Patents
Literature
382 results about "Glidant" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
A glidant is a substance that is added to a powder to improve its flowability. A glidant will only work at a certain range of concentrations. Above a certain concentration, the glidant will in fact function to inhibit flowability.
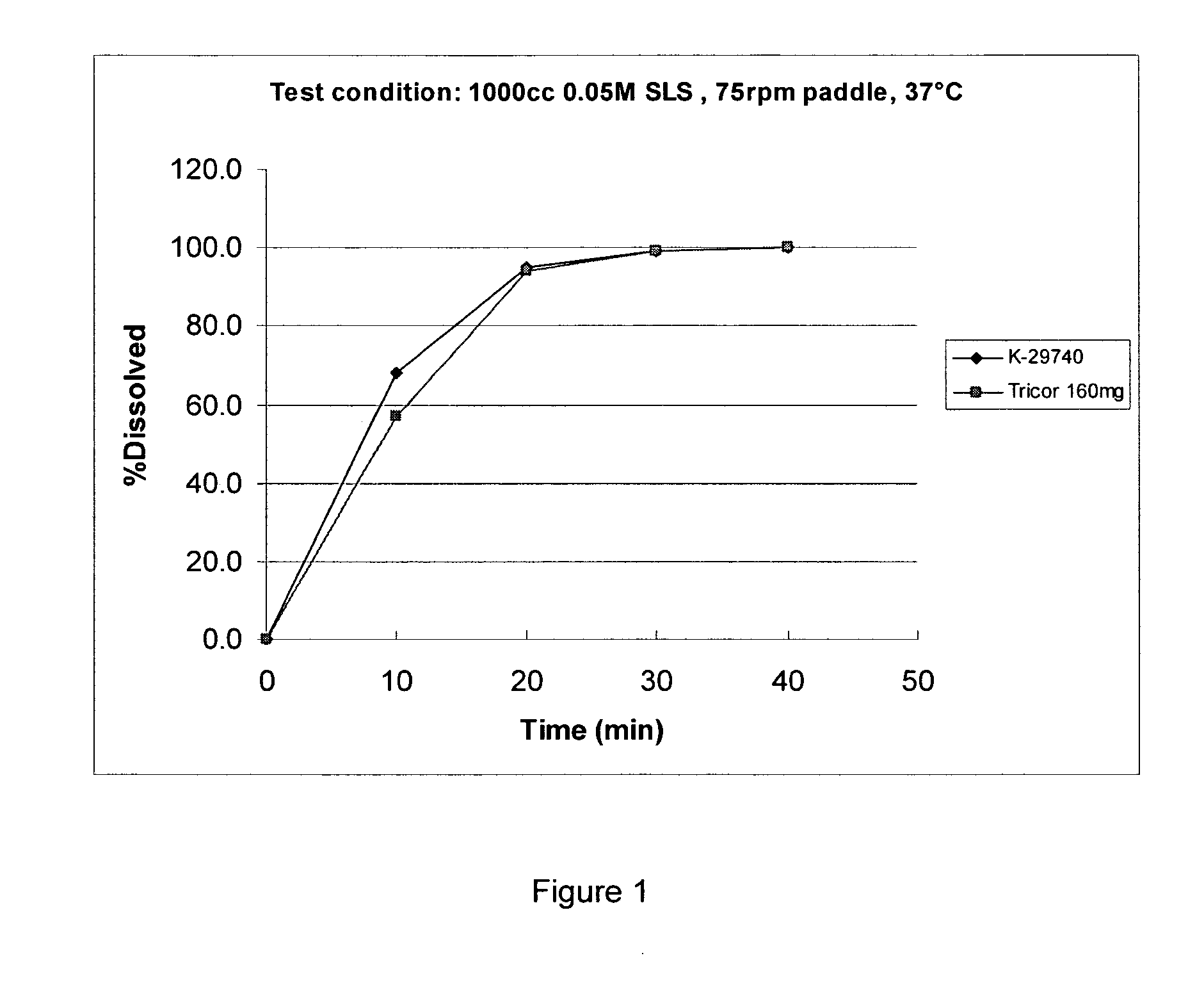
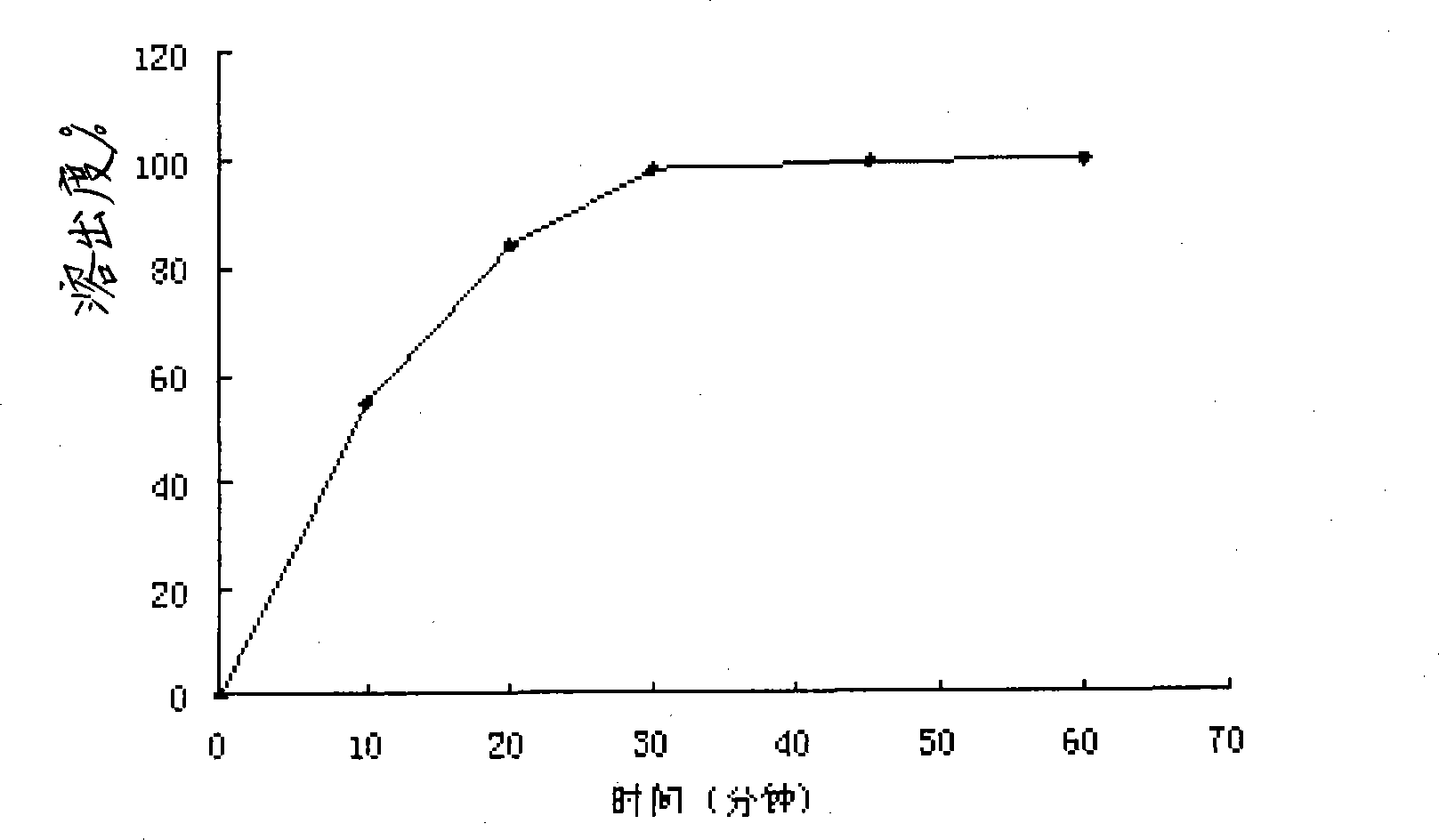
Pharmaceutical compositions of (r)-1-(2,2-difluorobenzo[d] [1,3]dioxol-5-yl)-n-(1-(2,3-dihydroxypropyl)-6-fluoro-2-(1-hydroxy-2-methylpropan-2-yl)-1h-indol-5-yl) cyclopropanecarboxamide and administration thereof
A pharmaceutical composition comprising Compound 1, (R)-1-(2,2-difluorobenzo[d][1,3]dioxol-5-yl)-N-(1-(2,3-dihydroxypropyl)-6-fluoro-2-(1-hydroxy-2-methylpropan-2-yl)-1H-indol-5-yl)cyclopropanecarboxamide, and at least one excipient selected from: a filler, a diluent, a disintegrant, a surfactant, a glidant and a lubricant, the composition being suitable for oral administration to a patient in need thereof to treat a CFTR mediated disease such as Cystic Fibrosis. Methods for treating a patient in need thereof include administering the pharmaceutical composition of Compound 1 are also disclosed.
Owner:VERTEX PHARMA INC
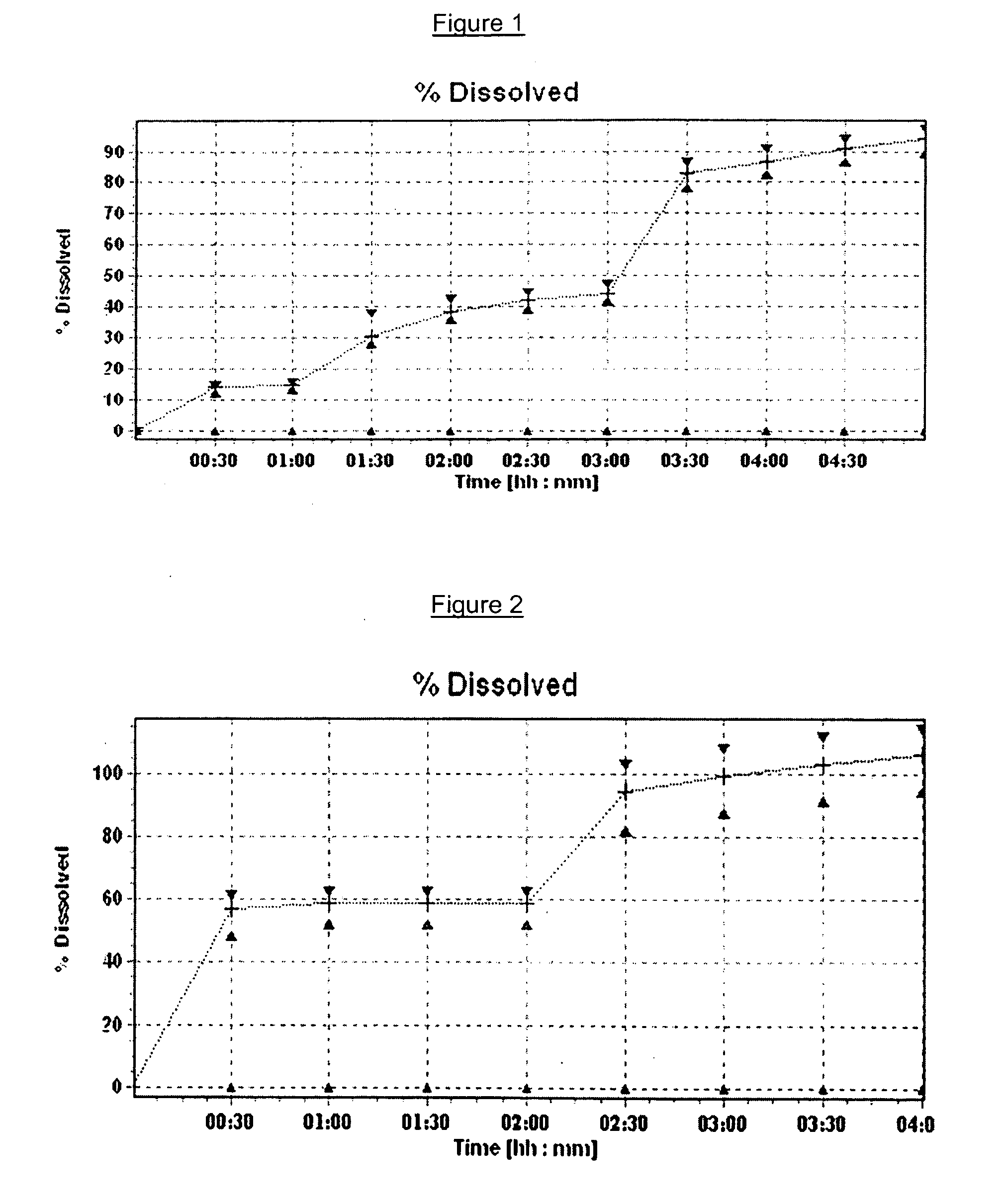
Pharmaceutical compositions of (R)-1-(2,2-difluorobenzo[D][1,3]dioxol-5-yl)-N-(1-(2,3-dihydroxypropyl)-6-fluoro-2-(1-hydroxy-2-methylpropan-2-yl)-1H-indol-5-yl)cyclopropanecarboxamide and administration thereof
A pharmaceutical composition comprising Compound 1, (R)-1-(2,2-difluorobenzo[d][1,3]dioxo-5-yl)-N-(1-(2,3-dihydroxypropyl)-6-fluoro-2-(1-hydroxy-2-methylpropan-2-yl)-1H-indol-5-yl)cyclopropanecarboxamide, and at least one excipient selected from: a filler, a disintegrant, a surfactant, a glidant and a lubricant, the composition being suitable for oral administration to a patient in need thereof to treat a CFTR mediated disease such as Cystic Fibrosis. Methods for treating a patient in need thereof include administering the pharmaceutical composition of Compound 1 are also disclosed.
Owner:VERTEX PHARMA INC
Apparatus and process for treating an aqueous solution containing chemical contaminants
Apparatus, process and article for treating an aqueous solution containing a chemical contaminant. The process includes contacting an aqueous solution containing a chemical contaminant with an aggregate composition comprising an insoluble rare earth-containing compound to form a solution depleted of chemical contaminants. The insoluble rare earth-containing compound can include one or more of cerium, lanthanum, or praseodymium. A suitable insoluble cerium-containing compound can be derived from a cerium carbonate, cerium oxalate and / or a cerium salt. The aggregate composition can include more than 10.01% by weight of the insoluble rare earth-containing compound, and in a particular embodiment consists essentially of one or more cerium oxides, and optionally a binder and / or flow aid. Although intended for a variety of fluid treatment applications, such applications specifically include removing or detoxifying chemical contaminants in water.
Owner:MOLYCORP MINERALS
Composition and process for making the composition
Owner:SECURE NATURAL RESOURCES LLC
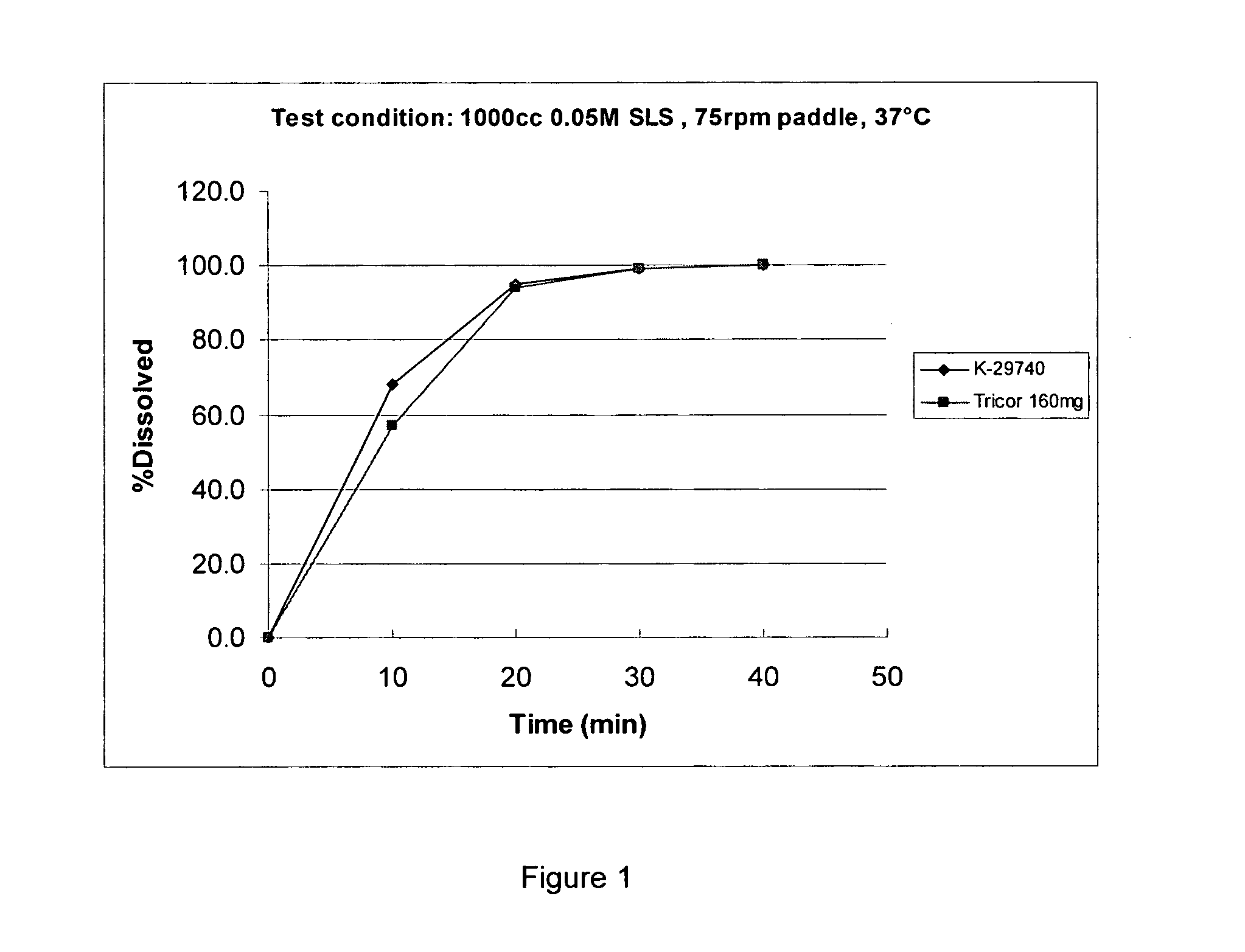
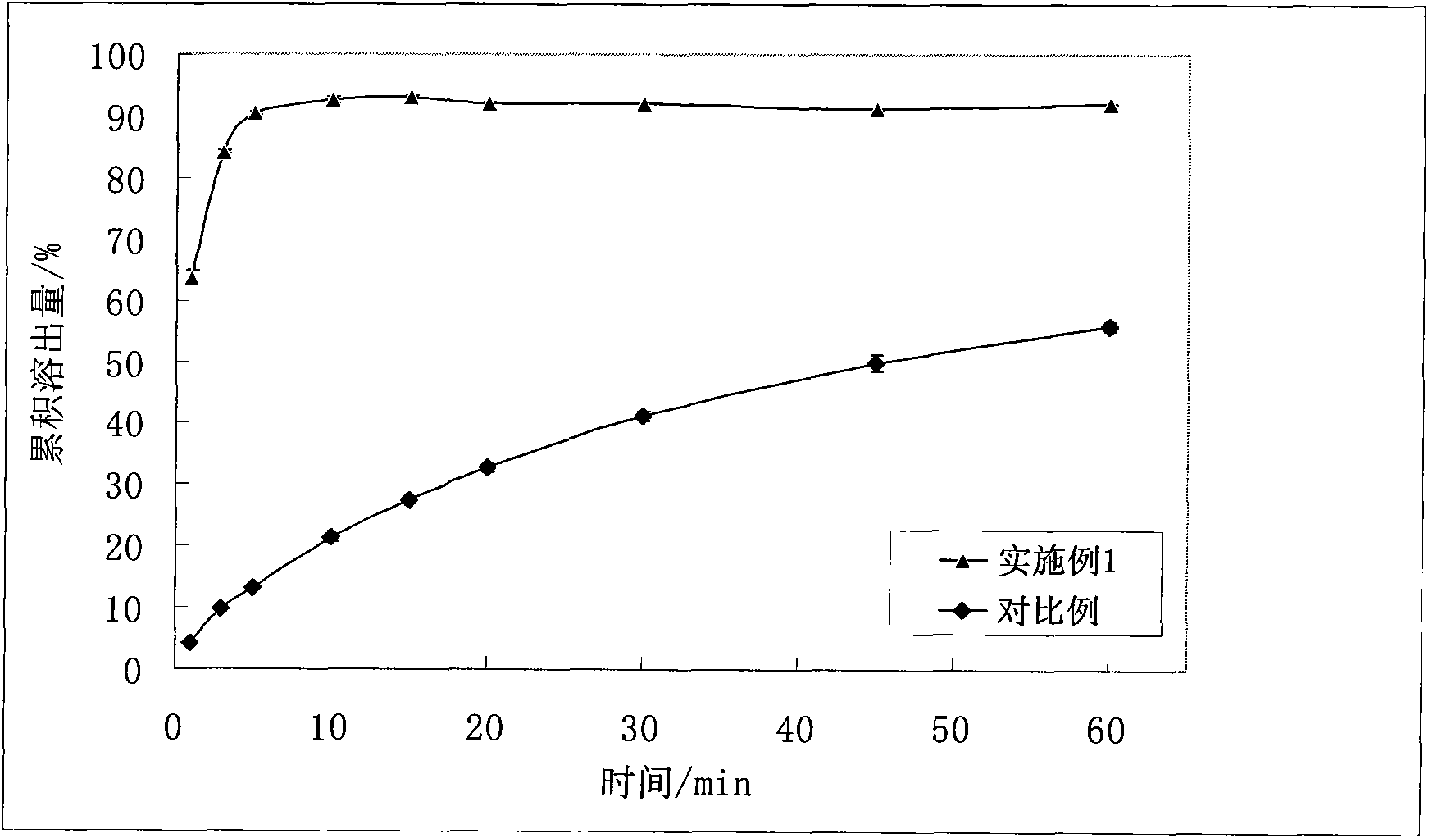
A kind of gefitinib dispersible tablet and its preparation method and application
ActiveCN102266300AImprove bioavailabilityPromote dissolutionOrganic active ingredientsPill deliveryMedicineActive agent
The invention discloses a gefitinib dispersible tablet, a preparation method and an application thereof. The gefitinib dispersible tablet of the invention comprises the following components by weight: 10-65% of gefitinib, 1-30% of fillers, 10-50% of disintegrants, 1-60% of acidifiers, 0.1-20% of adhesives, and 0.1-30% of lubricants and glidants. According to the invention, gefitinib is wrapped bythe acidifier or gefitinib and the acidifier are wrapped with each other so as to reach the embedding effect. Compared with commercially available common tablets, the gefitinib dispersible tablet of the invention does not contain surfactants, has good dissolvability, dispersibility and disintegrability, and can be disintegrated completely within one minute. The gefitinib dispersible tablet prepared by the method of the invention has a high dissolution rate, good bioavailability, rapid distribution in vivo, and stable quality, and the preparation method is simple and practical, and is applicable to industrial production.
Owner:GUANGDONG PHARMA UNIV
Apixaban tablet
InactiveCN102908324ASmall particle sizeIncrease dissolution rateOrganic active ingredientsPill deliveryMedicineDissolution
The invention discloses an Apixaban tablet, which comprises the following components in parts: 1 part of Apixaban, 10-20 parts of water-soluble carrier material, 30-60 parts of filling agent, 5-10 parts of disintegrating agent and 5-10 parts of glidant. The Apixaban tablet provided by the invention improves the deficiencies in the prior art that the extracorporeal dissolution rate and the bioavailability is low. The preparation method of the Apixaban tablet disclosed by the invention is simple to operate, has good reproducibility, and is suitable for large-scale production. The Apixaban tablet is mainly used for the anti-thrombosis purpose.
Owner:NANJING ZENKOM PHARMA
Solid waste red mud microfibers and production method thereof
InactiveCN101519272ALow costSave natural resourcesGlass making apparatusSolid waste disposalRed mudWhitening Agents
The invention relates to a technical method for producing microfibers by using industrial waste red mud, which comprises the following steps: adding a reactant in the industrial waste red mud to carry out a neutralization reaction; then adding a preparation agent, a glidant and a whitening agent and stirring the agents; after being stirred, feeding materials in a tube-type high-temperature melting furnace continuously; releasing the materials after melting; outputting fused masses continuously to pass through a high-speed roller to carry out centrifugal rejection wire; and splashing a cooling agent and a surface coating agent to produce the red mud microfibers. The production method is suitable for industrial production. The technical method endows the solid waste red mud with the functionality of the microfibers. The technical method can implement various applications, such as pulp production, the preparation of sound and heat insulating materials and energy-saving walling materials, the production of the indoor suspended ceiling of light fire-retardant construction and industrial filtering materials, and the like, and has significant economic value and social meaning.
Owner:EAST CHINA UNIV OF SCI & TECH
Dispersible tablet of colloid petcin
InactiveCN1634132AHeavy metal active ingredientsDigestive systemLow-substituted hydroxypropylcelluloseCross-linked polyethylene
The invention discloses a dispersible tablet of colloid pectin, which comprises (by weight ratio, in mg) colloid pectine bismuth 25-100 (by bismuth), filling agent 60-400, crumbling agent 42-280, flow aid 1-6, lubricant 0.25-5, the filling agent can be selected from lactose, white dextrine, pregelatine starch or / and starch. The crumbling agent can be selected from cross bonding polyvinylpyrrolidone, crystalline cellulose, cross-linked sodium carboxymethylstarch, low substituted methylcellulose propylene glycol ether, sodium carboxymethylstarch, the glidant can be selected from miropowdered silica gel, the lubricant can be selected from magnesium stearate or / and talcum powder.
Owner:HUNAN WARRANT PHARMA
Method for producing high-flowability pregelatinized starch
InactiveCN102134281AImprove liquidityLoose structurePharmaceutical non-active ingredientsFood preparationQuality controlMixed materials
The invention provides high-flowability pregelatinized starch and a method for preparing the same by mechanical activation, which belong to the technical field of starch modification processing. The method comprises the following steps: uniformly mixing starch and a certain amount of flow aid in a high-speed mixer uniformly, pre-drying the starch by using heat generated in a high-speed mixing process, and controlling the water content in the starch to be lower than 15 percent; and placing the uniformly mixed materials in a ball mill containing a milling medium, well regulating the temperature for constant-temperature dissolution in water, performing mechanical activation for a certain time period, taking the starch out, and sieving the starch with a 80 to 100-mesh sieve. The method has the advantages of simple process and equipment, small investment, environmentally-friendly process, low production cost, easy product quality control and the like. The prepared product has the characteristics of high flowability, loose structure, uniform particles and precision control over pregelatinization degree and can be widely used in industrials and fields of foods, pharmaceuticals, chemicals and the like.
Owner:GUANGXI UNIV +2
Stable pharmaceutical formulation comprising HMC-CoA reductase inhibitor
Lovastatin, pravastatin, simvastatin, mevastatin, atorvastatin, and derivatives and analogs thereof are known as HMG-CoA reductase inhibitors and are used as antihypercholesterolemic agents. The majority of them are produced by fermentation using microorganisms of different species identified as species belonging to Aspergillus, Monascus, Nocardia, Amycolatopsis, Mucor or Penicillium genus, and some are obtained by treating the fermentation products using the methods of chemical synthesis or they are the products of total chemical synthesis. The aforementioned active substances may be destabilised by the environmental factors, their degradation may also be accelerated by interactions with other pharmaceutical ingredients, such as fillers, binders, lubricants, glidants and disintegrating agents, therefore the pharmaceutical ingredients and the process for preparation of the pharmaceutical formulation should be meticulously chosen to avoid the aforementioned undesired interactions and reactions. The present invention relates to a stable solid pharmaceutical formulation for the treatment of hypercholesterolemia and hyperlipidemia. More precisely, the present invention relates to the new stable solid pharmaceutical formulation containing as an active ingredient a HMG-CoA reductase inhibitor, such as atorvastatin, pravastatin, fluvastatin and cerivastatin or pharmaceutically acceptable salts thereof.
Owner:LEK PHARMA & CHEM
Process for producing diabecron sustained release tablet
InactiveCN101428007AImprove bioavailabilityOrganic active ingredientsMetabolism disorderCelluloseMetformin Hydrochloride
The invention relates to a preparation method for metformin hydrochloride sustained-release tablets. The preparation method comprises the following steps: firstly, dissolving ethyl cellulose into ethanol solution, blending the ethyl cellulose with metformin hydrochloride to produce granules, and drying and sieving the granules at 45 to 55 DEG C; secondly, dissolving hydroxypropyl methylcellulose into ethanol solution to produce bonding agent, blending the granules prepared in step one, hydroxypropyl methylcellulose, ethyl cellulose and pore forming agent, adding the bonding agent, performing sieving and granulation, adding hydroxypropyl methylcellulose and lubricant, evenly blending, detecting the content, determining the tablet weight, and pressing into plain tablets; thirdly, dissolving film forming agent, plasticizer, masking agent and glidant for hydroxypropyl methylcellulose and ethyl cellulose into ethanol solution, completely stirring for more than 1 hour to produce sustained-release preparation coating solution, and finally obtaining the metformin hydrochloride sustained-release tablets by performing film coating to the plain tablets. The sustained-release preparation not only embodies the main characteristics of the sustained-release preparation for continuous sustained release of drug, but also presents high bioavailability.
Owner:上海天赐福生物工程有限公司
Jianweixiaoshi orally disintegrating tablets and preparation method
ActiveCN101185733AAvoid disadvantagesEasy to takeDigestive systemPill deliveryOrally disintegrating tabletTangerine Peel
The invention relates to an orally disintegrating tablet which is prepared by the Chinese medicinal materials of tangerine peel, common yam rhizome, heterophylly falsestarwort root, stir-fried malt and hawthorn fruit; the invention takes the Chinese medicinal materials as raw materials and uses certain proportion of pharmaceutically excipients for the preparation; the invention is prepared by the raw materials with the following weight percentages: 5 to 20 percent traditional Chinese medicine extract, 40 to 80 percent filler, 5 to 30 disintegrating agent, 0.5 to 5 percent flavoring agent, 0.5 to 3 percent glidant and 0.3 to 2 percent lubricant; the disintegrating tablet can be disintegrated in the oral cavity rapidly, the taste is good, so the invention is especially applicable to the elderly people, children and the patients with the difficulty in swallowing for administration.
Owner:JIANGZHONG PHARMA
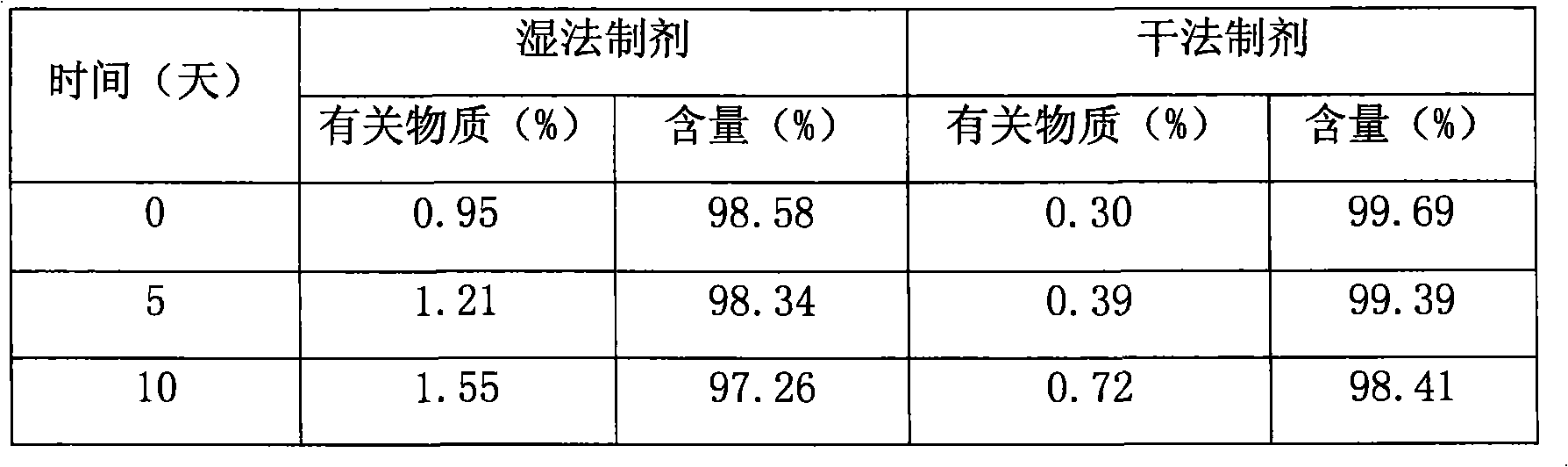
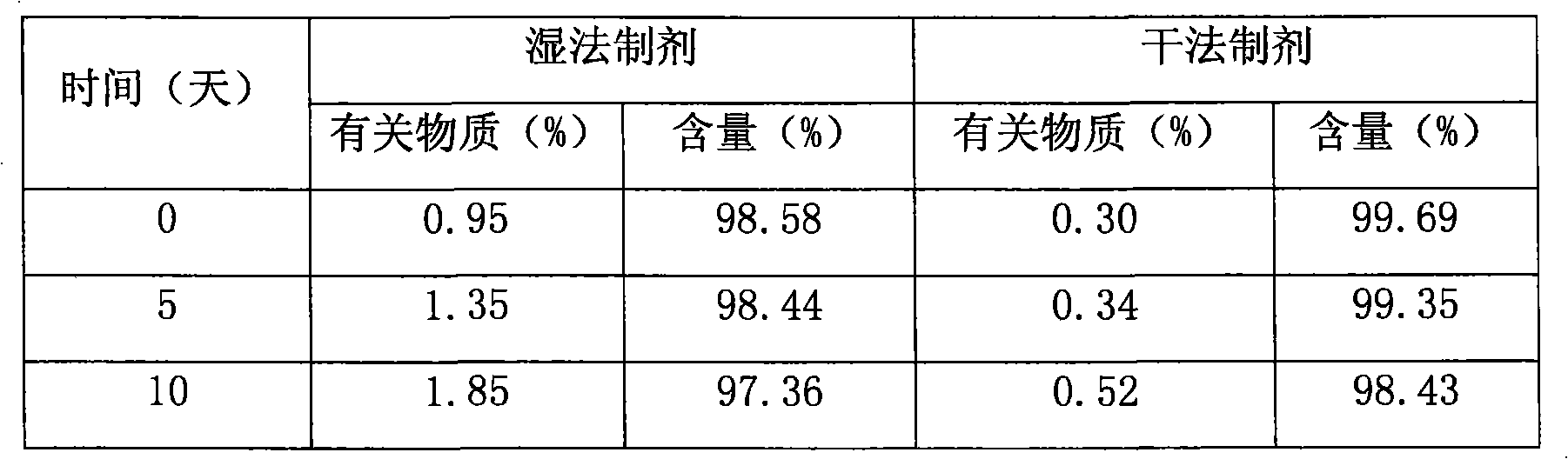
Pharmaceutical compositions of (R)-1-(2,2-difluorobenzo[D][1,3]dioxo1-5-y1)-N-(1-(2,3-dihydroxypropy1)-6-fluoro-2-(1-hydroxy-2-methylpropan-2-y1)-1H-indol-5-y1) cyclopropanecarbox-amide and administration thereof
A pharmaceutical composition comprising Compound 1, (R)-1-(2,2-difluorobenzo[d][1,3]dioxol-5-yl)-N-(1-(2,3-dihydroxypropyl)-6-fluoro-2-(1-hydroxy-2-methylpropan-2-yl)-1H-indol-5-yl)cyclopropanecarboxamide, and at least one excipient selected from: a filler, a disintegrant, a surfactant, a glidant and a lubricant, the composition being suitable for oral administration to a patient in need thereof to treat a CFTR mediated disease such as Cystic Fibrosis. Methods for treating a patient in need thereof include administering the pharmaceutical composition of Compound 1 are also disclosed.
Owner:VERTEX PHARMA INC
Novel granulation process
One of the objects of the invention relates to a pharmaceutical composition in the form of a granulate, wherein the granulates comprises an active pharmaceutical ingredient (API) having a poor water solubility intimately associated with at least one pharmaceutically acceptable sugar, and optionally or preferably at least one pharmaceutically acceptable excipient other than the at least one pharmaceutically acceptable sugar, wherein the active pharmaceutically ingredient has a water solubility less than about 20 mg / ml. The at least one pharmaceutically acceptable excipient other than the at least one pharmaceutically acceptable sugar is selected from the group consisting of disintegrants, wetting agents, diluents, binders, lubricants, glidants, coloring agents and flavoring agents. The at least one pharmaceutically acceptable sugar is preferably selected from pyranosyl pyranoses, such as lactose. Another object of the invention relates to a process for preparing a pharmaceutical granulate, comprising (a) combining an API having poor water solubility with a solution comprising at least one pharmaceutically acceptable sugar, for example a pyranosyl pyranose such as lactose, and a solvent, and optionally at least one pharmaceutically acceptable excipient other than the at least one pharmaceutically acceptable sugar to form a combined mixture; (b) drying the combined mixture of step (a); and (c) comminuting the product of step (b) to obtain the granulate.
Owner:TEVA PHARM USA INC
Drug delivery composition
A drug delivery composition that comprises extruded spheroids. The spheroids comprise at least one active pharmaceutical ingredient; at least one extrusion-spheronization aid; at least one superdisintegrant; and at least one glidant, at least one lubricant, and / or at least one oil. The spheroids may also be coated. In a further aspect, a drug delivery composition that comprises coated spheroids that have inert spheroids and at least one coating for the spheroids. The coating comprises at least one active pharmaceutical ingredient and at least one superdisintegrant.
Owner:INTELLIPHARMACEUTICS
Pharmaceutical combination containing Mosapride citrate and method of preparing the same
ActiveCN101273973ASolve technical problemsQuality improvementOrganic active ingredientsDigestive systemMedicineAdhesive
The invention relates to a pharmaceutical composition containing mosapride citrate which is applicable to the usage of direct powder compression for preparation and a preparation method thereof. The pharmaceutical composition further contains a disintegrant, a thinner, a lubricant, a glidant and an adhesive in addition to the active component of the mosapride citrate. The pharmaceutical composition is applicable to the preparation of dispersible tablets which can be fully disintegrated in 2 minutes in water under the temperature of 19 DEG C to 21 DEG C and pass through a No. 2 screen, the hardness is 8 to 11Kg, and the dissolution rate is in line with the relevant provisions, thus solving the problems of higher relevant substances, instable quality and difficult packaging of the mosapride citrate dispersible tablets prepared by wet granulation.
Owner:CHENGDU KANGHONG PHARMA GRP
Apparatus and process for treating an aqueous solution containing chemical contaminants
Owner:MOLYCORP MINERALS
Novel pharmaceutical granulate
One of the objects of the invention relates to a pharmaceutical composition in the form of a granulate, wherein the granulates comprises an active pharmaceutical ingredient (API) having a poor water solubility intimately associated with at least one pharmaceutically acceptable sugar, and optionally or preferably at least one pharmaceutically acceptable excipient other than the at least one pharmaceutically acceptable sugar, wherein the active pharmaceutically ingredient has a water solubility less than about 20 mg / ml. The at least one pharmaceutically acceptable excipient other than the at least one pharmaceutically acceptable sugar is selected from the group consisting of disintegrants, wetting agents, diluents, binders, lubricants, glidants, coloring agents and flavoring agents. The at least one pharmaceutically acceptable sugar is preferably selected from pyranosyl pyranoses, such as lactose. Another object of the invention relates to a process for preparing a pharmaceutical granulate, comprising (a) combining an API having poor water solubility with a solution comprising at least one pharmaceutically acceptable sugar, for example a pyranosyl pyranose such as lactose, and a solvent, and optionally at least one pharmaceutically acceptable excipient other than the at least one pharmaceutically acceptable sugar to form a combined mixture; (b) drying the combined mixture of step (a); and (c) comminuting the product of step (b) to obtain the granulate.
Owner:TEVA PHARM USA INC
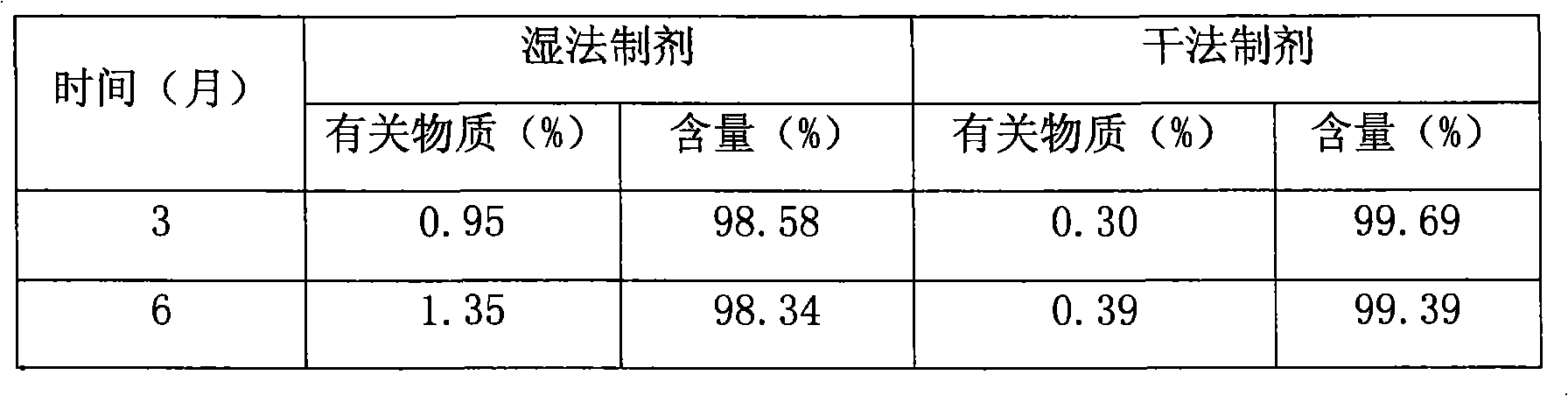
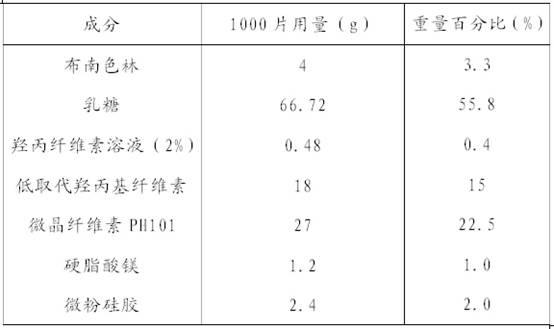
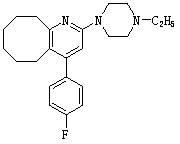
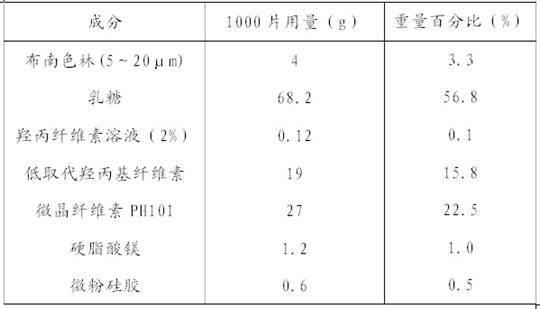
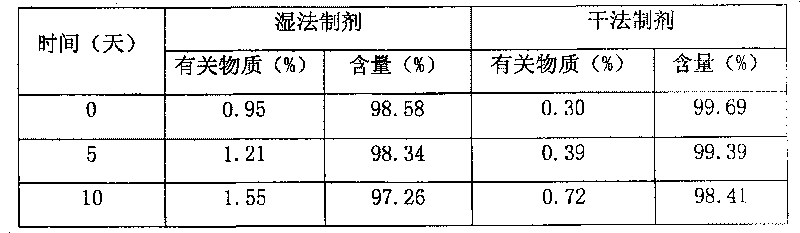
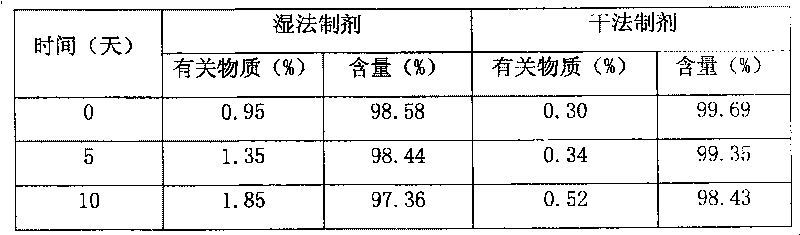
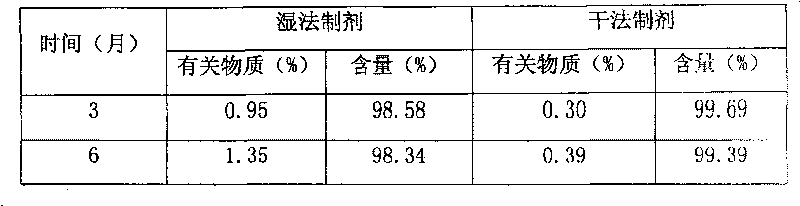
Blonanserin-containing medicinal composition and preparation method thereof
InactiveCN102078321ASolve solubilitySuitable for industrial productionOrganic active ingredientsNervous disorderBlonanserinAdhesive
The invention discloses a blonanserin-containing medicinal composition, which consists of the following components in percentage by weight: 3.3 to 20 percent of blonanserin, 60 to 85 percent of filler, 5 to 25 percent of disintegrating agent, 2 to 6 percent of adhesive, 0.25 to 5 percent of lubricant and 0.5 to 2 percent of fluidizer. The medicinal composition has the dissolution rate of over 75 percent in a dissolution medium with the pH of 4.0 to 6.0 so as to effectively solve the problem of dissolution rate of blonanserin medicaments.
Owner:泰州万全医药科技有限公司
Pharmaceutical combination containing Mosapride citrate
ActiveCN101273973BSolve the problem of highFix stability issuesOrganic active ingredientsDigestive systemActive componentAdhesive
The invention relates to a pharmaceutical composition containing mosapride citrate which is applicable to the usage of direct powder compression for preparation and a preparation method thereof. The pharmaceutical composition further contains a disintegrant, a thinner, a lubricant, a glidant and an adhesive in addition to the active component of the mosapride citrate. The pharmaceutical composition is applicable to the preparation of dispersible tablets which can be fully disintegrated in 2 minutes in water under the temperature of 19 DEG C to 21 DEG C and pass through a No. 2 screen, the hardness is 8 to 11Kg, and the dissolution rate is in line with the relevant provisions, thus solving the problems of higher relevant substances, instable quality and difficult packaging of the mosapridecitrate dispersible tablets prepared by wet granulation.
Owner:CHENGDU KANGHONG PHARMA GRP
Oral slow/controlled-release preparation containing febuxostat and preparation method thereof
ActiveCN101773498AImprove securityEffective plasma concentrationOrganic active ingredientsSkeletal disorderBlood concentrationBurst effect
The invention provides an oral slow / controlled-release preparation containing febuxostat and a preparation method thereof, which prepare the febuxostat into the long-acting oral slow / controlled-release preparation and can solve the problem that the incidence of an adverse reaction is increased because of the quicker dissolving-out and the burst effect of a common preparation existing in the prior art. The invention has the technical scheme that the oral slow / controlled-release preparation containing the febuxostat comprises the following components by weight percent: 5 to 60 percent of febuxostat, 10 to 50 percent of slow / controlled-release material, 20 to 80 percent of filling auxiliary material, 0.3 to 20 percent of adhesive and 0.1 to 7 percent of lubricant or glidant. Compared with a common quick-release preparation, the slow / controlled-release preparation can keep the effective and stable blood concentration for a longer time, avoids the burst effect of the quick-release preparation, lowers the incidence of the adverse reaction and enhances the application safety.
Owner:QINGDAO HUANGHAI PHARM CO LTD
Valsartan dispersible tablet and preparation method thereof
ActiveCN101167723AGood curative effectLittle side effectsPill deliveryMacromolecular non-active ingredientsValsartanMedicine
The invention provides valsartan dispersible tablets and a preparation method thereof, which contain effective doses of valsartan and pharmaceutical adjuvants, which include disintegrants, diluents, binders, lubricants, glidants and surface Active agent, based on 100 parts of valsartan, the dosage of disintegrating agent is 2-50 parts, the dosage of diluent is 10-150 parts, the dosage of binder is 2-25 parts, and the dosage of lubricant is 0.5-20 parts. The dosage of the liquid agent is 0.2-10 parts, and the surfactant is 0.1-2.5 parts; the present invention also provides the preparation method of the valsartan dispersible tablet. Compared with other dosage forms, the valsartan dispersible tablet has a good dispersion state , Short disintegration time, rapid drug dissolution, convenient administration, low production cost, no need for special equipment, convenient and stable carrying and transportation, etc.
Owner:HAINAN HUALON PHARM
Clopidogrel bisulfate tablet and preparation method thereof
ActiveCN102058550AImprove stabilitySolve problems in large-scale preparationOrganic active ingredientsPharmaceutical non-active ingredientsMedicineMedical prescription
The invention relates to a clopidogrel bisulfate tablet and a preparation method thereof. Each clopidogrel bisulfate tablet comprises 25-75 mg of clopidogrel bisulfate measured in the term of clopidogre, fillers, disintegrants, solubilizers, flow aids and lubricants. In the invention, the method of adding granular microcrystalline cellulose and aerosil through airflow crushing disposal to a prescription is adopted, therefore, the process of dry granulating is simple and feasible, reproducibility is good, prepared products have high stability, and the requirement of continuous large-scale production can be satisfied.
Owner:JIANGSU YABANG QIANGSHENG PHARMA
Ebstine solid oral preparation and its preparing method
ActiveCN101161233AImprove solubilityIncrease dissolution ratePill deliveryCapsule deliveryOrganic acidOrally disintegrating tablet
An ebastine solid oral preparation and preparation method are disclosed in the present invention. Made of ebastine, ethanol solution, cosolvent, diluent or filler, disintegrating agent, taste correcting agent, glidants or dispersing agent, effervescing agent, lubricant in proportion, its step is that firstly adding ebastine into ethanol solution, and then adding cosolvent organic acid, leading it to dissolve entirely; secondly mixing the diluent or filler, disintegrating agent, taste correcting agent, effervescing agent with dispersing agent, to obtain a mixer; third mixing the front two, preparing soft material, preparing granules, then adding disintegrating agent, effervescing agent, taste correcting agent, glidants and lubricant, tabletting or filling capsules, packing to obtain a solid oral preparation. The present invention preparing oral disintegrable tablet, dispersing tablet have short disintegrating time, good dispersing state. The obtained oral disintegrable tablet, dispersing tablet and chewing tablet have good taste and mouth feel, the obtained solid oral preparations dissolving out are all rapid. The method is easy, simple operation, without needing special equipment, and lower production cost.
Owner:HUBEI LIYI PHARM TECH CO LTD
Mifepristone medicinal preparation and preparation method thereof
InactiveCN101455671APromote dissolutionIncrease miscarriage rateOrganic active ingredientsSexual disorderMedicineAdhesive
The invention relates to a pharmaceutical preparation of mifepristone and preparation method thereof. The pharmaceutical preparation of mifepristone is prepared by the step of mixing the raw materials as follows according to mass ratio: 5-30 % of mifepristone particles, 10-40% of microcrystalline cellulose, 2-10% of adhesive, 20-40% of starch, 20-40% of dextrin, 05.-2% of lubricant, and 0.5-1% of glidant. The invention can improve dissolution and bioavailability of the mifepristone preparation having obviously increased action against early pregnancy of animals, comparatively simple preparation method, and low manufacturing cost.
Owner:HUBEI GEDIAN HUMANWELL PHARMACEUTICAL CO LTD
Lightweight component in hybrid construction
InactiveCN101735602AImprove bending abilityHigh torsional stabilitySeat framesSuperstructure subunitsThermoplasticEngineering
The present invention relates to lightweight components in hybrid construction, also referred to as hybrid components or hollow-chamber lightweight components, comprising a base body which is made by aluminum of which the surface is preprocessed and is reinforced by means of thermoplastics and is suitable for the transmission of high mechanical loads, wherein, the thermoplastics is added with a special glidant for improving physical property.
Owner:LANXESS DEUTDCHLAND GMBH
Preparation method of low melting point drug solid dispersion
InactiveCN101961306AReduce usageSmooth releasePharmaceutical delivery mechanismMacromolecular non-active ingredientsAdhesiveSolvent
The invention belongs to the pharmaceutical field and relates to a preparation method of a low melting point drug solid dispersion. The preparation method comprises the following steps: crushing pharmaceutical raw materials and auxiliary materials, passing through a 60-120-mesh sieve, then uniformly mixing, sealing, placing at the constant temperature of 45-140 DEG C for 0.5-4h, cooling to room temperature and obtaining the solid dispersion, wherein the pharmaceutical raw materials are insoluble drugs with the melting points in the range of 45-130 DEG C; and the auxiliary materials comprise a filling agent, a disintegrant, a dry adhesive, a lubricating agent, a glidant and a surfactant. The preparation method has the following advantages: simple operation, low equipment requirements and no solvent residue; the prepared solid dispersion is easy to crush and can be prepared into quick-release tablets, capsules, power or slow-release tablets; a quick-release preparation prepared through the preparation method has high drug loading, fast drug dissolution speed and high dissolution rate; and the prepared slow-release tablets have good uniformity and stable drug release.
Owner:BEIJING UNIV OF CHEM TECH
Lightweight component of hybrid design
InactiveUS20100173125A1Increase resistanceImprove stabilitySynthetic resin layered productsSeat framesThermoplasticEngineering
The present invention relates to lightweight components of hybrid design, also termed hybrid component or hollow-chamber lightweight component, composed of a parent body which is composed of surface-pretreated aluminium and which is reinforced by means of thermoplastics and is suitable for the transmission of high mechanical loads, where particular flow aids are added to the thermoplastic in order to improve its physical properties.
Owner:LANXESS DEUTDCHLAND GMBH
Brass flux-cored brazing filler metal with reducing agents and flow aids and preparation method thereof
InactiveCN104907722AImprove wettabilityImprove liquidityWelding/cutting media/materialsSoldering mediaIngot castingWeld seam
The invention discloses brass flux-cored brazing filler metal with reducing agents and flow aids. The brass flux-cored brazing filler metal comprises a brazing filler metal pipe with a spiral lap seam, the brazing filler metal pipe is formed by rotatably rolling strip-shaped brass base brazing filler metal, and a welding wire and a flux core prepared from brass brazing flux, the reducing agents and the flow aids are wrapped by the brazing filler metal pipe. In the preparation process, the brass base brazing filler metal is molten according to a conventional method for ingot casting and is processed into strip-shaped brazing filler metal, then the brazing filler metal is rotatably rolled to the brazing filler metal pipe with the spiral lap seam, and in the rolling process, the welding wire and the flux core are added into the brazing filler metal pipe. A brass flux-cored brazing filler metal wire or bar or brazing filler metal ring are manufactured through rolling or drawing. The brass flux-cored brazing filler metal has the advantages that Sn, Ni, Si and other elements are added into the brazing filler metal in the form of the welding wire, when the content of alloy elements in the brass brazing filler metal is low, the excellent processing performance can still be kept, the brazing filler metal can achieve good wettability and fluidity, meanwhile, weld strength, low-temperature impact toughness and the anti-cracking ability are improved, and ductile-brittle transition temperature is lowered.
Owner:ZHENGZHOU RES INST OF MECHANICAL ENG CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com
![Pharmaceutical compositions of (r)-1-(2,2-difluorobenzo[d] [1,3]dioxol-5-yl)-n-(1-(2,3-dihydroxypropyl)-6-fluoro-2-(1-hydroxy-2-methylpropan-2-yl)-1h-indol-5-yl) cyclopropanecarboxamide and administration thereof Pharmaceutical compositions of (r)-1-(2,2-difluorobenzo[d] [1,3]dioxol-5-yl)-n-(1-(2,3-dihydroxypropyl)-6-fluoro-2-(1-hydroxy-2-methylpropan-2-yl)-1h-indol-5-yl) cyclopropanecarboxamide and administration thereof](https://images-eureka.patsnap.com/patent_img/9bf554c5-b898-43aa-b4e5-83e553189b5a/US20120046330A1-20120223-D00001.png)
![Pharmaceutical compositions of (r)-1-(2,2-difluorobenzo[d] [1,3]dioxol-5-yl)-n-(1-(2,3-dihydroxypropyl)-6-fluoro-2-(1-hydroxy-2-methylpropan-2-yl)-1h-indol-5-yl) cyclopropanecarboxamide and administration thereof Pharmaceutical compositions of (r)-1-(2,2-difluorobenzo[d] [1,3]dioxol-5-yl)-n-(1-(2,3-dihydroxypropyl)-6-fluoro-2-(1-hydroxy-2-methylpropan-2-yl)-1h-indol-5-yl) cyclopropanecarboxamide and administration thereof](https://images-eureka.patsnap.com/patent_img/9bf554c5-b898-43aa-b4e5-83e553189b5a/US20120046330A1-20120223-D00002.png)
![Pharmaceutical compositions of (r)-1-(2,2-difluorobenzo[d] [1,3]dioxol-5-yl)-n-(1-(2,3-dihydroxypropyl)-6-fluoro-2-(1-hydroxy-2-methylpropan-2-yl)-1h-indol-5-yl) cyclopropanecarboxamide and administration thereof Pharmaceutical compositions of (r)-1-(2,2-difluorobenzo[d] [1,3]dioxol-5-yl)-n-(1-(2,3-dihydroxypropyl)-6-fluoro-2-(1-hydroxy-2-methylpropan-2-yl)-1h-indol-5-yl) cyclopropanecarboxamide and administration thereof](https://images-eureka.patsnap.com/patent_img/9bf554c5-b898-43aa-b4e5-83e553189b5a/US20120046330A1-20120223-D00003.png)
![Pharmaceutical compositions of (R)-1-(2,2-difluorobenzo[D][1,3]dioxol-5-yl)-N-(1-(2,3-dihydroxypropyl)-6-fluoro-2-(1-hydroxy-2-methylpropan-2-yl)-1H-indol-5-yl)cyclopropanecarboxamide and administration thereof Pharmaceutical compositions of (R)-1-(2,2-difluorobenzo[D][1,3]dioxol-5-yl)-N-(1-(2,3-dihydroxypropyl)-6-fluoro-2-(1-hydroxy-2-methylpropan-2-yl)-1H-indol-5-yl)cyclopropanecarboxamide and administration thereof](https://images-eureka.patsnap.com/patent_img/035f9415-566e-4b07-8bb0-7cbc2e5f286b/US09012496-20150421-D00001.PNG)
![Pharmaceutical compositions of (R)-1-(2,2-difluorobenzo[D][1,3]dioxol-5-yl)-N-(1-(2,3-dihydroxypropyl)-6-fluoro-2-(1-hydroxy-2-methylpropan-2-yl)-1H-indol-5-yl)cyclopropanecarboxamide and administration thereof Pharmaceutical compositions of (R)-1-(2,2-difluorobenzo[D][1,3]dioxol-5-yl)-N-(1-(2,3-dihydroxypropyl)-6-fluoro-2-(1-hydroxy-2-methylpropan-2-yl)-1H-indol-5-yl)cyclopropanecarboxamide and administration thereof](https://images-eureka.patsnap.com/patent_img/035f9415-566e-4b07-8bb0-7cbc2e5f286b/US09012496-20150421-D00002.PNG)
![Pharmaceutical compositions of (R)-1-(2,2-difluorobenzo[D][1,3]dioxol-5-yl)-N-(1-(2,3-dihydroxypropyl)-6-fluoro-2-(1-hydroxy-2-methylpropan-2-yl)-1H-indol-5-yl)cyclopropanecarboxamide and administration thereof Pharmaceutical compositions of (R)-1-(2,2-difluorobenzo[D][1,3]dioxol-5-yl)-N-(1-(2,3-dihydroxypropyl)-6-fluoro-2-(1-hydroxy-2-methylpropan-2-yl)-1H-indol-5-yl)cyclopropanecarboxamide and administration thereof](https://images-eureka.patsnap.com/patent_img/035f9415-566e-4b07-8bb0-7cbc2e5f286b/US09012496-20150421-D00003.PNG)

![Pharmaceutical compositions of (R)-1-(2,2-difluorobenzo[D][1,3]dioxo1-5-y1)-N-(1-(2,3-dihydroxypropy1)-6-fluoro-2-(1-hydroxy-2-methylpropan-2-y1)-1H-indol-5-y1) cyclopropanecarbox-amide and administration thereof Pharmaceutical compositions of (R)-1-(2,2-difluorobenzo[D][1,3]dioxo1-5-y1)-N-(1-(2,3-dihydroxypropy1)-6-fluoro-2-(1-hydroxy-2-methylpropan-2-y1)-1H-indol-5-y1) cyclopropanecarbox-amide and administration thereof](https://images-eureka.patsnap.com/patent_img/880710ea-c05a-427d-8935-c879e8a08209/US10058546-D00001.png)
![Pharmaceutical compositions of (R)-1-(2,2-difluorobenzo[D][1,3]dioxo1-5-y1)-N-(1-(2,3-dihydroxypropy1)-6-fluoro-2-(1-hydroxy-2-methylpropan-2-y1)-1H-indol-5-y1) cyclopropanecarbox-amide and administration thereof Pharmaceutical compositions of (R)-1-(2,2-difluorobenzo[D][1,3]dioxo1-5-y1)-N-(1-(2,3-dihydroxypropy1)-6-fluoro-2-(1-hydroxy-2-methylpropan-2-y1)-1H-indol-5-y1) cyclopropanecarbox-amide and administration thereof](https://images-eureka.patsnap.com/patent_img/880710ea-c05a-427d-8935-c879e8a08209/US10058546-D00002.png)
![Pharmaceutical compositions of (R)-1-(2,2-difluorobenzo[D][1,3]dioxo1-5-y1)-N-(1-(2,3-dihydroxypropy1)-6-fluoro-2-(1-hydroxy-2-methylpropan-2-y1)-1H-indol-5-y1) cyclopropanecarbox-amide and administration thereof Pharmaceutical compositions of (R)-1-(2,2-difluorobenzo[D][1,3]dioxo1-5-y1)-N-(1-(2,3-dihydroxypropy1)-6-fluoro-2-(1-hydroxy-2-methylpropan-2-y1)-1H-indol-5-y1) cyclopropanecarbox-amide and administration thereof](https://images-eureka.patsnap.com/patent_img/880710ea-c05a-427d-8935-c879e8a08209/US10058546-D00003.png)