Gene expression vaccine
a gene expression and vaccine technology, applied in the field of gene expression vaccines, can solve the problems of not only failing to develop a vaccine using a formalin-inactivated rsv vaccine, but also exacerbated the disease, and achieve the effect of significant attenuation of pulmonary inflammation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
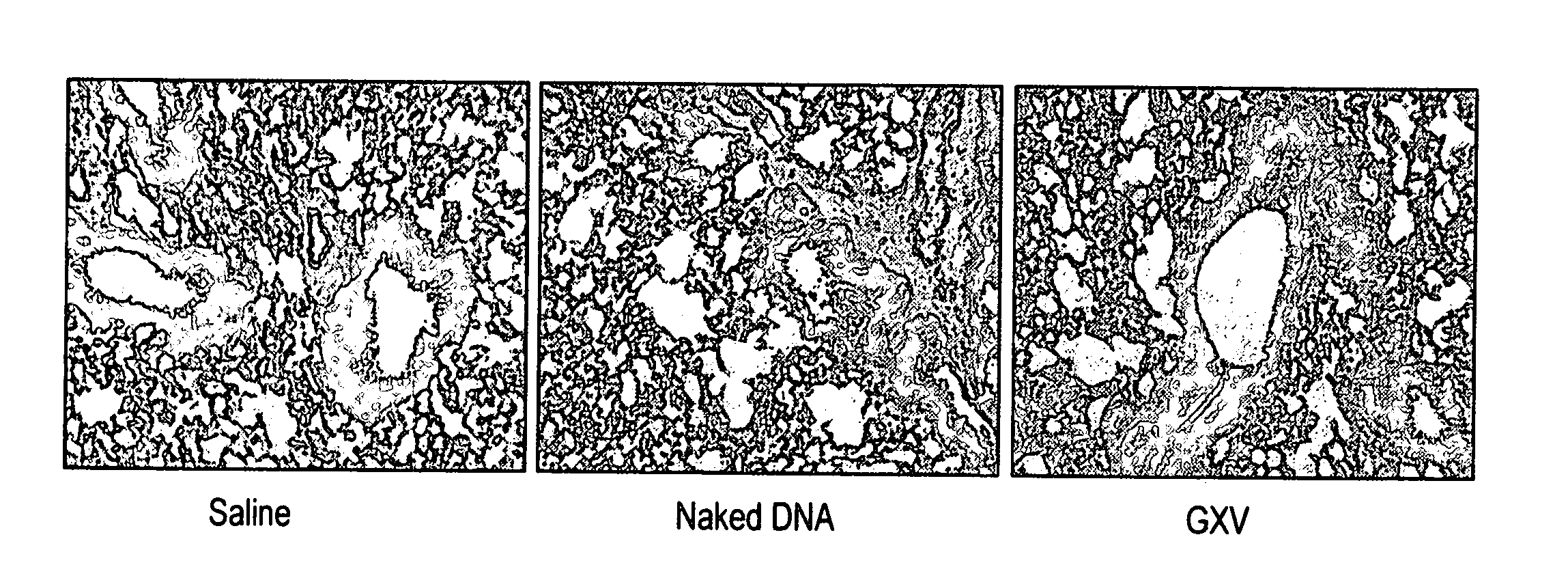
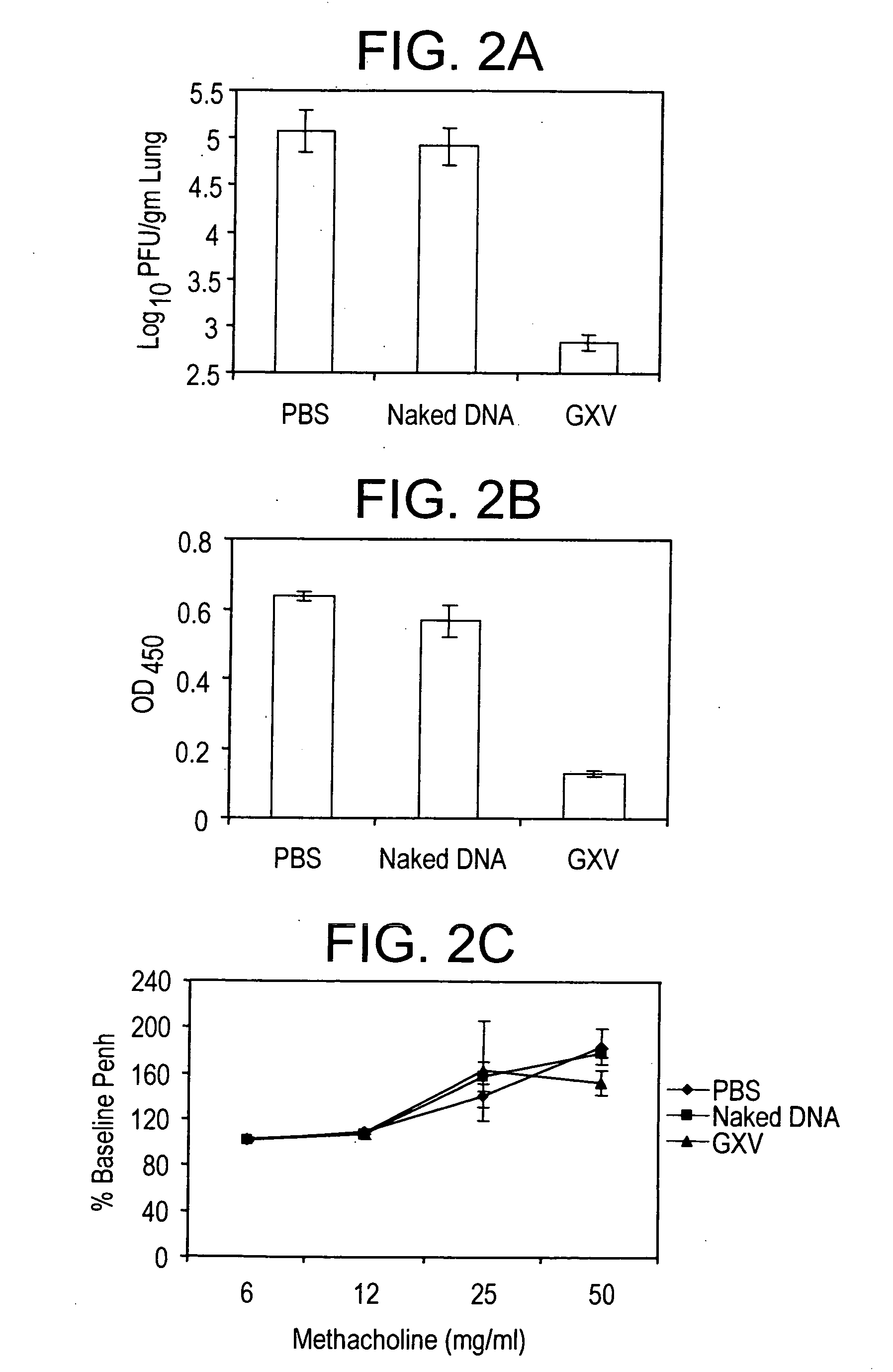
[0028] A RSV gene expression library is constructed in pVAX plasmid and the library is coacervated with chitosan to formulate nanospheres, referred to herein as RGCN vaccine.
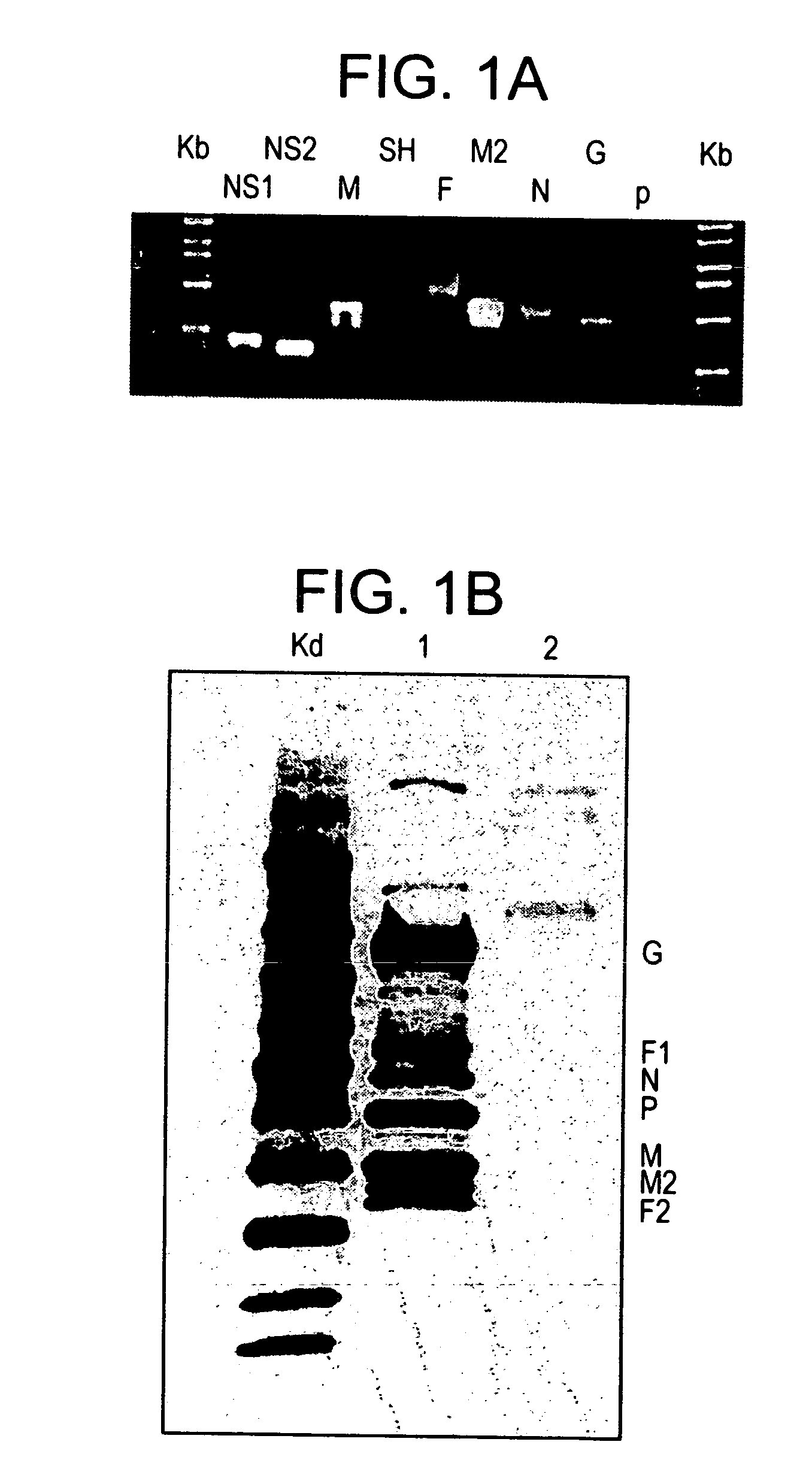
[0029] The present invention provides a gene expression vaccine (GXV) comprising a cocktail of plasmid DNAs encoding corresponding RSV antigens. The cocktail comprises combinations of the F, G, M, M2, SH, NS1, NS2, N, and P RSV antigens. The cocktail may contain a combination comprising the F, G and at least one of the M, M2, SH, NS1, NS2, N, and P RSV antigens. Alternatively, the cocktail may contain a combination comprising the M2 and at least one of the F, G, M, SH, NS1, NS2, N, and P RSV antigens. Also, alternatively, the cocktail may contain a combination comprising the F, G, M2 and at least one of the M, SH, NS1, NS2, N, and P RSV antigens. The GXV is formulated in the form of nanospheres with chitosan, a biodegradable, human-friendly, and cationic polymer that increases mucosal absorption of the vaccine ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| volume | aaaaa | aaaaa |
| pH | aaaaa | aaaaa |
| pH | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com