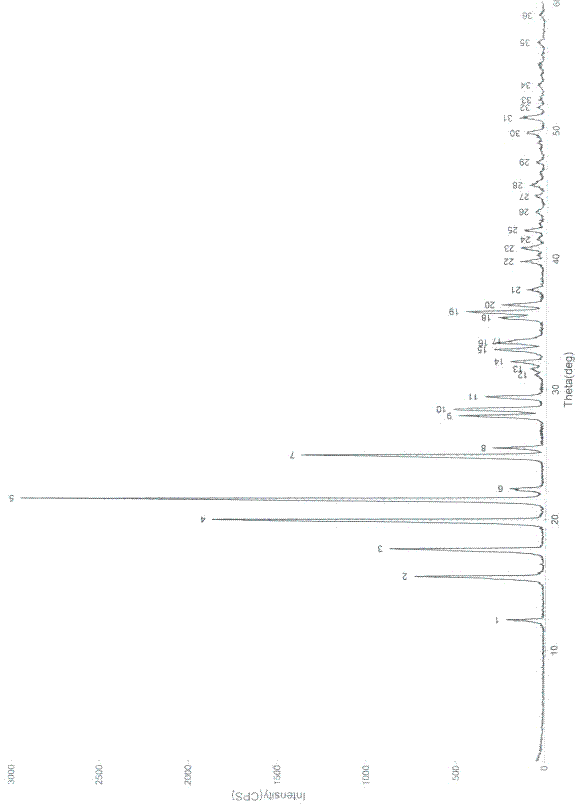
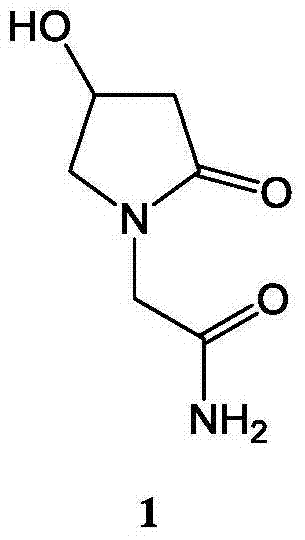
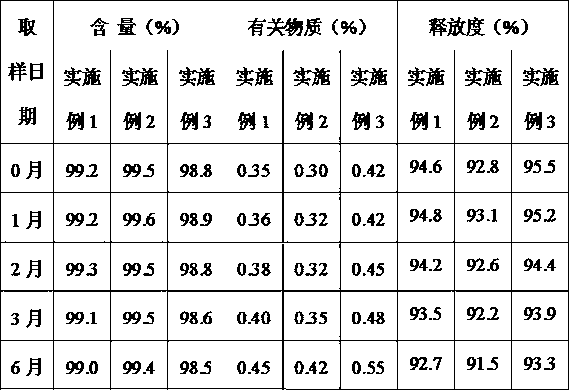
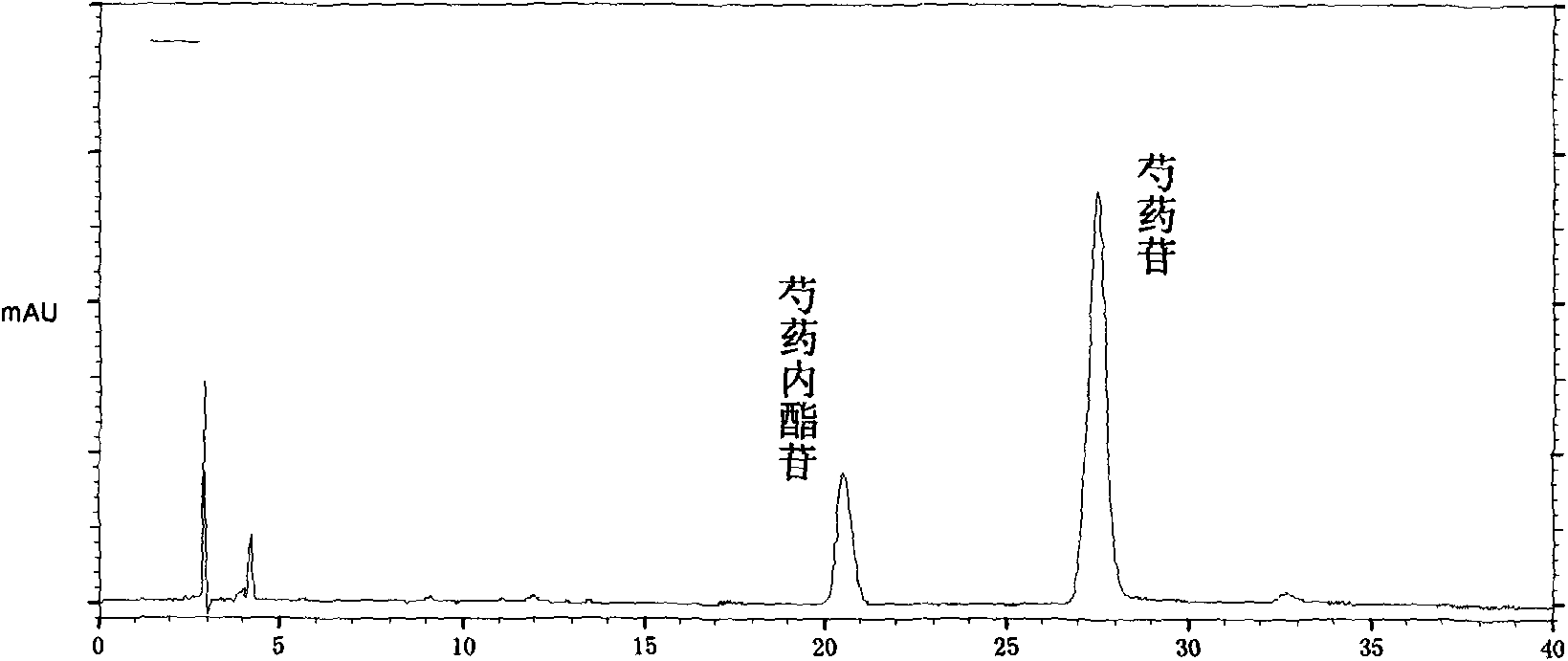
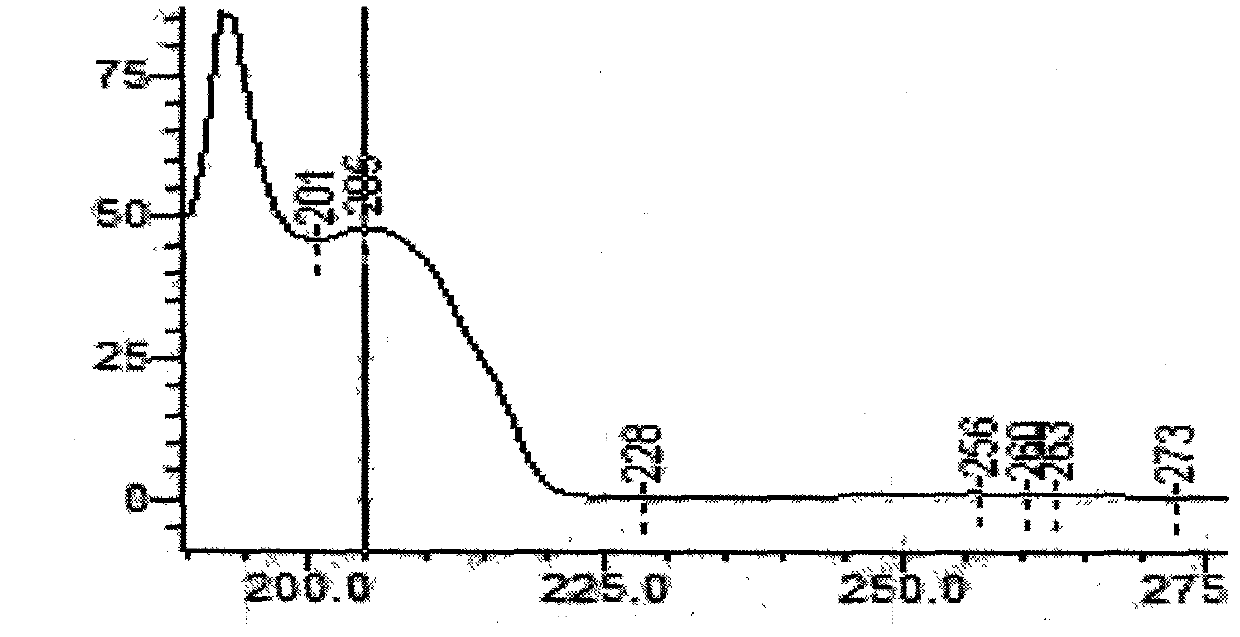
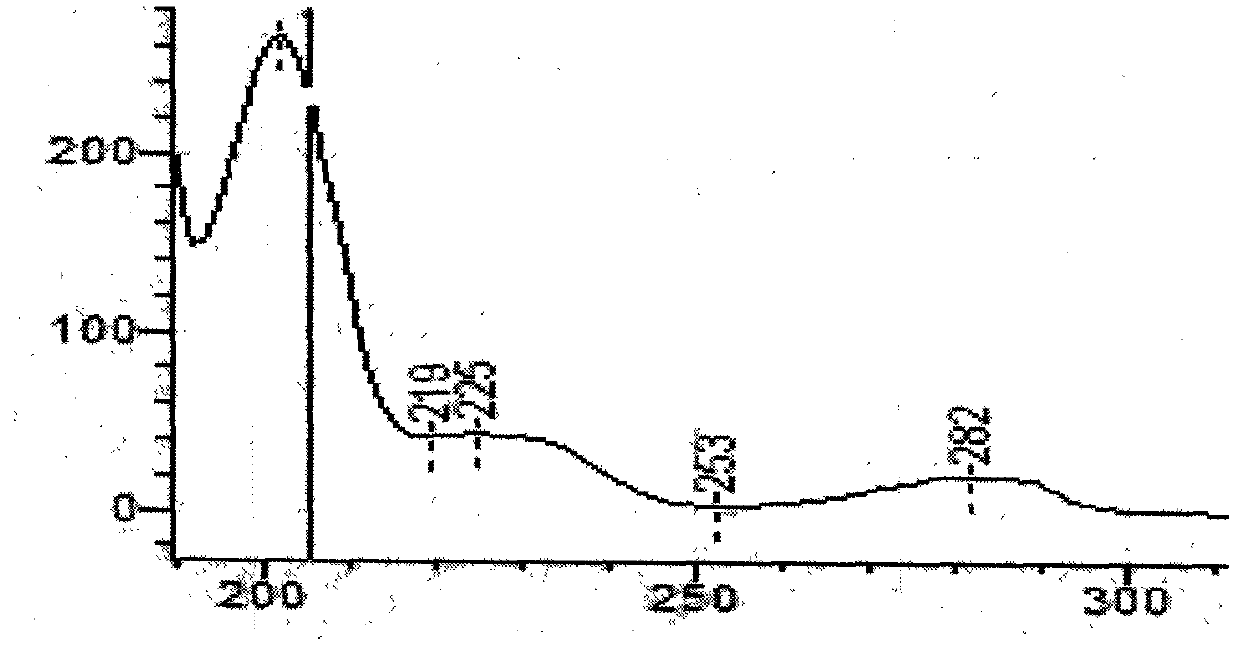
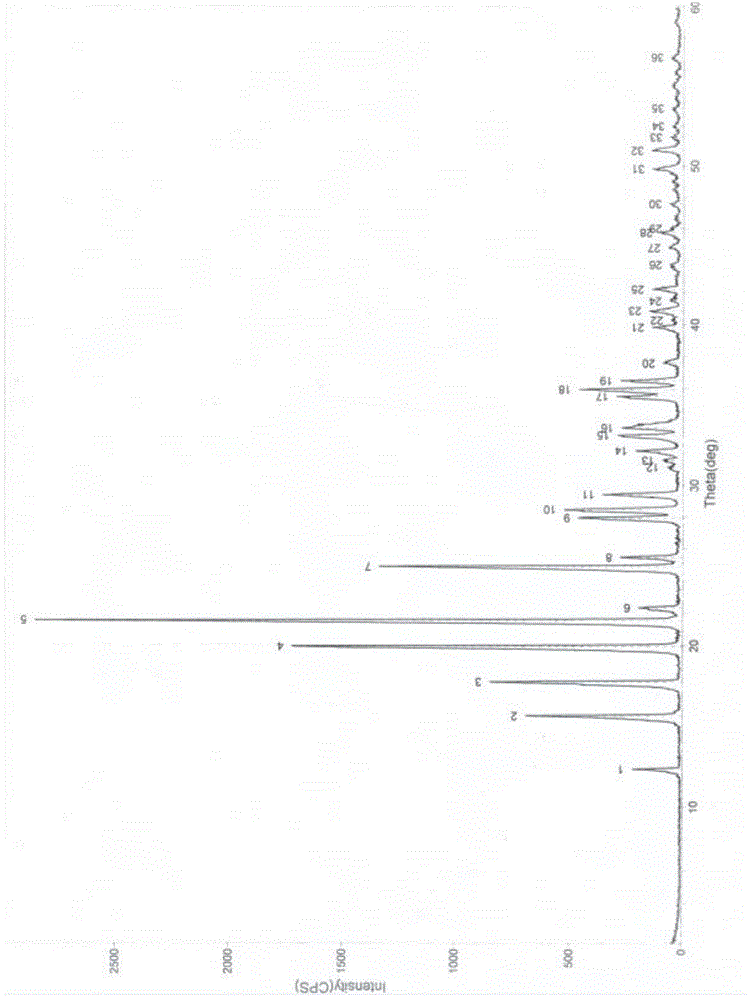
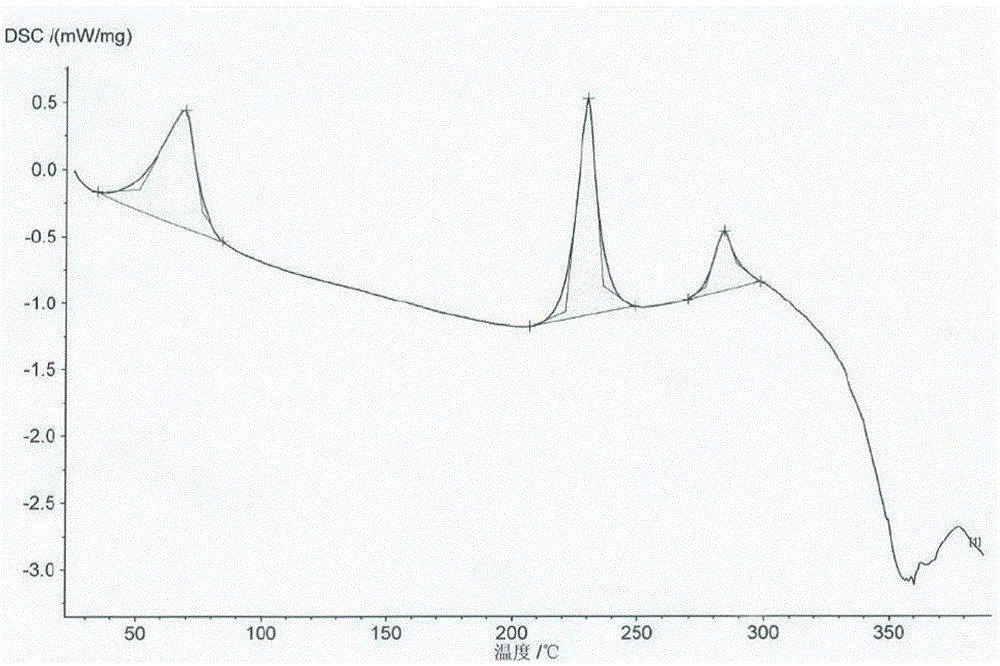
Patents
Literature
175results about How to "Guarantee safe and effective" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
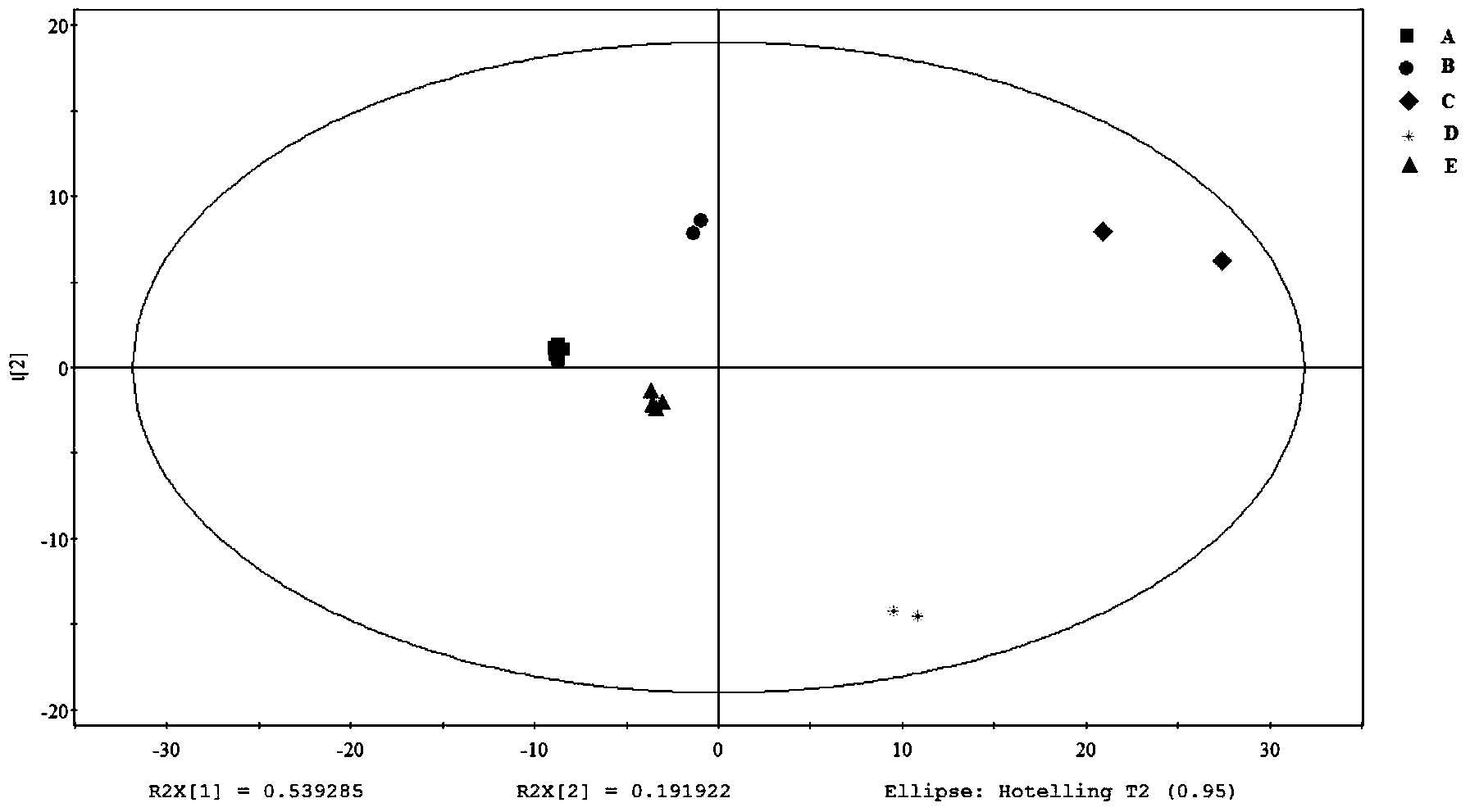
Method for evaluating quality of Chinese patent medicament by using metabonomics
InactiveCN102100706AGuarantee safe and effectiveThe internal quality is stable and controllableComponent separationPlant ingredientsEndogenous metabolismDisease
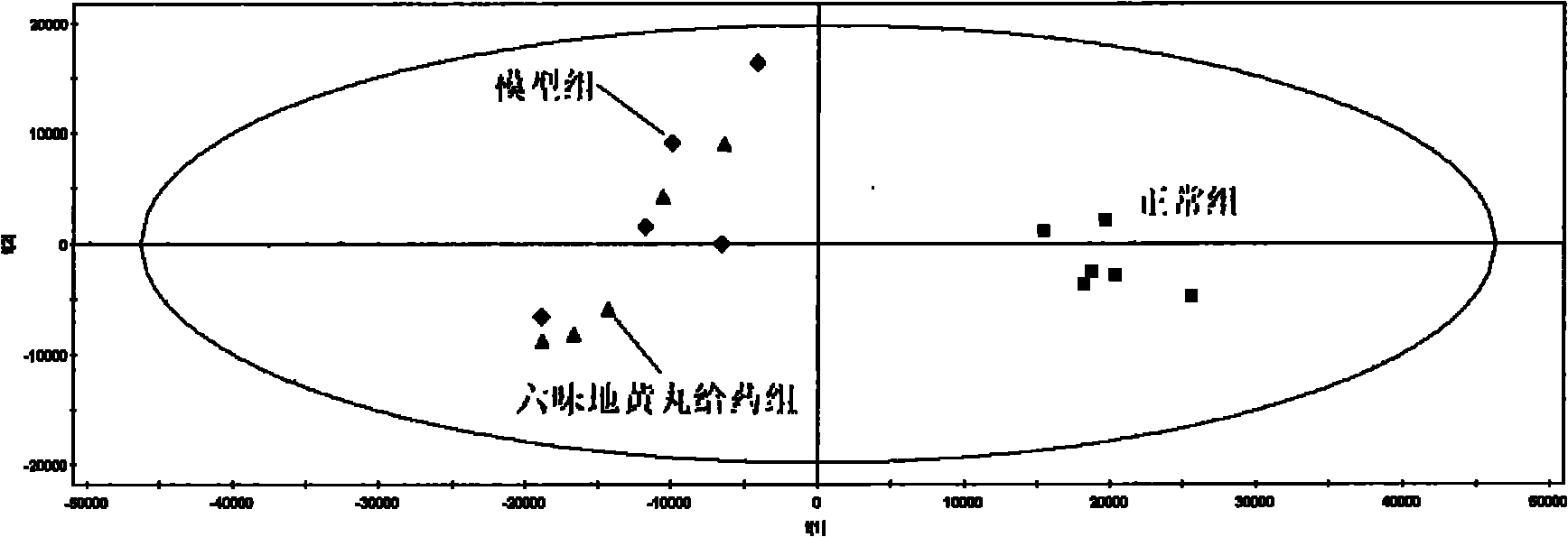
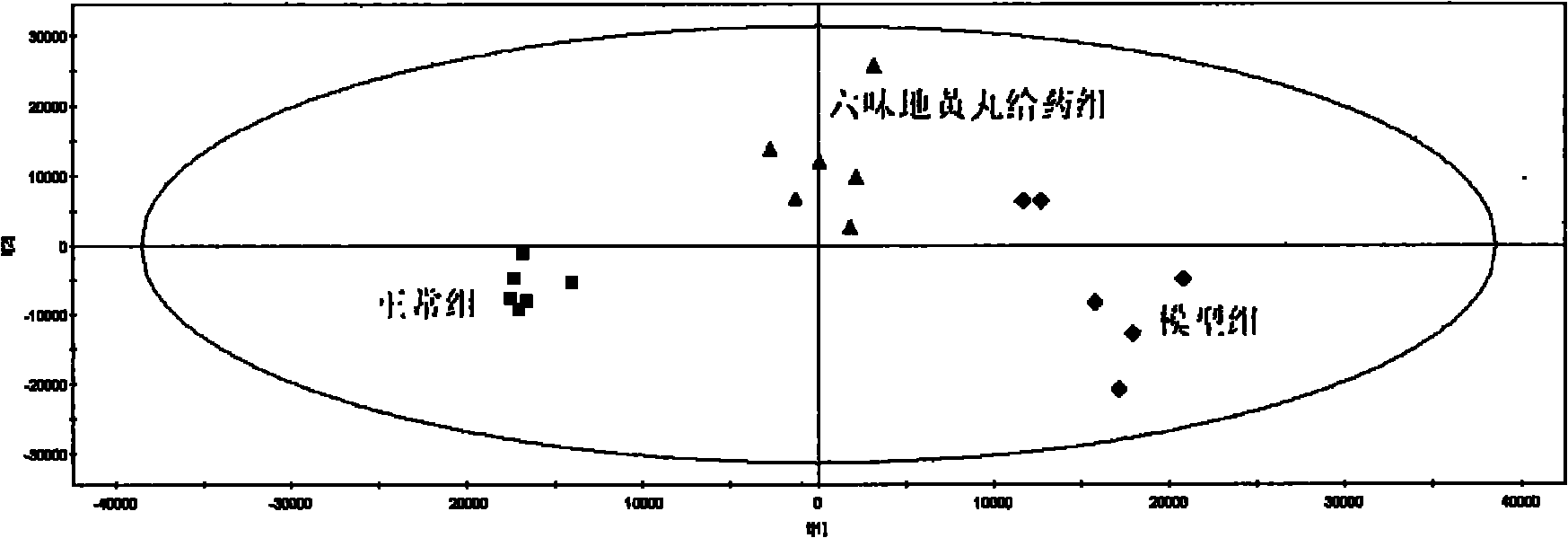
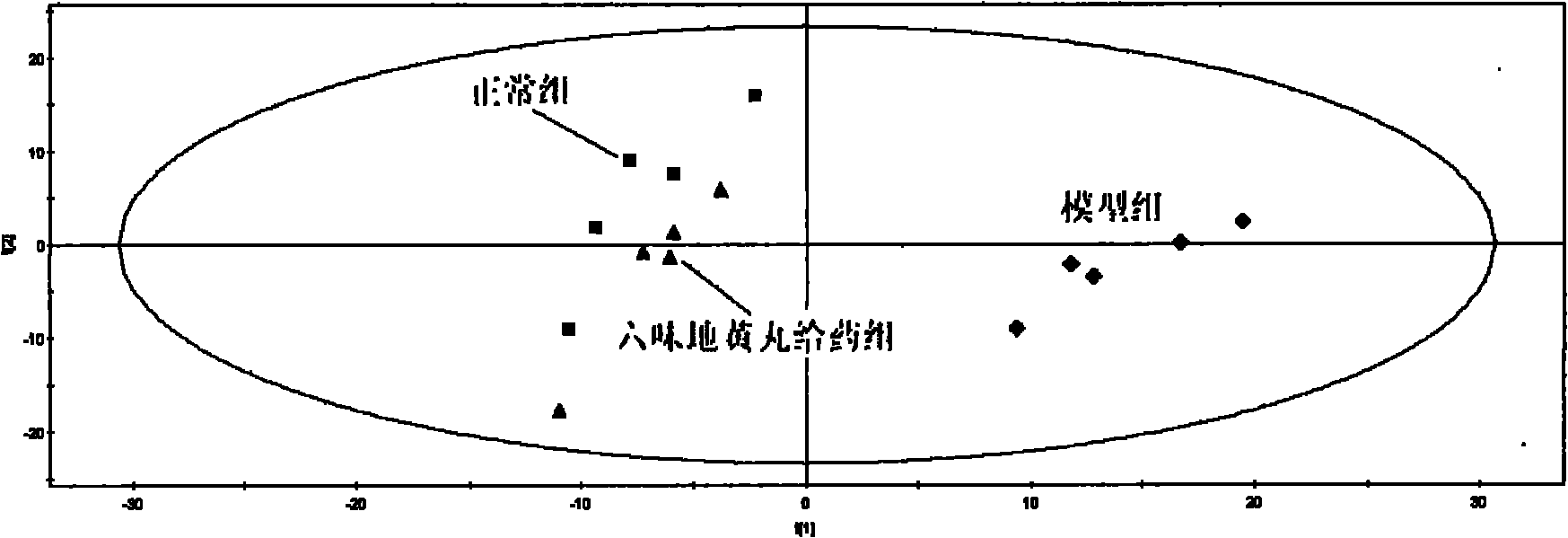
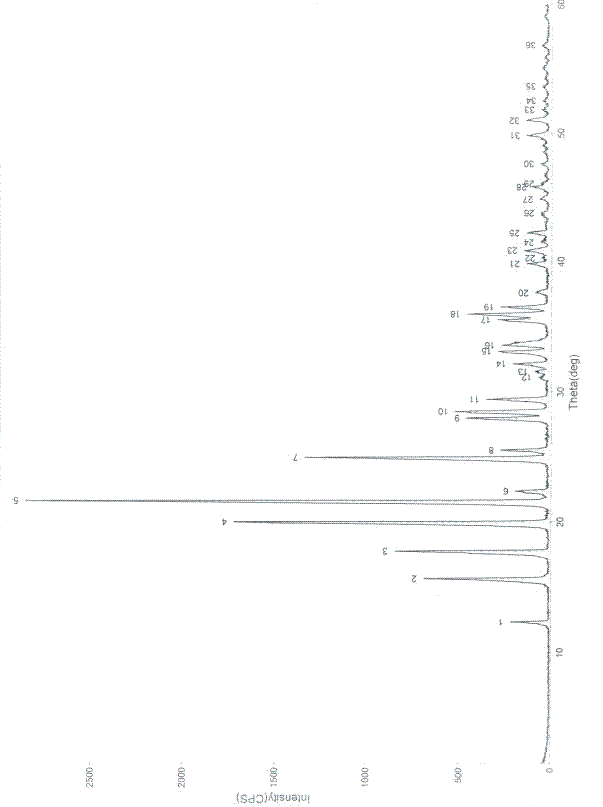
The invention provides a method for evaluating the quality of a Chinese patent medicament by using metabonomics. The method comprises the following steps: 1) according to the indication of traditional Chinese medicines, establishing a corresponding animal model and screening; 2) taking various of samples of the traditional Chinese medicines, and intervening a disease model animal by a corresponding route of administration; 3) collecting a corresponding biological sample and acquiring the metabolism finger-print of the sample; 4) carrying out pattern recognition on the metabolism fingerprints of a normal group and a disease group to obtain a metabolism biomarker group; 5) calculating the similarity of the metabolism biomarker group and the normal group in different administration groups, or carrying out PLS (partial least squares) regression analysis on differential endogenous metabolites, and observing the influence of medicament treatment on the endogenous metabolites; and 6) according to the similarity result or the result of the PLS regression analysis, evaluating the medicaments, wherein the more normal the control group trends to be, the better efficacy of the medicament is.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
Oxiracetam capsule and preparation method thereof
ActiveCN103494790AHigh purityLow impurity contentOrganic active ingredientsNervous disorderMedicinal chemistryImpurity
The invention relates to an oral capsule preparation of oxiracetam and a preparation method thereof. The oxiracetam capsule contains 80.0-85.5% by weight of oxiracetam, 9.7-15.2% by weight of xylitol and 4.8% by weight of an emollient, wherein the oxiracetam is in a crystal form. The oxiracetam in the crystal form is a good crystal with excellent mobility. Compared with the crystal form provided in the prior art, the oxiracetam in the crystal form has higher purity, less impurity, better quality stabilizer and better crystal form stability. The quality stability of the disclosed oxiracetam capsule is improved remarkably, the preparation process is simple, and the production cost is lowered.
Owner:CSPC OUYI PHARM CO LTD
Esomeprazole magnesium contained enteric-coated tablet and preparation method thereof
ActiveCN102940611AHigh safety complianceImprove stabilityOrganic active ingredientsDigestive systemCoated tabletsInsulation layer
The invention provides an esomeprazole magnesium contained enteric-coated tablet and a preparation method thereof. The enteric coated tablet is composed of an inner tablet core layer which uses esomeprazole as an active ingredient, an intermediate insulation layer and an enteric coating protection layer. According to the esomeprazole magnesium contained enteric-coated tablet, the conditions that basic remedies are instable and prone to be oxidized and decomposed are overcome, the prepared enteric-coated tablet is even in coating, compact in coating layer, stable and reliable in quality and capable of meeting large-scale production requirements of enterprises at the present stage and has good market development prospects, the releasing rate can reach over 90%, the bioavailability is obviously improved, and safe and effective drug using is guaranteed.
Owner:KAMP PHARMA
Quality control method of total glycosides single preparation of white paeony roots
ActiveCN102138985AQuality assuranceGuaranteed curative effectComponent separationPlant ingredientsClinical efficacyCurative effect
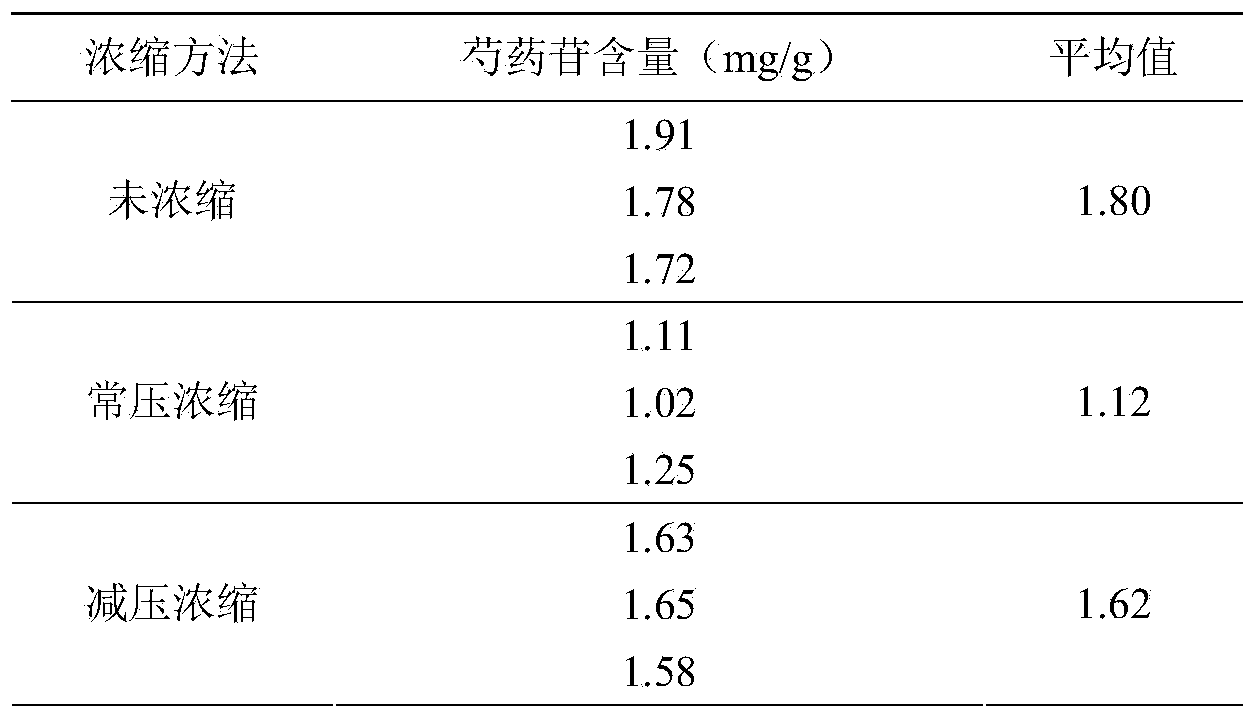
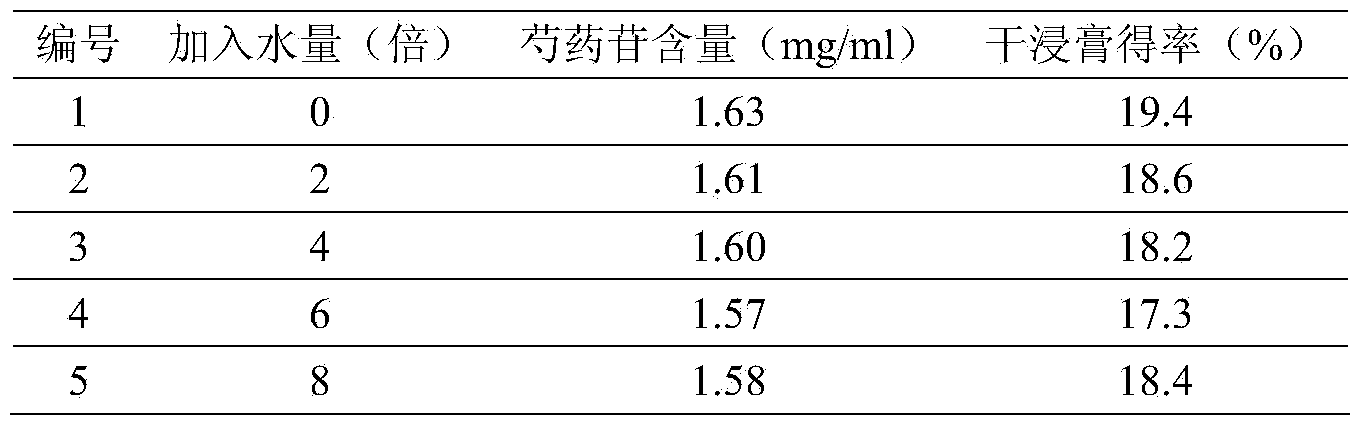
The invention provides a quality control method of a preparation of white paeony roots. The method comprises the following steps of: establishing a synchronous content measuring method of white paeony root herbs, white paeony root total glycosides and paeoniflorin and albiflorin in the white paeony root preparation by adopting the same liquid-phase chromatography condition; and accurately measuring the contents of the paeoniflorin and the albiflorin. The method is simple to preprocess a sample, keeps complete characteristic components and provides a stable sample solution and has higher accuracy, favorable reproduction and a certain specificity; characteristic peaks in the obtained fingerprint map have favorable separation effect, the fingerprint maps of the white paeony root herbs, the white paeony root total glycosides and the preparation have favorable relativity; the standard fingerprint map of the white paeony root herbs is established at a new angle by utilizing the relativity research of the fingerprint maps, can be used for identifying the qualities of the white paeony root herbs and improving the controllability of the production process of the white paeony root preparation and is favorable to ensuring the quality stability and the clinical curative effect of the white paeony root preparation.
Owner:NINGBO LIWAH PHARM CO LTD
System and method thereof for quickly detecting chemical components added in medicine, health-care food and food
ActiveCN101592613AAccurate detectionLow costMaterial analysis by observing effect on chemical indicatorComponent separationChemical compositionScreening method
The invention discloses a system and a method for quickly detecting chemical components added in medicine, health-care food and food. The detection system comprises a database of the added chemical components, a primary screening device or a kit, a confirmation instrument and an LC-MS (liquid chromatography-mass spectrography) instrument. The detection method comprises the following steps: establishing a database of the added chemical components, primarily screening the database of the added chemical components by using a quick screening method, further confirming the screening result, and finally arbitrating the screening result by using an LC-MS method. The system and the method realize effective supervision for illegally-added chemical components in Chinese patent medicine, health-care food and food, solve the difficult problem of supervision resource deficiency, comprehensively promote the capability and level of illegal addition detection for the Chinese patent medicament, health-care food and food, provide powerful technical support for legal supervision, form great deterrent for lawbreakers who sell fake medicament, and have quite important significance for ensuring the safety and the validity of diet medicament of people and protecting the fame of Chinese medicaments.
Owner:广东省药品检验所
Stomach detention sustained and controlled release medicament releasing system and preparation method
ActiveCN101371822AEasy to swallowImprove complianceSurface/boundary effectCarbohydrate active ingredientsControlled releaseOrganic acid
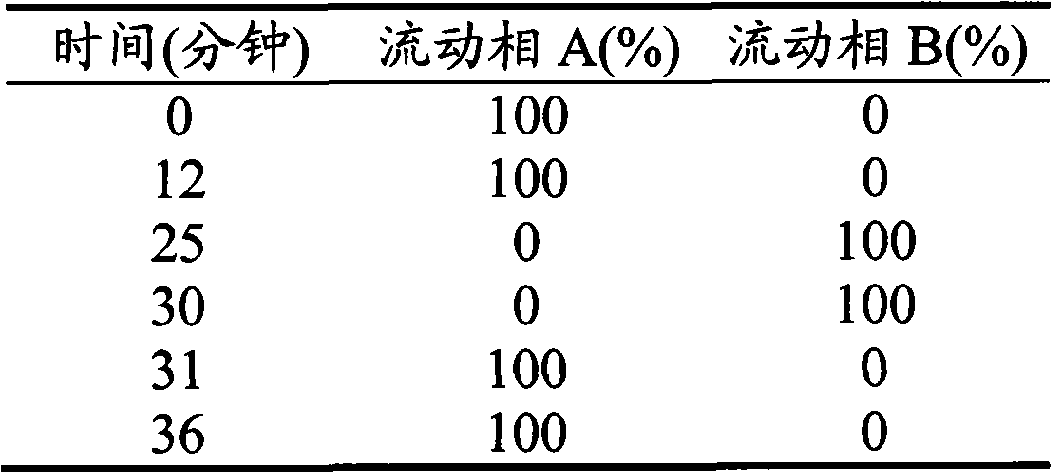
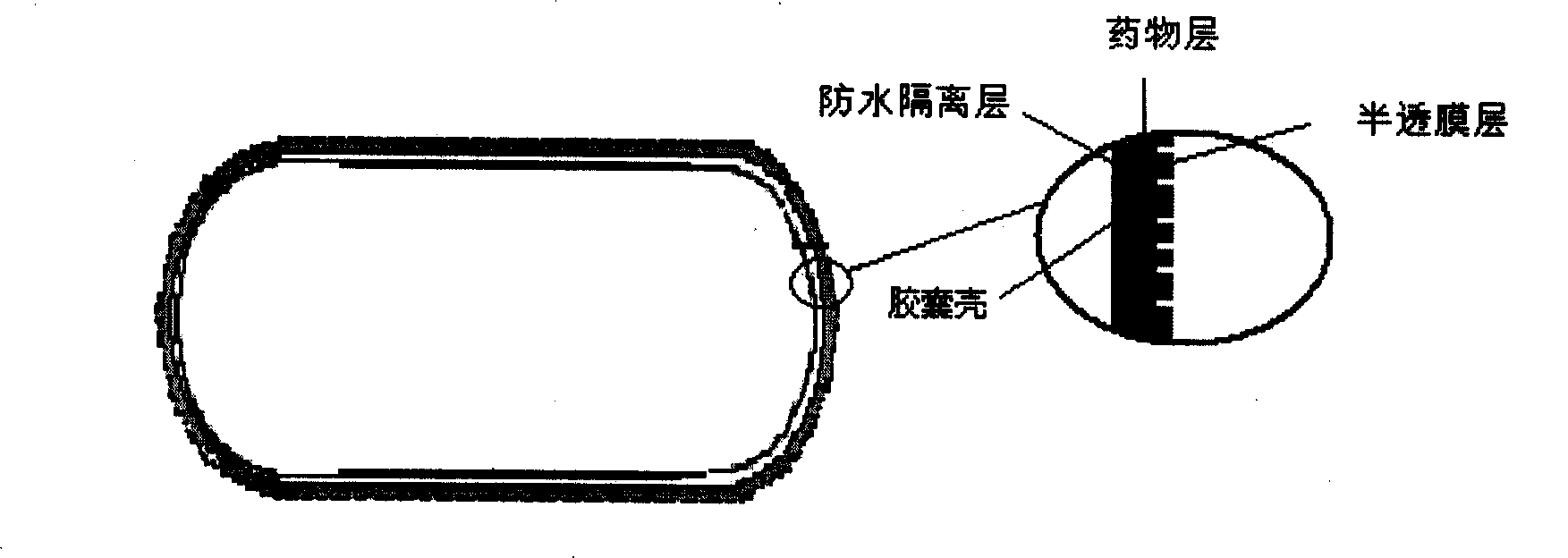
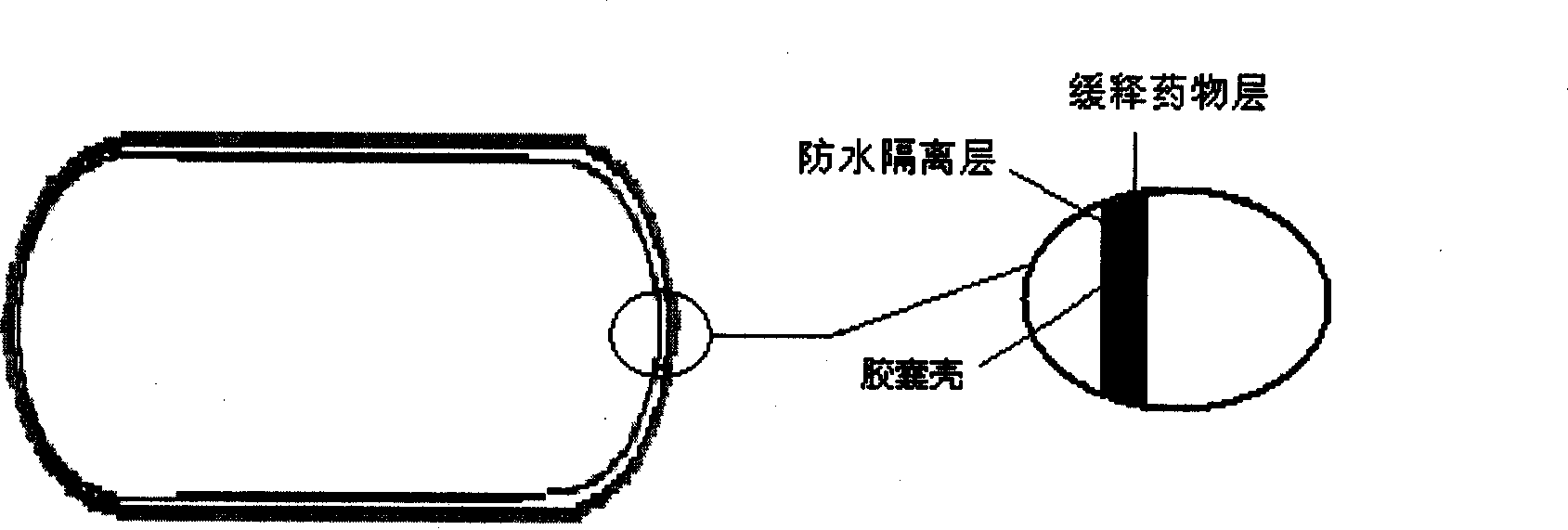
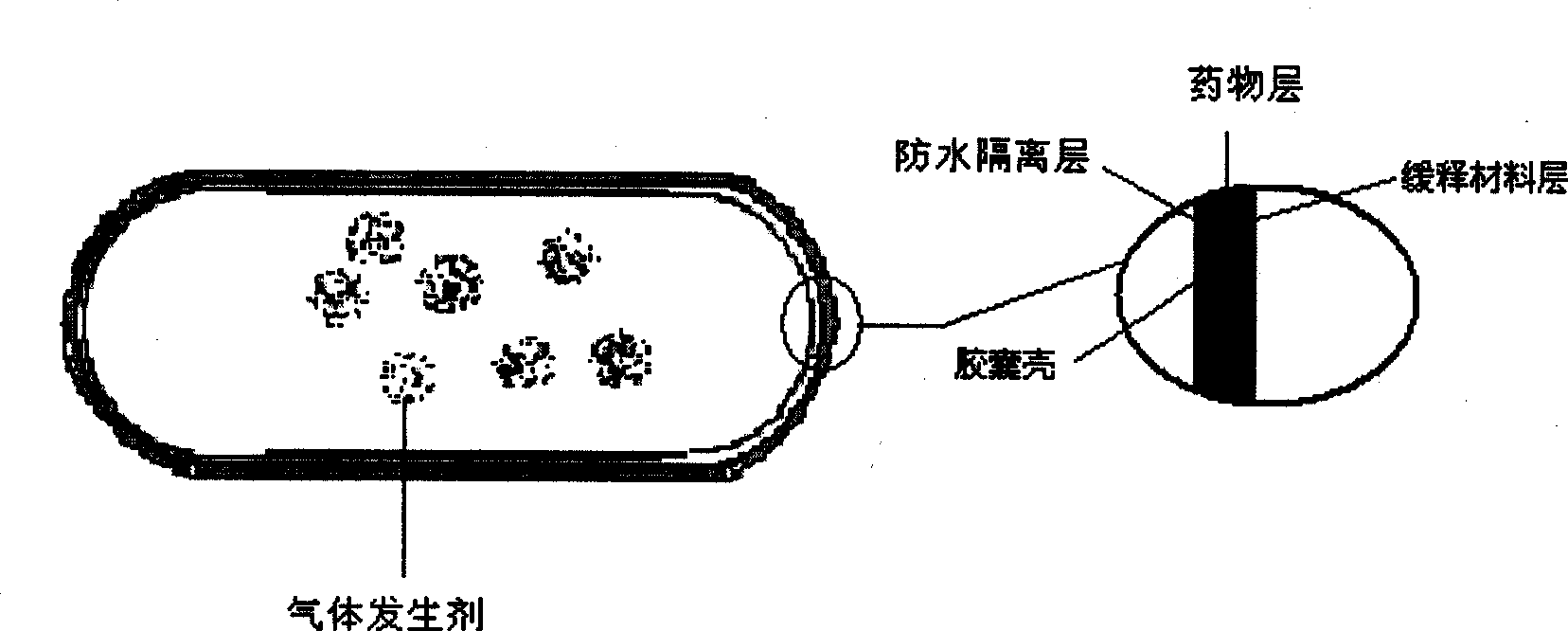
The invention provides a new air sac type gastric retention controlled-release medicine release system, including (1) an air sac which is formed by wrapping high molecular film-forming materials and hydrophobic materials outside a hollow sac; (2) a medicine containing layer which is composed of medicines and pharmaceutically acceptable excipient; the medicine containing layer covers the outside of the air sac and includes a medicine controlled release layer or a medicine slow release layer. If necessary, a quick release layer, which is composed of medicines and excipient and covers the outside of the controlled release layer or the slow release layer, can also be included. The air sac can also be internally filled with a certain amount of gas generant which includes carbonate and pharmaceutically acceptable organic acid. The average density of the whole formulation can be generally controlled below 0.6 / cm<3> and is obviously superior to the present gastro-floating formulation on sale, and the floating time in the stomach is rather longer than a common gastro-floating formulation.
Owner:北京天衡药物研究院有限公司
Effector cell combination for preventing and treating tumors and preparation method thereof
ActiveCN102641298ABreaking the rules of treatmentExpand the range of donorsMammal material medical ingredientsSkeletal/connective tissue cellsClinical efficacyMedicine
The invention relates to an effector cell combination for preventing and treating tumors. The effector cell combination comprises CIK (cytokine induced killer) cells and MSC (Mesenchymal Stem Cells) which are sequentially injected into a human body in the form of a cell suspension and are extracted and prepared from peripheral blood of exogenous healthy people in non-tumor patients. The invention also discloses a preparation method of the effector cell combination, and the method comprises the following steps of: blood mononuclear cell collection, CIK cell culture and MSC culture. According to the scheme disclosed by the invention, the CIK cells and the MSC are extracted and prepared from the peripheral blood of the exogenous healthy people in the non-tumor patients, thereby breaking the routine treatment of auto-CIK cells, improving the clinical effects of tumor biotherapy and especially the clinical effects of CIK cell therapy, and solving the problems in graft-versus-host reaction (GVHD).
Owner:祁岩超 +1
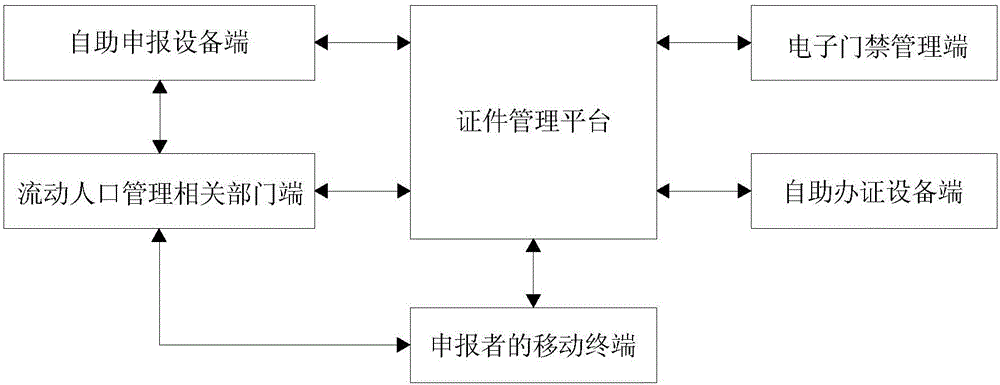
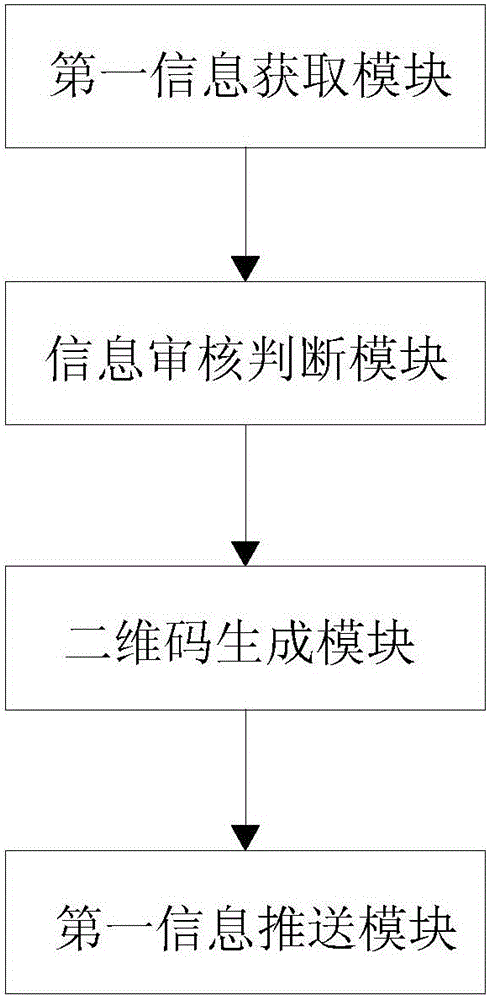
Certificate declaration management system and certificate declaration management method
ActiveCN106097223AEasy to useGuarantee safe and effectiveData processing applicationsIndividual entry/exit registersElectronic accessElectronic identification
The invention discloses a certificate declaration management system and a certificate declaration management method. The system comprises a certificate management platform, a declarant's mobile terminal, an electronic access management side, a self-service declaration equipment side, a self-service certificate handling equipment side, and a floating population management relevant department side. The method comprises the steps of getting declarant information from the floating population management relevant department side, judging whether the declarant information is approved by the floating population management relevant department side, generating a QR code according to the approved declarant information when judging that the declarant information is approved, and pushing the generated QR code and the declarant information to the declarant's mobile terminal, the electronic access management side, the self-service declaration equipment side, the self-service certificate handling equipment side and the floating population management relevant department side. According to the invention, a QR code used as the unique electronic identifier of a declarant can be generated according to the approved declarant information, use of certificates on the Internet is facilitated, and convenience in management and use is increased.
Owner:广州市华标科技发展有限公司
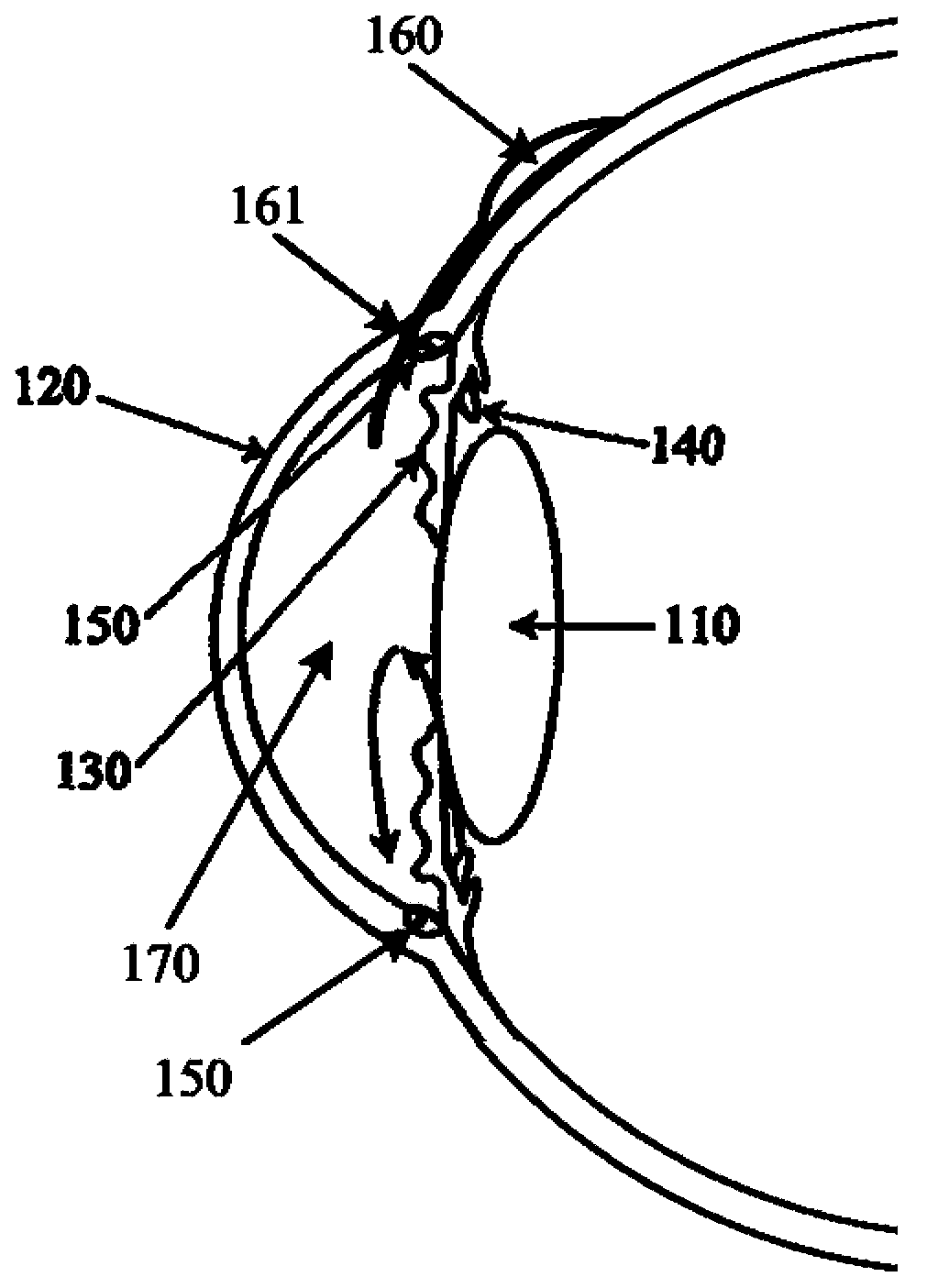
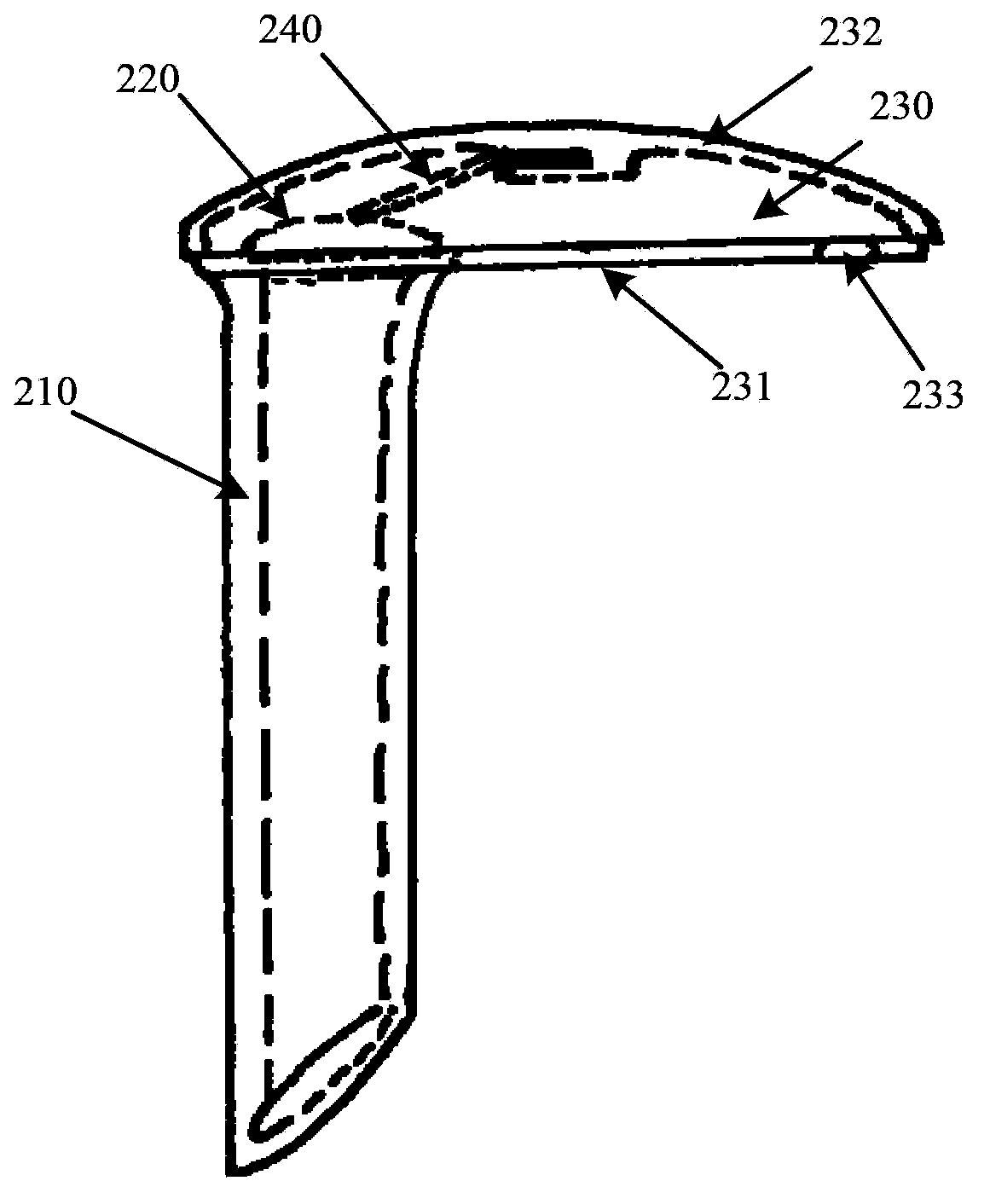
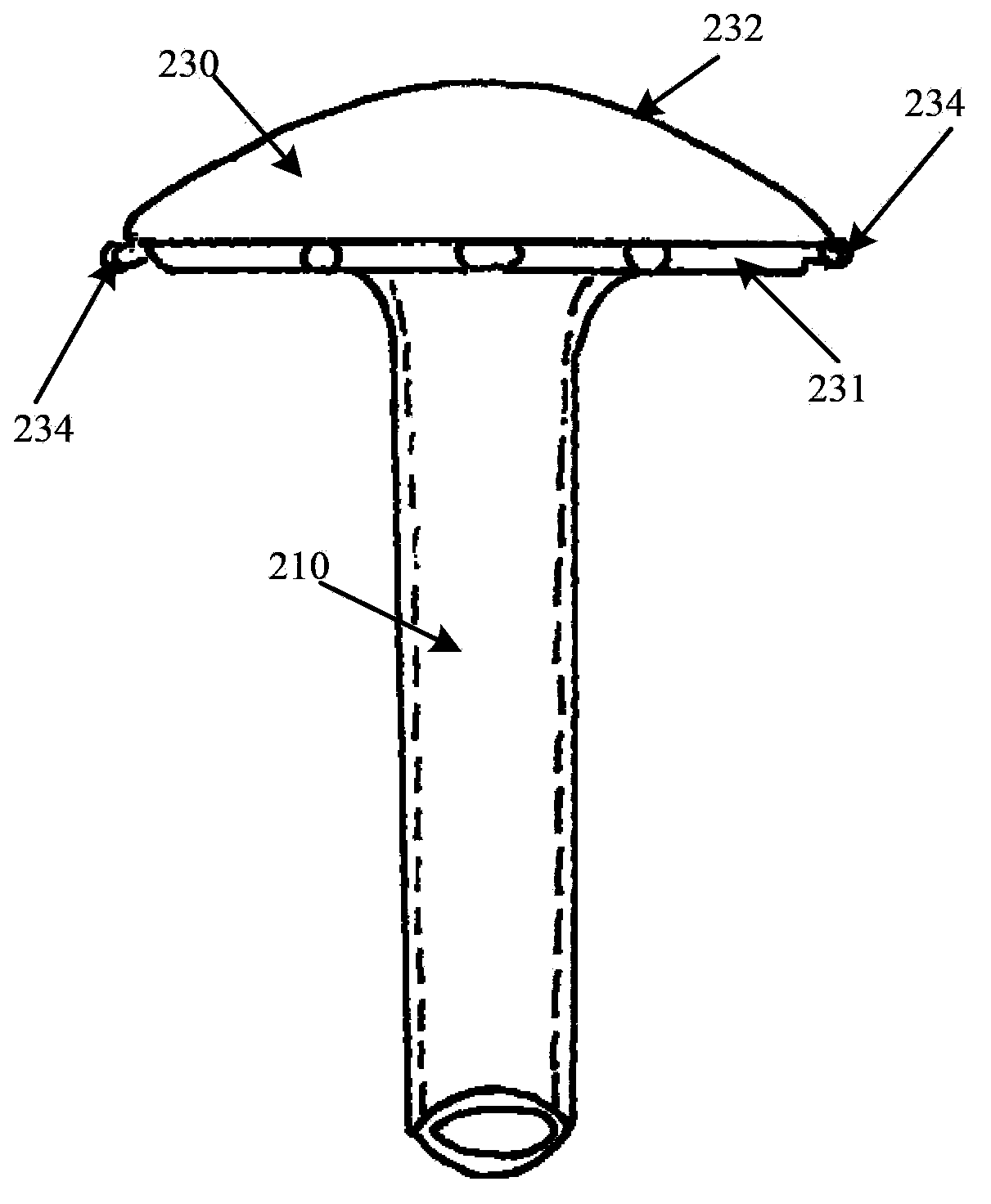
Rear stress-type glaucoma diversion valve
The invention provides a rear stress-type glaucoma diversion valve, relates to a method and a device for ophthalmosurgery and particularly relates to a rear glaucoma diversion embedding device for controlling intraocular pressure. The rear stress-type glaucoma diversion valve comprises a hollow diversion pipeline and a valve cover used for controlling the opening and closing of the diversion pipeline, wherein the outlet end of the diversion pipeline is provided with an openable and closable housing which consists of a diversion plate and a housing cover; the outlet end of the diversion pipeline is embedded in the diversion plate; the inlet end of the diversion pipeline is vertical to the lower surface of the diversion plate and downwards stretches out from the lower surface of the diversion plate; the diversion pipeline can enter from a pars plana corporis ciliaris by virtue of a 23G or 25G stab knife; the part, corresponding to the valve cover, of the inner side of the housing cover is provided with a spring pressurizing device; when the housing cover is closed, the spring pressurizing device applies a pressure to the valve cover. The spring pressurizing device is used for controlling the intraocular pressure within a normal and safe target intraocular pressure range; the inner cavity of the diversion pipeline is large in diameter, short in length and low in blockage possibility, thereby being conductive to ensuring the safety and effectiveness of the diversion valve and realizing long-acting control over the intraocular pressure.
Owner:SHANGHAI TONGJI HOSPITAL
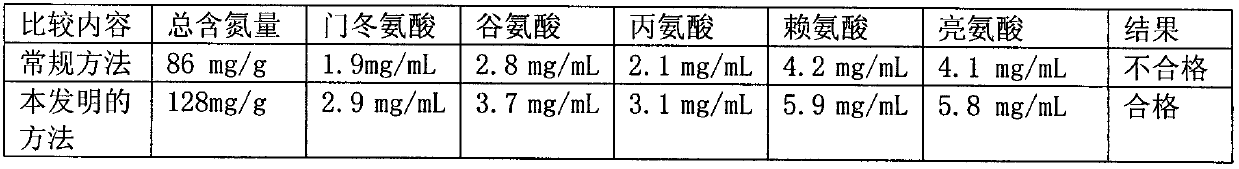
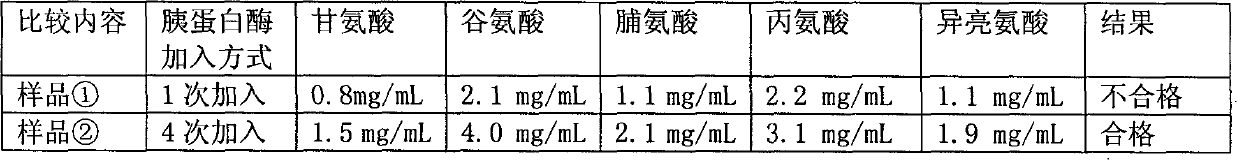
Denatured protein powder and brain protein hydrolyzate prepared from same
ActiveCN102718857AHigh in peptidesQuality assuranceAntibacterial agentsNervous disorderHydrolysateUltrafiltration
The invention relates to a denatured protein powder and a brain protein hydrolyzate prepared from the same. The total nitrogen content of the denatured protein powder is more than 120 mg / g; and the denatured protein powder is prepared by adding fresh lysozyme into a pig brain, homogenating, heating, and defatting with acetone. The denatured protein powder, as a raw material, is used for preparingthe brain protein hydrolyzate; the contents of amino acids in the prepared brain protein hydrolyzate meet national standards of brain protein hydrolyzate injections; and the content of small moleculepeptides with the molecular weight of less than 10000 Da is 25-35% of the brain protein hydrolyzate. The preparation method of the brain protein hydrolyzate mainly comprises the following steps: pre-treating, homogenizing, defatting with the acetone, performing dual enzymatic hydrolysis, centrifugating, refining by column chromatography, and performing ultrafiltration treatment. The invention discloses key technical points for controlling the total nitrogen index of the protein powder and the content of the small molecule peptides for the first time; the obtained brain protein hydrolyzate is a biologically active substance which is really extracted from the pig brain, without adding active components artificially, thus ensuring the efficacy and the safety of the brain protein hydrolyzate;and animal pharmacodynamic experiments show that the brain protein hydrolyzate is more effective than conventional products.
Owner:HEBEI ZHITONG BIOLOGICAL PHARMA
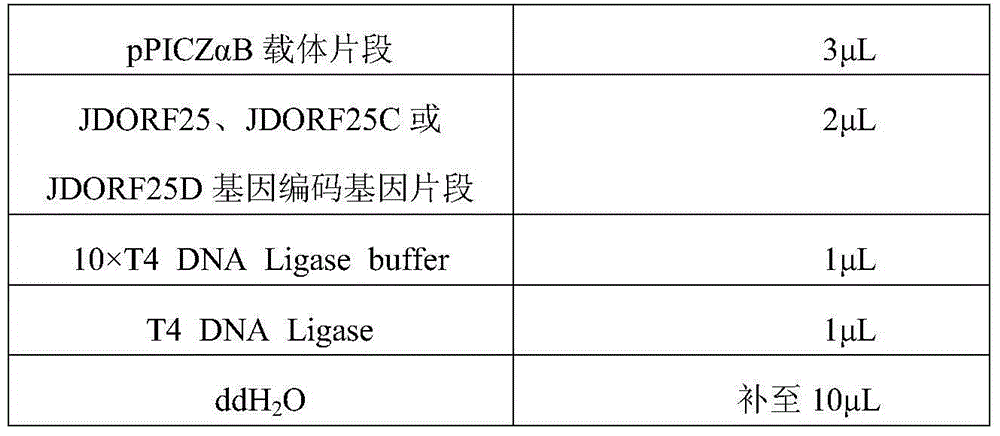
Crucian herpesvirus disease JDORF25 vaccine as well as preparation method and application thereof
ActiveCN104593388ASimple purification processReduce manufacturing costFungiVirus peptidesAntigenPichia pastoris
The invention discloses a crucian herpesvirus disease JDORF25 vaccine as well as a preparation method and an application thereof. The crucian herpesvirus disease vaccine is recombinant protein truncated, optimized and translated by crucian herpesvirus type II immuno-related ORF25 gene, wherein the sequence is shown in SEQ ID NO.2; the gene segment is synthesized, transferred to a yeast expression vector and screened by highly copying and cloning to finally obtain a high-expression recombinant protein pichia pastoris Km71 / CyHV-2-25 Pichia pastoris Km71 / CyHV-2-25, CCTCC NO: M2014570. The protein fermented by the yeast has the characteristics of high activity and high yield. The passive immunity test defines that the antigen protein can effectively prevent crucian herpesvirus disease and can also be mixed with adjuvants for highly effectively preventing crucian herpesvirus disease.
Owner:YANGTZE RIVER FISHERIES RES INST CHINESE ACAD OF FISHERY SCI
Crucian herpes virus disease compound vaccine preparation, preparation method and application
ActiveCN104524564AImprove securityNo genetic recombination issuesFungiVirus peptidesChannel catfish herpesvirusYeast
The invention discloses a crucian herpes virus disease compound vaccine preparation, a preparation method and application. An applicant provides three strains of recombinant saccharomycetes, including pichia pastoris Km71 / CyHV-2-25, CCTCC NO:M2014570, pichia pastoris Km71 / CyHV-2-25C, CCTCC NO:M2014571 and pichia pastoris Km71 / CyHV-2-25D, CCTCC NO:M2014572. Proteins produced by utilizing the saccharomycetes through fermentation have the characteristics of high activity and yield; and immunity protective experiments prove that the crucian herpes virus disease compound vaccine preparation prepared by mixing the three proteins is capable of effectively preventing crucian herpes virus diseases.
Owner:YANGTZE RIVER FISHERIES RES INST CHINESE ACAD OF FISHERY SCI
Quality inspection method of traditional Chinese medicine composition twenty-five-ingredient lung disease preparation
ActiveCN102749401AGuarantee safe and effectiveAdd inspection itemsComponent separationDiseaseCholic acid
The invention discloses a quality inspection method of a traditional Chinese medicine composition twenty-five-ingredient lung disease capsule and a preparation thereof. On the basis of the primary standard, high-performance liquid chromatography (HPLC) is additionally used to perform limit test to aconitine in the twenty-five-ingredient lung disease capsule, and simultaneously the high-performance liquid chromatography (HPLC) is adopted to perform quantitative detection to active ingredients of hydroxysafflor yellow A, swertiamarin and cholic acid, as well as differentiation to fructus terminaliae billericae, sandalwood, kummel, Baxiaga and licorice root. The method improves the product quality, and ensures that the preparation can guarantee the drug use safety and the effectiveness while curing diseases.
Owner:JINHE TIBETAN MEDICINE
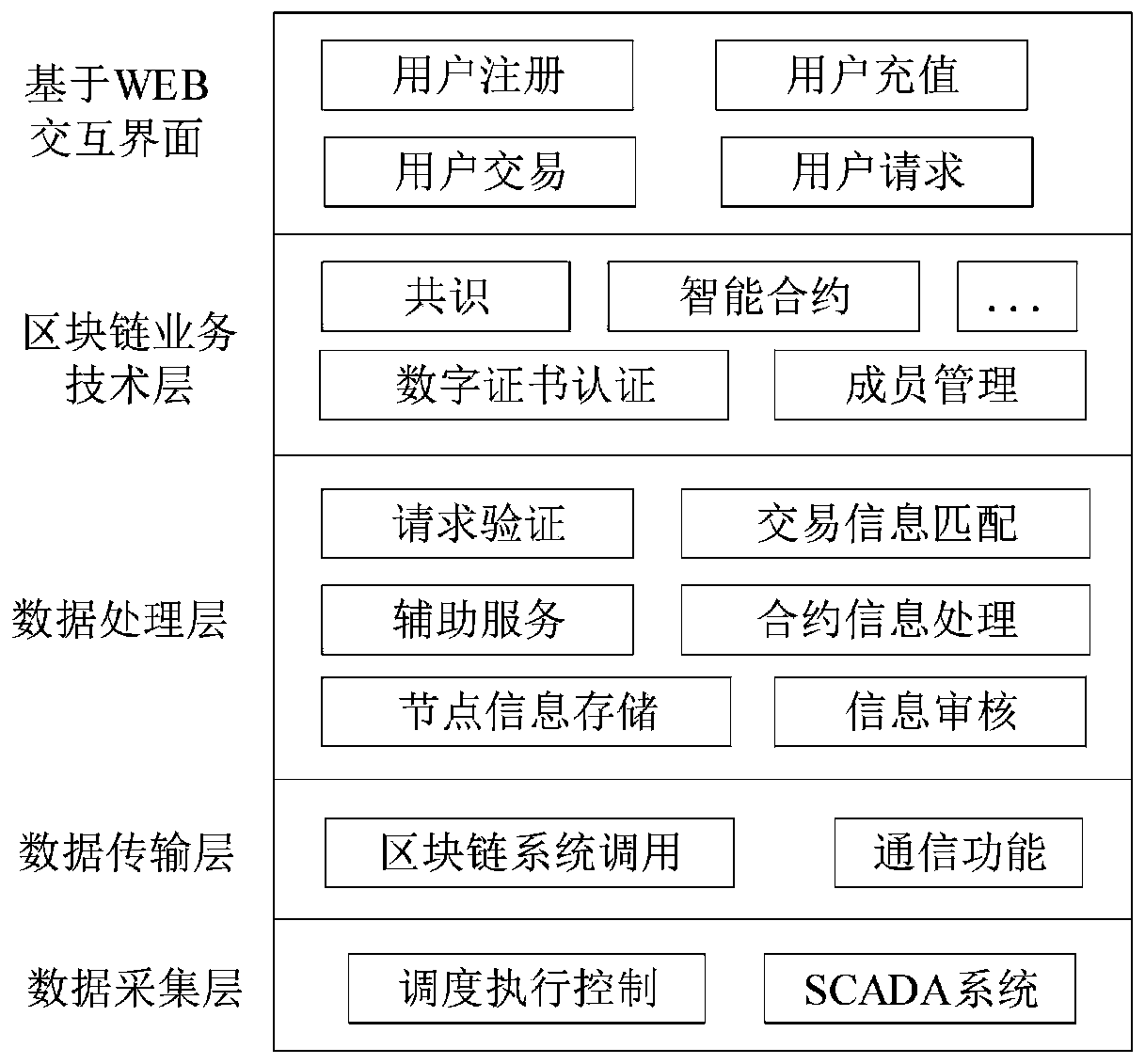
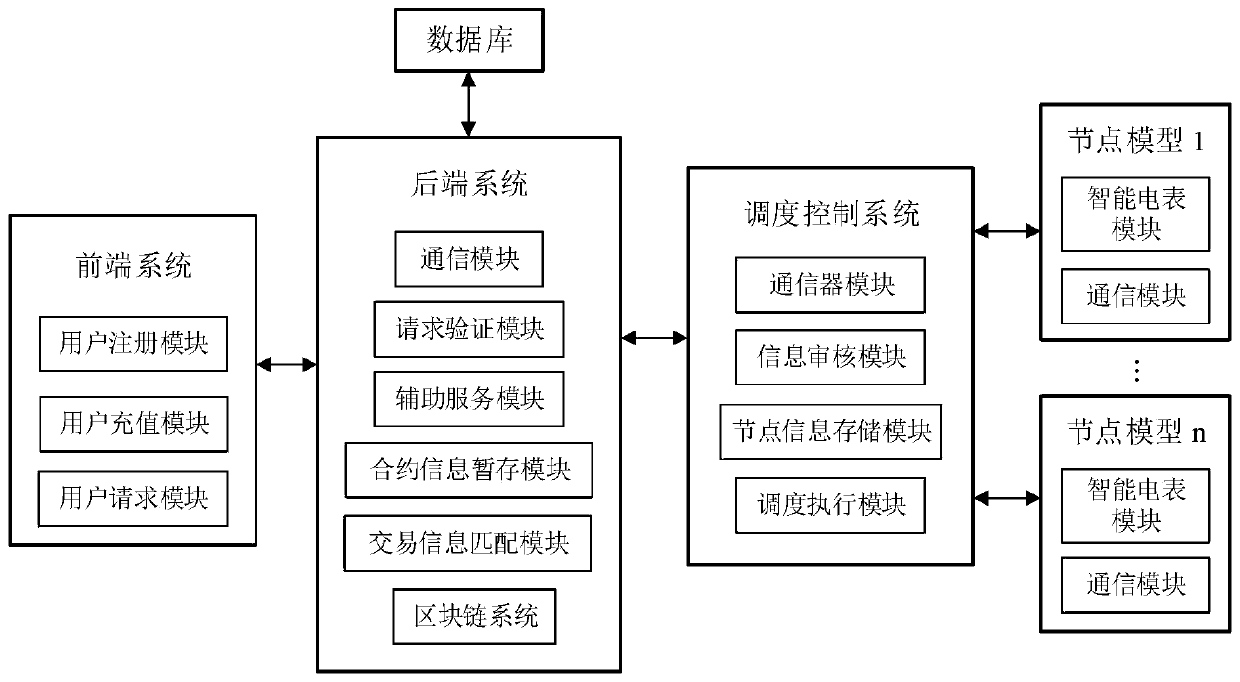
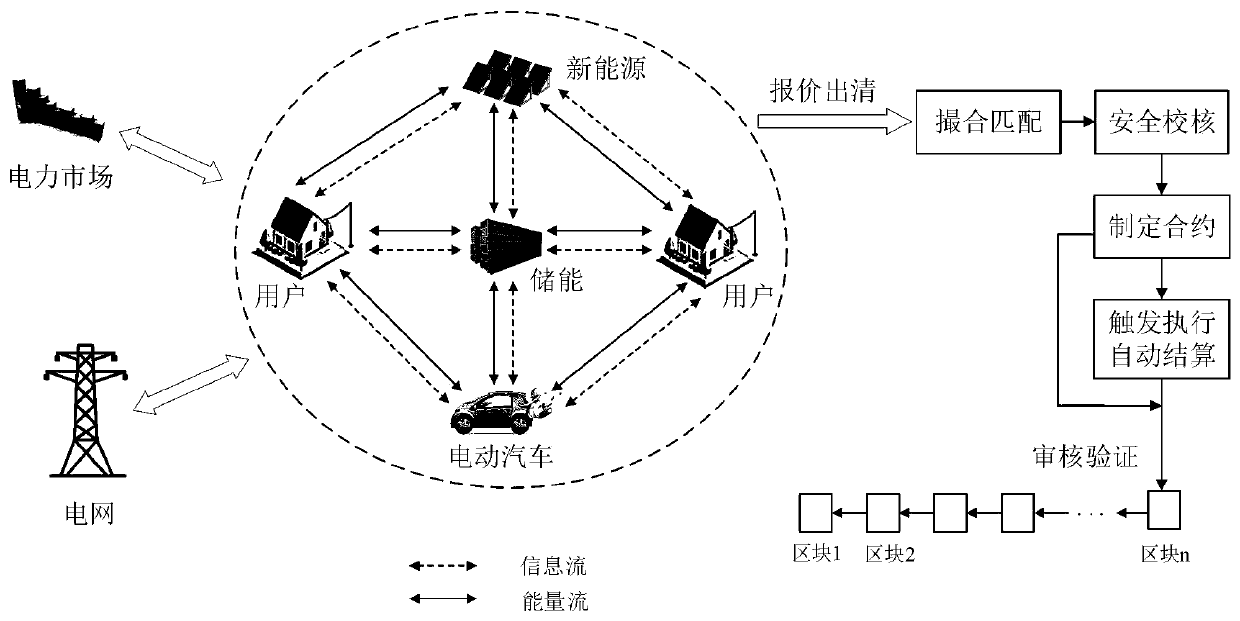
Virtual power plant regulation and control platform based on blockchain and operation method
PendingCN111563786AMeet diversityRealize free interactionBuying/selling/leasing transactionsInformation technology support systemBlockchainVirtual power plant
The invention discloses a virtual power plant regulation and control platform based on a blockchain and an operation method. The platform comprises a front-end system, a rear-end system, a blockchainsystem and a scheduling control system, wherein the front-end system is used for providing an interactive interface to complete interaction with a user and completing transaction processing by callingthe rear-end system; the back-end system is used for finishing core transaction processing comprising logic of related business processing; the blockchain system is used for storing data informationgenerated in the system operation process and automatically executing agreed transaction content; and the scheduling control system is used for determining whether a node has the capability of executing the request and receiving a decision from the blockchain system. The method has the beneficial effects that the trust problem of decentralization of the virtual power plant is solved, and credibleand reliable interconnection among the main bodies is realized; the authenticity and effectiveness of internal information are ensured; intelligentization of transaction is realized, and task execution efficiency is improved.
Owner:INST OF ELECTRICAL ENG CHINESE ACAD OF SCI
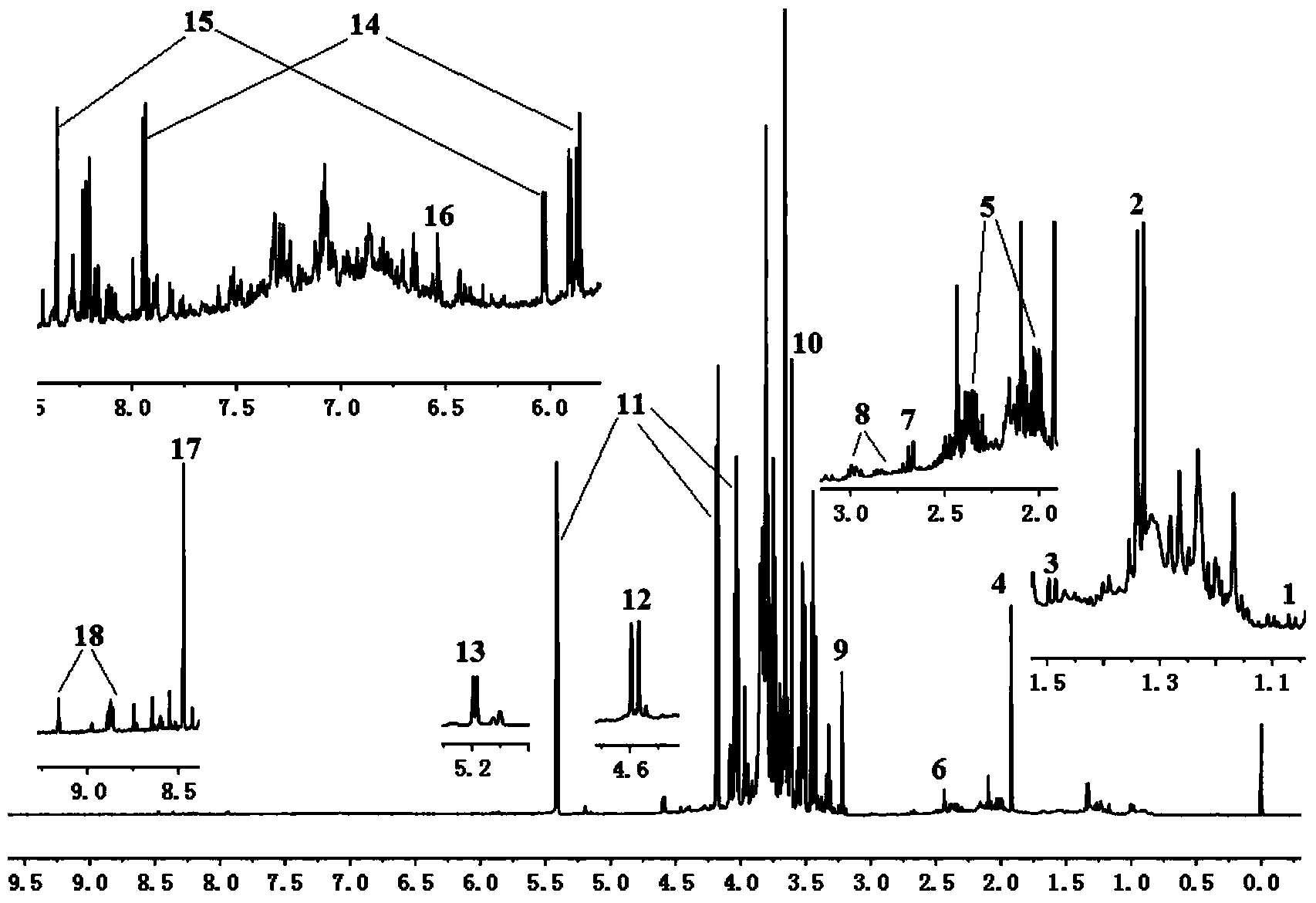
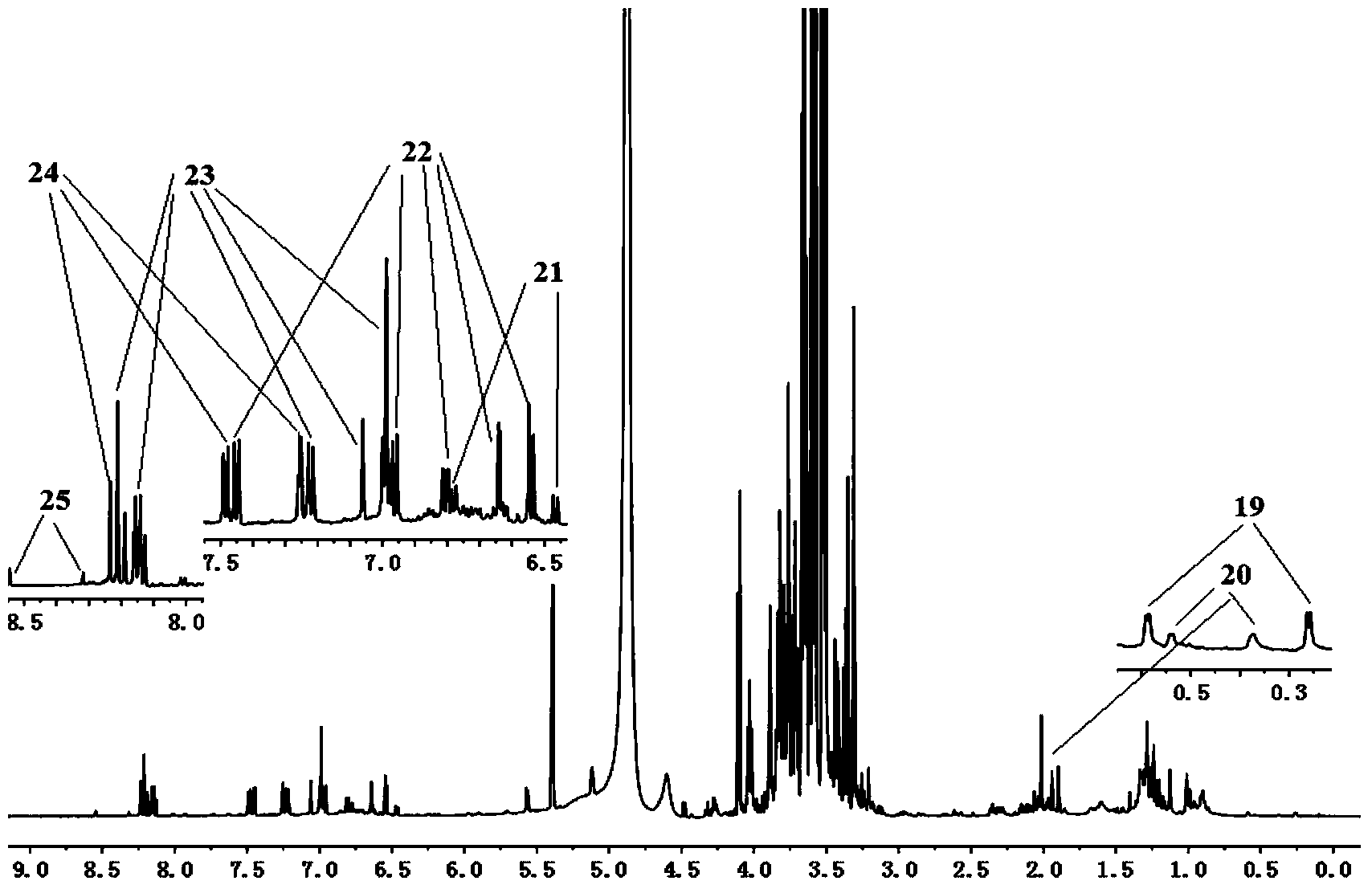
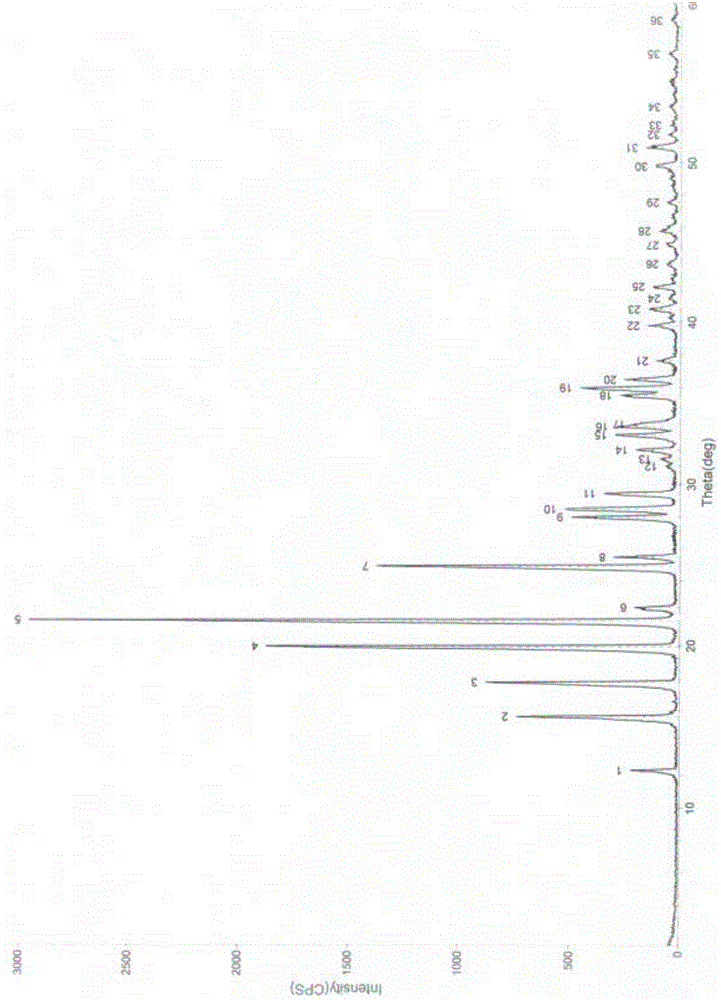
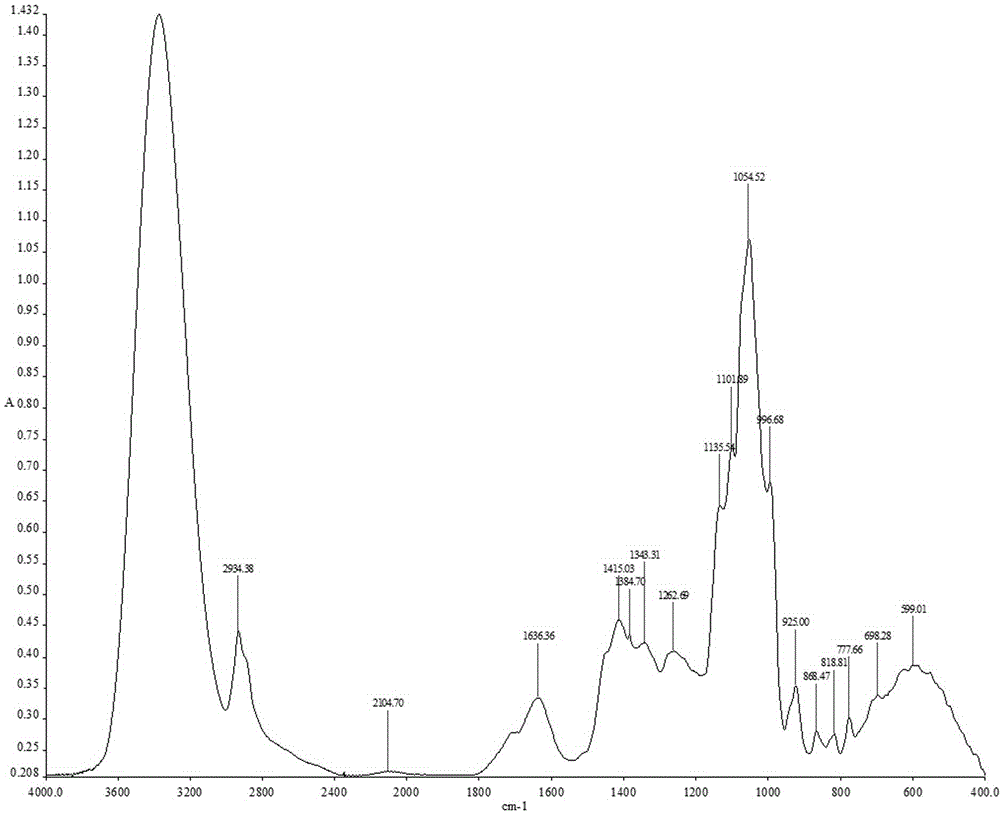
Construction method of <1>H-NMR fingerprint of radix astragali injection
ActiveCN104181186AGuarantee safe and effectiveAnalysis using nuclear magnetic resonanceNMR - Nuclear magnetic resonanceSecondary metabolite
The invention discloses a construction method of the <1>H-NMR fingerprint of a radix astragali injection, belonging to the field of quality control of traditional Chinese medicine injections. According to the construction method, the fingerprint and the characteristic peak of the fingerprint of the radix astragali injection are obtained by adopting the nuclear magnetic resonance technology, primary and secondary metabolites, namely totally 25 chemical components, in the radix astragali injection can be represented at the same time, the integral quality characteristic of the radix astragali injection can be accurately reflected, and thus the construction method can be used for quality control on radix astragali injection products and can ensure safe and effective clinical medication.
Owner:SHANXI UNIV
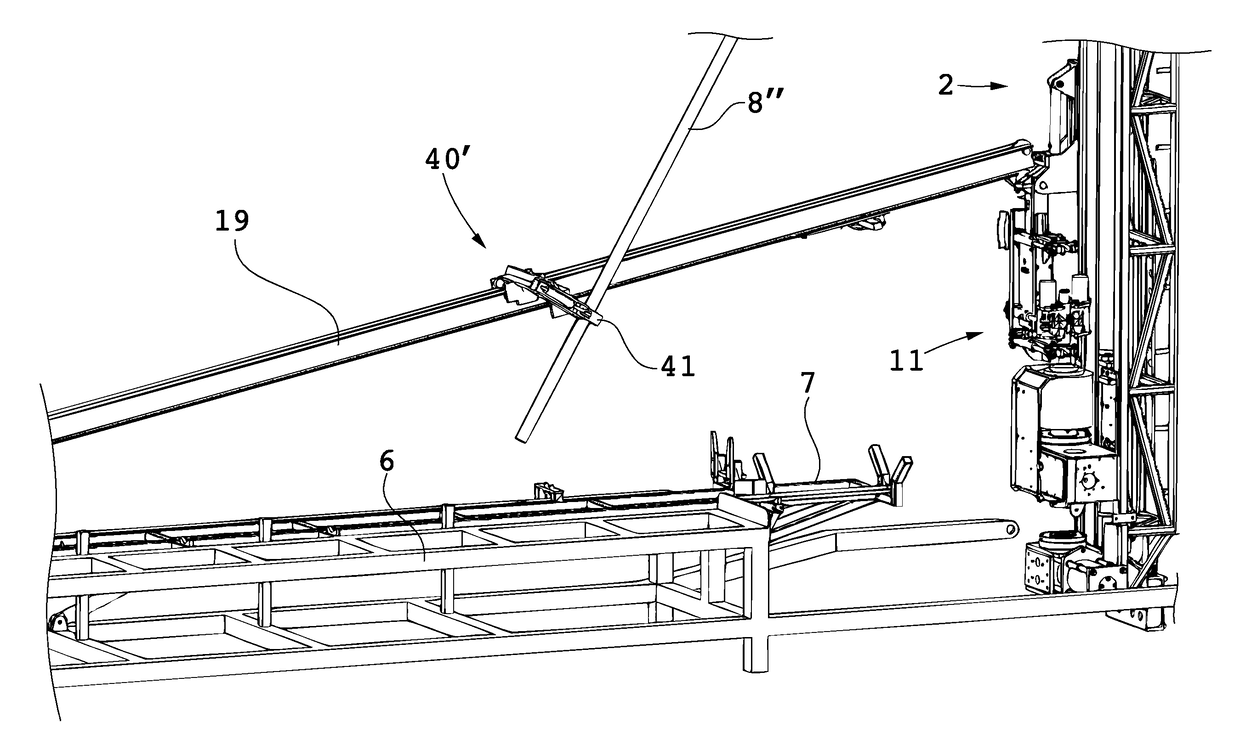
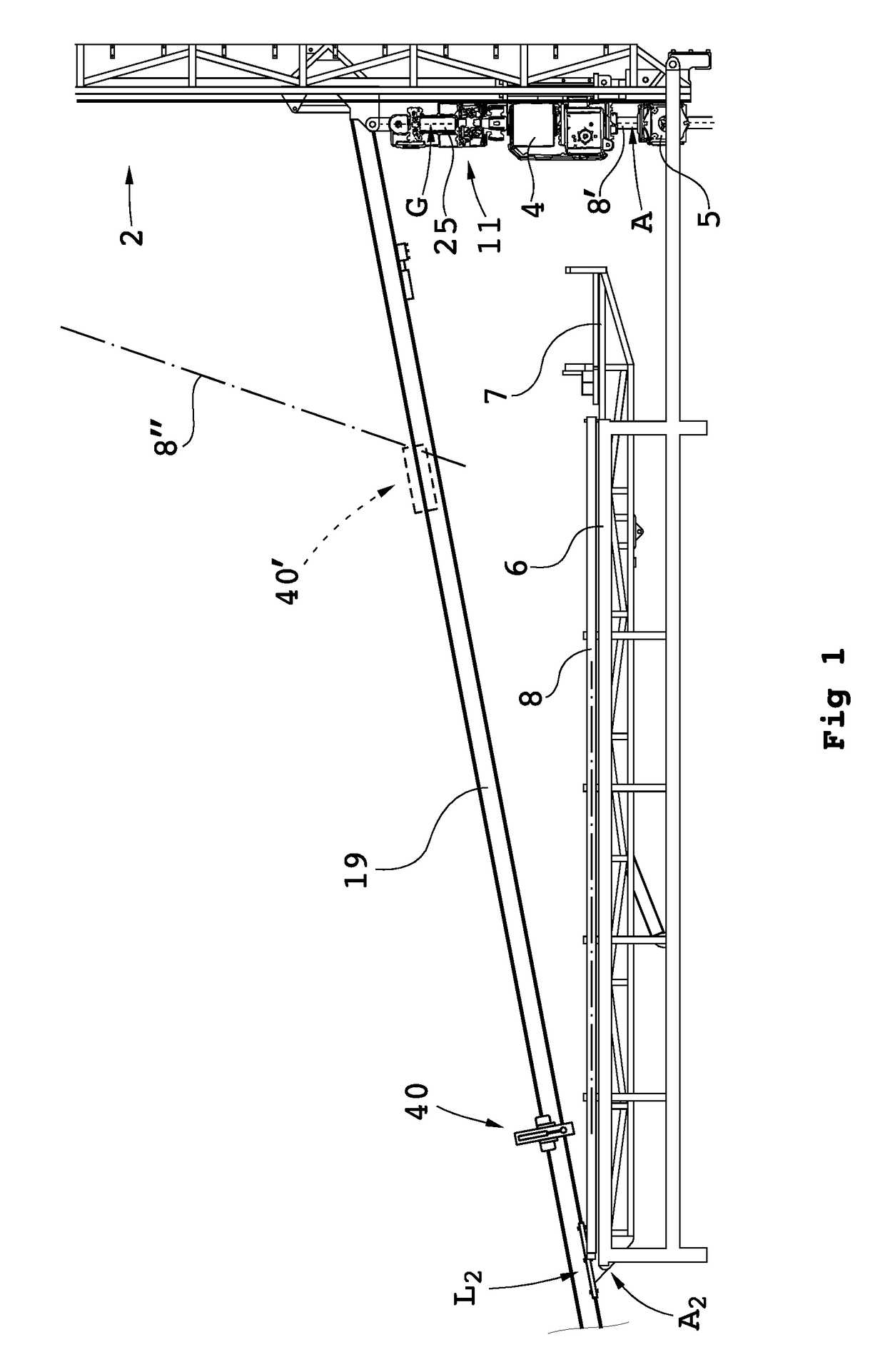
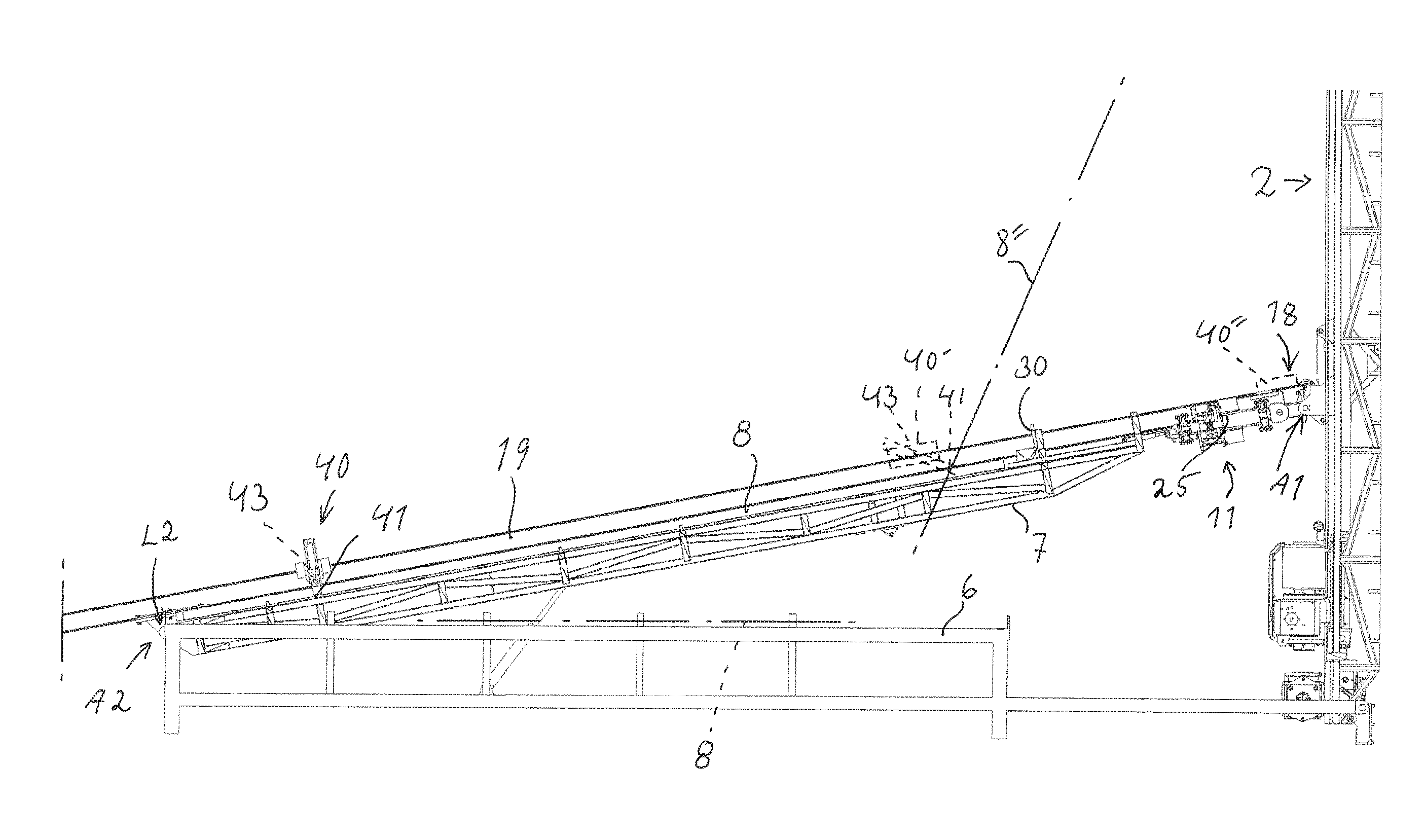
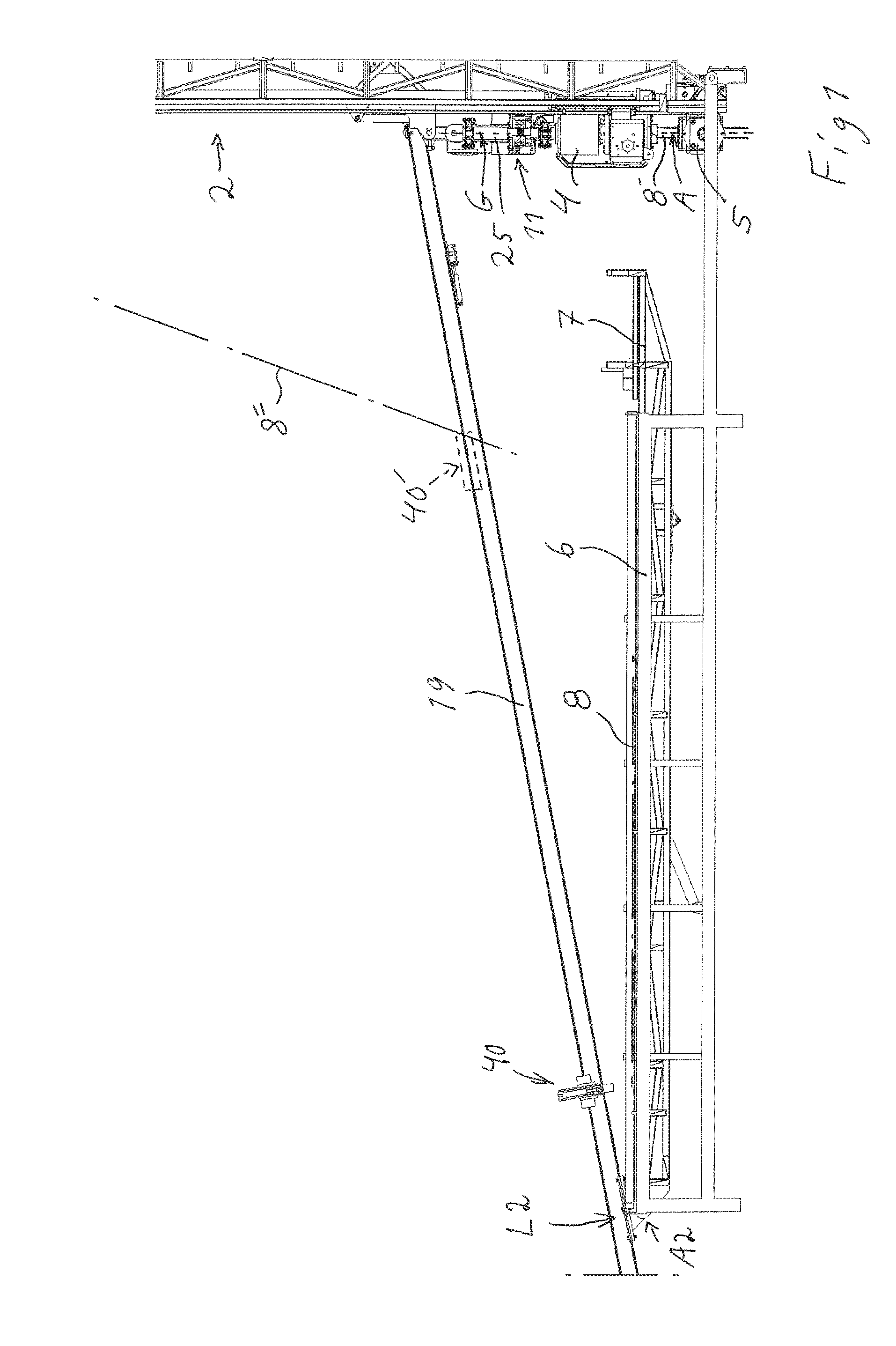
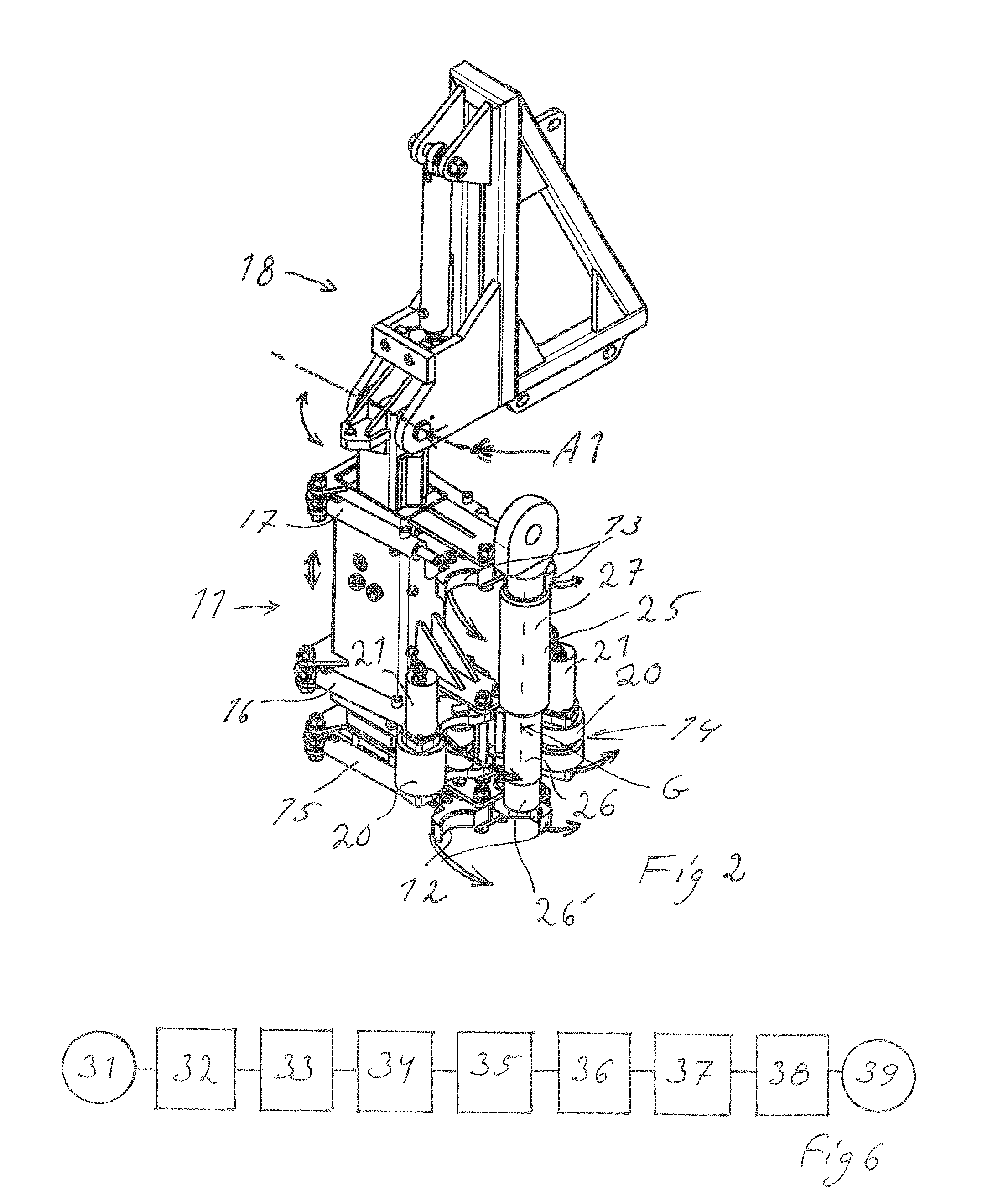
Handling device and method for handling drill string components in rock drilling and rock drill rig
A handling device for handling drill string components with respect to a rock drill rig including a rotator device supported on a feed beam and arranged to rotate and drive a drill string component. A gripper grips drill string components. The gripper is swingably supported around a first swing axis between: a first position aligned with the active drill string position, and a second position aligned with a delivering position for drill string components. A swing arm includes a support configured to support a drill string component and is swingable around a second swing axis. A guiding beam is fastenable at end regions in connection with respective regions of the first and second swing axis. The guiding beam forms mechanical stops for the gripper in the second position and for the swing arm in the delivering position. Also, a rock drill rig and a method.
Owner:EPIROC AB
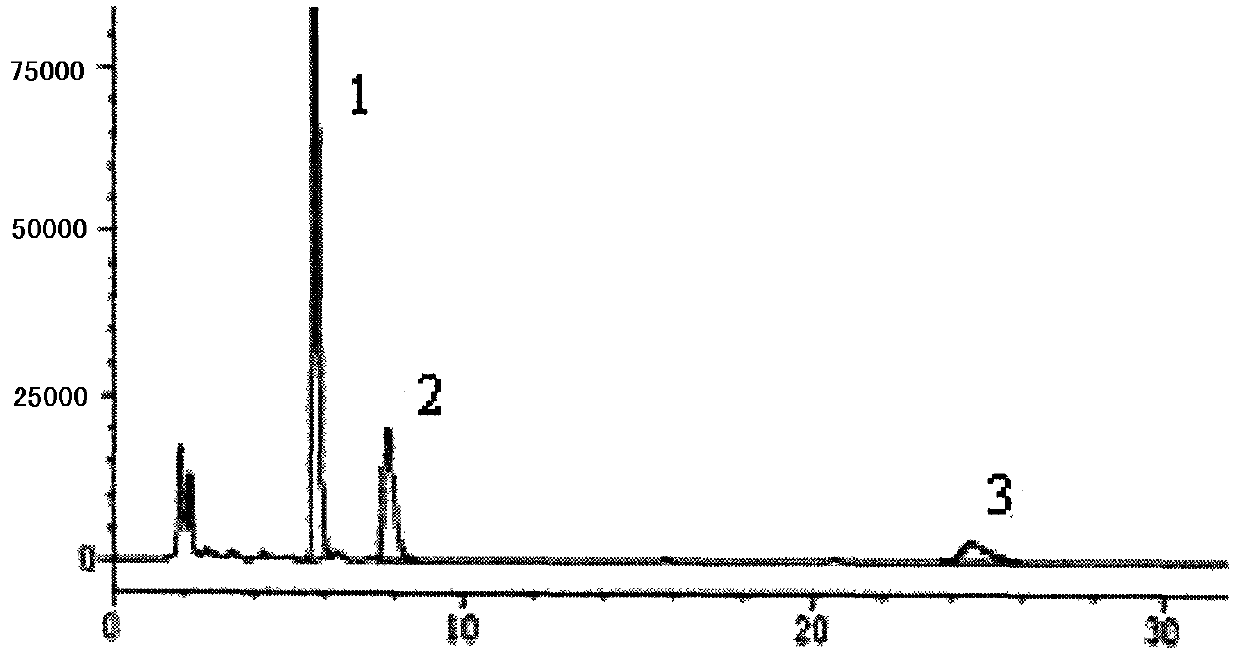
Method for simultaneously determining three alkaloids in granules for eliminating phlegm and stopping cough for children
ActiveCN102662024AQuality improvementGood peak separationComponent separationEmetine HydrochloridePhosphoric acid
The invention relates to a method for simultaneously determining three alkaloids in granules for eliminating the phlegm and stopping the cough for children. The method is characterized in that the HPLC (High Performance Liquid Chromatography) method is employed for the first time, an ordinary gradient elution and reversed-phase chromatographic column are adopted, and acetonitrile, methanol and 0.1% phosphoric acid in the volume ratio of (1.5-2.5) : (12.5-11) : (86-86.5) are taken as the mobile phase; the detection wavelength is 205 nm; and the contents of ephedrine hydrochloride, cephaeline hydrochloride and emetine hydrochloride in the granules for eliminating the phlegm and stopping the cough for children are determined simultaneously, so as to end the history of no HPLC determining method for emetine and no quantitative determining indexes for granules for eliminating the phlegm and stopping the cough for children. The method provided by the invention comprises the steps of performing an ultrasonic treatment to a sample with methanol, sucking a certain amount of subsequent filtrate, removing impurities with an alumina column, and determining. The method has the benefits that crest separation of the three alkaloids is excellent, the baseline is stable, and 30 min is required to finish the determining; the quality control aim of being simple, convenient, quick, scientific, standard and multi-component quantitive by one maker is realized; and safety and effectiveness of taking granules for eliminating the phlegm and stopping the cough for children are ensured.
Owner:JING JING PHARMA
Giant salamander iridescent virus vaccine, preparation method and application
ActiveCN104404057AImprove securityNo genetic recombination issuesFungiViral antigen ingredientsAntigenYeast
The invention discloses giant salamander iridescent virus vaccine, a preparation method and an application. The giant salamander iridescent virus vaccine is recombinant protein translated after truncating and optimizing a major capsid protein gene of a giant salamander iridescent virus, and has a sequence as shown as SEQIDNO.2 (Sequence Identifier Number 2). A nucleotide sequence corresponding to the protein is optimized according to preference of a yeast codon to synthesize a gene segment which is transferred to a yeast expression vector, and pichiapastoris Km71 / GSIV-MCP (i) Pichiapastoris(1 / i)Km71 / GSIV-MCP (CCTCC (China Center for Type Culture Collection) NO: M2014573) of the high expression recombinant protein is finally obtained via high copy and clone screening. The protein produced by fermentating the yeast has the characteristics of high activity and large yield. An immunoprotection experiment determines that the antigen protein can effectively prevent a giant salamander iridescent virus disease.
Owner:YANGTZE RIVER FISHERIES RES INST CHINESE ACAD OF FISHERY SCI
Flunarizine hydrochloride capsules and preparation method thereof
InactiveCN104382878AQuality improvementImprove stabilityOrganic active ingredientsCapsule deliveryMedicineFlunarizine Hydrochloride
The invention relates to an oral capsule preparation of flunarizine hydrochloride and a preparation method thereof, belonging to the technical field of medicines. The capsule comprises the following components in parts by weight: 58-60 parts of flunarizine hydrochloride, 114-115 parts of starch, 212.6-213 parts of 63 percent ethanol, 35.3-35.5 parts of sodium carboxymethyl starch and 11.6-12 parts of magnesium stearate. The flunarizine hydrochloride disclosed by the invention has high quality stability, the quality of the flunarizine hydrochloride capsules is stable and controllable, and safety and effectiveness of clinical application are ensured.
Owner:SHIJIAZHUANG HUAXIN PHARMA
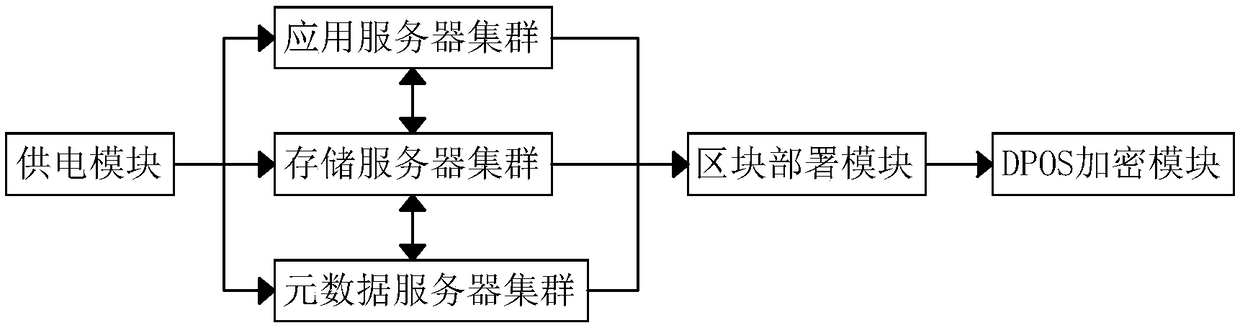
A block chain power data storage system
InactiveCN109033130AGuarantee safe and effectiveReduce the impact of security protection capabilitiesDigital data protectionSpecial data processing applicationsData validationApplication server
A block chain power data storage system comprises a power supply module, an application server cluster, a storage server cluster, a metadata server cluster, a block deployment module and a DPOS encryption module, wherein the three output terminals of the power supply module are respectively connected with the input terminals of the application server cluster, the storage server cluster and the metadata server cluster. The system consists of the application server cluster, the storage server cluster and the metadata server; through the function of the block deployment module and the DPOS encryption module, a distributed block chain structure and randomly selected nodes are used for data validation to ensure that an attacker cannot attack a network by breaking and controlling nodes and greatly reduce the influence of the terminal security performance on the whole network security protection ability. At the same time, 51% random node confirmation is needed for each data maintenance, the confirmation process needs to be carried out three times, and each node is different, which can ensure that the data is not tampered with and the data is safe and effective.
Owner:WENZHOU TUSHENG SCI & TECH +1
Monoclonal antibody for identifying PCV2 virus-like particles and application thereof in qualitative and quantitative detection of PCV2 virus-like particles
ActiveCN109536456AAccurate measurementSpecific determinationImmunoglobulins against virusesTissue cultureFluorescenceVirus-like particle
The invention discloses a monoclonal antibody for identifying PCV2 virus-like particles and an application thereof in qualitative and quantitative detection of PCV2 virus-like particles, wherein the monoclonal antibody capable of specifically identifying porcine circovirus type 2 virus-like particles is secreted by a hybridoma cell line with the preservation number of CGMCC NO.15793. The specificity of the obtained monoclonal antibody 3H9 is identified by indirect immunofluorescence assay, and the result indicates that the monoclonal antibody can specifically identified the PCV2 virus-like particles and can react with the PCV2 VLPs, but not with an ELISA plate coated with a linear cap protein. Therefore, a simple method for simultaneous qualitative and quantitative analysis of the PCV2 VLPs is established, and the problems of expensive instruments for detecting a structure of PCV2 VLP particles, difficult operation and inability of quantitative methods to distinguish non-specific proteins from broken subunits are solved.
Owner:HARBIN VETERINARY RES INST CHINESE ACADEMY OF AGRI SCI +1
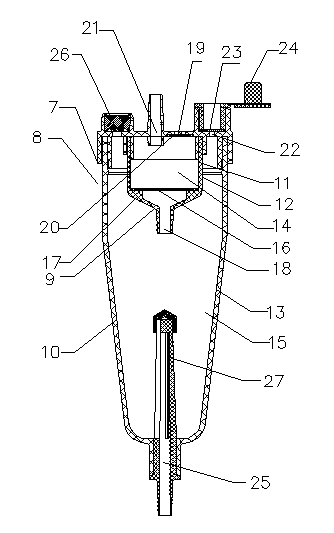
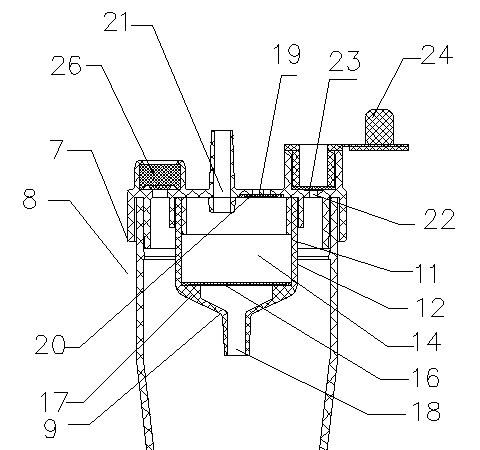
Integrated oxygen inhalation atomization device
InactiveCN103520818AReduce work intensityEnsure safetyRespiratorsMedical atomisersBottleIntensive care medicine
The invention discloses an integrated oxygen inhalation atomization device which comprises a connector, a humidification bottle and a flow meter, wherein the humidification bottle is connected with the connector and the flow meter is connected with the connector. The connecter is provided with an oxygen inlet, an atomization port and an oxygen inhalation port. An atomization oxygen inhalation conversion device connected with the oxygen inlet, the atomization port and the oxygen inhalation port is arranged in the connector. The integrated oxygen inhalation atomization device is simple in structure and easy to manufacture, solves the problem that oxygen inhalation and atomization cannot be converted easily in the prior art, so that a nurse carries out switching between atomization and oxygen inhalation freely according to needs and operation is very simple; a patient can be treated timely, effectively and safely, the labor intensity of the nurse is reduced, and working efficiency is improved.
Owner:周文婷 +1
Preparation method of traditional Chinese medicinal oral liquid for treating tourette syndrome of children
InactiveCN103751722AHigh clarityEnsure safetyNervous disorderAnthropod material medical ingredientsReflux extractionEthanol precipitation
A preparation method of traditional Chinese medicinal oral liquid for treating tourette syndrome of children includes performing reflux extraction on radix paeoniae alba, bombyx batryticatus, gastrodia elata, uncaria rhynchophylla, radix curcumae, pheretima and buthus martensii, filtering, recovering ethanol, concentrating, adding distilled water, standing, and refrigerating; mixing rehmannia glutinosa libosch and the medicinal residue, decocting in water, filtering, concentrating, adding ethanol, standing, collecting supernatant, and filtering to obtain water extraction / ethanol precipitation liquid; mixing water extraction / ethanol precipitation liquid and ethanol extraction / water precipitation liquid, recovering ethanol, heating, concentrating, standing, filtering to obtain filtrate; mixing 1 / 3 volume of the filtrate with steviosin, stirring, mixing with the rest 2 / 3 of the filtrate, adding ethylparaben, adding water, regulating pH value, and standing; and centrifuging, collecting supernatant, adding water to regulate total volume to 1,000 mL, stirring, subpackaging, and sterilizing. Through improvement on the basis of the original process, the preparation method provided by the invention improves preparation appearance, solves the problems of poor clarity and stability in the original process, and ensures safety and efficacy of medicament in children.
Owner:南京海思创新生物科技有限公司
Power data distributed security protection tool based on block chain
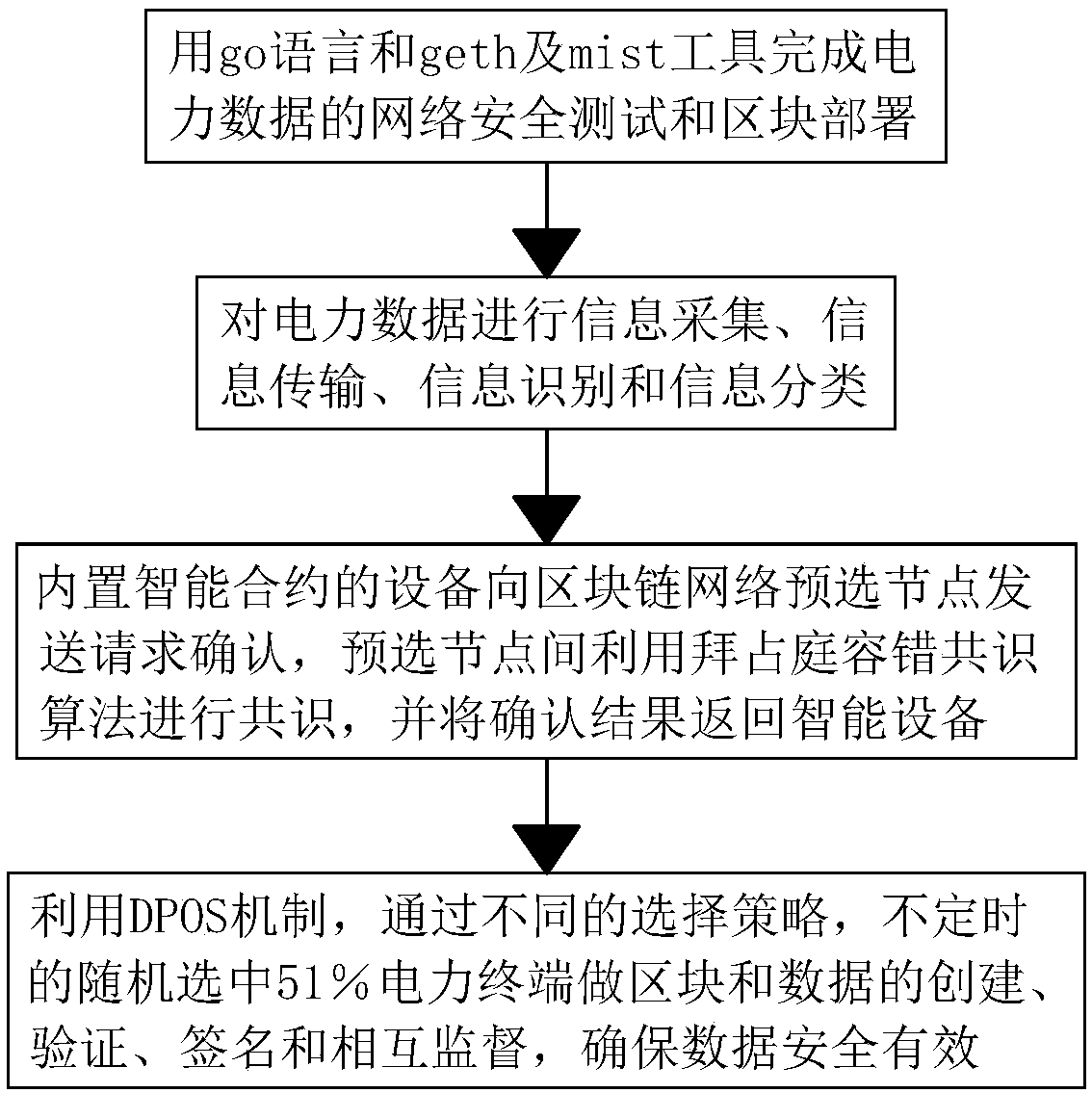
InactiveCN108848085AGuarantee safe and effectiveImpact on Security Protection CapabilitiesData switching networksData validationChain structure
The invention discloses a power data distributed security protection tool based on a block chain. A protection method comprises the following step of A, finishing network security test and block deployment of power data through utilization of a go language and geth and mint tools. According to the power data distributed security protection tool, through adoption of a decentralization characteristic, a central node (a sever, a database and so on) is cancelled in a traditional model, so an attacker cannot find an attack object, and an network attack is out of the question; a distributed block chain structure and randomly selected nodes carry out power data validation and it is ensured that the attacker cannot attack a network in a mode of breaking through and controlling nodes, so influenceof security performance of a terminal on whole network security protection capability is greatly reduced; and moreover, power data maintenance at each time requires validation of 51% of random nodes,a validation process needs to be carried out for three times, and the nodes at each time are different, so power data is prevented from being tampered, and the power data is guaranteed to be secure and valid.
Owner:WENZHOU TUSHENG SCI & TECH +1
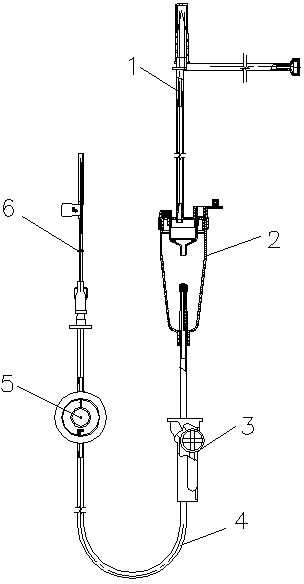
Safe infusion set
The invention discloses a safe infusion set which comprises a bottle stopper puncture device, a drip chamber, a flow regulator, an infusion conduit and an intravenous infusion needle. The safe infusion set is characterized in that the drip chamber comprises a drip chamber body and a drip chamber cover located at the upper part of the drip chamber body which is divided into an inner drip chamber and an outer drip chamber, the inner wall of the inner drip chamber is combined with the drip chamber cover to form an inner drip chamber cavity, an exhaust hole is matched in the top of the inner drip chamber cavity, a drip chamber liquid feed pipe is arranged on the drip chamber cover at the upper part of the inner drip chamber cavity, and a liquid stop filter membrane is arranged at the upper part of an inner drip chamber liquid outlet in the bottom end of the inner drip chamber; the inner wall of the outer drip chamber, the drip chamber cover and the outer wall of the inner drip chamber are combined to form an outer drip chamber cavity which is matched with an openable air inlet hole, and an automatic exhaust device is arranged at a drip chamber liquid outlet in the lower part of the outer drip chamber cavity. The safe infusion set has automatic exhaust and liquid stop functions, guarantees safe and effective infusion, is convenient to use by a medical worker and reduces the nursing intensity.
Owner:SHANDONG WEIGAO GROUP MEDICAL POLYMER
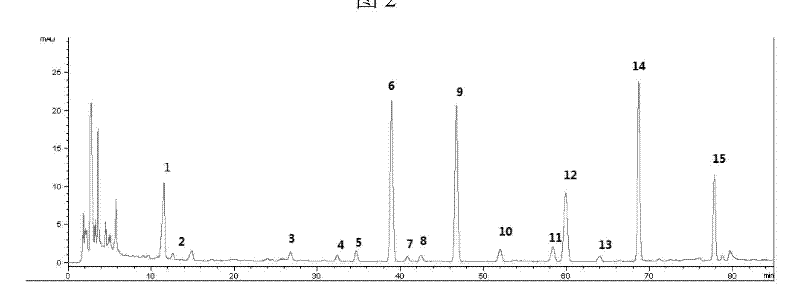
Method for preparing siwu decoction formula granules and quality control method thereof
ActiveCN105287875AEmbody conceptTo achieve the purpose of reducing toxicity and increasing efficiencyComponent separationMaterial analysis by optical meansBiotechnologyFormulary
The invention discloses a method for preparing siwu decoction formula granules and a quality control method thereof. The method for preparing the siwu decoction formula granules comprises steps: prepared rehmannia roots, angelica sinensis, radix paeoniae alba and ligusticum wallichii medicinal slices are added with water which is 5-15 times of the weight of total inventory, are extracted for two times, aromatic water is collected and is combined with two frying filter liquids, are decompressed and concentrated in vacuum, a concentrated solution is added with beta (Beta)-cyclodextrin and silicon dioxide to uniformly stir and obtains a clear paste, and the aromatic water and the aromatic water are sprayed and dried after being uniformly mixed, and are pelletized through a dry method to prepare the siwu decoction formula granules. The quality control method of the siwu decoction formula granules comprises qualitative identification of an infrared fingerprint spectrum and a thin layer and content determination of a high performance liquid chromatography (HPLC). The method for preparing siwu decoction formula granules and the quality control method thereof decoct in a combined mode according to a traditional method, can perfectly take full advantages of drug matching compared with an existing method that various medicinal odours are added when single formula particles are taken, reflects the overall concept of Chinese medicine, guarantees to achieve the purpose of reducing toxicity and enhancing efficacy, supplies novel selection for clinical medication, builds a perfect quality standard, controls quality by combining a power-spectral method and a chromatography, and can effectively control quality of complex granules from the overall to the more specific.
Owner:GUANGDONG YIFANG PHARMA
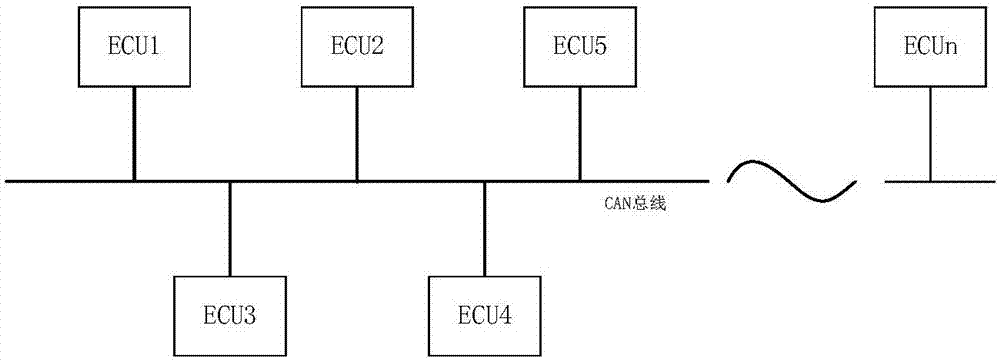
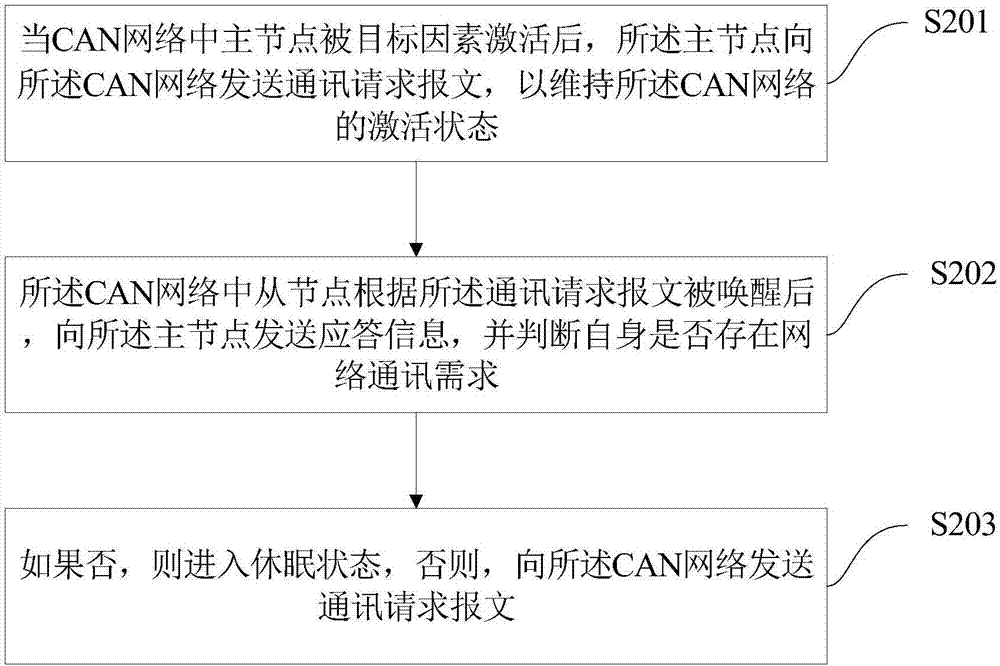
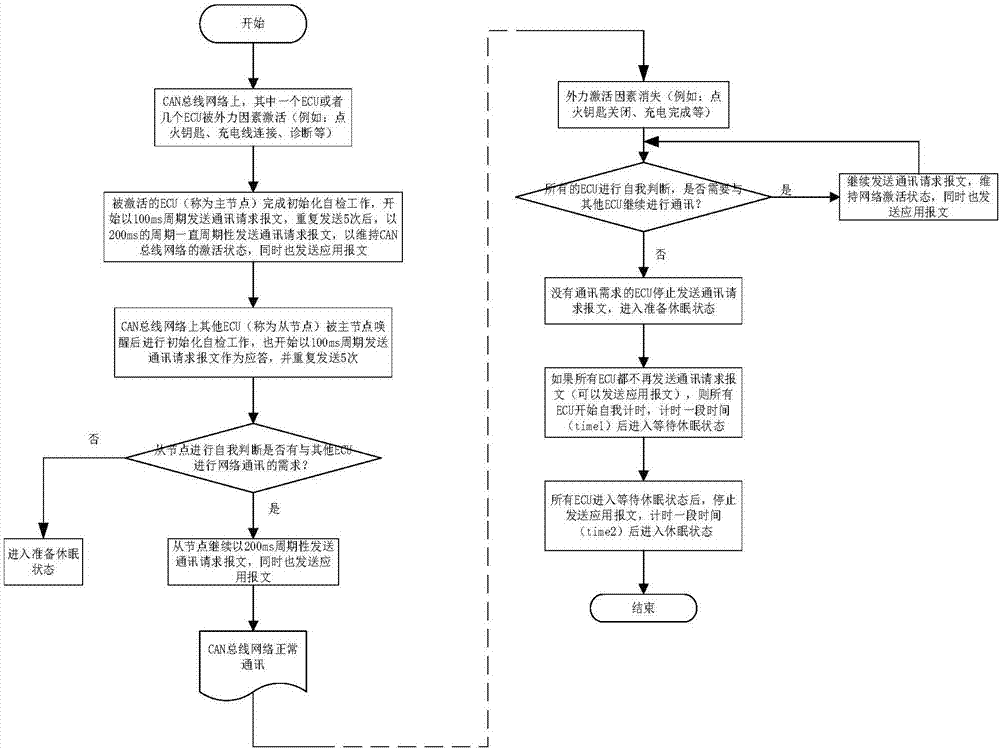
State control method of vehicle controller, system and vehicle thereof
InactiveCN107493215AFunctionally safe and effectiveSave energyElectric testing/monitoringData switching current supplyNetwork communicationBiological activation
The invention discloses a state control method of a vehicle controller, a system and a vehicle thereof. The method comprises the following steps that after being activated in a CAN network, a main node sends a communication request message to the CAN network so as to maintain an activation state of the CAN network; in the CAN network, a slave node is awakened according to the communication request message and then sends response information to the main node, and determines whether the slave node itself has a network communication demand; and if the slave node does not have the network communication demand, the slave node enters into a dormant state, otherwise, the slave node sends the communication request message to the CAN network. By using the state control method of the vehicle controller, the corresponding controller can be activated when a vehicle function is used, and the controller can enter into the dormant state when not being used so that energy is saved and the vehicle function can be ensured to be safe and effective.
Owner:BORGWARD AUTOMOTIVE CHINA CO LTD
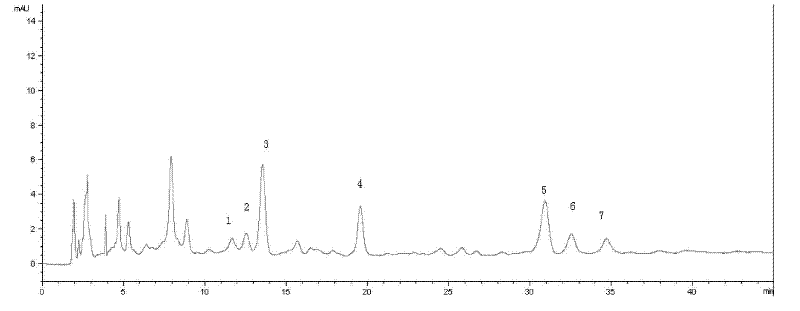
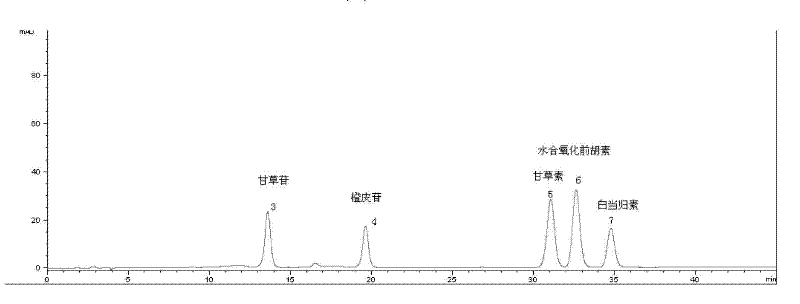
Huoxiang Zhengqi liquid quality control method
InactiveCN102539602AHigh repeatabilityGuarantee safe and effectiveComponent separationProcess engineeringQuality control
The invention discloses a Huoxiang Zhengqi liquid quality control method, which comprises the following steps of: preparing solution of products to be tested; testing a fingerprint map of solution of the products to be tested; and carrying out comparison with the set high-polarity part standard fingerprint map using liquiritin as reference objects and the medium and low-polarity part standard fingerprint map using nobiletin as reference objects for judging whether the products to be tested are qualified or not. According to the method, the major effective ingredients in the Huoxiang Zhengqi are used as the basis, a reasonable quality control method is built, and the product quality can be effectively controlled. The quality control method has the advantages that the precision, the stability and the repeatability are higher, and the stability and the uniformity of the product quality are ensured, so the safety and the effectiveness of the product are ensured.
Owner:GUANGDONG PHARMA UNIV
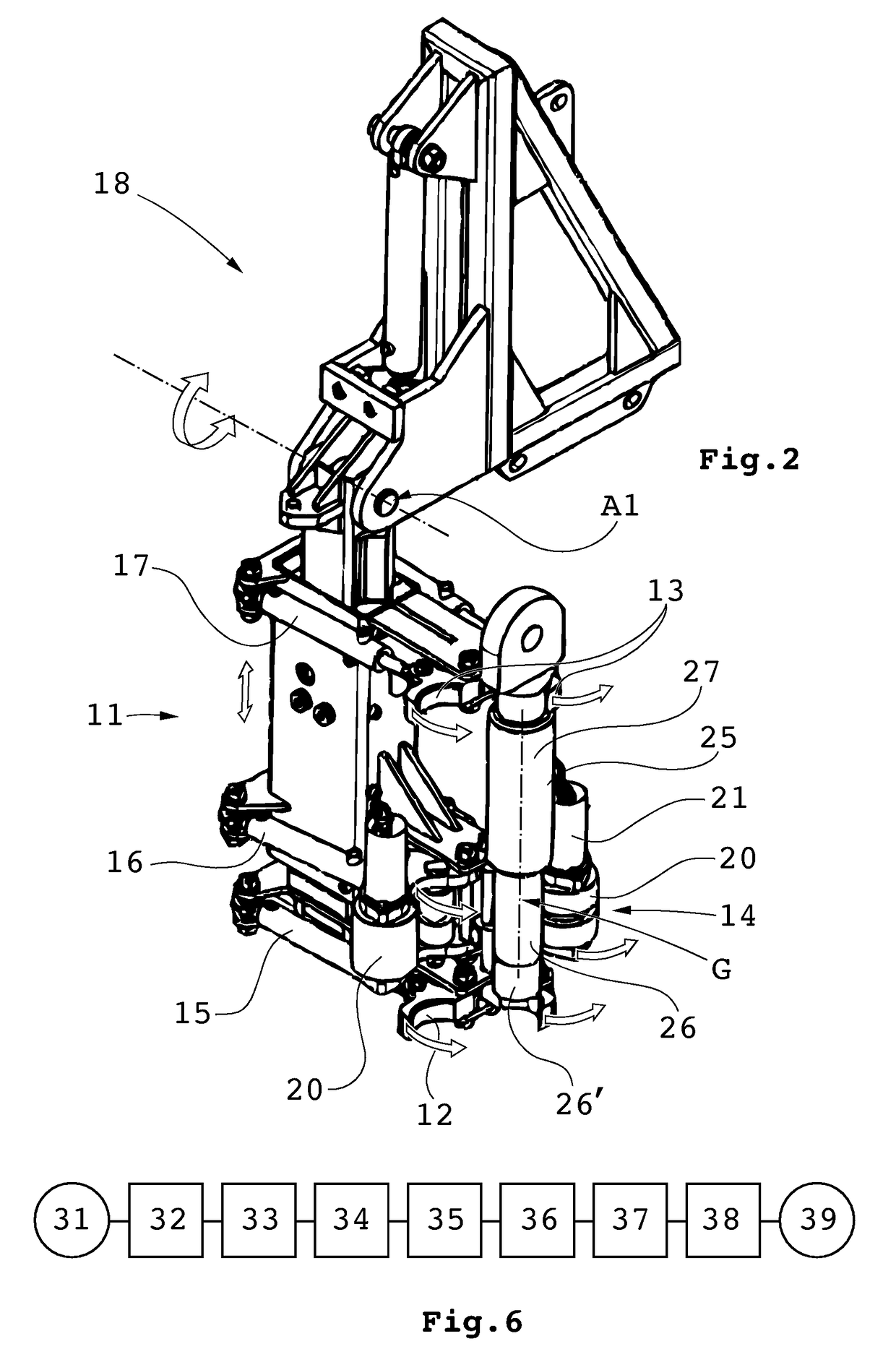
Handling device and method for handling drill string components in rock drilling and rock drill rig
ActiveUS20150144402A1Fully processedPrecise alignmentDrilling rodsDrilling casingsDrill stringEngineering
A handling device for handling drill string components with respect to a rock drill rig including a rotator device supported on a feed beam and arranged to rotate and drive a drill string component. A gripper grips drill string components. The gripper is swingably supported around a first swing axis between: a first position aligned with the active drill string position, and a second position aligned with a delivering position for drill string components. A swing arm includes a support configured to support a drill string component and is swingable around a second swing axis. A guiding beam is fastenable at end regions in connection with respective regions of the first and second swing axis. The guiding beam forms mechanical stops for the gripper in the second position and for the swing arm in the delivering position. Also, a rock drill rig and a method.
Owner:EPIROC AB
Y-shaped electrical connector used between tractor and trailer and power taking method thereof
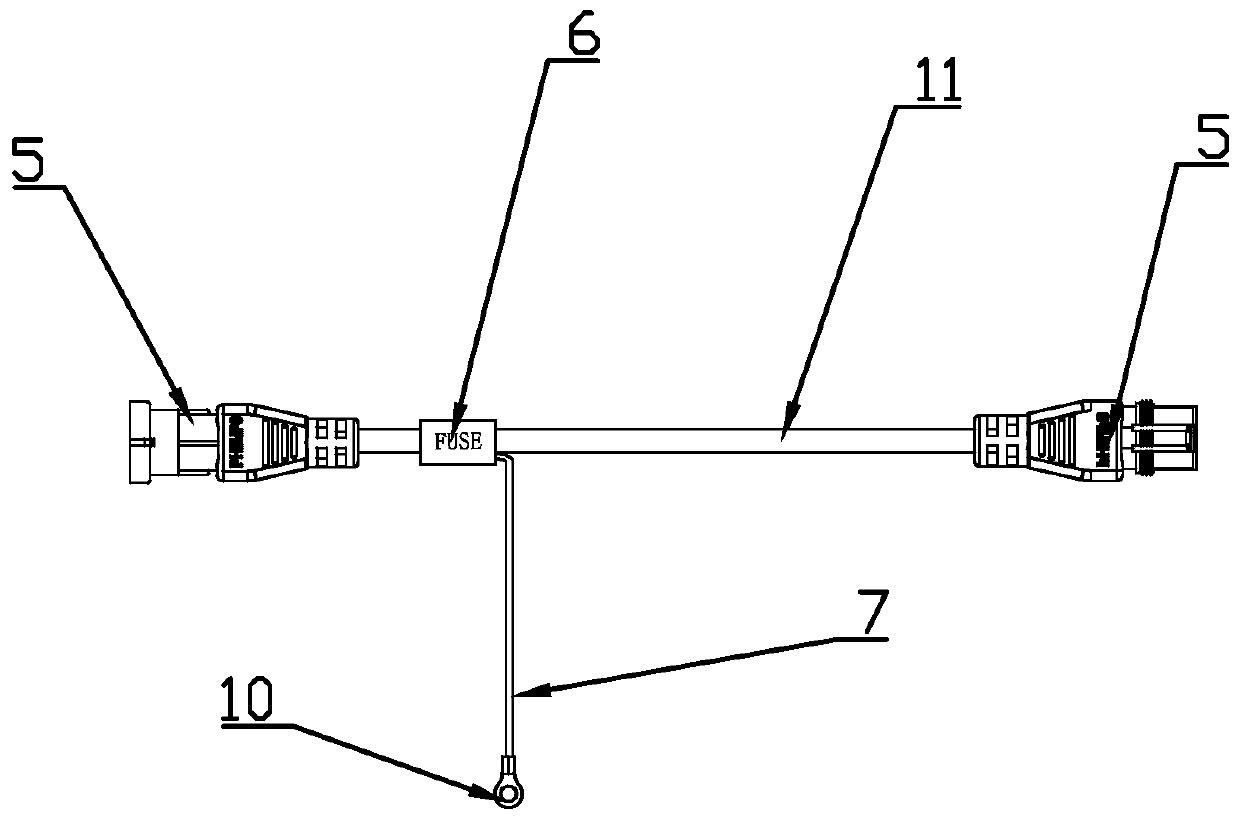
PendingCN110323643AGuarantee safe and effectiveAddresses insufficient, inability to power new devicesCoupling device connectionsVehicle connectorsElectricityElectrical connector
The invention provides a trailer Y-shaped branch power taking connector and relates to the field of continuous branch power taking connectors between tractors and trailers. A Y-shaped continuous powertaking connection device is arranged at the end of the trailer Y-shaped branch power taking connector. The trailer Y-shaped branch power taking connector comprises a seven-core-wire or five-core-wirepower plug socket which meets an ISO 7638 standard; a positive power wire connected with a second pin defined in the ISO 7638 standard is led out of the branch power taking connector; the tail end ofa connection wire of a branch power wire is equipped with a standard rapid connection socket; a negative wire is led out of a two-hole rapid connection socket and is bonded with a trailer to form anindependent current circuit so as to cause no influence on other circuit appliances. Compared with the prior art, the trailer Y-shaped branch power taking connector has the beneficial effects that theexisting trailer electrical connector is improved under the condition that the standards at home and abroad are met, a group of additional parallel circuits are added and are used for supplying powerto newly added intelligent devices; the problem that the existing electrical connector is not provided with sufficient ports and is incapable of supplying power to new devices is solved in the premise of ensuring that the new electrical connector is safe and effective.
Owner:宁波飞力普斯汽配工业有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com