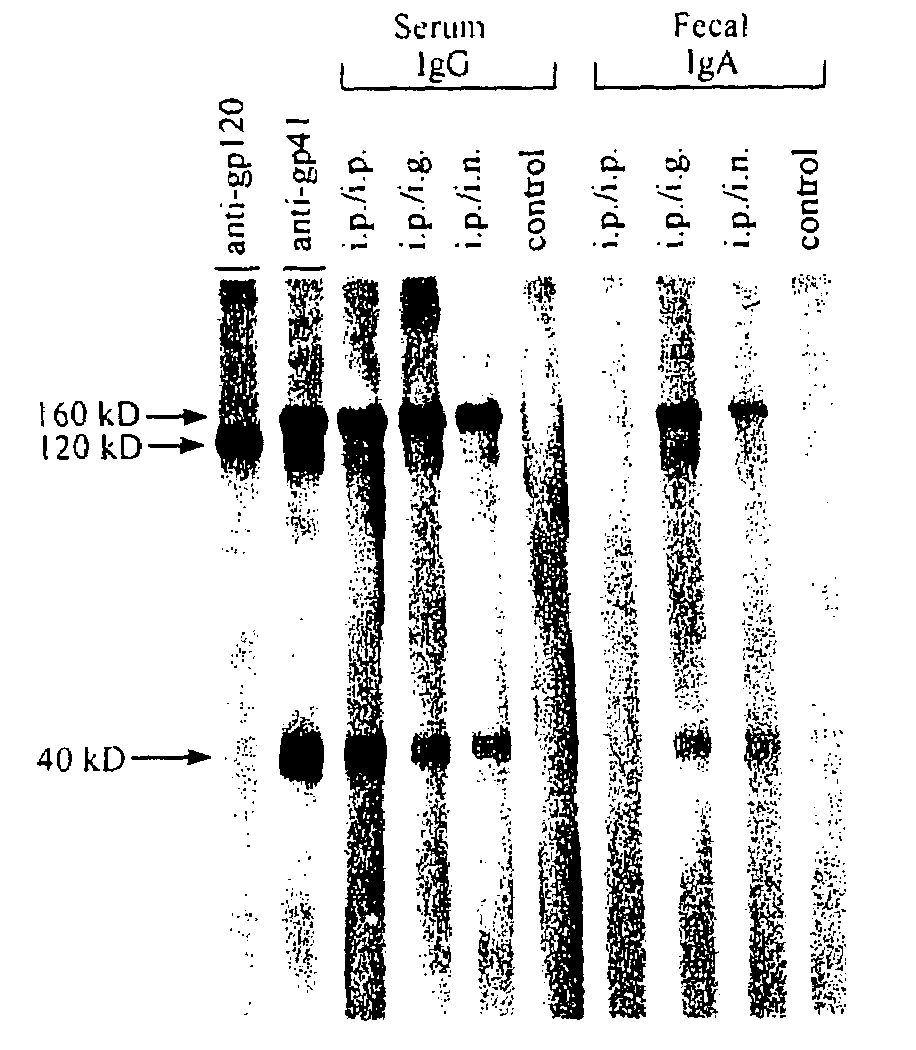
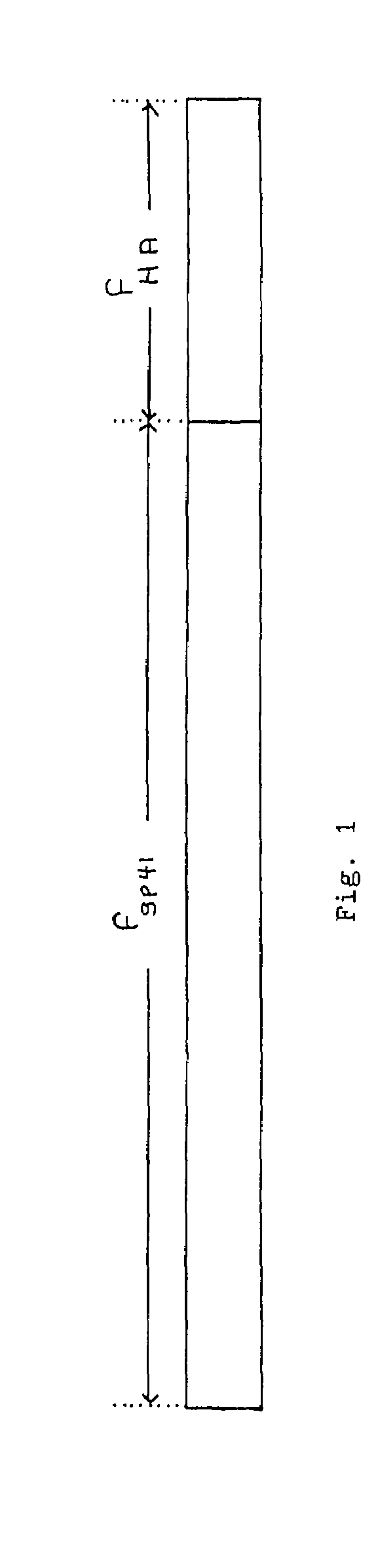
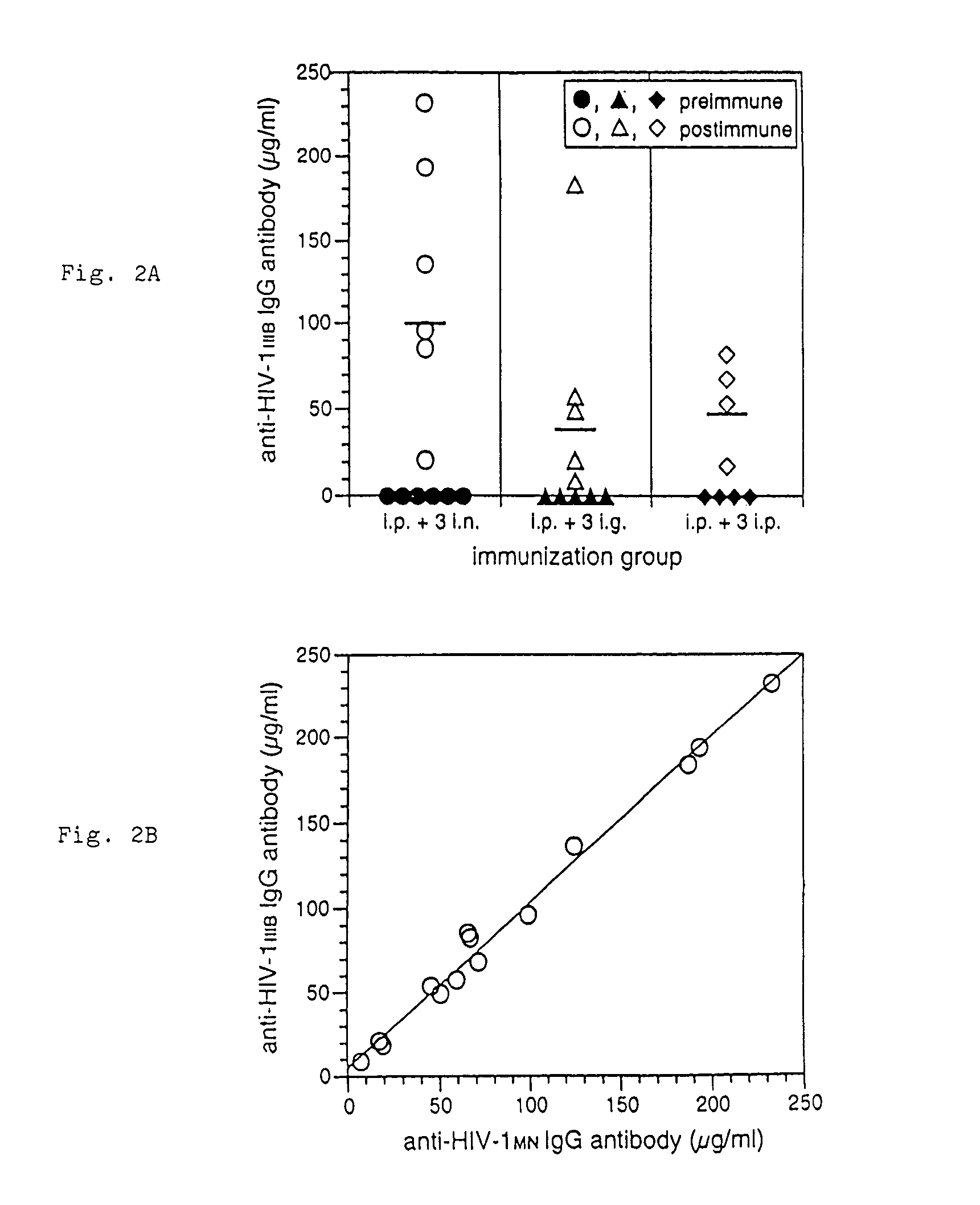
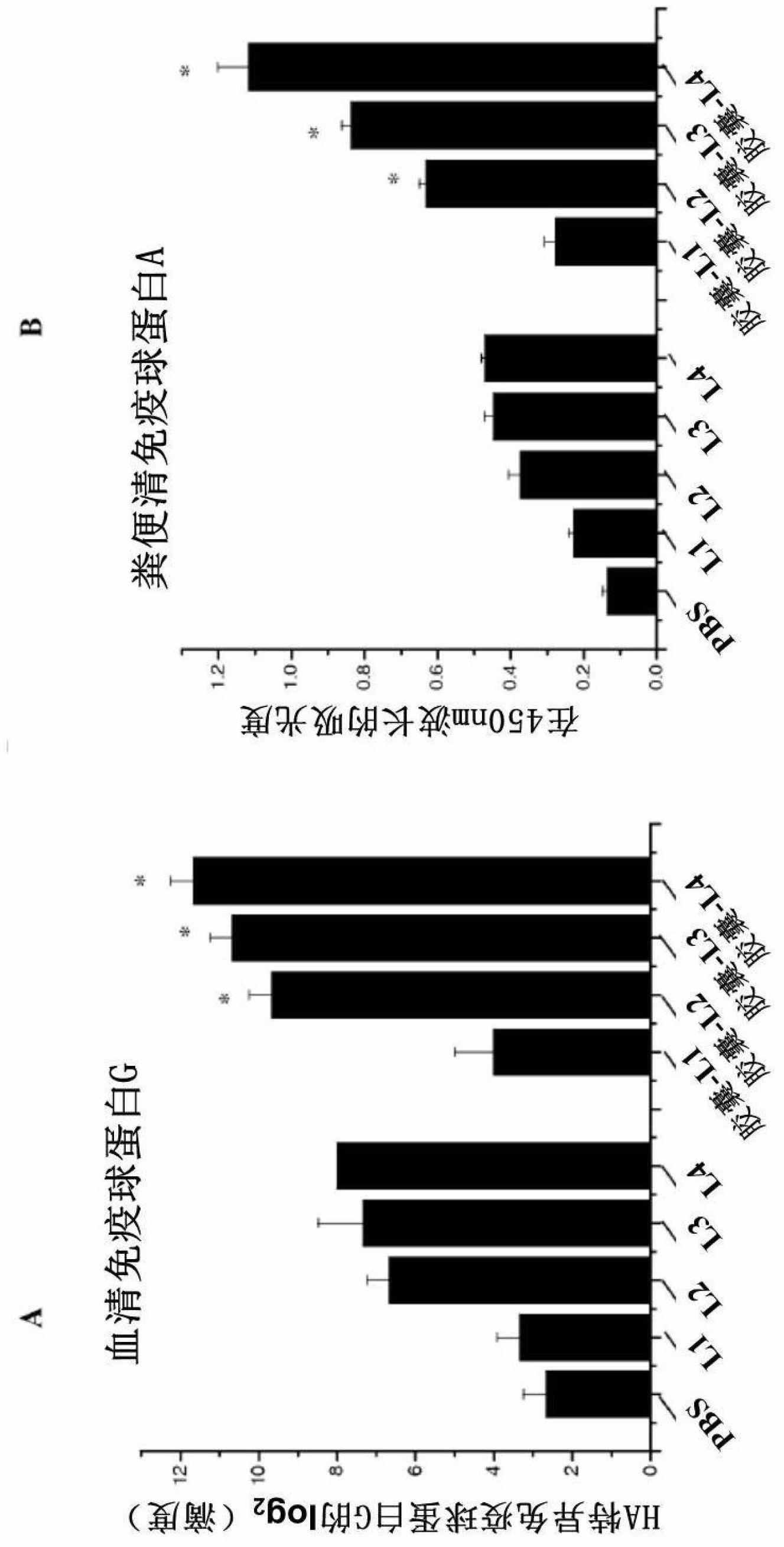
The present invention provides a fusion protein construct (gp41HA) consisting of the ectodomain of the HIV-1IIIB envelope glycoprotein gp41 fused to a fragment of the influenza virus HA2 hemagglutinin protein. Immunization in-vivo via an intraperitoneal prime followed by intranasal or intragastric boosts with gp41HA induces high concentrations of serum IgG antibodies and fecal IgA antibodies that reacted with gp41 in HIV-1IIIB viral lysate and are cross-reactive with gp41 in HIV-1MN lysate. Followup analyses by indirect immunofluorescence showed that both serum IgG and fecal IgA recognized human peripheral blood mononuclear cells infected with either syncytium-inducing (SI) or non-syncytium-inducing (NSI) North American HIV-1 field isolates, but not uninfected cells.
Owner:CHILDRENS MEDICAL CENT CORP
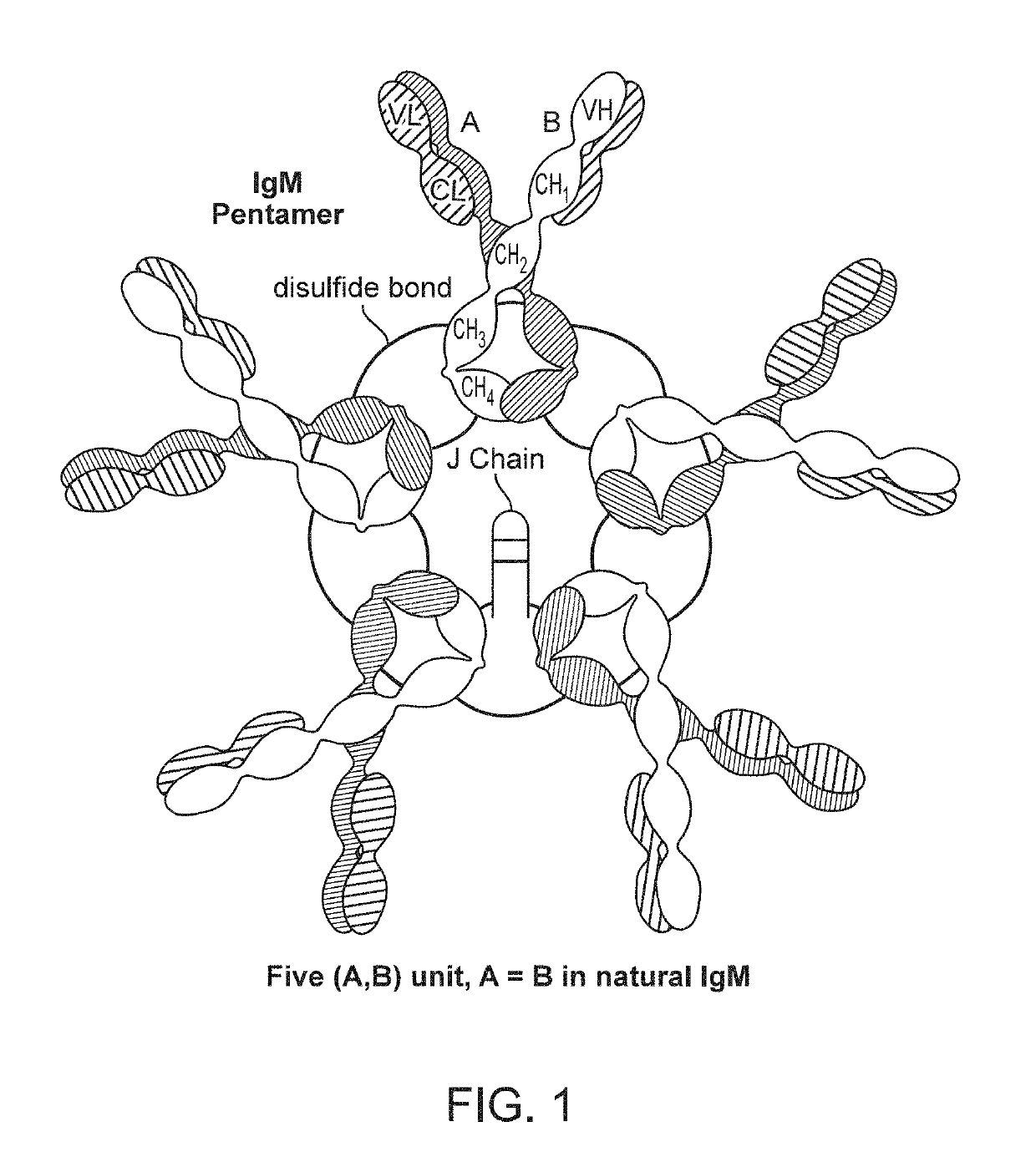
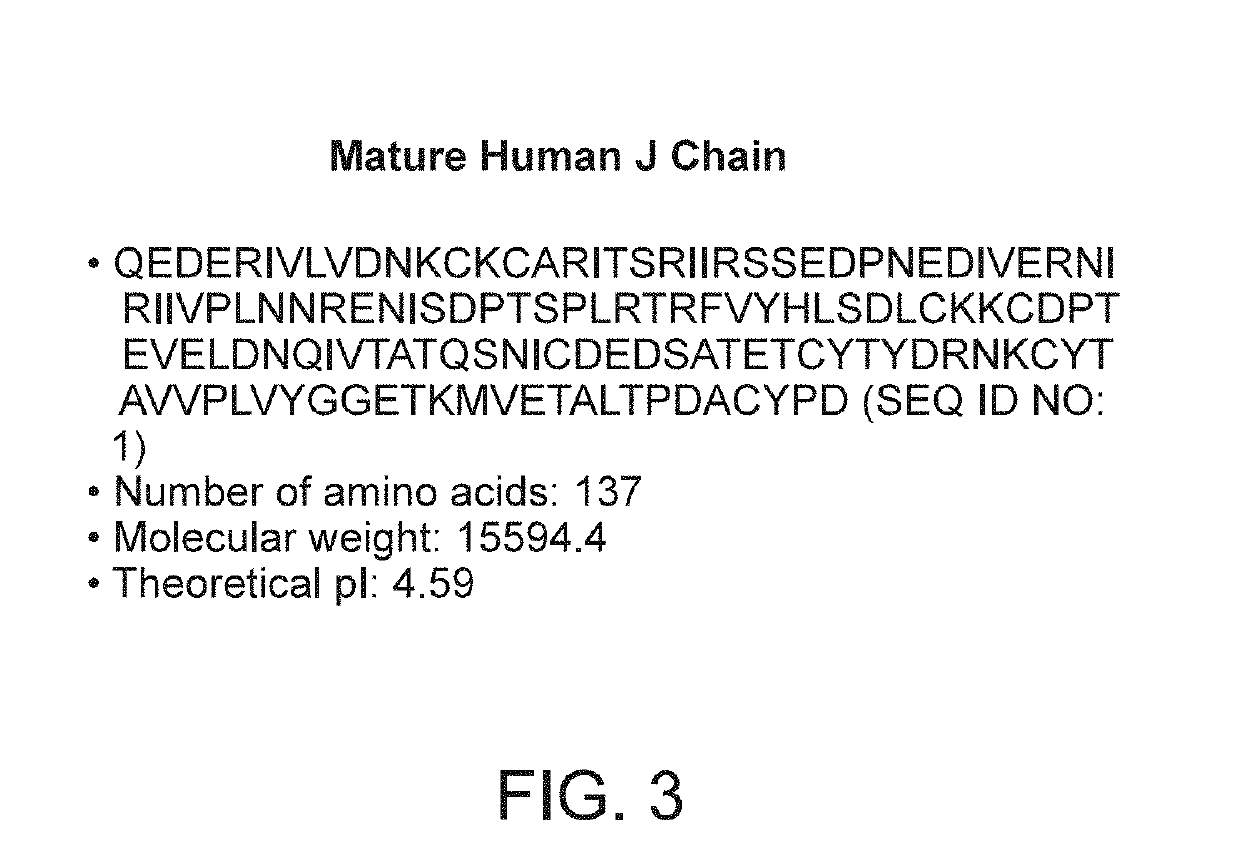
Binding molecules with modified j-chain
ActiveUS20190185570A1High affinityImprove bindingHybrid immunoglobulinsAntibody mimetics/scaffoldsT cellSignal pathway
The present invention provides binding molecules that include an IgM, IgA, IgG / IgM or IgG / IgA antibody with a modified J-chain that includes a binding moiety that antagonizes a T-cell inhibitory signaling pathway, and their uses.
Owner:IGM BIOSCI INC
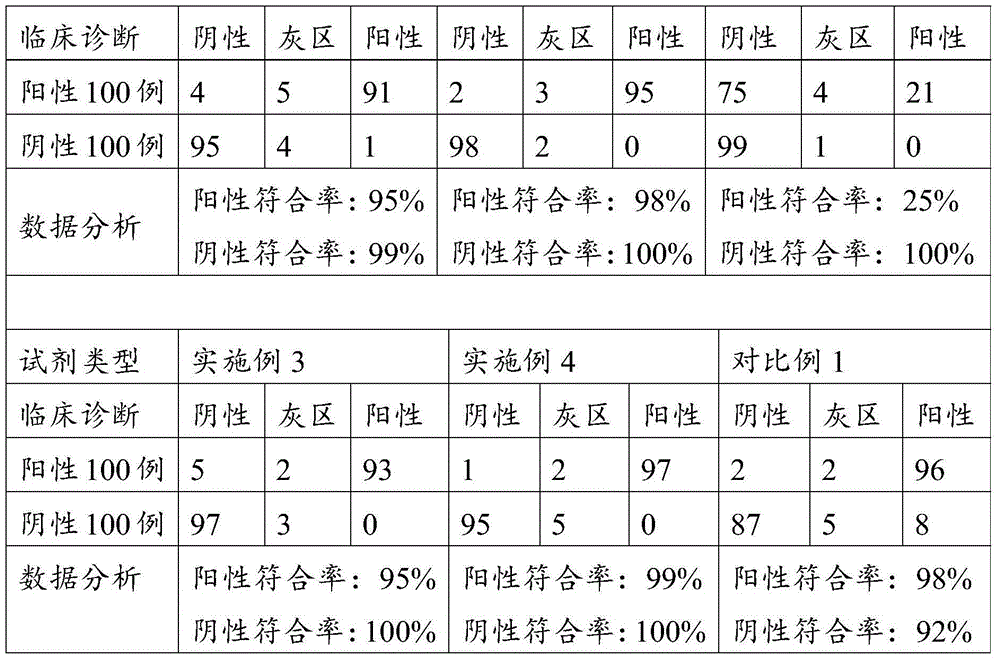
Kit for indirect ELISA (Enzyme-Linked Immunosorbent Assay) detection of IgG or IgA antibodies of PRRSV (Porcine reproductive and respiratory syndrome virus) of pigs
InactiveCN104330572AImprove immune activityImprove featuresBiological testingSerum igePositive control
The invention belongs to the field of biological detection reagents and relates to a kit for indirect ELISA (Enzyme-Linked Immunosorbent Assay) detection of IgG or IgA antibodies of PRRSV (Porcine reproductive and respiratory syndrome virus) of pigs. The kit is used for indirect ELISA detection of the IgG or IgA antibodies of the PRRSV in serum or saliva of pigs and comprises (a) an antibody detection board, (b) an HRP-labeled goat anti-pig IgG antibody solution or HRP-labeled goat anti-pig IgA, (c) a positive control, namely PRRSV standard positive serum or saliva, (d) a negative control, namely PRRSV negative serum or saliva, (e) sample diluting liquid, (f) 10* concentrated washing liquid, (g) substrate developing liquid, and (h) stopping liquid. The kit is good in PRRSV specificity and sensitivity when being used for detection of PRRSV IgG or IgA antibodies in serum or saliva of pigs. A repeatability test also shows that the kit is good in repeatability.
Owner:苏州市吴江区畜牧兽医站 +1
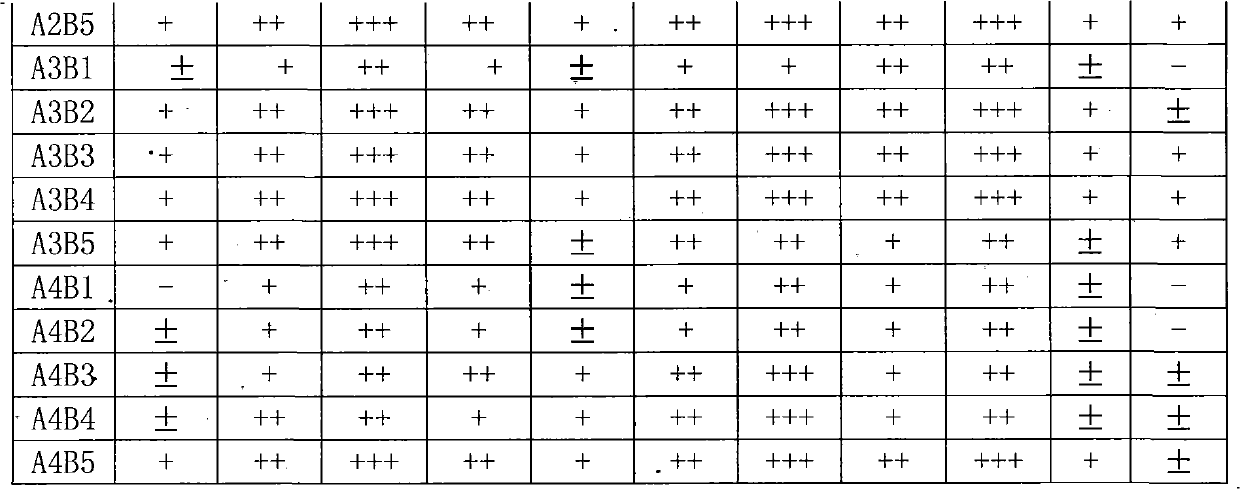
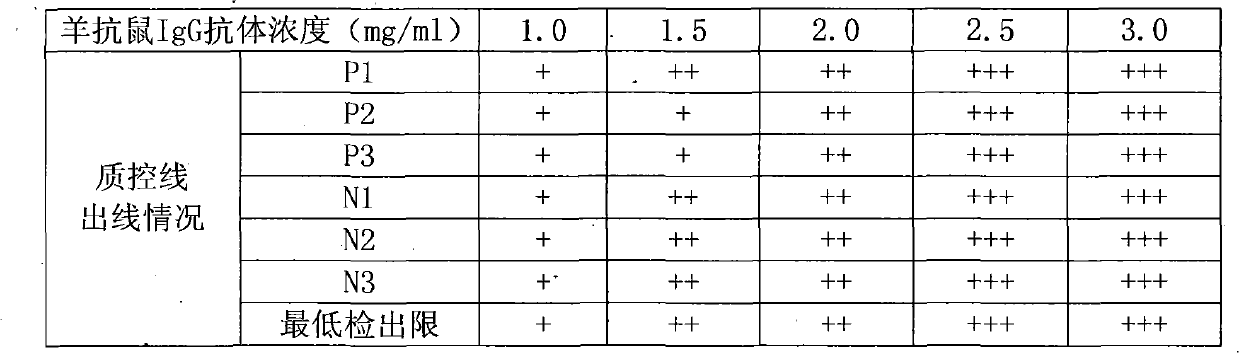
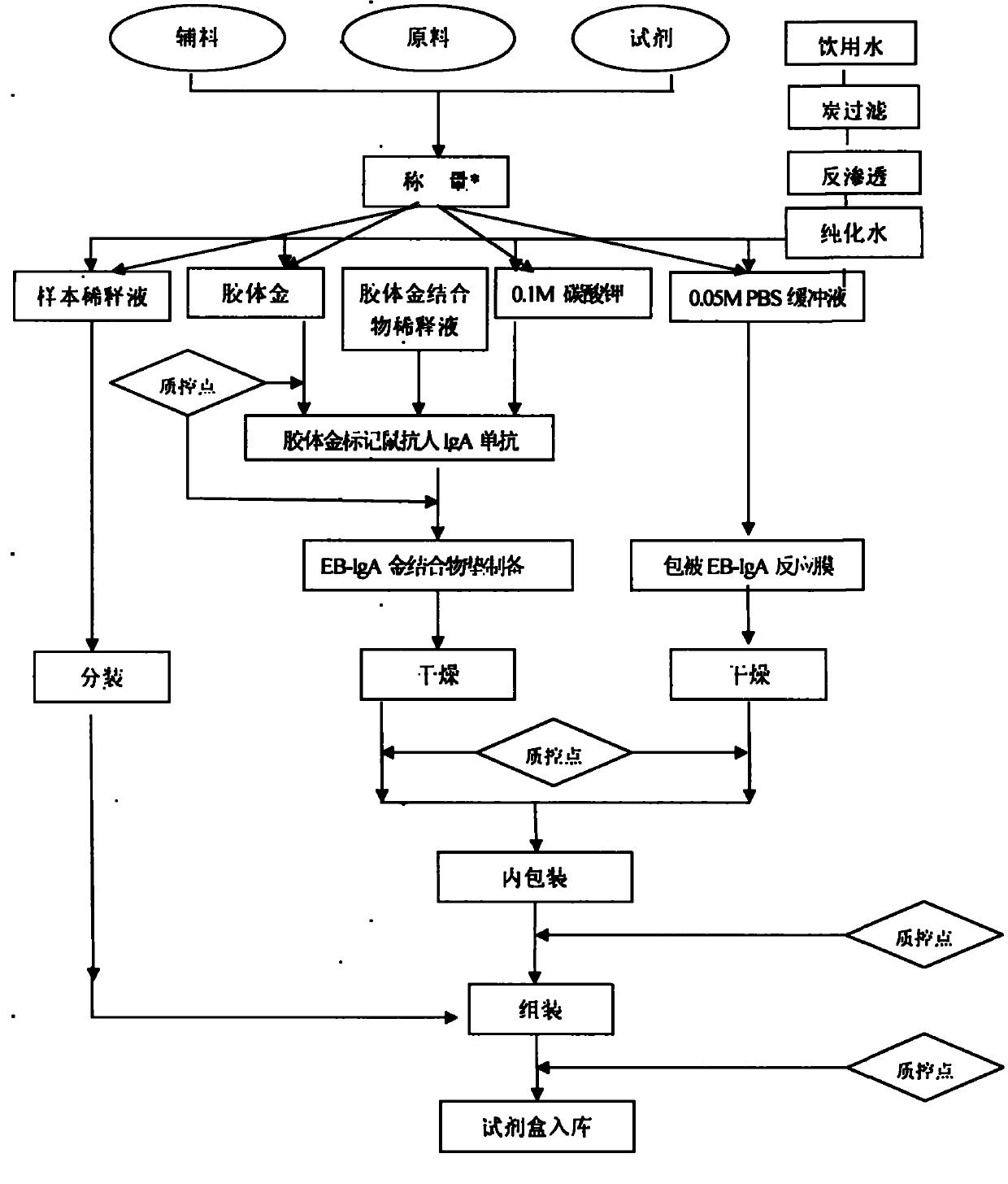
IgA (Immunoglobulin A) antibody detection reagent kit (colloidal gold method) for EB (Epstein-Barr) viruses and preparation method thereof
The invention relates to an IgA (Immunoglobulin A) antibody detection reagent kit (a colloidal gold method) for EB (Epstein-Barr) viruses and a preparation method thereof. The reagent kit comprises recombination antigen EB-NA1 coated by a nitrocellulose membrane detection line, a goat-anti-mouse IgG antibody coated on a quality control line and a mouse-anti-human IGA monoclonal antibody marked by colloidal gold and coated on a gold mark pad. The preparation method comprises the steps of: preparing a reaction membrane and a mouse-anti-human IGA monoclonal antibody gold combo pad, cutting and assembling to prepare the product. The invention has the advantages that: the IgA antibody detection reagent kit for the EB viruses has the characteristics of fast, simple and convenient detection, and high accuracy and sensitivity; the integrated operation time only requires 20 minutes to judge and read results; the colloidal gold is used for fast detecting test paper; a multi-epitope recombination antigen is used as a raw material; the method has the characteristics of simple and convenient operation, low cost, good specificity, high sensitivity, single portion detection and easy popularization; and the detection and control effect to the EB viruses is obvious.
Owner:北京中检安泰诊断科技有限公司
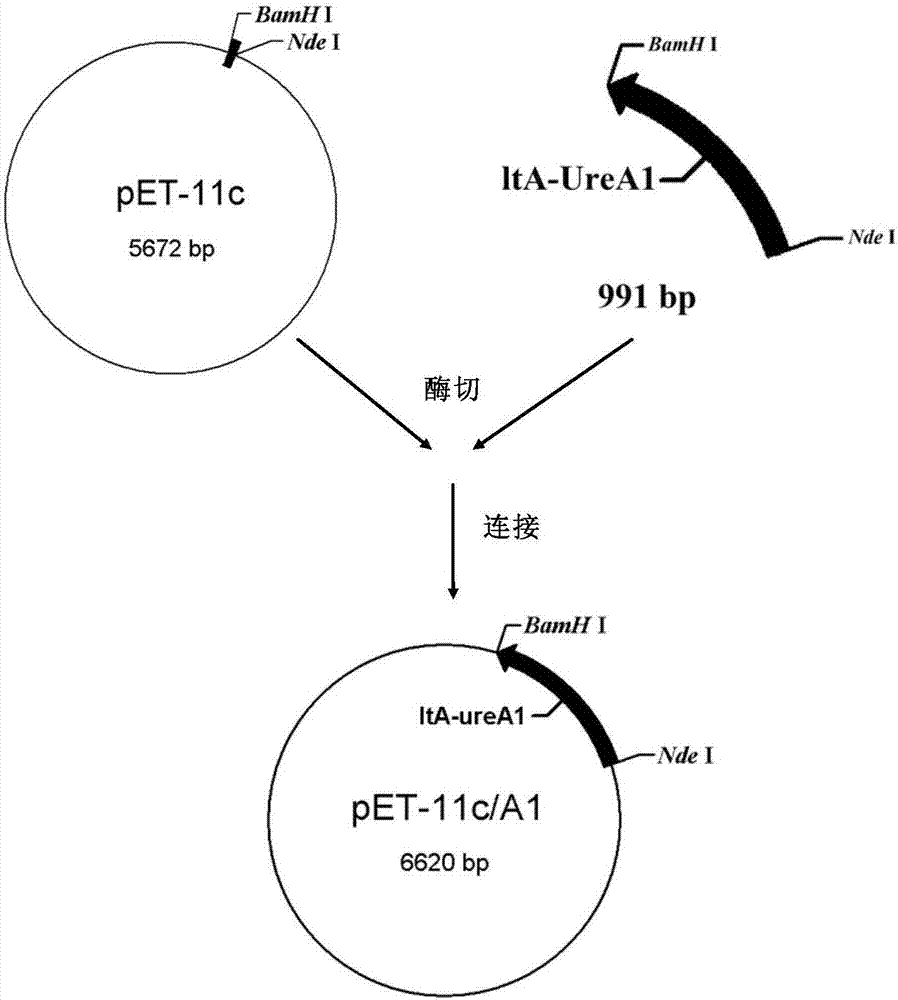
Recombinant helicobacter pylori protein vaccine and preparation method thereof
InactiveCN107298716ARetain the infrastructureRetain activityAntibacterial agentsBacterial antigen ingredientsAdjuvantVaccine antigen
The invention discloses a recombinant helicobacter pylori protein vaccine and a preparation method thereof. The active ingredient recombinant fusion protein of the vaccine consists of recombinant LTAl-Ureal protein and LTB protein, the amino acid sequence of the recombinant LTAl-Ureal protein is shown as Seq ID No.1, the amino acid sequence of the LTB protein is shown as Seq ID No.2. Epitope-containing gene segments of Hp urease A subunit is inserted in an LTA subunit-encoded gene, a toxic part-containing segment is replaced to prepare a recombinant plasmid so as to express and obtain recombinant fusion protein polymer as a vaccine antigen, the fusion antigen and LTB protein pentamer are combined to form a hexamer structure, so that not only can the structure basis and activity of an LT mucosa adjuvant be remained, but also the toxicity can be removed, the immune response of organism muscosa can be effectively induced through immunity of mucosa path, to generate specific IgA antibody. The recombinant helicobacter pylori protein vaccine provides a vaccine manner for preventing and treating infection of helicobacter pylori.
Owner:成都亿妙生物科技有限公司
Magnetic particle tagged blood bank reagents and techniques
InactiveUS20070059782A1Effect separationBiological testingMagnetic immunoreagent carriersMagnetic markerIsotope
Magnets and magnetic particle-labeled reagents are used to capture and / or release magnetic particle-tagged entities for immunohematology diagnostic testing purposes, especially tests performed in blood banking. The magnetic tagged entities may be tagged antibodies, tagged blood cells, tagged universal binding partners, especially tagged lectins and tagged Coombs reagent, and other binding agents such as biotin-avidin, Protein A or G, ligands and their receptors and the like. Separation of unbound material from bound material is effected through the use of one or both the magnetic field effect on the magnetic labeled reactants and the density gradients of layers of an assay construct. Constructs such as chromatographic strip lateral flow format, and liquid phase reactions in suitable vessels with end point determinations that do not require centrifugation to detect reacted entities. Readable labels such as enzymes, fluorophors, chemiluminescent materials, radioactive isotopes, and other labels may be attached to Coombs reagent to provide a readable product of the Coombs reagent with any antibody participating in the assay.
Owner:CHROME RED TECH
Novel coronavirus earlier-stage screening method
ActiveCN111366735AAppeared earlyThe test result is accurateChemiluminescene/bioluminescenceBiological testingMagnetic beadMorpholine
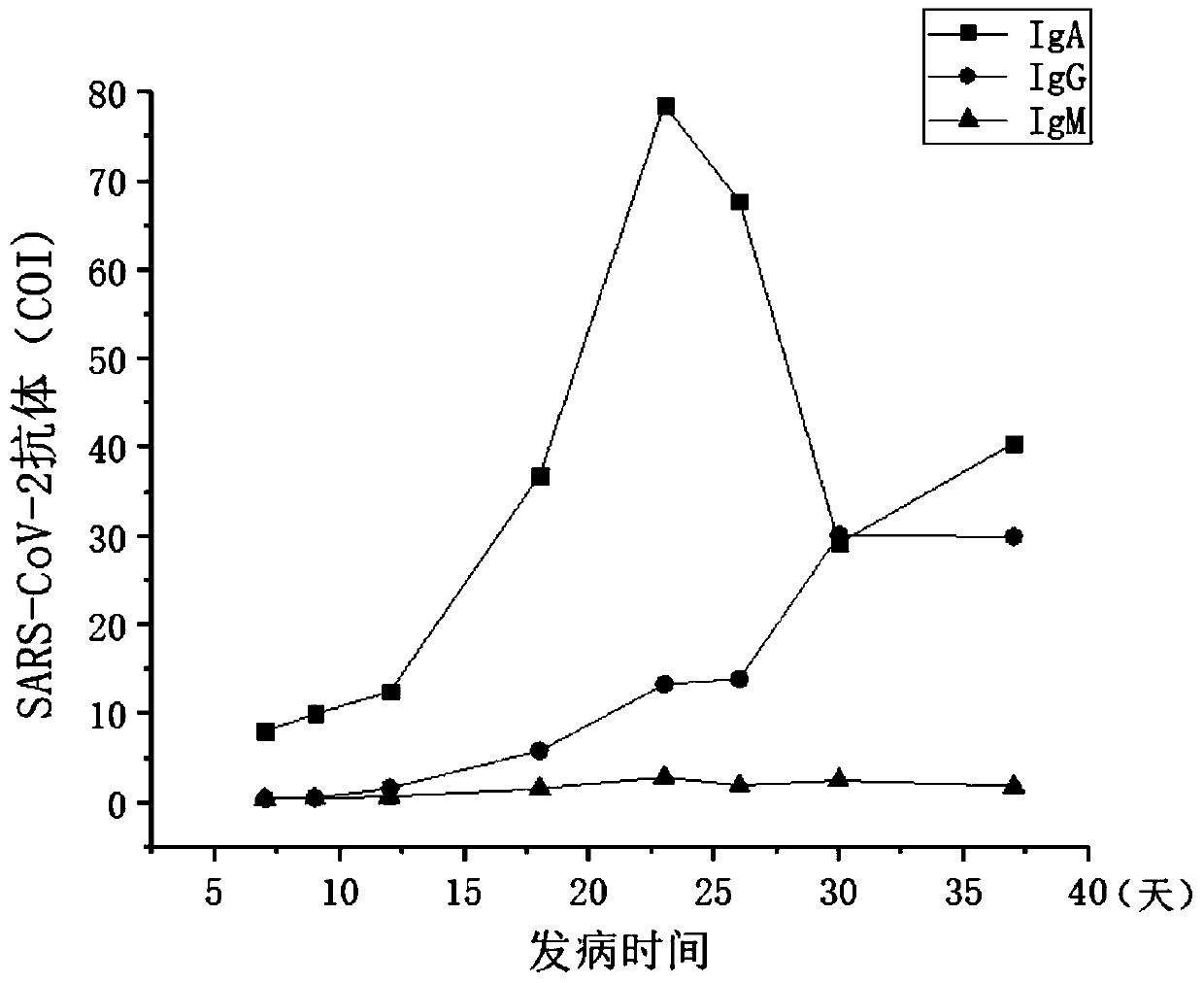
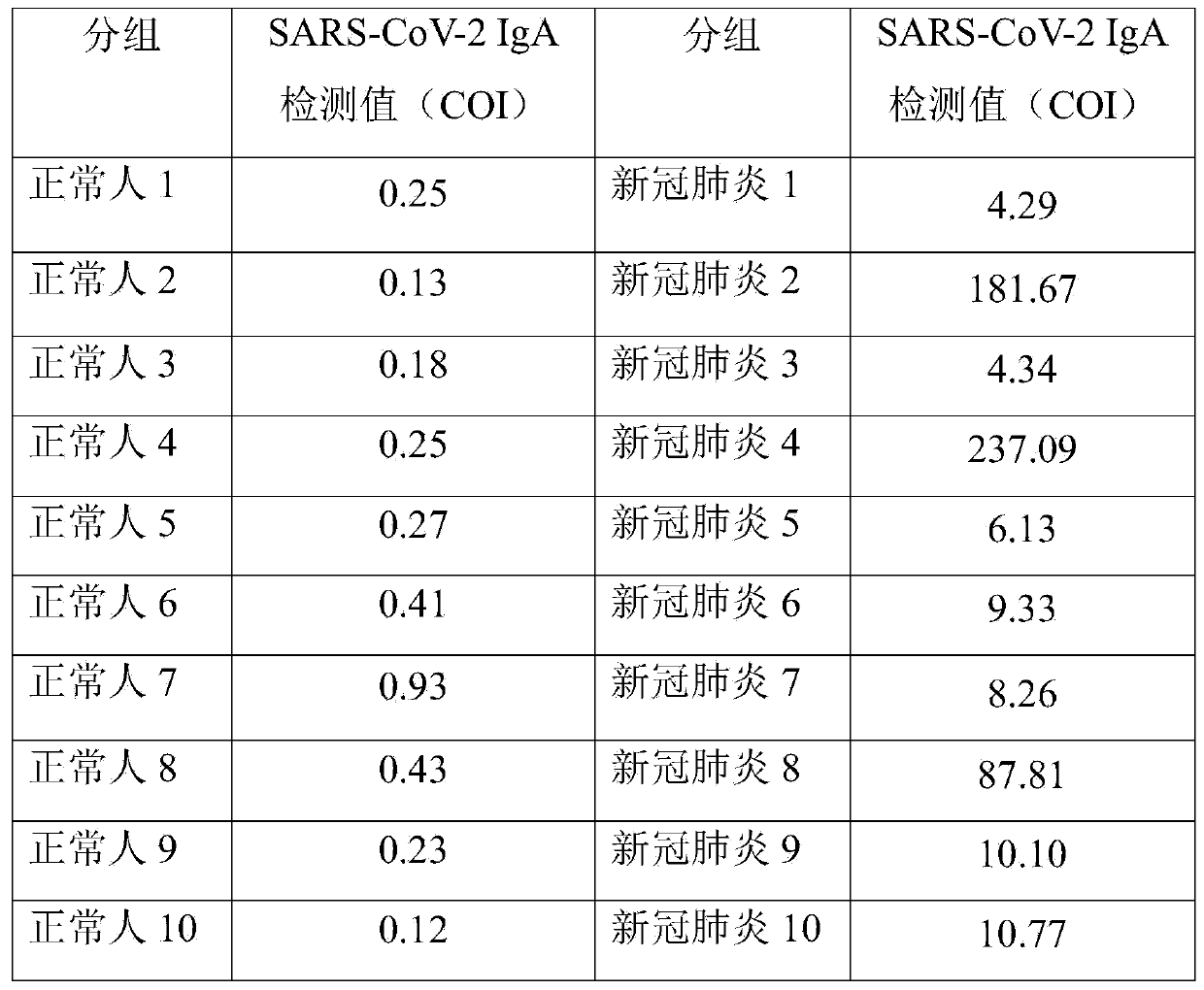
A novel coronavirus earlier-stage screening method disclosed by the invention can be used for detecting the novel coronavirus, and is particularly used for screening the SARS-CoV-2IgA antibody in theearly stage. A kit comprises an SARS-CoV-2 magnetic bead coating material used as R1, containing a magnetic bead coating material trihydroxymethyl aminomethane buffer solution coated by an SARS-CoV-2recombinant antigen, wherein the pH value is 7.1-7.4; and an acridinium ester marker of the anti-human IgA antibody as R2, wherein the acridinium ester marker is a 2-(N-morpholine) ethanesulfonic acidbuffer solution containing an acridinium ester labeled mouse anti-human IgA monoclonal antibody. The kit and the SARS-CoV-2IgA antibody immunoassay reagent disclosed by the invention can be used forsupplementing and diagnosing new coronal pneumonia at an earlier stage, and have the characteristic of accurate detection result.
Owner:GUANGZHOU KANGRUN BIOTECHNOLOGY CO LTD +1
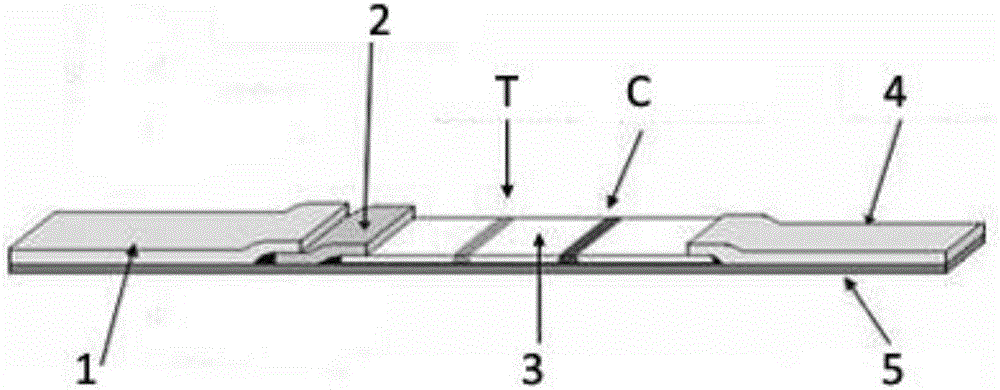
Novel coronavirus detection test strip as well as preparation method and application thereof
InactiveCN111426840AImprove accuracySimple and fast operationBiological testingImmunoassaysIgm antibodyMonoclonal antibody agent
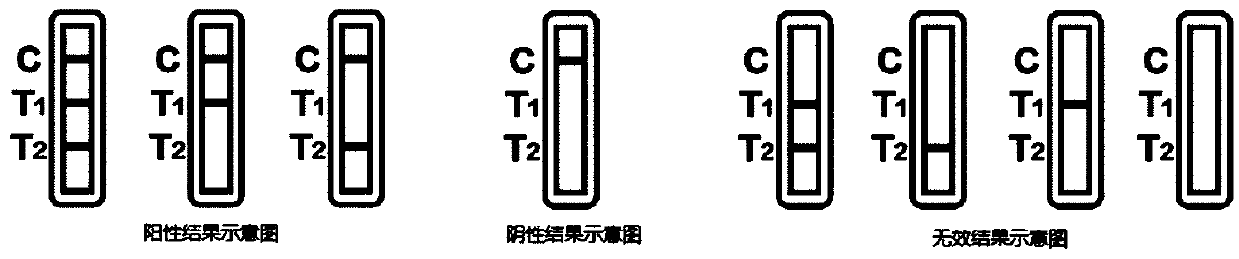
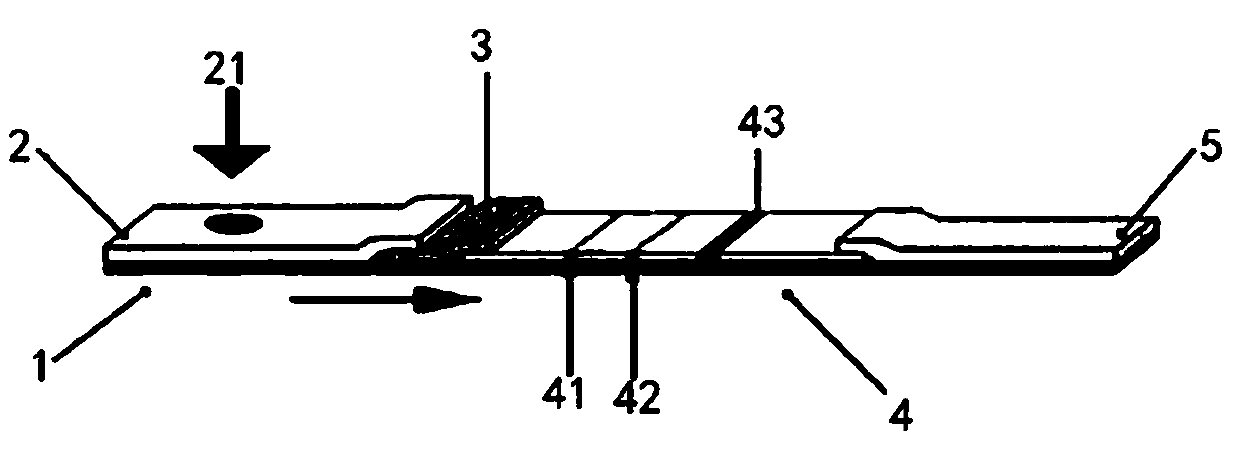
The invention provides a novel coronavirus detection test strip as well as a preparation method and application thereof. The test strip comprises a binding pad and an analysis membrane, and the binding pad is coated with a luminescent substance labeled 2019-nCoV recombinant antigen and a mouse anti-human HCG monoclonal antibody; a T2 detection line, a T1 detection line and a quality control line are sequentially arranged on the analysis membrane along the chromatography direction; the T2 detection line is coated with a mouse anti-human IgG monoclonal antibody, the T1 detection line is coated with a mouse anti-human IgA monoclonal antibody and a mouse anti-human IgM monoclonal antibody, and the quality control line is coated with a goat anti-mouse IgG polyclonal antibody. The test paper card can simultaneously determine the positive conditions of IgA antibody, IgM antibody and IgG antibody in serum of a patient, can more accurately detect the early antibody level condition in the body of the patient, assists in judging different periods of novel coronavirus infection of the patient, and improves the sensitivity and specificity of novel coronavirus detection.
Owner:北京中检安泰诊断科技有限公司
Colloidal gold test paper strip for detecting respiratory syncytial virus antibody and preparation method thereof
InactiveCN102253205ASimple methodThe result is clear and easy to distinguishMaterial analysisImmunopotencyRespiratory syncytial virus antibody
The invention provides a colloidal gold test paper strip for detecting a respiratory syncytial virus (RSV) antibody. In the colloidal gold test paper strip, the IgA antibody of the respiratory syncytial virus is detected by enveloping an antihuman IgA polyclonal antibody on a nitrocellulose membrane (NC membrane), and combining a respiratory syncytial virus specific recombinant antigen marked by colloidal gold and by using immune chromatography technology. The IgA antibody of the respiratory syncytial virus can be detected simply, conveniently, quickly and accurately by using the test paper strip, and a detection result is clear and easy to distinguish, so the test paper strip is suitable for batch detection and is suitable for grass-root screening and epidemiological survey, plays the role in assisting diagnosis of the early stage and medium stage of the infection of the respiratory syncytial virus and has a reference significance for judging whether children have the immunity of RSV resistance or not.
Owner:KUNMING BEIER ZUNSHENG TECH
Antibody detection kit (OmpC-IgA)
InactiveCN103091500AEasy to detectSensitive detectionChemiluminescene/bioluminescenceBiological testingImmunologic disordersAutoimmune disease
The invention relates to a detection kit and detection method for detecting Escherichia coli outer membrane protein C (OmpC) IgA antibody, belonging to the field of immunoassay detection. The OmpC antibody kit provided by the invention comprises a OmpC-coated microporous plate and an enzyme-labeled anti-human IgA antibody. The detection kit provided by the invention has the characteristics of high accuracy, high specificity, high sensitivity and the like, can be used for detecting human body tissue OmpC IgA antibody, and has important functions in diagnosing certain autoimmune diseases.
Owner:苏州和锐生物科技有限公司
Gene expression vaccine
InactiveUS7118888B2Safe and effective against RSVReduce inflammationOrganic active ingredientsPowder deliveryRSV InfectionsT lymphocyte
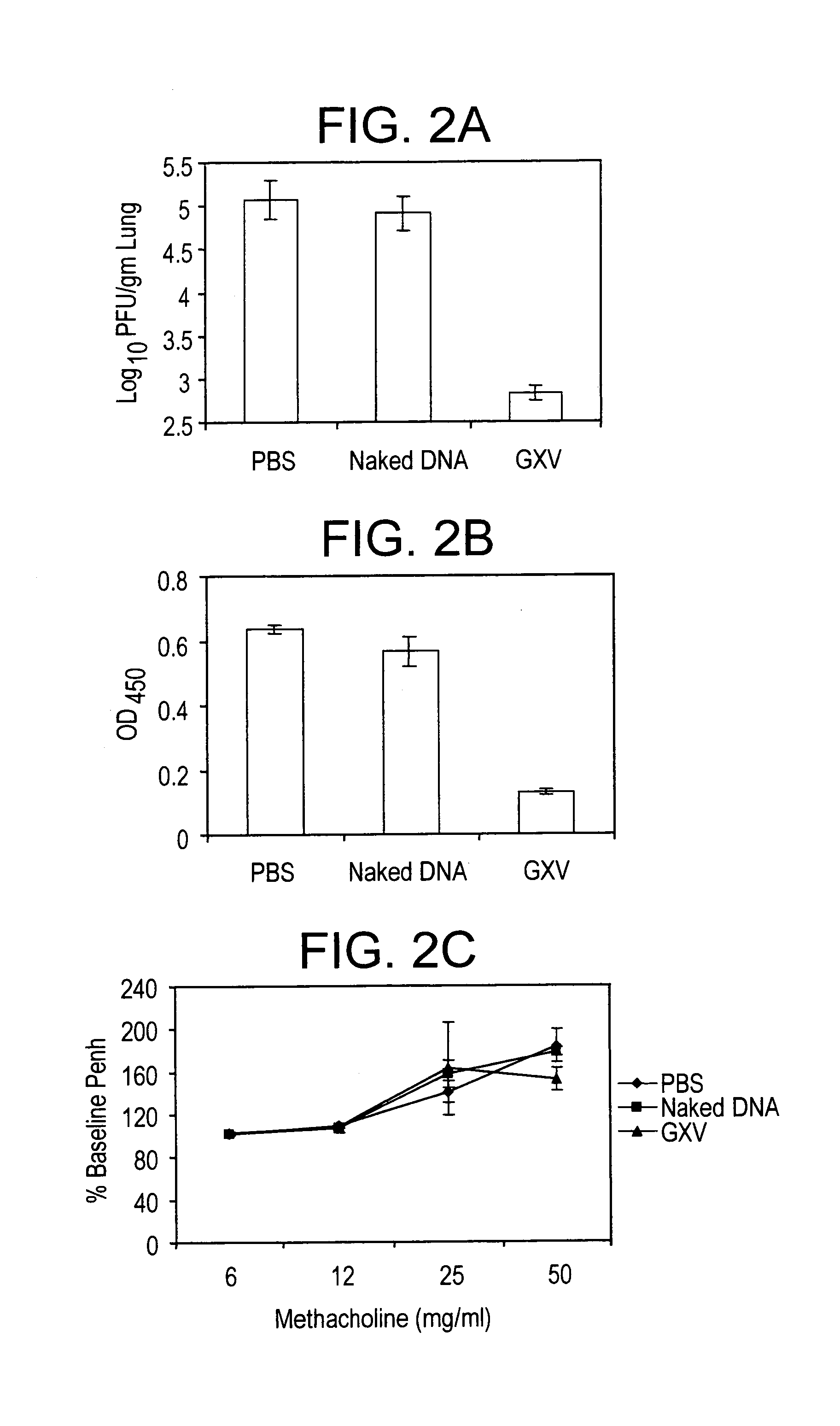
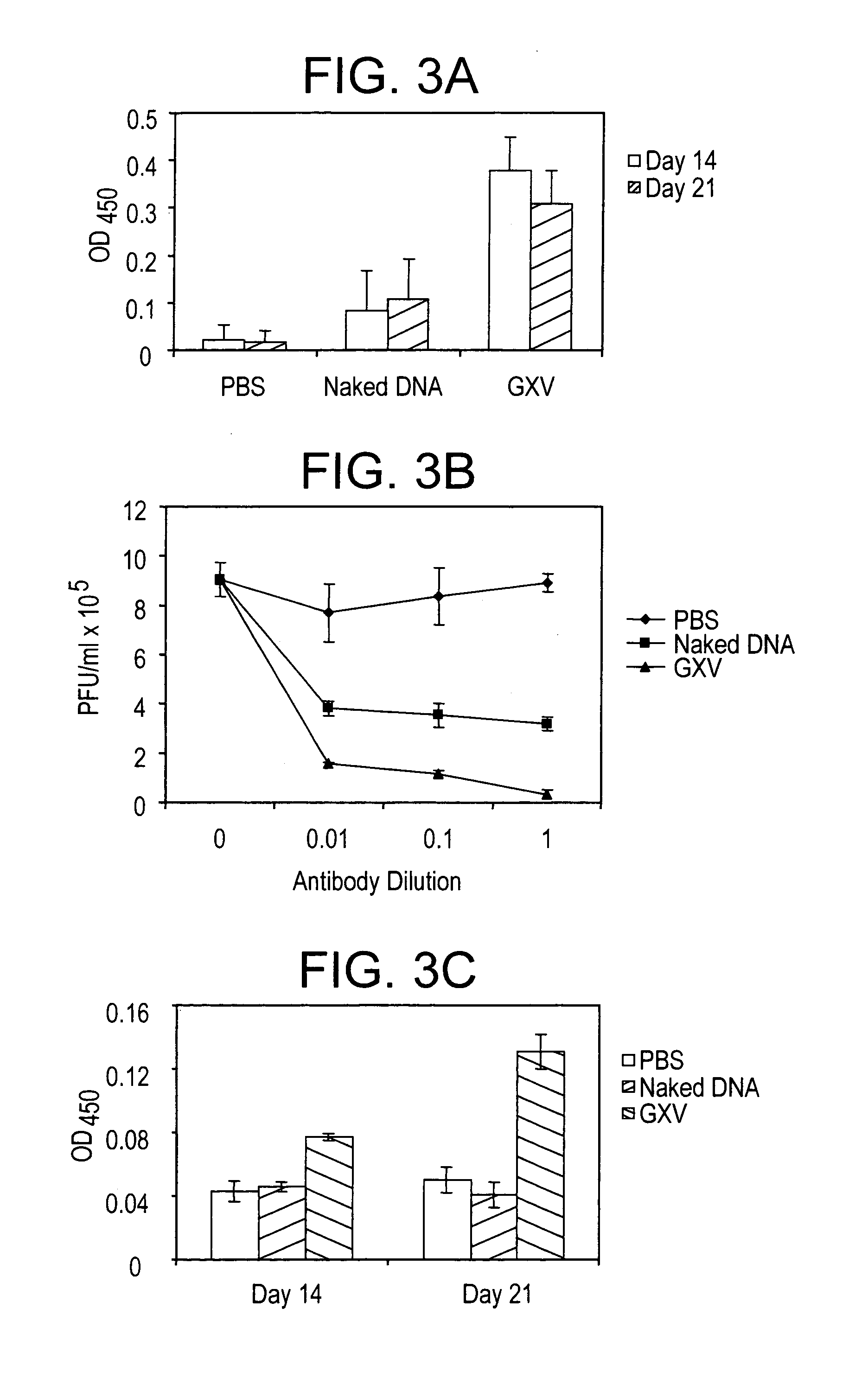
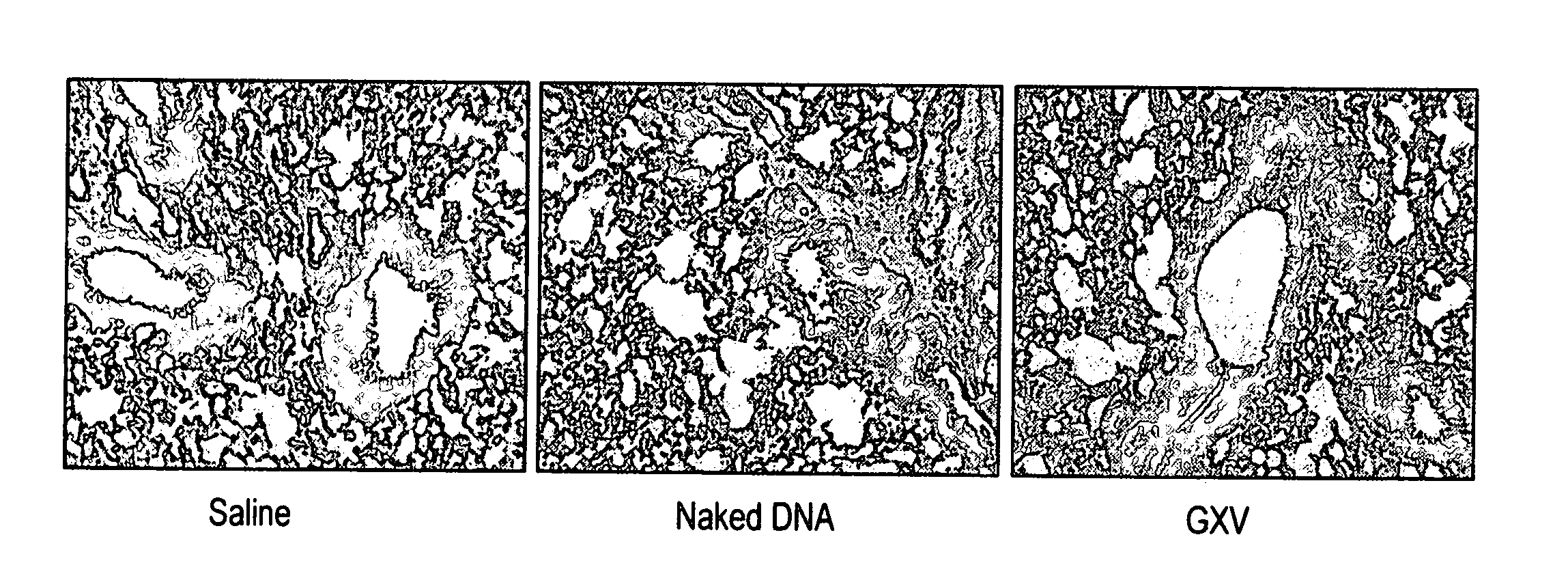
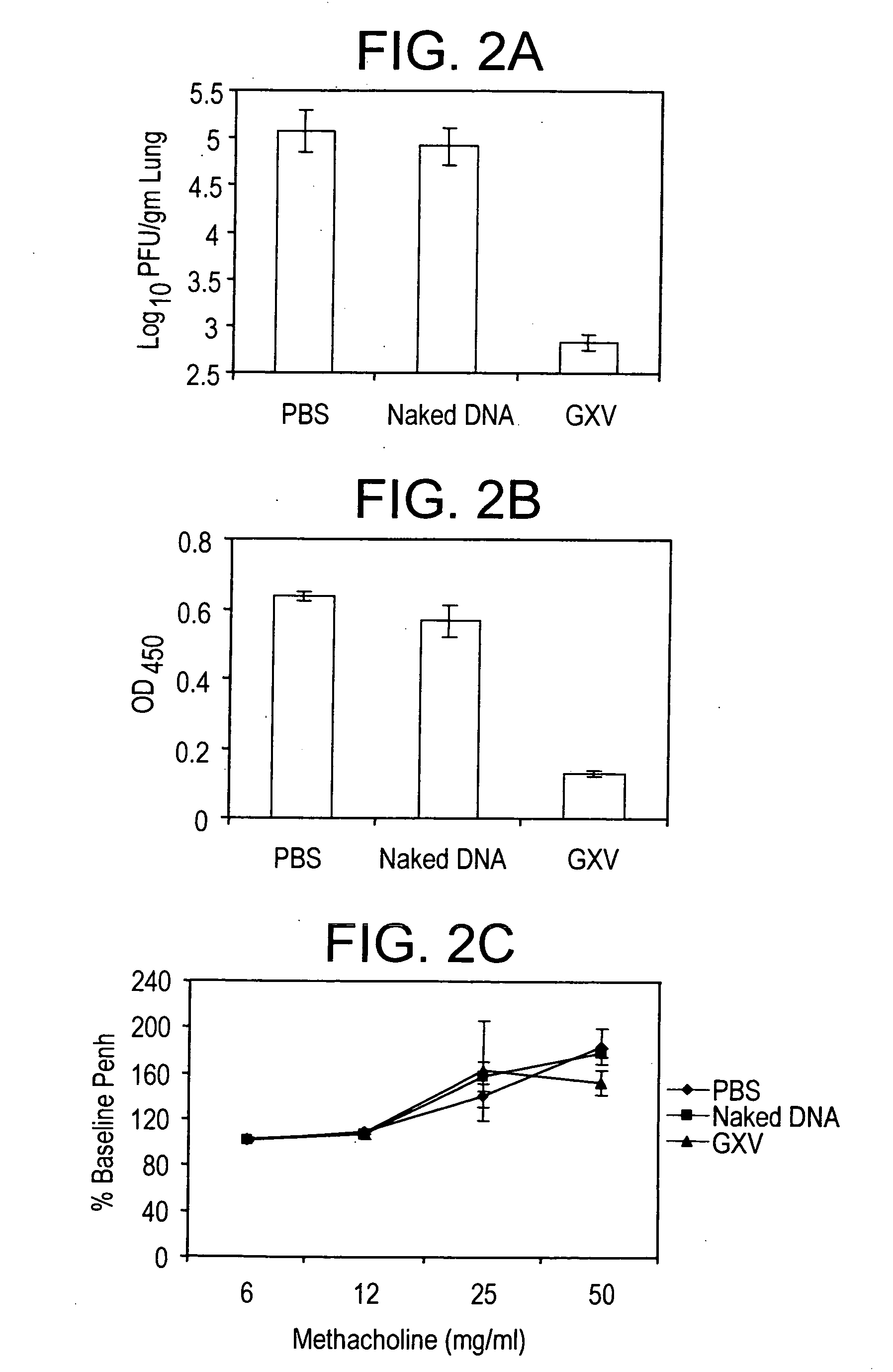
An effective prophylactic mucosal gene expression vaccine (GXV), made up of a cocktail of at least 4 different plasmid DNAs encoding corresponding RSV antigens, coacervated with chitosan to formulate nanospheres. In a murine model of RSV infection, intranasal administration with GXV results in significant induction of RSV-specific antibodies, nasal IgA antibodies, cytotoxic T lymphocytes, and IFN-γ production in the lung and splenocytes. A single dose of GXV induces a drastic reduction of viral titers.
Owner:THE JOHN HOPKINS UNIV SCHOOL OF MEDICINE +1
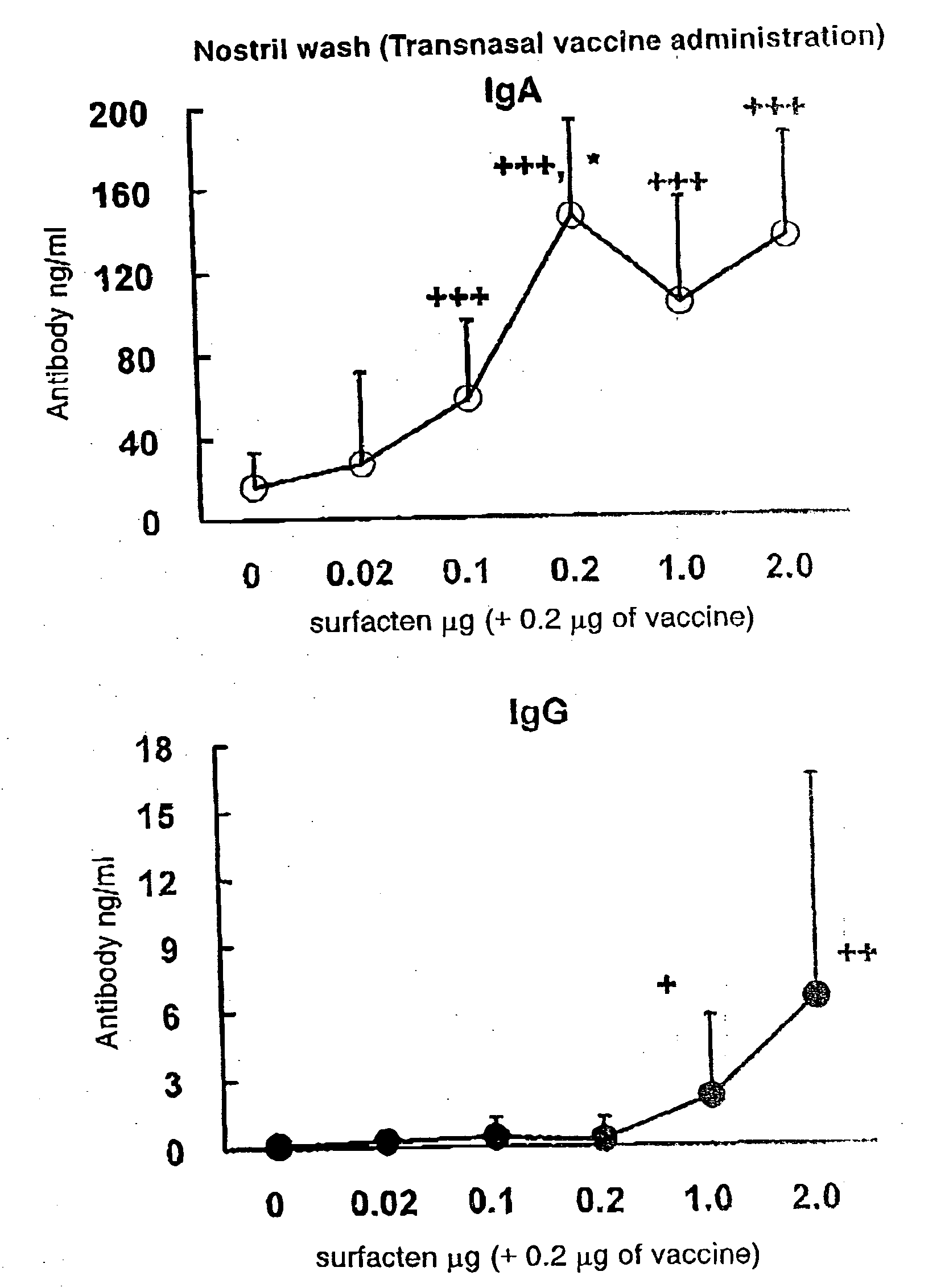
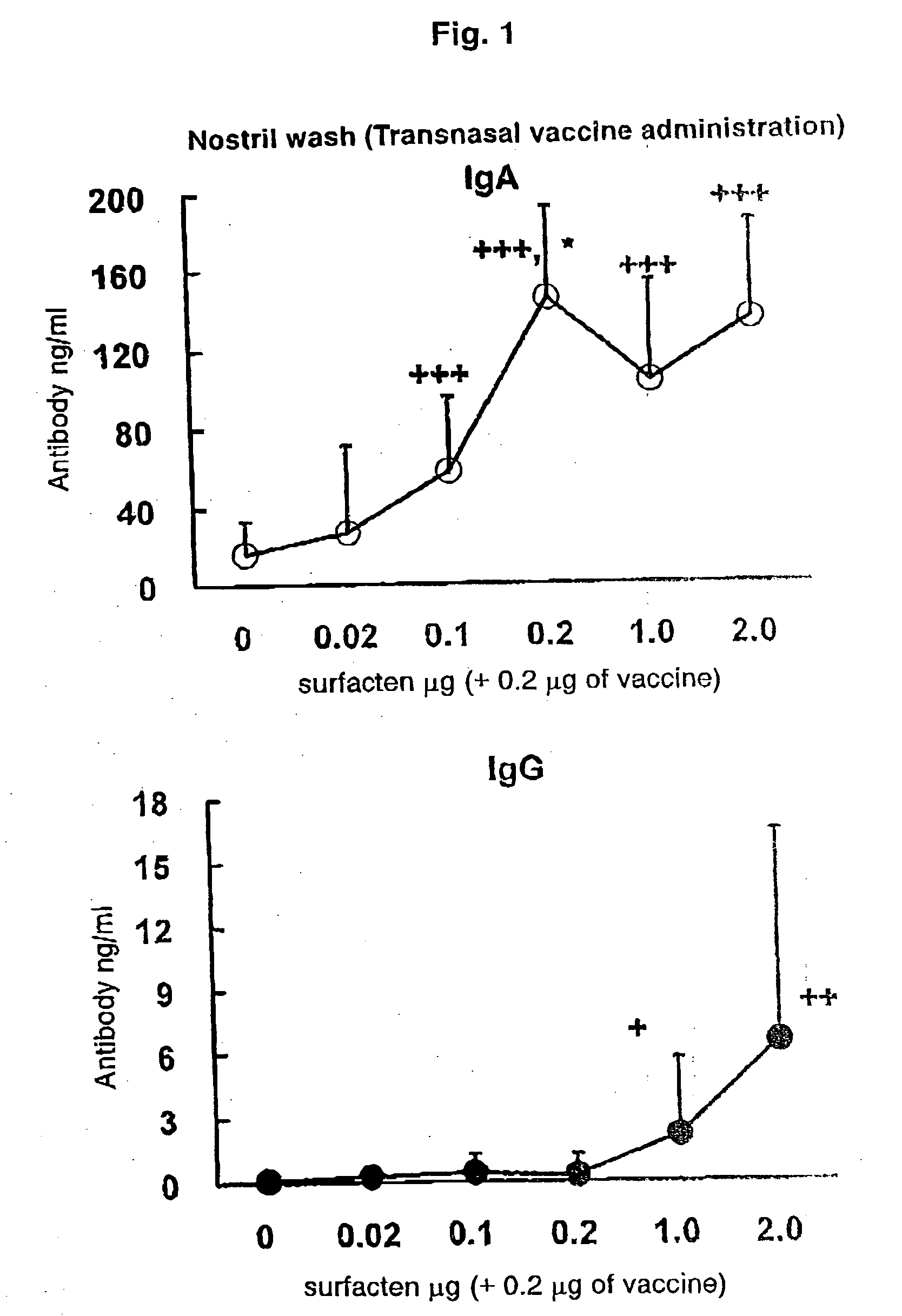
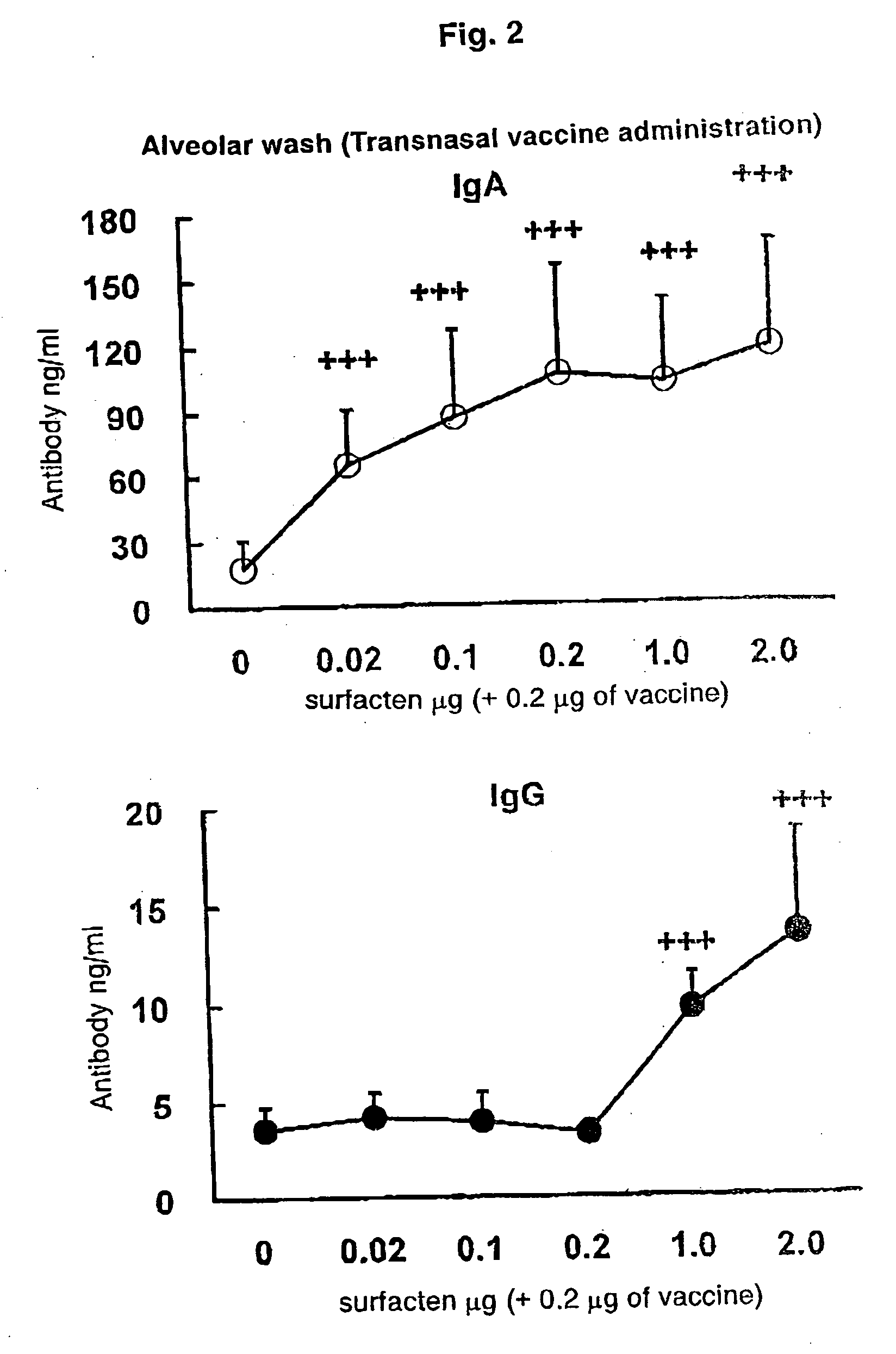
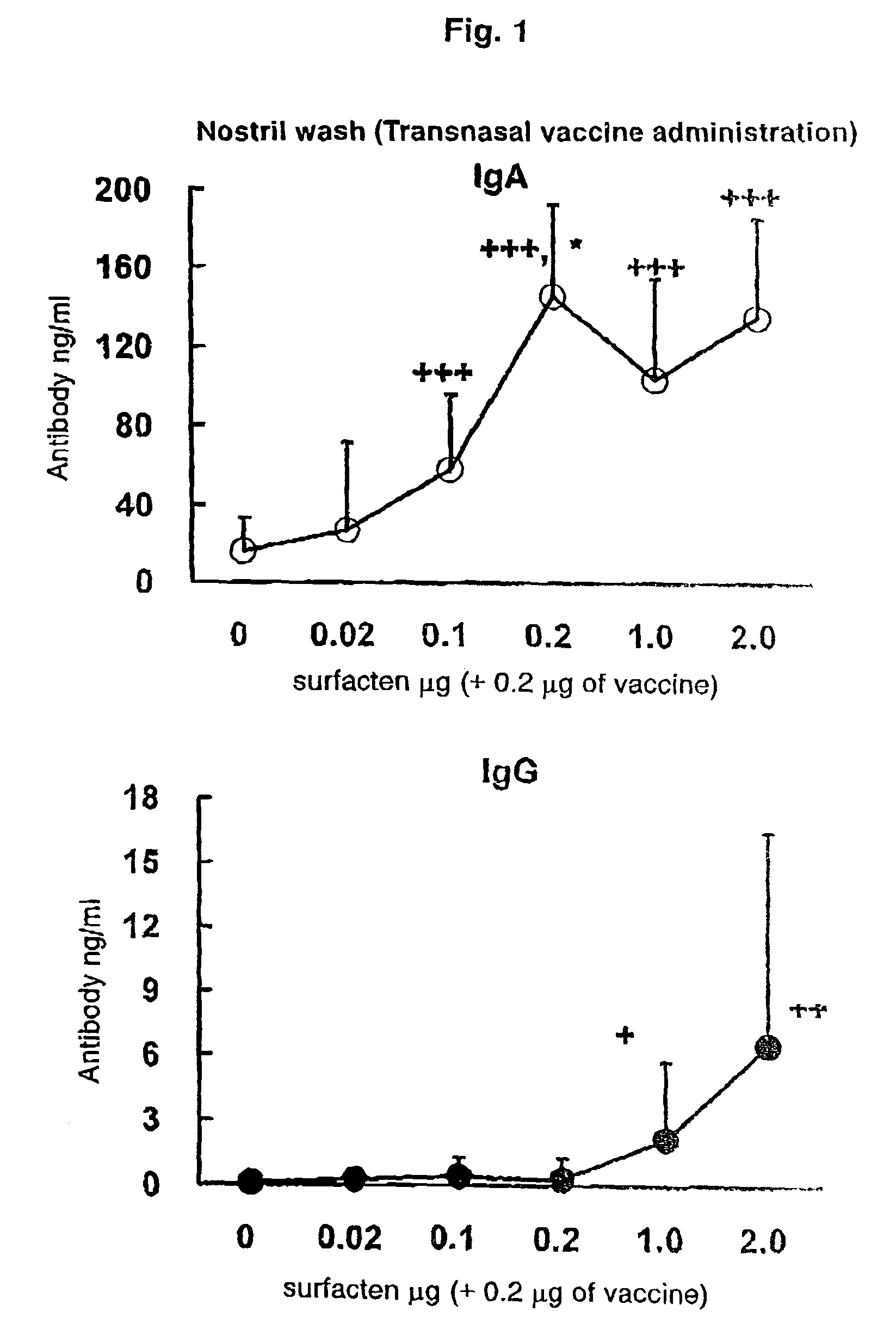
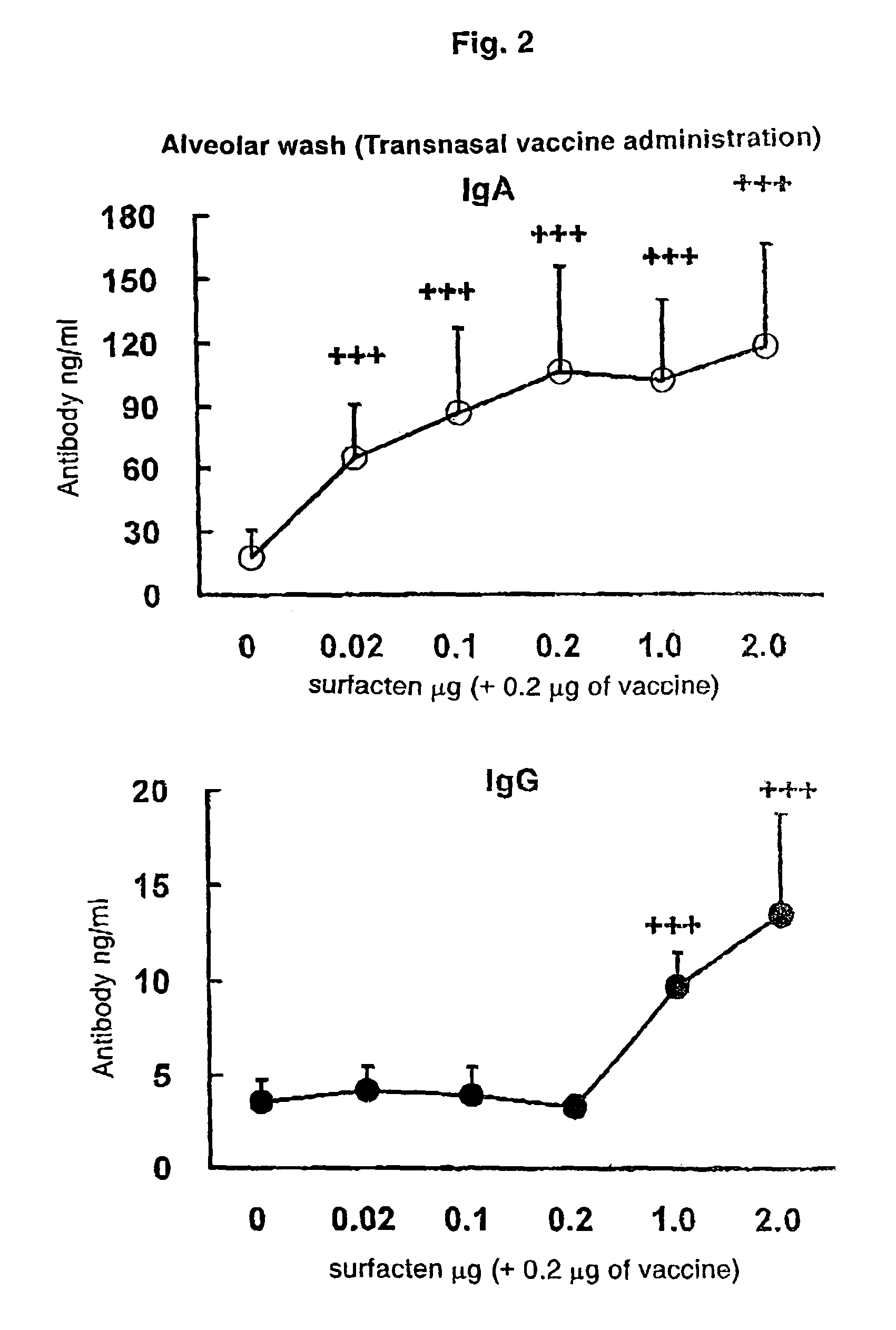
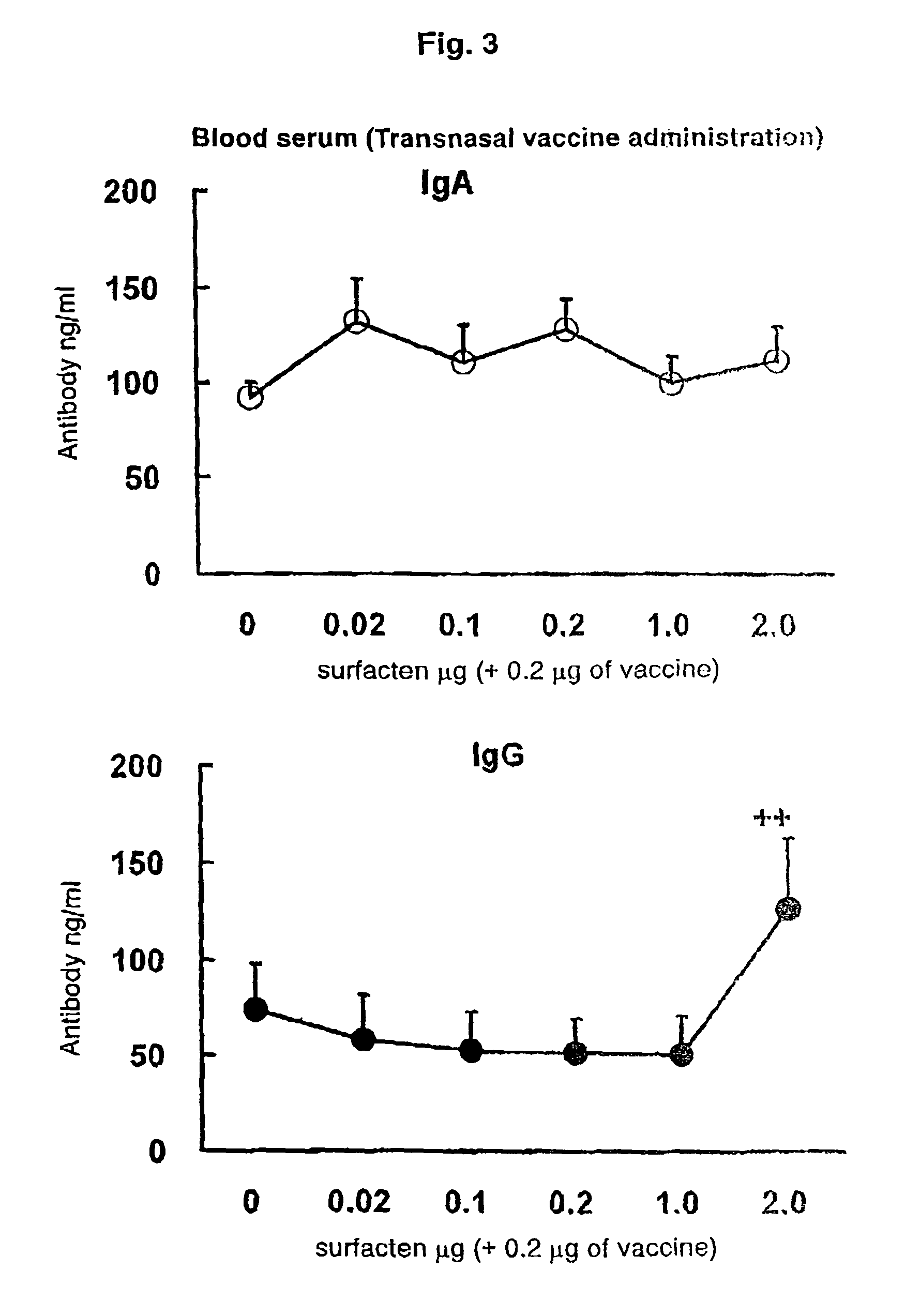
Antigen-Drug Vehicle Enabling Switch From Selective Production of IgA Antibody to Production of Both of IgA and IgG Antibodies and Transnasal/Mucosal Vaccine Using the Same
ActiveUS20090130131A1Easy to solveReinforces and promotes prophylactic/therapeutic effectSsRNA viruses negative-senseViral antigen ingredientsMucosal vaccineBody fluid
In the aim of practical utilization of a safe and effective transnasal / inactivated / mucosal vaccine and establishment of a technology for imparting capacity of producing both of IgA and IgG antibodies to a conventional inactivated vaccine, toxoid; allergen, or the like, a means for prevention and treatment of allergy, and the like, it is intended to provide an antigen-drug vehicle (AD vehicle) enabling transnasal, transmucosal, and transdermal administrations, an inactivated vaccine simultaneously inducing a mucosal immunity and humoral immunity by using the AD vehicle, a production method of the inactivated vaccine, an AD vehicle enabling a switch from induction of selective production of IgA antibody to induction of both of IgA and IgG antibodies, and a transnasal vaccine, a mucosal vaccine, a therapeutic / prophylactic agent for allergy, and the like using the AD vehicle.
Owner:UNIVERSITY OF TOKUSHIMA
Method for preparing rapid detection test paper of novel coronavirus IgA antibody
InactiveCN111233985AImprove stabilityHigh sensitivitySsRNA viruses positive-senseVirus peptidesSaliva sampleCoronavirus antibody
The invention provides a recombinant protein and test paper for detecting a novel coronavirus 2019-nCoV IgA antibody, and a preparation method and application, and belongs to the technical field of virus detection. The amino acid sequence of the recombinant protein is shown in SEQ ID No.1; the test paper comprises a bottom plate as well as a sample absorption pad, a fluorescent microsphere pad, anitrocellulose membrane and a water absorption pad which are in sequential lap joint and adhesion to the bottom plate; a 2019 novel coronavirus specific antigen marked by fluorescent microspheres is sprayed to the fluorescent microsphere pad; a detection zone and a quality control zone are fixed on the nitrocellulose membrane; an anti-human IgA antibody is sprayed to the detection zone; and a sheep anti-chicken IgY antibody is sprayed to the quality control zone. By detecting the IgA antibody in a saliva sample, the test paper provided by the invention is capable of simply, rapidly and accurate detecting novel coronaviruses, and early-stage detection on the novel coronaviruses can be achieved.
Owner:BEIJING DIAGREAT BIOTECH CO LTD
EB virus VCA-IgA antibody detection reagent and preparation method thereof
The invention provides an EB virus VCA-IgA antibody detection reagent which at least comprises a reactive film, an antigen pad and a gold-labeled pad, wherein a detection line and a quality control line are marked on the reactive film; the detection line contains a mouse anti-human IgA monoclonal antibody; the quality control line contains biotin; the antigen pad contains a biotin-labeled recombinant EB virus VCA antigen; and the gold-labeled pad contains an avidin complex labeled by colloidal gold. The detection kit disclosed by the invention has the characteristics of rapidness, simplicity, accuracy and high accuracy.
Owner:中山生物工程有限公司
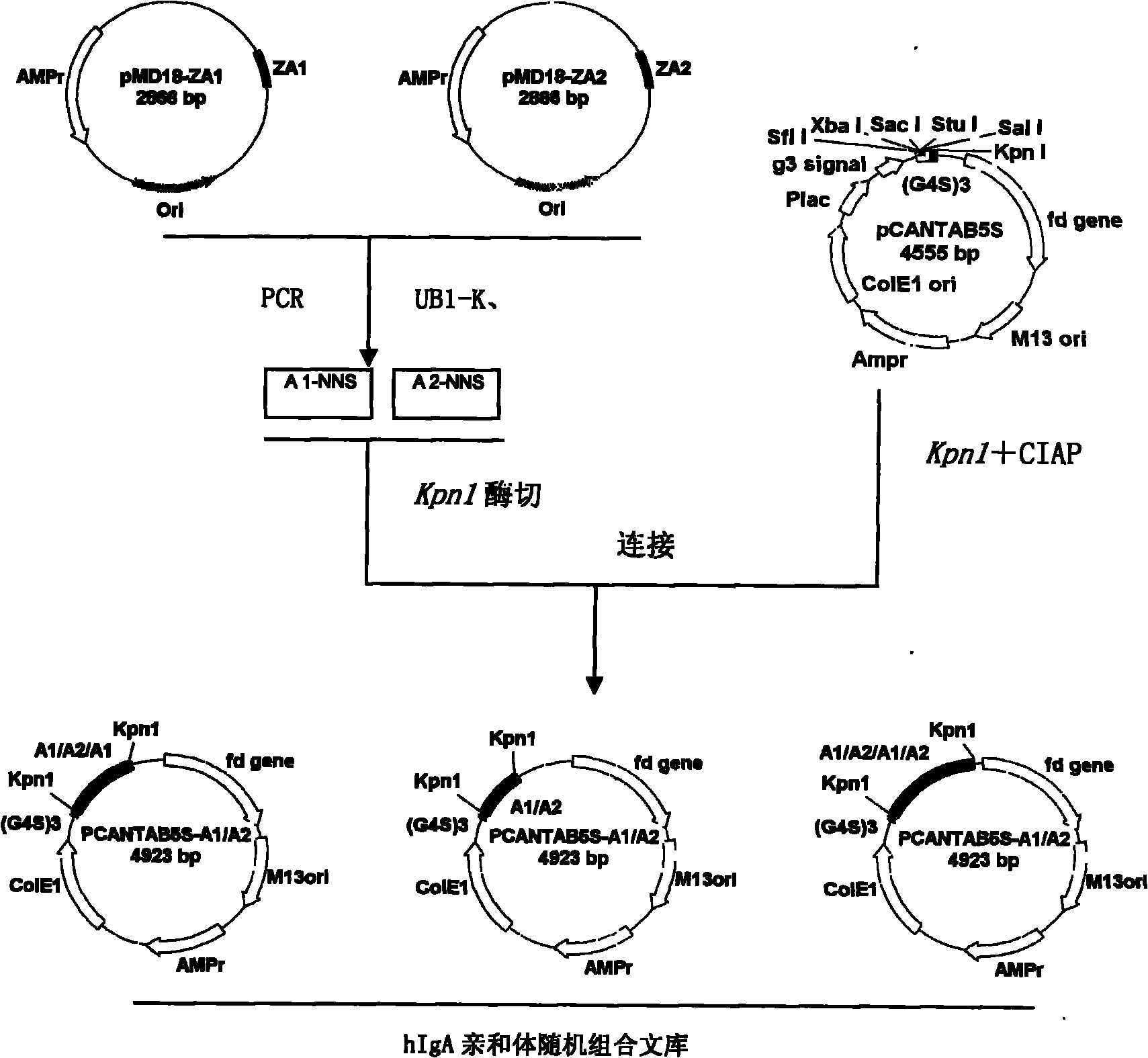
Human IgA immunoglobulin combination molecule having intramolecular affinity effect
InactiveCN102115497AHigh binding activityHigh activityBacteriaMicrobiological testing/measurementGlobin bindingBacteriophage
The invention discloses a human IgA immunoglobulin combination molecule having intramolecular affinity effect, a preparation method and an application thereof. The invention also discloses genes coding the human IgA immunoglobulin combination molecule, a preparation method and an application of the human IgA immunoglobulin combination molecule based on bacteriophage molecule evolution. The human IgA immunoglobulin combination molecule of the invention, especially repeated molecule of human IgA affibody, has intramolecular affinity effect when binding the human IgA, demonstrates very high human IgA binding activity, can be used for purification of high-specific IgA and research and development of detecting reagent, and purification for detection of human IgA antibody by ELISA adsorption method, immunity chromatography, immunohistochemical method and the like.
Owner:SECOND MILITARY MEDICAL UNIV OF THE PEOPLES LIBERATION ARMY
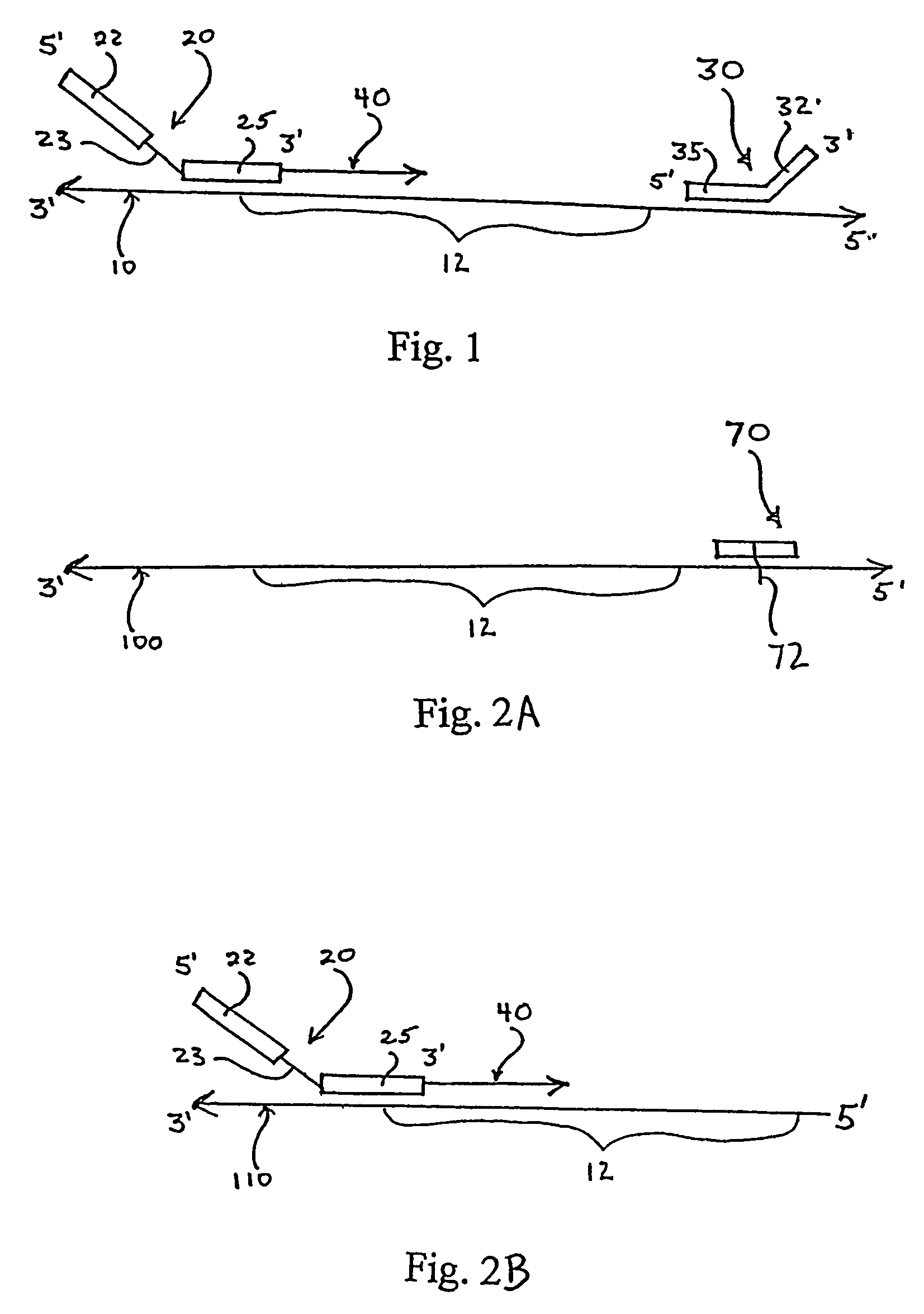
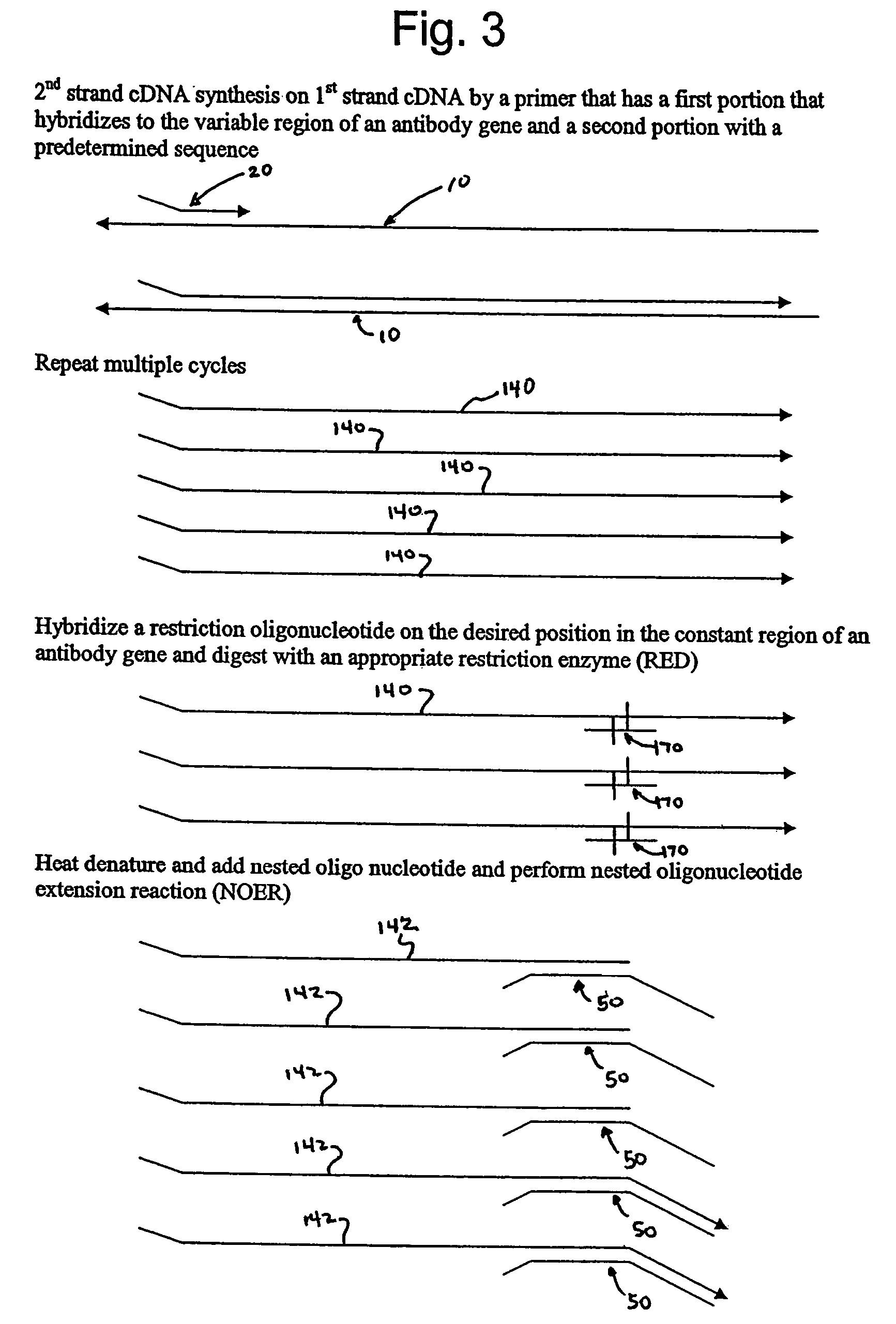
Engineered templates and their use in single primer amplification
InactiveUS7414111B2Peptide librariesMicrobiological testing/measurementNucleotideNucleic acid sequencing
Methods of amplifying nucleic acid have now been discovered which include the steps of: a) annealing a primer to a template nucleic acid sequence, the primer having a first portion which anneals to the template and a second portion of predetermined sequence; b) synthesizing a polynucleotide that anneals to and is complementary to the portion of the template between the location at which the first portion of the primer anneals to the template and the end of the template, the polynucleotide having a first end and a second end, wherein the first end incorporates the primer; c) separating the polynucleotide synthesized in step (b) from the template; d) annealing a nested oligonucleotide to the second end of the polynucleotide synthesized in step (b), the nested oligonucleotide having a first portion that anneals to the second end of the polynucleotide and a second portion having the same predetermined sequence as the second portion of the primer; e) extending the polynucleotide synthesized in step (b) to provide a terminal portion thereof that is complementary to the predetermined sequence; and f) amplifying the extended polynucleotide using a single primer having the predetermined sequence. In particularly useful embodiments, the methods are used to amplify a repertoire of IgA antibodies.
Owner:ALEXION PHARMA INC
Rheumatoid factor detection kit and detection method thereof
ActiveCN109406796AHigh sensitivityWide linear rangeDisease diagnosisBiological testingInorganic saltsPreservative
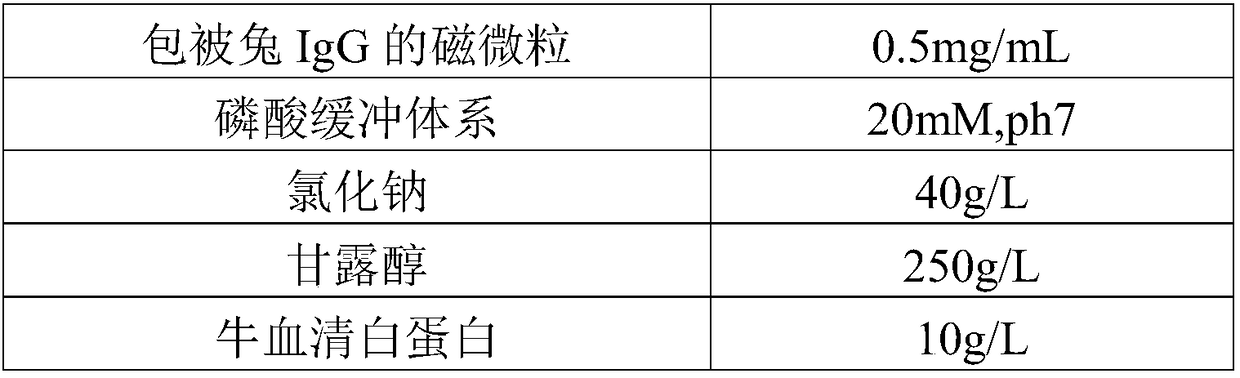
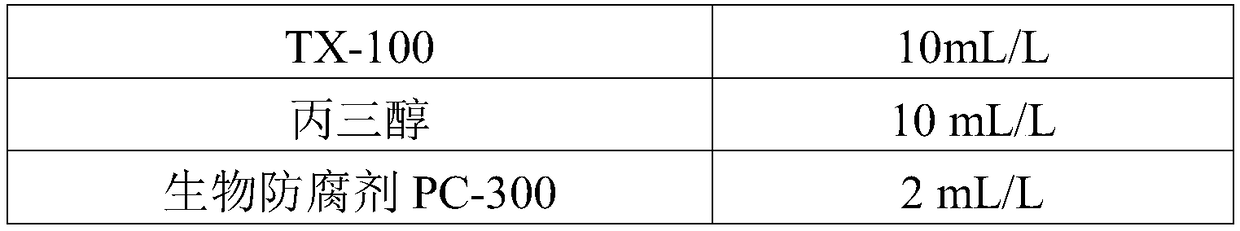
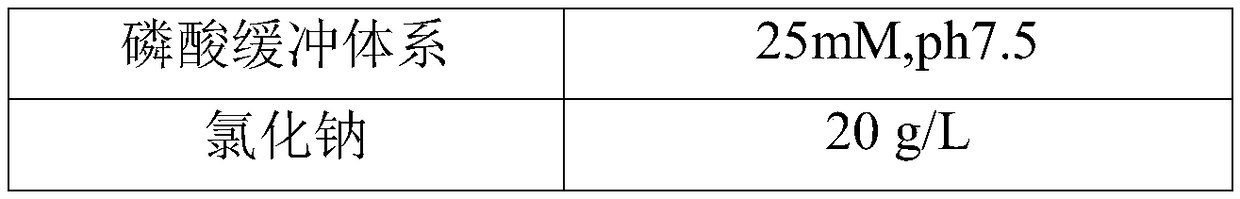
The invention discloses a rheumatoid factor detection kit and a preparation method thereof. The kit comprises a reagent R1, a reagent R2 and a reagent R3, wherein the reagent R1 contains magnetic particles coated with rabbit IgG; the reagent R2 contains markers of anti-human IgM / IgG / IgA antibodies or anti-human IgM antibodies or anti-human IgG antibodies or anti-human IgA antibodies; the reagent R3 is prepared by mixing a buffering system, inorganic salt, saccharides, proteins, a coloring agent, a nonionic surfactant and a preservative in a certain ratio. The rheumatoid factor detection kit ishigh in sensitivity, wide in linearity range, high in anti-interference capacity and capable of safely and quickly detecting rheumatoid factors.
Owner:SICHUAN MACCURA BIOTECH CO LTD
Detection method and detection kit for porcine type A foot-and-mouth disease virus specific IgA antibody
The invention discloses a detection method for a porcine type A foot-and-mouth disease virus specific IgA antibody; a prokaryotic expression system-expressed foot-and-mouth disease virus VP1 protein is used as a coating antigen, a mouse anti porcine IgA monoclonal antibody is used as a secondary antibody, an enzyme-labeled goat anti mouse IgG antibody is used as an indicator, a to-be-tested sample is subjected to reactions with the coating antigen, the monoclonal antibody and the enzyme-labeled antibody successively, and then is compared with a reference positive sample and a negative sample, and the result can be used for evaluating the level of the porcine foot-and-mouth disease virus specific IgA antibody; and a detection kit is provided. The detection method has the beneficial effects that the invention provides an effective method for evaluating the immune effect of foot-and-mouth disease mucosa and provides a new method for early diagnosis of infection of foot-and-mouth disease, and the foot-and-mouth disease virus specific IgA antibody in porcine nasal swabs can be quickly and accurately detected, sample collection operation is simple, manpower consumption is low, and the stress on animals is small.
Owner:LANZHOU INST OF VETERINARY SCI CHINESE ACAD OF AGRI SCI
Influenza virus A IgA antibody immunofluorescence detection test strip, and preparation method, detection method, and applications thereof
ActiveCN108254554AQuick screeningHigh sensitivityBiological material analysisCelluloseImmunofluorescence
The invention provides an influenza virus A IgA antibody immunofluorescence detection test strip, and a preparation method thereof. The influenza virus A IgA antibody immunofluorescence detection teststrip comprises a sample pad, a label pad, a cellulose nitrate membrane, a water absorbent paper, and a backboard; the cellulose nitrate membrane comprises a detection line of influenza virus A NP protein antigen polypeptide fragments, and a human IgA antibody control line; the label pad is provided with anti-human IgA antibody-fluorescence microsphere markers. The influenza virus A IgA antibodyimmunofluorescence detection test strip is extremely high in detection sensitivity; expensive PCR detector is not needed; detection results can be observed with naked eyes; rapid screening on influenza virus A is realized. The invention also provides a detection method and applications of the influenza virus A IgA antibody immunofluorescence detection test strip. The detection method and the stepsare simple; operation is convenient; results are obtained quickly; and the influenza virus A IgA antibody immunofluorescence detection test strip is worthy of wide popularization.
Owner:GUANGZHOU RHFAY BIOTECH CO LTD
Helicobacter pylori antibody detection kit, detection method and application
InactiveCN104614522AAccurate detectionHigh detection sensitivityChemiluminescene/bioluminescenceFluorescence/phosphorescencePre-DilutionMicrosphere
The invention discloses a helicobacter pylori antibody detection kit, comprising the following constituents: a magnetic microsphere solution directly coating or indirectly connecting helicobacter pylori antigen; and a marking tracer solution directly coating or indirectly connecting anti-human IgG antibody; and / or a marking tracer solution directly coating or indirectly connecting anti-human IgM antibody; and / or a marking tracer solution directly coating or indirectly connecting anti-human IgA antibody; and / or a marking tracer solution directly coating or indirectly connecting staphylococcus aureus A protein. The invention further discloses a corresponding helicobacter pylori antibody detection method and application thereof. The kit, the method and the application provided by the invention can detect various antibodies, including helicobacter pylori IgG antibody, helicobacter pylori IgM, helicobacter pylori IgA and total helicobacter pylori antibody, are applicable to complex conditions of helicobacter pylori infected person better, moreover, can accurately detect the helicobacter pylori antibody without pre-dilution to samples.
Owner:SHENZHEN NEW INDS BIOMEDICAL ENG
Mucosal vaccine enabling switching from production of IgA antibody to production of both of IgA and IgG antibodies
ActiveUS8211442B2Easy to solveReinforces and promotes effectSsRNA viruses negative-senseViral antigen ingredientsBody fluidMucosal vaccine
In the aim of practical utilization of a safe and effective transnasal / inactivated / mucosal vaccine and establishment of a technology for imparting capacity of producing both of IgA and IgG antibodies to a conventional inactivated vaccine, toxoid, allergen, or the like, a means for prevention and treatment of allergy, and the like, it is intended to provide an antigen-drug vehicle (AD vehicle) enabling transnasal, transmucosal, and transdermal administrations, an inactivated vaccine simultaneously inducing a mucosal immunity and humoral immunity by using the AD vehicle, a production method of the inactivated vaccine, an AD vehicle enabling a switch from induction of selective production of IgA antibody to induction of both of IgA and IgG antibodies, and a transnasal vaccine, a mucosal vaccine, a therapeutic / prophylactic agent for allergy, and the like using the AD vehicle.
Owner:UNIVERSITY OF TOKUSHIMA
Immune globulin M detection reagent
The invention discloses an immunoglobulin M detection reagent, which is provided with simple operation, high accuracy and good reproducibility and strong anti-interference ability; the sample is free from dilution and the detection reagent can be applicable to the immunoglobulin M (IgM) detection reagents of various automatic biochemical analyzers. The immunoglobulin M detection reagent comprises an IgA reagent, an anti-IgA antibody reagent and a liquid serotype constant value calibration liquid; wherein, the IgA reagent enables the IgA antigenic sites in the sample to be fully exposed so as to facilitate the full combination with the anti-IgA antibody reagent; the anti-IgA antibody reagent has high idiosyncrasy with the IgA antigens in human serum; and the liquid serotype constant value calibration liquid is compared with the sample for result calculation.
Owner:王贤理
EV71 virus IgA antibody detection test strip and application thereof
The invention relates to an EV71 virus IgA antibody detection test strip which comprises a sample pad, a label pad, a nitrocellulose membrane, absorbing paper and a back plate. The nitrocellulose membrane is provided with an EV71 virus VP1 protein antigen peptide fragment detection line and a human IgA antibody control line. The label pad is provided with an an-human IgA anti-body-colloidal gold marker. The EV71 virus IgA antibody detection test strip is used for saliva EV71 virus detection; by detecting an IgA anti-body in a saliva sample, EV71 viruses can be quickly screened, reliability of detection results can be improved, and false-negative conditions are reduced. A detection method is simple to operate, the results are quick, the collection of a detected sample has no invasiveness, and discomfort and cross infection caused by conventional blood sampling and collection of throat swab, anus swab samples and cerebrospinal fluid are avoided.
Owner:GUANGZHOU RHFAY BIOTECH CO LTD
Gene expression vaccine
InactiveUS20070009951A1Reduce inflammationInduces increases in specific IgG titersSsRNA viruses negative-senseOrganic active ingredientsEukaryotic plasmidsPlasmid dna
An effective prophylactic mucosal gene expression vaccine (GXV), made up of a cocktail of at least 4 different plasmid DNAs encoding corresponding RSV antigens, coacervated with chitosan to formulate nanospheres. In a murine model of RSV infection, intranasal administration with GXV results in significant induction of RSV-specific antibodies, nasal IgA antibodies, cytotoxic T lymphocytes, and IFN-γ production in the lung and splenocytes. A single dose of GXV induces a drastic reduction of viral titers.
Owner:UNIV OF SOUTH FLORIDA BOARD OF TRUSTEES +1
Immunoprotection by oral administration of recombinant lactococcus lactis mini-capsules
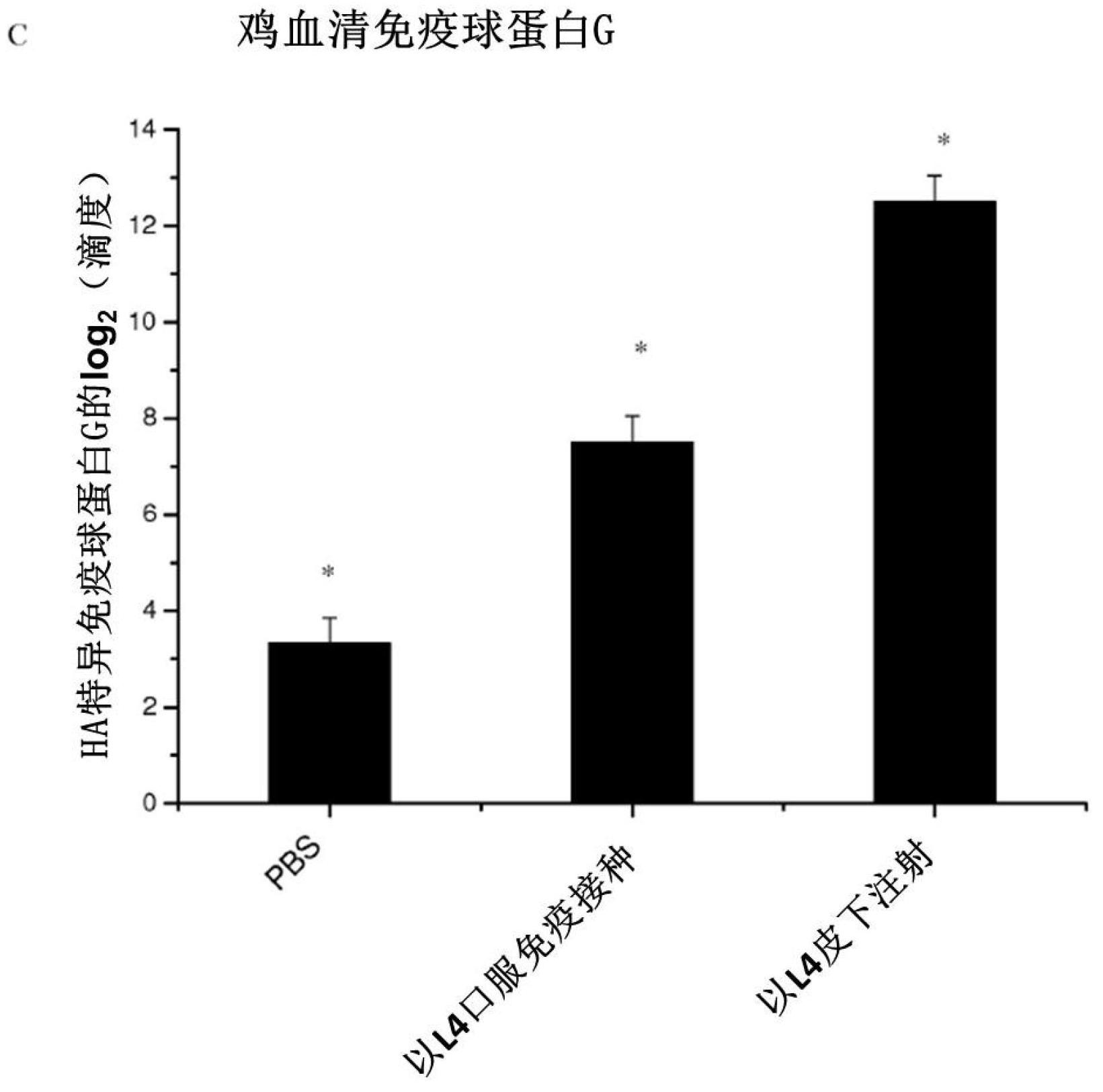
In one embodiment, the present invention provides for an edible mini-capsule form of live, non-persisting, recombinant Lactococcus lactis (L.lactis) vaccine against a pathogen such as the highly virulent influenza H5N1 strain. Enteric coated capsule of the present invention induced high levels of hemagglutinin-specific serum IgG and fecal IgA antibody production after oral administration in mice and chickens, and rendered complete protection against a lethal challenge of H5N1 virus in mice. The present invention thus demonstrates a broadly applicable platform technology for producing and administering edible vaccines against bacterial and viral infections.
Owner:VAXGENE
Immunochromatographic milk PEDV (porcine epidemic diarrhea virus) test strip and preparation method and application thereof
InactiveCN108761076AHigh detection sensitivityStrong specificityBiological material analysisFluorescence/phosphorescenceHuman bodyEpidemic diarrhea
The invention discloses an immunochromatographic milk PEDV (porcine epidemic diarrhea virus) test strip and a preparation method and application thereof. The immunochromatographic milk PEDV test stripcomprises a sample pad and a chromatographic strip which are overlapped onto a supporting material, wherein a detection line and a quality control line are arranged on the chromatographic strip; inactivated whole PEDV viruses are fixed to the detection line; a sheep anti-rabbit IgG antibody is fixed to the quality control line; a quantum dot-labeled S1 recombinant protein probe and a quantum dot-labeled rabbit IgG probe are sprayed on the sample pad. The immunochromatographic test strip fully combines the characteristics of the high sensitivity of fluorescent quantum dots and the antigen-antibody specificity, and can detect presence of an IgA antibody in a body fluid of an infected or immunized animal or human body or not, such as detection of the IgA antibody in milk of a sow infected orimmunized by the PEDV. The immunochromatographic test strip is high in detection sensitivity, good in specificity and short in cycle, can meet on-site detection needs, and is suitable for molecular biological detection of the porcine epidemic diarrhea viruses and large-scale epidemiological investigation.
Owner:SHENZHEN AUDAQUE DATA TECH +2
Respiratory syncytial virus IgA antibody detection test strip and detection method thereof
InactiveCN106124767AAvoid discomfortImprove work efficiencyBiological testingImmunoassaysRESPIRATORY SYNCYTIAL VIRUS - ELISABlood sampling
A respiratory syncytial virus IgA antibody detection test strip includes a sample pad, a labeling pad, a nitrocellulose membrane, water absorbent paper and a backing plate and is characterized in that the cellulose membrane comprises a detection line sprayed with a respiratory syncytial virus fusion protein antigen polypeptide fragment and a control line of a human IgA antibody. A saliva specimen is collected by aseptic degreasing cotton, then the saliva specimen is extruded by an injector, the IgA antibody in the saliva is detected by the respiratory syncytial virus IgA antibody rapid chromatography test strip, and the purpose of rapid diagnosis of patient infected respiratory syncytial virus is realized. The method is simple to operate, has rapid results, and is suitable for rapid diagnosis of respiratory syncytial virus infection in medical institutions at all levels. The collected specimen has no invasion, discomfort brought to patients by traditional blood sampling and nasopharyngeal swab collection is avoided, the work efficiency of health care workers is improved, and the promotion rate of respiratory syncytial virus diagnosis in clinic is improved.
Owner:GUANGZHOU RHFAY BIOTECH CO LTD
Methods for Detecting Antibodies
Methods for detection of any antibody utilizing a standardized approach applicable to any antibody which provides highly specific assays specific for individual or multiple antibodies. The methods enable improved pharmacokinetic analysis during development and clinical use of antibody-based therapies as well as determination of diagnostic and / or prognostic factors.
Owner:RGT UNIV OF CALIFORNIA
Novel coronavirus IgA antibody magnetic particle chemiluminiscence method detection kit
PendingCN112098645AChemiluminescene/bioluminescenceImmunoassaysAntiendomysial antibodiesChemiluminescence
The invention provides a novel coronavirus IgA antibody magnetic particle chemiluminiscence method detection kit. The novel coronavirus IgA antibody magnetic particle method detection kit comprises amagnetic particle anti-FITC antibody, an FITC labeled novel coronavirus recombinant antigen and an alkaline phosphatase or horseradish peroxidase labeled human IgA monoclonal antibody. The kit provided by the invention can well supplement diagnosis of a nucleic acid negative new crown infection patient, also shows a good value for diagnosis of an asymptomatic infection patient, can realize early diagnosis and early treatment, reduces a window period as much as possible, and has an important research value for early diagnosis of suspected cases.
Owner:BIOSCIENCE (CHONGQING) DIAGNOSTIC TECH CO LTD
Pertussis diagnostic kit and preparation method thereof
The invention discloses a pertussis diagnostic kit and a preparation method thereof. The kit comprises a detection probe of a nanogold marker mice anti-human IgA antibody, pertussis bacillus capsular antigen and a dot immunogold concentrated filtration device, wherein the centralized filtration device consists of a plastic capsule, a nitrocellulose membrane added with the detection probe of the nanogold marker mice anti-human IgA antibody and a water absorption layer; water absorption pads are arranged at a groove of the capsule; the nitrocellulose membrane is arranged between the water absorption pads and a cover of the capsule; and the water absorption layer is formed by three to five water absorption pads via stacking. According to the kit disclosed by the invention, the immunogold dots generated by the antigen-antibody reaction are concentrated and amplified again through the concentrated filtration device on the basis of a nanogold amplification technology, so that the detection sensitivity is improved, the limit of detection is enlarged and effective detection can be carried out on the incubation period, the convulsion period and the recovery period.
Owner:张明
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com