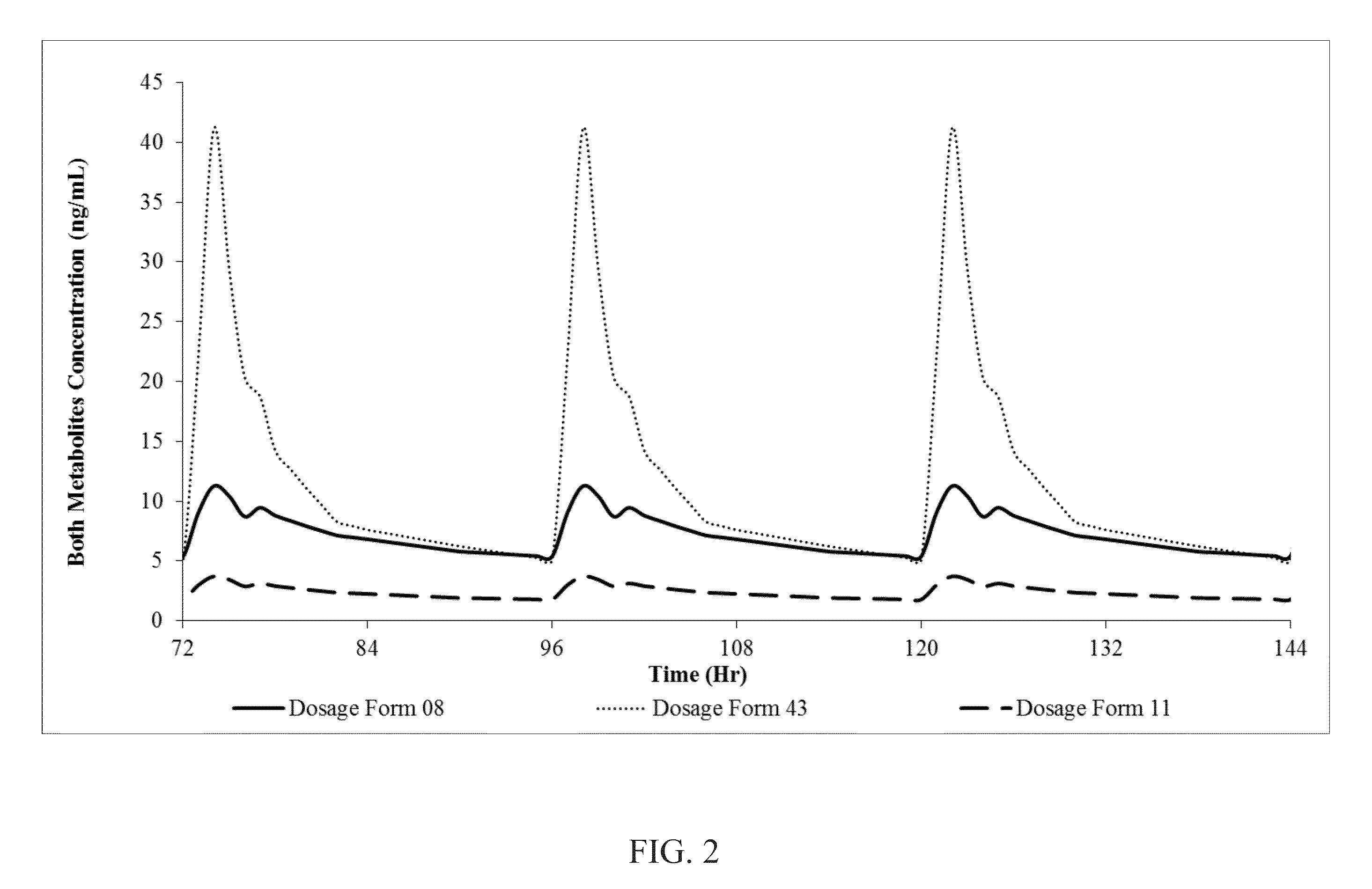
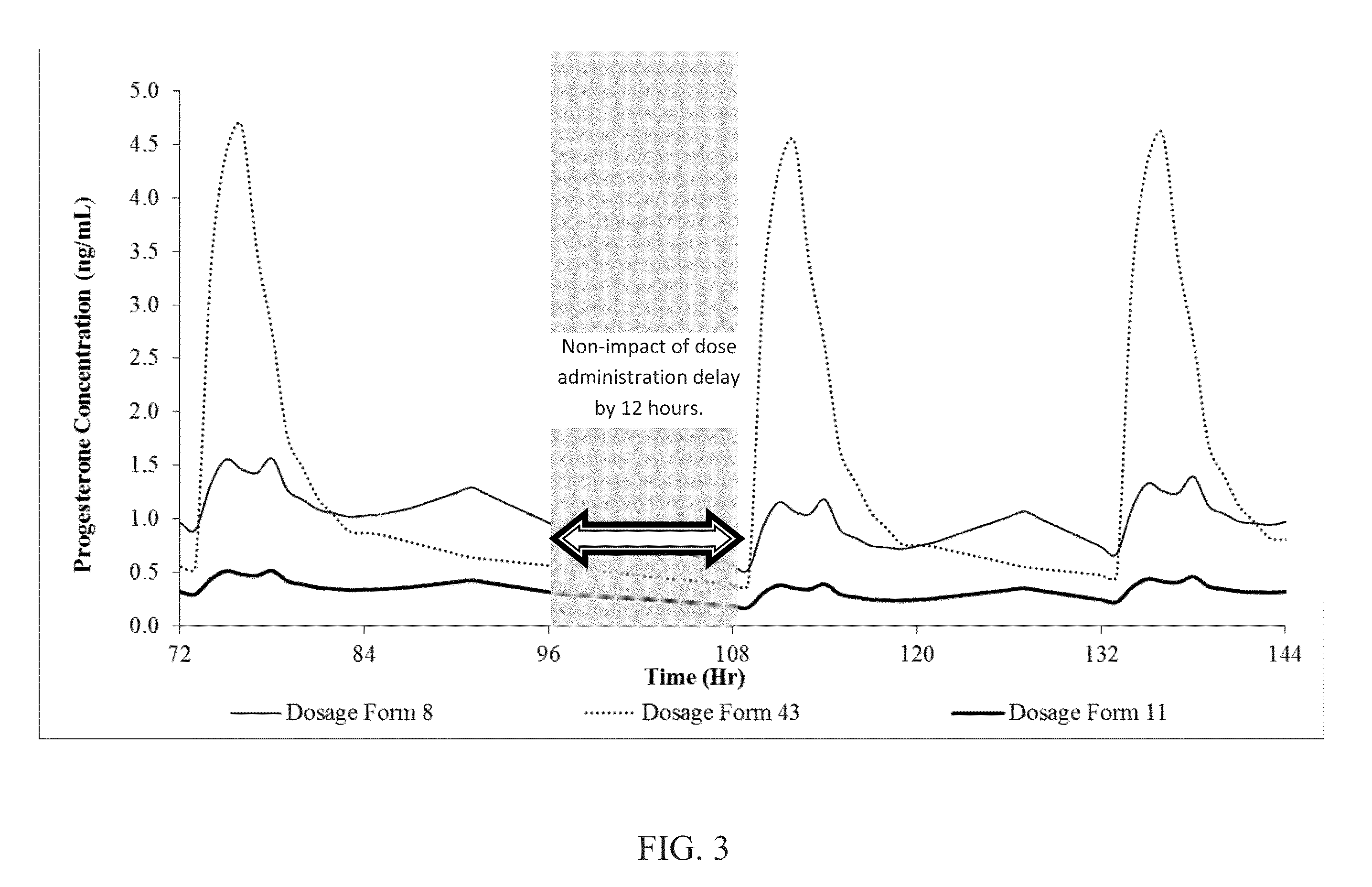
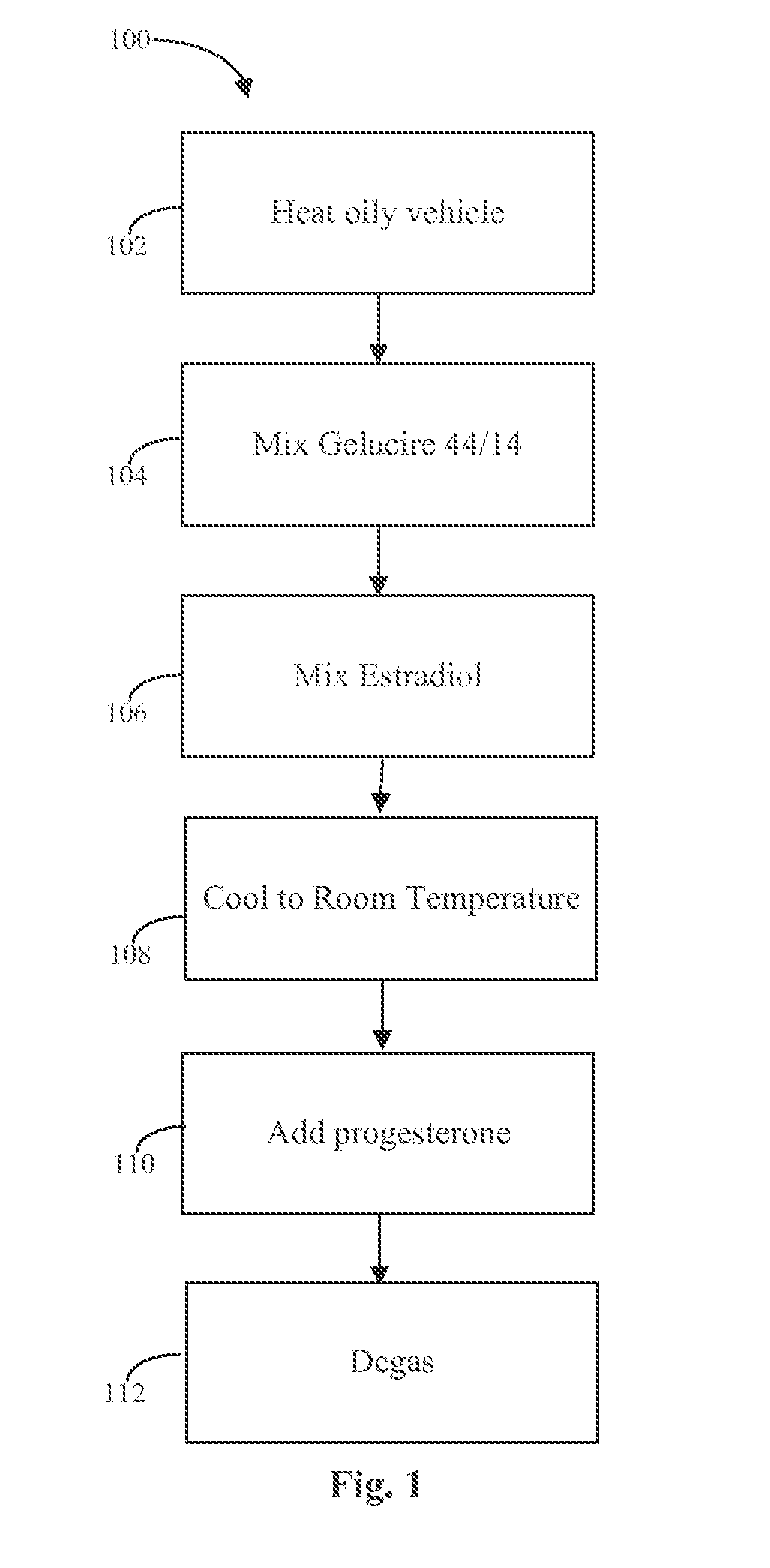
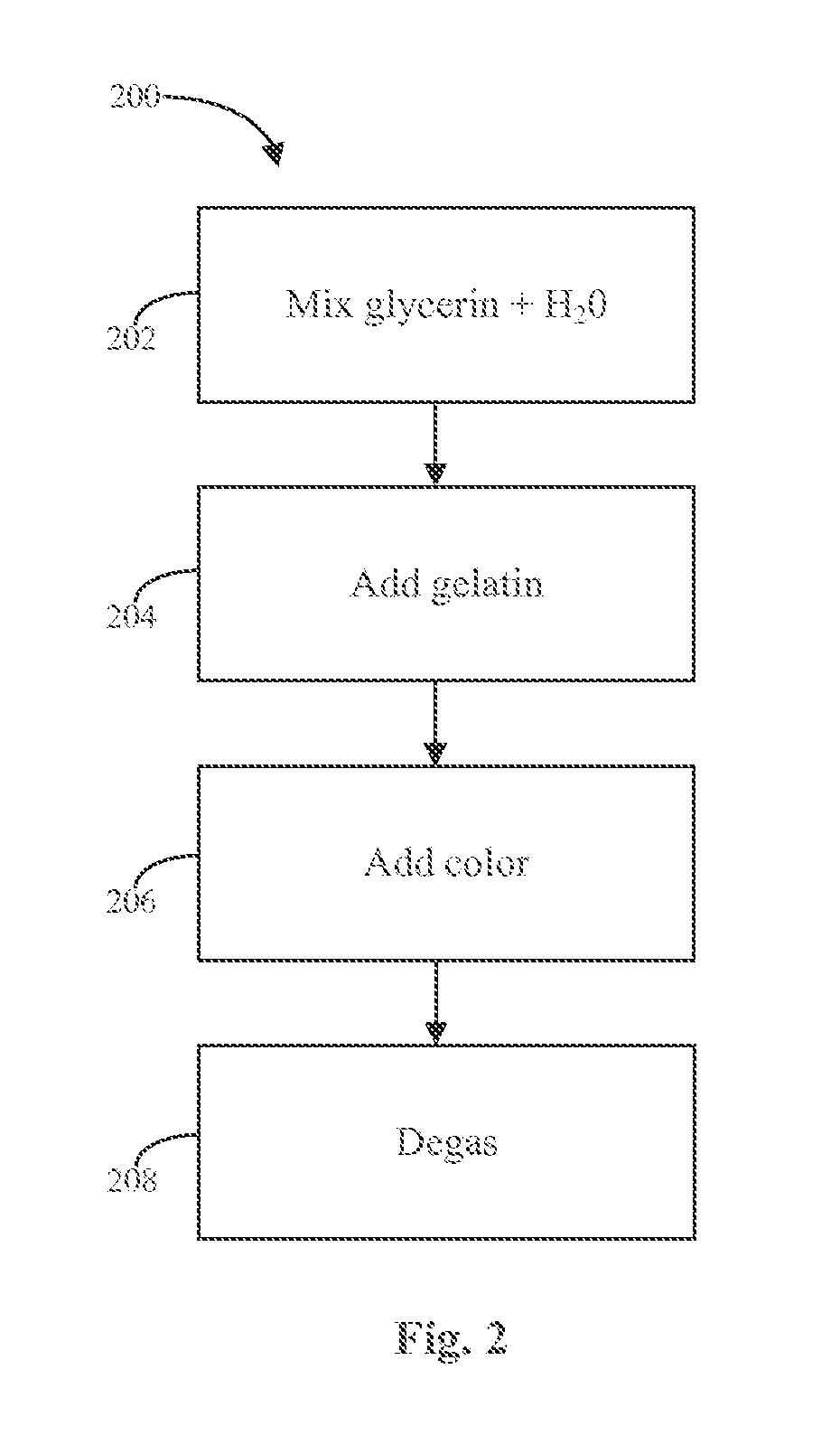
Patents
Literature
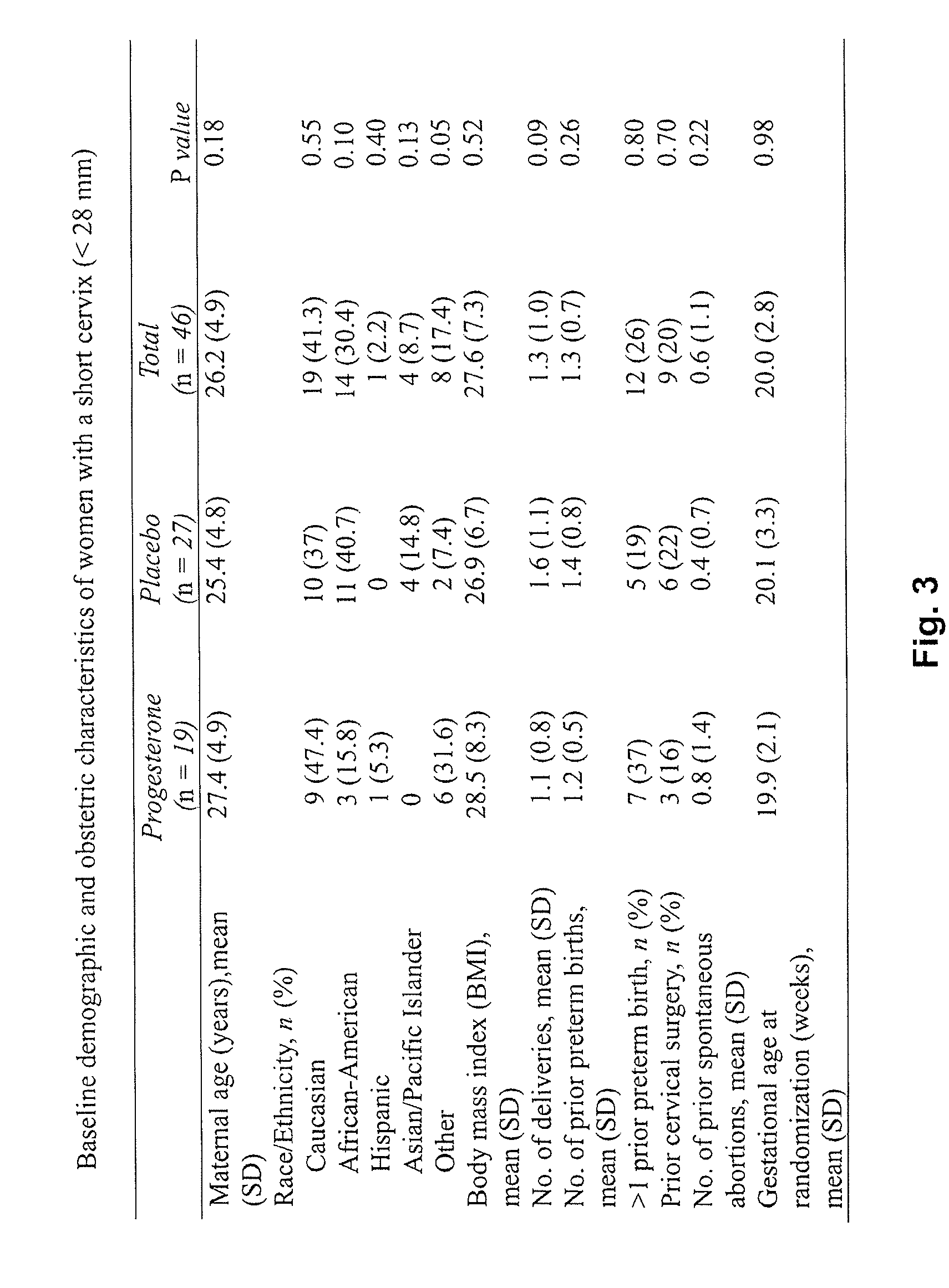
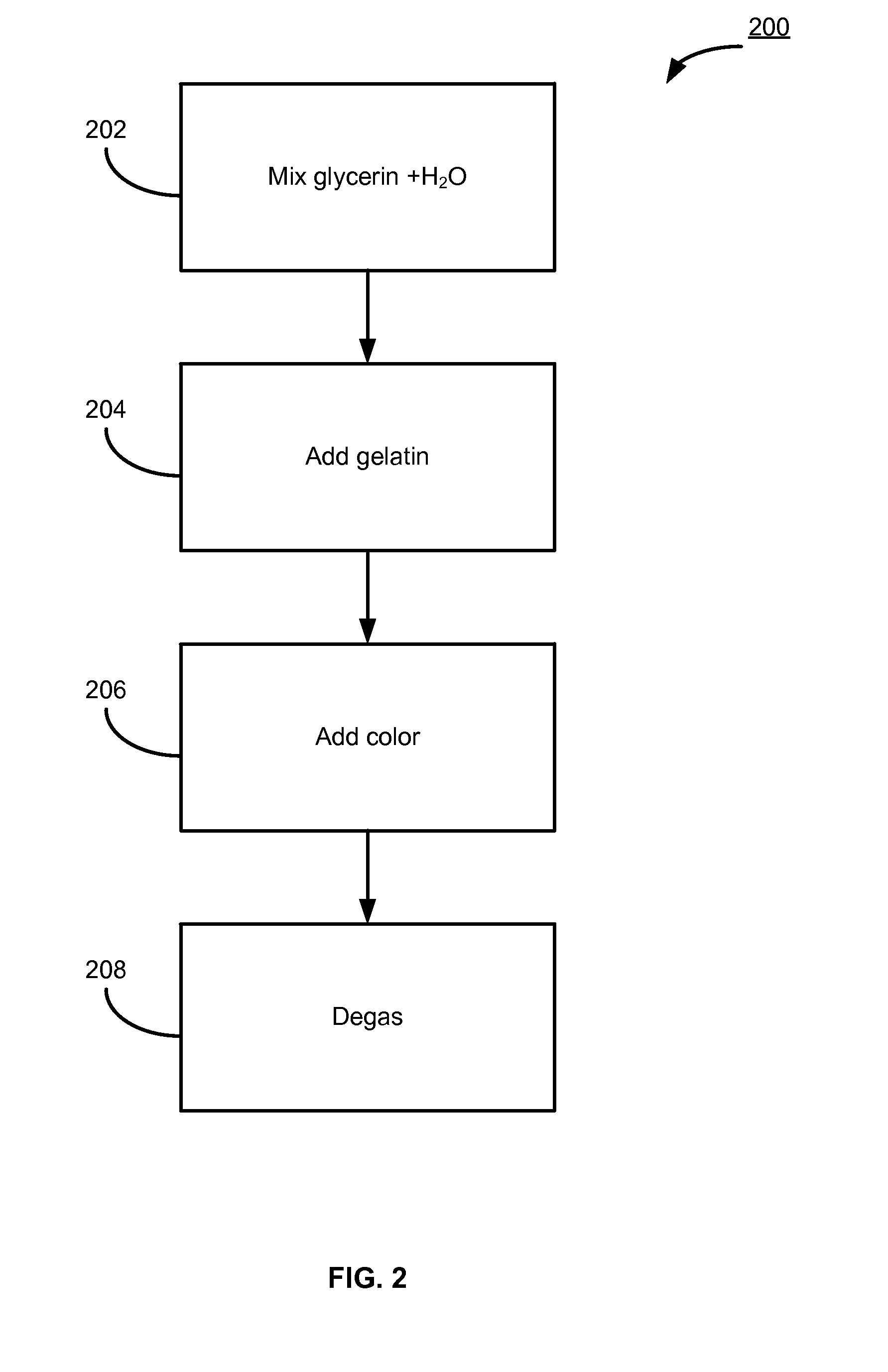
610 results about "Progesterones" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
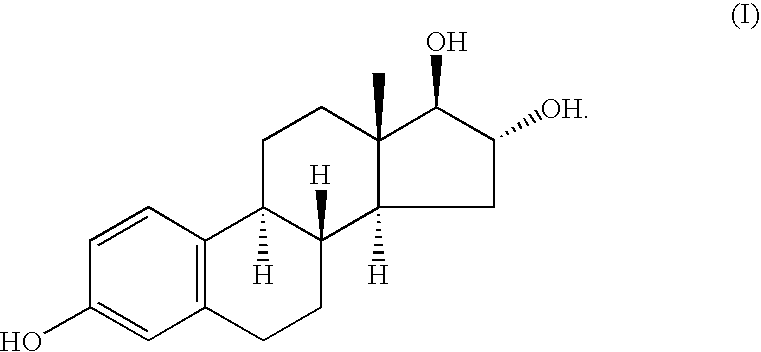
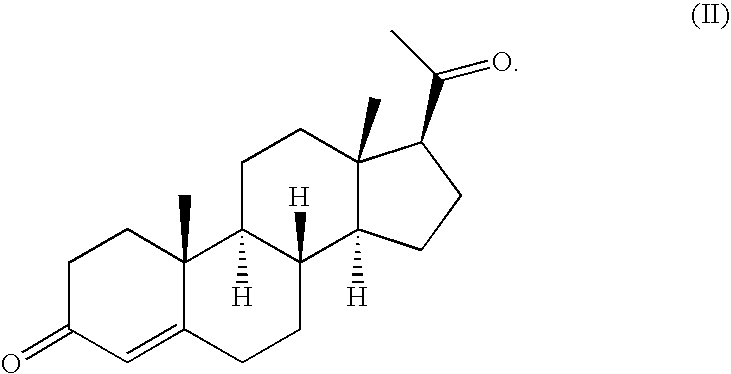
A type of female sex hormone
Molecular dispersions of drospirenone
InactiveUS20050220825A1Improve bioavailabilityGood chemical stabilityPowder deliveryPill deliveryParticulatesDrospirenone
Described are pharmaceutical compositions comprising at least one steroidal drug such as a progestin (e.g. drospirenone, progesterone, eplerenone, etonogestrel) and / or an estrogen (estradiol and esters thereof) in molecularly dispersed form. The composition comprises a steroidal drug, preferably drospirenone, which is present in the composition in a non-particulate form. Preferably, the drug is present in a dissolved state in the excipient. The molecularly dispersed drug will be released very fast as dissolution takes place instantly when the dosage unit has disintegrated. Also described are methods for preparing the pharmaceutical compositions and methods of using the compositions.
Owner:BAYER SCHERING PHARMA AG
Methods of neuroprotection using neuroprotective steroids and a vitamin d
InactiveUS20110306579A1Inhibiting neurodegenerationPromote physical recoveryBiocideOrganic active ingredientsNervous systemProgesterones
Described herein are compositions and methods for treating or preventing nervous system injury. In particular, the methods and compositions relate to the use of at least one neuroprotective steroid, such as progesterone, and vitamin D.
Owner:EMORY UNIVERSITY
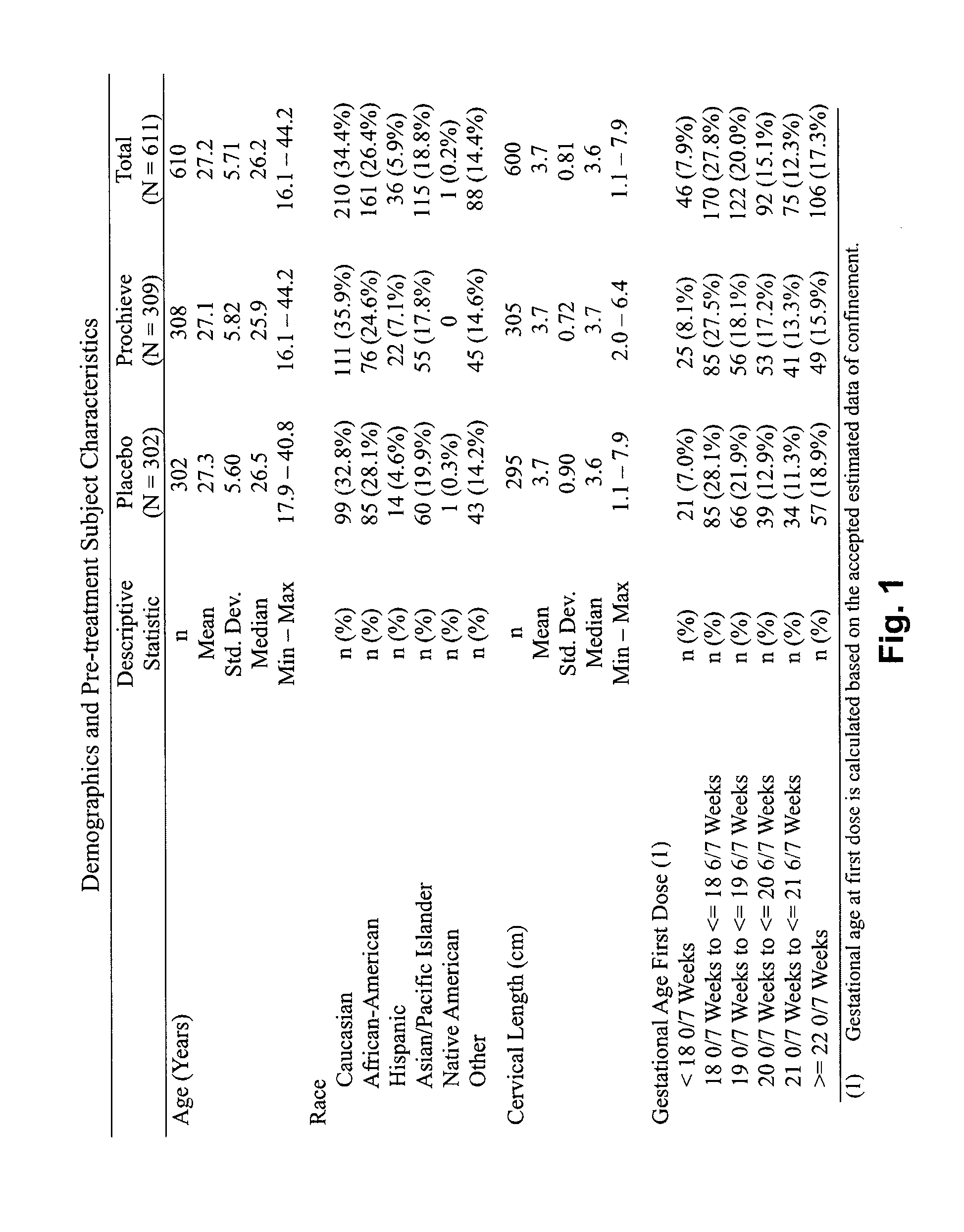
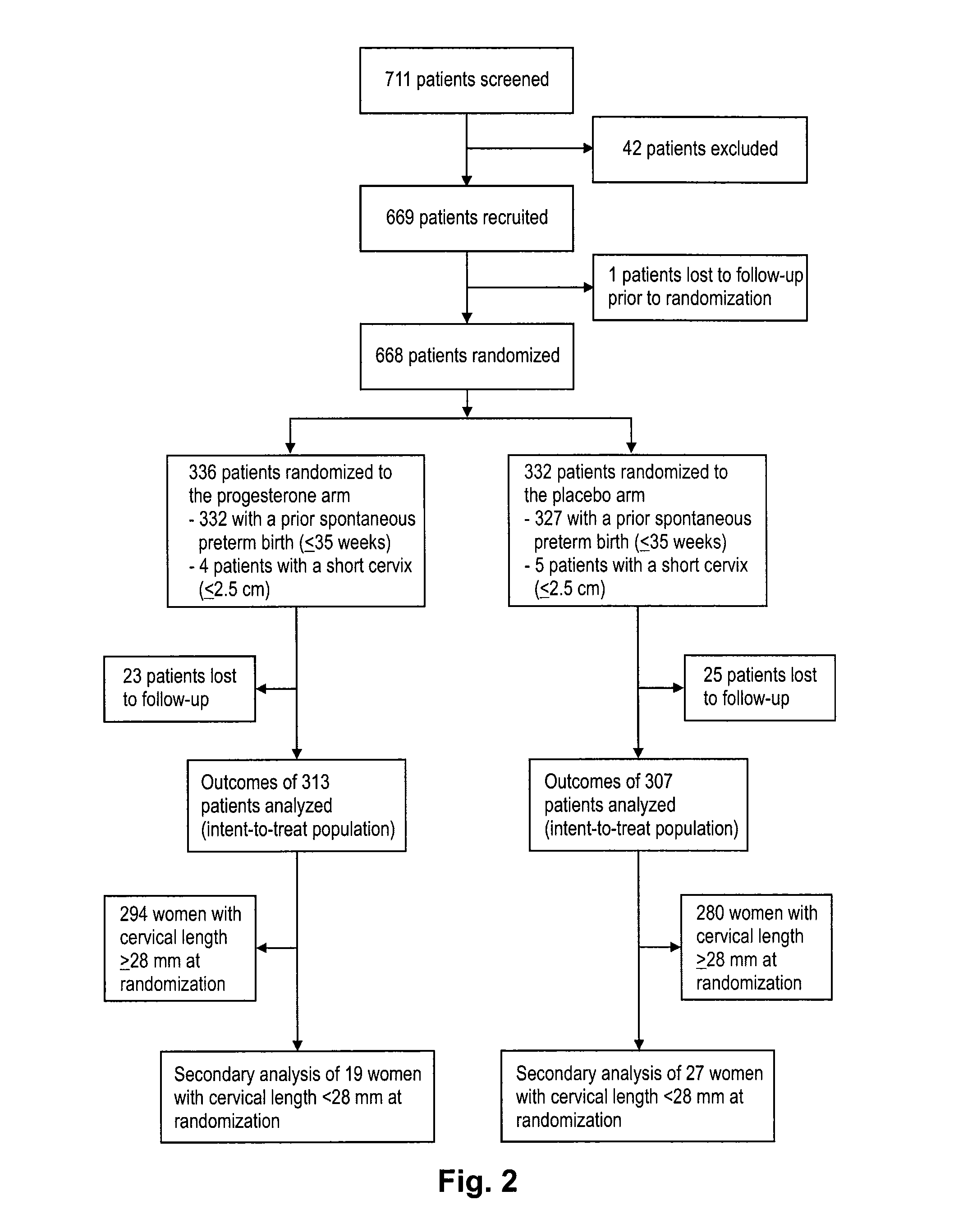
Progesterone for the treatment or prevention of spontaneous preterm birth
ActiveUS20080188829A1Minimize and delay shorteningMinimize and delay and effacingOrganic active ingredientsMedical devicesNeonatal morbidityPreterm Births
A method for treating or preventing spontaneous preterm birth in pregnant women and improving neonatal morbidity and mortality. The method includes administering to a pregnant woman in need thereof an effective amount of progesterone sufficient to prolong gestation by minimizing the shortening or effacing of her cervix. Treatment and prophylaxis with progesterone in pregnant women having symptoms of short cervix has been clinically shown to increase neonatal health.
Owner:ALLERGAN SALES LLC +3
Implantation rates after in vitro fertilization, treatment of infertility and early pregnancy loss with a nitric oxide donor alone or in combination with progesterone, and a method for contraception with nitric oxide inhibitors
InactiveUS6040340AImprove implantation rateSymptoms improvedHeavy metal active ingredientsBiocideEarly Pregnancy LossPhysiology
A method is provided for the improvement of implantation rates and / or pregnancy rates in a female mammal, comprising administering to a female mammal in whom pregnancy is desired an effective amount of (a) a nitric oxide synthase substrate, a nitric oxide donor, or both, optionally in combination with (b) a progestin, and, (c) optionally, in further combination with an estrogen. A method is also provided for fertility control for a female mammal, comprising administering to a female mammal in whom pregnancy is not desired and at risk for becoming pregnant an effective amount of nitric oxide synthase inhibitor in combination with an antiprogestin. Pharmaceutical compositions are also provided.
Owner:BOARD OF RGT THE UNIV OF TEXAS SYST
Oral dosage forms comprising progesterone and methods of making and using the same
InactiveUS20060275360A1Stable absorption profileOrganic active ingredientsCapsule deliveryProgesteronesEdible oil
The present invention relates to an oral pharmaceutical dosage form comprising micronized progesterone, an edible oil, a disintegrant, and a hydrophilic excipient. Particularly, the invention relates to a pharmaceutical dosage form wherein the dosage form is in a powder form and is contained in a pharmaceutically acceptable capsule. The present invention is also directed toward methods of making the dosage form, methods of using the dosage form, and kits comprising the dosage form.
Owner:TEVA WOMENS HEALTH
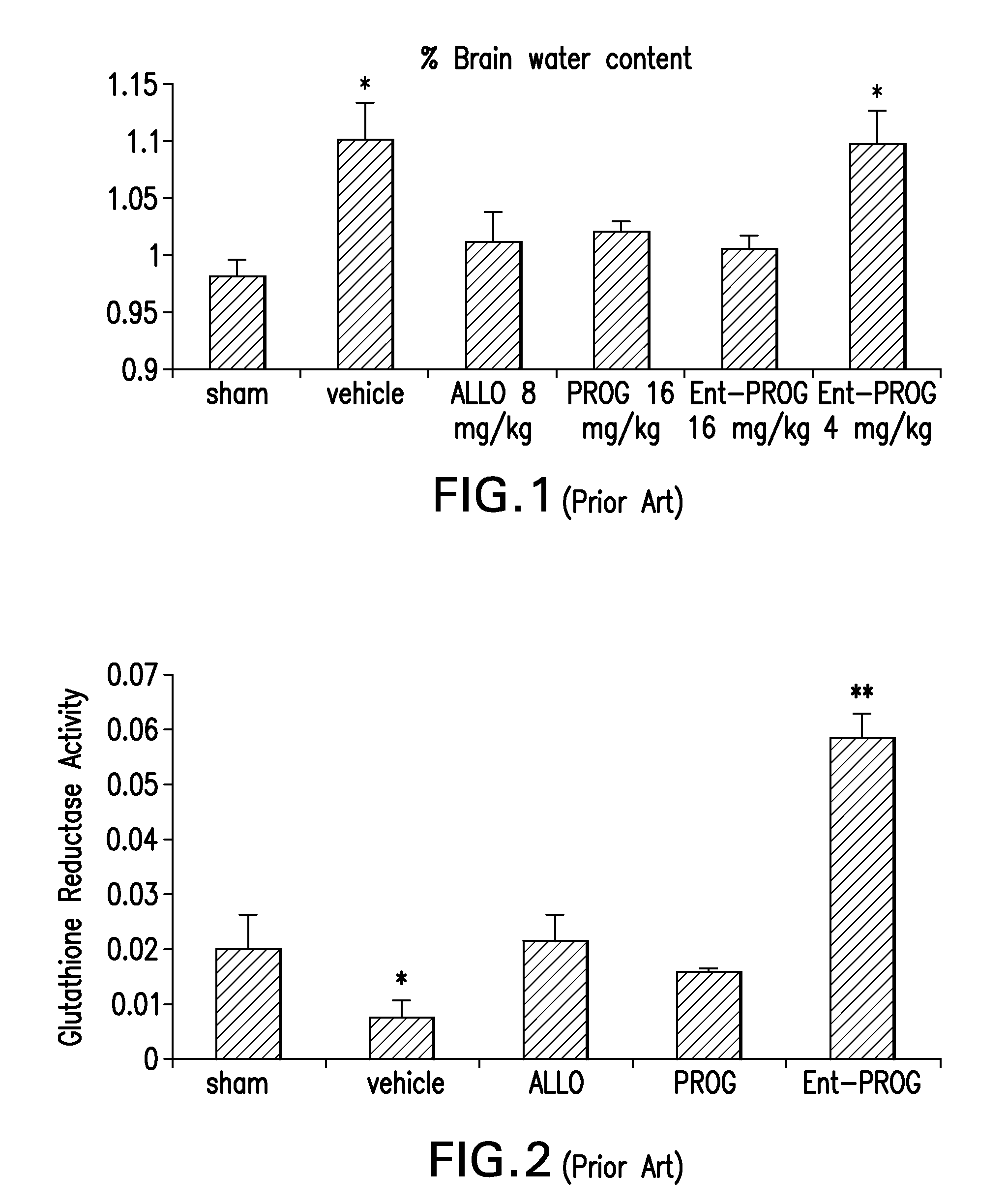
Neuroactive steroid compositions and methods of use therefor
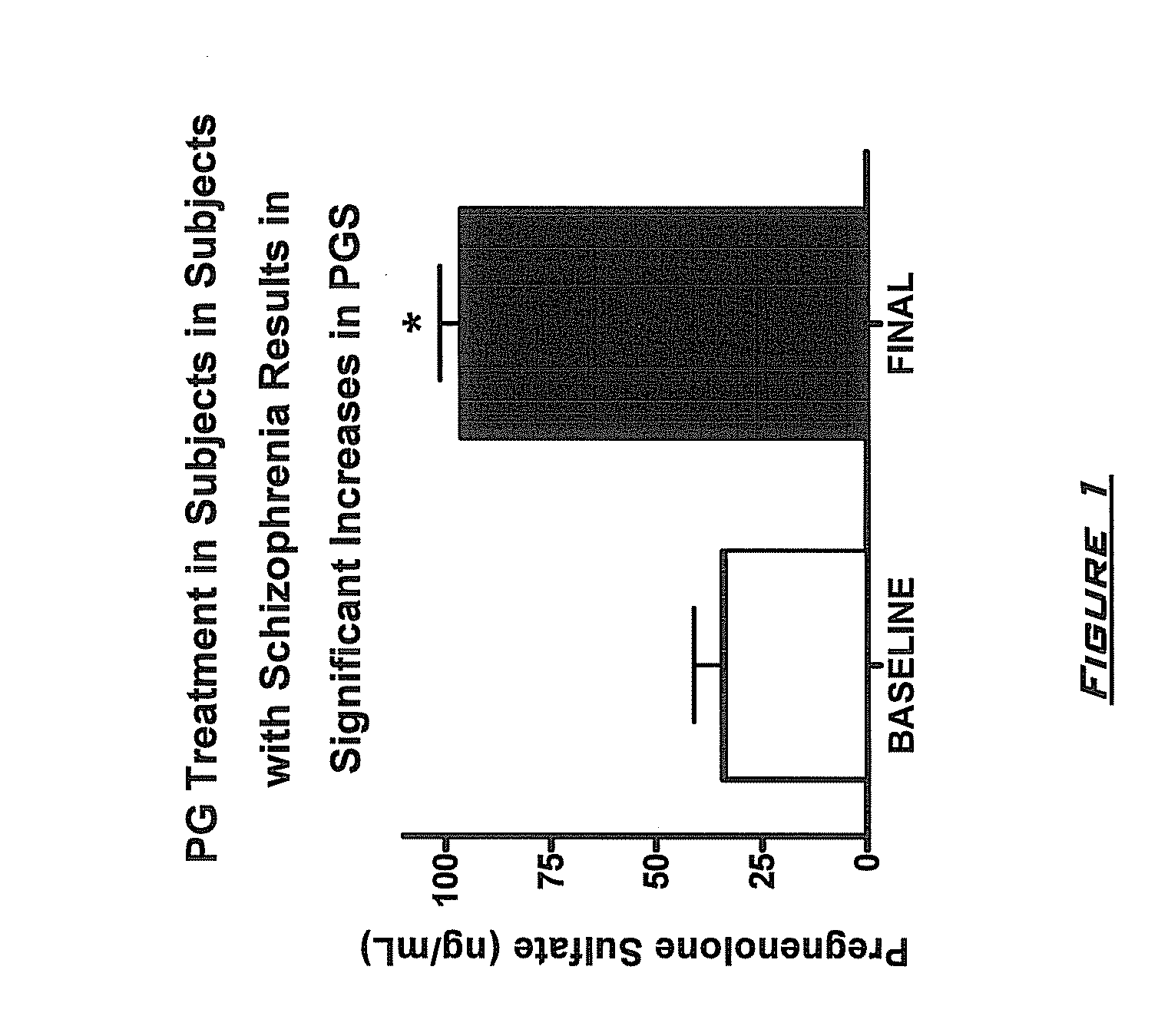
InactiveUS20090203658A1Improve cognitive functionPromote more developedOrganic active ingredientsBiocideMetaboliteSchizo-affective type
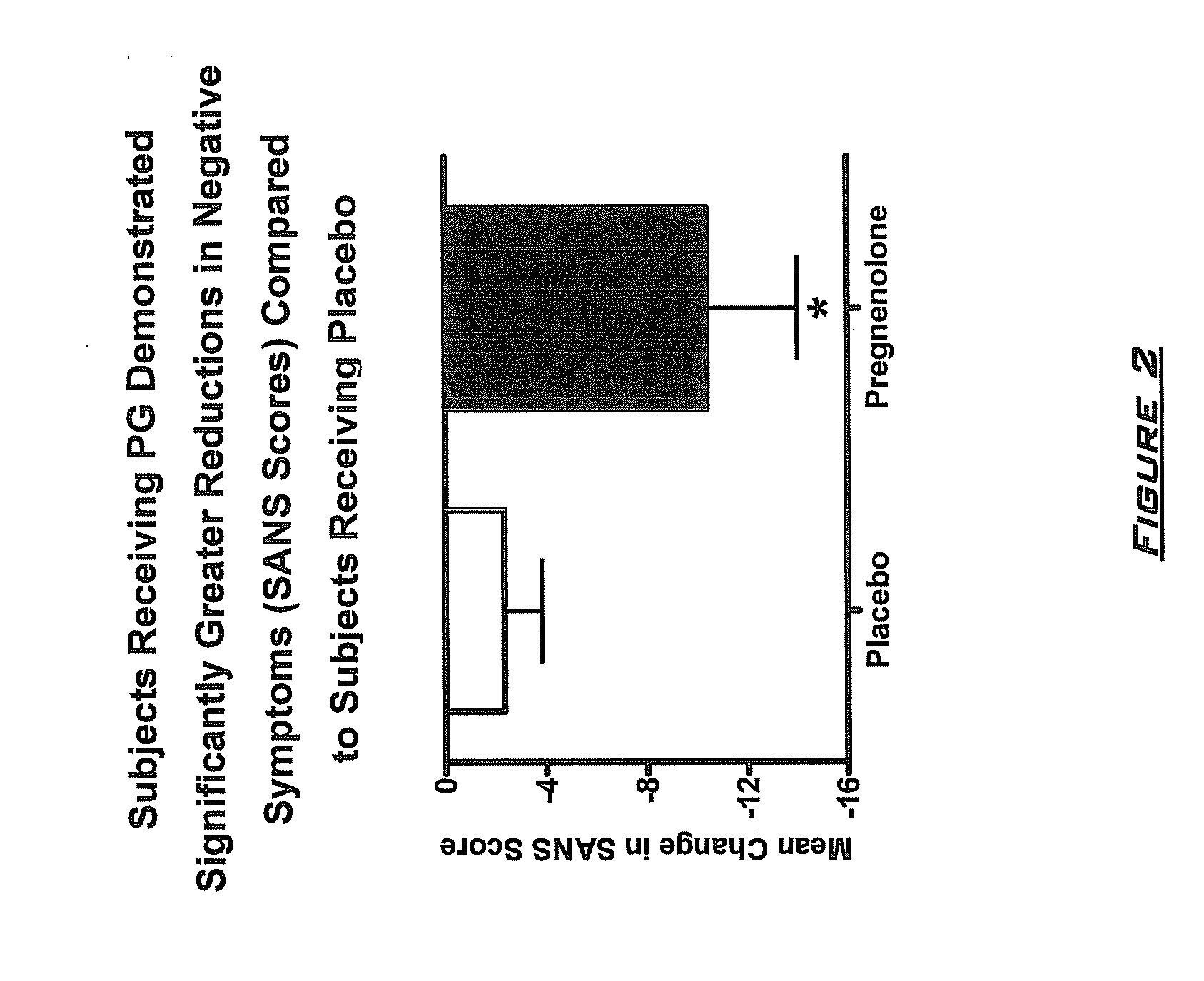
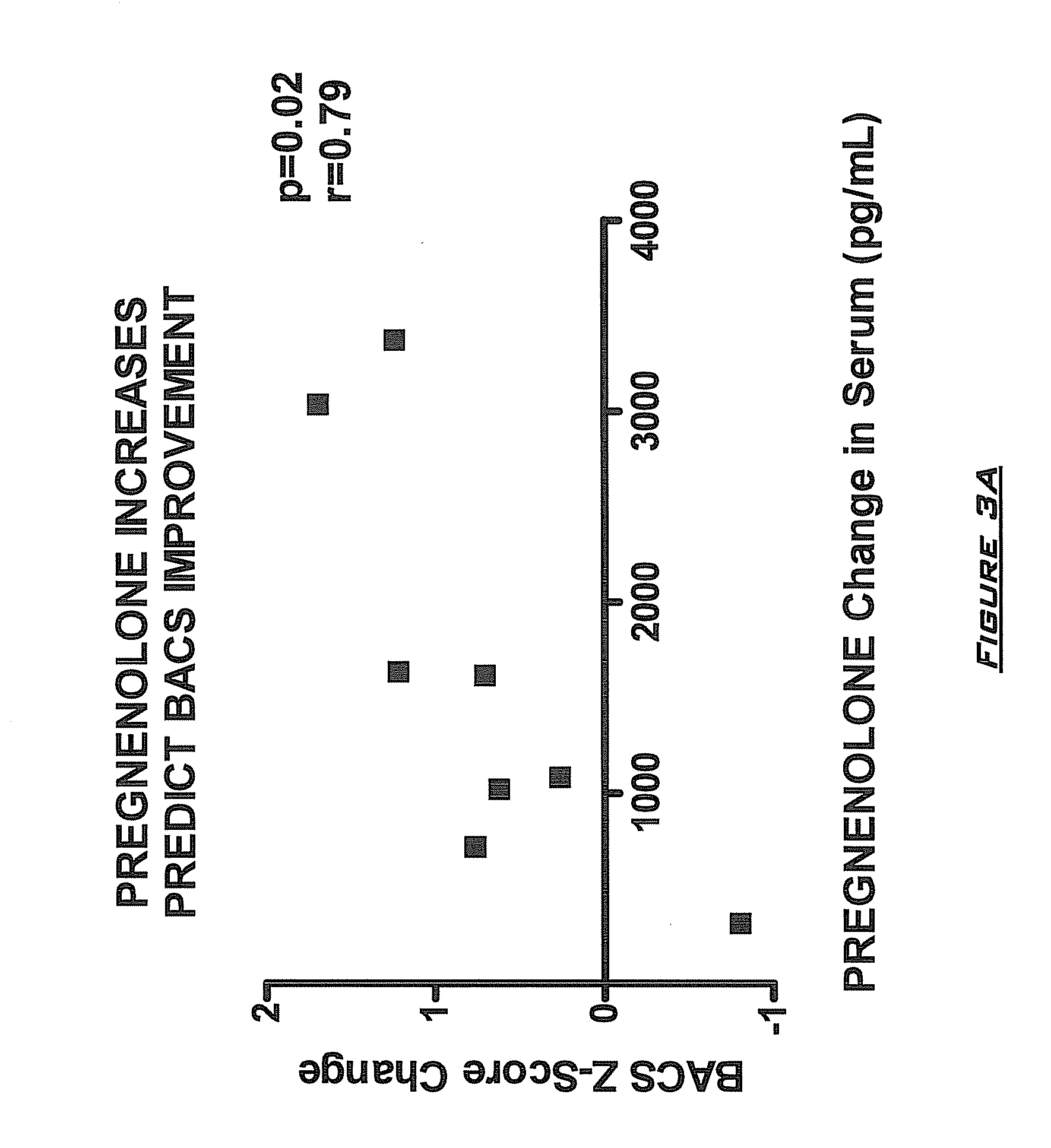
Provided are methods for ameliorating a symptom of a neuropsychiatric disorder in a subject. Also provided are methods for ameliorating at least one physical symptom or at least one psychological symptom resulting from tobacco cessation in a subject, methods for ameliorating a symptom of Alzheimer's disease or other cognitive disorder in a subject, methods for ameliorating a symptom of schizophrenia, schizoaffective disorder, or other psychotic disorder in a subject, methods for ameliorating a symptom of a depressive disorder in a subject, methods for ameliorating a symptom of bipolar disorder in a subject, methods for ameliorating a symptom of post-traumatic stress disorder or other anxiety disorder in a subject, methods for predicting a predisposition to suicide, suicidal ideation, suicidal behavior, or a combination thereof in a subject, methods for ameliorating a symptom of a pain disorder in a subject, methods for ameliorating a neurodegenerative disorder in a subject, methods for ameliorating a symptom of traumatic brain injury in a subject, methods for ameliorating a sleep disorder in a subject, and methods for improving cognitive functioning in a subject. In some embodiments, the methods include administering to a subject in need thereof an effective amount of a neuroactive steroid composition comprising pregnenolone (PG), allopregnanolone (ALLO), dehydroepiandrosterone (DHEA), progesterone (PROG), precursors thereof, metabolites thereof, pharmaceutically acceptable salts thereof, derivatives thereof, or combinations thereof.
Owner:DUKE UNIV
Pharmaceutical composition based on estrogen and progesterone
InactiveUS6165491AReduce riskEffective protectionOrganic active ingredientsFemale contraceptivesProgesteronesEstradiols
Owner:EFFIK BAT
Controlled release delivery system for nasal application of neurotransmitters
InactiveUS20090227550A1Improve bioavailabilityEffective serum levelOrganic active ingredientsBiocideNoseProgesterones
This invention relates to a galenical gel formulation for nasal administration of neurotransmitters / neuromodulators such as dopamine, serotonin or pregnenolone and progesterone. The special lipophilic or partly lipophilic system of the invention leads to high bioavailability of the active ingredient in plasma and brain caused by sustained serum levels and / or direct or partly direct transport from nose to the brain.
Owner:MATTERN PHARMA
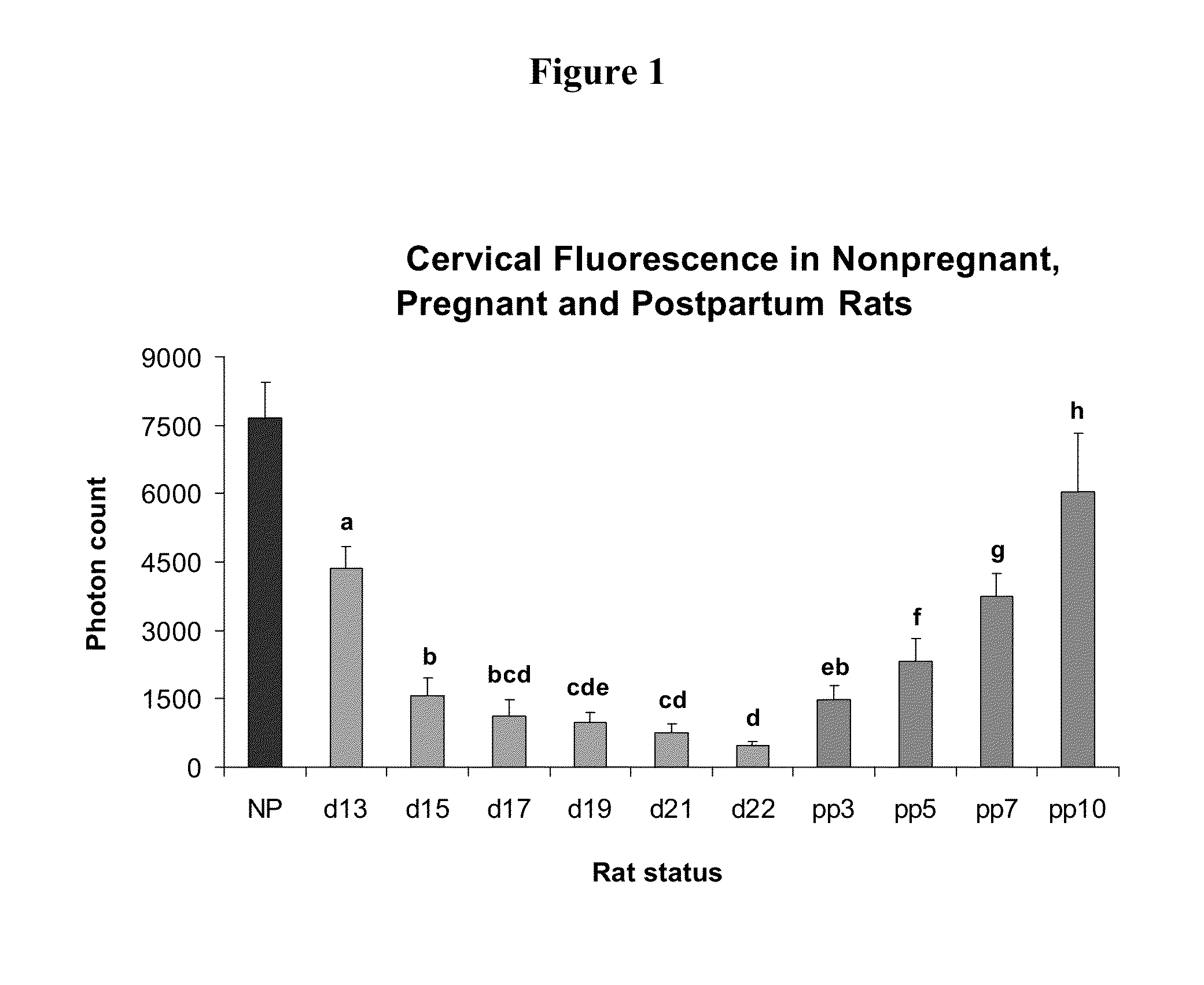
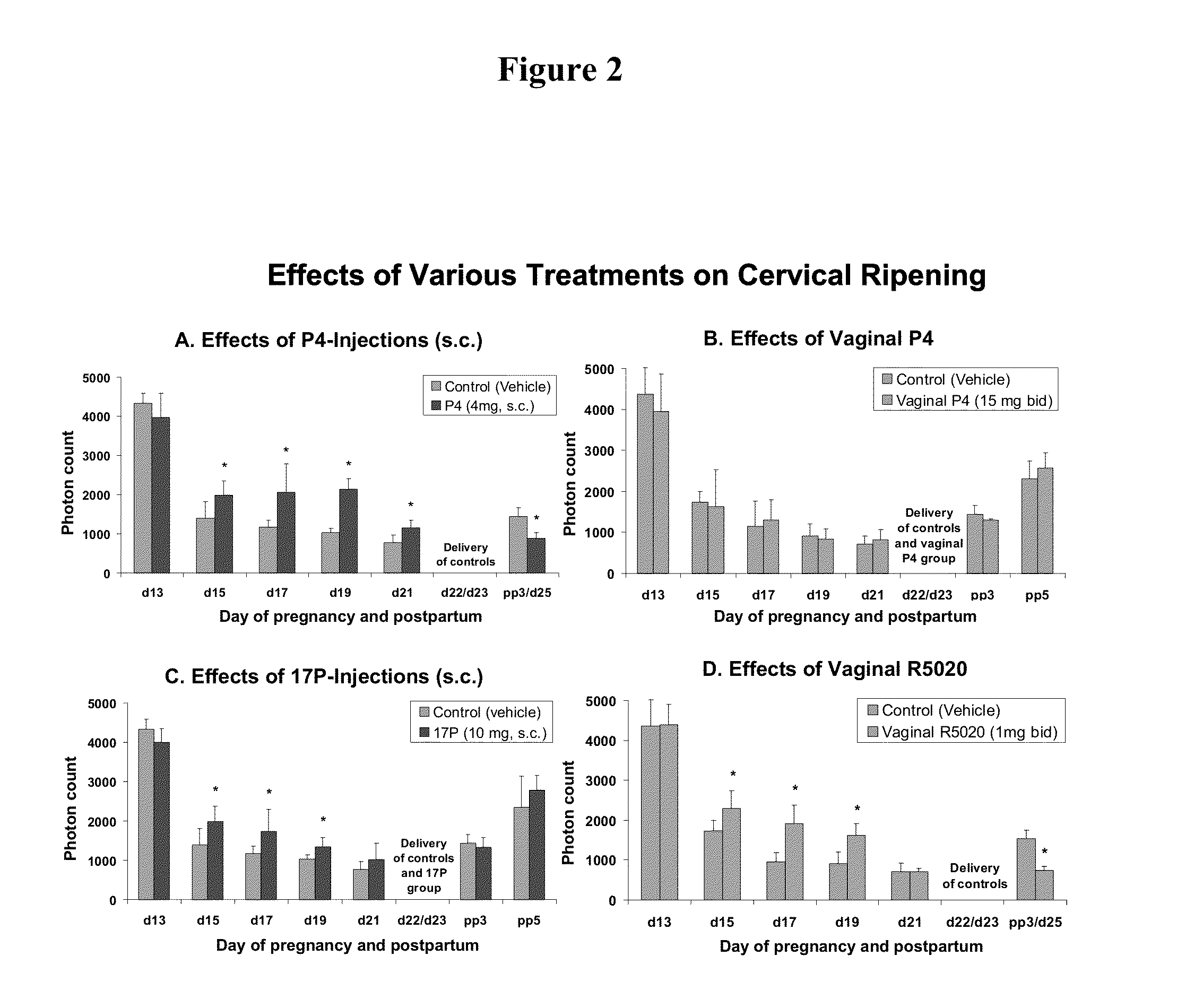
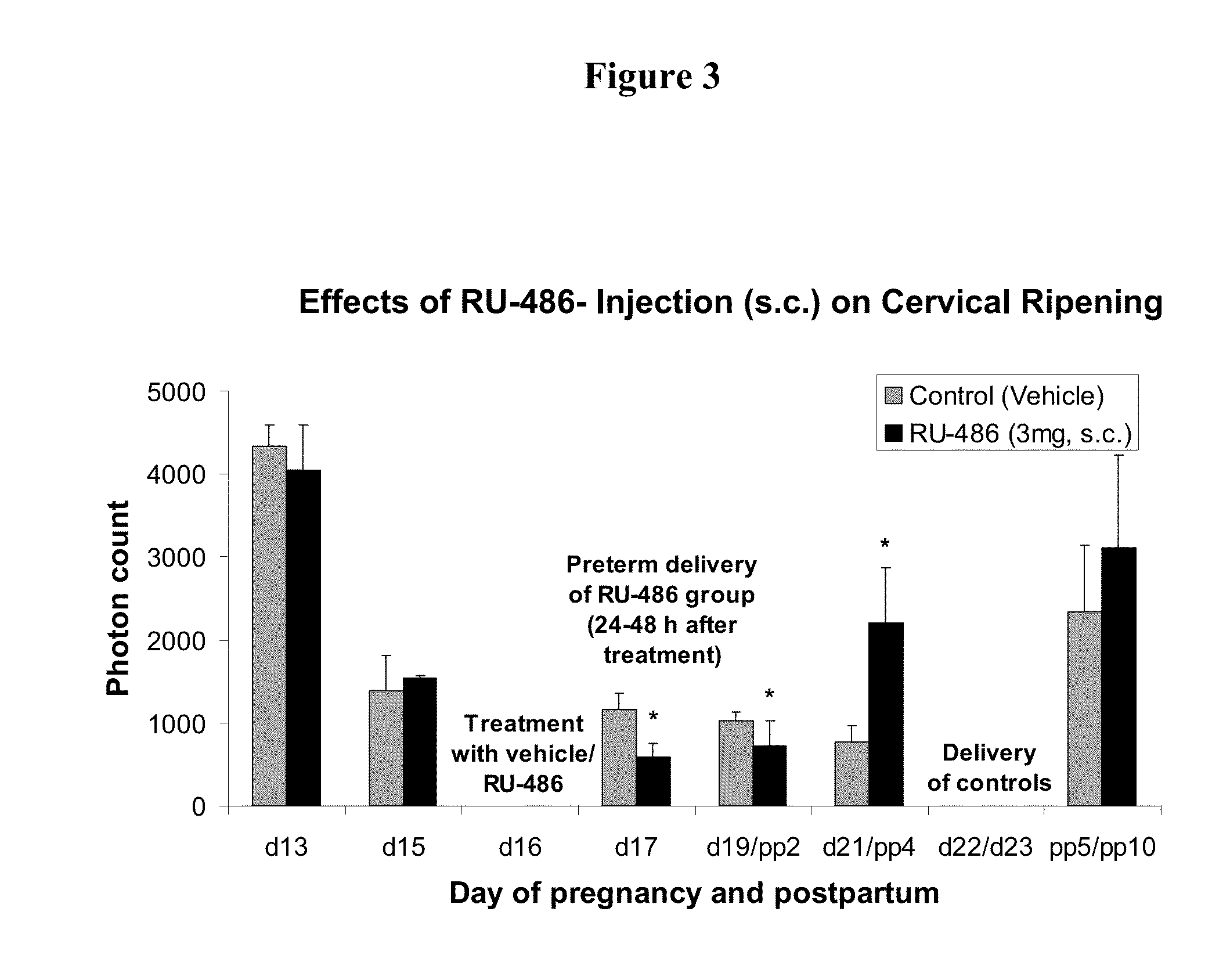
Methods for inhibiting preterm labor and uterine contractility disorders and preventing cervical ripening
InactiveUS20130023505A1Inhibit uterine contractilityInhibit cervical ripeningOrganic active ingredientsPharmaceutical delivery mechanismMyometrial contractilityGynecology
The invention relates to methods and pharmaceutical compositions for inhibiting or preventing preterm birth, inhibiting or delaying cervical ripening, inhibiting myometrial contractility and treating or inhibiting uterine contractility disorders. The methods comprise administering an effective amount of a composition comprising steroid hormones such as soluble progesterone.
Owner:DIGNITY HEALTH
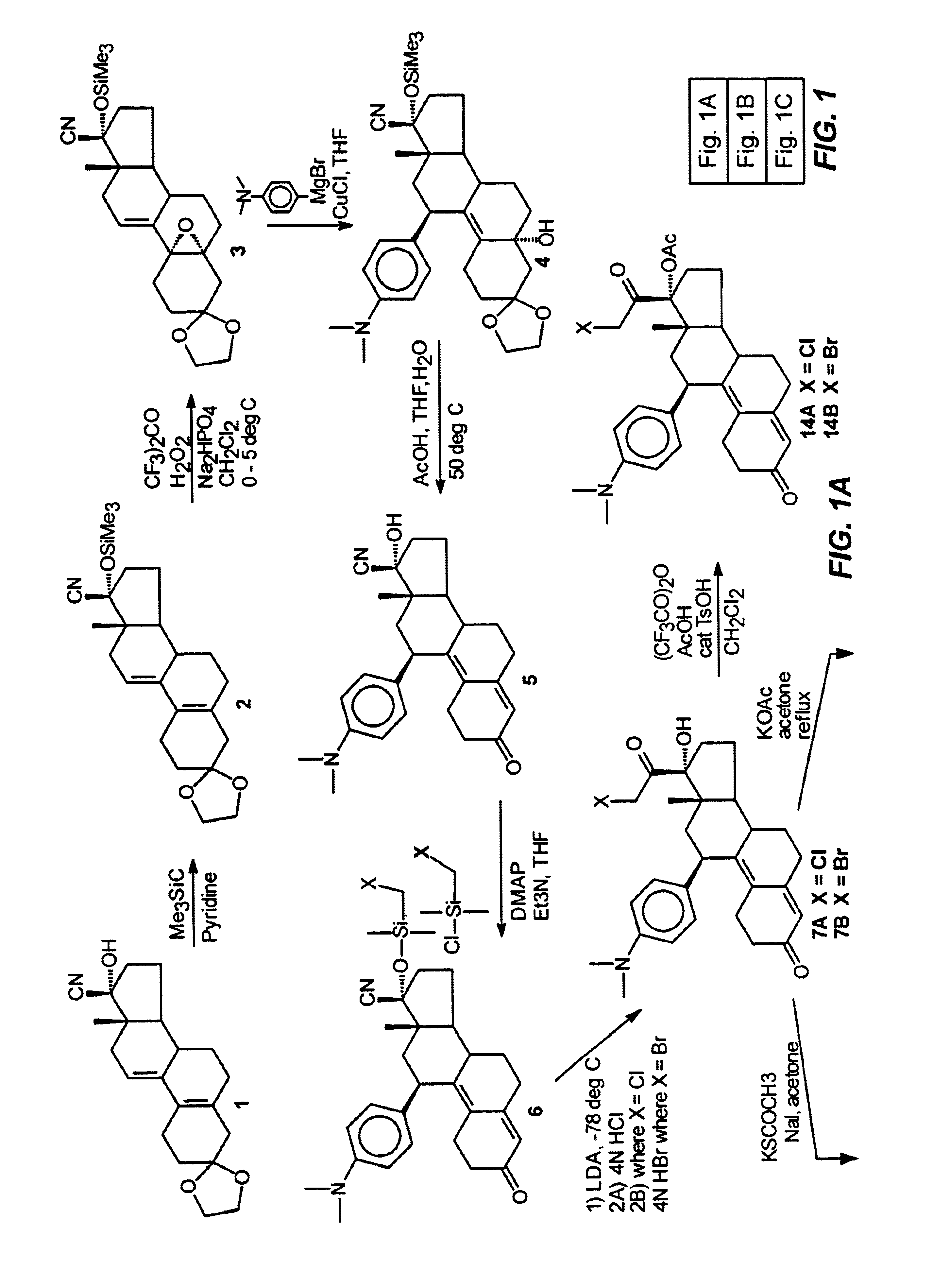
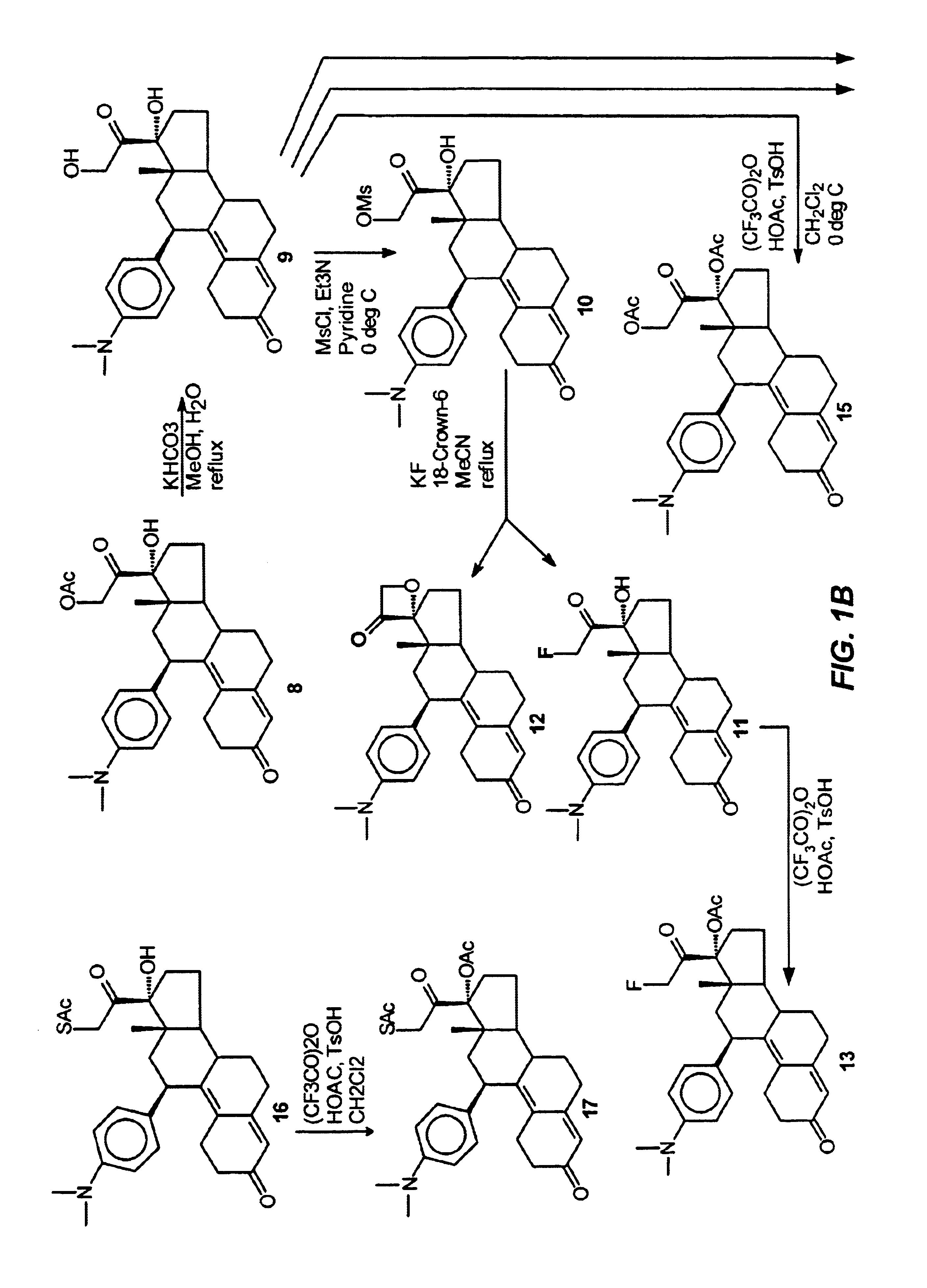
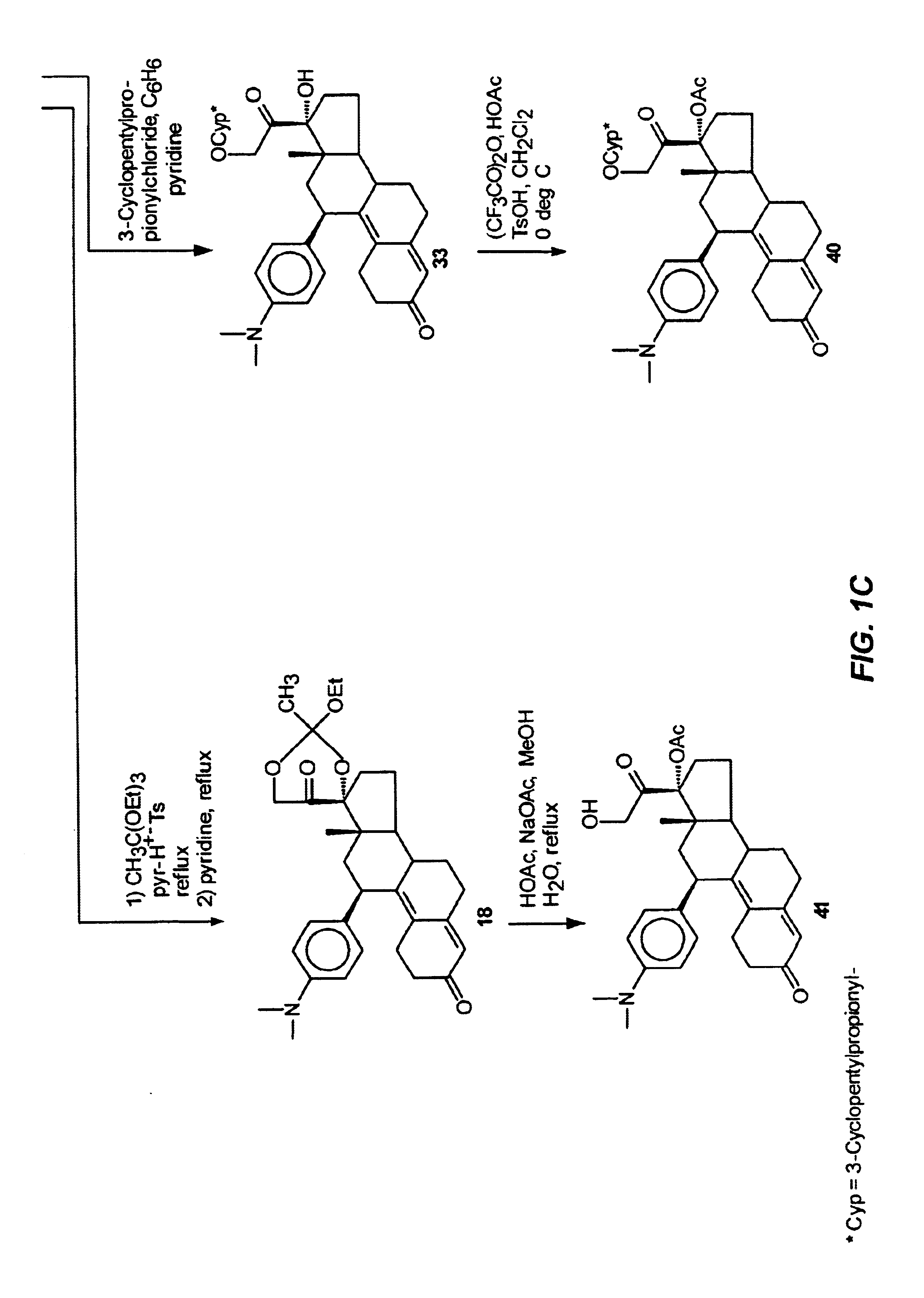
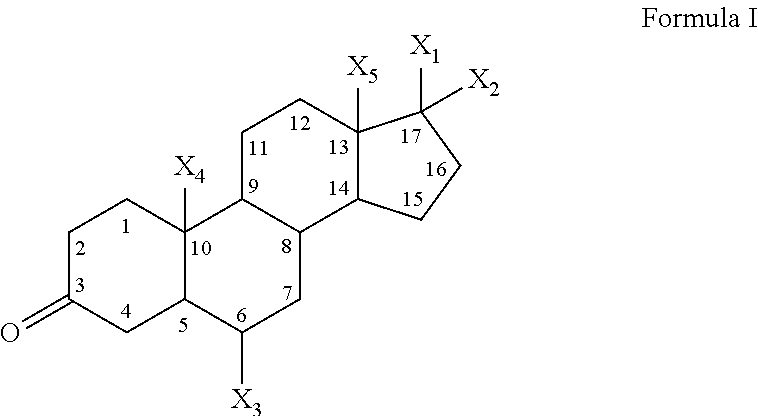
21-substituted progesterone derivatives as new antiprogestational agents
A compound having the general formula: in which: R1 is a member selected from the group consisting of —OCH3, —SCH3, —N(CH3)2, —NHCH3, —CHO, —COCH3 and —CHOHCH3; R2 is a member selected from the group consisting of halogen, alkyl, acyl, hydroxy, alkoxy, acyloxy, alkyl carbonate, cypionyloxy, S-alkyl and S-acyl; R3 is a member selected from the group consisting of alkyl, hydroxy, alkoxy and acyloxy; R4 is a member selected from the group consisting of hydrogen and alkyl; and X is a member selected from the group consisting of ═O and ═N—OR5, wherein R5 is a member selected from the group consisting of hydrogen and alkyl.In addition to providing the compounds of Formula I, the present invention provides methods wherein the compounds of Formula I are advantageously used, inter alia, to antagonize endogenous progesterone; to induce menses; to treat endometriosis; to treat dysmenorrhea; to treat endocrine hormone-dependent tumors; to treat uterine fibroids; to inhibit uterine endometrial proliferation; to induce labor; and for contraception.
Owner:DEPT OF HEALTH & HUMAN SERVICES THE GOVERNMENT OF THE UNITED STATES OF AMERICA AS REPRESENTED BY THE SEC OF THE
Endometriosis treatment protocol
The endometriosis treatment protocol provides for administering to a female patient in need of treatment for endometriosis a pharmaceutical composition in a form suitable for vaginal or rectal delivery having a pharmaceutically effective amount of an aromatase inhibitor, which may be either a steroid or non-steroidal. The pharmaceutical composition may be formed as a vaginal suppository, a rectal suppository, a vaginal gel, a rectal gel, a vaginal cream or a rectal cream. The pharmaceutical composition may optionally have pharmaceutically effective amounts of progesterone and calcitriol, and may be administered in combination with an oral COX-2 inhibitor. Alternatively, the pharmaceutical composition comprises an aromatase inhibitor administered vaginally or rectally and is administered in combination with oral calcitriol and the oral COX-2 inhibitor. The aromatase inhibitor is either steroidal or non-steroidal.
Owner:SHIPPEN EUGENE R
Progesterone for the treatment or prevention of spontaneous preterm birth
InactiveUS20090264395A1Reduce morbidityReduce mortalityOrganic active ingredientsOrganic chemistryNeonatal morbidityPreterm Births
A method for treating or preventing spontaneous preterm birth in pregnant women and improving neonatal morbidity and mortality. The method includes administering to a pregnant woman in need thereof an effective amount of progesterone sufficient to prolong gestation by minimizing the shortening or effacing of her cervix. Treatment and prophylaxis with progesterone in pregnant women having symptoms of short cervix has been clinically shown to increase neonatal health.
Owner:WATSON LAB INC +1
Low-oil pharmaceutical emulsion compositions comprising progestogen
ActiveUS20110262494A1High progesterone solubilityElevation in histamine releaseOrganic active ingredientsNervous disorderIUD with progestogenTG - Triglyceride
Described are sterile, ready-to-use, pharmaceutical oil-in-water emulsion compositions for parenteral administration comprising:0.015 to 0.5% wt / vol progesterone;0.5 to 10% wt / vol oil, wherein the oil comprises at least 85% wt. / wt. triglyceride;0.0425 to 4.1% wt / vol phospholipid;80-99.4% wt / vol aqueous medium;wherein the composition has an osmolality in the range of 200-1000 mOsm / kg.Also described are methods of making such compositions and method of using such compositions in therapeutic or prophylactic treatment, such as treatments comprising intravenous administration of the pharmaceutical composition.
Owner:BESINS HEALTHCARE LUXEMBOURG (LU)
Pharmaceutical composition and method for the transdermal delivery of magnesium
InactiveUS20050196434A1Reduce disadvantagesBiocideAerosol deliveryAutonomic bladder dysfunctionMagnesium salt
The present invention relates to a method and transdermal pharmaceutical composition for preventing magnesium deficiency or imbalances associated with magnesium deficiency including diabetes, hypertension, high cholesterol, cardiac arrhythmias, acute myocardial infarction, arteriosclerosis, atherosclerosis, preeclampsia, dysautonomia, mitral valve prolapse, asthma, constipation, irritable bowel syndrome, migraines, muscle spasms and cramping, premenstrual syndrome, osteoporosis, kidney stones, chronic fatigue syndrome, and fibromyalgia. The transdermal pharmaceutical composition includes a therapeutically effective amount of a pharmaceutically acceptable salt of magnesium and a pharmaceutically acceptable carrier. A therapeutically effective amount of a pharmaceutically acceptable salt of zinc a vitamin such as B-complex vitamin, a carotenoid, a mineral, or a combination thereof may also be included in the transdermal pharmaceutical composition. A therapeutically effective amount of progesterone may also be included in the transdermal pharmaceutical composition. The transdermal pharmaceutical composition may be topically administered to prevent magnesium deficiency or imbalances caused by magnesium deficiency.
Owner:BRIERRE BARBARA T
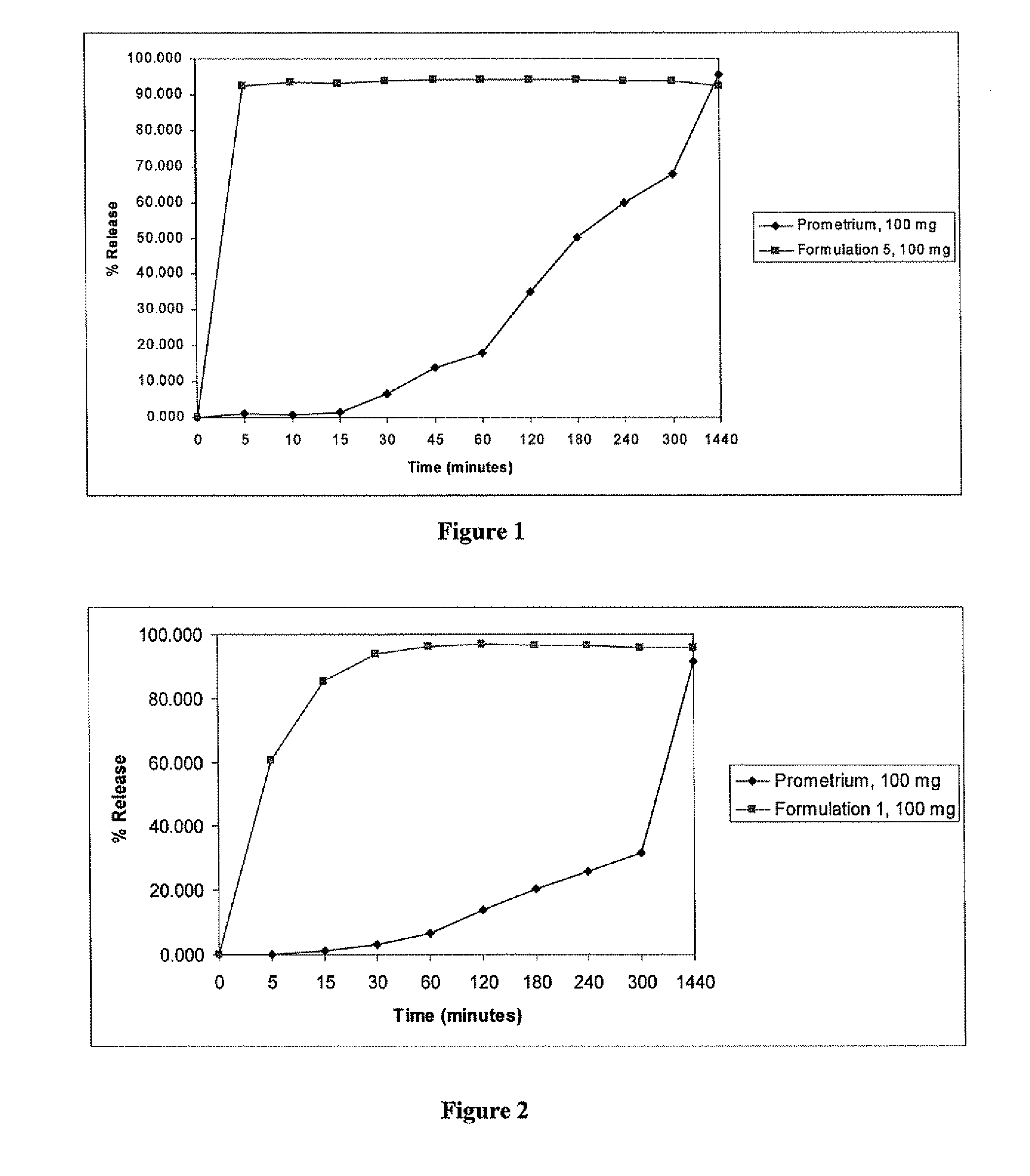
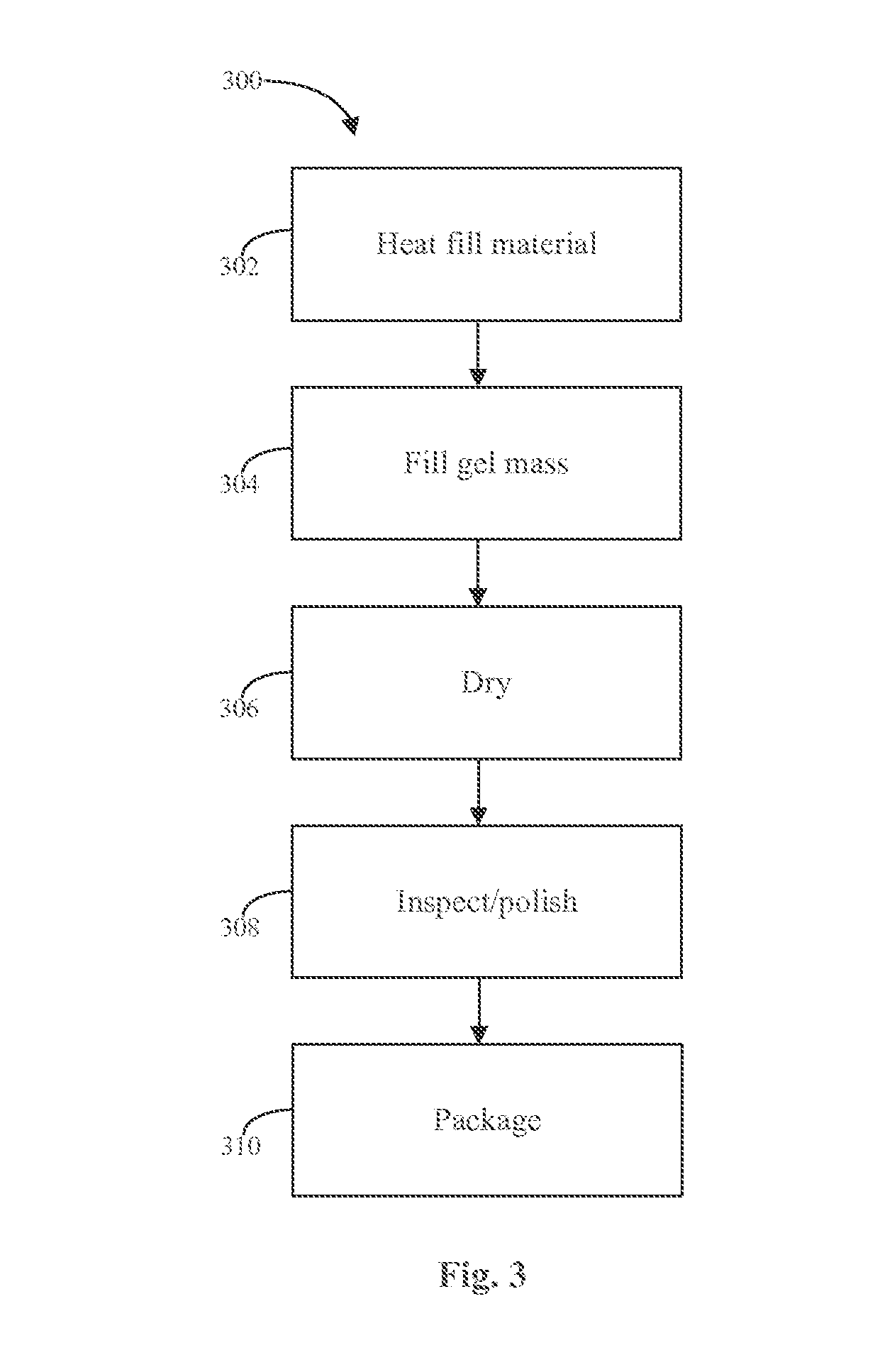
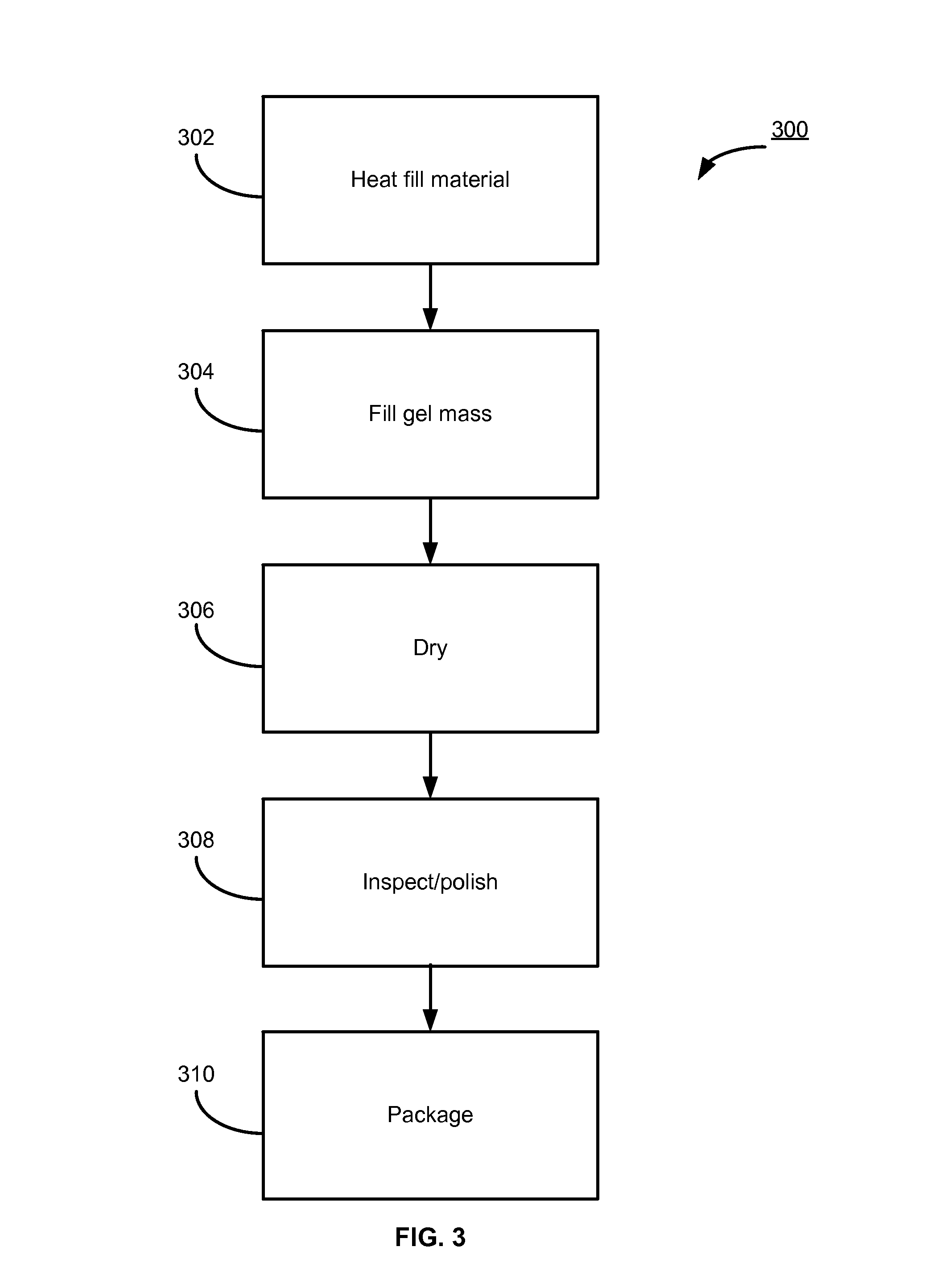
Progesterone Solutions for Increased Bioavailability
ActiveUS20100255085A1Improve bioavailabilityHigh dissolution rateOrganic active ingredientsPharmaceutical non-active ingredientsDissolutionProgesterones
Fill materials for hydrophobic drugs, such as progesterone, and methods of making and using thereof are described herein. The fill material contains the hydrophobic drug dissolved in one or more fatty acids. The concentration of the hydrophobic drug is typically from about 7% to about 50% by weight of the fill material. The concentration of the one or more fatty acids is from about 60% to about 95% by weight of the carrier. The formulation also contains an organic acid and one or both of one or more pharmaceutically acceptable alcohols and one or more pharmaceutically acceptable mono-, di-, or triesters of medium or long chain fatty acids. The fill material can be encapsulated in a hard or soft capsule. The formulations described herein have a higher dissolution rate and faster onset of dissolution compared to micronized progesterone suspended in an oil and thus should have increased bioavailability in vivo.
Owner:PATHEON SOFTGELS INC
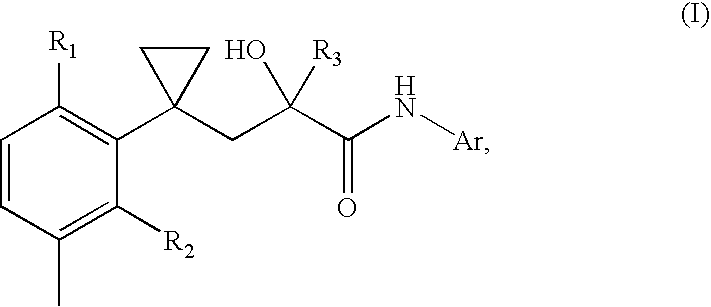
Transdermal pharmaceutical preparation with a progesterone A-specific ligand (PRASL) as active ingredient
InactiveUS20060134188A1Avoid reactionReduce riskBiocideOrganic active ingredientsTransdermal patchAdditive ingredient
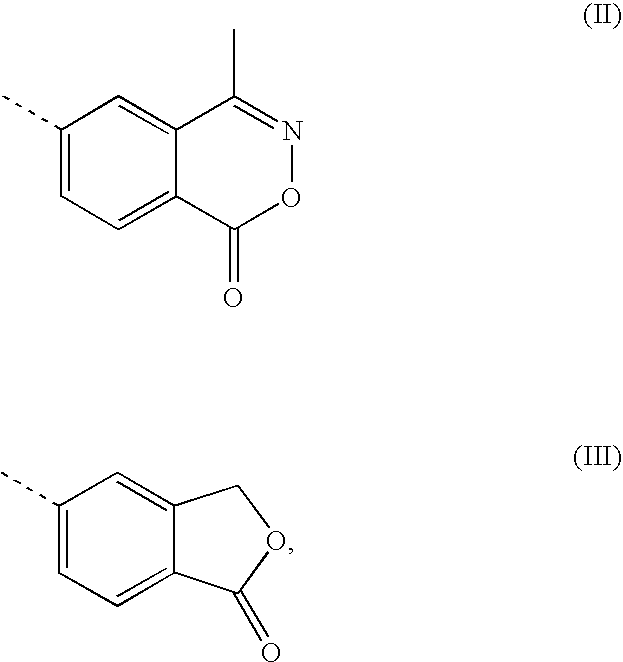
A transdermal patch for hormone therapy and fertility control has a backing layer, an effective-ingredient-containing adhesive layer adhering to the backing layer and a removable protective film. The adhesive layer includes a progestagenic effective ingredient and an estrogen in an adhesive matrix based on a silicone polymer, a polyisobutylene polymer (PIB), a polyacrylate polymer or a styrene block copolymer with butadiene or isoprene (SBS or SIS). The transdermal patch contains from 0.1 to 10%, based on a total weight of the adhesive matrix, of a progestagenic effective ingredient of formula I: wherein R1 and R2 each represent, independently of each other, H or F; R3 represents CH3 or CF3 and Ar is a group of formula II or III: or a pharmaceutically suitable derivative thereof.
Owner:SCHERING AG
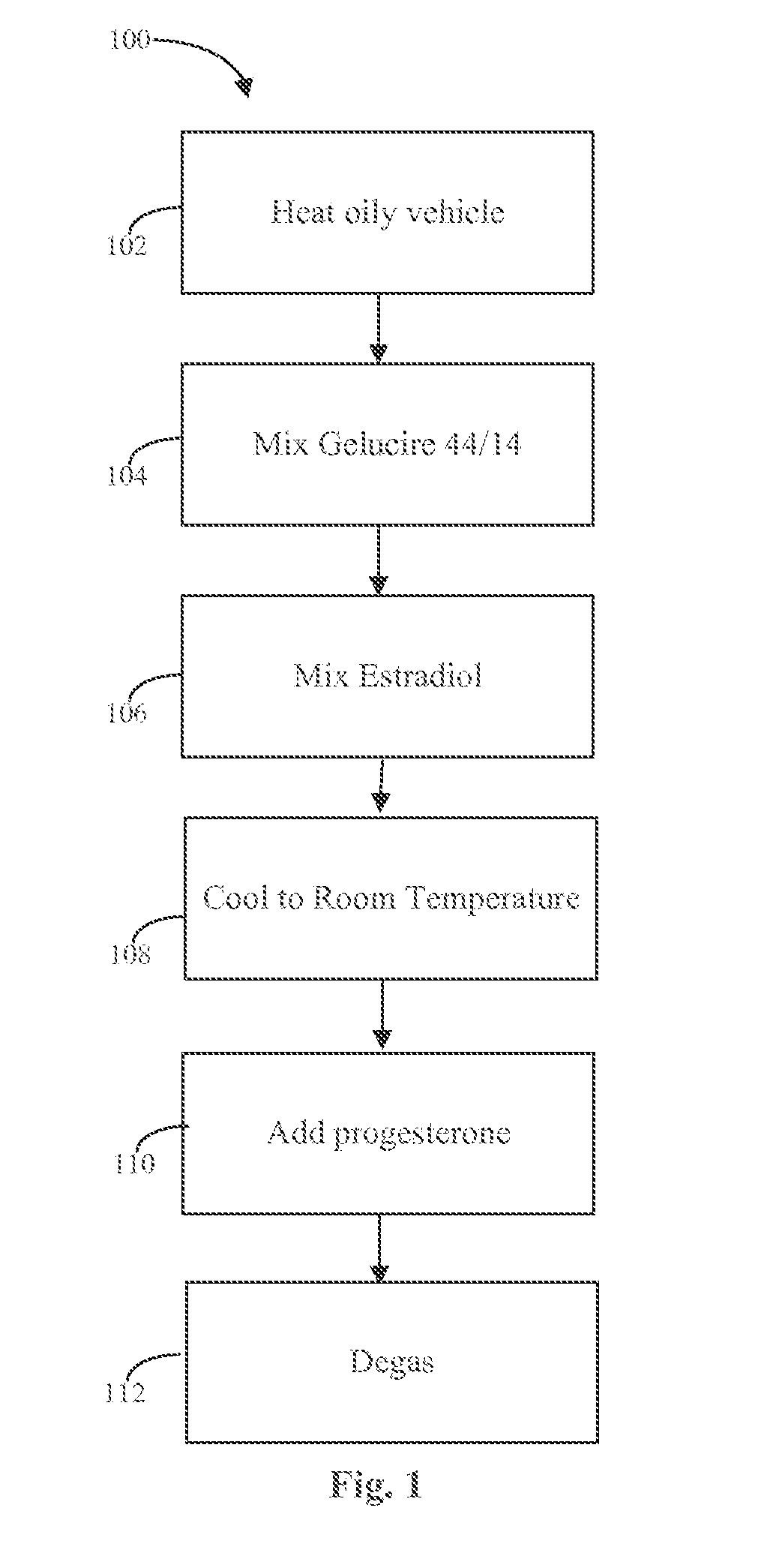
Natural combination hormone replacement formulations and therapies
ActiveUS20130129818A1Improve complianceOrganic active ingredientsBiocideHormone replacementEstradiol.free
Estrogen and progesterone replacement therapies are provided herein. Among others, the following formulations are provided herein: solubilized estradiol without progesterone; micronized progesterone without estradiol; micronized progesterone with partially solubilized progesterone; solubilized estradiol with micronized progesterone; solubilized estradiol with micronized progesterone in combination with partially solubilized progesterone; and solubilized estradiol with solubilized progesterone.
Owner:THERAPEUTICSMD
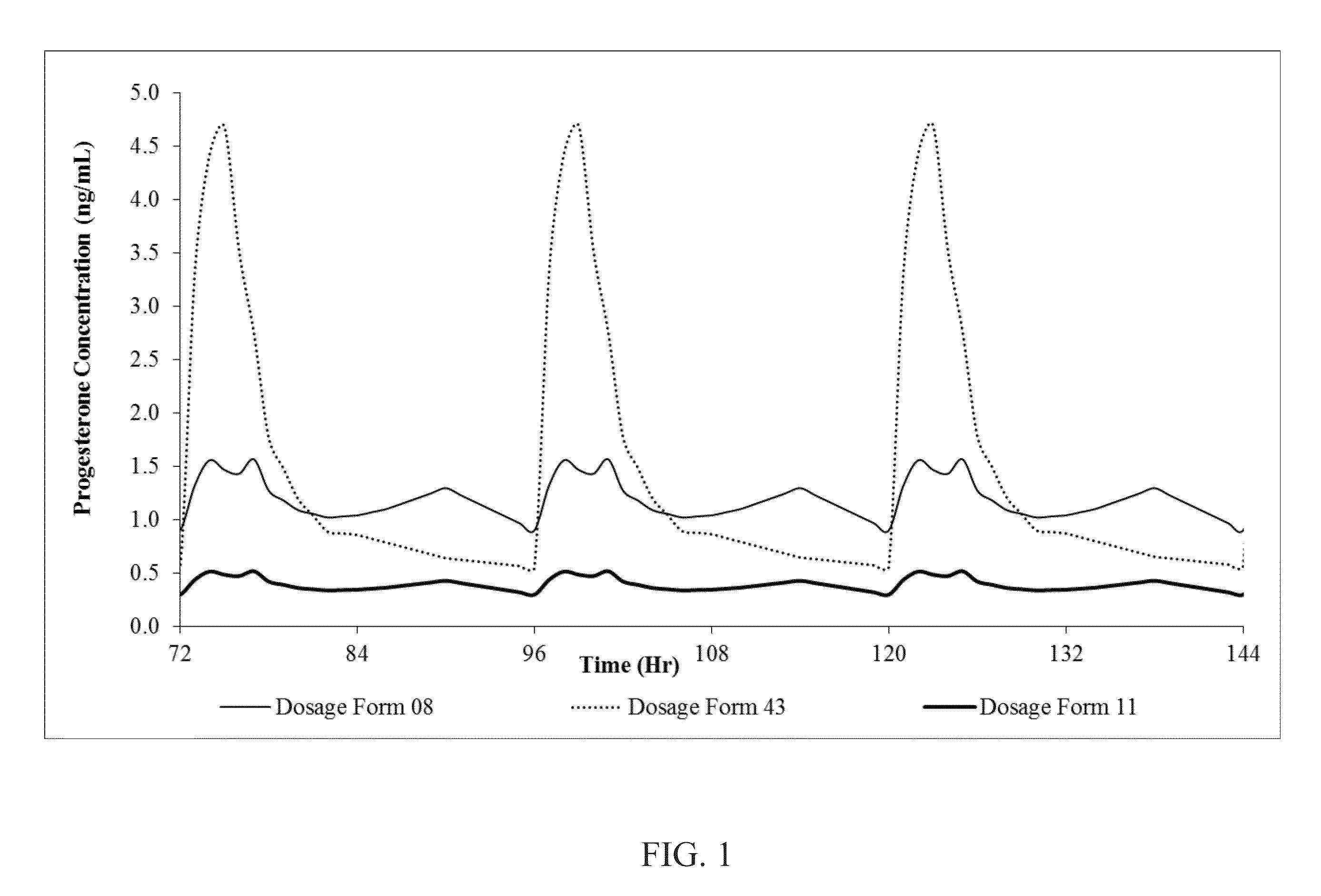
Progesterone containing oral dosage forms and kits
The present invention provides for progesterone containing pharmaceutical oral dosage forms, pharmaceutical kits, and related methods. In one embodiment, an oral dosage form formulated for on-going administration is provided. The oral dosage form includes an amount of progesterone and a pharmaceutically acceptable carrier. The oral dosage form is formulated such that upon single dose administration to a non-pregnant woman in follicular phase, the oral dosage form provides a serum progesterone C24h of at least 0.20 ng / mL.
Owner:LIPOCINE
Natural combination hormone replacement formulations and therapies
Estrogen and progesterone replacement therapies are provided herein. Among others, the following formulations are provided herein: solubilized estradiol without progesterone; micronized progesterone without estradiol; micronized progesterone with partially solubilized progesterone; solubilized estradiol with micronized progesterone; solubilized estradiol with micronized progesterone in combination with partially solubilized progesterone; and solubilized estradiol with solubilized progesterone.
Owner:THERAPEUTICSMD INC
Serum-free culture medium for mesenchymal stem cells
ActiveCN102827807AAvoid instabilityClear chemical compositionSkeletal/connective tissue cellsCell phenotypeSodium bicarbonate
The invention relates to the field of biology, and discloses a serum-free culture medium which essentially comprises an IMDM (Iscove Modified Dulbecco Medium), L-glutamine, sodium bicarbonate, Hepes, recombinant human insulin, recombinant human transferrin, recombinant human albumin, 2-mercaptoethanol, protocatechuic acid, lipid, amino acid, vitamins, trace elements, Pluronic F-68, hydrocortisone, vitamin C, bonding amine or recombinant human fibronectin, progesterone, putrescine, heparin, serotonin, epidermal growth factors (EGFs), b-fibroblast growth factors (FGF), platelet derive growth factor (PDGF)-BB and insulin-like growth factor (IGF)-I. The serum-free culture medium is clear in chemical components, free from animal sources and serum and safe and ideal in cell cultivation and avoids the doped animal components and unstable batches, and the results of the cultured mesenchymal stem cells show that the total cellular score, the cell phenotype and the secretory cell factors are normal, so that the serum-free culture medium has good industrial application prospect.
Owner:内蒙古干细胞医学工程技术研究中心
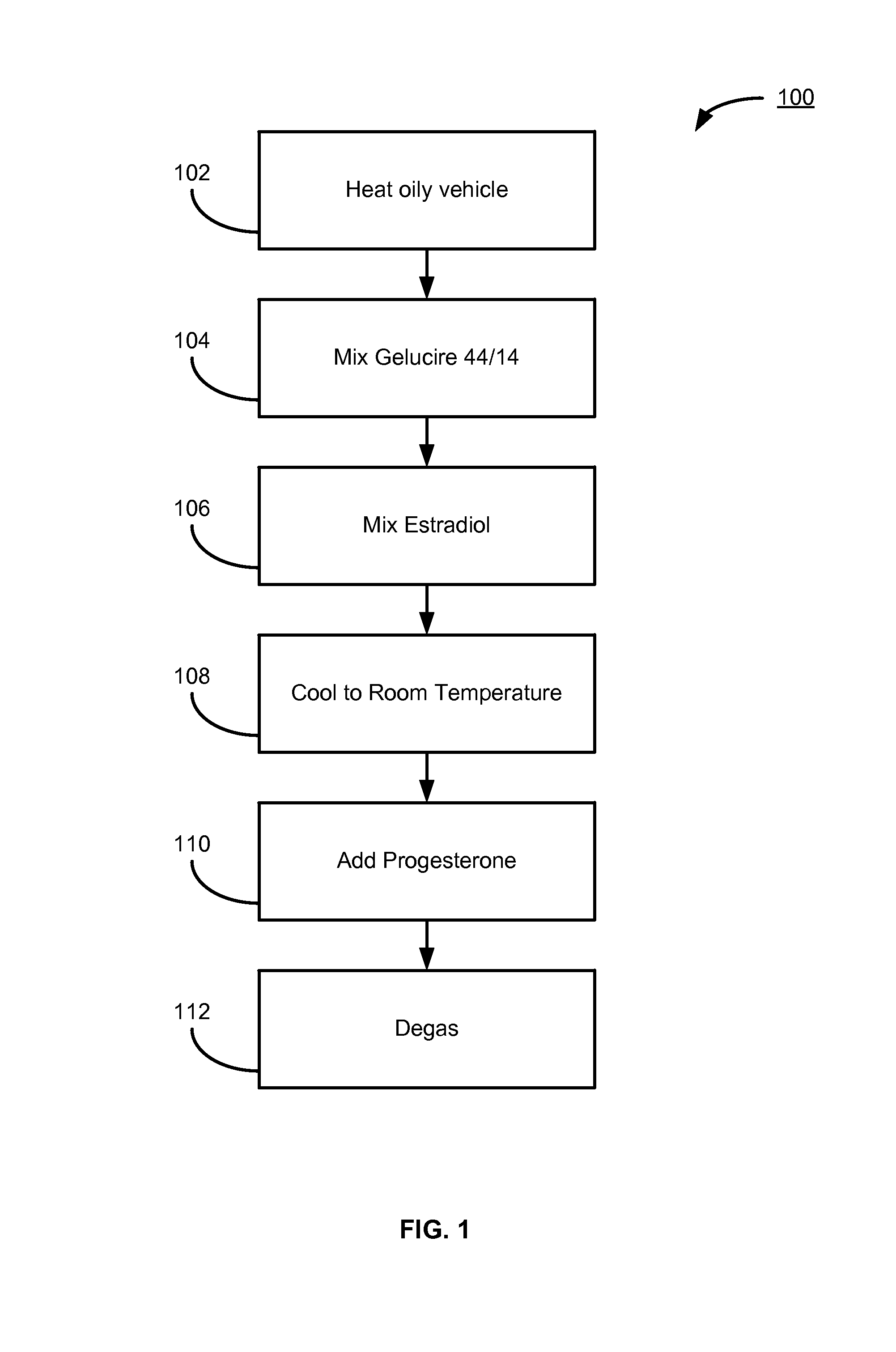
Pharmaceutical composition with a synthetic natural progesterone and oestradiol base and its preparation process
InactiveUS6656929B1Improve homogeneityExcellent characteristicsOrganic active ingredientsBiocideProgesteronesChemistry
Owner:BESINS HEALTHCARE SA
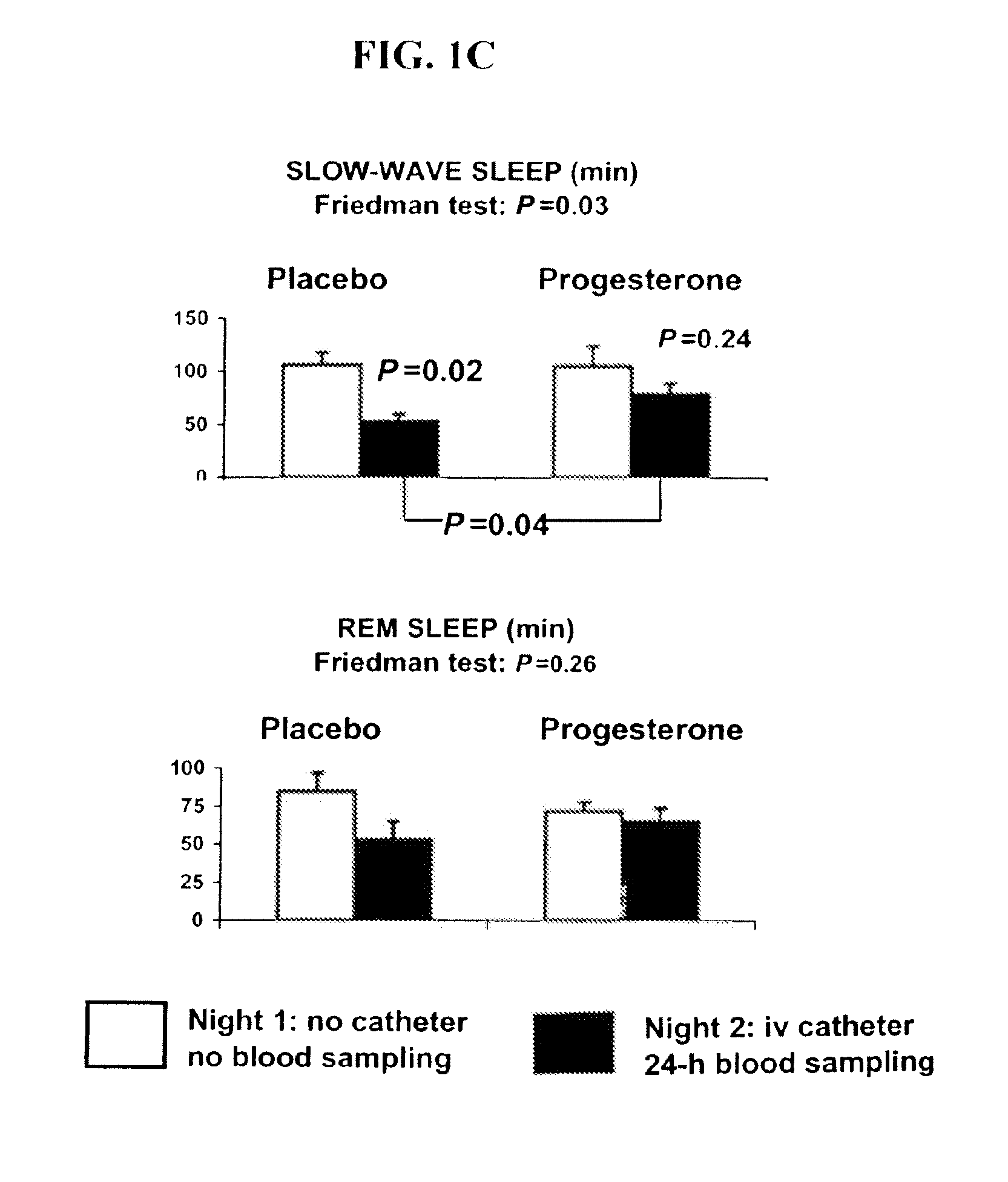
Progesterone treatment for improving sleep quality
ActiveUS20120302535A1Improve sleep qualityReduce wake up timeOrganic active ingredientsNervous disorderSleep patternsPhysiology
Described herein are therapeutic methods using progesterone and progesterone analogs to improve sleep quality. The methods may be particularly useful to treat subject with perturbed sleep patterns, such as subjects who suffer from mid-sleep period awakenings.
Owner:BESINS HEALTHCARE LUXEMBOURG (LU)
Method Of Treating Atrophic Vaginitis
InactiveUS20070264309A1Reducing concomitant liabilityBiocideOrganic active ingredientsGynecologyLong term treatments
This invention relates to a method and pharmaceutical composition useful in treating a condition responsive to hormone replacement therapy. Specifically, the invention is related to the long term treatment of symptoms associated with atrophic vaginitis. The composition contains effective amounts of an estrogen, a progesterone compound and a pharmaceutically accepted vehicle, carrier and / or diluent.
Owner:PEAR TREE WOMENS HEALTH CARE
Nasal delivery mechanism for prophylactic and post-acute use of progesterone and/or its enantiomer for use in treatment of mild traumatic brain injuries
Owner:FLORIDA STATE UNIV RES FOUND INC
Implantation rates after in vitro fertilization, and treatment of infertility and early pregnancy loss with a nitric oxide donor or substrate alone or in combination with progesterone, and a method for contraception with nitric oxide inhibitors in combination with antiprogestins or other agents
InactiveUS20020169205A1Improve implantation rateIncrease implantationBiocidePeptide/protein ingredientsEarly Pregnancy LossPhysiology
A method is provided for the improvement of implantation rates and / or pregnancy rates in a female mammal, comprising administering to a female mammal in whom pregnancy is desired an effective amount of (a) a nitric oxide synthase substrate, a nitric oxide donor, or both, optionally in combination with (b) a progestin, and, (c) optionally, in further combination with an estrogen. A method is also provided for fertility control for a female mammal, comprising administering to a female mammal in whom pregnancy is not desired and at risk for becoming pregnant an effective amount of nitric oxide synthase inhibitor in combination with an antiprogestin. Pharmaceutical compositions are also provided.
Owner:BOARD OF RGT THE UNIV OF TEXAS SYST
Intravaginal devices containing progesterone for estrus synchronization and related proceses
InactiveUS6939558B2Solve the lack of flexibilitySolving the Insufficiency of ElasticityAnimal reproductionPharmaceutical delivery mechanismMedicineEstrus synchronization
Embodiments of the present invention generally relate to devices and processes related to estrus synchronization. Particular embodiments of devices and processes of the present invention slowly release progesterone over a period of time for estrus synchronization.
Owner:AKZO NOBEL NV
Intravaginal devices containing progesterone for estrus synchronization and related proceses
InactiveUS20050021009A1Solve the lack of flexibilitySolving the Insufficiency of ElasticityAnimal reproductionFemale contraceptivesMedicineEstrus synchronization
Embodiments of the present invention generally relate to devices and processes related to estrus synchronization. Particular embodiments of devices and processes of the present invention slowly release progesterone over a period of time for estrus synchronization.
Owner:AKZO NOBEL NV
Trace elements
The invention discloses a trace element solution, which comprises at least one metal selected from the group comprising selenium, copper, zinc, manganese and chromium; and at least one component selected from the group comprising a vitamin, a vaccine, a growth stimulant, a dewormer, iron dextran, an antibiotic and a synchronization preparation. The synchronization preparation is a combination of injectable hormonal preparations, inplantable hormonal preparations, intravaginal hormonal preparation and other slow release hormonal preparation. The antibiotics include oral, injectable and implantable theurapeutic remedies. The vaccine includes antigens and a combination of antigens and adjuvents. The growth stimulants include zeranol, estradiol, testosterone, progesterone and trenbolone acetate. The dewormer includes macrocydic lactones, leramizoles, benzimidazoles and salicylanilides. The macrocydic lactones include doramectin, ivermectin, abamectin and moxidectin.
Owner:WARBURTON TECH
Progesterone Antagonist and Selective Progesterone Modulator in the Treatment of Excessive Uterine Bleeding
Progesterone antagonists and SPRM are useful to prepare a medication for the treatment or the prophylaxis of excessive uterine bleeding in women with spontaneous or iatrogenic coagulation disorders such as thrombopenia, coagulation factor deficiency or anti-coagulant therapy. Treatment will last from 1 day to 180 days.
Owner:PREGLEM
Natural combination hormone replacement formulations and therapies
Estrogen and progesterone replacement therapies are provided herein. Among others, the following formulations are provided herein: solubilized estradiol without progesterone; micronized progesterone without estradiol; micronized progesterone with partially solubilized progesterone; solubilized estradiol with micronized progesterone; solubilized estradiol with micronized progesterone in combination with partially solubilized progesterone; and solubilized estradiol with solubilized progesterone.
Owner:THERAPEUTICSMD INC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com