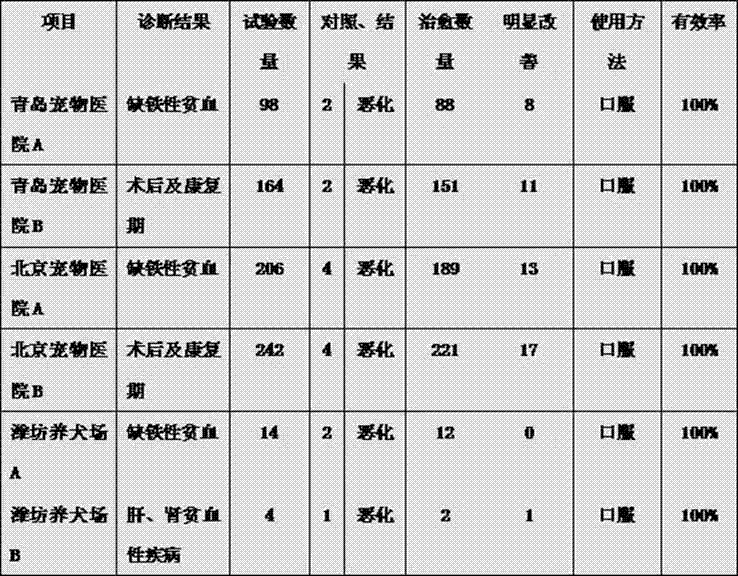
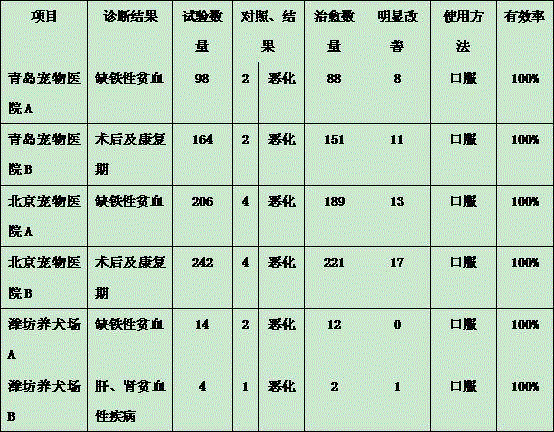
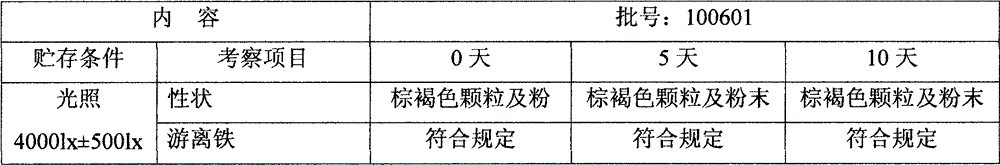
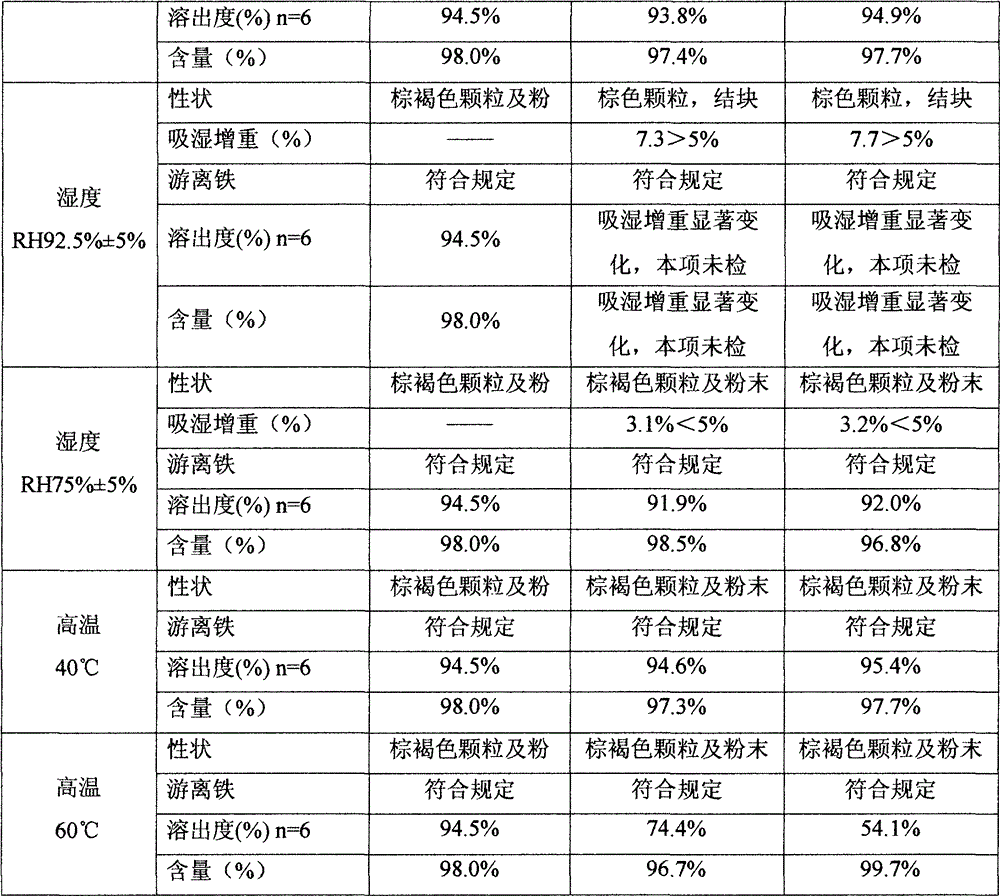
Patents
Literature
45 results about "Iron dextran" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Trace elements
The invention discloses a trace element solution, which comprises at least one metal selected from the group comprising selenium, copper, zinc, manganese and chromium; and at least one component selected from the group comprising a vitamin, a vaccine, a growth stimulant, a dewormer, iron dextran, an antibiotic and a synchronization preparation. The synchronization preparation is a combination of injectable hormonal preparations, inplantable hormonal preparations, intravaginal hormonal preparation and other slow release hormonal preparation. The antibiotics include oral, injectable and implantable theurapeutic remedies. The vaccine includes antigens and a combination of antigens and adjuvents. The growth stimulants include zeranol, estradiol, testosterone, progesterone and trenbolone acetate. The dewormer includes macrocydic lactones, leramizoles, benzimidazoles and salicylanilides. The macrocydic lactones include doramectin, ivermectin, abamectin and moxidectin.
Owner:WARBURTON TECH
Iron-dextran compound for use as a component in a therapeutical composition for prophylaxis or treatment of iron-deficiency
An iron-dextran compound for parenteral treatment of iron-deficiency anemia comprises hydrogenated dextran having a weight average molecular weight (Mw) between 700 and 1,400 Daltons, preferably approximately 1,000 Daltons, a number average molecular weight (Mn) of 400 to 1,400 Daltons and wherein 90% by weight of the dextran has molecular weights less than 2,700 Daltons and the Mw of the 10% by weight fraction of the dextran having the highest molecular weights is below 3,200 Daltons, said hydrogenated dextran having been subjected to purification by membrane processes having a cut-off value between 340 and 800 Daltons, in stable association with ferric oxyhydroxide. The compound is produced by using membrane processes to eliminate dextrans of higher molecular weights than approximately 2,700 Daltons and membrane processes to remove saccharides of molecular weights below approximately 340 Daltons from hydrogenated dextran before precipitating ferric hydroxide in the presence of said dextran followed by heat treatment and purification.
Owner:PHARMACOSMOS HLDG
Method of eliminating sodium chloride in iron-dextran complex compound water solution and device thereof
ActiveCN101108194AEliminate burningEliminate explosion hazardsOrganic active ingredientsMetabolism disorderSolventAqueous solution
The invention relates to a method and device for eliminating sodium chloride in the aqueous solution for the complex of ferrodextran, which is characterized in that: the ferrodextran is prepared with dextran and ferric trichloride. The sodium chloride in the ferrodextran is eliminated with an electrodialysis film, that is, eliminating the sodium chloride of the solvent by electrodialysis process in the ferrodextran complex solvent with iron content is more than or equal to 40mg / ml, sodium chloride content is more than or equal to 5.0 per cent wt and viscosity of 3.0mpa is multiplied by s (20 DEG C.) under the temperature of 10 to 90 DEG C. Compared with the technique of eliminating sodium chloride with alcohol precipitation, the invention prevents the safety hazard from flammable and combustible alcohol and reduces the production cost.
Owner:GUANGXI RES INST OF CHEM IND CO LTD
Process for producing an iron-dextran compound, iron-dextran compound produced according to said process, pharmaceutical composition for prophylaxis or treatment of iron-deficiency and use of said compound for the preparation of a parenterally administrable pharmaceutical composition
In a process for producing an iron-dextran compound for use in parenteral treatment of iron-deficiency in humans or animals a stable compound of desired relatively low molecular weight is obtained by using first hydrogenation and then oxidation to convert reducing terminal groups on the dextran molecules before reaction with the iron. By varying the ratio of hydrogenated groups to oxygenated groups the average molecular weight of the resulting iron-dextran compound can be varied.
Owner:PHARMACOSMOS HLDG
Iron dextran raw material for human intravenous injection and preparation method thereof
The invention relates to an iron dextran raw material for human intravenous injection and a preparation method thereof. The iron dextran for human intravenous injection is prepared from dextran through hydrolysis, ultra-filtration or gel column separation, oxidation, complexing, ultra-filtration refining and ethanol precipitation or spray drying. For human injection, the dextran is pretreated after hydrolysis, the small molecular dextran of which the absolute molecular weight is 5000-8000 is prepared by separation with an ultra-filtration device or a gel column, an oxidant and an oxidation process are adjusted, sodium peroxycarbonate is used as the oxidant for oxidation, a final product is refined by separation with the ultra-filtration device or the gel column, and then the iron dextran raw material which has the weight-average molecular weight of 135000-190000 and the molecular weight distribution of less than 2.0 and can meet the human intravenous injection is obtained.
Owner:HI NICE SHINE BEIJING PHARMA HLDG
Preparation method of iron-dextrin
ActiveCN107201387AUniform molecular weight distributionHigh quality raw materialFermentationSolubilityIron dextran
The invention discloses a preparation method of iron-dextrin. The preparation method comprises the following steps: preparing low molecular weight dextran through bi-enzyme (dextransucrase-dextranase) synergy, and then oxidizing dextran by taking sodium hypochlorite mixed liquor as an oxidizing agent, so as to obtain an oxidation-type dextran; simultaneously titrating and complexing with weak base and acid-base to prepare iron-dextrin and curing iron-dextrin under the conditions of high temperature and high pH value, so as to obtain cured iron-dextrin; filtering unreacted dextran and micromolecule iron-dextrin and ions therein with biological membranes with different molecular weights, so as to obtain purified iron-dextrin. The iron-dextrin prepared by the invention is uniform in distribution of the molecular weight, high in stability and less in content of impurities. Various indexes of iron-dextrin synthesized by the method conform to the requirements of Chinese pharmacopoeia, and the solubility of iron-dextrin is very good.
Owner:HEFEI UNIV OF TECH
Iron isomaltum oligosaccharide and preparing method thereof
The invention belongs to the saccharide derivative preparation technology field, in particular to an isomaltose oligosaccharide and preparation process. Isomaltose oligosaccharide is a novel stable chelating biological iron, and has the excellent effect of preventing iron-deficiency anemia of a human or livestock. The productive process comprises firstly preparing or choosing activating isomaltose oligosaccharide whose average molecular weight is 1500-2000 to be purified, conducting complex reaction of activating isomaltose oligosaccharide and iron hydroxide under a certain condition, wherein iron hydroxide is formed by the reaction of inorganic ferric salt and alkali before or in the process of the complex reaction, thirdly, purifying complex compound through adopting the methods such as organic solvent deposition, ion exchange, dialysis or hyper-filtration and the like. The product which is prepared by the invention has wider iron content range from 5% to 30% compared with current iron dextran products at home and abroad, and is superior to other product in other aspects such as flowability, viscosity, stability and absorption effect.
Owner:揭阳汉邦生物股份有限公司
Iron dextran bulk drug and preparation method thereof
The invention relates to an iron dextran bulk drug and a preparation method thereof. The preparation method comprises the steps of (1) alkalization of an iron salt; (2) decomposition and alkalization of dextran, comprising the procedures of dissolving the dextran in distilled water, adding an acid liquid, heating to a temperature of 80 DEG C-120 DEG C, hydrolyzing, adding an alkali liquid after the reaction is finished, and alkalizing at the temperature of 80 DEG C-120 DEG C to obtain a solution B; (3) complexation, comprising the procedures of adding a solution A obtained by the step (1) into the solution B obtained by the step (2), stirring and reacting at the temperature of 80 DEG C-120 DEG C to obtain a complexation solution; (4) alcohol precipitation; and (5) comprising procedures of performing solid-liquid separation on alcohol precipitation solution by a centrifugal machine, spray drying the solid phase and collecting powder to obtain a finished product of the iron dextran. The preparation method of the iron dextran is simple in process steps, has easily controllable reaction process, good product quality, high yield and low production cost, and is a preparation method of the iron dextran bulk drug, with relatively high innovativeness.
Owner:TIANJIN ZHONGAO BIOTECH
Iron-dextrin injection liquid for tonifying qi-blood and used for animals and preparation method
InactiveCN108498556AFast absorptionLong durationOrganic active ingredientsHeavy metal active ingredientsIron dextranVitamin B12
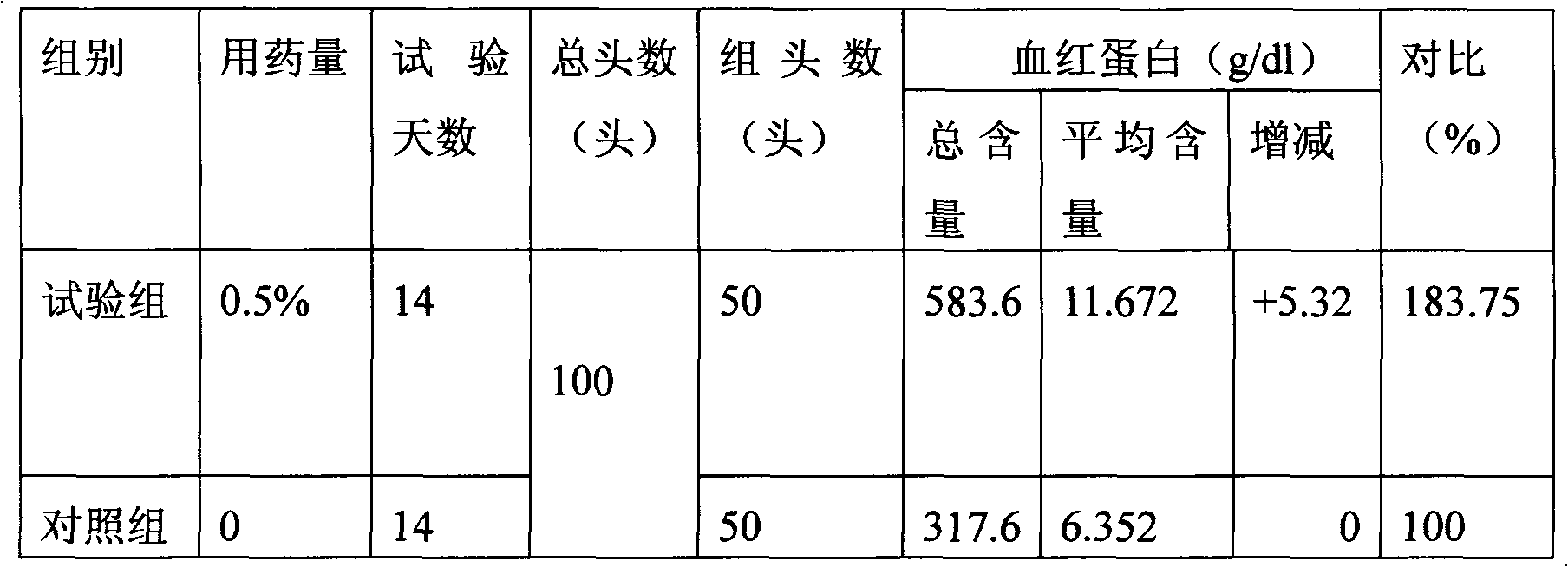
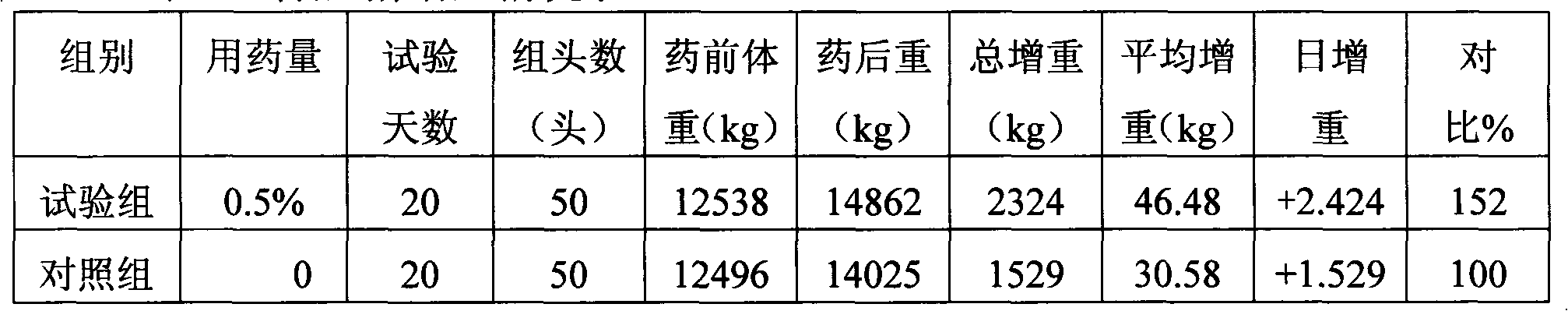
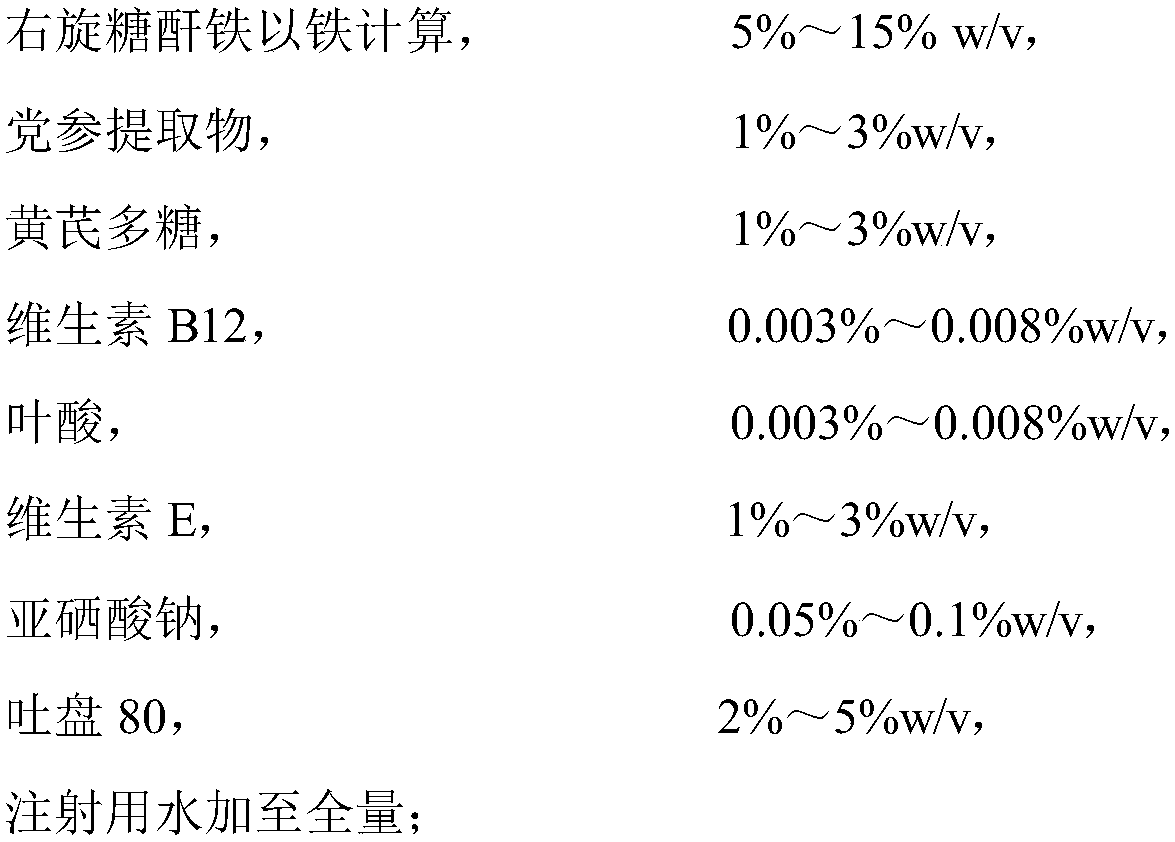
The invention discloses iron-dextrin injection liquid for tonifying qi-blood and used for animals. The liquid is prepared from, 5-15% w / v of iron-dextrin by iron, 1-3% w / v of radix codcnopsitis pilosulas extract, 1-3% w / v of astragalus polysaccharides, 0.003-0.008% w / v of vitamin B12, 0.003-0.008% w / v of folic acid, 1-3% w / v of vitamin E, 0.05-0.1% w / v of sodium selenite, 0.2-5% w / v of Twain-80 and the balance injection water; dextran in iron-dextrin is formed by mixing dextran with the weight average molecular weight of 5,000-6,000 Da, 6,000-6,500 Da and 6,500-7,500 Da. A preparation method of the iron-dextrin injection liquid comprises the steps that the set amount of Twain-80, folic acid and vitamin E are added to a container and evenly mixed, and then hot injection water with the amount of 10% of the total amount is added for dissolution; vitamin B12, sodium selenite, radix codcnopsitis pilosulas extract and astragalus polysaccharides are added and stirred and dissolved to obtain amixture; set iron-dextrin is added to the mixture and evenly stirred, and the injection water continues to be added. The iron-dextrin injection liquid has definite treatment effects on pregnant sowsand piglets and is worthy of popularization.
Owner:四川伴农动保生物技术有限公司
Composite organic iron supplementing agent
InactiveCN105192318AGood effectReduce the cost of farmingAnimal feeding stuffCompound organicIron dextran
The invention provides a composite organic iron supplementing agent. The composite organic iron supplementing agent comprises the following raw materials by weight: 20 to 30 parts of ferrous fumarate, 10 to 20 parts of iron amino acid chelate, 5 to 10 parts of iron-dextran and 10 to 20 parts of ferrous lactate, wherein the iron amino acid chelate is one or a composition of a group consisting of ferrous lysinate, ferrous methioninate, ferrous glycinate and ferrous DL-threoninate. The composite organic iron supplementing agent is used as an animal iron supplementing agent and has good treatment and prevention effects on iron-deficiency anemia caused by chronic hemorrhage, malnutrition, pregnancy, growth and development periods, etc.
Owner:南宁市泽威尔饲料有限责任公司
Iron dextran and preparation method thereof
ActiveCN104829745AHigh purityReduce contentBlood disorderExtracellular fluid disorderSolubilityUltrafiltration
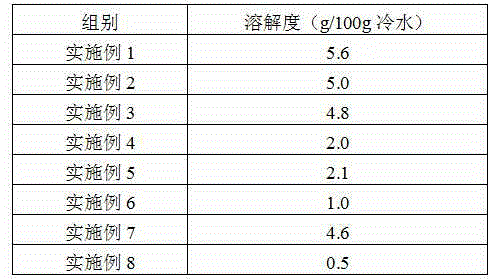
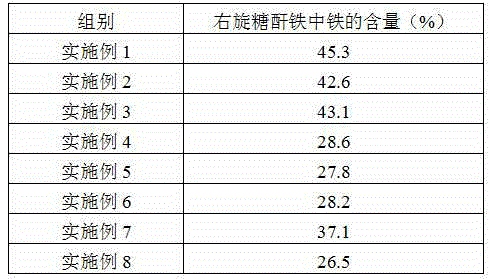
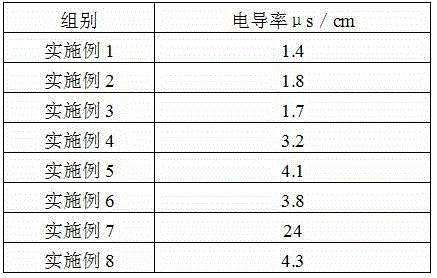
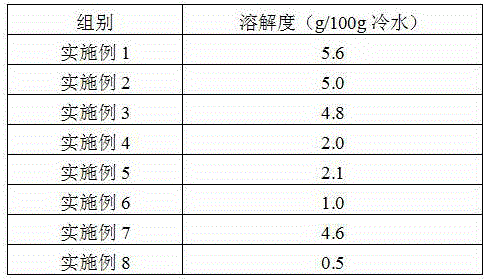
The invention provides a preparation method of iron dextran. In the provided preparation method, after the complexing liquid is obtained, the pH of the complexing liquid is adjusted so as to greatly improve the dissolving property of iron dextran, while during the process, intermediates, which has a molecular weight of about 180000 to 200000, and accounts for 5 to 8% of the total weight of the final product, are generated, a great amount of ions absorbs on the intermediates, because the molecular weight of the intermediates is similar to that of iron dextran, the iron dextran and intermediates cannot be separated easily through ultrafiltration, thus the ion content of the final product is high, and the iron content is influenced in some degree, so a treatment is carried out before the ultrafiltration so as to remove the intermediates. The iron content of the prepared iron dextran is much higher than the national standard (25%), and can reach 40% or more. The solubility of iron dextran in cold water can reach 4g / 100g (cold water) or more, moreover, the contents of ions such as sodium iron, chlorine iron, and the like are greatly reduced, and the purity of iron dextran is higher.
Owner:江西华太药业有限公司
Trace Elements
The invention discloses a trace element solution, which comprises at least one metal selected from the group comprising selenium, copper, zinc, manganese and chromium; and at least one component selected from the group comprising a vitamin, a vaccine, a growth stimulant, a dewormer, iron dextran, an antibiotic and a synchronisation preparation. The synchronisation preparation is a combination of injectable hormonal preparations, inplantable hormonal preparations, intravaginal hormonal preparation and other slow release hormonal preparation. The antibiotics include oral, injectable and implantable theurapeutic remedies. The vaccine includes antigens and a combination of antigens and adjuvents. The growth stimulants include zeranol, estradiol, testosterone, progesterone and trenbolone acetate. The dewormer includes macrocydic lactones, leramizoles, benzimidazoles and salicylanilides. The macrocydic lactones include doramectin, ivermectin, abamectin and moxidectin.
Owner:WARBURTON TECH
Feed premix containing nutrient elements of calcium and iron
The invention relates to a feed premix nutrient elements of calcium and iron. The premix comprises 1200-1600 parts of Fructus Psoraleae, 10-200 parts of calcium gluconate, 10-200 parts of cod liver oil, 5-10 parts of a bone strengthening factor and 10-20 parts of iron dextran. The additives added by the invention can help poultry or livestock to supplement rich calcium and iron and benefit fast growth and reproduction of livestock.
Owner:TIANJIN BIJIA PHARMA CO LTD
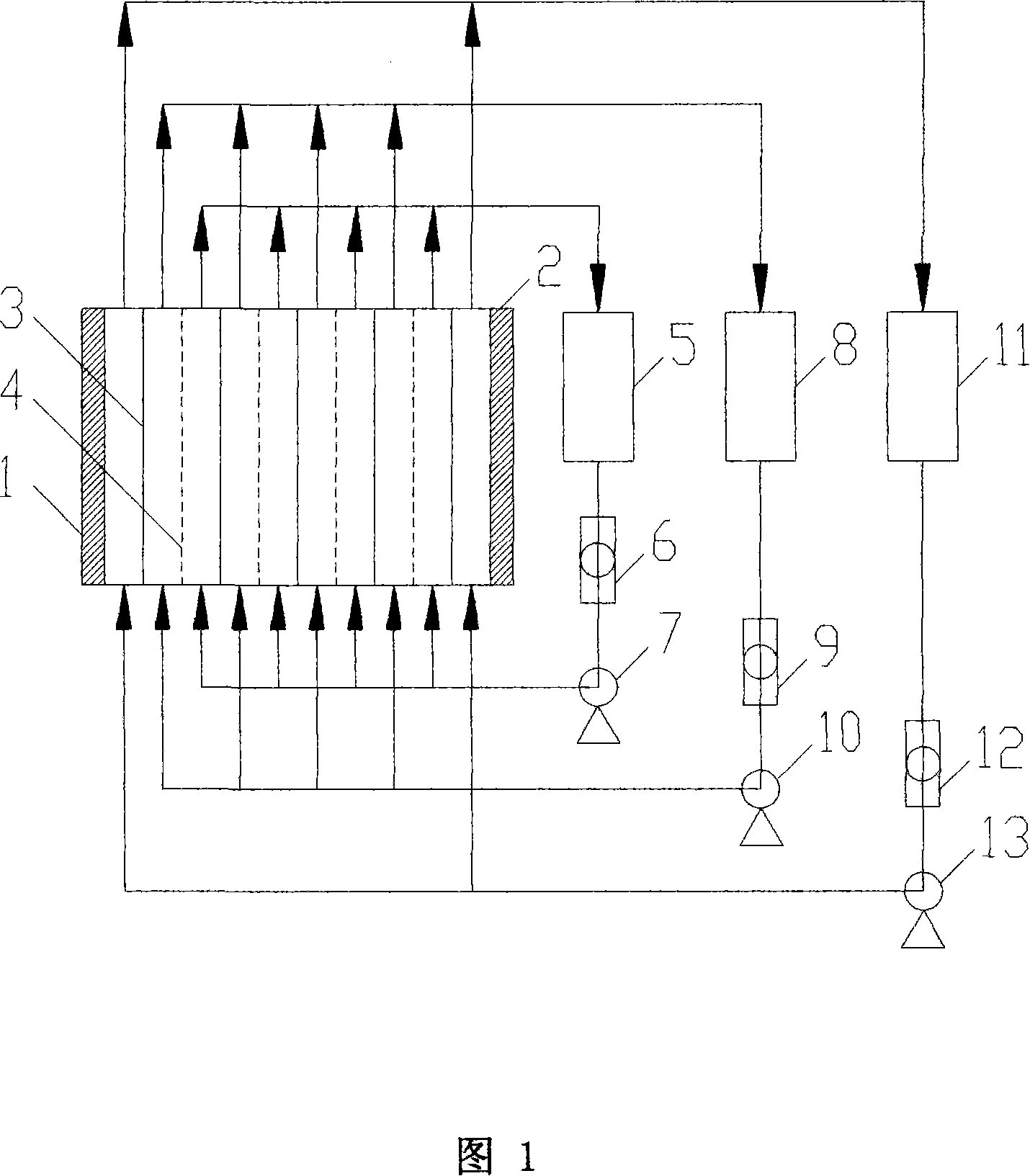
Iron dextran weight-average molecular weight and distribution coefficient stability control production method
InactiveCN112409505AQuality is easy to controlGuarantee safe and effectiveFerric hydroxideUltrafiltration
The invention discloses an iron dextran weight-average molecular weight and distribution coefficient stability control production method which comprises the following steps: S01, hydrolysis of dextran: hydrolyzing dextran under an acidic condition to reduce the molecular weight; s02, complexing: reacting ferric trichloride with sodium hydroxide to produce ferric hydroxide, and complexing ferric hydroxide with dextran to produce iron dextran; s03, nanofiltration: removing salt, heavy metals and free iron impurities in the complexing solution by adopting an ultrafiltration process, and concentrating the complexing solution; and S04, spray drying: adding the qualified ultrafiltration solution into spray drying equipment, and carrying out spray drying to obtain powder. The iron dextran with stable weight-average molecular weight and distribution coefficient is produced, and the weight-average molecular weight (Mw) of the iron dextran raw material and the preparation is accurately controlled to be about 6000, so that the internal quality and the clinical treatment effect of the iron dextran raw material and the preparation are improved.
Owner:SHANGHAI SHENYA ANIMAL HEALTH PROD FUYANG CO LTD
A kind of iron dextran raw material for human intravenous injection and preparation method thereof
ActiveCN104031170BMeet the requirements for injection usePrevent overflowIron dextranEthanol precipitation
The invention relates to an iron dextran raw material for human intravenous injection and a preparation method thereof. The iron dextran for human intravenous injection is prepared from dextran through hydrolysis, ultra-filtration or gel column separation, oxidation, complexing, ultra-filtration refining and ethanol precipitation or spray drying. For human injection, the dextran is pretreated after hydrolysis, the small molecular dextran of which the absolute molecular weight is 5000-8000 is prepared by separation with an ultra-filtration device or a gel column, an oxidant and an oxidation process are adjusted, sodium peroxycarbonate is used as the oxidant for oxidation, a final product is refined by separation with the ultra-filtration device or the gel column, and then the iron dextran raw material which has the weight-average molecular weight of 135000-190000 and the molecular weight distribution of less than 2.0 and can meet the human intravenous injection is obtained.
Owner:HI NICE SHINE BEIJING PHARMA HLDG
A kind of iron dextran and preparation method thereof
ActiveCN104829745BHigh purityReduce contentBlood disorderExtracellular fluid disorderSolubilityUltrafiltration
The invention provides a preparation method of iron dextran. In the provided preparation method, after the complexing liquid is obtained, the pH of the complexing liquid is adjusted so as to greatly improve the dissolving property of iron dextran, while during the process, intermediates, which has a molecular weight of about 180000 to 200000, and accounts for 5 to 8% of the total weight of the final product, are generated, a great amount of ions absorbs on the intermediates, because the molecular weight of the intermediates is similar to that of iron dextran, the iron dextran and intermediates cannot be separated easily through ultrafiltration, thus the ion content of the final product is high, and the iron content is influenced in some degree, so a treatment is carried out before the ultrafiltration so as to remove the intermediates. The iron content of the prepared iron dextran is much higher than the national standard (25%), and can reach 40% or more. The solubility of iron dextran in cold water can reach 4g / 100g (cold water) or more, moreover, the contents of ions such as sodium iron, chlorine iron, and the like are greatly reduced, and the purity of iron dextran is higher.
Owner:江西华太药业有限公司
Suspension injection of iron-dextran and ponazuril and preparation method thereof
PendingCN113209016AReduce stress responseImprove welfareHeavy metal active ingredientsMetabolism disorderIron dextranPharmaceutical medicine
The invention relates to a suspension injection containing iron-dextran and ponazuril. The suspension injection is further prepared from pharmaceutically acceptable silicone oil, lecithin, a metal ion complexing agent, n-butyl alcohol, povidone, water and an optional pH regulator. The suspension injection is stable, good in safety and high in bioavailability, and capable of preventing and treating coccidiosis infection and iron-deficiency anemia of animals at the same time.
Owner:HUBEI LONGXIANG PHARMA TECH CO LTD
A kind of compound iron dextran injection and preparation method thereof
ActiveCN103720706BImprove immunityTo promote metabolismOrganic active ingredientsMetabolism disorderDiseaseIron dextran
The invention discloses a compound iron-dextrin injection and a preparation method thereof. The injection is prepared from iron-dextrin, butafosfan and injection water. The preparation method comprises the following steps: (1) putting the prescribed butafosfan in a container, and adding a proper amount of injection water until the butafosfan is completely dissolved, regulating the pH to be 5.5-6.0 with a NaOH solution to obtain a butafosfan solution for later use; (2) adding a prescribed iron-dextrin solution into the butafosfan solution, uniformly mixing, and fixing the volume to the prescription with the injection water, sufficiently and uniformly mixing to obtain a mixed liquid; and (3) filtering and subpackaging the mixed liquid, filling, and sterilizing to obtain the compound iron-dextrin injection. The compound iron-dextrin injection can be used for preventing and treating secondary infection and other diseases caused by piglet anemia and low immunity, and promoting metabolism and stress resistance of piglets, so that the immunity of piglets can be strengthened, the energy utilization can be improved, and the slaughter weight of piglets can be increased.
Owner:HENAN HUITONG TIANXIA BIO ENG
Breeding method for improving growth speed of pigs
InactiveCN107006423AGuaranteed uniformityFast growthOrganic active ingredientsDigestive systemDiseaseWeight gaining
The invention relates to a breeding method for improving the growth speed of pigs, and belongs to the technical field of breeding. The method includes the following steps that 1, before newborn pigs are fed for the first time, each newborn pig is drenched with 1-2 ml of medicinal liquid, and the drenching is conducted twice a day for three days in a row; 2, on the third day, the seventh day, and the thirty-fifth day after the pigs are born, iron dextran is injected; 3, 28 days after being born, the pigs are weaned, 7 days before the weaning, the pigs are fed with creep feed, and on the eighth to the tenth days, creep feed and nursing early-stage feed are adopted to feed the pigs; after 11 days, weaning early-stage feed is adopted to feed the pigs till the weights of the pigs are 30 jin, and after the weights of the pigs reach 30 jin, the pigs eat feed freely. The breeding method for improving the growth speed of the pigs can prevent the occurrence of white and yellow scour of piglets, improve the immunity and disease resistance of the pigs and increase the growth speed of the pigs, the survival rate can be increased by 20%, the maximum weight gain rate can reach 25%, the pigs are ready for slaughter 8-12 days ahead of time, the feeding period is shortened, the labor cost is reduced, the feeding consumption is reduced, and the economic benefits of farmers are increased.
Owner:剑河县康科畜牧发展有限公司
Traditional Chinese medicine composition for weight gaining stress resistance and preparing method thereof
InactiveCN108261513AEffective treatmentGood nourishment and fatteningOrganic active ingredientsHeavy metal active ingredientsSide effectIron dextran
The invention relates to a traditional Chinese medicine composition for weight gaining stress resistance. The traditional Chinese medicine composition is prepared from, by weight, 10-30 parts of fructus crataegi, 10-40 parts of herba gynostemma pentaphylla, 10-20 parts of fructus hordei germinatus, 10-20 parts of soybeans and 1-10 parts of iron dextran. The formula composition is scientific and reasonable, replenishes qi and enriches the blood and supplements nutrients to accelerate weight gaining, has no toxic and side effect, has no drug residue, is convenient to use and low in cost, can effectively resist stress and has the efficacy and the effect of weight gaining.
Owner:TIANJIN REBATE SCI & TECH DEV
Feed for improving pork quality of fattening pigs and production method thereof
InactiveCN108522867ARaise the ratioPromote sugar metabolismFood processingAnimal feeding stuffLean meatDL-methionine
The technical scheme of the invention discloses feed for improving the pork quality of fattening pigs. The feed is prepared from the following raw materials: corns, barley with shells, third-class flour, soybean meal having the protein content of 46 percent, wheat bran, soybean oil, calcium carbonate, calcium hydrophosphate, vitamin complex, mineral elements, iron dextran, probiotics, lysine sulfate having a concentration of 70 percent, L-threonine having a concentration of 98 percent, DL-methionine having a concentration of 99 percent, L-tryptophan having a concentration of 98.5 percent, guanidinoacetic acid, choline chloride, baking soda and sodium chloride. According to the feed, by adding additives for promoting muscle protein deposition, improving glucose metabolism and protein anabolism of the fattening pigs, the lean meat percentage and daily gain of the fattening pigs can be improved, and the pork quality can be improved.
Owner:环山集团股份有限公司
Iron dextran compound oral liquid and preparation technology thereof
ActiveCN103479671ALong-actingEasy to usePharmaceutical delivery mechanismUnknown materialsDiseaseBenzoic acid
The invention discloses iron dextran compound oral liquid which is prepared from the following raw materials: 20-40kg of iron dextran, 1-5kg of donkey-hide gelatin, 5-13kg of taurine, 10-50kg of sucrose, 0.35kg of benzoic acid and a proper amount of purified water. The invention also provides a preparation technology of the iron dextran compound oral liquid, which comprises the steps of (A) fetching the purified water, adding the donkey-hide gelatin of the formula dosage, and standing until the donkey-hide gelatin is completely dissolved; (B) fetching the purified water, adding the taurine of the formula dosage, and completely dissolving the taurine; (C) fetching the iron dextran of the formula dosage and the mixed liquid in the step (B), sequentially adding into the prepared mixed liquid in the step (A), and mixing uniformly. The oral liquid disclosed by the invention is widely applied to the iron-deficiency anemia caused by chronic blood loss, malnutrition, developmental phase and the like, and also can be used as an adjuvant therapy for anemia diseases of operation recovery, the liver and kidney, the heart, the skin and the like.
Owner:山东亚华生物科技有限公司
Livestock traditional Chinese medicine composition having functions of nourishing, fattening, tonifying qi and nourishing blood and preparation method of the composition
InactiveCN103690838ANo residueEffective treatmentDigestive systemBlood disorderSide effectIron dextran
The invention discloses a livestock traditional Chinese medicine composition having functions of nourishing, fattening, tonifying qi and nourishing blood. The composition comprises following components by weight: 10-30 parts of basket fern rhizome, 10-40 parts of fleece-flower root, 10-20 parts of malt, 10-20 parts of soybean, and 1-10 parts of iron-dextrin. The formulation of the composition is scientific and reasonable. The composition has the functions of nourishing, fattening, tonifying qi and nourishing blood. The composition is free of toxic and side effects and medicine residues, and is convenient to use and low in cost. The composition can be used for treating common emaciation, slow growth, and the like for livestock.
Owner:TIANJIN REBATE SCI & TECH DEV
A kind of iron dextran compound oral liquid and its preparation process
ActiveCN103479671BLong-actingEasy to usePharmaceutical delivery mechanismUnknown materialsBenzoic acidDisease
The invention discloses iron dextran compound oral liquid which is prepared from the following raw materials: 20-40kg of iron dextran, 1-5kg of donkey-hide gelatin, 5-13kg of taurine, 10-50kg of sucrose, 0.35kg of benzoic acid and a proper amount of purified water. The invention also provides a preparation technology of the iron dextran compound oral liquid, which comprises the steps of (A) fetching the purified water, adding the donkey-hide gelatin of the formula dosage, and standing until the donkey-hide gelatin is completely dissolved; (B) fetching the purified water, adding the taurine of the formula dosage, and completely dissolving the taurine; (C) fetching the iron dextran of the formula dosage and the mixed liquid in the step (B), sequentially adding into the prepared mixed liquid in the step (A), and mixing uniformly. The oral liquid disclosed by the invention is widely applied to the iron-deficiency anemia caused by chronic blood loss, malnutrition, developmental phase and the like, and also can be used as an adjuvant therapy for anemia diseases of operation recovery, the liver and kidney, the heart, the skin and the like.
Owner:山东亚华生物科技有限公司
Iron dextran particles and preparation process thereof
ActiveCN104095818BImprove solubilityImprove toleranceOrganic active ingredientsGranular deliveryPrillAdhesive
Owner:TIANJIN HUAIREN PHARMA
Method for controlling feed ratio of iron-dextran production by measuring content of sugar anhydride
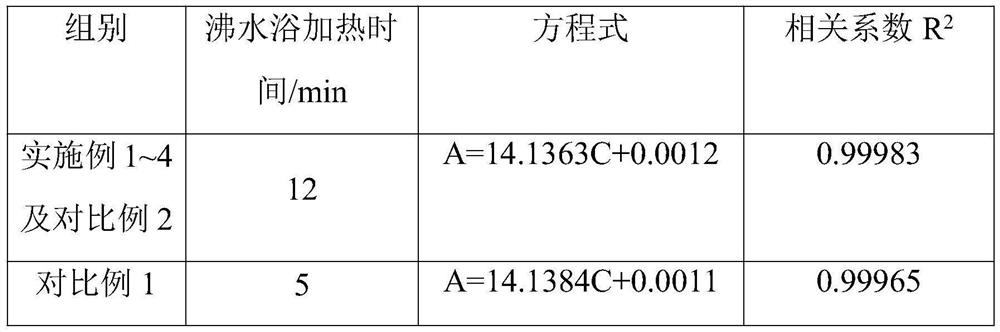
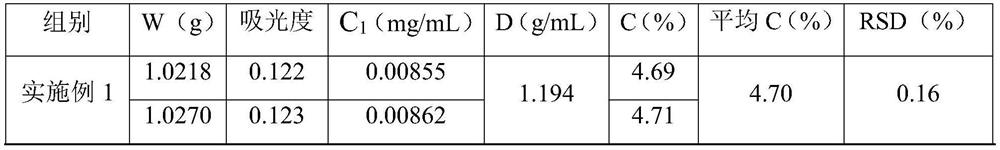
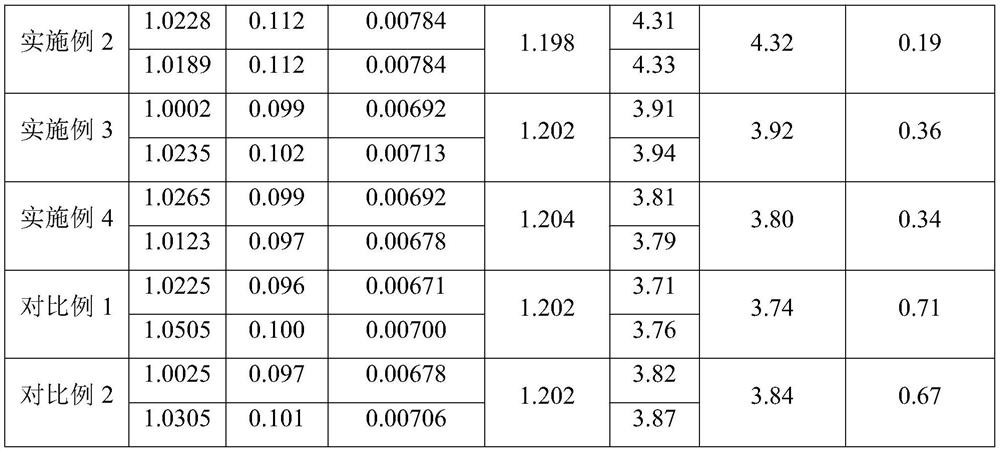
PendingCN113567372AControl production feed ratioImprove stabilityColor/spectral properties measurementsTest sampleSugar anhydride
The invention discloses a method for controlling the feed ratio of iron-dextran production by measuring the content of sugar anhydride. The method comprises the following steps of (1) detecting the iron content in an iron-dextran complexing solution, (2) preparing a test sample from the iron-dextran complexing solution, (3) sequentially cooling a sample to be tested, mixing the sample with an anthrone sulfuric acid solution, heating, cooling, and determining absorbance, (4) using water as blank control, (5) preparing the D-glucosum anhydricum into five standard solutions with different concentrations, (6) respectively measuring the absorbance of the five standard solutions with different concentrations, and making a standard curve according to the concentrations and the absorbance to obtain an equation, (7) calculating the absorbance of the sample to be tested according to the equation in the step (6) to obtain the concentration of glucose, and converting to obtain the content of the sugar anhydride, and (8) according to the iron content and the content of the sugar anhydride, converting the content of the sugar anhydride in the product with the corresponding specification, so as to adjust and control the feeding ratio of the sugar anhydride to the iron in the production process of the iron dextran.
Owner:广西南宁市桃源兽药厂
A kind of production method of iron dextran product that disintegrates quickly in pig oral cavity
ActiveCN108853053BSolve the problem of prone to stress responseRapid precipitationHeavy metal active ingredientsMetabolism disorderBiotechnologyOral medication
Owner:安徽万士生物制药有限公司
Short-T2-effect endorectal/endovaginal magnetic resonance imaging contrast agent and preparation method thereof
ActiveCN108514644AComposition is stableExtended shelf lifeEchographic/ultrasound-imaging preparationsNMR/MRI constrast preparationsCouplingT2 weighted
The invention discloses a short-T2-effect endorectal / endovaginal magnetic resonance imaging contrast agent and a preparation method thereof. The short-T2-effect endorectal / endovaginal magnetic resonance imaging contrast agent comprises iron dextran, an ultrasonic coupling agent and water. The short-T2-effect endorectal / endovaginal magnetic resonance imaging contrast agent has the beneficial effects that the iron dextran, the ultrasonic coupling agent and the water are mixed according to a specified proportion to prepare the contrast agent, so that the T2 relaxation time of the ultrasonic coupling agent during magnetic resonance imaging is shortened, the signal strength of the ultrasonic coupling agent on a T2-weighted imaging is reduced, and interference of the ultrasonic coupling agent tolesion display during endorectal / endovaginal magnetic resonance imaging is reduced; the contrast agent has attachment performance with cavity organs such as the rectum / vagina, is a non-liquid colloid, can be properly formed and is not required to be contained in a specific container, the cavity organs such as the rectum / vagina can be well filled, and the rectum or the vagina can be directly filled with the contrast agent; each component is stable, and the contrast agent is long in shelf life and convenient to store.
Owner:SHENZHEN PEOPLES HOSPITAL
Synthesis method of iron dextran and dispersible tablet thereof
ActiveCN113480678BHydrogen promotionOptimal complexation stateHeavy metal active ingredientsOrganic active ingredientsIron dextranSpray dried
The invention discloses a method for synthesizing iron dextran and its dispersible tablets, comprising the following steps: (1) preparing dextran 20 into a dextran hydrolyzate, weighing ferric chloride to prepare a ferric chloride solution; (2) preparing the dextran Add the hydrolyzate and ferric chloride solution into the reaction tank and mix evenly, adjust the pH of the obtained reaction solution to 10‑11, then heat to boiling state, and react to obtain the complex solution; (3) the obtained complex solution The pH of the solution is adjusted to 6.3-6.8 with hydrochloric acid solution, and then the finished product of iron dextran is obtained after being subjected to coarse filtration, medium filtration and ultrafiltration, and spray drying. The invention provides a method for synthesizing iron dextran and its dispersible tablets. Through the pH value adjustment before and after the complexation reaction and the design of the ceramic ultrafiltration membrane, the iron content and dissolution rate can be increased, the impurity content can be reduced, and the production efficiency can also be improved.
Owner:江西华太药业有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com