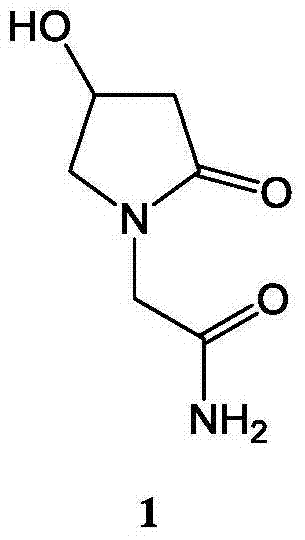
Oxiracetam capsule and preparation method thereof
A technology of capsules and micropowdered silica gel, which is applied in the field of medicine, can solve the problems of no investigation of long-term storage stability, no mention of preparation stability, and complicated preparation process steps, and achieve excellent fluidity, simple and environmentally friendly preparation process, and high quality. good stability effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
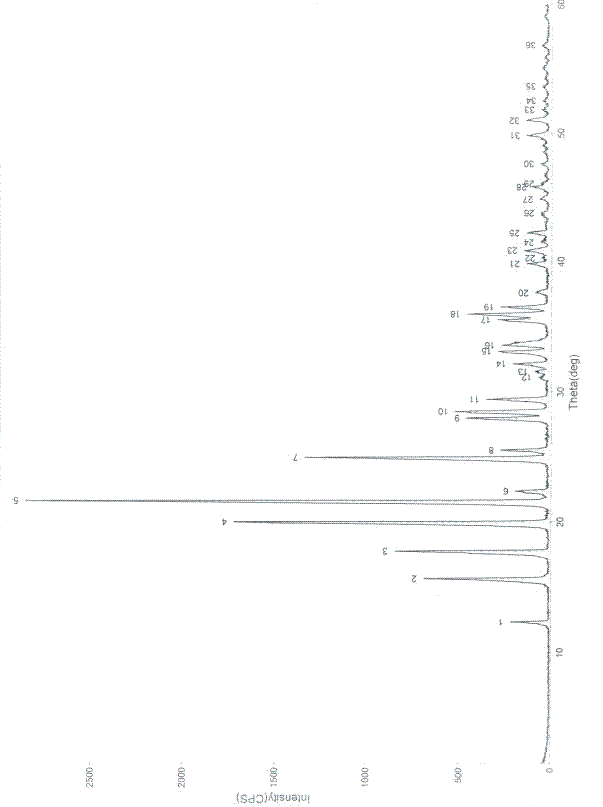
[0042] Example 1 Preparation of Oxiracetam Crystal Form
[0043] a) Take 100 g of the crude product of oxiracetam, add it to 200 ml of water, heat to dissolve it, and obtain an aqueous solution of oxiracetam;
[0044] b) Concentrate the above aqueous solution to 50ml under reduced pressure, the concentration temperature is 45-55°C, and the vacuum degree is -0.08MPa~-0.085MPa.
[0045] c) Cool the concentrated solution to 5°C, stir and crystallize for 1 hour at a stirring speed of 60 rpm, centrifuge to separate the crystals, and then vacuum-dry at a temperature of 45°C and a vacuum degree of -0.08MPa to -0.1MPa After 1 hour, 85.3 g of the finished crystal form of oxiracetam was obtained, the weight yield was 85.3%, the purity determined by HPLC was 99.89%, and the melting point was 168.2-170.0°C.
Embodiment 2
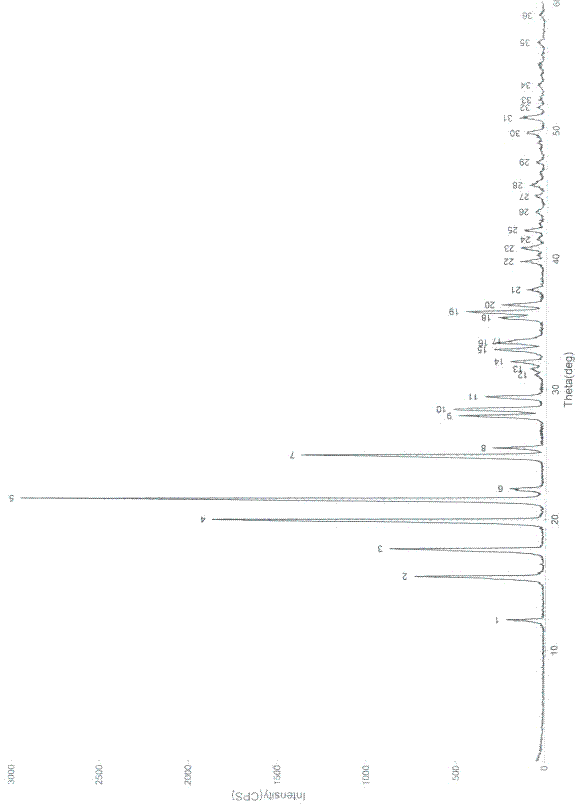
[0046] Example 2 Preparation of Oxiracetam Crystal Form
[0047] a) Take 100 g of the crude product of oxiracetam, add it to 600 ml of water, and dissolve it to obtain an aqueous solution of oxiracetam;
[0048] b) Add 5g of activated carbon to the above aqueous solution, stir and decolorize at 40-50°C for 0.5 hours, filter, and collect the filtrate;
[0049] c) Concentrate the above aqueous solution to 300ml under reduced pressure, the concentration temperature is 40-45°C, and the vacuum degree is -0.085MPa~-0.090MPa.
[0050] d) Cool the concentrated solution to 0°C, stir and crystallize for 6 hours at a stirring speed of 40 rpm, separate the crystals by filtration, and then vacuum dry at a temperature of 55°C and a vacuum degree of -0.08MPa to -0.1MPa After 4 hours, 73.5 g of the finished crystal form of oxiracetam was obtained, with a weight yield of 73.5%, a purity of 99.95% as determined by HPLC, and a melting point of 168.1-169.5°C.
Embodiment 3
[0051] Example 3 Preparation of Oxiracetam Crystal Form
[0052] a) Take 100 g of the crude product of oxiracetam, add it into 1000 ml of water, and dissolve it to obtain an aqueous solution of oxiracetam;
[0053] b) Concentrate the above aqueous solution to 200ml under reduced pressure, the concentration temperature is 35-40°C, and the vacuum degree is -0.090MPa~-0.095MPa.
[0054] c) Cool the concentrated solution to 2°C, stir and crystallize for 3 hours, and the stirring speed is 30 rpm; separate the crystals by pressure filtration, and then vacuum at a temperature of 65°C and a vacuum degree of -0.08MPa~-0.1MPa. After drying for 3 hours, 78.9 g of the finished oxiracetam crystal form was obtained, with a weight yield of 78.9%, a purity of 99.92% as determined by HPLC, and a melting point of 166.0-167.8°C.
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| angle of repose | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com